
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Cell Dev. Biol. , 07 April 2021
Sec. Cell Death and Survival
Volume 9 - 2021 | https://doi.org/10.3389/fcell.2021.653322
The phenomenon of mitochondria donation is found in various tissues of humans and animals and is attracting increasing attention. To date, numerous studies have described the transfer of mitochondria from stem cells to injured cells, leading to increased ATP production, restoration of mitochondria function, and rescue of recipient cells from apoptosis. Mitochondria transplantation is considered as a novel therapeutic approach for the treatment of mitochondrial diseases and mitochondrial function deficiency. Mitochondrial dysfunction affects cells with high energy needs such as neural, skeletal muscle, heart, and liver cells and plays a crucial role in type 2 diabetes, as well as Parkinson’s, Alzheimer’s diseases, ischemia, stroke, cancer, and age-related disorders. In this review, we summarize recent findings in the field of mitochondria donation and mechanism of mitochondria transfer between cells. We review the existing clinical trials and discuss advantages and disadvantages of mitochondrial transplantation strategies based on the injection of stem cells, isolated functional mitochondria, or EVs containing mitochondria.
Mitochondria are key players in the cell’s energy production, calcium homeostasis, signaling, and apoptosis (Rossi et al., 2019). Deficiency in mitochondrial function is observed in inherited mitochondrial diseases as well as cancer, diabetes, neurodegenerative diseases (including Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease), aging, and age-related metabolic disorders—so-called mitochondria-associated diseases (Bhatti et al., 2017). Replacement of non-functional mitochondria by transplantation of healthy mitochondria into injured cells is believed to potentially be a universal solution for the treatment of mitochondrial deficiency of different etiologies. Delivery of even a few healthy mitochondria can lead to the sustained restoration of mitochondria function in a recipient cell.
Detection of mitochondria donation from mesenchymal stromal cells also known as mesenchymal stem cells (MSCs) to recipient cells aroused great interest in the field of regenerative medicine. Mitochondrial donation leads to the rescue of injured cells, improved oxidative phosphorylation, increased ATP production, and restoration of mitochondrial function (Rustom, 2016). As a result of these findings, it is now generally accepted that the reparative function of MSCs is partly mediated by mitochondrial transfer, which seems to be an evolutionarily conserved phenomenon. Horizontal transfer of mitochondria between mammalian cells provides a novel therapeutic approach for the treatment of mitochondrial and mitochondria-associated diseases and stimulates development of mitochondria transplantation strategies.
The development of a robust mitochondria delivery protocol is a critical issue that needs to be addressed prior to any widespread clinical application of mitochondrial transplantation. Due to the immunogenic complications of using isolated mitochondria (Ishikawa et al., 2010; Zhang et al., 2010; Wang et al., 2018), MSC-based mitochondria transfer is considered as a promising therapeutic strategy. Since it was first described by Islam et al. (2012) discovered that BMSCs transfer mitochondria to injured lung alveolar epithelial cells. MSC-based mitochondria transfer has been used for the treatment of diverse mitochondria-associated disorders such as ischemic stroke (Liu et al., 2019), spinal cord injury (Li et al., 2019), kidney injury (Zou et al., 2018), cardiomyoblast ischemia model (Cselenyak et al., 2010), and respiratory system injury (Islam et al., 2012). A more recent shift in the field of regenerative medicine, from cell based to cell-free therapy (Gomzikova and Rizvanov, 2017), has led to the study of new approaches, such as mitochondria coating with biocompatible polymers and encapsulation into microvesicles.
In this review, we provide an overview of recent findings in the field of mitochondria donation and mechanisms of mitochondria transfer. We discuss a therapeutic strategy based on injection of isolated functional mitochondria and describe advances and challenges of mitochondrial transplantation strategies, based on injection of stem cells, isolated functional mitochondria, or EVs containing mitochondria.
The phenomenon of mitochondria transfer was first observed between endothelial progenitor cells and cardiac myocytes (Koyanagi et al., 2005). Subsequently, mitochondrial donation from MSCs was described by Spees et al. (2006). The authors demonstrated that after cocultivation of hMSCs with A549 ρ°cells (containing defective mtDNA), some A549 ρ°cells acquired functional mitochondria derived from donor hMSCs. Cells containing donor mitochondria showed active proliferation, decreased levels of reactive oxygen species, increased ATP production, membrane potential, and oxygen consumption (Spees et al., 2006). Furthermore, Plotnikov et al. (2008) also reported mitochondrial transfer when investigating the cocultivation of MSCs with rat cardiomyocytes or rat renal tubular cells (Plotnikov et al., 2010); the number of tunneling nanotubes (TNTs) significantly rose in correlation with the detection of mitochondrial transfer. Further studies have since confirmed the process of intercellular mitochondrial transfer in vitro (Acquistapace et al., 2011; Pankotai et al., 2012). With Islam et al. (2012) providing the first evidence of mitochondrial transfer in vivo (Islam et al., 2012), by demonstrating in an LPS-induced acute lung injury model, bone marrow-derived MSCs transfer mitochondria to the injured alveolar epithelial cells inducing generation of ATP and increasing mouse survival (Islam et al., 2012). In addition to mitochondrial donation from MSCs, mitochondrial donation has also been observed from endothelial cells to cancer cells (Pasquier et al., 2013), from astrocytes to neurons (Hayakawa et al., 2016), and from cancer-associated fibroblasts to prostate cancer cells (Ippolito et al., 2019).
Since these first studies reported the existence of intercellular mitochondria transfer, numerous studies have gone on to demonstrate that MSCs donate mitochondria leading to the rescue of the injured cell, improved aerobic respiration, and inhibited apoptosis. This has been demonstrated to occur in endothelial cells within in vitro ischemia–reperfusion models (Liu et al., 2014), as well as observations of attenuation of alveolar destruction and altered severity of fibrosis in models of cigarette smoke-induced damage (Li et al., 2014), neuroprotective effects and decline of infarct volume in the brain (Babenko et al., 2015), amelioration of acute renal ischemia reperfusion injury (Gu et al., 2016), recovery of mitochondrial function in rat cardiomyocytes in vitro after ischemia/reperfusion injury (Han et al., 2016), protection of corneal epithelial cells from Rotenone-induced oxidative damage (Jiang et al., 2016), and decreased mutation ratio and oxidative damage in cells derived from a patient with mitochondrial disease (MERRF syndrome) (Chuang et al., 2017).
MSCs are readily attracted to tumor stroma. Studies of the tumor microenvironment have also demonstrated that MSCs can donate mitochondria to cancer cells, inducing their chemoresistance (Pasquier et al., 2013; Moschoi et al., 2016) and restoring impaired mitochondria function (Lin et al., 2015). Transfer of normal mitochondria from human umbilical cord-derived MSCs into breast cancer MDA-MB-231 cells increased the proliferation and invasiveness of MDA-MB-231 cells as well as enhance cisplatin-induced apoptosis (Kheirandish-Rostami et al., 2020). However, in contrast to MSCs, mitochondria isolated from normal human astrocytes inhibited malignant proliferation of human glioma U87 cells, as well as increasing aerobic respiration, attenuating glycolysis, and enhancing radiosensitivity both in vitro and in vivo (Sun et al., 2019). This phenomenon underlines the complexity and significance of the tumor microenvironment in cancer progression. Future research of the mechanisms of mitochondrial transfer between tumor and tumor-associated cells will hopefully provide new insights into potential therapeutic targets.
