- 1State Key Laboratory of Reproductive Medicine, Department of Histology and Embryology, School of Basic Medical Sciences, Nanjing Medical University, Nanjing, China
- 2State Key Laboratory of Reproductive Medicine, Clinical Center of Reproductive Medicine, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China
The PIWI-interacting RNA (piRNA) pathway mainly consists of evolutionarily conserved protein factors. Intriguingly, many mutations of piRNA pathway factors lead to meiotic arrest during spermatogenesis. The majority of piRNA factor-knockout animals show arrested meiosis in spermatogenesis, and only a few show post-meiosis male germ cell arrest. It is still unclear whether the majority of piRNA factors expressed in spermatids are involved in long interspersed nuclear element-1 repression after meiosis, but future conditional knockout research is expected to resolve this. In addition, recent hamster knockout studies showed that a piRNA factor is necessary for oocytes—in complete contrast to the findings in mice. This species discrepancy allows researchers to reexamine the function of piRNA in female germ cells. This mini-review focuses on the current knowledge of protein factors derived from mammalian knockout studies and summarizes their roles in the biogenesis and function of piRNAs.
Introduction
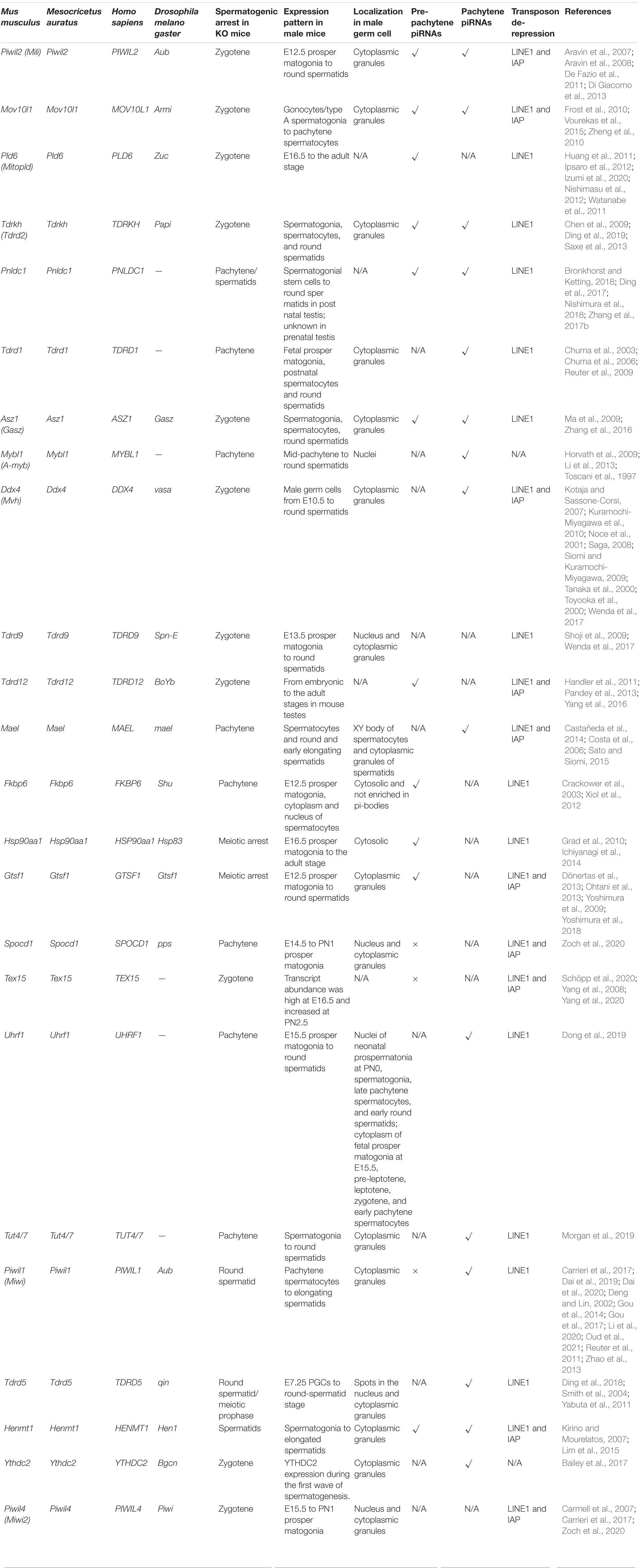
PIWI-interacting RNAs (piRNAs) are a distinct class of small RNAs [generally 24–31 nucleotides (nt) long] that are highly expressed in mouse testes. They are loaded onto PIWI proteins and function as an endogenous defense system against transposable elements (Aravin et al., 2004, 2006, 2007; Grivna et al., 2006; Kuramochi-Miyagawa et al., 2008). Some piRNAs are also involved in messenger RNA (mRNA) translation and mRNA/lncRNA elimination (Gou et al., 2014; Watanabe et al., 2015; Dai et al., 2019, 2020). Mice produce three types of germline piRNAs during spermatogenesis. Prenatal piRNAs first appear in the fetal testis and initiate transposon silencing via DNA methylation (Aravin et al., 2007, 2008; Carmell et al., 2007; Kuramochi-Miyagawa et al., 2008). The biogenesis of piRNAs in postnatal male germ cells is strikingly different from that in embryonic cells, as the majority of piRNAs are produced only by primary biogenesis after birth (Vourekas et al., 2012; Li et al., 2013). Postnatal piRNAs can be divided into pre-pachytene and pachytene piRNAs based on the timing of their expression and corresponding locus in the genome (Li et al., 2013). Because prenatal piRNA production and neonatal piRNA production involve continuous processes, they are rarely distinguishable during research. In most of the literature, prenatal piRNAs are classified as pre-pachytene piRNAs. Pachytene piRNAs are generally loaded onto MIWI (PIWIL1) or MILI (PIWIL2) (Vourekas et al., 2012; Li et al., 2013) and, unlike embryonic piRNAs, they have a strong 1U but no 10A bias, reflecting their primary biogenesis-dependent function (Vourekas et al., 2012; Li et al., 2013). These piRNA pathways are required during multiple stages of male germ cell development, including de novo DNA methylation, meiosis, and spermiogenesis (Kuramochi-Miyagawa et al., 2004; Carmell et al., 2007; Chuma and Nakano, 2013; Fu and Wang, 2014). In a review of piRNA pathway-knockout mice, meiosis arrest is described as the most common mouse phenotype and is mainly caused by abnormal piRNA production or retrotransposon DNA methylation in fetal testis (Yang and Wang, 2016).
Substantial past efforts have led to an understanding of piRNA biogenesis, which is thought to occur through either the primary or the secondary pathway. Primary piRNA biogenesis is coupled with a secondary piRNA amplification loop, the ping-pong cycle, in which piRNA pools, generated through primary processing, guide the MILI protein to slice transposon transcripts, providing substrates for piRNA generation and leading to the accelerated amplification of transposon-derived piRNAs (Brennecke et al., 2007; Gunawardane et al., 2007; Aravin et al., 2008). Primary piRNA biogenesis is initiated by the transcription of primary piRNA precursors derived from genomic regions called piRNA clusters—genomic regions mapped with a high density of piRNA sequences (Girard et al., 2006; Brennecke et al., 2007; Malone et al., 2009).
De novo DNA methylation occurs in prospermatogonia/gonocytes. During reprogramming, all DNA methylation marks are erased before being reset in germ cells, exposing the germline to essential challenge (Schaefer et al., 2007; Trasler, 2009). Loss of DNA methylation results in the activation of normally silenced transposable elements. Correct DNA methylation of transposons is vital for successful meiosis in male germ cells. Transposon demethylation was repeatedly observed in the testes of piRNA pathway mutants (Table 1), thus the pathway has been proposed to play a role in the de novo methylation of retrotransposons (Aravin et al., 2007; Carmell et al., 2007; Kuramochi-Miyagawa et al., 2008).
PIWI-interacting RNA pathway consists of many evolutionarily conserved protein factors. This mini-review focuses on our current knowledge of protein factors in mammals by summarizing their roles in the biogenesis and function of piRNAs based on research with gene-knockout models.
