- 1Laboratary for Bone Metabolism, Key Lab for Space Biosciences and Biotechnology, School of Life Sciences, Northwestern Polytechnical University, Xi’an, China
- 2Xi’an Key Laboratory of Special Medicine and Health Engineering, School of Life Sciences, Northwestern Polytechnical University, Xi’an, China
- 3Research Center for Special Medicine and Health Systems Engineering, School of Life Sciences, Northwestern Polytechnical University, Xi’an, China
- 4NPU-UAB Joint Laboratory for Bone Metabolism, School of Life Sciences, Northwestern Polytechnical University, Xi’an, China
Bone is crucial for supporting the body, protecting other organs, providing minerals, and secreting hormone to regulate other organ’s function. Bone disorders result in pain and disability, severely affecting human health, reducing the quality of life and increasing costs to society. With the rapid increase in the aging population worldwide, bone disorders have become one major disease. As a result, efficacious therapies of bone disorders have become the focus of attention worldwide. Mesenchymal stem cells (MSCs) have been widely explored as a new therapeutic method for numerous diseases. Recent evidence suggests that the therapeutic effects of MSCs are mainly mediated by their extracellular vesicles (EV). MSCs-derived extracellular vesicles (MSCs-EV) is indicated as a novel cell-free alternative to cell therapy with MSCs in regenerative medicine. Here, we review the current knowledge of EV and highlight the application studies of MSCs-EV in bone disorders by focusing on osteoarthritis (OA), rheumatoid arthritis (RA), osteoporosis (OP), and bone fracture. Moreover, we discuss the key issues and perspectives of MSCs-EV as a clinical therapeutic strategy for bone diseases.
Introduction
Bone, one most important supportive structure of human body, plays important roles in supporting body, protecting other organs, providing minerals and secreting hormone to regulate other organs. Bone disorders have a serious impact on human health and have become common diseases among the elderly. Therefore, appropriate and effective treatment of bone diseases is one of the greatest concerns in the world today.
Mesenchymal stem cells (MSCs) are multipotent fibroblast-like cells with the potential for self-renewal and multilineage differentiation. Moreover, MSCs possess the ability to migrate to the damaged sites, where they secret anti-inflammatory factors and growth factors to promote wound healing. Thus, MSCs have become a desirable cell source in regenerative medicine and immune therapy. MSCs transplant treatment (MSCT) is considered to be a promising therapy for a variety of human diseases and has drawn increasing attention. However, the direct use of MSCs for treating diseases faces many challenges, including genetic instability (Yoon et al., 2004; Tolar et al., 2007; Jeong et al., 2011), loss of function (Pak et al., 2003), pathogenicity (Furlani et al., 2009), and limited cell survival (Breitbach et al., 2007).
Recent evidence suggest that MSCs exert their therapeutic capabilities primarily through paracrine secretion of particles, rather than a cellular manner (Wang et al., 2015; Cosenza et al., 2017; Zhou Y. et al., 2019). The secreted particles are collectively referred to as extracellular vesicles (EV), which are usually classified as exosomes, microvesicles (MV), and apoptotic bodies based on different biogenesis way (Yáñez-Mó et al., 2015). Due to the lack of consensus on specific markers for EV subtypes, ISEV recommends the usage of EV, a collective term covering various EV subtypes (Thery et al., 2018). MSCs-derived extracellular vesicles (MSCs-EV) emerge as critical mediators of paracrine effects, with increasing evidence for their function in MSCs-mediated regeneration and immunotherapy (Li et al., 2013; Xin et al., 2013; Tan et al., 2014; Zhao et al., 2018; Biswas et al., 2019; Zhang S. et al., 2019). MSCs-EV regulate the function of recipient cells by transmitting information carried by lipids, nucleic acids and proteins, which are the main components of MSCs-EV (Yáñez-Mó et al., 2015). Compared with MSCT, MSCs-EV treatment show advantages of higher security, convenient storage, transportation and administration (Jing et al., 2018). Therefore, MSCs-EV has received increasing attention for its promising clinical application (Toh et al., 2017; Willis et al., 2018; Romanelli et al., 2019).
Here, we review the current knowledge of extracellular vesicles (EV) and MSCs-derived extracellular vesicles (MSCs-EV), and highlight the application studies of MSCs-EV for treatment of bone disorders by focusing on osteoarthritis (OA), rheumatoid arthritis (RA), osteoporosis (OP), and bone fracture. Furthermore, we discuss the key issues of MSCs-EV for clinical application.
Extracellular Vesicles (EV)
EV: Definition, Classification, and Function
Exosomes are a type of EV and the term “exosome” was first adopted by Trams et al. (1981) and refers to the EV formed by the endosomal system (Harding et al., 1983; Pan and Johnstone, 1983). Although the word “exosome” is commonly seen in literature, it is difficult to obtain purified exosomes, which make “exosome” ambiguous. Therefore, the International Society for Extracellular Vesicles (ISEV) has proposed minimal information for studies of extracellular vesicles 2018 (MISEV2018) in relation to the nomenclature of EV, which is defined for particles naturally released from the cell that are delimited by a lipid bilayer and cannot replicate (Thery et al., 2018). Since there is still lack of consensus on specific markers of EV subtypes, such as exosomes and ectosomes, the ISEV recommends the usage of EV, a collective term covering various subtypes of cell-released, membranous structures, called exosomes, microvesicles, microparticles, ectosomes, oncosomes, apoptotic bodies, and many other names (Thery et al., 2018). According to the uncertainty of what types of vesicles have been studied in many studies, in this review, we use the word “EV” and use the term as mentioned in the original work.
There is a different classification of EV proposed by different literature, ranging from two to six major different EV types (Cocucci et al., 2009; Thery et al., 2009; Beyer and Pisetsky, 2010; Mathivanan et al., 2010). However, due to the difficulty met in practice (e.g., the vesicles’ preparations are heterogeneous) and insufficient evidence for some EV types, van der Pol et al. (2012) have proposed four different types of eukaryotic cell-derived EV: (1) endosome-origin exosomes (50–100 nm); (2) plasma membrane-derived MV (20–1000 nm); (3) plasma membrane-derived membrane particles (50–600 nm); and (4) apoptotic vesicles (1000–5000 nm) from the plasma membrane and endoplasmic reticulum. However, at present, there is still no consensus on the classification of EV as stated by ISEV (Thery et al., 2018).
As particles naturally secreted from the cell, EV has been identified as vital mediators of paracrine communication (Batiz et al., 2015; Jing et al., 2018) and demonstrated a key role in different processes, such as angiogenesis (Qi et al., 2016), antigen presentation (Shenoda and Ajit, 2016), apoptosis (Qi et al., 2019), coagulation (Fricke et al., 2017), cellular homeostasis (Takahashi et al., 2017), inflammation (Zhang S. et al., 2019). EV is involved in numerous biological processes, including intercellular signaling, cell adhesion, waste management and protection against stress, coagulation, and vascular function and integrity, which has been summarized comprehensively by van der Pol et al. (2012).
Biogenesis and Components of EV
Different type of EV is generated through different way and contains different cargos, which depends on the cell types and the physiological conditions. The biogenesis of exosomes is well-studied, which involves the exocytosis of multivesicular endosomes (Figure 1). Generally, the biogenesis of exosome contains three phases: endocytosis, multivesicular body (MVB) development, and release (Figure 1) (Gurunathan et al., 2019). At the beginning, endocytic vesicles are produced from the plasma membrane (PM) to become early endosomes, which then develop into late endosomes via endosomal membrane budding inward to form intraluminal vesicles (ILVs). Subsequently, the late endosomes constantly bud inward and form ILVs, which keep accumulating and constitute MVBs. Finally, the MVBs may either be degraded by lysosome, or fuse with the plasma membrane to permit the ILVs to be released into the extracellular environment. The released vesicles are called exosomes (Figure 1) (van Niel et al., 2006; Dong et al., 2019). The exosomes play crucial roles in intercellular communication by regulating the recipient cell function. There are mainly three ways for exosomes regulating recipient cell function. First, exosomes interact with the membrane receptors of the recipient cell to activate the signaling cascade in the cell. Second, the endocytosis occurs in the recipient cell to ingurgitate the exosomes. Third, exosomes integrate own cargos with recipient cell membrane, then transfer the lipids, nucleic acids (mRNAs and miRNAs) and proteins through membrane fusion or by an endocytosis pathway, leading to numerous biological processes (Figure 1) (Yáñez-Mó et al., 2015).