MSC mitochondrial transfer has also been observed to regulate immune cell activity. MSCs can deliver mitochondria to activated T cells, improving their energy state and suppressing aberrant autophagy in systemic lupus erythematosus (SLE) patients (Chen et al., 2016). The authors suggested that regulation of the energy state of T cells by mitochondrial transfer could be a new therapeutic strategy in SLE treatment. Mitochondrial transfer from MSC to macrophages results in enhanced alveolar macrophages with increases in phagocytosis, oxidative phosphorylation, and antimicrobial effects in vivo in the context of acute respiratory distress syndrome (ARDS) (Jackson et al., 2016; Jackson and Krasnodembskaya, 2017). It is believed that mitochondrial donation is a novel mechanism of MSC-mediated antimicrobial effects, mediated by enhancement of macrophage phagocytic activity. Morrison et al. (2017) revealed a novel mechanism of modulation of macrophage polarization through mitochondrial donation. The authors showed that MSCs transfer functional mitochondria enclosed in EVs, inducing M2 macrophage polarization and enhancement of phagocytic capacity, protecting against endotoxin-induced lung injury and ameliorating lung injury in vivo (Morrison et al., 2017). Regulation of T cell function by mitochondrial transfer was observed under cocultivation of T helper 17 (Th17) cells with bone marrow-derived MSCs (Luz-Crawford et al., 2019). The authors showed that pro-inflammatory Th17 cells acquire an anti-inflammatory phenotype after the mitochondria were transferred, whereas a reduction of transferred mitochondria may contribute to the chronic inflammation seen in rheumatoid arthritis (RA) synovitis (Luz-Crawford et al., 2019). The concept of organelle−based therapy for the treatment of immune diseases was demonstrated using a graft versus host disease (GvHD) mouse model (Court et al., 2020). Transplantation of human T cells treated with mitochondria led to a significant improvement in survival and reduction in tissue damage (Court et al., 2020).
In recent years, the number of articles describing mitochondria transfer has increased tremendously. We have summarized the body of research in which mitochondrial transfer between stem cells and recipient cells was detected, as well as mitochondrial transplantation in various disease models in Supplementary Table 1.
Injury and stress signals were shown to trigger the transfer of mitochondria from MSCs to recipient cells. Mitochondrial donation by MSCs was observed in mtDNA-deficient cells and mitochondrial toxin-treated cells, whereas mitochondrial transfer was not detected in cells harboring pathogenic mutations (Cho et al., 2012). The process of mitochondrial donation by MSCs was triggered by damaged somatic cell-derived mitochondria (Mahrouf-Yorgov et al., 2017). Their uptake and degradation by MSCs led to the induction of the cytoprotective enzyme heme oxygenase-1 (HO-1) and stimulation of mitochondrial biogenesis (Mahrouf-Yorgov et al., 2017). Reactive oxygen species released by cells under oxidative stress and inflammation may also trigger mitochondrial donation (Paliwal et al., 2018). However, little is known about the intrinsic signaling mechanisms of mitochondrial transfer. It was shown that release of extracellular mitochondrial particles mediated by a calcium-dependent mechanism involving CD38 and cyclic ADP ribose signaling may play a key role (Hayakawa et al., 2016).
Based on published studies, it has been proposed that cell stress is required for organelle transfer. However, accumulating evidence suggests that mitochondrial transfer from MSCs also occurs under normal physiological conditions. Unidirectional transfer of intact mitochondria was observed from MSCs to PBMCs (Court et al., 2020), 56% in CD4+ cells, 17% in CD8+T cells, and 24% in B cells (Luz-Crawford et al., 2019), as well as to corneal endothelial cells (CECs), 661W cells (a photoreceptor cell line) and ARPE-19 cells (a retinal pigment epithelium cell line) (Jiang et al., 2020), primary astrocytes and neurons (Gao et al., 2019), and human umbilical cord vein endothelial cells (Feng et al., 2019) in coculture conditions.
Mitochondrial transfer from MSCs to recipient cells induced elevation of mitochondrial membrane potential, increased respiration, and improved energy metabolism as a result. Mitochondrial donation to the immune cells additionally led to metabolic and function alterations with acquisition of an anti-inflammatory phenotype. It is known that the mitochondrial metabolism influences stem cell fate and regulates pluripotency (Zhang et al., 2018). However, studies investigating the role of MSC-derived mitochondria on the morphology of recipient cells and their properties are few. Konari et al. (2019) observed that transfer of isolated mitochondria caused structural restoration of renal proximal tubular epithelial cells (PTECs) and the structure of the tubular basement membranes and brush borders in vivo. We believe that the influence of mitochondrial donation by MSCs on recipient cell morphology, physiological properties, and mitochondria-dependent metabolic reprogramming warrants further study in the future.
The ability of MSCs to transfer mitochondria may be enhanced by upregulation of Miro1 (adaptor protein participating in mitochondria moving along microtubules) (Ahmad et al., 2014), which can be primed in a number of ways including coculturing MSCs with the target cells (Babenko et al., 2015), under high level of TNFα-IP2 expression (Zhang et al., 2016), by antioxidant treatment (N-acetyl-L-cysteine and L-ascorbic acid 2-phosphate) of MSCs (Li et al., 2017), by TNF-α treatment (induce TNTs formation) (Melcher et al., 2017), or damaged somatic cell-derived mitochondria (Mahrouf-Yorgov et al., 2017). Modulation of mitochondrial donation capacity might be one route to increasing the therapeutic potential of MSCs.
In theory, even one functional mitochondrion transferred into a recipient cell may propagate, due to the evolutionarily conserved mechanism for the selective amplification of wild-type mtDNA (Hill et al., 2014). However, this assumption still needs to be experimentally verified. Recently, the fate of delivered foreign mitochondria in target cells was investigated by Jiang et al. (2020) using mitochondria Cyto-Tracer. The authors demonstrated that transferred mitochondria were either digested by the host lysosomes or expelled from the cell within 3–5-μm round bubbles after 8 days (Jiang et al., 2020). However, the main difficulty in the mitochondrial tracking studies is attenuation of fluorescent signal in recipient cells due to mitochondrial division. To detect the mitochondrial heteroplasmy in recipient cells and evaluate the lifespan of the foreign mitochondria in the recipient cells, more sensitive methods of detection such as sequencing and isotope labeling may provide a clearer picture.
Intercellular mitochondrial trafficking occurs via tunneling nanotubes (TNTs), extracellular vesicles (EVs), and cellular fusion. Recently, cell and cytoplasmic membrane-free respiratory competent mitochondria were observed in blood and conditioned cell culture medium (Al Amir Dache et al., 2020). Although the role of cell-free mitochondria in intercellular communication remains to be fully understood, the practical approaches aimed to transfer intact mitochondria into target cells have been previously developed. We summarize the known ways of mitochondrial transfer into recipient cells in Figure 1.
