
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell Dev. Biol., 22 July 2021
Sec. Developmental Epigenetics
Volume 9 - 2021 | https://doi.org/10.3389/fcell.2021.689624
In recent years, a number of studies focused on the role of epigenetics, including DNA methylation, in spermatogenesis and male infertility. We aimed to provide an overview of the knowledge concerning the gene and genome methylation and its regulation during spermatogenesis, specifically in the context of male infertility etiopathogenesis. Overall, the findings support the hypothesis that sperm DNA methylation is associated with sperm alterations and infertility. Several genes have been found to be differentially methylated in relation to impaired spermatogenesis and/or reproductive dysfunction. Particularly, DNA methylation defects of MEST and H19 within imprinted genes and MTHFR within non-imprinted genes have been repeatedly linked with male infertility. A deep knowledge of sperm DNA methylation status in association with reduced reproductive potential could improve the development of novel diagnostic tools for this disease. Further studies are needed to better elucidate the mechanisms affecting methylation in sperm and their impact on male infertility.
Male infertility affects about 15% of couples worldwide (Agarwal et al., 2015). In reproductive age, approximately 7% of males suffer from infertility (Cooper et al., 2009; Krausz, 2011; Rotondo et al., 2013). Male infertility is a multifactorial disease comprising a wide variety of disorders (Abrao et al., 2013; Stouffs et al., 2014; Contini et al., 2018; Tognon et al., 2020). Endocrine and immunological disorders, anatomical and genetic abnormalities as well as infections of the genital tract can affect the male reproductive potential (Abrao et al., 2013; Stouffs et al., 2014). Several other factors including age, stress, and lifestyle, such as obesity, smoking, and alcohol, have been associated with male infertility (Kovac et al., 2015; Craig et al., 2017; Ilacqua et al., 2018).
In recent years, a number of studies have focused on the role of epigenetics in spermatogenesis and male infertility (Seisenberger et al., 2012; Laurentino et al., 2015; Stuppia et al., 2015; Urdinguio et al., 2015; Laqqan et al., 2017b; Nasri et al., 2017; McSwiggin and O’Doherty, 2018). Epigenetics refers to the gene regulation process without changes in DNA sequence and includes DNA methylation, posttranslational histone modifications and microRNA (miRNA) regulation (Giacone et al., 2019; Stomper et al., 2021). Several specific epigenomic/epigenetic modifications are established during spermatogenesis to form highly specialized mature sperm cells, allowing significant reorganizations of sperm chromatin structure (Jenkins and Carrell, 2011). Therefore, spermatogenesis is particularly vulnerable to epigenetic alterations. Dysregulations in the DNA methylation process during spermatogenesis can result in the abnormal expression of target genes, which may lead to infertility (Cho et al., 2003; Aston et al., 2012). While many epigenetic abnormalities causing male reproductive dysfunction are still unknown, it is likely that most cases of idiopathic infertility could be accounted for underlying DNA methylation mechanisms (Rose and Klose, 2014).
This review provides an overview on gene and genome methylation and its regulation during spermatogenesis and the current knowledge of those DNA methylation defects potentially involved in the etiopathogenesis of male infertility.
We performed an investigation of the scientific literature by searching PubMed (Medline) database until March 2021. All studies investigating the relationship between DNA methylation and male infertility published from 1987 up to March 2021 were reviewed for specific topic areas, and the most relevant reports were included. The literature search was performed using the following keywords (alone and/or in combination): epigenomics, epigenetics, gene, methylation, DNA methylation, genome, methylome, hypermethylation, hypomethylation, regulation, genomic imprinting, imprinted genes, sperm, sperm cells, spermatozoa, semen parameters, spermatogenesis, infertility, subfertility, sterility, and male infertility. The reference lists of all publications included in this review have also been considered for additional relevant works.
DNA methylation is a biochemical process where a nucleotide is enzymatically methylated with a methyl group (–CH3) at the five-carbon position, usually cytosine (Moore et al., 2013; Rotondo et al., 2021). Cytosine methylation predominantly occurs at the CpG dinucleotide (Figure 1). Regions rich in CpGs are called CpG islands (CGI) and are usually found in gene promoters, where gene expression is regulated through methylation (Greenberg and Bourc’his, 2019; Rotondo et al., 2020b). CpG methylation in the promoters of genes typically leads to gene silencing (Rotondo et al., 2016, 2018a). The suppression of gene expression can occur by DNA methylation itself that, in some cases, can prevent the binding of transcriptional factors (Moore et al., 2013; Yin et al., 2017). Methylated DNA could also be recognized and bound by the methyl-binding proteins methyl CpG binding protein 2 and methyl-CpG-binding domain protein-1, -2, and -4, which recruit the enzymes histone methyltransferases and histone deacetylases to trigger histone modifications and chromatin packing, leading to the repression of gene expression (Parry and Clarke, 2011).
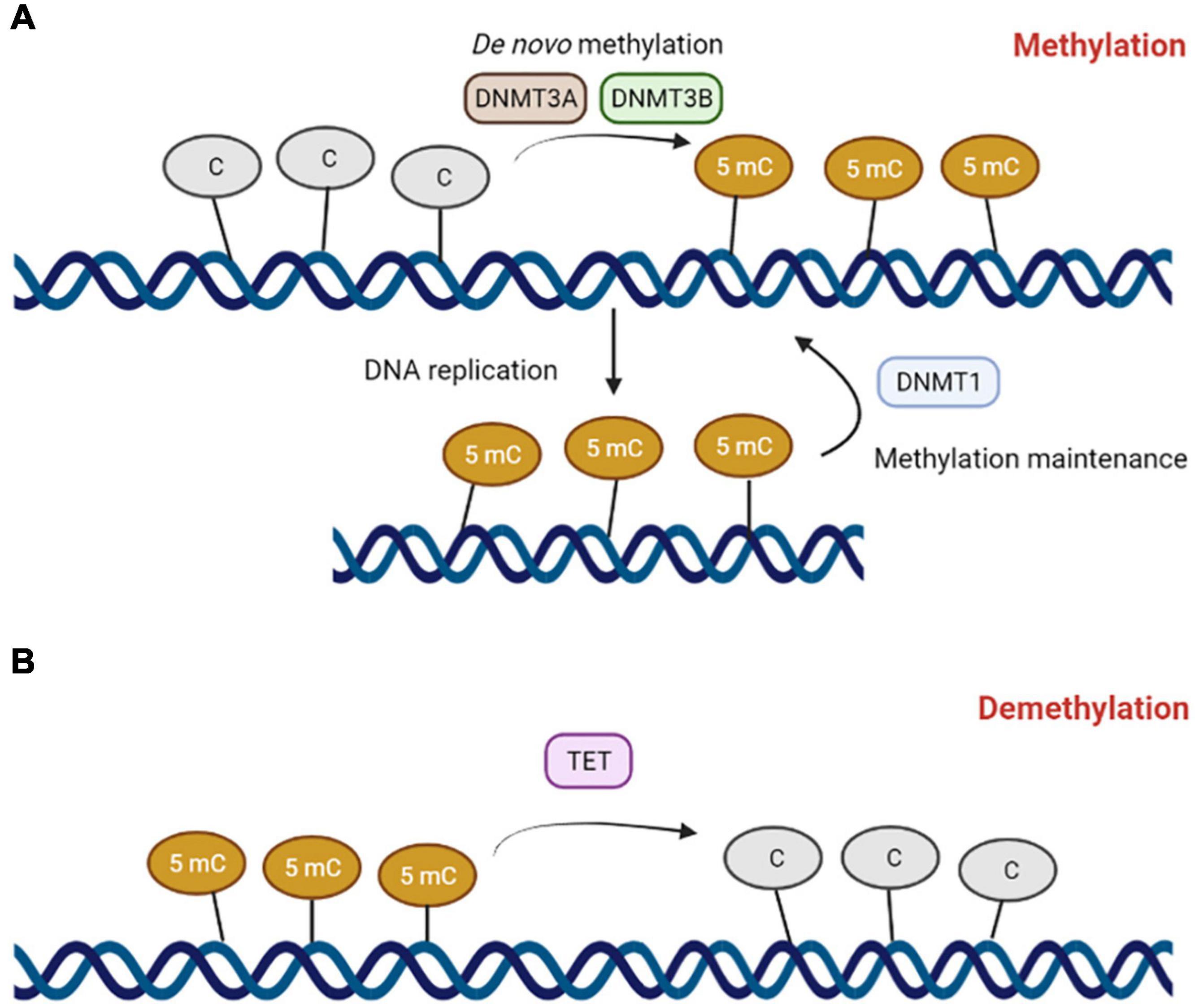
Figure 1. The DNA methylation machinery. (A) DNA methyltranferase 3A (DNMT3A) and 3B (DNMT3B) catalyze de novo methylation reactions on cytosines belonging to CpG dinucleotides to form 5-methylcytosine. DNA methyltransferase 1 (DNMT1) mediates DNA methylation maintenance. (B) Ten-eleven translocation (TET) enzymes mediates DNA demethylation reactions.
DNA methylation plays a pivotal role in regulating germ cell development (Reik and Walter, 2001; Castillo et al., 2018; Hanna et al., 2018; Lobo et al., 2019; Sharma et al., 2019; Wasserzug-Pash and Klutstein, 2019). During germ cell development, the genome is widely remodeled by waves of DNA demethylation and methylation (Figure 2; Reik and Walter, 2001; Li, 2002; Santos and Dean, 2004; Rousseaux et al., 2005; Biermann and Steger, 2007; Marcho et al., 2019). The wave of DNA demethylation occurs in the primordial germ cells (PGCs), which are precursors of male and female germ cells. Initially, the content of DNA methylation is equally distributed between PGCs and embryo somatic cells. Upon migration of PGCs from the epiblast to the gonadal ridge, the DNA methylation marks are extensively erased (Monk et al., 1987; Santos and Dean, 2004), resulting in lower methylation levels in PGCs compared to those in embryo somatic cells (Gkountela et al., 2015; Guo et al., 2015; Tang et al., 2015; Von Meyenn and Reik, 2015). These epigenetic modifications are essential for the genome reprogramming of PGCs, allowing sex-specific germ cell development during embryo development (Reik and Walter, 2001; Yao et al., 2015). De novo methylation proceeds in prospermatogonia or gonocytes arrested in mitosis, and it is firstly established at the repeated DNA sequences, such as retrotransposons, and then at the imprinted genes, leading to appropriate sex-specific methylation patterns in the germ cells.
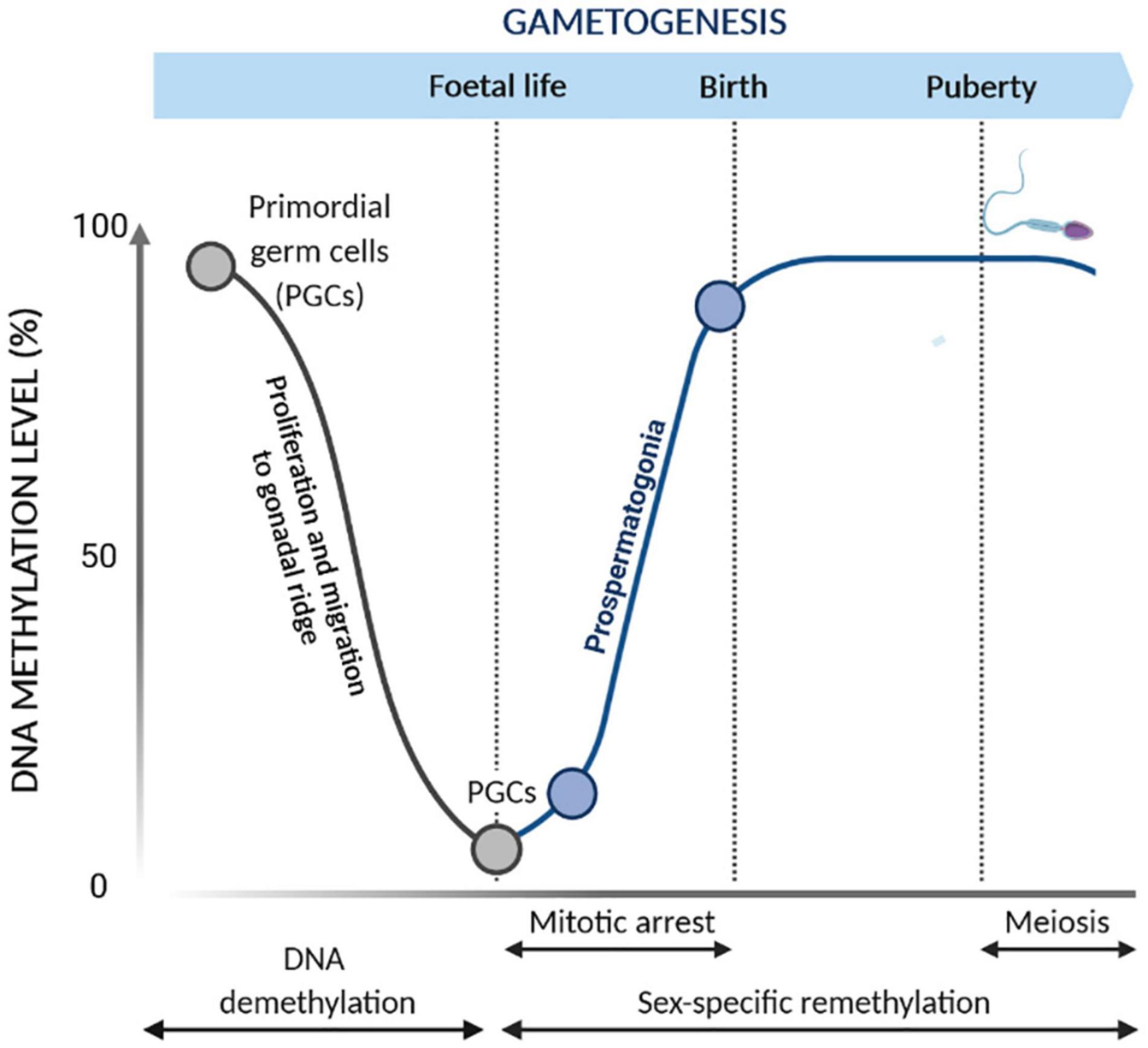
Figure 2. Epigenetic reprogramming during gametogenesis. Changes of DNA methylation occur during primordial germ cell (PGC) development. During the proliferation and migration into the gonadal ridge, PGCs undergo global demethylation to remove parental imprints. Subsequently, reestablishment of the male germ cell DNA methylation patterns occurs during gametogenesis. De novo methylation occurs prior to meiosis in mitotically arrested cells while being completed before birth.
DNA methylation establishment in the germline is of particular importance, as failure to establish correct methylation in retrotransposons and imprinted genes has serious consequences for embryo development. Indeed, retrotransposons, such as long interspersed nuclear elements 1 (LINE1), are interspersed repeated DNA sequences which are able to propagate throughout the genome. Lack of methylation in retrotransposons could allow their propagation throughout the genome, causing insertional mutagenesis and, in turn, several diseases, including male infertility (Solyom and Kazazian, 2012). Regarding the imprinted genes, methylation regulates the expression through an important process termed “genomic imprinting,” which leads to the expression of either the maternal or paternal allele. When maternal/paternal alleles undergo global demethylation upon fertilization, the imprinted genes preserve the methyl marks of the parental genome, leading to parental-origin patterns of mono-allelic gene expression. This process occurs in specific sequences called differentially methylated regions (DMRs), also known as imprinting control regions (ICRs) (Habib et al., 2019). For instance, the imprinted genes IGF2-H19 share common enhancers and ICR located downstream and upstream of the H19 gene, respectively. Under normal circumstances, ICR and H19 are methylated in sperm cells and unmethylated in oocytes, leading to the reciprocal expression of the maternal H19 allele and paternal IGF2 allele in somatic cells (Figure 3; Peters, 2000; Lanzillotti et al., 2021). Other paternally imprinted genes have been found to be similarly regulated in sperm cells, such as Ras Protein Specific Guanine Nucleotide Releasing Factor 1, Delta Like Non-Canonical Notch Ligand 1, and Zinc Finger DBF-Type Containing 2 (Arnaud, 2010; Gunes et al., 2016). Contrariwise, maternally imprinted genes are methylated in female germ cells, while they are expressed in male germ cells, including mesoderm-specific transcript (MEST), also known as paternally expressed gene 1 (PEG1), paternally expressed 3 (PEG3), and small nuclear ribonucleoprotein polypeptide N (SNRPN) (Mayer et al., 2000; Higashimoto et al., 2003; Broad et al., 2009; Hammoud et al., 2010; Zheng et al., 2011; Zhao et al., 2015; Bruno et al., 2018). However, failure in the maintenance of imprinted gene methylation patterns in the germline might occur, and this has been associated with low sperm quality and pregnancy rate and impaired post-fertilization development (Rotondo et al., 2013; Kitamura et al., 2015; McSwiggin and O’Doherty, 2018).