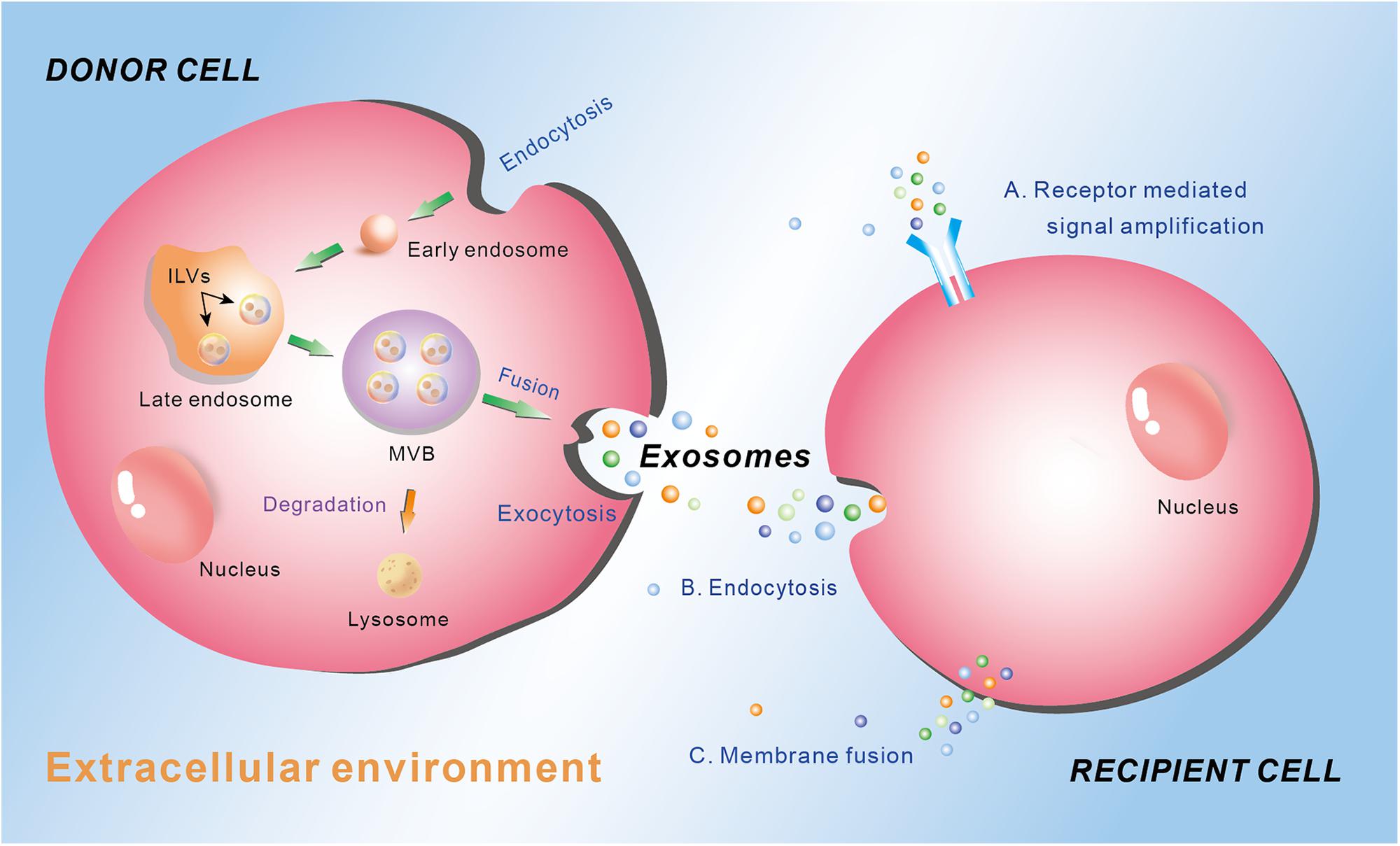
Figure 1. Schematic diagram of the biogenesis and secretion of exosomes, and their function ways to the recipient cell. Donor cell plasma membrane invaginate through endocytosis to form early endosome, which subsequently develop into late endosome via endosomal membrane budding inward to form intraluminal vesicles (ILVs). The late endosome constantly buds inward and form ILVs, which keep accumulating and constitute multivesicular body (MVB). MVB can either be degraded by lysosome or fuse with the plasma membrane to permit the ILVs to be released into the extracellular environment through exocytosis as exosomes. Exosomes mediate their effects on the recipient cells through three main manners: (A) receptor mediated signal amplification, (B) endocytosis, and (C) membrane fusion.
At present, the well-known mechanism of exosome generation is driven by the endosomal sorting complex required for transport (ESCRT), which consists of approximately 30 proteins that form four complexes (ESCRT-0, ESCRT-I, ESCRT-II, ESCRT-III) with associated proteins including vacuolar protein sorting-associated protein 4 (VPS4), vesicle trafficking 1 (VTA-1), and apoptosis-linked gene 2 (ALG-2)-interacting protein X (Alix) (Hanson and Cashikar, 2012). They give the exosomes membrane a flexible state, which make exosomes be transported through the cytoplasm. Moreover, ESCRT-independent mechanism has been demonstrated for exosome biogenesis and secretion, in which way, although the four subunits of ESCRTs are silent, MVBs can still be generated (Trajkovic et al., 2008; Frydrychowicz et al., 2015). The review written by Colombo et al. (2014) provides a comprehensive understanding of the mechanism of exosome biogenesis.
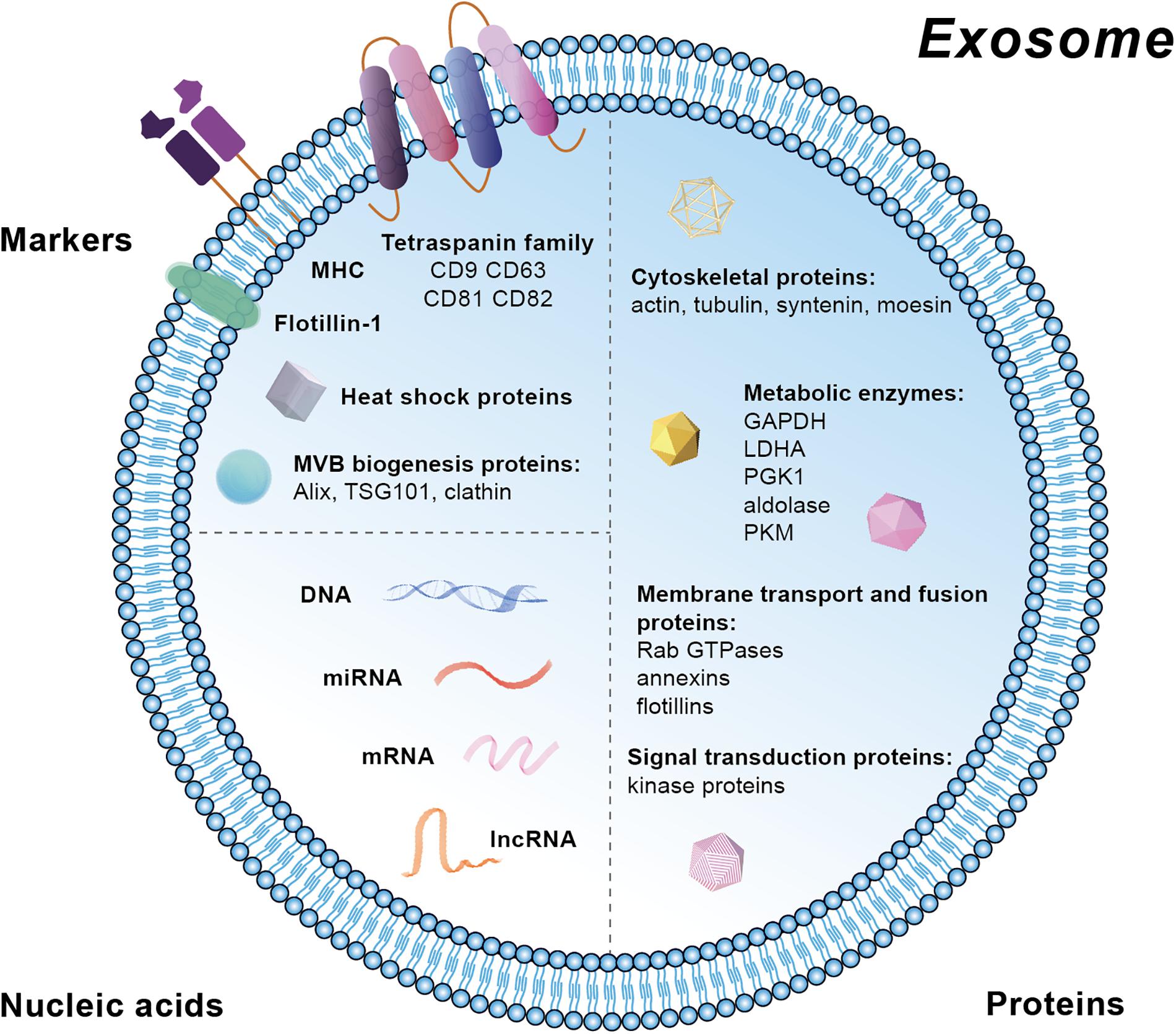
Extracellular vesicles contain markers from the parent cells and therefore their subtypes can be identified and characterized. Specific markers in exosomes are mainly located at the membrane, including the tetraspanin family (CD81, CD82, CD63, and CD9), major histocompatibility complex (MHC), and lipid raft such as Flotillin-1 (Figure 2). Moreover, heat shock proteins (HSP) and the proteins related with MVB biogenesis are also markers for exosomes (Figure 2). Besides these membrane molecules, there are other proteins and nucleic acids within the exosomes. The proteins are mainly related with MVBs biogenesis (Alix, TSG101, and clathrn), cytoskeleton (actin, tubulin, syntenin, and moesin), metabolism [GAPDH (Glyceraldehyde-3-phosphate dehydrogenase), LDHA (Lactic dehydrogenase A), PGK1 (Phosphoglycerate kinase 1), aldolase, and PKM (protein kinase)], membrane transport and fusion (Rab GTPases, annexins, and flotillins), and signal transduction (kinase proteins) (Chaput and Thery, 2011; Ohno et al., 2013; Raposo and Stoorvogel, 2013). The nucleic acids in exosomes are mainly mRNA, miRNA, long non-coding RNA (lncRNA), and DNA. Besides, various lipid compounds are found in exosomes, such as ceramide, sphingomyelin, phosphatidyl choline, phosphatidylserine, phosphatidyl ethanolamine, and cholesterol (Kowal et al., 2014) (Figure 2). Public on-line databases of EV composition are freely accessible at Exocarta1 and Vesiclepedia2.