Primary piRNA Biogenesis
Primary piRNA biogenesis is a stepwise process that starts with the transcription of long single-stranded precursor transcripts. A-MYB, which is the only transcription factor known to be involved in transcriptional regulation of pachytene piRNA precursor, also regulates the transcription of many pachytene piRNA pathway genes (Li et al., 2013). Through its ATP-dependent RNA helicase activity, MOV10L1 selectively binds to piRNA precursor transcripts and feeds them to MitoPLD, which catalyzes the first cleavage step of piRNA processing to generate piRNA intermediates. MOV10L1 is associated with MILI, MIWI, and MIWI2 (PIWIL4) in mouse testes; its expression emerges in prenatal gonocytes, peaks in pachytene spermatocytes, and ceases in post-meiotic spermatids. Disruption of Mov10l1 results in defects in both the transcriptional and posttranscriptional de-repression of transposons, consistent with the lack of retrotransposon-derived pre-pachytene piRNAs in Mov10l1 mutant testis (Zheng et al., 2010). Primary spermatocytes of Mov10l1–/– mice show the activation of long terminal repeat-containing retrotransposons and long interspersed nuclear element-1 (LINE1) retrotransposons, followed by cell death, causing infertility in males and the complete blockage of spermatogenesis at the zygotene stage of meiosis I prophase (Frost et al., 2010; Zheng et al., 2010; Vourekas et al., 2015).
MitoPLD is localized on the surface of the mitochondrial outer membrane in mouse germlines (Choi et al., 2006; Watanabe et al., 2011) and is a candidate for the nuclease that generates piRNA intermediates. In MitoPLD-mutant mouse testes, both primary and secondary piRNAs were significantly decreased, and piRNA biogenesis disruption was accompanied by a spike in LINE1 retrotransposon expression and genomic demethylation. MitoPLD-knockout mice showed arrested spermatogenesis at the meiosis zygotene stage (Huang et al., 2011; Watanabe et al., 2011), and MitoPLD has endoribonuclease activity on single-stranded RNAs in vitro (Ipsaro et al., 2012). A recent Bombyx mori study found that Zucchini (homolog of MitoPLD) requires Armi, GPAT1, and Gasz to cleave Siwi-loaded pre-pre-piRNAs in vitro (Izumi et al., 2020). In addition, the N6-methyadenosine (m6A) reader, YTHDC2, binds to specific piRNA precursors. P12 Ythdc2–/– mice exhibited much lower pachytene piRNA precursor levels than normal (Bailey et al., 2017).
MILI is one of three mouse homologs of the PIWI family that are defined by their conserved PAZ and Piwi domains. MILI, an important mediator of sense piRNA processing from retrotransposons and other cellular transcripts (Kuramochi-Miyagawa et al., 2004; Aravin et al., 2008), is expressed in the cytoplasm of testicular germline stem cells, spermatogonia, and early spermatocytes. In a mouse MILI-null mutant, spermatogenesis was completely blocked at the prophase of meiosis I from the zygotene to early pachytene (Kuramochi-Miyagawa et al., 2004). Acting as a piRNA-guided endonuclease, MILI initiates secondary piRNA biogenesis, which is vital for LINE1 and Intracisternal A particle (IAP) silencing (Aravin et al., 2007; De Fazio et al., 2011). Functions of MILI beyond piRNA biogenesis have been described recently. MILI forms a stable and RNA-independent complex with eIF3a and is associated with the eIF4E- and eIF4G-containing 5′-end 7-methylguanosine (m7G) cap-binding complex, which may positively regulate the translation of genes essential for germline stem cell self-renewal and differentiation (Unhavaithaya et al., 2009).
TDRKH, another mitochondria-anchored protein involved in primary piRNA biogenesis (Saxe et al., 2013), is a Tudor family protein that contains evolutionarily conserved Tudor and KH domains (Zhang et al., 2017a); it controls the entire MIWI/MIWI2-bound piRNA population and enables the trimming of MILI-bound piRNAs. Tdrkh mutants display meiotic arrest at the zygotene stage, with loss of DNA methylation of LINE1 retrotransposons and consequential retrotransposon de-repression (Saxe et al., 2013; Ding et al., 2019). Associated with MIWI and MIWI2 via the binding of symmetrically dimethylated arginine (sDMA), TDRKH is the scaffold for interactions between PIWI–piRNA complexes and PNLDC1. The exonuclease trims the 3′-end of piRNA intermediates to their mature length (Ding et al., 2017; Zhang et al., 2017b; Bronkhorst and Ketting, 2018; Nishimura et al., 2018). The 3′-end of mature piRNA is 2′-O-methylated by HENMT1, yet correct 3′ truncation is not necessary for 3′-end 2′-O-methylation (Yang et al., 2006; Zhai and Meyers, 2012; Peng et al., 2018). In addition, TUT4/7 mediates the 3′ uridylation of 30- to 31-nt-long piRNAs, but its effect is unknown (Morgan et al., 2019).
Secondary piRNA Biogenesis
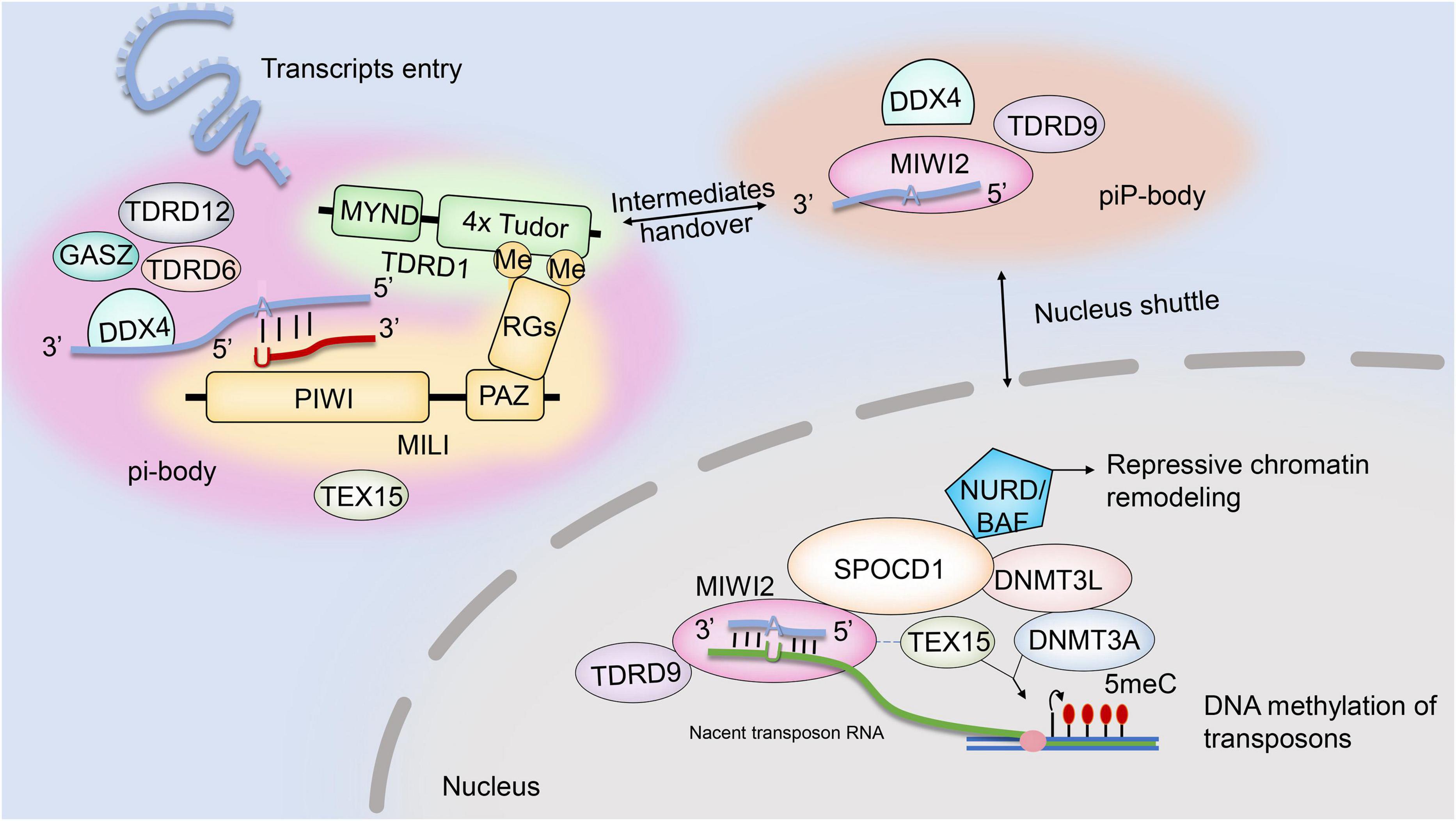
The piRNA pathway mediation of transposon posttranscriptional silencing is regulated by interactions between two RNA–protein complexes: pi-body and piP-body. While MILI–piRNA and MIWI2–piRNA complexes are key to the assembly and function of pi-body and piP-body, several other factors are also believed to be required. The existing evidence for the possible interactions and localizations of those factors is summarized in this review (Figure 1). Another member of the Piwi protein family, MIWI2, is coexpressed with MILI during embryonic testis development. Once loaded with secondary piRNAs, MIWI2 is shuttled from the cytoplasm to the nucleus to mediate repressive chromatin remodeling mainly via the promoter regions of transposons. However, it remains unclear whether MIWI2–piRNA complexes regulate the methylation patterns of other genomic regions (Schöpp et al., 2020). Loss of MIWI2 function affects the DNA methylation of LINE1 elements. Miwi2-deficient mice displayed zygotene-stage meiotic arrest, defective synapsis and double-strand break repair, and progressive loss of germ cells with age (Carmell et al., 2007).
