- State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, National Medical Center for Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
Hepatocellular carcinoma (HCC) is a common primary liver cancer with ∼750,000 annual incidence rates globally. PGE2, usually known as a pro-inflammatory cytokine, is over-expressed in various human malignancies including HCC. PGE2 binds to EP receptors in HCC cells to influence tumorigenesis or enhance tumor progression through multiple pathways such as EP1-PKC-MAPK, EP2-PKA-GSK3β, and EP4-PKA-CREB. In the progression of hepatocellular carcinoma, PGE2 can promote the proliferation and migration of liver cancer cells by affecting hepatocytes directly and the tumor microenvironment (TME) through ERK/COX-2/PGE2 signal pathway in hepatic stellate cells (HSC). For the treatment of hepatocellular carcinoma, there are drugs such as T7 peptide and EP1 antagonist ONO-8711 targeting Cox-2/PGE2 axis to inhibit tumor progression. In conclusion, PGE2 has been shown to be a traditional target with pleiotropic effects in tumorigenesis and progression of HCC that could be used to develop a new potential clinical impact. For the treatment study focusing on the COX-PGE2 axis, the exclusive usage of non-steroidal anti-inflammatory agents (NSAIDs) or COX-2-inhibitors may be replaced by a combination of selective EP antagonists and traditional anti-tumoral drugs to alleviate severe side effects and achieve better outcomes.
Introduction
Hepatocellular carcinoma (HCC) accounts for 90% of primary liver cancers with an annual incidence rate of ∼750,000, ranking as the fifth most common malignant tumor worldwide in men and seventh in women (Ferlay et al., 2010; Hepatocellular carcinoma, 2016). Due to complications such as gastrointestinal bleeding, liver failure, cachexia, and tumor rupture, HCC patients always have a short survival period (Leng et al., 2003). Surgical resection is considered as the best treatment method, but advanced-stage HCC, which is unqualified for surgery, remains a challenge. Compared to the natural history of advanced-stage liver cancer, systemic therapies based on tyrosine kinases (TKIs) and immune checkpoint inhibitors (ICIs) have improved patients’ outcomes, and prolonged life expectancy significantly in recent years (Yang et al., 2020). But there is still great demand for the new drugs due to the variability of hepatocellular carcinoma. Anti-inflammation is an important strategy for treatment as inflammation is a key progress in the tumorigenesis and progression of tumor. PGE2, usually known as a pro-inflammatory cytokine, is over-expressed in various human malignancies (Wang and Dubois, 2010). Inhibiting PGE2 synthesis has been an important anti-inflammatory strategy for more than 100 years (Vane and Botting, 2003). Non-steroidal anti-inflammatory agents (NSAIDs), such as aspirin, mainly take effect by inhibiting cyclooxygenase (COX) while prostaglandin E2 (PGE2) is the primary metabolic product in inflammation. Epidemiological observation of several common cancers shows a positive correlation between aspirin and lower death rate (Rothwell et al., 2011; Lin et al., 2018; Ma and Brusselaers, 2018; Cho et al., 2021), indicating that reduction of PGE2 may prevent solid-organ cancers such as HCC (Wu, 2006).
Biosynthesis of Prostaglandin E2
Prostaglandins (PGs) belong to the eicosanoid family and are synthesized by almost all kinds of cells in the human body, in which PGE2 is the most abundant (Park et al., 2006). When cells are stimulated by growth factors, cytokines, inflammatory mediators, or various cancer-promoting factors, and the expression of PGE2 can be significantly increased. The synthesis of PGE2 is derived from a polyunsaturated fatty acid Arachidonic Acid (AA), which is released from cell membrane by phospholipases A2 (PLA2). Then AA is oxidized to the prostaglandin G2 (PGG2) and turns into unstable intermediate prostaglandin H2 (PGH2) under the action of COX. PGH2 will be converted into PGE2 rapidly by the help of three distinct synthases: microsomal prostaglandin E2 synthase-1 (mPGES-1), microsomal prostaglandin E2 synthase-2 (mPGES-2), and cytosolic prostaglandin E2 synthase (cPGES). COX is the key enzyme in the AA metabolism pathway. COX has two kinds of isoforms, namely COX-1 and COX-2, which play different roles in physiological or pathological conditions. Generally, COX-1 is continuously expressed in most tissues, controlling physiological functions such as regulating vascular homeostasis or cellular responses to hormone stimulation. COX-2 is mainly expressed after growth factor action or inflammation stimulation, which often leads to the increasing synthesis of PGE2 in tumor tissue or inflammation tissue (Tanabe and Tohnai, 2002). Evidence suggests that there is preferential functional coupling in the interactions between COX and PGES, while mPGES-1 tends to couple with COX-2 and cPGES preferentially couples with COX-1 (Muthalif et al., 2001; Ouellet et al., 2002; Murakami et al., 2003). The key enzymes of the catabolic process of PGE2 include 15-ketoprostaglandin-13-reductase (13-PGR) and 15-hydroxy-prostaglandin dehydrogenase (15-PGDH) (Tai, 2011) (Figure 1).
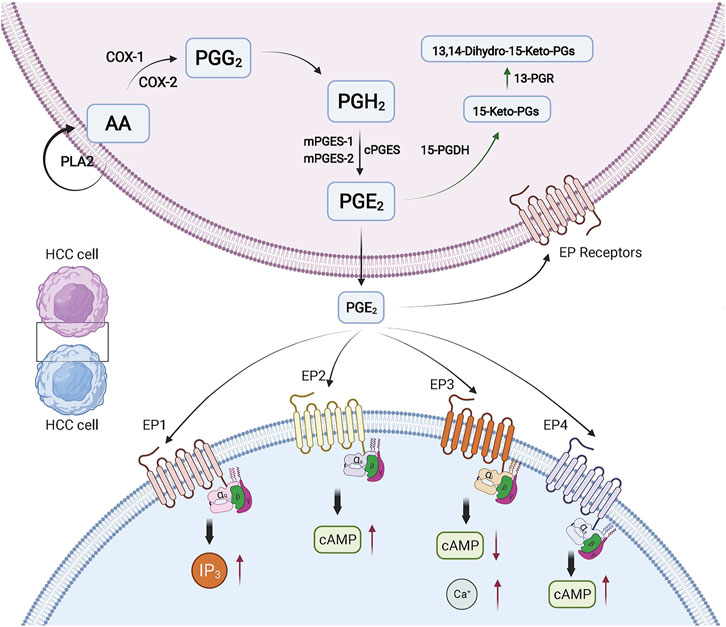
FIGURE 1. PGE2 in HCC. The biosynthesis and function of PGE2 in HCC is shown. The red arrows indicate the changed expression of molecules. The green arrows indicate the degradation pathway of PGE2. Created with BioRender.com. 15-PGDH, 15-hydroxyprostaglandin dehydrogenase; 13-PGR, 15-keto-prostaglandin-(13)-reductase.
