- Barts Cancer Institute, Queen Mary University of London, London, EC1M 6BQ, United Kingdom
Cell migration is crucial for efficient immune responses and is aberrantly used by cancer cells during metastatic dissemination. Amoeboid migrating cells use myosin II-powered blebs to propel themselves, and change morphology and direction. Immune cells use amoeboid strategies to respond rapidly to infection or tissue damage, which require quick passage through several barriers, including blood, lymph and interstitial tissues, with complex and varied environments. Amoeboid migration is also used by metastatic cancer cells to aid their migration, dissemination and survival, whereby key mechanisms are hijacked from professionally motile immune cells. We explore important parallels observed between amoeboid immune and cancer cells. We also consider key distinctions that separate the lifespan, state and fate of these cell types as they migrate and/or fulfil their function. Finally, we reflect on unexplored areas of research that would enhance our understanding of how tumour cells use immune cell strategies during metastasis, and how to target these processes.
Introduction
Immune cell populations are our body’s housekeepers and defence mechanisms. They must respond and relocate to distant sites and are adapted to cross diverse environments. They are professionally motile, and their localisation and timely responsiveness are critical to perform their functions effectively (Chaplin, 2010). Motility is therefore key for immune cell development, maintaining tissue homeostasis, immunosurveillance, responding to injury/infection and eliminating pathogens (Chaplin, 2010). While mesenchymal migration requires moderate levels of Rho-ROCK to contract the cell rear and retract protrusions, fast amoeboid migration relies on hyper-activation of Rho-ROCK-driven actomyosin contractility (Friedl and Wolf, 2009). Most leukocytes adopt amoeboid migration, while some cancer cells also use this mode to aid in metastatic spread (Madsen and Sahai, 2010). Amoeboid migration is conserved across different species, detected in early eukaryotes, such as the Dictyostelium genus (Barry and Bretscher, 2010) and across mammalian cells (Titus and Goodson, 2017). It is also observed during embryonic development (Richardson and Lehmann, 2010; Ruprecht et al., 2015), in primordial germ cells (Fujimoto et al., 1977) and in adult tissues, including neurons (Amini et al., 2022), satellite muscle stem cells (Otto et al., 2011), leukocytes and malignant cells (Lämmermann and Germain, 2014). Importantly, under physical confinement, both cancer and immune cells exhibit the fastest amoeboid migration described so far (Liu et al., 2015). We highlight up-to-date comparisons between amoeboid migration of immune and cancer cells, and suggest how cancer cell amoeboid migration could be targeted to prevent metastatic spread.
Cytoskeletal dynamics in cancer and immune amoeboid cells
Rho-ROCK-myosin II cytoskeletal regulation
During an immune response, leukocytes remodel their cytoskeleton to allow rapid amoeboid migration. This behaviour is observed for neutrophils, T cells, B cells, monocytes, macrophages and dendritic cells (Guenther, 2022), although macrophages and dendritic cells can also adopt mesenchymal migration (Friedl and Weigelin, 2008). Visualisation of leukocyte amoeboid migration has been possible using 3D models and in vivo imaging (Nourshargh and Alon, 2014), with neutrophils showing the highest speeds up to 30 μm/min (Friedl and Weigelin, 2008). Rho-ROCK-driven myosin contractility drives amoeboid leukocyte migration, with short-lived pseudopods at the cell front and a uropod at the rear (Bros et al., 2019; Eddy et al, 2000). In two dimensions (2D), cell migration has been described as a cyclical process: generation of actin-rich membrane protrusions, substrate-receptor engagement, actomyosin contraction of the cell rear and subsequent forward motion (Friedl and Weigelin, 2008). While cell polarity is key for directionality, leukocytes are guided by chemotactic gradients (Amano et al., 2010). Cytoskeletal polarisation is largely driven by signals mediated by G-protein coupled receptors (GPCRs) and receptor tyrosine kinases (RTKs). PI3K-PIP-Akt downstream signalling promotes actin polymerisation and pseudopods, as well as downstream T cell receptor (TCR) signalling involving Rac and Cdc42 activation (Enomoto et al., 2005; Gambardella and Vermeren, 2013). However, under confinement, leukocyte migration is largely adhesion-independent, utilising retrograde actin flow and Rho-ROCK-driven actomyosin contractility (Kameritsch and Renkawitz, 2020). This enhanced contractility allows cells to squeeze through small gaps whilst maintaining a stable cortex and cellular integrity (Bendix et al., 2008), but is also crucial for rear detachment, retraction (Alblas et al., 2001; Lämmermann and Sixt, 2009), differential integrin expression (Liu et al., 2002). Amoeboid leukocytes can still generate force and forward motion without strong substrate engagement (Reversat et al., 2020), where confinement and low adhesion can induce amoeboid behaviour (Liu et al., 2015). Low adhesion also allows amoeboid cells to migrate faster through increased cortical actin tension (Lämmermann et al., 2008). Integrins play a role in pseudopod selection (Andrew and Insall, 2007) and cellular contractility promotes uropod de-adhesion and rear retraction during amoeboid migration in several immune types (Worthylake et al., 2001; Sánchez-Madrid and Serrador, 2009). Consequently, myosin inhibition abrogates neutrophil rear retraction (Eddy et al., 2000), whilst reducing Rho-ROCK activity induces an amoeboid-to-mesenchymal transition (AMT) within macrophages (Gui et al., 2014). Therefore, actomyosin contractility is a crucial driver of leukocyte amoeboid migration.
Tumour cells can acquire amoeboid characteristics, which has been reported in several cancer types, including melanoma, breast cancer, lymphoma, leukaemia, liver cancer, sarcoma and prostate cancer (Crosas-Molist et al., 2017; Pan et al., 2018; López-Luque et al., 2019; Graziani et al., 2022). This amoeboid behaviour can be induced by cytokines (Sanz-Moreno et al., 2011; Georgouli et al., 2019) and mechanical cues (Liu et al., 2015; Khoo et al., 2019). Amoeboid cells harbour elevated levels of Rho-ROCK signalling, which support migration, invasion and metastasis (Crosas-Molist et al., 2021). Similarly to immune cells, enhanced contractility in cancer cells couples movement of the cell rear to the front, allowing cell body translocation, squeezing through confined environments and maintenance of cellular integrity (Sahai and Marshall, 2003; Wyckoff et al., 2004; Wyckoff et al., 2006; Liu et al., 2015). Both immune and tumour amoeboid cells use blebs as functional membrane protrusions (Paluch and Raz, 2013). Blebs are short-lived, formed due to increased hydrostatic pressure and can be induced by confinement (Ibo et al., 2016) and/or low-adhesion, which rely less on long-lasting substrate interactions (Schick and Raz, 2022). Amoeboid migration modes include ‘pseudopodial’ blebs governed by dynamic actin assembly in leukocytes (Lämmermann and Sixt, 2009), alongside leader or ‘stable’ blebs, which remain un-retracted during migration (Schick and Raz, 2022). However, less is known about how polarised bleb formation can drive directional migration. In summary, Rho-ROCK is a key driver of bleb-based amoeboid migration, enabling fast movement for both leukocytes and cancer cells.
Amoeboid migration and cellular plasticity
Amoeboid leukocytes display context-dependent migration strategies, guided by soluble (chemotaxis) or immobilised (haptotaxis) chemokines, in addition to haptokinesis (2D migration during vascular crawling), durotaxis (rigidity gradients) and tenertaxis (path of least resistance) (Schimmel et al., 2017). Cancer cells also adopt diverse migratory strategies, including collective or individual migration (De Pascalis and Etienne-Manneville, 2017), similarly guided by chemotaxis (Roussos et al., 2011), haptotaxis and haptokinesis (Lu et al., 2014; Oudin et al., 2016). However, it is unclear whether this is specific to amoeboid migration. Plasticity between individual mesenchymal and amoeboid migration has been observed and the mesenchymal-to-amoeboid transition (MAT) can be considered part of the epithelial-to-mesenchymal transition (EMT) spectrum (Graziani et al., 2022).
Leukocytes have evolved to display migratory plasticity to cross diverse barriers (Laurent et al., 2017). However, tumour cells lack this pre-programmed advantage, but instead hijack this migratory plasticity via transcriptional rewiring. Induction of cellular plasticity arises from aberrant mutations, involving the adaptability of migration strategies (Friedl and Wolf, 2009; Te Boekhorst et al., 2016). Mechanical constraints can trigger plasticity, where macrophage and cancer cell migration is influenced by matrix organisation (Van Goethem et al., 2010; Poltavets et al., 2018; Čermák et al., 2018). In tumours, the shift between elongated-mesenchymal and rounded-amoeboid migration modes are in part governed by the balance of Rho and Rac signalling (Sahai and Marshall, 2003; Sanz-Moreno et al., 2008), cytokine signalling (Sanz-Moreno et al., 2011; Cantelli et al., 2015; Georgouli et al., 2019) and mechanical sensing (Liu et al., 2015). Tumour cell mesenchymal migration has been associated with protease-dependence, while amoeboid migration can be protease-independent (Sahai and Marshall, 2003; Carragher et al., 2006; Sanz-Moreno et al., 2008) or protease-dependent (Orgaz et al., 2014). Leukocytes and tumour cells modulate this proteolytic dependency through the generation of actin-rich podosomes and invadosomes, respectively, which regulate points of ECM attachment and localised matrix metalloproteinase (MMP) release (Murphy and Courtneidge, 2011). Overall, both immune and tumour cells share parallels of cellular plasticity to overcome any barriers during dissemination.
Shared strategies for effective dissemination
Amoeboid-driven pro-inflammatory signalling
STAT3 (Yu et al., 2009; Kaplan, 2013) and NF-κB (Liu et al., 2017) act as central transcriptional hubs controlling inflammatory secretion in immune responses, and drive cancer progression and amoeboid dissemination (Pan et al., 2018; Taniguchi and Karin, 2018; Owen et al., 2019). Immune cells produce pro-inflammatory factors, including IFNγ and IL-1β, to activate immune responses (Lacy and Stow, 2011). While, regulatory T cells ensure timely immune response termination through secretion of immunomodulatory factors, namely IL-10 and TGF-β (Marrack et al., 2010; Kane et al., 2014). Furthermore, IL-6 regulates T cell recruitment through selective MAPK, PI3K and JAK/STAT activation (Weissenbach et al., 2004; Fielding et al., 2008). Comparatively, Rho-ROCK signalling sustains cancer amoeboid behaviour through cytokine secretion-driven positive feedback via IL-6 family cytokines/GP130-JAK1-STAT3 (Sanz-Moreno et al., 2011), TGF-β-SMAD2-CITED1 (Cantelli et al., 2015) and IL-1α-NF-κB (Georgouli et al., 2019). Therefore, a key difference is that immune responses are halted to resume homeostasis (Ruland, 2011), whereas this mechanism is lost in chronically inflamed cancers, which could be exploited for therapeutic interventions.