TDRD9, a TDRD family member, was also investigated as an essential partner of MIWI2. TDRD9 complexes with MIWI2 through its Tudor domain, which binds to sDMA sites of MIWI2. TDRD9 is expressed in the cytoplasm and nucleus of embryonic prospermatogonia, mitotic spermatogonia, meiotic spermatocytes, and haploid spermatids in the testis (Shoji et al., 2009). Abolishing TDRD9 expression caused male mouse sterility and meiotic arrest at the zygotene stage: the spermatogenic cells faithfully initiated meiotic DNA recombination, but homologous chromosomes failed to undergo synapsis (Shoji et al., 2009). TDRD9 participates in the biogenesis of secondary piRNAs by ensuring the proper selection of Line1 sequences for the ping-pong amplification loop (Shoji et al., 2009). Although dispensable for piRNA biogenesis, TDRD9 ATPase activity is indispensable for its nuclear localization and transcriptional silencing of transposable elements (Wenda et al., 2017).
Other TDRD family members, including TDRD1 and TDRD12, interact with MILI in the ping-pong cycle. TDRD1 recognizes arginine dimethylation in MILI (Chuma et al., 2003, 2006) and may regulate the entry of transcripts into piRNA biogenesis pathways. The loss of TDRD1 does not affect the abundance of MILI-bond piRNA but rather its constituents: ribosomal RNA- and genic-derived piRNA proportions increase, and transposon-derived piRNAs in MILI ribonucleoprotein (RNP) populations change substantially. In addition, the correct nuclear localization of Miwi2 needed for LINE1 transposon methylation was almost lost, and LINE1 transposons were repressed as consequences of TDRD1 knockout (Reuter et al., 2009). TDRD1 also draws ping-pong cycle factors together to promote their activity. DDX4 and FKBP6, components of the TDRD1 protein complex, are required for the loading of MIWI2-bound secondary piRNAs. FKBP6 may recruit HSP90AA1 for the loading of secondary piRNA intermediates onto MIWI2 (Xiol et al., 2012).
TDRD12 forms complexes with MILI piRNP in an RNA-dependent manner and is associated with TDRD1. TDRD12 might facilitate the RNP remodeling required for the inter-Piwi (MILI and MIWI2) exchange of piRNA intermediates essential for the biogenesis of MIWI2 piRNAs (Pandey et al., 2013). The biogenesis of piRNAs that associate with MILI appeared normal, with unchanged genome annotation profiles, in mice lacking TDRD12; however, MIWI2-bond piRNA biogenesis was almost absent. When TDRD12 was deficient, spermatogenesis stalled in the zygotene–pachytene transition stage of meiosis.
DDX4, which is expressed in the cytoplasm of various male germ cells (E10.5 to round spermatids) (Kuramochi-Miyagawa et al., 2010), has RNA helicase activity (Sengoku et al., 2006) and N terminal sDMAs characterized by Tudor domains (Kirino et al., 2010). Multiple mouse models have been adopted to investigate the roles of DDX4 in spermatogenesis and piRNA pathways. DDX4-knockout mice exhibited complete spermatogenic arrest at the zygotene stage, and mutation of the RNA-helicase domain of DDX4 (DDX4 was expressed normally but catalytically dead) also disrupted spermatogenesis. Ddx4–/catalytically dead mouse spermatogenesis did not proceed beyond meiotic pachytene in spermatocytes, while germ cells in Ddx4+/catalytically dead mice completed meiosis but uniformly arrested during the development of round spermatids (Wenda et al., 2017). The essential role of DDX4 in the piRNA pathway was recently revealed: DDX4 is required for RNP remodeling during the loading of secondary piRNA intermediates onto MIWI2. The endonucleolytic cleavage of a target transcript by cytosolic MILI generates a piRNA precursor, which is processed into phased pre-piRNA intermediates (Han et al., 2015; Mohn et al., 2015; Yang et al., 2016). Mice lacking catalytically active DDX4 were still able to generate MILI slicer products but failed to transfer pre-piRNA intermediates to the ping-pong biogenesis machinery. Therefore, no MIWI2-bound piRNA was detected in mice with catalytically dead DDX4, and MIWI2 failed to maintain the necessary DNA methylation of L1 retrotransposons (Wenda et al., 2017). Furthermore, catalytically dead DDX4 also trapped MILI and MIWI, pachytene piRNAs, and slicer products of transposon and genic mRNAs, suggesting it functions in posttranscriptional regulation in post-meiotic stages (Wenda et al., 2017). In addition, reduced GTSF1 protein, which co-localizes with TDRD9 and MIWI2 in piP-bodies, resulted in target RNA remaining unsliced at the cleavage site for MILI-directed secondary piRNA processing (Yoshimura et al., 2018).
Transposable Element Methylation by PIWI Pathway
Mael is highly expressed in mouse testes, and the protein’s location alternates throughout spermatogenesis. MAEL, found in the cytoplasm in spermatocytes and shuttled to the nucleus in spermatids (Soper et al., 2008; Pandey and Pillai, 2014), comprises a high-mobility group box and a MAEL domain that is predicted to adopt an RNase H-like fold. Meiotic entry was delayed in Mael-null spermatogenic cells (Soper et al., 2008). Although Mael-knockout mice phenocopied Mili- and Miwi2-knockout mice, pre-pachytene arrest was intact in Mael-null testes. MAEL is speculated to function in post-piRNA production steps by facilitating the nucleo-cytoplasmic trafficking of MIWI2–piRNA complexes (Soper et al., 2008; Pandey and Pillai, 2014). In post-meiotic spermatogenesis, MAEL interacts with MILI, MIWI, and TDRD6, binding pachytene piRNA precursors and enabling piRNA intermediate processing (Pandey and Pillai, 2014; Sato and Siomi, 2015).
A recent study revealed that TEX15, a nuclear protein, is an essential partner of MIWI2 in piRNA-directed de novo methylation and silencing of transposable elements in fetal gonocytes (Schöpp et al., 2020). TEX15 contains a DUF3715 domain, which is also found in other TE-silencing proteins (Tchasovnikarova et al., 2015; Liu et al., 2018). In TEX15-null spermatocytes, SPO11-mediated DSB formation was normal, but DSB repair was absent because of a failure in the DMC1 assembly, resulting in zygotene-stage meiotic arrest (Yang et al., 2008). Although TEX15 interacts with MILI in the cytoplasm, it is not required for primary or secondary piRNA biogenesis in mouse gonocytes. TEX15 also interacts with MIWI2 in the nucleus in an RNA/DNA-dependent manner, yet the nuclear localization of MIWI2 remains unchanged in TEX15-null gonocytes. Considering that loss of TEX15 causes demethylation in LINE1 and IAP transposon promoter regions, it may be a predominant nuclear executor of TE de novo methylation downstream of piRNA pathways (Schöpp et al., 2020; Yang et al., 2020).
SPOCD1, another MIWI2 interactome member, facilitates MIWI2 activity in the nucleus. Spocd1-null spermatocytes undergo early-pachytene-stage meiotic arrest, but both primary and secondary piRNA biogeneses remain. Loss of IAP and LINE1 transposon de novo DNA methylation and consequential transposon de-repression were observed in Spocd1-knockout testes. SPOCD1 engages with MIWI2 in an RNA/DNA-dependent manner and facilitates MIWI2 nuclear activity by summoning chromatin remodeling and DNA methylation machinery to the promoters of transcribing transposons (Zoch et al., 2020). SPOCD1 contains a SPOC domain, which was previously found to recruit transcriptional repressors (Ariyoshi and Schwabe, 2003; Mikami et al., 2014), and a nuclear localization signal. SPOCD1 co-immunoprecipitated with DNMT3L and DNMT3A, components of the de novo methylation machinery and the NURD (Kloet et al., 2015) and BAF (Mashtalir et al., 2018) repressive chromatin remodeling complexes.
