- 1Guangzhou University of Chinese Medicine, Guangzhou, China
- 2Department of Gynecology, The Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
- 3Shanghai Key Laboratory of Diabetes Mellitus, Center for Translational Medicine, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, China
- 4Academy of Integrative Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, China
The N6-methyladenosine (m6A) RNA modification regulates the expression of genes associated with various biological and pathological processes, including spontaneous abortion (SA). The aim of this study was to determine the role of the m6A demethylase fat mass and obesity (FTO)- associated protein in SA. The FTO,IGF2BP1 and IGF2BP2 mRNA levels were significantly lower in the chorionic villi obtained from spontaneously aborted pregnancies compared to that of normal pregnancies, while the expression levels of METTL3 and WTAP were significantly elevated. However, ALKBH5, YTHDF2, and IGF2BP3 were elevated with no statistical significance between groups. In addition, MDA was elevated and SOD levels were decreased in the villi tissues of the SA group compared to the normal group, which was indicative of placental oxidative stress in the former. Furthermore, the expression of FTO and HLA-G were significantly decreased in the trophoblasts of the SA patients compared to that of normal pregnant women, while that of m6A was markedly higher in the former. In addition, the HLA-G and VEGFR mRNA levels were downregulated in the SA versus the control group, and that of MMP2, MMP7, MMP9 and VEGFA were upregulated. Finally, The RIP assay showed significantly decreased levels of FTO-bound HLA-G, VEGFR and MMP9 RNA in SA patients (P < 0.05), which corresponded to an increase in transcripts enriched with the m6A antibody (P < 0.05). However, compared with normal pregnant women, the levels of HLA-G, VEGFA, VEGFR, and MMP2 mRNA bound by YTHDF2 were significantly decreased in SA patients. Compared to the normal pregnant women, both FTO- and m6A-bound MMP7 were significantly increased in SA patients (P < 0.05), but YTHDF2 almost unbound to MMP7 mRNA. In summary, the downregulation of FTO in the chorionic villi disrupts immune tolerance and angiogenesis at the maternal-fetal interface, resulting in aberrant methylation and oxidative stress that eventually leads to SA.
Introduction
Spontaneous abortion (SA) is the most common complication during the first trimester of pregnancy with an incidence rate of 10–15% (Sahin et al., 2001). The maternal-fetal interface, which is the site of nutrient exchange and circulation between the placenta and the growing fetus, has been implicated in miscarriage and preeclampsia. A dysfunctional maternal-fetal interface induces oxidative stress in the placenta and the subsequent loss of placental synthetic trophoblast cells, which contributes to the pathogenesis of abortion and eclampsia (Jauniaux et al., 2003). Studies increasingly show that normal progression of pregnancy relies on the dynamic balance between oxidases and antioxidant enzymes, and any disruption in this balance leads to pathological complications such as SA (Mentese et al., 2018).
Embryo development and implantation is a key stage in early pregnancy, and involves the chronological process of maturation, fertilization, cleavage, and blastocyst formation, along with synchronized cell proliferation and differentiation. Concomitantly, an immunotolerant state is maintained at the maternal-fetal interface by human leukocyte antigen-G (HLA-G). Women with lower HLA-G expression have a higher risk of recurrent spontaneous abortion (RSA) and preeclampsia (PE) (Cecati et al., 2011), which can be attributed to increased infiltration of maternal immune cells. The placenta at the maternal-fetal interface is formed by cytotrophoblast cells (CTB), which invade the uterine tissue and migrate to the uterine spiral artery, wherein they further differentiate into endovascular trophoblast cells (Sultana et al., 2018). Trophoblast invasion and migration mediate immunotolerance and remodeling of the uterine spiral artery at the maternal-fetal interface during embryo implantation (Ren et al., 2020). Dysfunctional trophoblast phenotypes can lead to adverse pregnancy outcomes such as SA. In addition to HLA-G, matrix metalloproteinases (MMPs) and vascular endothelial growth factor (VEGF) are also dysregulated during SA. However, the post-transcriptional regulation of the above genes in the context of trophoblast function and SA have not been studied in detail.
N6-methyladenosine (m6A) is the most abundant RNA modification (Csepany et al., 1990) that stabilizes mRNAs and also modulates their localization, transport, and post-translational regulation (Lence et al., 2019). The methylation of adenosine is mediated by the methyltransferase(also known as “writers”) complex consisting of METTL3, METTL14, WTAP, KIAA1429, and RBM15/RBM15B. The m6A modification can be reversed by demethylases(also known as “erasers”) such as Fat mass and Obesity-associated protein (FTO) and AlkB homologue 5 (ALKBH5). The m6A can influence post-transcriptional gene expression during transcription through specific recognition by m6-binding proteins (also known as “readers”) (Huang et al., 2020). Currently, the study of FTO and its expression products has attracted extensive interest from scientists due to the identification of FTO as the first m6A-demethylase modified by m6A (Jia et al., 2011). Whole genome association studies have shown that the FTO gene is closely related to diabetes and obesity (Gerken et al., 2007). In addition, there is increasing evidence that the m6A modification plays an important role in various pathological conditions such as nervous system diseases, metabolic diseases, reproductive dysfunction diseases and cancer (Zhen et al., 2019). Li et al. (2019) recently showed that AlKbH5 affects the stability of CYR61 mRNA in trophoblasts and is involved in the pathogenesis of SA. In addition, Andraweera et al. (2015) identified the FTO s9939609 single nucleotide polymorphism as a risk factor of SA in a cohort study of 202 Sinhalese women with a history of SA and 202 normal control women. Given the demethylase activity of FTO and aberrant trophoblast function in SA, we hypothesized that FTO is dysregulated in the trophoblasts during SA and results in the abnormal accumulation of transcripts with m6A. Our findings indicate that diminished FTO-mediated demethylation in trophoblasts is a likely pathological basis of SA.
Materials and Methods
Subjects
Eighty-four healthy women of average age 33 years and regular menstruation, including 49 with spontaneously aborted singleton pregnancies (SA) and 35 with voluntarily terminated pregnancies (normal), were recruited for the study. The SA group had received uterine curettage, and B-ultrasound at 8 weeks showed no fetal heart rate and fetal size inconsistent with the gestation time, which was indicative of spontaneous embryo abortion. In the normal group, the pregnancy sac and primitive heart tube were seen during the B-ultrasonic examination. There was no significant difference in age and number of pregnancies between the two groups. Chorionic villi samples (50–100 g) were collected from the SA group during the uterine curettage or at 6–9 weeks from the normal group, rinsed repeatedly with normal saline, and stored in liquid nitrogen. In addition, 5 mL blood samples were also collected from all subjects. This study was approved by the ethics committee of the First Affiliated Hospital of Guangzhou University of Traditional Chinese Medicine. All the patients signed the informed consent form.
Immunofluorescence Assay
The villi tissue was frozen in liquid nitrogen and then sliced using a cryotome. The cryosections were incubated with rabbit anti-FTO (1:500, ab126605, Abcam), mouse anti-HLA-G (1:500, ab7759, Abcam) and mouse anti-m6A (1:500, ab208577, Abcam) antibodies, washed thrice with PBS, and then probed with the secondary antibody for 1h. After washing thrice with PBS, the sections were sealed with the coverslips and gelvatol, and observed under a fluorescence microscope (Leica TCS-SP5).
Real-Time Quantitative Polymerase Chain Reaction (qRT-PCR)
The total RNA was extracted from frozen villi samples using Trizol reagent, and its purity was evaluated using a Nanophotometer (Implen, Germany). Following reverse transcription with the Thermo RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, United States), 10μl cDNA was amplified using Thermo Maxima SYBR Green qPCR Master Mix (Thermo Fisher Scientific, United States) on the CFX96 cycler (Bio-rad, United States). The RT-PCR parameters were as follows: 95°C for 10 min, followed by 40 cycles of 95°C for 10 s, 60°C for 34 s, and 95°C for 15 s. All primers were designed and synthesized by Sangon Bioengineering Company (Table 1). The expression levels of the target genes were calculated by the (2–ΔΔCT) method.
Enzyme Linked Immunosorbent Assay (ELISA)
The villi samples were homogenized in PBS and centrifuged at 2000 g for 10min at 4°C. The supernatants were collected, and the levels of MDA, SOD, and FTO were detected using specific ELISA kits (MDA, SOD from R&D, United States, and FTO kit from Biovision, United States).
