- 1University of Wuerzburg, Developmental Biochemistry, Biocenter, Wuerzburg, Germany
- 2INRA, UR1037, Fish Physiology and Genomics, Rennes, France
- 3State Key Laboratory of Developmental Biology of Freshwater Fish, College of Life Sciences, Hunan Normal University, Changsha, China
- 4Reproductive and Molecular Biology Group, Department of Morphology, Institute of Bioscience of Botucatu, São Paulo State University, Botucatu, Brazil
- 5Department of Human Genetics, University of Utah, Salt Lake City, UT, United States
- 6Xiphophorus Genetic Stock Center, Texas State University, San Marcos, TX, United States
Sex determination (SD) is a highly diverse and complex mechanism. In vertebrates, one of the first morphological differences between the sexes is the timing of initiation of the first meiosis, where its initiation occurs first in female and later in male. Thus, SD is intimately related to the responsiveness of the germ cells to undergo meiosis in a sex-specific manner. In some vertebrates, it has been reported that the timing for meiosis entry would be under control of retinoic acid (RA), through activation of Stra8. In this study, we used a fish model species for sex determination and lacking the stra8 gene, the Japanese medaka (Oryzias latipes), to investigate the connection between RA and the sex determination pathway. Exogenous RA treatments act as a stress factor inhibiting germ cell differentiation probably by activation of dmrt1a and amh. Disruption of the RA degrading enzyme gene cyp26a1 induced precocious meiosis and oogenesis in embryos/hatchlings of female and even some males. Transcriptome analyzes of cyp26a1–/–adult gonads revealed upregulation of genes related to germ cell differentiation and meiosis, in both ovaries and testes. Our findings show that germ cells respond to RA in a stra8 independent model species. The responsiveness to RA is conferred by sex-related genes, restricting its action to the sex differentiation period in both sexes.
Introduction
Sex determination is the decision whether the bipotential gonadal primordium will become a testis or an ovary (Capel, 2000; Devlin and Nagahama, 2002). In vertebrates, this complex and tightly controlled developmental determination process is characterized by a difference in the timing of meiotic initiation (Kimble and Page, 2007; Nishimura and Tanaka, 2014). In multicellular organisms the formation of the gametes is a key event for the production of future generations. In this process the germ cells take two crucial decisions, a temporal one, namely when meiosis entry happens, and a lineage decision to develop either to sperm or egg (Kimble and Page, 2007). However, the timing of the mitosis/meiosis decision and features of meiosis itself are often sex-specific, suggesting a close relationship between the mitosis/meiosis and sperm/egg decisions. In all so far studied vertebrates, initiation of meiosis occurs earlier in females than in males, and retinoic acid (RA) signaling has been assigned a crucial role in this process (Koubova et al., 2006; Bowles and Koopman, 2007; Kimble and Page, 2007).
RA is a vitamin A derivative responsible for activation of several genes during development, being important for the formation of the anterior-posterior axis, and proper development of many different organs and tissues (Sakai et al., 2001). The importance of RA in meiosis entry has been widely studied in mammals and other vertebrates (Smith et al., 2008; Wallacides et al., 2009; Dong et al., 2013). RA is small, polar and diffusible, and the concentration levels are fine-tuned by the balance between its synthesis and its oxidative degradation (Niederreither and Dolle, 2008; Shimozono et al., 2013). The main enzymes involved in the synthesis of RA are the retinaldehyde dehydrogenases (RALDHs). The enzymes responsible for its degradation are the cytochrome P450 family 26 (CYP26s) (Sakai et al., 2001; Yashiro et al., 2004; Deak et al., 2005; Emoto et al., 2005; White et al., 2007). In mice, RA was shown to make primordial germ cells (PGCs) in females entering meiosis by inducing Stra8 (stimulated by retinoic acid gene 8) expression and initiates oogenesis, while testis produce the CYP26B1 enzyme that catalyzes RA degradation, resulting in a delay of meiosis entry in males (Bowles et al., 2006, 2009; Bowles and Koopman, 2010). Therefore, the factors that are regulating the expression of Cyp26b1 are sex specific. In developing testes, Cyp26b1 is upregulated by the transcriptional activator SF1 in Leydig cells and by SF1 and SOX9 in Sertoli cells. In ovaries, its expression is suppressed by the female-specific transcription factor FOXL2 (Kashimada et al., 2011).
Teleost is the group of vertebrate with the highest diversity of species and simultaneously sex determination mechanism (Capel, 2017). Complexly, stra8 is absent in most teleost fish. In species containing stra8, the role of RA in meiosis seems to be conserved as in other vertebrates (Feng et al., 2015; Li et al., 2016). In medaka (Oryzias latipes), which lacks stra8, we have earlier shown that RA is implicated in meiosis regulation in the adult gonad. Expression analyses in embryos of medaka at the SD stage demonstrated that the RA synthesis gene (aldh1a2) is expressed in the early somatic gonad of both sexes. However, exogenous treatments with RA did not provide conclusive evidence for RA being involved in inducing the first meiosis as the primary step after SD (Adolfi et al., 2016).
Despite the high diversity of the genetic pathway, the role of RA and the sex-specific timing of germ cells meiosis entry first are conserved in fish and all vertebrates, which motivated to ask the following question: is there any conserved role of RA in the sex determination pathway? To analyze a possible connection between the mechanism of sex determination and meiosis entry we used the well-characterized sex model species medaka. This species has a well-characterized primary male master sex determination gene on the Y-chromosome. This gene, dmrt1bY, is a duplication of the autosomal copy, dmrt1a, a highly conserved gene generally involved in testis development and differentiation (Matsuda et al., 2002; Nanda et al., 2002). Here, we show that exogenous treatments with RA in early medaka embryos acts as stress factor leading to an increase in expression of important male sex-related genes, therefore blocking meiosis. Disruption of the cyp26a1 gene induced early meiosis in females and produced some males with transient oocytes in the gonad prior to testis differentiation. Our results indicate for the first time that the sex regulatory network may control the germ cell responsiveness to RA, which in turn regulates the different timing of meiosis entry of female and male in a stra8 independent model.
Materials and Methods
Animals
Laboratory reared medaka (Oryzias latipes, Class Actinopterygii, order Beloniformes, family Adrianichthyidae) were used. For detailed description of this model species and its features see (Kinoshita et al., 2009). All experiments were performed with fish of the Carbio strain. The animals were kept under standard photoperiod cycle of 14/10 h light/dark at 26°C (±1°C). Eggs were collected 1–2 h after starting the light cycle and raised at 26°C in Danieau's medium (17.4 mM NaCl, 0.21 mM KCl, 0.12 mM MgSO4, 0.18 mM Ca(NO3)2, 1.5 mM Hepes, pH 7.2). The stages of development were identified according to Iwamatsu (2004).
Animals for colony breeding and embryo production were kept and sampled in accordance with the applicable EU and national German legislation governing animal experimentation, in particular all experimental protocols were approved through an authorization (568/300-1870/13) of the Veterinary Office of the District Government of Lower Franconia, Germany, in accordance with the German Animal Protection Law (TierSchG).
In vivo Drugs Treatments
Treatments of embryos and dilutions of the drugs were made in Danieau's medium. To investigate an effect on regulation of sex-related genes, we performed long-term treatments from stage 29, before the sex determination period, and kept in the dark until 1 day after hatching (dah), first meiosis entry period in females. AM580 (10 nM), an agonist of the retinoic acid receptor alpha, and all-trans-retinoic acid ATRA (10 nM) were added to the medium and medium changed every 2 days. The exclude any effect of stress during the treatments, we co-treated the embryos with or without Metyrapone (5 μM, Sigma-Aldrich), a compound that inhibits endogenous cortisol synthesis. The selected drugs concentration for the treatments were based on previous studies (Adolfi et al., 2016, 2019). Specimen were collected at 1 dah and genotyped for sex by PCR for the Y-linked male determining gene dmrt1bY using genomic DNA as template.
Disruption of Cyp26a1 by TALEN
The genomic sequence of cyp26a1 (Ensembl gene number ENSORLG00000014516) was retrieved from the Ensembl medaka genome browser (http://www.ensembl.org/Oryzias_latipes). The construction of TALEN expression vectors (left, pCS2TAL3DDD, and right, pCS2TAL3RRR, with both vectors containing the respective TALE fragment, the FokI cleavage domain, and other necessary components) were developed following the standard procedure (Dahlem et al., 2012). The TALEN target sites of cyp26a1 were designed in the second exon, with the right binding site located at the junction of exon 2 and intron 2. The cyp26a1 TALEN recognition sequences were left TALEN 5′ –TCTCCAACATGCACGGAT- 3′ and right TALEN 5′ –TGGAGACTCACCTTTTT- 3′. Between the binding sites, an 18 bp spacer is included, where the FokI nuclease cuts.
