
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Cell Dev. Biol. , 10 January 2022
Sec. Molecular and Cellular Oncology
Volume 9 - 2021 | https://doi.org/10.3389/fcell.2021.755135
This article is part of the Research Topic Insights into Cancer of Unknown Primary (CUP) Research: Novel Diagnostic and Therapeutic Strategies View all 10 articles
The histological transformation from lung squamous cell carcinoma (LUSC) to lung adenocarcinoma (LUAD) and p. N771delinsGF mutations in EGFR exon 20 (ex20) are exceedingly rare in non–small cell lung carcinoma (NSCLC). EGFR ex20 mutations are insensitive to EGFR tyrosine kinase inhibitors in NSCLC. Here, we present a 76-year-old male smoker harboring LUAD with a novel p. N771delinsGF deletion/insertion mutation in EGFR ex20 transdifferentiating from advanced LUSC after chemoradiotherapy. The patient presented reduced hydrothorax and relieved tightness with the treatment of nivolumab plus docetaxel and carboplatin after the failure of second-line chemotherapy. The case highlights the importance of rebiopsy and molecular retesting after the progression of lung cancer and supports the idea that the combination of immune checkpoint blockade and chemotherapy may be an attractive option for patients with EGFR ex20 mutations associated with LUSC–LUAD transformation.
Lung cancer has the highest lethality rate among all cancers. Non–small cell lung carcinoma (NSCLC) is a major subtype of lung cancer, accounting for 85% of lung cancer cases, including lung adenocarcinoma (LUAD), lung squamous cell carcinoma (LUSC), and large cell carcinoma histologic subtypes. LUAD and LUSC harbor different features (Krause et al., 2020; Wang et al., 2020). Accumulating evidence from animal experiments and clinical observations indicates phenotypic plasticity in lung cancer cells, such as transdifferentiation of LUAD to LUSC or to small cell lung cancer. This phenotypic transition is a novel cellular mechanism for drug resistance in chemotherapy and in EGFR-targeted therapy (Hou et al., 2017). However, information regarding the transformation from LUSC to LUAD remains limited.
Studies have shown that approximately 12% of patients with EGFR-mutant NSCLC harbor insertion mutations in exon 20 (ex20ins), which are insensitive to first- and second-generation EGFR tyrosine kinase inhibitors (TKIs) (Riess et al., 2018). LUAD and LUSC of adenosquamous carcinomas (ASCs) also share a monoclonal origin, with EGFR mutations being the most common oncogenic driver in the Asian population (Lin et al., 2020). However, no information is available regarding the relationship between EGFR ex20ins mutations and the molecular mechanisms of driving LUSC transdifferentiating to LUAD.
Here, we describe a case of a patient with LUAD harboring a novel p. N771delinsGF deletion/insertion (delins) mutation in EGFR ex20 who experienced transdifferentiation from LUSC after failure of chemotherapy and subsequently responded favorably to immune checkpoint blockade (ICB) plus chemotherapy.
In June 2019, a 76-year-old male smoker weighing 50 kg with no notable medical, family, or psychosocial history was admitted to the hospital with a complaint of intermittent cough with sputum for >1 month. Chest computed tomography (CT) revealed a left upper lobe lung mass with multiple metastases involving the left pleura, left pleural effusion, and left mediastinal and hilar lymph node enlargement. Lung fine-needle aspiration biopsy confirmed the diagnosis of low-differentiated LUSC at stage IVA (cT4N2M1a) (Figures 1A, 2A). The immunohistochemistry of pulmonary biopsy samples was positive for PCK, P40, CK7, CK5/6, and PD-L1 (40%) and was negative for TTF1. Genetic abnormalities such as amplification of CCDN1, CCDN2, FGF19, FGF3, FGF4, FGFR1, KRAS, and PIK3CA; ex2 missense mutation of CDKN2A; and ex5 code-shift mutation of TP53 were observed in the primary lung cancer lesion by using next-generation sequencing (NGS; 520-gene panel, Oncoscreen plus, Burning Rock Biotech, Guangzhou, China). Plasma NGS demonstrated amplification of CCDN1, FGF19, FGF3, and FGF4; ex2 missense mutation of CDKN2A; ex5 shift mutation of TP53; and 11.1 mutations/Mb (Table 1).
