
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell Dev. Biol. , 05 February 2021
Sec. Molecular and Cellular Pathology
Volume 9 - 2021 | https://doi.org/10.3389/fcell.2021.625560
This article is part of the Research Topic Genetic Mutations Associated with Ocular Diseases View all 19 articles
 Imen Habibi1*
Imen Habibi1* Yosra Falfoul2
Yosra Falfoul2 Hoai Viet Tran3
Hoai Viet Tran3 Khaled El Matri2
Khaled El Matri2 Ahmed Chebil2
Ahmed Chebil2 Leila El Matri2
Leila El Matri2 Daniel F. Schorderet1,4,5
Daniel F. Schorderet1,4,5Retinal dystrophies (RD) are a group of Mendelian disorders caused by rare genetic variations leading to blindness. A pathogenic variant may manifest in both dominant or recessive mode and clinical and genetic heterogeneity makes it difficult to establish a precise diagnosis. In this study, families with autosomal dominant RD in successive generations were identified, and we aimed to determine the disease's molecular origin in these consanguineous families. Whole exome sequencing was performed in the index patient of each family. The aim was to determine whether these cases truly represented examples of dominantly inherited RD, or whether another mode of inheritance might be applicable. Six potentially pathogenic variants in four genes were identified in four families. In index patient with enhanced S-cone syndrome in F1, we identified a new digenetic combination: a heterozygous variant p.[G51A];[=] in RHO and a homozygous pathogenic variant p.[R311Q];[R311Q] in NR2E3. Helicoid subretinal fibrosis associated with recessive NR2E3 variant p.[R311Q];[R311Q] was identified in F2. A new frameshift variant c.[105delG];[105delG] in RDH12 was found in F3 with cone-rod dystrophy. In F4, the compound heterozygous variants p.[R964*];[W758*] were observed in IMPG2 with a retinitis pigmentosa (RP) phenotype. We showed that both affected parents and the offspring, were homozygous for the same variants in all four families. Our results provide evidence that in consanguineous families, autosomal recessive can be transmitted as pseudodominant inheritance in RD patients, and further extend our knowledge of pathogenic variants in RD genes.
Retinal dystrophies (RD), a group of heterogeneous hereditary diseases, are caused by perturbed photoreceptor function. It presents significant genetic heterogeneity and contribute notably to the etiology of blindness around the world (den Hollander et al., 2010). To date, more than 300 disease associated genes have been implicated in the pathogenesis of the disease, most of which are involved in the development and normal function of photoreceptor and cells from the retinal pigment epithelium (RPE) (den Hollander et al., 2008, 2010). Clinical symptoms vary widely among different RD subtypes and disease genes (Ellingford et al., 2016). RD are generally classified based on the type of photoreceptor cells affected, i.e., rods or cones, and thus on the location, macula or peripheral retina (Nash et al., 2015).
The described modes of inheritance in RD are recessive (ar) or dominant (ad) and autosomal or X-linked. A few rare cases of RD exist, in which mitochondrial mutations, digenic inheritance, and pseudodominant transmission have been documented (Lewis et al., 1999; Liu et al., 2019).
In this study, we used whole exome sequencing (WES) and Sanger sequencing to investigate four consanguineous families with several members affected by RD, inherited in an apparent ad pattern. We aimed to identify potential gene variants underlining these cases and described the genotypic and phenotypic findings in these complicated RD pedigrees. Distinct inheritance patterns and disease-causing variants were identified in all families.
This study was approved by the Local Ethics Committee of the Hedi Rais Institute in Tunisia. Informed consent was obtained from all participants in the study. Analyses were done in accordance with local guidelines.
Our study contains four Tunisian families with non-syndromic RD (Table 1). All patients underwent detailed clinical examinations and their family history was collected. A comprehensive ophthalmological examination was performed at the Department B of Hedi Rais Institute of Ophthalmology, Tunis (Tunisia), including best-corrected visual acuity (BCVA), slit lamp, dilated fundus examination, and full-field electroretinography according to the International Society for Clinical Electrophysiology of Vision (ISCEV) standards (Métrovision, France), swept source optical coherence tomography (SS-OCT, Topcon, Swept Source DRI-OCT Triton, Japan), and fundus autofluorescence (FAF) imaging (Heidelberg, HRA 2 Spectralis, Germany).
