
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell Dev. Biol. , 17 December 2020
Sec. Stem Cell Research
Volume 8 - 2020 | https://doi.org/10.3389/fcell.2020.547653
This article is part of the Research Topic Current Progress in Mesenchymal Stem/Stromal Cell Research View all 18 articles
 Roya Ramezankhani1,2†
Roya Ramezankhani1,2† Shukoofeh Torabi1,2†
Shukoofeh Torabi1,2† Neda Minaei1,2
Neda Minaei1,2 Hoda Madani1,2
Hoda Madani1,2 Siamak Rezaeiani1,3
Siamak Rezaeiani1,3 Seyedeh Nafiseh Hassani3,4
Seyedeh Nafiseh Hassani3,4 Adrian P. Gee5
Adrian P. Gee5 Massimo Dominici6
Massimo Dominici6 Daniela Nascimento Silva7,8
Daniela Nascimento Silva7,8 Hossein Baharvand3,4,9
Hossein Baharvand3,4,9 Ensiyeh Hajizadeh-Saffar1,2,4*
Ensiyeh Hajizadeh-Saffar1,2,4*The introduction of advanced therapy medicinal products (ATMPs) to the global pharma market has been revolutionizing the pharmaceutical industry and has opened new routes for treating various types of cancers and incurable diseases. In the past two decades, a noticeable part of clinical practices has been devoting progressively to these products. The first step to develop such an ATMP product is to be familiar with other approved products to obtain a general view about this industry trend. The present paper depicts an overall perspective of approved ATMPs in different countries, while reflecting the degree of their success in a clinical point of view and highlighting their main safety issues and also related market size as a whole. In this regard, published articles regarding safety, efficacy, and market size of approved ATMPs were reviewed using the search engines PubMed, Scopus, and Google Scholar. For some products which the related papers were not available, data on the relevant company website were referenced. In this descriptive study, we have introduced and classified approved cell, gene, and tissue engineering-based products by different regulatory agencies, along with their characteristics, manufacturer, indication, approval date, related regulatory agency, dosage, product description, price and published data about their safety and efficacy. In addition, to gain insights about the commercial situation of each product, we have gathered accessible sale reports and market size information that pertain to some of these products.
Based on Directive 2001/83/EC, medicinal products in Europe have been defined as any substance or combination of substances that have the capability to treat or prevent diseases in humans or may be used with the purpose to restore, correct, or modify physiological functions in conjunction with the capability to be used for medical diagnosis in humans. With the advent of new gene and/or cell therapies and in order to assure their appropriate quality, safety, and efficacy, these therapies were introduced into the European medicinal product legislation in June 2003 as a new class of medicinal products, which were later called ATMPs. In 2007, Regulation (EC) No. 1394/2007, a specific regulation for ATMPs, was established by the EU Commission. This regulation divides ATMPs into four distinct types: GTMPs, SCTMPs, TEPs, and the combined ATMPs (cATMPs). GTMPs are products directly related to therapeutic, prophylactic, or diagnostic effects with a recombinant nucleic acid sequence. SCTMPs are products that contain substantially manipulated cells or tissues, or the cells or tissues not intended to be used for the same essential function(s) in the recipient and the donor. TEPs are engineered cells or tissues that have the properties of regenerating, repairing, or replacing human tissue, all in accordance with the medicinal products general definition and finally cATMPs comprise another type of these products and contain one or several medical devices that are an integral part of the GTMPs, SCTMPs, or TEPs (Hanna et al., 2016a; Detela and Lodge, 2019). Also, each regulatory authority may provide a certain type of definition for ATMPs. For example, in US according to FDA, advanced therapies are regulated as biologic products, similar to EU classification. Biological products consist of allergenic products that includes allergen extracts, allergen patch tests, and antigen skin tests, blood and blood products, vaccines, xenotrasplants, and ATMPs which constitutes two sub-categories: CGTs (Integra, 2019). “Cellular immunotherapies, cancer vaccines, and other types of both autologous and allogeneic cells for certain therapeutic indications, including hematopoietic stem cells and adult and embryonic stem cells that have been subject to substantial ex vivo manipulation constitute cellular therapy based products, while modifying the expression of a gene or changing the biological properties of living cells for therapeutic use compose human gene therapy based products” (Genzyme, 2019). Moreover, “combination products include products that are comprised of two or more regulated components, i.e., drug/device, biologic/device, drug/biologic, or drug/device/biologic.” The MFDS in South Korea also define the cell therapy product as “a medicinal product manufactured through physical, chemical, and/or biological manipulation, such as in vitro culture of autologous, allogeneic, or xenogeneic cells. However, this definition does not apply to a case where a medical doctor performs minimal manipulation (e.g., simple separation, washing, freezing, thawing, and other manipulations, while maintaining biological properties) that does not cause safety problems of the cells in the course of surgical operation or treatment at a medical center.” And a gene therapy product is defined as “a genetic material or a medicinal product containing such genetic material intended to be administered to human beings for treatment of disease (Choi et al., 2015). The regulatory guidelines regarding the (pre)submission, details of approval procedures, marketing authorization etc. have described thoroughly elsewhere (Detela and Lodge, 2019; EU, 2020; Luria et al., 2020). The need to establish effective therapeutic approaches to treat incurable diseases, notably, inherited genetic conditions, blood related disorders, malignancies, neurodegenerative diseases, tissue regeneration, and provide a bridge for patients awaiting organ transplantation has encouraged the increased use of ATMPs in medical sciences. Interestingly, a significant growth in the research and development phase along with the clinical use of ATMPs has been observed in recent years. In this regard, based on the results of three clinical trials databases: ClinicalTrials.gov, the International Clinical Trials Registry Platform (ICTRP) of the World Health Organization (WHO), and EudraCT, 939 clinical trials of ATMPs conducted between 1999 and June 2015 (Hanna et al., 2016b). This would indicate an increase in investment by big pharma sponsors for ATMPs (Ten Ham et al., 2018). Of note, potential challenges that exist in terms of the development of ATMPs include the specific requirements for high-technology equipment, difficulty with manufacturing processes, complicated trial design, establishment of robust assays for validation of identity and functionality, achieving an expected high efficacy, avoidance of probable long-term adverse events, regulatory considerations in terms of regulatory cost burden and timelines etc., and, in particular, financial issues that provide situations where the product cannot be sold at a sufficiently high price to establish a commercially viable product (Mount et al., 2015; Elsanhoury et al., 2017; Lee, 2018). ATMPs are based on a diverse set of most advanced technologies (Elsanhoury et al., 2017), therefore, there is an increased need for the technical/academic personnel involved directly and professionally in ATMP development (Lee, 2018). Besides, regarding the rare nature of the diseases that ATMPs are mostly developed for, there are concerns in relation with trial design such as the low number of patients, insufficient knowledge respecting the disease pathogenesis and some issues with the interpretation of endpoints for new indications (Lee, 2018). Also, the statistical analysis of safety and efficacy is affected by the limited number of participants (Viganò et al., 2018). On the other hand, validating these products particularly with regard to identity, purity, and potency is of great importance. The restricted accessible appropriate standards and reference material along with an inadequacy in certain guidelines are the other challenges in this regard (McConaghie, 2017).
Financial issues may be one of the main challenges that can negatively influence the company and consumers. A well-known example, Glybera, is a gene therapy based drug for a rare familial LPLD (European Medicines Agency, 2020b). Its marketing authorization expired on October 28, 2017 following a decision by the marketing authorization holder to not apply for a renewal. The drug was proven to be a commercial failure because a single dose treatment cost over one million euro per patient, in addition to the low market size due to the fact that LPLD is a ultra-rare disease (Senior, 2017; Cuende et al., 2018).
Cuende et al. (2018) previously described cell therapy products with market authorization (Food and Drug Administration, 2019a), in this extensive review thanks to available information in the regulatory agencies and related company’s web resources, articles, and other data sources, we in-depth dissected and classified cell, gene, and tissue engineering products (Tables 1–3). Data are presented in detailed tables that has been categorized in terms of product’s definition, manufacturer, indication, approval date and related regulatory agency, product dosage and description, price, and related references. In addition, based on clinical trials data, we have further discussed each ATMP’s safety and efficacy points, categorized by the common indication within each group. Also included is a definition of the available market sizes and sale reports for the related products in an attempt to clarify the commercial point of view for each of the GTMPs, SCTMPs, and TEPs fields.
To achieving this end, published articles regarding the characteristics, safety and efficacy, and market size of approved ATMPs were reviewed using the search engines PubMed, Scopus, and Google Scholar. For some products which the related papers were not available, data on the relevant company website was used as reference. The type of documents used to obtain the data were original articles, review articles, HTML documents, and official websites of each product manufacturer. Search terms included MeSH (Medical Subject Headings) terms, “ATMP” “CTMP” “GTMP” “TEP” and also “product name” in addition to each terms of “efficacy” “safety” “adverse events” “price” and “market size.” The cut-off date for the data search was May 2020.
Collectively, this paper aims to provide a comprehensive insight for development of other cell therapy products for stakeholders, sponsors, manufacturing companies, regulatory agencies, and researchers interested in entering this research pathway.
To the best of our knowledge, worldwide, there are 64 approved ATMPs by taking into consideration that Prochymal, a CTMP product, approved by both the FDA and Health Canada, and the GTMP product, Kymriah, is being approved by the FDA, EMA, and Health Canada. In addition, the FDA and EMA both approved another GTMP product, Yescarta. Obviously, the CTMP group, with 34 products, is the largest class. The TEP and GTMP groups, with 20 and 10 products, follow in order. Figure 1 shows that United States, by authorizing 23 ATMPs (11 CTMPs, 7 TEPs, and 5 GTMPs), is the pioneer country in this field followed by South Korea with 15 ATMPs (13 CTMPs and 2 TEPs). Figure 2 shows the indications of each of the ATMP categories, which emphasizes the importance of the indications related to hematologic along with skin and soft tissue disorders (Figure 2A), skin and soft tissue related disorders (Figure 2B), and oncology (Figure 2C) in the CTMPs, TEPs, and GTMPs, respectively. It can be concluded that most CTMPs and GTMPs have an autologous source (Figure 2A,C), while TEPs involve 45 percent of allogeneic and 40 percent of autologous products (Figure 2B).
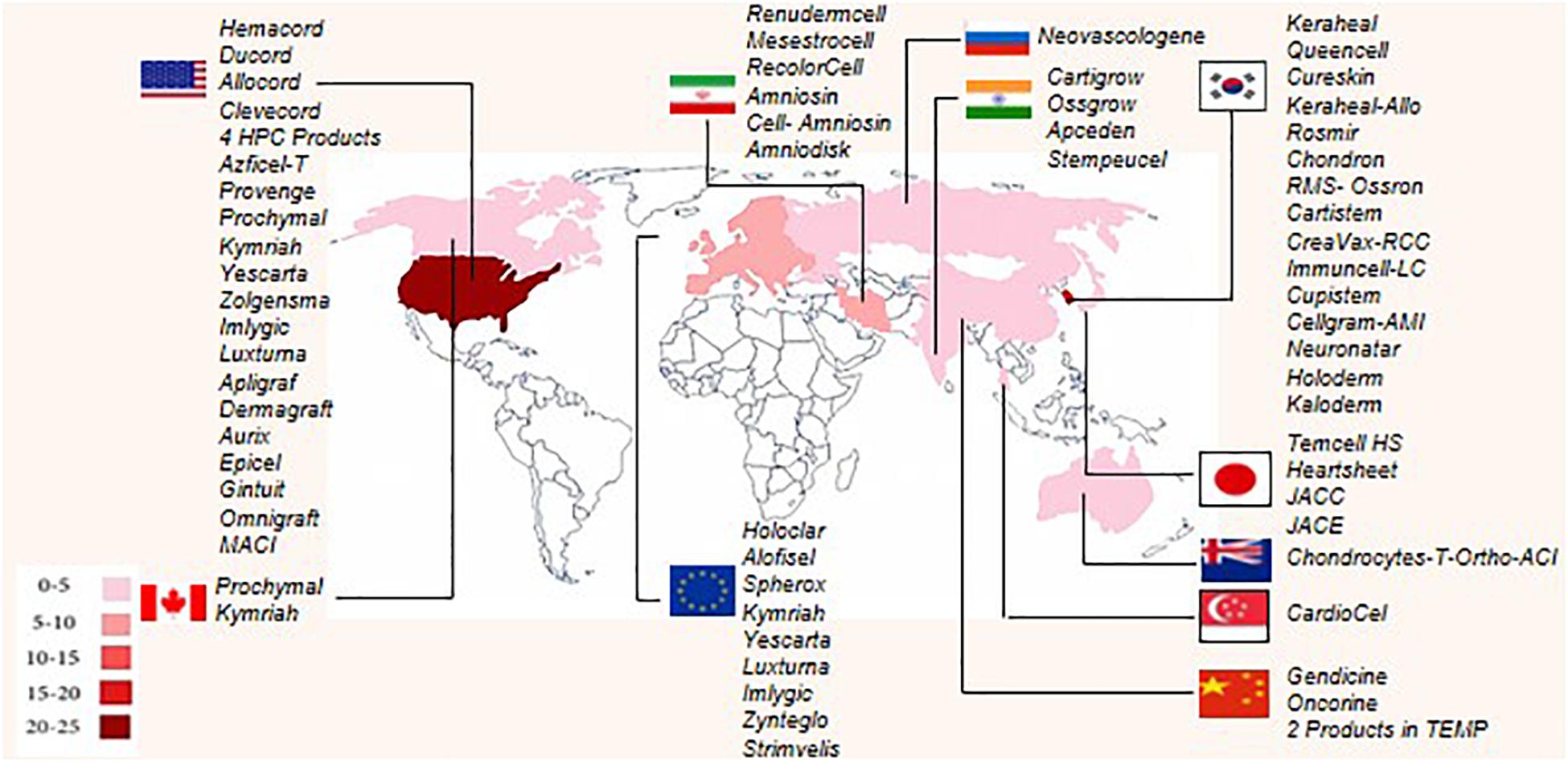
Figure 1. Number of authorized ATMPs worldwide, according to country. Each country has been determined by specific color, and based on the number of authorized ATMPs. United States, South Korea, and the European Union have the highest number of approved ATMPs.
