
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Cell Dev. Biol. , 20 September 2021
Sec. Cellular Biochemistry
Volume 9 - 2021 | https://doi.org/10.3389/fcell.2021.733627
This article is part of the Research Topic Biology and Pharmacological Effects of Extracellular Vesicles in Cancer View all 16 articles
Primary tumors selectively modify the microenvironment of distant organs such as the lung, liver, brain, bone marrow, and lymph nodes to facilitate metastasis. This supportive metastatic microenvironment in distant organs was termed the pre-metastatic niche (PMN) that is characterized by increased vascular permeability, extracellular matrix remodeling, bone marrow-derived cells recruitment, angiogenesis, and immunosuppression. Extracellular vesicles (EVs) are a group of cell-derived membranous structures that carry various functional molecules. EVs play a critical role in PMN formation by delivering their cargos to recipient cells in target organs. We provide an overview of the characteristics of the PMN in different organs promoted by cancer EVs and the underlying mechanisms in this review.
Primary tumors selectively modify the microenvironment of distant organs before metastasis (Hiratsuka et al., 2002; Kaplan et al., 2005). This supportive metastatic microenvironment in distant organs was first termed the pre-metastatic niche (PMN) by Kaplan et al. (2005). In the last decade, PMN induced by various cancers has been identified in the lung (Hiratsuka et al., 2002; Hoshino et al., 2015; Liu et al., 2016; Tyagi et al., 2021), liver (Hoshino et al., 2015; Zhang and Wang, 2015; Houg and Bijlsma, 2018; Sun et al., 2021), brain (You et al., 2020), bone (Kaplan et al., 2007; Xu et al., 2017), and other organs. The PMN is characterized by increased vascular permeability (Gupta et al., 2007; Huang et al., 2009; Zhou et al., 2014), a modified extracellular matrix (ECM) (Aguado et al., 2016; Kim et al., 2019; Mohan et al., 2020), recruited bone marrow-derived cells (BMDCs) (Kitamura et al., 2015; Wang et al., 2019), and immunosuppression (Chen et al., 2011; Tacke et al., 2012; Giles et al., 2016) in the future metastatic organs.
Extracellular vesicles (EVs) are a heterogeneous group of nano-sized membranous structures that are released by almost all cells into extracellular spaces and have many different physiological and pathophysiological functions (Raposo and Stoorvogel, 2013; Colombo et al., 2014). Different EV types, including exosomes, microvesicles, apoptotic bodies, oncosomes, and megasomes have been characterized on the basis of their biogenesis pathways and sizes. Among them, exosomes and microvesicles are the most intensively studied types. Exosomes have an endocytic origin and form by the fusion between multivesicular bodies and the plasma membrane, whereas microvesicles are generated by plasma membrane shedding (van Niel et al., 2018). However, it is difficult to classify EVs according to their biogenic origin once they are secreted into the extracellular space. Therefore, size-based or density-based nomenclature is recommended by the International Society of Extracellular Vesicles (Thery et al., 2018). EVs contain various functional molecules (proteins, mRNAs, miRNAs, long non-coding RNAs, and double stranded DNA, etc.) that can be trafficked between cells as a means of intercellular communication at both paracrine and systemic levels (Valadi et al., 2007; Balaj et al., 2011; Choi et al., 2013; Thakur et al., 2014). EV cargos are protected by the membrane during the delivery process, which is critical for the communication between primary tumors and distant organs. EVs can be internalized by recipient cells via different mechanisms, including phagocytosis, macropinocytosis, endocytosis, and direct membrane fusion (van Niel et al., 2018). In addition, ligands on EV membrane can interact with receptors on the recipient cell surface and elicit biological responses directly.
In this mini-review, we provide an overview of the characteristics of the PMN in different organs promoted by cancer EVs. The terms, exosomes, microvesicles, or EVs were used to describe their roles in PMN formation to ensure consistency with the original articles.
The characteristics of the PMN formed in various organs by EVs are summarized in Figure 1 and Table 1. The lung is the most commonly involved organ, followed by the liver, bone, brain, and lymph nodes (LNs). As shown in Figure 1, EVs produced by tumor and stromal cells enter the circulation and arrive at distant organs, where they trigger a sequence of local changes including increased vascular permeability, ECM remodeling, BMDC recruitment, angiogenesis, and immunosuppression.
