- Bone Biology and Disease Laboratory, School of Biomedical Sciences, The University of Western Australia, Nedlands, WA, Australia
During bone resorption, the osteoclast must sustain an extraordinarily low pH environment, withstand immense ionic pressures, and coordinate nutrient and waste exchange across its membrane to sustain its unique structural and functional polarity. To achieve this, osteoclasts are equipped with an elaborate set of membrane transport proteins (pumps, transporters and channels) that serve as molecular ‘gatekeepers’ to regulate the bilateral exchange of ions, amino acids, metabolites and macromolecules across the ruffled border and basolateral domains. Whereas the importance of the vacuolar-ATPase proton pump and chloride voltage-gated channel 7 in osteoclasts has long been established, comparatively little is known about the contributions of other membrane transport proteins, including those categorized as secondary active transporters. In this Special Issue review, we provide a contemporary update on the ‘ins and outs’ of membrane transport proteins implicated in osteoclast differentiation, function and bone homeostasis and discuss their therapeutic potential for the treatment of metabolic bone diseases.
Introduction
Osteoclasts (OCs) are large bone-digesting (resorbing) cells that play a central role in the regulation of skeletal bone mass and bone pathologies such as osteoporosis. These multinucleated giants arise from the fusion of mononuclear progenitor cells of the monocyte/macrophage lineage in response to macrophage-colony stimulating factor (M-CSF) and receptor activator of nuclear factor-κB ligand (RANKL) (for review see Teitelbaum and Ross (2003)). Upon contact with mineralized bone, OCs adopt a polarized anatomy that denotes their active bone-resorbing status (Figure 1). First, the bone-facing ‘apical’ membrane is hermetically sealed to the bone surface via podosomes; a dense network of actin filaments connected to transmembrane adhesion proteins (e.g., β3-integrin). The membrane entrapped within this ‘sealing zone’, in turn, assumes a highly convoluted morphology termed the ruffled border. The ruffled border serves as the OC bone-resorbing apparatus and is formed upon the rapid fusion of secretory lysosomes with the bone-apposed plasmalemma (reviewed in Ng et al. (2019)). Thus, the composition of the ruffled border shares close analogy with endolysosomal membranes (Weivoda and Oursler, 2014; Roy and Roux, 2018) and together with the underlying resorptive space (hemivacuole or resorptive pit) is viewed akin to a giant digestive ‘extracellular lysosome’. Reflecting this, the resorptive hemivacuole is highly acidified (pH ∼4.5). Acidification is driven by the efflux of protons (H+) and chloride ions (Cl–) across the OC ruffled border membrane. This process is requisite to dissolve the inorganic phase of bone (hydroxyapatite) and the activation of acidic hydrolysases (chiefly cathepsin K) that digest the underlying organic collagen (Type 1a) matrix.
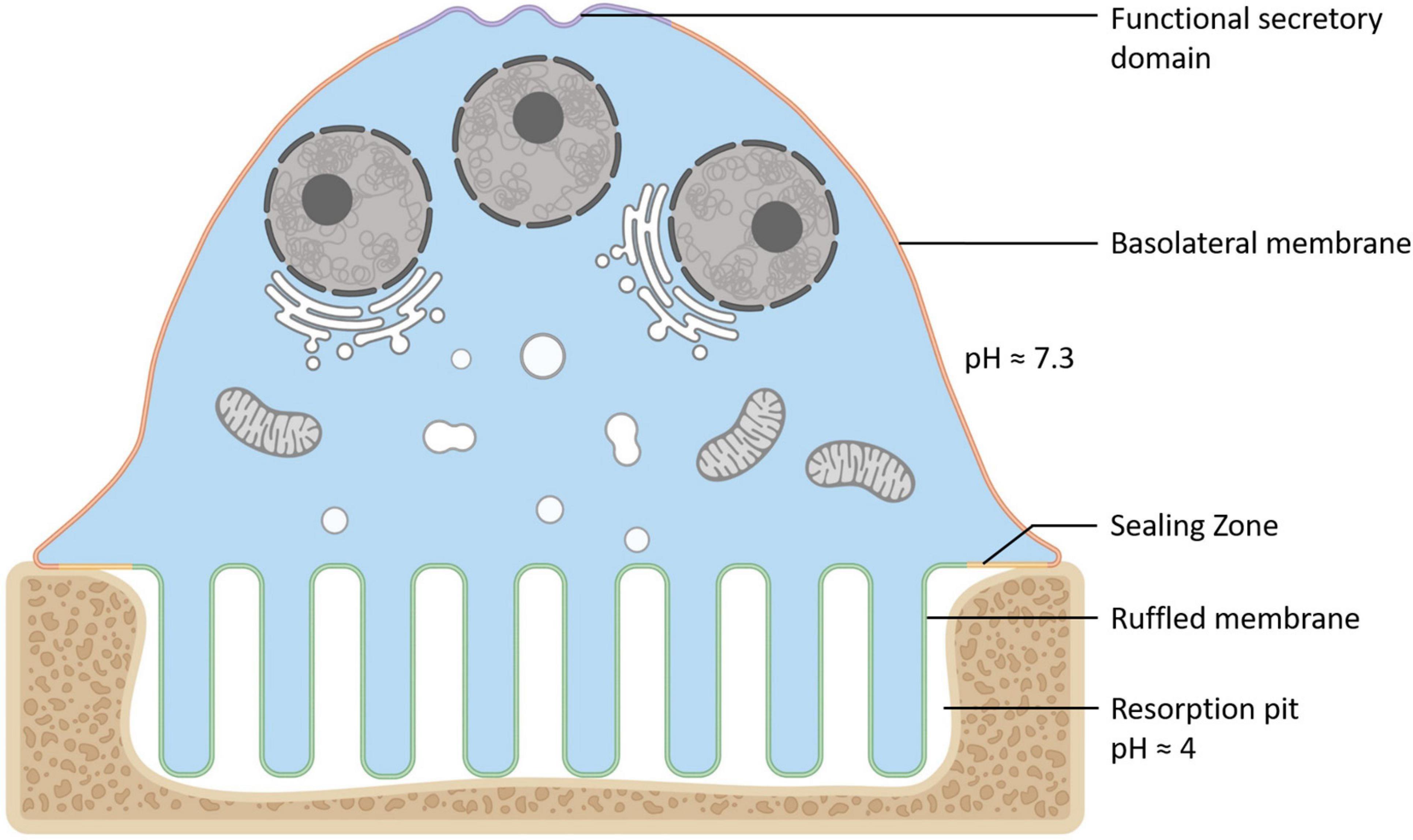
Figure 1. Anatomy of the Osteoclast. Illustration of the unique configuration and membrane organization of an osteoclast during active bone resorption. The osteoclast’s specialized plasma membrane domains are labeled and color-coded: Purple = the functional secretory domain, Orange = the basolateral membrane, Yellow = the sealing zone and Green = the ruffled border membrane. Created with BioRender.com.
Being semipermeable, the ruffled border permits passive diffusion of small non-charged molecules (e.g., oxygen, carbon dioxide and water) across its membrane in-folds but restricts the influx of charged ions such as H+ and Cl–, leading to increased membrane potential and ionic pressure within the resorptive space. In addition, the dissolution of hydroxyapatite crystals (Ca10(PO4)6(OH)2) back into its elemental forms further exposes the ruffled border membrane to high ambient concentrations of calcium ions (Ca2+) and inorganic phosphate (Pi). In order to sustain OC cell volume and polarity, these resorption by-products must be removed and transported across the OC ruffled border and basolateral membranes. Some of these materials (e.g., degraded collagen) are internalized by bulk endocytosis at the ruffled border and transported apico-basolaterally via transcytotic carriers before being expelled at the functional secretory domain (FSD) (Nesbitt and Horton, 1997; Salo et al., 1997). By comparison, other by-products, such as ions and metabolites, are translocated by sets of structurally and functionally diverse membrane transport proteins, enriched on the opposing ruffled border and basolateral membranes (Figure 2). In their simplest form, membrane transport proteins are considered ‘gatekeepers’ of molecular exchanges across biological membranes and are operationally categorized according to their mode, direction and molecule of transport: i.e., (i) channels, (ii) gated channels, (iii) primary active transporters and (iv) secondary active transporters (uniporters, symporters and antiporters, inclusive) (Figure 2A). These integral transmembrane proteins can be further subdivided into four main superfamilies: (i) the ATP-binding cassette (ABC) transporters, (ii) ATPases, (iii) ion channels, and (iv) solute carrier proteins (SLCs) (reviewed extensively in (Pizzagalli et al., 2020)).
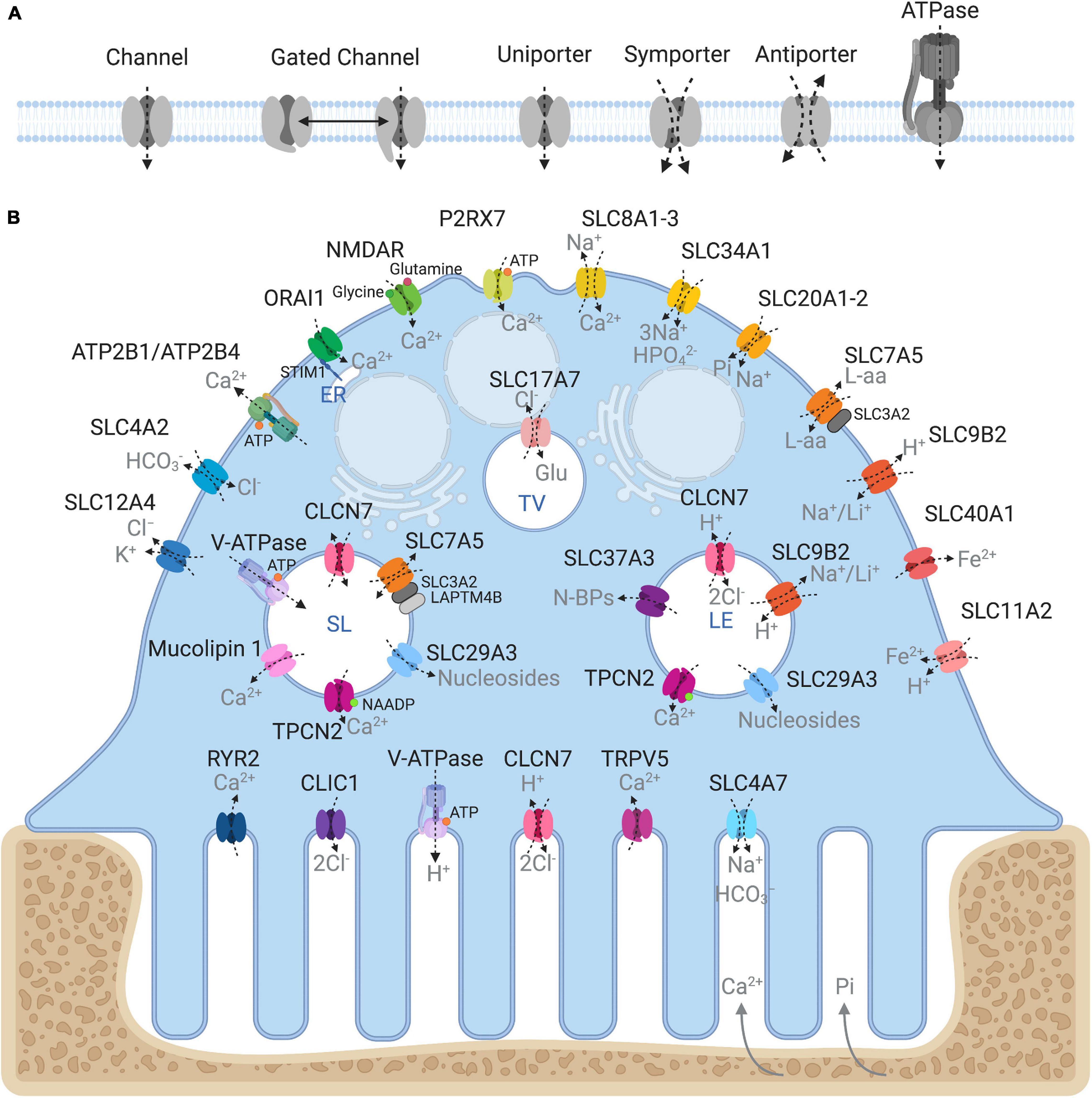
Figure 2. Ins and outs of membrane transport proteins in osteoclasts. (A) Schema depicting the major types of transport proteins and their modes of substrate movement across biological membranes. (B) Model summarizing the reported membrane localization of all the major membrane transport proteins expressed in osteoclasts. Known substrates are indicated in gray. Intracellular compartments correspond to: ER, endoplasmic reticulum; SL, Secretory lysosome; LE, Late endosome; TV, Transcytotic vesicle. Created with BioRender.com.
Collectively, membrane transport proteins not only facilitate complex and rich exchanges at the interface between the OC ruffled border or the basolateral surface with the extracellular milieu but also between membranes of intracellular organelles (such as lysosomes, the endoplasmic reticulum and mitochondria) and the cytosol (Figure 2B). In this way, they ensure that every functional unit of the OC receives its necessary complement of ions, metabolites and nutrients required for cellular homeostasis. In a giant energetic cell so heavily subjected to large ionic fluxes and membrane turnover, it is therefore unsurprising that an increasing number of mutations in membrane transport proteins have been implicated in the pathogenesis of bone diseases, in particular bone sclerosing (high bone mass) diseases associated with OC dysfunction (Table 1). For example, most of the known forms of OC-rich autosomal recessive osteopetrosis (ARO) have been linked to mutations in subunits of the multimolecular vacuolar-ATPase (V-ATPase) proton pump and chloride voltage-gated channel 7 (CLCN7), both indispensable components of the OC acidification machinery as described herein.
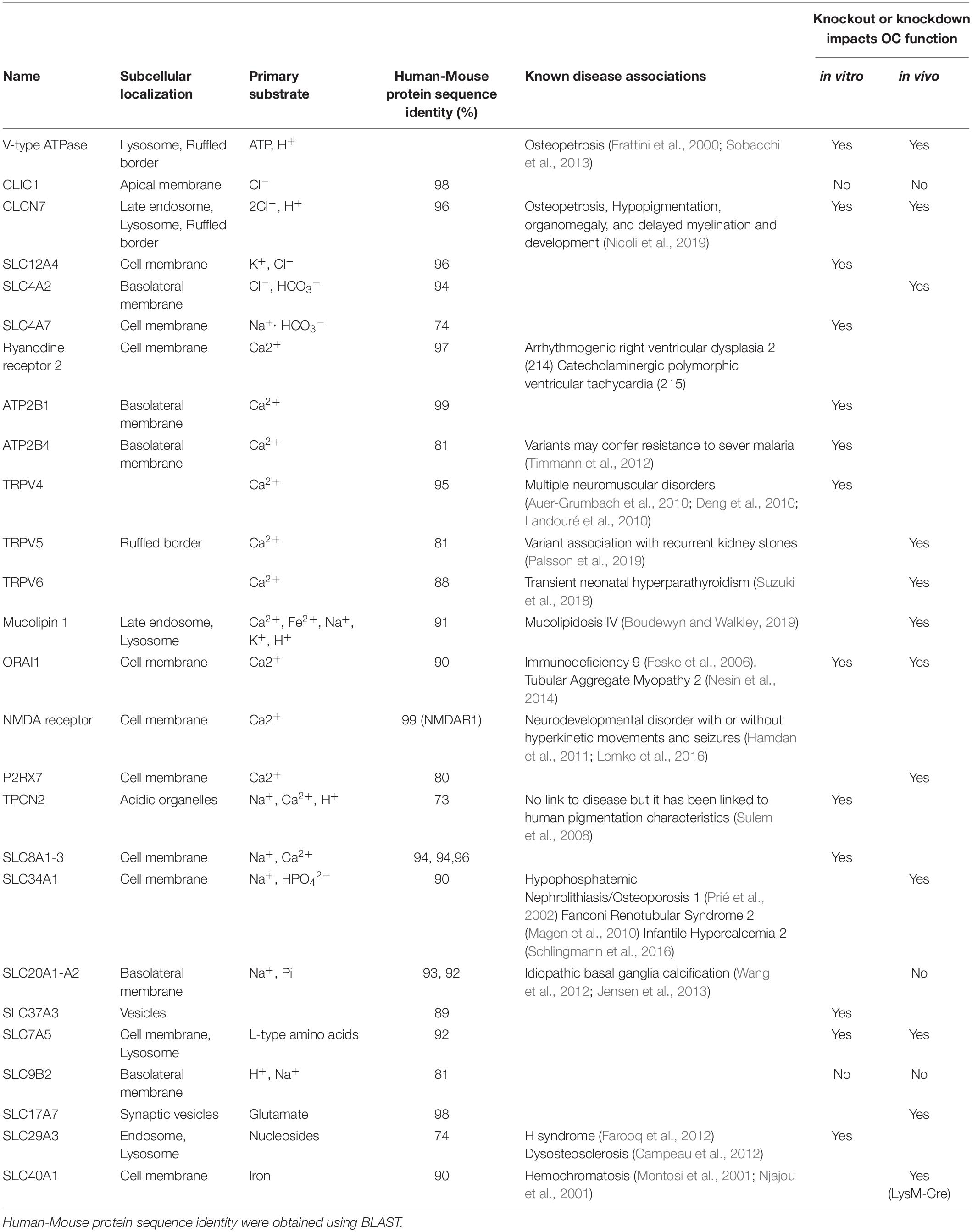
Table 1. Summary of known osteoclast membrane transport proteins, their localizations, substrates, associated disease phenotypes and impacts on osteoclasts.
In this Special Issue, we briefly review the ‘ins and outs’ of membrane transport proteins in OCs. We highlight the contributions of established OC membrane transporters and channels (e.g., V-ATPases and CLCN7) as well as shed light on lesser-known membrane transport proteins whose physiological contributions in OCs and bone health have only just begun to emerge. We describe the structure, localization and function of these membrane transport proteins in OCs and bone as well as discuss their therapeutic utility as molecular targets for the treatment of metabolic bone diseases such as osteoporosis.
Proton Transport in Osteoclasts: The V-ATPase Complex
The transport of protons (H+) across biological membranes is one of the most important physiological functions of cellular homeostasis, governing the regulation of intracellular pH, facilitating the generation of pH gradients in localized regions of cells, and maintaining the pH of the extracellular fluid. Aberrant regulation of pH in tissues and in cells leads to conditions such as acidosis and has been increasingly linked to the onset and progression of cancer (Parks et al., 2013; Aoi and Marunaka, 2014).
