- 1Department of Laboratory Medicine, Shanghai Tongji Hospital, School of Medicine, Tongji University, Shanghai, China
- 2School of Clinical Medcine, Ningxia Medical University, Yinchuan, Ningxia, China
- 3Department of Laboratory Medicine, Suzhou Hospital of Integrated Traditional Chinese and Western Medicine, Suzhou, China
- 4Department of Laboratory Medicine, The Second People’s Hospital of Lianyungang & Department of Laboratory Medicine, The Oncology Hospitals of Lianyungang, Lianyungang, China
Exosomes are tiny vesicles produced by a wide range of cells that contain complex RNA and protein. In the diagnosis, treatment, and prevention of illness, they offer great potential. In vitro engineering technique modifies exosomes to produce designed exosomes that include nucleic acids, proteins, and medicines, and are targeted to particular tissues or cells. Their applications range from tumor imaging and gene therapy to vaccine production and regenerative medicine to targeted medication delivery. Many disciplines have promising futures for using this technology. In this review, we’ll look at the potential therapeutic usefulness and use of engineered exosomes in a variety of human illnesses with various systemic manifestations.
Introduction
Exosomes (Exos) are extracellular vesicles with a diameter of 30–100 nm that are released by a wide range of cells and contain a wide range of biological components such as nucleic acids, lipids, metabolites, and proteins (Pegtel and Gould, 2019). Exosomes originate from the endosomal pathway by the formation of the early sorting endosomes, late sorting endosomes, and ultimately multivesicular body (MVBs), which contain intraluminal vesicles. When MVBs fuse with the plasma membrane, exosomes are released (size range ∼40–160 nm) (Kalluri and LeBleu, 2020). Exos may be found in a wide range of human bodily fluids, including blood, urine, saliva, etc., and other secretions, as well as in immune and cancer cells (Adriano et al., 2021). Other biological functions of Exos include signal transduction, chemical transfer, and angiogenesis promotion. Although Exos come from a variety of sources and contain a wide range of different components, this heterogeneity means that Exos can play a variety of roles in the development of disease and in the diagnosis and treatment of disease (Kalluri and LeBleu, 2020).
Exos are extremely permeable to tissues, blood, and the blood-brain barrier (BBB) (Zheng et al., 2019). Exos from patients have a better histocompatibility and lower toxicity than other medication carriers (Quah and O' Neill, 2005). Exos produced from cells have undergone modifications to enhance their functionality and expand the range of possible therapeutic uses (Yeo et al., 2013). A popular approach is to employ genetic engineering technology to make recipient cells strongly express specific proteins or polypeptides, resulting in designed Exos that are then sent to the membrane surface or the cell cavity of the receiver, we define modified Exos as Engineered exosomes (Eexos) (Xu et al., 2021). Based on the molecular transport capacity and targeting characteristics of exosomes, researchers endow exosomes with cell and tissue targeting specificity by modifying exosomes surface molecules, so as to deliver exosomes loaded with protein/RNA/small molecule transporters to the corresponding disease regions or cells (Xu et al., 2021). Target and accumulate in particular cells or tissues by acquiring this substance’s targeting and accumulation capabilities (Xu et al., 2021).
Exosomes are multiple vesicles secreted by most cells, and these exosomes secreted from different cells have the same common components, and studies have shown that there is no mechanism to show that these common components change with the physiological state of cells (Sterzenbach et al., 2017). These exosomes for engineering exosomes can be extracted from a variety of sources, including immune and cancer cell lines, blood, tears, urine, saliva, milk, ascites, eukaryotes, prokaryotes, herbs, etc (Xu et al., 2021).
Exos that have been modified may be loaded with miRNA, siRNA, DNA, medicinal medicines, and other substances and directed to particular tissues or cells to exercise their effects (Ortega et al., 2020). Studies have shown that loading substances into exosomes can be done in a variety of ways, such as fusion, electroporation, ultrasound, co-incubation, and so on. For example, the selected drug is suspended with the exosome under the condition that the drug enters the exosome through the hole generated by the electric field, and an electric field is applied (Gilligan and Dwyer, 2017). The exosome concentration range of each electroperforation is 0.07 - 0.5 μg/μL, which has been shown to be successful uptake at different voltages (Gilligan and Dwyer, 2017). Secondly, engineered exosomes can also be prepared by co-incubating the drug with the exosome if the drug size can pass through the exosome membrane (Gilligan and Dwyer, 2017). Engineered exosomes can also be modified using chemical methods, such as lipidization (Gilligan and Dwyer, 2017). Modified engineered exosomes can be injected intravenously, nasally or intraperitoneally (Nojehdehi et al., 2018; Ortega et al., 2020; Xu et al., 2021).
In addition to the ease with which Exos’ membrane structure may be labeled, a number of engineering methods have been utilized to alter Exos to improve their targeting capabilities, allowing modified Exos to be more useful for illness detection and therapy (Xu et al., 2021). Modified engineered exosomes have been widely used in gene therapy (Bai et al., 2020), targeted drug delivery (Kim et al., 2021), vaccine preparation (Ferrantelli et al., 2018; Di Bonito et al., 2019; Du et al., 2021), regenerative medicine (Liu et al., 2015; Li et al., 2018; Yang et al., 2021), tumor imaging (Srivastava et al., 2018; Chen et al., 2021) and other fields, mainly playing a role of targeted delivery of contents. In this review, we will discuss the clinical use of modified Exos in illnesses affecting many systems in the human body and their potential application to develop novel therapeutic methods for disease detection and treatment.
Respiratory system
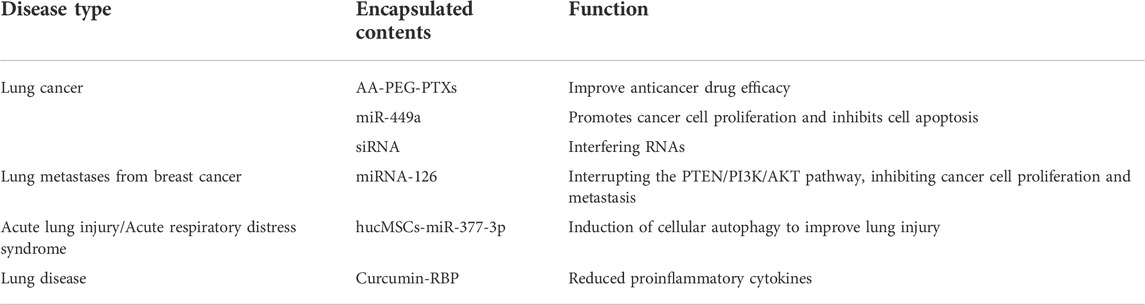
It is possible to use eexos to deliver a wide range of therapeutics, including as miRNA, siRNA, medicines, and more, to patients with a variety of respiratory disorders (Table 1). A great number of experimental research are required to demonstrate Eexos availability and value since most studies have demonstrated that Eexos is largely employed in clinical therapy, particularly cancer. Many discoveries are still in the laboratory stage.
PTX-Exo can be used to treat mice lung metastases and the addition of aminoethyl anisamide-polyethylene glycol (AA-PEG) to PTX-Exo produced AA-PEG-PTX-exo, which further improved lung cancer therapy (Kim et al., 2018; Bai et al., 2020; Zhou et al., 2021). Non-small cell lung cancer mice were treated with Eexos-miR-449a, a potent exosome with high targeting and delivery efficiency, which suppressed cancer cell proliferation and induced apoptosis to slow tumor growth in the animals (Zhou et al., 2021). It has been shown that a new tLyp-1 exosome may carry siRNA to cancer cells such as non small-cell lung cancer (NSCLC), functioning as a gene silencing therapy (Bai et al., 2020).
