- State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangzhou, China
Glaucoma is the most substantial cause of irreversible blinding, which is accompanied by progressive retinal ganglion cell damage. Retinal ganglion cells are energy-intensive neurons that connect the brain and retina, and depend on mitochondrial homeostasis to transduce visual information through the brain. As cofactors that regulate many metabolic signals, iron and zinc have attracted increasing attention in studies on neurons and neurodegenerative diseases. Here, we summarize the research connecting iron, zinc, neuronal mitochondria, and glaucomatous injury, with the aim of updating and expanding the current view of how retinal ganglion cells degenerate in glaucoma, which can reveal novel potential targets for neuroprotection.
Introduction
Glaucoma involves irreversible optic nerve injury, which remains an urgent clinical challenge affecting 3–4% of people over 40 years of age; the global prevalence of glaucoma is expected to escalate to approximately 112 million people by 2040 (Tham et al., 2014). Patients with glaucoma often experience vision loss, which negatively impacts their independence and quality of life (Fenwick et al., 2020). One hallmark of glaucoma is progressive damage to retinal ganglion cells (RGCs) (Gupta and Yucel, 2007). However, the mechanisms of RGC degeneration in glaucoma are not yet completely understood. Medical and surgical intraocular pressure (IOP) control is the most common treatment for glaucoma at present (Kass et al., 2002; Osborne, 2008). However, these traditional therapies can neither maintain the long-term survival of RGCs nor promote optic nerve regeneration, strongly suggesting the presence of unknown molecular mechanisms (Osborne, 2011).
Recent evidence points to mitochondrial dysfunction in the retina, especially in RGCs, as an emerging hypothesis for glaucoma pathogenesis, offering a potential novel target for intervention. In the retinal structure, the somata of RGCs are located in the ganglion cell layer, with dendrites projecting to the inner plexiform layer. The long axons extend through the optic nerve. Through synapses, the dendrites and axons connect with other retinal neurons and cellular partners in the brain, respectively. These synapses require large amounts of energy for neurotransmitter synthesis, synaptic vesicle assembly, ion gradient formation, and calcium buffering (Vos et al., 2010). Mitochondria in RGCs play an essential role in meeting this high energy demand (Chidlow et al., 2019). Mitochondria play a pivotal role in metabolism and cell death (e.g., tricarboxylic acid cycle, ATP production, and apoptosis), and dysfunctional mitochondria can result in various diseases (Nicholls and Budd, 2000; Devine and Kittler, 2018; Pfanner et al., 2019). Mitochondria maintain homeostasis and quality control through fission, fusion, mitophagy, and biogenesis (Youle and van der Bliek, 2012; Dorn, 2019). Although studies have unveiled the many critical molecules and pathways related to the roles of mitochondria in neurons, there are many unsolved questions regarding the existence and functions of these molecules and pathways in RGCs.
Metal ions such as iron and zinc are essential for normal cellular function, especially in the synapses of the nervous system, and both ions are critical cofactors of neurotransmitter synthesis (Rouault, 2013). In particular, zinc modulates synaptic activities (Sourkes, 1972; McAllister and Dyck, 2017) and acts as an intracellular second messenger (Yamasaki et al., 2007). In addition to the free form ion, metal ion signals are transduced by metalloproteins to affect specific intracellular functions. The crucial participation of these metal ions in the pathogenesis and progression of neurodegenerative diseases such as Alzheimer’s disease (Liu et al., 2019) and Parkinson’s disease (Mocchegiani et al., 2005; Derry et al., 2020), has attracted increasing interest. Although only a few studies have directly focused on the roles of metal ions in glaucoma, we can infer from the existing literature that dysregulation of mitochondrial metal ions is the primary pathogenic cause of glaucomatous injury, rendering these ions as the most promising targets for therapy. In this review, we briefly describe the coordination among mitochondria, iron, and zinc; elucidate the mechanism underlying the mitochondrial homeostasis of RGCs in glaucoma; and discuss the emerging roles of iron and zinc in the mitochondria-related pathogenesis of glaucomatous RGCs.
Metal Ions and Mitochondria
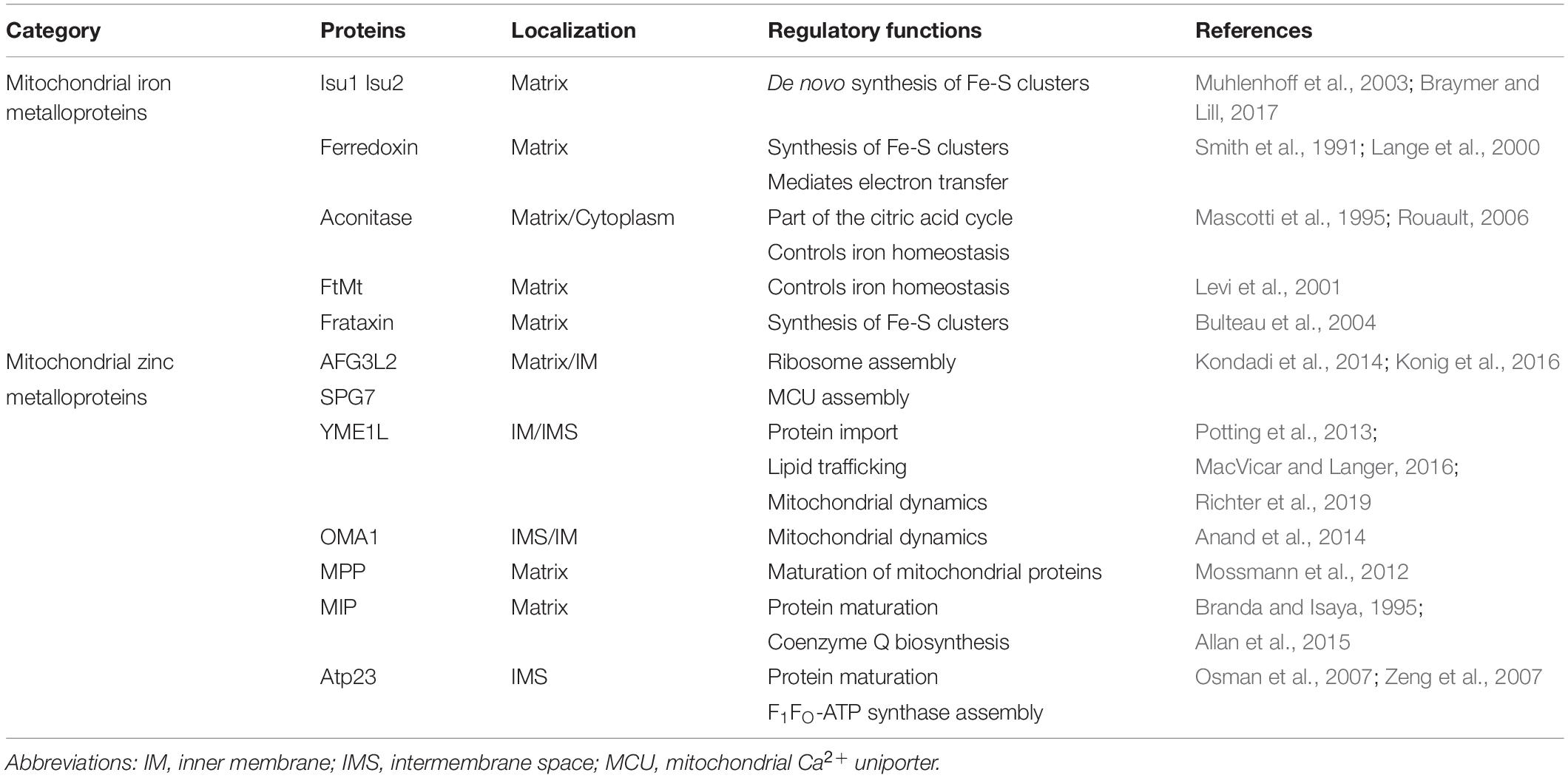
More than 1000 biochemical reactions responsible for cellular functions occur in mitochondria, which extend far beyond their well-known function of ATP synthesis. These vital functions depend on the structural composition of mitochondria (Figure 1). Mitochondria are incredibly dynamic double-membrane-bound subcellular organelles, comprising more than 1200 proteins, for which the composition varies substantially in different tissues or at different developmental stages. Many of these proteins are metalloproteins (Table 1).
Figure 1. The structure of mitochondria. The mitochondrial double membrane is a tubular reticulum, including an outer membrane (OM) and inner membrane (IM). The OM is porous and freely permits the diffusion of molecules smaller than 5 kD. The IM is a barrier to free ion diffusion and contains several cations and metabolite transporters. The invaginations of the IM that increase the surface area are called cristae. The inner mitochondrial membrane encloses the mitochondrial matrix. An intermembrane space (IMS) exists between the OM and IM, and its composition is close to that of the cytoplasmic matrix. The crista junction represents the architecture by which the cristae are attached to the inner membrane via narrow stems (Panek et al., 2020). The MICOS complex and OPA1 are involved in shaping the cristae. OPA1 act as a tether that maintains “tight” junctions to sequester cytochrome c and oxidative-phosphorylation protein complexes within the crista membrane (Mannella, 2020). Abbreviations: ETC, electron transport chain; mtDNA, mitochondrial DNA; MICOS, mitochondrial contact site and cristae organization system.
Mitochondrial Iron Metalloproteins
Iron is one of the most abundant metals in mitochondria, which is stored in mitochondrial ferritin (FtMt) within the mitochondrial matrix (Levi et al., 2001). Mitochondria require iron for the respiratory chain polynuclear sulfur-bridged iron-sulfur (Fe/S) centers residing in the cristae membrane. Fe/S clusters exist in respiratory complexes I, II, and III, and are critical for oxidative phosphorylation, which is the process by which electrons from NADH and FADH2 are transferred to O2 molecules through a series of electron carriers/protein complexes. This process generates potential energy in the form of a pH gradient and an electrical potential across the membrane to synthesize ATP from ADP to meet the cell’s energetic needs (Harper et al., 2018). Fe/S centers of different nuclearities are present in numerous proteins, in addition to those associated with the iron-sulfur cluster machinery within the mitochondrial matrix, such as the Fe/S scaffold proteins Isu1 and Isu2 (Andrew et al., 2008). These proteins include ferredoxin, biotin synthase, and aconitase (Lill and Muhlenhoff, 2008).
Mitochondrial Zinc Metalloproteins
Many zinc metalloproteins function in the mitochondria, including iAAA, mAAA, OMA1, mitochondrial processing peptidase (MPP), mitochondrial intermediate peptidase (MIP), and Atp23. Mitochondrial proteins are encoded by both nuclear DNA and mitochondrial DNA (mtDNA), which must be transported into the mitochondria for correct folding. Because mitochondrial electron transport chains can generate toxic reactive oxygen species (ROS), mitochondrial proteins are easily damaged. These processes make proteases particularly important. Mitoproteases are divided into four functional categories as follows: ATP-dependent peptidases, processing peptidases, oligopeptidases, and other mitochondrial peptidases (Deshwal et al., 2020).
ATP-dependent proteases, including iAAA and mAAA, perform quality control and regulatory functions in the mitoprotease system. The iAAA protease consists of six mobile zinc (Zn2+)-binding yeast mtDNA escape 1-like (YME1L) subunits and is active at the intermembrane space (IMS) side (Leonhard et al., 2000; Puchades et al., 2017). iAAA regulates mitochondrial protein degradation, lipid trafficking, and mitochondrial dynamics (Shi et al., 2016; MacVicar et al., 2019; Sprenger et al., 2019). The mAAA protease is composed of AFG3-like subunit 2 (AFG3L2) or spastic paraplegia 7-homolog (SPG7) subunits (Yta10/Afg3l2 and Yta12/Rca1 in yeast, respectively, each requiring Zn2+ for activity) and is active on the matrix side (Koppen et al., 2007). mAAA might function together with iAAA and OMA1 to regulate optic atrophy 1 (OPA1), a vital factor in mitochondrial dynamics (Consolato et al., 2018).
MPP cleaves mitochondrial-targeting sequences in the matrix for the maturation of nucleus-encoded mitochondrial proteins. The mitochondrial intermediate peptidase MIP (Oct1 in yeast) cleaves off the N-terminal octapeptide of some proteins for their stabilization (Vogtle et al., 2011). Atp23 forms an inner membrane protease to mediate the maturation of some proteins such as F1F0-ATP synthase into the IMS (Osman et al., 2007). However, protein processing removes the targeting sequences and acts as a regulatory mechanism that determines the activity and localization of mitochondrial proteins. As mentioned above, the inner membrane metalloendopeptidase OMA1 has some joint functions with mAAA to mediate proteolytic processes (Rainbolt et al., 2016; Consolato et al., 2018). The relationship between zinc-dependent proteases and mitochondrial dynamics has received increasing attention (further discussion in section “Effects of Zinc on Mitochondria”).
Movement of Metal Ions
As mentioned above, metalloproteins are distributed and function throughout the mitochondria. Therefore, in this section, we summarize the acquisition, distribution, transportation, storage, and exportation of metal ions in a cell and mitochondria.
Cellular and Mitochondrial Iron
As for the acquirement of cellular iron. The extracellular ferric form of iron combined with transferrin binds to transferrin receptor 1 and enters the cell via endocytosis (Picard et al., 2020). Transferrin receptors in neurons are expressed in the soma and dendrites but not in the axon (West et al., 1997). Upon maturation and acidification, endosomes release iron, which is reduced to the ferrous form. Divalent metal transporter-1 (DMT1, also known as NRAMP2, DCT1, or SLC11A2), transient receptor potential mucolipin 1 (TRPML1), and ZRT/IRT-like protein (ZIP14) mediate iron transport from the endosome to the cytoplasm (Upadhyay and Agarwal, 2020). Extracellular non-transferrin-bound Fe2+ can be directly internalized to the labile iron pool (LIP) by DMT1 on the cell surface (Moos et al., 2007). As for the storage of cellular iron. Ferritin, the principal iron-storage protein, is comprised of heavy (H)- and light (L)-chain monomers. The H-chain subunit oxidizes Fe2+ to Fe3+ via its ferroxidase activity to enhance iron sequestration by ferritin (Muhoberac and Vidal, 2013), whereas the L-subunit stores more iron by facilitating iron core formation (Ashraf et al., 2018). Fe2+ in the cytoplasm transiently enters the LIP. Notably, the ferrous form can cause molecular and cellular dysfunction by catalyzing the formation of hydroxyl free radicals (⋅OH) via the Fenton reaction (Ueda et al., 2018). However, the studies focused on cellular iron efflux are limited. Ferroportin (Fpn) is the only known cellular iron exporter that requires Ca2+ as a cofactor (Deshpande et al., 2018). Collectively, these studies suggest that RGCs take up iron at the cell body and dendrites, and ferritin and Fpn combine to export iron both within the axon and elsewhere.
