
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell Dev. Biol., 05 July 2021
Sec. Cellular Biochemistry
Volume 9 - 2021 | https://doi.org/10.3389/fcell.2021.685364
This article is part of the Research TopicTransport of Nutrients, Metabolites and Ions linked to Bioenergetics: Relevance to Human PathologyView all 16 articles
Iron overload, a high risk factor for many diseases, is seen in almost all human chronic and common diseases. Iron chelating agents are often used for treatment but, at present, most of these have a narrow scope of application, obvious side effects, and other disadvantages. Recent studies have shown that flavonoids can affect iron status, reduce iron deposition, and inhibit the lipid peroxidation process caused by iron overload. Therefore, flavonoids with iron chelating and antioxidant activities may become potential complementary therapies. In this study, we not only reviewed the research progress of iron overload and the regulation mechanism of flavonoids, but also studied the structural basis and potential mechanism of their function. In addition, the advantages and disadvantages of flavonoids as plant iron chelating agents are discussed to provide a foundation for the prevention and treatment of iron homeostasis disorders using flavonoids.
Iron overload is a long-standing problem that has aroused great interest in the field of chronic diseases (Fernández-Real and Manco, 2014). Iron, one of the essential metal elements, maintains normal physiological activities in human body, which is mainly involved in important physiological processes such as oxygen transport, electron transport, DNA synthesis, and many enzymatic reactions. Iron is absorbed through the gastrointestinal tract, delivered into the bloodstream, distributed throughout the body, and there are no physiologically routes of excretion after absorption (Fleming and Ponka, 2012). When there is too much iron in the body, excess iron can be deposited in tissues and organs, produce lipid peroxidation, affects cell damage, which can lead to cancer, hematological diseases, and other chronic and commonly encountered diseases. In addition, iron overload is a characteristic of ferroptosis that is a form of regulated cell death, leading to accumulation of lethal levels of lipid hydroperoxides (Wu et al., 2021). The review of iron overload is helpful for us to better understand the occurrence and progress mechanism of ferroptosis.
Studies on iron overload mainly focus on liver fibrosis, liver cancer (Molina-Sánchez and Lujambio, 2019), atherosclerosis (Wunderer et al., 2020), and hematological diseases (Franke et al., 2020). Deferrioxamine, deferiprone, and deferasirox are usually used to treat iron overload as iron chelators, but they are prone to side effects. Flavonoids, with their special structure, maybe have good iron chelation, antioxidant properties and less toxic. Based on this, flavonoids have become a hot topic as plant iron chelators.
Recent researches on the treatment of iron overload by flavonoids mainly concentrated on the effect of animal or cell experiments of a single compound, and there was a lack of macroscopic summing up and sorting out from the same kind of compounds. The mechanisms involved in different studies are different or complicated, some of the experimental results are controversial and contradictory, and lack of systematic summary. In this paper, the research progress on the regulation mechanism of iron overload and flavonoids was reviewed, and the potential molecular cytological mechanism was generalized according to their structural basis. These findings provide a solid evidence base for flavonoids as plant iron chelating agents.
Iron metabolism is a process in which iron is absorbed, regulated in the organism, and eventually excreted (Figure 1A). Fe3+ in the intestinal cavity is reduced to Fe2+ by duodenal cytochrome B, and then transported by divalent metal ion transporter 1 to the absorption cells of the intestinal mucosa epithelium. Some of this combines with apoferritin to form ferritin, which is stored in cells, and the remaining Fe2+ is released from the basal end of absorbing cells and enters the blood circulation, mediated by ferroportin (FPN), and is oxidized to Fe3+ by hephaestin. Excess ferritin is lost when cells are shed in the gut (Ramakrishnan et al., 2018). At the same time, the regulation of iron involves the combined action of various proteins and pathways. Among these, hepcidin plays a central regulatory role in the maintenance of iron homeostasis (Lee and Beutler, 2009).
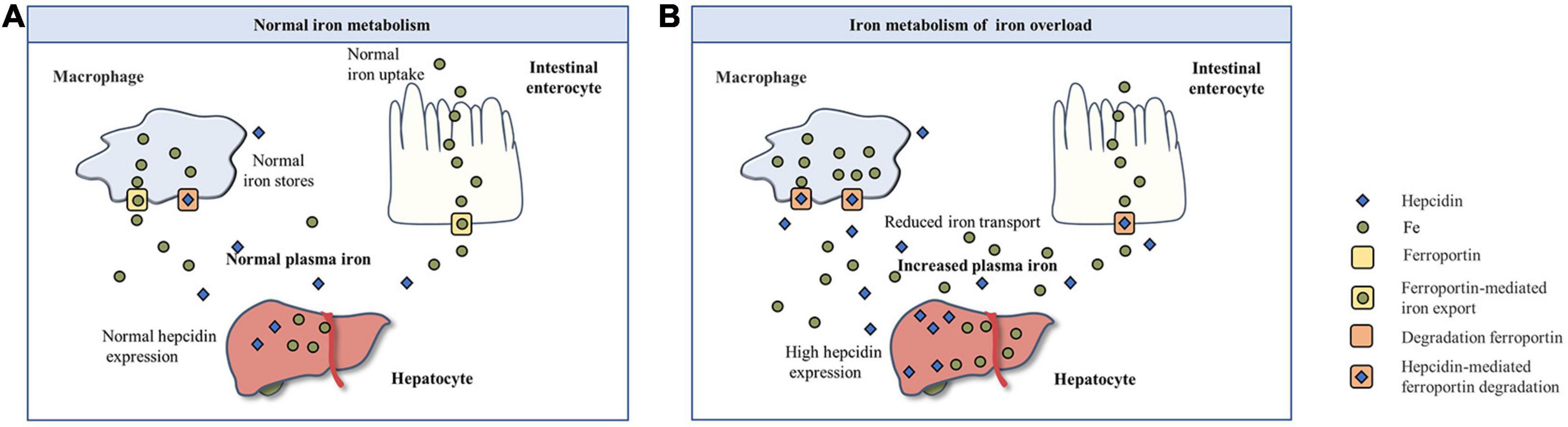
Figure 1. The basic process of iron metabolism. (A) Normal iron metabolism. (B) Iron metabolism of iron overload.
Iron overload or iron deficiency are the mainly iron dyshomeostasis (Muñoz et al., 2011). Iron deficiency is the most common public nutrition problem worldwide (Pasricha et al., 2020), but less attention is paid to iron overload. Iron overload is a pathological phenomenon in which the supply of iron in the body exceeds the need for iron, which leads to an increase in iron storage in some tissues and organs (Sousa et al., 2020). The human body has many absorption, transport, and storage mechanisms for iron, but there is no mechanism for excretion of excessive iron. Excessive iron can also have toxic effects on the body. An increasing amount of studies have shown that iron overload is related to the occurrence and development of many diseases. With respect to iron metabolism in the case of iron overload (Figure 1B), free iron exceeds the binding limit of transferrin (Tf), and iron absorption efficiency decreases relatively, but absolute values increase. At the same time, hepcidin levels increase, thereby accelerating the degradation of FPN, closing the iron transport outlet to the blood, and reducing the transport of iron from small intestinal epithelial cells and macrophages to the blood (Yan et al., 2018). Iron overload in the body can stimulate the synthesis of ferritin and capture excessive iron (Pickart et al., 2014).