Western Blotting
The villi tissues were lysed, and the amount of protein in the lysates was determined using the BCA Kit (Beyotime Biotechnology, China). Equal amounts of protein per sample were denatured in the loading buffer at 100°C for 5 min. The samples were electrophoresed in SDS-PA gel, and transferred to NC membrane. After blocking with 5% skimmed milk at room temperature for 1 h, the blots were incubated overnight with anti-FTO monoclonal antibody (ab126605) at 4°C, washed thrice with TBST, and incubated with secondary antibody for 1h at room temperature. The following antibodies were used for WB:METTL3 (ab195352), WTAP (ab195380), YTHDF2 (ab220163), IGF2BP1 (ab184305), and IGF2BP2 (ab124930). The positive bands were developed using the enhanced luminescent reagent (Bio-Rad, United States) and observed on an imaging system (Bio-Rad, United States).
RNA Immunoprecipitation
RNA Immunoprecipitation was performed using the EZ-Magna RIPTM RNA-Binding Protein Immunoprecipitation Kit (Merck, Germany) according to the manufacturer’s protocol. Briefly, the villi tissues were washed thrice with ice-cold PBS and centrifuged. The pelleted cells were resuspended in 200 μl RIP buffer and incubated with magnetic antibody-conjugated A/G beads at 4°C for 3 h. One hundred microliter of the supernatant was diluted with 900 μl RIP buffer and incubated with proteinase K. The FTO-bound RNA was then extracted using the phenol/chloroform method for RIP-Seq, and the levels of HLA-G, MMP2, MMP7, MMP9, VEGFA, and VEGFR mRNAs were quantified by qRT-PCR. The following antibodies were used for RIP and MeRIP: FTO (ab126605), m6A (ab208577), YTHDF2(ab220163), and rabbit anti-human IgG (1: 100, 10285-1-AP). The cells were incubated with the antibodies at room temperature for 30 min. The primers for RT-qPCR are listed in Table 1.
RNA m6A Dotblot Assays
Firstly, the total RNA of chorionic villi was extracted with trizol reagen and treated with DNase I to remove possible DNA contamination. The ratio of Poly (A) + RNA was diluted to 2 and 60 ng and spotted onto the nylon film (GE Healthcare, China). Then UV-crosslinked, blocking buffer was used to seal. The cells were incubated with m6A antibody (ab232905) overnight, then incubated with horseradish peroxidase labeled Goat Anti-Rabbit IgG H&L (ab6721) for 1 h, and finally detected by DAB peroxidase substrate kit (Yeasen Biotechnology, China).
Statistical Analysis
SPSS19.0 and GraphPad Prism6.0 were used for all statistical analysis. The measurement data were expressed as mean ± standard deviation ( ± s) of three independent experiments. One-way analysis of variance and LSD were used to compare groups with normally distributed data. Non-parametric test (Kruskal-Wallis test) was used for data with non-normal distribution. P < 0.05 was considered statistically significant.
Results
Placental Oxidative Stress in SA Is Associated With Aberrant FTO Expression in the Chorionic Villi and Trophoblasts
Compared to normal pregnancies, MDA levels were significantly higher in the villi of the SA group (P < 0.05) and the SOD level was markedly decreased (Figure 1). Furthermore, The FTO mRNA and protein levels were significantly decreased in the chorionic villi of the SA group compared to the controls (Figures 2A–C). Moreover, ELISA was performed to detect the FTO expression in larger samples of patients in villi of two groups, showing the FTO expression was significantly downregulated in SA patients (Figure 2D).
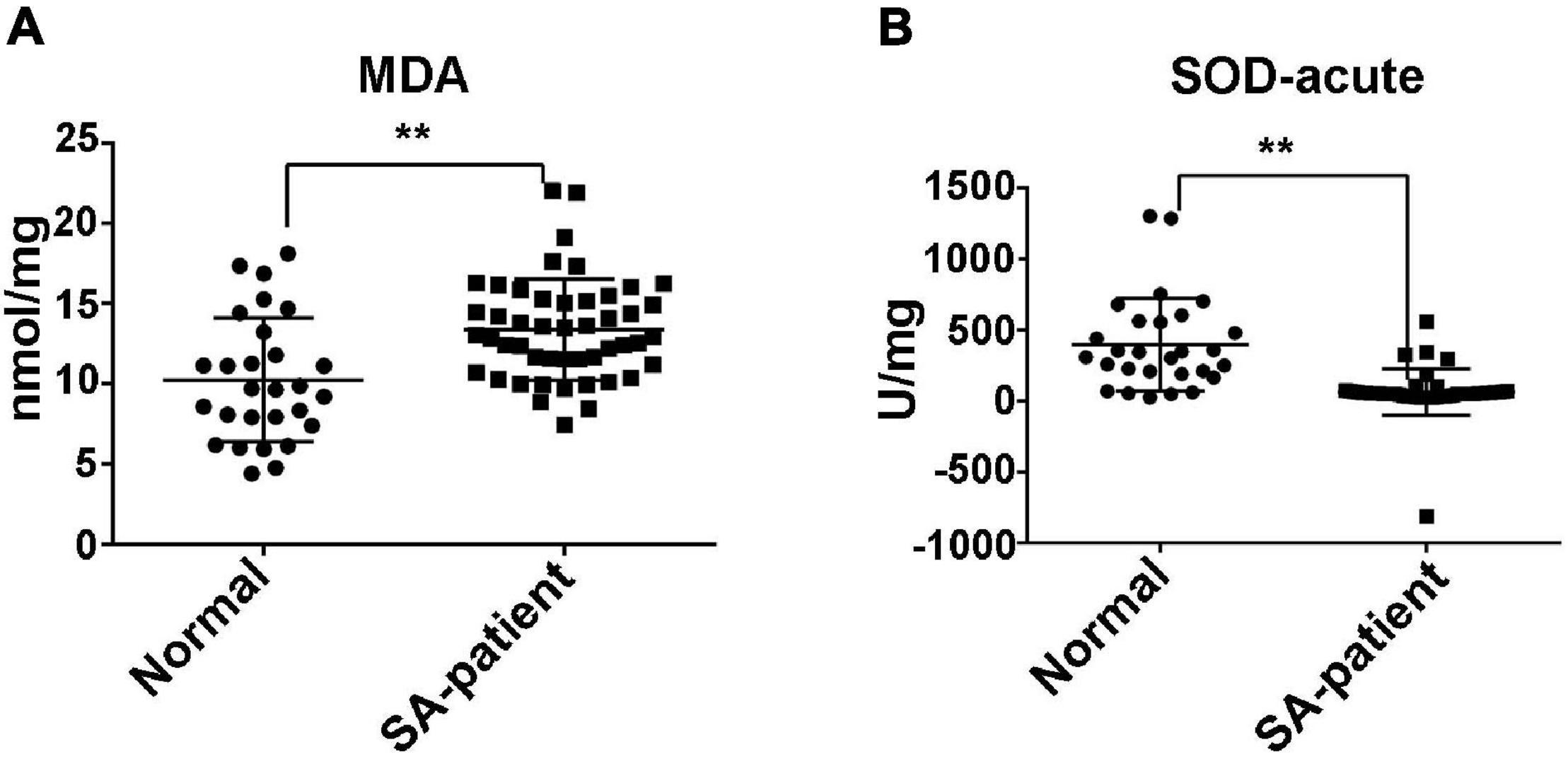
Figure 1. (A) The expression of MDA in the villi of N and SA groups. (B) The expression of SOD in the villi of N and SA groups (N, normal, n = 28; SA, spontaneous abortion, n = 48), **P < 0.01.
