- 1Department of Orthopaedics, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 2Key Laboratory of Musculoskeletal System Degeneration and Regeneration Translational Research of Zhejiang Province, Hangzhou, China
- 3Department of Orthopaedics, Shanghai Key Laboratory of Orthopaedic Implant, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
A Corrigendum on
Oxymatrine attenuates osteoclastogenesis via modulation of ROS-mediated SREBP2 signaling and counteracts ovariectomy-induced osteoporosis
by Jiang C, Ma Q, Wang S, Shen Y, Qin A, Fan S and Jie Z (2021). Front. Cell Dev. Biol. 9:684007. doi: 10.3389/fcell.2021.684007
In the original article, there were mistakes in Figures 1, 3 as published. The scale bars of TRAP staining images in Figures 1D, 3J were incorrect. Furthermore, we applied a mismatched picture for Figure 1F. The corrected Figures 1, 3 appear below.
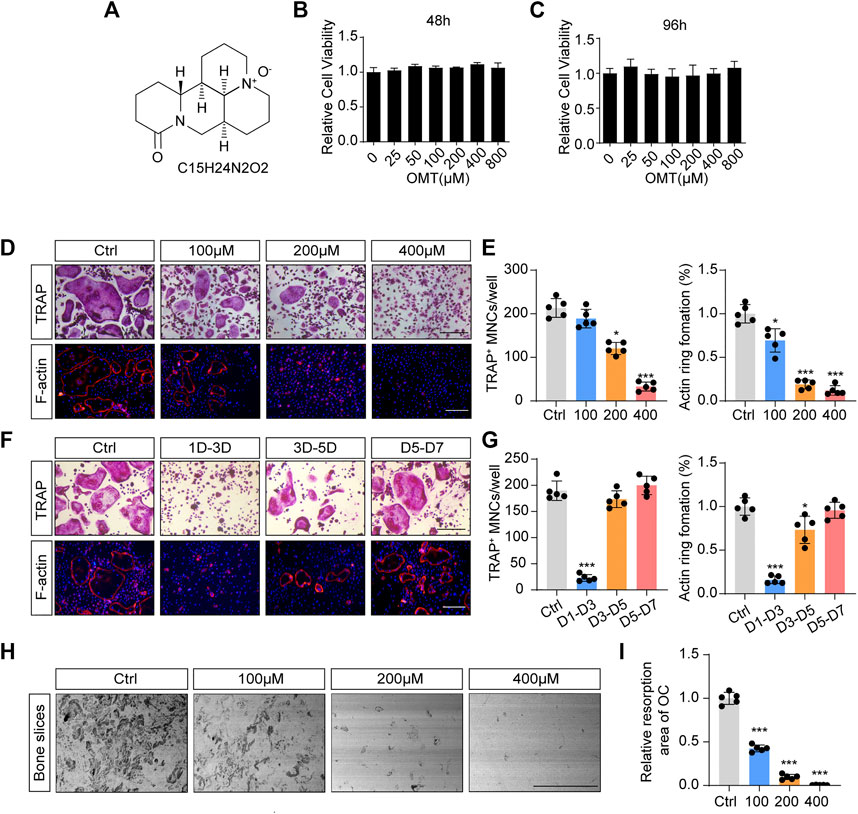
FIGURE 1. OMT inhibits RANKL-induced osteoclast formation and activity in vitro. (A) The chemical structure and formula of OMT. (B,C) Cell viability of OMT-treated BMMs at 48 and 96 h (D) BMMs were stimulated by 30 ng/mL M-CSF and 50 ng/mL RANKL, and treated with indicated concentrations of OMT for 5 days. Representative images of TRAP staining and F-actin staining were shown. Scale bar = 200 µm. (E) Quantification of TRAP-positive multinuclear cells and F-actin ring formation rate. (F) BMMs were stimulated with 30 ng/mL M-CSF and 50 ng/mL RANKL for 7 days, and treated with 200 µM OMT for the indicated days. TRAP staining and F-actin ring staining were performed. Scale bar = 200 µm. (G) Quantification of TRAP-positive multinuclear cells and F-actin ring formation rate. (H) Representative images of bone resorption pits. Scale bar = 500 µm. (I) Quantification of resorption pit area in each group. Data were presented as means ± SD of 5 independent experiments. *p < 0.05, ***p < 0.001.
