
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell Dev. Biol. , 30 November 2021
Sec. Molecular and Cellular Pathology
Volume 9 - 2021 | https://doi.org/10.3389/fcell.2021.791585
This article is part of the Research Topic Aptamer-Based Structural Biology, Computational Modelling, Translational Research and Drug Discovery, Volume II View all 6 articles
 Xueman Zhou1,2
Xueman Zhou1,2 Wenxiu Yuan1,2
Wenxiu Yuan1,2 Xin Xiong1
Xin Xiong1 Zhenzhen Zhang1,2
Zhenzhen Zhang1,2 Jiaqi Liu1,2
Jiaqi Liu1,2 Yingcheng Zheng1,2
Yingcheng Zheng1,2 Jun Wang1*
Jun Wang1* Jin Liu2*
Jin Liu2*Osteoporosis is a prevalent bone disorder characterized by bone mass reduction and deterioration of bone microarchitecture leading to bone fragility and fracture risk. In recent decades, knowledge regarding the etiological mechanisms emphasizes that inflammation, oxidative stress and senescence of bone cells contribute to the development of osteoporosis. Studies have demonstrated that heme oxygenase 1 (HO-1), an inducible enzyme catalyzing heme degradation, exhibits anti-inflammatory, anti-oxidative stress and anti-apoptosis properties. Emerging evidence has revealed that HO-1 is critical in the maintenance of bone homeostasis, making HO-1 a potential target for osteoporosis treatment. In this Review, we aim to provide an introduction to current knowledge of HO-1 biology and its regulation, focusing specifically on its roles in bone homeostasis and osteoporosis. We also examine the potential of HO-1-based pharmacological therapeutics for osteoporosis and issues faced during clinical translation.
Osteoporosis, the most common bone disorder, is characterized by decreased bone mineral density (BMD), deterioration of bone microarchitecture and poor mechanical properties, resulting in increased vulnerability to fractures (NIH Consensus Development Panel on Osteoporosis Prevention and Therapy, 2000; van den Bergh et al., 2012; Vestergaard et al., 2007). Osteoporosis affects more than 200 million patients worldwide and its complications, especially osteoporotic fractures, can markedly reduce mobility and quality of life, increasing mortality, thus causing huge social and economic burdens (Blume and Curtis, 2011).
Pathologically, osteoporosis is the result of an imbalance in bone remodeling which is a dynamic process that involves both bone formation and resorption (Langdahl et al., 2016). For this reason, current pharmacological treatments of osteoporosis primarily are antiresorptive (inhibiting the osteoclasts, e.g., estrogen and bisphosphonates), bone forming (stimulating the osteoblasts, e.g., parathyroid hormone) or dual acting (e.g., romosozumab) (Langdahl, 2021). However, there is growing concern about the risk of adverse skeletal effects such as atypical femoral fractures and osteonecrosis of the jaw, as well as off-target effects in long-term use (Davis et al., 2016; Kim et al., 2016; Lv et al., 2020). Therefore, continuing efforts remain focused on filling the unmet need for safe and effective preventative and/or therapeutic strategies. In recent decades, extensive studies regarding the etiological mechanisms underpinning osteoporosis have emphasized that oxidative stress, inflammation and cellular senescence contribute to the progression of osteoporosis, indicating a new class of treatment strategies (Callaway and Jiang, 2015; Hendrickx et al., 2015; Farr and Khosla, 2019; Saxena et al., 2021).
Heme oxygenases (HO) are thes enzyme responsible for the degradation of heme into free iron, which is rapidly exported from cells via ferroportin 1 (FPN1) or sequestered into ferritin for storage; biliverdin, which is converted to bilirubin by biliverdin reductase; and carbon monoxide (CO) (Tenhunen et al., 1968; Kumar and Bandyopadhyay, 2005). Heme oxygenase-1 (HO-1), is an inducible form of HO that is highly expressed in tissues responsible for heme metabolism, including bone marrow (Gozzelino et al., 2010). Mounting studies have suggested that activated HO-1 is associated with the prevention of various diseases, including cancer, diabetes, cardiovascular diseases and osteoarthritis (Ayer et al., 2016; Chiang et al., 2018; Rochette et al., 2018; Alcaraz and Ferrándiz, 2020). These beneficial effects might be attributed to the anti-inflammatory, antioxidative, antiapoptotic and cell-cycle regulatory effects of HO-1 through its metabolites. Recently, it has been postulated that management of the expression and activity of HO-1 could represent provide a new idea for osteoporosis treatment (Che et al., 2021). With these points in mind, this review discusses the current knowledge of HO-1 biology, focusing specifically on its roles in bone homeostasis and osteoporosis. We also highlight the potential pharmacological interventions under investigation that could alleviate osteoporosis by targeting HO-1.
Traditionally, osteoporosis is classified into primary and secondary types (Marcus et al., 2013). Primary osteoporosis is further divided into two subtypes: type I (postmenopausal osteoporosis), which is caused primarily by estrogen deficiency due to menopause, and type II (senile osteoporosis), which is primarily caused by aging. Secondary osteoporosis refers to bone disorders secondary to other medical conditions (renal osteodystrophy, diabetes-related osteoporosis, etc.) or adverse results of therapeutic interventions (glucocorticoid-induced osteoporosis etc.). (Riggs et al., 2001; Feng and McDonald, 2011). Regardless of type, the disease can be characterized as a disorder of bone remodeling resulting from the imbalance between bone-forming osteoblasts and bone-resorbing osteoclasts, and there are common underlying molecular mechanisms.
Accumulating studies have revealed that, to a certain extent, osteoporosis can be regarded as an inflammatory disease (Arron and Choi, 2000; Lorenzo, 2000). On the one hand, there is a close association between an increased risk of osteoporosis and inflammatory conditions, such as rheumatoid arthritis, ankylosing spondylitis, and inflammatory bowel disease (Haugeberg et al., 2004; Mitra et al., 2000; Moschen and R, 2005). On the other hand, during osteoporosis, inflammatory mediators such as pro-inflammatory cytokines act on the skeletal cells directly or indirectly to promote the development of osteoporosis (McLean, 2009). Besides, recent evidence suggests both innate and adaptive immunocytes contribute to osteoporosis (Saxena et al., 2021; Wu et al., 2021).
Clinical studies have revealed that menopause results in elevation of pro-inflammatory cytokines while in the case of old age, senescent cells secrete a wide range of inflammatory cytokines, such as IL-1, IL-6, IL-8, TNF-α and IFN-γ, which correlate with the progression of osteoporotic bone loss (Pacifici, 1998; De Cecco et al., 2019). It has been reported that human peripheral-blood monocytes (PBMCs) from osteoporosis patients have 29–67% higher secretion of IL-1β, IL-6, and TNF-α in whole blood culture compared with healthy control subjects (Pacifici et al., 1987). Zheng et al. (Zheng et al., 1997) found statistically significant negative correlations between PBMC secretion of IL-1β, IL-6, and TNF-α and lumbar spine BMD, while a study in healthy population similarly showed an association between reduced BMD and inflammatory markers in the circulation system, especially IL-6 (Heinrich et al., 2003). Animal models also supported the pathological role for inflammation in osteoporosis as both TNF and TNF receptor 1 deficient mice present resistance to ovariectomy-induced bone loss (Roggia et al., 2001; Iqbal et al., 2006).
In osteoporosis models, elevated pro-inflammatory cytokines, including IL-6, IL-1 and TNF-α can induce bone loss by regulating osteoclastic differentiation and activation both directly and indirectly (Hofbauer et al., 2000). Specifically, IL-6 promotes osteoclastogenesis by increasing RANKL production in osteocytes and osteoblasts (Liu et al., 2005). IL-6 also helps osteoclast precursors to transmigrate from the bone marrow to the blood leading to systemic bone loss by upregulating S1PR2 [Sphingosine-1-phosphate (S1P)] receptor (Tanaka et al., 2014). Besides, IL-6 hampers WNT/β-catenin pathway by enhansing its antagonists, Dickkopf-related protein 1 (DKK1) and sclerostin (SOST), which inhibit osteoblast differentiation (Ohori et al., 2019; Li S. et al., 2020). Moreover, IL-6 appears to mediate TNF-α and IL-1β induced bone resorption (Devlin et al., 1998). TNF-α and IL-1β, both of which are pro-inflammatory cytokines, also play pro-osteoclastogenic and anti-osteogenic roles, especially in post-menopausal osteoporosis (Du et al., 2018; Luo et al., 2018). On the one hand, they promote RANKL dependent osteoclastogenesis via activation of transcription factors NF-κB, AP-1 and PI3k/AKT pathway (Lee et al., 2017; Luo et al., 2018). TNF-α also triggers SOST expression, which induces RANKL expression in osteocytes and further boosts osteoclastogenesis, while IL-1β increases CCR7 to enhance osteoclast migration and activation (Kim et al., 2012; Lee et al., 2017). On the other hand, both TNF-α and IL-1β inhibit the proliferation, differentiation and activity of osteoblasts (Ruscitti et al., 2015; Du et al., 2018). Additionally, other inflammatory cytokines, such as IFN-γ and IL-7 indirectly promote bone loss by activating T cells and increasing the levels of IL-1 and TNF-α (Takayanagi et al., 2000; Baek et al., 2006; Tang et al., 2018). All these findings support the notion that inflammation contributes to the development of osteoporosis.
Oxidative stress (OS) is caused by the accumulation of free radicals mainly due to inflammation and mitochondrial dysfunction (Sies et al., 2017). A growing amount of evidence suggests that OS, which increases with aging or menopause, can adversely affect bone homeostasis by favoring a pro-resorptive environment, and it is often detected in the bone tissue of osteoporosis patients (Manolagas, 2010; Marie, 2014). Reactive oxygen species (ROS), especially hydrogen peroxide and superoxide ions, are thought to affect the bone environment mainly by two means: increasing osteoclastic activity and suppressing osteoblastic functions (Wauquier et al., 2009).
Primarily, ROS promote osteoclast formation and activity by stimulating RANKL-induced NF-kB and MAPK activation (An et al., 2019). Secondarily, they induce the excessive production of osteoclastogenic cytokines such as IL-1, IL-6, TNF-α and IL-7 (Hyeon et al., 2013). It has been reported that p66shc, a redox enzyme responsible for the reduction of O2 to H2O2, is a critical mediator of the stimulating effects of OS on the of activation NF-κB, cytokine production, and osteoclastogenesis (Almeida et al., 2007b). Further, OS also affects the function of osteoblasts. ROS trigger the activation of FOXOs, a subset of forkhead proteins contributing to cell cycle arrest, and suppresses the WNT/β-catenin pathway in MSCs, thus impairing osteogenic differentiation and increasing the expression and activity of peroxisome proliferator-activated receptor (PPAR) γ, which increases adipogenesis at the expense of osteogenesis (Almeida et al., 2007a; Takada et al., 2009). Apart from FOXO/WNT signaling, in murine primary bone marrow-derived and other MSC cell lines, OS also inhibits hedgehog signaling to suppress osteogenic differentiation (Kim et al., 2010). Further, increased OS in bone stimulates apoptosis of osteoblasts and osteoblast progenitors (Ambrogini et al., 2010). These facts convincingly demonstrate that OS advances the occurrence of osteoporosis.
Cellular senescence is a cell fate that involves irreversible cell cycle arrest, profound chromatin changes, apoptosis resistance and senescence-associated secretory phenotype (SASP) (Hayflick, 1965; Acosta et al., 2013). SASP is characterized by an increase in protein synthesis and secretion, including pro-inflammatory cytokines and chemokines, which has deleterious paracrine effects and is regarded as an essential mechanism of many age-related diseases (Coppé et al., 2010). In the past few years, there has been growing evidence suggesting that cellular senescence plays a vital role in the pathogenesis of osteoporosis (Liu and Wan, 2019). Firstly, markers of senescence p21, p16Ink4a, and p53 have been identified not only in mice but in aged bones from human biopsies (Dimri et al., 1995; Hernandez-Segura et al., 2019). Senescent cells (SnCs), including MSCs, osteoprogenitors, osteoblasts, osteocytes and immunocytes accumulate in the bone or bone marrow of aged mice and human (Farr et al., 2016; Piemontese et al., 2017). These SnCs, especially senescent osteoblasts and osteocytes acquire SASP to stimulate RANKL production, leading to enhanced osteoclastogenesis and the development of osteoporosis (Chen et al., 2013; Akkaoui et al., 2021). Elimination of senescent cells can be achieved pharmacologically by long-term senolytic treatment, by clearance of p16-positive genetic cells in INK-ATTAC transgenic mice, or by blocking SASP production through the targeted inhibition of Janus kinase pathway; each of these strategies can increase bone mass and improve microarchitecture in aged osteoporotic mice (Farr et al., 2017; Chandra et al., 2020; Sharma et al., 2020). Furthermore, DNA damage-induced cell cycle arrest leads to functional decline of osteoblasts by decreasing proliferation, limiting osteogenic differentiation, and impairing cell function (Abdallah et al., 2006; Kim et al., 2017). These findings point to targeting senescence as a novel strategy to alleviate osteoporosis.
