- 1Key Laboratory of Anti-inflammatory and Immune Medicine, Ministry of Education, Collaborative Innovation Center of Anti-inflammatory and Immune Medicine, Institute of Clinical Pharmacology, Anhui Medical University, Hefei, China
- 2Department of Pharmacology, University of California, Davis, Davis, CA, United States
- 3VA Northern California Health Care System, Mather, CA, United States
G protein-coupled receptors (GPCRs), as the largest family of receptors in the human body, are involved in the pathological mechanisms of many diseases. Heterotrimeric G proteins represent the main molecular switch and receive cell surface signals from activated GPCRs. Growing evidence suggests that Gα12 subfamily (Gα12/13)-mediated signaling plays a crucial role in cellular function and various pathological processes. The current research on the physiological and pathological function of Gα12/13 is constantly expanding, Changes in the expression levels of Gα12/13 have been found in a wide range of human diseases. However, the mechanistic research on Gα12/13 is scattered. This review briefly describes the structural sequences of the Gα12/13 isoforms and introduces the coupling of GPCRs and non-GPCRs to Gα12/13. The effects of Gα12/13 on RhoA and other signaling pathways and their roles in cell proliferation, migration, and immune cell function, are discussed. Finally, we focus on the pathological impacts of Gα12/13 in cancer, inflammation, metabolic diseases, fibrotic diseases, and circulatory disorders are brought to focus.
Introduction
G protein-coupled receptors (GPCRs) family are a superfamily of membrane receptors responsible for signal transduction in cells. GPCRs are extensively studied drug targets because they participate in a broad range of human physiological and pathological processes. There are currently 481 drugs (about 34% of all drugs approved by the FDA) acting on 107 unique GPCRs to treat different diseases, including neurological disorders, metabolic and cardiovascular diseases, cancer, and inflammation (Hauser et al., 2017; Wang et al., 2020). Heterotrimeric G proteins are sensors for GPCR active conformations and trigger intracellular signal transduction (Maziarz et al., 2020). Heterotrimeric G proteins are composed of Gα, Gβ, and Gγ subunits, which are mainly located on the inner leaflet of the plasma membrane (Bondar and Lazar, 2021). Gα proteins are divided into four categories based on sequence homology and downstream effectors: Gαs (s stands for stimulation), Gαi/o (i stands for inhibition), Gαq/11, and Gα12/13 (Hilger et al., 2018; Yang et al., 2020). The function of Gαs, Gαi/o, and Gαq have been well documented. Meanwhile, the progress in understanding the function of the Gα12/13 family, which was discovered in the early 1990s, has been relatively slow (Kim et al., 2018a). Nevertheless, with the development of new research tools (for example, constitutively active mutants, fusion proteins, and gene knockout, etc.), progress has been made in further to understanding the function of Gα12/13 in recent years (Worzfeld et al., 2008).
Gα12/13 subunits are expressed in most cell types and are able to induce diversified cellular signaling and responses that are important players in health and disease. Gα12 and Gα13 share 67% of their amino acid sequence and have many downstream signaling targets in commm (Montgomery et al., 2014; Stecky et al., 2020). Some of the pathways that are triggered by both Gα12 and Gα13 include phospholipase C (PLC)-ε and phospholipase D, mitogen-activated protein kinase (MAPK), and Na/H-exchange which promote cytoskeletal alterations, carcinogenic responses, and apoptosis (Litosch, 2012; Dusaban et al., 2013; Xie et al., 2016). Moreover, Gα12/13 interacts with specific guanine nucleotide exchange factors (GEFs) (e.g., p115RhoGEF, leukemia-related RhoGEF, and PDZ-RhoGEF) to activate downstream effectors, including ras homolog family member A (RhoA), PLC, adenylate cyclase, and a variety of ion channels (Mikelis et al., 2013). These effectors, in turn, regulate the concentration of secondary messengers in the cells, such as diglycerides, cyclic adenosine monophosphate (cAMP), sodium ions, and calcium ions. Together, the activated RhoA and sencodary messengers eventually lead to physiological responses (Jiang et al., 2008; Kalwa et al., 2015; Wang et al., 2019). Furthermore, Gα12/13 subunits also regulate the activity of a variety of transcription factors, such as signal transducer and activator of transcription 3, serum response factor (SRF), activator protein 1 (AP-1), and activated T cell nuclear factor (NFAT) (Kumar et al., 2006; Lee et al., 2009; Song et al., 2018; Yagi et al., 2019). Abnormally elevated upstream stimuli promote the incidence and development of diseases by increasing the corresponding receptor coupling to Gα12 and/or Gα13 (Yang et al., 2020; Rasheed et al., 2021). In this review, the structure of Gα12 and Gα13 is reviewed along with their roles in GPCR signal pathways, cell function, and disease pathogenesis.
The Amino Acid Structure of Gα12 and Gα13
The Gα12/13 subfamily consists of two α subunits encoded by GNA12 and GNA13. The Gα12/13 subunits were originally discovered based on the amino acid sequence similarity with other Gα subunits and their insensitivity to pertussis toxin (Arang and Gutkind, 2020). The structure of Gα12/13 subunit consists of an amino-terminal α-helical domain and a Ras-like GTPase domain. There is a link between these two domains involved in binding to GDP and GTP (Syrovatkina et al., 2016; Smrcka and Fisher, 2019). In response to activation of GPCR, the conformation of GDP-bound inactive Gα12/13 is transformed into the active form with GTP binding, triggering the dissociation of Gα12/13 from Gβγ and activation of downstream effectors (Arthofer et al., 2016). After dissociation, free Gβγ subunits transmit signals through regulating canonical effectors, including adenylate cyclase, PLC, and various ion channels (Senarath et al., 2018). Gβγ subunits also regulate a series of non-canonical effectors, such as the nuclear import of the extracellular regulated protein kinases (ERK) 1/2, oxidative phosphorylation, and mRNA processing (Khan et al., 2016). The large number of Gβγ subunits in mammal cells define much of the diversity that occurs within GPCR signaling with resepect to spatial and temporal bias and are extensively involved in the pathogenesis of diseases (Masuho et al., 2021). Gβγ signaling has been previously well summarized.
Although Gβγ-mediated effects of GPCR signaling are diverse, the assorted isoforms of Gα also have a wide variety of influences. These varied functions are highly related to their structures. Under present consideration, Gα12 has four isoforms, of which isoform 1 is the longest isoform containing 381 amino acids (Figure 1). Compared with isoform 1, the encoded isoform 2 (305 amino acids) and isoform 3 (322 amino acids) are shorter and have different N-termini. Both Gα12 isoform 2 and 3 have distinct 5′ untranslated region and 5′ coding region for different N-termini. The isoform 4 of Gα12 lacks in-frame exons in the 3′ coding region relative to the isoform 1; the encoded isoform 4 (364 amino acids) is also shorter than isoform 1. Gα13 has two isoforms, of which isoform 1 is the longer isoform containing 377 amino acids. The isoform 2 of Gα13 uses an alternative 5′ exons resulting in a downstream start codon AUG. Thus, the encoded isoform 2 of Gα13 has a shorter N-terminus than the isoform 1. The isoform sequences of Gα12 or Gα13 show similar structural domains, including an adenylyl cyclase binding site, a β-γ complex binding site, a switch I region (one of two surface loops that undergo conformational changes upon GTP binding), a switch II region, and a putative receptor binding site, respectively, (Lambright et al., 1996; Sunahara et al., 1997; Tesmer et al., 1997). Additional difference in the amino acid sequence of each isoform may provide tissue specific expression and cellular localization to fulfill the broad functional roles of the Gα12 or Gα13.
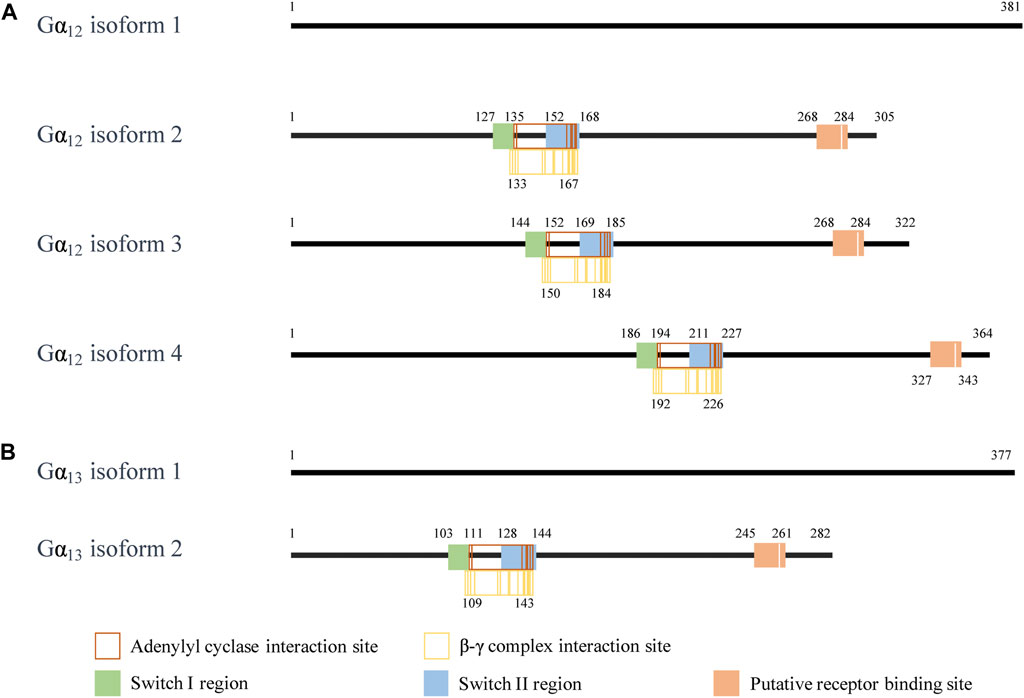
FIGURE 1. The interaction site of the Gα12/13 isoform. (A) The interaction sites on the amino acid fragment of Gα12 isoforms. (B) The interaction sites on the amino acid fragment of Gα13 isoforms. The information on the interaction sites of Gα12 and Gα13 is from NCBI. In NCBI reference sequence database, the human GNA12 isoforms are NP_031379.2, NP_001269369.1, NP_001269370.1, and NP_001280021.1. (https://www.ncbi.nlm.nih.gov/gene/2768). The human GNA13 isoforms are NP_006563.2 and NP_001269354.1. (https://www.ncbi.nlm.nih.gov/gene/10672).
While literature clearly show that Gα12/13 has different functions from other Gα subtypes, the functional difference between the isoforms of Gα12 and Gα13 has not been reported. Future studies on the structure-functional relationship between these isoforms will help understanding their roles in cells and diseases.
Regulation Network of Gα12/13
Gα12/13 has been shown to couple to more than 30 GPCRs. Activated by various upstream stimuli, Gα12/13 can transmit to divergent downstream signaling pathways and regulates cell function in pathophysiological processes (Ackerman et al., 2015; Yung et al., 2017; Yanagida et al., 2018; Spoerri et al., 2020). Gα12 and Gα13 are broadly expressed, yet gene deficiency in mice shows that they are not interchangeable (Yang et al., 2020). Some GPCRs preferentially couple to either Gα12 or Gα13 (Ayoub and Pin, 2013; Yung et al., 2017; Mackenzie et al., 2019). Moreover, non-GPCRs are also shown to couple to Gα12 and Gα13 (Shen et al., 2013; Shen et al., 2015; Piccinin et al., 2019) (Table 1).
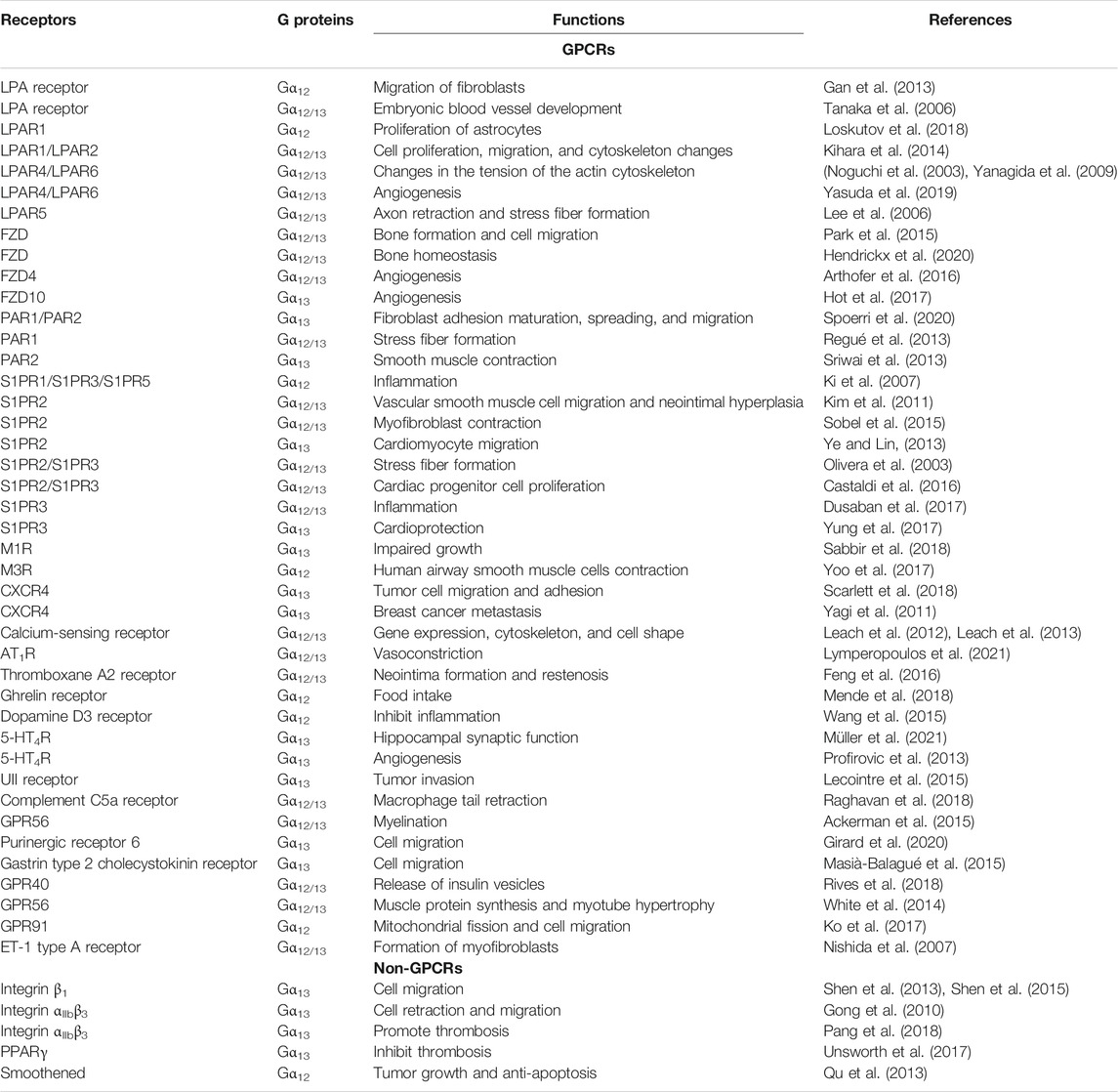
TABLE 1. List of biologically significant Gα12/13-associated receptors and their physiological functions.
GPCRs Triggered Gα12/13 Signaling
Lysophosphatidic Acid Receptors
Lysophosphatidic acid (LPA) is a biologically active phospholipid, which mediates various biological functions through six homologous LPA receptors (LAPRs) (Plastira et al., 2020). Activated LPARs promote Gα12, but not Gα13, association with v-raf murine sarcoma viral oncogene homolog A which than activates ERK. The activated ERK promotes ring finger and FYVE like domain-containing E3 ubiquitin protein ligase transcription and fibroblast migration (Gan et al., 2013). LPAR-Gα12/13 signaling also plays an important role in embryonic blood vessel development (Tanaka et al., 2006). Additionally, LPAR1/2 couple with Gαi/o, Gαq/11, as well as Gα12/13 to activate a variety of downstream pathways, such as protein kinase B (PKB, commonly known as AKT), RhoA, MAPK, and phosphatidylinositol 3-kinase (PI3K), and regulates cell proliferation, migration, and cytoskeleton rearrangement (Kihara et al., 2014). Previous studies have identified that LPAR4/6 can effectively couple to Gα12/13 to stimulate Rho GTPase, which subsequently activates Rho-associated protein kinase (ROCK) I/II, leading to changes in the tension of the actin cytoskeleton (Noguchi et al., 2003; Yanagida et al., 2009). The LPAR4/6-activated Gα12/13-Rho-ROCK signal also promotes nuclear translocation of Yes-associated protein/transcriptional coactivator with PDZ-binding motif (YAP/TAZ) (Yasuda et al., 2019). YAP/TAZ promotes the proliferation of cancer cells (such as liver, bladder, and lung cancers) and accelerates the progress of cancer (Yagi et al., 2016; Maziarz et al., 2020). Activation of LPAR5 also induces axon retraction and stress fiber formation through an LPA-LPAR5-Gα12/13 pathway (Lee et al., 2006). As of today, there is no evidence supporting a coupling between LPAR3 and Gα12/13.
