
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell Dev. Biol., 28 September 2021
Sec. Molecular and Cellular Pathology
Volume 9 - 2021 | https://doi.org/10.3389/fcell.2021.737003
This article is part of the Research TopicFibrosis and Inflammation in Tissue PathophysiologyView all 28 articles
 Hongjian Hou1,2†
Hongjian Hou1,2† Gabriel Komla Adzika1†
Gabriel Komla Adzika1† Qi Wu1
Qi Wu1 Tongtong Ma1
Tongtong Ma1 Yanhong Ma1
Yanhong Ma1 Juan Geng1
Juan Geng1 Mingjin Shi1
Mingjin Shi1 Lu Fu1
Lu Fu1 Ruqayya Rizvi3
Ruqayya Rizvi3 Zheng Gong4
Zheng Gong4 Hong Sun1,3*
Hong Sun1,3*Clinical demographics have demonstrated that postmenopausal women are predisposed to chronic stress-induced cardiomyopathy (CSC) and this has been associated with the decrease of estrogen. Meanwhile, recent studies have implicated unsolved myocardial proinflammatory responses, which are characterized by enormous CD86+ macrophage infiltrations as an underlying disease mechanism expediting the pathological remodeling of the heart during chronic stress. However, we had previously demonstrated that estrogen confers cardioprotection via the modulation of cardiomyocytes β2-adrenoceptors (β2AR)-Gs/Gi pathways during stress to lessen the incidence of stress-induced cardiovascular diseases in premenopausal women. Intriguingly, macrophages express β2AR profoundly as well; as such, we sought to elucidate the possibilities of estrogen modulating β2AR-Gs/Gi pathway to confer cardioprotection during stress via immunomodulation. To do this, ovariectomy (OVX) and sham operations (Sham) were performed on female Sprague-Dawley (SD) rats. Two weeks after OVX, the rats were injected with 40 μg/kg/day of estradiol (E2). Next, on day 36 after OVX, chronic stress was induced by a daily subcutaneous injection of 5 mg/kg/day of isoproterenol (ISO). The effect of E2 on relevant clinical cardiac function indexes (LVSP, LVEDP, + dp/dt and −dp/dt), myocardial architecture (cardiomyocyte diameter and fibrosis), β2AR alterations, and macrophage (CD86+ and CD206+) infiltrations were assessed. In vitro, peritoneal macrophages (PMΦ) were isolated from wild-type and β2AR-knockout female mice. The PMΦ were treated with ISO, E2, and β2AR blocker ICI 118,551 for 24 h, and flow cytometric evaluations were done to assess their phenotypic expression. E2 deficiency permitted the induction of CSC, which was characterized by cardiac dysfunctions, maladaptive myocardial hypertrophy, unresolved proinflammatory responses, and fibrosis. Nonetheless, E2 presence/supplementation during stress averted all the aforementioned adverse effects of chronic stress while preventing excessive depletion of β2AR. Also, we demonstrated that E2 facilitates timely resolution of myocardial proinflammation to permit reparative functions by enhancing the polarization of CD86+ to CD206+ macrophages. However, this adaptive immunomodulation is hampered when β2AR is inhibited. Taken together, the outcomes of this study show that E2 confers cardioprotection to prevent CSC via adaptive immunomodulation of macrophage phenotypes, and β2AR-mediated signaling is crucial for the polarizations of CD86+ to CD206+ macrophages.
Similar to other cardiovascular diseases (CVDs), chronic stress-induced cardiomyopathy (CSC) has been clinically shown to be predominant in males of all age cohorts, while females are mostly predisposed to its occurrence during their menopausal period (Boese et al., 2017; Ndzie Noah et al., 2021). Recent attempts to elucidate the underlying disease mechanisms have revealed crucial roles played by estrogen during stress to sustain good cardiac health in premenopausal women, besides its reproductive functions (Mori et al., 2011; Boese et al., 2017).
Typically, the clinical manifestations of CSC patients are severe left ventricular (LV) diastolic dysfunction (LVDD) and systolic dysfunctions (LVSD) (Medeiros et al., 2014). The adverse structural remodeling includes excessive LV hypertrophy and massive interstitial fibrosis, which results in the stiffening of the myocardia and causes these cardiac dysfunctions. Also, recent studies have demonstrated that unresolved myocardial inflammatory responses characterized by the enormous influx of proinflammatory macrophages exacerbates CSC and aggravates adverse cardiac remodeling (Wilson et al., 2018; Scally et al., 2019).
Under physiological state, inotropic and chronotropic functions of the heart are mediated by β-adrenergic receptors (βARs) via G stimulatory protein (Gs), mainly β1AR and β2AR. However, hyperstimulation of the receptors during chronic stress results in the downregulation of β1AR mostly, while β2AR traffics the stimuli signal via G inhibitory protein (Gi) to prevent cardiac insults (Paur et al., 2012). The homologous desensitization of βARs which results in the downregulation is induced by G protein-coupled receptor kinases 2 (GRK2) phosphorylation and β-arrestin-1 recruitment (Adzika et al., 2019). Nonetheless, it was demonstrated in previous studies that estrogen ameliorates stress-induced cardiac insults by preventing excessive depletion of β2ARs and also facilitating a balance in the Gi/Gs signaling pathways (Cao et al., 2015; Hou et al., 2018). The estrogenic effects of sustaining β2AR activities during stress might be likely due to its inhibitory effects on GRK2 (Abraham et al., 2018). Intriguingly, β2AR are profoundly expressed on macrophages; hence, estrogen may indirectly modulate their activities and possibly their polarizations into proinflammatory (CD86+ macrophages) or anti-inflammatory (CD206+ macrophages) phenotypes in the myocardia. It is hypothesized that the possible exertion of the aforementioned estrogenic immunoregulation might prevent extensive pathological cardiac remodeling during chronic stress by subduing maladaptive myocardial hypertrophy, fibrosis, and proinflammatory responses.
Herein, we sought to explore the cardioprotective mechanisms employed by estrogen via immunomodulation to decrease the incidence of CSC in female rat models, as understanding these mechanisms will provide the basis for further translational research into preventing CSC in postmenopausal women.
The wild-type and β2AR knockout FVB female mice (donated by Professor Daniel Bernstein, Stanford University—United States) and adult female Sprague-Dawley (SD) rats (180–200 g) were used for the experiments (n = 4–8 rats/group). All standard animal house boundary protocols were observed. After ensuring the SD rats were in the same menstrual phase through vaginal mucus examination, bilateral ovariectomy (OVX) and sham surgeries were done as we previously described (Zhang et al., 2021).
As illustrated (Figure 1A), 2 weeks after ovariectomy, the rats were intraperitoneally injected with 40 μg/kg/day of estradiol (E2) (E2758; Sigma, St. Louis, MO, United States) for 31 days, as done previously (Zhang et al., 2021). Olive oils of equivalent amounts were administered as a placebo to the control groups. On day 36 after ovariectomy, chronic stress was induced in rats that were being treated with either E2 or the placebo by subcutaneous injections of isoproterenol (ISO) (160504; Sigma) at 5 mg/kg/day for 10 days, as previously done (Lin et al., 2016; Zhou et al., 2017). Also, the Sham surgery rats had similar ISO and placebo treatments. In total, in vivo experiments included the following six groups; (i) Sham group, (ii) OVX group, (iii) OVX + E2 group, (iv) Sham + ISO group, (v) OVX + ISO group, and (vi) OVX + ISO + E2 group.
The dosage of E2 employed was to mimic its physiological levels in rats, as we had demonstrated previously (Liu et al., 2012; Zhang et al., 2021). Also, rather than the high dosage of ISO used in acute stress models, as done previously (Youssef et al., 2021), a relatively milder dosage was used due to the prolonged duration (10 days) of the catecholamine treatment (Zhang et al., 2021).
After properly sedating rats (n = 7 rats/group), they were fixed in the supine position, and longitudinal incisions (about 2 cm in length) were made in the mid-neck. By using blunt hemostatic forceps, the fascia and aponeurosis were separated to reveal 1–1.5 cm of the right common carotid artery. The distal end of the right common carotid artery was ligated, and the proximal end of the right common carotid artery was clamped with an arterial clamp. Next, an ophthalmic scissor was used to nick the artery (in a V-shaped), a heparin-filled catheter attached to a pressure transducer was carefully inserted into the left ventricle. The left ventricular systolic and end-diastolic pressures and electrocardiography (ECG) were recorded with PowerLab data acquisition system (ADInstruments, North America, COlorado Springs, CO, United States).