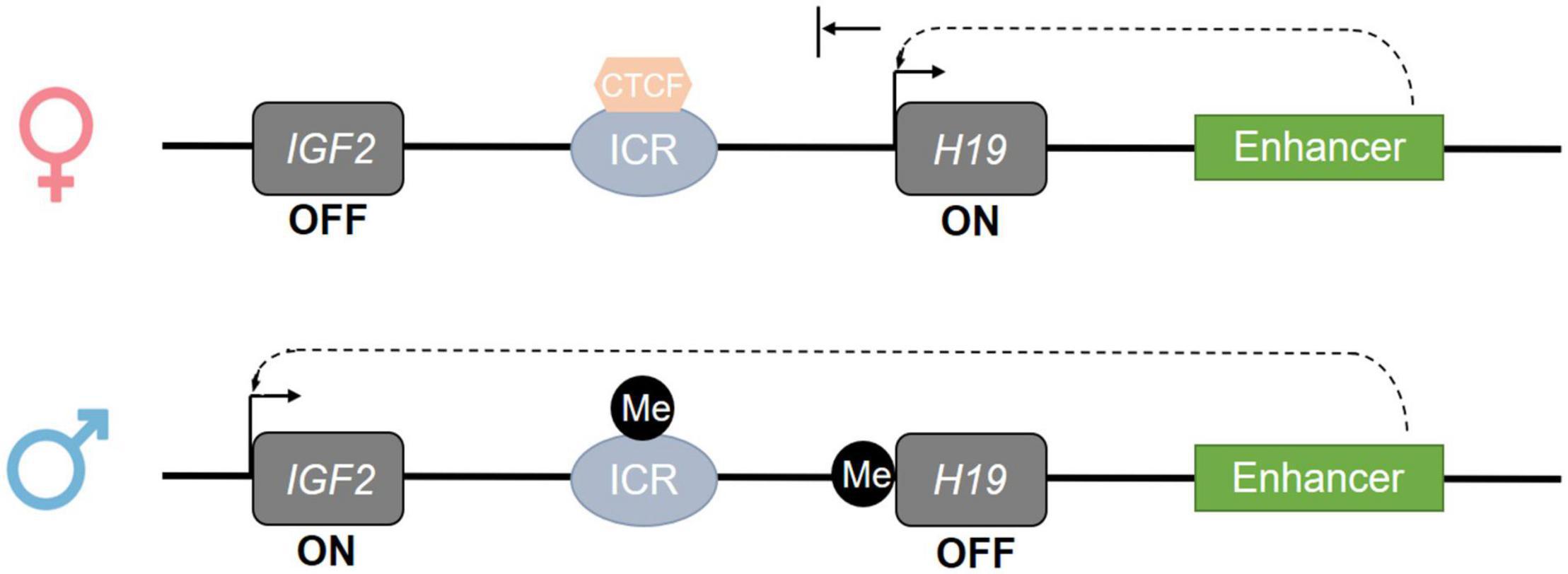
Figure 3. Epigenetic regulation of the imprinted IGF2-H19 gene cluster. The regulation of the imprinted genes is mediated by imprinting control regions (ICR) whose methylation regulates the imprinted genes located at the same gene cluster. On the maternal allele, at the IGF2-H19 locus, the ICR is de-methylated; this allows CTCF binding and promotes the expression of H19 and silencing of IGF2 through down-stream enhancer activity. On the paternal allele, at the IGF2-H19 locus, the ICR and H19 are methylated; this prevents CTCF binding and induces the inactivation of H19 and the expression of IGF2. Consistently, IGF2 is expressed in the sperm cell (Cannarella et al., 2019).
DNA methylation is carefully coordinated by a family of enzymes named DNA methyltransferases (DNMTs). Four members of this family, DNMT1, DNMT3A, DNMT3B, and DNMT3C, are endowed with catalytic activity (Lopomo et al., 2016). DNMT1, also known as the maintenance DNMT, copies pre-existing methylation marks onto the new strands following DNA replication (Baylin and Jones, 2011). DNMT3A and DNMT3B are de novo methyltransferases, as they are able to methylate previously unmethylated DNA sequences (Baylin and Jones, 2011). DNMT3C, along with P-element induced wimpy testis/interacting RNA (PIWI/piRNA) (Yang and Wang, 2016; Nagamori et al., 2018), is responsible for the promoter methylation of retrotransposons in the male germline (Barau et al., 2016). piRNAs are small non-coding RNAs that form RNA–protein complexes through interaction with PIWI proteins, a gonad-specific class of Argonaute proteins (Yang and Wang, 2016). These RNA–protein complexes are essential for the LINE1 methylation processes in male germ cells (Aravin et al., 2008; Kuramochi-Miyagawa et al., 2008). DNA methylation is a reversable chemical modification, and active demethylation processes are mediated by erasing DNA methylation mechanisms, mainly controlled by Ten-Eleven Translocation (TET) enzymes, such as TET1, -2, and -3, (Melamed et al., 2018). TET enzymes specifically convert 5mC to 5-hydroxymethylcytosine (5hmC). The TET-mediated oxidation of 5mC induces DNA demethylation by passive or active processing. As 5hmC is not recognized by DNMT1 during DNA replication, passive DNA demethylation can occur in proliferating cells (Valinluck and Sowers, 2007). In the active process, TETs convert 5hmC to 5-formylcytosine (5fC) and then 5fC into 5-carboxylcytosine (5cC). 5fC and 5cC are both recognized and excised by the base-excision DNA repair machinery and subsequently replaced with cytosine (Williams et al., 2012; Jin et al., 2015).
DNA methylation plays a critical role during spermatogenesis (Gunes et al., 2018b; Fend-Guella et al., 2019; Franzago et al., 2019; Huang et al., 2019; Pisarska et al., 2019). The correct methylation of DNA ensures proper chromatin condensation in the sperm head, enabling sperm maturation and its ability in fertilization and post-fertilization events (Carrell, 2019). Contrariwise, incomplete or abnormal condensation of the sperm chromatin results in damaged DNA, leading to the impairment of egg cell fertilization and/or reduction in pregnancy rates (Benchaib et al., 2005; Miller et al., 2009; McSwiggin and O’Doherty, 2018). Considering these aspects, several studies have analyzed the gene and genome methylation levels of sperm DNA in association with male reproductive dysfunctions (Houshdaran et al., 2007; Rotondo et al., 2012, 2013; Das et al., 2017; Alkhaled et al., 2018). These studies have mainly, but not only, investigated associations between improper DNA methylation in spermatozoa genome and negative variations in semen parameters, such as concentration (oligozoospermia), morphology (teratozoospermia), and progressive motility (asthenozoospermia), alone or in combinations, i.e., oligoasthenozoospermia (OA), oligoteratozoospermia, asthenoteratozoospermia (AT), and oligoasthenoteratozoospermia (OAT). Semen samples are considered normal when the concentration, motility, and morphology are ≥15 × 106 sperm/ml, ≥32% (sperm progressive motility), and ≥4% normal, respectively (Cooper et al., 2009).
Several genes carrying defective methylation have been associated with abnormalities in semen parameters (Table 1). One of the most highly studied genes is methylenetetrahydrofolate reductase (MTHFR) (Haggarty, 2015; Saraswathy et al., 2018; Coppedè et al., 2019; Mishra et al., 2019; Kulac et al., 2021), which is a key regulatory enzyme involved in folate metabolism and in DNA synthesis and methylation (Födinger et al., 2000). In male mice testes, the activity of MTHFR is five times higher than in other organs (Chen, 2001), whereas its inactivation results in hyperhomocysteinemia, decreased methylation capacity, and the arrest of spermatogenesis due to sperm DNA hypomethylation (Chen, 2001). In humans, a number of studies have pointed out that impairment of the MTHFR gene can contribute to disease, including male infertility (Stangler Herodež et al., 2013; Tara et al., 2015; Coppedè et al., 2016, 2019; Lévesque et al., 2016; Asim et al., 2017). Mutations/polymorphisms in MTHFR gene are broadly known causes of reduced MTHFR enzyme activity in sperm cells, resulting in reduced methionine availability and decreased DNA methylation (Gupta et al., 2011; Montjean et al., 2011; Murphy et al., 2011; Safarinejad et al., 2011; Choi et al., 2016; Poorang et al., 2018; Ullah et al., 2019). The improper methylation of MTHFR gene in sperm germ cells may likewise result in decreased MTHFR enzyme activity, leading to aberrant methylation of sperm DNA. Hypermethylation of MTHFR has been found in testes from males affected by non-obstructive azoospermia and in sperm cells from males affected by oligozoospermia and teratozoospermia as well as in idiopathic infertile males (Khazamipour et al., 2009; Wu et al., 2010; Botezatu et al., 2014; Karaca et al., 2017). In our previous studies, hypermethylation at the MTHFR promoter has been found to be associated with aberrant concentration, motility, and morphology in males from infertile couples affected by recurrent spontaneous abortion (Rotondo et al., 2012). In addition, we have found a correlation between MTHFR gene promoter hypermethylation and extensive methylation defects at the paternal imprinted gene H19 in sperm DNAs from infertile males with both normal and abnormal semen parameters. These results have suggested that MTHFR hypermethylation-induced inactivation may affect methylation at the imprinted loci of sperm cells, leading to impaired fertilization (Rotondo et al., 2013). Contrariwise, no correlation was found between methylation errors at H19 and MEST genes and inactivating MTHFR C677T single-nucleotide polymorphism (SNP) [a cytosine (C) to a thymine (T) substitution at position 677] in sperm DNA from infertile patients with defective semen parameters. However, a higher incidence trend of aberrant DNA methylation among severe oligozoospermic infertile males carrying C677T genotype was observed (Louie et al., 2016).
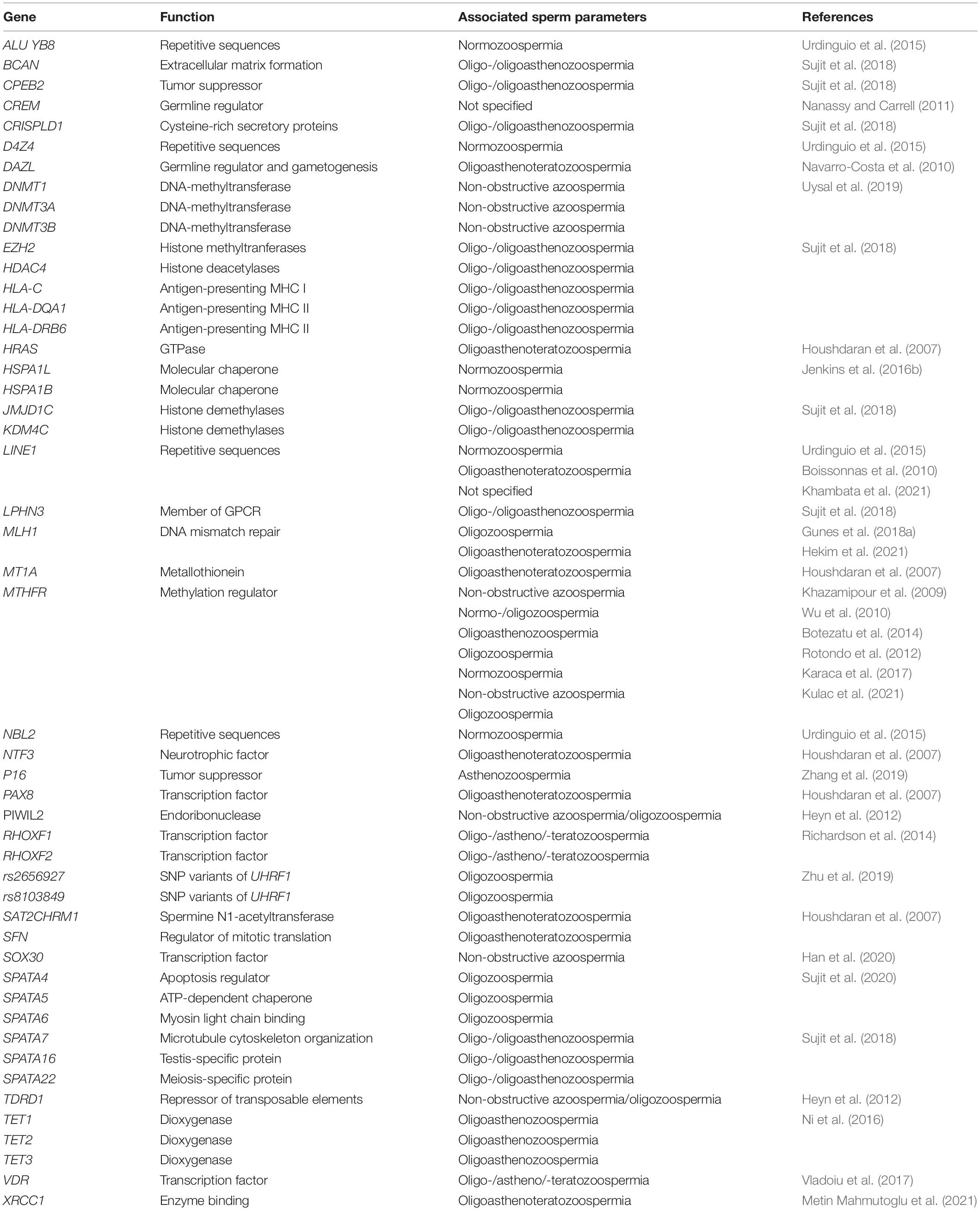
Table 1. Aberrant methylation of non-imprinted genes associated with abnormalities of semen parameters.
Germline mutations arise from replication errors, potentially resulting in reproductive health impairment (Gao et al., 2019; Altakroni et al., 2021). Accordingly, the genetic defects of DNA mismatch repair mechanisms have been related to impaired spermatogenesis and male infertility (Gunes et al., 2015; Hekim et al., 2021; Metin Mahmutoglu et al., 2021). A recent study has investigated the methylation profile of the DNA mismatch repair gene MutL alpha (MLH1) in sperm DNA from oligozoospermic patients and normozoospermic males enrolled as the control group (Gunes et al., 2018a). The results indicated an association between MLH1 hypermethylation and male infertility, suggesting that this gene might be under epigenetic regulation during sperm cell development (Gunes et al., 2018a). High methylation levels of the DNA repair X-ray repair cross-complementing protein 1 gene have been found in OAT males, thereby suggesting that the improper methylation of this gene may have a role in sperm chromatin condensation and OAT (Metin Mahmutoglu et al., 2021). Contrariwise, two additional well-known DNA repair genes, i.e., breast cancer type 1 susceptibility protein (BRCA1) and BRCA2, did not show defective methylation in infertile men with OAT (Kabartan et al., 2019).
Several other genes found aberrantly methylated in altered sperm samples from infertile males have been investigated at a single-study level (Table 1). Overall, several abnormally methylated genes have been found to be associated to altered sperm parameters, mainly oligozoospermia, indicating a potential involvement of DNA methylation in male infertility. All, but MTHFR, gene investigations have been carried out at a single-study level and need further studies to be confirmed. On the contrary, improper MTHFR promoter methylation, such as hypermethylation, has been repeatedly found in aberrant sperm samples, suggesting the methylation-induced MTHFR dysregulation as a potential causal factor of abnormal sperm changes. Interestingly, MTHFR hypermethylation has been found in normal sperm samples of males from idiopathic infertile couples. This may indicate that MTHFR dysfunction could play a role in changing sperm function and health, possibly by lowering CpG methylation content in imprinted genes, which, in turn, may affect embryo development and pregnancy outcome (Rotondo et al., 2013). In view of finding gene methylation hallmarks in male infertility, these data are interesting and will deserve further investigations. For instance, large studies of different infertile populations along with a functional analysis of hypermethylation are needed in order to elucidate the role and the mechanisms of the MTHFR gene in human spermatogenesis and the related pathologies.