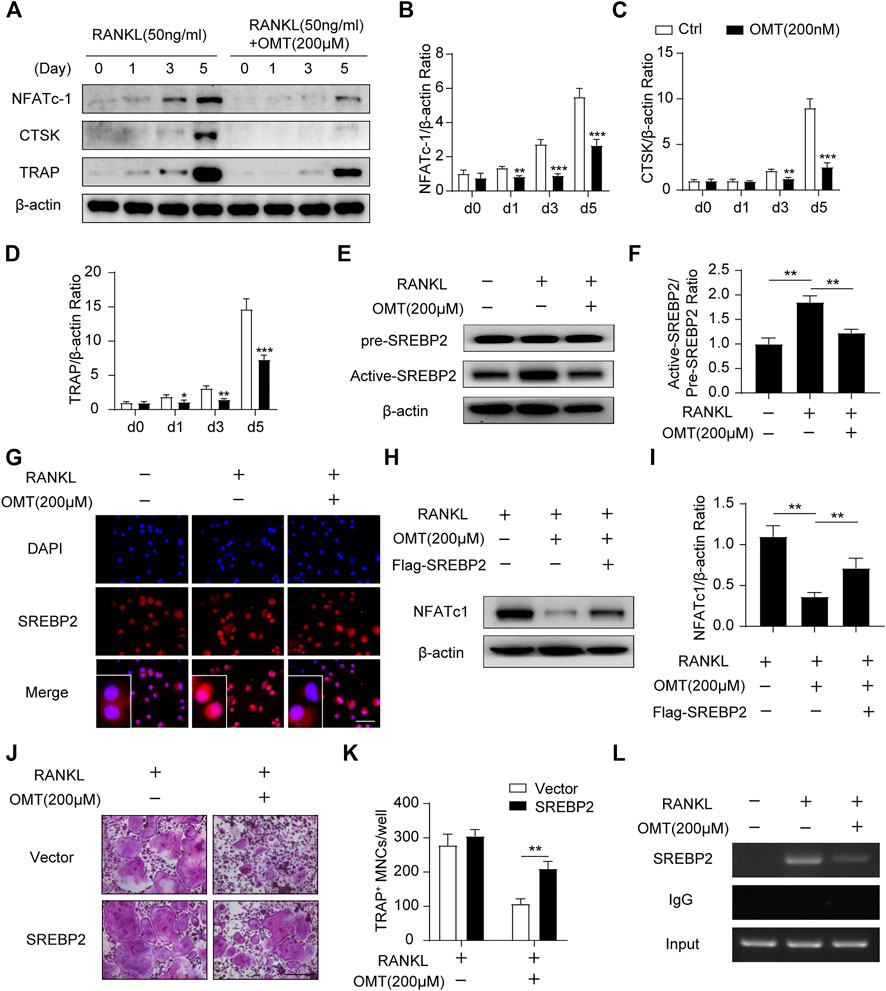
FIGURE 3. OMT attenuates SREBP2 activity and downstream NFATc1 expression during osteoclastogenesis. (A) BMMs were stimulated with RANKL, with or without 200 µM OMT for 0, 1, 3, 5 days, the expression of NFATc1, CTSK and TRAP was tested by western blots. (B–D) Quantification of the ratios of band intensity of NFATc1, TRAP, and CTSK relative to β-actin (n = 3 per group). (E) BMMs were treated with RANKL and 200 µM OMT as indicated, western blot was used to detect the level of pre-SREBP2 and active-SREBP2. (F) Quantification of active-SREBP2/pre-SREBP2 ratio (n = 3 per group). (G) RAW264.7 cells were treated with RANKL and OMT as indicated, immunofluorescence assay was performed to detect SREBP2 translocation. Scale bar = 100 µm. (H) BMMs were transfected with Flag-SREBP2 plasmid or empty vector, then treated with RANKL and OMT as indicated, the expression of NFATc1 was examined. (I) Quantification of NFATc1/β-actin ratio (n = 3 per group). (J) BMMs were transfected with Flag-SREBP2 plasmid or empty vector, then treated with RANKL and OMT as indicated. Representative images of TRAP staining were shown. Scale bar = 200 µm. (K) Quantification of TRAP-positive multinuclear cells per well (n = 5 per group). (L) ChIP assay was performed on BMMs, treated with RANKL and OMT as indicated. Data were presented as means ± SD. *p < 0.05, **p < 0.01, ***p < 0.001.
The authors apologize for these errors and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: oxymatrine, osteoclast, ROS, SREBP2, osteoporosis
Citation: Jiang C, Ma Q, Wang S, Shen Y, Qin A, Fan S and Jie Z (2022) Corrigendum: Oxymatrine attenuates osteoclastogenesis via modulation of ROS-mediated SREBP2 signaling and counteracts ovariectomy-induced osteoporosis. Front. Cell Dev. Biol. 10:1002132. doi: 10.3389/fcell.2022.1002132
Received: 25 July 2022; Accepted: 18 August 2022;
Published: 09 September 2022.
Edited and reviewed by:
Claudia Fiorillo, University of Florence, ItalyCopyright © 2022 Jiang, Ma, Wang, Shen, Qin, Fan and Jie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiwei Jie, amllemhpd2VpQHpqdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Chao Jiang1,2†
Chao Jiang1,2† Zhiwei Jie
Zhiwei Jie