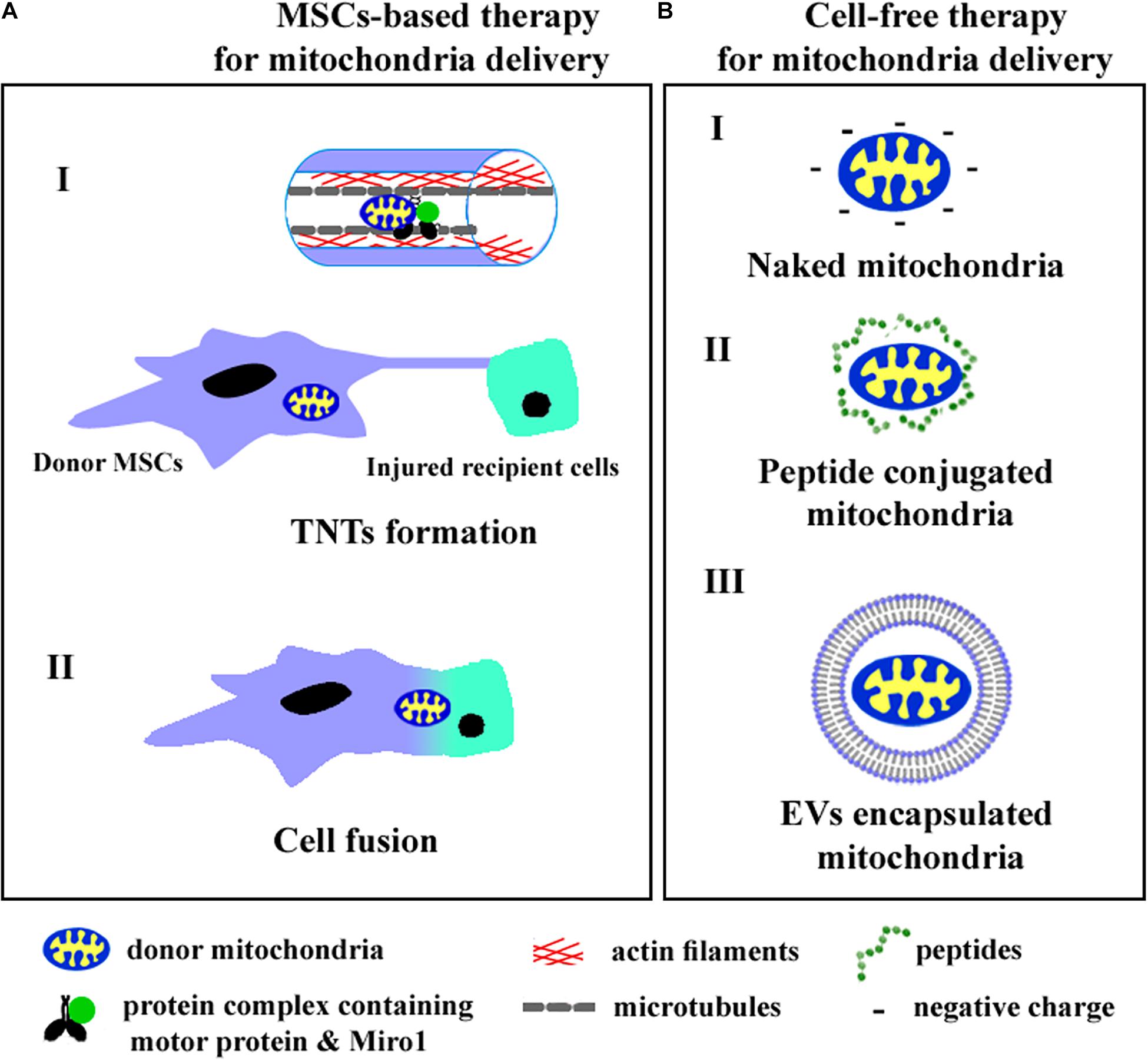
Figure 1. The cell-based (A) and cell-free (B) strategies of mitochondria delivery into recipient cells. A (I)—mitochondria transfer through TNTs, A (II)—mitochondria exchange after cell fusion, B (I)—injection of isolated mitochondria, B (II)—application of peptide conjugated mitochondria, B (III)—delivery of mitochondria encapsulated into EVs.
TNTs are intercellular, actin, or microtubule-based cytoplasmic channels enveloped by a cytoplasmic membrane, connecting cells and forming intercellular transport networks (Vignais et al., 2017; Jash et al., 2018). TNTs can be formed by thin filaments of F-actin and a thicker subset (0.7 μm) of both F-actin and microtubules (Onfelt et al., 2004; Wang et al., 2018). The first type are called actin-based TNTs (AC-TNTs); the last type are microtubules containing TNTs (MT-TNTs) (Rustom, 2016). AC-TNTs are characterized by a limited lifespan and transfer of small molecules, organelles, and ions, whereas MT-TNTs have an increased diameter, have a prolonged lifespan, and transfer larger organelles such as mitochondria (Rustom, 2016). MT-TNTs were first described by Wang et al. in the context of a long-distance transport of mitochondria from control cells to rescue of apoptotic pheochromocytoma (PC12) cells, stressed by UV radiation (Wang and Gerdes, 2015; Rustom, 2016). Since then, numerous investigations have shown mitochondrial donation via TNTs, these are summarized in Table 1.
Ahmad et al. (2014) investigated the molecular mechanisms of mitochondrial donation, demonstrating that Miro1 is essential for mitochondrial transport. The lack of Miro1 retarded mitochondrial movement through TNTs and abolished the MSC therapeutic effect. Yao et al. (2018) showed that connexin 43 regulates TNT formation, since its knockdown diminished TNT formation in human-induced pluripotent stem cells (iPSCs) and derived MSCs. Mitochondrial transfer was reduced in all cocultures after microtubule/TNT or endocytosis inhibition (Sinclair et al., 2016; Jackson and Krasnodembskaya, 2017).
EVs are a heterogeneous group of bilipid membrane vesicles, encapsulating proteins and genetic material, as well as organelles, including mitochondria (Islam et al., 2012), ribosomes (Court et al., 2008), and proteasomes (Yu et al., 2014). EVs transfer biomolecules and organelles to target cells to mediate long-distance intercellular cross talk. Large EVs 100–1,000 nm in size (microparticles) are able to encapsulate mitochondria which are on average nearly 500 nm in size (Vignais et al., 2017). Falchi et al. (2013) also described large shedding vesicles (1–8 μm in diameter) that contained mitochondria in cultures of human fetal astrocytes.
Islam et al. (2012) first described the EV-mediated transfer of mitochondria from BMSCs to injured lung alveolar epithelial cells in a model of LPS-induced acute lung injury. The same LPS-induced acute lung injury model was also used by Morrison et al. (2017) to demonstrate the mechanism of action of MSC-derived EVs in the amelioration of lung injury. The authors showed that the transfer of mitochondria from MSCs to macrophages was mediated by EVs (Morrison et al., 2017). Hayakawa et al. (2016) also observed the presence of extracellular particles containing mitochondria in conditioned medium from rat cortical astrocytes in vitro. The authors also demonstrated that astrocytes in mice can release functional mitochondria that enter neurons in a mouse model of focal cerebral ischemia (Hayakawa et al., 2016). Phinney et al. (2015) showed that encapsulating of mitochondria into EVs might be a rescue mechanism from oxidative stress and clearance of depolarized mitochondria.