Rho-ROCK regulates secretion of pro-inflammatory factors, IL-1α and IL-8, and immunosuppressive factors, TGF-β and IL-10. This amoeboid cancer cell secretome supports endothelium attachment and vascular permeability, alongside the induction of tumour-promoting macrophages (Cantelli et al., 2015; Georgouli et al., 2019). Moreover, tumour and immune cells utilise cytokine and chemokine signalling to regulate their microenvironment and invasiveness (Sokol and Luster, 2015). However, evidence linking cytokine signalling to immune cell amoeboid migration and ROCK signalling is missing. Whether chemokines regulate Rho-ROCK actomyosin in amoeboid cancer cells is also not fully understood. Therefore, a better understanding of these pathways could present another avenue for targeting amoeboid cancer cells. Delineating cancer-specific pro-inflammatory signalling and non-canonical roles of these central transcription nodes will be crucial in tackling cancer-specific programmes (Baud and Karin, 2009; Siveen et al., 2014; Yu et al., 2020).
Interstitial and transendothelial migration
During interstitial migration, leukocytes use tenertaxis to migrate towards preferential crossing points to minimise the tissue remodelling required (Muller, 2013). Interstitial migration is further directed by chemotactic gradients, including CXCL8-CXCR2, CXCL2-CXCR2, and CCL21-CCR7 chemokine signalling (Lam and Huttenlocher, 2013), whereas amoeboid cancer cell chemotaxis is reliant on CXCL12-CXCR4 (Wyse et al., 2017) and CCL25-CCR9 (Zhang et al., 2011), while CCL2-CCR2 activates MEK-ROCK2-myosin II axis (Wong et al., 2020). CXCL12-CXCR4 drives RhoA-dependent bleb-based migration (Wyse et al., 2017) which promotes rapid amoeboid cancer cell interstitial migration, also driven independently of integrin β1 (Tozluoğlu et al., 2013). In leukocytes, integrins are not essential for interstitial migration; instead, cells become reliant on increased actin polymerisation and actomyosin contractility (Nourshargh et al., 2010). However, adhesions in amoeboid cells can be induced by inflammatory signals, such as TNFα, to aid in context-dependent stoppage and retention (Lokuta and Huttenlocher, 2005). Generally, amoeboid cancer cells are less reliant on adhesion (Carragher et al., 2006), while β1 integrins are required for their adhesion to collagen I (Pinner and Sahai, 2008b; Sanz-Moreno et al., 2008). Furthermore, leukocytes and tumour cells use podosomes (Linder and Aepfelbacher, 2003; Wiesner et al., 2014) and invadopodia, respectively (Linder and Kopp, 2005; Linder, 2009), for interstitial tissue migration and to cross the endothelium (Bravo-Cordero et al., 2012). Podosomes mediate ECM attachment and act as active hubs of MMP release (Murphy and Courtneidge, 2011), while invadopodia, which are typically longer-lived, also function in degrading matrix (Spuul et al., 2014). Amoeboid cancer migration is less reliant on proteolysis as enhanced contractility allows matrix deformation, but Rho-ROCK signalling in amoeboid cancer cells still supports MMP secretion and matrix degradation (Orgaz et al., 2014). Whether amoeboid cancer cells can form invadosomes in certain conditions is unknown, however it has been shown that RhoC-ROCK promotes ovarian carcinoma invasion through cortactin/cofilin-mediated invadosome formation and MMP-mediated degradation (Semprucci et al., 2016).
When moving between dense interstitial tissue and vasculature, cells use local signals to identify suitable intravasation and extravasation sites (Subramanian et al., 2020) and adapt their cytoskeleton (Mihlan et al., 2022). Rho-ROCK signalling promotes the transendothelial migration (TEM) process (Honing et al., 2004). Establishing interactions with endothelium is also key for cancer cells and leukocytes to cross the vasculature. Integrins, CAMs, selectins and N-cadherin on the surface of endothelial and transmigrating cells are key for this process (Ley et al., 2007). Leukocytes rely on integrins, such as α4β1, αLβ2, α4β7 and αMβ2 for firm adhesion and crawling along the endothelium before extravasation (Mitroulis et al., 2015). Whilst cancer cells use similar mechanisms to cross the endothelium, they are not as reliant on integrins (Miles et al., 2008). For example, leukocytes use PSGL-1, LFA-1, JAM-C, PECAM-1, and CD-99, whereas cancer cells rely on PSGL-1, MUC1, P-, L- and E-selectins (Strell and Entschladen, 2008; Muller, 2011). Furthermore, leukocytes maintain adhesion molecule expression alongside chemokine-driven “inside-out” signalling to activate integrins and promote adhesion to endothelium (Nourshargh and Alon, 2014). On the other hand, CXCR4 expression (Jin et al., 2012) and TGF-β-driven Rho-ROCK-myosin II (Lamouille et al., 2014; Cantelli et al., 2015) aid cancer cell-endothelial adhesion. Both cancer and immune cells then use these interactions to alter endothelial cell cytoskeleton to aid TEM (Schimmel et al., 2017; Rodenburg and van Buul, 2021). When leaving the vasculature, extravasation sites are selected by leukocytes (Nourshargh and Alon, 2014) and tumour cells (Sökeland and Schumacher, 2019) based on the production of chemotactic factors by inflamed tissue. Consequently, chemokine receptor expression also influences leukocyte tissue tropism (Olson and Ley, 2002) and organ-specific metastasis tropism (Marcuzzi et al., 2018). Therefore, targeting chemokine and/or Rho-ROCK signalling in amoeboid cancer cells could prevent cancer-endothelium interactions, TEM and metastatic dissemination to peripheral secondary sites. However, a better understanding of the amoeboid cancer cell adhesome will allow specific targeting of amoeboid cancer cell-endothelium interactions, whilst sparing leukocyte-endothelium interactions.
Survival in circulation
In circulation, leukocytes rely on their plastic nature to survive via metabolic adaptation, integrin-dependent adhesions (Alon and van Buul, 2017) and extensive nuclear and cytoplasmic deformations to cope with shear stress (Majidpoor and Mortezaee, 2021; Perea Paizal et al., 2021). Comparatively, tumour cells struggle to survive in circulation, with an estimated half-life of only 1–2.4 hours (Meng et al., 2004) and <0.01% of circulating tumour cells successfully extravasate to secondary organs (Langley and Fidler, 2011). This represents a significant discrepancy to leukocytes, particularly monocytes that can survive in circulation for days (Patel et al., 2017). However, tumours cells that survive in circulation show increased actomyosin signalling (Moose et al., 2020) and upregulate pro-survival and proliferation signalling pathways, such as FAK, ERK, and Akt (Alanko et al., 2015; Douma et al., 2004). This is achieved through increased adhesion-dependent ‘shielding’ with platelets (Palumbo et al., 2005) and monocytes/macrophages (Gil-Bernabé, et al., 2012), to protect from shear stress and immune responses (Strilic and Offermanns, 2017). Targeting these pathways could hinder survival in circulation and perturb secondary site seeding and metastasis.
Differences between amoeboid immune and cancer cells
We have explained how immune cells paradoxically share a panel of similarities with tumour cells. In the search for cancer amoeboid biomarkers, features and pathways that are unique to cancer cells and absent in anti-tumoural immune cells would be interesting. Genetic mutations, lifespan and differentiation status are unique characteristics of tumour cells.
Mutational status
Normal cells harbour a lower mutation burden than cancers originating in the same organs, however certain immune cells, such as T cells and B cells, generate programmed somatic mutations to create the antigen repertoire necessary to exert their functions (Machado et al., 2022). Random mutations can also occur in immune cells and these can lead to lymphoid malignancies or other pathological conditions (Abplanalp et al., 2021). Tumours harbour mutation patterns caused by random errors occurring during DNA replication, either inherited or by environmental factors (Tomasetti et al., 2017). Frequently altered genes across tumour types contribute to tumour fitness (Bailey et al., 2018; Buisson et al., 2019) and, in certain tumours, lead to aberrant activation of Rho-ROCK-myosin II signalling, supporting a cancer amoeboid phenotype (Graziani et al., 2022).
BRAF and NRAS cooperate with Rho-ROCK (Qiu et al., 1995; Sahai et al., 1998) for transformation, and as such BRAF- and NRAS-mutant melanomas harbour amoeboid characteristics (Orgaz et al., 2020; Rodriguez-Hernandez et al., 2020). Inhibition of either BRAFV600E or MEK results in loss of amoeboid behaviour by reducing myosin activity (Orgaz et al., 2020). BRAF inhibitors are available, yet resistance is a clinical challenge (Flaherty et al., 2012; Sanchez et al., 2018). BRAF-resistant melanomas adapt to therapy by altering cytoskeletal gene expression, which leads to myosin II activity restoration (Orgaz et al., 2020). Rho-ROCK-myosin II axis also cooperates with MYC oncogenes (Dyberg et al., 2017; Talamillo et al., 2017). MYC-dependent Rho-ROCK-myosin II activation sustains glioblastoma growth and invasion (Talamillo et al., 2017), while increased ROCK2 expression characterises high-risk neuroblastoma and correlates with poor patient survival (Dyberg et al., 2017). In acute myeloid leukaemia (AML), the oncogenic forms of FLT3, BCR-ABL, and KIT drive PI3K-Rho-ROCK-myosin II activation and in vivo tumour growth (Mali et al., 2011). In diffuse gastric cancer, RhoAY42C gain-of-function mutations are characterised by a 12-fold increased capability to bind ROCK (Kakiuchi et al., 2014). Nonetheless, whether MYC, RhoAY42C, FLT3, BCR-ABL, and KIT oncogenes promote amoeboid behaviour is unknown.
Importantly, the cancer amoeboid cellular state cannot solely be explained by oncogenic signalling. Environmental factors such as hypoxia (Lehmann et al., 2017), production of arachidonic acid by cells under confinement (Lomakin et al., 2020) and low levels of reactive oxygen species (ROS) (Herraiz et al., 2016; Rodriguez-Hernandez et al., 2020) support amoeboid behaviour. Moreover, some cancer cells present constitutive amoeboid behaviour, suggesting an ‘amoeboid cellular memory’ regulated at the epigenetic level (Graziani et al., 2022).