Lung metastases are a promising application for Eexos in addition to its involvement in original lung malignancies. Integrin four on the exosome surface recognizes and internalizes breast cancer (MDA-MB-223) Exos, and lung metastasis occurs. However, Exos loaded with miRNA-126 block the cancer cell metastasis-related pathway PTEN/PI3K/AKT and inhibit cancer cell proliferation and metastasis, effectively inhibiting the formation of lung metastasis in a mouse model (Nie et al., 2020; Wei et al., 2020; Kim et al., 2021).
There are two major types of acute lung injury and respiratory failure: acute respiratory distress syndrome (ARDS) and acute lung injury (ALI). One study discovered a novel way to treat lung illness by releasing miR-377-3p from human umbilical cord blood-derived mesenchymal stem cells (hucMSCs) and inducing cell autophagy, which reduced lipopolysaccharide-induced acute lung damage (Wei et al., 2020). To deliver the anti-inflammatory peptide-RBP to the Exos, it bound to exosomal membrane integrins and formed an RBP-Exos complex with anti-inflammatory properties. Curcumin loading into RBP-Exo (Cur-RBP-Exos) enhances curcumin transport efficiency and enhances curcumin’s anti-inflammatory activity. When given to mice with acute lung damage, RBP-Exo supplemented with curcumin decreased the production of pro-inflammatory cytokines and controlled the inflammatory response more efficiently (Kim et al., 2021).
Lung cancer is the cancer with the highest mortality rate in the world. Eexos have been used in the treatment of lung cancer by loading drugs, miRNA, siRNA, etc., to enhance the therapeutic effect. In addition, engineered exosomes have been investigated for the treatment of other respiratory diseases. Modified engineered exosomes play a therapeutic role by inhibiting the growth and proliferation of tumor cells, regulating tumor-related signaling pathways, regulating immune cells, and targeting drug delivery.
Digestive system
Because of the digestive system’s complexity and the wide range of disorders it might affect, Eexos has continuously improved its therapy capabilities for those conditions (Table 2). Diagnosis and therapy of digestive system malignancies in complicated systems benefit greatly from using Eexos’ highly focused ability to precisely detect the lesion location and prevent harm to normal tissues.
Anti-cancer medicine 5-fluorouracil (5-FU) and miR-21 inhibitor oligonucleotide (miR-21i) were loaded into Exos (5-FU/miR-21i-Exos) from colorectal cancer cells HCT-116 and then delivered to the cells via Exos (Liang et al., 2020a). According to the findings, the Exos effectively promoted cancer cell uptake of the anti-cancer drug 5-FU and miR-21i, and significantly reduced the expression of miR-21, which mediates drug resistance, and enhanced the cytotoxicity of 5-FU for cancer cells. This suggests Eexos offers a therapeutic approach for drug resistance in cancer cells (Liang et al., 2020a). Cancer cells that have acquired oxaliplatin resistance can no longer be treated with oxaliplatin. Studies have shown that Exos encapsulating oxaliplatin and PGM5 antisense RNA (PGM5-AS1) (oxaliplatin/PGM5-AS1-Exos) can reverse cancer cell resistance, increasing the effectiveness of oxaliplatin and effectively inhibiting cancer cell metastasis (Hui et al., 2021). Oxaliplatin resistance is common in metastatic cases of colorectal cancer.
Dendritic cells activated by tumor lysate have been shown to stimulate immune responses in mice, slow tumor growth, and aid in the treatment of hepatocellular carcinoma. This study found that human HepG2 cell-derived Exos stimulated immune responses in mice, which improved the tumor microenvironment, resulting in specific lysis of hepatitis C tumor cells. Delivery of Erastin Er and Rose Bengal RB, two drugs that cause iron death in tumor cells, to the liver is an efficient way to kill liver cells (Rao et al., 2016). When Erastin and Rose Benga were loaded into Exos using ultrasound, it formed Er/RB-ExoCD47, which had CD47 molecules on the exosome surface. This allowed the Exos to avoid being phagocytosed by the mononuclear phagocytic system. When mice are injected with drug-laden Exos, only hepatocellular carcinoma cells die, while no other organs are harmed (Du et al., 2021).
One of the most important risk factors for pancreatic cancer is a GTPase KRAS mutation. Short interfering Eexos designed to target mutant sites in pancreatic cancer in normal fibroblast-like mesenchymal cells dramatically inhibited pancreatic cancer in mice, enhanced survival and offered a novel strategy to target mutated sites for pancreatic cancer therapy in mice (Aspe et al., 2014; Anderson and Morrow, 2017; Kase et al., 2021). Tetracycline-modified and loaded Exos from human melanoma cells were used to study the effects of an apoptosis inhibitor protein dominant inactivating mutant on pancreatic cancer cell growth in mice. This mutant, Survivin-T34A, was found to significantly increase apoptosis in the pancreatic cancer cell line, suggesting that it could be used to treat pancreatic cancer (Aspe et al., 2014). Through the use of an electroporation approach, Exos that are specifically targeting oral squamous cell carcinoma cells may be loaded with siLCP1 (siLCP1-Exos) and delivered to the cancer cells to inhibit them (Kase et al., 2021).
The incidence and mortality rates of liver cancer, colorectal cancer and gastric cancer in the digestive system are among the first few. Targeted delivery of therapeutic drugs and siRNA by Eexos can regulate the occurrence and development of tumors, immune microenvironment and tumor drug resistance, which provides a new idea for the treatment of digestive system cancers.
Cardiovascular system
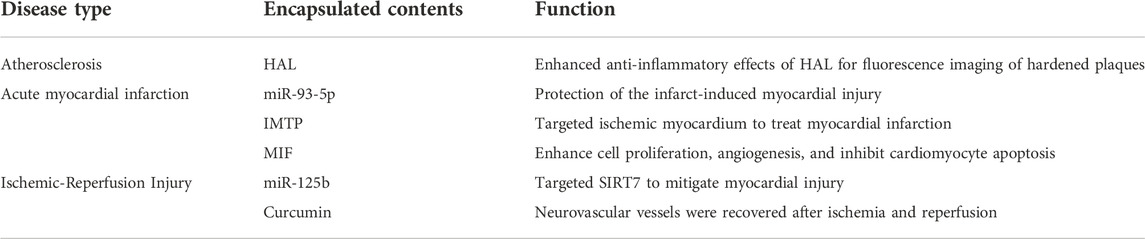
Cardiovascular illnesses continue to be among the world’s deadliest and most debilitating conditions, and Eexos therapy may help protect the heart, lessen the severity of the disease, and lessen the consequences it causes (Table 3).
Heart disease is caused by atherosclerosis, which may be effectively treated by decreasing inflammation. A research has shown anti-inflammatory properties in Exos generated from macrophage M2, including the ability to bind anti-inflammatory substances released by M2. It was discovered that electroporating HAL (hexyl 5-aminolevulinic acid hydrochloride) into the M2-derived Exos (HAL-Exos) improved the anti-inflammatory effects and reduced atherosclerosis by using HLA’s potential to create anti-inflammatory carbon monoxide and bilirubin. Another way in which HLA contributes to heme production is via protoporphyrin IX (PpI IX), an intermediate metabolite that allows for fluorescence imaging of atherosclerosis (Wu et al., 2020).