UHRF1 maintains the crosstalk between the PIWI pathway and repressive chromatin remodeling machinery. UHRF1 was found to be abundant in the nuclei of neonatal prospermatonia at P0, as well as spermatogonia, late pachytene spermatocytes, and early round spermatids, and shifted into the cytoplasm of fetal prospermatogonia during spermatocyte E15.5, pre-leptotene, leptotene, zygotene, and early pachytene. The conditional deletion of Uhrf1 in differentiating spermatogonia led to pachytene-stage meiotic arrest. UHRF1 interacts with PRMT5 (Kirino et al., 2009; Zhao et al., 2009; Wang et al., 2015), an arginine methyltransferase, to regulate repressive histone arginine modifications (H4R3me2s and H3R2me2s) (Ancelin et al., 2006; Migliori et al., 2012) and piRNA biogenesis by controlling the localization of PIWI pathway proteins (MILI, MIWI, and TDRKH). UHRF1 depletion also induces global loss of DNA methylation during spermatogenesis. UHRF1 appears to play essential roles in the crosstalk between the piRNA pathway and repressive epigenetic pathways, providing new clues to piRNA pathway functions (Dong et al., 2019).
Repression of LINE1 Retrotransposons in Germ Cells
LINE1 retrotransposons are members of the most abundant class of transposable elements in mammals, accounting for ∼20% of mouse and human genomes. Up to 3,000 and 100 copies of LINE1 are intact and active in mice (Deberardinis et al., 1998) and humans (Sassaman et al., 1997; Mandal and Kazazian, 2008), respectively. In male piRNA pathway mutants, LINE1 activated late embryonic germ cells or early and mid-pachytene spermatocytes (Yang and Wang, 2016). Most male mouse piRNA pathway mutants exhibit meiotic arrest and sterility, but this effect is not observed in females (Yang and Wang, 2016). Notably, LINE1 de-repression in spermatocytes does not necessarily lead to meiotic arrest, such as in Henmt1-knockout animals (Lim et al., 2015). Some mouse mutants of Miwi (Deng and Lin, 2002), Pnldc1 (Ding et al., 2017; Zhang et al., 2017c; Bronkhorst and Ketting, 2018; Nishimura et al., 2018), Tdrd5 (Yabuta et al., 2011; Ding et al., 2018), and Henmt1 (Lim et al., 2015), etc., still produce post-meiotic germ cells. Interestingly, although a large proportion of MIWI-piRNAs were thought to originate from non-transposon-related regions (Vourekas et al., 2012), LINE1 de-repression was found in Miwi-knockout mouse spermatids (Reuter et al., 2011). MIWI slicer activity involved in the direct cleavage of transposon mRNAs in spermatids (Reuter et al., 2011) is also chromatoid body location dependent but may not be piRNA dependent (Ding et al., 2019). In a Pnldc1 mutant, dramatically reduced MIWI protein and MIWI-piRNAs, without spermatid LINE1 de-repression, were seen (Ding et al., 2017; Zhang et al., 2017c; Nishimura et al., 2018), and the remaining MIWI in the mutant possibly played a role in LINE1 repression (Ding et al., 2019). Spermatids in Henmt1-knockout mice also showed activated LINE1 that was unassociated with MIWI slicer activity (Lim et al., 2015). These results suggest that LINE1 repression also occurs in spermatids. Most piRNA factor knockouts display meiotic arrest; therefore, there is a lack of information on LINE1 repression after meiosis. Pachytene piRNA cluster is usually non-repeat origin, thus the mechanism of LINE1 repression after meiosis needs further exploration. The active LINE1 ORF1p is often found in the cytoplasm of spermatocytes but is more commonly seen in round spermatid nuclei, although the reason for this is unknown. A recent conditional knockout (cKO) study provided examples of how this process can be explored; TdrkhcKO driven by Stra8-Cre, but not Mov10l1cKO, showed obvious LINE1 de-repression in spermatids (Ding et al., 2019). This raises questions about whether piRNA factor genes expressed in spermatids, such as Tdrd1, Asz1, Mybl1, Ddx4, Tdrd9, Mael, Gtsf1, Uhrf1, Tut4/7, and Tdrd5, are involved in LINE1 inhibition after meiosis (Table 1).
Because of knockout mouse studies, piRNA pathway is believed to be unnecessary in mammalian female germ cells (Yang and Wang, 2016). In mouse oocytes, the ribonuclease MARF1, which is not associated with piRNA, is considered to be involved in LINE1 inhibition in oocytes (Su et al., 2012a,b; Yao et al., 2018). This phenomenon suggests that a transposon inhibition system other than piRNA may function in mouse oocytes. Apart from mice, most mammals have four PIWI genes. PIWIL3, which is not expressed in mice, binds to a class of piRNAs of 19 and 20 nt in hamster and human oocytes, respectively (Yang et al., 2019; Ishino et al., 2021). PIWIL3-deficient female hamsters have reduced fertility (Hasuwa et al., 2021). Furthermore, abolishing piRNA factors PIWIL1, PLD6, and MOV10L1 in golden hamsters led to female infertility, with embryos arresting at the two-cell stage (Ishino et al., 2021; Zhang et al., 2021). Therefore, the function of piRNA in oocytes may be significantly different among mammalian species.
Conclusion
Previous studies using knockout mice have revealed the formation of piRNA in mammals and its role in male germ cells. Most piRNA factor knockouts showed spermatogenesis arrest in meiosis, but a few showed male germ cell arrest after meiosis. It is unclear whether the majority of piRNA factors expressed in spermatids are involved in LINE1 repression after meiosis, and future cKO research is required. In addition, in recent hamster gene-knockout studies, a piRNA factor was found to be necessary for oocytes, a complete contrast to findings in mice. This species difference allows researchers to reexamine the function of piRNA in female germ cells, which should broaden our knowledge on female infertility in humans.
Author Contributions
ML, YZ, and YL: conceptualization. YL and YZ: literature search. ML, YZ, and YL: writing—original draft preparation. ML and YL: writing—review and editing. YL: visualization of histological structures. All authors read and approved the final version of manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (2016YFA0500902 to ML), the Natural Science Foundation of China (32070842 and 31771654 to ML and 82001614 to YZ), the Natural Science Foundation of Jiangsu Province (Grant No. BK20190081 to ML), and the Qing Lan Project (ML).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Ancelin, K., Lange, U. C., Hajkova, P., Schneider, R., Bannister, A. J., Kouzarides, T., et al. (2006). Blimp1 associates with Prmt5 and directs histone arginine methylation in mouse germ cells. Nat. Cell Biol. 8, 623–630. doi: 10.1038/ncb1413
Aravin, A., Gaidatzis, D., Pfeffer, S., Lagos-Quintana, M., Landgraf, P., Iovino, N., et al. (2006). A novel class of small RNAs bind to MILI protein in mouse testes. Nature 442, 203–207. doi: 10.1038/nature04916
Aravin, A. A., Klenov, M. S., Vagin, V. V., Bantignies, F., Cavalli, G., and Gvozdev, V. A. (2004). Dissection of a natural RNA silencing process in the Drosophila melanogaster germ line. Mol. Cell Biol. 24, 6742–6750. doi: 10.1128/mcb.24.15.6742-6750.2004
Aravin, A. A., Sachidanandam, R., Bourc’his, D., Schaefer, C., Pezic, D., Toth, K. F., et al. (2008). A piRNA pathway primed by individual transposons is linked to de novo DNA methylation in mice. Mol. Cell. 31, 785–799. doi: 10.1016/j.molcel.2008.09.003
Aravin, A. A., Sachidanandam, R., Girard, A., Fejes-Toth, K., and Hannon, G. J. (2007). Developmentally regulated piRNA clusters implicate MILI in transposon control. Science 316, 744–747. doi: 10.1126/science.1142612
Ariyoshi, M., and Schwabe, J. W. (2003). A conserved structural motif reveals the essential transcriptional repression function of Spen proteins and their role in developmental signaling. Genes Dev. 17, 1909–1920. doi: 10.1101/gad.266203
Bailey, A. S., Batista, P. J., Gold, R. S., Chen, Y. G., De Rooij, D. G., Chang, H. Y., et al. (2017). The conserved RNA helicase YTHDC2 regulates the transition from proliferation to differentiation in the germline. eLife 6:e26116.