PGE2 Receptors in Hepatocellular Carcinoma
PGE2 execute physiological or pathological activities by binding to seven transmembrane G-protein coupled receptors (GPCRs), which are known as prostaglandin E (EP) receptors. EP receptors can divide into four isoforms: EP1-4. Heterotrimeric G proteins couple with EP receptors containing stimulatory subunit (GαS) or inhibitory subunit (Gαi) which can regulate the levels of cytoplasmic cyclic AMP (cAMP), Ca2+, and inositol phosphate to activate different downstream signaling pathways (Figure 2). Normally PGE2 binds to the EP receptors in immunocyte, playing a pro-inflammatory or anti-inflammatory role due to the type of EP receptor and immunocyte (An et al., 1993; Reinold et al., 2005; Sugimoto and Narumiya, 2007). But in hepatocellular carcinoma cell line and tumor, the activation of EP receptors in HCC cell influence tumorigenesis, and tumor progression through multiple pathways (Table 1).
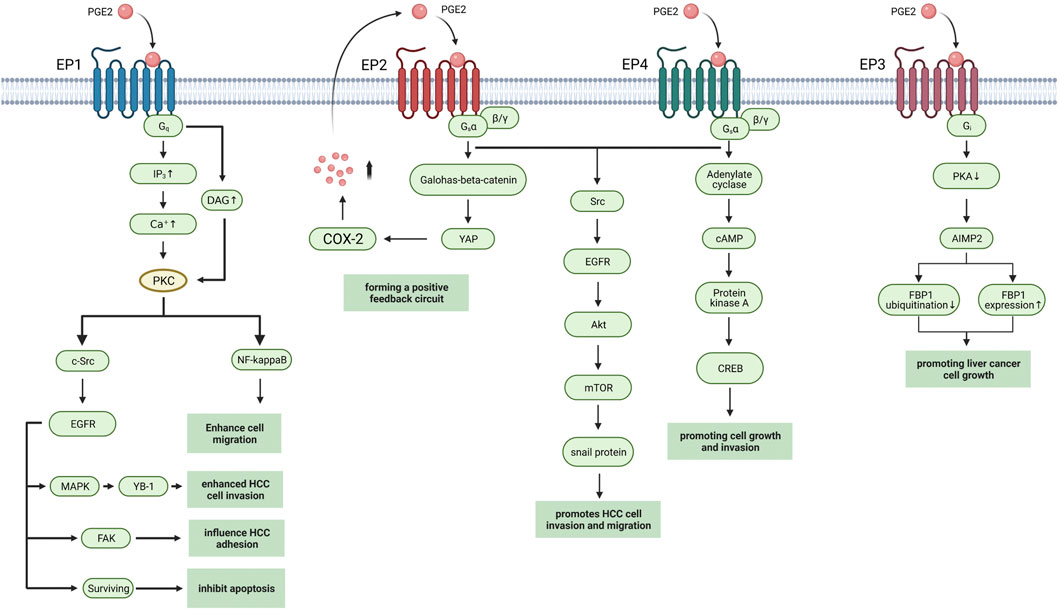
FIGURE 2. EP receptors in HCC. The EP receptors with downstream pathways in HCC are shown. Created with BioRender.com. YB-1, Y box-binding protein 1; FAK, Focal adhesion kinase; YAP, Yes-associated protein; AIMP2, aminoacyl-tRNA synthetase interacting multifunctional protein 2, also known as JTV1; FBP1, FUSE-binding protein 1.
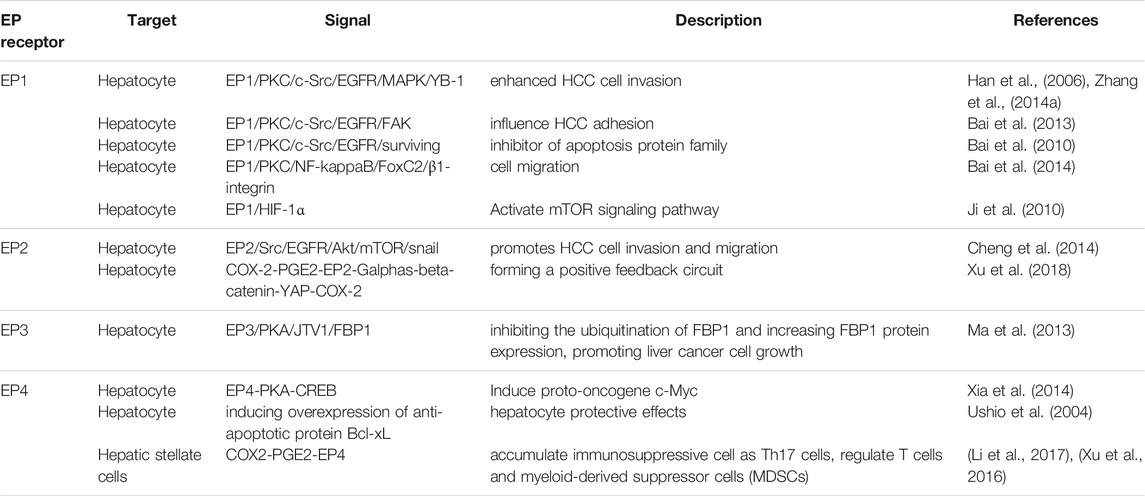
EP1 receptor usually couples with the Gαq protein subunit, which increases the level of intracellular Ca2+ and activate protein kinase C (PKC), thus inducing gene transcription through regulating the MAPK, nuclear factor-kappaB (NFκB), and nuclear factor of activated T cells (NFAT) pathways (Sugimoto and Narumiya, 2007). Clinical studies show that high EP1 receptor expression in tumor is associated with poor overall survival (OS) and poor differentiation of tumor (Yang et al., 2016). In HCC cell line, researchers found that EP1/PKC/c-Src signaling pathway can modulate the activation of epidermal growth factor receptor (EGFR) (Han et al., 2006). Then activated EGFR induced the phosphorylation of p44/42 MAPK, which in turn up-regulated Y box-binding protein 1 (YB-1) to promote cell invasion (Zhang et al., 2014a). The other targets of EGFR include focal adhesion kinase (FAK) which influences cell adhesion and survival by inhibiting apoptosis progress (Bai et al., 2010; Bai et al., 2014) Activation of PKC also induced β1-integrin over-expression and cell migration via NF-kappa B/FoxC2 signaling pathway through EP1 receptor to up-regulate β1-integrin expression and cell migration (Bai et al., 2014). The mTOR signaling pathway is also activated while EP1 agonist up-regulates HIF-1 alpha in HCC cell line (Ji et al., 2010).