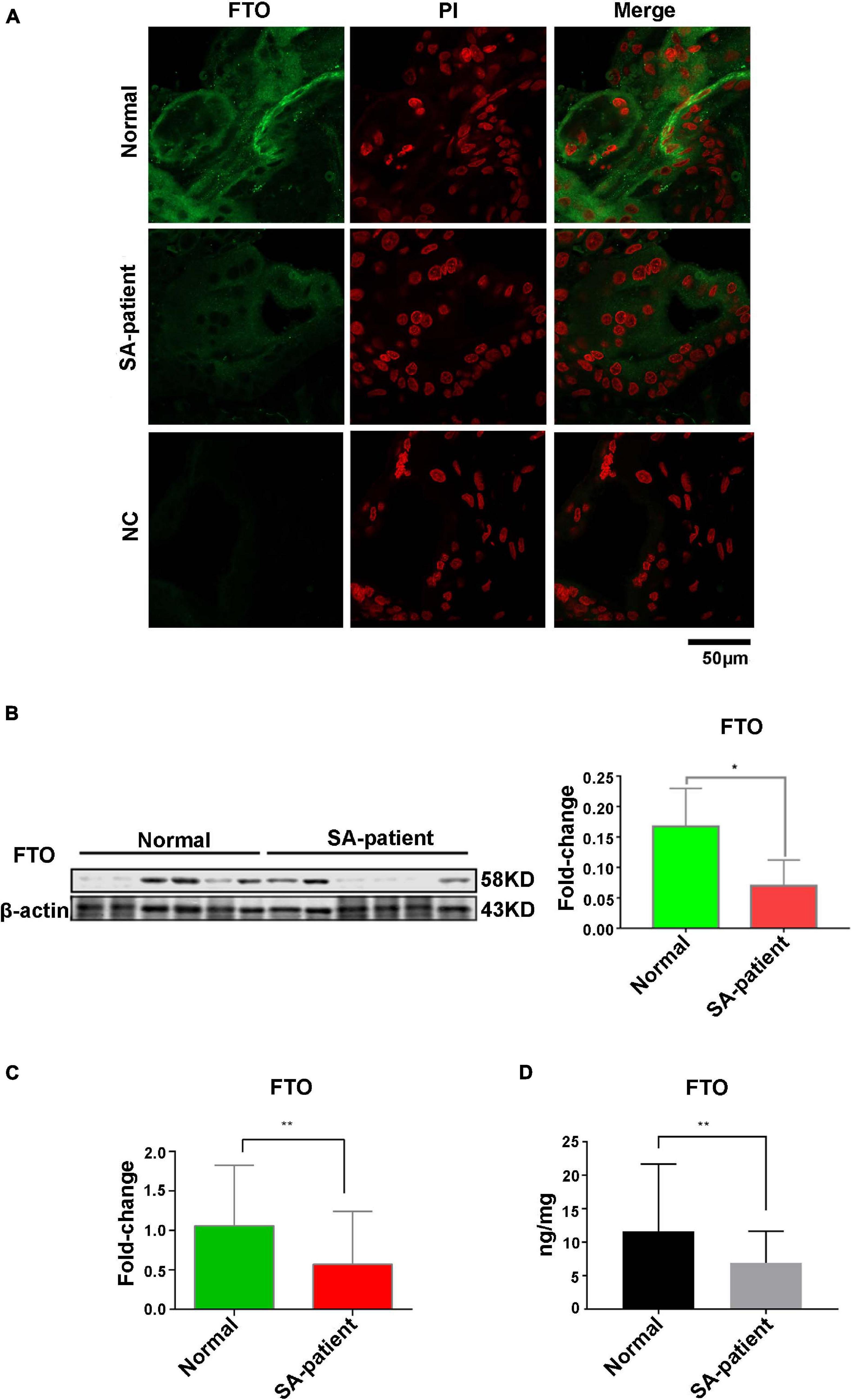
Figure 2. (A) Representative immunofluorescence images of chorionic villi showing in situ expression of FTO in both groups. Green, FTO; Red, PI counterstained nuclei. Normal n = 3; Patient n = 3; NC, negative control n = 3. (B) Immunoblot showing expression level of FTO protein in the villi of two groups (Normal n = 6; Patient n = 6), *P<0.05. (C) The expression level of FTO mRNA in villi of two groups (Normal n = 30; Patient n = 67), **P<0.01. (D) The expression level of FTO in the villi of two groups as detected by ELISA (Normal n = 28; Patient n = 48), **P<0.01.
Abnormal Gene Expression Levels of RNA m6A Modification-Related Proteins in SA Patients
To explore whether mRNA m6A methylation is involved in the pathogenesis of SA, we screened samples with differential FTO expression according to the FTO levels at enrolment and analyzed the expression of m6A “writers,” “erasers,” and “readers” in the villi tissues in the above SA patients (n = 8) and normal (n = 8) using qRT-PCR and Western blot (Figures 3H). Compared to the normal patients, the expression levels of METTL3 and WTAP were significantly elevated (p < 0.05, Figures 3B,C,L,M) while the expression levels of IGF2BP1, IGF2BP2 were significantly decreased (p < 0.05, Figures 3E,F,I,J) in SA patients. However, ALKBH5, YTHDF2, and IGF2BP3 were elevated with no statistical significance between groups (Figures 3A,D,G,K).
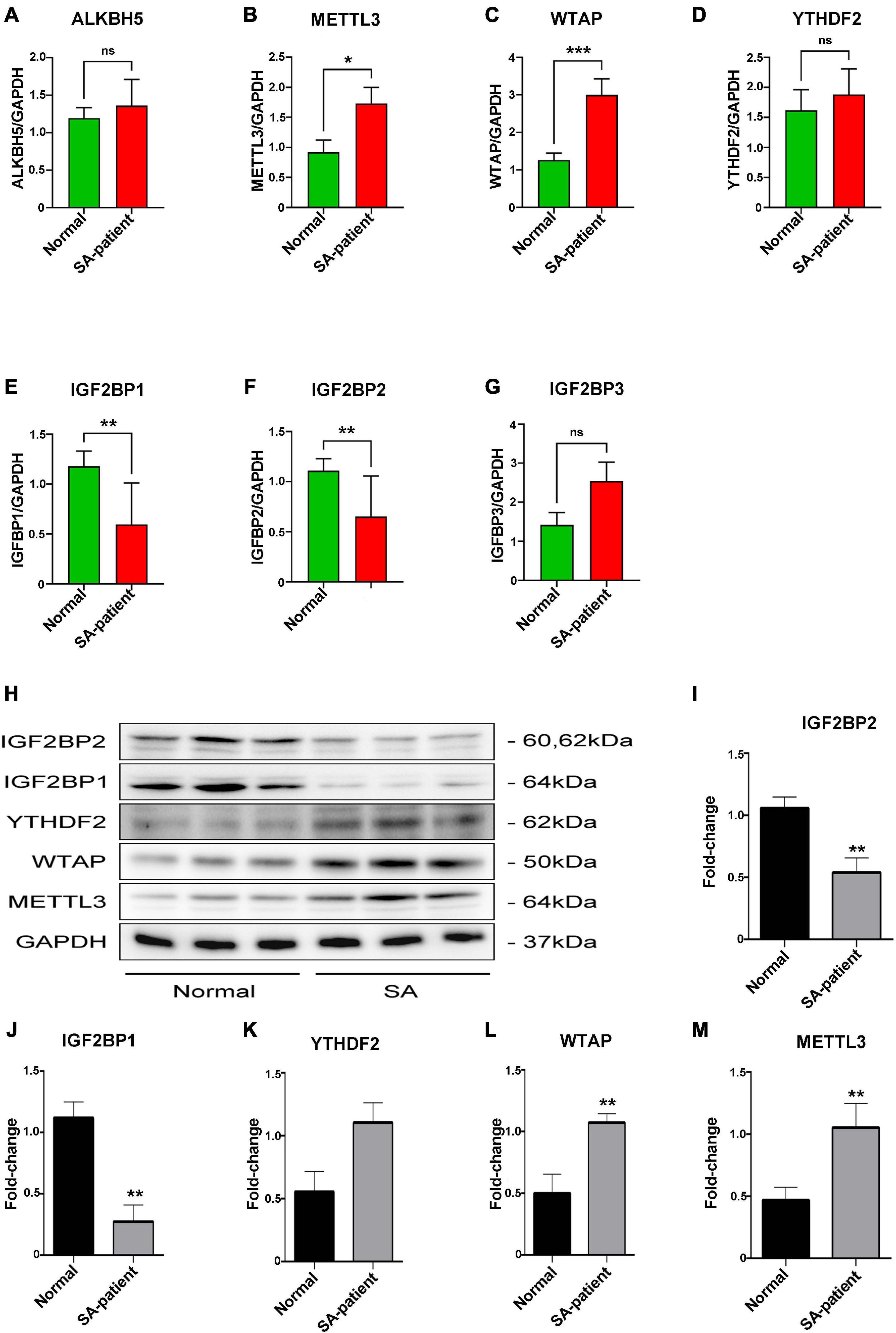
Figure 3. (A) ALKBH5, (B) METTL3, (C) WTAP, (D) YTHDF2, (E) IGF2BP1, (F) IGF2BP2, and (G) IGF2BP3 mRNA levels in the indicated groups (Normal n = 8; Patient n = 8, *P<0.05, **P<0.01). (H) Immunoblot showing expression level of target protein in the villi of two groups. (I) IGF2BP2, (J) IGF2BP1, (K) YTHDF2, (L) WTAP, and (M) METTL3 protein expression level in the villi of two groups (Normal n = 6; Patient n = 6). *P<0.05, **P<0.01, ***P < 0.001.
Upregulation of m6A Modification in the Villi of SA Patients
Fat mass and obesity can catalyse and mediate the demethylation of m6A, therefore, downregulated FTO in the villi of SA patients may lead to the disorder of m6A modification. Double-immunofluorescence staining (FTO and m6A) was performed for each clinical sample and revealed that the FTO expression in the villi of SA patients was downregulated, while the m6A expression was upregulated, with a negative correlation between them (Figure 4A). Then, we extracted the RNA of chorionic villi from each sample of SA patients and normal pregnancy control group and performed RNA m6A Dotblot test after multiple dilution. We found that the content of m6A in Patients group was significantly up-regulated while keeping the same amount of samples (Figure 4B).