Brennecke, J., Aravin, A. A., Stark, A., Dus, M., Kellis, M., Sachidanandam, R., et al. (2007). Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell 128, 1089–1103. doi: 10.1016/j.cell.2007.01.043
Bronkhorst, A. W., and Ketting, R. F. (2018). Trimming it short: PNLDC1 is required for piRNA maturation during mouse spermatogenesis. EMBO Rep. 19:e45824.
Carmell, M. A., Girard, A., Van De Kant, H. J., Bourc’his, D., Bestor, T. H., De Rooij, D. G., et al. (2007). MIWI2 is essential for spermatogenesis and repression of transposons in the mouse male germline. Dev. Cell 12, 503–514. doi: 10.1016/j.devcel.2007.03.001
Carrieri, C., Comazzetto, S., Grover, A., Morgan, M., Buness, A., Nerlov, C., et al. (2017). A transit-amplifying population underpins the efficient regenerative capacity of the testis. J. Exp. Med. 214, 1631–1641. doi: 10.1084/jem.20161371
Castañeda, J., Genzor, P., Van Der Heijden, G. W., Sarkeshik, A., Yates, J. R. III, Ingolia, N. T., et al. (2014). Reduced pachytene piRNAs and translation underlie spermiogenic arrest in Maelstrom mutant mice. EMBO J. 33, 1999–2019. doi: 10.15252/embj.201386855
Chen, C., Jin, J., James, D. A., Adams-Cioaba, M. A., Park, J. G., Guo, Y., et al. (2009). Mouse Piwi interactome identifies binding mechanism of Tdrkh Tudor domain to arginine methylated Miwi. Proc. Natl. Acad. Sci. U.S.A. 106, 20336–20341. doi: 10.1073/pnas.0911640106
Choi, S. Y., Huang, P., Jenkins, G. M., Chan, D. C., Schiller, J., and Frohman, M. A. (2006). A common lipid links Mfn-mediated mitochondrial fusion and SNARE-regulated exocytosis. Nat. Cell Biol. 8, 1255–1262. doi: 10.1038/ncb1487
Chuma, S., Hiyoshi, M., Yamamoto, A., Hosokawa, M., Takamune, K., and Nakatsuji, N. (2003). Mouse Tudor Repeat-1 (MTR-1) is a novel component of chromatoid bodies/nuages in male germ cells and forms a complex with snRNPs. Mech. Dev. 120, 979–990. doi: 10.1016/s0925-4773(03)00181-3
Chuma, S., Hosokawa, M., Kitamura, K., Kasai, S., Fujioka, M., Hiyoshi, M., et al. (2006). Tdrd1/Mtr-1, a tudor-related gene, is essential for male germ-cell differentiation and nuage/germinal granule formation in mice. Proc. Natl. Acad. Sci. U.S.A. 103, 15894–15899. doi: 10.1073/pnas.0601878103
Chuma, S., and Nakano, T. (2013). piRNA and spermatogenesis in mice. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368:20110338. doi: 10.1098/rstb.2011.0338
Costa, Y., Speed, R. M., Gautier, P., Semple, C. A., Maratou, K., Turner, J. M., et al. (2006). Mouse MAELSTROM: the link between meiotic silencing of unsynapsed chromatin and microRNA pathway? Hum. Mol. Genet. 15, 2324–2334. doi: 10.1093/hmg/ddl158
Crackower, M. A., Kolas, N. K., Noguchi, J., Sarao, R., Kikuchi, K., Kaneko, H., et al. (2003). Essential role of Fkbp6 in male fertility and homologous chromosome pairing in meiosis. Science 300, 1291–1295. doi: 10.1126/science.1083022
Dai, P., Wang, X., Gou, L. T., Li, Z. T., Wen, Z., Chen, Z. G., et al. (2019). A translation-activating function of MIWI/piRNA during mouse spermiogenesis. Cell 179, 1566.e16–1581.e16.
Dai, P., Wang, X., and Liu, M. F. (2020). A dual role of the PIWI/piRNA machinery in regulating mRNAs during mouse spermiogenesis. Sci. China Life Sci. 63, 447–449. doi: 10.1007/s11427-020-1632-5
De Fazio, S., Bartonicek, N., Di Giacomo, M., Abreu-Goodger, C., Sankar, A., Funaya, C., et al. (2011). The endonuclease activity of Mili fuels piRNA amplification that silences LINE1 elements. Nature 480, 259–263. doi: 10.1038/nature10547
Deberardinis, R. J., Goodier, J. L., Ostertag, E. M., and Kazazian, H. H. Jr. (1998). Rapid amplification of a retrotransposon subfamily is evolving the mouse genome. Nat. Genet. 20, 288–290. doi: 10.1038/3104
Deng, W., and Lin, H. (2002). miwi, a murine homolog of piwi, encodes a cytoplasmic protein essential for spermatogenesis. Dev. Cell 2, 819–830. doi: 10.1016/s1534-5807(02)00165-x
Di Giacomo, M., Comazzetto, S., Saini, H., De Fazio, S., Carrieri, C., Morgan, M., et al. (2013). Multiple epigenetic mechanisms and the piRNA pathway enforce LINE1 silencing during adult spermatogenesis. Mol. Cell 50, 601–608. doi: 10.1016/j.molcel.2013.04.026
Ding, D., Liu, J., Dong, K., Melnick, A. F., Latham, K. E., and Chen, C. (2019). Mitochondrial membrane-based initial separation of MIWI and MILI functions during pachytene piRNA biogenesis. Nucleic Acids Res. 47, 2594–2608. doi: 10.1093/nar/gky1281
Ding, D., Liu, J., Dong, K., Midic, U., Hess, R. A., Xie, H., et al. (2017). PNLDC1 is essential for piRNA 3’ end trimming and transposon silencing during spermatogenesis in mice. Nat. Commun. 8:819.
Ding, D., Liu, J., Midic, U., Wu, Y., Dong, K., Melnick, A., et al. (2018). TDRD5 binds piRNA precursors and selectively enhances pachytene piRNA processing in mice. Nat. Commun. 9:127.
Dönertas, D., Sienski, G., and Brennecke, J. (2013). Drosophila Gtsf1 is an essential component of the Piwi-mediated transcriptional silencing complex. Genes Dev. 27, 1693–1705. doi: 10.1101/gad.221150.113
Dong, J., Wang, X., Cao, C., Wen, Y., Sakashita, A., Chen, S., et al. (2019). UHRF1 suppresses retrotransposons and cooperates with PRMT5 and PIWI proteins in male germ cells. Nat. Commun. 10:4705.
Frost, R. J., Hamra, F. K., Richardson, J. A., Qi, X., Bassel-Duby, R., and Olson, E. N. (2010). MOV10L1 is necessary for protection of spermatocytes against retrotransposons by Piwi-interacting RNAs. Proc. Natl. Acad. Sci. U.S.A. 107, 11847–11852. doi: 10.1073/pnas.1007158107
Fu, Q., and Wang, P. J. (2014). Mammalian piRNAs: biogenesis, function, and mysteries. Spermatogenesis 4:e27889. doi: 10.4161/spmg.27889
Girard, A., Sachidanandam, R., Hannon, G. J., and Carmell, M. A. (2006). A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature 442, 199–202. doi: 10.1038/nature04917
Gou, L. T., Dai, P., Yang, J. H., Xue, Y., Hu, Y. P., Zhou, Y., et al. (2014). Pachytene piRNAs instruct massive mRNA elimination during late spermiogenesis. Cell Res. 24, 680–700. doi: 10.1038/cr.2014.41
Gou, L. T., Kang, J. Y., Dai, P., Wang, X., Li, F., Zhao, S., et al. (2017). Ubiquitination-deficient mutations in human piwi cause male infertility by impairing histone-to-protamine exchange during spermiogenesis. Cell 169, 1090.e13–1104.e13.