Lifespan and function
Most human cells are characterised by a finite replicative potential (Hahn et al., 1999) and immune cells present a limited lifespan (Nayar et al., 2015). Short-lived immune cells include neutrophil and monocytes, which have a half-life of 13–19 hours and lifespan of 1–7 days, respectively (Patel et al., 2021). Monocyte-derived macrophages have a short lifespan, while tissue macrophages survive for six weeks (Plowden et al., 2004). Memory T cells live for approximatively six months, whereas naive T cells can live for up to nine years (Borghans and Ribeiro, 2017). Conversely, cancer cells need to become immortal to form a neoplasm (Hahn et al., 1999). In most normal cells, each division results in telomeric DNA shortening, which eventually causes genomic instability, senescence and apoptosis (Hahn et al., 1999). Stem cells and cancers maintain stable telomere length due to telomerase reactivation or lengthening (Gunes and Rudolph, 2013). However, telomerase inhibitors have been unsuccessful in the clinic (Bar and Thum, 2017; Akincilar et al., 2021) and alternative strategies to halt tumour replicative immortality are needed. Interestingly, the actin cytoskeleton mechanically regulates telomeres in a length- and timescale-dependent manner (Jokhun et al., 2018), where cortical actomyosin-based contraction may influence replicative potential in amoeboid cells. However, whether ROCK plays a role in telomere lengthening is unknown. Amoeboid melanoma cells display cancer stem cell properties in vitro and in vivo (Rodriguez-Hernandez et al., 2020) and a pro-survival advantage during the acquisition of resistance to anti-melanoma therapies (Orgaz et al., 2020). Stemness is sustained by WNT11-FZD7-DAAM1 signalling and targeting this pathway could potentially inhibit self-renewal capabilities of melanoma amoeboid cells (Rodriguez-Hernandez et al., 2020).
Differentiation status
Immune cells differentiate to perform specific functions (Maslova et al., 2020), while tumours vary in differentiation status (Vega et al., 2019). Undifferentiated tumour cells are often characteristic of advanced disease and poor prognosis (Jogi et al., 2012). Tumour de-differentiation has been linked with EMT (Wang and Unternaehrer, 2019) and cancer amoeboid cells could be considered part of the EMT spectrum (Graziani et al., 2022). Consistently, amoeboid cells support tumour-initiating abilities in melanoma, where ALDH1 is a strong biomarker of self-renewal (Rodriguez-Hernandez et al., 2020) and podoplaninhigh amoeboid melanoma cells are linked to a clear de-differentiation state (de Winde et al., 2021). Therefore, differentiation therapies targeting amoeboid cancer stem cells could halt tumour progression and prevent relapse, but more work is needed in this field.
Undifferentiated tumours have been linked to immune suppression (Mo et al., 2016; Ahn et al., 2021; Yi et al., 2021). Checkpoint inhibitors have showed clinical activity in a variety of tumours, although resistance is still a challenge (Sharma et al., 2017). Immunotherapy (IT)-resistant melanomas harbour high actomyosin and amoeboid features, but combining IT with ROCK inhibitors improved responses (Orgaz et al., 2020). These effects are due in part to decreased immunosuppressive populations and unaffected CD4+CD8+ infiltration. Hence, there may be a therapeutic window in which ROCK inhibitors can be used without affecting anti-tumour immune responses while reducing pro-tumorigenic populations, such as macrophages and regulatory T cells (Orgaz et al., 2020). Strategies to eradicate amoeboid cells with stemness and immunosuppressive attributes should be considered for future therapeutic strategies while reliable ‘amoeboid biomarkers’ will be crucial.
Amoeboid behaviour in haematological malignancies
The immune system is a double-edged sword in cancer as it can boost or hinder tumour development (Lakshmi Narendra et al., 2013). When genetic alterations occur in immune cells, haematological malignancies can develop (Abplanalp et al., 2021). While adherent cells require optimal levels of myosin II to survive (Schipper et al., 2019), immune cells and haematological cancer cells exhibit hard-wired capacities to live in environments without strong adhesive interactions (Suresh, 2007; Yamada and Sixt, 2019). While in solid tumours the activation of Rho-ROCK-myosin II is coupled to metastatic potential, in haematological malignancies it is also linked with enhanced proliferation and survival (Crosas-Molist et al., 2021). In AML, PI3K-Rho-ROCK signalling is highly upregulated, whereby ROCK inhibition impairs their proliferative capacity (Mali et al., 2011). Moreover, Rho-ROCK promotes metastasis of acute lymphoblastic leukaemia (ALL) cells in response to CCL25 (Zhang et al., 2011), while KIF13A regulates RhoB vesicular recycling promotes bleb-based amoeboid migration in ALL (Gong et al., 2018). Moreover, in chronic lymphocytic leukaemia (CLL) the pro-survival protein ABL1 co-localises with F-actin structures to promote amoeboid migration (Hutchinson et al., 2014). On the other hand, STAT3 supports amoeboid migration in diffuse large B-cell lymphoma (DLBCL), via ARHGEF2-RhoA signalling, whereby JAK inhibition reduces dissemination (Pan et al., 2018). In chronic myelogenous leukaemia (CML), p210bcr/abl1 activates RhoA leading to amoeboid migration, where inactivation of RhoA is able to reverse this process (Daubon et al., 2008). This body of data suggests that Rho signalling is a promising target in haematological malignancies and inhibitors of this pathway should be explored.
Discussion and future directions
There are important parallels between amoeboid leukocyte and tumour cell migration, driven by both cytokine signalling and Rho-ROCK activation. The rapid nature of amoeboid migration often allows the spread of cancer before its detection. Finding unique vulnerabilities of cancer cells that will not affect immune function will be important. We have explored how Rho-ROCK signalling drives key strategies during both immune responses and cancer cell dissemination, while diverse oncogenic drivers converge to activate the Rho-ROCK-myosin II axis. Since myosin II is a tuneable switch, its contribution to tumourigenesis may be context-, tumour stage- and microenvironment-dependent (Wang et al., 2019). Thus, it is important to select patients that will benefit from Rho-ROCK-myosin II signalling inhibition.
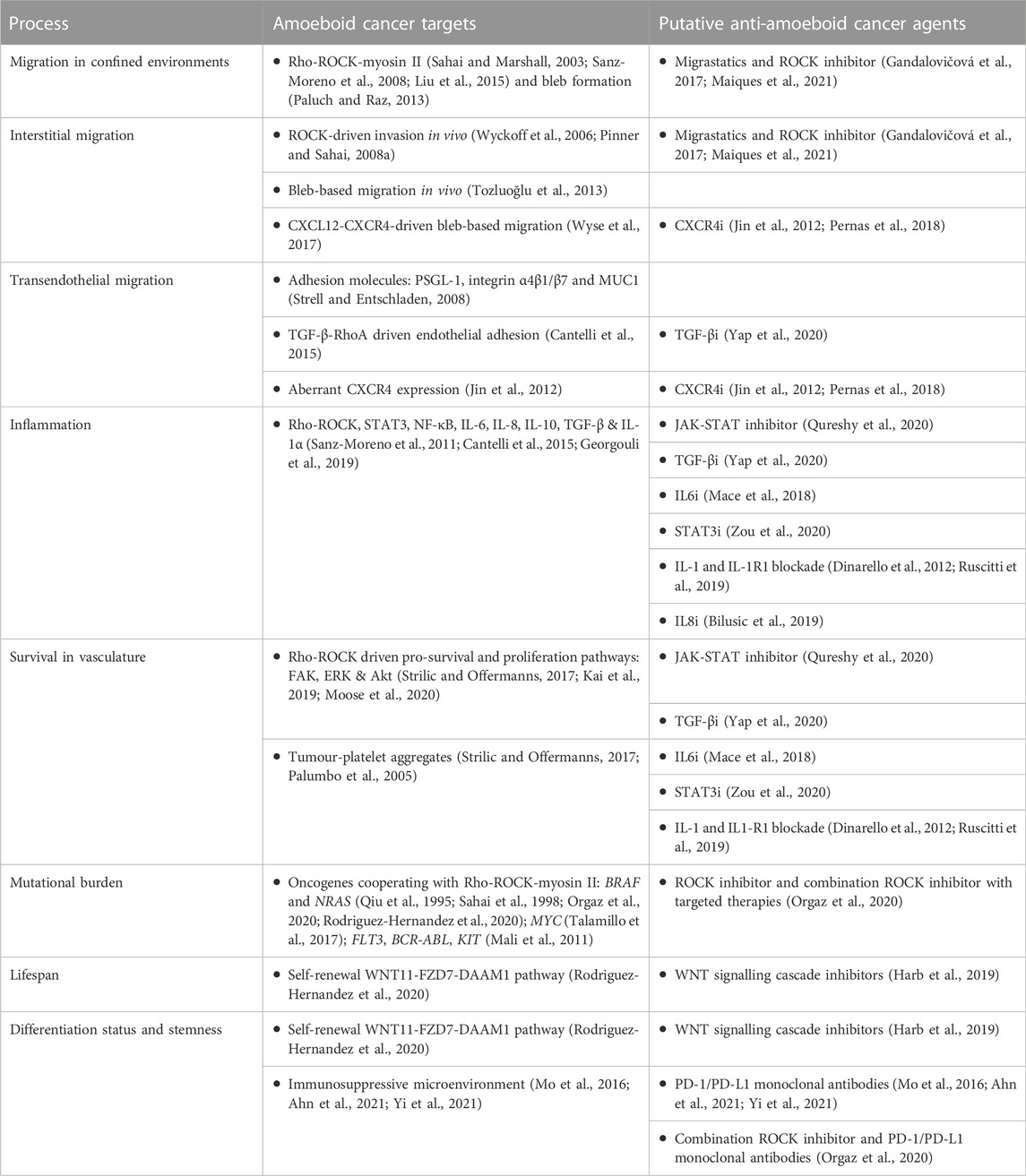
ROCK inhibitors show clear anti-tumour effects in in vivo mouse models (Liu et al., 2009; Georgouli et al., 2019; Orgaz et al., 2020; Rodriguez-Hernandez et al., 2020; Kim et al., 2021). Targeting Rho GTPases within immune cells has been explored for the treatment of chronic inflammatory disorders (Biro et al., 2014). However, more work is needed to understand if ROCK inhibitors affect anti-tumour immunity (Kim et al., 2021). Migrastatics, including ROCK inhibitors, could be used to prevent metastatic disease (Gandalovičová et al., 2017; Maiques et al., 2021), but this needs to be carefully considered within the context of complex tumour environments and administration routes (Rath and Olson, 2012). ROCK inhibitors are approved to treat glaucoma (Honjo and Tanihara, 2018), cerebral vasospasm (Zhao et al., 2006) and graft vs. host disease (Ali and Ilyas, 2022), and are currently being tested for numerous diseases, including cancer (Crosas-Molist et al., 2021). ROCK inhibitors could be utilised for combination therapies, for example ROCK inhibitor/BRAF inhibitor and ROCK inhibitor/αPD-1 (Orgaz et al., 2020), alongside other chemotherapeutic agents (Kim et al., 2021), or via dual target inhibition (McLeod et al., 2020). Alternatively, local application for skin cancers, drug delivery systems (e.g., nanoparticles), antibody drug conjugates or oncolytic viruses could be explored, although these avenues are in early stage development (Senapati et al., 2018; Krishnan and Mitragotri, 2020; Yao et al., 2020). ‘Soft’ ROCK inhibitors have also been put forward in an effort to reduce systemic exposure and side effects (Boland et al., 2015). These options all contribute to the arsenal of treatment options (Figure 1; Table 1).