The methylation status of LINE1 in sperm DNA from infertile males was initially evaluated by Kobayashi et al. (2007) who reported no correlation between LINE1 methylation and male reproductive function. No correlation has also been found in sperm DNA from idiopathic infertile males belonging to recurrent spontaneous miscarriage couples (Ankolkar et al., 2013). Low methylation levels at LINE1 have been shown in asthenospermic and OAT patients in an additional study, although no statistical significance was found between cases and controls (Boissonnas et al., 2010; Tian et al., 2014). A meta-analysis in 291 infertile patients and 198 fertile individuals from seven published works found no significant correlation with LINE1 methylation levels (Santi et al., 2017). Conversely, DNA methylation marks at LINE1 and the repetitive sequences ALU YB8, NBL2, and D4Z4 have been found to be significantly lower in spermatozoa from idiopathic infertile males than in somatic cells (Urdinguio et al., 2015). A significant decrease in global 5mC levels and LINE1 promoter methylation in spermatozoa of males from couples experiencing recurrent pregnancy loss has also been described (Khambata et al., 2021). Similarly, LINE1 hypomethylation along with promoter hypermethylation-associated silencing of PIWIL2 and Tudor Domain Containing 1 genes, two PIWI/piRNA pathway-related genes, has been found in sperm DNA from infertile males affected by spermatogenic disorders (Heyn et al., 2012). Animal model studies have reported similar results with other PIWI/piRNA pathway-related genes and satellite DNA regions (Capra et al., 2019; Zhang et al., 2020).
LINE1 methylation levels evaluated through sperm genome methylation analyses might be a good indicator of genome-wide alterations. Indeed, these repetitive sequences have frequently been considered as surrogate markers for global methylation changes (Pinto-Medel et al., 2017). However, the role of LINE1 methylation in male infertility is far to be elucidated. Few studies are currently available and often reporting contradictory data, probably due to the lack of homogeneity in experimental design and data processing among studies.
Over the years, with the development of molecular technology/methods (Park et al., 2012; Tognon et al., 2015; Rotondo et al., 2018b, 2020a; Mazzoni et al., 2020; Preti et al., 2020; Oton-Gonzalez et al., 2021), many studies have investigated the relationship between DNA methylation and male infertility on a genome-wide scale by employing high-throughput techniques (Houshdaran et al., 2007; Ferfouri et al., 2013; Schütte et al., 2013; Camprubí et al., 2016; Du et al., 2016; Jenkins et al., 2016a, b; Laqqan et al., 2017a; Lee et al., 2019). The first array-wide analysis was performed by Houshdaran et al. (2007) highlighting improper DNA methylation at repetitive element satellite 2, H-RAS, neurotrophin 3, paired-box gene 8, and Stratifin, 3′-nucleotidase, and metallothionein 1A genes in semen samples with abnormal sperm concentration, motility, and morphology. DNA methylation variations at over 9K CpGs spread throughout the testicular genome have been detected between obstructive and non-obstructive azoospermic patients (Ferfouri et al., 2013). The human methylation 450k array analysis in semen samples from couples who conceived and couples who did not showed significantly decreased and increased methylation in two and three genomic regions, respectively, in the “failure-to-conceive” group. Interestingly, the two sites with low methylation content were at closely related genes known to be expressed in the sperm, i.e., HSPA1L and HSPA1B (Jenkins et al., 2016b). A recent methylome and transcriptome study in euploid blastocysts derived from couples with OAT males reported significant alterations in approximately 1.1K CpGs compared to the cryopreserved euploid blastocysts used as controls (Denomme et al., 2018). A pathway analysis elucidated the genes involved in the regulation of cellular metabolic process as universally affected. This epigenetic dysregulation provided an explanation for the reduced reproductive potential in OAT patients despite euploid blastocyst transfers (Denomme et al., 2018).
Additional array-wide studies have been reported to show the following: (i) changes in the methylation of testis/epididymis-specific genes in the testis and epididymis of vasectomized males (Wu et al., 2013), (ii) loss of methylation in inflammation- and immune response-related genes in sperm samples from males belonging to infertile couples (Schütte et al., 2013), and (iii) gain of methylation in spermatogenesis-related genes in sperm samples derived from individuals undergoing assisted reproduction (Schütte et al., 2013).
In normospermic males, the DNA methylome of the sperm cell differs between infertile and fertile, and this difference may be predictive of embryo quality during in vitro fertilization (Aston et al., 2015). Nevertheless, differences in sperm epigenome within healthy subjects have been also detected, indicating intra-sample heterogeneity in DNA methylation patterns (Jenkins et al., 2014). Furthermore, additional studies have underlined potential epigenetic heterogeneity among, and within, sperm cells, spermatogonia, and gonocytes (Kuhtz et al., 2014; Laurentino et al., 2015, 2016).
The impairment of both sperm epigenome and gene expression in relation to male reproductive dysfunction was recently explored using high-throughput techniques. Hypermethylation-induced silencing at SRY-Box Transcription Factor 30 gene has been detected in association with non-obstructive azoospermia (Han et al., 2020). Two array-wide studies have described inverse correlations between DNA methylation and the mRNA expression of genes involved in different pathways, such as the response to hormone stimulus, activation of protein kinase activity, apoptosis, and reproduction, in sperm from both severe oligozoospermic and azoospermic patients (Li et al., 2017; Wu et al., 2020).
As high-throughput approaches are able to detect extensive variations in epigenetic marks, DNA methylation changes could potentially be used as a diagnostic tool to predict the risk of both male infertility and male infertility-related diseases (Aston et al., 2015; Fang et al., 2019; Patel et al., 2020). For instance, an array-based DNA methylation profile conducted on peripheral blood has revealed variations in DNA methylation patterns in infertile males compared to normozoospermic fertile controls, suggesting that epigenome-based blood markers can be used for diagnostic purposes (Sarkar et al., 2019). This has also been suggested by the results of another high-throughput study focused on congenital hypopituitarism (CH), which is a pituitary gland hormone deficiency that leads to metabolic disorders and male reproductive dysfunction. This study identified methylation at CpG sites on two spermatogenesis/testicular development-related genes in whole blood DNA isolated from a cohort of CH patients (Fang et al., 2019). In this context, the clinical application of these highly sensitive techniques cannot be ruled out. As high-throughput approaches can potentially support work on defining the forms of male infertility, these methods will deserve attention in andrological clinical practice (Ferlin and Foresta, 2014).
DNA methyltransferases and TET are two key regulative gene families involved in DNA methylation and demethylation pathways, respectively, during both embryogenesis and spermatogenesis (Nettersheim et al., 2013; Barišić et al., 2017; Li et al., 2019). Despite their critical role in sperm methylation, the DNMT and TET genes have been poorly investigated in the context of male infertility (Uysal et al., 2016, 2019; Tang et al., 2017, 2018). Decreased expression of DNMT1, -3A, and -3B enzymes in association with global DNA methylation changes has been detected in the testes of non-obstructive azoospermic patients, suggesting that the methylation-induced DNMT inactivation may be involved in male reproductive dysfunction (Uysal et al., 2019). The presence of DNMT1 SNP variant rs4804490 was found to be associated with an increased risk of idiopathic infertility in males with abnormal semen parameters (Tang et al., 2017). In the same study, no associations were afterward determined between additional four DNMT variants, i.e., DNMT1 (rs4804490), DNMT3A (rs1550117), DNMT3B (rs2424909), and DNMT3L (rs7354779), and aberrant DNA methylation at several imprinted genes, including H19, GNAS complex locus (GNAS), and GTP-binding protein Di-Ras3 (DIRAS3) (Tang et al., 2018). Conversely, two SNP variants, rs2656927/rs8103849, of ubiquitin-like, containing PHD and RING finger domains 1 (UHRF1) gene, which is tightly related to the DNMT1 pathway, have been identified in the blood of oligozoospermic infertile males (Zhu et al., 2019). Other recent evidence indicated that UHRF1 inactivation may have a negative impact on male fertility potential, as the conditional loss of this gene in germ cells leads to DNA hypomethylation, upregulation of retrotransposons, and DNA damage response activation (Dong et al., 2019). UHRF1 also cooperates with the PIWI/piRNA pathway during spermatogenesis, suggesting a molecular link between DNA methylation and the PIWI/piRNA pathway in the germline (Dong et al., 2019).
Ten-eleven translocation 1, -2, and -3 genes have been found to be consecutively expressed at different stages of spermatogenesis, and their expression levels have been positively correlated to male reproductive potential and pregnancy (Ni et al., 2016). Indeed sperms from oligozoospermic and/or asthenozoospermia males have significantly reduced TET1, -2 and -3 mRNAs compared to fertile donors, but not to normozoospermic males (Ni et al., 2016). TET1 protein deficiency in spermatogonia decreases the 5hmC levels and downregulates genes that are involved in several pathways, such as cell cycle, germ cell differentiation, meiosis, and reproduction, resulting in the reduction of spermatogonia stem cells and germ cell differentiation (Huang et al., 2020).
When taken together, these studies suggest that DNMT and TET impairment in sperm cells may represent a potential risk factor for male infertility. Therefore, further investigations are needed to assess the role of DNMT and TET enzymes in the male infertility phenotype.
Imbalances in the methylation patterns of imprinted genes could potentially impair spermatogenesis and/or favor the male infertility phenotype (Hartmann et al., 2006; Rotondo et al., 2012, 2013; Das et al., 2017; Laurentino et al., 2019; Åsenius et al., 2020a; Pohl et al., 2021). The methylation status of a number of imprinted genes has been investigated in relation to impaired spermatogenesis and/or reproductive dysfunction (Table 2). DNA methylation defects of three maternally imprinted genes, i.e., PLAGL1, MEST, and DIRAS3, have been identified in sperm DNAs from infertile males with a low semen concentration (Houshdaran et al., 2007). One of these genes, MEST, has been repeatedly found as hypermethylated in association with the male infertility phenotype (Marques et al., 2004; Hammoud et al., 2010; El Hajj et al., 2011; Montjean et al., 2013; Xu et al., 2016). Specifically, the improper DNA methylation at MEST has been (i) linked to low sperm concentration and motility as well as poor sperm morphology in idiopathic infertile males (Marques et al., 2008; Poplinski et al., 2010; Kläver et al., 2013), (ii) detected in primary spermatocytes from azoospermic patients presenting complete or incomplete maturation arrest (Marques et al., 2017), (iii) associated with decreased bi-testicular volume and increased follicle-stimulating hormone levels (Kläver et al., 2013), and (iv) associated with abnormal protamine ratio in oligozoospermic patients (Hammoud et al., 2010). These data have been further confirmed by a meta-analytic study (Santi et al., 2017). Recently, MEST hypermethylation has been found in the spermatozoa of male partners from couples experiencing recurrent pregnancy loss (Khambata et al., 2021).
Altered methylation in H19 has been broadly documented to be associated with infertile males affected by oligozoospermia, asthenozoospermia, teratozoospermia, OA, AT, and OAT (Marques et al., 2004, 2008, 2010; Kobayashi et al., 2007; Boissonnas et al., 2010; Minor et al., 2011; Rotondo et al., 2013; Li et al., 2016; Dong et al., 2017; Bruno et al., 2018; Peng et al., 2018). Idiopathic infertile males with normal semen parameters have also been found to carry methylation defects at H19 in their sperm (Poplinski et al., 2010; Ankolkar et al., 2013; Tang et al., 2018). Imprinting errors at H19 gene were detected in primary spermatocytes and elongated spermatids/spermatozoa with incomplete maturation arrest from two azoospermic patients, although the differences in H19 methylation levels between patients and controls were not statistically significant (Marques et al., 2017). In addition, abnormal methylation at H19 has been shown at the regulatory region CTCF-binding site 6 (CTCF6), located within the DMR of IGF2-H19, in sperm DNA from infertile males (Rotondo et al., 2013). Therefore, sperm cells carrying methylation defects at the IGF2-H19 CTCF6 region are at a high risk of causing biallelic inactivation of the IGF2 gene, which, in turn, could negatively impact embryo development and/or pregnancy outcome (Boissonnas et al., 2010; Santi et al., 2017; Bruno et al., 2018). Similarly, methylation defects at IGF2-H19 have been detected in placenta tissues from offspring conceived by assisted reproductive technologies (ART) (Pinborg et al., 2016; Hattori et al., 2019; Lou et al., 2019). Thus, these studies have pointed out that ART procedures may account for the epigenetic defects in the embryo.
Altered DNA methylation patterns at overlapping transcript 1 (LIT1) and SNRPN have been found in infertile males tested with abnormal protamine ratio in sperm cells (Hammoud et al., 2010). Aberrant methylation at the SNRPN gene has also been associated with altered sperm motility and morphology as well as with OA and AT (Botezatu et al., 2014; Peng et al., 2018). Interestingly, imprinting errors at the SNRPN gene and IGF2-H19 have also been found in spermatozoa from asthenozoospermia patients and in ART-conceived human fetuses (Lou et al., 2019). Hypermethylation at SNRPN, alongside other maternally imprinted genes involved in embryonic germ cell development, such as MEG3, PEG1 (also known as MEST), PEG3, LIT1, and PLAG1 like zinc finger 1 (ZAC), as well as loss of methylation at the paternal imprinted gene GTL2 has been detected in sperm DNA derived from patients affected by moderate/severe oligozoospermia (Kobayashi et al., 2007). Hypermethylation of PEG1, PEG3, PEG10, and ZAC genes has been shown in spermatozoa from male partners from couples experiencing recurrent pregnancy loss (Khambata et al., 2021). Contrariwise, the lack of altered methylation has been reported for PEG1, ZAC, and GTL2 in the sperm of male partners from recurrent spontaneous miscarriages (Ankolkar et al., 2013). Lastly, the maternally imprinted gene SNRPN upstream reading frame protein, which is related to the SNRPN pathway, has been detected as hypermethylated in association with oligozoospermia (Bruno et al., 2018).
An additional imprinted gene possibly involved in male reproductive dysfunction is zinc-finger CCHC-type containing 13 (ZCCHC13). ZCCHC13 is a novel imprinted gene involved in an epigenetic mechanism known as X-chromosome inactivation and has been detected both hypermethylated and down-regulated in testis biopsies isolated from non-obstructive azoospermia patients (Li et al., 2018). Low methylation at GNAS and DIRAS3 imprinted genes has also been found to be more prevalent in idiopathic infertile males, especially in oligozoospermic males, than in fertile males (Bruno et al., 2018; Tang et al., 2018). The evidence that improper DNA methylation at the GNAS gene is linked to male infertility is also supported by animal models (Congras et al., 2014).
Imprinting errors could also be related to DNA fragmentation in sperm cells. Indeed, the improper methylation of the paternally imprinted gene Potassium Voltage-Gated Channel Subfamily Q Member 1 (KCNQ1), along with that of IGF2, has been found to be associated with a high level of DNA fragmentation in semen samples (Ni et al., 2019). In addition, the centromeric (domain 2) differentially methylated region (KvDMR) located in exon 10 of KCNQ1OT1 has been found to be hypermethylated in the spermatozoa of male partners from couples experiencing recurrent pregnancy loss (Khambata et al., 2021).
A recent array-wide methylation analysis conducted on sperm DNA from a cohort of males identified a number of genes associated with infertility, including the maternally imprinted genes DLG associated protein 2 (DLGAP2) and GATA binding protein 3 as well as the paternally imprinted genes catenin alpha 3, membrane-associated guanylate kinase, and tumor protein 73 (Sujit et al., 2018). An additional gene, brevican, which is a non-imprinted gene involved in brain extracellular matrix formation, has been identified in this study. All these genes might be considered as novel potential candidates accounting for male infertility phenotype, and their roles in spermatogenesis need to be further investigated (Sujit et al., 2018).