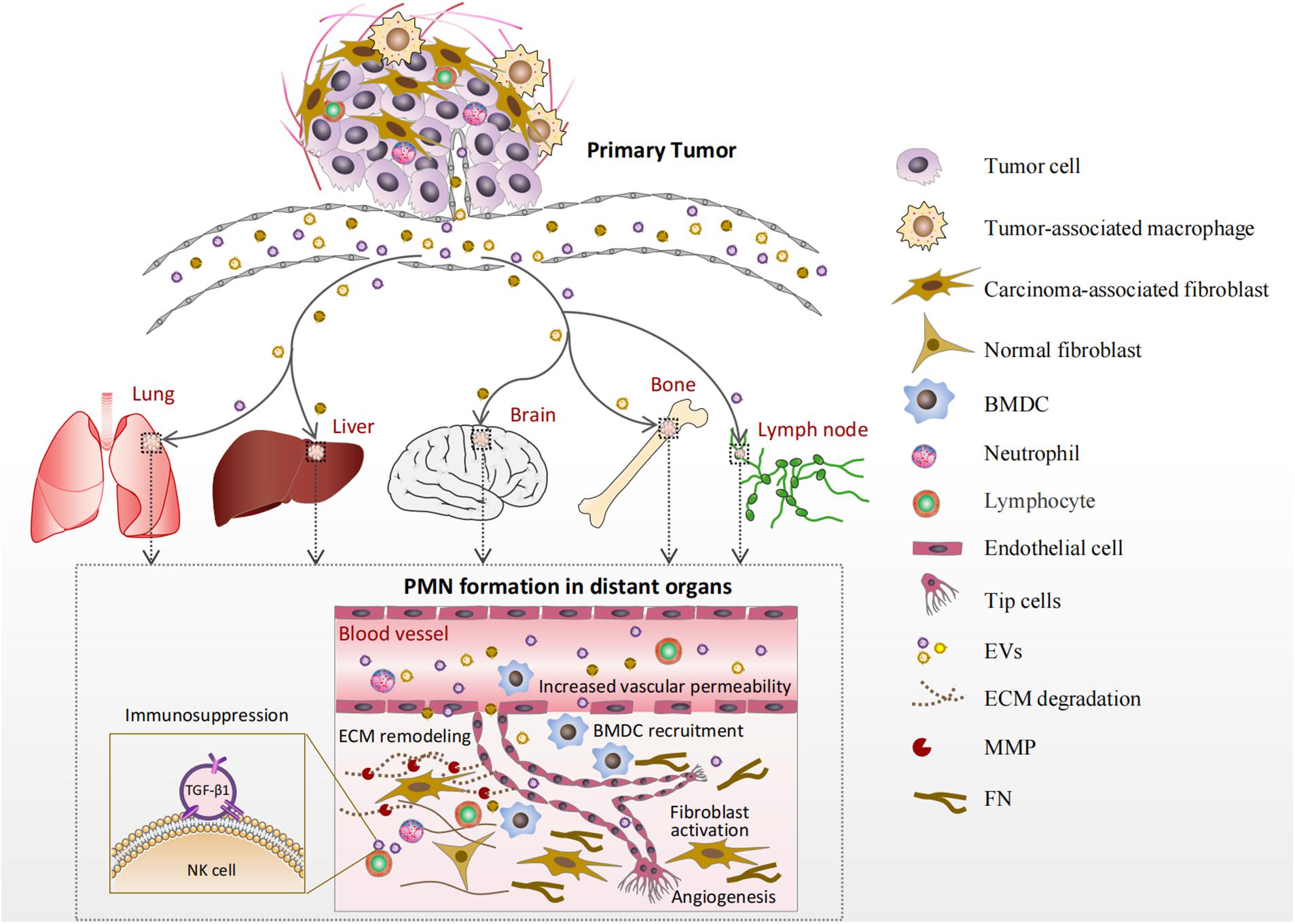
Figure 1. Formation of the pre-metastatic niche (PMN) in various organs. Extracellular vesicles (EVs) produced by tumor and stromal cells enter the circulation and arrive at distant organs such as the lung, liver, brain, bone marrow, and lymph nodes (LNs). Increased vascular permeability is an early event in PMN formation. Then, EVs cause extracellular matrix (ECM) remodeling principally by activating resident normal fibroblasts. The activated fibroblasts deposit new ECM components, such as FN, and produce matrix metalloproteinases (MMPs). Bone marrow-derived cells (BMDCs) are recruited into the target organs by EVs and involved in angiogenesis and/or immune responses. Furthermore, EVs can deliver their cargos to endothelial cells or immunocytes directly in the PMN to promote angiogenesis or create an immunosuppressive microenvironment.
Increased vascular permeability is an early event in PMN formation (Huang et al., 2009; Araldi et al., 2012). Vascular destabilization in the PMN promotes the extravasation of tumor cells and facilitates metastasis. Both EV-associated miRNAs and proteins contribute to vascular destabilization by destroying adhesion molecules between endothelial cells. Exosomal miR-25-3p derived from human colorectal cancer cells promotes vascular permeability in mouse models by regulating the expression of the tight junction proteins zonula occludens-1 (ZO-1), occludin, and claudin-5 in endothelial cells, thereby promoting cancer metastasis in the liver and lungs of mice (Zeng et al., 2018). In breast cancer, metastatic cancer cells secrete miR-105-rich exosomes that regulate vascular permeability and promote tumor metastasis by downregulating ZO-1 expression (Zhou et al., 2014). Melanoma-derived exosomes upregulate tumor necrosis factor-α expression in the lung, disrupting endothelial cell–cell junctions and increasing vascular permeability (Peinado et al., 2012). Vascular endothelial growth factor-A (VEGF-A) is carried by EVs derived from glioblastoma stem-like cells, increasing vascular permeability in vivo and the angiogenic potential of human brain endothelial cells (Treps et al., 2017). In addition, nucleoside diphosphate kinase B enriched in EVs derived from triple negative breast cancer cells (MDA-MB-231) enhances pulmonary blood vessel leakage and experimental lung metastasis (Duan et al., 2021).
Extracellular matrix remodeling, a key event in PMN formation, is characterized by the deposition of new ECM components and the expression of enzymes related to ECM modification. The remodeled ECM provides substrates for incoming cancer cells and increases matrix stiffness, which affects the properties of cancer cells (Erler et al., 2009; Cox et al., 2013; Wu et al., 2021). Several ECM components are involved in PMN formation, including fibronectin (Murgai et al., 2017), tenascin-C (Urooj et al., 2020), periostin (Malanchi et al., 2011; Fukuda et al., 2015; Wang et al., 2016), and versican (Kim et al., 2009; Gao et al., 2012). Fibronectin is reported to be upregulated in the livers of mice treated with exosomes from highly metastatic pancreatic cancer cells (Yu et al., 2017). Macrophage migration inhibitory factor (MIF) is highly expressed in pancreatic cancer cell-derived exosomes. Uptake of exosomal MIF by liver Kupffer cells causes transforming growth factor-β (TGF-β) secretion, leading to the activation of hepatic stellate cells. Fibronectin production by the activated hepatic stellate cells promotes the infiltration of bone marrow-derived macrophages and neutrophils in the liver, leading to the formation of the PMN (Costa-Silva et al., 2015). Insulin-like growth factor 2 mRNA-binding protein 1 is rich in melanoma cell EVs and promotes PMN formation in the lungs through the deposition of fibronectin and accumulation of CD45+ cells (Ghoshal et al., 2019). Exosomes from mutant p53-expressing pancreatic ductal adenocarcinoma cells affect the deposition and remodeling of the ECM by fibroblasts to generate a microenvironment highly supportive of tumor cell migration and invasion (Novo et al., 2018). Proteolytic enzymes play a critical role in ECM remodeling during PMN formation. Matrix metalloproteinases (MMPs) are a family of zinc-dependent endopeptidases that target ECM proteins, and MMP induction is one of the hallmarks of PMN formation. Deep et al. (2020) found that exosomes secreted by prostate cancer cells under hypoxia increase MMP activity and upregulate the expression of MMP-2, MMP-9, fibronectin, and collagen IV in the lung, liver, kidney, and spleen. A growing number of EV-associated miRNA have been involved in the regulation of ECM in regional sites. EV-associated miR-494 and miR-542-3p in metastatic rat adenocarcinoma cells regulate tumor-draining LNs and lung tissue by upregulating MMPs to form the PMN (Rana et al., 2013).
