- 1Department of Genetics and Bioinformatics, Dasman Diabetes Institute, Dasman, Kuwait
- 2Department of Animal and Imaging Core Facilities, Dasman Diabetes Institute, Dasman, Kuwait
- 3Department of Immunology and Microbiology, Dasman Diabetes Institute, Dasman, Kuwait
- 4Biointelligence Technology Systems S.L., Barcelona, Spain
The fields of regenerative medicine and stem cell-based tissue engineering have the potential of treating numerous tissue and organ defects. The use of adult stem cells is of particular interest when it comes to dynamic applications in translational medicine. Recently, dental pulp stem cells (DPSCs) have been traced in third molars of adult humans. DPSCs have been isolated and characterized by several groups. DPSCs have promising characteristics including self-renewal capacity, rapid proliferation, colony formation, multi-lineage differentiation, and pluripotent gene expression profile. Nevertheless, genotypic, and phenotypic heterogeneities have been reported for DPSCs subpopulations which may influence their therapeutic potentials. The underlying causes of DPSCs’ heterogeneity remain poorly understood; however, their heterogeneity emerges as a consequence of an interplay between intrinsic and extrinsic cellular factors. The main objective of the manuscript is to review the current literature related to the human DPSCs derived from the third molar, with a focus on their physiological properties, isolation procedures, culture conditions, self-renewal, proliferation, lineage differentiation capacities and their prospective advances use in pre-clinical and clinical applications.
Introduction
Dental pulp stem cells (DPSCs) are a unique population of cells embedded within the pulp cavity of the impacted third molars. DPSCs were initially isolated and characterized by Gronthos et al. (2000). Subsequently, several investigators have reported DPSCs’ isolation, characterization, differentiation, and banking (Atari et al., 2012; Ferro et al., 2012a; Tirino et al., 2012). In comparison to other adult stem cells, DPSCs are noted for their high recovery rate from the disposable dental pulp after occlusion management. Their isolation procedure involves non-invasive techniques and has no notable ethical constraints. Significantly, DPSCs’ stemness, viability, proliferation, and differentiating capabilities are not compromised after cryopreservation (Zhang et al., 2006; Pilbauerova et al., 2021b). Therefore, DPSCs have the potential to be a promising personalized patient-specific stem cells source for regenerative therapy. In this review article, we will discuss the tooth anatomy and dental stem cells with a particular interest on the current advances in adult human DPSCs including their origin, biological characteristics, heterogenicity, differentiation, and immunomodulatory potentials, as well as paracrine effects and pre-clinical and clinical applications.
Anatomical Structure of the Tooth
Teeth are viable organs made up of well-organized structures with numerous but defined specific shapes (Magnusson, 1968). Odontogenesis or teeth generation undergoes several complex developmental stages that are yet to be fully defined (Smith, 1998; Zheng et al., 2014; Rathee and Jain, 2021). Remarkably, the tooth tissues originate from different cell lineages. The enamel develops from cells derived from the ectoderm of the oral cavity, whereas the cementum, dentin, and pulp tissues are derived from neural crest-mesenchyme cells of ectodermal and mesodermal origins (Figure 1A; Miletich and Sharpe, 2004; Thesleff and Tummers, 2008; Caton and Tucker, 2009; Koussoulakou et al., 2009). The lineage diversities may explain the observed differences in tissue topography and physiological function. The enamel-producing cells and associated metabolites are lost during tooth eruption, whereas pulp cells are longevous and have the capacity to undergo remodeling and regeneration (Simon et al., 2014).
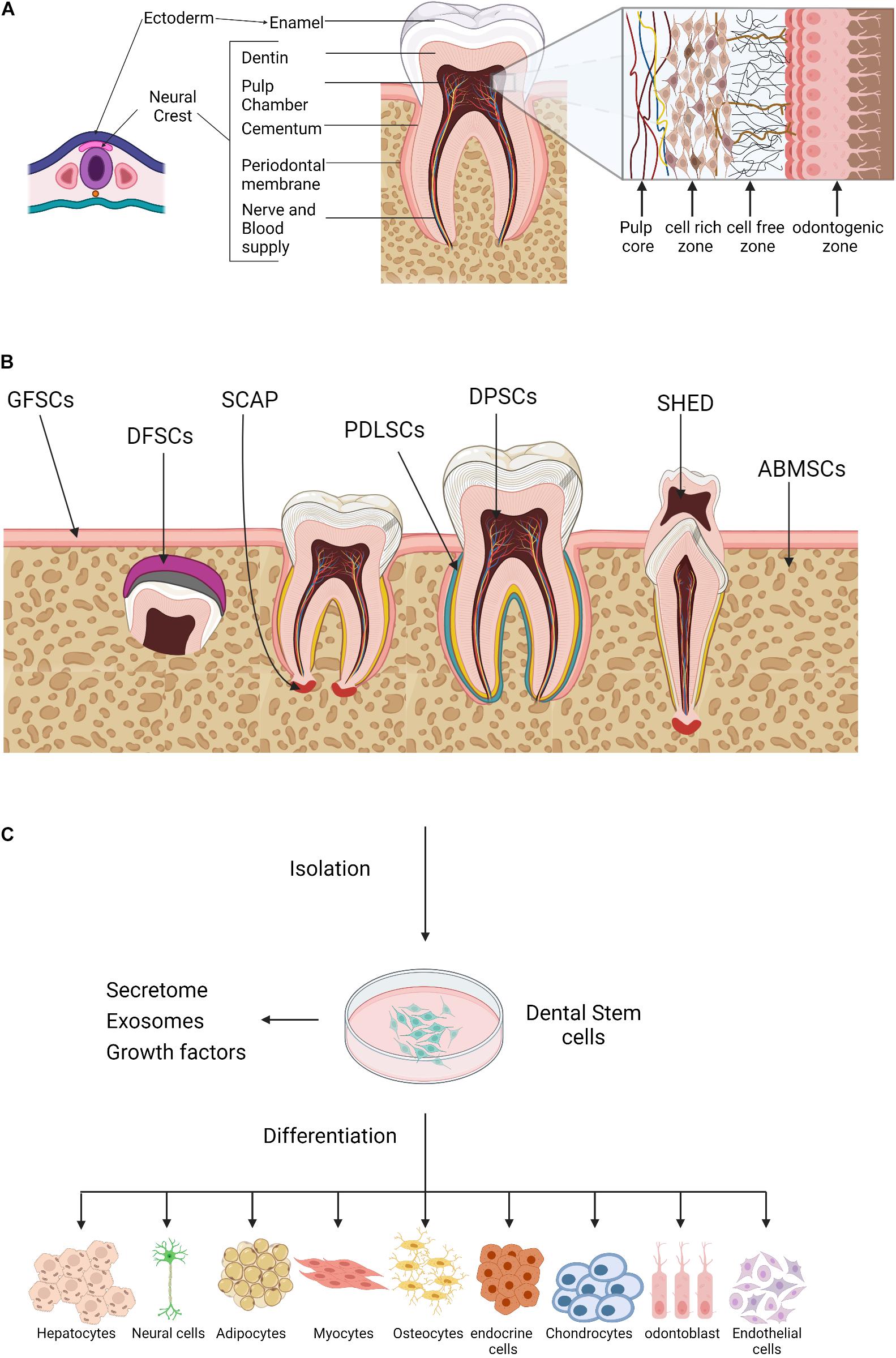
Figure 1. Oral tissue and dental stem cells. (A) A schematic outline for the tooth development. Ectoderm cells contribute to the formation of the tooth enamel only, whereas, neural crest cells generate the rest of the tooth tissues. (B) Sources of oral tissue and dental stem cells. GMSCs, gingiva-derived mesenchymal stem cells; DFPCs, dental follicle precursor cells; SCAP, stem cells from apical papilla; PDLSCs, periodontal ligament stem cells; DPSCs, dental pulp stem cells; SHED, stem cells from human exfoliated deciduous teeth, ABMSCs, alveolar bone–derived mesenchymal stem cell. (C) A schematic illustration of the dental stem cells multilineage differentiation potential. Created with BioRender.com.
The dental pulp is a highly vascularized connective tissue, consists of four zones, namely (1) the peripheral odontogenic zone, (2) intermediate cell-free zone, (3) cell-rich zone, and (4) the pulp core (Figure 1A, insert). Adjacent to the dentin layer, the peripheral odontogenic zone contains the specialized columnar odontoblast cells that produce dentin (Gotjamanos, 1969; Sunitha et al., 2008; Pang et al., 2016; Ghannam et al., 2021). Besides the extensive vascular and neuronal network, the cell-free zone or basal layer of Weil contains a small number of cells whereas, high density of specialized cells is observed in the underlying zones. The cell-rich zone contains fibroblasts, macrophages, capillaries and proliferating mesenchymal cells that can differentiate into odontoblasts. The pulp core is populated by multiple populations of dental mesenchymal cells, mesenchymal-like stem cells, macrophages and dendritic cells that maintain the dentin-pulp complex functionality and homeostasis (Berkovitz, 1989; Abd-Elmeguid and Yu, 2009; Farges et al., 2015).
Stem Cells in the Tooth Compartments
Like other organs, tooth compartments harbor a niche of heterogeneous stem/progenitor cell populations of the embryonic stem cells; however, the developmental stage for most dental stem cells has not been established yet and their precise role remains poorly understood (Kaukua et al., 2014; Krivanek et al., 2017). Several studies have indicated that in mild tooth trauma and post-inflammatory recovery, these cells regenerate dentin barrier to protect the pulp from infectious agents and demonstrate an immunomodulatory capacity, either via secreting proinflammatory cytokines or through crosstalk with immune cells (Lesot, 2000; Tomic et al., 2011; Hosoya et al., 2012; Leprince et al., 2012; Li et al., 2014).
The various sources of dental progenitor cells include the DPSCs (Gronthos et al., 2000), stem cells from human exfoliated deciduous teeth (SHED) (Miura et al., 2003), periodontal ligament stem cells (PDLSCs) (Seo et al., 2004), dental follicle stem cells (DFSCs) (Morsczeck et al., 2005), stem cells from apical papilla (SCAP) (Sonoyama et al., 2006, 2008), and gingival stem cells (GING SCs) (Mitrano et al., 2010; Figure 1B). Like bone marrow-derived mesenchymal stem cells (BM-MSCs), dental progenitor/stem cells exhibit self-renewal capacity and multilineage differentiation potential. In vitro studies have shown that dental stem cells generate clonogenic cell clusters, possess high proliferation rates and have the potential of multi-lineage differentiation into a wide spectrum of cell types from the three germ layers or, at least in part, express their specific markers under the appropriate culture conditions (Figure 1C). Despite being similar at a coarse level, the transcriptomic and proteomic profiles of oral stem cells reveal several molecular differences including differential expression of surface marker, structural proteins, growth hormones, and metabolites; indicating prospective developmental divergence (Hosmani et al., 2020; Krivanek et al., 2020), and also suggest that dental stem cells might be the optimal choice for tissue self-repair and regeneration.
Dental Stem Cell Lineage Tracing
The plasticity and multi-potential competency of oral stem cells owe to the fact that the dental pulp contains neuro-mesenchymal components. Genetic lineage tracing studies have identified the perivascular pericytes and glial cells as immediate ancestors of the dental stem cells. Utilizing mouse incisors’ regenerative capacity as a model for recovery from pulp/dentin injury, Feng et al. (2011) identified the pericytes NG+ cells as oligodendrocyte progenitors. Post trauma, pericytes NG+ cells proliferate rapidly and partially contribute to the generated odontoblasts (Pang et al., 2016). Later, Zhao et al. (2014) reported the role of neurovascular sensory cells in activating periarterial Gli1+ cells through sonic hedgehog signaling pathways, which were sufficient to maintain homeostasis and injury repair of the incisor mesenchyme. Notably, lineage tracing studies revealed that Gli1+ cells contributed to the entire pericytes NG+ cell population, but not vice versa (Zhao et al., 2014). Therefore, periarterial Gli1+ cells are believed to be the sole source of odontoblast derivation. These observations were further supported experimentally by combining a clonal color-coding technique with tracing of peripheral glia cells, in addition, quantification analysis revealed that both pericytes and glial cells contribute equally to the dynamics of tooth organogenesis, homeostasis, and growth (Kaukua et al., 2014; Sharpe, 2016; Shi et al., 2020).
Dental Pulp Stem Cells Isolation Procedures and Culture Conditions
Dental pulp stem cells constitute merely 5% of the pulp cells and they were first isolated and characterized by Gronthos et al. (2000). The quality of the isolated DPSCs primarily impacts their regenerative potential. The culturing method and accurate characterization are pivotal steps for the isolation of high-quality DPSCs. Following extraction of the third molar, further procedures include mechanical extraction of the soft pulp connective tissue, maceration, enzymatic digestion of extracellular matrix proteins (ECM), and cell growth in plastic tissue/cell culture plates. The various isolation and culture procedures used for the human DPSCs have been best reviewed by Rodas-Junco and Villicana (2017).
Here, we also describe the standard procedure used in our clinic and laboratory. Briefly, immediately after extraction, the third molar is thoroughly rinsed with ethanol and sterile distilled water. Using a cylindrical turbine bur, an incision is made between the enamel and the cement at the point of molar fracture. The fragmented tooth is refreshed in PBS in sterile tubes and rushed to the laboratory. Using aseptic techniques, the tooth is transferred to a petri dish and dental pulp tissue is isolated using a sterile nerve-puller file-15 and forceps, chopped into fine fragments and digested by collagenase type I for 60 min at 37°C. Single cell suspension is prepared by first passing cells through an insulin syringe and then passing through cell strainer with 40 μm APD, followed by centrifugation. The cell fraction is washed with sterile PBS, counted and cells are seeded in culture medium. For primary culture establishment, cells are seeded in fibronectin-coated culture dishes. At 60% confluency, DPSCs are passed at a cell density of 80–100 cells/cm2. DPSCs expansion medium consists of 60% DMEM-low glucose and 40% chick fibroblast basal medium MCDB-201, supplemented with a myriad of factors such as Insulin-Transferrin-Selenium (ITS), linoleic acid bovine serum albumin (LA-BSA), dexamethasone (dex), ascorbic acid 2-phosphate (Asc-2P), antibiotics (Penicillin/Streptomycin), human Platelet-Derived Growth Factor (hPDGF)-BB, human Epidermal Growth Factor (hEGF), human Leukemia Inhibitory Factor (hLIF), Chemically Defined Lipid Concentrate (CDLC), and β-mercaptoethanol (Atari et al., 2012; Martinez-Sarra et al., 2017; Nunez-Toldra et al., 2017a,b; Al Madhoun et al., 2018; Faruqu et al., 2020).