Frizzleds
Frizzled (FZD) receptors are unconventional GPCRs, which can be activated by the Wingless/Int-1 lipoglycoprotein (WNT) family (Janda et al., 2017). FZD4 interacts with Gα12/13 and does not interact with other subunits of the G protein family. The complex formed by FZD4 and Gα12/13 is dissociated under WNT stimulation. The FZD4-Gα12/13 signaling mediates cytoskeletal rearrangement and Rho signaling through p115RhoGEF, affecting angiogenesis in embryonic and tumor development (Arthofer et al., 2016). WNT5a/b and WNT3a bind to the receptor tyrosine kinase-like orphan receptor 1/2-FZD complex, activating Rho GTPases through Gα12/13. The activated Rho inhibits the activity of large tumor suppressor 1/2 (LATS1/2), leading to YAP/TAZ dephosphorylation and nuclear translocation and promoting bone formation and cell migration (Park et al., 2015). Interestingly, a similar mechanism has been found in brain endothelial cells. The FZD10-Gα13 complex dissociates under WNT5a/7a stimulation, and Gα13 transmits a signal to YAP/TAZ through Rho family members (Hot et al., 2017). In osteoblasts, WNT family member 16 binds to FZD receptors to activate canonical WNT signaling and non-canonical Gα12/13 signaling and regulate bone homeostasis (Hendrickx et al., 2020).
Protease-Activated Receptors
Protease-activated receptors (PARs) consist of four subtypes (PAR1-4). PARs play an important role in blood vessel development, cell proliferation, tumorigenesis, and thrombosis (Chandrabalan and Ramachandran, 2021). In fibroblasts, PAR1/2 send signals to integrin α5β1 through Gβγ and PI3K to induce fibronectin binding and initiate cell adhesion. PAR1/2 also send signals through Gα13, Gαi, ROCK, and Src to enhance integrin α5β1-mediated adhesion (Spoerri et al., 2020). The PAR1-mediated Gα12/13 activation stimulates RhoGEF and simultaneously activates the RhoA-ROCK pathway and myosin light chain (Flaumenhaft and De Ceunynck, 2017). Stimulation of PAR1 also initiates the assembly of F-actin through the Gα12/13-RhoA pathway, which induces YAP dephosphorylation and nuclear translocation, thereby promoting cell migration and invasion (Regué et al., 2013). PAR2 couples with Gαq, Gαi, and Gα13 to stimulate RhoA-ROCK activity independently of the cyclic adenosine monophosphate-protein kinase A pathway to induce smooth muscle contraction (Sriwai et al., 2013). An earlier study found that in endothelial cells, PAR3 directly interacts with PAR1 to change the binding conformation of PAR1/Gα13, induce the activation of downstream signaling pathways, and promote endothelial barrier dysfunction (McLaughlin et al., 2007). Presently, there is no clear evidence showing a direct interaction between PAR3/4 and Gα12/13.
Sphingosine 1-Phosphate Receptors
Sphingosine 1-phosphate (S1P) is a natural biologically active lipid molecule that binds to five different S1P receptors (S1PR1-5). Activated S1PRs couple with Gα12/13 to induce downstream signals (Zhang et al., 2020). An earlier study shows that S1PR2/S1PR3 couple to Gα12/13 and send an “inside-out” signal to mediate the formation of stress fibers (Olivera et al., 2003). After binding to S1P, S1PR1/3/5 couple with Gα12 and activate the c-Jun N-terminal kinase (JNK)-nuclear factor-kappa B (NF-κB) pathway to promote the expression of cyclooxygenase-2 and accelerate local inflammation (Ki et al., 2007). S1PR3 activates the Gα12/13-RhoA pathway and promotes the expression of inflammatory gene in astrocytes (Dusaban et al., 2017). S1PR3 activates Gα13-RhoA in cardiomyocytes and mediates cardio-protection during ischemia/reperfusion (I/R) (Yung et al., 2017). In vascular smooth muscle cells, S1PR2 couples to Gα12/13 to activate AP-1-dependent induction of cysteine-rich protein 61 and promote the migration of vascular smooth muscle cells and neointimal hyperplasia (Kim et al., 2011). S1PR2 also induces the contraction of myofibroblasts through the Gα12/13-Rho-ROCK pathway (Sobel et al., 2015). Moreover, Gα12, activated by S1PR2, is recruited to E-cadherin to form a complex after mechanical stress. Gα12 then recruits and activates p114RhoGEF, driving RhoA signaling and increasing the tensile strength of multicellular connections (Acharya et al., 2018).
Muscarinic Acetylcholine Receptors
Muscarinic acetylcholine receptors have five different subtypes (M1R-M5R) (Ruan et al., 2021). The increase of acetylcholine signal in neurons leads to the overexpression of M1R, promoting the polymerization of Gα13 and Gβγ (Sabbir et al., 2018). Gα13 disrupts the stability of tubulin polymer through the RhoA-ROCK signal, reducing mitochondrial transport and impairing growth in neurites (Sabbir et al., 2018). Early study has shown that M3R promotes the activity of phospholipase D through Gα12 in HEK-293 cells (Rümenapp et al., 2001). It has also been found that Gα12 binds to M3R in human airway smooth muscle cells. The M3R-Gα12 signaling is important in promoting the contraction of human airway smooth muscle cells by inducing PI3K-mediated ROCK activation in a RhoA-dependent manner (Yoo et al., 2017).
Chemokine Receptors
The binding of chemokine C-X-C motif chemokine 12 (CXCL12) to C-X-C chemokine receptor 4 (CXCR4) activates RhoA through Gα13, which leads to ROCK phosphorylation of myosin light chain and promotes cell migration and adhesion (Scarlett et al., 2018). In metastatic breast cancer cells, CXCR4 activates small Rho GTPases through Gα13 to initiate cell motility and trans-endothelial migration (Yagi et al., 2011).
Non-GPCRs Triggered Gα12/13 Signaling
Integrin “outside-in” signaling requires Gα13 and monomeric small G proteins (i.e., Rho) to mediate cell retraction, migration, and spreading (Shen et al., 2012). Gα13 directly binds to the ExE motif in the cytoplasmic domain of the integrin β3 subunits, which is important in transducing the “outside-in” integrin signaling (Shen et al., 2013). Gα13 also binds to the cytoplasmic domain of the integrin β1 subunit in platelets, which mediates the Src-dependent transient inhibition of RhoA, activates the Rac1 and PI3K pathways, and promotes cell migration (Shen et al., 2013; Shen et al., 2015). Interfering with the expression of Gα13 reduces αIIbβ3-dependent activation of c-Src, inhibits cell migration, and accelerates cell contraction, thereby spreading platelets on fibrinogen (Gong et al., 2010). Integrin αIIbβ3 also serves as a mechanical sensor that transmits “outside-in” signals through Gα13-Src-Rac1-dependent pathways in platelets and facilitates coagulation in vitro and intravascularly in vivo (Pang et al., 2018). Therefore, the Gα13-integrin interaction is important in thrombosis.
Additionally, peroxisome proliferator-activated receptor-γ (PPARγ), a member of the nuclear hormone superfamily, regulates lipid and glucose metabolism and homeostasis in many metabolic pathways (Piccinin et al., 2019). Treatment of platelets with PPARγ agonists leads to decreased binding between Gα13 and integrin β3, which prevents c-Src-dependent integrin β3 phosphorylation and talin dissociation and weakens the downstream signal transduction of integrin αIIbβ3, thereby regulating platelet activation and reducing thrombosis (Unsworth et al., 2017). Moreover, in diffuse large B-cell lymphoma (DLBCL), smoothened recruits Gαi and Gα12 and activates the protein kinase C (PKC)-caspase recruitment domain and membrane-associated guanylate kinase-like domain protein 1-dependent signaling cascade, which promotes activation of NF-κB, tumor growth, and anti-apoptosis (Qu et al., 2013).
Gα12/13 and Biased Signaling
Traditionally, each GPCR is thought to initiate the “canonical” signal transduction through a single homologous G protein class (Seyedabadi et al., 2019). With the advances in the study of the ligand-receptor-effector relationship, it has been found that specific ligands induce a GPCR to selectively bind to a particular G protein subunit, transducing biased intracellular signaling toward one of many downstream pathways. This phenomenon is called “biased signaling”; and functionally selective ligands are called “biased ligands” (Tan et al., 2018).
Up to now, only a few GPCRs have shown biased signaling, but it is still the early days of understanding the biased signaling mechanisms. The characteristics of biased ligands, which have strong or weak activity in different pathways, may provide significant clinical advantages for developing new drugs. However, attention should be paid to testing conditions, ligand verification, and patient and disease selection to achieve successful biased ligand therapy (Seyedabadi et al., 2019). So far a few of the receptor types seen to produce biased signaling involving Gα subunit switch include calcium-sensing receptors, angiotensin receptors, PARs, prostaglandin receptors, and ghrelin receptor.
The biased signaling can occur due to different underlying principles. The well-documented biased signaling is triggered by biased ligands. For instance, calcium-sensing receptor induces the activation of four G protein subfamilies (Gαq/11, Gαi/o, Gα12/13, and Gαs) (Abid et al., 2021). The ligands NPS-2143/NPS-R568 binds to a calcium-sensing receptor to specifically mediate the activation of Gα12/13-RhoA signal, which in turn activates various other signal checkpoints that regulate gene expression, cytoskeleton, and cell shape (Leach et al., 2012; Leach et al., 2013). Similarly, the prostaglandin F2α receptor, a Gαq-coupled GPCR, is activated by prostaglandin F2α (Pathe-neuschäfer-rube et al., 2005). PDC113.824 acts on the prostaglandin F2α receptor, which biasedly increases Gαq-PKC-ERK1/2 signaling while inhibiting Gα12-Rho-ROCK signaling, blocking cell contraction and skeletal reorganization, and inhibiting uterine contraction (Goupil et al., 2010). PAR2 is activated mainly by trypsin-like serine proteases and regulates various signaling pathways in coupling with Gαi/o, Gαq/11, and Gα12/13 (Kim et al., 2018b). Among PAR2 antagonists, I-287 inhibits Gαq, but biasedly activates Gα12/13 without affecting Gαi/o signaling and β-arrestin recruitment, thereby attenuating the PAR2-mediated inflammatory response (Avet et al., 2020). Likewise, the ghrelin receptor in the arcuate nucleus of the hypothalamus, activated by Ghrelin, induces an intracellular signaling cascade through Gαq, Gαi/o, Gα12/13, and β-arrestin (Hedegaard and Holst, 2020). The biased ligand YIL781 selectively activates Gαq/11 and Gα12 through the ghrelin receptor without intrinsic activity for β-arrestin recruitment, leading to an increased food intake and a reduced gastric emptying (Mende et al., 2018). However, other factors, including ionic strength, lipid environments, and downstream signaling partners, can also contribute to biased signaling observed in native cells and tissues. For instance, receptors can couple in a cell type-specific manner. The binding of angiotensin II (Ang II) to the Ang II type 1 receptor (AT1R) causes AT1R interaction with Gαq/11, Gα12/13, and Gαi, depending on cell type (Forrester et al., 2018). When Ang II stimulates vascular smooth muscle cells, AT1R binds to Gα12/13 instead of Gαq/11 to activate RhoA-ROCK and promote vasoconstriction (Lymperopoulos et al., 2021). Cleaving a receptor is another method that often leads to a change in downstream signaling. For PAR1, the endogenous ligand thrombin promotes PAR1 binding to heterotrimeric G proteins of the Gαq/11, Gα12/13, Gαi, and Gαs families. The activation of matrix metalloproteinase-1 in platelets cleaves the N-terminal extracellular domain of PAR1, activating the Gα12/13-Rho-MAPK signal instead of Gαq/11, and promotes cell shape changes and platelet thrombosis (Trivedi et al., 2009).
Gα12/13Signaling and Cell Function
As discussed above, the well-characterized downstream effector of active Gα12/13 is the Rho GTPases through stimulating RhoGEF, which regulates the actin cytoskeleton and participates in various cellular functions, including cell proliferation, migration, contractility, and gene expression (Bodmann et al., 2017) (Figure 2).
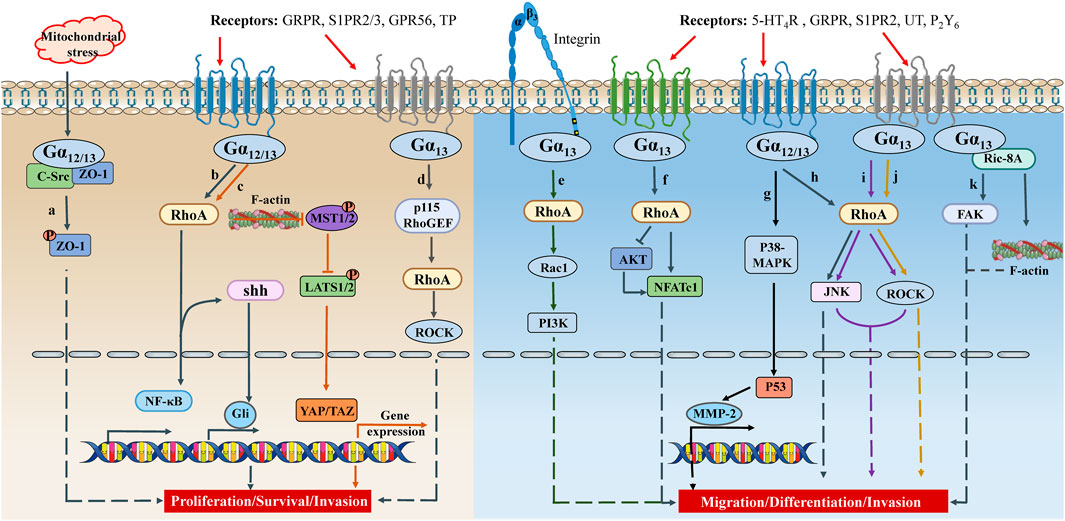
FIGURE 2. The crosstalk between Gα12/13 signaling and cell proliferation/migration. Under different physiological/pathological environments, GPCRs on the cell membrane receive different signals, couple Gα12 or Gα13, activate signal cascades, and promote synthesis of transcription factors or secretion of inflammatory factors. These signals ultimately lead to different cell activities, such as cell proliferation, survival, differentiation, migration, invasion, etc. TP, thromboxane A2 receptor; UT, urotensin II receptor; P2Y6, purinergic receptor 6; shh, sonic hedgehog; MST1/2, mammalian sterile 20-like kinases 1/2.
Cell Growth and Apoptosis
Gα12/13 was initially identified as an oncogene with the potential for tumor transformation of fibroblasts (Chan et al., 1993; Xu et al., 1993). Subsequent studies have shown that Gα12/13 can promote mitogenic response and cell growth by transducing a RhoA-dependent signal, which increases YAP/TAZ-dependent gene expression (Goldsmith and Dhanasekaran, 2007; Syrovatkina and Huang, 2019).
Subsequent publications have revealed that Gα12/13 coupling with several different receptor types promotes proliferation. S1P activates S1PR2/3 to trigger Gα12/13-RhoA signaling, leading to the proliferation of mouse cardiac progenitor cells and regulating gene transcription in hearts (Castaldi et al., 2016). Stimulation of the thromboxane A2 receptor also promotes the activity of Rho GTPase through Gα12/13. The activated Rho GTPase regulates actin cytoskeleton, increases nuclear translocation of YAP/TAZ, and promotes proliferation and migration of T/G HA-vascular smooth muscle cells (Feng et al., 2016). After the loss of primary cilia on human astrocytes, LPA promotes association between LPAR1 and Gα12/Gαq, augmenting mitogenic signaling and cell proliferation (Loskutov et al., 2018). Furthermore, the acetylcholine signaling via M1R activates Gα13 protein to disrupt tubulin polymerization in axons and inhibit mitochondrial transport, thereby limiting the growth of neurites (Sabbir et al., 2018). G protein-coupled receptor 56 (GPR56) interacts with Gα12/13 to mediate RhoA signaling and regulates zebrafish oligodendrocytes’ development and subsequent myelination (Ackerman et al., 2015).
