
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell Dev. Biol., 06 April 2022
Sec. Molecular and Cellular Oncology
Volume 10 - 2022 | https://doi.org/10.3389/fcell.2022.784968
This article is part of the Research TopicThe Role of ncRNAs (non-coding RNAs) in Regulating Tumor Immune MicroenvironmentView all 32 articles
miR-874 is located at 5q31.2, which is frequently deleted in cancer. miR-874 is downregulated in 22 types of cancers and aberrantly expressed in 18 types of non-cancer diseases. The dysfunction of miR-874 is not only closely related to the diagnosis and prognosis of tumor patients but also plays an important role in the efficacy of tumor chemotherapy drugs. miR-874 participates in the ceRNA network of long non-coding RNAs or circular RNAs, which is closely related to the occurrence and development of cancer and other non-cancer diseases. In addition, miR-874 is also involved in the regulation of multiple signaling pathways, including the Wnt/β-catenin signaling pathway, Hippo signaling pathway, PI3K/AKT signaling pathway, JAK/STAT signaling pathway, and Hedgehog signaling pathway. This review summarizes the molecular functions of miR-874 in the biological processes of tumor cell survival, apoptosis, differentiation, and tumorigenesis, and reveal the value of miR-874 as a cancer biomarker in tumor diagnosis and prognosis. Future work is necessary to explore the potential clinical application of miR-874 in chemotherapy resistance.
MicroRNA (miRNA) is a short RNA of 19–25 nucleotides in length that can regulate the post-transcriptional silencing of target genes. A single miRNA can target hundreds of mRNAs, thereby affecting the signal transduction of multiple pathways (Lu and Rothenberg, 2018). miRNA expression profiling shows that abnormal miRNA expression is related to the occurrence and progression of tumors and the response to anticancer drugs (Iorio and Croce, 2017). In addition, almost all developmental, physiological, and disease-related processes seem to be linked to miRNAs (Sun and Lai, 2013).
microRNA-874 (miR-874) is a new anti-cancer miRNA (Zhang et al., 2018b). It was originally obtained by sequencing a small RNA library (Song et al., 2016) and was first characterized in normal human cervical tissue samples (Xia et al., 2018). miR-874 is located on chromosome 5q31.2 that is often deleted in cancer (Zhang et al., 2018b). There are many target genes of miR-874 (Figure 1). By inhibiting the expression of these target genes, miR-874 is widely involved in cell proliferation, apoptosis, invasion, migration, cell cycle, epithelial-mesenchymal transition (EMT), and other cellular processes.
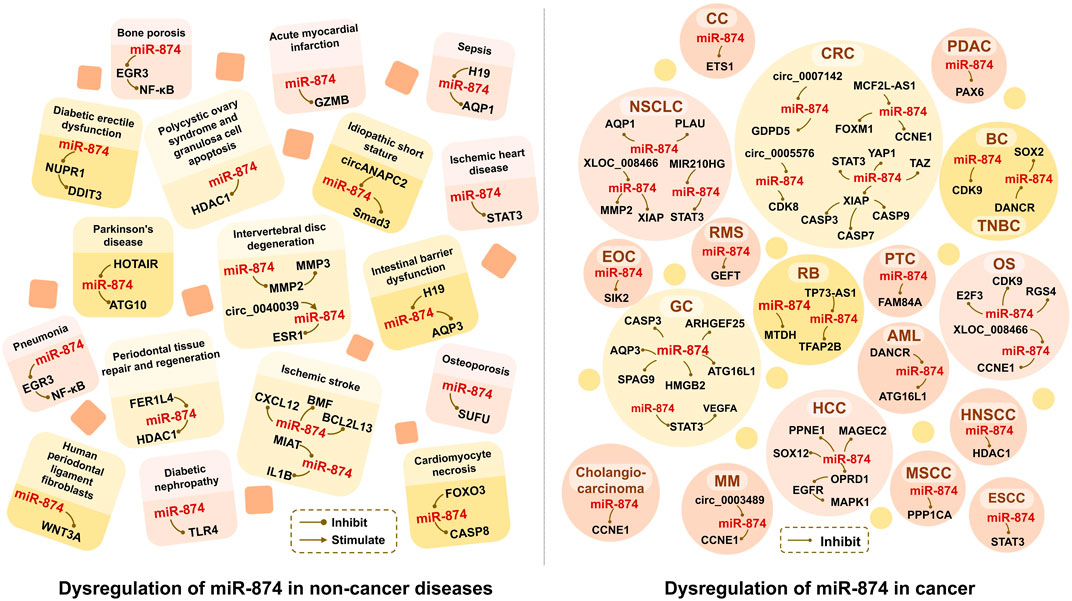
FIGURE 1. Dysregulation of miR-874 in human cancer and non-cancer diseases. HCC, Hepatocellular carcinoma; PDAC, Pancreatic ductal adenocarcinoma; NSCLC, Non-small cell lung cancer; OS, Osteosarcoma; EOC, Epithelial ovarian cancer; CC, Cervical cancer; TNBC, Triple negative breast cancer; r BC, Breast cancer; CRC, Colorectal cancer; ESCC, Esophageal squamous cell Squamous cell carcinoma; MSCC, Maxillary sinus squamous cell carcinoma; RB, Retinoblastoma; GC, Gastric cancer; RMS, Rhabdomyosarcoma; PTC, Papillary thyroid carcinoma; MM, myeloma; AML, acute myeloid leukemia; HNSCC, head and neck squamous cell carcinoma.
Since there is no comprehensive introduction of miR-874, this work summarizes the abnormal expression of miR-874 in cancer and non-cancerous diseases, and outlines the molecular mechanisms between it and protein-coding genes and non-coding RNAs.
As shown in Table 1, miR-874 is downregulated in at least 22 human malignancies, such as colorectal cancer (CRC) (Huang et al., 2020a; Yu et al., 2020; Que et al., 2017; Zhao and Dong, 2016; Han et al., 2016; Wang et al., 2021; Zhang et al., 2021c), gastric cancer (GC) (Huang et al., 2018; Sun et al., 2020; Yuan et al., 2020; Liu et al., 2017; Zhang et al., 2015; Jiang et al., 2014), hepatocellular carcinoma (HCC) (Zhang et al., 2018b; Jiang et al., 2017; Leong et al., 2017; Song et al., 2016), esophageal squamous cell carcinoma (ESCC) (Yuan et al., 2018), pancreatic ductal adenocarcinoma (PDAC) (Diao et al., 2018), non-small cell lung cancer (NSCLC) (Bu et al., 2020; Yang et al., 2017; Ahmad et al., 2015; Kesanakurti et al., 2013; Wang et al., 2020b), osteosarcoma (OS) (Tang et al., 2018; Liu et al., 2020; Dong et al., 2016; Ghosh et al., 2017), epithelial ovarian cancer (EOC) (Xia et al., 2018) cervical cancer (CC) (Liao et al., 2018; Liu et al., 2021a), retinoblastoma (RB) (Zhang et al., 2018a; Wang et al., 2020a), prostate cancer (PCa) (Pashaei et al., 2017) maxillary sinus squamous cell carcinoma (MSCC) (Nohata et al., 2011), breast cancer (BC) (Li et al., 2020; Zhang et al., 2017; Wang et al., 2014), triple-negative breast cancer (TNBC) (Wu et al., 2020), rhabdomyosarcoma (RMS) (Shang et al., 2019), papillary thyroid carcinoma (PTC) (Ding et al., 2021), endometrial cancer (Witek et al., 2021), cholangiocarcinoma (Pan et al., 2021), glioma (Li et al., 2021a), myeloma (MM) (Tian et al., 2021), head and neck squamous cell carcinoma (HNSCC) (Nohata et al., 2013) and acute myeloid leukemia (AML) (Zhang et al., 2021a).