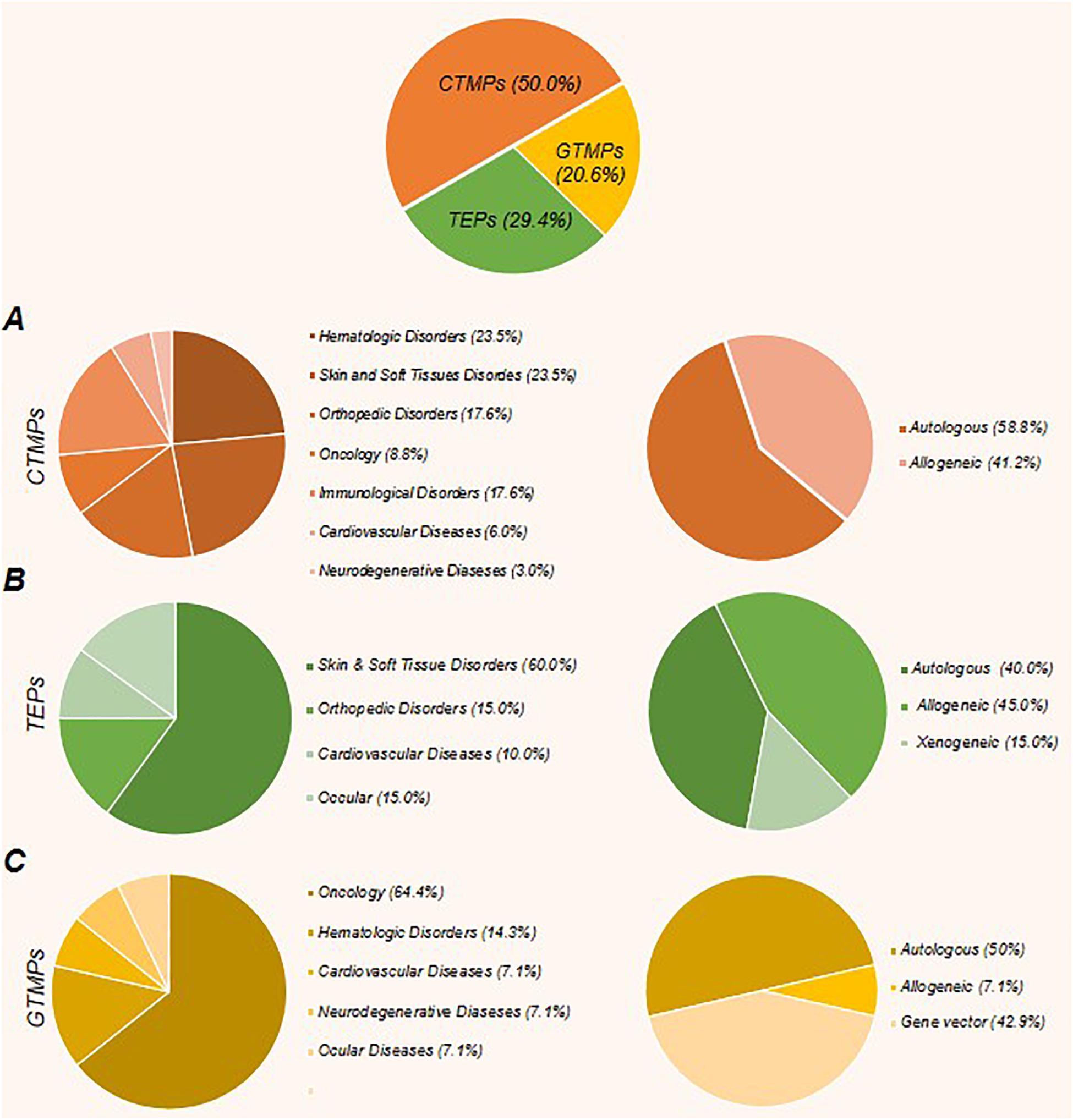
Figure 2. The percent of approved cell, gene, and tissue engineering products, along with their related indications and product type (autologous or allogeneic). (A) The CTMPs with 34 members constitute the largest class of ATMPs. Most of these products have an autologous source. Hematologic disorders, along with skin and soft tissue related disorders and orthopedic disorders constitute the main fields. (B) The TEPs, with 20 products, represent the next largest class of ATMPs. The most emphasized indications in this class are skin and soft tissue related disorders, orthopedic disorders, and cardiovascular diseases, respectively, and the percentage of allogeneic products are more than autologous products. (C) GTMPs with 10 products comprise the last class. The first common indications are oncology, hematologic disorders, and cardiovascular diseases, respectively. Similar to CTMPs, GTMP members mostly have autologous sources.
CTMPs can be divided into eight distinct groups with respect to the indication for which they have been developed: hematologic disorders, skin and soft tissue related issues, orthopedic diseases, oncology, and immunological, cardiovascular, neurological, and ocular related disorders.
For hematologic disorders, Hemacord (US FDA.2011), Ducord (US FDA.2012), Allocord (US FDA.2013), Clevecord (US FDA.2016), and four other HPC based products are approved for hematopoietic stem cell transplantations. Each product related efficacy has been compared against two studies: the COBLT study and another study with retrospective information from docket and public data (RxList, 2018; Food and Drug Administration, 2019b, c, d, e, f, g, h) with regards to neutrophil recovery at day 42, platelet recovery at day 100 (20,000/μL and 50,000/μL), and erythrocyte recovery at day 100. After receiving a TNC dose of ≥ 2.5 × 107/kg HPC, from multiple cord blood banks, the estimated values were as follows: neutrophils (76%), platelets (20,000/μL [57%] and 50,000/μL [46%]), and erythrocyte (65%) recovery in the COBLT study. The docket and public data information show an estimated neutrophil recovery of 77% and platelet recovery (50,000/μL) of 45%. On the other hand, the three parameters for neutrophil recovery and platelet recovery (20,000/μL and 50,000/μL) parameters were 88, 87, and 79% for Allocord; 96, 92, and 83% for Clevecord; 95, 92, and 71% for Ducord; 79, 62, and 55% for HPCs Cord Blood from Clinimmune Labs; 88.2, 73.6, and 43% for HPCs Cord Blood from MD Anderson Cord Blood Bank; 91, 95, and, 95% for HPCs Cord Blood from LifeSouth; and 82, 66, and 50% for HPCs Cord Blood from Bloodworks, respectively. In addition, the reported data related to the Hemacord study were 83% (neutrophil) and 77% platelet (20,000/μL) recovery. The median time for neutrophil recovery, platelet recovery (20,000/μL and 50,000/μL), and erythrocyte recovery were 27, 90, 113, and 64 days in the COBLT study, respectively. The median time for neutrophil recovery and platelet recovery (50,000/μL) were 25 and 122 days according to docket and public data information. The median time of neutrophil recovery and platelet recovery (20,000/μL and 50,000/μL) were 21, 48, and 56 days for Allocord; 18, 41, and 43 days for Clevecord; 21, 46, and 61 days for Ducord; 25, 55, and 49 days for HPCs Cord Blood from Clinimmune labs; 19, 47, and 65 days for HPCs Cord Blood from MD Anderson Cord Blood Bank; 22, 44, and 70 days from HPCs Cord Blood from LifeSouth; and 21.5, 46, and 53 days for HPCs Cord Blood from Bloodworks, respectively. Also, 20 days for neutrophil recovery and 45 days for platelet recovery (20,000/μL) were reported for Hemacord. The most important adverse events related to the safety of these products included hypersensitivity reactions, infusion reactions, graft-versus-host disease, engraftment syndrome and graft failure, malignancies of donor origin, transmission of serious infections, and transmission of rare genetic diseases.
For skin and soft tissue related disorders, CureSkin (South Korea MFDS.2010), Queencell (South Korea MFDS.2010), Azficel-T (US FDA.2011), Rosmir (South Korea MFDS.2017), and RenudermCell (Iran FDA.2018) are approved to treat acne scars and facial wrinkles, KeraHeal (South Korea MFDS.2006) and KeraHeal-allo (South Korea MFDS.2015) are approved for skin burns and finally RecolorCell (Iran FDA.2019) is indicated for different types of Vitiligo. The effectiveness of Azficel-T was demonstrated in two studies consist of 421 total patients at 3 and 6 months follow up. The self- and physician-reported assessments indicated 57 and 33% improvements in the first study, and 45 and 19% in the second study for patients who received Azficel-T. A two-point improvement in NLF wrinkles was reported after 6 months. The most common adverse events were injection-site redness, bruising, swelling, pain, hemorrhage, edema, nodules, papules, irritation, dermatitis, and pruritus (S-Biomedics Ltd., 2019a). Qualitative data regarding the efficacy of CureSkin shows its superior effect in the healing process of depressed acne scars (S-Biomedics Ltd., 2012). Moreover, according to the product’s brochure, the most common reported adverse events after repeated administration of CureSkin was erythema at the injection site. Common adverse events for Rosmir included eye irritation and allergic rhinitis (Yim et al., 2011; Tego Science, 2019a). Regarding KeraHeal efficacy, the take rate of 1:4–6 meshed autografts along with KeraHeal in 29 patients with burn injuries was estimated to be 96 and 100% at 2 and 4 weeks after treatment, respectively. A reduction in Vancouver burn scar scale at 8, 12, and 24 weeks following the treatment was observed (Yoon et al., 2017). The primary outcome used to show the KeraHeal-allo efficacy was the period of re-epithelialization, which occurred 2.5 or 2.8 days faster in the treated sites in comparison with the control. No associated adverse events were reported with these two products (Park et al., 2017).
For orthopedic disorders, Cartistem (South Korea MFDS.2012) and MesestroCell (Iran FDA.2018) are approved for knee osteoarthritis; Chondron (South Korea MFDS.2001), Cartigrow (India DCGI.2017), and Chondrocytes-T-Ortho-ACI (Australia TGA.2017) are approved for defective knee cartilage. Ossron (South Korea MFDS.2009) and Ossgrow (India DCGI.2017) are approved for repair of bone defects. The safety and efficacy of Cartistem was assessed in a phase I/II clinical trial with 7 years long−term follow−up in seven patients with osteoarthritis of the knee joint with Kellgren−Lawrence (K−L) grade 3 and painful full−thickness cartilage defects. Results have shown repaired tissue at 12-weeks post-transplantation, while arthroscopic examination and biopsy at 1 year showed stable regenerated cartilage. Furthermore, the 100-mm VAS score for pain and the IKDC subjective score were improved at 24 weeks post-transplantation and maintained for up to 7 years. A high glycosaminoglycan content in the regenerated cartilage at 3 years was determined through the mean relative change in R1 (ΔR1) index. Mild to moderate adverse events included arthralgia, back pain, and bladder distension. No particular adverse reactions were noted over 7 years of clinical follow-up (Choi et al., 2010). Following the Chondron transplantation in 98 patients with articular cartilage injury of the knee joint and 13∼25-month follow-up, assessments showed a notable improvement in the Knee Society Scoring system (tKSS)-A (pain) and tKSS-B (function) scores. A total of 2.04% of the patients experienced adverse events related to GACI due to ‘catching symptom’ (Bauer et al., 2012). The safety, tolerability, and efficacy of Chondrocytes-T-Ortho-ACI are reported from clinical trials of 1077 reported cases that showed significant improvements in the KOOS subscales and in the 6MWT during 36 months of post-surgery follow up in comparison with the pre-operative group. MRI analysis also showed significant post-operative progression; however, this observation was not sustained until the study end point. The most common adverse events encountered during these clinical trials included engraft failure, cartilage hypertrophy, incomplete drug effect, and graft delamination (Kim et al., 2009). Safety and efficacy of Ossron and Ossgrow have been assessed in a clinical trial of 64 patients with long-bone shaft fractures during 2 months. The average callus formation score was significantly higher in the experimental group at 1 and 2 months of follow up, while the osteoblast injection response was not statistically different between younger and older patients. No adverse effects were observed in association with the osteoblast injection (Kim et al., 2007).
In oncology, CreaVax-RCC (South Korea MFDS.2007) is approved for metastatic renal cell carcinoma, Immuncell-LC (South Korea MFDS.2007) is approved for post-surgical recurrence of hepatocellular carcinoma, Sipuleucel-T (Provenge) (US FDA.2010/EMA.2010) is approved for mCRPC, and finally, Apceden (India DCGI.2017) is approved for Prostate, ovarian, colorectal, and NSCLC. The safety and efficacy of CreaVax-RCC was assessed in nine patients suffering from metastatic renal cell carcinoma with a median follow-up of 17.5 months. This treatment had the ability to prompt an immune response against the tumor. Out of nine patients, one experienced a PR and a decrease in the size of lung metastases; five had stable disease; and three had evidence of progressive disease after one cycle of immunotherapy. The results of the DTH skin test with KLH or TL-pulsed DCs determined that three patients with no initial DTH reactivity and three patients with a positive initial DTH response to KLH- or TL-pulsed DC had positive reactions to both after immunotherapy and experienced raised skin reactions after the vaccination. In addition, there was an elevation in the number of tumor specific interferon gamma (IFN-γ)-producing cells after one cycle of the vaccination. No severe adverse effects have been reported (Lee et al., 2015). In the Immuncell-LC phase III clinical trial, 230 patients with HCC were assigned randomly to receive immunotherapy 16 times during 60 weeks or no adjuvant therapy (controls). RFS and RFS rates were considered to be the primary outcomes. Overall survival (OS), cancer-specific survival, the OS rate, and cancer-specific survival rate criteria accounted for secondary outcomes. The RFS time for the immunotherapy group was 44 months in comparison with 30 months in the control group. The RFS rate for both groups declined during 12, 24, 36, and 48 months post-treatment; however, the amount of statistically significant rates per month were higher in the immunotherapy group. Both the OS rate and cancer-specific survival rate decreased in the immunotherapy and control groups during 12, 24, 36, and 48 months. Again, the amounts of the statistical rates per month were higher in the treatment group. Serious adverse events were reported in the immunotherapy group and included hemorrhage from esophageal varices, hepatic vein stenosis, herpes zoster, laceration, meniscus lesion, humerus fracture, foot fracture, bladder neoplasm, and high frequency ablation (Kantoff et al., 2010). In a phase III clinical trial, 341 patients with mCRPC received Stempeucel-T (Provenge). There was a relative decline of 22% in the risk of death observed in the sipuleucel-T group compared with the placebo group. This reduction resulted in a 4.1-month improvement in median patient survival. The most common adverse events were chills, fever, and headache. These adverse events were more frequent in the sipuleucel-T group than in the placebo group (Bapsy et al., 2014). Safety and efficacy of Apceden was assessed in a multicenter phase II clinical trial in India that enrolled 51 patients with refractory solid cancers. A significant improvement regarding QOL and overall median survival in patients with objective response was observed. The TTP analysis showed a notable delay in the onset of disease development. There was an increase in the mean CD4:CD8 ratio in the immune response evaluation, along with an ORR of 28.9 and 42.1% by RECIST and irRC, respectively. One adverse event, an episode of rigors together with mild fever during a single infusion was reported (Prasad et al., 2011).
Immunological disorders have four approved products. Prochymal (Health Canada.2012, US FDA.2015) together with Temcell HS (Japan PMDA.2015) are produced to treat GvHD. Cupistem (South Korea MFDS.2012) and Alofisel (EMA.2018) are two other products in this group. First one is indicated for reducing the inflammation in Crohn’s Fistula and the latter is indicated for treatment of complex perianal fistulas in adult patients with non-active/mildly active luminal Crohn’s disease. In one trial, Prochymal was used to treat refractory grades III and IV acute GvHD in 12 children. The results indicated that allogeneic HSCT was well-tolerated. The survival rate was 42% after a median follow up of 611 days. The OS for patients who achieved CR was estimated to be 68% at 2 years (Muroi et al., 2016). Temcell HS was evaluated in a phase I/II study of 25 patients with steroid-refractory grade III or IV acute GvHD. A statistically significant consistent CR for grade III or IV steroid-refractory acute GvHD was shown from 4 to 52 weeks of follow up. At 52 weeks, 48% of the patients who achieved CR were still alive. In addition, the survival of patients who had an OR, which is the sum of the CR and PR, was substantially higher than those with no OR. Responses in children were better than adults. The most common adverse events were leukocytopenia, thrombocytopenia, anemia, sepsis, hypertension, microangiopathy, liver dysfunction, and chronic GvHD (European Medicines Agency, 2020a).
The assessment efficacy and safety of Alofisel compared to placebo was considered in a pivotal Phase III clinical trial. In this study 212 perianal fistulising CD patients (107 receiving Alofisel Cx601 and 105 receiving placebo) was screened over 24, 52, and 104 weeks. Full analysis of the efficacy data showed combined remission of perianal fistulising CD and absence of collections > 2 cm of the treated fistula confirmed by MRI images, at week 24. The combined remission in the active group was 49.5% (53/107) and in the placebo group were 34.5% (36/105). Presented data from week 52 showed statistically significant effects in favor of Alofisel treatment, and finally in patients who entered the 104 weeks follow-up (25 Alofisel, 15 placebo), the rate of clinical remission was 56 and 40% in the active and placebo group, respectively (Cho et al., 2015). In a phase II clinical trial fistula healing was evaluated 24 months after the administration of Cupistem in 43 patients. Cupistem seemed to be efficient considering the results of the mPP analysis 24 months after transplantation where 80.8% of the patients showed complete fistula healing. In order to assess the sustainability of the initial response, complete closure in 83.3% of the 24 patients who had evidence of complete closure at the 8th week after the injection still had complete closure at year 2. The most common adverse reactions were abdominal pain, eczema and exacerbation of crohn’s disease, anal inflammation, diarrhea, and fever. None of the observed adverse events were considered to be related to the product (Kim et al., 2018).