Isolation and Characterization of EV
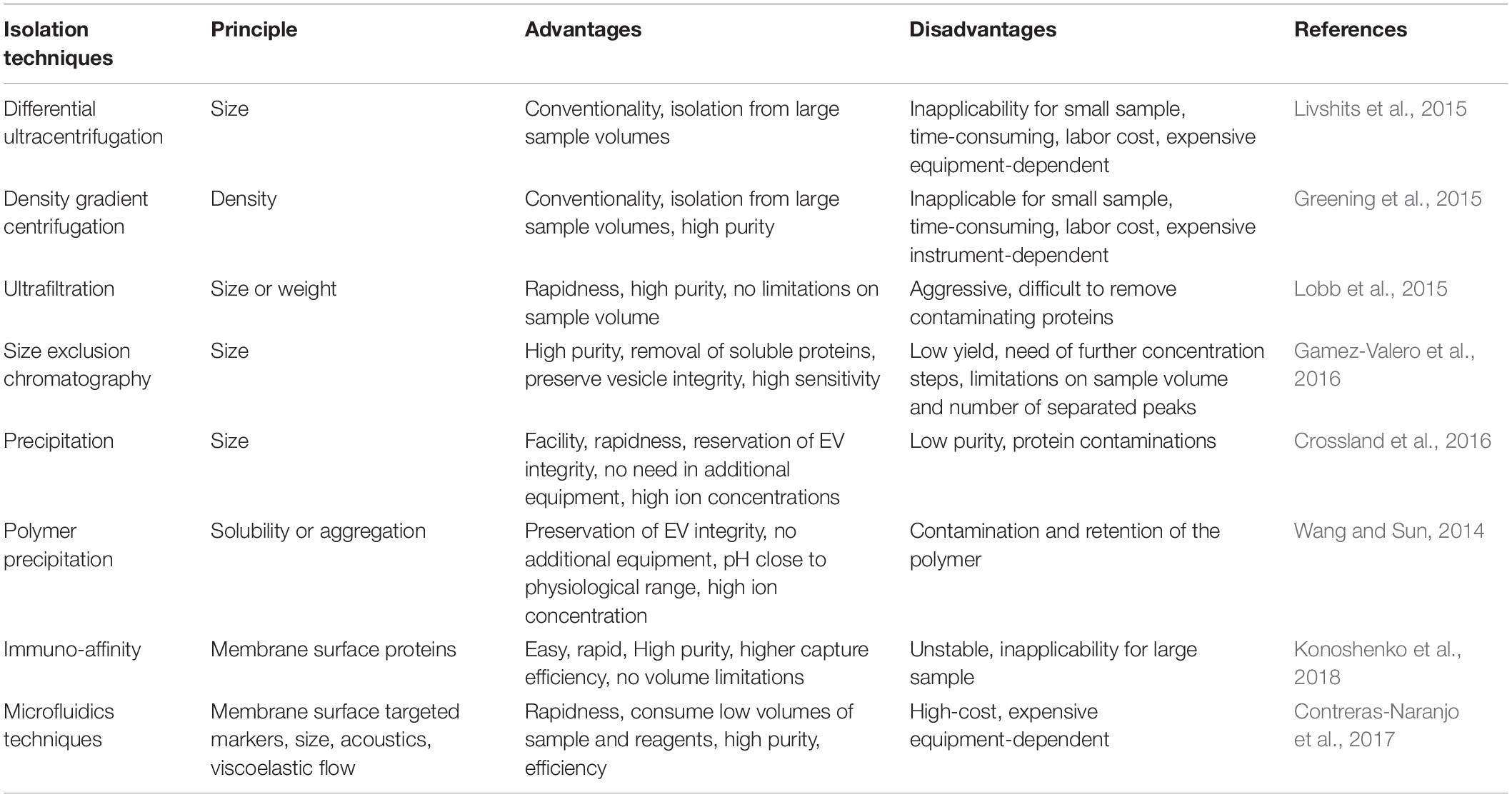
Isolation of EV from specific tissues or cells is one important step toward further investigation and applications (Figure 3). Various techniques have been adopted to facilitate the isolation of EV based on different principles (Table 1). Traditional methods used for EV isolation are mainly based on EV properties (e.g., size and density), including ultracentrifugation, ultrafiltration, chromatography and precipitation. The ultracentrifugation process requires high centrifugal forces, up to 1,000,000 g. Ultracentrifugation is suitable for large sample volumes, but not for small volumes because it is time-consuming, labor-intensive and requires expensive equipment (Witwer et al., 2013). In addition, protein, aggregates, apoptotic bodies, and other non-exosomal particles may be present in EV obtained by ultracentrifugation, which is the major shortcoming of this method (Konoshenko et al., 2018). There are two types of ultracentrifugation: differential ultracentrifugation and density gradient ultracentrifugation. These two methods are used in order to increase the efficiency of particle separation and to obtain purer EV (Fernandez-Llama et al., 2010). Another isolation method is ultrafiltration, which merely depends on size or molecular weight. Ultrafiltration is rapid and does not require expensive devices, but it is difficult to remove contaminating proteins (Zeringer et al., 2015). Size-exclusion chromatography allows the separation of exosomes from proteins, but not from MV, protein aggregates, lipoparticles, macromolecules, or particulate matter (Gurunathan et al., 2019). Size-exclusion chromatography can be used in combination with ultracentrifugation for higher yields of EV (Lai et al., 2010). Precipitation isolates EV by capturing a certain size, and using simple, rapid, low-speed centrifugation on the bench top at 1,500 g in “polymer nets” (Gurunathan et al., 2019). Because it is easily operated and does not require specialized equipment, precipitation allows to be integrated into clinical usage and it can be applied for large sample sizes (Konoshenko et al., 2018). The disadvantage of this method is that there is no specificity for non-exosomal material, such as protein aggregates, which may be co-isolated with the exosomes resulting in low purity (Peterson et al., 2015). In addition, polymer-based precipitation is also used for EV isolation based on the changes in EV solubility and/or aggregation (Zeringer et al., 2015). System Biosciences, LLC. offers a proprietary reagent named ExoQuick, which can be used to purify exosomes from a wide variety of tissue culture media, and certain biofluids3. In order to isolate more specific EV populations, immunological methods are used based on highly specific interactions with the molecules (e.g., lipids, proteins, and polysaccharides) exposed on the EV surface. This approach is particularly useful when the protein expressed on the EV surface lacks a soluble counterpart (Gurunathan et al., 2019). Immuno-affinity is simple, rapid, and compatible with the laboratory equipment, while it is unstable and not suited for isolating EV from large quantities of biological samples (Konoshenko et al., 2018). Moreover, a new method utilizing aqueous two-phase system is adopted to isolate high-purity EV by preventing the protein contamination in the EV fraction (Kim et al., 2015). Recently, microfluidics-based technologies have become a trend for EV isolation, especially for microscale isolation, detection, and analysis of exosomes (Konoshenko et al., 2018; Gurunathan et al., 2019). Microfluidic devices utilizes the usual separation determinants and innovative sorting principles, mainly including: (a) trapping exosomes with an immune-affinity approach (microfluidic chip, “Exochip,” magnetic capture beads) (Chen C. et al., 2010; Kanwar et al., 2014; Shao et al., 2015); (b) membrane-based filtration (double filtration) (Liang et al., 2017); (c) trapping exosomes on porous structures (nanowire micropillars) (Wang et al., 2013); (d) acoustics (acoustic nano-filter system); (e) lateral displacement (nanoscale lateral displacement arrays) (Wunsch et al., 2016); and (f) viscoelastic flow (field-free microfluidic sorting) (Zhou J. et al., 2019).