In vitro transcription of TALENs was carried out with the Sp6mMESSAGEmMACHINE Kit (Ambion). The resulting mRNA was purified by phenol/chloroform-extraction and then quantified using NanoDrop-2000 (Thermo Scientific). The left and right arm mRNA of each TALEN pair was then mixed at a molar ratio of 1:1, with a final concentration of 100 ng/μL mRNA of each arm, and stored at −80°C until use. About 200 to 600 pg of the mRNA mixture was directly microinjected into medaka embryos at the one-cell stage. The injected embryos were cultivated at 26°C and 10 animals collected at stage 1 dah to extract DNA for mutation efficiency analysis.
Genotyping of Embryos and Adult Fish
To determine the genotypic sex of embryos and adult fish and the presence and absence of mutations, genomic DNA was extracted. Caudal fin clips of the adult fish or whole hatchling were incubated for 1 h at 95°C in 100 μL of Base Solution (25 mM NaOH, 0,2 mM EDTA, pH = 12) and shaking. The solution was cooled down on ice, 100 μL of Neutralization Solution (40 mM Tris-HCl pH = 5.0) added and vortexed. Two microliter of the total volume was used in a 25 μL PCR reaction. The PCR products were resolved on 1% agarose gels.
For determination of genotypic sex, a pair of primers (Supplementary Table 1) was used that amplifies fragments of both dmrt1a (1,100 bp) and dmrt1bY (900 bp), yielding one PCR product (dmrt1a) in XX genotypes, and two PCR products (dmrt1a and dmrt1bY) in XY genotypes. To detect cyp26a1 TALEN mutants, primers were designed flanking the region where the mutations are expected (exon2). PCR product were purified using GenElute™ Gel Extraction Kit (Sigma-Aldrich) according to the manufacturer's instructions and sequenced using the PCR amplification primers.
Luciferase Reporter Assays
HEK 293 cells were cultured in Dulbecco's modified Eagle's medium and 10% fetal calf serum, and maintained at 37°C, 5% CO2 with 100% humidity. To analyze transcriptional regulation of dmrt1a, an 11875 bp fragment upstream of the start codon was isolated and cloned into pGL4.20 vector containing the firefly luciferase gene (Dmrt1aprom::LucFF) as described (Adolfi et al., 2019). In addition, the responsiveness of the HEK 293 cells to ATRA and AM580 was tested using the pGL3-RARE-luciferase plasmid (Addgene, Cat. 13458), which contains the retinoic acid responsive element (RARE) upstream of the firefly luciferase gene.
Transient transfections in HEK 293 were done at 80% confluency by a polyethylenimine-based procedure. The empty pGL4.20 vector containing the tk promoter and the firefly luciferase gene (pGL4.20-tkmini) was used as negative control. To normalize firefly activity, cells were co-transfected with a Renilla luciferase expressing plasmid (pGL4.74) (Regneri et al., 2015).
For luciferase assays, single wells of a 24-well plate were co-transfected with firefly and Renilla luciferase reporter constructs in a 5:1 molar ratio. The concentration of each construct was calculated in order to obtain a total DNA concentration of 0.5 μg per well. pGL4.20-tkmini and Dmrt1a-prom::LucFF reporter constructs were used with and without co-transfection of the transcriptional activator SF1 of medaka (100 ng). The SF1 expression vector (pcDNA3.1::medakaSF1) was kindly donated from Yann Guiguen (INRA, France). After 16–18 h (day 1), medium was changed. On day 2, cells were incubated for 24 h and with 1 μM ATRA, 10 nM AM580 or DMSO for control. On day 3, cells were harvested in 100 μl of 1 X PLB (Promega).
Renilla and firefly luciferase activities were quantified using the Dual-Luciferase® Reporter Assay System from Promega and the TriStar LB941 microplate multimode reader (Berthold Technologies). Experiments result from at least three replicates and error bars represent the standard error of the mean.
RNA Sequencing
Three individual ovaries and three pools of three testes from wildtype Carbio strain and cyp26a1–/–of medaka were homogenized in TRIzol® reagent (Invitrogen). The total RNA phase was isolated using chloroform and purified using RNeasy® Mini kit (Qiagen) following the manufacturer's instructions. The RNA quality was assessed by measuring the RNA Integrity Number (RIN) using an Agilent 2100 Electrophoresis Bioanalyzer Instrument (Agilent Technologies 2100 Expert). RNA samples with RIN > 8 were used for sequencing.
RNA sequencing libraries were constructed following the standard TruSeq Illumina mRNA library preparation protocol (www.illumina.com; Illumina Inc., BGI, Hong Kong). Read length = 150, sequencing depth for paired end: 65–71 million reads.
Transcriptome Analysis
Transcriptome sequences were mapped to the O. latipes reference genome (Ensembl Release 93) using the RNA-sequence aligner STAR (https://github.com/alexdobin/STAR/releases). Transcripts were quantified as expected read counts using RSEM (http://deweylab.github.io/RSEM). Differentially expressed genes between testis and ovary were detected by DESeq2 (Love et al., 2014) (Bioconductor/R) for wildtype and mutants. Genes were considered to be differentially expressed, if p.value <= 0.05 AND log2FC ≤ −2 (higher expression in female) and log2FC ≥ +2 (higher expression in male). Histograms for genes with log2FC > 2 AND baseMean > 100 were plotted and genes showing comparable regulation between male and female wildtype and mutant samples were selected. Functional clustering was made using DAVID Bioinformatics Resources 6.8 (https://david.ncifcrf.gov/).
Real Time Quantitative RT-PCR
Wildtype and mutant organs of adult (4 months after hatching) males and females and whole embryos of different developmental stages were collected. Total RNA was extracted from 3 pools of adult fish organs (n = 4) or whole embryos (n = 20) using the TRIZOL reagent (Invitrogen) according to the supplier's recommendation. After DNase treatment, reverse transcription was done from 2 μg total RNA using RevertAid First Strand Synthesis kit (Fermentas) and random primers. Real-time quantitative PCR was carried out with SYBR Green reagent and amplifications were detected with a Mastercycler® ep realplex (Eppendorf). All results are averages of at least three independent RT reactions from three independent RNA preparations. Transcript levels of the target genes were normalized against the medaka elongation factor-1 alpha (ef1a) gene (Supplementary Table 1). The ΔCt values presented as means ± standard error of the mean (SEM), were analyzed by one-way ANOVA, Tukey's and Student's t-test. A significance level of P < 0.05 was used for all tests.
Light Microscopy
Whole larvae and gonads from adult fish were dissected and fixed in Karnovski solution (2% glutaraldehyde and 4% paraformaldehyde in Sörensen buffer [0.1 M, pH 7.2]) for 24 h at 4°C. Then, samples were washed in water, dehydrated in increasing concentrations of ethanol, and embedded in Historesin Technovit 7100 (Kulzer, Hanau, Germany). Serial sections of 2 μm thickness were obtained and counterstained with hematoxylin & eosin (HE).
Results
Induction of Sex Determination Genes After RA Induction
We performed treatments of medaka embryos at different time points with ATRA and AM580 to activate the RA pathway. From the treated embryos, we analyzed expression of genes known to be involved in sex determination or gonad differentiation. Long-term treatments (stage 29 until 1 dah) of BACdmrt1a::GFP transgenic fish with ATRA resulted in a strong induction of reporter gene expression exclusively in the somatic gonad at hatching stage in both sexes (Figure 1A). Gene expression levels of male-related genes were determined from whole embryos after long-term treatment with AM580 (Figure 1B). The dmrt1bY expression levels were unaffected in males. However, amh and dmrt1a showed significantly increased mRNA levels in both sexes.
