
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Cell Dev. Biol., 19 February 2021
Sec. Stem Cell Research
Volume 9 - 2021 | https://doi.org/10.3389/fcell.2021.635340
This article is part of the Research TopicHair Follicle Stem Cell Regeneration in AgingView all 21 articles
During aging, the skin undergoes changes in architecture and composition. Skin aging phenotypes occur due to accumulated changes in the genome/epigenome, cytokine/cell adhesion, cell distribution/extracellular matrix (ECM), etc. Here we review data suggesting that tissue mechanics also plays a role in skin aging. While mouse and human skin share some similarities, their skin architectures differ in some respects. However, we use recent research in haired murine skin because of the available experimental data. Skin suffers from changes in both its appendages and inter-appendage regions. The elderly exhibit wrinkles and loose dermis and are more likely to suffer from wounds and superficial abrasions with poor healing. They also have a reduction in the number of skin appendages. While telogen is prolonged in aging murine skin, hair follicle stem cells can be rejuvenated to enter anagen if transplanted to a young skin environment. We highlight recent single-cell analyses performed on epidermis and aging human skin which identified new basal cell subpopulations that shift in response to wounding. This may be due to alterations of basement membrane stiffness which would change tissue mechanics in aging skin, leading to altered homeostatic dynamics. We propose that the extracellular matrix (ECM) may play a key role as a chemo-mechanical integrator of the multi-layered senescence-associated signaling pathways, dictating the tissue mechanical landscape of niche microenvironments in aging phenotypes. We show examples where failed chemo-mechanical signaling leads to deteriorating homeostasis during skin aging and suggest potential therapeutic strategies to guide future research to delay the aging processes.
As skin ages, it undergoes physiological and pathological changes causing a decline in both structure and function (Gilchrest, 1989; Tobin, 2017). These changes affect many aspects of skin biology, including (1) compromised barrier function and mechanical protection, (2) dampened immune responses, (3) impaired thermoregulation, (4) decreased sweat and sebum production, (5) delayed hair cycling (Chueh et al., 2013; Chen et al., 2016), and (6) delayed wound healing (Gosain and DiPietro, 2004). Aging is complex involving both intrinsic and extrinsic factors. Earlier work by Hayflick suggests cells have a fixed cellular lifespan, leading to the identification of roles of telomere in aging (Kameda et al., 2020). Recent epigenetic studies identified unique age-related methylome changes associated with the aging epigenome (Zhang et al., 2020). Environmental stresses such as somatic mutations, free radical damage, senescence-associated secretory phenotype, autophagy, chronic inflammation, etc. can also promote senescence (Di Micco et al., 2020; Tabibzadeh, 2021). These findings have identified new concepts and therapeutic targets fostering the hope that aging can be delayed and perhaps tissues can be rejuvenated by modifying the epigenome (Guarasci et al., 2019) or by activating resident tissue stem cells (Fuchs and Blau, 2020). While these are important aspects of the aging process, in this mini-review, we discuss the role of tissue mechanics in the aging skin. Tensional homeostasis, a process that balances the extracellular forces exerted on cells by extracellular matrix (ECM) or neighboring cells and the reciprocal forces, is essential to maintain cell function and tissue remodeling. While we consider this concept has a role in both human and mouse aging skin, we will focus more on the haired murine skin because of the available reports. During mouse skin aging, the tissue mechanics landscape shifts greatly, as skin homeostasis declines. Based on these reports, we suggest some potential therapeutic strategies at the end.
Manifestations of the aging skin include rough skin textures, wrinkles, laxity, atrophy, pigmentary changes, loss of underlying fat, dry and itchy skin, inability to perspire sufficiently, hair graying, hair loss, thinning of nail plates amongst others (Farage et al., 2013). Recent reports show these traits are affected by both intrinsic (chronological) and extrinsic (non-genetic) factors (Parsons, 1996; Gunn et al., 2009; Tobin, 2017).
Intrinsically aged skin appears dry and pale with fine wrinkles and increased laxity. This results from gradual physiologic changes include reductions of cell number, collagen production, blood flow, lipids, and rete ridges (Montagna et al., 1989; Tobin, 2017). Extrinsic human skin aging entails changes caused by environmental factors, including ultraviolet light (UV). UV damage upregulates matrix metalloproteases (MMP) (Fisher et al., 1996; Quan et al., 2013) and impacts collagen degradation and synthesis (Varani et al., 2006), as well as the production of elastotic materials in the skin. This creates a microenvironment of fragmented collagen and leads to aberrant collagen homeostasis. There is controversy regarding the effect of aging, whether intrinsic or extrinsic, on epidermal thickness (El-Domyati et al., 2002). Some studies have suggested that intrinsic aging tends to cause a slight overall thinning of the viable epidermis (Lavker, 1979; Lavker and Kligman, 1988; Branchet et al., 1990), while others have found that extrinsic aging tends to cause irregular thickening of the epidermis (Gilchrest, 1989). Recently, single-cell RNA analyses revealed a progressive accumulation of photoaging-related changes and increased chronic inflammation in aging human eyelid skin (Zou et al., 2020). Hair follicular stem cells (HFSC) were shown to be nearly normal in human androgenetic alopecia; the problem is the failure of the niche to convert bulge stem cells (SC) into hair germs, hence resulting in prolonged telogen (Garza et al., 2011). These changes also contribute to hair follicle miniaturization and lowered hair density (Matsumura et al., 2016) and the competition of HFSC fates (Liu et al., 2019).
Solar elastosis, characterized by the replacement of normal elastic fibers with a disordered mass of elastotic material near the dermal-epidermal junction, is the hallmark of photodamaged dermis (Yaar and Gilchrest, 2007). Senile purpura, characterized by recurrent formation of ecchymoses on the sun-exposed extensor surfaces of the extremities, is another disease related to reduction of connective tissue in the dermis. Age-related skin thinning and sun-induced damage of the dermal connective tissue results in inadequate support, increased fragility and rupture of the microvasculature (Fenske and Lober, 1986) as well as decreased wound tensile strength (Thomas, 2001); its cosmetic concerns can negatively impact a patient’s quality of life (McKnight et al., 2015).