To maintain intracellular pH homeostasis, cells have evolved sophisticated proton extrusion mechanisms and complementary buffering systems (e.g., CA2 and SLC4A2 reviewed below). Of these, the vacuolar H+-ATPase (V-ATPase) is among the most conspicuous features of eukaryotic endocytic and secretory organelle membranes and is arguably the keystone transporter for OC acidification and bone resorptive function. This large macromolecular complex is powered by the hydrolysis of ATP to extrude hydrogen ions (H+) across biological membranes. In OCs, the V-ATPase complex is enriched on the OC ruffled border membrane and decorates the surface of secretory lysosomes (Baron et al., 1985). Here, it facilitates the transport of protons necessary for acidification of the underlying resorptive space and activation of acidic hydrolysases (e.g., cathepsin K). Structurally, the V-ATPase proton pump is composed of 14 subunits and is the sum of two domains: (i) the ATP-hydrolytic domain (V1) and (ii) the proton-translocation domain (V0) (Figure 3). The V1 domain is composed of eight subunits (A to H) and is anchored indirectly to membranes through its interaction with the V0 domain. The V0 domain contains six subunits named a, d, e, c, c′, and c″, that assemble into a complex embedded into the membrane (Figure 3; Qin et al., 2012b; Duan et al., 2018). Following assembly, hydrolysis of ATP by the V1 domain generates the energy required to initiate V0 domain rotation. It is this active rotational movement that drives proton-translocation across the membrane (Forgac, 2007; Jefferies et al., 2008). For an extensive review see (Qin et al., 2012b).
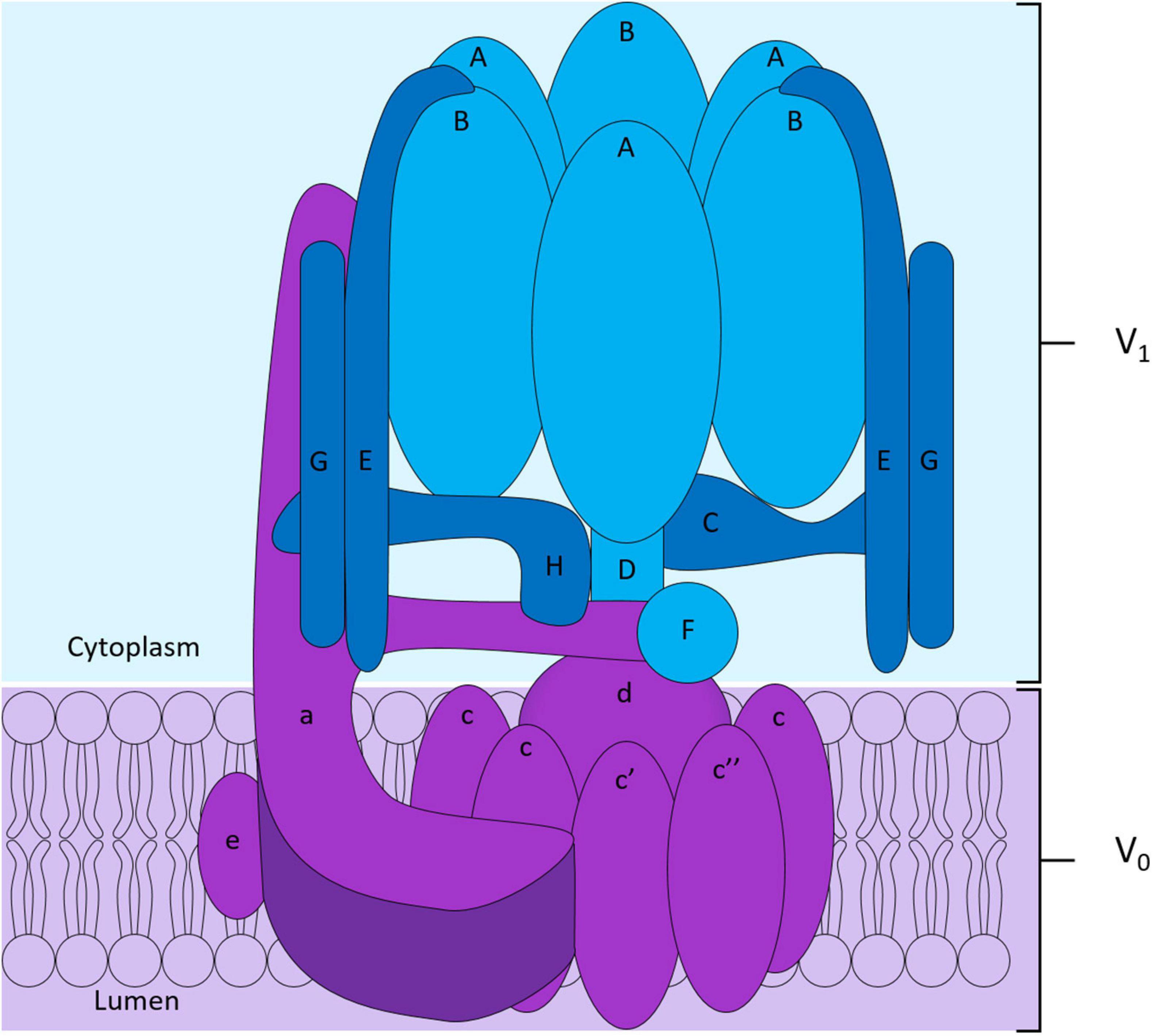
Figure 3. Structure of the V-ATPase proton pump. The V-ATPase complex is composed of two domains: the peri-membranous V1 domain composed of subunits A to H responsible for the hydrolysis of ATP shown in blue, and the intramembranous V0 domain who allows the translocation of protons across the membrane shown in purple in the diagram. The V0 domain is composed of the subunits a, e, d and of a hexameric ring formed by subunits c, c′ and c″.
The V-ATPase complex is obligatory for OC function and bone homeostasis. Accordingly, the gene expression levels of V-ATPase subunits are robustly amplified during RANKL-induced OC differentiation (Toyomura et al., 2000; Manolson et al., 2003). Transcriptionally, this is regulated by MITF (Microphthalmia-associated transcription factor) (Zhang et al., 2015), a transcription factor of the MiT-TFE family of basic helix-loop-helix leucine-zipper transcription factors that serve collectively as master regulators of lysosomal biogenesis, autophagy and osteoclastogenesis (Hershey and Fisher, 2004). Owing to its unique demands for acidification, the OC has also evolved cell-specific V-ATPase subunits. In particular, the OC-specific a3 subunit of the V0 domain encoded by TCIRG1 in humans or ATP6i in mice, respectively. The a3 subunit has been shown to facilitate trafficking of the V-ATPase pump to the ruffled border (Sun-Wada et al., 2000; Futai et al., 2019) in collaboration with the small regulatory GTPases Rab7 and Rab27A (Matsumoto et al., 2018; Futai et al., 2019). Not surprisingly, mutations in TCIRG1 account for ∼50% of OC-rich ARO in humans (Frattini et al., 2000; Sobacchi et al., 2013), a skeletal phenotype that is recapitulated in mice upon genetic ablation of ATP6i (Li et al., 1999).
Neutralizing Membrane Potentials with Chloride Ions During Bone Resorption
The electrogenic efflux of H+ into the bone resorptive space generates a large membrane potential across the OC ruffled border (Kuno, 2018). To balance this charge, the OC must transport negatively charged counter-ions (anions) such as chloride (Cl–) across the ruffled border membrane. Failure to neutralize this membrane potential inhibits the acidification of the underlying resorption pit (Kornak et al., 2001). To achieve this, the OC furnishes its ruffled border membrane with complementary chloride-proton antiporters and secondary active transporters, described herein, that cooperatively facilitate Cl– exchange across membranes.
CLIC1
Chloride intracellular channel 1 (CLIC1) is a highly conserved chloride ion channel (Ulmasov et al., 2007) that exists intracellularly in a soluble state but undergoes reversible conformational changes that give rise to a dimeric integral membrane channel (Varela et al., 2019). The precise mechanisms governing CLIC1 membrane insertion remain unclear although oxidizing conditions, pH, Ca2+ and Zn2+ ion availability and membrane cholesterol have all been reported to influence its membrane integration (Valenzuela et al., 2013; Hossain et al., 2019). In OCs, the physiological role of CLIC1 has been contentious. Initial studies by Schaller et al. (2004) demonstrated that the chloride channel inhibitor NS3736 protected against ovariectomy-induced bone loss in rats owing to a dose-dependent inhibition of OC bone resorption. In keeping with this position, NS3736 was found to block OC acidification and bone resorption under in vitro settings. Based on these observations, the authors speculated that NS3736 inhibits the transport activity of either CLIC1 or CLCN7. Closer inspection of the expression levels of CLIC1 and CLCN7 revealed that whereas CLCN7 is highly expressed in OCs, CLIC1 is expressed widely in peripheral tissues, implying that NS3736 acted primarily on CLCN7 rather than CLIC1. Consistently, mice deficient in CLIC1 lack an overt bone phenotype but rather exhibit bleeding abnormalities owing to platelet dysfunction (Qiu et al., 2010). Collectively, these data point to a physiological role for CLIC1 outside of the skeleton.
CLCN7
Unlike CLIC1, the chloride voltage-gated channel 7 (CLCN7) (also known as CLC-7, OPTA2, and PPP1R63) is indispensable for OC function and bone homeostasis (Kornak et al., 2001). Intracellularly, CLCN7 exists as a homodimer residing on the membranes of late endosomes, lysosomes and the ruffled border where it functions as a 2Cl–/H+ antiporter with a fixed stoichiometry of 2Cl– for each H+ transported (Graves et al., 2008). Unique among mammalian CLCN transporters, CLCN7 requires an obligatory β-subunit: osteoclastogenesis associated transmembrane protein 1 (Ostm1) (also known as CLCN7 accessory beta subunit) (Lange et al., 2006). This association is critical for the stability of CLCN7 and Ostm1 expression and Cl–/H+ exchange (Stauber and Jentsch, 2010). Similar to the V-ATPase proton pump subunits, the expression of both CLCN7 and Ostm1 is regulated by the transcription factor MITF (Meadows et al., 2007). CLCN7 traffics to late endosomes and lysosomes via interaction of its [DE]XXXL[LI] dileucine lysosomal targeting motifs with cognate adaptor proteins (Stauber and Jentsch, 2010). The mechanisms by which Ostm1 and CLCN7 cooperate on lysosome membranes has long remained unclear. However, recent cryoelectron microscopy studies by two independent groups demonstrated that the highly glycosylated Ostm1 serves as a molecular shield by covering the luminal surface of CLCN7 thereby protecting it from proteolysis in acidic environments that occur inside lysosomes and the resorptive space (Schrecker et al., 2020; Zhang et al., 2020). The importance of this intimate molecular association is exemplified in the skeleton where mutations in either CLCN7 or Ostm1 correspond with severe osteopetrosis in humans and in mice (Kornak et al., 2001; Chalhoub et al., 2003). The G215R mutation in CLCN7, for example, leads to autosomal dominant osteopetrosis type 2 that does not abolish the transport function of the channel but rather causes a severe trafficking defect with the G215R-CLCN7 mutation rendering CLCN7 retention in the ER (Schulz et al., 2010). OCs derived from both CLCN7 and Ostm1 mutant mice form normally but fail to form a ruffled border and resorb bone (Kornak et al., 2001; Chalhoub et al., 2003).
In addition to CLCN7, CLCN3 and CLCN5 have also been reported to reside on late endosomes in OCs (Okamoto et al., 2008) and mineralizing osteoblasts (Larrouture et al., 2015). Genetic ablation and siRNA-mediated depletion of Clcn3 was found to reduce the acidification and bone resorptive capabilities of mouse OCs cultured in vitro (Okamoto et al., 2008). Clcn3 knockout mice lack a bone phenotype although, like their Clcn7 knockout counterparts, they exhibit severe neurodegeneration (Yoshikawa et al., 2002; Kasper et al., 2005; Okamoto et al., 2008). This indicates that CLCN3 is dispensable for OC function and skeletal homeostasis.
SLC12A4
Outside the CLCN family, solute carrier family 12 member 4 (SLC12A4), also known as the electroneutral potassium-chloride cotransporter 1 (KCC1), has been shown to facilitate Cl– extrusion in OCs. SLC12A4 localizes exclusively to the OC basolateral surface where it has been proposed to function in the cotransport of K+ and Cl– simultaneously in the outward direction. Unlike other members of the SLC12 family, SLC12A4 K+/Cl– cotransport is not dependent on the presence of Na+ (Hebert et al., 2004). Structurally, SLC12A4 is composed of 1085 amino acids forming a 12 transmembrane domain structure with a large extracellular loop containing potential N-linked glycosylation sites, and cytoplasmic N- and C-terminal domains (Gillen et al., 1996). To enable transporter function, SLC12A4 dimerises through links in both transmembrane and extracellular domains (Liu et al., 2019). Although ubiquitously expressed, the function of Slc12a4 has been explored in mouse OCs in in vitro settings (Kajiya et al., 2006). Studies by Kajiya et al. (2006) demonstrated that Slc12a4 is the primary potassium-chloride transporter expressed in OCs. Moreover, treatment of OCs with the KCC transporter inhibitor R(+)-butylindazone (DIOA), or knockdown of Slc12a4 expression using siRNA, decreased Cl– extrusion resulting in increased OC alkalinisation and, in turn, reduced capacity to resorb bone (Kajiya et al., 2006). It remains to be seen whether these in vitro defects in OC activity extend to physiological impacts on the skeleton in intact mice.
Internal Buffering and HCO3– Transport in Osteoclasts
To sustain acidification during ongoing cycles of bone resorption, the V-ATPase pump requires a continuous supply of protons. Protons are supplied to the V-ATPase pump by the cytoplasmic carbonic anhydrase 2 (CA2), an enzyme that catalyses the reversible hydration of carbon dioxide along the following equation: CO2 + H2O → H+ + HCO3–. Unsurprisingly, defects in CA2 activity lead to osteopetrosis and extra-skeletal disturbances including renal tubular acidosis and cerebral calcification, reminiscent of the clinical features observed in patients harboring mutations in TCIRG1. At the same time, CA2 stabilizes intracellular pH (pHi) and alkalinisation in OCs through the liberation of HCO3– ions. HCO3– ions are, in turn, exchanged for Cl– by select members of the solute carrier protein family 4 (SLC4), thereby ensuring an electroneutral supply of Cl– to the OC (Teti et al., 1989).
SLC4A2
SLC4A2, previously known as EPB3L1 or AE2, is a member of the SLC4 family of Cl–/HCO3– exchangers. SLC4 family members are important for the modulation of pHi and have been implicated in the regulation of cell volume, migration, and transepithelial movement of ions in various tissues (Alper, 2006). The SLC4 family comprises 10 members divided into three major clades; (i) the Na+-independent electroneutral Cl–/HCO3– exchangers (e.g., SLC4A1), (ii) the Na+-dependent HCO3– transporters (e.g., SLC4A4) and, (iii) borate transporters comprised of a singular member in mammals, SLC4A11 (Cordat and Casey, 2008; Liu et al., 2015). In mice, due to different promoter regions Slc4a2 can be transcribed as five different N-terminal variants: Slc4a2a, Slc4a2b1, Slc4a2b2, Slc4a2c1, and Slc4a2c2. Slc4a2a and Slc4a2b are ubiquitously expressed while Slc4a2c expression is restricted to the stomach. In OCs, Slc4a2 expression is upregulated during differentiation in an NFATc1-dependent manner (Wu et al., 2008) and has been localized to the basolateral plasma membrane. Slc4a2 knockout mice are viable and have been used as a model to study the role of the transporter in different tissues including the stomach, intestines, colon, biliary epithelial cells, testes, and ameloblasts (Rossmann et al., 2000; Medina et al., 2003; Lyaruu et al., 2008; Gawenis et al., 2010; Concepcion et al., 2013).
In bone, four independent studies have investigated the impact of Slc4a2 deletion on skeletal homeostasis in mice. The first by Wu et al. (2008) assessed the bone phenotypes of mice globally lacking all Slc4a2 isoforms. In this study Slc4a2-deficient mice were shown to manifest osteopetrosis with growth retardation and clubbing of the long bones. The authors went on to show that this disruption in bone homeostasis was related to a cell-autonomous defect in OCs and concluded that the primary function of Slc4a2 in OCs is to facilitate bone resorption by suppressing OC apoptosis (Wu et al., 2008). The second, by Josephsen et al. employed a global deletion mouse model of Slc4a2. In this instance, the knockout mice presented with an osteopetrotic phenotype affecting the skull and long bones. This high bone mass phenotype was attributed to defects in OCs, which where morphologically enlarged and possessed rudimentary ruffled borders. Soon after, Jansen et al. (2009) confirmed the osteopetrotic phenotypes of long bones in Slc4a2a-b knockout mice. However, in contrast to global Slc4a2-deficient mice, calvarias of Slc4a2a-b knockout mice are indistinguishable from wild-type littermates implying partial compensation from untargeted Slc4 isoforms. More recent studies by Coury et al. (2013) used an OC-specific conditional deletion of Slc4a2 in mice. In this study, specific loss of Slc4a2 in OCs corresponded with a high bone mass phenotype and dysfunctional OCs which exhibited impaired bone resorption capacity owing to a dysregulation of calpain-dependent podosomal disassembly (Coury et al., 2013). The importance of SLC4A2 is not limited to mice, but has also been shown to underpin the osteopetrotic phenotype observed in Red Angus cattle (Meyers et al., 2010). Taken together, these findings highlight the indispensable role for the SLC4A2 transporter in buffering HCO3– / Cl– exchange during OC bone resorption. A future challenge will be deciphering the exact contribution of each SLC4A2 variant in OCs as well as exploiting this transporter family for therapeutic targeting. A recent study demonstrating that the anion exchange inhibitor 4, 4′-diisothiocyano-2,2′-stilbenedisulfonic acid (DIDS) is capable of suppressing OC activity in a mouse model of wear-particle-induced osteolysis (Wu et al., 2018) lends ‘proof of principal’ toward this.