HO-1, as a stress-inducible isozyme, catalyzes the degradation of heme into biliverdin (BV), carbon monoxide (CO), and free iron (Fe2+), releasing NADP+ and H2O (Maines, 1997). Under homeostatic conditions, HO-1 primarily maintains a low expression level or is absent from the body; however, it is highly upregulated in response to oxidative stress and provides protection against oxidative damage (Ryter and Choi, 2009). The induction of HO-1 exerts pleiotropic protective effects that are ascribed mainly to the biological activities of individual or cooperative effects of the metabolites (shown in Figure 1). Firstly, bilirubin (BR), derived by BV reduction, is a potent antioxidant able to scavenge ROS, thus preventing protein and lipid peroxidation. BR is also a key player in the control of inflammation as well as in the suppression of adaptive immunoreaction (McClung et al., 2021). Secondly, experimental evidence has established a firm basis for cytoprotective effects of CO involving the attenuation of inflammation, modulation of cell apoptosis and proliferation, as well as other cellular processes (Otterbein et al., 2016). Free iron, the third product of heme degradation induced by HO-1, reacts with hydrogen peroxide or lipid peroxides and produces numbers of reactive radicals, resulting in increased risk of many diseases and tissue injuries. However, it can be stored intracellularly by ferritin heavy chain (Van Lenten et al., 1995; Soares and Hamza, 2016). This means that ferritin serves as an antioxidant by binding and detoxifying ferrous iron.
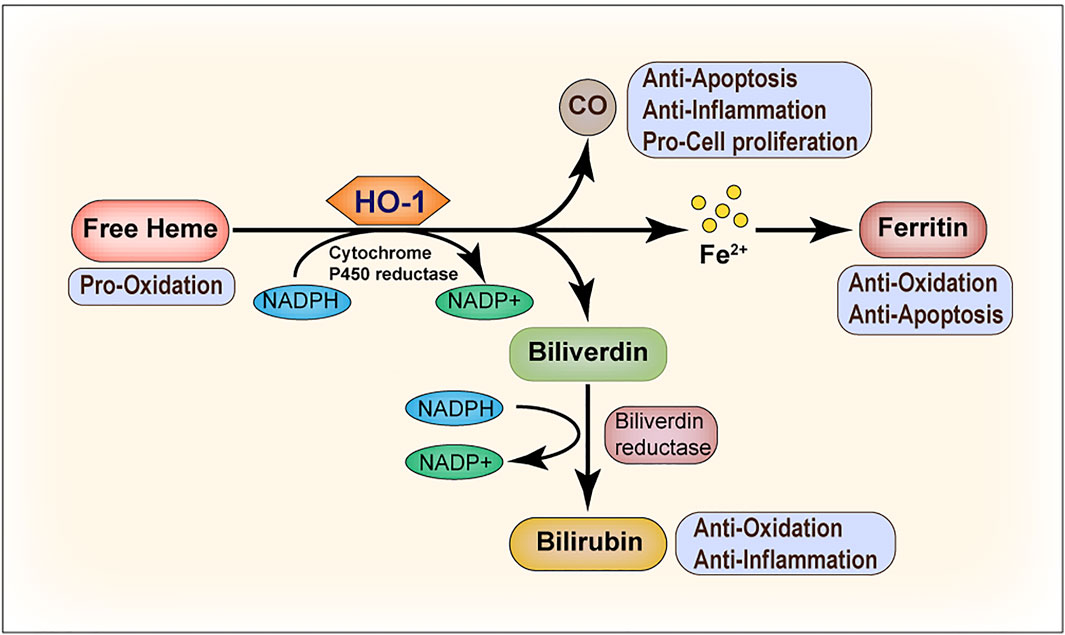
FIGURE 1. Heme oxygenase-1 (HO-1) catalyzes heme degradation. Free heme is degraded by HO-1, leading to the production of biliverdin, carbon monoxide (CO), and ferrous iron (Fe2+). Biliverdin is subsequently converted to bilirubin by biliverdin reductase, and Fe2+ is sequestered by ferritin. The degradation of heme and the conversion of biliverdin to bilirubin requires NADPH as the reducing agent. All the three end products, that is, biliverdin/bilirubin, CO, and Fe/ferritin, are cytoprotective. Under most conditions, biliverdin and bilirubin act as antioxidants. CO mainly inhibits the production of anti-inflammatory cytokines and upregulates the anti-apoptotic effectors. Ferritin serves as an antioxidant and suppresses cell apoptosis by binding and detoxifying ferrous iron.
Given its essential role, understanding the regulatory mechanism of HO-1 expression has been the focus of considerable research. Current knowledge regarding the regulation of HO-1 activity depends heavily upon changes at the transcriptional level but also involves a range of post- or co-transcriptional regulated events (shown in Figure 2). The HMOX1 gene which encodes HO-1 in mammals contains a motif known as Antioxidant Response Element (ARE) in its promoter site that can be recognized by a dimer composed of the BTB and CNC Homology 1 (Bach1) transcription factor together with Maf proteins. Under basal conditions, the Bach1-Maf dimer bounds to the ARE motif and represses the transcription of HO-1 while during OS, Bach1 is dislocated from ARE and exported from the nucleus to be degraded (Sun et al., 2002; Suzuki et al., 2004; Zenke-Kawasaki et al., 2007). Evidence coming from structure and sequence analysis of the promoter suggests that a group of redox-sensitive transcription factors activate HO-1, especially nuclear factor erythroid 2-related factor 2 (Nrf2) (Zhang et al., 2007). Under basal conditions, Nrf2 is sequestered in the cytoplasm by the kelch-like ECH-associated protein (Keap1) that hinders Nrf2 activity by facilitating the ubiquitylation and degradation of Nrf2 by the proteasome (Figure 2A) (Kim et al., 2013; Taguchi et al., 2011). However, in the presence of oxidants, ROS dissociate Keap1 from Nrf2 and translocate Nrf2 to the nucleus to bind AREs, promoting the expression of HO-1 (Korytina et al., 2019). Thus, the cellular induction of HO-1 is tightly regulated by extracellular conditions through this Nrf2/Keap1/Bach1 system. Other transcription factors are also known to bind ARE to stimulate HO-1 expression, such as activator protein-1 (AP-1), nuclear factor-kappa B (NF-κB) and hypoxia-inducible factor 1α (HIF1α) (Figure 2B) (Lavrovsky et al., 1994; Alam and Cook, 2007; Medina et al., 2020). Apart from the direct interaction with transcription factors, emerging evidence shows that microRNAs (miRs), the small noncoding RNAs involved in post-transcriptional modulation of gene expression, are also involved in the regulation of HMOX1 gene expression either directly by decreasing the stability or translation of messenger RNA or indirectly by regulating the expression of upstream factors (e.g., Nrf2, Keap1, Bach1, etc.), which opens up a brand new horizon for the research of HO-1 regulation (Cheng et al., 2013; Kozakowska et al., 2014; Reziwan et al., 2019; Zhang et al., 2019).
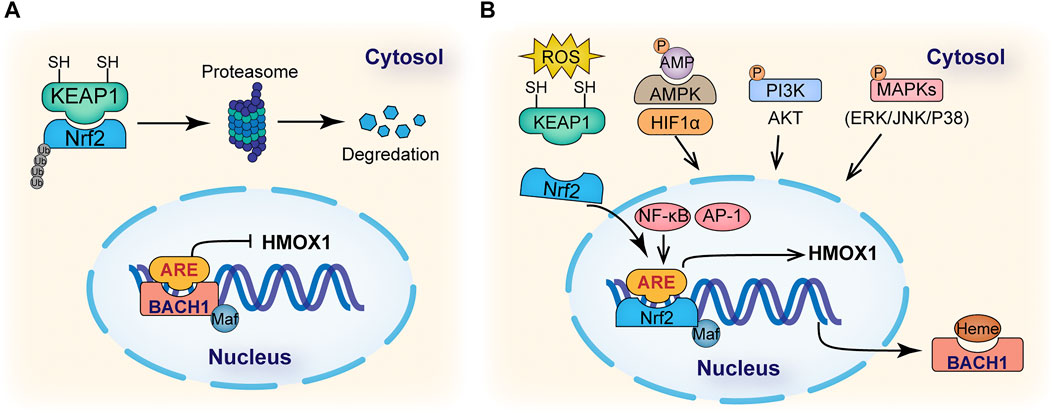
FIGURE 2. Mechanisms of HO-1 regulation. (A) Under basal conditions, nuclear factor erythroid 2- related factor 2 (NRF2) in cell cytosol binds to Kelch-like ECH- associated protein 1 (KEAP1), which promotes the ubiquitination and degradation of NRF2 in proteasomes. In the nucleus, BACH1 is bound to the ARE region in HMOX1 gene promoter and represses its transcription. (B) Under stress, binding of heme molecules to BACH1 promotes its dissociation from the small Maf protein and ARE motif in the HMOX1 gene promoter. ROS induces changes in KEAP1 cysteine residues, promoting the nucleus translocation of NRF2 to bind the ARE motif in HMOX1 gene promoting its expression. Signaling cascades, such as AMP- activated protein kinase (AMPK), phosphatidylinositol 3-kinase (PI3K), mitogen-activated protein kinases (MAPKs), and transcription factors, such as hypoxia-inducible factor 1α (HIF1α), AP-1 and NF-κB, have also been reported to be involved in the regulation of HO-1 expression.
It has been implied that many inducers regulate HO-1 expression via intermediate protein kinase pathways. For example, the mitogen-activated protein kinases (MAPKs), including extracellular signal-regulated kinase (ERK), JUN amino-terminal kinase (JNK) and p38 all act in regulating HO-1 expression (Martin et al., 2004; Mense and Zhang, 2006). The phosphatidylinositol 3- kinase (PI3K)-AKT pathway can also regulate HO-1 expression in response to oxidative stimuli and some alternative HO-1 inducers (Lin et al., 2007). Recently, AMP- activated protein kinase (AMPK) has been characterized as an HO-1 enhancer that interacts with Nrf2 when stimulated by many metabolic regulators and HO-1 inducers (Figure 2B) (Liu et al., 2011; Cho et al., 2018). In summary, induction of HO-1 can be achieved through various regulatory mechanisms under stress conditions.
HO-1 is expressed in bone cells involved in the maintenance of bone homeostasis physiologically (Anselmino et al., 2020). A prior clinical study reported that total bilirubin, a metabolite of HO-1, positively correlated with serum calcium and BMD, and subjects with osteoporosis had a significantly lower total bilirubin level (Bian et al., 2013). Another study showed that in rheumatoid arthritis patients, serous bilirubin levels were much lower in those suffering bone damage than in those without bone loss (Peng et al., 2017). These clinical data suggest that HO-1 plays a key role in the maintenance of bone mass in humans.
Pioneering work on the role of HO-1 in bone biology was conducted in mice with global gene knockout (KO) of Homx1, Nrf2 or Bach1 (Florczyk-Soluch et al., 2018; Hama et al., 2012). Compared to wild-type controls, global Homx1 KO mice revealed significantly decreased bone volume with a decline in the number of osteoblasts and osteogenic parameters. Also, plasma of Homx1−/− mice contained higher levels of C-telopeptide and tartrate-resistant acid phosphatase (TRAP) accompanied by an increase in active osteoclasts (Florczyk-Soluch et al., 2018; Ke et al., 2015; Zwerina et al., 2005). In the serum of Nrf2−/− mice, where HO-1 expression was inhibited, levels of RANKL significantly increased while osteocalcin decreased (Maicas et al., 2011). Besides, Nrf2 genetic deficiency boosted RANKL-induced osteoclastogenesis of bone marrow macrophages leading to bone resorption (Ibáñez et al., 2014). In Bach1 -KO mice, in which the expression of HO-1 was upregulated, the activity and mineralization of osteoblasts increased while osteoclastogenesis-induced bone resorption in vivo was repressed (Sudan et al., 2019). All these findings support that HO-1 functions in bone remodeling. Meanwhile, multiple studies have revealed that HO-1 deficient mice display increased inflammation accompanied by tissue iron accumulation and increased susceptibility to OS (Poss and Tonegawa, 1997a, b). Interestingly, the expression of HO-1 decreases with age. Induction of HO-1 in either MSCs or osteoblasts can reduce the features of senescence and restore the regenerative function of SnCs (Liu et al., 2017; Szade et al., 2020).
So far, there remains a lack of knowledge concerning the specific role of HO-1 in the bone tissues in vivo due to a lack of bone-specific conditional gene knockout animal models. To fill this research gap and to examine how HO-1 exerts its effect on bone metabolism through actions on bone cells, a battery of in vitro models with pharmacological treatment (such as hemin and cobalt protoporphyrin IX (CoPP)) or genetic modification to inhibit or overexpress HO-1 in osteogenic or osteoclast cell linages have been developed (Chae et al., 2006; Vanella et al., 2010; Nowak et al., 2018; Pan et al., 2018; Ma et al., 2020). Generally, the activation of HO-1 positively controls bone metabolism by maintaining an intracellular redox balance and cellular defenses to inflammation (Kensler et al., 2007; Rana et al., 2012). Under OS, which is induced by inflammatory cytokines such as TNF-α or by metabolic disorders such as diabetes-induced high glucose conditions, upregulation of HO-1 is required for maintaining mitochondrial homeostasis and protecting osteoblasts from apoptosis (Chae et al., 2006; Takanche et al., 2020; Zheng et al., 2021). Studies have shown that HO-1 induction in bone marrow mesenchymal stem cells (BMSCs) enhances the expression of osteogenic differentiation-related markers such as Runx2, bone morphogenetic protein-2 (Bmp2), osteocalcin (Bglap), and collagen 1A (Col1a), while also increasing the ratio of OPG/RANKL (Lin et al., 2010). Baebagallo et al. (Barbagallo et al., 2010) inhibited the expression of HO-1 in BMSCs by siRNA and detected an increase in the adipogenesis marker, PPARγ, whereas HO-1 overexpression promoted osteogenic differentiation and reduced the adipogenic differentiation. Meanwhile, the activity of senescence-associated β-galactosidase and the expression of the senescence markers were significantly decreased upon HO-1 induction, indicating that HO-1 levels could be strategically manipulated to protect osteoblast from senescence to restore cell function (Clérigues et al., 2012). These findings above all underline that HO-1 plays an indispensable, positive role in the process of bone formation.