Excised hearts (n = 6 hearts/group) were properly washed with prechilled PBS, blotted with filter paper, and fixed in 4% paraformaldehyde for more than 48 h. Next, the heart specimens were embedded in paraffin, sectioned at 4 μm thickness, and preserved for histological assessments.
The myocardial sectionings were deparaffinized before performing Masson’s trichrome (Maxim Biotechnologies, Fuzhou, China), hematoxylin and eosin (H&E), and immunohistochemical (IHC) staining as previously described (Zhang et al., 2021). The trichome staining were done to ascertain the collagen volume fraction (CVF) while H&E staining were done to assess cardiomyocyte diameters and help depict the extent of myocardial hypertrophy. Also, IHC staining with CD68 (Abcam, Cambridge, United Kingdom; ab955), CD86 (Bioss, Woburn, MA, United States; BS-1035R), and CD206 (Abcam; ab8918) was done to assess the extent of myocardial infiltrations of inflammatory cells.
Imaging of all stained sections were done at × 400 magnification (IX 71, Olympus, Tokyo, Japan) and analyzed using ImageJ (1.53a version; National Institute of Health, Bethesda, MD, United States).
Trizol (Invitrogen Co., Carlsbad, CA, United States) was used to extract RNAs from homogenized myocardia (n = 4 hearts/group). After the normalization of RNA concentrations, cDNAs were synthesized using Revertra ace® qPCR rt kit (Toyobo, Osaka, Japan). By using SYBR Green Master Mix (Vazyme Biotech, Nanjing, China), the following gene primers (Sangon Biotech, Shanghai, China) were used to evaluate mRNA expressions; (1) Tumor necrosis factor-alpha (TNF-α), Forward GAAAGCATGATCCGAGATGTG; Reverse: CACGAGCAGGAATGAGAAGAG, (2) transforming growth factor-beta (TGF-β1), Forward: ATGGTGGACCGCA ACAACGC; Reverse: CTGGCACTGCTTCCCGAATGTC, (3) inducible nitric oxide synthase (iNOS), Forward: TCTTGGAGCGAGTTGTGGATTGT; Reverse: TAGGTGAGG GCTTGCCTGAGTG, (4) arginase 1 (Arg-1), Forward: CGTTG ACCTTGTCTTGTTTTGG; Reverse: CTGGTTCTGTTCGGT TTGCTG, (5) glyceraldehyde 3-phosphate dehydrogenase (GAPDH), Forward: TCCTGCACCACCAACTGCTTAG; Reverse: AGTGGCAGTGATGGCATGGACT.
The 2–ΔΔCt analysis method was used to evaluate the relative mRNA levels as described (Gold et al., 2012) and have been graphically presented as fold changes compared with the Sham group.
Proteins were extracted from myocardial apexes (n = 4 hearts/group), treated with reducing agents, denatured at 100°C, and separated by gel electrophoresis as previously described (Hou et al., 2018). Next, the proteins were transferred onto polyvinylidene fluoride (PVDF) membranes, blocked with 1% bovine serum albumin, and incubated in the following primary antibodies at 4°C overnight; ANP (1:1,000, Santa Cruz Biotechnology, Dallas, TX, United States; sc-515701), BNP (1:1,000, Santa Cruz Biotechnology; sc-271185), β2AR (1:1,000, Abcam; ab182136), GAPDH (1:4,000, Proteintech, Manchester, United Kingdom; 10494-1-AP). Visualizations of immunoblots were done with enhanced chemiluminescence (Tanon, Shanghai, China). The protein bands were quantified and evaluated by the relative expressions with their GAPDH.
Peritoneal macrophages (PMΦ) (n ≤ 2 * 106 cells) were harvested from wild-type (WT) and β2AR knockout (β2AR-KO) FVB female mice by using methods previously demonstrated (Ray and Dittel, 2010). In brief, the mice peritoneum were exposed under aseptic conditions. Five to 10 ml of prewarmed (37°C) 3% fetal bovine serum (FBS) were injected into the peritoneal cavity. Cell suspensions were collected after softly massaging for 5 min and centrifuged at 1,500 rpm for 10 min, and the obtained cell pellets were resuspended and cultured with 10% FBS at 37°C and 5% CO2 for 48 h. Next, 24 h in vitro treatments of cultured PMΦ included; ISO (10 μM), E2 (1 nM), and β2AR blocker ICI 118,551 (55 nM) (Figure 1B). These treatments were preceded by E2 pretreatments for 1 h, in groups where the estrogenic effects were to be ascertained.
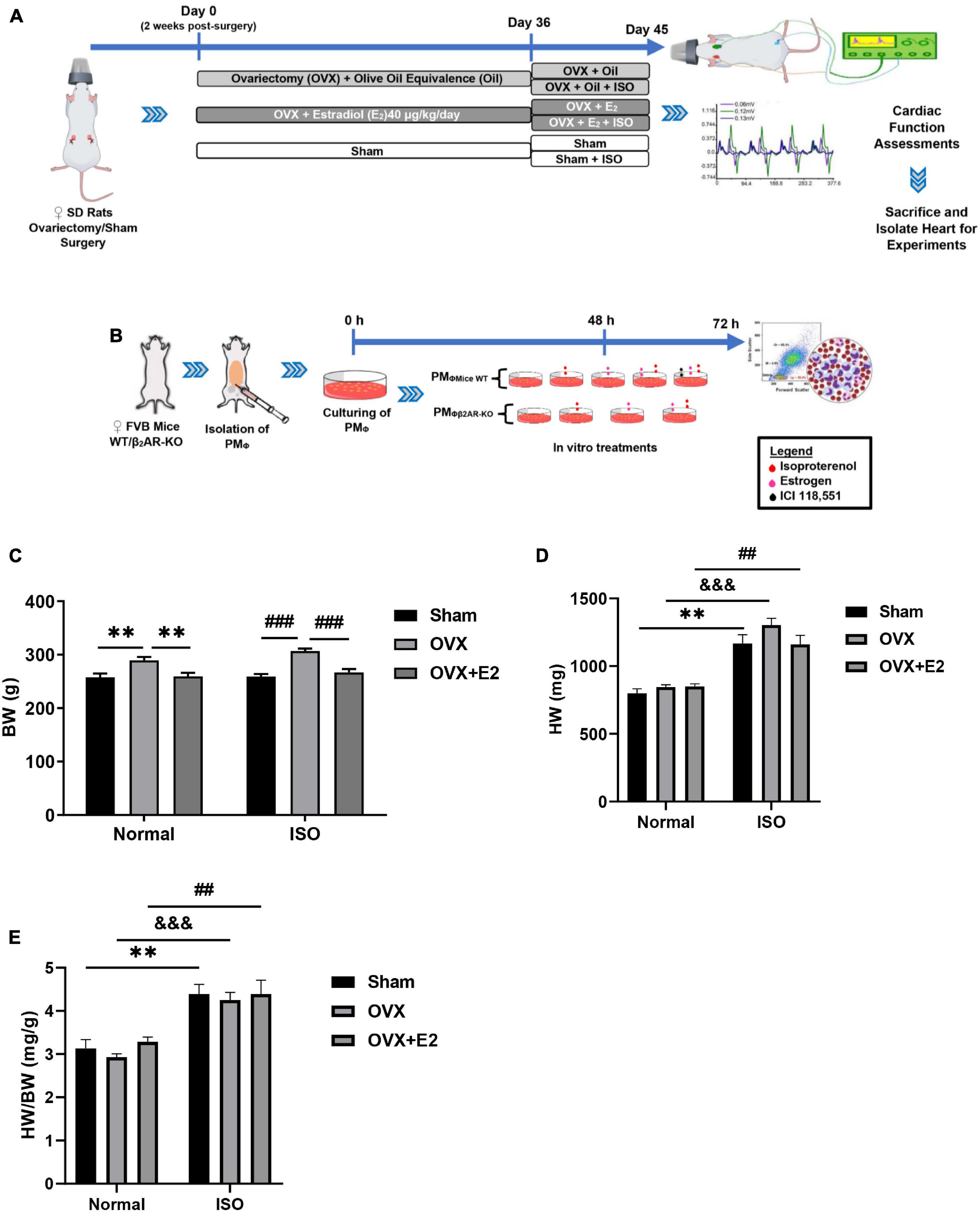
Figure 1. (A,B) Illustration of the experiment timeline for making in vivo and in vitro models, respectively. (C–E) Graphical presentations of morphometric data demonstrate alterations in body weight (BW), heart weight (HW), and HW/BW coefficient during chronic stress and estrogen deficiency (n = 8 rats/group). **p < 0.01; ##p < 0.01 and ###p < 0.001; $$$p < 0.001. Data are presented as mean ± SEM. Data were analyzed using two-way ANOVA, followed by Sidak’s post hoc analysis.
