
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell Dev. Biol. , 20 August 2021
Sec. Molecular and Cellular Oncology
Volume 9 - 2021 | https://doi.org/10.3389/fcell.2021.710165
Colorectal cancer (CRC) is a common cancer worldwide with complex etiology. Fusobacterium nucleatum (F. nucleatum), an oral symbiotic bacterium, has been linked with CRC in the past decade. A series of gut microbiota studies show that CRC patients carry a high abundance of F. nucleatum in the tumor tissue and fecal, and etiological studies have clarified the role of F. nucleatum as a pro-carcinogenic bacterium in various stages of CRC. In this review, we summarize the biological characteristics of F. nucleatum and the epidemiological associations between F. nucleatum and CRC, and then highlight the mechanisms by which F. nucleatum participates in CRC progression, metastasis, and chemoresistance by affecting cancer cells or regulating the tumor microenvironment (TME). We also discuss the research gap in this field and give our perspective for future studies. These findings will pave the way for manipulating gut F. nucleatum to deal with CRC in the future.
Colorectal cancer (CRC), known for its high morbidity and mortality, is prevalent worldwide and increasing the global health burden (Keum and Giovannucci, 2019). In 2020, CRC was responsible for the second-highest number of cancer-related deaths in men and women (Siegel et al., 2020). Though it has shown a decline in overall morbidity and ranks third among all cancers in recent years, the number of affected individuals is rapidly shifting from elderly people to young and middle-aged people (Hofseth et al., 2020; Siegel et al., 2020; Akimoto et al., 2021). The causes of CRC are complex and varied, and the interaction between genetic background and environmental stimuli contributes to the development of CRC (Lichtenstein et al., 2000). It is estimated that genetic factors account for only 10–30% of the CRC risk; therefore, environmental factors may play a significant role in causing sporadic CRC (Lichtenstein et al., 2000; Jasperson et al., 2010; Jiao et al., 2014; Graff et al., 2017). A high fat and low fiber diet, smoking, heavy drinking, and physical inactivity all could increase the risk of CRC (Song et al., 2020; Yue et al., 2021). Gut microbiota, which mainly resides in the colon, has been recognized as an essential contributor to CRC in recent years.
The gut microbiota consisting of bacteria, archaea, viruses, fungi, protozoa, and helminths reflects a micro-ecosystem that intensely interacts with the host (Lynch and Pedersen, 2016). Physically, the microbiota performs many functions, such as fiber digestion and immune modulation (Adak and Khan, 2019). However, several factors, such as gut inflammation and changes in dietary habits, may alter the composition and function of gut microbiota, which is commonly termed dysbiosis (Weiss and Hennet, 2017; Illiano et al., 2020). This gut microbiota dysbiosis plays a role in intestinal and extraintestinal diseases, such as inflammatory bowel disease and cardiovascular diseases (Glassner et al., 2020; Jin et al., 2020). With the advancement of 16S rRNA and shotgun metagenomics, researchers have confirmed that CRC patients are accompanied by gut microbiota dysbiosis, characterized by an increase in cancer-associated bacteria such as polyketide synthase Escherichia coli (pks+ E. coli), enterotoxigenic B. fragilis (ETBF), Fusobacterium nucleatum (F. nucleatum) and others, whereas protective or beneficial bacteria such as Clostridium Faecalibacterium are reduced (Janney et al., 2020). While, F. nucleatum has attracted more attention in CRC studies (Castellarin et al., 2012; Kostic et al., 2012). In this review, we first introduce the biological characteristics of F. nucleatum, and then summarize the clinical findings of F. nucleatum in kinds of CRC patients’ samples. We also highlight its role as a pro-carcinogenic bacterium in various CRC models and the specific mechanisms. Finally, we put forward feasible insights for further studies in F. nucleatum and CRC.
Fusobacterium nucleatum is a fusiform or spindle-shaped gram-negative anaerobe that mainly colonizes the oral mucosa. As a symbiotic bacterium, F. nucleatum serves as a structural support for other bacteria to form the oral biofilms, which are essential for the normal oral microenvironment (Lamont et al., 2018; Zhang et al., 2018). On the other hand, since it has been isolated from clinical infections and multiple tumor samples, such as periodontitis (Kim E. H. et al., 2020), adverse pregnancy (Vander Haar et al., 2018; Figuero et al., 2020), appendicitis (Hattori et al., 2019), CRC (Castellarin et al., 2012; Kostic et al., 2012), and breast cancer (Parhi et al., 2020), it has been regarded as an opportunistic pathogen and a tumor-associated bacterium. To further explore its mechanisms to promote CRC, we first introduce four basic biological characteristics associated with pathogenicity.
First and foremost, F. nucleatum is an adhesive bacterium that expresses multiple adhesins on its surface, including RadD, Aid1, and FomA, which can mediate the coaggregation of bacteria to form biofilms (Liu et al., 2010; Kaplan et al., 2014; Guo et al., 2017). Not only in the oral cavity (Bowen et al., 2018), but also in CRC, bacterial biofilms composed of F. nucleatum are widely present, especially in right-sided colon cancer (Dejea et al., 2014; Drewes et al., 2017; Li et al., 2017).
Second, F. nucleatum can adhere to and even invade various host cells, including epithelial cells, endothelial cells, and fibroblasts (Han, 2015). The downstream responses of host cells induced by adherence and invasion are the primary conditions for F. nucleatum to exert its virulence and pathogenicity. At present, the well-known virulence factors/adhesins that connect F. nucleatum to host cells are as follows. (1) FadA, a complex composed of immature pre-FadA and surface mature mFadA in different proportions, is the specific and best-characterized virulence factor of F. nucleatum (Xu et al., 2007). The invasion of F. nucleatum is mainly attributed to FadA, and once FadA binds to the corresponding ligands, it can activate the zipper mechanism to transport F. nucleatum into cells (Fardini et al., 2011; Rubinstein Mara et al., 2013). (2) Fap2, the largest outer membrane protein of F. nucleatum, is a galactose-inhibitable adhesin (Coppenhagen-Glazer et al., 2015). It is the primary molecule that regulates F. nucleatum adherence to various cancer cells, such as colorectal and breast cancer cells, which usually overexpress its specific ligand, Gal-GalNAc (Abed et al., 2016; Parhi et al., 2020).
Third, F. nucleatum has vast metabolic potential. On the one hand, F. nucleatum has multiple enzymes that produce hydrogen sulfide (H2S) from L-cysteine (Basic et al., 2017; Kezuka et al., 2018; Blachier et al., 2019). H2S, which is the cause of oral malodor (Hampelska et al., 2020), can stimulate cell proliferation in CRC (Szabo et al., 2013). On the other hand, F. nucleatum is identified as a potential butyrate producer (Vital et al., 2014; Llama-Palacios et al., 2020; Dahlstrand Rudin et al., 2021). Unlike other bacteria, it does not rely on polysaccharides to produce butyrate but uses amino acids (Vital et al., 2014). The role of butyrate in CRC has always been the focus of controversy (Guan et al., 2021), and whether butyrate from F. nucleatum plays a role in the CRC-associated microenvironment is still unknown. Furthermore, its outer membrane contains lipopolysaccharide (LPS), which can play a vital role in causing inflammation or tumorigenesis through LPS receptors, such as Toll-like receptor 4 (TLR4) (Chen et al., 2017).
Fourth, like other gram-negative bacteria, F. nucleatum can release extracellular vesicles (EVs) or outer membrane vesicles (OMVs) (Liu et al., 2019; Liu L. et al., 2021), which contain a large number of bioactive substances and participate in bacteria-bacteria or bacteria-host cells communications (Macia et al., 2019). By isolating and purifying F. nucleatum (ATCC 23726)-derived EVs, researchers verified that EVs’ surface protein, FomA, could bind to TLR2 on the intestinal epithelial cells and then modulate innate immunity (Martin-Gallausiaux et al., 2020). F. nucleatum EVs’ can also disrupt the intestinal epithelial barrier by activating the FADD-RIPK1-caspase3 signaling pathway to promote necrosis of intestinal epithelial cells (Liu L. et al., 2021). Furthermore, Engevik et al. (2021) used OMVs isolated from F. nucleatum (ATCC 10953) to stimulate colonic epithelial cells and found the production of the proinflammatory cytokines IL-8 and TNF increased. These studies suggest that EVs or OMVs are also a form of pathogenic effect.
Before exploring the associations between F. nucleatum and CRC, it is necessary to determine the existence and abundance of F. nucleatum in different sample types. There are numerous qualitative and quantitative methods for studying F. nucleatum, including whole-genome shotgun sequencing, quantitative PCR (qPCR), 16S rRNA sequence analysis, fluorescence in situ hybridization (FISH), RNA sequencing, and bacterial culture. Moreover, with the development of technology, droplet digital PCR (ddPCR) and fluorescence quantitative PCR (FQ-PCR) have gradually become new quantitative methods. Among these methods, bacterial culture and FISH provide the strongest evidence, as they can identify the presence of viable F. nucleatum and its position, respectively (Table 1).
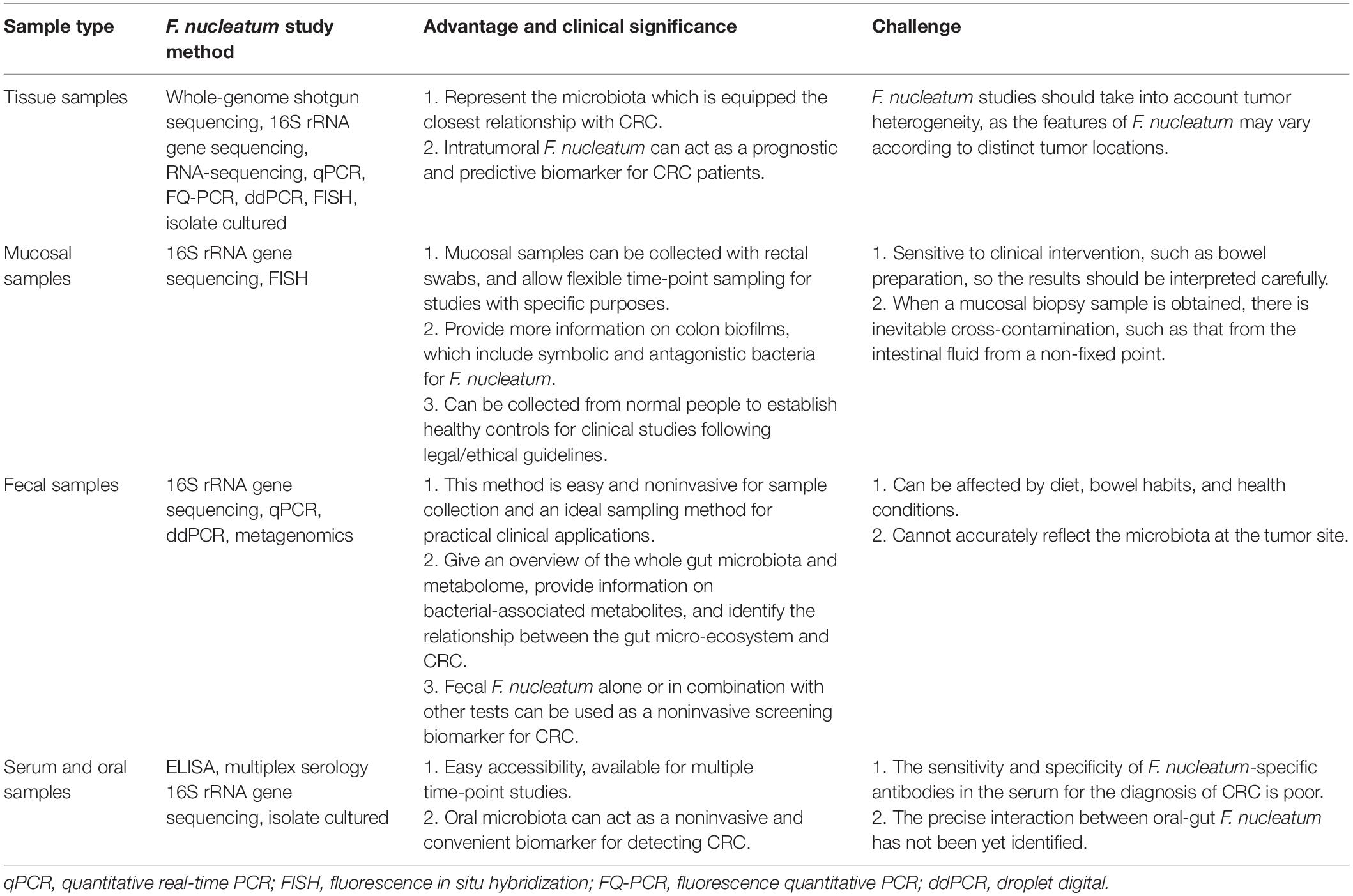
Table 1. The advantages, clinical significance, and challenges of different sample types for F. nucleatum studies.