EP2 receptor mainly couples with the Gαq protein subunit that activates adenylate cyclase to generate cAMP. The increasing cAMP in plasma activates the protein kinase A (PKA) to mediate glycogen synthase kinase 3β (GSK3β)-β catenin pathways (Narumiya et al., 1999).
Researchers found that activation of EP2 receptor enhances Hep3B human hepatocellular carcinoma cell line proliferation while antagonism of EP2 blocked growth and invasion induced by PGE2 (Guo et al., 2011; Zang et al., 2017). And activation of EP2 upregulated snail protein through Src-EGFR-Akt-mTOR pathway to enhance tumor cell migration (Cheng et al., 2014). In HCC cell line, EP2 also mediated the transcriptional induction of Yes-associated protein (YAP) via Wnt/β-Catein pathway while YAP can interact with the COX-2 promoter to increase COX-2 expression, thus forming a positive feedback circuit (Xu et al., 2018).
EP3 receptor is a G-protein-coupled receptor with multiple C-terminal tails due to alternative mRNA splicing (Kotani et al., 1997). Cell experiments show that EP3 can down-regulate the expression of aminoacyl-tRNA synthetase interacting multifunctional protein 2 (AIMP2) to inhibit the ubiquitination of FUSE-binding protein 1(FBP1) and increase FBP1 protein expression through Gs protein and PKA pathway, thus promoting cell growth (Ma et al., 2013). But further study of EP3 in HCC has produced unclear results.
Activation of EP4 also upregulates snail protein via EGFR to promote migration in hepatoma cells (Zhang et al., 2014b). Proto-oncogene c-Myc is induced through EP4-PKA-CREB signaling pathway (Xia et al., 2014). EP4-receptor agonist (PGE2R-A) shows hepatocyte protective effects in HepG2 HCC cells by inducing overexpression of anti-apoptotic protein Bcl-xL (Ushio et al., 2004). The PGE2 synthesized by Hepatic stellate cells (HSCs) accumulates immunosuppressive cells, such as Th17 cells, and regulates T cells and myeloid-derived suppressor cells (MDSCs) via EP4 receptor (Xu et al., 2016; Li et al., 2017).
Overexpression of PGE2 in Hepatocellular Carcinoma
Clinical research has discovered that PGE2 in the peripheral blood of patients with hepatocellular carcinoma has increased significantly. A further study found that COX-2 expression in HCC tissues is higher than in adjacent tissues and in normal liver tissues (Cervello and Montalto, 2006). As for mPGES-1, which synthesize PGE2 directly by binding to Cox-2, this also increased in HCC tissue (Zang et al., 2013), indicating that increased PGE2 in HCC patients is synthesized by HCC tissues. The increased expression of PGE2-related synthase in HCC is closely related to various carcinogenic factors.
Viral hepatitis is one of the main causes of hepatocellular carcinoma. HBV and HCV can promote the expression of PGE2 in liver cancer tissues in different ways. HBx protein is one of the HBV virus proteins that has a wide range of transactivation functions and plays an important role in cell proliferation, apoptosis, and genetic stability of liver cells.
Transfected with HBx, Hep3B hepatocellular carcinoma cells overexpress COX-2, and generates more PGE2 (Cheng et al., 2004). In HBx-positive cells of chronic hepatitis B patients, HBx protein reduces the function of DNA methyltransferase, which in turn increases methylation site in the CpG dinucleotide of the COX-2 promoter. Methylation increases binding affinity of the COX-2 promoter to increase COX-2 expression in hepatocytes, which ultimately leads to higher level of circulating PGE2 in peripheral blood (Yue et al., 2011). HBx protein is also positively correlated with mPGES-1 expression in cancer tissues of HBV-related HCC patients. The researchers believe that HBx may also promote the expression of PGE2 by increasing the level of early growth response 1 (EGR1) that binds to the transcription site of the mPGES-1 promoter (Liu et al., 2014). HCV can increase PGE2 synthesis by non-structural protein NS3 which can enhance the activity of the COX-2 gene promoter in HepG2 cells through a transcription factor NF-κB-dependent pathway (Lu et al., 2008).
In addition to viral hepatitis, there are a variety of factors that induce the production of prostaglandin-related synthase in non-viral non-cirrhotic HCC. Obesity is one of the most important pathogenic factors. The intestinal microflora of obese people secretes lipoprotein phosphate (LTA) and intestinal microbial metabolite deoxycholic acid (DCA), which synergistically enhances hepatic stellate cells (HSC) aging-related secreted phenotype (SASP), through Toll -Like receptor 2 (TLR2), up-regulating the expression of COX-2, thereby promoting the synthesis of PGE2 (Loo et al., 2017). The LPS secreted by abnormal intestinal flora can interact with TLR4 in HCC to induce PGE2 expression through COX-2/PGE2/STAT3 positive feedback loop.
At the same time, the LPS secreted by the abnormal intestinal flora can also activate the COX-2/PGE2/STAT3 positive feedback loop through the functional expression of TLR4 on HCC cells (Lin et al., 2016).
Overexpression of PGE2 is also related to the severity and prognosis of HCC patients. Correlation analysis found that the patients with higher circulating PGE2 levels have larger tumor size and shorter overall survival (Tai et al., 2019; Pelizzaro et al., 2021). Patients with portal vein thrombosis, non-enveloped, and advanced stage disease according to Barcelona Clinic Liver Cancer (BCLC) have higher PGE2 levels in their cancer tissues, suggesting that PGE2 may be related to the aggressiveness of liver cancer (Zang et al., 2017). There is also a significant positive correlation between the level of mPGES-1 and the clinical liver cancer stage of BCLC (Zang et al., 2013), while patients with higher COX-2 expression are more likely to suffer from lymphatic infiltration and distant metastasis of HCC (Tai et al., 2019).
Two Opinions About Effect of PGE2 on Tumorigenesis of Hepatocellular Carcinoma
The tumorigenesis of hepatocellular carcinoma is a complex multi-step process involving persistent inflammatory damage, hepatocyte necrosis, regeneration, and fibrosis deposition. Nearly 70–90% of hepatocellular carcinoma cases have a history of chronic liver disease or cirrhosis (El–Serag and Rudolph, 2007), while PGE2 shows high levels in chronic hepatitis patient and even higher levels in patients with cirrhosis, suggesting that PGE2 may promote the tumorigenesis of hepatocellular carcinoma. But there is also some evidence which shows the tumorigenesis of hepatocellular carcinoma is unrelated to PGE2.