In conclusion, many imprinted genes have been investigated for methylation in aberrant semen samples, such as oligozoospermia, asthenozoospermia, and teratozoospermia. H19 and MEST were the most investigated genes in aberrant sperm samples, showing repetitively aberrant loss or gain of methylation, respectively, which, in turn, may be causal factors in the development of male infertility. Of note is the fact that aberrant methylations have also been found in infertile males with normal sperm parameters. This aspect may have important implications in the diagnosis of male infertility, which, to date, lacks explanation in 30% of infertile males. It is also hoped that further studies will shed light into the etiology of these aberrant imprinting patterns in paternally or maternally imprinted genes in idiopathic infertility cases. Indeed the loss of methylation of paternally imprinted genes may be due to a deficiency of DNMTs, whereas hypermethylation of maternally imprinted genes might result from erroneous de novo methylation or a failure to erase maternal imprint in the male germ cell genomes (Barlow and Bartolomei, 2014; Uysal et al., 2019).
Habits and lifestyle factors, such as smoking, alcohol consumption, and diet, have been investigated in an effort to gain insight into the causes of aberrant sperm DNA methylation in relation to male infertility (El Khoury et al., 2019). A recent pilot study conducted on sperm DNA from a cohort of infertile males who underwent a supervised yoga practice regimen reported DNA methylation changes at nearly 400 genes, suggesting a link between positive lifestyle practices and male reproductive health (Bisht et al., 2020).
Tobacco smoke has a strong negative impact on sperm DNA methylation in a number of genes, also demonstrated in animal models (Xu et al., 2013; Dai et al., 2016; Dong et al., 2017; Laqqan et al., 2017a; Hamad et al., 2018; Wyck et al., 2018; Fragou et al., 2019). The imprinted gene SNRPN has been found to be aberrantly methylated in asthenozoospermia and teratozoospermic smokers (Dong et al., 2017). Other studies on humans and/or animal models have reported modifications to sperm DNA methylation following cannabis use (Murphy et al., 2018; Reece and Hulse, 2019). Improper DLGAP2 methylation has recently been reported in sperm from cannabis users, thereby suggesting the potentially negative effect of cannabis use prior to conception on imprinting marks (Schrott et al., 2020). In this context, the impact of parental drug addiction on sperm DNA methylation in drug-sired offspring needs to be more deeply explored (Jarred et al., 2018; Nieto and Kosten, 2019). Moreover, both alcohol and nicotine consumption seem to impair DNA methylation in several genes and genomic regions, leading to male infertility. Indeed data on DNA methylation changes at cyclin-dependent kinase inhibitor 2A and LINE1 and an increased risk of male reproductive dysfunction have been reported (Zhang et al., 2019). More recently, evidence has also indicated that DNA methylation defects at MEST and GNAS genes, along with altered sperm motility, morphology, and concentration, could be linked to alcohol and nicotine exposure (Zhang et al., 2019).
Paternal diet is able to alter sperm epigenome and has been associated with negative pregnancy outcomes in mice (Lambrot et al., 2013). In addition, sperm from mice under a folate-deficient diet showed differential DNA methylation marks at genes implicated in development, diabetes, autism, and schizophrenia (Lambrot et al., 2013). In humans, several studies have reported on the relationship between paternal and/or maternal diet and sperm DNA methylation in male infertility (Shukla et al., 2014; Hoek et al., 2020; Toschi et al., 2020). A recent study conducted on infertile males who underwent oral supplementation with micronutrients in support of folate, B vitamins, zinc, and cysteines reported an increase in DNA methylation levels, resulting in improved sperm nuclear maturation and antioxidant defenses, with a possible positive effect on reproductive function (Bassiri et al., 2020). Furthermore, evidences on sperm DNA from infertile males indicate a high methylation of vitamin D receptor promoter in vitamin D-deficient conditions, thus suggesting the role of diet in epigenetic modifications and male reproductive dysfunction (Vladoiu et al., 2017). Similarly, one investigation indicated that parental diet can modulate the methylation levels of several genes, including liver-specific genes involved in cholesterol/lipid metabolism, whereas no effect of paternal diet on sperm DNA methylation was found in other studies (Carone et al., 2010; Rando and Simmons, 2015; Shea et al., 2015; Whitelaw, 2015). Similarly, parental pre-conceptional obesity could potentially impact on the establishment of imprinting marks during embryogenesis (Soubry et al., 2015; Ou et al., 2019; Åsenius et al., 2020b; Keyhan et al., 2021). Changes in methylation in two imprinted genes, PLAGL1 and MEG3, have been detected in umbilical cord blood leukocytes in a group of newborns from obese mothers, making a link between maternal overnutrition and the impairment of imprinting marks in the offspring (Soubry et al., 2015). In males, imprinted genes involved in growth and development, including PEG3 and neuronatin alongside MEST, have been found to be hypomethylated in DNA derived from the blood of children of obese fathers. The relationship between parental obesity and the methylation status of the offspring clearly indicates that spermatogenesis may be susceptible to environmental factors from an epigenetic point of view (Soubry et al., 2015). A recent animal model study supported and extended this conclusion by reporting data about environmental toxicant exposure and transgenerational inheritance of epigenetic marks (Sadler-Riggleman et al., 2019). Additional animal models have shown similar results, reporting that epigenetic alterations at PEG3 and H19 can occur in sperm cells in offspring from diabetic and/or obese mice (Ge et al., 2014). These studies, when taken together, highlight that both defective DNA methylation and imprinting marks may be transferred through generations, potentially affecting the health of adult offspring (Grossniklaus et al., 2013; Ge et al., 2014; Soubry et al., 2015; Blanco Rodríguez and Camprubí Sánchez, 2019). However, this assumption has been questioned in a recent meta-analysis of coordinated epigenome-wide association studies of paternal prenatal body mass index (BMI) in relation to DNA methylation (Sharp et al., 2021). The study conclusions do not support the hypothesis that paternal BMI around the time of pregnancy is linked with offspring-blood DNA methylation, even at the imprinted regions (Sharp et al., 2021). Considering the data available, more research is needed to address the role of paternal diet on sperm DNA methylation in infertile males.
This review provides an overview of gene and genome methylation, its regulation during the spermatogenesis process, and the current knowledge on those DNA methylation defects which are known to be involved in male reproductive dysfunctions.
Most of the aforementioned studies have added sufficient strength to the hypothesis that sperm methylation is associated with sperm alterations and infertility. In spite of these reports, the causative role of improper DNA methylation marks in inducing male infertility remains poorly characterized, particularly due to the lack of studies on the mechanisms involving DNA methylation in sperm cells.
It is convincible that aberrations in methylation at specific target genes may reflect whole methylome defects due to altered DNMT and TET activities during sperm cell development and spermatogenesis. On the other hand, several risk factors, such as lifestyle, drugs, hormones, and diet, may account for the gain or loss of methylation in key genes in sperm cells. New functional studies on this topic are needed to better elucidate the mechanisms affecting methylation in sperm. Nevertheless, extensive knowledge of sperm DNA methylation status in association with reduced reproductive capability is useful in developing novel diagnostic tools for male infertility (Balasubramanian et al., 2019; Sarkar et al., 2019). The current primary diagnostic protocol for identifying infertile males relies on the assessment of sperm number, motility, and morphology. This diagnostic approach has several limitations in differentiating fertile and infertile males, as abnormalities causing male reproductive dysfunction are still unknown in a fraction of males. In addition, this approach also provides limited information on male reproductive potential and cannot be employed as a prognostic tool in predicting pregnancy success and possible outcome (Kläver and Gromoll, 2014). When taken together, these drawbacks in the current protocols make DNA methylation evaluation a putative useful tool in clinical practice (Sarkar et al., 2019). A large number of studies have already demonstrated that aberrant DNA methylation in spermatozoa is linked to defective sperm parameters and male infertility phenotype as well as a negative pregnancy outcome. In this context, sperm DNA methylation could become a novel diagnostic and prognostic parameter for assessing male infertility and pregnancy outcome, respectively (Kläver and Gromoll, 2014). This approach has also been reported in monitoring other diseases (Luján et al., 2019). Thanks to high-throughput technology, which is rapidly increasing in epigenetic studies, methylome analyses may soon allow the methylation differences between infertile and fertile males to be characterized, thereby greatly improving the current knowledge on the relationship between sperm DNA methylation and male infertility (Ferlin and Foresta, 2014). Hopefully, the translation of this technology into clinical andrological practice will improve the diagnostic and prognostic assessment of infertile males (Kläver and Gromoll, 2014).
The role of DNA methylation in spermatogenesis, sperm function, and male infertility is an important research area that deserves attention. Although recent evidence seems to indicate a possible overestimation of aberrant DNA methylation in male infertility (Camprubí et al., 2012; Leitão et al., 2020; Åsenius et al., 2020b), the aforementioned studies have provided significant associations between improper DNA methylation in sperm and infertile males.
A number of genes have been found to be differentially methylated in relation to impaired spermatogenesis and/or reproductive dysfunction. DNA methylation defects in genes, including MEST and H19 within imprinted genes and MTHFR within non-imprinted genes, have been previously identified in a wide number of studies, and their defective marks could be potentially employed as useful tools in clinical practice for assessing male infertility (Marques et al., 2004, 2008, 2010; Kobayashi et al., 2007; Boissonnas et al., 2010; Hammoud et al., 2010; El Hajj et al., 2011; Minor et al., 2011; Rotondo et al., 2012, 2013; Montjean et al., 2013; Haggarty, 2015; Dai et al., 2016; Xu et al., 2016; Dong et al., 2017; Li et al., 2017; Bruno et al., 2018; Saraswathy et al., 2018; Coppedè et al., 2019; Mishra et al., 2019; Sarkar et al., 2019).
The lack of studies for several of the additional genes reported herein and/or the conflicting results for others make the use of defective DNA methylation marks as a diagnostic tool still unlikely. Further studies are necessary to identify novel genes that could potentially be employed as tools in clinical practice.
As suggested in several works, the dysregulation of epigenetic mechanisms, including the aberrant methylation of DNA, may play an important role in the development of infertility with unknown etiology in a high fraction of males. However, further research is needed to epigenetically explore the etiology of this disease. Indeed the use of DNA methylation as a marker or cause of sperm abnormalities in the field of fertility is only beginning to be explored thoroughly and is currently not completely clarified. Moreover, the use of methylation changes in DNA as a marker to identify the male infertility factor is difficult, as these changes may have little or even no significant biological impact or multiple different changes may be necessary to establish infertile phenotypes. In this effort, methylation signatures in normal sperm from fertile individuals should provide useful knowledge for elucidating these topics. Moreover, as aging is correlated with changes in DNA methylation, investigating sperm DNA methylation in age-stratified normal fertile individuals should also be taken into account as well as to improve our knowledge in this field (Johnson et al., 2012). Another point deserving attention in the context of methodological approaches is the potential contamination by somatic cells during epigenetic analyses, such as blood, white, and epithelial cells, as a result of incorrect sperm cell isolation/processing (Jenkins et al., 2017). Standardized and detailed protocols for processing sperm samples should be used in studying DNA methylation in male infertility, as even only a few contaminated somatic cells might alter the epigenetic signatures of sperm cells. Research into sperm DNA methylation in male infertility might be an important future area of study. We thus encourage further studies focused on the role of DNA methylation in spermatogenesis and male reproductive potential as well as embryo development and pregnancy outcome. Such newly acquired data could improve the diagnostic and prognostic setup of male infertility phenotypes and pregnancy outcomes, respectively, with high health benefits for humans.
JR, MT, and FM contributed to conceptualization and writing—review and editing. JR contributed to writing—original draft preparation. CM and CL contributed to visualization. MT and FM contributed to supervision, contributed to funding acquisition, and project administration. All the authors have read and agreed to the published version of the manuscript.
This work was supported by the University of Ferrara, Fondo di Ateneo per la Ricerca (FAR) grants.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Georgia Emma Gili revised the English text of the manuscript.
ARTs, assisted reproductive technologies; AT, asthenoteratozoospermia; CH, congenital hypopituitarism; CGIs, CpG islands; CTCF6, CTCF-binding site 6; DMRs, differentially methylated regions; DNMTs, DNA methyltransferases; ICEs, imprint control elements; ICRs, imprinting control regions; LINEs, long interspersed nuclear elements; OA, oligoasthenozoospermia; OT, oligoteratozoospermia; OAT, oligoasthenoteratozoospermia; PGCs, primordial germ cells; TET, ten-eleven translocation.