Considering that microbiota in direct contact with epithelial cells is likely to influence the progression of CRC, microbiome analysis of tissue samples can provide highly useful data. The tumor tissue samples examined include postoperative fresh frozen tissues (Castellarin et al., 2012; Kostic et al., 2012; Li et al., 2016; Wei et al., 2016; Ye et al., 2017; Yamaoka et al., 2018), formalin-fixed paraffin-embedded (FFPE) tissue blocks (Ito et al., 2015; Mima et al., 2015, 2016a,b; Park et al., 2017; Liu et al., 2018), and patient-derived tumor xenografts (PDXs) (Bullman et al., 2017). Castellarin et al. (2012) and Kostic et al. (2012) first suggested the existence of intratumoral F. nucleatum, and this conclusion was fully confirmed in multiple samples and cohorts. At the level of subcellular localization, FISH revealed that F. nucleatum was located close to the lumen and ulcerated regions, predominantly associated with malignant cells (Kostic et al., 2012; Bullman et al., 2017). Transmission electron microscopy even showed that F. nucleatum invaded cancer cells and existed in a vesicle structure (Bullman et al., 2017). As a result, intratumoral F. nucleatum is considered invasive. Except for CRC tissues, F. nucleatum can be detected in secondary distal metastases such as liver (Bullman et al., 2017), lung (Abed et al., 2016), and lymph node (Abed et al., 2016) as well as in PDX models (Bullman et al., 2017).
The abundance of F. nucleatum in F. nucleatum-positive CRC is based on the following characteristics. (1) F. nucleatum is significantly enriched in tumor tissues compared to adjacent normal tissues, even a mean 415 times higher (Castellarin et al., 2012). (2) The abundance of F. nucleatum varies and increases along with the adenoma-carcinoma sequence (Flanagan et al., 2014; Nakatsu et al., 2015). (3) Patients with metastasis or recurrence have more intratumoral F. nucleatum than patients without metastasis or recurrence (Chen Y. et al., 2020; Yu et al., 2017). (4) Even in the same tumor tissues, the abundance of F. nucleatum varies at different sampling sites (Liu W. et al., 2021).
Based on the high amount of intratumoral F. nucleatum and the clinical, pathological, and molecular features of CRC, association studies revealed critical roles for F. nucleatum. (1) Several studies revealed that patients with higher intratumoral F. nucleatum usually had shorter survival times and were more likely to experience recurrence, which suggested using F. nucleatum as a prognostic and predictive biomarker (Flanagan et al., 2014; Mima et al., 2016b; Yu et al., 2017). (2) Pathological connections seem to suggest that F. nucleatum participates in the occurrence and development of CRC, as F. nucleatum is related to different tumor phenotypes, such as autophagy and glycolysis (Yu et al., 2017; Hong et al., 2020). (3) Regarding its relationship with molecular features, intratumoral F. nucleatum is associated with high microsatellite instability, the CpG island methylator phenotype (CIMP) status, and certain gene mutations (Tahara et al., 2014). In addition to the cancer cells, F. nucleatum is also associated with low CD3+ T cells (Mima et al., 2015; Borowsky et al., 2021).
Mucosal samples examined include rectal swab samples (Chen et al., 2012), colonoscopy mucosal biopsy samples (Nakatsu et al., 2015; Tunsjo et al., 2019), and postoperative tissue samples with the mucus layer preserved (Dejea et al., 2014). With the methods mentioned at the beginning of the paragraph, F. nucleatum can be detected in all three mucosal samples of CRC patients, and its abundance is higher than that in healthy controls. Mucosal samples contain the microbiota in the superficial layer of the tissue, which is called “mucosa-adherent microbiota” (Chen et al., 2012). To emphasize their association with tumor tissues, the mucosa-adherent microbiota, and tissue microbiota are collectively called the “mucosa-associated microbiota” (Chen et al., 2012). Rectal swabs come in close contact with feces, so their microbiota structure is similar to that in feces in some degree (Chen et al., 2012). However, rectal swabs can allow flexible time-point sampling for studies with specific purposes. As for biopsy samples, although the sampling area is slightly small, the sampling process is inevitably contaminated, and bowel preparation before inspection may destroy the microbiota (Tang et al., 2020), they have the advantage of allowing the inclusion of healthy controls, which is a limitation of clinical studies because the collection of tissue samples is restricted by ethical requirements. Tissue samples with a preserved mucus layer are mainly used to study colon biofilms, which are essential to understand the impacts of multimicrobiota interactions on CRC (Drewes et al., 2017).
Although the microbiota in the feces is easily affected by diet, bowel habits, and other factors (Falony et al., 2018), cohort studies in multiple countries have confirmed that both CRC and advanced adenoma patients have an increased abundance of F. nucleatum in feces (Wu et al., 2013; Yu et al., 2015; Eklof et al., 2017; Suehiro et al., 2017; Wong et al., 2017a; Guo et al., 2018), although there are some studies that cannot confirm this (Amitay et al., 2017). Fecal samples usually represent gut luminal microbiota, but the structure of the microbiota differs between various lumen locations (Tanca et al., 2017). Therefore, fecal samples cannot truly reflect the luminal microbiota corresponding to tumor tissues (Flanagan et al., 2014), which is a great limitation to study their direct pathogenic effects on CRC. However, fecal samples not only provide an overview of the whole gut microbiota changes, but also provide information on bacterial-associated metabolites, and reveal the relationship between the gut micro-ecosystem and CRC (Kim et al., 2020). Moreover, their easy accessibility makes them better screening biomarkers than tissue or mucosal samples. Studies have shown that the combination of the fecal immunochemical test (FIT) and quantitation of fecal F. nucleatum or the ratio of F. nucleatum to Faecalibacterium prausnitzii can improve the screening sensitivity and specificity of advanced adenoma and CRC (Wong et al., 2017a; Guo et al., 2018). Moreover, the fecal F. nucleatum test may be an effective non-invasive predictor for metachronous adenoma after endoscopic polypectomy (Xue et al., 2021).
Since F. nucleatum infection can stimulate the body to produce specific antibodies, several researchers used ELISA and multiplex serology to detect F. nucleatum-specific IgA and IgG antibodies in the serum and then evaluated their sensitivity and specificity to diagnose CRC. However, the results were disappointing. Kurt and Yumuk (2021) revealed that the diagnostic accuracy of IgA and IgG was poor, and Wang et al. (2016) suggested that only combining IgA, CEA, and CA199 could improve the diagnostic sensitivity. Furthermore, numerous diseases in addition to CRC can cause an increase in antibodies of F. nucleatum in the serum (Yamamura et al., 2017), so the results should be interpreted carefully. However, in a prospective cohort study, researchers found that serum F. nucleatum antibody level was not associated with CRC incidence, which indicated that F. nucleatum infection did not increase the risk of CRC (Butt et al., 2019; Lo et al., 2021). In contrast to the cross-sectional clinical studies, this study gives some hints for the cause-effect relationship between F. nucleatum and CRC.
Oral samples examined mainly include saliva, oral wash, tongue coating, and oral swabs (Zhang et al., 2020). The oral microbiota has been associated with several cancers, such as pancreatic cancer (Fan et al., 2018), esophageal cancer (Peters et al., 2017), and CRC (Han, 2014). Flemer et al. (2018) found that the oral swab microbiota of CRC patients was significantly different from that of healthy controls. Using the oral swab microbiota alone or in combination with the fecal microbiota can distinguish CRC patients from healthy controls, so the oral microbiota may act as a non-invasive biomarker for detecting CRC (Flemer et al., 2018). Zhang et al. (2020) reached a similar conclusion in a Chinese cohort. However, Flemer et al. (2018) detected the abundance of Fusobacterium in both oral and CRC tissues, finally found that there was no association between them in quantity.
In summary, the abundance of F. nucleatum in tissue, mucosal biopsy, and fecal samples of CRC is higher. Considering that each kind of sample has specific characteristics and scientific investigation values, researchers should apply the appropriate sampling methods according to the aim of the study (Table 1).
As F. nucleatum is rarely found in the healthy gut, researchers have tried to explore the origin of F. nucleatum and how it migrates to and colonizes CRC tissues. For this purpose, Flemer et al. (2018) analyzed the microbiota in oral swabs, colonic mucosa, and feces, and ultimately found a similar network between colonic mucosal bacteria and oral bacteria. Komiya et al. (2019) and Abed et al. (2020) studied this problem in-depth and suggested that gut F. nucleatum originated from the oral cavity. Not only was the oral cavity the main habitat of F. nucleatum, but identical strains of F. nucleatum existed in CRC tissues and saliva specimens obtained from the same patient. However, several studies have shown that the virulence of F. nucleatum varies from the oral cavity to the gut, and the latter seems to be more virulent (Chen et al., 2018).
There are two possible routes for F. nucleatum to translocate from the oral cavity to the gut. Abed et al. (2016) injected F. nucleatum via the tail vein into orthotopic CRC mouse models and found F. nucleatum in tumors, which verified that blood-borne F. nucleatum could localize to CRC tissues. This is consistent with the clinical phenomenon that patients with F. nucleatum bacteremia were at an increased risk of CRC (Kwong et al., 2018). Another way is by swallowing through the digestive tract. Kostic et al. (2013) gave F. nucleatum to Apc Min/+ mice by gavage every day and harvested more and larger tumors from these mice than control mice. Simultaneously, they found F. nucleatum in the tumor tissues by qPCR and FISH. By comparing these two routes, Abed et al. (2020) were more inclined to support the blood-borne route because compared to oral F. nucleatum, intravenous F. nucleatum was more effective at colonizing tumors. Furthermore, blood-borne F. nucleatum can avoid the attack of the digestive tract. However, although previous studies showed that F. nucleatum was present in liver and lymph node metastases (even viable F. nucleatum in liver metastases) (Abed et al., 2016; Bullman et al., 2017), the mechanisms that mediate the transport of F. nucleatum to distal metastases are still unclear. One possible mechanism is that F. nucleatum invades cancer cells and reaches the liver or lymph nodes by blood circulation or lymphatic circulation. Alternatively, during CRC, F. nucleatum may migrate from the oral cavity to the liver through the blood circulation and participate in the development of liver metastases.
