- 1The Center for Stem Cell Biology, Developmental Biology Program, Sloan-Kettering Institute for Cancer Research, New York, NY, United States
- 2Developmental Biology Program, Sloan-Kettering Institute for Cancer Research, New York, NY, United States
- 3Neuroscience Graduate Program of Weill Cornell Graduate School of Biomedical Sciences, Weill Cornell Medicine, New York, NY, United States
In Parkinson’s disease (PD), there are currently no effective therapies to prevent or slow down disease progression. Cell replacement therapy using human pluripotent stem cell (hPSC)-derived dopamine neurons holds considerable promise. It presents a novel, regenerative strategy, building on the extensive history of fetal tissue grafts and capturing the potential of hPSCs to serve as a scalable and standardized cell source. Progress in establishing protocols for the direct differentiation to midbrain dopamine (mDA) neurons from hPSC have catalyzed the development of cell-based therapies for PD. Consequently, several groups have derived clinical-grade mDA neuron precursors under clinical good manufacture practice condition, which are progressing toward clinical testing in PD patients. Here we will review the current status of the field, discuss the remaining key challenges, and highlight future areas for further improvements of hPSC-based technologies in the clinical translation to PD.
Introduction
A key promise of human pluripotent stem cells (hPSCs), both human embryonic stem cells (hESCs) and induced pluripotent stem cells (hiPSCs), is their ability to access unlimited number of specialized cell types for application in cell-based therapies for neurodegenerative diseases such as Parkinson’s disease (PD; Fox et al., 2014; Tabar and Studer, 2014; Blau and Daley, 2019). The characteristic, patho-physiological feature of PD is the specific loss of midbrain dopamine (mDA) neurons in the substantia nigra (SN; Mingote et al., 2015), leading to motor symptoms such as bradykinesia, tremor, and rigidity (Lees et al., 2009). Drug treatments, such as L-dopa, for restoring the dopamine deficiency is widely used to relieve disease symptoms, but long-term use shows decreased efficacy and can trigger debilitating side effects including motor complications as well as psychiatric problems (Poewe et al., 2017).
Given the quite selective mDA neuron loss in PD, cell replacement may represent an attractive therapeutic strategy. There has been experience using various sources of cells for transplantation including adrenal medullary tissue and more importantly, human fetal midbrain tissue, starting with human clinical trials more than 30 years ago (Backlund et al., 1985; Goetz et al., 1989; Lindvall et al., 1990; Freed et al., 1992; Widner et al., 1992). More than 300 patients were transplanted using human fetal tissue world-wide, and despite variable results overall, some patients showed remarkable recovery obviating the need for any L-dopa treatment (Kefalopoulou et al., 2014), and robust graft survival has been demonstrated histologically up to 24 years after transplantation (Li et al., 2016). However, the use of human fetal tissue has several limitations such as transplantation of a heterogenous cell population, non-standardized tissue-processing, ethical issues associated with the routine use of fetal tissue, and importantly, the limited availability of suitable tissues. Therefore, alternative cell sources are urgently needed. The use of hPSCs is particularly attractive due to their ability to yield defined lineages such as mDA neurons at scale. Furthermore, hPSCs may provide a more practical and ethically acceptable cell source (Steinbeck and Studer, 2015; Barker et al., 2017; Parmar et al., 2020). The initial challenge in the use of hPSCs was the ability to direct their broad potential toward the restricted production of mDA neuronal lineages. Over the years, several groups have developed suitable protocols for mDA neuron production, and in ongoing or completed developments those protocols have been adapted to generate authentic and functional mDA neurons or precursors under clinical good manufacture practice (cGMP) grade conditions suitable for early stage human trials. Here, we discuss the tremendous progress made over the last decade, review the current bottlenecks, and provide a perspective for the future of developing cell replacement strategies in PD.
Brief History on mDA Neuron Protocol Development
The initial approach to generate mDA neurons from hPSCs was based on adapting protocols from mouse ESC, which generate the neuronal-rosette like intermediates by co-culturing with feeder such as MS5 and PA6 and then further differentiate mDA neurons (Kawasaki et al., 2000; Perrier et al., 2004; Sonntag et al., 2007). While the rosette-based protocols could yield dopamine neurons that express TH, the rate-limiting enzyme for dopamine production, and showed dopamine release in vitro, those cells unlikely represented the correct cell type of origin as they barely expressed floorplate markers, such as FOXA2 and LMX1A. Importantly, rosette-derived dopamine (DA) neuron protocols displayed a considerable risk of neural overgrowth (Brederlau et al., 2006; Roy et al., 2006; Elkabetz et al., 2008), and resulted in only limited in vivo DA neuron survival and function (Perrier et al., 2004; Park et al., 2005).
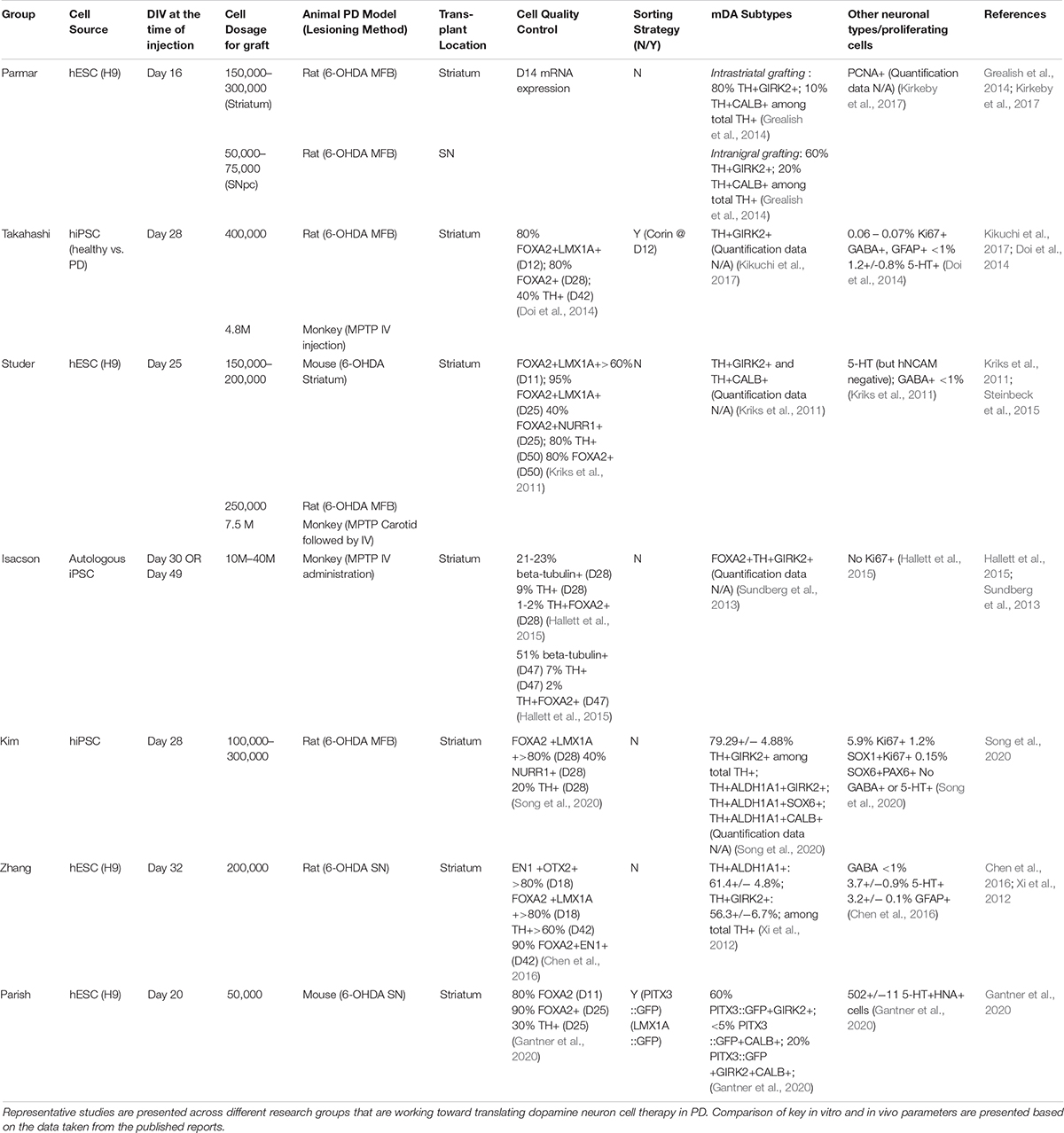
The realization that mDA neurons originate from the midbrain floor-plate (Ono et al., 2007; Bonilla et al., 2008) and the development of improved strategies to drive neural differentiation from hPSCs ignited the development of a new class of protocols. Those protocols used dual SMAD inhibition (inhibition of BMP and TGFβ signaling) for neural induction (Chambers et al., 2009) together with patterning factors activating SHH (Sonic hedgehog), WNT, and FGF8b signaling (Kriks et al., 2011; Figure 1). The resulting floor-plate derived mDA cells showed the biochemical and electrophysiological properties of mDA neurons in vitro and resulted in more robust survival and function in vivo while reducing the risk of neural overgrowth or teratoma formation (Kriks et al., 2011; Table 1). Since those initial studies, several groups have developed independent mDA neuron differentiation paradigms (Kirkeby et al., 2012, 2017; Sundberg et al., 2013; Doi et al., 2014; Hallett et al., 2014; Steinbeck et al., 2015; Chen et al., 2016; Niclis et al., 2017; Gantner et al., 2020; Song et al., 2020) but all of those are based on the specification of mDA neurons via a floor-plate intermediate (Figure 1).
