
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CORRECTION article
Front. Cell Dev. Biol. , 23 June 2021
Sec. Molecular and Cellular Pathology
Volume 9 - 2021 | https://doi.org/10.3389/fcell.2021.671532
This article is part of the Research Topic Intracellular Mechanisms of α-Synuclein Processing View all 18 articles
This article is a correction to:
Cathepsin D Variants Associated With Neurodegenerative Diseases Show Dysregulated Functionality and Modified α-Synuclein Degradation Properties
 Josina Bunk1
Josina Bunk1 Susy Prieto Huarcaya1,2
Susy Prieto Huarcaya1,2 Alice Drobny1,2
Alice Drobny1,2 Jan Philipp Dobert1,2
Jan Philipp Dobert1,2 Lina Walther1
Lina Walther1 Stefan Rose-John1
Stefan Rose-John1 Philipp Arnold3,4
Philipp Arnold3,4 Friederike Zunke1,2*
Friederike Zunke1,2*A Corrigendum on
Cathepsin D Variants Associated With Neurodegenerative Diseases Show Dysregulated Functionality and Modified α-Synuclein Degradation Properties
by Bunk, J., Prieto Huarcaya, S., Drobny, A., Dobert, J. P., Walther, L., Rose-John, S., et al. (2021). Front. Cell Dev. Biol. 9:581805. doi: 10.3389/fcell.2021.581805
In the original article, there was a mistake in Figure 1C as published. In the Coomassie brilliant blue (CBB) protein staining, which was used as additional loading control (besides GAPDH), 11 instead of 10 sample lanes (+ protein ladder) were shown in the original Figure 1C. This mistake resulted from an additional sample (transfection control), that was loaded on the far right. This lane was not shown in both immunoblots (CTSD and GAPDH), but accidentally was still shown in the CBB control. The corrected Figure 1C appears below.
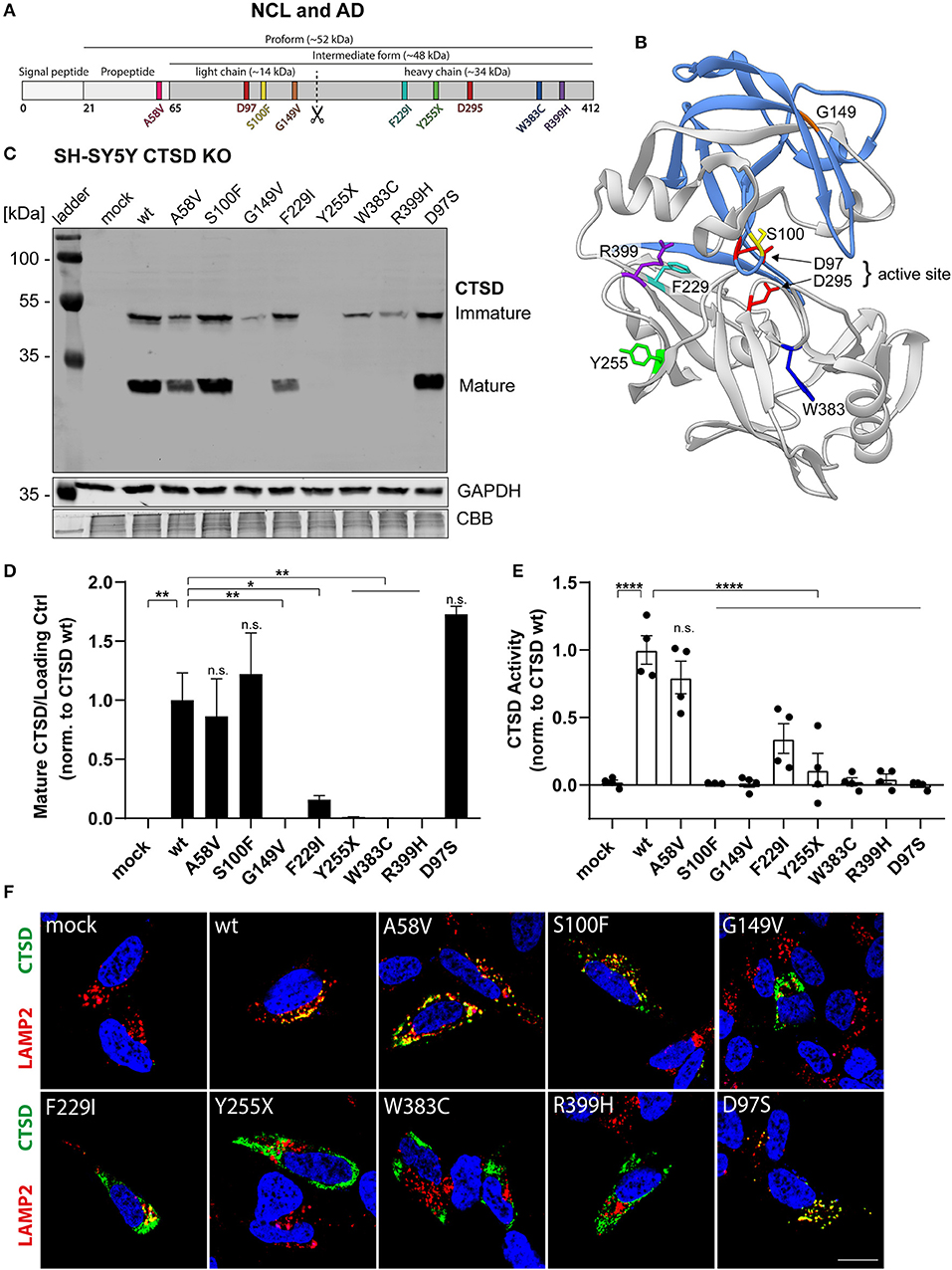
Figure 1. Characterization of CTSD variants associated with neurodegenerative diseases in SH-SY5Y CTSD KO cells. (A) Schematic overview of NCL- and AD-associated CTSD variants analyzed in this study. During protein maturation, the signal peptide (20 aa) and the propeptide (44 aa) are removed, generating an intermediate form (~48 kDa) that is further processed in the lysosome into a double-chain mature form comprised of a light chain (~14 kDa) and a heavy chain (~34 kDa; symbolized by scissor symbol). Both chains remain associated by hydrophobic interactions. Point mutations found in NCL- and AD- patients located within different protein parts are shown in different colors (A58V, pink, propeptide; S100F, yellow, light chain; G149V, orange, light chain; F229I, light blue, heavy chain; Y255X, green, heavy chain; W383C, dark blue, heavy chain; R399H, purple, heavy chain). Aspartates D97 and D295 as part the