Cardiovascular diseases are the next category in the field of CTMPs. Cellgram-AMI (South Korea MFDS.2011) for AMI and Stempeucel (India DCGI.2016) for CLI due to thromboangiitis obliterans (Buerger’s disease) are in this group. In a clinical study, for evaluating the safety and efficacy of Cellgram-AMI, 26 patients with successful PCI for acute ST-segment elevation anterior wall myocardial infarction were assigned to either a control group (n = 12) or Cellgram group (n = 14) and were follow-up for 4-month. Patients who received Cellgram-AMI had improved Left ventricular function as shown by a substantial progress in overall LVEF, measured by SPECT and echocardiography 3 months after the BM-MSC injection and 4 months after PCI. This improvement continued to the 12th month follow up as assessed by echocardiography. However, the baseline and 4-month LVEDV and LVESV values did not significantly change. No adverse events, in-stent restenosis, or proarrhythmic effects were noted in both groups during the 4 and 12-month follow up periods (Gupta et al., 2017). A phase II study in India assessed the safety and efficacy of Stempeucel. This study placed 90 patients with CLI due to Buerger’s disease into two dose groups. The rest pain and ulcer size per month were the primary outcomes. Both decreased in comparison with the SOC group. The secondary outcomes of ABPI, amount of total walking distance, and QOL activity score of units per month increased for both doses. Skin ulcer and gangrene in the 1 × 106 and 2 × 106cells/kg groups, respectively, were the most frequently reported TEAEs. They were considered either remotely related or unrelated to Stempeucel (Oh et al., 2015).
The only related neurological disorder was ALS for which the product Neuronata-R (South Korea MFDS.2014) has been approved. In a phase I clinical trial to evaluate the safety of of Neuronata-R, seven patients with definite or probable ALS received two intrathecal injections of Neuronata-R and were follow-up for 12 months. The ALSFRS score, AALS score, and FVC were used to assess treatment efficacy. It was shown that none of the mentioned parameters declined rapidly and that the decrease in ALSFRS-R score during the 6 months follow up was more gradual than the observed decrease in the lead-in period, while these scores remained persistent for 6 months after the first injection of MSCs. None of the patients experienced serious adverse events during the 12-month follow-up period (Zhang et al., 2018).
The GTMPs have been developed for oncology, hematologic, cardiovascular, neurodegenerative, and ocular diseases.
In oncology related indications, Gendicine (CFDA.2003) is the first approved gene therapy product to treat head and neck squamous cell carcinoma, Imlygic (US FDA/EMA.2015) is approved for melanoma treatment, and Kymriah (US FDA.2017-EMA/Health Canada.2018) and Yescarta (US FDA.2017-EMA.2018) are two products used for hematologic malignancies. To evaluate the safety and efficacy of Gendicine, there are over 30 clinical study related publications. This product was assessed in a large number of clinical studies that included more than 30,000 patients. The results showed remarkable safety records along with improvements in efficacy, including tumor shrinkage and an enhanced QOL. The average response rate for CR and PR reached 90%. After the 5-year follow up, a large group of patients were still alive. The most common adverse events include fever, arthralgia, and myalgia (Andtbacka et al., 2015). In a phase III clinical trial, an intralesional injection of Imlygic was compared with subcutaneous administration of GM-CSF in patients with advanced melanoma. The mean treatment duration was 23 in the Imlygic group and 10 weeks in the GM-CSF group. The median OS was determined to be 23.3 in the patients treated with Imlygic compared with 18.9 months in patients who received GM-CSF. The DRR and ORR were considerably higher in the Imlygic arm. Additional efficacy criteria included the median TTF and median time to response which were 8.2 and 4.1 months in Imlygic treated group versus 2.9 and 3.7 months in the GM-CSF arm. The most common reported adverse events were fatigue, chills, and pyrexia (Maude et al., 2018). In a phase II clinical trial, 75 pediatric and young adults with relapsed or refractory B-ALL received Kymriah. The ORR was 81% at 3 months of follow-up. EFS and OS rates were calculated 73 and 90% at 6 months, and 50 and 76% at 12 months, respectively. The median duration of remission and EFS were not reached, while the rate for RFS was 80 and 59% at 6 and 12 months, respectively, among patients who responded to treatment. The most common non-hematological adverse reaction in 77% of patients was cytokine release syndrome. Also, neurologic events occurred in 40% of patients (Schuster et al., 2019). In another phase II clinical trial, to evaluate the efficacy of Kymriah therapy, 93 patients with relapsed or refractory DLBCL were infused with Kymriah and the median follow up time was 14 months. The best OR was 52%, 40% of the patients had complete responses and 12% had partial responses in a median of 2 months. After 12 months of the initial response, the rate of RFS was estimated to be 65% (79% among patients with a complete response). Also, cytokine release syndrome (58%), anemia (48%), and pyrexia (35%) were the most common adverse events of any grade (Neelapu et al., 2017). In a phase II clinical trial, Yescarta was administrated to 101 patients with DLBCL and PMBCL. The ORR and the complete response rate were 82 and 54%, respectively. The OS rates at 6, 12, and 18 months were 78, 59, and 52%. CAR T cell levels in the blood peaked within 14 days and were detectable in most patients at 180 days after the Yescarta infusion. The most serious adverse reactions included cytokine release syndrome and neurologic events (Cicalese et al., 2016).
For hematologic disorders, Strimvelis (EMA.2016) is developed to treat adenosine deaminase deficiency derived severe combined immunodeficiency (ADA-SCID) and Zynteglo (EMA.2019) is approved to treat Patients up to 12 years old with beta thalassemia who require regular blood transfusions.
In a phase II clinical trial, 18 patients with ADA-SCID received Strimvelis. The OS in a median follow-up of 6.9 years was 100% and there were increased numbers of CD3 +, CD4 +, CD8 + T cells, and CD16 + CD56 + NK cells as an outcome of immune reconstitution. A slower increase in CD19 + B-cells was reported. The TREC and lymphocyte ADA enzyme activity both increased in peripheral blood lymphocytes after treatment. Venous red blood cell deoxyadenosine nucleotide levels were <100 nmol/ml. The most frequent adverse events were respiratory and gastrointestinal tract infections (Karponi and Zogas, 2019). Five completed and ongoing clinical trials are existed regarding Zynteglo, HGB-205, HGB-204, HGB-207, HGB-212, and LTF-303. During HGB-204 and HGB-205 clinical trials, it was revealed that 11 of 18 and all of 4 enrolled patients with transfusion-dependent beta (β)-thalassemia (TDT) in each study met the primary endpoint which was the elimination of RBC transfusion requirement, respectively. HGB-207, HGB-212 clinical trials and a long-term follow-up study named LTF-303 constitute the ongoing clinical trials. So far, 17 of 20 and 6 of 11 enrolled patients in HGB-207, HGB-212 clinical trials have shown transfusion independency, respectively (European Medicines Agency, 2020e). Thrombocytopenia constituted the only serious adverse reaction related to Zynteglo. Moreover, there were common adverse reactions attributed to Zynteglo containing leukopenia, neutropenia, hot flush, dyspnea, pain in extremity, non-cardiac chest pain, and one very common adverse reaction as abdominal pain (ClinicalTrials, 2020).
Some of the common side effects reported in patients receiving ZyntegloTM during the clinical trials were a low count of thrombocytes, numbness in hands and feet, pain in the bone, nausea, headache, and low blood calcium levels (Deev et al., 2018).
A phase I/IIa clinical trial for Neovasculgen (Russian MOH.2011), as the single product in the cardiovascular diseases class, verified the safety of this product. In phase IIb/III trials that enrolled 100 patients (75 in the treatment and 25 in the control group), PWD was estimated to have increased significantly by 110% at 6 months in the treatment group. Moreover, PWD increased in the Neovasculgen treated group by 167% at 1 year and 191% at 2 years after treatment. There were no reported adverse events (Deev et al., 2015a, 2017; NOVARTIS, 2019a). Finally, the safety and efficacy of Zolgensma (US FDA.2019) one of the recent gene therapy products for neurodegenerative related diseases is being evaluated in an ongoing phase III STR1VE trial that enrolled 21 SMA pediatric patients with biallelic mutations in the survival motor neuron (SMN1) gene. As of the March 2019 data cutoff, remarkable survival rates, improved rapid motor function, and the capability to sit without support were among the most momentous results related to the efficacy of this product. The most common adverse events were elevated aminotransferases and vomiting (Russell et al., 2017). Luxturna (US FDA.2017/EMA.2018), the only gene product related to ocular diseases was assessed in a phase III clinical trial of 31 patients with RPE65-mediated inherited retinal dystrophy. The mean bilateral MLMT score was 1.8 light levels in the intervention group and 0.2 in the control group. Mean FST improved by more than two log units by day 30 in the intervention group, whereas the control group had no meaningful change. However, BCVA showed a numerical improvement among both groups. The most common adverse events included increased intraocular pressure, cataracts, retinal tears, and eye inflammation (Edmonds, 2009).
Skin and soft tissue related disorders, orthopedic, cardiovascular and ocular disorders are four indications for authorized TEPs.
For skin and soft tissue disorders, Apligraf (US FDA.2000), Dermagraft (US FDA.2001), Aurix (US FDA.2007), Omnigraft (US FDA.2016), Amniocin (Iran FDA.2017), and Cell-Amniosin (Iran FDA.2017) are developed to treat Chronic VLU and/or DFU. Holoderm (South Korea MFDS.2002), Epicel (US FDA.2007), and JACE (Japan PMDA.2007) are approved for skin burns and finally, Kaloderm (South Korea MFDS.2005, for burns/2010, DFU) is approved for both DFU and deep 2nd degree burns. The safety and efficacy Apligraf compared to standard therapy was assessed in 106 patients with neuropathic DFUs during 12 weeks. Kaplan–Meier curves indicated that the Apligraf treated group had a significantly faster complete wound closure in comparison with the standard treatment; after 12 weeks, 51.5% of patients who received Apligraf had achieved complete wound closure compared with 26.3% in the control group. The reported related adverse events consist of suspected wound local infection, cellulitis, and exudate (Marston et al., 2003). A clinical study of 314 patients with chronic DFUs evaluated the safety and efficacy of Dermagraft. The results showed a trend toward a shorter time for complete wound healing using Dermagraft. In addition, 30.0% of Dermagraft patients achieved complete wound closure compared with 18.3% of control patients after 12 weeks. No specific related adverse events were reported and the incidence of ulcer infection, cellulitis, or osteomyelitis was significantly lower in the Dermagraft treated patients versus the control patients (Driver et al., 2006). A clinical study of Aurix for 72 patients who suffered from non-healing DFU showed that 91% of long-term non-healing wounds responded to treatment with a 64% reduction in volume during 15 days or less. They researchers observed that 81.3% of Aurix treated wounds, which were less than 7 cm2, healed completely within 6.2 weeks in comparison with saline gel. In addition, the Kaplan–Meier time-to-healing was significantly better in the Aurix group. No product-related serious adverse events were reported (Driver et al., 2015). The results of the study that evaluated the safety and efficacy of Omnigraft in comparison to standard wound care in 307 patients with neuropathic DFU demonstrated improved life quality, approximately 5 weeks faster wound closure, and 19% increased healing rate in patients treated with Omnigraft (Tego Science, 2019b). Allergic reaction was the most concern about the safety of Omnigraft. We were unable to locate any data that pertained to the efficacy of Holoderm; however, according to the product brochure, abnormal cellular responses such as dyskeratosis or parakeratosis may occur following the use of Holoderm-derived epidermis (Food and Drug Administration, 2019i). The survival rate of Epicel was assessed in three studies. In the first study, the OS rate was 86.6% for overall patients and 89.3% for pediatric patients at 3 months after the initial implantation. In the second study, an OS rate of 88.3% in pediatric patients compared with 81.3% in the total population was reported. In the third study, the treatment group had a 90% survival rate compared to 37.5% for the control group. The most common adverse reactions were infections, graft shear, blisters, drainage, sepsis, graft detachment, and renal failure (Matsumura et al., 2016). The CEA JACE safety and efficacy was evaluated through a 6-year multicenter clinical trial for treatment of burns that covered more than 30% of the TBSA. The mean CEA take rate at 4 weeks post-engraftment was 66%, while the use of combined treatments such as artificial dermis or a wide split-thickness auto or a patch graft significantly elevated this rate. The most common adverse events were skin ulcers or auto graft detachment; however, death and sepsis, which were reported as serious adverse events during later periods, did not appear to be related to CEA (Tego Science, 2019c). Treatment of DFU with Kaloderm has shown that 12 weeks after treatment, all patients in the keratinocyte-treated group and 69% of patients in the control group experienced complete wound healing. No adverse events were reported in relation with the wound dressings. In terms of skin burns, the product packaging insert for Kaloderm stated that, no adverse reaction has been reported other than a possible occasional infection at the site, dermatitis, exudate formation, weak edema, hypersensitivity, and pain. In addition, Kaloderm can promote the re-epithelialization of deep abdominal cavity burns (McGuire et al., 2011; You et al., 2012).
Gintuit (US FDA.2012) is approved for surgically created vascular wound in the treatment of mucogingival conditions, the results of a clinical trial with 96 patients during 6 months follow up showed that the LCC mediated ≥ 2 mm regenerated keratinized gingiva in 81 of 85 patients and ≥ 1 mm in all patients, while the color and texture was similar to the adjacent tissue. The most common adverse events included sinusitis, nasopharyngitis, respiratory tract infections, aphthous stomatitis, and the local effects of oral surgery (Tohyama et al., 2009).
In orthopedics disorders, JACC (Japan PMDA.2012), MACI (US FDA.2016/EMA.2013) and Spherox (EMA.2017), are used to treat cartilage defects. JACC was studied through a multi-center clinical trial for transplanting autologous cultured chondrocytes in 27 patients with cartilage lesions who were evaluated at 3, 6, 12, and 24 months after the implantation surgery. Elimination of locked knee together with decreased pain were observed following the transplantation. Also, substantial progression of the original knee-function scale and the clinical scores based on the Lysholm Knee Scoring Scale, and observation of the natural appearance in 92% of patients as indicated by arthroscopic assessment showed restorative promotion of articular cartilage in the knees (Saris et al., 2014). There were few adverse events, except for two cases of graft detachment. The efficiency of a MACI implant has been assessed and its superiority versus microfracture treatment was evaluated in the SUMMIT clinical trial of 144 patients. The results at week 104 revealed significant improvement with MACI in the three KOOS subscales of pain, SRA, and ADL when compared with the microfracture group. Serious adverse reactions reported for MACI were arthralgia, cartilage injury, meniscus injury, treatment failure, and osteoarthritis (Armoiry et al., 2018). In a phase III clinical trial, Spherox was compared with the microfracture treatment in 102 patients. The mean overall KOOS in patients treated with Spherox increased from 56.6 ± 15.4 at baseline to 78.7 ± 18.6 after 12 months, with a further increase to 81.5 ± 17.3 after 24 months. However, the MOCART scores did not change significantly among the two groups (Becher et al., 2017). In a phase II clinical trial, 73 patients with cartilage defects received transplants of three different doses (low, medium, and high) and were subsequently followed until 36 months. Severe adverse events included meniscus lesion with the low dose; syncope and joint effusion with the medium dose; and arthralgia, joint effusion, and chondropathy with the high dose (Nordmeyer et al., 2018).