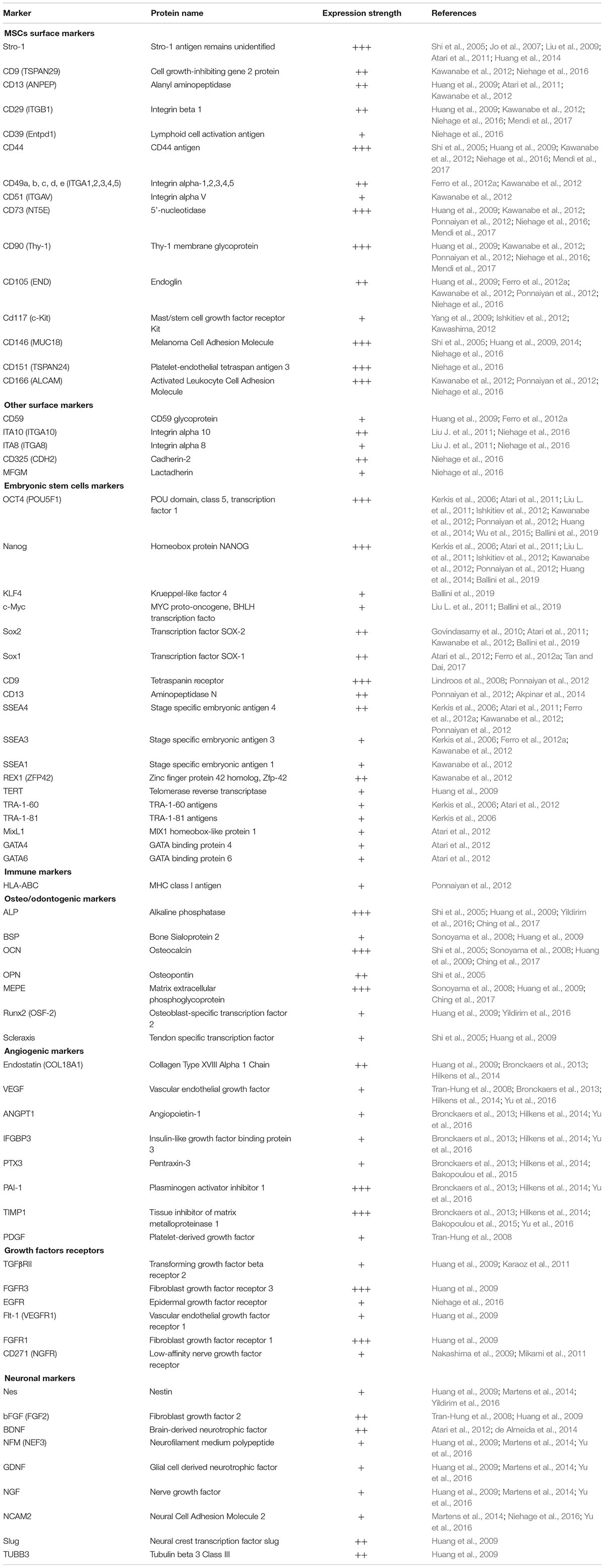
Dental Pulp Stem Cells Markers
Dental pulp stem cells are a heterogeneous mixture of cell populations with no distinct cell surface antigens (Kawashima, 2012). DPSCs display characteristics that are much similar to those of MSCs such as the abilities for self-renewal and multilineage differentiation. According to the minimal criteria defined by the International Society of Cellular Therapy (ISCT) for the human MSCs, these cells adhere to plastic, express CD29, CD44, CD49a-f, CD51, CD73 (SH3), CD90, CD105 (SH2), CD106, and CD166, and lack the expression of the hemopoietic surface antigens including CD11b, CD14, CD19, CD34, CD45, CD79a, and human leukocyte antigen-DR isotype (HLA-DR) (Dominici et al., 2006; Sonoyama et al., 2006; Huang et al., 2009; Wu et al., 2015). DPSCs express a wide spectrum of other surface markers also as shown in Table 1. However, notable complexity and divergence in their expression levels have been reported by several groups (Laino et al., 2005; Yamada et al., 2010; Hilkens et al., 2013; Niehage et al., 2016; Alraies et al., 2020) which could be attributed, at least in part, to their heterogenicity. DPSCs can be enriched by using different isolation procedures and cell culture conditions. For example, their surface marker expression may vary depending on the serum concentrations and/or the addition of growth factors to the basal culture media. Martens et al. (2012) have documented expression of the neural markers (nestin, vimentin, synaptophysin, S100, and βIII-tubulin) on undifferentiated DPSCs that were cultured in media containing 10% FBS. Longoni et al. (2020) reported fibrous cartilage tissue conversion (expression of aggrecan, glycosaminoglycan, elevated expression of collagen type I, and limited expression of collagen type II) of DPSCs using chondro-inductive growth factors such as insulin-like growth factor (IGF)-1, transforming growth factor (TGF)-β3, and bone morphogenetic protein (BMP)-2, -6, -7. Notably, Zhang et al. (2008) have reported adipogenic, myogenic, and odontogenic plasticity of the DPSCs using respective lineage-specific pre-inductions media in vitro.
Antibody-based methods, proteomics and RNA transcriptomics are the main procedures used for DPSCs immunophenotyping. Besides the MSCs markers, DPSCs possess the embryonic stem cell-specific markers (Table 1). In addition, DPSCs express a variety of antigens associated with cell adhesion, growth factors, transcription regulation and multiple lineage-specific markers related to perivascular tissue, endothelium, immunogenic, neuronal and osteo/odontogenic tissues (Table 1). It is also noteworthy to mention that DPSCs express Major Histocompatibility Complex (MHC) class I antigens, but they do not express the immune co-stimulating molecules such as MHC class II antigen HLA-DR, CD40, CD80, and CD86 (Wada et al., 2009; Bhandi et al., 2021; Pilbauerova et al., 2021a).
Heterogeneity of Dental Pulp Stem Cells
The heterogeneity of the DPSCs subpopulations isolated from different donors is mainly influenced by donor health, age, genetic, and environmental factors (Kellner et al., 2014; Wu et al., 2015; Alraies et al., 2017; Kobayashi et al., 2020; Longoni et al., 2020). Alternatively, the intra-population heterogeneity refers to the DPSCs subpopulations found within the preparation from a single individual. The evidence demonstrating that DPSCs populations are functionally heterogeneous comes largely from their surface antigen profile or expression patterns of a variety of markers that are associated with progenitors of different lineages. DPSCs show surface expression of STRO-1, CD13, CD29, CD44, CD73, CD90, CD105, CD146, and CD166 – a profile which is reminiscent of BM-MSCs (Table 1), while DPSCs lack the expression of hematopoietic (CD34 and CD45) and monocytic (CD14) markers (Yamada et al., 2010). Additionally, DPSCs express various pluripotency markers, such as Oct-3/4, Nanog, and Sox-2, i.e., the stemness-related markers observed in embryonic stem cells (Table 1) which explains, at least in part, their self-renewal potential (Atari et al., 2011, 2012; Ferro et al., 2012b; Faruqu et al., 2020). As neuronal crest-derived cells, DPSCs express several neural stem cell markers, including nestin, neuronal nuclei antigen, vimentin, synaptophysin, musashi-1, Galactosyl-ceramidase, S100 calcium binding protein B, neurofilament heavy (NFH) chain, class III β-tubulin, and neurofilaments (Table 1). Thus, DPSCs are comprised of the progenitor cells that are marked by diverse characteristics, such as clonal heterogeneity, multi-lineage differentiation, self-renewal capacity, and phenotypic complexity.
Notably, specific conditions and media components used may act as a source of potential phenotypic and functional changes in the freshly extracted DPSCs. The isolation procedures may also influence their heterogeneity. Whereas, DPSCs isolation by enzymatic digestion provides a large number of cells at low passage rate, the tissue explants enable the isolation of a more homogeneous cell population (Bronckaers et al., 2013; Raoof et al., 2014). Furthermore, DPSCs heterogeneity is impacted by the culture media components, serum concentration, and growth factors supplements, all of which have been well-reviewed by Rodas-Junco and Villicana (2017). Moreover, long-term and large-scale expansion in culture may also impact the heterogeneity, survival, and differentiation potential of DPSCs. The selection and expansion of different DPSCs subpopulations driven by specific culture conditions, media supplementation and 2D/3D culture systems may collectively alter the cellular profile, homeostasis, plasticity, and regenerative potential, as well as immunomodulatory properties of DPSCs (further elaborated below).
Dental Pulp Stem Cells Crosstalk With Microenvironment in Homeostasis
Dental pulp stem cells are located within a heterogenic niche. Homeostatic regulation of the DPSCs niche, DPSCs’ proliferation and differentiation implicate a complex network of bioactive molecules, growth factors, ECM, and key signaling pathways (Scheller et al., 2008; Mitsiadis et al., 2011; Tsutsui, 2020; Deng et al., 2021). Nevertheless, the signals that regulate DPSC fate are not only the biochemical cues, but also the biophysical cues (mechanical signals) that play a crucial role in influencing DPSC fate since orthodontic mechanical tension or stresses are exerted to teeth and transmitted into the dental pulp tissue by jaw movement during the process of normal mastication (Tatullo et al., 2016). Thus, DPSCs are mechanosensitive cells by default with the capacity to recognize mechanical signals and transform these stimuli into various cellular responses to sustain niche homeostasis (Han et al., 2008, 2010; Hata et al., 2013). Importantly, Marrelli et al. (2018) have presented an excellent review of the mechanobiology and mechanoresponsiveness of DPSCs, deciphering how the mechanical stimuli might regulate behavior, fate, and homeostasis of DPSCs. These studies may enhance our understanding and improve approaches to the DPSC-based tissue engineering applications.
Dental Pulp Stem Cells Potential Role in Tissue Repair and Flourishment
Dental pulp stem cells could be valuable source for cell therapy and advancing the current regenerative medicine strategies. DPSCs plasticity to surrounding environment has made them a notable source for disease treatment, though the full understanding of DPSCs tissue repair mechanisms is still in their preliminary stages. In this section, we will review the current advances in DPSCs in vitro differentiation potentials and the capability to secrete growth factors that may contribute to their role in tissue repair.
Differentiation Potential of Dental Pulp Stem Cells
Due to their potential to differentiate into several cell lineages (Figure 1C), DPSCs have received extensive attention in the field of regenerative medicine and tissue engineering. DPSCs have the potential to differentiate into endodermal (respiratory and gastrointestinal tracts, liver, pancreas, thyroid, prostate, and bladder lineages), mesodermal (adipogenic, osteogenic, and chondrogenic lineages) and ectodermal (skin and neural lineages) (Yamada et al., 2019). In addition, DPSCs were shown to differentiate into myocytes, cardiomyocytes, hepatocyte-like cells, melanocytes, and active neurons (Figure 1C; Stevens et al., 2008; Patil et al., 2014). As a thumb rule, a substantial improvement in the efficacy DPSCs differentiation was observed using defined conditioned media.
Several recent reviews have documented the current knowledge and understanding of DPSCs’ differentiation into vital lineages including their angiogenic and neurogenic potential (Ratajczak et al., 2016; Mortada et al., 2018; Mattei et al., 2021), odontogenic and chondrogenic potential (Nuti et al., 2016; Ching et al., 2017; Mortada and Mortada, 2018), and periodontal and dental tissue regeneration (Hu et al., 2018; Zhai et al., 2019); Therefore, we will focus in this section in summarizing the current knowledge regarding DPSCs hepatogenic and pancreatic β-cells differentiation capacities.
Differentiation of Dental Pulp Stem Cells Into Hepatocytes
Implementing defined, serum-free, and stepwise differentiation protocols that mimic the developmental stages of hepatocytes during embryogenesis were found to be sufficient for inducing hepatogenesis (summarized in Table 2). Using this approach, Ishkitiev et al. (2010) were the pioneers to demonstrate the hepatogenic differentiation potential of DPSCs. Initially, they developed the Ishkitiev et al. (2010) developed the two-stage conditioned media that contained low percentage of fetal bovine serum (FBS) but was enriched with essential hepatogenic inducers (see Table 2). Later, they used a serum-free conditioned medium to generate the hepatocyte-like cells from a CD117+ DPSCs subpopulation (Ishkitiev et al., 2012). The latter hepatogenesis protocol utilized three developmental stages, i.e., cell specification, differentiation, and maturation to generate cells with phenotypical, and functional characteristics similar to hepatocytes. This approach was further improved and implemented in later studies (Table 2; Ferro et al., 2012a; Kumar et al., 2017). Recently, we implemented a similar approach to differentiate a pluripotent-like subpopulation of DPSCs into hepatocyte-like cells with detailed characterization of each differentiation stage and associated markers (Gil-Recio et al., 2020).
