
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Cell Dev. Biol. , 05 December 2022
Sec. Molecular and Cellular Pathology
Volume 10 - 2022 | https://doi.org/10.3389/fcell.2022.1089668
 Xiaoman Lv1†
Xiaoman Lv1† Ting Zhao2†
Ting Zhao2† Youwu Dai1†
Youwu Dai1† Mingqin Shi1
Mingqin Shi1 Xiaoyi Huang1
Xiaoyi Huang1 Yuanyuan Wei1
Yuanyuan Wei1 Jiayan Shen2
Jiayan Shen2 Xiaoyu Zhang2
Xiaoyu Zhang2 Zhaohu Xie1
Zhaohu Xie1 Qi Wang2*
Qi Wang2* Zhaofu Li1*
Zhaofu Li1* Dongdong Qin1*
Dongdong Qin1*Autophagy is an intracellular degradation system that maintains the stable state of cell energy metabolism. Some recent findings have indicated that autophagy dysfunction is an important driving factor for the occurrence and development of osteoarthritis (OA). The decrease of autophagy leads to the accumulation of damaged organelles and macromolecules in chondrocytes, which affects the survival of chondrocytes and ultimately leads to OA. An appropriate level of autophagic activation may be a new method to prevent articular cartilage degeneration in OA. This minireview discussed the mechanism of autophagy and OA, key autophagy targets regulating OA progression, and evaluated therapeutic applications of drugs targeting autophagy in preclinical and clinical research. Some critical issues worth paying attention to were also raised to guide future research efforts.
Osteoarthritis (OA), the most common musculoskeletal disorder, is complex, multifaceted, and characterized by the degradation of articular cartilage and alterations in other joint tissues (Miyaki & Asahara, 2012). It is a highly prevalent disease, particularly in population over 65 years of age worldwide (Motta, Barone, Sica, & Selmi, 2022). Age is considered the strongest risk factor, injury, lifestyle, and congenital abnormality may further increase the risk of OA as well (Brumat et al., 2022). Cartilage degeneration is considered as the primary pathological change at the tissue level related to OA symptoms. The main pathogenesis of OA is the disorder of synthesis and degradation of articular cartilage and extracellular matrix (ECM). Articular cartilage is a kind of connective tissue composed of chondrocytes and ECM. Cartilage ECM is synthesized and secreted by chondrocytes and mainly consists of collagens (essentially type II) and aggregating proteoglycans (Eyre, 2002; Duan, Xie, & Liu, 2020). The abnormal expression of a set of aggrecanases is the main reason for cartilage degradation (e.g., a disintegrin and metalloproteinase with thrombospondin motifs ADAMTS-4 and -5) and collagenases (e.g., matrix metalloproteases, MMP-1, -3, -8, and -13), which are upregulated in OA (Charlier et al., 2016).
Studies have found that the degeneration of articular chondrocytes may be related to autophagy, which directly or indirectly affects the occurrence and development of OA (Takayama et al., 2014). Autophagy is an adaptive response, protecting cells from stress (Lamark & Johansen, 2012; Caramés, Olmer, Kiosses, & Lotz, 2015). During autophagy, parts of the cytoplasm and intracellular organelles are sequestered within characteristic double- or multi-membraned autophagic vacuoles and are finally delivered to lysosomes for bulk degradation (Almonte-Becerril et al., 2010; Yang & Klionsky, 2010). Three types of autophagy have been distinguished on the basis of the mechanism of cargo sequestration, including microautophagy, chaperone-mediated autophagy, and macroautophagy (Hansen, Rubinsztein, & Walker, 2018). Among them, macroautophagy, degradation of cytosolic material via sequestration into double-membrane vesicles called autophagosomes that subsequently fuse with lysosomes, is the most reported regarding the pathogenesis of OA (Jeon & Im, 2017). About 35 different conserved autophagy-related (ATG) genes encode proteins involved in the main steps of the macro-autophagy process (Parzych & Klionsky, 2014). Normal human cartilage can express high levels of autophagy regulators, including Unc-51-like kinase 1 (ULK1), beclin1, and LC3-II (Caramés, Taniguchi, Otsuki, Blanco, & Lotz, 2010; Zhang et al., 2015). The inhibition of autophagy caused OA-related gene expression changes in human chondrocytes, while the induction of autophagy prevented this (Sasaki et al., 2012). Targeted deletion of ATG in chondrocytes can promote cell death (Vuppalapati et al., 2015). Furthermore, the loss of proteoglycan is associated with reduced autophagic markers in OA (Kao et al., 2022). As essential organelles in chondrocytes, mitochondria supply energy and play vital roles in cellular metabolism and proliferation. Mitochondrial autophagy (also called mitophagy) is a specific type of autophagy that selectively removes damaged or depolarized mitochondria to maintain mitochondrial quality control (Yamashita & Kanki, 2017; Liu et al., 2022). Defective mitophagy leads to the accumulation of damaged mitochondria in the cytoplasm and cellular dysfunction (Lou et al., 2020). A study has found that mitophagy-related genes are highly expressed in OA patients’ cartilage (Shin et al., 2019). In addition, promoting mitophagy can protect the cartilage of OA patients (Jin et al., 2022). Therefore, insufficient autophagy can be associated with mitochondrial change in the pathogenesis of OA. Activating autophagy in degenerated cells may be a feasible and effective method to slow articular cartilage degeneration.
This minireview discussed the mechanism of autophagy and OA, key autophagy targets regulating OA progression, and evaluated therapeutic applications of drugs targeting chondrocyte autophagy in preclinical and clinical research. Some critical issues worth paying attention to were also raised to guide future research efforts.
Autophagy could promote either chondrocyte survival or death depending on the age, the presence and stage of OA, and the type of autophagy induction (Almonte-Becerril et al., 2010; Hwang, Yang, Park, & Kim, 2015). The pathogenesis of OA is mostly about aging (Terman, Kurz, Navratil, Arriaga, & Brunk, 2010; Bouderlique et al., 2016). The imbalance of catabolism and anabolism in the ECM is related to aging because the more vulnerable joint cannot afford damage from outside (Rahmati, Nalesso, Mobasheri, & Mozafari, 2017). Further, aging has a significant impact on autophagy-mediated chondrocyte survival. Studies have confirmed that autophagy-related proteins, such as Unc-51-like kinase 1 (ULK1), beclin-1, and LC3, were highly expressed in human chondrocyte clusters, whereas a reduced expression of these proteins was observed in the aged population (Caramés et al., 2010). Decrease of autophagy leads to the accumulation of intracellular damaged organelles and macromolecules, affecting chondrocyte survival, and ultimately leading to age-related OA (Bouderlique et al., 2016) (Figure 1). Aging may accelerate chondrocytes’ death by inhibiting chondrocytes’ autophagy, which promotes the development of OA.