Within the aging mouse skin, the cell number and turnover rate decrease across all cell types (Farage et al., 2013), which impacts epidermal thickness and barrier integrity (Minematsu et al., 2011). Langerhans cells also decrease in number, leading to impaired immune responses (Wulf et al., 2004). Melanocytes lose their enzyme activity, resulting in uneven pigmentation (Nishimura et al., 2005). The flattening of the dermo-epidermal junction alters the epidermal anchoring system (Le Varlet et al., 1998), and hence the skin becomes more vulnerable to dermo-epidermal separation. In the aging dermis, the cellularity, vascularity, innervation, and ECM content also decrease (Makrantonaki and Zouboulis, 2007). The structure of sweat glands becomes distorted and the number of functional sweat glands decreases (Fenske and Lober, 1986). The loss of adipose tissue hinders thermoregulatory function and other signaling roles (Zwick et al., 2018). Interestingly, aging-associated decreases in dermal thickness corresponds with increases in dermal white adipose tissue (dWAT) thickness (Kruglikov et al., 2019b; Zhang et al., 2021). Mature dermal adipocytes de-differentiate and release free fatty acids into the surrounding ECM, which further changes the metabolism and strongly modulates the physiology of adjacent dermal fibroblasts, significantly influencing their collagen expression (Kruglikov and Scherer, 2016). The reduced number and activity of fibroblasts were implicated as the cause of decreased dermal ECM synthesis, leading to deranged ECM support and adequate functions for the skin and skin appendages (Cole et al., 2018). Low fibroblast activity also affects collagen fibril crosslinking (Yang et al., 2008), resulting in a rigid but weakened dermis (Silver et al., 2003). Disorganized ECM also decreases the amount of mechanical loading experienced by the dermal fibroblasts and a possibly diminished ability of the skin to respond to mechanical stimuli, creating a vicious cycle that further deteriorates the homeostasis and physiological functioning of the aged skin (Wu et al., 2011). The structural changes in aging skin and the multilayer control of skin homeostasis (Figures 1A,B), wound healing, and HFSC interaction with the surrounding ECM will be further discussed.
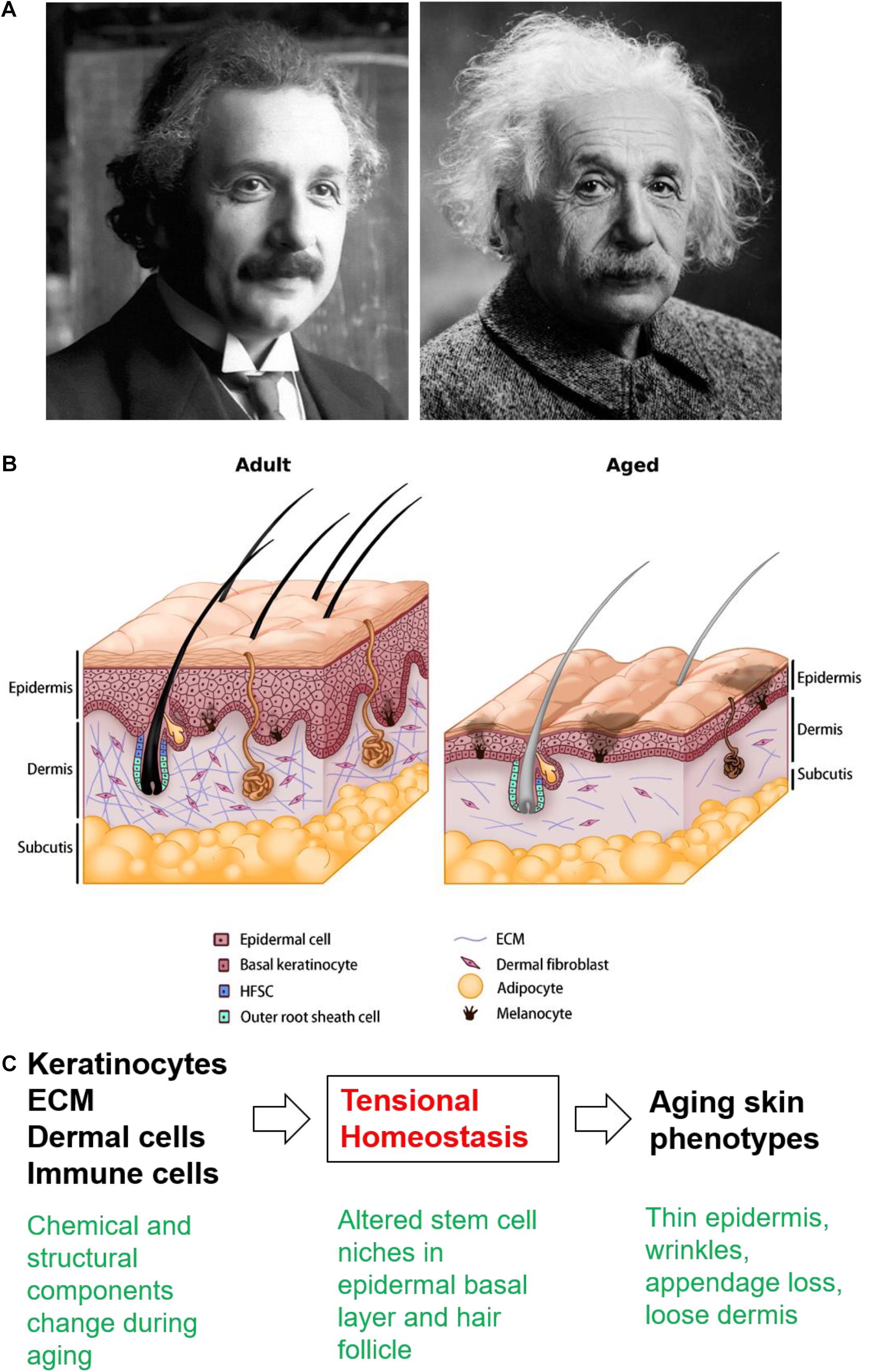
Figure 1. The aging skin. (A) Young and old Albert Einstein used to illustrate the changes in the appearance of aging skin. Source: Pixabay. (B) Schematic drawing showing skin architecture in the adult and aged skin. In aged skin, the overall thickness decreases progressively across all layers, accompanied by wrinkles, pigmentary changes, loss of underlying fat, hair graying, hair loss, and decreased sebaceous gland function. The reduced number and altered activity of fibroblasts have been implicated as the underlying cause of decreased dermal ECM synthesis, leading to deranged ECM support and affects collagen fibril crosslinking. The changes in decreasing cell numbers and altered ECM production and assembly also contribute to hair follicle miniaturization and lowered hair density in aged skin. (C) Cellular/molecular events reviewed in the text present potential new therapeutic targets.
