- Department of Molecular Microbiology and Immunology, Saint Louis University School of Medicine, St. Louis, MO, United States
Gastric cancer is a leading cause of mortality worldwide. The risk of developing gastric adenocarcinoma, which comprises >90% of gastric cancers, is multifactorial, but most associated with Helicobacter pylori infection. Autoimmune gastritis is a chronic autoinflammatory syndrome where self-reactive immune cells are activated by gastric epithelial cell autoantigens. This cause of gastritis is more so associated with the development of neuroendocrine tumors. However, in both autoimmune and infection-induced gastritis, high risk metaplastic lesions develop within the gastric mucosa. This warrants concern for carcinogenesis in both inflammatory settings. There are many similarities and differences in disease progression between these two etiologies of chronic gastritis. Both diseases have an increased risk of gastric adenocarcinoma development, but each have their own unique comorbidities. Autoimmune gastritis is a primary cause of pernicious anemia, whereas chronic infection typically causes gastrointestinal ulceration. Both immune responses are driven by T cells, primarily CD4+ T cells of the IFN-γ producing, Th1 phenotype. Neutrophilic infiltrates help clear H. pylori infection, but neutrophils are not necessarily recruited in the autoimmune setting. There have also been hypotheses that infection with H. pylori initiates autoimmune gastritis, but the literature is far from definitive with evidence of infection-independent autoimmune gastric disease. Gastric cancer incidence is increasing among young women in the United States, a population at higher risk of developing autoimmune disease, and H. pylori infection rates are falling. Therefore, a better understanding of these two chronic inflammatory diseases is needed to identify their roles in initiating gastric cancer.
Introduction
Gastric cancer is the fourth leading cause of cancer related mortality with the fifth highest incidence rate worldwide (Hyuna Sung et al., 2021). The discrepancy with lower incidence than mortality rate may be linked to the fact that most gastric cancers are diagnosed late in disease progression due to limited premalignant signs and symptoms. In the United States, late diagnosis contributes to a 5-year survival rate of only 31% for gastric cancer (Matsuzaka et al., 2016). In addition, stomach cancer research has been identified as an underfunded field given its high mortality rate (Carter and Nguyen, 2012). Gastric adenocarcinomas which derive from epithelial cells in the gastric glands make up more than 90% of all gastric cancers. A combination of environmental, host behavior, genetic, and microbial factors contribute to the risk of developing gastric adenocarcinoma (Rawla and Barsouk, 2019). Chronic infection with Helicobacter pylori (Hp) is recognized as a major risk factor associated with gastric adenocarcinoma development. Autoimmune gastritis (AIG) is another risk factor that is more commonly associated with neuroendocrine tumor development. More thorough investigation is needed into how chronic inflammation, triggered by infection or autoimmunity, causes epithelial cells to undergo premalignant changes that can lead to gastric cancer. The high mortality and poor survival associated with gastric adenocarcinoma make it imperative to better understand disease progression to improve preventative and therapeutic strategies.
Gastric Cancer Burden in the United States
Within the United States, gastric cancer incidence rates have been decreasing for several decades. However, when incidence is considered by the age at diagnosis (early-onset < 50 years, late-onset >50 years), early-onset incidence has shown a two-fold increase in the last 2 decades (Bergquist et al., 2019). Studies have indicated that this increase in incidence of early-onset individuals is primarily due to an increase in young females (Anderson et al., 2018). Autoimmune disease shows a female-specific predominance and there is a strong possibility AIG follows this trend (Cabrera de León et al., 2012). As well, it has been shown that within the United States the prevalence of Hp-positive gastric cancer cases has been continually declining since 2007 and North America is one of the regions with the lowest incidence rates of Hp infection (Hooi et al., 2017; Nguyen et al., 2020). Given the low Hp infection rates and the specific increase in gastric cancer incidence among young women in the United States, it can be inferred that AIG may be the culprit behind this rise in gastric cancer incidence. Unfortunately, due to underdiagnosis and a lack in research, there is no definitive evidence supporting an increase in AIG incidence among young women over time to confirm this theory (Carmel, 1996; Neumann et al., 2013). However, the incidence rates of autoimmune diseases associated with AIG, autoimmune thyroiditis and type I diabetes, have shown increases in incidence over time (McLeod and Cooper, 2012; Kahaly and Hansen, 2016; Rodriguez-Castro et al., 2018; Mobasseri et al., 2020). As well, benign neuroendocrine tumors in the stomach, associated with AIG, have shown a significant increase in incidence over time and are more common in women (Yao et al., 2008). Combining these facts, it is likely that AIG may also be on the rise and therefore responsible for the increased gastric cancer incidence in young women. It is necessary to learn the pathophysiology behind this etiology of gastric inflammation compared to Hp infection to improve early diagnostic and management strategies in patients with a high risk of developing gastric cancer.
Helicobacter pylori-Induced Gastritis
It has been almost 40 years since Barry Marshall and Robin Warren isolated and identified a spiral bacterium present in the stomachs of gastritis patients, now known as Helicobacter pylori (Hp) (Marshall and Warren, 1984). After personally ingesting a culture of Hp, Marshall and others discovered this bacterium could not only colonize the stomach, but also induced an inflammatory response in the epithelium (Marshall et al., 1985; Morris and Nicholson, 1987). Thanks to these pioneers, it is now well established that Hp is a Gram negative spirochete capable of chronically colonizing the inhospitable superficial gastric mucosa, leading to diseases like peptic ulceration and chronic gastritis.
Hp infection has been estimated to contribute to almost 90% of gastric adenocarcinoma cases (Plummer et al., 2015). Hp currently infects more than half of the world’s population, however less than 3% of individuals infected with Hp will go on to develop gastric adenocarcinoma (Uemura et al., 2001; Hooi et al., 2017). Prevalence is higher than 70% in countries such as Brazil, Nigeria, and Pakistan, and lower than 40% in places such as Switzerland, Australia, and the United States (Hooi et al., 2017) Countries with high prevalence have difficulties controlling infection due to socioeconomic factors like limited access to clean drinking water and health care, especially for children (Malaty and Graham, 1994). Long-term infection with Hp can lead to outcomes like dyspepsia, chronic gastritis, peptic ulcer disease, mucosa-associated lymphoid tissue (MALT) lymphoma, gastric metaplasia, and gastric adenocarcinoma (Kusters et al., 2006). Chronic gastritis induced by infection follows a stepwise progression of disease culminating in gastric cancer (Figure 1), originally described as the Correa Cascade (Correa, 1988). Along this pathway, prolonged inflammation leads to oxyntic atrophy: the loss of corpus glands containing acid-secreting parietal cells and digestive enzyme-producing chief cells. Oxyntic atrophy can trigger metaplastic transformations in remaining epithelial cells that over time evolve into dysplasia and cancer (Sáenz and Mills, 2018). Research in vaccination strategies for infection prevention have been underway for decades, but most studies are still in early and preclinical stages of development (Dos Santos Viana et al., 2021). Currently, there are effective Hp treatments available as combinations of antibiotics and proton pump inhibitors with/without bismuth subsalicylate (Schistosomes liver flukes and Heclicobacter Pylori, 1994). Downfalls with antibiotics, including resistance, side effects, and inability to prevent reinfection, have promoted research into alternative treatment approaches like probiotics and vaccination, but combination therapy still remains the first line option (Abadi, 2016). Combination therapy administered prior to the development of metaplasia has been reported to reverse progression through the pre-metaplastic cascade (Koulis et al., 2019). Eradication of Hp has also been shown to decrease gastric cancer incidence, so clinical guidelines recommend testing for and treating infection in patients with identified gastric lesions (e.g., gastritis, atrophy, metaplasia, dysplasia) followed by confirmation of eradication testing (Lee et al., 2016; Gupta et al., 2020). In fact, confirmed eradication of Hp has been found to decrease the recurrence of dysplastic lesions in patients with previously resected gastric neoplasms compared to those who were not treated or failed treatment (Shiotani et al., 2014; Shin et al., 2015). Due to effective treatments and enhanced standard of living, Hp infection rates in the United States are low, but other countries around the world still struggle to control the spread of Hp.