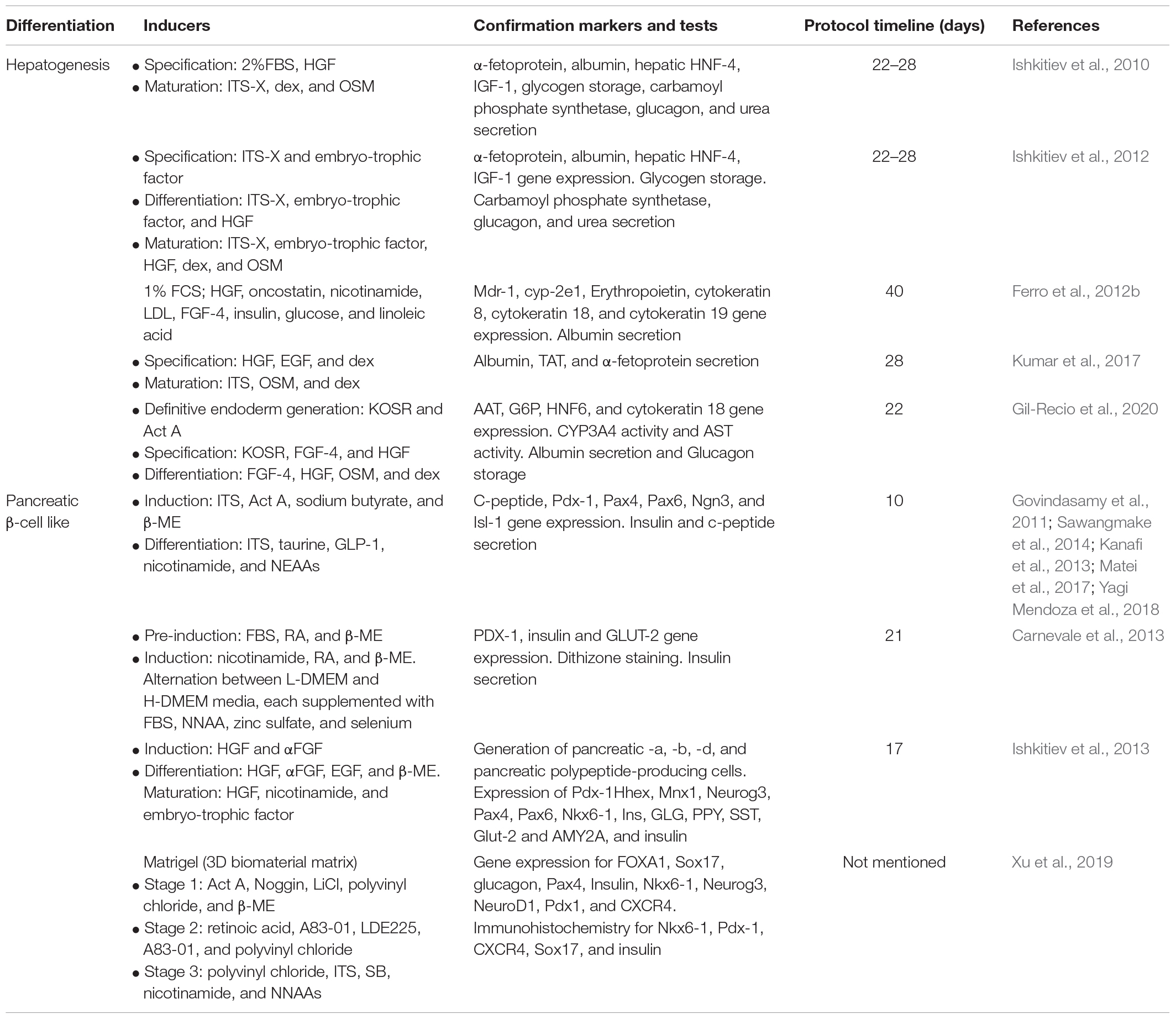
Table 2. Protocols and composition of media used by different studies to generate hepatocytes and insulin producing pancreatic β-cells from DPSCs.
Differentiation of Dental Pulp Stem Cells Into Pancreatic Insulin-Producing Cells
There are several reports (summarized in Table 2) describing the differentiation of DPSCs into glucose-responsive pancreatic insulin-producing β-cells (IPCs). Using a three-step differentiation procedure, IPCs induction and functionality were confirmed by insulin secretion and C-peptide expression in a glucose-dependent manner (Table 2; Govindasamy et al., 2011; Carnevale et al., 2013; Sawangmake et al., 2014). Interestingly, another study demonstrated that DPSCs-derived IPCs were physiologically functional and, as expected, reversed hyperglycemia to the normal level in streptozotocin (STZ)-induced diabetic mice (Kanafi et al., 2013). Furthermore, Matei et al. (2017) observed that hydrogen sulfide exposure increases insulin and C-peptide secretions, protects against glucotoxicity, and enhances the expression insulin and PI3K/AKT pathway. As a proof of principle, Ishkitiev et al. (2013) confirmed that CD117+ DPSCs subpopulation generated a heterogeneous population of cells that expressed pancreatic-specific endocrine and exocrine markers (Table 2). Notably, comparative studies between 2D and 3D culture systems revealed that IPCs in 3D models mimic in vivo cell growth and possess phenotypical structures like native pancreatic islets (Yagi Mendoza et al., 2018; Xu et al., 2019). Importantly, Xu et al. (2020) documented that coating IPCs with Matrigel, a basement membrane matrix, improves cell survival after orthotopic injection into the pancreatic parenchyma of Sprague Dawley (SD) rats (Table 3).
Paracrine Activity of Dental Pulp Stem Cells: Secretome and Exosomes
Currently, accumulating evidence indicates that a great deal of therapeutic benefit of primary DPSCs exists in their paracrine activity, that is, the ability to modulate their microenvironment through the release of bioactive molecules. These factors can be released directly into the surrounding microenvironment known as secretome or they can be embedded within the membrane-bound extracellular nanovesicles (∼30–150 nm in diameter), known as exosome (Thery et al., 2002). These factors include cytokines, chemokines, growth factors, angiogenic mediators, hormones, and regulatory nucleic acid molecules (Wei et al., 2020). In general, secretome and exosomes participate in the processes of tissue replenishment, cellular homeostasis, anti-inflammation, immunomodulation, and other functions (Tang et al., 2021).
In relation to tumor tropism, Altanerova et al. (2016) engineered DPSCs to express the fused yeast suicide gene cytosine deaminase::uracil phosphoribosyl transferase (yCD::UPRT), a gene that converts the nontoxic 5-fluorocytosine (5-FC) into the toxic 5-fluoro-20-deoxyuridine-50-monophosphate (5-FdUMP) (Graepler et al., 2005). Exosomes released from these engineered DPSCs were sufficient to integrate human tumor cells transplanted into the brain of rat models and were able to induce apoptosis in the presence of pro-drug 5-FC (Altanerova et al., 2016).
Secretome of DPSCs contains neurotrophic factors (NDNF, NT-3, NGF, and GDNF) and TGF-β, possessing the therapeutic potential for neurodegenerative diseases (de Almeida et al., 2011). In Alzheimer’s disease (AD) cell model, DPSCs’ secretome reduced the amyloid beta (Aβ) peptide-mediated cell cytotoxicity and apoptosis by stimulating the endogenous survival factor Bcl-2 and decreasing the apoptotic regulator Bax (Ahmed Nel et al., 2016). DPSCs secretome promoted proliferation, migration, and survival in Schwann peripheral glial cells (Yamamoto et al., 2016) and SH-SY5Y neuroblastoma cells (Gervois et al., 2017). Furthermore, conditioned media from DPSCs improved neuromuscular junction innervation and motor neuron survival in a mouse model of amyotrophic lateral sclerosis (ALS) (Wang et al., 2019), and promoted the survival of retinal ganglion cells in a rat model for optic nerve injury (Mead et al., 2014).
Dental pulp stem cells secrete angiogenic growth factors including VEGF, bFGF, and PDGF which are sufficient to mediate the formation of a network of tubular structures of endothelial cells, an indicator of angiogenic stimulation (Zhou H. et al., 2020). Furthermore, conditioned media from cultured DPSCs promoted wound healing, angiogenesis and soft-tissue regeneration in a mouse model of excisional wound healing (Yang et al., 2013).
Taken together, DPSCs secretome and exosome are rich in trophic factors that can mediate tissue regeneration and proliferation, which could be highly useful for prospective cell-free regenerative medicine and advantageous over interventions involving cell transplantation (Vizoso et al., 2017). Nevertheless, a proper characterization of the DPSCs’ secretome and exosome is required since they are significantly influenced by culture conditions (Chin et al., 2021), hypoxia (Aranha et al., 2010), insult (Mattei et al., 2021), DPSCs passage (Faruqu et al., 2020), subpopulation (Nakashima et al., 2009), and stage of differentiation (Huang et al., 2016).
Immunomodulatory Properties of Dental Pulp Stem Cells
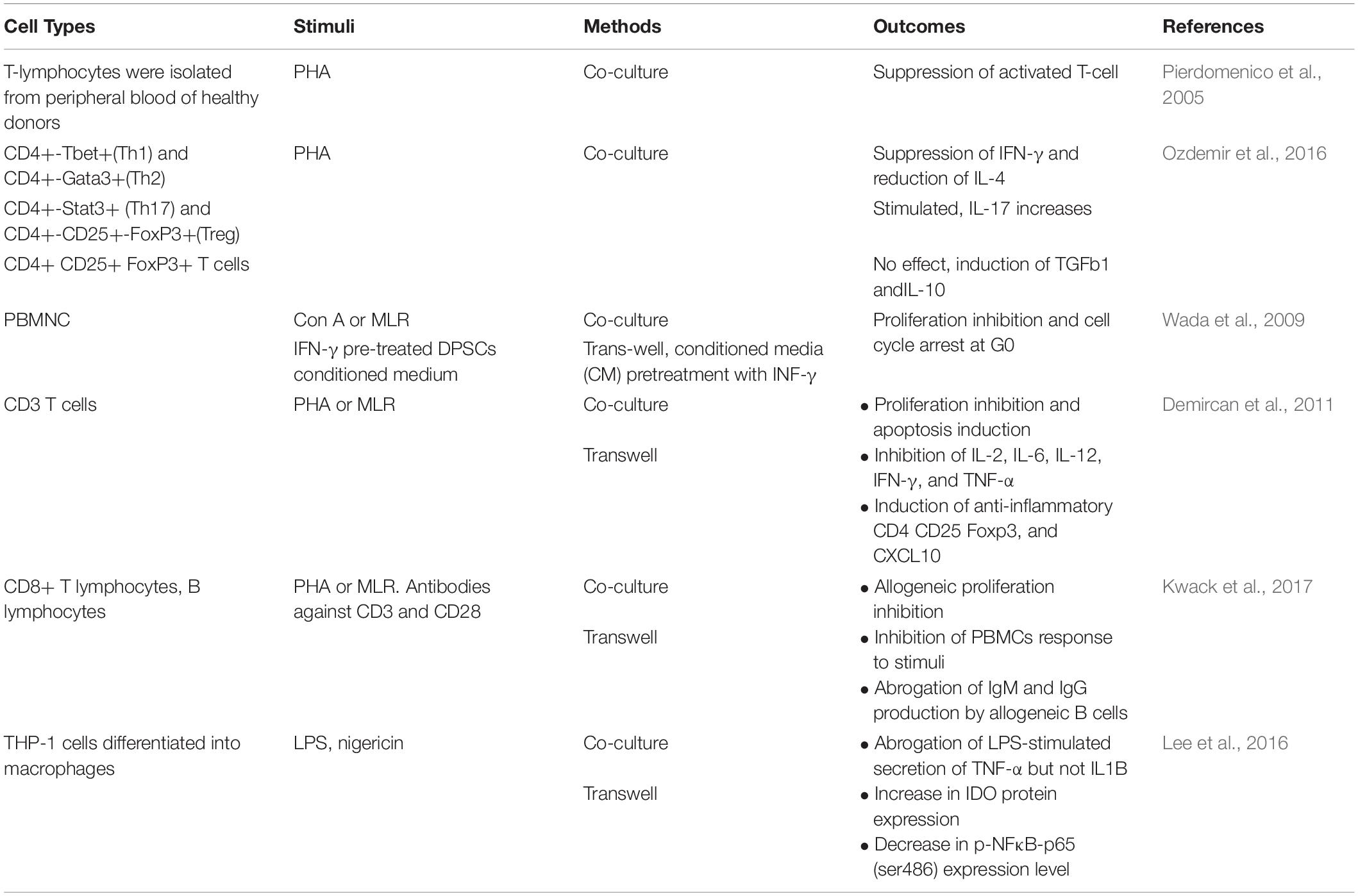
The crosstalk between DPSCs and immune cell subsets impacts the functioning of both the innate and adaptive immune systems, implying that DPSCs have immunomodulatory properties – an exciting field that needs to be further investigated [best reviewed in Li et al. (2014) and Andrukhov et al. (2019)]. Immunomodulatory phenotype of DPSCs is primarily attributed to in vitro cell culture approaches and conditions, such as enzymatic stimuli, soluble factors secretions, and cell-to-cell contacts. As though, all of these attributes may not precisely mimic the complexity of the in vivo microenvironment, nevertheless, the data generated are valid, at least in part, with respect to the studied immune cell subsets.
Co-culture cell models have revealed that DPSCs mediate G0/G1 cell cycle arrest of the chemically-activated T cells (Table 3; Pierdomenico et al., 2005); while, other studies also show induction of differential T-cell subset responses. Co-cultures of DPSCs with CD3+, CD4+, or CD8+ T cells mediated differential proliferation arrest, apoptosis and/or induction of regulatory T cells (Treg) (Table 3; Demircan et al., 2011; Zhao et al., 2012; Ozdemir et al., 2016; Kwack et al., 2017). Interestingly, proliferation inhibition of the peripheral blood mononuclear cells (PBMCs) was observed in cultures with conditioned medium from DPSCs pre-treated with interferon (INF)-γ (Wada et al., 2009). Taken together, T lymphocyte activation and INF-γ production is a prerequisite for the induction of immunomodulatory DPSCs and secretion of IL-10/TGF-β1, expression of soluble factors inducing Treg formation and lymphocytes proliferation arrest (Ding et al., 2015; Kwack et al., 2017). Furthermore, co-culture of the DPSCs isolated from symptomatic irreversible pulpitis with macrophages suppresses the LPS-stimulated secretion of TNF-α, via TNF-α/IDO (indoleamine 2,3-dioxygenase) axis mechanism (Lee et al., 2016). In a rat model of diabetic neuropathy, DPSCs transplantation led to the anti-inflammatory M2-type macrophage polarization and ameliorated diabetic polyneuropathy (Omi et al., 2016). In addition, DPSCs were reported to express the complement cascade receptors C3aR and C5aR, and the treatment with C3a or C5a augmented DPSCs’ proliferation and mobilization (Chmilewsky et al., 2014; Rufas et al., 2016).