HNSCC is the sixth most common cancer in the world (Nohata et al., 2013). The expression of miR-874-3p is reduced in HNSCC, and miR-874-3p can be upregulated after 5-Aza-dC treatment, indicating that the downregulation of miR-874-3p in HNSCC may be due to the methylation of its upstream CpG island (Nohata et al., 2013). Histone deacetylase 1 (HDAC1) belongs to the HDAC family. HDAC removes acetyl groups from histones and other nuclear proteins that induce chromatin condensation and transcriptional inhibition. HDAC plays an important role in the abnormal epigenetic changes associated with human cancer (Witt et al., 2009). In HNSCC, miR-874-3p can directly target HDAC1, significantly inhibit cell proliferation, and induce cell cycle arrest and apoptosis (Nohata et al., 2013).
Also, miR-874 plays an important role in non-cancer diseases. Low expression of miR-874 is associated with the risk of ischemic stroke (IS) (Jiang et al., 2019; Xie et al., 2020; Zhang et al., 2021b), ischemic heart disease (IHD) (Chen et al., 2019), cardiomyocyte necrosis (Wang et al., 2013), diabetic erectile dysfunction (DMED) (Huo et al., 2020), diabetic nephropathy (DN) (Yao et al., 2018), sepsis (Fang et al., 2018), osteoporosis (Lin et al., 2018), Parkinson’s disease (PD) (Zhao et al., 2020), acute myocardial infarction (AMI) (Yan et al., 2017), intestinal barrier dysfunction (Su et al., 2016), periodontal tissue repair and regeneration (Huang et al., 2020b), idiopathic short stature (ISS) (Liu et al., 2021b), intervertebral disc degeneration (IDD) (Song et al., 2021a), pneumonia (Yang et al., 2021), and human periodontal ligament fibroblasts (Song et al., 2021b) (Table 2).
Highly expressed miR-874-3p is associated with the risk of bone porous (Kushwaha et al., 2016) and polycystic ovary syndrome (PCOS) (Wei et al., 2021), IDD (Li et al., 2021b). miR-874-3p in maternal osteoblasts increased 4–6 times during the child’s weaning period. Increasing the expression of miR-874-3p could enhance bone formation and restore the mother’s bone quality after pregnancy and lactation (Kushwaha et al., 2016). In granulosa cells, testosterone promotes p53 acetylation and expression by upregulating the expression of miR-874-3p and induces granulosa cell apoptosis (Wei et al., 2021), thereby promoting the occurrence and development of PCOS (Wei et al., 2021) (Table 2).
However, the results of the association between miR-874-3p expression and IDD are divergent. The expression level of miR-874-3p in the NP tissues of IDD patients was significantly reduced, thereby upregulating the expression of MMP2 and MMP3, eventually leading to the occurrence of IDD (Song et al., 2021a). In nucleus pulposus cells (NPCs), circ_0040039 can increase the stability of miR-874-3p and upregulate the miR-874-3p/ESR1 pathway to aggravate IDD (Li et al., 2021b). The different effects of miR-874-3p in IDD may be related to the tested sample types. It is worth noting that the sample size of IDD-related studies is small, and there is a lack of follow-up experiments to further explore the in vivo function of miR-874-3p. In the future, more samples and in vivo experiments are needed to confirm the mechanism of miR-874-3p in IDD.
In patients of HCC, OS, or RMS, decreased expression of miR-874 is associated with tumor size, vascular infiltration, lymph node metastasis, tumor-node-metastasis (TNM) staging, clinical staging, and tumor differentiation (Dong et al., 2016; Zhang et al., 2018b; Shang et al., 2019). Subsequent cell function experiments revealed the tumor suppressor effects of miR-874, including inhibition of proliferation, invasion, metastasis, and promotion of apoptosis (Diao et al., 2018) (Table 3).
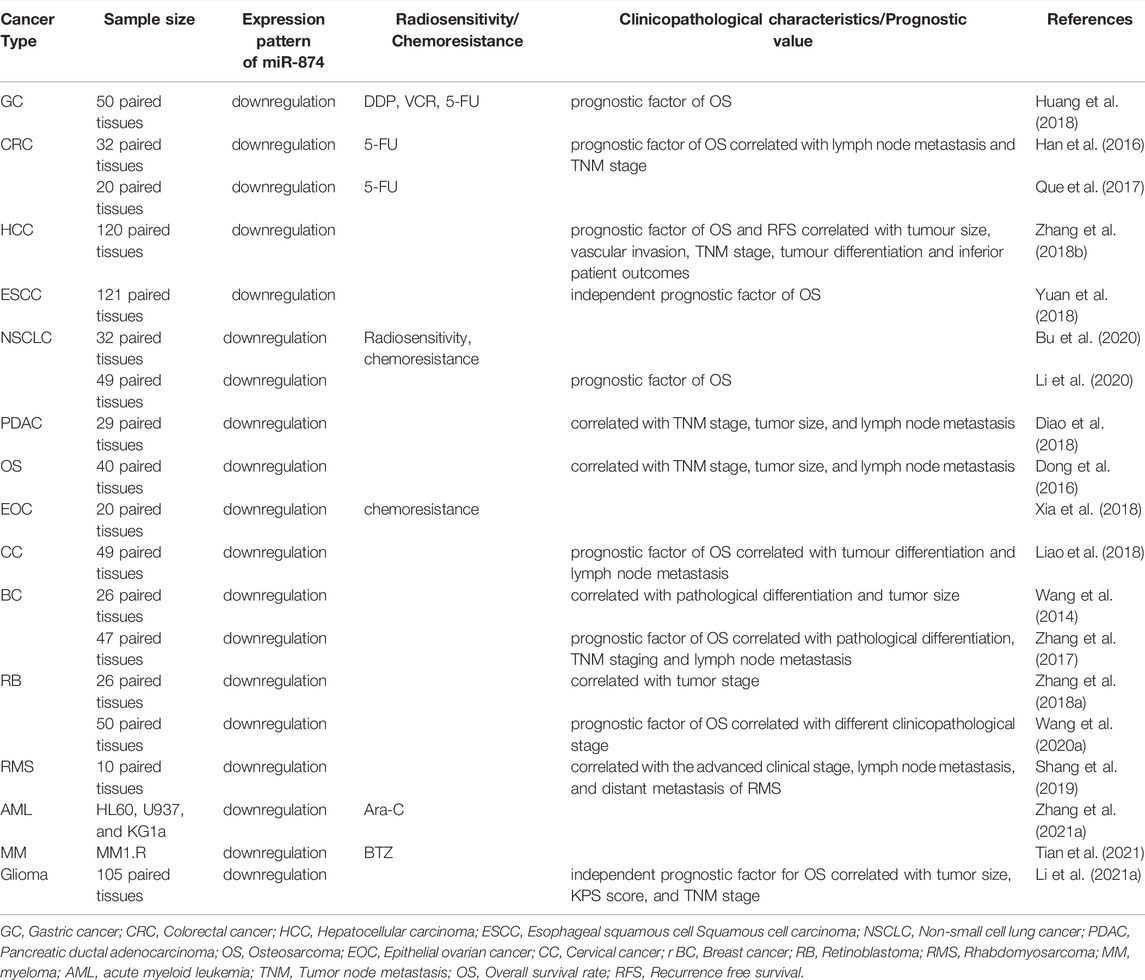
TABLE 3. The contribution of miR-874 to the treatment, clinicopathological characteristics, and prognosis in cancer.
