- 1Hepatobiliary Center, The First Affiliated Hospital of Nanjing Medical University, Key Laboratory of Liver Transplantation, Chinese Academy of Medical Sciences, NHC Key Laboratory of Living Donor Liver Transplantation, Nanjing Medical University, Nanjing, China
- 2Department of General Surgery, Nanjing First Hospital, Nanjing Medical University, Nanjing, China
Immune associated cells in the microenvironment have a significant impact on the development and progression of hepatocellular carcinoma (HCC) and have received more and more attention. Different types of immune-associated cells play different roles, including promoting/inhibiting HCC and several different types that are controversial. It is well known that immune escape of HCC has become a difficult problem in tumor therapy. Therefore, in recent years, a large number of studies have focused on the immune microenvironment of HCC, explored many mechanisms worth identifying tumor immunosuppression, and developed a variety of immunotherapy methods as targets, laying the foundation for the final victory in the fight against HCC. This paper reviews recent studies on the immune microenvironment of HCC that are more reliable and important, and provides a more comprehensive view of the investigation of the immune microenvironment of HCC and the development of more immunotherapeutic approaches based on the relevant summaries of different immune cells.
Introduction
Hepatocellular carcinoma (HCC) is the most common type of liver cancer, accounting for the sixth most common type of cancer and the second leading cause of death among all cancers (Ferlay et al., 2015). Due to changes in environmental factors, immunization and people’s lifestyle, the incidence of HCC and HCC-related mortality are increasing all over the world. The latest research indicates that HCC accounts for approximately 85% of patients diagnosed with liver cirrhosis. Its 5-year survival rate is only 18%, second only to pancreatic cancer (Asafo-Agyei and Samant, 2021). With the improvement of the treatment level for HCC, a variety of treatment options such as liver transplantation, surgical removal, systemic therapy and liver targeted therapy are constantly enhanced and created. At present, only surgical treatment is considered as a potential radical treatment for HCC. But only 15% of HCC patients have the opportunity to have surgery, most patients are found in the advanced stage (Roxburgh and Evans, 2008). Sorafenib is the only systemic medication approved by the FDA for advanced HCC. However, because of the overexpression of dihydropyrimine dehydrogenase, the multi-drug resistance gene MDR-1and p-glycoprotein gene products, HCC is regarded a chemotherapy-resistant tumour, and how to execute effective chemotherapy is still a major difficulty (Soini et al., 1996; Jiang et al., 1997; Kato et al., 2001).
Seven essential characteristics of cancer have been identified as crosstalk between cells and immune cells, self-sufficiency of signals for growth, unrestricted replication potential, apoptosis avoidance, growth signals insensitivity and continuous angiogenesis and invasion/metastasis of tissue (Hanahan and Weinberg, 2000; Hanahan and Weinberg, 2011). Tumor cells, immune cells, stromal cells, endothelial cells, and cancer-related fibroblasts are all found in the tumour microenvironment (TME), according to current research. Malignant tumour cells can evade immune monitoring and kill, as well as impair the human body, via a range of intricate ways (Hanahan and Weinberg, 2011). Due to the limitations of traditional chemotherapy regimens in the treatment of HCC, a variety of immunotherapy methods for HCC have been developed. Immunotherapy mostly employs immune cells within or outside of the TME to specifically target and assault cancer cells, with the benefits of high specificity and low side effects (Yost et al., 2019). More crucially, thanks to advances in tools such as mass spectrometry and single-cell RNA sequencing, We can map immunological cells in TMEs at the single-cell level (Spitzer and Nolan, 2016; Zheng et al., 2017; Papalexi and Satija, 2018; Wang et al., 2019a). We outline the significance of tumor-associated immune cells in the HCC tumour microenvironment and highlight their relevance in HCC cancer immunotherapy in this study.
The Immune Cells in TME
Tumor-associated immune cells are broadly classified into two types: tumor-promoting immune cells and tumor-antagonistic immune cells. At different stages of tumour formation, these two types of cells play different functions and impact each other (Figure 1). Because the significance of tumor-associated B cells in tumour growth is debatable, we shall introduce B cells additionally.
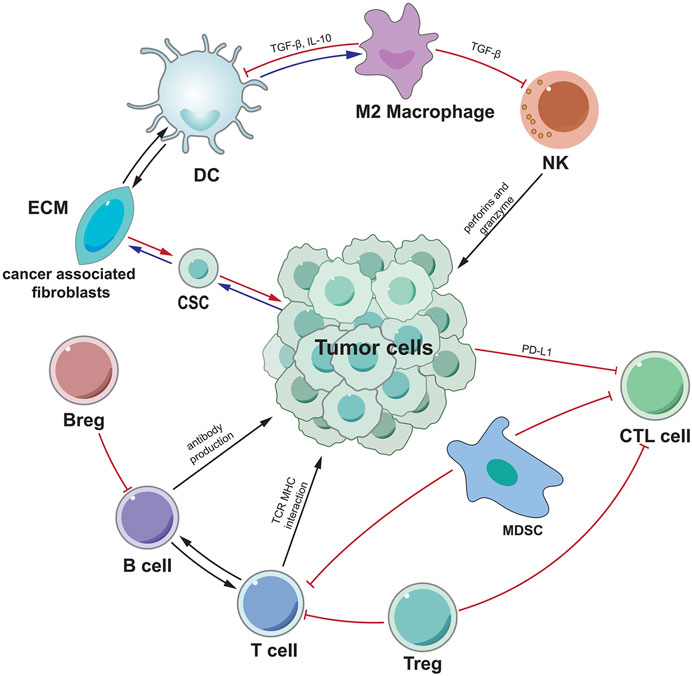
FIGURE 1. A schematic overview of the most important mechanisms and interactions of the tumor microenvironment. Tumor cells interact with other cells in various ways. CSC indicates cancer stem cell, ECM indicates extracellular matrix, CTL indicates cytotoxic T lymphocytes.
Tumor-Antagonizing Immune Cells
Effector T Cells
Current studies suggest that CD8+ cytotoxic T cells (CTLs) are the main lymphocytes that kill cancer cells. When CD8+ T cells recognize antibodies on DC, CD80−CD86 and CD70 ligands on DC connect to CD27 and CD28 receptors on CD8+ T cells, and CD8+ T cells are modified to become cytotoxic effector CD8+ T cells (Tanaka et al., 1999; Farhood et al., 2019). Furthermore, CD4+ T cells can activate CD8+ T cells through CD40−CD40L interaction, and CD4+ T cells can produce IL-2 to enhance CD8+ T cell proliferation. CD4+ T cells are also important in the maturation of CD8+ T cells into memory cells (Bennett et al., 1997; Mackey et al., 1997; Bennett et al., 1998; Mackey et al., 1998; Schoenberger et al., 1998; Bourgeois et al., 2002; Cheng et al., 2002; Borst et al., 2018). CTL kills target cells through granular exocytosis and apoptotic induction mediated by FasL ligand (FasL) in working state. CTL can also produce interferon- (IFN-) and tumour necrosis factor (TNF-) to cause cancer cell cytotoxicity (Farhood et al., 2019). Activation and regulation of CTL requires signals from T cell receptors (TCR) and immune checkpoints (Rogler et al., 1999). For example, cancer cells inhibit CTL activity through the expression of a ligand that binds to an inhibitory checkpoint, such as PD-L1 (Iwai et al., 2002). A significant number of studies have established the function of CD8+ T cells and CD4+ T cells in the formation and progression of HCC, including diagnosis/treatment/prognosis, and so on.
Chang et al. 's study confirmed that NanoMnSor improves the effectiveness of anti-PD-1 antibodies and whole-cell cancer vaccine immunotherapy by encouraging macrophage polarization to an immunostimulating M1 phenotype, decreasing hypoxica-induced tumor infiltration of tumor-associated macrophages, and raising the number of CD8 cytotoxic T cells in tumors, thereby reprogramming immunosuppressive TME (Chang et al., 2020). Xie et al. 's research proposed that PD-L1 overexpression is mostly triggered by pre-existing activated CD8 (+) cytotoxic T cells in the HCC environment, rather than being produced constitutively by tumor cells, and that it is a good prognostic factor for HCC (Xie et al., 2016). The frequency of circulating PD-1 (+) CD8 (+) T cells increases as the illness develops from LC to HCC. PD-1 expression was shown to be much higher in tumor-infiltrating CD8 (+) T cells. In vitro, CD8 (+) T cells promoted the production of PD-L1 on HCC cells in an IFN-dependent way, increasing CD8 (+) T cell death, whereas inhibiting PD-L1 reversed this effect (Shi et al., 2011).
Both in vitro restimulation and in vivo depletion studies have indicated that CD4+ and CD8+ lymphocytes contribute to anticancer activity. ASPH activation resulted in considerable production of antigen-specific CD4+ T cells in PBMC from healthy volunteers and HCC patients (Shimoda et al., 2012). Lack of recovered CD19+, CD3+, CD4+, and especially CD8+ T cells is associated with poor survival in patients (Carr and Metes, 2012). Zhou et al. ‘s revealed that antibodies against CD274 (PD-L1), LAG3, or TIM3 boost CD4+ and CD8+ TIL proliferation and cytokine secretion in response to polyclonal antigens or TAA stimulation (Zhou et al., 2017). More research results on the effect of Effector T cells in HCC are summarized in Table 1. It is clear that Effector T cells play a critical role in the immunological milieu of HCC. Many studies have shown that targeting these cells is effective in patients with HCC.
NK Cells
NK cells are an essential anti-tumor immune cell that primarily mediates immune surveillance of malignancies. It performs a similar function as CD8+ T cells: NK cells regulate the killing response of tumor cells by releasing perforin and granulein, triggering apoptosis in target cells. In addition, to improve their anticancer activity, NK cells can produce proinflammatory cytokines and chemokines (Voskoboinik et al., 2006; Guillerey et al., 2016; Habif et al., 2019). Existing studies have confirmed the value of NK cells in the development, targeted therapy, prognosis of HCC. Sprinzl et al. 's research confirmed that Sorafenib can promote the pro-inflammatory response of tumor-associated macrophages in HCC, and then activate the anti-tumor NK cell response through the cytokine and NF-κB pathway (Sprinzl et al., 2013). Senescence monitoring necessitates the recruitment and maturation of CCR2 myeloid cells, and CCR2 deficiency promotes HCC growth. Conversely, HCC cells suppress the maturation of recruited myeloid progenitors, which promotes mice HCC growth and worsens prognosis and survival in human HCC patients via NK cell inhibition (Eggert et al., 2016). Kohga et al. 's revealed that natural killer (NK) cells had stronger cytolytic activity on ADAM9KD-HCC cells than on control cells, and that this cytotoxicity is enhanced by the MICA/B and NK group 2, D pathways. Sorafenib treatment resulted in a decrease in ADAM9 expression in HCC cells, an increase in membrane-bound MICA expression, and a decrease in the quantity of soluble MICA. Sorafenib increased HCC cell NK sensitivity by boosting the expression of membrane-bound MICA (Kohga et al., 2010a). Table 2 summarizes current research on NK cells in HCC, confirming the importance of NK cells in immune escape and anti-HCC therapy.
Dendritic cells
DC cells, as specialized antigen-presenting cells in the human body, can present antigens to T cells and produce costimulatory signals for T cell activation. According to the current study, mature DC cells can penetrate tumor cells and limit tumor incidence and progression. Under many severe conditions, this inhibition effect will be avoided by tumors through certain means. Therefore, targeting at DC cells, some studies have reported its role in the occurrence, development, immunotherapy, diagnosis and prognosis of HCC. For example, In mice, combining DC vaccination and PD-L1 inhibitor treatment can result in longer overall life, reduced tumor volume, and increased tumor cell apoptosis. As a new therapy method for HCC, combined treatment with DC vaccination and PD-L1 inhibitor may offer promising results (Teng et al., 2020). Ali et al. 's research clarified that the combination of PEI or RFTA with active antigen-specific immunotherapy using DCS is a promising approach to induce a sustained anti-tumor immune response aimed at reducing tumor recurrence and metastasis in patients with HCC. Table 3 summarizes the current role of DC cells in HCC.
M1-Polarized Macrophages
Another important type of immune cell in TME is macrophages derived from circulating monocytes, which can generally be divided into M2 polarization and M1 polarization (Figure 2). M1-polarized macrophages, for example, can create pro-inflammatory cytokines and reactive oxygen species/nitrogen to prevent the formation and progression of malignancies (Aras and Zaidi, 2017). There are limited investigations on the function of M1-polarized macrophages in HCC at the moment. Studies have shown that M1-polarized macrophages in the S3 subclass in HCC increased, and the prognosis was good. Memory B cells, Total B cells, M1 macrophages and T follicle helper cells, were linked with strong total immune cell infiltration into HCC, whereas resting mast cells, neutrophils, and NK cells were associated with poor infiltration (Rohr-Udilova et al., 2018). Table 4 summarizes the current role of M1-polarized macrophages in HCC.
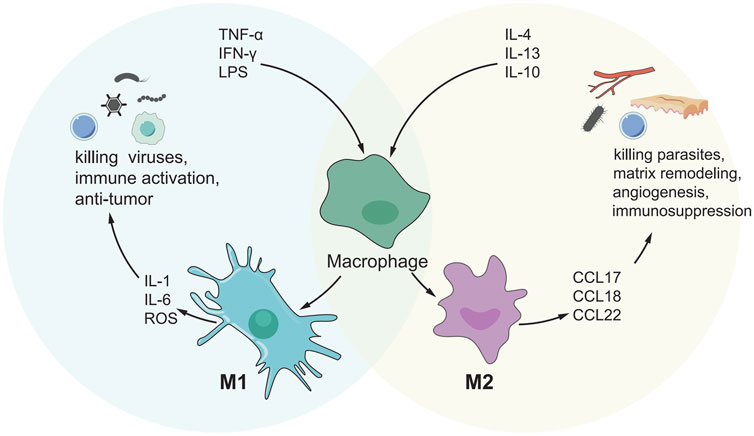
FIGURE 2. M1/M2 model of macrophage activation. M1 cells exert an inflammatory phenotype and are involved in killing bacteria, viruses and tumor cells, while M2 cells are involved in killing encapsulated parasites, immunosuppression, angiogenesis, etc.
