
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
HYPOTHESIS AND THEORY article
Front. Cell Dev. Biol. , 13 May 2021
Sec. Membrane Traffic and Organelle Dynamics
Volume 9 - 2021 | https://doi.org/10.3389/fcell.2021.664295
This article is part of the Research Topic Does the Golgi Complex Enable Oncogenesis? View all 6 articles
Cancer cells exhibit increased glycolytic flux and adenosine triphosphate (ATP) hydrolysis. These processes increase the acidic burden on the cells through the production of lactate and protons. Nonetheless, cancer cells can maintain an alkaline intracellular pH (pHi) relative to untransformed cells, which sets the stage for optimal functioning of glycolytic enzymes, evasion of cell death, and increased proliferation and motility. Upregulation of plasma membrane transporters allows for H+ and lactate efflux; however, recent evidence suggests that the acidification of organelles can contribute to maintenance of an alkaline cytosol in cancer cells by siphoning off protons, thereby supporting tumor growth. The Golgi is such an acidic organelle, with resting pH ranging from 6.0 to 6.7. Here, we posit that the Golgi represents a “proton sink” in cancer and delineate the proton channels involved in Golgi acidification and the ion channels that influence this process. Furthermore, we discuss ion channel regulators that can affect Golgi pH and Golgi-dependent processes that may contribute to pHi homeostasis in cancer.
Cancer cells require large amounts of energy in the form of adenosine triphosphate (ATP) to drive rapid proliferation and support cellular processes including the activation of cell signaling pathways, membrane transport, and DNA and protein synthesis (Alberts, 2015; Zhu and Thompson, 2019). Interestingly, cancer cells frequently switch to a less efficient glucose metabolism pathway for ATP production, compared to untransformed cells (Heiden et al., 2009; Liberti and Locasale, 2016). This cancer hallmark phenomenon is known as the Warburg effect. Untransformed cells mostly rely on mitochondrial oxidative phosphorylation (OXPHOS) for energy production, which is a highly efficient process where oxygen and glucose-derived pyruvate fuel the TCA cycle and drive the electron transport chain to produce 36 ATPs per glucose molecule (Heiden et al., 2009; Liberti and Locasale, 2016). Although cancer cells are generally equipped with a functional OXPHOS pathway, glucose metabolism is frequently switched to aerobic glycolysis where pyruvate is fermented to lactate, even when oxygen is available. This switch in metabolism is thought to provide essential building blocks for the rapid production of biomass and to facilitate rapid proliferation. However, the pathway leads to the production of only two ATPs per glucose molecule and thus requires the cells to boost glucose consumption to meet energy demands. A byproduct of this metabolic rewiring is the increased production of lactate and a surge in H+. Further contributing to the surge in H+ is the hydrolysis of ATP that supports cellular processes and accelerated proliferation. High lactate and H+ levels can have devastating effects on cellular fitness by lowering the intracellular pH (pHi). Surprisingly though, the pHi of cancer cells is not acidic, but is even more alkaline relative to untransformed cells (Webb et al., 2011; Corbet and Feron, 2017; Zheng et al., 2020). The maintenance of this alkaline pHi provides cancer cells with an optimal environment for glycolytic enzyme activity and cellular advantages to proliferate, migrate, and withstand cell death cues. To avoid the accumulation of cytosolic H+, cancer cells express multiple families of transporters on the plasma membrane, including vacuolar H+-ATPases, sodium-hydrogen exchangers, and monocarboxylate transporters, all of which can extrude protons (Figure 1). However, increasing evidence shows that the regulation of pH homeostasis in cancer is not as straightforward as extrusion of protons at the plasma membrane level, as acidification of organelles, such as lysosomes and the Golgi, contributes to the maintenance of an alkaline cytosol (Liu et al., 2018; Funato et al., 2020; Galenkamp et al., 2020). These organelles therefore function as repositories for H+ storage or means to extrude protons through an alternative pathway. Hence, because of their role in siphoning off cytosolic protons, these organelles can be considered the “proton sinks” of the cell.
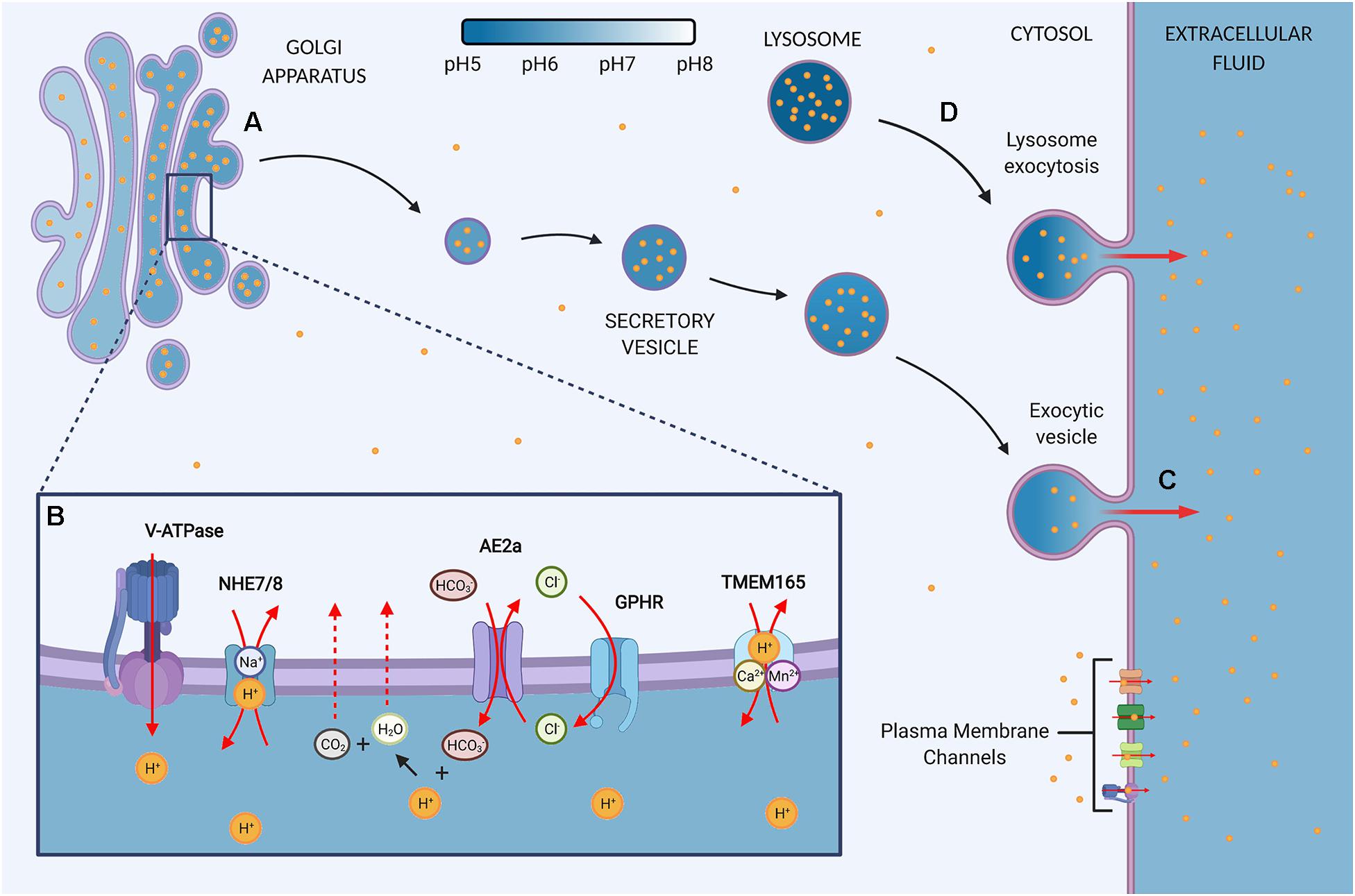
Figure 1. The Golgi contains proton channels and inherent properties that may convert the organelle into a proton sink in cancer. (A) The Golgi is an acidic organelle that shows a decreasing pH gradient along the sub-compartments, eventually leading to the formation of acidic secretory vesicles and granules. The luminal proton concentration is 10–100 times higher as the cytosol and thus the organelle may function as a proton repository that contributes to the upkeep of an alkaline intracellular pH (pHi) in cancer cells. The resting pH of the Golgi and vesicles is thought to be mediated by proton loading and counter ion conductance. Additionally, a proton leak pathway allows for reducing luminal proton content, but the pathway is suggested to be absent in secretory vesicles. (B) Ion channels at the Golgi regulate the luminal H+ content. V-ATPase: Vacuolar H+-ATPases load the lumen constitutively with protons in an ATP-dependent manner. NHE7/8: Sodium-hydrogen exchanger 7 (NHE7) has been implicated in proton loading by exchanging protons for luminal Na+. However, the directionality of NHEs at the Golgi is debated since other studies propose that NHE7 and NHE8 function as a proton leak pathway. AE2a: Anion exchanger 2a is a Golgi-residing AE2 isoform that buffers the Golgi through HCO3– loading in exchange for Cl–. The buffering presents a sought-after proton leak pathway by providing means to neutralize luminal protons through the production of water and carbon dioxide, which can exit the lumen through diffusion. The directionality of AE2 is reversible and therefore bicarbonate influx is gradient-dependent. GPHR: Golgi pH regulator loads the lumen with Cl– in a voltage-gated manner. Hence, it provides the chloride ions required for counterion conductance to sustain the constitutive activity of V-ATPases. In addition, GHPR is thought to provide the chloride ions to allow for AE2a-mediated HCO3– buffering. TMEM165: Transmembrane protein 165 ion selectivity and directionality is still under investigation but data points toward Ca2+/Mn2+ transport in exchange for H+. (C) Secretory vesicles are targeted for exocytosis and thus present a pathway by which the Golgi may target protons to the extracellular space and convert the Golgi into a proton sink by siphoning off cytosolic H+. Secretion is upregulated in cancer, but the role in regulating pHi homeostasis remains to be determined. (D) In response to an acidic extracellular environment, lysosomes have been shown to be targeted for exocytosis, thereby maintaining an alkaline pHi and protecting the cells from extracellular acid.