Gα12/13 is also very important in the proliferation of tumor cells. The activation of Gα12/13 promotes cell growth and tumor development of hepatocellular, small cell lung carcinoma, and ovarian cancer cells, but not in breast and prostate cancer cells (Grzelinski et al., 2010; Rasheed et al., 2013; Yagi et al., 2016; Syrovatkina and Huang, 2019). The synthetic ligand, Clozapine N-oxide, stimulates GPCR-Gα12/13 signaling and promotes the proliferation of ovarian cancer cells by activating YAP1 (Yagi et al., 2016). Bombesin secreted by small cell lung carcinoma cells activates the gastrin-releasing peptide receptor (GRPR)-Gα12/13-Rho-NF-κB signaling cascade. Subsequently, the activated NF-κB increases the production of Sonic Hedgehog, which activates the Gli transcription factor and promotes cell proliferation, survival, blood vessel generation, and local invasion (Castellone et al., 2015).
Meanwhile, the combination of muscle-restricted coiled-coil protein and caveolin-1 promotes Gα13-mediated p115RhoGEF activation, leading to subsequent activation of the Rho-ROCK signal and enhancing the proliferation and migration of human pulmonary artery smooth muscle cells (Nakanishi et al., 2016). Moreover, Gα13 dynamically regulates the RhoA signaling through the combination of RhoGEF GTPase and integrin β1, promoting integrin β1-mediated proliferation of CHO cell lines (Shen et al., 2015). In addition to autophagy-mediated mitochondrial damage and oxidative stress, lysosomal dysfunction in cystopathy stimulates Gα12/Src-mediated phosphorylation of zona occludin-1. The activated zona occludin-1-related signaling cascade promotes the proliferation of epithelial cells and disrupts the cell lining along the proximal tubules of mouse kidneys (Festa et al., 2018).
Studies have also found that Gα12 and Gα13 regulate apoptosis. In Madin-Darby canine kidney cells, the activation of endogenous Gα12 and thrombin stimulation increase the activity of JNK1, inhibit the activity of NF-κB, and promote cell apoptosis (Yanamadala et al., 2007). In melanoma cells, US28, a GPCR encoded by human cytomegalovirus, is coupled with Gα13 to induce cell apoptosis; silencing Gα13 inhibits cell apoptosis driven by US28 (Joshi et al., 2015). Notably, this event has been only observed in human melanoma cell lines but not in murine cells. The emerging discovery of ligand-GPCR-Gα12/13 signals will offer insight into the regulation of apoptosis on cancer cells.
Cell Migration
The ability of cell migration is essential for cell growth, proliferation, the inputs and outputs of nutrients and signal intermediates, and normal cell physiology. Cell movement also enhances tumor cell invasion and metastasis (Svitkina, 2018). The process of cell migration is usually accompanied by changes in the actin cytoskeleton. Gα12/13 activates RhoA through their respective RhoGEF effectors and promotes the dynamic changes of cell shape controlled by actin cytoskeleton reorganization (Castillo-Kauil et al., 2020).
Gα12/13 plays pivotal roles in cell migration that occurs during development. For instance, the 5-hydroxytryptamine type 4 receptor (5-HT4R) triggers Gα13-mediated RhoA signal transduction, promoting the reorganization of filamentous actin and the morphology of mouse astrocytes, and enhancing hippocampal synaptic function (Müller et al., 2021). The 5-HT4R also mediates human endothelia cell migration and angiogenesis through the Gα13-RhoA-ROCK pathway in vitro (Profirovic et al., 2013). In zebrafish development, the S1PR2-Gα13-RhoGEF signal is necessary for the convergent movement of the endoderm by promoting myocardial migration at all stages of heart development (Ye et al., 2015). Disrupting the S1PR2-Gα13-RhoGEF pathway jeopardizes the endoderm’s convergence and the myocardium’s migration during the segmentation process (Ye and Lin, 2013). With the high concentrations of urotensin II stimulation, urotensin II receptors recruit Gα13 to activate the Rho-ROCK pathway and promote actin polymerization, which contributes to glioma cells invasion and new blood vessel formation (Lecointre et al., 2015). Gα12 participates in cell differentiation through the nuclear factor of activated T-cell c1 (NFATc1) and regulates cell migration and resorption through RhoA in the process of osteoclast formation in mice (Song et al., 2018). Resistance to inhibitors of cholinesterase 8A (Ric-8A) is a guanine nucleotide exchange factor of Gα subunits and an important partner of Gαi, Gαq, and Gα13 proteins (Papasergi-Scott et al., 2018). In Xenopus cranial neural crest cells, the Ric-8A-Gα13-focal adhesion kinase (FAK) signal regulates focal adhesion dynamics and neurite formation to control cell migration (Toro-Tapia et al., 2018). The activated Ric-8A catalyzes the nucleotide exchange on Gα13, induces the reorganization of the actin cytoskeleton, and promotes the migration of mouse embryonic fibroblasts (Wang et al., 2011).
Cell migration that is mediated by Gα12/13 is also important in metastasis. For example, the activated purinergic receptor 6 increases the number of filopodia and adhesions of human lung cancer cells (A549) and colorectal cancer cells (Caco-2) through both Gαq-Ca2+-PKCα and Gα13-ROCK signals, regulating cell migration (Girard et al., 2020). The GRPR-Gα13-RhoA-ROCK signaling is necessary for GRP to stimulate the migration of human colon cancer cells. This signaling also promotes the expression of COX-2, which contributes to cell migration (Patel et al., 2014). Similarly, Gα12/13 signal facilitates the invasion of human cervical cancer cells through a RhoA-ROCK-JNK signal axis (Yuan et al., 2016). Gα12/13 increases the gene expression of metalloproteinase-2 through p53, which is necessary for inducing the invasion and migration phenotypic changes of untransformed human breast epithelial cells (Kim et al., 2010). Gα12, which is overexpressed in many hepatocellular carcinoma patients, relieves the regulation of p53-responsive miRNA and promotes the metastasis and invasion of tumor cells (Yang et al., 2015). The Gα12-mediated pathway promotes metastasis and invasion of human nasopharyngeal carcinoma cells by regulating the reorganization of the actin cytoskeleton (Liu et al., 2009).
Increased Gα12/13 mediated cell migration also greatly contributes to primary solid tumor growth. Take as example that Gα13, which is up-regulated in breast cancer, inhibits the transcription of kallikreins through the RhoA-ROCK pathway and promotes the invasion and metastasis of breast cancer cells (Teo et al., 2016). The expression of GNA13 in breast cancer cells is primarily regulated by MicroRNA (miR)-31, and the absence of miR-31 increases the expression of GNA13 and the invasion of cancer cells (Rasheed et al., 2015). Similarly, Gα13 is overexpressed in pancreatic ductal adenocarcinoma and enhances the invasion of human pancreatic cancer cells in three-dimensional collagen by destroying cell adhesion; however, this process does not go through ROCK signal transduction (Chow et al., 2016). Gα13 is also involved in cell fusion (Carloni et al., 2013). Disintegrins and metalloproteinases stimulate Gα13 to activate RhoA. The up-regulated RhoA activity causes dephosphorylation of ezrin/radixin/moesin proteins and destruction of plasma membrane-cortical actin interaction, promoting fusion of human metastatic colon cancer cells and cell acquisition of drug resistance (Carloni et al., 2013).
These observations highlight the importance of Gα12/13-RhoA signaling in cell proliferation or migration. The expression of Gα12/13 and the downstream signaling are often elevated in cancer cells. However, in some disease states (especially cancer), the upstream ligand and receptor of Gα12/13 remain unknown.
Immune Cell Function
Due to the lack of specific inhibitors of Gα12/13, the research of Gα12/13 in immune cells was stagnant for many years. However, with various transgenic models, the studies on the role of Gα12/13 in immune cells are accelerating.
T Cells
After naive T cells are activated, CD4+ T cells differentiate into different effector subsets: T helper (Th) 1, Th2, Th17, and T follicular helper (Tfh) cells, which perform their specific auxiliary functions (Dong, 2021). During T cell activation, Gα12/13 regulates actin polymerization and contributes to cell adhesion and migration (Wang et al., 2013). A recent study has found that Gα13-RhoA-ROCK2 signaling plays a key regulatory role in the differentiation and function of early Tfh cells. The Gα13-deficient Tfh cells impair the function of adhering B cells to form conjugates and stimulating B cells to produce immunoglobulins (Kuen et al., 2021). Morover, receptors coupled to Gα12/13 have been demonstrated to be essential for T cell adhesion, differentiation, and retention in lymph nodes (Moriyama et al., 2014; Mathew et al., 2019). For example, S1PR2 promotes the maturation of Tfh cells by directing co-localization with B cells in the germinal center; and the deficiency of S1PR2 in Tfh cells inhibits the retention in lymph nodes (Moriyama et al., 2014). In response to the stimulation of CXCL12, the Gα13-Rho signal in human T cells mediates the endosomal trafficking of CXCR4 (Kumar et al., 2011). CXCL12 also promotes the migration of Jurkat T cells through the CXCR4-Gα13-Rho signal axis (Tan et al., 2006). Meanwhile, Gα12/13 negatively regulates the activation state of integrin leukocyte-function-antigen-1 to modulate CD4+T cells trafficking and proliferation and susceptibility to immune diseases (Herroeder et al., 2009). In response to the activation of T cell receptor signals, the interleukin-2 inducible T cell kinase directly interacts with Gα13 to mediate the activation of SRF transcriptional activity (Huang et al., 2013). Conversely, Gα12 is a key mediator of T cell receptor-mediated interleukin-2 production and controls the differentiation of Th2 and Th17 cells (Won et al., 2010). Additionally, Gα13, but not Gα12, mediated signal transduction is necessary for early thymocyte proliferation and survival (McNeil Coffield et al., 2004).
B Cells
Mature B lymphocytes express LPAR2/5, LPA negatively regulates B cell receptor signaling through the LPAR5–Gα12/13–Arhgef1 pathway, inhibiting the release of calcium stored in the cell and the antibody response (Hu et al., 2014). Gα12/13 also regulates the maturation, migration, and polarization of marginal zone B cells. In mice with depletion of Gα12/13 in B cells, the number of zone B cells and zone B cell precursors are significantly reduced, but the formation of pseudopods is increased (Rieken et al., 2006).
Knockout of Gα13 also results in the loss of restriction of B cells in the germinal center, thus spreading to lymph nodes and blood (Muppidi et al., 2014). Meanwhile, Gα13 plays a direct role in the growth inhibition of B cells, affecting their survival and differentiation. It has been noted that the impaired phosphorylation of AKT at the Ser473 site is related to Gα13 activity and may affect the growth and survival of B cells (Green et al., 2011; O’Hayre et al., 2016). The mechanism by which the Gα12/13-RhoA axis inhibits the growth of B cells needs to be further explored.
Macrophages
In macrophages, complement C5a couples to Gα12/13 to activate Rho GTPases and tail retraction in migrating cells. Macrophages without Gα12/13 show complete chemotaxis but increased migration speed and a moderately impaired tail contraction (van den Bos et al., 2020). Thrombin, a major platelet activator, selectively induces the expression of CD36, a plasma membrane fatty acid transporter, and foam cell formation of RAW264.7 cells through PAR1-Gα12 signaling and facilitates atherosclerosis (Raghavan et al., 2018). Thrombin also induces the migration of monocytes or macrophages through the PAR1-Gα12 pathway (Gadepalli et al., 2013). The loss of Ric-8A in lymphocytes and bone marrow-derived macrophages leads to a decrease in the expression of Gαi2/3, Gαq, and Gα13, but not Gα12, leading to anemia and leukocytosis (Boularan et al., 2015).
The above studies suggest that dysregulation of Gα12 or Gα13 in immune cells may lead to pathophysiological consequences, such as cancer and autoimmunity. So far, the research of Gα12/13 in immune cells is relatively scarce. More research is needed to bring new therapeutic targets for cancers and autoimmune diseases.
Gα12/13 Signaling and Diseases
Gα12/13 plays an important role in multiple stages of disease development in different tissues and organs. An increasing number of cancers have shown overexpressed Gα12/13, which is correlated to abnormal cell proliferation, metastasis, and invasion (Montgomery et al., 2014). The high abundance or constitutive activation of Gα12 and Gα13 are effective stimulators of oncogenic transformation. Gα12/13 also interacts with cell surface GPCRs and participates in the inflammation regulation (Dusaban et al., 2013; Hou et al., 2021). Meanwhile, Gα12/13 levels in metabolic organs, including liver and muscle, are altered in metabolic diseases (Koo et al., 2017; Kim et al., 2019). However, the mechanism by which Gα12/13 regulates the progression of these diseases has not been fully elucidated.
Gα12/13 and Cancer
Gα12/13 are called the gep proto-oncogenes and are usually overexpressed in cancers (Yagi et al., 2016). Besides the functional roles in cancer cell migration and invasion, Gα12/13 and Rho GTPases exert pro- or anti-cancer effects in a cancer type and background-dependent manner.
The results of Gene Expression Omnibus and The Cancer Genome Atlas database analysis have shown that GNA12 can be used as a biomarker for the personalized treatment of head and neck squamous cell carcinoma (HNSCC) patients and one of the prognostic genes for patients with HNSCC (Liu et al., 2021). GNA13 is also a biomarker for the prognosis and metastasis of solid tumors, including HNSCC, ovarian cancer, lung cancer, and gastric cancer (Cerami et al., 2012; Gao et al., 2013; Cancer Genome Atlas, 2015). GNA13 mutations are present at a high frequency in gastric cancer, nasopharyngeal cancer, prostate cancer, breast cancer, lymphoma, and bladder cancer (Muppidi et al., 2014; Wu et al., 2019; Arang and Gutkind, 2020).
The increased expression and activity of Gα12/13 contribute to the pathogenesis of different cancers. The copy number of GNA12 is significantly increased in ovarian cancer, which enhances the function of Gα12 to promote cancer growth and metastasis (Wu et al., 2019). In two prostate cancer cell lines (C4-2B and PC3), both Gα12 and Gα13 are abundantly expressed (El-Haibi et al., 2013). The highly expressed GNA13 also acts through Rho GTPase to drive the NF-κB transcription program and induce the expression of CXCL5 (Lim et al., 2019). A study on HNSCC has demonstrated that GNA13 promotes the aggressive phenotype and drug resistance of tumor-initiating cells through the MAPK/AP-1 and NF-κB pathways (Rasheed et al., 2018). In bladder cancer, the Arg-200 (residue required to hydrolyze GTP) mutation of Gα13 strongly activates YAP/TAZ-dependent TEAD and myocardin-related transcription factor-A/B-dependent SRF transcriptional activities through the RhoGEF-Rho GTPase cascade (Maziarz et al., 2020). Additionally, the low expression of MiR-30b-5p in renal cell carcinoma up-regulates the activity of Gα13, which promotes cell proliferation, metastasis, and epithelial cell-mesenchymal transition (Liu et al., 2017). The reduction of regulators of G protein signaling protein (RGS) 12 promotes the Gα12/13-RhoA-YAP pathway and Ezrin expression, which leads to the growth and progression of osteosarcoma and lung metastasis (Li et al., 2021). Moreover, Gα13-mediated down-regulation of LATS1 promotes phenotypic changes of epithelial cell-mesenchymal transition in ovarian cancer cells (Yagi et al., 2019). These findings identify the Gα13-Hippo signaling as a potential target for cancer therapeutic interventions.
A few GPCR ligands have been identified upstream of Gα12/13 in cancers. LPA promotes the combination of Gα12 and EFA6 through LPAR2 and activates the ADP-ribosylation factors 6 mesenchymal pathway, thereby promoting the invasion, metastasis, and drug resistance of renal cancer cells (Hashimoto et al., 2016). High concentrations of LPA are also accumulated in the ascites of patients with ovarian cancer (Yu et al., 2016). The heterodimerization of LPAR1 and CD97 amplifies the LPA-mediated Gα12/13-Rho signal transduction and promotes the invasion of prostate cancer cells (Ward et al., 2011). Gastrin induces the activation of paxillin and FAK through the cholecystokinin B receptor-Gα12/13-RhoA-ROCK signaling pathway, thereby promoting the redirection of the Golgi apparatus and the directional migration of pancreatic cancer cells (Mu et al., 2018). Gastrin also promotes the activation of Gα13 through the gastrin type 2 cholecystokinin receptor in colon cancer cells. The recruitment of p190RhoGEF by Gα13 enhances the phosphorylation of FAK and paxillin, leading to RhoA activation and promoting the migration of cancer cells (Masià-Balagué et al., 2015). In addition, the down-regulated GPR65 in a variety of hematological malignancies promotes Gα13/Rho signal transduction, which leads to the decrease of c-myc oncogene expression and promotes growth, migration, and metastasis of blood cancer cells (Justus et al., 2017). Gα13 binds to CXCR5 in response to CXCL13 treatment and promotes prostate cancer cell movements; however, silencing Gα13 does not affect CXCL13-dependent cell invasion (El-Haibi et al., 2013).