In addition, ferroptosis is closely related to iron metabolism under iron overload. Ferroptosis, as a kind of programmed apoptosis, is characterized by excessive accumulation of lipid peroxides and reactive oxygen species (Zhai et al., 2021). Ferroptosis, often accompanied with iron overload, caused the tissue damage mainly driven by iron overload and lipid peroxidation. At the same time, ferroptosis causes ferritin degradation, affects iron metabolism and leads to iron overload (Chen et al., 2021). The occurrence of ferroptosis leads to normal tissue and organ damage and loss of function, which is directly involved in the occurrence, development and prognosis of some chronic and common diseases (Mao et al., 2020).
When there is too much iron in the body, the body will mount a defensive response, but after excess iron exceeds the binding capacity of serum FPN, it becomes labile plasma iron (LPI), labile cellular iron (LCI), or non-transferrin bound iron (NTBI). Unbound free iron leads to the formation of dangerous free radicals, which lead to lipid peroxidation. The accumulation of unstable iron (LPI and LCI) and NTBI will inevitably lead to excessive iron deposition in tissues and organs, ultimately causing multiple target organ damage, seriously affecting the quality of life and survival time of patients. In addition, iron overload affects the electron transfer of oxygen (Ali-Rahmani et al., 2014). Tf continues to transport iron ions to cells, and this may eventually lead to aging, chronic anemia, or cancer (Figure 2).
Excessive iron is widely deposited in the parenchymal cells of some organs and tissues of the human body, resulting in multiple organ function damage. For example, iron ions cannot only act on the gastrointestinal mucosa, causing gastrointestinal damage, but also severely damage hepatocytes, causing lipid peroxidation, cell swelling, and tissue necrosis, leading to liver fibrosis or liver cancer (Jang et al., 2014). On the other hand, inhalation of oxide-containing iron can cause toxic reactions in the respiratory tract. Inhaled iron oxide particles gather in the lungs and are swallowed by macrophages, leading to siderosis (Kim and Wessling-Resnick, 2012). Excessive deposition in the pancreas can cause diabetes, in the heart it can cause cardiomyopathy and atherosclerosis, and in bones and joints it can cause osteoporosis and joint pain. These varied diseases may be due to the ability of different cells to synthesize antioxidants or ferritin. Phytochelators such as flavonoids bind to excess iron, decrease the concentration of iron ions in serum and, due to their antioxidant effects, might reduce iron deposits.
The most direct effect of iron overload on cell damage is caused by lipid peroxidation can triggered by free iron ions. In the presence of free iron ions, hydrogen peroxide (H2O2) and superoxide (O2–) produce OH (hydroxyl radicals) via the Fenton and Haber-Weiss reactions (Choi et al., 2020). OH produced in vivo is the most important chemical substance that initiates lipid peroxidation and causes DNA damage (Afsar et al., 2016). It can extract hydrogen from lipids to form lipid free radicals. Lipid free radicals react rapidly with oxygen at a high rate to form lipid peroxyl radicals. Lipid peroxides can continue to extract hydrogen to form other lipid free radicals, which become lipid peroxides themselves, and a series of chain reactions ensues to form a large number of lipid peroxides (Chen et al., 2009). In the presence of Fe2+/Fe3+, iron ions near membrane lipids react with lipid peroxides to form new lipid peroxides and lipid oxygen free radicals, which further accelerate the chain reaction and eventually lead to cross-linking, polymerization, and inactivation of some lipids and functional macromolecular compounds (Choi et al., 2020). This results in damage to DNA, proteins, and lipids in cells, leading to cancer, hypertension, hyperlipidemia, or atherosclerosis. Antioxidants can block lipid chain autoxidation and provide hydrogen to free radicals, especially lipid peroxides, thus forming stable free radicals that do not initiate or induce further lipid oxidation (Ben Salah et al., 2019). The antioxidant properties of phytochemicals, such as flavonoids, may have a similar effect.
Iron chelating agents are the main treatment options for iron overload diseases. At present, three iron chelating agents, deferrioxamine, deferiprone, and deferasirox, are mainly used in clinical practice (Yang et al., 2018). However, they are currently only used to treat thalassemia iron overload caused by excessive blood transfusions (Kontoghiorghes and Kontoghiorghe, 2020). In addition to the narrow scope of application, they also have some disadvantages, such as frequent use and high prices, obvious side effects, and poor patient compliance.
With the increased understanding of the pathways related to iron homeostasis in the human body, novel iron chelators have been widely studied. For example, hepcidin supplementation can reduce intestinal iron absorption (Swinkels and Drenth, 2008; Katsarou and Pantopoulos, 2018). Tf extracted from human serum, or genetically engineered (Bobik et al., 2019), can be used as a natural iron-chelating agent to treat iron overload in specific parts of the body. However, as far as current technology is concerned, these are difficult to obtain and have low yields and poor activity, which are sufficient for scientific research but not clinical application. The search for an ideal iron chelator has become a popular pursuit.
Flavonoids, which have the capacity to inhibit reactive oxygen species, scavenge free radicals and regulate iron homeostasis, are less expensive and have fewer side effects, and are promising novel iron chelators (Lesjak and Srai, 2019).
Flavonoids are widely distributed in fruits, vegetables, tea, wine, seeds, and plant roots (Batiha et al., 2020). Many flavonoids have antitussive, expectorant, antiasthmatic, and antibacterial properties. In addition, flavonoids exert the same effect as phytoestrogens. They also have iron chelating and antioxidative properties. At present, iron overload is an important killer that endangers human health. Some phytochemicals, such as flavonoids, may provide a basis for new therapeutic approaches.
Flavonoids are a type of yellow pigment derived from two benzene rings with phenolic hydroxyl groups connected by three carbon atoms, that is, a series of compounds with C6-C3-C6 as the basic carbon structure. According to the different connection modes of the C3, flavonoids can be divided into several sub-types, including flavones, flavanones, flavonols, flavanols, isoflavones, chalcones, aurones, and anthocyanidins (Huynh et al., 2014; Table 1). Compared with other phytochemicals, flavonoids are highly iron chelation ability and reliable antioxidants. It is speculated that flavonoids can regulate iron metabolism and be used to treat iron overload. Two influential structures of flavonoids treating iron overload were presented as follows.
One is iron chelating properties of flavonoids. Studies have shown that flavonoids contain a variety of iron binding sites (Table 2), such as the 6, 7-dihydroxy structure, B ring catechol, and 2, 3-double bond. However, isolated ketone, hydroxyl, methoxy, or ortho-methoxy groups are not associated with the chelation of iron at all (Mladěnka et al., 2011). The 6, 7-dihydroxy structure is the most effective iron-binding site (Borges et al., 2016). Baicalein and baicalin, which have this structure, have strong iron-chelating properties. However, Vlachodimitropoulou et al. (2011) believe that the most effective is the catechin B ring catechol in quercetin and luteolin, but it may not play an important role in acidic conditions (Mladěnka et al., 2011). In addition, the chelation of metal ions generally requires the presence of 3- or 5-hydroxy groups. Rutin and quercetin contain these structures (Khalili et al., 2015). Furthermore, the 2, 3-double bonds of flavones and flavanols may also be important sites affecting iron binding (Braakhuis, 2019).