As shown in Table 3, compared with cancer patients with high miR-874 expression, patients with low miR-874 expression have a significantly worse prognosis. These cancers include GC (Huang et al., 2018), CRC (Han et al., 2016), HCC (Zhang et al., 2018b), ESCC (Yuan et al., 2018), NSCLC (Li et al., 2020), CC (Liao et al., 2018), BC (Zhang et al., 2017), RB (Wang et al., 2020a), and glioma (45).
In GC, CRC, NSCLC, and EOC, miR-874 can reduce the drug resistance of cancer cells (Huang et al., 2018; Han et al., 2016; Que et al., 2017; Bu et al., 2020; Xia et al., 2018). Among them, the upregulation of miR-874 expression in CRC cells can increase the sensitivity to 5-FU (Han et al., 2016; Que et al., 2017). The overexpression of miR-874 significantly enhanced the sensitivity of GC cells to DDP, VCR, and 5-FU (Huang et al., 2018). The MIR210HG/miR-874/STAT3 axis plays a carcinogenic regulatory role in the radiosensitivity and drug resistance of NSCLC (Bu et al., 2020). miR-874-3p and miR-874-5p can enhance the chemical sensitivity of EOC cells (Xia et al., 2018).
Cancer cells use autophagy to provide energy and develop resistance to anti-cancer drugs; therefore, inhibiting autophagy may promote cancer cell death and help overcome drug resistance (Levy et al., 2017). Autophagy involved in miR-874-3p is an important mechanism for regulating chemotherapy resistance in AML (Zhang et al., 2021a). In AML, the DANCR/miR-874-3p/ATG16L1 axis can promote autophagy, thereby enhancing the resistance of human AML cells to Ara-C (Zhang et al., 2021a). In addition, knocking out circ_0003489 can upregulate miR-874-3p and inhibit HDAC1, thereby prompting MM cells to switch from autophagy to apoptosis, and reducing the growth of MM cells (Tian et al., 2021).
The above findings indicate that miR-874 can be developed as a new diagnostic and prognositc biomarker for patients with the above cancer types and suggest a potential value of miR-874 in cancer drug resistance.
Non-coding RNA can regulate gene expression, thereby affecting cell proliferation, survival, and migration, and is related to genome stability and malignant transformation of inflammatory cells (Zhang et al., 2021a). There are interactions between non-coding RNAs. For example, lncRNAs and circRNAs can be used as ceRNAs to sponge miRNAs (Zhang et al., 2021a). The ceRNA network centered on miR-874 is involved with at least 10 lncRNAs or 12 circRNAs. The dysfunction of miR-874 is closely related to the occurrence and development of tumors and other diseases (Figure 2).
In TNBC, lncRNA DANCR acts as a ceRNA for miR-874-3p, thereby regulating the derepression of SOX2 and promoting the EMT in TNBC (Wu et al., 2020). In CRC, the MCF2LAS1/miR-874-3p/FOXM1 axis (Liao et al., 2018) and MCF2LAS1/miR-874-3p/CCNE1 axis (Huang et al., 2020a) can promote cancer cell apoptosis, inhibit cancer cell proliferation, invasion, migration and EMT process. Also, the circ_0005576/miR-874/CDK8 axis can promote the malignant progression of CRC (Yu et al., 2020). In CRC cells, circ_0007142 can regulate the level of GDPD5 by sponging miR-874-3p (12). Knock-down of circ_0007142 can induce ferroptosis through the circ_0007142/miR-874-3p/GDPD5 axis, thereby increasing the effectiveness of chemotherapy or radiotherapy and inhibiting the malignant progression of CRC (Wang et al., 2021). In RB, the expression of lncRNA TP73-AS1 is upregulated, and the downregulated TP73-AS1/miR-874-3p/TFAP2B axis can inhibit the Wnt/β-catenin signaling pathway, thereby inhibiting tumor progression (Wang et al., 2020a). In NSCLC, the miR210HG/miR-874-3p/STAT3 axis plays a role in the progression of NSCLC cells (Bu et al., 2020). Besides, XLOC_008466, as the ceRNA of miR-874-3p, can increase the expression of MMP2 and XIAP and affect NSCLC cell proliferation, apoptosis, and invasion (Yang et al., 2017; Bu et al., 2020). In OS, the downregulated lncRNA XLOC_008466/miR-874-3p/CCNE1 axis can also inhibit tumor growth (Ghosh et al., 2017). The expression of lncRNA DCST1-AS1 increases in cervical cancer tissues and cells, and inhibition of DCST1-AS1 can increase the expression of miR-874-3p, thereby inhibiting the proliferation, migration and invasion of cervical cancer cells (Liu et al., 2021a). In PTC, miR-874-3p can inhibit FAM84A and exert carcinogenic effects through the Wnt/β-catenin signal transduction (Ding et al., 2021). LncRNA DANCR is a promising tumor-related lncRNA that can enhance cancer cell proliferation, stemness, invasion, and metastasis (Zhang et al., 2021a). In AML, DANCR regulates autophagy by promoting the miR-874-3p/ATG16L1 axis, thereby reducing Ara-C resistance in human AML cells (Zhang et al., 2021a). BTZ is a first-class proteasome inhibitor approved by the FDA for the treatment of newly diagnosed and relapsed MM patients. In MM, the circ_0003489/miR-874-3p/HDAC1 axis plays a crucial role in controlling the balance of autophagy and apoptosis in MM cells. Downregulation of circ_0003489 can increase the inhibition of miR-874-3p on HDAC1, and improve the efficacy of BTZ in the treatment of MM (Tian et al., 2021).
Besides, we found that the ceRNA network of miR-874 also plays an important role in non-cancer diseases. The H19/miR-874/AQP1 axis can help restore inflammatory response to lipopolysaccharide (LPS) and inflammation associated with sepsis-induced myocardial dysfunction (Fang et al., 2018). Also, the H19/miR-874/AQP3 axis plays an important role in maintaining the intestinal barrier function (Su et al., 2016). During the continuous osteogenic differentiation of periodontal ligament stromal cells (PDLSCs), the FER1L4/miR-874-3p/VEGFA axis can positively regulate the osteogenic differentiation of PDLSCs (Huang et al., 2020b). In PD, the HOTAIR/miR-874-5p/ATG10 axis can promote MPP+-induced neuronal damage (Zhao et al., 2020). miR-874-3p can reduce the levels of TNF-α, IL-1, IL-6, and IL-8, increase the level of IL-10, reduce neuronal apoptosis, and significantly inhibit brain inflammation in the IS model mice. LncRNA MIAT can sponge miR-874-3p to increase the risk of IS (Zhang et al., 2021b). In addition, circANAPC2 is upregulated in ISS patients, and it can inhibit the proliferation, hypertrophy, and endochondral ossification of chondrocytes through the circANAPC2/miR-874-3p/SMAD3 axis in vitro (Liu et al., 2021b).
It is worth noting that circRNA-mediated regulation of miRNA expression consists of two modes (Piwecka et al., 2017). One is the classical sponge mechanism, in which circRNA inhibits or does not affect miRNA expression. The other is a stabilization mechanism in which circRNAs increase the expression of miRNAs. In IDD, circ_0040039 can stabilize miR-874-3p and inhibit the expression level of ESR1, thereby promoting the apoptosis of the NPCs and inhibiting the growth of NPCs (Li et al., 2021b).