It is critical to recognize and treat acute myocardial infarction early in order to prevent mortality and morbidity (Anderson and Morrow, 2017). It is a frequent cardiac emergency. Studies have revealed that ADSCs secreting miR-93-5p-loaded Exos (miR-93-5p-Exos) from adipose-derived stromal cells protect against infarct-induced cardiac damage more effectively (Liu et al., 2018). After a heart attack, the use of Exos fused to the ischemia cardiac targeting protein (IMTP) has been shown to have a therapeutic impact on heart attack victims with an acute myocardial infarction (Wang et al., 2018). It was found that loading the macrophage migration inhibitory factor (MIF) into human umbilical cord blood MSC-derived Exos, known as MIF-Exo, increased proliferation and angiogenesis in the human umbilical vein endothelial cells (HUVEC) and inhibited apoptosis, showing good cardioprotective effects in an acute myocardial infarction rat model (Zhu et al., 2021).
Exos generated from bone marrow mesenchymal cells containing miR-125b (miR125b-Exos) have been shown in a rat model to reduce cardiac tissue damage by focusing on SIRT7 and exerting cardioprotective effects. In the case of ischemia-reperfusion damage, the complex generated by mixing embryonic stem cell Exos with curcumin may aid neurovascular repair by achieving effective therapeutic effects (Kalani et al., 2016).
To sum up, Eexos have been used to treat common cardiovascular diseases such as atherosclerosis, acute myocardial infarction and ischemia-reperfusion injury by regulating inflammatory response and immune cells to target the delivery of drugs (such as curcumin).
Genitourinary system
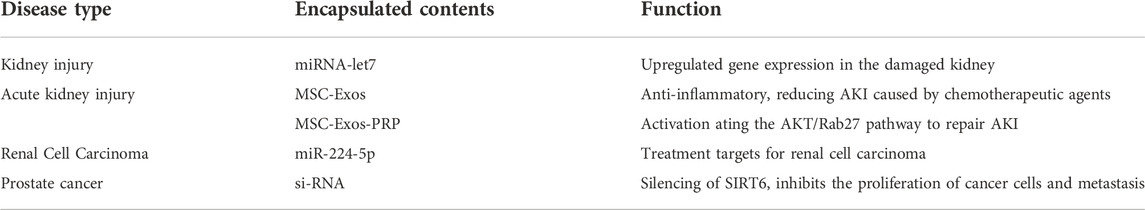
The whole urinary system may be affected by diseases of the kidneys, bladder, prostate, and other organs. Eexos containing therapeutic medications are increasingly being used to treat illnesses effectively (Table 4).
MiRNAs may be used to treat a damaged kidney. Gene expression may be regulated selectively by MSCs overexpressing miRNA-let7, homing to the injured kidney to upregulate. This procedure is mostly carried out in mice by the MSCs secreting Exos (miRNA-let7-Exos), which act on the recipient cells. To repair damaged kidneys, researchers are now using modified MSC-secreted Exos that carry miRNAs to specific locations in the body (Wang et al., 2016).
It is typical for hospitalized patients to suffer from acute kidney injury. MSCs are regarded an excellent treatment for tissue damage, and Exos generated from MSCs may be used to treat acute kidney injury. Researchers found that MSC-Exos were more efficiently taken up by tubular epithelial cells in a mouse model of acute kidney injury and showed efficient anti-inflammatory capacity, showing great potential in the treatment of acute kidney injury caused by chemotherapeutic agents using a three-dimensional culture system with a hollow fiber bioreactor (Cao et al., 2020). Serum urea nitrogen and creatinine levels may be dramatically reduced by using PRP-MSCs, as well as the histopathological kidney damage reversed. Exos produced from adipose tissue-derived MSCs rescue mice from acute renal damage (Gao et al., 2020a).
Cancer-associated fibroblasts (CAF) in the cancer stroma release Exos that may impact tumor growth in clear cell renal cell carcinoma. Exos from CAF were shown to transport miR-224-5p (miR-224-5p-Exos) into renal cell carcinoma cells, increasing the course of the disease. This suggests that miR-224-5p might be a therapeutic target for renal cell carcinoma (Liu et al., 2021). In another work, Exos loaded with siRNA silenced SIRT6 in vitro and in vivo, resulting in SIRT6 deletion and a substantial reduction in prostate cancer cell growth and metastasis in mice (Han et al., 2021).
Gynecology
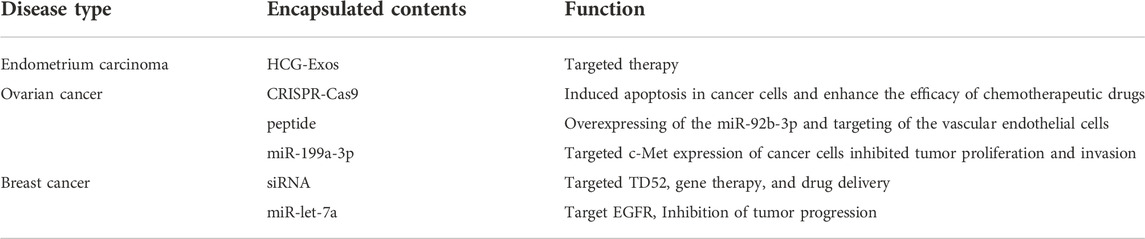
Gynecological disorders, particularly cancers of the uterus and fallopian tubes, have grown to be a significant threat to women’s health. Cancer cell proliferation and invasion may be inhibited by using Exos, which regulate hormone secretion in the body, regulate the tumor microenvironment, and regulate hormone secretion via the gynecological system (Table 5).
There are studies showing that Human Chorionic Gonadotropin (HCG)-carrying Exos can be delivered into the endometrium to increase endometrial tolerance by loading HCG into the endoscopic Exos through freeze-thaw cycles and ultrasound treatment, and that endoscopic Exos can also carry endometrial therapeutic agents to achieve targeted endometrial treatment (Hajipour et al., 2021).
To trigger apoptosis in ovarian cancer cells and increase the susceptibility of ovarian cancer cells to cisplatin-based chemotherapeutic drugs, cancer cell-derived Exos may convey the CRISPR-Cas9 system to SKOV3 cells (Kim et al., 2017). Exos derived from ovarian cancer cells increased the angiogenic and migratory capacity of vascular endothelial cells in an unrelated mouse model, which promoted tumor-associated angiogenesis. This showed the potential of artificially generated Exos overexpressing miR-92b-3p as anti-angiogenic agents, which may provide a new approach for the treatment of ovarian cancer that is anti-angiogenic. Using Exos and peptides to create miR-92b-3p overexpressed peptide-engineered Exos (miR-92b-3p-Exos) that target vascular endothelial cells to decrease angiogenesis and tumor development To test whether Eexos may be utilized for targeted treatment, researchers employed Exos loaded with miR-199a-3p to decrease tumor growth and invasion in a mouse model of ovarian cancer. (Kobayashi et al., 2020; Wang et al., 2021).
Exos loaded with siRNAs targeting TD52 are delivered to HER2-positive breast cancer cells to modulate the expression of the TD52 gene for gene therapy and medication delivery (Limoni et al., 2019). Breast cancer is now the most common cancer in women. Exos may carry miRNAs in the form of miR-Exos, which are loaded into cells. Intravenous administration of miR-let-7a-loaded Exos to mice had inhibitory effects on tumor development in the presence of breast cancer cells expressing the epidermal growth factor receptor (EGFR) (Ohno et al., 2013).
Women ovarian cancer, breast cancer, the male urogenital system such as prostate cancer, kidney cancer incidence continues to increase, research shows that Eexos shows great potential in cancer treatment, development by regulating tumor cells, and the ingredients in the tumor microenvironment, compared to the pure drug therapy, further enhance the therapeutic effect.
Nervous system
The central nervous system (CNS), which includes the brain and spinal cord, is the body’s top organ for regulating and controlling numerous functional processes. The peripheral nervous system connects it to other bodily systems and organs. Because many medications cannot cross the blood-brain barrier to treat CNS diseases, therapy options are restricted (Srikanth and Kessler, 2012). Many biological barriers may be crossed by Exos, making drug-loaded Eexos a promising therapy option for CNS diseases, according to research (Srikanth and Kessler, 2012) (Table 6).