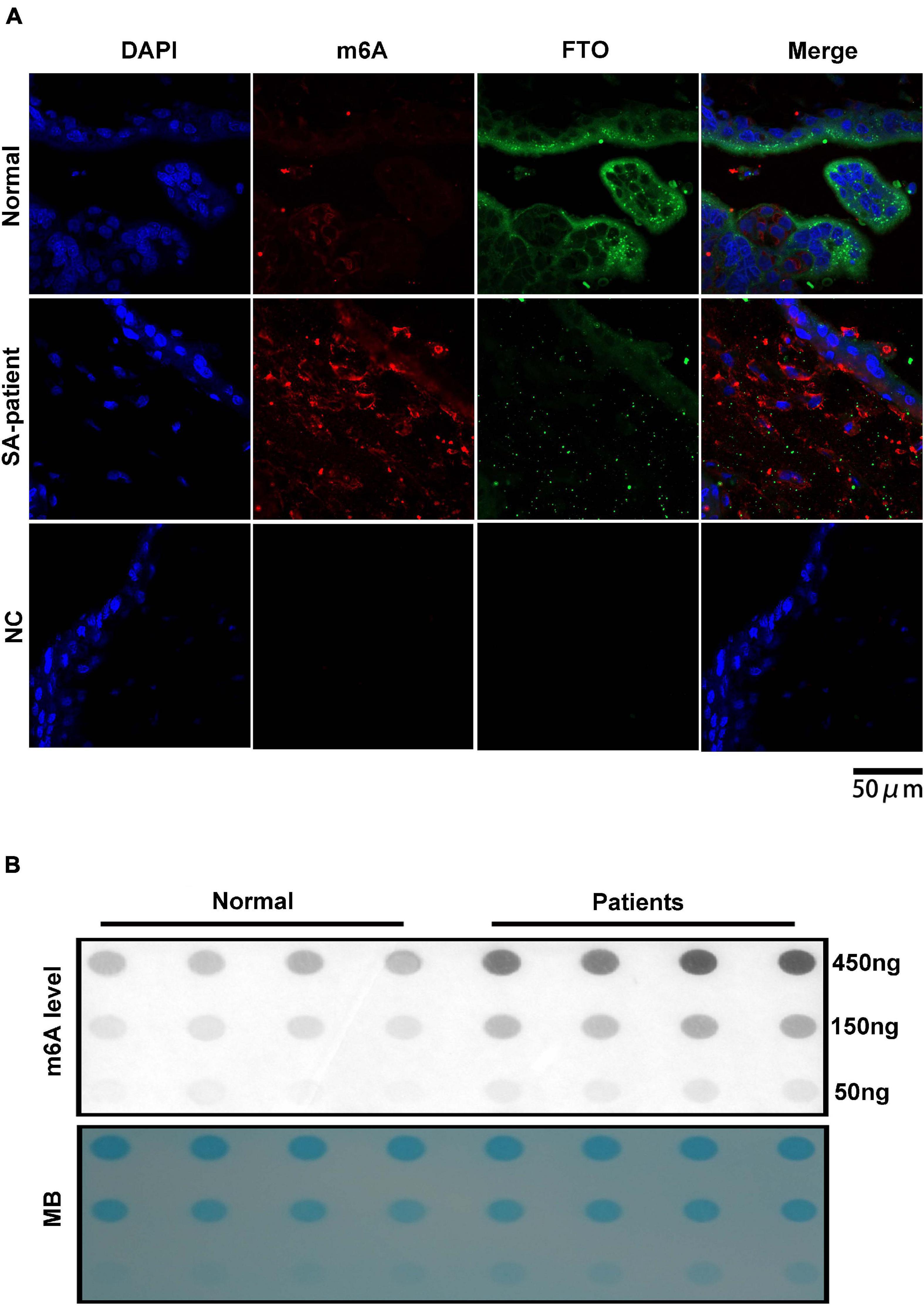
Figure 4. (A) Representative immunofluorescence images of chorionic villi showing in situ expression of FTO and m6A in both groups (Normal n = 3; Patient n = 3; NC:negative control n = 3). Blue – FTO and red – m6A. (B) Poly(A)+ RNAs isolated from chorionic villi were used in dot blot analyses with m6A antibody. RNA was loaded by equal dilution method (Normal n = 4; Patient n = 4).
Phenotypic Differences of Villi Between SA Patients and Normal Pregnant Women
As shown in Figure 5A, the in situ expression of FTO and HLA-G was significantly lower in the trophoblasts of the SA patients, and the expression pattern of both were similar. Finally, HLA-G and VEGFR mRNA levels were downregulated in the SA group (p < 0.05, Figures 5G,F), and MMP7 and MMP9 were upregulated (p < 0.05, Figures 5C,D). MMP2 and VEGFA mRNA levels were also higher in the SA group, although the difference was not statistically significant (Figures 5B,E).
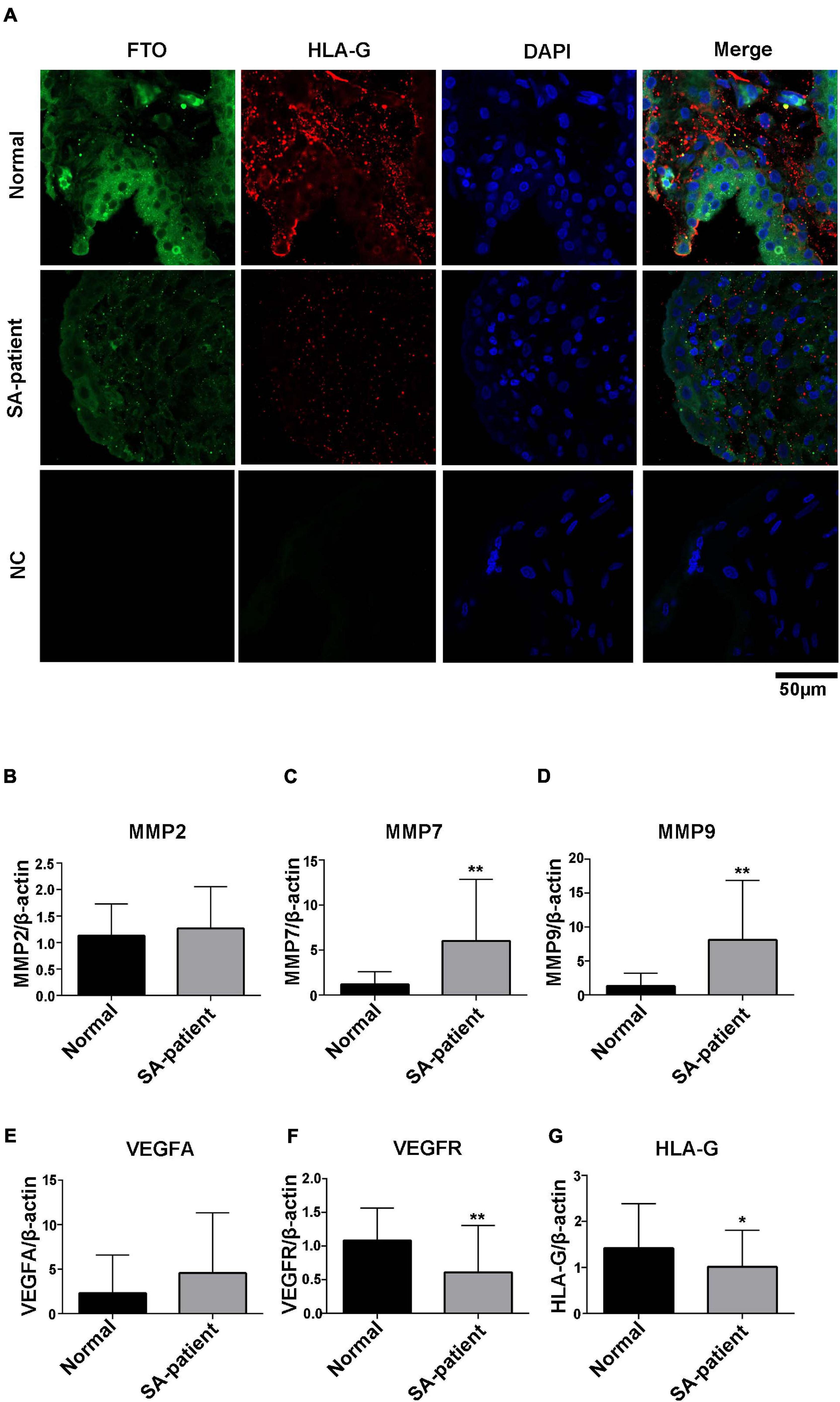
Figure 5. (A) Representative immunofluorescence images of chorionic villi showing in situ expression of FTO and HLA-G in both groups (Normal n = 3; Patient n = 3; NC:negative control n = 3), green – FTO and red – HLA-G. (B) MMP2, (C) MMP7, (D) MMP9, (E) VEGFA, (F) VEGFR, and (G) HLA-G mRNA levels in the indicated groups. (Normal n = 35; Patient n = 49, *P<0.05 and **P<0.01).