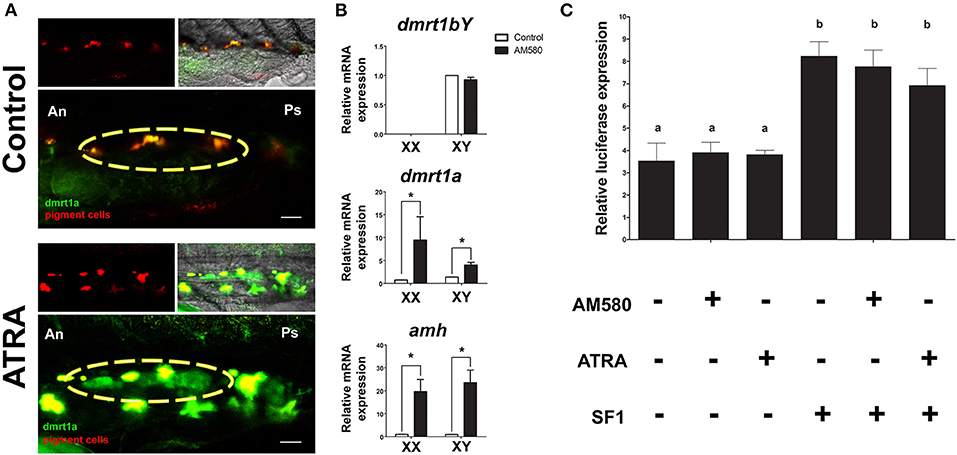
Figure 1. Regulation of sexual development genes after RA pathway activation. (A) Long-term treatments of BACdmrt1a::GFP medaka embryos led to gonad (yellow circle) specific induction of dmrt1a at 1 dah. (B) Expression levels of dmrt1bY, dmrt1a, and amh at 1 dah after long-term treatments with AM580. No effect on dmrt1bY expression is observed, however, significant upregulation of dmrt1a and amh occurred in embryos of both sexes. Values are expressed as arbitrary mRNA units normalized against the expression levels of ef1a amplified from the same template and relative to the average expression of control male and female embryos. Asterisk indicates a significant difference (p < 0.05) after Student's t-test comparing the expression between control and AM580 treatments. (C) The dmrt1a promoter activity was higher when co-transfected with medaka SF1, but no significant luciferase signal was observed after treatments with both ATRA and AM580. Scale bar = 60 μm.
To date, the responsiveness of dmrt1a to RA is unknown. Hence, to check whether the treatments had a direct effect by activating dmrt1a transcription, we analyzed the 11,8 kb promoter of dmrt1a after treatments with ATRA or AM580 in HEK 293 cells. The HEK 293 cells were shown to be capable to respond to both ATRA and AM580 when compared to control (DMSO), indicating that the retinoic acid receptors (RAR/RXR) are endogenously produced in this cell line (Supplementary Figure 1). Cells co-transfected with the cofactor SF1 showed a significant increase of the dmrt1a promoter activity. However, treatments with RA had no effect on the promoter of dmrt1a in vitro (Figure 1C).
Cyp26a1–/–Medaka Gonad Development
We showed earlier that cyp26a1 is differently expressed in gonads of medaka, with much higher transcript levels in females than in males (Adolfi et al., 2016) This indicated that cyp26a1 in medaka may perform the role of CYP26B1 in mammals. To evaluate a possible role of cyp26a1 in the timing of meiosis entry and consequently in sex determination/differentiation, we generated knockout cyp26a1 medaka.
To molecularly characterize the cyp26a1 TALEN induced mutations, we amplified the expected target site from F0 embryos and adult male and female founders and sequenced the 544 bp PCR product (Supplementary Figure 2A). We obtained three different mutant lines (Supplementary Figure 2B) with deletions at the target site. However, only the Δ5 mutation conceptually translates into a protein with a predicted compromised function (frameshift and, premature termination), while the other two presented an in-frame deletion of two or three aa that still could lead to fully functional enzyme (Supplementary Figure 2C). All three mutants present deletions or substitutions within the P450 superfamily domain, however, the Δ5 mutation is predicted to translate into a shorter protein that lacks the Cytochrome P450 cysteine heme-iron ligand signature. Therefore, we generated cyp26a1–/– animals and performed the mutants analyzes only from the Δ5 mutation line.
We then followed the gonad development of wildtype and cyp26a1–/–in male and female larvae at the early meiosis stages (Figure 2). Already at 5 dah, differences in the germ cells are observed in females, in which the cyp26a1–/– present more proliferating germ cells compared to the wildtype (Figures 2A,B), while in male no morphological differences were observed until 15 dah (Figures 2G–J). Mutant females at 10 dah apparently contain more pre-vitellogenic oocytes than wildtype females, indicating increased oogenesis and meiosis entry in the mutant at this stage (Figures 2C,D). At 15 dah, the gonads of both wildtype and cyp26a1–/–females presented no apparent morphological difference anymore (Figures 2E,F). Strikingly, 2 out of 10 15 dah males of cyp26a1–/–had an isolated pre-vitellogenic oocytes inside the undifferentiated gonad, and no sign of germ cell proliferation could be observed (Figures 2K,L). Comparing 4 months old wildtype and mutant mature gonads of medaka no apparent differences in morphology were observed in both sexes (Supplementary Figure 3). Despite the development of oocytes at 15 dah in males of cyp26a1–/– genotype, no sign of any female structure was observed in adult testis.
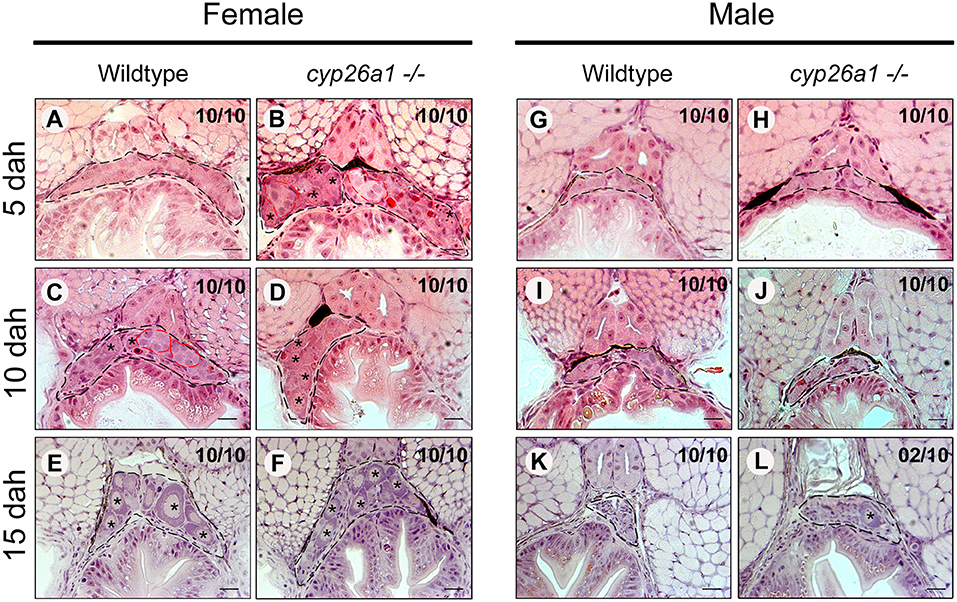
Figure 2. Germ cell proliferation and differentiation during male and female gonad development in wildtype and cyp26a1–/–medaka. Gonad (black dashed lines) of cyp26a1–/–female present higher amounts of differentiated germ cells (red dashed lines) at 5 days after hatching (dah) in comparison with the wildtype (A,B). At 10 dah, differentiated germ cells are more preeminent in the wildtype female, while in the mutant, the higher amount of pre-vitellogenic oocytes (star) indicates more advanced stage of oogenesis (C,D). At 15 dah, no apparent differences were observed in female gonads (E,F). In males, no differences were observed between wildtype and mutant at 5 dah (G,H) and 10 dah (I,J). At 15 dah, no sign of germ cells differentiation is observed, by comparing with the wildtype gonad (K,L). However, some cyp26–/–males presented pre-vitellogenic oocytes (star). Scale bar = 20 μm.
Treatments of Wildtype and Cyp26a1–/–Medaka Embryos
We performed treatments of cyp26a1 KO embryos with AM580 from embryo stage 29 until 1 dah. Control females of cyp26a1–/–medaka presented morphologically differentiated germ cells already at 1 dah, showing commitment to gametogenesis (Figure 3A). However, XX mutants treated with AM580 had only undifferentiated germ cells (Figure 3B). The developing gonads of both control and treated XY mutants show no sign of germ cell differentiation (Figures 3D,E), indicating that AM580 delays germ cell commitment to gametogenesis in cyp26a1 mutants.