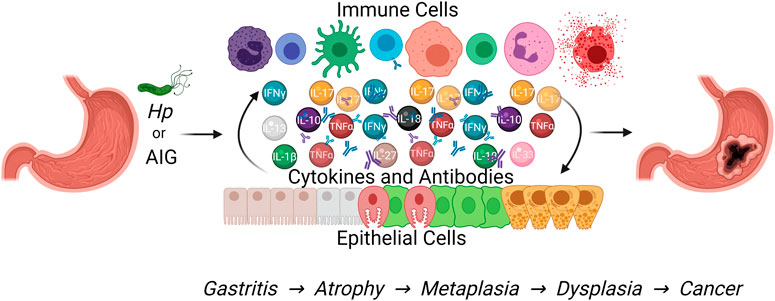
FIGURE 1. Both Hp infection and autoimmune gastritis cause chronic gastric inflammation that can progress to gastric metaplasia and increase the risk of gastric adenocarcinoma development. This Figure was created using BioRender.com.
The chronic gastric inflammation and pathology induced by Hp is mediated by immune cell infiltration into the gastric mucosa, producing inflammatory molecules that interact with resident epithelial cells. The cytokines TNFα, IFN-γ, IL-1β, IL-6, IL-8, IL-10, and IL-18 have all shown increased expression measured within gastric biopsies of Hp-infected compared to healthy patients (Lindholm et al., 1998; Graham et al., 2004; Moyat and Velin, 2014). There is a prominent lymphocytic infiltrate in Hp gastric inflammation, with specific increases in CD4+ and CD8+ T cells and IgM- and IgG-producing B cells present in the gastric mucosa of Hp-infected over uninfected individuals (Nurgalieva et al., 2005). It is typical that the anti-bacterial immune response promotes differentiation of CD4+ T cells into Th1 cells that produce IFN-γ and TNFα, as opposed to Th2 cells that produce IL-4 and IL-13 usually in response to parasitic infection or allergy. In fact, it has been established that Hp-specific CD4+ T cells are predominantly differentiated into Th1 cells in response to Hp antigens (e.g., CagA, FlaA, VacA, UreB). IFN-γ, but not IL-4, is reported to be important for promoting gastric inflammation (Nurgalieva et al., 2005; Smythies et al., 2000; D'Elios et al., 1997). Some hypothesize that early parasitic infection skews an individual’s immune response toward Th2, decreasing the risk of Hp associated gastric cancer; however others have found increased levels of the type 2 cytokine, IL-13, in chronically Hp infected individuals to be associated with enhanced disease progression beyond inflammation (Holcombe, 1992; Marotti et al., 2008). Therefore, it is likely that the immune response to Hp is heterogenous, but the early inflammatory response is skewed Th1 over Th2. Recent studies have shown that both Th1 and Th17 (IL-17 producing) cells play important roles in the induced response against infection. One study found that Th17 cells precede Th1 and are important for inducing the Th1 response (Shi et al., 2010). IL-17 also plays a role in recruiting neutrophils to the site of infection (DeLyria et al., 2009). In mice infected with Helicobacter species, neutrophil depletion slowed clearance and limited inflammation-mediated pathology (Ismail et al., 2003; DeLyria et al., 2009). A proposed mechanism of neutrophil-induced pathology is Hp phagosome escape strategies causing reactive oxygen species to leak into the extracellular environment and elicit tissue damage (Allen et al., 2005). Other cells, including eosinophils and mast cells, have also been identified within the gastric mucosa of Hp-infected individuals, although their exact contributions to disease progression and/or infection clearance remains unclear (Nakajima et al., 1997; Moorchung et al., 2006).
Antigen-presenting cells are important for Hp-mediated inflammation through the activation of autoreactive CD4+ T cells. Dendritic cells presenting Hp antigen have been found to stimulate CD4+ T cells in vitro to produce IFN-γ, TNFα, and IL-17 (Hafsi et al., 2004; Khamri et al., 2010). In contrast, classical dendritic cells expressing Programmed Death Ligand 1 (PDL1) were recently found in the gastric submucosa of Hp-infected individuals interacting with effector T cells to promote tolerance and limit gastric pathology (Go et al., 2021). Epithelial cells within the inflamed gastric tissue may also be responsible for lymphocyte activation. Studies have demonstrated that molecules like HLA-DR (MHC II), ICAM-1 (lymphocytic adhesion), B7-1 and B7-2 (T cell co-stimulation), and Fas/FasL (receptor mediated apoptosis) have increased expression on epithelial cells in Hp-infected tissue compared to healthy controls (Ye et al., 1997; Archimandritis et al., 2000; Wang et al., 2000). These molecules contribute to immune cell-mediated epithelial cell apoptosis and epithelial cell-mediated T cell proliferation and activation. In summary, infection with Hp causes an infiltration of immune cells, which leads to chronic inflammation and inflammation-mediated pathology. A more in-depth understanding of the specific immune response influencing the risk of gastric cancer is still needed.
Autoimmune Gastritis
Autoimmune gastritis (AIG) is the consequence of an individual’s immune system lacking appropriate tolerance to self-antigens. This allows autoreactive T cells to become activated against gastric parietal cells, leading to chronic gastritis and oxyntic atrophy. Due to inefficient tolerance by the immune system to self-antigens, AIG can occur as part of multiple autoimmune syndrome, in which a person is diagnosed with three or more distinct autoimmune diseases (Cojocaru et al., 2010). Commonly, these individuals have diseases like autoimmune thyroiditis and type one diabetes mellitus alongside autoimmune gastritis (Kahaly and Hansen, 2016; Rodriguez-Castro et al., 2018). Immune destruction of parietal cells in AIG can cause a loss of intrinsic factor, a protein produced by parietal cells required for Vitamin B12 absorption in humans. The resulting megaloblastic anemia due to Vitamin B12 deficiency is referred to as pernicious anemia (PA) (Toh et al., 1997). Since Vitamin B12 has long term storage in the liver, an absorptive deficiency may go undetected for years, allowing time for gastric inflammation, atrophy, and metaplasia to develop, as shown in Figure 1 (Lenti et al., 2019). Patients diagnosed with PA require lifelong Vitamin B12 supplementation to prevent severe clinical manifestations of deficiency (Green et al., 2017). Another outcome from the loss of acid-secreting parietal cells is hypochlorhydria. Increased gastric pH enhances gastrin production from G cells in the stomach. Gastrin acts on and induces hyperplasia of neuroendocrine ECL cells, which promote acid secretion from parietal cells (Walsh, 1990). This type of hyperplasia is a common feature of AIG and can lead to the development of generally benign neuroendocrine tumors (Müller et al., 1987; Coati et al., 2015). The combination of the insidious nature of PA’s disease progression, the accepted association of AIG with neuroendocrine tumors, and a focus on Hp as the major risk factor for gastric adenocarcinoma may have contributed to a lack of appreciation for the gastric cancer risk associated with AIG. However, recent studies have shown that AIG patients have a significantly increased risk of developing gastric cancer (Landgren et al., 2011; Hemminki et al., 2012). In one such study, Vannella et al. estimates that patients suffering from AIG have a seven-fold increased relative risk which is equivalent to the relative risk associated with Hp infection (Kamangar et al., 2006; Vannella et al., 2013).
It is well-established that the primary etiology of autoimmune gastritis is the targeted atrophy of Parietal cells via the activation of parietal cell-specific T and B cells in the stomach. Antibodies against parietal cells and intrinsic factor can be found in the serum of most AIG patients (Taylor et al., 1962; Rusak et al., 2016). Autoreactive CD4+ T cells mainly target the H+/K+ ATPase proton pump found on parietal cells and predominantly differentiate into the Th1 phenotype, producing IFN-γ upon stimulation with this antigen (D'Elios et al., 2001). These Th1 cells are also capable of inducing IgM, IgG and IgA production from autologous B cells in vitro and can induce pathology via perforin/granzyme or Fas/FasL-mediated cell death. Dendritic cells (DC) have also been identified in gastric biopsies from AIG patients and in mice an increase in gastric DCs correlated to enhanced pathology (Ninomiya et al., 2000). In a prospective study, women who developed AIG were found to have T cell and macrophage infiltration in their stomach biopsies as well as increased HLA-DR (MHC II) expression on epithelial cells (Burman et al., 1992). Mast cells and eosinophils have also been identified in the setting of AIG, but their contribution to disease and the significance of infiltration have not yet been determined (Park et al., 2013; Bockerstett et al., 2020).