The identification and subtyping of isolated PMΦ after culturing or treatments were done by flow cytometry BD LSR II (BD Biosciences, San Jose, CA, United States). APC anti-F4/80 (123116; BioLegend, San Diego, CA, United States) and FITC anti-CD11b (101206; BioLegend) antibodies were used to identify the macrophages while PerCP anti-CD86 (105028; BioLegend) and PE anti-CD206 (141706; BioLegend) antibodies were used to differentially identify M1 macrophages and M2 macrophages, respectively. Preparations of cultured or treated PMΦ for flow cytometry were done as previously described (Zhu et al., 2017). Acquired data were analyzed with FlowJo software (v10; FlowJo LLC, Oregon, OR, United States).
Statistical analysis was performed with GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, United States). All data were presented as mean ± SEM and compared by two-way ANOVA. p-values < 0.05 were deemed statistically significant.
Analysis of the morphometrics of rats demonstrated that E2-deficient (OVX) rats gained significant body weights (BW). This phenomenon is shown to have been further aggravated by chronic stress (ISO) and is accompanied by increases in heart weights (HW) (Figures 1C,D). However, the supplementation with exogenous E2 (E2Exo) in the OVX + E2 group and endogenous E2 (E2Endo) in the Sham group helped to significantly prevent BW gains and slight decrease HW (without statistical significance on comparing among the stress groups). Furthermore, it is shown that the HW/BW coefficient variation between physiological and stress states is more significant in OVX rats (Figure 1E).
E2 deficiency during chronic stress resulted in decreased heart rates (HR) in OVX rats. The supplementation of E2Exo in OVX rats and the presence of E2Endo in Sham rats prevented a significant decrease in HR during chronic stress (Figure 2A). Furthermore, the cardiac function index; LVSP, LVEDP, the rate of pressure development (+dp/dt), and the rate of pressure development decay (−dp/dt) were assessed to ascertain for any occurring dysfunctionalities. It was demonstrated that E2 deficiency during chronic stress resulted in depressions in LVSP, LVEDP, +dp/dt, and −dp/dt. However, E2Endo and E2Exo prevented significant alterations in these cardiac function indexes during stress (Figures 2B–E).
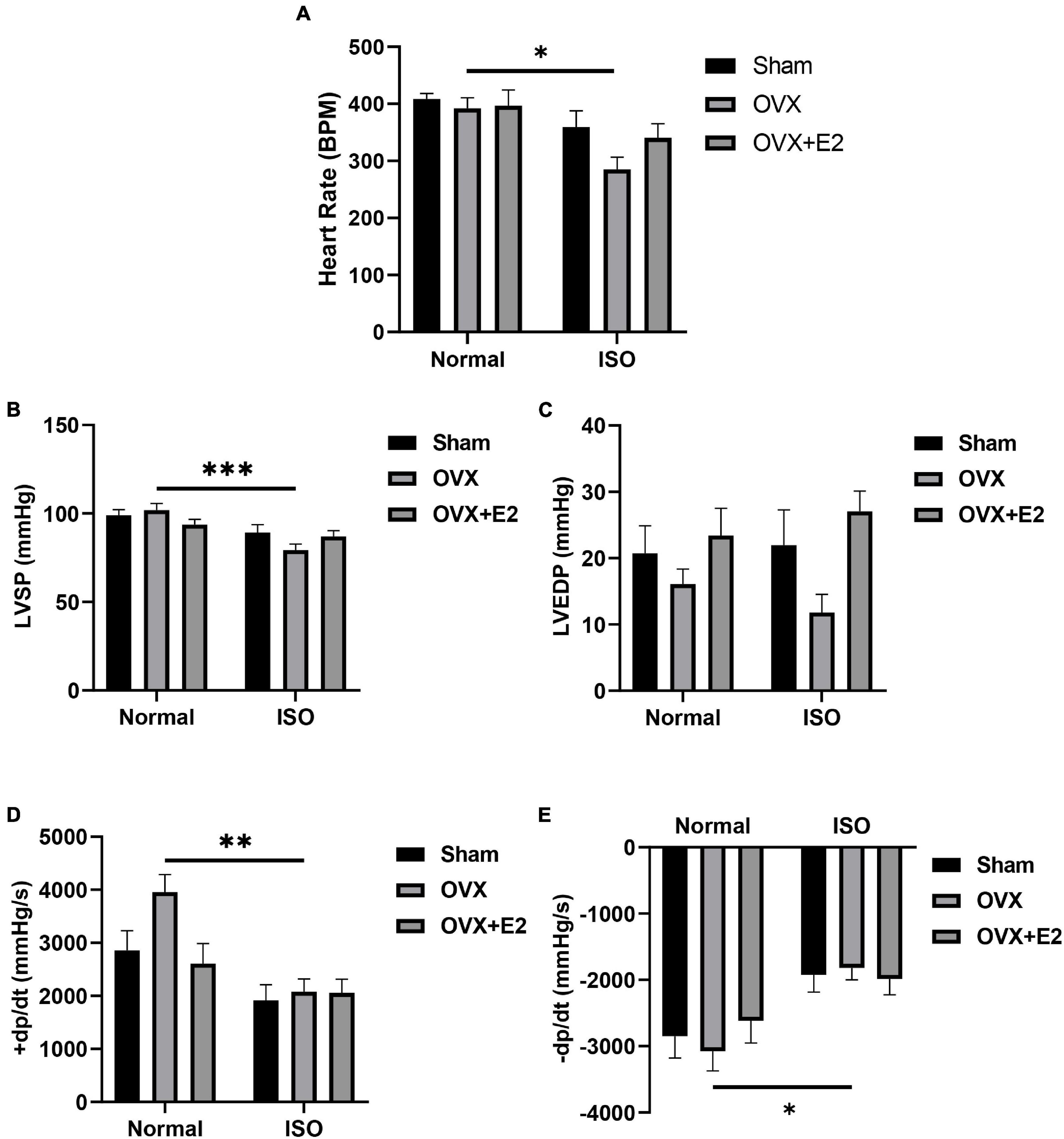
Figure 2. Estrogen deficiency permits cardiac dysfunction chronic stress. (A) Graphical representation of heart rates (HR). (B,C) Left ventricular systolic pressure (LVSP) and left ventricular end-diastolic pressure (LVEDP) recordings depict cardiac dysfunction in OVX + ISO rats. (D,E) Rate of pressure development (+ dp/dt) and the rate of pressure development decay (−dp/dt) further validate cardiac dysfunction in OVX + ISO rats. (n = 7 rats/group). *p < 0.05, **p < 0.01, and ***p < 0.001. Data are presented as mean ± SEM. Data were analyzed using two-way ANOVA and Bonferroni’s multiple comparisons test.
To ascertain the impact of chronic stress on the myocardial architecture, H&E and trichrome staining were done to evaluate the extent of cardiomyocyte hypertrophy and interstitial collagen deposition, respectively. The measurements of cardiomyocyte diameters from H&E-stained myocardia across all groups demonstrated that, under physiological state, the deficiency of E2 does not affect the cell sizes. However, E2 deficiency (in OVX rats) during chronic stress permits excessive cardiomyocyte hypertrophy. Also, the obtained results showed that, while E2 in general inhibited excessive cardiomyocyte hypertrophy during stress in both Sham and OVX + E2 groups, E2Endo (in Sham) exhibited much more potent antihypertrophic effects than E2Exo (in OVX + E2) did (Figures 3A,B). Next, immunoblotting of atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) depicted the maladaptive nature of the resulting cardiomyocyte hypertrophy when E2 is deficient during stress. E2Endo relatively decreased the expressions of both natriuretic peptides; whereas, E2Exo only affected ANP upregulations (Figures 3C–F).