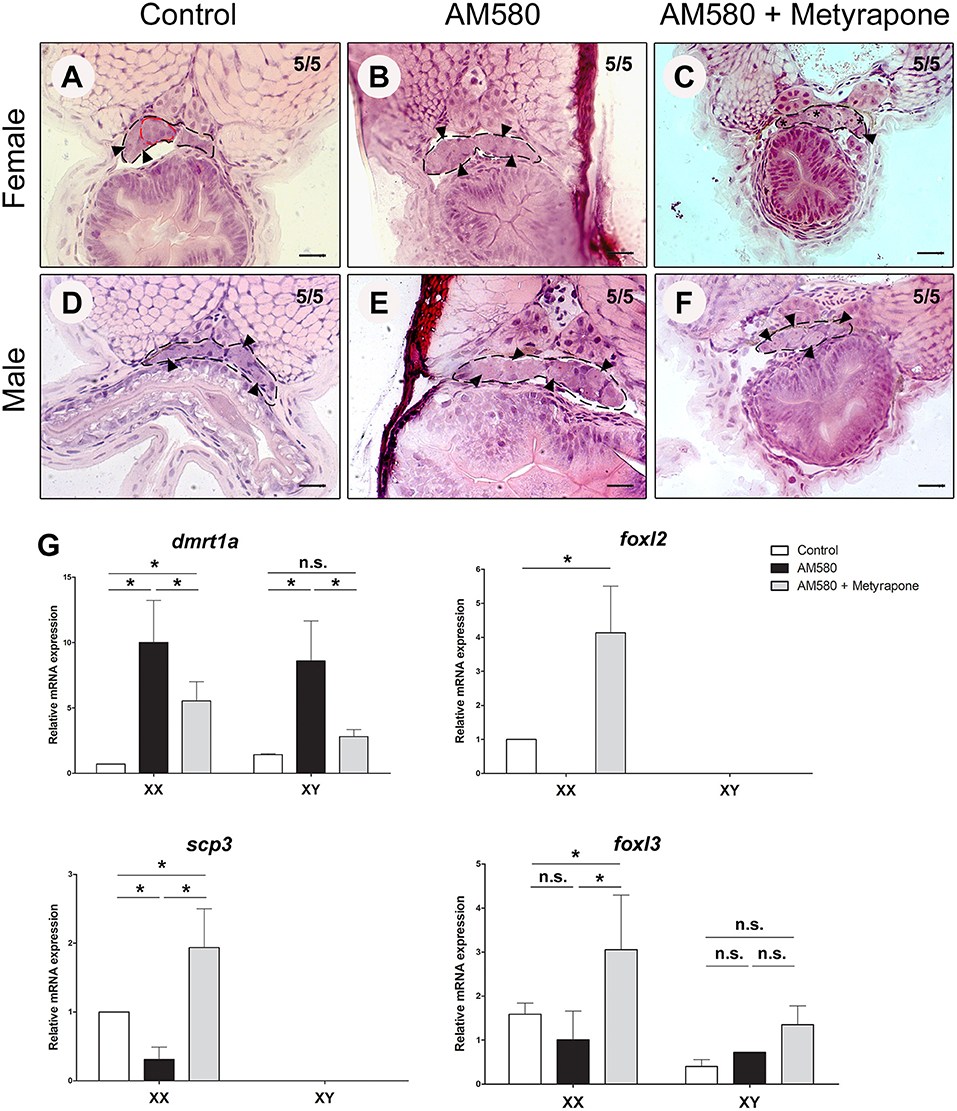
Figure 3. Exogenous treatment of AM580 and Metyrapone in cyp26a1–/–embryos during the sex determination period. Gonad (black dashed lines) of cyp26a1–/–female containing differentiated germ cells (red dashed lines) at 1 days after hatching (dah) in the control embryos (A). After treatments, XX embryos present only undifferentiated germ cells (arrowhead) (B). Co-treatment with AM580 and Metyrapone showed increase of early oogenesis stage germ cells (star) in females (C). No morphological differences in the gonads of XY mutants were observed in the control (D) and in treated embryos with AM580 (E) and both AM580 and Metyrapone (F), all containing only undifferentiated germ cells (arrowhead) Scale bar = 20 μm. Expression of dmrt1a, foxl2, foxl3, and scp3 in cyp26a1–/–embryos after treatment with AM580 and Metyrapone. After Student's t-test (p < 0.05), significant (asterisk) and not significant (n.s.) expression differences were observed between control and treatment groups (G).
Retinoic acid is an important morphogen, and treatments with AM580 performed during the sex determination period led to several malformations in the embryos (data not shown). Our recent study showed that temperature stress and cortisol treatments lead to masculinization of XX medaka, possibly through a direct activation of dmrt1a in the gonad and repressing germ cell differentiation (Adolfi et al., 2019). Hence, such stress factors in our treatments could possibly interfere with the effect of AM580 in meiosis initiation. To test this hypothesis, we co-treated the cyp26a1 KO embryos with AM580 and Metyrapone, a compound that inhibits the endogenous production of cortisol. Early differentiating oocytes (stage I) were observed in treated females (Figure 3C), and only undifferentiated germ cells were present in males in the same conditions (Figure 3F) showing that RA leads to germ cell differentiation in females.
Expression analyzes of mutant embryos showed that treatments with AM580 resulted in presented upregulation of the male-related gene dmrt1a in both sexes, while the female-related foxl2 gene expression is extremely reduced in females (Figure 3G). Treatments with both AM580 and Metyrapone presented less dmrt1a expression compared to those treated with AM580 alone, while foxl2, foxl3 (oogenesis inducer) and the meiosis marker scp3 were upregulated in females when compared to the control (Figure 3G).
Transcriptome Analyzes of Adult Cyp26a1–/–Medaka Gonads
Despite the effect in the early gonad of the cyp26a1 mutants, no apparent effect was seen in the adult animals on the cellular level. Nevertheless, the transcriptome analyses showed significant differences between adult wildtype and KO gonads of both male and female (Figure 4). Differential gene expression analysis (LogFC > 2) revealed that the mutation of cyp26a1 regulated more genes in female than male gonads (Figure 4A; Supplementary Table 2).
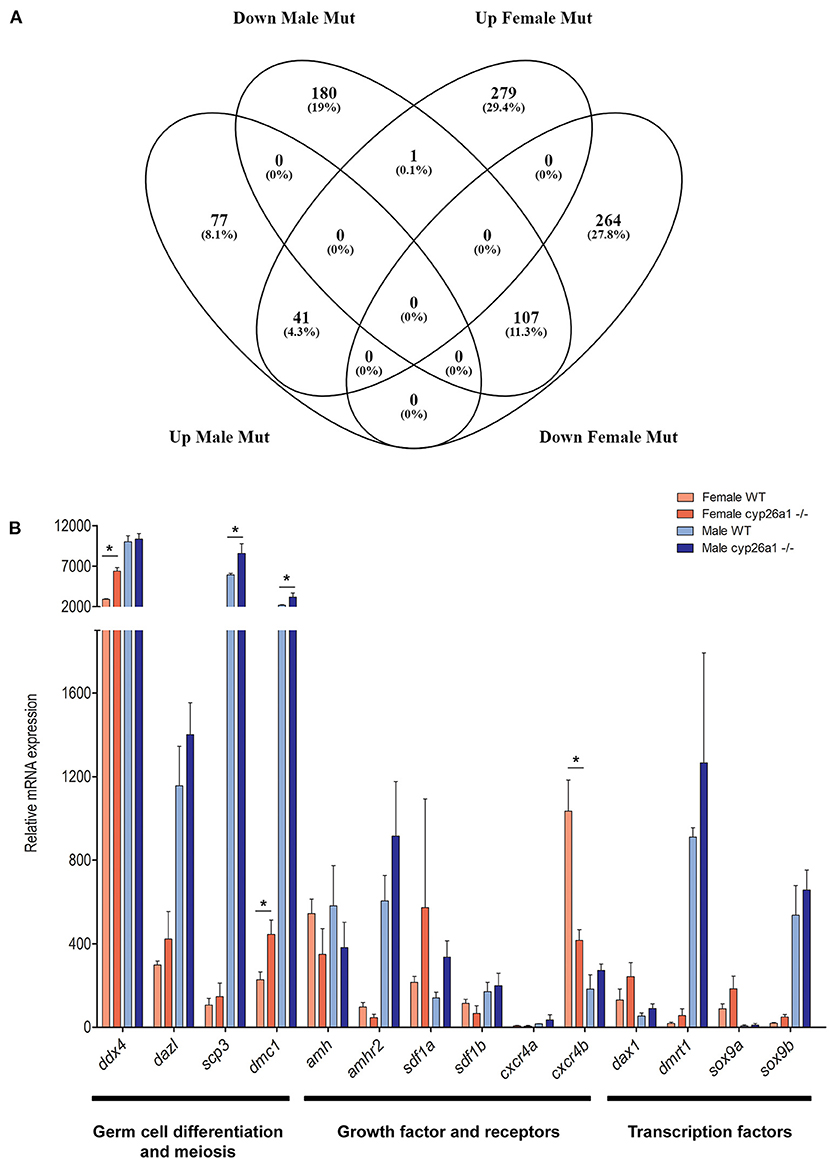
Figure 4. RNA-seq analyzes of adult gonad of wildtype and cyp26a1–/–medaka. (A) Venn diagram showing the number of regulated genes in both male and female mutants compared to the wildtype gonads. (B) Expression levels of sex-related genes known to be involved in germ cell migration, proliferation, differentiation and meiosis. Asterisk indicates a significant difference (p < 0.05) after Student's t-test.
