
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell Dev. Biol. , 19 November 2020
Sec. Stem Cell Research
Volume 8 - 2020 | https://doi.org/10.3389/fcell.2020.545126
This article is part of the Research Topic Current Progress in Mesenchymal Stem/Stromal Cell Research View all 18 articles
 Armel H. Nwabo Kamdje1*
Armel H. Nwabo Kamdje1* Paul F. Seke Etet1,2
Paul F. Seke Etet1,2 Richard Simo Tagne1
Richard Simo Tagne1 Lorella Vecchio2
Lorella Vecchio2 Kiven Erique Lukong3
Kiven Erique Lukong3 Mauro Krampera4
Mauro Krampera4The role of mesenchymal stromal cells (MSCs) in the tumor microenvironment is well described. Available data support that MSCs display anticancer activities, and that their reprogramming by cancer cells in the tumor microenvironment induces their switch toward pro-tumorigenic activities. Here we discuss the recent evidence of pro-tumorigenic effects of stromal cells, in particular (i) MSC support to cancer cells through the metabolic reprogramming necessary to maintain their malignant behavior and stemness, and (ii) MSC role in cancer cell immunosenescence and in the establishment and maintenance of immunosuppression in the tumor microenvironment. We also discuss the mechanisms of tumor microenvironment mediated reprogramming of MSCs, including the effects of hypoxia, tumor stiffness, cancer-promoting cells, and tumor extracellular matrix. Finally, we summarize the emerging strategies for reprogramming tumor MSCs to reactivate anticancer functions of these stromal cells.
Mesenchymal stromal cells (MSCs) are multipotent stem cells capable of differentiating into various cell types of the mesodermal lineage, including adipocytes, endothelial cells, fibroblasts, chondrocytes, osteoblasts, and myocytes (Dominici et al., 2006), and possibly into non-mesodermal cell types, such as neural, pancreatic, hepatic, and gastric cells (Oswald et al., 2004; Cislo-Pakuluk and Marycz, 2017; Luo et al., 2020; Xuan et al., 2020). They are a heterogeneous mesenchymal cell population, which resides in the stroma of various tissues and organs and expressing the membrane markers CD105, CD73, and CD90, but not HLA-DR, CD14, CD19, CD31, CD34, and CD45 (Dominici et al., 2006; Nwabo Kamdje et al., 2011). MSCs are a key tool in tissue engineering and regenerative medicine, because they are easily collected and thanks to their ability to migrate and home into damaged tissues where they (i) interact with the microenvironment to drive tissue repair; (ii) transdifferentiate into new cells to restore and/or replace damaged tissues; (iii) rescue organ functions, thanks to their high proliferation, adhesion, migration, differentiation, and immunoregulatory properties (Barberini et al., 2014; Chi et al., 2014; Li et al., 2015). MSC properties are mainly dependent of components of their secretome including numerous factors favoring tissue repair, such as angiopoietin-1 (Ang1), vascular epidermal growth factor (EGF), endothelial growth factor (VEGF), transforming growth factor-beta (TGF-β), hepatocyte growth factor (HGF), fibroblast growth factor (FGF), granulocyte-colony stimulating factor (G-CSF), platelet-derived growth factor (PDGF), interleukin 6 (IL-6) and IL-12, chemokine (C-C motif) ligand 7 (CCL7) and CCL25, and C-X-C motif chemokine 8 (CXCL8), CXCL9, CXCL16, and CCL20 (Ishiki et al., 1992; Li et al., 2013; Wang et al., 2013; Liu et al., 2014; Windmolders et al., 2014; Sesia et al., 2015; Rolandsson Enes et al., 2016; Tsiklauri et al., 2018; Gyukity-Sebestyen et al., 2019; Ozdemir et al., 2019).
However, MSCs also reside in the tumor microenvironment, where they were reported to promote pivotal tumorigenic processes such as: (i) malignant transformation; (ii) cancer cell maintenance and stemness; (iii) cancer stem cell niche formation, including angiogenesis and neovascularization; (iv) metastasis formation; and (v) resistance to anticancer drugs [for review see Seke Etet et al. (2013), Nwabo Kamdje et al. (2014), Atiya et al. (2020), Osman et al. (2020)]. On the other hand, MSC-derived stromal cells restraining cancer growth have been reported in the tumor microenvironment (Bu et al., 2019; Mizutani et al., 2019; Tew et al., 2019) and growing evidence supports that the pro-tumorigenic effects of MSCs emerge from cell reprogramming by the tumor microenvironment (Coffman et al., 2019; Mandal et al., 2019; Al-Jawadi et al., 2020; Boada et al., 2020). Herein, we provide an overview and discuss emerging data supporting MSC reprogramming by the tumor microenvironment and recent reports supporting the existence of stromal cells restraining cancer growth in the tumor microenvironment.
Some of the most reported pro-tumorigenic effects of MSCs include their roles in the metabolic and cellular senescence-like changes, typically observed in various cancers and in the tumor microenvironment-mediated immunosuppression (Figure 1).
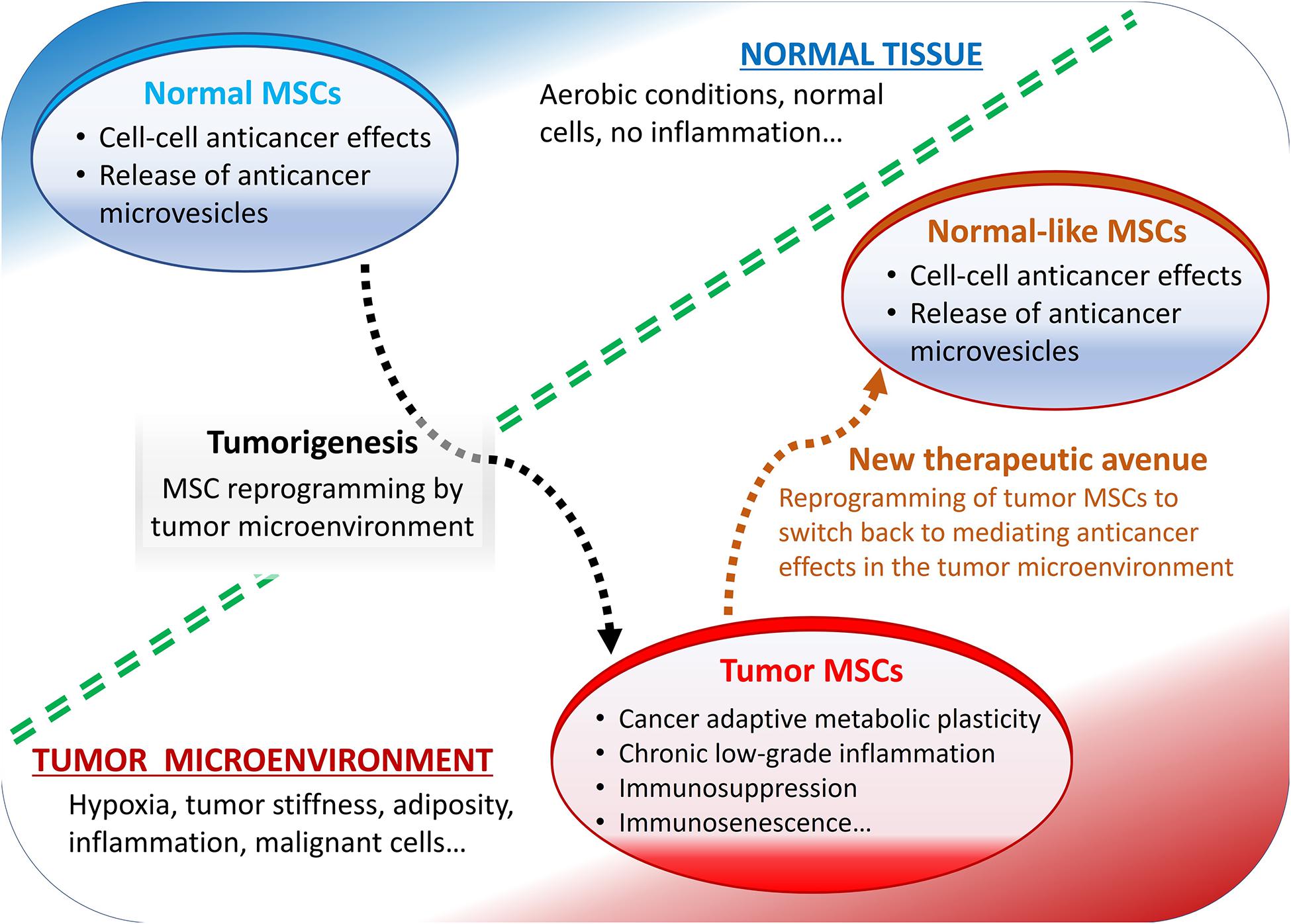
Figure 1. Summary of mesenchymal stromal cells (MSCs) role in the tumor microenvironment. Normally, MSCs mediate anticancer effects but reprogrammed by tumor microenvironment they display pro-tumorigenic effects.
The Warburg effect, a metabolic hallmark of tumor cells, is the fact that cancer cells produce most of their ATP via glycolysis, even under aerobic conditions, although it is a less efficient pathway compared to oxidative phosphorylation and despite their greater need for energy [for review see Xu et al. (2015), Fu et al. (2017)]. For instance, pancreatic cancer cells can utilize “metabolic reprogramming,” through the enhancement of glycolysis with increased lactate production and glycolytic enzyme overexpression, to satisfy their energy demand and support malignant behavior, despite a hypoxic and nutrient-deficient microenvironment (Yang et al., 2020). Growing evidence supports that stromal cells trigger the Warburg effect in cancer cells. For instance, the bone marrow (BM)-derived MSCs co-cultured with leukemia cells under normoxic conditions revealed reduced mitochondrial membrane potential and pyruvate metabolism in the both cell types (Samudio et al., 2008). Interestingly, mitochondrial membrane potential reduction was mediated in leukemia cells by an uncoupling protein 2 (UCP2)-dependent mechanism, suggesting that MSCs facilitated the Warburg effect in cancer cells by activating highly conserved mammalian UCPs. On the same hand, a study carried out on bevacizumab-resistant glioblastoma, suggested that inside the hypoxic microenvironment, chemoresistance in cancer cells occurs through: (i) metabolic reprogramming, characterized with suppressed oxidative phosphorylation and upregulated glycolysis; (ii) perivascular invasiveness along remaining blood vessels in a VEGF- and neo-angiogenesis-independent manner; and (iii) enrichment of tumor-initiating stem cells residing in the perivascular niche close to residual blood vessels (Chandra et al., 2020). Such observations are the basis of studies aiming at targeting signaling molecules pivotal for cancer cell glycolysis [for review see Xu et al. (2015)].