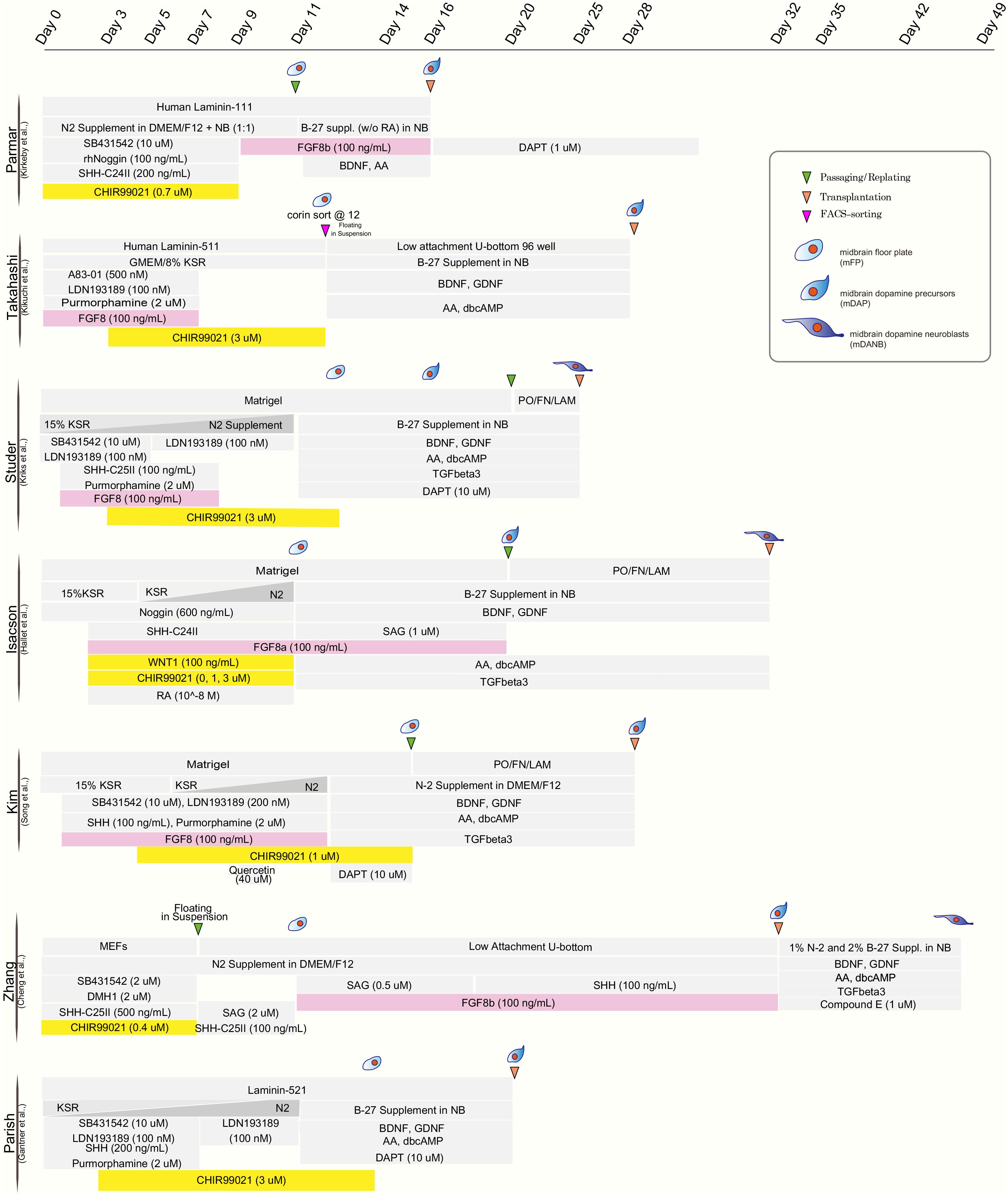
Figure 1. Comparison of published differentiation protocols for dopamine neuron derivation from human pluripotent stem cells. While all protocols use comparable strategies for neural induction (dual-SMAD inhibition) and for midbrain floor plate induction (activation of SHH pathway and WNT pathway), there are differences in the use of FGF8 (highlighted in pink) and the timing and concentration of the WNT activating compounds (typically CHIR99021; highlighted in yellow).
One interesting comparator to assess the potency of hPSC-derived mDA neurons was their preclinical assessment in direct comparison to the efficacy of human fetal tissue grafts showing comparable results in rescuing dopamine deficits in a PD rat model and in achieving proper target-specific neurite-outgrowth (Grealish et al., 2014). In another set of studies, in vivo graft function of mDA cells was explored in preclinical PD animal models using more sophisticated technologies such as optogenetics (Steinbeck et al., 2015) and chemogenetic manipulation (Chen et al., 2016). Those studies demonstrated conclusively that the recovery from PD behavior is dependent on neuronal activity and activity-dependent dopamine release from transplanted mDA cells. Finally, differentiation protocols to generate floor-plate-derived mDA neurons demonstrated their robustness and reproducibility by developing mDA cell products under conditions that should be suitable for cGMP manufacturing (Kikuchi et al., 2017; Kirkeby et al., 2017; Figure 1). In an effort to promote the safe transition of hPSC-derived mDA neurons toward clinical testing in PD patients, a global effort, G-Force-PD was initiated in 2015 (Barker et al., 2015). G-Force-PD includes several groups from the United States, Europe, and Japan which all are leading independent efforts to bring hESC or hiPSC-derived mDA neurons to the clinic, but are committed to sharing their ongoing experience, discuss unexpected challenges and possible solutions, and propose guidelines ranging from cell manufacturing to clinical trials design (Barker et al., 2017). One of the groups within G-Force-PD has already started a first in human clinical cell transplantation trial in PD patients using iPSC-derived mDA neurons, with the first patient grafted in October 2018 (Normile, 2018)1. Another group has just recently published the feasibility of transplanting one single patient with its own iPSC-derived mDA neurons (Schweitzer et al., 2020).
What Cell Type-Related Factors Are Critical for Clinical Translation?
Proper Patterning of mDA Precursors Beyond Establishing Floor-Plate Precursor Identity
Although most protocols have been able to generate floor-plate precursors at high efficiency, various groups have used different timing, duration, and concentration of patterning factors, specially CHIR99021 (CHIR), the most widely used GSK3-inhibitor for triggering canonical WNT activation and recombinant factor FGF8 which is added in some but not other protocols for distinct time periods of differentiation (Figure 1). The precise titration of CHIR is critical for proper rostro-caudal pattering during mDA neuron specification from hPSC. Work by the Parmar lab (Kirkeby et al., 2012) demonstrated that different levels of WNT activation trigger dose-dependent changes in the regional identity of neuronal progenitor cells ranging from telencephalon (low WNT activation) to hindbrain (high WNT activation) fates, with the optimized CHIR concentration for mDA neuron induction defined as (0.7–0.8 μM). Furthermore, single cell RNA sequencing of Lmx1a+ precursors in the mouse suggests that the midbrain floor-plate markers FOXA2 and LMX1A are not specific to just mDA progenitors, but also mark the more anterior, subthalamic nucleus (STN) precursors which go on to give rise to glutamatergic neurons (Kee et al., 2017). Those results are in agreement with the idea that the midbrain-diencephalic floor-plate region is subdivided into two distinct domains by En1 for posterior, midbrain (mDA) identity, and Dbx1 for STN neuron population (Nouri and Awatramani, 2017). Importantly, lowering CHIR exposure (0.4–0.6 μM) resulted in a bias toward Lmx1a+/Pitx2+ positive cells (STN neuron) while the percentage of STN neurons was greatly decreased at higher concentrations of CHIR (0.8–1 μM) with increased efficiencies of mDA neuron differentiation (Kee et al., 2017). Nevertheless, a broad range of concentrations and durations of CHIR treatment have been proposed across for mDA neuron derivation from hPSCs as detailed in Figure 1. There are several possibilities for those discrepancies across protocols. In particular, the basal media composition (KSR versus N2 versus E6 media), coating substrates (Matrigel versus LAM511) and the total lengths of CHIR treatment likely contribute to those differences.