Adult skin SC are vital for replacing cells in tissues, but their capacity declines with age. SC are regulated by both macroenvironmental signals (Chen and Chuong, 2012; Chen et al., 2016) and their microenvironmental niche (Ge et al., 2020). On the macroenvironment level, the hair cycle in aged mouse skin shows higher and longer expression of HFSC activation inhibitors such as BMPs, DKK1, and SFRPs in the dermis (Chen et al., 2014). On the cellular level, HFSC from aged skin show defects in Nfatc1 (Keyes et al., 2013) and FoxC1 (Lay et al., 2016; Wang et al., 2016). Microenvironmental factors including ECM (Ge et al., 2020), immune, sensory nerves and arrector pili (Shwartz et al., 2020) all take part in regulating HFSC activity. Expression and maintenance of the hemidesmosome component Col17A1 are critical for skin homeostasis and skin epithelial stem cell retention (Matsumura et al., 2016; Liu et al., 2019). Tenascin C (Tnc), an element of the ECM, is shown to promote ECM integrity and is reduced in aged skin (Choi et al., 2020). Thus, both intrinsic and extrinsic factors play a role in the aging of HFSC (Lei and Chuong, 2016).
Tissue mechanics (Kenedi et al., 1975; Hsu et al., 2018a) can provide a new approach in contemplating stem cell regeneration in the aged skin.
The skin cells interact dynamically with the basement membrane (BM), ECM, and the dermis to shape the structure and function of the skin (Fiore et al., 2020). The dermis contains the greatest volume of ECM, which interacts with the dermal cells that generate contractile forces, especially in face of injury (Hsu et al., 2018a; Harn et al., 2019). The amount, content, and organization of ECM being synthesized and remodeled by the MMPs in the dermis is in an intricate balance to maintain the homeostasis of the skin and in aging (Naylor et al., 2011; Tracy et al., 2016). In turn, the ECM provides not only biochemical but also mechanical cues to impact cell behavior (Watt and Fujiwara, 2011). Cells sense mechanical stimuli via mechanosensors (e.g., integrin, hemidesmosomes, adherent junctions, stretch-sensitive ion channels) and translocate these signals into the nucleus for transcriptional response in the mechanotransduction process (Wong et al., 2011). Tensional homeostasis, a concept introduced by Weaver and colleagues to describe the balance between the extracellular forces exerted on the cells by neighboring cells, ECM and reciprocal forces generated by the cells (Paszek et al., 2005), is also essential for maintaining cell function and tissue remodeling in the skin (Harn et al., 2019).
Recent single-cell analyses identify new sub-populations of basal cells that specifically localize at the top or bottom of rete ridges (Wang et al., 2020), suggesting a mechanical control. Interfollicular epithelial cell differentiation is best described as a single step gradualistic process with a large number of transition cells between the basal and spinous layer (Lin et al., 2020). Their homeostatic states also shift in response to wounding into four different cell states that govern cell proliferation or differentiation (Haensel et al., 2020). We speculate this flexibility of cell states shown during homeostasis is also reflected on cell mechanics; the dynamic changes of cell states also create physical changes to cellular forces that drive cell migration and collective morphological changes.
Rete ridge reductions and tissue mechanical landscape changes in aging skin can either be the cause or consequence of changes in these basal layer sub-populations. Additionally, the basement membrane (BM) adjacent to HFSC serves as an arrector pili muscle (APM) niche (Fujiwara et al., 2011), which together with the sympathetic nerve can form a dual-component niche to modulate HFSC activity (Shwartz et al., 2020). ECM dysregulation that accumulates during skin aging could also affect BM properties chemically and mechanically, which in turn alters APM homeostasis and HFSC regulation.
The changes in ECM synthesis, assembly and dampened cellular response to mechanical stimuli in aged skin lead to low proliferation, discontinued migration, prolonged cell-cycle arrest, and eventually cell death (Sherratt, 2009; Wu et al., 2011). Kruglikov and Scherer reported wrinkling as a mechanical phenomenon where the mismatch of mechanical modules between adjacent epidermal-dermal or dermal-subcutaneous junctions, leads to structural instabilities inside the skin (Kruglikov and Scherer, 2018). We view wrinkles as an excessively stiff apical surface area (stratified epidermis) with a decreased basal dimension and resistance (reduced ECM, dermal cell, adipose tissue) that leads to tissue folding (Varner and Nelson, 2014). In multilayered epithelial tissue, softening, and enhanced remodeling of the BM promote tumor budding, while stiffening of the BM promotes tissue folding (Fiore et al., 2020). In parallel, what factors could contribute to the reduced rete ridges and increased wrinkles in aging skin? We propose 3 possible scenarios: (1) BM stiffness increases in aging skin, (2) loss of Col17A1 causes an increase in the ratio of differentiated cells to basal progenitor cells, and (3) decreases in dermis stiffness and length force the attached apical epidermis to bend and fold upward. The precise mechanical changes of these constituents in aging skin remains to be investigated.
Wound healing in a healthy, aged individual is delayed but not defective (Gerstein et al., 1993). This is due to their dampened cellular activity and asynchronization of pro and anti-inflammatory responses to injury (Ashcroft et al., 2002). The reduced number, activity, and migration of keratinocytes and fibroblasts in aged skin lead to delayed re-epithelialization, wound closure, and tissue reformation (Moulin et al., 2000; Ashcroft et al., 2002). On the other hand, although aged skin shows little capacity to regenerate, it is less prone to form hypertrophic scars and keloids (Hsu et al., 2018b), whose etiology is mechanically sensitive (Harn et al., 2015, 2016). Aging skin is characterized by reduced ECM deposition brought about by (1) low skin tension, (2) dampened cellular responses to mechanical stimuli coupled with (3) low pro-inflammatory signaling, and (4) the reduced capacity of dermal adipocytes to transdifferentiate into myofibroblasts (Varani et al., 2006; Zhang et al., 2019) which could underlie reduced scarring after wounding.
Perturbation to tissue mechanics or disruption of tensional homeostasis impacts physiological functioning of the skin, including hair cycling and wound healing (Hsu et al., 2018a; Harn et al., 2019).
Generally, increasing skin tension or stiffness leads to higher cell activity and ECM synthesis. Molecules responsible for mechanotransduction are also important in regulating tensional homeostasis. Epidermal-specific deletion of integrin or focal adhesion kinase (FAK) results in disfigured hair follicles and dysregulated hair cycle propagation (Essayem et al., 2006). Activation of TRPV1, a transient receptor potential cation channel involved in mechano-transduction, inhibits hair shaft lengthening and induces premature catagen (Biro et al., 2006). Tension across the wound has been demonstrated as a key inducer of scarring in hypertrophic scars and keloids (Harn et al., 2019). FAK and integrins could be important mediators of these mechanotransduction processes (Wong et al., 2012), although the reduced capacity of dermal adipocytes to de- and transdifferentiate into myofibroblasts could also be an underlying factor (Zhang et al., 2019). In contrast, the African Spiny mice skin expresses a high collagen III to I ratio producing an exceptionally soft substrate (Seifert et al., 2012). Spiny mice can regenerate their skin and all of their appendages after full-thickness wounding (Jiang et al., 2019).