Mitochondria acquire iron directly from both endosomes and the cytosol. Iron utilizes voltage-dependent anion channels and DMT1 to cross the outer membrane (Szabo and Zoratti, 2014; Wolff et al., 2018). The transport of Fe2+ across the inner membrane and its import into the matrix requires the mitoferrins Mfrn1 and Mfrn2 (orthologs in yeast are Mrs3/4), also known as SLC25A37 and SLC25A28, respectively (Grillo et al., 2017). Mitoferrin deficiency impairs iron import into the mitochondrial matrix via disruption of Mfrn1 and Mfrn2 in mammalian cells and of Mrs3 and Mrs4 in yeast, resulting in impaired iron metabolism and mitochondrial [Fe-S] cluster biogenesis (Chung et al., 2014). FtMt is an iron-storage protein that specifically functions in the mitochondria and cooperates with cytosolic ferritin to regulate iron homeostasis in both the cytoplasm and mitochondria (Drysdale et al., 2002). FtMt can suppress Fe2+-induced mitochondrial ROS production (Yang et al., 2013). Moreover, FtMt overexpression has been shown to ameliorate several neurodegenerative diseases (Gao and Chang, 2014). The neuroprotection mechanism of FtMt is considered to involve inhibiting the elevation of LIP levels and ferroptosis, as a new type of iron-dependent regulated cell death (Wang et al., 2016). However, there are few reports on the channels responsible for iron export from the mitochondria. Overexpression of Mmt1/2 in yeast leads to a low-iron transcriptional response, which can also be seen in Mrs3- and Mrs4-knockout cells. Therefore, Li et al. (2014) hypothesized that Mmt1/2 functions as a mitochondrial iron exporter. Mmt1 and Mmt2 expression is transcriptionally regulated by the low iron–sensing transcription factor Aft1 and the oxidant-sensing transcription factor Yap1 to accommodate changes in cytoplasmic and mitochondrial iron (Tuncay et al., 2019).
Cellular and Mitochondrial Zinc
Zinc transporters SLC39s/ZIPs (ZRT/IRT-like proteins) increase cytoplasmic zinc concentrations by translocating Zn2+ from the extracellular space or organelles into the cytoplasm (Eide, 2006; Baltaci and Yuce, 2018). In addition to ZIPs, the uptake of extracellular Zn2+ also occurs through amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) (Yin and Weiss, 1995; Sensi et al., 1999), voltage-gated calcium channels (VGCCs) (Kerchner et al., 2000), and N-methyl-D-aspartate receptors (NMDARs) (Koh and Choi, 1994). The translocated Zn2+ then activates numerous physiological and pathophysiological signaling processes. The majority (∼90%) of cellular zinc in neurons is found in a tightly protein-bound form (Huang, 1997). Under various injurious stimuli, zinc liberation from cytosolic zinc metalloproteins can lead to an increase in the intracellular Zn2+ level. The accumulated Zn2+ has three potential fates: (1) transport into the mitochondria and other subcellular compartments such as the endoplasmic reticulum; (2) formation of a labile zinc pool in the cytosol; and (3) enter the synaptic vesicles, followed by its transportation out of the cell to post-synaptic neurons. As for zinc export, SLC30s/ZnTs (zinc transporters) reduce the cytoplasmic zinc concentration by translocating Zn2+ from the cytoplasm into the extracellular space or organelles (Jackson et al., 2007). ZnT-3 is responsible for loading Zn2+ into the synaptic vesicles of glutamatergic, monoaminergic, and GABAergic neurons (Palmiter et al., 1996). In the retina, ZnT-3 is also a key regulator of Zn2+ transport to the synaptic vesicles of amacrine cells and melanopsin-containing RGCs (Li et al., 2017; Moshirpour et al., 2020).
The zinc ion transport channel in neural mitochondria has not yet been identified. Previous studies have shown that Zn2+ enters the mitochondria through the mitochondrial calcium uniporter (MCU) to trigger mitochondrial dysfunction (Gazaryan et al., 2007; Ji et al., 2019). A South Korean research team found that ZIP1, located in the mitochondrial outer membrane, and MCU, located in the mitochondrial inner membrane, work together to mediate zinc ion entry to the mitochondrial matrix after dynamin-1-like protein (DRP1) is activated in primary rat cortical neurons (Cho et al., 2019). This mechanism links zinc with the mitochondrial membrane potential and mitochondrial division, which provides new insight into the role of mitophagy in neurodegenerative diseases (Cho et al., 2019; Ji et al., 2020). ZIP1 is widely expressed in the central nervous system, and is localized in both the cytoplasmic membrane and cytoplasm (Tang et al., 2006; Qian et al., 2011). Tuncay et al. (2019) provided an essential description of the roles of ZIP7 and ZnT7, demonstrating that the proteins are localized in both the mitochondria and the sarco(endo)plasmic reticulum, and contribute to cellular Zn2+ exchange between subcellular compartments in cardiomyocytes under hyperglycemia or hypertrophy by affecting sarco(endo)plasmic reticulum-mitochondria coupling (Tuncay et al., 2019). Increasing ZnT7 and decreasing ZIP7 levels in mitochondria induces higher mitochondrial free Zn2+ levels, ROS production, and a depolarized mitochondrial membrane potential (Tuncay et al., 2019). However, the existence of these mechanisms in neurons or RGCs should be confirmed in further studies.
Metal Homeostasis and the Fate of RGCs
Retinal and humoral metal levels have been assessed in the context of glaucoma and other neurodegenerative diseases. Inductively coupled plasma-mass spectrometry analysis showed that retina and optic nerve samples from the DBA/2J glaucoma mouse model had lower iron concentrations than those of the retina from age-matched C57BL/6J control mice. Moreover, the retina of pre-glaucomatous DBA/2J mice showed over twofold higher zinc concentrations than those in 10-month-old DBA/2J retina (DeToma et al., 2014). Nevertheless, consuming iron above a threshold can increase the risk of developing glaucoma (Wang et al., 2012). Synchrotron X-ray fluorescence of the choroidal stroma in aged old-world primates confirmed the focal accumulation of iron (Ugarte et al., 2018). Compared with the control retina, glaucomatous retina of monkeys and humans show increased mRNA expression levels of iron-regulating genes (Farkas et al., 2004). Similarly, high serum ferritin levels were reported to be independently associated with a greater risk for human glaucoma (Gye et al., 2016).
With a greater understanding of the relationships among glaucoma, iron, and zinc, it is important to further elucidate the mechanism of metal dyshomeostasis in RGCs and the subsequent cellular pathophysiological signaling processes.
Iron Homeostasis and the Fate of RGCs
The iron steady state regulated by mitochondrial iron-binding proteins is essential for RGC survival. Frataxin (FXN) is a highly conserved nuclear-encoded mitochondrial protein among metabolically active eukaryotes (Adinolfi et al., 2009). FXN is an iron chaperone protein that protects against aconitase [4Fe-4S]2+ disassembly and promotes enzyme reactivation, preserving mitochondrial iron homeostasis and Kreb cycle functions. FXN stores iron within the mitochondria and promotes Fe2+ availability. Aconitase is highly susceptible to oxidation and inactivation following the release of solvent-exposed Fe-α and the formation of a [3Fe-4S]1+ cluster (Bulteau et al., 2004). Friedreich’s ataxia is a neurodegenerative and cardiac disorder characterized by FXN deficiency, resulting in mitochondrial iron accumulation and impaired activity of aconitase along with other mitochondrial iron-sulfur proteins (Ast et al., 2019). The transient elevation of IOP causes retinal endogenous FXN upregulation (Schultz et al., 2016). Whole-body or Müller cells overexpressing FXN protect RGCs after acute ischemia/reperfusion (I/R) injury (Schultz et al., 2016, 2018). Improved RGC survival is associated with increased antioxidative responsivity and mitochondrial functional maintenance.
Iron homeostasis regulated by neurotransmission is essential for RGC survival. Glutamate-mediated neurotransmission translates visual information from photoreceptors to bipolar cells, RGCs, and brain centers (Lukasiewicz, 2005). Excess glutamate in the retina underlies common neurodegenerative disorders such as retinal artery occlusion and glaucoma (Pang and Clark, 2020). The mechanism of excitotoxic injury involves the binding of excess glutamate to cell-surface NMDARs, resulting in a toxic influx of calcium, ultimately leading to RGC death (Almasieh et al., 2012). Moreover, glutamate-induced NMDAR activation was found to increase iron uptake through the iron import channel DMT1 (Cheah et al., 2006; Chen Y. et al., 2013). A more recent study demonstrated that intravitreal NMDA causes iron accumulation in RGCs and triggers apoptosis in these neurons (Sakamoto et al., 2018). Iron-chelating agents such as deferoxamine (DFO) and deferasirox (DFX) protect RGCs against excitoneurotoxicity or IOP perturbations by reducing the intracellular iron content and oxidative stress in rats (Liu et al., 2014; Sakamoto et al., 2018).
In addition to excitotoxicity, oxidative stress leads to RGC death in experimental models of optic nerve injury and in human glaucoma. The intracellular ROS triggered by axonal injury has also been proposed to be a key death signal leading to RGC apoptosis (Almasieh et al., 2012). As discussed earlier, intracellular ferrous iron catalyzes the conversion of hydrogen peroxide to hydroxyl radicals (⋅OH). Fe2+ also catalyzes the oxidation of lipid peroxide to lipid alkoxyl radicals via the Fenton reaction (Pollitt, 1999) as a key process of ferroptosis. However, under oxidative stress, superoxide also induces Fe2+ release from iron metalloproteins, including iron-sulfur clusters and ferritin (Aliaga et al., 2011). The degradation of ferritin via ferritinophagy is mediated by nuclear receptor coactivator 4, which increases the cytosolic LIP to ultimately enhance ferroptosis (Mancias et al., 2014). Thus, the cellular redox state and Fe2+ availability mutually interact to develop a positive feedback loop in RGCs.
These studies indicate that metallochaperones such as FXN, iron transporters such as DMT1, and the cellular redox state collectively regulate iron homeostasis. Thus, dysfunction in a metallochaperone, activation of iron import channels, and/or oxidative stress will result in iron dyshomeostasis and subsequent death of RGCs (Figure 2A).
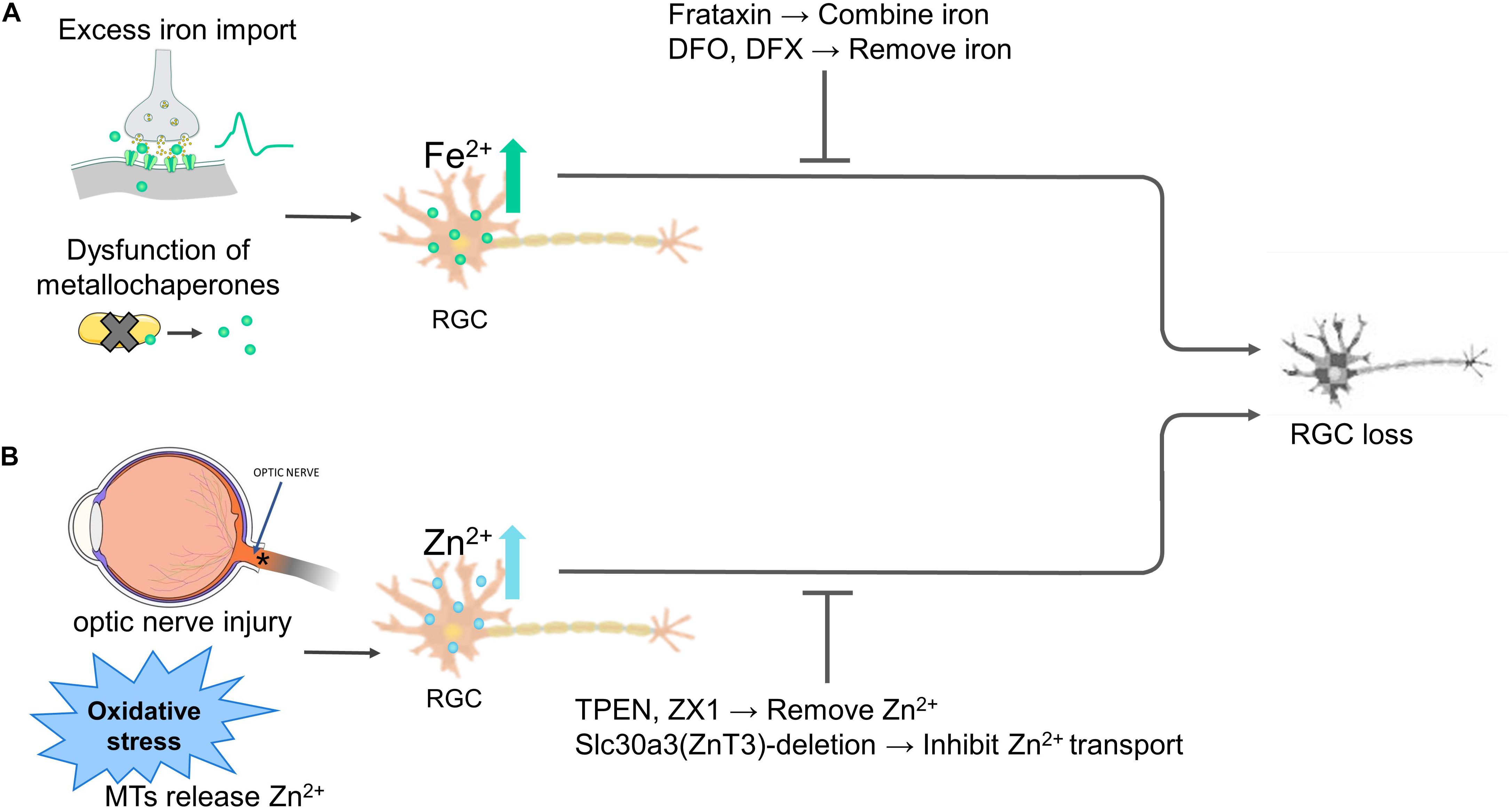
Figure 2. Iron and zinc homeostasis regulate the fate of retinal ganglion cells (RGCs). (A) Overly extracellular iron import, iron-binding proteins dysfunction disturbs intracellular iron levels, both causes RGC death. Overexpressing frataxin or the use of iron-chelating agents deferoxamine (DFO) and deferasirox (DFX) to stabilize or remove the excessive Fe2+ alleviates RGC death. (B) Optic nerve injury and various stimuli such as oxidative stress liberate Zn2+ from MTs, inhibiting RGC survival and axon regeneration. Zinc transporter Slc30a3-deletion or the Zn2+ chelator TPEN and ZX1 treatment inhibit transport or chelate Zn2+, protecting RGC survival and axon regeneration. Abbreviations: MTs, metallothioneins.