Regarding colonization, CRC is an essential precondition for F. nucleatum colonization. Abed et al. (2016) unveiled that adhesin Fap2 bound to Gal-GalNAc, a sugar residue overexpressed on the surface of CRC cells, and then mediated the attachment of F. nucleatum. Moreover, in metastatic lesions, such as liver and omentum, Gal-GalNAc is also overexpressed, which suggests that F. nucleatum colonization here depends on Fap2-Gal-GalNAc as well. F. nucleatum strains with Fap2 absent or mutated can reduce this colonization. The combination of adhesin FadA with E-cadherin, which presents on the surface of CRC cells, can also promote colonization (Rubinstein Mara et al., 2013). However, unlike Gal-GalNAc, since E-cadherin is not specifically overexpressed on cancer cells, the attachment of F. nucleatum may be largely attributed to Fap2-Gal-GalNAc.
During the initial exploration of the gut microbiota in CRC, researchers transplanted fecal or biofilm samples into mice and successfully accelerated tumorigenesis (Wong et al., 2017b; Tomkovich et al., 2019). Tumorigenic mechanisms, such as inflammation, immune regulation, genotoxins, and harmful metabolites, have been completely reviewed in other studies (Janney et al., 2020). However, fecal or biofilm samples include not only a variety of microbiota but also multiple metabolites. As such, there are great limitations to precisely manipulate fecal or biofilm samples, and using antibiotics to abolish the whole microbiota will cause unpredictable effects. It is urgent to identify the specific microbiota that plays a key role in tumorigenesis.
Since many studies showed that F. nucleatum was enriched in the tumor tissue and even F. nucleatum can invade into the tumor cell of CRC, it is reasonable to suspect F. nucleatum could influence tumorigenesis. Therefore, many studies attempted to prove the tumorigenic ability of F. nucleatum with various experimental models. These models are roughly classified into three categories, in vitro co-culture system, 3D intestinal organoids, and CRC mouse models. The co-culture with cancer cells in vitro is the simplest and classical way to study carcinogenic changes, but there are still some limitations to applying the results to the in vivo environment. The organoid is a model which plays an important role in stimulating the gastrointestinal environment in vitro (Drost and Clevers, 2018). The application of the organoid model not only proved that the pks+ E. coli could cause the formation of CRC (Pleguezuelos-Manzano et al., 2020), but also proved that certain substances secreted by F. nucleatum could stimulate organoids to secrete TNF and increase intestinal inflammation (Engevik et al., 2021). Considering that the native host physiological condition is a critical feature for a successful tumor model, the mouse model is still the ideal model for cancer studies, so we summarized the mouse models that were used in the studies on F. nucleatum and CRC progression. In addition, since F. nucleatum has been found to exist in metastatic and recurrent CRC tissues, its effects on promoting tumor metastasis and inducing chemoresistance have also been investigated (Table 2).
It needs to be mentioned that, though F. nucleatum has the potential to influence the progression, metastasis, and chemoresistance of CRC, its role in CRC initiation still needs to be further investigated. On the one hand, in some models, such as colitis-associated mice and germ-free APC Min+ mice, F. nucleatum intervention did not cause or accelerate tumorigenesis (Kostic et al., 2013; Tomkovich et al., 2017). On the other hand, even though clinical observations have found that the abundance of F. nucleatum was enriched up-raise in precancerous lesions before carcinogenesis, but this temporal precedence is only one of the criteria that are essential to build up the cause-effect relationship between F. nucleatum and CRC. Besides, unlike other well-known CRC tumor-causing bacteria, one clinical prospective cohort study found that serum F. nucleatum antibodies do not relate to CRC incidence rate (Butt et al., 2019, 2021; Lo et al., 2021). All these results indicate that F. nucleatum may not have an effect on CRC initiation, but further studies are needed to give a clear conclusion.
Researchers have provided multiple mechanisms by which F. nucleatum participates in CRC progression, metastasis, and chemoresistance. In essence, F. nucleatum may influence CRC development by affecting cancer cells or their tumor microenvironment (TME) in different ways. Therefore, to clarify the mechanisms systematically, we will discuss two aspects: one is the mechanisms by which F. nucleatum reprograms cancer cells, and the other is the mechanisms by which F. nucleatum reprograms the TME to play a pro-carcinogenic role (Figure 1).
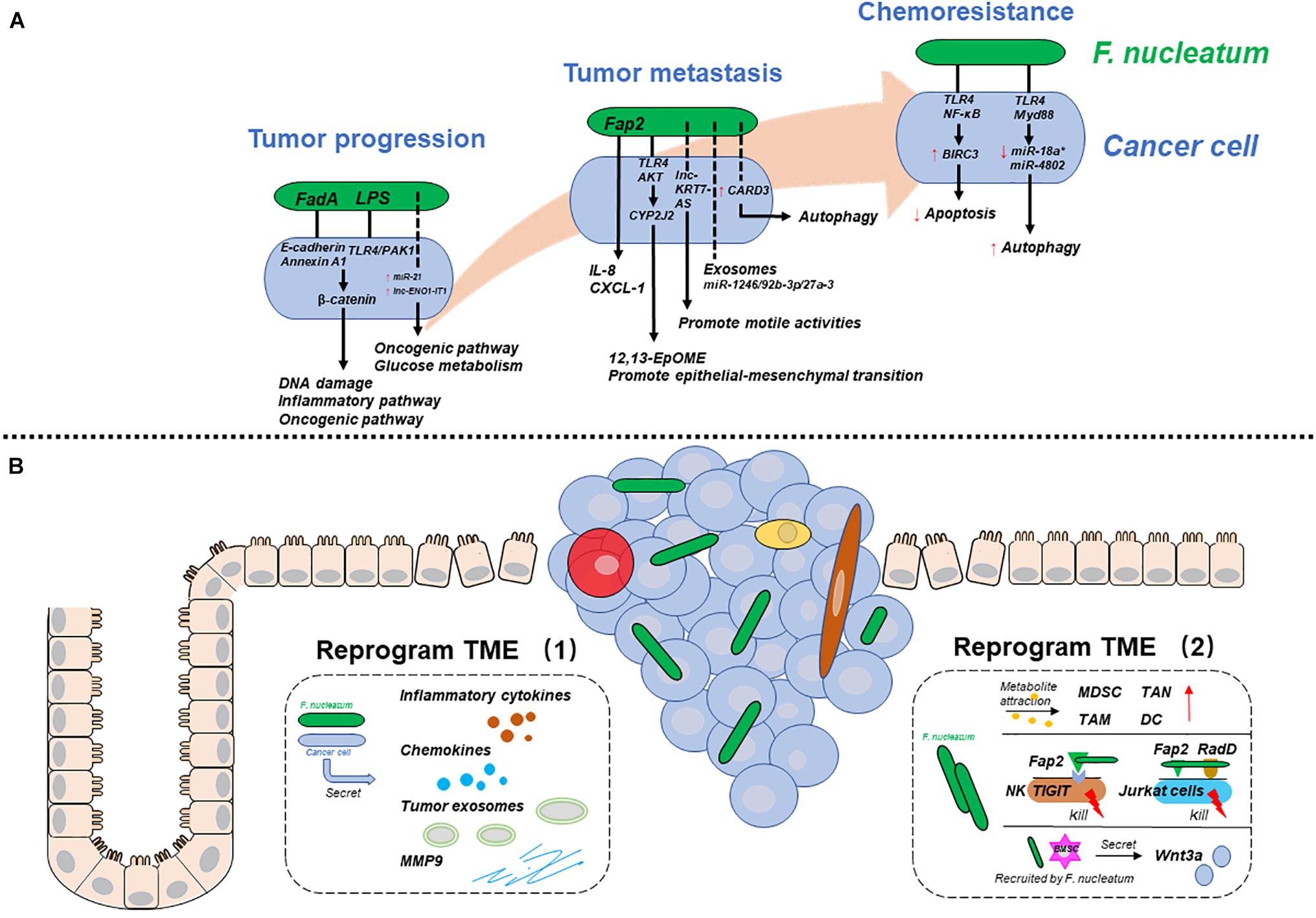
Figure 1. The mechanisms by which F. nucleatum is involved in various CRC stages. (A) F. nucleatum reprograms cancer cells during tumor progression, metastasis, and chemoresistance. (B) F. nucleatum reprograms the tumor microenvironment (TME) by interacting with cancer cells (left), immune cells and others (right). Solid lines represent proven mechanisms, and dotted lines represent unclear mechanisms.
In addition to mediating adhesion and invasion, FadA also plays a key role in cellular oncogenesis (Rubinstein Mara et al., 2013; Rubinstein et al., 2019; Guo P. et al., 2020). Briefly, the binding of FadA and E-cadherin promotes the phosphorylation and internalization of the latter. At the same time, downstream β-catenin phosphorylation is reduced, and excess β-catenin accumulates in the cytoplasm and then translocates into the nucleus. Rubinstein Mara et al. (2013) verified that once the FadA/E-cadherin/β-catenin pathway was activated, the expression of inflammatory genes (such as NF-κB) and oncogenes (such as Myc and Cyclin D1) was increased. However, though both inflammatory genes and oncogenes were regulated by FadA/E-cadherin/β-catenin, activation of the former did not depend on the internalization of E-cadherin, while the latter required both its phosphorylation and internalization. Guo P. et al. (2020) also showed that F. nucleatum increased DNA damage and accelerated tumor growth through the FadA/E-cadherin/β-catenin pathway. This active pathway causes the overexpression of chk2, which is a multifunctional enzyme related to DNA damage. As a result, chk2 upregulation facilitates DNA damage and tumor growth. This is not the first study to find that F. nucleatum can affect DNA damage. Geng et al. (2020) reported that F. nucleatum induced DNA double-strand breaks in oral cancer cells, but there was no in-depth discussion on which factors of F. nucleatum cause this phenomenon. However, as E-cadherin is expressed on nearly all epithelial cells, it is difficult to attribute oncogenesis to FadA/E-cadherin. In vitro experiments showed that FadA bound to noncancerous cells expressing E-cadherin and promoted the expression of inflammatory genes, but it did not stimulate tumor growth (Rubinstein et al., 2019). This oncogenesis is specific to cancerous cells. Rubinstein et al. (2019) reported that in contrast to noncancerous cells, there was a special membrane protein called Annexin A1 on the surface of cancerous cells. Previous studies have verified that Annexin A1 is associated with the activation of Cyclin D1 and can stimulate CRC cell proliferation (Foo et al., 2019). To explore the relationship between FadA and Annexin A1, researchers used confocal microscopy and found that FadA, E-cadherin, Annexin A1, and β-catenin formed a complex on the surface of cancerous cells. There is a functional interaction among the components of the complex. On the one hand, Annexin A1 enhances the efficiency of FadA adhesion and invasion. On the other hand, FadA and E-cadherin increase Annexin A1 expression in CRC cells. Finally, through E-cadherin and Annexin A1, FadA causes β-catenin to enter the nucleus, which activates the expression of inflammatory genes and oncogenes, such as Cyclin D1, and then accelerates cell proliferation and tumor growth. Therefore, Annexin A1 is a critical component of FadA that has a stimulatory effect (Rubinstein et al., 2019). Approximately 90% of CRCs are associated with the activation of β-catenin signaling, and F. nucleatum is the main activator (Matly et al., 2021). Not only FadA but also the LPS produced by F. nucleatum can activate β-catenin signaling. Chen et al. (2017) reported that F. nucleatum and LPS induced β-catenin nuclear accumulation in CRC cells via the TLR4/PAK1 pathway. Although it is theoretically believed that the activation of β-catenin can promote cell proliferation, this study did not report whether LPS treatment alone could accelerate tumor growth. Therefore, the function of LPS alone is still unclear.