An early study aimed at determining whether the Warburg effect is due mainly to the hypoxic microenvironment, or to inherent metabolic alterations in transformed MSCs, revealed that aerobic glycolysis results from MSC oncogenic adaptation to bioenergetic requirements. Authors observed that in some circumstances, transformed MSCs may also rely on increased in oxidative phosphorylation (Funes et al., 2007). However, the study revealed a reversible increase in the transcription of glycolytic enzymes genes, in tumors generated by transformed MSCs, indicating a metabolic support of MSCs for surrounding cells of the tumor to its microenvironment. Similar observations led to the development of a new hypothesis for stromal cell support to cancer cell metabolism, the reverse Warburg effect [for review see Xu et al. (2015), Fu et al. (2017)]. Basically, cancer cells induce oxidative stress in neighboring stromal cells such as MSC-derived fibroblasts by secreting reactive oxygen species (ROS). In stromal cells, ROS trigger aerobic glycolysis and the production of lactate, pyruvate, and other high energy metabolites. Finally, the latter are transported to adjacent cancer cells where they sustain the energy need and various signaling pathways driving tumor progression, metastasis, and chemoresistance (Fu et al., 2017). This new approach focused on tumor cell metabolism, pointed out many targets in tumor microenvironment and cancer cell machinery for anticancer therapy.
A wealth of other reports also supports the involvement of stromal cells in the metabolic changes supporting tumorigenic processes. For instance, In breast cancer, depending on ROS, hypoxia, and glucose availability in the microenvironment, tumor-initiating cells are capable to switch toward oxidative phosphorylation and glycolysis. This adaptative metabolic switch is controlled at least in part by stromal cells to confer a survival advantage to malignant cells (Walsh et al., 2019). Lung et al. (2019) reported that the expression of estrogen receptor-α (ER), the target of endocrine therapies in breast cancer, is regulated by the BM microenvironment. In this study, the induction of ESR1 mRNA and ER protein downregulation, through a MAPK-independent mechanism, was achieved by the treatment of breast cancer cells with conditioned culture media from either cancer-activated BM stromal cells or HS5 BM stromal cell line (Lung et al., 2019). In addition, thyroid hormones, which are well-established pro-tumorigenic players, may stimulate tumor growth and neovascularization in various solid cancers by activating MSCs through a non-classical integrin αvβ3 signaling (Schmohl et al., 2019). Moreover, the EGF-like superfamily member EGFL6 would promote tumor growth by mediating a cross-talk between stromal and cancer cells that would contribute to stemness and epithelial-mesenchymal transition (EMT), an important tumorigenic mechanism where epithelial cells become MSCs by losing their cell polarity and adhesion ability, and by gaining migratory and invasive properties (An et al., 2019). Altogether, these observations confirm that targeting cancer cell energy metabolism is still a sound anticancer strategy, and point out stromal cells as major players in cancer cell energy metabolism.
Immunosuppression and immunosenescence are two major immunological phenomena observed in the tumor microenvironment. Like aging processes, cancers environment is characterized by a chronic inflammation (“inflammaging”) and cellular senescence (“immunosenescence”). The role of stromal cells’ immunomodulation in shaping a senescent microenvironment in broad spectrum of human malignancies, especially tumorigenesis, has been documented extensively [for review see Salminen et al. (2020), Thomas et al. (2020)]. For instance, BM stromal cells from patients with myelodysplastic syndrome display a senescence phenotype induced by S100A9-induced Toll-like receptor 4 (TLR4), NLRP3 inflammasome activation and IL-1β secretion (Shi et al., 2019). Senescent breast luminal cells promoted carcinogenesis by activating MSC-derived fibroblasts through the inflammatory cytokine IL-8 (Al-Khalaf et al., 2019). Acute myeloid leukemia (AML) blasts induced a senescence-associated secretory phenotype (SASP) in BM stromal cells through a p16INK4a-dependent mechanism, which encompassed the irreversible arrest of cell proliferation and the secretion of a set of chemokines, proinflammatory cytokines, and growth factors (Abdul-Aziz et al., 2019). MSCs promoted the progression of gastric cancer cells through the release of CXCL16, which activates STAT3-mediated expression of Ror1 in the cancer cells (Ikeda et al., 2020). In oral mucosa carcinogenesis, MSCs increased immunosuppressive functions on T cell proliferation and pro-tumorigenic effects of tumor resident MSCs correlated with higher expressions of cellular proliferative status indicator Ki67 (Chen et al., 2019a). STAT4 over-expression in gastric cancer cells made normal fibroblasts acquire cancer-associated fibroblast (CAF)-like features through wnt/β-catenin-dependent signaling (Zhou et al., 2019a). A recent study using MSCs expanded from BM and prostate cancer tissue from independent donors showed that tumor-infiltrating MSCs are major drivers of the immunosuppressive tumor microenvironment in prostate cancer (Krueger et al., 2019). The authors reported the ability of prostate cancer-infiltrating MSCs to suppress T-cell proliferation through immunosuppressive properties comparable to canonical BM-derived MSCs. The suppression of proliferation mediated by prostate cancer-infiltrating MSCs was dose-dependent, and the expressions of PD-L1 and PD-L2 were upregulated on T-cells in the presence of IFN-γ and TNF-α (Krueger et al., 2019). In another study, the transcriptome analysis of MSCs from multiple myeloma patients revealed constitutive abnormalities in immune system activation, cell cycle progression, and osteoblastogenesis that were maintained even in the absence of tumor cells, thus strongly suggesting that MSCs may contribute to the immune evasion and bone lesions frequently found in multiple myeloma (Fernando et al., 2019). Altogether, these observations also point out stromal cells as major players in tumorigenesis and reveal more targets for pharmacological anticancer therapy.
Although it is well-established that MSCs are major drivers of the immunomodulation observed in solid tumor microenvironment, many others cell components can cooperate with MSCs to modulate immune response. In AML, CXCL8 supports the survival and proliferation of leukemic cells via the PI3K/AKT signaling pathway. In the affected BM microenvironment CXCL8 is mainly secreted by MSCs (Cheng et al., 2019). Study on other bone marrow disorders show that and MSCs to shape the Microenvironment at least partly by inducing suppressive monocytes and dampening NK cell functions (Sarhan et al., 2020).
A growing body of evidence supports that stromal cells follow the program dictated by their microenvironment.
Early studies addressing the composition of the tumor microenvironment, reported an atypical cellular and molecular microenvironment supporting carcinogenesis and chemoresistance (Garcia-Hernandez et al., 2017; Pelizzo et al., 2018). Recently, Coffman et al. (2019) reported that ovarian carcinoma-associated MSCs, which are critical stromal progenitor cells promoting tumor cell growth, cancer stemness and chemoresistance, arose from a process of tumor-mediated reprogramming of local tissue MSCs. This study also provided strong evidence that tumor-mediated MSC conversion is tissue- and cancer-type dependent, requiring tumor-secreted factors and hypoxia (Coffman et al., 2019). In other studies, gene expression signatures and mesenchymal shift in quiescent glioblastoma cells, a source of tumor recurrence in highly malignant glioblastoma, was observed following their interactions with niche microenvironment (Tejero et al., 2019). Breast tumor microenvironment transformed naive MSCs into tumor-forming cells in nude mice. Indeed, MSCs pre-exposed to conditioned medium or purified exosomes derived from breast cancer cells (MDA-MB-231) formed a tumor-like mass rich in stromal tissue by 14 weeks when injected into mammary glands of nude mice (Worner et al., 2019). Similarly, CCL5 secreted by classic Hodgkin lymphoma cells recruited MSCs and monocytes, enhancing MSC proliferation and CCL5 secretion. Conditioned medium from these educated MSCs increased tumor cell growth and monocyte migration (Casagrande et al., 2019). Similarly TLR4 signaling educated MSCs to promote tumor microenvironment transformation in multiple myeloma (Giallongo et al., 2019).
Exosomes including extracellular vesicles (EVs) represent a mean used by tumor cells to educate MSCs in the microenvironment. In chronic myeloid leukemia (CML), leukemia cells altered the cellular and immune-related properties of BM-MSCs and macrophages in vitro by the mean of exosomes (Jafarzadeh et al., 2019). Consistently, Zannoni et al. (2019) reported that EVs released by monocytes from chronic myelomonocytic leukemia patients conferred a procoagulant state favorable for cancer progression, through a tissue factor-dependent mechanism mediated by MSCs. In glioma, exosomes from cancer cells induced a tumor-like phenotype in MSCs by activating glycolysis (Ma et al., 2019). In gastric cancer, tumor cell-derived exosomes affected the immunomodulatory functions of MSCs by activating the NF-kB signaling pathway, which in turn mediates support to tumor growth by maintaining the inflammatory environment and enhancing the ability of MSCs to activate immune cells (Shen et al., 2019).
In addition, pre-metastatic niche in distant organs may be created, at least in part, by the transfer of EVs secreted by tumor-associated macrophages (TAMs) to stromal cells, such as fibroblasts, peritoneal mesothelial cells (PMCs), and endothelial cells (Umakoshi et al., 2019). Long-term culture of human MDA-MB-231 breast cancer cells with normal human MSCs was associated with the formation of three-dimensional (3D) tumor spheroids in vitro, with a 14-fold enhanced expression of the breast tumor marker urokinase plasminogen activator (uPA; Melzer et al., 2019). Similarly, MSCs cultured with colorectal cancer cells showed increased invasiveness and proliferative abilities due to increased TGF-β1 and decreased p53 levels (Oh et al., 2020). In another study, TGF-β1 promoted the migration and invasion of HCT116 and HT29 colorectal cancer cells, and induced the differentiation of MSCs into CAFs through a JAK/STAT3 signaling-depended mechanism (Tan et al., 2019).
The available data also support detrimental cross-talks between stromal and cancer cells. For instance, reciprocal reprogramming of cancer stem cells (CSCs) and associated MSCs may promote tumor progression in gastric cancer (Shamai et al., 2019). Similarly, asporin, a factor secreted by MSCs following cellular interactions within the tumor microenvironment, altered the tumor microenvironment and inhibited MSC differentiation to drive metastatic progression through CD49d/CD29 signaling (Hughes et al., 2019). Moreover, Dabbah et al. (2019) reported that microvesicles derived from BM MSCs of multiple myeloma patients increased the tumorigenicity of MM cells (Hughes et al., 2019). In this study, CD49d and CD29 integrin overexpression in MM-MSC microvesicles were associated with patient staging and response to treatment. The concomitant inhibition of these molecules resulted in reduced uptake of EVs by MM-MSC (but not normal donor MSC microvesicles), and downregulation of aggressiveness markers, thus enhancing response to chemotherapy (Dabbah et al., 2019). Interestingly, this study also suggested that the reciprocal interactions of malignant cells and MSCs in breast cancer microenvironment may result in the transformation of naive MSCs into cells capable of forming explants in nude mice (Dabbah et al., 2019).