Zinc Homeostasis and the Fate of RGCs
Intracellular zinc is stored within the retina in RGCs, horizontal cells, and amacrine cells (Akagi et al., 2001; Kaneda et al., 2005). Most of the intracellular zinc is tightly bound, compartmentalized, and sequestered with high affinity by proteins, including metallothioneins (MTs), facilitating specific processes. As a consequence of these high-affinity binding events, the concentration of available zinc, referred to as “free,” “labile,” “mobile,” or “exchangeable” zinc, for metabolic processes is tightly regulated (Gilbert et al., 2019). Upon perturbation, cytosolic zinc metalloproteins respond to transient cytosolic zinc ion concentration changes, which is termed “zinc muffling” (Tuncay et al., 2019).
Excess intracellular zinc promotes the loss of RGCs. The Zn2+ levels of RGCs can vary depending on many factors, including Zn2+ release from subcellular zinc stores and zinc ion influx via channel activity. Under oxidative stress conditions, ROS and peroxynitrite oxidize residues on the metal-binding sites of metal-binding proteins and release the mobile zinc (Hidalgo et al., 2001). Peroxynitrite is an oxidant produced by the reaction between nitric oxide (NO) and superoxide radicals. Interestingly, the production of NO after optic nerve injury might act upstream of zinc liberation, leading to accumulation in amacrine cell terminals (Sergeeva et al., 2021). The accumulation of Zn2+ in the synaptic contacts between the amacrine cells and dendrites of RGCs is one of the earliest events following optic nerve injury (Benowitz et al., 2017). Li et al. (2017) observed that retinal amacrine cell Zn2+ concentrations increase by several-fold following optic nerve injury, and the excess is secreted in vesicles to RGCs. Zn2+ accumulation in RGCs is a newly discovered cause of axonal degeneration and apoptosis. However, zinc chelation reduces intracellular zinc concentrations, promoting RGC survival and axon regeneration (Li et al., 2017). These results show that excess intracellular zinc promotes the loss of RGCs.
The high levels of cellular zinc make RGCs susceptible to excitotoxic apoptosis. In addition to NMDARs, GABA/glycine receptors exist in retinal ganglion cells, which inhibit neurotransmission and protect RGCs against excitotoxic episodes (Hadj-Said et al., 2017). At concentrations below 10 μM (Kaneda et al., 2005), Zn2+ binds to the high-affinity site of glycine receptors, which facilitates glycine binding. As the concentration increases to 50 μM or more (Kaneda et al., 2005), Zn2+ binds to the low-affinity site. This binding inhibits Zn2+ binding to high-affinity sites on glycine receptors. The low-affinity site is located near the glycine-binding pocket, acting as a competitive inhibitor of glycine receptors (Han and Wu, 1999).
These studies indicate that oxidative stress, optic nerve injury, dysfunction of zinc-binding proteins result in zinc dysregulation and subsequent susceptibility to excitotoxic and death of RGCs (Figure 2B).
Mitochondria of RGCs: Critical Target of Metal Ions
The mitochondria are highly interconnected, and their synchronous intracellular functions make them unique among organelles. Mitochondria are continually recycled in a dynamic balance termed mitochondrial homeostasis, including mitochondrial genesis, movement, fission, fusion, and mitophagy.
Mitochondrial Biogenesis, Motility, Dynamics, and Mitophagy
Mitochondria produce ∼1300 proteins from the nuclear genome, and 13 proteins are solely encoded by vertebrate mitochondrial DNA, reflecting the truly symbiotic nature of this organelle (Schapira, 2012; Alston et al., 2021). Mitochondrial biogenesis mainly occurs near the nucleus to ensure the incorporation of only correctly synthesized molecules. Due to their bacterial nature, new mitochondria result from the fission of older mitochondria. Hence, some mitochondria remain in the neuronal soma to generate new mitochondria.
Other mitochondria must be trafficked to axons and dendrites, and positioned carefully to meet the demand of energy-consuming sites such as the pre-synaptic terminals. However, as mitochondrial transport can be accomplished in ∼0.5 μm/s and many nuclear-encoded mitochondrial proteins have short half-lives, we speculate that non-canonical local mitogenesis must also take place within neurons (Kaplan et al., 2009; Price et al., 2010), and that both mitogenic events are regulated by peroxisome proliferator-activated γ coactivator 1α (PGC-1α) (Fernandez-Marcos and Auwerx, 2011). However, the exact relationship between mitochondrial soma biogenesis and local biogenesis is not yet clear.
Because of the heterogeneous distribution and synthesis of mitochondria, in RGCs, one group of mitochondria undergoes anterograde and retrograde transport under control of the kinesin-1 motor protein family (Kif5B in mammals) and dynein proteins, respectively (Shanmughapriya et al., 2020). The membrane-anchored Miro (also called RhoT1/2) and its motor-binding partner Milton (also called TRAK1/2) form a Miro-Milton complex that cooperates with the kinesin-1 motor and dynein to mediate anterograde and retrograde transport (Schwarz, 2013). Another group of mitochondria remains anchored to the microtubules via syntaphilin, a mitochondrial outer membrane-attached protein, and an actin cytoskeleton component (Pathak et al., 2010). Interestingly, syntaphilin is only expressed in the soma and not in the axon of rat RGCs, suggesting that axons regulate their stationary pool of mitochondria differently from other neurons (Miki et al., 2014). Mitochondrial trafficking can be visualized via video microscopy using a fluorescent dye such as tetramethylrhodamine or fluorescent protein-labeled mitochondria.
Mitochondrial fission and fusion are highly dependent on cellular stress response signaling pathways (Lackner, 2013; Giacomello et al., 2020). Mitochondrial fission is regulated by the evolutionarily conserved dynamin-1-like protein (DRP1). In addition, the endoplasmic reticulum can wrap around mitochondria with actin and the DRP1 receptor, causing the release of mitochondrial fission factor, which promotes fission (Labbe et al., 2014). Mitochondrial fission can isolate damaged components, which can then be degraded by mitophagy (Ito and Di Polo, 2017). This mechanism is essential for the quality control of mitochondria (Das et al., 2020).
Mitophagy is the most compelling hypothesis for the selective autophagy of an entire mitochondrion and fragmented mitochondria. Autophagosomes recognize and endocytose dysfunctional mitochondria for their degradation. Mitophagy is induced by accumulating PTEN-induced putative kinase 1 (PINK1) and parkin (Narendra et al., 2008, 2010). PINK1 frequently translocates from the cytoplasm to the mitochondrial outer membrane. In the healthy mitochondria, PINK1 is constitutively repressed via its import into the inner mitochondrial membrane and is degraded by the rhomboid protease PARL (one of the mAAAs) (Yamano and Youle, 2013). When a mitochondrion becomes damaged, the import of PINK1 is prevented, and it therefore accumulates on the outer mitochondrial membrane (Meissner et al., 2015).
PINK1 on the outer mitochondrial membrane recruits parkin from the cytosol to ubiquitinate the impaired mitochondria and induces mitophagy (Eiyama and Okamoto, 2015). Optineurin is a parkin-mediated mitophagy receptor, and its recruitment to damaged mitochondria is an important downstream signal of parkin-mediated mitophagy (Lazarou et al., 2015). PINK1 accumulation is determined by severe mitochondrial depolarization. Therefore, when fission generates mitochondrial fragments, all of them are depolarized. The damaged fragments cannot restore their membrane potential and undergo mitophagy, whereas any healthy mitochondria maintain oxidative phosphorylation, restore their membrane potential, and undergo mitochondrial fusion to avoid mitophagy (Cho et al., 2019). Thus, this mechanism could be a novel model for mitochondrial quality surveillance.
Mitochondrial fusion consists of outer membrane fusion and inner membrane fusion. Membrane-anchored dynamin family members Mfn1 and Mfn2 mediate the fusion between mitochondrial outer membranes, whereas a single dynamin family member, optic atrophy 1 (OPA1), mediates fusion between mitochondrial inner membranes (Youle and van der Bliek, 2012). Enhancing mitochondrial fusion in glaucoma patients might ameliorate sub-clinical mitochondria damage by promoting the fusion with healthy mitochondria. Fused mitochondria are more capable of supplying ATP and are resistant to environmental stressors (Hoppins, 2014). Williams et al. (2012) demonstrated that OPA1 maintains the synaptic architecture and RGC connectivity. Further, OPA1 upregulation restores dysfunctional mitochondrial morphology and protects neurons against excitotoxic injury (Jahani-Asl et al., 2011).
In contrast, OPA1 deficiency leads to mitochondrial fragmentation, respiratory impairment, and calcium disturbances (Kushnareva et al., 2013; Sun et al., 2020). However, damaged mitochondria contaminate other mitochondria if they fuse with the mitochondrial network excessively before they are eliminated by autophagy (Youle and van der Bliek, 2012). Some scholars refer to these processes as the “dance between fusion and fission.”
Defect of Mitochondrial Biogenesis, Motility, Dynamics, and Mitophagy in Glaucoma
Numerous studies have demonstrated mitochondrial abnormalities occurring in the neurodegeneration accompanying glaucoma in patients and animal ocular hypertension (OHT) models (Ju et al., 2008; Williams et al., 2017; Hass and Barnstable, 2019; Tribble et al., 2019, 2021). Previous studies have demonstrated that mitochondrial dysfunction is an early driver of neuronal dysfunction preceding clinically observable neurodegeneration (Williams et al., 2017).
Many studies have demonstrated that inhibiting neuronal PGC-1α activity impairs mitogenesis, thereby promoting neurodegeneration, as in Alzheimer’s disease and Parkinson’s disease (Pirooznia et al., 2020; Singulani et al., 2020). In vitro and in vivo experiments proved that enhanced AMPK/PGC-1α signaling pathway activity and PGC-1α expression protect RGCs in the RGC-5 cell line, rat primary RGCs, and rat chronic ocular hypertension models (Chen S. et al., 2013; Zhang et al., 2018; Uchida et al., 2019). A recent study showed that zinc is essential for PGC-1α transcription and increases antioxidant stress in human primary endometrial stromal cells (Lu et al., 2020). However, more research is needed to elucidate the role of zinc in PGC-1α signaling in neurons and RGCs.
The detection of real-time mitochondrial motility in human RGCs is limited owing to technical challenges. The explant model of the mouse eye and optic nerve enables the image analysis of the living optic nerve head, showing that the percentage of mitochondria in motion significantly decreases with an acute and chronic IOP elevation (Kimball et al., 2017, 2018). Nicotinamide, which protects the RGCs of DBA/2J mice, increases mitochondrial size and motility in primary RGC cultures (Tribble et al., 2021).
Mitochondrial defects drive many degenerative retinal diseases, and mitochondrial transplant restores function to RGCs in the retina with defective mitochondria (Jiang et al., 2019; Ferrington et al., 2020). However, the long-term consequences of manipulating the balance of mitochondrial dynamics to protect RGCs are unknown, which is a source of controversy when designing glaucoma treatments. Some studies indicate that increasing fission and mitophagy to flush out unhealthy mitochondria protects RGCs from glaucoma. Similarly, mitochondrial uncoupling protein 2 (UCP2) knock-out promotes mitophagy and decreases the death of RGCs in a chronic OHT mouse model (Hass and Barnstable, 2019). This assertion is easy to accept because autophagy is beneficial to longevity in most cases.
Dai et al. (2018) proposed that in early hypertensive rats, mitophagy is increased to compensate for the change in pressure, but the damage to RGCs progresses as mitophagy is impaired owing to lysosome dysfunction. Interestingly, parkin overexpression downregulates mitophagy in the first 3 days following IOP elevation and promotes mitophagy 2 weeks following IOP elevation, reducing RGC death in chronic hypertensive glaucoma rats (Dai et al., 2018). Parkin overexpression affects mitophagy via two different mechanisms in early and later stages of IOP perturbations, suggesting that manipulating mitophagy might be harmful to some patients. Mitophagic hyperactivity can result in an inadequate ATP supply and eventually trigger neuronal cell death, which has attracted increasing attention (Doxaki and Palikaras, 2020).
In humans with glaucoma and mouse glaucomatous models (including DBA/2J mice), the mitochondria in the soma, dendrites, and axon of RGCs are smaller, more rounded, and more fragmented than those in healthy humans or mice, suggesting a defect in mitochondrial fusion (Ju et al., 2008; Coughlin et al., 2015; Kim et al., 2015; Tribble et al., 2019). Some studies consider that the high fission and mitophagy in glaucomatous RGCs result from damage instead of successful adaptation, recommending caution when attempting therapeutic manipulations of these processes. This view might result from the phenomenon that cristae of fragmented mitochondria are structurally disrupted and less capable of producing ATP compared with whole mitochondria. For example, decreased fission mediated by DRP1 inhibition or increased fusion mediated by OPA1 overexpression rescues RGCs and their axons by preserving mitochondrial integrity (Park et al., 2011; Kim et al., 2015; Hu et al., 2018).