There have been multiple reports of EVs carrying and delivering mitochondria to a wide range of cell types. EVs of myeloid-derived regulatory cells (MDRCs) delivered mitochondria to recipient T cells in vitro (Hough et al., 2018). EV-mediated mitochondrial transfer was detected from renal scattered tubular cells to tubular epithelial cells in vitro and to stenotic kidney in vivo causing a protective effect, restoring mitochondrial function in vitro, and improving perfusion and oxygenation in vivo (Zou et al., 2018). Zhang et al. (2020) showed that an average 83.11% of HSC-derived EVs, B cell-derived EVs, and T cell-derived EVs carry respiring mitochondria. Neural stem cells (NSC) deliver functional mitochondria to target cells via Mito-EVs increasing Rho0 cell survival in vitro and ameliorating clinical deficits in a mouse model of autoimmune encephalomyelitis (Peruzzotti-Jametti et al., 2020). However, the mechanisms of how mitochondria are encapsulated into EVs remains insufficiently investigated.
Acquistapace et al. (2011) showed that under coculture of mouse-differentiated cardiomyocytes with human multipotent adipose-derived stem cells, cell fusion occurs. The authors showed that as a result of mitochondrial transfer into cardiomyocytes, the resulting hybrid cells were reprogrammed to a progenitor-like state (Acquistapace et al., 2011). To date, several studies have shown that stem cells can fuse with neurons (Cusulin et al., 2012) and hepatocytes (Terada et al., 2002), forming hybrid cells which recapitulate traits specific for stem and differentiated cells (Murray and Krasnodembskaya, 2019). It was shown that length of intercellular connection is inversely proportional to the number of transferred mitochondria: elongation of the distance between cells led to fewer mitochondria being transferred (Wada et al., 2017). Cell fusion results in massive mitochondrial delivery into recipient cells. Cell fusion is a rare event under normal conditions, but hypoxia-induced apoptosis (Noubissi et al., 2015), chronic inflammation (Weimann et al., 2003), or irradiation (Alvarez-Dolado et al., 2003) markedly increased it. Increased cell fusion events between MSCs and differentiated cells as a consequence increase the mitochondria transfer and tissue restoration with heterokaryons detected in regenerated tissue.
Replacement of damaged mitochondria with isolated functional mitochondria which could be internalized by targeted cells has been proposed to treat mitochondrial diseases. Autologous respiration competent mitochondria (naked mitochondria) isolated from non-ischemic tissue and injected directly into the ischemic myocardium can protect the heart from ischemia–reperfusion injury (Masuzawa et al., 2013). In neurodevelopmental diseases, systemic administration of isolated mitochondria improved the endurance of mice and prevented the progression of Parkinson disease by increasing the activity of the electron transport chain, decreasing reactive oxygen species levels, and preventing cell apoptosis and necrosis (Shi et al., 2017). Animal models of schizophrenia show that intra-prefrontal cortex injection of isolated mitochondria prevents the decrease of mitochondrial potential and attentional deficit at adulthood (Robicsek et al., 2018). The introduction of isolated mitochondria in rats with doxorubicin-mediated nephrotoxicity found that mitochondrial transplantation in the renal cortex decreased cellular oxidative stress and promoted regeneration of tubular cells (Kubat et al., 2020). Even xenogenic mitochondria restored the motor activity and mitigated the brain infarct area and neuronal cell death (Huang et al., 2016).
The first clinical application of mitochondrial autotransplantation was carried out in 2017 in Boston Children’s Hospital (United States) to treat myocardial ischemia–reperfusion injury in pediatric patients (Emani et al., 2017). Mitochondria were isolated from the patients’ non-ischemic skeletal muscle and injected directly into the injured myocardium. The authors observed improvement of ventricular function and no adverse complications (i.e., arrhythmia, intramyocardial hematoma, or scarring) (Emani et al., 2017). A second clinical trial was initiated in 2018 in Sun Yat-sen University (China) to improve oocyte quality. The procedure included the microinjection of autologous mitochondria from bone marrow MSCs into human sex cells (oocyte and sperm) (ClinicalTrials.gov Identifier: NCT03639506).
In general, naked mitochondria show low internalization ratios into target cells due to the negative surface charge. Therefore, peptide-mediated mitochondrial delivery (Chang et al., 2013), magnetic nanoparticles (Macheiner et al., 2016), and centrifugation-based (Kim et al., 2018) approaches were applied to enhance the efficiency of naked mitochondrial delivery. Peptide labeling of mitochondria was applied by Chang et al. (2016) before injection into rat brains. The authors showed significant enhancement of the survival of dopaminergic neurons and support of mitochondrial function after mitochondria were injected into a mouse model of Parkinson’s disease (Chang et al., 2016). More recently, biocompatible polymers (dextran with lipophilic cation triphenylphosphonium) have been suggested as a more effective strategy of coating of isolated mitochondria to improve uptake (Wu et al., 2018).
Since mitochondrial donation by stem cells has been demonstrated to play a significant role in rescuing injured cells and tissues, stem cell transplantation was suggested as one of the perspective approaches for mitochondrial delivery. Joerg et al. showed that allogeneic hematopoietic stem cell transplantation restored mitochondrial function and improved clinical symptoms in patients with mitochondrial neurogastrointestinal encephalomyopathy (Halter et al., 2015). According to ClinicalTrials.gov, there were the following ongoing clinical trials of cell therapy for the treatment of mitochondrial dysfunction and mitochondria-associated diseases: Pearson syndrome (NCT03384420), ophthalmic pathology (including age-related macular degeneration, glaucoma) (NCT03011541), inherited metabolic disorders (including mitochondrial neurogastrointestinal encephalopathy) (NCT02171104)1. However, a major challenge of any stem cell-based therapy is oncogenic transformation, undesired differentiation, and blood vessel occlusion, which have to date limited their clinical use (Gomzikova et al., 2019).
Stability of naked mitochondria in serum was a key question for its successful therapeutic application. Shi et al. (2017) showed that incubation of naked mitochondria in serum did not significantly impair the membrane potential of mitochondria during at least 2 h of observation. Results obtained by Ishikawa et al. (2010) raised the question of mitochondrial immunogenicity. The authors demonstrated that tumor cell transplants with polymorphisms of mtDNA (mitochondria were replaced) were rejected from the host mice by the innate immune system with suppression of tumor formation. In addition, it was shown that circulating mitochondrial formyl peptides and mtDNA are recognized as damage-associated molecular patterns (DAMPs) and cause inflammatory responses identical to those activated in sepsis (Zhang et al., 2010; Wang et al., 2018).
Findings in recent years have demonstrated that EVs derived from MSCs could be the most suitable instruments for the delivery of mitochondria into damaged tissues. The membrane of EVs keeps the integrity and functional activity of the mitochondria intact, increasing their lifespan in the bloodstream. However, for the clinical development of EV-mediated mitochondrial delivery, it is necessary to overcome the challenge of obtaining sufficient quantities of EVs containing mitochondria.
MSCs are an attractive source for EV isolation due to their non- or low immunogenicity and ability to proliferate well in vitro. MSCs may be grown in sufficient quantities for the subsequent isolation or enrichment of EVs containing mitochondria (Wang et al., 2018). However, this is an often time-consuming and expensive approach. There are a number of approaches that can increase the enrichment of EVs containing mitochondria; these include using magnetic separation (Hubbard et al., 2019), differential centrifugation (Djafarzadeh and Jakob, 2017), or centrifugation in density gradient (Kristian, 2010) to separate those larger EVs capable of carrying intact mitochondria. There are also a number of techniques that induce the artificial production of EVs from MSCs, which are capable of carrying mitochondria and are reliably produced in much greater quantities. These approaches have been reviewed in detail previously (Gomzikova et al., 2019). Combining the approaches of increased EV isolation with enrichment would enable the creation of a therapeutic mitochondrial treatment with the potential for robust clinical application.