The presence of acidic organelles and proton pumping into their lumen can conceptually contribute to the upkeep of an alkaline cytosol by sequestering protons or targeting them for the extracellular space through exocytosis. While it is conceivable that both normal and cancer cells might exploit organelle-mediated sequestering of protons for pHi maintenance, the available literature specifically points to this process as being selective to cancer cells (Liu et al., 2018; Funato et al., 2020; Galenkamp et al., 2020). This idea is consistent with the notion that cancer cells produce more acidic moieties relative to untransformed cells due to their altered metabolism.
Acidic organelles in mammalian cells belong to the endocytic and secretory pathway and each of these organelles has a distinct resting pH (Paroutis et al., 2004; Casey et al., 2010). Notably, the Golgi pH gradually descends through the sub-compartments; starting at pH 6.7 at the cis-Golgi, reaching pH 6.0 at the trans-Golgi network and ultimately ending in the formation of secretory vesicles and granules which can reach pH values as low as 5.2. Lysosomes are the cell’s most acidic organelle with a resting pH that ranges between pH 4.7 and 5.5, and endosomes acidify as they mature; early endosomes have a resting pH of 6.3–6.5 that decreases to pH 5.5 in late endosomes. The luminal pH of these organelles is required for proper organelle function and regulation of the organelle pH is important for maintaining cellular fitness. For instance, in the Golgi, luminal pH levels regulate the activity of glycosyltransferases and vesicular trafficking (Axelsson et al., 2001; Kornak et al., 2008; Maeda et al., 2008; Hucthagowder et al., 2009; Rivinoja et al., 2009), while in the lysosomes the pH activates hydrolases and mediates cargo degradation (Chen et al., 2020).
The pH scale is a negative logarithm and thus these organelles contain proton concentrations that are 10–1,000 times higher than those found in the cytosol of cancer cells, which has pH values of 7.4 and higher (Webb et al., 2011, 2021; Corbet and Feron, 2017; Zheng et al., 2020). Lysosomes, endosomes, and the Golgi each occupy 3% or less of the total volume of a cell (Alberts, 2015; Valm et al., 2017). But due to these high luminal proton concentrations, these organelles may function as proton repositories that contribute to maintaining an alkaline pHi in cancer. Even small changes in the pH of these organelles can translate to high molar quantities of H+ ions at the cytosolic level. For instance, in cervical cancer, with lysosomes showing a resting pH of 4.6, an increase of ∼0.7 pH units in the lysosome pH was able to bring about a ∼0.4 pH unit decrease in the cytosol (Liu et al., 2018). Moreover, in pancreatic cancer, a ∼0.5 pH unit increase in trans-Golgi network pH resulted in a ∼0.5 pH unit decrease in pHi and ablation of the Golgi through Brefeldin A administration produced similar effects on pHi homeostasis (Galenkamp et al., 2020). In these cases of induced pH alteration of the lysosome and Golgi, the increase in organelle pH and the concomitant decrease in cytosolic pH reduced the viability of the cells, marking the importance of these organelles in maintaining an alkaline pHi and cell fitness in cancer cells.
In addition to storing protons, the Golgi and lysosomes can potentially target protons to the extracellular space through exocytosis, which adds an extra layer by which these organelles can regulate pHi homeostasis (Figure 1; Jaiswal et al., 2009; Rivera-Molina and Toomre, 2013; Deng et al., 2016; Nugues et al., 2018; Funato et al., 2020). Interestingly, exocytosis may be further stimulated in cancer cells by the acidification of the extracellular surroundings and alkalinization of the cytosol. During tumor acidosis, the extracellular fluid can reach pH 6.5 through increased proton and lactate extrusion (Webb et al., 2011; Corbet and Feron, 2017; Zheng et al., 2020). As an adaptation to this acidic environment, lysosomes are targeted for fusion with the plasma membrane to protect the cells from acidity, but thereby also contribute to maintaining an alkaline cytosol (Steffan et al., 2009; Damaghi et al., 2015; Funato et al., 2020). Moreover, cytosolic acidification was discovered to inhibit Golgi to plasma membrane trafficking (Cosson et al., 1989), while an alkaline pHi promotes exocytosis (Pernas-Sueiras et al., 2005; Huck et al., 2007). An alkaline cytosol was recently shown to change the protonation status of PI4P, a phosphatidylinositol that predominantly localizes to the Golgi and is required for secretory vesicle formation (Dippold et al., 2009; Rahajeng et al., 2019; Waugh, 2019; Shin et al., 2020). Using a yeast model system, it was demonstrated that reduced PI4P protonation in response to cytosolic alkalinization increased PI4P protein binding (Shin et al., 2020). It remains to be determined whether this protonation status promotes secretory vesicle formation. Nonetheless, PI4P is an important docking station for GOLPH3, a Golgi-localizing oncoprotein frequently upregulated in cancer, which is described to increase secretory vesicle formation and Golgi-to-plasma membrane trafficking (Halberg et al., 2016; Waugh, 2019; Sechi et al., 2020). Multiple cancer types have been found to display increased and malignant Golgi-dependent secretion that drives metastasis, a process enhanced by extracellular acidification (Webb et al., 2011; Halberg et al., 2016; Corbet and Feron, 2017; Capaci et al., 2020; Gupta et al., 2020; Tan et al., 2020; Zheng et al., 2020). Whether the malignant secretion presents a pathway to boost proton extrusion through the Golgi and whether that contributes to the metastatic potential of cancer cells and pHi homeostasis is a concept that requires further investigation (Figure 1).
Organelle pH levels are tightly regulated to support cell function; however, changes in organelle pH are detected in cancer. Multiple cancer types display a decreased lysosome pH compared to tissue-matched untransformed cells (Webb et al., 2021). Lysosomes of triple negative breast cancer cells are significantly more acidic than lysosomes of untransformed mammary epithelial or benign breast cancer cells. Similarly, the lysosomes of pancreatic ductal adenocarcinoma (PDAC) cells with mutant KRAS show a >0.5 pH unit decrease compared to normal human pancreatic duct epithelial cells or PDAC cells lacking oncogenic KRAS. Transformation of normal kidney or mammary cells with mutant KRAS or mutant HRAS, respectively, results in a lysosome acidification similar to cancer cells. Interestingly, mutant RAS transformation-mediated reduction in lysosome pH coincides with cytosol alkalinization, but a direct link between these observations remains to be established. Nonetheless, a direct link between alkaline pHi and lysosome pH was found for cervical cancer cells, where loss of lysosome acidification coincides with a reduction in cytosolic pH, which points to lysosomes functioning as a proton sink (Liu et al., 2018). The lysosome as a proton sink in cancer is extensively reviewed by Chen et al. (2020). The medial/trans-Golgi is proposed to become more alkaline in cancer, as determined by comparing various human cancer cell lines to canine kidney epithelial cells and human and monkey fibroblasts (Rivinoja et al., 2006; Kokkonen et al., 2019; Khosrowabadi et al., 2021). However, no differences in luminal pH were observed when comparing the trans-Golgi network of Chinese hamster ovary (CHO) and cervical cancer cells (Demaurex et al., 1998). If there is indeed an increase in Golgi pH in cancer cells, this might not necessarily be linked to reduced proton loading since organelle pH also relies on buffering capacity. Such a buffering effect could result in increased luminal pH, while still allowing for the capture of protons from the cytosol. The alkalinization in the Golgi is proposed to occur through exchanger-mediated bicarbonate buffering that reduces the luminal proton content by converting HCO3– and H+ into H2O and CO2, which can readily occur at the resting pH of the Golgi since the pKa of HCO3– is 6.4 (Khosrowabadi et al., 2021). With this increased pH buffering capacity, the Golgi in cancer cells is possibly better equipped to tolerate proton sequestration and allow for additional proton loading relative to normal cells, which may contribute to clearing cytosolic protons. However, additional studies are required to confirm this potential.