Under the use of different animal models, the complexity of spatio-temporal regulation, and the lack of standard mitophagy flux assay, there are multifaceted results reported on the role of mitochondrial dynamics and mitophagy in insulted RGCs, with mitochondrial fission and mitophagy either protecting or promoting cell death. Accordingly, the molecular mechanism of hyperactive mitophagy-induced loss of RGCs remains unclear and requires further research. Zhou et al. (2019) showed that dysregulated mitophagy is toxic to the body when mitochondrial permeability increases. In acute IOP elevation models, inhibiting the opening of the mitochondrial permeability transition pore (mPTP) reduces mitochondrial permeability and promotes RGC survival (Kim et al., 2014). In addition, excess iron and zinc have been shown to be the triggers for mPTP opening (Jiang et al., 2001; Rauen et al., 2004; Sripetchwandee et al., 2014). These findings might help us to better understand the role of mitophagy in glaucoma.
It is worth noting that the state of RGCs likely differs depending on the status of glaucoma. For example, widespread mPTP opening occurs shortly after reperfusion, which results in detrimental mitophagic cell death (Ong et al., 2015). The use of chloroquine, which inhibits autophagic flux, rescues the early-phase I/R injury in cells. However, in the late phase of reperfusion, the mPTP closes in mitochondria to restore functionality (Ma et al., 2015). Hence, mitophagy plays a beneficial role by selectively degrading dysfunctional mitochondria and improving cellular homeostasis in the late phase of I/R injury. It is thus necessary to comprehensively consider the state of mitochondria and select appropriate protective measures for glaucoma.
So far, we have summarized (1) the critical functions of iron and zinc in mitochondria; (2) the kinetics of these metal ions in cells and mitochondria; (3) the relationship of iron, zinc, and RGCs; and (4) the relationship of mitochondria and glaucoma. Since studies directly focusing the roles of iron and zinc in mitochondria of glaucomatous RGCs are limited, the detailed impacts of iron and zinc on the mitochondria of RGCs in glaucoma is far from clear. In the following sections, we provide an overview of discoveries of how metal ions affect mitochondria in the RGCs and the other neurons, which can help to infer the potential molecular mechanisms involved in glaucoma.
Effects of Iron on Mitochondria
Iron is crucial for mitochondrial biogenesis, motility, and dynamics in RGCs. The use of iron chelators is protective to RGCs in OHT and optic nerve injury models (Thaler et al., 2010; Cui et al., 2020). Previous studies demonstrated that iron deficiency caused by treating cells with iron chelators decreases mitochondrial biogenesis, increases mitochondrial mobility, and inhibits anterograde movement, which impair dendritic outgrowth and synapse formation during neuronal development (Bastian et al., 2019; Upadhyay and Agarwal, 2020). Excess iron accumulation increases intracellular Ca2+ and activates calcineurin, inducing mitochondrial fragmentation by dephosphorylating DRP1 (Park et al., 2015). Use of an iron chelator leads to mitochondrial elongation by decreasing Fis1 expression, a mitochondrial fission modulator (Yoon et al., 2006). Both iron overload and deficiency result in mitochondrial dysfunction. A detailed treatment window for iron chelators must be explored.
In glaucoma, an increasing number of studies have offered evidence that ferroptosis contributes to RGC death due to the dysregulation of iron. Gao et al. (2019) proved that mitochondrial metabolism plays a crucial role in cysteine deprivation-induced ferroptosis. Ferroptosis induces a pathogenic mitochondrial morphology, including rupture of the outer mitochondrial membrane, reduction or disappearance of mitochondrial cristae, and changes in membrane potential (Vanden Berghe et al., 2014; Xie et al., 2016). Dexras1, essential for iron import in glutamate-NMDA neurotoxicity, promotes ferroptosis (Cheah et al., 2006; Peng et al., 2020). Deletion of Dexras1 in mice attenuates RGC death in NMDA/NO-mediated experimental glaucoma and optic neuritis (Chen Y. et al., 2013; Khan et al., 2019). FXN, a mitochondrial iron chaperone protein, is involved in ferroptosis by modulating iron homeostasis and mitochondrial function (Du et al., 2020). Suppressing FXN expression in RGCs results in enhanced mitochondrial fragmentation, undetectable cristae, impeded Fe-S cluster assembly, and enhanced ferroptosis. Further, FXN overexpression blocks erastin-induced ferroptosis (Du et al., 2020). Nevertheless, whether the protective role of FXN blocks experimental glaucoma owing to the suppression of ferroptosis remains unknown (Figure 3).
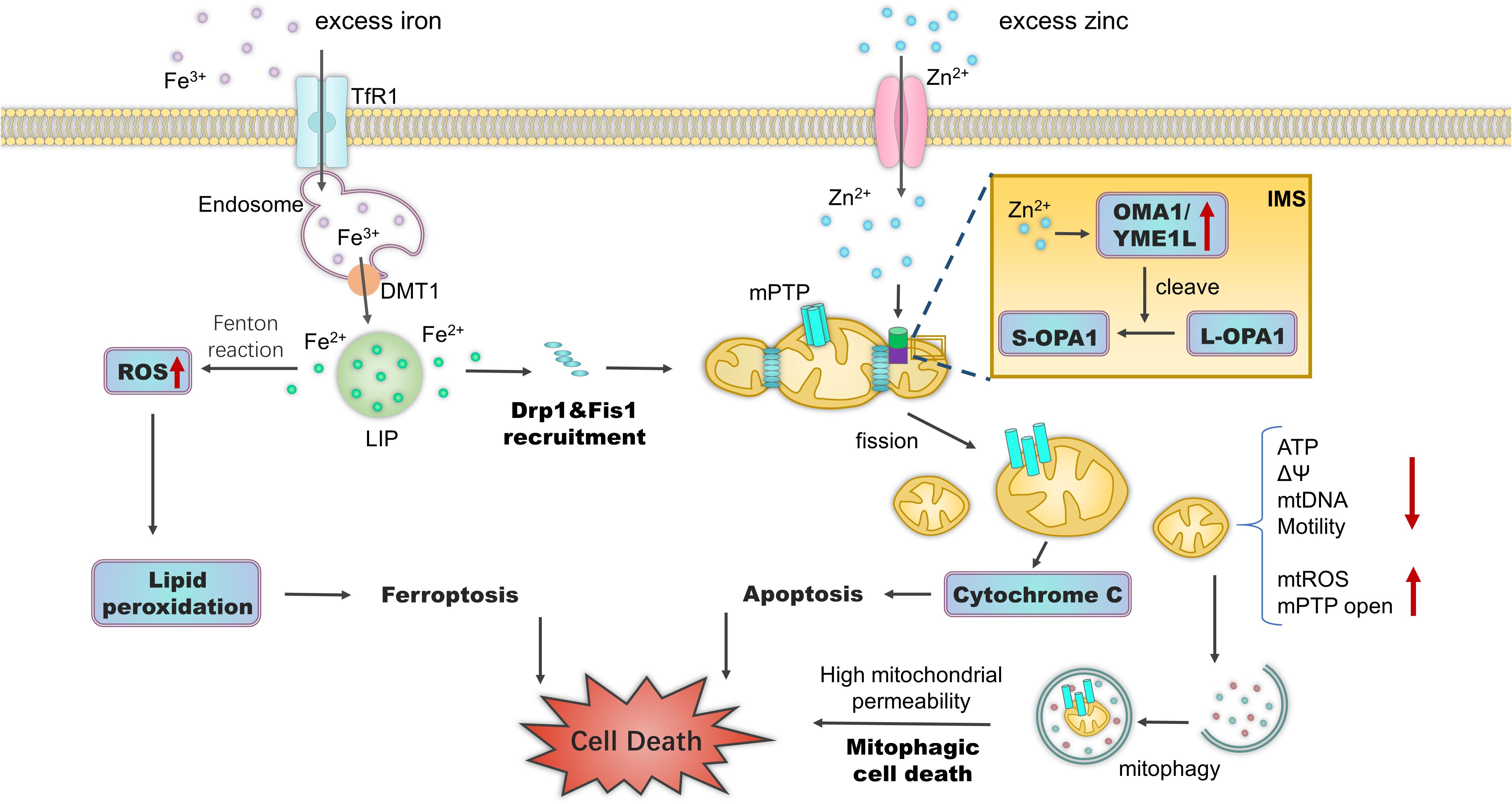
Figure 3. Effects of iron and zinc on mitochondria. Excess intracellular iron accumulation triggers mitochondrial fragmentation by increasing the expression of mitochondrial fission modulators Drp1 and Fis1. Fe2+ accumulation leads to the Fenton reaction and ferroptosis. Zn2+ accumulation and import into mitochondria via ion channels trigger mitochondrial depolarization, Drp1-dependent fission, and increased permeability. Under a range of stress stimuli, Zn2+ in the mitochondrial IMS activates zinc metallopeptidases OMA1 and YME1L to cleave long, fusion-active L-OPA1 to fusion-inactive S-OPA1 isoforms. This results in mitochondrial fragmentation and mitochondrial bioenergetic deficiency. During mPTP opening, fragmented mitochondria trigger mitophagic cell death. Zn2+ in the cytoplasm can also disrupt iron metabolism. Subsequent ferroptosis can occur via the over-accumulation of lipid ROS. Abbreviations: TfR1, transferrin receptor 1; DMT1, divalent metal transporter-1; LIP, labile iron pool; IMS, intermembrane space; ΔΨ, mitochondrial membrane potential; mtROS, mitochondrial reactive oxygen species.
Effects of Zinc on Mitochondria
In glaucoma, Zn2+ import into mitochondria then leads to mitochondrial depolarization, fission, and the increase of permeability, which could be involved in RGC loss. Zn2+ accumulation, mPTP opening, and mitochondrial fission occur before neuronal apoptosis (Martin et al., 2011). As mentioned above, DRP1 activates the ZIP1-MCU complex, which imports Zn2+ into mitochondria, resulting in mitochondrial depolarization along with mitochondrial fission and Zn2+ accumulation in RGCs after optic nerve injury (Li et al., 2017). Recently, Ji et al. (2020) reported that using MCU knock-out or pharmacologic blockers significantly reduced mitochondrial Zn2+ accumulation, and attenuated Zn2+-triggered mitochondrial dysfunction and cortical neuron cell death. Using structured illumination microscopy and a new single Zn2+ fluorescent probe, Fang et al. (2021) revealed that CCCP-induced mitophagy in living HeLa cells was associated with mobile Zn2+ enhancement. It would be interesting to further explore whether mitophagy is deleterious under this probable mechanism in the early state of glaucoma.
There are studies provide links between zinc-induced ferroptosis and glaucomatous injury. Intracellular Zn2+ accumulation perturbs iron homeostasis and induces ferroptosis (Palmer et al., 2019). Chen et al. (2021) used genome-wide RNA interference screens to show that the zinc transporters ZIP7 and ZnT8, which control zinc movement between mitochondria and different cell compartments are essential for ferroptosis. The underlying mechanism was found to be related to mitochondrial ROS that activate AMPK-ULK1 signaling, triggering ferritinophagy (Qin et al., 2021). Ferritinophagy increases intracellular iron levels and subsequently results in oxidative injury via the Fenton reaction.
Zinc metallopeptidases regulate the mitochondrial dynamics of RGCs in glaucoma. The zinc metallopeptidases YME1L and OMA1 regulate the balance between long (L-OPA1) and short (S-OPA1) OPA1 protein forms through alternative splicing and proteolytic processing (Ehses et al., 2009). The generation of membrane-anchored L-OPA1 depends on zinc metallopeptidase MPP activity, which cleaves the OPA1 mitochondrial targeting sequence (Ehses et al., 2009). OMA1 cooperates with YME1L and converts L-OPA1 into the soluble S-OPA1 at the S1 and S2 sites, respectively (MacVicar and Langer, 2016). L-OPA1 induces mitochondrial fusion, whereas S-OPA1 does not. Moderately active OMA1 and YME1L maintain a steady-state balance between fusion and fission. Stress insults or metabolic cues can activate OMA1 or YME1L in RGCs (Rodriguez-Graciani et al., 2020). During aging and in diseases, increased OPA1 processing limits the content of fusion-active L-OPA1 and triggers mitochondrial fragmentation (Baburamani et al., 2015). OPA1 mutation is a cause of primary open-angle glaucoma (Huang et al., 2014). As discussed above, the upregulation of OPA1 protects RGCs in glaucoma (Hu et al., 2018). Epigallocatechin gallate (EGCG) supplementation was also shown to play a neuroprotective role on RGCs in vitro and in mouse models of retinal I/R and chronic glaucoma (Zhang et al., 2008; Shen et al., 2015). Indeed, EGCG directly decreased OMA1 activity by inhibiting the self-cleavage of OMA1, attenuating L-OPA1 cleavage, and maintaining mitochondrial function (Nan et al., 2019). However, it is unknown whether the increase in Zn2+ levels during the course of optic nerve injury can directly activate these zinc metallopeptidases. The zinc chelator TPEN significantly inhibits OMA1 activity in L-OPA1 cleaving, suggesting that zinc is necessary for enzymatic activity (Tobacyk et al., 2019). The association between OPA1 and mitochondrial zinc metallopeptidases can be another potential research focus with respect to providing insight into the roles of zinc and the mitochondria of RGCs in glaucoma (Figure 3).
Challenges and Future Directions
Collectively, the findings summarized in this review indicate that metal homeostasis, whether involving metalloproteins or metal ions, is deterministic for the fate of RGCs. The overload of iron and zinc ions leads to the loss of RGCs, which can be alleviated by chelator treatment. In addition to metal ion levels, the forms and distribution of iron and zinc within cells are crucial for the normal function of RGCs, such as FXN and FtMt, which tune mitochondrial iron. Accumulating evidence indicates that mitochondrial abnormalities caused by iron or zinc accumulation are the underlying mechanism of glaucomatous injury. Including mitochondrial biogenesis and fusion deficiency, along with fission and mitophagy increases, among other processes, might participate in the loss process of RGCs.
However, there remains much to discover about the roles of iron and zinc in RGCs. Several questions still need to be solved, such as obtaining direct evidence of changes in iron and zinc levels in glaucomatous RGCs, the source of increased metal ions, where the ions go, and how they impact mitochondria. Importantly, for development of an effective glaucoma treatment, it is also important to determine the specificity of metal chelators, whether low metal concentrations would impair the synthesis and function of metalloproteins in the short or long term, and whether the surviving RGCs are still functional, which will necessitate further direct and detailed research. Moreover, how these essential metal ions, not only iron and zinc, interfere with each other in different cellular circumstances (for example, calcium assists ferroportin exporting iron) remains a challenge.