Mitochondrial transplantation strategies based on the systemic injection of isolated functional mitochondria, stem cells, or EVs still do not possess a specificity of delivery and will affect a variety of cells, such as blood cells and vessel-rich organs such as the lung and liver. Previously, it was shown that the intravenous administration of isolated mitochondria caused the mitochondria to become trapped in the lungs (Zhu et al., 2016), while the therapeutic efficacy of mitochondrial transplantation for the treatment of tissue injury or mitochondria-associated disorders will benefit from the targeted delivery of mitochondria into a specific tissue or organs. Due to the inherited characteristics of mitochondrial diseases and the presence of defective mitochondria in every cell of an organism, specificity of mitochondrial delivery is not always strictly necessary. In these cases, achieving sufficient systemic distribution remains a clear obstacle. We suppose that a major focus of future research will be the development of delivery strategies or vectors to target specific cells or overcome the challenges of systemic distribution.
Mitochondrial transfer is a prospective strategy for the treatment of tissue injury, mitochondrial diseases, and mitochondria-associated disorders. Single healthy delivered mitochondria can cause the amplification of functional mitochondria in recipient cells and rescue the phenotype of mitochondrial deficient cells. Development of efficient mitochondrial delivery protocols is a key task for the translation of recent findings into appropriate clinical applications.
MG conceived the idea, wrote the manuscript, and created the tables and the figure. VJ edited the manuscript. AR provided financial support and final approval of manuscript. All authors contributed to the article and approved the submitted version.
This work was funded by a grant of the President of the Russian Federation for state support of leading scientific schools of the Russian Federation NSh-2603.2020.4. The study was partially accomplished in the Center of the National Technology Initiative at the M.M. Shemyakin–Yu.A. Ovchinnikov Institute of Bioorganic Chemistry of the Russian Academy of Sciences. The work was performed according to the Russian Government Program of Competitive Growth of Kazan Federal University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2021.653322/full#supplementary-material
Acquistapace, A., Bru, T., Lesault, P. F., Figeac, F., Coudert, A. E., le Coz, O., et al. (2011). Human mesenchymal stem cells reprogram adult cardiomyocytes toward a progenitor-like state through partial cell fusion and mitochondria transfer. Stem Cells 29, 812–824. doi: 10.1002/stem.632
Ahmad, T., Mukherjee, S., Pattnaik, B., Kumar, M., Singh, S., Kumar, M., et al. (2014). Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J. 33, 994–1010.
Al Amir Dache, Z., Otandault, A., Tanos, R., Pastor, B., Meddeb, R., et al. (2020). Blood contains circulating cell-free respiratory competent mitochondria. FASEB J. 34, 3616–3630. doi: 10.1096/fj.201901917rr
Alvarez-Dolado, M., Pardal, R., Garcia-Vardugo, J. M., Fike, J. R., Lee, H. O., Pfeffer, K., et al. (2003). Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature 425, 968–973. doi: 10.1038/nature02069
Babenko, V. A., Silachev, D. N., Zorova, L. D., Pevzner, I. B., Khutornenko, A. A., Plotnikov, E. Y., et al. (2015). Improving the post-stroke therapeutic potency of mesenchymal multipotent stromal cells by cocultivation with cortical neurons: the role of crosstalk between cells. Stem Cell Transl Med. 4, 1011–1020. doi: 10.5966/sctm.2015-0010
Bhatti, J. S., Bhatti, G. K., and Reddy, P. H. (2017). Mitochondrial dysfunction and oxidative stress in metabolic disorders - a step towards mitochondria based therapeutic strategies. Biochim Biophys. Acta Mol. Basis Dis. 1863, 1066–1077. doi: 10.1016/j.bbadis.2016.11.010
Chang, J. C., Liu, K. H., Li, Y. C., Kou, S. J., Wei, Y. H., Chuang, C. S., et al. (2013). Functional recovery of human cells harbouring the mitochondrial DNA mutation MERRF A8344G via peptide-mediated mitochondrial delivery. Neurosignals 21, 160–173.
Chang, J. C., Wu, S. L., Liu, K. H., Chen, Y. H., Chuang, C. S., Cheng, F. C., et al. (2016). Allogeneic/xenogeneic transplantation of peptide-labeled mitochondria in Parkinson’s disease: restoration of mitochondria functions and attenuation of 6-hydroxydopamine-induced neurotoxicity. Transl. Res. 170:e3.
Chen, J. Y., Wang, Q., Feng, X. B., Zhang, Z. Y., Geng, L. Y., Xu, T., et al. (2016). Umbilical cord-derived mesenchymal stem cells suppress autophagy of T cells in patients with systemic lupus erythematosus via transfer of mitochondria. Stem Cells Int. 2016:4062789.
Cho, Y. M., Kim, J. H., Kim, M., Park, S. J., Koh, S. H., Ahn, H. S., et al. (2012). Mesenchymal stem cells transfer mitochondria to the cells with virtually no mitochondrial function but not with pathogenic mtDNA mutations. PLoS One 7:e32778. doi: 10.1371/journal.pone.0032778
Chuang, Y. C., Liou, C. W., Chen, S. D., Wang, P. W., Chuang, J. H., Tiao, M. M., et al. (2017). Mitochondrial transfer from wharton’s jelly mesenchymal stem cell to MERRF cybrid reduces oxidative stress and improves mitochondrial bioenergetics. Oxid. Med. Cell Longev. 2017:5691215.
Court, A. C., Le-Gatt, A., Luz-Crawford, P., Parra, E., Aliaga-Tobar, V., Batiz, L. F., et al. (2020). Mitochondrial transfer from MSCs to T cells induces Treg differentiation and restricts inflammatory response. EMBO Rep. 21:e48052.
Court, F. A., Hendriks, W. T., MacGillavry, H. D., Alvarez, J., and van Minnen, J. (2008). Schwann cell to axon transfer of ribosomes: toward a novel understanding of the role of glia in the nervous system. J. Neurosci. 28, 11024–11029. doi: 10.1523/jneurosci.2429-08.2008
Cselenyak, A., Pankotai, E., Horvath, E. M., Kiss, L., and Lacza, Z. (2010). Mesenchymal stem cells rescue cardiomyoblasts from cell death in an in vitro ischemia model via direct cell-to-cell connections. BMC Cell Biol. 11:29. doi: 10.1186/1471-2121-11-29
Cusulin, C., Monni, E., Ahlenius, H., Wood, J., Brune, J. C., Lindvall, O., et al. (2012). Embryonic stem cell-derived neural stem cells fuse with microglia and mature neurons. Stem Cells 30, 2657–2671. doi: 10.1002/stem.1227
Djafarzadeh, S., and Jakob, S. M. (2017). Isolation of intact mitochondria from skeletal muscle by differential centrifugation for high-resolution respirometry measurements. J. Vis. Exp. 121:55251. doi: 10.3791/55251
Emani, S. M., Piekarski, B. L., Harrild, D., Del Nido, P. J., and McCully, J. D. (2017). Autologous mitochondrial transplantation for dysfunction after ischemia-reperfusion injury. J. Thorac. Cardiovasc. Surg. 154, 286–289. doi: 10.1016/j.jtcvs.2017.02.018
Falchi, A. M., Sogos, V., Saba, F., Piras, M., Congiu, T., and Piludu, M. (2013). Astrocytes shed large membrane vesicles that contain mitochondria, lipid droplets and ATP. Histochem. Cell Biol. 139, 221–231. doi: 10.1007/s00418-012-1045-x
Feng, Y., Zhu, R., Shen, J., Wu, J., Lu, W., et al. (2019). Human bone marrow mesenchymal stem cells rescue endothelial cells experiencing chemotherapy stress by mitochondrial transfer via tunneling nanotubes. Stem Cells Dev. 28, 674–682. doi: 10.1089/scd.2018.0248
Gao, L., Zhang, Z., Lu, J., and Pei, G. (2019). Mitochondria are dynamically transferring between human neural cells and alexander disease-associated GFAP mutations impair the astrocytic transfer. Front. Cell Neurosci. 13:316. doi: 10.3389/fncel.2019.00316
Gomzikova, M. O., James, V., and Rizvanov, A. A. (2019). Therapeutic application of mesenchymal stem cells derived extracellular vesicles for immunomodulation. Front. Immunol. 10:2663. doi: 10.3389/fimmu.2019.02663
Gomzikova, M. O., and Rizvanov, A. A. (2017). Current trends in regenerative medicine: from cell to cell-free therapy. Bionanoscience 7, 240–245. doi: 10.1007/s12668-016-0348-0
Gu, D., Zou, X. Y., Ju, G. Q., Zhang, G. Y., Bao, E. D., and Zhu, Y. J. (2016). Mesenchymal stromal cells derived extracellular vesicles ameliorate acute renal ischemia reperfusion injury by inhibition of mitochondrial fission through miR-30. Stem Cells Int. 2016:2093940.