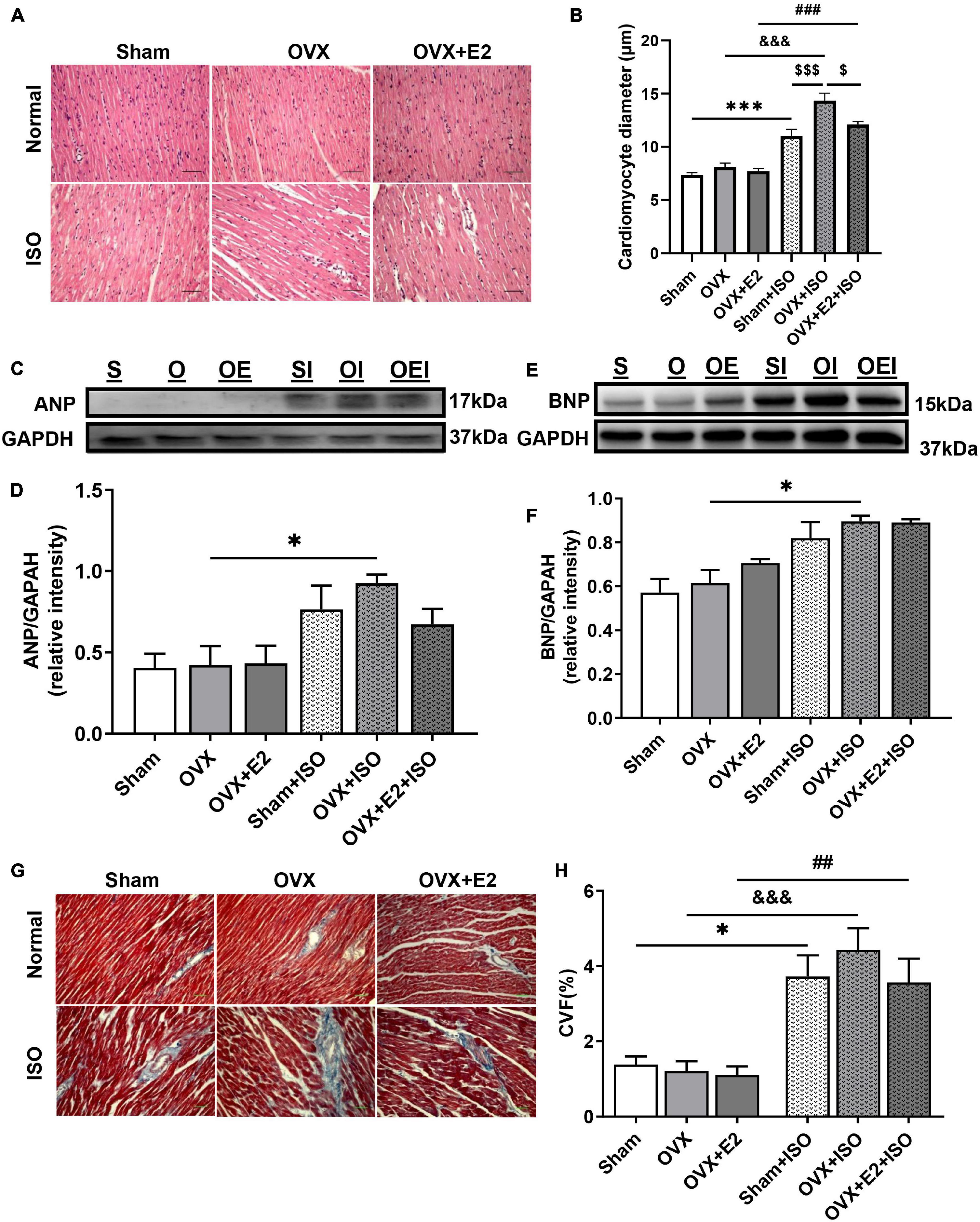
Figure 3. Estrogen deficiency promotes myocardial hypertrophy and fibrosis during chronic stress. (A,B) Representative H&E staining and graphical presentation of measured cardiomyocyte diameters, respectively (n = 10–12 cells/5 field of view/6–8 sections/6 hearts/group). (C–F) Representative immunoblots and graphical presentations of assessed cardiac hypertrophy markers, atrial natriuretic peptide (ANP), and brain natriuretic peptide (BNP) (n = 4 hearts/group). (G,H) Representative Masson’s trichrome staining and graphical presentation of evaluated collagen volume fractions to assess the extent of fibrosis (n = 5–7 field of view/6–8 sections/6 hearts/group). *p < 0.05 and ***p < 0.001; ##p < 0.01 and ###p < 0.001; $p < 0.05 and $$$p < 0.001; &&&p < 0.001. Data are presented as mean ± SEM. Data were analyzed using two-way ANOVA and Bonferroni’s multiple comparisons test.
Assessed CVF demonstrated that myocardial interstitial fibrosis increases during chronic stress in OVX rats; however, the results trend showed that the presence of E2 does ameliorate its severity but without statistical significance on comparing with OVX + E2 + ISO and Sham + ISO groups (Figures 3G,H).
Myocardial inflammation during chronic stress contributes to aggravated cardiac remodeling (Hulsmans et al., 2018). Hence, we assessed the potentials of E2 in exerting adaptive immunoregulation in the myocardia during stress. CD68-positive IHC staining demonstrated that, under physiological state, the amount of macrophages infiltrating the myocardia are slightly elevated when E2 is deficient (in OVX rats). Also, although CD68-positive cell infiltrations were generally increased during chronic stress, significant upregulations only resulted in OVX + ISO rats. The presence of E2Endo and E2Exo in Sham + ISO and OVX + E2 + ISO, respectively, prevented enormous CD68-positive cell infiltration into the myocardia during stress (Figures 4A,B). Furthermore, by using CD86 and CD206 IHC staining, it is shown that majority of the inflammatory cells infiltrating the myocardia during stress when E2 is deficient are CD86-positive (proinflammatory) cells, while CD206-positive (anti-inflammatory) cell infiltrations are significantly hampered. However, the contrast of this phenomenon is demonstrated by E2Endo and E2Exo presence in Sham + ISO and OVX + E2 + ISO, respectively, during stress. The anti-inflammatory cell infiltrations are significantly increased while proinflammatory cell infiltrations were dampened in these groups. Also, it is observed that E2Endo was potent than E2Exo in the adaptive modulation of myocardial inflammatory cell infiltrations (Figures 4C–F). To validate the adaptive immunoregulation exerted by E2, mRNAs of proinflammatory (TNF-α and iNOS) and anti-inflammatory (TGF-β1 and Arg-1) biomarkers were assessed from the myocardia. During chronic stress, E2 deficiency (in OVX rats) permitted upregulations of TNF-α and iNOS while TGF-β1 and Arg-1 expressions were downregulated. Conversely, E2 enhanced the expressions of TGF-β1 and Arg-1 and decreased TNF-α and iNOS levels (Figures 4G–J).