The target genes of miR-874 are involved in at least five classical signaling pathways, including the Wnt/β-catenin pathway [TFAP2B (Wang et al., 2020a), CDK8 (Yu et al., 2020), HMGB2 (Yuan et al., 2020), CXCL12 (Xie et al., 2020), and FAM84A (Ding et al., 2021)], the Hippo pathway [YAP1 and TAZ (Que et al., 2017)], the PI3K/AKT pathway [AQP3 (Li et al., 2020)], the JAK/STAT pathway [STAT3 (Zhang et al., 2015; Li et al., 2020; Zhao and Dong, 2016; Bu et al., 2020; Yuan et al., 2018; Shang et al., 2019)] and the Hedgehog signaling pathway [SUFU (Lin et al., 2018)] (Figure 3).
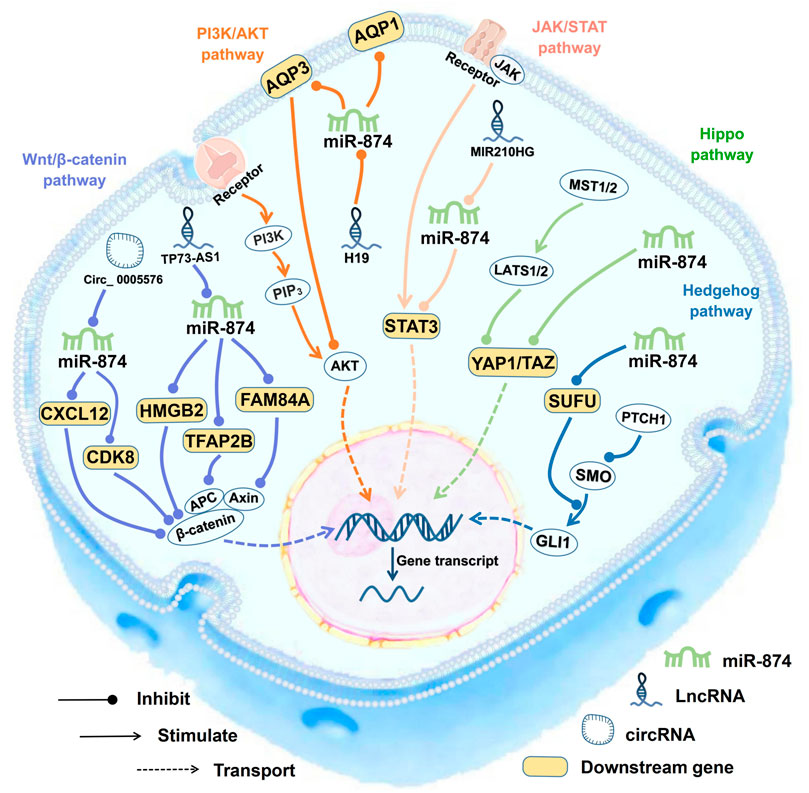
FIGURE 3. miR-874 regulate many biological processes within the cell. The target genes of miR-874 are involved in at least five classical signaling pathways, including the Wnt/β-catenin signaling pathway, P13/AKT signaling pathway, Hippo signaling pathway, JAK/STAT signaling pathway, and Hedgehog signaling pathway. EMT, epithelial-mesenchymal transition.
The close relationship between miR-874 and Wnt/β-catenin signaling pathway is of great significance not only for tumor diseases but also for IS (Figure 3). Wnt/β-catenin signaling pathway plays a key role in regulating cell growth, cell development, and normal stem cell differentiation. Constitutive activation of the Wnt/β-catenin signaling pathway has been found in many human cancers (Yao et al., 2011). In RB tissues and cells, the TP73-AS1/miR-874-3p/TFAP2B axis can activate the Wnt/β-catenin signaling pathway and enhance the expression of downstream tumor-related factors TCF4, BCL2, CCND1, and MYC (Wang et al., 2020a).
Abnormal activation of the Wnt/β-catenin signaling pathway occurs in almost all CRC (Wang et al., 2020a). In CRC, the circ_0005576/miR-874-3p/CDK8 axis can cause the abnormal activation of the Wnt/β-catenin signaling pathway and the proliferation of CRC cells (Yu et al., 2020). In GC cell lines the downregulation of the miR-874-3p/HMGB2 axis can upregulate the expression of β-catenin, CCND1, and MYC, which shows that the abnormal activation Wnt/β-catenin signaling pathway may be regulated by the miR-874-3p/HMGB2 axis in GC (Yuan et al., 2020).
The expression of serum CXCL12 in patients with IS was higher than that in healthy controls. CXCL12 can act as a ligand for CXC motif chemokine receptor 4 (CXCR4) and is a downstream target gene of miR-874-3p (Xie et al., 2020). In mice with IS, the Wnt/β-catenin signaling pathway is inhibited, and downregulation of CXCL12 can activate the Wnt/β-catenin signaling pathway, thereby promoting angiogenesis and inhibiting the brain tissue apoptosis in mice with IS (Xie et al., 2020). This suggests that the miR-874-3p/CXCL12 axis can activate the Wnt/β-catenin signaling pathway, which provides a new hint for the treatment of IS (Xie et al., 2020).
In PTC, FAM84A can activate EMT and the Wnt/β-catenin signaling pathway, thereby inducing tumorigenesis of thyroid cancer (Ding et al., 2021). miR-874-3p can target the 3′UTR of FAM84A, thereby reducing the expression of FAM84A. Attenuation of miR-874-3p/FAM84A/Wnt/β-catenin axis can inhibit PTC tumor progression (Ding et al., 2021).
In addition, miR-874-3p/WNT/β-catenin axis can inhibit the osteogenic differentiation of hPDLF. During osteogenic differentiation of hPDLF, the downregulation of miR-874-3p corresponds to the increase in WNT3A expression, while overexpression of miR-874-3p can inhibit WNT3A expression, thereby upregulating the expression of the β-catenin protein (Song et al., 2021b).
The Hippo signaling pathway can regulate cell growth, differentiation, aging, contact inhibition, and other biological processes, and plays an important role in maintaining cell growth and maintaining the stability of apoptosis balance (Que et al., 2017). YAP1 and TAZ are downstream transcriptional effectors of the Hippo signaling pathway, which can promote cell growth, invasion, and migration (Que et al., 2017). In CRC cells, the ectopic expression of miR-874-3p can inhibit the expression of YAP1 and TAZ, and by downregulating the expression of BCL2 and BCL2L1, increasing the activity of CASP9 and CASP3, thereby promoting 5-FU-induced apoptosis (Que et al., 2017). Downregulation of miR-874-3p can inactivate the Hippo signaling pathway, thereby increasing the resistance of cells to 5-FU chemotherapy (Que et al., 2017) (Figure3).
The PI3K/AKT signaling pathway is downstream of many growth factor receptors. It promotes the proliferation and malignant transformation of tumor cells and inhibits tumor cell apoptosis through the phosphorylation of PI3K and AKT proteins (Lu et al., 2019). Downregulation of miR-874 in NSCLC tissues and cell lines can increase the expression of its target gene AQP3, promote p-PI3K and p-AKT phosphorylation, and activate the PI3K/AKT signaling pathway (Wang et al., 2020b). The above implies that miR-874 deactivates the PI3K/AKT signaling pathway by targeting AQP3 and exerts its tumor suppressor effect (Wang et al., 2020b) (Figure 3).