PGE2 Promote the Tumorigenesis of Hepatocellular Carcinoma
In clinical practice, non-steroidal anti-inflammatory drugs such as aspirin are widely used. The epidemiological studies on HCC have shown that the long-term use of small doses of aspirin can significantly reduce the risk of HCC. PGE2 is one of the most abundant COX-dependent prostaglandins in acute and chronic inflammation, indicating that PGE2 might be the major factor influencing the occurrence of HCC treated with long-term use of aspirin.
In animal experiments, the use of different doses of selective COX-2 inhibitors can significantly reduce the level of serum biochemical parameters of HCC induced by nitrosamine diethyl nitrosamine (DEN) in rats. Stained sections of rat liver tissues treated with high-dose COX-2 inhibitors are almost like normal tissues (Afzal et al., 2019). Deletion of the EP2 receptor gene leads to a reduction in the number and size of intestinal polyps in human familial adenomatous polyposis model mice (Sonoshita et al., 2001).
At the genetic level, the occurrence of hepatocellular carcinoma is not only related to epigenetic modifications, but also the result of cumulative changes in the genome of the hepatocyte. When changes in normal liver tissue affect the expression of oncogenes and tumor suppressor genes, it can further evolve the abnormal liver cell monoclonal population into HCC. Studies have shown that PGE2 can significantly up-regulate C-myc expression at both mRNA and protein levels, while knocking down C-myc can block PGE2-induced HCC cell growth and human hepatoma cell line Huh7 invasive ability; this process may be achieved through the EP4/GS/AC/cAMP/PKA/CREB signaling pathway (Xia et al., 2014).
PGE2 Is Unrelated to the Tumorigenesis of Hepatocellular Carcinoma
But there is also some evidence to disprove the assumption that PGE2 promotes the tumorigenesis of hepatocellular carcinoma. Although long-term low-dose aspirin can reduce the incidence of HCC, the study of other NSAIDs such as ibuprofen found that its application has no significant relationship with the incidence of HCC (Petrick et al., 2015). In addition, aspirin inhibits the effects of COX-1 and COX-2 at the same time, but in the chronic hepatitis process that is closely related to cancer, it is generally believed that COX-2 plays a major role. However, no studies have reported that selective COX- 2 inhibitors are statistically related to HCC risk. These all suggest that the mechanism by which aspirin reduces the risk of HCC may be non-COX-dependent. The level of COX does not affect the pathogenesis of HCC. In the latest prospective study, researchers saved urine samples of 18,244 people based on prostaglandin E2 metabolite (PGE-M), a stable final metabolite, after catabolism of PGE2 mainly excreted in urine, and which represents the level of PGE2 synthesis in the human body over a period of time. After 28 years of follow-up, the number of liver cancer cases was counted, and the PGE-M level of corresponding urine was detected. There is no significant evidence to show the association between urine PGE-M level and the risk of HCC (Yuan et al., 2019). In animal model, studies on COX-2 transgenic mice with chemical carcinogens induced by diethylnitrosamine (DEN) have shown that overexpression of COX-2 is beneficial to the growth of pre-tumor tumors, but it will not affect their transformation into malignant tumors. (Llorente Izquierdo et al., 2011).
In general, PGE2 has shown the ability to promote the transformation of liver cells into cancer cells in vitro and vivo, but further clinical studies suggested that PGE2 may not play a significant role in the tumorigenesis of liver cancer. The discrepancies between two opinions are due to the unappropriated indicator PGE-M in clinical study, which reflects the PGE2 catabolism of multiple organs instead of just the liver. And mice model with chemical carcinogens induced by DEN is also not a qualified model to reflect PGE2 effect on tumorigenesis of liver cancer, as PGE2 is known as an inflammatory mediator.
PGE2 Enhance Hepatocellular Carcinoma Progress
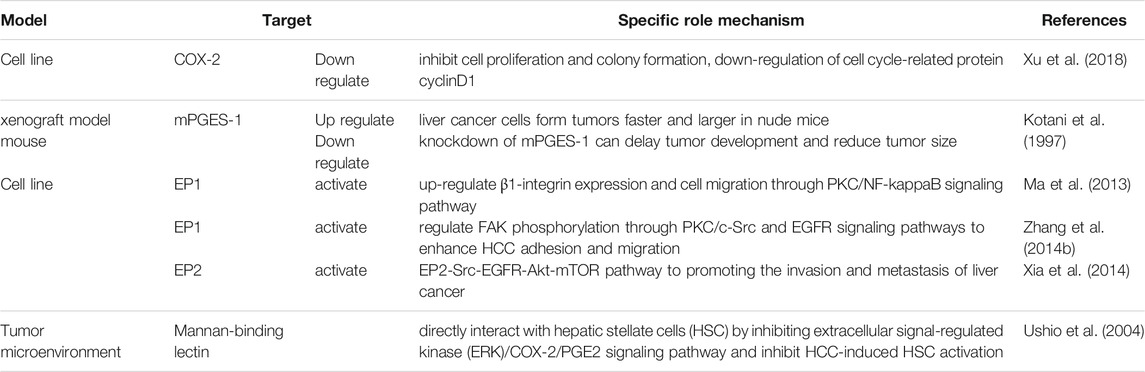
Evidence shows that PGE2 can promote HCC progression through autocrine and paracrine mechanisms by multiple ways (Table 2). In vitro, the downregulation of COX-2 expression can significantly inhibit HCC cell proliferation and colony formation, the downregulation of cell cycle-related protein cyclin D1, and lead to cell cycle arrest in vitro (Lv et al., 2019). In the mouse xenograft model, mPGES-1 overexpressed liver cancer cells form tumors faster and larger in nude mice, knockdown of mPGES-1 can delay tumor development and reduce tumor size (Lu et al., 2012), while research shows that the expression level of mPGES-1 in non-cancerous liver tissues can be used as a statistically important independent predictor of early recurrence after HCC (Nonaka et al., 2010).
PGE2 can not only directly affect hepatocyte but also promote the proliferation and migration of liver cancer cells by affecting the tumor microenvironment (TME). Mannan-binding lectin (MBL) can activate the complement lectin pathway and prevent infection as part of TME. Moreover, Cox-2/PGE2/EP/VEGF pathway may also contribute to tumor angiogenesis in HCC (Zhao et al., 2007).