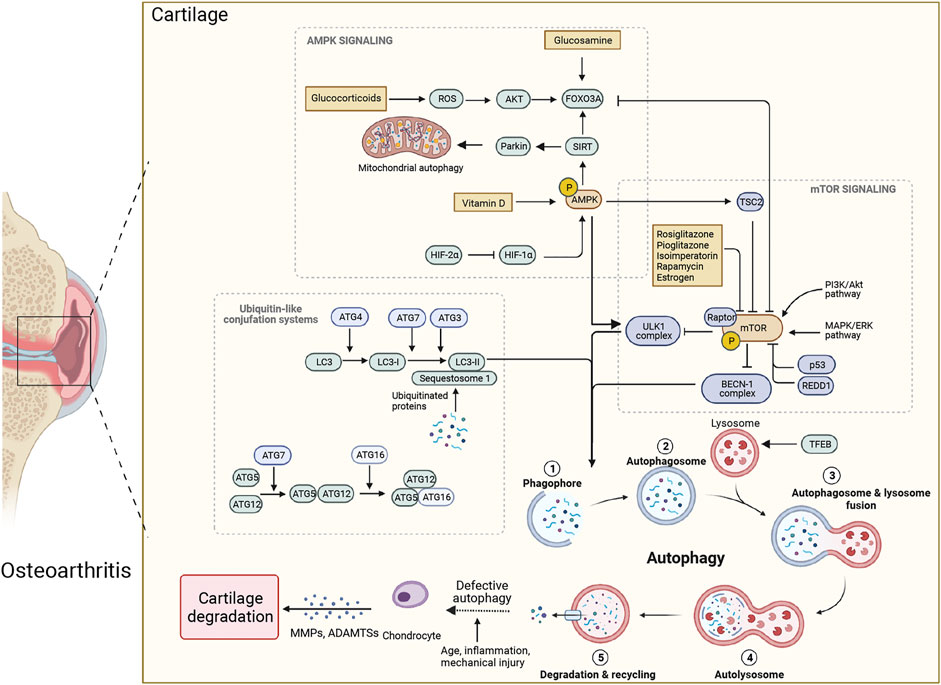
FIGURE 1. Therapeutic modulators of autophagy and associated mechanisms in osteoarthritis (OA). Autophagy is a multistep process that includes: ① phagophore formation; ② expansion elongation of phagophores to produce autophagosome; ③ autophagosomes fuse with endosomes and lysosomes; ④ autolysosome formation; ⑤ degradation of sequestered cargo in the autolysosome and recycling. Defective autophagy can lead to extracellular matrix degradation, resulting in OA. The mammalian target of rapamycin (mTOR) and AMP-activated kinase (AMPK) are the main regulators of chondrocyte autophagy in OA, with mTOR acting as an inhibitor and AMPK as an activator. The AMPK could phosphorylate and activate the sirtuin1 (SIRT1) and forkhead box class O 3a (FOXO3a), triggering autophagic flux through unc-51 like autophagy activating kinase 1 (ULK1), then suppressing mTOR. ULK1-mediated phosphorylation of AMPK constitutes a negative regulatory feedback loop. The mTOR can inhibit ULK1 and beclin-1 complexes, causing activation of autophagy. In addition, the transition from phagophore to autophagosome depends on the activity of two ubiquitin-like conjugation systems, the ATG5–ATG12 and LC3 system. The ubiquitin-like protein of ATG12 attaches to ATG5 with a covalent bond, and combines with ATG16 to elongate the pre-autophagosomal membrane. LC3-II is transformed from LC3-I through the lipidation of the ubiquitin-like reaction and binds to autophagic vesicles. Glucosamine can promote autophagy in cartilage through activating the dephosphorylation of FOXO3a. In contrast, glucocorticoids can stimulate FOXO3a and activate autophagy through a higher level of reactive oxygen species. Vitamin D can alleviate OA through AMPK-mTOR pathway. The rosiglitazone, pioglitazone, rapamycin, estrogen and isoimperatorin can inhibit mTOR activity, making them good candidates for potential therapies of OA.
In the early phase of OA, chondrocytes proliferate, giving rise to OA-typical clusters (Kim & Blanco, 2007). Autophagy is activated as an adaptive response to sublethal conditions, with the aim to avoid cell death (Klionsky, 2005; Levine & Yuan, 2005). Some studies have found that the ULK1, beclin-1, LC3 protein expression is decreased in the superficial zone, while these proteins are strongly expressed in the middle and deep zone (Kao et al., 2022). In later stages, there is an all-layered autophagic genetic change, including not only reduced ULK1, LC3, and beclin-1 but also fewer ATG3, ATG5, and ATG12. In later stage of OA, chondrocytes showed decreased autophagy and increased apoptosis. Apoptosis occurs when autophagy causes excessive consumption of intracellular proteins and organelles, which makes cells unable to survive. The reduction in key regulators of chondrocyte autophagy is a combination between classical apoptosis and autophagy (Almonte-Becerril et al., 2010). Autophagy is chondroprotective by regulating apoptosis, which is thought to be in balance with apoptosis when increased chondrocyte apoptosis occurs with lower expression of autophagy regulators in OA (Caramés et al., 2010).
In addition, autophagy can function to promote cell survival or cell death, depending on the type of cellular stress. Starvation and catabolic stress can increase autophagy in chondrocytes (Sasaki et al., 2012). However, autophagy related proteins (LC3-II, an autophagosomal marker in mammals) appear to decrease in mechanically injured cartilage (Caramés et al., 2012b; Vinatier, Domínguez, Guicheux, & Caramés, 2018). Additionally, mitochondrial dysfunction of chondrocytes showed an early increase in autophagy, which is a compensatory mechanism. However, when long-term pressure exceeds cell compensation, mitochondria in chondrocytes are damaged, leading to a massive increase in reactive oxygen species (ROS) and eventually OA (López de Figueroa, Lotz, Blanco, & Caramés, 2015). Therefore, autophagy has an essential role in protecting chondrocytes from different stressors. However, the relationship between autophagy and chondrocyte death has not been fully understood, and further research is needed to verify it.
Many molecules participate in the regulation of autophagic activity to affect the occurrence and development of OA, such as the mammalian target of rapamycin (mTOR), AMP-activated protein kinase (AMPK), non-coding RNA (ncRNA), etc. Studies have confirmed that the mTOR is the core target in regulating autophagy, which plays a vital role in cartilage growth and development and in altering articular cartilage homeostasis (Pal, Endisha, Zhang, & Kapoor, 2015). Furthermore, the specific knockdown of peroxisome proliferator activated receptor γ (PPARγ) can accelerate OA associated with aberrant mTOR signaling in the articular cartilage (Vasheghani et al., 2015). The inhibition of the PI3K/AKT/mTOR signaling pathway can significantly promote the autophagy level of OA chondrocytes (Chen, Crawford, & Xiao, 2013; Sun et al., 2020). In addition, tRNA-derived fragment tRF-5009A has been reported to have critical regulatory roles in OA, which regulates autophagy and cartilage degeneration by targeting mTOR (Deng et al., 2022). Regulated in development and DNA damage response 1 (REDD1), an endogenous mTOR inhibitor, was found to be decreased in OA cartilage (Charlier et al., 2016). It is highly expressed in normal human cartilage and reduced in OA patients (Alvarez-Garcia et al., 2016). Research has confirmed that REDD1 can regulate autophagy and mitochondrial biosynthesis to maintain the viability of chondrocytes (Alvarez-Garcia et al., 2017). REDD1 deficiency exacerbates the severity of injury-induced OA. Reduced REDD1 expression thus represents a novel mechanism for the defective autophagy observed in OA (Figure 1).