As for the formation and activation of osteoclasts, HO-1 acts as a suppressor. When RANKL is combined with its receptor, the downstream signaling cascades such as NF-κB and MAPKs are activated, resulting in the sequential activation of nuclear factor of activated T cells cytoplasmic 1 (NFATc1) and c-Fos, known as master regulators of osteoclast differentiation and maturation (Boyle et al., 2003). It is worth noting that RANKL stimulation can upregulate Keap1 and induce separation of Nrf2 from the nucleus, resulting in reduction of downstream HO-1 (Kanzaki et al., 2013). The inhibition of HO-1 in bone marrow-derived macrophages (BMMs) consequently leads to impaired osteoclast differentiation (Sakai et al., 2012). Thus, HO-1 downregulation is a critical step of RANKL-induced osteoclastogenesis. Meanwhile, a series of studies have identified HO-1 as a negative regulator of osteoclast differentiation. In vitro, hemin-induced HO-1 upregulation downregulates the expression of cfms, RANK, TRAF-6, and c-fos mediated by MAPK inhibition, resulting in a compromised response of osteoclast to RANKL (Zwerina et al., 2005). HO-1 activation also decreases NF-κB translocation and preventes bone loss accompanied by a significant decrease in the ratio of RANKL/OPG (Kim et al., 2019). HO-1 also reduces intracellular ROS levels of the osteoclast precursors by suppressing expression of NOX1 and TRAF6 (Ke et al., 2015). Furthermore, metabolic products of HO-1, including CO and bilirubin can reduce RANKL-induced osteoclastogenesis via inhibiting the ROS/NF-κB pathway (Bak et al., 2017). In vivo, increasing HO-1 expression alleviates loss of bone mass in OVX osteoporotic mice (Xiao et al., 2020). Besides direct inhibition, HO-1 may exert immunomodulatory effects on the production of immune and inflammatory factors, and negatively regulate the differentiation or function of osteoclasts (Castejón et al., 2017; Alcaraz and Ferrándiz, 2020).
Growing evidence shows that iron plays an important role in the regulation of bone metabolism. Iron deficiency negatively affects collagen synthesis and vitamin D metabolism (Toxqui and Vaquero, 2015). However, iron overload, a state of excessive iron storage seen especially in patients with thalassemia, hemochromatosis, or sickle cell disease, is closely related to osteoporosis by promoting osteoclast differentiation and suppressing the proliferation and differentiation of osteoblasts (Jeney, 2017). In this regard, iron which is induced by HO-1-mediated heme degradation should be immediately seized by ferritin to avoid excess release and deposition. An in vitro study showed that HO-1 decreased the apoptotic rate of BMMSCs with iron overload through reducing intracellular ROS (Yu et al., 2018), indicating that HO-1 might participate in the regulation of iron metabolic homeostasis or attenuate iron-induced toxicity through a more comprehensive mechanism.
In summary, by alleviating inflammation and oxidative stress, HO-1 provides a favorable remodeling microenvironment and maintains a positive net balance of bone via dual-regulation of both osteoblasts and osteoclasts (shown in Figure 3).
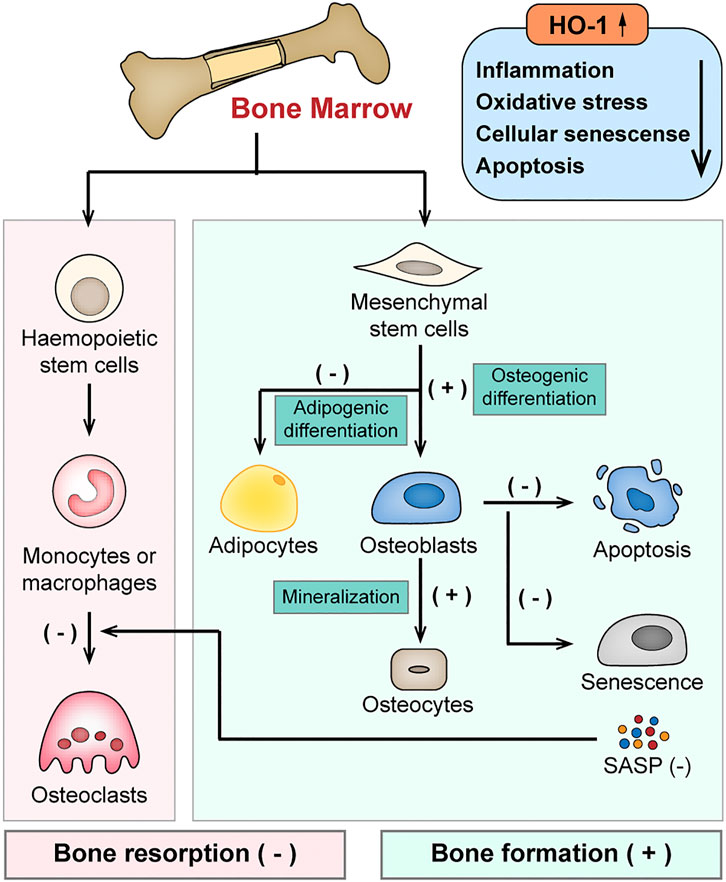
FIGURE 3. Schematic diagram of the protective effects of HO-1 in bone remodeling. HO-1 maintains a positive net balance of bone remodeling via dual-regulation of both osteoblasts and osteoclasts by alleviating inflammation, oxidative stress in the bone microenvironment. HO-1 regulates the differentiation of bone mesenchymal stem cells (BMSCs), enhances osteoblast function and inhibits the apoptosis and senescence of osteoblasts. It also negatively regulates the differentiation or function of osteoclasts.
Despite the studies mentioned above supporting the notion that HO-1 is a novel therapeutic target for bone diseases, especially osteoporosis, it remains challenging for HO-1-based therapies to go into clinical application. Traditional HO-1 inducers such as metalloporphyrin are unsuitable for clinical use due to the significant toxicity (Suresh et al., 2003). Furthermore, clinical use of HO-1 metabolites, such as CO, is also hindered by concerns about toxicity and bioavailability (Bauer and Pannen, 2009). Thus, it is urgent to develop safer and more tolerable alternatives to HO-1 inducers and metabolites. In the following sections, we select some promising candidates and discuss their biological effects, as revealed by in vitro or in vivo studies (see Table 1 and Table 2).
Phytochemicals are secondary metabolites found in various plants and herbal substances which have been widely studied as antioxidant and anti-inflammatory agents (Islam et al., 2016; Pandey et al., 2018; Shin et al., 2020). Certain phytochemicals, employed alone or combined with other agents appear to be safe and effective disease-modifying drugs. Many phytochemicals have been reported to exert protective effects on bone cells and osteoporosis animal models through activation of HO-1 (Haines et al., 2012; Su et al., 2013). Among them, the most promising drug candidates are curcumin and resveratrol, both of which have been suggested to be effective in the treatment of osteoporosis in laboratory, translational and clinical studies (see Table 3) (Deng et al., 2021; Moschen and Shakibaei, 2013; Sharan et al., 2009). In a double-blind randomized controlled trial (RCT), postmenopausal osteoporotic women treated with the combination of curcumin and alendronate showed significant increases in total hip, lumbar spine and femoral neck BMDs accompanied by increased bone turnover markers (Khanizadeh et al., 2018). Similarly, a 24 months, two-period crossover clinical RCT was conducted recently to evaluate whether resveratrol supplementation could strengthen bones in postmenopausal women. The results showed that resveratrol (75 mg, twice daily) positively augmented BMD in the lumbar spine and femoral neck together with a 7.24% reduction in CTx (C-telopeptide of type I collagen, a marker of bone resorption). Further, the increased BMD in the femoral neck accounted for a reduction in the 10 years probability of hip fracture risk (Wong et al., 2020). Another RCT evaluating effects of resveratrol treatment on bone in obese men with metabolic syndrome revealed that high-dose resveratrol supplementation (Oral treatment with 1,000 mg daily) stimulated bone formation or mineralization (Ornstrup et al., 2014). However, several considerations should not be ignored. Firstly, despite their effectiveness, some of these compounds can modulate different signaling pathways and do not have selectivity for HO-1, which might lead to undesired side effects. For instance, many phytochemicals have low bioavailability, partially due to their poor stability and solubility in the digestive tract, which ultimately compromises their clinical use (Dos Santos et al., 2011; Mukkavilli et al., 2017; Wong et al., 2021). Secondly, these phytochemicals appear to be effective only at supraphysiological concentrations far exceeding those achievable through a daily diet. To overcome the shortcomings of phytochemicals, structural modification and catalyst compound-based approaches including novel delivery systems have been used (reviewed by Dei Cas, Ghidoni and McClements (Dei Cas and Ghidoni, 2019; McClements, 2020)). In addition, nanotechnology-based formulations have been shown to be useful as therapeutic agents for preventing and treating osteoporosis (Heo et al., 2014). Even so, it is necessary for much more attention to be paid to questions of bioavailability, route of administration and effective dosages before phytochemical HO-1 inducers go into clinical translation.
Some existing drugs currently used for the treatment of inflammation have been revealed to activate NRF2 and/or upregulate HO-1. For example, 5-aminolevulinic acid (5- ALA) has been widely applied in photodynamic therapy for the treatment of skin diseases and some tumors (Shi et al., 2021). NRF2 activation and HO-1 upregulation by 5-ALA also present therapeutic potential for osteoporosis. In vitro, 5-ALA suppresses RANKL-mediated nuclear translocation of Bach1 and upregulates nuclear NRF2, inducing HO-1 expression in mouse primary peritoneal macrophages and in RAW264.7 cells, in turn increasing antioxidant activity. This results in decreased osteoclastogenesis and inhibited bone resorption (Kanzaki et al., 2017). Similarly, dimethyl fumarate (DMF) has been reported to trigger Nrf2 signaling to induce HO-1 and has been applied in clinical trials for skin diseases and neurodegenerative diseases (Foresti et al., 2013; Lehmann et al., 2007). In macrophages, DMF attenuates RANKL-induced intracellular ROS, inhibits RANKL-mediated osteoclastogenesis and suppresses osteoclast function, thus protecting bone from destruction (Yamaguchi et al., 2018). Melatonin significantly improves the osteogenic capacity of MC3T3-E1 cells by reducing the level of ferroptosis through via Nrf2/HO-1 pathway and augments bone mass in type 2 diabetic osteoporotic rats (Ma et al., 2020). Through enhancing HO-1, simvastatin, a potent hypolipidemic drug, ameliorated H2O2-induced intracellular OS and cell apoptosis while increasing alkaline phosphatase (ALP) activity in MG-63 human osteoblastic cells. In addition, simvastatin inhibits nitric oxide synthase (NOS) activity and iNOS expression under OS to protect against osteoporosis in aged and OVX rats (Yin et al., 2012). These drugs could be suitable candidates for osteoporosis treatment as HO-1 inducers considering their existing regulatory approval and safety data.
CO-releasing molecules (CORMs) are spatially and temporally controlled CO releasers that can target specific tissues and present an alternative to CO gas inhalation. The biological effects of CORMs have also been observed in various animal studies and preclinical trials; they act via upregulation of HO-1 and can prevent inflammation and apoptosis (Alcaraz et al., 2008). When pre-treated with CORM-2, MSCs’ resistance to H2O2-mediated apoptosis is significantly increased (Cremers et al., 2014). It has been shown that, both in RAW264.7 cells and BMMs, CORM-2 treatment inhibits RANKL induced osteoclastogenesis and osteoclastic resorption activity by reducing RANKL-induced NFATc1 expression via inhibition of IKK-dependent NF-κB activation and ROS production (Bak et al., 2017). In vivo, administration of CORM-3 in OVX osteoporotic mice strongly induces HO-1 expression and shows a potent protection of BMD and bone mass as well as microarchitecture. The protective effects could be attributed to an anti-inflammatory effect, as indicated by lower levels of serous inflammatory cytokines, including TNF-α and IL-6 (Ibanez et al., 2012). However, heavy metal-based carrier presents toxicity concerns and there remains uncertainty concerning what constitutes a safe and effective dose, so further research is required before CORMs are eventually translated into clinical use.
Recently, there has been increasing focus on identification of novel HO-1 inducers that may hold potential as anti-osteoporosis therapies. RTA-408, a novel NRF2 activator, has shown clinical therapeutic potential for dermatitis (Reisman et al., 2014), solid tumors (Creelan et al., 2017) and mitochondrial myopathies (A et al., 2016). A recent report revealed that RTA-408 effectively attenuates OVX-induced bone loss in mice by inhibiting STING-dependent NF-κB signaling and subsequent osteoclastogenesis (Sun et al., 2020). Itaconate, an endogenous metabolite, has been demonstrated to activate NRF2 to induce downstream HO-1 through alkylation of KEAP1, and controls inflammation control in both murine and human macrophages (Lampropoulou et al., 2016; Mills et al., 2018). Itaconate also ameliorates the severity of bone loss in a mouse model of OVX-induced osteoporosis and restrains ROS production, inflammatory responses and osteoclastogenesis via inhibition of the E3 ubiquitin ligase (Hrd1) to dislocate Nrf2 from ubiquitin (Sun et al., 2019), suggesting it could be a promising candidate for osteoporosis treatment in the future.