There is limited data addressing the specific immune cell responses leading to autoimmune gastritis in humans, however there are experimental models that have expanded upon this topic. Neonatal thymectomy of mice can induce autoimmune gastritis amongst other autoimmune diseases. In this model, the thymus is removed prior to day three of life, preventing the development of regulatory T cells (Tregs) that promote tolerance and limit autoimmunity (Suri-Payer et al., 1998; Fontenot et al., 2005). In these animals autoreactive T cells escape tolerance and enter the periphery to be activated by self-antigens. The gastritis in these thymectomized mice is caused by parietal cell specific CD4+ T cells infiltrating the gastric mucosa and initiating disease, followed by autoreactive B cells producing anti-parietal cell antibodies (Fukuma et al., 1988).
McHugh et al. isolated CD4+ T cells from neonatally thymectomized mice that developed AIG and cloned a T cell receptor (TCR) specific for a peptide from the parietal cell H+/K+ ATPase α chain, the major autoantigen in both mice and humans. This TCR sequence was used to generate a CD4+ TCR transgenic mouse model of AIG referred to as the TxA23 mouse (McHugh et al., 2001). In this model, the transgenic CD4+ T cells initiate disease progression which follows the same pattern seen in humans: gastritis progressing to atrophic gastritis by 2 months of age, metaplasia by 4 months of age, and then mice begin to show signs of gastric dysplasia by 12 months of age (McHugh et al., 2001; Nguyen and Dipaolo, 2013). TxA23 mice generate an adaptive immune response against parietal cells similar to humans, producing parietal cell autoantibodies and similar inflammatory cytokines like IFN-γ and IL-17 (Nguyen et al., 2013). IL-17 and IFN-γ in this model have been found to induce parietal cell atrophy initiating pathology in the corpus of the stomach (Bockerstett et al., 2018; Osaki et al., 2019). The type two cytokine IL-13 was also found to have a profound impact on progressing gastric pathology beyond atrophic gastritis in TxA23 animals, driving metaplastic transformation in the tissue (Noto et al., 2021). These mice also show significant increases in pSTAT3 and IL-6, two molecules known to be associated with human carcinomas (Nguyen et al., 2013). IL-27, produced by macrophages in the gastric mucosa, is protective against inflammation and metaplastic development by acting on CD4+ T cells to dampen the inflammatory response (Bockerstett et al., 2020). Although these and other recent studies of immune responses during autoimmune gastritis have increased our understanding of the pathogenesis of AIG, the progression from gastritis to gastric cancer in AIG is still incompletely understood.
Comparison of Helicobacter pylori and Autoimmune Gastritis
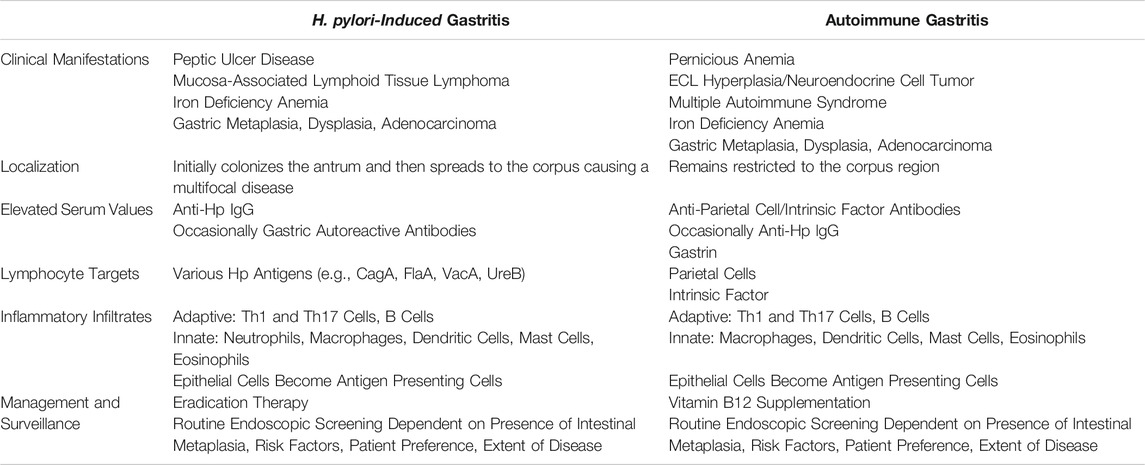
There are established similarities and differences between AIG and Hp-induced gastritis which are summarized in Table 1. As previously stated, both Hp and AIG cause inflammation-mediated pathology that can lead to the appearance of metaplastic gastric glands, chronic gastritis, parietal cell atrophy, and eventually gastric adenocarcinoma. Prominent unique late manifestations are pernicious anemia, ECL cell hyperplasia, and neuroendocrine tumors in AIG and peptic ulceration and MALT lymphoma in Hp. Iron deficiency anemia, a microcytic anemia, can emerge early on in both settings, but pernicious anemia, a macrocytic anemia, is primarily associated with late-stage AIG (Park et al., 2013; Amieva and Peek, 2016; Kulnigg-Dabsch, 2016). Surprisingly, studies have found that 92% of patients with MALT lymphoma, a primary B cell lymphoma, are infected with Hp, but no strong correlation between this type of lymphoma and AIG has been identified (Wotherspoon et al., 1991). In AIG, the targeted loss of acid-secreting parietal cells causes a rise in gastric pH and increased gastrin production, triggering hyperplasia of acid-promoting neuroendocrine cells from which neuroendocrine tumors can arise (Walsh, 1990; Park et al., 2013). Measured gastric pH can be high, low, or normal in Hp patients depending on the localization, length of infection, and extent of inflammation and atrophy, but there is no strong association between Hp infection and neuroendocrine tumor development (McColl et al., 1998). Differences in extent of parietal cell atrophy and impact on gastric pH probably contribute to the divergence in clinical manifestations, driving prominent ECL cell hyperplasia and neuroendocrine tumor development in AIG but not Hp. AIG initiates and remains restricted to the corpus of the stomach primarily targeting parietal cells, whereas Hp initially colonizes the antrum and spreads through to the corpus after long-standing infection, causing a patchy or multifocal disease (Graham et al., 2019).
As previously detailed, the immune responses triggered by AIG and Hp in the gastric mucosa have prominent similarities with some notable variance, but directed studies are needed to establish where these immune responses diverge. Remarkable similarities include a considerable lymphocytic infiltration with predominant Th1- and Th17-differentiated effectors and inflammation-responding epithelial cells upregulating MHC II molecules to aid in CD4+ T cell activation (Smythies et al., 2000; Shi et al., 2010; Archimandritis et al., 2000; D'Elios et al., 2001; Burman et al., 1992; Nguyen et al., 2013). While type two cytokines, IL-4 and IL-13, are not responsible for initiating inflammation in response to either Hp or AIG, studies have found them to be critical for promoting severe metaplastic and even dysplastic lesions as disease progresses beyond gastritis (Marotti et al., 2008; Gabitass et al., 2011; Miska et al., 2018; Petersen et al., 2018; De Salvo et al., 2021; Noto et al., 2021). Cells like dendritic cells, macrophages, mast cells, and eosinophils have also been identified in both disease settings (Burman et al., 1992; Nakajima et al., 1997; Ninomiya et al., 2000; Suzuki et al., 2002; Hafsi et al., 2004; Moorchung et al., 2006; Khamri et al., 2010; Park et al., 2013; Bockerstett et al., 2020; Go et al., 2021). Key immunological differences include an abundance of autoantibodies in the serum of AIG patients and a clear neutrophilic gastric infiltrate in Hp infection important for inducing pathology and clearance (Taylor et al., 1962; Ismail et al., 2003; DeLyria et al., 2009; Rusak et al., 2016). ROS producing neutrophils may be responsible for inducing peptic ulceration in Hp but not AIG. Autoimmune gastritis directly targets parietal cells and induces the production of parietal cell and intrinsic factor autoantibodies, while in Hp various anti-Hp IgGs can be detected in the serum (Taylor et al., 1962; Pan et al., 2014). In some Hp-infected patients, autoreactive antibodies can also be detected (Faller et al., 1997; Ayesh et al., 2013). In comparing the immunologic responses, several factors distinguish or relate AIG- and Hp-mediated inflammation.