Another is antioxidant properties of flavonoids. As compounds containing two phenolic hydroxyl groups, flavonoids are effective antioxidants and free radical scavengers (Silva et al., 2020). The more phenolic hydroxyl groups there are, the more sites can be oxidized and the stronger the ability to scavenge oxygen free radicals (Table 2). For example, based on the structure, the oxidation resistance of luteolin is greater than that of apigenin, and that of luteolin is less than quercetin (Supplementary Figure 1).
Based on the previous research results, we summarized the underlying mechanisms of flavonoids for treating iron overload according to the following three mechanisms: amelioration of iron status by various proteins and pathways, chelation of iron to reduce iron deposition, and resistance to oxidation to reduce iron overload-induced oxidative damage (Figure 3A).
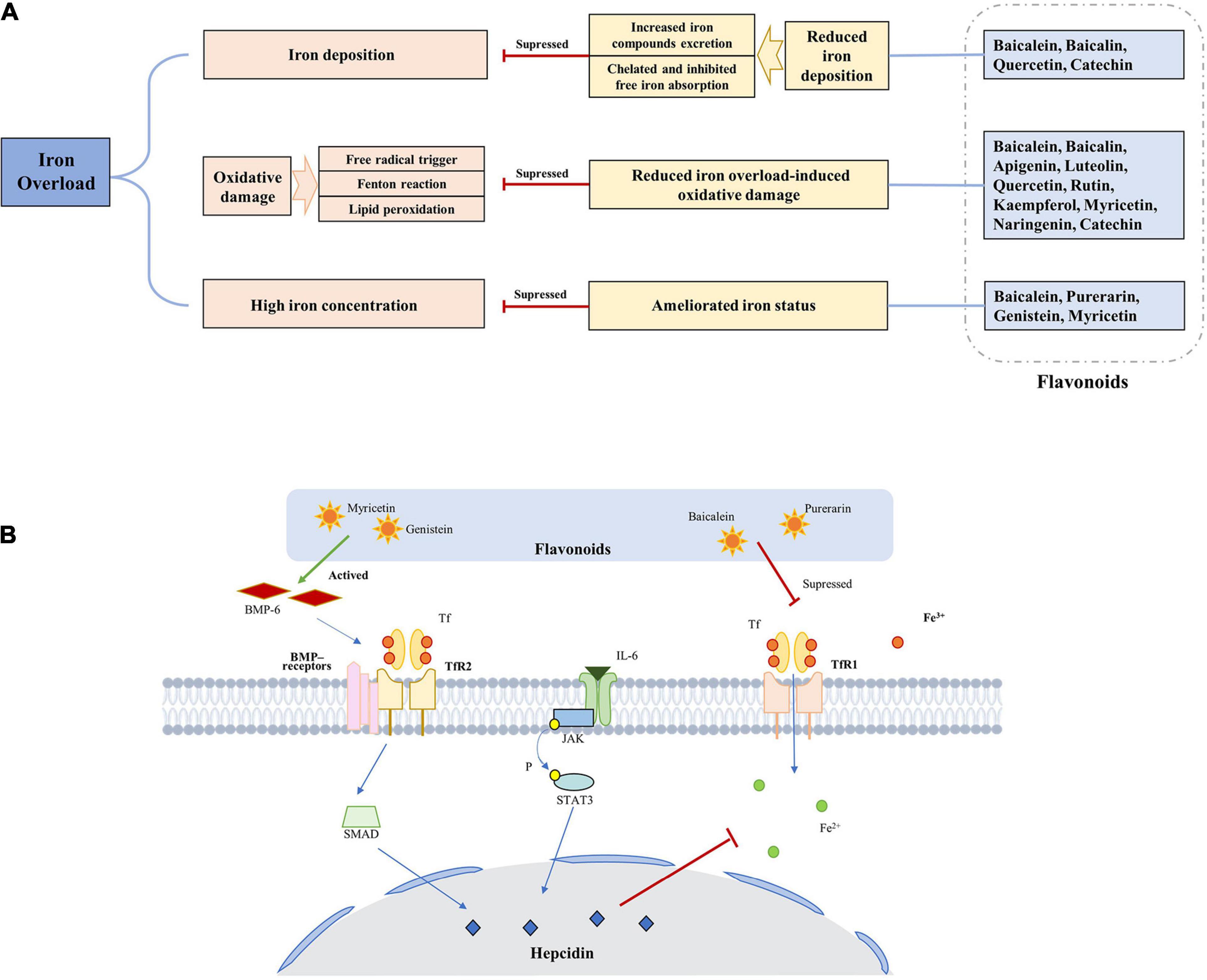
Figure 3. Underlying mechanisms of flavonoids for treating iron overload. (A) The basic process of underlying mechanisms. (B) The main pathways of flavonoids can ameliorate iron status.
Flavonoids can ameliorate iron status by various proteins and pathways, which is a indirect way to reduce the saturation of iron (Figure 3B), such as ferritin, Tf, and hepcidin. The mechanism may involve inhibition of the expression of TfR1 and ferroportin 1, promoting the expression of divalent metal transporter 1, increasing hepcidin transcript levels and promoter activity. Furthermore, the regulation of hepcidin and its regulatory elements by flavonoids is related to the STAT3-binding site.
Flavonoids can chelate iron to reduce iron accumulation, which is a direct way to reduce the saturation of iron. On account of flavonoids have iron-binding sites (Table 2) that are the structural basis of iron chelation. According to the relevant literature, baicalein, baicalin, quercetin, and rutin have the strongest iron chelating activity. When the bodily iron is overloaded, flavonoids inhibit the absorption and redistribution of iron in to some extent by chelating iron, thus reducing the iron content. In addition to inhibiting absorption, they can also increase excretion, and may combine with iron to form flavonoid-iron complexes that are excreted through feces.
Flavonoids can resist oxidation to reduce iron overload-induced oxidative damage. Flavonoids are a class of polyphenolic compounds, have strong reducibility, and can be used as antioxidants. The mechanism of action can be summarized in the following three stages: reaction with superoxide anion radical to prevent free radical trigger, inhibition of the Fenton reaction to prevent hydroxyl radical generation, and reaction with lipid peroxidation groups to prevent lipid peroxidation.
A number of in vitro and in vivo experiments have provided compelling evidence that flavonoids have iron chelating activity and strong antioxidant ability, which can reduce the damage caused by iron overload. These experiments indicate that flavonoids may subsequently be used as potential therapies for iron overload syndromes. Herein, we focus on representative flavonoid compounds and summarize the experimental results regarding the treatment of iron overload-related diseases. We also discuss the related mechanisms of plant iron-chelating agents (Table 3).