Abrao, M. S., Muzii, L., and Marana, R. (2013). Anatomical causes of female infertility and their management. Int. J. Gynaecol. Obstet. 123 Suppl 2, S18–S24. doi: 10.1016/j.ijgo.2013.09.008
Agarwal, A., Mulgund, A., Hamada, A., and Chyatte, M. R. (2015). A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 13:37. doi: 10.1186/s12958-015-0032-1
Alkhaled, Y., Laqqan, M., Tierling, S., Lo Porto, C., and Hammadeh, M. E. (2018). DNA methylation level of spermatozoa from subfertile and proven fertile and its relation to standard sperm parameters. Andrologia 50:e13011. doi: 10.1111/and.13011
Altakroni, B., Nevin, C., Carroll, M., Murgatroyd, C., Horne, G., Brison, D. R., et al. (2021). The marker of alkyl DNA base damage, N7-methylguanine, is associated with semen quality in men. Sci. Rep. 11:3121. doi: 10.1038/s41598-021-81674-x
Ankolkar, M., Salvi, V., Warke, H., Vundinti, B. R., and Balasinor, N. H. (2013). Methylation status of imprinted genes DLK1-GTL2, MEST (PEG1), ZAC (PLAGL1), and LINE-1 elements in spermatozoa of normozoospermic men, unlike H19 imprinting control regions, is not associated with idiopathic recurrent spontaneous miscarriages. Fertil. Steril. 99, 1668–1673. doi: 10.1016/j.fertnstert.2013.01.107
Aravin, A. A., Sachidanandam, R., Bourc’his, D., Schaefer, C., Pezic, D., Toth, K. F., et al. (2008). A piRNA pathway primed by individual transposons is linked to de novo DNA methylation in mice. Mol. Cell 31, 785–799. doi: 10.1016/j.molcel.2008.09.003
Arnaud, P. (2010). Genomic imprinting in germ cells: imprints are under control. Reproduction 140, 411–423. doi: 10.1530/REP-10-0173
Åsenius, F., Danson, A. F., and Marzi, S. J. (2020a). DNA methylation in human sperm: a systematic review. Hum. Reprod. Update 26, 841–873. doi: 10.1093/humupd/dmaa025
Åsenius, F., Gorrie-Stone, T. J., Brew, A., Panchbhaya, Y., Williamson, E., Schalkwyk, L. C., et al. (2020b). The DNA methylome of human sperm is distinct from blood with little evidence for tissue-consistent obesity associations. PLoS Genet. 16:e1009035. doi: 10.1371/journal.pgen.1009035
Asim, A., Agarwal, S., Panigrahi, I., Saiyed, N., and Bakshi, S. (2017). MTHFR promoter hypermethylation may lead to congenital heart defects in down syndrome. Intractable Rare Dis. Res. 6, 295–298. doi: 10.5582/irdr.2017.01068
Aston, K. I., Punj, V., Liu, L., and Carrell, D. T. (2012). Genome-wide sperm deoxyribonucleic acid methylation is altered in some men with abnormal chromatin packaging or poor in vitro fertilization embryogenesis. Fertil. Steril. 97, 285–292. doi: 10.1016/j.fertnstert.2011.11.008
Aston, K. I., Uren, P. J., Jenkins, T. G., Horsager, A., Cairns, B. R., Smith, A. D., et al. (2015). Aberrant sperm DNA methylation predicts male fertility status and embryo quality. Fertil. Steril. 104, 1388–1397. doi: 10.1016/j.fertnstert.2015.08.019
Balasubramanian, A., Thirumavalavan, N., and Pastuszak, A. W. (2019). DNA methylation profiling of peripheral blood samples is a promising new approach to screen for male infertility. Fertil. Steril. 112, 32–33. doi: 10.1016/j.fertnstert.2019.04.019
Barau, J., Teissandier, A., Zamudio, N., Roy, S., Nalesso, V., Hérault, Y., et al. (2016). The DNA methyltransferase DNMT3C protects male germ cells from transposon activity. Science 354, 909–912. doi: 10.1126/science.aah5143
Barišić, A., Pereza, N., Hodžić, A., Ostojić, S., and Peterlin, B. (2017). A single nucleotide polymorphism of DNA methyltransferase 3B gene is a risk factor for recurrent spontaneous abortion. Am. J. Reprod. Immunol. 78:e12765. doi: 10.1111/aji.12765
Barlow, D. P., and Bartolomei, M. S. (2014). Genomic imprinting in mammals. Cold Spring Harb. Perspect. Biol. 6:a018382. doi: 10.1101/cshperspect.a018382
Bassiri, F., Tavalaee, M., Dattilio, M., and Nasr-Esfahani, M. H. (2020). Micronutrients in support to the carbon cycle activate antioxidant defences and reduce sperm DNA damage in infertile men attending assisted reproductive technology programs: clinical trial study. Int. J. Fertil. Steril. 14, 57–62. doi: 10.22074/ijfs.2020.6084
Baylin, S. B., and Jones, P. A. (2011). A decade of exploring the cancer epigenome-biological and translational implications. Nat. Rev. Cancer 11, 726–734. doi: 10.1038/nrc3130
Benchaib, M., Braun, V., Ressnikof, D., Lornage, J., Durand, P., Niveleau, A., et al. (2005). Influence of global sperm DNA methylation on IVF results. Hum. Reprod. 30, 768–773. doi: 10.1093/humrep/deh684
Biermann, K., and Steger, K. (2007). Epigenetics in male germ cells. J. Androl. 28, 466–480. doi: 10.2164/jandrol.106.002048
Bisht, S., Banu, S., Srivastava, S., Pathak, R. U., Kumar, R., Dada, R., et al. (2020). Sperm methylome alterations following yoga-based lifestyle intervention in patients of primary male infertility: a pilot study. Andrologia 2:e13551. doi: 10.1111/and.13551
Blanco Rodríguez, J., and Camprubí Sánchez, C. (2019). Epigenetic transgenerational inheritance. Adv. Exp. Med. Biol. 1166, 57–74. doi: 10.1007/978-3-030-21664-1_4
Boissonnas, C. C., El Abdalaoui, H., Haelewyn, V., Fauque, P., Dupont, J. M., Gut, I., et al. (2010). Specific epigenetic alterations of IGF2-H19 locus in spermatozoa from infertile men. Eur. J. Hum. Genet. 18, 73–80. doi: 10.1038/ejhg.2009.117
Botezatu, A., Socolov, R., Socolov, D., Iancu, I. V., and Anton, G. (2014). Methylation pattern of methylene tetrahydrofolate reductase and small nuclear ribonucleoprotein polypeptide N promoters in oligoasthenospermia: a case-control study. Reprod. Biomed. Online 28, 225–231. doi: 10.1016/j.rbmo.2013.10.010
Broad, K. D., Curley, J. P., and Keverne, E. B. (2009). Increased apoptosis during neonatal brain development underlies the adult behavioral deficits seen in mice lacking a functional paternally expressed gene 3 (Peg3). Dev. Neurobiol. 69, 314–325. doi: 10.1002/dneu.20702
Bruno, C., Blagoskonov, O., Barberet, J., Guilleman, M., Daniel, S., Tournier, B., et al. (2018). Sperm imprinting integrity in seminoma patients? Clin. Epigenetics 10:125. doi: 10.1186/s13148-018-0559-z
Camprubí, C., Pladevall, M., Grossmann, M., Garrido, N., Pons, M. C., and Blanco, J. (2012). Semen samples showing an increased rate of spermatozoa with imprinting errors have a negligible effect in the outcome of assisted reproduction techniques. Epigenetics 7, 1115–1124. doi: 10.4161/epi.21743
Camprubí, C., Salas-Huetos, A., Aiese-Cigliano, R., Godo, A., Pons, M. C., Castellano, G., et al. (2016). Spermatozoa from infertile patients exhibit differences of DNA methylation associated with spermatogenesis-related processes: an array-based analysis. Reprod. Biomed. Online 33, 709–719. doi: 10.1016/j.rbmo.2016.09.001
Cannarella, R., Condorelli, R. A., La Vignera, S., Bellucci, C., Luca, G., Calafiore, R., et al. (2019). IGF2 and IGF1R mRNAs are detectable in human spermatozoa. World J. Mens Health 1, 37–47. doi: 10.5534/wjmh.190070
Capra, E., Lazzari, B., Turri, F., Cremonesi, P., Portela, A. M. R., Ajmone-Marsan, P., et al. (2019). Epigenetic analysis of high and low motile sperm populations reveals methylation variation in satellite regions within the pericentromeric position and in genes functionally related to sperm DNA organization and maintenance in Bos taurus. BMC Genomics 20:940. doi: 10.1186/s12864-019-6317-6
Carone, B. R., Fauquier, L., Habib, N., Shea, J. M., Hart, C. E., Li, R., et al. (2010). Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell 143, 1084–1096. doi: 10.1016/j.cell.2010.12.008
Carrell, D. T. (2019). The sperm epigenome: implications for assisted reproductive technologies. Adv. Exp. Med. Biol. 1166, 47–57. doi: 10.1007/978-3-030-21664-1_3
Castillo, J., Jodar, M., and Oliva, R. (2018). The contribution of human sperm proteins to the development and epigenome of the preimplantation embryo. Hum. Reprod. Update 24, 535–555. doi: 10.1093/humupd/dmy017
Chen, Z. (2001). Mice deficient in methylenetetrahydrofolate reductase exhibit hyperhomocysteinemia and decreased methylation capacity, with neuropathology and aortic lipid deposition. Hum. Mol. Genet. 10, 433–443. doi: 10.1093/hmg/10.5.433
Cho, C., Jung-Ha, H., Willis, W. D., Goulding, E. H., Stein, P., Xu, Z., et al. (2003). Protamine 2 deficiency leads to sperm DNA damage and embryo death in Mice1. Biol. Reprod. 69, 211–217. doi: 10.1095/biolreprod.102.015115
Choi, Y., Kim, J. O., Shim, S. H., Lee, Y., Kim, J. H., Jeon, Y. J., et al. (2016). Genetic variation of methylenetetrahydrofolate reductase (MTHFR) and thymidylate synthase (TS) genes is associated with idiopathic recurrent implantation failure. PLoS One 11:e0160884. doi: 10.1371/journal.pone.0160884
Congras, A., Yerle-Bouissou, M., Pinton, A., Vignoles, F., Liaubet, L., Ferchaud, S., et al. (2014). Sperm DNA methylation analysis in swine reveals conserved and species-specific methylation patterns and highlights an altered methylation at the GNAS locus in infertile boars1. Biol. Reprod. 91:137. doi: 10.1095/biolreprod.114.119610
Contini, C., Rotondo, J. C., Magagnoli, F., Maritati, M., Seraceni, S., Graziano, A., et al. (2018). Investigation on silent bacterial infections in specimens from pregnant women affected by spontaneous miscarriage. J. Cell. Physiol. 234, 100–107. doi: 10.1002/jcp.26952
Cooper, T. G., Noonan, E., von Eckardstein, S., Auger, J., Baker, H. W. G., Behre, H. M., et al. (2009). World Health Organization reference values for human semen characteristics. Hum. Reprod. Update 16, 231–246. doi: 10.1093/humupd/dmp048
Coppedè, F., Denaro, M., Tannorella, P., and Migliore, L. (2016). Increased MTHFR promoter methylation in mothers of down syndrome individuals. Mutat. Res. 787, 1–6. doi: 10.1016/j.mrfmmm.2016.02.008
Coppedè, F., Stoccoro, A., Tannorella, P., Gallo, R., Nicolì, V., and Migliore, L. (2019). Association of polymorphisms in genes involved in one-carbon metabolism with MTHFR methylation levels. Int. J. Mol. Sci. 20:3754. doi: 10.3390/ijms20153754
Craig, J. R., Jenkins, T. G., Carrell, D. T., and Hotaling, J. M. (2017). Obesity, male infertility, and the sperm epigenome. Fertil. Steril. 107, 848–859. doi: 10.1016/j.fertnstert.2017.02.115
Dai, J., Xu, W., Zhao, X., Zhang, M., Zhang, D., Nie, D., et al. (2016). Protein profile screening: reduced expression of sord in the mouse epididymis induced by nicotine inhibits tyrosine phosphorylation level in capacitated spermatozoa. Reproduction 151, 227–237. doi: 10.1530/REP-15-0370
Das, L., Parbin, S., Pradhan, N., Kausar, C., and Patra, S. K. (2017). Epigenetics of reproductive infertility. Front. Biosci. (Schol. Ed.) 9:509–535. doi: 10.2741/s497
Denomme, M. M., McCallie, B. R., Parks, J. C., Booher, K., Schoolcraft, W. B., and Katz-Jaffe, M. G. (2018). Inheritance of epigenetic dysregulation from male factor infertility has a direct impact on reproductive potential. Fertil. Steril. 110, 419–428. doi: 10.1016/j.fertnstert.2018.04.004
Dong, H., Wang, Y., Zou, Z., Chen, L., Shen, C., Xu, S., et al. (2017). Abnormal methylation of imprinted genes and cigarette smoking: assessment of their association with the risk of male infertility. Reprod. Sci. 24, 114–123. doi: 10.1177/1933719116650755
Dong, J., Wang, X., Cao, C., Wen, Y., Sakashita, A., Chen, S., et al. (2019). UHRF1 suppresses retrotransposons and cooperates with PRMT5 and PIWI proteins in male germ cells. Nat. Commun. 17:4705. doi: 10.1038/s41467-019-12455-4
Du, Y., Li, M., Chen, J., Duan, Y., Wang, X., Qiu, Y., et al. (2016). Promoter targeted bisulfite sequencing reveals DNA methylation profiles associated with low sperm motility in asthenozoospermia. Hum. Reprod. 31, 24–33. doi: 10.1093/humrep/dev283
El Hajj, N., Zechner, U., Schneider, E., Tresch, A., Gromoll, J., Hahn, T., et al. (2011). Methylation status of imprinted genes and repetitive elements in sperm DNA from infertile males. Sex. Dev. 5, 60–69. doi: 10.1159/000323806
El Khoury, D., Fayjaloun, S., Nassar, M., Sahakian, J., and Aad, P. Y. (2019). Updates on the effect of mycotoxins on male reproductive efficiency in mammals. Toxins 11:515. doi: 10.3390/toxins11090515
Fang, X., Chen, C., Cai, J., Xiang, E., Li, J., and Chen, P. (2019). Genome-wide methylation study of whole blood cells DNA in men with congenital hypopituitarism disease. Int. J. Mol. Med. 43, 155–166. doi: 10.3892/ijmm.2018.3945
Fend-Guella, D. L., Von Kopylow, K., Spiess, A. N., Schulze, W., Salzbrunn, A., Diederich, S., et al. (2019). The DNA methylation profile of human spermatogonia at single-cell- and single-allele-resolution refutes its role in spermatogonial stem cell function and germ cell differentiation. Mol. Hum. Reprod. 25, 283–294. doi: 10.1093/molehr/gaz017
Ferfouri, F., Boitrelle, F., Ghout, I., Albert, M., Molina Gomes, D., Wainer, R., et al. (2013). A genome-wide DNA methylation study in azoospermia. Andrology 1, 815–821. doi: 10.1111/j.2047-2927.2013.00117.x
Ferlin, A., and Foresta, C. (2014). New genetic markers for male infertility. Curr. Opin. Obstet. Gynecol. 3, 193–198. doi: 10.1097/GCO.0000000000000061
Födinger, M., Hörl, W. H., and Sunder-Plassmann, G. (2000). Molecular biology of 5,10-methylenetetrahydrofolate reductase. J. Nephrol. 13, 20–33.