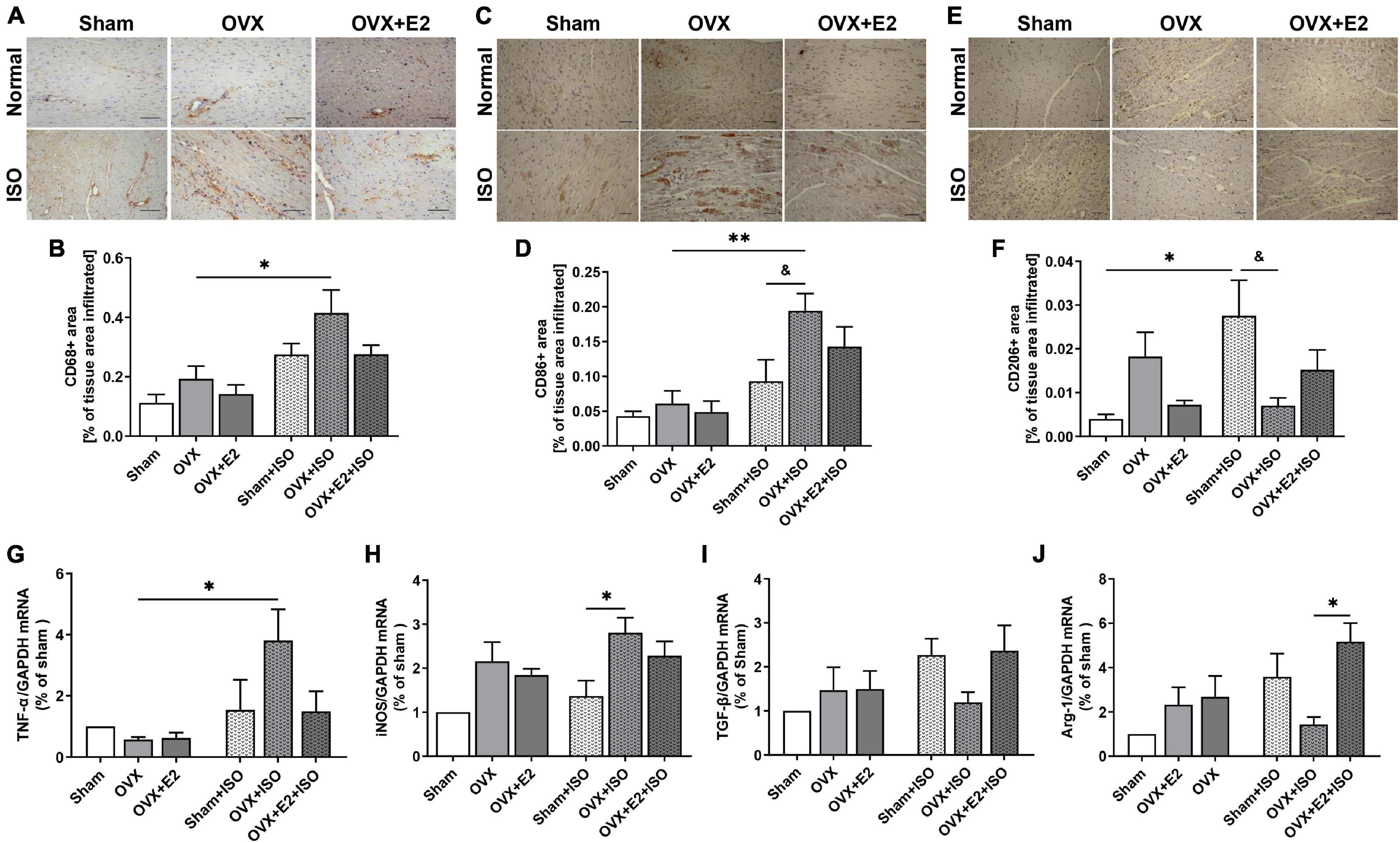
Figure 4. Estrogen attenuates maladaptive myocardial inflammatory responses chronic stress. (A,B) Representative immunohistochemical staining and graphical presentation of CD68-positive cells (whole macrophages) assessed from the myocardia. (C,D) Representative immunohistochemical staining and graphical presentation of CD86-positive cells (proinflammatory phenotype/M1 macrophages) assessed from the myocardia. (E,F) Representative immunohistochemical staining and graphical presentation of CD206-positive cells (anti-inflammatory phenotype/M2 macrophages) assessed from the myocardia (n = 6–8 field of view/6–8 sections/6 hearts/group). (G,H) Graphical presentation of M1 macrophage markers, tumor necrosis factor-alpha (TNF-α), and inducible nitric oxide synthase (iNOS) mRNA expressions assessed by RT-qPCR (n = 4 hearts/group). (I,J) Graphical presentation of M2 macrophage markers, transforming growth factor-beta (TGF-β1), and arginase 1 (Arg-1) mRNA expressions assessed by RT-qPCR (n = 5 hearts). *p < 0.05 and **p < 0.01; &p < 0.05. Data are presented as mean ± SEM. Data were analyzed using two-way ANOVA and Bonferroni’s multiple comparisons test.
In our previous studies (Hou et al., 2018), it was demonstrated that E2 conferred cardioprotective effects via the modulation of β2AR-Gαs/Gαi-mediated signaling cascades during stress. Hence, to elucidate the underlying mechanism employed by E2 to facilitate timely resolutions of myocardial proinflammatory responses, we again investigated the likely involvement of β2AR since they are well expressed in both cardiomyocytes and macrophages. Immunoblotting results showed a significant decrease in β2AR expression in OVX + ISO rats (Figures 5A,B). However, the extent of β2AR downregulations in Sham + ISO and OVX + E2 + ISO was relatively lower than OVX + ISO, which showed statistical significance when compared with OVX. Flowcytometry evaluations of PMΦ isolated from WT and β2AR-KO and treated with ISO (10 μM) and/or E2 (1 nM) along with or without β2AR blocker ICI 118,551 (55 nM), demonstrated that the inhibition or obliteration of β2AR abolished the adaptive immunoregulatory effects exerted by E2 during chronic stress. Typically, it is shown that during stress, E2 enhanced PMΦ polarizations into more CD206+ macrophages (anti-inflammatory phenotype) than CD86+ macrophages (proinflammatory phenotype) when β2ARs are not inhibited. However, obliteration of β2AR activities (by its KO or blocker ICI 118,551) obstructs the initially observed estrogenic phenomenon and consequently causes an increase in M1 macrophage phenotype (Figures 5C,D).

Figure 5. Estrogenic adaptive immunoregulation of macrophage polarization involves modulation of β2AR signaling activities. (A,B) Representative immunoblots and graphical presentation of β2AR expressions evaluated from the myocardia. S, Sham; O, OVX; OE, OVX + E2; SI, Sham + ISO; OI, OVX + ISO; OEI, OVX + E2 + ISO (n = 4 hearts/group). (C,D) Representative flow cytometry plots of gated macrophage phenotypes and graphical presentation of their M1 and M2 expression ratios (n ≤ 1 * 106 cells). The phenotypic populations of macrophages were quantified using FlowJo. *p < 0.05; $$$p < 0.001 vs. CD86+ (ISO + E2); ###p < 0.001 vs. CD206+ (ISO + E2 + ICI118551). Data are presented as mean ± SEM. Data were analyzed using two-way ANOVA, followed by Sidak’s post hoc analysis.
Unresolved myocardial inflammatory responses have been clinically demonstrated as an underlying factor expediting the pathological remodeling of the heart during stress (Mori et al., 2011; Wilson et al., 2018; Scally et al., 2019). The homeostatic balance between cardiac proinflammatory and anti-inflammatory macrophage phenotypes is crucial for resolving myocardial inflammation and proper heart functioning (Mouton et al., 2018). However, clinical studies have shown that the myocardia of CSC patients have massive bias infiltrations of proinflammatory macrophages, which prolongs inflammation without timely resolutions to permit reparative functions of anti-inflammatory macrophages. Hence, in post-stress–induced cardiac injuries, the maladaptive proinflammatory responses in the myocardia drives the pathological remodeling of the heart, which is evident by marked fibrosis (Mori et al., 2011; Mouton et al., 2018).
Herein, we demonstrate the mechanistic roles employed by E2 to protect the heart during chronic stress from an immunoregulatory perspective. Morphometric evaluations revealed significant gains in BW resulting from the deficiency of estrogen in the OVX rats under physiological and chronic stress states. This finding provides supporting evidence that E2 is crucial for efficient lipid metabolism. In fact, previous studies have demonstrated that E2 maintains a healthy lipid profile by upregulating bloodstream levels of high-density lipoprotein (HDL) and lowering low-density lipoprotein receptors (LDL) (Lee et al., 2015; Fu et al., 2019; Ndzie Noah et al., 2021). As such, the deficiency of E2 scaffolded disorders in lipid metabolism that caused weight gain as it permitted increased circulation LDL (bad cholesterol) level which deposited as adipose all over the body as well as in and around vascular tissues and circulatory organs (Lee et al., 2015; Kozakowski et al., 2017). Similar to the previous work of Ren et al. (2003), BW was increased in OVX rats. Also, it was observed that the combination of E2 deficiency and stress increased HW and HW/BW coefficient more in OVX rats. The HW and HW/BW coefficient increases may be due to increased epicardial adipose and cardiomyocyte hypertrophy. Intriguingly, epicardial adipose has been shown to be a reservoir for macrophages which infiltrates the myocardia to hasten maladaptive inflammatory responses (Mori et al., 2011). Therefore, the increased accumulation of epicardial adipose resulting from E2 deficiency predisposes the heart to sustained myocardial inflammation should there be any cardiac insult during stress. Overall, consistent with early findings (Ren et al., 2003; Mori et al., 2011; Michalson et al., 2018), it was demonstrated that E2Endo and its supplementation (E2Exo) prevents excessive weight gains, which ultimately impacts positively on cardiac health.
Clinically, demographics clearly show that normally, females have higher heart rates (HR) and cardiac outputs than males of the same age cohort (Wheatley et al., 2014). In menopause, there is a further increase in HR, which results in short-term arrhythmias (heart palpitations) and are attributed to the loss of E2 and possibly β2AR signaling dysregulation (Carpenter et al., 2021). Interestingly, the contrary was found in this study. The obliteration of E2 via ovariectomy resulted in a slight decrease in HR under normal state; however, chronic stress in these OVX rats caused a significant reduction in HR. The possible explanation for this outcome is that inotropy and chronotropic functions of the heart are mediated by β1AR and β2AR; meanwhile, E2 prevents their dysregulations and substantial depletion during stress (Hou et al., 2018; Ndzie Noah et al., 2021). Therefore, E2 deficiency might have permitted dysfunctionalities and downregulation of the β2ARs during stress, hence the significant decrease in HR. Also, consistent with previous reports, it was found that the cardiac function index; LVSP, LVEDP, +dp/dt, and −dp/dt were unaffected by E2 deficiency under physiological state (Mori et al., 2011; Ribeiro et al., 2013). Even so, chronic stimulation of βARs by ISO during E2 deficiency demonstrated overt cardiac dysfunctionalities. In contrast, it was demonstrated that E2Endo and E2Exo in the Sham + ISO and OVX + E2 + ISO rats, respectively, ameliorated these heart dysfunctions to sustain cardiac output during stress.