Current gastric precancerous diagnostics use the severity and extent of inflammation, atrophy, and metaplasia present in both the antrum and corpus to grade and stage disease progression, placing the emphasis for cancer risk on gastric pathology, as opposed to etiology of disease. This equates the risk of adenocarcinoma development attributed to AIG and Hp once metaplastic lesions arise (Rugge and Genta, 2005; Rugge et al., 2016). However, there is ongoing debate about how frequently, if at all, patients with metaplastic lesions should be routinely surveyed (Pimentel-Nunes et al., 2019; Gupta et al., 2020). For patients in the United States with a late disease stage and compounding risk factors (e.g., racial/ethnic minorities, immigrants from high incidence countries, family history) guidelines suggest the decision for routine endoscopic screening be left up to the patient. Controversy surrounds these guidelines since other premalignant lesions with similar associated cancer risk, like in the esophagus, have stricter surveillance recommendations (Huang and Hwang, 2020). In other countries, such as the United Kingdom, routine endoscopic screening is recommended every 3 years in patients with extensive premalignant lesions and even retroactively in healthy individuals at increased risk of gastric cancer development (Banks et al., 2019). In high incidence countries like Japan and South Korea national gastric screening programs exist (Rawla and Barsouk, 2019). While screening guidelines and practices vary greatly throughout the world, it is widely accepted that gastric metaplasia is a risk factor for gastric adenocarcinoma. Overall, these identified differences and similarities between AIG and Hp-induced gastritis are crucial and require further investigation to fully understand the progression to gastric adenocarcinoma arising from either disease setting.
Helicobacter pylori Inducing Autoimmune Gastritis
There are studies suggesting that infection with Hp can trigger an autoimmune response within the gastric mucosa. Around 65% of Hp-infected individuals have detectable levels of autoreactive gastric antibodies (Negrini et al., 1996). These autoantibodies were most frequently specific to parietal cells and an increase in autoantibodies positively correlated with gastric disease severity (Negrini et al., 1996; Faller et al., 1997). Hp-infected people with serum-detectable gastric autoantibodies showed increased corpus atrophy, decreased stomach acid production, and increases in routine AIG diagnostic markers. In addition to AIG, studies have found Hp infection to be associated with a variety of other autoimmune diseases (Smyk et al., 2014).
Molecular mimicry has been suggested as a potential mechanism for developing autoimmunity out of infection. A major Hp surface protein, β urease, has 72% sequence homology with the β chain of the parietal cell specific H+/K+ ATPase and has been suggested as one possible antigen activating Hp effector cells against gastric tissue (Uibo et al., 1995). In Hp infected patients who developed AIG, CD4+ T cells were isolated from gastric biopsies, cloned, and stimulated in the presence of H+/K+ ATPase peptides or Hp peptides from lysate (Amedei et al., 2003). Several clones were cross-reactive against peptides from parietal cells and Hp lysate and stimulation in the presence of both antigens induced T cells to produce large and equivalent amounts of IFN-γ (Smythies et al., 2000; D'Elios et al., 1997; D'Elios et al., 2001). These cross-reactive cells were differentiated into the Th1 cells typically present in both AIG and Hp-induced gastritis. Also, in Hp infection, it has been shown that epithelial cells increase antigen presentation capabilities by upregulating MHC II molecules on their surface, increasing the possibility for self-peptide presentation (Archimandritis et al., 2000). This could be another way to induce autoreactive cells without the need for molecular mimicry.
Overall, worldwide prevalence of Hp infection is around 50%, but this frequency can range between 40–60% depending on age, social class, and geographic region, among other factors (Sitas et al., 1991; Hooi et al., 2017; Wang et al., 2019). Around 60% of pernicious anemia (PA) patients have serologic and/or histologic evidence of Hp infection, close to the frequency found in the general population (Annibale et al., 2000; Presotto et al., 2003). This infection prevalence among PA patients does not show a correlation between Hp infection and PA. Other studies have shown that PA patients have a lower frequency of Hp positivity than the general population. Some speculate that atrophy endured from AIG may deter Hp colonization as even fewer PA patients with severe gastric body atrophy show evidence of infection (Pérez-Pérez, 1997; Presotto et al., 2003). However, a recent study by Saenz et al. showed that in Hp-infected mice an atrophy-induced increase in gastric pH promotes Hp corpus colonization and that Hp preferentially binds deeper within antralized metaplastic corpus glands (Sáenz et al., 2019). This may make it more difficult to detect Hp in severely diseased, shallow human biopsy samples. It has also been shown that Hp-infected individuals can have seroconversion, or the loss of detectable serologic anti-Hp immunoglobulin after years of infection with and without successful eradication therapy (Valle et al., 1996; Perez-Perez et al., 2002; Veijola et al., 2007). Severe gastritis leading to clearance or deep binding of bacteria, combined with a loss of serologic positivity, could explain why PA patients don’t show higher rates of Hp, although these ideas still do not prove that AIG is triggered by infection. A decrease in Hp prevalence among the United States population and a rise in gastric cancer incidence in young American females possibly points to the independent development of autoimmune gastritis (Anderson et al., 2018; Bergquist et al., 2019). There has been clear documentation of AIG patients without evidence of Hp infection that typically have comorbidities with other autoimmune diseases and have unique diagnostic criteria more associated with Hp-negative AIG (Venerito et al., 2016). To date, there have been no studies definitively showing that Hp induces AIG, and therefore no confident conclusions can yet be drawn.
Further experimentation is needed to prove a link between Hp and AIG. To definitively establish that Hp infection induces AIG, a prospective study would need to be conducted on chronically Hp infected patients. Patients would get sequential blood draws to measure if/when gastric autoantibodies can be detected in the serum. Finding patients for this study would be extremely difficult considering they would need to be untreated or treatment resistant. This would require a multi-national approach which would be logistically very difficult and expensive to perform. In the animal model it would be feasible to infect mice and serially measure serum for the emergence of autoantibodies, but this would not definitely establish the link in humans. It is also a challenge to establish the significance of these studies, as the results would not necessarily change anything about patient management. Overall a combination of high expense, low significance, and difficultly conducting the experiments may leave the question of whether Hp induces AIG unanswered.
Conclusion
Gastric cancer is a world leading cause of cancer related mortality (Hyuna Sung et al., 2021). It has been well-established that gastric infection with Hp is the major risk factor for developing gastric cancer. This bacterium still infects a large portion of the world population, but infection rates in the United States are low and incidence of Hp-negative gastric cancer is increasing (Hooi et al., 2017; Nguyen et al., 2020). As well in the United States, gastric cancer incidence rates are increasing specifically among young females (Hooi et al., 2017; Anderson et al., 2018; Bergquist et al., 2019). These trends converge to the likelihood that United States gastric cancer may arise out of AIG. While no definitive evidence exists for a rise in AIG incidence to corroborate this hypothesis, increases in related diseases (type I diabetes, autoimmune thyroiditis, gastric neuroendocrine tumors) suggest this may be the case (Yao et al., 2008; McLeod and Cooper, 2012; Kahaly and Hansen, 2016; Rodriguez-Castro et al., 2018; Mobasseri et al., 2020). In the past, AIG has been missed as a significant risk factor for gastric cancer, but the risk associated with AIG may be just as high as with Hp infection and the major pathology-inducing immune cells are consistent in both disease settings (Smythies et al., 2000; D'Elios et al., 1997; Vannella et al., 2013; D'Elios et al., 2001). Some unanswered questions between these two etiologies remain. For example, it is not definitively known why neuroendocrine tumors do not arise out of Hp infection and peptic ulcers do not develop in AIG patients. Differences in the extent of parietal cell atrophy and subsets of infiltrating immune cells may contribute to these divergent manifestations. Speculation on whether the immune response to Hp infection can induce autoimmune gastritis is based on correlative studies, but there is no definitive evidence to prove that Hp infection initiates the autoimmune response found in AIG. Studies needed to prove this link would be challenging and costly. This review aims to highlight two risk factors for gastric cancer, Hp and AIG, and compile what is currently in the literature for how these inflammatory triggers lead to gastric cancer. Further immunological studies on the progression from healthy tissue through inflammation to cancer is required for these two etiologies of gastritis to diminish the global burden of gastric cancer.