Medications may have a hard time getting into the brain because of the blood-brain barrier. Anticancer medicines delivered through brain endothelium-derived Exos decreased cancer cell fluorescence and tumor growth indicators considerably, demonstrating that Exos may transfer pharmaceuticals across the blood-brain barrier and display beneficial therapeutic effects as a drug delivery mechanism (Yang et al., 2015). The blood-brain barrier may be repaired using endothelial colony-forming cells (ECFC) obtained from human umbilical cord blood. There is evidence to suggest that tissue-resident endothelial cells (EC) produced from the ECFC increased proliferation and migration of this kind of EC in mice’s brain, therefore repairing the damaged blood-brain barrier. Certain origin Exos have been shown to pass the blood-brain barrier and have favorable impacts on the blood-brain barrier’s overall integrity (Gao et al., 2018).
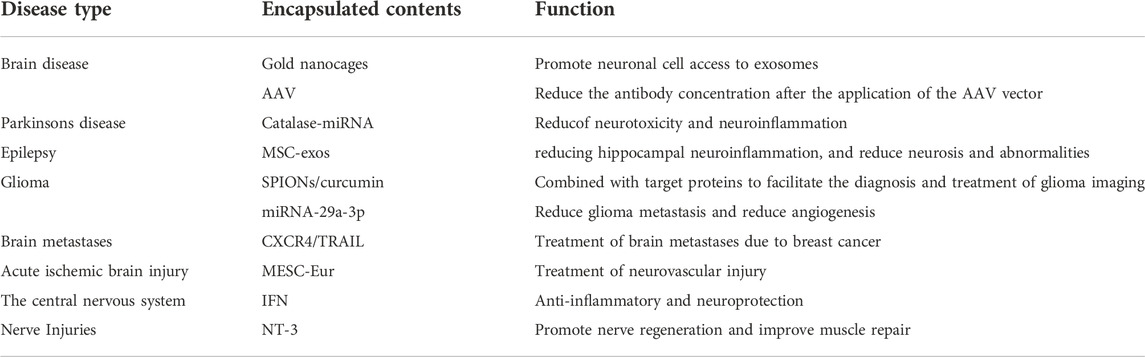
Using gold nanoparticles, researchers loaded markers into bone marrow mesenchymal cell-derived Exos (MSC-exos) and tracked the distribution of these Eexos in mice with various neurological disorders such as Parkinson’s, Alzheimer’s, and stroke using conventional X-ray computed tomography CT techniques. The results showed that MSC-Exos can be acquired by neuronal cells in disease areas to track the severity of the condition and can be used for targeted drug (Perets et al., 2019). Toxicities of adeno-associated virus (AAV) can be reduced by combining Exos with AAV, and this combination can also slow the rapid clearance of AAV vectors. Moreover, expression of brain-targeting peptides on the surface of Exos can improve transduction, and this can help reduce the amount of anti-AAV antibodies produced following AAV administration (György et al., 2014).
Neuroinflammation has been linked to Parkinson’s disease, which is a degenerative condition. To minimize neurotoxicity and inflammation, Exos loaded with catalase mRNA may be delivered to the brain (Kojima et al., 2018). Using MSC-exo has been shown to have anti-inflammatory effects, and after 6 h of intranasal injection of MSC-exo into epileptic mice, it was found that Exos reached the hippocampus, where they reduced inflammation and prevented neurodegeneration and abnormalities from arising in the hippocampal region (Long et al., 2017).
Treatment for glioma is complicated because the blood-brain barrier prevents therapeutic drugs from entering the brain. Loading SPIONs and curcumin into Exos (SPIONS/Cur-Exos), which can easily cross the blood-brain barrier and bind Exos to target proteins chemically, offers a new approach to the diagnosis and treatment of glioma (Jia et al., 2018). Exos overexpressing mesenchymal stem cell miRNA-29a-3p can diminish glioma metastasis and angiogenesis by inhibiting their migration and angiogenesis in vitro (Zhang et al., 2021).
A new therapeutic strategy for treating brain metastases caused by breast cancer may be formed by using genetic engineering to prepare CXCR4/TRAIL Exos, which can selectively induce apoptosis in cancer-transformed cells. These Exos can cross the blood-brain barrier (BBB) and can be used to treat brain metastases (Liu et al., 2020). Acute ischemic brain damage activates vascular endothelial cells, which may then stimulate neural progenitor cell proliferation and migration. Vascular endothelial cells (VEC) Exos stimulate proliferation and migration of neural progenitor cells and decrease apoptosis, indicating that Exos are critical for neuronal reconstruction and brain protection in acute ischemia damage (Gao et al., 2020b; Zhou et al., 2020). When embryonic stem cells loaded with curcumin were used to treat cerebral ischemia-induced neurovascular loss, it was shown that this MESC-exo helped in the regeneration of the brain’s blood vessels (Kalani et al., 2016). Researchers put Neurotrophin-3(NT-3) miRNA into adipose stem cell ADCS-derived Exos, which were then loaded into nerve-guided catheters in rats for the repair of neurological abnormalities. NT-3 is a chemical that contributes to peripheral nerve regeneration. AdCS-exo enhanced nerve regeneration and improved muscular recovery in rats, according to the findings (Yang et al., 2021).
Bone and joint system
Various complicated motor functions in the human body include the bone and joint system, which has a crucial role to play in preserving the body’s structure and function. Bone and joint illnesses, as well as bone and joint restoration, may benefit from the use of exos, which contain medications or gene expression modulators (Table 7).
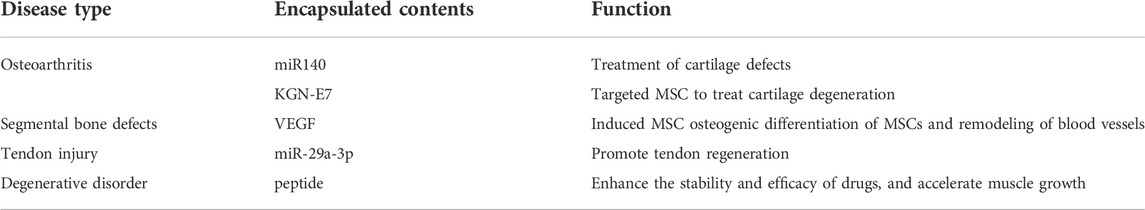
MiRNAs that target chondrocytes in Exos may be utilized to treat cartilage damage using eexos produced from these miRNAs. Exos expressing the cartilage-affinity peptide CAP on their surface may be used to convey miR-140-loaded Exos to deep cartilage areas, according to research. Interarticular injection of CAPExoMiR140 can be used to treat cartilage abnormalities than unmodified Exos, thereby slowing osteoarthritis development, and might be employed as an innovative cell-free therapy (Liang et al., 2020b). Studies have shown that MiR-214 regulates osteoblast function by targeting specific molecular pathways and the expression of various osteoblast-related genes, while promoting osteoclast activity by targeting phosphatase and tensin homolog (Pten) (Sun et al., 2018). The exosomal miRNA paracrine mechanism is used to mediate osteoclast-osteoblast intercellular crosstalk, and osteoclast-derived exosomal miR-214 can be transferred to osteoblasts to inhibit bone formation, which can be used to treat bone diseases with reduced bone formation (Li et al., 2016; Zhu et al., 2018).
