- 1Department of Research, Affiliated Tumor Hospital, Guangxi Medical University, Nanning, China
- 2Shenzhen People’s Hospital (The Second Clinical Medical College, Jinan University, The First Affiliated Hospital, Southern University of Science and Technology), Shenzhen, China
Aim: We aimed to develop and validate a comprehensive nomogram containing pre-treatment plasma HSP90AA1 to predict the risk of breast cancer onset and metastasis.
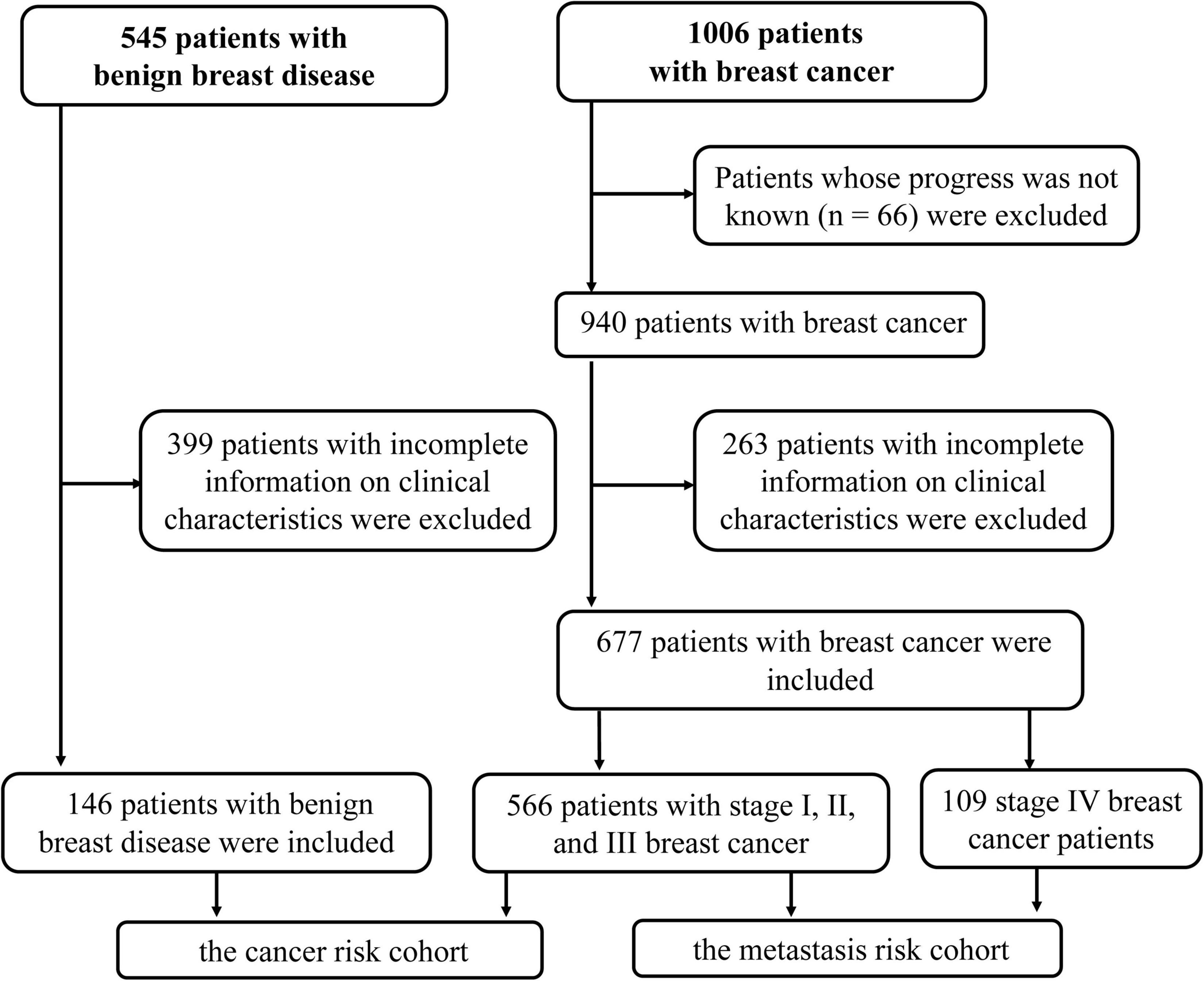
Methods: We assessed the expression of HSP90s in breast cancer patients using an online database. To verify the results, 677 patients diagnosed with breast cancer and 146 patients with benign breast disease between 2014 and 2019 were selected from our hospital and were divided into cancer risk and metastasis risk cohorts. We focused on HSP90AA1 to elucidate the risks of onset and metastasis in the cohorts.
Results: Expression levels of HSP90AA1, HSP90AA2, HSP90AB1, HSP90B1, and TRAP1 were linked to disease progression. Survival analysis using the GEPIA and OncoLnc databases indicated that the upregulation of HSP90AA1 and HSP90AB1 was related to poor overall survival. In the cancer risk cohort, carcinoembryonic antigen (CEA), carbohydrate antigen 153 (CA153), HSP90AA1, T cells%, natural killer cells%, B cells%, neutrophil count, monocyte count, and d-dimer were incorporated into the nomogram. A high Harrell’s concordance index (C-index) value of 0.771 [95% confidence interval (CI), 0.725–0.817] could still be reached in the interval validation. In the metastasis risk cohort, predictors contained in the prediction nomogram included the use of CEA, CA153, HSP90AA1, carbohydrate antigen 125 (CA125), natural killer cells%, B cells%, platelet count, monocyte count, and d-dimer. The C-index was 0.844 (95% CI, 0.801–0.887) and it was well-calibrated. HSP90AA1 raised net clinical benefit of breast cancer onset and metastasis risk prediction nomogram in a range of risk thresholds (5–92%) and (1–90%).
Conclusion: Our study revealed that pretreatment plasma HSP90AA1 combined with other markers could conveniently predict the risk of breast cancer onset and metastasis.
Introduction
Breast cancer is one of the most common malignant tumors worldwide, with high incidence and mortality rates. It is the most frequent cancer and the second leading cause of cancer-related death in females (Bray et al., 2018; Siegel et al., 2018). Surgery, endocrine therapy, and chemotherapy are the primary treatment approaches for breast cancer, and breast cancer that is detected early-on always has a better prognosis after surgery. The 5-year survival rate of early stage breast cancer patients is estimated to be as high as 90%, and declines to 27% once metastasis has occurred (Siegel et al., 2018). Early and timely diagnosis is crucial for the treatment and prognosis of breast cancer patients. Hence, it is urgent to explore non-invasive diagnostic biomarkers that can allow clinicians to distinguish between benign and malignant tumors as well as to detect the stage of cancer in order to develop an individualized treatment plan.
Heat shock protein 90-alpha (HSP90AA1), an essential molecular chaperon that is highly conserved in evolution, is efficiently expressed under the stimulatory conditions of trauma, infection, and tumors. Newly formed HSP90AA1 can be secreted into the extracellular environment and can also enter the nucleus to stimulate the formation of immune memory and participate in tumor formation (Frydman, 2001; Condelli et al., 2019). HSP90AA1 plays an essential role in DNA damage regulation, cell cycle regulation, gene expression, and carcinogenesis (Condelli et al., 2019). Previous research has demonstrated that in cancer cells, HSP90AA1 activates many oncogenic client proteins, thereby stimulating cell survival, growth, and invasiveness (Eustace et al., 2004; Chehab et al., 2015; Wu et al., 2017). These results suggest that HSP90AA1 could serve as a biomarker for cancer. Furthermore, many experiments have shown that HSP90AA1 is able to promote cancer cell proliferation, metastasis, invasion, and epithelial-to-mesenchymal transition in several diseases, suggesting that HSP90AA1 could be a potential target for the treatment of cancers (Wu et al., 2017; Tian et al., 2019).
Biomarkers in peripheral blood are considered widely accepted and convenient predictors of cancer. Carbohydrate antigen 153 (CA153) is now regarded as the most critical tumor marker for breast cancer, although its sensitivity and specificity are not sufficiently high. Recently, some studies have indicated that overexpression of HSP90AA1 is related to various malignant tumors. For example, previous studies have confirmed that HSP90AA1 is a sensitive biomarker of lung cancer and is valuable in predicting the response of lung cancer patients to surgery or chemotherapy (Shi et al., 2014). It can also be used as an initial diagnosis in patients with hepatocellular carcinoma (Fu et al., 2017; Wei et al., 2020). Other studies have reported that high expression of HSP90AA1 is associated with poor prognosis in patients with colorectal cancer (Zhang et al., 2019). In a multi-center clinical study of 1,558 patients (39 cases of breast cancer), plasma HSP90AA1 can be used as a biomarker for cancer (Liu et al., 2019). It has been reported that elevated plasma HSP90AA1 levels are specific to malignant tumors and patients with metastatic liver or breast tumors have higher plasma HSP90AA1 levels than those without metastasis (Wang et al., 2009). Thus, HSP90AA1 is considered a potential tumor biomarker, but the utility of measuring plasma HSP90AA1 in breast cancer clinical treatment has not yet been investigated. Nomogram is a common tool in oncology and medicine; one of its main advantages is the ability to estimate individual risk based on patient and disease characteristics (Balachandran et al., 2015). In this study, we aimed to examine the diagnostic value of plasma HSP90AA1 in breast cancer by using an online database and clinical parameters. We also developed prediction nomogram models for assessing the risk of breast cancer and breast cancer metastasis.
Materials and Methods
Bioinformatics Data Mining
The online tool “ONCOMINE”1 was used to evaluate the expression patterns of HSP90 family genes (HSP90s) in different cancers. Further, the online tool “Gene Expression Profiling Interactive Analysis” (GEPIA)2 was used to draw the correlation between the expression of HSP90s and overall survival (OS). “UALCAN”3 was used toextract the tumor grade in the Cancer Genome Atlas (TCGA) breast cancer dataset. We used mass spectrometry data in the Human Protein Atlas (THPA)4 to analyze the expression of HSP90 gene-encoded proteins in patient plasma samples.
Selection of Breast Cancer Patients
The participants in this retrospective study were selected from the Guangxi Medical University Cancer Hospital between May 2014 and August 2019. The inclusion criteria were as follows: (1) women with primary breast cancer or breast benign disease; (2) breast cancer was confirmed histologically; and (3) patients whose clinical characteristics were complete. The exclusion criteria were as follows: (1) patients with more than one malignant tumor in the same period; (2) patients with severe systemic infection, acute or chronic hematologic or autoimmune diseases; and (3) incomplete information on any of these clinical characteristics. This study followed the ethical guidelines of the 2008 Declaration of Helsinki and was approved by the Ethics Committee of Guangxi Medical University Cancer Hospital (LW2020065).