Halter, J. P., Schupbach, W. M. M., Mandel, H., Casali, C., Orchard, K., Collin, M., et al. (2015). Allogeneic haematopoietic stem cell transplantation for mitochondrial neurogastrointestinal encephalomyopathy. Brain 138, 2847–2858.
Han, H., Hu, J. Q., Yan, Q., Zhu, J. Z., Zhu, Z. B., Chen, Y. J., et al. (2016). Bone marrow-derived mesenchymal stem cells rescue injured H9c2 cells via transferring intact mitochondria through tunneling nanotubes in an in vitro simulated ischemia/reperfusion model. Mol. Med. Rep. 13, 1517–1524. doi: 10.3892/mmr.2015.4726
Hayakawa, K., Esposito, E., Wang, X., Terasaki, Y., Liu, Y., Xing, C., et al. (2016). Transfer of mitochondria from astrocytes to neurons after stroke. Nature 535, 551–555. doi: 10.1038/nature18928
Hill, J. H., Chen, Z., and Xu, H. (2014). Selective propagation of functional mitochondrial DNA during oogenesis restricts the transmission of a deleterious mitochondrial variant. Nat. Genet. 46, 389–392. doi: 10.1038/ng.2920
Hough, K. P., Trevor, J. L., Strenkowski, J. G., Wang, Y., Chacko, B. K., Tousif, S., et al. (2018). Exosomal transfer of mitochondria from airway myeloid-derived regulatory cells to T cells. Redox Biol. 18, 54–64. doi: 10.1016/j.redox.2018.06.009
Huang, P. J., Kuo, C. C., Lee, H. C., Shen, C. I., Cheng, F. C., Wu, S. F., et al. (2016). Transferring xenogenic mitochondria provides neural protection against ischemic stress in ischemic rat brains. Cell Transpl. 25, 913–927. doi: 10.3727/096368915x689785
Hubbard, W. B., Harwood, C. L., Prajapati, P., Springer, J. E., Saatman, K. E., and Sullivan, P. G. (2019). Fractionated mitochondrial magnetic separation for isolation of synaptic mitochondria from brain tissue. Sci. Rep. 9:9656.
Ippolito, L., Morandi, A., Taddei, M. L., Parri, M., Comito, G., Iscaro, A., et al. (2019). Cancer-associated fibroblasts promote prostate cancer malignancy via metabolic rewiring and mitochondrial transfer. Oncogene 38, 5339–5355. doi: 10.1038/s41388-019-0805-7
Ishikawa, K., Toyama-Sorimachi, N., Nakada, K., Morimoto, M., Imanishi, H., Yoshizaki, M., et al. (2010). The innate immune system in host mice targets cells with allogenic mitochondrial DNA. J. Exp. Med. 207, 2297–2305. doi: 10.1084/jem.20092296
Islam, M. N., Das, S. R., Emin, M. T., Wei, M., Sun, L., Westphalen, K., et al. (2012). Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat. Med. 18, 759–765. doi: 10.1038/nm.2736
Jackson, M. V., and Krasnodembskaya, A. D. (2017). Analysis of mitochondrial transfer in direct co-cultures of human Monocyte-derived Macrophages (MDM) and Mesenchymal Stem Cells (MSC). Bio-Protocol 7: e2255.
Jackson, M. V., Morrison, T. J., Doherty, D. F., McAuley, D. F., Matthay, M. A., Kissenpfennig, A., et al. (2016). Mitochondrial transfer via tunneling nanotubes is an important mechanism by which mesenchymal stem cells enhance macrophage phagocytosis in the in vitro and in vivo models of ARDS. Stem cells 34, 2210–2223. doi: 10.1002/stem.2372
Jash, E., Prasad, P., Kumar, N., Sharma, T., Goldman, A., and Sehrawat, S. (2018). Perspective on nanochannels as cellular mediators in different disease conditions. Cell Commun. Signal. : CCS 16, 76.
Jiang, D., Chen, F. X., Zhou, H., Lu, Y. Y., Tan, H., Yu, S. J., et al. (2020). Bioenergetic crosstalk between mesenchymal stem cells and various ocular cells through the intercellular trafficking of mitochondria. Theranostics 10, 7260–7272. doi: 10.7150/thno.46332
Jiang, D., Gao, F., Zhang, Y., Wong, D. S., Li, Q., Tse, H. F., et al. (2016). Mitochondrial transfer of mesenchymal stem cells effectively protects corneal epithelial cells from mitochondrial damage. Cell Death Dis. 7:e2467. doi: 10.1038/cddis.2016.358
Kheirandish-Rostami, M., Roudkenar, M. H., Jahanian-Najafabadi, A., Tomita, K., Kuwahara, Y., Sato, T., et al. (2020). Mitochondrial characteristics contribute to proliferation and migration potency of MDA-MB-231 cancer cells and their response to cisplatin treatment. Life Sci. 244:117339. doi: 10.1016/j.lfs.2020.117339
Kim, M. J., Hwang, J. W., Yun, C. K., Lee, Y., and Choi, Y. S. (2018). Delivery of exogenous mitochondria via centrifugation enhances cellular metabolic function. Sci. Rep. 8:3330.
Konari, N., Nagaishi, K., Kikuchi, S., and Fujimiya, M. (2019). Mitochondria transfer from mesenchymal stem cells structurally and functionally repairs renal proximal tubular epithelial cells in diabetic nephropathy in vivo. Sci. Rep. 9:5184.
Koyanagi, M., Brandes, R. P., Haendeler, J., Zeiher, A. M., and Dimmeler, S. (2005). Cell-to-cell connection of endothelial progenitor cells with cardiac myocytes by nanotubes: a novel mechanism for cell fate changes? Circul. Res. 96, 1039–1041. doi: 10.1161/01.res.0000168650.23479.0c
Kristian, T. (2010). Isolation of mitochondria from the CNS. Curr. Protoc. Neurosci. Chapter 7:Unit 7.22.