Author Contributions
SH substantially contributed to the concept and outline of the work. SH also drafted and revised the work for publication. CN read and revised drafts of the work. RD revised, read, and approved the manuscript for submission.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abadi, A. T. B. (2016). Vaccine againstHelicobacter Pylori: Inevitable Approach. Wjg 22, 3150–3157. doi:10.3748/wjg.v22.i11.3150
Allen, L.-A. H., Beecher, B. R., Lynch, J. T., Rohner, O. V., and Wittine, L. M. (2005). Helicobacter pyloriDisrupts NADPH Oxidase Targeting in Human Neutrophils to Induce Extracellular Superoxide Release. J. Immunol. 174, 3658–3667. doi:10.4049/jimmunol.174.6.3658
Amedei, A., Bergman, M. P., Appelmelk, B. J., Azzurri, A., Benagiano, M., Tamburini, C., et al. (2003). Molecular Mimicry between Helicobacter pylori Antigens and H+,K+-Adenosine Triphosphatase in Human Gastric Autoimmunity. J. Exp. Med. 198, 1147–1156. doi:10.1084/jem.20030530
Amieva, M., and Peek, R. M. (2016). Pathobiology of Helicobacter Pylori-Induced Gastric Cancer. Gastroenterology 150, 64–78. doi:10.1053/j.gastro.2015.09.004
Anderson, W. F., Rabkin, C. S., Turner, N., Fraumeni, J. F., Rosenberg, P. S., and Camargo, M. C. (2018). The Changing Face of Noncardia Gastric Cancer Incidence Among United States Non-hispanic Whites. J. Natl. Cancer Inst. 110, 608–615. doi:10.1093/jnci/djx262
Annibale, B., Lahner, E., Bordi, C., Martino, G., Caruana, P., Grossi, C., et al. (2000). Role of Helicobacter pylori Infection in Pernicious Anaemia. Dig. Liver Dis. 32, 756–762. doi:10.1016/s1590-8658(00)80351-5
Archimandritis, A., Sougioultzis, S., Foukas, P. G., Tzivras, M., Davaris, P., and Moutsopoulos, H. M. (2000). Expression of HLA-DR, Costimulatory Molecules B7-1, B7-2, Intercellular Adhesion Molecule-1 (ICAM-1) and Fas Ligand (FasL) on Gastric Epithelial Cells in Helicobacter pylori Gastritis; Influence of H. pylori Eradication. Clin. Exp. Immunol. 119, 464–471. doi:10.1046/j.1365-2249.2000.01164.x
Ayesh, M. H., Jadalah, K., Al Awadi, E., Alawneh, K., and Khassawneh, B. (2013). Association between Vitamin B12 Level and Anti-parietal Cells and Anti-intrinsic Factor Antibodies Among Adult Jordanian Patients with Helicobacter pylori Infection. Braz. J. Infect. Dis. 17, 629–632. doi:10.1016/j.bjid.2013.01.009
Banks, M., Graham, D., Jansen, M., Gotoda, T., Coda, S., di Pietro, M., et al. (2019). British Society of Gastroenterology Guidelines on the Diagnosis and Management of Patients at Risk of Gastric Adenocarcinoma. Gut 68, 1545–1575. doi:10.1136/gutjnl-2018-318126
Bergquist, J. R., Leiting, J. L., Habermann, E. B., Cleary, S. P., Kendrick, M. L., Smoot, R. L., et al. (2019). Early-onset Gastric Cancer Is a Distinct Disease with Worrisome Trends and Oncogenic Features. Surgery 166, 547–555. doi:10.1016/j.surg.2019.04.036
Bockerstett, K. A., Osaki, L. H., Petersen, C. P., Cai, C. W., Wong, C. F., Nguyen, T.-L. M., et al. (2018). Interleukin-17A Promotes Parietal Cell Atrophy by Inducing Apoptosis. Cell Mol. Gastroenterol. Hepatol. 5, 678–690. doi:10.1016/j.jcmgh.2017.12.012
Bockerstett, K. A., Petersen, C. P., Noto, C. N., Kuehm, L. M., Wong, C. F., Ford, E. L., et al. (2020). Interleukin 27 Protects from Gastric Atrophy and Metaplasia during Chronic Autoimmune Gastritis. Cell Mol. Gastroenterol. Hepatol. 10, 561–579. doi:10.1016/j.jcmgh.2020.04.014
Burman, P., Kämpe, O., Kraaz, W., Lööfv, L., Smolka, A., Karlsson, A., et al. (1992). A Study of Autoimmune Gastritis in the Postpartum Period and at a 5-year Follow-Up. Gastroenterology 103, 934–942. doi:10.1016/0016-5085(92)90027-v
Cabrera de León, A., Almeida González, D., Almeida, A. A., Hernández, A. G., Carretero Pérez, M., Cristo Rodríguez Pérez, M. D., et al. (2012). Factors Associated with Parietal Cell Autoantibodies in the General Population. Immunol. Lett. 147, 63–66. doi:10.1016/j.imlet.2012.06.004
Carmel, R. (1996). Prevalence of Undiagnosed Pernicious Anemia in the Elderly. Arch. Intern. Med. 156, 1097–1100. doi:10.1001/archinte.1996.00040041097008
Carter, A. J., and Nguyen, C. N. (2012). A Comparison of Cancer burden and Research Spending Reveals Discrepancies in the Distribution of Research Funding. BMC Public Health 12, 526. doi:10.1186/1471-2458-12-526
Coati, I., Fassan, M., Farinati, F., Graham, D. Y., Genta, R. M., and Rugge, M. (2015). Autoimmune Gastritis: Pathologist's Viewpoint. Wjg 21, 12179–12189. doi:10.3748/wjg.v21.i42.12179
Cojocaru, M., Cojocaru, I. M., and Silosi, I. (2010). Multiple Autoimmune Syndrome. Maedica (Bucur) 5, 132–134.
D'Elios, M. M., Manghetti, M., De Carli, M., Costa, F., Baldari, C. T., Burroni, D., et al. (1997). T Helper 1 Effector Cells Specific for Helicobacter pylori in the Gastric Antrum of Patients with Peptic Ulcer Disease. J. Immunol. 158, 962–967.
D'Elios, M. M., Bergman, M. P., Azzurri, A., Amedei, A., Benagiano, M., De Pont, J. J., et al. (2001). H+,K+-ATPase (Proton Pump) Is the Target Autoantigen of Th1-type Cytotoxic T Cells in Autoimmune Gastritis. Gastroenterology 120, 377–386. doi:10.1053/gast.2001.21187
De Salvo, C., Pastorelli, L., Petersen, C. P., Buttò, L. F., Buela, K.-A., Omenetti, S., et al. (2021). Interleukin 33 Triggers Early Eosinophil-dependent Events Leading to Metaplasia in a Chronic Model of Gastritis-Prone Mice. Gastroenterology 160, 302–316.e7. doi:10.1053/j.gastro.2020.09.040
DeLyria, E. S., Redline, R. W., and Blanchard, T. G. (2009). Vaccination of Mice against H Pylori Induces a strong Th-17 Response and Immunity that Is Neutrophil Dependent. Gastroenterology 136, 247–256. doi:10.1053/j.gastro.2008.09.017
Dos Santos Viana, I., Cordeiro Santos, M. L., Santos Marques, H., Lima de Souza Gonçalves, V., Bittencourt de Brito, B., França da Silva, F. A., et al. (2021). Vaccine Development against Helicobacter pylori: from Ideal Antigens to the Current Landscape. Expert Rev. Vaccin. 20, 989–999. doi:10.1080/14760584.2021.1945450
Faller, G., Steininger, H., Kränzlein, J., Maul, H., Kerkau, T., Hensen, J., et al. (1997). Antigastric Autoantibodies in Helicobacter Pyloriinfection: Implications of Histological and Clinical Parameters of Gastritis. Gut 41, 619–623. doi:10.1136/gut.41.5.619
Fontenot, J. D., Dooley, J. L., Farr, A. G., and Rudensky, A. Y. (2005). Developmental Regulation of Foxp3 Expression during Ontogeny. J. Exp. Med. 202, 901–906. doi:10.1084/jem.20050784
Fukuma, K., Sakaguchi, S., Kuribayashi, K., Chen, W.-L., Morishita, R., Sekita, K., et al. (1988). Immunologic and Clinical Studies on Murine Experimental Autoimmune Gastritis Induced by Neonatal Thymectomy. Gastroenterology 94, 274–283. doi:10.1016/0016-5085(88)90413-1
Gabitass, R. F., Annels, N. E., Stocken, D. D., Pandha, H. A., and Middleton, G. W. (2011). Elevated Myeloid-Derived Suppressor Cells in Pancreatic, Esophageal and Gastric Cancer Are an Independent Prognostic Factor and Are Associated with Significant Elevation of the Th2 Cytokine Interleukin-13. Cancer Immunol. Immunother. 60, 1419–1430. doi:10.1007/s00262-011-1028-0