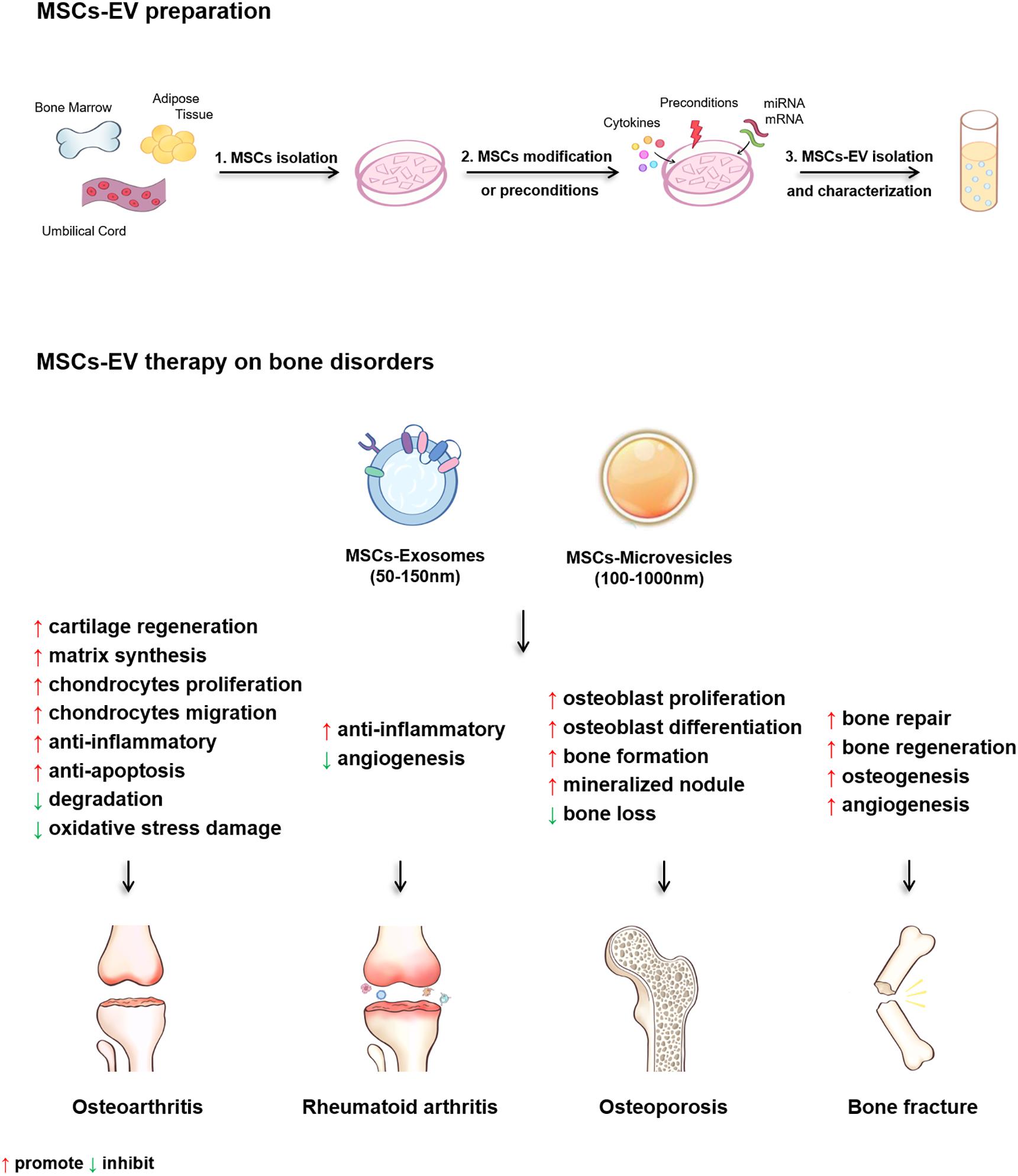
Figure 3. The diagram of the MSCs-EV preparation and the therapeutic effects of MSCs-EV on osteoarthritis (OA), rheumatoid arthritis (RA), osteoporosis (OP) and bone fracture.
Characterization of EV is essential for determining their biochemical properties and biological functions. The characterization mainly includes the evaluation of the size, structure, surface biochemical markers, concentration and contents of EV. There are numerous approaches for characterizing different biochemical properties of EV. To detect EV biochemical markers, western blotting, flow cytometry, microfluidic-based technique and nanoparticle-tracking analyses (NTA) are normally used. EV components, including proteins, mRNA, miRNA, and lipid subsets can be detected by conventional test method such as western blotting, PCR and flow cytometry. As technology advances, high throughput transcriptomic studies have identified a wide range of mRNA and miRNA data sets based on microarray and next-generation sequencing (NGS) analyses leading to a comprehensive data classification. For detecting size and structure of EV, NTA, Tunable-resistive pulse sensing (TRPS), Dynamic-light scattering (DLS), Photon-correlation spectroscopy (PCS), Atomic-force microscopy (AFM), Transmission electron microscopy (TEM), Raman spectroscopy and Flow cytometry are used. Meanwhile, NTA and TRPS can be used to detect concentration.
Mesenchymal Stem Cells (MSCs) and MSCs-Derived EV (MSCs-EV)
Mesenchymal Stem Cells (MSCs)
MSCs, also known as mesenchymal stromal cells (MSCs), are multipotent fibroblast-like cells with the potential for self-renewal and multilineage differentiation (Hu et al., 2018). The Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy (ISCT) has proposed the minimal criteria to define human MSCs (Dominici et al., 2006). First, MSCs must be plastic-adherent when maintained in standard culture conditions. Second, MSCs must express CD105, CD73, and CD90, and lack expression of CD45, CD34, CD14 or CD11b, CD79 alpha or CD19 and HLA-DR surface molecules. Third, MSCs must differentiate to osteoblasts, adipocytes and chondroblasts in vitro.
MSCs reside in almost all post-natal organs and tissues such as bone marrow, adipose, liver, lung, spleen, and muscle (da Silva Meirelles et al., 2006). MSCs have the ability to proliferate extensively and maintain the ability to differentiate into multiple cell types (e.g., osteoblasts, adipocytes, chondrocytes, and fibroblasts), which endow MSCs with stem cell nature (Pittenger et al., 1999). Besides, MSCs can migrate to the sites of damage and secrete anti-inflammatory and growth factors to maintain cellular homeostasis and promote damage repair.
With the above characteristics, MSCs are commonly used in cell therapy for regenerative medicine and immunotherapy (Squillaro et al., 2016; Galipeau and Sensebe, 2018). MSCT has caused increasing attention and is regarded as a potential new therapy for a variety of human diseases associated with brain, liver, kidney, bone, and so on (Hu et al., 2018; Chakari-Khiavi et al., 2019; Hu et al., 2019; Missoum, 2020). However, the direct use of MSCs for treating diseases encounters some problems. For example, MSCs inoculated into damage site show low survival rate and loss of function after 1 week (Pak et al., 2003; Breitbach et al., 2007; Rani et al., 2015). Besides, intraarterial MSCs administration may lead to occlusion in the distal vasculature due to their relatively large cell size (Furlani et al., 2009). In addition, MSCs may genetically unstable and undergo chromosomal abnormalities, even develop malignant tumor or generated calcification (Yoon et al., 2004; Tolar et al., 2007; Jeong et al., 2011). These issues may lower its security for treating human disease.
Recently, numerous studies demonstrate the therapeutic effect of MSCs is primarily mediated by the paracrine secretion of cytokines, growth factors and EV (Wang et al., 2015; Zhou Y. et al., 2019). MSCs-EV are identified as key mediators for the paracrine effects and gradually become the focus in regenerative medicine and disease treatment (Yang et al., 2017; Chang et al., 2018).