Noncoding RNAs (ncRNAs), such as microRNAs and long ncRNAs, are crucial regulators of epigenetic genes, and their expression is dysregulated in cancer cells (Dragomir et al., 2020). Recent studies based on pathogenic bacteria have revealed that ncRNAs are a crosstalk factor between bacteria and host cells (Aguilar et al., 2019; Dong et al., 2019). Therefore, F. nucleatum may promote CRC by regulating gene expression through ncRNAs. Yang et al. (2017) found that the high expression of miR-21 in CRC was specifically induced by F. nucleatum. miR-21 reduces the expression of RAS1, which activates the MAPK pathway and enhances cell proliferation. Moreover, TLR4/Myd88/NF-κB may be an upstream pathway connecting F. nucleatum and miR-21. Although other bacteria such as E. coli can also activate TLR4/Myd88/NF-κB in cancer cells, they do not upregulate the expression of miR-21. This finding suggests that miR-21 is a F. nucleatum-specific oncogenic ncRNA. Hong et al. (2020) demonstrated that F. nucleatum enhanced abnormal glycolysis in cancer cells, which can provide more energy to promote cell proliferation and tumor growth. Mechanistically, F. nucleatum induces the transcriptional activation of the lncRNA ENO1-IT1, which binds to and interacts with KAT7, a subunit of the histone acetyltransferase complexes. The ENO1-IT1/KAT7 complex increases its target gene transcription by promoting histone acetylation. One of the target genes, ENO1, a key component of the glycolysis pathway, is transactivated and then alters the metabolic pathway of cancer cells.
Cancer cells secrete cytokines during bacterial infections. Some cytokines affect cell proliferation and migration through autocrine mechanisms, and some act on adjacent cancer cells or noncancerous cells through paracrine mechanisms, which also play a crucial role in the development of tumors (Briukhovetska et al., 2021). Casasanta et al. (2020) treated HCT116 cells with F. nucleatum and detected cytokines in the culture medium. F. nucleatum induced the secretion of IL-8 and CXCL1, which are proinflammatory and prometastatic cytokines. Medium rich in IL-8 and CXCL1 enhanced the migration of uninfected cancer cells in a paracrine manner. However, the deletion of adhesin Fap2 caused F. nucleatum to lose the above functions. This research showed that Fap2 might be indispensable for the production of cytokines to enhance the migration ability of cancer cells.
Noncoding RNAs may be another type of molecule through which F. nucleatum promotes migration and metastasis. Chen S. et al. (2020) performed RNA sequencing to detect the expression of ncRNAs in LoVo cells after incubation with F. nucleatum. Among the variable ncRNAs, the expression of the lncRNA KRT7-AS increased significantly, as did its target gene KRT7. Intracellular KRT7-AS promoted cell migration by enhancing the stability of KRT7. However, when KRT7-AS was depleted, the metastatic effect induced by F. nucleatum was abolished, which suggested that F. nucleatum promoted migration by upregulating KRT7-AS and KRT7. Mechanistically, F. nucleatum infection can activate the NF-κB signaling pathway, which acts as a transcription factor in the nucleus and promotes the transcription of KRT7-AS. In addition to playing a role intracellularly, ncRNAs, which are delivered by cancer cells then loaded into exosomes, also play a role in metastasis. Guo S. et al. (2020) revealed that F. nucleatum-infected associated tumor exosomes were larger than control exosomes and rich in ncRNAs. Among the ncRNAs, miR-1246/92b-3p/27a-3 expression was significantly increased and was absorbed by adjacent uninfected cancer cells. miR-1246/92b-3p/27a-3 can target GSK3β and activate the Wnt/β-catenin pathway to promote CRC cell migration in vitro. In summary, exosomes may magnify the effect of F. nucleatum on cancer cells. This conclusion, i.e., that F. nucleatum-infected exosomes facilitate CRC metastasis to intrahepatic blood vessels and the lung, has also been confirmed in vivo.
In addition to these means, Chen Y. et al. (2020) demonstrated that F. nucleatum might promote tumor metastasis by activating the autophagy pathway and that the autophagy inhibitor, chloroquine (CQ), could reverse this phenomenon in vivo and in vitro. The reason for this observation was that F. nucleatum infection upregulated the expression of CARD3, a protein thought to be involved in bacterial infection-related autophagy, and activated autophagy then promoted migration. Moreover, this study also showed that F. nucleatum might promote metastasis by regulating epithelial-mesenchymal transition (EMT), though there was no in-depth exploration of the mechanism involved. In another study, researchers suggested that F. nucleatum promoted EMT by altering cancer cell metabolism. Mechanistically, F. nucleatum can active the TLR4/Keap1/NRF2 pathway then up-regulates CYP2J2, a cytochrome P450. Then 12,13-EPOME, a metabolite of CYP2J2, can activate EMT in vitro and promote metastasis of CRC in vivo (Kong et al., 2021).
After radical surgery, CRC patients usually receive combination chemotherapy, including oxaliplatin, 5-fluorouracil (5-FU), and others (Chiorean et al., 2020). However, some patients are not sensitive to chemotherapeutic drugs, which eventually leads to tumor recurrence. Previous studies have proven that the gut microbiota plays a positive or negative role in antitumor therapy (Helmink et al., 2019), so intratumor bacteria may be the cause of CRC chemoresistance. Some studies have focused on the impact of F. nucleatum on the chemoresistance of CRC. Zhang et al. (2019) suggested that F. nucleatum upregulated the expression of BIRC3 via the TLR4/NF-κB pathway in CRC cells. Through BIRC3, a protein that can directly inhibit the caspase cascade and reduce cell apoptosis, F. nucleatum reduced the responsiveness of CRC cells to 5-FU. Moreover, Yu et al. (2017) suggested that F. nucleatum induced the resistance of CRC cells to oxaliplatin and 5-FU by selectively losing miR-18a∗ and miR-4802, then activating the autophagy pathway and preventing cells from posttreatment apoptosis, and finally promoting recurrence. Although F. nucleatum had been founded to contribute to CRC chemoresistance by inducing cancer cell autophagy and apoptosis, the exact virulence factors or metabolites of F. nucleatum responsible for this have not been identified, and the mechanisms during this process still need further investigation.
The TME consists of immune-inflammatory cells, stromal cells, secreted products, metabolites, and the extracellular matrix (Maman and Witz, 2018). In addition, recent studies have proven that the existence of microbiota in various tumor tissues is not special but a common phenomenon (Nejman et al., 2020). Therefore, the intratumor microbiota is also considered a part of the TME. The TME plays a vital role in the development of CRC; for instance, it impacts tumors through angiogenesis and immune regulation. Moreover, there are complex interactions between the TME and cancer cells (Maman and Witz, 2018).
The development of CRC is often accompanied by inflammation (Schmitt and Greten, 2021). Inflammatory cells and inflammatory cytokines have been considered active tumor factors since they can induce angiogenesis, and stimulate cancer cells proliferation and invasion (West et al., 2015; Galdiero et al., 2018). In addition to inflammatory cells, cancer cells stimulated by F. nucleatum can also produce a variety of inflammatory cytokines, such as IL-6, IL-8, and TNF-α, which are related to activation of the NF-κB pathway (Kostic et al., 2013; Rubinstein Mara et al., 2013; Koi et al., 2018). These inflammatory cytokines can act on cells that express their specific receptors in the TME and promote tumor progression in a variety of ways.
In addition to inflammatory cytokines, cancer cells infected with F. nucleatum can secrete chemokines. As mentioned earlier, IL-8 and CXCL1 not only stimulated the migration of noninfected HCT116 cells but also recruited immune cells, particularly neutrophils, to the tumor site and increased inflammation (Casasanta et al., 2020). Moreover, F. nucleatum stimulates cancer cells to produce CCL-20. Once CCL-20 binds to its ligand CCR6, it can recruit CCR6+ immune cells, including subsets of IL17-expressing T helper cells (Th17), regulatory T cells, and dendritic cells (Ye et al., 2017).
Tumor-derived exosomes (TEXs) are important components of the TME, as they can deliver ncRNAs and proteins to recipient cells, which contribute to intercellular communication and molecular transfer (Xu et al., 2018). In addition to being internalized by uninfected cancer cells mentioned above, they may also be internalized by noncancerous cells to reprogram the TME, though Guo S. et al. (2020) did not show this phenomenon. They can even enter body fluids or the blood, mediating remote regulation between organs (Xu et al., 2018).
The success of metastasis depends not only on the metastatic capacity of cancer cells but also on the assistance of the TME (Hinshaw and Shevde, 2019). For instance, the initial stage of local tumor spread requires hydrolysis of the extracellular matrix to overcome the tissue barrier. Some researches have shown that in breast and oral cancers, F. nucleatum can induce the secretion of MMP9, which can degrade basement membrane collagen and provide conditions for metastasis (Gholizadeh et al., 2016; Gonzalez-Avila et al., 2019; Parhi et al., 2020). However, this phenomenon has not been reported in CRC.
The most abundant cells in the TME are infiltrating immune cells and mesenchymal support cells (Maman and Witz, 2018). Though the interaction between F. nucleatum and mesenchymal cells, such as cancer-associated fibroblasts, has rarely been studied in CRC, the modulation of the tumor-immune microenvironment by F. nucleatum is considered to be an essential component of its tumorigenesis. There are two types of tumor-infiltrating immune cells: antitumor cells, such as natural killer cells (NK cells) and cytotoxic CD8+ T cells, and tumor-permissive immunosuppressive cells, such as myeloid-derived stem cells (MDSCs) and tumor-associated macrophages (TAMs). Overall, F. nucleatum reshapes the tumor-immune microenvironment and accelerates CRC progression by increasing the number and function of immunosuppressive cells and inhibiting antitumor cells.
Kostic et al. (2013) provided compelling evidence that F. nucleatum selectively expanded MDSCs, TAMs, tumor-associated neutrophils (TANs), and dendritic cells (DCs) in APC Min+ mouse models. These cells not only suppress immunity but also participate in the biological behaviors of tumors. For instance, M2-like TAMs not only inhibit T cells through the expression of arginase-1 (Arlauckas et al., 2018), but also promote proliferation, metastasis, and angiogenesis by secreting numerous growth factors and chemokines (Galdiero et al., 2018). Researchers attributed this selection expansion to the metabolites of F. nucleatum, such as SCFAs and formyl-methionyl-leucyl-phenylalanine, which act as chemoattractants of MDSCs (Kostic et al., 2013), though no experiments were conducted to confirm this hypothesis. Another study also showed that F. nucleatum promoted the M2 polarization of macrophages via the TLR4/IL-6/p-STAT3/c-MYC cascade (Chen et al., 2018).
Epidemiological studies have revealed that a high abundance of F. nucleatum is associated with a low level of CD3+ T cells (Mima et al., 2015), specifically CD3+ CD4+ CD45RO+ cells (Borowsky et al., 2021). This may be because F. nucleatum has a direct or indirect inhibitory effect on CD3+ T cells. Kaplan et al. (2010) demonstrated that F. nucleatum used its outer membrane proteins Fap2 and RadD to induce the death of Jurkat cells, human lymphocytes, in a manner that doesn’t depend on the complete bacterial structure (Kaplan et al., 2010). In addition, Fap2 binds to TIGIT receptors on NK cells and other tumor-infiltrating T cells. TIGIT can inhibit immune cytotoxicity and protect F. nucleatum and cancer cells from being killed (Gur et al., 2015).