Peripheral blood samples were obtained from 4 index patients and 13-related individuals, including parents and affected siblings. DNA was extracted from leukocytes according to the salting-out method (Miller et al., 1988).
We sequenced the exome in the index patient, then variants were confirmed by Sanger sequencing and segregation was established in all families.
Next-generation sequencing was done at Sophia Genetics (SOPHiA GENETICS SA, Saint-Sulpice, Switzerland) using the clinical exome solution v.2 (CES2). The complete list of genes analyzed in CES2 can be obtained at www.sophiagenetics.com. ~98.95% of target regions were covered at least 25×.
All variants were first filtered against several public databases for the minor allele frequency (MAF) of <1%. dbSNP database served as a reference to exclude any known frequent variants occurring in coding (missense) and regulatory regions including splice site and promoter regions. Table 2 lists the criteria to classify the variants described here.
A variant was considered novel if it has not been described in the medical literature or was not present in the Human Mutation Database (www.hgmd.cf.ac.uk/ac). The variant frequency in the control population was evaluated using gnomAD (http://gnomad.broadinstitute.org/).
All variants were confirmed by Sanger sequencing. PCR reactions and amplification conditions were performed using the following primers in Table 3.
The index patient is a 19-year-old girl from consanguineous marriage with the diagnosis of enhanced S-cone syndrome. She had nyctalopia since the age of 6 years along with visual impairment. Her BCVA was limited to 1/20 in both eyes (OU). On fundus examination, she had mid-peripheral nummular pigment clumping, atrophy and cystoid macular edema better visualized on SS-OCT. FAF showed hyper FAF dots in the posterior pole (Figures 1A–C). In full-field ERG, reduced cone and rod responses with similar waveforms under photopic and scotopic conditions were detected (Figure 2). Affected mother had severe visual impairment with cataract and diffuse retinal atrophy (Figures 1D,E).
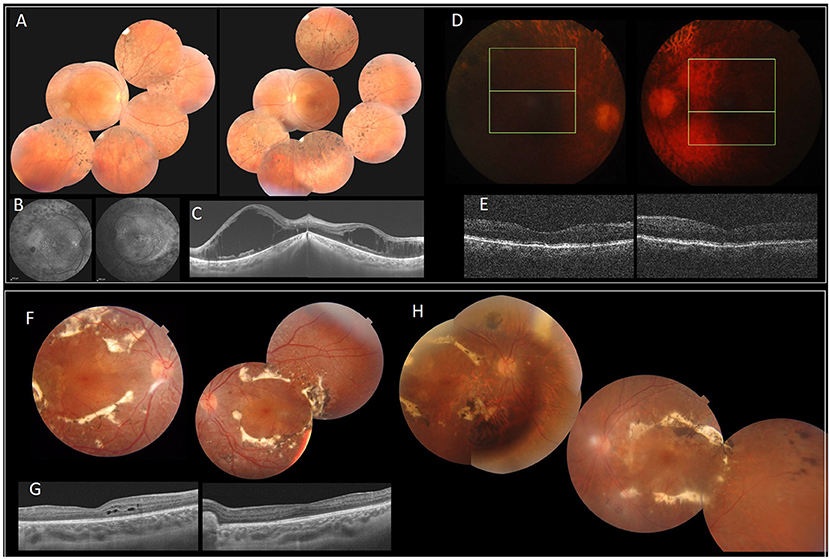
Figure 1. Clinical and imaging features of patients with NR2E3 mutations. F1 (A–E). (A) FP of the index patient with peripheral nummular spicule deposits and atrophy. (B) FAF with peripheral hypo-autofluorescence spots and heterogeneous autofluorescence of the posterior pole. (C) SS-OCT. Bilateral cystoid macular edema. (D,E) Diffuse retinal atrophy. F2 (F–H). (F,H) FP of the index patient (II.1) (F) and his father (I.1) (H) with circumferential fibrosis and nummular pigment clumping and peripheral spicule deposits. (G) SS-OCT. intraretinal cysts (II.1). FP, Fundus photography.