The JAK/STAT signaling pathway includes a family of receptor-associated cytoplasmic tyrosine kinases (JAKs) that phosphorylate tyrosine residues in STAT homologs (Wang et al., 2019). The JAK/STAT signaling pathway plays an inhibitory role in various physiological processes, such as cell development and differentiation (Wang et al., 2019).
miR-874-3p can inhibit the JAK/STAT signaling pathway by inhibiting STAT3 (Figure 3). As an anti-apoptotic factor, STAT3 plays an important role in the regulation of gene expression and mitochondrial electron transport during cellular stress (Wang et al., 2019). miR-874-3p can inhibit STAT3 in several cancers, including GC (Zhang et al., 2015), CRC (Zhao and Dong, 2016), NSCLC (Bu et al., 2020), and ESCC (Yuan et al., 2018). In gastric cancer, constitutive STAT3 activation promotes VEGF-A expression and stimulates tumor angiogenesis. miR-874 can bind to the 3′-UTR of STAT3 and downregulate STAT3 expression, thereby inhibiting angiogenesis (Zhang et al., 2015). In CRC, miR-874 inhibits STAT3 expression by targeting its mRNA 3′UTR, thereby inhibiting cell growth and inducing apoptosis (Zhao and Dong, 2016). In NSCLC cells, miR210HG can downregulate the expression of miR-874, thereby promoting the expression of STAT3 (Bu et al., 2020). In ESCC, the overexpression of miR-874 can inhibit tumor development by targeting STAT3. Besides, in IHD, inhibiting miR-874-3p can activate the JAK2/STAT3 signaling pathway, thereby inhibiting the expression of BAX, upregulating BCL2, reducing cardiomyocyte apoptosis, and ultimately reducing the risk of ischaemia/reperfusion (I/R) damage in mice (Chen et al., 2019).
The Hedgehog signaling pathway is conservative and it is involved in the proliferation and differentiation of a variety of cells (Lin et al., 2018). SUFU is a negative regulator of the Hedgehog signaling pathway in vertebrates. SUFU can inhibit the GLI transcription factor and induce skeletal dysplasia, osteoarthritis, or chondroma (Lin et al., 2018). By inhibiting SUFU and activating the Hedgehog signaling pathway, miR-874 can promote osteoblast proliferation, increase alkaline phosphatase activity and calcium nodules, and inhibit osteoblast apoptosis (Lin et al., 2018).
miR-874 is downregulated in many cancers and non-cancer diseases, suggesting that it plays a key role in the physiological and pathological processes of human disease. miR-874 plays an important role in the progression of malignant tumors by regulating a complex ceRNA network. The ceRNA network centered on miR-874 includes at least 10 ncRNAs and 12 protein-coding genes. miR-874 has also been shown to participate in at least 4 important signaling pathways, including the Hippo signaling pathway, Wnt/β-catenin signaling pathway, JAK/STAT signaling pathway, and Hedgehog signaling pathway.
It is worth noting that in the relevant research of IDD, the expression of miR-874-3p is inconsistent. This may be related to the cell state and type, and these differences need to be further verified in large-scale experiments. In IDD, circ_0040039 can enhance miR-874-3p through a stabilization mechanism. In the future, further exploration of miR-874-related stabilization mechanisms will help to understand the ceRNA network of miR-874 and the clinical effectiveness of targeting miR-874.
The abnormal expression of miR-874 is closely related to the clinicopathological characteristics of 15 cancers. Therefore, miR-874 can be used as a potential biomarker for the early prediction of cancer. In addition, in AML and MM, miR-874 participates in the regulation of autophagy-related functions and affects drug resistance of cells, which provides new ideas for overcoming drug resistance. However, the current research of miR-874 is focused on the exploration of the mechanism of its upstream and downstream genes. The potential clinical application of miR-874 in cancer prognosis and chemotherapy resistance is still lacking.
In existing studies, miR-874 is downregulated in all cancers studied and is related to the clinicopathological characteristics of cancer. Therefore, miR-874 is promising as a potential biomarker for the early prediction of cancer. In addition, in recent years, more and more non-cancer diseases have also recognized the evidence related to miR-874, but the specific regulatory mechanism of miR-874 in non-cancer diseases remains to be revealed. Future work is necessary to explore the mechanism of miR-874-related ceRNA network in cancer and non-cancer disease.
SD, WG and QZ contributed to the conception, design and final approval of the submitted version. QZ, QY and CZ collected and analyzed literature. QZ, CZ, L-hZ, WG, and SD contributed to manuscript writing. All the authors conceived and gave the approval of the final manuscript.
The research was supported by the grant of Zhejiang Provincial Health Department Project (No. 2020KY680).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ahmad, A., Sayed, A., Ginnebaugh, K. R., Sharma, V., Suri, A., and Saraph, A. (2015). Molecular Docking and Inhibition of Matrix Metalloproteinase-2 by Novel Difluorinatedbenzylidene Curcumin Analog. Am. J. Transl Res. 7 (2), 298–308.
Bu, L., Zhang, L., Tian, M., Zheng, Z., Tang, H., and Yang, Q. (2020). LncRNA MIR210HG Facilitates Non-small Cell Lung Cancer Progression through Directly Regulation of miR-874/STAT3 Axis. Dose Response 18 (3), 1559325820918052. doi:10.1177/1559325820918052
Chen, P. J., Shang, A. Q., Yang, J. P., and Wang, W. W. (2019). microRNA-874 Inhibition Targeting STAT3 Protects the Heart from Ischemia-Reperfusion Injury by Attenuating Cardiomyocyte Apoptosis in a Mouse Model. J. Cel Physiol 234 (5), 6182–6193. doi:10.1002/jcp.27398
Diao, J., Su, X., Cao, L., Yang, Y., and Liu, Y. (2018). MicroRNA874 Inhibits Proliferation and Invasion of Pancreatic Ductal Adenocarcinoma Cells by Directly Targeting Paired Box 6. Mol. Med. Rep. 18 (1), 1188–1196. doi:10.3892/mmr.2018.9069
Ding, Y., Wu, L., Zhuang, X., Cai, J., Tong, H., Si, Y., et al. (2021). The Direct miR-874-3p-Target FAM84A Promotes Tumor Development in Papillary Thyroid Cancer. Mol. Oncol. 15 (5), 1597–1614. doi:10.1002/1878-0261.12941