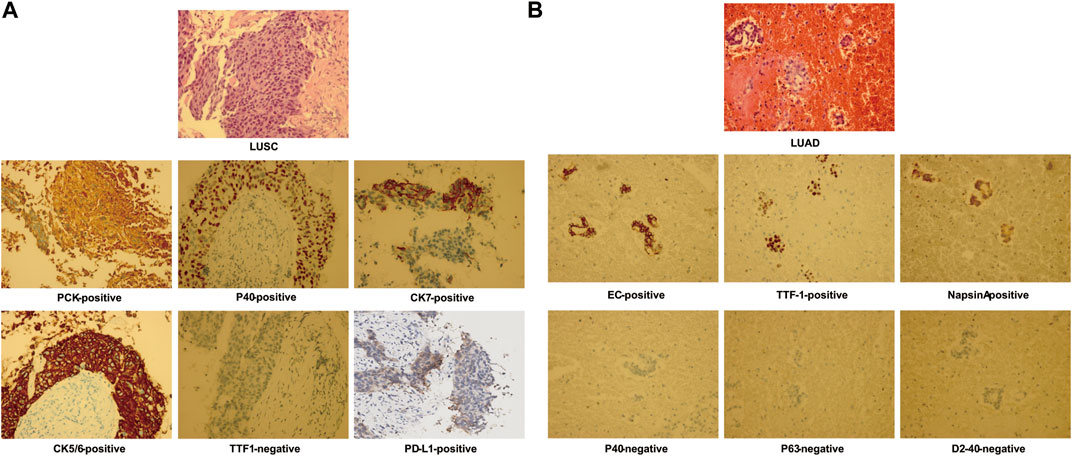
FIGURE 1. Hematoxylin–eosin staining and immunohistochemistry staining from pulmonary biopsy shows lung squamous cell carcinoma (LUSC) (A) and of pleural fluid–exfoliated cells shows lung adenocarcinoma (LUAD) (B).
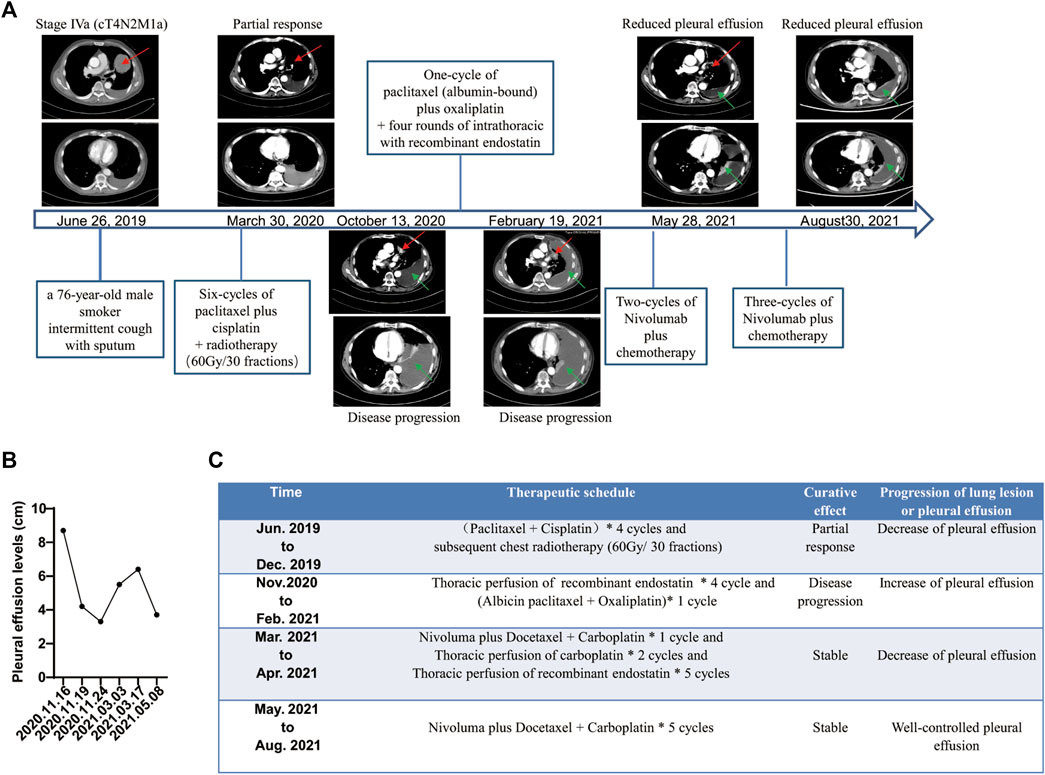
FIGURE 2. Summary of treatment schedule and associated therapeutic effects. (A) Computed tomography (CT) from June 26, 2019, shows a lesion in the left lung and enlarged left mediastinal and hilar lymph nodes (red arrows). CT from March 30, 2020, shows partial response after six cycles of paclitaxel plus cisplatin chemotherapy and subsequent radiotherapy (red arrows). CT from October 13, 2020 shows progressive spread of the tumor within the chest associated with left pleural