SLC4A7
Solute carrier family 4 member 7 (SLC4A7), also known as NBC2, NBC3 and NBCn1, is responsible for the electroneutral cotransport of Na+ and HCO3– with a stoichiometry of one for one (Pushkin et al., 1999; Choi et al., 2000). SLC4A7 is expressed in many human tissues including the kidneys and gastrointestinal tract where it localizes to basolateral and apical cell membranes and participates in net cellular acid extrusion. The transporter is predicted to homodimerise and is understood to have 14 transmembrane domains with a long intracellular N-terminal and short C-terminal presenting a PDZ domain. SLC4A7 possesses a dileucine motif in its cytoplasmic C-terminal tail indicating potential lysosomal residency supported by reports of co-localization with LAMP1. The C-terminal of the protein has also been shown to interact with RACK1. In addition, some putative binding candidates for this terminal have been identified and include LAMP1, LAMP2, AP2A1, AP2B1, Rab5A, 5B, 5C, 8A, 11, and SNX1, 2, 5, 6, 27 (Olesen et al., 2018). The PDZ domain of Slc4a7 has been shown to interact with NHERF-1, PSD-95, and CFTR. There is also evidence for interactions between the C-terminal PDZ domain and V-ATPase subunits in renal tissues (Pushkin et al., 2003) and CA2, consequently recruiting CA2 to the plasma membrane (Loiselle et al., 2004).
Slc4a7 expression has been detected at both the mRNA and protein level in neonatal rat OCs and murine OC-like cells where it has been suggested to function as a key participant in M-CSF-induced cellular alkalinisation (Bouyer et al., 2007). Indeed, Slc4a7 expression is upregulated during osteoclastogenesis and had been localized to the ruffled border membrane (Riihonen et al., 2010). Consistently, knockdown of Slc4a7 expression using a shRNA lentivirus-mediated delivery impairs OC bone resorption and cytosolic pH homeostasis but does not alter OC formation or survival. To date, Slc4a7 knockout mice have been generated although their skeletal phenotype remains unknown (Lee et al., 2016).
Calcium Transporters in Osteoclast Signaling, Differentiation and Function
Ca2+ is a universal second messenger required for cell signaling and plays multiple roles in OC formation, survival and activity (Robinson et al., 2010). It is liberated from bone during resorption by OCs, which, in turn, exposes the ruffled border membrane to high concentrations of the ion (up to 40mM Ca2+) (Silver et al., 1988). Aforementioned, some of this Ca2+ is removed by transcytotic mechanisms or through passive leakage from the resorptive compartment during OC migration. However, a portion of this solubilized Ca2+ is exchanged transcellularly across OC membranes via a number of structurally diverse calcium transport proteins embedded on the surface of the OC ruffled border and basolateral domain that are described here (Berger et al., 2001).
Ryanodine Receptor 2
Being enriched in the OC plasma membrane, the Ryanodine receptor 2 (RYR2) was initially proposed to be the major Ca2+channel controlling Ca2+ influx in OCs, a position supported by several lines of biophysical, pharmacological and immunochemical evidence in the early 2000s (Zaidi et al., 1995, 1999; Baljit et al., 2002; Datta and Horrocks, 2003). The role of the RYR2 in OCs has been reviewed extensively in Robinson et al. (2010) and thus will not be discussed further herein. Instead, we will focus on the contribution of more recently identified transporters and channels that have been implicated in modulating the exchange of Ca2+ across OC membranes.
ATP2B1 and ATP2B4
ATP2B1 and ATP2B4, also known as PMCA1 and PMCA4 respectively, belong to the P-type Ca2+ ATPase family that maintains intracellular homeostasis by exporting Ca2+ from the cytoplasm to the extracellular space. These pumps are powered by the hydrolysis of ATP, with one Ca2+ ion removed from the cell for each molecule of ATP hydrolysed. Calmodulin increases the affinity of the proteins’ alternatively spliced Ca2+-binding site and also increases the rate at which the pump extrudes Ca2+ (Keeton et al., 1993; Tidow et al., 2012). ATP2B1 and ATP2B4 are highly expressed in OCs and transcriptionally regulated by NFATc1 (Kim et al., 2012). Both ATP2B1 and ATP2B4 pumps reside on the basolateral membrane of OCs where they have been proposed to function to extrude superfluous intracellular Ca2+ and thus prevent Ca2+-induced apoptosis. Studies by Kim et al. (2012) demonstrated that siRNA-mediated double knockdown of ATP2B1 and ATP2B4 led to a significantly reduced bone volume to trabecular volume ratio and a significant increase in OC surface to bone surface ratio using a mouse calvarial injection model. Genetic ablation of Atp2b1 in mice is lethal during early embryonic development and thus far has precluded assessment of the skeleton (Okunade et al., 2004). Similarly, there has been no reported bone phenotype for Atp2b4 knockout mice, however males are infertile owing to reduced sperm motility (Okunade et al., 2004; Schuh et al., 2004).
TRPV4-6
In addition to Ca2+ ATPases, OCs possess an array of Ca2+ channels including several members belonging to the transient receptor potential (TRP) channel family. TRP channels are a class of 6-membrane-spanning helices ion channels primarily located on the plasma membrane of several mammalian cell types. They can be divided into Groups 1 and Group 2 channels; with each possessing their own subfamilies. TRP channels mediate several sensory functions including pain, temperature, taste, pressure, and vision but have also been linked to regulatory mechanisms. Trpv1-6 (Transient receptor potential cation channel subfamily V member 1 to 6) are members for the TRPV subfamily of Group 1 TRP channels. Several TRPV members including TRPV4, 5 and 6 are known to be expressed in OCs. TRPV4 mRNA and protein expression has been reported to be upregulated during OC differentiation. In vitro, TRPV4 has been implicated in OC formation, with overexpression or knockdown of the channel correlating to increased and decreased OC numbers, respectively (Cao et al., 2019).
In comparison, TRPV5 is primarily expressed in the kidney, intestines and OCs where it plays a central role in the systemic regulation of Ca2+ homeostasis by facilitating: (i) Ca2+ reabsorption in the kidneys, (ii) absorption of dietary Ca2+ by intestinal cells, and (iii) the release of the ion by active OCs. The importance of TRPV5 in Ca2+ homeostasis is exemplified in Trpv5 knockout mice, which exhibit reduced renal Ca2+ reabsorption leading to hypercalciuria-induced polyuria coupled with urine acidification (Hoenderop et al., 2003). The Ca2+ lost through urine in Trpv5 knockout mice is compensated by hyperabsorption of dietary Ca2+, which, in turn, leads to reduced bone cortical and trabecular thickness. In these mice, serum peptide hormones (PTH), Ca2+ and K+ levels are normal despite increased 1,25-(OH)2D3, implying that the decreased cortical and trabecular bone thickness observed in Trpv5 knockout mice is an indirect consequence of prolonged elevations in 1,25-(OH)2D3 (Hoenderop et al., 2003). However, studies by the same group later reported a cell-autonamous defect in Trpv5-deficient OCs. In OCs, Trpv5 localizes to the ruffled border suggestive of a role in modulating bone resorption. Closer inspection of the bones of Trpv5 knockout mice revealed that loss of Trpv5 was associated with increased OC numbers and an increase in the number of nuclei per OC. Moreover, Trpv5 knockout OCs exhibited reduced bone resorptive capacity in line with a intrinsic cellular defect (van der Eerden et al., 2005). Further, alendronate has been shown to normalize the reduced bone thickness in Trpv5 knockout mice (Nijenhuis et al., 2008). It is noteworthy that Econazole (Yan et al., 2011), TH-1177 (Landowski et al., 2011), and selected cannabinoids (Janssens et al., 2018) have been reported to modulate TRPV5 but have potencies in the mid-micromolar range and are not selective for TRPV5 over its close homolog TRPV6. Hughes et al. (2019) identified three novel TRPV5 inhibitors through structural-based virtual screening. One of which was selective for TRPV5 over TRPV6 as revealed through the identification of a previously uncharacterised TRPV5 binding site by cryoelectron microscopy (Hughes et al., 2019). The apparent importance of TRPV5 in OC function along with recent development of selective TRPV5 inhibitors makes this channel a potential candidate for the therapeutic modulation of bone homeostasis.
Finally, similar to TRPV5, Trvp6 knockout mice manifest a low bone mass (osteopenia) phenotype owing to increased OC numbers, and hence a net increase in OC bone resorptive activity in vivo (Chen et al., 2014).
Mucolipin-1
Mucolipin-1 (also known as TRPML1) is the first member of the transient receptor potential cation channel, mucolipin subfamily and is widely recognized as a major calcium release channel residing on lysosomal membranes (Vergarajauregui and Puertollano, 2006; Thompson et al., 2007). Mucolipin-1 plays critical roles in a variety of membrane trafficking processes such as retrograde trafficking of lysosomes to the trans-Golgi network, autophagic maturation and lysosomal homeostasis (Haoxing and Dejian, 2015; Scotto Rosato et al., 2019). This non-selective cation channel is modulated by changes in Ca2+ concentration, phosphoinositides and pH. Mutations in MCOLN1 lead to Mucolipidosis type IV, an autosomal recessive neurodegenerative lysosomal storage disorder (Bargal et al., 2000). The importance of Mucolipin-1 extends to bone. Genetic deletion of Mucolipin-1 in mice correlates with impaired osteoclastogenesis, altered lysosomal homeostasis and attenuated OC bone resorption pointing to an indispensable and physiological role for this cation channel in bone remodeling (Erkhembaatar et al., 2017). For an extensive review see (Wang et al., 2014).
ORAI1
ORAI calcium release-activated calcium modulator 1 (ORAI1), also known as CRACM1 and TMEM142A, is a plasma membrane localized Ca2+ channel. It associates with stromal interaction molecule 1 (STIM1), an endoplasmic reticulum transmembrane protein, to modulate Ca2+ cellular entry through a process termed calcium release-activated calcium (CRAC) (Lunz et al., 2019). Upon depletion of luminal endoplasmic reticulum Ca2+ stores, the CRAC assembly formed by ORAI1 and STIM1 opens to refill depleted stores. To achieve channel opening, the cytoplasmic portion of STIM1 must come into contact with the intracellular portion of ORAI1 after which ORAI1, in turn, allows extracellular Ca2+ influx into the cell to replenish stores. Studies by Zhou et al. (2011) reported a decline in both ORAI1 and STIM1 expression during osteoclastogenesis in human cells. Moreover, they found that siRNA-mediated knockdown of ORAI1 decreased OC formation. The authors further demonstrated that pharmacological inhibition of the CRAC channel assembly prevented OC differentiation. Using a shRNA knockdown approach, Hwang and Putney (2012) similarly reported that depletion of Orai1 impairs OC differentiation in vitro owing to a decreased in NFATc1 transcritpional activity and associated downstream genes. Importantly, Orai1 knockout mice exhibit a clear skeletal phenotype (Robinson et al., 2012). Orai1 knockout mice are anatomically smaller than their wild-type counterparts and show less post-natal growth. In addition, they exhibit tooth abnormalities and bone defects, including the existence of unresorbed cartilage remnants and decreased bone mineralization. Accordingly, OCs from Orai1 knockout mice are morphologically smaller (mononuclear) and osteoblastic maturation was impaired. Thus, the skeletal abnormalities observed in Orai1 knockout mice appear to arise from cell-autonomous defects in both OCs and osteoblasts highlighting a general role for this Ca2+ channel in bone homeostasis.
NMDAR
The N-methyl-D-aspartate receptor (NMDAR) is a glutamate receptor and ion channel found on the plasma membrane of OCs whose physiological function has long been debated. The channel is composed of three subunits: NMDAR1 associates with one or more NMDAR2 and/or NMDAR3 subunits. The multiple receptor subunit combinations along with the existence of 8 different NMDAR1 splice variants allows diversity in glutamate receptor function (Spencer et al., 2007). When activated by the binding of glutamate and glycine, the NMDAR allows for the flow of Ca2+ across the membrane. At present the role of NMDAR in OCs physiology remains contentious with several conflicting reports in the literature (Spencer et al., 2007). For instance, Chenu et al. (1998) reported that MK-801, an NMDAR inhibitor, had no effect on adhesion or survival of OCs but caused the disruption of actin ring formation. In contrast, studies by Peet et al. (1999) found no effect of MK-801 on OC actin ring formation but instead showed that MK-801 prevented OC differentiation and bone resorption in a co-culture model in vitro. Further, Merle et al. (2003) reported that MK-801 inhibited osteoclastogenesis and that activation of NMDAR induced the nuclear translocation of NFκB. Thus, further study using in vivo models will be required to resolve the physiological contribution of this Ca2+ channel in OCs and bone homeostasis.
P2RX7
ATP-activated Ca2+ channel Purinergic receptor P2X 7 (P2RX7), also known under the alias P2X7, is a member of the Purinergic receptors P2X group that mediate Ca2+ fluxes across membranes in response to extracellular ATP. Following activation, P2RX7 forms a homotrimeric pore that facilitates the movement of organic ions including N-methyl-D-glucamine, choline and fluorescent dyes such as ethidium and YO-PRO-12 across membranes (Alves et al., 2014; Sluyter, 2017). P2RX7 is the major PX2 family member expressed in OCs (Naemsch et al., 2001) and its role in skeletal and joint diseases has been extensively reviewed (Zeng et al., 2019). P2RX7 has been implicated in OC fusion in both mice (Ke et al., 2003) and humans (Pellegatti et al., 2011). In humans, the formation of OCs is inhibited by an anti-P2RX7 monoclonal antibody and by specific P2RX7 pharmacological antagonists A740003 and AZ11645363 (Pellegatti et al., 2011). There is also accumulated evidence implicating several P2RX7 SNPs in postmenopausal osteoporosis (Ohlendorff et al., 2007; Gartland et al., 2012; Jørgensen et al., 2012). In keeping with this position, genetic deletion of P2RX7 in mice corresponds with a significant reduction in total and cortical bone content and periosteal circumference in femurs, and reduced periosteal bone formation and increased trabecular bone resorption in tibias (Ke et al., 2003). Consistently, Wang et al. (2018a) reported increased OC formation in P2RX7 knockout mice compared to wild-type littermates following ovariectomy (OVX)-induced bone loss. Further, whereas both the cortical and trabecular bone volume fractions were significantly decreased in the tibias of P2RX7 knockout mice compared to knockout sham controls at 6-weeks post-OVX, no statistically significant change was observed in the corresponding bone parameters in OVX and sham operated wild-type mice 6-weeks post-surgery (Wang et al., 2018a).
TPCN2
Two pore segment channel 2 (TPCN2), also known as TPC2, is a member of the Two pore channel family of intracellular voltage-gated and ligand-gated cation-selective channels that enables Ca2+ ion release in response to NAADP binding. Intracellularly, TPCN2 localizes to lysosome-related organelles via a lysosomal targeting motif (Brailoiu et al., 2010; Schieder et al., 2010; Ambrosio et al., 2015) where it has been shown to contribute to the regulation of their biogenesis (Ambrosio et al., 2015). In OCs, Tpcn2 mRNA expression is upregulated in response to RANKL and has been proposed to play a role in maintaining intracellular Mg2+ rather than Ca2+ homeostasis. At steady-state Mg2+ levels, siRNA-mediated knockdown of Tpcn2 has been shown to impair OC differentiation and bone resorption in vitro. Conversely, under low Mg2+ culture conditions, OC formation was accelerated suggesting that Tpcn2 functions to regulates OC differentiation and activity in response to circulating Mg2+ levels (Notomi et al., 2012, 2017).
SLC8A1-3
Solute carrier family 8 member A1, A2 and A3 (SLC8A1, SLC8A2, SLC8A3), previously known as NCX1, NCX2 and NCX3, respectively, are Na+/Ca2+ antiporters from the SLC8 family. All three SLC8A transporters are expressed in OCs and are reported to localize to the basolateral membrane. Of these transporters, SLC8A1 has received the most attention in OCs (Li et al., 2007; Albano et al., 2017). The use of chemical inhibitors or siRNA to inhibit SLC8A1 and SLC8A3 leads to reduced bone resorption in vitro (Moonga et al., 2001; Li et al., 2007). Using an OC-specific Slc8a1 knockout mouse model Albano et al. (2017) reported that Slc8a1 knockout OCs form normally but resorb significantly more bone than their wild-type counterparts in vitro. However, no obvious skeletal changes were detected in Slc8a1 knockout mice at 3 months of age with only very minor differences appreciable at 6 months of age. The findings in vivo suggest that Slc8a1 plays an accessory but not essential role in OC Ca2+ homeostasis.
Phosphate Handling in the Osteoclast
Inorganic phosphate (Pi) is an essential molecule for cellular homeostasis and is the major anionic component of hydroxyapatite. Therefore, in addition to Ca2+, OCs are exposed to high ambient concentrations of Pi during bone resorption. Although the exact Pi concentration liberated into the resorption pit remains to be established, it is predicted to reach upward of 20 mM (Kuno, 2018). High ambient Pi has been shown to influence OC differentiation and resorptive activity underlying the importance of appropriate Pi handling by the cell (Kanatani et al., 2003). It has also been hypothesized that part of the Pi released from bone may be utilized by the OC to maintain cellular ATP during the energy requiring cyclical processes of migration, attachment, and resorption (Gupta et al., 1997). Typically, Pi influx has been reported to require extensive V-ATPase activity and thus a large amount of energy. Evidence of the existence of Na+-dependent Pi transport systems in OCs was first demonstrated by Gupta et al. (1996) and later confirmed by the Miyamoto and Gupta laboratories who demonstrated the presence of Pi influx and efflux systems in OC-like cells derived from RAW264.7 macrophages and primary mouse OCs (Khadeer et al., 2003; Mikiko et al., 2005). The expression of several Na+-dependent Pi transporters have been assessed in OCs including SLC17A1 (previously NPT1), SLC34A1 (previously Npt2a or SLC17A2), SLC34A2 (previously Npt2b), SLC34A3 (previously Npt2c), SLC20A1 (previously Pit-1), SLC20A2 (previously Pit-2) and SLC37A3. Of these, SLC34A1, SLC20A1/2 and SLC37A3 have been functionally characterized in OCs.