Effect of PGE2 on the Tumor Microenvironment in Hepatocellular Carcinoma
The tumor microenvironment (TME) in hepatocellular carcinoma plays a critical role in hepatocarcinogenesis, liver fibrosis, tumor invasion, and metastasis. A series of studies have revealed the importance of TME components, such as tumor-associated macrophages (TAMs), hepatic stellate cells, and regulatory and cytotoxic T cells, in tumorigenesis and tumor progression (Mizuno et al., 2019). Here, we summarize the role of COX-2/PGE2 axis played in each component of HCC tumor microenvironment.
TAMs inhibit anti-tumor immunity and promote tumor progression by expressing cytokines and chemokines. High COX-2-expressing HCC cell lines can induce anti-tumor abilities’ exhaustion in activated CD8+ T cell through alternative (M2) tumor-associated macrophages polarization and TGF-β pathway. COX-2 inhibitors may reduce the inhibitory effect on CD8+ T cells through regulating TAMs in tumor immune microenvironment, thus enhancing the T cell-based cytotoxicity and improving the prognosis of HCC patients (Xun et al., 2021). Studies have found that ERK/COX-2/PGE2 signaling pathway is related to the activation of hepatic stellate cells (HSCs) (Li et al., 2019). And the HSCs-induced myeloid-derived suppressor cells (MDSC) accumulation and HCC growth is mediated by COX2-PGE2-EP4 pathway (Xu et al., 2016). In patients with advanced stage HBV-related liver fibrosis, researchers found that regulatory T cells and Th17 cells are upregulated by Hepatic stellate cells via PGE2-EP2 and PGE2-EP4 pathway (Li et al., 2017). In conclusion, PGE2 usually plays an anti-inflammation role on the tumor microenvironment in HCC to promote tumor invasion and metastasis.
The Treatment Strategy Based on Cox-2/PGE2 Axis on Hepatocellular Carcinoma
As the Cox-2/PGE2 axis plays an important role in the progress of hepatocellular carcinoma, there are a number of experiments focusing on the treatment of HCC based on it. Due to the abundant effect of PGE2 in physiological and pathological progress, the single use of antagonist of EP receptor or abnormal dose inhibitor of synthesis enzyme for PGE2 is always accompanied with side effects. But combination use of drugs targeting Cox-2/PGE2 axis and traditional antitumor drugs show great potentials in HCC. T7 peptide is the N-terminal part of tumstatin, an endogenous angiogenic. Compared to tumstatin, T7 peptide has low molecular weight and shares similar activity in suppressing the proliferation, migration, and promotion of the apoptosis of endothelial cells. Combination use of selective Cox-2 inhibitor meloxicam and T7 peptide shows a greater anti-tumor effect against HCC tumor in mice (Yang et al., 2021). EP1 antagonist ONO-8711 enhanced the effect of EGCG, Epigallocatechin gallate, in inhibiting PGE2-induced HCC proliferation (Yang et al., 2019). The combination uses of drugs based on COX-2/PGE2 axis and traditional antitumor drugs show great potentials to diminish tumor progression of HCC in vivo and vitro, indicating that it might be a possible strategy for treatment of advanced primary hepatocellular carcinoma.
Discussion
PGE2 is known as an important factor in inflammatory milieu that influence malignant tumor outset and progression. Researchers have found that PGE2 can promote various kinds of cancer cell growth by regulating immune response and enhancing resistance to apoptosis. Extensive clinical and epidemiological studies show that reduction of PGE2 level in tumor can rebuild tumor microenvironment by reprograming anti-tumor immunity, thus inhibiting tumor growth and metastasis. For example, a new study found that the selective COX-2 inhibitor celecoxib can be used in vitro, synergistically enhancing the inhibitory effect of sorafenib on cancer cell growth and AKT activation and inducing cancer cell apoptosis [70].
The clinical study of the anti-tumoral effect of PGE2 in cancer by using NSAIDs or COX-2 inhibitor has been performed with failed outcomes due to severe side effects (Mizuno et al., 2019). This suggests that drugs based on the COX-PGE2 axis may be used in combination with traditional anti-tumoral drugs to avoid serious side effects caused by high-dose medication alone. In HCC, evidence shows the pivotal role PGE2 played in the progression of tumor in vitro. But there are few clinical studies focusing of the COX-2/PGE2 axis in HCC. As more small-molecule ligands targeting EP receptors have been developed, therapy based on a combination of EP receptor and traditional anti-tumoral drugs is being considered.
In conclusion, PGE2 shows a traditional target with pleiotropic effects in tumorigenesis and progression of HCC to create a new potential clinical impact. For the treatment study focusing on the COX-PGE2 axis, the exclusive usage of NSAIDs or COX-2-inhibitors may be replaced by a combination of selective EP antagonists and traditional anti-tumoral drugs to alleviate severe side effects and achieve better outcomes.