Stromal ECM proteins are mainly produced by fibroblasts. ECM remodeling by activated fibroblasts in the PMN has been reported by several groups. Wu et al. (2020) demonstrated that EVs from nasopharyngeal carcinoma package latent membrane protein 1 and activate the conversion of normal fibroblasts into carcinoma-associated fibroblasts (CAFs), thus, increasing the levels of typical PMN biomarkers, including fibronectin, S100A8, and VEGFR1 in lung and liver tissues. Ji et al. (2020) reported that colorectal cancer cells release integrin beta-like 1 (ITGBL1)-rich EVs that activate fibroblasts through the EVs-ITGBL1-CAFs-TNFAIP3-NF-κB axis in the liver and lung; the activated fibroblasts promote PMN formation by secreting pro-inflammatory factors. Fang et al. (2018) found that the highly metastatic hepatocellular carcinoma cell-derived exosomal miR-1247-3p induces the activation of normal fibroblasts into CAFs, which secrete inflammatory cytokines such as IL-6 and IL-8 to promote PMN formation in the lung, thus, promoting lung metastasis of liver cancer. Mazumdar et al. (2020b) provided strong evidence that osteosarcoma cell-derived EVs can activate fibroblasts into CAF phenotypes in the lung PMN through EV-associated TGF-β1 and SMAD2 pathway activation.
One mechanism by which tumor factors promote PMN formation is by mobilizing BMDCs to establish a suitable environment in specific secondary organs. The proto-oncoprotein MET is a receptor tyrosine kinase involved in cancer cell growth and invasion. Exosomes from highly metastatic melanomas reprogram BMDCs toward a pro-vasculogenic phenotype by transferring EV-associated MET, and thus, increasing the metastatic behavior of primary tumors (Peinado et al., 2012). Osteosarcoma-derived EVs can increase CD11b+ myeloid cell infiltration in the lungs (Mazumdar et al., 2020a). In pancreatic cancer, highly metastatic pancreatic cancer cell-derived exosomes recruit CD11b+ and CD45+ hematopoietic progenitor cells at the PMN (Yu et al., 2017). An opposite example of tumor EVs promoting PMN formation is the inhibition of lung metastasis in melanoma cells with low metastatic potential (Plebanek et al., 2017). These low-metastatic tumor cell-derived exosomes can amplify Ly6Clow patrolling monocytes in the bone marrow and trigger a wide range of innate immune responses. The pigment epithelial-derived factor on the external surface of exosomes plays a critical role in the process.
Angiogenesis is a prominent characteristic of the PMN. It was demonstrated that treating immunodeficient mice with epiregulin-enriched exosomes derived from salivary adenoid cystic carcinoma greatly enhanced tumor metastasis to the lung. Epiregulin-enriched exosomes upregulate the expression of VEGF, FGF-2, IL-8, and VEGFR1 in lung vascular endothelial cells, thus, contributing to angiogenesis (Yang et al., 2017). Human renal cancer stem cells promote angiogenesis and the formation of a PMN in the lung; the process involves the induction of pro-angiogenic mRNAs and miRNAs by CD105+ microvesicles in the whole organ (Grange et al., 2011). Nidogen 1 in metastatic hepatocellular carcinoma cell-derived EVs is reported to promote PMN formation in the lung by enhancing angiogenesis and pulmonary endothelial permeability to facilitate colonization of tumor cells. EV-associated nidogen 1 activates fibroblasts to secrete tumor necrosis factor receptor 1, thereby facilitating lung colonization of tumor cells (Mao et al., 2020). Annexin II is one of the most highly expressed proteins in exosomes. Maji et al. (2017) showed that exosomal Annexin II generates a PMN to facilitate breast cancer metastasis in distant organs by promoting tissue plasminogen activator-dependent angiogenesis.
The PMN is an immunosuppressive microenvironment comprising T cells, natural killer (NK) cells, neutrophils, monocytes, and macrophages (Seubert et al., 2015; Patel et al., 2018). Wen et al. (2016) reported that exosomes derived from highly metastatic murine breast cancer cells are distributed predominantly to the lungs of mice, where they suppress T-cell proliferation and inhibit NK cell cytotoxicity, likely suppressing the anticancer immune response in premetastatic organs. EVs isolated from pancreatic cancer induce a dysfunctional phenotype in NK cells, which contributes to an immunosuppressive microenvironment in the liver, and ultimately results in PMN formation. The study provided evidence that pancreatic cancer-derived EVs induce Smad2/3 phosphorylation and downregulate NKG2D in NK cells by delivering TGF-β1 to NK cells, which contributes to PMN formation in liver (Zhao et al., 2019). It is demonstrated that lung alveolar epithelial cells are stimulated by tumor exosomal RNAs via Toll-like receptor 3, which triggers neutrophil recruitment and lung metastatic niche formation (Liu et al., 2016). EVs secreted by brain metastases cells cause low-density lipoprotein aggregation, which accelerates EVs uptake by monocytes and macrophages. These monocytes and macrophages secrete immunosuppressive factors such as interleukin 10, chemokine ligand 2, and TGF-β, which contribute to PMN formation (Busatto et al., 2020). Maus et al. (2019) investigated lymphatic EVs of melanoma patients and showed that EVs traffic from the primary tumor microenvironment to the sentinel LNs and regulate the immune microenvironment in LNs, thereby promoting PMN formation.