SLC34A1
Solute carrier family 34 member 1 (SLC34A1), previously known as NPT2a or SLC17A2 is an 80-90 kDa sodium cation/divalent phosphate cotransporter with a stoichiometry of three Na+ to one HPO42– (Forster et al., 1999). It resides on cell membranes where it preferentially cotransports Na+ and HPO42–, although it has also been reported to transport Li+ as a driving cation (Olga et al., 2012; Schlingmann et al., 2016). SLC34A1 sequentially binds two Na+ cations, a HPO42– and a third Na+ before undergoing conformational changes allowing the release of the ions on the opposite side of the membrane (Forster, 2019).
Several independent studies have implicated a role for SLC34A1 in OC differentiation, bone resorption and skeletal homeostasis albeit with conflicting reports (Gupta et al., 2001; Khadeer et al., 2003; Albano et al., 2015). On one hand, studies by Albano et al. (2015) reported that SLC34A1 is dispensable for OC differentiation and bone resorption. On the other, studies by Gupta et al. (2001) reported that Slc34a1 knockout mice exhibit an age-dependent bone phenotype associated with a reduction in OC numbers that accompanied increases in bone formation. The precise reasons for these discrepancies is unclear but may reflect differences in the targeting approach and mouse strains used. Alternatively, they might point to extra-skeletal disturbances in Pi homeostasis influencing the magnitude of bone phenotypes observed. For instance, outside of bone, SLC34A1 is highly expressed in the kidneys. Inactivating mutations in SLC34A1 have been associated with idiopathic infantile hypercalcemia 2 (IIH2), a disease usually attributed to mutations in CYP24A1 (Schlingmann et al., 2011; De Paolis et al., 2019). Thus, a major role of SLC34A1 is to facilitate reabsorption of glomerular-filtered phosphate in the proximal tubule, with disruption of this transporter leading to hypophosphatemia. Mutations in SLC34A1 result in surplus active vitamin D in the body. This vitamin D excess, in turn, increases calcium absorption into the bloodstream, resulting in hypercalcemia (Schlingmann et al., 2016). Consistently, global Slc34a1 knockout mice manifest hypophosphatemia and hyperphosphaturia associated with hypercalcemia and hypercalciuria all of which are known to influence bone mass.
SLC20A1 and SLC20A2
SLC20A1 (also known as PiT-1 and Glvr-1) and SLC20A2 (also known as PiT-2, Glvr-2 and Ram-1) are sodium-phosphate (Na+/Pi) electrogenic cotransporters that are widely expressed. These transporters share 60% of their amino acid sequence although their precise stoichiometry remains to be established (Kavanaugh and Kabat, 1996; Bai et al., 2000). In OCs, both SLC20A isoforms are constitutively expressed throughout osteoclastogenesis (Khadeer et al., 2003; Albano et al., 2015). Of these, SLC20A1 has been localized to the basolateral membrane of bone-resorbing OCs (Khadeer et al., 2003). Global genetic ablation of Slc20a1 in mice leads to embryonic lethality. However, a Slc20a1 variant mouse in which both alleles code for a gene with 85% reduced expression (knockdown) are viable but manifest multiple abnormalities that do not extend to bone (Beck et al., 2010; Bourgine et al., 2013). Interestingly, Slc20a1 knockdown mice exhibit an upregulation of Slc20a2 implying functional redundancy. Slc20a2 knockdown mice, however, show no overt bone phenotype but rather manifest features consistent with familial idiopathic infantile ganglia calcification as observed in humans harboring mutations in SLC20A2 (Wang et al., 2012; Jensen et al., 2013). Future generation of a double Slc20a1-Slc20a2 OC-specific knockout model might prove valuable toward resolving the ambiguity around the physiological importance of these two Na+/Pi transporters in OC function.
SLC37A3
Solute carrier family 37 member 3 (SLC37A3) is a little studied member of the SLC37 family of glucose-6-phosphate (G6P)/Pi antiporters (Chou et al., 2013; Cappello et al., 2018). SLC37A3 is highly expressed in neutrophils and the pancreas implying an important role for this transporter in these systems. SLC37A3 has been shown to localize to the endoplasmic reticulum but unlike other SLC37 family members does not transport G6P (Pan et al., 2011). Instead, SLC37A3 has been recently implicated in the translocation of nitrogen-containing bisphosphonates (N-BPs) from lysosomes to the cytosol (Drake et al., 2008). Using a CRISPRi-mediated genome-wide screen, Yu et al. (2018) identified SLC37A3 among the top genes to confer resistance to N-BPs. They further demonstrated that SLC37A3 interacts with ATRAID, a type I transmembrane protein on lysosomes, that stabilizes SLC37A3 expression thereby allowing the transport of N-BPs into the cytosol. In keeping with this position, deletion of SLC37A3 and/or ATRAID conferred resistance to alendronate in OC-like cells derived from RAW 264.7 cells. While SLC37A3 appears to facilitate efflux of N-BPs from the lysosome, its endogenous substrate remains to be defined.
Other Solute Carrier Transporters in Osteoclasts
Among the multitude of membrane transporters known to exist in OCs (Figure 2), a growing number of members of the solute carrier (SLC) transporter superfamily have recently been identified in OCs and implicated in bone health and disease. SLC proteins constitute a large group of transmembrane transporters spanning 65 gene families and having more than 400 putatively functional protein-coding genes (He et al., 2009; Schlessinger et al., 2013). These transporters are organized into families based on predicted protein sequence homology and predicted substrate specificity (Chou et al., 2013). Some of these transporters are ubiquitously expressed while others are a specialized feature of terminally differentiated cell types like neurons and OCs. All members of this superfamily have a preferred substrate and subcellular localization allowing for a vast number of varied exchanges across biological membranes intracellularly and with the extracellular environment (Pizzagalli et al., 2020). A number of diverse SLC transporters have been recently linked to OC function involved in the exchange of a range of fundamental biological substrates including amino acids (SLC7A5/LAT1, SLC9B, SLC17A7/VGLUT1), nucleosides (SLC29A3) and iron (SLC40A1 and SLC11A2) that are described briefly herein.
Amino Acid and Nucleoside Transporters
Amino acids and nucleosides are utilized as the building blocks for proteins, DNA and RNA, respectively, as well as serving critical roles in a variety of cellular signaling functions including synaptic neurotransmitter in neurons (e.g., the glutamate transporter SLC17A7/VGLUT1). Dysregulation of amino acid and nucleoside transporters have been increasingly implicated in various human metabolic diseases as well as cancer pathogenesis (Kandasamy et al., 2018; Pastor-Anglada and érez-Torras, 2018). Thus, it is not surprising that the importance of these membrane transporters has been extended to OC formation and function.
SLC7A5
The solute carrier family 7 member 5 (SLC7A5), also known as L-type amino acid transporter (LAT1), mediates the cellular uptake of phenylalanine, tyrosine, L-DOPA, leucine, histidine, methionine and tryptophan. SLC7A5 forms a heterodimer through the formation of a disulfide bond with the solute carrier family 3 member 2 (SLC3A2). The heterodimer functions as a Na+-independent large neutral L-amino acid exchanger where SLC7A5 is the transport component (Napolitano et al., 2015). Whereas the heterodimer localizes to the plasma membrane, it translocates to lysosomes upon heterotrimerisation with LAPTM4B. At the lysosomal membrane, the heterotrimer allows for entry of amino acids into the lysosomal lumen leading to the V-ATPase mediated activation of mTORC1 (Milkereit et al., 2015).
Slc7a5 has been linked to the pathogenesis of various cancers (e.g., highly proliferative breast cancer subtypes and small cell lung cancer) where it is thought to modulate cell proliferation (El Ansari et al., 2018). More recently, the role of Slc7a5 has been extended to bone homeostasis. Studies by Ozaki et al. (2019) demonstrated that Slc7a5 is expressed in both OCs and osteoblasts, with expression of the transporter found to decline in preosteoclasts of ovariectomized mice. Conditional deletion of Slc7a5 in OCs yields a low bone mass phenotype associated with an elevation in the bone resorption marker CTx, whereas bone formation markers remained unchanged. Moreover, loss of Slc7a5 accelerates osteoclastogenesis and bone resorption in an mTORC1-dependent manner that, in turn, contributes to the nuclear accumulation of the pro-osteoclastogenic transcription factor NFATc1.
SLC9B2
Solute carrier family 9 member 2 (SLC9B2), also known as FLJ23984 or NHA2 and NHEDC2, is a 547 amino acid antiporter that regulates the exchange of extracellular H+ for cytosolic Na+ or Li+. SLC9B2 is highly expressed in OCs and is transcriptionally regulated by TFEB (Sardiello et al., 2009). This transporter possesses an N-terminal lysosomal targeting motif and accordingly is localized to late endosomes as well as the OC basolateral membrane (Pham et al., 2007; Battaglino et al., 2008; Hilton et al., 2008; Lee et al., 2008). Despite its abundance in OCs, Slc9b2 knockout mice lack a prominent skeletal phenotype (Hofstetter et al., 2010). Further, OCs from Slc9b2 deficient mice differentiate normally and are capable of bone resorption in vitro, suggesting that SLC9B2 is dispensable in OCs or may be compensated by a hitherto unidentified transporter that fulfills its function (Pizzagalli et al., 2020).
SLC17A7
SLC17A7, also known as BNPI or VGLUT1, is an electrogenic Cl– dependent glutamate transporter of the SLC17 family. SLC17A7 is best recognized for its function in mediating the uptake of glutamate into synaptic vesicles in neurons, with a preference for L-glutamate over D-glutamate (Voglmaier et al., 2006). The importance of glutamate in bone homeostasis has long been recognized (Serre et al., 1999; Hinoi et al., 2001; Skerry and Taylor, 2001; Mason, 2004). In OCs, glutamate is secreted through the process of transcytosis. Studies by Morimoto et al. (2006) were the first to demonstrate the existence of the glutamate transporter in OCs where it was localized to transcytotic vesicles. Using OCs from Slc17a7 knockout mice they further demonstrated that whereas the number and morphology of Slc17a7 knockout OCs are comparable to those derived from wild-type littermates, loss of the transporter corresponded with altered glutamate signaling and significantly increased OC bone resorption. Accordingly, Slc17a7 knockout mice exhibit reduced bone mass at 4 months of age (Morimoto et al., 2006).
SLC29A3
Solute carrier family 29 member 3 (SLC29A3), also known as ENT3 or FLJ11160, is one of the four members of the SLC29 family. It is highly expressed in macrophages and OCs and encodes an equilibrative nucleoside transporter. SLC29A3 possesses an N-terminal dileucine motif necessary for its targeting to endosomal and lysosomal membranes (Baldwin et al., 2005). Various IDELS of SLC29A3 have been linked to diseases including Faisalabad histiocytosis (FHC), H syndrome, sinus histiocytosis with massive lymphadenopathy (also known as familial Rosai-Dorfman disease), pigmented hypertrichosis and insulin-dependent diabetes have all been proposed to form part of a continuous ’SLC29A3 spectrum disorder’ (Morgan et al., 2010).
Campeau et al. (2012) identified SLC29A3 as the causative gene underpinning two cases of dysosteosclerosis, a rare bone sclerosing dysplasia. OCs derived from peripheral blood mononuclear cells isolated from these patients exhibited reduced differentiation and resorption capacity in vitro (Campeau et al., 2012). Howaldt et al. (2019) also reported sclerosing bone dysplasias in two patients with SLC29A3 mutations.
Iron Transporters
In addition to Ca2+ and Pi, iron (ferritin) is an important element for OC differentiation and bone homeostasis. This is exemplified in patients with iron overload conditions such as hemochromatosis, thalassemia and sickle cell disease, which frequently suffer osteoporosis and associated fragility fractures (Guggenbuhl et al., 2005; Fung et al., 2008). Hemochromatosis, in particular, has been reported to arise from mutations in SLC40A1 (Zhang et al., 2017; Ka et al., 2018; Yin et al., 2019). SLC40A1, also known as Ferroportin 1 and SLC11A3, is a 571-amino acid transporter localized to cell membranes where it functions to facilitate iron export (Hentze et al., 2004).
SLC40A1
Global Slc40a1 knockout, myeloid lineage-specific (LysM-Cre) and mature OC-specific (Ctsk-Cre) conditional Slc40a1 knockout mice have been generated and characterized by several independent groups (Wang et al., 2018b; Pereira et al., 2020). Global deletion of Slc40a1 in mice leads to embryonic lethality (Donovan et al., 2005). On the other hand, myeloid lineage-specific and mature OC conditional Slc40a1 knockout mice are viable but exhibit mild or little bone phenotypes, respectively, which also depend on the sex, age and Cre-driver employed. For instance, LysM-Cre Slc40a1 conditional knockout mice exhibit decreased trabecular bone mass of both long bones and vertebrae in female, but not in male littermates at 2-months of age. By comparison, specific deletion of Slc40a1 in the late stages of OC differentiation by Ctsk-Cre has no obvious effects on either trabecular or cortical bone mass of both the long bones and vertebrae, suggesting that the importance of Slc40a1 in OC bone remodeling occurs indirectly through myeliod projenitors. In keeping with this position, Slc40a1-deficient OC precurors (from female mice) have increased intracellular iron accumulation and exhibit accelerated OC formation in vitro but this was not observed in Ctsk-Cre conditional Slc40a1 knockout mice.
In contrast, independent studies by Pereira et al. (2020) recently reported a high bone mass phenotype in 16 week-old female and male LysM-Cre Slc40a1 conditional knockout mice associated with reduced OC formation in vivo. While the precise reasons for these discrepancies warrant further study, it may reflect differences in the age at which mice were phenotyped and the strain of Slc40a1 floxed mice investigated (C57BL/6N-Slc40a1tm1c(EUCOMM)Hmgu/H in the Pereira study compared to 129S-Slc40a1tm2Nca/J by Wang et al., 2018b) (Pereira et al., 2020). Regardless, together these findings highlight a role for Slc40a1 in the regulation of bone structure and strength via direct actions in the macrophage-OC lineage. In keeping with the importance of Slc40a1 in iron handling during OC differentiation, hepcidin, an upstream modulator of Slc40a1 has also been reported to modulate osteoclastogenesis in vitro (Xie et al., 2016).
SLC11A2
Finally, like SLC40A1, the SLC11A2 transporter (also known as DMT1) has been shown to regulate iron uptake (Knutson, 2017). Currently, very little is known about the role of SLC11A2 in OCs. Nonetheless, at steady-state conditions, the expression of SLC11A2 has been shown to be upregulated during OC maturation. Conversly, the expression of SLC11A2 is downregulated in mature OCs following addition of exogenous iron to cell culture media in vitro (Xie et al., 2016).
Therapeutic Potential of Membrane Transport Proteins for Metabolic Bone Diseases
Over the past few decades, the advent of genomic sequencing coupled with genome-wide association studies has rapidly expanded our knowledge of the number of membrane transport proteins underpinning the pathophysiology of various human diseases ranging from cancer to metabolic diseases such as obesity, type 2 diabetes and osteoporosis (reviewed extensively in Lin et al. (2015a), Zhang Y. et al. (2018), Schumann et al. (2020)). Not surprisingly, unraveling the substrates transported and molecular mechanisms regulating transporter activity has been the focus of intense scientific and pharmaceutical inquiry in recent years, in pursuit of lucrative therapeutic targets against these critical yet largely untapped protein superfamilies (ésar-Razquin et al., 2015). In fact, outside of G-protein coupled receptors (GPCRs), the most intensively studied drug targets, membrane transport proteins constitute the largest but most understudied group of potential new drug targets (Schumann et al., 2020). For example, more than 30 transporters of the SLC superfamily have recently been presented as current, prospective, or potential drug targets (Lin et al., 2015b; Schumann et al., 2020; Wang et al., 2020) and spawned strategic partnerships between pharmaceutical industry and academia, the largest conglomerate called RESOLUTE (Research Empowerment on Solute Carriers) (Pizzagalli et al., 2020). In addition, multiple drug classes targeting membrane transporters have already reached the market, with several additional drugs in phase II clinical trials, many aimed at targeting metabolic diseases (Lin et al., 2015b; Zhang Y. et al., 2018; Schumann et al., 2020).
Osteoporosis, defined as low bone mass associated with skeletal fragility and increased bone fracture risk (Lane, 2006), is by far the most prominent of all OC-mediated metabolic bone diseases. Frontline treatments for osteoporosis use remedies aimed at reducing further bone loss termed ‘anti-resorptive’ agents such as bisphosphonates and antibodies directed against the key OC differentiation cytokine RANKL (i.e., Denosumab) (Langdahl, 2020). Alternate treatments that help stimulate new bone formation (anabolic agents) are also available but the approved arsenal is small. These include parathyroid/parathyroid-related peptide hormones (PTH/rP) and the recently approved anti-sclerostin therapy “EVENITY” (Romosozumab-aqqg). While each drug is capable of building new bone, they remain (i) expensive; (ii) require subcutaneous injection (PTH/rP-daily; EVENITY-monthly); (iii) have limited efficacy (PTH/rP ∼18-months; EVENITY 12-doses, switching to standard anti-resorptives thereafter); (iv) carry increased treatment risks (PTH/rP-osteosarcoma; EVENITY-cardiovascular) and thus; (v) are restricted to patients with the most severe osteoporosis. Similarly, despite several anti-resorptive drugs on the market, issues remain concerning patient compliance and rare but unwanted side effects such as osteonecrosis of the jaw or atypical sub-trochanteric femoral fractures with prolonged use (Langdahl, 2020). Moreover, current anti-resorptive agents suppress OC differentiation and survival. This results in arrested bone turnover, as OC help regulate bone remodeling by communicating with osteoblasts and encouraging their recruitment, a process termed ‘coupling’ (Sims and Martin, 2020). Therefore, there remains an unmet need for alternative and better next-generation therapies for which membrane transporter proteins may serve as a promising and tractable drug target.