Clinical and Hematology Test Data Collection
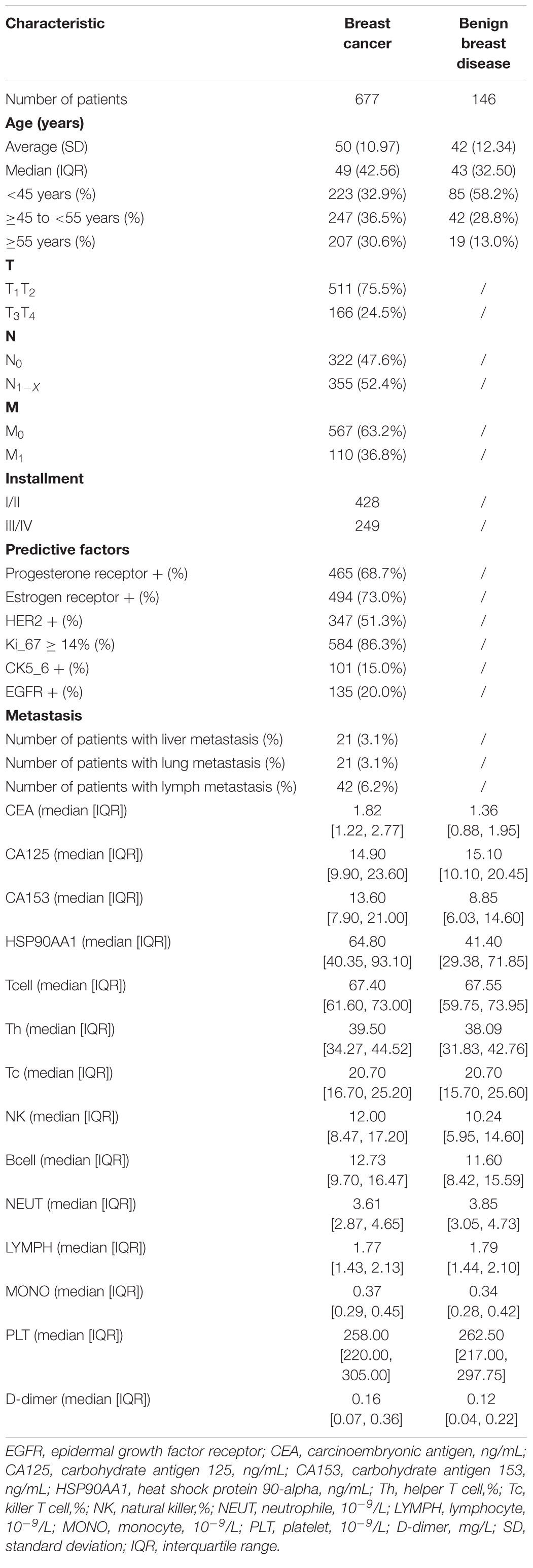
In this retrospective study, the data on the clinical-pathological features and biomarkers in the peripheral blood of the selected patients diagnosed with breast disease were collected. These included carcinoembryonic antigen (CEA), carbohydrate antigen 125 (CA125), carbohydrate antigen 153 (CA153), HSP90AA1, d-dimer, estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor-2 (HER2), Ki-67, CK5-6, epidermal growth factor receptor (EGFR), TNM stage, tumor location, and tumor metastasis sites. Based on the previous studies, we assumed that tumor occurrence and prognosis may be related to cellular immunity (T cells, helper T cells (Th), killer T cells (Tc), natural killer (NK) cells, B cells, neutrophils, monocytes, and platelets (Liu et al., 2016; Tang et al., 2020). Therefore, this article also included cellular immunity-related indicators. All blood samples were collected before treatment.
Clinical Molecular Typing
The division of hormone receptor (HR) negative/positive patients: either estrogen receptor (ER) or progesterone receptor (PR) positive or double-positive is HR-positive (528 cases), and double-negative is HR negative (149 cases). Human epidermal growth factor receptor-2 (HER2) negative/positive expression classifies patients as HER2-positive/negative (347/330 cases).
Assessment of HSP90AA1 Levels
The levels of plasma HSP90AA1 were measured using an ELISA kit for HSP90AA1 protein (Yantai Protgen Biotechnology Development Co., Ltd., Yantai, China). Fresh blood samples (2 mL) were collected from patients and controls and were combined with EDTA-K2 anticoagulant. All steps were performed according to the manufacturer’s instructions. The fresh blood samples were first preincubated at 37°C for 30 min, then centrifuged at 3,000 rpm for 10 min, and diluted 20 times with the diluent solution provided. Then, the standards were loaded together with the quality controls, and the prepared samples (50 μL of each) were added to 96-well plates followed by the addition of 50 μL of the anti–HSP90AA1HRP-conjugated antibody. These were incubated at 37°C, and samples were subjected to gentle shaking for 1 h. Next, the plates were washed six times using the washing buffer provided in this kit, which was followed by the chromogenic reaction; 50 μL of the peroxide and 50 μL of 3, 3, 5, 5′-tetramethylbenzidine were added the samples, which were then incubated at 37°C for 20 min. The reaction was terminated by the addition of an acid stop buffer. Finally, the optical density was measured using a spectrophotometer using an excitation of 450 nm and a detection wavelength of 620 nm as the reference wavelength. The concentration of HSP90AA1 protein in each sample was calculated according to a standard curve of the optical density values.
The levels of plasma HSP90AA1 were measured using Western blotting. Erasin of abundance proteins, including immunoglobulin G (IgG) and albumin, from plasma samples, was performed using Spin Albumin and IgG Erasin Kit [Sangon Biotech (Shanghai) Co., Ltd.] according to the manufacturer’s instructions. Next, Put a spin column into a 2 mL collection tube. Turn the reagent bottle containing the resin upside down, mix well to form a uniform suspension, and add 340 μL of the suspension to the spin column. Centrifuge at 7,500 rpm (10,000 g) for 1 min, pour out the liquid in the collection tube and then put the resin-containing spin column into the collection tube again. Add 300 μL of binding/washing solution to the spin column, close the lid, turn upside down 3∼5 times or vortex for 10 s, then centrifuge at 7,500 rpm (10,000 g) for 1 min and pour out the liquid in the collection tube. Repeat this step 2∼3 times until the liquid flowing out is almost colorless. Take a clean centrifuge tube and add 10 μL of plasma sample to 200 μL of pre-chilled binding/washing solution and mix well. Add the above mixture to the spin column containing the resin, close the lid, invert 3– 5 times, or vortex for 10 s. Shake on a shaker at 4°C at medium speed for 15 min or vortex for 15 min. Then transfer to a refrigerated centrifuge, centrifuge at 7,500 rpm (10,000 g) at 4°C for 1 min, and collect the filtrate. Transfer the filtrate in the collection tube to the spin column containing the resin again, and vortex for 15 min at 4°C on a shaker or vortex for 10–15 min. Transfer to a refrigerated centrifuge, centrifuge at 7,500 rpm (10,000 g) for 1 min at 4°C, and add 200 μL of pre-cooled binding/washing solution to the spin column containing the resin. Centrifuge at 4°C at 7,500 rpm (10,000 g) for 1 min. At this time, the collection tube contains about 400 μL of filtrate. This filtrate is a sample from which plasma albumin and IgG antibodies have been removed. Samples mixed with loading buffer were denatured at 95°C for 5 min and separated on SDS−PAGE gels as previously described. Separated on 8% SDS−PAGE gels, followed by transfer to Nitrocellulose membrane (NC). The target proteins were probed with the indicated primary antibodies (Cat no: 13171-1-AP, Proteintech Group, Inc.) overnight at 4°C, then incubated with horseradish peroxidase (HRP)−conjugated secondary antibodies (Abcam (Shanghai) Trading Co., Ltd.) for 1 h at room temperature, and visualized using a common enhanced chemiluminescence technique (Abcam) according to the manufacturer’s protocol.
Construction of the Nomograms
The least absolute shrinkage and selection operator (LASSO) regression model was used to select the relevant factors of breast cancer diagnosis and metastasis. The logistic regression analysis was then used to establish a convincing prediction model for cancer risk and metastasis risk by merging the features selected in the LASSO method. Clinicopathological features and biomarkers in the peripheral blood with a p-value of less than 0.05 were included in the model. All selected predictors were used to develop a predictive nomogram model for cancer risk and metastasis risk in breast cancer patients.
Validation and Calibration
The nomograms were subjected to 1,000 bootstrap resamples for internal validation of the two cohorts. The concordance index (C-index) between the predicted probability and response was used to assess the discrimination performance of the nomograms (Wolbers et al., 2009). The value of the C-index ranges from 0.5 to 1.0, with 0.5 indicating random chance and 1.0 indicating perfectly corrected discrimination (Pencina et al., 2008). Calibration is the ability of a model to make unbiased estimates of the outcome. The marginal estimate versus the average predictive probability of the models was used to construct calibration curves. For a well-calibrated model, the predictions were expected to fall on a 45° diagonal line.
Statistical Analyses
Statistical analyses were performed using R statistical software (version 4.0.2). Differences between discrete variables were examined by box plots. Receiver operating characteristic (ROC) curves were established to determine the optimal cut-off thresholds and diagnostic accuracies of the continuous variables. P-values (all two-sided) less than 0.05 were considered statistically significant.
Results
HSP90AA1 Is More Suitable as a Potential Clinical Target and Prognostic Biomarker
The mRNA levels of HSP90AA1 in breast cancer tissues were higher than those in normal tissues (Figures 1A,B). Although the mRNA levels of HSP90AB1, HSP90B1, and TRAP1 were increased in breast cancer tissues compared with those in normal tissues, the mRNA levels were not significant. We further used Oncomine to verify these findings (Supplementary Figure 1). The UALCAN results suggested that advanced breast cancer patients were more inclined to show higher HSP90s expression levels (Supplementary Figure 2A). Further, the GEPIA results revealed that increased expression levels of HSP90AA1 and HSP90AB1 were strongly associated with poor OS (Supplementary Figure 2B). HSP90s protein target was detected in plasma for the reference set (Peptide atlas) using mass spectrometry; the protein expression level of HSP90AA1 in the plasma was 94 μg/L (Figure 2).