The gradual loss of RGCs in glaucoma, most of which is the result of apoptosis, is a chronic progressive process. Some patients still suffer from progressive loss of RGCs when using medication or surgery to maintain the IOP within acceptable limits. That is, RGCs undergo a long period of chronic stress before apoptosis occurs. Because mitochondria determine cell metabolism and fate in neurodegenerative diseases, the mitochondria of RGCs can play a vital role in this period. Specifying how metal ions influence the mitochondria might provide much needed insight into the bottleneck of glaucoma.
Conclusion
This is an exciting time for research in glaucoma mitochondrial biology, with the emergence of several intriguing findings regarding mitochondrial biogenesis, dynamics, and quality control. RGCs are extremely dependent on mitochondria, and the function of mitochondria in glaucoma patients is of particular interest. Moreover, an increasing number of new studies has shown that both metalloproteins and metal ions participate in mitochondrial homeostasis and in the pathogenesis of glaucoma. It is important to investigate the roles of metals such as iron and zinc in the mitochondria of glaucomatous RGCs, despite many open questions remaining about the fundamental mechanisms underlying these processes. By utilizing advancements in single-cell and subcellular visualization technologies, it is expected that the mechanisms by which metals affect the mitochondria of RGCs in the development of glaucoma will be uncovered.
Author Contributions
All authors, wrote and edited the manuscript, contributed to the article, and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (Grant Nos. 81870657 and 81870658), the Natural Science Foundation of Guangdong Province, China (Grant No. 2018A030313049), the Medical Scientific Research Foundation of Guangdong Province (A2018052), and the Fundamental Research Funds for the Youth Scholars of Sun Yat-sen University (18ykpy32).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Zhaoran Zhang and Editage (www.editage.cn) for English language editing.
References
Adinolfi, S., Iannuzzi, C., Prischi, F., Pastore, C., Iametti, S., Martin, S. R., et al. (2009). Bacterial frataxin CyaY is the gatekeeper of iron-sulfur cluster formation catalyzed by IscS. Nat. Struct. Mol. Biol. 16, 390–396. doi: 10.1038/nsmb.1579
Akagi, T., Kaneda, M., Ishii, K., and Hashikawa, T. (2001). Differential subcellular localization of zinc in the rat retina. J. Histochem. Cytochem. 49, 87–96. doi: 10.1177/002215540104900109
Aliaga, M. E., Carrasco-Pozo, C., Lopez-Alarcon, C., Olea-Azar, C., and Speisky, H. (2011). Superoxide-dependent reduction of free Fe3+ and release of Fe2+ from ferritin by the physiologically-occurring Cu(I)-glutathione complex. Bioorg. Med. Chem. 19, 534–541. doi: 10.1016/j.bmc.2010.10.064
Allan, C. M., Awad, A. M., Johnson, J. S., Shirasaki, D. I., Wang, C., Blaby-Haas, C. E., et al. (2015). Identification of Coq11, a new coenzyme Q biosynthetic protein in the CoQ-synthome in Saccharomyces cerevisiae. J. Biol. Chem. 290, 7517–7534. doi: 10.1074/jbc.M114.633131
Almasieh, M., Wilson, A. M., Morquette, B., Cueva Vargas, J. L., and Di Polo, A. (2012). The molecular basis of retinal ganglion cell death in glaucoma. Prog. Retin. Eye Res. 31, 152–181. doi: 10.1016/j.preteyeres.2011.11.002
Alston, C. L., Stenton, S. L., Hudson, G., Prokisch, H., and Taylor, R. W. (2021). The genetics of mitochondrial disease: dissecting mitochondrial pathology using multi-omic pipelines. J. Pathol. 254, 430–442. doi: 10.1002/path.5641
Anand, R., Wai, T., Baker, M. J., Kladt, N., Schauss, A. C., Rugarli, E., et al. (2014). The i-AAA protease YME1L and OMA1 cleave OPA1 to balance mitochondrial fusion and fission. J. Cell Biol. 204, 919–929. doi: 10.1083/jcb.201308006
Andrew, A. J., Song, J. Y., Schilke, B., and Craig, E. A. (2008). Posttranslational regulation of the scaffold for Fe-S cluster biogenesis, Isu. Mol. Biol. Cell 19, 5259–5266. doi: 10.1091/mbc.E08-06-0622
Ashraf, A., Clark, M., and So, P. W. (2018). The Aging of Iron Man. Front. Aging Neurosci. 10:65. doi: 10.3389/fnagi.2018.00065
Ast, T., Meisel, J. D., Patra, S., Wang, H., Grange, R. M. H., Kim, S. H., et al. (2019). Hypoxia Rescues Frataxin Loss by Restoring Iron Sulfur Cluster Biogenesis. Cell 177, 1507–1521.e16. doi: 10.1016/j.cell.2019.03.045
Baburamani, A. A., Hurling, C., Stolp, H., Sobotka, K., Gressens, P., Hagberg, H., et al. (2015). Mitochondrial Optic Atrophy (OPA) 1 Processing Is Altered in Response to Neonatal Hypoxic-Ischemic Brain Injury. Int. J. Mol. Sci. 16, 22509–22526. doi: 10.3390/ijms160922509
Baltaci, A. K., and Yuce, K. (2018). Zinc Transporter Proteins. Neurochem. Res. 43, 517–530. doi: 10.1007/s11064-017-2454-y
Bastian, T. W., von Hohenberg, W. C., Georgieff, M. K., and Lanier, L. M. (2019). Chronic Energy Depletion due to Iron Deficiency Impairs Dendritic Mitochondrial Motility during Hippocampal Neuron Development. J. Neurosc. 39, 802–813. doi: 10.1523/Jneurosci.1504-18.2018
Benowitz, L. I., He, Z., and Goldberg, J. L. (2017). Reaching the brain: advances in optic nerve regeneration. Exp. Neurol. 287, 365–373. doi: 10.1016/j.expneurol.2015.12.015
Branda, S. S., and Isaya, G. (1995). Prediction and identification of new natural substrates of the yeast mitochondrial intermediate peptidase. J. Biol. Chem. 270, 27366–27373. doi: 10.1074/jbc.270.45.27366
Braymer, J. J., and Lill, R. (2017). Iron-sulfur cluster biogenesis and trafficking in mitochondria. J. Biol. Chem. 292, 12754–12763. doi: 10.1074/jbc.R117.787101
Bulteau, A. L., O’Neill, H. A., Kennedy, M. C., Ikeda-Saito, M., Isaya, G., and Szweda, L. I. (2004). Frataxin acts as an iron chaperone protein to modulate mitochondrial aconitase activity. Science 305, 242–245. doi: 10.1126/science.1098991
Cheah, J. H., Kim, S. F., Hester, L. D., Clancy, K. W., Patterson, S. E. III, Papadopoulos, V., et al. (2006). NMDA receptor-nitric oxide transmission mediates neuronal iron homeostasis via the GTPase Dexras1. Neuron 51, 431–440. doi: 10.1016/j.neuron.2006.07.011
Chen, P. H., Wu, J. L., Xu, Y. T., Ding, C. K. C., Mestre, A. A., Lin, C. C., et al. (2021). Zinc transporter ZIP7 is a novel determinant of ferroptosis. Cell Death Dis. 12:198. doi: 10.1038/s41419-021-03482-5
Chen, S., Fan, Q., Li, A., Liao, D., Ge, J., Laties, A. M., et al. (2013). Dynamic mobilization of PGC-1alpha mediates mitochondrial biogenesis for the protection of RGC-5 cells by resveratrol during serum deprivation. Apoptosis 18, 786–799. doi: 10.1007/s10495-013-0837-3
Chen, Y., Khan, R. S., Cwanger, A., Song, Y., Steenstra, C., Bang, S., et al. (2013). Dexras1, a Small GTPase, Is Required for Glutamate-NMDA Neurotoxicity. J. Neurosci. 33, 3582–3587. doi: 10.1523/Jneurosci.1497-12.2013
Chidlow, G., Wood, J. P. M., Sia, P. I., and Casson, R. J. (2019). Distribution and Activity of Mitochondrial Proteins in Vascular and Avascular Retinas: implications for Retinal Metabolism. Invest. Ophthalmol. Vis. Sci. 60, 331–344. doi: 10.1167/iovs.18-25536
Cho, H. M., Ryu, J. R., Jo, Y., Seo, T. W., Choi, Y. N., Kim, J. H., et al. (2019). Drp1-Zip1 Interaction Regulates Mitochondrial Quality Surveillance System. Mol. Cell 73, 364–376.e8. doi: 10.1016/j.molcel.2018.11.009
Chung, J., Anderson, S. A., Gwynn, B., Deck, K. M., Chen, M. J., Langer, N. B., et al. (2014). Iron regulatory protein-1 protects against mitoferrin-1-deficient porphyria. J. Biol. Chem. 289, 7835–7843. doi: 10.1074/jbc.M114.547778
Consolato, F., Maltecca, F., Tulli, S., Sambri, I., and Casari, G. (2018). m-AAA and i-AAA complexes coordinate to regulate OMA1, the stress-activated supervisor of mitochondrial dynamics. J. Cell Sci. 131:jcs213546. doi: 10.1242/jcs.213546
Coughlin, L., Morrison, R. S., Horner, P. J., and Inman, D. M. (2015). Mitochondrial morphology differences and mitophagy deficit in murine glaucomatous optic nerve. Invest. Ophthalmol. Vis. Sci. 56, 1437–1446. doi: 10.1167/iovs.14-16126
Cui, Q. N., Bargoud, A. R., Ross, A. G., Song, Y., and Dunaief, J. L. (2020). Oral administration of the iron chelator deferiprone protects against loss of retinal ganglion cells in a mouse model of glaucoma. Exp. Eye Res. 193:107961. doi: 10.1016/j.exer.2020.107961
Dai, Y., Hu, X., and Sun, X. (2018). Overexpression of parkin protects retinal ganglion cells in experimental glaucoma. Cell Death Dis. 9:88. doi: 10.1038/s41419-017-0146-9
Das, A., Bell, C. M., Berlinicke, C. A., Marsh-Armstrong, N., and Zack, D. J. (2020). Programmed switch in the mitochondrial degradation pathways during human retinal ganglion cell differentiation from stem cells is critical for RGC survival. Redox Biol. 34:101465. doi: 10.1016/j.redox.2020.101465
Derry, P. J., Hegde, M. L., Jackson, G. R., Kayed, R., Tour, J. M., Tsai, A. L., et al. (2020). Revisiting the intersection of amyloid, pathologically modified tau and iron in Alzheimer’s disease from a ferroptosis perspective. Prog. Neurobiol. 184:101716. doi: 10.1016/j.pneurobio.2019.101716
Deshpande, C. N., Ruwe, T. A., Shawki, A., Xin, V., Vieth, K. R., Valore, E. V., et al. (2018). Calcium is an essential cofactor for metal efflux by the ferroportin transporter family. Nat. Commun. 9:3075. doi: 10.1038/s41467-018-05446-4
Deshwal, S., Fiedler, K. U., and Langer, T. (2020). Mitochondrial Proteases: multifaceted Regulators of Mitochondrial Plasticity. Annu. Rev. Biochem. 89, 501–528. doi: 10.1146/annurev-biochem-062917-012739
DeToma, A. S., Dengler-Crish, C. M., Deb, A., Braymer, J. J., Penner-Hahn, J. E., van der Schyf, C. J., et al. (2014). Abnormal metal levels in the primary visual pathway of the DBA/2J mouse model of glaucoma. Biometals 27, 1291–1301. doi: 10.1007/s10534-014-9790-z
Devine, M. J., and Kittler, J. T. (2018). Mitochondria at the neuronal presynapse in health and disease. Nat. Rev. Neurosci. 19, 63–80. doi: 10.1038/nrn.2017.170
Dorn, G. W. II (2019). Evolving Concepts of Mitochondrial Dynamics. Annu. Rev. Physiol. 81, 1–17. doi: 10.1146/annurev-physiol-020518-114358
Doxaki, C., and Palikaras, K. (2020). Neuronal Mitophagy: friend or Foe? Front. Cell Dev. Biol. 8:611938. doi: 10.3389/fcell.2020.611938
Drysdale, J., Arosio, P., Invernizzi, R., Cazzola, M., Volz, A., Corsi, B., et al. (2002). Mitochondrial ferritin: a new player in iron metabolism. Blood Cells Mol. Dis. 29, 376–383. doi: 10.1006/bcmd.2002.0577
Du, J., Zhou, Y., Li, Y. C., Xia, J., Chen, Y. J., Chen, S. F., et al. (2020). Identification of Frataxin as a regulator of ferroptosis. Redox Biol. 32:101483. doi: 10.1016/j.redox.2020.101483
Ehses, S., Raschke, I., Mancuso, G., Bernacchia, A., Geimer, S., Tondera, D., et al. (2009). Regulation of OPA1 processing and mitochondrial fusion by m-AAA protease isoenzymes and OMA1. J. Cell Biol. 187, 1023–1036. doi: 10.1083/jcb.200906084
Eide, D. J. (2006). Zinc transporters and the cellular trafficking of zinc. Biochim. Biophys. Acta 1763, 711–722. doi: 10.1016/j.bbamcr.2006.03.005