As detailed above, OCs possess a multitude of membrane transporters, many that are indispensable for OC formation and function. However, despite their obvious importance, to date very few have been targeted therapeutically for OC-mediated diseases. This is partly due to the technical limitations when designing inhibitors against integral membrane proteins. First, membrane transporters are very difficult to express and purify biochemically and thus the majority lack available high-resolution crystal structures required to inform the design of small molecule inhibitors and channel blockers. Second, there remain many membrane transport proteins for which the substrate transported is unknown, i.e., ‘orphans’ making drug targeting difficult. Third, the development and delivery of specific inhibitors that can readily penetrate OC membranes and target to specific intracellular transporters also remains a considerable challenge. This is perhaps best exemplified by drugs targeting the V-ATPase complex. Despite having attracted intense interest by bone researchers and spawned the development of a vast number of pharmacological V-ATPase inhibitors (summarized in Table 2), so far all have lacked targeting specificity to OCs and bone, precluding there clinical use for the treatment of OC-mediated diseases like osteoporosis. Similarly, given the high expression levels of CLCN7 in OCs and its indispensable role in bone homeostasis, CLCN7 has also received considerable attention as a potential therapeutic target for the treatment of osteoporosis. However, thus far pharmacological compounds targeting CLCN7 have been limited to preclinical studies owing to safety concerns regarding their extra-skeletal effects on retinal cells and the central nervous system given that CLCN7 knockout mice also manifest severe retinal and neuronal degeneration (Li et al., 1999). Nonetheless, CLCN7 inhibitors have been trialed successfully in vitro and in ovariectomized rat models and found to attenuate bone resorption while maintaining bone formation rates (Chalhoub et al., 2003; Valenzuela et al., 2013).
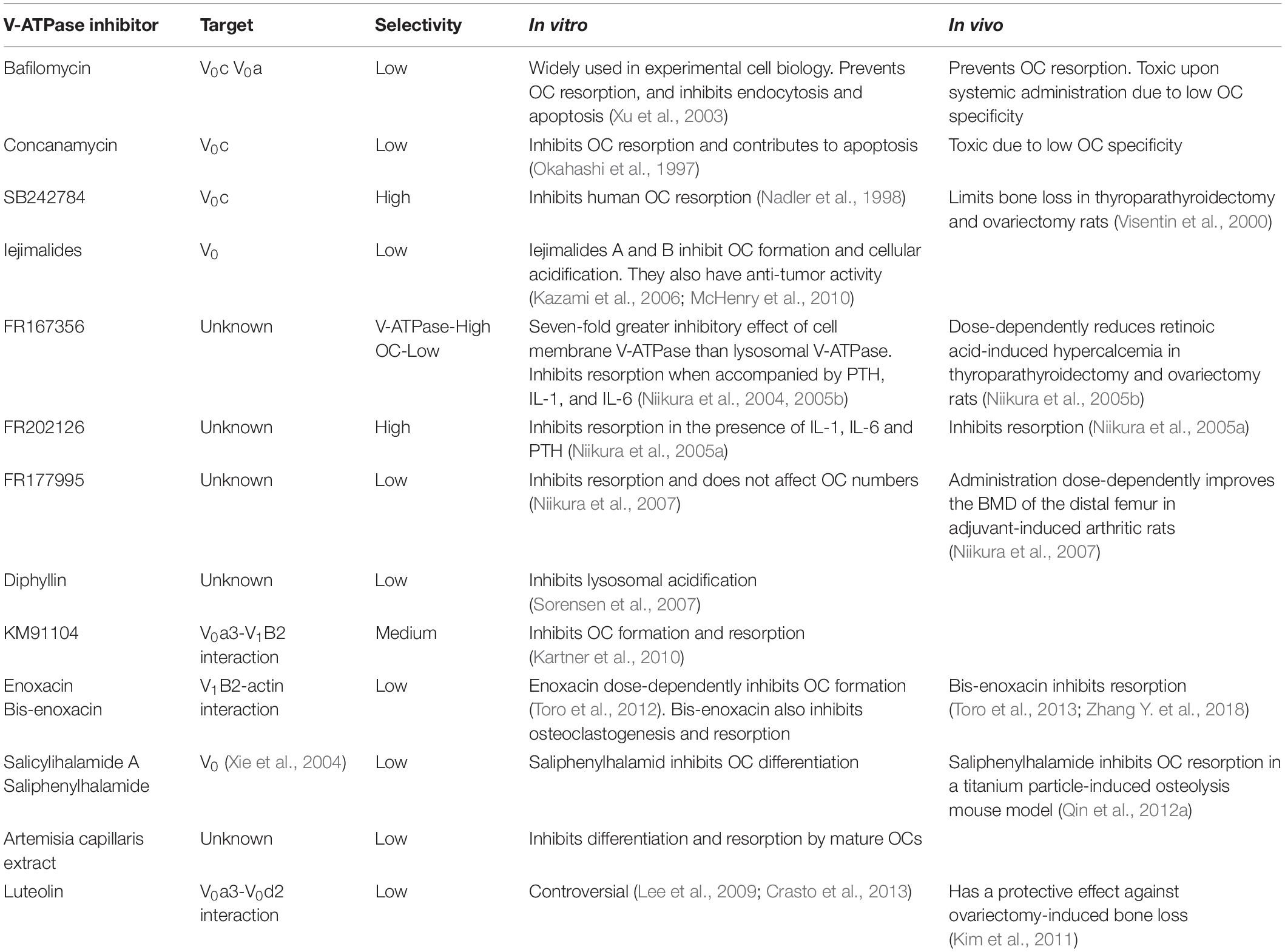
Table 2. V-ATPase inhibitors, targeting selectively and their impact on osteoclasts in vitro and in vivo.
We anticipate therefore that new and enabling technologies, together with increased recognition of membrane transport protein importance, will bring about significant advances in the ability to develop new and selective next-generation therapies targeting specific OC membrane transport proteins that may serve as an alternative to conventional anti-osteoporosis therapies.
Summary and Perspectives
In this review, we have summarized the ‘ins and outs’ of the main families of membrane transport proteins that regulate OC homeostasis. Briefly, the well-established V-ATPase proton pump is responsible for acidification and the formation of a membrane potential at the ruffled border that is neutralized by Cl– transport while CA2 and SLC4 family members offer an internal buffering system for H+ and Cl– levels. Mass release of Ca2+ by the bone is handled by a disproportionately large number of Ca2+ channels compared to only a few known Pi handling proteins. To date, intensive research has focused on understanding the transport of H+, Cl– and Ca2+ across OC membranes, whereas our understanding of other small molecule transporters, including secondary active transporters (e.g., SLCs), remains comparatively underrepresented.
In spite of considerable progress over the past few decades toward the identification and expansion of the OC membrane transport protein inventory, very few transporters have been characterized functionally in OCs. This is, in part, due to several inherent challenges encountered when working with proteins embedded deeply in cellular membranes, which are not readily accessible to standard biochemical methods, together with a giant cell that is notoriously impervious to conventional cell biology manipulations such as transfection. Further, attempts to identify and characterize new membrane transport proteins in OCs have thus far been largely unsystematic, informed instead by research in more advanced polarized systems such as neurons and epithelial cells. It is likely, however, that OCs possess their own unique complement of membrane transport proteins necessary to fulfill their specialized requirement for rapid molecular exchanges during bone resorption. In this regard, an unbiased approach to identify OC-specific membrane transport proteins would therefore be a valuable future line of inquiry. Recent developments in ‘omics’ technologies coupled with genome-wide association studies may prove useful toward this goal.
Author Contributions
AR contributed to the writing, tables, and accompanying figures. NP contributed to the conceptualization and writing and editing of the manuscript. PN contributed to the editing of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported, in part, by funding from the National Health and Medical Research Council of Australia (NHMRC) (APP1143921) to NP. Arthritis Australia to NP and PN and a Faculty of Health and Medical Sciences Research Grant Scheme (SE Ohman Medical Research Fund) to NP and PN. AR is supported by Australian Government Research Training Program Scholarship.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We apologize to colleagues whose work could not be cited owing to space limitations. We thank members of Bone Biology and Disease Laboratory for their enthusiastic input and insightful discussions. In particular, we also thank Laila Abudulai for her valuable feedback.
Abbreviations
OC, osteoclast; V1, V-ATPase ATP-hydrolytic domain; V0, V-ATPase proton-translocation domain; BMD, bone mineral density; ARO, autosomal recessive osteopetrosis.
References
Albano, G., Dolder, S., Siegrist, M., Mercier-Zuber, A., Auberson, M., Stoudmann, C., et al. (2017). Increased bone resorption by osteoclast-specific deletion of the sodium/calcium exchanger isoform 1 (NCX1). Pflugers Arch. 469, 225–233. doi: 10.1007/s00424-016-1923-5
Albano, G., Moor, M., Dolder, S., Siegrist, M., Wagner, C. A., Biber, J., et al. (2015). Sodium-dependent phosphate transporters in osteoclast differentiation and function. PLoS One 10:e0125104. doi: 10.1371/journal.pone.0125104
Alper, S. L. (2006). Molecular physiology of SLC4 anion exchangers. Exp. Physiol. 91, 153–161. doi: 10.1113/expphysiol.2005.031765
Alves, L. A., de Melo Reis, R. A., de Souza, C. A., de Freitas, M. S., Teixeira, P. C., Ferreira, D. N. M., et al. (2014). The P2X7 receptor: shifting from a low- to a high-conductance channel - an enigmatic phenomenon? Biochim. Biophys. Acta 1838, 2578–2587. doi: 10.1016/j.bbamem.2014.05.015
Ambrosio, A. L., Boyle, J. A., and Di Pietro, S. M. (2015). TPC2 mediates new mechanisms of platelet dense granule membrane dynamics through regulation of Ca2+ release. Mol. Biol. Cell 26, 3263–3274. doi: 10.1091/mbc.e15-01-0058
Aoi, W., and Marunaka, Y. (2014). Importance of pH homeostasis in metabolic health and diseases: crucial role of membrane proton transport. BioMed Res. Int. 2014:598986.
Auer-Grumbach, M., Olschewski, A., Papić, L., Kremer, H., McEntagart, M. E., Uhrig, S., et al. (2010). Alterations in the ankyrin domain of TRPV4 cause congenital distal SMA, scapuloperoneal SMA and HMSN2C. Nat. Genet. 42, 160–164. doi: 10.1038/ng.508
Bai, L., Collins, J. F., and Ghishan, F. K. (2000). Cloning and characterization of a type III Na-dependent phosphate cotransporter from mouse intestine. Am. J. Physiol. Cell Physiol. 279, C1135–C1143.
Baldwin, S. A., Yao, S. Y., Hyde, R. J., Ng, A. M., Foppolo, S., Barnes, K., et al. (2005). Functional characterization of novel human and mouse equilibrative nucleoside transporters (hENT3 and mENT3) located in intracellular membranes. J. Biol. Chem. 280, 15880–15887. doi: 10.1074/jbc.m414337200
Baljit, S. M., Sun, L., Jameel, I., Robert, D., Vijai, S. S., Peter, J. R. B., et al. (2002). Ca2+ influx through the osteoclastic plasma membrane ryanodine receptor. Am. J. Physiol. Renal Physiol. 282, F921–F932.
Bargal, R., Avidan, N., Ben-Asher, E., Olender, Z., Zeigler, M., Frumkin, A., et al. (2000). Identification of the gene causing mucolipidosis type IV. Nat. Genet. 26, 118–123. doi: 10.1038/79095
Baron, R., Neff, L., Louvard, D., and Courtoy, P. J. (1985). Cell-mediated extracellular acidification and bone resorption: evidence for a low pH in resorbing lacunae and localization of a 100-kD lysosomal membrane protein at the osteoclast ruffled border. J. Cell Biol. 101, 2210–2222. doi: 10.1083/jcb.101.6.2210
Battaglino, R. A., Pham, L., Morse, L. R., Vokes, M., Sharma, A., Odgren, P. R., et al. (2008). NHA-oc/NHA2: a mitochondrial cation-proton antiporter selectively expressed in osteoclasts. Bone 42, 180–192. doi: 10.1016/j.bone.2007.09.046
Beck, L., Leroy, C., Beck-Cormier, S., Forand, A., Salaün, C., Paris, N., et al. (2010). The phosphate transporter PiT1 (Slc20a1) revealed as a new essential gene for mouse liver development. PLoS One 5:e9148. doi: 10.1371/journal.pone.0009148
Berger, C. E., Rathod, H., Gillespie, J. I., Horrocks, B. R., and Datta, H. K. (2001). Scanning electrochemical microscopy at the surface of bone-resorbing osteoclasts: evidence for steady-state disposal and intracellular functional compartmentalization of calcium. J. Bone Miner. Res. 16, 2092–2102. doi: 10.1359/jbmr.2001.16.11.2092
Boudewyn, L. C., and Walkley, S. U. (2019). Current concepts in the neuropathogenesis of mucolipidosis type IV. J. Neurochem. 148, 669–689. doi: 10.1111/jnc.14462
Bourgine, A., Pilet, P., Diouani, S., Sourice, S., Lesoeur, J., Beck-Cormier, S., et al. (2013). Mice with hypomorphic expression of the sodium-phosphate cotransporter PiT1/Slc20a1 have an unexpected normal bone mineralization. PLoS One 8:e65979. doi: 10.1371/journal.pone.0065979
Bouyer, P., Sakai, H., Itokawa, T., Kawano, T., Fulton, C. M., Boron, W. F., et al. (2007). Colony-stimulating factor-1 increases osteoclast intracellular pH and promotes survival via the electroneutral Na/HCO3 cotransporter NBCn1. Endocrinology 148, 831–840. doi: 10.1210/en.2006-0547
Brailoiu, E., Rahman, T., Churamani, D., Prole, D. L., Brailoiu, G. C., Hooper, R., et al. (2010). An NAADP-gated two-pore channel targeted to the plasma membrane uncouples triggering from amplifying Ca2+ signals. J. Biol. Chem. 285, 38511–38516. doi: 10.1074/jbc.m110.162073
Campeau, P. M., Lu, J. T., Sule, G., Jiang, M. M., Bae, Y., Madan, S., et al. (2012). Whole-exome sequencing identifies mutations in the nucleoside transporter gene SLC29A3 in dysosteosclerosis, a form of osteopetrosis. Hum. Mol. Genet. 21, 4904–4909. doi: 10.1093/hmg/dds326
Cao, B., Dai, X., and Wang, W. (2019). Knockdown of TRPV4 suppresses osteoclast differentiation and osteoporosis by inhibiting autophagy through Ca(2+) -calcineurin-NFATc1 pathway. J. Cell Physiol. 234, 6831–6841. doi: 10.1002/jcp.27432
Cappello, A. R., Curcio, R., Lappano, R., Maggiolini, M., and Dolce, V. (2018). The physiopathological role of the exchangers belonging to the SLC37 family. Front. Chem. 6:122. doi: 10.3389/fchem.2018.00122
Chalhoub, N., Benachenhou, N., Rajapurohitam, V., Pata, M., Ferron, M., Frattini, A., et al. (2003). Grey-lethal mutation induces severe malignant autosomal recessive osteopetrosis in mouse and human. Nat. Med. 9, 399–406. doi: 10.1038/nm842
Chen, F., Ni, B., Yang, Y. O., Ye, T., and Chen, A. (2014). Knockout of TRPV6 causes osteopenia in mice by increasing osteoclastic differentiation and activity. Cell Physiol. Biochem. 33, 796–809. doi: 10.1159/000358653
Chenu, C., Serre, C. M., Raynal, C., Burt-Pichat, B., and Delmas, P. D. (1998). Glutamate receptors are expressed by bone cells and are involved in bone resorption. Bone 22, 295–299. doi: 10.1016/s8756-3282(97)00295-0
Choi, I., Aalkjaer, C., Boulpaep, E. L., and Boron, W. F. (2000). An electroneutral sodium/bicarbonate cotransporter NBCn1 and associated sodium channel. Nature 405, 571–575. doi: 10.1038/35014615
Chou, J. Y., Sik Jun, H., and Mansfield, B. C. (2013). The SLC37 family of phosphate-linked sugar phosphate antiporters. Mol. Aspects Med. 34, 601–611. doi: 10.1016/j.mam.2012.05.010
Concepcion, A. R., Lopez, M., Ardura-Fabregat, A., and Medina, J. F. (2013). Role of AE2 for pHi regulation in biliary epithelial cells. Front. Physiol. 4:413. doi: 10.3389/fphys.2013.00413
Cordat, E., and Casey, J. R. (2008). Bicarbonate transport in cell physiology and disease. Biochem. J. 417, 423–439. doi: 10.1042/bj20081634
Coury, F., Zenger, S., Stewart, A. K., Stephens, S., Neff, L., Tsang, K., et al. (2013). SLC4A2-mediated Cl-/HCO3- exchange activity is essential for calpain-dependent regulation of the actin cytoskeleton in osteoclasts. Proc. Natl. Acad. Sci. U.S.A. 110, 2163–2168. doi: 10.1073/pnas.1206392110
Crasto, G. J., Kartner, N., Yao, Y., Li, K., Bullock, L., Datti, A., et al. (2013). Luteolin inhibition of V-ATPase a3-d2 interaction decreases osteoclast resorptive activity. J. Cell Biochem. 114, 929–941. doi: 10.1002/jcb.24434
Datta, H. K., and Horrocks, B. R. (2003). Mechanisms of calcium disposal from osteoclastic resorption hemivacuole. J. Endocrinol. 176, 1–5. doi: 10.1677/joe.0.1760001
De Paolis, E., Scaglione, G. L., De Bonis, M., Minucci, A., and Capoluongo, E. (2019). CYP24A1 and SLC34A1 genetic defects associated with idiopathic infantile hypercalcemia: from genotype to phenotype. Clin. Chem. Lab. Med. 57, 1650–1667. doi: 10.1515/cclm-2018-1208
Deng, H.-X., Klein, C. J., Yan, J., Shi, Y., Wu, Y., Fecto, F., et al. (2010). Scapuloperoneal spinal muscular atrophy and CMT2C are allelic disorders caused by alterations in TRPV4. Nat. Genet. 42, 165–169. doi: 10.1038/ng.509
Donovan, A., Lima, C. A., Pinkus, J. L., Pinkus, G. S., Zon, L. I., Robine, S., et al. (2005). The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 1, 191–200. doi: 10.1016/j.cmet.2005.01.003
Drake, M. T., Clarke, B. L., and Khosla, S. (2008). Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin. Proc. 83, 1032–1045. doi: 10.4065/83.9.1032
Duan, X., Yang, S., Zhang, L., and Yang, T. (2018). V-ATPases and osteoclasts: ambiguous future of V-ATPases inhibitors in osteoporosis. Theranostics 8, 5379–5399. doi: 10.7150/thno.28391
El Ansari, R., Craze, M. L., Miligy, I., Diez-Rodriguez, M., Nolan, C. C., Ellis, I. O., et al. (2018). The amino acid transporter SLC7A5 confers a poor prognosis in the highly proliferative breast cancer subtypes and is a key therapeutic target in luminal B tumours. Breast Cancer Res. 20:21.