catalytic site are highlighted in red. (B) Crystal structure model of mature CTSD consisting of the light chain (blue) and the heavy chain (gray) [PDB-ID: 4OBZ (Gradler et al., 2014)]. The active site, consisting of the two aspartates D97 and D295, is shown in red. Other colors indicate disease-associated point mutations within the CTSD protein (same color code as in A). (C) Representative immunoblot of transiently overexpressed CTSD wildtype (wt) and NCL-/AD-associated CTSD variants, as well as enzymatically inactive control (D97S) in SH-SY5Y CTSD KO cells. An anti-CTSD antibody was used for the detection of immature (pro- and intermediate form) as well as mature CTSD (heavy chain, 34 kDa). GAPDH and coomassie brilliant blue (CBB) were used as a loading control. (D) Quantification of western blot signal intensity of mature CTSD (heavy chain) normalized to GAPDH and expressed relative to CTSD wt (n = 4). (E) Analysis of CTSD activity assessed in whole cell lysates utilizing a fluorogenic CTSD peptide cleavage assay. The activity was normalized to CTSD wt (n = 4). (F) Representative immunofluorescence pictures of SH-SY5Y CTSD KO cells expressing CTSD wt or NCL-/AD-associated variants. Cells were visualized by staining of CTSD (green), the lysosomal associated membrane protein LAMP2 (red), and DAPI as nuclear staining (blue). Scale bar: 20 mm. Confocal images showing the single channels can be found in Supplementary Figure 2. All statistical analyses were performed using a one-way ANOVA followed by a Tukey's multiple comparison test. *p < 0.05, **p < 0.01, ****p < 0.0001, n.s., not significant in comparison to wt.
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
Keywords: lysosomal degradation, molecular dynamics simulation, Parkinson's disease, neuronal ceroid lipofuscinoses, lysosomes, alpha-synuclein, cathepsin D
Citation: Bunk J, Prieto Huarcaya S, Drobny A, Dobert JP, Walther L, Rose-John S, Arnold P and Zunke F (2021) Corrigendum: Cathepsin D Variants Associated With Neurodegenerative Diseases Show Dysregulated Functionality and Modified α-Synuclein Degradation Properties. Front. Cell Dev. Biol. 9:671532. doi: 10.3389/fcell.2021.671532
Received: 24 February 2021; Accepted: 07 June 2021;
Published: 23 June 2021.
Edited by:
Masaru Katoh, National Cancer Centre, JapanReviewed by:
Claudia Fiorillo, University of Florence, ItalyCopyright © 2021 Bunk, Prieto Huarcaya, Drobny, Dobert, Walther, Rose-John, Arnold and Zunke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Friederike Zunke, ZnJpZWRlcmlrZS56dW5rZUBmYXUuZGU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.