FTO Downregulation Increases the m6A Modification of Several Genes During SA
Immunoprecipitation and qPCR experiments showed a significant decrease in the levels of FTO-bound HLA-G, VEGFR and MMP9 mRNA (Figures 6A,D,F), and markedly higher enrichment of FTO-bound MMP7 (Figure 6C) in the SA patients compared to the normal pregnant women. No significant difference was observed in the levels of co-precipitated MMP2 and VEGFA between the two groups (Figures 6B,E). Consistent with this, the SA samples showed have a higher enrichment ratio of HLA-G, VEGFR, MMP7 and MMP9 mRNA with m6A modification (Figures 7A,C,D,F), while that of the modified MMP2 and VEGFA mRNAs were unaffected (Figures 7B,E). Linear correlation analysis showed that the levels of FTO-bound HLA-G, MMP2,MMP7,MMP9,VEGFA and VEGFR mRNA was correlated with these mRNA with m6A modification (Figures 8A–F). Immunoprecipitation and qPCR experiments both showed that the target protein YTHDF2 directly bound to HLA-G, VEGFA, VEGFR, MMP9, and MMP2 mRNA, but almost unbound to MMP7 mRNA (Figure 9C). Compared with normal pregnant women, the levels of HLA-G, VEGFA, VEGFR, and MMP2 mRNA bound by YTHDF2 were significantly decreased in SA patients (Figures 9A,B,E,F). However, the level of co-precipitated MMP9 was not significantly different between groups (Figure 9D).
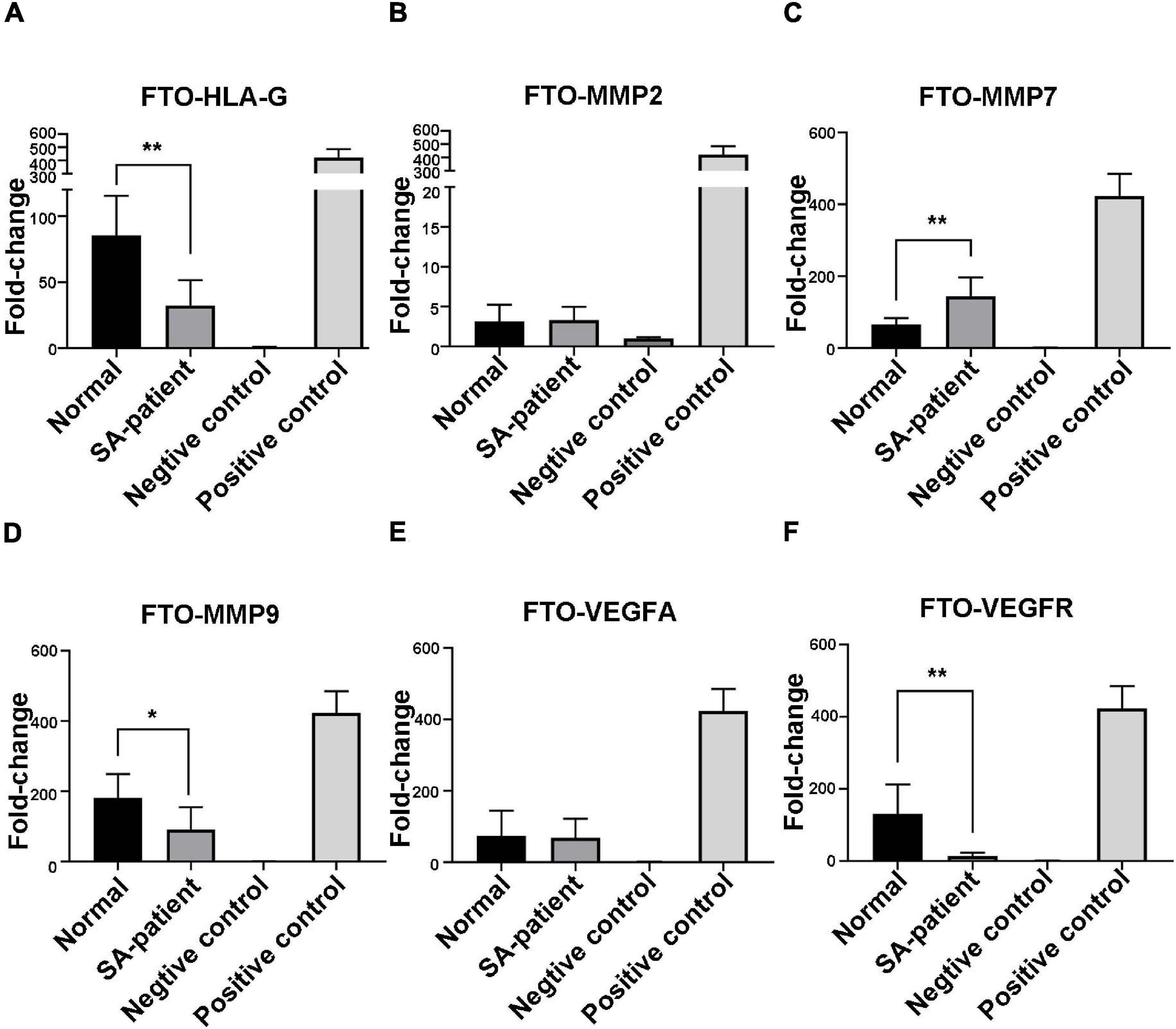
Figure 6. Fold-change in FTO-bound (A) HLA-G, (B) MMP2, (C) MMP7, (D) MMP9, (E) VEGFA, and (F) VEGFR mRNA in the chorionic villi of the indicated groups (Normal n = 9; Patient n = 9, *P < 0.05, **P < 0.01).
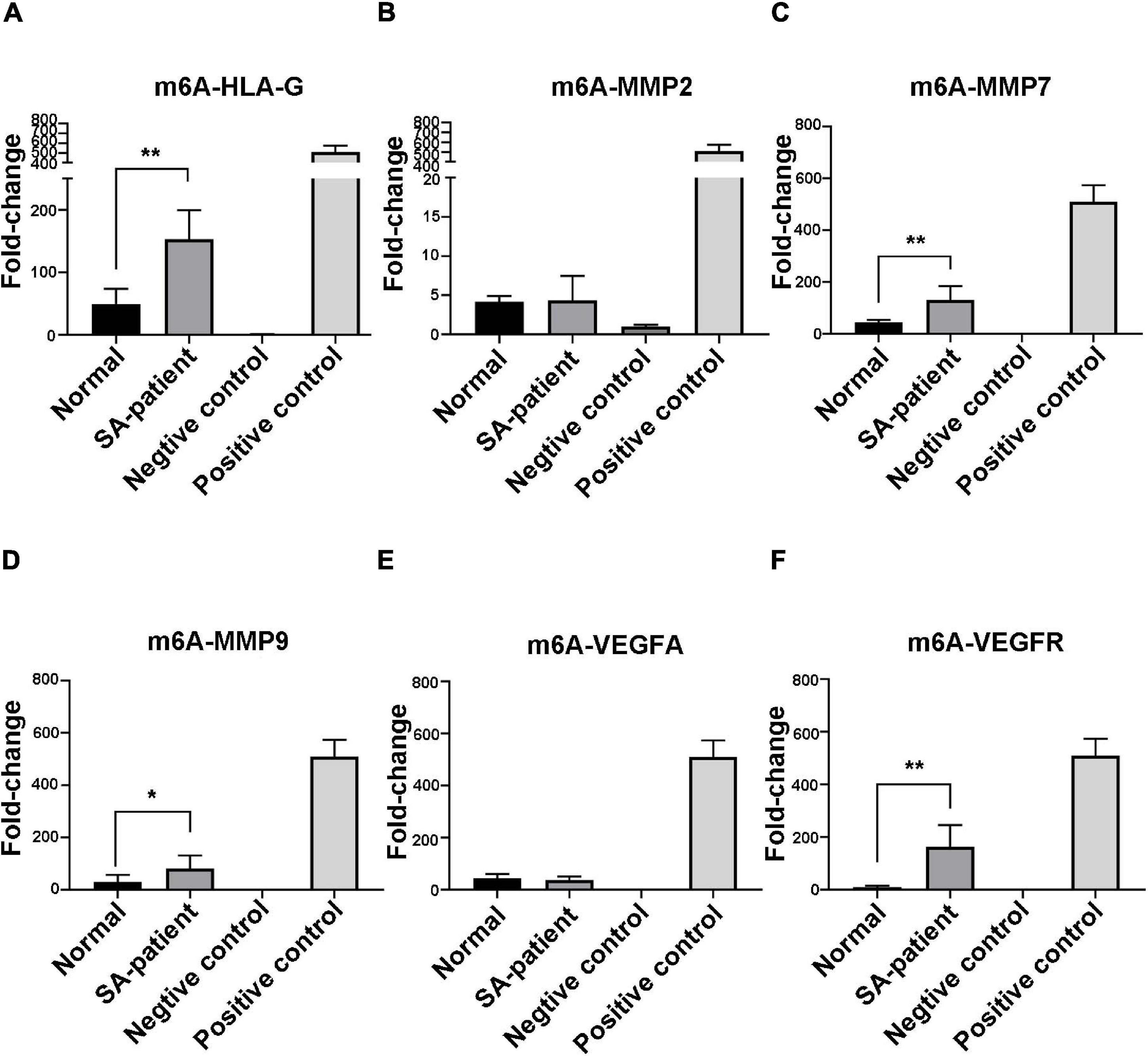
Figure 7. Fold-change in m6A-bound (A) HLA-G, (B) MMP2, (C) MMP7, (D) MMP9, (E) VEGFA, and (F) VEGFR mRNA in the chorionic villi of the indicated groups (Normal n = 9; Patient n = 9, *P < 0.05, **P < 0.01).