Dong, D., Gong, Y., Zhang, D., Bao, H., and Gu, G. (2016). miR-874 Suppresses the Proliferation and Metastasis of Osteosarcoma by Targeting E2F3. Tumour Biol. 37 (5), 6447–6455. doi:10.1007/s13277-015-4527-3
Fang, Y., Hu, J., Wang, Z., Zong, H., Zhang, L., Zhang, R., et al. (2018). LncRNA H19 Functions as an Aquaporin 1 Competitive Endogenous RNA to Regulate microRNA-874 Expression in LPS Sepsis. Biomed. Pharmacother. 105, 1183–1191. doi:10.1016/j.biopha.2018.06.007
Ghosh, T., Varshney, A., Kumar, P., Kaur, M., Kumar, V., Shekhar, R., et al. (2017). MicroRNA-874-mediated Inhibition of the Major G1/S Phase Cyclin, CCNE1, Is Lost in Osteosarcomas. J. Biol. Chem. 292 (52), 21264–21281. doi:10.1074/jbc.M117.808287
Han, J., Liu, Z., Wang, N., and Pan, W. (2016). MicroRNA-874 Inhibits Growth, Induces Apoptosis and Reverses Chemoresistance in Colorectal Cancer by Targeting X-Linked Inhibitor of Apoptosis Protein. Oncol. Rep. 36 (1), 542–550. doi:10.3892/or.2016.4810
Huang, F. K., Zheng, C. Y., Huang, L. K., Lin, C. Q., Zhou, J. F., and Wang, J. X. (2020a). Long Non-coding RNA MCF2L-AS1 Promotes the Aggressiveness of Colorectal Cancer by Sponging miR-874-3p and Thereby Up-Regulating CCNE1. J. Gene Med. 36, e3285. doi:10.1002/jgm.3285
Huang, H., Tang, J., Zhang, L., Bu, Y., and Zhang, X. (2018). miR-874 Regulates Multiple-Drug Resistance in Gastric Cancer by Targeting ATG16L1. Int. J. Oncol. 53 (6), 2769–2779. doi:10.3892/ijo.2018.4593
Huang, Y., Han, Y., Guo, R., Liu, H., Li, X., Jia, L., et al. (2020b). Long Non-coding RNA FER1L4 Promotes Osteogenic Differentiation of Human Periodontal Ligament Stromal Cells via miR-874-3p and Vascular Endothelial Growth Factor A. Stem Cel Res Ther 11 (1), 5. doi:10.1186/s13287-019-1519-z
Huo, W., Li, H., Zhang, Y., and Li, H. (2020). Epigenetic Silencing of microRNA-874-3p Implicates in Erectile Dysfunction in Diabetic Rats by Activating the Nupr1/Chop-Mediated Pathway. FASEB J. 34 (1), 1695–1709. doi:10.1096/fj.201902086R
Iorio, M. V., and Croce, C. M. (2017). MicroRNA Dysregulation in Cancer: Diagnostics, Monitoring and Therapeutics. A Comprehensive Review. EMBO Mol. Med. 9 (6), 852. doi:10.15252/emmm.201707779
Jiang, B., Li, Z., Zhang, W., Wang, H., Zhi, X., Feng, J., et al. (2014). miR-874 Inhibits Cell Proliferation, Migration and Invasion through Targeting Aquaporin-3 in Gastric Cancer. J. Gastroenterol. 49 (6), 1011–1025. doi:10.1007/s00535-013-0851-9
Jiang, D., Sun, X., Wang, S., and Man, H. (2019). Upregulation of miR-874-3p Decreases Cerebral Ischemia/reperfusion Injury by Directly Targeting BMF and BCL2L13. Biomed. Pharmacother. 117, 108941. doi:10.1016/j.biopha.2019.108941
Jiang, T., Guan, L. Y., Ye, Y. S., Liu, H. Y., and Li, R. (2017). MiR-874 Inhibits Metastasis and Epithelial-Mesenchymal Transition in Hepatocellular Carcinoma by Targeting SOX12. Am. J. Cancer Res. 7 (6), 1310–1321.
Kesanakurti, D., Maddirela, D. R., Chittivelu, S., Rao, J. S., and Chetty, C. (2013). Suppression of Tumor Cell Invasiveness and In Vivo Tumor Growth by microRNA-874 in Non-small Cell Lung Cancer. Biochem. Biophys. Res. Commun. 434 (3), 627–633. doi:10.1016/j.bbrc.2013.03.132
Kushwaha, P., Khedgikar, V., Sharma, D., Yuen, T., Gautam, J., Ahmad, N., et al. (2016). MicroRNA 874-3p Exerts Skeletal Anabolic Effects Epigenetically during Weaning by Suppressing Hdac1 Expression. J. Biol. Chem. 291 (8), 3959–3966. doi:10.1074/jbc.M115.687152
Leong, K. W., Cheng, C. W., Wong, C. M., Ng, I. O., Kwong, Y. L., and Tse, E. (2017). miR-874-3p Is Down-Regulated in Hepatocellular Carcinoma and Negatively Regulates PIN1 Expression. Oncotarget 8 (7), 11343–11355. doi:10.18632/oncotarget.14526
Levy, J. M. M., Towers, C. G., and Thorburn, A. (2017). Targeting Autophagy in Cancer. Nat. Rev. Cancer 17 (9), 528–542. doi:10.1038/nrc.2017.53
Li, Y., Chen, X., Xue, W., Liang, J., and Wang, L. (2021a). MiR-874 Inhibits Cell Proliferation, Migration, and Invasion of Glioma Cells and Correlates with Prognosis of Glioma Patients. Neuromolecular Med. 23 (2), 247–255. doi:10.1007/s12017-020-08608-0
Li, Y. L., Wang, X. M., Qiao, G. D., Zhang, S., Wang, J., Cong, Y. Z., et al. (2020). Up-regulated Lnc-Lung Cancer Associated Transcript 1 Enhances Cell Migration and Invasion in Breast Cancer Progression. Biochem. Biophys. Res. Commun. 521 (2), 271–278. doi:10.1016/j.bbrc.2019.08.040
Li, Y., Wang, X., Xu, H., Li, G., Huo, Z., Du, L., et al. (2021b). Circ_0040039 May Aggravate Intervertebral Disk Degeneration by Regulating the MiR-874-3p-ESR1 Pathway. Front. Genet. 12, 656759. doi:10.3389/fgene.2021.656759
Liao, H., Pan, Y., Pan, Y., Shen, J., Qi, Q., Zhong, L., et al. (2018). MicroRNA874 Is Downregulated in Cervical Cancer and Inhibits Cancer Progression by Directly Targeting ETS1. Oncol. Rep. 40 (4), 2389–2398. doi:10.3892/or.2018.6624
Lin, J. C., Liu, Z. G., Yu, B., and Zhang, X. R. (2018). MicroRNA-874 Targeting SUFU Involves in Osteoblast Proliferation and Differentiation in Osteoporosis Rats through the Hedgehog Signaling Pathway. Biochem. Biophys. Res. Commun. 506 (1), 194–203. doi:10.1016/j.bbrc.2018.09.187
Liu, B., Li, F., Zhao, H. P., Chen, J. B., Li, Y. P., and Yu, H. H. (2017). miR-874 Inhibits Metastasis-Relevant Traits via Targeting SH2B Adaptor Protein 1 (SH2B1) in Gastric Cancer. Int. J. Clin. Exp. Pathol. 10 (8), 8577–8584.
Liu, J., Zhang, J., Hu, Y., Zou, H., Zhang, X., and Hu, X. (2021a). Inhibition of lncRNA DCST1-AS1 Suppresses Proliferation, Migration and Invasion of Cervical Cancer Cells by Increasing miR-874-3p Expression. J. Gene Med. 23 (1), e3281. doi:10.1002/jgm.3281
Liu, W. G., Zhuo, L., Lu, Y., Wang, L., Ji, Y. X., and Guo, Q. (2020). miR-874-3p Inhibits Cell Migration through Targeting RGS4 in Osteosarcoma. J. Gene Med. 22 (9), e3213. doi:10.1002/jgm.3213
Liu, X., Du, Z., Yi, X., Sheng, T., Yuan, J., and Jia, J. (2021b). Circular RNA circANAPC2 Mediates the Impairment of Endochondral Ossification by miR-874-3p/SMAD3 Signalling Pathway in Idiopathic Short Stature. J. Cel Mol Med 25 (7), 3408–3426. doi:10.1111/jcmm.16419
Lu, T. X., and Rothenberg, M. E. (2018). MicroRNA. J. Allergy Clin. Immunol. 141 (4), 1202–1207. doi:10.1016/j.jaci.2017.08.034
Lu, Y., Li, L., Wu, G., Zhuo, H., Liu, G., and Cai, J. (2019). Effect of PI3K/Akt Signaling Pathway on PRAS40Thr246 Phosphorylation in Gastric Cancer Cells. Iran J. Public Health 48 (12), 2196–2204.