Altered glucose metabolism, a hallmark of cancer, is characterized by increased glycolysis and glucose uptake. Fong et al. (2015) demonstrated that EV-associated microRNA-122 secreted by breast cancer cells can be transferred to normal cells (lung fibroblasts, brain astrocytes, and neurons) in the PMN, leading to reduced glucose uptake in these cells. Thereby, the niche accommodates a massive energy for cancer cell metastatic growth by suppressing the nutrient utilization in other cell types.
Our understanding of PMN biology is mostly based on studies of lung metastasis. Tumor EVs can target lung endothelial cells and cause vascular leakage and angiogenesis at pre-metastatic sites (Grange et al., 2011; Peinado et al., 2012; Zhou et al., 2014; Maji et al., 2017; Yang et al., 2017; Zeng et al., 2018; Mao et al., 2020). Lung fibroblasts are another common target of tumor EVs at PMN sites. Tumor EVs induce lung fibroblast reprogramming, by which fibroblasts are activated and differentiate into myofibroblast/CAF phenotypes, resulting in ECM remodeling, angiogenesis, secretion of pro-inflammatory cytokines, and immunosuppression (Fang et al., 2018; Novo et al., 2018; Ji et al., 2020; Mazumdar et al., 2020b; Wu et al., 2020). Alveolar epithelial cells released from tumor EVs secrete chemokines and neutrophils in the lungs to promote the formation of the microenvironment before lung metastasis (Liu et al., 2016). In addition, BMDCs (CD45+) from tumor exosomes in the lung inhibit the proliferation of T cells and the cytotoxicity of NK cells to form an immunosuppressive microenvironment (Wen et al., 2016). Overall, EVs target lung endothelial cells, fibroblasts, and alveolar epithelial cells to construct an inflammatory and immunosuppressive niche for the colonization of circulating tumor cells.
Tumor EVs can be internalized by Kupffer cells (F4/80 positive) and hepatic stellate cells (alpha smooth muscle actin and desmin positive) in the liver and activate downstream signaling pathways. Costa-Silva et al. showed that macrophage MIF is highly expressed in pancreatic cancer-derived exosomes. Uptake of these exosomes by liver Kupffer cells causes TGF-β secretion, leading to activation of hepatic stellate cells. Fibronectin production by activated hepatic stellate cells promotes the infiltration of bone marrow-derived macrophages and neutrophils in the liver, leading to the formation of the PMN (Costa-Silva et al., 2015). In gastric cancer cells, epidermal growth factor receptor-containing exosomes target Kupffer cells and hepatic stellate cells to favor the development of a liver-like microenvironment, thereby promoting liver-specific metastasis (Zhang et al., 2017). In addition, cancer-derived EVs may target NK cells in the liver. Zhao et al. (2019) demonstrated that pancreatic cancer-derived EVs induce a dysfunctional phenotype of NK cells, which contributes to an immunosuppressive microenvironment in the liver, and ultimately results in PMN formation. These studies indicate that EVs establish a fibrotic and immunosuppressive liver PMN mainly through Kupffer cells and hepatic stellate cells, but not hepatocytes. It suggests that targeting circulation EVs and/or the fibrotic niche may provide an early prevention and therapeutic intervention for liver metastasis.
Disruption of the blood-brain barrier is related to metastatic initiation and progression. Treps et al. (2017) reported that VEGF-A is carried by EVs derived from glioblastoma stem-like cells, and VEGF-A-enriched EVs promote PMN formation in the brain by targeting endothelial cells and increasing vascular permeability. In the same study, they demonstrated that EV-associated VEGF-A exerts pro-angiogenic activity on brain endothelial cells to stimulate angiogenesis. In addition, EVs may target non-endothelial cells in the brain to promote cancer brain metastasis. Busatto et al. revealed that brain metastasis cell-derived EVs interact with low-density lipoprotein and accelerate EV uptake by monocytes. These monocytes are key components in the brain niche and secrete immunosuppressive factors, such as interleukin 10, chemokine ligand 2, and TGF-β, which contribute to PMN formation (Busatto et al., 2020).
Bone marrow myeloid cells and osteoblasts may contribute to PMN formation in the bone marrow. Henrich et al. characterized EV-mediated communication between prostate cancer cells and bone marrow myeloid cells, and demonstrated that cholesterol homeostasis in bone marrow myeloid cells regulates pro-metastatic EV signaling and metastasis. The phospholipase D (PLD) isoforms PLD1/2 regulate tumor progression and metastasis by catalyzing the hydrolysis of phosphatidylcholine to yield phosphatidic acid (Henrich et al., 2020). Borel et al. (2020) demonstrated that PLD2 is present in EVs of prostate cancer cells and activates proliferation and differentiation of osteoblasts by stimulating ERK 1/2 phosphorylation, leading to the formation of a microenvironment before bone metastasis. By contrast, Valencia et al. reported that lung cancer cells release miR-192-enriched EVs, which target endothelial cells and show antimetastatic activity in vivo. EV-associated miR-192 is internalized by endothelial cells and inhibits the expression of proangiogenic factors including IL-8, ICAM, and CXCL1 to reduce metastatic colonization (Valencia et al., 2014). Bone marrow is a complex microenvironment and contains multiple cell types such as osteoblasts, osteoclasts, myeloid cells, fibroblasts, macrophages, adipocytes, and endothelial cells. More studies are required to elucidate how these cells cooperate in PMN formation in the bone marrow.