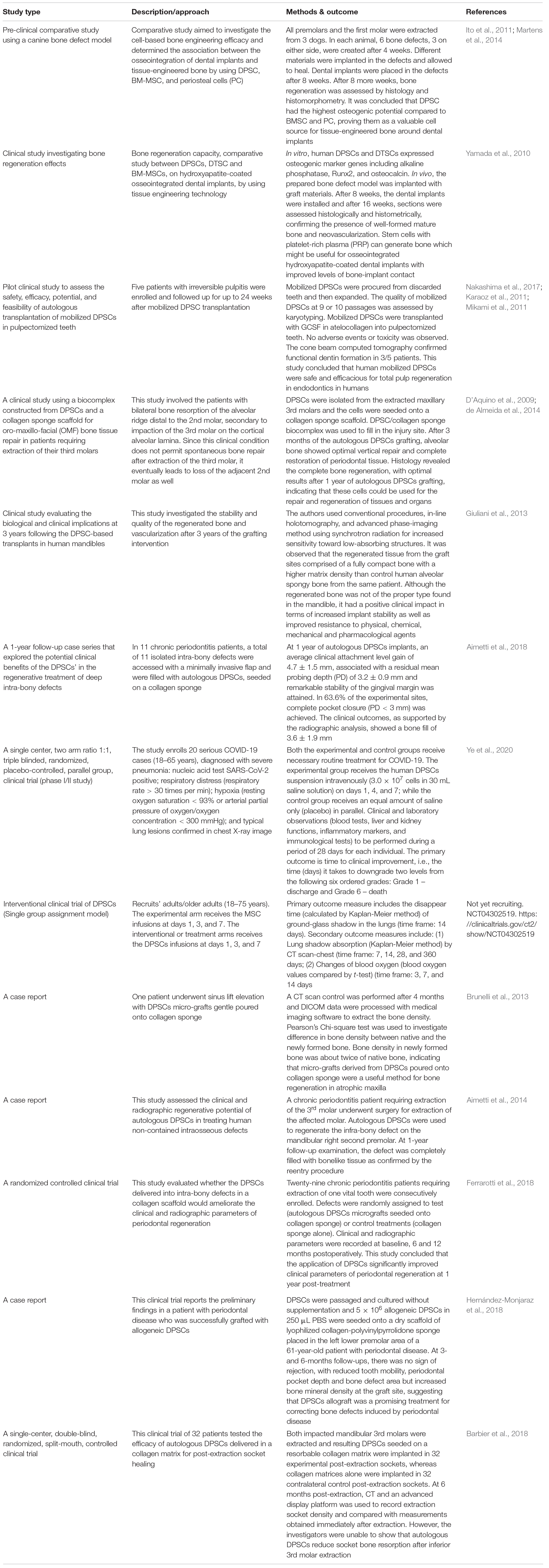
Pre-Clinical and Clinical Applications of Human Dental Pulp Stem Cells
Dental pulp stem cells have remarkable potential as alternative sources to multipotent MSCs and due mainly to their immunomodulatory properties as discussed earlier, DPSCs constitute a highly valuable source for cell therapy of a variety of inflammatory diseases and other disorders. Briefly, in regard to basic or pre-clinical studies in experimental animal models, the human DPSCs-based therapies, implicating cells or secretome/conditioned media, have been successfully used in several disease conditions, which has been extensively reviewed by Anitua et al. (2018) including diabetes (Govindasamy et al., 2011; Datta et al., 2017), neuropathy (Makino et al., 2019), hepatic diseases (Cho et al., 2015; Kim et al., 2016), oculopathies (Syed-Picard et al., 2015; Kushnerev et al., 2016; Mead et al., 2016), spinal cord injury (Sakai et al., 2012; Yang et al., 2017), peripheral nerve injury (Sasaki et al., 2011; Sanen et al., 2017), AD (Nakashima et al., 2009; Mita et al., 2015; Wang et al., 2017), cerebral ischemia (Leong et al., 2012; Song et al., 2017), muscular dystrophy (Kerkis et al., 2008; Pisciotta et al., 2015; Martinez-Sarra et al., 2017), myocardial infarction (Gandia et al., 2008), Parkinson’s disease (Nesti et al., 2011; Gnanasegaran et al., 2017a,b), lung injury (Wakayama et al., 2015), and stroke (Yang et al., 2009; Leong et al., 2012; Song et al., 2015). It is noteworthy that not many clinical trials have so far published their results. Table 4 summarizes the clinical investigations that have ingeniously tested the regenerative potentials and multifaceted benefits of human DPSCs in various trials.
Of note, owing to their anti-inflammatory properties and regenerative potentials, DPSCs are also being tested for their therapeutic benefits in coronavirus disease 2019 (COVID-19) patients. Like in acute respiratory distress syndrome (ARDS), COVID-19 patients show loss of alveolar structures and invasion/accumulation of proinflammatory M1 macrophages, resulting in the release of proinflammatory cytokines/mediators and enhanced tissue fibrosis. Interestingly, Wakayama et al. (2015) demonstrated earlier in a mouse model study of acute lung injury that intravenous infusion of DPSC/SHED or the conditioned media potentiated the anti-inflammatory effects via M2 macrophage activation and ameliorated the disease pathophysiology. Currently, few clinical trials are in progress to test out the safety and efficacy of DPSCs-based therapies in COVID-19 patients (Table 4).
Concluding Remarks and Future Prospectives
Dental pulp is a promising source of DPSCs, which are multipotent stem cells with potentials of self-renewal, multilineage differentiation, and immunomodulatory functions. These stem cells offer the advantage of more comprehensive clinical applications as compared to MSCs derived from other sources like the peripheral blood, adipose tissue, umbilical cord, and bone marrow. Other intriguing aspects that make the use of DPSCs more attractive is their easy access from the discarded third molar tooth and the minimal ethical concerns are involved in the procurement process, as well as the fact that cryopreserved DPSCs will retain their ability for multilineage differentiation into osteogenic, chondrogenic, dentinogenic, myogenic, neurogenic, and adipogenic lineages. Not surprisingly, DPSCs-based stem cell therapy approaches are currently being exhaustively investigated.
However, it is noteworthy that dental pulp has the element of heterogeneity involved as it is a mixture of different cell types that can differentiate into multiple lineages. Of note, first, dental pulp-derived single-cell suspensions need to be cultured to allow the development of individual clones. Next, individual clones are isolated and single cell types are enriched by extended culture expansion, followed by immunophenotyping to characterize the subpopulations based on molecular and phenotypic markers that regulate their differentiation potential into multiple lineages using either 2- or 3-dimension culture conditions in defined media which promote the desired cell lineage specification (Al Madhoun et al., 2016, 2018). Although, fluorescence-activated cell sorting techniques are applicable and improve single-cell population purity, these approaches have several limitations including the exposure of cells to electric charge which may alter their integrity (Tirino et al., 2011). DPSCs’ capacities to differentiate into various lineages are driven by donor age, genetics, and epigenetic factors such as growth and differentiation factors and culture settings used (Zhou D. et al., 2020). Indeed, further research toward standardization of DPSCs’ isolation and culture protocols is still needed. There is also a pressing need for identifying the markers that more specifically and consistently represent DPSCs. Moreover, it is speculated that the identification of such markers will facilitate the direct purification techniques which will minimize DPSCs’ exposure to culture conditions needed for their prospective medical applications. Regarding cell-banking aspects, new cryopreservation media and optimized methods may have to be established to maintain the viability and immunobiological characteristics of the DPSCs over long term use. Similarly, further studies will also be required to better understand the molecular mechanisms that regulate interactions between DPSCs and various biomaterials. The studies employing transmission electron microscopy may help characterizing different phenotypes of the heterogeneous progenitor cells that populate dental stem cell niches and a rigorous testing of the endothelial-mesenchymal transformation will be required to assess their potential of replenishing these niches.
Last but not least, DPSCs-related cellular or secretome based therapeutic interventions used in pre-clinical and clinical trials have yielded promising outcomes. However, there is a growing need for conducting more clinical trials to further establish the safety and efficacy of the DPSCs-based interventions as a powerful therapeutic tool and to lead development in regenerative medicine. Not surprisingly, major challenges still remain before the DPSCs-based interventions can be translated into clinical application to patients (Yamada et al., 2019). Nevertheless, innovated procedures have been used to develop immortalized DPSCs such as mutant baculovirus-based piggyBac system (Li et al., 2020), and DPSCs transduction with CDK4R24C (Orimoto et al., 2020), cyclin D1 or telomerase reverse transcriptase (Wilson et al., 2015). Furthermore, CRISPR gene editing technology has been recently applied to study the functional role of genetic variations in patient-derived DPSCs such as the role of TRPV4 polymorphism (c.1855C > T), a gene known to be implicated in metatropic dysplasia disease (Kang et al., 2012; Nonaka et al., 2019). Nonaka et al. (2019) studies revealed that this gain of function mutation is associated with alternations in calcium/NFATc1 signaling pathway, which in turn accelerates chondrogenic and osteogenic differentiation of DPSCs causing the congenital skeletal disease (Han et al., 2020). Taken together, these outstanding approaches highlight the importance of DPSCS as an adult stem cell model for prospective contribution in the future development of treatment strategies for human diseases.
Author Contributions
AA, SS, and RA wrote first draft of the manuscript. DH prepared the figures and tables. DH, MA, and FA-M were involved in discussing, drafting, and editing the manuscript. All authors contributed to the drafting and critical review of the manuscript and approved the final draft.
Funding
This study was funded by Kuwait Foundation for the Advancement of Sciences (KFAS) and Dasman Diabetes Institute (DDI), Grant Number RA-2013-009.
Conflict of Interest
MA is employed by Biointelligence Technology Systems S.L., Barcelona, Spain.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank Kuwait University and Dasman Diabetes Institute for providing the financial support and laboratory space.
Abbreviations
HGF, hepatocyte growth factor; EGF, epidermal growth factor; VEGF, vascular endothelial growth factor; bFGF, basic fibroblast growth factor; PDGF, platelet-derived growth factor; dex, dexamethasone; ITS, insulin-transferrin-selenium; ITS-X, insulin-transferrin-selenium-ethanolamine; LDL, low density lipoprotein; FGF-4, fibroblast growth factor-4; HNF-4, nuclear factor-4 alpha; IFG-1, insulin-like growth factor-1; TAT, tyrosine amino transferase; KOSR, knock-out serum replacement; GLP-1, glucagon-like peptide; NEAAs, non-essential amino acids;.; PHA, phytohemagglutinin; MLR, mixed lymphocyte reaction; LPS, lipopolysaccharide; PBMC, peripheral blood mononuclear cell; Mdr-1, multidrug resistance protein-I; cyp-2e1, cytochrome P450-2E1; Epo, erythropoietin; Pdx-1, pancreas/duodenum homeobox protein 1; Hhex, homeobox protein HEX; MNX1, motor neuron and pancreas homeobox protein 1; Pax-4/-6, paired box protein Pax-4 and 6; NKX6-1, homeobox protein Nkx-6.1; NDNF, neuron-derived neurotrophic factor; NT-3, neurotrophin-3; NGF, beta-nerve growth factor; GDNF, glial cell line-derived neurotrophic factor; FoxA1, forkhead box A1; HNF-3- α, hepatocyte nuclear factor 3-alpha; FoxA2, forkhead box A2; HNF-3- β, hepatocyte nuclear factor 3-beta; GLUT-2, glucose transporter-2; OSM, oncostatin M; β-ME, beta-mercaptoethanol; AMY2A, pancreatic alpha-amylase-PA; Act A, activin A.
References
Abd-Elmeguid, A., and Yu, D. C. (2009). Dental pulp neurophysiology: part 1. Clinical and diagnostic implications. J. Can. Dent. Assoc. 75, 55–59.
Ahmed Nel, M., Murakami, M., Hirose, Y., and Nakashima, M. (2016). Therapeutic potential of dental pulp stem cell secretome for Alzheimer’s disease treatment: an in vitro study. Stem Cells Int. 2016, 8102478. doi: 10.1155/2016/8102478
Aimetti, M., Ferrarotti, F., Cricenti, L., Mariani, G. M., and Romano, F. (2014). Autologous dental pulp stem cells in periodontal regeneration: a case report. Int. J. Periodontics Restorative Dent. 34, s27–s33.
Aimetti, M., Ferrarotti, F., Gamba, M. N., Giraudi, M., and Romano, F. (2018). Regenerative treatment of periodontal intrabony defects using autologous dental pulp stem cells: a 1-year follow-up case series. Int. J. Periodontics Restorative Dent. 38, 51–58.