Nohata, N., Hanazawa, T., Kikkawa, N., Sakurai, D., Fujimura, L., Chiyomaru, T., et al. (2011). Tumour Suppressive microRNA-874 Regulates Novel Cancer Networks in Maxillary Sinus Squamous Cell Carcinoma. Br. J. Cancer 105 (6), 833–841. doi:10.1038/bjc.2011.311
Nohata, N., Hanazawa, T., Kinoshita, T., Inamine, A., Kikkawa, N., Itesako, T., et al. (2013). Tumour-suppressive microRNA-874 Contributes to Cell Proliferation through Targeting of Histone Deacetylase 1 in Head and Neck Squamous Cell Carcinoma. Br. J. Cancer 108 (8), 1648–1658. doi:10.1038/bjc.2013.122
Pan, X., Wang, G., and Wang, B. (2021). Ectopic Expression of microRNA-874 Represses Epithelial Mesenchymal Transition through the NF-kappaB Pathway via CCNE1 in Cholangiocarcinoma. Cell Signal 82, 109927. doi:10.1016/j.cellsig.2021.109927
Pashaei, E., Pashaei, E., Ahmady, M., Ozen, M., and Aydin, N. (2017). Meta-analysis of miRNA Expression Profiles for Prostate Cancer Recurrence Following Radical Prostatectomy. PLoS One 12 (6), e0179543. doi:10.1371/journal.pone.0179543
Piwecka, M., Glazar, P., Hernandez-Miranda, L. R., Memczak, S., Wolf, S. A., Rybak-Wolf, A., et al. (2017). Loss of a Mammalian Circular RNA Locus Causes miRNA Deregulation and Affects Brain Function. Science 357 (6357). doi:10.1126/science.aam8526
Que, K., Tong, Y., Que, G., Li, L., Lin, H., Huang, S., et al. (2017). Downregulation of miR-874-3p Promotes Chemotherapeutic Resistance in Colorectal Cancer via Inactivation of the Hippo Signaling Pathway. Oncol. Rep. 38 (6), 3376–3386. doi:10.3892/or.2017.6041
Shang, H., Liu, Y., Li, Z., Liu, Q., Cui, W., Zhang, L., et al. (2019). MicroRNA-874 Functions as a Tumor Suppressor in Rhabdomyosarcoma by Directly Targeting GEFT. Am. J. Cancer Res. 9 (4), 668–681.
Song, Q., Zhang, F., Wang, K., Chen, Z., Li, Q., Liu, Z., et al. (2021a). MiR-874-3p Plays a Protective Role in Intervertebral Disc Degeneration by Suppressing MMP2 and MMP3. Eur. J. Pharmacol. 895, 173891. doi:10.1016/j.ejphar.2021.173891
Song, S., Yan, Z., and Wu, W. (2021b). MiR-874-3p Inhibits Osteogenic Differentiation of Human Periodontal Ligament Fibroblasts through Regulating Wnt/beta-Catenin Pathway. J. Dent Sci. 16 (4), 1146–1153. doi:10.1016/j.jds.2021.02.006
Song, X., Song, W., Wang, Y., Wang, J., Li, Y., Qian, X., et al. (2016). MicroRNA-874 Functions as a Tumor Suppressor by Targeting Cancer/Testis Antigen HCA587/MAGE-C2. J. Cancer 7 (6), 656–663. doi:10.7150/jca.13674
Su, Z., Zhi, X., Zhang, Q., Yang, L., Xu, H., and Xu, Z. (2016). LncRNA H19 Functions as a Competing Endogenous RNA to Regulate AQP3 Expression by Sponging miR-874 in the Intestinal Barrier. FEBS Lett. 590 (9), 1354–1364. doi:10.1002/1873-3468.12171
Sun, K., and Lai, E. C. (2013). Adult-specific Functions of Animal microRNAs. Nat. Rev. Genet. 14 (8), 535–548. doi:10.1038/nrg3471
Sun, Q. H., Yin, Z. X., Li, Z., Tian, S. B., Wang, H. C., Zhang, F. X., et al. (2020). miR-874 Inhibits Gastric Cancer Cell Proliferation by Targeting SPAG9. BMC Cancer 20 (1), 522. doi:10.1186/s12885-020-06994-z
Tang, W., Wang, W., Zhao, Y., and Zhao, Z. (2018). MicroRNA-874 Inhibits Cell Proliferation and Invasion by Targeting Cyclin-dependent Kinase 9 in Osteosarcoma. Oncol. Lett. 15 (5), 7649–7654. doi:10.3892/ol.2018.8294
Tian, F. Q., Chen, Z. R., Zhu, W., Tang, M. Q., Li, J. H., Zhang, X. C., et al. (2021). Inhibition of Hsa_circ_0003489 Shifts Balance from Autophagy to Apoptosis and Sensitizes Multiple Myeloma Cells to Bortezomib via miR-874-3p/HDAC1 axis. J. Gene Med. 23 (9), e3329. doi:10.1002/jgm.3329
Wang, K., Liu, F., Zhou, L. Y., Ding, S. L., Long, B., Liu, C. Y., et al. (2013). miR-874 Regulates Myocardial Necrosis by Targeting Caspase-8. Cell Death Dis 4, e709. doi:10.1038/cddis.2013.233
Wang, L., Gao, W., Hu, F., Xu, Z., and Wang, F. (2014). MicroRNA-874 Inhibits Cell Proliferation and Induces Apoptosis in Human Breast Cancer by Targeting CDK9. FEBS Lett. 588 (24), 4527–4535. doi:10.1016/j.febslet.2014.09.035
Wang, L., Wang, C., Wu, T., and Sun, F. (2020a). Long Non-coding RNA TP73-AS1 Promotes TFAP2B-Mediated Proliferation, Metastasis and Invasion in Retinoblastoma via Decoying of miRNA-874-3p. J. Cel Commun Signal 14 (2), 193–205. doi:10.1007/s12079-020-00550-x
Wang, S., Wu, Y., Yang, S., Liu, X., Lu, Y., Liu, F., et al. (2020b). miR-874 Directly Targets AQP3 to Inhibit Cell Proliferation, Mobility and EMT in Non-small Cell Lung Cancer. Thorac. Cancer 11 (6), 1550–1558. doi:10.1111/1759-7714.13428
Wang, W., Li, J., Ding, Z., Li, Y., Wang, J., Chen, S., et al. (2019). Tanshinone I Inhibits the Growth and Metastasis of Osteosarcoma via Suppressing JAK/STAT3 Signalling Pathway. J. Cel Mol Med 23 (9), 6454–6465. doi:10.1111/jcmm.14539
Wang, Y., Chen, H., and Wei, X. (2021). Circ_0007142 Downregulates miR-874-3p-Mediated GDPD5 on Colorectal Cancer Cells. Eur. J. Clin. Invest. 51 (7), e13541. doi:10.1111/eci.13541
Wei, Y., Wang, Z., Wei, L., Li, S., Qiu, X., and Liu, C. (2021). MicroRNA-874-3p Promotes Testosterone-Induced Granulosa Cell Apoptosis by Suppressing HDAC1-Mediated P53 Deacetylation. Exp. Ther. Med. 21 (4), 359. doi:10.3892/etm.2021.9790
Witek, L., Janikowski, T., Gabriel, I., Bodzek, P., and Olejek, A. (2021). Analysis of microRNA Regulating Cell Cycle-Related Tumor Suppressor Genes in Endometrial Cancer Patients. Hum. Cel 34 (2), 564–569. doi:10.1007/s13577-020-00451-6