Author Contributions
HZ contributed to conception and design of the review. CC, XG, and QC wrote sections of the manuscript. JG organized the database. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This study was supported by Grants awarded by the National Science and Technology Major Project of China (NO 2018ZX10302206) and the Science and Technology Major Projects of Zhejiang Province (NO 2018C04016).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Afzal, M., Bhardwaj, D. P., Khan, R., Kazmi, I., Saleem, S., Al-Abbasi, F. A., et al. (2019). Antineoplastic Influence of Nimesulide in Chemically Induced Hepatocellular Carcinoma by Inhibition of DNA Synthesis. Inflammopharmacol 27, 89–98. doi:10.1007/s10787-018-0481-1
An, S. Z., Yang, J. H., Xia, M. H., and Goetzl, E. J. (1993). Cloning and Expression of the EP2 Subtype of Human Receptors for Prostaglandin E2. Biochem. biophysical Res. Commun. 197, 263–270. doi:10.1006/bbrc.1993.2470
Bai, X.-M., Jiang, H., Ding, J.-X., Peng, T., Ma, J., Wang, Y.-H., et al. (2010). Prostaglandin E2 Upregulates Survivin Expression via the EP1 Receptor in Hepatocellular Carcinoma Cells. Life Sci. 86, 214–223. doi:10.1016/j.lfs.2009.12.009
Bai, X., Wang, J., Guo, Y., Pan, J., Yang, Q., Zhang, M., et al. (2014). Prostaglandin E2 Stimulates β1-integrin Expression in Hepatocellular Carcinoma through the EP1 receptor/PKC/NF-κB Pathway. Sci. Rep. 4, 6538. doi:10.1038/srep06538
Bai, X., Wang, J., Zhang, L., Ma, J., Zhang, H., Xia, S., et al. (2013). Prostaglandin E2 Receptor EP1-Mediated Phosphorylation of Focal Adhesion Kinase Enhances Cell Adhesion and Migration in Hepatocellular Carcinoma Cells. Int. J. Oncol. 42, 1833–1841. doi:10.3892/ijo.2013.1859
Cervello, M., and Montalto, G. (2006). Cyclooxygenases in Hepatocellular Carcinoma. Wjg 12, 5113–5121. doi:10.3748/wjg.v12.i32.511310.3748/wjg.12.5113
Cheng, A. S.-L., Chan, H. L.-Y., Leung, W. K., To, K. F., Go, M. Y.-Y., Chan, J. Y.-H., et al. (2004). Expression of HBx and COX-2 in Chronic Hepatitis B, Cirrhosis and Hepatocellular Carcinoma: Implication of HBx in Upregulation of COX-2. Mod. Pathol. 17, 1169–1179. doi:10.1038/modpathol.3800196
Cheng, S.-Y., Zhang, H., Zhang, M., Xia, S.-K., Bai, X.-M., Zhang, L., et al. (2014). Prostaglandin E2 Receptor EP2 Mediates Snail Expression in Hepatocellular Carcinoma Cells. Oncol. Rep. 31, 2099–2106. doi:10.3892/or.2014.3074
Cho, M. H., Yoo, T. G., Jeong, S.-M., and Shin, D. W. (2021). Association of Aspirin, Metformin, and Statin Use with Gastric Cancer Incidence and Mortality: A Nationwide Cohort Study. Cancer Prev. Res. 14, 95–104. doi:10.1158/1940-6207.Capr-20-0123
El–Serag, H. B., and Rudolph, K. L. (2007). Hepatocellular Carcinoma: Epidemiology and Molecular Carcinogenesis. Gastroenterology 132, 2557–2576. doi:10.1053/j.gastro.2007.04.061
Ferlay, J., Shin, H.-R., Bray, F., Forman, D., Mathers, C., and Parkin, D. M. (2010). Estimates of Worldwide burden of Cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 127, 2893–2917. doi:10.1002/ijc.25516
Guo, D., Chen, N. N., Hou, L. B., and Lei, L. S. (2011). Prostaglandin E2 Promotes Hepatocellular Carcinoma Cell Proliferation through EP2 Prostanoid Receptor. Nan Fang Yi Ke Da Xue Xue Bao 31, 1564–1567.
Han, C., Michalopoulos, G. K., and Wu, T. (2006). Prostaglandin E2 Receptor EP1 Transactivates EGFR/MET Receptor Tyrosine Kinases and Enhances Invasiveness in Human Hepatocellular Carcinoma Cells. J. Cel. Physiol. 207, 261–270. doi:10.1002/jcp.20560
Ji, R., Chou, C.-L., Xu, W., Chen, X.-B., Woodward, D. F., and Regan, J. W. (2010). EP1 Prostanoid Receptor Coupling to Gi/oUp-Regulates the Expression of Hypoxia-Inducible Factor-1α through Activation of a Phosphoinositide-3 Kinase Signaling Pathway. Mol. Pharmacol. 77, 1025–1036. doi:10.1124/mol.110.063933
Kotani, M., Tanaka, I., Ogawa, Y., Usui, T., Tamura, N., Mori, K., et al. (1997). Structural Organization of the Human Prostaglandin EP3Receptor Subtype Gene (PTGER3). Genomics 40, 425–434. doi:10.1006/geno.1996.4585
Leng, J., Han, C., Demetris, A. J., Michalopoulos, G. K., and Wu, T. (2003). Cyclooxygenase-2 Promotes Hepatocellular Carcinoma Cell Growth through Akt Activation: Evidence for Akt Inhibition in Celecoxib-Induced Apoptosis. Hepatology 38, 756–768. doi:10.1053/jhep.2003.50380
Li, J., Li, H., Yu, Y., Liu, Y., Liu, Y., Ma, Q., et al. (2019). Mannan-binding Lectin Suppresses Growth of Hepatocellular Carcinoma by Regulating Hepatic Stellate Cell Activation via the ERK/COX-2/PGE2 Pathway. Oncoimmunology 8, e1527650. doi:10.1080/2162402x.2018.1527650
Li, X., Su, Y., Hua, X., Xie, C., Liu, J., Huang, Y., et al. (2017). Levels of Hepatic Th17 Cells and Regulatory T Cells Upregulated by Hepatic Stellate Cells in Advanced HBV-Related Liver Fibrosis. J. Transl Med. 15, 75. doi:10.1186/s12967-017-1167-y
Lin, A., Wang, G., Zhao, H., Zhang, Y., Han, Q., Zhang, C., et al. (2016). TLR4 Signaling Promotes a COX-2/PGE2/STAT3 Positive Feedback Loop in Hepatocellular Carcinoma (HCC) Cells. Oncoimmunology 5, e1074376. doi:10.1080/2162402x.2015.1074376
Lin, Y.-S., Yeh, C.-C., Huang, S.-F., Chou, Y.-S., Kuo, L.-T., Sung, F.-C., et al. (2018). Aspirin Associated with Risk Reduction of Secondary Primary Cancer for Patients with Head and Neck Cancer: A Population-Based Analysis. PloS one 13, e0199014. doi:10.1371/journal.pone.0199014