For cardiovascular diseases, CardioCel (Singapore HAS.2014) is approved to treat ASD and VSD and Heartsheet (Japan PMDA.2015) is developed for severe heart failure due to ischemic heart disease. In a study, CardioCel patches were applied on 40 patients for 2 years. While the probability of stopping the development of the combined end point that consisted of death, additional surgery, and a moderate degree of aortic valve dysfunction after AVR was 92 ± 5% at 12 months, this probability reduced to 28 ± 9% at 36 months after surgery. In this study, 23% of patients experienced an event during follow up, which included death and additional surgery due to stenosis, aortic valve insufficiency, and aortic valve endocarditis (Imamura et al., 2016). Heartsheet was evaluated in a phase II multi-center clinical trial of autologous skeletal myoblast sheet transplantation in seven patients with advanced heart failure compared with a control group receiving CRT in a 1 year follow upLVRR and heart failure symptoms improved in the treatment group and a lower rate of cardiac death during 800 days of follow up was observed. Common adverse events experienced by all of the patients during the study included arrhythmia, wound complications, hypokalemia, and post-operative fever (Rama et al., 2010). For ocular disorder, Amniodisk (Iran FDA.2020) is approved for Corneal ulcer, conjunctival and epithelial damage and Holoclar (EMA.2015) is approved for severe limbal stem cell deficiency. In a retrospective case series study, 106 patients with corneal damage received Holoclar. As the human limbal stem cells are recognized through p63 transcription factor expression, the clinical outcome assessment was conducted according to the percentage of p63-bright holoclone-forming stem cells in culture. If this percentage was greater than 3%, then the transplantation was considered successful. The success, partial success, and failure rates in the transplantation process were 76.6, 13.1, and 10.3% of the treated eyes, respectively (European Medicines Agency, 2015). The most common adverse reactions were blepharitis and corneal epithelium defects (Market Research, 2020).
Price and market size are two main issues that should be emphasized for guarantee of product survival in the market. We have attempted to provide information of the market size of most products introduced in this paper by preferably accessing the appropriate company‘s available data on the Internet. All product prices are presented in USD to be comparable. The information regarding the market size of each mentioned product was collected by using company’s IR book, website, and market research websites. The disease markets were considered based on the related CAGRs, following with the data respecting each product.
Thus far, the area of diseases that is targeted by ATMPs are divided into hematological disorders, skin and soft tissue disorders, orthopedic disorders, immunological disorders, cardiovascular diseases, neurodegenerative diseases, ocular diseases, and cancers. The market size of each mentioned diseases can be classified based on the industrial analysis and CAGR. According to Mordorintelligence, the largest predicted CAGR from 2020 till 2025 belongs to dermatology (Cancer Therapy Market, 2020) therapeutics market (8.95%) and cancers (Autoimmune Disease Diagnostics Market, 2020), autoimmune disorders (Orthopedic Devices Market, 2020), orthopedic disorders (Cardiovascular Devices Market, 2020), cardiovascular diseases (CellTech, 2019) and the hematology (Hematology Market, 2020) field come in the next places with the CAGRs of 8.37, 8.3, 7.2, 6.2, and 5%, respectively. Also, predicted CAGRs for markets of soft tissue (Soft Tissue Repair Market, 2020) repair, neurodegenerative diseases (Neurodegenerative Disease Market, 2020), and ophthalmic disorders (Ophthalmic Drugs Market, 2020) from 2019 till 2024 are 5.8, 5.5, and 4.6%. Therefore, beginning with dermatology therapeutics, Fibrocell Science has announced that the sale of Azficel-T increased approximately $0.3 million, or 63.4%, for the year ending December 31, 2016 compared to 2015 (Fibrocell Science Inc., 2020) Apligraf and Dermagraft have experienced strong sales growth, with more than one million units of each one, shipped for patient use until April 2016 (Organogenesis Inc., 2019a) along with approximately one million patient units for Apligraf until September 2017 (Organogenesis Inc., 2019b). Nuo Therapeutics estimated a turn in the Aurix sales from $0.495 million in 2014, to peak sales over $50 million (Napodano, 2015). Based on Vericel investor reports, Epicel has been administered to approximately 100 patients in the U.S. annually, and it could achieve $23.1 million net revenue by December 31, 2018 (Vericel Corporation, 2019). According to the company’s IR book, the operating margin of Tego Science Company for Kaloderm and Holoderm products was $10.9 million in 2006, which reached $23.4 million in 2013. Sales of Kaloderm were lower in 2006; however, by 2013, sales of Kaloderm were significantly higher than Holoderm (Tego Science, 2019d). As stated by Biosolution Company’s IR book, 15% of the market in 2008 in South Korea belonged to KeraHeal in comparison with competitive products. This amount rose to more than 70% in 2012. Regarding the market size, the number of patients in 2017 was approximately 410 with the sales percent of nearly $9.5 million. The number of patients is expected to reach approximately 450 in 2021 with an estimated sales of $42.6 million (Bio Solution Co Ltd., 2019). In 2017, the first year that KeraHeal-Allo was released, it occupied 20% of the market. It was estimated that this product would experience rapid growth in sales during 2019. Regarding market size, in 2017 there were approximately 203,000 persons with a sale of $7.5 million. The numbers of patients are expected to reach 217,000 persons in 2021 with an estimated sales of $56 million (Bio Solution Co Ltd., 2019). The J-TEC company in 2009 has estimated that the market size of JACE would be approximately 100 to 300 million US dollars only in Japan (Joo-sung, 2016). In the cancer area, the Green Cross Cell company IR book estimated that the market potential for Immuncell-LC for only the US will be about $6 billion, with the targeted market consisting of liver cancer, brain tumors, and pancreatic cancer (Green Cross Cell, 2019). Also, several approved GTMP therapeutics, Kymriah, Yescarta, Imlygic, Zalmoxis, Gendicine, and Oncorine are indicated for oncology related diseases. Kymriah grew strongly in Europe and US in the first quarter of 2020, with a net sale of $93 million (Globenewswire, 2020). Also, Yescarta’s sale during the first quarter of 2019 was $96 million compared to $40 million for the same period in 2018 (Business Wire, 2020). Amgen, the manufacturer of Imlygic, has not disclosed Imlygic sales in its quarterly results presentations. However, EvaluatePharma reported consensus analyst forecasts of $45 million and $250 million for worldwide Imlygic sales in 2016 and 2022, respectively (Imlygic, 2017). Financial reports in 2012 revealed that over 6000 patients diagnosed with various types of solid cancers in China received Gendicin, which had a CAGR from 2004 to 2011 of 68.3% (Kudrin, 2018). Sales of Gendicin were $3.6 million in 2007 and $16 million in 2008. However, the sales of Oncorine were unsatisfactory in China (<$1.2) due to its high price in 2009 (Kudrin, 2018). Products indicted for immunological disorders show that Alofisel is forecasted by Evaluate Pharma that the worldwide consensus sales will reach $529 million in 2024 (Mesoblast Limited, 2020). In terms of the Cupistem market in South Korea, the number of CD patients increased from approximately 15,000 in 2010 to almost 20,000 in 2014, while an increased prevalence has also been observed (Green Cross Cell, 2019). Sales of Temcell HS were $14.3 million in FY 2017, which showed a 124.3% increase compared to previous fiscal years (HIYAKU, 2019). For orthopedic disorders, in the beginning of 2019, Cartistem sold $11.1 million and the revenues for its sale accounted for 34.4% of the total sales in the first 6 months of 2019 (Korea Biomedical, 2020). Chondron sales for cartilage cell therapy in 2012 were approximately $13.8 million and increased to approximately $17.8 million in 2014 (Mksvc, 2019). In addition, Cartigrow has been used in 140,000 knee replacement cases annually (Das, 2018). According to Orthocel, Chondrocytes-T-Ortho-ACI is currently marketed in Australia, New Zealand, Hong Kong, Singapore, the United States, Europe, and China. Total revenues for its sale reached $739,100 during the 2018 fiscal year (FY). Moreover, the revenue forecast for Chondrocytes-T-Ortho-ACI until the FY 2028 is an estimated $340,203,044 (Hice, 2018). According to a 2018 report published in the standard business website, Ossgrow is used in 108,000 cases of total hip replacement in India each year (Das, 2018). TMACI, Spherox, and JACC are other approved therapeutics for orthopedic disorders. Vericel Company announced that MACI generated net revenues of approximately $67.7 million in the year ending December 31, 2018 (Vericel Corporation, 2019). CO.DON AG, launched Spherox for distribution in European countries in September 2017, starting from Germany. In Germany, the market volume addressed by CO.DON AG, approximated 20,000 annual treatments. With a population of 82.2 million, this meant that in Germany about 0.025% of the population could receive treatment with Spherox per year. Expressed in a conservative perspective, the potential for more than 115,000 knee-joint treatments per year was calculated in Europe. Marketing approval of Spherox was received in May 2017 from EMA (CODON, 2019). According to the J-TEC company’s IR book, the sales of JACC between FY 2011–2012 were reported to be $10.8 million compared to $371280 at its launch in 2003 (PMDA, 2011). In the field of cardiovascular diseases, according to the FCB Pharmicell Company IR book for Cellgram-AMI, in the South Korea the number of patients with AMI in 2015 was 87,984. The total amount of medical care costs for AMI in the same year was $1749 million (Pharmicell Ltd., 2020). Assessment of Neovasculogen, a product for cardiovascular diseases in Russia, indicated that 5 million people have been diagnosed with PAD. Also, the number of patients with CLI annually amounts up to 145,000, of which around 25% die (Deev et al., 2015b; NOVARTIS, 2019a). Also, the market size for Stempeucel is approximately $1.5 billion worldwide (Lane, 2016). Moreover, the annual reports from Admedus indicated that they achieved revenues of $6.9M million by selling their ADAPT®, bio-scaffold tissue technology, realted products (including CardioCel® Neo, VascuCel®) in North America, Europe, the MENA region, Asia, Australia, and New Zealand (Cardiocel, 2017). Products based on human cord blood derived HPCs, cover the market of hematologic disorders. Cord blood, as stem cell sources for patients without a donor, has its own market with more than 2000 cord blood hematopoietic stem cell transplants performed each year (WHO, 2013). The other approved product in this field is Zalmoxis. In 2018, Molmed Company projected peak sales of around 100 $ million to be achieved by 2026 for Zalmoxis (Spark Therapeutics Inc., 2019). However, very recently MolMed decided to the withdraw the drug’s conditional marketing authorization (CMA) after Phase III clinical trial results showed that the drug offered no benefit on DFS (Sharma, 2019) and stopped investing in the product. Revenues for Zalmoxis in 2018 is estimated at approximately $4.6 million (MolMed, 2018). In the neurological disorders, by taking into account the production of Nuronata-r in January 2015 and based on sales for June 2015 that were approximately $1.6 million, 2016 sales were estimated to be approximately $4.8 million with a rapid sales growth compared to other stem cell treatments. Also, the domestic patient sales in 2018 were expected to reach near $16.6 million which, together with overseas patients, the final number would be approximately $37.4 million (Commercialized Stem Cell Corestem Inc., 2020). Finally, Spark Therapeutics reported that in the year ended December 31, 2018, they recognized $64.7 million in total revenue, of which $27.0 million was net product sales of LUXTURNA (Spark Therapeutics Inc., 2019).
Overall, also it is worthy to note that according to the Seoane- Vazquez et al. (2019), the treatment cost of Kymriah, sipuleucel-T, Imlygic, and autologous cultured chondrocytes was higher in the United States than in Europe.
Cellular therapies have attracted tremendous attention from numerous researchers, clinicians and from industry, specifically for incurable diseases. As it can be concluded from Tables 1–3, most of the approved products in the recent years belong to the CTMPs, although a decrease in the total amount of approved products occurred in 2013 and 2014, which may be occurred due to financial and regulatory restriction issues. For the next years, there was an increased trend with five products approved in 2017 (Figure 3A). The year 2007 witnessed an increase in the number of approved TEP products; however, in the next years this amount decreased and this field has not experienced any evident growth trend, which can be seen in two other products (Figure 3B). GTMPs have faced an obvious progressive trend regarding the number of approved products and this is clearly apparent from the growth in the trend of these products, which may be due to entrance of big pharma in this field to develop a treatment for refractory conditions or rare diseases which do not have an effective pharmaceutical drug. The latest approved product, Zynteglo, is placed in this class (Figure 3C). It is expected that with the current advances in genetic engineering, products in this field would experience significant growth in following years. The origin of these products is an important subject since the use of autologous, allogeneic or xenogeneic cell sources would have a substantial effect on the financial policies of the companies that produce the regenerative medicine product and the related regulatory agencies. The allogeneic based products have a preferred advantage regarding their capability to be adapted for scale up strategies and also catching large market through massive export. However, as mentioned before, most members of CTMPs and GTMPs have an autologous source, due to the medicinal constraints in regards with immune rejection and the extensive required safety tests for allogeneic cells, while the cells used in tissue engineering based products with local indications, have the higher percentage for allogeneic rather than autologous products and have a greater market and higher sales (Kim et al., 2019). Altogether, it seems that with release of guidelines in different regulatory agencies the regulatory problem could be overcome in next years and trend of allogeneic products would raise noticeably. Market size and price are two main determinants of a product success or failure. Glybera with a both low market size and high price is one example of how these two factors can affect the survival of a certain ATMP in the market. Generally, the average prices for GTMPs are higher in comparison to CTMPs and TEPs. ATMPs related treatments despite their great therapeutic potential are very costly and there are challenges regarding their reimbursement issues. The high price of ATMPs is the result of several factors; high manufacturing costs, complex quality control tests, expensive raw materials, requirement of cold chains for transfer, intellectual property rules, small target populations (in some of cases) and strict regulatory inspections are among the most important reasons.
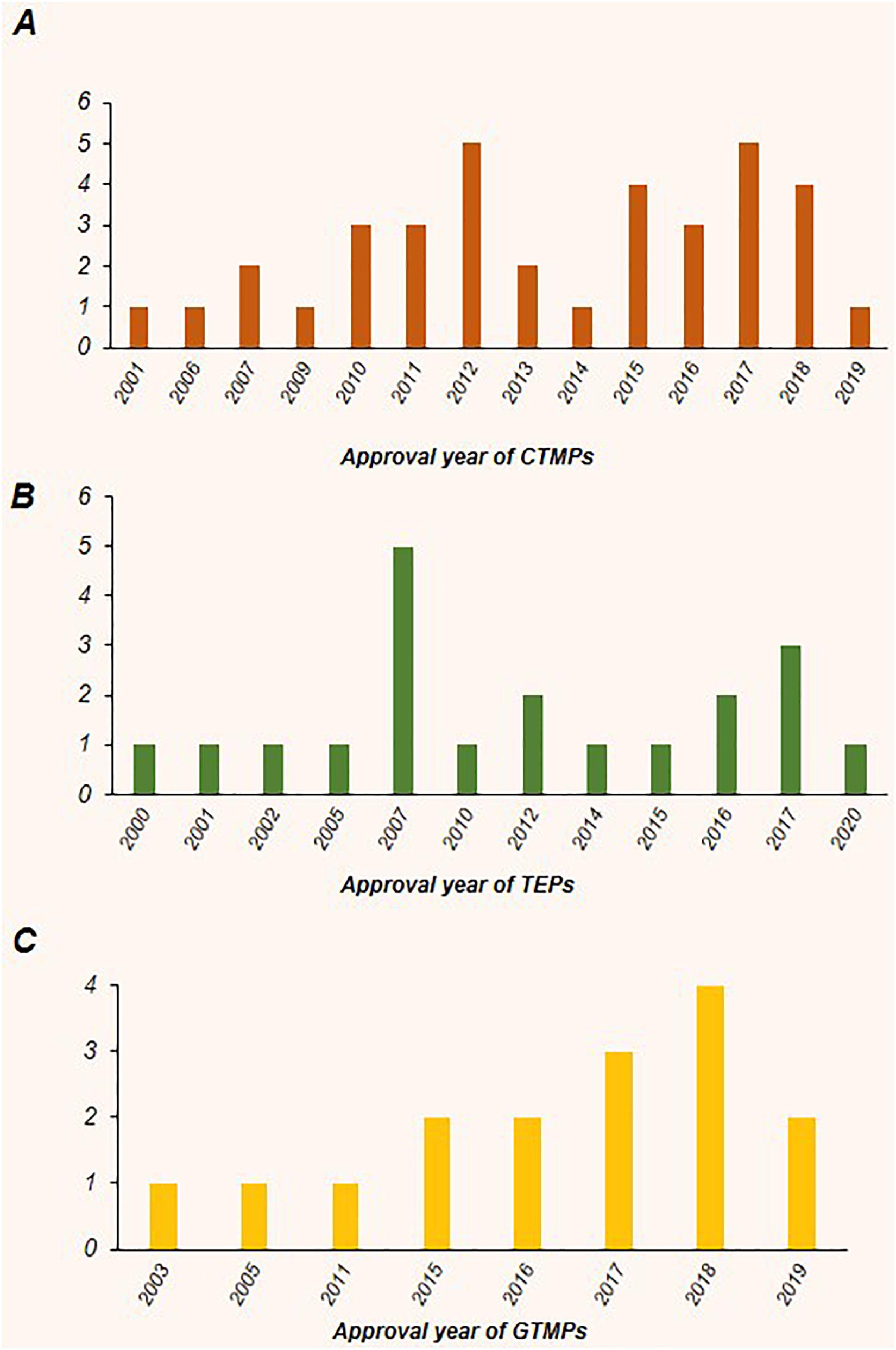
Figure 3. The annual trend of approval for CTMPs, GTMPs, and TEPs worldwide. Most of the products were recently approved. (A) In terms of CTMPs, despite a decrease in the number of approved products in 2013 and 2014, a second increased trend can be observed with five products approved in 2017. (B) For TEPs, the year 2007 with five approved products showed an increase in the total trend; however, there was a subsequent decrease in the following years. (C) There is a noticeable growth trend with respect to gene therapy medicinal products (GTMPs).