FIGURE 1. Targeting amoeboid cancer cells during tumour progression. Both leukocytes and tumour cells utilise amoeboid migration for their motility. We can aim to target distinct hallmarks of amoeboid cancer cells within the tumour microenvironment, whilst leaving homeostatic leukocytes intact. The effect of migrastatics on tumour-immune infiltrate is less understood, and should also be considered. Targeting approaches for amoeboid cancer cells are highlighted, from migrastatics, transcriptome and secretome inhibitors, immunotherapies and combination therapies, alongside future considerations for therapies such as chemokine receptor inhibitors.
Alternative avenues could target amoeboid immunosuppressive secretion, including TGF-β (Cantelli et al., 2015; Giannelli et al., 2020; Yap et al., 2020). Beyond targeting individual amoeboid-dependent secreted factors, it could be possible to target related signalling nodes, such as the JAK-STAT and NF-κB pathways (Qureshy et al., 2020). Recently, combining STAT3 inhibitors and immunotherapy has shown encouraging results (Zou et al., 2020), while IL-1/IL-1R1 also represent potential targeting options (Dinarello et al., 2012; Ruscitti et al., 2019). Furthermore, immunotherapy could be combined with chemokine signalling inhibition, for example CXCR4 antagonist (Balixafortide) with Eribulin in metastatic breast cancers (Pernas et al., 2018), and CCR2 inhibition (CCX872) with αPD-1 treatment in pancreatic adenocarcinoma tumours (Jung et al., 2016). Therefore, these strategies could represent the most effective way to target amoeboid cancer-dependent secretion and chemotactic migration. However, a better understanding of chemokine signalling in amoeboid cancer cells is required to elucidate whether chemokine signalling inhibition would be effective. Finally, amoeboid cancer cells also retain cancer stem cell-like properties (Rodriguez-Hernandez et al., 2020). Developing targeted therapies to eradicate cancer stem cells is challenging because they share key features with normal stem cells (O'Brien et al., 2010). However, different classes of WNT signalling cascade inhibitors could be tested in cancer (Harb et al., 2019).
Overall, we have discussed hallmarks of amoeboid-specific cancer cell programs, and how these can be teased apart from their homeostatic functions within leukocyte populations. Additionally, it is important to define a window of opportunity for anti-amoeboid cancer therapy in which tumours have already been infiltrated. Moreover, treatment in the neo-adjuvant setting could be an option to prevent amoeboid cell-mediated recurrence. In conclusion, identifying targetable attributes of amoeboid cancer cells will be key to prevent cancer metastasis and therapy resistance.
Author contributions
SG, JM, VG, and VS-M wrote the manuscript. The figure was created with BioRender.com.
Funding
This work was supported by Cancer Research UK (CRUK) C33043/A24478 (VS-M.) CRUK-Barts PhD studentship, (JM), World Wide Cancer Research 22-0329 (VS-M and VG.), and Barts Charity (VS-M and SG.).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abplanalp, W. T., Cremer, S., John, D., Hoffmann, J., Schuhmacher, B., Merten, M., et al. (2021). Clonal hematopoiesis-driver DNMT3A mutations alter immune cells in heart failure. Circ. Res. 128, 216–228.
Ahn, A., Rodger, E. J., Motwani, J., Gimenez, G., Stockwell, P. A., Parry, M., et al. (2021). Transcriptional reprogramming and constitutive PD-L1 expression in melanoma are associated with dedifferentiation and activation of interferon and tumour necrosis factor signalling pathways. Cancers (Basel) 13.
Akincilar, S. C., Chan, C. H. T., Ng, Q. F., Fidan, K., and Tergaonkar, V. (2021). Non-canonical roles of canonical telomere binding proteins in cancers. Cell Mol. Life Sci. 78, 4235–4257. doi:10.1007/s00018-021-03783-0
Alanko, J., Mai, A., Jacquemet, G., et al. (2015). Integrin endosomal signalling suppresses anoikis. Nat Cell Biol. 17, 1412–1421. doi:10.1038/ncb3250
Alblas, J., Ulfman, L., Hordijk, P., and Koenderman, L. (2001). Activation of RhoA and ROCK are essential for detachment of migrating leukocytes. Mol. Biol. Cell 12, 2137–2145. doi:10.1091/mbc.12.7.2137
Ali, F., and Ilyas, A. (2022). Belumosudil with ROCK-2 inhibition: Chemical and therapeutic development to FDA approval for the treatment of chronic graft-versus-host disease. Curr. Res. Transl. Med. 70, 103343. doi:10.1016/j.retram.2022.103343
Alon, R., and Van Buul, J. D. (2017). Leukocyte breaching of endothelial barriers: The actin link. Trends Immunol. 38, 606–615. doi:10.1016/j.it.2017.05.002
Amano, M., Nakayama, M., and Kaibuchi, K. (2010). Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton 67, 545–554. doi:10.1002/cm.20472
Amini, R., Bhatnagar, A., SchlüßLER, R., Möllmert, S., Guck, J., and Norden, C. (2022). Amoeboid-like migration ensures correct horizontal cell layer formation in the developing vertebrate retina. eLife 11, e76408. doi:10.7554/eLife.76408
Andrew, N., and Insall, R. H. (2007). Chemotaxis in shallow gradients is mediated independently of PtdIns 3-kinase by biased choices between random protrusions. Nat. Cell Biol. 9, 193–200. doi:10.1038/ncb1536
Bailey, M. H., Tokheim, C., Porta-Pardo, E., Sengupta, S., Bertrand, D., Weerasinghe, A., et al. (2018). Comprehensive characterization of cancer driver genes and mutations. Cell 173, 1034–1035. doi:10.1016/j.cell.2018.07.034
Bar, C., and Thum, T. (2017). Changing direction: From therapeutic telomerase inhibition to activation? Circ. Res. 120, 1393–1395. doi:10.1161/CIRCRESAHA.116.310316
Barry, N. P., and Bretscher, M. S. (2010). Dictyostelium amoebae and neutrophils can swim. Proc. Natl. Acad. Sci. U. S. A. 107, 11376–11380. doi:10.1073/pnas.1006327107
Baud, V., and Karin, M. (2009). Is NF-κB a good target for cancer therapy? Hopes and pitfalls. Nat. Rev. Drug Discov. 8, 33–40. doi:10.1038/nrd2781
Bendix, P. M., Koenderink, G. H., Cuvelier, D., Dogic, Z., Koeleman, B. N., Brieher, W. M., et al. (2008). A quantitative analysis of contractility in active cytoskeletal protein networks. Biophysical J. 94, 3126–3136. doi:10.1529/biophysj.107.117960
Bilusic, M., Heery, C. R., Collins, J. M., Donahue, R. N., Palena, C., Madan, R. A., et al. (2019). Phase I trial of HuMax-IL8 (BMS-986253), an anti-IL-8 monoclonal antibody, in patients with metastatic or unresectable solid tumors. J. Immunother. Cancer 7, 240. doi:10.1186/s40425-019-0706-x
Biro, M., Munoz, M. A., and Weninger, W. (2014). Targeting Rho-GTPases in immune cell migration and inflammation. Br. J. Pharmacol. 171, 5491–5506. doi:10.1111/bph.12658
Boland, S., Bourin, A., Alen, J., Geraets, J., Schroeders, P., Castermans, K., et al. (2015). Design, synthesis, and biological evaluation of novel, highly active soft ROCK inhibitors. J. Med. Chem. 58, 4309–4324. doi:10.1021/acs.jmedchem.5b00308
Borghans, J., and Ribeiro, R. M. (2017). The maths of memory. Elife 6, e26754. doi:10.7554/eLife.26754
Bravo-Cordero, J. J., Hodgson, L., and Condeelis, J. (2012). Directed cell invasion and migration during metastasis. Curr. Opin. Cell Biol. 24, 277–283. doi:10.1016/j.ceb.2011.12.004
Bros, M., Haas, K., Moll, L., and Grabbe, S. (2019). RhoA as a key regulator of innate and adaptive immunity. Cells 8, 733. doi:10.3390/cells8070733
Buisson, R., Langenbucher, A., Bowen, D., Kwan, E. E., Benes, C. H., Zou, L., et al. (2019). Passenger hotspot mutations in cancer driven by APOBEC3A and mesoscale genomic features. Science 364, eaaw2872. doi:10.1126/science.aaw2872
Cantelli, G., Orgaz, J. O. S. E. L., Rodriguez-Hernandez, I., Karagiannis, P., Maiques, O., Matias-Guiu, X., et al. (2015). TGF-β-Induced transcription sustains amoeboid melanoma migration and dissemination. Curr. Biol. 25, 2899–2914. doi:10.1016/j.cub.2015.09.054
Carragher, N. O., Walker, S. M., Scott Carragher, L. A., Harris, F., Sawyer, T. K., Brunton, V. G., et al. (2006). Calpain 2 and src dependence distinguishes mesenchymal and amoeboid modes of tumour cell invasion: A link to integrin function. Oncogene 25, 5726–5740. doi:10.1038/sj.onc.1209582
Čermák, V., Gandalovičová, A., Merta, L., Fučíková, J., Špíšek, R., Rösel, D., et al. (2018). RNA-seq of macrophages of amoeboid or mesenchymal migratory phenotype due to specific structure of environment. Sci. Data 5, 180198. doi:10.1038/sdata.2018.198
Chaplin, D. D. (2010). Overview of the immune response. J. Allergy Clin. Immunol. 125, S3–S23. doi:10.1016/j.jaci.2009.12.980
Crosas-Molist, E., Bertran, E., Rodriguez-Hernandez, I., Herraiz, C., Cantelli, G., Fabra, À., et al. (2017). The NADPH oxidase NOX4 represses epithelial to amoeboid transition and efficient tumour dissemination. Oncogene 36, 3002–3014. doi:10.1038/onc.2016.454
Crosas-Molist, E., Samain, R., Kohlhammer, L., Orgaz, J. L., George, S. L., Maiques, O., et al. (2021). Rho GTPase signaling in cancer progression and dissemination. Physiol. Rev. 102, 455–510. doi:10.1152/physrev.00045.2020
Daubon, T., Chasseriau, J., Ali, A. E., Rivet, J., Kitzis, A., Constantin, B., et al. (2008). Differential motility of p190bcr-abl- and p210bcr-abl-expressing cells: Respective roles of vav and bcr-abl GEFs. Oncogene 27, 2673–2685. doi:10.1038/sj.onc.1210933
De Pascalis, C., and Etienne-Manneville, S. (2017). Single and collective cell migration: The mechanics of adhesions. Mol. Biol. Cell 28, 1833–1846. doi:10.1091/mbc.E17-03-0134