effusion (green arrows). CT from February 19, 2021, shows increased pleural effusion after one cycle of second-line chemotherapy (green arrows). CT from May 28, 2021, to August 30, 2021, shows well-controlled pleural effusion after five courses of nivolumab plus chemotherapy and four rounds of intrathoracic chemotherapy with recombinant endostatin followed by two rounds of that with recombinant endostatin plus carboplatin (green arrows). (B) Pleural effusion levels before and after treatment with nivolumab plus chemotherapy by B-mode ultrasound. (C) Treatment course and results.
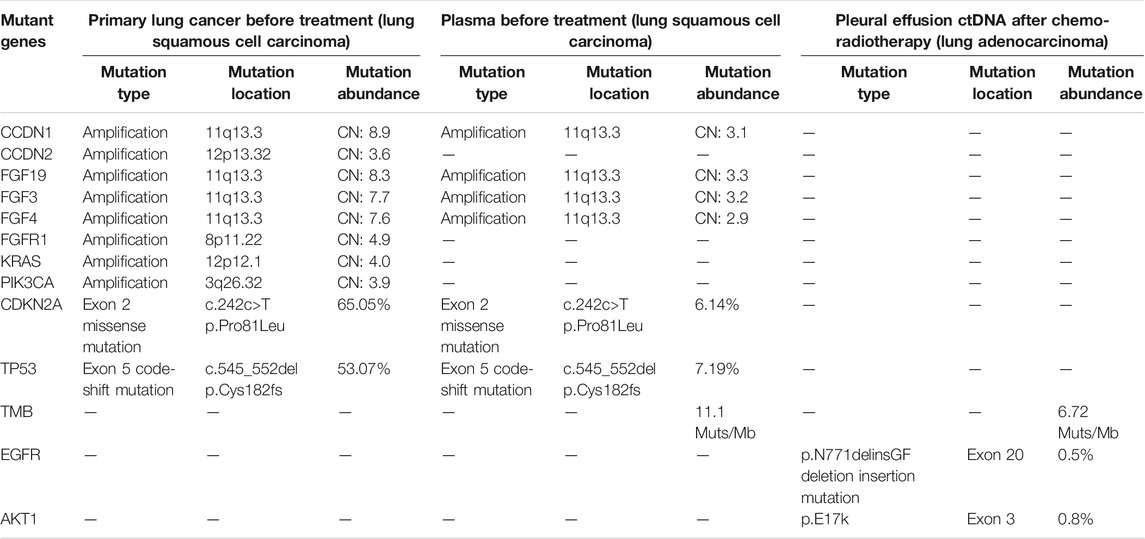
TABLE 1. Next-generation sequencing results of pulmonary biopsy and plasma before treatments and of pleural fluid–exfoliated cells after chemoradiotherapy.