Studies, using bulk-RNA sequencing of mDA neurons at the time of grafting demonstrated a correlation of improved in vivo graft outcome, across >30 batches, for mDA neuron populations expressing more caudal floor-plate markers including expression of EN1 (Kirkeby et al., 2017). Furthermore, the same study showed that FGF8b treatment after floor-plate induction could more reliably induce caudal marker expression. These results were consistent with previous work suggesting the need for FGF8 treatment at later time points of differentiation during non-human primate mDA neuron induction (Xi et al., 2012; Figure 1). In contrast, earlier exposure of FGF8b during mDA patterning may not impact the robustness of midbrain marker expression (Kriks et al., 2011). One challenge of protracted FGF8b treatment is the fact that FGF8b is highly expressed in the hindbrain during early development (Liu et al., 2003). Therefore, induction of hindbrain markers, such as HOXA2 and GBX2, can occur following high dose FGF8b treatment (Kirkeby et al., 2017). Thus, the use of FGF8 needs to be very carefully timed and titrated to avoid hindbrain and potentially other FGF8-driven proliferative contaminants. Alternatively, it may be possible to find alternative strategies such as the use of midbrain-specific FGFs (Liu et al., 2003) to substitute for late FGF8b treatment or to develop strategies to selectively enrich for the desired phenotype for translational applications.
Another remaining challenge is to selectively generate populations of specific mDA neuron subtypes including A9 (SN) or A10 (Ventral tegmental area; VTA) mDA neurons. A9 mDA neurons are particularly vulnerable in PD (Damier et al., 1999; Surmeier et al., 2017) and are the main cell type of interest for cell therapy. Thus far, most studies used immunolabeling for two widely used markers, GIRK2 for A9 and CALBINDIN for A10 (Chung et al., 2005) to characterize mDA neurons subtypes in vitro or upon transplantation. Several studies reported the presence of ∼60–80% of GIRK2 + TH + neurons and ∼10–20% of CALB1 + TH+ in dorso-striatal grafts after 4.5–6 months post hPSC-mDA neuron transplantation (Table 1). However, those two markers are likely insufficient to segregate A9 versus A10 identity, and they may be particularly less reliable at early, prenatal stages of development. Importantly, neither marker is suitable to prospectively distinguish mDA neuron subtypes at the time of transplantation.
Other subtype selective markers of interest include ALDH1A1, which has been defined as an A9 type marker (Chung et al., 2011; Xi et al., 2012; Song et al., 2020). Both ALDH1A1 and the transcription factor SOX6 have been reported as specific molecular determinants expressed at the mDA neuron progenitor stage, marking precursors that later develop into ventro-lateral A9-type SN neurons (Panman et al., 2014; Blaess and Ang, 2015). Accordingly, several studies strived to induce the expression of ALDH1A1 and SOX6 during hPSC-differentiation. R-Spondin 2 (Gyllborg et al., 2018) and BMP5/7 (Jovanovic et al., 2018) were reported as candidate inducers of ALDH1A1 and SOX6 respectively in vitro. R-Spondin 2 treatment during floor-plate patterning increased ALDH1A1 expression by 5-fold compared to control by gene expression, but it remained unclear how those gene expression changes correspond to protein expression. Therefore, it will be important to determine the total numbers of ALDH1A1+/TH+ neurons that can be achieved under those conditions and the level of their functionality in vivo (Gyllborg et al., 2018). Similarly, despite promising data in the mouse, where BMP signaling mutants showed reduced numbers of A9 neurons postnatally, BMP5/7 exposure appeared to have minimal effects on enriching A9 mDA neurons in vitro and without clear information on the percentages of SOX6 + TH + neurons that can be obtained under those conditions (Jovanovic et al., 2018).
Despite many years of work using GIRK2, SOX6, and ALDH1A1 as individual A9 markers to characterize mDA neurons, the growing number of scRNAseq studies on mDA neuron subtypes (Poulin et al., 2014; La Manno et al., 2016; Tiklova et al., 2019) indicate that it is essential to multiplex markers for reliable subtype identification. For example, ALDH1A1 and SOX6 single positive cells can be found both in VTA and SN domains, whereas co-expression should identify SN neurons at the ventro-lateral location within the midbrain (Poulin et al., 2020). Given the availability of multiple protocols to efficiently derive floor-plate-derived mDA precursors, a key next step is to define the signaling cascades that restrict those precursors into pure mDA neurons (versus other floor-plate-derived neuronal lineages) and further into selective mDA neuron subtypes. Those efforts should go hand in hand with improved strategies to identify markers that capture stage and subtype identity during early developmental stages, stages relevant for cell transplantation.
Homogenous mDA Cell Population
The application of hPSC derivatives in human patients has progressed slowly due to potential risks associated with the use of pluripotent stem cells, where a few contaminating hPSCs could proliferate and develop into a teratoma or into early stage neuroepithelial tumors (Brederlau et al., 2006; Sonntag et al., 2007; Elkabetz et al., 2008). Furthermore, the transplantation of heterogenous neuronal population may entail the risk of side effects such as graft-induced dyskinesia (Dorsey et al., 2018) which have been attributed to the presence of serotonergic neurons in fetal grafts, as documented in grafted human PD patients by histological analysis and by functional studies using pharmacological and PET based assays (Politis et al., 2010). Without understanding the potential risks and side-effects that could come from “off-target” neuronal or non-neuronal populations, most groups strive to produce highly enriched and defined mDA neuron populations and to eliminate any contaminating cells to assure safety for clinical translation. Several strategies have been proposed to avoid unwanted cells either via enriching floor-plate or later stage mDA precursors using a surface marker (Doi et al., 2014; Samata et al., 2016; Lehnen et al., 2017) or via eliminating remaining undifferentiated hPSCs by exposing cultures during mDA neuron differentiation with natural compound, quercetin (Song et al., 2020). Those studies reported that purified mDA progenitors resulted in more homogenous graft size and mDA neuron density as well as in improved recovering motor dysfunctions in PD animal models than non-sorted cells without evidence of any tumor formation (Doi et al., 2014; Samata et al., 2016; Lehnen et al., 2017). However, while some markers such as Corin can enrich for floor-plate precursors, and NCAM or ALCAM (Bye et al., 2015) can enrich for certain neural cells, none of the currently available markers seems to be truly specific for mDA neuron lineage. Accordingly, there is currently no consensus on any strategy to enrich for mDA neurons reliably and new technologies will be needed for successful clinical translation. One strategy that appears capable of generating near homogenous populations of mDA neurons is to use genetic reporter PSC lines that mark either mDA progenitors or their post-mitotic progeny. We have recently reported that NURR1 (NR4A2) can serve as a reliable marker of early post-mitotic mDA neurons under floor-plate differentiation conditions. An NURR1:H2B-GFP reporter line allowed enrichment of mDA neurons in vitro that yielded nearly pure populations based on mDA markers such as FOXA2, LMX1A, NURR1 and TH including by using flow based and sc-RT-qPCR analyses (Riessland et al., 2019). While genetic engineering and FACS-based purification of neurons presents major challenges of human translation, it may be possible to adapt this approach for MACS-based or genetic selection strategies more suitable or translation. In either case, the use of genetic reporters is a powerful tool to define the appropriate stage of mDA neuron development for transplantation (see below). Similarly, once we better define early markers that can segregate mDA neurons, we can also apply those and other novel markers to selectively isolate A9-versus A10 type mDA neurons in vitro.
Stage of mDA Cell for Grafting
Over the last decades, fetal tissue transplantation studies have provided tremendous insights how to reconstruct the damaged brain of a PD patient. One such aspect was the discovery of the optimal window for isolating mesencephalic cells from fetal tissue. Human mDA neurogenesis occurs during a narrow window of early CNS development at week 6–8.5 p.c. (Olson et al., 1973; Freeman et al., 1991). Interestingly, human fetal grafts derived from tissue later than 9 weeks showed reduced survival, particularly when injected as cell suspension, and did not reliably yield functional mDA neurons in vivo (Freeman et al., 1995; Almqvist et al., 1996; Olanow et al., 1996). In contrast, tissue derived from fetuses at 5.5–8 weeks p.c. has yielded the best results in pre-clinical and clinical results with robust survival of mDA neuron rich grafts. One additional important point regarding the age of the donor may be the differential yield in production of mDA neuronal subtypes. Rodent studies have implicated that the younger E10 tissue gave rise to ∼75% GIRK2 + mDA neurons in intrastriatal grafts whereas older E12 tissue contributed 60% of GIRK2 + mDA neurons possibly due to staggered birth timing of A9 versus A10 mDA neurons (Gates et al., 2006; Bye et al., 2012).
Given the extensive push to develop floor-plate derived mDA neurons from hPSCs, it is essential to address questions about optimal stage of in vitro-derived cells for transplantation. From a simple, regenerative medicine perspective, one could argue that it makes the most sense to replace what is lost in PD, which are mature mDA neurons. However, as learned from the fetal tissue experience, mature mDA neurons may not survive upon transplantation into the adult brain, and thereby do not represent a viable option.