This review reveals the important roles of stress-induced HO-1 activity in bone homeostasis and disorders, most notably osteoporosis. HO-1 can effectively restore the balance of bone remodeling through directly regulating the survival, differentiation, and function of bone cells, as well as by exerting anti-inflammatory, anti-oxidative and immunoregulatory effects to modulate the bone remodeling microenvironment. It is therefore a promising novel target for the development of anti-osteoporotic therapies. Up to now, some phytochemicals, existing drugs, and CORMs as well as novel Nrf2 inducers have been reported to prevent bone loss by upregulating HO-1 expression. In addition, the accumulated preclinical evidence and ongoing clinical trials have the lain the foundation for HO-1 inducers to be used as anti-osteoporotic drugs. However, despite significant progress, from a clinical perspective, the therapeutic potential of HO-1 is yet to be realized, and many questions about how to optimize the efficacy and minimize undesired effects of HO-1 inducers remain to be answered. Thus, further studies should be pursued to investigate novel or alternative HO-1 inducers, as well as to repurpose existing drugs as HO-1 stimulators.
XZ and JW conceived the idea. XZ, WY, and JaL wrote the manuscript, YZ created the table, XZ, ZZ, and XX prepared the figures. JW and JnL revised the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by National Natural Science Foundation of China (No. 81771114 and No. 81970967 to JW) and Sichuan Science and Technology Program (No. 2020YFS0173 to JW).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdallah, B. M., Haack-Sørensen, M., Fink, T., and Kassem, M. (2006). Inhibition of Osteoblast Differentiation but Not Adipocyte Differentiation of Mesenchymal Stem Cells by Sera Obtained from Aged Females. Bone 39, 181–188. doi:10.1016/j.bone.2005.12.082
Acosta, J. C., Banito, A., Wuestefeld, T., Georgilis, A., Janich, P., Morton, J. P., et al. (2013). A Complex Secretory Program Orchestrated by the Inflammasome Controls Paracrine Senescence. Nat. Cel Biol. 15, 978–990. doi:10.1038/ncb2784
Ai, Z., Wu, Y. o., Yu, M., Li, J., and Li, S. (2020). Theaflavin-3, 3′-Digallate Suppresses RANKL-Induced Osteoclastogenesis and Attenuates Ovariectomy-Induced Bone Loss in Mice. Front. Pharmacol. 11, 803. doi:10.3389/fphar.2020.00803
Akkaoui, J., Yamada, C., Duarte, C., Ho, A., Vardar-Sengul, S., Kawai, T., et al. (2021). Contribution of Porphyromonas Gingivalis Lipopolysaccharide to Experimental Periodontitis in Relation to Aging. GeroScience 43, 367–376. doi:10.1007/s11357-020-00258-1
Alam, J., and Cook, J. L. (2007). How many Transcription Factors Does it Take to Turn on the Heme Oxygenase-1 Gene? Am. J. Respir. Cel Mol. Biol. 36, 166–174. doi:10.1165/rcmb.2006-0340TR
Alcaraz, M., Guillen, M., Ferrandiz, M., Megias, J., and Motterlini, R. (2008). Carbon Monoxide-Releasing Molecules: a Pharmacological Expedient to Counteract Inflammation. Cpd 14, 465–472. doi:10.2174/138161208783597362
Alcaraz, M. J., and Ferrándiz, M. L. (2020). Relevance of Nrf2 and Heme Oxygenase-1 in Articular Diseases. Free Radic. Biol. Med. 157, 83–93. doi:10.1016/j.freeradbiomed.2019.12.007
Almeida, M., Han, L., Martin-Millan, M., O'Brien, C. A., and Manolagas, S. C. (2007a). Oxidative Stress Antagonizes Wnt Signaling in Osteoblast Precursors by Diverting β-Catenin from T Cell Factor- to Forkhead Box O-Mediated Transcription. J. Biol. Chem. 282, 27298–27305. doi:10.1074/jbc.M702811200
Almeida, M., Han, L., Martin-Millan, M., Plotkin, L. I., Stewart, S. A., Roberson, P. K., et al. (2007b). Skeletal Involution by Age-Associated Oxidative Stress and its Acceleration by Loss of Sex Steroids. J. Biol. Chem. 282, 27285–27297. doi:10.1074/jbc.M702810200
Ambrogini, E., Almeida, M., Martin-Millan, M., Paik, J.-H., Depinho, R. A., Han, L., et al. (2010). FoxO-mediated Defense against Oxidative Stress in Osteoblasts Is Indispensable for Skeletal Homeostasis in Mice. Cel Metab. 11, 136–146. doi:10.1016/j.cmet.2009.12.009
An, Y., Zhang, H., Wang, C., Jiao, F., Xu, H., Wang, X., et al. (2019). Activation of ROS/MAPK S/NF‐ κ B/NLRP3 and Inhibition of Efferocytosis in Osteoclast‐mediated Diabetic Osteoporosis. FASEB j. 33, 12515–12527. doi:10.1096/fj.201802805RR
Anselmino, N., Starbuck, M., Labanca, E., Cotignola, J., Navone, N., Gueron, G., et al. (2020). Heme Oxygenase-1 Is a Pivotal Modulator of Bone Turnover and Remodeling: Molecular Implications for Prostate Cancer Bone Metastasis. Antioxid. Redox Signaling 32, 1243–1258. doi:10.1089/ars.2019.7879
Arron, J. R., and Choi, Y. (2000). Bone versus Immune System. Nature 408, 535–536. doi:10.1038/35046196
Ayer, A., Zarjou, A., Agarwal, A., and Stocker, R. (2016). Heme Oxygenases in Cardiovascular Health and Disease. Physiol. Rev. 96, 1449–1508. doi:10.1152/physrev.00003.2016
Baek, K. H., Oh, K. W., Lee, W. Y., Tae, H. J., Rhee, E. J., Han, J. H., et al. (2006). Changes in the Serum Sex Steroids, IL-7 and RANKL-OPG System after Bone Marrow Transplantation: Influences on Bone and mineral Metabolism. Bone 39, 1352–1360. doi:10.1016/j.bone.2006.06.011
Bak, S.-U., Kim, S., Hwang, H.-J., Yun, J.-A., Kim, W.-S., Won, M.-H., et al. (2017). Heme Oxygenase-1 (HO-1)/carbon Monoxide (CO) axis Suppresses RANKL-Induced Osteoclastic Differentiation by Inhibiting Redox-Sensitive NF-Κb Activation. BMB Rep. 50, 103–108. doi:10.5483/bmbrep.2017.50.2.220
Barbagallo, I., Vanella, A., Peterson, S. J., Kim, D. H., Tibullo, D., Giallongo, C., et al. (2010). Overexpression of Heme Oxygenase-1 Increases Human Osteoblast Stem Cell Differentiation. J. Bone Miner. Metab. 28, 276–288. doi:10.1007/s00774-009-0134-y
Bauer, I., and Pannen, B. H. (2009). Bench-to-bedside Review: Carbon Monoxide - from Mitochondrial Poisoning to Therapeutic Use. Crit. Care 13, 220. doi:10.1186/cc7887
Bhattarai, G., Poudel, S. B., Kook, S.-H., and Lee, J.-C. (2016). Resveratrol Prevents Alveolar Bone Loss in an Experimental Rat Model of Periodontitis. Acta Biomater. 29, 398–408. doi:10.1016/j.actbio.2015.10.031
Bian, L.-Q., Li, R.-Z., Zhang, Z.-Y., Jin, Y.-J., Kang, H.-W., Fang, Z.-Z., et al. (2013). Effects of Total Bilirubin on the Prevalence of Osteoporosis in Postmenopausal Women without Potential Liver Disease. J. Bone Miner. Metab. 31, 637–643. doi:10.1007/s00774-013-0452-y
Blume, S. W., and Curtis, J. R. (2011). Medical Costs of Osteoporosis in the Elderly Medicare Population. Osteoporos. Int. 22, 1835–1844. doi:10.1007/s00198-010-1419-7
Boyle, W. J., Simonet, W. S., and Lacey, D. L. (2003). Osteoclast Differentiation and Activation. Nature 423, 337–342. doi:10.1038/nature01658
Bukhari, S. N. A., Hussain, F., Thu, H. E., and Hussain, Z. (2019). Synergistic Effects of Combined Therapy of Curcumin and Fructus Ligustri Lucidi for Treatment of Osteoporosis: Cellular and Molecular Evidence of Enhanced Bone Formation. J. Integr. Med. 17, 38–45. doi:10.1016/j.joim.2018.08.003
Callaway, D. A., and Jiang, J. X. (2015). Reactive Oxygen Species and Oxidative Stress in Osteoclastogenesis, Skeletal Aging and Bone Diseases. J. Bone Miner. Metab. 33, 359–370. doi:10.1007/s00774-015-0656-4
Castejón, M. L., Rosillo, M. Á., Montoya, T., González-Benjumea, A., Fernández-Bolaños, J. M., and Alarcón-de-la-Lastra, C. (2017). Oleuropein Down-Regulated IL-1β-induced Inflammation and Oxidative Stress in Human Synovial Fibroblast Cell Line SW982. Food Funct. 8, 1890–1898. doi:10.1039/c7fo00210f
Chae, H. J., Chin, H. Y., Lee, G. Y., Park, H. R., Yang, S. K., Chung, H. T., et al. (2006). Carbon Monoxide and Nitric Oxide Protect against Tumor Necrosis Factor-α-Induced Apoptosis in Osteoblasts: HO-1 Is Necessary to Mediate the protection. Clinica Chim. Acta 365, 270–278. doi:10.1016/j.cca.2005.09.011
Chaea, H.-J., Kim, H.-R., Kang, Y. J., Hyun, K. C., Kim, H. J., Seo, H. G., et al. (2007). Heme Oxygenase-1 Induction by (S)-enantiomer of YS-51 (YS-51S), a Synthetic Isoquinoline Alkaloid, Inhibits Nitric Oxide Production and Nuclear Factor-Κb Translocation in ROS 17/2.8 Cells Activated with Inflammatory Stimulants. Int. Immunopharmacology 7, 1559–1568. doi:10.1016/j.intimp.2007.07.023
Chandra, A., Lagnado, A. B., Farr, J. N., Monroe, D. G., Park, S., Hachfeld, C., et al. (2020). Targeted Reduction of Senescent Cell Burden Alleviates Focal Radiotherapy‐Related Bone Loss. J. Bone Miner Res. 35, 1119–1131. doi:10.1002/jbmr.3978
Che, J., Yang, J., Zhao, B., and Shang, P. (2021). HO-1: A New Potential Therapeutic Target to Combat Osteoporosis. Eur. J. Pharmacol. 906, 174219. doi:10.1016/j.ejphar.2021.174219
Chen, Q., Liu, K., Robinson, A. R., Clauson, C. L., Blair, H. C., Robbins, P. D., et al. (2013). DNA Damage Drives Accelerated Bone Aging via an NF-κb-dependent Mechanism. J. Bone Miner Res. 28, 1214–1228. doi:10.1002/jbmr.1851
Chen, Z., Xue, J., Shen, T., Ba, G., Yu, D., and Fu, Q. (2016a). Curcumin Alleviates Glucocorticoid-Induced Osteoporosis by Protecting Osteoblasts from Apoptosisin Vivoandin Vitro. Clin. Exp. Pharmacol. Physiol. 43, 268–276. doi:10.1111/1440-1681.12513
Chen, Z., Xue, J., Shen, T., Mu, S., and Fu, Q. (2016b). Curcumin Alleviates Glucocorticoid-Induced Osteoporosis through the Regulation of the Wnt Signaling Pathway. Int. J. Mol. Med. 37, 329–338. doi:10.3892/ijmm.2015.2432
Cheng, J., Wang, H., Zhang, Z., and Liang, K. (2019). Stilbene Glycoside Protects Osteoblasts against Oxidative Damage via Nrf2/HO-1 and NF-Κb Signaling Pathways. aoms 15, 196–203. doi:10.5114/aoms.2018.79937
Cheng, X., Ku, C.-H., and Siow, R. C. M. (2013). Regulation of the Nrf2 Antioxidant Pathway by microRNAs: New Players in Micromanaging Redox Homeostasis. Free Radic. Biol. Med. 64, 4–11. doi:10.1016/j.freeradbiomed.2013.07.025
Chiang, S.-K., Chen, S.-E., and Chang, L.-C. (2018). A Dual Role of Heme Oxygenase-1 in Cancer Cells. Ijms 20, 39. doi:10.3390/ijms20010039
Cho, R.-L., Lin, W.-N., Wang, C.-y., Yang, C.-C., Hsiao, L.-D., Lin, C.-C., et al. (2018). Heme Oxygenase-1 Induction by Rosiglitazone via PKCα/AMPKα/p38 MAPKα/SIRT1/PPARγ Pathway Suppresses Lipopolysaccharide-Mediated Pulmonary Inflammation. Biochem. Pharmacol. 148, 222–237. doi:10.1016/j.bcp.2017.12.024