Flavones mostly form glycosides with sugars and are stored in plants. More than 30 types of glycoside belonging to the flavone group have been found, among which baicalein, baicalin apigenin, and luteolin are widely distributed.
Baicalein and baicalin are the main compounds isolated from the roots of the traditional Chinese medicinal herb Scutellaria baicalensis Georgi, and can be present at the same time. Animal studies have shown that Baicalein is an antioxidant or iron chelator, and it can significantly reduce the oxidation levels of lipids and proteins (Czapski et al., 2012).
One study showed that baicalein could significantly improve white blood cells and hemoglobin in the bone marrow of anemic mice with regenerative disorder, and had a protective effect on apoptosis induced by iron overload. Baicalein had a limited effect on platelet recovery, which was better than deferrioxamine; however, in reducing iron deposition, baicalein is superior to deferrioxamine and its mechanism may be related to the up regulation of hepcidin and its regulators bone morphogenetic protein 6 (BMP-6), SMAD family member 4 (SMAD4), and transferrin receptor 2 (TfR2) at the protein and mRNA levels (Dijiong et al., 2019). Studies by Perez et al. (2009) suggested that baicalin strongly inhibits iron-promoted Fenton chemistry through a combination of chelation and free radical scavenging mechanisms, whereas baicalin only partially protects against free radical damage. Regulation of the metal dynamic balance and inhibition of Fenton chemistry may be two of the mechanisms of herbal medicine.
Baicalin is the 7-hydroxyl group of baicalein, followed by glucuronide. After entering the animal body, baicalein is rapidly transformed into baicalin and other metabolites in the blood. Baicalin is not easily absorbed orally and can be absorbed into the blood only after enzymatic hydrolysis into baicalein in the intestine, which is rapidly transformed into baicalin in the body. The structures of baicalin and baicalein are similar and have similar functions.
The results of Zhao et al. (2005) showed that baicalin has a protective effect on the liver of iron-overload mice, and its mechanism may be due to both the antioxidant and chelating iron activity. Xu et al. (2012) found that baicalin inhibits oxidative stress induced by the combination of alcohol and iron via iron chelation.
The antioxidation and iron chelation properties of baicalin may be related to the following reasons: baicalin promotes the transfer of electrons from Fe2+ to dissolved oxygen, so that most of the Fe2+ is transformed into Fe3+, subsequently inhibiting the formation of hydroxyl radicals (Nishizaki and Iwahashi, 2015). In addition, baicalin positively regulates the expression of divalent metal transporter 1 and negatively regulates the expression of ferroportin 1, which reduces the accumulation of iron in different brain regions (Xiong et al., 2012; Guo et al., 2014).
The main botanical sources of apigenin stem from Chamomile, Petroselinum crispum, and Apium graveolens L. have the ability to scavenge free radicals, chelate iron, and inhibit lipoxygenase. Compared with other flavonoids (quercetin and kaempferol), it has the characteristics of low toxicity and non-mutagenicity (Simunkova et al., 2019). Danciu et al. (2018) confirmed that apigenin has the ability to scavenge free radicals, chelate iron, and inhibit lipoxygenase. In addition, they found that apigenin from different sources had different apoptosis-promoting and immune-activating potentials.
Luteolin is found in various plants in the form of glycosides. These plants have a high content of Capsicum annuum L., Dendranthema indicum, Lonicera japonica Thunb., and Perilla frutescens (L.) Britt. Luteolin, which has various pharmacological activities, reduces lipid oxidation of emulsions by scavenging radicals and chelating iron (Kim and Choe, 2018).
Flavonols include more than 60 types of aglycones, among which quercetin and kaempferol are widely distributed, although rutin and myricetin are also common.
Quercetin is a flavonoid that is widely distributed in the flowers, leaves, and fruits of plants and is found in large quantities in Chinese medicine, vegetables, fruits, and red wine. It has the ability to scavenge free radicals and chelate iron.
Gholampour et al. (Gholampour and Saki, 2019) found that quercetin could inhibit hepatorenal toxicity induced by ferrous sulfate and reduce the degree of liver and renal tissue injury in rats. Glei et al. (2002) confirmed through experiments that quercetin might reduce the risk of colon cancer by protecting the oxidative damage to DNA induced by iron. Li et al. (2020) approved that quercetin lowered the iron level particularly in the islet in type 2 diabetes (T2DM) mice and abolished partially oxidative stress in pancreatic tissue. In addition, clinical experiments (Sajadi Hezaveh et al., 2019) indicated that quercetin could significantly reduce the saturation of high-sensitivity C-reactive protein, iron, ferritin, and Tf saturation, and increase Tf. Quercetin has also been shown to improve iron status.
The mechanism of quercetin in the treatment of iron overload mainly depends on its antioxidant properties, which reduce lipid peroxidation and iron chelation. In addition, quercetin may reduce iron overload-induced injury through the ROS/ADMA/DDAH and II/eNOS/NO pathways (Chen et al., 2020). It also has the characteristic of iron-shuttling, and a quercetin concentration of <1 μM can facilitate chelatable iron-shuttling through GLUT1 in any direction on the cell membrane (Vlachodimitropoulou et al., 2011). Quercetin and baicalin can release iron from the liver, which is eventually excreted through feces (Zhang et al., 2006).
These factors make it possible for quercetin to be effectively used in chelotherapy under conditions of iron overload. However, an experiment showed that, in contrast to the traditional antioxidant mechanism of quercetin, quercetin has dual effects on the hemoglobin (Hb) redox reaction in vitro. Quercetin significantly aggravates Hb–H2O2-induced protein oxidation at low concentrations and exhibits protective effects at high concentrations, which may provide new insights into the physiological and pharmacological implications of quercetin in iron overload diseases (Lu et al., 2013).
Quercetin reacts with rutinose to form rutin. It is an effective component of the dried flowers and mature fruits of Sophora japonica L. Rutin is a well-known antioxidant and could be an efficient protective agent against iron overload (Aziza et al., 2014).
Aziza et al. (2014) found that the protective effect of rutin on the liver of iron-loaded rats might be related to its antioxidant and metal-chelating activities. One possible mechanism is that the formation of the iron-rutin complex cannot catalyze the conversion of superoxide ions to reactive hydroxyl radicals, which is the main process of the free radical-mediated toxicity of iron overload.
The fruit extract of Prunus nepalensis Ser. (Chaudhuri et al., 2015), Drosera burmanni i (Ghate et al., 2015), and Pleurocybella porrigens (Khalili et al., 2015) contain rutin and other phytochemicals and contribute to their free radical scavenging and iron chelation activity, potentially offering new natural alternatives to treat patients with iron overload. More studies are needed to determine which compounds are responsible for these biological activities.
Kaempferol is mainly derived from the rhizome of Kaempferia galanga L. and widely exists in a variety of fruits, vegetables, and beverages. It has attracted attention because of its anti-cancer, anti-inflammatory, anti-oxidative, anti-viral, and other effects.