Overall, together with evidence of MSC role in cancer metabolism discussed in section 1.1, these data suggest that tumorigenesis is triggered and driven by a bidirectional cross-talks between MSCs and tumor environment. Therefore, unraveling the signaling molecules involved in these pro-tumorigenic cross-talks may lead to the identification of novel targets for anti-cancer therapy. Promising reports using this approach include a recent study that addressed the potential roles and mechanisms of long non-coding RNAs in EMT and in the maintenance of CSC-like properties in non-small cell lung cancer (NSCLC). Using A549 and H1299 human NSCLC cell lines, L9981 and 95D highly metastatic NSCLC cell lines, and NL9980 and 95C low-metastatic NSCLC cell lines., the authors observed that knockdown of long non-coding RNA linc-ITGB1 inhibited the expression of various markers of cancer stemness and CSC formation by reducing the expression of the EMT-related transcription factor Snail. Rewardingly, the overexpression of Snail reversed the inhibitory effects of linc-ITGB1 knockdown (Guo et al., 2019). Further studies should characterize and target the signaling pathways supporting the reprogramming of stromal cells by cancer cells, as well as other interactions between these cells that support tumorigenesis.
Tumor stiffness and hypoxia are key conditions of the solid tumor microenvironment, known to promote tumor survival, progression and metastasis. Hypoxia-driven phosphorylated glycoprotein such as osteopontin, promoted stem cell-like properties and EMT in pancreatic cancer cells in a paracrine manner, through integrin αvβ3-Akt/Erk- forkhead box protein M1 (FOXM1) signaling (Cao et al., 2019). Hypoxia-induced EMT was observed in non-small-cell lung cancer (Chen et al., 2019b) where hypoxia induced the acquisition of cancer stem cell features through CXCR4 activation (Kang et al., 2019). In fact earlier reports pointed out HIF-1 as a link between hypoxia, inflammation, and cancer [for review see Balamurugan (2016), Shi et al. (2018)]. Growing evidence suggests that stromal cells mediate the pro-tumorigenic effects of hypoxia and tumor stiffness. Notably, MSC-derived CAFs were suggested as the link between biophysical forces and pro-metastatic signaling in colon cancer, as they respond to increased stiffness of the tumor microenvironment by activating TGF-β family members and the signaling of the strong pro-metastatic cytokine activin A (Bauer et al., 2020). On the same hand, microvesicles derived from human BM-MSCs supported human osteosarcoma (U2OS) cell growth under hypoxia conditions both in vitro and in vivo through PI3K/AKT and HIF-1α-dependent mechanisms (Lin et al., 2019). In addition, interactions of cancer cells and stromal cells in hypoxic microenvironment were found to drive EMT through NOTCH and c-MET signaling, inducing an immunosuppressive response within the microenvironment in pancreatic ductal adenocarcinoma (PDA; Daniel et al., 2019).
In a study addressing the end-stage of myeloma cell mobilization from the BM into peripheral blood (PB), hypoxic BM niches, together with a pro-inflammatory microenvironment resulting from the interactions between tumor cells and BM stromal cells, were able to induce an arrest in proliferation, thus forcing tumor cells to circulate into the peripheral blood to seek other BM niches (Garces et al., 2020). These observations suggest that hypoxic BM niches are key players in metastatic processes. In agreement with this view, it has been observed in an in vivo mouse syngeneic tumor model, that hypoxic BM stromal cells-derived exosomal miRNAs promoted metastasis of lung cancer cells via STAT3-induced EMT (Zhang et al., 2019a,b). Consistently, Saforo et al. (2019) described an in vitro cell culturing system incorporating elements of the in vivo lung environment, including physiological hypoxia (5% O2) and lung fibroblast-derived extracellular matrix. Through this culture system, a rapid expansion of stromal progenitors from patient’s lung tumor resections was achieved. These progenitor cells retained the secretion of factors associated with cancer progression, the expression of pluripotency markers, and the ability to enhance tumor cell growth and metastasis (Saforo et al., 2019). The ability of hypoxia-conditioned MSCs to promote cancer progression was also observed in hepatocellular carcinoma but the effect was dependent of yes-associated protein (YAP)-mediated lipogenesis reprogramming (Liu et al., 2019). In Glioblastoma, the glioblastoma stem-like cells (GSCs) phenotype, the worst prognostic marker of Glioblastoma, was reported to persist due to hypoxic microenvironment-dependent release of extracellular adenosine, which in turn, promote cell migration, invasion and tumor recurrence through the activation of the A3 Adenosine Receptor (A3AR; Torres et al., 2019).
Altogether, these data suggest that hypoxia, tumor stiffness, and inflammation are among the major drivers of the pro-tumorigenic reprogramming of stromal cells in the tumor microenvironment.
Emerging data strongly suggest that the tumor extracellular matrix (ECM) also contributes to tumor microenvironment effects on stromal cells. For example, after showing that multiple myeloma (MM) cells, cultured with BM-MSCs, co-modulated the phenotype of MM cells in a MAPKs/translation initiation (TI)-dependent manner, Ibraheem et al. (2019) reported that even the decellularized ECM of BM-MSCs from MM patients was able to induce comparable pro-tumorigenic effects (Ref). A number of changes in microRNAs was shown to affect MM phenotype and the activation of MAPK/TI, EMT, proliferation, and CXCR4, with a role for BM-MSCs secretome and microvesicles. On the other hand, the decellularized ECM of BM-MSCs from normal donors mediated anticancer effects, including a rapid and persistent decrease in MAPK/TI activation, proliferation, cell count, viability, migration, and invasion (Ibraheem et al., 2019). These authors also provided evidence of a synergism between the ECM and microvesicles in the modulation of MM cell response to chemotherapy as well as in the hierarchy and interdependence of MAPKs/TI/autophagy/phenotype cascade. These observations suggested that to reprogram MSCs for pro-tumorigenic effects, the ECM also needs to be reprogrammed by cancer-promoting cells. For example, senescent MSCs actively remodeled the surrounding ECM to drive breast cancer cells to a more-invasive phenotype (Ghosh et al., 2020). Consistently, matrix metalloproteinase-9 (MMP-9) produced by leukemia cells facilitated tumor progression via remodeling of the ECM of the BM microenvironment. This is supported by the fact that MMP-9-deficiency in the BM microenvironment reduced leukemia-initiating cells and prolonged survival of mice with BCR-ABL1-positive B-cell acute lymphoblastic leukemia (B-ALL; Verma et al., 2020).
Three-dimensional culture studies with cancer and stromal cells in ECM and multiplex quantitative analysis method, reprensent majors tool to tacle signaling molecules and mechanisms used by reprogrammed ECM to drive MSC pro-tumorigenic effects, (Hwang et al., 2019; Maliszewska-Olejniczak et al., 2019). Therefore, a recent study using such approach in hepatocellular carcinoma (HCC), showed that cell repopulation of cirrhotic scaffolds displayed a unique up-regulation of genes related to EMT and TGF-β signaling as well as high concentration of endogenous TGF-β1 in comparison to healthy scaffolds and TGF-β1-induced phosphorylation of canonical proteins Smad2/3 (Mazza et al., 2019). This study characterized the inherent features of ECM microenvironment from human cirrhotic liver acting as key pro-tumorigenic components in HCC development.
It is well-established that fat tissue overgrowth in obesity promotes tumor progression [for reviews of earlier reports see Park et al. (2014), Iyengar et al. (2016), Quail and Dannenberg (2019)]. Using a xenograft model of early multiple myeloma, it has been shown that bone niche switching towards a “fatty” marrow supports the development of malignant cells during carcinogenesis. In this study, MSCs mainly gave rise to adipocytes supporting tumor growth by increasing the survival and chemoresistance of malignant cells (Berlier et al., 2019). Su et al. (2019) compared lean and obese mice grafted with prostate tumors and showed that obesity promotes EMT in cancer cells and tumor invasion into the surrounding fat tissue. In this study, adipose stromal cells induced EMT in prostate cancer cells and rendered them more migratory and chemo-resistant. By contrast, interference of adipose stromal cell capabilities suppressed both EMT and chemoresistance to docetaxel, cabazitaxel, and cisplatin chemotherapy in human prostate cancer cells (Su et al., 2019). It has been suggested that that adipose-derived factors may play a role in MSC-mediated pro-tumorigenic effects. For instance, the adipokine chemerin, a cell differentiation promoter and leukocyte chemoattractant factor established as a major player in obesity-mediated support of cancer progression, was reported to promote the growth, proliferation migration, invasion, and metastasis of cancer cells. The effects of the adipokine chemerin were achieved through the recruitment of tumor-associated MSCs and the stimulation of angiogenesis pathways in endothelial cells through chemerin receptor 1 (CMKLR1), chemerin receptor 2 (GPR1), and CCLR2 signaling (Goralski et al., 2019). Interestingly, in a culture system established to investigate the paracrine effects of MSCs on the migration and invasion potential of this aggressive breast cancer cell line, human adipose-derived MSCs promoted EMT in MCF7 breast cancer cells by cross-interacting with the TGF-β/Smad and PI3K/AKT signaling pathways, suggesting that stromal cells are key players in obesity-mediated tumor progression (Wu et al., 2019). There is probably a pro-tumorigenic cross-talk between adipose tissue and tumor stromal cells, particularly in obesity-like contexts. Adipose-derived signaling molecules might be among the drivers of pro-tumorigenic reprogramming of stromal cells by cancer cells. The evidence of stromal cell programming by tumor microenvironment suggest that non programmed cells could mediate anticancer effects in contrast with their educated counterpart.
Emerging data support that non-tumor associated MSCs mediate anticancer effects and suggest the existence of stromal cells mediating anticancer effects in the tumor microenvironment, notably, MSCs slowing tumor progression and cancer-restraining CAFs.