Choi, E. M., Suh, K. S., Kim, Y. J., Hong, S. M., Park, S. Y., and Chon, S. (2016). Glabridin Alleviates the Toxic Effects of Methylglyoxal on Osteoblastic MC3T3-E1 Cells by Increasing Expression of the Glyoxalase System and Nrf2/HO-1 Signaling and Protecting Mitochondrial Function. J. Agric. Food Chem. 64, 226–235. doi:10.1021/acs.jafc.5b05157
Clérigues, V., Guillén, M. I., Castejón, M. A., Gomar, F., Mirabet, V., and Alcaraz, M. J. (2012). Heme Oxygenase-1 Mediates Protective Effects on Inflammatory, Catabolic and Senescence Responses Induced by Interleukin-1β in Osteoarthritic Osteoblasts. Biochem. Pharmacol. 83, 395–405. doi:10.1016/j.bcp.2011.11.024
Coppé, J.-P., Desprez, P.-Y., Krtolica, A., and Campisi, J. (2010). The Senescence-Associated Secretory Phenotype: the Dark Side of Tumor Suppression. Annu. Rev. Pathol. Mech. Dis. 5, 99–118. doi:10.1146/annurev-pathol-121808-102144
Creelan, B., Gabrilovich, D., Gray, J., Williams, C., Tanvetyanon, T., Haura, E., et al. (2017). Safety, Pharmacokinetics, and Pharmacodynamics of Oral Omaveloxolone (RTA 408), a Synthetic Triterpenoid, in a First-In-Human Trial of Patients with Advanced Solid Tumors. Ott 10, 4239–4250. doi:10.2147/ott.S136992
Cremers, N., Lundvig, D., van Dalen, S., Schelbergen, R., van Lent, P., Szarek, W., et al. (2014). Curcumin-Induced Heme Oxygenase-1 Expression Prevents H2O2-Induced Cell Death in Wild Type and Heme Oxygenase-2 Knockout Adipose-Derived Mesenchymal Stem Cells. Ijms 15, 17974–17999. doi:10.3390/ijms151017974
Davis, S., Martyn-St James, M., Sanderson, J., Stevens, J., Goka, E., Rawdin, A., et al. (2016). A Systematic Review and Economic Evaluation of Bisphosphonates for the Prevention of Fragility Fractures. Health Technol. Assess. 20, 1–406. doi:10.3310/hta20780
De Cecco, M., Ito, T., Petrashen, A. P., Elias, A. E., Skvir, N. J., Criscione, S. W., et al. (2019). L1 Drives IFN in Senescent Cells and Promotes Age-Associated Inflammation. Nature 566, 73–78. doi:10.1038/s41586-018-0784-9
Dei Cas, M., and Ghidoni, R. (2019). Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 11, 2147. doi:10.3390/nu11092147
Deng, J., Golub, L. M., Lee, H.-M., Raja, V., Johnson, F., Kucine, A., et al. (2021). A Novel Modified-Curcumin Promotes Resolvin-like Activity and Reduces Bone Loss in Diabetes-Induced Experimental Periodontitis. Jir 14, 5337–5347. doi:10.2147/jir.S330157
Devlin, R. D., Reddy, S. V., Savino, R., Ciliberto, G., and Roodman, G. D. (1998). IL-6 Mediates the Effects of IL-1 or TNF, but Not PTHrP or 1,25(OH)2D3, on Osteoclast-like Cell Formation in normal Human Bone Marrow Cultures. J. Bone Miner. Res. 13, 393–399. doi:10.1359/jbmr.1998.13.3.393
Dimri, G. P., Lee, X., Basile, G., Acosta, M., Scott, G., Roskelley, C., et al. (1995). A Biomarker that Identifies Senescent Human Cells in Culture and in Aging Skin In Vivo. Proc. Natl. Acad. Sci. 92, 9363–9367. doi:10.1073/pnas.92.20.9363
Dos Santos, C., Buera, M. P., and Mazzobre, M. F. (2011). Phase Solubility Studies and Stability of Cholesterol/β-Cyclodextrin Inclusion Complexes. J. Sci. Food Agric. 91, 2551–2557. doi:10.1002/jsfa.4425
Du, D., Zhou, Z., Zhu, L., Hu, X., Lu, J., Shi, C., et al. (2018). TNF-α Suppresses Osteogenic Differentiation of MSCs by Accelerating P2Y2 Receptor in Estrogen-Deficiency Induced Osteoporosis. Bone 117, 161–170. doi:10.1016/j.bone.2018.09.012
Farr, J. N., Fraser, D. G., Wang, H., Jaehn, K., Ogrodnik, M. B., Weivoda, M. M., et al. (2016). Identification of Senescent Cells in the Bone Microenvironment. J. Bone Miner. Res. 31, 1920–1929. doi:10.1002/jbmr.2892
Farr, J. N., and Khosla, S. (2019). Cellular Senescence in Bone. Bone 121, 121–133. doi:10.1016/j.bone.2019.01.015
Farr, J. N., Xu, M., Weivoda, M. M., Monroe, D. G., Fraser, D. G., Onken, J. L., et al. (2017). Targeting Cellular Senescence Prevents Age-Related Bone Loss in Mice. Nat. Med. 23, 1072–1079. doi:10.1038/nm.4385
Feng, X., and McDonald, J. M. (2011). Disorders of Bone Remodeling. Annu. Rev. Pathol. Mech. Dis. 6, 121–145. doi:10.1146/annurev-pathol-011110-130203
Florczyk-Soluch, U., Józefczuk, E., Stępniewski, J., Bukowska-Strakova, K., Mendel, M., Viscardi, M., et al. (2018). Various Roles of Heme Oxygenase-1 in Response of Bone Marrow Macrophages to RANKL and in the Early Stage of Osteoclastogenesis. Sci. Rep. 8, 10797. doi:10.1038/s41598-018-29122-1
Foresti, R., Bains, S. K., Pitchumony, T. S., de Castro Brás, L. E., Drago, F., Dubois-Randé, J.-L., et al. (2013). Small Molecule Activators of the Nrf2-HO-1 Antioxidant axis Modulate Heme Metabolism and Inflammation in BV2 Microglia Cells. Pharmacol. Res. 76, 132–148. doi:10.1016/j.phrs.2013.07.010
French, D. L., Muir, J. M., and Webber, C. E. (2008). The Ovariectomized, Mature Rat Model of Postmenopausal Osteoporosis: an Assessment of the Bone Sparing Effects of Curcumin. Phytomedicine 15, 1069–1078. doi:10.1016/j.phymed.2008.06.007
Gozzelino, R., Jeney, V., and Soares, M. P. (2010). Mechanisms of Cell protection by Heme Oxygenase-1. Annu. Rev. Pharmacol. Toxicol. 50, 323–354. doi:10.1146/annurev.pharmtox.010909.105600
Haines, D. D., Lekli, I., Teissier, P., Bak, I., and Tosaki, A. (2012). Role of Haeme Oxygenase-1 in Resolution of Oxidative Stress-Related Pathologies: Focus on Cardiovascular, Lung, Neurological and Kidney Disorders. Acta Physiol. (Oxf.) 204, 487–501. doi:10.1111/j.1748-1716.2011.02387.x
Hama, M., Kirino, Y., Takeno, M., Takase, K., Miyazaki, T., Yoshimi, R., et al. (2012). Bach1 Regulates Osteoclastogenesis in a Mouse Model via Both Heme Oxygenase 1-dependent and Heme Oxygenase 1-independent Pathways. Arthritis Rheum. 64, 1518–1528. doi:10.1002/art.33497
Han, D., Gu, X., Gao, J., Wang, Z., Liu, G., Barkema, H. W., et al. (2019). Chlorogenic Acid Promotes the Nrf2/HO-1 Anti-oxidative Pathway by Activating p21Waf1/Cip1 to Resist Dexamethasone-Induced Apoptosis in Osteoblastic Cells. Free Radic. Biol. Med. 137, 1–12. doi:10.1016/j.freeradbiomed.2019.04.014
Haugeberg, G., Lodder, M., Lems, W., Uhlig, T., Ørstavik, R., Dijkmans, B., et al. (2004). Hand Cortical Bone Mass and its Associations with Radiographic Joint Damage and Fractures in 50-70 Year Old Female Patients with Rheumatoid Arthritis: Cross Sectional Oslo-Truro-Amsterdam (OSTRA) Collaborative Study. Ann. Rheum. Dis. 63, 1331–1334. doi:10.1136/ard.2003.015065
Hayflick, L. (1965). The Limited In Vitro Lifetime of Human Diploid Cell Strains. Exp. Cel Res. 37, 614–636. doi:10.1016/0014-4827(65)90211-9
He, T., Shen, H., Zhu, J., Zhu, Y., He, Y., Li, Z., et al. (2019). Geniposide Attenuates Cadmium-induced O-xidative S-tress I-njury via Nrf2 S-ignaling in O-steoblasts. Mol. Med. Rep. 20, 1499–1508. doi:10.3892/mmr.2019.10396
Heinrich, P. C., Behrmann, I., Haan, S., Hermanns, H. M., Müller-newen, G., and Schaper, F. (2003). Principles of Interleukin (IL)-6-type Cytokine Signalling and its Regulation. Biochem. J. 374, 1–20. doi:10.1042/bj20030407
Hendrickx, G., Boudin, E., and Van Hul, W. (2015). A Look behind the Scenes: the Risk and Pathogenesis of Primary Osteoporosis. Nat. Rev. Rheumatol. 11, 462–474. doi:10.1038/nrrheum.2015.48
Heo, D. N., Ko, W.-K., Moon, H.-J., Kim, H.-J., Lee, S. J., Lee, J. B., et al. (2014). Inhibition of Osteoclast Differentiation by Gold Nanoparticles Functionalized with Cyclodextrin Curcumin Complexes. ACS Nano 8, 12049–12062. doi:10.1021/nn504329u
Hernandez-Segura, A., Rubingh, R., and Demaria, M. (2019). Identification of Stable Senescence-Associated Reference Genes. Aging Cell 18, e12911. doi:10.1111/acel.12911
Hofbauer, L. C., Khosla, S., Dunstan, C. R., Lacey, D. L., Boyle, W. J., and Riggs, B. L. (2000). The Roles of Osteoprotegerin and Osteoprotegerin Ligand in the Paracrine Regulation of Bone Resorption. J. Bone Miner. Res. 15, 2–12. doi:10.1359/jbmr.2000.15.1.2
Hong, J., Shi, Z., Li, C., Ji, X., Li, S., Chen, Y., et al. (2021). Virtual Screening Identified Natural Keap1-Nrf2 PPI Inhibitor Alleviates Inflammatory Osteoporosis through Nrf2-Mir214-Traf3 axis. Free Radic. Biol. Med. 171, 365–378. doi:10.1016/j.freeradbiomed.2021.05.020
Hussan, F., Ibraheem, N. G., Kamarudin, T. A., Shuid, A. N., Soelaiman, I. N., and Othman, F. (20122012). Curcumin Protects against Ovariectomy-Induced Bone Changes in Rat Model. Evidence-Based Complement. Altern. Med. 2012, 1–7. doi:10.1155/2012/174916
Hyeon, S., Lee, H., Yang, Y., and Jeong, W. (2013). Nrf2 Deficiency Induces Oxidative Stress and Promotes RANKL-Induced Osteoclast Differentiation. Free Radic. Biol. Med. 65, 789–799. doi:10.1016/j.freeradbiomed.2013.08.005
Ibáñez, L., Alcaraz, M. J., Maicas, N., Guede, D., Caeiro, J. R., Motterlini, R., et al. (2012). Downregulation of the Inflammatory Response by CORM-3 Results in Protective Effects in a Model of Postmenopausal Arthritis. Calcif. Tissue Int. 91, 69–80. doi:10.1007/s00223-012-9612-7
Ibáñez, L., Ferrándiz, M. L., Brines, R., Guede, D., Cuadrado, A., and Alcaraz, M. J. (20142014). Effects of Nrf2 Deficiency on Bone Microarchitecture in an Experimental Model of Osteoporosis. Oxidative Med. Cell Longevity 2014, 1–9. doi:10.1155/2014/726590
Iqbal, J., Sun, L., Kumar, T. R., Blair, H. C., and Zaidi, M. (2006). Follicle-stimulating Hormone Stimulates TNF Production from Immune Cells to Enhance Osteoblast and Osteoclast Formation. Proc. Natl. Acad. Sci. 103, 14925–14930. doi:10.1073/pnas.0606805103
Islam, M. A., Alam, F., Solayman, M., Khalil, M. I., Kamal, M. A., and Gan, S. H. (20162016). Dietary Phytochemicals: Natural Swords Combating Inflammation and Oxidation-Mediated Degenerative Diseases. Oxidative Med. Cell Longevity 2016, 1–25. doi:10.1155/2016/5137431
Jeney, V. (2017). Clinical Impact and Cellular Mechanisms of Iron Overload-Associated Bone Loss. Front. Pharmacol. 8, 77. doi:10.3389/fphar.2017.00077
Jeon, W.-J., Kim, K.-M., Kim, E.-J., and Jang, W.-G. (2017). Costunolide Increases Osteoblast Differentiation via ATF4-dependent HO-1 Expression in C3H10T1/2 Cells. Life Sci. 178, 94–99. doi:10.1016/j.lfs.2017.04.012
Kanzaki, H., Shinohara, F., Itohiya, K., Yamaguchi, Y., Katsumata, Y., Matsuzawa, M., et al. (2017). RANKL Induces Bach1 Nuclear Import and Attenuates Nrf2‐mediated Antioxidant Enzymes, Thereby Augmenting Intracellular Reactive Oxygen Species Signaling and Osteoclastogenesis in Mice. FASEB j. 31, 781–792. doi:10.1096/fj.201600826R