From July to December 2019, the patient was treated with six cycles of paclitaxel plus cisplatin chemotherapy and subsequent radiotherapy (60 Gy/30 fractions at 2 Gy per day). He experienced an initial partial response with a decrease in tumor size (from 8.5 × 9.6 × 10.4 cm to unmeasurable). However, he was readmitted to our hospital with breathlessness and tightness of the chest. Chest CT in October 2020 showed that the amount of pleural effusion in his left chest had notably increased. LUAD cells (EC-positive, TTF-1–positive, napsin A–positive, P40-negative, P63-negative, D2-40–negative) were found by cytological examination of pleural effusion (Figure 1B). Unfortunately, the patient showed poor compliance resulting from the side effects of kidney function and gastrointestinal symptoms of cisplatin during first-line chemotherapy. We have demonstrated the safety and efficacy of the combination of paclitaxel plus oxaliplatin in pretreated advanced NSCLC (Xie et al., 2004); oxaliplatin was administered in the second-line treatment instead of cisplatin.
However, after one cycle of second-line treatment (albumin-bound paclitaxel plus oxaliplatin) and four rounds of recombinant endostatin (an antiangiogenesis agent), the disease continued to progress. Chest CT and B-mode ultrasound revealed an increase in the amount of pleural effusion (from 4.2 cm in November 2020 to 6.4 cm in February 2021; Figures 2A, B). A novel p. N771delinsGF indel mutation in EGFR ex20, a p. E17k mutation in AKT1 ex3 and 6.72 mutations/Mb were identified in the pleural fluid ctDNA by a hybrid-capture NGS method using a 1,021-gene panel (Table 1).
Five cycles of nivolumab plus docetaxel and carboplatin therapy as third-line therapy were initiated on March 19, 2021. The amount of pleural effusion then decreased (from 6.4 cm in March to 3.7 cm in May by B-mode ultrasound, Figure 2B) with well-controlled symptoms of breathlessness and chest tightness, and the lung lesion appeared stable. Moreover, an improved Eastern Cooperative Oncology Group performance status score (from 2 to 0) was observed in the patient after treatment with nivolumab plus chemotherapy. Therapeutic response was stable until the last follow-up on August 31, 2021, according to the well-controlled and unmeasurable lung lesion, decreased pleural effusion tested by chest CT, and well-controlled symptoms (Figure 2A). During the whole process of treatment with immunochemotherapy, the patient experienced no severe side effects.
We report a patient who was initially diagnosed with LUSC and was then found to have LUAD cells in the pleural effusion after chemotherapy resistance. One explanation for this is that the LUAD cells may have arisen from an original LUAD component of ASCs in our case. However, the patient had no shared mutations between the two genomic profiles of LUAD and LUSC detected by NGS. This is not in line with previous studies suggesting that LUAD and LUSC of ASCs share a monoclonal origin (Lin et al., 2020). Such a case may present more like two different pathological types. Recently, emerging data have provided convincing evidence supporting a phenotypic transition in lung cancer, such as adenocarcinoma–to–squamous cell transdifferentiation (AST) (Hou et al., 2017). Animal studies suggest that the mechanisms of AST are associated with Kras activation and Lkb1 deletion. Moreover, AST is observed during the development of chemotherapy resistance, suggesting that the transdifferentiation of lung cancer represents a novel mechanism of drug resistance (Han et al., 2014). Thus, we suggest that there may have been a transdifferentiation from LUSC to LUAD which led to chemotherapy resistance in our case. Although this hypothesis needs to be further confirmed, the high plasticity of lung cancer indicates this possibility.
Notably, LUSC and LUAD in our report were diagnosed using bronchoscopy biopsy specimens and cytological examination of exfoliated cells in pleural effusion, respectively. This highlights the importance of repetitive and comprehensive sampling and molecular testing, including that of tissues (biopsy specimens or surgery), plasma, and body fluid, both before and after treatments. The overall genomic profiles of both LUAD and LUSC in our case were detected by NGS, which allows sequencing of a high number of nucleotides (Mosele et al., 2020) and facilitated identification of the novel EGFR ex20 p. N771delinsGF mutation in our case. However, it has been reported that each fluid type (blood, urine, or saliva) still retains its own characteristic signature based on its unique molecules (El-Mogy et al., 2018). For instance, cell-free DNA from body fluid supernatants has a higher detection rate and sensitivity for tumor-specific mutations than cell-free DNA from sedimented tumor cells and plasma from body fluid (Guo et al., 2019; Zhang et al., 2019). LUAD was detected by pleural effusion in our case, indicating that more information may be revealed from a pleural effusion sample compared with LUSC diagnosed by biopsy specimens and plasma before treatment.