Further investigations sought to characterize the impact of chronic stress on the myocardial structure during E2 deficiency. It was observed that cardiomyocyte diameters generally increased during stress; however, the E2 deficiency permitted maladaptive hypertrophy, which distorted the typical myocardial architecture. This was further proven by the significant upregulations of ANP and BNP in the hearts of OVX rats during chronic stress. Nevertheless, E2Endo (in the Sham rats) showed much potency at minimizing the upregulations of both ANP and BNP during stress, while E2Exo (in the OVX + E2 rats) was unable to downregulate the latter substantially. In conformity with our findings, Goncalves et al. (2018) and others had early demonstrated that E2 exerts antihypertrophic effects via GPER (Goldstein et al., 2004). Also, the discrepancies observed between the antihypertrophic effect of E2Endo and E2Exo might have occurred because other ovarian secretions such as vascular endothelial growth factor (VEGF) may complement the efforts of E2 in preventing maladaptive cardiomyocyte hypertrophy (Cai et al., 2015). Besides, as suggested by Zhang et al. (2021), unlike the E2Exo treatment dose, which remained constant during CSC modeling, the levels of E2Endo are altered due to the estrous cycle in the Sham and could have also contributed to the observed differences in the antihypertrophic effect of E2. In addition, it was found that obliteration of E2 in OVX rats permitted induction of massive interstitial fibrosis; nevertheless, its presence/restoration ameliorated this adverse outcome. We showed that comparatively, E2Endo in the Sham and its supplementation (E2Exo) lessened the extent of fibrosis, just as demonstrated earlier (Mori et al., 2011; Michalson et al., 2018).
The homeostatic balance between proinflammatory and anti-inflammatory macrophages in the myocardia during steady state is crucial for cardiac function, as is the timely trafficking of either of them during injury/cell clearance or reparative process, respectively, essential for preventing adverse heart remodeling (Lavine et al., 2014; Mouton et al., 2018). However, as demonstrated from the postmortem examination of the hearts from CSC patients, proinflammatory macrophages were abundant in the myocardia and were shown to have exacerbated myocardial proinflammatory responses, which may have resulted from stress-induced cardiac insults. The observed biased infiltration of CD86+ macrophages (proinflammatory) hastened the pathological cardiac remodeling as autopsied hearts had marked fibrosis (Wilson et al., 2018; Scally et al., 2019). Similar to these clinical findings, it has been shown in rats that stress causes augmentation of myocardial CD86+ macrophage infiltrations, and the phenomena are worsened by E2 deficiency (Mori et al., 2011). Following up on these previous studies, consistent findings were made. CD68-positive cell infiltration into the myocardia were increased only under stress conditions; however, E2 deficiency augmented their infiltration significantly. Nonetheless, E2Endo and its supplementation (E2Exo) to the rats during stress minimized CD68-positive cell infiltration. Assessing the phenotypic ratios with CD86 and CD206 immunostaining revealed the majority of the CD68-positive cells infiltrating the myocardia when E2 is deficient during stress are CD86-positive cells, while CD206-positive cells are less present. Nevertheless, E2Endo and E2Exo reversed these phenomena by enhancing anti-inflammatory responses in the hearts during stress via increasing CD206+ macrophage presence, as similarly reported previously (Xing et al., 2009; Bolego et al., 2013). Validations of the aforementioned findings were done by assessing the mRNA expressions of proinflammatory (TNF-α and iNOS) and anti-inflammatory macrophage (TGF-β and Arg-1) markers from the myocardia of all experimental groups. Similar to the observations of the histological evaluations, TNF-α and iNOS were upregulated during stress and were further elevated significantly when E2 is deficient. Also, TGF-β and Arg-1 mRNA expressions were downregulated in the myocardia due to E2 deficiency. Conversely, E2Endo exerted anti-inflammatory effects by enhancing TGF-β and Arg-1 while decreasing TNF-α and iNOS mRNA expressions during stress. Although E2Exo upregulated TGF-β and Arg-1 and inhibited TNF-α similarly to E2Endo, it was not as potent as E2Endo in downregulating iNOS. The possible explanation of the phenomenon is that ovarian secretions of progesterone might have complimented the inhibitory effects of E2Endo, as it has been reported that besides E2, progesterone decreases iNOS levels in non-cardiac tissue (Menzies et al., 2011). However, progesterone is obliterated in OVX + E2 + ISO rats; hence, it might account for iNOS being significantly downregulated in Sham + ISO than OVX + E2 + ISO. Nonetheless, the estrogenic anti-inflammatory effects demonstrated here have been similarly reported by Villa et al. (2015) and others (Chen et al., 2021).
Similar to cardiomyocytes, macrophages have profound expressions of β2AR and estrogen receptors (ERs), and the cardioprotective effects conferred by E2 have been demonstrated to mostly involved the synergy of ERs and β2AR signaling cascades (Kang et al., 2012; Hou et al., 2018; Machuki et al., 2019; Ndzie Noah et al., 2021). Hence, to elucidate the immunoregulatory mechanisms employed by E2 to facilitate more CD206+ macrophage polarizations to accelerate the resolution of myocardial inflammation during stress, we investigated the possible involvement of β2AR signaling modulation by E2-induced cascades. In conformity with our initial speculations, β2AR expressions from apical myocardia (constituting cardiomyocytes and infiltrated macrophages) were found to be significantly depleted during chronic stress due to E2 deficiency, as the presence of E2Endo in the Sham and the supplementation of E2Exo in OVX rats showed a minimal reduction in the expression of the receptor under the same stress condition. Further investigations of β2AR involvement deployed the isolations of PMΦ from female WT and β2AR-KO mice as well as the use of β2AR blocker ICI 118,551 to ascertain if E2 induced any variations in the phenotypic ratios of the macrophages during stress was affected by impeding β2AR signaling. We report that the estrogenic signaling facilitates adaptive immunoregulation by ensuring CD206+ macrophage polarizations to timely resolve inflammation as reported by others (Keselman et al., 2017). However, for the first time, we show the underlying mechanism involves interplays of E2, ERs and β2AR signaling during stress. Flow cytometric evaluations show that E2 treatments during stress increased CD206+ macrophage polarizations against CD86+ macrophages; however, the deletion/inhibition of β2AR impaired this phenomenon. These observations are possibly because the bioavailability of nitric oxide (NO), which is produced via β2AR-Gai-PI3K-Akt–mediated signaling cascade, is crucial for the polarization of macrophages from proinflammatory to the anti-inflammatory phenotype (De Nigris and Prattichizzo, 2021). As such, blockade of β2AR signaling disrupts NO bioavailability and abolishes this adaptive immunoregulatory mechanism. Also, E2 had been shown previously to exert these anti-inflammatory effects primarily via estrogen receptor alpha (ERα) (Bolego et al., 2013; Campbell et al., 2014), and although we have demonstrated here the essential involvement of β2AR to facilitate the polarizations of CD206+ macrophages, there are apparent interplays among E2, ERs, and β2AR to ensure this immunomodulation during stress. Intriguingly, E2 and ER activities downregulate GRK2, which otherwise would have induced the homologous desensitization and downregulation of β2AR during stress (Abraham et al., 2018; Arcones et al., 2021). Therefore, E2 and ERs indirectly sustain the bioavailability of NO via β2AR-Gai-PI3K-Akt signaling by preventing dysregulation of the receptor during stress and enhancing the β2AR-mediated CD206+ macrophage polarization.