The Golgi pH is thought to be regulated at multiple levels through proton pumping, proton leakage, buffering, and counter ion transport. Significant advances have been made in the understanding of these processes, but a comprehensive model for luminal Golgi pH regulation in cancer largely remains unresolved. Regulators of Golgi pH have mainly been identified in non-cancerous models and the variety of cancer types and their genomic differences may contribute to alternative regulation. Moreover, cancer frequently displays changes in ion channel expression levels and localization and is therefore proposed as a channelopathy, which, given the multitude of ion channels that can set off pH changes, beclouds the direct translation of non-cancerous findings to cancer models (Litan and Langhans, 2015; Prevarskaya et al., 2018). Notably, it should be considered that changes in expression levels do not necessarily translate to changes in pH levels since ion channel activity can also be driven by membrane potential and ion gradients. Here, we will outline the regulators of Golgi pH homeostasis thus far identified (Figure 1).
The Vacuolar H+-ATPase (V-ATPases) is the main acidifier of organelles along the endocytic and secretory pathway and its activity is required for both endocytosis and exocytosis (Vasanthakumar and Rubinstein, 2020). The importance of the proton pump in Golgi acidification is well illustrated by studies from Llopis et al. (1998) who showed that V-ATPase inhibition with bafilomycin A1 led to Golgi alkalinization, reaching pH values close to the cytosolic pH levels. The proton pump is a multi-subunit protein complex that transports H+ ions across the membrane using ATP as an energy source. Expression levels of the different subunits are tissue-, cell-, and organelle-specific, and, in cancer, the subunits are frequently differentially expressed (Couto-Vieira et al., 2020). The localization of V-ATPase to the Golgi and endosomes is thought to occur through the V0a2 subunit, and its role in proper Golgi function is illustrated by the Golgi dysfunction observed in cutis laxa patients, which harbor a mutation in ATP6V0A2 (Kornak et al., 2008; Hucthagowder et al., 2009). In cancer, V0a2 subunit expression is mostly unaffected at the transcriptional level (ATP6V0A2,1) (Tang et al., 2019), but the subunit may show differential localization to the plasma membrane, as observed in ovarian cancer (Kulshrestha et al., 2015). Here, knockdown of the subunit results in cytosolic acidification, indicating that V-ATPase activity is important for pHi homeostasis, but additional studies are required to determine if the Golgi is involved (Kulshrestha et al., 2016).
The V-ATPase proton pump has been demonstrated to transport protons constitutively into the Golgi lumen, where the resting pH is dictated by a balance between proton pumping and proton leakage (Schapiro and Grinstein, 2000; Wu et al., 2001). Inhibition of V-ATPases causes luminal alkalinization of the Golgi and secretory vesicles, highlighting the role for V-ATPase in acidifying these organelles. However, artificial luminal acidification in combination with V-ATPase inhibition only results in gradual alkalinization of the Golgi and is not detected in secretory vesicles (Wu et al., 2001). These results indicate the possible presence of a proton leak pathway at the Golgi, which is non-existent or minimal in secretory vesicles. Interestingly, at the lysosome, V-ATPase is shown to be regulated by the oncoprotein STAT3, which increases proton pump activity in response to sensing cytosolic acidification, thereby restoring the alkaline pHi in cancer (Liu et al., 2018). The role of STAT3 in regulating Golgi acidification seems to be absent, since the study did not find evidence for STAT3 localization at the Golgi. Nevertheless, a large number of proteins have been identified to interact with the V-ATPase proton pump, which could possibly affect its Golgi acidification capacity (Merkulova et al., 2015).
Sodium-hydrogen exchangers (NHEs) are a family of electroneutral membrane ion transporters that transfer protons across membranes in a 1:1 exchange for Na+, and in some cases Li+ and K+ (Pedersen and Counillon, 2019). The family consists of nine isoforms that share a conserved architecture and can be classified into two groups: the plasma membrane NHEs (NHE1-5) and the endomembrane NHEs (NHE6-9), although NHE8 can be considered a separate group as it exerts its function at both the plasma membrane and endomembranes. Both NHE7 and NHE8 have been identified to localize to the Golgi and have been implicated in the regulation of the Golgi resting pH (Numata and Orlowski, 2001; Nakamura et al., 2005). NHE7 expression is frequently increased in cancer (SLC9A7,1 (Tang et al., 2019) and overexpression of NHE7 in a breast cancer cell line enhances cell adhesion, invasion, and anchorage-independent growth (Onishi et al., 2012). No significant differences are observed for NHE8 expression in tumors relative to tissue-matched controls and no data is available for its role in cancer (SLC9A8,1) (Tang et al., 2019).
The NHE7 and NHE8 exchangers were originally proposed to function as a Golgi leak pathway for protons (Numata and Orlowski, 2001; Nakamura et al., 2005), allowing H+ to flow out of the lumen through the ion gradient formed by the elevated H+ levels relative to the cytosol. The notion that sodium-hydrogen exchangers act as a proton leak pathway was supported by the original findings that exogenous NHE8 expression in monkey fibroblasts increased the pH of the Golgi and NHE7 expression in CHO cells increased the Na+ influx into endomembrane structures (Numata and Orlowski, 2001; Nakamura et al., 2005). However, CHO cells lacking plasma membrane NHE1 did not confirm the NHE7 leak pathway since the Golgi resting pH was unaltered after exogenous NHE7 expression (Khayat et al., 2019). Nevertheless, exogenous expression of an NHE7 mutant linked to intellectual disability did cause alkalinization of the Golgi. According to the proton-leak hypothesis, the authors proposed a model in which the mutation transformed the exchanger into a hyperactive proton leak responsible for the luminal alkalization (Khayat et al., 2019). Subsequent studies on NHE8 have pointed toward a role in regulating the function and morphology of multivesicular bodies, with no observed changes in luminal pH (Lawrence et al., 2010). The proton-leak hypothesis warrants further scrutiny, especially since an increase of cytosolic Na+ to 103 and 140 mM, concentrations similar to physiological extracellular concentrations known to drive NHE1-mediated proton extrusion, had minimal effects on the Golgi pH, suggesting that the Na+/H+ leak activity at the Golgi is insignificant (Demaurex et al., 1998; Schapiro and Grinstein, 2000).
An opposing hypothesis for the function of NHE7 at the Golgi is in a role as a proton loader. NHE7 expression is frequently increased in cancer, as is the case for PDAC (Galenkamp et al., 2020). Here, endogenous NHE7 localization was confirmed at the trans-Golgi network and knockdown of NHE7 resulted in alkalization of the organelle and a concomitant increase in cytosolic pH. A thorough assessment of NHE7 features by Milosavljevic et al. (2014) revealed that the exchanger functions as an acid loader. Importantly, NHE7 was shown to display non-reversible proton transport from the cytosol to the lumen, arguing against NHE7 being able to function as a proton leak pathway at the Golgi. Moreover, the study indicated that NHE7-mediated proton loading is only effectuated by high Na+ concentrations, not by K+, and is constitutively activated by cytosolic H+.
The anion exchangers (AEs) family contains membrane transporters that electroneutrally and reversibly exchange Cl– for HCO3– (Romero et al., 2013). The exchangers mediate bicarbonate buffering that contributes to pH homeostasis by sequestering H+ at acidic pH levels and which leads to the conversion of protons and bicarbonate into water and CO2. Both products can readily escape the lumen of organelles through diffusion and, possibly, aquaporin water channels (Nozaki et al., 2008; Alberts, 2015). Of special interest is the Golgi-localized AE2 isoform AE2a (Holappa et al., 2001). AE2 gene transcription is upregulated in multiple cancer types and is linked to promoting cell viability, proliferation, migration and invasion of cancer cells (SLC4A2,1) (Hwang et al., 2009, 2019; Zhang et al., 2017; Celay et al., 2018; Tang et al., 2019; Khosrowabadi et al., 2021). The AE2a isoform is reported to increase the Golgi resting pH and therefore represents a likely candidate for the proton leak observed at the Golgi (Wu et al., 2001; Khosrowabadi et al., 2021).