Erkhembaatar, M., Gu, D. R., Lee, S. H., Yang, Y. M., Park, S., Muallem, S., et al. (2017). Lysosomal Ca(2+) signaling is essential for osteoclastogenesis and bone remodeling. J. Bone Miner. Res. 32, 385–396. doi: 10.1002/jbmr.2986
ésar-Razquin, A. C., Snijder, B., Frappier-Brinton, T., Isserlin, R., Gyimesi, G., Bai, X., et al. (2015). A call for systematic research on solute carriers. Cell 162, 478–487. doi: 10.1016/j.cell.2015.07.022
Farooq, M., Moustafa, R. M., Fujimoto, A., Fujikawa, H., Abbas, O., Kibbi, A. G., et al. (2012). Identification of two novel mutations in SLC29A3 encoding an equilibrative nucleoside transporter (hENT3) in two distinct Syrian families with H syndrome: expression studies of SLC29A3 (hENT3) in human skin. Dermatology 224, 277–284. doi: 10.1159/000338886
Feske, S., Gwack, Y., Prakriya, M., Srikanth, S., Puppel, S.-H., Tanasa, B., et al. (2006). A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441, 179–185.
Forgac, M. (2007). Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat. Rev. Mol. Cell Biol. 8, 917–929. doi: 10.1038/nrm2272
Forster, I. C. (2019). The molecular mechanism of SLC34 proteins: insights from two decades of transport assays and structure-function studies. Pflugers Arch. 471, 15–42. doi: 10.1007/s00424-018-2207-z
Forster, I. C., Loo, D. D., and Eskandari, S. (1999). Stoichiometry and Na+ binding cooperativity of rat and flounder renal type II Na+-Pi cotransporters. Am. J. Physiol. 276, F644–F649.
Frattini, A., Orchard, P. J., Sobacchi, C., Giliani, S., Abinun, M., Mattsson, J. P., et al. (2000). Defects in TCIRG1 subunit of the vacuolar proton pump are responsible for a subset of human autosomal recessive osteopetrosis. Nat. Genet. 25, 343–346. doi: 10.1038/77131
Fung, E. B., Harmatz, P. R., Milet, M., Coates, T. D., Thompson, A. A., Ranalli, M., et al. (2008). Fracture prevalence and relationship to endocrinopathy in iron overloaded patients with sickle cell disease and thalassemia. Bone 43, 162–168. doi: 10.1016/j.bone.2008.03.003
Futai, M., Sun-Wada, G. H., Wada, Y., Matsumoto, N., and Nakanishi-Matsui, M. (2019). Vacuolar-type ATPase: a proton pump to lysosomal trafficking. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 95, 261–277. doi: 10.2183/pjab.95.018
Gartland, A., Skarratt, K. K., Hocking, L. J., Parsons, C., Stokes, L., Jørgensen, N. R., et al. (2012). Polymorphisms in the P2X7 receptor gene are associated with low lumbar spine bone mineral density and accelerated bone loss in post-menopausal women. Eur. J. Hum. Genet. 20, 559–564. doi: 10.1038/ejhg.2011.245
Gawenis, L. R., Bradford, E. M., Alper, S. L., Prasad, V., and Shull, G. E. (2010). AE2 Cl-/HCO3- exchanger is required for normal cAMP-stimulated anion secretion in murine proximal colon. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G493–G503.
Gillen, C. M., Brill, S., Payne, J. A., and Forbush, B. III (1996). Molecular cloning and functional expression of the K-Cl cotransporter from rabbit, rat, and human. A new member of the cation-chloride cotransporter family. J. Biol. Chem. 271, 16237–16244. doi: 10.1074/jbc.271.27.16237
Graves, A. R., Curran, P. K., Smith, C. L., and Mindell, J. A. (2008). The Cl-/H+ antiporter ClC-7 is the primary chloride permeation pathway in lysosomes. Nature 453, 788–792. doi: 10.1038/nature06907
Guggenbuhl, P., Deugnier, Y., Boisdet, J. F., Rolland, Y., Perdriger, A., Pawlotsky, Y., et al. (2005). Bone mineral density in men with genetic hemochromatosis and HFE gene mutation. Osteoporos Int. 16, 1809–1814. doi: 10.1007/s00198-005-1934-0
Gupta, A., Guo, X. L., Alvarez, U. M., and Hruska, K. A. (1997). Regulation of sodium-dependent phosphate transport in osteoclasts. J. Clin. Invest. 100, 538–549. doi: 10.1172/jci119563
Gupta, A., Miyauchi, A., Fujimori, A., and Hruska, K. A. (1996). Phosphate transport in osteoclasts: a functional and immunochemical characterization. Kidney Int. 49, 968–974. doi: 10.1038/ki.1996.137
Gupta, A., Tenenhouse, H. S., Hoag, H. M., Wang, D., Khadeer, M. A., Namba, N., et al. (2001). Identification of the type II Na(+)-Pi cotransporter (Npt2) in the osteoclast and the skeletal phenotype of Npt2-/- mice. Bone 29, 467–476. doi: 10.1016/s8756-3282(01)00601-9
Hamdan, F. F., Gauthier, J., Araki, Y., Lin, D.-T., Yoshizawa, Y., Higashi, K., et al. (2011). Excess of de novo deleterious mutations in genes associated with glutamatergic systems in nonsyndromic intellectual disability. Am. J. Hum. Genet. 88, 306–316. doi: 10.1016/j.ajhg.2011.02.001
Haoxing, X., and Dejian, R. (2015). Lysosomal physiology. Annu. Rev. Physiol. 77, 57–80. doi: 10.1146/annurev-physiol-021014-071649
He, L., Vasiliou, K., and Nebert, D. W. (2009). Analysis and update of the human solute carrier (SLC) gene superfamily. Hum. Genomics 3, 195–206. doi: 10.1186/1479-7364-3-2-195
Hebert, S. C., Mount, D. B., and Gamba, G. (2004). Molecular physiology of cation-coupled Cl- cotransport: the SLC12 family. Pflugers Arch. 447, 580–593. doi: 10.1007/s00424-003-1066-3
Hentze, M. W., Muckenthaler, M. U., and Andrews, N. C. (2004). Balancing acts: molecular control of mammalian iron metabolism. Cell 117, 285–297.
Hershey, C. L., and Fisher, D. E. (2004). Mitf and Tfe3: members of a b-HLH-ZIP transcription factor family essential for osteoclast development and function. Bone 34, 689–696. doi: 10.1016/j.bone.2003.08.014
Hilton, M. J., Tu, X., Wu, X., Bai, S., Zhao, H., Kobayashi, T., et al. (2008). Notch signaling maintains bone marrow mesenchymal progenitors by suppressing osteoblast differentiation. Nat. Med. 14, 306–314. doi: 10.1038/nm1716
Hinoi, E., Fujimori, S., Nakamura, Y., and Yoneda, Y. (2001). Group III metabotropic glutamate receptors in rat cultured calvarial osteoblasts. Biochem. Biophys. Res. Commun. 281, 341–346. doi: 10.1006/bbrc.2001.4355
Hoenderop, J. G., van Leeuwen, J. P., van der Eerden, B. C., Kersten, F. F., van der Kemp, A. W., Merillat, A. M., et al. (2003). Renal Ca2+ wasting, hyperabsorption, and reduced bone thickness in mice lacking TRPV5. J. Clin. Invest. 112, 1906–1914. doi: 10.1172/jci200319826
Hofstetter, W., Siegrist, M., Simonin, A., Bonny, O., and Fuster, D. G. (2010). Sodium/hydrogen exchanger NHA2 in osteoclasts: subcellular localization and role in vitro and in vivo. Bone 47, 331–340. doi: 10.1016/j.bone.2010.04.605
Hossain, K. R., Turkewitz, D. R., Holt, S. A., Herson, L., Brown, L. J., Cornell, B. A., et al. (2019). A conserved GXXXG motif in the transmembrane domain of CLIC proteins is essential for their cholesterol-dependant membrane interaction. Biochim. Biophys. Acta Gen. Subj. 8:8.
Howaldt, A., Nampoothiri, S., Quell, L.-M., Ozden, A., Fischer-Zirnsak, B., Collet, C., et al. (2019). Sclerosing bone dysplasias with hallmarks of dysosteosclerosis in four patients carrying mutations in SLC29A3 and TCIRG1. Bone 120, 495–503. doi: 10.1016/j.bone.2018.12.002
Hughes, T. E., Del Rosario, J. S., Kapoor, A., Yazici, A. T., Yudin, Y., and Fluck, E. C. III, et al. (2019). Structure-based characterization of novel TRPV5 inhibitors. Elife 8:e49572.
Hwang, S. Y., and Putney, J. W. (2012). Orai1-mediated calcium entry plays a critical role in osteoclast differentiation and function by regulating activation of the transcription factor NFATc1. FASEB J. 26, 1484–1492. doi: 10.1096/fj.11-194399
Jansen, I. D. C., Mardones, P., Lecanda, F., de Vries, T. J., Recalde, S., Hoeben, K. A., et al. (2009). Ae2a,b-Deficient mice exhibit osteopetrosis of long bones but not of calvaria. FASEB J. 23, 3470–3481. doi: 10.1096/fj.08-122598
Janssens, A., Silvestri, C., Martella, A., Vanoevelen, J. M., Di Marzo, V., and Voets, T. (2018). Delta(9)-tetrahydrocannabivarin impairs epithelial calcium transport through inhibition of TRPV5 and TRPV6. Pharmacol. Res. 136, 83–89. doi: 10.1016/j.phrs.2018.08.021
Jefferies, K. C., Cipriano, D. J., and Forgac, M. (2008). Function, structure and regulation of the vacuolar (H+)-ATPases. Arch. Biochem. Biophys. 476, 33–42. doi: 10.1016/j.abb.2008.03.025
Jensen, N., Schrøder, H. D., Hejbøl, E. K., Füchtbauer, E.-M., de Oliveira, J. R. M., and Pedersen, L. (2013). Loss of function of Slc20a2 associated with familial idiopathic basal ganglia calcification in humans causes brain calcifications in mice. J. Mol. Neurosci. 51, 994–999. doi: 10.1007/s12031-013-0085-6
Jørgensen, N. R., Husted, L. B., Skarratt, K. K., Stokes, L., Tofteng, C. L., Kvist, T., et al. (2012). Single-nucleotide polymorphisms in the P2X7 receptor gene are associated with post-menopausal bone loss and vertebral fractures. Eur. J. Hum. Genet. 20, 675–681. doi: 10.1038/ejhg.2011.253
Ka, C., Guellec, J., Pepermans, X., Kannengiesser, C., Ged, C., Wuyts, W., et al. (2018). The SLC40A1 R178Q mutation is a recurrent cause of hemochromatosis and is associated with a novel pathogenic mechanism. Haematologica 103, 1796–1805. doi: 10.3324/haematol.2018.189845
Kajiya, H., Okamoto, F., Li, J.-P., Nakao, A., and Okabe, K. (2006). Expression of mouse osteoclast K-Cl Co-transporter-1 and its role during bone resorption. J. Bone Mineral Res. 21, 984–992. doi: 10.1359/jbmr.060407
Kanatani, M., Sugimoto, T., Kano, J., Kanzawa, M., and Chihara, K. (2003). Effect of high phosphate concentration on osteoclast differentiation as well as bone-resorbing activity. J. Cell. Physiol. 196, 180–189. doi: 10.1002/jcp.10270
Kandasamy, P., Gyimesi, G., Kanai, Y., and Hediger, M. A. (2018). Amino acid transporters revisited: new views in health and disease. Trends Biochem. Sci. 43, 752–789. doi: 10.1016/j.tibs.2018.05.003
Kartner, N., Yao, Y., Li, K., Crasto, G. J., Datti, A., and Manolson, M. F. (2010). Inhibition of osteoclast bone resorption by disrupting vacuolar H+-ATPase a3-B2 subunit interaction. J. Biol. Chem. 285, 37476–37490. doi: 10.1074/jbc.m110.123281
Kasper, D., Planells-Cases, R., Fuhrmann, J. C., Scheel, O., Zeitz, O., Ruether, K., et al. (2005). Loss of the chloride channel ClC-7 leads to lysosomal storage disease and neurodegeneration. EMBO J. 24, 1079–1091. doi: 10.1038/sj.emboj.7600576
Kavanaugh, M. P., and Kabat, D. (1996). Identification and characterization of a widely expressed phosphate transporter/retrovirus receptor family. Kidney Int. 49, 959–963. doi: 10.1038/ki.1996.135
Kazami, S., Muroi, M., Kawatani, M., Kubota, T., Usui, T., Kobayashi, J., et al. (2006). Iejimalides show anti-osteoclast activity via V-ATPase inhibition. Biosci. Biotechnol. Biochem. 70, 1364–1370. doi: 10.1271/bbb.50644
Ke, H. Z., Qi, H., Weidema, A. F., Zhang, Q., Panupinthu, N., Crawford, D. T., et al. (2003). Deletion of the P2X7 nucleotide receptor reveals its regulatory roles in bone formation and resorption. Mol. Endocrinol. 17, 1356–1367. doi: 10.1210/me.2003-0021
Keeton, T. P., Burk, S. E., and Shull, G. E. (1993). Alternative splicing of exons encoding the calmodulin-binding domains and C termini of plasma membrane Ca (2+)-ATPase isoforms 1, 2, 3, and 4. J. Biol. Chem. 268, 2740–2748. doi: 10.1016/s0021-9258(18)53836-9
Khadeer, M. A., Tang, Z., Tenenhouse, H. S., Eiden, M. V., Murer, H., Hernando, N., et al. (2003). Na+-dependent phosphate transporters in the murine osteoclast: cellular distribution and protein interactions. Am. J. Physiol. Cell Physiol. 284, C1633–C1644.
Kim, H. J., Prasad, V., Hyung, S. W., Lee, Z. H., Lee, S. W., Bhargava, A., et al. (2012). Plasma membrane calcium ATPase regulates bone mass by fine-tuning osteoclast differentiation and survival. J. Cell Biol. 199, 1145–1158. doi: 10.1083/jcb.201204067
Kim, T. H., Jung, J. W., Ha, B. G., Hong, J. M., Park, E. K., Kim, H. J., et al. (2011). The effects of luteolin on osteoclast differentiation, function in vitro and ovariectomy-induced bone loss. J. Nutr. Biochem. 22, 8–15. doi: 10.1016/j.jnutbio.2009.11.002
Knutson, M. D. (2017). Iron transport proteins: gateways of cellular and systemic iron homeostasis. J. Biol. Chem. 292, 12735–12743. doi: 10.1074/jbc.r117.786632
Kornak, U., Kasper, D., Bösl, M. R., Kaiser, E., Schweizer, M., Schulz, A., et al. (2001). Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell 104, 205–215. doi: 10.1016/s0092-8674(01)00206-9
Kuno, M. (2018). Cooperative electrogenic proton transport pathways in the plasma membrane of the proton-secreting osteoclast. Pflügers Archiv. Eur. J. Physiol. 470, 851–866. doi: 10.1007/s00424-018-2137-9
Landouré, G., Zdebik, A. A., Martinez, T. L., Burnett, B. G., Stanescu, H. C., Inada, H., et al. (2010). Mutations in TRPV4 cause charcot-marie-tooth disease type 2C. Nat. Genet. 42, 170–174.
Landowski, C. P., Bolanz, K. A., Suzuki, Y., and Hediger, M. A. (2011). Chemical inhibitors of the calcium entry channel TRPV6. Pharm. Res. 28, 322–330. doi: 10.1007/s11095-010-0249-9
Lane, N. E. (2006). Epidemiology, etiology, and diagnosis of osteoporosis. Am. J. Obstet. Gynecol. 194, S3–S11.
Langdahl, B. L. (2020). Overview of treatment approaches to osteoporosis. Br. J. Pharmacol. doi: 10.1111/bph.15024 [Epub ahead of print].
Lange, P. F., Wartosch, L., Jentsch, T. J., and Fuhrmann, J. C. (2006). ClC-7 requires Ostm1 as a β-subunit to support bone resorption and lysosomal function. Nature 440, 220–223. doi: 10.1038/nature04535
Larrouture, Q. C., Nelson, D. J., Robinson, L. J., Liu, L., Tourkova, I., Schlesinger, P. H., et al. (2015). Chloride–hydrogen antiporters ClC-3 and ClC-5 drive osteoblast mineralization and regulate fine-structure bone patterning in vitro. Physiol. Rep. 3:e12607. doi: 10.14814/phy2.12607
Lee, J. W., Ahn, J. Y., Hasegawa, S., Cha, B. Y., Yonezawa, T., Nagai, K., et al. (2009). Inhibitory effect of luteolin on osteoclast differentiation and function. Cytotechnology 61, 125–134. doi: 10.1007/s10616-010-9253-5
Lee, S., Axelsen, T. V., Andersen, A. P., Vahl, P., Pedersen, S. F., and Boedtkjer, E. (2016). Disrupting Na+, HCO3–-cotransporter NBCn1 (Slc4a7) delays murine breast cancer development. Oncogene 35, 2112–2122. doi: 10.1038/onc.2015.273
Lee, S. H., Kim, T., Park, E. S., Yang, S., Jeong, D., Choi, Y., et al. (2008). NHE10, an osteoclast-specific member of the Na+/H+ exchanger family, regulates osteoclast differentiation and survival [corrected]. Biochem. Biophys. Res. Commun. 369, 320–326. doi: 10.1016/j.bbrc.2008.01.168
Lemke, J. R., Geider, K., Helbig, K. L., Heyne, H. O., Schütz, H., Hentschel, J., et al. (2016). Delineating the GRIN1 phenotypic spectrum. a distinct genetic NMDA receptor encephalopathy. Neurology 86, 2171–2178. doi: 10.1212/wnl.0000000000002740
Li, J. P., Kajiya, H., Okamoto, F., Nakao, A., Iwamoto, T., and Okabe, K. (2007). Three Na+/Ca2+ exchanger (NCX) variants are expressed in mouse osteoclasts and mediate calcium transport during bone resorption. Endocrinology 148, 2116–2125. doi: 10.1210/en.2006-1321
Li, Y. P., Chen, W., Liang, Y., Li, E., and Stashenko, P. (1999). Atp6i-deficient mice exhibit severe osteopetrosis due to loss of osteoclast-mediated extracellular acidification. Nat. Genet. 23, 447–451. doi: 10.1038/70563
Lin, L., Yee, S. W., Kim, R. B., and Giacomini, K. M. (2015a). SLC transporters as therapeutic targets: emerging opportunities. Nat. Rev. Drug Discov. 14, 543–560. doi: 10.1038/nrd4626
Lin, L., Yee, S. W., Kim, R. B., and Giacomini, K. M. (2015b). SLC transporters as therapeutic targets: emerging opportunities. Nat. Rev. Drug Discov. 14, 543–560.