AMPK is a positive autophagy regulator, inhibiting mTOR from activating autophagy. After activation of AMPK, ULK1 and beclin1-VPS34 complex are phosphorylated and activated to promote the induction and formation of autophagosomes (Li & Chen, 2019). The increase of AMPK may be related to a change in the chondrocyte energy charge, which may promote autophagy. Dysfunction of AMPK activity has been associated with reduced autophagy, impaired mitochondrial function, excessive ROS generation, and inflammation in joint tissue, which leads to articular cartilage degeneration and synovial inflammation (Wang J. et al., 2020). Silent information regulator T1 (SIRT1) and forkhead box class O 3a (FOXO3a), as the signaling molecule downstream of AMPK, have been shown to trigger the formation of autophagosomes and activate autophagy by regulating the expression of ATG proteins (Hu et al., 2019; Wang J. et al., 2020). SIRT1 regulates autophagy by interacting with autophagy-related ATG7, which may become a more critical target in OA treatment (Liao et al., 2021). SIRT1-conditional knockout mice exhibit increased MMP13 and ADAMTS5 levels (Vinatier et al., 2018). SIRT3 can maintain the normal function of mitochondria and protect chondrocytes. The intra-articular SIRT3 overexpression alleviated the severity of OA-induced joint damage (Xu et al., 2021). Further, the FOXO may play a crucial role in postnatal cartilage development, maturation, and homeostasis and protects against OA-associated cartilage damage (Matsuzaki et al., 2018).
In addition, the putative kinase protein 1 (PINK1) is a serine kinase that can target mitochondria. Parkin is an E3 ubiquitin ligase that eliminates damaged mitochondria in OA chondrocytes (Ansari, Khan, Ahmad, & Haqqi, 2018). The PINK1-Parkin pathway can target to clear damaged mitochondria, reduce cell damage caused by oxidative stress, and improve chondrocyte survival rate (Wang et al., 2019). The transcription factor EB, a master regulator for autophagic flux, can alleviate articular cartilage degeneration and enhance autophagic activity (Zheng et al., 2018). Hypoxia-inducible factor-1α (HIF-1α) mediated mitophagy has a protective role in OA. The expression of HIF-1α was increased in human and mouse OA cartilage (Hu et al., 2020). HIF-1 inhibits mTOR signaling and ultimately enhances the autophagy activity of chondrocytes under hypoxia (Bohensky, Leshinsky, Srinivas, & Shapiro, 2010). Suppressing HIF-1α degradation can promote mitochondrial autophagy and alleviate cartilage degeneration (Hu et al., 2020). In contrast, HIF-2 is a potent negative regulator of autophagy in maturing chondrocytes, which promotes the degradation of chondrocyte ECM and is elevated in OA cartilage (Duan et al., 2020) (Figure 1).
For note, ncRNA has been confirmed to mediate autophagy in chondrocytes, such as microRNA, long non-coding RNA (lncRNA), and circular RNA (circRNA). Many microRNAs are commonly involved in the process of autophagy in OA (44–47). MiR-155 is an inhibitor in chondrocyte autophagy, which can alleviate key autophagy regulators contributing to the autophagy defects of OA (D'Adamo et al., 2016). MiR-9, MiR-34a, and miR-449 have been demonstrated to significantly reduce the expression of SIRT1 in chondrocytes (D'Adamo et al., 2017; Park et al., 2016; Yan et al., 2016). MiR-27a can enhance the autophagy and apoptosis of IL-1β-treated chondrocytes through PI3K/AKT/mTOR signaling (Cai et al., 2019). In addition, lncRNAs have been noticed to participate in OA through autophagy. LncRNA-GAS5 expresses highly in OA cartilage tissues, which is able to bind to miR-144 and regulate the expression of mTOR, inhibiting autophagy of OA chondrocytes (Ji, Qiao, Liu, & Wang, 2021). Hox transcript antisense intergenic RNA-induced apoptosis is mediated by sponging miR-130a-3p to repress chondrocyte autophagy in OA (He & Jiang, 2020). LncRNA-POU3F3 can inhibit chondrocytes, restraining autophagy and alleviating the pathogenesis of OA by regulating the miR-29a-3p/FOXO3 axis (Shi et al., 2022). Mesenchymal stem cell-derived exosome-mediated lncRNA KLF3-AS1 can repress autophagy of chondrocytes in OA (Wen, Lin, Zou, Lin, & Liu, 2022). Sex-determining region Y-box 4tbox4-activated lncRNA-MCM3AP antisense RNA 1 aggravated OA progression by modulating autophagy and ECM degradation via targeting miR-149-5p/Notch1 axis (Xu et al., 2022). Additionally, the circRNAs have also been involved in OA microenvironment for control of autophagy to perturb the situation of inflammation such as hsa_circ_0005567, ciRS-7, circPan3, has_circ_0037658, circMELK, and circRHOT1 (Zhang, Cheng, Rong, Tang, & Gui, 2020; Zhou et al., 2020; Sui, Liu, Que, Xu, & Hu, 2021; Zeng et al., 2021; Man et al., 2022; Zhang et al., 2022). Therefore, taking transcription factors, microRNA, lncRNA, circRNA, and autophagy inhibitors as entry points can serve as potential therapeutic targets for OA and represent a surprising new lead in the search for drugs to treat OA. However, more high-quality evidence is needed to confirm the appropriate therapeutic target of OA in clinical practice.
A variety of drugs regulating autophagic activity are used to treat OA. Rapamycin affected the mTOR signaling pathway in mouse knee joints as indicated by the inhibition of ribosomal protein S6 phosphorylation, a target of mTOR, and activation of LC3 (Caramés et al., 2012a). Rapamycin can prevent cell death and increase the expression of aggrecan and type II collagen while decreasing MMP-13 and ADAMTS5 in OA chondrocytes (Caramés et al., 2012a; Zhang et al., 2015). The intra-articular injection of rapamycin in a murine model could reduce vascular endothelial growth factor, collagen type X alpha 1, and MMP13 expression, leading to a delay in articular cartilage degradation (Takayama et al., 2014). However, long-term use of rapamycin may cause adverse events, such as headache, nausea, dizziness and epistaxis (Figure 1).
2-amino-2-deoxy-β-d-glucopyranose (glucosamine) is widely used to improve the symptoms and to delay the structural progression of OA (Conrozier & Lohse, 2022). Glucosamine is an effective autophagy activator, and autophagy enhancement mainly depends on the Akt/FOXO/mTOR pathway (Caramés et al., 2013). It has been found that the dual role of glucosamine in autophagy in human chondrocytes depends on exposure time (Kang et al., 2015). The exposure of glucosamine to chondrocytes activated autophagy, peroxidation, and pexophagy. However, long-time exposure of glucosamine may have the opposite effects due to the accumulation of peroxisomal dysfunction and long-chain fatty acids. Treatment of chondrocytes with glucosamine exerts exposure time-dependent dual effects on autophagy (Hochberg et al., 2012; Kang et al., 2015; Bruyère, Altman, & Reginster, 2016).