Liu, C., Chen, S., Wang, X., Chen, Y., and Tang, N. (2014). 15d-PGJ2 Decreases PGE2 Synthesis in HBx-Positive Liver Cells by Interfering EGR1 Binding to mPGES-1 Promoter. Biochem. Pharmacol. 91, 337–347. doi:10.1016/j.bcp.2014.07.032
Llorente Izquierdo, C., Mayoral, R., Flores, J. M., García-Palencia, P., Cucarella, C., Boscá, L., et al. (2011). Transgenic Mice Expressing Cyclooxygenase-2 in Hepatocytes Reveal a Minor Contribution of This Enzyme to Chemical Hepatocarcinogenesis. Am. J. Pathol. 178, 1361–1373. doi:10.1016/j.ajpath.2010.11.074
Llovet, J. M., Zucman-Rossi, J., Pikarsky, E., Sangro, B., Schwartz, M., Sherman, M., et al. (2016). Hepatocellular Carcinoma. Nat. Rev. Dis. Primers 2, 16018. doi:10.1038/nrdp.2016.18
Loo, T. M., Kamachi, F., Watanabe, Y., Yoshimoto, S., Kanda, H., Arai, Y., et al. (2017). Gut Microbiota Promotes Obesity-Associated Liver Cancer through PGE2-Mediated Suppression of Antitumor Immunity. Cancer Discov. 7, 522–538. doi:10.1158/2159-8290.cd-16-0932
Lu, D., Han, C., and Wu, T. (2012). Microsomal Prostaglandin E Synthase-1 Promotes Hepatocarcinogenesis through Activation of a Novel EGR1/β-Catenin Signaling axis. Oncogene 31, 842–857. doi:10.1038/onc.2011.287
Lu, L., Wei, L., Peng, G., Mu, Y., Wu, K., Kang, L., et al. (2008). NS3 Protein of Hepatitis C Virus Regulates Cyclooxygenase-2 Expression through Multiple Signaling Pathways. Virology 371, 61–70. doi:10.1016/j.virol.2007.09.025
Lv, X., Chen, Z., Li, S., and Xie, H. (2019). Knockdown of Cyclooxygenase-2 Leads to Growth Inhibition and Cell Cycle Arrest in Hepatocellular Carcinoma Cells. Ott Vol. 12, 4341–4349. doi:10.2147/ott.s196822
Ma, J., Chen, M., Xia, S.-K., Shu, W., Guo, Y., Wang, Y.-H., et al. (2013). Prostaglandin E2 Promotes Liver Cancer Cell Growth by the Upregulation of FUSE-Binding Protein 1 Expression. Int. J. Oncol. 42, 1093–1104. doi:10.3892/ijo.2013.1782
Ma, Y., and Brusselaers, N. (2018). Maintenance Use of Aspirin or Other Non-steroidal Anti-inflammatory Drugs (NSAIDs) and Prostate Cancer Risk. Prostate Cancer Prostatic Dis. 21, 147–152. doi:10.1038/s41391-017-0021-x
Mizuno, R., Kawada, K., and Sakai, Y. (2019). Prostaglandin E2/EP Signaling in the Tumor Microenvironment of Colorectal Cancer. Ijms 20, 6254. doi:10.3390/ijms20246254
Murakami, M., Nakashima, K., Kamei, D., Masuda, S., Ishikawa, Y., Ishii, T., et al. (2003). Cellular Prostaglandin E2 Production by Membrane-Bound Prostaglandin E Synthase-2 via Both Cyclooxygenases-1 and -2. J. Biol. Chem. 278, 37937–37947. doi:10.1074/jbc.M305108200
Muthalif, M. M., Hefner, Y., Canaan, S., Harper, J., Zhou, H., Parmentier, J.-H., et al. (2001). Functional Interaction of Calcium-/Calmodulin-dependent Protein Kinase II and Cytosolic Phospholipase A2. J. Biol. Chem. 276, 39653–39660. doi:10.1074/jbc.M103136200
Narumiya, S., Sugimoto, Y., and Ushikubi, F. (1999). Prostanoid Receptors: Structures, Properties, and Functions. Physiol. Rev. 79, 1193–1226. doi:10.1152/physrev.1999.79.4.1193
Nonaka, K., Fujioka, H., Takii, Y., Abiru, S., Migita, K., Ito, M., et al. (2010). mPGES-1 Expression in Non-cancerous Liver Tissue Impacts on Postoperative Recurrence of HCC. Wjg 16, 4846–4853. doi:10.3748/wjg.v16.i38.4846
Ouellet, M., Falgueyret, J.-P., Hien Ear, P., Pen, A., Mancini, J. A., Riendeau, D., et al. (2002). Purification and Characterization of Recombinant Microsomal Prostaglandin E Synthase-1. Protein Expr. Purif. 26, 489–495. doi:10.1016/s1046-5928(02)00566-1
Park, J. Y., Pillinger, M. H., and Abramson, S. B. (2006). Prostaglandin E2 Synthesis and Secretion: the Role of PGE2 Synthases. Clin. Immunol. 119, 229–240. doi:10.1016/j.clim.2006.01.016
Pelizzaro, F., Kitenge, M. P., Cardin, R., Ponzoni, A., Cillo, U., Vitale, A., et al. (2021). Circulating Prostaglandin E2: a Novel Potential Prognostic Biomarker in Patients with Hepatocellular Carcinoma. Clin. Exp. Med. 21, 675–682. doi:10.1007/s10238-021-00705-z
Petrick, J. L., Sahasrabuddhe, V. V., Chan, A. T., Alavanja, M. C., Beane-Freeman, L. E., Buring, J. E., et al. (2015). NSAID Use and Risk of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma: The Liver Cancer Pooling Project. Cancer Prev. Res. 8, 1156–1162. doi:10.1158/1940-6207.CAPR-15-0126
Reinold, H., Ahmadi, S., Depner, U. B., Layh, B., Heindl, C., Hamza, M., et al. (2005). Spinal Inflammatory Hyperalgesia Is Mediated by Prostaglandin E Receptors of the EP2 Subtype. J. Clin. Invest. 115, 673–679. doi:10.1172/jci23618
Rothwell, P. M., Fowkes, F. G. R., Belch, J. F., Ogawa, H., Warlow, C. P., and Meade, T. W. (2011). Effect of Daily Aspirin on Long-Term Risk of Death Due to Cancer: Analysis of Individual Patient Data from Randomised Trials. The Lancet 377, 31–41. doi:10.1016/s0140-6736(10)62110-1
Sonoshita, M., Takaku, K., Sasaki, N., Sugimoto, Y., Ushikubi, F., Narumiya, S., et al. (2001). Acceleration of Intestinal Polyposis through Prostaglandin Receptor EP2 in ApcΔ716 Knockout Mice. Nat. Med. 7, 1048–1051. doi:10.1038/nm0901-1048
Sugimoto, Y., and Narumiya, S. (2007). Prostaglandin E Receptors. J. Biol. Chem. 282, 11613–11617. doi:10.1074/jbc.R600038200
Tai, H.-H. (2011). Prostaglandin Catabolic Enzymes as Tumor Suppressors. Cancer Metastasis Rev. 30, 409–417. doi:10.1007/s10555-011-9314-z