Due to the significant therapeutic potential of ATMPs for serious conditions in comparison with conventional drugs, as one of the major growth drivers for market, their market share is anticipated to be increasing. Respecting to the new slow shift toward personalized medicine while ATMPs are one of the high potential players in this regard, increasing the value of ATMPs global market size is highly expected. This idea is strongly supported by entrance of big pharma to this field in recent years. Moreover, the market interest has largely affected the number of developed products. This can be seen, for instance, in the percent of approved products for skin related application, as they are placed in the first position of TEPs (60%) and also CTMPs (23.5%).
Overall, the criteria regarding the efficacy of each ATMPs, shows optimistic results and the total numbers of adverse events are not dramatic. Cellular therapy is expected to be a promising area used for treatment of a noticeable quantity of incurable disorders. However, limiting factors regarding the development and uses of ATMPs should be still overcome, including the demand for high-technology systems for cell manufacturing and delivery (reducing production times and costs), vectors for gene modification availability and production, establishment of assays to validate products potency to ultimately improve treatment efficacy while avoiding adverse events and, last but not least the costs of these products and their reimbursability. On the other hand, since regenerative medicine strategies might become a solution for treatment of still incurable diseases and due to massive public and private investments, it is rational to claim that this field has the potential to overcome many of the mentioned limitations in a near future to reach a revolutionary phase in both the pharmaceutical industry and in the clinics.
HB, ST, EH-S, and RR conceived the manuscript concept, wrote and final edited the manuscript. All authors participated in the literature search, wrote the manuscript parts, prepared the figures and tables, and read and approved the final manuscript.
We gratefully appreciate supportive colleagues in Royan Advanced Therapy Medicinal Product Technology Development Center (ATMP-TDC) and Dr. Massoud Vosough, who helped us in this project. This study was financially supported by the grant from the “Italian Ministry of Research and University – Dipartimenti Eccellenti 2017 (MD)” and “Royan Institute and Ministry of Health and Medical Education (MoH, no. 700/147)”.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
6MWT, 6-minute walk test; AALS, Appel ALS; AAV, adeno-associated virus; ABPI, ankle brachial pressure index; ACKFBC, Allogeneic Cultured Keratinocytes and Fibroblasts in Bovine Collagen; ADA-SCID, adenosine deaminase severe combined immunodeficiency; ADC, adipose tissue-derived cell; ADL, activities of daily living; AL, allogeneic; ALS, amyotrophic lateral sclerosis; ALSFRS, Amyotrophic Lateral Sclerosis Functional Rating Scale; AMDDC, autologous monocyte-derived mature dendritic cell; AMI, acute myocardial infarction; ASD, atrial septal defect; AT, autologous; ATMP, advanced therapy medicinal product; AT-MSC, adipose tissue-derived mesenchymal stem cell; AVN, avascular necrosis; AVR, aortic valve reconstruction; B-ALL, B-cell precursor acute lymphoblastic leukemia; BCVA, best-corrected visual acuity; BM-MSC, bone marrow-derived mesenchymal stem cell; CAGR, compound annual growth rate; CAR, chimeric antigen receptor; CD, Crohn’s disease; CEA, cultured epidermal autograft; CFDA, China Food and Drug Administration; CLI, critical limb ischemia; COBLT, cord blood transplantation; CR, complete remission; CRT, cardiac resynchronization therapy; CTLA4Ig, cytotoxic T-lymphocyte associated protein 4 immunoglobulin; DB, dermal burn; DC, dendritic cell; DCGI, Drug Controller General of India; DDB, deep dermal burn; DFS, disease-free survival; DFU, diabetic foot ulcer; DLBCL, diffuse large B-cell lymphoma; DRR, durable response rate; DTH, delayed type hypersensitivity; EFS, event-free survival; EMA, European Medicines Agency; FDA, Food and Drug Administration; FST, full-field light sensitivity testing; FVC, forced vital capacity; GACI, gel-type autologous chondrocyte implantation; GM-CSF, granulocyte macrophage colony stimulating factor; GTMP, Gene Therapy Medicinal Product; GvHD, graft-versus-host disease; HAS, Heath Administration of Singapore; HCEpC, human corneal epithelial cell; HDFn, human dermal fibroblasts, neonatal; HEKn, human epidermal keratinocytes, neonatal; HPC, hematopoietic progenitor cell; HNSCC, head and neck squamous cell carcinoma; hRPE65, human retinal pigment epithelium 65 kDa; HSCT, hematopoietic stem cell transplantation; HSV, herpes simplex virus; ICRS, International Cartilage Repair Society; IFN-γ, interferon gamma; IKDC, International Knee Documentation Committee; irRC, immune-related response criteria; KLH, keyhole limpet hemocyanin; KOOS, knee injury and osteoarthritis outcome score; KSS, Knee Society Scoring system; LCC, living cellular construct; LPLD, lipoprotein lipase deficiency; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, end-systolic volume; LVRR, left ventricular reverse remodeling; mCRPC, metastatic castration-resistant prostate cancer; MFDS, Ministry of Food and Drug Safety; MLMT, multi-luminance mobility test; MOCART, Magnetic Resonance Observation of Cartilage Repair Tissue; MOH, Ministry of Health; mPP, modified per protocol; MSC, mesenchymal stem cell; MSS, MRI Scoring of Severity; NGFR, nerve growth factor receptor; NLF, nasolabilal fold; NRM, non-relapse mortality; NSCLC, non-small cell lung carcinoma; OA, osteoarthritis; OCD, osteochondritis dissecans; OR, overall response; ORR, objective response rate; ORR, overall response rate; ORR, overall remission rate; OS, overall survival; PAD, peripheral artery disease; PAP, prostatic acid phosphatase; PBMNC, peripheral-blood mononuclear cell; Pca, prostate cancer; PCI, percutaneous coronary intervention; PFU, plaque forming unit; PMBCL, primary mediastinal large B-cell lymphoma; PMDA, Pharmaceuticals and Medical Devices Agency; PR, partial remission; PRP, platelet-rich plasma; PWD, pain-free walking distance; QOL, quality of life; r/r, relapsed or refractory; RECIST, Response Evaluation Criteria in Solid Tumor; RFS, recurrence free survival; RFS, relapse-free survival; SCTMP, somatic cell therapy medicinal product; SDCS, skeletal myoblast-derived cell sheet; SOC, standard of care; SPECT, single-photon emission computed tomography; SRA, sports and recreational activity; TBSA, total body surface area; TEAEs, treatment emergent adverse events; TEP, tissue engineered product; TGA, Therapeutic Goods Administration; TL-pulsed DC, tumor lysate-pulsed dendritic cell; TNC, total nucleated cell; TREC, T-cell receptor excision circle; TTF, time to treatment failure; TTP, time to progression; UCCBB, University of Colorado Cord Blood Bank; SMA, spinal muscular atrophy; SMN, survival motor neuron; VAS, visual analog scale; VEGF, vascular endothelial growth factor; VG, vector genome; VLU, venous leg ulcer; VP, viral particle; VSD, ventricular septal defect; Xn, xenogeneic.
Andtbacka, R. H. I., Kaufman, H. L., Collichio, F., Amatruda, T., Senzer, N., Chesney, J., et al. (2015). Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 33, 2780–2788.
Anteris Technologies Limited (2019). CardioCel Admedus. Available online at: https://admedus.com/solutions/adapt/cardiocel/ (accessed May 10, 2019).
Anterogen Co. (2019). 안트로젠. Available online at: http://anterogen.com/main/en/sub02_01.html?type= (accessed Jun 23, 2019).
Anterogen Co. (2020). 안트로젠. Available online at: http://anterogen.com/main/en/sub02_01_02.html?type=2 (accessed May 15, 2020).
Apac Biotech (2019). Approved Immunotherapy Treatment Centre India. Available online at: http://apacbiotech.com/ (accessed May 9, 2019).
Armoiry, X., Cummins, E., Connock, M., Metcalfe, A., Royle, P., and Johnston, R. (2018). Autologous Chondrocyte Implantation with Chondrosphere for Treating Articular Cartilage Defects in the Knee: An Evidence Review Group Perspective of a NICE Single Technology Appraisal. Pharmacoeconomics. Available online at: http://www.ncbi.nlm.nih.gov/pubmed/30426462 (accessed May 10, 2019).
Autoimmune Disease Diagnostics Market (2020). Growth, Trends, and Forecasts. Available online at: https://www.mordorintelligence.com/industry-reports/global-autoimmune-disease-diagnostics-market (accessed May 24, 2020).
Bapsy, P. P., Sharan, B., Kumar, C., Das, R. P., Rangarajan, B., Jain, M., et al. (2014). Open-label, multi-center, non-randomized, single-arm study to evaluate the safety and efficacy of dendritic cell immunotherapy in patients with refractory solid malignancies, on supportive care. Cytotherapy 16, 234–244. doi: 10.1016/j.jcyt.2013.11.013
Bauer, S., Khan, R. J. K., Ebert, J. R., Robertson, W. B., Breidahl, W., Ackland, T. R., et al. (2012). Knee joint preservation with combined neutralising High tibial osteotomy (HTO) and matrix-induced autologous chondrocyte implantation (MACI) in younger patients with medial knee osteoarthritis: a case series with prospective clinical and MRI follow-up over 5years. Knee 19, 431–439. doi: 10.1016/j.knee.2011.06.005
Becher, C., Laute, V., Fickert, S., Zinser, W., Niemeyer, P., John, T., et al. (2017). Safety of three different product doses in autologous chondrocyte implantation: results of a prospective, randomised, controlled trial. J. Orthop Surg. Res. 12:71.
Berkrot, B. (2018). Spark’s Price for Luxturna Blindness Gene Therapy Too High: ICER - Reuters. Reuters Helath News. Available online at: https://www.reuters.com/article/us-spark-icer/sparks-price-for-luxturna-blindness-gene-therapy-too-high-icer-idUSKBN1F1298 (accessed May 10, 2019).
Bio Solution Co Ltd. (2019). Improve the Quality of Your Life by Cell-Based Biotechnology BIOSOLUTION. Investor Relations 2. Available online at: https://file.irgo.co.kr/data/BOARD/ATTACH_PDF/5dc0bf4035c46328f53a815b9d3d8bf7.pdf (accessed May 11, 2019).
Biomedic (2019). S-Biomedic. Available online at: https://www.sbiomedic.com/ (accessed Jun 23,2019).
S-Biomedics Ltd. (2012). 제정일자. rosmir. Available online at: http://sthepharm.com/download/cure_skin_kr.pdf (accessed June 29, 2012).
Biosolution Ltd. (2019a). (Cell therapy Products) Biosolution Co., Ltd. View|Biological Products | Minisry of Food and Drug Safety. Available online at: https://www.mfds.go.kr/eng/brd/m_30/view.do?seq=71374 (accessed Jun 23, 2019).
Biosolution Ltd. (2019b). 바이오솔루션. Available online at: http://www.biosolutions.co.kr/eng/product/medicine1.asp (accessed Jun 23, 2019).
Biosolution Ltd. (2019c). (Cell therapy products) Biosolution Co., Ltd. View|Biological Products | Minisry of Food and Drug Safety. Available online at: http://www.mfds.go.kr/eng/brd/m_30/view.do?seq=71374 (accessed May 9, 2019).
Bloomberg Businessweek (2018). Michelle Fay Cortez. This Implant Helps Heal Knees With a Patient’s Own Cartilage - Bloomberg. Bloomberg Businessweek. Available online at: https://www.bloomberg.com/news/articles/2018-09-22/this-implant-helps-heal-knees-with-a-patient-s-own-cartilage (accessed May 10, 2019).
Business Wire (2020). Gilead Sciences Announces First Quarter 2019 Financial Results | Business Wire. Available online at: https://www.businesswire.com/news/home/20190502005790/en/Gilead-Sciences-Announces-Quarter-2019-Financial-Results (accessed May 24, 2020).
Cade Hildreth (2018). Pricing of Approved Cell Therapy Products. Bioinformant. Available online at: https://bioinformant.com/price-of-cell-therapy-pro. Available online at: https://bioinformant.com/price-of-cell-therapy-pro (accessed March 22, 2018).
Cancer Therapy Market (2020). Growth, Trends, and Forecast (2020 - 2025). Available online at: https://www.mordorintelligence.com/industry-reports/cancer-therapy-market (accessed May 24, 2020).
Cardiocel (2017). Admedus Annual Report 2. Available online at: https://cdn.admedus.com/2018/08/10114914/2016-2017-Annual-Report.pdf (accessed August 8, 2017).
Cardiovascular Devices Market (2020). Growth, Trends, and Forecasts (2020 - 2025). Available online at: https://www.mordorintelligence.com/industry-reports/global-cardiovascular-devices-market-industry (accessed May 24, 2020).
CellTech (2019). Products - CellTech. Available online at: http://en.celltech.ir/products/ (accessed May 9, 2019).
Cheng, B., Lu, S., and Fu, X. (2016). Regenerative medicine in China: main progress in different fields. Mil. Med. Res. 3:24.
Cho, Y. B., Park, K. J., Yoon, S. N., Song, K. H., Kim, D. S., and Jung, S. H. (2015). Long-term results of adipose-derived stem cell therapy for the treatment of crohn’s fistula. Stem Cells Transl. Med. 4, 532–537. doi: 10.5966/sctm.2014-0199
Choi, M., Han, E., Lee, S., Kim, T., and Shin, W. (2015). Regulatory Oversight of Gene Therapy and Cell Therapy Products in Korea. Advances in Experimental Medicine and Biology. New York, NY: Springer, 163.
Choi, N.-Y., Kim, B.-W., Yeo, W.-J., Kim, H.-B., Suh, D.-S., Kim, J.-S., et al. (2010). Gel-type autologous chondrocyte (ChondronTM) implantation for treatment of articular cartilage defects of the knee. BMC Musculoskelet Disord 11:103.