The relationship between kaempferol and iron absorption remains unclear. Some researchers believe that kaempferol inhibits iron absorption, while Hart and colleagues (Hart et al., 2015, 2020) believe that it promotes iron absorption. These inconsistencies may be related to the concentration of kaempferol and the pH of the environment. Kaempferol, as a plant iron-chelating agent for the treatment of iron overload-related diseases, requires further study.
However, kaempferol has good antioxidant activity. Cho et al. (2019) observed that kaempferol pretreatment could block the production of ROS induced by arachidonic acid and iron, reverse glutathione depletion, and reduce cell death.
Myricetin mainly comes from the extract of the leaves, bark, and roots of Myrica rubra (Lour.), and it has been reported to have the biological functions of anti-oxidation, iron absorption inhibition, regulation of hepcidin, and chelation of iron ions.
Studies by Hart et al. (2015) showed that myricetin inhibits iron uptake by Caco-2 cells. Wang et al. (2017) suggested that myricetin reduces iron content by inhibiting the expression of TfR1. A study (Abalea et al., 1999) showed that myricetin antagonizes iron-induced genotoxicity by stimulating the process of DNA repair, preventing lipid peroxidation and the accumulation of oxidation products in DNA, in order to inhibit iron overload produced by liver cancer. In contrast, Mu et al. (2016) demonstrated that myricetin significantly inhibits hepcidin expression in vitro and in vivo by inhibiting the BMP/SMAD pathway and increasing the expression of FPN and the level of serum iron.
In addition, myricetin is generally shown to be an antioxidant, but sometimes may also be a pro-oxidant. In the presence of ascorbic acid, myricetin has antioxidative ability; in the absence of ascorbic acid, the pro-oxidative activity is dominant, and these two effects are enhanced when it forms a complex with iron (Chobot and Hadacek, 2011).
Although it is unclear whether myricetin can be used as an iron chelator, these results suggest that myricetin may play a key role in regulating iron homeostasis.
Flavanones are double-bonded hydrogenated derivatives of flavonoids at the C2-3 position, and most of these plant components have hydroxyl or methoxy groups. They exist in free form or in combined forms in the plant kingdom. The most common compound is naringenin.
Naringenin mainly originates from the buds of Prunus yedoensis Mate and the core and shell of Anacardium occidentale. It has antibacterial, anti-inflammatory, anti-cancer, antispasmodic, and cholagogic effects.
Chtourou et al. (2014) suggested that naringenin could increase the activities of antioxidant enzymes and reduce the oxidative damage observed in the cerebral cortex of iron-treated rats. In addition, they also found that it could improve the anxiogenic-like behavior impairment induced by excessive iron in rats, and emphasized that adding this flavonoid to the diet may prevent brain damage associated with iron load (Chtourou et al., 2015). These results suggest that naringin has antioxidant and iron-chelating properties and can protect nerve tissues.
Flavanols are reduced from flavanonols and can be regarded as flavanonols after the removal of four carboxyl oxygen atoms. Catechin is a common flavanol.
The derivatives of Flavan-3-ol is catechin, which is a type of active phenolic substance mainly extracted from tea and other natural plants. The catechin in Prunus nepalensis Ser. (Steud) (Chaudhuri et al., 2015) and Phyllostachys nigra Bamboo stems (henosis variety) (Yang et al., 2019) have been studied, and it was suggested that iron-mediated oxidative stress could be improved, thereby increasing cell survival.
In addition, Farsetia hamiltonii Royle (Basu et al., 2019) and Drosera burmannii Vahl. (Ghate et al., 2015) catechin have good antioxidant activity, can remove a variety of reactive oxygen and nitrogen, and chelate iron. However, additional research is needed to determine whether catechins could be used to treat iron overload diseases.
Isoflavones are a type of flavonoids mainly found in legumes. Daidzein is an isoflavone that has excellent antioxidant properties, although it has been proven unsuitable as a plant iron-chelating agent, as it does not chelate Fe3+ (Dowling et al., 2010). Puerarin and genistein may be potential iron chelating agents.
Puerarin is a crown-expanding isoflavone derivative separated from the roots of Pueraria lobata (Willd) Ohwi and Pueraria thunbergiana Benth.
Experimental data generated by Song et al. (2020) confirmed that puerarin reduces the retinal damage caused by iron overload. The possible mechanism might be associated with the regulation of iron-treated proteins, enhancement of antioxidant capacity (Zhang et al., 2018), inhibition of the activation and apoptosis of MAPK, and STAT3 under iron overload (Song et al., 2020).
Genistein is derived from Genista tinctoria Linn. and Sophora subprostrata Chun et T. Chen root, which have antioxidant properties.
Zhen et al. (2013) confirmed that genistein treatment of HepG2 cells could increase both hepcidin promoter activity and transcript levels. This may be related to the BMP response element or the STAT3-binding site in the hepcidin promoter.
There have been few studies on chalcones and aurones associated with iron overload and their basic mechanisms are still poorly understood.
Many flavonoids have specific metal binding sites, and the multiple phenolic hydroxyl structures they contain also have good oxygen reduction properties. Therefore, flavonoids can chelate iron to form iron complexes for excretion and reduce iron accumulation in tissues and organs. At the same time, they strongly inhibit active oxygen and scavenge free radicals, and can reduce lipid peroxidation caused by iron overload, which results in cell damage. Flavonoids have the effect of regulating iron homeostasis and can be used as plant iron chelating agents. Compared with existing iron-chelating agents, they are very safe and inexpensive. In recent s, a large number of studies have shown that flavonoids can play an important role in the regulation of iron overload and are expected to be developed as natural drugs for the treatment of iron homeostasis disorders. Among them, baicalein, baicalin, quercetin, and rutin have shown outstanding results.
Researchers have made great efforts to explore the molecular mechanisms of flavonoids in iron overload. Although some progress has been made in the study of flavonoids in iron overload, research has mainly focused on in vitro studies and l sufficient in vivo and clinical evidence is lacking, which are important considerations in evidence-based medicine. Furthermore, the use of flavonoids as iron-chelating agents requires risk and benefit assessment and comparison with existing treatments. In this respect, it is difficult to determine whether flavonoids have significant advantages.
It is also worth exploring whether flavonoids that chelate iron and inhibit lipid peroxidation can play an important role in ferroptosis. In addition, flavonoids also face a series of problems, such as low oral utilization rates and poor water solubility. New drug delivery approaches or dissolution methods for flavonoids have become a hot research topic. For example, the use of nanomaterials may be a viable solution strategy (Xia et al., 2011; Kang et al., 2019). The ability of flavonoids to chelate iron is affected by their concentration and environmental pH, and impacts the dosage and conditions of use. These factors should be the focus of subsequent studies (Abotaleb et al., 2018).
Iron overload can affect the clinical course of a variety of diseases and we suggest that patients with chronic and common diseases, such as cancer and hematological diseases, consume fruits, vegetables, and beverages that contain abundant flavonoids to prevent iron overload damage. Although the amount of flavonoids consumed in the diet for achieving a therapeutic effect remains to be determined, this field continues to hold much promise.