Go, D.-M., Lee, S. H., Lee, S.-H., Woo, S.-H., Kim, K., Kim, K., et al. (2021). Programmed Death Ligand 1-Expressing Classical Dendritic Cells Mitigate -Induced Gastritis. Cell Mol. Gastroenterol. Hepatol. 12, 715–739. doi:10.1016/j.jcmgh.2021.04.007
Graham, D. Y., Opekun, A. R., Osato, M. S., El-Zimaity, H. M. T., Lee, C. K., Yamaoka, Y., et al. (2004). Challenge Model for Helicobacter pylori Infection in Human Volunteers. Gut 53, 1235–1243. doi:10.1136/gut.2003.037499
Graham, D. Y., Rugge, M., and Genta, R. M. (2019). Diagnosis. Curr. Opin. Gastroenterol. 35, 535–543. doi:10.1097/mog.0000000000000576
Green, R., Allen, L. H., Bjørke-Monsen, A.-L., Brito, A., Guéant, J.-L., Miller, J. W., et al. (2017). Vitamin B12 Deficiency. Nat. Rev. Dis. Primers 3, 17040. doi:10.1038/nrdp.2017.40
Gupta, S., Li, D., El Serag, H. B., Davitkov, P., Altayar, O., Sultan, S., et al. (2020). AGA Clinical Practice Guidelines on Management of Gastric Intestinal Metaplasia. Gastroenterology 158, 693–702. doi:10.1053/j.gastro.2019.12.003
Hafsi, N., Voland, P., Schwendy, S., Rad, R., Reindl, W., Gerhard, M., et al. (2004). Human Dendritic Cells Respond toHelicobacter Pylori, Promoting NK Cell and Th1-Effector Responses In Vitro. J. Immunol. 173, 1249–1257. doi:10.4049/jimmunol.173.2.1249
Hemminki, K., Liu, X., Ji, J., Sundquist, J., and Sundquist, K. (2012). Effect of Autoimmune Diseases on Mortality and Survival in Subsequent Digestive Tract Cancers. Ann. Oncol. 23, 2179–2184. doi:10.1093/annonc/mdr590
Holcombe, C. (1992). Helicobacter pylori: the African enigma. Gut 33, 429–431. doi:10.1136/gut.33.4.429
Hooi, J. K. Y., Lai, W. Y., Ng, W. K., Suen, M. M. Y., Underwood, F. E., Tanyingoh, D., et al. (2017). Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 153, 420–429. doi:10.1053/j.gastro.2017.04.022
Huang, R. J., and Hwang, J. H. (2020). The Management of Gastric Intestinal Metaplasia in the United States: A Controversial Topic. Gastroenterology 159, 402–403. doi:10.1053/j.gastro.2020.02.066
Hyuna Sung, J. F., Siegel, Rebecca. L., Laversanne, Mathieu., Soerjomataram, Isabelle., Jemal, Ahmedin., and Bray, Freddie. (2021). Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Ismail, H. F., Fick, P., Zhang, J., Lynch, R. G., and Berg, D. J. (2003). Depletion of Neutrophils in IL-10−/−Mice Delays Clearance of GastricHelicobacterInfection and Decreases the Th1 Immune Response toHelicobacter. J. Immunol. 170, 3782–3789. doi:10.4049/jimmunol.170.7.3782
Kahaly, G. J., and Hansen, M. P. (2016). Type 1 Diabetes Associated Autoimmunity. Autoimmun. Rev. 15, 644–648. doi:10.1016/j.autrev.2016.02.017
Kamangar, F., Dawsey, S. M., Blaser, M. J., Perez-Perez, G. I., Pietinen, P., Newschaffer, C. J., et al. (2006). Opposing Risks of Gastric Cardia and Noncardia Gastric Adenocarcinomas Associated with Helicobacter pylori Seropositivity. J. Natl. Cancer Inst. 98, 1445–1452. doi:10.1093/jnci/djj393
Khamri, W., Walker, M. M., Clark, P., Atherton, J. C., Thursz, M. R., Bamford, K. B., et al. (2010). Helicobacter pylori Stimulates Dendritic Cells to Induce Interleukin-17 Expression from CD4 + T Lymphocytes. Infect. Immun. 78, 845–853. doi:10.1128/iai.00524-09
Koulis, A., Buckle, A., and Boussioutas, A. (2019). Premalignant Lesions and Gastric Cancer: Current Understanding. Wjgo 11, 665–678. doi:10.4251/wjgo.v11.i9.665
Kulnigg-Dabsch, S. (2016). Autoimmune Gastritis. Wien Med. Wochenschr 166, 424–430. doi:10.1007/s10354-016-0515-5
Kusters, J. G., van Vliet, A. H. M., and Kuipers, E. J. (2006). Pathogenesis of Helicobacter pylori Infection. Clin. Microbiol. Rev. 19, 449–490. doi:10.1128/cmr.00054-05
Landgren, A. M., Landgren, O., Gridley, G., Dores, G. M., Linet, M. S., and Morton, L. M. (2011). Autoimmune Disease and Subsequent Risk of Developing Alimentary Tract Cancers Among 4.5 Million United States Male Veterans. Cancer 117, 1163–1171. doi:10.1002/cncr.25524
Lee, Y.-C., Chiang, T.-H., Chou, C.-K., Tu, Y.-K., Liao, W.-C., Wu, M.-S., et al. (2016). Association between Helicobacter pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-Analysis. Gastroenterology 150, 1113–1124. doi:10.1053/j.gastro.2016.01.028
Lenti, M. V., Miceli, E., Cococcia, S., Klersy, C., Staiani, M., Guglielmi, F., et al. (2019). Determinants of Diagnostic Delay in Autoimmune Atrophic Gastritis. Aliment. Pharmacol. Ther. 50, 167–175. doi:10.1111/apt.15317
Lindholm, C., Quiding-Järbrink, M., Lönroth, H., Hamlet, A., and Svennerholm, A.-M. (1998). Local Cytokine Response in Helicobacter pylori -Infected Subjects. Infect. Immun. 66, 5964–5971. doi:10.1128/iai.66.12.5964-5971.1998
Malaty, H. M., and Graham, D. Y. (1994). Importance of Childhood Socioeconomic Status on the Current Prevalence of Helicobacter pylori Infection. Gut 35, 742–745. doi:10.1136/gut.35.6.742
Marotti, B., Rocco, A., De Colibus, P., Compare, D., de Nucci, G., Staibano, S., et al. (2008). Interleukin-13 Mucosal Production in Helicobacter Pylori-Related Gastric Diseases. Dig. Liver Dis. 40, 240–247. doi:10.1016/j.dld.2007.11.021
Marshall, B. J., Armstrong, J. A., McGechie, D. B., and Clancy, R. J. (1985). Attempt to Fulfil Koch's Postulates for Pyloric Campylobacter. Med. J. Aust. 142, 436–439. doi:10.5694/j.1326-5377.1985.tb113443.x
Marshall, B., and Warren, J. R. (1984). Unidentified Curved Bacilli in the Stomach of Patients with Gastritis and Peptic Ulceration. The Lancet 323, 1311–1315. doi:10.1016/s0140-6736(84)91816-6
Matsuzaka, M., Tanaka, R., and Sasaki, Y. (2016). High Mortality Rate of Stomach Cancer Caused Not by High Incidence but Delays in Diagnosis in Aomori Prefecture, Japan. Asian Pac. J. Cancer Prev. 17, 4723–4727. doi:10.22034/apjcp.2016.17.10.4723
McColl, K. E. L., el-Omar, E., and Gillen, D. (1998). Interactions between H. pylori Infection, Gastric Acid Secretion and Anti-secretory Therapy. Br. Med. Bull. 54, 121–138. doi:10.1093/oxfordjournals.bmb.a011663
McHugh, R. S., Shevach, E. M., Margulies, D. H., and Natarajan, K. (2001). A T Cell Receptor Transgenic Model of Severe, Spontaneous Organ-specific Autoimmunity. Eur. J. Immunol. 31, 2094–2103. doi:10.1002/1521-4141(200107)31:7<2094:aid-immu2094>3.0.co;2-s
McLeod, D. S. A., and Cooper, D. S. (2012). The Incidence and Prevalence of Thyroid Autoimmunity. Endocrine 42, 252–265. doi:10.1007/s12020-012-9703-2
Miska, J., Lui, J. B., Toomer, K. H., Devarajan, P., Cai, X., Houghton, J., et al. (2018). Initiation of Inflammatory Tumorigenesis by CTLA4 Insufficiency Due to Type 2 Cytokines. J. Exp. Med. 215, 841–858. doi:10.1084/jem.20171971
Mobasseri, M., Shirmohammadi, M., Amiri, T., Vahed, N., Hosseini Fard, H., and Ghojazadeh, M. (2020). Prevalence and Incidence of Type 1 Diabetes in the World: a Systematic Review and Meta-Analysis. Health Promot. Perspect. 10, 98–115. doi:10.34172/hpp.2020.18
Moorchung, N., Srivastava, A. N., Gupta, N. K., Malaviya, A. K., Achyut, B. R., and Mittal, B. (2006). The Role of Mast Cells and Eosinophils in Chronic Gastritis. Clin. Exper.Med. 6, 107–114. doi:10.1007/s10238-006-0104-9
Morris, A., and Nicholson, G. (1987). Ingestion of Campylobacter Pyloridis Causes Gastritis and Raised Fasting Gastric pH. Am. J. Gastroenterol. 82, 192–199.