Akpinar, G., Kasap, M., Aksoy, A., Duruksu, G., Gacar, G., and Karaoz, E. (2014). Phenotypic and proteomic characteristics of human dental pulp derived mesenchymal stem cells from a natal, an exfoliated deciduous, and an impacted third molar tooth. Stem Cells Int. 2014, 457059. doi: 10.1155/2014/457059
Al Madhoun, A., Ali, H., AlKandari, S., Atizado, V. L., Akhter, N., Al-Mulla, F., et al. (2016). Defined three-dimensional culture conditions mediate efficient induction of definitive endoderm lineage from human umbilical cord Wharton’s jelly mesenchymal stem cells. Stem Cell Res. Ther. 7:165. doi: 10.1186/s13287-016-0426-9
Al Madhoun, A., Alkandari, S., Ali, H., Carrio, N., Atari, M., Bitar, M. S., et al. (2018). Chemically defined conditions mediate an efficient induction of mesodermal lineage from human umbilical cord- and bone marrow- mesenchymal stem cells and dental pulp pluripotent-like stem cells. Cell Reprogram 20, 9–16. doi: 10.1089/cell.2017.0028
Alraies, A., Alaidaroos, N. Y., Waddington, R. J., Moseley, R., and Sloan, A. J. (2017). Variation in human dental pulp stem cell ageing profiles reflect contrasting proliferative and regenerative capabilities. BMC Cell Biol. 18:12. doi: 10.1186/s12860-017-0128-x
Alraies, A., Waddington, R. J., Sloan, A. J., and Moseley, R. (2020). Evaluation of dental pulp stem cell heterogeneity and behaviour in 3D Type I collagen gels. Biomed. Res. Int. 2020:3034727. doi: 10.1155/2020/3034727
Altanerova, U., Benejova, K., Altanerova, V., Tyciakova, S., Rychly, B., Szomolanyi, P., et al. (2016). Dental pulp mesenchymal stem/stromal cells labeled with iron sucrose release exosomes and cells applied intra-nasally migrate to intracerebral glioblastoma. Neoplasma 63, 925–933. doi: 10.4149/neo_2016_611
Andrukhov, O., Behm, C., Blufstein, A., and Rausch-Fan, X. (2019). Immunomodulatory properties of dental tissue-derived mesenchymal stem cells: implication in disease and tissue regeneration. World J. Stem Cells 11, 604–617. doi: 10.4252/wjsc.v11.i9.604
Anitua, E., Troya, M., and Zalduendo, M. (2018). Progress in the use of dental pulp stem cells in regenerative medicine. Cytotherapy 20, 479–498. doi: 10.1016/j.jcyt.2017.12.011
Aranha, A. M., Zhang, Z., Neiva, K. G., Costa, C. A., Hebling, J., and Nor, J. E. (2010). Hypoxia enhances the angiogenic potential of human dental pulp cells. J. Endod. 36, 1633–1637. doi: 10.1016/j.joen.2010.05.013
Atari, M., Barajas, M., Hernandez-Alfaro, F., Gil, C., Fabregat, M., Ferres Padro, E., et al. (2011). Isolation of pluripotent stem cells from human third molar dental pulp. Histol. Histopathol. 26, 1057–1070.
Atari, M., Gil-Recio, C., Fabregat, M., Garcia-Fernandez, D., Barajas, M., Carrasco, M. A., et al. (2012). Dental pulp of the third molar: a new source of pluripotent-like stem cells. J. Cell Sci. 125, 3343–3356. doi: 10.1242/jcs.096537
Bakopoulou, A., Kritis, A., Andreadis, D., Papachristou, E., Leyhausen, G., Koidis, P., et al. (2015). Angiogenic potential and secretome of human apical papilla mesenchymal stem cells in various stress microenvironments. Stem Cells Dev. 24, 2496–2512. doi: 10.1089/scd.2015.0197
Ballini, A., Cantore, S., Scacco, S., Perillo, L., Scarano, A., Aityan, S. K., et al. (2019). A comparative study on different stemness gene expression between dental pulp stem cells vs. dental bud stem cells. Eur. Rev. Med. Pharmacol. Sci. 23, 1626–1633.
Barbier, L., Ramos, E., Mendiola, J., Rodriguez, O., Santamaria, G., Santamaria, J., et al. (2018). Autologous dental pulp mesenchymal stem cells for inferior third molar post-extraction socket healing: a split-mouth randomised clinical trial. Med. Oral Patol. Oral Cir. Bucal 23, e469–e477. doi: 10.4317/medoral.22466
Bhandi, S., Al Kahtani, A., Mashyakhy, M., Alsofi, L., Maganur, P. C., Vishwanathaiah, S., et al. (2021). Modulation of the dental pulp stem cell secretory profile by hypoxia induction using cobalt chloride. J. Pers. Med. 11:247. doi: 10.3390/jpm11040247
Bronckaers, A., Hilkens, P., Fanton, Y., Struys, T., Gervois, P., Politis, C., et al. (2013). Angiogenic properties of human dental pulp stem cells. PLoS One 8:e71104. doi: 10.1371/journal.pone.0071104
Brunelli, G., Motroni, A., Graziano, A., D’Aquino, R., Zollino, I., and Carinci, F. (2013). Sinus lift tissue engineering using autologous pulp micro-grafts: a case report of bone density evaluation. J. Indian Soc. Periodontol. 17, 644–647.
Carnevale, G., Riccio, M., Pisciotta, A., Beretti, F., Maraldi, T., Zavatti, M., et al. (2013). In vitro differentiation into insulin-producing beta-cells of stem cells isolated from human amniotic fluid and dental pulp. Dig. Liver Dis. 45, 669–676. doi: 10.1016/j.dld.2013.02.007
Caton, J., and Tucker, A. S. (2009). Current knowledge of tooth development: patterning and mineralization of the murine dentition. J. Anat. 214, 502–515. doi: 10.1111/j.1469-7580.2008.01014.x
Chin, Y. T., Liu, C. M., Chen, T. Y., Chung, Y. Y., Lin, C. Y., Hsiung, C. N., et al. (2021). 2,3,5,4’-tetrahydroxystilbene-2-O-beta-D-glucoside-stimulated dental pulp stem cells-derived conditioned medium enhances cell activity and anti-inflammation. J. Dent. Sci. 16, 586–598. doi: 10.1016/j.jds.2020.10.014
Ching, H. S., Luddin, N. I, Rahman, A., and Ponnuraj, K. T. (2017). Expression of odontogenic and osteogenic markers in DPSCs and SHED: a review. Curr. Stem Cell Res. Ther. 12, 71–79. doi: 10.2174/1574888X11666160815095733
Chmilewsky, F., Jeanneau, C., Laurent, P., and About, I. (2014). Pulp fibroblasts synthesize functional complement proteins involved in initiating dentin-pulp regeneration. Am. J. Pathol. 184, 1991–2000. doi: 10.1016/j.ajpath.2014.04.003
Cho, Y. A., Noh, K., Jue, S. S., Lee, S. Y., and Kim, E. C. (2015). Melatonin promotes hepatic differentiation of human dental pulp stem cells: clinical implications for the prevention of liver fibrosis. J. Pineal Res. 58, 127–135. doi: 10.1111/jpi.12198
D’Aquino, R., De Rosa, A., Lanza, V., Tirino, V., Laino, L., Graziano, A., et al. (2009). Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur. Cells Mater. 18, 75–83.
Datta, I., Bhadri, N., Shahani, P., Majumdar, D., Sowmithra, S., Razdan, R., et al. (2017). Functional recovery upon human dental pulp stem cell transplantation in a diabetic neuropathy rat model. Cytotherapy 19, 1208–1224. doi: 10.1016/j.jcyt.2017.07.009
de Almeida, F. M., Marques, S. A., Ramalho Bdos, S., Rodrigues, R. F., Cadilhe, D. V., Furtado, D., et al. (2011). Human dental pulp cells: a new source of cell therapy in a mouse model of compressive spinal cord injury. J. Neurotrauma 28, 1939–1949. doi: 10.1089/neu.2010.1317
de Almeida, J. F., Chen, P., Henry, M. A., and Diogenes, A. (2014). Stem cells of the apical papilla regulate trigeminal neurite outgrowth and targeting through a BDNF-dependent mechanism. Tissue Eng. Part A 20, 3089–3100. doi: 10.1089/ten.tea.2013.0347
Demircan, P. C., Sariboyaci, A. E., Unal, Z. S., Gacar, G., Subasi, C., and Karaoz, E. (2011). Immunoregulatory effects of human dental pulp-derived stem cells on T cells: comparison of transwell co-culture and mixed lymphocyte reaction systems. Cytotherapy 13, 1205–1220. doi: 10.3109/14653249.2011.605351
Deng, Z., Yan, W., Dai, X., Chen, M., Qu, Q., Wu, B., et al. (2021). N-cadherin regulates the odontogenic differentiation of dental pulp stem cells via beta-catenin activity. Front. Cell Dev. Biol. 9:661116. doi: 10.3389/fcell.2021.661116
Ding, G., Niu, J., and Liu, Y. (2015). Dental pulp stem cells suppress the proliferation of lymphocytes via transforming growth factor-beta1. Hum. Cell 28, 81–90. doi: 10.1007/s13577-014-0106-y
Dominici, M., Le Blanc, K., Mueller, I., Slaper-Cortenbach, I., Marini, F., Krause, D., et al. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 8, 315–317. doi: 10.1080/14653240600855905
Farges, J. C., Alliot-Licht, B., Renard, E., Ducret, M., Gaudin, A., Smith, A. J., et al. (2015). Dental pulp defence and repair mechanisms in dental caries. Med. Inflamm. 2015:230251. doi: 10.1155/2015/230251
Faruqu, F. N., Zhou, S., Sami, N., Gheidari, F., Lu, H., and Al-Jamal, K. T. (2020). Three-dimensional culture of dental pulp pluripotent-like stem cells (DPPSCs) enhances Nanog expression and provides a serum-free condition for exosome isolation. FASEB Bioadv. 2, 419–433. doi: 10.1096/fba.2020-00025
Feng, J., Mantesso, A., De Bari, C., Nishiyama, A., and Sharpe, P. T. (2011). Dual origin of mesenchymal stem cells contributing to organ growth and repair. Proc. Natl. Acad. Sci. U.S.A. 108, 6503–6508. doi: 10.1073/pnas.1015449108
Ferrarotti, F., Romano, F., Gamba, M. N., Quirico, A., Giraudi, M., Audagna, M., et al. (2018). Human intrabony defect regeneration with micrografts containing dental pulp stem cells: a randomized controlled clinical trial. J. Clin. Periodontol. 45, 841–850.
Ferro, F., Spelat, R., Beltrami, A. P., Cesselli, D., and Curcio, F. (2012a). Isolation and characterization of human dental pulp derived stem cells by using media containing low human serum percentage as clinical grade substitutes for bovine serum. PLoS One 7:e48945. doi: 10.1371/journal.pone.0048945
Ferro, F., Spelat, R., D’Aurizio, F., Puppato, E., Pandolfi, M., Beltrami, A. P., et al. (2012b). Dental pulp stem cells differentiation reveals new insights in Oct4A dynamics. PLoS One 7:e41774. doi: 10.1371/journal.pone.0041774
Gandia, C., Arminan, A., Garcia-Verdugo, J. M., Lledo, E., Ruiz, A., Minana, M. D., et al. (2008). Human dental pulp stem cells improve left ventricular function, induce angiogenesis, and reduce infarct size in rats with acute myocardial infarction. Stem Cells 26, 638–645. doi: 10.1634/stemcells.2007-0484
Gervois, P., Wolfs, E., Dillen, Y., Hilkens, P., Ratajczak, J., Driesen, R. B., et al. (2017). Paracrine maturation and migration of SH-SY5Y cells by dental pulp stem cells. J. Dent. Res. 96, 654–662. doi: 10.1177/0022034517690491
Ghannam, M. G., Alameddine, H., and Bordoni, B. (2021). Anatomy, Head and Neck, Pulp (Tooth). Treasure Island, FL: StatPearls.
Gil-Recio, C., Montori, S., Vallejo, C., Demour, S. A., Ferrés-Padró, E., Barajas, M., et al. (2020). Direct differentiation of dental pulp pluripotent-like stem cells differentiation into hepatocyte-like Cells. bioRxiv [Preprint] bioRxiv: 2020.12.09.418780, doi: 10.1101/2020.12.09.418780
Giuliani, N., Lisignoli, G., Magnani, M., Racano, C., Bolzoni, M., Dalla Palma, B., et al. (2013). New insights into osteogenic and chondrogenic differentiation of human bone marrow mesenchymal stem cells and their potential clinical applications for bone regeneration in pediatric orthopaedics. Stem Cells Int. 2013:312501.