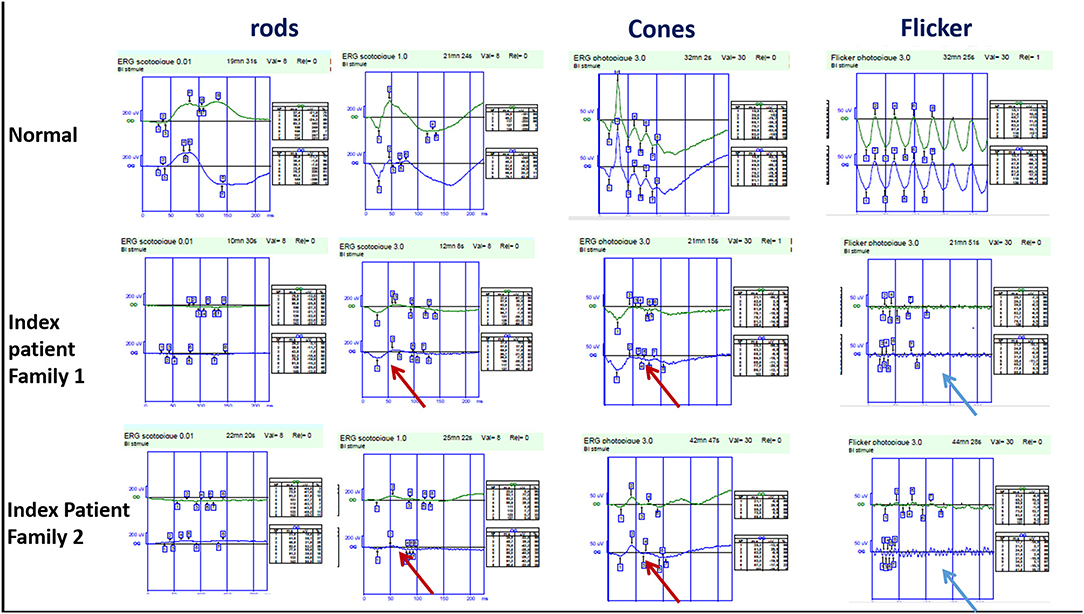
Figure 2. Full-field ERG findings in enhanced-S cone syndrome patients from families 1 and 2 showing similar waveforms in both scotopic and photopic conditions (red arrow) with markedly reduced flicker (blue arrow).
The index patient was a 13-year-old boy from consanguineous marriage presenting with retinal dystrophy. His BCVA was limited to 3/10 OU. On fundus examination, he had circumferential fibrosis, nummular pigment clumping on the posterior pole and peripheral spicule deposits with preserved macular region (Figures 1F,G). In full-field ERG, there were similar cone and rod responses under photopic and scotopic conditions with markedly reduced flicker, which allowed us to retain the diagnosis of enhanced S-cone syndrome (Figure 2). Affected father had similar retinal phenotype with bilateral cataract (Figure 1H).
The index patient is a 13-year-old girl with impaired visual acuity (BCVA: 1/10 OU) and nyctalopia since her first decade of life. She had cone-rod dystrophy with macular atrophy and spicule deposits in the mid peripheral retina (Figure 3A). In full-field ERG, there were reduced cone and rod responses. Her mother had similar phenotype with BCVA limited to light perception and diffuse spicule deposits in fundus examination (Figure 3B).