de Winde, C. M., George, S. L., Crosas-Molist, E., Hari-Gupta, Y., Arp, A. B., Benjamin, A. C., et al. (2021). Podoplanin drives dedifferentiation and amoeboid invasion of melanoma. iScience 24 (9), 102976. doi:10.1016/j.isci.2021.102976
Douma, S., van Laar, T., Zevenhoven, J., et al. (2004). Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature 430, 1034–1039. doi:10.1038/nature02765
Dinarello, C. A., Simon, A., and Vandermeer, J. W. M. (2012). Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 11, 633–652. doi:10.1038/nrd3800
Dyberg, C., Fransson, S., Andonova, T., Sveinbjornsson, B., Lannerholm-Palm, J., Olsen, T. K., et al. (2017). Rho-associated kinase is a therapeutic target in neuroblastoma. Proc. Natl. Acad. Sci. U. S. A. 114, E6603–E6612. doi:10.1073/pnas.1706011114
Eddy, R. J., Pierini, L. M., Matsumura, F., and Maxfield, F. R. (2000). Ca2+-dependent myosin II activation is required for uropod retraction during neutrophil migration. J. Cell Sci. 113, 1287–1298. doi:10.1242/jcs.113.7.1287
Enomoto, A., Murakami, H., Asai, N., Morone, N., Watanabe, T., Kawai, K., et al. (2005). Akt/PKB regulates actin organization and cell motility via girdin/APE. Dev. Cell 9, 389–402. doi:10.1016/j.devcel.2005.08.001
Fielding, C. A., Mcloughlin, R. M., Mcleod, L., Colmont, C. S., Najdovska, M., Grail, D., et al. (2008). IL-6 regulates neutrophil trafficking during acute inflammation via STAT3. J. Immunol. 181, 2189–2195. doi:10.4049/jimmunol.181.3.2189
Flaherty, K. T., Infante, J. R., Daud, A., Gonzalez, R., Kefford, R. F., Sosman, J., et al. (2012). Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N. Engl. J. Med. 367, 1694–1703. doi:10.1056/NEJMoa1210093
Friedl, P., and Weigelin, B. (2008). Interstitial leukocyte migration and immune function. Nat. Immunol. 9, 960–969. doi:10.1038/ni.f.212
Friedl, P., and Wolf, K. (2009). Plasticity of cell migration: A multiscale tuning model. J. Cell Biol. 188, 11–19. doi:10.1083/jcb.200909003
Fujimoto, T., Miyayama, Y., and Fuyuta, M. (1977). The origin, migration and fine morphology of human primordial germ cells. Anatomical Rec. 188, 315–330. doi:10.1002/ar.1091880305
Gambardella, L., and Vermeren, S. (2013). Molecular players in neutrophil chemotaxis—Focus on PI3K and small GTPases. J. Leukoc. Biol. 94, 603–612. doi:10.1189/jlb.1112564
Gandalovičová, A., Rosel, D., Fernandes, M., Veselý, P., Heneberg, P., Čermák, V., et al. (2017). Migrastatics—anti-metastatic and anti-invasion drugs: Promises and challenges. Trends Cancer 3, 391–406. doi:10.1016/j.trecan.2017.04.008
Georgouli, M., Herraiz, C., Crosas-Molist, E., Fanshawe, B., Maiques, O., Perdrix, A., et al. (2019). Regional activation of myosin II in cancer cells drives tumor progression via a secretory cross-talk with the immune microenvironment. Cell 176, 757–774. e23. doi:10.1016/j.cell.2018.12.038
Giannelli, G., Santoro, A., Kelley, R. K., Gane, E., Paradis, V., Cleverly, A., et al. (2020). Biomarkers and overall survival in patients with advanced hepatocellular carcinoma treated with TGF-βRI inhibitor galunisertib. PLoS One 15, e0222259. doi:10.1371/journal.pone.0222259
Gil-Bernabé, A. M., Ferjancic, S., Tlalka, M., Zhao, L., Allen, P. D., Im, J. H., et al. (2012). Recruitment of monocytes/macrophages by tissue factor-mediated coagulation is essential for metastatic cell survival and premetastatic niche establishment in mice. Blood 119 (13), 3164–3175. doi:10.1182/blood-2011-08-376426
Gong, X., Didan, Y., Lock, J. G., and Strömblad, S. (2018). KIF13A-regulated RhoB plasma membrane localization governs membrane blebbing and blebby amoeboid cell migration. EMBO J. 37, e98994. doi:10.15252/embj.201898994
Graziani, V., Rodriguez-Hernandez, I., Maiques, O., and Sanz-Moreno, V. (2022). The amoeboid state as part of the epithelial-to-mesenchymal transition programme. Trends Cell Biol. 32, 228–242. doi:10.1016/j.tcb.2021.10.004
Guenther, C. (2022). β2-Integrins – regulatory and executive bridges in the signaling network controlling leukocyte trafficking and migration. Front. Immunol. 13, 809590. doi:10.3389/fimmu.2022.809590
Gui, P., Labrousse, A., VAN Goethem, E., Besson, A., Maridonneau-Parini, I., and LE Cabec, V. (2014). Rho/ROCK pathway inhibition by the CDK inhibitor p27kip1 participates in the onset of macrophage 3D-mesenchymal migration. J. Cell Sci. 127, 4009–4023. doi:10.1242/jcs.150987
Gunes, C., and Rudolph, K. L. (2013). The role of telomeres in stem cells and cancer. Cell 152, 390–393. doi:10.1016/j.cell.2013.01.010
Hahn, W. C., Stewart, S. A., Brooks, M. W., York, S. G., Eaton, E., Kurachi, A., et al. (1999). Inhibition of telomerase limits the growth of human cancer cells. Nat. Med. 5, 1164–1170. doi:10.1038/13495
Harb, J., Lin, P. J., and Hao, J. (2019). Recent development of wnt signaling pathway inhibitors for cancer therapeutics. Curr. Oncol. Rep. 21, 12. doi:10.1007/s11912-019-0763-9
Herraiz, C., Calvo, F., Pandya, P., Cantelli, G., Rodriguez-Hernandez, I., Orgaz, J. L., et al. (2016). Reactivation of p53 by a cytoskeletal sensor to control the balance between DNA damage and tumor dissemination. J. Natl. Cancer Inst. 108, 1.
Honing, H., Van den berg, T. K., Vanderpol, S. M. A., Dijkstra, C. D., Vanderkammen, R. A., Collard, J. G., et al. (2004). RhoA activation promotes transendothelial migration of monocytes via ROCK. J. Leukoc. Biol. 75, 523–528. doi:10.1189/jlb.0203054
Honjo, M., and Tanihara, H. (2018). Impact of the clinical use of ROCK inhibitor on the pathogenesis and treatment of glaucoma. Jpn. J. Ophthalmol. 62, 109–126. doi:10.1007/s10384-018-0566-9
Hutchinson, C. V., Natarajan, S., Johnson, S. M., Adams, J. A., Rees-Unwin, K. S., and Burthem, J. (2014). Lymphocytes from chronic lymphocytic leukaemia undergo ABL1-linked amoeboid motility and homotypic interaction as an early adaptive change to ex vivo culture. Exp. Hematol. Oncol. 3, 7. doi:10.1186/2162-3619-3-7
Ibo, M., Srivastava, V., Robinson, D. N., and Gagnon, Z. R. (2016). Cell blebbing in confined microfluidic environments. PLOS ONE 11, e0163866. doi:10.1371/journal.pone.0163866
Jin, F., Brockmeier, U., Otterbach, F., and Metzen, E. (2012). New insight into the SDF-1/CXCR4 axis in a breast carcinoma model: Hypoxia-induced endothelial SDF-1 and tumor cell CXCR4 are required for tumor cell intravasation. Mol. Cancer Res. 10, 1021–1031. doi:10.1158/1541-7786.MCR-11-0498
Jogi, A., Vaapil, M., Johansson, M., and Pahlman, S. (2012). Cancer cell differentiation heterogeneity and aggressive behavior in solid tumors. Ups. J. Med. Sci. 117, 217–224. doi:10.3109/03009734.2012.659294
Jokhun, D. S., Shang, Y., and Shivashankar, G. V. (2018). Actin dynamics couples extracellular signals to the mobility and molecular stability of telomeres. Biophys. J. 115, 1166–1179. doi:10.1016/j.bpj.2018.08.029
Jung, H., Ertl, L., Janson, C., Schall, T., and Charo, I. (2016). Abstract A107: Inhibition of CCR2 potentiates the checkpoint inhibitor immunotherapy in pancreatic cancer. Cancer Immunol. Res. 4, A107. doi:10.1158/2326-6066.imm2016-a107
Kai, F., Drain, A. P., and Weaver, V. M. (2019). The extracellular matrix modulates the metastatic journey. Dev. Cell 49, 332–346. doi:10.1016/j.devcel.2019.03.026
Kakiuchi, M., Nishizawa, T., Ueda, H., Gotoh, K., Tanaka, A., Hayashi, A., et al. (2014). Recurrent gain-of-function mutations of RHOA in diffuse-type gastric carcinoma. Nat. Genet. 46, 583–587. doi:10.1038/ng.2984
Kameritsch, P., and Renkawitz, J. (2020). Principles of leukocyte migration strategies. Trends Cell Biol. 30, 818–832. doi:10.1016/j.tcb.2020.06.007
Kane, B. A., Bryant, K. J., Mcneil, H. P., and Tedla, N. T. (2014). Termination of immune activation: An essential component of healthy host immune responses. J. Innate Immun. 6, 727–738. doi:10.1159/000363449
Khoo, A. S., Valentin, T. M., Leggett, S. E., Bhaskar, D., Bye, E. M., Benmelech, S., et al. (2019). Breast cancer cells transition from mesenchymal to amoeboid migration in tunable three-dimensional silk–collagen hydrogels. ACS Biomaterials Sci. Eng. 5, 4341–4354. doi:10.1021/acsbiomaterials.9b00519
Kim, S., Kim, S. A., Han, J., and Kim, I.-S. (2021). Rho-Kinase as a target for cancer therapy and its immunotherapeutic potential. Int. J. Mol. Sci. 22, 12916. doi:10.3390/ijms222312916
Krishnan, V., and Mitragotri, S. (2020). Nanoparticles for topical drug delivery: Potential for skin cancer treatment. Adv. Drug Deliv. Rev. 153, 87–108. doi:10.1016/j.addr.2020.05.011
Lacy, P., and Stow, J. L. (2011). Cytokine release from innate immune cells: Association with diverse membrane trafficking pathways. Blood 118, 9–18. doi:10.1182/blood-2010-08-265892
Lakshmi Narendra, B., Eshvendar Reddy, K., Shantikumar, S., and Ramakrishna, S. (2013). Immune system: A double-edged sword in cancer. Inflamm. Res. 62, 823–834. doi:10.1007/s00011-013-0645-9
Lam, P.-Y., and Huttenlocher, A. (2013). Interstitial leukocyte migration in vivo. Curr. Opin. Cell Biol. 25, 650–658. doi:10.1016/j.ceb.2013.05.007
Lämmermann, T., Bader, B. L., Monkley, S. J., Worbs, T., Wedlich-Söldner, R., Hirsch, K., et al. (2008). Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature 453, 51–55. doi:10.1038/nature06887
Lämmermann, T., and Germain, R. N. (2014). The multiple faces of leukocyte interstitial migration. Semin Immunopathol. 36, 227–251. doi:10.1007/s00281-014-0418-8