Metastasis to LNs is common in various cancers. Melanoma-derived exosomes home to sentinel LNs and can convert a remote LN into a PMN before tumor cell colonization by inducing the expression of factors responsible for cell recruitment, matrix remodeling, and angiogenesis (Hood et al., 2011). Maus et al. (2019) investigated lymphatic EVs of melanoma patients and showed that EVs traffic from the primary tumor microenvironment to the sentinel LNs, where they regulate the immune microenvironment, thereby initiating PMN formation. However, these studies did not identify the definite target cells of tumor EVs in LNs.
Studies show that various cancers can promote PMN formation in the lung, liver, brain, bone, and LNs by targeting endothelial cells, fibroblasts, alveolar epithelial cells, Kupffer cells, hepatic stellate cells, monocytes, and macrophages in these organs. Tumor EVs play a critical role in the communication between the primary tumor and distant organs by precisely delivering tumor products to target cells. Thus, tumor EVs in circulation should be a potential target of liquid biopsy to predict metastasis. Blocking the delivery of tumor EVs to target cells may prevent cancer metastasis. However, the biomarkers of tumor EVs that promote PMN formation have not been fully identified, and the mechanisms underlying the uptake of tumor EVs by target cells remain unclear. Except tumor cell-derived EVs, our group demonstrated that EVs secreted by CAFs can promote PMN formation in the lung by activating lung fibroblasts (Kong et al., 2019). Further study is necessary to determine whether EVs from other stromal cells in the primary tumor can induce PMN formation in more distant organs. Potential future studies in the field may focus on the following: (1) the different roles of EVs secreted by tumor and stromal cells in PMN formation; (2) the biomarkers of tumor or stromal EVs that promote PMN formation in various cancers; (3) the mechanisms by which recipient cells take up tumor or stromal EVs. The identification of PMN characteristics and a better understanding of the roles of EVs in PMN formation in various organs may help prevent cancer metastasis at an early stage.
QD, XL, KC, JS, and JK collected the literatures. TL and QD prepared the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by National Natural Science Foundation of China (82073001), LiaoNing Revitalization Talent Program (XLYC1805006), and Dalian Science and Technology Innovation Project (2020JJ26SN053).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aguado, B. A., Caffe, J. R., Nanavati, D., Rao, S. S., Bushnell, G. G., Azarin, S. M., et al. (2016). Extracellular matrix mediators of metastatic cell colonization characterized using scaffold mimics of the pre-metastatic niche. Acta Biomater. 33, 13–24. doi: 10.1016/j.actbio.2016.01.043
Araldi, E., Kramer-Albers, E. M., Hoen, E. N., Peinado, H., Psonka-Antonczyk, K. M., Rao, P., et al. (2012). International Society for Extracellular Vesicles: first annual meeting, April 17-21, 2012: ISEV-2012. J. Extracell. Vesicles 1:19995. doi: 10.3402/jev.v1i0.19995
Balaj, L., Lessard, R., Dai, L., Cho, Y. J., Pomeroy, S. L., Breakefield, X. O., et al. (2011). Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat. Commun. 2:180. doi: 10.1038/ncomms1180
Borel, M., Lollo, G., Magne, D., Buchet, R., Brizuela, L., and Mebarek, S. (2020). Prostate cancer-derived exosomes promote osteoblast differentiation and activity through phospholipase D2. Biochim. Biophys. Acta Mol. Basis Dis. 1866:165919. doi: 10.1016/j.bbadis.2020.165919
Busatto, S., Yang, Y., Walker, S. A., Davidovich, I., Lin, W. H., Lewis-Tuffin, L., et al. (2020). Brain metastases-derived extracellular vesicles induce binding and aggregation of low-density lipoprotein. J. Nanobiotechnol. 18:162. doi: 10.1186/s12951-020-00722-2
Chen, Q., Zhang, X. H., and Massague, J. (2011). Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell 20, 538–549. doi: 10.1016/j.ccr.2011.08.025
Choi, D. S., Kim, D. K., Kim, Y. K., and Gho, Y. S. (2013). Proteomics, transcriptomics and lipidomics of exosomes and ectosomes. Proteomics 13, 1554–1571. doi: 10.1002/pmic.201200329
Colombo, M., Raposo, G., and Thery, C. (2014). Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 30, 255–289. doi: 10.1146/annurev-cellbio-101512-122326
Costa-Silva, B., Aiello, N. M., Ocean, A. J., Singh, S., Zhang, H., Thakur, B. K., et al. (2015). Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 17, 816–826. doi: 10.1038/ncb3169