LUAD with a novel p. N771delinsGF mutation occurring in EGFR ex20 was found in our case. The EGFR ex20ins mutations are diverse, accounting for approximately 12% of patients with EGFR mutations. The V769_D770insASV is the most common in EGFR ex20ins mutation (23.0%) (Tsiambas et al., 2016; Riess et al., 2018). Similar to other EGFR mutation subtypes, EGFR ex20in functions as an oncogenic driver. However, compared with those with EGFR ex19 deletion or ex21 L858R mutation, NSCLC patients with EGFR ex20ins mutations have reduced affinity for EGFR-TKIs. They experienced a shorter median survival than patients with EGFR-TKI–sensitive mutations (16.5 vs. 33.0 months) during EGFR-TKI therapy (Naidoo et al., 2015; Yang et al., 2020). Nowadays, targeted therapeutics such as amivantamab, mobocertinib, and poziotinib have shown initial efficacy in NSCLC patients with EGFR ex20ins (Köhler and Jänne, 2021); however, no targeted therapeutics have been approved for EGFR ex20ins-driven NSCLC by the US Food and Drug Administration. Currently, chemotherapy remains the suitable treatment for patients harboring these mutations in China (Naidoo et al., 2015). One study showed that metastatic NSCLC patients with EGFR ex20ins treated with platinum chemotherapy presented a more favorable overall survival (OS; median 20 vs. 12 months) compared with NSCLC without targetable alterations (Choudhury et al., 2021). In a single-institution retrospective study (n = 29) of NSCLC with EGFR ex20ins, the median progression-free survival (PFS) with platinum-based chemotherapy was 7.1 months (95% confidence interval [CI], 6.3–13.7), and the median OS was 3.2 years (95% CI, 1.92–not reached) (Shah et al., 2021). These data suggest that LUAD patients with EGFR 20ins mutation may respond to chemotherapy. However, the present patient’s disease remained unresponsive to second-line chemotherapy agents alone in our case, indicating an urgent need for the development of new therapeutic strategies.
Immunotherapy, such as ICB, has changed the treatment landscape in metastatic and recurrent NSCLC. Chemotherapy plus immunotherapy has shown particularly promising efficacy in advanced NSCLC patients (Leonetti et al., 2019). Although compared with EGFR wide-type LUAD, cases of NSCLC with EGFR 19del or 21exon L858R mutations have a lower clinical response rate to ICB combined with chemotherapy. NSCLC patients with EGFR ex20ins mutation achieved both better PFS and OS than NSCLC patients with no targetable oncogenes after ICB and chemotherapy treatment (Remon et al., 2020). Therefore, chemotherapy in combination with nivolumab was administered to our patient, resulting in a significant reduction of pleural effusion and relieved dyspnea for more than 5 months. Likewise, in a retrospective single-center case series of patients with NSCLC and EGFR ex20ins mutation, two patients received platinum-doublet chemotherapy in combination with pembrolizumab, and both had stable disease (Shah et al., 2021). Thus, according to these reports and our case, ICB combined with chemotherapy can be considered an effective therapeutic strategy in NSCLC patients with EGFR ex20 p. N771delinsGF mutation. However, there is a lack of clinical trials to validate this hypothesis, and future clinical trials could be conducted to clarify the efficacy of ICB in combination with chemotherapy in NSCLC patients harboring EGFR ex20ins mutation.
In conclusion, our case suggests that phenotypic transition between LUSC and LUAD may occur in lung cancer during treatment. When lung cancer progresses, rebiopsy is helpful in accurately understanding and treating the disease. Moreover, NSCLC with an EGFR ex20 p. N771delinsGF mutation can benefit from nivolumab plus docetaxel and carboplatin. ICB plus chemotherapy may be a favorable strategy for NSCLC with EGFR ex20 mutation.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by West China Hospital of Sichuan University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
LZ, YL, HG, and JL collected the clinical information, diagnostic information, therapeutic information, and images of the patients. LZ wrote the manuscript. LZ and YL identified the case and submitted the manuscript. FL, QZ, and HG revised the manuscript. FL and QZ proofread the manuscript. LZ and YL contributed equally to this work. All authors contributed to the article and approved the submitted version.