Lämmermann, T., and Sixt, M. (2009). Mechanical modes of ‘amoeboid’ cell migration. Curr. Opin. Cell Biol. 21, 636–644. doi:10.1016/j.ceb.2009.05.003
Lamouille, S., Xu, J., and Derynck, R. (2014). Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 15, 178–196. doi:10.1038/nrm3758
Langley, R. R., and Fidler, I. J. (2011). The seed and soil hypothesis revisited-the role of tumor-stroma interactions in metastasis to different organs. Int. J. Cancer 128, 2527–2535. doi:10.1002/ijc.26031
Laurent, P., Jolivel, V., Manicki, P., Chiu, L., Contin-Bordes, C., Truchetet, M.-E., et al. (2017). Immune-mediated repair: A matter of plasticity. Front. Immunol. 8, 454. doi:10.3389/fimmu.2017.00454
Ley, K., Laudanna, C., Cybulsky, M., et al. (2007). Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 7, 678–689. doi:10.1038/nri2156
Lehmann, S., Te Boekhorst, V., Odenthal, J., Bianchi, R., VAN Helvert, S., Ikenberg, K., et al. (2017). Hypoxia induces a HIF-1-Dependent transition from collective-to-amoeboid dissemination in epithelial cancer cells. Curr. Biol. 27, 392–400. doi:10.1016/j.cub.2016.11.057
Linder, S., and Aepfelbacher, M. (2003). Podosomes: Adhesion hot-spots of invasive cells. Trends Cell Biol. 13, 376–385. doi:10.1016/s0962-8924(03)00128-4
Linder, S., and Kopp, P. (2005). Podosomes at a glance. J. Cell Sci. 118, 2079–2082. doi:10.1242/jcs.02390
Liu, L., Schwartz, B. R., Lin, N., Winn, R. K., and Harlan, J. M. (2002). Requirement for RhoA kinase activation in leukocyte de-adhesion. J. Immunol. 169, 2330–2336. doi:10.4049/jimmunol.169.5.2330
Liu, S., Goldstein, R. H., Scepansky, E. M., and Rosenblatt, M. (2009). Inhibition of rho-associated kinase signaling prevents breast cancer metastasis to human bone. Cancer Res. 69, 8742–8751. doi:10.1158/0008-5472.CAN-09-1541
Liu, T., Zhang, L., Joo, D., and Sun, S.-C. (2017). NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2, 17023. doi:10.1038/sigtrans.2017.23
Liu, Y.-J., LE Berre, M., Lautenschlaeger, F., Maiuri, P., Callan-Jones, A., Heuzé, M., et al. (2015). Confinement and low adhesion induce fast amoeboid migration of slow mesenchymal cells. Cell 160, 659–672. doi:10.1016/j.cell.2015.01.007
Lokuta, M. A., and Huttenlocher, A. (2005). TNF-α promotes a stop signal that inhibits neutrophil polarization and migration via a p38 MAPK pathway. J. Leukoc. Biol. 78, 210–219. doi:10.1189/jlb.0205067
Lomakin, A. J., Cattin, C. J., Cuvelier, D., Alraies, Z., Molina, M., Nader, G. P. F., et al. (2020). The nucleus acts as a ruler tailoring cell responses to spatial constraints. Science 370, eaba2894. doi:10.1126/science.aba2894
López-Luque, J., Bertran, E., Crosas-Molist, E., Maiques, O., Malfettone, A., Caja, L., et al. (2019). Downregulation of epidermal growth factor receptor in hepatocellular carcinoma facilitates transforming growth factor-β-induced epithelial to amoeboid transition. Cancer Lett. 464, 15–24. doi:10.1016/j.canlet.2019.08.011
Lu, J., Zhou, S., Siech, M., Habisch, H., Seufferlein, T., and Bachem, M. G. (2014). Pancreatic stellate cells promote hapto-migration of cancer cells through collagen I-mediated signalling pathway. Br. J. Cancer 110, 409–420. doi:10.1038/bjc.2013.706
Mace, T. A., Shakya, R., Pitarresi, J. R., Swanson, B., Mcquinn, C. W., Loftus, S., et al. (2018). IL-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer. Gut 67, 320–332. doi:10.1136/gutjnl-2016-311585
Machado, H. E., Mitchell, E., Obro, N. F., Kubler, K., Davies, M., Leongamornlert, D., et al. (2022). Diverse mutational landscapes in human lymphocytes. Nature 608, 724–732. doi:10.1038/s41586-022-05072-7
Madsen, C. D., and Sahai, E. (2010). Cancer dissemination—lessons from leukocytes. Dev. Cell 19, 13–26. doi:10.1016/j.devcel.2010.06.013
Maiques, O., Fanshawe, B., Crosas-Molist, E., Rodriguez-Hernandez, I., Volpe, A., Cantelli, G., et al. (2021). A preclinical pipeline to evaluate migrastatics as therapeutic agents in metastatic melanoma. Br. J. Cancer 125, 699–713. doi:10.1038/s41416-021-01442-6
Majidpoor, J., and Mortezaee, K. (2021). Steps in metastasis: An updated review. Med. Oncol. 38, 3–17. doi:10.1007/s12032-020-01447-w
Mali, R. S., Ramdas, B., Ma, P., Shi, J., Munugalavadla, V., Sims, E., et al. (2011). Rho kinase regulates the survival and transformation of cells bearing oncogenic forms of KIT, FLT3, and BCR-ABL. Cancer Cell 20, 357–369. doi:10.1016/j.ccr.2011.07.016
Mammadova-Bach, E., Mangin, P., Lanza, F., and Gachet, C. (2015). Platelets in cancer. From basic research to therapeutic implications. Hamostaseologie 35, 325–336. doi:10.5482/hamo-14-11-0065
Marcuzzi, E., Angioni, R., Molon, B., and Calì, B. (2018). Chemokines and chemokine receptors: Orchestrating tumor metastasization. Int. J. Mol. Sci. 20, 96. doi:10.3390/ijms20010096
Marrack, P., Scott-Browne, J., and Macleod, M. K. (2010). Terminating the immune response. Immunol. Rev. 236, 5–10. doi:10.1111/j.1600-065X.2010.00928.x
Maslova, A., Ramirez, R. N., Ma, K., Schmutz, H., Wang, C., Fox, C., et al. (2020). Deep learning of immune cell differentiation. Proc. Natl. Acad. Sci. U. S. A. 117, 25655–25666. doi:10.1073/pnas.2011795117
Mcleod, R., Kumar, R., Papadatos-Pastos, D., Mateo, J., Brown, J. S., Garces, A. H. I., et al. (2020). First-in-Human study of AT13148, a dual ROCK-AKT inhibitor in patients with solid tumors. Clin. Cancer Res. 26, 4777–4784. doi:10.1158/1078-0432.CCR-20-0700
Meng, S., Tripathy, D., Frenkel, E. P., Shete, S., Naftalis, E. Z., Huth, J. F., et al. (2004). Circulating tumor cells in patients with breast cancer dormancy. Clin. Cancer Res. 10, 8152–8162. doi:10.1158/1078-0432.CCR-04-1110
Mihlan, M., Glaser, K. M., Epple, M. W., and Lämmermann, T. (2022). Neutrophils: Amoeboid migration and swarming dynamics in tissues. Front. Cell Dev. Biol. 10, 871789. doi:10.3389/fcell.2022.871789
Miles, F. L., Pruitt, F. L., VAN Golen, K. L., and Cooper, C. R. (2008). Stepping out of the flow: Capillary extravasation in cancer metastasis. Clin. Exp. Metastasis 25, 305–324. doi:10.1007/s10585-007-9098-2
Mitroulis, I., Alexaki, V. I., Kourtzelis, I., Ziogas, A., Hajishengallis, G., and Chavakis, T. (2015). Leukocyte integrins: role in leukocyte recruitment and as therapeutic targets in inflammatory disease. Pharmacol. Ther. 147, 123–135. doi:10.1016/j.pharmthera.2014.11.008
Mo, Z., Liu, J., Zhang, Q., Chen, Z., Mei, J., Liu, L., et al. (2016). Expression of PD-1, PD-L1 and PD-L2 is associated with differentiation status and histological type of endometrial cancer. Oncol. Lett. 12, 944–950. doi:10.3892/ol.2016.4744
Moose, D. L., Krog, B. L., Kim, T. H., Zhao, L., Williams-Perez, S., Burke, G., et al. (2020). Cancer cells resist mechanical destruction in circulation via RhoA/actomyosin-dependent mechano-adaptation. Cell Rep. 30, 3864–3874. e6. doi:10.1016/j.celrep.2020.02.080
Muller, W. A. (2013). Getting leukocytes to the site of inflammation. Veterinary Pathol. 50, 7–22. doi:10.1177/0300985812469883
Muller, W. A. (2011). Mechanisms of leukocyte transendothelial migration. Annu. Rev. Pathology Mech. Dis. 6, 323–344. doi:10.1146/annurev-pathol-011110-130224
Murphy, D. A., and Courtneidge, S. A. (2011). The 'ins' and 'outs' of podosomes and invadopodia: Characteristics, formation and function. Nat. Rev. Mol. Cell Biol. 12, 413–426. doi:10.1038/nrm3141
Nayar, S., Dasgupta, P., and Galustian, C. (2015). Extending the lifespan and efficacies of immune cells used in adoptive transfer for cancer immunotherapies-A review. Oncoimmunology 4, e1002720. doi:10.1080/2162402X.2014.1002720
Nourshargh, S., and Alon, R. (2014). Leukocyte migration into inflamed tissues. Immunity 41, 694–707. doi:10.1016/j.immuni.2014.10.008
Nourshargh, S., Hordijk, P. L., and Sixt, M. (2010). Breaching multiple barriers: Leukocyte motility through venular walls and the interstitium. Nat. Rev. Mol. Cell Biol. 11, 366–378. doi:10.1038/nrm2889
O'brien, C. A., Kreso, A., and Jamieson, C. H. (2010). Cancer stem cells and self-renewal. Clin. Cancer Res. 16, 3113–3120. doi:10.1158/1078-0432.CCR-09-2824
Olson, T. S., and Ley, K. (2002). Chemokines and chemokine receptors in leukocyte trafficking. Am. J. Physiol. Regul. Integr. Comp. Physiol. 283, R7–R28. doi:10.1152/ajpregu.00738.2001
Orgaz, J. L., Crosas-Molist, E., Sadok, A., Perdrix-Rosell, A., Maiques, O., Rodriguez-Hernandez, I., et al. (2020). Myosin II reactivation and cytoskeletal remodeling as a hallmark and a vulnerability in melanoma therapy resistance. Cancer Cell 37, 85–103. e9. doi:10.1016/j.ccell.2019.12.003
Orgaz, J. L., Pandya, P., Dalmeida, R., Karagiannis, P., Sanchez-Laorden, B., Viros, A., et al. (2014). Diverse matrix metalloproteinase functions regulate cancer amoeboid migration. Nat. Commun. 5, 4255. doi:10.1038/ncomms5255
Otto, A., Collins-Hooper, H., Patel, A., Dash, P. R., and Patel, K. (2011). Adult skeletal muscle stem cell migration is mediated by a blebbing/amoeboid mechanism. Rejuvenation Res. 14, 249–260. doi:10.1089/rej.2010.1151
Oudin, M. J., Jonas, O., Kosciuk, T., Broye, L. C., Guido, B. C., Wyckoff, J., et al. (2016). Tumor cell–driven extracellular matrix remodeling drives haptotaxis during metastatic progression. Cancer Discov. 6, 516–531. doi:10.1158/2159-8290.CD-15-1183
Owen, K. L., Brockwell, N. K., and Parker, B. S. (2019). JAK-STAT signaling: A double-edged sword of immune regulation and cancer progression, Cancers [Online]. 1. 11.