Liu, S., Chang, S., Han, B., Xu, L., Zhang, M., Zhao, C., et al. (2019). Cryo-EM structures of the human cation-chloride cotransporter KCC1. Science 366, 505–508. doi: 10.1126/science.aay3129
Liu, Y., Yang, J., and Chen, L. M. (2015). Structure and function of SLC4 family [Formula: see text] transporters. Front. Physiol. 6:355. doi: 10.3389/fphys.2015.00355
Loiselle, F. B., Morgan, P. E., Alvarez, B. V., and Casey, J. R. (2004). Regulation of the human NBC3 Na+/HCO3- cotransporter by carbonic anhydrase II and PKA. Am. J. Physiol. Cell Physiol. 286, C1423–C1433.
Lunz, V., Romanin, C., and Frischauf, I. (2019). STIM1 activation of Orai1. Cell Calcium 77, 29–38. doi: 10.1016/j.ceca.2018.11.009
Lyaruu, D. M., Bronckers, A. L., Mulder, L., Mardones, P., Medina, J. F., Kellokumpu, S., et al. (2008). The anion exchanger Ae2 is required for enamel maturation in mouse teeth. Matrix Biol. 27, 119–127. doi: 10.1016/j.matbio.2007.09.006
Magen, D., Berger, L., Coady, M. J., Ilivitzki, A., Militianu, D., Tieder, M., et al. (2010). A loss-of-function mutation in NaPi-IIa and renal fanconi’s syndrome. N. Engl. J. Med. 362, 1102–1109. doi: 10.1056/nejmoa0905647
Manolson, M. F., Yu, H., Chen, W., Yao, Y., Li, K., Lees, R. L., et al. (2003). The a3 isoform of the 100-kDa V-ATPase subunit is highly but differentially expressed in large (= 10 nuclei) and small (= 5 nuclei) osteoclasts. J. Biol. Chem. 278, 49271–49278. doi: 10.1074/jbc.m309914200
Mason, D. J. (2004). Glutamate signalling and its potential application to tissue engineering of bone. Eur. Cell. Mater. 7, 12–25;discussion25–6.
Matsumoto, N., Sekiya, M., Tohyama, K., Ishiyama-Matsuura, E., Sun-Wada, G. H., Wada, Y., et al. (2018). Essential role of the a3 Isoform of V-ATPase in secretory lysosome trafficking via rab7 recruitment. Sci. Rep. 8:6701.
McHenry, P., Wang, W. L., Devitt, E., Kluesner, N., Davisson, V. J., McKee, E., et al. (2010). Iejimalides A and B inhibit lysosomal vacuolar H+-ATPase (V-ATPase) activity and induce S-phase arrest and apoptosis in MCF-7 cells. J. Cell Biochem. 109, 634–642.
Meadows, N. A., Sharma, S. M., Faulkner, G. J., Ostrowski, M. C., Hume, D. A., and Cassady, A. I. (2007). The expression of Clcn7 and Ostm1 in osteoclasts is coregulated by microphthalmia transcription factor. J. Biol. Chem. 282, 1891–1904. doi: 10.1074/jbc.m608572200
Medina, J. F., Recalde, S., Prieto, J., Lecanda, J., Saez, E., Funk, C. D., et al. (2003). Anion exchanger 2 is essential for spermiogenesis in mice. Proc. Natl. Acad. Sci. U.S.A. 100, 15847–15852. doi: 10.1073/pnas.2536127100
Merle, B., Itzstein, C., Delmas, P. D., and Chenu, C. (2003). NMDA glutamate receptors are expressed by osteoclast precursors and involved in the regulation of osteoclastogenesis. J. Cell. Biochem. 90, 424–436. doi: 10.1002/jcb.10625
Meyers, S. N., McDaneld, T. G., Swist, S. L., Marron, B. M., Steffen, D. J., O’Toole, D., et al. (2010). A deletion mutation in bovine SLC4A2 is associated with osteopetrosis in Red Angus cattle. BMC Genomics 11:337. doi: 10.1186/1471-2164-11-337
Mikiko, I., Naoko, M., Michiyo, I., Sakiko, H., Yuko, S., Rie, N., et al. (2005). Characterization of inorganic phosphate transport in osteoclast-like cells. Am. J. Physiol. Cell Physiol. 288, C921–C931.
Milkereit, R., Persaud, A., Vanoaica, L., Guetg, A., Verrey, F., and Rotin, D. (2015). LAPTM4b recruits the LAT1-4F2hc Leu transporter to lysosomes and promotes mTORC1 activation. Nat. Commun. 6:7250.
Montosi, G., Donovan, A., Totaro, A., Garuti, C., Pignatti, E., Cassanelli, S., et al. (2001). Autosomal-dominant hemochrom-atosis is associated with a mutation in the ferroportin (SLC11A3) gene. J. Clin. Invest. 108, 619–623. doi: 10.1172/jci200113468
Moonga, B. S., Davidson, R., Sun, L., Adebanjo, O. A., Moser, J., Abedin, M., et al. (2001). Identification and characterization of a sodium/calcium exchanger, NCX-1, in osteoclasts and its role in bone resorption. Biochem. Biophys. Res. Commun. 283, 770–775. doi: 10.1006/bbrc.2001.4870
Morgan, N. V., Morris, M. R., Cangul, H., Gleeson, D., Straatman-Iwanowska, A., Davies, N., et al. (2010). Mutations in SLC29A3, encoding an equilibrative nucleoside transporter ENT3, cause a familial histiocytosis syndrome (Faisalabad histiocytosis) and familial Rosai-Dorfman disease. PLoS Genet. 6:e1000833. doi: 10.1371/journal.pgen.1000833
Morimoto, R., Uehara, S., Yatsushiro, S., Juge, N., Hua, Z., Senoh, S., et al. (2006). Secretion of L-glutamate from osteoclasts through transcytosis. Embo J. 25, 4175–4186. doi: 10.1038/sj.emboj.7601317
Nadler, G., Morvan, M., Delimoge, I., Belfiore, P., Zocchetti, A., James, I., et al. (1998). (2Z,4E)-5-(5,6-dichloro-2-indolyl)-2-methoxy-N-(1,2,2,6,6- pentamethylpiperidin-4-yl)-2,4-pentadienamide, a novel, potent and selective inhibitor of the osteoclast V-ATPase. Bioorg. Med. Chem. Lett. 8, 3621–3626. doi: 10.1016/s0960-894x(98)00660-x
Naemsch, L. N., Dixon, S. J., and Sims, S. M. (2001). Activity-dependent development of P2X7 current and Ca2+ entry in rabbit osteoclasts. J. Biol. Chem. 276, 39107–39114. doi: 10.1074/jbc.m105881200
Napolitano, L., Scalise, M., Galluccio, M., Pochini, L., Albanese, L. M., and Indiveri, C. (2015). LAT1 is the transport competent unit of the LAT1/CD98 heterodimeric amino acid transporter. Int. J. Biochem. Cell Biol. 67, 25–33. doi: 10.1016/j.biocel.2015.08.004
Nesbitt, S. A., and Horton, M. A. (1997). Trafficking of matrix collagens through bone-resorbing osteoclasts. Science 276, 266–269. doi: 10.1126/science.276.5310.266
Nesin, V., Wiley, G., Kousi, M., Ong, E. C., Lehmann, T., Nicholl, D. J., et al. (2014). Activating mutations in STIM1 and ORAI1 cause overlapping syndromes of tubular myopathy and congenital miosis. Proc. Nat. Acad. Sci. U.S.A. 111, 4197–4202. doi: 10.1073/pnas.1312520111
Ng, P. Y., Ribet, A. B. P., and Pavlos, N. J. (2019). Membrane trafficking in osteoclasts and implications for osteoporosis. Biochem. Soc. Trans. 47, 639–650. doi: 10.1042/bst20180445
Nicoli, E. R., Weston, M. R., Hackbarth, M., Becerril, A., Larson, A., Zein, W. M., et al. (2019). Lysosomal storage and albinism due to effects of a de novo CLCN7 variant on lysosomal acidification. Am. J. Hum. Genet. 104, 1127–1138.
Niikura, K., Nakajima, S., Takano, M., and Yamazaki, H. (2007). FR177995, a novel vacuolar ATPase inhibitor, exerts not only an inhibitory effect on bone destruction but also anti-immunoinflammatory effects in adjuvant-induced arthritic rats. Bone 40, 888–894. doi: 10.1016/j.bone.2006.10.015
Niikura, K., Takano, M., and Sawada, M. (2004). A novel inhibitor of vacuolar ATPase, FR167356, which can discriminate between osteoclast vacuolar ATPase and lysosomal vacuolar ATPase. Br. J. Pharmacol. 142, 558–566. doi: 10.1038/sj.bjp.0705812
Niikura, K., Takeshita, N., and Chida, N. (2005a). A novel inhibitor of vacuolar ATPase, FR202126, prevents alveolar bone destruction in experimental periodontitis in rats. J. Toxicol. Sci. 30, 297–304. doi: 10.2131/jts.30.297
Niikura, K., Takeshita, N., and Takano, M. (2005b). A vacuolar ATPase inhibitor, FR167356, prevents bone resorption in ovariectomized rats with high potency and specificity: potential for clinical application. J. Bone Miner. Res. 20, 1579–1588. doi: 10.1359/jbmr.050517
Nijenhuis, T., van der Eerden, B. C., Hoenderop, J. G., Weinans, H., van Leeuwen, J. P., and Bindels, R. J. (2008). Bone resorption inhibitor alendronate normalizes the reduced bone thickness of TRPV5(-/-) mice. J. Bone Miner. Res. 23, 1815–1824. doi: 10.1359/jbmr.080613
Njajou, O. T., Vaessen, N., Joosse, M., Berghuis, B., van Dongen, J. W. F., Breuning, M. H., et al. (2001). A mutation in SLC11A3 is associated with autosomal dominant hemochromatosis. Nat. Genet. 28, 213–214. doi: 10.1038/90038
Notomi, T., Ezura, Y., and Noda, M. (2012). Identification of two-pore channel 2 as a novel regulator of osteoclastogenesis. J. Biol. Chem. 287, 35057–35064. doi: 10.1074/jbc.m111.328930
Notomi, T., Kuno, M., Hiyama, A., Nozaki, T., Ohura, K., Ezura, Y., et al. (2017). Role of lysosomal channel protein TPC2 in osteoclast differentiation and bone remodeling under normal and low-magnesium conditions. J. Biol. Chem. 292, 20998–21010. doi: 10.1074/jbc.m117.780072
Ohlendorff, S. D., Tofteng, C. L., Jensen, J. E., Petersen, S., Civitelli, R., Fenger, M., et al. (2007). Single nucleotide polymorphisms in the P2X7 gene are associated to fracture risk and to effect of estrogen treatment. Pharm. Genomics 17, 555–567. doi: 10.1097/fpc.0b013e3280951625
Okahashi, N., Nakamura, I., Jimi, E., Koide, M., Suda, T., and Nishihara, T. (1997). Specific inhibitors of vacuolar H(+)-ATPase trigger apoptotic cell death of osteoclasts. J. Bone Miner. Res. 12, 1116–1123. doi: 10.1359/jbmr.1997.12.7.1116
Okamoto, F., Kajiya, H., Toh, K., Uchida, S., Yoshikawa, M., Sasaki, S., et al. (2008). Intracellular ClC-3 chloride channels promote bone resorption in vitro through organelle acidification in mouse osteoclasts. Am. J. Physiol. Cell Physiol. 294, C693–C701.
Okunade, G. W., Miller, M. L., Pyne, G. J., Sutliff, R. L., O’Connor, K. T., Neumann, J. C., et al. (2004). Targeted ablation of plasma membrane Ca2+-ATPase (PMCA) 1 and 4 indicates a major housekeeping function for PMCA1 and a critical role in hyperactivated sperm motility and male fertility for PMCA4. J. Biol. Chem. 279, 33742–33750. doi: 10.1074/jbc.m404628200
Olesen, C. W., Vogensen, J., Axholm, I., Severin, M., Schnipper, J., Pedersen, I. S., et al. (2018). Trafficking, localization and degradation of the Na(+),HCO3(-) co-transporter NBCn1 in kidney and breast epithelial cells. Sci. Rep. 8:7435.
Olga, A., Anne-Kristine, M., Chiara, G., Heini, M., and Ian, C. F. (2012). Lithium interactions with Na+-coupled inorganic phosphate cotransporters: insights into the mechanism of sequential cation binding. Am. J. Physiol. Cell Physiol. 302, C539–C554.
Ozaki, K., Yamada, T., Horie, T., Ishizaki, A., Hiraiwa, M., Iezaki, T., et al. (2019). The L-type amino acid transporter LAT1 inhibits osteoclastogenesis and maintains bone homeostasis through the mTORC1 pathway. Sci. Signal. 12:eaaw3921. doi: 10.1126/scisignal.aaw3921
Palsson, R., Indridason, O. S., Edvardsson, V. O., and Oddsson, A. (2019). Genetics of common complex kidney stone disease: insights from genome-wide association studies. Urolithiasis 47, 11–21. doi: 10.1007/s00240-018-1094-2
Pan, C.-J., Chen, S.-Y., Jun, H. S., Lin, S. R., Mansfield, B. C., and Chou, J. Y. (2011). SLC37A1 and SLC37A2 are phosphate-linked, glucose-6-phosphate antiporters. PLoS One 6:e23157. doi: 10.1371/journal.pone.0023157
Parks, S. K., Chiche, J., and Pouysségur, J. (2013). Disrupting proton dynamics and energy metabolism for cancer therapy. Nat. Rev. Cancer 13, 611–623. doi: 10.1038/nrc3579
Pastor-Anglada, M., and érez-Torras, S. P. (2018). Who is who in adenosine transport. Front. Pharmacol. 9:627. doi: 10.3389/fphar.2018.00627
Peet, N. M., Grabowski, P. S., Laketić-Ljubojević, I. R. A., and Skerry, T. M. (1999). The glutamate receptor antagonist MK801 modulates bone resorption in vitro by a mechanism predominantly involving osteoclast differentiation. FASEB J. 13, 2179–2185. doi: 10.1096/fasebj.13.15.2179
Pellegatti, P., Falzoni, S., Donvito, G., Lemaire, I., and Di Virgilio, F. (2011). P2X7 receptor drives osteoclast fusion by increasing the extracellular adenosine concentration. FASEB J. 25, 1264–1274. doi: 10.1096/fj.10-169854
Pereira, M., Ko, J. H., Logan, J., Protheroe, H., Kim, K. B., Tan, A. L. M., et al. (2020). A trans-eQTL network regulates osteoclast multinucleation and bone mass. Elife 19:55549.
Pham, L., Purcell, P., Morse, L., Stashenko, P., and Battaglino, R. A. (2007). Expression analysis of nha-oc/NHA2: a novel gene selectively expressed in osteoclasts. Gene Expr. Patterns 7, 846–851. doi: 10.1016/j.modgep.2007.07.002
Pizzagalli, M. D., Bensimon, A., and Superti-Furga, G. (2020). A guide to plasma membrane solute carrier proteins. FEBS J.
Prié, D., Huart, V., Bakouh, N., Planelles, G., Dellis, O., Gérard, B., et al. (2002). Nephrolithiasis and osteoporosis associated with hypophosphatemia caused by mutations in the type 2a sodium–phosphate cotransporter. N. Engl. J. Med. 347, 983–991. doi: 10.1056/nejmoa020028
Pushkin, A., Abuladze, N., Lee, I., Newman, D., Hwang, J., and Kurtz, I. (1999). Cloning, tissue distribution, genomic organization, and functional characterization of NBC3, a new member of the sodium bicarbonate cotransporter family. J. Biol. Chem. 274, 16569–16575. doi: 10.1074/jbc.274.23.16569
Pushkin, A., Abuladze, N., Newman, D., Muronets, V., Sassani, P., Tatishchev, S., et al. (2003). The COOH termini of NBC3 and the 56-kDa H+-ATPase subunit are PDZ motifs involved in their interaction. Am. J. Physiol. Cell Physiol. 284, C667–C673.
Qin, A., Cheng, T. S., Lin, Z., Cao, L., Chim, S. M., Pavlos, N. J., et al. (2012a). Prevention of wear particle-induced osteolysis by a novel V-ATPase inhibitor saliphenylhalamide through inhibition of osteoclast bone resorption. PLoS One 7:e34132. doi: 10.1371/journal.pone.0034132
Qin, A., Cheng, T. S., Pavlos, N. J., Lin, Z., Dai, K. R., and Zheng, M. H. (2012b). V-ATPases in osteoclasts: structure, function and potential inhibitors of bone resorption. Int. J. Biochem. Cell Biol. 44, 1422–1435. doi: 10.1016/j.biocel.2012.05.014
Qiu, M. R., Jiang, L., Matthaei, K. I., Schoenwaelder, S. M., Kuffner, T., Mangin, P., et al. (2010). Generation and characterization of mice with null mutation of the chloride intracellular channel 1 gene. Genesis 48, 127–136.
Riihonen, R., Nielsen, S., Vaananen, H. K., Laitala-Leinonen, T., and Kwon, T. H. (2010). Degradation of hydroxyapatite in vivo and in vitro requires osteoclastic sodium-bicarbonate co-transporter NBCn1. Matrix Biol. 29, 287–294. doi: 10.1016/j.matbio.2010.01.003
Robinson, L. J., Blair, H. C., Barnett, J. B., Zaidi, M., and Huang, C. L. (2010). Regulation of bone turnover by calcium-regulated calcium channels. Ann. N. Y. Acad. Sci. 1192, 351–357. doi: 10.1111/j.1749-6632.2009.05219.x
Robinson, L. J., Mancarella, S., Songsawad, D., Tourkova, I. L., Barnett, J. B., Gill, D. L., et al. (2012). Gene disruption of the calcium channel Orai1 results in inhibition of osteoclast and osteoblast differentiation and impairs skeletal development. Lab. Invest. 92, 1071–1083. doi: 10.1038/labinvest.2012.72
Rossmann, H., Alper, S. L., Nader, M., Wang, Z., Gregor, M., and Seidler, U. (2000). Three 5′-variant mRNAs of anion exchanger AE2 in stomach and intestine of mouse, rabbit, and rat. Ann. N. Y. Acad. Sci. 915, 81–91. doi: 10.1111/j.1749-6632.2000.tb05226.x
Roy, M., and Roux, S. (2018). Rab GTPases in osteoclastic endomembrane systems. BioMed Res. Int. 2018:4541538.