This work was supported by the Wu Jieping Medical Foundation, China (312150082), Post-Doctor Research Project of West China Hospital and Sichuan University (LZ).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, orclaim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We sincerely appreciate Yan Wang, Ting Cao, and Hongying Chen from the Core Facility, West China Hospital of Sichuan University for their assistance and guidance. We also thank Yang Yang and Xiaoting Chen from the Animal Experimental Center of West China Hospital for their suggestions.
Choudhury, N. J., Schoenfeld, A. J., Flynn, J., Falcon, C. J., Rizvi, H., Rudin, C. M., et al. (2021). Response to Standard Therapies and Comprehensive Genomic Analysis for Patients with Lung Adenocarcinoma with EGFR Exon 20 Insertions. Clin. Cancer Res. 27 (10), 2920–2927. doi:10.1158/1078-0432.CCR-20-4650
El-Mogy, M., Lam, B., Haj-Ahmad, T. A., McGowan, S., Yu, D., Nosal, L., et al. (2018). Diversity and Signature of Small RNA in Different Bodily Fluids Using Next Generation Sequencing. BMC Genomics 19 (1), 408. doi:10.1186/s12864-018-4785-8
Guo, Z., Xie, Z., Shi, H., Du, W., Peng, L., Han, W., et al. (2019). Malignant Pleural Effusion Supernatant Is an Alternative Liquid Biopsy Specimen for Comprehensive Mutational Profiling. Thorac. Cancer 10 (4), 823–831. doi:10.1111/1759-7714.13006
Han, X., Li, F., Fang, Z., Gao, Y., Li, F., Fang, R., et al. (2014). Transdifferentiation of Lung Adenocarcinoma in Mice with Lkb1 Deficiency to Squamous Cell Carcinoma. Nat. Commun. 5, 3261. doi:10.1038/ncomms4261
Hou, S., Zhou, S., Qin, Z., Yang, L., Han, X., Yao, S., et al. (2017). Evidence, Mechanism, and Clinical Relevance of the Transdifferentiation from Lung Adenocarcinoma to Squamous Cell Carcinoma. Am. J. Pathol. 187 (5), 954–962. doi:10.1016/j.ajpath.2017.01.009
Köhler, J., and Jänne, P. A. (2021). Amivantamab: Treating EGFR Exon 20-Mutant Cancers with Bispecific Antibody-Mediated Receptor Degradation. Jco 39 (30), 3403–3406. doi:10.1200/JCO.21.01494
Krause, A., Roma, L., Lorber, T., Habicht, J., Lardinois, D., De Filippo, M. R., et al. (2020). Deciphering the Clonal Relationship between Glandular and Squamous Components in Adenosquamous Carcinoma of the Lung Using Whole Exome Sequencing. Lung Cancer 150, 132–138. doi:10.1016/j.lungcan.2020.10.013
Leonetti, A., Wever, B., Mazzaschi, G., Assaraf, Y. G., Rolfo, C., Quaini, F., et al. (2019). Molecular Basis and Rationale for Combining Immune Checkpoint Inhibitors with Chemotherapy in Non-small Cell Lung Cancer. Drug Resist. Updates 46, 100644. doi:10.1016/j.drup.2019.100644
Lin, G., Li, C., Li, P. S., Fang, W. Z., Xu, H. P., Gong, Y. H., et al. (2020). Genomic Origin and EGFR-TKI Treatments of Pulmonary Adenosquamous Carcinoma. Ann. Oncol. 31 (4), 517–524. doi:10.1016/j.annonc.2020.01.014
Mosele, F., Remon, J., Mateo, J., Westphalen, C. B., Barlesi, F., Lolkema, M. P., et al. (2020). Recommendations for the Use of Next-Generation Sequencing (NGS) for Patients with Metastatic Cancers: a Report from the ESMO Precision Medicine Working Group. Ann. Oncol. 31 (11), 1491–1505. doi:10.1016/j.annonc.2020.07.014