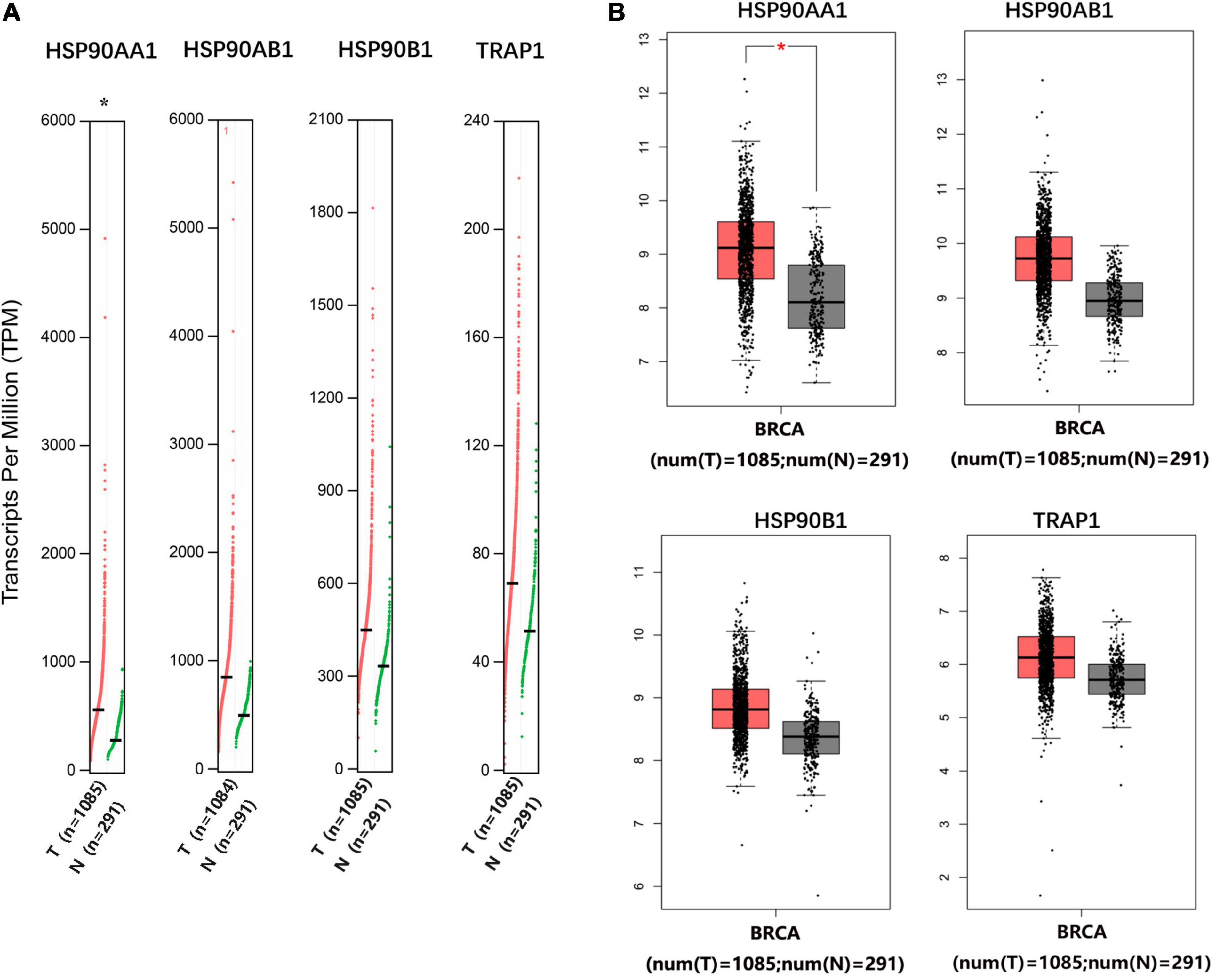
Figure 1. Gene Expression Profiling Interactive Analysis of the expression profiles of HSP90AA1, HSP90AB1, HSP90B1, and TRAP1 in breast cancer patients. The profile shows the tissue-wise expression of heat shock protein 90 kDa family gene signatures in breast cancer using (A) a dot plot and (B) a box plot match of TCGA normal and GTEx data. *Indicates a statistically significant difference (p < 0.05).
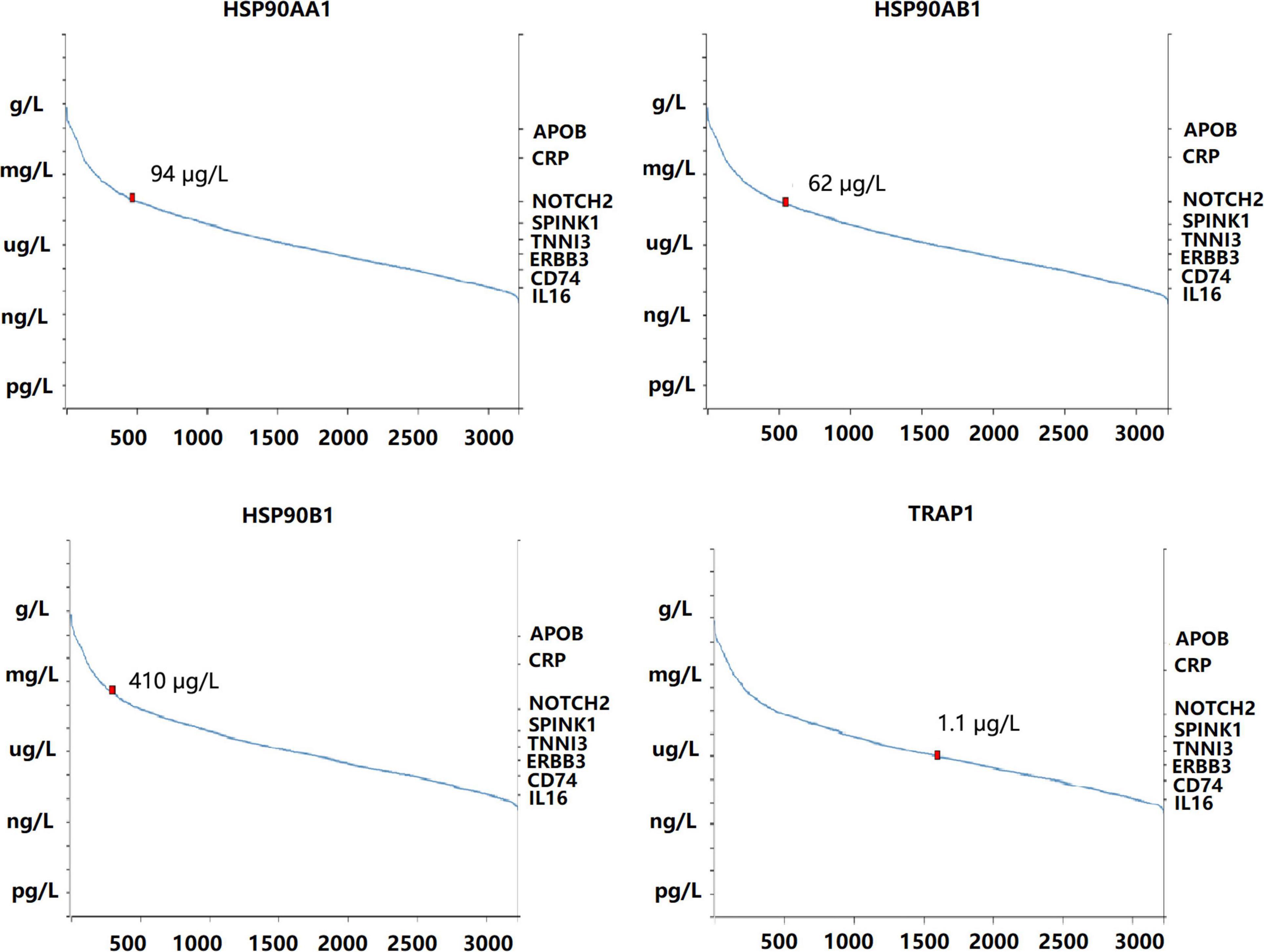
Figure 2. Concentration of heat shock protein 90 in the blood quantified by mass spectrometry-based plasma proteomics.
Clinical Characteristics
In order to verify the above results and analyze the expression pattern of HSP90AA1 in breast cancer, a total of 677 breast cancer patients and 146 breast benign disease patients were selected after applying the inclusion and exclusion criteria, and all enrolled patients were female. We selected 146 patients with benign breast disease and 566 patients with stage I, II, and III breast cancer as the cancer risk cohort. Among breast cancer patients, we included 566 stage I-III patients and 109 stage IV patients as the metastasis risk cohort (Figure 3). The characteristics of these patients are summarized in Table 1. We visualized the correlation matrix of all clinical biomarkers in the two cohorts. There was no significant correlation between the expression of plasma HSP90AA1 and any of the clinical indicators (Figure 4).