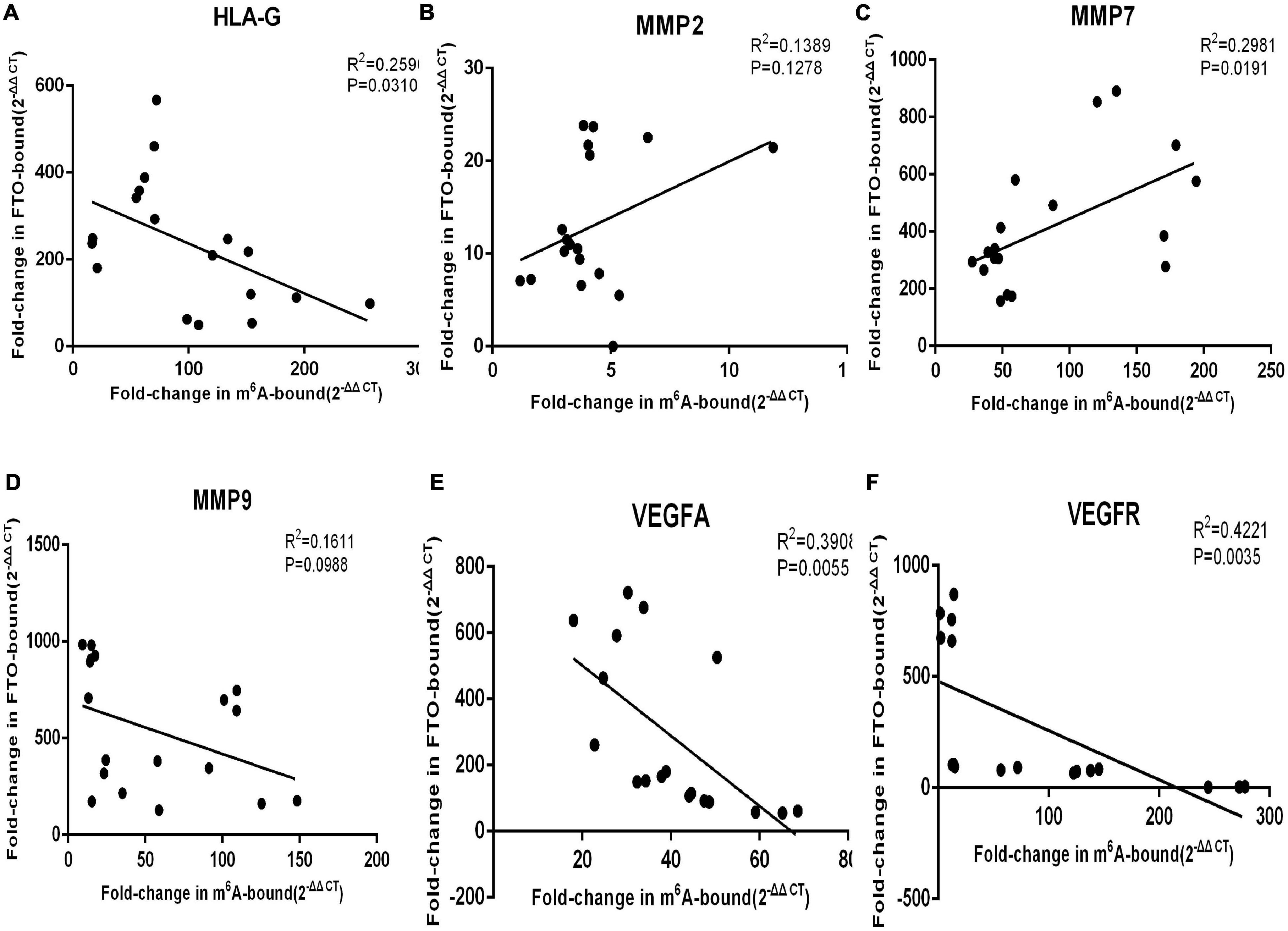
Figure 8. The levels of FTO-bound (A) HLA-G, (B) MMP2, (C) MMP7, (D) MMP9, (E) VEGFA, and (F) VEGFR mRNA was correlated to these mRNA with m6A modification.
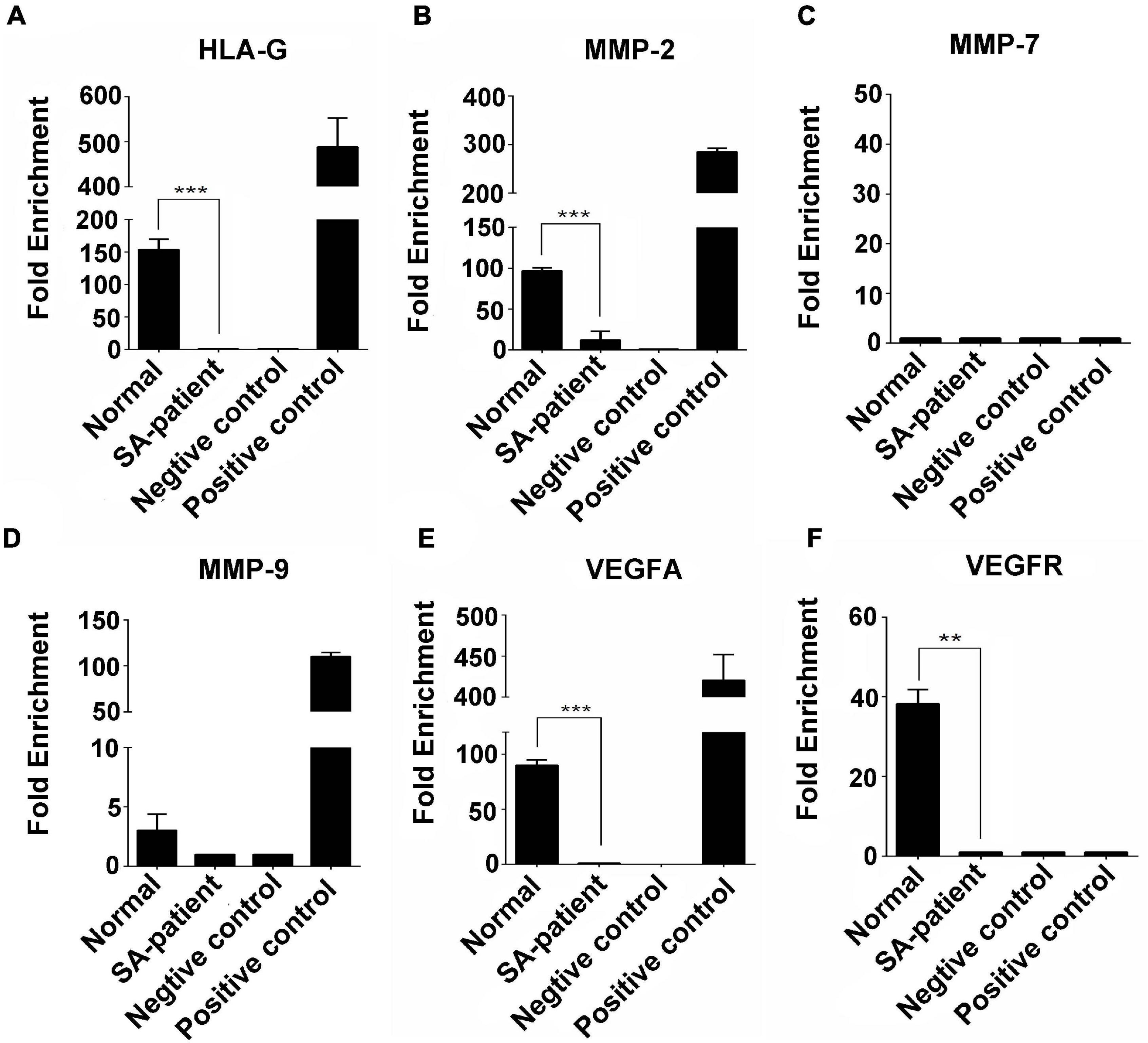
Figure 9. Fold-change in YTHDF2-bound (A) HLA-G, (B) MMP2, (C) MMP7, (D) MMP9, (E) VEGFA, and (F) VEGFR mRNA in the chorionic villi of the indicated groups. (Normal n = 9; Patient n = 9, **P < 0.01, ***P < 0.001).