Glucocorticosteroid drugs have been used to treat early-stage OA, which may also lead to autophagy initiation. However, multiple administrations of glucocorticosteroid drugs may destroy the articular cartilage, induce human chondrocytes’ mitochondrial dysfunction, and increase ROS (Li, Chen, Li, Zhang, & Chen, 2022). In turn, dexamethasone may significantly attenuate the expression of MMP-13 in human OA chondrocytes through an mitogen-activated protein kinase phosphatase-1 and p38 mitogen-activated protein kinases-dependent manner (Lehtola et al., 2022). A study found that increased autophagy is an adaptive response to protect chondrocytes from short-term glucocorticosteroid exposure, whereas prolonged glucocorticosteroid drug treatment decreases autophagy and increases apoptosis (Liu, Wang, Zhao, Zhang, & Song, 2014). Thus, autophagy may be one of the essential mechanisms of glucocorticoids in the treatment of OA.
Many small molecule compounds and natural plant components play protective roles in OA by activating autophagy, such as isoimperatorin, delphinidin, celastrol, curcumin and astragaloside IV (Liu, Meng, Jing, & Zhou, 2017; Ouyang, Jiang, Fang, Cui, & Cai, 2017; Lee et al., 2020; Xiao et al., 2020; Dai et al., 2021). Baicalin protects chondrocytes against the degradation of ECM through activating autophagy via miR-766-3p/AIFM1 axis (Li, Cheng, & Liu, 2020). Dihydroartemisinin can suppress the levels of inflammatory factors in chondrocytes by promoting autophagy via inhibition of nuclear factor kappa-B pathway (Jiang et al., 2016). Trehalose can ameliorate endoplasmic reticulum stress and oxidative stress-mediated mitochondrial dysfunction via autophagic flux restoration and selective autophagy stimulation (Tang et al., 2017). Resveratrol can inhibit OA disease progression by activating SIRT1 (Deng et al., 2019).
In addition, the chemical autophagy inducer Torin 1 is an mTOR inhibitor, inhibiting the degenerative changes of experimental OA by activating autophagy (Cheng, Guo, & Meng, 2016). A study has demonstrated that fenofibrate, a PPARα agonist used for dyslipidaemias in humans, reduced both senescence and inflammation, increased autophagic flux, and increased autophagy in both ageing human and OA chondrocytes (Nogueira-Recalde et al., 2019). Metformin increased phosphorylated levels of AMPKα and upregulated SIRT1 protein expression, leading to an increase in autophagy, which may aid the development of novel therapeutic approaches for OA treatment (Wang C. et al., 2020). Parathyroid hormone-(1–34) can alleviate knee OA progression in rats via autophagy (Chen et al., 2018). Active vitamin D could be crucial in autophagosome aggregating and activates chondrocyte autophagy to reduce OA via mediating the AMPK-mTOR signaling (Kong et al., 2020). Estrogen may prevent articular cartilage destruction by promoting chondrocyte autophagy via the ERK-mammalian target of mTOR signaling (Ge et al., 2019). The PPARγ agonists rosiglitazone and pioglitazone also indirectly inhibit mTOR activity, making them good candidates for potential therapies in OA.
Autophagy dysfunction is an important driving factor for the occurrence and development of OA. Autophagy is a relatively balanced state under physiological conditions. When there is external stressor, the damaged organelles and the wrong proteins (such as drug stimulation, oxidative stress, diseases, etc.) are removed to improve the cell survival rate. Furthermore, when the cells are “weak,” they have no or deficient ability to autophagy. The cells are damaged by toxic substances, making survival difficult. An appropriate level of autophagic activation can effectively remove damaged organelles and macromolecules that need to be degraded to a certain extent, preventing chondrocyte damage and providing a good intracellular environment to promote the survival of chondrocytes. Given the critical role of autophagy in the pathogenesis of OA, the targeted autophagy pathway provides a new direction for the treatment of OA.
The relationship between chondrocyte autophagy targets autophagic activity in OA needs to be elucidated. Nevertheless, it means the likelihood of broadening the search for OA-related autophagy targets, which can serve as more comprehensive therapeutic targets or diagnostic biomarkers. Meanwhile, the research on drug regulation of OA autophagy focuses on animal research. Clinical studies are still dominated by single-center studies, lacking multicenter, large sample randomized controlled studies to evaluate drug efficacy. Therefore, further studies are needed to reduce sample heterogeneity and evaluate the effectiveness and safety of drugs in regulating autophagic signals. Nowadays, nanoplatforms combined to test therapeutic potential have become more common, which may have plenty of opportunities for the progress of autophagy-related treatment in OA. It is worth noting that the pathogenesis of OA is complex and is closely related to inflammation, aging, proliferation, and apoptosis. These pathogenesis have different autophagy needs in chondrocyte function and cell fate determination. Therefore, before the clinical application of autophagic active drugs to target the treatment of OA, it is necessary to conduct a comprehensive study on the relationship between crosstalk effects and multitargeting relationships.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