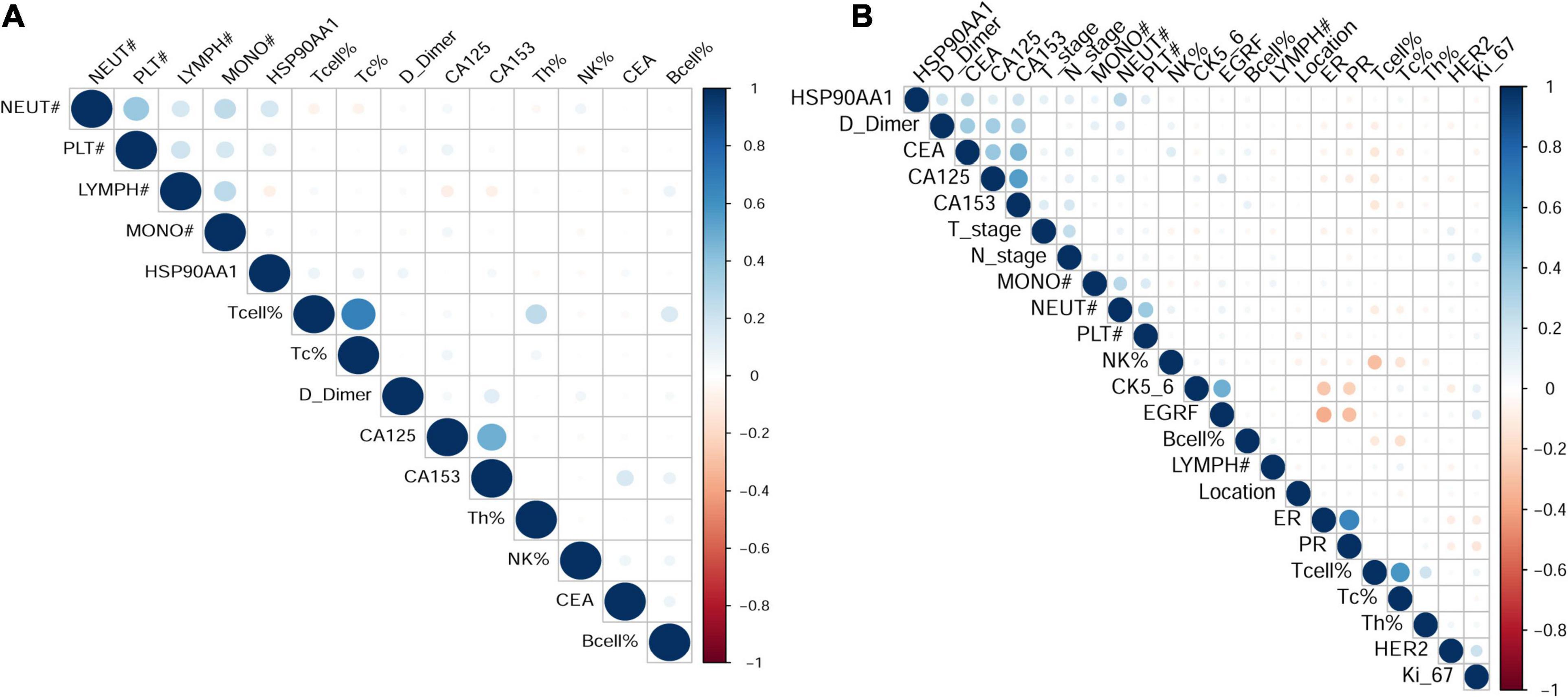
Figure 4. Visualization of the correlation matrix of all clinical biomarkers including HSP90AA1. (A) Benign breast disease and stage I-III breast cancer; (B) breast cancer stages I-III and IV.
The levels of different detection indicators in the plasma were compared between patients with breast cancer and benign breast disease (Supplementary Figure 3A). HSP90AA1 was significantly elevated in breast cancer patients (Figure 5A; p = 6.807e-07; Wilcoxon test). Moreover, HSP90AA1 expression was significantly different between patients in different subgroups sorted by clinical stage (p = 8.623e-06, Kruskal–Wallis test), histological N grade (p = 1.549e-04, Wilcoxon test), and histological M grade (p = 5.599e-06, Wilcoxon test) (Figure 5B). However, HSP90AA1 expression showed no difference among other clinical groups (Supplementary Figure 3B).
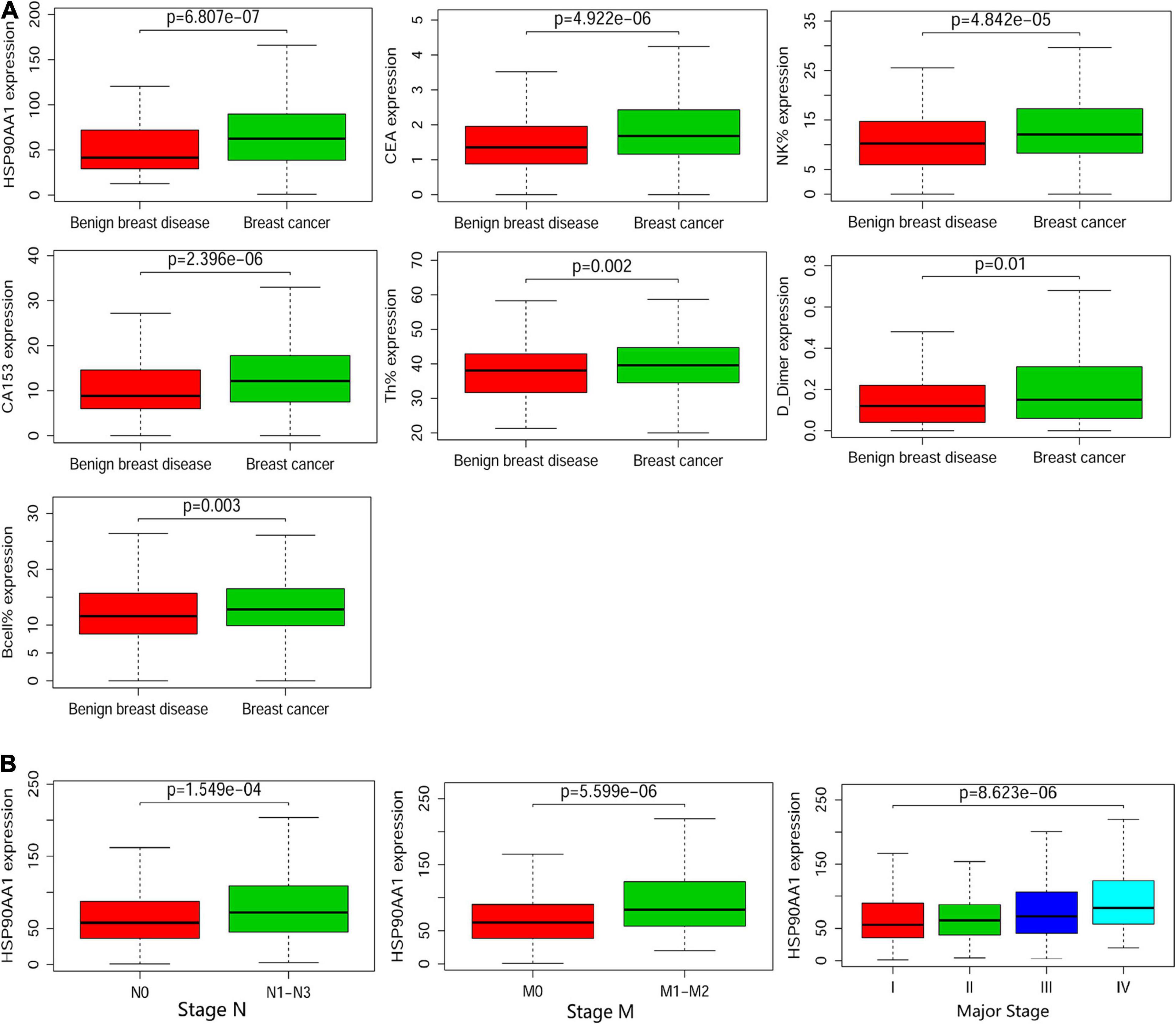
Figure 5. Boxplots of the cancer and metastasis risks according to the cohort. (A) Boxplots of the significantly elevated clinical indicators in benign breast disease and in stage I, II, and III breast cancer. (B) Boxplots of HSP90α according to clinical significance in breast cancer patients. Differences between groups were estimated using the Mann–Whitney U or Kruskal–Wallis test, as appropriate.
Receiver Operating Characteristic (ROC) Curves
We compared the area under the curve (AUC) values between all of the detection indicators in the two cohorts. The AUC value of HSP90AA1 was significantly higher than that of other markers in the cancer risk cohort. Next, we used ROC curve analysis (indexed by Youden’s index) to determine the optimal cut-off value for the cancer risk cohort (HSP90AA1: 50.35, Figure 6A). Next, we investigated the significance of HSP90AA1 levels in the metastasis of breast cancer. ROC curves were drawn to analyze the optimal cut-off values, diagnostic sensitivities, and specificities (HSP90AA1: 75.75, Figure 6B).
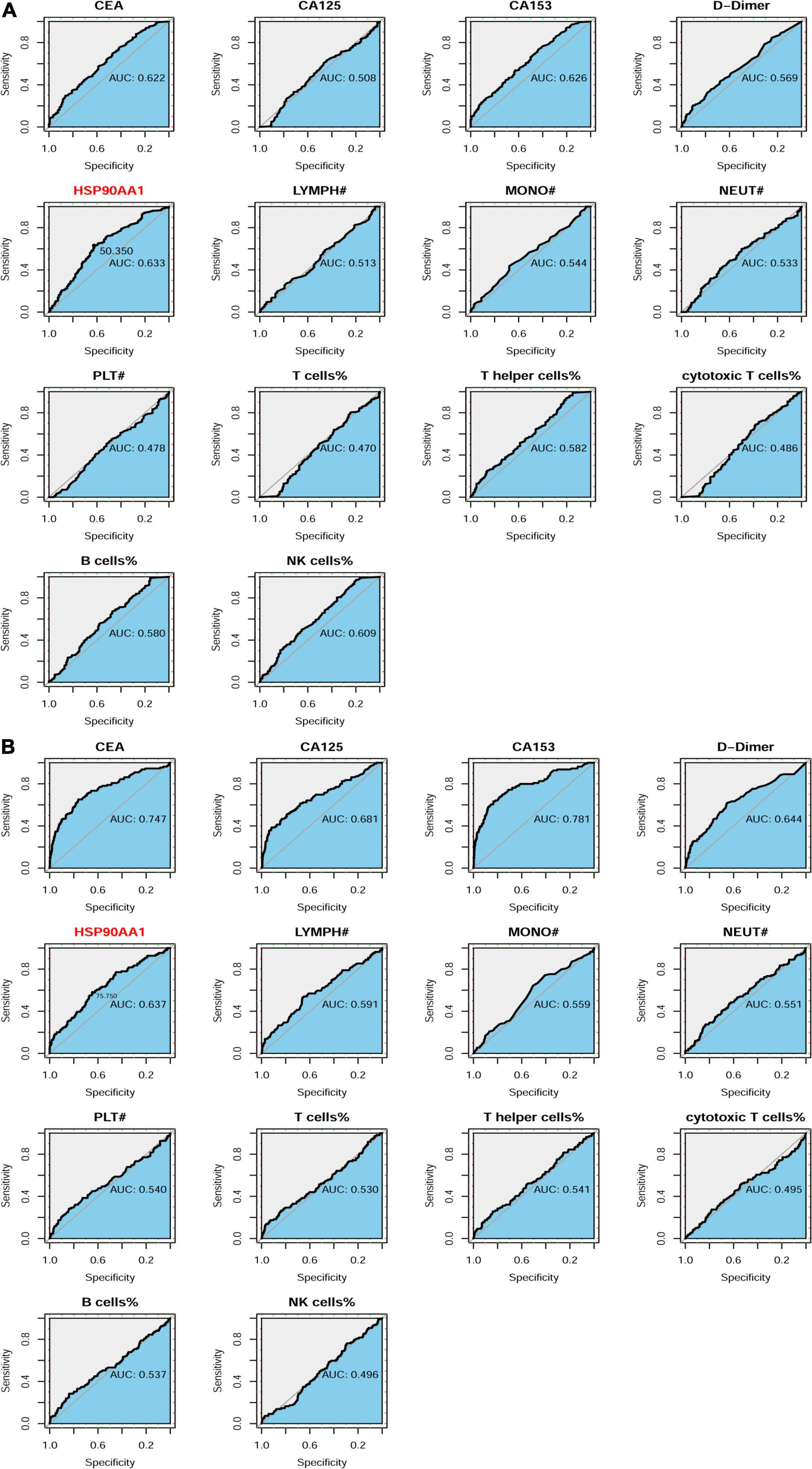
Figure 6. Patient enrollment in this study and ROC curve analyses. (A) Receiver operating characteristic (ROC) curve analysis to evaluate the predictive value of each marker for definite tumor diagnosis. HSP90AA1 shows the highest accuracy for the prediction of breast cancer compared with that of other markers. (B) ROC curve analysis to evaluate the predictive value of each marker for the definite diagnosis of breast cancer metastasis.