Eiyama, A., and Okamoto, K. (2015). PINK1/Parkin-mediated mitophagy in mammalian cells. Curr. Opin. Cell Biol. 33, 95–101. doi: 10.1016/j.ceb.2015.01.002
Fang, H., Geng, S., Hao, M., Chen, Q., Liu, M., Liu, C., et al. (2021). Simultaneous Zn(2+) tracking in multiple organelles using super-resolution morphology-correlated organelle identification in living cells. Nat. Commun. 12:109. doi: 10.1038/s41467-020-20309-7
Farkas, R. H., Chowers, I., Hackam, A. S., Kageyama, M., Nickells, R. W., Otteson, D. C., et al. (2004). Increased expression of iron-regulating genes in monkey and human glaucoma. Invest. Ophthalmol. Vis. Sci. 45, 1410–1417. doi: 10.1167/iovs.03-0872
Fenwick, E. K., Man, R. E., Aung, T., Ramulu, P., and Lamoureux, E. L. (2020). Beyond intraocular pressure: optimizing patient-reported outcomes in glaucoma. Prog. Retin. Eye Res. 76:100801. doi: 10.1016/j.preteyeres.2019.100801
Fernandez-Marcos, P. J., and Auwerx, J. (2011). Regulation of PGC-1alpha, a nodal regulator of mitochondrial biogenesis. Am. J. Clin. Nutr. 93, 884S–890S. doi: 10.3945/ajcn.110.001917
Ferrington, D. A., Fisher, C. R., and Kowluru, R. A. (2020). Mitochondrial Defects Drive Degenerative Retinal Diseases. Trends Mol. Med. 26, 105–118. doi: 10.1016/j.molmed.2019.10.008
Gao, G., and Chang, Y. Z. (2014). Mitochondrial ferritin in the regulation of brain iron homeostasis and neurodegenerative diseases. Front. Pharmacol. 5:19. doi: 10.3389/fphar.2014.00019
Gao, M., Yi, J., Zhu, J., Minikes, A. M., Monian, P., Thompson, C. B., et al. (2019). Role of Mitochondria in Ferroptosis. Mol. Cell 73, 354–363e353. doi: 10.1016/j.molcel.2018.10.042
Gazaryan, I. G., Krasinskaya, I. P., Kristal, B. S., and Brown, A. M. (2007). Zinc irreversibly damages major enzymes of energy production and antioxidant defense prior to mitochondrial permeability transition. J. Biol. Chem. 282, 24373–24380. doi: 10.1074/jbc.M611376200
Giacomello, M., Pyakurel, A., Glytsou, C., and Scorrano, L. (2020). The cell biology of mitochondrial membrane dynamics. Nat. Rev. Mol. Cell Biol. 21, 204–224. doi: 10.1038/s41580-020-0210-7
Gilbert, R., Peto, T., Lengyel, I., and Emri, E. (2019). Zinc Nutrition and Inflammation in the Aging Retina. Mol. Nutr. Food Res. 63:e1801049. doi: 10.1002/mnfr.201801049
Grillo, A. S., SantaMaria, A. M., Kafina, M. D., Cioffi, A. G., Huston, N. C., Han, M., et al. (2017). Restored iron transport by a small molecule promotes absorption and hemoglobinization in animals. Science 356, 608–616. doi: 10.1126/science.aah3862
Gupta, N., and Yucel, Y. H. (2007). Glaucoma as a neurodegenerative disease. Curr. Opin. Ophthalmol. 18, 110–114. doi: 10.1097/ICU.0b013e3280895aea
Gye, H. J., Kim, J. M., Yoo, C., Shim, S. H., Won, Y. S., Sung, K. C., et al. (2016). Relationship between high serum ferritin level and glaucoma in a South Korean population: the Kangbuk Samsung health study. Br. J. Ophthalmol. 100, 1703–1707. doi: 10.1136/bjophthalmol-2015-307678
Hadj-Said, W., Fradot, V., Ivkovic, I., Sahel, J.-A., Picaud, S., and Froger, N. (2017). Taurine Promotes Retinal Ganglion Cell Survival Through GABAB Receptor Activation. Adv. Exp. Med. Biol. 975, 687–701. doi: 10.1007/978-94-024-1079-2_54
Han, Y., and Wu, S. M. (1999). Modulation of glycine receptors in retinal ganglion cells by zinc. Proc. Natl. Acad. Sci. U. S. A. 96, 3234–3238. doi: 10.1073/pnas.96.6.3234
Harper, J. W., Ordureau, A., and Heo, J. M. (2018). Building and decoding ubiquitin chains for mitophagy. Nat. Rev. Mol. Cell Biol. 19, 93–108. doi: 10.1038/nrm.2017.129
Hass, D. T., and Barnstable, C. J. (2019). Mitochondrial Uncoupling Protein 2 Knock-out Promotes Mitophagy to Decrease Retinal Ganglion Cell Death in a Mouse Model of Glaucoma. J. Neurosci. 39, 3582–3596. doi: 10.1523/JNEUROSCI.2702-18.2019
Hidalgo, J., Aschner, M., Zatta, P., and Vasak, M. (2001). Roles of the metallothionein family of proteins in the central nervous system. Brain Res. Bull. 55, 133–145. doi: 10.1016/s0361-9230(01)00452-x
Hoppins, S. (2014). The regulation of mitochondrial dynamics. Curr. Opin. Cell Biol. 29, 46–52. doi: 10.1016/j.ceb.2014.03.005
Hu, X., Dai, Y., Zhang, R., Shang, K., and Sun, X. (2018). Overexpression of Optic Atrophy Type 1 Protects Retinal Ganglion Cells and Upregulates Parkin Expression in Experimental Glaucoma. Front. Mol. Neurosci. 11:350. doi: 10.3389/fnmol.2018.00350
Huang, E. P. (1997). Metal ions and synaptic transmission: think zinc. Proc. Natl. Acad. Sci. U. S. A. 94, 13386–13387. doi: 10.1073/pnas.94.25.13386
Huang, X., Li, M., Guo, X., Li, S., Xiao, X., Jia, X., et al. (2014). Mutation analysis of seven known glaucoma-associated genes in Chinese patients with glaucoma. Invest. Ophthalmol. Vis. Sci. 55, 3594–3602. doi: 10.1167/iovs.14-13927
Ito, Y. A., and Di Polo, A. (2017). Mitochondrial dynamics, transport, and quality control: a bottleneck for retinal ganglion cell viability in optic neuropathies. Mitochondrion 36, 186–192. doi: 10.1016/j.mito.2017.08.014
Jackson, K. A., Helston, R. M., McKay, J. A., O’Neill, E. D., Mathers, J. C., and Ford, D. (2007). Splice variants of the human zinc transporter ZnT5 (SLC30A5) are differentially localized and regulated by zinc through transcription and mRNA stability. J. Biol. Chem. 282, 10423–10431. doi: 10.1074/jbc.M610535200
Jahani-Asl, A., Pilon-Larose, K., Xu, W., MacLaurin, J. G., Park, D. S., McBride, H. M., et al. (2011). The mitochondrial inner membrane GTPase, optic atrophy 1 (Opa1), restores mitochondrial morphology and promotes neuronal survival following excitotoxicity. J. Biol. Chem. 286, 4772–4782. doi: 10.1074/jbc.M110.167155
Ji, S. G., Medvedeva, Y. V., Wang, H. L., Yin, H. Z., and Weiss, J. H. (2019). Mitochondrial Zn2+ Accumulation: a Potential Trigger of Hippocampal Ischemic Injury. Neuroscientist 25, 126–138. doi: 10.1177/1073858418772548
Ji, S. G., Medvedeva, Y. V., and Weiss, J. H. (2020). Zn2+ entry through the mitochondrial calcium uniporter is a critical contributor to mitochondrial dysfunction and neurodegeneration. Exp. Neurol. 325:113161. doi: 10.1016/j.expneurol.2019.113161
Jiang, D., Sullivan, P. G., Sensi, S. L., Steward, O., and Weiss, J. H. (2001). Zn(2+) induces permeability transition pore opening and release of pro-apoptotic peptides from neuronal mitochondria. J. Biol. Chem. 276, 47524–47529. doi: 10.1074/jbc.M108834200
Jiang, D., Xiong, G., Feng, H., Zhang, Z., Chen, P., Yan, B., et al. (2019). Donation of mitochondria by iPSC-derived mesenchymal stem cells protects retinal ganglion cells against mitochondrial complex I defect-induced degeneration. Theranostics 9, 2395–2410. doi: 10.7150/thno.29422
Ju, W. K., Kim, K. Y., Lindsey, J. D., Angert, M., Duong-Polk, K. X., Scott, R. T., et al. (2008). Intraocular pressure elevation induces mitochondrial fission and triggers OPA1 release in glaucomatous optic nerve. Invest. Ophthalmol. Vis. Sci. 49, 4903–4911. doi: 10.1167/iovs.07-1661
Kaneda, M., Ishii, K., Akagi, T., Tatsukawa, T., and Hashikawa, T. (2005). Endogenous zinc can be a modulator of glycinergic signaling pathway in the rat retina. J. Mol. Histol. 36, 179–185. doi: 10.1007/s10735-005-1693-4
Kaplan, B. B., Gioio, A. E., Hillefors, M., and Aschrafi, A. (2009). Axonal protein synthesis and the regulation of local mitochondrial function. Results Probl. Cell Differ. 48, 225–242. doi: 10.1007/400_2009_1
Kass, M. A., Heuer, D. K., Higginbotham, E. J., Johnson, C. A., Keltner, J. L., Miller, J. P., et al. (2002). The Ocular Hypertension Treatment Study - A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch. Ophthalmol. 120, 701–713. doi: 10.1001/archopht.120.6.701
Kerchner, G. A., Canzoniero, L. M. T., Yu, S. P., Ling, C., and Choi, D. W. (2000). Zn2+ current is mediated by voltage-gated Ca2+ channels and enhanced by extracellular acidity in mouse cortical neurones. J. Physiol. 528, 39–52. doi: 10.1111/j.1469-7793.2000.00039.x
Khan, R. S., Baumann, B., Dine, K., Song, Y., Dunaief, J. L., Kim, S. F., et al. (2019). Dexras1 Deletion and Iron Chelation Promote Neuroprotection in Experimental Optic Neuritis. Sci. Rep. 9:11664. doi: 10.1038/s41598-019-48087-3
Kim, K. Y., Perkins, G. A., Shim, M. S., Bushong, E., Alcasid, N., Ju, S., et al. (2015). DRP1 inhibition rescues retinal ganglion cells and their axons by preserving mitochondrial integrity in a mouse model of glaucoma. Cell Death Dis. 6:e1839. doi: 10.1038/cddis.2015.180
Kim, S. Y., Shim, M. S., Kim, K. Y., Weinreb, R. N., Wheeler, L. A., and Ju, W. K. (2014). Inhibition of cyclophilin D by cyclosporin A promotes retinal ganglion cell survival by preventing mitochondrial alteration in ischemic injury. Cell Death Dis. 5:e1105. doi: 10.1038/cddis.2014.80
Kimball, E. C., Jefferys, J. L., Pease, M. E., Oglesby, E. N., Nguyen, C., Schaub, J., et al. (2018). The effects of age on mitochondria, axonal transport, and axonal degeneration after chronic IOP elevation using a murine ocular explant model. Exp. Eye Res. 172, 78–85. doi: 10.1016/j.exer.2018.04.001
Kimball, E. C., Pease, M. E., Steinhart, M. R., Oglesby, E. N., Pitha, I., Nguyen, C., et al. (2017). A mouse ocular explant model that enables the study of living optic nerve head events after acute and chronic intraocular pressure elevation: focusing on retinal ganglion cell axons and mitochondria. Exp. Eye Res. 160, 106–115. doi: 10.1016/j.exer.2017.04.003
Koh, J. Y., and Choi, D. W. (1994). Zinc toxicity on cultured cortical neurons: involvement of N-methyl-D-aspartate receptors. Neuroscience 60, 1049–1057. doi: 10.1016/0306-4522(94)90282-8
Kondadi, A. K., Wang, S. Y., Montagner, S., Kladt, N., Korwitz, A., Martinelli, P., et al. (2014). Loss of the m-AAA protease subunit AFG3L2 causes mitochondrial transport defects and tau hyperphosphorylation. EMBO J. 33, 1011–1026. doi: 10.1002/embj.201387009
Konig, T., Troder, S. E., Bakka, K., Korwitz, A., Richter-Dennerlein, R., Lampe, P. A., et al. (2016). The m-AAA Protease Associated with Neurodegeneration Limits MCU Activity in Mitochondria. Mol. Cell 64, 148–162. doi: 10.1016/j.molcel.2016.08.020
Koppen, M., Metodiev, M. D., Casari, G., Rugarli, E. I., and Langer, T. (2007). Variable and tissue-specific subunit composition of mitochondrial m-AAA protease complexes linked to hereditary spastic paraplegia. Mol. Cell Biol. 27, 758–767. doi: 10.1128/MCB.01470-06
Kushnareva, Y. E., Gerencser, A. A., Bossy, B., Ju, W. K., White, A. D., Waggoner, J., et al. (2013). Loss of OPA1 disturbs cellular calcium homeostasis and sensitizes for excitotoxicity. Cell Death Differ. 20, 353–365. doi: 10.1038/cdd.2012.128
Labbe, K., Murley, A., and Nunnari, J. (2014). Determinants and functions of mitochondrial behavior. Annu. Rev. Cell Dev. Biol. 30, 357–391. doi: 10.1146/annurev-cellbio-101011-155756
Lackner, L. L. (2013). Determining the shape and cellular distribution of mitochondria: the integration of multiple activities. Curr. Opin. Cell Biol. 25, 471–476. doi: 10.1016/j.ceb.2013.02.011
Lange, H., Kaut, A., Kispal, G., and Lill, R. (2000). A mitochondrial ferredoxin is essential for biogenesis of cellular iron-sulfur proteins. Proc. Natl. Acad. Sci. U. S. A. 97, 1050–1055. doi: 10.1073/pnas.97.3.1050