Taken together, the findings from this study demonstrate the immunoregulatory mechanisms employed by E2 to confer cardioprotection and lower the incidence of CSC in premenopausal women as compared with postmenopausal women and males of all age cohorts. E2 exerts this immunoregulatory myocardia protection to prevent pathological cardiac remodeling during stress by ensuring the timely resolution of myocardial proinflammatory responses and enhancing reparative functions of CD206+ macrophage. More importantly, we demonstrate here that the adaptive modulation of macrophage phenotypes by E2 during stress requires the mediation of β2AR signaling. The classical interplays among E2, ERs, and β2AR discussed by Ndzie Noah et al. (2021) are also shown here, as E2 and ER activities are in turn required to prevent β2AR dysregulations and dysfunctionalities during stress. From a therapeutic standpoint, the findings from this study reechoes the essence of E2 replacement therapy (E2RT) in postmenopausal women, as it reduces the incidence of CSC. However, it is recommended that E2RT is initiated within 5–6 years after menopause so as to explore its therapeutic benefits fully while circumventing the adverse outcomes reported by the Women’s Health Initiative from their randomized controlled trial (Rossouw et al., 2002; Michalson et al., 2018; Ndzie Noah et al., 2021). Finally, it is deemed necessary to point out the limitations of this study due to its clinical significance. β1ARs are essential for myocardial functions and might play other immunologic roles facilitated by E2, but they have not been elucidated previously nor in this study. Also, in some instances (Figures 3E,F), (Figures 4C–J), it is shown that E2Exo did not confer anti-inflammatory effects as E2Endo did. However, the fact that other ovarian secretions such as progesterone can complement the anti-inflammatory effects of E2Endo but are obliterated by ovariectomy in the E2Exo treatment group might explain the observed differences. The estrous cycle in the Shams causing alterations in E2Endo levels while E2Exo treatment dosage used remained constant might also account for the shown slight variations in E2Endo and E2Exo effects. Therefore, we stand with Zhang et al. (2021) in suggesting that E2RT should be given at dosages that mimic the concentrations of the estrous cycle to eliminate the observed variations in its cardioprotection efficacy. This will enhance the exploitation of the therapeutic potentials of E2RT in attenuation/prevention of CSC via immunomodulation in postmenopausal women.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The animal study was reviewed and approved by the Experimental Animal Centre of Xuzhou Medical University and the Animal Ethics Committee of Xuzhou Medical University (permit no: xz11-12540).
HH conceived the experiment idea. HS, HH, GKA, and QW designed the experiments. HH and GKA isolated and cultured PMϕ. HH, TM, and YM made animal models. HH, JG, MS, and LF performed cardiac function and histological assessments. HH, GKA, QW, and HS analyzed and interpreted the results. Based on the contributions of all authors, HH drafted the initial manuscript and GKA revised it entirely. HH, GKA, QW, TM, YM, JG, MS, LF, RR, and ZG proofread and approved the manuscript in its current form.
This study was funded by the National Natural Science Foundation of China (grant nos. 81370329 and 81461138036), the Postgraduate Research and Practice Innovation Program of Jiangsu Province (grant no. KYCX18-2167), the Scientific Research Start-up Project of Shangqiu normal University (grant no. 7001/700216), and the Natural Science Foundation of The Jiangsu Higher Education Institutes of China (grant no. 17KJB180016).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abraham, A. D., Schattauer, S. S., and Reichard, K. L. (2018). Estrogen regulation of GRK2 inactivates kappa opioid receptor signaling mediating analgesia, but not aversion. J. Neurosci. 38, 8031–8043. doi: 10.1523/JNEUROSCI.0653-18.2018
Adzika, G. K., Machuki, J. O., Shang, W., Hou, H., Ma, T., Wu, L., et al. (2019). Pathological cardiac hypertrophy: the synergy of adenylyl cyclases inhibition in cardiac and immune cells during chronic catecholamine stress. J. Mol. Med. 97, 897–907. doi: 10.1007/s00109-019-01790-0
Arcones, A. C., Martínez-Cignoni, M. R., Vila-Bedmar, R., Yáñez, C., and Lladó, I. (2021). Cardiac GRK2 protein levels show sexual dimorphism during aging and are regulated by ovarian hormones. Cells 10:673. doi: 10.3390/cells10030673
Boese, A. C., Kim, S. C., Yin, K. J., Lee, J. P., and Hamblin, M. H. (2017). Sex differences in vascular physiology and pathophysiology: estrogen and androgen signaling in health and disease. Am. J. Physiol. Heart Circ. Physiol. 313, H524–H545. doi: 10.1152/ajpheart.00217.2016
Bolego, C., Cignarella, A., Staels, B., and Chinetti-Gbaguidi, G. (2013). Macrophage function and polarization in cardiovascular disease: a role of estrogen signaling? Arterioscler. Thromb. Vasc. Biol. 33, 1127–1134. doi: 10.1161/ATVBAHA.113.301328
Cai, B., Tan, X., Zhang, Y., Li, X., Wang, X., Zhu, J., et al. (2015). Mesenchymal stem cells and cardiomyocytes interplay to prevent myocardial hypertrophy. Stem Cells Transl. Med. 4, 1425–1435. doi: 10.5966/sctm.2015-0032
Campbell, L., Emmerson, E., Williams, H., Saville, C. R., Krust, A., Chambon, P., et al. (2014). Estrogen receptor-alpha promotes alternative macrophage activation during cutaneous repair. J. Invest. Dermatol. 134, 2447–2457. doi: 10.1038/jid.2014.175
Cao, X., Zhou, C., Chong, J., Fu, L., Zhang, L., Sun, D., et al. (2015). Estrogen resisted stress-induced cardiomyopathy through increasing the activity of β2AR-Gαs signal pathway in female rats. Int. J. Cardiol. 187, 377–386. doi: 10.1016/j.ijcard.2015.02.113
Carpenter, J. S., Sheng, Y., Elomba, C. D., Alwine, J. S., Yue, M., Pike, C. A., et al. (2021). A systematic review of palpitations prevalence by menopausal status. Curr. Obstet. Gynecol. Rep. 10, 7–13. doi: 10.1007/s13669-020-00302-z
Chen, Q., Qi, X., Zhang, W., Zhang, Y., Bi, Y., Meng, Q., et al. (2021). Catalpol inhibits macrophage polarization and prevents postmenopausal atherosclerosis through regulating estrogen receptor alpha. Front. Pharmacol. 12:655081. doi: 10.3389/fphar.2021.655081
De Nigris, V., and Prattichizzo, F. (2021). DPP-4 inhibitors have different effects on endothelial low-grade inflammation and on the M1-M2 macrophage polarization under hyperglycemic conditions. Diabetes Metab. Syndr. Obes. 14, 1519–1531. doi: 10.2147/DMSO.S302621
Fu, W., Gao, X. P., Zhang, S., Dai, Y. P., Zou, W. J., and Yue, L. M. (2019). 17β-estradiol inhibits PCSK9-mediated LDLR degradation through GPER/PLC activation in HepG2 Cells. Front. Endocrinol. 10:930. doi: 10.3389/fendo.2019.00930
Gold, J. I., Gao, E., Shang, X., Premont, R. T., and Koch, W. J. (2012). Determining the absolute requirement of G protein-coupled receptor kinase 5 for pathological cardiac hypertrophy: short communication. Circ. Res. 111, 1048–1053. doi: 10.1161/CIRCRESAHA.112.273367
Goldstein, J., Sites, C. K., and Toth, M. J. (2004). Progesterone stimulates cardiac muscle protein synthesis via receptor-dependent pathway. Fertil. Steril. 82, 430–436. doi: 10.1016/j.fertnstert.2004.03.018
Goncalves, G. K., Scalzo, S., Alves, A. P., Agero, U., Guatimosim, S., and Reis, A. M. (2018). Neonatal cardiomyocyte hypertrophy induced by endothelin-1 is blocked by estradiol acting on GPER. Am. J. Physiol. Cell Physiol. 314, C310–C322. doi: 10.1152/ajpcell.00060.2017
Hou, H., Zhao, Z., Machuki, J. O., Zhang, L., Zhang, Y., Fu, L., et al. (2018). Estrogen deficiency compromised the β(2)AR-Gs/Gi coupling: implications for arrhythmia and cardiac injury. Pflugers Arch. 470, 559–570. doi: 10.1007/s00424-017-2098-4