Bone mesenchymal stem cells (BMSCs), pluripotent stem cells with the abilities to self-renew and differentiate, are involved in the development of CRC (Koliaraki et al., 2017). Lin et al. (2020) explored the effect of BMSCs on F. nucleatum-induced CRC. The authors verified that F. nucleatum induced BMSCs to transplant to the submucosa and mucosal layer and simultaneously activated the Wnt signaling pathway. Furthermore, after stimulation with F. nucleatum, BMSCs accelerated the tumorigenesis of CRC by increasing the secretion of the Wnt3a protein. In summary, F. nucleatum modulates the TME to accelerate tumorigenesis by BMSCs.
Although extensive studies have deeply explored the relationship between F. nucleatum and CRC, there is still much unresolved. First, it will make sense to find a more convenient method for patients using F. nucleatum as a biomarker for CRC. Considering that patients with F. nucleatum bacteremia were at high risk of CRC (Kwong et al., 2018), whether the blood test for F. nucleatum DNA alone could be developed as an early screening technique for CRC is still an open question. Second, FadA, Fap2, and LPS, which have been widely accepted to contribute to the pathogenicity of F. nucleatum, that is FadA and Fap2 enable F. nucleatum to adhere to cells and LPS regulate the inflammation signal through the innate immune pathway, so is there any other specific cell components or metabolites of F. nucleatum realize its pathogenetic role still need further investigation (Zanzoni et al., 2017; Cochrane et al., 2020). In addition, whether F. nucleatum would affect other important phenomena in the entire TME is also worth exploring, such as abnormal metabolism, tumor angiogenesis, and hardness of the tumor matrix (Nia et al., 2020). Emerging research techniques, like multi-omics joint analysis, single bacteria/cell sequencing technology may help solve these problems. Third, experimental results should be interpreted more carefully, because we cannot define F. nucleatum as totally a harmful or a pro-carcinogenetic bacterium. The role of F. nucleatum to CRC depends on the genetic background of the host, the heterogeneity of the tumor, the associated TME, and other environmental factors. So, the traditional in vitro or in vivo experiments hardly take into consideration of these confounding factors. More ingenious or specific experiments should be carried out, such as organoid (Lau et al., 2020), PDX model (Invrea et al., 2020), and organ-on-a-chip (Del Piccolo et al., 2021; Puschhof et al., 2021), which somehow gives the results closer to the real world. Fourth, chemotherapy/immunotherapy resistance is still a tough problem for the oncologist, and few studies claim that F. nucleatum leads to chemoresistance by regulating autophagy or apoptosis (Yu et al., 2017; Zhang et al., 2019), but the exact mechanism is still needed to be figured out. The effects of F. nucleatum on pharmacotherapy come down to three aspects. Firstly, F. nucleatum regulates the cancer cells directly to equip them with resistance. F. nucleatum could also affect the metabolite process of pharmaceutical molecules directly or indirectly in bodies. Finally, the therapeutic effects no doubt depends on the TME (Binnewies et al., 2018), so F. nucleatum could regulate the components of TME to realize the resistance to therapy.
In summary, precisely defining mechanisms by which F. nucleatum is involved in the progression, metastasis, and treatment response of CRC will potentially become the cornerstone of the establishment of CRC management methods based on F. nucleatum in the future.
SW and YW conceived the review. SW and YL searched the literature and drafted the manuscript. JL and LZ polished the language. WY drew the figure. BL and XG made the tables. YW edited the manuscript. All authors read and approved its final version.
This work was supported by the National Natural Science Foundation of China Grant (81970466).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abed, J., Emgard, J. E., Zamir, G., Faroja, M., Almogy, G., Grenov, A., et al. (2016). Fap2 mediates Fusobacterium nucleatum colorectal adenocarcinoma enrichment by binding to tumor-expressed Gal-GalNAc. Cell Host Microb. 20, 215–225. doi: 10.1016/j.chom.2016.07.006
Abed, J., Maalouf, N., Manson, A. L., Earl, A. M., Parhi, L., Emgard, J. E. M., et al. (2020). Colon cancer-associated Fusobacterium nucleatum may originate from the oral cavity and reach colon tumors via the circulatory system. Front. Cell. Infect. Microbiol. 10:400. doi: 10.3389/fcimb.2020.00400
Adak, A., and Khan, M. R. (2019). An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. 76, 473–493. doi: 10.1007/s00018-018-2943-4
Aguilar, C., Mano, M., and Eulalio, A. (2019). MicroRNAs at the host-bacteria interface: host defense or bacterial offense. Trends Microbiol. 27, 206–218. doi: 10.1016/j.tim.2018.10.011
Akimoto, N., Ugai, T., Zhong, R., Hamada, T., Fujiyoshi, K., Giannakis, M., et al. (2021). Rising incidence of early-onset colorectal cancer - a call to action. Nat. Rev. Clin. Oncol. 18, 230–243. doi: 10.1038/s41571-020-00445-1
Amitay, E. L., Werner, S., Vital, M., Pieper, D. H., Hofler, D., Gierse, I. J., et al. (2017). Fusobacterium and colorectal cancer: causal factor or passenger? Results from a large colorectal cancer screening study. Carcinogenesis 38, 781–788. doi: 10.1093/carcin/bgx053
Arlauckas, S. P., Garren, S. B., Garris, C. S., Kohler, R. H., Oh, J., Pittet, M. J., et al. (2018). Arg1 expression defines immunosuppressive subsets of tumor-associated macrophages. Theranostics 8, 5842–5854. doi: 10.7150/thno.26888
Basic, A., Blomqvist, M., Dahlen, G., and Svensater, G. (2017). The proteins of Fusobacterium spp. involved in hydrogen sulfide production from L-cysteine. BMC Microbiol. 17:61. doi: 10.1186/s12866-017-0967-9
Binnewies, M., Roberts, E. W., Kersten, K., Chan, V., Fearon, D. F., Merad, M., et al. (2018). Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 24, 541–550. doi: 10.1038/s41591-018-0014-x
Blachier, F., Beaumont, M., and Kim, E. (2019). Cysteine-derived hydrogen sulfide and gut health: a matter of endogenous or bacterial origin. Curr. Opin. Clin. Nutr. Metab. Care 22, 68–75. doi: 10.1097/MCO.0000000000000526
Borowsky, J., Haruki, K., Lau, M. C., Dias Costa, A., Vayrynen, J. P., Ugai, T., et al. (2021). Association of Fusobacterium nucleatum with Specific T Cell subsets in the colorectal carcinoma microenvironment. Clin. Cancer Res. 27, 2816–2826. doi: 10.1158/1078-0432.CCR-20-4009
Bowen, W. H., Burne, R. A., Wu, H., and Koo, H. (2018). Oral biofilms: pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 26, 229–242. doi: 10.1016/j.tim.2017.09.008
Briukhovetska, D., Dorr, J., Endres, S., Libby, P., Dinarello, C. A., and Kobold, S. (2021). Interleukins in cancer: from biology to therapy. Nat. Rev. Cancer 2021, 1–19. doi: 10.1038/s41568-021-00363-z
Bullman, S., Pedamallu, C. S., Sicinska, E., Clancy, T. E., Zhang, X., Cai, D., et al. (2017). Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science 358, 1443–1448. doi: 10.1126/science.aal5240
Butt, J., Jenab, M., Pawlita, M., Overvad, K., Tjonneland, A., Olsen, A., et al. (2019). Antibody responses to Fusobacterium nucleatum proteins in prediagnostic blood samples are not associated with risk of developing colorectal cancer. Cancer Epidemiol. Biomarkers Prev. 28, 1552–1555. doi: 10.1158/1055-9965.EPI-19-0313
Butt, J., Jenab, M., Werner, J., Fedirko, V., Weiderpass, E., Dahm, C. C., et al. (2021). Association of Pre-diagnostic antibody responses to Escherichia coli and Bacteroides fragilis toxin proteins with colorectal cancer in a European Cohort. Gut Microb. 13, 1–14. doi: 10.1080/19490976.2021.1903825
Casasanta, M. A., Yoo, C. C., Udayasuryan, B., Sanders, B. E., Umana, A., Zhang, Y., et al. (2020). Fusobacterium nucleatum host-cell binding and invasion induces IL-8 and CXCL1 secretion that drives colorectal cancer cell migration. Sci. Signal. 13:eaba9157. doi: 10.1126/scisignal.aba9157
Castellarin, M., Warren, R. L., Freeman, J. D., Dreolini, L., Krzywinski, M., Strauss, J., et al. (2012). Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 22, 299–306. doi: 10.1101/gr.126516.111
Chen, S., Su, T., Zhang, Y., Lee, A., He, J., Ge, Q., et al. (2020). Fusobacterium nucleatum promotes colorectal cancer metastasis by modulating KRT7-AS/KRT7. Gut Microb. 11, 511–525. doi: 10.1080/19490976.2019.1695494
Chen, T., Li, Q., Wu, J., Wu, Y., Peng, W., Li, H., et al. (2018). Fusobacterium nucleatum promotes M2 polarization of macrophages in the microenvironment of colorectal tumours via a TLR4-dependent mechanism. Cancer Immunol. Immunother. 67, 1635–1646. doi: 10.1007/s00262-018-2233-x
Chen, W., Liu, F., Ling, Z., Tong, X., and Xiang, C. (2012). Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer. PLoS One 7:e39743. doi: 10.1371/journal.pone.0039743
Chen, Y., Chen, Y., Zhang, J., Cao, P., Su, W., Deng, Y., et al. (2020). Fusobacterium nucleatum promotes metastasis in colorectal cancer by activating autophagy signaling via the upregulation of CARD3 expression. Theranostics 10, 323–339. doi: 10.7150/thno.38870
Chen, Y., Peng, Y., Yu, J., Chen, T., Wu, Y., Shi, L., et al. (2017). Invasive Fusobacterium nucleatum activates beta-catenin signaling in colorectal cancer via a TLR4/P-PAK1 cascade. Oncotarget 8, 31802–31814. doi: 10.18632/oncotarget.15992
Chiorean, E. G., Nandakumar, G., Fadelu, T., Temin, S., Alarcon-Rozas, A. E., Bejarano, S., et al. (2020). Treatment of patients with late-stage colorectal cancer: ASCO resource-stratified guideline. JCO Glob. Oncol. 6, 414–438. doi: 10.1200/JGO.19.00367