The Golgi pH regulator (GPHR) is a voltage-gated chloride channel that regulates Golgi pH through counter ion conductance (Maeda et al., 2008). The influx of chloride ions into the Golgi allows for H+ pumping by reducing the membrane potential, which increases through continuous H+ pumping by the V-ATPases (Llopis et al., 1998; Maeda et al., 2008). Moreover, GPHR can conceivably provide the chloride ions required for anion exchanger-mediated bicarbonate transport at the Golgi (Becker and Deitmer, 2020). GPHR is mainly localized to the Golgi and the introduction of an inactivating mutation or downregulation increases the Golgi resting pH, causes disruption of Golgi integrity, impairs glycosylation, and Golgi to plasma membrane trafficking (Maeda et al., 2008; Vavassori et al., 2013; Sou et al., 2019). Little is known about the role of GPHR in cancer and its expression is largely unaffected by transformation (GPR89A/B,1) (Tang et al., 2019).
The ion specificity of transmembrane protein 165 (TMEM165) is still debated, but research findings point to the function of a Ca2+/Mn2+ transporter in exchange for H+ (Lebredonchel et al., 2019; Stribny et al., 2020; Wang et al., 2020). TMEM165 localizes to the Golgi and knockdown of TMEM165 in normal liver cells results in Golgi acidification, suggesting TMEM165 is a proton leak pathway (Foulquier et al., 2012; Wang et al., 2020). However, in cervical cancer cells knockdown of TMEM165 was shown to cause acidification of lysosomes (Demaegd et al., 2013). Nonetheless, the role of TMEM165 as a possible regulator of Golgi pH is supported by glycosylation abnormalities found in patients harboring a TMEM165 mutation, which is tightly linked to Golgi pH homeostasis (Foulquier et al., 2012). TMEM165 is upregulated in a few cancer types and is linked to promoting migration and invasion (TMEM165,1) (Lee et al., 2018; Tang et al., 2019; Murali et al., 2020).
The role of cystic fibrosis transmembrane conductance regulator (CFTR) in regulating Golgi pH is controversial since studies have described seemingly contradictory findings. Moreover, CFTR is not a Golgi-resident protein per se but traffics through the Golgi to reach the cell surface. Nonetheless, this counterion channel has been shown to change Golgi pH in a cystic fibrosis model (Poschet et al., 2001). CFTR is a cAMP-activated Cl–/HCO3– channel best known for causing the life-limiting cystic fibrosis disease through the F508del mutation, but mutations and differential expression are also linked to cancer predisposition (Amaral et al., 2020). Gene expression is increased or reduced depending on the cancer type (CFTR,1) (Tang et al., 2019). The F508del mutation causes CFTR misfolding, ER retention, and degradation (Cheng et al., 1990; Denning et al., 1992). In a PDAC cell line derived from a cystic fibrosis patient, the F508del mutation was shown to cause Golgi dispersion which was reverted by wild-type CFTR expression (Hollande et al., 2005). Cells harboring the mutation show hyperacidification of the Golgi, which is counteracted by restoring Δ508-CFTR folding or reintroduction of wild-type CFTR (Chandy et al., 2001; Poschet et al., 2001). However, reduced Cl– influx by decreased expression of CFTR at the Golgi cannot explain the increased acidity, since Cl– is a H+ counterion that reduces the membrane potential. This led to the proposal of a model in which Na+ efflux from the organelle is increased in the absence of CFTR, allowing for additional H+ pumping (Poschet et al., 2002). The CFTR chloride channel represses sodium efflux by inhibiting the epithelial sodium channel, ENaC, resulting in Na+ build up due to the action of Na+/K+-ATPases. This leads to reduced proton pumping in response to the increase in membrane potential caused by Na+ ions. Nonetheless, the role of CFTR in regulating Golgi pH remains controversial as administration of cAMP had little effect on Golgi pH of CFTR mutant and wild-type cells (Llopis et al., 1998; Chandy et al., 2001). This in contrast to previous studies where cAMP was found to alkalinize the Golgi, but where overexpression of CFTR did not significantly change the pH of the organelle (Seksek et al., 1995, 1996).
The concept of the Golgi as a proton sink remains to be fully explored and requires more extensive studies that specifically address the contribution of the Golgi to maintenance of cytosolic pH. Moreover, plasma membrane transporters are historically thought to predominately regulate pHi and the role of organelles in this homeostatic process needs additional scrutinization (Paroutis et al., 2004; Casey et al., 2010; Webb et al., 2011; Corbet and Feron, 2017; Zheng et al., 2020). It would be interesting to examine the level of contribution that alternative pathways have on H+ efflux, such as exocytosis and proton loading of lysosomes and the Golgi, and whether these pathways’ contributions are distinctive for cancer vs. untransformed cells. In cancer, both the perturbation of Golgi and lysosome pH has been shown to affect the pH of the cytosol, but equivalent analyses of normal cells is lacking (Liu et al., 2018; Funato et al., 2020; Galenkamp et al., 2020). As an acidic organelle, the Golgi subtracts protons from the cytosol and, as a part of the secretory pathway, might target H+ for the extracellular space. Conceptually, it may be possible that the Golgi can contribute to pHi homeostasis in both cancer and normal cells. However, one observation that this might not be the case is that the depletion of NHE7 in normal pancreatic cells did not affect the cytosolic pH, while loss of NHE7 in PDAC cells resulted in alkalinization of the Golgi that caused a decrease in cytosolic pH (Galenkamp et al., 2020). A likely explanation is that organelle acidification plays a greater role in cancer cells due to the acidic burden that these cells have to withstand in response to amplified aerobic glycolysis and ATP hydrolysis (Webb et al., 2011; Corbet and Feron, 2017; Zheng et al., 2020). Alternatively, acidic organelles in cancer may exhibit increased or altered activity that exacerbates the effect on cytosolic pH when perturbed. Indeed, cancer cells show increased Golgi-mediated secretion which would increase the number of protons secreted through this pathway (Halberg et al., 2016; Capaci et al., 2020; Gupta et al., 2020; Tan et al., 2020). Given this enhanced secretory flux, perturbation of the secretory pathway might result in greater accumulation of protons in the cytosol, relative to untransformed cells. Additionally, cancer cells display altered luminal ion levels, and differential transporter levels or activity, which could potentially bring about changes in proton loading (Litan and Langhans, 2015; Prevarskaya et al., 2018).
The contribution of additional Golgi proton loaders or leak pathways and their physiological relevance alongside V-ATPase, which is the main acidifier of secretory and endocytic organelles, in cancer and normal cells is limitedly studied. In cancer, the Golgi is proposed to display elevated buffering capacity via increased AE2a expression and bicarbonate loading (Khosrowabadi et al., 2021). A conceivable source of bicarbonate for transport into the Golgi lumen are the carbonic anhydrases present at the cell surface, which are upregulated in cancer and convert carbon dioxide and water into H+ and HCO3– (Mboge et al., 2018). In turn, bicarbonate is imported by the cell through Na+/HCO3– cotransporters that utilize the existing Na+ gradient between the cytosol and extracellular fluid (Becker and Deitmer, 2020). Additionally, the cytosolic carbonic anhydrase CAII may provide HCO3– as it has been determined to localize to the Golgi (Alvarez et al., 2001). Importantly, CAII and AE2 are able to form a transport metabolon, a complex between the anion exchanger and carbonic anhydrase, which is proposed to be required for full bicarbonate transport activity by AE2 (Vince and Reithmeier, 2000; Sterling et al., 2001; Gonzalez-Begne et al., 2007; Becker and Deitmer, 2020). Luminal chloride ions required for the counter ion transport in the HCO3– exchange are most likely transported to the lumen through chloride channels, such as GPHR, present at the Golgi (Maeda et al., 2008). Although AE2a does not directly mediate proton leakage, but provides a means for proton neutralization, it presents a plausible option for the proton leak pathway that had previously been identified, but for which thus far a compelling candidate is lacking (Wu et al., 2001). Given that the pKa of bicarbonate is 6.4, in the Golgi lumen, bicarbonate and H+ can convert to water and carbon dioxide without requiring carbonic anhydrases (Mboge et al., 2018). This alkalinization of the Golgi by increased bicarbonate buffering may provide means for additional subtraction of protons from the cytosol. The contribution of the pathway to cytosolic pH alkalinization in cancer ultimately depends on the fate of the carbon dioxide and whether it remains within the cell and converts back to bicarbonate in a carbonic anhydrase-dependent or independent manner, or whether it leaves the cell and thereby leads to the neutralization of intracellular protons.