Gnanasegaran, N., Govindasamy, V., Mani, V., and Abu Kasim, N. H. (2017a). Neuroimmunomodulatory properties of DPSCs in an in vitro model of Parkinson’s disease. IUBMB Life 69, 689–699. doi: 10.1002/iub.1655
Gnanasegaran, N., Govindasamy, V., Simon, C., Gan, Q. F., Vincent-Chong, V. K., Mani, V., et al. (2017b). Effect of dental pulp stem cells in MPTP-induced old-aged mice model. Eur. J. Clin. Invest. 47, 403–414. doi: 10.1111/eci.12753
Gotjamanos, T. (1969). Cellular organization in the subodontoblastic zone of the dental pulp. I. A study of cell-free and cell-rich layers in pulps of adult rat and deciduous monkey teeth. Arch. Oral. Biol. 14, 1007–1010. doi: 10.1016/0003-9969(69)90070-3
Govindasamy, V., Abdullah, A. N., Ronald, V. S., Musa, S., Ab Aziz, Z. A., Zain, R. B., et al. (2010). Inherent differential propensity of dental pulp stem cells derived from human deciduous and permanent teeth. J. Endod. 36, 1504–1515. doi: 10.1016/j.joen.2010.05.006
Govindasamy, V., Ronald, V. S., Abdullah, A. N., Nathan, K. R., Ab Aziz, Z. A., Abdullah, M., et al. (2011). Differentiation of dental pulp stem cells into islet-like aggregates. J. Dent. Res. 90, 646–652. doi: 10.1177/0022034510396879
Graepler, F., Lemken, M. L., Wybranietz, W. A., Schmidt, U., Smirnow, I., Gross, C. D., et al. (2005). Bifunctional chimeric SuperCD suicide gene -YCD: YUPRT fusion is highly effective in a rat hepatoma model. World J. Gastroenterol. WJG 11, 6910–6919. doi: 10.3748/wjg.v11.i44.6910
Gronthos, S., Mankani, M., Brahim, J., Robey, P. G., and Shi, S. (2000). Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. U.S.A. 97, 13625–13630. doi: 10.1073/pnas.240309797
Han, M.-J., Seo, Y.-K., Yoon, H.-H., Song, K.-Y., and Park, J.-K. (2008). Effect of mechanical tension on the human dental pulp cells. Biotechnol. Bioprocess Eng. 13, 410–417. doi: 10.1007/s12257-008-0146-9
Han, M.-J., Seo, Y.-K., Yoon, H.-H., Song, K.-Y., and Park, J.-K. (2010). Upregulation of bone-like extracellular matrix expression in human dental pulp stem cells by mechanical strain. Biotechnol. Bioprocess Eng. 15, 572–579. doi: 10.1007/s12257-009-0102-3
Han, X., Kato, H., Sato, H., Hirofuji, Y., Fukumoto, S., and Masuda, K. (2020). Accelerated osteoblastic differentiation in patient-derived dental pulp stem cells carrying a gain-of-function mutation of TRPV4 associated with metatropic dysplasia. Biochem. Biophys. Res. Commun.. 523, 841–846. doi: 10.1016/j.bbrc.2019.12.123
Hata, M., Naruse, K., Ozawa, S., Kobayashi, Y., Nakamura, N., Kojima, N., et al. (2013). Mechanical stretch increases the proliferation while inhibiting the osteogenic differentiation in dental pulp stem cells. Tissue Eng. Part A 19, 625–633. doi: 10.1089/ten.tea.2012.0099
Hernández-Monjaraz, B., Santiago-Osorio, E., Ledesma-Martínez, E., Alcauter-Zavala, A., and Mendoza-Núñez, V. M. (2018). Retrieval of a periodontally compromised tooth by allogeneic grafting of mesenchymal stem cells from dental pulp: a case report. J. Int. Med. Res. 46, 2983–2993. doi: 10.1177/0300060518773244
Hilkens, P., Fanton, Y., Martens, W., Gervois, P., Struys, T., Politis, C., et al. (2014). Pro-angiogenic impact of dental stem cells in vitro and in vivo. Stem Cell Res. 12, 778–790. doi: 10.1016/j.scr.2014.03.008
Hilkens, P., Gervois, P., Fanton, Y., Vanormelingen, J., Martens, W., Struys, T., et al. (2013). Effect of isolation methodology on stem cell properties and multilineage differentiation potential of human dental pulp stem cells. Cell Tissue Res. 353, 65–78. doi: 10.1007/s00441-013-1630-x
Hosmani, J., Assiri, K., Almubarak, H. M., Mannakandath, M. L., Al-Hakami, A., Patil, S., et al. (2020). Proteomic profiling of various human dental stem cells – a systematic review. World J. Stem Cells 12, 1214–1236. doi: 10.4252/wjsc.v12.i10.1214
Hosoya, A., Yukita, A., Yoshiba, K., Yoshiba, N., Takahashi, M., and Nakamura, H. (2012). Two distinct processes of bone-like tissue formation by dental pulp cells after tooth transplantation. J. Histochem. Cytochem. 60, 861–873. doi: 10.1369/0022155412459741
Hu, L., Liu, Y., and Wang, S. (2018). Stem cell-based tooth and periodontal regeneration. Oral Dis. 24, 696–705. doi: 10.1111/odi.12703
Huang, C. C., Narayanan, R., Alapati, S., and Ravindran, S. (2016). Exosomes as biomimetic tools for stem cell differentiation: applications in dental pulp tissue regeneration. Biomaterials 111, 103–115. doi: 10.1016/j.biomaterials.2016.09.029
Huang, C. E., Hu, F. W., Yu, C. H., Tsai, L. L., Lee, T. H., Chou, M. Y., et al. (2014). Concurrent expression of Oct4 and Nanog maintains mesenchymal stem-like property of human dental pulp cells. Int. J. Mol. Sci. 15, 18623–18639. doi: 10.3390/ijms151018623
Huang, G. T., Gronthos, S., and Shi, S. (2009). Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J. Dent. Res. 88, 792–806. doi: 10.1177/0022034509340867
Ishkitiev, N., Yaegaki, K., Calenic, B., Nakahara, T., Ishikawa, H., Mitiev, V., et al. (2010). Deciduous and permanent dental pulp mesenchymal cells acquire hepatic morphologic and functional features in vitro. J. Endod. 36, 469–474. doi: 10.1016/j.joen.2009.12.022
Ishkitiev, N., Yaegaki, K., Imai, T., Tanaka, T., Nakahara, T., Ishikawa, H., et al. (2012). High-purity hepatic lineage differentiated from dental pulp stem cells in serum-free medium. J. Endod. 38, 475–480. doi: 10.1016/j.joen.2011.12.011
Ishkitiev, N., Yaegaki, K., Kozhuharova, A., Tanaka, T., Okada, M., Mitev, V., et al. (2013). Pancreatic differentiation of human dental pulp CD117(+) stem cells. Regen. Med. 8, 597–612. doi: 10.2217/rme.13.42
Ito, K., Yamada, Y., Nakamura, S., and Ueda, M. (2011). Osteogenic potential of effective bone engineering using dental pulp stem cells, bone marrow stem cells, and periosteal cells for osseointegration of dental implants. Int. J. Oral Maxillofac. Implants 26, 947–954.
Jo, Y. Y., Lee, H. J., Kook, S. Y., Choung, H. W., Park, J. Y., Chung, J. H., et al. (2007). Isolation and characterization of postnatal stem cells from human dental tissues. Tissue Eng. 13, 767–773. doi: 10.1089/ten.2006.0192
Kanafi, M. M., Rajeshwari, Y. B., Gupta, S., Dadheech, N., Nair, P. D., Gupta, P. K., et al. (2013). Transplantation of islet-like cell clusters derived from human dental pulp stem cells restores normoglycemia in diabetic mice. Cytotherapy 15, 1228–1236. doi: 10.1016/j.jcyt.2013.05.008
Kang, S. S., Shin, S. H., Auh, C. K., and Chun, J. (2012). Human skeletal dysplasia caused by a constitutive activated transient receptor potential vanilloid 4 (TRPV4) cation channel mutation. Exp. Mol. Med. 44, 707–722. doi: 10.3858/emm.2012.44.12.080
Karaoz, E., Demircan, P. C., Saglam, O., Aksoy, A., Kaymaz, F., and Duruksu, G. (2011). Human dental pulp stem cells demonstrate better neural and epithelial stem cell properties than bone marrow-derived mesenchymal stem cells. Histochem. Cell Biol. 136, 455–473. doi: 10.1007/s00418-011-0858-3
Kaukua, N., Shahidi, M. K., Konstantinidou, C., Dyachuk, V., Kaucka, M., Furlan, A., et al. (2014). Glial origin of mesenchymal stem cells in a tooth model system. Nature 513, 551–554. doi: 10.1038/nature13536
Kawanabe, N., Murata, S., Fukushima, H., Ishihara, Y., Yanagita, T., Yanagita, E., et al. (2012). Stage-specific embryonic antigen-4 identifies human dental pulp stem cells. Exp. Cell Res. 318, 453–463. doi: 10.1016/j.yexcr.2012.01.008
Kawashima, N. (2012). Characterisation of dental pulp stem cells: a new horizon for tissue regeneration? Arch. Oral Biol. 57, 1439–1458. doi: 10.1016/j.archoralbio.2012.08.010
Kellner, M., Steindorff, M. M., Strempel, J. F., Winkel, A., Kuhnel, M. P., and Stiesch, M. (2014). Differences of isolated dental stem cells dependent on donor age and consequences for autologous tooth replacement. Arch. Oral Biol. 59, 559–567. doi: 10.1016/j.archoralbio.2014.02.014
Kerkis, I., Ambrosio, C. E., Kerkis, A., Martins, D. S., Zucconi, E., Fonseca, S. A., et al. (2008). Early transplantation of human immature dental pulp stem cells from baby teeth to golden retriever muscular dystrophy (GRMD) dogs: local or systemic? J. Transl. Med. 6:35. doi: 10.1186/1479-5876-6-35
Kerkis, I., Kerkis, A., Dozortsev, D., Stukart-Parsons, G. C., Gomes Massironi, S. M., Pereira, L. V., et al. (2006). Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs. 184, 105–116. doi: 10.1159/000099617
Kim, H. J., Cho, Y. A., Lee, Y. M., Lee, S. Y., Bae, W. J., and Kim, E. C. (2016). PIN1 suppresses the hepatic differentiation of pulp stem cells via wnt3a. J. Dent. Res. 95, 1415–1424. doi: 10.1177/0022034516659642
Kobayashi, T., Torii, D., Iwata, T., Izumi, Y., Nasu, M., and Tsutsui, T. W. (2020). Characterization of proliferation, differentiation potential, and gene expression among clonal cultures of human dental pulp cells. Hum. Cell 33, 490–501. doi: 10.1007/s13577-020-00327-9
Koussoulakou, D. S., Margaritis, L. H., and Koussoulakos, S. L. (2009). A curriculum vitae of teeth: evolution, generation, regeneration. Int. J. Biol. Sci. 5, 226–243. doi: 10.7150/ijbs.5.226
Krivanek, J., Adameyko, I., and Fried, K. (2017). Heterogeneity and developmental connections between cell types inhabiting teeth. Front. Physiol. 8:376. doi: 10.3389/fphys.2017.00376
Krivanek, J., Soldatov, R. A., Kastriti, M. E., Chontorotzea, T., Herdina, A. N., Petersen, J., et al. (2020). Dental cell type atlas reveals stem and differentiated cell types in mouse and human teeth. Nat. Commun. 11:4816. doi: 10.1038/s41467-020-18512-7
Kumar, A., Kumar, V., Rattan, V., Jha, V., Pal, A., and Bhattacharyya, S. (2017). Molecular spectrum of secretome regulates the relative hepatogenic potential of mesenchymal stem cells from bone marrow and dental tissue. Sci. Rep. 7:15015. doi: 10.1038/s41598-017-14358-0
Kushnerev, E., Shawcross, S. G., Sothirachagan, S., Carley, F., Brahma, A., Yates, J. M., et al. (2016). Regeneration of corneal epithelium with dental pulp stem cells using a contact lens delivery system. Invest. Ophthalmol. Vis. Sci. 57, 5192–5199. doi: 10.1167/iovs.15-17953
Kwack, K. H., Lee, J. M., Park, S. H., and Lee, H. W. (2017). Human dental pulp stem cells suppress alloantigen-induced immunity by stimulating t cells to release transforming growth factor beta. J. Endod. 43, 100–108. doi: 10.1016/j.joen.2016.09.005
Laino, G., d’Aquino, R., Graziano, A., Lanza, V., Carinci, F., Naro, F., et al. (2005). A new population of human adult dental pulp stem cells: a useful source of living autologous fibrous bone tissue (LAB). J. Bone Miner. Res. 20, 1394–1402. doi: 10.1359/JBMR.050325
Lee, S., Zhang, Q. Z., Karabucak, B., and Le, A. D. (2016). DPSCs from inflamed pulp modulate macrophage function via the TNF-alpha/IDO Axis. J. Dent. Res. 95, 1274–1281. doi: 10.1177/0022034516657817
Leong, W. K., Henshall, T. L., Arthur, A., Kremer, K. L., Lewis, M. D., Helps, S. C., et al. (2012). Human adult dental pulp stem cells enhance poststroke functional recovery through non-neural replacement mechanisms. Stem Cells Transl. Med. 1, 177–187. doi: 10.5966/sctm.2011-0039
Leprince, J. G., Zeitlin, B. D., Tolar, M., and Peters, O. A. (2012). Interactions between immune system and mesenchymal stem cells in dental pulp and periapical tissues. Int. Endod. J. 45, 689–701. doi: 10.1111/j.1365-2591.2012.02028.x
Lesot, H. (2000). Odontoblast differentiation and tooth morphogenesis. J. Dent. Res. 79, 1640–1644. doi: 10.1177/00220345000790090101
Li, X., Wang, L., Su, Q., Ye, L., Zhou, X., Song, D., et al. (2020). Highly proliferative immortalized human dental pulp cells retain the odontogenic phenotype when combined with a beta-tricalcium phosphate scaffold and BMP2. Stem Cells Int. 2020:4534128. doi: 10.1155/2020/4534128
Li, Z., Jiang, C. M., An, S., Cheng, Q., Huang, Y. F., Wang, Y. T., et al. (2014). Immunomodulatory properties of dental tissue-derived mesenchymal stem cells. Oral Dis. 20, 25–34. doi: 10.1111/odi.12086
Lindroos, B., Maenpaa, K., Ylikomi, T., Oja, H., Suuronen, R., and Miettinen, S. (2008). Characterisation of human dental stem cells and buccal mucosa fibroblasts. Biochem. Biophys. Res. Commun. 368, 329–335. doi: 10.1016/j.bbrc.2008.01.081
Liu, J., Jin, T. C., Chang, S., Czajka-Jakubowska, A., and Clarkson, B. H. (2011). Adhesion and growth of dental pulp stem cells on enamel-like fluorapatite surfaces. J. Biomed. Mater. Res. A 96, 528–534. doi: 10.1002/jbm.a.33002
Liu, L., Ling, J., Wei, X., Wu, L., and Xiao, Y. (2009). Stem cell regulatory gene expression in human adult dental pulp and periodontal ligament cells undergoing odontogenic/osteogenic differentiation. J. Endod. 35, 1368–1376. doi: 10.1016/j.joen.2009.07.005
Liu, L., Wei, X., Ling, J., Wu, L., and Xiao, Y. (2011). Expression pattern of Oct-4, Sox2, and c-Myc in the primary culture of human dental pulp derived cells. J. Endod. 37, 466–472. doi: 10.1016/j.joen.2010.12.012
Longoni, A., Utomo, L. I, van Hooijdonk, E., Bittermann, G. K., Vetter, V. C., Kruijt Spanjer, E. C., et al. (2020). The chondrogenic differentiation potential of dental pulp stem cells. Eur. Cell Mater. 39, 121–135. doi: 10.22203/eCM.v039a08
Magnusson, B. (1968). Tissue changes during molar tooth eruption. Trans. R. Sch. Dent. Stockh. Umea 13, 1–122.