Tumor-Promoting Immune Cells
In the immune microenvironment of HCC, some cells could promote the occurrence and development of HCC, and we will review them one by one.
Regulatory T cells(Tregs)
Tregs play a vital role in immunological homeostasis and immune self-tolerance, and they can express the CD4+ marker and the Foxp3 marker (Samstein et al., 2012). Foxp3+ Treg acts as a switch for all levels of immune response, and its effects appear to be two-sided. First, Treg can inhibit harmful immune responses and thus inhibit the occurrence of autoimmune diseases (Berod et al., 2012). Second, Treg suppresses protective immune responses against invading pathogens or tumors, leading to further progression of the disease (Sakaguchi et al., 2010). How does Treg play a role in tumors, including how does Treg infiltrate and metastasize to tumor sites or how does Treg help tumors evade immune monitoring, has become a hot research topic in recent years. Many ideas have been put forward. Tregs were found in much higher numbers in HCC patients than in healthy controls. In addition, patients with high Treg(III) levels after TACE had a significantly lower progression-free survival than patients with low Treg(III) levels after TACE (Park et al., 2020). Other studies have shown that tumor Treg upregulated the expression of the glucocorticoid-induced tumor necrosis factor receptor (GITR). Treatment with soluble GITR ligand (GITRL) reduced inhibition caused by activated tumor infiltrating Treg and restored CD4+ CD25-T cell proliferation and cytokine production. (Pedroza-Gonzalez et al., 2013). In addition to that, the proportion of Tregs cells in HCC patients was significantly higher than that in healthy and cirrhosis controls, and was related to various clinical indicators of HCC patients. In HCC patients with BCLC stage C, the proportion of Treg cells was more pronounced than in BCLC stage B patients. One to 2 weeks after surgery, the fraction of Treg cells was much lower than before GSMS-TACE. Three to 5 weeks following surgery, the proportion of Treg cells continued to decline (Ren et al., 2021). Table 5 summarizes the most credible studies related to the role of Treg in HCC.
Myeloid-Derived Suppressor cells
MDSC is thought to be a cancer-promoting immune cell in the HCC tumor microenvironment and was discovered a decade ago (Gabrilovich et al., 2007). MDSC is divided into granulocyte or multinuclear MDSC(PMN MDSC) and mononuclear MDSC(M-MDSC) (Veglia et al., 2018). MDSCs enhance angiogenesis by producing vascular endothelial growth factor (VEGF), actuator 2 and MMP9. They can also cause cancer cells to migrate to endothelial cells and encourage metastasis (Zhou et al., 2018a). MDSC also suppresses T cell activity by secreting immunosuppressive cytokines, inducible nitric oxide synthase, and argininase (Gabrilovich et al., 2012; Gabrilovich, 2017). MDSCs can have a dual influence on immune cells via distinct methods. B cells, T cells, DCs and NK cells, are all inhibited by MDSCs. MDSCs, on the other hand, can stimulate Th17 cells, Tregs, and TAMs, as well as tumor angiogenesis and metastasis (Figure 3). There has been a great deal of research on the processes through which MDSC supports the advancement of HCC, and many therapeutic pathways have been developed for MDSC as a target of HCC. For example, myeloid-derived suppressor cells (MDSCs) are drawn to the tumor microenvironment by PIWIL1-overexpressed HCC cells. MDSCs consumption reduced the proliferation and growth of PIWIL1-overexpressed HCC tumors. Complement C3 stimulates HCC cell secretion via PIWIL1 and mediates the contact between HCC cells and MDSC via p38 MAPK activation in MD38, and then initiates the expression of immunosuppressive cytokine IL10. PIWIL1, which is expressed by tumor cells, could be a viable target for the development of new HCC treatments (Wang et al., 2021a). Tumor-infiltrating LY6G MDSCs from orthotopic liver tumors treated with sorafenib dramatically increased CD4 T cells expressing IL-10 and TGF-and decreased CD8 T cell cytotoxicity. In vitro, IL-6 protects LY6G MDSC from sorafenib-induced cell death. Combining sorafenib and anti-IL-6 antibody or anti-LY6G antibody dramatically decreased the cell proportion of LY6G MDSCs in orthotopic liver tumors, synergistically boosted sorafenib’s therapeutic efficacy and increased T cell proliferation (Chang et al., 2018). Icaritin blocks MDSC production by blocking the attenuation of EMH, thereby inhibiting the immunosuppressive effect of the tumor, thereby triggering an anti-tumor immune response. Therefore, Icaritin can be used as a new adjuvant or even as an independent therapeutic agent for the effective treatment of HCC(Tao et al., 2021). In Table 6, we show the relevant role of MDSC in HCC immune microenvironment in recent years.
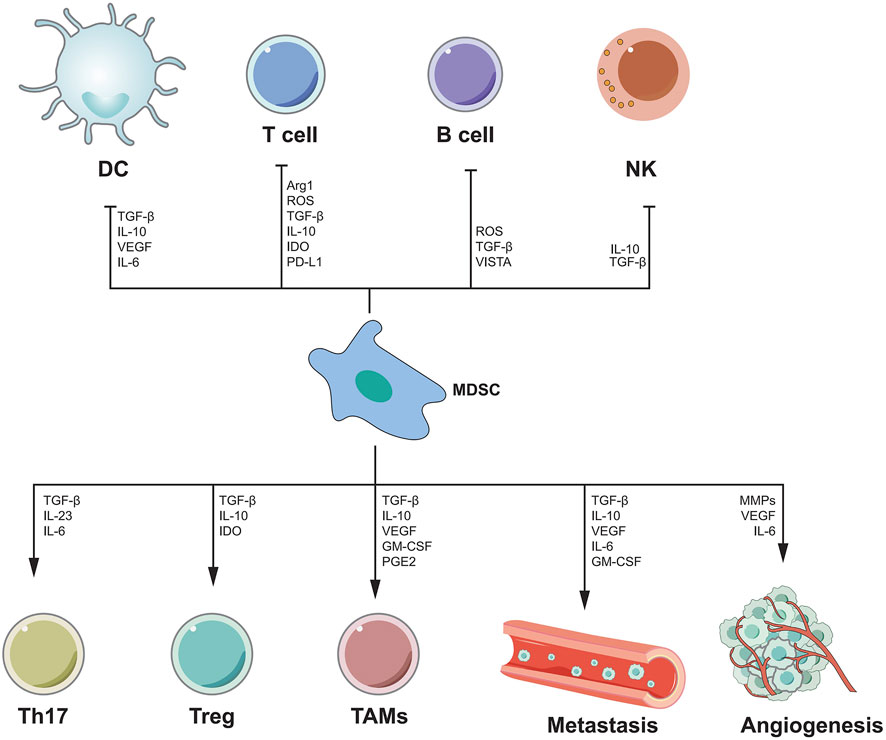
FIGURE 3. The mechanism of MDSC-mediated immunosuppression. MDSC inhibits the functions of DC, T cells, B cells and NK cells by secreting various cytokines, while promoting the functions of Th17, Treg, TAMs cells, and can promote angiogenesis and metastasis.
Cancer-Associated fibroblasts
CAF is not an immune cell, but it plays an important role in the tumor microenvironment, so we will introduce it in detail here. CAF exists as a prominent component of the tumor stroma between various inflammatory cells and components in the tumor microenvironment (Kalluri, 2016). As a result, CAF possesses functions that normal fibroblasts do not. A vast number of prior studies have demonstrated that CAF plays a critical function in changing the tumor microenvironment and driving the development of a variety of cancers (Jiang et al., 2017a; Zhao et al., 2017a; Deng et al., 2017; Pistore et al., 2017). CAF is also significant in HCC. Many studies have revealed the great role of CAF in the pathogenesis, progression, prognosis, treatment and other aspects of HCC. CAF-derived cardioctonutrient-like cytokine 1 (CLCF1) has been found in studies to stimulate the release of TGF-β and CXCL6 in tumor cells, consequently increasing tumor stem cell development in HCC-TME (Song et al., 2021). Other research have found that CCN2, EMA, and FAP expression may be involved in the activation of CAF in HCC, resulting in aggressive behavior. The substantial association between EMA-expressing tumor cells and FAP-expressing CAF, as well as their topographical proximity, suggests that there may be interplay between tumor epithelial and stromal cells in the HCC tumor microenvironment (Kim et al., 2014). Table 7 summarizes the current role of CAF in HCC.
M2-Polarized Macrophages
M2 polarized macrophages, as opposed to M1 polarized macrophages, have anti-inflammatory and pro-tumor actions (Figure 4). M2 macrophages are further differentiated into M2a, M2b, M2c, and M2d subsets. Th2 cytokines such as IL-13 and IL-4 can trigger macrophage transformation to the M2A phenotype, whereas TLR and immune complex activation induces M2B macrophages, the M2C subtype polarized by IL-10 (Hao et al., 2012; Sica and Mantovani, 2012). Despite the fact that there have been few research on M2-polarized macrophages in HCC, the function of them in the occurrence and development of HCC has been confirmed. According to several studies, arsenite raises miR-15b levels and causes M2 polarization in THP-1 cells. Increased miR-15b in Evs transfer from arsenite-treated THP-1 (AS-THP-1) cells to HCC cells via miR-15b. By targeting LATS1, it can reduce Hippo pathway activation while still accelerating the invasion and metastasis of growing HCC cells (Li et al., 2021). The potential therapeutic potential of M2 polarized macrophages has also been pointed out that Tumor cell-derived Wnt ligands induce M2-like polarization of TAM via traditional Wnt/-catenin signaling, resulting in tumor migration, development, immunosuppression and metastasis in HCC(Yang et al., 2018a). We summarize the current relevant research progress in Table 8.
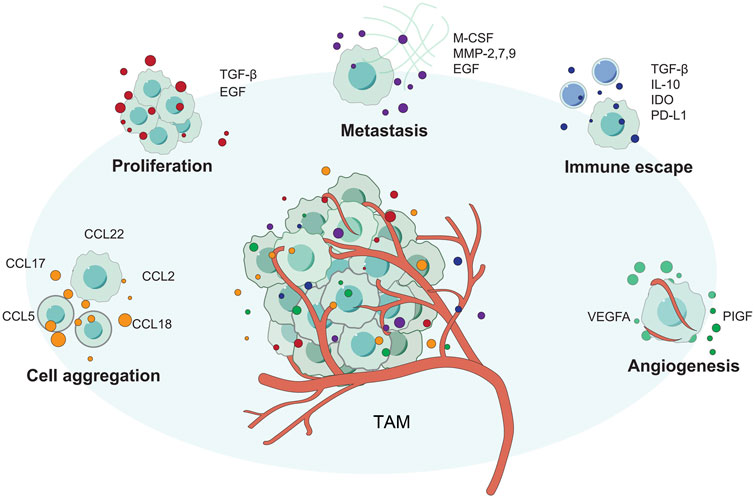
FIGURE 4. The different ways that TAM supports tumor growth. TAM promotes tumor proliferation, invasion, angiogenesis, and immune escape through various methods.
The Controversial Immune Cell Type in Cancer: B Cells
One type of immune cells that cannot be ignored in the HCC tumor microenvironment is B cells. According to the current relevant studies, it is not clear whether B cells are “good” or “bad”. Some studies have reported that B cells promote HCC, while others have reported the opposite effect. B cells, on the one hand, release cytokines that comport with CTL activity and serve as potent antigen-presenting cells (APCs). On the other hand, they may be tumorigenic due to the production of cytokines that attract MDSC and promote angiogenesis (de Visser et al., 2005; Tsou et al., 2016). Studies have shown that CCL20 derived from tumor cells interacts with CD19CD5 B cells overexpressed by CCR6 to promote the development of HCC, possibly through enhanced angiogenesis (He et al., 2017). Liu et al. 's study confirmed that Selective recruitment of CXCR3 (+) B cells Bridges the pro-inflammatory interleukin-17 response and the polarization of tumorigenic macrophages in the tumor environment, and blocking the migration or function of CXCR3+ B cells may help to overcome HCC (Liu et al., 2015). Therefore, B cells are involved in both the development and inhibition of HCC. Table 9 highlights the most recent reliable research on the involvement of B cells in HCC.
Conclusions and Perspectives
With the development of single-cell sequencing and other technologies, we have the opportunity to further explore TME. Immunotherapy, a new tumor treatment method, also has a better and broader application prospect. However, at present, it is still too early for immunotherapy to replace traditional chemotherapeutic therapy, and there is still a long way to go in the process of clinical application. However, immunotherapy can be regarded as a good alternative therapy for patients with chemotherapy resistance. As mentioned in this paper, immunotherapies for tumor suppressor related immune cells, such as effect DCs, as well as tumor promoting immune cells, such as MDSC/Treg, are being developed one after another.
Compared with traditional chemotherapy, immunotherapy has many advantages. For example, immunotherapy has fewer overall side effects than chemotherapy and, once effective, may lead to long-term survival and even clinical cure. In addition, immunotherapy can be used as an important anti-tumor adjuvant therapy in addition to chemotherapy, radiotherapy and surgery. Appropriate immunotherapy can kill the tiny residual tumor cells after chemotherapy or some tumor cells resistant to chemotherapy. Immunotherapy should be considered for patients who are intolerant to chemotherapy or have extensive metastasis and cannot undergo surgery, radiotherapy or chemotherapy (Yang, 2015).