Naidoo, J., Sima, C. S., Rodriguez, K., Busby, N., Nafa, K., Ladanyi, M., et al. (2015). Epidermal Growth Factor Receptor Exon 20 Insertions in Advanced Lung Adenocarcinomas: Clinical Outcomes and Response to Erlotinib. Cancer 121 (18), 3212–3220. doi:10.1002/cncr.29493
Remon, J., Hendriks, L. E. L., Cardona, A. F., and Besse, B. (2020). EGFR Exon 20 Insertions in Advanced Non-small Cell Lung Cancer: A New History Begins. Cancer Treat. Rev. 90, 102105. doi:10.1016/j.ctrv.2020.102105
Riess, J. W., Gandara, D. R., Frampton, G. M., Madison, R., Peled, N., Bufill, J. A., et al. (2018). Diverse EGFR Exon 20 Insertions and Co-occurring Molecular Alterations Identified by Comprehensive Genomic Profiling of NSCLC. J. Thorac. Oncol. 13 (10), 1560–1568. doi:10.1016/j.jtho.2018.06.019
Shah, M. P., Aredo, J. V., Padda, S. K., Ramchandran, K. J., Wakelee, H. A., Das, M. S., et al. (2021). EGFR Exon 20 Insertion NSCLC and Response to Platinum-Based Chemotherapy. Clin. Lung Cancer 162, 140. doi:10.1016/j.cllc.2021.07.001
Tsiambas, E., Lefas, A. Y., Georgiannos, S. N., Ragos, V., Fotiades, P. P., Grapsa, D., et al. (2016). EGFR Gene Deregulation Mechanisms in Lung Adenocarcinoma: A Molecular Review. Pathol. - Res. Pract. 212 (8), 672–677. doi:10.1016/j.prp.2016.06.005
Wang, B.-Y., Huang, J.-Y., Chen, H.-C., Lin, C.-H., Lin, S.-H., Hung, W.-H., et al. (2020). The Comparison between Adenocarcinoma and Squamous Cell Carcinoma in Lung Cancer Patients. J. Cancer Res. Clin. Oncol. 146 (1), 43–52. doi:10.1007/s00432-019-03079-8
Xie, C. Y., Wu, S. X., and Zhang, P. (2004). Efficacy of Combined Paclitaxel and Oxaliplatin Therapy in Patients with Pretreated Advanced Non-small Cell Lung Cancer. Ai Zheng 23 (8), 947–950.
Yang, G., Li, J., Xu, H., Yang, Y., Yang, L., Xu, F., et al. (2020). EGFR Exon 20 Insertion Mutations in Chinese Advanced Non-small Cell Lung Cancer Patients: Molecular Heterogeneity and Treatment Outcome from Nationwide Real-World Study. Lung Cancer 145, 186–194. doi:10.1016/j.lungcan.2020.03.014
Keywords: non-small cell lung cancer, immune checkpoint blockade, EGFR exon 20 mutation, histological transformation, immune therapy
Citation: Zhu L, Liu Y, Gao H, Liu J, Zhou Q and Luo F (2022) Case Report: Partial Response Following Nivolumab Plus Docetaxel in a Patient With EGFR Exon 20 Deletion/Insertion (p.N771delinsGF) Mutant Lung Adenocarcinoma Transdifferentiated From Squamous Cell Carcinoma. Front. Cell Dev. Biol. 9:755135. doi: 10.3389/fcell.2021.755135
Received: 08 August 2021; Accepted: 03 December 2021;
Published: 10 January 2022.
Edited by:
Qinghua Xu, The Canhelp Genomics Research Center, ChinaReviewed by:
Luis Corrales, Cancer Research and Management Center (CIMCA), Costa RicaCopyright © 2022 Zhu, Liu, Gao, Liu, Zhou and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qinghua Zhou, cHJvZl9xaF96aG91QDEyNi5jb20=; Feng Luo, bHVvZmVuZzY2NjY2QHNpbmEuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.