Kanzaki, H., Shinohara, F., Kajiya, M., and Kodama, T. (2013). The Keap1/Nrf2 Protein axis Plays a Role in Osteoclast Differentiation by Regulating Intracellular Reactive Oxygen Species Signaling. J. Biol. Chem. 288, 23009–23020. doi:10.1074/jbc.M113.478545
Ke, K., Safder, M. A., Sul, O.-J., Kim, W.-K., Suh, J.-H., Joe, Y., et al. (2015). Hemeoxygenase-1 Maintains Bone Mass via Attenuating a Redox Imbalance in Osteoclast. Mol. Cell Endocrinol. 409, 11–20. doi:10.1016/j.mce.2015.03.022
Kensler, T. W., Wakabayashi, N., and Biswal, S. (2007). Cell Survival Responses to Environmental Stresses via the Keap1-Nrf2-ARE Pathway. Annu. Rev. Pharmacol. Toxicol. 47, 89–116. doi:10.1146/annurev.pharmtox.46.120604.141046
Khanizadeh, F., Rahmani, A., Asadollahi, K., and Ahmadi, M. R. H. (2018). Combination Therapy of Curcumin and Alendronate Modulates Bone Turnover Markers and Enhances Bone mineral Density in Postmenopausal Women with Osteoporosis. Arch. Endocrinol. Metab. 62, 438–445. doi:10.20945/2359-3997000000060
Kim, B.-J., Bae, S. J., Lee, S.-Y., Lee, Y.-S., Baek, J.-E., Park, S.-Y., et al. (2012). TNF-α Mediates the Stimulation of Sclerostin Expression in an Estrogen-Deficient Condition. Biochem. Biophysical Res. Commun. 424, 170–175. doi:10.1016/j.bbrc.2012.06.100
Kim, H.-N., Chang, J., Shao, L., Han, L., Iyer, S., Manolagas, S. C., et al. (2017). DNA Damage and Senescence in Osteoprogenitors Expressing Osx1 May Cause Their Decrease with Age. Aging Cell 16, 693–703. doi:10.1111/acel.12597
Kim, H.-N., Han, L., Iyer, S., de Cabo, R., Zhao, H., O'Brien, C. A., et al. (2015). Sirtuin1 Suppresses Osteoclastogenesis by Deacetylating FoxOs. Mol. Endocrinol. 29, 1498–1509. doi:10.1210/me.2015-1133
Kim, J.-H., Yu, S., Chen, J. D., and Kong, A. N. (2013). The Nuclear Cofactor RAC3/AIB1/SRC-3 Enhances Nrf2 Signaling by Interacting with Transactivation Domains. Oncogene 32, 514–527. doi:10.1038/onc.2012.59
Kim, S., Choi, S.-I., Kim, G.-H., and Imm, J.-Y. (2019). Anti-inflammatory Effect of Ecklonia Cava Extract on Porphyromonas Gingivalis Lipopolysaccharide-Stimulated Macrophages and a Periodontitis Rat Model. Nutrients 11, 1143. doi:10.3390/nu11051143
Kim, S. C., Kim, D. H., Mogun, H., Eddings, W., Polinski, J. M., Franklin, J. M., et al. (2016). Impact of the U.S. Food and Drug Administration's Safety-Related Announcements on the Use of Bisphosphonates after Hip Fracture. J. Bone Miner Res. 31, 1536–1540. doi:10.1002/jbmr.2832
Kim, W.-K., Meliton, V., Bourquard, N., Hahn, T. J., and Parhami, F. (2010). Hedgehog Signaling and Osteogenic Differentiation in Multipotent Bone Marrow Stromal Cells Are Inhibited by Oxidative Stress. J. Cel. Biochem. 111, 1199–1209. doi:10.1002/jcb.22846
Kim, W. K., Ke, K., Sul, O. J., Kim, H. J., Kim, S. H., Lee, M. H., et al. (2011). Curcumin Protects against Ovariectomy-Induced Bone Loss and Decreases Osteoclastogenesis. J. Cel. Biochem. 112, 3159–3166. doi:10.1002/jcb.23242
Korytina, G. F., Akhmadishina, L. Z., Aznabaeva, Y. G., Kochetova, O. V., Zagidullin, N. S., Kzhyshkowska, J. G., et al. (2019). Associations of the NRF2/KEAP1 Pathway and Antioxidant Defense Gene Polymorphisms with Chronic Obstructive Pulmonary Disease. Gene 692, 102–112. doi:10.1016/j.gene.2018.12.061
Kozakowska, M., Szade, K., Dulak, J., and Jozkowicz, A. (2014). Role of Heme Oxygenase-1 in Postnatal Differentiation of Stem Cells: a Possible Cross-Talk with microRNAs. Antioxid. Redox Signaling 20, 1827–1850. doi:10.1089/ars.2013.5341
Kumar, S., and Bandyopadhyay, U. (2005). Free Heme Toxicity and its Detoxification Systems in Human. Toxicol. Lett. 157, 175–188. doi:10.1016/j.toxlet.2005.03.004
Lampropoulou, V., Sergushichev, A., Bambouskova, M., Nair, S., Vincent, E. E., Loginicheva, E., et al. (2016). Itaconate Links Inhibition of Succinate Dehydrogenase with Macrophage Metabolic Remodeling and Regulation of Inflammation. Cel Metab. 24, 158–166. doi:10.1016/j.cmet.2016.06.004
Langdahl, B., Ferrari, S., and Dempster, D. W. (2016). Bone Modeling and Remodeling: Potential as Therapeutic Targets for the Treatment of Osteoporosis. Ther. Adv. Musculoskelet. 8, 225–235. doi:10.1177/1759720X16670154
Langdahl, B. L. (2021). Overview of Treatment Approaches to Osteoporosis. Br. J. Pharmacol. 178, 1891–1906. doi:10.1111/bph.15024
Lavrovsky, Y., Schwartzman, M. L., Levere, R. D., Kappas, A., and Abraham, N. G. (1994). Identification of Binding Sites for Transcription Factors NF-Kappa B and AP-2 in the Promoter Region of the Human Heme Oxygenase 1 Gene. Proc. Natl. Acad. Sci. 91, 5987–5991. doi:10.1073/pnas.91.13.5987
Lee, J., Park, C., Kim, H. J., Lee, Y. D., Lee, Z. H., Song, Y. W., et al. (2017). Stimulation of Osteoclast Migration and Bone Resorption by C-C Chemokine Ligands 19 and 21. Exp. Mol. Med. 49, e358. doi:10.1038/emm.2017.100
Lehmann, J. C. U., Listopad, J. J., Rentzsch, C. U., Igney, F. H., von Bonin, A., Hennekes, H. H., et al. (2007). Dimethylfumarate Induces Immunosuppression via Glutathione Depletion and Subsequent Induction of Heme Oxygenase 1. J. Invest. Dermatol. 127, 835–845. doi:10.1038/sj.jid.5700686
Li, H., Huang, C., Zhu, J., Gao, K., Fang, J., and Li, H. (2018). Lutein Suppresses Oxidative Stress and Inflammation by Nrf2 Activation in an Osteoporosis Rat Model. Med. Sci. Monit. 24, 5071–5075. doi:10.12659/msm.908699
Li, S., Yin, Y., Yao, L., Lin, Z., Sun, S., Zhang, J., et al. (2020a). TNF-α T-reatment I-ncreases DKK1 P-rotein L-evels in P-rimary O-steoblasts via U-pregulation of DKK1 mRNA L-evels and D-ownregulation of miR-335-5p. Mol. Med. Rep. 22, 1017–1025. doi:10.3892/mmr.2020.11152
Li, X., Chen, Y., Mao, Y., Dai, P., Sun, X., Zhang, X., et al. (2020b). Curcumin Protects Osteoblasts from Oxidative Stress-Induced Dysfunction via GSK3β-Nrf2 Signaling Pathway. Front. Bioeng. Biotechnol. 8, 625. doi:10.3389/fbioe.2020.00625
Liang, Y., Zhu, B., Li, S., Zhai, Y., Yang, Y., Bai, Z., et al. (2020). Curcumin Protects Bone Biomechanical Properties and Microarchitecture in Type2 Diabetic Rats with Osteoporosis via the TGFβ/Smad2/3 Pathway. Exp. Ther. Med. 20, 2200–2208. doi:10.3892/etm.2020.8943
Lin, C.-C., Chiang, L.-L., Lin, C.-H., Shih, C.-H., Liao, Y.-T., Hsu, M.-J., et al. (2007). Transforming Growth Factor-Β1 Stimulates Heme Oxygenase-1 Expression via the PI3K/Akt and NF-Κb Pathways in Human Lung Epithelial Cells. Eur. J. Pharmacol. 560, 101–109. doi:10.1016/j.ejphar.2007.01.025
Lin, T.-H., Tang, C.-H., Hung, S.-Y., Liu, S.-H., Lin, Y.-M., Fu, W.-M., et al. (2009). Upregulation of Heme Oxygenase-1 Inhibits the Maturation and Mineralization of Osteoblasts. J. Cel. Physiol. 222, a–n. doi:10.1002/jcp.22008
Liu, H., Dong, Y., Gao, Y., Zhao, L., Cai, C., Qi, D., et al. (2019). Hesperetin Suppresses RANKL‐induced Osteoclastogenesis and Ameliorates Lipopolysaccharide‐induced Bone Loss. J. Cel. Physiol. 234, 11009–11022. doi:10.1002/jcp.27924
Liu, J.-T., Chen, H.-Y., Chen, W.-C., Man, K.-M., and Chen, Y.-H. (2017). Red Yeast rice Protects Circulating Bone Marrow-Derived Proangiogenic Cells against High-Glucose-Induced Senescence and Oxidative Stress: the Role of Heme Oxygenase-1. Oxidative Med. Cell Longevity 2017, 1–11. doi:10.1155/2017/3831750
Liu, X., Ward, K., Wu, H., Jann, J., Clark, A. F., Pang, I. H., et al. (2016). The Novel Triterpenoid RTA 408 Protects Human Retinal Pigment Epithelial Cells against H2O2-Induced Cell Injury via NF-E2-Related Factor 2 (Nrf2) Activation. Redox Biol. 8, 98–109. doi:10.1016/j.redox.2015.12.005,
Liu, X.-H., Kirschenbaum, A., Yao, S., and Levine, A. C. (2005). Cross-Talk between the Interleukin-6 and Prostaglandin E2 Signaling Systems Results in Enhancement of Osteoclastogenesis through Effects on the Osteoprotegerin/Receptor Activator of Nuclear Factor-Κb (RANK) Ligand/RANK System. Endocrinology 146, 1991–1998. doi:10.1210/en.2004-1167
Liu, X.-m., Peyton, K. J., Shebib, A. R., Wang, H., Korthuis, R. J., and Durante, W. (2011). Activation of AMPK Stimulates Heme Oxygenase-1 Gene Expression and Human Endothelial Cell Survival. Am. J. Physiology-Heart Circulatory Physiol. 300, H84–H93. doi:10.1152/ajpheart.00749.2010
Liu, X., and Wan, M. (2019). A Tale of the Good and Bad: Cell Senescence in Bone Homeostasis and Disease. Int. Rev. Cel Mol. Biol. 346, 97–128. doi:10.1016/bs.ircmb.2019.03.005
Lorenzo, J. (2000). Interactions between Immune and Bone Cells: New Insights with many Remaining Questions. J. Clin. Invest. 106, 749–752. doi:10.1172/jci11089
Lu, S.-H., Chen, T.-H., and Chou, T.-C. (2015). Magnolol Inhibits RANKL-Induced Osteoclast Differentiation of Raw 264.7 Macrophages through Heme Oxygenase-1-dependent Inhibition of NFATc1 Expression. J. Nat. Prod. 78, 61–68. doi:10.1021/np500663y
Luo, G., Li, F., Li, X., Wang, Z. G., and Zhang, B. (2018). TNF-α and RANKL P-romote O-steoclastogenesis by U-pregulating RANK via the NF-κB P-athway. Mol. Med. Rep. 17, 6605–6611. doi:10.3892/mmr.2018.8698
Lv, F., Cai, X., Yang, W., Gao, L., Chen, L., Wu, J., et al. (2020). Denosumab or Romosozumab Therapy and Risk of Cardiovascular Events in Patients with Primary Osteoporosis: Systematic Review and Meta- Analysis. Bone 130, 115121. doi:10.1016/j.bone.2019.115121
Ma, H., Wang, X., Zhang, W., Li, H., Zhao, W., Sun, J., et al. (2020). Melatonin Suppresses Ferroptosis Induced by High Glucose via Activation of the Nrf2/HO-1 Signaling Pathway in Type 2 Diabetic Osteoporosis. Oxidative Med. Cell Longevity 2020, 1–18. doi:10.1155/2020/9067610
Maicas, N., Ferrándiz, M. L., Brines, R., Ibáñez, L., Cuadrado, A., Koenders, M. I., et al. (2011). Deficiency of Nrf2 Accelerates the Effector Phase of Arthritis and Aggravates Joint Disease. Antioxid. Redox Signaling 15, 889–901. doi:10.1089/ars.2010.3835
Maines, M. D. (1997). The Heme Oxygenase System:a Regulator of Second Messenger Gases. Annu. Rev. Pharmacol. Toxicol. 37, 517–554. doi:10.1146/annurev.pharmtox.37.1.517
Manolagas, S. C. (2010). From Estrogen-Centric to Aging and Oxidative Stress: a Revised Perspective of the Pathogenesis of Osteoporosis. Endocr. Rev. 31, 266–300. doi:10.1210/er.2009-0024
Marcus, R., Dempster, D. W., and Bouxsein, M. L. (2013). The Nature of Osteoporosis. Osteoporosis. Fourth Edition. New York: Academic Press.