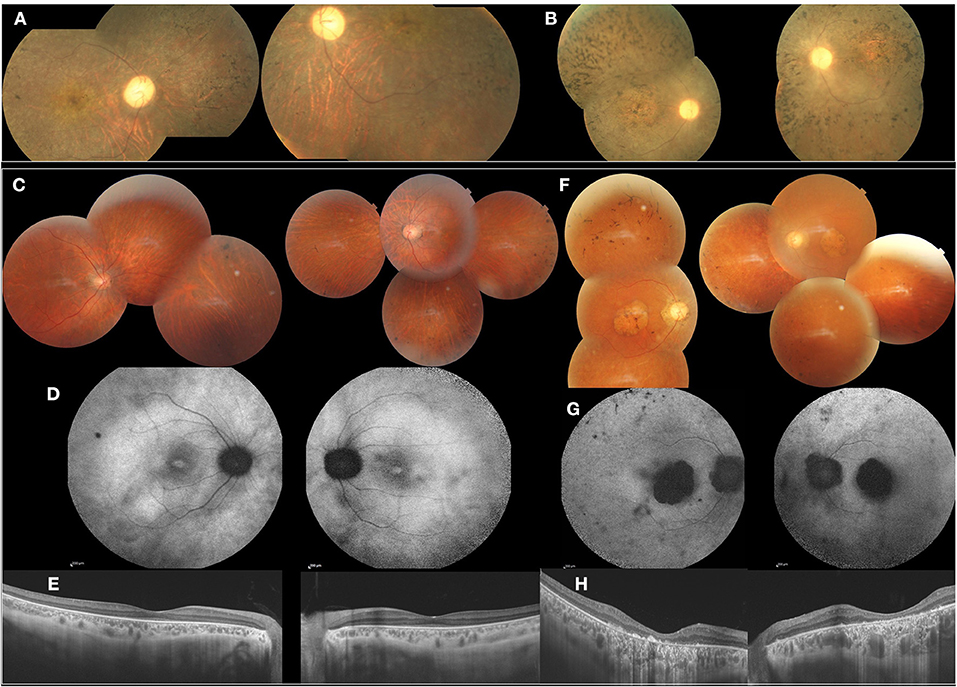
Figure 3. Clinical and imaging features of affected patients from F3 and F4, F3 (A,B). FP of the index patient (II.1) and her mother (I.2) showed cone-rod dystrophy with macular atrophy and spicule deposits in the mid peripheral retina. F4 (C–H). (C) FP of the index patient (II.2) with peripheral spicule migrations and preserved posterior pole. (D) Foveolar hyperautofluorescence, peripheral hypo-autofluorescence spots. (E) SS-OCT. Preserved retinal macular layers. (F) FP of the mother (I.2) showing macular atrophy and peripheral spicule deposits. (G) FAF with lack of autofluorescence in the macula. (H) SS-OCT. Severe and diffuse macular atrophy.
The index patient is a 38-year-old male diagnosed with familial retinitis pigmentosa (RP). BCVA was 5/10 s. On fundus examination, there were peripheral spicule migrations and preserved posterior pole (Figures 3C–E). The same phenotype was present in three-affected sisters. The mother, had BCVA limited to 1/20 OU with macular atrophy and peripheral spicule deposits (Figures 3F–H).
Six potentially pathogenic variants in four genes were identified in four families (Figure 4).
In the index patient with enhanced S-cone syndrome in F1, we identified, a new digenetic combination: a heterozygous likely benign variant p.[G51A];[=] in RHO and homozygous LP variant p.[R311Q];[R311Q] in NR2E3. Helicoid subretinal fibrosis associated with a recessive NR2E3 variant p.[R311Q];[R311Q] was identified in F2. New frameshift P variant c.[105delG];[105delG] in RDH12 was found in the index patient of F3 with cone-rod dystrophy (CRD). Compound heterozygous LP variants p.[R964*];[W758*] in IMPG2 were observed in the index patient with RP in F4. We have shown that both, the affected parents and the offspring, are homozygous for the same variant in the four families. These variants are classified as pathogenic according to the American College of Medical Genetics (ACMG) guidelines.
In this report, molecular testing revealed the coexistence of pathogenic variants affecting distinct RD causing genes in four RD families. Among the five pathogenic variants identified in these families, one was novel (RDH12 c.105delG, p.Q36Sfs*6) and four were recurrent (RHO p.G51A, NR2E3 p.R311Q and IMPG2 p.W758*, p.R964*) (Macke et al., 1993; Haider et al., 2000; Bandah-Rozenfeld et al., 2010; Patel et al., 2016).
Two families, F1 and F2, carried the most frequent variant p.R311Q in NR2E3. Gerber et al. have previously identified this in patients who may have Enhanced S-cone syndrome (ESCS) (Gerber et al., 2000). Later, Haider et al. reported the same p.R311Q variant with 44.8% frequency in patients with ESCS (Haider et al., 2000) and it was also reported in a patient with Goldmann-Favre syndrome (GFS) (Bernal et al., 2008). The particularity in F1 is the presence of a second heterozygous variant p.G51A in RHO. Two diseases are associated with RHO variants, RP and congenital stationary night blindness (CSNB). However, in F1 the index patient presented with ESCS, which is more related to NR2E3. Interesting, and although RHO: p.G51A has been involved in retinitis pigmentosa (Macke et al., 1993), GnomAD mentions a frequency of 0.1% in the general population and ClinVar classifies this variant as benign/likely benign. Family F1 would favor the latter classification.