Taken together, SA is associated with increased methylation of genes involved in immunotolerance, immune cell infiltration and angiogenesis at the maternal-fetal interface.
Discussion
We found that the RNA demethylase FTO was downregulated in the chorionic villi of women that underwent SA, and correlated with oxidative stress and aberrant m6A accumulation at the maternal-fetal interface. During the implantation of embryos, genetic material is transferred through the dynamic regulation of methylation and demethylation (Qin et al., 2020). Typically, RNA methylation regulates gene expression after transcription. Interestingly, we found that the m6A-modified writer proteins, including METTL3 and WTAP, were elevated when the demethylase FTO was downregulated in SA patients. Meanwhile, another eraser protein (ALKBH5) did not affect the abnormal accumulation of m6A. Therefore, we suggest that the aberrant accumulation of m6A in SA was also related with the dysregulation of other modifiers. It is generally believed that when m6A RNA is methylated, demethylase FTO will play a role after methylated transferase (Mathiyalagan et al., 2019). Recent studies have correlated changes in m6A levels with human reproductive disorders (Kaspi et al., 2018). Given that the m6A modification occurs at the 3’-UTR near the transcription termination site (Lan et al., 2019), accumulation of hypermethylated mRNAs due to aberrant FTO expression during SA likely affects the expression levels of crucial genes.
Oxidative stress in the placenta and synthetic trophoblast cells can induce abortion (Fortis et al., 2018). We detected lower levels of the antioxidant enzyme SOD in the homologous villi tissue of the SA group compared to the controls, which is consistent with the findings of Popovia et al. (El-Far et al., 2007). In agreement with Hempstock et al. (El-Far et al., 2009; Zhao et al., 2020), MDA levels were significantly higher in the chorionic villi of the SA group. MDA is a by-product of lipid peroxidation, and its elevated content reflects excessive production of lipid peroxides or impaired antioxidant defence mechanisms. A previous study showed that aberrant m6A modification disrupted the antioxidant system in preadolescent testicular injury (Hernández-Vargas et al., 2020). Likewise, the oxidative stress during early and late SA (Costello et al., 2019) has been correlated with decreased expression of HLA-G and VEGF along with elevated MMPs in the chorionic villi, which can impair immunotolerance, trophoblastic invasion and angiogenesis at the maternal-fetal interface. Consistent with previous reports, HLA-G and VEGFR were significantly downregulated in the SA group, while MMP7 and 9 were upregulated.
To further elucidate whether impaired demethylation due to FTO inhibition contributes to the pathogenesis of SA, we analyzed the levels of FTO- and m6A-bound HLA-G, VEGFR and MMP9 mRNA in the trophoblasts. As expected, the mRNAs showed decreased binding to FTO, which was accompanied by increased methylation as indicated by anti-m6A antibody-mediated enrichment of the specific transcripts. Since m6A accumulation in the 5’UTR region of mRNA inhibits translation (Zhou et al., 2018; Sun et al., 2020), the downregulation of these genes observed in SA samples can be attributed to their hypermethylated state.
As mentioned before, m6A from SA is abnormally deposited on RNA transcripts during transcription due to dysregulation of writer and eraser, and therefore affects gene expression post-transcriptionally by altering the specific recognition of m6A binding proteins (also called readers) (Shi et al., 2019). The IGF2BP family protects m6A-modified mRNA from degradation and promotes mRNA translation (Huang et al., 2018), whereas we found that compared to normal individuals, IGF2BP1/2 was less expressed using qPCR and m6A itself is abnormally accumulated in SA patients. Thus, it is hard to tell that the abnormalities of the phenotypic genes in SA patients were due to the reduced IGF2BPs or hypermethylation of m6A. Accordingly, we did not select IGF2BPs for RIP-qPCR. On the other hand, YTHDF2 functions to bring m6A-modified translatable mRNAs to the decay site of mRNAs and mediates the decay of mRNAs (Lee et al., 2020). Our analysis revealed reduced binding of YTHDF2 to HLA-G, VEGFR, and MMP9 in SA patients, accompanied by increased m6A methylation. Besides, qPCR found no difference of YTHDF2 expression between normal and SA patients, suggesting that the downregulation of these genes in SA patients is due to their hypermethylation status.
Interestingly, the levels of both FTO- and m6A-bound MMP7 were significantly increased in the SA group, which indicates that MMP7 methylation is regulated by other mechanisms. At the same time, we found elevated IGF2BP1-2 in both SA groups, suggesting that the abnormal accumulation of methylation in MMP7 is largely responsible for the abnormal elevated recognition of writer proteins. Whether the level of FTO and m6A RNA methylation in RNA transcripts affect the recognition and functions of different m6A reader proteins remains elusive (Shi et al., 2019). In addition, the changes in MMP2 and VEGFA methylation levels were not significantly affected in the SA samples, which also points to other regulatory mechanisms. Therefore, it is necessary to identify the core pathological genes of SA, and to explore the function of each m6A-regulated gene to explore the potential molecular mechanisms. Nevertheless, our findings show that impaired mRNA demethylation at the maternal-fetal interface due to low FTO levels can at least partly mediate SA pathogenesis. A dysfunctional placenta can significantly increase the risk of preeclampsia, fetal growth restriction (FGR) and early SA (Rana et al., 2020; Yang et al., 2021). Based on our results, we recommend analysis of placental FTO demethylase levels in patients with late SA in order to determine whether these epigenetic changes are sudden or related to implantation. Beside, FTO downregulation increases the m6A modification tends to be incomplete, and further studies of the mechanism of FTO and m6A methylation in vitro experiments is recommended.
Conclusion
The villi of SA patients are impaired in immune tolerance, invasion and migration, and angiogenesis, manifested as decreased HLA-G and VEGFR mRNA expression and increased MMP7 and MMP9 mRNA expression. The villi of SA patients were in a state of oxidative stress, with downregulated FTO. Meanwhile, the combination of relevant phenotypic functional genes was downregulated but the m6A modification was increased. In conclusion, inhibition of the RNA demethylase FTO leads to hypermethylation of crucial genes and oxidative stress in the maternal-fetal interface during SA.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou University of Traditional Chinese. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
WQ, SL, and JG conceived and designed the experiments, and wrote the manuscript. WQ, YZ, HW, XL, ZR, and LY performed the experiments. QY, LZ, HT, and JL analyzed the data. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Project of Fok Ying Tung Education Foundation for Young Teachers in Colleges and Universities (Grant No. 151042), National Natural Science Foundation Project of China (Grant No. 81774358), Guangdong Science and Technology Department Planning Project (Grant No. 2017A020215106), Scientific Research Team Training Project of GZUCM (Grant No. 2019KYTD202), and Guangdong Provincial Key R&D Program “Modernization of Traditional Chinese Medicine in Lingnan” (Grant No. 2020B1111100003).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the National Key Discipline and the Gynecology Laboratory of Guangzhou University of Chinese Medicine for providing associated facilities.