Marie, P. J. (2014). Bone Cell Senescence: Mechanisms and Perspectives. J. Bone Miner Res. 29, 1311–1321. doi:10.1002/jbmr.2190
Martin, D., Rojo, A. I., Salinas, M., Diaz, R., Gallardo, G., Alam, J., et al. (2004). Regulation of Heme Oxygenase-1 Expression through the Phosphatidylinositol 3-kinase/Akt Pathway and the Nrf2 Transcription Factor in Response to the Antioxidant Phytochemical Carnosol. J. Biol. Chem. 279, 8919–8929. doi:10.1074/jbc.M309660200
McClements, D. J. (2020). Advances in Nanoparticle and Microparticle Delivery Systems for Increasing the Dispersibility, Stability, and Bioactivity of Phytochemicals. Biotechnol. Adv. 38, 107287. doi:10.1016/j.biotechadv.2018.08.004
McClung, J. A., Levy, L., Garcia, V., Stec, D. E., Peterson, S. J., and Abraham, N. G. (202110797). Heme-oxygenase and Lipid Mediators in Obesity and Associated Cardiometabolic Diseases: Therapeutic Implications. Pharmacol. Ther., 107975. doi:10.1016/j.pharmthera.2021.107975
McLean, R. R. (2009). Proinflammatory Cytokines and Osteoporosis. Curr. Osteoporos. Rep. 7, 134–139. doi:10.1007/s11914-009-0023-2
Medina, M. V., Sapochnik, D., Garcia Solá, M., and Coso, O. (2020). Regulation of the Expression of Heme Oxygenase-1: Signal Transduction, Gene Promoter Activation, and beyond. Antioxid. Redox Signaling 32, 1033–1044. doi:10.1089/ars.2019.7991
Mense, S. M., and Zhang, L. (2006). Heme: a Versatile Signaling Molecule Controlling the Activities of Diverse Regulators Ranging from Transcription Factors to MAP Kinases. Cell Res 16, 681–692. doi:10.1038/sj.cr.7310086
Messer, J. G., La, S., Hopkins, R. G., and Kipp, D. E. (2016). Quercetin Partially Preserves Development of Osteoblast Phenotype in Fetal Rat Calvaria Cells in an Oxidative Stress Environment. J. Cel. Physiol. 231, 2779–2788. doi:10.1002/jcp.25392
Mills, E. L., Ryan, D. G., Prag, H. A., Dikovskaya, D., Menon, D., Zaslona, Z., et al. (2018). Itaconate Is an Anti-inflammatory Metabolite that Activates Nrf2 via Alkylation of KEAP1. Nature 556, 113–117. doi:10.1038/nature25986
Mitra, D., Elvins, D. M., Speden, D. J., and Collins, A. J. (2000). The Prevalence of Vertebral Fractures in Mild Ankylosing Spondylitis and Their Relationship to Bone mineral Density. Rheumatology 39, 85–89. doi:10.1093/rheumatology/39.1.85
Moschen, A. R., and Shakibaei, A. (2013). The RANKL/OPG System Is Activated in Inflammatory Bowel Disease and Relates to the State of Bone Loss. Gut 54, 479–487. doi:10.1136/gut.2004.044370
Mukkavilli, R., Yang, C., Singh Tanwar, R., Ghareeb, A., Luthra, L., and Aneja, R. (2017). Absorption, Metabolic Stability, and Pharmacokinetics of Ginger Phytochemicals. Molecules 22, 553. doi:10.3390/molecules22040553
Ni, S., Qian, Z., Yuan, Y., Li, D., Zhong, Z., Ghorbani, F., et al. (2020). Schisandrin A Restrains Osteoclastogenesis by Inhibiting Reactive Oxygen Species and Activating Nrf2 Signalling. Cell Prolif. 53, e12882. doi:10.1111/cpr.12882
Nih Consensus Development Panel on Osteoporosis Prevention, (2001). NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy, March 7-29, 2000: Highlights of the conferenceOsteoporosis Prevention, Diagnosis, and Therapy. South. Med. J. 94, 569–573. doi:10.1001/jama.285.6.785
Nowak, W. N., Taha, H., Kachamakova-Trojanowska, N., Stępniewski, J., Markiewicz, J. A., Kusienicka, A., et al. (2018). Murine Bone Marrow Mesenchymal Stromal Cells Respond Efficiently to Oxidative Stress Despite the Low Level of Heme Oxygenases 1 and 2. Antioxid. Redox Signaling 29, 111–127. doi:10.1089/ars.2017.7097
Ohori, F., Kitaura, H., Marahleh, A., Kishikawa, A., Ogawa, S., Qi, J., et al. (2019). Effect of TNF-α-Induced Sclerostin on Osteocytes during Orthodontic Tooth Movement. J. Immunol. Res. 2019, 1–10. doi:10.1155/2019/9716758
Ornstrup, M. J., Harsløf, T., Kjær, T. N., Langdahl, B. L., and Pedersen, S. B. (2014). Resveratrol Increases Bone mineral Density and Bone Alkaline Phosphatase in Obese Men: a Randomized Placebo-Controlled Trial. J. Clin. Endocrinol. Metab. 99, 4720–4729. doi:10.1210/jc.2014-2799
Otterbein, L. E., Foresti, R., and Motterlini, R. (2016). Heme Oxygenase-1 and Carbon Monoxide in the Heart. Circ. Res. 118, 1940–1959. doi:10.1161/circresaha.116.306588
Pacifici, R. (1998). Editorial: Cytokines, Estrogen, and Postmenopausal Osteoporosis-The Second Decade. Endocrinology 139, 2659–2661. doi:10.1210/endo.139.6.6087
Pacifici, R., Rifas, L., Teitelbaum, S., Slatopolsky, E., McCracken, R., Bergfeld, M., et al. (1987). Spontaneous Release of Interleukin 1 from Human Blood Monocytes Reflects Bone Formation in Idiopathic Osteoporosis. Proc. Natl. Acad. Sci. 84, 4616–4620. doi:10.1073/pnas.84.13.4616
Pan, Y., Song, J., Ma, L., Zong, X., Chen, H., Zhao, B., et al. (2018). Carbon Monoxide Releasing Molecule 3 Inhibits Osteoclastogenic Differentiation of RAW264.7 Cells by Heme Oxygenase-1. Cell Physiol Biochem 50, 1988–2003. doi:10.1159/000494891
Pandey, M. K., Gupta, S. C., Karelia, D., Gilhooley, P. J., Shakibaei, M., and Aggarwal, B. B. (2018). Dietary Nutraceuticals as Backbone for Bone Health. Biotechnol. Adv. 36, 1633–1648. doi:10.1016/j.biotechadv.2018.03.014
Peng, Y.-F., Wang, J.-L., and Pan, G.-G. (2017). The Correlation of Serum Bilirubin Levels with Disease Activity in Patients with Rheumatoid Arthritis. Clinica Chim. Acta 469, 187–190. doi:10.1016/j.cca.2017.04.006
Piemontese, M., Almeida, M., Robling, A. G., Kim, H.-N., Xiong, J., Thostenson, J. D., et al. (2017). Old Age Causes De Novo Intracortical Bone Remodeling and Porosity in Mice. JCI Insight 2, e93771. doi:10.1172/jci.insight.93771
Poss, K. D., and Tonegawa, S. (1997a). Heme Oxygenase 1 Is Required for Mammalian Iron Reutilization. Proc. Natl. Acad. Sci. 94, 10919–10924. doi:10.1073/pnas.94.20.10919
Poss, K. D., and Tonegawa, S. (1997b). Reduced Stress Defense in Heme Oxygenase 1-deficient Cells. Proc. Natl. Acad. Sci. 94, 10925–10930. doi:10.1073/pnas.94.20.10925
Qi, D., Liu, H., Sun, X., Luo, D., Zhu, M., Tao, T., et al. (2020). Pristimerin Suppresses RANKL-Induced Osteoclastogenesis and Ameliorates Ovariectomy-Induced Bone Loss. Front. Pharmacol. 11, 621110. doi:10.3389/fphar.2020.621110
Rana, T., Schultz, M. A., Freeman, M. L., and Biswas, S. (2012). Loss of Nrf2 Accelerates Ionizing Radiation-Induced Bone Loss by Upregulating RANKL. Free Radic. Biol. Med. 53, 2298–2307. doi:10.1016/j.freeradbiomed.2012.10.536
Reisman, S. A., Lee, C.-Y. I., Meyer, C. J., Proksch, J. W., Sonis, S. T., and Ward, K. W. (2014). Topical Application of the Synthetic Triterpenoid RTA 408 Protects Mice from Radiation-Induced Dermatitis. Radiat. Res. 181, 512–520. doi:10.1667/rr13578.1
Reziwan, K., Sun, D., Zhang, B., and Zhao, Z. (2019). MicroRNA‐1225 Activates Keap1‐Nrf2‐HO‐1 Signalling to Inhibit TNFα‐induced Osteoclastogenesis by Mediating ROS Generation. Cell Biochem. Funct. 37, 256–265. doi:10.1002/cbf.3394
Riggs, B. L., Khosla, S., and Melton, L. J. (2001). in “The Type I/Type II Model for Involutional Osteoporosis: Update and Modification Based on New Observations” in the Osteoporosis. Editor R. Marcus. Second Edition (New York: Academic Press), 49–58. doi:10.1016/b978-012470862-4/50039-8
Rochette, L., Zeller, M., Cottin, Y., and Vergely, C. (2018). Redox Functions of Heme Oxygenase-1 and Biliverdin Reductase in Diabetes. Trends Endocrinol. Metab. 29, 74–85. doi:10.1016/j.tem.2017.11.005
Roggia, C., Gao, Y., Cenci, S., Weitzmann, M. N., Toraldo, G., Isaia, G., et al. (2001). Up-regulation of TNF-Producing T Cells in the Bone Marrow: a Key Mechanism by Which Estrogen Deficiency Induces Bone Loss In Vivo. Proc. Natl. Acad. Sci. 98, 13960–13965. doi:10.1073/pnas.251534698
Ruscitti, P., Cipriani, P., Carubbi, F., Liakouli, V., Zazzeroni, F., Di Benedetto, P., et al. (2015). The Role of IL-1βin the Bone Loss during Rheumatic Diseases. Mediators Inflamm. 2015, 1–10. doi:10.1155/2015/782382
Ryter, S. W., and Choi, A. M. K. (2009). Heme Oxygenase-1/Carbon Monoxide. Am. J. Respir. Cel Mol. Biol. 41, 251–260. doi:10.1165/rcmb.2009-0170TR
Sakai, E., Shimada-Sugawara, M., Nishishita, K., Fukuma, Y., Naito, M., Okamoto, K., et al. (2012). Suppression of RANKL-dependent Heme Oxygenase-1 Is Required for High Mobility Group Box 1 Release and Osteoclastogenesis. J. Cel. Biochem. 113, 486–498. doi:10.1002/jcb.23372
Saxena, Y., Routh, S., and Mukhopadhaya, A. (2021). Immunoporosis: Role of Innate Immune Cells in Osteoporosis. Front. Immunol. 12, 687037. doi:10.3389/fimmu.2021.687037
Sharan, K., Siddiqui, J., Swarnkar, G., Maurya, R., and Chattopadhyay, N. (2009). Role of Phytochemicals in the Prevention of Menopausal Bone Loss: Evidence from In Vitro and In Vivo, Human Interventional and Pharmacokinetic Studies. Cmc 16, 1138–1157. doi:10.2174/092986709787581806
Sharma, A. K., Roberts, R. L., Benson, R. D., Pierce, J. L., Yu, K., Hamrick, M. W., et al. (2020). The Senolytic Drug Navitoclax (ABT-263) Causes Trabecular Bone Loss and Impaired Osteoprogenitor Function in Aged Mice. Front. Cel Dev. Biol. 8, 354. doi:10.3389/fcell.2020.00354
Shi, L., Wang, H., Chen, K., Yan, J., Yu, B., Wang, S., et al. (2021). Chinese Guidelines on the Clinical Application of 5-aminolevulinic Acid-Based Photodynamic Therapy in Dermatology (2021 Edition). Photodiagnosis Photodynamic Ther. 35, 102340. doi:10.1016/j.pdpdt.2021.102340
Shin, S. A., Joo, B. J., Lee, J. S., Ryu, G., Han, M., Kim, W. Y., et al. (2020). Phytochemicals as Anti-inflammatory Agents in Animal Models of Prevalent Inflammatory Diseases. Molecules 25, 5932. doi:10.3390/molecules25245932
Sies, H., Berndt, C., and Jones, D. P. (2017). Oxidative Stress. Annu. Rev. Biochem. 86, 715–748. doi:10.1146/annurev-biochem-061516-045037