New pathogenic homozygous variant c.[105delG];[105delG], p.[Q36Sfs*6;[Q36Sfs*6] in RDH12 was detected in F3. Variants in RDH12 have been previously linked to Leber congenital amaurosis (LCA) and ad-RP. Retinal pathologies resulting from variants in RDH12 gene can be inherited in either ad or ar mode (Kumaran et al., 2017; Sarkar et al., 2020). Ar biallelic variants in RDH12 were first identified in three consanguineous Austrian families with severe retinal dystrophy (Janecke et al., 2004).
F4 harbored compound heterozygous variants p.[W758*];[R964*] in IMPG2. This is the first time the association of these two variants is described. Biallelic variants in IMPG2 have been shown to underlie recessive childhood-onset rod-cone dystrophy with early macular involvement in several families (Khan and Al Teneiji, 2019). At the heterozygous state, IMPG2 variants have been associated with dominant vitelliform macular dystrophy (Brandl et al., 2017). In F4, the affected mother carried compound likely pathogenic variants, the unaffected father carried a p.W758* heterozygous variant and the affected children a combination of these variants. Pseudodominant inheritance occurs when an individual with a known recessive disorder has a clinically unaffected partner, but then unexpectedly gives birth to children who are affected with the same recessive disorder as the affected parent (Thompson and Thompson, 1986). Although all the parents in the described families were first cousins, each disease followed a dominant pattern of inheritance, with one affected parent and one or several affected children. Molecular analyses allowed us to correctly classify the mode of inheritance as pseudo-dominant, a mode usually associated with a high mutant frequency, like in hemochromatosis.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
The studies involving human participants were reviewed and approved by Local Ethics Committee of the Hedi Rais Institute in Tunisia. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
DS and LE designed the research protocol. IH and DS performed the analyses. YF, HT, KE, AC, and LE performed the clinical evaluation. IH, YF, LE, and DS wrote the manuscript. All authors reviewed the manuscript.
Institute for Research in Ophthalmology, Sion, Switzerland Fondation pour la Recherche sur les Maladies Héréditaires.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank the family members for their invaluable participation and co-operation. We acknowledge the help provided by the ophthalmologists in this study, and the colleagues who referred patients to us. We thank Denisa Dzulova for reading the manuscript.
Bandah-Rozenfeld, D., Collin, R. W. J., Banin, E., Van den Born, L. I., Karlien, L. M. C., Siemiatkowska, A. M., et al. (2010). Mutations in IMPG2, encoding interphotoreceptor matrix proteoglycan 2, cause autosomal-recessive retinitis pigmentosa. Am. J. Hum. Genet. 1, 199–208. doi: 10.1016/j.ajhg.2010.07.004
Bernal, S., Solans, T., Gamundi, M. J., Hernan, I., de Jorge, L., Carballo, M., et al. (2008). Analysis of the involvement of the NR2E3 gene in autosomal recessive retinal dystrophies. Clin. Genet. 73, 360–366. doi: 10.1111/j.1399-0004.2008.00963.x
Brandl, C., Schulz, H. L., Issa, P. C., Birtel, J., Bergholz, R., Lange, C., et al. (2017). Mutations in the genes for interphotoreceptor matrix proteoglycans, IMPG1 and IMPG2, in patients with vitelliform macular lesions. Genes 8:170. doi: 10.3390/genes8070170
den Hollander, A. I., Black, A., Bennett, J., and Cremers, F. P. (2010). Lighting a candle in the dark: advances in genetics and gene therapy of recessive retinal dystrophies. J. Clin. Invest. 120, 3042–3053. doi: 10.1172/JCI42258
den Hollander, A. I., Roepman, R., Koenekoop, R. K., and Cremers, F. P. (2008). Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog. Retin. Eye Res. 27, 391–419. doi: 10.1016/j.preteyeres.2008.05.003
