
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CORRECTION article
Front. Cell Dev. Biol. , 24 February 2021
Sec. Signaling
Volume 9 - 2021 | https://doi.org/10.3389/fcell.2021.639486
This article is a correction to:
The Reciprocal Causation of the ASK1-JNK1/2 Pathway and Endoplasmic Reticulum Stress in Diabetes-Induced Cognitive Decline
 Yanqing Wu1,2†
Yanqing Wu1,2† Yuan Yuan2†
Yuan Yuan2† Chengbiao Wu3†
Chengbiao Wu3† Ting Jiang2
Ting Jiang2 Beini Wang2
Beini Wang2 Jun Xiong2
Jun Xiong2 Peipei Zheng2
Peipei Zheng2 Yiyang Li2
Yiyang Li2 Jingyu Xu1
Jingyu Xu1 Ke Xu1
Ke Xu1 Yaqian Liu2
Yaqian Liu2 Xiaokun Li2
Xiaokun Li2 Jian Xiao2*
Jian Xiao2*A Corrigendum on
The Reciprocal Causation of the ASK1-JNK1/2 Pathway and Endoplasmic Reticulum Stress in Diabetes-Induced Cognitive Decline
by Wu, Y., Yuan, Y., Wu, C., Jiang, T., Wang, B., Xiong, J., et al. (2020). Front. Cell Dev. Biol. 8:602. doi: 10.3389/fcell.2020.00602
In the original article, there were some mistakes in “Figure 1G, (Figures 2A, C, and D) and Figure 6B” as published. The image of representative swimming track of db/m mice in the probe trial (24 h after training) (Figure 1G), and the images of immunohistochemical staining of Aβ1−42 and immunofluorescence staining of p-Tau in the CA1 region of db/m mice (Figure 2A) should be replaced with appropriate images. The last result of quantitative analysis in Figure 6B should be the quantitative analysis of CHOP. The labels in Figures 2C and D of Synapthysin-1 and Synapsin should be Synapthysin and Synapsin-1. The corrected Figures appears below.
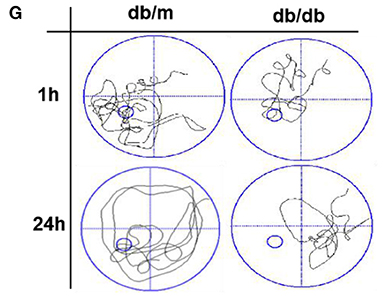
Figure 1. Diabetes significantly induces cognitive decline of mice. (A) The learning curve of the training period of mice during six blocks in the Morris water maze test. (B) Representative swimming track of mice at block 1 and block 6 during the training period. (C) Number of crossings over the original platform location of mice in the probe trial (1 h after training). (D) Latency to find the platform of mice in the probe trial (1 h after training). (E) Number of crossings over the original platform location of mice in the probe trial (24 h after training). (F) Latency to find the platform of mice in the probe trial (24 h after training). (G) Representative swimming track of mice in the probe trial (1 and 24 h after training). (H) Percentage of residence time in each quadrant. The quadrant with the platform was designated as TQ and the quadrant from which the mice started their swimming was designated as OP for “opposite”. The quadrant on the left side of OP was designated as AL for “adjacent left” and the quadrant on the right side of OP was designated as AR for “adjacent right”. *p < 0.05, **p < 0.01 vs db/m, n = 10.