The sodium-hydrogen exchanger NHE7 had previously been proposed as a proton leak pathway at the Golgi, but more recent data has pointed toward NHE7 functioning as a proton loader (Numata and Orlowski, 2001; Milosavljevic et al., 2014; Galenkamp et al., 2020). Endogenous NHE7 localizes to the trans-Golgi network; however, the transporter can be present on post-Golgi vesicles, endosomes, and traffic to the plasma membrane when overexpressed or when endocytosis is inhibited (Numata and Orlowski, 2001; Lin et al., 2005, 2007; Nakamura et al., 2005; Fukura et al., 2010; Onishi et al., 2012; Khayat et al., 2019; Galenkamp et al., 2020; Lopez-Hernandez et al., 2020). Whether NHE7 is required for the formation of post-Golgi vesicles and endosomes, or whether it is a passenger, and if it regulates post-Golgi vesicle acidification has not been carefully assessed. When forced to express at the plasma membrane, NHE7 is shown to function as a proton loader of endosomes, with no leak activity (Milosavljevic et al., 2014). However, cells with and without NHE7 expression displayed similar steady-state endosomal pH levels. Inhibition of NHE7 with the pan-NHE inhibitor EIPA reduced acidification of endosomes to a similar extent as bafilomycin A1, whereas the effect of EIPA was absent in cells without NHE7 expression. These data indicate that NHE7 can function alongside V-ATPases in mediating luminal acidification and that the steady-state pH might involve both transporters. NHE7 knockdown in PDAC cells was shown to alkalinize the Golgi, but a direct evaluation of Na+-mediated proton loading of the Golgi lumen remains to be carried out (Galenkamp et al., 2020). Nonetheless, the involvement of NHEs in Golgi acidification in PDAC was confirmed by treatment with EIPA. Altogether, the data fits a model in which NHE7 acidifies the trans-Golgi network, possibly alongside V-ATPases. It cannot be completely ruled out that the downregulation or inhibition of NHE7 affects V-ATPase activity or that proton extrusion at the plasma membrane partially contributes to the observed effects. NHE7 constitutively binds protons (Km = 2.5 10–7 M), but has a low affinity for Na+ (Km = 240 mM) and thus needs high Na+ concentrations to drive proton transport against the gradient between the Golgi lumen and the cytosol (Milosavljevic et al., 2014). These findings suggest that, if NHE7 functions as an acid loader at the Golgi, a source of luminal Na+ should be present. Thus far the concentrations of Na+ at the Golgi have not been determined and might be differentially regulated in cancer. Na+/K+-ATPases present at the Golgi are likely not the source of Na+, since their inhibition did not affect Golgi pH in cervical cancer cells (Llopis et al., 1998) and reduced Golgi resting pH in cystic fibrosis control cells (Poschet et al., 2001). However, luminal Na+ concentration could be driven by retrograde transport from endosomes to the trans-Golgi network, which delivers extracellular Na+ obtained through endocytosis (Tu et al., 2020). This process is thought to contribute to the high Na+ concentrations observed in lysosomes, where Na+ is the predominant cation at a concentration of ∼150 mM (Wang et al., 2012; Xu and Ren, 2015). A role for this pathway in Na+ delivery to the Golgi has yet to be assessed.
Altogether, the available data indicates that additional transporters besides V-ATPase can co-regulate the luminal pH of the Golgi and contribute to the extraction of protons from the cytosol. The role of each of these transporters and whether the correct physiological conditions are met to allow for each of these transporters to function remains to be determined on a contextual basis and could be differentially regulated in untransformed cells vs. cancer.
Cancer is a pathological state in which cells are exposed to chronic stresses that can alter cellular processes in order to provide growth benefits despite the harsh tumor microenvironment (Webb et al., 2011; Corbet and Feron, 2017; Zheng et al., 2020). One such stress is the increased production of H+ ions, which can lead to cellular acidification and tumor acidosis. Despite these conditions, cancer cells maintain an alkaline pHi that allows proliferation to thrive. The Golgi is shown to contribute to the upkeep of this alkaline cytosolic pH in cancer by functioning as a proton sink (Galenkamp et al., 2020). Whether the Golgi also plays a role in the maintenance of cytosolic pH in normal cells remains to be determined. In addition to the proton storage capacity, the Golgi contains inherent properties, the secretory pathway, to target protons for the extracellular space. This pathway becomes malignant in cancer and may promote proton extrusion and provide means to drive metastasis (Halberg et al., 2016; Capaci et al., 2020; Gupta et al., 2020; Tan et al., 2020). The limited understanding of the players involved in Golgi pH homeostasis in cancer impedes the targeting of this pathway as a therapeutic strategy. Further investigation is warranted to fully comprehend the contribution of the Golgi ion channels to Golgi pH and pHi homeostasis in cancer. It would be beneficial to scrutinize how ion channels in the Golgi are regulated, as they may be affected by changes in expression levels or subcellular localization, or through activation cascades, such as observed in the WNK signaling pathway, and by secondary messengers, such a cAMP and PDGF (Seksek et al., 1995; Alessi et al., 2014). Such examinations may provide valuable insights into ways to therapeutically disrupt pH homeostasis in cancer and may open new avenues for pharmacological intervention, which are eagerly needed to improve clinical outcomes for cancer patients.
Both authors conceived, organized, and wrote the manuscript.
This work was supported by a Department of Defense Career Developmental Award (W81XWH-17-10316 to CC). KG is the recipient of a TRDRP Postdoctoral Fellowship Award (T30FT0952).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The figure was created with BioRender.com.
Alberts, B. (2015). Molecular biology of the cell. New York, NY: Garland Science, Taylor and Francis Group.
Alessi, D. R., Zhang, J. W., Khanna, A., Hochdorfer, T., Shang, Y. Z., and Kahle, K. T. (2014). The WNK-SPAK/OSR1 pathway: Master regulator of cation-chloride cotransporters. Sci. Signal. 7:334. doi: 10.1126/scisignal.2005365
Alvarez, L., Fanjul, M., Carter, N., and Hollande, E. (2001). Carbonic anhydrase II associated with plasma membrane in a human pancreatic duct cell line (CAPAN-1). J. Histochem. Cytochem. 49, 1045–1053. doi: 10.1177/002215540104900812
Amaral, M. D., Quaresma, M. C., and Pankonien, I. (2020). What Role Does CFTR Play in Development, Differentiation, Regeneration and Cancer? Int. J. Mole. Sci. 21:21093133. doi: 10.3390/ijms21093133
Axelsson, M. A. B., Karlsson, N. G., Steel, D. M., Ouwendijk, J., Nilsson, T., and Hansson, G. C. (2001). Neutralization of pH in the Golgi apparatus causes redistribution of glycosyltransferases and changes in the O-glycosylation of mucins. Glycobiology 11, 633–644. doi: 10.1093/glycob/11.8.633
Becker, H. M., and Deitmer, J. W. (2020). Transport Metabolons and Acid/Base Balance in Tumor Cells. Cancers 12:12040899. doi: 10.3390/cancers12040899
Capaci, V., Bascetta, L., Fantuz, M., Beznoussenko, G. V., Sommaggio, R., Cancila, V., et al. (2020). Mutant p53 induces Golgi tubulo-vesiculation driving a prometastatic secretome. Nat. Comm. 11:5. doi: 10.1038/s41467-020-17596-5
Casey, J. R., Grinstein, S., and Orlowski, J. (2010). Sensors and regulators of intracellular pH. Nat. Rev. Mole. Cell Biol. 11, 50–61. doi: 10.1038/nrm2820
Celay, J., Lozano, T., Concepcion, A. R., Beltran, E., Rudilla, F., Garcia-Barchino, M. J., et al. (2018). Targeting the anion exchanger 2 with specific peptides as a new therapeutic approach in B lymphoid neoplasms. Haematologica 103, 1065–1072. doi: 10.3324/haematol.2017.175687
Chandy, G., Grabe, M., Moore, H. P. H., and Machen, T. E. (2001). Proton leak and CFTR in regulation of Golgi pH in respiratory epithelial cells. Am. J. Physiol. Cell Physiol. 281, C908–C921.