Cochrane, K., Robinson, A. V., Holt, R. A., and Allen-Vercoe, E. (2020). A survey of Fusobacterium nucleatum genes modulated by host cell infection. Microb. Genom. 6:e000300. doi: 10.1099/mgen.0.000300
Coppenhagen-Glazer, S., Sol, A., Abed, J., Naor, R., Zhang, X., Han, Y. W., et al. (2015). Fap2 of Fusobacterium nucleatum is a galactose-inhibitable adhesin involved in coaggregation, cell adhesion, and preterm birth. Infect. Immun. 83, 1104–1113. doi: 10.1128/IAI.02838-14
Dahlstrand Rudin, A., Khamzeh, A., Venkatakrishnan, V., Basic, A., Christenson, K., and Bylund, J. (2021). Short chain fatty acids released by Fusobacterium nucleatum are neutrophil chemoattractants acting via free fatty acid receptor 2 (FFAR2). Cell. Microbiol. 23:e13348. doi: 10.1111/cmi.13348
Dejea, C. M., Wick, E. C., Hechenbleikner, E. M., White, J. R., Mark Welch, J. L., Rossetti, B. J., et al. (2014). Microbiota organization is a distinct feature of proximal colorectal cancers. Proc. Natl. Acad. Sci. U S A. 111, 18321–18326. doi: 10.1073/pnas.1406199111
Del Piccolo, N., Shirure, V. S., Bi, Y., Goedegebuure, S. P., Gholami, S., Hughes, C. C. W., et al. (2021). Tumor-on-chip modeling of organ-specific cancer and metastasis. Adv. Drug Deliv. Rev. 175:113798. doi: 10.1016/j.addr.2021.05.008
Dong, J., Tai, J. W., and Lu, L. F. (2019). miRNA-microbiota interaction in gut homeostasis and colorectal cancer. Trends Cancer 5, 666–669. doi: 10.1016/j.trecan.2019.08.003
Dragomir, M. P., Kopetz, S., Ajani, J. A., and Calin, G. A. (2020). Non-coding RNAs in GI cancers: from cancer hallmarks to clinical utility. Gut 69, 748–763. doi: 10.1136/gutjnl-2019-318279
Drewes, J. L., White, J. R., Dejea, C. M., Fathi, P., Iyadorai, T., Vadivelu, J., et al. (2017). High-resolution bacterial 16S rRNA gene profile meta-analysis and biofilm status reveal common colorectal cancer consortia. NPJ Biofilms Microb. 3:34. doi: 10.1038/s41522-017-0040-3
Drost, J., and Clevers, H. (2018). Organoids in cancer research. Nat. Rev. Cancer 18, 407–418. doi: 10.1038/s41568-018-0007-6
Eklof, V., Lofgren-Burstrom, A., Zingmark, C., Edin, S., Larsson, P., Karling, P., et al. (2017). Cancer-associated fecal microbial markers in colorectal cancer detection. Int. J. Cancer 141, 2528–2536. doi: 10.1002/ijc.31011
Engevik, M. A., Danhof, H. A., Ruan, W., Engevik, A. C., Chang-Graham, A. L., Engevik, K. A., et al. (2021). Fusobacterium nucleatum secretes outer membrane vesicles and promotes intestinal inflammation. mBio 12:e2706–20. doi: 10.1128/mBio.02706-20
Falony, G., Vieira-Silva, S., and Raes, J. (2018). Richness and ecosystem development across faecal snapshots of the gut microbiota. Nat. Microbiol. 3, 526–528. doi: 10.1038/s41564-018-0143-5
Fan, X., Alekseyenko, A. V., Wu, J., Peters, B. A., Jacobs, E. J., Gapstur, S. M., et al. (2018). Human oral microbiome and prospective risk for pancreatic cancer: a population-based nested case-control study. Gut 67, 120–127. doi: 10.1136/gutjnl-2016-312580
Fardini, Y., Wang, X., Temoin, S., Nithianantham, S., Lee, D., Shoham, M., et al. (2011). Fusobacterium nucleatum adhesin FadA binds vascular endothelial cadherin and alters endothelial integrity. Mol. Microbiol. 82, 1468–1480. doi: 10.1111/j.1365-2958.2011.07905.x
Figuero, E., Han, Y. W., and Furuichi, Y. (2020). Periodontal diseases and adverse pregnancy outcomes: Mechanisms. Periodontol 2000 83, 175–188. doi: 10.1111/prd.12295
Flanagan, L., Schmid, J., Ebert, M., Soucek, P., Kunicka, T., Liska, V., et al. (2014). Fusobacterium nucleatum associates with stages of colorectal neoplasia development, colorectal cancer and disease outcome. Eur. J. Clin. Microbiol. Infect. Dis. 33, 1381–1390. doi: 10.1007/s10096-014-2081-3
Flemer, B., Warren, R. D., Barrett, M. P., Cisek, K., Das, A., Jeffery, I. B., et al. (2018). The oral microbiota in colorectal cancer is distinctive and predictive. Gut 67, 1454–1463. doi: 10.1136/gutjnl-2017-314814
Foo, S. L., Yap, G., Cui, J., and Lim, L. H. K. (2019). Annexin-A1 - A blessing or a curse in cancer? Trends Mol. Med. 25, 315–327. doi: 10.1016/j.molmed.2019.02.004
Galdiero, M. R., Marone, G., and Mantovani, A. (2018). Cancer inflammation and cytokines. Cold Spring Harb. Perspect. Biol. 10:a028662. doi: 10.1101/cshperspect.a028662
Geng, F., Zhang, Y., Lu, Z., Zhang, S., and Pan, Y. (2020). Fusobacterium nucleatum caused DNA damage and promoted cell proliferation by the Ku70/p53 pathway in oral cancer cells. DNA Cell. Biol. 39, 144–151. doi: 10.1089/dna.2019.5064
Gholizadeh, P., Eslami, H., Yousefi, M., Asgharzadeh, M., Aghazadeh, M., and Kafil, H. S. (2016). Role of oral microbiome on oral cancers, a review. Biomed. Pharmacother. 84, 552–558. doi: 10.1016/j.biopha.2016.09.082
Glassner, K. L., Abraham, B. P., and Quigley, E. M. M. (2020). The microbiome and inflammatory bowel disease. J. Allergy Clin. Immunol. 145, 16–27. doi: 10.1016/j.jaci.2019.11.003
Gonzalez-Avila, G., Sommer, B., Mendoza-Posada, D. A., Ramos, C., Garcia-Hernandez, A. A., and Falfan-Valencia, R. (2019). Matrix metalloproteinases participation in the metastatic process and their diagnostic and therapeutic applications in cancer. Crit. Rev. Oncol. Hematol. 137, 57–83. doi: 10.1016/j.critrevonc.2019.02.010
Graff, R. E., Moller, S., Passarelli, M. N., Witte, J. S., Skytthe, A., Christensen, K., et al. (2017). Familial risk and heritability of colorectal cancer in the nordic twin study of cancer. Clin. Gastroenterol. Hepatol. 15, 1256–1264. doi: 10.1016/j.cgh.2016.12.041
Guan, X., Li, W., and Meng, H. (2021). A double-edged sword: Role of butyrate in the oral cavity and the gut. Mol. Oral Microbiol. 36, 121–131. doi: 10.1111/omi.12322
Guo, L., Shokeen, B., He, X., Shi, W., and Lux, R. (2017). Streptococcus mutans SpaP binds to RadD of Fusobacterium nucleatum ssp. polymorphum. Mol. Oral Microbiol. 32, 355–364. doi: 10.1111/omi.12177
Guo, P., Tian, Z., Kong, X., Yang, L., Shan, X., Dong, B., et al. (2020). FadA promotes DNA damage and progression of Fusobacterium nucleatum-induced colorectal cancer through up-regulation of chk2. J. Exp. Clin. Cancer Res. 39:202. doi: 10.1186/s13046-020-01677-w
Guo, S., Chen, J., Chen, F., Zeng, Q., Liu, W. L., and Zhang, G. (2020). Exosomes derived from Fusobacterium nucleatum-infected colorectal cancer cells facilitate tumour metastasis by selectively carrying miR-1246/92b-3p/27a-3p and CXCL16. Gut 2020:321187. doi: 10.1136/gutjnl-2020-321187
Guo, S., Li, L., Xu, B., Li, M., Zeng, Q., Xiao, H., et al. (2018). A simple and novel fecal biomarker for colorectal cancer: ratio of Fusobacterium Nucleatum to probiotics populations, based on their antagonistic effect. Clin. Chem. 64, 1327–1337. doi: 10.1373/clinchem.2018.289728
Gur, C., Ibrahim, Y., Isaacson, B., Yamin, R., Abed, J., Gamliel, M., et al. (2015). Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 42, 344–355. doi: 10.1016/j.immuni.2015.01.010
Hampelska, K., Jaworska, M. M., Babalska, Z. L., and Karpinski, T. M. (2020). The role of oral microbiota in intra-oral halitosis. J. Clin. Med. 9:2484. doi: 10.3390/jcm9082484
Han, Y. W. (2014). Commentary: Oral bacteria as drivers for colorectal cancer. J. Periodontol. 85, 1155–1157. doi: 10.1902/jop.2014.140039
Han, Y. W. (2015). Fusobacterium nucleatum: a commensal-turned pathogen. Curr. Opin. Microbiol. 23, 141–147. doi: 10.1016/j.mib.2014.11.013
Hattori, T., Yuasa, N., Ikegami, S., Nishiyama, H., Takeuchi, E., Miyake, H., et al. (2019). Culture-based bacterial evaluation of the appendix lumen in patients with and without acute appendicitis. J. Infect. Chemother. 25, 708–713. doi: 10.1016/j.jiac.2019.03.021
Helmink, B. A., Khan, M. A. W., Hermann, A., Gopalakrishnan, V., and Wargo, J. A. (2019). The microbiome, cancer, and cancer therapy. Nat. Med. 25, 377–388. doi: 10.1038/s41591-019-0377-7
Hinshaw, D. C., and Shevde, L. A. (2019). The tumor microenvironment innately modulates cancer progression. Cancer Res. 79, 4557–4566. doi: 10.1158/0008-5472.CAN-18-3962
Hofseth, L. J., Hebert, J. R., Chanda, A., Chen, H., Love, B. L., Pena, M. M., et al. (2020). Early-onset colorectal cancer: initial clues and current views. Nat. Rev. Gastroenterol. Hepatol. 17, 352–364. doi: 10.1038/s41575-019-0253-4
Hong, J., Guo, F., Lu, S. Y., Shen, C., Ma, D., Zhang, X., et al. (2020). F. nucleatum targets lncRNA ENO1-IT1 to promote glycolysis and oncogenesis in colorectal cancer. Gut 2020:322780. doi: 10.1136/gutjnl-2020-322780
Illiano, P., Brambilla, R., and Parolini, C. (2020). The mutual interplay of gut microbiota, diet and human disease. FEBS J. 287, 833–855. doi: 10.1111/febs.15217
Invrea, F., Rovito, R., Torchiaro, E., Petti, C., Isella, C., and Medico, E. (2020). Patient-derived xenografts (PDXs) as model systems for human cancer. Curr. Opin. Biotechnol. 63, 151–156. doi: 10.1016/j.copbio.2020.01.003
Ito, M., Kanno, S., Nosho, K., Sukawa, Y., Mitsuhashi, K., Kurihara, H., et al. (2015). Association of Fusobacterium nucleatum with clinical and molecular features in colorectal serrated pathway. Int. J. Cancer 137, 1258–1268. doi: 10.1002/ijc.29488
Janney, A., Powrie, F., and Mann, E. H. (2020). Host-microbiota maladaptation in colorectal cancer. Nature 585, 509–517. doi: 10.1038/s41586-020-2729-3
Jasperson, K. W., Tuohy, T. M., Neklason, D. W., and Burt, R. W. (2010). Hereditary and familial colon cancer. Gastroenterology 138, 2044–2058. doi: 10.1053/j.gastro.2010.01.054
Jiao, S., Peters, U., Berndt, S., Brenner, H., Butterbach, K., Caan, B. J., et al. (2014). Estimating the heritability of colorectal cancer. Hum. Mol. Genet. 23, 3898–3905. doi: 10.1093/hmg/ddu087
Jin, L., Shi, X., Yang, J., Zhao, Y., Xue, L., Xu, L., et al. (2020). Gut microbes in cardiovascular diseases and their potential therapeutic applications. Protein Cell 12, 346–359. doi: 10.1007/s13238-020-00785-9