Cicalese, M. P., Ferrua, F., Castagnaro, L., Pajno, R., Barzaghi, F., Giannelli, S., et al. (2016). Update on the safety and efficacy of retroviral gene therapy for immunodeficiency due to adenosine deaminase deficiency. Blood 128, 45–54.
Clarke, T., and Berkrot, B. (2019). FOOD AND DRUG ADMINISTRATION Approves Gilead Cancer Gene therapy; Price set at $373,000 - Reuters. Reuters Helath News. Available online at: https://www.reuters.com/article/us-gilead-sciences-Food and Drug Administration/Food and Drug Administration-approves-gilead-cancer-gene-therapy-price-set-at-373000-idUSKBN1CN35H/ (accessed May 10, 2019).
ClinicalTrials (2020). Zynteglo for Transfusion-Dependent Beta-Thalassemia, Europe. Available online at: https://www.clinicaltrialsarena.com/projects/zynteglo-beta-thalassemia/ (accessed May 27, 2020).
CODON (2019). Financial Reports. Interim Consolidated Financial Statements 1 January to 30 June 2. Available online at: https://www.codon.de/en/investoren/financial-reports (accessed May 15, 2020).
Commercialized Stem Cell Corestem Inc. (2020). Available online at: https://dokumen.tips/documents/the-commercialized-stem-cell-corestem-inc-ir-bookpdf-bbc-research.html (accessed May 13, 2020).
Corestem Inc. (2019). (Cell Therapy Products) Corestem, Inc. View|Biological Products | Minisry of Food and Drug Safety. Available online at: http://www.mfds.go.kr/eng/brd/m_30/view.do?seq=70956 (accessed May 9, 2019).
Cuende, N., Rasko, J. E. J., Koh, M. B. C., Dominici, M., and Ikonomou, L. (2018). Cell, tissue and gene products with marketing authorization in 2018 worldwide. Cytotherapy 20, 1401–1401. doi: 10.1016/j.jcyt.2018.09.010
Das, S. (2018). Mumbai’s Regrow Biosciences Develops Cell Therapy for Bone Joint Disorders | Business Standard News. Available online at: https://www.business-standard.com/article/companies/mumbai-s-regrow-biosciences-develops-cell-therapy-for-bone-joint-disorders-118091000783_1.html (accessed May 11, 2019).
Deev, R. V., Bozo, I. Y., Mzhavanadze, N. D., Voronov, D. A., Gavrilenko, A. V., Chervyakov, Y. V., et al. (2015a). pCMV- vegf165 intramuscular gene transfer is an effective method of treatment for patients with chronic lower limb ischemia. J. Cardiovasc Pharmacol. Ther. 20, 473–482. doi: 10.1177/1074248415574336
Deev, R. V., Drobyshev, A. Y., Bozo, I. Y., and Isaev, A. A. (2015b). Ordinary and activated bone grafts: applied classification and the main features. Biomed. Res. Int. 2015, 1–19. doi: 10.1155/2015/365050
Deev, R., Plaksa, I., Bozo, I., and Isaev, A. (2017). Results of an international postmarketing surveillance study of pl-VEGF165 safety and efficacy in 210 patients with peripheral arterial disease. Am. J. Cardiovasc. Drugs 17, 235–242. doi: 10.1007/s40256-016-0210-3
Deev, R., Plaksa, I., Bozo, I., Mzhavanadze, N., Suchkov, I., Chervyakov, Y., et al. (2018). Results of 5-year follow-up study in patients with peripheral artery disease treated with PL-VEGF165 for intermittent claudication. Ther. Adv. Cardiovasc. Dis. 12, 237–246. doi: 10.1177/1753944718786926
Detela, G., and Lodge, A. (2019). EU regulatory pathways for ATMPs: standard, accelerated and adaptive pathways to marketing authorisation. Mol. Ther. Methods Clin. Dev. 13, 205–232. doi: 10.1016/j.omtm.2019.01.010
Doo-hyun, A. (2018). Cell Therapy Rosmir to Target Anti-Wrinkle Market - Korea Biomedical Review. Koreabiomed. Available online at: http://www.koreabiomed.com/news/articleView.html?idxno=2617 (accessed May 15, 2020).
Doo-hyun, N. (2019). Will Korea’s First Stem Cell Therapy Pass Reexamination? - Korea Biomedical Review. Koreabiomed. Available online at: http://www.koreabiomed.com/news/articleView.html?idxno=585 (accessed Jun 23, 2019).
Drew, S. W. (2015). JACE. Available online at: https://haseloff.plantsci.cam.ac.uk/resources/SynBio_reports/BEM-FinalReport-Web.pdf (accessed March 6, 2015).
Driver, V. R., Hanft, J., Fylling, C. P., and Beriou, J. M. (2006). Autologel diabetic foot ulcer study group. a prospective, randomized, controlled trial of autologous platelet-rich plasma gel for the treatment of diabetic foot ulcers. Ostomy Wound Manage 52, 68–70.
Driver, V. R., Lavery, L. A., Reyzelman, A. M., Dutra, T. G., Dove, C. R., Kotsis, S. V., et al. (2015). A clinical trial of integra template for diabetic foot ulcer treatment. Wound Repair Regen 23, 891–900. doi: 10.1111/wrr.12357
Edmonds, M. (2009). European and australian apligraf diabetic foot ulcer study group. apligraf in the treatment of neuropathic diabetic foot ulcers. Int. J. Low Extrem Wounds 8, 11–18. doi: 10.1177/1534734609331597
Elsanhoury, A., Sanzenbacher, R., Reinke, P., and Abou-El-Enein, M. (2017). Accelerating Patients’ Access to Advanced Therapies in the EU, Molecular Therapy - Methods and Clinical Development, Cambridge, MA: Cell Press, 15–19.
EMC Inc. (2020). Holoclar - Summary of Product Characteristics (SmPC) - (emc). Available online at: https://www.medicines.org.uk/emc/product/8033/smpc (accessed May 11, 2020).
EU (2020). Study on the Regulation of Advanced Therapies in Selected Jurisdictions - Publications Office of the EU. Available online at: https://op.europa.eu/en/publication-detail/-/publication/78af6082-bc4a-11e6-a237-01aa75ed71a1 (accessed May 24, 2020).
European Medicines Agency (2015). Holoclar. Available online at: https://www.ema.europa.eu/en/medicines/human/EPAR/holoclar (accessed March 2, 2015).
European Medicines Agency (2019). kymriah2. Available online at: https://www.ema.europa.eu/en/medicines/human/EPAR/kymriah#overview-section (accessed June 15, 2019).
European Medicines Agency (2020a). Alofisel | European Medicines Agency. Available online at: https://www.ema.europa.eu/en/medicines/human/EPAR/alofisel (accessed May 11, 2020).
European Medicines Agency (2020b). Glybera | European Medicines Agency. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/glybera. [Accessed on 2020 Nov 19].
European Medicines Agency (2020c). Holoclar | European Medicines Agency. Available online at: https://www.ema.europa.eu/en/medicines/human/EPAR/holoclar (accessed May 11, 2020).
European Medicines Agency (2020d). Spherox. Available online at: https://www.ema.europa.eu/en/medicines/human/EPAR/spherox 31 (January 6, 2020).
European Medicines Agency. (2020e). Zynteglo | European Medicines Agency. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/zynteglo. [accessed on Nov 19, 2020].
FAQ (2019). Patient FAQ’S FOR CARTIGROW. Available online at: https://www.regrow.in/pdf/FAQ-FOR-CARTIGROW.PDF (accessed May 9, 2019).
Fibrocell Science Inc. (2013). EXTON P. Fibrocell Science Pursues Premium Aesthetic Market Position for LAVIV® | Business Wire. -Fibrocell Science, Inc. Available online at: https://www.businesswire.com/news/home/20130424006764/en/Fibrocell-Science-Pursues-Premium-Aesthetic-Market-Position (accessed May 9, 2019).
Fibrocell Science Inc. (2020). Annual Reports. Available online at: http://www.annualreports.com/HostedData/AnnualReportArchive/f/NASDAQ_FCSC_2013.pdf (accessed May 24, 2020).
Food and Drug Administration (2019a). Cord Blood - MD Anderson Cord Blood Bank. Available online at: https://www.FoodandDrugAdministration.gov/vaccines-blood-biologics/cellular-gene-therapy-products/hpc-cord-blood-md-anderson-cord-blood-bank (accessed May 9, 2019).
Food and Drug Administration (2019b). Cord Blood - Bloodworks | FOOD AND DRUG ADMINISTRATION. Available online at: https://www.Food and Drug Administration.gov/vaccines-blood-biologics/cellular-gene-therapy-products/hpc-cord-blood-bloodworks (accessed May 9, 2019).
Food and Drug Administration (2019c). CLEVECORD (HPC Cord Blood). Available online at: https://www.Food and Drug Administration.gov/vaccines-blood-biologics/cellular-gene-therapy-products/clevecord-hpc-cord-blood (accessed May 9, 2019).
Food and Drug Administration (2019d). DUCORD (HPC Cord Blood). Available online at: https://www.Food and Drug Administration.gov/vaccines-blood-biologics/cellular-gene-therapy-products/ducord-hpc-cord-blood (accessed May 9, 2019).
Food and Drug Administration (2019e). HPC, Cord Blood. Available online at: https://www.Food and Drug Administration.gov/vaccines-blood-biologics/cellular-gene-therapy-products/hpc-cord-blood (accessed May 9, 2019).
Food and Drug Administration (2019f). HEMACORD (HPC, cord blood). Available online at: https://www.Food and Drug Administration.gov/vaccines-blood-biologics/cellular-gene-therapy-products/hemacord-hpc-cord-blood (accessed May 9, 2019).
Food and Drug Administration (2019g). HPC, Cord Blood - LifeSouth. Available online at: https://www.Food and Drug Administration.gov/vaccines-blood-biologics/cellular-gene-therapy-products/hpc-cord-blood-lifesouth (accessed May 9, 2019).
Food and Drug Administration (2019h). Laviv (Azficel-T) | Food And Drug AdministratioN. Available online at: https://www.Food and Drug Administration.gov/vaccines-blood-biologics/cellular-gene-therapy-products/laviv-azficel-t (accessed May 9, 2019).
Food and Drug Administration (2019i). Epicel (Cultured Epidermal Autografts) | Food And Drug Administration. Available online at: https://www.Food and Drug Administration.gov/vaccines-blood-biologics/approved-blood-products/epicel-cultured-epidermal-autografts (accessed May 10, 2019).
Food and Drug Administration (2019j). PROVENGE (sipuleucel-T) | FOOD AND DRUG ADMINISTRATION. Available online at: https://www.Food and Drug Administration.gov/vaccines-blood-biologics/cellular-gene-therapy-products/provenge-sipuleucel-t (accessed May 9, 2019).
Food and Drug Administration (2019k). KYMRIAH (tisagenlecleucel). Available online at: https://www.Food and Drug Administration.gov/vaccines-blood-biologics/cellular-gene-therapy-products/kymriah-tisagenlecleucel (accessed May 10, 2019).
Food and Drug Administration (2019l). YESCARTA (axicabtagene ciloleucel). Available online at: https://www.Food and Drug Administration.gov/vaccines-blood-biologics/cellular-gene-therapy-products/yescarta-axicabtagene-ciloleucel (accessed May 10, 2019).
Food and Drug Administration (2019m). ZOLGENSMA | FOOD AND DRUG ADMINISTRATION. Available online at: https://www.Food and Drug Administration.gov/vaccines-blood-biologics/zolgensma (accessed June 23, 2019).
Food and Drug Administration (2019n). Package Insert - LUXTURNA (voretigene neparvovec-rzyl). Available online at: www.Food and Drug Administration.gov/medwatch. (accessed May 10, 2019).
Food and Drug Administration (2019o). GINTUIT (Allogeneic Cultured Keratinocytes and Fibroblasts in Bovine Collagen). Available online at: https://www.Food and Drug Administration.gov/vaccines-blood-biologics/cellular-gene-therapy-products/gintuit-allogeneic-cultured-keratinocytes-and-fibroblasts-bovine-collagen (accessed May 10, 2019).
Food and Drug Administration (2019p). Integra® OmnigraftTM Dermal Regeneration Matrix-Smart Solutions for Serious Wounds. Patient Guide to Healing Diabetic Foot Ulcers. Available online at: https://www.accessdata.Food and Drug Administration.gov/cdrh_docs/pdf/P900033S042c.pdf (accessed May 10, 2019).
Food and Drug Administration (2019q). MACI (Autologous Cultured Chondrocytes on a Porcine Collagen Membrane). Available online at: https://www.Food and Drug Administration.gov/vaccines-blood-biologics/cellular-gene-therapy-products/maci-autologous-cultured-chondrocytes-porcine-collagen-membrane (accessed May 10, 2019).
Food and Drug Administration (2020a). Cellular & Gene Therapy Products | FOOD AND DRUG ADMINISTRATION. Available online at: https://www.Food and Drug Administration.gov/vaccines-blood-biologics/cellular-gene-therapy-products (accessed May 11, 2020).
Food and Drug Administration (2020b). yeskarta. Available online at: https://www.Food and Drug Administration.gov/vaccines-blood-biologics/cellular-gene-therapy-products/yescarta-axicabtagene-ciloleucel (accessed may 28, 2020).
Food and Drug Administration (2020c). IMLYGIC (talimogene Laherparepvec) | FOOD AND DRUG ADMINISTRATION. Available online at: https://www.Food and Drug Administration.gov/vaccines-blood-biologics/cellular-gene-therapy-products/imlygic-talimogene-laherparepvec (accessed May 14, 2020).
Genzyme (2019). Biosurgery Genzyme. Epicel® (cultured epidermal autografts) HDE# BH990200 Patient Information. Available online at: https://www.Food and Drug Administration.gov/media/103055/download (accessed May 10, 2019).
Global Sources (2019). KALODERM | Global Sources. Available online at: https://www.globalsources.com/si/AS/Tego-Science/6008850646004/pdtl/KALODERM/1131606547.htm (accessed May 10, 2019).
Globenewswire (2020). Novartis Operational Performance. Available online at: https://www.globenewswire.com/news-release/2020/04/28/2022974/0/en/Novartis-maintains-strong-operational-performance-in-Q1-confirms-FY-2020-guidance-at-this-time-and-advances-a-broad-range-of-efforts-to-support-the-global-response-to-COVID-19.html (accessed April 28, 2020).
Green Cross Cell (2019). 세포치료 전문기업 GC 녹십자셀 2018년 7월 24일 [Internet]. Available online at: https://file.irgo.co.kr/data/BOARD/ATTACH_PDF/da48452112f0f845a6b9e1ffd028dab3.pdf (accessed May 11, 2019).
Gupta, P. K., Krishna, M., Chullikana, A., Desai, S., Murugesan, R., Dutta, S., et al. (2017). Administration of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells in critical limb ischemia due to buerger’s disease: phase ii study report suggests clinical efficacy. Stem Cells Transl. Med. 6, 689–699. doi: 10.5966/sctm.2016-0237
Hanna, E., Rémuzat, C., Auquier, P., and Toumi, M. (2016a). Advanced therapy medicinal products: current and future perspectives. J. Mark Access. Heal Policy 4:31.
Hanna, E., Rémuzat, C., Auquier, P., and Toumi, M. (2016b). Risk of discontinuation of advanced therapy medicinal products clinical trials. J. Mark Access Heal Policy. 16:367.
Han-soo, L. (2018). Corestem’s NeuroNata-R Marks Increase in Foreign Patients - Korea Biomedical Review. Koreabiomed. Available online at: http://www.koreabiomed.com/news/articleView.html?idxno=2138 (accessed May 9, 2019).
Hematology Market (2020). Growth, Trends, and Forecast (2020-2025). Available online at: https://www.mordorintelligence.com/industry-reports/global-hematology-market-industry (accessed May 24, 2020).