This work was supported by the National Natural Science Foundation of China (31960178, 82160923, 81960870, 81560781, and 81760822); Construction Project of National Traditional Chinese Medicine Clinical Research Base (2018 No. 131); Clinical Cooperative Project of Chinese and Western Medicine for Major and Knotty Diseases; Yunnan Provincial Key Laboratory Construction Project Funding; Yunnan Provincial Key Laboratory of Chinese Medicine Rheumatology and Immunology; Yunnan Provincial Ten Thousands Program Famous Doctor Special; Yunnan Province Qingguo Wang Expert Workstation Construction Project (202005AF150017); Yunnan Applied Basic Research Projects-Yunnan University of Chinese Medicine Union Foundation (202101AZ070001-247); Yunnan Applied Basic Research Projects-Union Foundation (2019FF002(-031)); Applied Basic Research Programs of Science and Technology Commission Foundation of Yunnan Province (2019FA007); Key Laboratory of Traditional Chinese Medicine for Prevention and Treatment of Neuropsychiatric Diseases, Yunnan Provincial Department of Education; Scientific Research Projects for High-level Talents of Yunnan University of Chinese Medicine (2019YZG01); Young Top-Notch Talent in 10,000 Talent Program of Yunnan Province (YNWR-QNBJ-2019-235); National Science and Technology Innovation 2030 Major Program (2021ZD0200900); Yunnan Key Research and Development Program (202103AC100005); Yunnan Province Fabao Gao Expert Workstation Construction Project (202105AF150037); Yunnan Engineering Research Center of Drug Development for Bone Diseases.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Almonte-Becerril, M., Navarro-Garcia, F., Gonzalez-Robles, A., Vega-Lopez, M. A., Lavalle, C., and Kouri, J. B. (2010). Cell death of chondrocytes is a combination between apoptosis and autophagy during the pathogenesis of Osteoarthritis within an experimental model. Apoptosis 15 (5), 631–638. doi:10.1007/s10495-010-0458-z
Alvarez-Garcia, O., Matsuzaki, T., Olmer, M., Plate, L., Kelly, J. W., and Lotz, M. K. (2017). Regulated in development and DNA damage response 1 deficiency impairs autophagy and mitochondrial biogenesis in articular cartilage and increases the severity of experimental osteoarthritis. Arthritis Rheumatol. 69 (7), 1418–1428. doi:10.1002/art.40104
Alvarez-Garcia, O., Olmer, M., Akagi, R., Akasaki, Y., Fisch, K. M., Shen, T., et al. (2016). Suppression of REDD1 in osteoarthritis cartilage, a novel mechanism for dysregulated mTOR signaling and defective autophagy. Osteoarthr. Cartil. 24 (9), 1639–1647. doi:10.1016/j.joca.2016.04.015
Ansari, M. Y., Khan, N. M., Ahmad, I., and Haqqi, T. M. (2018). Parkin clearance of dysfunctional mitochondria regulates ROS levels and increases survival of human chondrocytes. Osteoarthr. Cartil. 26 (8), 1087–1097. doi:10.1016/j.joca.2017.07.020
Bohensky, J., Leshinsky, S., Srinivas, V., and Shapiro, I. M. (2010). Chondrocyte autophagy is stimulated by HIF-1 dependent AMPK activation and mTOR suppression. Pediatr. Nephrol. 25 (4), 633–642. doi:10.1007/s00467-009-1310-y
Bouderlique, T., Vuppalapati, K. K., Newton, P. T., Li, L., Barenius, B., and Chagin, A. S. (2016). Targeted deletion of Atg5 in chondrocytes promotes age-related osteoarthritis. Ann. Rheum. Dis. 75 (3), 627–631. doi:10.1136/annrheumdis-2015-207742
Brumat, P., Kunšič, O., Novak, S., Slokar, U., Pšenica, J., Topolovec, M., et al. (2022). The surgical treatment of osteoarthritis. Life (Basel) 12 (7), 982. doi:10.3390/life12070982
Bruyère, O., Altman, R. D., and Reginster, J. Y. (2016). Efficacy and safety of glucosamine sulfate in the management of osteoarthritis: Evidence from real-life setting trials and surveys. Semin. Arthritis Rheum. 45 (4), S12–S17. doi:10.1016/j.semarthrit.2015.11.011
Cai, C., Min, S., Yan, B., Liu, W., Yang, X., Li, L., et al. (2019). MiR-27a promotes the autophagy and apoptosis of IL-1β treated-articular chondrocytes in osteoarthritis through PI3K/AKT/mTOR signaling. Aging (Albany NY) 11 (16), 6371–6384. doi:10.18632/aging.102194
Caramés, B., Hasegawa, A., Taniguchi, N., Miyaki, S., Blanco, F. J., and Lotz, M. (2012a). Autophagy activation by rapamycin reduces severity of experimental osteoarthritis. Ann. Rheum. Dis. 71 (4), 575–581. doi:10.1136/annrheumdis-2011-200557
Caramés, B., Kiosses, W. B., Akasaki, Y., Brinson, D. C., Eap, W., Koziol, J., et al. (2013). Glucosamine activates autophagy in vitro and in vivo. Arthritis Rheum. 65 (7), 1843–1852. doi:10.1002/art.37977
Caramés, B., Olmer, M., Kiosses, W. B., and Lotz, M. K. (2015). The relationship of autophagy defects to cartilage damage during joint aging in a mouse model. Arthritis Rheumatol. 67 (6), 1568–1576. doi:10.1002/art.39073
Caramés, B., Taniguchi, N., Otsuki, S., Blanco, F. J., and Lotz, M. (2010). Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 62 (3), 791–801. doi:10.1002/art.27305
Caramés, B., Taniguchi, N., Seino, D., Blanco, F. J., D'Lima, D., and Lotz, M. (2012b). Mechanical injury suppresses autophagy regulators and pharmacologic activation of autophagy results in chondroprotection. Arthritis Rheum. 64 (4), 1182–1192. doi:10.1002/art.33444
Charlier, E., Relic, B., Deroyer, C., Malaise, O., Neuville, S., Collée, J., et al. (2016). Insights on molecular mechanisms of chondrocytes death in osteoarthritis. Int. J. Mol. Sci. 17 (12), 2146. doi:10.3390/ijms17122146
Chen, C. H., Ho, M. L., Chang, L. H., Kang, L., Lin, Y. S., Lin, S. Y., et al. (2018). Parathyroid hormone-(1-34) ameliorated knee osteoarthritis in rats via autophagy. J. Appl. Physiol. 124 (5), 1177–1185. doi:10.1152/japplphysiol.00871.2017
Chen, J., Crawford, R., and Xiao, Y. (2013). Vertical inhibition of the PI3K/Akt/mTOR pathway for the treatment of osteoarthritis. J. Cell. Biochem. 114 (2), 245–249. doi:10.1002/jcb.24362
Cheng, N. T., Guo, A., and Meng, H. (2016). The protective role of autophagy in experimental osteoarthritis, and the therapeutic effects of Torin 1 on osteoarthritis by activating autophagy. BMC Musculoskelet. Disord. 17, 150. doi:10.1186/s12891-016-0995-x
Conrozier, T., and Lohse, T. (2022). Glucosamine as a treatment for osteoarthritis: What if it's true? Front. Pharmacol. 13, 820971. doi:10.3389/fphar.2022.820971
D'Adamo, S., Alvarez-Garcia, O., Muramatsu, Y., Flamigni, F., and Lotz, M. K. (2016). MicroRNA-155 suppresses autophagy in chondrocytes by modulating expression of autophagy proteins. Osteoarthr. Cartil. 24 (6), 1082–1091. doi:10.1016/j.joca.2016.01.005
D'Adamo, S., Cetrullo, S., Guidotti, S., Borzì, R. M., and Flamigni, F. (2017). Hydroxytyrosol modulates the levels of microRNA-9 and its target sirtuin-1 thereby counteracting oxidative stress-induced chondrocyte death. Osteoarthr. Cartil. 25 (4), 600–610. doi:10.1016/j.joca.2016.11.014
Dai, S., Fan, J., Zhang, Y., Hao, Z., Yu, H., and Zhang, Z. (2021). Celastrol promotes chondrocyte autophagy by regulating mTOR expression. Chin. Med. J. 135 (1), 92–94. doi:10.1097/cm9.0000000000001552