Fragou, D., Pakkidi, E., Aschner, M., Samanidou, V., and Kovatsi, L. (2019). Smoking and DNA methylation: correlation of methylation with smoking behavior and association with diseases and fetus development following prenatal exposure. Food Chem. Toxicol. 129, 312–327. doi: 10.1016/j.fct.2019.04.059
Franzago, M., La Rovere, M., Franchi, P. G., Vitacolonna, E., and Stuppia, L. (2019). Epigenetics and human reproduction: the primary prevention of the noncommunicable diseases. Epigenomics 11, 1441–1460. doi: 10.2217/epi-2019-0163
Gao, Z., Moorjani, P., Sasani, T. A., Pedersen, B. S., Quinlan, A. R., Jorde, L. B., et al. (2019). Overlooked roles of DNA damage and maternal age in generating human germline mutations. Proc. Natl. Acad. Sci. U. S. A. 116, 9491–9500. doi: 10.1073/pnas.1901259116
Ge, Z. J., Liang, Q. X., Hou, Y., Han, Z. M., Schatten, H., Sun, Q. Y., et al. (2014). Maternal obesity and diabetes may cause DNA methylation alteration in the spermatozoa of offspring in mice. Reprod. Biol. Endocrinol. 12:29. doi: 10.1186/1477-7827-12-29
Giacone, F., Cannarella, R., Mongioì, L. M., Alamo, A., Condorelli, R. A., Calogero, A. E., et al. (2019). Epigenetics of male fertility: effects on assisted reproductive techniques. World J. Mens Health 37, 148–156. doi: 10.5534/wjmh.180071
Gkountela, S., Zhang, K. X., Shafiq, T. A., Liao, W. W., Hargan-Calvopiña, J., Chen, P. Y., et al. (2015). DNA demethylation dynamics in the human prenatal germline. Cell 161, 1425–1436. doi: 10.1016/j.cell.2015.05.012
Greenberg, M. V. C., and Bourc’his, D. (2019). The diverse roles of DNA methylation in mammalian development and disease. Nat. Rev. Mol. Cell Biol. 20, 590–607. doi: 10.1038/s41580-019-0159-6
Grossniklaus, U., Kelly, B., Ferguson-Smith, A. C., Pembrey, M., and Lindquist, S. (2013). Transgenerational epigenetic inheritance: how important is it? Nat. Rev. Genet. 14, 228–235. doi: 10.1038/nrg3435
Gunes, S., Agarwal, A., Henkel, R., Mahmutoglu, A. M., Sharma, R., Esteves, S. C., et al. (2018a). Association between promoter methylation of MLH1 and MSH2 and reactive oxygen species in oligozoospermic men—a pilot study. Andrologia 50:e12903. doi: 10.1111/and.12903
Gunes, S., Al-Sadaan, M., and Agarwal, A. (2015). Spermatogenesis, DNA damage and DNA repair mechanisms in male infertility. Reprod. Biomed. Online 31, 309–319. doi: 10.1016/j.rbmo.2015.06.010
Gunes, S., Arslan, M. A., Hekim, G. N. T., and Asci, R. (2016). The role of epigenetics in idiopathic male infertility. J. Assist. Reprod. Genet. 33, 553–569. doi: 10.1007/s10815-016-0682-8
Gunes, S., Kablan, A., Agarwal, A., and Henkel, R. (2018b). “Epigenetics, spermatogenesis, and male infertility,” in Reproductomics: The -Omics Revolution and Its Impact on Human Reproductive Medicine, eds J. A. Horcajadas and J. Gosálvez (Cambridge, MA: Academic Press), 171–187. doi: 10.1016/B978-0-12-812571-7.00011-3
Guo, G., Chmielecki, J., Goparaju, C., Heguy, A., Dolgalev, I., Carbone, M., et al. (2015). Whole-exome sequencing reveals frequent genetic alterations in BAP1, NF2, CDKN2A, and CUL1 in malignant pleural mesothelioma. Cancer Res. 75, 264–269. doi: 10.1158/0008-5472.CAN-14-1008
Gupta, N., Gupta, S., Dama, M., David, A., Khanna, G., Khanna, A., et al. (2011). Strong association of 677 C>T substitution in the MTHFR gene with male infertility – a study on an Indian population and a meta-analysis. PLoS One 6:e22277. doi: 10.1371/journal.pone.0022277
Habib, W. A., Brioude, F., Azzi, S., Rossignol, S., Linglart, A., Sobrier, M. L., et al. (2019). Transcriptional profiling at the DLK1/MEG3 domain explains clinical overlap between imprinting disorders. Sci. Adv. 5:9425. doi: 10.1126/sciadv.aau9425
Haggarty, P. (2015). Genetic and metabolic determinants of human epigenetic variation. Curr. Opin. Clin. Nutr. Metab. Care 18, 334–338. doi: 10.1097/MCO.0000000000000194
Hamad, M. F., Dayyih, W. A. A., Laqqan, M., AlKhaled, Y., Montenarh, M., and Hammadeh, M. E. (2018). The status of global DNA methylation in the spermatozoa of smokers and non-smokers. Reprod. Biomed. Online 37, 581–589. doi: 10.1016/j.rbmo.2018.08.016
Hammoud, S. S., Purwar, J., Pflueger, C., Cairns, B. R., and Carrell, D. T. (2010). Alterations in sperm DNA methylation patterns at imprinted loci in two classes of infertility. Fertil. Steril. 94, 1728–1733. doi: 10.1016/j.fertnstert.2009.09.010
Han, F., Jiang, X., Li, Z., Zhuang, X., Zhang, X., Ouyang, W., et al. (2020). Epigenetic inactivation of SOX30 is associated with male infertility and offers a therapy target for non-obstructive azoospermia. Mol. Ther. Nucleic Acids 6, 72–83. doi: 10.1016/j.omtn.2019.10.038
Hanna, C. W., Demond, H., and Kelsey, G. (2018). Epigenetic regulation in development: is the mouse a good model for the human? Hum. Reprod. Update 24, 556–576. doi: 10.1093/humupd/dmy021
Hartmann, S., Bergmann, M., Bohle, R. M., Weidner, W., and Steger, K. (2006). Genetic imprinting during impaired spermatogenesis. Mol. Hum. Reprod. 12, 407–411. doi: 10.1093/molehr/gal040
Hattori, H., Hiura, H., Kitamura, A., Miyauchi, N., Kobayashi, N., Takahashi, S., et al. (2019). Association of four imprinting disorders and ART. Clin. Epigenetics 11:21. doi: 10.1186/s13148-019-0623-3
Hekim, N., Gunes, S., Asci, R., Henkel, R., and Abur, U. (2021). Semiquantitative promoter methylation of MLH1 and MSH2 genes and their impact on sperm DNA fragmentation and chromatin condensation in infertile men. Andrologia 53:e13827. doi: 10.1111/and.13827
Heyn, H., Ferreira, H. J., Bassas, L., Bonache, S., Sayols, S., Sandoval, J., et al. (2012). Epigenetic disruption of the PIWI pathway in human spermatogenic disorders. PLoS One 6, 1–18. doi: 10.1371/journal.pone.0047892
Higashimoto, K., Urano, T., Sugiura, K., Yatsuki, H., Joh, K., Zhao, W., et al. (2003). Loss of CpG methylation is strongly correlated with loss of histone H3 Lysine 9 methylation at DMR-LIT1 in patients with beckwith-wiedemann syndrome. Am. J. Hum. Genet. 73, 948–956. doi: 10.1086/378595
Hoek, J., Steegers-Theunissen, R. P. M., Willemsen, S. P., and Schoenmakers, S. (2020). Paternal folate status and sperm quality, pregnancy outcomes, and epigenetics: a systematic review and meta-analysis. Mol. Nutr. Food Res. 64:e1900696. doi: 10.1002/mnfr.201900696
Houshdaran, S., Cortessis, V. K., Siegmund, K., Yang, A., Laird, P. W., and Sokol, R. Z. (2007). Widespread epigenetic abnormalities suggest a broad DNA methylation erasure defect in abnormal human sperm. PLoS One 2:e1289. doi: 10.1371/journal.pone.0001289
Huang, G., Liu, L., Wang, H., Gou, M., Gong, P., Tian, C., et al. (2020). Tet1 deficiency leads to premature reproductive aging by reducing spermatogonia stem cells and germ cell differentiation. iScience 23:100908. doi: 10.1016/j.isci.2020.100908
Huang, Y., Liu, H., Du, H., Zhang, W., Kang, X., Luo, Y., et al. (2019). Developmental features of DNA methylation in CpG islands of human gametes and preimplantation embryos. Exp. Ther. Med. 4447–4456. doi: 10.3892/etm.2019.7523
Ilacqua, A., Izzo, G., Pietro Emerenziani, G., Baldari, C., and Aversa, A. (2018). Lifestyle and fertility: the influence of stress and quality of life on male fertility. Reprod. Biol. Endocrinol. 16:115. doi: 10.1186/s12958-018-0436-9
Jarred, E. G., Bildsoe, H., and Western, P. S. (2018). Out of sight, out of mind? Germ cells and the potential impacts of epigenomic drugs [version 1; referees: 3 approved]. F1000Res. 7:F1000. doi: 10.12688/f1000research.15935.1
Jenkins, T. G., Aston, K. I., Hotaling, J. M., Shamsi, M. B., Simon, L., and Carrell, D. T. (2016a). Teratozoospermia and asthenozoospermia are associated with specific epigenetic signatures. Andrology 4, 843–849. doi: 10.1111/andr.12231
Jenkins, T. G., Aston, K. I., James, E. R., and Carrell, D. T. (2017). Sperm epigenetics in the study of male fertility, offspring health, and potential clinical applications. Syst. Biol. Reprod. Med. 63, 69–76. doi: 10.1080/19396368.2016.1274791
Jenkins, T. G., Aston, K. I., Meyer, T. D., Hotaling, J. M., Shamsi, M. B., Johnstone, E. B., et al. (2016b). Decreased fecundity and sperm DNA methylation patterns. Fertil. Steril. 105, 51–57. doi: 10.1016/j.fertnstert.2015.09.013
Jenkins, T. G., Aston, K. I., Trost, C., Farley, J., Hotaling, J. M., and Carrell, D. T. (2014). Intra-sample heterogeneity of sperm DNA methylation. Mol. Hum. Reprod. 21, 313–319. doi: 10.1093/molehr/gau115
Jenkins, T. G., and Carrell, D. T. (2011). The paternal epigenome and embryogenesis: poising mechanisms for development. Asian J. Androl. 13, 76–80. doi: 10.1038/aja.2010.61
Jin, C., Qin, T., Barton, M. C., Jelinek, J., and Issa, J. P. J. (2015). Minimal role of base excision repair in TET-induced global DNA demethylation in HEK293T cells. Epigenetics 10, 1006–1013. doi: 10.1080/15592294.2015.1091145
Johnson, A. A., Akman, K., Calimport, S. R. G., Wuttke, D., Stolzing, A., and de Magalhães, J. P. (2012). The role of DNA methylation in aging, rejuvenation, and age-related disease. Rejuvenation Res. 15, 483–494. doi: 10.1089/rej.2012.1324
Kabartan, E., Gunes, S., Arslan, M. A., and Asci, R. (2019). Investigating the relationship between BRCA1 and BRCA2 genes methylation profile and sperm DNA fragmentation in infertile men. Andrologia 51:e13308. doi: 10.1111/and.13308
Karaca, M. Z., Konac, E., Yurteri, B., Bozdag, G., Sogutdelen, E., and Bilen, C. Y. (2017). Association between methylenetetrahydrofolate reductase (MTHFR) gene promoter hypermethylation and the risk of idiopathic male infertility. Andrologia 49:e12698. doi: 10.1111/and.12698
Keyhan, S., Burke, E., Schrott, R., Huang, Z., Grenier, C., Price, T., et al. (2021). Male obesity impacts DNA methylation reprogramming in sperm. Clin. Epigenetics 13:17. doi: 10.1186/s13148-020-00997-0
Khambata, K., Raut, S., Deshpande, S., Mohan, S., Sonawane, S., Gaonkar, R., et al. (2021). DNA methylation defects in spermatozoa of male partners from couples experiencing recurrent pregnancy loss. Hum. Reprod. Oxf. Engl. 36, 48–60. doi: 10.1093/humrep/deaa278
Khazamipour, N., Noruzinia, M., Fatehmanesh, P., Keyhanee, M., and Pujol, P. (2009). MTHFR promoter hypermethylation in testicular biopsies of patients with non-obstructive azoospermia: the role of epigenetics in male infertility. Hum. Reprod. 24, 2361–2364. doi: 10.1093/humrep/dep194
Kitamura, A., Miyauchi, N., Hamada, H., Hiura, H., Chiba, H., Okae, H., et al. (2015). Epigenetic alterations in sperm associated with male infertility. Congenit. Anom. 55, 133–144. doi: 10.1111/cga.12113
Kläver, R., and Gromoll, J. (2014). Bringing epigenetics into the diagnostics of the andrology laboratory: challenges and perspectives. Asian J. Androl. 16, 669–674. doi: 10.4103/1008-682X.125412
Kläver, R., Tüttelmann, F., Bleiziffer, A., Haaf, T., Kliesch, S., and Gromoll, J. (2013). DNA methylation in spermatozoa as a prospective marker in andrology. Andrology 1, 731–740. doi: 10.1111/j.2047-2927.2013.00118.x
Kobayashi, H., Sato, A., Otsu, E., Hiura, H., Tomatsu, C., Utsunomiya, T., et al. (2007). Aberrant DNA methylation of imprinted loci in sperm from oligospermic patients. Hum. Mol. Genet. 16, 2542–2551. doi: 10.1093/hmg/ddm187
Kovac, J. R., Khanna, A., and Lipshultz, L. I. (2015). The effects of cigarette smoking on male fertility. Postgrad. Med. 127, 338–341. doi: 10.1080/00325481.2015.1015928
Krausz, C. (2011). Male infertility: pathogenesis and clinical diagnosis. Best Pract. Res. Clin. Endocrinol. Metab. 127, 271–285. doi: 10.1016/j.beem.2010.08.006
Kuhtz, J., Schneider, E., El Hajj, N., Zimmermann, L., Fust, O., Linek, B., et al. (2014). Epigenetic heterogeneity of developmentally important genes in human sperm: implications for assisted reproduction outcome. Epigenetics 9, 1648–1658. doi: 10.4161/15592294.2014.988063
Kulac, T., Hekim, N., Kocamanoglu, F., Beyaz, C., Gunes, S., and Asci, R. (2021). Methylation patterns of methylenetetrahydrofolate reductase gene promoter in infertile males. Andrologia 53:e13942. doi: 10.1111/and.13942
Kuramochi-Miyagawa, S., Watanabe, T., Gotoh, K., Totoki, Y., Toyoda, A., Ikawa, M., et al. (2008). DNA methylation of retrotransposon genes is regulated by Piwi family members MILI and MIWI2 in murine fetal testes. Genes Dev. 22, 908–917. doi: 10.1101/gad.1640708
Lambrot, R., Xu, C., Saint-Phar, S., Chountalos, G., Cohen, T., Paquet, M., et al. (2013). Low paternal dietary folate alters the mouse sperm epigenome and is associated with negative pregnancy outcomes. Nat. Commun. 4:2889. doi: 10.1038/ncomms3889
Lanzillotti, C., De Mattei, M., Mazziotta, C., Taraballi, F., Rotondo, J. C., Tognon, M., et al. (2021). Long non-coding RNAs and microRNAs interplay in osteogenic differentiation of mesenchymal stem cells. Front. Cell Dev. Biol. 9:646032. doi: 10.3389/fcell.2021.646032
Laqqan, M., Tierling, S., Alkhaled, Y., Lo Porto, C., Solomayer, E. F., and Hammadeh, M. (2017a). Spermatozoa from males with reduced fecundity exhibit differential DNA methylation patterns. Andrology 5, 971–978. doi: 10.1111/andr.12362
Laqqan, M., Tierling, S., Alkhaled, Y., Lo Porto, C., Solomayer, E. F., and Hammadeh, M. E. (2017b). Aberrant DNA methylation patterns of human spermatozoa in current smoker males. Reprod. Toxicol. 71, 126–133. doi: 10.1016/j.reprotox.2017.05.010
Laurentino, S., Beygo, J., Nordhoff, V., Kliesch, S., Wistuba, J., Borgmann, J., et al. (2015). Epigenetic germline mosaicism in infertile men. Hum. Mol. Genet. 24, 1295–1304. doi: 10.1093/hmg/ddu540
Laurentino, S., Borgmann, J., and Gromoll, J. (2016). On the origin of sperm epigenetic heterogeneity. Reproduction 151, 71–78. doi: 10.1530/REP-15-0436
Laurentino, S., Heckmann, L., Di Persio, S., Li, X., Zu Hörste, G. M., Wistuba, J., et al. (2019). High-resolution analysis of germ cells from men with sex chromosomal aneuploidies reveals normal transcriptome but impaired imprinting. Clin. Epigenetics 11:127.