Hice, R. (2018). orthoACI. Global Equity Research led. Available online at: https://www.orthocell.com.au/s/Orthocell-Limited-Report-April-3-2018-gpnh.pdf (accessed April 3, 2018).
HIYAKU (2019). quot;HIYAKU". Available online at: http://www.jcrpharm.co.jp/wp2/wp-content/uploads/2018/09/ee53eae8e85c0d1d22ce14ee9f0c91a5.pdf (accessed May 11, 2019).
Iglesias-López, C., Agustí, A., Obach, M., and Vallano, A. (2019). Regulatory framework for advanced therapy medicinal products in europe and united states. Front. Pharmacol. 10:569.
Imamura, T., Kinugawa, K., Sakata, Y., Miyagawa, S., Sawa, Y., Yamazaki, K., et al. (2016). Improved clinical course of autologous skeletal myoblast sheet (TCD-51073) transplantation when compared to a propensity score-matched cardiac resynchronization therapy population. J. Artif. Organs 19, 80–86. doi: 10.1007/s10047-015-0862-9
Imlygic (2017). Viralytics. Available online at: https://www.edisoninvestmentresearch.com/?ACT=18&ID=18566&LANG= (accessed April 19, 2017).
Integra (2019). DFU4041S, Integra OmniGraft Dermal Regeneration Matrix Kit w/Stapler 4cm x 4cm - eSutures. Available online at: https://www.esutures.com/product/1-expired/105-integra/848-duraplasty/46286263-integra-omnigraft-dermal-regeneration-matrix-kit-wstapler-4cm-x-4cm-DFU4041S/ (accessed May 10, 2019).
Jae-hyeon, S. (2018). Will Tego Science Win reimbursement for Diabetic foot Ulcer Treatment? - Korea Biomedical Review. Koreabiomed. Available online at: http://www.koreabiomed.com/news/articleView.html?idxno=3971 (accessed May 10, 2019).
Japan Tissue Engineering Co Ltd. (2020). Autologous Cultured Cartilage (Regenerative Medicine Business)?:Japan Tissue Engineering Co., Ltd. (J-TEC). Available from: http://www.jpte.co.jp/english/business/Regenerative/cultured_cartilage.html [accessed on Nov 19, 2020].
Japan Tissue Engineering Ltd. (2009). Biotech Gate (Company Database). Available online at: http://www.jpte.co.jp/english/ir/BIOTECH_report.pdf (acessed May 14, 2020).
Jayaraman, K. S. (2016). Stempeucel. Natureasia. Available online at: https://www.natureasia.com/en/nindia/article/10.10 (accessed 15 May 2016).
Jcr Pharmaceuticals Co. (2020). Products | JCR Pharmaceuticals Co., Ltd. Available online at: https://www.jcrpharm.co.jp/en/site/en/biopharmaceutical/product_tem.html (accessed May 14, 2020).
Joo-sung, K. (2016). 식약처-심평원은 왜 이 약에 집착할까? [Internet]. Available online at: http://www.pressian.com/news/article/?no=140360 (accessed May 9, 2019).
Kantoff, P. W., Higano, C. S., Shore, N. D., Berger, E. R., Small, E. J., Penson, D. F., et al. (2010). Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 363, 411–422.
Karponi, G., and Zogas, N. (2019). Gene Therapy for Beta-Thalassemia: Updated Perspectives, Application of Clinical Genetics, Vol. 12.? Macclesfield: Dove Medical Press Ltd, 167.
Kim, J. H., Lee, Y., Bae, Y.-S., Kim, W. S., Kim, K., and Im, H. Y. (2007). Phase I/II study of immunotherapy using autologous tumor lysate-pulsed dendritic cells in patients with metastatic renal cell carcinoma. Clin. Immunol. 125, 257–267. doi: 10.1016/j.clim.2007.07.014
Kim, J.-S. (2014). 화상세포치료제 급여산정 무슨일 있었나? - 메디칼업저버. Medical Observer. Available online at: http://www.monews.co.kr/news/articleView.html?idxno=70997 (accessed May 10, 2019).
Kim, S. H., Cho, J. H., Lee, Y. H., Lee, J. H., Kim, S. S., Kim, M. Y., et al. (2018). Improvement in left ventricular function with intracoronary mesenchymal stem cell therapy in a patient with anterior wall st-segment elevation myocardial infarction. Cardiovasc Drugs Ther. 32, 329–338. doi: 10.1007/s10557-018-6804-z
Kim, S.-J., Shin, Y.-W., Yang, K.-H., Kim, S.-B., Yoo, M.-J., Han, S.-K., et al. (2009). A multi-center, randomized, clinical study to compare the effect and safety of autologous cultured osteoblast (OssronTM) injection to treat fractures. BMC Musculoskelet Disord 10:20.
Kim, Y. S., Smoak, M. M., Melchiorri, A. J., and Mikos, A. G. (2019). An overview of the tissue engineering market in the United States from 2011 to 2. Tissue Eng. Part A 25, 1–8. doi: 10.1089/ten.tea.2018.0138
Korea Biomedical (2020). Sae-im J. Medipost’s Stem Cell Therapy, Cord Blood Bank Show Solid Growth - Korea Biomedical Review. Available online at: http://www.koreabiomed.com/news/articleView.html?idxno=6289 (accessed May 14, 2020).
Kudrin, A. (2018). Business models and opportunities for cancer vaccine developers. Hum. Vaccin. Immunother. 8, 1431–1438. doi: 10.4161/hv.20629
Lane, E. (2016). Stempeutics’ Buerger’s Disease Cell Therapy Gets Conditional Nod in India | FiercePharma. Pharma Asia. Available online at: https://www.fiercepharma.com/pharma-asia/stempeutics-buerger-s-disease-cell-therapy-gets-conditional-nod-india (accessed May 14, 2020).
Lee, B. (2018). 9 Challenges Keeping Cell And Gene Therapy Executives Up At Night. RepliCel Life Sciences Inc. Available online at: https://www.cellandgene.com/doc/challenges-keeping-cell-and-gene-therapy-executives-up-at-night-0001 (accessed May 27, 2020).
Lee, J. H., Lee, J.-H., Lim, Y.-S., Yeon, J. E., Song, T.-J., and Yu, S. J. (2015). Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology 148, 1383–1391. doi: 10.1053/j.gastro.2015.02.055
Luria, B. S. X. R., Castelo, F. F., Vandromme, M., Monteiro, S., Portuguesa, B. A., Rogelio, P. C. A., et al. (2020). BIOREG (SOE3/P1/E750)-Co-financed by the INTERREG IVB SUDOE Program with ERDF funds Handbook about Regulatory Guidelines and Procedures for the Preclinical and Clinical Stages of Advanced Therapy Medicinal Products (ATMPs) Reviewers: Editor. Available online at: www.ddrmedic.com (accessed May 24, 2020).
Market Research (2020). Consulting, Reports, Advisory, Sizing | Consulting - Client Research, Market Analysis | Competitive Landscape Analysis | Global Strategic Business Reports | Custom Market Research - Mordor Intelligence. Available online at: https://www.mordorintelligence.com/industry-reports/dermatological-therapeutics-market (accessed May 23, 2020).
Marston, W. A., Hanft, J., Norwood, P., and Pollak, R. (2003). Dermagraft diabetic foot ulcer study group. the efficacy and safety of dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care 26, 1701–1705. doi: 10.2337/diacare.26.6.1701
Matsumura, H., Matsushima, A., Ueyama, M., and Kumagai, N. (2016). Application of the cultured epidermal autograft “JACE®” for treatment of severe burns: results of a 6-year multicenter surveillance in Japan. Burns 42, 769–776. doi: 10.1016/j.burns.2016.01.019
Maude, S. L., Laetsch, T. W., Buechner, J., Rives, S., Boyer, M., Bittencourt, H., et al. (2018). Tisagenlecleucel in children and young adults with b-cell lymphoblastic leukemia. N. Engl. J. Med. 378, 439–448.
McConaghie, A. (2017). Glybera, The Most Expensive Drug in the World, to be Withdrawn After Commercial Flop -. Pharmaphorum. Available online at: https://pharmaphorum.com/news/glybera-expensive-drug-world-withdrawn-commercial-flop/ (accessed May 14, 2020).
McGuire, M. K., Scheyer, E. T., Nevins, M. L., Neiva, R., Cochran, D. L., Mellonig, J. T., et al. (2011). Living Cellular construct for increasing the width of keratinized gingiva: results from a randomized, within-patient, controlled trial. J. Periodontol. 82, 1414–1423. doi: 10.1902/jop.2011.100671
Medical observer (2019). 말 많던 화상약제 케라힐, 결국 감사원 감사 결정 - 메디칼업저버. Available online at: http://www.monews.co.kr/news/articleView.html?idxno=75292 (accessed Jun 23, 2019).
Medipost (2019). The Future of Biotechnology. Available online at: http://www.medi-post.com/front/eng/stemcell/cartistem.do (accessed Jun 23, 2019).
Mesoblast Limited (2020). (ASX:MSB) - Msb, Page-2 - HotCopper | ASX Share Prices, Stock Market & Share Trading Forum. Available online at: https://hotcopper.co.nz/threads/msb.4694211/page-2 (accessed May 17, 2020).
Miller, J. (2018). Novartis wins EU Approval for Blood Cancer Therapy Kymriah - Reuters. Reuters Helath News. Available online at: https://www.reuters.com/article/us-novartis-cancer/novartis-wins-eu-approval-for-blood-cancer-therapy-kymriah-idUSKCN1LC0CU/ (accessed May 10, 2019).
Mills, R. (2019). Counting Coup: Is Osiris Losing Faith in Prochymal? | SCSI Stem Cell Stock Index. Osiris Therapeutics. Available online at: http://busaconsultingllc.com/scsi/organelles/counting_coup_prochymal.php (accessed May 9, 2019).
Ministry of Food and Drug Safety (2019a). (Cell therapy products) JW CreaGene Corporation View|Biological Products | Minisry of Food and Drug Safety. Available online at: http://www.mfds.go.kr/eng/brd/m_30/view.do?seq=71375 (accessed May 9, 2019).
Ministry of Food and Drug Safety (2019b). (Cell Therapy Prodcuts) Green Cross Cell Corp. View|Biological Products |. Available online at: https://www.mfds.go.kr/eng/brd/m_30/view.do?seq=70951 (accessed Jun 23, 2019).
Ministry of Food and Drug Safety (2019c). Minisry of Food and Drug Safety. Available online at: https://www.mfds.go.kr/eng/index.do (accessed Jun 23, 2019)Google Scholar
Ministry of Food and Drug Safety (2020). (Cell Therapy Products) ANTEROGEN View|Biological Products | Minisry of Food and Drug Safety. Available online at: https://www.mfds.go.kr/eng/brd/m_30/view.do?seq=71337&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=1 (accessed May 15, 2020).
Mksvc (2019). 플랜트사업부 수주 증가로 턴어라운드. Available online at: http://file.mk.co.kr/imss/write/20150724104734_mksvc01_00.pdf (accessed May 11, 2019).
MolMed (2018). Annual Report as of December 31st, 2018. Available online at: https://www.molmed.com/sites/default/files/2019-11/EN 31 12 2018_ last.pdf (accessed May 27, 2020).
MolMed (2019). MolMed S.p.A. Available online at: https://www.molmed.com/search/node/Zalmoxis?page=2 (accessed May 10, 2019).
Mount, N. M., Ward, S. J., Kefalas, P., and Hyllner, J. (2015). Cell-Based Therapy Technology Classifications and Translational Challenges, Philosophical Transactions of the Royal Society B: Biological Sciences, London: Royal Society, 370.
Mullin, E. (2019). A Year After Approval, Gene-Therapy Cure Gets Its First Customer - MIT Technology Review. MIT thechnology review. Available online at: https://www.technologyreview.com/s/604295/a-year-after-approval-gene-therapy-cure-gets-its-first-customer/ (accessed May 10, 2019).
Muroi, K, Miyamura, K, Okada, M, Yamashita, T, Murata, M, Ishikawa, T, et al. (2016). Bone marrow-derived mesenchymal stem cells (JR-031) for steroid-refractory grade III or IV acute graft-versus-host disease: a phase II/III study. Int. J. Hematol. 103, 243–50.
Napodano, J. (2015). aurix. Nuo Therapeutics, Inc. Available online at: http://s1.q4cdn.com/460208960/files/May-15-2015_NUOT_Napodano_v001_v296b7.pdf (accessed May 15, 2015).
Naver (2019). 콘드론 : 네이버 블로그. Available online at: https://m.blog.naver.com/azure0321/150107641730 (accessed Jun 23, 2019).
Neelapu, S. S., Locke, F. L., Bartlett, N. L., Lekakis, L. J., Miklos, D. B., Jacobson, C. A., et al. (2017). Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 377, 2531–2544.
Neurodegenerative Disease Market (2020). Growth, Trends, and Forecast (2019-2024). Available online at: https://www.mordorintelligence.com/industry-reports/neurodegenerative-disease-market (accessed May 24, 2020).
NICE (2019). 2 Information About Darvadstrocel | Darvadstrocel for Treating Complex Perianal Fistulas in Crohn’s Disease | Guidance | NICE. National Institute for Health and Care Excellence. Available online at: https://www.nice.org.uk/guidance/ta556/chapter/2-Information-about-darvadstrocel (accessed May 11, 2020).
Nordmeyer, S., Murin, P., Schulz, A., Danne, F., Nordmeyer, J., Kretzschmar, J., et al. (2018). Results of aortic valve repair using decellularized bovine pericardium in congenital surgery. Eur. J. Cardio. Thoracic Surg. 54, 986–992. doi: 10.1093/ejcts/ezy181
NOVARTIS (2019a). MEDIA RELEASE COMMUNIQUE AUX MEDIAS MEDIENMITTEILUNG AveXis receives Food And Drug Administration approval for Zolgensma®, the First and Only Gene Therapy for Pediatric Patients with Spinal Muscular Atrophy (SMA). Available online at: http://www.novartis.com (accessed Jun 23, 2019).
Novartis. (2019b). Novartis Receives Health Canada approval of its CAR-T cell therapy, KymriahTM (tisagenlecleucel) | Novartis Canada. Available online at: https://www.novartis.ca/en/news/media-releases/novartis-receives-health-canada-approval-its-car-t-cell-therapy-kymriah (accessed May 10, 2019).
Nuo Therapeutics Inc. (2019). Treatment For Tunneling, Sinus Tracts & More - Aurix System. Available online at: http://www.nuot.com/aurix-system/ (accessed May 10, 2019).
Oh, K.-W., Moon, C., Kim, H. Y., Oh, S., Park, J., Lee, J. H., et al. (2015). Phase I trial of repeated intrathecal autologous bone marrow-derived mesenchymal stromal cells in amyotrophic lateral sclerosis. Stem Cells Transl. Med. 4, 590–597. doi: 10.5966/sctm.2014-0212
Ophthalmic Drugs Market (2020). Growth, Trends, and Forecast (2019-2024). Available online at: https://www.mordorintelligence.com/industry-reports/global-opthalmic-drugs-market (accessed May 24, 2020).
Organogenesis Inc. (2019a). Canton, Mass / San Diego C. Organogenesis Secures $30 Million in Financing to Fund Aggressive Expansion of Commercial Operations and Product Portfolio | Organogenesis Holdings Inc. Available online at: https://investors.organogenesis.com/news-releases/news-release-details/organogenesis-secures-30-million-financing-fund-aggressive (accessed May 11, 2019).
Organogenesis Inc. (2019b). CANTON M. Organogenesis Names New Chief Commercial Officer and Chief Operating Officer | Organogenesis Holdings Inc. Available online at: https://investors.organogenesis.com/news-releases/news-release-details/organogenesis-names-new-chief-commercial-officer-and-chief (accessed May 11, 2019).
Organogenesis Inc. (2019c). Apligraf?: What is Apligraf? Available online at: http://www.apligraf.com/professional/what_is_apligraf/index.html (accessed May 10, 2019).