At present, the mainstream immunotherapy mainly includes CAR T therapy/immune checkpoint inhibitor therapy (PD-1, PD-L1, etc.)/tumor vaccine. CAR-T is the T cells, biological engineering, when the cancer has an immune deficiency, immune surveillance, give play to the role of the case, through the biological engineering to determine the targets of leukemia, it specifically chimeric in T cells, to attack the leukemia cells, the effect is significant, but easy to appear “storm” cells, serious and even cause death (Feins et al., 2019). Immune checkpoint inhibitor therapy has the advantage of long-term survival and relatively small adverse reactions. This therapy activates tumor-specific immune cells in the body by removing or attenuating the negative regulatory factors of immunoreactive cells, but it is not suitable for all patients. The higher the mutation load, the better the treatment response. Therefore, biomarkers should be used to screen the dominant population. The most common predictive indicators of PD-1/PD-L1 immune checkpoint inhibitors are microsatellite instability, PD-L1 and tumor mutation load (Pinato et al., 2019). The treatment of cancer vaccines is still incomplete.
Immunotherapy is not the end of tumor therapy; on the contrary, tumor immunotherapy represented by immune checkpoints has just opened a new chapter in tumor therapy. Combined with the basic and characteristics of immunotherapy, with the deepening of human understanding of tumors, tumors as a chronic disease that can be cured are no longer so far out of reach.
Author Contributions
The manuscript had three first authors who made equal contributions to the project. XH, GS, and YZ were responsible for collecting information and design reviews of relevant studies. XK and DR are responsible for drawing the pictures. In addition, we have three corresponding authors in this manuscript. WT, JS, and XW contributed to the interpretation, editing and critical revision of the manuscript.
Funding
We are grateful for the grants from the National Natural Science Key Foundation of China (Grant No. 31930020) and National Natural Science Foundation of China (Grant No. 81771716).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, orclaim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to express our sincere thanks to Magdalens Klink, the author of the book “Interaction of immune and cancer cells.” Our pictures have been partially changed and repainted on the basis of this book. At the same time, this book has also given us a lot of writing inspiration.
References
Ali, M. Y., Grimm, C. F., Ritter, M., Mohr, L., Allgaier, H.-P., Weth, R., et al. (2005). Activation of Dendritic Cells by Local Ablation of Hepatocellular Carcinoma. J. Hepatol. 43, 817–822. doi:10.1016/j.jhep.2005.04.016
Anson, M., Crain-Denoyelle, A.-M., Baud, V., Chereau, F., Gougelet, A., Terris, B., et al. (2012). Oncogenic β-catenin Triggers an Inflammatory Response that Determines the Aggressiveness of Hepatocellular Carcinoma in Mice. J. Clin. Invest. 122, 586–599. doi:10.1172/jci43937
Aras, S., and Zaidi, M. R. (2017). TAMeless Traitors: Macrophages in Cancer Progression and Metastasis. Br. J. Cancer 117, 1583–1591. doi:10.1038/bjc.2017.356
Arihara, F., Mizukoshi, E., Kitahara, M., Takata, Y., Arai, K., Yamashita, T., et al. (2013). Increase in CD14+HLA-DR−/low Myeloid-Derived Suppressor Cells in Hepatocellular Carcinoma Patients and its Impact on Prognosis. Cancer Immunol. Immunother. 62, 1421–1430. doi:10.1007/s00262-013-1447-1
Barajas, M., Mazzolini, G., Genove, G., Bilbao, R., Narvaiza, I., Schmitz, V., et al. (2001). Gene Therapy of Orthotopic Hepatocellular Carcinoma in Rats Using Adenovirus Coding for Interleukin 12. Hepatology 33, 52–61. doi:10.1053/jhep.2001.20796
Bennett, S. R. M., Carbone, F. R., Karamalis, F., Flavell, R. A., Miller, J. F. A. P., and Heath, W. R. (1998). Help for Cytotoxic-T-Cell Responses Is Mediated by CD40 Signalling. Nature 393, 478–480. doi:10.1038/30996
Bennett, S. R. M., Carbone, F. R., Karamalis, F., Miller, J. F. A. P., and Heath, W. R. (1997). Induction of a CD8+ Cytotoxic T Lymphocyte Response by Cross-Priming Requires Cognate CD4+ T Cell Help. J. Exp. Med. 186, 65–70. doi:10.1084/jem.186.1.65
Berod, L., Puttur, F., Huehn, J., and Sparwasser, T. (2012). Tregs in Infection and Vaccinology: Heroes or Traitors? Microb. Biotechnol. 5, 260–269. doi:10.1111/j.1751-7915.2011.00299.x
Borst, J., Ahrends, T., Bąbała, N., Melief, C. J. M., and Kastenmüller, W. (2018). CD4+ T Cell Help in Cancer Immunology and Immunotherapy. Nat. Rev. Immunol. 18, 635–647. doi:10.1038/s41577-018-0044-0
Bourgeois, C., Rocha, B., and Tanchot, C. (2002). A Role for CD40 Expression on CD8+ T Cells in the Generation of CD8+ T Cell Memory. Science 297, 2060–2063. doi:10.1126/science.1072615
Brown, Z. J., Fu, Q., Ma, C., Kruhlak, M., Zhang, H., Luo, J., et al. (2018). Carnitine Palmitoyltransferase Gene Upregulation by Linoleic Acid Induces CD4+ T Cell Apoptosis Promoting HCC Development. Cell Death Dis 9, 620. doi:10.1038/s41419-018-0687-6
Cabrera, R., Ararat, M., Xu, Y., Brusko, T., Wasserfall, C., Atkinson, M. A., et al. (2013). Immune Modulation of Effector CD4+ and Regulatory T Cell Function by Sorafenib in Patients with Hepatocellular Carcinoma. Cancer Immunol. Immunother. 62, 737–746. doi:10.1007/s00262-012-1380-8
Cai, M.-Y., Xu, Y.-F., Qiu, S.-J., Ju, M.-J., Gao, Q., Li, Y.-W., et al. (2009). Human Leukocyte Antigen-G Protein Expression Is an Unfavorable Prognostic Predictor of Hepatocellular Carcinoma Following Curative Resection. Clin. Cancer Res. 15, 4686–4693. doi:10.1158/1078-0432.ccr-09-0463
Cao, M., Huang, W., Chen, Y., Li, G., Liu, N., Wu, Y., et al. (2021). Chronic Restraint Stress Promotes the Mobilization and Recruitment of Myeloid-Derived Suppressor Cells through Beta-Adrenergic-Activated CXCL5-CXCR2-Erk Signaling Cascades. Int. J. Cancer 1, 1.
Carr, B. I., and Metes, D. M. (2012). Peripheral Blood Lymphocyte Depletion after Hepatic Arterial 90Yttrium Microsphere Therapy for Hepatocellular Carcinoma. Int. J. Radiat. Oncology*Biology*Physics 82, 1179–1184. doi:10.1016/j.ijrobp.2010.10.042
Chang, C.-C., Dinh, T. K., Lee, Y.-A., Wang, F.-N., Sung, Y.-C., Yu, P.-L., et al. (2020). Nanoparticle Delivery of MnO2 and Antiangiogenic Therapy to Overcome Hypoxia-Driven Tumor Escape and Suppress Hepatocellular Carcinoma. ACS Appl. Mater. Inter. 12, 44407–44419. doi:10.1021/acsami.0c08473
Chang, C.-J., Yang, Y.-H., Chiu, C.-J., Lu, L.-C., Liao, C.-C., Liang, C.-W., et al. (2018). Targeting Tumor-Infiltrating Ly6G+ Myeloid Cells Improves Sorafenib Efficacy in Mouse Orthotopic Hepatocellular Carcinoma. Int. J. Cancer 142, 1878–1889. doi:10.1002/ijc.31216
Chaoul, N., Mancarella, S., Lupo, L., Giannelli, G., and Dituri, F. (2020). Impaired Anti-tumor T Cell Response in Hepatocellular Carcinoma. Cancers (Basel) 12, 1. doi:10.3390/cancers12030627
Chen, K., Wu, Z., Zhao, H., Wang, Y., Ge, Y., Wang, D., et al. (2020). XCL1/Glypican-3 Fusion Gene Immunization Generates Potent Antitumor Cellular Immunity and Enhances Anti-PD-1 Efficacy. Cancer Immunol. Res. 8, 81–93. doi:10.1158/2326-6066.cir-19-0210
Chen, L., Zhou, S., Qin, J., Hu, H., Ma, H., Liu, B., et al. (2013). Combination of SLC Administration and Tregs Depletion Is an Attractive Strategy for Targeting Hepatocellular Carcinoma. Mol. Cancer 12, 153. doi:10.1186/1476-4598-12-153
Chen, M.-L., Yan, B.-S., Lu, W.-C., Chen, M.-H., Yu, S.-L., Yang, P.-C., et al. (2014). Sorafenib Relieves Cell-Intrinsic and Cell-Extrinsic Inhibitions of Effector T Cells in Tumor Microenvironment to Augment Antitumor Immunity. Int. J. Cancer 134, 319–331. doi:10.1002/ijc.28362
Chen, Y., Hao, X., Sun, R., Wei, H., and Tian, Z. (2019). Natural Killer Cell-Derived Interferon‐Gamma Promotes Hepatocellular Carcinoma through the Epithelial Cell Adhesion Molecule-Epithelial‐to‐Mesenchymal Transition Axis in Hepatitis B Virus Transgenic Mice. Hepatology 69, 1735–1750. doi:10.1002/hep.30317
Chen, Y., Yang, D., Li, S., Gao, Y., Jiang, R., Deng, L., et al. (2012). Development of a Listeria Monocytogenes-Based Vaccine against Hepatocellular Carcinoma. Oncogene 31, 2140–2152. doi:10.1038/onc.2011.395
Cheng, L., Du, X., Wang, Z., Ju, J., Jia, M., Huang, Q., et al. (2014). Hyper-IL-15 Suppresses Metastatic and Autochthonous Liver Cancer by Promoting Tumour-specific CD8+ T Cell Responses. J. Hepatol. 61, 1297–1303. doi:10.1016/j.jhep.2014.07.004
Cheng, L. E., Ohlen, C., Nelson, B. H., and Greenberg, P. D. (2002). Enhanced Signaling through the IL-2 Receptor in CD8+ T Cells Regulated by Antigen Recognition Results in Preferential Proliferation and Expansion of Responding CD8+ T Cells rather Than Promotion of Cell Death. Proc. Natl. Acad. Sci. 99, 3001–3006. doi:10.1073/pnas.052676899
Cheng, Y., Li, H., Deng, Y., Tai, Y., Zeng, K., Zhang, Y., et al. (2018). Cancer-associated Fibroblasts Induce PDL1+ Neutrophils through the IL6-STAT3 Pathway that foster Immune Suppression in Hepatocellular Carcinoma. Cell Death Dis 9, 422. doi:10.1038/s41419-018-0458-4
Chew, V., Tow, C., Teo, M., Wong, H. L., Chan, J., Gehring, A., et al. (2010). Inflammatory Tumour Microenvironment Is Associated with superior Survival in Hepatocellular Carcinoma Patients. J. Hepatol. 52, 370–379. doi:10.1016/j.jhep.2009.07.013
Chiu, D. K.-C., Tse, A. P.-W., Xu, I. M.-J., Di Cui, J., Lai, R. K.-H., Li, L. L., et al. (2017). Hypoxia Inducible Factor HIF-1 Promotes Myeloid-Derived Suppressor Cells Accumulation through ENTPD2/CD39L1 in Hepatocellular Carcinoma. Nat. Commun. 8, 517. doi:10.1038/s41467-017-00530-7
Chiu, D. K.-C., Yuen, V. W.-H., Cheu, J. W.-S., Wei, L. L., Ting, V., Fehlings, M., et al. (2020). Hepatocellular Carcinoma Cells Up-Regulate PVRL1, Stabilizing PVR and Inhibiting the Cytotoxic T-Cell Response via TIGIT to Mediate Tumor Resistance to PD1 Inhibitors in Mice. Gastroenterology 159, 609–623. doi:10.1053/j.gastro.2020.03.074