The mutant ovaries presented higher number of exclusive upregulated genes (279), some of which are related to retinoic acid metabolism (e.g., aldh1a2, aldh8a1, and rarb), and gametogenesis (spata7, aqp3a, and zar1). In the genes exclusively downregulated in mutant females (264), some factors related to the TGF-beta signaling (smad1, inhbb, and lefty1), retinol metabolism and steroidogenesis (cyp1a) were affected. Interestingly, the gata4 gene, known to be required for gonad formation and testis development in mice (Hu et al., 2013), was strongly downregulated. Despite of a fewer number of genes regulated exclusively in male mutants, the nr4a1 gene, important in testis function (Daems et al., 2014), was highly upregulated.
The genes that were downregulated in the mutant gonads of both sexes (107) are enriched for functions related to mitochondrial electron transport (e.g., cox1, cox2, and cox7a2) and response to oxidative stress (e.g., rsp29). On the other hand, the genes that were upregulated in the mutants of both sexes (41) are related to immune response (e.g., c6) and peptidase activity (e.g., cela1, ela2, and prss1).
The induction of sex-related genes by AM580 treatments already indicated a possible effect of RA on gametogenesis and germ cell differentiation. This could also be observed by the genome wide expression analysis: genes which have a crucial function in germ cell differentiation (ddx4 and dazl) and meiosis (scp3 and dmc1) were slightly upregulated in the mutants of both sexes (Figure 4B). Similarly, growth factors (e.g., amh/amhr2, and sdf1/cxcr4) and transcription factors (e.g., dmrt1 and sox9) related to germ cell differentiation, proliferation and survival were regulated in mutants, especially in females (Figure 4B).
Discussion
The network and factors involved in sex determination appear to be more complex and diverse than previously thought (Herpin and Schartl, 2015). Recent studies suggest that neither the master sex determination gene nor the downstream regulatory network of gonad determination is conserved (Herpin et al., 2013). However, the timing of meiosis entry is conserved in vertebrates, with PGCs proliferating in female embryos first and then enter meiosis immediately afterwards, much earlier than in males (Bowles and Koopman, 2007; Morinaga et al., 2007; Saito and Tanaka, 2009; Wallacides et al., 2009). Therefore, to characterize the relation between the action of the sex determining genetic network and meiosis entry and a possible role of retinoic acid in this process, we performed treatments with RA agonists and analyzed possible changes in the process and sex determination.
At the sex determining stage, the male-related genes dmrt1a and amh were induced by RA treatment in both sexes. Overexpression of amh in both sexes after AM580 treatment can also be correlated to blocking of germ cell differentiation, since AMH signaling acts in the supporting cells of the undifferentiated gonad of medaka to promote proliferation of the mitotically active germ cells, which subsequently enter meiosis (Nakamura et al., 2012). Importantly, the upregulation of dmrt1a occurs specifically in the gonad, and this gene is known to have a main role in testis differentiation and maintenance (Masuyama et al., 2012). We showed recently that early induction of dmrt1a in medaka embryos results in sex-reversed XX males and blocks germ cell proliferation and differentiation (Adolfi et al., 2019). In medaka, the mechanism by which dmrt1a and dmrt1bY block germ cell differentiation is unknown, but in mice it has been shown that DMRT1 restricts RA responsiveness by repressing Stra8 transcription, preventing meiosis and promoting spermatogonial development in adult testis (Matson et al., 2010, 2011; Krentz et al., 2013). However, medaka has no stra8 gene, suggesting that dmrt1 would still have the same role for the germ cells, but through a different mechanism than in mammals. It was hypothesized that DMRT1 and retinoic acid receptor (RARα) are antagonistic regulators of some key feminizing genes (Huang et al., 2017), indicating that the presence of DMRT1 in the germ cells would be regulating their responsiveness to RA.
We have previously demonstrated that activation of the RA pathway could not induce meiosis in early embryos, but only in gonads that already started sexual differentiation and gametogenesis (Adolfi et al., 2016). Here we demonstrate that the same activation leads to overexpression of genes related to inhibition of germ cell differentiation (amh and dmrt1a) in the gonad, preventing the germ cells to differentiate and enter meiosis. The dmrt1a promoter activity experiments showed that treatments with ATRA and AM580 had no significant effect on the promoter activity of dmrt1a, indicating that RA upregulates dmrt1a only indirectly but not by direct transcriptional activation in vivo.
We showed that exogenous RA treatments can increase both cyp26a1 and cyp26b1 expression, the former being more sensitive to the treatments (Adolfi et al., 2016). This could lead to degradation of the drug and camouflage its direct effect. In order to remove the possible effect of cyp26a1 upregulation in the gonad, and increase the effect of RA in germ cell differentiation, we generated a full KO of the cyp26a1 gene. A cyp26a1 deficiency in Tilapia and catfish did not affect normal development of the animals and induced earlier initiation of meiosis for both XX and XY fish, but it was still earlier in females than in males (Feng et al., 2015; Li et al., 2016). Similarly, we observed more noticeable germ cell in meiosis, and early oogenesis in female cyp26a1 knockouts, but only after the sex determination stage. Despite the minor effect noted in adult gonads, the transcriptome analyzes show that ovaries of cyp26a1–/– medakas presented more regulated genes than other groups, since wildtype ovaries had the highest expression of cyp26a1 compared to other tissues (Adolfi et al., 2016; Biscotti et al., 2018). Consistently, strong upregulation was observed in mutant ovaries of genes known to be related to gametogenesis regulation like zar1, spata7, and aqp3a, the latter two genes already being known to be regulated by retinoic acid (Liu et al., 2005; Huang et al., 2006; Bellemere et al., 2008; Wang et al., 2009). The zar1 gene was described to have a conserved evolutionary role in ovarian follicle development and in the oocyte-to-embryo transition, but not correlation between RA and zar1 was demonstrated so far (Wu et al., 2003).
Interestingly, strong downregulation of gata4, ihnbb and cxcr4b in mutant ovaries was observed. The gata4 gene was reported to be a key transcriptional regulator of ovarian somatic cell function in both fetal and adult mice (Kyronlahti et al., 2011; Efimenko et al., 2013). In addition, gata4 was proposed to be important for gonadal development and maturation in both sexes of Tilapia (Li et al., 2012). Analyzes in vitro demonstrated that Gata4 is upregulated in murine embryonic stem cells (ESCs) after ATRA treatments (Mauney et al., 2010). The inhbb gene is known to play a role in regulating steroid hormone production during follicular development (Luisi et al., 2005), and testis of vitamin A-deficient rats showed low levels of the Inhibin alpha-subunit, which increases after retinol administration (Zhuang et al., 1997). The SDF1/CXCR4 signaling is known to be required for the maintenance of mouse spermatogonial stem cells, and inhibition of CXCR4 signaling increases the responsiveness of the germ cells to RA (Yang et al., 2013). In addition, study in Orange-spotted grouper demonstrated that the expression of cxcr4b gene is sharply decreased in mature ovary (Lu et al., 2018). In cyp26a1 mutant testis, the orphan nuclear receptor 4A1 (nr4a1) was strongly upregulated. This transcription factor can heterodimerize with the retinoid X receptor (RXR) (Zetterstrom et al., 1996). In mammals, NR4A1 is strongly and rapidly induced in Leydig cells, and it regulates several steroidogenic genes including Star, Hsd3b1, and Cyp17a1 (Daems et al., 2014).