Lee, S. W., Hwang, H. H., Hsu, P. W. C., Chuang, T. Y., Liu, C. W., and Wu, L. S. H. (2019). Whole-genome methylation profiling from PBMCs in acute-exacerbation COPD patients with good and poor responses to corticosteroid treatment. Genomics 111, 1381–1386. doi: 10.1016/j.ygeno.2018.09.010
Leitão, E., Di Persio, S., Laurentino, S., Wöste, M., Dugas, M., Kliesch, S., et al. (2020). The sperm epigenome does not display recurrent epimutations in patients with severely impaired spermatogenesis. Clin. Epigenetics 12:61. doi: 10.1186/s13148-020-00854-0
Lévesque, N., Leclerc, D., Gayden, T., Lazaris, A., De Jay, N., Petrillo, S., et al. (2016). Murine diet/tissue and human brain tumorigenesis alter Mthfr/MTHFR 5′-end methylation. Mamm. Genome 27, 122–134. doi: 10.1007/s00335-016-9624-0
Li, E. (2002). Chromatin modification and epigenetic reprogramming in mammalian development. Nat. Rev. Genet. 3, 662–673. doi: 10.1038/nrg887
Li, X. P., Hao, C. L., Wang, Q., Yi, X. M., and Jiang, Z. S. (2016). H19 gene methylation status is associated with male infertility. Exp. Ther. Med. 12, 451–456. doi: 10.3892/etm.2016.3314
Li, Z., Chen, S., Yang, Y., Zhuang, X., and Tzeng, C. M. (2018). Novel biomarker ZCCHC13 revealed by integrating DNA methylation and mRNA expression data in non-obstructive azoospermia. Cell Death Discov. 4, doi: 10.1038/s41420-018-0033-x
Li, Z., Fang, F., Zhao, Q., Li, H., and Xiong, C. (2019). Supplementation of vitamin C promotes early germ cell specification from human embryonic stem cells. Stem Cell Res. Ther. 10:324. doi: 10.1186/s13287-019-1427-2
Li, Z., Zhuang, X., Zeng, J., and Tzeng, C. M. (2017). Integrated analysis of DNA methylation and mRNA expression profiles to identify key genes in severe oligozoospermia. Front. Physiol. 8:261. doi: 10.3389/fphys.2017.00261
Lobo, J., Nunes, S. P., Gillis, A. J. M., Barros-Silva, D., Miranda-Gonçalves, V., van den Berg, A., et al. (2019). XIST-promoter demethylation as tissue biomarker for testicular germ cell tumors and spermatogenesis quality. Cancers 11:1385. doi: 10.3390/cancers11091385
Lopomo, A., Ricciardi, R., Maestri, M., Rosa, A., Melfi, F., Lucchi, M., et al. (2016). Gene-specific methylation analysis in thymomas of patients with myasthenia gravis. Int. J. Mol. Sci. 17:2121. doi: 10.3390/ijms17122121
Lou, H., Le, F., Hu, M., Yang, X., Li, L., Wang, L., et al. (2019). Aberrant DNA methylation of IGF2-H19 locus in human fetus and in spermatozoa from assisted reproductive technologies. Reprod. Sci. 26, 997–1004. doi: 10.1177/1933719118802052
Louie, K., Minor, A., Ng, R., Poon, K., Chow, V., and Ma, S. (2016). Evaluation of DNA methylation at imprinted DMRs in the spermatozoa of oligozoospermic men in association with MTHFR C677T genotype. Andrology 4, 825–831. doi: 10.1111/andr.12240
Luján, S., Caroppo, E., Niederberger, C., Arce, J. C., Sadler-Riggleman, I., Beck, D., et al. (2019). Sperm DNA methylation epimutation biomarkers for male infertility and FSH therapeutic responsiveness. Sci. Rep. 14:16786. doi: 10.1038/s41598-019-52903-1
Marcho, C., Oluwayiose, O. A., and Pilsner, J. R. (2019). The preconception environment and sperm epigenetics. Andrology 8, 924–942. doi: 10.1111/andr.12753
Marques, C. J., Carvalho, F., Sousa, M., and Barros, A. (2004). Genomic imprinting in disruptive spermatogenesis. Lancet 363, 1700–1702. doi: 10.1016/S0140-6736(04)16256-9
Marques, C. J., Costa, P., Vaz, B., Carvalho, F., Fernandes, S., Barros, A., et al. (2008). Abnormal methylation of imprinted genes in human sperm is associated with oligozoospermia. Mol. Hum. Reprod. 14, 67–74. doi: 10.1093/molehr/gam093
Marques, C. J., Francisco, T., Sousa, S., Carvalho, F., Barros, A., and Sousa, M. (2010). Methylation defects of imprinted genes in human testicular spermatozoa. Fertil. Steril. 94, 585–594. doi: 10.1016/j.fertnstert.2009.02.051
Marques, P. I., Fernandes, S., Carvalho, F., Barros, A., Sousa, M., and Marques, C. J. (2017). DNA methylation imprinting errors in spermatogenic cells from maturation arrest azoospermic patients. Andrology 5, 451–459. doi: 10.1111/andr.12329
Mayer, W., Hemberger, M., Frank, H. G., Grümmer, R., Winterhager, E., Kaufmann, P., et al. (2000). Expression of the imprinted genes MEST/Mest in human and murine placenta suggests a role in angiogenesis. Dev. Dyn. 217, 1–10. doi: 10.1002/(SICI)1097-0177(200001)217:1<1::AID-DVDY1<3.0.CO;2-4
Mazzoni, E., Pellegrinelli, E., Mazziotta, C., Lanzillotti, C., Rotondo, J. C., Bononi, I., et al. (2020). Mother-to-child transmission of oncogenic polyomaviruses BKPyV, JCPyV and SV40. J. Infect. 80, 563–570. doi: 10.1016/j.jinf.2020.02.006
McSwiggin, H. M., and O’Doherty, A. M. (2018). Epigenetic reprogramming during spermatogenesis and male factor infertility. Reproduction 156, 9–21. doi: 10.1530/REP-18-0009
Melamed, P., Yosefzon, Y., David, C., Tsukerman, A., and Pnueli, L. (2018). Tet enzymes, variants, and differential effects on function. Front. Cell Dev. Biol. 6:22. doi: 10.3389/fcell.2018.00022
Metin Mahmutoglu, A., Gunes, S., Asci, R., Henkel, R., and Aydin, O. (2021). Association of XRCC1 and ERCC2 promoters’ methylation with chromatin condensation and sperm DNA fragmentation in idiopathic oligoasthenoteratozoospermic men. Andrologia 53:e13925. doi: 10.1111/and.13925
Miller, D., Brinkworth, M., and Iles, D. (2009). Paternal DNA packaging in spermatozoa: more than the sum of its parts? Reprod. Camb. Engl. 139, 287–301. doi: 10.1530/REP-09-0281
Minor, A., Chow, V., and Ma, S. (2011). Aberrant DNA methylation at imprinted genes in testicular sperm retrieved from men with obstructive azoospermia and undergoing vasectomy reversal. Reproduction 141, 749–757. doi: 10.1530/REP-11-0008
Mishra, J., Talwar, S., Kaur, L., Chandiok, K., Yadav, S., Puri, M., et al. (2019). Differential global and MTHFR gene specific methylation patterns in preeclampsia and recurrent miscarriages: a case-control study from North India. Gene 704, 68–73. doi: 10.1016/j.gene.2019.04.036
Monk, M., Boubelik, M., and Lehnert, S. (1987). Temporal and regional changes in DNA methylation in the embryonic, extraembryonic and germ cell lineages during mouse embryo development. Development 99, 371–382.
Montjean, D., Benkhalifa, M., Dessolle, L., Cohen-Bacrie, P., Belloc, S., Siffroi, J. P., et al. (2011). Polymorphisms in MTHFR and MTRR genes associated with blood plasma homocysteine concentration and sperm counts. Fertil. Steril. 95, 635–640. doi: 10.1016/j.fertnstert.2010.08.054
Montjean, D., Ravel, C., Benkhalifa, M., Cohen-Bacrie, P., Berthaut, I., Bashamboo, A., et al. (2013). Methylation changes in mature sperm deoxyribonucleic acid from oligozoospermic men: assessment of genetic variants and assisted reproductive technology outcome. Fertil. Steril. 100, 1241–1247. doi: 10.1016/j.fertnstert.2013.06.047
Moore, L. D., Le, T., and Fan, G. (2013). DNA methylation and its basic function. Neuropsychopharmacology 38, 23–38. doi: 10.1038/npp.2012.112
Murphy, L. E., Mills, J. L., Molloy, A. M., Qian, C., Carter, T. C., Strevens, H., et al. (2011). Folate and vitamin B12 in idiopathic male infertility. Asian J. Androl. 13, 856–861. doi: 10.1038/aja.2011.96
Murphy, S. K., Itchon-Ramos, N., Visco, Z., Huang, Z., Grenier, C., Schrott, R., et al. (2018). Cannabinoid exposure and altered DNA methylation in rat and human sperm. Epigenetics 13, 1559–1230. doi: 10.1080/15592294.2018.1554521
Nagamori, I., Kobayashi, H., Nishimura, T., Yamagishi, R., Katahira, J., Kuramochi-Miyagawa, S., et al. (2018). Relationship between PIWIL4-mediated H3K4me2 demethylation and piRNA-dependent DNA methylation. Cell Rep. 25, 350–356. doi: 10.1016/j.celrep.2018.09.017
Nanassy, L., and Carrell, D. T. (2011). Abnormal methylation of the promoter of CREM is broadly associated with male factor infertility and poor sperm quality but is improved in sperm selected by density gradient centrifugation. Fertil. Steril. 95, 2310–2314. doi: 10.1016/j.fertnstert.2011.03.096
Nasri, F., Gharesi-Fard, B., Namavar Jahromi, B., Farazi-fard, M. A., Banaei, M., Davari, M., et al. (2017). Sperm DNA methylation of H19 imprinted gene and male infertility. Andrologia 49:e12766. doi: 10.1111/and.12766
Navarro-Costa, P., Nogueira, P., Carvalho, M., Leal, F., Cordeiro, I., Calhaz-Jorge, C., et al. (2010). Incorrect DNA methylation of the DAZL promoter CpG island associates with defective human sperm. Hum. Reprod. Oxf. Engl. 25, 2647–2654. doi: 10.1093/humrep/deq200
Nettersheim, D., Heukamp, L. C., Fronhoffs, F., Grewe, M. J., Haas, N., Waha, A., et al. (2013). Analysis of TET expression/activity and 5mC oxidation during normal and malignant germ cell development. PLoS One 8:e82881. doi: 10.1371/journal.pone.0082881
Ni, K., Dansranjavin, T., Rogenhofer, N., Oeztuerk, N., Deuker, J., Bergmann, M., et al. (2016). TET enzymes are successively expressed during human spermatogenesis and their expression level is pivotal for male fertility. Hum. Reprod. 31, 1411–1424. doi: 10.1093/humrep/dew096
Ni, W., Pan, C., Pan, Q., Fei, Q., Huang, X., and Zhang, C. (2019). Methylation levels of IGF2 and KCNQ1 in spermatozoa from infertile men are associated with sperm DNA damage. Andrologia 51:e13239. doi: 10.1111/and.13239
Nieto, S. J., and Kosten, T. A. (2019). Who’s your daddy? Behavioral and epigenetic consequences of paternal drug exposure. Int. J. Dev. Neurosci. 78, 109–121. doi: 10.1016/j.ijdevneu.2019.07.002
Oton-Gonzalez, L., Rotondo, J. C., Cerritelli, L., Malagutti, N., Lanzillotti, C., Bononi, I., et al. (2021). Association between oncogenic human papillomavirus type 16 and Killian polyp. Infect. Agent. Cancer 16:3. doi: 10.1186/s13027-020-00342-3
Ou, X. H., Zhu, C. C., and Sun, S. C. (2019). Effects of obesity and diabetes on the epigenetic modification of mammalian gametes. J. Cell. Physiol. 234, 7847–7855. doi: 10.1002/jcp.27847
Park, E., Gong, E.-Y., Romanelli, M. G., and Lee, K. (2012). Suppression of estrogen receptor-alpha transactivation by thyroid transcription factor-2 in breast cancer cells. Biochem. Biophys. Res. Commun. 421, 532–537. doi: 10.1016/j.bbrc.2012.04.039
Parry, L., and Clarke, A. R. (2011). The roles of the methyl-CpG binding proteins in cancer. Genes Cancer 2, 618–630. doi: 10.1177/1947601911418499
Patel, D. P., Jenkins, T. G., Aston, K. I., Guo, J., Pastuszak, A. W., Hanson, H. A., et al. (2020). Harnessing the full potential of reproductive genetics and epigenetics for male infertility in the era of “big data.” Fertil. Steril. 113, 478–488. doi: 10.1016/j.fertnstert.2020.01.001
Peng, H., Zhao, P., Liu, J., Zhang, J., Zhang, J., Wang, Y., et al. (2018). Novel epigenomic biomarkers of male infertility identified by methylation patterns of CpG sites within imprinting control regions of H19 and SNRPN genes. OMICS J. Integr. Biol. 22, 354–364. doi: 10.1089/omi.2018.0019
Pinborg, A., Loft, A., Romundstad, L. B., Wennerholm, U. B., Söderström-Anttila, V., Bergh, C., et al. (2016). Epigenetics and assisted reproductive technologies. Acta Obstet. Gynecol. Scand. 15, 12–25. doi: 10.1111/aogs.12799
Pinto-Medel, M. J., Oliver-Martos, B., Urbaneja-Romero, P., Hurtado-Guerrero, I., Ortega-Pinazo, J., Serrano-Castro, P., et al. (2017). Global methylation correlates with clinical status in multiple sclerosis patients in the first year of IFNbeta treatment. Sci. Rep. 7:8727. doi: 10.1038/s41598-017-09301-2
Pisarska, M. D., Chan, J. L., Lawrenson, K., Gonzalez, T. L., and Wang, E. T. (2019). Genetics and epigenetics of infertility and treatments on outcomes. J. Clin. Endocrinol. Metab. 104, 1871–1886. doi: 10.1210/jc.2018-01869
Pohl, E., Gromoll, J., Wistuba, J., and Laurentino, S. (2021). Healthy ageing and spermatogenesis. Reprod. Camb. Engl. 161, R89–R101. doi: 10.1530/REP-20-0633
Poorang, S., Abdollahi, S., Anvar, Z., Tabei, S. M. B., Jahromi, B. N., Moein-Vaziri, N., et al. (2018). The impact of methylenetetrahydrofolate reductase (MTHFR) sperm methylation and variants on semen parameters and the chance of recurrent pregnancy loss in the couple. Clin. Lab. 67, 1121–1128. doi: 10.7754/Clin.Lab.2018.171231
Poplinski, A., Tüttelmann, F., Kanber, D., Horsthemke, B., and Gromoll, J. (2010). Idiopathic male infertility is strongly associated with aberrant methylation of MEST and IGF2/H19 ICR1. Int. J. Androl. 33, 642–649. doi: 10.1111/j.1365-2605.2009.01000.x
Preti, M., Rotondo, J. C., Holzinger, D., Micheletti, L., Gallio, N., McKay-Chopin, S., et al. (2020). Role of human papillomavirus infection in the etiology of vulvar cancer in Italian women. Infect. Agent. Cancer 15:20. doi: 10.1186/s13027-020-00286-8
Rando, O. J., and Simmons, R. A. (2015). I’m eating for two: parental dietary effects on offspring metabolism. Cell 161, 93–105. doi: 10.1016/j.cell.2015.02.021
Reece, A. S., and Hulse, G. K. (2019). Impacts of cannabinoid epigenetics on human development: reflections on Murphy et al. ‘cannabinoid exposure and altered DNA methylation in rat and human sperm’ epigenetics 2018; 13: 1208-1221. Epigenetics 14, 1041–1056. doi: 10.1080/15592294.2019.1633868
Reik, W., and Walter, J. (2001). Genomic imprinting: parental influence on the genome. Nat. Rev. Genet. 2, 21–32. doi: 10.1038/35047554
Richardson, M. E., Bleiziffer, A., Tüttelmann, F., Gromoll, J., and Wilkinson, M. F. (2014). Epigenetic regulation of the RHOX homeobox gene cluster and its association with human male infertility. Hum. Mol. Genet. 23, 12–23. doi: 10.1093/hmg/ddt392
Rose, N. R., and Klose, R. J. (2014). Understanding the relationship between DNA methylation and histone lysine methylation. Biochim. Biophys. Acta Gene Regul. Mech. 1839, 1362–1372. doi: 10.1016/j.bbagrm.2014.02.007
Rotondo, J. C., Aquila, G., Oton-Gonzalez, L., Selvatici, R., Rizzo, P., De Mattei, M., et al. (2021). Methylation of SERPINA1 gene promoter may predict chronic obstructive pulmonary disease in patients affected by acute coronary syndrome. Clin. Epigenetics 13:79. doi: 10.1186/s13148-021-01066-w
Rotondo, J. C., Borghi, A., Selvatici, R., Magri, E., Bianchini, E., Montinari, E., et al. (2016). Hypermethylation-induced inactivation of the IRF6 gene as a possible early event in progression of vulvar squamous cell carcinoma associated with lichen sclerosus. JAMA Dermatol. 152, 928–933. doi: 10.1001/jamadermatol.2016.1336
Rotondo, J. C., Borghi, A., Selvatici, R., Mazzoni, E., Bononi, I., Corazza, M., et al. (2018a). Association of retinoic acid receptor β gene with onset and progression of lichen sclerosus-associated vulvar squamous cell carcinoma. JAMA Dermatol. 154, 819–823. doi: 10.1001/jamadermatol.2018.1373
Rotondo, J. C., Bosi, S., Bazzan, E., Di Domenico, M., De Mattei, M., Selvatici, R., et al. (2012). Methylenetetrahydrofolate reductase gene promoter hypermethylation in semen samples of infertile couples correlates with recurrent spontaneous abortion. Hum. Reprod. 27, 3632–3638. doi: 10.1093/humrep/des319