Chen, R., Jaattela, M., and Liu, B. (2020). Lysosome as a Central Hub for Rewiring PH Homeostasis in Tumors. Cancers 12, doi: 10.3390/cancers12092437
Cheng, S. H., Gregory, R. J., Marshall, J., Paul, S., Souza, D. W., White, G. A., et al. (1990). Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell 63, 827–834. doi: 10.1016/0092-8674(90)90148-8
Corbet, C., and Feron, O. (2017). Tumour acidosis: from the passenger to the driver’s seat. Nat. Rev. Cancer 17, 577–593. doi: 10.1038/nrc.2017.77
Cosson, P., de Curtis, I., Pouyssegur, J., Griffiths, G., and Davoust, J. (1989). Low cytoplasmic pH inhibits endocytosis and transport from the trans-Golgi network to the cell surface. J. Cell Biol. 108, 377–387. doi: 10.1083/jcb.108.2.377
Couto-Vieira, J., Nicolau-Neto, P., Costa, E. P., Figueira, F. F., Simao, T. D., and Okorokova-Facanha, A. L., et al. (2020). Multi-cancer V-ATPase molecular signatures: A distinctive balance of subunit C isoforms in esophageal carcinoma. Ebiomedicine 51:42. doi: 10.1016/j.ebiom.2019.11.042
Damaghi, M., Tafreshi, N. K., Lloyd, M. C., Sprung, R., Estrella, V., Wojtkowiak, J. W., et al. (2015). Chronic acidosis in the tumour microenvironment selects for overexpression of LAMP2 in the plasma membrane. Nat. Comm. 6:9752. doi: 10.1038/ncomms9752
Demaegd, D., Foulquier, F., Colinet, A. S., Gremillon, L., Legrand, D., Mariot, P., et al. (2013). Newly characterized Golgi- localized family of proteins is involved in calcium and pH homeostasis in yeast and human cells. Proc. Natl. Acad. Sci. U S A 110, 6859–6864. doi: 10.1073/pnas.1219871110
Demaurex, N., Furuya, W., D’Souza, S., Bonifacino, J. S., and Grinstein, S. (1998). Mechanism of acidification of the trans-Golgi network (TGN) - In situ measurements of pH using retrieval of TGN38 and furin from the cell surface. J. Biol. Chem. 273, 2044–2051. doi: 10.1074/jbc.273.4.2044
Deng, Y. Q., Rivera-Molina, F. E., Toomre, D. K., and Burd, C. G. (2016). Sphingomyelin is sorted at the trans Golgi network into a distinct class of secretory vesicle. Proc. Natl. Acad. Sci. U S A 113, 6677–6682. doi: 10.1073/pnas.1602875113
Denning, G. M., Anderson, M. P., Amara, J. F., Marshall, J., Smith, A. E., and Welsh, M. J. (1992). Processing Of Mutant Cystic-Fibrosis Transmembrane Conductance Regulator Is Temperature-Sensitive. Nature 358, 761–764. doi: 10.1038/358761a0
Dippold, H. C., Ng, M. M., Farber-Katz, S. E., Lee, S. K., Kerr, M. L., Peterman, M. C., et al. (2009). GOLPH3 Bridges Phosphatidylinositol-4-Phosphate and Actomyosin to Stretch and Shape the Golgi to Promote Budding. Cell 139, 337–351. doi: 10.1016/j.cell.2009.07.052
Foulquier, F., Amyere, M., Jaeken, J., Zeevaert, R., Schollen, E., Race, V., et al. (2012). TMEM165 Deficiency Causes a Congenital Disorder of Glycosylation. Am. J. Hum. Genet. 91, 15–26. doi: 10.1016/j.ajhg.2012.05.002
Fukura, N., Ohgaki, R., Matsushita, M., Nakamura, N., Mitsui, K., and Kanazawa, H. (2010). A Membrane-Proximal Region in the C-Terminal Tail of NHE7 Is Required for Its Distribution in the Trans-Golgi Network, Distinct from NHE6 Localization at Endosomes. J. Membr. Biol. 234, 149–158. doi: 10.1007/s00232-010-9242-9
Funato, Y., Yoshida, A., Hirata, Y., Hashizume, O., Yamazaki, D., and Miki, H. (2020). The Oncogenic PRL Protein Causes Acid Addiction of Cells by Stimulating Lysosomal Exocytosis. Dev. Cell 55, 387. doi: 10.1016/j.devcel.2020.08.009
Galenkamp, K. M. O., Sosicka, P., Jung, M., Recouvreux, M. V., Zhang, Y. J., Moldenhauer, M. R., et al. (2020). Golgi Acidification by NHE7 Regulates Cytosolic pH Homeostasis in Pancreatic Cancer Cells. Cancer Discov. 10, 822–835. doi: 10.1158/2159-8290.Cd-19-1007
Gonzalez-Begne, M., Nakamoto, T., Nguyen, H. V., Stewart, A. K., Alper, S. L., and Melvin, J. E. (2007). Enhanced formation of a HCO3- transport metabolon in exocrine cells of Nhe1(-/-) mice. J. Biol. Chem. 282, 35125–35132. doi: 10.1074/jbc.M707266200
Gupta, R., Malvi, P., Parajuli, K. R., Janostiak, R., Bugide, S., Cai, G. P., et al. (2020). KLF7 promotes pancreatic cancer growth and metastasis by up-regulating ISG expression and maintaining Golgi complex integrity. Proc. Natl. Acad. Sci. U S A 117, 12341–12351. doi: 10.1073/pnas.2005156117
Halberg, N., Sengelaub, C. A., Navrazhina, K., Molina, H., Uryu, K., and Tavazoie, S. F. (2016). PITPNC1 Recruits RAB1B to the Golgi Network to Drive Malignant Secretion. Can. Cell 29, 339–353. doi: 10.1016/j.ccell.2016.02.013
Heiden, M. G. V., Cantley, L. C., and Thompson, C. B. (2009). Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 324, 1029–1033. doi: 10.1126/science.1160809
Holappa, K., Suokas, M., Soininen, P., and Kellokumpu, S. (2001). Identification of the full-length AE2 (AE2a) isoform as the Golgi-associated anion exchanger in fibroblasts. J. Histochem. Cytochem. 49, 259–269. doi: 10.1177/002215540104900213
Hollande, E., Salvador-Cartier, C., Alvarez, L., and Fanjul, M. (2005). Expression of a wild-type CFTR maintains the integrity of the biosynthetic/secretory pathway in human cystic fibrosis pancreatic duct cells. J. Histochem. Cytochem. 53, 1539–1552. doi: 10.1369/jhc.4A6587.2005
Huck, V., Niemeyer, A., Goerge, T., Schnaeker, E. M., Ossig, R., Rogge, P., et al. (2007). Delay of acute intracellular pH recovery after acidosis decreases endothelial cell activation. J. Cell. Physiol. 211, 399–409. doi: 10.1002/jcp.20947
Hucthagowder, V., Morava, E., Kornak, U., Lefeber, D. J., Fischer, B., Dimopoulou, A., et al. (2009). Loss-of-function mutations in ATP6V0A2 impair vesicular trafficking, tropoelastin secretion and cell survival. Hum. Mole. Genet. 18, 2149–2165. doi: 10.1093/hmg/ddp148
Hwang, J. M., Kao, S. H., Hsieh, Y. H., Li, K. L., Wang, P. H., Hsu, L. S., et al. (2009). Reduction of anion exchanger 2 expression induces apoptosis of human hepatocellular carcinoma cells. Mole. Cell. Biochem. 327, 135–144. doi: 10.1007/s11010-009-0051-3
Hwang, S., Shin, D. M., and Hong, J. H. (2019). Drug Repurposing as an Antitumor Agent: Disulfiram-Mediated Carbonic Anhydrase 12 and Anion Exchanger 2 Modulation to Inhibit Cancer Cell Migration. Molecules 24:18. doi: 10.3390/molecules24183409
Jaiswal, J. K., Rivera, V. M., and Simon, S. M. (2009). Exocytosis of Post-Golgi Vesicles Is Regulated by Components of the Endocytic Machinery. Cell 137, 1308–1319. doi: 10.1016/j.cell.2009.04.064
Khayat, W., Hackett, A., Shaw, M., Ilie, A., Dudding-Byth, T., Kalscheuer, V. M., et al. (2019). A recurrent missense variant in SLC9A7 causes nonsyndromic X-linked intellectual disability with alteration of Golgi acidification and aberrant glycosylation. Hum. Mole. Genet. 28, 598–614. doi: 10.1093/hmg/ddy371
Khosrowabadi, E., Rivinoja, A., Risteli, M., Tuomisto, A., Salo, T., Mäkinen, M. J., et al. (2021). PREPRINT: SLC4A2 Anion Exchanger Promotes Tumor Cell Malignancy via Enhancing H+ Leak across Golgi Membranes. bioRxiv 2021:428406. doi: 10.1101/2021.02.09.428406