The impact of skin “loosening” during aging can be deduced from the effects of mechanical force on the homeostasis of HFSC. The amount and duration of skin mechanical stretching result in HFSC proliferation (Chu et al., 2019). This depends on three major components. First, the counterbalance between Wnt and BMP-2; second, M2 subtype macrophage polarization; third, growth factor secretion, especially HGF and IGF-1 (Chu et al., 2019). However, this mechanical activation of hair cells seems compromised in the stiff and less extendable aged mice skin (Lynch et al., 2017; Meador et al., 2020) which has difficulty in eliciting a desirable regeneration response via stretching. How mechanical force, ECM and immune cells contribute to aging skin’s inadequate regenerative activity remains to be explored.
External mechanical forces such as hair plucking can also trigger the immune response-mediated regenerative process. Properly arranged hair plucking can trigger an efficient regenerative response mediated through a two-step immune response cascade, called quorum sensing (Chen et al., 2015). These examples demonstrate the importance of tissue mechanics on maintaining homeostasis of the skin and its response to injury.
Although currently there is no prominent strategy for developing new drugs against skin aging, there are a few chemical and mechanical approaches that have demonstrated potential.
Platelet-rich plasma (PRP) has been adopted to treat many degenerative disorders including hair loss (Li et al., 2012; Khatu et al., 2014) with reasonable success despite variations in current protocols (Ayatollahi et al., 2017; Picard et al., 2017). In principle, this approach is based on the various growth factors released (Gentile et al., 2017); however, its precise mechanism has not been elucidated. Charles-de-Sa et al. demonstrated that dWAT completely disappears after PRP injection and is replaced by fibrotic tissue, which is very similar to the effect observed after Bleomycin injection (Charles-de-Sa et al., 2018).
Replenishing effective growth factors or activating specific downstream genes such as Wnt-follistatin and follistatin-like 1 protein have been tested as a treatment for alopecia (Zimber et al., 2011; Chen et al., 2014). Pharmacological activation of HES1 alleviated the cellular senescence of aged dermal fibroblasts (Zou et al., 2020). Adipose-derived stem cells (ADSC) have shown effects related to dermal fibroblast activation via secretion of growth factors (Kim et al., 2011). Subcutaneous injection of ADSC significantly increased collagen density, dermal thickness, and fibroblast number in hairless mice (Fukuoka et al., 2017), and also stimulated collagen synthesis of human dermal fibroblasts (Kim et al., 2009).
The adipocytes in the dWAT have high plasticity and undergo reversible dedifferentiation, in which adipocyte-derived preadipocytes trans-differentiate into myoblasts and are involved not only in the physiological hair cycle but also under pathological conditions such as hypertrophic scarring, systemic sclerosis and androgenetic alopecia (Zhang et al., 2019). During proliferation, differentiation and dedifferentiation, various extracellular vesicles are secreted to the nearby HF and in turn stimulate its hair cycle (Kruglikov et al., 2019b). Lastly, Caveolin-1 (Cav-1) is upregulated in aged skin and is associated with altered dermal thickness, dWAT expansion and ECM expression, which sequentially influences the mechanical properties of the skin layers (Kruglikov et al., 2019a,b). This makes Cav-1 a potential candidate for anti-aging treatment especially since Mevastatin has been shown to target Cav-1 as an effective treatment for chronic wound healing (Sawaya et al., 2019).
Although the effectiveness of stretching-induced HF activation is less pronounced in aging mice (Chu et al., 2019), interestingly, subcutaneous injection of cross-linked hyaluronic acid stimulates collagen synthesis and partially restores dermal ECM that are lost in photodamaged skin (Bukhari et al., 2018; Yamada and Prow, 2020). It is speculated that this effect is achieved by mechanical stretching of the dermis and the activation of dermal fibroblasts (Chu et al., 2019). It also was demonstrated that cross-linked HA can significantly influence dWAT and subcutaneous WAT which modifies the dermal-subcutaneous junction (Kruglikov and Wollina, 2015), and directly but differentially affects the mature adipocytes and preadipocytes (Nadra et al., unpublished). Other studies have also focused on securing collagen integrity via suppressing MMP production and activity (Oh et al., 2020a), particularly in alleviating UVA-mediated suppression of collagen expression by stimulating TGF-β/Smad signaling (Oh et al., 2020b).
We review the physiological changes associated with skin aging (Table 1 and Figure 1C) and highlight the potential role of tensional homeostasis. Utilizing and providing a chemically or mechanically induced young environment might rescue aging phenotypes in the skin. This approach could potentially rescue basal layer homeostasis to control (1) epidermal thickness, (2) wound healing, (3) activation of HFSC, and (4) cell reprogramming during wound-induced hair neogenesis (Bhoopalam et al., 2020). While the haired murine skin provides an experimental model that reveals new molecular understanding summarized here, aging human skin has features that do not exist in the mouse model and await further exploration.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
CCC and SPW contributed more to the clinical aspect. HICH, ML, and CMC contributed more to the basic research elements. All authors contributed to the writing, organization, and editing of the manuscript. The manuscript was proofread by Dr. R. B. Widelitz.