To address the antitumor potential of non-tumor associated-MSCs, Francois et al. (2019), treated immunocompetent rat models of colorectal carcinogenesis with non-tumor BM-derived MSCs, observing inhibition of cancer progression. This effect was partially due to the control of the tumor microenvironmental immunity as shown by (i) the modulation of effector cells, such as regulatory T cells (Tregs), CD8+ cells and NK cells; (ii)macrophage reprogramming into regulatory cells performing phagocytosis with reduced production of proinflammatory cytokines; (iii) the restoration ofTh17 activity and (iv) 50% decrease in the infiltration rate of CD68+ cells, and two-fold increase of CD3+ cells (Francois et al., 2019). In another study, intra-BM but not the systemic administration of BM MSCs from healthy donors reduced tumor burden and prolonged survival of the leukemia-bearing mice (Xia et al., 2020). In this study, the MSC senescence observed during disease progression was stopped and the BM microenvironment was restored, with functional recovery of host myelopoiesis and improvement of thrombopoiesis. Moreover, in a bioluminescence imaging study monitoring the effects of human umbilical cord-derived MSCs in mouse hepatoma tumor models with H7402 cell line, MSC microenvironment effectively inhibited the growth of cancer cells (Liu et al., 2019). Interestingly, human BM MSC-derived exosomes overexpressing miR-34a inhibited glioblastoma development (Wang et al., 2019). Two other microRNAs, associated with the capacity of MSCs to attenuate cancer growth have also been identified, namely miR-150 and miR-7 (Wang et al., 2019). Mandal et al. (2019) reported that perinatal tissue MSCs encapsulated with the sodium alginate biomaterial for isolation from tumor microenvironment displayed: (i) increased proliferation with enhanced expressions of pluripotency genes, EMT, immune-modulation and angiogenesis; (ii) increased expression of the tumor invasion suppressor protein E-cadherin; (iii) and increased secretions of VEGF, TGF-β, TNF-α, IFN-γ, IL-10 and IL-6, and IL-3β. Furthermore, treatment of CSCs derived from MDA-MB-231 and MCF7 breast cancer cell lines with encapsulated MSCs lowered CSC viability and migration, with downregulation of markers related to angiogenesis, EMT and proliferation, and upregulation of Wnt antagonists sFRP4 and DKK1 (Mandal et al., 2019). Taken together, these data suggest that non-tumor associated MSCs mediate anticancer effects and support that MSC pro-tumorigenic effects result from tumor reprogramming.
Early clinical and experimental studies in mouse models suggested the existence of at least two types of MSC-derived CAFs: they extensively studied cancer-promoting CAFs and the cancer-restraining CAFs, which were poorly investigated due to the lack of markers [for review see Bu et al. (2019)]. In a recent study using stromal cell lines derived from central nervous system (CNS) metastasis of breast and lung cancer patients, a cell population with tumor inhibitory functions, expressing high levels of collagen and displaying gene expression signatures of CAFs, MSCs, and EMT, was isolated and characterized in cancer metastasis microenvironment (Tew et al., 2019). Some very interesting recent reports have proposed markers to identify cancer-restraining CAFs. The study of Mizutani et al. (2019) has reported the glycosylphosphatidylinositol-anchored protein Meflin as a potential marker of cancer-restraining CAFs. These authors observed that the tissue infiltration of Meflin-positive CAFs correlated with favorable patient outcome in pancreatic ductal adenocarcinoma. Meflin deficiency or downregulated resulted in markedly faster tumor progression in a pancreatic ductal adenocarcinoma mouse model. Consistently, the overexpression of Meflin in CAFs or the delivery of a Meflin-expressing lentivirus into the tumor stroma were sufficient to suppress the growth of xenograft tumors (Mizutani et al., 2019). This new marker paves the way to isolation and further characterization of CAFs exerting anti-tumoral effects.
Overall, in vitro studies and studies using naïve MSCs, i.e., MSCs that were not in contact with tumor microenvironment, support the anti-tumor effects of MSCs. But these anti-cancer functions can be markedly reduced by the direct crosstalk with tumor bulk or tumor stromal elements. Interestingly, Early studies addressing the immunological hallmarks of MSCs in the tumor microenvironment revealed various molecular mechanisms through which MSCs may modulate the immune response in the cancer microenvironment. This indicated that it may be possible to convert the microenvironment from immunosuppressive to immunostimulant feature [for review see Turley et al. (2015), Poggi et al. (2018)]. These reports paved the way for studies attempting to reprogram tumor stromal cells for anticancer effects.
A bulk of recent reports propose promising strategies for reprogramming microenvironmental stromal cells to mediate only anticancer effects. For instance, treatments with various flavonoids and non-flavonoid polyphenolic compounds from medicinal plants alleviated multidrug resistance in breast, prostate, lung and colorectal cancer with survival benefits in patients. These effects were achieve through the modulation of inflammatory responses, their antioxidant capacity, and the inactivation of oncogenes, the inhibition of angiogenesis, proliferation, survival, and metastasis [for review see Costea et al. (2020)]. On the same hand, unlike conditioned medium from human adipose MSCs, eicosapentanoic acid-treated adipose MSCs reduced mRNA levels of the tumor-associated genes FASN, STAT3, cIAP-2 in MDA-MB-231 and MCF-7 breast cancer cell lines. Functionaly, cancer cell lines treated in these conditions displayed reduced glycolysis, inflammation and motility in vivo (Al-Jawadi et al., 2020). In addition, treatment with 5-Azacytidine restored IL-6-increased production in MSCs collected from myelodysplastic patients (Boada et al., 2020). Engineered human placenta-derived MSCs, armed with a double fusion gene containing the herpes simplex virus truncated thymidine kinase and firefly luciferase, inhibited the tumorigenesis mediated by the HT29 colon cancer cell line in nude mice (Yang et al., 2019). Similarly, as compared to short-culture CAFs, prolonged culture of heterogeneous prostatic CAFs resulted in marked decreases in the expression of proliferative endothelial cell surface marker endoglin (CD105), and loss of their tumor expansion potential in 3D-cultures and patient-derived xenograft tissues (Kato et al., 2019).
Furthermore, irradiated endothelial cells decreased the malignancy of liver cancer cells in a culture system using conditioned medium from endothelial cells, suggesting that irradiated endothelial cells are key players in the therapeutic effects of radiotherapy (Kim et al., 2019). Similarly, a study addressing the response of human MSCs to low-dose photodynamic therapy revealed that this treatment may increase MSC immunogenicity and promote angiogenic potential (Udartseva et al., 2019). In this in vitro study, low-dose photodynamic therapy: (i) induced the reorganization of MSC cytoskeleton, with decrease in cell motility; (ii) induced the inhibition of GSK-3 and the activation of Erk1/2 signaling in MSCs; (iii) significantly upregulated the secretion of VEGF-A, IL-8, PAI-1, MMP-9, and other proangiogenic factors by MSCs; (iv) dramatically inhibited the secretion of pro-tumorigenic macrophage infiltration marker CCL2 (MCP-1) by MSCs and decreased MSC viability and immunogenicity when cultured with lymphocytes. In another study, MSCs loaded with photosensitizer MnO2@Ce6 successfully shipped these nanoparticles into lung cancer tumor sites, enhancing the effects of photodynamic therapy in vivo (Cao et al., 2020). In sarcomas, tumor-initiating cells are thought to derive from MSCs, modified MSC were successfully used to deliver TNF-related apoptosis-inducing ligand (TRAIL) to induce tumor apoptosis, open novel therapeutic opportunities (Grisendi et al., 2015). Altogether, these observations confirm that it is possible to reprogram tumor stromal cells to mediate anticancer effects, and warrant further studies aimed at developing therapies using this approach. We propose that reprogrammed tumor MSCs loaded with photosensitizer MnO2@Ce6 or other nanoparticles with anticancer effects could display strong anticancer effects in vivo, as this approach will couple the MSC anticancer effects with the anticancer effects of nanoparticles (Ref).
Beyond the fact that MSC effects in the tumor microenvironment depend on the interactions with the adipose tissue and malignant cells (pro-tumorigenic when there are cross-talks and anticancer effects when there are poor interactions) and with the ECM programming (pro- or anticancer) to support differences between in vitro and in vivo studies, controversies on the roles of stromal cells in the tumor microenvironment may also emerge from MSC origin and the cancer type. This hypothesis is fully supported by a report of Quach et al. (2019) where the inhibition of the glypican-1 (GPC-1) prostate cancer biomarker in the aggressive prostate cancer cell line PC-3 decreased cell growth and migration in vitro, but increased PC-3 tumor size in NCr nude mice xenografts. Authors also observed that GPC-1 inhibition in an aggressive prostate cancer cell line, the DU-145 cells, increased cancer cell proliferation and migration, suggesting that GPC-1 accounts among the factors that drive cancer cell line-dependent responses to stromal cells Reduced cell growth observed in GPC-1 knockdown PC-3 cells was rescued by culturing the cells with MSCs and CAFs. Further, the treatment of these stromal cells with tumor-conditioned media from PC-3 cells transfected with GPC-1 shRNA increased the expression of extracellular matrix components, endocrine and paracrine biomolecules, and migration markers (Quach et al., 2019). In another study, despite in vivo observations revealing the ability of IGF/IGF-IR signaling to induce drug resistance and influence the ability to form metastasis via the induction of EMT in pancreatic cancer, the activation of this signaling pathway by stromal cells failed to induce EMT in cultures with MiaPaCa-2, AsPC-1, Capan-2, BxPC-3, and Panc1 pancreatic cancer cell lines (Kopantzev et al., 2019), suggesting a key role for tumor microenvironment for the pro-tumorigenic effects of this MSC-activated signaling pathway.
When assessing how breast cancer cells from different stages of the metastatic cascade convert MSCs into tumor-associated MSCs, it was observed that only MDA-MB-231 breast cancer secretomes, but not MCF-7 cells or sublines isolated from bone, lung, and brain metastases, converted MSCs into tumor-associated MSCs in bioengineered 3D microenvironments (Blache et al., 2019). These observations further confirm that MSCs from tumor microenvironment are reprogrammed by cancer-initiating cells and primary tumor ECM to mediate pro-tumorigenic effects, and that without such reprogramming the stromal cells may rather mediate anticancer effects. On the same hand, in co-cultures with stem cell-like (CD133+) cells from urinary bladder cancer cell lines, adipose-derived MSCs produced soluble mediators that: (i) increased the phosphorylation of molecules involved in cancer progression and drug resistance, such as p70 S6K, ERK1/2, and AKT1/2/3 in CD133+ cells (5,637 cell line); but instead, (ii) decreased the phosphorylation of those involved in PI3K/Akt and MAPK signaling molecules in CD133+ cells (HB-CLS-1 cell line; Maj et al., 2019). In this study, there seemed to be a controversy on the effect of MSCs on urinary bladder cancer lines in vitro, as MSCs induced pro-tumorigenic effects in culture with 5637 cell line and anticancer effects in culture with HB-CLS-1 cell line. However, this difference may actually suggest that unlike the first, the latter cell line was not able to reprogram MSCs for pro-tumorigenic effects, hence, MSCs mediated anticancer effects.