Makino, E., Nakamura, N., Miyabe, M., Ito, M., Kanada, S., Hata, M., et al. (2019). Conditioned media from dental pulp stem cells improved diabetic polyneuropathy through anti-inflammatory, neuroprotective and angiogenic actions: cell-free regenerative medicine for diabetic polyneuropathy. J. Diabetes Invest. 10, 1199–1208. doi: 10.1111/jdi.13045
Marrelli, M., Codispoti, B., Shelton, R. M., Scheven, B. A., Cooper, P. R., Tatullo, M., et al. (2018). Dental pulp stem cell mechanoresponsiveness: effects of mechanical stimuli on dental pulp stem cell behavior. Front. Physiol. 9:1685. doi: 10.3389/fphys.2018.01685
Martens, W., Sanen, K., Georgiou, M., Struys, T., Bronckaers, A., Ameloot, M., et al. (2014). Human dental pulp stem cells can differentiate into Schwann cells and promote and guide neurite outgrowth in an aligned tissue-engineered collagen construct in vitro. FASEB J. 28, 1634–1643. doi: 10.1096/fj.13-243980
Martens, W., Wolfs, E., Struys, T., Politis, C., Bronckaers, A., and Lambrichts, I. (2012). Expression pattern of basal markers in human dental pulp stem cells and tissue. Cells Tissues Organs 196, 490–500. doi: 10.1159/000338654
Martinez-Sarra, E., Montori, S., Gil-Recio, C., Nunez-Toldra, R., Costamagna, D., Rotini, A., et al. (2017). Human dental pulp pluripotent-like stem cells promote wound healing and muscle regeneration. Stem Cell Res Ther. 8:175. doi: 10.1186/s13287-017-0621-3
Matei, I. V., Ii, H., and Yaegaki, K. (2017). Hydrogen sulfide enhances pancreatic beta-cell differentiation from human tooth under normal and glucotoxic conditions. Regen. Med. 12, 125–141. doi: 10.2217/rme-2016-0142
Mattei, V., Martellucci, S., Pulcini, F., Santilli, F., Sorice, M., and Delle Monache, S. (2021). Regenerative potential of DPSCs and revascularization: direct, paracrine or autocrine effect? Stem Cell Rev. Rep. doi: 10.1007/s12015-021-10162-6
Mead, B., Hill, L. J., Blanch, R. J., Ward, K., Logan, A., Berry, M., et al. (2016). Mesenchymal stromal cell-mediated neuroprotection and functional preservation of retinal ganglion cells in a rodent model of glaucoma. Cytotherapy 18, 487–496. doi: 10.1016/j.jcyt.2015.12.002
Mead, B., Logan, A., Berry, M., Leadbeater, W., and Scheven, B. A. (2014). Dental pulp stem cells, a paracrine-mediated therapy for the retina. Neural Regen. Res. 9, 577–578. doi: 10.4103/1673-5374.130089
Mendi, A., Yagci, B. G., Kiziloglu, M., Sarac, N., Yilmaz, D., Ugur, A., et al. (2017). Effects of Syzygium aromaticum, Cinnamomum zeylanicum, and Salvia triloba extracts on proliferation and differentiation of dental pulp stem cells. J. Appl. Oral Sci. 25, 515–522. doi: 10.1590/1678-7757-2016-0522
Mikami, Y., Ishii, Y., Watanabe, N., Shirakawa, T., Suzuki, S., Irie, S., et al. (2011). CD271/p75(NTR) inhibits the differentiation of mesenchymal stem cells into osteogenic, adipogenic, chondrogenic, and myogenic lineages. Stem Cells Dev. 20, 901–913. doi: 10.1089/scd.2010.0299
Miletich, I., and Sharpe, P. T. (2004). Neural crest contribution to mammalian tooth formation. Birth Defects Res. C Embryo Today 72, 200–212. doi: 10.1002/bdrc.20012
Mita, T., Furukawa-Hibi, Y., Takeuchi, H., Hattori, H., Yamada, K., Hibi, H., et al. (2015). Conditioned medium from the stem cells of human dental pulp improves cognitive function in a mouse model of Alzheimer’s disease. Behav. Brain Res. 293, 189–197. doi: 10.1016/j.bbr.2015.07.043
Mitrano, T. I., Grob, M. S., Carrion, F., Nova-Lamperti, E., Luz, P. A., Fierro, F. S., et al. (2010). Culture and characterization of mesenchymal stem cells from human gingival tissue. J. Periodontol. 81, 917–925. doi: 10.1902/jop.2010.090566
Mitsiadis, T. A., Feki, A., Papaccio, G., and Caton, J. (2011). Dental pulp stem cells, niches, and notch signaling in tooth injury. Adv. Dent. Res. 23, 275–279. doi: 10.1177/0022034511405386
Miura, M., Gronthos, S., Zhao, M., Lu, B., Fisher, L. W., Robey, P. G., et al. (2003). SHED: stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. U.S.A 100, 5807–5812. doi: 10.1073/pnas.0937635100
Morsczeck, C., Gotz, W., Schierholz, J., Zeilhofer, F., Kuhn, U., Mohl, C., et al. (2005). Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 24, 155–165. doi: 10.1016/j.matbio.2004.12.004
Mortada, I., and Mortada, R. (2018). Dental pulp stem cells and osteogenesis: an update. Cytotechnology 70, 1479–1486. doi: 10.1007/s10616-018-0225-5
Mortada, I., Mortada, R., and Al Bazzal, M. (2018). Dental pulp stem cells and neurogenesis. Adv. Exp. Med. Biol. 1083, 63–75. doi: 10.1007/5584_2017_71
Nakashima, M., Iohara, K., and Sugiyama, M. (2009). Human dental pulp stem cells with highly angiogenic and neurogenic potential for possible use in pulp regeneration. Cytokine Growth Factor Rev. 20, 435–440. doi: 10.1016/j.cytogfr.2009.10.012
Nakashima, M., Iohara, K., Murakami, M., Nakamura, H., Sato, Y., Ariji, Y., et al. (2017). Pulp regeneration by transplantation of dental pulp stem cells in pulpitis: a pilot clinical study. Stem Cell Res. Ther. 8:61.
Nesti, C., Pardini, C., Barachini, S., D’Alessandro, D., Siciliano, G., Murri, L., et al. (2011). Human dental pulp stem cells protect mouse dopaminergic neurons against MPP+ or rotenone. Brain Res. 1367, 94–102. doi: 10.1016/j.brainres.2010.09.042
Niehage, C., Karbanova, J., Steenblock, C., Corbeil, D., and Hoflack, B. (2016). Cell surface proteome of dental pulp stem cells identified by label-free mass spectrometry. PLoS One 11:e0159824. doi: 10.1371/journal.pone.0159824
Nonaka, K., Han, X., Kato, H., Sato, H., Yamaza, H., Hirofuji, Y., et al. (2019). Novel gain-of-function mutation of TRPV4 associated with accelerated chondrogenic differentiation of dental pulp stem cells derived from a patient with metatropic dysplasia. Biochem. Biophys. Rep. 19:100648. doi: 10.1016/j.bbrep.2019.100648
Nunez-Toldra, R., Dosta, P., Montori, S., Ramos, V., Atari, M., and Borros, S. (2017a). Improvement of osteogenesis in dental pulp pluripotent-like stem cells by oligopeptide-modified poly(beta-amino ester)s. Acta Biomater. 53, 152–164. doi: 10.1016/j.actbio.2017.01.077
Nunez-Toldra, R., Martinez-Sarra, E., Gil-Recio, C., Carrasco, M. A., Al Madhoun, A., Montori, S., et al. (2017b). Dental pulp pluripotent-like stem cells (DPPSC), a new stem cell population with chromosomal stability and osteogenic capacity for biomaterials evaluation. BMC Cell Biol. 18:21. doi: 10.1186/s12860-017-0137-9
Nuti, N., Corallo, C., Chan, B. M., Ferrari, M., and Gerami-Naini, B. (2016). Multipotent differentiation of human dental pulp stem cells: a literature review. Stem Cell Rev. Rep. 12, 511–523. doi: 10.1007/s12015-016-9661-9
Omi, M., Hata, M., Nakamura, N., Miyabe, M., Kobayashi, Y., Kamiya, H., et al. (2016). Transplantation of dental pulp stem cells suppressed inflammation in sciatic nerves by promoting macrophage polarization towards anti-inflammation phenotypes and ameliorated diabetic polyneuropathy. J. Diabetes Invest. 7, 485–496. doi: 10.1111/jdi.12452
Orimoto, A., Kyakumoto, S., Eitsuka, T., Nakagawa, K., Kiyono, T., and Fukuda, T. (2020). Efficient immortalization of human dental pulp stem cells with expression of cell cycle regulators with the intact chromosomal condition. PLoS One 15:e0229996. doi: 10.1371/journal.pone.0229996
Ozdemir, A. T., Ozgul Ozdemir, R. B., Kirmaz, C., Sariboyaci, A. E., Unal Halbutogllari, Z. S., Ozel, C., et al. (2016). The paracrine immunomodulatory interactions between the human dental pulp derived mesenchymal stem cells and CD4 T cell subsets. Cell Immunol. 310, 108–115. doi: 10.1016/j.cellimm.2016.08.008
Pang, Y. W., Feng, J., Daltoe, F., Fatscher, R., Gentleman, E., Gentleman, M. M., et al. (2016). Perivascular stem cells at the tip of mouse incisors regulate tissue regeneration. J. Bone Miner. Res. 31, 514–523. doi: 10.1002/jbmr.2717
Patil, R., Kumar, B. M., Lee, W. J., Jeon, R. H., Jang, S. J., Lee, Y. M., et al. (2014). Multilineage potential and proteomic profiling of human dental stem cells derived from a single donor. Exp. Cell Res. 320, 92–107. doi: 10.1016/j.yexcr.2013.10.005
Pierdomenico, L., Bonsi, L., Calvitti, M., Rondelli, D., Arpinati, M., Chirumbolo, G., et al. (2005). Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation 80, 836–842. doi: 10.1097/01.tp.0000173794.72151.88
Pilbauerova, N., Soukup, T., Suchankova Kleplova, T., Schmidt, J., and Suchanek, J. (2021b). The effect of cultivation passaging on the relative telomere length and proliferation capacity of dental pulp stem cells. Biomolecules 11:464. doi: 10.3390/biom11030464
Pilbauerova, N., Schmidt, J., Soukup, T., Koberova Ivancakova, R., and Suchanek, J. (2021a). The effects of cryogenic storage on human dental pulp stem cells. Int. J. Mol. Sci. 22:4432. doi: 10.3390/ijms22094432
Pisciotta, A., Riccio, M., Carnevale, G., Lu, A., De Biasi, S., Gibellini, L., et al. (2015). Stem cells isolated from human dental pulp and amniotic fluid improve skeletal muscle histopathology in mdx/SCID mice. Stem Cell Res Ther. 6:156. doi: 10.1186/s13287-015-0141-y
Ponnaiyan, D., Bhat, K. M., and Bhat, G. S. (2012). Comparison of immuno-phenotypes of stem cells from human dental pulp and periodontal ligament. Int. J. Immunopathol. Pharmacol. 25, 127–134. doi: 10.1177/039463201202500115
Raoof, M., Yaghoobi, M. M., Derakhshani, A., Kamal-Abadi, A. M., Ebrahimi, B., Abbasnejad, M., et al. (2014). A modified efficient method for dental pulp stem cell isolation. Dent. Res. J. (Isfahan) 11, 244–250.