The early meiosis entry in cyp26a1–/–females, together with the regulation of genes related to gametogenesis in adult mutants, confirm the role of RA in germ cell differentiation after the sex-determining period. However, the timing of meiosis entry is still different between male and female, being much later in males. Interestingly, germ cells of some males entered oogenesis around the period of male meiosis initiation. However, neither sex reversals nor ovotestes were observed in the adult gonad. This striking result demonstrates a tight correlation between RA in regulating gametogenesis and possibly germ cells sex identity in a stra8 independent model species. The formation of the testis and absence of oocytes in adult cyp26a1–/–males could be related to the late initiation of the male sex differentiation pathway, which occurs around 30–45 dah (Nishimura and Tanaka, 2014), and overrides the initiation of female gametogenesis. Testicular germ cell transplantation into female undifferentiated embryonic gonad of rainbow trout produces functional egg, demonstrating the outstanding capacity of the germ cell to respond to the gonad environment (Okutsu et al., 2006). Hence, we hypothesize that, in for those few cyp26a1 –/– medaka, the germ cells start oogenesis until the time of testicular differentiation period, which leads to the development of normal male gonads and regression of the already formed oocytes.
Treatments with AM580 were expected to strongly induce gametogenesis in cyp26a1–/–medaka, since the main RA degrading enzyme is absent. However, our data from the mutants demonstrate on the contrary that AM580 blocks germ cell differentiation, like in wildtype medaka. The lack of meiosis induction in the cyp26a1 mutants could be explained by a possible gene compensation of cyp26b1. On the other side, our result is in line with the induction of dmrt1a and amh in wildtype embryos after RA treatments, indicating an activation of the male pathway, which is marked by a reduced germ cell proliferation and differentiation. On the other hand, our previous experiments demonstrated that increased cortisol levels induce masculinization of XX medaka by direct activation of the dmrt1a promoter (Adolfi et al., 2019). Hence, another explanation for the activation of dmrt1a after RA treatment could have been that this is simply due to a stress condition, since all embryos showed malformation after treatments with this morphogen. The treatments with both AM580 and Metyrapone (cortisol synthesis inhibitor) demonstrated that the germ cells respond to RA physiologically leading to increased meiosis and early oogenesis in females. Males, however, did not show oogenesis nor spermatogenesis stimulation after treatments at 1 dah. In mammals, DMRT1 allows Sertoli cells to participate in RA signaling while avoiding consequent cell fate reprogramming (Huang et al., 2017). At the time when meiosis initiates in females, in males dmrt1bY is expressed in the gonads (Nishimura et al., 2014), and possibly restrains the germ cells from entering differentiation. Hence, despite of mutating the main RA degrading enzyme, and treating the embryos with exogenous RA, the timing of meiosis entry is still different between males and females likely due to expression of dmrt1bY, dmrt1a, or even other male development promoting factors in the somatic gonads of males (Figure 5).
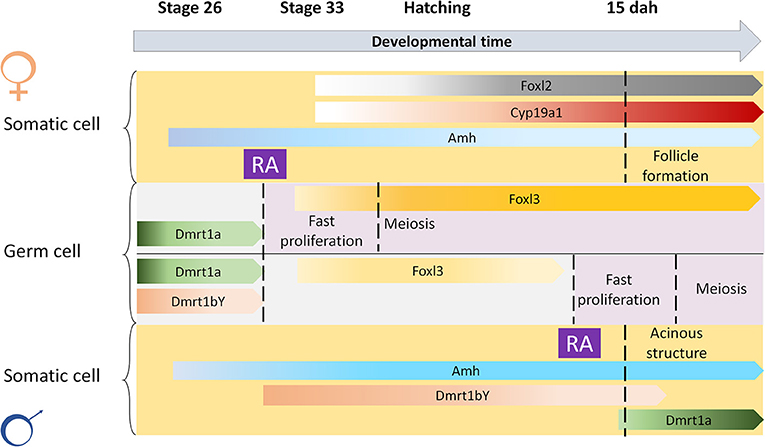
Figure 5. Scheme for spatial-temporal crosstalk between retinoic acid and sex-related genes in meiosis induction. Retinoic acid (RA) is important to induce gametogenesis in both sexes. The restrain of RA action at early stages in the germ cells of males is possibly regulated by male-related factors such as Dmrt1a, Dmrt1bY, or Amh, that are known to inhibit germ cell differentiation.
In summary, we showed that RA has an important role in regulating meiosis and gametogenesis but only after the sex determination stage. Exogenous treatments with ATRA and AM580 in wildtype embryos reduced the meiosis entry by activation of male-related genes, probably due to the stress conditions. Full knockouts of the main RA degrading Cyp26a1 led to an increase of meiosis in female embryos and to the regulation of genes related to gametogenesis and meiosis entry in adult gonads. In males, despite some mutants showing oocytes in the early gonad, the timing of meiosis entry is still later than in females. This makes us to suggest that in medaka the differential expression between male and female of sexual development related genes regulates the responsiveness of the germ cells to RA independent of stra8 regulation. Hence, the sex determination network limits the action of RA to a time after the sex determination period.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by Veterinary Office of the District Government of Lower Franconia, Germany (568/300-1870/13).
Author Contributions
MA carried out the mutant line production, sampling, treatments, histology, imaging, molecular analysis, and drafted the manuscript. AH coordinated part of the study and helped to review the manuscript. AM-B extracted the RNA samples from gonads of wildtype medakas for sequencing. SK carried out the bioinformatic analyses of the transcriptomes. MR carried out part of the qRT-PCR and luciferase assay. DG designed and provided the cyp26a1 TALEN plasmids for mRNA synthesis. MS defined and designed the study, coordinated all steps of the research, and reviewed all versions of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft by a grant (SCHA 408/12-1; HE 7135/2-1) to MS and AH. AH was also funded by the AquaCRISPR (ANR-16-COFA-0004-01), TUNESAL (Research Project- HAVBRUK2, PN: 294971), AQUAEXCEL 3.0 European Project, and 111 Project (China, Grant no. D20007) projects. MA was supported by Graduate School of Life Sciences (GSLS) Post-Doc Plus Funding.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Marcel Prögler for synthesizing the mRNAs for injection. This publication was supported by the Open Access Publication Fund of the University of Wuerzburg. The data presented in this article partially overlaps with the results contained in the doctoral thesis of MA (Adolfi, 2016).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2020.613497/full#supplementary-material
Supplementary Figure 1. Responsiveness of HEK 293 cells to exogenous treatments with ATRA and AM580. Transfection of plasmids containing retinoic acid responsive elements (RARE) in HEK 293 cells resulted increased luciferase activity after treatments with both ATRA and AM580.
Supplementary Figure 2. Genomic structure of the medaka cyp26a1 gene and mutant sequences induced by TALENs. (A) The cyp26a1 TALENs were designed to target the second exon of the gene. The right binding site was located at the junction of exon 2 and intron 2. Underlined bases indicate the left and right recognition sequences of the TALENs. Forward and reverse primers were designed to amplify the fragment for sequencing. (B) Wildtype and mutant cyp26a1sequences. (C) Predicted protein sequences. Amino acid substitutions are labeled in red. Deletions are indicated by dashes. E, exon; F, forward; R, reverse; WT, wildtype.
Supplementary Figure 3. Morphology of adult gonads of wildtype and cyp26a1–/–medaka. Both mature wildtype (A) and mutant ovaries (B) presented big vitellogenic oocytes (Vo). Scale bar = 400 μm. In mature males, greater amounts of spermatozoa (star) are observed inside the testicular ducts in wildtype (C, scale bar = 50 μm) than in the mutant (D, scale bar = 100 μm).
Supplementary Table 1. Sequences of the oligos used in the present study.
Supplementary Table 2. List of genes strongly regulared in cyp26a1–/–(−2 < LogFC > 2).
References
Adolfi, M. C. (2016). Sex Determination and Meiosis in Medaka: The Role of Retinoic Acid. Würzburg: University of Würzburg.