Construction of the Cancer Risk and Metastasis Risk Nomograms Containing HSP90AA1
Of the cancer risk cohort, 14 functional markers were all included and had non-zero coefficients in the LASSO regression model (Figures 7A,B). Nomograms were constructed based on the logistic regression analysis among these independent predictors (Supplementary Figure 4A). Based on the nomograms, HSP90AA1, B cells, and NK cells contribute the most to diagnosis, followed by CEA, CA153, cytotoxic T cells, neutrophils, monocytes, and d-dimers (Figure 7C).
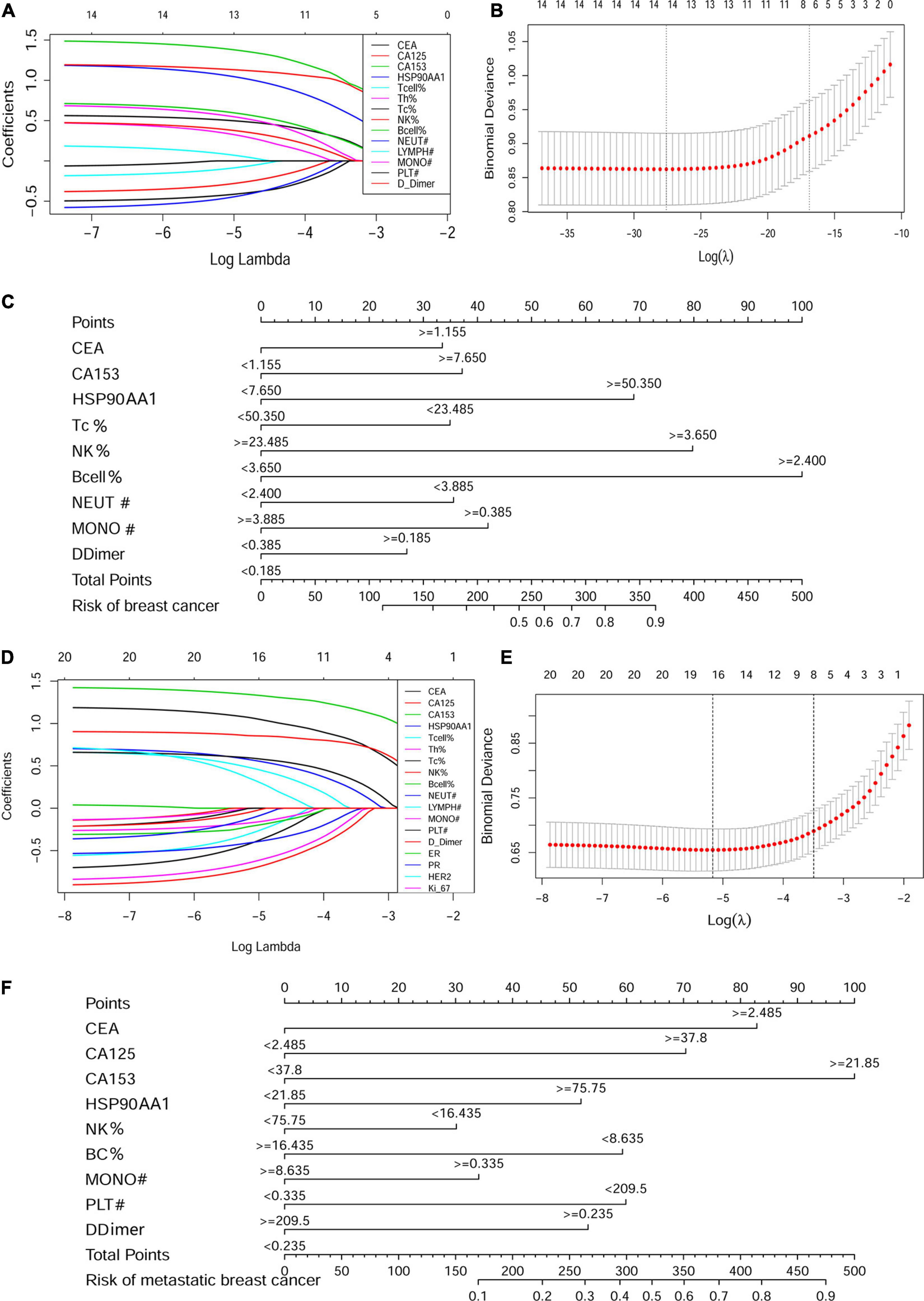
Figure 7. Construction of the cancer risk and metastasis risk nomograms containing HSP90AA1. LASSO coefficient profiles of the (A) 14 factors in the cancer risk cohort and (D) 21 factors in the metastasis risk cohort. A coefficient profile plot was produced against the logλ sequence. (B,E) Selection results of the LASSO model. The partial likelihood deviance (binomial deviance) curve is plotted versus logλ. Dotted vertical lines are drawn at the optimal values by using the minimum criteria and the 1 SE of the minimum standards (the 1-SE rules). Development of the (C) breast cancer risk nomogram and (F) metastasis risk nomogram. LASSO, least absolute shrinkage and selection operator.
Next, in the metastasis risk cohort, 21 clinical-pathological features and biomarkers in the peripheral blood were reduced to 14 possible predictors, which had non-zero coefficients in the LASSO regression model (Figures 7D,E). The results of the logistic regression analysis of these independent predictors are shown in Supplementary Figure 4B. The predicted model, which contained CEA, CA125, CA153, HSP90AA1, NK cells, B cells, monocytes, platelets, and d-dimers, was established and presented in the form of a nomogram (Figure 7F).
Calibration and Validation
The calibration curve of the cancer risk nomogram and metastatic risk nomogram for the prediction of cancer risk and metastasis risk in females demonstrated significant agreement in these cohorts (Figures 8A,D). The AUCs were 77.1% [95% CI: 72.5–81.7%] and 84.4% [95% CI: 80.1–88.7%], which were higher than that of the AUC of any single factor (Figures 8B,E). The C-indexes for the prediction nomogram were 0.77 and 0.84, which were confirmed to be 0.75 and 0.83 through bootstrapping validation. Decision curve analysis and clinical impact plot were used to determine the clinical utility of risk prediction nomograms. The decision curve analysis showed that if patients and doctors’ threshold probabilities are 5–92% and 1 –90%, respectively, this progression nomogram could add more benefit than the scheme to predict the risk of onset and metastasis (Figures 8C,F). The results indicated that HSP90AA1 was not a decisive factor in diagnosing metastasis in breast cancer; however, it improved diagnostic accuracy. The clinical impact plot provided corresponding verification (Supplementary Figure 5).
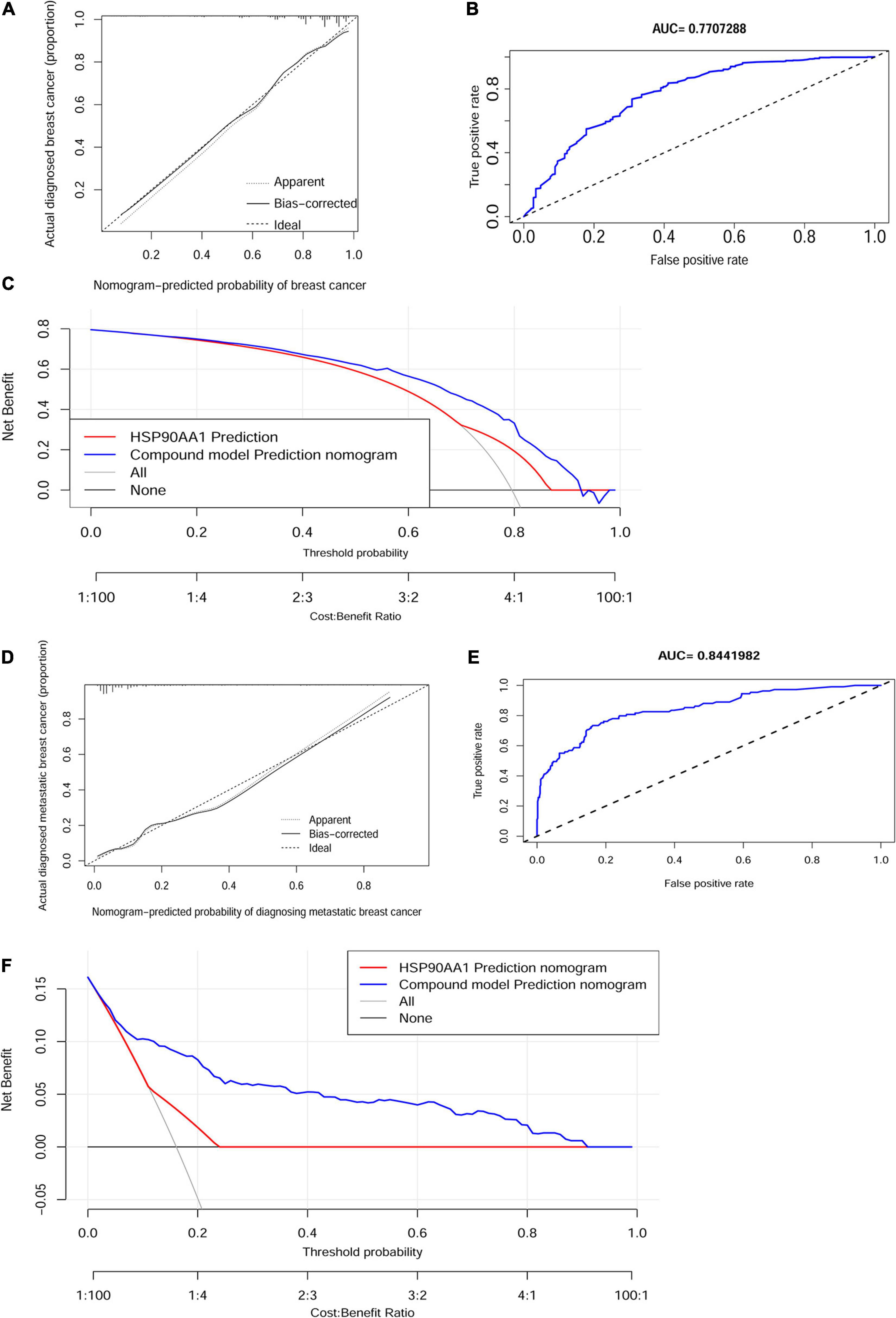
Figure 8. Calibration and validation. Calibration curves of the non-adherence nomogram prediction for (A) cancer risk and (D) metastasis risk. The x-axis represents the predicted risk whereas the y-axis represents the diagnosed value. The diagonal dotted line represents perfect prediction by an ideal model. The solid line represents the performance of the nomogram, of which a closer fit to the diagonal dotted line represents better prediction. Receiver operating characteristic curve of (B) the cancer risk model and (E) metastasis risk. Decision curve analysis for (C) the breast cancer risk nomogram and (F) the metastasis risk nomogram. The X-axis is the risk threshold probability that changes from 0 to 1, and the Y-axis is the calculated net benefit for a given threshold probability. AUC, area under the curve.