References
Andraweera, P. H., Dekker, G. A., Jayasekara, R. W., Dissanayake, V. H. W., and Roberts, C. T. (2015). The obesity-related FTO gene variant associates with the risk of recurrent miscarriage. Acta Obstet. Gynecol. Scand. 94, 722–726. doi: 10.1111/aogs.12640
Cecati, M., Giannubilo, S. R., Emanuelli, M., Tranquilli, A. L., and Saccucci, F. (2011). HLA-G and pregnancy adverse outcomes. Med. Hypotheses 76, 782–784. doi: 10.1016/j.mehy.2011.02.017
Costello, A., Lao, N. T., Barron, N., and Clynes, M. (2019). Improved yield of rhEPO in CHO cells with synthetic 5’ UTR. Biotechnol. Lett. 41, 231–239. doi: 10.1007/s10529-018-2632-2
Csepany, T., Lin, A., Baldick, C. J. Jr., and Beemon, K. (1990). Sequence specificity of mRNA N6-adenosine methyltransferase. J. Biol. Chem. 265, 20117–20122. doi: 10.1016/S0021-9258(17)30477-5
El-Far, M., El-Motwally, A. E. G., Abou Hashem, I., and Bakry, N. (2009). Biochemical role of intravaginal sildenafil citrate as a novel antiabortive agent in unexplained recurrent spontaneous miscarriage: first clinical study of four case reports from Egypt. Clin. Chem. Lab. Med. 47, 1433–1438. doi: 10.1515/CCLM.2009.311
El-Far, M., El-Sayed, I. H., El-Motwally, A. E. G., Hashem, I. A., and Bakry, N. (2007). Tumor necrosis factor-α and oxidant status are essential participating factors in unexplained recurrent spontaneous abortions. Clin. Chem. Lab. Med. 45, 879–883. doi: 10.1515/CCLM.2007.138
Fortis, M. F., Fraga, L. R., Boquett, J. A., Kowalski, T. W., Dutra, C. G., Gonçalves, R. O., et al. (2018). Angiogenesis and oxidative stress-related gene variants in recurrent pregnancy loss. Reprod. Fertil. Dev. 30, 498–506. doi: 10.1071/RD17117
Gerken, T., Girard, C. A., Tung, Y. C. L., Webby, C. J., Saudek, V., Hewitson, K. S., et al. (2007). The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science. 318, 1469–1472. doi: 10.1126/science.1151710
Hernández-Vargas, P., Muñoz, M., and Domínguez, F. (2020). Identifying biomarkers for predicting successful embryo implantation: applying single to multi-OMICs to improve reproductive outcomes. Hum. Reprod. Update 26, 264–301. doi: 10.1093/humupd/dmz042
Huang, H., Weng, H., and Chen, J. (2020). m6 A Modification in Coding and Non-coding RNAs: roles and Therapeutic Implications in Cancer. Cancer Cell 37, 270–288. doi: 10.1016/j.ccell.2020.02.004
Huang, H., Weng, H., Sun, W., Qin, X., Shi, H., Wu, H., et al. (2018). Recognition of RNA N-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat. Cell Biol. 20, 285–295. doi: 10.1038/s41556-018-0045-z
Jauniaux, E., Hempstock, J., Greenwold, N., and Burton, G. J. (2003). Trophoblastic oxidative stress in relation to temporal and regional differences in maternal placental blood flow in normal and abnormal early pregnancies. Am. J. Surg. Pathol. 162, 115–125. doi: 10.1016/S0002-9440(10)63803-5
Jia, G., Fu, Y., Zhao, X., Dai, Q., Zheng, G., Yang, Y., et al. (2011). N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 7, 885–887. doi: 10.1038/nchembio.687
Kaspi, A., Khurana, I., Ziemann, M., Connor, T., Spolding, B., Zimmet, P., et al. (2018). Diet during pregnancy is implicated in the regulation of hypothalamic RNA methylation and risk of obesity in offspring. Mol. Nutr. Food Res. 62:1800134. doi: 10.1002/mnfr.201800134
Lan, Q., Liu, P. Y., Haase, J., Bell, J. L., Hüttelmaier, S., and Liu, T. (2019). The critical role of RNA m6A methylation in cancer. Cancer Res. 79, 1285–1292. doi: 10.1158/0008-5472.CAN-18-2965
Lee, Y., Choe, J., Park, O. H., and Kim, Y. K. (2020). Molecular Mechanisms Driving mRNA Degradation by mA Modification. Trends Genet. 36, 177–188. doi: 10.1016/j.tig.2019.12.007
Lence, T., Paolantoni, C., Worpenberg, L., and Roignant, J. Y. (2019). Mechanistic insights into m6A RNA enzymes. Biochim. Biophys. Acta Gene Regul. Mech. 1862, 222–229. doi: 10.1016/j.bbagrm.2018.10.014
Li, X. C., Jin, F., Wang, B. Y., Yin, X. J., Hong, W., and Tian, F. J. (2019). The m6A demethylase ALKBH5 controls trophoblast invasion at the maternal-fetal interface by regulating the stability of CYR61 mRNA. Theranostics 9: 3853. doi: 10.7150/thno.31868
Mathiyalagan, P., Adamiak, M., Mayourian, J., Sassi, Y., Liang, Y., Agarwal, N., et al. (2019). FTO-dependent N6-methyladenosine regulates cardiac function during remodeling and repair. Circulation 139, 518–532. doi: 10.1161/CIRCULATIONAHA.118.033794
Mentese, A., Güven, S., Demir, S., Sümer, A., Yaman, S. Ö, Alver, A., et al. (2018). Circulating parameters of oxidative stress and hypoxia in normal pregnancy and HELLP syndrome. Adv. Clin. Exp. Med. 27, 1567–1572. doi: 10.17219/acem/74653
Qin, L., Min, S., Shu, L., Pan, H., Zhong, J., Guo, J., et al. (2020). Genetic analysis of N6-methyladenosine modification genes in Parkinson’s disease. Neurobiol. Aging 93, 143.e9–143.e13. doi: 10.1016/j.neurobiolaging.2020.03.018
Rana, S., Burke, S. D., and Karumanchi, S. A. (2020). Imbalances in circulating angiogenic factors in the pathophysiology of preeclampsia and related disorders. Am. J. Obstet. Gynecol. [Online ahead of print] doi: 10.1016/j.ajog.2020.10.022
Ren, Y., Xu, Y., Wang, Y., Jiang, Y., and Wen, J. (2020). Regulation of miR-375 and Sonic hedgehog on vascular endothelial growth factor in preeclampsia rats and its effect on trophoblast cells. Biosci. Rep. [Online ahead of print] doi: 10.1042/BSR20200613
Sahin, H. G., Sahin, H. A., and Kocer, M. (2001). Randomized outpatient clinical trial of medical evacuation and surgical curettage in incomplete miscarriage. Eur. J. Contracept. Reprod. Health Care 6, 141–144. doi: 10.1080/ejc.6.3.141.144
Shi, H., Wei, J., and He, C. (2019). Where, When, and How: context-Dependent Functions of RNA Methylation Writers, Readers, and Erasers. Mol. Cell 74, 640–650. doi: 10.1016/j.molcel.2019.04.025
Sultana, Z., Maiti, K., Dedman, L., and Smith, R. (2018). Is there a role for placental senescence in the genesis of obstetric complications and fetal growth restriction? Am. J. Obstet. Gynecol. 218, S762–S773. doi: 10.1016/j.ajog.2017.11.567
Sun, F., Wang, S., and Du, M. (2020). Functional Regulation of Decidual Macrophages during Pregnancy. J. Reprod. Immunol. 143:103264. doi: 10.1016/j.jri.2020.103264
Yang, Y., Xu, P., Zhu, F., Liao, J., Wu, Y., Hu, M., et al. (2021). The potent antioxidant MitoQ protects against preeclampsia during late gestation but increases the risk of preeclampsia when administered in early pregnancy. Antioxid. Redox Signal. 34, 118–136. doi: 10.1089/ars.2019.7891
Zhao, T. X., Wang, J. K., Shen, L. J., Long, C. L., Liu, B., Wei, Y., et al. (2020). Increased m6A RNA modification is related to the inhibition of the Nrf2-mediated antioxidant response in di-(2-ethylhexyl) phthalate-induced prepubertal testicular injury. Environ. Pollut. 259:113911. doi: 10.1016/j.envpol.2020.113911
Zhen, B., Youhua, L., and Yuanling, Z. Yongxi, Y., Ruifan, W., Qing, L., et al. (2019). A dynamic reversible RNA N -methyladenosine modification: current status and perspectives. J. Cell Physiol. 234, 7948–7956. doi: 10.1002/jcp.28014
Keywords: villous, spontaneous abortion, FTO, m6A, RNA methylation
Citation: Qiu W, Zhou Y, Wu H, Lv X, Yang L, Ren Z, Tian H, Yu Q, Li J, Lin W, Zhao L, Luo S and Gao J (2021) RNA Demethylase FTO Mediated RNA m6A Modification Is Involved in Maintaining Maternal-Fetal Interface in Spontaneous Abortion. Front. Cell Dev. Biol. 9:617172. doi: 10.3389/fcell.2021.617172
Received: 14 October 2020; Accepted: 28 June 2021;
Published: 19 July 2021.
Edited by:
Kyoko Yokomori, University of California, Irvine, United StatesReviewed by:
Meena Parvatha Balakrishnan, Baylor College of Medicine, United StatesJiangbo Wei, University of Chicago, United States
Copyright © 2021 Qiu, Zhou, Wu, Lv, Yang, Ren, Tian, Yu, Li, Lin, Zhao, Luo and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Songping Luo, c29uZ3BpbmdsdW9AaG90bWFpbC5jb20=; Jie Gao, Z2pma3RzQHFxLmNvbQ==
†These authors have contributed equally to this work
 Weiyu Qiu
Weiyu Qiu Yuexi Zhou1†
Yuexi Zhou1† Ling Zhao
Ling Zhao