Ratajczak, J., Bronckaers, A., Dillen, Y., Gervois, P., Vangansewinkel, T., Driesen, R. B., et al. (2016). The Neurovascular properties of dental stem cells and their importance in dental tissue engineering. Stem Cells Int. 2016, 9762871. doi: 10.1155/2016/9762871
Rodas-Junco, B. A., and Villicana, C. (2017). Dental pulp stem cells: current advances in isolation, expansion and preservation. Tissue Eng. Regen. Med. 14, 333–347. doi: 10.1007/s13770-017-0036-3
Rufas, P., Jeanneau, C., Rombouts, C., Laurent, P., and About, I. (2016). Complement C3a mobilizes dental pulp stem cells and specifically guides pulp fibroblast recruitment. J. Endod. 42, 1377–1384. doi: 10.1016/j.joen.2016.06.011
Sakai, K., Yamamoto, A., Matsubara, K., Nakamura, S., Naruse, M., Yamagata, M., et al. (2012). Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J. Clin. Invest. 122, 80–90. doi: 10.1172/JCI59251
Sanen, K., Martens, W., Georgiou, M., Ameloot, M., Lambrichts, I., and Phillips, J. (2017). Engineered neural tissue with Schwann cell differentiated human dental pulp stem cells: potential for peripheral nerve repair? J. Tissue Eng. Regen. Med. 11, 3362–3372. doi: 10.1002/term.2249
Sasaki, R., Aoki, S., Yamato, M., Uchiyama, H., Wada, K., Ogiuchi, H., et al. (2011). PLGA artificial nerve conduits with dental pulp cells promote facial nerve regeneration. J. Tissue Eng. Regen. Med. 5, 823–830. doi: 10.1002/term.387
Sawangmake, C., Nowwarote, N., Pavasant, P., Chansiripornchai, P., and Osathanon, T. (2014). A feasibility study of an in vitro differentiation potential toward insulin-producing cells by dental tissue-derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 452, 581–587. doi: 10.1016/j.bbrc.2014.08.121
Scheller, E. L., Chang, J., and Wang, C. Y. (2008). Wnt/beta-catenin inhibits dental pulp stem cell differentiation. J. Dent. Res. 87, 126–130. doi: 10.1177/154405910808700206
Seo, B. M., Miura, M., Gronthos, S., Bartold, P. M., Batouli, S., Brahim, J., et al. (2004). Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364, 149–155. doi: 10.1016/S0140-6736(04)16627-0
Sharpe, P. T. (2016). Dental mesenchymal stem cells. Development 143, 2273–2280. doi: 10.1242/dev.134189
Shi, S., Bartold, P. M., Miura, M., Seo, B. M., Robey, P. G., and Gronthos, S. (2005). The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod. Craniofac. Res. 8, 191–199. doi: 10.1111/j.1601-6343.2005.00331.x
Shi, X., Mao, J., and Liu, Y. (2020). Pulp stem cells derived from human permanent and deciduous teeth: Biological characteristics and therapeutic applications. Stem Cells Transl. Med. 9, 445–464. doi: 10.1002/sctm.19-0398
Simon, S. R., Tomson, P. L., and Berdal, A. (2014). Regenerative endodontics: regeneration or repair? J. Endod. 40, S70–S75. doi: 10.1016/j.joen.2014.01.024
Smith, C. E. (1998). Cellular and chemical events during enamel maturation. Crit. Rev. Oral Biol. Med. 9, 128–161. doi: 10.1177/10454411980090020101
Song, M., Jue, S. S., Cho, Y. A., and Kim, E. C. (2015). Comparison of the effects of human dental pulp stem cells and human bone marrow-derived mesenchymal stem cells on ischemic human astrocytes in vitro. J. Neurosci. Res. 93, 973–983. doi: 10.1002/jnr.23569
Song, M., Lee, J. H., Bae, J., Bu, Y., and Kim, E. C. (2017). Human dental pulp stem cells are more effective than human bone marrow-derived mesenchymal stem cells in cerebral ischemic injury. Cell Transl. 26, 1001–1016. doi: 10.3727/096368916X694391
Sonoyama, W., Liu, Y., Fang, D., Yamaza, T., Seo, B. M., Zhang, C., et al. (2006). Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One 1:e79. doi: 10.1371/journal.pone.0000079
Sonoyama, W., Liu, Y., Yamaza, T., Tuan, R. S., Wang, S., Shi, S., et al. (2008). Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J. Endod. 34, 166–171. doi: 10.1016/j.joen.2007.11.021
Stevens, A., Zuliani, T., Olejnik, C., LeRoy, H., Obriot, H., Kerr-Conte, J., et al. (2008). Human dental pulp stem cells differentiate into neural crest-derived melanocytes and have label-retaining and sphere-forming abilities. Stem Cells Dev. 17, 1175–1184. doi: 10.1089/scd.2008.0012
Sunitha, V. R., Emmadi, P., Namasivayam, A., Thyegarajan, R., and Rajaraman, V. (2008). The periodontal – endodontic continuum: a review. J. Conserv. Dent. 11, 54–62. doi: 10.4103/0972-0707.44046
Syed-Picard, F. N., Du, Y., Lathrop, K. L., Mann, M. M., Funderburgh, M. L., and Funderburgh, J. L. (2015). Dental pulp stem cells: a new cellular resource for corneal stromal regeneration. Stem Cells Transl. Med. 4, 276–285. doi: 10.5966/sctm.2014-0115
Tan, X., and Dai, Q. (2017). Characterization of microRNAs expression profiles in human dental-derived pluripotent stem cells. PLoS One 12:e0177832. doi: 10.1371/journal.pone.0177832
Tang, Y., Zhou, Y., and Li, H. J. (2021). Advances in mesenchymal stem cell exosomes: a review. Stem Cell Res. Ther. 12:71. doi: 10.1186/s13287-021-02138-7
Tatullo, M., Marrelli, M., Falisi, G., Rastelli, C., Palmieri, F., Gargari, M., et al. (2016). Mechanical influence of tissue culture plates and extracellular matrix on mesenchymal stem cell behavior: a topical review. Int. J. Immunopathol. Pharmacol. 29, 3–8. doi: 10.1177/0394632015617951
Thery, C., Zitvogel, L., and Amigorena, S. (2002). Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2, 569–579. doi: 10.1038/nri855
Thesleff, I., and Tummers, M. (2008). Tooth Organogenesis And Regeneration. Cambridge, MA: StemBook. doi: 10.3824/stembook.1.37.1
Tirino, V., Paino, F., d’Aquino, R., Desiderio, V., De Rosa, A., and Papaccio, G. (2011). Methods for the identification, characterization and banking of human DPSCs: current strategies and perspectives. Stem Cell Rev. Rep. 7, 608–615. doi: 10.1007/s12015-011-9235-9
Tirino, V., Paino, F., De Rosa, A., and Papaccio, G. (2012). Identification, isolation, characterization, and banking of human dental pulp stem cells. Methods Mol. Biol. 879, 443–463. doi: 10.1007/978-1-61779-815-3_26
Tomic, S., Djokic, J., Vasilijic, S., Vucevic, D., Todorovic, V., Supic, G., et al. (2011). Immunomodulatory properties of mesenchymal stem cells derived from dental pulp and dental follicle are susceptible to activation by toll-like receptor agonists. Stem Cells Dev. 20, 695–708. doi: 10.1089/scd.2010.0145
Tran-Hung, L., Laurent, P., Camps, J., and About, I. (2008). Quantification of angiogenic growth factors released by human dental cells after injury. Arch. Oral Biol. 53, 9–13. doi: 10.1016/j.archoralbio.2007.07.001
Tsutsui, T. W. (2020). Dental pulp stem cells: advances to applications. Stem Cells Cloning 13, 33–42. doi: 10.2147/SCCAA.S166759
Vizoso, F. J., Eiro, N., Cid, S., Schneider, J., and Perez-Fernandez, R. (2017). Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int. J. Mol. Sci. 18:1852. doi: 10.3390/ijms18091852
Wada, N., Menicanin, D., Shi, S., Bartold, P. M., and Gronthos, S. (2009). Immunomodulatory properties of human periodontal ligament stem cells. J. Cell. Physiol. 219, 667–676. doi: 10.1002/jcp.21710
Wakayama, H., Hashimoto, N., Matsushita, Y., Matsubara, K., Yamamoto, N., Hasegawa, Y., et al. (2015). Factors secreted from dental pulp stem cells show multifaceted benefits for treating acute lung injury in mice. Cytotherapy 17, 1119–1129. doi: 10.1016/j.jcyt.2015.04.009
Wang, F., Jia, Y., Liu, J., Zhai, J., Cao, N., Yue, W., et al. (2017). Dental pulp stem cells promote regeneration of damaged neuron cells on the cellular model of Alzheimer’s disease. Cell Biol. Int. 41, 639–650. doi: 10.1002/cbin.10767
Wang, J., Zuzzio, K., and Walker, C. L. (2019). Systemic dental pulp stem cell secretome therapy in a mouse model of amyotrophic lateral sclerosis. Brain Sci. 9:165. doi: 10.3390/brainsci9070165
Wei, W., Ao, Q., Wang, X., Cao, Y., Liu, Y., Zheng, S. G., et al. (2020). Mesenchymal stem cell-derived exosomes: a promising biological tool in nanomedicine. Front. Pharmacol. 11:590470. doi: 10.3389/fphar.2020.590470
Wilson, R., Urraca, N., Skobowiat, C., Hope, K. A., Miravalle, L., Chamberlin, R., et al. (2015). Assessment of the tumorigenic potential of spontaneously immortalized and hTERT-Immortalized cultured dental pulp stem cells. Stem Cells Transl. Med. 4, 905–912. doi: 10.5966/sctm.2014-0196
Wu, W., Zhou, J., Xu, C. T., Zhang, J., Jin, Y. J., and Sun, G. L. (2015). Derivation and growth characteristics of dental pulp stem cells from patients of different ages. Mol. Med. Rep. 12, 5127–5134. doi: 10.3892/mmr.2015.4106
Xu, B., Fan, D., Zhao, Y., Li, J., Wang, Z., Wang, J., et al. (2019). Three-dimensional culture promotes the differentiation of human dental pulp mesenchymal stem cells into insulin-producing cells for improving the diabetes therapy. Front. Pharmacol. 10:1576. doi: 10.3389/fphar.2019.01576
Xu, B., Yuan, F. Z., Lin, L., Ye, J., Fan, B. S., Zhang, J. Y., et al. (2020). The higher inherent therapeutic potential of biomaterial-based hDPSCs and hEnSCs for pancreas diseases. Front. Bioeng. Biotechnol. 8:636. doi: 10.3389/fbioe.2020.00636
Yagi Mendoza, H., Yokoyama, T., Tanaka, T., Ii, H., and Yaegaki, K. (2018). Regeneration of insulin-producing islets from dental pulp stem cells using a 3D culture system. Regen. Med. 13, 673–687. doi: 10.2217/rme-2018-0074
Yamada, Y., Nakamura, S., Ito, K., Sugito, T., Yoshimi, R., Nagasaka, T., et al. (2010). A feasibility of useful cell-based therapy by bone regeneration with deciduous tooth stem cells, dental pulp stem cells, or bone-marrow-derived mesenchymal stem cells for clinical study using tissue engineering technology. Tissue Eng. Part A 16, 1891–1900. doi: 10.1089/ten.tea.2009.0732
Yamada, Y., Nakamura-Yamada, S., Kusano, K., and Baba, S. (2019). Clinical potential and current progress of dental pulp stem cells for various systemic diseases in regenerative medicine: a concise review. Int. J. Mol. Sci. 20:1132. doi: 10.3390/ijms20051132
Yamamoto, T., Osako, Y., Ito, M., Murakami, M., Hayashi, Y., Horibe, H., et al. (2016). Trophic effects of dental pulp stem cells on schwann cells in peripheral nerve regeneration. Cell Trans. 25, 183–193. doi: 10.3727/096368915X688074
Yang, C., Li, X., Sun, L., Guo, W., and Tian, W. (2017). Potential of human dental stem cells in repairing the complete transection of rat spinal cord. J. Neural Eng. 14:026005. doi: 10.1088/1741-2552/aa596b
Yang, H., Shin, S., Ahn, J., Choi, Y., Kim, K. H., and Chung, C. J. (2013). Local injection of pulp cells enhances wound healing during the initial proliferative phase through the stimulation of host angiogenesis. J. Endod. 39, 788–794. doi: 10.1016/j.joen.2013.01.011
Yang, K. L., Chen, M. F., Liao, C. H., Pang, C. Y., and Lin, P. Y. (2009). A simple and efficient method for generating Nurr1-positive neuronal stem cells from human wisdom teeth (tNSC) and the potential of tNSC for stroke therapy. Cytotherapy 11, 606–617. doi: 10.1080/14653240902806994
Ye, Q., Wang, H., Xia, X., Zhou, C., Liu, Z., Xia, Z. E., et al. (2020). Safety and efficacy assessment of allogeneic human dental pulp stem cells to treat patients with severe COVID-19: structured summary of a study protocol for a randomized controlled trial (Phase I / II). Trials 21:520.
Yildirim, S., Zibandeh, N., Genc, D., Ozcan, E. M., Goker, K., and Akkoc, T. (2016). The comparison of the immunologic properties of stem cells isolated from human exfoliated deciduous teeth, dental pulp, and dental follicles. Stem Cells Int. 2016:4682875. doi: 10.1155/2016/4682875
Yu, S., Zhao, Y., Ma, Y., and Ge, L. (2016). Profiling the secretome of human stem cells from dental apical papilla. Stem Cells Dev. 25, 499–508. doi: 10.1089/scd.2015.0298
Zhai, Q., Dong, Z., Wang, W., Li, B., and Jin, Y. (2019). Dental stem cell and dental tissue regeneration. Front. Med. 13:152–159. doi: 10.1007/s11684-018-0628-x
Zhang, W., Walboomers, X. F., Shi, S., Fan, M., and Jansen, J. A. (2006). Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng. 12, 2813–2823. doi: 10.1089/ten.2006.12.2813
Zhang, W., Walboomers, X. F., Van Kuppevelt, T. H., Daamen, W. F., Van Damme, P. A., Bian, Z., et al. (2008). In vivo evaluation of human dental pulp stem cells differentiated towards multiple lineages. J. Tissue Eng. Regen. Med. 2, 117–125. doi: 10.1002/term.71
Zhao, H., Feng, J., Seidel, K., Shi, S., Klein, O., Sharpe, P., et al. (2014). Secretion of shh by a neurovascular bundle niche supports mesenchymal stem cell homeostasis in the adult mouse incisor. Cell Stem Cell 14, 160–173. doi: 10.1016/j.stem.2013.12.013
Zhao, Y., Wang, L., Jin, Y., and Shi, S. (2012). Fas ligand regulates the immunomodulatory properties of dental pulp stem cells. J. Dent. Res. 91, 948–954. doi: 10.1177/0022034512458690
Zheng, L., Ehardt, L., McAlpin, B., About, I., Kim, D., Papagerakis, S., et al. (2014). The tick tock of odontogenesis. Exp. Cell Res. 325, 83–89. doi: 10.1016/j.yexcr.2014.02.007
Zhou, D., Gan, L., Peng, Y., Zhou, Y., Zhou, X., Wan, M., et al. (2020). Epigenetic regulation of dental pulp stem cell fate. Stem Cells Int. 2020:8876265. doi: 10.1155/2020/8876265
Keywords: dental pulp stem cells, surface markers, heterogeneity, immunomodulation, hepatogenic and pancreatic differentiation, human DPSCs
Citation: Al Madhoun A, Sindhu S, Haddad D, Atari M, Ahmad R and Al-Mulla F (2021) Dental Pulp Stem Cells Derived From Adult Human Third Molar Tooth: A Brief Review. Front. Cell Dev. Biol. 9:717624. doi: 10.3389/fcell.2021.717624
Received: 31 May 2021; Accepted: 15 September 2021;
Published: 12 October 2021.
Edited by:
Jianyong Xu, Shenzhen University, ChinaReviewed by:
Marco Tatullo, University of Bari Medical School, ItalyMiguel Barajas, Public University of Navarre, Spain
Yan Xu, Third Affiliated Hospital of Sun Yat-sen University, China
Copyright © 2021 Al Madhoun, Sindhu, Haddad, Atari, Ahmad and Al-Mulla. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ashraf Al Madhoun, YXNocmFmLm1hZGhvdW5AZGFzbWFuaW5zdGl0dXRlLm9yZw==; Rasheed Ahmad, cmFzaGVlZC5haG1hZEBkYXNtYW5pbnN0aXR1dGUub3Jn
†These authors have contributed equally to this work
 Ashraf Al Madhoun
Ashraf Al Madhoun Sardar Sindhu
Sardar Sindhu Dania Haddad
Dania Haddad Maher Atari
Maher Atari Rasheed Ahmad
Rasheed Ahmad Fahd Al-Mulla
Fahd Al-Mulla