Metastasis Risk Nomogram in Patients With Different Molecular Types of Breast Cancer
In HR negative/positive and HER2 negative/positive patients, 21 clinicopathological characteristics and biomarkers in peripheral blood were screened and a nomogram for metastasis prediction was established (Table 1 for the number of patients in each group). We found that the nomograms of patients with different molecular types are different. Among them, the predictive nomogram of HR positive/negative patients is consistent with the metastasis cohort (Figure 9A), but the predictive ability of HR negative patients is reduced (Figure 9C). The metastasis prediction model for HER2-negative patients includes CEA, CA153, HSP90AA1, B cells, and d-dimers (Figure 9E). HSP90AA1 was not included in the metastasis prediction model for HER2-positive patients (Figure 9G). But the AUCs of the four models are all greater than 80% (Figures 9B,D,F,H). The model calibration and verification results are shown in Supplementary Figure 6.
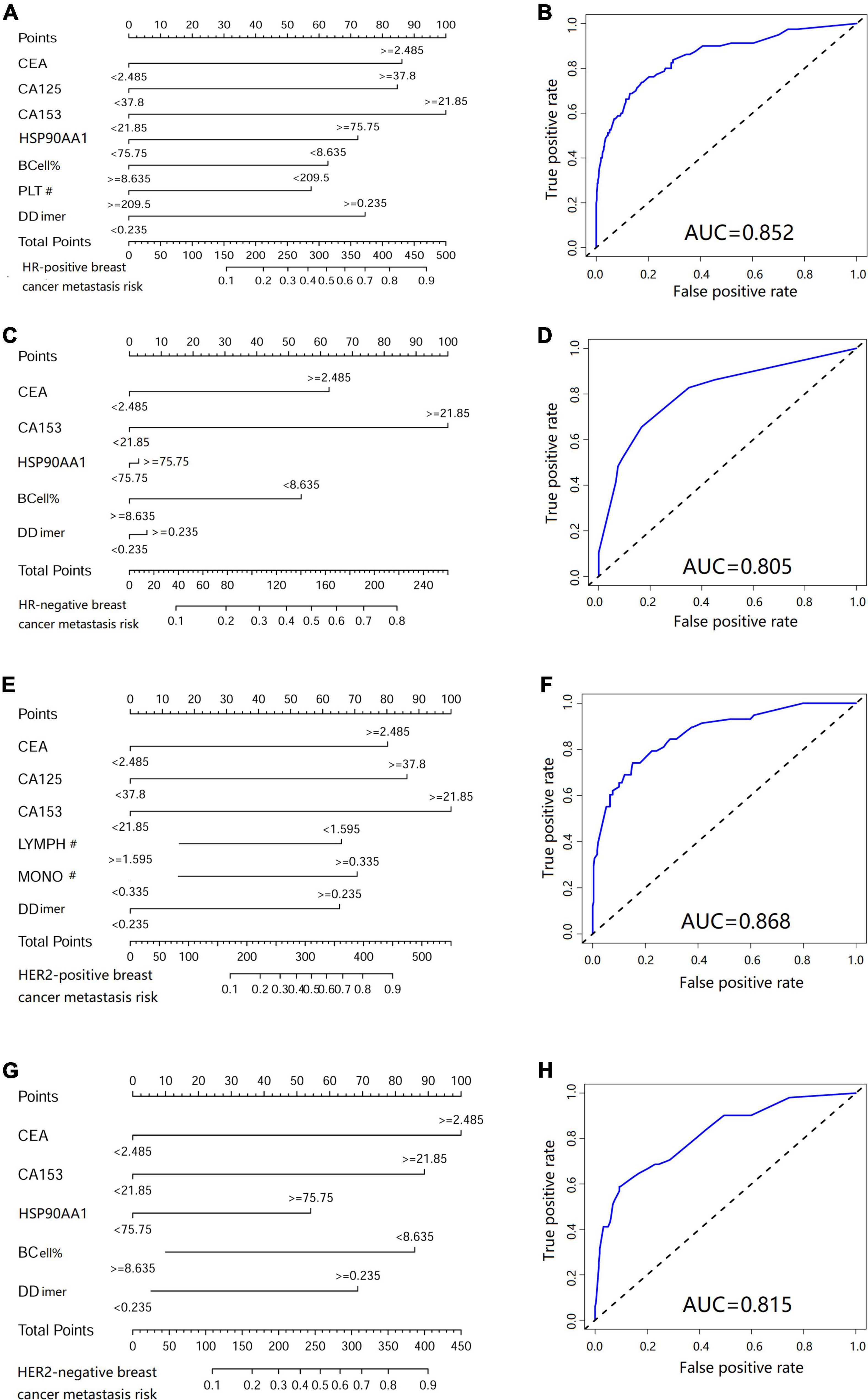
Figure 9. Metastasis risk nomogram in patients with different molecular types of breast cancer. Metastasis risk nomograms of (A) hormone receptor (HR) positive and (C) negative patients. Metastasis risk nomograms of (E) human epidermal growth factor receptor-2 (HER2) positive and (G) negative patients. Receiver operating characteristic curve of HR (B) positive, (D) negative, HER2 (F) positive and (H) negative patients.
Western Blotting Methods Verification Levels of Plasma Hsp90AA1 From Partial ELSIA Negative Patients
Finally, Because Wei’s reported the uncertainty of the diagnosis of plasma Hsp90AA1 and the heterogeneity of the HSP90AA1 protein secreted by tumor cells, we randomly screened the plasma of 32 ELISA-negative breast cancer patients. We used the polyclonal antibodies with clear characteristics for western blotting. It was found that in some ELISA-negative breast cancer patients, the secretion level of plasma Hsp90AA1 detected by western blotting was higher than the critical value (Supplementary Figure 7A). Still, the overall results were consistent with the ELISA test results (Supplementary Figure 7B).
Discussion
In this study, we investigated the expression patterns and prognostic values of different HSP90 family members (HSP90AA1, HSP90AA2, HSP90AB1, HSP90B1, and TRAP1) in breast cancer. Further, we included 677 patients with breast cancer and 146 with breast benign disease who underwent surgery and medical treatment. These findings may provide the means to improve the accuracy of the diagnosis and prognosis of breast cancer patients. The primary role of heat shock proteins (HSPs) in tumorigenesis is to stabilize abnormally expressed tumor-related genes (Calderwood and Gong, 2016). HSPs are released from cancer cells and affect their properties and functions through receptor-mediated signal transduction (Ono et al., 2018). Previous reports have shown that HSPs are overexpressed in melanoma and colon cancer and are related to the excessive activation of the Wnt signaling pathway (Skrzypczak et al., 2010). HSP90 is important for the stabilization and activation of more than 200 proteins, many of which are essential for cell signaling and adaptive responses to stress (Schopf et al., 2017). HSP90 forms a dynamic complex of HSP90 chaperons with other HSPs (Young et al., 2003). Therefore, HSP90 is considered an essential promoter of cancer cell survival, especially in breast cancer (Whitesell and Lindquist, 2005; Vartholomaiou et al., 2017). In our study, we confirmed that the expression of HSP90s in breast cancer tissues was significantly higher than that in normal tissues. We also observed a significant correlation between the expression of HSP90AA1 and tumor stage in breast cancer patients. The OS of breast cancer patients with high HSP90AA1 expression was low.
Accumulating studies have demonstrated that genes within the HSP90 family are potentially involved in the pathogenesis of human malignancies. Although previous research has shown that they can predict tumor development, the expression levels of HSP90s are rarely used as tumor diagnostic biomarkers (Trepel et al., 2010). Some studies believe that some HSP90 family genes such as HSP90AA1 and TRAP1 are not necessary for breast tumors to develop and metastasize; however, they have surprising regulatory effects (Vartholomaiou et al., 2017). HSP90AA2 gene polymorphism has been reported to be related to certain immune diseases (Zhang et al., 2018), but there is no report related to tumors. HSP90AB1 is a closed homolog of HSP90AA1, which is necessary for large-scale cellular processes and, therefore, is essential for cell survival (Haase and Fitze, 2016). Studies have suggested that HSP90AA1, HSP90AB1 gene products, and their associated chaperon proteins (Aha1, Cdc37, p23, and Tpr2) as well as HSP90-dependent transcription factor HSF1 are overexpressed in a variety of cancers (McDowell et al., 2009). High mRNA expression driven by amplification of the chromosome coding region of HPS90AB1 was found to be associated with poor prognosis of HER2-negative/ER-positive breast cancer (Cheng et al., 2012). In our study, the ONCOMINE and TCGA data sets showed that the expression of HPS90AB1 in breast cancer tissues was higher than that in healthy tissues, but the difference was not significant. The high expression of HPS90AB1 was closely related to the overall poor survival in breast cancer patients who were followed up with for more than 250 months. HSP90B1 is an HSP90 paralog found in the endoplasmic reticulum; it plays critical roles in folding proteins in the secretory pathway, such as Toll-like receptors and integrins (Randow and Seed, 2001). Dejean and Liu showed that the expression level of HSP90B1 in recurrent human breast cancer was higher than that of its matched primary tumor. Further, it was found to be an independent and unfavorable prognostic indicator of breast cancer survival (Dejeans et al., 2012; Liu et al., 2018). Our research suggested that the imbalance of HSP90B1 was not closely related to the occurrence or development of breast cancer, and had no effect on the prognosis of breast cancer. In summary, our results indicated that although gene expression levels of HSP90AA1, HSP90AA2, HSP90AB1, HSP90B1, and TRAP1 were upregulated in breast cancer patients, only the expression of HSP90AA1 was related to the tumor stage and prognostic survival.