Adolfi, M. C., Fischer, P., Herpin, A., Regensburger, M., Kikuchi, M., Tanaka, M., et al. (2019). Increase of cortisol levels after temperature stress activates dmrt1a causing female-to-male sex reversal and reduced germ cell number in medaka. Mol. Reprod. Dev. 86, 1405–1417. doi: 10.1002/mrd.23177
Adolfi, M. C., Herpin, A., Regensburger, M., Sacquegno, J., Waxman, J. S., and Schartl, M. (2016). Retinoic acid and meiosis induction in adult versus embryonic gonads of medaka. Sci. Rep. 6:34281. doi: 10.1038/srep34281
Bellemere, G., Von Stetten, O., and Oddos, T. (2008). Retinoic acid increases aquaporin 3 expression in normal human skin. J. Invest. Dermatol. 128, 542–548. doi: 10.1038/sj.jid.5701047
Biscotti, M. A., Adolfi, M. C., Barucca, M., Forconi, M., Pallavicini, A., Gerdol, M., et al. (2018). A comparative view on sex differentiation and gametogenesis genes in lungfish and coelacanths. Genome Biol. Evol. 10, 1430–1444. doi: 10.1093/gbe/evy101
Bowles, J., Feng, C. W., Knight, D., Smith, C. A., Roeszler, K. N., Bagheri-Fam, S., et al. (2009). Male-specific expression of Aldh1a1 in mouse and chicken fetal testes: implications for retinoid balance in gonad development. Dev. Dyn. 238, 2073–2080. doi: 10.1002/dvdy.22024
Bowles, J., Knight, D., Smith, C., Wilhelm, D., Richman, J., Mamiya, S., et al. (2006). Retinoid signaling determines germ cell fate in mice. Science 312, 596–600. doi: 10.1126/science.1125691
Bowles, J., and Koopman, P. (2007). Retinoic acid, meiosis and germ cell fate in mammals. Development 134, 3401–3411. doi: 10.1242/dev.001107
Bowles, J., and Koopman, P. (2010). Sex determination in mammalian germ cells: extrinsic versus intrinsic factors. Reproduction 139, 943–958. doi: 10.1530/REP-10-0075
Capel, B. (2000). The battle of the sexes. Mech. Dev. 92, 89–103. doi: 10.1016/S0925-4773(99)00327-5
Capel, B. (2017). Vertebrate sex determination: evolutionary plasticity of a fundamental switch. Nat. Rev. Genet. 18, 675–689. doi: 10.1038/nrg.2017.60
Daems, C., Martin, L. J., Brousseau, C., and Tremblay, J. J. (2014). MEF2 is restricted to the male gonad and regulates expression of the orphan nuclear receptor NR4A1. Mol. Endocrinol. 28, 886–898. doi: 10.1210/me.2013-1407
Dahlem, T. J., Hoshijima, K., Jurynec, M. J., Gunther, D., Starker, C. G., Locke, A. S., et al. (2012). Simple methods for generating and detecting locus-specific mutations induced with TALENs in the zebrafish genome. PLoS Genet. 8:e1002861. doi: 10.1371/journal.pgen.1002861
Deak, K. L., Dickerson, M. E., Linney, E., Enterline, D. S., George, T. M., Melvin, E. C., et al. (2005). Analysis of ALDH1A2, CYP26A1, CYP26B1, CRABP1, and CRABP2 in human neural tube defects suggests a possible association with alleles in ALDH1A2. Birth Defects Res. Part A Clin. Mol. Teratol. 73, 868–875. doi: 10.1002/bdra.20183
Devlin, R., and Nagahama, Y. (2002). Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture 208, 191–364. doi: 10.1016/S0044-8486(02)00057-1
Dong, R., Yang, S., Jiao, J., Wang, T., Shi, H., Zhou, L., et al. (2013). Characterization of Stra8 in Southern catfish (Silurus meridionalis): evidence for its role in meiotic initiation. BMC Mol. Biol. 14:11. doi: 10.1186/1471-2199-14-11
Efimenko, E., Padua, M. B., Manuylov, N. L., Fox, S. C., Morse, D. A., and Tevosian, S. G. (2013). The transcription factor GATA4 is required for follicular development and normal ovarian function. Dev. Biol. 381, 144–158. doi: 10.1016/j.ydbio.2013.06.004
Emoto, Y., Wada, H., Okamoto, H., Kudo, A., and Imai, Y. (2005). Retinoic acid-metabolizing enzyme Cyp26a1 is essential for determining territories of hindbrain and spinal cord in zebrafish. Dev. Biol. 278, 415–427. doi: 10.1016/j.ydbio.2004.11.023
Feng, R., Fang, L., Cheng, Y., He, X., Jiang, W., Dong, R., et al. (2015). Retinoic acid homeostasis through aldh1a2 and cyp26a1 mediates meiotic entry in Nile tilapia (Oreochromis niloticus). Sci. Rep. 5:10131. doi: 10.1038/srep10131
Herpin, A., Adolfi, M. C., Nicol, B., Hinzmann, M., Schmidt, C., Klughammer, J., et al. (2013). Divergent expression regulation of gonad development genes in medaka shows incomplete conservation of the downstream regulatory network of vertebrate sex determination. Mol. Biol. Evol. 30, 2328–2346. doi: 10.1093/molbev/mst130
Herpin, A., and Schartl, M. (2015). Plasticity of gene-regulatory networks controlling sex determination: of masters, slaves, usual suspects, newcomers, and usurpators. EMBO Rep. 16, 1260–1274. doi: 10.15252/embr.201540667
Hu, Y. C., Okumura, L. M., and Page, D. C. (2013). Gata4 is required for formation of the genital ridge in mice. PLoS Genet. 9:e1003629. doi: 10.1371/journal.pgen.1003629
Huang, H. F., He, R. H., Sun, C. C., Zhang, Y., Meng, Q. X., and Ma, Y. Y. (2006). Function of aquaporins in female and male reproductive systems. Hum. Reprod. Update 12, 785–795. doi: 10.1093/humupd/dml035
Huang, S., Ye, L., and Chen, H. (2017). Sex determination and maintenance: the role of DMRT1 and FOXL2. Asian J. Androl. 19, 619–624. doi: 10.4103/1008-682X.194420
Iwamatsu, T. (2004). Stages of normal development in the medaka Oryzias latipes. Mech. Dev. 121, 605–618. doi: 10.1016/j.mod.2004.03.012
Kashimada, K., Svingen, T., Feng, C. W., Pelosi, E., Bagheri-Fam, S., Harley, V. R., et al. (2011). Antagonistic regulation of Cyp26b1 by transcription factors SOX9/SF1 and FOXL2 during gonadal development in mice. FASEB J. 25, 3561–3569. doi: 10.1096/fj.11-184333
Kimble, J., and Page, D. C. (2007). The mysteries of sexual identity. The germ cell's perspective. Science 316, 400–401. doi: 10.1126/science.1142109
Kinoshita, M., Murata, K., Naruse, K., and Tanaka, M. (2009). Medaka: Biology, Management, and Experimental Protocols. Ames, IA: Wiley-Blackwell.