Cicinnati, V. R., Zhang, X., Yu, Z., Ferencik, S., Schmitz, K. J., Dworacki, G., et al. (2006). Increased Frequencies of CD8+ T Lymphocytes Recognizing Wild-type P53-Derived Epitopes in Peripheral Blood Correlate with Presence of Epitope Loss Tumor Variants in Patients with Hepatocellular Carcinoma. Int. J. Cancer 119, 2851–2860. doi:10.1002/ijc.22251
de Visser, K. E., Korets, L. V., and Coussens, L. M. (2005). De Novo carcinogenesis Promoted by Chronic Inflammation Is B Lymphocyte Dependent. Cancer Cell 7, 411–423. doi:10.1016/j.ccr.2005.04.014
Deng, Y., Cheng, J., Fu, B., Liu, W., Chen, G., Zhang, Q., et al. (2017). Hepatic Carcinoma-Associated Fibroblasts Enhance Immune Suppression by Facilitating the Generation of Myeloid-Derived Suppressor Cells. Oncogene 36, 1090–1101. doi:10.1038/onc.2016.273
Dhanasekaran, R., Park, J., Yevtodiyenko, A., Bellovin, D. I., Adam, S. J., Kd, A. R., et al. (2020). MYC ASO Impedes Tumorigenesis and Elicits Oncogene Addiction in Autochthonous Transgenic Mouse Models of HCC and RCC. Mol. Ther. - Nucleic Acids 21, 850–859. doi:10.1016/j.omtn.2020.07.008
Duffy, A. G., Ulahannan, S. V., Makorova-Rusher, O., Rahma, O., Wedemeyer, H., Pratt, D., et al. (2017). Tremelimumab in Combination with Ablation in Patients with Advanced Hepatocellular Carcinoma. J. Hepatol. 66, 545–551. doi:10.1016/j.jhep.2016.10.029
Eggert, T., Wolter, K., Ji, J., Ma, C., Yevsa, T., Klotz, S., et al. (2016). Distinct Functions of Senescence-Associated Immune Responses in Liver Tumor Surveillance and Tumor Progression. Cancer Cell 30, 533–547. doi:10.1016/j.ccell.2016.09.003
Elinav, E., Abd-Elnabi, A., Pappo, O., Bernstein, I., Klein, A., Engelhardt, D., et al. (2006). Suppression of Hepatocellular Carcinoma Growth in Mice via Leptin, Is Associated with Inhibition of Tumor Cell Growth and Natural Killer Cell Activation. J. Hepatol. 44, 529–536. doi:10.1016/j.jhep.2005.08.013
Evdokimova, V. N., Liu, Y., Potter, D. M., and Butterfield, L. H. (2007). AFP-specific CD4+ Helper T-Cell Responses in Healthy Donors and HCC Patients. J. Immunother. 30, 425–437. doi:10.1097/cji.0b013e31802fd8e2
Faggioli, F., Palagano, E., Di Tommaso, L., Donadon, M., Marrella, V., Recordati, C., et al. (2018). B Lymphocytes Limit Senescence-Driven Fibrosis Resolution and Favor Hepatocarcinogenesis in Mouse Liver Injury. Hepatology 67, 1970–1985. doi:10.1002/hep.29636
Fan, Y., Gao, Y., Rao, J., Wang, K., Zhang, F., and Zhang, C. (2017). YAP-1 Promotes Tregs Differentiation in Hepatocellular Carcinoma by Enhancing TGFBR2 Transcription. Cell Physiol Biochem 41, 1189–1198. doi:10.1159/000464380
Farhood, B., Najafi, M., and Mortezaee, K. (2019). CD8 + Cytotoxic T Lymphocytes in Cancer Immunotherapy: A Review. J. Cell Physiol 234, 8509–8521. doi:10.1002/jcp.27782
Feins, S., Kong, W., Williams, E. F., Milone, M. C., and Fraietta, J. A. (2019). An Introduction to Chimeric Antigen Receptor (CAR) T‐cell Immunotherapy for Human Cancer. Am. J. Hematol. 94, S3–S9. doi:10.1002/ajh.25418
Ferlay, J., Soerjomataram, I., Dikshit, R., Eser, S., Mathers, C., Rebelo, M., et al. (2015). Cancer Incidence and Mortality Worldwide: Sources, Methods and Major Patterns in GLOBOCAN 2012. Int. J. Cancer 136, E359–E386. doi:10.1002/ijc.29210
Fu, J., Xu, D., Liu, Z., Shi, M., Zhao, P., Fu, B., et al. (2007). Increased Regulatory T Cells Correlate with CD8 T-Cell Impairment and Poor Survival in Hepatocellular Carcinoma Patients. Gastroenterology 132, 2328–2339. doi:10.1053/j.gastro.2007.03.102
Gabrielson, A., Wu, Y., Wang, H., Jiang, J., Kallakury, B., Gatalica, Z., et al. (2016). Intratumoral CD3 and CD8 T-Cell Densities Associated with Relapse-free Survival in HCC. Cancer Immunol. Res. 4, 419–430. doi:10.1158/2326-6066.cir-15-0110
Gabrilovich, D. I., Bronte, V., Chen, S.-H., Colombo, M. P., Ochoa, A., Ostrand-Rosenberg, S., et al. (2007). The Terminology Issue for Myeloid-Derived Suppressor Cells. Cancer Res. 67, 425. doi:10.1158/0008-5472.can-06-3037
Gabrilovich, D. I. (2017). Myeloid-Derived Suppressor Cells. Cancer Immunol. Res. 5, 3–8. doi:10.1158/2326-6066.cir-16-0297
Gabrilovich, D. I., Ostrand-Rosenberg, S., and Bronte, V. (2012). Coordinated Regulation of Myeloid Cells by Tumours. Nat. Rev. Immunol. 12, 253–268. doi:10.1038/nri3175
Gao, Q., Qiu, S.-J., Fan, J., Zhou, J., Wang, X.-Y., Xiao, Y.-S., et al. (2007). Intratumoral Balance of Regulatory and Cytotoxic T Cells Is Associated with Prognosis of Hepatocellular Carcinoma after Resection. Jco 25, 2586–2593. doi:10.1200/jco.2006.09.4565
Garnelo, M., Tan, A., Her, Z., Yeong, J., Lim, C. J., Chen, J., et al. (2017). Interaction between Tumour-Infiltrating B Cells and T Cells Controls the Progression of Hepatocellular Carcinoma. Gut 66, 342–351. doi:10.1136/gutjnl-2015-310814
Goyal, L., Zheng, H., Abrams, T. A., Miksad, R., Bullock, A. J., Allen, J. N., et al. (2019). A Phase II and Biomarker Study of Sorafenib Combined with Modified FOLFOX in Patients with Advanced Hepatocellular Carcinoma. Clin. Cancer Res. 25, 80–89. doi:10.1158/1078-0432.ccr-18-0847
Guillerey, C., Huntington, N. D., and Smyth, M. J. (2016). Targeting Natural Killer Cells in Cancer Immunotherapy. Nat. Immunol. 17, 1025–1036. doi:10.1038/ni.3518
Habif, G., Crinier, A., André, P., Vivier, E., and Narni-Mancinelli, E. (2019). Targeting Natural Killer Cells in Solid Tumors. Cell Mol Immunol 16, 415–422. doi:10.1038/s41423-019-0224-2
Hage, C., Hoves, S., Strauss, L., Bissinger, S., Prinz, Y., Pöschinger, T., et al. (2019). Sorafenib Induces Pyroptosis in Macrophages and Triggers Natural Killer Cell-Mediated Cytotoxicity against Hepatocellular Carcinoma. Hepatology 70, 1280–1297. doi:10.1002/hep.30666
Han, Q., Wang, Y., Pang, M., and Zhang, J. (2017). STAT3-blocked Whole-Cell Hepatoma Vaccine Induces Cellular and Humoral Immune Response against HCC. J. Exp. Clin. Cancer Res. 36, 156. doi:10.1186/s13046-017-0623-0
Hanahan, D., and Weinberg, R. A. (2011). Hallmarks of Cancer: the Next Generation. Cell 144, 646–674. doi:10.1016/j.cell.2011.02.013
Hanahan, D., and Weinberg, R. A. (2000). The Hallmarks of Cancer. Cell 100, 57–70. doi:10.1016/s0092-8674(00)81683-9
Hao, N. B., Lü, M. H., Fan, Y. H., Cao, Y. L., Zhang, Z. R., and Yang, S. M. (2012). Macrophages in Tumor Microenvironments and the Progression of Tumors. Clin. Dev. Immunol. 2012, 948098. doi:10.1155/2012/948098
He, H., Wu, J., Zang, M., Wang, W., Chang, X., Chen, X., et al. (2017). CCR6+ B Lymphocytes Responding to Tumor Cell-Derived CCL20 Support Hepatocellular Carcinoma Progression via Enhancing Angiogenesis. Am. J. Cancer Res. 7, 1151–1163.
Hiroishi, K., Eguchi, J., Baba, T., Shimazaki, T., Ishii, S., Hiraide, A., et al. (2010). Strong CD8+ T-Cell Responses against Tumor-Associated Antigens Prolong the Recurrence-free Interval after Tumor Treatment in Patients with Hepatocellular Carcinoma. J. Gastroenterol. 45, 451–458. doi:10.1007/s00535-009-0155-2
Hoechst, B., Ormandy, L. A., Ballmaier, M., Lehner, F., Krüger, C., Manns, M. P., et al. (2008). A New Population of Myeloid-Derived Suppressor Cells in Hepatocellular Carcinoma Patients Induces CD4+CD25+Foxp3+ T Cells. Gastroenterology 135, 234–243. doi:10.1053/j.gastro.2008.03.020
Hoechst, B., Voigtlaender, T., Ormandy, L., Gamrekelashvili, J., Zhao, F., Wedemeyer, H., et al. (2009). Myeloid Derived Suppressor Cells Inhibit Natural Killer Cells in Patients with Hepatocellular Carcinoma via the NKp30 Receptor. Hepatology 50, 799–807. doi:10.1002/hep.23054
Homma, S., Komita, H., Sagawa, Y., Ohno, T., and Toda, G. (2005). Antitumour Activity Mediated by CD4+ Cytotoxic T Lymphocytes against MHC Class II-Negative Mouse Hepatocellular Carcinoma Induced by Dendritic Cell Vaccine and Interleukin-12. Immunology 115, 451–461. doi:10.1111/j.1365-2567.2005.02179.x
Hu, Z., Chen, J., Zhou, S., Yang, N., Duan, S., Zhang, Z., et al. (2017). Mouse IP-10 Gene Delivered by Folate-Modified Chitosan Nanoparticles and Dendritic/tumor Cells Fusion Vaccine Effectively Inhibit the Growth of Hepatocellular Carcinoma in Mice. Theranostics 7, 1942–1952. doi:10.7150/thno.16236
Huang, F., Chen, J., Lan, R., Wang, Z., Chen, R., Lin, J., et al. (2018). δ-Catenin Peptide Vaccines Repress Hepatocellular Carcinoma Growth via CD8+ T Cell Activation. Oncoimmunology 7, e1450713. doi:10.1080/2162402x.2018.1450713
Irie, M., Homma, S., Komita, H., Zeniya, M., Kufe, D., Ohno, T., et al. (2004). Inhibition of Spontaneous Development of Liver Tumors by Inoculation with Dendritic Cells Loaded with Hepatocellular Carcinoma Cells in C3H/HeNCRJ Mice. Int. J. Cancer 111, 238–245. doi:10.1002/ijc.20247
Ishiyama, K., Ohdan, H., Ohira, M., Mitsuta, H., Arihiro, K., and Asahara, T. (2006). Difference in Cytotoxicity against Hepatocellular Carcinoma between Liver and Periphery Natural Killer Cells in Humans. Hepatology 43, 362–372. doi:10.1002/hep.21035
Iwai, Y., Ishida, M., Tanaka, Y., Okazaki, T., Honjo, T., and Minato, N. (2002). Involvement of PD-L1 on Tumor Cells in the Escape from Host Immune System and Tumor Immunotherapy by PD-L1 Blockade. Proc. Natl. Acad. Sci. 99, 12293–12297. doi:10.1073/pnas.192461099
Jia, C., Wang, G., Wang, T., Fu, B., Zhang, Y., Huang, L., et al. (2020). Cancer-associated Fibroblasts Induce Epithelial-Mesenchymal Transition via the Transglutaminase 2-dependent IL-6/IL6R/STAT3 axis in Hepatocellular Carcinoma. Int. J. Biol. Sci. 16, 2542–2558. doi:10.7150/ijbs.45446
Jiang, J., Ye, F., Yang, X., Zong, C., Gao, L., Yang, Y., et al. (2017). Peri-tumor Associated Fibroblasts Promote Intrahepatic Metastasis of Hepatocellular Carcinoma by Recruiting Cancer Stem Cells. Cancer Lett. 404, 19–28. doi:10.1016/j.canlet.2017.07.006
Jiang, R., Tang, J., Chen, Y., Deng, L., Ji, J., Xie, Y., et al. (2017). The Long Noncoding RNA Lnc-EGFR Stimulates T-Regulatory Cells Differentiation Thus Promoting Hepatocellular Carcinoma Immune Evasion. Nat. Commun. 8, 15129. doi:10.1038/ncomms15129
Jiang, W., Lu, Z., He, Y., and Diasio, R. B. (1997). Dihydropyrimidine Dehydrogenase Activity in Hepatocellular Carcinoma: Implication in 5-Fluorouracil-Based Chemotherapy. Clin. Cancer Res. 3, 395–399.