Osteoarthritis cartilage degradation may be treated by mesenchymal stem cells from synovial fluid (SF-MSC). To induce SF-MSC differentiation into chondrocytes, researchers used Kartogenin (KGN). KGN bound to E7-Exo, a peptide that targets MSC, forming Exos that target MSC and delivering it to the surface of SF-MSC to promote differentiation. This produced more significant therapeutic effects and made KGN an advanced stem cell therapy (Kim et al., 2017). When ATDC5-derived Exos are used to make Eexos, they may be used to stimulate osteogenesis and vascular regeneration in MSCs. These Exos can release VEGF genes, which can then be used to remodel the vascular system and aid in vascularized bone repair (Zha et al., 2021). Treatment of tendon injuries using exosomal HUMSC-Exo from human umbilical cord blood MSCs has been shown to be more successful, and genetically editing HUMSC-Exo overexpressing miR-29a-3p has also suggested that Eexos may be employed (Yao et al., 2021). Functional dystrophin deficiency causes muscle atrophy in people with Duchenne muscular dystrophy (DMD). The serum stability and efficiency of the pre-muscle growth inhibitor peptide are much improved by attaching it to the surface of Exos. Exos attached with myostatin pro-peptide given to mice on a regular basis promote muscle development, offering a novel medication delivery mechanism for the treatment of DMD (Ran et al., 2020).
Eexos also have great application potential in regenerative medicine, such as inducing osteogenic differentiation and vascular remodeling of bone marrow mesenchymal stem cells, promoting tendon regeneration and so on, which broaden new ideas for the treatment of bone and joint diseases.
Immune system
Multiple body systems are affected when the immune system is weakened because of the immune system’s role in immunological surveillance, defense, and regulatory actions. It's possible that eexos-mediated immune system regeneration might be a novel way to boost treatment’s effectiveness (Table 8).
In the case of rheumatoid arthritis (RA), MSC-Exos was investigated as a potential anti-rheumatic drug. When Exos derived from DS-modified MSCs are administered to inflamed areas of RA, they reduce inflammation and block the activity of pro-inflammatory cells, leading in a novel cell-free therapeutic method for the treatment of RA. RA may be lessened by intra-articular autotolerant dendritic cells (tolDC) (Bell et al., 2017). It is possible that ROS, as a physiological stimulus in the RA microenvironment, can alleviate the severity of the disease and represent a new therapeutic approach by inducing the T-cell differentiation center (tlDC) to release Exos that reduce pro-inflammatory factor secretion (Lee et al., 2021). Human immunodeficiency virus (HIV) Research shows that HIV causes immune system abnormalities and a zinc-lipid protein (ZEP-362) targets the HIV-1 promoter, reduces HIV epigenetic expression and limits viral infection by constructing Exos that encode a zinc-lipid protein for HIV-1 and encapsulate it to decrease viral expression (Kalani et al., 2016).
Endocrine system
Multiple substances interact to exert regulatory effects on the endocrine system’s functions (Table 9). There are substantial health consequences to untreated diabetes mellitus, which is an endocrine system disorder characterized by inadequate insulin secretion or activity.
In diabetic individuals, LncRNA-H19 levels were shown to be considerably lower, and this may have an effect on angiogenesis. Diabetic wounds are a typical consequence of diabetes. Therapy delivery of LncRNA-H19-exo dramatically accelerated wound healing, indicating that LncRNA-H19-exo has the potential to counteract hyperglycemia and limit vascular regeneration, giving an efficient therapeutic impact on diabetic wounds (Tao et al., 2018). These mesenchymal stem cells have immunomodulatory effects on T-cell inflammation and may enhance autoimmune responses when administered as Exos. T1DM in a mouse model has been demonstrated to be improved by boosting regulatory T cells and their products without modifying the lymphocyte proliferation index, which makes AD-MSC-exo a more effective and realistic technique. AD-MSC-exo has been proven to improve autoimmune T1DM (Nojehdehi et al., 2018). A common complication of diabetes is diabetic peripheral neuropathy, and studies using Eexos loaded with miR-146a and injected into mice found that miR-146a-exo significantly inhibited the activation of peripheral blood nucleated cells by inhibiting the TLR4/NF-B signaling pathway, helping to relieve peripheral neurovascular dysfunction and improve functional recovery (Fan et al., 2021).
Hematological system
Eexos may be used to deliver therapeutic medications that block the development of a class of illnesses characterized by anomalies in the blood and hematopoietic organs, therefore postponing disease progression and introducing a novel therapeutic method into clinical practice (Table 10).
Chronic myeloid leukemia (CML) parent cells overexpress IL-3 receptors in chronic myeloid leukemia CML. We fused IL-3 with Exos containing chemotherapeutic agents or BCR-ABL-siRNA and targeted the fusion Exos to CML mother cells in order to more effectively limit cancer cell proliferation and give improved therapeutic effects (Bellavia et al., 2017). By encapsulating GP350 in Exos, this Eexos targets malignant B-blast cells in patients with B chronic lymphocytic leukemia, conferring on malignant B-blast cells the immunogenicity of EBV, stimulating EBV-specific T cells to generate an immune response, and thereby providing a novel immunotherapeutic approach for B- Chronic lymphocytic leukemia (B-CLL) (Ruiss et al., 2011).
Compared with other systemic diseases, diseases of the immune system, endocrine system and blood system require less traumatic treatment methods such as drug regulation, molecular therapy and cell therapy. Exos can be loaded with corresponding drugs and molecules to enhance their targeted delivery, so as to enhance therapeutic effect and reduce side effects.
Others
HPV16-E7 is a human papillomavirus (HPV) tumor-associated antigen that activates cytotoxic T-lymphocytes (CTLs). Endogenous Eexos is created by coupling Exos with HPV16-E7 and administering it intramuscularly to produce an efficient anti-HPV16-E7 immune response. This suggests that Eexos may be exploited for vaccine development (Di Bonito et al., 2019) (Table 11).
Conclusion
There are several methods to employ Exos in the human body, including illness detection, vector transfer, disease therapy, and liquid biopsy. Exos are found in a wide range of cells and bodily fluids and have become a major source of disease biomarkers. Biochemical engineering, physical engineering and other engineering technologies can process and modify Exos to load miRNA, siRNA, proteins and other substances for tumor imaging, gene therapy, targeted drug delivery and vaccine preparation as well as non-cytotoxicity, low immunogenicity and other fields (Figure 1). It's becoming a popular method for both diagnosing and treating illness. Eexos, a cell-free therapy for cancer, has shown remarkable promise and usefulness in various studies, especially in the area of cancer. The human body is a complex system, and Exos play a part in many of these systems, highlighting the relevance of Exos and the necessity to continue our research in specific areas like the genesis and function of Exos in order to better understand this important cellular component.
Despite the fact that Eexos has a lot of promise in a wide range of disciplines, there are still numerous obstacles to overcome. Since stable Exos are critical to the production of Eexos, the extraction and purification of Exos remain a difficult problem to solve for the time being. Another problem is that Eexos is still in the experimental stage, which means further clinical trials are required to demonstrate its efficacy in human applications. To sum it up, if Eexos is to be utilized in the clinic, it will need to be studied further in terms of cost and affordability, as well as how the Exos are supplied, their half-life in vivo, and their negative effects when combined with other medications.
However challenging it may be, Eexos has a lot of potential in the field of illness therapy. With the benefits that Exos have over other nanocarriers, further research into Eexos for clinical applications is needed to improve their clinical relevance and usefulness in the future. Exosome loading efficiency, such as lowering drug loss, enhancing usage, and reducing contamination during reproduction, may still be improved. However, in clinical applications, undesirable effects or side effects of Exos must also be taken into account. All things considered, Eexos has tremendous promise for use in the diagnosis and treatment of illness, as well as in future clinical trials.