Kaplan, A., Kaplan, C. W., He, X., McHardy, I., Shi, W., and Lux, R. (2014). Characterization of aid1, a novel gene involved in Fusobacterium nucleatum interspecies interactions. Microb. Ecol. 68, 379–387. doi: 10.1007/s00248-014-0400-y
Kaplan, C. W., Ma, X., Paranjpe, A., Jewett, A., Lux, R., Kinder-Haake, S., et al. (2010). Fusobacterium nucleatum outer membrane proteins Fap2 and RadD induce cell death in human lymphocytes. Infect. Immun. 78, 4773–4778. doi: 10.1128/IAI.00567-10
Keum, N., and Giovannucci, E. (2019). Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 16, 713–732. doi: 10.1038/s41575-019-0189-8
Kezuka, Y., Ishida, T., Yoshida, Y., and Nonaka, T. (2018). Structural insights into the catalytic mechanism of cysteine (hydroxyl) lyase from the hydrogen sulfide-producing oral pathogen, Fusobacterium nucleatum. Biochem. J. 475, 733–748. doi: 10.1042/bcj20170838
Kim, E. H., Kim, S., Kim, H. J., Jeong, H. O., Lee, J., Jang, J., et al. (2020). Prediction of chronic periodontitis severity using machine learning models based on salivary bacterial copy number. Front. Cell. Infect. Microbiol. 10:571515. doi: 10.3389/fcimb.2020.571515
Kim, M., Vogtmann, E., Ahlquist, D. A., Devens, M. E., Kisiel, J. B., Taylor, W. R., et al. (2020). Fecal metabolomic signatures in colorectal adenoma patients are associated with gut microbiota and early events of colorectal cancer pathogenesis. mBio 11:e3186–19. doi: 10.1128/mBio.03186-19
Koi, M., Okita, Y., and Carethers, J. M. (2018). Fusobacterium nucleatum infection in colorectal cancer: linking inflammation, DNA mismatch repair and genetic and epigenetic alterations. J. Anus. Rectum. Colon. 2, 37–46. doi: 10.23922/jarc.2017-055
Koliaraki, V., Pallangyo, C. K., Greten, F. R., and Kollias, G. (2017). Mesenchymal cells in colon cancer. Gastroenterology 152, 964–979. doi: 10.1053/j.gastro.2016.11.049
Komiya, Y., Shimomura, Y., Higurashi, T., Sugi, Y., Arimoto, J., Umezawa, S., et al. (2019). Patients with colorectal cancer have identical strains of Fusobacterium nucleatum in their colorectal cancer and oral cavity. Gut 68, 1335–1337. doi: 10.1136/gutjnl-2018-316661
Kong, C., Yan, X., Zhu, Y., Zhu, H., Luo, Y., Liu, P., et al. (2021). Fusobacterium nucleatum promotes the development of colorectal cancer by activating a cytochrome P450/epoxyoctadecenoic acid axis via TLR4/Keap1/NRF2 signaling. Cancer Res. 2021:453. doi: 10.1158/0008-5472.CAN-21-0453
Kostic, A. D., Chun, E., Robertson, L., Glickman, J. N., Gallini, C. A., Michaud, M., et al. (2013). Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microb. 14, 207–215. doi: 10.1016/j.chom.2013.07.007
Kostic, A. D., Gevers, D., Pedamallu, C. S., Michaud, M., Duke, F., Earl, A. M., et al. (2012). Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 22, 292–298. doi: 10.1101/gr.126573.111
Kurt, M., and Yumuk, Z. (2021). Diagnostic accuracy of Fusobacterium nucleatum IgA and IgG ELISA test in colorectal cancer. Sci. Rep. 11:1608. doi: 10.1038/s41598-021-81171-1
Kwong, T. N. Y., Wang, X., Nakatsu, G., Chow, T. C., Tipoe, T., Dai, R. Z. W., et al. (2018). Association between bacteremia from specific microbes and subsequent diagnosis of colorectal cancer. Gastroenterology 155, 383–390. doi: 10.1053/j.gastro.2018.04.028
Lamont, R. J., Koo, H., and Hajishengallis, G. (2018). The oral microbiota: dynamic communities and host interactions. Nat. Rev. Microbiol. 16, 745–759. doi: 10.1038/s41579-018-0089-x
Lau, H. C. H., Kranenburg, O., Xiao, H., and Yu, J. (2020). Organoid models of gastrointestinal cancers in basic and translational research. Nat. Rev. Gastroenterol. Hepatol. 17, 203–222. doi: 10.1038/s41575-019-0255-2
Li, S., Konstantinov, S. R., Smits, R., and Peppelenbosch, M. P. (2017). Bacterial biofilms in colorectal cancer initiation and progression. Trends Mol. Med. 23, 18–30. doi: 10.1016/j.molmed.2016.11.004
Li, Y. Y., Ge, Q. X., Cao, J., Zhou, Y. J., Du, Y. L., Shen, B., et al. (2016). Association of Fusobacterium nucleatum infection with colorectal cancer in Chinese patients. World J. Gastroenterol. 22, 3227–3233. doi: 10.3748/wjg.v22.i11.3227
Lichtenstein, P., Holm, N. V., Verkasalo, P. K., Iliadou, A., Kaprio, J., Koskenvuo, M., et al. (2000). Environmental and heritable factors in the causation of cancer–analyses of cohorts of twins from Sweden, Denmark, and Finland. N. Engl. J. Med. 343, 78–85. doi: 10.1056/NEJM200007133430201
Lin, R., Han, C., Ding, Z., Shi, H., He, R., Liu, J., et al. (2020). Knock down of BMSC-derived Wnt3a or its antagonist analogs attenuate colorectal carcinogenesis induced by chronic Fusobacterium nucleatum infection. Cancer Lett. 495, 165–179. doi: 10.1016/j.canlet.2020.08.032
Liu, J., Hsieh, C. L., Gelincik, O., Devolder, B., Sei, S., Zhang, S., et al. (2019). Proteomic characterization of outer membrane vesicles from gut mucosa-derived fusobacterium nucleatum. J. Proteomics 195, 125–137. doi: 10.1016/j.jprot.2018.12.029
Liu, L., Liang, L., Yang, C., Zhou, Y., and Chen, Y. (2021). Extracellular vesicles of Fusobacterium nucleatum compromise intestinal barrier through targeting RIPK1-mediated cell death pathway. Gut Microb. 13, 1–20. doi: 10.1080/19490976.2021.1902718
Liu, L., Tabung, F. K., Zhang, X., Nowak, J. A., Qian, Z. R., Hamada, T., et al. (2018). Diets that promote colon inflammation associate with risk of colorectal carcinomas that contain Fusobacterium nucleatum. Clin. Gastroenterol. Hepatol. 16, 1622–1631. doi: 10.1016/j.cgh.2018.04.030
Liu, P. F., Shi, W., Zhu, W., Smith, J. W., Hsieh, S. L., Gallo, R. L., et al. (2010). Vaccination targeting surface FomA of Fusobacterium nucleatum against bacterial co-aggregation: Implication for treatment of periodontal infection and halitosis. Vaccine 28, 3496–3505. doi: 10.1016/j.vaccine.2010.02.047
Liu, W., Zhang, X., Xu, H., Li, S., Cheuk-Hay Lau, H., Chen, Q., et al. (2021). Microbial community heterogeneity within colorectal neoplasia and its correlation with colorectal carcinogenesis. Gastroenterology 160, 2395–2408. doi: 10.1053/j.gastro.2021.02.020
Llama-Palacios, A., Potupa, O., Sanchez, M. C., Figuero, E., Herrera, D., and Sanz, M. (2020). Proteomic analysis of Fusobacterium nucleatum growth in biofilm versus planktonic state. Mol. Oral. Microbiol. 35, 168–180. doi: 10.1111/omi.12303
Lo, C. H., Blot, W. J., Teras, L. R., Visvanathan, K., Le Marchand, L., Haiman, C. A., et al. (2021). Prediagnostic antibody responses to Fusobacterium nucleatum proteins are not associated with risk of colorectal cancer in a large U.S. consortium. Cancer Epidemiol. Biomarkers Prev. 30, 1279–1282. doi: 10.1158/1055-9965.EPI-20-1471
Lynch, S. V., and Pedersen, O. (2016). The human intestinal microbiome in health and disease. N. Engl. J. Med. 375, 2369–2379. doi: 10.1056/NEJMra1600266
Macia, L., Nanan, R., Hosseini-Beheshti, E., and Grau, G. E. (2019). Host- and microbiota-derived extracellular vesicles, immune function, and disease development. Int. J. Mol. Sci. 21:107. doi: 10.3390/ijms21010107
Maman, S., and Witz, I. P. (2018). A history of exploring cancer in context. Nat. Rev. Cancer 18, 359–376. doi: 10.1038/s41568-018-0006-7
Martin-Gallausiaux, C., Malabirade, A., Habier, J., and Wilmes, P. (2020). Fusobacterium nucleatum extracellular vesicles modulate gut epithelial cell innate immunity via FomA and TLR2. Front. Immunol. 11:583644. doi: 10.3389/fimmu.2020.583644
Matly, A., Quinn, J. A., McMillan, D. C., Park, J. H., and Edwards, J. (2021). The relationship between beta-catenin and patient survival in colorectal cancer systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 163:103337. doi: 10.1016/j.critrevonc.2021.103337
Mima, K., Cao, Y., Chan, A. T., Qian, Z. R., Nowak, J. A., Masugi, Y., et al. (2016a). Fusobacterium nucleatum in colorectal carcinoma tissue according to tumor location. Clin. Transl. Gastroenterol. 7:e200. doi: 10.1038/ctg.2016.53
Mima, K., Nishihara, R., Qian, Z. R., Cao, Y., Sukawa, Y., Nowak, J. A., et al. (2016b). Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut 65, 1973–1980. doi: 10.1136/gutjnl-2015-310101
Mima, K., Sukawa, Y., Nishihara, R., Qian, Z. R., Yamauchi, M., Inamura, K., et al. (2015). Fusobacterium nucleatum and T Cells in Colorectal Carcinoma. JAMA Oncol. 1, 653–661. doi: 10.1001/jamaoncol.2015.1377
Nakatsu, G., Li, X., Zhou, H., Sheng, J., Wong, S. H., Wu, W. K., et al. (2015). Gut mucosal microbiome across stages of colorectal carcinogenesis. Nat. Commun. 6:8727. doi: 10.1038/ncomms9727
Nejman, D., Livyatan, I., Fuks, G., Gavert, N., Zwang, Y., Geller, L. T., et al. (2020). The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 368, 973–980. doi: 10.1126/science.aay9189
Nia, H. T., Munn, L. L., and Jain, R. K. (2020). Physical traits of cancer. Science 370:6516. doi: 10.1126/science.aaz0868
Parhi, L., Alon-Maimon, T., Sol, A., Nejman, D., Shhadeh, A., Fainsod-Levi, T., et al. (2020). Breast cancer colonization by Fusobacterium nucleatum accelerates tumor growth and metastatic progression. Nat. Commun. 11:3259. doi: 10.1038/s41467-020-16967-2
Park, H. E., Kim, J. H., Cho, N. Y., Lee, H. S., and Kang, G. H. (2017). Intratumoral Fusobacterium nucleatum abundance correlates with macrophage infiltration and CDKN2A methylation in microsatellite-unstable colorectal carcinoma. Virchows Arch. 471, 329–336. doi: 10.1007/s00428-017-2171-6
Peters, B. A., Wu, J., Pei, Z., Yang, L., Purdue, M. P., Freedman, N. D., et al. (2017). Oral microbiome composition reflects prospective risk for Esophageal Cancers. Cancer Res. 77, 6777–6787. doi: 10.1158/0008-5472.CAN-17-1296
Pleguezuelos-Manzano, C., Puschhof, J., Huber, A. R., van Hoeck, A., Wood, H. M., Nomburg, J., et al. (2020). Mutational signature in colorectal cancer caused by genotoxic pks(+) E. coli. Nature 580, 269–273. doi: 10.1038/s41586-020-2080-8