Deng, Z., Li, Y., Liu, H., Xiao, S., Li, L., Tian, J., et al. (2019). The role of sirtuin 1 and its activator, resveratrol in osteoarthritis. Biosci. Rep. 39 (5), BSR20190189. doi:10.1042/bsr20190189
Deng, Z., Long, D., Liu, H., Xu, Y., Xin, R., Liao, H., et al. (2022). tRNA-derived fragment tRF-5009a regulates autophagy and degeneration of cartilage in osteoarthritis via targeting mTOR. Oxid. Med. Cell. Longev. 2022, 5781660. doi:10.1155/2022/5781660
Duan, R., Xie, H., and Liu, Z. Z. (2020). The role of autophagy in osteoarthritis. Front. Cell Dev. Biol. 8, 608388. doi:10.3389/fcell.2020.608388
Ge, Y., Zhou, S., Li, Y., Wang, Z., Chen, S., Xia, T., et al. (2019). Estrogen prevents articular cartilage destruction in a mouse model of AMPK deficiency via ERK-mTOR pathway. Ann. Transl. Med. 7 (14), 336. doi:10.21037/atm.2019.06.77
Hansen, M., Rubinsztein, D. C., and Walker, D. W. (2018). Autophagy as a promoter of longevity: Insights from model organisms. Nat. Rev. Mol. Cell Biol. 19 (9), 579–593. doi:10.1038/s41580-018-0033-y
He, B., and Jiang, D. (2020). HOTAIR-induced apoptosis is mediated by sponging miR-130a-3p to repress chondrocyte autophagy in knee osteoarthritis. Cell Biol. Int. 44 (2), 524–535. doi:10.1002/cbin.11253
Hochberg, M. C., Altman, R. D., April, K. T., Benkhalti, M., Guyatt, G., McGowan, J., et al. (2012). American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 64 (4), 465–474. doi:10.1002/acr.21596
Hu, S., Zhang, C., Ni, L., Huang, C., Chen, D., Shi, K., et al. (2020). Stabilization of HIF-1α alleviates osteoarthritis via enhancing mitophagy. Cell Death Dis. 11 (6), 481. doi:10.1038/s41419-020-2680-0
Hu, X., Lu, Z., Yu, S., Reilly, J., Liu, F., Jia, D., et al. (2019). CERKL regulates autophagy via the NAD-dependent deacetylase SIRT1. Autophagy 15 (3), 453–465. doi:10.1080/15548627.2018.1520548
Hwang, H. S., Yang, C. M., Park, S. J., and Kim, H. A. (2015). Monosodium urate crystal-induced chondrocyte death via autophagic process. Int. J. Mol. Sci. 16 (12), 29265–29277. doi:10.3390/ijms161226164
Jeon, H., and Im, G. I. (2017). Autophagy in osteoarthritis. Connect. Tissue Res. 58 (6), 497–508. doi:10.1080/03008207.2016.1240790
Ji, Q., Qiao, X., Liu, Y., and Wang, D. (2021). Expression of long-chain noncoding RNA GAS5 in osteoarthritis and its effect on apoptosis and autophagy of osteoarthritis chondrocytes. Histol. Histopathol. 36 (4), 475–484. doi:10.14670/hh-18-312
Jiang, L. B., Meng, D. H., Lee, S. M., Liu, S. H., Xu, Q. T., Wang, Y., et al. (2016). Dihydroartemisinin inhibits catabolism in rat chondrocytes by activating autophagy via inhibition of the NF-κB pathway. Sci. Rep. 6, 38979. doi:10.1038/srep38979
Jin, Z., Chang, B., Wei, Y., Yang, Y., Zhang, H., Liu, J., et al. (2022). Curcumin exerts chondroprotective effects against osteoarthritis by promoting AMPK/PINK1/Parkin-mediated mitophagy. Biomed. Pharmacother. 151, 113092. doi:10.1016/j.biopha.2022.113092
Kang, Y. H., Park, S., Ahn, C., Song, J., Kim, D., and Jin, E. J. (2015). Beneficial reward-to-risk action of glucosamine during pathogenesis of osteoarthritis. Eur. J. Med. Res. 20, 89. doi:10.1186/s40001-015-0176-7
Kao, W. C., Chen, J. C., Liu, P. C., Lu, C. C., Lin, S. Y., Chuang, S. C., et al. (2022). The role of autophagy in osteoarthritic cartilage. Biomolecules 12 (10), 1357. doi:10.3390/biom12101357
Kim, H. A., and Blanco, F. J. (2007). Cell death and apoptosis in osteoarthritic cartilage. Curr. Drug Targets 8 (2), 333–345. doi:10.2174/138945007779940025
Klionsky, D. J. (2005). The molecular machinery of autophagy: Unanswered questions. J. Cell Sci. 118 (1), 7–18. doi:10.1242/jcs.01620
Kong, C., Wang, C., Shi, Y., Yan, L., Xu, J., and Qi, W. (2020). Active vitamin D activates chondrocyte autophagy to reduce osteoarthritis via mediating the AMPK-mTOR signaling pathway. Biochem. Cell Biol. 98 (3), 434–442. doi:10.1139/bcb-2019-0333
Lamark, T., and Johansen, T. (2012). Aggrephagy: Selective disposal of protein aggregates by macroautophagy. Int. J. Cell Biol. 2012, 736905. doi:10.1155/2012/736905
Lee, D. Y., Park, Y. J., Song, M. G., Kim, D. R., Zada, S., and Kim, D. H. (2020). Cytoprotective effects of delphinidin for human chondrocytes against oxidative stress through activation of autophagy. Antioxidants (Basel) 9 (1), 83. doi:10.3390/antiox9010083
Lehtola, T., Nummenmaa, E., Tuure, L., Hämäläinen, M., Nieminen, R. M., Moilanen, T., et al. (2022). Dexamethasone attenuates the expression of MMP-13 in chondrocytes through MKP-1. Int. J. Mol. Sci. 23 (7), 3880. doi:10.3390/ijms23073880
Levine, B., and Yuan, J. (2005). Autophagy in cell death: An innocent convict? J. Clin. Invest. 115 (10), 2679–2688. doi:10.1172/jci26390
Li, Q., Chen, H., Li, Z., Zhang, F., and Chen, L. (2022). Glucocorticoid caused lactic acid accumulation and damage in human chondrocytes via ROS-mediated inhibition of Monocarboxylate Transporter 4. Bone 155, 116299. doi:10.1016/j.bone.2021.116299
Li, Y., and Chen, Y. (2019). AMPK and autophagy. Adv. Exp. Med. Biol. 1206, 85–108. doi:10.1007/978-981-15-0602-4_4
Li, Z., Cheng, J., and Liu, J. (2020). Baicalin protects human OA chondrocytes against IL-1β-induced apoptosis and ECM degradation by activating autophagy via MiR-766-3p/AIFM1 Axis. Drug Des. devel. Ther. 14, 2645–2655. doi:10.2147/dddt.S255823
Liao, F. X., Huang, F., Ma, W. G., Qin, K. P., Xu, P. F., Wu, Y. F., et al. (2021). The new role of Sirtuin1 in human osteoarthritis chondrocytes by regulating autophagy. Cartilage 13 (2), 1237S–1248S. doi:10.1177/1947603519847736
Liu, D., Cai, Z. J., Yang, Y. T., Lu, W. H., Pan, L. Y., Xiao, W. F., et al. (2022). Mitochondrial quality control in cartilage damage and osteoarthritis: New insights and potential therapeutic targets. Osteoarthr. Cartil. 30 (3), 395–405. doi:10.1016/j.joca.2021.10.009
Liu, J., Meng, Q., Jing, H., and Zhou, S. (2017). Astragaloside IV protects against apoptosis in human degenerative chondrocytes through autophagy activation. Mol. Med. Rep. 16 (3), 3269–3275. doi:10.3892/mmr.2017.6980
Liu, N., Wang, W., Zhao, Z., Zhang, T., and Song, Y. (2014). Autophagy in human articular chondrocytes is cytoprotective following glucocorticoid stimulation. Mol. Med. Rep. 9 (6), 2166–2172. doi:10.3892/mmr.2014.2102