Paluch, E. K., and Raz, E. (2013). The role and regulation of blebs in cell migration. Curr. Opin. Cell Biol. 25, 582–590. doi:10.1016/j.ceb.2013.05.005
Palumbo, J. S., Talmage, K. E., Massari, J. V., La Jeunesse, C. M., Flick, M. J., Kombrinck, K. W., et al. (2005). Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood 105 (1), 178–185. doi:10.1182/blood-2004-06-2272
Pan, Y.-R., Chen, C.-C., Chan, Y.-T., Wang, H.-J., Chien, F.-T., Chen, Y.-L., et al. (2018). STAT3-coordinated migration facilitates the dissemination of diffuse large B-cell lymphomas. Nat. Commun. 9, 3696. doi:10.1038/s41467-018-06134-z
Patel, A. A., Ginhoux, F., and Yona, S. (2021). Monocytes, macrophages, dendritic cells and neutrophils: An update on lifespan kinetics in health and disease. Immunology 163, 250–261. doi:10.1111/imm.13320
Patel, A. A., Zhang, Y., Fullerton, J. N., Boelen, L., Rongvaux, A., Maini, A. A., et al. (2017). The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J. Exp. Med. 214, 1913–1923. doi:10.1084/jem.20170355
Perea Paizal, J., Au, S. H., and Bakal, C. (2021). Squeezing through the microcirculation: Survival adaptations of circulating tumour cells to seed metastasis. Br. J. Cancer 124, 58–65. doi:10.1038/s41416-020-01176-x
Pernas, S., Martin, M., Kaufman, P. A., Gil-Martin, M., Gomez Pardo, P., Lopez-Tarruella, S., et al. (2018). Balixafortide plus eribulin in HER2-negative metastatic breast cancer: A phase 1, single-arm, dose-escalation trial. Lancet Oncol. 19, 812–824. doi:10.1016/S1470-2045(18)30147-5
Pinner, S., and Sahai, E. (2008a). Imaging amoeboid cancer cell motility in vivo. J. Microsc. 231, 441–445. doi:10.1111/j.1365-2818.2008.02056.x
Pinner, S., and Sahai, E. (2008b). PDK1 regulates cancer cell motility by antagonising inhibition of ROCK1 by RhoE. Nat. Cell Biol. 10, 127–137. doi:10.1038/ncb1675
Plowden, J., Renshaw-Hoelscher, M., Engleman, C., Katz, J., and Sambhara, S. (2004). Innate immunity in aging: Impact on macrophage function. Aging Cell 3, 161–167. doi:10.1111/j.1474-9728.2004.00102.x
Poltavets, V., Kochetkova, M., Pitson, S. M., and Samuel, M. S. (2018). The role of the extracellular matrix and its molecular and cellular regulators in cancer cell plasticity. Front. Oncol. 8, 431. doi:10.3389/fonc.2018.00431
Qiu, R. G., Chen, J., Mccormick, F., and Symons, M. (1995). A role for Rho in Ras transformation. Proc. Natl. Acad. Sci. U. S. A. 92, 11781–11785. doi:10.1073/pnas.92.25.11781
Qureshy, Z., Johnson, D. E., and Grandis, J. R. (2020). Targeting the JAK/STAT pathway in solid tumors. J. Cancer Metastasis Treat. 6, 27.
Rath, N., and Olson, M. F. (2012). Rho-associated kinases in tumorigenesis: Re-considering ROCK inhibition for cancer therapy. EMBO Rep. 13, 900–908. doi:10.1038/embor.2012.127
Reversat, A., Gaertner, F., Merrin, J., Stopp, J., Tasciyan, S., Aguilera, J., et al. (2020). Cellular locomotion using environmental topography. Nature 582, 582–585. doi:10.1038/s41586-020-2283-z
Richardson, B. E., and Lehmann, R. (2010). Mechanisms guiding primordial germ cell migration: Strategies from different organisms. Nat. Rev. Mol. Cell Biol. 11, 37–49. doi:10.1038/nrm2815
Rodenburg, W. S., and Van Buul, J. D. (2021). Rho GTPase signalling networks in cancer cell transendothelial migration. Vasc. Biol. 3, R77–R95. doi:10.1530/VB-21-0008
Rodriguez-Hernandez, I., Maiques, O., Kohlhammer, L., Cantelli, G., Perdrix-Rosell, A., Monger, J., et al. (2020). WNT11-FZD7-DAAM1 signalling supports tumour initiating abilities and melanoma amoeboid invasion. Nat. Commun. 11, 5315. doi:10.1038/s41467-020-18951-2
Roussos, E. T., Condeelis, J. S., and Patsialou, A. (2011). Chemotaxis in cancer. Nat. Rev. Cancer 11, 573–587. doi:10.1038/nrc3078
Ruland, J. (2011). Return to homeostasis: Downregulation of NF-κB responses. Nat. Immunol. 12, 709–714. doi:10.1038/ni.2055
Ruprecht, V., Wieser, S., Callan-Jones, A., Smutny, M., Morita, H., Sako, K., et al. (2015). Cortical contractility triggers a stochastic switch to fast amoeboid cell motility. Cell 160, 673–685. doi:10.1016/j.cell.2015.01.008
Ruscitti, P., Masedu, F., Alvaro, S., Airò, P., Battafarano, N., Cantarini, L., et al. (2019). Anti-interleukin-1 treatment in patients with rheumatoid arthritis and type 2 diabetes (track): A multicentre, open-label, randomised controlled trial. PLOS Med. 16, e1002901. doi:10.1371/journal.pmed.1002901
Sahai, E., Alberts, A. S., and Treisman, R. (1998). RhoA effector mutants reveal distinct effector pathways for cytoskeletal reorganization, SRF activation and transformation. EMBO J. 17, 1350–1361. doi:10.1093/emboj/17.5.1350
Sahai, E., and Marshall, C. J. (2003). Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat. Cell Biol. 5, 711–719. doi:10.1038/ncb1019
Sanchez, J. N., Wang, T., and Cohen, M. S. (2018). BRAF and MEK inhibitors: Use and resistance in BRAF-mutated cancers. Drugs 78, 549–566. doi:10.1007/s40265-018-0884-8
Sánchez-Madrid, F., and Serrador, J. M. (2009). Bringing up the rear: Defining the roles of the uropod. Nat. Rev. Mol. Cell Biol. 10, 353–359. doi:10.1038/nrm2680
Sanz-Moreno, V., Gadea, G., Ahn, J., Paterson, H., Marra, P., Pinner, S., et al. (2008). Rac activation and inactivation control plasticity of tumor cell movement. Cell 135, 510–523. doi:10.1016/j.cell.2008.09.043
Sanz-Moreno, V., Gaggioli, C., Yeo, M., Albrengues, J., Wallberg, F., Viros, A., et al. (2011). ROCK and JAK1 signaling cooperate to control actomyosin contractility in tumor cells and stroma. Cancer Cell 20, 229–245. doi:10.1016/j.ccr.2011.06.018
Schick, J., and Raz, E. (2022). Blebs—formation, regulation, positioning, and role in amoeboid cell migration. Front. Cell Dev. Biol. 10, 926394. doi:10.3389/fcell.2022.926394
Schimmel, L., Heemskerk, N., and VAN Buul, J. D. (2017). Leukocyte transendothelial migration: A local affair. Small GTPases 8, 1–15. doi:10.1080/21541248.2016.1197872
Schipper, K., Seinstra, D., Paulien Drenth, A., Vanderburg, E., Ramovs, V., Sonnenberg, A., et al. (2019). Rebalancing of actomyosin contractility enables mammary tumor formation upon loss of E-cadherin. Nat. Commun. 10, 3800. doi:10.1038/s41467-019-11716-6
Semprucci, E., Tocci, P., Cianfrocca, R., Sestito, R., Caprara, V., Veglione, M., et al. (2016). Endothelin A receptor drives invadopodia function and cell motility through the β-arrestin/PDZ-RhoGEF pathway in ovarian carcinoma. Oncogene 35, 3432–3442. doi:10.1038/onc.2015.403
Senapati, S., Mahanta, A. K., Kumar, S., and Maiti, P. (2018). Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 3, 7. doi:10.1038/s41392-017-0004-3
Sharma, P., Hu-Lieskovan, S., Wargo, J. A., and Ribas, A. (2017). Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 168, 707–723. doi:10.1016/j.cell.2017.01.017
Siveen, K. S., Sikka, S., Surana, R., Dai, X., Zhang, J., Kumar, A. P., et al. (2014). Targeting the STAT3 signaling pathway in cancer: Role of synthetic and natural inhibitors. Biochimica Biophysica Acta (BBA) - Rev. Cancer 1845, 136–154. doi:10.1016/j.bbcan.2013.12.005
Sökeland, G., and Schumacher, U. (2019). The functional role of integrins during intra- and extravasation within the metastatic cascade. Mol. Cancer 18, 12. doi:10.1186/s12943-018-0937-3
Sokol, C. L., and Luster, A. D. (2015). The chemokine system in innate immunity. Cold Spring Harb. Perspect. Biol. 7, a016303. doi:10.1101/cshperspect.a016303
Spuul, P., Ciufici, P., Veillat, V., Leclercq, A., Daubon, T., Kramer, I., et al. (2014). Importance of RhoGTPases in formation, characteristics, and functions of invadosomes. Small GTPases 5, e28195. doi:10.4161/sgtp.28713