Hulsmans, M., Sager, H. B., Roh, J. D., and Valero-Muñoz, M. (2018). Cardiac macrophages promote diastolic dysfunction. J. Exp. Med. 215, 423–440. doi: 10.1084/jem.20171274
Kang, S., Liu, Y., Sun, D., Zhou, C., Liu, A., Xu, C., et al. (2012). Chronic activation of the G protein-coupled receptor 30 with agonist G-1 attenuates heart failure. PLoS One 7:e48185. doi: 10.1371/journal.pone.0048185
Keselman, A., Fang, X., and White, P. B. (2017). Estrogen signaling contributes to sex differences in macrophage polarization during asthma. J. Immunol. 199, 1573–1583. doi: 10.4049/jimmunol.1601975
Kozakowski, J., Gietka-Czernel, M., Leszczyńska, D., and Majos, A. (2017). Obesity in menopause – our negligence or an unfortunate inevitability? Prz. Menopauzalny 16, 61–65. doi: 10.5114/pm.2017.68594
Lavine, K. J., Epelman, S., Uchida, K., Weber, K. J., Nichols, C. G., Schilling, J. D., et al. (2014). Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc. Natl. Acad. Sci. U.S.A. 111, 16029–16034. doi: 10.1073/pnas.1406508111
Lee, J. Y., Hyun, H. S., Park, H. G., Seo, J. H., Lee, E. Y., Lee, J. S., et al. (2015). Effects of hormone therapy on serum lipid levels in postmenopausal Korean women. J. Menopausal Med. 21, 104–111. doi: 10.6118/jmm.2015.21.2.104
Lin, Y., Zhang, X., Xiao, W., Li, B., Wang, J., Jin, L., et al. (2016). Endoplasmic reticulum stress is involved in DFMO attenuating isoproterenol-induced cardiac hypertrophy in rats. Cell. Physiol. Biochem. 38, 1553–1562. doi: 10.1159/000443096
Liu, A., Gao, L., Kang, S., Liu, Y., Xu, C., Sun, H., et al. (2012). Testosterone enhances estradiol’s cardioprotection in ovariectomized rats. J. Endocrinol. 212, 61–69. doi: 10.1530/JOE-11-0181
Machuki, J. O., Zhang, H. Y., Geng, J., Fu, L., Adzika, G. K., Wu, L., et al. (2019). Estrogen regulation of cardiac cAMP-L-type Ca(2+) channel pathway modulates sex differences in basal contraction and responses to β(2)AR-mediated stress in left ventricular apical myocytes. Cell Commun. Signal. 17:34. doi: 10.1186/s12964-019-0346-2
Medeiros, K., O’Connor, M. J., Baicu, C. F., Fitzgibbons, T. P., Shaw, P., Tighe, D. A., et al. (2014). Systolic and diastolic mechanics in stress cardiomyopathy. Circulation 129, 1659–1667. doi: 10.1161/CIRCULATIONAHA.113.002781
Menzies, F. M., Henriquez, F. L., Alexander, J., and Roberts, C. W. (2011). Selective inhibition and augmentation of alternative macrophage activation by progesterone. Immunology 134, 281–291. doi: 10.1111/j.1365-2567.2011.03488.x
Michalson, K. T., Groban, L., Howard, T. D., Shively, C. A., Sophonsritsuk, A., Appt, S. E., et al. (2018). Estradiol treatment initiated early after ovariectomy regulates myocardial gene expression and inhibits diastolic dysfunction in female cynomolgus monkeys: potential roles for calcium homeostasis and extracellular matrix remodeling. J. Am. Heart Assoc. 7:e009769. doi: 10.1161/JAHA.118.009769
Mori, T., Kai, H., Kajimoto, H., Koga, M., Kudo, H., Takayama, N., et al. (2011). Enhanced cardiac inflammation and fibrosis in ovariectomized hypertensive rats: a possible mechanism of diastolic dysfunction in postmenopausal women. Hypertens. Res. 34, 496–502. doi: 10.1038/hr.2010.261
Mouton, A. J., DeLeon-Pennell, K. Y., Rivera Gonzalez, O. J., Flynn, E. R., Freeman, T. C., Saucerman, J. J., et al. (2018). Mapping macrophage polarization over the myocardial infarction time continuum. Basic Res. Cardiol. 113:26. doi: 10.1007/s00395-018-0686-x
Ndzie Noah, M. L., Adzika, G. K., Mprah, R., Adekunle, A. O., Adu-Amankwaah, J., and Sun, H. (2021). Sex–gender disparities in cardiovascular diseases: the effects of estrogen on eNOS, lipid profile, and NFATs during catecholamine stress. Front. Cardiovasc. Med. 8:639946. doi: 10.3389/fcvm.2021.639946
Paur, H., Wright, P. T., Sikkel, M. B., Tranter, M. H., Mansfield, C., O’Gara, P., et al. (2012). High levels of circulating epinephrine trigger apical cardiodepression in a β2-adrenergic receptor/Gi-dependent manner: a new model of Takotsubo cardiomyopathy. Circulation 126, 697–706. doi: 10.1161/CIRCULATIONAHA.112.111591
Ray, A., and Dittel, B. N. (2010). Isolation of mouse peritoneal cavity cells. J. Vis. Exp. 35:1488. doi: 10.3791/1488
Ren, J., Hintz, K. K., Roughead, Z. K., Duan, J., Colligan, P. B., Ren, B. H., et al. (2003). Impact of estrogen replacement on ventricular myocyte contractile function and protein kinase B/Akt activation. Am. J. Physiol. Heart Circ. Physiol. 284, H1800–H1807. doi: 10.1152/ajpheart.00866.2002
Ribeiro, R. F. Jr., Potratz, F. F., Pavan, B. M., Forechi, L., Lima, F. L., Fiorim, J., et al. (2013). Carvedilol prevents ovariectomy-induced myocardial contractile dysfunction in female rat. PLoS One 8:e53226. doi: 10.1371/journal.pone.0053226
Rossouw, J. E., Anderson, G. L., Prentice, R. L., LaCroix, A. Z., Kooperberg, C., Stefanick, M. L., et al. (2002). Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s health initiative randomized controlled trial. JAMA 288, 321–333. doi: 10.1001/jama.288.3.321
Scally, C., Abbas, H., Ahearn, T., Srinivasan, J., Mezincescu, A., Rudd, A., et al. (2019). Myocardial and systemic inflammation in acute stress-induced (Takotsubo) cardiomyopathy. Circulation 139, 1581–1592. doi: 10.1161/CIRCULATIONAHA.118.037975
Villa, A., Rizzi, N., Vegeto, E., Ciana, P., and Maggi, A. (2015). Estrogen accelerates the resolution of inflammation in macrophagic cells. Sci. Rep. 5:15224. doi: 10.1038/srep15224
Wheatley, C. M., Snyder, E. M., Johnson, B. D., and Olson, T. P. (2014). Sex differences in cardiovascular function during submaximal exercise in humans. Springerplus 3:445. doi: 10.1186/2193-1801-3-445
Wilson, H. M., Cheyne, L., Brown, P. A. J., Kerr, K., Hannah, A., Srinivasan, J., et al. (2018). Characterization of the myocardial inflammatory response in acute stress-induced (Takotsubo) cardiomyopathy. JACC Basic Transl. Sci. 3, 766–778. doi: 10.1016/j.jacbts.2018.08.006
Xing, D., Nozell, S., Chen, Y. F., Hage, F., and Oparil, S. (2009). Estrogen and mechanisms of vascular protection. Arterioscler. Thromb. Vasc. Biol. 29, 289–295. doi: 10.1161/ATVBAHA.108.182279
Youssef, M. E., El-Mas, M. M., Abdelrazek, H. M., and El-Azab, M. F. (2021). α7-nAChRs-mediated therapeutic angiogenesis accounts for the advantageous effect of low nicotine doses against myocardial infarction in rats. Eur. J. Pharmacol. 898:173996. doi: 10.1016/j.ejphar.2021.173996
Zhang, L., Li, C., Yang, L., Adzika, G. K., Machuki, J. O. A., Shi, M., et al. (2021). Estrogen protects vasomotor functions in rats during catecholamine stress. Front. Cardiovasc. Med. 8:679240. doi: 10.3389/fcvm.2021.679240
Zhou, R., Ma, P., Xiong, A., Xu, Y., Wang, Y., and Xu, Q. (2017). Protective effects of low-dose rosuvastatin on isoproterenol-induced chronic heart failure in rats by regulation of DDAH-ADMA-NO pathway. Cardiovasc. Ther. 35:e12241. doi: 10.1111/1755-5922.12241
Keywords: chronic stress-induced cardiomyopathy, myocardial inflammation, estrogen, β2-adrenoceptors, macrophage polarization
Citation: Hou H, Adzika GK, Wu Q, Ma T, Ma Y, Geng J, Shi M, Fu L, Rizvi R, Gong Z and Sun H (2021) Estrogen Attenuates Chronic Stress-Induced Cardiomyopathy by Adaptively Regulating Macrophage Polarizations via β2-Adrenergic Receptor Modulation. Front. Cell Dev. Biol. 9:737003. doi: 10.3389/fcell.2021.737003
Received: 06 July 2021; Accepted: 31 August 2021;
Published: 28 September 2021.
Edited by:
Susanne Sattler, Imperial College London, United KingdomReviewed by:
Mahmoud El-Mas, Alexandria University, EgyptCopyright © 2021 Hou, Adzika, Wu, Ma, Ma, Geng, Shi, Fu, Rizvi, Gong and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Sun, c3VuaEB4emhtdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.