Lazarou, M., Sliter, D. A., Kane, L. A., Sarraf, S. A., Wang, C., Burman, J. L., et al. (2015). The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 524, 309–314. doi: 10.1038/nature14893
Leonhard, K., Guiard, B., Pellecchia, G., Tzagoloff, A., Neupert, W., and Langer, T. (2000). Membrane protein degradation by AAA proteases in mitochondria: extraction of substrates from either membrane surface. Mol. Cell 5, 629–638. doi: 10.1016/S1097-2765(00)80242-7
Levi, S., Corsi, B., Bosisio, M., Invernizzi, R., Volz, A., Sanford, D., et al. (2001). A human mitochondrial ferritin encoded by an intronless gene. J. Biol. Chem. 276, 24437–24440. doi: 10.1074/jbc.C100141200
Li, L., Miao, R., Jia, X., Ward, D. M., and Kaplan, J. (2014). Expression of the yeast cation diffusion facilitators Mmt1 and Mmt2 affects mitochondrial and cellular iron homeostasis: evidence for mitochondrial iron export. J. Biol. Chem. 289, 17132–17141. doi: 10.1074/jbc.M114.574723
Li, Y., Andereggen, L., Yuki, K., Omura, K., Yin, Y., Gilbert, H. Y., et al. (2017). Mobile zinc increases rapidly in the retina after optic nerve injury and regulates ganglion cell survival and optic nerve regeneration. Proc. Natl. Acad. Sci. U. S. A. 114, E209–E218. doi: 10.1073/pnas.1616811114
Lill, R., and Muhlenhoff, U. (2008). Maturation of iron-sulfur proteins in eukaryotes: mechanisms, connected processes, and diseases. Annu. Rev. Biochem. 77, 669–700. doi: 10.1146/annurev.biochem.76.052705.162653
Liu, P., Zhang, M., Shoeb, M., Hogan, D., Tang, L., Syed, M. F., et al. (2014). Metal chelator combined with permeability enhancer ameliorates oxidative stress-associated neurodegeneration in rat eyes with elevated intraocular pressure. Free Radic. Biol. Med. 69, 289–299. doi: 10.1016/j.freeradbiomed.2014.01.039
Liu, Y., Nguyen, M., Robert, A., and Meunier, B. (2019). Metal Ions in Alzheimer’s Disease: a Key Role or Not? Acc. Chem. Res. 52, 2026–2035. doi: 10.1021/acs.accounts.9b00248
Lu, X. D., Zhang, Q., Xu, L., Lin, X. Y., Fu, J. H., Wang, X., et al. (2020). Zinc is essential for the transcription function of the PGC-1 alpha/Nrf2 signaling pathway in human primary endometrial stromal cells. Am. J. Physiol. Cell Physiol. 318, C640–C648. doi: 10.1152/ajpcell.00152.2019
Lukasiewicz, P. D. (2005). Synaptic mechanisms that shape visual signaling at the inner retina. Prog. Brain Res. 147, 205–218. doi: 10.1016/S0079-6123(04)47016-2
Ma, S., Wang, Y., Chen, Y., and Cao, F. (2015). The role of the autophagy in myocardial ischemia/reperfusion injury. Biochim. Biophys. Acta 1852, 271–276. doi: 10.1016/j.bbadis.2014.05.010
MacVicar, T., and Langer, T. (2016). OPA1 processing in cell death and disease - the long and short of it. J. Cell Sci. 129, 2297–2306. doi: 10.1242/jcs.159186
MacVicar, T., Ohba, Y., Nolte, H., Mayer, F. C., Tatsuta, T., Sprenger, H. G., et al. (2019). Lipid signalling drives proteolytic rewiring of mitochondria by YME1L. Nature 575, 361–365. doi: 10.1038/s41586-019-1738-6
Mancias, J. D., Wang, X. X., Gygi, S. P., Harper, J. W., and Kimmelman, A. C. (2014). Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature 509, 105–109. doi: 10.1038/nature13148
Mannella, C. A. (2020). Consequences of Folding the Mitochondrial Inner Membrane. Front. Physiol. 11:536. doi: 10.3389/fphys.2020.00536
Martin, L. J., Adams, N. A., Pan, Y., Price, A., and Wong, M. (2011). The mitochondrial permeability transition pore regulates nitric oxide-mediated apoptosis of neurons induced by target deprivation. J. Neurosci. 31, 359–370. doi: 10.1523/JNEUROSCI.2225-10.2011
Mascotti, D. P., Rup, D., and Thach, R. E. (1995). Regulation of iron metabolism: translational effects mediated by iron, heme, and cytokines. Annu. Rev. Nutr. 15, 239–261. doi: 10.1146/annurev.nu.15.070195.001323
McAllister, B. B., and Dyck, R. H. (2017). Zinc transporter 3 (ZnT3) and vesicular zinc in central nervous system function. Neurosci. Biobehav. Rev. 80, 329–350. doi: 10.1016/j.neubiorev.2017.06.006
Meissner, C., Lorenz, H., Hehn, B., and Lemberg, M. K. (2015). Intramembrane protease PARL defines a negative regulator of PINK1- and PARK2/Parkin-dependent mitophagy. Autophagy 11, 1484–1498. doi: 10.1080/15548627.2015.1063763
Miki, A., Kanamori, A., Nakamura, M., Matsumoto, Y., Mizokami, J., and Negi, A. (2014). The expression of syntaphilin is down-regulated in the optic nerve after axonal injury. Exp. Eye Res. 129, 38–47. doi: 10.1016/j.exer.2014.10.017
Mocchegiani, E., Bertoni-Freddari, C., Marcellini, F., and Malavolta, M. (2005). Brain, aging and neurodegeneration: role of zinc ion availability. Prog. Neurobiol. 75, 367–390. doi: 10.1016/j.pneurobio.2005.04.005
Moos, T., Rosengren Nielsen, T., Skjorringe, T., and Morgan, E. H. (2007). Iron trafficking inside the brain. J. Neurochem. 103, 1730–1740. doi: 10.1111/j.1471-4159.2007.04976.x
Moshirpour, M., Nakashima, A. S., Sehn, N., Smith, V. M., Thackray, S. E., Dyck, R. H., et al. (2020). Examination of Zinc in the Circadian System. Neuroscience 432, 15–29. doi: 10.1016/j.neuroscience.2020.02.016
Mossmann, D., Meisinger, C., and Vogtle, F. N. (2012). Processing of mitochondrial presequences. Biochim. Biophys. Acta 1819, 1098–1106. doi: 10.1016/j.bbagrm.2011.11.007
Muhlenhoff, U., Gerber, J., Richhardt, N., and Lill, R. (2003). Components involved in assembly and dislocation of iron-sulfur clusters on the scaffold protein Isu1p. EMBO J. 22, 4815–4825. doi: 10.1093/emboj/cdg446
Muhoberac, B. B., and Vidal, R. (2013). Abnormal iron homeostasis and neurodegeneration. Front. Aging Neurosci. 5:32. doi: 10.3389/fnagi.2013.00032
Nan, J. L., Nan, C. J., Ye, J., Qian, L., Geng, Y., Xing, D. W., et al. (2019). EGCG protects cardiomyocytes against hypoxia-reperfusion injury through inhibition of OMA1 activation. J. Cell Sci. 132:jcs220871. doi: 10.1242/jcs.220871
Narendra, D., Tanaka, A., Suen, D. F., and Youle, R. J. (2008). Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 183, 795–803. doi: 10.1083/jcb.200809125
Narendra, D. P., Jin, S. M., Tanaka, A., Suen, D. F., Gautier, C. A., Shen, J., et al. (2010). PINK1 Is Selectively Stabilized on Impaired Mitochondria to Activate Parkin. PLoS Biol. 8:e1000298. doi: 10.1371/journal.pbio.1000298
Nicholls, D. G., and Budd, S. L. (2000). Mitochondria and neuronal survival. Physiol. Rev. 80, 315–360. doi: 10.1152/physrev.2000.80.1.315
Ong, S. B., Samangouei, P., Kalkhoran, S. B., and Hausenloy, D. J. (2015). The mitochondrial permeability transition pore and its role in myocardial ischemia reperfusion injury. J. Mol. Cell Cardiol. 78, 23–34. doi: 10.1016/j.yjmcc.2014.11.005
Osborne, N. N. (2008). Pathogenesis of ganglion “cell death” in glaucoma and neuroprotection: focus on ganglion cell axonal mitochondria. Prog. Brain Res. 173, 339–352. doi: 10.1016/S0079-6123(08)01124-2
Osborne, N. N. (2011). Mitochondria: their role in ganglion cell death and survival in primary open angle glaucoma. Exp. Eye Res. 93, 750–757. doi: 10.1016/j.exer.2010.03.008
Osman, C., Wilmes, C., Tatsuta, T., and Langer, T. (2007). Prohibitins interact genetically with Atp23, a novel processing peptidase and chaperone for the F1Fo-ATP synthase. Mol. Biol. Cell 18, 627–635. doi: 10.1091/mbc.e06-09-0839
Palmer, L. D., Jordan, A. T., Maloney, K. N., Farrow, M. A., Gutierrez, D. B., Gant-Branum, R., et al. (2019). Zinc intoxication induces ferroptosis in A549 human lung cells. Metallomics 11, 982–993. doi: 10.1039/c8mt00360b
Palmiter, R. D., Cole, T. B., Quaife, C. J., and Findley, S. D. (1996). ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc. Natl. Acad. Sci. U. S. A. 93, 14934–14939. doi: 10.1073/pnas.93.25.14934
Panek, T., Elias, M., Vancova, M., Lukes, J., and Hashimi, H. (2020). Returning to the Fold for Lessons in Mitochondrial Crista Diversity and Evolution. Curr. Biol. 30, R575–R588. doi: 10.1016/j.cub.2020.02.053
Pang, I. H., and Clark, A. F. (2020). Inducible rodent models of glaucoma. Prog. Retin. Eye Res. 75:100799. doi: 10.1016/j.preteyeres.2019.100799
Park, J., Lee, D. G., Kim, B., Park, S. J., Kim, J. H., Lee, S. R., et al. (2015). Iron overload triggers mitochondrial fragmentation via calcineurin-sensitive signals in HT-22 hippocampal neuron cells. Toxicology 337, 39–46. doi: 10.1016/j.tox.2015.08.009
Park, S. W., Kim, K. Y., Lindsey, J. D., Dai, Y., Heo, H., Nguyen, D. H., et al. (2011). A selective inhibitor of drp1, mdivi-1, increases retinal ganglion cell survival in acute ischemic mouse retina. Invest. Ophthalmol. Vis. Sci. 52, 2837–2843. doi: 10.1167/iovs.09-5010
Pathak, D., Sepp, K. J., and Hollenbeck, P. J. (2010). Evidence That Myosin Activity Opposes Microtubule-Based Axonal Transport of Mitochondria. J. Neurosci. 30, 8984–8992. doi: 10.1523/Jneurosci.1621-10.2010
Peng, J. J., Song, W. T., Yao, F., Zhang, X., Peng, J., Luo, X. J., et al. (2020). Involvement of regulated necrosis in blinding diseases: focus on necroptosis and ferroptosis. Exp. Eye Res. 191:107922. doi: 10.1016/j.exer.2020.107922
Pfanner, N., Warscheid, B., and Wiedemann, N. (2019). Mitochondrial proteins: from biogenesis to functional networks. Nat. Rev. Mol. Cell Biol. 20, 267–284. doi: 10.1038/s41580-018-0092-0
Picard, E., Daruich, A., Youale, J., Courtois, Y., and Behar-Cohen, F. (2020). From Rust to Quantum Biology: the Role of Iron in Retina Physiopathology. Cells 9:705. doi: 10.3390/cells9030705
Pirooznia, S. K., Yuan, C., Khan, M. R., Karuppagounder, S. S., Wang, L., Xiong, Y., et al. (2020). PARIS induced defects in mitochondrial biogenesis drive dopamine neuron loss under conditions of parkin or PINK1 deficiency. Mol. Neurodegener. 15:17. doi: 10.1186/s13024-020-00363-x
Pollitt, E. (1999). Early iron deficiency anemia and later mental retardation. Am. J. Clin. Nutr. 69, 4–5. doi: 10.1093/ajcn/69.1.4
Potting, C., Tatsuta, T., Konig, T., Haag, M., Wai, T., Aaltonen, M. J., et al. (2013). TRIAP1/PRELI complexes prevent apoptosis by mediating intramitochondrial transport of phosphatidic acid. Cell Metab. 18, 287–295. doi: 10.1016/j.cmet.2013.07.008
Price, J. C., Guan, S., Burlingame, A., Prusiner, S. B., and Ghaemmaghami, S. (2010). Analysis of proteome dynamics in the mouse brain. Proc. Natl. Acad. Sci. U. S. A. 107, 14508–14513. doi: 10.1073/pnas.1006551107
Puchades, C., Rampello, A. J., Shin, M., Giuliano, C. J., Wiseman, R. L., Glynn, S. E., et al. (2017). Structure of the mitochondrial inner membrane AAA plus protease YME1 gives insight into substrate processing. Science 358:eaao0464. doi: 10.1126/science.aao0464
Qian, J., Xu, K., Yoo, J., Chen, T. T., Andrews, G., and Noebels, J. L. (2011). Knockout of Zn transporters Zip-1 and Zip-3 attenuates seizure-induced CA1 neurodegeneration. J. Neurosci. 31, 97–104. doi: 10.1523/JNEUROSCI.5162-10.2011
Qin, X., Zhang, J., Wang, B., Xu, G., Yang, X., Zou, Z., et al. (2021). Ferritinophagy is involved in the zinc oxide nanoparticles-induced ferroptosis of vascular endothelial cells. Autophagy 1–20. doi: 10.1080/15548627.2021.1911016 [Epub ahead of print].