XW and YL: conceptualization, data curation, and writing – original draft preparation. LH: data curation and investigation. JL and CL: supervision, validation, and writing – review and editing. CS: conceptualization and writing – review and editing. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2021.685364/full#supplementary-material
Abalea, V., Cillard, J., Dubos, M., Sergent, O., Cillard, P., and Morel, I. (1999). Repair of iron-induced DNA oxidation by the flavonoid myricetin in primary rat hepatocyte cultures. Free Rad. Biol. Med. 26, 1457–1466. doi: 10.1016/s0891-5849(99)00010-6
Abotaleb, M., Samuel, S., Varghese, E., Varghese, S., Kubatka, P., Liskova, A., et al. (2018). Flavonoids in cancer and apoptosis. Cancers 11:28. doi: 10.3390/cancers11010028
Afsar, T., Razak, S., Khan, M., Mawash, S., Almajwal, A., Shabir, M., et al. (2016). Evaluation of antioxidant, anti-hemolytic and anticancer activity of various solvent extracts of Acacia hydaspica R. Parker aerial parts. BMC Complement. Altern. Med. 16:258. doi: 10.1186/s12906-016-1240-8
Ali-Rahmani, F., Schengrund, C., and Connor, J. (2014). HFE gene variants, iron, and lipids: a novel connection in Alzheimer’s disease. Front. Pharmacol. 5:165. doi: 10.3389/fphar.2014.00165
Aziza, S., Azab, M.-S., and El-Shall, S. (2014). Ameliorating role of rutin on oxidative stress induced by iron overload in hepatic tissue of rats. Pak. J. Biol. Sci. 17, 964–977. doi: 10.3923/pjbs.2014.964.977
Basu, T., Kumar, B., Shendge, A., Panja, S., Chugh, H., Gautam, H., et al. (2019). An Indian desert shrub ‘Hiran Chabba’, Farsetia hamiltonii royle, exhibits potent antioxidant and hepatoprotective effect against iron- overload induced liver toxicity in Swiss albino mice. Curr. Drug Discov. Technol. 16, 210–222. doi: 10.2174/1570163815666180418150123
Batiha, G., Beshbishy, A., Ikram, M., Mulla, Z., El-Hack, M., Taha, A., et al. (2020). The pharmacological activity, biochemical properties, and pharmacokinetics of the major natural Polyphenolic flavonoid: quercetin. Foods 9:374. doi: 10.3390/foods9030374
Ben Salah, H., Smaoui, S., Abdennabi, R., and Allouche, N. (2019). LC-ESI-MS/MS phenolic profile of Volutaria lippii (L.) Cass. extracts and evaluation of their antioxidant, antiacetylcholinesterase, antidiabetic, and antibacterial activities. Evid. Based Complement. Altern. Med. 2019:9814537.
Bobik, T., Popov, R., Aliev, T., Mokrushina, Y., Shamborant, O., Khurs, E., et al. (2019). Production of recombinant human transferrin in eukaryotic Pichia pastoris expression system. Bull. Exp. Biol. Med. 167, 335–338. doi: 10.1007/s10517-019-04521-0
Borges, A., Abreu, A., Dias, C., Saavedra, M., Borges, F., and Simões, M. (2016). New perspectives on the use of phytochemicals as an emergent strategy to control bacterial infections including biofilms. Molecules 21:877. doi: 10.3390/molecules21070877
Braakhuis, A. (2019). Evidence on the health benefits of supplemental propolis. Nutrients 11:2705. doi: 10.3390/nu11112705
Chaudhuri, D., Ghate, N., Panja, S., Das, A., and Mandal, N. (2015). Wild edible fruit of Prunus nepalensis Ser. (Steud), a potential source of antioxidants, ameliorates iron overload-induced hepatotoxicity and liver fibrosis in mice. PLoS One 10:e0144280. doi: 10.1371/journal.pone.0144280
Chen, B., Caballero, S., Seo, S., Grant, M., and Lewin, A. (2009). Delivery of antioxidant enzyme genes to protect against ischemia/reperfusion-induced injury to retinal microvasculature. Invest. Ophthalmol. Vis. Sci. 50, 5587–5595. doi: 10.1167/iovs.09-3633
Chen, X., Comish, P., Tang, D., and Kang, R. (2021). Characteristics and biomarkers of ferroptosis. Front. Cell Dev. Biol. 9:637162. doi: 10.3389/fcell.2021.637162
Chen, X., Li, H., Wang, Z., Zhou, Q., Chen, S., Yang, B., et al. (2020). Quercetin protects the vascular endothelium against iron overload damages via ROS/ADMA/DDAH II/eNOS/NO pathway. Eur. J. Pharmacol. 868:172885. doi: 10.1016/j.ejphar.2019.172885
Cho, S., Yang, J., Seo, K., Shin, S., Park, E., Cho, S., et al. (2019). Cudrania Tricuspidata extract and its major constituents inhibit oxidative stress-induced liver injury. J. Med. Food 22, 602–613. doi: 10.1089/jmf.2018.4322
Chobot, V., and Hadacek, F. (2011). Exploration of pro-oxidant and antioxidant activities of the flavonoid myricetin. Redox Rep. 16, 242–247. doi: 10.1179/1351000211y.0000000015
Choi, J., Min, K., Jeon, S., Kim, N., Pack, J., and Song, K. (2020). Continuous exposure to 1.7 GHz LTE electromagnetic fields increases intracellular reactive oxygen species to decrease human cell proliferation and induce senescence. Sci. Rep. 10:9238.
Chtourou, Y., Fetoui, H., and Gdoura, R. (2014). Protective effects of naringenin on iron-overload-induced cerebral cortex neurotoxicity correlated with oxidative stress. Biol. Trace Element Res. 158, 376–383. doi: 10.1007/s12011-014-9948-0
Chtourou, Y., Slima, A., Gdoura, R., and Fetoui, H. (2015). Naringenin mitigates iron-induced anxiety-like behavioral impairment, mitochondrial dysfunctions, Ectonucleotidases and Acetylcholinesterase alteration activities in rat Hippocampus. Neurochem. Res. 40, 1563–1575. doi: 10.1007/s11064-015-1627-9
Czapski, G., Czubowicz, K., and Strosznajder, R. (2012). Evaluation of the antioxidative properties of lipoxygenase inhibitors. Pharmacol. Rep. 64, 1179–1188. doi: 10.1016/s1734-1140(12)70914-3
Danciu, C., Zupko, I., Bor, A., Schwiebs, A., Radeke, H., Hancianu, M., et al. (2018). Botanical Therapeutics: phytochemical screening and biological assessment of chamomile, parsley and celery extracts against A375 human melanoma and dendritic cells. Int. J. Mol. Sci. 19:3624. doi: 10.3390/ijms19113624
Dijiong, W., Xiaowen, W., Linlong, X., Wenbin, L., Huijin, H., Baodong, Y., et al. (2019). Iron chelation effect of curcumin and baicalein on aplastic anemia mouse model with iron overload. Iran. J. Basic Med. Sci. 22, 660–668.