Interestingly, the Gα13-Rho GTPase signaling has also been shown to alleviate hematological malignancies (Justus et al., 2017). In malignant tumors of the hematopoietic and lymphatic system, GNA13 and RhoA mutations are present in B-cell lymphomas, mainly in DLBCL and Burkitt’s lymphoma (Justus et al., 2017). The mutation of GNA13 in germinal center B cells is resistant to programmed cell death (Healy et al., 2016). These B cells are differentiated, facilitating genetic instability through continuous somatic hypermutation (Muppidi et al., 2014). Over time, the accumulation of driver mutations in persistent germinal center B cells may cause lymphoma. Loss of Gα13 facilitates germinal center B cells to spread to the lymph node and blood, leading to the pathogenesis of DLBCL (Healy et al., 2016). Moreover, in the absence of Gα13 signaling, S1P acts through S1PR3 to promote mouse germinal center B cells migration into the circulation (Muppidi et al., 2015). S1PR2 signals through Gα13 to induce tumor cell apoptosis and exert its tumor suppressor function (Flori et al., 2016). However, in DLBCL, the forkhead box protein 1 directly inhibits S1PR2 and promotes tumor cell survival (Flori et al., 2016). The restoration and activation of the Gα13-Rho pathway help reduce tumor growth and progression, supporting that the Gα13-RhoA axis has a tumor suppressor effect (Justus et al., 2017). Meanwhile, analyzing the genome of tumor tissues from patients with classical Hodgkin lymphoma has revealed that GNA13 is one of the genes repeatedly mutated (Tiacci et al., 2018). GNA13 variants are mostly heterozygous, including nonsense, frameshift, and missense mutations, like the mutation patterns in DLBCL and Burkitt’s lymphoma (Muppidi et al., 2014; Justus et al., 2017).
Despite the emerging evidence supporting the critical roles of Gα12/13 in cancers, many questions remain about how the ligands and receptors trigger Gα12 or Gα13 signaling in a cancer type-specific manner. A comprehensive understanding of these mechanisms may provide a promising prospect for targeting specific Gα12/13 signaling processes in cancer therapies.
Gα12/13 and Inflammation
Gα12/13 has been implicated in both induction and suppression of inflammation. In the context of inflammation, thrombin, LPA, and S1P transmit signals through Gα12/13-coupled receptors to amplify inflammatory responses (Gavard and Gutkind, 2008; Dusaban et al., 2017; Lee et al., 2019).
Early studies have found a direct connection between Gα12/13 signaling and arachidonic acid in cells (Dermott et al., 1999; Mariggiò et al., 2006). NIH-3T3 cells transformed with Gα12QL show increased secretion of arachidonic acid and transcriptional activation of cyclooxygenase-2 (Dermott et al., 1999). Thrombin stimulates CHO cells to activate the Gα13-RhoA-ERK1/2 signaling through PAR1, leading to the phosphorylation of cytosolic phospholipase A2 and the increase of arachidonic acid (Mariggiò et al., 2006). The activation of LPAR and S1PR also promotes the expression of inflammatory genes (such as cyclooxygenase-2) and the proliferation of astrocytes through Gα12/13-RhoA-PLCɛ-protein kinase D (PKD)-NF-κB signaling, which contributes to neuroinflammation (Dusaban et al., 2013).
Recent work has shown that S1P promotes the NLRP3 inflammasome-mediated inflammatory response and the secretion of pro-inflammatory cytokines such as interleukin-1β and interleukin-18 through the S1PR2-Gα12/13-MAPK pathway (Hou et al., 2021). Meanwhile, a variety of pro-inflammatory ligands promote the interaction of Gα13 to VE-cadherin, inducing Src activation and VE-cadherin phosphorylation, leading to the internalization of VE-cadherin, the loss of endothelial barrier function, and vascular inflammation (Gong et al., 2014). The deficiency of Gα13 in leukocytes and platelets inhibits thrombosis and inflammation and significantly optimizes the survival rate of septic mice (Cheng et al., 2021).
Gα12 and Gα13 have opposite effects on osteoclasts. The silencing of Gα13 enhances the AKT-GSK3β-NFATc1 signal cascade by inhibiting RhoA, strongly promoting the formation and size of osteoclasts (Wu et al., 2017). This observation depicts that Gα13 is the main endogenous negative switch for osteoclast production. Gα13 also protects against various bone loss disease models from inflammation (Wu et al., 2017; Nakano et al., 2019). In comparison, a study has shown Gα12 is involved in the pathophysiology of bone diseases such as osteoporosis or rheumatoid arthritis (Song et al., 2018). Interestingly, the volume of trabecular bone increases in Gα12 knockout mice, whereas the number of osteoclasts decreases, contrary to the phenotype of Gα13 deletion (Song et al., 2018). The underlying mechanisms of these opposite phenotypes are unclear, possibly because that Gα12-/- mice are whole body knocked out, whereas Gα13-/- mice undergo an osteoclast lineage-specific conditional knockout.
Gα12/13 and Metabolic Diseases
Gα12 is extensively expressed in metabolic organs, for instance, the liver (Strathmann and Simon, 1991; Kim et al., 2018a). Fasting of normal mice for 24–48 h significantly enhances the expression of Gα12 in the liver (Kim et al., 2018a). GNA12-knockout mice are prone to hepatic steatosis and obesity after being fed a high-fat diet due to reduced energy consumption (Kim et al., 2018a). A study using cDNA microarray analysis with the liver of GNA12-knockout mice has found that Gα12 regulates mitochondrial respiration through the Sirtuin 1/PPARα network (Kim et al., 2018a). Sirtuin 1, a class III histone deacetylases activated by NAD+, is an important regulator involved in the fatty acid oxidation transcription network (Kalliora et al., 2019). Gα12 induces ubiquitin-specific peptidase 22 through HIF-1α to promote the stability of Sirtuin 1, controlling lipid metabolism and mitochondrial respiration (Kim et al., 2018a). Interestingly, Gα12 also exists in the endoplasmic reticulum and participates in the endoplasmic reticulum export (Subramanian et al., 2019). With the coat protein II subunit Sec24 binds to the cargo, it acts as a GEF to activate Gα12 at the export site of the endoplasmic reticulum, promoting cargo export and inhibiting protein synthesis (Subramanian et al., 2019).
The role of Gα13 in energy metabolism has opposite effects in skeletal muscle (prodiabetic) and liver (antidiabetic) (Koo et al., 2017; Kim et al., 2019). Gα13 is more highly expressed in skeletal muscle than other metabolic organs (Koo et al., 2017). The knockout Gα13 in skeletal muscles contributes to systemic energy homeostasis, increasing glucose metabolism and insulin sensitivity and inhibiting diet-induced obesity and hepatic steatosis (Koo et al., 2017). The level of Gα13 in skeletal muscle is reduced by exercise but is increased under conditions of metabolic diseases. Loss of Gα13 in skeletal muscle inhibits the RhoA-ROCK2 pathway and activates NFATc1, which induces the conversion of skeletal muscle fibers into oxidized form and enhances energy metabolism (Koo et al., 2017). On the contrary, the lack of Gα13 in the liver of mice leads to the overproduction of inter-α-trypsin inhibitor heavy chain 1, which exacerbates systemic insulin resistance (Kim et al., 2019).
GPR40 is a clinically proven molecular target for the treatment of diabetes. A GPR40 allosteric full agonist promotes glucose-stimulated insulin secretion in pancreatic β cells through GPR40-mediated Gα12 signaling (Rives et al., 2018). In response to mechanical overload, GPR56 elevates the expression of the mammalian target of rapamycin and insulin-like growth factor 1 through Gα12/13 and promotes muscle protein synthesis and myotube hypertrophy (White et al., 2014). Succinate activates the Gα12-PKC-p38-dynamin-related protein 1 signaling through GPR91 to increase mitochondrial fission, enhances ATP production and membrane potential of mitochondria, and promote the migration of human mesenchymal stem cells (Ko et al., 2017). LPA is a key product of fatty acid metabolism. LPAR4 is selectively coupled with Gα12/13 in adipocytes to limit the remodeling and healthy expansion of white adipose tissue in high-fat diet mice (Yanagida et al., 2018). The above studies have shown that the GPCR-Gα12/13 axis can be an attractive target for treating metabolic diseases.
Gα12/13 and Fibrotic Diseases
Gα12/13 has a pro-fibrotic effect mainly mediated by the RhoA-ROCK activation (Haak et al., 2020). Early studies have demonstrated that Gα12/13 play a key role in regulating the phenotype of cardiac fibroblasts (Fujii et al., 2005; Nishida et al., 2007). The stimulation of Ang II induces the production of reactive oxygen species (ROS) through the AT1R-Gα12/13-Rac pathway. The ROS-mediated JNK activation promotes the activity of AP-1 and NFAT, leading to the proliferation of cardiac fibroblasts (Fujii et al., 2005). Recently, it has been found that targeted inhibition of the Gα12/13-RhoA-ROCK pathway successfully alleviates Ang II-induced cardiac dysfunction and the fibrotic response of cardiac fibroblasts (He et al., 2017). Endothelin-1 activates the Endothelin-1 type A receptor-Gα12/13 axis in cardiac fibroblasts and promotes the formation of myofibroblasts through Rac-dependent ROS production and JNK activation (Nishida et al., 2007). The osteoglycin in the heart binds to LPAR3 and mediates the Gα12/13-Rho-ROCK signal to attenuate the transactivation of epidermal growth factor receptors, thus inhibiting the proliferation and migration of cardiac myofibroblasts and negatively regulating cardiac fibrotic remodeling (Zuo et al., 2018).
Among numerous G protein members, Gα12 is expressed abundantly in hepatic stellate cells of the fibrotic liver, whereas Gα13 is not (Kim et al., 2018c). The imbalance of miR-16 in hepatic stellate cells leads to the overexpression of Gα12, which promotes autophagy through the transcription of JNK-dependent autophagy-related genes 12-5 and accelerates the progression of liver fibrosis (Kim et al., 2018c). Meanwhile, deficiency of Gα12 significantly blocks bleomycin-induced pulmonary fibrosis in mice. LPA stimulates the phosphorylation and activation of PKC-δ through Gα12 and the mammalian target of rapamycin complex 2, which is important for fibroblast migration and the development of pulmonary fibrosis (Gan et al., 2012).
In short, the role of Gα12 or Gα13 in fibrotic diseases is unclear. Using various omics (e.g., genomics, proteomics) to detect the expression of Gα12/13 in specific tissues/cells of fibrotic diseases, exploring its upstream receptors and downstream signals may lead to a better understanding of the pathological mechanisms of the fibrotic diseases.
Gα12/13 and Circulatory and Renal Disorders
Gα13 is involved in heart remodeling induced by pressure overload and plays a central role in the transition to heart failure (Takefuji et al., 2012). The Ang II-AT1R-Gα13-RhoA-myocardin-related transcription factor signal cascade regulates the expression of hypertrophy and fibrosis genes in cardiomyocytes (Takefuji et al., 2012). In response to low concentrations of thrombin, disabled-2, a linker protein, regulates Gα12/13-mediated RhoA-ROCK activation and enhances ADP release, which increases the activity of AKT and mammalian target of rapamycin and promotes platelet aggregation and thrombosis (Tsai et al., 2014). Thrombin also stimulates platelets to promote the interaction of Gα13 and receptor-interacting protein kinase 3. Receptor-interacting protein kinase 3 participates in the integrin-Gα13 signal to promote platelet activation and thrombosis (Zhang et al., 2017). The newly developed high-load ExE peptide nanoparticles, based on the Gα13 binding ExE motif on the cytoplasmic domain of integrin β3, inhibit thrombosis and protect mice from cardiac I/R injury (Pang et al., 2020). This study supports that the integrin-Gα13-RhoA-YAP pathway can be targeted for anti-atherosclerosis therapy (Wang et al., 2016). Inhibiting proteins that specifically interact with Gα13 is a promising approach for the treatment of diseases such as macular degeneration, atherosclerosis, and tumor angiogenesis (Wang et al., 2017). In the process of angiogenesis, RGS5 converts Gαq/11-mediated calcium-dependent contraction to Gα12/13-mediated RhoA activation, leading to the formation of stress fibers in the vascular smooth muscle cells of the artery and the process of vascular remodeling (Arnold et al., 2014). Gα12/13 are also essential for blood vessel formation. Gα13 interacts with Abl1 to form a complex, which regulates actin cytoskeleton reorganization, remodeling, and endothelial cell migration and promotes blood vessel formation (Wang et al., 2017).
Gα12 also plays a key role in inducing renal I/R injury through various mechanisms, such as destruction of tight cell connections, oxidative stress, inflammation, and cell apoptosis. Gα12 knockout mice are almost completely protected from I/R injury (Yu et al., 2012). In addition, kidney injury molecule-1 prevents tissue damage by blocking the binding of GTP to Gα12 in renal I/R (Ismail et al., 2015). Gα12 is also necessary for the development of a mouse renal cyst model induced by polycystin-1 mutation. The lack of polycystin-1 promotes the activation of Gα12, leading to alteration in the form of N-cadherin, destroying cell-matrix/cell adhesion, inhibiting FAK, but promoting the formation of stress fibers (Wu et al., 2016). The activation of Gα12 in mouse podocytes also leads to the imbalance of collagen expression in glomeruli, age-dependent proteinuria, and focal glomerular sclerosis (Boucher et al., 2012). However, a study has found that the activation of the dopamine D3 receptor increases coupling to Gα12, leading to the reduction in ROS production and inflammation, ultimately preventing renal I/R injury (Wang et al., 2015).
The coupling of different GPCRs to Gα12/13 and the impacts on the pathophysiology have not been fully elucidated. The identification of the reciprocal binding domains of Gα12/13 to related cellular proteins will facilitate the development of more specific inhibitors to selectively disrupt the interaction. A comprehensive understanding of the complex regulatory mechanisms of Gα12/13 signaling may lead to novel therapeutics targeting specific functions of Gα12 or Gα13 in a disease-specific manner.
Conclusion
The mechanisms of signal transduction through Gα12/13 are still one of the most enchanting problems in biology. Many crucial questions remain to be addressed: what determines the specificity of the receptor-Gα12/13 interaction (Montgomery et al., 2014; Lymperopoulos et al., 2021)? What determines the specific selectivity of the receptor to Gα12 or Gα13 (Corbisier et al., 2015; Mackenzie et al., 2019)? Do receptor and Gα12/13 form a pre-coupling complex (Ayoub et al., 2012; Zhang et al., 2017)? The importance of Gα12/13 in mediating the complexity of signals, affecting the diversity of cell functions, and participating in the pathogenesis of diseases are continuously being explored. Gα12/13 are pathologically relevant to cancer, bone diseases, liver steatosis, and pulmonary fibrosis. Gα12/13 are considered an attractive biomarker and target for diagnosing and treating related diseases. However, it is unwise to directly stimulate or inhibit the activity of Gα12/13 due to the complexity of its regulatory network, the diversity of functions, and the high likelihood of off-target effects. In comparison, stimulating/inhibiting the upstream receptor/downstream pathways of Gα12 or Gα13 in specific organs/cells may offer a chance of successful therapy. A better understanding of the ligand-receptor-Gα12/13-downstream signaling networks may guide new drug development in the future.
Author Contributions
PG wrote the manuscript; YT and MW drew the figures; HS made the table; LZ and WW gave comments; QW and YX gave instructions and revised the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (81973314, 81202541, and 81973332), the Anhui Provincial Natural Science Foundation for Distinguished Young Scholars (1808085J28), Collaborative Innovation Project of Key Scientific Research Platform in Anhui Universities (GXXT-2020-066), Program for Upgrading Scientific Research Level of Anhui Medical University (2019xkjT008), Academic Funding for Top-notch Talents in University Disciplines (Majors) of Anhui Province (gxbjZD2021047), National Institute of Health R01HL147263, and Veteran Affair 1I01BX005100 and IK6BX005753.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We appreciate Huijuan Cheng, Tiantian Su, Chunru Jiang, and Zhenduo Zhu for the literature search.