Kokkonen, N., Khosrowabadi, E., Hassinen, A., Harrus, D., Glumoff, T., Kietzmann, T., et al. (2019). Abnormal Golgi pH Homeostasis in Cancer Cells Impairs Apical Targeting of Carcinoembryonic Antigen by Inhibiting Its Glycosyl-Phosphatidylinositol Anchor-Mediated Association with Lipid Rafts. Antiox. Redox Signal. 30, 5–21. doi: 10.1089/ars.2017.7389
Kornak, U., Reynders, E., Dimopoulou, A., van Reeuwijk, J., Fischer, B., Rajab, A., et al. (2008). Impaired glycosylation and cutis laxa caused by mutations in the vesicular H+-ATPase subunit ATP6V0A2. Nat. Genet. 40, 32–34. doi: 10.1038/ng.2007.45
Kulshrestha, A., Katara, G. K., Ginter, J., Pamarthy, S., Ibrahim, S. A., Jaiswal, M. K., et al. (2016). Selective inhibition of tumor cell associated Vacuolar-ATPase ‘a2’ isoform overcomes cisplatin resistance in ovarian cancer cells. Mole. Oncol. 10, 789–805. doi: 10.1016/j.molonc.2016.01.003
Kulshrestha, A., Katara, G. K., Ibrahim, S., Pamarthy, S., Jaiswal, M. K., Sachs, A. G., et al. (2015). Vacuolar ATPase ‘a2’ isoform exhibits distinct cell surface accumulation and modulates matrix metalloproteinase activity in ovarian cancer. Oncotarget 6, 3797–3810. doi: 10.18632/oncotarget.2902
Lawrence, S. P., Bright, N. A., Luzio, J. P., and Bowers, K. (2010). The Sodium/Proton Exchanger NHE8 Regulates Late Endosomal Morphology and Function. Mole. Biol. Cell 21, 3540–3551. doi: 10.1091/mbc.E09-12-1053
Lebredonchel, E., Houdou, M., Potelle, S., de Bettignies, G., Schulz, C., Recchi, M. A. K., et al. (2019). Dissection of TMEM165 function in Golgi glycosylation and its Mn2+ sensitivity. Biochimie 165, 123–130. doi: 10.1016/j.biochi.2019.07.016
Lee, J. S., Kim, M. Y., Park, E. R., Shen, Y. N., Jeon, J. Y., Cho, E. H., et al. (2018). TMEM165, a Golgi transmembrane protein, is a novel marker for hepatocellular carcinoma and its depletion impairs invasion activity. Oncol. Rep. 40, 1297–1306. doi: 10.3892/or.2018.6565
Liberti, M. V., and Locasale, J. W. (2016). The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 41, 211–218. doi: 10.1016/j.tibs.2015.12.001
Lin, P. J. C., Williams, W. P., Kobiljski, J., and Numata, M. (2007). Caveolins bind to (Na+, K+)/H+ exchanger NHE7 by a novel binding module. Cell. Signal. 19, 978–988. doi: 10.1016/j.cellsig.2006.11.006
Lin, P. J. C., Williams, W. P., Luu, Y., Molday, R. S., Orlowski, J., and Numata, M. (2005). Secretory carrier membrane proteins interact and regulate trafficking of the organellar (Na+,K+)/H+ exchanger NHE7. J. Cell Sci. 118, 1885–1897. doi: 10.1242/jcs.02315
Litan, A., and Langhans, S. A. (2015). Cancer as a channelopathy: ion channels and pumps in tumor development and progression. Front. Cell. Neurosci. 9:00086. doi: 10.3389/fncel.2015.00086
Liu, B., Palmfeldt, J., Lin, L., Colaco, A., Clemmensen, K. K. B., Huang, J., et al. (2018). STAT3 associates with vacuolar H+-ATPase and regulates cytosolic and lysosomal pH. Cell Res. 28, 996–1012. doi: 10.1038/s41422-018-0080-0
Llopis, J., McCaffery, J. M., Miyawaki, A., Farquhar, M. G., and Tsien, R. Y. (1998). Measurement of cytosolic, mitochondrial, and Golgi pH in single living cells with green fluorescent proteins. Proc. Natl. Acad. Sci. U S A 95, 6803–6808. doi: 10.1073/pnas.95.12.6803
Lopez-Hernandez, T., Puchkov, D., Krause, E., Maritzen, T., and Haucke, V. (2020). Endocytic regulation of cellular ion homeostasis controls lysosome biogenesis. Nat. Cell Biol. 22:815. doi: 10.1038/s41556-020-0535-7
Maeda, Y., Ide, T., Koike, M., Uchiyama, Y., and Kinoshita, T. (2008). GPHR is a novel anion channel critical for acidification and functions of the Golgi apparatus. Nat. Cell Biol. 10, 1135–1145. doi: 10.1038/ncb1773
Mboge, M. Y., Mahon, B. P., McKenna, R., and Frost, S. C. (2018). Carbonic Anhydrases: Role in pH Control and Cancer. Metabolites 8:8010019. doi: 10.3390/metabo8010019
Merkulova, M., Paunescu, T. G., Azroyan, A., Marshansky, V., Breton, S., and Brown, D. (2015). Mapping the H+ (V)-ATPase interactome: identification of proteins involved in trafficking, folding, assembly and phosphorylation. Sci. Rep. 5:14827. doi: 10.1038/srep14827
Milosavljevic, N., Monet, M., Lena, I., Brau, F., Lacas-Gervais, S., Feliciangeli, S., et al. (2014). The Intracellular Na+/H+ Exchanger NHE7 Effects a Na+-Coupled, but Not K+-Coupled Proton-Loading Mechanism in Endocytosis. Cell Rep. 7, 689–696. doi: 10.1016/j.celrep.2014.03.054
Murali, P., Johnson, B. P., Lu, Z., Climer, L., Scott, D. A., Foulquier, F., et al. (2020). Novel role for the Golgi membrane protein TMEM165 in control of migration and invasion for breast carcinoma. Oncotarget 11, 2747–2762. doi: 10.18632/oncotarget.27668
Nakamura, N., Tanaka, S., Teko, Y., Mitsui, K., and Kanazawa, H. (2005). Four Na+/H+ exchanger isoforms are distributed to Golgi and post-Golgi compartments and are involved in organelle pH regulation. J. Biol. Chem. 280, 1561–1572. doi: 10.1074/jbc.M410041200
Nozaki, K., Ishii, D., and Ishibashi, K. (2008). Intracellular aquaporins: clues for intracellular water transport? Pflugers Archiv-Eur. J. Physiol. 456, 701–707. doi: 10.1007/s00424-007-0373-5
Nugues, C., Helassa, N., Rajamanoharan, D., Burgoyne, R. D., and Haynes, L. P. (2018). PREPRINT: Lysosome exocytosis is required for mitosis. bioRxiv 2018, 375816. doi: 10.1101/375816
Numata, M., and Orlowski, J. (2001). Molecular cloning and characterization of a novel (Na+,K+)/H+ exchanger localized to the trans-Golgi network. J. Biol. Chem. 276, 17387–17394. doi: 10.1074/jbc.M101319200
Onishi, I., Lin, P. J. C., Numata, Y., Austin, P., Cipollone, J., Roberge, M., et al. (2012). Organellar (Na+, K+)/H+ exchanger NHE7 regulates cell adhesion, invasion and anchorage-independent growth of breast cancer MDA-MB-231 cells. Oncol. Rep. 27, 311–317. doi: 10.3892/or.2011.1542
Paroutis, P., Touret, N., and Grinstein, S. (2004). The pH of the secretory pathway: Measurement, determinants, and regulation. Physiology 19, 207–215. doi: 10.1152/physiol.00005.2004
Pedersen, S. F., and Counillon, L. (2019). The Slc9a-C Mammalian Na+/H+ Exchanger Family: Molecules. Mechanisms, and physiology. Physiol. Rev. 99, 2015–2113. doi: 10.1152/physrev.00028.2018
Pernas-Sueiras, O., Alfonso, A., Vieytes, M. R., and Botana, L. M. (2005). Mast cell exocytosis can be triggered by ammonium chloride with just a cytosolic alkalinization and no calcium increase. J. Cell. Physiol. 204, 775–784. doi: 10.1002/jcp.20334
Poschet, J., Perkett, E., and Deretic, V. (2002). Hyperacidification in cystic fibrosis: links with lung disease and new prospects for treatment. Trends Mole. Med. 8, 512–519. doi: 10.1016/s1471-4914(02)02414-0
Poschet, J. F., Boucher, J. C., Tatterson, L., Skidmore, J., Van Dyke, R. W., and Deretic, V. (2001). Molecular basis for defective glycosylation and Pseudomonas pathogenesis in cystic fibrosis lung. Proc. Natl. Acad. Sci. U S A 98, 13972–13977. doi: 10.1073/pnas.241182598
Prevarskaya, N., Skryma, R., and Shuba, Y. (2018). Ion channels in cancer: are cancer hallmarks oncochannelopathies? Physiol. Rev. 98, 559–621. doi: 10.1152/physrev.00044.2016