Based on the above findings, it is expected that HSP90AA1 could act as a potential diagnostic and prognostic biomarker for breast cancer. According to the definition of the term “biomarker” by the National Institutes of Health Biomarker Definition Working Group (Boers et al., 2014), HSP90AA1 is currently recognized as a signature that is independent of conventional classifications (Fu et al., 2017). In our research, we did not find that HSP90AA1 expression was highly correlated with other detection indicators; HSP90AA1 is expected to predict a patient’s response or adverse effect to a specific treatment by improving their prognosis and quality of life (Boers et al., 2014). HSP90AA1 is inexpensive, readily available, simplified, and used objective approaches to inform clinical decision-making and stratify patients into different risk groups (Boers et al., 2014; Carethers and Jung, 2015; Fu et al., 2017; Okugawa et al., 2020). In this study, the expression of HSP90AA1 was significantly different between tumors and precancerous lesions. Further analysis of tumor patients revealed that HSP90AA1 was highly correlated with N and M stages, showing its distinguishing power for metastatic patients. Subsequently, we used the ROC curve analysis to adopt two different cut-off points for the risk of disease and the risk of metastasis. Compared with the traditional medical reference values, they showed higher accuracy despite the established evidence for B-cell, CEA, and CA153 for assessing the risk of recurrence and prognosis in breast cancer patients (Coronella-Wood and Hersh, 2003; Dai et al., 2016). Using established biomarkers for breast cancer which are currently available in clinical practice, we first developed a predictive model including HSP90AA1, which can be used to calculate breast cancer risk for breast disease and risk of distant metastasis in breast cancer. We explored the predictive value of pre-therapeutic plasma HSP90AA1 levels and developed nomograms that include HSP90AA1. These novel nomograms may help to facilitate breast cancer risk prediction and metastasis risk prediction in breast cancer patients.
The strengths of this study include its broadly clinically applicable prospects. The protein levels of HSP90AA1 were significantly elevated in tumors compared to normal mammary glands (Vartholomaiou et al., 2017). This was also observed in a breast cancer diagnostic study; they used fluorescein HS-27 in combination with HSP90 to provide a cost-effective and easy-to-implement diagnostic platform (Crouch et al., 2019). The biochemical assessment may lead to savings of almost 50% compared with medical imaging techniques (Robertson et al., 1995). Therefore, we developed and validated a novel prediction tool for breast cancer risk using a few readily available variables. Incorporating tumor markers, inflammation markers, and cell-mediated immunity markers into an easy-to-use nomogram can facilitate the prediction of risk. This study provided a relatively accurate prediction tool. Internal validation in the cohort demonstrated good discrimination and calibration power; in particular, our high C-indexes (0.77 and 0.84) for interval validation identified that this nomogram could be widely used (Wei et al., 2017). Seven indicators including HSP90, CEA, CA153, NK, B-cell, monocyte count, and d-dimer were incorporated into both models. NK, B-cell, monocyte count and d-dimer have also been reported to be related to tumor metastasis in previous studies (Liu et al., 2016; Tang et al., 2020). To the best of our knowledge, this study is the first to combine nomograms with commonly used tumor markers and HSP90 for breast cancer risk assessment and metastasis. Through this convenient evaluation method, both clinicians and high-risk patients can quickly obtain personalized risk predictions. Further, this method may be beneficial in estimating high-risk groups and guiding follow-up treatment. Although it has been reported that the expression of HSP90AA1 in tissues is significantly correlated with HR and HER2 (Klimczak et al., 2019), the expression of free HSP90AA1 is not significantly different in patients with HR (ER, PR) and HER2 positive/negative subgroups (Supplementary Figure 3B). Furthermore, in the HER2-positive subgroup, free HSP90AA1 is not a risk factor for distant metastasis in this group than other HR and HER2-negative subgroups.
Our retrospective study also has some limitations. First, our study relied exclusively on a single institutional database, although eligibility criteria were formulated to minimize selection bias. Second, the accuracy of our nomograms should be assessed via external validation, which would help to evaluate whether our nomograms are appropriate for a new population; if so, they could be generalized to other people. Further clinical trials (including a prospective cohort study) are required to illustrate and improve the validity of this model for therapeutic decision-making for breast cancer. In addition, for the experimental method we use, the ELISA kit only quantifies the target protein through the exposed epitopes in the solution instead of detecting the total protein. The interaction between the target antigen and antibody in the kit can also be affected by autoantibodies, other binding proteins, and even post-translational modification. These will lead to a decline in the diagnostic performance of the current ELISA kit (Liu et al., 2019), which will affect the application value of the model in this article. In the later stage, we will use Western blot and proteomics methods to improve our results.
Conclusion
In conclusion, our systematic and comprehensive analysis determined that HSP90AA1 of the HSP90 family can be a good diagnostic marker for breast cancer disease and metastasis. HSP90AA1 is a new disease and metastasis risk evaluation index for breast cancer patients is essential. Our study revealed that appropriate use of pretreatment plasma HSP90AA1 levels in combination with other markers could more effectively predict the patient’s cancer and metastasis rates. Quantification of preoperative HSP90AA1 may help physicians to more effectively manage risk and to determine optimal postoperative oncological follow-up strategies for patients with breast cancer.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://figshare.com/articles/figure/Plasma_HSP90AA1_Predicts_the_Risk_of_Breast_Cancer_Onset_and_Distant_Metastasis/14349362.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Guangxi Medical University Cancer Hospital (LW2020065). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
HL, KL, and LZ designed the study. ZZ, YH, JL, and WW performed the experiments. HL and SN analyzed the data. HL wrote the manuscript. All authors approved the final version of the manuscript.
Funding
This study was supported by grants from the Natural Science Foundation of Guangxi (Grant Nos. 2017GXNSFAA198155 and 2018GXNSFBA281049), the Guangxi Scientific Research and Technical Planning Project (Grant No. Gui ke AB19110018), Self-funded scientific research project of Guangxi Zhuang Autonomous Region Health Committee (Grant No. 20191022), and the Scientific Research and Technical Development Project of Qingxiu District, Nanning City, Guangxi Province (Grant Nos. 2017029, 2017036, and 2016051). This work was supported by the Guangxi Cancer Hospital Biological Resource Bank and the Shenzhen Public Service Platform on Tumor Precision Medicine and Molecular Diagnosis.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2021.639596/full#supplementary-material
Abbreviations
AUC, area under the curve; B cell, B lymphocytes%; CA125, carbohydrate antigen 125; CA153, carbohydrate antigen 153; CEA, carcinoembryonic antigen; CI, confidence interval; C-index, concordance index; D_Dimer, d-dimer; EGFR, epidermal growth factor receptor; ER, estrogen receptor; GEPIA, Gene Expression Profiling Interactive Analysis; HER2, human epidermal growth factor receptor 2; HSP90s, HSP90 family genes; LASSO, least absolute shrinkage and selection operator; LYMPH#, lymphocyte count; MONO#, monocyte count; NEUT#, neutrophil count; NK cell, natural killer lymphocytes%; OS, overall survival; PLT#, platelet count; PR, progesterone receptor; ROC, receiver operating characteristic; T cell, T lymphocytes%; Tc, cytotoxic T cells%; TCGA, the Cancer Genome Atlas; Th, helper T cell%; THPA, the Human Protein Atlas.