Moyat, M., and Velin, D. (2014). Immune Responses toHelicobacter Pyloriinfection. Wjg 20, 5583–5593. doi:10.3748/wjg.v20.i19.5583
Müller, J., Kirchner, T., and Müller-Hermelink, H. K. (1987). Gastric Endocrine Cell Hyperplasia and Carcinoid Tumors in Atrophic Gastritis Type A. Am. J. Surg. Pathol. 11, 909–917.
Nakajima, S., Krishnan, B., Ota, H., Segura, A., Hattori, T., Graham, D., et al. (1997). Mast Cell Involvement in Gastritis with or without Helicobacter pylori Infection. Gastroenterology 113, 746–754. doi:10.1016/s0016-5085(97)70167-7
Negrini, R., Savio, A., Poiesi, C., Appelmelk, B., Buffoli, F., Paterlini, A., et al. (1996). Antigenic Mimicry between Helicobacter pylori and Gastric Mucosa in the Pathogenesis of Body Atrophic Gastritis. Gastroenterology 111, 655–665. doi:10.1053/gast.1996.v111.pm8780570
Neumann, W. L., Coss, E., Rugge, M., and Genta, R. M. (2013). Autoimmune Atrophic Gastritis-Pathogenesis, Pathology and Management. Nat. Rev. Gastroenterol. Hepatol. 10, 529–541. doi:10.1038/nrgastro.2013.101
Nguyen, T.-L. M., and Dipaolo, R. J. (2013). A New Mouse Model of Inflammation and Gastric Cancer. Oncoimmunology 2, e25911. doi:10.4161/onci.25911
Nguyen, T.-L. M., Khurana, S. S., Bellone, C. J., Capoccia, B. J., Sagartz, J. E., Kesman, R. A., et al. (2013). Autoimmune Gastritis Mediated by CD4+ T Cells Promotes the Development of Gastric Cancer. Cancer Res. 73, 2117–2126. doi:10.1158/0008-5472.can-12-3957
Nguyen, T. H., Mallepally, N., Hammad, T., Liu, Y., Thrift, A. P., El-Serag, H. B., et al. (2020). Prevalence of Helicobacter pylori Positive Non-cardia Gastric Adenocarcinoma Is Low and Decreasing in a United States Population. Dig. Dis. Sci. 65, 2403–2411. doi:10.1007/s10620-019-05955-2
Ninomiya, T., Matsui, H., Akbar, S. M., Murakami, fnm., and Onji, fnm. (2000). Localization and Characterization of Antigen-Presenting Dendritic Cells in the Gastric Mucosa of Murine and Human Autoimmune Gastritis. Eur. J. Clin. Invest. 30, 350–358. doi:10.1046/j.1365-2362.2000.00629.x
Noto, C. N., Hoft, S. G., Bockerstett, K. A., Jackson, N. M., Ford, E. L., Vest, L. S., et al. (2021). IL-13 Acts Directly on Gastric Epithelial Cells to Promote Metaplasia Development during Chronic Gastritis. Cell Mol Gastroenterol Hepatol. doi:10.1016/j.jcmgh.2021.09.012
Nurgalieva, Z. Z., Conner, M. E., Opekun, A. R., Zheng, C. Q., Elliott, S. N., Ernst, P. B., et al. (2005). B-cell and T-Cell Immune Responses to Experimental Helicobacter pylori Infection in Humans. Infect. Immun. 73, 2999–3006. doi:10.1128/iai.73.5.2999-3006.2005
Osaki, L. H., Bockerstett, K. A., Wong, C. F., Ford, E. L., Madison, B. B., DiPaolo, R. J., et al. (2019). Interferon-γ Directly Induces Gastric Epithelial Cell Death and Is Required for Progression to Metaplasia. J. Pathol. 247, 513–523. doi:10.1002/path.5214
Pan, K.-F., Formichella, L., Zhang, L., Zhang, Y., Ma, J.-L., Li, Z.-X., et al. (2014). Helicobacter Pyloriantibody Responses and Evolution of Precancerous Gastric Lesions in a Chinese Population. Int. J. Cancer 134, 2118–2125. doi:10.1002/ijc.28560
Park, J. Y., Lam-Himlin, D., and Vemulapalli, R. (2013). Review of Autoimmune Metaplastic Atrophic Gastritis. Gastrointest. Endosc. 77, 284–292. doi:10.1016/j.gie.2012.09.033
Pérez-Pérez, G. I. (1997). Role of Helicobacter pylori Infection in the Development of Pernicious Anemia. Clin. Infect. Dis. 25, 1020–1022. doi:10.1086/516088
Perez-Perez, G. I., Salomaa, A., Kosunen, T. U., Daverman, B., Rautelin, H., Aromaa, A., et al. (2002). Evidence that cagA+Helicobacter pylori Strains Are Disappearing More Rapidly Than cagA- Strains. Gut 50, 295–298. doi:10.1136/gut.50.3.295
Petersen, C. P., Meyer, A. R., De Salvo, C., Choi, E., Schlegel, C., Petersen, A., et al. (2018). A Signalling cascade of IL-33 to IL-13 Regulates Metaplasia in the Mouse Stomach. Gut 67, 805–817. doi:10.1136/gutjnl-2016-312779
Pimentel-Nunes, P., Libânio, D., Marcos-Pinto, R., Areia, M., Leja, M., Esposito, G., et al. (2019). Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy 51, 365–388. doi:10.1055/a-0859-1883
Plummer, M., Franceschi, S., Vignat, J., Forman, D., and de Martel, C. (2015). Global burden of Gastric Cancer Attributable toHelicobacterpylori. Int. J. Cancer 136, 487–490. doi:10.1002/ijc.28999
Presotto, F., Sabini, B., Cecchetto, A., Plebani, M., Lazzari, F. D., Pedini, B., et al. (2003). Helicobacter pylori Infection and Gastric Autoimmune Diseases: Is There a Link? Helicobacter 8, 578–584. doi:10.1111/j.1523-5378.2003.00187.x
Rawla, P., and Barsouk, A. (2019). Epidemiology of Gastric Cancer: Global Trends, Risk Factors and Prevention. pg 14, 26–38. doi:10.5114/pg.2018.80001
Rodriguez-Castro, K. I., Franceschi, M., Miraglia, C., Russo, M., Nouvenne, A., Leandro, G., et al. (2018). Autoimmune Diseases in Autoimmune Atrophic Gastritis. Acta Biomed. 89, 100–103. doi:10.23750/abm.v89i8-S.7919
Rugge, M., Genta, R. M., Graham, D. Y., Di Mario, F., Vaz Coelho, L. G., Kim, N., et al. (2016). Chronicles of a Cancer Foretold: 35 Years of Gastric Cancer Risk Assessment. Gut 65, 721–725. doi:10.1136/gutjnl-2015-310846
Rugge, M., and Genta, R. M. (2005). Staging and Grading of Chronic Gastritis. Hum. Pathol. 36, 228–233. doi:10.1016/j.humpath.2004.12.008
Rusak, E., Chobot, A., Krzywicka, A., and Wenzlau, J. (2016). Anti-parietal Cell Antibodies - Diagnostic Significance. Adv. Med. Sci. 61, 175–179. doi:10.1016/j.advms.2015.12.004
Sáenz, J. B., and Mills, J. C. (2018). Acid and the Basis for Cellular Plasticity and Reprogramming in Gastric Repair and Cancer. Nat. Rev. Gastroenterol. Hepatol. 15, 257–273. doi:10.1038/nrgastro.2018.5
Sáenz, J. B., Vargas, N., and Mills, J. C. (2019). Tropism for Spasmolytic Polypeptide-Expressing Metaplasia Allows Helicobacter pylori to Expand its Intragastric Niche. Gastroenterology 156, 160–174.e7. doi:10.1053/j.gastro.2018.09.050
Schistosomes liver flukes and Helicobacter pylori (1994). IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr. Eval. Carcinog Risks Hum. 61, 1–241.