López de Figueroa, P., Lotz, M. K., Blanco, F. J., and Caramés, B. (2015). Autophagy activation and protection from mitochondrial dysfunction in human chondrocytes. Arthritis Rheumatol. 67 (4), 966–976. doi:10.1002/art.39025
Lou, G., Palikaras, K., Lautrup, S., Scheibye-Knudsen, M., Tavernarakis, N., and Fang, E. F. (2020). Mitophagy and neuroprotection. Trends Mol. Med. 26 (1), 8–20. doi:10.1016/j.molmed.2019.07.002
Man, G., Yang, H., Shen, K., Zhang, D., Zhang, J., Wu, H., et al. (2022). Circular RNA RHOT1 regulates miR-142-5p/CCND1 to participate in chondrocyte autophagy and proliferation in osteoarthritis. J. Immunol. Res. 2022, 4370873. doi:10.1155/2022/4370873
Matsuzaki, T., Alvarez-Garcia, O., Mokuda, S., Nagira, K., Olmer, M., Gamini, R., et al. (2018). FoxO transcription factors modulate autophagy and proteoglycan 4 in cartilage homeostasis and osteoarthritis. Sci. Transl. Med. 10 (428), eaan0746. doi:10.1126/scitranslmed.aan0746
Miyaki, S., and Asahara, H. (2012). Macro view of microRNA function in osteoarthritis. Nat. Rev. Rheumatol. 8 (9), 543–552. doi:10.1038/nrrheum.2012.128
Motta, F., Barone, E., Sica, A., and Selmi, C. (2022). Inflammaging and osteoarthritis. Clin. Rev. Allergy Immunol. doi:10.1007/s12016-022-08941-1
Nogueira-Recalde, U., Lorenzo-Gómez, I., Blanco, F. J., Loza, M. I., Grassi, D., Shirinsky, V., et al. (2019). Fibrates as drugs with senolytic and autophagic activity for osteoarthritis therapy. EBioMedicine 45, 588–605. doi:10.1016/j.ebiom.2019.06.049
Ouyang, J., Jiang, H., Fang, H., Cui, W., and Cai, D. (2017). Isoimperatorin ameliorates osteoarthritis by downregulating the mammalian target of rapamycin C1 signaling pathway. Mol. Med. Rep. 16 (6), 9636–9644. doi:10.3892/mmr.2017.7777
Pal, B., Endisha, H., Zhang, Y., and Kapoor, M. (2015). mTOR: a potential therapeutic target in osteoarthritis? Drugs R. D. 15 (1), 27–36. doi:10.1007/s40268-015-0082-z
Park, K. W., Lee, K. M., Yoon, D. S., Park, K. H., Choi, W. J., Lee, J. W., et al. (2016). Inhibition of microRNA-449a prevents IL-1β-induced cartilage destruction via SIRT1. Osteoarthr. Cartil. 24 (12), 2153–2161. doi:10.1016/j.joca.2016.07.002
Parzych, K. R., and Klionsky, D. J. (2014). An overview of autophagy: Morphology, mechanism, and regulation. Antioxid. Redox Signal. 20 (3), 460–473. doi:10.1089/ars.2013.5371
Rahmati, M., Nalesso, G., Mobasheri, A., and Mozafari, M. (2017). Aging and osteoarthritis: Central role of the extracellular matrix. Ageing Res. Rev. 40, 20–30. doi:10.1016/j.arr.2017.07.004
Sasaki, H., Takayama, K., Matsushita, T., Ishida, K., Kubo, S., Matsumoto, T., et al. (2012). Autophagy modulates osteoarthritis-related gene expression in human chondrocytes. Arthritis Rheum. 64 (6), 1920–1928. doi:10.1002/art.34323
Shi, M., Sun, M., Wang, C., Shen, Y., Wang, Y., and Yan, S. (2022). Therapeutic potential of POU3F3, a novel long non-coding RNA, alleviates the pathogenesis of osteoarthritis by regulating the miR-29a- 3p/FOXO3 Axis. Curr. Gene Ther. 22 (5), 427–438. doi:10.2174/1566523222666220309150722
Shin, H. J., Park, H., Shin, N., Kwon, H. H., Yin, Y., Hwang, J. A., et al. (2019). Pink1-Mediated chondrocytic mitophagy contributes to cartilage degeneration in osteoarthritis. J. Clin. Med. 8 (11), 1849. doi:10.3390/jcm8111849
Sui, C., Liu, D., Que, Y., Xu, S., and Hu, Y. (2021). Knockdown of hsa_circ_0037658 inhibits the progression of osteoarthritis via inducing autophagy. Hum. Cell 34 (1), 76–85. doi:10.1007/s13577-020-00440-9
Sun, K., Luo, J., Guo, J., Yao, X., Jing, X., and Guo, F. (2020). The PI3K/AKT/mTOR signaling pathway in osteoarthritis: A narrative review. Osteoarthr. Cartil. 28 (4), 400–409. doi:10.1016/j.joca.2020.02.027
Takayama, K., Kawakami, Y., Kobayashi, M., Greco, N., Cummins, J. H., Matsushita, T., et al. (2014). Local intra-articular injection of rapamycin delays articular cartilage degeneration in a murine model of osteoarthritis. Arthritis Res. Ther. 16 (6), 482. doi:10.1186/s13075-014-0482-4
Tang, Q., Zheng, G., Feng, Z., Chen, Y., Lou, Y., Wang, C., et al. (2017). Trehalose ameliorates oxidative stress-mediated mitochondrial dysfunction and ER stress via selective autophagy stimulation and autophagic flux restoration in osteoarthritis development. Cell Death Dis. 8 (10), e3081. doi:10.1038/cddis.2017.453
Terman, A., Kurz, T., Navratil, M., Arriaga, E. A., and Brunk, U. T. (2010). Mitochondrial turnover and aging of long-lived postmitotic cells: The mitochondrial-lysosomal axis theory of aging. Antioxid. Redox Signal. 12 (4), 503–535. doi:10.1089/ars.2009.2598
Vasheghani, F., Zhang, Y., Li, Y. H., Blati, M., Fahmi, H., Lussier, B., et al. (2015). PPARγ deficiency results in severe, accelerated osteoarthritis associated with aberrant mTOR signalling in the articular cartilage. Ann. Rheum. Dis. 74 (3), 569–578. doi:10.1136/annrheumdis-2014-205743
Vinatier, C., Domínguez, E., Guicheux, J., and Caramés, B. (2018). Role of the inflammation-autophagy-senescence integrative network in osteoarthritis. Front. Physiol. 9, 706. doi:10.3389/fphys.2018.00706
Vuppalapati, K. K., Bouderlique, T., Newton, P. T., Kaminskyy, V. O., Wehtje, H., Ohlsson, C., et al. (2015). Targeted deletion of autophagy genes Atg5 or Atg7 in the chondrocytes promotes caspase-dependent cell death and leads to mild growth retardation. J. Bone Min. Res. 30 (12), 2249–2261. doi:10.1002/jbmr.2575
Wang, C., Yang, Y., Zhang, Y., Liu, J., Yao, Z., and Zhang, C. (2019). Protective effects of metformin against osteoarthritis through upregulation of SIRT3-mediated PINK1/Parkin-dependent mitophagy in primary chondrocytes. Biosci. Trends 12 (6), 605–612. doi:10.5582/bst.2018.01263
Wang, C., Yao, Z., Zhang, Y., Yang, Y., Liu, J., Shi, Y., et al. (2020a). Metformin mitigates cartilage degradation by activating AMPK/SIRT1-Mediated autophagy in a mouse osteoarthritis model. Front. Pharmacol. 11, 1114. doi:10.3389/fphar.2020.01114
Wang, J., Li, J., Song, D., Ni, J., Ding, M., Huang, J., et al. (2020b). Ampk: Implications in osteoarthritis and therapeutic targets. Am. J. Transl. Res. 12 (12), 7670–7681.