Tai, Y., Zhang, L.-H., Gao, J.-H., Zhao, C., Tong, H., Ye, C., et al. (2019). Suppressing Growth and Invasion of Human Hepatocellular Carcinoma Cells by Celecoxib through Inhibition of Cyclooxygenase-2. Cmar Vol. 11, 2831–2848. doi:10.2147/cmar.s183376
Tanabe, T., and Tohnai, N. (2002). Cyclooxygenase Isozymes and Their Gene Structures and Expression. Prostaglandins & Other Lipid Mediators 68-69, 95–114. doi:10.1016/s0090-6980(02)00024-2
Ushio, A., Takikawa, Y., Lin, S., Miyamoto, Y., and Suzuki, K. (2004). Induction of Bcl-xL Is a Possible Mechanism of Anti-apoptotic Effect by Prostaglandin E2 EP4-Receptor Agonist in Human Hepatocellular Carcinoma HepG2 Cells. Hepatol. Res. 29, 173–179. doi:10.1016/j.hepres.2004.03.001
Vane, J. R., and Botting, R. M. (2003). The Mechanism of Action of Aspirin. Thromb. Res. 110, 255–258. doi:10.1016/s0049-3848(03)00379-7
Wang, D., and Dubois, R. N. (2010). Eicosanoids and Cancer. Nat. Rev. Cancer 10, 181–193. doi:10.1038/nrc2809
Wu, T. (2006). Cyclooxygenase-2 in Hepatocellular Carcinoma. Cancer Treat. Rev. 32, 28–44. doi:10.1016/j.ctrv.2005.10.004
Xia, S., Ma, J., Bai, X., Zhang, H., Cheng, S., Zhang, M., et al. (2014). Prostaglandin E2 Promotes the Cell Growth and Invasive Ability of Hepatocellular Carcinoma Cells by Upregulating C-Myc Expression via EP4 Receptor and the PKA Signaling Pathway. Oncol. Rep. 32, 1521–1530. doi:10.3892/or.2014.3393
Xu, G., Wang, Y., Li, W., Cao, Y., Xu, J., Hu, Z., et al. (2018). COX-2 Forms Regulatory Loop with YAP to Promote Proliferation and Tumorigenesis of Hepatocellular Carcinoma Cells. Neoplasia 20, 324–334. doi:10.1016/j.neo.2017.12.004
Xu, Y., Zhao, W., Xu, J., Li, J., Hong, Z., Yin, Z., et al. (2016). Activated Hepatic Stellate Cells Promote Liver Cancer by Induction of Myeloid-Derived Suppressor Cells through Cyclooxygenase-2. Oncotarget 7, 8866–8878. doi:10.18632/oncotarget.6839
Xun, X., Zhang, C., Wang, S., Hu, S., Xiang, X., Cheng, Q., et al. (2021). Cyclooxygenase-2 Expressed Hepatocellular Carcinoma Induces Cytotoxic T Lymphocytes Exhaustion through M2 Macrophage Polarization. Am. J. Transl Res. 13, 4360
Yang, H.-J., Jiang, J.-H., Yang, Y.-T., Yang, X.-D., Guo, Z., Qi, Y.-P., et al. (2016). Cyclooxygenase-2 Expression Is Associated with Initiation of Hepatocellular Carcinoma, while Prostaglandin Receptor-1 Expression Predicts Survival. Wjg 22, 8798–8805. doi:10.3748/wjg.v22.i39.8798
Yang, H., Wang, M., Sun, H., Zhu, S., and Jin, J. (2019). Synergetic Effect of EP1 Receptor Antagonist and (-)-Epigallocatechin-3-Gallate in Hepatocellular Carcinoma. Pharmacology 104, 267–275. doi:10.1159/000502076
Yang, J., Zhong, J., Zhou, M., Zhou, Y., Xiu, P., Liu, F., et al. (2021). Targeting of the COX-2/PGE2 axis Enhances the Antitumor Activity of T7 Peptide In Vitro and In Vivo. Drug Deliv. 28, 844–855. doi:10.1080/10717544.2021.1914776
Yang, X., Wang, D., Lin, J., Yang, X., and Zhao, H. (2020). Atezolizumab Plus Bevacizumab for Unresectable Hepatocellular Carcinoma. Lancet Oncol. 21, e412. doi:10.1016/s1470-2045(20)30430-7
Yuan, J.-M., Grouls, M., Carmella, S. G., Wang, R., Heskin, A., Jiang, Y., et al. (2019). Prediagnostic Levels of Urinary 8-Epi-Prostaglandin F2α and Prostaglandin E2 Metabolite, Biomarkers of Oxidative Damage and Inflammation, and Risk of Hepatocellular Carcinoma. Carcinogenesis 40, 989–997. doi:10.1093/carcin/bgy180
Yue, X., Yang, F., Yang, Y., Mu, Y., Sun, W., Li, W., et al. (2011). Induction of Cyclooxygenase-2 Expression by Hepatitis B Virus Depends on Demethylation-Associated Recruitment of Transcription Factors to the Promoter. Virol. J. 8, 118. doi:10.1186/1743-422x-8-118
Zang, S., Ma, X., Wu, Y., Liu, W., Cheng, H., Li, J., et al. (2017). PGE 2 Synthesis and Signaling in Malignant Transformation and Progression of Human Hepatocellular Carcinoma. Hum. Pathol. 63, 120–127. doi:10.1016/j.humpath.2017.02.018
Zang, S., Ni, M., Lian, Y., Zhang, Y., Liu, J., and Huang, A. (2013). Expression of Microsomal Prostaglandin E2 Synthase-1 and its Role in Human Hepatocellular Carcinoma. Hum. Pathol. 44, 1681–1687. doi:10.1016/j.humpath.2013.04.007
Zhang, H., Cheng, S., Zhang, M., Ma, X., Zhang, L., Wang, Y., et al. (2014a). Prostaglandin E2 Promotes Hepatocellular Carcinoma Cell Invasion through Upregulation of YB-1 Protein Expression. Int. J. Oncol. 44, 769–780. doi:10.3892/ijo.2013.2234
Zhang, M., Zhang, H., Cheng, S., Zhang, D., Xu, Y., Bai, X., et al. (2014b). Prostaglandin E2 Accelerates Invasion by Upregulating Snail in Hepatocellular Carcinoma Cells. Tumor Biol. 35, 7135–7145. doi:10.1007/s13277-014-1963-4
Keywords: prostaglandin E2, EP receptor, hepatocellular carcinoma, tumorigenesis, tumor progression, tumor treatment
Citation: Chen C, Guan J, Gu X, Chu Q and Zhu H (2022) Prostaglandin E2 and Receptors: Insight Into Tumorigenesis, Tumor Progression, and Treatment of Hepatocellular Carcinoma. Front. Cell Dev. Biol. 10:834859. doi: 10.3389/fcell.2022.834859
Received: 13 December 2021; Accepted: 07 February 2022;
Published: 10 March 2022.
Edited by:
Mitsugu Fujita, Kindai University, JapanReviewed by:
Liya Pi, Tulane University, United StatesJordi Muntané, Spanish National Research Council (CSIC), Spain
Copyright © 2022 Chen, Guan, Gu, Chu and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haihong Zhu, emh1aGg3MkB6anUuZWR1LmNu
 Chao Chen
Chao Chen Haihong Zhu
Haihong Zhu