HICH was supported by the Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE), The Excellent Research Center Program by the Ministry of Science and Technology (MOST 107-3017-F-006-002) in Taiwan, and NIGMS, NIH. CCC was funded by Ministry of Science and Technology (109-2314-B-010 -014 -MY3). ML was supported by the National Natural Science Foundation of China (82003384), Fundamental Research Funds for the Central Universities (2020CDJYGSG003), Chongqing Talents Program (CQYC2020058022), and Scientific Research Foundation from Chongqing University (02210011044110). CMC was funded by NIGMS, NIH (5R01GM 125322-02).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ashcroft, G. S., Mills, S. J., and Ashworth, J. J. (2002). Ageing and wound healing. Biogerontology 3, 337–345. doi: 10.1023/a:1021399228395
Ayatollahi, A., Hosseini, H., Gholami, J., Mirminachi, B., Firooz, F., and Firooz, A. (2017). Platelet rich plasma for treatment of non-scarring hair loss: systematic review of literature. J. Dermatolog. Treat. 28, 574–581. doi: 10.1080/09546634.2017.1303571
Bhoopalam, M., Garza, L. A., and Reddy, S. K. (2020). Wound induced hair neogenesis – a novel paradigm for studying regeneration and aging. Front. Cell Dev. Biol. 8:582346. doi: 10.3389/fcell.2020.582346
Biro, T., Bodo, E., Telek, A., Geczy, T., Tychsen, B., Kovacs, L., et al. (2006). Hair cycle control by vanilloid receptor-1 (TRPV1): evidence from TRPV1 knockout mice. J. Invest. Dermatol. 126, 1909–1912. doi: 10.1038/sj.jid.5700321
Branchet, M. C., Boisnic, S., Frances, C., and Robert, A. M. (1990). Skin thickness changes in normal aging skin. Gerontology 36, 28–35. doi: 10.1159/000213172
Bukhari, S. N. A., Roswandi, N. L., Waqas, M., Habib, H., Hussain, F., Khan, S., et al. (2018). Hyaluronic acid, a promising skin rejuvenating biomedicine: a review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int. J. Biol. Macromol. 120(Pt B), 1682–1695. doi: 10.1016/j.ijbiomac.2018.09.188
Charles-de-Sa, L., Gontijo-de-Amorim, N. F., Takiya, C. M., Borojevic, R., Benati, D., Bernardi, P., et al. (2018). Effect of use of platelet-rich plasma (PRP) in skin with intrinsic aging process. Aesthet. Surg. J. 38, 321–328. doi: 10.1093/asj/sjx137
Chen, C. C., and Chuong, C. M. (2012). Multi-layered environmental regulation on the homeostasis of stem cells: the saga of hair growth and alopecia. J. Dermatol. Sci. 66, 3–11. doi: 10.1016/j.jdermsci.2012.02.007
Chen, C. C., Murray, P. J., Jiang, T. X., Plikus, M. V., Chang, Y. T., Lee, O. K., et al. (2014). Regenerative hair waves in aging mice and extra-follicular modulators follistatin, dkk1, and sfrp4. J. Invest. Dermatol. 134, 2086–2096. doi: 10.1038/jid.2014.139
Chen, C. C., Plikus, M. V., Tang, P. C., Widelitz, R. B., and Chuong, C. M. (2016). The modulatable stem cell niche: tissue interactions during hair and feather follicle regeneration. J. Mol. Biol. 428, 1423–1440. doi: 10.1016/j.jmb.2015.07.009
Chen, C. C., Wang, L., Plikus, M. V., Jiang, T. X., Murray, P. J., Ramos, R., et al. (2015). Organ-level quorum sensing directs regeneration in hair stem cell populations. Cell 161, 277–290. doi: 10.1016/j.cell.2015.02.016
Choi, Y. E., Song, M. J., Hara, M., Imanaka-Yoshida, K., Lee, D. H., Chung, J. H., et al. (2020). Effects of tenascin C on the integrity of extracellular matrix and skin aging. Int. J. Mol. Sci. 21:8693. doi: 10.3390/ijms21228693
Chu, S. Y., Chou, C. H., Huang, H. D., Yen, M. H., Hong, H. C., Chao, P. H., et al. (2019). Mechanical stretch induces hair regeneration through the alternative activation of macrophages. Nat. Commun. 10:1524. doi: 10.1038/s41467-019-09402-8
Chueh, S. C., Lin, S. J., Chen, C. C., Lei, M., Wang, L. M., Widelitz, R., et al. (2013). Therapeutic strategy for hair regeneration: hair cycle activation, niche environment modulation, wound-induced follicle neogenesis, and stem cell engineering. Expert Opin. Biol. Ther. 13, 377–391. doi: 10.1517/14712598.2013.739601
Cole, M. A., Quan, T., Voorhees, J. J., and Fisher, G. J. (2018). Extracellular matrix regulation of fibroblast function: redefining our perspective on skin aging. J. Cell Commun. Signal. 12, 35–43. doi: 10.1007/s12079-018-0459-1
Di Micco, R., Krizhanovsky, V., Baker, D., and d’Adda di Fagagna, F. (2020). Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 22, 75–95. doi: 10.1038/s41580-020-00314-w
El-Domyati, M., Attia, S., Saleh, F., Brown, D., Birk, D. E., Gasparro, F., et al. (2002). Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp. Dermatol. 11, 398–405. doi: 10.1034/j.1600-0625.2002.110502.x
Essayem, S., Kovacic-Milivojevic, B., Baumbusch, C., McDonagh, S., Dolganov, G., Howerton, K., et al. (2006). Hair cycle and wound healing in mice with a keratinocyte-restricted deletion of FAK. Oncogene 25, 1081–1089. doi: 10.1038/sj.onc.1209130
Farage, M. A., Miller, K. W., Elsner, P., and Maibach, H. I. (2013). Characteristics of the aging skin. Adv. Wound Care (New Rochelle) 2, 5–10. doi: 10.1089/wound.2011.0356
Fenske, N. A., and Lober, C. W. (1986). Structural and functional changes of normal aging skin. J. Am. Acad. Dermatol. 15(4 Pt 1), 571–585. doi: 10.1016/s0190-9622(86)70208-9
Fiore, V. F., Krajnc, M., Quiroz, F. G., Levorse, J., Pasolli, H. A., Shvartsman, S. Y., et al. (2020). Mechanics of a multilayer epithelium instruct tumour architecture and function. Nature 585, 433–439. doi: 10.1038/s41586-020-2695-9
Fisher, G. J., Datta, S. C., Talwar, H. S., Wang, Z. Q., Varani, J., Kang, S., et al. (1996). Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature 379, 335–339. doi: 10.1038/379335a0
Fuchs, E., and Blau, H. M. (2020). Tissue stem cells: architects of their niches. Cell Stem Cell 27, 532–556. doi: 10.1016/j.stem.2020.09.011
Fujiwara, H., Ferreira, M., Donati, G., Marciano, D. K., Linton, J. M., Sato, Y., et al. (2011). The basement membrane of hair follicle stem cells is a muscle cell niche. Cell 144, 577–589. doi: 10.1016/j.cell.2011.01.014
Fukuoka, H., Narita, K., and Suga, H. (2017). Hair regeneration therapy: application of adipose-derived stem cells. Curr. Stem Cell Res. Ther. 12, 531–534. doi: 10.2174/1574888X12666170522114307
Garza, L. A., Yang, C. C., Zhao, T., Blatt, H. B., Lee, M., He, H., et al. (2011). Bald scalp in men with androgenetic alopecia retains hair follicle stem cells but lacks CD200-rich and CD34-positive hair follicle progenitor cells. J. Clin. Invest. 121, 613–622. doi: 10.1172/JCI44478
Ge, Y., Miao, Y., Gur-Cohen, S., Gomez, N., Yang, H., Nikolova, M., et al. (2020). The aging skin microenvironment dictates stem cell behavior. Proc. Natl. Acad. Sci. U.S.A. 117, 5339–5350. doi: 10.1073/pnas.1901720117
Gentile, P., Cole, J. P., Cole, M. A., Garcovich, S., Bielli, A., Scioli, M. G., et al. (2017). evaluation of not-activated and activated PRP in hair loss treatment: role of growth factor and cytokine concentrations obtained by different collection systems. Int. J. Mol. Sci. 18:408. doi: 10.3390/ijms18020408
Gerstein, A. D., Phillips, T. J., Rogers, G. S., and Gilchrest, B. A. (1993). Wound healing and aging. Dermatol. Clin. 11, 749–757.