Author contributions
Writing—Original Draft: GL and AS; Figure design: JW; Data collection: JW and GC; Searching: AS; Review & Editing: All authors. GL and JW contributed equally to this work. All authors read and approved the final manuscript.
Funding
This work was supported by the Shanghai Yiyuan Rising Star Young Medical Talents Training Grant Program (2018-JY), the Postdoctoral Science Foundation of China (2020M681399);The Medical Research Project of Jiangsu Provincial Health and Health Commission (2019179).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adriano, B., Cotto, N. M., Chauhan, N., Jaggi, M., Chauhan, S. C., and Yallapu, M. M. (2021). Milk exosomes: Nature's abundant nanoplatform for theranostic applications. Bioact. Mat. 6 (8), 2479–2490. doi:10.1016/j.bioactmat.2021.01.009
Anderson, J. L., and Morrow, D. A. (2017). Acute myocardial infarction. N. Engl. J. Med. 376 (21), 2053–2064. doi:10.1056/NEJMra1606915
Aspe, J. R., Diaz Osterman, C. J., Jutzy, J. M., Deshields, S., Whang, S., and Wall, N. R. (2014). Enhancement of Gemcitabine sensitivity in pancreatic adenocarcinoma by novel exosome-mediated delivery of the Survivin-T34A mutant. J. Extracell. Vesicles 3, 23244. doi:10.3402/jev.v3.23244
Bai, J., Duan, J., Liu, R., Du, Y., Luo, Q., Cui, Y., et al. (2020). Engineered targeting tLyp-1 exosomes as gene therapy vectors for efficient delivery of siRNA into lung cancer cells. Asian J. Pharm. Sci. 15 (4), 461–471. doi:10.1016/j.ajps.2019.04.002
Bell, G. M., Anderson, A. E., Diboll, J., Reece, R., EltheringtOn, O., Harry, R. A., et al. (2017). Autologous tolerogenic dendritic cells for rheumatoid and inflammatory arthritis. Ann. Rheum. Dis. 76 (1), 227–234. doi:10.1136/annrheumdis-2015-208456
Bellavia, D., Raimondo, S., Calabrese, G., Forte, S., Cristaldi, M., Patinella, A., et al. (2017). Interleukin 3- receptor targeted exosomes inhibit in vitro and in vivo Chronic Myelogenous Leukemia cell growth. Theranostics 7 (5), 1333–1345. doi:10.7150/thno.17092
Cao, J., Wang, B., Tang, T., Lv, L., Ding, Z., Li, Z., et al. (2020). Three-dimensional culture of MSCs produces exosomes with improved yield and enhanced therapeutic efficacy for cisplatin-induced acute kidney injury. Stem Cell Res. Ther. 11 (1), 206. doi:10.1186/s13287-020-01719-2
Chen, M. L., He, F., Yin, B. C., and Ye, B. C. (2021). Simultaneous imaging of cancer biomarkers in live cells based on DNA-engineered exosomes. Analyst 146 (5), 1626–1632. doi:10.1039/d0an02353a
Di Bonito, P., Accardi, L., Galati, L., Ferrantelli, F., and Federico, M. (2019). Anti-cancer vaccine for HPV-associated neoplasms: Focus on a therapeutic HPV vaccine based on a novel tumor antigen delivery method using endogenously engineered exosomes. Cancers (Basel) 11 (2), E138. doi:10.3390/cancers11020138
Du, J., Wan, Z., Wang, C., Lu, F., Wei, M., Wang, D., et al. (2021). Designer exosomes for targeted and efficient ferroptosis induction in cancer via chemo-photodynamic therapy. Theranostics 11 (17), 8185–8196. doi:10.7150/thno.59121
Fan, B., Chopp, M., Zhang, Z. G., and Liu, X. S. (2021). Treatment of diabetic peripheral neuropathy with engineered mesenchymal stromal cell-derived exosomes enriched with microRNA-146a provide amplified therapeutic efficacy. Exp. Neurol. 341, 113694. doi:10.1016/j.expneurol.2021.113694
Ferrantelli, F., Manfredi, F., Chiozzini, C., Anticoli, S., Olivetta, E., Arenaccio, C., et al. (2018). DNA vectors generating engineered exosomes potential CTL vaccine candidates against AIDS, hepatitis B, and tumors. Mol. Biotechnol. 60 (11), 773–782. doi:10.1007/s12033-018-0114-3
Gao, B., Zhou, S., Sun, C., Cheng, D., Zhang, Y., Li, X., et al. (2020). Brain endothelial cell-derived exosomes induce neuroplasticity in rats with ischemia/reperfusion injury. ACS Chem. Neurosci. 11 (15), 2201–2213. doi:10.1021/acschemneuro.0c00089
Gao, F., Zuo, B., Wang, Y., Li, S., Yang, J., and Sun, D. (2020). Protective function of exosomes from adipose tissue-derived mesenchymal stem cells in acute kidney injury through SIRT1 pathway. Life Sci. 255, 117719. doi:10.1016/j.lfs.2020.117719
Gao, W., Li, F., Liu, L., Xu, X., Zhang, B., Wu, Y., et al. (2018). Endothelial colony-forming cell-derived exosomes restore blood-brain barrier continuity in mice subjected to traumatic brain injury. Exp. Neurol. 307, 99–108. doi:10.1016/j.expneurol.2018.06.001
Gilligan, K. E., and Dwyer, R. M. (2017). Engineering exosomes for cancer therapy. Int. J. Mol. Sci. 18 (6), E1122. doi:10.3390/ijms18061122
György, B., Fitzpatrick, Z., Crommentuijn, M. H., Mu, D., and Maguire, C. A. (2014). Naturally enveloped AAV vectors for shielding neutralizing antibodies and robust gene delivery in vivo. Biomaterials 35 (26), 7598–7609. doi:10.1016/j.biomaterials.2014.05.032
Hajipour, H., Farzadi, L., Roshangar, L., Latifi, Z., Kahroba, H., Shahnazi, V., et al. (2021). A human chorionic gonadotropin (hCG) delivery platform using engineered uterine exosomes to improve endometrial receptivity. Life Sci. 275, 119351. doi:10.1016/j.lfs.2021.119351
Han, Q., Xie, Q. R., Li, F., Cheng, Y., Wu, T., Zhang, Y., et al. (2021). Targeted inhibition of SIRT6 via engineered exosomes impairs tumorigenesis and metastasis in prostate cancer. Theranostics 11 (13), 6526–6541. doi:10.7150/thno.53886
Hui, B., Lu, C., Wang, J., Xu, Y., Yang, Y., Ji, H., et al. (2021). Engineered exosomes for co-delivery of PGM5-AS1 and oxaliplatin to reverse drug resistance in colon cancer. J. Cell. Physiol. 237, 911–933. doi:10.1002/jcp.30566
Jia, G., Han, Y., An, Y., Ding, Y., He, C., Wang, X., et al. (2018). NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials 178, 302–316. doi:10.1016/j.biomaterials.2018.06.029
Kalani, A., Chaturvedi, P., Kamat, P. K., Maldonado, C., Bauer, P., Joshua, I. G., et al. (2016). Curcumin-loaded embryonic stem cell exosomes restored neurovascular unit following ischemia-reperfusion injury. Int. J. Biochem. Cell Biol. 79, 360–369. doi:10.1016/j.biocel.2016.09.002
Kalluri, R., and LeBleu, V. S. (2020). The biology, function, and biomedical applications of exosomes. Science 367 (6478), eaau6977. doi:10.1126/science.aau6977