MSCs-EV
Over the years, of a wide array of bioactive molecules, MSCs secretions such as growth factors, cytokines and chemokines have been proved to play vital biological roles. Among these secretory products, none of them, by itself, can fully decipher the mechanism of MSCs (Meirelles Lda et al., 2009). In 2009, MSCs-derived MV were found to alleviate glycerol-induced acute tubular injury. The size of these MV ranges from 80 nm to 1 μm, with a mean value of 135 nm (Bruno et al., 2009). Subsequently, Lai et al. isolated and characterized MSCs-derived exosome for the first time from conditioned medium of human embryonic-derived MSCs (hESC-MSCs) (Lai et al., 2010). These membrane vesicles feature a diameter of 50–100 nm with an endosomal origin, housing abundant exosome-associated proteins including Alix, TSG101 and tetraspanins (Lai et al., 2010). MSCs-exosomes have special membrane binding proteins such as CD73, CD44, and CD29 compared with other EV (Lai et al., 2012). To date, more than 850 unique gene products and 150 miRNAs have been identified in MSCs-exosomes (Chen T.S. et al., 2010; Lai et al., 2012). In addition, MSCs have been demonstrated as the most prolific producer of mass exosomes (Yeo et al., 2013). They can produce large numbers of exosomes and generate permanent cell lines through cell immortalization, whose yield is not affected in quantity or quality, thus ensuring a sustainable and reproducible production of exosomes from MSCs (Yeo et al., 2013).
Previous studies have shown that the function of MSCs-EV may vary depending on the source of the MSCs, thus affecting the biological effects of MSCs-EV (Baglio et al., 2015; Del Fattore et al., 2015; Lopez-Verrilli et al., 2016; Borger et al., 2017). Thus, MSCs-EV derived from different MSCs may show different clinical efficacy. It is important to determine the optimal source of MSCs. In addition, the environment of MSCs can change the content of EV derived from MSCs, thus affecting and changing the tissue environment. MSCs-EV may have the versatility and capacity to interact with multiple cell types within the immediate vicinity and remote areas to elicit appropriate cellular responses to keep the maintenance of a dynamic and homeostatic tissue microenvironment (Lai et al., 2015).
MSCs-EV and Osteoarthritis (OA)
Osteoarthritis is the most common chronic joint disease around the world, with an incidence of 10–20% in the population over 50 years old. As the population ages and obesity increases, the incidence of OA is expected to double within the next 30 years (Blanco et al., 2011). It has become the fastest growing major health concern worldwide. OA is characterized by the degradation of the articular cartilage, the degeneration of menisci and ligaments, the thickening of the subchondral bone, and the formation of osteophytes (Loeser et al., 2012). The pathogenesis of OA is complex, involving multiple factors (e.g., age, body mass index, and genetics), multiple tissues, and processes.
There is still currently no effective treatment for OA. Most treatments are applied to manage pain, stiffness and swelling to improve joint mobility, and joint replacement is the only option for treating the entire joint dysfunction. Although the current physiotherapy or pharmacological therapy can temporarily relieve the clinical symptoms, it is difficult to fully restore joint function and also has a high risk of instability and infection (Keith, 2012; Stiehler et al., 2015; Xin-Wei et al., 2015).
The potential of MSCs to treat OA has been extensively studied (Qi et al., 2012; Vega et al., 2015; Davatchi et al., 2016). Recent studies have discovered that MSCs function in a paracrine manner by secreting cytokines, growth factors and EV (D’souza et al., 2015; Giebel et al., 2017; Fan et al., 2020) and exosomes derived from MSCs have shown the capability to protect cartilage and bone from degradation in OA through reinducing the expression of chondrocyte markers (type II collagen, aggrecan), inhibiting catabolic (MMP13 (matrix metalloproteinase 13), ADAMTS5) and inflammatory markers, inhibiting macrophage activation, and protecting chondrocytes from apoptosis, which show anti-inflammatory and cartilage protection effects (Cosenza et al., 2017).
Anti-inflammatory Effect of MSCs-EV in OA
Inflammation is an important factor in the onset and progression of OA (Nold et al., 2015; Burrello et al., 2016). In OA patients, activated macrophages and other innate immune cells release inflammatory cytokines and promote cartilage damage (Liu-Bryan, 2015). Infiltrations of B lymphocyte, T lymphocyte, plasma cells, T-helper cells, and Human Leukocyte Antigen-antigen D Related (HLA-DR)-positive dendritic-like cell were observed in the inflamed synovium (Lindblad and Hedfors, 1987). Moreover, catabolic factors [e.g., interleukin-1α (IL-1α), tumor necrosis factor-α (TNF-α)] are present in OA joints and inhibit the chondrogenesis of stem cells (Heldens et al., 2012).
Recent evidence suggest that adipose tissue-derived mesenchymal stem cells (AD-MSCs)-exosomes (size of 104 ± 19 nm) and MV (size of 279 ± 94 nm) show great potential in anti-inflammatory and preventing degeneration processes in OA (Tofiño-Vian et al., 2018). Exosomes (∼120 nm) and MV/microparticles (>150 nm) derived from bone marrow mesenchymal stem cells (BM-MSCs) show anti-inflammatory function through inhibiting T lymphocyte proliferation, stimulating macrophage polarization toward anti-inflammatory phenotype, and decreasing the percentage of CD4+ and CD8+ T cell subsets (Cosenza et al., 2018). In addition, BM-MSCs-exosomes (30–250 nm) induce conversion of T helper type 1 (Th1) cells into T helper type 2 (Th2) cells and reduce the potential of T cells to differentiate into interleukin 17-producing effector T cells (Th17) (Chen et al., 2016). These findings demonstrate that MSCs-EV suppress pro-inflammatory response by reducing inflammation and promoting anti-inflammatory responses that maintain the immune balance. Furthermore, in the rat model of temporomandibular joint osteoarthritis (TMJ-OA), hESC-MSCs-exosomes (size of 100–200 nm, density of 1.10–1.19 g/ml) can relieve pain and repair osteoarthritic TMJ. MSCs-exosomes increase a well-coordinated response of attenuating inflammation, enhance matrix synthesis, while reduce OA joint cellular apoptosis and matrix degradation to achieve overall joint homeostasis, which finally promote TMJ repair and regeneration (Zhang S. et al., 2019). MSCs-exosomes function through adenosine receptor-mediated adenosine activation of protein-serine-threonine kinase (AKT), extracellular regulated protein kinases (ERK) and Adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK) phosphorylation to reduce inflammation and restore matrix homeostasis in OA. These therapeutic effects were achieved by enhancing sulfated glycosaminoglycan (s-GAG) matrix synthesis impeded by interleukin-1β (IL-1β), while suppressing IL-1β-induced nitric oxide and MMP13 production (Zhang S. et al., 2019). Cosenza et al. (2017) also found that exosomes (112 ± 6.6 nm) and microparticles (223 ± 15.6 nm) from murine BM-MSCs exerted similar functional effect in vitro by re-establishing chondrocyte homeostatic state, protecting chondrocytes from apoptosis and stimulating macrophage polarization toward anti-inflammatory phenotype. Therefore, MSCs-EV possess the immunomodulatory properties and accelerate the recovery of cartilage and joint in OA.
Cartilage Protection and Regeneration Effect of MSCs-EV in OA
The main pathology of early stage OA is the degeneration of chondrocytes, resulting in damage to articular cartilage. Metabolic and structural changes in articular cartilage play a major role in the initiation and progression of OA. MSCs-EV exert important therapeutic effect on OA by protecting cartilage from degradation and promoting cartilage regeneration, which is now the focus of clinical therapy.
The efficacy of hESC-MSCs-exosomes (a modal size of 100 nm) on cartilage repair was firstly reported in 2016 (Zhang S. et al., 2016). After treatment with exosomes, the rat model of osteochondral defect displayed almost complete neotissue coverage with good surface regularity and complete integration with the surrounding cartilage. hESC-MSCs-exosomes accelerated neotissue filling and enhanced matrix synthesis of type II collagen and s-GAG, demonstrating the capacity of MSCs-exosomes in cartilage repair and regeneration (Zhang S. et al., 2016). It has also been demonstrated that MSCs-EV protect chondrocytes from apoptosis, balance the anabolic and catabolic processes and re-establish chondrocyte homeostatic state via balancing the synthesis and degradation of cartilage matrix, thus protect cartilage and bone from degradation (Cosenza et al., 2017; Wang et al., 2017; Wu et al., 2019). All these observations demonstrate the therapeutic effects of MSCs-EV on OA by re-establishing cartilage homeostasis, protecting and regenerating cartilage.