Soares, M. P., and Hamza, I. (2016). Macrophages and Iron Metabolism. Immunity 44, 492–504. doi:10.1016/j.immuni.2016.02.016
Su, Z.-Y., Shu, L., Khor, T. O., Lee, J. H., Fuentes, F., and Kong, A.-N. T. (2012). A Perspective on Dietary Phytochemicals and Cancer Chemoprevention: Oxidative Stress, Nrf2, and Epigenomics. Top. Curr. Chem. 329, 133–162. doi:10.1007/128_2012_340
Sudan, K., Vijayan, V., Madyaningrana, K., Gueler, F., Igarashi, K., Foresti, R., et al. (2019). TLR4 Activation Alters Labile Heme Levels to Regulate BACH1 and Heme Oxygenase-1 Expression in Macrophages. Free Radic. Biol. Med. 137, 131–142. doi:10.1016/j.freeradbiomed.2019.04.024
Sun, J., Hoshino, H., Takaku, K., Nakajima, O., Muto, A., Suzuki, H., et al. (2002). Hemoprotein Bach1 Regulates Enhancer Availability of Heme Oxygenase-1 Gene. EMBO J. 21, 5216–5224. doi:10.1093/emboj/cdf516
Sun, X., Xie, Z., Hu, B., Zhang, B., Ma, Y., Pan, X., et al. (2020). The Nrf2 Activator RTA-408 Attenuates Osteoclastogenesis by Inhibiting STING Dependent NF-Κb Signaling. Redox Biol. 28, 101309. doi:10.1016/j.redox.2019.101309
Sun, X., Zhang, B., Pan, X., Huang, H., Xie, Z., Ma, Y., et al. (2019). Octyl Itaconate Inhibits Osteoclastogenesis by Suppressing Hrd1 and Activating Nrf2 Signaling. FASEB j. 33, 12929–12940. doi:10.1096/fj.201900887RR
Suresh, G., Martin, C. L., and Soll, R. (2003). Metalloporphyrins for Treatment of Unconjugated Hyperbilirubinemia in Neonates. Cochrane Database Syst. Rev., CD004207. doi:10.1002/14651858.Cd004207
Suzuki, H., Tashiro, S., Hira, S., Sun, J., Yamazaki, C., Zenke, Y., et al. (2004). Heme Regulates Gene Expression by Triggering Crm1-dependent Nuclear export of Bach1. EMBO J. 23, 2544–2553. doi:10.1038/sj.emboj.7600248
Szade, K., Zukowska, M., Szade, A., Nowak, W., Skulimowska, I., Ciesla, M., et al. (2020). Heme Oxygenase‐1 Deficiency Triggers Exhaustion of Hematopoietic Stem Cells. EMBO Rep. 21, e47895. doi:10.15252/embr.201947895
Taguchi, K., Motohashi, H., and Yamamoto, M. (2011). Molecular Mechanisms of the Keap1-Nrf2 Pathway in Stress Response and Cancer Evolution. Genes Cells 16, 123–140. doi:10.1111/j.1365-2443.2010.01473.x
Takada, I., Kouzmenko, A. P., and Kato, S. (2009). Wnt and PPARγ Signaling in Osteoblastogenesis and Adipogenesis. Nat. Rev. Rheumatol. 5, 442–447. doi:10.1038/nrrheum.2009.137
Takanche, J. S., Kim, J.-E., Han, S.-H., and Yi, H.-K. (2020). Effect of Gomisin A on Osteoblast Differentiation in High Glucose-Mediated Oxidative Stress. Phytomedicine 66, 153107. doi:10.1016/j.phymed.2019.153107
Takayanagi, H., Ogasawara, K., Hida, S., Chiba, T., Murata, S., Sato, K., et al. (2000). T-cell-mediated Regulation of Osteoclastogenesis by Signalling Cross-Talk between RANKL and IFN-γ. Nature 408, 600–605. doi:10.1038/35046102
Tanaka, K., Hashizume, M., Mihara, M., Yoshida, H., Suzuki, M., and Matsumoto, Y. (2014). Anti-interleukin-6 Receptor Antibody Prevents Systemic Bone Mass Loss via Reducing the Number of Osteoclast Precursors in Bone Marrow in a Collagen-Induced Arthritis Model. Clin. Exp. Immunol. 175, 172–180. doi:10.1111/cei.12201
Tang, M., Tian, L., Luo, G., and Yu, X. (2018). Interferon-gamma-mediated Osteoimmunology. Front. Immunol. 9, 1508. doi:10.3389/fimmu.2018.01508
Tenhunen, R., Marver, H. S., and Schmid, R. (1968). The Enzymatic Conversion of Heme to Bilirubin by Microsomal Heme Oxygenase. Proc. Natl. Acad. Sci. 61, 748–755. doi:10.1073/pnas.61.2.748
Toxqui, L., and Vaquero, M. (2015). Chronic Iron Deficiency as an Emerging Risk Factor for Osteoporosis: a Hypothesis. Nutrients 7, 2324–2344. doi:10.3390/nu7042324
Van den Bergh, J. P., van Geel, T. A., and Geusens, P. P. (2012). Osteoporosis, Frailty and Fracture: Implications for Case Finding and Therapy. Nat. Rev. Rheumatol. 8, 163–172. doi:10.1038/nrrheum.2011.217
Van Lenten, B. J., Prieve, J., Navab, M., Hama, S., Lusis, A. J., and Fogelman, A. M. (1995). Lipid-induced Changes in Intracellular Iron Homeostasis In Vitro and In Vivo. J. Clin. Invest. 95, 2104–2110. doi:10.1172/jci117898
Vanella, L., Kim, D. H., Asprinio, D., Peterson, S. J., Barbagallo, I., Vanella, A., et al. (2010). HO-1 Expression Increases Mesenchymal Stem Cell-Derived Osteoblasts but Decreases Adipocyte Lineage. Bone 46, 236–243. doi:10.1016/j.bone.2009.10.012
Vestergaard, P., Rejnmark, L., and Mosekilde, L. (2007). Increased Mortality in Patients with a Hip Fracture-Effect of Pre-morbid Conditions and post-fracture Complications. Osteoporos. Int. 18, 1583–1593. doi:10.1007/s00198-007-0403-3
Wang, N., Xin, H., Xu, P., Yu, Z., and Shou, D. (2019). Erxian Decoction Attenuates TNF-α Induced Osteoblast Apoptosis by Modulating the Akt/Nrf2/HO-1 Signaling Pathway. Front. Pharmacol. 10, 988. doi:10.3389/fphar.2019.00988
Wauquier, F., Leotoing, L., Coxam, V., Guicheux, J., and Wittrant, Y. (2009). Oxidative Stress in Bone Remodelling and Disease. Trends Mol. Med. 15, 468–477. doi:10.1016/j.molmed.2009.08.004
Wong, R. H., Thaung Zaw, J. J., Xian, C. J., and Howe, P. R. (2020). Regular Supplementation with Resveratrol Improves Bone Mineral Density in Postmenopausal Women: A Randomized, Placebo‐Controlled Trial. J. Bone Miner Res. 35, 2121–2131. doi:10.1002/jbmr.4115
Wong, S. C., Kamarudin, M. N. A., and Naidu, R. (2021). Anticancer Mechanism of Curcumin on Human Glioblastoma. Nutrients 13, 950. doi:10.3390/nu13030950
Wu, D., Cline-Smith, A., Shashkova, E., Perla, A., Katyal, A., and Aurora, R. (2021). T-cell Mediated Inflammation in Postmenopausal Osteoporosis. Front. Immunol. 12, 687551. doi:10.3389/fimmu.2021.687551
Xiao, L., Zhong, M., Huang, Y., Zhu, J., Tang, W., Li, D., et al. (2020). Puerarin Alleviates Osteoporosis in the Ovariectomy-Induced Mice by Suppressing Osteoclastogenesis via Inhibition of TRAF6/ROS-dependent MAPK/NF-κB Signaling Pathways. Aging 12, 21706–21729. doi:10.18632/aging.103976
Xin, M., Yang, Y., Zhang, D., Wang, J., Chen, S., and Zhou, D. (2015). Attenuation of Hind-Limb Suspension-Induced Bone Loss by Curcumin Is Associated with Reduced Oxidative Stress and Increased Vitamin D Receptor Expression. Osteoporos. Int. 26, 2665–2676. doi:10.1007/s00198-015-3153-7
Xu, Y., Guan, J., Xu, J., Chen, S., and Sun, G. (2019). Z-guggulsterone Attenuates Glucocorticoid-Induced Osteoporosis through Activation of Nrf2/HO-1 Signaling. Life Sci. 224, 58–66. doi:10.1016/j.lfs.2019.03.051
Yamaguchi, Y., Kanzaki, H., Katsumata, Y., Itohiya, K., Fukaya, S., Miyamoto, Y., et al. (2018). Dimethyl Fumarate Inhibits Osteoclastsviaattenuation of Reactive Oxygen Species Signalling by Augmented Antioxidation. J. Cel. Mol. Med. 22, 1138–1147. doi:10.1111/jcmm.13367
Yang, H., Gu, Q., Huang, C., Cai, Y., and Shi, Q. (2012). Curcumin Increases Rat Mesenchymal Stem Cell Osteoblast Differentiation but Inhibits Adipocyte Differentiation. Phcog Mag. 8, 202–208. doi:10.4103/0973-1296.99285
Yin, H., Shi, Z.-G., Yu, Y.-S., Hu, J., Wang, R., Luan, Z.-P., et al. (2012). Protection against Osteoporosis by Statins Is Linked to a Reduction of Oxidative Stress and Restoration of Nitric Oxide Formation in Aged and Ovariectomized Rats. Eur. J. Pharmacol. 674, 200–206. doi:10.1016/j.ejphar.2011.11.024
Yu, Z. Y., Ma, D., He, Z. C., Liu, P., Huang, J., Fang, Q., et al. (2018). Heme Oxygenase-1 Protects Bone Marrow Mesenchymal Stem Cells from Iron Overload through Decreasing Reactive Oxygen Species and Promoting IL-10 Generation. Exp. Cel Res. 362, 28–42. doi:10.1016/j.yexcr.2017.10.029
Zenke-Kawasaki, Y., Dohi, Y., Katoh, Y., Ikura, T., Ikura, M., Asahara, T., et al. (2007). Heme Induces Ubiquitination and Degradation of the Transcription Factor Bach1. Mol. Cel. Biol. 27, 6962–6971. doi:10.1128/mcb.02415-06
Zhang, J., Cai, W., Fan, Z., Yang, C., Wang, W., Xiong, M., et al. (2019). MicroRNA-24 Inhibits the Oxidative Stress Induced by Vascular Injury by Activating the Nrf2/Ho-1 Signaling Pathway. Atherosclerosis 290, 9–18. doi:10.1016/j.atherosclerosis.2019.08.023
Zhang, J., Hosoya, T., Maruyama, A., Nishikawa, K., Maher, J. M., Ohta, T., et al. (2007). Nrf2 Neh5 Domain Is Differentially Utilized in the Transactivation of Cytoprotective Genes. Biochem. J. 404, 459–466. doi:10.1042/bj20061611
Zheng, S. X., Vrindts, Y., Lopez, M., De Groote, D., Zangerle, P. F., Collette, J., et al. (1997). Increase in Cytokine Production (IL-1β, IL-6, TNF-α but Not IFN-γ, GM-CSF or LIF) by Stimulated Whole Blood Cells in Postmenopausal Osteoporosis. Maturitas 26, 63–71. doi:10.1016/s0378-5122(96)01080-8
Zheng, Y.-h., Yang, J.-j., Tang, P.-j., Zhu, Y., Chen, Z., She, C., et al. (2021). A Novel Keap1 Inhibitor iKeap1 Activates Nrf2 Signaling and Ameliorates Hydrogen Peroxide-Induced Oxidative Injury and Apoptosis in Osteoblasts. Cell Death Dis 12, 679. doi:10.1038/s41419-021-03962-8
Zhu, Z., Wang, X., Wang, Z., Zhao, Z., Zhou, P., and Gao, X. (2021). Neobavaisoflavone Protects Osteoblasts from Dexamethasone‐induced Oxidative Stress by Upregulating the CRNDE ‐mediated Nrf2/HO ‐1 Signaling Pathway. Drug Dev. Res. doi:10.1002/ddr.21811
Keywords: bone remodeling, osteoporosis, pharmacological therapeutics, HO-1 inducer, heme oxygenase 1 (HO-1)
Citation: Zhou X, Yuan W, Xiong X, Zhang Z, Liu J, Zheng Y, Wang J and Liu J (2021) HO-1 in Bone Biology: Potential Therapeutic Strategies for Osteoporosis. Front. Cell Dev. Biol. 9:791585. doi: 10.3389/fcell.2021.791585
Received: 08 October 2021; Accepted: 12 November 2021;
Published: 30 November 2021.
Edited by:
Cheng Yang, Fudan University, ChinaReviewed by:
Stefan W. Ryter, Harvard Medical School, United StatesCopyright © 2021 Zhou, Yuan, Xiong, Zhang, Liu, Zheng, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin Liu, bGl1amluQHNjdS5lZHUuY24=; Jun Wang, d2FuZ2p1bnZAc2N1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.