Grad, I., Cederroth, C. R., Walicki, J., Grey, C., Barluenga, S., Winssinger, N., et al. (2010). The molecular chaperone Hsp90α is required for meiotic progression of spermatocytes beyond pachytene in the mouse. PLoS One 5:e15770. doi: 10.1371/journal.pone.0015770
Grivna, S. T., Beyret, E., Wang, Z., and Lin, H. (2006). A novel class of small RNAs in mouse spermatogenic cells. Genes Dev. 20, 1709–1714. doi: 10.1101/gad.1434406
Gunawardane, L. S., Saito, K., Nishida, K. M., Miyoshi, K., Kawamura, Y., Nagami, T., et al. (2007). A slicer-mediated mechanism for repeat-associated siRNA 5’ end formation in Drosophila. Science 315, 1587–1590. doi: 10.1126/science.1140494
Han, B. W., Wang, W., Li, C., Weng, Z., and Zamore, P. D. (2015). Noncoding RNA. piRNA-guided transposon cleavage initiates Zucchini-dependent, phased piRNA production. Science 348, 817–821. doi: 10.1126/science.aaa1264
Handler, D., Olivieri, D., Novatchkova, M., Gruber, F. S., Meixner, K., Mechtler, K., et al. (2011). A systematic analysis of Drosophila TUDOR domain-containing proteins identifies Vreteno and the Tdrd12 family as essential primary piRNA pathway factors. EMBO J. 30, 3977–3993. doi: 10.1038/emboj.2011.308
Hasuwa, H., Iwasaki, Y. W., Wan Kin, A. Y., Ishino, K., Masuda, H., Sasaki, H., et al. (2021). Production of functional oocytes requires maternally expressed PIWI genes and piRNAs in golden hamsters. bioRxiv [Preprint]. doi: 10.1101/2021.01.27.428354v2
Horvath, G. C., Kistler, M. K., and Kistler, W. S. (2009). RFX2 is a candidate downstream amplifier of A-MYB regulation in mouse spermatogenesis. BMC Dev. Biol. 9:63. doi: 10.1186/1471-213X-9-63
Huang, H., Gao, Q., Peng, X., Choi, S. Y., Sarma, K., Ren, H., et al. (2011). piRNA-associated germline nuage formation and spermatogenesis require MitoPLD profusogenic mitochondrial-surface lipid signaling. Dev. Cell 20, 376–387. doi: 10.1016/j.devcel.2011.01.004
Ichiyanagi, T., Ichiyanagi, K., Ogawa, A., Kuramochi-Miyagawa, S., Nakano, T., Chuma, S., et al. (2014). HSP90α plays an important role in piRNA biogenesis and retrotransposon repression in mouse. Nucleic Acids Res. 42, 11903–11911. doi: 10.1093/nar/gku881
Ipsaro, J. J., Haase, A. D., Knott, S. R., Joshua-Tor, L., and Hannon, G. J. (2012). The structural biochemistry of Zucchini implicates it as a nuclease in piRNA biogenesis. Nature 491, 279–283. doi: 10.1038/nature11502
Ishino, K., Hasuwa, H., Yoshimura, J., Iwasaki, Y. W., Nishihara, H., Seki, N. M., et al. (2021). Hamster PIWI proteins bind to piRNAs with stage-specific size variations during oocyte maturation. Nucleic Acids Res. 49, 2700–2720. doi: 10.1093/nar/gkab059
Izumi, N., Shoji, K., Suzuki, Y., Katsuma, S., and Tomari, Y. (2020). Zucchini consensus motifs determine the mechanism of pre-piRNA production. Nature 578, 311–316. doi: 10.1038/s41586-020-1966-9
Kirino, Y., Kim, N., De Planell-Saguer, M., Khandros, E., Chiorean, S., Klein, P. S., et al. (2009). Arginine methylation of Piwi proteins catalysed by dPRMT5 is required for Ago3 and Aub stability. Nat. Cell Biol. 11, 652–658. doi: 10.1038/ncb1872
Kirino, Y., and Mourelatos, Z. (2007). The mouse homolog of HEN1 is a potential methylase for Piwi-interacting RNAs. RNA 13, 1397–1401. doi: 10.1261/rna.659307
Kirino, Y., Vourekas, A., Kim, N., De Lima Alves, F., Rappsilber, J., Klein, P. S., et al. (2010). Arginine methylation of vasa protein is conserved across phyla. J. Biol. Chem. 285, 8148–8154. doi: 10.1074/jbc.m109.089821
Kloet, S. L., Baymaz, H. I., Makowski, M., Groenewold, V., Jansen, P. W., Berendsen, M., et al. (2015). Towards elucidating the stability, dynamics and architecture of the nucleosome remodeling and deacetylase complex by using quantitative interaction proteomics. FEBS J. 282, 1774–1785. doi: 10.1111/febs.12972
Kotaja, N., and Sassone-Corsi, P. (2007). The chromatoid body: a germ-cell-specific RNA-processing centre. Nat. Rev. Mol. Cell Biol. 8, 85–90. doi: 10.1038/nrm2081
Kuramochi-Miyagawa, S., Kimura, T., Ijiri, T. W., Isobe, T., Asada, N., Fujita, Y., et al. (2004). Mili, a mammalian member of piwi family gene, is essential for spermatogenesis. Development 131, 839–849. doi: 10.1242/dev.00973
Kuramochi-Miyagawa, S., Watanabe, T., Gotoh, K., Takamatsu, K., Chuma, S., Kojima-Kita, K., et al. (2010). MVH in piRNA processing and gene silencing of retrotransposons. Genes Dev. 24, 887–892. doi: 10.1101/gad.1902110
Kuramochi-Miyagawa, S., Watanabe, T., Gotoh, K., Totoki, Y., Toyoda, A., Ikawa, M., et al. (2008). DNA methylation of retrotransposon genes is regulated by Piwi family members MILI and MIWI2 in murine fetal testes. Genes Dev. 22, 908–917. doi: 10.1101/gad.1640708
Li, F., Yuan, P., Rao, M., Jin, C. H., Tang, W., Rong, Y. F., et al. (2020). piRNA-independent function of PIWIL1 as a co-activator for anaphase promoting complex/cyclosome to drive pancreatic cancer metastasis. Nat. Cell Biol. 22, 425–438. doi: 10.1038/s41556-020-0486-z
Li, X. Z., Roy, C. K., Dong, X., Bolcun-Filas, E., Wang, J., Han, B. W., et al. (2013). An ancient transcription factor initiates the burst of piRNA production during early meiosis in mouse testes. Mol. Cell 50, 67–81. doi: 10.1016/j.molcel.2013.02.016
Lim, S. L., Qu, Z. P., Kortschak, R. D., Lawrence, D. M., Geoghegan, J., Hempfling, A. L., et al. (2015). HENMT1 and piRNA stability are required for adult male germ cell transposon repression and to define the spermatogenic program in the mouse. PLoS Genet. 11:e1005620. doi: 10.1371/journal.pgen.1005620
Liu, N., Lee, C. H., Swigut, T., Grow, E., Gu, B., Bassik, M. C., et al. (2018). Selective silencing of euchromatic L1s revealed by genome-wide screens for L1 regulators. Nature 553, 228–232. doi: 10.1038/nature25179
Ma, L., Buchold, G. M., Greenbaum, M. P., Roy, A., Burns, K. H., Zhu, H., et al. (2009). GASZ is essential for male meiosis and suppression of retrotransposon expression in the male germline. PLoS Genet. 5:e1000635. doi: 10.1371/journal.pgen.1000635
Malone, C. D., Brennecke, J., Dus, M., Stark, A., Mccombie, W. R., Sachidanandam, R., et al. (2009). Specialized piRNA pathways act in germline and somatic tissues of the Drosophila ovary. Cell 137, 522–535. doi: 10.1016/j.cell.2009.03.040
Mashtalir, N., D’avino, A. R., Michel, B. C., Luo, J., Pan, J., Otto, J. E., et al. (2018). Modular organization and assembly of SWI/SNF family chromatin remodeling complexes. Cell 175, 1272.e20–1288.e20.
Migliori, V., Müller, J., Phalke, S., Low, D., Bezzi, M., Mok, W. C., et al. (2012). Symmetric dimethylation of H3R2 is a newly identified histone mark that supports euchromatin maintenance. Nat. Struct. Mol. Biol. 19, 136–144. doi: 10.1038/nsmb.2209
Mikami, S., Kanaba, T., Takizawa, N., Kobayashi, A., Maesaki, R., Fujiwara, T., et al. (2014). Structural insights into the recruitment of SMRT by the corepressor SHARP under phosphorylative regulation. Structure 22, 35–46. doi: 10.1016/j.str.2013.10.007
Mohn, F., Handler, D., and Brennecke, J. (2015). Noncoding RNA. piRNA-guided slicing specifies transcripts for Zucchini-dependent, phased piRNA biogenesis. Science 348, 812–817. doi: 10.1126/science.aaa1039
Morgan, M., Kabayama, Y., Much, C., Ivanova, I., Di Giacomo, M., Auchynnikava, T., et al. (2019). A programmed wave of uridylation-primed mRNA degradation is essential for meiotic progression and mammalian spermatogenesis. Cell Res. 29, 221–232. doi: 10.1038/s41422-018-0128-1
Nishimasu, H., Ishizu, H., Saito, K., Fukuhara, S., Kamatani, M. K., Bonnefond, L., et al. (2012). Structure and function of Zucchini endoribonuclease in piRNA biogenesis. Nature 491, 284–287. doi: 10.1038/nature11509
Nishimura, T., Nagamori, I., Nakatani, T., Izumi, N., Tomari, Y., Kuramochi-Miyagawa, S., et al. (2018). PNLDC1, mouse pre-piRNA Trimmer, is required for meiotic and post-meiotic male germ cell development. EMBO Rep. 19:e44957.