Rainbolt, T. K., Lebeau, J., Puchades, C., and Wiseman, R. L. (2016). Reciprocal Degradation of YME1L and OMA1 Adapts Mitochondrial Proteolytic Activity during Stress. Cell Rep. 14, 2041–2049. doi: 10.1016/j.celrep.2016.02.011
Rauen, U., Petrat, F., Sustmann, R., and de Groot, H. (2004). Iron-induced mitochondrial permeability transition in cultured hepatocytes. J. Hepatol. 40, 607–615. doi: 10.1016/j.jhep.2003.12.021
Richter, F., Dennerlein, S., Nikolov, M., Jans, D. C., Naumenko, N., Aich, A., et al. (2019). ROMO1 is a constituent of the human presequence translocase required for YME1L protease import. J. Cell Biol. 218, 598–614. doi: 10.1083/jcb.201806093
Rodriguez-Graciani, K. M., Chapa-Dubocq, X. R., MacMillan-Crow, L. A., and Javadov, S. (2020). Association Between L-OPA1 Cleavage and Cardiac Dysfunction During Ischemia-Reperfusion Injury in Rats. Cell Physiol. Biochem. 54, 1101–1114. doi: 10.33594/000000303
Rouault, T. A. (2006). The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat. Chem. Biol. 2, 406–414. doi: 10.1038/nchembio807
Rouault, T. A. (2013). Iron metabolism in the CNS: implications for neurodegenerative diseases. Nat. Rev. Neurosci. 14, 551–564. doi: 10.1038/nrn3453
Sakamoto, K., Suzuki, T., Takahashi, K., Koguchi, T., Hirayama, T., Mori, A., et al. (2018). Iron-chelating agents attenuate NMDA-Induced neuronal injury via reduction of oxidative stress in the rat retina. Exp. Eye Res. 171, 30–36. doi: 10.1016/j.exer.2018.03.008
Schapira, A. H. (2012). Mitochondrial diseases. Lancet 379, 1825–1834. doi: 10.1016/S0140-6736(11)61305-6
Schultz, R., Krug, M., Precht, M., Wohl, S. G., Witte, O. W., and Schmeer, C. (2018). Frataxin overexpression in Muller cells protects retinal ganglion cells in a mouse model of ischemia/reperfusion injury in vivo. Sci. Rep. 8:4846. doi: 10.1038/s41598-018-22887-5
Schultz, R., Witte, O. W., and Schmeer, C. (2016). Increased Frataxin Levels Protect Retinal Ganglion Cells After Acute Ischemia/Reperfusion in the Mouse Retina In Vivo. Invest. Ophthalmol. Vis Sci. 57, 4115–4124. doi: 10.1167/iovs.16-19260
Schwarz, T. L. (2013). Mitochondrial trafficking in neurons. Cold Spring Harb. Perspect. Biol. 5:a011304. doi: 10.1101/cshperspect.a011304
Sensi, S. L., Yin, H. Z., Carriedo, S. G., Rao, S. S., and Weiss, J. H. (1999). Preferential Zn2+ influx through Ca2+-permeable AMPA/kainate channels triggers prolonged mitochondrial superoxide production. Proc. Natl. Acad. Sci. U. S. A. 96, 2414–2419. doi: 10.1073/pnas.96.5.2414
Sergeeva, E. G., Rosenberg, P. A., and Benowitz, L. I. (2021). Non-Cell-Autonomous Regulation of Optic Nerve Regeneration by Amacrine Cells. Front. Cell. Neurosci. 15:666798. doi: 10.3389/fncel.2021.666798
Shanmughapriya, S., Langford, D., and Natarajaseenivasan, K. (2020). Inter and Intracellular mitochondrial trafficking in health and disease. Ageing Res. Rev. 62:101128. doi: 10.1016/j.arr.2020.101128
Shen, C., Chen, L., Jiang, L., and Lai, T. Y. (2015). Neuroprotective effect of epigallocatechin-3-gallate in a mouse model of chronic glaucoma. Neurosci. Lett. 600, 132–136. doi: 10.1016/j.neulet.2015.06.002
Shi, H., Rampello, A. J., and Glynn, S. E. (2016). Engineered AAA plus proteases reveal principles of proteolysis at the mitochondrial inner membrane. Nat. Commun. 7:13301. doi: 10.1038/ncomms13301
Singulani, M. P., Pereira, C. P. M., Ferreira, A. F. F., Garcia, P. C., Ferrari, G. D., Alberici, L. C., et al. (2020). Impairment of PGC-1alpha-mediated mitochondrial biogenesis precedes mitochondrial dysfunction and Alzheimer’s pathology in the 3xTg mouse model of Alzheimer’s disease. Exp. Gerontol. 133:110882. doi: 10.1016/j.exger.2020.110882
Smith, E. T., Tomich, J. M., Iwamoto, T., Richards, J. H., Mao, Y., and Feinberg, B. A. (1991). A totally synthetic histidine-2 ferredoxin: thermal stability and redox properties. Biochemistry 30, 11669–11676. doi: 10.1021/bi00114a009
Sourkes, T. L. (1972). Influence of specific nutrients on catecholamine synthesis and metabolism. Pharmacol. Rev. 24, 349–359.
Sprenger, H. G., Wani, G., Hesseling, A., Konig, T., Patron, M., MacVicar, T., et al. (2019). Loss of the mitochondrial i-AAA protease YME1L leads to ocular dysfunction and spinal axonopathy. EMBO Mol. Med. 11:e9288. doi: 10.15252/emmm.201809288
Sripetchwandee, J., KenKnight, S. B., Sanit, J., Chattipakorn, S., and Chattipakorn, N. (2014). Blockade of mitochondrial calcium uniporter prevents cardiac mitochondrial dysfunction caused by iron overload. Acta Physiol. 210, 330–341. doi: 10.1111/apha.12162
Sun, S., Erchova, I., Sengpiel, F., and Votruba, M. (2020). Opa1 Deficiency Leads to Diminished Mitochondrial Bioenergetics With Compensatory Increased Mitochondrial Motility. Invest. Ophthalmol. Vis. Sci. 61:42. doi: 10.1167/iovs.61.6.42
Szabo, I., and Zoratti, M. (2014). Mitochondrial channels: ion fluxes and more. Physiol. Rev. 94, 519–608. doi: 10.1152/physrev.00021.2013
Tang, Z., Sahu, S. N., Khadeer, M. A., Bai, G., Franklin, R. B., and Gupta, A. (2006). Overexpression of the ZIP1 zinc transporter induces an osteogenic phenotype in mesenchymal stem cells. Bone 38, 181–198. doi: 10.1016/j.bone.2005.08.010
Thaler, S., Fiedorowicz, M., Rejdak, R., Choragiewicz, T. J., Sulejczak, D., Stopa, P., et al. (2010). Neuroprotective effects of tempol on retinal ganglion cells in a partial optic nerve crush rat model with and without iron load. Exp. Eye Res. 90, 254–260. doi: 10.1016/j.exer.2009.10.013
Tham, Y. C., Li, X., Wong, T. Y., Quigley, H. A., Aung, T., and Cheng, C. Y. (2014). Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 121, 2081–2090. doi: 10.1016/j.ophtha.2014.05.013
Tobacyk, J., Parajuli, N., Shrum, S., Crow, J. P., and MacMillan-Crow, L. A. (2019). The first direct activity assay for the mitochondrial protease OMA1. Mitochondrion 46, 1–5. doi: 10.1016/j.mito.2019.03.001
Tribble, J. R., Otmani, A., Sun, S., Ellis, S. A., Cimaglia, G., Vohra, R., et al. (2021). Nicotinamide provides neuroprotection in glaucoma by protecting against mitochondrial and metabolic dysfunction. Redox Biol. 43:101988. doi: 10.1016/j.redox.2021.101988
Tribble, J. R., Vasalauskaite, A., Redmond, T., Young, R. D., Hassan, S., Fautsch, M. P., et al. (2019). Midget retinal ganglion cell dendritic and mitochondrial degeneration is an early feature of human glaucoma. Brain Commun. 1:fcz035. doi: 10.1093/braincomms/fcz035
Tuncay, E., Bitirim, C. V., Olgar, Y., Durak, A., Rutter, G. A., and Turan, B. (2019). Zn2+-transporters ZIP7 and ZnT7 play important role in progression of cardiac dysfunction via affecting sarco(endo)plasmic reticulum-mitochondria coupling in hyperglycemic cardiomyocytes. Mitochondrion 44, 41–52. doi: 10.1016/j.mito.2017.12.011
Uchida, T., Ueta, T., Honjo, M., and Aihara, M. (2019). The Neuroprotective Effect of the Adiponectin Receptor Agonist AdipoRon on Glutamate-Induced Cell Death in Rat Primary Retinal Ganglion Cells. J. Ocul. Pharmacol. Ther. 35, 535–541. doi: 10.1089/jop.2018.0152
Ueda, K., Kim, H. J., Zhao, J., Song, Y., Dunaief, J. L., and Sparrow, J. R. (2018). Iron promotes oxidative cell death caused by bisretinoids of retina. Proc. Natl. Acad. Sci. U. S. A. 115, 4963–4968. doi: 10.1073/pnas.1722601115
Ugarte, M., Geraki, K., and Jeffery, G. (2018). Aging results in iron accumulations in the non-human primate choroid of the eye without an associated increase in zinc, copper or sulphur. Biometals 31, 1061–1073. doi: 10.1007/s10534-018-0147-x
Upadhyay, M., and Agarwal, S. (2020). Ironing the mitochondria: relevance to its dynamics. Mitochondrion 50, 82–87. doi: 10.1016/j.mito.2019.09.007
Vanden Berghe, T., Linkermann, A., Jouan-Lanhouet, S., Walczak, H., and Vandenabeele, P. (2014). Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat. Rev. Mol. Cell Biol. 15, 135–147. doi: 10.1038/nrm3737
Vogtle, F. N., Prinz, C., Kellermann, J., Lottspeich, F., Pfanner, N., and Meisinger, C. (2011). Mitochondrial protein turnover: role of the precursor intermediate peptidase Oct1 in protein stabilization. Mol. Biol. Cell 22, 2135–2143. doi: 10.1091/mbc.E11-02-0169
Vos, M., Lauwers, E., and Verstreken, P. (2010). Synaptic mitochondria in synaptic transmission and organization of vesicle pools in health and disease. Front. Synaptic Neurosci. 2:139. doi: 10.3389/fnsyn.2010.00139
Wang, S. Y., Singh, K., and Lin, S. C. (2012). The Association between Glaucoma Prevalence and Supplementation with the Oxidants Calcium and Iron. Invest. Ophthalmol. Vis. Sci. 53, 725–731. doi: 10.1167/iovs.11-9038
Wang, Y. Q., Chang, S. Y., Wu, Q., Gou, Y. J., Jia, L., Cui, Y. M., et al. (2016). The Protective Role of Mitochondrial Ferritin on Erastin-Induced Ferroptosis. Front. Aging Neurosci. 8:308. doi: 10.3389/fnagi.2016.00308
West, A. E., Neve, R. L., and Buckley, K. M. (1997). Identification of a somatodendritic targeting signal in the cytoplasmic domain of the transferrin receptor. J. Neurosci. 17, 6038–6047. doi: 10.1523/jneurosci.17-16-06038.1997
Williams, P. A., Harder, J. M., Foxworth, N. E., Cochran, K. E., Philip, V. M., Porciatti, V., et al. (2017). Vitamin B3 modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science 355, 756–760. doi: 10.1126/science.aal0092
Williams, P. A., Piechota, M., von Ruhland, C., Taylor, E., Morgan, J. E., and Votruba, M. (2012). Opa1 is essential for retinal ganglion cell synaptic architecture and connectivity. Brain 135, 493–505. doi: 10.1093/brain/awr330
Wolff, N. A., Garrick, M. D., Zhao, L., Garrick, L. M., Ghio, A. J., and Thevenod, F. (2018). A role for divalent metal transporter (DMT1) in mitochondrial uptake of iron and manganese. Sci. Rep. 8:211. doi: 10.1038/s41598-017-18584-4
Xie, Y., Hou, W., Song, X., Yu, Y., Huang, J., Sun, X., et al. (2016). Ferroptosis: process and function. Cell Death Differ. 23, 369–379. doi: 10.1038/cdd.2015.158
Yamano, K., and Youle, R. J. (2013). PINK1 is degraded through the N-end rule pathway. Autophagy 9, 1758–1769. doi: 10.4161/auto.24633
Yamasaki, S., Sakata-Sogawa, K., Hasegawa, A., Suzuki, T., Kabu, K., Sato, E., et al. (2007). Zinc is a novel intracellular second messenger. J. Cell Biol. 177, 637–645. doi: 10.1083/jcb.200702081
Yang, H., Yang, M., Guan, H., Liu, Z., Zhao, S., Takeuchi, S., et al. (2013). Mitochondrial ferritin in neurodegenerative diseases. Neurosci. Res. 77, 1–7. doi: 10.1016/j.neures.2013.07.005
Yin, H. Z., and Weiss, J. H. (1995). Zn(2+) permeates Ca(2+) permeable AMPA/kainate channels and triggers selective neural injury. Neuroreport 6, 2553–2556. doi: 10.1097/00001756-199512150-00025
Yoon, Y. S., Yoon, D. S., Lim, I. K., Yoon, S. H., Chung, H. Y., Rojo, M., et al. (2006). Formation of elongated giant mitochondria in DFO-induced cellular senescence: involvement of enhanced fusion process through modulation of Fis1. J. Cell. Physiol. 209, 468–480. doi: 10.1002/jcp.20753
Youle, R. J., and van der Bliek, A. M. (2012). Mitochondrial fission, fusion, and stress. Science 337, 1062–1065. doi: 10.1126/science.1219855
Zeng, X. M., Neupert, W., and Tzagoloff, A. (2007). The metalloprotease encoded by ATP23 has a dual function in processing and assembly of subunit 6 of mitochondrial ATPase. Mol. Biol. Cell 18, 617–626. doi: 10.1091/mbc.E06-09-0801
Zhang, B., Rusciano, D., and Osborne, N. N. (2008). Orally administered epigallocatechin gallate attenuates retinal neuronal death in vivo and light-induced apoptosis in vitro. Brain Res. 1198, 141–152. doi: 10.1016/j.brainres.2007.12.015
Zhang, X., Feng, Y., Wang, Y., Wang, J., Xiang, D., Niu, W., et al. (2018). Resveratrol ameliorates disorders of mitochondrial biogenesis and dynamics in a rat chronic ocular hypertension model. Life Sci. 207, 234–245. doi: 10.1016/j.lfs.2018.06.010
Keywords: glaucoma, retinal ganglion cell, mitochondria, mitophagy, iron, zinc, ferroptosis
Citation: Tang J, Zhuo Y and Li Y (2021) Effects of Iron and Zinc on Mitochondria: Potential Mechanisms of Glaucomatous Injury. Front. Cell Dev. Biol. 9:720288. doi: 10.3389/fcell.2021.720288
Received: 04 June 2021; Accepted: 22 July 2021;
Published: 10 August 2021.
Edited by:
Hao Chen, Guangdong Academy of Medical Sciences, ChinaReviewed by:
Colin Barnstable, The Pennsylvania State University, United StatesYi Dai, Fudan University, China
Guy Perkins, University of California, San Diego, United States
Makoto Ishikawa, Tohoku University, Japan
Copyright © 2021 Tang, Zhuo and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yiqing Li, bGl5aXFpbmczQG1haWwuc3lzdS5lZHUuY24=
 Jiahui Tang
Jiahui Tang Yehong Zhuo
Yehong Zhuo Yiqing Li
Yiqing Li