Strell, C., and Entschladen, F. (2008). Extravasation of leukocytes in comparison to tumor cells. Cell Commun. Signal. 6, 10. doi:10.1186/1478-811X-6-10
Strilic, B., and Offermanns, S. (2017). Intravascular survival and extravasation of tumor cells. Cancer Cell 32, 282–293. doi:10.1016/j.ccell.2017.07.001
Subramanian, B. C., Melis, N., Chen, D., Wang, W., Gallardo, D., Weigert, R., et al. (2020). The LTB4–BLT1 axis regulates actomyosin and β2-integrin dynamics during neutrophil extravasation. J. Cell Biol. 219, e201910215. doi:10.1083/jcb.201910215
Suresh, S. (2007). Biomechanics and biophysics of cancer cells. Acta Biomater. 3, 413–438. doi:10.1016/j.actbio.2007.04.002
Takemoto, A., Miyata, K., and Fujita, N. (2017). Platelet-activating factor podoplanin: From discovery to drug development. Cancer Metastasis Rev. 36, 225–234. doi:10.1007/s10555-017-9672-2
Talamillo, A., Grande, L., Ruiz-Ontanon, P., Velasquez, C., Mollinedo, P., Torices, S., et al. (2017). ODZ1 allows glioblastoma to sustain invasiveness through a Myc-dependent transcriptional upregulation of RhoA. Oncogene 36, 1733–1744. doi:10.1038/onc.2016.341
Taniguchi, K., and Karin, M. (2018). NF-κB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 18, 309–324. doi:10.1038/nri.2017.142
Te Boekhorst, V., Preziosi, L., and Friedl, P. (2016). Plasticity of cell migration in vivo and in silico. Annu. Rev. Cell Dev. Biol. 32, 491–526. doi:10.1146/annurev-cellbio-111315-125201
Titus, M. A., and Goodson, H. V. (2017). An evolutionary perspective on cell migration: Digging for the roots of amoeboid motility. J. Cell Biol. 216, 1509–1511. doi:10.1083/jcb.201704112
Tomasetti, C., Li, L., and Vogelstein, B. (2017). Stem cell divisions, somatic mutations, cancer etiology, and cancer prevention. Science 355, 1330–1334. doi:10.1126/science.aaf9011
Tozluoğlu, M., Tournier, A. L., Jenkins, R. P., Hooper, S., Bates, P. A., and Sahai, E. (2013). Matrix geometry determines optimal cancer cell migration strategy and modulates response to interventions. Nat. Cell Biol. 15, 751–762. doi:10.1038/ncb2775
Van Goethem, E., Poincloux, R., Gauffre, F., Maridonneau-Parini, I., and LE Cabec, V. (2010). Matrix architecture dictates three-dimensional migration modes of human macrophages: Differential involvement of proteases and podosome-like structures. J. Immunol. 184, 1049–1061. doi:10.4049/jimmunol.0902223
Vega, F. M., Colmenero-Repiso, A., Gomez-Munoz, M. A., Rodriguez-Prieto, I., Aguilar-Morante, D., Ramirez, G., et al. (2019). CD44-high neural crest stem-like cells are associated with tumour aggressiveness and poor survival in neuroblastoma tumours. EBioMedicine 49, 82–95. doi:10.1016/j.ebiom.2019.10.041
Wang, H., and Unternaehrer, J. J. (2019). Epithelial-mesenchymal transition and cancer stem cells: At the crossroads of differentiation and dedifferentiation. Dev. Dyn. 248, 10–20. doi:10.1002/dvdy.24678
Wang, Y., Liu, S., Zhang, Y., and Yang, J. (2019). Myosin heavy chain 9: Oncogene or tumor suppressor gene? Med. Sci. Monit. 25, 888–892. doi:10.12659/MSM.912320
Weissenbach, M., Clahsen, T., Weber, C., Spitzer, D., Wirth, D., Vestweber, D., et al. (2004). Interleukin-6 is a direct mediator of T cell migration. Eur. J. Immunol. 34, 2895–2906. doi:10.1002/eji.200425237
Wiesner, C., LE-Cabec, V., EL Azzouzi, K., Maridonneau-Parini, I., and Linder, S. (2014). Podosomes in space: Macrophage migration and matrix degradation in 2D and 3D settings. Cell Adhesion Migr. 8, 179–191. doi:10.4161/cam.28116
Winer, A., Adams, S., and Mignatti, P. (2018). Matrix metalloproteinase inhibitors in cancer therapy: Turning past failures into future successes. Mol. Cancer Ther. 17, 1147–1155. doi:10.1158/1535-7163.MCT-17-0646
Wong, P.-P., Muñoz-Félix, J. M., Hijazi, M., Kim, H., Robinson, S. D., DE Luxán-Delgado, B., et al. (2020). Cancer burden is controlled by mural cell-β3-integrin regulated crosstalk with tumor cells. Cell 181, 1346–1363. e21. doi:10.1016/j.cell.2020.02.003
Worthylake, R. A., Lemoine, S., Watson, J. M., and Burridge, K. (2001). RhoA is required for monocyte tail retraction during transendothelial migration. J. Cell Biol. 154, 147–160. doi:10.1083/jcb.200103048
Wyckoff, J. B., Pinner, S. E., Gschmeissner, S., Condeelis, J. S., and Sahai, E. (2006). ROCK- and myosin-dependent matrix deformation enables protease-independent tumor-cell invasion in vivo. Curr. Biol. 16, 1515–1523. doi:10.1016/j.cub.2006.05.065
Wyckoff, J., Wang, W., Lin, E. Y., Wang, Y., Pixley, F., Stanley, E. R., et al. (2004). A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 64, 7022–7029. doi:10.1158/0008-5472.CAN-04-1449
Wyse, M. M., Goicoechea, S., Garcia-Mata, R., Nestor-Kalinoski, A. L., and Eisenmann, K. M. (2017). mDia2 and CXCL12/CXCR4 chemokine signaling intersect to drive tumor cell amoeboid morphological transitions. Biochem. Biophys. Res. Commun. 484, 255–261. doi:10.1016/j.bbrc.2017.01.087
Yamada, K. M., and Sixt, M. (2019). Mechanisms of 3D cell migration. Nat. Rev. Mol. Cell Biol. 20, 738–752. doi:10.1038/s41580-019-0172-9
Yao, Y., Zhou, Y., Liu, L., Xu, Y., Chen, Q., Wang, Y., et al. (2020). Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front. Mol. Biosci. 7, 193. doi:10.3389/fmolb.2020.00193
Yap, T. A., Lakhani, N. J., Araujo, D. V., Ahnert, J. R., Chandana, S. R., Sharma, M., et al. (2020). AVID200, first-in-class TGF-beta 1 and 3 selective and potent inhibitor: Safety and biomarker results of a phase I monotherapy dose-escalation study in patients with advanced solid tumors. J. Clin. Oncol. 38, 3587. doi:10.1200/jco.2020.38.15_suppl.3587
Yi, M., Niu, M., Xu, L., Luo, S., and Wu, K. (2021). Regulation of PD-L1 expression in the tumor microenvironment. J. Hematol. Oncol. 14, 10. doi:10.1186/s13045-020-01027-5
Yu, H., Lin, L., Zhang, Z., Zhang, H., and Hu, H. (2020). Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target. Ther. 5, 209. doi:10.1038/s41392-020-00312-6
Yu, H., Pardoll, D., and Jove, R. (2009). STATs in cancer inflammation and immunity: A leading role for STAT3. Nat. Rev. Cancer 9, 798–809. doi:10.1038/nrc2734
Zhang, L., Yu, B., Hu, M., Wang, Z., Liu, D., Tong, X., et al. (2011). Role of Rho-ROCK signaling in MOLT4 cells metastasis induced by CCL25. Leukemia Res. 35, 103–109. doi:10.1016/j.leukres.2010.07.039
Zhao, J., Zhou, D., Guo, J., Ren, Z., Zhou, L., Wang, S., et al. (2006). Effect of fasudil hydrochloride, a protein kinase inhibitor, on Cerebral vasospasm and delayed cerebral ischemic symptoms after aneurysmal subarachnoid hemorrhage.
Keywords: amoeboid migration, cancer, leukocyte, metastasis, migrastatics
Citation: George S, Martin JAJ, Graziani V and Sanz-Moreno V (2023) Amoeboid migration in health and disease: Immune responses versus cancer dissemination. Front. Cell Dev. Biol. 10:1091801. doi: 10.3389/fcell.2022.1091801
Received: 07 November 2022; Accepted: 15 December 2022;
Published: 05 January 2023.
Edited by:
Verena Ruprecht, Centre for Genomic Regulation (CRG), SpainReviewed by:
Miguel Vicente-Manzanares, Spanish National Research Council (CSIC), SpainCopyright © 2023 George, Martin, Graziani and Sanz-Moreno. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Victoria Sanz-Moreno, di5zYW56LW1vcmVub0BxbXVsLmFjLnVr
†These authors have contributed equally to this work.
 Samantha George
Samantha George Joshua Alexander James Martin†
Joshua Alexander James Martin† Victoria Sanz-Moreno
Victoria Sanz-Moreno