To systematically determine the optimal stage for transplantation, we previously reported in mouse ESCs on a side-by-side comparison of the engraftment potential of Hes5:GFP + progenitors, vs Nurr1 + neuroblasts vs Pitx3 + mDA neurons in vivo (Ganat et al., 2012). Pitx3 + mDA neurons survived only poorly resulting in smaller grafts with lower numbers of total mDA neurons and with worse behavioral data in drug-induced rotation tests as compared to both Hes5 + or Nurr1 + grafts. Our results indicated that Nurr1 may capture a suitable stage for transplantation while Pitx3 marks mDA neurons that are too mature for efficient in vivo engraftment. Interestingly, the Pitx3 finding was recently confirmed in an independent study using human ESC genetic reporter lines for PITX3 and for LMX1A (de Luzy et al., 2019). While PITX3 + mDA neuron showed very poor survival, LMX1A + cells at the floor-plate precursor stage showed efficient engraftment, albeit the resulting grafts (similar to Corin + grafts) were far from representing pure mDA neuron grafts (Gantner et al., 2020).
The identification of Nurr1 + stage a suitable for grafting has been translated into human PSC-based differentiation studies where mDA neurons were isolated at day 25 of differentiation, the stage of high NURR1 expression when most cells have started to exit cell cycle. Such early post-mitotic neurons appear to produce rich mDA neuron grafts and have resulted in successful rescue of complex motor abnormalities in animal PD models including mice, rat, and monkey (Kriks et al., 2011; Ganat et al., 2012; Steinbeck et al., 2015). Other teams such as the group by Isacson and Kim used similar strategy to transplant their cells after expanding mDA progenitors for a certain period (Song et al., 2020). Jun Takahashi’s group transplanted later, day 28 mDA “progenitors,” which are differentiated further starting from floor-plate progenitor stage sorted by CORIN at day 12 (Doi et al., 2014; Kikuchi et al., 2017). In contrast, the Parmar team demonstrated that relatively early stage, day 16 mDA progenitors, just beyond floor-plate precursor stage are also suitable for intracerebral transplantations (Kirkeby et al., 2012, 2017; Grealish et al., 2014, 2015; Cardoso et al., 2018; Adler et al., 2019). Despite the relatively earlier time point for transplantation, the protocol strives to transition the progenitors toward a “neural” fate via concurrent treatment of FGF8b (Kirkeby et al., 2017; Nolbrant et al., 2017), BDNF, and ascorbic acid (AA) (Yan et al., 2001) for about a week to achieve progenitor cell expansion and in vitro differentiation toward mDA neuron stage. In conclusion, the transplantation of the cells starting from late midbrain floor-plate to an mDA neuroblast stage or early mDA neuron may be all suitable for robust survival and function, but additional side-by-side transplantation studies using human PSCs will be required to fully explore this point, particularly for the mDA stage-dependent extent of DA fiber outgrowth and synaptic integration.
A parallel strategy to further improve graft composition is the generation of extensive molecular data on hPSC-derived mDA cells prior to transplantation and correlating those data with the subsequent performance of grafted cells (Kirkeby et al., 2017; Tiklova et al., 2020). This strategy should allow the iterative optimization of graft composition to fine-tune both subtype identity and stage of the grafted cells for optimal functional results. Such technology can also be further combined with the use of prospective lineage tracking and barcoding methods to link cell identity to graft outcome.
Location of the Cell Injected
Cell transplantation in PD has mainly focused on ectopic placement of cells within the striatal target region, far away from the site of degeneration in the SN. The rationale for this approach arose from the concern that the extent of the mDA axonal outgrowth from the graft may not suffice to efficiently innervate the human caudate or putamen. Additionally, in some of the studies, DA neurons did not survive as well when grafted in the midbrain compared to striatum (Thompson and Bjorklund, 2012). Furthermore, grafting studies over the last decade have demonstrated that intra-striatal grafting of fetal tissue is sufficient for restoring striatal dopamine release and inducing recovery from PD-relevant symptoms at least a subset of patients (Kefalopoulou et al., 2014). Ectopic transplantation, however, raises the concern that grafted cells may lack major afferent inputs of endogenous mDA neurons in SN, inputs known to play important roles in phasic regulation of nigrostriatal neuron activity. This lack of afferent control may restrict the ability of the cells to improve more complex motor behaviors (Winkler et al., 2000; Björklund et al., 2003). Furthermore, there is increasing evidence that DA released from dendrites locally in the SN may have important physiological functions distinct from striatal DA release (Cheramy et al., 1981). Therefore, several groups have pursued a long-term goal of orthotopic transplantation using hPSCs-derived mDA cells into the SN.
The Parmar group demonstrated that mDA progenitors grafted into the murine SN can extend long axons over 10 mm from the graft core innervating various rat brain structures including caudate-putamen, nucleus accumbens, and amygdala (Grealish et al., 2015). Using pseudo rabies-virus-based retrograde, monosynaptic tracing technology (Grealish et al., 2015; Cardoso et al., 2018), they demonstrated the establishment of afferent inputs on the grafted cells from the host starting by 6 weeks post transplantation (Grealish et al., 2015). Those inputs appear largely dependent on graft placement (Grealish et al., 2015; Cardoso et al., 2018). However, it remains unclear to what extent differences in afferent synaptic inputs, such as exaggerated thalamic but reduced hypothalamic and raphe inputs, affect the functional behavior of striatal versus nigral placed mDA cell grafts (Adler et al., 2019).
Furthermore, the behavioral readouts in intranigral grafting studies were typically limited to drug-induced rotation tests, which are not dependent on afferent input of the transplant (Winkler et al., 2000). Therefore, it would be interesting to test whether intranigral grafting can trigger rescue of more complex motor behaviors and whether those are dependent on afferent input or possibly on nigral DA release. Additionally, the human brain is much larger than rodent or primate brains, and thus homotopic grafting may be more challenging to achieve meaningful projections from the SN to appropriate regions within the striatum such as the post-commissural putamen. In human fetal tissue grafting studies, SN injections have been attempted but only in combination with striatal injections (Mendez et al., 2005), which makes it difficult to assess the relative contribution of each site to any clinical parameters. However, the technology to deliver hPSC-derived mDA progenitors in the SN will likely improve, and optimization of graft composition, the addition of growth promoting factors or even exercise of the host brain may contribute to achieve reliable restoration of the nigro-striatal circuit (Torikoshi et al., 2020).
mDA Cell Dosage for Transplantation
Typically, 200,000–420,000 dopamine neurons reside in human midbrain, and it is estimated that 50% loss of those DA neurons leads to the PD symptom (Brichta and Greengard, 2014). According to preclinical studies using fetal tissue or hPSC-derived mDA cell, 1200–2400 surviving TH + neurons in rat, 13,000 in primate, 40,000–80,000 in the human brain may be required to achieve a meaningful therapeutic effect (Hallett et al., 2015; Harris et al., 2020). The current bottleneck in delivering cells to the brain is that typically less than 10% of grafted mDA neurons survive following transplantation (Brundin et al., 2000; Thompson and Bjorklund, 2012; Hallett et al., 2014). Multiple factor may contribute to poor mDA neuron survival including mechanical trauma, growth factor deprivation, initial lack of vascularization, hypoxia and free radical production, or excessive extracellular concentrations of excitatory amino acids in the host brain (Brundin et al., 2000).
The Takahashi group embarked on the first human clinical trial by injecting 2.4 million iPSC-derived mDA cells in 2018. The Kim group injected 4 million iPSC-derived mDA precursors in one patient (Schweitzer et al., 2020). Upcoming human clinical trials in New York and Europe propose two different starting doses – high and low – for their proposed early stage (Phase I/IIa) clinical trials to assess the feasibility and safety of various dosages (Barker et al., 2017). Several studies have been proposed to enhance fetal or PSC-derived mDA neuron grafts such as by promoting their survival or function. Examples include treatment with pifithrin-alpha, increase of polysialic acid levels (Chou et al., 2011; Battista et al., 2014), or delivery of neurotrophic factors such as glial cell line-derived neurotrophic factor (GDNF) which may provide benefits to facilitate graft integration and functional recovery (Rosenblad et al., 1996; Redmond et al., 2009; Gantner et al., 2020).
Next Generation of mDA Cell Products
Immuno-Compatible mDA Neuron
Past clinical fetal and hPSC-based studies have mostly focused on the use of allogenic cell sources for transplantation. Given the relatively mild reaction to allogenic grafts in the CNS, the use of only transient immunosuppression offers long-term graft survival for >20 years (Li et al., 2016). In fact, some groups have performed fetal transplantation without any immunosuppression and showed long-term graft survival, albeit possibly with reduced mDA neuron numbers. Studies in the mouse (Tabar et al., 2008) and in primates (Morizane et al., 2017) showed concordant results suggesting that allografting results in mDA neuron survival but at lower rates, particularly if there is a considerable mismatch between host and graft. While those differences seem to disappear in the presence of transient immunosuppression (Morizane et al., 2017) grafting matched cells back into non-human primate hosts has been demonstrated by several groups (Hallett et al., 2015; Kikuchi et al., 2017) and most recently in one human individual (Schweitzer et al., 2020).