Cox, T. R., Bird, D., Baker, A. M., Barker, H. E., Ho, M. W., Lang, G., et al. (2013). LOX-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer Res. 73, 1721–1732. doi: 10.1158/0008-5472.CAN-12-2233
Deep, G., Jain, A., Kumar, A., Agarwal, C., Kim, S., Leevy, W. M., et al. (2020). Exosomes secreted by prostate cancer cells under hypoxia promote matrix metalloproteinases activity at pre-metastatic niches. Mol. Carcinog. 59, 323–332. doi: 10.1002/mc.23157
Duan, S., Nordmeier, S., Byrnes, A. E., and Buxton, I. L. O. (2021). Extracellular Vesicle-Mediated Purinergic Signaling Contributes to Host Microenvironment Plasticity and Metastasis in Triple Negative Breast Cancer. Int. J. Mol. Sci. 22:597. doi: 10.3390/ijms22020597
Erler, J. T., Bennewith, K. L., Cox, T. R., Lang, G., Bird, D., Koong, A., et al. (2009). Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell 15, 35–44. doi: 10.1016/j.ccr.2008.11.012
Fang, T., Lv, H., Lv, G., Li, T., Wang, C., Han, Q., et al. (2018). Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat. Commun. 9:191. doi: 10.1038/s41467-017-02583-0
Fong, M. Y., Zhou, W., Liu, L., Alontaga, A. Y., Chandra, M., Ashby, J., et al. (2015). Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat. Cell Biol. 17, 183–194. doi: 10.1038/ncb3094
Fukuda, K., Sugihara, E., Ohta, S., Izuhara, K., Funakoshi, T., Amagai, M., et al. (2015). Periostin Is a Key Niche Component for Wound Metastasis of Melanoma. PLoS One 10:e0129704. doi: 10.1371/journal.pone.0129704
Gao, D., Joshi, N., Choi, H., Ryu, S., Hahn, M., Catena, R., et al. (2012). Myeloid progenitor cells in the premetastatic lung promote metastases by inducing mesenchymal to epithelial transition. Cancer Res. 72, 1384–1394. doi: 10.1158/0008-5472.CAN-11-2905
Ghoshal, A., Rodrigues, L. C., Gowda, C. P., Elcheva, I. A., Liu, Z., Abraham, T., et al. (2019). Extracellular vesicle-dependent effect of RNA-binding protein IGF2BP1 on melanoma metastasis. Oncogene 38, 4182–4196. doi: 10.1038/s41388-019-0797-3
Giles, A. J., Reid, C. M., Evans, J. D., Murgai, M., Vicioso, Y., Highfill, S. L., et al. (2016). Activation of Hematopoietic Stem/Progenitor Cells Promotes Immunosuppression Within the Pre-metastatic Niche. Cancer Res. 76, 1335–1347. doi: 10.1158/0008-5472.CAN-15-0204
Grange, C., Tapparo, M., Collino, F., Vitillo, L., Damasco, C., Deregibus, M. C., et al. (2011). Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 71, 5346–5356. doi: 10.1158/0008-5472.CAN-11-0241
Gupta, G. P., Nguyen, D. X., Chiang, A. C., Bos, P. D., Kim, J. Y., Nadal, C., et al. (2007). Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature 446, 765–770. doi: 10.1038/nature05760
Henrich, S. E., McMahon, K. M., Plebanek, M. P., Calvert, A. E., Feliciano, T. J., Parrish, S., et al. (2020). Prostate cancer extracellular vesicles mediate intercellular communication with bone marrow cells and promote metastasis in a cholesterol-dependent manner. J. Extracell. Vesicles 10:e12042. doi: 10.1002/jev2.12042
Hiratsuka, S., Nakamura, K., Iwai, S., Murakami, M., Itoh, T., Kijima, H., et al. (2002). MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell 2, 289–300. doi: 10.1016/s1535-6108(02)00153-8
Hood, J. L., San, R. S., and Wickline, S. A. (2011). Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 71, 3792–3801. doi: 10.1158/0008-5472.CAN-10-4455
Hoshino, A., Costa-Silva, B., Shen, T. L., Rodrigues, G., Hashimoto, A., Tesic Mark, M., et al. (2015). Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335. doi: 10.1038/nature15756
Houg, D. S., and Bijlsma, M. F. (2018). The hepatic pre-metastatic niche in pancreatic ductal adenocarcinoma. Mol. Cancer 17:95. doi: 10.1186/s12943-018-0842-9
Huang, Y., Song, N., Ding, Y., Yuan, S., Li, X., Cai, H., et al. (2009). Pulmonary vascular destabilization in the premetastatic phase facilitates lung metastasis. Cancer Res. 69, 7529–7537. doi: 10.1158/0008-5472.CAN-08-4382
Ji, Q., Zhou, L., Sui, H., Yang, L., Wu, X., Song, Q., et al. (2020). Primary tumors release ITGBL1-rich extracellular vesicles to promote distal metastatic tumor growth through fibroblast-niche formation. Nat. Commun. 11:1211. doi: 10.1038/s41467-020-14869-x
Kaplan, R. N., Psaila, B., and Lyden, D. (2007). Niche-to-niche migration of bone-marrow-derived cells. Trends Mol. Med. 13, 72–81. doi: 10.1016/j.molmed.2006.12.003
Kaplan, R. N., Riba, R. D., Zacharoulis, S., Bramley, A. H., Vincent, L., Costa, C., et al. (2005). VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827. doi: 10.1038/nature04186
Kim, H., Chung, H., Kim, J., Choi, D. H., Shin, Y., Kang, Y. G., et al. (2019). Macrophages-Triggered Sequential Remodeling of Endothelium-Interstitial Matrix to Form Pre-Metastatic Niche in Microfluidic Tumor Microenvironment. Adv. Sci. 6:1900195. doi: 10.1002/advs.201900195