However, considering that naïve MSCs promoted anticancer effects in most reports, treatment of MDAMB231 and MCF7 human breast cancer cells with medium containing EVs from naïve MSC cultures promoted the in vitro proliferation and migration of cancer cells through ERK signaling (Zhou et al., 2019b). We hypothesize that these effects may be due to differences in the origin of MSCs, as unlike in most reports, in this study human umbilical cord MSCs, and not BM or adipose-derived MSCs were used. A comparative study of subcutaneous and visceral adipose-derived MSCs revealed various functional similarities and differences, despite similar surface markers (Ritter et al., 2019). Notably, visceral MSCs secreted higher levels of inflammatory cytokines (IL-6, IL-8, and TNF-α) and had more active sonic hedgehog pathway than subcutaneous MSCs. Moreover, fetal and adult lung MSCs possess lung-specific properties, unlike BM MSC (Rolandsson Enes et al., 2016). A study profiling the transcriptomes of 361 single MSCs derived from two umbilical cords (UC-MSCs), harvested at different passages and stimulated with or without inflammatory cytokines, revealed that UC-MSCs are a well-organized population with limited heterogeneity, as compared to other MSC types (Huang et al., 2019). These data support strong differences between MSC lines based on their origin, and even raise caution for the therapeutic use of some MSC lines in cancer context.
Because MSCs are able to increase cancer cell malignancy in vitro, early studies raised the danger of the application of human MSCs in regenerative medicine for patients with history of breast cancer, small cell ovarian cancer and other malignancies (Kucerova et al., 2011; Yang et al., 2015). Although subsequent reports from in vitro studies provided encouraging results for potential use of MSCs from patients for post-anticancer therapy tissue regeneration, there are still some concerns. For instance, an in vitro biosafety profile evaluation of MSCs derived from the BM of sarcoma patients showed that the in vitro expansion of MSCs from osteosarcoma (OS) and Ewing sarcoma (EWS) patients does not favor malignant transformation, but instead of that these MSCs displayed comparable morphology, immunophenotype, differentiation potential, proliferation rate, and telomerase activity to MSCs from healthy donors, indicating that OS and EWS patients may benefit from an autologous MSCs-based bone reconstruction after anticancer chemotherapy (Lucarelli et al., 2014). However, these promising findings, which need to be confirmed in vivo, were mitigated by the observation of chromosomal aberrations in MSCs after culture, raising caution and confirming the need for rigorous phenotypic, genetic and functional evaluation of the biosafety of MSCs from patients before clinical use. Interestingly, reports from exploratory studies in mice confirmed the therapeutic potential of MSCs for repairing damaged tissues after anticancer chemotherapy in vivo, thus after elimination of most of the primary tumor tissue that could have reprogrammed MSCs to mediate pro-tumorigenic effects [for review see Bussard et al. (2016), Baghban et al. (2020)]. Notably, human adipose-derived MSCs displayed repairing properties in damaged thymus following chemotherapy in mouse models of blood cancer, with improvements in the thymic structure and functions, as shown by the proportion of circulating and splenic Treg cells and the recovery of T-cell subpopulations (Zhan et al., 2019). However, the caution remains, not because of the possibility of reprogramming of MSCs for pro-tumorigenic effects, considering that anticancer chemotherapy normally eliminates most of the primary tumor tissue, but because we still need studies proving phenotypic, genetic and functional biosafety of MSCs in cancer context. Autologous MSC use may require a biosafety evaluation for each patient considering the clinical implications of using damaged MSCs.
The available data support that stromal cells normally have anticancer cancer effects. MSCs reprogrammed by cancer cells in the tumor microenvironment undergo a switch towards pro-tumorigenic activities, including their support to cancer cells in part through the metabolic reprogramming necessary to satisfy the energy demand and malignant behavior of the latter in a hypoxic and nutrient-deficient microenvironment. Tumor microenvironment reprograms MSCs thanks to hypoxia and the extracellular matrix cross-talks with MSCs. Interestingly, promising emerging reports suggest strategies for reprogramming microenvironmental stromal cells, which in turn switch back to naïve MSCs capable to function as anti-cancer agents. These reprogramming treatment include MSCs treatment with polyphenolic compounds from medicinal plants, with eicosapentanoic acid, or with 5-Azacytidine…. Taken together, the available data suggests that targeting the tumor microenvironment could be a promising therapeutic strategy in cancer, and that it is possible to reprogram tumor stromal cells to revert back to anticancer effects. These strategies should be further developed in the search for anticancer therapies, in particular for refractory cancers.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abdul-Aziz, A. M., Sun, Y., Hellmich, C., Marlein, C. R., Mistry, J., Forde, E., et al. (2019). Acute myeloid leukemia induces protumoral p16INK4a-driven senescence in the bone marrow microenvironment. Blood 133, 446–456. doi: 10.1182/blood-2018-04-845420
Al-Jawadi, A., Rasha, F., Ramalingam, L., Alhaj, S., Moussa, H., Gollahon, L., et al. (2020). Protective effects of eicosapentaenoic acid in adipocyte-breast cancer cell cross talk. The Journal of nutritional biochemistry 75, 108244. doi: 10.1016/j.jnutbio.2019.108244
Al-Khalaf, H. H., Ghebeh, H., Inass, R., and Aboussekhra, A. (2019). Senescent Breast Luminal Cells Promote Carcinogenesis through Interleukin-8-Dependent Activation of Stromal Fibroblasts. Molecular and cellular biology 39
An, J., Du, Y., Fan, X., Wang, Y., Ivan, C., Zhang, X. G., et al. (2019). EGFL6 promotes breast cancer by simultaneously enhancing cancer cell metastasis and stimulating tumor angiogenesis. Oncogene 38, 2123–2134. doi: 10.1038/s41388-018-0565-9
Atiya, H., Frisbie, L., Pressimone, C., and Coffman, L. (2020). Mesenchymal Stem Cells in the Tumor Microenvironment. Advances in experimental medicine and biology 1234, 31–42. doi: 10.1007/978-3-030-37184-5_3
Baghban, R., Roshangar, L., Jahanban-Esfahlan, R., Seidi, K., Jaymand, M., Kolahian, S., et al. (2020). Tumor microenvironment complexity and therapeutic implications at a glance. Cell communication and signaling : CCS 18, 59.
Balamurugan, K. (2016). HIF-1 at the crossroads of hypoxia, inflammation, and cancer. International journal of cancer 138, 1058–1066. doi: 10.1002/ijc.29519
Barberini, D. J., Freitas, N. P., Magnoni, M. S., Maia, L., Listoni, A. J., Heckler, M. C., et al. (2014). Equine mesenchymal stem cells from bone marrow, adipose tissue and umbilical cord: immunophenotypic characterization and differentiation potential. Stem cell research & therapy 5, 25. doi: 10.1186/scrt414
Bauer, J., Emon, A. B. M., Staudacher, J. J., Thomas, A. L., Mancinelli, G., Krett, N., et al. (2020). Increased stiffness of the tumor microenvironment in colon cancer stimulates cancer associated fibroblast-mediated prometastatic activin A signaling. Scientific reports 10, 50.
Berlier, J. L., Rethnam, M., Banu Binte Abdul Majeed, A., and Suda, T. (2019). Modification of the bone marrow MSC population in a xenograft model of early multiple myeloma. Biochemical and biophysical research communications 508, 1175–1181. doi: 10.1016/j.bbrc.2018.11.178
Blache, U., Horton, E. R., Xia, T., Schoof, E. M., Blicher, L. H., Schonenberger, A., et al. (2019). Mesenchymal stromal cell activation by breast cancer secretomes in bioengineered 3D microenvironments. Life science alliance 2
Boada, M., Echarte, L., Guillermo, C., Diaz, L., Tourino, C., and Grille, S. (2020). 5-Azacytidine restores interleukin 6-increased production in mesenchymal stromal cells from myelodysplastic patients. Hematology, transfusion and cell therapy
Bu, L., Baba, H., Yoshida, N., Miyake, K., Yasuda, T., Uchihara, T., et al. (2019). Biological heterogeneity and versatility of cancer-associated fibroblasts in the tumor microenvironment. Oncogene 38, 4887–4901. doi: 10.1038/s41388-019-0765-y
Bussard, K. M., Mutkus, L., Stumpf, K., Gomez-Manzano, C., and Marini, F. C. (2016). Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast cancer research : BCR 18, 84.
Cao, J., Li, J., Sun, L., Qin, T., Xiao, Y., Chen, K., et al. (2019). Hypoxia-driven paracrine osteopontin/integrin alphavbeta3 signaling promotes pancreatic cancer cell epithelial-mesenchymal transition and cancer stem cell-like properties by modulating forkhead box protein M1. Molecular oncology 13, 228–245. doi: 10.1002/1878-0261.12399
Cao, W., Liu, B., Xia, F., Duan, M., Hong, Y., Niu, J., et al. (2020). MnO2@Ce6-loaded mesenchymal stem cells as an “oxygen-laden guided-missile” for the enhanced photodynamic therapy on lung cancer. Nanoscale 12, 3090–3102. doi: 10.1039/c9nr07947e
Casagrande, N., Borghese, C., Visser, L., Mongiat, M., Colombatti, A., and Aldinucci, D. (2019). CCR5 antagonism by maraviroc inhibits Hodgkin lymphoma microenvironment interactions and xenograft growth. Haematologica 104, 564–575. doi: 10.3324/haematol.2018.196725
Chandra, A., Jahangiri, A., Chen, W., Nguyen, A. T., Yagnik, G., Pereira, M. P., et al. (2020). Clonal ZEB1-driven mesenchymal transition promotes targetable oncologic anti-angiogenic therapy resistance. Cancer research
Chen, Y., Wang, X., Fang, J., Song, J., Ma, D., Luo, L., et al. (2019a). Mesenchymal stem cells participate in oral mucosa carcinogenesis by regulating T cell proliferation. Clinical immunology (Orlando, Fla.) 198, 46–53. doi: 10.1016/j.clim.2018.12.001
Chen, Y. L., Zhang, Y., Wang, J., Chen, N., Fang, W., Zhong, J., et al. (2019b). A 17 gene panel for non-small-cell lung cancer prognosis identified through integrative epigenomic-transcriptomic analyses of hypoxia-induced epithelial-mesenchymal transition. Molecular oncology 13, 1490–1502. doi: 10.1002/1878-0261.12491
Cheng, J., Li, Y., Liu, S., Jiang, Y., Ma, J., Wan, L., et al. (2019). CXCL8 derived from mesenchymal stromal cells supports survival and proliferation of acute myeloid leukemia cells through the PI3K/AKT pathway. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 33, 4755–4764. doi: 10.1096/fj.201801931r
Chi, Y., Han, Z. B., Xu, F. Y., Wang, Y. W., Feng, X. M., Chen, F., et al. (2014). Adipogenic potentials of mesenchymal stem cells from human bone marrow, umbilical cord and adipose tissue are different. Zhongguo shi yan xue ye xue za zhi 22, 588–594.