Koubova, J., Menke, D. B., Zhou, Q., Capel, B., Griswold, M. D., and Page, D. C. (2006). Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc. Natl. Acad. Sci. U.S.A. 103, 2474–2479. doi: 10.1073/pnas.0510813103
Krentz, A. D., Murphy, M. W., Zhang, T., Sarver, A. L., Jain, S., Griswold, M. D., et al. (2013). Interaction between DMRT1 function and genetic background modulates signaling and pluripotency to control tumor susceptibility in the fetal germ line. Dev. Biol. 377, 67–78. doi: 10.1016/j.ydbio.2013.02.014
Kyronlahti, A., Vetter, M., Euler, R., Bielinska, M., Jay, P. Y., Anttonen, M., et al. (2011). GATA4 deficiency impairs ovarian function in adult mice. Biol. Reprod. 84, 1033–1044. doi: 10.1095/biolreprod.110.086850
Li, J., Chen, W., Wang, D., Zhou, L., Sakai, F., Guan, G., et al. (2012). GATA4 is involved in the gonadal development and maturation of the teleost fish tilapia, Oreochromis niloticus. J. Reprod. Dev. 58, 237–242. doi: 10.1262/jrd.11-131S
Li, M., Feng, R., Ma, H., Dong, R., Liu, Z., Jiang, W., et al. (2016). Retinoic acid triggers meiosis initiation via stra8-dependent pathway in Southern catfish, Silurus meridionalis. Gen. Comp. Endocrinol. 232, 191–198. doi: 10.1016/j.ygcen.2016.01.003
Liu, S., Liu, B., He, S., Zhao, Y., and Wang, Z. (2005). Cloning and characterization of zebra fish SPATA4 gene and analysis of its gonad specific expression. Biochem. Biokhim. 70, 638–644. doi: 10.1007/s10541-005-0163-7
Love, M. I., Huber, W., and Anders, S. (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15:550. doi: 10.1186/s13059-014-0550-8
Lu, W. J., Zhou, L., Gao, F. X., Sun, Z. H., Li, Z., Liu, X. C., et al. (2018). Divergent expression patterns and function of Two cxcr4 paralogs in hermaphroditic Epinephelus coioides. Int. J. Mol. Sci. 19:2943. doi: 10.3390/ijms19102943
Luisi, S., Florio, P., Reis, F. M., and Petraglia, F. (2005). Inhibins in female and male reproductive physiology: role in gametogenesis, conception, implantation and early pregnancy. Hum. Reprod. Update 11, 123–135. doi: 10.1093/humupd/dmh057
Masuyama, H., Yamada, M., Kamei, Y., Fujiwara-Ishikawa, T., Todo, T., Nagahama, Y., et al. (2012). Dmrt1 mutation causes a male-to-female sex reversal after the sex determination by Dmy in the medaka. Chromosome Res. 20, 163–176. doi: 10.1007/s10577-011-9264-x
Matson, C. K., Murphy, M. W., Griswold, M. D., Yoshida, S., Bardwell, V. J., and Zarkower, D. (2010). The mammalian doublesex homolog DMRT1 is a transcriptional gatekeeper that controls the mitosis versus meiosis decision in male germ cells. Dev. Cell 19, 612–624. doi: 10.1016/j.devcel.2010.09.010
Matson, C. K., Murphy, M. W., Sarver, A. L., Griswold, M. D., Bardwell, V. J., and Zarkower, D. (2011). DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature 476, 101–104. doi: 10.1038/nature10239
Matsuda, M., Nagahama, Y., Shinomiya, A., Sato, T., Matsuda, C., Kobayashi, T., et al. (2002). DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature 417, 559–563. doi: 10.1038/nature751
Mauney, J. R., Ramachandran, A., Yu, R. N., Daley, G. Q., Adam, R. M., and Estrada, C. R. (2010). All-trans retinoic acid directs urothelial specification of murine embryonic stem cells via GATA4/6 signaling mechanisms. PLoS ONE 5:e11513. doi: 10.1371/journal.pone.0011513
Morinaga, C., Saito, D., Nakamura, S., Sasaki, T., Asakawa, S., Shimizu, N., et al. (2007). The hotei mutation of medaka in the anti-Mullerian hormone receptor causes the dysregulation of germ cell and sexual development. Proc. Natl. Acad. Sci. U.S.A. 104, 9691–9696. doi: 10.1073/pnas.0611379104
Nakamura, S., Watakabe, I., Nishimura, T., Picard, J. Y., Toyoda, A., Taniguchi, Y., et al. (2012). Hyperproliferation of mitotically active germ cells due to defective anti-Mullerian hormone signaling mediates sex reversal in medaka. Development 139, 2283–2287. doi: 10.1242/dev.076307
Nanda, I., Kondo, M., Hornung, U., Asakawa, S., Winkler, C., Shimizu, A., et al. (2002). A duplicated copy of DMRT1 in the sex-determining region of the Y chromosome of the medaka, Oryzias latipes. Proc. Natl. Acad. Sci. U.S.A. 99, 11778–11783. doi: 10.1073/pnas.182314699
Niederreither, K., and Dolle, P. (2008). Retinoic acid in development: towards an integrated view. Nat. Rev. Genet. 9, 541–553. doi: 10.1038/nrg2340
Nishimura, T., Herpin, A., Kimura, T., Hara, I., Kawasaki, T., Nakamura, S., et al. (2014). Analysis of a novel gene, Sdgc, reveals sex chromosome-dependent differences of medaka germ cells prior to gonad formation. Development 141, 3363–3369. doi: 10.1242/dev.106864
Nishimura, T., and Tanaka, M. (2014). Gonadal development in fish. Sex Dev. 8, 252–261. doi: 10.1159/000364924
Okutsu, T., Suzuki, K., Takeuchi, Y., Takeuchi, T., and Yoshizaki, G. (2006). Testicular germ cells can colonize sexually undifferentiated embryonic gonad and produce functional eggs in fish. Proc. Natl. Acad. Sci. U.S.A. 103, 2725–2729. doi: 10.1073/pnas.0509218103
Regneri, J., Volff, J. N., and Schartl, M. (2015). Transcriptional control analyses of the Xiphophorus melanoma oncogene. Comp. Biochem. Physiol. Toxicol. Pharmacol. 178, 116–127. doi: 10.1016/j.cbpc.2015.09.001
Saito, D., and Tanaka, M. (2009). Comparative aspects of gonadal sex differentiation in medaka: a conserved role of developing oocytes in sexual canalization. Sex Dev. 3, 99–107. doi: 10.1159/000223075
Sakai, Y., Meno, C., Fujii, H., Nishino, J., Shiratori, H., Saijoh, Y., et al. (2001). The retinoic acid-inactivating enzyme CYP26 is essential for establishing an uneven distribution of retinoic acid along the anterio-posterior axis within the mouse embryo. Genes Dev. 15, 213–225. doi: 10.1101/gad.851501
Shimozono, S., Iimura, T., Kitaguchi, T., Higashijima, S., and Miyawaki, A. (2013). Visualization of an endogenous retinoic acid gradient across embryonic development. Nature 496, 363–366. doi: 10.1038/nature12037
Smith, C. A., Roeszler, K. N., Bowles, J., Koopman, P., and Sinclair, A. H. (2008). Onset of meiosis in the chicken embryo; evidence of a role for retinoic acid. BMC Dev. Biol. 8:85. doi: 10.1186/1471-213X-8-85
Wallacides, A., Chesnel, A., Chardard, D., Flament, S., and Dumond, H. (2009). Evidence for a conserved role of retinoic acid in urodele amphibian meiosis onset. Dev. Dyn. 238, 1389–1398. doi: 10.1002/dvdy.21948
Wang, H., den Hollander, A. I., Moayedi, Y., Abulimiti, A., Li, Y., Collin, R. W., et al. (2009). Mutations in SPATA7 cause Leber congenital amaurosis and juvenile retinitis pigmentosa. Am. J. Hum. Genet. 84, 380–387. doi: 10.1016/j.ajhg.2009.02.005
White, R. J., Nie, Q., Lander, A. D., and Schilling, T. F. (2007). Complex regulation of cyp26a1 creates a robust retinoic acid gradient in the zebrafish embryo. PLoS Biol. 5:e304. doi: 10.1371/journal.pbio.0050304
Wu, X., Wang, P., Brown, C. A., Zilinski, C. A., and Matzuk, M. M. (2003). Zygote arrest 1 (Zar1) is an evolutionarily conserved gene expressed in vertebrate ovaries. Biol. Reprod. 69, 861–867. doi: 10.1095/biolreprod.103.016022
Yang, Q. E., Kim, D., Kaucher, A., Oatley, M. J., and Oatley, J. M. (2013). CXCL12-CXCR4 signaling is required for the maintenance of mouse spermatogonial stem cells. J. Cell Sci. 126(Pt. 4), 1009–1020. doi: 10.1242/jcs.119826
Yashiro, K., Zhao, X., Uehara, M., Yamashita, K., Nishijima, M., Nishino, J., et al. (2004). Regulation of retinoic acid distribution is required for proximodistal patterning and outgrowth of the developing mouse limb. Dev. Cell 6, 411–422. doi: 10.1016/S1534-5807(04)00062-0
Zetterstrom, R. H., Solomin, L., Mitsiadis, T., Olson, L., and Perlmann, T. (1996). Retinoid X receptor heterodimerization and developmental expression distinguish the orphan nuclear receptors NGFI-B, Nurr1, and Nor1. Mol. Endocrinol. 10, 1656–1666. doi: 10.1210/mend.10.12.8961274
Keywords: sex determination, retinoic acid, meiosis, gametogenesis, medaka
Citation: Adolfi MC, Herpin A, Martinez-Bengochea A, Kneitz S, Regensburger M, Grunwald DJ and Schartl M (2021) Crosstalk Between Retinoic Acid and Sex-Related Genes Controls Germ Cell Fate and Gametogenesis in Medaka. Front. Cell Dev. Biol. 8:613497. doi: 10.3389/fcell.2020.613497
Received: 05 October 2020; Accepted: 21 December 2020;
Published: 18 January 2021.
Edited by:
Taisen Iguchi, Graduate University for Advanced Studies (Sokendai), JapanReviewed by:
Tapas Chakraborty, Kyushu University, JapanShinichi Miyagawa, Tokyo University of Science, Japan
Copyright © 2021 Adolfi, Herpin, Martinez-Bengochea, Kneitz, Regensburger, Grunwald and Schartl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mateus C. Adolfi, bWF0ZXVzLmFkb2xmaUBiaW96ZW50cnVtLnVuaS13dWVyemJ1cmcuZGU=
 Mateus C. Adolfi
Mateus C. Adolfi Amaury Herpin
Amaury Herpin Anabel Martinez-Bengochea
Anabel Martinez-Bengochea Susanne Kneitz1
Susanne Kneitz1 Manfred Schartl
Manfred Schartl