However, an isogenic mDA neuron approach raises the issue whether those patient-specific neurons may have a genetic predisposition that will make them more prone to succumb to disease after transplantation. In addition, such an approach is labor intensive and costly which will complicate future clinical implementation. In particular, it will be more difficult to establish extensive safety data for the cells in each individual patient and thereby increase clinical risk. Instead, transplantation of human leucocyte antigen (HLA)-matched iPSC-derived mDA cells has been proposed to minimize the risk of allograft rejection (Taylor et al., 2012). There are several ongoing efforts world-wide to establish HLA-homozygous iPSC lines that can match large proportions of the overall population. In fact, the first human clinical trial in Japan made use of one such HLA-homozygous lines for transplantation in PD (Normile, 2018), albeit still using transient immunosuppression.
Alternatively, there is considerable excitement in generating universal hPSC lines, which may provide immune tolerance without any immunosuppression. Using such an approach, theoretically, mDA neurons from a single universal hPSC line could be administrated to any PD patient worldwide. Proof-of-concept in establishing universal hPSC has achieved either by the combination of B2M gene knock-out with HLA-E overexpression (Gornalusse et al., 2017) or by knock-out of major histocompatibility complex (MHC) class I/II with CD47 (do not eat me signal) overexpression (Deuse et al., 2019). Given the potential of such engineered, universal cells to escape immune surveillance, implanting these cells should be pursued very carefully to avoid concerns regarding safety. In fact, those strategies are typically combined with integrating a drug-induced suicide switch in the cells as a failsafe (Gornalusse et al., 2017; Deuse et al., 2019).
Pathology-Resistant mDA Neuron
Lewy body formation is well known as one of the neuro-pathological hallmarks in PD (Braak et al., 2003), Interestingly, while no disease pathology was observed in PD patients over the first 10 years after transplantation, Lewy bodies have been identified in human fetal tissue grafts starting 11–16 years after the cell therapy with increasing percentages of affected cells by >20 years post grafting. As grafted cells were fetal tissue-derived and unlikely to have a PD predisposition, such Lewy body pathology suggests host-to-graft disease propagation (Kordower et al., 2008; Li et al., 2008). While progressing very slowly, such transmission could ultimately be toxic to transplanted mDA cells and limit the long-term efficacy of the cell therapy. Previous work suggests that both cytoplasmic insoluble and endogenous soluble alpha-synuclein protein is necessary to form the Lewy body and exert toxic effects on mDA neurons (Luk et al., 2009). Therefore, SNCA (alpha-synuclein) knockout (KO) or knock-down hPSC lines (Chen et al., 2019) may be an attractive strategy to yield pathology-resistant mDA neurons. The feasibility of this approach is further supported by the lack of major functional deficits in SNCA KO mice (Abeliovich et al., 2000).
Pure Substantia Nigra (A9-type) mDA Neuron
Recently, single cell gene profiling has been used to define subtype compositions during mouse and human midbrain development (Poulin et al., 2014; La Manno et al., 2016; Kee et al., 2017; Tiklova et al., 2019). Such technology extended the previous molecular definition of mDA neurons and enabled to further divide SN and VTA region into seven distinct molecular clusters (Poulin et al., 2020). However, to what extent these molecular findings can be translated into hPSC-derived mDA neuron development remains unexplored. This is illustrated when profiling of hPSC-derived mDA neurons in La Manno et al., which seem to recapitulate key stages of in vivo ventral midbrain development. However, those cell preparations expressed many poorly defined radial glial and neuroblast markers and differed from the in vivo phenotypes in gene expression (La Manno et al., 2016). Additionally, ALDH1A1 was not expressed in any of those PSC-derived mDA cells. This result may be due to the in vitro culture environment that does not fully support mDA neuron development or lack of proper induction of certain subtype-specific genes. In either case, those results indicate that there is considerable room for further improvements in mDA neuron derivation and maturation strategies.
The developmental ontogeny of distinct mDA neuron subtypes remains a long-standing question in the field. Now with rapidly evolving high throughput sequencing technology and lineage tracking tools, it becomes possible to revisit questions about ontogeny and to monitor the developmental trajectory at a single cell resolution from the acquisition of mDA precursor stage both in vitro to a fully functional mDA neurons many months after transplantation in vivo. Such data should enable a next generation of mDA neuron protocols to reap the full benefit of this approach for human clinical transplantation studies of the future.
Conclusion
Given the extensive history of cell replacement therapy using human fetal tissue and the rapid recent advances in human stem cell technology, there is considerable current activity around establishing and translating clinical-grade protocols into early stage trials in PD patients. However, despite the progress made thus far and results from the first clinical studies emerging soon, the scientific effort to develop improved grafting strategies should not stop here. It remains essential to carefully consider and address remaining bottlenecks in the field as reviewed in this article. Next generation of mDA cell products should address those remaining barriers on the road to making cell replacement possibly a routine therapeutic strategy that could become available to PD patients world-wide. Only if basic and translational efforts continue hand in hand, we will be able to capture the full potential of this approach in PD and pave the way for other regenerative approaches in brain repair.
Author Contributions
All authors contributed to the writing, reviewing, and editing the manuscript, and to the preparation of figures and tables.
Funding
Our own work discussed in this article was funded by a contract from NYSTEM and by grants from NINDS, the Starr Foundation, the Lise & Jeffrey Wilks Family Foundation, and the MJ Fox Foundation and the core grant P30CA008748.
Conflict of Interest
LS is a scientific founder and paid consultant of BlueRock Therapeutics and an inventor on patents related to the differentiation of dopamine neurons from pluripotent stem cells.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
- ^ https://www.sciencemag.org/news/2018/07/first-its-kind-clinical-trial-will-use-reprogramed-adult-stem-cells-treat-parkinson-s
References
Abeliovich, A., Schmitz, Y., Farinas, I., Choi-Lundberg, D., Ho, W. H., Castillo, P. E., et al. (2000). Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25, 239–252. doi: 10.1016/s0896-6273(00)80886-7
Adler, A. F., Cardoso, T., Nolbrant, S., Mattsson, B., Hoban, D. B., Jarl, U., et al. (2019). hESC-derived dopaminergic transplants integrate into basal ganglia circuitry in a preclinical model of parkinson’s disease. Cell Rep. 28, 3462–3473.e3465.
Almqvist, P. M., Akesson, E., Wahlberg, L. U., Pschera, H., Seiger, A., and Sundstrom, E. (1996). First trimester development of the human nigrostriatal dopamine system. Exp. Neurol. 139, 227–237. doi: 10.1006/exnr.1996.0096
Backlund, E. O., Granberg, P. O., Hamberger, B., Knutsson, E., Martensson, A., Sedvall, G., et al. (1985). Transplantation of adrenal medullary tissue to striatum in parkinsonism. First clinical trials. J. Neurosurg. 62, 169–173. doi: 10.3171/jns.1985.62.2.0169
Barker, R. A., Parmar, M., Studer, L., and Takahashi, J. (2017). Human trials of stem cell-derived dopamine neurons for parkinson’s disease: dawn of a new era. Cell Stem. Cell 21, 569–573. doi: 10.1016/j.stem.2017.09.014
Barker, R. A., Studer, L., Cattaneo, E., Takahashi, J., and Consortium, G.-F. P. (2015). G-Force PD: a global initiative in coordinating stem cell-based dopamine treatments for Parkinson’s disease. Npj. Parkinson Dis. 1:15017.
Battista, D., Ganat, Y., El Maarouf, A., Studer, L., and Rutishauser, U. (2014). Enhancement of polysialic acid expression improves function of embryonic stem-derived dopamine neuron grafts in Parkinsonian mice. Stem. Cells Transl. Med. 3, 108–113. doi: 10.5966/sctm.2013-0084
Björklund, A., Dunnett, S. B., Brundin, P., Stoessl, A. J., Freed, C. R., Breeze, R. E., et al. (2003). Neural transplantation for the treatment of Parkinson’s disease. Lancet Neurol. 2, 437–445.
Blaess, S., and Ang, S. L. (2015). Genetic control of midbrain dopaminergic neuron development. Wiley Interdiscip. Rev. Dev. Biol. 4, 113–134. doi: 10.1002/wdev.169
Blau, H. M., and Daley, G. Q. (2019). Stem cells in the treatment of disease. N. Engl. J. Med. 380, 1748–1760.