Salo, J., Lehenkari, P., Mulari, M., Metsikkö, K., and Väänänen, H. K. (1997). Removal of osteoclast bone resorption products by transcytosis. Science 276, 270–273. doi: 10.1126/science.276.5310.270
Sardiello, M., Palmieri, M., di Ronza, A., Medina, D. L., Valenza, M., Gennarino, V. A., et al. (2009). A gene network regulating lysosomal biogenesis and function. Science 325, 473–477. doi: 10.1126/science.1174447
Schaller, S., Henriksen, K., Sveigaard, C., Heegaard, A. M., Helix, N., Stahlhut, M., et al. (2004). The chloride channel inhibitor NS3736 [corrected] prevents bone resorption in ovariectomized rats without changing bone formation. J. Bone Miner. Res. 19, 1144–1153. doi: 10.1359/jbmr.040302
Schieder, M., Rötzer, K., Brüggemann, A., Biel, M., and Wahl-Schott, C. A. (2010). Characterization of two-pore channel 2 (TPCN2)-mediated Ca2+ currents in isolated lysosomes. J. Biol. Chem. 285, 21219–21222. doi: 10.1074/jbc.c110.143123
Schlessinger, A., Yee, S. W., Sali, A., and Giacomini, K. M. (2013). SLC classification: an update. Clin. Pharmacol. Ther. 94, 19–23. doi: 10.1038/clpt.2013.73
Schlingmann, K. P., Kaufmann, M., Weber, S., Irwin, A., Goos, C., John, U., et al. (2011). Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N. Engl. J. Med. 365, 410–421.
Schlingmann, K. P., Ruminska, J., Kaufmann, M., Dursun, I., Patti, M., Kranz, B., et al. (2016). Autosomal-recessive mutations in SLC34A1 encoding sodium-phosphate cotransporter 2A cause idiopathic infantile hypercalcemia. J. Am. Soc. Nephrol. 27, 604–614. doi: 10.1681/asn.2014101025
Schrecker, M., Korobenko, J., and Hite, R. K. (2020). Cryo-EM structure of the lysosomal chloride-proton exchanger CLC-7 in complex with OSTM1. Elife 9:e59555.
Schuh, K., Cartwright, E. J., Jankevics, E., Bundschu, K., Liebermann, J., Williams, J. C., et al. (2004). Plasma membrane Ca2+ ATPase 4 is required for sperm motility and male fertility. J. Biol. Chem. 279, 28220–28226. doi: 10.1074/jbc.m312599200
Schulz, P., Werner, J., Stauber, T., Henriksen, K., and Fendler, K. (2010). The G215R mutation in the Cl-/H+-antiporter ClC-7 found in ADO II osteopetrosis does not abolish function but causes a severe trafficking defect. PLoS One 5:e12585. doi: 10.1371/journal.pone.0012585
Schumann, T., König, J., Henke, C., Willmes, D. M., Bornstein, S. R., Jordan, J., et al. (2020). Solute carrier transporters as potential targets for the treatment of metabolic disease. Pharmacol. Rev. 72, 343–379. doi: 10.1124/pr.118.015735
Scotto Rosato, A., Montefusco, S., Soldati, C., Di Paola, S., Capuozzo, A., Monfregola, J., et al. (2019). TRPML1 links lysosomal calcium to autophagosome biogenesis through the activation of the CaMKKβ/VPS34 pathway. Nat. Commun. 10:5630.
Serre, C. M., Farlay, D., Delmas, P. D., and Chenu, C. (1999). Evidence for a dense and intimate innervation of the bone tissue, including glutamate-containing fibers. Bone 25, 623–629. doi: 10.1016/s8756-3282(99)00215-x
Silver, I. A., Murrills, R. J., and Etherington, D. J. (1988). Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp. Cell Res. 175, 266–276. doi: 10.1016/0014-4827(88)90191-7
Sims, N. A., and Martin, T. J. (2020). Osteoclasts provide coupling signals to osteoblast lineage cells through multiple mechanisms. Annu. Rev. Physiol. 82, 507–529. doi: 10.1146/annurev-physiol-021119-034425
Skerry, T. M., and Taylor, A. F. (2001). Glutamate signalling in bone. Curr. Pharm. Des. 7, 737–750. doi: 10.2174/1381612013397771
Sobacchi, C., Schulz, A., Coxon, F. P., Villa, A., and Helfrich, M. H. (2013). Osteopetrosis: genetics, treatment and new insights into osteoclast function. Nat. Rev. Endocrinol. 9, 522–536. doi: 10.1038/nrendo.2013.137
Sorensen, M. G., Henriksen, K., Neutzsky-Wulff, A. V., Dziegiel, M. H., and Karsdal, M. A. (2007). Diphyllin, a novel and naturally potent V-ATPase inhibitor, abrogates acidification of the osteoclastic resorption lacunae and bone resorption. J. Bone Miner. Res. 22, 1640–1648. doi: 10.1359/jbmr.070613
Spencer, G. J., McGrath, C. J., and Genever, P. G. (2007). Current perspectives on NMDA-type glutamate signalling in bone. Int. J. Biochem. Cell Biol. 39, 1089–1104. doi: 10.1016/j.biocel.2006.11.002
Stauber, T., and Jentsch, T. J. (2010). Sorting motifs of the endosomal/lysosomal CLC chloride transporters. J. Biol. Chem. 285, 34537–34548. doi: 10.1074/jbc.m110.162545
Sulem, P., Gudbjartsson, D. F., Stacey, S. N., Helgason, A., Rafnar, T., Jakobsdottir, M., et al. (2008). Two newly identified genetic determinants of pigmentation in Europeans. Nat. Genet. 40, 835–837. doi: 10.1038/ng.160
Sun-Wada, G., Murata, Y., Yamamoto, A., Kanazawa, H., Wada, Y., and Futai, M. (2000). Acidic endomembrane organelles are required for mouse postimplantation development. Dev. Biol. 228, 315–325. doi: 10.1006/dbio.2000.9963
Suzuki, Y., Chitayat, D., Sawada, H., Deardorff, M. A., McLaughlin, H. M., Begtrup, A., et al. (2018). TRPV6 variants interfere with maternal-fetal calcium transport through the placenta and cause transient neonatal hyperparathyroidism. Am. J. Hum. Genet. 102, 1104–1114. doi: 10.1016/j.ajhg.2018.04.006
Teitelbaum, S. L., and Ross, F. P. (2003). Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 4, 638–649. doi: 10.1038/nrg1122
Teti, A., Blair, H. C., Teitelbaum, S. L., Kahn, A. J., Koziol, C., Konsek, J., et al. (1989). Cytoplasmic pH regulation and chloride/bicarbonate exchange in avian osteoclasts. J. Clin. Invest. 83, 227–233. doi: 10.1172/jci113863
Thompson, E. G., Schaheen, L., Dang, H., and Fares, H. (2007). Lysosomal trafficking functions of mucolipin-1 in murine macrophages. BMC Cell Biol. 8:54. doi: 10.1186/1471-2121-8-54
Tidow, H., Poulsen, L. R., Andreeva, A., Knudsen, M., Hein, K. L., Wiuf, C., et al. (2012). A bimodular mechanism of calcium control in eukaryotes. Nature 491, 468–472. doi: 10.1038/nature11539
Timmann, C., Thye, T., Vens, M., Evans, J., May, J., Ehmen, C., et al. (2012). Genome-wide association study indicates two novel resistance loci for severe malaria. Nature 489, 443–446. doi: 10.1038/nature11334
Toro, E., Zuo, J., Guiterrez, A., La Rosa, R., Gawron, A., Bradaschia-Correa, V., et al. (2013). Bis-enoxacin inhibits bone resorption and orthodontic tooth movement. J. Dental Res. 92, 925–931. doi: 10.1177/0022034513501876
Toro, E. J., Zuo, J., Ostrov, D. A., Catalfamo, D., Bradaschia-Correa, V., Arana-Chavez, V., et al. (2012). Enoxacin directly inhibits osteoclastogenesis without inducing apoptosis. J. Biol. Chem. 287, 17894–17904. doi: 10.1074/jbc.m111.280511
Toyomura, T., Oka, T., Yamaguchi, C., Wada, Y., and Futai, M. (2000). Three subunit a isoforms of mouse vacuolar H+-ATPase preferential expression of the a3 isoform during osteoclast differentiation. J. Biol. Chem. 275, 8760–8765. doi: 10.1074/jbc.275.12.8760
Ulmasov, B., Bruno, J., Woost, P. G., and Edwards, J. C. (2007). Tissue and subcellular distribution of CLIC1. BMC Cell Biol. 8:8. doi: 10.1186/1471-2121-8-8
Valenzuela, S. M., Alkhamici, H., Brown, L. J., Almond, O. C., Goodchild, S. C., Carne, S., et al. (2013). Regulation of the membrane insertion and conductance activity of the metamorphic chloride intracellular channel protein CLIC1 by cholesterol. PLoS One 8:e56948. doi: 10.1371/journal.pone.0056948
van der Eerden, B. C., Hoenderop, J. G., de Vries, T. J., Schoenmaker, T., Buurman, C. J., Uitterlinden, A. G., et al. (2005). The epithelial Ca2+ channel TRPV5 is essential for proper osteoclastic bone resorption. Proc. Natl. Acad. Sci. U.S.A. 102, 17507–17512. doi: 10.1073/pnas.0505789102
Varela, L., Hendry, A. C., Medina-Carmona, E., Cantoni, D., and Ortega-Roldan, J. L. (2019). Membrane insertion of soluble CLIC1 into active chloride channels is triggered by specific divalent cations. bioRxiv [Preprint] doi: 10.1101/638080
Vergarajauregui, S., and Puertollano, R. (2006). Two di-leucine motifs regulate trafficking of mucolipin-1 to lysosomes. Traffic 7, 337–353. doi: 10.1111/j.1600-0854.2006.00387.x
Visentin, L., Dodds, R. A., Valente, M., Misiano, P., Bradbeer, J. N., Oneta, S., et al. (2000). A selective inhibitor of the osteoclastic V-H(+)-ATPase prevents bone loss in both thyroparathyroidectomized and ovariectomized rats. J. Clin. Invest. 106, 309–318. doi: 10.1172/jci6145
Voglmaier, S. M., Kam, K., Yang, H., Fortin, D. L., Hua, Z., Nicoll, R. A., et al. (2006). Distinct endocytic pathways control the rate and extent of synaptic vesicle protein recycling. Neuron 51, 71–84. doi: 10.1016/j.neuron.2006.05.027
Wang, C., Li, Y., Shi, L., Ren, J., Patti, M., Wang, T., et al. (2012). Mutations in SLC20A2 link familial idiopathic basal ganglia calcification with phosphate homeostasis. Nat. Genet. 44, 254–256.
Wang, L., Fang, B., Fujiwara, T., Krager, K., Gorantla, A., Li, C., et al. (2018b). Deletion of ferroportin in murine myeloid cells increases iron accumulation and stimulates osteoclastogenesis in vitro and in vivo. J. Biol. Chem. 293, 9248–9264. doi: 10.1074/jbc.ra117.000834
Wang, N., Agrawal, A., Jørgensen, N. R., and Gartland, A. (2018a). P2X7 receptor regulates osteoclast function and bone loss in a mouse model of osteoporosis. Sci. Rep. 8:3507.
Wang, W., Zhang, X., Gao, Q., and Xu, H. (2014). TRPML1: an ion channel in the lysosome. Handb. Exp. Pharmacol. 222, 631–645. doi: 10.1007/978-3-642-54215-2_24
Wang, W. W., Gallo, L., Jadhav, A., Hawkins, R., and Parker, C. G. (2020). The druggability of solute carriers. J. Med. Chem. 63, 3834–3867. doi: 10.1021/acs.jmedchem.9b01237
Weivoda, M. M., and Oursler, M. J. (2014). The roles of small GTPases in osteoclast biology. Orthop. Muscular Syst. Curr. Res. 3:1000161.
Wu, C., Liu, X., Sun, R., Qin, Y., Liu, Z., Yang, S., et al. (2018). Targeting anion exchange of osteoclast, a new strategy for preventing wear particles induced- osteolysis. Front. Pharmacol. 9:1291. doi: 10.3389/fphar.2018.01291
Wu, J., Glimcher, L. H., and Aliprantis, A. O. (2008). HCO3-/Cl- anion exchanger SLC4A2 is required for proper osteoclast differentiation and function. Proc. Natl. Acad. Sci. U.S.A. 105, 16934–16939. doi: 10.1073/pnas.0808763105
Xie, W., Lorenz, S., Dolder, S., and Hofstetter, W. (2016). Extracellular Iron is a modulator of the differentiation of osteoclast lineage cells. Calcif. Tissue Int. 98, 275–283. doi: 10.1007/s00223-015-0087-1
Xie, X. S., Padron, D., Liao, X., Wang, J., Roth, M. G., and De Brabander, J. K. (2004). Salicylihalamide A inhibits the V0 sector of the V-ATPase through a mechanism distinct from bafilomycin A1. J. Biol. Chem. 279, 19755–19763. doi: 10.1074/jbc.m313796200
Xu, J., Feng, H. T., Wang, C., Yip, K. H., Pavlos, N., Papadimitriou, J. M., et al. (2003). Effects of Bafilomycin A1: an inhibitor of vacuolar H (+)-ATPases on endocytosis and apoptosis in RAW cells and RAW cell-derived osteoclasts. J. Cell Biochem. 88, 1256–1264. doi: 10.1002/jcb.10477
Yan, P., Li, T., Bo, M., Die, L., and Xing, L. (2011). Inhibition of bone resorption by econazole in rat osteoclast-like cells through suppressing TRPV5. Arch. Pharm. Res. 34, 1007–1013. doi: 10.1007/s12272-011-0618-x
Yin, X., Zhang, Y., Gao, H., Jin, Q. L., and Wen, X. Y. (2019). A case report of hereditary hemochromatosis caused by mutation of SLC40A1 gene. Medicine (Baltimore) 98:e17526. doi: 10.1097/md.0000000000017526
Yoshikawa, M., Uchida, S., Ezaki, J., Rai, T., Hayama, A., Kobayashi, K., et al. (2002). CLC-3 deficiency leads to phenotypes similar to human neuronal ceroid lipofuscinosis. Genes Cells 7, 597–605. doi: 10.1046/j.1365-2443.2002.00539.x
Yu, Z., Surface, L. E., Park, C. Y., Horlbeck, M. A., Wyant, G. A., Abu-Remaileh, M., et al. (2018). Identification of a transporter complex responsible for the cytosolic entry of nitrogen-containing bisphosphonates. Elife 7:e36620.
Zaidi, M., Moonga, B. S., and Adebanjo, O. A. (1999). Novel mechanisms of calcium handling by the osteoclast: a review–hypothesis. Proc. Assoc. Am. Phys. 111, 319–327. doi: 10.1046/j.1525-1381.1999.99233.x
Zaidi, M., Shankar, V. S., Tunwell, R., Adebanjo, O. A., Mackrill, J., Pazianas, M., et al. (1995). A ryanodine receptor-like molecule expressed in the osteoclast plasma membrane functions in extracellular Ca2+ sensing. J. Clin. Invest. 96, 1582–1590. doi: 10.1172/jci118197
Zeng, D., Yao, P., and Zhao, H. (2019). P2X7, a critical regulator and potential target for bone and joint diseases. J. Cell. Physiol. 234, 2095–2103. doi: 10.1002/jcp.27544
Zhang, S., Liu, Y., Zhang, B., Zhou, J., Li, T., Liu, Z., et al. (2020). Molecular insights into the human CLC-7/Ostm1 transporter. Sci. Adv. 6:eabb4747. doi: 10.1126/sciadv.abb4747
Zhang, T., Zhou, Q., Ogmundsdottir, M. H., Moller, K., Siddaway, R., Larue, L., et al. (2015). Mitf is a master regulator of the v-ATPase, forming a control module for cellular homeostasis with v-ATPase and TORC1. J. Cell Sci. 128, 2938–2950. doi: 10.1242/jcs.173807
Zhang, W., Lv, T., Huang, J., and Ou, X. (2017). Type 4B hereditary hemochromatosis associated with a novel mutation in the SLC40A1 gene: a case report and a review of the literature. Medicine (Baltimore) 96:e8064. doi: 10.1097/md.0000000000008064
Zhang, X., Xu, X., Liu, X., Mao, C., Qin, A., and Lu, E. (2018). Bisenoxacin blocks alveolar bone resorption in rats with ovariectomyinduced osteoporosis. Mol. Med. Rep. 17, 3232–3238.
Zhang, Y., Zhang, Y., Sun, K., Meng, Z., and Chen, L. (2018). The SLC transporter in nutrient and metabolic sensing, regulation, and drug development. J. Mol. Cell Biol. 11, 1–13. doi: 10.1093/jmcb/mjy052
Keywords: osteoclast, membrane transporter, V-ATPase, bone disease, ion channel, CLCN7, solute carrier, osteoporosis
Citation: Ribet ABP, Ng PY and Pavlos NJ (2021) Membrane Transport Proteins in Osteoclasts: The Ins and Outs. Front. Cell Dev. Biol. 9:644986. doi: 10.3389/fcell.2021.644986
Received: 22 December 2020; Accepted: 09 February 2021;
Published: 26 February 2021.
Edited by:
Yankel Gabet, Tel Aviv University, IsraelReviewed by:
Wei Zou, Washington University in St. Louis, United StatesMariafrancesca Scalise, University of Calabria, Italy
Copyright © 2021 Ribet, Ng and Pavlos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nathan J. Pavlos, bmF0aGFuLnBhdmxvc0B1d2EuZWR1LmF1
 Amy B. P. Ribet
Amy B. P. Ribet Pei Ying Ng
Pei Ying Ng Nathan J. Pavlos
Nathan J. Pavlos