Noce, T., Okamoto-Ito, S., and Tsunekawa, N. (2001). Vasa homolog genes in mammalian germ cell development. Cell Struct. Funct. 26, 131–136. doi: 10.1247/csf.26.131
Ohtani, H., Iwasaki, Y. W., Shibuya, A., Siomi, H., Siomi, M. C., and Saito, K. (2013). DmGTSF1 is necessary for Piwi-piRISC-mediated transcriptional transposon silencing in the Drosophila ovary. Genes Dev. 27, 1656–1661. doi: 10.1101/gad.221515.113
Oud, M. S., Volozonoka, L., Friedrich, C., Kliesch, S., Nagirnaja, L., Gilissen, C., et al. (2021). Lack of evidence for a role of PIWIL1 variants in human male infertility. Cell 184, 1941–1942. doi: 10.1016/j.cell.2021.03.001
Pandey, R. R., and Pillai, R. S. (2014). Primary piRNA biogenesis: caught up in a Maelstrom. EMBO J. 33, 1979–1980. doi: 10.15252/embj.201489670
Pandey, R. R., Tokuzawa, Y., Yang, Z., Hayashi, E., Ichisaka, T., Kajita, S., et al. (2013). Tudor domain containing 12 (TDRD12) is essential for secondary PIWI interacting RNA biogenesis in mice. Proc. Natl. Acad. Sci. U.S.A. 110, 16492–16497. doi: 10.1073/pnas.1316316110
Peng, L., Zhang, F., Shang, R., Wang, X., Chen, J., Chou, J. J., et al. (2018). Identification of substrates of the small RNA methyltransferase Hen1 in mouse spermatogonial stem cells and analysis of its methyl-transfer domain. J. Biol. Chem. 293, 9981–9994. doi: 10.1074/jbc.ra117.000837
Reuter, M., Berninger, P., Chuma, S., Shah, H., Hosokawa, M., Funaya, C., et al. (2011). Miwi catalysis is required for piRNA amplification-independent LINE1 transposon silencing. Nature 480, 264–267. doi: 10.1038/nature10672
Reuter, M., Chuma, S., Tanaka, T., Franz, T., Stark, A., and Pillai, R. S. (2009). Loss of the Mili-interacting Tudor domain-containing protein-1 activates transposons and alters the Mili-associated small RNA profile. Nat. Struct. Mol. Biol. 16, 639–646. doi: 10.1038/nsmb.1615
Saga, Y. (2008). Mouse germ cell development during embryogenesis. Curr. Opin. Genet. Dev. 18, 337–341. doi: 10.1016/j.gde.2008.06.003
Sassaman, D. M., Dombroski, B. A., Moran, J. V., Kimberland, M. L., Naas, T. P., Deberardinis, R. J., et al. (1997). Many human L1 elements are capable of retrotransposition. Nat. Genet. 16, 37–43. doi: 10.1038/ng0597-37
Sato, K., and Siomi, M. C. (2015). Functional and structural insights into the piRNA factor Maelstrom. FEBS Lett. 589, 1688–1693. doi: 10.1016/j.febslet.2015.03.023
Saxe, J. P., Chen, M., Zhao, H., and Lin, H. (2013). Tdrkh is essential for spermatogenesis and participates in primary piRNA biogenesis in the germline. EMBO J. 32, 1869–1885. doi: 10.1038/emboj.2013.121
Schaefer, C. B., Ooi, S. K., Bestor, T. H., and Bourc’his, D. (2007). Epigenetic decisions in mammalian germ cells. Science 316, 398–399. doi: 10.1126/science.1137544
Schöpp, T., Zoch, A., Berrens, R. V., Auchynnikava, T., Kabayama, Y., Vasiliauskaitė, L., et al. (2020). TEX15 is an essential executor of MIWI2-directed transposon DNA methylation and silencing. Nat. Commun. 11:3739.
Sengoku, T., Nureki, O., Nakamura, A., Kobayashi, S., and Yokoyama, S. (2006). Structural basis for RNA unwinding by the DEAD-box protein Drosophila vasa. Cell 125, 287–300. doi: 10.1016/j.cell.2006.01.054
Shoji, M., Tanaka, T., Hosokawa, M., Reuter, M., Stark, A., Kato, Y., et al. (2009). The TDRD9-MIWI2 complex is essential for piRNA-mediated retrotransposon silencing in the mouse male germline. Dev. Cell 17, 775–787. doi: 10.1016/j.devcel.2009.10.012
Siomi, M. C., and Kuramochi-Miyagawa, S. (2009). RNA silencing in germlines–exquisite collaboration of Argonaute proteins with small RNAs for germline survival. Curr. Opin. Cell Biol. 21, 426–434. doi: 10.1016/j.ceb.2009.02.003
Smith, J. M., Bowles, J., Wilson, M., Teasdale, R. D., and Koopman, P. (2004). Expression of the tudor-related gene Tdrd5 during development of the male germline in mice. Gene Expr. Patterns 4, 701–705. doi: 10.1016/j.modgep.2004.04.002
Soper, S. F., Van Der Heijden, G. W., Hardiman, T. C., Goodheart, M., Martin, S. L., De Boer, P., et al. (2008). Mouse maelstrom, a component of nuage, is essential for spermatogenesis and transposon repression in meiosis. Dev. Cell 15, 285–297. doi: 10.1016/j.devcel.2008.05.015
Su, Y. Q., Sugiura, K., Sun, F., Pendola, J. K., Cox, G. A., Handel, M. A., et al. (2012a). MARF1 regulates essential oogenic processes in mice. Science 335, 1496–1499. doi: 10.1126/science.1214680
Su, Y. Q., Sun, F., Handel, M. A., Schimenti, J. C., and Eppig, J. J. (2012b). Meiosis arrest female 1 (MARF1) has nuage-like function in mammalian oocytes. Proc. Natl. Acad. Sci. U.S.A. 109, 18653–18660. doi: 10.1073/pnas.1216904109
Tanaka, S. S., Toyooka, Y., Akasu, R., Katoh-Fukui, Y., Nakahara, Y., Suzuki, R., et al. (2000). The mouse homolog of Drosophila Vasa is required for the development of male germ cells. Genes Dev. 14, 841–853.
Tchasovnikarova, I. A., Timms, R. T., Matheson, N. J., Wals, K., Antrobus, R., Göttgens, B., et al. (2015). GENE SILENCING. Epigenetic silencing by the HUSH complex mediates position-effect variegation in human cells. Science 348, 1481–1485. doi: 10.1126/science.aaa7227
Toscani, A., Mettus, R. V., Coupland, R., Simpkins, H., Litvin, J., Orth, J., et al. (1997). Arrest of spermatogenesis and defective breast development in mice lacking A-myb. Nature 386, 713–717. doi: 10.1038/386713a0
Toyooka, Y., Tsunekawa, N., Takahashi, Y., Matsui, Y., Satoh, M., and Noce, T. (2000). Expression and intracellular localization of mouse Vasa-homologue protein during germ cell development. Mech. Dev. 93, 139–149. doi: 10.1016/s0925-4773(00)00283-5
Trasler, J. M. (2009). Epigenetics in spermatogenesis. Mol. Cell Endocrinol. 306, 33–36. doi: 10.1016/j.mce.2008.12.018
Unhavaithaya, Y., Hao, Y., Beyret, E., Yin, H., Kuramochi-Miyagawa, S., Nakano, T., et al. (2009). MILI, a PIWI-interacting RNA-binding protein, is required for germ line stem cell self-renewal and appears to positively regulate translation. J. Biol. Chem. 284, 6507–6519. doi: 10.1074/jbc.m809104200
Vourekas, A., Zheng, K., Fu, Q., Maragkakis, M., Alexiou, P., Ma, J., et al. (2015). The RNA helicase MOV10L1 binds piRNA precursors to initiate piRNA processing. Genes Dev. 29, 617–629. doi: 10.1101/gad.254631.114
Vourekas, A., Zheng, Q., Alexiou, P., Maragkakis, M., Kirino, Y., Gregory, B. D., et al. (2012). Mili and Miwi target RNA repertoire reveals piRNA biogenesis and function of Miwi in spermiogenesis. Nat. Struct. Mol. Biol. 19, 773–781. doi: 10.1038/nsmb.2347
Wang, Y., Zhu, T., Li, Q., Liu, C., Han, F., Chen, M., et al. (2015). Prmt5 is required for germ cell survival during spermatogenesis in mice. Sci. Rep. 5:11031.