Bonilla, S., Hall, A. C., Pinto, L., Attardo, A., Gotz, M., Huttner, W. B., et al. (2008). Identification of midbrain floor plate radial glia-like cells as dopaminergic progenitors. Glia 56, 809–820. doi: 10.1002/glia.20654
Braak, H., Del Tredici, K., Rub, U., de Vos, R. A. I., Steur, E. N. H. J., and Braak, E. (2003). Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 24, 197–211. doi: 10.1016/s0197-4580(02)00065-9
Brederlau, A., Correia, A. S., Anisimov, S. V., Elmi, M., Paul, G., Roybon, L., et al. (2006). Transplantation of human embryonic stem cell-derived cells to a rat model of Parkinson’s disease: effect of in vitro differentiation on graft survival and teratoma formation. Stem. Cells 24, 1433–1440. doi: 10.1634/stemcells.2005-0393
Brichta, L., and Greengard, P. (2014). Molecular determinants of selective dopaminergic vulnerability in Parkinson’s disease: an update. Front. Neuroanat. 8:152.
Brundin, P., Karlsson, J., Emgard, M., Schierle, G. S., Hansson, O., Petersen, A., et al. (2000). Improving the survival of grafted dopaminergic neurons: a review over current approaches. Cell Transplant. 9, 179–195.
Bye, C. R., Jonsson, M. E., Bjorklund, A., Parish, C. L., and Thompson, L. H. (2015). Transcriptome analysis reveals transmembrane targets on transplantable midbrain dopamine progenitors. Proc. Natl. Acad. Sci. U.S.A. 112, E1946–E1955.
Bye, C. R., Thompson, L. H., and Parish, C. L. (2012). Birth dating of midbrain dopamine neurons identifies A9 enriched tissue for transplantation into parkinsonian mice. Exp. Neurol. 236, 58–68. doi: 10.1016/j.expneurol.2012.04.002
Cardoso, T., Adler, A. F., Mattsson, B., Hoban, D. B., Nolbrant, S., Wahlestedt, J. N., et al. (2018). Target-specific forebrain projections and appropriate synaptic inputs of hESC-derived dopamine neurons grafted to the midbrain of parkinsonian rats. J. Compar. Neurol. 526, 2133–2146. doi: 10.1002/cne.24500
Chambers, S. M., Fasano, C. A., Papapetrou, E. P., Tomishima, M., Sadelain, M., and Studer, L. (2009). Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 27, 275–280. doi: 10.1038/nbt.1529
Chen, Y., Dolt, K. S., Kriek, M., Baker, T., Downey, P., Drummond, N. J., et al. (2019). Engineering synucleinopathy-resistant human dopaminergic neurons by CRISPR-mediated deletion of the SNCA gene. Eur. J. Neurosci. 49, 510–524. doi: 10.1111/ejn.14286
Chen, Y., Xiong, M., Dong, Y., Haberman, A., Cao, J., Liu, H., et al. (2016). Chemical control of grafted human PSC-Derived neurons in a mouse model of parkinson’s disease. Cell Stem. Cell 18, 817–826. doi: 10.1016/j.stem.2016.03.014
Cheramy, A., Leviel, V., and Glowinski, J. (1981). Dendritic release of dopamine in the substantia nigra. Nature 289, 537–542.
Chou, J., Greig, N. H., Reiner, D., Hoffer, B. J., and Wang, Y. (2011). Enhanced survival of dopaminergic neuronal transplants in hemiparkinsonian rats by the p53 inactivator PFT-alpha. Cell Transplant 20, 1351–1359.
Chung, C. Y., Seo, H., Sonntag, K. C., Brooks, A., Lin, L., and Isacson, O. (2005). Cell type-specific gene expression of midbrain dopaminergic neurons reveals molecules involved in their vulnerability and protection. Hum. Mol. Genet. 14, 1709–1725. doi: 10.1093/hmg/ddi178
Chung, S., Moon, J. I., Leung, A., Aldrich, D., Lukianov, S., Kitayama, Y., et al. (2011). ES cell-derived renewable and functional midbrain dopaminergic progenitors. Proc. Natl. Acad. Sci. U.S.A. 108, 9703–9708. doi: 10.1073/pnas.1016443108
Damier, P., Hirsch, E. C., Agid, Y., and Graybiel, A. M. (1999). The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain 122(Pt 8), 1437–1448.
de Luzy, I. R., Niclis, J. C., Gantner, C. W., Kauhausen, J. A., Hunt, C. P. J., Ermine, C., et al. (2019). Isolation of LMX1a ventral midbrain progenitors improves the safety and predictability of human pluripotent stem cell-derived neural transplants in parkinsonian disease. J. Neurosci. 39, 9521–9531. doi: 10.1523/jneurosci.1160-19.2019
Deuse, T., Hu, X., Gravina, A., Wang, D., Tediashvili, G., De, C., et al. (2019). Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat. Biotechnol. 37, 252–258. doi: 10.1038/s41587-019-0016-3
Doi, D., Samata, B., Katsukawa, M., Kikuchi, T., Morizane, A., Ono, Y., et al. (2014). Isolation of human induced pluripotent stem cell-derived dopaminergic progenitors by cell sorting for successful transplantation. Stem. Cell Rep. 2, 337–350. doi: 10.1016/j.stemcr.2014.01.013
Dorsey, E. R., Elbaz, A., Nichols, E., Abd-Allah, F., Abdelalim, A., Adsuar, J. C., et al. (2018). Global, regional, and national burden of Parkinson’s disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 17, 939–953.
Elkabetz, Y., Panagiotakos, G., Al Shamy, G., Socci, N. D., Tabar, V., and Studer, L. (2008). Human ES cell-derived neural rosettes reveal a functionally distinct early neural stem cell stage. Genes Dev. 22, 152–165. doi: 10.1101/gad.1616208
Fox, I. J., Daley, G. Q., Goldman, S. A., Huard, J., Kamp, T. J., and Trucco, M. (2014). Stem cell therapy. Use of differentiated pluripotent stem cells as replacement therapy for treating disease. Science 345, 1247391. doi: 10.1126/science.1247391
Freed, C. R., Breeze, R. E., Rosenberg, N. L., Schneck, S. A., Kriek, E., Qi, J. X., et al. (1992). Survival of implanted fetal dopamine cells and neurologic improvement 12 to 46 months after transplantation for parkinsons-disease. New. Engl. J. Med. 327, 1549–1555. doi: 10.1056/nejm199211263272202
Freeman, T. B., Sanberg, P. R., Nauert, G. M., Boss, B. D., Spector, D., Olanow, C. W., et al. (1995). The influence of donor age on the survival of solid and suspension intraparenchymal human embryonic nigral grafts. Cell Transplant 4, 141–154. doi: 10.1016/0963-6897(94)00048-o
Freeman, T. B., Spence, M. S., Boss, B. D., Spector, D. H., Strecker, R. E., Olanow, C. W., et al. (1991). Development of dopaminergic neurons in the human substantia nigra. Exp. Neurol. 113, 344–353.
Ganat, Y. M., Calder, E. L., Kriks, S., Nelander, J., Tu, E. Y., Jia, F., et al. (2012). Identification of embryonic stem cell-derived midbrain dopaminergic neurons for engraftment. J. Clin. Invest. 122, 2928–2939. doi: 10.1172/jci58767
Gantner, C. W., De Luzy, I. R., Kauhausen, J. A., Moriarty, N., Niclis, J. C., Bye, C. R., et al. (2020). Viral Delivery of GDNF promotes functional integration of human stem cell grafts in parkinson’s disease. Cell Stem Cell 26, 511–526.e515.
Gates, M. A., Torres, E. M., White, A., Fricker-Gates, R. A., and Dunnett, S. B. (2006). Re-examining the ontogeny of substantia nigra dopamine neurons. Eur. J. Neurosci. 23, 1384–1390. doi: 10.1111/j.1460-9568.2006.04637.x
Goetz, C. G., Olanow, C. W., Koller, W. C., Penn, R. D., Cahill, D., Morantz, R., et al. (1989). Multicenter study of autologous adrenal medullary transplantation to the corpus striatum in patients with advanced Parkinson’s disease. N. Engl. J. Med. 320, 337–341. doi: 10.1056/nejm198902093200601
Gornalusse, G. G., Hirata, R. K., Funk, S. E., Riolobos, L., Lopes, V. S., Manske, G., et al. (2017). HLA-E-expressing pluripotent stem cells escape allogeneic responses and lysis by NK cells. Nat. Biotechnol. 35, 765–772. doi: 10.1038/nbt.3860
Grealish, S., Diguet, E., Kirkeby, A., Mattsson, B., Heuer, A., Bramoulle, Y., et al. (2014). Human ESC-derived dopamine neurons show similar preclinical efficacy and potency to fetal neurons when grafted in a rat model of Parkinson’s disease. Cell Stem Cell 15, 653–665. doi: 10.1016/j.stem.2014.09.017
Grealish, S., Heuer, A., Cardoso, T., Kirkeby, A., Jonsson, M., Johansson, J., et al. (2015). Monosynaptic tracing using modified rabies virus reveals early and extensive circuit integration of human embryonic stem cell-derived neurons. Stem Cell Rep. 4, 975–983. doi: 10.1016/j.stemcr.2015.04.011
Gyllborg, D., Ahmed, M., Toledo, E. M., Theofilopoulos, S., Yang, S., Ffrench-Constant, C., et al. (2018). The matricellular protein R-Spondin 2 promotes midbrain dopaminergic neurogenesis and differentiation. Stem Cell Rep. 11, 651–664. doi: 10.1016/j.stemcr.2018.07.014
Hallett, P. J., Cooper, O., Sadi, D., Robertson, H., Mendez, I., and Isacson, O. (2014). Long-term health of dopaminergic neuron transplants in Parkinson’s disease patients. Cell Rep. 7, 1755–1761. doi: 10.1016/j.celrep.2014.05.027
Hallett, P. J., Deleidi, M., Astradsson, A., Smith, G. A., Cooper, O., Osborn, T. M., et al. (2015). Successful function of autologous iPSC-derived dopamine neurons following transplantation in a non-human primate model of Parkinson’s disease. Cell Stem Cell 16, 269–274. doi: 10.1016/j.stem.2015.01.018
Harris, J. P., Burrell, J. C., Struzyna, L. A., Chen, H. I., Serruya, M. D., Wolf, J. A., et al. (2020). Emerging regenerative medicine and tissue engineering strategies for Parkinson’s disease. NPJ. Parkinsons Dis. 6:4.