Cislo-Pakuluk, A., and Marycz, K. (2017). A Promising Tool in Retina Regeneration: Current Perspectives and Challenges When Using Mesenchymal Progenitor Stem Cells in Veterinary and Human Ophthalmological Applications. Stem cell reviews and reports 13, 598–602. doi: 10.1007/s12015-017-9750-4
Coffman, L. G., Pearson, A. T., Frisbie, L. G., Freeman, Z., Christie, E., Bowtell, D. D., et al. (2019). Ovarian Carcinoma-Associated Mesenchymal Stem Cells Arise from Tissue-Specific Normal Stroma. Stem cells (Dayton, Ohio) 37, 257–269. doi: 10.1002/stem.2932
Costea, T., Vlad, O. C., Miclea, L. C., Ganea, C., Szollosi, J., and Mocanu, M. M. (2020). Alleviation of Multidrug Resistance by Flavonoid and Non-Flavonoid Compounds in Breast, Lung, Colorectal and Prostate Cancer. International journal of molecular sciences 21
Dabbah, M., Jarchowsky-Dolberg, O., Attar-Schneider, O., Tartakover Matalon, S., Pasmanik-Chor, M., and Drucker, L. (2019). Multiple myeloma BM-MSCs increase the tumorigenicity of MM cells via transfer of VLA4 enriched microvesicles. Carcinogenesis
Daniel, S. K., Sullivan, K. M., Labadie, K. P., and Pillarisetty, V. G. (2019). Hypoxia as a barrier to immunotherapy in pancreatic adenocarcinoma. Clinical and translational medicine 8, 10.
Dominici, M., Le Blanc, K., Mueller, I., Marini, F., Krause, D., and Deans, R. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement, Cytotherapy 8, 315–317. doi: 10.1080/14653240600855905
Fernando, R. C., Mazzotti, D. R., Azevedo, H., Sandes, A. F., Rizzatti, E. G., and Dalboni, M. A. (2019). Transcriptome Analysis of Mesenchymal Stem Cells from Multiple Myeloma Patients Reveals Downregulation of Genes Involved in Cell Cycle Progression. Immune Response, and Bone Metabolism, Scientific reports 9, 1056.
Francois, S., Usunier, B., Benderitter, M., Douay, L., Gorin, N. C., Larsen, A. K., et al. (2019). Mesenchymal Stem Cell Administration Attenuates Colon Cancer Progression by Modulating the Immune Component within the Colorectal Tumor Microenvironment. Stem cells translational medicine 8, 285–300. doi: 10.1002/sctm.18-0117
Fu, Y., Liu, S., Yin, S., Niu, W., Xiong, W., Tan, M., et al. (2017). The reverse Warburg effect is likely to be an Achilles’ heel of cancer that can be exploited for cancer therapy. Oncotarget 8, 57813–57825. doi: 10.18632/oncotarget.18175
Funes, J. M., Quintero, M., Henderson, S., Martinez, D., Qureshi, U., Westwood, C., et al. (2007). Transformation of human mesenchymal stem cells increases their dependency on oxidative phosphorylation for energy production. Proceedings of the National Academy of Sciences of the United States of America 104, 6223–6228. doi: 10.1073/pnas.0700690104
Garces, J. J., Simicek, M., Vicari, M., Brozova, L., Burgos, L., Bezdekova, R., et al. (2020). Transcriptional profiling of circulating tumor cells in multiple myeloma: a new model to understand disease dissemination. Leukemia 34, 589–603. doi: 10.1038/s41375-019-0588-4
Garcia-Hernandez, M. L., Uribe-Uribe, N. O., Espinosa-Gonzalez, R., Kast, W. M., Khader, S. A., and Rangel-Moreno, J. (2017). A Unique Cellular and Molecular Microenvironment Is Present in Tertiary Lymphoid Organs of Patients with Spontaneous Prostate Cancer Regression. Frontiers in immunology 8:563.
Ghosh, D., Mejia Pena, C., Quach, N., Xuan, B., Lee, A. H., and Dawson, M. R. (2020). Senescent mesenchymal stem cells remodel extracellular matrix driving breast cancer cells to a more-invasive phenotype. Journal of cell science 133
Giallongo, C., Tibullo, D., Camiolo, G., Parrinello, N. L., Romano, A., Puglisi, F., et al. (2019). TLR4 signaling drives mesenchymal stromal cells commitment to promote tumor microenvironment transformation in multiple myeloma. Cell death & disease 10, 704.
Goralski, K. B., Jackson, A. E., McKeown, B. T., and Sinal, C. J. (2019). More Than an Adipokine: The Complex Roles of Chemerin Signaling in Cancer. International journal of molecular sciences 20
Grisendi, G., Spano, C., Rasini, V., Veronesi, E., Prapa, M., Petrachi, T., et al. (2015). Mesenchymal progenitors expressing TRAIL induce apoptosis in sarcomas. Stem cells (Dayton, Ohio) 33, 859–869. doi: 10.1002/stem.1903
Guo, L., Sun, C., Xu, S., Xu, Y., Dong, Q., Zhang, L., et al. (2019). Knockdown of long non-coding RNA linc-ITGB1 inhibits cancer stemness and epithelial-mesenchymal transition by reducing the expression of Snail in non-small cell lung cancer. Thoracic cancer 10, 128–136. doi: 10.1111/1759-7714.12911
Gyukity-Sebestyen, E., Harmati, M., Dobra, G., Nemeth, I. B., Mihaly, J., Zvara, A., et al. (2019). Melanoma-Derived Exosomes Induce PD-1 Overexpression and Tumor Progression via Mesenchymal Stem Cell Oncogenic Reprogramming. Frontiers in immunology 10:2459.
Huang, Y., Li, Q., Zhang, K., Hu, M., Wang, Y., Du, L., et al. (2019). Single cell transcriptomic analysis of human mesenchymal stem cells reveals limited heterogeneity. Cell death & disease 10, 368.
Hughes, R. M., Simons, B. W., Khan, H., Miller, R., Kugler, V., Torquato, S., et al. (2019). Asporin Restricts Mesenchymal Stromal Cell Differentiation, Alters the Tumor Microenvironment, and Drives Metastatic Progression. Cancer research 79, 3636–3650. doi: 10.1158/0008-5472.can-18-2931
Hwang, H. J., Oh, M. S., Lee, D. W., and Kuh, H. J. (2019). Multiplex quantitative analysis of stroma-mediated cancer cell invasion, matrix remodeling, and drug response in a 3D co-culture model of pancreatic tumor spheroids and stellate cells. Journal of experimental & clinical cancer research : CR 38, 258.
Ibraheem, A., Attar-Schneider, O., Dabbah, M., Dolberg Jarchowsky, O., Tartakover Matalon, S., and Lishner, M. (2019). BM-MSCs-derived ECM modifies multiple myeloma phenotype and drug response in a source-dependent manner. Translational research : the journal of laboratory and clinical medicine 207, 83–95. doi: 10.1016/j.trsl.2019.01.003
Ikeda, T., Nishita, M., Hoshi, K., Honda, T., Kakeji, Y., and Minami, Y. (2020). Mesenchymal stem cell-derived CXCL16 promotes progression of gastric cancer cells by STAT3-mediated expression of Ror1. Cancer science
Ishiki, Y., Ohnishi, H., Muto, Y., Matsumoto, K., and Nakamura, T. (1992). Direct evidence that hepatocyte growth factor is a hepatotrophic factor for liver regeneration and has a potent antihepatitis effect in vivo. Hepatology (Baltimore, Md.) 16, 1227–1235. doi: 10.1016/0270-9139(92)90019-6
Iyengar, N. M., Gucalp, A., Dannenberg, A. J., and Hudis, C. A. (2016). Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 34, 4270–4276. doi: 10.1200/jco.2016.67.4283
Jafarzadeh, N., Safari, Z., Pornour, M., Amirizadeh, N., Forouzandeh Moghadam, M., and Sadeghizadeh, M. (2019). Alteration of cellular and immune-related properties of bone marrow mesenchymal stem cells and macrophages by K562 chronic myeloid leukemia cell derived exosomes. Journal of cellular physiology 234, 3697–3710. doi: 10.1002/jcp.27142
Kang, N., Choi, S. Y., Kim, B. N., Yeo, C. D., Park, C. K., Kim, Y. K., et al. (2019). Hypoxia-induced cancer stemness acquisition is associated with CXCR4 activation by its aberrant promoter demethylation. BMC cancer 19:148.
Kato, M., Madhav, A., Haldar, S., Tripathi, M., Billet, S., Mishra, R., et al. (2019). Heterogeneous cancer-associated fibroblast population potentiates neuroendocrine differentiation and castrate resistance in a CD105-dependent manner. Oncogene 38, 716–730. doi: 10.1038/s41388-018-0461-3
Kim, S. D., Yi, J. M., and Park, M. T. (2019). Irradiated endothelial cells modulate the malignancy of liver cancer cells. Oncology letters 17, 2187–2196.
Kopantzev, E. P., Kopantseva, M. R., Grankina, E. V., Mikaelyan, A., Egorov, V. I., and Sverdlov, E. D. (2019). Activation of IGF/IGF-IR signaling pathway fails to induce epithelial-mesenchymal transition in pancreatic cancer cells. Pancreatology : official journal of the International Association of Pancreatology (IAP). [et al.] 19, 390–396. doi: 10.1016/j.pan.2019.01.010
Krueger, T. E., Thorek, L. J. D., Meeker, A. K., Isaacs, J. T., and Brennen, W. N. (2019). Tumor-infiltrating mesenchymal stem cells: Drivers of the immunosuppressive tumor microenvironment in prostate cancer? The Prostate 79, 320–330. doi: 10.1002/pros.23738
Kucerova, L., Kovacovicova, M., Polak, S., Bohac, M., Fedeles, J., Palencar, D., et al. (2011). Interaction of human adipose tissue-derived mesenchymal stromal cells with breast cancer cells. Neoplasma 58, 361–370. doi: 10.4149/neo_2011_05_361
Li, C. Y., Wu, X. Y., Tong, J. B., Yang, X. X., Zhao, J. L., Zheng, Q. F., et al. (2015). Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem cell research & therapy 6, 55.