References
Abid, H. A., Inoue, A., and Gorvin, C. M. (2021). Heterogeneity of G Protein Activation by the Calcium-Sensing Receptor. J. Mol. Endocrinol. 67, 41–53. doi:10.1530/jme-21-0058
Acharya, B. R., Nestor-Bergmann, A., Liang, X., Gupta, S., Duszyc, K., Gauquelin, E., et al. (2018). A Mechanosensitive RhoA Pathway that Protects Epithelia against Acute Tensile Stress. Developmental Cell 47, 439–452. doi:10.1016/j.devcel.2018.09.016
Ackerman, S. D., Garcia, C., Piao, X., Gutmann, D. H., and Monk, K. R. (2015). The Adhesion GPCR Gpr56 Regulates Oligodendrocyte Development via Interactions with Gα12/13 and RhoA. Nat. Commun. 6, 6122. doi:10.1038/ncomms7122
Arang, N., and Gutkind, J. S. (2020). G Protein‐Coupled Receptors and Heterotrimeric G Proteins as Cancer Drivers. FEBS Lett. 594, 4201–4232. doi:10.1002/1873-3468.14017
Arnold, C., Feldner, A., Pfisterer, L., Hödebeck, M., Troidl, K., Genové, G., et al. (2014). RGS 5 Promotes Arterial Growth during Arteriogenesis. EMBO Mol. Med. 6, 1075–1089. doi:10.15252/emmm.201403864
Arthofer, E., Hot, B., Petersen, J., Strakova, K., Jäger, S., Grundmann, M., et al. (2016). WNT Stimulation Dissociates a Frizzled 4 Inactive-State Complex with Gα12/13. Mol. Pharmacol. 90, 447–459. doi:10.1124/mol.116.104919
Avet, C., Sturino, C., Grastilleur, S., Gouill, C. L., Semache, M., Gross, F., et al. (2020). The PAR2 Inhibitor I-287 Selectively Targets Gαq and Gα12/13 Signaling and Has Anti-inflammatory Effects. Commun. Biol. 3, 719. doi:10.1038/s42003-020-01453-8
Ayoub, M. A., Al-Senaidy, A., and Pin, J.-P. (2012). Receptor-G Protein Interaction Studied by Bioluminescence Resonance Energy Transfer: Lessons from Protease-Activated Receptor 1. Front. Endocrin. 3, 82. doi:10.3389/fendo.2012.00082
Ayoub, M. A., and Pin, J.-P. (2013). Interaction of Protease-Activated Receptor 2 with G Proteins and β-Arrestin 1 Studied by Bioluminescence Resonance Energy Transfer. Front. Endocrinol. 4, 196. doi:10.3389/fendo.2013.00196
Bodmann, E. L., Krett, A. L., and Bünemann, M. (2017). Potentiation of Receptor Responses Induced by Prolonged Binding of Gα 13 and Leukemia‐associated RhoGEF. FASEB j. 31, 3663–3676. doi:10.1096/fj.201700026r
Bondar, A., and Lazar, J. (2021). Optical Sensors of Heterotrimeric G Protein Signaling. FEBS J. 288, 2570–2584. doi:10.1111/febs.15655
Boucher, I., Yu, W., Beaudry, S., Negoro, H., Tran, M., Pollak, M. R., et al. (2012). Gα12 Activation in Podocytes Leads to Cumulative Changes in Glomerular Collagen Expression, Proteinuria and Glomerulosclerosis. Lab. Invest. 92, 662–675. doi:10.1038/labinvest.2011.198
Boularan, C., Hwang, I.-Y., Kamenyeva, O., Park, C., Harrison, K., Huang, Z., et al. (2015). B Lymphocyte-specific Loss of Ric-8A Results in a Gα Protein Deficit and Severe Humoral Immunodeficiency. J.I. 195, 2090–2102. doi:10.4049/jimmunol.1500523
Cancer Genome Atlas, N. (2015). Comprehensive Genomic Characterization of Head and Neck Squamous Cell Carcinomas. Nature 517, 576–582. doi:10.1038/nature14129
Carloni, V., Mazzocca, A., Mello, T., Galli, A., and Capaccioli, S. (2013). Cell Fusion Promotes Chemoresistance in Metastatic colon Carcinoma. Oncogene 32, 2649–2660. doi:10.1038/onc.2012.268
Castaldi, A., Chesini, G. P., Taylor, A. E., Sussman, M. A., Brown, J. H., and Purcell, N. H. (2016). Sphingosine 1-phosphate Elicits RhoA-dependent Proliferation and MRTF-A Mediated Gene Induction in CPCs. Cell Signal. 28, 871–879. doi:10.1016/j.cellsig.2016.04.006
Castellone, M. D., Laukkanen, M. O., Teramoto, H., Bellelli, R., Alì, G., Fontanini, G., et al. (2015). Cross Talk between the Bombesin Neuropeptide Receptor and Sonic Hedgehog Pathways in Small Cell Lung Carcinoma. Oncogene 34, 1679–1687. doi:10.1038/onc.2014.104
Castillo-Kauil, A., García-Jiménez, I., Cervantes-Villagrana, R. D., Adame-García, S. R., Beltrán-Navarro, Y. M., Gutkind, J. S., et al. (2020). Gαs Directly Drives PDZ-RhoGEF Signaling to Cdc42. J. Biol. Chem. 295, 16920–16928. doi:10.1074/jbc.ac120.015204
Cerami, E., Gao, J., Dogrusoz, U., Gross, B. E., Sumer, S. O., Aksoy, B. A., et al. (2012). The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data: Figure 1. Cancer Discov. 2, 401–404. doi:10.1158/2159-8290.cd-12-0095
Chan, A. M., Fleming, T. P., McGovern, E. S., Chedid, M., Miki, T., and Aaronson, S. A. (1993). Expression cDNA Cloning of a Transforming Gene Encoding the Wild-type G Alpha 12 Gene Product. Mol. Cell. Biol. 13, 762–768. doi:10.1128/mcb.13.2.762
Chandrabalan, A., and Ramachandran, R. (2021). Molecular Mechanisms Regulating Proteinase‐Activated Receptors (PARs). FEBS J. 288, 2697–2726. doi:10.1111/febs.15829
Cheng, N., Zhang, Y., Delaney, M. K., Wang, C., Bai, Y., Skidgel, R. A., et al. (2021). Targeting Gα13-Integrin Interaction Ameliorates Systemic Inflammation. Nat. Commun. 12, 3185. doi:10.1038/s41467-021-23409-0
Chow, C. R., Ebine, K., Knab, L. M., Bentrem, D. J., Kumar, K., and Munshi, H. G. (2016). Cancer Cell Invasion in Three-Dimensional Collagen Is Regulated Differentially by Gα13 Protein and Discoidin Domain Receptor 1-Par3 Protein Signaling. J. Biol. Chem. 291, 1605–1618. doi:10.1074/jbc.m115.669606
Corbisier, J., Galès, C., Huszagh, A., Parmentier, M., and Springael, J.-Y. (2015). Biased Signaling at Chemokine Receptors. J. Biol. Chem. 290, 9542–9554. doi:10.1074/jbc.m114.596098
Dermott, J. M., Reddy, M. R., Onesime, D., Reddy, E. P., and Dhanasekaran, N. (1999). Oncogenic Mutant of Gα12 Stimulates Cell Proliferation through Cycloxygenase-2 Signaling Pathway. Oncogene 18, 7185–7189. doi:10.1038/sj.onc.1203345
Dong, C. (2021). Cytokine Regulation and Function in T Cells. Annu. Rev. Immunol. 39, 51–76. doi:10.1146/annurev-immunol-061020-053702
Dusaban, S. S., Chun, J., Rosen, H., Purcell, N. H., and Brown, J. H. (2017). Sphingosine 1-phosphate Receptor 3 and RhoA Signaling Mediate Inflammatory Gene Expression in Astrocytes. J. Neuroinflammation 14, 111. doi:10.1186/s12974-017-0882-x
Dusaban, S. S., Purcell, N. H., Rockenstein, E., Masliah, E., Cho, M. K., Smrcka, A. V., et al. (2013). Phospholipase Cɛ Links G Protein-Coupled Receptor Activation to Inflammatory Astrocytic Responses. Proc. Natl. Acad. Sci. USA 110, 3609–3614. doi:10.1073/pnas.1217355110
El-Haibi, C. P., Sharma, P., Singh, R., Gupta, P., Taub, D. D., Singh, S., et al. (2013). Differential G Protein Subunit Expression by Prostate Cancer Cells and Their Interaction with CXCR5. Mol. Cancer 12, 64. doi:10.1186/1476-4598-12-64
Feng, X., Liu, P., Zhou, X., Li, M.-T., Li, F.-L., Wang, Z., et al. (2016). Thromboxane A2 Activates YAP/TAZ Protein to Induce Vascular Smooth Muscle Cell Proliferation and Migration. J. Biol. Chem. 291, 18947–18958. doi:10.1074/jbc.m116.739722
Festa, B. P., Chen, Z., Berquez, M., Debaix, H., Tokonami, N., Prange, J. A., et al. (2018). Impaired Autophagy Bridges Lysosomal Storage Disease and Epithelial Dysfunction in the Kidney. Nat. Commun. 9, 161. doi:10.1038/s41467-017-02536-7
Flaumenhaft, R., and De Ceunynck, K. (2017). Targeting PAR1: Now what? Trends Pharmacol. Sci. 38, 701–716. doi:10.1016/j.tips.2017.05.001
Flori, M., Schmid, C. A., Sumrall, E. T., Tzankov, A., Law, C. W., Robinson, M. D., et al. (2016). The Hematopoietic Oncoprotein FOXP1 Promotes Tumor Cell Survival in Diffuse Large B-Cell Lymphoma by Repressing S1PR2 Signaling. Blood 127, 1438–1448. doi:10.1182/blood-2015-08-662635
Forrester, S. J., Booz, G. W., Sigmund, C. D., Coffman, T. M., Kawai, T., Rizzo, V., et al. (2018). Angiotensin II Signal Transduction: An Update on Mechanisms of Physiology and Pathophysiology. Physiol. Rev. 98, 1627–1738. doi:10.1152/physrev.00038.2017
Fujii, T., Onohara, N., Maruyama, Y., Tanabe, S., Kobayashi, H., Fukutomi, M., et al. (2005). Gα12/13-mediated Production of Reactive Oxygen Species Is Critical for Angiotensin Receptor-Induced NFAT Activation in Cardiac Fibroblasts. J. Biol. Chem. 280, 23041–23047. doi:10.1074/jbc.m409397200
Gadepalli, R., Kotla, S., Heckle, M. R., Verma, S. K., Singh, N. K., and Rao, G. N. (2013). Novel Role for P21-Activated Kinase 2 in Thrombin-Induced Monocyte Migration. J. Biol. Chem. 288, 30815–30831. doi:10.1074/jbc.m113.463414
Gan, X., Wang, C., Patel, M., Kreutz, B., Zhou, M., Kozasa, T., et al. (2013). Different Raf Protein Kinases Mediate Different Signaling Pathways to Stimulate E3 Ligase RFFL Gene Expression in Cell Migration Regulation. J. Biol. Chem. 288, 33978–33984. doi:10.1074/jbc.m113.477406
Gan, X., Wang, J., Wang, C., Sommer, E., Kozasa, T., Srinivasula, S., et al. (2012). PRR5L Degradation Promotes mTORC2-Mediated PKC-δ Phosphorylation and Cell Migration Downstream of Gα12. Nat. Cell Biol 14, 686–696. doi:10.1038/ncb2507
Gao, J., Aksoy, B. A., Dogrusoz, U., Dresdner, G., Gross, B., Sumer, S. O., et al. (2013). Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci. Signal. 6, pl1. doi:10.1126/scisignal.2004088
Gavard, J., and Gutkind, J. S. (2008). Protein Kinase C-Related Kinase and ROCK Are Required for Thrombin-Induced Endothelial Cell Permeability Downstream from Gα12/13 and Gα11/q. J. Biol. Chem. 283, 29888–29896. doi:10.1074/jbc.m803880200
Girard, M., Dagenais Bellefeuille, S., Eiselt, É., Brouillette, R., Placet, M., Arguin, G., et al. (2020). The P2Y 6 Receptor Signals through Gα Q/Ca 2+/PKCα and Gα 13/ROCK Pathways to Drive the Formation of Membrane Protrusions and Dictate Cell Migration. J. Cell Physiol 235, 9676–9690. doi:10.1002/jcp.29779
Goldsmith, Z. G., and Dhanasekaran, D. N. (2007). G Protein Regulation of MAPK Networks. Oncogene 26, 3122–3142. doi:10.1038/sj.onc.1210407
Gong, H., Gao, X., Feng, S., Siddiqui, M. R., Garcia, A., Bonini, M. G., et al. (2014). Evidence of a Common Mechanism of Disassembly of Adherens Junctions through Gα13 Targeting of VE-Cadherin. J. Exp. Med. 211, 579–591. doi:10.1084/jem.20131190
Gong, H., Shen, B., Flevaris, P., Chow, C., Lam, S. C.-T., Voyno-Yasenetskaya, T. A., et al. (2010). G Protein Subunit Gα 13 Binds to Integrin α IIb β 3 and Mediates Integrin "Outside-In" Signaling. Science 327, 340–343. doi:10.1126/science.1174779
Goupil, E., Tassy, D., Bourguet, C., Quiniou, C., Wisehart, V., Pétrin, D., et al. (2010). A Novel Biased Allosteric Compound Inhibitor of Parturition Selectively Impedes the Prostaglandin F2α-Mediated Rho/ROCK Signaling Pathway. J. Biol. Chem. 285, 25624–25636. doi:10.1074/jbc.m110.115196
Green, J. A., Suzuki, K., Cho, B., Willison, L. D., Palmer, D., Allen, C. D. C., et al. (2011). The Sphingosine 1-phosphate Receptor S1P2 Maintains the Homeostasis of Germinal center B Cells and Promotes Niche Confinement. Nat. Immunol. 12, 672–680. doi:10.1038/ni.2047
Grzelinski, M., Pinkenburg, O., Büch, T., Gold, M., Stohr, S., Kalwa, H., et al. (2010). Critical Role of Gα12 and Gα13 for Human Small Cell Lung Cancer Cell Proliferation In Vitro and Tumor Growth In Vivo. Clin. Cancer Res. 16, 1402–1415. doi:10.1158/1078-0432.ccr-09-1873
Haak, A. J., Ducharme, M. T., Diaz Espinosa, A. M., and Tschumperlin, D. J. (2020). Targeting GPCR Signaling for Idiopathic Pulmonary Fibrosis Therapies. Trends Pharmacol. Sci. 41, 172–182. doi:10.1016/j.tips.2019.12.008
Hashimoto, S., Mikami, S., Sugino, H., Yoshikawa, A., Hashimoto, A., Onodera, Y., et al. (2016). Lysophosphatidic Acid Activates Arf6 to Promote the Mesenchymal Malignancy of Renal Cancer. Nat. Commun. 7, 10656. doi:10.1038/ncomms10656
Hauser, A. S., Attwood, M. M., Rask-Andersen, M., Schiöth, H. B., and Gloriam, D. E. (2017). Trends in GPCR Drug Discovery: New Agents, Targets and Indications. Nat. Rev. Drug Discov. 16, 829–842. doi:10.1038/nrd.2017.178
He, Z., Yang, Y., Wen, Z., Chen, C., Xu, X., Zhu, Y., et al. (2017). CYP2J2 Metabolites, Epoxyeicosatrienoic Acids, Attenuate Ang II-Induced Cardiac Fibrotic Response by Targeting Gα12/13. J. Lipid Res. 58, 1338–1353. doi:10.1194/jlr.m074229
Healy, J. A., Nugent, A., Rempel, R. E., Moffitt, A. B., Davis, N. S., Jiang, X., et al. (2016). GNA13 Loss in Germinal center B Cells Leads to Impaired Apoptosis and Promotes Lymphoma In Vivo. Blood 127, 2723–2731. doi:10.1182/blood-2015-07-659938
Hedegaard, M. A., and Holst, B. (2020). The Complex Signaling Pathways of the Ghrelin Receptor. Endocrinology 161, 161. doi:10.1210/endocr/bqaa020
Hendrickx, G., Boudin, E., Verbeek, M., Fransen, E., Mortier, G., and Van Hul, W. (2020). WNT16 Requires Gα Subunits as Intracellular Partners for Both its Canonical and Non-canonical WNT Signalling Activity in Osteoblasts. Calcif Tissue Int. 106, 294–302. doi:10.1007/s00223-019-00633-x
Herroeder, S., Reichardt, P., Sassmann, A., Zimmermann, B., Jaeneke, D., Hoeckner, J., et al. (2009). Guanine Nucleotide-Binding Proteins of the G12 Family Shape Immune Functions by Controlling CD4+ T Cell Adhesiveness and Motility. Immunity 30, 708–720. doi:10.1016/j.immuni.2009.02.010