Jovanovic, V. M., Salti, A., Tilleman, H., Zega, K., Jukic, M. M., Zou, H., et al. (2018). BMP/SMAD pathway promotes neurogenesis of midbrain dopaminergic neurons in vivo and in human induced pluripotent and neural stem cells. J. Neurosci. 38, 1662–1676. doi: 10.1523/jneurosci.1540-17.2018
Kawasaki, H., Mizuseki, K., Nishikawa, S., Kaneko, S., Kuwana, Y., Nakanishi, S., et al. (2000). Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron 28, 31–40. doi: 10.1016/s0896-6273(00)00083-0
Kee, N., Volakakis, N., Kirkeby, A., Dahl, L., Storvall, H., Nolbrant, S., et al. (2017). Single-cell analysis reveals a close relationship between differentiating dopamine and subthalamic nucleus neuronal lineages. Cell Stem. Cell 20, 29–40. doi: 10.1016/j.stem.2016.10.003
Kefalopoulou, Z., Politis, M., Piccini, P., Mencacci, N., Bhatia, K., Jahanshahi, M., et al. (2014). Long-term clinical outcome of fetal cell transplantation for Parkinson disease: two case reports. JAMA Neurol. 71, 83–87.
Kikuchi, T., Morizane, A., Doi, D., Magotani, H., Onoe, H., Hayashi, T., et al. (2017). Human iPS cell-derived dopaminergic neurons function in a primate Parkinson’s disease model. Nature 548, 592–596. doi: 10.1038/nature23664
Kirkeby, A., Grealish, S., Wolf, D. A., Nelander, J., Wood, J., Lundblad, M., et al. (2012). Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Rep. 1, 703–714. doi: 10.1016/j.celrep.2012.04.009
Kirkeby, A., Nolbrant, S., Tiklova, K., Heuer, A., Kee, N., Cardoso, T., et al. (2017). Predictive markers guide differentiation to improve graft outcome in clinical translation of hesc-based therapy for parkinson’s disease. Cell Stem Cell 20, 135–148. doi: 10.1016/j.stem.2016.09.004
Kordower, J. H., Chu, Y., Hauser, R. A., Freeman, T. B., and Olanow, C. W. (2008). Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat. Med. 14, 504–506. doi: 10.1038/nm1747
Kriks, S., Shim, J. W., Piao, J., Ganat, Y. M., Wakeman, D. R., Xie, Z., et al. (2011). Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature 480, 547–551. doi: 10.1038/nature10648
La Manno, G., Gyllborg, D., Codeluppi, S., Nishimura, K., Salto, C., Zeisel, A., et al. (2016). Molecular diversity of midbrain development in mouse, human, and stem cells. Cell 167, 566–580.e519.
Lehnen, D., Barral, S., Cardoso, T., Grealish, S., Heuer, A., Smiyakin, A., et al. (2017). IAP-based cell sorting results in homogeneous transplantable dopaminergic precursor cells derived from human pluripotent stem cells. Stem Cell Rep. 9, 1207–1220. doi: 10.1016/j.stemcr.2017.08.016
Li, J. Y., Englund, E., Holton, J. L., Soulet, D., Hagell, P., Lees, A. J., et al. (2008). Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat. Med. 14, 501–503. doi: 10.1038/nm1746
Li, W., Englund, E., Widner, H., Mattsson, B., Van Westen, D., Latt, J., et al. (2016). Extensive graft-derived dopaminergic innervation is maintained 24 years after transplantation in the degenerating parkinsonian brain. Proc. Natl. Acad. Sci. U.S.A. 113, 6544–6549. doi: 10.1073/pnas.1605245113
Lindvall, O., Brundin, P., Widner, H., Rehncrona, S., Gustavii, B., Frackowiak, R., et al. (1990). Grafts of fetal dopamine neurons survive and improve motor function in parkinsons-disease. Science 247, 574–577. doi: 10.1126/science.2105529
Liu, A., Li, J. Y., Bromleigh, C., Lao, Z., Niswander, L. A., and Joyner, A. L. (2003). FGF17b and FGF18 have different midbrain regulatory properties from FGF8b or activated FGF receptors. Development 130, 6175–6185. doi: 10.1242/dev.00845
Luk, K. C., Song, C., O’Brien, P., Stieber, A., Branch, J. R., Brunden, K. R., et al. (2009). Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc. Natl. Acad. Sci. U.S.A. 106, 20051–20056. doi: 10.1073/pnas.0908005106
Mendez, I., Sanchez-Pernaute, R., Cooper, O., Vinuela, A., Ferrari, D., Bjorklund, L., et al. (2005). Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson’s disease. Brain 128, 1498–1510. doi: 10.1093/brain/awh510
Mingote, S., Chuhma, N., Kusnoor, S. V., Field, B., Deutch, A. Y., and Rayport, S. (2015). Functional connectome analysis of dopamine neuron glutamatergic connections in forebrain regions. J. Neurosci. 35, 16259–16271. doi: 10.1523/jneurosci.1674-15.2015
Morizane, A., Kikuchi, T., Hayashi, T., Mizuma, H., Takara, S., Doi, H., et al. (2017). MHC matching improves engraftment of iPSC-derived neurons in non-human primates. Nat. Commun. 8:385.
Niclis, J. C., Gantner, C. W., Hunt, C. P. J., Kauhausen, J. A., Durnall, J. C., Haynes, J. M., et al. (2017). A PITX3-EGFP reporter line reveals connectivity of dopamine and non-dopamine neuronal subtypes in grafts generated from human embryonic stem cells. Stem Cell Rep. 9, 868–882. doi: 10.1016/j.stemcr.2017.08.002
Nolbrant, S., Heuer, A., Parmar, M., and Kirkeby, A. (2017). Generation of high-purity human ventral midbrain dopaminergic progenitors for in vitro maturation and intracerebral transplantation. Nat. Protoc. 12, 1962–1979. doi: 10.1038/nprot.2017.078
Normile, D. (2018). First-of-its-kind clinical trial will use reprogrammed adult stem cells to treat Parkinson’s. Science 355, 1109–1110. doi: 10.1126/science.aau9466
Nouri, N., and Awatramani, R. (2017). A novel floor plate boundary defined by adjacent En1 and Dbx1 microdomains distinguishes midbrain dopamine and hypothalamic neurons. Development 144, 916–927. doi: 10.1242/dev.144949
Olanow, C. W., Kordower, J. H., and Freeman, T. B. (1996). Fetal nigral transplantation as a therapy for Parkinson’s disease. Trends Neurosci. 19, 102–109. doi: 10.1016/s0166-2236(96)80038-5
Olson, L., Boréus, L. O., and Seiger, Å (1973). Histochemical demonstration and mapping of 5-hydroxytryptamine- and catecholamine-containing neuron systems in the human fetal brain. Zeitschr. Anat. Entwicklung. 139, 259–282. doi: 10.1007/bf00519968
Ono, Y., Nakatani, T., Sakamoto, Y., Mizuhara, E., Minaki, Y., Kumai, M., et al. (2007). Differences in neurogenic potential in floor plate cells along an anteroposterior location: midbrain dopaminergic neurons originate from mesencephalic floor plate cells. Development 134, 3213–3225. doi: 10.1242/dev.02879
Panman, L., Papathanou, M., Laguna, A., Oosterveen, T., Volakakis, N., Acampora, D., et al. (2014). Sox6 and Otx2 control the specification of substantia nigra and ventral tegmental area dopamine neurons. Cell Rep. 8, 1018–1025. doi: 10.1016/j.celrep.2014.07.016
Park, C. H., Minn, Y. K., Lee, J. Y., Choi, D. H., Chang, M. Y., Shim, J. W., et al. (2005). In vitro and in vivo analyses of human embryonic stem cell-derived dopamine neurons. J. Neurochem. 92, 1265–1276. doi: 10.1111/j.1471-4159.2004.03006.x
Parmar, M., Grealish, S., and Henchcliffe, C. (2020). The future of stem cell therapies for Parkinson disease. Nat. Rev. Neurosci. 21, 103–115. doi: 10.1038/s41583-019-0257-7
Perrier, A. L., Tabar, V., Barberi, T., Rubio, M. E., Bruses, J., Topf, N., et al. (2004). Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc. Natl. Acad. Sci. U.S.A. 101, 12543–12548. doi: 10.1073/pnas.0404700101
Poewe, W., Seppi, K., Tanner, C. M., Halliday, G. M., Brundin, P., Volkmann, J., et al. (2017). Parkinson disease. Nat. Rev. Dis. Primers 3:17013.