Jinushi, M., Takehara, T., Tatsumi, T., Hiramatsu, N., Sakamori, R., Yamaguchi, S., et al. (2005). Impairment of Natural Killer Cell and Dendritic Cell Functions by the Soluble Form of MHC Class I-Related Chain A in Advanced Human Hepatocellular Carcinomas. J. Hepatol. 43, 1013–1020. doi:10.1016/j.jhep.2005.05.026
Kakita, N., Kanto, T., Itose, I., Kuroda, S., Inoue, M., Matsubara, T., et al. (2012). Comparative Analyses of Regulatory T Cell Subsets in Patients with Hepatocellular Carcinoma: A Crucial Role of CD25−FOXP3−T Cells. Int. J. Cancer 131, 2573–2583. doi:10.1002/ijc.27535
Kakumu, S., Ito, S., Ishikawa, T., Mita, Y., Tagaya, T., Fukuzawa, Y., et al. (2000). Decreased Function of Peripheral Blood Dendritic Cells in Patients with Hepatocellular Carcinoma with Hepatitis B and C Virus Infection. J. Gastroenterol. Hepatol. 15, 431–436. doi:10.1046/j.1440-1746.2000.02161.x
Kalathil, S. G., Hutson, A., Barbi, J., Iyer, R., and Thanavala, Y. (2019). Augmentation of IFN-Γ+ CD8+ T Cell Responses Correlates with Survival of HCC Patients on Sorafenib Therapy. JCI Insight 4. doi:10.1172/jci.insight.130116
Kalathil, S. G., Lugade, A. A., Miller, A., Iyer, R., and Thanavala, Y. (2016). PD-1+ and Foxp3+ T Cell Reduction Correlates with Survival of HCC Patients after Sorafenib Therapy. JCI Insight 1. doi:10.1172/jci.insight.86182
Kalathil, S. G., Wang, K., Hutson, A., Iyer, R., and Thanavala, Y. (2020). Tivozanib Mediated Inhibition of C-Kit/SCF Signaling on Tregs and MDSCs and Reversal of Tumor Induced Immune Suppression Correlates with Survival of HCC Patients. Oncoimmunology 9, 1824863. doi:10.1080/2162402x.2020.1824863
Kalluri, R. (2016). The Biology and Function of Fibroblasts in Cancer. Nat. Rev. Cancer 16, 582–598. doi:10.1038/nrc.2016.73
Kato, A., Miyazaki, M., Ambiru, S., Yoshitomi, H., Ito, H., Nakagawa, K., et al. (2001). Multidrug Resistance Gene (MDR-1) Expression as a Useful Prognostic Factor in Patients with Human Hepatocellular Carcinoma after Surgical Resection. J. Surg. Oncol. 78, 110–115. doi:10.1002/jso.1129
Kim, G. J., Rhee, H., Yoo, J. E., Ko, J. E., Lee, J. S., Kim, H., et al. (2014). Increased Expression of CCN2, Epithelial Membrane Antigen, and Fibroblast Activation Protein in Hepatocellular Carcinoma with Fibrous Stroma Showing Aggressive Behavior. PLoS One 9, e105094. doi:10.1371/journal.pone.0105094
Kohga, K., Takehara, T., Tatsumi, T., Ishida, H., Miyagi, T., Hosui, A., et al. (2010). Sorafenib Inhibits the Shedding of Major Histocompatibility Complex Class I-Related Chain A on Hepatocellular Carcinoma Cells by Down-Regulating a Disintegrin and Metalloproteinase 9. Hepatology 51, 1264–1273. doi:10.1002/hep.23456
Kohga, K., Tatsumi, T., Takehara, T., Tsunematsu, H., Shimizu, S., Yamamoto, M., et al. (2010). Expression of CD133 Confers Malignant Potential by Regulating Metalloproteinases in Human Hepatocellular Carcinoma. J. Hepatol. 52, 872–879. doi:10.1016/j.jhep.2009.12.030
Kou, T., Marusawa, H., Kinoshita, K., Endo, Y., Okazaki, I.-m., Ueda, Y., et al. (2007). Expression of Activation-Induced Cytidine Deaminase in Human Hepatocytes during Hepatocarcinogenesis. Int. J. Cancer 120, 469–476. doi:10.1002/ijc.22292
Langhans, B., Nischalke, H. D., Krämer, B., Dold, L., Lutz, P., Mohr, R., et al. (2019). Role of Regulatory T Cells and Checkpoint Inhibition in Hepatocellular Carcinoma. Cancer Immunol. Immunother. 68, 2055–2066. doi:10.1007/s00262-019-02427-4
Lau, E. Y. T., Lo, J., Cheng, B. Y. L., Ma, M. K. F., Lee, J. M. F., Ng, J. K. Y., et al. (2016). Cancer-Associated Fibroblasts Regulate Tumor-Initiating Cell Plasticity in Hepatocellular Carcinoma through C-Met/FRA1/HEY1 Signaling. Cell Rep. 15, 1175–1189. doi:10.1016/j.celrep.2016.04.019
Li, B., He, X., Pang, X., Zhang, H., Chen, J., and Chen, W. (2004). Elicitation of Both CD4+ and CD8+ T-Cell-Mediated Specific Immune Responses to HCA587 Protein by Autologous Dendritic Cells. Scand. J. Immunol. 60, 506–513. doi:10.1111/j.0300-9475.2004.01503.x
Li, C., Song, B., Santos, P. M., and Butterfield, L. H. (2019). Hepatocellular Cancer-Derived Alpha Fetoprotein Uptake Reduces CD1 Molecules on Monocyte-Derived Dendritic Cells. Cell Immunol. 335, 59–67. doi:10.1016/j.cellimm.2018.10.011
Li, C. X., Ling, C. C., Shao, Y., Xu, A., Li, X. C., Ng, K. T.-P., et al. (2016). CXCL10/CXCR3 Signaling Mobilized-Regulatory T Cells Promote Liver Tumor Recurrence after Transplantation. J. Hepatol. 65, 944–952. doi:10.1016/j.jhep.2016.05.032
Li, G., Liu, D., Cooper, T. K., Kimchi, E. T., Qi, X., Avella, D. M., et al. (2017). Successful Chemoimmunotherapy against Hepatocellular Cancer in a Novel Murine Model. J. Hepatol. 66, 75–85. doi:10.1016/j.jhep.2016.07.044
Li, J., Xue, J., Ling, M., Sun, J., Xiao, T., Dai, X., et al. (2021). MicroRNA-15b in Extracellular Vesicles from Arsenite-Treated Macrophages Promotes the Progression of Hepatocellular Carcinomas by Blocking the LATS1-Mediated Hippo Pathway. Cancer Lett. 497, 137–153. doi:10.1016/j.canlet.2020.10.023
Li, Y. M., Liu, Z. Y., Wang, J. C., Yu, J. M., Li, Z. C., Yang, H. J., et al. (2019). Receptor‐Interacting Protein Kinase 3 Deficiency Recruits Myeloid‐Derived Suppressor Cells to Hepatocellular Carcinoma through the Chemokine (C‐X‐C Motif) Ligand 1-Chemokine (C‐X‐C Motif) Receptor 2 Axis. Hepatology 70, 1564–1581. doi:10.1002/hep.30676
Li, Y., Wang, R., Xiong, S., Wang, X., Zhao, Z., Bai, S., et al. (2019). Cancer-associated Fibroblasts Promote the Stemness of CD24+ Liver Cells via Paracrine Signaling. J. Mol. Med. 97, 243–255. doi:10.1007/s00109-018-1731-9
Liang, C.-m., Ye, S.-l., Zhong, C.-p., Zheng, N., Bian, W., Sun, R.-x., et al. (2007). More Than Chemotaxis: a New Anti-tumor DC Vaccine Modified by rAAV2-SLC. Mol. Immunol. 44, 3797–3804. doi:10.1016/j.molimm.2007.03.026
Liao, R., Sun, J., Wu, H., Yi, Y., Wang, J.-X., He, H.-W., et al. (2013). High Expression of IL-17 and IL-17RE Associate with Poor Prognosis of Hepatocellular Carcinoma. J. Exp. Clin. Cancer Res. 32, 3. doi:10.1186/1756-9966-32-3
Lim, C. J., Lee, Y. H., Pan, L., Lai, L., Chua, C., Wasser, M., et al. (2019). Multidimensional Analyses Reveal Distinct Immune Microenvironment in Hepatitis B Virus-Related Hepatocellular Carcinoma. Gut 68, 916–927. doi:10.1136/gutjnl-2018-316510
Lin, L., Chen, S., Wang, H., Gao, B., Kallakury, B., Bhuvaneshwar, K., et al. (2021). SPTBN1 Inhibits Inflammatory Responses and Hepatocarcinogenesis via the Stabilization of SOCS1 and Downregulation of P65 in Hepatocellular Carcinoma. Theranostics 11, 4232–4250. doi:10.7150/thno.49819
Lin, W., Chen, Y. L., Jiang, L., and Chen, J. K. (2011). Reduced Expression of Chemerin Is Associated with a Poor Prognosis and a Lowed Infiltration of Both Dendritic Cells and Natural Killer Cells in Human Hepatocellular Carcinoma. Clin. Lab. 57, 879–885.
Lin, Y., Yang, X., Liu, W., Li, B., Yin, W., Shi, Y., et al. (2017). Chemerin Has a Protective Role in Hepatocellular Carcinoma by Inhibiting the Expression of IL-6 and GM-CSF and MDSC Accumulation. Oncogene 36, 3599–3608. doi:10.1038/onc.2016.516
Liu, B., Ye, S., He, P., Zheng, N., Zhao, Y., Sun, R., et al. (2001). Study of the Cytotoxity against Human Hepatocellular Carcinoma Cells Induced by the MAGE-1 Gene Modified Dendritic Cells. Zhonghua Gan Zang Bing Za Zhi 9, 151–153.
Liu, C., Liu, L., Chen, X., Cheng, J., Zhang, H., Zhang, C., et al. (2018). LSD1 Stimulates Cancer-Associated Fibroblasts to Drive Notch3-dependent Self-Renewal of Liver Cancer Stem-like Cells. Cancer Res. 78, 938–949. doi:10.1158/0008-5472.can-17-1236
Liu, D., Li, G., Avella, D. M., Kimchi, E. T., Kaifi, J. T., Rubinstein, M. P., et al. (2017). Sunitinib Represses Regulatory T Cells to Overcome Immunotolerance in a Murine Model of Hepatocellular Cancer. Oncoimmunology 7, e1372079. doi:10.1080/2162402x.2017.1372079
Liu, G., Sun, J., Yang, Z.-F., Zhou, C., Zhou, P.-Y., Guan, R.-Y., et al. (2021). Cancer-associated Fibroblast-Derived CXCL11 Modulates Hepatocellular Carcinoma Cell Migration and Tumor Metastasis through the circUBAP2/miR-4756/IFIT1/3 axis. Cell Death Dis 12, 260. doi:10.1038/s41419-021-03545-7
Liu, J., Chen, S., Wang, W., Ning, B.-F., Chen, F., Shen, W., et al. (2016). Cancer-associated Fibroblasts Promote Hepatocellular Carcinoma Metastasis through Chemokine-Activated Hedgehog and TGF-β Pathways. Cancer Lett. 379, 49–59. doi:10.1016/j.canlet.2016.05.022
Liu, M., Zhou, J., Liu, X., Feng, Y., Yang, W., Wu, F., et al. (2020). Targeting Monocyte-Intrinsic Enhancer Reprogramming Improves Immunotherapy Efficacy in Hepatocellular Carcinoma. Gut 69, 365–379. doi:10.1136/gutjnl-2018-317257
Liu, R.-X., Wei, Y., Zeng, Q.-H., Chan, K.-W., Xiao, X., Zhao, X.-Y., et al. (2015). Chemokine (C-X-C Motif) Receptor 3-positive B Cells Link Interleukin-17 Inflammation to Protumorigenic Macrophage Polarization in Human Hepatocellular Carcinoma. Hepatology 62, 1779–1790. doi:10.1002/hep.28020
Liu, Y.-T., Tseng, T.-C., Soong, R.-S., Peng, C.-Y., Cheng, Y.-H., Huang, S.-F., et al. (2018). A Novel Spontaneous Hepatocellular Carcinoma Mouse Model for Studying T-Cell Exhaustion in the Tumor Microenvironment. J. Immunotherapy Cancer 6, 144. doi:10.1186/s40425-018-0462-3
Liu, Z., Chen, M., Zhao, R., Huang, Y., Liu, F., Li, B., et al. (2020). CAF-induced Placental Growth Factor Facilitates Neoangiogenesis in Hepatocellular Carcinoma. Acta Biochim. Biophys. Sin(shanghai). 52, 18–25. doi:10.1093/abbs/gmz134
Liu, Z., Wang, J., Liu, L., Yuan, H., Bu, Y., Feng, J., et al. (2020). Chronic Ethanol Consumption and HBV Induce Abnormal Lipid Metabolism through HBx/SWELL1/arachidonic Acid Signaling and Activate Tregs in HBV-Tg Mice. Theranostics 10, 9249–9267. doi:10.7150/thno.46005
Lo Re, O., Mazza, T., Giallongo, S., Sanna, P., Rappa, F., Vinh Luong, T., et al. (2020). Loss of Histone macroH2A1 in Hepatocellular Carcinoma Cells Promotes Paracrine-Mediated Chemoresistance and CD4+CD25+FoxP3+ Regulatory T Cells Activation. Theranostics 10, 910–924. doi:10.7150/thno.35045
Long, J., Zhou, B., Li, H., Dai, Q., Zhang, B., Xing, S., et al. (2013). Improvement of HBsAg Gene-Modified Dendritic Cell-Based Vaccine Efficacy by Optimizing Immunization Method or the Application of β-glucosylceramide. Immunological Invest. 42, 137–155. doi:10.3109/08820139.2012.744418
Lv, B., Zhu, W., and Feng, C. (2020). Coptisine Blocks Secretion of Exosomal circCCT3 from Cancer-Associated Fibroblasts to Reprogram Glucose Metabolism in Hepatocellular Carcinoma. DNA Cell Biol 8, 1. doi:10.1089/dna.2020.6058
Ma, J., Zheng, B., Goswami, S., Meng, L., Zhang, D., Cao, C., et al. (2019). PD1Hi CD8+ T Cells Correlate with Exhausted Signature and Poor Clinical Outcome in Hepatocellular Carcinoma. J. Immunotherapy Cancer 7, 331. doi:10.1186/s40425-019-0814-7
Ma, L., Luo, L., Qiao, H., Dong, X., Pan, S., Jiang, H., et al. (2007). Complete Eradication of Hepatocellular Carcinomas by Combined Vasostatin Gene Therapy and B7H3-Mediated Immunotherapy. J. Hepatol. 46, 98–106. doi:10.1016/j.jhep.2006.07.031
Mackey, M. F., Gunn, J. R., Ting, P. P., Kikutani, H., Dranoff, G., Noelle, R. J., et al. (1997). Protective Immunity Induced by Tumor Vaccines Requires Interaction between CD40 and its Ligand, CD154. Cancer Res. 57, 2569–2574.