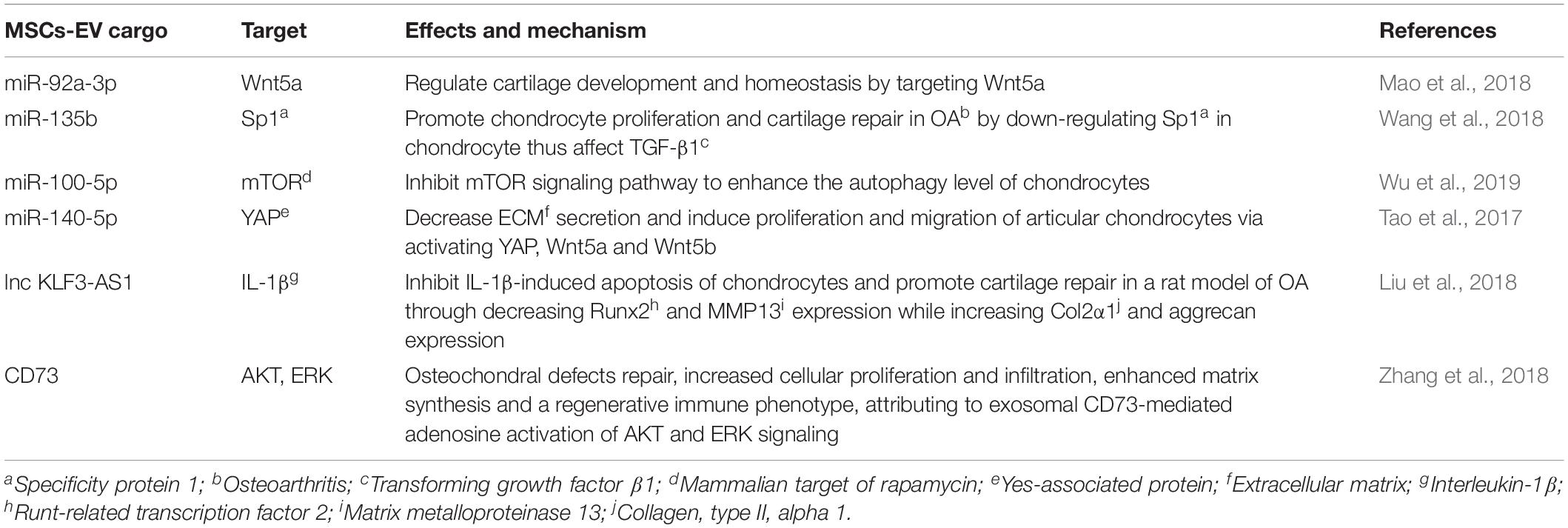
Several key cargo components of MSCs-EV have shown important roles in mediating the therapeutic effects of MSCs-EV. MiRNAs, a large cargo of MSCs-EV (Chen T.S. et al., 2010), are identified as key regulators in mediating the therapeutic roles of MSCs-exosomes on OA by targeting different molecules or signaling pathways (Toh et al., 2017). AD-MSCs-EV (ranged in size from 40–50 nm to 300–400 nm) have been demonstrated to affect fibroblast-like synoviocytes (FLS) behavior in a model of chronic inflammation through direct interaction with hyaluronan matrix and miRNA release (Ragni et al., 2019). They reduced the expression of pro-inflammatory cytokines and chemokines in a chronic model of FLS inflammation. Through bioinformatics analysis, EV-embedded miRNAs regulate the main pathways, which is strictly associated with synovial inflammation in OA (Ragni et al., 2019). Further research revealed AD-MSCs-EV (50–400 nm in diameter)-embedded miRNAs alter cartilage homeostasis and macrophage polarization, supporting the protective and pro-regenerative effects in joint (Ragni et al., 2020). miR-92a-3p derived from BM-MSCs-exosomes (50–150 nm) regulates cartilage development and homeostasis through targeting Wnt5a in both chondrogenesis and OA pathogenesis as a negative regulator (Mao et al., 2018). miR-135b derived from hESC-MSCs-exosomes assists transforming growth factor β1 (TGF-β1) to promote chondrocyte proliferation by down-regulating Specificity Protein 1 (Sp1), thus promotes cartilage repair in OA (Wang et al., 2018). Infrapatellar fat pad (IPFP)-derived mesenchymal stem cells (IPFP-MSCs)-exosomes (average size of 121.9 nm) significantly enhance the autophagy level of chondrocytes via miR100-5p-mediated inhibition of mammalian target of rapamycin (mTOR) signaling pathway (Wu et al., 2019). Exosomes (30–150 nm) derived from miR-140-5p overexpressing synovial MSCs (SMSCs) activate yes-associated protein (YAP), decrease extracellular matrix (ECM) secretion, and promote proliferation and migration of articular chondrocytes via Wnt5a and Wnt5b, which in turn ameliorate OA (Tao et al., 2017). These studies demonstrate that MSCs-EV attenuate OA progression through the delivery of miRNAs, which exhibit as potential targets for future therapy (Table 2).
In addition to miRNA, a few lncRNAs have been found as main components of EV and to function as novel biomarkers and therapeutic targets for various diseases (Naderi-Meshkin et al., 2019). Exosomal lncRNA KLF3-AS1 derived from human MSCs (hMSCs) has been demonstrated to inhibit IL-1β induced apoptosis of chondrocytes and promote cartilage repair in a OA rat model by decreasing Runt-related transcription factor 2 (Runx2) and MMP13 expression while increasing collagen, type II, alpha 1 (Col2a1) and aggrecan expression (Liu et al., 2018). This suggests the important role of lncRNA in mediating the therapeutic effect of MSCs-exosomes on OA, demonstrating a new possible mechanism. In addition, protein components in MSCs-exosomes have been suggested to be linked with OA recovery. Zhang et al. (2018) demonstrated the therapeutic effect of vesicular CD73 in cartilage repair and regeneration (Zhang et al., 2018). hESC-MSCs-Exosomes (a modal size of 100 nm, density of 1.10–1.19 g/ml) mediated repair of osteochondral defects, which was characterized by increased cellular proliferation and infiltration, enhanced matrix synthesis and a regenerative immune phenotype. These could attribute to exosomal CD73-mediated adenosine AKT and ERK signaling (Zhang et al., 2018) (Table 2).
As mitochondrial dysfunction is one important cause of OA (Blanco et al., 2011) and MSCs-EV are important for intercellular mitochondria communication (Cao et al., 2020), suggesting the possibility of MSCs-EV in treating OA by regulating mitochondria function. Mitochondrial dysfunction and oxidative stress damage are associated with apoptosis, senescence and various pathological conditions, which are hallmarks of OA. In OA, chondrocytes lose their metabolic flexibility, decrease their cellular mitochondrial biogenesis, and increase their mitochondrial DNA (mtDNA) damage (Chen et al., 2019). Qi et al. (2019) have found that BM-MSCs-exosomes (50–150 nm) suppress IL-1β-induced chondrocyte apoptosis, which was partly due to mitochondrial dysfunction, through inhibiting the phosphorylation of p38 and ERK1/2, and stimulating the phosphorylation of AKT signaling pathway. Moreover, Chen et al. (2019) have reported that BM-MSCs-exosomes (40–110 nm) restore mitochondrial function and oxidative stress damage in degenerative cartilage and balance the energy metabolism, which promote cartilage regeneration. Based on these findings, they furtherly fabricated a 3D printed cartilage extracellular matrix/gelatin methacrylate/exosome (ECM/GelMA/exosome) scaffold with radially oriented channels and found it significantly facilitated the cartilage regeneration in the animal model (Chen et al., 2019).
MSCs-EV and Rheumatoid Arthritis (RA)
Arthritis is a chronic autoimmune disease involving joints. It is characterized by persistent inflammation of joints. In RA, auto-reactive T cells and B cells are activated because of defective immune regulation. These cells proliferate and differentiate into pathological T cells and plasma B cells, which produce auto-reactive antibodies, eventually leading to inflammation and degradation of joints (Pozsgay et al., 2017). As mentioned in OA section that MSCs-EV exert anti-inflammatory effects, they have also exerted an immunomodulatory function in RA and show therapeutic effects on joint destruction (Chen et al., 2018; Cosenza et al., 2018).
Collagen-induced arthritis (CIA) is one of the most accepted animal models of RA for its capacity to simulate RA inflammatory symptoms clinically and biologically. Cosenza et al. (2018) have demonstrated that Exosomes (∼120 nm) and MV/microparticles (>150 nm) derived from BM-MSCs exert an indirect inhibitory effect on T lymphocyte and B lymphocyte proliferation in CIA murine model, providing the first evidence that MSCs-exosomes exert an immunomodulatory function in RA. Chen et al. (2018) have also found that BM-MSCs-exosomes show therapeutic effects on joint destruction in RA, partly due to the expression of miR-150-5p in exosomes. After being treated with MSCs-derived miR-150-5p exosomes (Exo-150), the expression of the RA markers including matrix metalloproteinase 14 (MMP14) and vascular endothelial growth factor (VEGF) in CIA mice was down-regulated. Exo-150 inhibited migration of FLS from RA, and angiogenesis in vitro, and alleviated arthritis in CIA mice in vivo. These findings indicate that EV facilitate the direct intracellular transfer of miRNAs and represent a potential therapeutic strategy for RA (Chen et al., 2018).