Politis, M., Wu, K., Loane, C., Quinn, N. P., Brooks, D. J., Rehncrona, S., et al. (2010). Serotonergic neurons mediate dyskinesia side effects in parkinson’s patients with neural transplants. Sci. Transl. Med. 2:8ra46.
Poulin, J. F., Gaertner, Z., Moreno-Ramos, O. A., and Awatramani, R. (2020). Classification of midbrain dopamine neurons using single-cell gene expression profiling approaches. Trends Neurosci. 43, 155–169. doi: 10.1016/j.tins.2020.01.004
Poulin, J. F., Zou, J., Drouin-Ouellet, J., Kim, K. Y., Cicchetti, F., and Awatramani, R. B. (2014). Defining midbrain dopaminergic neuron diversity by single-cell gene expression profiling. Cell Rep. 9, 930–943. doi: 10.1016/j.celrep.2014.10.008
Redmond, D. E. Jr., Elsworth, J. D., Roth, R. H., Leranth, C., Collier, T. J., Blanchard, B., et al. (2009). Embryonic substantia nigra grafts in the mesencephalon send neurites to the host striatum in non-human primate after overexpression of GDNF. J. Compar. Neurol. 515, 31–40. doi: 10.1002/cne.22028
Riessland, M., Kolisnyk, B., Kim, T. W., Cheng, J., Ni, J., Pearson, J. A., et al. (2019). Loss of SATB1 Induces a p21 dependent cellular senescence phenotype in dopaminergic neurons. Cell Stem Cell 10:30340. doi: 10.30310.31016/j.stem.32019.30308.30013
Rosenblad, C., Martinez-Serrano, A., and Bjorklund, A. (1996). Glial cell line-derived neurotrophic factor increases survival, growth and function of intrastriatal fetal nigral dopaminergic grafts. Neuroscience 75, 979–985.
Roy, N. S., Cleren, C., Singh, S. K., Yang, L., Beal, M. F., and Goldman, S. A. (2006). Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat. Med. 12, 1259–1268. doi: 10.1038/nm1495
Samata, B., Doi, D., Nishimura, K., Kikuchi, T., Watanabe, A., Sakamoto, Y., et al. (2016). Purification of functional human ES and iPSC-derived midbrain dopaminergic progenitors using LRTM1. Nat. Commun. 7:13097.
Schweitzer, J. S., Song, B., Herrington, T. M., Park, T. Y., Lee, N., Ko, S., et al. (2020). Personalized iPSC-derived dopamine progenitor cells for parkinson’s disease. N. Engl. J. Med. 382, 1926–1932.
Song, B., Cha, Y., Ko, S., Jeon, J., Lee, N., Seo, H., et al. (2020). Human autologous iPSC-derived dopaminergic progenitors restore motor function in Parkinson’s disease models. J. Clin. Invest. 130, 904–920. doi: 10.1172/jci130767
Sonntag, K. C., Pruszak, J., Yoshizaki, T., van Arensbergen, J., Sanchez-Pernaute, R., and Isacson, O. (2007). Enhanced yield of neuroepithelial precursors and midbrain-like dopaminergic neurons from human embryonic stem cells using the bone morphogenic protein antagonist noggin. Stem Cells 25, 411–418. doi: 10.1634/stemcells.2006-0380
Steinbeck, J. A., Choi, S. J., Mrejeru, A., Ganat, Y., Deisseroth, K., Sulzer, D., et al. (2015). Optogenetics enables functional analysis of human embryonic stem cell-derived grafts in a Parkinson’s disease model. Nat. Biotechnol. 33, 204–209. doi: 10.1038/nbt.3124
Steinbeck, J. A., and Studer, L. (2015). Moving stem cells to the clinic: potential and limitations for brain repair. Neuron 86, 187–206. doi: 10.1016/j.neuron.2015.03.002
Sundberg, M., Bogetofte, H., Lawson, T., Jansson, J., Smith, G., Astradsson, A., et al. (2013). Improved cell therapy protocols for Parkinson’s disease based on differentiation efficiency and safety of hESC-, hiPSC-, and non-human primate iPSC-derived dopaminergic neurons. Stem Cells 31, 1548–1562. doi: 10.1002/stem.1415
Surmeier, D. J., Obeso, J. A., and Halliday, G. M. (2017). Selective neuronal vulnerability in parkinson disease. Nat. Rev. Neurosci. 18, 101–113. doi: 10.1038/nrn.2016.178
Tabar, V., and Studer, L. (2014). Pluripotent stem cells in regenerative medicine: challenges and recent progress. Nat. Rev. Genet. 15, 82–92. doi: 10.1038/nrg3563
Tabar, V., Tomishima, M., Panagiotakos, G., Wakayama, S., Menon, J., Chan, B., et al. (2008). Therapeutic cloning in individual parkinsonian mice. Nat. Med. 14, 379–381. doi: 10.1038/nm1732
Taylor, C. J., Peacock, S., Chaudhry, A. N., Bradley, J. A., and Bolton, E. M. (2012). Generating an iPSC bank for HLA-matched tissue transplantation based on known donor and recipient HLA types. Cell Stem Cell 11, 147–152. doi: 10.1016/j.stem.2012.07.014
Thompson, L., and Bjorklund, A. (2012). Survival, differentiation, and connectivity of ventral mesencephalic dopamine neurons following transplantation. Prog. Brain Res. 200, 61–95. doi: 10.1016/b978-0-444-59575-1.00004-1
Tiklova, K., Bjorklund, A. K., Lahti, L., Fiorenzano, A., Nolbrant, S., Gillberg, L., et al. (2019). Single-cell RNA sequencing reveals midbrain dopamine neuron diversity emerging during mouse brain development. Nat. Commun. 10:581.
Tiklova, K., Nolbrant, S., Fiorenzano, A., Bjorklund, A. K., Sharma, Y., Heuer, A., et al. (2020). Single cell transcriptomics identifies stem cell-derived graft composition in a model of Parkinson’s disease. Nat. Commun. 11:2434.
Torikoshi, S., Morizane, A., Shimogawa, T., Samata, B., Miyamoto, S., and Takahashi, J. (2020). Exercise promotes neurite extensions from grafted dopaminergic neurons in the direction of the dorsolateral striatum in parkinson’s disease model rats. J. Parkinsons Dis. 10, 511–521. doi: 10.3233/jpd-191755
Widner, H., Tetrud, J., Rehncrona, S., Snow, B., Brundin, P., Gustavii, B., et al. (1992). Bilateral fetal mesencephalic grafting in two patients with parkinsonism induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). N. Engl. J. Med. 327, 1556–1563. doi: 10.1056/nejm199211263272203
Winkler, A. S., Baethmann, A., Peters, J., Kempski, O., and Staub, F. (2000). Mechanisms of arachidonic acid induced glial swelling. Brain Res. Mol. Brain Res. 76, 419–423. doi: 10.1016/s0169-328x(00)00017-6
Xi, J., Liu, Y., Liu, H., Chen, H., Emborg, M. E., and Zhang, S. C. (2012). Specification of midbrain dopamine neurons from primate pluripotent stem cells. Stem Cells 30, 1655–1663. doi: 10.1002/stem.1152
Keywords: pluripotent stem cells, Parkinson’s disease, neural transplantation, directed differentiation, dopamine neuron, midbrain development, regenerative medicine
Citation: Kim TW, Koo SY and Studer L (2020) Pluripotent Stem Cell Therapies for Parkinson Disease: Present Challenges and Future Opportunities. Front. Cell Dev. Biol. 8:729. doi: 10.3389/fcell.2020.00729
Received: 04 June 2020; Accepted: 15 July 2020;
Published: 06 August 2020.
Edited by:
Sandra Blaess, University of Bonn, GermanyReviewed by:
Nilima Prakash, Hamm-Lippstadt University of Applied Sciences, GermanyClare Parish, The University of Melbourne, Australia
Copyright © 2020 Kim, Koo and Studer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lorenz Studer, c3R1ZGVybEBtc2tjYy5vcmc=
†These authors have contributed equally to this work
 Tae Wan Kim
Tae Wan Kim So Yeon Koo
So Yeon Koo Lorenz Studer
Lorenz Studer