Ellingford, J. M., Barton, S., Bhaskar, S., O'Sullivan, J., Williams, S. G., Lamb, J. A., et al. (2016). Molecular findings from 537 individuals with inherited retinal disease. J. Med. Genet. 53, 761–767. doi: 10.1136/jmedgenet-2016-103837
Gerber, S., Rozet, J. M., Takezawa, S. I., dos Santos, L. C., Lopes, L., Gribouval, O., et al. (2000). The photoreceptor cell-specific nuclear receptor gene (PNR) accounts for retinitis pigmentosa in the Crypto-Jews from Portugal (Marranos), survivors from the Spanish Inquisition. Hum. Genet. 107, 276–284. doi: 10.1007/s004390000350
Haider, N. B., Jacobson, S. G., Cideciyan, A. V., Swiderski, R., Streb, L. M., Searby, C., et al. (2000). Mutation of a nuclear receptor gene, NR2E3, causes enhanced S cone syndrome, a disorder of retinal cell fate. Nat. Genet. 24, 127–131. doi: 10.1038/72777
Janecke, A. R., Thompson, D. A., Utermann, G., Becker, C., Hubner, C. A., Schmid, E., et al. (2004). Mutations in RDH12 encoding a photoreceptor cell retinol dehydrogenase cause childhood-onset severe retinal dystrophy. Nat. Genet. 36, 850–854. doi: 10.1038/ng1394
Khan, A. O., and Al Teneiji, A. M. (2019). Homozygous and heterozygous retinal phenotypes in families harbouring IMPG2 mutations. Ophthalmic Genet. 40, 247–251. doi: 10.1080/13816810.2019.1627467
Kumaran, N., Moore, A. T., Weleber, R. G., and Michaelides, M. (2017). Leber congenital amaurosis/early-onset severe retinal dystrophy: clinical features, molecular genetics and therapeutic interventions. Br. J. Ophthalmol. 101, 1147–1154. doi: 10.1136/bjophthalmol-2016-309975
Lewis, R. A., Shroyer, N. F., Singh, N., Allikmets, R., Hutchinson, A., Li, Y., et al. (1999). Genotype/phenotype analysis of a photoreceptor-specific ATP-binding cassette transporter gene, ABCR, in Stargardt disease. Am. J. Hum. Genet. 64, 422–434. doi: 10.1086/302251
Liu, H. Y., Huang, J., Xiao, H., Zhang, M. J., Shi, F. F., Jiang, Y. H., et al. (2019). Pseudodominant inheritance of autosomal recessive congenital stationary night blindness in one family with three co-segregating deleterious GRM6 variants identified by next-generation sequencing. Mol. Genet. Genomic Med. 7:e952. doi: 10.1002/mgg3.952
Macke, J. P., Davenport, C. M., Jacobson, S. G., Hennessey, J. C., Gonzalez-Fernandez, F., Conway, B. P., et al. (1993). Identification of novel rhodopsin mutations responsible for retinitis pigmentosa: implications for the structure and function of rhodopsin. Am. J. Hum. Genet. 53, 80–89.
Miller, S. A., Dykes, D. D., and Polesky, H. F. (1988). A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 16:1215. doi: 10.1093/nar/16.3.1215
Nash, B. M., Wright, D. C., Grigg, J. R., Bennetts, B., and Jamieson, R. V. (2015). Retinal dystrophies, genomic applications in diagnosis and prospects for therapy. Transl. Pediatr. 4, 139–163. doi: 10.3978/j.issn.2224-4336.2015.04.03
Patel, N., Aldahmesh, M. A., and Alkuraya, F. S. (2016). Expanding the clinical, allelic, and locus heterogeneity of retinal dystrophies. Gene. Med. 18, 554–562. doi: 10.1038/gim.2015.127
Keywords: retinal dystrophies, whole exome sequencing, pathogenic variants, pseudodominant inheritance, retinitis pigmentosa
Citation: Habibi I, Falfoul Y, Tran HV, El Matri K, Chebil A, El Matri L and Schorderet DF (2021) Different Phenotypes in Pseudodominant Inherited Retinal Dystrophies. Front. Cell Dev. Biol. 9:625560. doi: 10.3389/fcell.2021.625560
Received: 03 November 2020; Accepted: 11 January 2021;
Published: 05 February 2021.
Edited by:
Wei He, He Eye Hospital, ChinaReviewed by:
Xiaoyan Ding, Sun Yat-sen University, ChinaCopyright © 2021 Habibi, Falfoul, Tran, El Matri, Chebil, El Matri and Schorderet. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Imen Habibi, aGFiaWJpaW1lbkBob3RtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.