Kim, S., Takahashi, H., Lin, W. W., Descargues, P., Grivennikov, S., Kim, Y., et al. (2009). Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature 457, 102–106. doi: 10.1038/nature07623
Kitamura, T., Qian, B. Z., and Pollard, J. W. (2015). Immune cell promotion of metastasis. Nat. Rev. Immunol. 15, 73–86. doi: 10.1038/nri3789
Kong, J., Tian, H., Zhang, F., Zhang, Z., Li, J., Liu, X., et al. (2019). Extracellular vesicles of carcinoma-associated fibroblasts creates a pre-metastatic niche in the lung through activating fibroblasts. Mol. Cancer 18:175. doi: 10.1186/s12943-019-1101-4
Liu, Y., Gu, Y., Han, Y., Zhang, Q., Jiang, Z., Zhang, X., et al. (2016). Tumor Exosomal RNAs Promote Lung Pre-metastatic Niche Formation by Activating Alveolar Epithelial TLR3 to Recruit Neutrophils. Cancer Cell. 30, 243–256. doi: 10.1016/j.ccell.2016.06.021
Maji, S., Chaudhary, P., Akopova, I., Nguyen, P. M., Hare, R. J., Gryczynski, I., et al. (2017). Exosomal Annexin II Promotes Angiogenesis and Breast Cancer Metastasis. Mol. Cancer Res. 15, 93–105. doi: 10.1158/1541-7786.MCR-16-0163
Malanchi, I., Santamaria-Martinez, A., Susanto, E., Peng, H., Lehr, H. A., Delaloye, J. F., et al. (2011). Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 481, 85–89. doi: 10.1038/nature10694
Mao, X., Tey, S. K., Yeung, C. L. S., Kwong, E. M. L., Fung, Y. M. E., Chung, C. Y. S., et al. (2020). Nidogen 1-Enriched Extracellular Vesicles Facilitate Extrahepatic Metastasis of Liver Cancer by Activating Pulmonary Fibroblasts to Secrete Tumor Necrosis Factor Receptor 1. Adv. Sci. 7:2002157. doi: 10.1002/advs.202002157
Maus, R. L. G., Jakub, J. W., Hieken, T. J., Nevala, W. K., Christensen, T. A., Sutor, S. L., et al. (2019). Identification of novel, immune-mediating extracellular vesicles in human lymphatic effluent draining primary cutaneous melanoma. Oncoimmunology 8:e1667742. doi: 10.1080/2162402X.2019.1667742
Mazumdar, A., Urdinez, J., Boro, A., Arlt, M. J. E., Egli, F. E., Niederost, B., et al. (2020a). Exploring the Role of Osteosarcoma-Derived Extracellular Vesicles in Pre-Metastatic Niche Formation and Metastasis in the 143-B Xenograft Mouse Osteosarcoma Model. Cancers 12:3457. doi: 10.3390/cancers12113457
Mazumdar, A., Urdinez, J., Boro, A., Migliavacca, J., Arlt, M. J. E., Muff, R., et al. (2020b). Osteosarcoma-Derived Extracellular Vesicles Induce Lung Fibroblast Reprogramming. Int. J. Mol. Sci. 21:5451. doi: 10.3390/ijms21155451
Mohan, V., Das, A., and Sagi, I. (2020). Emerging roles of ECM remodeling processes in cancer. Semin. Cancer Biol. 62, 192–200. doi: 10.1016/j.semcancer.2019.09.004
Murgai, M., Ju, W., Eason, M., Kline, J., Beury, D. W., Kaczanowska, S., et al. (2017). KLF4-dependent perivascular cell plasticity mediates pre-metastatic niche formation and metastasis. Nat. Med. 23, 1176–1190. doi: 10.1038/nm.4400
Novo, D., Heath, N., Mitchell, L., Caligiuri, G., MacFarlane, A., Reijmer, D., et al. (2018). Mutant p53s generate pro-invasive niches by influencing exosome podocalyxin levels. Nat. Commun. 9:5069. doi: 10.1038/s41467-018-07339-y
Patel, S., Fu, S., Mastio, J., Dominguez, G. A., Purohit, A., Kossenkov, A., et al. (2018). Unique pattern of neutrophil migration and function during tumor progression. Nat. Immunol. 19, 1236–1247. doi: 10.1038/s41590-018-0229-5
Peinado, H., Aleckovic, M., Lavotshkin, S., Matei, I., Costa-Silva, B., Moreno-Bueno, G., et al. (2012). Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 18, 883–891. doi: 10.1038/nm.2753
Plebanek, M. P., Angeloni, N. L., Vinokour, E., Li, J., Henkin, A., Martinez-Marin, D., et al. (2017). Pre-metastatic cancer exosomes induce immune surveillance by patrolling monocytes at the metastatic niche. Nat. Commun. 8:1319. doi: 10.1038/s41467-017-01433-3
Rana, S., Malinowska, K., and Zoller, M. (2013). Exosomal tumor microRNA modulates premetastatic organ cells. Neoplasia 15, 281–295. doi: 10.1593/neo.122010
Raposo, G., and Stoorvogel, W. (2013). Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 200, 373–383. doi: 10.1083/jcb.201211138
Seubert, B., Grunwald, B., Kobuch, J., Cui, H., Schelter, F., Schaten, S., et al. (2015). Tissue inhibitor of metalloproteinases (TIMP)-1 creates a premetastatic niche in the liver through SDF-1/CXCR4-dependent neutrophil recruitment in mice. Hepatology 61, 238–248. doi: 10.1002/hep.27378
Sun, H., Meng, Q., Shi, C., Yang, H., Li, X., Wu, S., et al. (2021). Hypoxia-inducible exosomes facilitate liver-tropic pre-metastatic niche in colorectal cancer. Hepatology doi: 10.1002/hep.32009 [Epub ahead of print].