Puschhof, J., Pleguezuelos-Manzano, C., and Clevers, H. (2021). Organoids and organs-on-chips: Insights into human gut-microbe interactions. Cell Host Microbe 29, 867–878. doi: 10.1016/j.chom.2021.04.002
Rubinstein, M. R., Baik, J. E., Lagana, S. M., Han, R. P., Raab, W. J., Sahoo, D., et al. (2019). Fusobacterium nucleatum promotes colorectal cancer by inducing Wnt/beta-catenin modulator Annexin A1. EMBO Rep. 20:e47638. doi: 10.15252/embr.201847638
Rubinstein Mara, R., Wang, X., Liu, W., Hao, Y., Cai, G., and Han Yiping, W. (2013). Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-Cadherin/β-Catenin signaling via its FadA Adhesin. Cell Host Microb. 14, 195–206. doi: 10.1016/j.chom.2013.07.012
Schmitt, M., and Greten, F. R. (2021). The inflammatory pathogenesis of colorectal cancer. Nat. Rev. Immunol. doi: 10.1038/s41577-021-00534-x ∗∗pg& Vol,
Siegel, R. L., Miller, K. D., Goding Sauer, A., Fedewa, S. A., Butterly, L. F., Anderson, J. C., et al. (2020). Colorectal cancer statistics, 2020. CA Cancer J. Clin. 70, 145–164. doi: 10.3322/caac.21601
Song, M., Chan, A. T., and Sun, J. (2020). Influence of the gut microbiome, diet, and environment on risk of colorectal cancer. Gastroenterology 158, 322–340. doi: 10.1053/j.gastro.2019.06.048
Suehiro, Y., Sakai, K., Nishioka, M., Hashimoto, S., Takami, T., Higaki, S., et al. (2017). Highly sensitive stool DNA testing of Fusobacterium nucleatum as a marker for detection of colorectal tumours in a Japanese population. Ann. Clin. Biochem. 54, 86–91. doi: 10.1177/0004563216643970
Szabo, C., Coletta, C., Chao, C., Modis, K., Szczesny, B., Papapetropoulos, A., et al. (2013). Tumor-derived hydrogen sulfide, produced by cystathionine-beta-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc. Natl. Acad. Sci. U S A. 110, 12474–12479. doi: 10.1073/pnas.1306241110
Tahara, T., Yamamoto, E., Suzuki, H., Maruyama, R., Chung, W., Garriga, J., et al. (2014). Fusobacterium in colonic flora and molecular features of colorectal carcinoma. Cancer Res. 74, 1311–1318. doi: 10.1158/0008-5472.CAN-13-1865
Tanca, A., Manghina, V., Fraumene, C., Palomba, A., Abbondio, M., Deligios, M., et al. (2017). Metaproteogenomics reveals taxonomic and functional changes between cecal and fecal microbiota in mouse. Front. Microbiol. 8:391. doi: 10.3389/fmicb.2017.00391
Tang, Q., Jin, G., Wang, G., Liu, T., Liu, X., Wang, B., et al. (2020). Current sampling methods for gut microbiota: a call for more precise devices. Front. Cell. Infect. Microbiol. 10:151. doi: 10.3389/fcimb.2020.00151
Tomkovich, S., Dejea, C. M., Winglee, K., Drewes, J. L., Chung, L., Housseau, F., et al. (2019). Human colon mucosal biofilms from healthy or colon cancer hosts are carcinogenic. J. Clin. Invest. 130, 1699–1712. doi: 10.1172/JCI124196
Tomkovich, S., Yang, Y., Winglee, K., Gauthier, J., Muhlbauer, M., Sun, X., et al. (2017). Locoregional effects of microbiota in a preclinical model of colon carcinogenesis. Cancer Res. 77, 2620–2632. doi: 10.1158/0008-5472.CAN-16-3472
Tunsjo, H. S., Gundersen, G., Rangnes, F., Noone, J. C., Endres, A., and Bemanian, V. (2019). Detection of Fusobacterium nucleatum in stool and colonic tissues from Norwegian colorectal cancer patients. Eur. J. Clin. Microbiol. Infect. Dis. 38, 1367–1376. doi: 10.1007/s10096-019-03562-7
Vander Haar, E. L., So, J., Gyamfi-Bannerman, C., and Han, Y. W. (2018). Fusobacterium nucleatum and adverse pregnancy outcomes: epidemiological and mechanistic evidence. Anaerobe 50, 55–59. doi: 10.1016/j.anaerobe.2018.01.008
Vital, M., Howe, A. C., and Tiedje, J. M. (2014). Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. mBio 5:e00889. doi: 10.1128/mBio.00889-14
Wang, H. F., Li, L. F., Guo, S. H., Zeng, Q. Y., Ning, F., Liu, W. L., et al. (2016). Evaluation of antibody level against Fusobacterium nucleatum in the serological diagnosis of colorectal cancer. Sci. Rep. 6:33440. doi: 10.1038/srep33440
Wei, Z., Cao, S., Liu, S., Yao, Z., Sun, T., Li, Y., et al. (2016). Could gut microbiota serve as prognostic biomarker associated with colorectal cancer patients’ survival? A pilot study on relevant mechanism. Oncotarget 7, 46158–46172. doi: 10.18632/oncotarget.10064
Weiss, G. A., and Hennet, T. (2017). Mechanisms and consequences of intestinal dysbiosis. Cell. Mol. Life Sci. 74, 2959–2977. doi: 10.1007/s00018-017-2509-x
West, N. R., McCuaig, S., Franchini, F., and Powrie, F. (2015). Emerging cytokine networks in colorectal cancer. Nat. Rev. Immunol. 15, 615–629. doi: 10.1038/nri3896
Wong, S. H., Kwong, T. N. Y., Chow, T. C., Luk, A. K. C., Dai, R. Z. W., Nakatsu, G., et al. (2017a). Quantitation of faecal Fusobacterium improves faecal immunochemical test in detecting advanced colorectal neoplasia. Gut 66, 1441–1448. doi: 10.1136/gutjnl-2016-312766
Wong, S. H., Zhao, L., Zhang, X., Nakatsu, G., Han, J., Xu, W., et al. (2017b). Gavage of fecal samples from patients with colorectal cancer promotes intestinal carcinogenesis in germ-free and conventional mice. Gastroenterology 153, 1621–1633. doi: 10.1053/j.gastro.2017.08.022
Wu, N., Yang, X., Zhang, R., Li, J., Xiao, X., Hu, Y., et al. (2013). Dysbiosis signature of fecal microbiota in colorectal cancer patients. Microb. Ecol. 66, 462–470. doi: 10.1007/s00248-013-0245-9
Wu, Y., Wu, J., Chen, T., Li, Q., Peng, W., Li, H., et al. (2018). Fusobacterium nucleatum potentiates intestinal tumorigenesis in mice via a toll-like receptor 4/p21-Activated Kinase 1 Cascade. Dig. Dis. Sci. 63, 1210–1218. doi: 10.1007/s10620-018-4999-2
Xu, M., Yamada, M., Li, M., Liu, H., Chen, S. G., and Han, Y. W. (2007). FadA from Fusobacterium nucleatum utilizes both secreted and nonsecreted forms for functional oligomerization for attachment and invasion of host cells. J. Biol. Chem. 282, 25000–25009. doi: 10.1074/jbc.M611567200
Xu, R., Rai, A., Chen, M., Suwakulsiri, W., Greening, D. W., and Simpson, R. J. (2018). Extracellular vesicles in cancer - implications for future improvements in cancer care. Nat. Rev. Clin. Oncol. 15, 617–638. doi: 10.1038/s41571-018-0036-9
Xue, J. H., Xie, Y. H., Zou, T. H., Qian, Y., Kang, Z. R., Zhou, C. B., et al. (2021). Fecal Fusobacterium nucleatum as a predictor for metachronous colorectal adenoma after endoscopic polypectomy. J. Gastroenterol. Hepatol. 2021:15559. doi: 10.1111/jgh.15559
Yamamura, K., Baba, Y., Miyake, K., Nakamura, K., Shigaki, H., Mima, K., et al. (2017). Fusobacterium nucleatum in gastroenterological cancer: Evaluation of measurement methods using quantitative polymerase chain reaction and a literature review. Oncol. Lett. 14, 6373–6378. doi: 10.3892/ol.2017.7001
Yamaoka, Y., Suehiro, Y., Hashimoto, S., Hoshida, T., Fujimoto, M., Watanabe, M., et al. (2018). Fusobacterium nucleatum as a prognostic marker of colorectal cancer in a Japanese population. J. Gastroenterol. 53, 517–524. doi: 10.1007/s00535-017-1382-6
Yang, Y., Weng, W., Peng, J., Hong, L., Yang, L., Toiyama, Y., et al. (2017). Fusobacterium nucleatum increases proliferation of colorectal cancer cells and tumor development in mice by activating toll-like receptor 4 signaling to nuclear factor-kappaB, and Up-regulating expression of MicroRNA-21. Gastroenterology 152, 851–866. doi: 10.1053/j.gastro.2016.11.018
Ye, X., Wang, R., Bhattacharya, R., Boulbes, D. R., Fan, F., Xia, L., et al. (2017). Fusobacterium Nucleatum subspecies animalis influences proinflammatory cytokine expression and monocyte activation in human colorectal tumors. Cancer Prev. Res. (Phila) 10, 398–409. doi: 10.1158/1940-6207.CAPR-16-0178
Yu, T., Guo, F., Yu, Y., Sun, T., Ma, D., Han, J., et al. (2017). Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell 170, 548–563. doi: 10.1016/j.cell.2017.07.008
Yu, Y. N., Yu, T. C., Zhao, H. J., Sun, T. T., Chen, H. M., Chen, H. Y., et al. (2015). Berberine may rescue Fusobacterium nucleatum-induced colorectal tumorigenesis by modulating the tumor microenvironment. Oncotarget 6, 32013–32026. doi: 10.18632/oncotarget.5166
Yue, Y., Hur, J., Cao, Y., Tabung, F. K., Wang, M., Wu, K., et al. (2021). Prospective evaluation of dietary and lifestyle pattern indices with risk of colorectal cancer in a cohort of younger women. Ann. Oncol. 32, 778–786. doi: 10.1016/j.annonc.2021.03.200
Zanzoni, A., Spinelli, L., Braham, S., and Brun, C. (2017). Perturbed human sub-networks by Fusobacterium nucleatum candidate virulence proteins. Microbiome 5:89. doi: 10.1186/s40168-017-0307-1
Zhang, S., Kong, C., Yang, Y., Cai, S., Li, X., Cai, G., et al. (2020). Human oral microbiome dysbiosis as a novel non-invasive biomarker in detection of colorectal cancer. Theranostics 10, 11595–11606. doi: 10.7150/thno.49515
Zhang, S., Yang, Y., Weng, W., Guo, B., Cai, G., Ma, Y., et al. (2019). Fusobacterium nucleatum promotes chemoresistance to 5-fluorouracil by upregulation of BIRC3 expression in colorectal cancer. J. Exp. Clin. Cancer Res. 38:14. doi: 10.1186/s13046-018-0985-y
Keywords: colorectal cancer, Fusobacterium nucleatum, tumor microenvironment, metastasis, chemoresistance
Citation: Wang S, Liu Y, Li J, Zhao L, Yan W, Lin B, Guo X and Wei Y (2021) Fusobacterium nucleatum Acts as a Pro-carcinogenic Bacterium in Colorectal Cancer: From Association to Causality. Front. Cell Dev. Biol. 9:710165. doi: 10.3389/fcell.2021.710165
Received: 15 May 2021; Accepted: 16 July 2021;
Published: 20 August 2021.
Edited by:
Rui Manuel Reis, Barretos Cancer Hospital, BrazilReviewed by:
Christian Martin Stock, Hannover Medical School, GermanyCopyright © 2021 Wang, Liu, Li, Zhao, Yan, Lin, Guo and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunwei Wei, aHlkd3l3MTFAaG90bWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.