Li, D., Wang, N., Zhang, L., Hanyu, Z., Xueyuan, B., Fu, B., et al. (2013). Mesenchymal stem cells protect podocytes from apoptosis induced by high glucose via secretion of epithelial growth factor. Stem cell research & therapy 4, 103. doi: 10.1186/scrt314
Lin, S., Zhu, B., Huang, G., Zeng, Q., and Wang, C. (2019). Microvesicles derived from human bone marrow mesenchymal stem cells promote U2OS cell growth under hypoxia: the role of PI3K/AKT and HIF-1alpha. Human cell 32, 64–74. doi: 10.1007/s13577-018-0224-z
Liu, X., Chen, H., Zhu, W., Chen, H., Hu, X., Jiang, Z., et al. (2014). Transplantation of SIRT1-engineered aged mesenchymal stem cells improves cardiac function in a rat myocardial infarction model. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation 33, 1083–1092. doi: 10.1016/j.healun.2014.05.008
Liu, Y., Ren, H., Zhou, Y., Shang, L., Zhang, Y., Yang, F., et al. (2019). The hypoxia conditioned mesenchymal stem cells promote hepatocellular carcinoma progression through YAP mediated lipogenesis reprogramming. Journal of experimental & clinical cancer research : CR 38, 228.
Lucarelli, E., Bellotti, C., Mantelli, M., Avanzini, M. A., Maccario, R., Novara, F., et al. (2014). In vitro biosafety profile evaluation of multipotent mesenchymal stem cells derived from the bone marrow of sarcoma patients. Journal of translational medicine 12, 95. doi: 10.1186/1479-5876-12-95
Lung, D. K., Warrick, J. W., Hematti, P., Callander, N. S., Mark, C. J., Miyamoto, S., et al. (2019). Bone Marrow Stromal Cells Transcriptionally Repress ESR1 but Cannot Overcome Constitutive ESR1 Mutant Activity. Endocrinology 160, 2427–2440. doi: 10.1210/en.2019-00299
Luo, Y., Kishi, S., Sasaki, T., Ohmori, H., Mori, S., Goto, K., et al. (2020). Targeting claudin-4 enhances chemosensitivity in breast cancer. Cancer science 1, 1.
Ma, Z., Cui, X., Lu, L., Chen, G., Yang, Y., Hu, Y., et al. (2019). Exosomes from glioma cells induce a tumor-like phenotype in mesenchymal stem cells by activating glycolysis. Stem cell research & therapy 10, 60.
Maj, M., Kokocha, A., Bajek, A., and Drewa, T. (2019). The effects of adipose-derived stem cells on CD133-expressing bladder cancer cells. Journal of cellular biochemistry
Maliszewska-Olejniczak, K., Brodaczewska, K. K., Bielecka, Z. F., Solarek, W., Kornakiewicz, A., Szczylik, C., et al. (2019). Development of extracellular matrix supported 3D culture of renal cancer cells and renal cancer stem cells. Cytotechnology 71, 149–163. doi: 10.1007/s10616-018-0273-x
Mandal, S., Arfuso, F., Sethi, G., Dharmarajan, A., and Warrier, S. (2019). Encapsulated human mesenchymal stem cells (eMSCs) as a novel anti-cancer agent targeting breast cancer stem cells: Development of 3D primed therapeutic MSCs. The international journal of biochemistry & cell biology 110, 59–69. doi: 10.1016/j.biocel.2019.02.001
Mazza, G., Telese, A., Frenguelli, L., Levi, A., Marrali, M., Longato, L., et al. (2019). Cirrhotic Human Liver Extracellular Matrix 3D Scaffolds Promote Smad-Dependent TGF-beta1 Epithelial Mesenchymal Transition. Cells 9
Melzer, C., von der Ohe, J., Otterbein, H., Ungefroren, H., and Hass, R. (2019). Changes in uPA, PAI-1, and TGF-beta Production during Breast Cancer Cell Interaction with Human Mesenchymal Stroma/Stem-Like Cells (MSC). International journal of molecular sciences 20
Mizutani, Y., Kobayashi, H., Iida, T., Asai, N., Masamune, A., Hara, A., et al. (2019). Meflin-Positive Cancer-Associated Fibroblasts Inhibit Pancreatic Carcinogenesis. Cancer research 79, 5367–5381. doi: 10.1158/0008-5472.can-19-0454
Nwabo Kamdje, A. H., Mosna, F., Bifari, F., Lisi, V., Bassi, G., Malpeli, G., et al. (2011). Notch-3 and Notch-4 signaling rescue from apoptosis human B-ALL cells in contact with human bone marrow-derived mesenchymal stromal cells. Blood 118, 380–389. doi: 10.1182/blood-2010-12-326694
Nwabo Kamdje, A. H., Seke Etet, P. F., Vecchio, L., Muller, J. M., Krampera, M., and Lukong, K. E. (2014). Signaling pathways in breast cancer: therapeutic targeting of the microenvironment. Cellular signalling 26, 2843–2856. doi: 10.1016/j.cellsig.2014.07.034
Oh, I. R., Raymundo, B., Kim, M., and Kim, C. W. (2020). Mesenchymal stem cells co-cultured with colorectal cancer cells showed increased invasive and proliferative abilities due to its altered p53/TGF-beta1 levels. Bioscience, biotechnology, and biochemistry 84, 256–267. doi: 10.1080/09168451.2019.1676692
Osman, A., Afify, S. M., Hassan, G., Fu, X., Seno, A., and Seno, M. (2020). Revisiting Cancer Stem Cells as the Origin of Cancer-Associated Cells in the Tumor Microenvironment: A Hypothetical View from the Potential of iPSCs. Cancers 12
Oswald, J., Boxberger, S., Jorgensen, B., Feldmann, S., Ehninger, G., Bornhauser, M., et al. (2004). Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem cells (Dayton, Ohio) 22, 377–384. doi: 10.1634/stemcells.22-3-377
Ozdemir, B. O. R., Ozdemir, A. T., Sariboyaci, A. E., Uysal, O., Tuglu, M. I., and Kirmaz, C. (2019). The investigation of immunomodulatory effects of adipose tissue mesenchymal stem cell educated macrophages on the CD4 T cells. Immunobiology 224, 585–594. doi: 10.1016/j.imbio.2019.04.002
Park, J., Morley, T. S., Kim, M., Clegg, D. J., and Scherer, P. E. (2014). Obesity and cancer–mechanisms underlying tumour progression and recurrence. Nature reviews. Endocrinology 10, 455–465. doi: 10.1038/nrendo.2014.94
Pelizzo, G., Veschi, V., Mantelli, M., Croce, S., Maltese, A., Catenacci, L., et al. (2018). Microenvironment in neuroblastoma: isolation and characterization of tumor-derived mesenchymal stromal cells. BMC cancer 18:1176.
Poggi, A., Varesano, S., and Zocchi, M. R. (2018). How to Hit Mesenchymal Stromal Cells and Make the Tumor Microenvironment Immunostimulant Rather Than Immunosuppressive. Frontiers in immunology 9:262.
Quach, N. D., Kaur, S. P., Eggert, M. W., Ingram, L., Ghosh, D., Sheth, S., et al. (2019). Paradoxical Role of Glypican-1 in Prostate Cancer Cell and Tumor Growth. Scientific reports 9, 11478.
Quail, D. F., and Dannenberg, A. J. (2019). The obese adipose tissue microenvironment in cancer development and progression. Nature reviews. Endocrinology 15, 139–154. doi: 10.1038/s41574-018-0126-x
Ritter, A., Friemel, A., Roth, S., Kreis, N. N., Hoock, S. C., Safdar, B. K., et al. (2019). Subcutaneous and Visceral Adipose-Derived Mesenchymal Stem Cells: Commonality and Diversity. Cells 8
Rolandsson Enes, S., Andersson Sjoland, A., Skog, I., Hansson, L., Larsson, H., Eriksson, L., et al. (2016). MSC from fetal and adult lungs possess lung-specific properties compared to bone marrow-derived MSC. Scientific reports 6, 29160.
Saforo, D., Omer, L., Smolenkov, A., Barve, A., Casson, L., Boyd, N., et al. (2019). Primary lung cancer samples cultured under microenvironment-mimetic conditions enrich for mesenchymal stem-like cells that promote metastasis. Scientific reports 9, 4177.