Wen, C., Lin, L., Zou, R., Lin, F., and Liu, Y. (2022). Mesenchymal stem cell-derived exosome mediated long non-coding RNA KLF3-AS1 represses autophagy and apoptosis of chondrocytes in osteoarthritis. Cell Cycle 21 (3), 289–303. doi:10.1080/15384101.2021.2019411
Xiao, L., Ding, B., Gao, J., Yang, B., Wang, J., and Xu, H. (2020). Curcumin prevents tension-induced endplate cartilage degeneration by enhancing autophagy. Life Sci. 258, 118213. doi:10.1016/j.lfs.2020.118213
Xu, F., Hu, Q. F., Li, J., Shi, C. J., Luo, J. W., Tian, W. C., et al. (2022). SOX4-activated lncRNA MCM3AP-AS1 aggravates osteoarthritis progression by modulating miR-149-5p/Notch1 signaling. Cytokine 152, 155805. doi:10.1016/j.cyto.2022.155805
Xu, K., He, Y., Moqbel, S. A. A., Zhou, X., Wu, L., and Bao, J. (2021). SIRT3 ameliorates osteoarthritis via regulating chondrocyte autophagy and apoptosis through the PI3K/Akt/mTOR pathway. Int. J. Biol. Macromol. 175, 351–360. doi:10.1016/j.ijbiomac.2021.02.029
Yamashita, S. I., and Kanki, T. (2017). How autophagy eats large mitochondria: Autophagosome formation coupled with mitochondrial fragmentation. Autophagy 13 (5), 980–981. doi:10.1080/15548627.2017.1291113
Yan, S., Wang, M., Zhao, J., Zhang, H., Zhou, C., Jin, L., et al. (2016). MicroRNA-34a affects chondrocyte apoptosis and proliferation by targeting the SIRT1/p53 signaling pathway during the pathogenesis of osteoarthritis. Int. J. Mol. Med. 38 (1), 201–209. doi:10.3892/ijmm.2016.2618
Yang, Z., and Klionsky, D. J. (2010). Eaten alive: A history of macroautophagy. Nat. Cell Biol. 12 (9), 814–822. doi:10.1038/ncb0910-814
Zeng, J., Zhang, Z., Liao, Q., Lu, Q., Liu, J., Yuan, L., et al. (2021). CircPan3 promotes the ghrelin system and chondrocyte autophagy by sponging miR-667-5p during rat osteoarthritis pathogenesis. Front. Cell Dev. Biol. 9, 719898. doi:10.3389/fcell.2021.719898
Zhang, J., Cheng, F., Rong, G., Tang, Z., and Gui, B. (2020). Hsa_circ_0005567 activates autophagy and suppresses IL-1β-induced chondrocyte apoptosis by regulating miR-495. Front. Mol. Biosci. 7, 216. doi:10.3389/fmolb.2020.00216
Zhang, Y., Lu, R., Huang, X., Yin, E., Yang, Y., Yi, C., et al. (2022). Circular RNA MELK promotes chondrocyte apoptosis and inhibits autophagy in osteoarthritis by regulating MYD88/NF-κB signaling Axis through MicroRNA-497-5p. Contrast Media Mol. Imaging 2022, 7614497. doi:10.1155/2022/7614497
Zhang, Y., Vasheghani, F., Li, Y. H., Blati, M., Simeone, K., Fahmi, H., et al. (2015). Cartilage-specific deletion of mTOR upregulates autophagy and protects mice from osteoarthritis. Ann. Rheum. Dis. 74 (7), 1432–1440. doi:10.1136/annrheumdis-2013-204599
Zheng, G., Zhan, Y., Li, X., Pan, Z., Zheng, F., Zhang, Z., et al. (2018). TFEB, a potential therapeutic target for osteoarthritis via autophagy regulation. Cell Death Dis. 9 (9), 858. doi:10.1038/s41419-018-0909-y
Zhou, X., Li, J., Zhou, Y., Yang, Z., Yang, H., Li, D., et al. (2020). Down-regulated ciRS-7/up-regulated miR-7 axis aggravated cartilage degradation and autophagy defection by PI3K/AKT/mTOR activation mediated by IL-17A in osteoarthritis. Aging (Albany NY) 12 (20), 20163–20183. doi:10.18632/aging.103731
Keywords: autophagy, osteoarthritis, mechanism, therapeutic applications, preclinical and clinical research
Citation: Lv X, Zhao T, Dai Y, Shi M, Huang X, Wei Y, Shen J, Zhang X, Xie Z, Wang Q, Li Z and Qin D (2022) New insights into the interplay between autophagy and cartilage degeneration in osteoarthritis. Front. Cell Dev. Biol. 10:1089668. doi: 10.3389/fcell.2022.1089668
Received: 08 November 2022; Accepted: 28 November 2022;
Published: 05 December 2022.
Edited by:
Liu Yang, Fourth Military Medical University, ChinaReviewed by:
Dongsheng Guo, University of Massachusetts Medical School, United StatesCopyright © 2022 Lv, Zhao, Dai, Shi, Huang, Wei, Shen, Zhang, Xie, Wang, Li and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi Wang, d2FuZ3FpbmV0QDE2My5jb20=; Zhaofu Li, bHpmMDgxN0AxMjYuY29t; Dongdong Qin, cWluZG9uZzEwOEAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.