Rahajeng, J., Kuna, R. S., Makowski, S. L., Tran, T. T. T., Buschman, M. D., Li, S., et al. (2019). Efficient Golgi Forward Trafficking Requires GOLPH3-Driven, PI4P-Dependent Membrane Curvature. Dev. Cell 50:573. doi: 10.1016/j.devcel.2019.05.038
Rivera-Molina, F., and Toomre, D. (2013). Live-cell imaging of exocyst links its spatiotemporal dynamics to various stages of vesicle fusion. J. Cell Biol. 201, 673–680. doi: 10.1083/jcb.201212103
Rivinoja, A., Hassinen, A., Kokkonen, N., Kauppila, A., and Kellokumpu, S. (2009). Elevated Golgi pH Impairs Terminal N-Glycosylation by Inducing Mislocalization of Golgi Glycosyltransferases. J. Cell. Physiol. 220, 144–154. doi: 10.1002/jcp.21744
Rivinoja, A., Kokkonen, N., Kellokumpu, I., and Kellokumpu, S. (2006). Elevated Golgi pH in breast and colorectal cancer cells correlates with the expression of oncofetal carbohydrate T-antigen. J. Cell. Physiol. 208, 167–174. doi: 10.1002/jcp.20653
Romero, M. F., Chen, A. P., Parker, M. D., and Boron, W. F. (2013). The SLC4 family of bicarbonate (HCO3-) transporters. Mole. Aspects Med. 34, 159–182. doi: 10.1016/j.mam.2012.10.008
Schapiro, F. B., and Grinstein, S. (2000). Determinants of the pH of the Golgi complex. J. Biol. Chem. 275, 21025–21032. doi: 10.1074/jbc.M002386200
Sechi, S., Frappaolo, A., Karimpour-Ghahnavieh, A., Piergentili, R., and Giansanti, M. G. (2020). Oncogenic Roles of GOLPH3 in the Physiopathology of Cancer. Int. J. Mole Sci. 21:3. doi: 10.3390/ijms21030933
Seksek, O., Biwersi, J., and Verkman, A. S. (1995). Direct Measurement Of Trans-Golgi Ph In Living Cells And Regulation By 2nd Messengers. J. Biol. Chem. 270, 4967–4970. doi: 10.1074/jbc.270.10.4967
Seksek, O., Biwersi, J., and Verkman, A. S. (1996). Evidence against defective trans-Golgi acidification in cystic fibrosis. J. Biol. Chem. 271, 15542–15548. doi: 10.1074/jbc.271.26.15542
Shin, J. J. H., Liu, P., Chan, L. J., Ullah, A., Pan, J. X., Borchers, C. H., et al. (2020). pH Biosensing by PI4P Regulates Cargo Sorting at the TGN. Dev. Cell 52:461. doi: 10.1016/j.devcel.2019.12.010
Sou, Y. S., Kakuta, S., Kamikubo, Y., Niisato, K., Sakurai, T., Parajuli, L. K., et al. (2019). Cerebellar Neurodegeneration and Neuronal Circuit Remodeling in Golgi pH Regulator-Deficient Mice. Eneuro 6:3. doi: 10.1523/eneuro.0427-18.2019
Steffan, J. J., Snider, J. L., Skalli, O., Welbourne, T., and Cardelli, J. A. (2009). Na+/H+ Exchangers and RhoA Regulate Acidic Extracellular pH-Induced Lysosome Trafficking in Prostate Cancer Cells. Traffic 10, 737–753. doi: 10.1111/j.1600-0854.2009.00904.x
Sterling, D., Reithmeier, R. A. F., and Casey, J. R. (2001). A transport metabolon. Functional interaction of carbonic anhydrase II and chloride/bicarbonate exchangers. J. Biol. Chem. 276, 47886–47894. doi: 10.1074/jbc.M105959200
Stribny, J., Thines, L., Deschamps, A., Goffin, P., and Morsomme, P. (2020). The human Golgi protein TMEM165 transports calcium and manganese in yeast and bacterial cells. J. Biol. Chem. 295, 3865–3874. doi: 10.1074/jbc.RA119.012249
Tan, X. C., Banerjee, P., Pham, E. A., Rutaganira, F. U. N., Basu, K., Bota-Rabassedas, N., et al. (2020). PI4KIII beta is a therapeutic target in chromosome 1q-amplified lung adenocarcinoma. Sci. Transl. Med. 12:527. doi: 10.1126/scitranslmed.aax3772
Tang, Z. F., Kang, B. X., Li, C. W., Chen, T. X., and Zhang, Z. M. (2019). GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 47, W556–W560. doi: 10.1093/nar/gkz430
Tu, Y. F., Zhao, L., Billadeau, D. D., and Jia, D. (2020). Endosome-to-TGN Trafficking: Organelle-Vesicle and Organelle-Organelle Interactions. Front. Cell Dev. Biol. 8:00163. doi: 10.3389/fcell.2020.00163
Valm, A. M., Cohen, S., Legant, W. R., Melunis, J., Hershberg, U., Wait, E., et al. (2017). Applying systems-level spectral imaging and analysis to reveal the organelle interactome. Nature 546:162. doi: 10.1038/nature22369
Vasanthakumar, T., and Rubinstein, J. L. (2020). Structure and Roles of V-type ATPases. Trends Biochem. Sci. 45, 295–307. doi: 10.1016/j.tibs.2019.12.007
Vavassori, S., Cortini, M., Masui, S., Sannino, S., Anelli, T., Caserta, I. R., et al. (2013). A pH-Regulated Quality Control Cycle for Surveillance of Secretory Protein Assembly. Mole. Cell 50, 783–792. doi: 10.1016/j.molcel.2013.04.016
Vince, J. W., and Reithmeier, R. A. F. (2000). Identification of the carbonic anhydrase II binding site in the Cl-/HCO3- anion exchanger AE1. Biochemistry 39, 5527–5533. doi: 10.1021/bi992564p
Wang, H., Yang, Y. Y., Huang, F., He, Z. X., Li, P., Zhang, W., et al. (2020). In Situ Fluorescent and Photoacoustic Imaging of Golgi pH to Elucidate the Function of Transmembrane Protein 165. Anal. Chem. 92, 3103–3110. doi: 10.1021/acs.analchem.9b04709
Wang, X., Zhang, X. L., Dong, X. P., Samie, M., Li, X. R., Cheng, X. P., et al. (2012). TPC Proteins Are Phosphoinositide-Activated Sodium-Selective Ion Channels in Endosomes and Lysosomes. Cell 151, 372–383. doi: 10.1016/j.cell.2012.08.036
Waugh, M. G. (2019). The Great Escape: How phosphatidylinositol 4-kinases and PI4P promote vesicle exit from the Golgi (and drive cancer) (vol 476, pg 2321, 2019). Biochem. J. 476, 3067–3067. doi: 10.1042/bcj20180622_cor
Webb, B. A., Aloisio, F. M., Charafeddine, R. A., Cook, J., Wittmann, T., and Barber, D. L. (2021). pHLARE: a new biosensor reveals decreased lysosome pH in cancer cells. Mole. Biol. Cell 32, 131–142. doi: 10.1091/mbc.E20-06-0383
Webb, B. A., Chimenti, M., Jacobson, M. P., and Barber, D. L. (2011). Dysregulated pH: a perfect storm for cancer progression. Nat. Rev. Cancer 11, 671–677. doi: 10.1038/nrc3110
Wu, M. M., Grabe, M., Adams, S., Tsien, R. Y., Moore, H. P. H., and Machen, T. E. (2001). Mechanisms of pH regulation in the regulated secretory pathway. J. Biol. Chem. 276, 33027–33035. doi: 10.1074/jbc.M103917200
Zhang, L. J., Lu, R. Q., Song, Y. N., Zhu, J. Y., Xia, W., Zhang, M., et al. (2017). Knockdown of anion exchanger 2 suppressed the growth of ovarian cancer cells via mTOR/p70S6K1 signaling. Sci. Rep. 7:062472. doi: 10.1038/s41598-017-06472-w
Zheng, T. Y., Jaattela, M., and Liu, B. (2020). pH gradient reversal fuels cancer progression. Int. J. Biochem. Cell Biol. 125:105796. doi: 10.1016/j.biocel.2020.105796
Keywords: Golgi pH, proton sink, pH homeostasis, ion transport, intracellular pH, cancer
Citation: Galenkamp KMO and Commisso C (2021) The Golgi as a “Proton Sink” in Cancer. Front. Cell Dev. Biol. 9:664295. doi: 10.3389/fcell.2021.664295
Received: 04 February 2021; Accepted: 21 April 2021;
Published: 13 May 2021.
Edited by:
Daniel Ungar, University of York, United KingdomReviewed by:
Sakari Kellokumpu, University of Oulu, FinlandCopyright © 2021 Galenkamp and Commisso. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Koen M. O. Galenkamp, a2dhbGVua2FtcEBzYnBkaXNjb3Zlcnkub3Jn; Cosimo Commisso, Y2NvbW1pc3NvQHNicGRpc2NvdmVyeS5vcmc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.