Salminen, A., Kaarniranta, K., and Kauppinen, A. (2020). ER stress activates immunosuppressive network: implications for aging and Alzheimer’s disease. Journal of molecular medicine (Berlin, Germany) 98, 633–650. doi: 10.1007/s00109-020-01904-z
Samudio, I., Fiegl, M., McQueen, T., Clise-Dwyer, K., and Andreeff, M. (2008). The warburg effect in leukemia-stroma cocultures is mediated by mitochondrial uncoupling associated with uncoupling protein 2 activation. Cancer research 68, 5198–5205. doi: 10.1158/0008-5472.can-08-0555
Sarhan, D., Wang, J., Hallstrom, C., Verneris, M. R., Grzywacz, B., Warlick, E., et al. (2020). Mesenchymal stromal cells shape the MDS microenvironment by inducing suppressive monocytes that dampen NK cell function. JCI insight
Schmohl, K. A., Mueller, A. M., Dohmann, M., Spellerberg, R., Urnauer, S., Schwenk, N., et al. (2019). Integrin alphavbeta3-Mediated Effects of Thyroid Hormones on Mesenchymal Stem Cells in Tumor Angiogenesis. Thyroid : official journal of the American Thyroid Association 29, 1843–1857. doi: 10.1089/thy.2019.0413
Seke Etet, P. F., Vecchio, L., Bogne Kamga, P., Nchiwan Nukenine, E., Krampera, M., and Nwabo Kamdje, A. H. (2013). Normal hematopoiesis and hematologic malignancies: role of canonical Wnt signaling pathway and stromal microenvironment. Biochimica et biophysica acta 1835, 1–10. doi: 10.1016/j.bbcan.2012.08.002
Sesia, S. B., Duhr, R., Todorov, A., Schaeren, S., Padovan, E., Spagnoli, G., et al. (2015). Anti-inflammatory/tissue repair macrophages enhance the cartilage-forming capacity of human bone marrow-derived mesenchymal stromal cells. Journal of cellular physiology 230, 1258–1269. doi: 10.1002/jcp.24861
Shamai, Y., Alperovich, D. C., Yakhini, Z., Skorecki, K., and Tzukerman, M. (2019). Reciprocal Reprogramming of Cancer Cells and Associated Mesenchymal Stem Cells in Gastric Cancer. Stem cells (Dayton, Ohio) 37, 176–189. doi: 10.1002/stem.2942
Shen, Y., Xue, C., Li, X., Ba, L., Gu, J., Sun, Z., et al. (2019). Effects of Gastric Cancer Cell-Derived Exosomes on the Immune Regulation of Mesenchymal Stem Cells by the NF-kB Signaling Pathway. Stem cells and development 28, 464–476. doi: 10.1089/scd.2018.0125
Shi, L., Zhao, Y., Fei, C., Guo, J., Jia, Y., Wu, D., et al. (2019). Cellular senescence induced by S100A9 in mesenchymal stromal cells through NLRP3 inflammasome activation. Aging 11, 9626–9642. doi: 10.18632/aging.102409
Shi, Y., Wang, Y., Li, Q., Liu, K., Hou, J., Shao, C., et al. (2018). Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nature reviews. Nephrology 14, 493–507. doi: 10.1038/s41581-018-0023-5
Su, F., Ahn, S., Saha, A., DiGiovanni, J., and Kolonin, M. G. (2019). Adipose stromal cell targeting suppresses prostate cancer epithelial-mesenchymal transition and chemoresistance. Oncogene 38, 1979–1988. doi: 10.1038/s41388-018-0558-8
Tan, H. X., Cao, Z. B., He, T. T., Huang, T., Xiang, C. L., and Liu, Y. (2019). TGFbeta1 is essential for MSCs-CAFs differentiation and promotes HCT116 cells migration and invasion via JAK/STAT3 signaling. OncoTargets and therapy 12, 5323–5334. doi: 10.2147/ott.s178618
Tejero, R., Huang, Y., Katsyv, I., Kluge, M., Lin, J. Y., Daviaud, N., et al. (2019). Gene signatures of quiescent glioblastoma cells reveal mesenchymal shift and interactions with niche microenvironment. EBioMedicine 42, 252–269. doi: 10.1016/j.ebiom.2019.03.064
Tew, B. Y., Legendre, C., Gooden, G. C., Johnson, K. N., Martinez, R. A., Kiefer, J., et al. (2019). Isolation and characterization of patient-derived CNS metastasis-associated stromal cell lines. Oncogene 38, 4002–4014. doi: 10.1038/s41388-019-0680-2
Thomas, R., Wang, W., and Su, D. M. (2020). Contributions of Age-Related Thymic Involution to Immunosenescence and Inflammaging. Immunity & ageing : I & A 17, 2.
Torres, A., Erices, J. I., Sanchez, F., Ehrenfeld, P., Turchi, L., Virolle, T., et al. (2019). Extracellular adenosine promotes cell migration/invasion of Glioblastoma Stem-like Cells through A3 Adenosine Receptor activation under hypoxia. Cancer letters 446, 112–122. doi: 10.1016/j.canlet.2019.01.004
Tsiklauri, L., Werner, J., Kampschulte, M., Frommer, K. W., Berninger, L., Irrgang, M., et al. (2018). Visfatin alters the cytokine and matrix-degrading enzyme profile during osteogenic and adipogenic MSC differentiation. Osteoarthritis and cartilage 26, 1225–1235. doi: 10.1016/j.joca.2018.06.001
Turley, S. J., Cremasco, V., and Astarita, J. L. (2015). Immunological hallmarks of stromal cells in the tumour microenvironment. Nature reviews. Immunology 15, 669–682. doi: 10.1038/nri3902
Udartseva, O. O., Zhidkova, O. V., Ezdakova, M. I., Ogneva, I. V., Andreeva, E. R., Buravkova, L. B., et al. (2019). Low-dose photodynamic therapy promotes angiogenic potential and increases immunogenicity of human mesenchymal stromal cells. Journal of photochemistry and photobiology. B, Biology 199, 111596. doi: 10.1016/j.jphotobiol.2019.111596
Umakoshi, M., Takahashi, S., Itoh, G., Kuriyama, S., Sasaki, Y., Yanagihara, K., et al. (2019). Macrophage-mediated transfer of cancer-derived components to stromal cells contributes to establishment of a pro-tumor microenvironment. Oncogene 38, 2162–2176. doi: 10.1038/s41388-018-0564-x
Verma, D., Zanetti, C., Godavarthy, P. S., Kumar, R., Minciacchi, V. R., Pfeiffer, J., et al. (2020). Bone marrow niche-derived extracellular matrix-degrading enzymes influence the progression of B-cell acute lymphoblastic leukemia. Leukemia
Walsh, H. R., Cruickshank, B. M., Brown, J. M., and Marcato, P. (2019). The Flick of a Switch: Conferring Survival Advantage to Breast Cancer Stem Cells Through Metabolic Plasticity. Frontiers in oncology 9:753.
Wang, B., Wu, Z. H., Lou, P. Y., Chai, C., Han, S. Y., Ning, J. F., et al. (2019). Human bone marrow-derived mesenchymal stem cell-secreted exosomes overexpressing microRNA-34a ameliorate glioblastoma development via down-regulating MYCN. Cellular oncology (Dordrecht) 42, 783–799. doi: 10.1007/s13402-019-00461-z
Wang, N., Zhang, R., Wang, S. J., Zhang, C. L., Mao, L. B., Zhuang, C. Y., et al. (2013). Vascular endothelial growth factor stimulates endothelial differentiation from mesenchymal stem cells via Rho/myocardin-related transcription factor–a signaling pathway. The international journal of biochemistry & cell biology 45, 1447–1456. doi: 10.1016/j.biocel.2013.04.021
Windmolders, S., De Boeck, A., Koninckx, R., Daniels, A., Bracke, M., Hendrikx, M., et al. (2014). Mesenchymal stem cell secreted platelet derived growth factor exerts a pro-migratory effect on resident Cardiac Atrial appendage Stem Cells. Journal of molecular and cellular cardiology 66, 177–188. doi: 10.1016/j.yjmcc.2013.11.016
Worner, P. M., Schachtele, D. J., Barabadi, Z., Srivastav, S., Chandrasekar, B., Izadpanah, R., et al. (2019). Breast Tumor Microenvironment Can Transform Naive Mesenchymal Stem Cells into Tumor-Forming Cells in Nude Mice. Stem cells and development 28, 341–352. doi: 10.1089/scd.2018.0110
Wu, S., Wang, Y., Yuan, Z., Wang, S., Du, H., Liu, X., et al. (2019). Human adiposederived mesenchymal stem cells promote breast cancer MCF7 cell epithelialmesenchymal transition by cross interacting with the TGFbeta/Smad and PI3K/AKT signaling pathways. Molecular medicine reports 19, 177–186.
Xia, C., Wang, T., Cheng, H., Dong, Y., Weng, Q., Sun, G., et al. (2020). Mesenchymal stem cells suppress leukemia via macrophage-mediated functional restoration of bone marrow microenvironment. Leukemia
Xu, X. D., Shao, S. X., Jiang, H. P., Cao, Y. W., Wang, Y. H., Yang, X. C., et al. (2015). Warburg effect or reverse Warburg effect? A review of cancer metabolism. Oncology research and treatment 38, 117–122. doi: 10.1159/000375435
Xuan, W., Khan, M., and Ashraf, M. (2020). Extracellular Vesicles From Notch Activated Cardiac Mesenchymal Stem Cells Promote Myocyte Proliferation and Neovasculogenesis. Frontiers in cell and developmental biology 8:11.
Yang, J., Lv, K., Sun, J., and Guan, J. (2019). Anti-tumor effects of engineered mesenchymal stem cells in colon cancer model. Cancer management and research 11, 8443–8450. doi: 10.2147/cmar.s209880
Yang, J., Ren, B., Yang, G., Wang, H., Chen, G., You, L., et al. (2020). The enhancement of glycolysis regulates pancreatic cancer metastasis. Cellular and molecular life sciences : CMLS 77, 305–321. doi: 10.1007/s00018-019-03278-z
Yang, Y., Bucan, V., Baehre, H., von der Ohe, J., Otte, A., and Hass, R. (2015). Acquisition of new tumor cell properties by MSC-derived exosomes. International journal of oncology 47, 244–252. doi: 10.3892/ijo.2015.3001
Zannoni, J., Mauz, N., Seyve, L., Meunier, M., Brault, J., Jouzier, C., et al. (2019). Tumor microenvironment and clonal monocytes from chronic myelomonocytic leukemia induce a procoagulant climate. Blood advances 3, 1868–1880. doi: 10.1182/bloodadvances.2018026955
Zhan, Y., Wang, L., Liu, G., Zhang, X., Yang, J., Pan, Y., et al. (2019). The Reparative Effects of Human Adipose-Derived Mesenchymal Stem Cells in the Chemotherapy-Damaged Thymus. Stem cells and development 28, 186–195. doi: 10.1089/scd.2018.0142
Zhang, L. N., Huang, Y. H., and Zhao, L. (2019b). Fusion of macrophages promotes breast cancer cell proliferation, migration and invasion through activating epithelial-mesenchymal transition and Wnt/beta-catenin signaling pathway. Archives of biochemistry and biophysics 676, 108137. doi: 10.1016/j.abb.2019.108137
Zhang, X., Sai, B., Wang, F., Wang, L., Wang, Y., Zheng, L., et al. (2019a). Hypoxic BMSC-derived exosomal miRNAs promote metastasis of lung cancer cells via STAT3-induced EMT. Molecular cancer 18, 40.
Zhou, X., Li, T., Chen, Y., Zhang, N., Wang, P., Liang, Y., et al. (2019b). Mesenchymal stem cellderived extracellular vesicles promote the in vitro proliferation and migration of breast cancer cells through the activation of the ERK pathway. International journal of oncology 54, 1843–1852.
Keywords: stromal cells, tumorigenic effects, anticancer effects, tumor microenvironment, reprogramming
Citation: Nwabo Kamdje AH, Seke Etet PF, Simo Tagne R, Vecchio L, Lukong KE and Krampera M (2020) Tumor Microenvironment Uses a Reversible Reprogramming of Mesenchymal Stromal Cells to Mediate Pro-tumorigenic Effects. Front. Cell Dev. Biol. 8:545126. doi: 10.3389/fcell.2020.545126
Received: 24 March 2020; Accepted: 28 October 2020;
Published: 19 November 2020.
Edited by:
Lindolfo da Silva Meirelles, Universidade Luterana do Brazil, BrazilReviewed by:
Gianandrea Pasquinelli, University of Bologna, ItalyCopyright © 2020 Nwabo Kamdje, Seke Etet, Simo Tagne, Vecchio, Lukong and Krampera. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Armel H. Nwabo Kamdje, YXJtZWwubndhYm9AZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.