Mackey, M. F., Barth, R. J., and Noelle, R. J. (1998). The Role of CD40/CD154 Interactions in the Priming, Differentiation, and Effector Function of Helper and Cytotoxic T Cells. J. Leukoc. Biol. 63, 418–428. doi:10.1002/jlb.63.4.418
Mano, Y., Yoshio, S., Shoji, H., Tomonari, S., Aoki, Y., Aoyanagi, N., et al. (2019). Bone Morphogenetic Protein 4 Provides Cancer-Supportive Phenotypes to Liver Fibroblasts in Patients with Hepatocellular Carcinoma. J. Gastroenterol. 54, 1007–1018. doi:10.1007/s00535-019-01579-5
Mantovani, S., Oliviero, B., Lombardi, A., Varchetta, S., Mele, D., Sangiovanni, A., et al. (2019). Deficient Natural Killer Cell NKp30-Mediated Function and Altered NCR3 Splice Variants in Hepatocellular Carcinoma. Hepatology 69, 1165–1179. doi:10.1002/hep.30235
Menne, S., Tumas, D. B., Liu, K. H., Thampi, L., AlDeghaither, D., Baldwin, B. H., et al. (2015). Sustained Efficacy and Seroconversion with the Toll-like Receptor 7 Agonist GS-9620 in the Woodchuck Model of Chronic Hepatitis B. J. Hepatol. 62, 1237–1245. doi:10.1016/j.jhep.2014.12.026
Mizukoshi, E., Yamashita, T., Arai, K., Terashima, T., Kitahara, M., Nakagawa, H., et al. (2016). Myeloid-derived Suppressor Cells Correlate with Patient Outcomes in Hepatic Arterial Infusion Chemotherapy for Hepatocellular Carcinoma. Cancer Immunol. Immunother. 65, 715–725. doi:10.1007/s00262-016-1837-2
Morén, A., Bellomo, C., Tsubakihara, Y., Kardassis, D., Mikulits, W., Heldin, C.-H., et al. (2019). LXRα Limits TGFβ-dependent Hepatocellular Carcinoma Associated Fibroblast Differentiation. Oncogenesis 8, 36. doi:10.1038/s41389-019-0140-4
Mossanen, J. C., Kohlhepp, M., Wehr, A., Krenkel, O., Liepelt, A., Roeth, A. A., et al. (2019). CXCR6 Inhibits Hepatocarcinogenesis by Promoting Natural Killer T- and CD4+ T-cell-dependent Control of Senescence. Gastroenterology 156, 1877–1889. doi:10.1053/j.gastro.2019.01.247
Ning, J., Ye, Y., Bu, D., Zhao, G., Song, T., Liu, P., et al. (2021). Imbalance of TGF-Β1/bmp-7 Pathways Induced by M2-Polarized Macrophages Promotes Hepatocellular Carcinoma Aggressiveness. Mol. Ther. 29, 2067–2087. doi:10.1016/j.ymthe.2021.02.016
O'Beirne, J., Farzaneh, F., and Harrison, P. M. (2010). Generation of Functional CD8+ T Cells by Human Dendritic Cells Expressing Glypican-3 Epitopes. J. Exp. Clin. Cancer Res. 29, 48. doi:10.1186/1756-9966-29-48
Ormandy, L. A., Farber, A., Cantz, T., Petrykowska, S., Wedemeyer, H., Horning, M., et al. (2006). Direct Ex Vivo Analysis of Dendritic Cells in Patients with Hepatocellular Carcinoma. Wjg 12, 3275–3282. doi:10.3748/wjg.v12.i20.3275
Ouyang, F.-Z., Wu, R.-Q., Wei, Y., Liu, R.-X., Yang, D., Xiao, X., et al. (2016). Dendritic Cell-Elicited B-Cell Activation Fosters Immune Privilege via IL-10 Signals in Hepatocellular Carcinoma. Nat. Commun. 7, 13453. doi:10.1038/ncomms13453
Pang, P. H.-S., Chan, K.-T., Tse, L. Y.-W., Chan, R. C.-F., Cheung, Y.-K., Sin, F. W.-Y., et al. (2007). Induction of Cytotoxic T Cell Response against HCA661 Positive Cancer Cells through Activation with Novel HLA-A∗0201 Restricted Epitopes. Cancer Lett. 256, 178–185. doi:10.1016/j.canlet.2007.06.002
Papalexi, E., and Satija, R. (2018). Single-cell RNA Sequencing to Explore Immune Cell Heterogeneity. Nat. Rev. Immunol. 18, 35–45. doi:10.1038/nri.2017.76
Park, H., Jung, J. H., Jung, M. K., Shin, E.-C., Ro, S. W., Park, J. H., et al. (2020). Effects of Transarterial Chemoembolization on Regulatory T Cell and its Subpopulations in Patients with Hepatocellular Carcinoma. Hepatol. Int. 14, 249–258. doi:10.1007/s12072-020-10014-4
Pedroza-Gonzalez, A., Verhoef, C., Ijzermans, J. N. M., Peppelenbosch, M. P., Kwekkeboom, J., Verheij, J., et al. (2013). Activated Tumor-Infiltrating CD4+ Regulatory T Cells Restrain Antitumor Immunity in Patients with Primary or Metastatic Liver Cancer. Hepatology 57, 183–194. doi:10.1002/hep.26013
Peng, B. G., He, Q., Liang, L. I., Xie, B. H., Hua, Y. P., Chen, Z. B., et al. (2006). Induction of Cytotoxic T-Lymphocyte Responses Using Dendritic Cells Transfected with Hepatocellular Carcinoma mRNA. Br. J. Biomed. Sci. 63, 123–128. doi:10.1080/09674845.2006.11732731
Peng, H., Xue, R., Ju, Z., Qiu, J., Wang, J., Yan, W., et al. (2020). Cancer-associated Fibroblasts Enhance the Chemoresistance of CD73+ Hepatocellular Carcinoma Cancer Cells via HGF-Met-Erk1/2 Pathway. Ann. Transl Med. 8, 856. doi:10.21037/atm-20-1038
Pinato, D. J., Howlett, S., Ottaviani, D., Urus, H., Patel, A., Mineo, T., et al. (2019). Association of Prior Antibiotic Treatment with Survival and Response to Immune Checkpoint Inhibitor Therapy in Patients with Cancer. JAMA Oncol. 5, 1774–1778. doi:10.1001/jamaoncol.2019.2785
Pistore, C., Giannoni, E., Colangelo, T., Rizzo, F., Magnani, E., Muccillo, L., et al. (2017). DNA Methylation Variations Are Required for Epithelial-To-Mesenchymal Transition Induced by Cancer-Associated Fibroblasts in Prostate Cancer Cells. Oncogene 36, 5551–5566. doi:10.1038/onc.2017.159
Qin, W.-H., Yang, Z.-S., Li, M., Chen, Y., Zhao, X.-F., Qin, Y.-Y., et al. (2020). High Serum Levels of Cholesterol Increase Antitumor Functions of Nature Killer Cells and Reduce Growth of Liver Tumors in Mice. Gastroenterology 158, 1713–1727. doi:10.1053/j.gastro.2020.01.028
Qiu, S.-J., Lu, L., Qiao, C., Wang, L., Wang, Z., Xiao, X., et al. (2005). Induction of Tumor Immunity and Cytotoxic T Lymphocyte Responses Using Dendritic Cells Transduced by Adenoviral Vectors Encoding HBsAg: Comparison to Protein Immunization. J. Cancer Res. Clin. Oncol. 131, 429–438. doi:10.1007/s00432-004-0616-1
Ren, Z., Yue, Y., Zhang, Y., Dong, J., Liu, Y., Yang, X., et al. (2021). Changes in the Peripheral Blood Treg Cell Proportion in Hepatocellular Carcinoma Patients after Transarterial Chemoembolization with Microparticles. Front. Immunol. 12, 624789. doi:10.3389/fimmu.2021.624789
Ritter, M., Ali, M. Y., Grimm, C. F., Weth, R., Mohr, L., Bocher, W. O., et al. (2004). Immunoregulation of Dendritic and T Cells by Alpha-Fetoprotein in Patients with Hepatocellular Carcinoma. J. Hepatol. 41, 999–1007. doi:10.1016/j.jhep.2004.08.013
Rogler, G., Hausmann, M., Spöttl, T., Vogl, D., Aschenbrenner, E., Andus, T., et al. (1999). T-cell Co-stimulatory Molecules Are Upregulated on Intestinal Macrophages from Inflammatory Bowel Disease Mucosa. Eur. J. Gastroenterol. Hepatol. 11, 1105–1112. doi:10.1097/00042737-199910000-00006
Rohr-Udilova, N., Klinglmüller, F., Schulte-Hermann, R., Stift, J., Herac, M., Salzmann, M., et al. (2018). Deviations of the Immune Cell Landscape between Healthy Liver and Hepatocellular Carcinoma. Sci. Rep. 8, 6220. doi:10.1038/s41598-018-24437-5
Roxburgh, P., and Evans, T. R. J. (2008). Systemic Therapy of Hepatocellular Carcinoma: Are We Making Progress? Adv. Ther. 25, 1089–1104. doi:10.1007/s12325-008-0113-z
Sakaguchi, S., Miyara, M., Costantino, C. M., and Hafler, D. A. (2010). FOXP3+ Regulatory T Cells in the Human Immune System. Nat. Rev. Immunol. 10, 490–500. doi:10.1038/nri2785
Samstein, R. M., Arvey, A., Josefowicz, S. Z., Peng, X., Reynolds, A., Sandstrom, R., et al. (2012). Foxp3 Exploits a Pre-existent Enhancer Landscape for Regulatory T Cell Lineage Specification. Cell 151, 153–166. doi:10.1016/j.cell.2012.06.053
Santos, P. M., Menk, A. V., Shi, J., Tsung, A., Delgoffe, G. M., and Butterfield, L. H. (2019). Tumor-Derived α-Fetoprotein Suppresses Fatty Acid Metabolism and Oxidative Phosphorylation in Dendritic Cells. Cancer Immunol. Res. 7, 1001–1012. doi:10.1158/2326-6066.cir-18-0513
Sayem, M. A., Tomita, Y., Yuno, A., Hirayama, M., Irie, A., Tsukamoto, H., et al. (2016). Identification of Glypican-3-Derived Long Peptides Activating Both CD8+and CD4+T Cells; Prolonged Overall Survival in Cancer Patients with Th Cell Response. Oncoimmunology 5, e1062209. doi:10.1080/2162402x.2015.1062209
Schmitz, V., Barajas, M., Wang, L., Peng, D., Duarte, M., Prieto, J., et al. (2001). Adenovirus-mediated CD40 Ligand Gene Therapy in a Rat Model of Orthotopic Hepatocellular Carcinoma. Hepatology 34, 72–81. doi:10.1053/jhep.2001.25757
Schoenberger, S. P., Toes, R. E. M., van der Voort, E. I. H., Offringa, R., and Melief, C. J. M. (1998). T-cell Help for Cytotoxic T Lymphocytes Is Mediated by CD40-Cd40l Interactions. Nature 393, 480–483. doi:10.1038/31002
Sconocchia, G., Eppenberger, S., Spagnoli, G. C., Tornillo, L., Droeser, R., Caratelli, S., et al. (2014). NK Cells and T Cells Cooperate during the Clinical Course of Colorectal Cancer. Oncoimmunology 3, e952197. doi:10.4161/21624011.2014.952197
Shao, Y., Lo, C. M., Ling, C. C., Liu, X. B., Ng, K. T.-P., Chu, A. C. Y., et al. (2014). Regulatory B Cells Accelerate Hepatocellular Carcinoma Progression via CD40/CD154 Signaling Pathway. Cancer Lett. 355, 264–272. doi:10.1016/j.canlet.2014.09.026
Shen, Y., Wei, Y., Wang, Z., Jing, Y., He, H., Yuan, J., et al. (2015). TGF-β Regulates Hepatocellular Carcinoma Progression by Inducing Treg Cell Polarization. Cell Physiol Biochem 35, 1623–1632. doi:10.1159/000373976
Shi, F., Shi, M., Zeng, Z., Qi, R.-Z., Liu, Z.-W., Zhang, J.-Y., et al. (2011). PD-1 and PD-L1 Upregulation Promotes CD8+ T-Cell Apoptosis and Postoperative Recurrence in Hepatocellular Carcinoma Patients. Int. J. Cancer 128, 887–896. doi:10.1002/ijc.25397
Shimoda, M., Tomimaru, Y., Charpentier, K. P., Safran, H., Carlson, R. I., and Wands, J. (2012). Tumor Progression-Related Transmembrane Protein Aspartate-β-Hydroxylase Is a Target for Immunotherapy of Hepatocellular Carcinoma. J. Hepatol. 56, 1129–1135. doi:10.1016/j.jhep.2011.12.016
Sica, A., and Mantovani, A. (2012). Macrophage Plasticity and Polarization: In Vivo Veritas. J. Clin. Invest. 122, 787–795. doi:10.1172/jci59643
Soini, Y., Virkajarvi, N., Raunio, H., and Paakko, P. (1996). Expression of P-Glycoprotein in Hepatocellular Carcinoma: a Potential Marker of Prognosis. J. Clin. Pathol. 49, 470–473. doi:10.1136/jcp.49.6.470
Song, M., He, J., Pan, Q. Z., Yang, J., Zhao, J., Zhang, Y. J., et al. (2021). Cancer‐Associated Fibroblast‐Mediated Cellular Crosstalk Supports Hepatocellular Carcinoma Progression. Hepatology 73, 1717–1735. doi:10.1002/hep.31792
Spitzer, M. H., and Nolan, G. P. (2016). Mass Cytometry: Single Cells, Many Features. Cell 165, 780–791. doi:10.1016/j.cell.2016.04.019
Sprinzl, M. F., Reisinger, F., Puschnik, A., Ringelhan, M., Ackermann, K., Hartmann, D., et al. (2013). Sorafenib Perpetuates Cellular Anticancer Effector Functions by Modulating the Crosstalk between Macrophages and Natural Killer Cells. Hepatology 57, 2358–2368. doi:10.1002/hep.26328
Subleski, J. J., Scarzello, A. J., Alvord, W. G., Jiang, Q., Stauffer, J. K., Kronfli, A., et al. (2015). Serum-based Tracking of De Novo Initiated Liver Cancer Progression Reveals Early Immunoregulation and Response to Therapy. J. Hepatol. 63, 1181–1189. doi:10.1016/j.jhep.2015.06.021
Suk, K.-T., Mederacke, I., Gwak, G.-Y., Cho, S. W., Adeyemi, A., Friedman, R., et al. (2016). Opposite Roles of Cannabinoid Receptors 1 and 2 in Hepatocarcinogenesis. Gut 65, 1721–1732. doi:10.1136/gutjnl-2015-310212
Sukowati, C. H. C., Anfuso, B., Crocé, L. S., and Tiribelli, C. (2015). The Role of Multipotent Cancer Associated Fibroblasts in Hepatocarcinogenesis. BMC Cancer 15, 188. doi:10.1186/s12885-015-1196-y
Sun, H., Huang, Q., Huang, M., Wen, H., Lin, R., Zheng, M., et al. (2019). Human CD96 Correlates to Natural Killer Cell Exhaustion and Predicts the Prognosis of Human Hepatocellular Carcinoma. Hepatology 70, 168–183. doi:10.1002/hep.30347
Sun, L., Wang, Y., Wang, L., Yao, B., Chen, T., Li, Q., et al. (2019). Resolvin D1 Prevents Epithelial-Mesenchymal Transition and Reduces the Stemness Features of Hepatocellular Carcinoma by Inhibiting Paracrine of Cancer-Associated Fibroblast-Derived COMP. J. Exp. Clin. Cancer Res. 38, 170. doi:10.1186/s13046-019-1163-6
Takata, Y., Nakamoto, Y., Nakada, A., Terashima, T., Arihara, F., Kitahara, M., et al. (2011). Frequency of CD45RO+ Subset in CD4+CD25high Regulatory T Cells Associated with Progression of Hepatocellular Carcinoma. Cancer Lett. 307, 165–173. doi:10.1016/j.canlet.2011.03.029
Tanaka, H., Yoshizawa, H., Yamaguchi, Y., Ito, K., Kagamu, H., Suzuki, E., et al. (1999). Successful Adoptive Immunotherapy of Murine Poorly Immunogenic Tumor with Specific Effector Cells Generated from Gene-Modified Tumor-Primed Lymph Node Cells. J. Immunol. 162, 3574–3582.