Hilger, D., Masureel, M., and Kobilka, B. K. (2018). Structure and Dynamics of GPCR Signaling Complexes. Nat. Struct. Mol. Biol. 25, 4–12. doi:10.1038/s41594-017-0011-7
Hot, B., Valnohova, J., Arthofer, E., Simon, K., Shin, J., Uhlén, M., et al. (2017). FZD10-Gα13 Signalling axis Points to a Role of FZD10 in CNS Angiogenesis. Cell Signal. 32, 93–103. doi:10.1016/j.cellsig.2017.01.023
Hou, L., Zhang, Z., Yang, L., Chang, N., Zhao, X., Zhou, X., et al. (2021). NLRP3 Inflammasome Priming and Activation in Cholestatic Liver Injury via the Sphingosine 1-phosphate/S1P Receptor 2/Gα(12/13)/MAPK Signaling Pathway. J. Mol. Med. 99, 273–288. doi:10.1007/s00109-020-02032-4
Hu, J., Oda, S. K., Shotts, K., Donovan, E. E., Strauch, P., Pujanauski, L. M., et al. (2014). Lysophosphatidic Acid Receptor 5 Inhibits B Cell Antigen Receptor Signaling and Antibody Response. J.I. 193, 85–95. doi:10.4049/jimmunol.1300429
Huang, W., Morales, J. L., Gazivoda, V. P., Lai, J., Qi, Q., and August, A. (2013). The Zinc-Binding Region of IL-2 Inducible T Cell Kinase (Itk) Is Required for Interaction with Gα13 and Activation of Serum Response Factor. Int. J. Biochem. Cell Biol. 45, 1074–1082. doi:10.1016/j.biocel.2013.02.011
Ismail, O. Z., Zhang, X., Wei, J., Haig, A., Denker, B. M., Suri, R. S., et al. (2015). Kidney Injury Molecule-1 Protects against Gα12 Activation and Tissue Damage in Renal Ischemia-Reperfusion Injury. Am. J. Pathol. 185, 1207–1215. doi:10.1016/j.ajpath.2015.02.003
Janda, C. Y., Dang, L. T., You, C., Chang, J., de Lau, W., Zhong, Z. A., et al. (2017). Surrogate Wnt Agonists that Phenocopy Canonical Wnt and β-catenin Signalling. Nature 545, 234–237. doi:10.1038/nature22306
Jiang, L. I., Collins, J., Davis, R., Fraser, I. D., and Sternweis, P. C. (2008). Regulation of cAMP Responses by the G12/13 Pathway Converges on Adenylyl Cyclase VII. J. Biol. Chem. 283, 23429–23439. doi:10.1074/jbc.m803281200
Joshi, S., Wels, C., Beham-Schmid, C., Fukunaga-Kalabis, M., Holmen, S. L., Otte, M., et al. (2015). Gα13 Mediates Human Cytomegalovirus-Encoded Chemokine Receptor US28-Induced Cell Death in Melanoma. Int. J. Cancer 137, 1503–1508. doi:10.1002/ijc.29506
Justus, C. R., Sanderlin, E. J., Dong, L., Sun, T., Chi, J.-T., Lertpiriyapong, K., et al. (2017). Contextual Tumor Suppressor Function of T Cell Death-Associated Gene 8 (TDAG8) in Hematological Malignancies. J. Transl Med. 15, 204. doi:10.1186/s12967-017-1305-6
Kalliora, C., Kyriazis, I. D., Oka, S. I., Lieu, M. J., Yue, Y., Area-Gomez, E., et al. (2019). Dual Peroxisome-Proliferator-Activated-Receptor-Alpha/gamma Activation Inhibits SIRT1-PGC1alpha axis and Causes Cardiac Dysfunction. JCI Insight 5, e129556. doi:10.1172/jci.insight.129556
Kalwa, H., Storch, U., Demleitner, J., Fiedler, S., Mayer, T., Kannler, M., et al. (2015). Phospholipase C Epsilon (PLCε) Induced TRPC6 Activation: A Common but Redundant Mechanism in Primary Podocytes. J. Cell. Physiol. 230, 1389–1399. doi:10.1002/jcp.24883
Khan, S. M., Sung, J. Y., and Hébert, T. E. (2016). Gβγ Subunits-Different Spaces, Different Faces. Pharmacol. Res. 111, 434–441. doi:10.1016/j.phrs.2016.06.026
Ki, S. H., Choi, M. J., Lee, C. H., and Kim, S. G. (2007). Gα12 Specifically Regulates COX-2 Induction by Sphingosine 1-Phosphate. J. Biol. Chem. 282, 1938–1947. doi:10.1074/jbc.m606080200
Kihara, Y., Maceyka, M., Spiegel, S., and Chun, J. (2014). Lysophospholipid Receptor Nomenclature Review: IUPHAR Review 8. Br. J. Pharmacol. 171, 3575–3594. doi:10.1111/bph.12678
Kim, E.-S., Jeong, J.-B., Kim, S., Lee, K.-M., Ko, E., Noh, D.-Y., et al. (2010). The G12 Family Proteins Upregulate Matrix Metalloproteinase-2 via P53 Leading to Human Breast Cell Invasion. Breast Cancer Res. Treat. 124, 49–61. doi:10.1007/s10549-009-0697-2
Kim, K., Lee, J., and Ghil, S. (2018). The Regulators of G Protein Signaling RGS 16 and RGS 18 Inhibit Protease‐activated Receptor 2/Gi/o Signaling through Distinct Interactions with Gα in Live Cells. FEBS Lett. 592, 3126–3138. doi:10.1002/1873-3468.13220
Kim, K. M., Han, C. Y., Kim, J. Y., Cho, S. S., Kim, Y. S., Koo, J. H., et al. (2018). Gα12 Overexpression Induced by miR-16 Dysregulation Contributes to Liver Fibrosis by Promoting Autophagy in Hepatic Stellate Cells. J. Hepatol. 68, 493–504. doi:10.1016/j.jhep.2017.10.011
Kim, T. H., Koo, J. H., Heo, M. J., Han, C. Y., Kim, Y. I., Park, S. Y., et al. (2019). Overproduction of Inter-α-trypsin Inhibitor Heavy Chain 1 after Loss of Gα13 in Liver Exacerbates Systemic Insulin Resistance in Mice. Sci. Transl Med. 11, eaan4735. doi:10.1126/scitranslmed.aan4735
Kim, T. H., Yang, Y. M., Han, C. Y., Koo, J. H., Oh, H., Kim, S. S., et al. (2018). Gα12 Ablation Exacerbates Liver Steatosis and Obesity by Suppressing USP22/SIRT1-Regulated Mitochondrial Respiration. J. Clin. Invest. 128, 5587–5602. doi:10.1172/jci97831
Kim, Y. M., Lim, S.-C., Han, C. Y., Kay, H. Y., Cho, I. J., Ki, S. H., et al. (2011). Gα 12/13 Induction of CYR61 in Association with Arteriosclerotic Intimal Hyperplasia. Arterioscler Thromb. Vasc. Biol. 31, 861–869. doi:10.1161/atvbaha.110.218552
Ko, S. H., Choi, G. E., Oh, J. Y., Lee, H. J., Kim, J. S., Chae, C. W., et al. (2017). Succinate Promotes Stem Cell Migration through the GPR91-dependent Regulation of DRP1-Mediated Mitochondrial Fission. Sci. Rep. 7, 12582. doi:10.1038/s41598-017-12692-x
Koo, J. H., Kim, T. H., Park, S.-Y., Joo, M. S., Han, C. Y., Choi, C. S., et al. (2017). Gα13 Ablation Reprograms Myofibers to Oxidative Phenotype and Enhances Whole-Body Metabolism. J. Clin. Invest. 127, 3845–3860. doi:10.1172/jci92067
Kuen, D. S., Park, M., Ryu, H., Choi, G., Moon, Y. H., Kim, J. O., et al. (2021). Critical Regulation of Follicular Helper T Cell Differentiation and Function by Gα13 Signaling. Proc. Natl. Acad. Sci. U S A. 118, 118. doi:10.1073/pnas.2108376118
Kumar, A., Kremer, K. N., Dominguez, D., Tadi, M., and Hedin, K. E. (2011). Gα13 and Rho Mediate Endosomal Trafficking of CXCR4 into Rab11+ Vesicles upon Stromal Cell-Derived Factor-1 Stimulation. J.I. 186, 951–958. doi:10.4049/jimmunol.1002019
Kumar, R. N., Shore, S. K., and Dhanasekaran, N. (2006). Neoplastic Transformation by the Gep Oncogene, Gα12, Involves Signaling by STAT3. Oncogene 25, 899–906. doi:10.1038/sj.onc.1209132
Lambright, D. G., Sondek, J., Bohm, A., Skiba, N. P., Hamm, H. E., and Sigler, P. B. (1996). The 2.0 Å crystal Structure of a Heterotrimeric G Protein. Nature 379, 311–319. doi:10.1038/379311a0
Leach, K., Wen, A., Cook, A. E., Sexton, P. M., Conigrave, A. D., and Christopoulos, A. (2013). Impact of Clinically Relevant Mutations on the Pharmacoregulation and Signaling Bias of the Calcium-Sensing Receptor by Positive and Negative Allosteric Modulators. Endocrinology 154, 1105–1116. doi:10.1210/en.2012-1887
Leach, K., Wen, A., Davey, A. E., Sexton, P. M., Conigrave, A. D., and Christopoulos, A. (2012). Identification of Molecular Phenotypes and Biased Signaling Induced by Naturally Occurring Mutations of the Human Calcium-Sensing Receptor. Endocrinology 153, 4304–4316. doi:10.1210/en.2012-1449
Lecointre, C., Desrues, L., Joubert, J. E., Perzo, N., Guichet, P.-O., Le Joncour, V., et al. (2015). Signaling Switch of the Urotensin II Vasosactive Peptide GPCR: Prototypic Chemotaxic Mechanism in Glioma. Oncogene 34, 5080–5094. doi:10.1038/onc.2014.433
Lee, C.-W., Rivera, R., Gardell, S., Dubin, A. E., and Chun, J. (2006). GPR92 as a New G12/13- and Gq-Coupled Lysophosphatidic Acid Receptor that Increases cAMP, LPA5. J. Biol. Chem. 281, 23589–23597. doi:10.1074/jbc.m603670200
Lee, J. H., Kim, D., Oh, Y. S., and Jun, H. S. (2019). Lysophosphatidic Acid Signaling in Diabetic Nephropathy. Int. J. Mol. Sci. 20. doi:10.3390/ijms20112850
Lee, S. J., Yang, J. W., Cho, I. J., Kim, W. D., Cho, M. K., Lee, C. H., et al. (2009). The Gep Oncogenes, Gα12 and Gα13, Upregulate the Transforming Growth Factor-β1 Gene. Oncogene 28, 1230–1240. doi:10.1038/onc.2008.488
Li, Y., Liu, M., Yang, S., Fuller, A. M., Karin Eisinger-Mathason, T. S., and Yang, S. (2021). RGS12 Is a Novel Tumor Suppressor in Osteosarcoma that Inhibits YAP-TEAD1-Ezrin Signaling. Oncogene 40, 2553–2566. doi:10.1038/s41388-020-01599-z
Lim, W. K., Chai, X., Ghosh, S., Ray, D., Wang, M., Rasheed, S. A. K., et al. (2019). Gα-13 Induces CXC Motif Chemokine Ligand 5 Expression in Prostate Cancer Cells by Transactivating NF-κB. J. Biol. Chem. 294, 18192–18206. doi:10.1074/jbc.ra119.010018
Litosch, I. (2012). Negative Feedback Regulation of Gq Signaling by Protein Kinase C Is Disrupted by Diacylglycerol Kinase ζ in COS-7 Cells. Biochem. Biophysical Res. Commun. 417, 956–960. doi:10.1016/j.bbrc.2011.12.037
Liu, B., Su, Q., Ma, J., Chen, C., Wang, L., Che, F., et al. (2021). Prognostic Value of Eight-Gene Signature in Head and Neck Squamous Carcinoma. Front. Oncol. 11, 657002. doi:10.3389/fonc.2021.657002
Liu, S.-C., Jen, Y.-M., Jiang, S. S., Chang, J.-L., Hsiung, C. A., Wang, C.-H., et al. (2009). Gα12-Mediated Pathway Promotes Invasiveness of Nasopharyngeal Carcinoma by Modulating Actin Cytoskeleton Reorganization. Cancer Res. 69, 6122–6130. doi:10.1158/0008-5472.can-08-3435
Liu, W., Li, H., Wang, Y., Zhao, X., Guo, Y., Jin, J., et al. (2017). MiR-30b-5p Functions as a Tumor Suppressor in Cell Proliferation, Metastasis and Epithelial-To-Mesenchymal Transition by Targeting G-Protein Subunit α-13 in Renal Cell Carcinoma. Gene 626, 275–281. doi:10.1016/j.gene.2017.05.040
Loskutov, Y. V., Griffin, C. L., Marinak, K. M., Bobko, A., Margaryan, N. V., Geldenhuys, W. J., et al. (2018). LPA Signaling Is Regulated through the Primary Cilium: a Novel Target in Glioblastoma. Oncogene 37, 1457–1471. doi:10.1038/s41388-017-0049-3
Lymperopoulos, A., Borges, J. I., Carbone, A. M., Cora, N., and Sizova, A. (2021). Cardiovascular Angiotensin II Type 1 Receptor Biased Signaling: Focus on Non-gq-, Non-βarrestin-dependent Signaling. Pharmacol. Res. 174, 105943. doi:10.1016/j.phrs.2021.105943
Mackenzie, A. E., Quon, T., Lin, L. C., Hauser, A. S., Jenkins, L., Inoue, A., et al. (2019). Receptor Selectivity between the G Proteins Gα 12 and Gα 13 Is Defined by a Single Leucine‐to‐isoleucine Variation. FASEB j. 33, 5005–5017. doi:10.1096/fj.201801956r
Mariggiò, S., Bavec, A., Natale, E., Zizza, P., Salmona, M., Corda, D., et al. (2006). Gα13 Mediates Activation of the Cytosolic Phospholipase A2α through fine Regulation of ERK Phosphorylation. Cell Signal. 18, 2200–2208. doi:10.1016/j.cellsig.2006.05.003
Masià-Balagué, M., Izquierdo, I., Garrido, G., Cordomí, A., Pérez-Benito, L., Miller, N. L. G., et al. (2015). Gastrin-stimulated Gα13 Activation of Rgnef Protein (ArhGEF28) in DLD-1 Colon Carcinoma Cells. J. Biol. Chem. 290, 15197–15209. doi:10.1074/jbc.m114.628164
Masuho, I., Skamangas, N. K., Muntean, B. S., and Martemyanov, K. A. (2021). Diversity of the Gβγ Complexes Defines Spatial and Temporal Bias of GPCR Signaling. Cell Syst. 12, 324–337. doi:10.1016/j.cels.2021.02.001
Mathew, D., Kremer, K. N., and Torres, R. M. (2019). ARHGEF1 Deficiency Reveals Gα13-Associated GPCRs Are Critical Regulators of Human Lymphocyte Function. J. Clin. Invest. 129, 965–968. doi:10.1172/jci125893
Maziarz, M., Federico, A., Zhao, J., Dujmusic, L., Zhao, Z., Monti, S., et al. (2020). Naturally Occurring Hotspot Cancer Mutations in Gα13 Promote Oncogenic Signaling. J. Biol. Chem. 295, 16897–16904. doi:10.1074/jbc.ac120.014698
McLaughlin, J. N., Patterson, M. M., and Malik, A. B. (2007). Protease-activated Receptor-3 (PAR3) Regulates PAR1 Signaling by Receptor Dimerization. Proc. Natl. Acad. Sci. 104, 5662–5667. doi:10.1073/pnas.0700763104
McNeil Coffield, V., Helms, W. S., Jiang, Q., and Su, L. (2004). Gα13 Mediates a Signal that Is Essential for Proliferation and Survival of Thymocyte Progenitors. J. Exp. Med. 200, 1315–1324. doi:10.1084/jem.20040944
Mende, F., Hundahl, C., Plouffe, B., Skov, L. J., Sivertsen, B., Madsen, A. N., et al. (2018). Translating Biased Signaling in the Ghrelin Receptor System into Differential In Vivo Functions. Proc. Natl. Acad. Sci. USA 115, E10255–E10264. doi:10.1073/pnas.1804003115
Mikelis, C. M., Palmby, T. R., Simaan, M., Li, W., Szabo, R., Lyons, R., et al. (2013). PDZ-RhoGEF and LARG Are Essential for Embryonic Development and Provide a Link between Thrombin and LPA Receptors and Rho Activation. J. Biol. Chem. 288, 12232–12243. doi:10.1074/jbc.m112.428599
Montgomery, E. R., Temple, B. R. S., Peters, K. A., Tolbert, C. E., Booker, B. K., Martin, J. W., et al. (2014). Gα12 Structural Determinants of Hsp90 Interaction Are Necessary for Serum Response Element-Mediated Transcriptional Activation. Mol. Pharmacol. 85, 586–597. doi:10.1124/mol.113.088443
Moriyama, S., Takahashi, N., Green, J. A., Hori, S., Kubo, M., Cyster, J. G., et al. (2014). Sphingosine-1-phosphate Receptor 2 Is Critical for Follicular Helper T Cell Retention in Germinal Centers. J. Exp. Med. 211, 1297–1305. doi:10.1084/jem.20131666