Witt, O., Deubzer, H. E., Milde, T., and Oehme, I. (2009). HDAC Family: What Are the Cancer Relevant Targets? Cancer Lett. 277 (1), 8–21. doi:10.1016/j.canlet.2008.08.016
Wu, G., Zhou, H., Li, D., Zhi, Y., Liu, Y., Li, J., et al. (2020). LncRNA DANCR Upregulation Induced by TUFT1 Promotes Malignant Progression in Triple Negative Breast Cancer via miR-874-3p-SOX2 axis. Exp. Cel Res 396 (2), 112331. doi:10.1016/j.yexcr.2020.112331
Xia, B., Lin, M., Dong, W., Chen, H., Li, B., Zhang, X., et al. (2018). Upregulation of miR-874-3p and miR-874-5p Inhibits Epithelial Ovarian Cancer Malignancy via SIK2. J. Biochem. Mol. Toxicol. 32 (8), e22168. doi:10.1002/jbt.22168
Xie, K., Cai, Y., Yang, P., Du, F., and Wu, K. (2020). Upregulating microRNA-874-3p Inhibits CXCL12 Expression to Promote Angiogenesis and Suppress Inflammatory Response in Ischemic Stroke. Am. J. Physiol. Cel Physiol 319 (3), C579–C588. doi:10.1152/ajpcell.00001.2020
Yan, Y., Song, X., Li, Z., Zhang, J., Ren, J., Wu, J., et al. (2017). Elevated Levels of Granzyme B Correlated with miR-874-3p Downregulation in Patients with Acute Myocardial Infarction. Biomark Med. 11 (9), 761–767. doi:10.2217/bmm-2017-0144
Yang, H., Dong, Y., Zhou, Y., and Li, H. (2021). Overexpression of miR-874-3p Alleviates LPS-Induced Apoptosis and Inflammation in Alveolar Epithelial Cell by Targeting EGR3/NF-kappaB. Acta Biochim. Pol. 68 (2), 231–238. doi:10.18388/abp.2020_5523
Yang, R., Li, P., Zhang, G., Lu, C., Wang, H., and Zhao, G. (2017). Long Non-coding RNA XLOC_008466 Functions as an Oncogene in Human Non-small Cell Lung Cancer by Targeting miR-874. Cell Physiol Biochem 42 (1), 126–136. doi:10.1159/000477121
Yao, H., Ashihara, E., and Maekawa, T. (2011). Targeting the Wnt/beta-Catenin Signaling Pathway in Human Cancers. Expert Opin. Ther. Targets 15 (7), 873–887. doi:10.1517/14728222.2011.577418
Yao, T., Zha, D., Gao, P., Shui, H., and Wu, X. (2018). MiR-874 Alleviates Renal Injury and Inflammatory Response in Diabetic Nephropathy through Targeting Toll-like Receptor-4. J. Cel Physiol 234 (1), 871–879. doi:10.1002/jcp.26908
Yu, C., Li, S., and Hu, X. (2020). Circ_0005576 Promotes Malignant Progression through miR-874/CDK8 Axis in Colorectal Cancer. Onco Targets Ther. 13, 7793–7805. doi:10.2147/OTT.S249494
Yuan, F., Zhao, Z. T., Jia, B., Wang, Y. P., and Lei, W. (2020). TSN Inhibits Cell Proliferation, Migration, Invasion, and EMT through Regulating miR-874/HMGB2/beta-Catenin Pathway in Gastric Cancer. Neoplasma 67 (5), 1012–1021. doi:10.4149/neo_2020_190919N931
Yuan, R. B., Zhang, S. H., He, Y., Zhang, X. Y., and Zhang, Y. B. (2018). MiR-874-3p Is an Independent Prognostic Factor and Functions as an Anti-oncomir in Esophageal Squamous Cell Carcinoma via Targeting STAT3. Eur. Rev. Med. Pharmacol. Sci. 22 (21), 7265–7273. doi:10.26355/eurrev_201811_16261
Zhang, H., Liu, L., Chen, L., Liu, H., Ren, S., and Tao, Y. (2021a). Long Noncoding RNA DANCR Confers Cytarabine Resistance in Acute Myeloid Leukemia by Activating Autophagy via the miR-874-3P/ATG16L1 axis. Mol. Oncol. 15 (4), 1203–1216. doi:10.1002/1878-0261.12661
Zhang, L., Yan, D. L., Yang, F., Wang, D. D., Chen, X., Wu, J. Z., et al. (2017). DNA Methylation Mediated Silencing of microRNA-874 Is a Promising Diagnosis and Prognostic Marker in Breast Cancer. Oncotarget 8 (28), 45496–45505. doi:10.18632/oncotarget.17569
Zhang, S., Zhang, Y., Wang, N., Wang, Y., Nie, H., Zhang, Y., et al. (2021b). Long Non-coding RNA MIAT Impairs Neurological Function in Ischemic Stroke via Up-Regulating microRNA-874-3p-Targeted IL1B. Brain Res. Bull. 175, 81–89. doi:10.1016/j.brainresbull.2021.07.005
Zhang, X., Tang, J., Zhi, X., Xie, K., Wang, W., Li, Z., et al. (2015). miR-874 Functions as a Tumor Suppressor by Inhibiting Angiogenesis through STAT3/VEGF-A Pathway in Gastric Cancer. Oncotarget 6 (3), 1605–1617. doi:10.18632/oncotarget.2748
Zhang, Y., Wang, X., and Zhao, Y. (2018a). MicroRNA874 Prohibits the Proliferation and Invasion of Retinoblastoma Cells by Directly Targeting Metadherin. Mol. Med. Rep. 18 (3), 3099–3105. doi:10.3892/mmr.2018.9295
Zhang, Y., Wei, Y., Li, X., Liang, X., Wang, L., Song, J., et al. (2018b). microRNA-874 Suppresses Tumor Proliferation and Metastasis in Hepatocellular Carcinoma by Targeting the DOR/EGFR/ERK Pathway. Cel Death Dis 9 (2), 130. doi:10.1038/s41419-017-0131-3
Zhang, Z., Yang, W., Li, N., Chen, X., Ma, F., Yang, J., et al. (2021c). LncRNA MCF2L-AS1 Aggravates Proliferation, Invasion and Glycolysis of Colorectal Cancer Cells via the Crosstalk with miR-874-3p/FOXM1 Signaling axis. Carcinogenesis 42 (2), 263–271. doi:10.1093/carcin/bgaa093
Zhao, B., and Dong, A. S. (2016). MiR-874 Inhibits Cell Growth and Induces Apoptosis by Targeting STAT3 in Human Colorectal Cancer Cells. Eur. Rev. Med. Pharmacol. Sci. 20 (2), 269–277.
Keywords: miR-874, cancer, tumor suppressor, non-cancer, diagnosis, prognosis, ceRNA, pathway
Citation: Zhang Q, Zhong C, Yan Q, Zeng L-h, Gao W and Duan S (2022) miR-874: An Important Regulator in Human Diseases. Front. Cell Dev. Biol. 10:784968. doi: 10.3389/fcell.2022.784968
Received: 28 September 2021; Accepted: 23 March 2022;
Published: 06 April 2022.
Edited by:
Zong Sheng Guo, Roswell Park Comprehensive Cancer Center, United StatesReviewed by:
Mario Cioce, Campus Bio-Medico University, ItalyCopyright © 2022 Zhang, Zhong, Yan, Zeng, Gao and Duan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Gao, d2VpZ0B6dWNjLmVkdS5jbg==; Shiwei Duan, ZHVhbnN3QHp1Y2MuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.