Rotondo, J. C., Giari, L., Guerranti, C., Tognon, M., Castaldelli, G., Fano, E. A., et al. (2018b). Environmental doses of perfluorooctanoic acid change the expression of genes in target tissues of common carp. Environ. Toxicol. Chem. 37, 942–948. doi: 10.1002/etc.4029
Rotondo, J. C., Oton-Gonzalez, L., Mazziotta, C., Lanzillotti, C., Iaquinta, M. R., Tognon, M., et al. (2020a). Simultaneous detection and viral DNA load quantification of different Human Papillomavirus types in clinical specimens by the high analytical droplet digital PCR method. Front. Microbiol. 11:591452. doi: 10.3389/fmicb.2020.591452 in press
Rotondo, J. C., Oton-Gonzalez, L., Selvatici, R., Rizzo, P., Pavasini, R., Campo, G. C., et al. (2020b). SERPINA1 gene promoter is differentially methylated in peripheral blood mononuclear cells of pregnant women. Front. Cell Dev. Biol. 8:550543. doi: 10.3389/fcell.2020.550543
Rotondo, J. C., Selvatici, R., Di Domenico, M., Marci, R., Vesce, F., Tognon, M., et al. (2013). Methylation loss at H19 imprinted gene correlates with methylenetetrahydrofolate reductase gene promoter hypermethylation in semen samples from infertile males. Epigenetics 8, 990–997. doi: 10.4161/epi.25798
Rousseaux, S., Caron, C., Govin, J., Lestrat, C., Faure, A. K., and Khochbin, S. (2005). Establishment of male-specific epigenetic information. Gene 345, 139–153. doi: 10.1016/j.gene.2004.12.004
Sadler-Riggleman, I., Klukovich, R., Nilsson, E., Beck, D., Xie, Y., Yan, W., et al. (2019). Epigenetic transgenerational inheritance of testis pathology and Sertoli cell epimutations: generational origins of male infertility. Environ. Epigenetics 29:dvz013. doi: 10.1093/eep/dvz013
Safarinejad, M. R., Shafiei, N., and Safarinejad, S. (2011). Relationship between genetic polymorphisms of methylenetetra-hydrofolate reductase (C677T, A1298C, and G1793A) as risk factors for idiopathic male infertility. Reprod. Sci. 18, 304–315. doi: 10.1177/1933719110385135
Santi, D., De Vincentis, S., Magnani, E., and Spaggiari, G. (2017). Impairment of sperm DNA methylation in male infertility: a meta-analytic study. Andrology 5, 695–703. doi: 10.1111/andr.12379
Santos, F., and Dean, W. (2004). Epigenetic reprogramming during early development in mammals. Reproduction 127, 643–651. doi: 10.1530/rep.1.00221
Saraswathy, K., Kaur, L., Talwar, S., Mishra, J., Huidrom, S., Sachdeva, M., et al. (2018). Methylenetetrahydrofolate reductase gene-specific methylation and recurrent miscarriages: a case-control study from North India. J. Hum. Reprod. Sci. 11, 142–147. doi: 10.4103/jhrs.JHRS_145_17
Sarkar, S., Sujit, K. M., Singh, V., Pandey, R., Trivedi, S., Singh, K., et al. (2019). Array-based DNA methylation profiling reveals peripheral blood differential methylation in male infertility. Fertil. Steril. 112, 61–72. doi: 10.1016/j.fertnstert.2019.03.020
Schrott, R., Acharya, K., Itchon-Ramos, N., Hawkey, A. B., Pippen, E., Mitchell, J. T., et al. (2020). Cannabis use is associated with potentially heritable widespread changes in autism candidate gene DLGAP2 DNA methylation in sperm. Epigenetics 15, 161–173. doi: 10.1080/15592294.2019.1656158
Schütte, B., El Hajj, N., Kuhtz, J., Nanda, I., Gromoll, J., Hahn, T., et al. (2013). Broad DNA methylation changes of spermatogenesis, inflammation and immune response-related genes in a subgroup of sperm samples for assisted reproduction. Andrology 1, 822–829. doi: 10.1111/j.2047-2927.2013.00122.x
Seisenberger, S., Andrews, S., Krueger, F., Arand, J., Walter, J., Santos, F., et al. (2012). The dynamics of genome-wide DNA methylation reprogramming in mouse primordial germ cells. Mol. Cell 48, 849–862. doi: 10.1016/j.molcel.2012.11.001
Sharma, P., Ghanghas, P., Kaushal, N., Kaur, J., and Kaur, P. (2019). Epigenetics and oxidative stress: a twin-edged sword in spermatogenesis. Andrologia 5:e13432. doi: 10.1111/and.13432
Sharp, G. C., Alfano, R., Ghantous, A., Urquiza, J., Rifas-Shiman, S. L., Page, C. M., et al. (2021). Paternal body mass index and offspring DNA methylation: findings from the PACE consortium. Int. J. Epidemiol. doi: 10.1093/ije/dyaa267
Shea, J. M., Serra, R. W., Carone, B. R., Shulha, H. P., Kucukural, A., Ziller, M. J., et al. (2015). Genetic and epigenetic variation, but not diet, shape the sperm methylome. Dev. Cell 35, 750–758. doi: 10.1016/j.devcel.2015.11.024
Shukla, K. K., Chambial, S., Dwivedi, S., Misra, S., and Sharma, P. (2014). Recent scenario of obesity and male fertility. Andrology 2, 809–819. doi: 10.1111/andr.270
Solyom, S., and Kazazian, H. H. (2012). Mobile elements in the human genome: implications for disease. Genome Med. 4:12. doi: 10.1186/gm311
Soubry, A., Murphy, S. K., Wang, F., Huang, Z., Vidal, A. C., Fuemmeler, B. F., et al. (2015). Newborns of obese parents have altered DNA methylation patterns at imprinted genes. Int. J. Obes. 39, 650–657. doi: 10.1038/ijo.2013.193
Stangler Herodež, Š, Zagradišnik, B., Erjavec Škerget, A., Zagorac, A., Takač, I., Vlaisavljević, V., et al. (2013). MTHFR C677T and A1298C genotypes and haplotypes in slovenian couples with unexplained infertility problems and in embryonic tissues from spontaneous abortions. Balkan J. Med. Genet. 16, 31–40. doi: 10.2478/bjmg-2013-0015
Stomper, J., Rotondo, J. C., Greve, G., and Lübbert, M. (2021). Hypomethylating agents (HMA) for the treatment of acute myeloid leukemia and myelodysplastic syndromes: mechanisms of resistance and novel HMA-based therapies. Leukemia doi: 10.1038/s41375-021-01218-0 [Epub ahead of print]
Stouffs, K., Seneca, S., and Lissens, W. (2014). Genetic causes of male infertility. Ann. Endocrinol. 75, 109–111. doi: 10.1016/j.ando.2014.03.004
Stuppia, L., Franzago, M., Ballerini, P., Gatta, V., and Antonucci, I. (2015). Epigenetics and male reproduction: the consequences of paternal lifestyle on fertility, embryo development, and children lifetime health. Clin. Epigenetics 7:120. doi: 10.1186/s13148-015-0155-4
Sujit, K. M., Sarkar, S., Singh, V., Pandey, R., Agrawal, N. K., Trivedi, S., et al. (2018). Genome-wide differential methylation analyses identifies methylation signatures of male infertility. Hum. Reprod. 33, 2256–2267. doi: 10.1093/humrep/dey319
Sujit, K. M., Singh, V., Trivedi, S., Singh, K., Gupta, G., and Rajender, S. (2020). Increased DNA methylation in the spermatogenesis-associated (SPATA) genes correlates with infertility. Andrology 8, 602–609. doi: 10.1111/andr.12742
Tang, Q., Chen, Y., Wu, W., Ding, H., Xia, Y., Chen, D., et al. (2017). Idiopathic male infertility and polymorphisms in the DNA methyltransferase genes involved in epigenetic marking. Sci. Rep. 7:11219. doi: 10.1038/s41598-017-11636-9
Tang, Q., Pan, F., Yang, J., Fu, Z., Lu, Y., Wu, X., et al. (2018). Idiopathic male infertility is strongly associated with aberrant DNA methylation of imprinted loci in sperm: a case-control study. Clin. Epigenetics 10:134. doi: 10.1186/s13148-018-0568-y
Tang, W. W. C., Dietmann, S., Irie, N., Leitch, H. G., Floros, V. I., Bradshaw, C. R., et al. (2015). A unique gene regulatory network resets the human germline epigenome for development. Cell 161, 1453–1467. doi: 10.1016/j.cell.2015.04.053
Tara, S. S., Ghaemimanesh, F., Zarei, S., Reihani-Sabet, F., Pahlevanzadeh, Z., Modarresi, M. H., et al. (2015). Methylenetetrahydrofolate reductase C677T and A1298C polymorphisms in male partners of recurrent miscarriage couples. J. Reprod. Infertil. 16, 193–198.
Tian, M., Bao, H., Martin, F. L., Zhang, J., Liu, L., Huang, Q., et al. (2014). Association of DNA methylation and mitochondrial DNA copy number with human semen Quality1. Biol. Reprod. 91, 1–8. doi: 10.1095/biolreprod.114.122465
Tognon, M., Luppi, M., Corallini, A., Taronna, A., Barozzi, P., Rotondo, J. C., et al. (2015). Immunologic evidence of a strong association between non-Hodgkin lymphoma and simian virus 40. Cancer 121, 2618–2626. doi: 10.1002/cncr.29404
Tognon, M., Tagliapietra, A., Magagnoli, F., Mazziotta, C., Oton-Gonzalez, L., Lanzillotti, C., et al. (2020). Investigation on spontaneous abortion and human papillomavirus infection. Vaccines 8:473.
Toschi, P., Capra, E., Anzalone, D. A., Lazzari, B., Turri, F., Pizzi, F., et al. (2020). Maternal peri-conceptional undernourishment perturbs offspring sperm methylome. Reprod. Camb. Engl. 159, 513–523. doi: 10.1530/REP-19-0549
Ullah, N., Mansoor, A., Micheal, S., Mirza, B., Qamar, R., Mazhar, K., et al. (2019). MTHFR polymorphisms as risk for male infertility in Pakistan and its comparison with socioeconomic status in the world. Pers. Med. 16, 35–49. doi: 10.2217/pme-2018-0045
Urdinguio, R. G., Bayón, G. F., Dmitrijeva, M., Toraño, E. G., Bravo, C., Fraga, M. F., et al. (2015). Aberrant DNA methylation patterns of spermatozoa in men with unexplained infertility. Hum. Reprod. 30, 1014–1028. doi: 10.1093/humrep/dev053
Uysal, F., Akkoyunlu, G., and Ozturk, S. (2016). DNA methyltransferases exhibit dynamic expression during spermatogenesis. Reprod. Biomed. Online 33, 690–702. doi: 10.1016/j.rbmo.2016.08.022
Uysal, F., Akkoyunlu, G., and Ozturk, S. (2019). Decreased expression of DNA methyltransferases in the testes of patients with non-obstructive azoospermia leads to changes in global DNA methylation levels. Reprod. Fertil. Dev. doi: 10.1071/rd18246 [Epub ahead of print]
Valinluck, V., and Sowers, L. C. (2007). Endogenous cytosine damage products alter the site selectivity of human DNA maintenance methyltransferase DNMT1. Cancer Res. 67, 946–950. doi: 10.1158/0008-5472.CAN-06-3123
Vladoiu, S., Botezatu, A., Anton, G., Manda, D., Paun, D. L., Oros, S., et al. (2017). The involvement of vdr promoter methylation, CDX-2 VDR polymorphism and vitamin d levels in male infertility. Acta Endocrinol. (Copenh.) 13, 294–301. doi: 10.4183/aeb.2017.294
Von Meyenn, F., and Reik, W. (2015). Forget the parents: epigenetic reprogramming in human germ cells. Cell 161, 1248–1251. doi: 10.1016/j.cell.2015.05.039
Wasserzug-Pash, P., and Klutstein, M. (2019). Epigenetic changes in mammalian gametes throughout their lifetime: the four seasons metaphor. Chromosoma 128, 423–441. doi: 10.1007/s00412-019-00704-w
Whitelaw, E. (2015). Sperm DNA methylation: not a vehicle for dietary reprogramming of offspring? Dev. Cell 25, 668–669. doi: 10.1016/j.devcel.2015.12.005
Williams, K., Christensen, J., and Helin, K. (2012). DNA methylation: TET proteins-guardians of CpG islands? EMBO Rep. 13, 28–35. doi: 10.1038/embor.2011.233
Wu, C., Ding, X., Li, H., Zhu, C., and Xiong, C. (2013). Genome-wide promoter methylation profile of human testis and epididymis: identified from cell-free seminal DNA. BMC Genomics 14:288. doi: 10.1186/1471-2164-14-288
Wu, W., Shen, O., Qin, Y., Niu, X., Lu, C., Xia, Y., et al. (2010). Idiopathic male infertility is strongly associated with aberrant promoter methylation of methylenetetrahydrofolate reductase (MTHFR). PLoS One 5:e13884. doi: 10.1371/journal.pone.0013884
Wu, X., Luo, C., Hu, L., Chen, X., Chen, Y., Fan, J., et al. (2020). Unraveling epigenomic abnormality in azoospermic human males by WGBS, RNA-Seq, and transcriptome profiling analyses. J. Assist. Reprod. Genet. 37, 789–802. doi: 10.1007/s10815-020-01716-7
Wyck, S., Herrera, C., Requena, C. E., Bittner, L., Hajkova, P., Bollwein, H., et al. (2018). Oxidative stress in sperm affects the epigenetic reprogramming in early embryonic development. Epigenetics Chromatin 11:60. doi: 10.1186/s13072-018-0224-y
Xu, J., Zhang, A., Zhang, Z., Wang, P., Qian, Y., He, L., et al. (2016). DNA methylation levels of imprinted and nonimprinted genes DMRs associated with defective human spermatozoa. Andrologia 48, 1027–1035. doi: 10.1111/and.12535
Xu, W., Fang, P., Zhu, Z., Dai, J., Nie, D., Chen, Z., et al. (2013). Cigarette smoking exposure alters Pebp1 DNA methylation and protein profile involved in MAPK signaling pathway in mice Testis1. Biol. Reprod. 89, 1–11. doi: 10.1095/biolreprod.113.111245
Yang, F., and Wang, P. J. (2016). Multiple LINEs of retrotransposon silencing mechanisms in the mammalian germline. Semin. Cell Dev. Biol. 59, 118–125. doi: 10.1016/j.semcdb.2016.03.001
Yao, C., Liu, Y., Sun, M., Niu, M., Yuan, Q., Hai, Y., et al. (2015). MicroRNAs and DNA methylation as epigenetic regulators of mitosis, meiosis and spermiogenesis. Reproduction 150, 25–34. doi: 10.1530/REP-14-0643
Yin, Y., Morgunova, E., Jolma, A., Kaasinen, E., Sahu, B., Khund-Sayeed, S., et al. (2017). Impact of cytosine methylation on DNA binding specificities of human transcription factors. Science 356:eaaj2239. doi: 10.1126/science.aaj2239
Zhang, G. W., Wang, L., Chen, H., Guan, J., Wu, Y., Zhao, J., et al. (2020). Promoter hypermethylation of PIWI/piRNA pathway genes associated with diminished pachytene piRNA production in bovine hybrid male sterility. Epigenetics 15, 914–931. doi: 10.1080/15592294.2020.1738026
Zhang, W., Li, M., Sun, F., Xu, X., Zhang, Z., Liu, J., et al. (2019). Association of sperm methylation at LINE-1, four candidate genes, and nicotine/alcohol exposure with the risk of infertility. Front. Genet. 18:1001. doi: 10.3389/fgene.2019.01001
Zhao, L., Zhang, S., An, X., Tan, W., Tang, B., Zhang, X., et al. (2015). Sodium fluoride affects DNA methylation of imprinted genes in mouse early embryos. Cytogenet. Genome Res. 147, 41–47. doi: 10.1159/000442067
Zheng, H. Y., Shi, X. Y., Wu, F. R., Wu, Y. Q., Wang, L. L., and Chen, S. L. (2011). Assisted reproductive technologies do not increase risk of abnormal methylation of PEG1/MEST in human early pregnancy loss. Fertil. Steril. 96, 84–89. doi: 10.1016/j.fertnstert.2011.04.021
Keywords: DNA methylation, sperm DNA, male infertility, imprinting, infertility, epigenomics, sperm, imprinted genes
Citation: Rotondo JC, Lanzillotti C, Mazziotta C, Tognon M and Martini F (2021) Epigenetics of Male Infertility: The Role of DNA Methylation. Front. Cell Dev. Biol. 9:689624. doi: 10.3389/fcell.2021.689624
Received: 01 April 2021; Accepted: 17 June 2021;
Published: 22 July 2021.
Edited by:
Ozren Bogdanovic, Garvan Institute of Medical Research, AustraliaReviewed by:
José María Santos-Pereira, Andalusian Center for Development Biology (CABD), SpainCopyright © 2021 Rotondo, Lanzillotti, Mazziotta, Tognon and Martini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: John Charles Rotondo, cnRuam5jQHVuaWZlLml0; Mauro Tognon, dGdtQHVuaWZlLml0; Fernanda Martini, bXJmQHVuaWZlLml0
†ORCID: John Charles Rotondo, orcid.org/0000-0001-5179-1525; Carmen Lanzillotti, orcid.org/0000-0003-3253-5985; Chiara Mazziotta, orcid.org/0000-0002-5091-0497; Mauro Tognon, orcid.org/0000-0001-8269-7937; Fernanda Martini, orcid.org/0000-0001-9137-0805
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.