Tao, H., Liu, M., Wang, Y., Luo, S., Xu, Y., Ye, B., et al. (2021). Icaritin Induces Anti-tumor Immune Responses in Hepatocellular Carcinoma by Inhibiting Splenic Myeloid-Derived Suppressor Cell Generation. Front. Immunol. 12, 609295. doi:10.3389/fimmu.2021.609295
Tao, Z., Ruan, H., Sun, L., Kuang, D., Song, Y., Wang, Q., et al. (2019). Targeting the YB-1/pd-L1 Axis to Enhance Chemotherapy and Antitumor Immunity. Cancer Immunol. Res. 7, 1135–1147. doi:10.1158/2326-6066.cir-18-0648
Teng, C. F., Wang, T., Wu, T. H., Lin, J. H., Shih, F. Y., Shyu, W. C., et al. (2020). Combination Therapy with Dendritic Cell Vaccine and Programmed Death Ligand 1 Immune Checkpoint Inhibitor for Hepatocellular Carcinoma in an Orthotopic Mouse Model. Ther. Adv. Med. Oncol. 12, 1758835920922034. doi:10.1177/1758835920922034
Tipton, K. N., Sullivan, N., Bruening, W., Inamdar, R., Launders, J., Uhl, S., et al. (2011). Stereotactic Body Radiation Therapy. Rockville(MD): Mayo Foundation for Medical Education and Research.
Tseng, H.-c., Xiong, W., Badeti, S., Yang, Y., Ma, M., Liu, T., et al. (2020). Efficacy of Anti-cd147 Chimeric Antigen Receptors Targeting Hepatocellular Carcinoma. Nat. Commun. 11, 4810. doi:10.1038/s41467-020-18444-2
Tsou, P., Katayama, H., Ostrin, E. J., and Hanash, S. M. (2016). The Emerging Role of B Cells in Tumor Immunity. Cancer Res. 76, 5597–5601. doi:10.1158/0008-5472.can-16-0431
Veglia, F., Perego, M., and Gabrilovich, D. (2018). Myeloid-derived Suppressor Cells Coming of Age. Nat. Immunol. 19, 108–119. doi:10.1038/s41590-017-0022-x
Vogt, A., Sievers, E., Lukacs-Kornek, V., Decker, G., Raskopf, E., Meumann, N., et al. (2014). Improving Immunotherapy of Hepatocellular Carcinoma (HCC) Using Dendritic Cells (DC) Engineered to Express IL-12 In Vivo. Liver Int. 34, 447–461. doi:10.1111/liv.12284
Voskoboinik, I., Smyth, M. J., and Trapani, J. A. (2006). Perforin-mediated Target-Cell Death and Immune Homeostasis. Nat. Rev. Immunol. 6, 940–952. doi:10.1038/nri1983
Wang, C., Shang, C., Gai, X., Song, T., Han, S., Liu, Q., et al. (2021). Sulfatase 2-Induced Cancer-Associated Fibroblasts Promote Hepatocellular Carcinoma Progression via Inhibition of Apoptosis and Induction of Epithelial-To-Mesenchymal Transition. Front. Cell Dev. Biol. 9, 631931. doi:10.3389/fcell.2021.631931
Wang, D., Li, X., Li, J., Lu, Y., Zhao, S., Tang, X., et al. (2019). APOBEC3B Interaction with PRC2 Modulates Microenvironment to Promote HCC Progression. Gut 68, 1846–1857. doi:10.1136/gutjnl-2018-317601
Wang, F., Jing, X., Li, G., Wang, T., Yang, B., Zhu, Z., et al. (2012). Foxp3+ Regulatory T Cells Are Associated with the Natural History of Chronic Hepatitis B and Poor Prognosis of Hepatocellular Carcinoma. Liver Int. 32, 644–655. doi:10.1111/j.1478-3231.2011.02675.x
Wang, H., Wang, X., Li, X., Fan, Y., Li, G., Guo, C., et al. (2014). CD68+HLA-DR+ M1-like Macrophages Promote Motility of HCC Cells via NF-Κb/FAK Pathway. Cancer Lett. 345, 91–99. doi:10.1016/j.canlet.2013.11.013
Wang, J., Sanmamed, M. F., Datar, I., Su, T. T., Ji, L., Sun, J., et al. (2019). Fibrinogen-like Protein 1 Is a Major Immune Inhibitory Ligand of LAG-3. Cell 176, 334–347. doi:10.1016/j.cell.2018.11.010
Wang, K., Nie, X., Rong, Z., Fan, T., Li, J., Wang, X., et al. (2017). B Lymphocytes Repress Hepatic Tumorigenesis but Not Development in Hras12V Transgenic Mice. Int. J. Cancer 141, 1201–1214. doi:10.1002/ijc.30823
Wang, N., Tan, H.-Y., Lu, Y., Chan, Y.-T., Wang, D., Guo, W., et al. (2021). PIWIL1 Governs the Crosstalk of Cancer Cell Metabolism and Immunosuppressive Microenvironment in Hepatocellular Carcinoma. Sig Transduct Target. Ther. 6, 86. doi:10.1038/s41392-021-00485-8
Wang, Y. H., Chuang, Y. H., Wu, C. F., Jan, M. C., Wu, W. J., Lin, C. L., et al. (2019). Smoking and Hepatitis B Virus-Related Hepatocellular Carcinoma Risk: The Mediating Roles of Viral Load and Alanine Aminotransferase. Hepatology 69, 1412–1425. doi:10.1002/hep.30339
Wang, Y., Liu, T., Tang, W., Deng, B., Chen, Y., Zhu, J., et al. (2016). Hepatocellular Carcinoma Cells Induce Regulatory T Cells and Lead to Poor Prognosis via Production of Transforming Growth Factor-Β1. Cell Physiol Biochem 38, 306–318. doi:10.1159/000438631
Wei, Y., Lao, X.-M., Xiao, X., Wang, X.-Y., Wu, Z.-J., Zeng, Q.-H., et al. (2019). Plasma Cell Polarization to the Immunoglobulin G Phenotype in Hepatocellular Carcinomas Involves Epigenetic Alterations and Promotes Hepatoma Progression in Mice. Gastroenterology 156, 1890–1904. e16. doi:10.1053/j.gastro.2019.01.250
Williams, M., Liu, X., Zhang, Y., Reske, J., Bahal, D., Gohl, T. G., et al. (2020). NCOA5 Deficiency Promotes a Unique Liver Protumorigenic Microenvironment through p21WAF1/CIP1 Overexpression, Which Is Reversed by Metformin. Oncogene 39, 3821–3836. doi:10.1038/s41388-020-1256-x
Wolf, M. J., Adili, A., Piotrowitz, K., Abdullah, Z., Boege, Y., Stemmer, K., et al. (2014). Metabolic Activation of Intrahepatic CD8+ T Cells and NKT Cells Causes Nonalcoholic Steatohepatitis and Liver Cancer via Cross-Talk with Hepatocytes. Cancer Cell 26, 549–564. doi:10.1016/j.ccell.2014.09.003
Wong, Y. Q., Qiu, S. J., Tang, Z. Y., Ye, S. L., Liu, Y. K., Fan, J., et al. (2005). Changes in the Immune Function of Dendritic Cells (DC) Derived from HBV-Related Hepatocellular Carcinoma (HCC) Patient's Peripheral Blood Monocytes (PBMC) Pulsed with Tumor Antigen. Zhonghua Gan Zang Bing Za Zhi 13, 339–342.
Wu, C.-J., Tsai, Y.-T., Lee, I.-J., Wu, P.-Y., Lu, L.-S., Tsao, W.-S., et al. (2018). Combination of Radiation and Interleukin 12 Eradicates Large Orthotopic Hepatocellular Carcinoma through Immunomodulation of Tumor Microenvironment. Oncoimmunology 7, e1477459. doi:10.1080/2162402x.2018.1477459
Wu, J.-J., Yang, Y., Peng, W.-T., Sun, J.-C., Sun, W.-Y., and Wei, W. (2019). G Protein-Coupled Receptor Kinase 2 Regulating β2-adrenergic Receptor Signaling in M2-Polarized Macrophages Contributes to Hepatocellular Carcinoma Progression. Ott 12, 5499–5513. doi:10.2147/ott.s209291
Wu, Q., Zhou, W., Yin, S., Zhou, Y., Chen, T., Qian, J., et al. (2019). Blocking Triggering Receptor Expressed on Myeloid Cells‐1‐Positive Tumor‐Associated Macrophages Induced by Hypoxia Reverses Immunosuppression and Anti‐Programmed Cell Death Ligand 1 Resistance in Liver Cancer. Hepatology 70, 198–214. doi:10.1002/hep.30593
Wu, Y., Kuang, D.-M., Pan, W.-D., Wan, Y.-L., Lao, X.-M., Wang, D., et al. (2013). Monocyte/macrophage-elicited Natural Killer Cell Dysfunction in Hepatocellular Carcinoma Is Mediated by CD48/2B4 Interactions. Hepatology 57, 1107–1116. doi:10.1002/hep.26192
Xiao, X., Lao, X.-M., Chen, M.-M., Liu, R.-X., Wei, Y., Ouyang, F.-Z., et al. (2016). PD-1hi Identifies a Novel Regulatory B-Cell Population in Human Hepatoma that Promotes Disease Progression. Cancer Discov. 6, 546–559. doi:10.1158/2159-8290.cd-15-1408
Xie, Q.-K., Zhao, Y.-J., Pan, T., Lyu, N., Mu, L.-W., Li, S.-L., et al. (2016). Programmed Death Ligand 1 as an Indicator of Pre-existing Adaptive Immune Responses in Human Hepatocellular Carcinoma. Oncoimmunology 5, e1181252. doi:10.1080/2162402x.2016.1181252
Xing, W., Wu, S., Yuan, X., Chen, Q., Shen, X., He, F., et al. (2009). The Anti-tumor Effect of Human Monocyte-Derived Dendritic Cells Loaded with HSV-TK/GCV Induced Dying Cells. Cell Immunol. 254, 135–141. doi:10.1016/j.cellimm.2008.08.004
Xiong, S., Wang, R., Chen, Q., Luo, J., Wang, J., Zhao, Z., et al. (2018). Cancer-associated Fibroblasts Promote Stem Cell-like Properties of Hepatocellular Carcinoma Cells through IL-6/STAT3/Notch Signaling. Am. J. Cancer Res. 8, 302–316.