At present, only a few studies have reported the therapeutic effect of MSCs-EV on RA and the mechanism is unclear. As MSCs-EV play roles in immunoregulation and cartilage protection and regeneration, this may partially contribute to the therapeutic effect of MSCs-EV on RA. More research is necessary to prove the effects of MSCs-EV in treating RA.
MSCs-EV and Osteoporosis (OP)
Osteoporosis is an age-related systemic bone disease characterized by reduced bone mass, destroyed bone microstructure, weakened bone strength, which causes increased bone brittleness and fracture risk. As one major disease worldwide, osteoporosis seriously affects people’s health and increases society’s financial burden. Therefore, efficient therapy of osteoporosis is one main concern of the world.
MSCs-EV have been indicated as a novel therapeutic method for osteoporosis. Qi et al. (2016) have found that exosomes (50–150 nm) secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells (hiPSC-MSCs) effectively promote osteoblast proliferation, differentiation and bone formation, thus improve bone regeneration in osteoporotic rats. Zuo et al. (2019) have reported that exosomes (40–100 nm) derived from BM-MSCs alleviate radiation-induced bone loss in a rat model. After irradiation to rats, BM-MSCs-exosomes were intravenously injected into the rats immediately. The results showed that BM-MSCs-exosomes reduced intracellular reactive oxygen species (ROS) to protect cells from damage, accelerated DNA repair in the recipient BM-MSCs and partially rescued cell proliferation. Moreover, BM-MSCs-exosomes decreased the senescence-associated protein expression and restored the differentiation potential of irradiated BM-MSCs (Zuo et al., 2019). Meanwhile, BM-MSCs-exosomes reduced adipogenic gene expression and increased osteogenic gene expression of recipient BM-MSCs (Zuo et al., 2019). These results demonstrate that MSCs-EV promote bone regeneration via reducing ROS, accelerating DNA repair, restoring cell (especially osteoblast) proliferation and differentiation potential, decreasing the senescence-associated protein and adipogenic gene expression and increasing osteogenic gene expression, thus alleviate osteoporosis.
Present studies demonstrate that MSCs-EV alleviate osteoporosis by activating several signaling pathways. Wnt/β-catenin signaling is a key pathway for bone development and homeostasis (Baron and Kneissel, 2013; Zhong et al., 2014). The activation of Wnt/β-catenin signaling promotes preosteoblast to differentiate into osteoblasts. Zuo et al. (2019) have found that BM-MSCs-exosomes promotes osteogenesis, reduced the decrease in bone mass induced by irradiation by activating Wnt/β-catenin pathway in the recipient BM-MSCs. Zhao et al. (2018) found that BM-MSCs-exosomes (about 40 nm) alleviated the osteoporosis progression by promoting the osteoblast proliferation through mitogen-activated protein kinase (MAPK) signaling pathway. Moreover, Liu et al. have demonstrated the therapeutic function of BM-MSCs-exosomes on osteopenia in Fas-deficient-MRL/lpr mice (Liu et al., 2015). The Fas-deficient-MRL/lpr mice exhibit Fas-deficiency that causes elevation of intracellular miR-29b levels and downregulation of DNA methyltransferase 1 (Dnmt1) in BM-MSCs, which causes hypomethylation of the Notch1 promoter and activation of Notch signaling, thus results in impaired osteogenic differentiation. MSCs-exosomes reduce intracellular miR-29b levels by transfer Fas to the recipient MRL/lpr BM-MSCs and recover Dnmt1-mediated Notch1 promoter hypomethylation, elevate mineralized nodule formation, expression of Runx2 and alkaline phosphatase (ALP) of MRL/lpr BM-MSCs, thus increase bone formation, trabecular bone volume, bone mineral density (BMD), bone volume/total volume (BV/TV), mineral apposition rate (MAR) and bone formation rate per bone Surface (BFR/BS) in Fas-deficient-MRL/lpr mice (Liu et al., 2015). This study demonstrates that BM-MSCs-exosomes function through Fas/miR-29b/Dnmt1/Notch epigenetic cascade to regulate the recipient cell function.
MSCs-EV and Bone Fracture
Besides the critical role in alleviating osteoporosis, MSCs-EV show key roles in fracture healing. In order to ensure bone fracture healing, two basic conditions must be met: good blood transport and stable fixation. hMSCs-exosomes (80–100 mm) have been found to promote bone regeneration by enhancing angiogenesis. MSCs-exosomes increased hMSC migration, induced mineral deposition and enhanced the differentiation potential of hMSCs into osteoblasts. In addition, MSCs-exosomes enhanced the expression of osteogenesis-related and angiogenesis-related genes, such as COL I, ALP, and VEGF, and promoted bone formation in vivo (Takeuchi et al., 2019). The CD9–/– mouse, which is an established strain with low-exosome productivity, shows a significant delay in endochondral ossification and fracture healing compared with the wild-type mouse. However, injection of BM-MSCs-exosomes (approximately 80 nm) rescues the fracture healing speed in CD9–/– mice and increases that in the wild-type mice, indicating the potential therapeutic role of MSCs-exosomes in fracture healing (Furuta et al., 2016). In addition, MSCs-exosomes contain bone repair related cytokines such as monocyte chemotactic protein 1 (MCP-1), monocyte chemotactic protein 3 (MCP-3), stromal cell-derived factor-1 (SDF-1) and angiogenic factors, which accelerate fracture healing. Meanwhile, miRNAs derived from MSCs-exosomes are key regulators for fracture healing. miR-21 accelerates osteogenic bone formation, osteogenesis and angiogenesis, which promote fracture healing (Furuta et al., 2016). Furthermore, Zhang et al. have also demonstrated the therapeutic effect of MSCs-exosomes (approximately 100 nm) on bone fracture healing (Zhang Y. et al., 2019). They delivered umbilical cord mesenchymal stem cells (uMSCs)-exosomes carried by a HyStem-HP hydrogel to the fracture site in a rat model of femoral fracture and found that 14 days treatment significantly increased callus volumes, BMD, BV and BV/TV (Zhang Y. et al., 2019). In a separate study, hiPSC-MSCs-exosomes enhance the osteoinductivity of β-tricalcium phosphate (β-TCP) through activating the phosphatidylinositol-3-kinases/protein-serine-threonine kinase (PI3K/AKT) signaling pathway in hBMSCs. Based on the above findings, they designed hiPSC-MSCs-Exos functionalized β-TCP scaffold can effectively promote bone repair and regeneration in a rat model of calvarial bone defects (Zhang J. Y. et al., 2016). In a latest study, hypoxic uMSCs-exosomes (50–150 nm) promote fracture healing by transfering miR-126 (Liu et al., 2020). Compared with regular exosomes, hypo-exos function better in promoting angiogenesis, proliferation and migration of endothelial cells to a greater extent. Moreover, hypoxia preconditioning mediated enhanced production of exosomal miR-126 through the activation of hypoxia inducible factor 1 (HIF-1α). Hypoxia preconditioning represents a promising method for optimizing the therapeutic actions of MSC- exosomes in bone fracture healing (Liu et al., 2020). These findings verify the vital regulatory role of MSCs-EV in bone fracture healing, identifying it as a potential therapeutic method for bone fracture.
In summary, present studies demonstrate that MSCs-EV are effective for treating OA, RA, OP, and bone fracture both in vitro and in animal models (Table 3 and Figure 3). Several regulatory pathways have been demonstrated to function in MSCs-EV treating OA, RA, OP, and bone fracture, including AKT, ERK, AMPK, mTOR-autophagy, Wnt/β-catenin, MAPK, and Notch signaling pathways. For the treatment of OA, MSCs-EV reduce inflammation by activating adenosine of AKT and ERK, and the phosphorylation of AMPK. Moreover, MSCs-EV significantly enhance the autophagy level of chondrocytes through inhibiting mTOR signaling pathway. In terms of cartilage protection and regeneration, MSCs-EV activate YAP, decrease ECM secretion, and promote articular chondrocytes proliferation and migration via Wnt5a and Wnt5b. In addition, MSCs-EV suppress IL-1β-induced chondrocyte apoptosis, and enhance s-GAG matrix synthesis through inhibiting the phosphorylation of p38 and ERK1/2 thus restore matrix homeostasis and ameliorate OA (Table 3 and Figure 3). In the treatment of bone loss and fracture healing, MSCs-EV promote osteogenesis, reduced the decrease in bone mass by activating Wnt/β-catenin pathway, and alleviate the osteoporosis progression by promoting the osteoblast proliferation through MAPK signaling pathway. Furthermore, MSCs-EV recover Dnmt1-mediated Notch1 promoter hypomethylation, enhance mineralized nodule formation, elevate the expression of Runx2 and ALP, thus increase bone formation (Table 3 and Figure 3). These findings suggest potential clinical application of MSCs-EV in the treatment of bone disorders and also reveal the possible regulatory pathways that can be targeted for the clinical translation of MSCs-EV in treating bone disorders (Table 3 and Figure 3).