Kubat, G. B., Ozler, M., Ulger, O., Ekinci, O., Atalay, O., Celik, E., et al. (2020). The effects of mesenchymal stem cell mitochondrial transplantation on doxorubicin-mediated nephrotoxicity in rats. J. Biochem. Mol. Toxicol. 35:e22612.
Li, C. J., Chen, P. K., Sun, L. Y., and Pang, C. Y. (2017). Enhancement of mitochondrial transfer by antioxidants in human mesenchymal stem cells. Oxid Med. Cell Longev. 2017:8510805.
Li, H., Wang, C., He, T., Zhao, T., Chen, Y. Y., Shen, Y. L., et al. (2019). Mitochondrial transfer from bone marrow mesenchymal stem cells to motor neurons in spinal cord injury rats via gap junction. Theranostics 9, 2017–2035. doi: 10.7150/thno.29400
Li, X., Zhang, Y. L., Yeung, S. C., Liang, Y. M., Liang, X. T., Ding, Y., et al. (2014). Mitochondrial transfer of induced pluripotent stem cell-derived mesenchymal stem cells to airway epithelial cells attenuates cigarette smoke-induced damage. Am. J. Resp. Cell Mol. 51, 455–465. doi: 10.1165/rcmb.2013-0529oc
Lin, H. Y., Liou, C. W., Chen, S. D., Hsu, T. Y., Chuang, J. H., Wang, P. W., et al. (2015). Mitochondrial transfer from Wharton’s jelly-derived mesenchymal stem cells to mitochondria-defective cells recaptures impaired mitochondrial function. Mitochondrion 22, 31–44. doi: 10.1016/j.mito.2015.02.006
Liu, K., Guo, L., Zhou, Z., Pan, M., and Yan, C. (2019). Mesenchymal stem cells transfer mitochondria into cerebral microvasculature and promote recovery from ischemic stroke. Microvasc. Res. 123, 74–80. doi: 10.1016/j.mvr.2019.01.001
Liu, K. M., Ji, K. Q., Guo, L., Wu, W., Lu, H. X., Shan, P. Y., et al. (2014). Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia-reperfusion model via tunneling nanotube like structure-mediated mitochondrial transfer. Microvasc. Res. 92, 10–18. doi: 10.1016/j.mvr.2014.01.008
Luz-Crawford, P., Hernandez, J., Djouad, F., Luque-Campos, N., Caicedo, A., Carrere-Kremer, S., et al. (2019). Mesenchymal stem cell repression of Th17 cells is triggered by mitochondrial transfer. Stem Cell Res. Ther. 10:232.
Macheiner, T., Fengler, V. H., Agreiter, M., Eisenberg, T., Madeo, F., Kolb, D., et al. (2016). Magnetomitotransfer: an efficient way for direct mitochondria transfer into cultured human cells. Sci. Rep. 6:35571.
Mahrouf-Yorgov, M., Augeul, L., Da Silva, C. C., Jourdan, M., Rigolet, M., Manin, S., et al. (2017). Mesenchymal stem cells sense mitochondria released from damaged cells as danger signals to activate their rescue properties. Cell Death Differ. 24, 1224–1238. doi: 10.1038/cdd.2017.51
Marlein, C. R., Zaitseva, L., Piddock, R. E., Robinson, S. D., Edwards, D. R., Shafat, M. S., et al. (2017). NADPH oxidase-2 derived superoxide drives mitochondrial transfer from bone marrow stromal cells to leukemic blasts. Blood 130, 1649–1660. doi: 10.1182/blood-2017-03-772939
Masuzawa, A., Black, K. M., Pacak, C. A., Ericsson, M., Barnett, R. J., Drumm, C., et al. (2013). Transplantation of autologously derived mitochondria protects the heart from ischemia-reperfusion injury. Am. J. Physiol-Heart C 304, H966–H982.
Melcher, M., Danhauser, K., Seibt, A., Degistirici, O., Baertling, F., Kondadi, A. K., et al. (2017). Modulation of oxidative phosphorylation and redox homeostasis in mitochondrial NDUFS4 deficiency via mesenchymal stem cells. Stem Cell Res. Ther. 8:150.
Morrison, T. J., Jackson, M. V., Cunningham, E. K., Kissenpfennig, A., McAuley, D. F., O’Kane, C. M., et al. (2017). Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am. J. Respir. Crit. Care Med. 196, 1275–1286. doi: 10.1164/rccm.201701-0170oc
Moschoi, R., Imbert, V., Nebout, M., Chiche, J., Mary, D., Prebet, T., et al. (2016). Protective mitochondrial transfer from bone marrow stromal cells to acute myeloid leukemic cells during chemotherapy. Blood 128, 253–264. doi: 10.1182/blood-2015-07-655860
Murray, L. M. A., and Krasnodembskaya, A. D. (2019). Concise review: intercellular communication via organelle transfer in the biology and therapeutic applications of stem cells. Stem Cells 37, 14–25. doi: 10.1002/stem.2922
Noubissi, F. K., Harkness, T., Alexander, C. M., and Ogle, B. M. (2015). Apoptosis-induced cancer cell fusion: a mechanism of breast cancer metastasis. FASEB J. 29, 4036–4045. doi: 10.1096/fj.15-271098
Onfelt, B., Nedvetzki, S., Yanagi, K., and Davis, D. M. (2004). Cutting edge: membrane nanotubes connect immune cells. J. Immunol. 173, 1511–1513. doi: 10.4049/jimmunol.173.3.1511
Paliwal, S., Chaudhuri, R., Agrawal, A., and Mohanty, S. (2018). Regenerative abilities of mesenchymal stem cells through mitochondrial transfer. J. Biomed. Sci. 25:31.
Pankotai, E., Cselenyak, A., Ratosi, O., Lorincz, J., Kiss, L., and Lacza, Z. (2012). The role of mitochondria in direct cell-to-cell connection dependent rescue of postischemic cardiomyoblasts. Mitochondrion 12, 352–356. doi: 10.1016/j.mito.2011.09.008
Pasquier, J., Guerrouahen, B. S., Al Thawadi, H., Ghiabi, P., Maleki, M., Abu-Kaoud, N., et al. (2013). Preferential transfer of mitochondria from endothelial to cancer cells through tunneling nanotubes modulates chemoresistance. J. Transl. Med. 11:94. doi: 10.1186/1479-5876-11-94
Peruzzotti-Jametti, L., Bernstock, J. D., Manferrari, G., Rogall, R., Fernandez-Vizarra, E., Williamson, J. C., et al. (2020). Neural stem cells traffic functional mitochondria via extracellular vesicles to correct mitochondrial dysfunction in target cells. bioRxiv [preprint]. doi: 10.1101/2020.01.29.923441 preprint
Phinney, D. G., Di Giuseppe, M., Njah, J., Sala, E., Shiva, S., and Croix, C.M. St, et al. (2015). Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat. Commun. 6:8472.