Xu, Y., Fang, F., Jiao, H., Zheng, X., Huang, L., Yi, X., et al. (2019). Activated Hepatic Stellate Cells Regulate MDSC Migration through the SDF-1/CXCR4 axis in an Orthotopic Mouse Model of Hepatocellular Carcinoma. Cancer Immunol. Immunother. 68, 1959–1969. doi:10.1007/s00262-019-02414-9
Yamamoto, T., Nagano, H., Sakon, M., Wada, H., Eguchi, H., Kondo, M., et al. (2004). Partial Contribution of Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL)/TRAIL Receptor Pathway to Antitumor Effects of Interferon-Α/5-Fluorouracil against Hepatocellular Carcinoma. Clin. Cancer Res. 10, 7884–7895. doi:10.1158/1078-0432.ccr-04-0794
Yamanaka, T., Harimoto, N., Yokobori, T., Muranushi, R., Hoshino, K., Hagiwara, K., et al. (2021). Conophylline Inhibits HCC by Inhibiting Activated Cancer-Associated Fibroblasts through Suppression of G Protein-Coupled Receptor 68. Mol. Cancer Ther. 20, 1019–1028. doi:10.1158/1535-7163
Yang, D., Li, T., Li, Y., Zhang, S., Li, W., Liang, H., et al. (2019). H2S Suppresses Indoleamine 2, 3-dioxygenase 1 and Exhibits Immunotherapeutic Efficacy in Murine Hepatocellular Carcinoma. J. Exp. Clin. Cancer Res. 38, 88. doi:10.1186/s13046-019-1083-5
Yang, F., Wei, Y., Han, D., Li, Y., Shi, S., Jiao, D., et al. (2020). Interaction with CD68 and Regulation of GAS6 Expression by Endosialin in Fibroblasts Drives Recruitment and Polarization of Macrophages in Hepatocellular Carcinoma. Cancer Res. 80, 3892–3905. doi:10.1158/0008-5472.CAN-19-2691
Yang, J., Lu, Y., Lin, Y.-Y., Zheng, Z.-Y., Fang, J.-H., He, S., et al. (2016). Vascular Mimicry Formation Is Promoted by Paracrine TGF-β and SDF1 of Cancer-Associated Fibroblasts and Inhibited by miR-101 in Hepatocellular Carcinoma. Cancer Lett. 383, 18–27. doi:10.1016/j.canlet.2016.09.012
Yang, L., Shao, X., Jia, S., Zhang, Q., and Jin, Z. (2019). Interleukin-35 Dampens CD8+ T Cells Activity in Patients with Non-viral Hepatitis-Related Hepatocellular Carcinoma. Front. Immunol. 10, 1032. doi:10.3389/fimmu.2019.01032
Yang, M., Zhang, Z., Chen, J., Xu, M., Huang, J., Wang, M., et al. (2019). Soluble Fibrinogen-like Protein 2 Promotes the Growth of Hepatocellular Carcinoma via Attenuating Dendritic Cell-Mediated Cytotoxic T Cell Activity. J. Exp. Clin. Cancer Res. 38, 351. doi:10.1186/s13046-019-1326-5
Yang, T., Zhang, W., Wang, L., Xiao, C., Wang, L., Gong, Y., et al. (2018). Co-culture of Dendritic Cells and Cytokine-Induced Killer Cells Effectively Suppresses Liver Cancer Stem Cell Growth by Inhibiting Pathways in the Immune System. BMC Cancer 18, 984. doi:10.1186/s12885-018-4871-y
Yang, X. H., Yamagiwa, S., Ichida, T., Matsuda, Y., Sugahara, S., Watanabe, H., et al. (2006). Increase of CD4+CD25+ Regulatory T-Cells in the Liver of Patients with Hepatocellular Carcinoma. J. Hepatol. 45, 254–262. doi:10.1016/j.jhep.2006.01.036
Yang, Y. (2015). Cancer Immunotherapy: Harnessing the Immune System to Battle Cancer. J. Clin. Invest. 125, 3335–3337. doi:10.1172/jci83871
Yang, Y., Ye, Y.-C., Chen, Y., Zhao, J.-L., Gao, C.-C., Han, H., et al. (2018). Crosstalk between Hepatic Tumor Cells and Macrophages via Wnt/β-Catenin Signaling Promotes M2-like Macrophage Polarization and Reinforces Tumor Malignant Behaviors. Cell Death Dis 9, 793. doi:10.1038/s41419-018-0818-0
Yao, R.-R., Li, J.-H., Zhang, R., Chen, R.-X., and Wang, Y.-H. (2018). M2-polarized Tumor-Associated Macrophages Facilitated Migration and Epithelial-Mesenchymal Transition of HCC Cells via the TLR4/STAT3 Signaling Pathway. World J. Surg. Onc 16, 9. doi:10.1186/s12957-018-1312-y
Ye, L., Zhang, Q., Cheng, Y., Chen, X., Wang, G., Shi, M., et al. (2018). Tumor-derived Exosomal HMGB1 Fosters Hepatocellular Carcinoma Immune Evasion by Promoting TIM-1+ Regulatory B Cell Expansion. J. Immunotherapy Cancer 6, 145. doi:10.1186/s40425-018-0451-6
Yost, K. E., Satpathy, A. T., Wells, D. K., Qi, Y., Wang, C., Kageyama, R., et al. (2019). Clonal Replacement of Tumor-specific T Cells Following PD-1 Blockade. Nat. Med. 25, 1251–1259. doi:10.1038/s41591-019-0522-3
Yu, S. J., Ma, C., Heinrich, B., Brown, Z. J., Sandhu, M., Zhang, Q., et al. (2019). Targeting the Crosstalk between Cytokine-Induced Killer Cells and Myeloid-Derived Suppressor Cells in Hepatocellular Carcinoma. J. Hepatol. 70, 449–457. doi:10.1016/j.jhep.2018.10.040
Yu, Z., Zhao, H., Feng, X., Li, H., Qiu, C., Yi, X., et al. (2019). Long Non-coding RNA FENDRR Acts as a miR-423-5p Sponge to Suppress the Treg-Mediated Immune Escape of Hepatocellular Carcinoma Cells. Mol. Ther. - Nucleic Acids 17, 516–529. doi:10.1016/j.omtn.2019.05.027
Yugawa, K., Yoshizumi, T., Mano, Y., Itoh, S., Harada, N., Ikegami, T., et al. (2021). Cancer-associated Fibroblasts Promote Hepatocellular Carcinoma Progression through Downregulation of Exosomal miR-150-3p. Eur. J. Surg. Oncol. 47, 384–393. doi:10.1016/j.ejso.2020.08.002
Zhang, D. Y., and Friedman, S. L. (2012). Fibrosis-dependent Mechanisms of Hepatocarcinogenesis. Hepatology 56, 769–775. doi:10.1002/hep.25670
Zhang, H.-G., Chen, H.-S., Peng, J.-R., Shang, X.-Y., Zhang, J., Xing, Q., et al. (2007). Specific CD8+ T Cell Responses to HLA-A2 Restricted MAGE-A3 P271-279 Peptide in Hepatocellular Carcinoma Patients without Vaccination. Cancer Immunol. Immunother. 56, 1945–1954. doi:10.1007/s00262-007-0338-8
Zhang, Q., Huang, H., Zheng, F., Liu, H., Qiu, F., Chen, Y., et al. (2020). Resveratrol Exerts Antitumor Effects by Downregulating CD8+CD122+ Tregs in Murine Hepatocellular Carcinoma. Oncoimmunology 9, 1829346. doi:10.1080/2162402x.2020.1829346
Zhang, X. D., Wang, Y., and Ye, L. H. (2014). Hepatitis B Virus X Protein Accelerates the Development of Hepatoma. Cancer Biol. Med. 11, 182–190. doi:10.7497/j.issn.2095-3941.2014.03.004
Zhang, Z., Li, X., Sun, W., Yue, S., Yang, J., Li, J., et al. (2017). Loss of Exosomal miR-320a from Cancer-Associated Fibroblasts Contributes to HCC Proliferation and Metastasis. Cancer Lett. 397, 33–42. doi:10.1016/j.canlet.2017.03.004
Zhang, Z., Ma, L., Goswami, S., Ma, J., Zheng, B., Duan, M., et al. (2019). Landscape of Infiltrating B Cells and Their Clinical Significance in Human Hepatocellular Carcinoma. Oncoimmunology 8, e1571388. doi:10.1080/2162402x.2019.1571388
Zhao, J., Chu, F., Xu, H., Guo, M., Shan, S., Zheng, W., et al. (2020). C/EBPα/miR-7 Controls CD4+ T Cell Activation and Function and Orchestrates Experimental Autoimmune Hepatitis in Mice. Hepatology 1, 1. doi:10.1002/hep.31607
Zhao, Q., Huang, Z.-L., He, M., Gao, Z., and Kuang, D.-M. (2016). BTLA Identifies Dysfunctional PD-1-Expressing CD4+ T Cells in Human Hepatocellular Carcinoma. Oncoimmunology 5, e1254855. doi:10.1080/2162402x.2016.1254855
Zhao, T., Jia, H., Cheng, Q., Xiao, Y., Li, M., Ren, W., et al. (2017). Nifuroxazide Prompts Antitumor Immune Response of TCL-Loaded DC in Mice with Orthotopically-Implanted Hepatocarcinoma. Oncol. Rep. 37, 3405–3414. doi:10.3892/or.2017.5629
Zhao, X.-L., Lin, Y., Jiang, J., Tang, Z., Yang, S., Lu, L., et al. (2017). High-mobility Group Box 1 Released by Autophagic Cancer-Associated Fibroblasts Maintains the Stemness of Luminal Breast Cancer Cells. J. Pathol. 243, 376–389. doi:10.1002/path.4958
Zhao, Z., Bai, S., Wang, R., Xiong, S., Li, Y., Wang, X., et al. (2019). Cancer-associated Fibroblasts Endow Stem-like Qualities to Liver Cancer Cells by Modulating Autophagy. Cmar 11, 5737–5744. doi:10.2147/cmar.s197634
Zheng, B. H., Ma, J. Q., Tian, L. Y., Dong, L. Q., Song, G. H., Pan, J. M., et al. (2020). The Distribution of Immune Cells within Combined Hepatocellular Carcinoma and Cholangiocarcinoma Predicts Clinical Outcome. Clin. Translational Med. 10, 45–56. doi:10.1002/ctm2.11
Zheng, B., Wang, D., Qiu, X., Luo, G., Wu, T., Yang, S., et al. (2020). Trajectory and Functional Analysis of PD‐1 High CD4 + CD8 + T Cells in Hepatocellular Carcinoma by Single‐Cell Cytometry and Transcriptome Sequencing. Adv. Sci. 7, 2000224. doi:10.1002/advs.202000224
Zheng, C., Zheng, L., Yoo, J.-K., Guo, H., Zhang, Y., Guo, X., et al. (2017). Landscape of Infiltrating T Cells in Liver Cancer Revealed by Single-Cell Sequencing. Cell 169, 1342–1356. doi:10.1016/j.cell.2017.05.035
Zhou, G., Sprengers, D., Boor, P. P. C., Doukas, M., Schutz, H., Mancham, S., et al. (2017). Antibodies against Immune Checkpoint Molecules Restore Functions of Tumor-Infiltrating T Cells in Hepatocellular Carcinomas. Gastroenterology 153, 1107–1119. doi:10.1053/j.gastro.2017.06.017
Zhou, J., Ding, T., Pan, W., Zhu, L.-y., Li, L., and Zheng, L. (2009). Increased Intratumoral Regulatory T Cells Are Related to Intratumoral Macrophages and Poor Prognosis in Hepatocellular Carcinoma Patients. Int. J. Cancer 125, 1640–1648. doi:10.1002/ijc.24556
Zhou, J., Liu, M., Sun, H., Feng, Y., Xu, L., Chan, A. W. H., et al. (2018). Hepatoma-intrinsic CCRK Inhibition Diminishes Myeloid-Derived Suppressor Cell Immunosuppression and Enhances Immune-Checkpoint Blockade Efficacy. Gut 67, 931–944. doi:10.1136/gutjnl-2017-314032
Zhou, J., Nefedova, Y., Lei, A., and Gabrilovich, D. (2018). Neutrophils and PMN-MDSC: Their Biological Role and Interaction with Stromal Cells. Semin. Immunol. 35, 19–28. doi:10.1016/j.smim.2017.12.004
Zhou, L., Fu, J.-L., Lu, Y.-Y., Fu, B.-Y., Wang, C.-P., An, L.-J., et al. (2010). Regulatory T Cells Are Associated with post-cryoablation Prognosis in Patients with Hepatitis B Virus-Related Hepatocellular Carcinoma. J. Gastroenterol. 45, 968–978. doi:10.1007/s00535-010-0243-3
Zhu, H.-f., Liu, Y.-p., Liu, D.-l., Ma, Y.-d., Hu, Z.-y., Wang, X.-y., et al. (2019). Role of TGFβ3-Smads-Sp1 axis in DcR3-Mediated Immune Escape of Hepatocellular Carcinoma. Oncogenesis 8, 43. doi:10.1038/s41389-019-0152-0
Keywords: HCC, TAM, NK cell, cancer immunotherapy, challenge, development
Citation: Hao X, Sun G, Zhang Y, Kong X, Rong D, Song J, Tang W and Wang X (2021) Targeting Immune Cells in the Tumor Microenvironment of HCC: New Opportunities and Challenges. Front. Cell Dev. Biol. 9:775462. doi: 10.3389/fcell.2021.775462
Received: 14 September 2021; Accepted: 19 October 2021;
Published: 12 November 2021.
Edited by:
Dechun Feng, National Institute on Alcohol Abuse and Alcoholism (NIAAA), United StatesReviewed by:
Talib Hassan Ali, University of ThiQar, IraqSaisha Nalawade, Baylor College of Medicine, United States
Copyright © 2021 Hao, Sun, Zhang, Kong, Rong, Song, Tang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuehao Wang, d2FuZ3hoQG5qbXUuZWR1LmNu; Weiwei Tang, MTI0Mzc3MzQ3M3R3d3dAc2luYS5jb20=; Jinhua Song, amluaHVhc29uZ25hbmpAMTYzLmNvbQ==
†These authors share first authorship
 Xiaopei Hao1†
Xiaopei Hao1† Guangshun Sun
Guangshun Sun Yao Zhang
Yao Zhang Weiwei Tang
Weiwei Tang