Kase, Y., Uzawa, K., Wagai, S., Yoshimura, S., Yamamoto, J. I., Toeda, Y., et al. (2021). Engineered exosomes delivering specific tumor-suppressive RNAi attenuate oral cancer progression. Sci. Rep. 11 (1), 5897. doi:10.1038/s41598-021-85242-1
Kim, G., Lee, Y., Ha, J., Han, S., and Lee, M. (2021). Engineering exosomes for pulmonary delivery of peptides and drugs to inflammatory lung cells by inhalation. J. Control. Release 330, 684–695. doi:10.1016/j.jconrel.2020.12.053
Kim, M. S., Haney, M. J., Zhao, Y., Yuan, D., Deygen, I., Klyachko, N. L., et al. (2018). Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: in vitro and in vivo evaluations. Nanomedicine 14 (1), 195–204. doi:10.1016/j.nano.2017.09.011
Kim, S. M., Yang, Y., Oh, S. J., Hong, Y., Seo, M., and Jang, M. (2017). Cancer-derived exosomes as a delivery platform of CRISPR/Cas9 confer cancer cell tropism-dependent targeting. J. Control. Release 266, 8–16. doi:10.1016/j.jconrel.2017.09.013
Kobayashi, M., Sawada, K., Miyamoto, M., Shimizu, A., Yamamoto, M., Kinose, Y., et al. (2020). Exploring the potential of engineered exosomes as delivery systems for tumor-suppressor microRNA replacement therapy in ovarian cancer. Biochem. Biophys. Res. Commun. 527 (1), 153–161. doi:10.1016/j.bbrc.2020.04.076
Kojima, R., Bojar, D., Rizzi, G., Hamri, G. C. E., El-Baba, M. D., Saxena, P., et al. (2018). Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson's disease treatment. Nat. Commun. 9 (1), 1305. doi:10.1038/s41467-018-03733-8
Lee, E. S., Sul, J. H., Shin, J. M., Shin, S., Lee, J. A., Kim, H. K., et al. (2021). Reactive oxygen species-responsive dendritic cell-derived exosomes for rheumatoid arthritis. Acta Biomater. 128, 462–473. doi:10.1016/j.actbio.2021.04.026
Li, D., Liu, J., Guo, B., Liang, C., Dang, L., Lu, C., et al. (2016). Osteoclast-derived exosomal miR-214-3p inhibits osteoblastic bone formation. Nat. Commun. 7, 10872. doi:10.1038/ncomms10872
Li, W., Liu, Y., Zhang, P., Tang, Y., Zhou, M., Jiang, W., et al. (2018). Tissue-engineered bone immobilized with human adipose stem cells-derived exosomes promotes bone regeneration. ACS Appl. Mat. Interfaces 10 (6), 5240–5254. doi:10.1021/acsami.7b17620
Liang, G., Zhu, Y., Ali, D. J., Tian, T., Xu, H., Si, K., et al. (2020). Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J. Nanobiotechnology 18 (1), 10. doi:10.1186/s12951-019-0563-2
Liang, Y., Xu, X., Li, X., Xia, J., Wang, D., Duan, L., et al. (2020). Chondrocyte-targeted MicroRNA delivery by engineered exosomes toward a cell-free osteoarthritis therapy. ACS Appl. Mater. Interfaces. doi:10.1021/acsami.0c10458
Limoni, S. K., Moghadam, M. F., Moazzeni, S. M., Gomari, H., and Salimi, F. (2019). Engineered exosomes for targeted transfer of siRNA to HER2 positive breast cancer cells. Appl. Biochem. Biotechnol. 187 (1), 352–364. doi:10.1007/s12010-018-2813-4
Liu, J., Jiang, M., Deng, S., Lu, J., Huang, H., Zhang, Y., et al. (2018). miR-93-5p-Containing exosomes treatment attenuates acute myocardial infarction-induced myocardial damage. Mol. Ther. Nucleic Acids 11, 103–115. doi:10.1016/j.omtn.2018.01.010
Liu, M., Hu, Y., and Chen, G. (2020). The antitumor effect of gene-engineered exosomes in the treatment of brain metastasis of breast cancer. Front. Oncol. 10, 1453. doi:10.3389/fonc.2020.01453
Liu, S., Liu, D., Chen, C., Hamamura, K., Moshaverinia, A., Yang, R., et al. (2015). MSC transplantation improves osteopenia via epigenetic regulation of notch signaling in lupus. Cell Metab. 22 (4), 606–618. doi:10.1016/j.cmet.2015.08.018
Liu, Y., Fu, W., Cao, X., Li, S., Xiong, T., Zhang, X., et al. (2021). Delivery of miR-224-5p by exosomes from cancer-associated fibroblasts potentiates progression of clear cell renal cell carcinoma. Comput. Math. Methods Med. 2021, 5517747. doi:10.1155/2021/5517747
Long, Q., Upadhya, D., Hattiangady, B., Kim, D. K., Shuai, B., An, S. Y., et al. (2017). Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc. Natl. Acad. Sci. U. S. A. 114 (17), E3536–E3545. doi:10.1073/pnas.1703920114
Nie, H., Xie, X., Zhang, D., Zhou, Y., Li, B., Li, F., et al. (2020). Use of lung-specific exosomes for miRNA-126 delivery in non-small cell lung cancer. Nanoscale 12 (2), 877–887. doi:10.1039/c9nr09011h
Nojehdehi, S., Soudi, S., Hesampour, A., Rasouli, S., Soleimani, M., and Hashemi, S. M. (2018). Immunomodulatory effects of mesenchymal stem cell-derived exosomes on experimental type-1 autoimmune diabetes. J. Cell. Biochem. 119 (11), 9433–9443. doi:10.1002/jcb.27260
Ohno, S., Takanashi, M., Sudo, K., Ueda, S., Ishikawa, A., Matsuyama, N., et al. (2013). Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol. Ther. 21 (1), 185–191. doi:10.1038/mt.2012.180
Ortega, A., Martinez-Arroyo, O., Forner, M. J., and Cortes, R. (2020). Exosomes as drug delivery systems: endogenous nanovehicles for treatment of systemic lupus erythematosus. Pharmaceutics 13 (1), E3. doi:10.3390/pharmaceutics13010003
Pegtel, D. M., and Gould, S. J. (2019). Exosomes. Annu. Rev. Biochem. 88, 487–514. doi:10.1146/annurev-biochem-013118-111902
Perets, N., Betzer, O., Shapira, R., Brenstein, S., Angel, A., Sadan, T., et al. (2019). Golden exosomes selectively target brain pathologies in neurodegenerative and neurodevelopmental disorders. Nano Lett. 19 (6), 3422–3431. doi:10.1021/acs.nanolett.8b04148
Quah, B. J., and O' Neill, H. C. (2005). The immunogenicity of dendritic cell-derived exosomes. Blood Cells Mol. Dis. 35, 94–110. doi:10.1016/j.bcmd.2005.05.002
Ran, N., Gao, X., Dong, X., Li, J., Lin, C., Geng, M., et al. (2020). Effects of exosome-mediated delivery of myostatin propeptide on functional recovery of mdx mice. Biomaterials 236, 119826. doi:10.1016/j.biomaterials.2020.119826
Rao, Q., Zuo, B., Lu, Z., Gao, X., You, A., Wu, C., et al. (2016). Tumor-derived exosomes elicit tumor suppression in murine hepatocellular carcinoma models and humans in vitro. Hepatology 64 (2), 456–472. doi:10.1002/hep.28549
Ruiss, R., Jochum, S., Mocikat, R., Hammerschmidt, W., and Zeidler, R. (2011). EBV-gp350 confers B-cell tropism to tailored exosomes and is a neo-antigen in normal and malignant B cells-a new option for the treatment of B-CLL. PLoS One 6 (10), e25294. doi:10.1371/journal.pone.0025294