Gilchrest, B. A. (1989). Skin aging and photoaging: an overview. J. Am. Acad. Dermatol. 21(3 Pt 2), 610–613. doi: 10.1016/s0190-9622(89)70227-9
Gosain, A., and DiPietro, L. A. (2004). Aging and wound healing. World J. Surg. 28, 321–326. doi: 10.1007/s00268-003-7397-6
Guarasci, F., D’Aquila, P., Montesanto, A., Corsonello, A., Bellizzi, D., and Passarino, G. (2019). Individual DNA methylation profile is correlated with age and can be targeted to modulate healthy aging and longevity. Curr. Pharm. Des. 25, 4139–4149. doi: 10.2174/1381612825666191112095655
Gunn, D. A., Rexbye, H., Griffiths, C. E., Murray, P. G., Fereday, A., Catt, S. D., et al. (2009). Why some women look young for their age. PLoS One 4:e8021. doi: 10.1371/journal.pone.0008021
Haensel, D., Jin, S., Sun, P., Cinco, R., Dragan, M., Nguyen, Q., et al. (2020). Defining epidermal basal cell states during skin homeostasis and wound healing using single-cell transcriptomics. Cell Rep. 30, e3932–e3936. doi: 10.1016/j.celrep.2020.02.091
Harn, H. I., Hsu, C. K., Wang, Y. K., Huang, Y. W., Chiu, W. T., Lin, H. H., et al. (2016). Spatial distribution of filament elasticity determines the migratory behaviors of a cell. Cell Adh. Migr. 10, 368–377. doi: 10.1080/19336918.2016.1156825
Harn, H. I., Ogawa, R., Hsu, C. K., Hughes, M. W., Tang, M. J., and Chuong, C. M. (2019). The tension biology of wound healing. Exp. Dermatol. 28, 464–471. doi: 10.1111/exd.13460
Harn, H. I., Wang, Y. K., Hsu, C. K., Ho, Y. T., Huang, Y. W., Chiu, W. T., et al. (2015). Mechanical coupling of cytoskeletal elasticity and force generation is crucial for understanding the migrating nature of keloid fibroblasts. Exp. Dermatol. 24, 579–584. doi: 10.1111/exd.12731
Hsu, C. K., Lin, H. H., Harn, H. I., Hughes, M. W., Tang, M. J., and Yang, C. C. (2018a). Mechanical forces in skin disorders. J. Dermatol. Sci. 90, 232–240. doi: 10.1016/j.jdermsci.2018.03.004
Hsu, C. K., Lin, H. H., Harn, H. I., Ogawa, R., Wang, Y. K., Ho, Y. T., et al. (2018b). Caveolin-1 controls hyperresponsiveness to mechanical stimuli and fibrogenesis-associated RUNX2 activation in keloid fibroblasts. J. Invest. Dermatol. 138, 208–218. doi: 10.1016/j.jid.2017.05.041
Jiang, T. X., Harn, H. I., Ou, K. L., Lei, M., and Chuong, C. M. (2019). Comparative regenerative biology of spiny (Acomys cahirinus) and laboratory (Mus musculus) mouse skin. Exp. Dermatol. 28, 442–449. doi: 10.1111/exd.13899
Kameda, M., Mikawa, T., Yokode, M., Inagaki, N., and Kondoh, H. (2020). Senescence research from historical theory to future clinical application. Geriatr. Gerontol. Int. doi: 10.1111/ggi.14121 [Epub ahead of print].
Kenedi, R. M., Gibson, T., Evans, J. H., and Barbenel, J. C. (1975). Tissue mechanics. Phys. Med. Biol. 20, 699–717. doi: 10.1088/0031-9155/20/5/001
Keyes, B. E., Segal, J. P., Heller, E., Lien, W. H., Chang, C. Y., Guo, X., et al. (2013). Nfatc1 orchestrates aging in hair follicle stem cells. Proc. Natl. Acad. Sci. U.S.A. 110, E4950–E4959. doi: 10.1073/pnas.1320301110
Khatu, S. S., More, Y. E., Gokhale, N. R., Chavhan, D. C., and Bendsure, N. (2014). Platelet-rich plasma in androgenic alopecia: myth or an effective tool. J. Cutan. Aesthet. Surg. 7, 107–110. doi: 10.4103/0974-2077.138352
Kim, J. H., Jung, M., Kim, H. S., Kim, Y. M., and Choi, E. H. (2011). Adipose-derived stem cells as a new therapeutic modality for ageing skin. Exp. Dermatol. 20, 383–387. doi: 10.1111/j.1600-0625.2010.01221.x
Kim, W. S., Park, B. S., Park, S. H., Kim, H. K., and Sung, J. H. (2009). Antiwrinkle effect of adipose-derived stem cell: activation of dermal fibroblast by secretory factors. J. Dermatol. Sci. 53, 96–102. doi: 10.1016/j.jdermsci.2008.08.007
Kruglikov, I. L., and Scherer, P. E. (2016). Dermal adipocytes: from irrelevance to metabolic targets? Trends Endocrinol. Metab. 27, 1–10. doi: 10.1016/j.tem.2015.11.002
Kruglikov, I. L., and Scherer, P. E. (2018). Skin aging as a mechanical phenomenon: the main weak links. Nutr. Healthy Aging 4, 291–307. doi: 10.3233/NHA-170037
Kruglikov, I. L., and Wollina, U. (2015). Soft tissue fillers as non-specific modulators of adipogenesis: change of the paradigm? Exp. Dermatol. 24, 912–915. doi: 10.1111/exd.12852
Kruglikov, I. L., Zhang, Z., and Scherer, P. E. (2019a). Caveolin-1 in skin aging – from innocent bystander to major contributor. Ageing Res. Rev. 55:100959. doi: 10.1016/j.arr.2019.100959
Kruglikov, I. L., Zhang, Z., and Scherer, P. E. (2019b). The role of immature and mature adipocytes in hair Cycling. Trends Endocrinol. Metab. 30, 93–105. doi: 10.1016/j.tem.2018.11.004
Lavker, R. M. (1979). Structural alterations in exposed and unexposed aged skin. J. Invest. Dermatol. 73, 59–66. doi: 10.1111/1523-1747.ep12532763
Lavker, R. M., and Kligman, A. M. (1988). Chronic heliodermatitis: a morphologic evaluation of chronic actinic dermal damage with emphasis on the role of mast cells. J. Invest. Dermatol. 90, 325–330. doi: 10.1111/1523-1747.ep12456193
Lay, K., Kume, T., and Fuchs, E. (2016). FOXC1 maintains the hair follicle stem cell niche and governs stem cell quiescence to preserve long-term tissue-regenerating potential. Proc. Natl. Acad. Sci. U.S.A. 113, E1506–E1515. doi: 10.1073/pnas.1601569113