Tacke, R. S., Lee, H. C., Goh, C., Courtney, J., Polyak, S. J., Rosen, H. R., et al. (2012). Myeloid suppressor cells induced by hepatitis C virus suppress T-cell responses through the production of reactive oxygen species. Hepatology 55, 343–353. doi: 10.1002/hep.24700
Thakur, B. K., Zhang, H., Becker, A., Matei, I., Huang, Y., Costa-Silva, B., et al. (2014). Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 24, 766–769. doi: 10.1038/cr.2014.44
Thery, C., Witwer, K. W., Aikawa, E., Alcaraz, M. J., Anderson, J. D., Andriantsitohaina, R., et al. (2018). Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 7:1535750. doi: 10.1080/20013078.2018.1535750
Treps, L., Perret, R., Edmond, S., Ricard, D., and Gavard, J. (2017). Glioblastoma stem-like cells secrete the pro-angiogenic VEGF-A factor in extracellular vesicles. J. Extracell. Vesicles 6:1359479. doi: 10.1080/20013078.2017.1359479
Tyagi, A., Sharma, S., Wu, K., Wu, S. Y., Xing, F., Liu, Y., et al. (2021). Nicotine promotes breast cancer metastasis by stimulating N2 neutrophils and generating pre-metastatic niche in lung. Nat. Commun. 12:474. doi: 10.1038/s41467-020-20733-9
Urooj, T., Wasim, B., Mushtaq, S., Shah, S. N. N., and Shah, M. (2020). Cancer Cell-derived Secretory Factors in Breast Cancer-associated Lung Metastasis: their Mechanism and Future Prospects. Curr. Cancer Drug Targets 20, 168–186. doi: 10.2174/1568009620666191220151856
Valadi, H., Ekstrom, K., Bossios, A., Sjostrand, M., Lee, J. J., and Lotvall, J. O. (2007). Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659. doi: 10.1038/ncb1596
Valencia, K., Luis-Ravelo, D., Bovy, N., Anton, I., Martinez-Canarias, S., Zandueta, C., et al. (2014). miRNA cargo within exosome-like vesicle transfer influences metastatic bone colonization. Mol. Oncol. 8, 689–703. doi: 10.1016/j.molonc.2014.01.012
van Niel, G., D’Angelo, G., and Raposo, G. (2018). Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 19, 213–228. doi: 10.1038/nrm.2017.125
Wang, Y., Ding, Y., Guo, N., and Wang, S. (2019). MDSCs: key Criminals of Tumor Pre-metastatic Niche Formation. Front. Immunol. 10:172. doi: 10.3389/fimmu.2019.00172
Wang, Z., Xiong, S., Mao, Y., Chen, M., Ma, X., Zhou, X., et al. (2016). Periostin promotes immunosuppressive premetastatic niche formation to facilitate breast tumour metastasis. J. Pathol. 239, 484–495. doi: 10.1002/path.4747
Wen, S. W., Sceneay, J., Lima, L. G., Wong, C. S., Becker, M., Krumeich, S., et al. (2016). The Biodistribution and Immune Suppressive Effects of Breast Cancer-Derived Exosomes. Cancer Res. 76, 6816–6827. doi: 10.1158/0008-5472.CAN-16-0868
Wu, S., Xing, X., Wang, Y., Zhang, X., Li, M., Wang, M., et al. (2021). The pathological significance of LOXL2 in pre-metastatic niche formation of HCC and its related molecular mechanism. Eur. J. Cancer 147, 63–73. doi: 10.1016/j.ejca.2021.01.011
Wu, X., Zhou, Z., Xu, S., Liao, C., Chen, X., Li, B., et al. (2020). Extracellular vesicle packaged LMP1-activated fibroblasts promote tumor progression via autophagy and stroma-tumor metabolism coupling. Cancer Lett. 478, 93–106. doi: 10.1016/j.canlet.2020.03.004
Xu, W. W., Li, B., Guan, X. Y., Chung, S. K., Wang, Y., Yip, Y. L., et al. (2017). Cancer cell-secreted IGF2 instigates fibroblasts and bone marrow-derived vascular progenitor cells to promote cancer progression. Nat. Commun. 8:14399. doi: 10.1038/ncomms14399
Yang, W. W., Yang, L. Q., Zhao, F., Chen, C. W., Xu, L. H., Fu, J., et al. (2017). Epiregulin Promotes Lung Metastasis of Salivary Adenoid Cystic Carcinoma. Theranostics 7, 3700–3714. doi: 10.7150/thno.19712
You, H., Baluszek, S., and Kaminska, B. (2020). Supportive roles of brain macrophages in CNS metastases and assessment of new approaches targeting their functions. Theranostics 10, 2949–2964. doi: 10.7150/thno.40783
Yu, Z., Zhao, S., Ren, L., Wang, L., Chen, Z., Hoffman, R. M., et al. (2017). Pancreatic cancer-derived exosomes promote tumor metastasis and liver pre-metastatic niche formation. Oncotarget 8, 63461–63483. doi: 10.18632/oncotarget.18831
Zeng, Z., Li, Y., Pan, Y., Lan, X., Song, F., Sun, J., et al. (2018). Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat. Commun. 9:5395. doi: 10.1038/s41467-018-07810-w
Zhang, H., Deng, T., Liu, R., Bai, M., Zhou, L., Wang, X., et al. (2017). Exosome-delivered EGFR regulates liver microenvironment to promote gastric cancer liver metastasis. Nat. Commun. 8:15016. doi: 10.1038/ncomms15016
Zhang, Y., and Wang, X. F. (2015). A niche role for cancer exosomes in metastasis. Nat. Cell Biol. 17, 709–711. doi: 10.1038/ncb3181
Zhao, J., Schlosser, H. A., Wang, Z., Qin, J., Li, J., Popp, F., et al. (2019). Tumor-Derived Extracellular Vesicles Inhibit Natural Killer Cell Function in Pancreatic Cancer. Cancers 11:874. doi: 10.3390/cancers11060874
Keywords: pre-metastatic niche (PMN), extracellular vesicles (EVs), vascular permeability, extracellular matrix (ECM), bone marrow-derived cells (BMDCs), immunosuppression
Citation: Dong Q, Liu X, Cheng K, Sheng J, Kong J and Liu T (2021) Pre-metastatic Niche Formation in Different Organs Induced by Tumor Extracellular Vesicles. Front. Cell Dev. Biol. 9:733627. doi: 10.3389/fcell.2021.733627
Received: 30 June 2021; Accepted: 01 September 2021;
Published: 20 September 2021.
Edited by:
Dongmei Zhang, Jinan University, ChinaReviewed by:
Laurent Counillon, University of Nice Sophia Antipolis, FranceCopyright © 2021 Dong, Liu, Cheng, Sheng, Kong and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tingjiao Liu, dGluZ2ppYW9fbGl1QGZ1ZGFuLmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.