Dowling, S., Regan, F., and Hughes, H. (2010). The characterisation of structural and antioxidant properties of isoflavone metal chelates. J. Inorganic Biochem. 104, 1091–1098. doi: 10.1016/j.jinorgbio.2010.06.007
Fernández-Real, J., and Manco, M. (2014). Effects of iron overload on chronic metabolic diseases. Lance. Diabetes Endocrinol. 2, 513–526. doi: 10.1016/s2213-8587(13)70174-8
Franke, G., Kubasch, A., Cross, M., Vucinic, V., and Platzbecker, U. (2020). Iron overload and its impact on outcome of patients with hematological diseases. Mol. Aspects Med. 75:100868. doi: 10.1016/j.mam.2020.100868
Ghate, N., Chaudhuri, D., Das, A., Panja, S., and Mandal, N. (2015). An antioxidant extract of the insectivorous plant Drosera burmannii Vahl. Alleviates iron-induced oxidative stress and hepatic injury in mice. PLoS One 10:e0128221. doi: 10.1371/journal.pone.0128221
Gholampour, F., and Saki, N. (2019). Hepatic and renal protective effects of quercetin in ferrous sulfateinduced toxicity. Gen. Physiol. Biophys. 38, 27–38. doi: 10.4149/gpb_2018038
Glei, M., Latunde-Dada, G., Klinder, A., Becker, T., Hermann, U., Voigt, K., et al. (2002). Iron-overload induces oxidative DNA damage in the human colon carcinoma cell line HT29 clone 19A. Mutat. Res. 519, 151–161. doi: 10.1016/s1383-5718(02)00135-3
Guo, C., Chen, X., and Xiong, P. (2014). Baicalin suppresses iron accumulation after substantia nigra injury: relationship between iron concentration and transferrin expression. Neural Regen. Res. 9, 630–636. doi: 10.4103/1673-5374.130108
Hart, J., Tako, E., Kochian, L., and Glahn, R. (2015). Identification of Black Bean (Phaseolus vulgaris L.) Polyphenols that inhibit and promote iron uptake by Caco-2 cells. J. Agric. Food Chem. 63, 5950–5956. doi: 10.1021/acs.jafc.5b00531
Hart, J., Tako, E., Wiesinger, J., and Glahn, R. (2020). Polyphenolic profiles of yellow bean seed coats and their relationship with iron bioavailability. J. Agric. Food Chem. 68, 769–778. doi: 10.1021/acs.jafc.9b05663
Huynh, N., Van Camp, J., Smagghe, G., and Raes, K. (2014). Improved release and metabolism of flavonoids by steered fermentation processes: a review. Int. J. Mol. Sci. 15, 19369–19388. doi: 10.3390/ijms151119369
Jang, H., Do, G., Lee, H., Ok, H., Shin, J., and Kwon, O. (2014). Schisandra Chinensis Baillon regulates the gene expression of phase II antioxidant/detoxifying enzymes in hepatic damage induced rats. Nutr. Res. Pract. 8, 272–277. doi: 10.4162/nrp.2014.8.3.272
Kang, H., Han, M., Xue, J., Baek, Y., Chang, J., Hu, S., et al. (2019). Renal clearable nanochelators for iron overload therapy. Nat. Commun. 10:5134.
Katsarou, A., and Pantopoulos, K. (2018). Hepcidin therapeutics. Pharmaceuticals 11:127. doi: 10.3390/ph11040127
Khalili, M., Ebrahimzadeh, M., and Kosaryan, M. (2015). In vivo iron-chelating activity and phenolic profiles of the Angel’s wings mushroom, Pleurotus porrigens (Higher Basidiomycetes). Int. J. Med. Mushr. 17, 847–856. doi: 10.1615/intjmedmushrooms.v17.i9.50
Kim, J., and Choe, E. (2018). Effect of the pH on the lipid oxidation and polyphenols of soybean oil-in-water emulsion with added peppermint (Mentha piperita) extract in the presence and absence of iron. Food Sci. Biotechnol. 27, 1285–1292. doi: 10.1007/s10068-018-0324-2
Kim, J., and Wessling-Resnick, M. (2012). The role of iron metabolism in lung inflammation and injury. J. Allergy Ther. 3:004. doi: 10.4172/2155-6121.S4-004
Kontoghiorghes, G., and Kontoghiorghe, C. (2020). Iron and chelation in biochemistry and medicine: new approaches to controlling iron metabolism and treating related diseases. Cells 9:1456. doi: 10.3390/cells9061456
Lee, P., and Beutler, E. (2009). Regulation of hepcidin and iron-overload disease. Ann. Rev. Pathol. 4, 489–515. doi: 10.1146/annurev.pathol.4.110807.092205
Lesjak, M., and Srai, S. K. S. (2019). Role of dietary flavonoids in iron homeostasis. Pharmaceuticals 12:119. doi: 10.3390/ph12030119
Li, D., Jiang, C., Mei, G., Zhao, Y., Chen, L., Liu, J., et al. (2020). Quercetin alleviates ferroptosis of pancreatic β cells in Type 2 diabetes. Nutrients 12:2954. doi: 10.3390/nu12102954
Lu, N., Chen, C., He, Y., Tian, R., Xiao, Q., and Peng, Y. (2013). Effects of quercetin on hemoglobin-dependent redox reactions: relationship to iron-overload rat liver injury. J. Asian Nat. Products Res. 15, 1265–1276. doi: 10.1080/10286020.2013.838952
Mao, L., Zhao, T., Song, Y., Lin, L., Fan, X., Cui, B., et al. (2020). The emerging role of ferroptosis in non-cancer liver diseases: hype or increasing hope? Cell Death Dis. 11:518.
Mladěnka, P., Macáková, K., Filipský, T., Zatloukalová, L., Jahodáø, L., Bovicelli, P., et al. (2011). In vitro analysis of iron chelating activity of flavonoids. J. Inorganic Biochem. 105, 693–701. doi: 10.1016/j.jinorgbio.2011.02.003
Molina-Sánchez, P., and Lujambio, A. (2019). Iron overload and liver cancer. J. Exp. Med. 216, 723–724. doi: 10.1084/jem.20190257
Mu, M., An, P., Wu, Q., Shen, X., Shao, D., Wang, H., et al. (2016). The dietary flavonoid myricetin regulates iron homeostasis by suppressing hepcidin expression. J. Nutr. Biochem. 30, 53–61. doi: 10.1016/j.jnutbio.2015.10.015
Muñoz, M., García-Erce, J., and Remacha, Á (2011). Disorders of iron metabolism. Part II: iron deficiency and iron overload. J. Clin. Pathol. 64, 287–296. doi: 10.1136/jcp.2010.086991
Nishizaki, D., and Iwahashi, H. (2015). Baicalin inhibits the fenton reaction by enhancing electron transfer from Fe (2+) to dissolved oxygen. Am. J. Chin. Med. 43, 87–101. doi: 10.1142/s0192415x15500068
Pasricha, S., Tye-Din, J., Muckenthaler, M., and Swinkels, D. (2020). Iron deficiency. Lancet 397, 233–248.