Shi, Y., Liu, X.-F., Zhuang, Y., Zhang, J.-Y., Liu, T., Yin, Z., et al. (2010). Helicobacter Pylori-Induced Th17 Responses Modulate Th1 Cell Responses, Benefit Bacterial Growth, and Contribute to Pathology in Mice. J.I. 184, 5121–5129. doi:10.4049/jimmunol.0901115
Shin, S. H., Jung, D. H., Kim, J.-H., Chung, H. S., Park, J. C., Shin, S. K., et al. (2015). Helicobacter pylori Eradication Prevents Metachronous Gastric Neoplasms after Endoscopic Resection of Gastric Dysplasia. PLoS One 10, e0143257. doi:10.1371/journal.pone.0143257
Shiotani, A., Haruma, K., and Graham, D. Y. (2014). Metachronous Gastric Cancer after successfulHelicobacter Pylorieradication. Wjg 20, 11552–11559. doi:10.3748/wjg.v20.i33.11552
Sitas, F., Forman, D., Yarnell, J. W., Burr, M. L., Elwood, P. C., Pedley, S., et al. (1991). Helicobacter pylori Infection Rates in Relation to Age and Social Class in a Population of Welsh Men. Gut 32, 25–28. doi:10.1136/gut.32.1.25
Smyk, D. S., Koutsoumpas, A. L., Mytilinaiou, M. G., et al. (2014). Helicobacter Pyloriand Autoimmune Disease: Cause or Bystander. Wjg 20, 613–629. doi:10.3748/wjg.v20.i3.613
Smythies, L. E., Waites, K. B., Lindsey, J. R., Harris, P. R., Ghiara, P., and Smith, P. D. (2000). Helicobacter Pylori-Induced Mucosal Inflammation Is Th1 Mediated and Exacerbated in IL-4, but Not IFN-γ, Gene-Deficient Mice. J. Immunol. 165, 1022–1029. doi:10.4049/jimmunol.165.2.1022
Suri-Payer, E., Amar, A. Z., Thornton, A. M., and Shevach, E. M. (1998). CD4+CD25+ T Cells Inhibit Both the Induction and Effector Function of Autoreactive T Cells and Represent a Unique Lineage of Immunoregulatory Cells. J. Immunol. 160, 1212–1218.
Suzuki, T., Kato, K., Ohara, S., Noguchi, K., Sekine, H., Nagura, H., et al. (2002). Localization of Antigen-Presenting Cells in Helicobacter Pylori-Infected Gastric Mucosa. Pathol. Int. 52, 265–271. doi:10.1046/j.1440-1827.2002.01347.x
Taylor, K. B., Roitt, I. M., Doniach, D., Couchman, K. G., and Shapland, C. (1962). Autoimmune Phenomena in Pernicious Anaemia: Gastric Antibodies. Bmj 2, 1347–1352. doi:10.1136/bmj.2.5316.1347
Toh, B.-H., van Driel, I. R., and Gleeson, P. A. (1997). Pernicious Anemia. N. Engl. J. Med. 337, 1441–1448. doi:10.1056/nejm199711133372007
Uemura, N., Okamoto, S., Yamamoto, S., Matsumura, N., Yamaguchi, S., Yamakido, M., et al. (2001). Helicobacter pyloriInfection and the Development of Gastric Cancer. N. Engl. J. Med. 345, 784–789. doi:10.1056/nejmoa001999
Uibo, R., Vorobjova, T., Metsküla, K., Kisand, K., Wadström, T., and Kivik, T. (1995). Association ofHelicobacter Pyloriand Gastric Autoimmunity: A Population-Based Study. FEMS Immunol. Med. Microbiol. 11, 65–68. doi:10.1111/j.1574-695x.1995.tb00079.x
Valle, J., Kekki, M., Sipponen, P., Ihamäki, T., and Siurala, M. (1996). Long-Term Course and Consequences ofHelicobacter Pylorigastritis Results of a 32-Year Follow-Up Study. Scand. J. Gastroenterol. 31, 546–550. doi:10.3109/00365529609009126
Vannella, L., Lahner, E., Osborn, J., and Annibale, B. (2013). Systematic Review: Gastric Cancer Incidence in Pernicious Anaemia. Aliment. Pharmacol. Ther. 37, 375–382. doi:10.1111/apt.12177
Veijola, L., Oksanen, A., Linnala, A., Sipponen, P., and Rautelin, H. (2007). Persisting Chronic Gastritis and Elevated Helicobacter pylori Antibodies after Successful Eradication Therapy. Helicobacter 12, 605–608. doi:10.1111/j.1523-5378.2007.00549.x
Venerito, M., Varbanova, M., Röhl, F.-W., Reinhold, D., Frauenschläger, K., Jechorek, D., et al. (2016). Oxyntic Gastric Atrophy inHelicobacter Pylorigastritis Is Distinct from Autoimmune Gastritis. J. Clin. Pathol. 69, 677–685. doi:10.1136/jclinpath-2015-203405
Walsh, J. H. (1990). Role of Gastrin as a Trophic Hormone. Digestion 47 Suppl 1 (Suppl. 1), 11–52. discussion 49-52. doi:10.1159/000200509
Wang, J., Fan, X., Lindholm, C., Bennett, M., O'Connoll, J., Shanahan, F., et al. (2000). Helicobacter pylori Modulates Lymphoepithelial Cell Interactions Leading to Epithelial Cell Damage through Fas/Fas Ligand Interactions. Infect. Immun. 68, 4303–4311. doi:10.1128/iai.68.7.4303-4311.2000
Wang, W., Jiang, W., Zhu, S., Sun, X., Li, P., Liu, K., et al. (2019). Assessment of Prevalence and Risk Factors of helicobacter Pylori Infection in an Oilfield Community in Hebei, China. BMC Gastroenterol. 19, 186. doi:10.1186/s12876-019-1108-8
Wotherspoon, A., Ortiz-Hidalgo, C., Falzon, M. R., et al. (1991). Helicobacter Pylori-Associated Gastritis and Primary B-Cell Gastric Lymphoma. The Lancet 338, 1175–1176. doi:10.1016/0140-6736(91)92035-z
Yao, J. C., Hassan, M., Phan, A., Dagohoy, C., Leary, C., Mares, J. E., et al. (2008). One Hundred Years after "carcinoid": Epidemiology of and Prognostic Factors for Neuroendocrine Tumors in 35,825 Cases in the United States. Jco 26, 3063–3072. doi:10.1200/jco.2007.15.4377
Ye, G., Barrera, C., Fan, X., Gourley, W. K., Crowe, S. E., Ernst, P. B., et al. (1997). Expression of B7-1 and B7-2 Costimulatory Molecules by Human Gastric Epithelial Cells: Potential Role in CD4+ T Cell Activation during Helicobacter pylori Infection. J. Clin. Invest. 99, 1628–1636. doi:10.1172/jci119325
Keywords: immunology, gastric cancer, gastric adenocarcinoma, H. pylori, AIG = autoimmune gastritis, autoimmunity, gastritis
Citation: Hoft SG, Noto CN and DiPaolo RJ (2021) Two Distinct Etiologies of Gastric Cancer: Infection and Autoimmunity. Front. Cell Dev. Biol. 9:752346. doi: 10.3389/fcell.2021.752346
Received: 02 August 2021; Accepted: 12 November 2021;
Published: 26 November 2021.
Edited by:
Sophie Mouillet-Richard, Institut National de la Santé et de la Recherche Médicale (INSERM), FranceReviewed by:
Laurence Vernis, Institut National de la Santé Et de la Recherche Médicale (INSERM), FranceJose Saenz, Washington University in St. Louis, United States
Copyright © 2021 Hoft, Noto and DiPaolo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stella G. Hoft, c3RlbGxhLmhvZnRAaGVhbHRoLnNsdS5lZHU=; Richard J. DiPaolo, cmljaGFyZC5kaXBhb2xvQGhlYWx0aC5zbHUuZWR1
 Stella G. Hoft
Stella G. Hoft Christine N. Noto
Christine N. Noto Richard J. DiPaolo
Richard J. DiPaolo