Le Varlet, B., Chaudagne, C., Saunois, A., Barre, P., Sauvage, C., Berthouloux, B., et al. (1998). Age-related functional and structural changes in human dermo-epidermal junction components. J. Investig. Dermatol. Symp. Proc. 3, 172–179. doi: 10.1038/jidsymp.1998.34
Lei, M., and Chuong, C. M. (2016). Stem cells. aging, alopecia, and stem cells. Science 351, 559–560. doi: 10.1126/science.aaf1635
Li, Z. J., Choi, H. I., Choi, D. K., Sohn, K. C., Im, M., Seo, Y. J., et al. (2012). Autologous platelet-rich plasma: a potential therapeutic tool for promoting hair growth. Dermatol. Surg. 38(7 Pt 1), 1040–1046. doi: 10.1111/j.1524-4725.2012.02394.x
Lin, Z., Jin, S., Chen, J., Li, Z., Lin, Z., Tang, L., et al. (2020). Murine interfollicular epidermal differentiation is gradualistic with GRHL3 controlling progression from stem to transition cell states. Nat. Commun. 11:5434. doi: 10.1038/s41467-020-19234-6
Liu, N., Matsumura, H., Kato, T., Ichinose, S., Takada, A., Namiki, T., et al. (2019). Stem cell competition orchestrates skin homeostasis and ageing. Nature 568, 344–350. doi: 10.1038/s41586-019-1085-7
Lynch, B., Bonod-Bidaud, C., Ducourthial, G., Affagard, J. S., Bancelin, S., Psilodimitrakopoulos, S., et al. (2017). How aging impacts skin biomechanics: a multiscale study in mice. Sci. Rep. 7:13750. doi: 10.1038/s41598-017-13150-4
Makrantonaki, E., and Zouboulis, C. C. (2007). Molecular mechanisms of skin aging: state of the art. Ann. N. Y. Acad. Sci. 1119, 40–50. doi: 10.1196/annals.1404.027
Matsumura, H., Mohri, Y., Binh, N. T., Morinaga, H., Fukuda, M., Ito, M., et al. (2016). Hair follicle aging is driven by transepidermal elimination of stem cells via COL17A1 proteolysis. Science 351:aad4395. doi: 10.1126/science.aad4395
McKnight, B., Seidel, R., and Moy, R. (2015). Topical human epidermal growth factor in the treatment of senile purpura and the prevention of dermatoporosis. J. Drugs Dermatol. 14, 1147–1150.
Meador, W. D., Sugerman, G. P., Story, H. M., Seifert, A. W., Bersi, M. R., Tepole, A. B., et al. (2020). The regional-dependent biaxial behavior of young and aged mouse skin: a detailed histomechanical characterization, residual strain analysis, and constitutive model. Acta Biomater. 101, 403–413. doi: 10.1016/j.actbio.2019.10.020
Minematsu, T., Yamamoto, Y., Nagase, T., Naito, A., Takehara, K., Iizaka, S., et al. (2011). Aging enhances maceration-induced ultrastructural alteration of the epidermis and impairment of skin barrier function. J. Dermatol. Sci. 62, 160–168. doi: 10.1016/j.jdermsci.2011.03.005
Montagna, W., Kirchner, S., and Carlisle, K. (1989). Histology of sun-damaged human skin. J. Am. Acad. Dermatol. 21(5 Pt 1), 907–918. doi: 10.1016/s0190-9622(89)70276-0
Moulin, V., Auger, F. A., Garrel, D., and Germain, L. (2000). Role of wound healing myofibroblasts on re-epithelialization of human skin. Burns 26, 3–12. doi: 10.1016/s0305-4179(99)00091-1
Naylor, E. C., Watson, R. E., and Sherratt, M. J. (2011). Molecular aspects of skin ageing. Maturitas 69, 249–256. doi: 10.1016/j.maturitas.2011.04.011
Nishimura, E. K., Granter, S. R., and Fisher, D. E. (2005). Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science 307, 720–724. doi: 10.1126/science.1099593
Oh, J. H., Joo, Y. H., Karadeniz, F., Ko, J., and Kong, C. S. (2020a). Syringaresinol inhibits UVA-induced MMP-1 expression by suppression of MAPK/AP-1 Signaling in HaCaT keratinocytes and human dermal fibroblasts. Int. J. Mol. Sci. 21:3981. doi: 10.3390/ijms21113981
Oh, J. H., Karadeniz, F., Kong, C. S., and Seo, Y. (2020b). Antiphotoaging effect of 3,5-dicaffeoyl-epi-quinic acid against UVA-induced skin damage by protecting human dermal fibroblasts in vitro. Int. J. Mol. Sci. 21:7756. doi: 10.3390/ijms21207756
Parsons, P. A. (1996). The limit to human longevity: an approach through a stress theory of ageing. Mech. Ageing Dev. 87, 211–218. doi: 10.1016/0047-6374(96)01710-1
Paszek, M. J., Zahir, N., Johnson, K. R., Lakins, J. N., Rozenberg, G. I., Gefen, A., et al. (2005). Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254. doi: 10.1016/j.ccr.2005.08.010
Picard, F., Hersant, B., Niddam, J., and Meningaud, J. P. (2017). Injections of platelet-rich plasma for androgenic alopecia: a systematic review. J. Stomatol. Oral Maxillofac. Surg. 118, 291–297. doi: 10.1016/j.jormas.2017.06.011
Quan, T., Little, E., Quan, H., Qin, Z., Voorhees, J. J., and Fisher, G. J. (2013). Elevated matrix metalloproteinases and collagen fragmentation in photodamaged human skin: impact of altered extracellular matrix microenvironment on dermal fibroblast function. J. Invest. Dermatol. 133, 1362–1366. doi: 10.1038/jid.2012.509
Sawaya, A. P., Jozic, I., Stone, R. C., Pastar, I., Egger, A. N., Stojadinovic, O., et al. (2019). Mevastatin promotes healing by targeting caveolin-1 to restore EGFR signaling. JCI Insight 4:e129320. doi: 10.1172/jci.insight.129320
Seifert, A. W., Kiama, S. G., Seifert, M. G., Goheen, J. R., Palmer, T. M., and Maden, M. (2012). Skin shedding and tissue regeneration in African spiny mice (Acomys). Nature 489, 561–565. doi: 10.1038/nature11499