Srikanth, M., and Kessler, J. A. (2012). Nanotechnology-novel therapeutics for CNS disorders. Nat. Rev. Neurol. 8 (6), 307–318. doi:10.1038/nrneurol.2012.76
Srivastava, A., Amreddy, N., Razaq, M., Towner, R., Zhao, Y. D., Ahmed, R. A., et al. (2018). Exosomes as theranostics for lung cancer. Adv. Cancer Res. 139, 1–33. doi:10.1016/bs.acr.2018.04.001
Sterzenbach, U., Putz, U., Low, L. H., Silke, J., Tan, S. S., and Howitt, J. (2017). Engineered exosomes as vehicles for biologically active proteins. Mol. Ther. 25 (6), 1269–1278. doi:10.1016/j.ymthe.2017.03.030
Sun, Y., Kuek, V., Liu, Y., Tickner, J., Yuan, Y., Chen, L., et al. (2018). MiR-214 is an important regulator of the musculoskeletal metabolism and disease. J. Cell. Physiol. 234 (1), 231–245. doi:10.1002/jcp.26856
Tao, S. C., Rui, B. Y., Wang, Q. Y., Zhou, D., Zhang, Y., and Guo, S. C. (2018). Extracellular vesicle-mimetic nanovesicles transport LncRNA-H19 as competing endogenous RNA for the treatment of diabetic wounds. Drug Deliv. 25 (1), 241–255. doi:10.1080/10717544.2018.1425774
Wang, B., Yao, K., Huuskes, B. M., Shen, H. H., Zhuang, J., Godson, C., et al. (2016). Mesenchymal stem cells deliver exogenous MicroRNA-let7c via exosomes to attenuate renal fibrosis. Mol. Ther. 24 (7), 1290–1301. doi:10.1038/mt.2016.90
Wang, J., Wang, C., Li, Y., Li, M., Zhu, T., Shen, Z., et al. (2021). Potential of peptide-engineered exosomes with overexpressed miR-92b-3p in anti-angiogenic therapy of ovarian cancer. Clin. Transl. Med. 11 (5), e425. doi:10.1002/ctm2.425
Wang, X., Chen, Y., Zhao, Z., Meng, Q., Yu, Y., Sun, J., et al. (2018). Engineered exosomes with ischemic myocardium-targeting peptide for targeted therapy in myocardial infarction. J. Am. Heart Assoc. 7 (15), e008737. doi:10.1161/JAHA.118.008737
Wei, X., Yi, X., Lv, H., Sui, X., Lu, P., Li, L., et al. (2020). MicroRNA-377-3p released by mesenchymal stem cell exosomes ameliorates lipopolysaccharide-induced acute lung injury by targeting RPTOR to induce autophagy. Cell Death Dis. 11 (8), 657. doi:10.1038/s41419-020-02857-4
Wu, G., Zhang, J., Zhao, Q., Zhuang, W., Ding, J., Zhang, C., et al. (2020). Molecularly engineered macrophage-derived exosomes with inflammation tropism and intrinsic heme biosynthesis for atherosclerosis treatment. Angew. Chem. Int. Ed. Engl. 59 (10), 4068–4074. doi:10.1002/anie.201913700
Xu, M., Feng, T., Liu, B., Qiu, F., Xu, Y., Zhao, Y., et al. (2021). Engineered exosomes: desirable target-tracking characteristics for cerebrovascular and neurodegenerative disease therapies. Theranostics 11 (18), 8926–8944. doi:10.7150/thno.62330
Yang, T., Martin, P., Fogarty, B., Brown, A., Schurman, K., Phipps, R., et al. (2015). Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm. Res. 32 (6), 2003–2014. doi:10.1007/s11095-014-1593-y
Yang, Z., Yang, Y., Xu, Y., Jiang, W., Shao, Y., Xing, J., et al. (2021). Biomimetic nerve guidance conduit containing engineered exosomes of adipose-derived stem cells promotes peripheral nerve regeneration. Stem Cell Res. Ther. 12 (1), 442. doi:10.1186/s13287-021-02528-x
Yao, Z., Li, J., Xiong, H., Cui, H., Ning, J., Wang, S., et al. (2021). MicroRNA engineered umbilical cord stem cell-derived exosomes direct tendon regeneration by mTOR signaling. J. Nanobiotechnology 19 (1), 169. doi:10.1186/s12951-021-00906-4
Yeo, R. W., Lai, R. C., Zhang, B., Tan, S. S., Yin, Y., Teh, B. J., et al. (2013). Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv. Drug Deliv. Rev. 65 (3), 336–341. doi:10.1016/j.addr.2012.07.001
Zha, Y., Li, Y., Lin, T., Chen, J., Zhang, S., and Wang, J. (2021). Progenitor cell-derived exosomes endowed with VEGF plasmids enhance osteogenic induction and vascular remodeling in large segmental bone defects. Theranostics 11 (1), 397–409. doi:10.7150/thno.50741
Zhang, Z., Guo, X., Guo, X., Yu, R., Qian, M., Wang, S., et al. (2021). MicroRNA-29a-3p delivery via exosomes derived from engineered human mesenchymal stem cells exerts tumour suppressive effects by inhibiting migration and vasculogenic mimicry in glioma. Aging (Albany NY) 13 (4), 5055–5068. doi:10.18632/aging.202424
Zheng, M., Huang, M., Ma, X., Chen, H., and Gao, X. (2019). Harnessing exosomes for the development of brain drug delivery systems. Bioconjug. Chem. 30 (4), 994–1005. doi:10.1021/acs.bioconjchem.9b00085
Zhou, S., Gao, B., Sun, C., Bai, Y., Cheng, D., Zhang, Y., et al. (2020). Vascular endothelial cell-derived exosomes protect neural stem cells against ischemia/reperfusion injury. Neuroscience 441, 184–196. doi:10.1016/j.neuroscience.2020.05.046
Zhou, W., Xu, M., Wang, Z., and Yang, M. (2021). Engineered exosomes loaded with miR-449a selectively inhibit the growth of homologous non-small cell lung cancer. Cancer Cell Int. 21 (1), 485. doi:10.1186/s12935-021-02157-7
Zhu, S., Yao, F., Qiu, H., Zhang, G., Xu, H., and Xu, J. (2018). Coupling factors and exosomal packaging microRNAs involved in the regulation of bone remodelling. Biol. Rev. Camb. Philos. Soc. 93 (1), 469–480. doi:10.1111/brv.12353
Zhu, W., Sun, L., Zhao, P., Liu, Y., Zhang, J., Zhang, Y., et al. (2021). Macrophage migration inhibitory factor facilitates the therapeutic efficacy of mesenchymal stem cells derived exosomes in acute myocardial infarction through upregulating miR-133a-3p. J. Nanobiotechnology 19 (1), 61. doi:10.1186/s12951-021-00808-5
Keywords: engineered exosomes, diagnostic, treatment, clinical value, application prospects
Citation: Liu G, Wu J, Chen G and Shang A (2022) The potential therapeutic value and application prospect of engineered exosomes in human diseases. Front. Cell Dev. Biol. 10:1051380. doi: 10.3389/fcell.2022.1051380
Received: 30 September 2022; Accepted: 18 November 2022;
Published: 01 December 2022.
Edited by:
Jiake Xu, University of Western Australia, AustraliaReviewed by:
Sipin Zhu, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, ChinaBaosheng Boris GUO, Nanjing University, China
Copyright © 2022 Liu, Wu, Chen and Shang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anquan Shang, c2hhbmdhbnF1YW5AdG9uZ2ppLmVkdS5jbg==
†These authors have contributed equally to this work
 Gege Liu
Gege Liu Junlu Wu
Junlu Wu Guofei Chen
Guofei Chen Anquan Shang
Anquan Shang