Footnotes
- ^ https://www.oncomine.org
- ^ http://gepia2.cancer-pku.cn
- ^ http://ualcan.path.uab.edu/index.html
- ^ https://www.proteinatlas.org
References
Balachandran, V. P., Gonen, M., Smith, J. J., and DeMatteo, R. P. (2015). Nomograms in oncology: more than meets the eye. Lancet Oncol. 16, e173–e180. doi: 10.1016/S1470-2045(14)71116-7
Boers, M., Idzerda, L., Kirwan, J. R., Beaton, D., Escorpizo, R., Boonen, A., et al. (2014). Toward a generalized framework of core measurement areas in clinical trials: a position paper for omeract 11. J. Rheumatol. 41, 978–985. doi: 10.3899/jrheum.131307
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., and Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424. doi: 10.3322/caac.21492
Calderwood, S. K., and Gong, J. (2016). Heat shock proteins promote cancer: it’s a protection racket. Trends Biochem. Sci. 41, 311–323. doi: 10.1016/j.tibs.2016.01.003
Carethers, J. M., and Jung, B. H. (2015). Genetics and genetic biomarkers in sporadic colorectal cancer. Gastroenterology 149, 1177–1190.e3. doi: 10.1053/j.gastro.2015.06.047
Chehab, M., Caza, T., Skotnicki, K., Landas, S., Bratslavsky, G., Mollapour, M., et al. (2015). Targeting Hsp90 in urothelial carcinoma. Oncotarget 6, 8454–8473. doi: 10.18632/oncotarget.3502
Cheng, Q., Chang, J. T., Geradts, J., Neckers, L. M., Haystead, T., Spector, N. L., et al. (2012). Amplification and high-level expression of heat shock protein 90 marks aggressive phenotypes of human epidermal growth factor receptor 2 negative breast cancer. Breast Cancer Res. 14:R62. doi: 10.1186/bcr3168
Condelli, V., Crispo, F., Pietrafesa, M., Lettini, G., Matassa, D. S., Esposito, F., et al. (2019). HSP90 molecular chaperones, metabolic rewiring, and epigenetics: impact on tumor progression and perspective for anticancer therapy. Cells 8:532. doi: 10.3390/cells8060532
Coronella-Wood, J. A., and Hersh, E. M. (2003). Naturally occurring B-cell responses to breast cancer. Cancer Immunol. Immunother. 52, 715–738. doi: 10.1007/s00262-003-0409-4
Crouch, B. T., Gallagher, J., Wang, R., Duer, J., Hall, A., Soo, M. S., et al. (2019). Exploiting heat shock protein expression to develop a non-invasive diagnostic tool for breast cancer. Sci. Rep. 9:3461. doi: 10.1038/s41598-019-40252-y
Dai, D., Chen, B., Tang, H., Wang, B., Zhao, Z., Xie, X., et al. (2016). Nomograms for predicting the prognostic value of pre-therapeutic CA15-3 and CEA serum levels in TNBC patients. PLoS One 11:e0161902. doi: 10.1371/journal.pone.0161902
Dejeans, N., Glorieux, C., Guenin, S., Beck, R., Sid, B., Rousseau, R., et al. (2012). Overexpression of GRP94 in breast cancer cells resistant to oxidative stress promotes high levels of cancer cell proliferation and migration: implications for tumor recurrence. Free Radic. Biol. Med. 52, 993–1002. doi: 10.1016/j.freeradbiomed.2011.12.019
Eustace, B. K., Sakurai, T., Stewart, J. K., Yimlamai, D., Unger, C., Zehetmeier, C., et al. (2004). Functional proteomic screens reveal an essential extracellular role for hsp90α in cancer cell invasiveness. Nat. Cell Biol. 6, 507–514. doi: 10.1038/ncb1131
Frydman, J. (2001). Folding of newly translated proteins in vivo: the role of molecular chaperones. Annu. Rev. Biochem. 70, 603–647. doi: 10.1146/annurev.biochem.70.1.603
Fu, Y., Xu, X., Huang, D., Cui, D., Liu, L., Liu, J., et al. (2017). Plasma heat shock protein 90alpha as a biomarker for the diagnosis of liver cancer: an official, large-scale, and multicenter clinical trial. EBioMedicine 24, 56–63. doi: 10.1016/j.ebiom.2017.09.007
Haase, M., and Fitze, G. (2016). HSP90AB1: helping the good and the bad. Gene 575, 171–186. doi: 10.1016/j.gene.2015.08.063
Klimczak, M., Biecek, P., Zylicz, A., and Zylicz, M. (2019). Heat shock proteins create a signature to predict the clinical outcome in breast cancer. Sci. Rep. 9:7507. doi: 10.1038/s41598-019-43556-1
Liu, H. Z., Deng, W., Li, J. L., Tang, Y. M., Zhang, L. T., Cui, Y., et al. (2016). Peripheral blood lymphocyte subset levels differ in patients with hepatocellular carcinoma. Oncotarget 7, 77558–77564. doi: 10.18632/oncotarget.13041
Liu, S., Li, R., Zuo, S., Luo, R., Fang, W., and Xie, Y. (2018). GRP94 overexpression as an indicator of unfavorable outcomes in breast cancer patients. Int. J. Clin. Exp. Pathol. 11, 3061–3067.
Liu, W., Li, J., Zhang, P., Hou, Q., Feng, S., Liu, L., et al. (2019). A novel pan-cancer biomarker plasma heat shock protein 90alpha and its diagnosis determinants in clinic. Cancer Sci. 110, 2941–2959. doi: 10.1111/cas.14143
McDowell, C. L., Bryan Sutton, R., and Obermann, W. M. J. (2009). Expression of Hsp90 chaperome proteins in human tumor tissue. Int. J. Biol. Macromol. 45, 310–314. doi: 10.1016/j.ijbiomac.2009.06.012
Okugawa, Y., Toiyama, Y., Yamamoto, A., Shigemori, T., Ide, S., Kitajima, T., et al. (2020). Lymphocyte-C-reactive protein ratio as promising new marker for predicting surgical and oncological outcomes in colorectal cancer. Ann. Surg. 272, 342–351. doi: 10.1097/SLA.0000000000003239
Ono, K., Eguchi, T., Sogawa, C., Calderwood, S. K., Futagawa, J., Kasai, T., et al. (2018). HSP-enriched properties of extracellular vesicles involve survival of metastatic oral cancer cells. J. Cell. Biochem. 119, 7350–7362. doi: 10.1002/jcb.27039
Pencina, M. J., D’Agostino, R. B., D’Agostino, R. B., and Vasan, R. S. (2008). Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat. Med. 27, 157–172. doi: 10.1002/sim.2929
Randow, F., and Seed, B. (2001). Endoplasmic reticulum chaperone gp96 is required for innate immunity but not cell viability. Nat. Cell Biol. 3, 891–896. doi: 10.1038/ncb1001-891
Robertson, J. F. R., Whynes, D. K., Dixon, A., and Blamey, R. W. (1995). Potential for cost economies in guiding therapy in patients with metastatic breast cancer. Br. J. Cancer 72, 174–177. doi: 10.1038/bjc.1995.297
Schopf, F. H., Biebl, M. M., and Buchner, J. (2017). The HSP90 chaperone machinery. Nat. Rev. Mol. Cell Biol. 18, 345–360. doi: 10.1038/nrm.2017.20
Shi, Y., Liu, X., Lou, J., Han, X., Zhang, L., Wang, Q., et al. (2014). Plasma levels of heat shock protein 90 alpha associated with lung cancer development and treatment responses. Clin. Cancer Res. 20, 6016–6022. doi: 10.1158/1078-0432.CCR-14-0174
Siegel, R. L., Miller, K. D., and Jemal, A. (2018). Cancer statistics, 2018. CA Cancer J. Clin. 68, 7–30. doi: 10.3322/caac.21442
Skrzypczak, M., Goryca, K., Rubel, T., Paziewska, A., Mikula, M., Jarosz, D., et al. (2010). Modeling oncogenic signaling in colon tumors by multidirectional analyses of microarray data directed for maximization of analytical reliability. PLoS One 5:e13091. doi: 10.1371/journal.pone.0013091
Tang, Y. P., Xie, M. Z., Li, K. Z., Li, J. L., Cai, Z. M., and Hu, B. L. (2020). Prognostic value of peripheral blood natural killer cells in colorectal cancer. BMC Gastroenterol. 20:31. doi: 10.1186/s12876-020-1177-8
Tian, Y., Wang, C., Chen, S., Liu, J., Fu, Y., and Luo, Y. (2019). Extracellular Hsp90á and clusterin synergistically promote breast cancer epithelial-to-mesenchymal transition and metastasis via LRP1. J. Cell Sci. 132:jcs228213. doi: 10.1242/JCS.228213
Trepel, J., Mollapour, M., Giaccone, G., and Neckers, L. (2010). Targeting the dynamic HSP90 complex in cancer. Nat. Rev. Cancer 10, 537–549. doi: 10.1038/nrc2887
Vartholomaiou, E., Madon-Simon, M., Hagmann, S., Mühlebach, G., Wurst, W., Floss, T., et al. (2017). Cytosolic Hsp90a and its mitochondrial isoform Trap1 are differentially required in a breast cancer model. Oncotarget 8, 17428–17442. doi: 10.18632/oncotarget.15659
Wang, X., Song, X., Zhuo, W., Fu, Y., Shi, H., Liang, Y., et al. (2009). The regulatory mechanism of Hsp90α secretion and its function in tumor malignancy. Proc. Natl. Acad. Sci. U.S.A. 106, 21288–21293. doi: 10.1073/pnas.0908151106
Wei, L., Champman, S., Li, X., Li, X., Li, S., Chen, R., et al. (2017). Beliefs about medicines and non-adherence in patients with stroke, diabetes mellitus and rheumatoid arthritis: a cross-sectional study in China. BMJ Open 7:e017293. doi: 10.1136/bmjopen-2017-017293
Wei, W., Liu, M., Ning, S., Wei, J., Zhong, J., Li, J., et al. (2020). Diagnostic value of plasma HSP90α levels for detection of hepatocellular carcinoma. BMC Cancer 20:6. doi: 10.1186/s12885-019-6489-0
Whitesell, L., and Lindquist, S. L. (2005). HSP90 and the chaperoning of cancer. Nat. Rev. Cancer 5, 761–772. doi: 10.1038/nrc1716
Wolbers, M., Koller, M. T., Witteman, J. C. M., and Steyerberg, E. W. (2009). Prognostic models with competing risks methods and application to coronary risk prediction. Epidemiology 20, 555–561. doi: 10.1097/EDE.0b013e3181a39056
Wu, J., Liu, T., Rios, Z., Mei, Q., Lin, X., and Cao, S. (2017). Heat shock proteins and cancer. Trends Pharmacol. Sci. 38, 226–256. doi: 10.1016/j.tips.2016.11.009
Young, J. C., Hoogenraad, N. J., and Hartl, F. U. (2003). Molecular chaperones Hsp90 and Hsp70 deliver preproteins to the mitochondrial import receptor Tom70. Cell 112, 41–50. doi: 10.1016/S0092-8674(02)01250-3
Zhang, M., Li, S. S., Xie, Q. M., Xu, J. H., Sun, X. X., Pan, F. M., et al. (2018). Associations of HSP90AA2 gene polymorphisms with disease susceptibility, glucocorticoids efficacy and health-related quality of life in Chinese systemic lupus erythematosus patients. Genes Genomics 40, 1069–1079. doi: 10.1007/s13258-018-0714-0
Keywords: breast cancer, metastasis, heat shock protein-90, prognosis, nomogram
Citation: Liu H, Zhang Z, Huang Y, Wei W, Ning S, Li J, Liang X, Liu K and Zhang L (2021) Plasma HSP90AA1 Predicts the Risk of Breast Cancer Onset and Distant Metastasis. Front. Cell Dev. Biol. 9:639596. doi: 10.3389/fcell.2021.639596
Received: 09 December 2020; Accepted: 03 May 2021;
Published: 24 May 2021.
Edited by:
Shazib Pervaiz, National University of Singapore, SingaporeReviewed by:
Gregory Lucien Bellot, National University of Singapore, SingaporeTongzheng LIU, Jinan University, China
Copyright © 2021 Liu, Zhang, Huang, Wei, Ning, Li, Liang, Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kaisheng Liu, a2Fpc2hlbmdfbGl1QDE2My5jb20=; Litu Zhang, emhhbmdsaXR1QGdtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Haizhou Liu
Haizhou Liu Zihan Zhang
Zihan Zhang Yi Huang1†
Yi Huang1† Jilin Li
Jilin Li Kaisheng Liu
Kaisheng Liu Litu Zhang
Litu Zhang