Watanabe, T., Cheng, E. C., Zhong, M., and Lin, H. (2015). Retrotransposons and pseudogenes regulate mRNAs and lncRNAs via the piRNA pathway in the germline. Genome Res. 25, 368–380. doi: 10.1101/gr.180802.114
Watanabe, T., Chuma, S., Yamamoto, Y., Kuramochi-Miyagawa, S., Totoki, Y., Toyoda, A., et al. (2011). MITOPLD is a mitochondrial protein essential for nuage formation and piRNA biogenesis in the mouse germline. Dev. Cell 20, 364–375. doi: 10.1016/j.devcel.2011.01.005
Wenda, J. M., Homolka, D., Yang, Z., Spinelli, P., Sachidanandam, R., Pandey, R. R., et al. (2017). Distinct roles of RNA helicases MVH and TDRD9 in PIWI slicing-triggered mammalian piRNA biogenesis and function. Dev. Cell 41, 623.e9–637.e9.
Xiol, J., Cora, E., Koglgruber, R., Chuma, S., Subramanian, S., Hosokawa, M., et al. (2012). A role for Fkbp6 and the chaperone machinery in piRNA amplification and transposon silencing. Mol. Cell 47, 970–979. doi: 10.1016/j.molcel.2012.07.019
Yabuta, Y., Ohta, H., Abe, T., Kurimoto, K., Chuma, S., and Saitou, M. (2011). TDRD5 is required for retrotransposon silencing, chromatoid body assembly, and spermiogenesis in mice. J. Cell Biol. 192, 781–795. doi: 10.1083/jcb.201009043
Yang, F., Eckardt, S., Leu, N. A., Mclaughlin, K. J., and Wang, P. J. (2008). Mouse TEX15 is essential for DNA double-strand break repair and chromosomal synapsis during male meiosis. J. Cell Biol. 180, 673–679. doi: 10.1083/jcb.200709057
Yang, F., Lan, Y., Pandey, R. R., Homolka, D., Berger, S. L., Pillai, R. S., et al. (2020). TEX15 associates with MILI and silences transposable elements in male germ cells. Genes Dev. 34, 745–750. doi: 10.1101/gad.335489.119
Yang, F., and Wang, P. J. (2016). Multiple LINEs of retrotransposon silencing mechanisms in the mammalian germline. Semin. Cell Dev. Biol. 59, 118–125. doi: 10.1016/j.semcdb.2016.03.001
Yang, Q., Li, R., Lyu, Q., Hou, L., Liu, Z., Sun, Q., et al. (2019). Single-cell CAS-seq reveals a class of short PIWI-interacting RNAs in human oocytes. Nat. Commun. 10:3389.
Yang, Z., Chen, K. M., Pandey, R. R., Homolka, D., Reuter, M., Janeiro, B. K., et al. (2016). PIWI slicing and EXD1 drive biogenesis of nuclear piRNAs from cytosolic targets of the mouse piRNA pathway. Mol. Cell 61, 138–152. doi: 10.1016/j.molcel.2015.11.009
Yang, Z., Ebright, Y. W., Yu, B., and Chen, X. (2006). HEN1 recognizes 21-24 nt small RNA duplexes and deposits a methyl group onto the 2′ OH of the 3′ terminal nucleotide. Nucleic Acids Res. 34, 667–675. doi: 10.1093/nar/gkj474
Yao, Q., Cao, G., Li, M., Wu, B., Zhang, X., Zhang, T., et al. (2018). Ribonuclease activity of MARF1 controls oocyte RNA homeostasis and genome integrity in mice. Proc. Natl. Acad. Sci. U.S.A. 115, 11250–11255. doi: 10.1073/pnas.1809744115
Yoshimura, T., Toyoda, S., Kuramochi-Miyagawa, S., Miyazaki, T., Miyazaki, S., Tashiro, F., et al. (2009). Gtsf1/Cue110, a gene encoding a protein with two copies of a CHHC Zn-finger motif, is involved in spermatogenesis and retrotransposon suppression in murine testes. Dev. Biol. 335, 216–227. doi: 10.1016/j.ydbio.2009.09.003
Yoshimura, T., Watanabe, T., Kuramochi-Miyagawa, S., Takemoto, N., Shiromoto, Y., Kudo, A., et al. (2018). Mouse GTSF1 is an essential factor for secondary piRNA biogenesis. EMBO Rep. 19:e42054.
Zhai, J., and Meyers, B. C. (2012). Deep sequencing from hen1 mutants to identify small RNA 3’ modifications. Cold Spring Harb. Symp. Quant. Biol. 77, 213–219. doi: 10.1101/sqb.2013.77.014779
Zhang, H., Liu, K., Izumi, N., Huang, H., Ding, D., Ni, Z., et al. (2017a). Structural basis for arginine methylation-independent recognition of PIWIL1 by TDRD2. Proc. Natl. Acad. Sci. U.S.A. 114, 12483–12488. doi: 10.1073/pnas.1711486114
Zhang, Y., Guo, R., Cui, Y., Zhu, Z., Wu, H., Zheng, B., et al. (2017b). An essential role for PNLDC1 in piRNA 3’ end trimming and male fertility in mice. Cell Res. 27, 1392–1396. doi: 10.1038/cr.2017.125
Zhang, Y., Guo, R., Cui, Y., Zhu, Z., Zhang, Y., Wu, H., et al. (2017c). An essential role for PNLDC1 in piRNA 3’ end trimming and male fertility in mice. Cell Res. 27, 1392–1396.
Zhang, H., Zhang, F., Chen, J., Li, M., Lv, X., Xiao, Y., et al. (2021). piRNA pathway is essential for generating functional oocytes in golden hamster. bioRxiv [Preprint]. doi: 10.1101/2021.03.21.434510v1
Zhang, J., Wang, Q., Wang, M., Jiang, M., Wang, Y., Sun, Y., et al. (2016). GASZ and mitofusin-mediated mitochondrial functions are crucial for spermatogenesis. EMBO Rep. 17, 220–234. doi: 10.15252/embr.201540846
Zhao, Q., Rank, G., Tan, Y. T., Li, H., Moritz, R. L., Simpson, R. J., et al. (2009). PRMT5-mediated methylation of histone H4R3 recruits DNMT3A, coupling histone and DNA methylation in gene silencing. Nat. Struct. Mol. Biol. 16, 304–311. doi: 10.1038/nsmb.1568
Zhao, S., Gou, L. T., Zhang, M., Zu, L. D., Hua, M. M., Hua, Y., et al. (2013). piRNA-triggered MIWI ubiquitination and removal by APC/C in late spermatogenesis. Dev. Cell 24, 13–25. doi: 10.1016/j.devcel.2012.12.006
Zheng, K., Xiol, J., Reuter, M., Eckardt, S., Leu, N. A., Mclaughlin, K. J., et al. (2010). Mouse MOV10L1 associates with Piwi proteins and is an essential component of the Piwi-interacting RNA (piRNA) pathway. Proc. Natl. Acad. Sci. U.S.A. 107, 11841–11846. doi: 10.1073/pnas.1003953107
Keywords: piRNA, meiosis, male infertility, RBPs, RNA binding proteins
Citation: Li Y, Zhang Y and Liu M (2021) Knockout Gene-Based Evidence for PIWI-Interacting RNA Pathway in Mammals. Front. Cell Dev. Biol. 9:681188. doi: 10.3389/fcell.2021.681188
Received: 16 March 2021; Accepted: 08 June 2021;
Published: 14 July 2021.
Edited by:
Akira Shinohara, Osaka University, JapanReviewed by:
Yuka W. Iwasaki, Keio University, JapanSatomi Kuramochi-Miyagawa, Osaka University, Japan
Copyright © 2021 Li, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingxi Liu, bWluZ3hpLmxpdUBuam11LmVkdS5jbg==
†These authors have contributed equally to this work
 Yinuo Li
Yinuo Li Yue Zhang
Yue Zhang Mingxi Liu
Mingxi Liu