Perez, C., Wei, Y., and Guo, M. (2009). Iron-binding and anti-Fenton properties of baicalein and baicalin. J. Inorganic Biochem. 103, 326–332. doi: 10.1016/j.jinorgbio.2008.11.003
Pickart, L., Vasquez-Soltero, J., and Margolina, A. (2014). GHK and DNA: resetting the human genome to health. Biomed. Res. Int. 2014:151479.
Ramakrishnan, L., Pedersen, S., Toe, Q., Quinlan, G., and Wort, S. (2018). Pulmonary arterial hypertension: iron matters. Front. Physiol. 9:641. doi: 10.3389/fphys.2018.00641
Sajadi Hezaveh, Z., Azarkeivan, A., Janani, L., Hosseini, S., and Shidfar, F. (2019). The effect of quercetin on iron overload and inflammation in β-thalassemia major patients: a double-blind randomized clinical trial. Complement. Ther. Med. 46, 24–28. doi: 10.1016/j.ctim.2019.02.017
Silva, T., Cavalcanti Filho, J., Barreto Fonsêca, M., Santos, N., Barbosa da Silva, A., Zagmignan, A., et al. (2020). Products derived Buchenavia tetraphylla from leaves have in vitro antioxidant activity and protect larvae against -induced injury. Pharmaceuticals (Basel, Switzerland) 13:46. doi: 10.3390/ph13030046
Simunkova, M., Alwasel, S., Alhazza, I., Jomova, K., Kollar, V., Rusko, M., et al. (2019). Management of oxidative stress and other pathologies in Alzheimer’s disease. Arch. Toxicol. 93, 2491–2513.
Song, Q., Zhao, Y., Li, Q., Han, X., and Duan, J. (2020). Puerarin protects against iron overload-induced retinal injury through regulation of iron-handling proteins. Biomed. Pharmacother. 122:109690. doi: 10.1016/j.biopha.2019.109690
Sousa, L., Oliveira, M., Pessôa, M., and Barbosa, L. (2020). Iron overload: effects on cellular biochemistry. Clin. Chim. Acta 504, 180–189. doi: 10.1016/j.cca.2019.11.029
Swinkels, D., and Drenth, J. (2008). Hepcidin in the management of patients with mild non-hemochromatotic iron overload: fact or fiction? J. Hepatol. 49, 680–685. doi: 10.1016/j.jhep.2008.08.004
Vlachodimitropoulou, E., Sharp, P., and Naftalin, R. (2011). Quercetin-iron chelates are transported via glucose transporters. Free Rad. Biol. Med. 50, 934–944. doi: 10.1016/j.freeradbiomed.2011.01.005
Wang, B., Zhong, Y., Gao, C., and Li, J. (2017). Myricetin ameliorates scopolamine-induced memory impairment in mice via inhibiting acetylcholinesterase and down-regulating brain iron. Biochem. Biophys. Res. Commun. 490, 336–342. doi: 10.1016/j.bbrc.2017.06.045
Wu, X., Li, Y., Zhang, S., and Zhou, X. (2021). Ferroptosis as a novel therapeutic target for cardiovascular disease. Theranostics 11, 3052–3059. doi: 10.7150/thno.54113
Wunderer, F., Traeger, L., Sigurslid, H., Meybohm, P., Bloch, D., and Malhotra, R. (2020). The role of hepcidin and iron homeostasis in atherosclerosis. Pharmacol. Res. 153:104664. doi: 10.1016/j.phrs.2020.104664
Xia, G., Chen, B., Ding, J., Gao, C., Lu, H., Shao, Z., et al. (2011). Effect of magnetic Fe3O4 nanoparticles with 2-methoxyestradiol on the cell-cycle progression and apoptosis of myelodysplastic syndrome cells. Int. J. Nanomed. 6, 1921–1927. doi: 10.2147/ijn.s24078
Xiong, P., Chen, X., Guo, C., Zhang, N., and Ma, B. (2012). Baicalin and deferoxamine alleviate iron accumulation in different brain regions of Parkinson’s disease rats. Neural Regen. Res. 7, 2092–2098.
Xu, Y., Feng, Y., Li, H., and Gao, Z. (2012). Ferric citrate CYP2E1-independently promotes alcohol-induced apoptosis in HepG2 cells via oxidative/nitrative stress which is attenuated by pretreatment with baicalin. Food Chem. Toxicol. 50, 3264–3272. doi: 10.1016/j.fct.2012.05.061
Yan, H., Liu, Z., Guan, Z., and Guo, C. (2018). Deferoxamine ameliorates adipocyte dysfunction by modulating iron metabolism in ob/ob mice. Endocr. Connect. 7, 604–616. doi: 10.1530/ec-18-0054
Yang, J., Choi, M., Na, C., Cho, S., Kim, J., Ku, S., et al. (2019). Bamboo Stems (Phyllostachys nigra variety henosis) containing polyphenol mixtures activate Nrf2 and attenuate phenylhydrazine-induced oxidative stress and liver injury. Nutrients 11:114. doi: 10.3390/nu11010114
Yang, J., Zhang, G., Dong, D., and Shang, P. (2018). Effects of iron overload and oxidative damage on the musculoskeletal system in the space environment: data from spaceflights and ground-based simulation models. Int. J. Mol. Sci. 19:2608. doi: 10.3390/ijms19092608
Zhai, Z., Zou, P., Liu, F., Xia, Z., and Li, J. (2021). Ferroptosis is a potential novel diagnostic and therapeutic target for patients with cardiomyopathy. Front. Cell Dev. Biol. 9:649045. doi: 10.3389/fcell.2021.649045
Zhang, Y., Kong, W., and Chai, X. (2018). Compound of icariin, astragalus, and puerarin mitigates iron overload in the cerebral cortex of Alzheimer’s disease mice. Neural Regen. Res. 13, 731–736. doi: 10.4103/1673-5374.230302
Zhang, Y., Li, H., Zhao, Y., and Gao, Z. (2006). Dietary supplementation of baicalin and quercetin attenuates iron overload induced mouse liver injury. Eur. J. Pharmacol. 535, 263–269. doi: 10.1016/j.ejphar.2006.01.067
Zhao, Y., Li, H., Gao, Z., and Xu, H. (2005). Effects of dietary baicalin supplementation on iron overload-induced mouse liver oxidative injury. Eur. J. Pharmacol. 509, 195–200. doi: 10.1016/j.ejphar.2004.11.060
Keywords: flavonoids, iron overload, iron metabolism, iron balance, plant iron chelator
Citation: Wang X, Li Y, Han L, Li J, Liu C and Sun C (2021) Role of Flavonoids in the Treatment of Iron Overload. Front. Cell Dev. Biol. 9:685364. doi: 10.3389/fcell.2021.685364
Received: 25 March 2021; Accepted: 10 June 2021;
Published: 05 July 2021.
Edited by:
Piotr Koprowski, Nencki Institute of Experimental Biology (PAS), PolandReviewed by:
Ming He, Nanchang University, ChinaCopyright © 2021 Wang, Li, Han, Li, Liu and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changgang Sun, c2NnZG9jdG9yQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.