Mu, G., Ding, Q., Li, H., Zhang, L., Zhang, L., He, K., et al. (2018). Gastrin Stimulates Pancreatic Cancer Cell Directional Migration by Activating the Gα12/13-RhoA-ROCK Signaling Pathway. Exp. Mol. Med. 50, 1–14. doi:10.1038/s12276-018-0081-6
Müller, F. E., Schade, S. K., Cherkas, V., Stopper, L., Breithausen, B., Minge, D., et al. (2021). Serotonin Receptor 4 Regulates Hippocampal Astrocyte Morphology and Function. Glia 69, 872–889. doi:10.1002/glia.23933
Muppidi, J. R., Lu, E., and Cyster, J. G. (2015). The G Protein-Coupled Receptor P2RY8 and Follicular Dendritic Cells Promote Germinal center Confinement of B Cells, whereas S1PR3 Can Contribute to Their Dissemination. J. Exp. Med. 212, 2213–2222. doi:10.1084/jem.20151250
Muppidi, J. R., Schmitz, R., Green, J. A., Xiao, W., Larsen, A. B., Braun, S. E., et al. (2014). Loss of Signalling via Gα13 in Germinal centre B-Cell-Derived Lymphoma. Nature 516, 254–258. doi:10.1038/nature13765
Nakanishi, N., Ogata, T., Naito, D., Miyagawa, K., Taniguchi, T., Hamaoka, T., et al. (2016). MURC Deficiency in Smooth Muscle Attenuates Pulmonary Hypertension. Nat. Commun. 7, 12417. doi:10.1038/ncomms12417
Nakano, S., Inoue, K., Xu, C., Deng, Z., Syrovatkina, V., Vitone, G., et al. (2019). G-protein Gα13 Functions as a Cytoskeletal and Mitochondrial Regulator to Restrain Osteoclast Function. Sci. Rep. 9, 4236. doi:10.1038/s41598-019-40974-z
Nishida, M., Onohara, N., Sato, Y., Suda, R., Ogushi, M., Tanabe, S., et al. (2007). Gα12/13-mediated Up-Regulation of TRPC6 Negatively Regulates Endothelin-1-Induced Cardiac Myofibroblast Formation and Collagen Synthesis through Nuclear Factor of Activated T Cells Activation. J. Biol. Chem. 282, 23117–23128. doi:10.1074/jbc.m611780200
Noguchi, K., Ishii, S., and Shimizu, T. (2003). Identification of p2y9/GPR23 as a Novel G Protein-Coupled Receptor for Lysophosphatidic Acid, Structurally Distant from the Edg Family. J. Biol. Chem. 278, 25600–25606. doi:10.1074/jbc.m302648200
O'Hayre, M., Inoue, A., Kufareva, I., Wang, Z., Mikelis, C. M., Drummond, R. A., et al. (2016). Inactivating Mutations in GNA13 and RHOA in Burkitt's Lymphoma and Diffuse Large B-Cell Lymphoma: a Tumor Suppressor Function for the Gα13/RhoA axis in B Cells. Oncogene 35, 3771–3780. doi:10.1038/onc.2015.442
Olivera, A., Rosenfeldt, H. M., Bektas, M., Wang, F., Ishii, I., Chun, J., et al. (2003). Sphingosine Kinase Type 1 Induces G12/13-Mediated Stress Fiber Formation, yet Promotes Growth and Survival Independent of G Protein-Coupled Receptors. J. Biol. Chem. 278, 46452–46460. doi:10.1074/jbc.m308749200
Pang, A., Cheng, N., Cui, Y., Bai, Y., Hong, Z., Delaney, M. K., et al. (2020). High-loading Gα13-Binding EXE Peptide Nanoparticles Prevent Thrombosis and Protect Mice from Cardiac Ischemia/reperfusion Injury. Sci. Transl Med. 12. doi:10.1126/scitranslmed.aaz7287
Pang, A., Cui, Y., Chen, Y., Cheng, N., Delaney, M. K., Gu, M., et al. (2018). Shear-induced Integrin Signaling in Platelet Phosphatidylserine Exposure, Microvesicle Release, and Coagulation. Blood 132, 533–543. doi:10.1182/blood-2017-05-785253
Papasergi-Scott, M. M., Stoveken, H. M., MacConnachie, L., Chan, P. Y., Gabay, M., Wong, D., et al. (2018). Dual Phosphorylation of Ric-8A Enhances its Ability to Mediate G Protein α Subunit Folding and to Stimulate Guanine Nucleotide Exchange. Sci. Signal. 11, eaap8113. doi:10.1126/scisignal.aap8113
Park, H. W., Kim, Y. C., Yu, B., Moroishi, T., Mo, J.-S., Plouffe, S. W., et al. (2015). Alternative Wnt Signaling Activates YAP/TAZ. Cell 162, 780–794. doi:10.1016/j.cell.2015.07.013
Patel, M., Kawano, T., Suzuki, N., Hamakubo, T., Karginov, A. V., and Kozasa, T. (2014). Gα13/PDZ-RhoGEF/RhoA Signaling Is Essential for Gastrin-Releasing Peptide Receptor-Mediated Colon Cancer Cell Migration. Mol. Pharmacol. 86, 252–262. doi:10.1124/mol.114.093914
Pathe-neuschäfer-rube, A., Neuschäfer-rube, F., and Püschel, G. P. (2005). Role of the ERC Motif in the Proximal Part of the Second Intracellular Loop and the C-Terminal Domain of the Human Prostaglandin F2α Receptor (hFP-R) in G-Protein Coupling Control. Biochem. J. 388, 317–324. doi:10.1042/bj20041321
Piccinin, E., Villani, G., and Moschetta, A. (2019). Metabolic Aspects in NAFLD, NASH and Hepatocellular Carcinoma: the Role of PGC1 Coactivators. Nat. Rev. Gastroenterol. Hepatol. 16, 160–174. doi:10.1038/s41575-018-0089-3
Plastira, I., Bernhart, E., Joshi, L., Koyani, C. N., Strohmaier, H., Reicher, H., et al. (2020). MAPK Signaling Determines Lysophosphatidic Acid (LPA)-induced Inflammation in Microglia. J. Neuroinflammation 17, 127. doi:10.1186/s12974-020-01809-1
Profirovic, J., Strekalova, E., Urao, N., Krbanjevic, A., Andreeva, A. V., Varadarajan, S., et al. (2013). A Novel Regulator of Angiogenesis in Endothelial Cells: 5-hydroxytriptamine 4 Receptor. Angiogenesis 16, 15–28. doi:10.1007/s10456-012-9296-7
Qu, C., Liu, Y., Kunkalla, K., Singh, R. R., Blonska, M., Lin, X., et al. (2013). Trimeric G Protein-CARMA1 axis Links Smoothened, the Hedgehog Receptor Transducer, to NF-κB Activation in Diffuse Large B-Cell Lymphoma. Blood 121, 4718–4728. doi:10.1182/blood-2012-12-470153
Raghavan, S., Singh, N. K., Gali, S., Mani, A. M., and Rao, G. N. (2018). Protein Kinase Cθ via Activating Transcription Factor 2-Mediated CD36 Expression and Foam Cell Formation of Ly6C Hi Cells Contributes to Atherosclerosis. Circulation 138, 2395–2412. doi:10.1161/circulationaha.118.034083
Rasheed, S. A. K., Leong, H. S., Lakshmanan, M., Raju, A., Dadlani, D., Chong, F.-T., et al. (2018). GNA13 Expression Promotes Drug Resistance and Tumor-Initiating Phenotypes in Squamous Cell Cancers. Oncogene 37, 1340–1353. doi:10.1038/s41388-017-0038-6
Rasheed, S. A. K., Subramanyan, L. V., Lim, W. K., Udayappan, U. K., Wang, M., and Casey, P. J. (2021). The Emerging Roles of Galpha12/13 Proteins on the Hallmarks of Cancer in Solid Tumors. Oncogene 41, 147–158. doi:10.1038/s41388-021-02069-w
Rasheed, S. A. K., Teo, C. R., Beillard, E. J., Voorhoeve, P. M., and Casey, P. J. (2013). MicroRNA-182 and MicroRNA-200a Control G-Protein Subunit α-13 (GNA13) Expression and Cell Invasion Synergistically in Prostate Cancer Cells. J. Biol. Chem. 288, 7986–7995. doi:10.1074/jbc.m112.437749
Rasheed, S. A. K., Teo, C. R., Beillard, E. J., Voorhoeve, P. M., Zhou, W., Ghosh, S., et al. (2015). MicroRNA-31 Controls G Protein Alpha-13 (GNA13) Expression and Cell Invasion in Breast Cancer Cells. Mol. Cancer 14, 67. doi:10.1186/s12943-015-0337-x
Regué, L., Mou, F., and Avruch, J. (2013). G Protein-Coupled Receptors Engage the Mammalian Hippo Pathway through F-Actin. Bioessays 35, 430–435. doi:10.1002/bies.201200163
Rieken, S., Sassmann, A., Herroeder, S., Wallenwein, B., Moers, A., Offermanns, S., et al. (2006). G12/G13 Family G Proteins Regulate Marginal Zone B Cell Maturation, Migration, and Polarization. J. Immunol. 177, 2985–2993. doi:10.4049/jimmunol.177.5.2985
Rives, M.-L., Rady, B., Swanson, N., Zhao, S., Qi, J., Arnoult, E., et al. (2018). GPR40-Mediated Gα12 Activation by Allosteric Full Agonists Highly Efficacious at Potentiating Glucose-Stimulated Insulin Secretion in Human Islets. Mol. Pharmacol. 93, 581–591. doi:10.1124/mol.117.111369
Ruan, Y., Patzak, A., Pfeiffer, N., and Gericke, A. (2021). Muscarinic Acetylcholine Receptors in the Retina-Therapeutic Implications. Int. J. Mol. Sci. 22, 4989. doi:10.3390/ijms22094989
Rümenapp, U., Asmus, M., Schablowski, H., Woznicki, M., Han, L., Jakobs, K. H., et al. (2001). The M3 Muscarinic Acetylcholine Receptor Expressed in HEK-293 Cells Signals to Phospholipase D via G12 but Not Gq-type G Proteins. J. Biol. Chem. 276, 2474–2479. doi:10.1074/jbc.m004957200
Sabbir, M. G., Calcutt, N. A., and Fernyhough, P. (2018). Muscarinic Acetylcholine Type 1 Receptor Activity Constrains Neurite Outgrowth by Inhibiting Microtubule Polymerization and Mitochondrial Trafficking in Adult Sensory Neurons. Front. Neurosci. 12, 402. doi:10.3389/fnins.2018.00402
Scarlett, K. A., White, E.-S. Z., Coke, C. J., Carter, J. R., Bryant, L. K., and Hinton, C. V. (2018). Agonist-induced CXCR4 and CB2 Heterodimerization Inhibits Gα13/RhoA-Mediated Migration. Mol. Cancer Res. 16, 728–739. doi:10.1158/1541-7786.mcr-16-0481
Senarath, K., Kankanamge, D., Samaradivakara, S., Ratnayake, K., Tennakoon, M., and Karunarathne, A. (2018). Regulation of G Protein βγ Signaling. Int. Rev. Cell Mol Biol 339, 133–191. doi:10.1016/bs.ircmb.2018.02.008
Seyedabadi, M., Ghahremani, M. H., and Albert, P. R. (2019). Biased Signaling of G Protein Coupled Receptors (GPCRs): Molecular Determinants of GPCR/transducer Selectivity and Therapeutic Potential. Pharmacol. Ther. 200, 148–178. doi:10.1016/j.pharmthera.2019.05.006
Shen, B., Delaney, M. K., and Du, X. (2012). Inside-out, Outside-In, and Inside-Outside-In: G Protein Signaling in Integrin-Mediated Cell Adhesion, Spreading, and Retraction. Curr. Opin. Cell Biol. 24, 600–606. doi:10.1016/j.ceb.2012.08.011
Shen, B., Estevez, B., Xu, Z., Kreutz, B., Karginov, A., Bai, Y., et al. (2015). The Interaction of Gα13 with Integrin β1 Mediates Cell Migration by Dynamic Regulation of RhoA. MBoC 26, 3658–3670. doi:10.1091/mbc.e15-05-0274
Shen, B., Zhao, X., O’Brien, K. A., Stojanovic-Terpo, A., Delaney, M. K., Kim, K., et al. (2013). A Directional Switch of Integrin Signalling and a New Anti-thrombotic Strategy. Nature 503, 131–135. doi:10.1038/nature12613
Smrcka, A. V., and Fisher, I. (2019). G-protein βγ Subunits as Multi-Functional Scaffolds and Transducers in G-Protein-Coupled Receptor Signaling. Cell. Mol. Life Sci. 76, 4447–4459. doi:10.1007/s00018-019-03275-2
Sobel, K., Monnier, L., Menyhart, K., Bolinger, M., Studer, R., Nayler, O., et al. (2015). FTY720 Phosphate Activates Sphingosine-1-Phosphate Receptor 2 and Selectively Couples to Gα12/13/Rho/ROCK to Induce Myofibroblast Contraction. Mol. Pharmacol. 87, 916–927. doi:10.1124/mol.114.097261
Song, M. K., Park, C., Lee, Y. D., Kim, H., Kim, M. K., Kwon, J. O., et al. (2018). Gα12 Regulates Osteoclastogenesis by Modulating NFATc1 Expression. J. Cell Mol Med 22, 849–860. doi:10.1111/jcmm.13370
Spoerri, P. M., Strohmeyer, N., Sun, Z., Fässler, R., and Müller, D. J. (2020). Protease-activated Receptor Signalling Initiates α5β1-integrin-mediated Adhesion in Non-haematopoietic Cells. Nat. Mater. 19, 218–226. doi:10.1038/s41563-019-0580-4
Sriwai, W., Mahavadi, S., Al-Shboul, O., Grider, J. R., and Murthy, K. S. (2013). Distinctive G Protein-dependent Signaling by Protease-Activated Receptor 2 (PAR2) in Smooth Muscle: Feedback Inhibition of RhoA by cAMP-independent PKA. PLoS One 8, e66743. doi:10.1371/journal.pone.0066743
Stecky, R. C., Quick, C. R., Fleming, T. L., Mull, M. L., Vinson, V. K., Whitley, M. S., et al. (2020). Divergent C-Terminal Motifs in Gα12 and Gα13 Provide Distinct Mechanisms of Effector Binding and SRF Activation. Cell Signal. 72, 109653. doi:10.1016/j.cellsig.2020.109653
Strathmann, M. P., and Simon, M. I. (1991). G Alpha 12 and G Alpha 13 Subunits Define a Fourth Class of G Protein Alpha Subunits. Proc. Natl. Acad. Sci. 88, 5582–5586. doi:10.1073/pnas.88.13.5582
Subramanian, A., Capalbo, A., Iyengar, N. R., Rizzo, R., di Campli, A., Di Martino, R., et al. (2019). Auto-regulation of Secretory Flux by Sensing and Responding to the Folded Cargo Protein Load in the Endoplasmic Reticulum. Cell 176, 1461–1476. doi:10.1016/j.cell.2019.01.035
Sunahara, R. K., Tesmer, J. J. G., Gilman, A. G., and Sprang, S. R. (1997). Crystal Structure of the Adenylyl Cyclase Activator G S α. Science 278, 1943–1947. doi:10.1126/science.278.5345.1943
Svitkina, T. (2018). The Actin Cytoskeleton and Actin-Based Motility. Cold Spring Harb Perspect. Biol. 10, a018267. doi:10.1101/cshperspect.a018267
Syrovatkina, V., Alegre, K. O., Dey, R., and Huang, X.-Y. (2016). Regulation, Signaling, and Physiological Functions of G-Proteins. J. Mol. Biol. 428, 3850–3868. doi:10.1016/j.jmb.2016.08.002
Syrovatkina, V., and Huang, X. Y. (2019). Signaling Mechanisms and Physiological Functions of G‐protein Gα 13 in Blood Vessel Formation, Bone Homeostasis, and Cancer. Protein Sci. 28, 305–312. doi:10.1002/pro.3531
Takefuji, M., Wirth, A., Lukasova, M., Takefuji, S., Boettger, T., Braun, T., et al. (2012). G 13 -Mediated Signaling Pathway Is Required for Pressure Overload-Induced Cardiac Remodeling and Heart Failure. Circulation 126, 1972–1982. doi:10.1161/circulationaha.112.109256
Tan, L., Yan, W., McCorvy, J. D., and Cheng, J. (2018). Biased Ligands of G Protein-Coupled Receptors (GPCRs): Structure-Functional Selectivity Relationships (SFSRs) and Therapeutic Potential. J. Med. Chem. 61, 9841–9878. doi:10.1021/acs.jmedchem.8b00435
Tan, W., Martin, D., and Gutkind, J. S. (2006). The Gα13-Rho Signaling Axis Is Required for SDF-1-Induced Migration through CXCR4. J. Biol. Chem. 281, 39542–39549. doi:10.1074/jbc.m609062200