Sherratt, M. J. (2009). Tissue elasticity and the ageing elastic fibre. Age (Dordr) 31, 305–325. doi: 10.1007/s11357-009-9103-6
Shwartz, Y., Gonzalez-Celeiro, M., Chen, C. L., Pasolli, H. A., Sheu, S. H., Fan, S. M., et al. (2020). Cell types promoting goosebumps form a niche to regulate hair follicle stem cells. Cell 182, 578–593.e19. doi: 10.1016/j.cell.2020.06.031
Silver, F. H., Siperko, L. M., and Seehra, G. P. (2003). Mechanobiology of force transduction in dermal tissue. Skin Res. Technol. 9, 3–23. doi: 10.1034/j.1600-0846.2003.00358.x
Tabibzadeh, S. (2021). Cell-centric hypotheses of aging. Front. Biosci. (Landmark Ed.) 26, 1–49. doi: 10.2741/4888
Thomas, D. R. (2001). Age-related changes in wound healing. Drugs Aging 18, 607–620. doi: 10.2165/00002512-200118080-00005
Tobin, D. J. (2017). Introduction to skin aging. J. Tissue Viability 26, 37–46. doi: 10.1016/j.jtv.2016.03.002
Tracy, L. E., Minasian, R. A., and Caterson, E. J. (2016). Extracellular matrix and dermal fibroblast function in the healing wound. Adv. Wound Care (New Rochelle) 5, 119–136. doi: 10.1089/wound.2014.0561
Varani, J., Dame, M. K., Rittie, L., Fligiel, S. E., Kang, S., Fisher, G. J., et al. (2006). Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am. J. Pathol. 168, 1861–1868. doi: 10.2353/ajpath.2006.051302
Wang, L., Siegenthaler, J. A., Dowell, R. D., and Yi, R. (2016). Foxc1 reinforces quiescence in self-renewing hair follicle stem cells. Science 351, 613–617. doi: 10.1126/science.aad5440
Wang, S., Drummond, M. L., Guerrero-Juarez, C. F., Tarapore, E., MacLean, A. L., Stabell, A. R., et al. (2020). Single cell transcriptomics of human epidermis identifies basal stem cell transition states. Nat. Commun. 11:4239. doi: 10.1038/s41467-020-18075-7
Watt, F. M., and Fujiwara, H. (2011). Cell-extracellular matrix interactions in normal and diseased skin. Cold Spring Harb. Perspect. Biol. 3:a005124. doi: 10.1101/cshperspect.a005124
Wong, V. W., Akaishi, S., Longaker, M. T., and Gurtner, G. C. (2011). Pushing back: wound mechanotransduction in repair and regeneration. J. Invest. Dermatol. 131, 2186–2196. doi: 10.1038/jid.2011.212
Wong, V. W., Longaker, M. T., and Gurtner, G. C. (2012). Soft tissue mechanotransduction in wound healing and fibrosis. Semin. Cell Dev. Biol. 23, 981–986. doi: 10.1016/j.semcdb.2012.09.010
Wu, M., Fannin, J., Rice, K. M., Wang, B., and Blough, E. R. (2011). Effect of aging on cellular mechanotransduction. Ageing Res. Rev. 10, 1–15. doi: 10.1016/j.arr.2009.11.002
Wulf, H. C., Sandby-Moller, J., Kobayasi, T., and Gniadecki, R. (2004). Skin aging and natural photoprotection. Micron 35, 185–191. doi: 10.1016/j.micron.2003.11.005
Yaar, M., and Gilchrest, B. A. (2007). Photoageing: mechanism, prevention and therapy. Br. J. Dermatol. 157, 874–887. doi: 10.1111/j.1365-2133.2007.08108.x
Yamada, M., and Prow, T. W. (2020). Physical drug delivery enhancement for aged skin, UV damaged skin and skin cancer: translation and commercialization. Adv. Drug Deliv. Rev. 153, 2–17. doi: 10.1016/j.addr.2020.04.008
Yang, L., van der Werf, K. O., Fitie, C. F., Bennink, M. L., Dijkstra, P. J., and Feijen, J. (2008). Mechanical properties of native and cross-linked type I collagen fibrils. Biophys. J. 94, 2204–2211. doi: 10.1529/biophysj.107.111013
Zhang, W., Qu, J., Liu, G. H., and Belmonte, J. C. I. (2020). The ageing epigenome and its rejuvenation. Nat. Rev. Mol. Cell Biol. 21, 137–150. doi: 10.1038/s41580-019-0204-5
Zhang, Z., Kruglikov, I., Zhao, S., Zi, Z., Gliniak, C. M., Li, N., et al. (2021). Dermal adipocytes contribute to the metabolic regulation of dermal fibroblasts. Exp. Dermatol. 30, 102–111. doi: 10.1111/exd.14181
Zhang, Z., Shao, M., Hepler, C., Zi, Z., Zhao, S., An, Y. A., et al. (2019). Dermal adipose tissue has high plasticity and undergoes reversible dedifferentiation in mice. J. Clin. Invest. 129, 5327–5342. doi: 10.1172/JCI130239
Zimber, M. P., Ziering, C., Zeigler, F., Hubka, M., Mansbridge, J. N., Baumgartner, M., et al. (2011). Hair regrowth following a Wnt- and follistatin containing treatment: safety and efficacy in a first-in-man phase 1 clinical trial. J. Drugs Dermatol. 10, 1308–1312.
Zou, Z., Long, X., Zhao, Q., Zheng, Y., Song, M., Ma, S., et al. (2020). A single-cell transcriptomic atlas of human skin aging. Dev. Cell doi: 10.1016/j.devcel.2020.11.002 [Epub ahead of print].
Keywords: skin aging, extracellular matrix, macroenvironment, hair regeneration, skin tension, tissue mechanics, tensional homeostasis, microenvironment
Citation: Harn HI, Chen CC, Wang SP, Lei M and Chuong CM (2021) Tissue Mechanics in Haired Murine Skin: Potential Implications for Skin Aging. Front. Cell Dev. Biol. 9:635340. doi: 10.3389/fcell.2021.635340
Received: 30 November 2020; Accepted: 01 February 2021;
Published: 19 February 2021.
Edited by:
Masatake Osawa, Gifu University, JapanReviewed by:
Ilja L. Kruglikov, Wellcomet GmbH, GermanyCopyright © 2021 Harn, Chen, Wang, Lei and Chuong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hans I-Chen Harn, aGFybmhudkBnbWFpbC5jb20=; Cheng-Ming Chuong, Y21jaHVvbmdAbWVkLnVzYy5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.