Organogenesis Inc. (2019d). About Dermagraft. Available online at: http://www.dermagraft.com/patient/about-dermagraft/ (accessed May 10, 2019).
Organogenesis Inc. (2020). apligraft. Available online at: http://www.apligraf.com/professional/pdf/PaymentRateSheetHospitalOutpatient.pdf (accessed May 5, 2020).
Orthocell (2018). (ASX: OCC) Growing with CelGro®. NDF research. Available online at: https://static1.squarespace.com/static/55d2ae4ce4b0e20eb51007ce/t/5aafccb4575d1ff97c019043/1521470660673/2018+03+20+Orthocell+report+from+NDF+Research_Final.pdf (accessed May 9, 2019).
Orthocell (2019). Cartilage Regeneration — Orthocell. Available online at: https://www.orthocell.com.au/ortho-aci/ (accessed May 9, 2019).
Orthopedic Devices Market (2020). Growth, Trends, and Forecasts (2020 - 2025). Available online at: https://www.mordorintelligence.com/industry-reports/global-orthopedic-devices-market-industry (accessed May 24, 2020).
Park, Y.-B., Ha, C.-W., Lee, C.-H., Yoon, Y. C., and Park, Y.-G. (2017). Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: results from a clinical trial for safety and proof-of-concept with 7 years of extended follow-Up. Stem Cells Transl. Med. 6, 613–621. doi: 10.5966/sctm.2016-0157
Pharmaceuticals and Medical Devices Agency (2019). List of Approved Products | Pharmaceuticals and Medical Devices Agency. Available online at: https://www.pmda.go.jp/english/review-services/reviews/approved-information/0002.html (accessed May 10, 2019).
Pharmicell Ltd. (2020). Available online at: http://www.pharmicell.com/ (accessed May 24, 2020).
PMDA (2011). Hata D S Ph D Ken ichiro Hata DDS KD. PMDA 4th International Symposium on Biologics PMDA 4th International Symposium on Biologics J-TEC’s Perspective: The Clinical Development of the Autologous cell/Tissue-Based Medicinal Products cell/Tissue Based Medicinal Products. Available online at: https://www.pmda.go.jp/files/000152967.pdf (accessed May 14, 2020).
Prasad, V. K., Lucas, K. G., Kleiner, G. I., Talano, J. A. M., Jacobsohn, D., Broadwater, G., et al. (2011). Efficacy and Safety of ex vivo cultured adult human mesenchymal stem cells (prochymaltm) in pediatric patients with severe refractory acute graft-versus-host disease in a compassionate use study. Biol. Blood Marrow Transplant 17, 534–541. doi: 10.1016/10.1016/j.bbmt.2010.04.014
Pricing of Approved Cell Therapy (2019). Available online at: https://bioinformant.com/price-of-cell-therapy-products/ (accessed May 9, 2019).
Rama, P., Matuska, S., Paganoni, G., Spinelli, A., De Luca, M., and Pellegrini, G. (2010). Limbal stem-cell therapy and long-term corneal regeneration. N. Engl. J. Med. 363, 147–155. doi: 10.1056/nejmoa0905955
Rattue, P. (2012). Prochymal–First Stem Cell Drug Approved. Medical News Today. Available online at: https://www.medicalnewstoday.com/articles/245704.php (accessed May 9, 2019).
Regrow Biosciences Pvt.Ltd. (2019). Available online at: https://www.regrow.in/ (accessed May 9, 2019).
Rosen, L. (2012). Gene Therapy Product Gets Green Light With More Comng Soon. Available online at: https://www.21stcentech.com/biomedicine-update-commercial-gene-therapy-treatments-starting-2013/ (accessed May 10, 2019).
Russell, S., Bennett, J., Wellman, J. A., Chung, D. C., Yu, Z.-F., Tillman, A., et al. (2017). Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65 -mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet 390, 849–860.
RxList (2018). Allocord (Cord Blood Injectable Suspension for Intravenous Use): Side Effects, Interactions, Warning, Dosage & Uses. Available online at: https://www.rxlist.com/allocord-drug.html (accessed May 9, 2019).
Safer (2019). Cancer Therapy Trials in India Show Promise. Available online at: http://www.delhidailynews.com/news/Safer-cancer-therapy-trials-in-India-show-promise-1400044111/ (accessed May 9, 2019).
Sang-jun, P. (2010). 자가세포 이용 흉터 치료제 ‘큐어스킨’ 출시 - 메디칼업저버. Medical observer. Available online at: http://www.monews.co.kr/news/articleView.html?idxno=36759 (accessed Jun 23, 2019).
Saris, D., Price, A., Widuchowski, W., Bertrand-Marchand, M., Caron, J., Drogset, J. O., et al. (2014). Matrix-applied characterized autologous cultured chondrocytes versus microfracture. Am. J. Sports Med. 42, 1384–1394. doi: 10.1177/0363546514528093
S-Biomedics Ltd. (2019a). 체세포 치료제 - S.BIOMEDICS [Internet]. Available online at: http://sbiomedics.com/체세포-치료제 (accessed May 10, 2019).
S-Biomedics Ltd. (2019b). (Cell therapy products) S.Biomedics Co., Ltd. View|Biological Products | Minisry of Food and Drug Safety. Available online at: https://www.mfds.go.kr/eng/brd/m_30/view.do?seq=71390 (accessed Jun 23, 2019).
Schlatter, S. (2019). Epicel Skin Grafts. Available online at: http://www.Food and Drug Administration.gov/cdrh/mda/docs/H990002.html (accessed May 10, 2019).
Schuster, S. J., Bishop, M. R., Tam, C. S., Waller, E. K., Borchmann, P., McGuirk, J. P., et al. (2019). Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N. Engl. J. Med. 380, 45–56.
SCT (2019). Stem-art. Available online at: http://www.stem-art.com/Library/Miscellaneous/SCT products Sheet 1.pdf (accessed April 5, 2019).
Senior, M. (2017). After Glybera’s withdrawal, what’s next for gene therapy? Nat. Biotechnol. 35, 491–492. doi: 10.1038/nbt0617-491
Seoane- Vazquez, E., Shukla, V., and Rodriguez- Monguio, R. (2019). Innovation and competition in advanced therapy medicinal products. EMBO Mol Med. 11:3.
Sewon Cellontech Ltd. (2019a). (Cell therapy products) Sewon Cellontech Co., LTD. View|Biological Products | Minisry of Food and Drug Safety. Available online at: https://www.mfds.go.kr/eng/brd/m_30/view.do?seq=70954&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=1 (accessed Jun 23, 2019).
Sewon Cellontech Ltd. (2019b). (Cell therapy products) Sewon Cellontech Co., LTD. View|Biological Products | Minisry of Food and Drug Safety. Available online at: http://www.mfds.go.kr/eng/brd/m_30/view.do?seq=70954 (accessed May 9, 2019).
Sharma, V. (2019). Disappointing End For MolMed’s Zalmoxis Cell Therapy In EU?:: Pink Sheet. Pharmaintelligence. Available online at: https://pink.pharmaintelligence.informa.com/PS140998/Disappointing-End-For-MolMeds-Zalmoxis-Cell-Therapy-In-EU (accessed Jan 13, 2020).
Sibiono (2019). Weblet Importer. Available online at: http://www.sibiono.com/en/index.aspx (accessed May 10, 2019).
SinaCell Enterprise Knowledge Management (2019). Home English - SinaCell Co. Available online at: http://sinacellco.com/?lang=en (accessed May 10, 2019).
Soft Tissue Repair Market (2020). Growth, Trends, and Forecast (2019-2024). Available online at: https://www.mordorintelligence.com/industry-reports/soft-tissue-repair-market (accesed May 24, 2020).
Spark Therapeutics Inc. (2019). Spark Therapeutics Reports Second Quarter 2018 Financial Results and Recent Business Progress | Spark Therapeutics Inc. – IR Site. Available online at: http://ir.sparktx.com/news-releases/news-release-details/spark-therapeutics-reports-second-quarter-2018-financial-results (accessed May 11, 2019).
Specialist pharmecy service (2020). Darvadstrocel – Medicines – SPS - Specialist Pharmacy Service – The First stop for Professional Medicines Advice. Specialist Pharmecy Service. Available online at: https://www.sps.nhs.uk/medicines/darvadstrocel/ (accessed May 11, 2020).
Startseite. (2020). Startseite. Available from: https://www.codon.de/. (accessed on Nov 19, 2020).
Stem Cell Research (2019). Stem Cell Blog - Cell Therapy 2016 – Year in Review (part 1) | Cell Trials. Available online at: http://celltrials.info/2016/12/31/cell-therapy-2016-year-review-1/ (accessed May 10, 2019).
Stempeutics Research Pvt Ltd. (2019). Stem Cell, stempeucel, stempeutron, stempeucare, USA Patents, Cuticera, Manipal Group, Cipla. Available online at: http://www.stempeutics.com/ (accessed May 9, 2019).
StreetInsider (2015). Mesoblast’s (MESO) Japan Licensee Receives Pricing for TEMCELL HS Inj. For Treatment of aGVHD. Streetinsider. Available online at: https://www.streetinsider.com/Corporate+News/Mesoblasts+%28MESO%29+Japan+Licensee+Receives+Pricing+for+TEMCELL+HS+Inj.+For+Treatment+of+aGVHD/11109517.html (accessed May 14, 2020).
Sun-kyu, K. (2016). 더벨 - 국내 최고 자본시장 (Capital Markets) 미디어. Available online at: http://www.thebell.co.kr/free/content/ArticleView.asp?key=201602030100007350000448&lcode=00 (accessed Jun 23, 2019).
SunWay Biotech (2019). 上海三维生物技术有限公司. Available online at: http://www.sunwaybio.com.cn/en/product.html (accessed May 10, 2019).
Taylor and Francis Group (2015). cardiocel. Available online at: https://www.tandfonline.com/doi/full/10.1586/17434440.2015.985651?scroll=top&needAccess=true (accessed April 10, 2015).
Tego Science (2019a). 로스미르® CELL THERAPY?:: TECO SCIENCE [Internet]. Available online at: http://www.tegoscience.com/kor/product/view.do?prdSeq=13 (accessed May 10, 2019).
Tego Science (2019b). 홀로덤® CELL THERAPY?:: TECO SCIENCE [Internet]. Available online at: http://www.tegoscience.com/kor/product/view.do?prdSeq=7 (accessed May 10, 2019).
Tego Science (2019c). 칼로덤® CELL THERAPY?:: TECO SCIENCE [Internet]. Available online at: http://www.tegoscience.com/kor/product/view.do?prdSeq=12 (accessed May 10, 2019).
Tego Science (2019d). 본 자료는 제안된 IPO 공모와 관련하여 기관투자가와 일반투자자들을 대상으로 실시되는 PRESENTATION 에서의 정보제공을 목적으로 테고사이언스㈜. [Internet]. INVESTOR RELATIONS 2. Available online at: http://w3.kirs.or.kr/download/broadcast/테고사이언스_IR Book.pdf (accessed May 11, 2019).
Tego Science (2019e). (Cell therapy products) Tego Science, Inc View|Biological Products | Minisry of Food and Drug Safety. Available online at: http://www.mfds.go.kr/eng/brd/m_30/view.do?seq=71359 (accessed May 10, 2019).
Tego Science (2020a). 홍보 < PR/IR?:: TECO SCIENCE. Available online at: http://www.tegoscience.com/kor/board/view.do?seq=309 (accessed May 15, 2020).
Tego Science (2020b). (Cell therapy products) Tego Science, Inc View|Biological Products | Minisry of Food and Drug Safety. Available online at: https://www.mfds.go.kr/eng/brd/m_30/view.do?seq=71359 (accessed May 15, 2020).
Ten Ham, R. M. T., Hoekman, J., Hövels, A. M., Broekmans, A. W., Leufkens, H. G. M., and Klungel, O. H. (2018). Challenges in advanced therapy medicinal product development: a survey among companies in Europe. Mol. Ther. Methods Clin. Dev. 11, 121–130. doi: 10.1016/j.omtm.2018.10.003
Timmerman, L. (2010). Xconomy: Dendreon Sets Provenge Price at $93,000, Says Only 2,000 People Will Get it in First Year. Available online at: https://xconomy.com/seattle/2010/04/29/dendreon-sets-provenge-price-at-93000-says-only-2000-people-will-get-it-in-first-year/ (accessed May 9, 2019).
Tohyama, H., Yasuda, K., Minami, A., Majima, T., Iwasaki, N., Muneta, T., et al. (2009). Atelocollagen-associated autologous chondrocyte implantation for the repair of chondral defects of the knee: a prospective multicenter clinical trial in Japan. J. Orthop. Sci. 14, 579–588. doi: 10.1007/s00776-009-1384-1
Vericel Corporation (2019). Seeking Alpha. Available online at: https://seekingalpha.com/filing/4373677 (accessed May 11, 2019).
Viganò, M., Budelli, S., Lavazza, C., Montemurro, T., Montelatici, E., De Cesare, S., et al. (2018). Tips and tricks for validation of quality control analytical methods in good manufacturing practice mesenchymal stromal cell production. Stem Cells Int. 2018:3038565.
Woo, S. J. (2007). 약업닷컴 (약업신문) - 뉴스 :: 민ㆍ관 협력 항암제 개발 개가 [Internet]. Available online at: http://m.yakup.com/?m=n&mode=view&nid=90918 (accessed May 9, 2019).
Yeh, A. C., Khan, M. A., Harlow, J., Biswas, A. R., Akter, M., Ferdous, J., et al. (2018). Hematopoietic stem-cell transplantation in the resource-limited setting: establishing the first bone marrow transplantation unit in bangladesh. J. Glob. Oncol. 4, 1–10. doi: 10.1007/978-3-319-59358-6_1
Yim, H., Yang, H. T., Cho, Y. S., Seo, C. H., Lee, B. C., and Ko, J. H. (2011). Clinical study of cultured epithelial autografts in liquid suspension in severe burn patients. Burns 37, 1067–1071. doi: 10.1016/j.burns.2011.03.018
Yoon, J., Yang, H.-T., Yim, H., Cho, Y.-S., Kym, D., and Hur, J. (2017). Effectiveness and safety of a thermosensitive hydrogel cultured epidermal allograft for burns. Adv Skin Wound Care 30, 559–564. doi: 10.1097/01.asw.0000526882.14740.01
You, H.-J., Han, S.-K., Lee, J.-W., and Chang, H. (2012). Treatment of diabetic foot ulcers using cultured allogeneic keratinocytes-A pilot study. Wound Repair Regen 20:334.
Zalmoxis (2016). Available online at: https://www.ema.europa.eu/en/medicines/human/EPAR/zalmoxis (accessed September 5, 2016).
Keywords: advanced therapy medicinal products, ATMP, safety, efficacy, market size
Citation: Ramezankhani R, Torabi S, Minaei N, Madani H, Rezaeiani S, Hassani SN, Gee AP, Dominici M, Silva DN, Baharvand H and Hajizadeh-Saffar E (2020) Two Decades of Global Progress in Authorized Advanced Therapy Medicinal Products: An Emerging Revolution in Therapeutic Strategies. Front. Cell Dev. Biol. 8:547653. doi: 10.3389/fcell.2020.547653
Received: 31 March 2020; Accepted: 16 September 2020;
Published: 17 December 2020.
Edited by:
Marcela F. Bolontrade, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), ArgentinaReviewed by:
Patrick Celis, European Medicines Agency, United KingdomCopyright © 2020 Ramezankhani, Torabi, Minaei, Madani, Rezaeiani, Hassani, Gee, Dominici, Silva, Baharvand and Hajizadeh-Saffar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ensiyeh Hajizadeh-Saffar, SGFqaXphZGVoLmVoc0Byb3lhbmluc3RpdHV0ZS5vcmc=; aGFqaXphZGVoLmVoc0BnbWFpbC5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.