Tanaka, M., Okudaira, S., Kishi, Y., Ohkawa, R., Iseki, S., Ota, M., et al. (2006). Autotaxin Stabilizes Blood Vessels and Is Required for Embryonic Vasculature by Producing Lysophosphatidic Acid. J. Biol. Chem. 281, 25822–25830. doi:10.1074/jbc.m605142200
Teo, C. R., Casey, P. J., and Rasheed, S. A. K. (2016). The GNA13-RhoA Signaling axis Suppresses Expression of Tumor Protective Kallikreins. Cell Signal. 28, 1479–1488. doi:10.1016/j.cellsig.2016.07.001
Tesmer, J. J. G., Sunahara, R. K., Gilman, A. G., and Sprang, S. R. (1997). Crystal Structure of the Catalytic Domains of Adenylyl Cyclase in a Complex with G Sα ·GTPγS. Science 278, 1907–1916. doi:10.1126/science.278.5345.1907
Tiacci, E., Ladewig, E., Schiavoni, G., Penson, A., Fortini, E., Pettirossi, V., et al. (2018). Pervasive Mutations of JAK-STAT Pathway Genes in Classical Hodgkin Lymphoma. Blood 131, 2454–2465. doi:10.1182/blood-2017-11-814913
Toro-Tapia, G., Villaseca, S., Beyer, A., Roycroft, A., Marcellini, S., Mayor, R., et al. (2018). The Ric-8A/Gα13/FAK Signalling cascade Controls Focal Adhesion Formation during Neural Crest Cell Migration in Xenopus. Development 145, 145. doi:10.1242/dev.164269
Trivedi, V., Boire, A., Tchernychev, B., Kaneider, N. C., Leger, A. J., O'Callaghan, K., et al. (2009). Platelet Matrix Metalloprotease-1 Mediates Thrombogenesis by Activating PAR1 at a Cryptic Ligand Site. Cell 137, 332–343. doi:10.1016/j.cell.2009.02.018
Tsai, H.-J., Huang, C.-L., Chang, Y.-W., Huang, D.-Y., Lin, C.-C., Cooper, J. A., et al. (2014). Disabled-2 Is Required for Efficient Hemostasis and Platelet Activation by Thrombin in Mice. Arterioscler Thromb. Vasc. Biol. 34, 2404–2412. doi:10.1161/atvbaha.114.302602
Unsworth, A. J., Kriek, N., Bye, A. P., Naran, K., Sage, T., Flora, G. D., et al. (2017). PPAR γ Agonists Negatively Regulate α II Bβ3 Integrin Outside‐in Signaling and Platelet Function through Up‐regulation of Protein Kinase A Activity. J. Thromb. Haemost. 15, 356–369. doi:10.1111/jth.13578
van den Bos, E., Ambrosy, B., Horsthemke, M., Walbaum, S., Bachg, A. C., Wettschureck, N., et al. (2020). Knockout Mouse Models Reveal the Contributions of G Protein Subunits to Complement C5a Receptor-Mediated Chemotaxis. J. Biol. Chem. 295, 7726–7742. doi:10.1074/jbc.ra119.011984
Wang, J., Hua, T., and Liu, Z.-J. (2020). Structural Features of Activated GPCR Signaling Complexes. Curr. Opin. Struct. Biol. 63, 82–89. doi:10.1016/j.sbi.2020.04.008
Wang, L., Guo, D., Xing, B., Zhang, J. J., Shu, H.-B., Guo, L., et al. (2011). Resistance to Inhibitors of cholinesterase-8A (Ric-8A) Is Critical for Growth Factor Receptor-Induced Actin Cytoskeletal Reorganization. J. Biol. Chem. 286, 31055–31061. doi:10.1074/jbc.m111.253427
Wang, L., Luo, J.-Y., Li, B., Tian, X. Y., Chen, L.-J., Huang, Y., et al. (2016). Integrin-YAP/TAZ-JNK cascade Mediates Atheroprotective Effect of Unidirectional Shear Flow. Nature 540, 579–582. doi:10.1038/nature20602
Wang, L., Wang, D., Xing, B., Tan, Y.-c., Huang, J., Liu, B., et al. (2017). G-protein Gα13 Functions with Abl Kinase to Regulate Actin Cytoskeletal Reorganization. J. Mol. Biol. 429, 3836–3849. doi:10.1016/j.jmb.2017.10.020
Wang, N., Huang, H., Xiong, Q., Chen, N., Xi, N., Wu, P., et al. (2019). GNAQ Negatively Regulates Antiviral Innate Immune Responses in a Calcineurin-dependent Manner. J.I. 203, 1288–1297. doi:10.4049/jimmunol.1900427
Wang, Y., Li, Y., and Shi, G. (2013). The Regulating Function of Heterotrimeric G Proteins in the Immune System. Arch. Immunol. Ther. Exp. 61, 309–319. doi:10.1007/s00005-013-0230-5
Wang, Z., Guan, W., Han, Y., Ren, H., Tang, X., Zhang, H., et al. (2015). Stimulation of Dopamine D3 Receptor Attenuates Renal Ischemia-Reperfusion Injury via Increased Linkage with Gα12. Transplantation 99, 2274–2284. doi:10.1097/tp.0000000000000762
Ward, Y., Lake, R., Yin, J. J., Heger, C. D., Raffeld, M., Goldsmith, P. K., et al. (2011). LPA Receptor Heterodimerizes with CD97 to Amplify LPA-Initiated RHO-dependent Signaling and Invasion in Prostate Cancer Cells. Cancer Res. 71, 7301–7311. doi:10.1158/0008-5472.can-11-2381
White, J. P., Wrann, C. D., Rao, R. R., Nair, S. K., Jedrychowski, M. P., You, J.-S., et al. (2014). G Protein-Coupled Receptor 56 Regulates Mechanical Overload-Induced Muscle Hypertrophy. Proc. Natl. Acad. Sci. 111, 15756–15761. doi:10.1073/pnas.1417898111
Won, H. Y., Min, H. J., Lee, W. H., Kim, S. G., and Hwang, E. S. (2010). Gα12 Is Critical for TCR-Induced IL-2 Production and Differentiation of T Helper 2 and T Helper 17 Cells. Biochem. Biophysical Res. Commun. 394, 811–816. doi:10.1016/j.bbrc.2010.03.079
Worzfeld, T., Wettschureck, N., and Offermanns, S. (2008). G12/G13-mediated Signalling in Mammalian Physiology and Disease. Trends Pharmacol. Sci. 29, 582–589. doi:10.1016/j.tips.2008.08.002
Wu, M., Chen, W., Lu, Y., Zhu, G., Hao, L., and Li, Y.-P. (2017). Gα13 Negatively Controls Osteoclastogenesis through Inhibition of the Akt-GSK3β-NFATc1 Signalling Pathway. Nat. Commun. 8, 13700. doi:10.1038/ncomms13700
Wu, V., Yeerna, H., Nohata, N., Chiou, J., Harismendy, O., Raimondi, F., et al. (2019). Illuminating the Onco-GPCRome: Novel G Protein-Coupled Receptor-Driven Oncocrine Networks and Targets for Cancer Immunotherapy. J. Biol. Chem. 294, 11062–11086. doi:10.1074/jbc.rev119.005601
Wu, Y., Xu, J. X., El-Jouni, W., Lu, T., Li, S., Wang, Q., et al. (2016). Gα12 Is Required for Renal Cystogenesis Induced by Pkd1 Inactivation. J. Cell Sci 129, 3675–3684. doi:10.1242/jcs.190496
Xie, S., Mason, F. M., and Martin, A. C. (2016). Loss of Gα12/13 Exacerbates Apical Area Dependence of Actomyosin Contractility. MBoC 27, 3526–3536. doi:10.1091/mbc.e16-05-0305
Xu, N., Bradley, L., Ambdukar, I., and Gutkind, J. S. (1993). A Mutant Alpha Subunit of G12 Potentiates the Eicosanoid Pathway and Is Highly Oncogenic in NIH 3T3 Cells. Proc. Natl. Acad. Sci. 90, 6741–6745. doi:10.1073/pnas.90.14.6741
Yagi, H., Tan, W., Dillenburg-Pilla, P., Armando, S., Amornphimoltham, P., Simaan, M., et al. (2011). A Synthetic Biology Approach Reveals a CXCR4-G13-Rho Signaling axis Driving Transendothelial Migration of Metastatic Breast Cancer Cells. Sci. Signal. 4, ra60. doi:10.1126/scisignal.2002221
Yagi, H., Asanoma, K., Ohgami, T., Ichinoe, A., Sonoda, K., and Kato, K. (2016). GEP Oncogene Promotes Cell Proliferation through YAP Activation in Ovarian Cancer. Oncogene 35, 4471–4480. doi:10.1038/onc.2015.505
Yagi, H., Onoyama, I., Asanoma, K., Hori, E., Yasunaga, M., Kodama, K., et al. (2019). Gα13-mediated LATS1 Down-Regulation Contributes to Epithelial-Mesenchymal Transition in Ovarian Cancer. FASEB J. 33, 13683–13694. doi:10.1096/fj.201901278r
Yanagida, K., Igarashi, H., Yasuda, D., Kobayashi, D., Ohto-Nakanishi, T., Akahoshi, N., et al. (2018). The Gα12/13-Coupled Receptor LPA4 Limits Proper Adipose Tissue Expansion and Remodeling in Diet-Induced Obesity. JCI Insight 3, e97293. doi:10.1172/jci.insight.97293
Yanagida, K., Masago, K., Nakanishi, H., Kihara, Y., Hamano, F., Tajima, Y., et al. (2009). Identification and Characterization of a Novel Lysophosphatidic Acid Receptor, p2y5/LPA6. J. Biol. Chem. 284, 17731–17741. doi:10.1074/jbc.m808506200
Yanamadala, V., Negoro, H., Gunaratnam, L., Kong, T., and Denker, B. M. (2007). Gα12 Stimulates Apoptosis in Epithelial Cells through JNK1-Mediated Bcl-2 Degradation and Up-Regulation of IκBα. J. Biol. Chem. 282, 24352–24363. doi:10.1074/jbc.m702804200
Yang, Y. M., Kuen, D.-S., Chung, Y., Kurose, H., and Kim, S. G. (2020). Gα12/13 Signaling in Metabolic Diseases. Exp. Mol. Med. 52, 896–910. doi:10.1038/s12276-020-0454-5
Yang, Y. M., Lee, W. H., Lee, C. G., An, J., Kim, E.-S., Kim, S. H., et al. (2015). Gα12 Gep Oncogene Deregulation of P53-Responsive microRNAs Promotes Epithelial-Mesenchymal Transition of Hepatocellular Carcinoma. Oncogene 34, 2910–2921. doi:10.1038/onc.2014.218
Yasuda, D., Kobayashi, D., Akahoshi, N., Ohto-Nakanishi, T., Yoshioka, K., Takuwa, Y., et al. (2019). Lysophosphatidic Acid-Induced YAP/TAZ Activation Promotes Developmental Angiogenesis by Repressing Notch Ligand Dll4. J. Clin. Invest. 129, 4332–4349. doi:10.1172/jci121955
Ye, D., and Lin, F. (2013). S1pr2/Gα13 Signaling Controls Myocardial Migration by Regulating Endoderm Convergence. Development 140, 789–799. doi:10.1242/dev.085340
Ye, D., Xie, H., Hu, B., and Lin, F. (2015). Endoderm Convergence Controls Subduction of the Myocardial Precursors during Heart-Tube Formation. Development 142, 2928–2940. doi:10.1242/dev.113944
Yoo, E. J., Cao, G., Koziol-White, C. J., Ojiaku, C. A., Sunder, K., Jude, J. A., et al. (2017). Gα12facilitates Shortening in Human Airway Smooth Muscle by Modulating Phosphoinositide 3-Kinase-Mediated Activation in a RhoA-dependent Manner. Br. J. Pharmacol. 174, 4383–4395. doi:10.1111/bph.14040
Yu, W., Beaudry, S., Negoro, H., Boucher, I., Tran, M., Kong, T., et al. (2012). H2O2 Activates G Protein, 12 to Disrupt the Junctional Complex and Enhance Ischemia Reperfusion Injury. Proc. Natl. Acad. Sci. 109, 6680–6685. doi:10.1073/pnas.1116800109
Yu, X., Zhang, Y., and Chen, H. (2016). LPA Receptor 1 Mediates LPA-Induced Ovarian Cancer Metastasis: an In Vitro and In Vivo Study. BMC Cancer 16, 846. doi:10.1186/s12885-016-2865-1
Yuan, B., Cui, J., Wang, W., and Deng, K. (2016). Gα12/13 Signaling Promotes Cervical Cancer Invasion through the RhoA/ROCK-JNK Signaling axis. Biochem. Biophysical Res. Commun. 473, 1240–1246. doi:10.1016/j.bbrc.2016.04.048
Yung, B. S., Brand, C. S., Xiang, S. Y., Gray, C. B. B., Means, C. K., Rosen, H., et al. (2017). Selective Coupling of the S1P 3 Receptor Subtype to S1P-Mediated RhoA Activation and Cardioprotection. J. Mol. Cell Cardiol. 103, 1–10. doi:10.1016/j.yjmcc.2016.12.008
Zhang, L., Dong, Y., Wang, Y., Hu, W., Dong, S., and Chen, Y. (2020). Sphingosine‐1‐phosphate (S1P) Receptors: Promising Drug Targets for Treating Bone‐related Diseases. J. Cell Mol Med 24, 4389–4401. doi:10.1111/jcmm.15155
Zhang, Y., Zhang, J., Yan, R., Tian, J., Zhang, Y., Zhang, J., et al. (2017). Receptor-interacting Protein Kinase 3 Promotes Platelet Activation and Thrombosis. Proc. Natl. Acad. Sci. USA 114, 2964–2969. doi:10.1073/pnas.1610963114
Zuo, C., Li, X., Huang, J., Chen, D., Ji, K., Yang, Y., et al. (2018). Osteoglycin Attenuates Cardiac Fibrosis by Suppressing Cardiac Myofibroblast Proliferation and Migration through Antagonizing Lysophosphatidic Acid 3/matrix Metalloproteinase 2/epidermal Growth Factor Receptor Signalling. Cardiovasc. Res. 114, 703–712. doi:10.1093/cvr/cvy035
Glossary
5-HT4R 5-hydroxytryptamine type 4 receptor
AKT protein kinase B
Ang II angiotensin II
AP-1 activator protein 1
AT1R Ang II type 1 receptor
CXCL12 C-X-C motif chemokine 12
CXCR4 C-X-C chemokine receptor 4
DLBCL diffuse large B-cell lymphoma
ERK extracellular regulated protein kinases
FAK focal adhesion kinase
FZD Frizzled
GEF guanine nucleotide exchange factors
GPCRs G protein-coupled receptors
GRP gastrin-releasing peptide
GPR56 g protein-coupled receptor 56
HNSCC head and neck squamous cell carcinoma
I/R ischemia/reperfusion
JNK c-Jun N-terminal kinase
LATS1/2 large tumor suppressor 1/2
LPA lysophosphatidic acid
LPAR LPA receptor
MAPK mitogen-activated protein kinase
MRTF myocardin-related transcription factor
NFAT activated T cell nuclear factor
NFATc1 nuclear factor of activated T-cell c1
NF-κB nuclear factor-kappa B
PARs protease-activated receptors
PI3K phosphatidylinositol 3-kinase
PKC protein kinase C
PLC phospholipase C
PPARγ peroxisome proliferator-activated receptor-γ
RGS regulators of G protein signaling
RhoGEF Rho guanine nucleotide exchange factor
RhoA ras homolog family member A
Ric-8A resistance to inhibitors of cholinesterase 8A
ROCK Rho-associated protein kinase
ROS reactive oxygen species
S1P sphingosine 1-phosphate
SRF serum response factor
Tfh T follicular helper
Th T helper
WNT Wingless/Int-1 lipoglycoprotein
YAP/TAZ yes-associated protein/transcriptional coactivator with PDZ-binding motif
Keywords: G protein-coupled receptor, Gα12, Gα13, cell pathophysiology, diseases
Citation: Guo P, Tai Y, Wang M, Sun H, Zhang L, Wei W, Xiang YK and Wang Q (2022) Gα12 and Gα13: Versatility in Physiology and Pathology. Front. Cell Dev. Biol. 10:809425. doi: 10.3389/fcell.2022.809425
Received: 05 November 2021; Accepted: 17 January 2022;
Published: 14 February 2022.
Edited by:
Zhiqiang Lin, Masonic Medical Research Institute (MMRI), United StatesReviewed by:
Amanda Mackenzie, BioAscent, United KingdomHenry Alexander Dunn, Western University, Canada
Copyright © 2022 Guo, Tai, Wang, Sun, Zhang, Wei, Xiang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang K. Xiang, eWt4aWFuZ0B1Y2RhdmlzLmVkdQ==; Qingtong Wang, aGZ3cXQ3MjdAMTYzLmNvbQ==
 Paipai Guo
Paipai Guo Yu Tai1
Yu Tai1 Manman Wang
Manman Wang Hanfei Sun
Hanfei Sun Yang K. Xiang
Yang K. Xiang Qingtong Wang
Qingtong Wang