Plotnikov, E. Y., Khryapenkova, T. G., Galkina, S. I., Sukhikh, G. T., and Zorov, D. B. (2010). Cytoplasm and organelle transfer between mesenchymal multipotent stromal cells and renal tubular cells in co-culture. Exp. Cell Res. 316, 2447–2455. doi: 10.1016/j.yexcr.2010.06.009
Plotnikov, E. Y., Khryapenkova, T. G., Vasileva, A. K., Marey, M. V., Galkina, S. I., Isaev, N. K., et al. (2008). Cell-to-cell cross-talk between mesenchymal stem cells and cardiomyocytes in co-culture. J. Cell Mol. Med. 12, 1622–1631. doi: 10.1111/j.1582-4934.2007.00205.x
Robicsek, O., Ene, H. M., Karry, R., Ytzhaki, O., Asor, E., McPhie, D., et al. (2018). Isolated mitochondria transfer improves neuronal differentiation of schizophrenia-derived induced pluripotent stem cells and rescues deficits in a rat model of the disorder. Schizophr Bull. 44, 432–442. doi: 10.1093/schbul/sbx077
Rossi, A., Pizzo, P., and Filadi, R. (2019). Calcium, mitochondria and cell metabolism: a functional triangle in bioenergetics. Biochim Biophys. Acta Mol. Cell Res. 1866, 1068–1078. doi: 10.1016/j.bbamcr.2018.10.016
Rustom, A. (2016). The missing link: does tunnelling nanotube-based supercellularity provide a new understanding of chronic and lifestyle diseases? Open Biol. 6:160057. doi: 10.1098/rsob.160057
Shi, X. X., Zhao, M., Fu, C., and Fu, A. L. (2017). Intravenous administration of mitochondria for treating experimental Parkinson’s disease. Mitochondrion 34, 91–100. doi: 10.1016/j.mito.2017.02.005
Sinclair, K. A., Yerkovich, S. T., Hopkins, P. M., and Chambers, D. C. (2016). Characterization of intercellular communication and mitochondrial donation by mesenchymal stromal cells derived from the human lung. Stem Cell Res. Therapy 7:91.
Spees, J. L., Olson, S. D., Whitney, M. J., and Prockop, D. J. (2006). Mitochondrial transfer between cells can rescue aerobic respiration. Proc. Natl. Acad. Sci. U S A. 103, 1283–1288. doi: 10.1073/pnas.0510511103
Sun, C., Liu, X., Wang, B., Wang, Z., Liu, Y., Di, C., et al. (2019). Endocytosis-mediated mitochondrial transplantation: transferring normal human astrocytic mitochondria into glioma cells rescues aerobic respiration and enhances radiosensitivity. Theranostics 9, 3595–3607. doi: 10.7150/thno.33100
Terada, N., Hamazaki, T., Oka, M., Hoki, M., Mastalerz, D. M., Nakano, Y., et al. (2002). Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature 416, 542–545. doi: 10.1038/nature730
Vignais, M. L., Caicedo, A., Brondello, J. M., and Jorgensen, C. (2017). Cell connections by tunneling nanotubes: effects of mitochondrial trafficking on target cell metabolism, homeostasis, and response to therapy. Stem Cells Int. 2017:6917941.
Wada, K. I., Hosokawa, K., Ito, Y., and Maeda, M. (2017). Quantitative control of mitochondria transfer between live single cells using a microfluidic device. Biol. Open 6, 1960–1965. doi: 10.1242/bio.024869
Wang, J. Y., Li, H. Y. Z., Yao, Y., Zhao, T. F., Chen, Y. Y., Shen, Y. L., et al. (2018). Stem cell-derived mitochondria transplantation: a novel strategy and the challenges for the treatment of tissue injury. Stem Cell Res. Ther. 9:106.
Wang, X., and Gerdes, H. H. (2015). Transfer of mitochondria via tunneling nanotubes rescues apoptotic PC12 cells. Cell Death Differ. 22, 1181–1191. doi: 10.1038/cdd.2014.211
Weimann, J. M., Johansson, C. B., Trejo, A., and Blau, H. M. (2003). Stable reprogrammed heterokaryons form spontaneously in Purkinje neurons after bone marrow transplant. Nat. Cell Biol. 5, 959–966. doi: 10.1038/ncb1053
Wu, S., Zhang, A., Li, S., Chatterjee, S., Qi, R., Segura-Ibarra, V., et al. (2018). Polymer functionalization of isolated mitochondria for cellular transplantation and metabolic phenotype alteration. Adv. Sci. (Weinh) 5:1700530. doi: 10.1002/advs.201700530
Yang, Y., Ye, G., Zhang, Y. L., He, H. W., Yu, B. Q., Hong, Y. M., et al. (2020). Transfer of mitochondria from mesenchymal stem cells derived from induced pluripotent stem cells attenuates hypoxia-ischemia-induced mitochondrial dysfunction in PC12 cells. Neural Regen. Res. 15, 464–472. doi: 10.4103/1673-5374.266058
Yao, Y., Fan, X. L., Jiang, D., Zhang, Y., Li, X., Xu, Z. B., et al. (2018). Connexin 43-Mediated mitochondrial transfer of iPSC-MSCs alleviates asthma inflammation. Stem Cell Rep. 11, 1120–1135. doi: 10.1016/j.stemcr.2018.09.012
Yasuda, K., Khandare, A., Burianovskyy, L., Maruyama, S., Zhang, F., Nasjletti, A., et al. (2011). Tunneling nanotubes mediate rescue of prematurely senescent endothelial cells by endothelial progenitors: exchange of lysosomal pool. Aging 3, 597–608.
Yu, B., Zhang, X., and Li, X. (2014). Exosomes derived from mesenchymal stem cells. Int. J. Mol. Sci. 15, 4142–4157.
Zhang, H., Menzies, K. J., and Auwerx, J. (2018). The role of mitochondria in stem cell fate and aging. Development 145:dev143420.
Zhang, Q., Raoof, M., Chen, Y., Sumi, Y., Sursal, T., Junger, W., et al. (2010). Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464, 104–107.
Zhang, X., Hubal, M. J., and Kraus, V. B. (2020). Immune cell extracellular vesicles and their mitochondrial content decline with ageing. Immun. Ageing 17:1.
Zhang, Y. L., Yu, Z. D., Jiang, D., Liang, X. T., Liao, S. Y., Zhang, Z., et al. (2016). iPSC-MSCs with high intrinsic MIRO1 and sensitivity to TNF-alpha yield efficacious mitochondrial transfer to rescue anthracycline-induced cardiomyopathy. Stem Cell Rep. 7, 749–763.
Zhu, L., Zhang, J., Zhou, J., Lu, Y., Huang, S., Xiao, R., et al. (2016). Mitochondrial transplantation attenuates hypoxic pulmonary hypertension. Oncotarget 7, 48925–48940.
Keywords: mitochondria donation, mitochondria transplantation, tunneling nanotubes, extracellular vesicles, cell fusion, isolated mitochondria
Citation: Gomzikova MO, James V and Rizvanov AA (2021) Mitochondria Donation by Mesenchymal Stem Cells: Current Understanding and Mitochondria Transplantation Strategies. Front. Cell Dev. Biol. 9:653322. doi: 10.3389/fcell.2021.653322
Received: 15 January 2021; Accepted: 09 March 2021;
Published: 07 April 2021.
Edited by:
Jochen H. M. Prehn, Royal College of Surgeons in Ireland, IrelandReviewed by:
Yuan Wei, Sun Yat-sen University, ChinaCopyright © 2021 Gomzikova, James and Rizvanov. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marina O. Gomzikova, TU9Hb216aWtvdmFAa3BmdS5ydQ==; bWFyaW5hLmdvbXppa292YS5nbW9AZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.