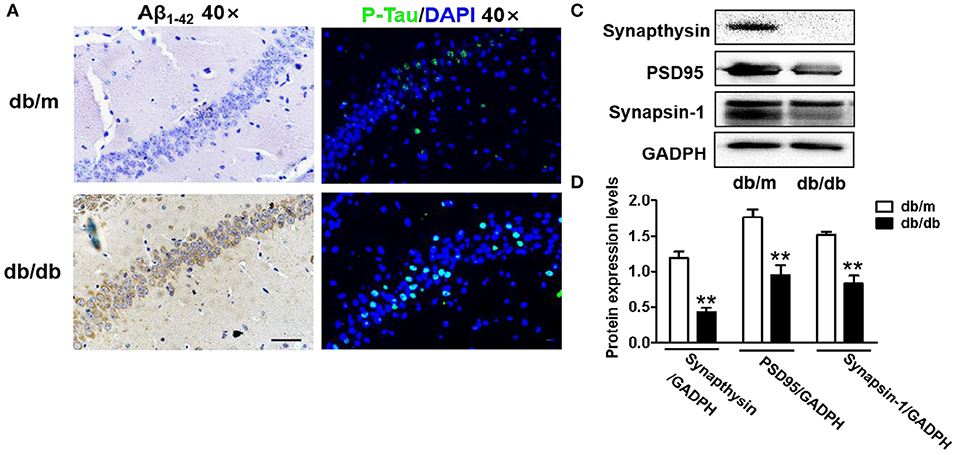
Figure 2. Diabetes significantly leads to morphological changes, Aβ1-42 deposition, hyperphosphorylation of Tau and synaptic dysfunction in the hippocampus during DICD. (A) The representative images of H&E staining, Nissl staining, immunohistochemical staining of Aβ1-42, and immunofluorescence staining of p-Tau in the CA1 region of the hippocampus of db/m mice and db/db mice (scale bar = 15 μm). (B) Westere blotting and quantitative analysis of Aβ1-42 in the hippocampus of db/m mice and db/db mice. (C,D) Western blotting and quantitative analysis of synaptic function-related protein expression (PSD95, synaptophysin, and synapsin-1) in the hippocampus of db/m mice and db/db mice. *p < 0.05, **p < 0.01 vs db/m mice, n = 3.
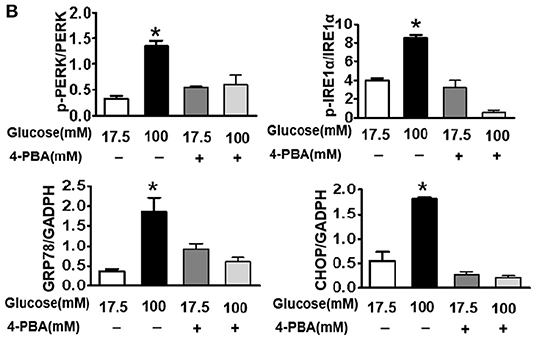
Figure 6. Suppressing ER stress by 4-PBA blocks HG-triggered ASK1-JNK1/2 signaling activation and excessive apoptosis in SH-SY5Y cells. Glucose (100 μM) was used as the HG condition. 4-PBA (2 mM) was used to inhibit ER stress. (A,B) Western blotting and quantitative analysis of p-PERK, p-IRE1α, GRP78, and CHOP expression in SH-SY5Y cells under HG with or without 4-PBA. (C,D) Western blotting and quantitative analysis of p-ASK1, TRAF2, p-JNK1/2, and p-FoxO3a expression in SH-SY5Y cells under HG with or without 4-PBA. (E,F) Western blotting and quantitative analysis of Bax and cleaved caspase-3 expression in SH-SY5Y cells under HG with or without 4-PBA. (G) Representative images of the TUNEL assay showing apoptotic cells (green signal) in SH-SY5Y cells under HG with or without 4-PBA. Cell nuclei were stained with DAPI (blue) (scale bar = 15 μm). HG: high glucose; 4-PBA: 4-phenylbutyric acid. *p < 0.05 vs the other group, n = 3.
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
Keywords: diabetes-induced cognitive decline (DICD), hippocampus, neuronal apoptosis, apoptosis signal-regulating kinase 1 (ASK1), endoplasmic reticulum (ER) stress
Citation: Wu Y, Yuan Y, Wu C, Jiang T, Wang B, Xiong J, Zheng P, Li Y, Xu J, Xu K, Liu Y, Li X and Xiao J (2021) Corrigendum: The Reciprocal Causation of the ASK1-JNK1/2 Pathway and Endoplasmic Reticulum Stress in Diabetes-Induced Cognitive Decline. Front. Cell Dev. Biol. 9:639486. doi: 10.3389/fcell.2021.639486
Received: 09 December 2020; Accepted: 08 February 2021;
Published: 24 February 2021.
Edited and reviewed by: José Lozano, University of Malaga, Spain
Copyright © 2021 Wu, Yuan, Wu, Jiang, Wang, Xiong, Zheng, Li, Xu, Xu, Liu, Li and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Xiao, eGZ4ajIwMDBAMTI2LmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.