Conclusion and Future Perspectives
Bone is the specific organ for supporting the body, protecting other organs, and storing minerals. Bone disorders normally results in pain and disability, severely reduces the quality of life and increases the burden on society. Therefore, the efficacious therapies for bone diseases have become the major concern worldwide. More recently, MSCs-EV have been shown to mediate the therapeutic effects of MSCs in various diseases and have been applied to clinical trials for several diseases, such as type I diabetes mellitus (trial NCT02138331), macular holes (NCT03437759) and acute ischemic stroke (NCT03384433)7. Thus, the role of MSCs-EV in the treatment of bone diseases has become a research focus. Here, we focus on the application of MSCs-EV in four major bone disorders, including OA, RA, OP, and bone fracture.
MSCs-EV, lipid-bilayer spheroids, are characterized by possessing immunomodulatory and regenerative properties, similar to their producing cells. As MSCs-EV mediate both intercellular communication and the interactions with the cellular microenvironments, they have become fascinating to be a novel cell-free therapeutic strategy for treating diseases, including bone disorders. The currently in vitro and in vivo studies reveal the efficacy of MSCs-EV in treating OA, RA, OP, and bone fracture. MSCs-EV may alleviate the pain, promote overall joint repair and regeneration in OA by suppressing inflammation, promoting cartilage repair and regeneration, and restoring joint homeostasis. They also show immunomodulatory function in RA by regulating the function of T lymphocytes and B lymphocytes and inhibiting angiogenesis via downregulating MMP14 and VEGF, exerting therapeutic effects on joint destruction of RA. Moreover, MSCs-EV improve the osteoporosis by promoting cell proliferation, osteogenic differentiation, and reducing cell senescence of BM-MSCs or osteoblast. Furthermore, the therapeutic effect of MSCs-EV is also observed in bone fracture healing. MSCs-EV orchestrate the process of osteogenesis and angiogenesis to enhance fracture healing. Overall, the present findings suggest MSCs-EV as a new cell-free therapeutic method for bone disorders.
The findings at current stage are very encouraging; however, further research is still needed to make MSCs-EV a routine clinical treatment for bone disease. There are several critical issues to consider, including: (1) The optimal source of MSCs. Since there are many sources of MSCs (e.g., BM-MSCs and AD-MSCs) and MSCs-EV produced by different cell source contain different cargos, it is important to determine the optimal source of MSCs, which may be bioengineered for producing MSCs-EV or their specific cargos according to specific requirements. (2) Standard methods for large-scale production and isolation of MSCs-EV. Because the culture condition affects the MSCs-EV production, the production methods of MSCs-EV should be optimized and controllable for large-scale and specific type of MSCs-EV production. Moreover, there is an urgent need to develop standardized methods for the isolation and purification of MSCs-EV, although establishing a universal isolation method is unlikely due to the complexity of MSC-EV. The isolation methods should not disrupt the structure and functions of MSCs-EV. (3) Methods for rapid and accurate quantification and characterization of MSCs-EV, which are critical for MSCs-EV clinical application. (4) Safe and effective approaches to deliver MSCs-EV to the body or to target sites for treatment. (5) The pharmacokinetics (e.g., tissue distribution, half-life period) of MSCs-EV in the body. (6) Safe and optimal dosage of MSCs-EV for treating different bone diseases. MSCs-exosomes execute synergetic biological functions through combinatorial effects together with their large diverse proteomic and RNA cargo (Lai et al., 2016). It has to be taken into consideration that exosomes do not harbor many copies of miRNA molecules. On average, 100 exosomes would be needed to transfer one copy of a given abundant miRNA. In contrast, MSCs-exosomes proteins in a typical therapeutic dose have the potential to trigger a biologically relevant response (Toh et al., 2018). Moreover, as individual MSCs-EV cargo is not equally or sufficiently efficacious in ameliorating tissue injury (Lai et al., 2012), a safe and optimal dosage needs to be considered. (7) Uncovery of the action mechanisms of MSCs-EV in treating bone disorders by characterizing their functional cargos. Current available publications have demonstrated the involvement of some key molecules and signaling pathways in the therapeutic effects of MSCs-EV on bone disorders, such as Wnts, YAP, AKT, ERK, AMPK, mTOR-autophagy, Wnt/β-catenin, MAPK, and Notch signaling pathways. However, most studies have only identified the mechanism of action of MSCs-EV as a whole, without identifying their specific components. For illustrating the function and mechanism of the MSCs-EV cargo components, current available publications mainly focus on miRNAs. Therefore, the molecular mechanisms of functional components such as MSCs-EV proteins still need to be further investigated. Moreover, current findings show that MSCs-EV exert different effects on angiogenesis in the treatment of RA and bone fracture. MSCs-EV inhibit angiogenesis and the expression of VEGF in treating RA while promote angiogenesis and VEGF expression in treating bone fracture. The molecular mechanism of MSCs-EV is still unclear, further investigation is necessary to make them more suitable for clinical application. (8) The possibility of modifying MSCs and artificially synthesizing MSCs-EV or their specific cargos for treating bone diseases. Biophysical and biochemical methods can be used to modify the properties of MSCs, thereby influencing EV composition and secretion. It leaves a wide range of conditions to be explored in attempts to increase MSCs-EV yields and their therapeutic potential (Park et al., 2019). In addition, understanding the molecular mechanisms of MSCs-EV for the treatment of bone diseases will prevent some side effects and make the clinical application of MSCs-EV more precise and safer. (9) Clinical studies are needed to prove the efficacy of MSCs-EV for the treatment of bone diseased. Clarification of these key issues will make MSCs-EV a more fantastic novel cell-free therapeutic strategy for bone disorders treatment in clinics.
Author Contributions
SLiu, XX, and SLia drafted the manuscript. ZC and YZ revised the manuscript. SLiu designed the figure. AQ and LH revised and approved the manuscript. All the authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (81772017), the China Postdoctoral Science Foundation (2018T111099), Young Talent Fund of University Association for Science and Technology in Shaanxi, China (20170401), the Natural Science Foundation of Shaanxi Province (2018JM3040), and the Seed Foundation of Innovation and Creation for Graduate Students in Northwestern Polytechnical University (CX2020264).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
β-TCP, β-tricalcium phosphate; AD-MSCs, adipose tissue-derived mesenchymal stem cells; AFM, atomic-force microscopy; AKT, phosphorylation of protein-serine-threonine kinase; ALP, alkaline phosphatase; AMPK, adenosine 5’-monophosphate (AMP)-activated protein kinase; BFR/BS, bone formation rate per bone Surface; BMD, bone mineral density; BM-MSCs, bone marrow mesenchymal stem cells; BV/TV, bone volume/total volume; CIA, collagen-induced arthritis; Col2a1, collagen, type II, alpha 1; Dnmt1, DNA methyltransferase 1; DLS, dynamic-light scattering; ECM, extracellular matrix; ECM/GelMA/exosome, extracellular matrix/gelatin methacrylate/exosome; ERK 1/2, extracellular regulated protein kinases 1/2; ESCRT, endosomal sorting complex required for transport; EV, extracellular vesicles; FLS, fibroblast-like synoviocytes; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; hESC-MSCs, human embryonic-derived mesenchymal stem cells; hiPSC, human-induced pluripotent stem cell; hMSC, human mesenchymal stem cells; HLA-DR, Antigen-antigen D Related; HSP, heat shock proteins; IL-1 α, interleukin-1 α; IL-1 β, interleukin-1 β; ILVs, intraluminal vesicles; IPFP-MSCs, infrapatellar fat pad (IPFP)-derived mesenchymal stem cells; LDHA, lactic dehydrogenase A; lncRNA, long non-coding RNA; MAPK, mitogen-activated protein kinase; MAR, mineral apposition rate; MCP-1, monocyte chemotactic protein 1; MCP-3, monocyte chemotactic protein 3; MHC, major histocompatibility complex; miRNAs, microRNAs; MMP13, matrix metalloproteinase 13; MMP14, matrix metalloproteinase 14; mRNAs, message RNAs; MSCs, mesenchymal stem cells; MSCT, MSCs transplant treatment; mtDNA, mitochondrial DNA; mTOR, mammalian target of rapamycin; MV, microvesicles; MVBs, multivesicular bodies; NTA, nanoparticle-tracking analyses; NGS, next-generation sequencing; OA, osteoarthritis; OP, osteoporosis; PGK1, phosphoglycerate kinase 1; PI3K/AKT, phosphatidylinositol-3-kinases/protein-serine-threonine kinase; PKM, protein kinase; PM, plasma membrane; PCS, photon-correlation spectroscopy; RA, rheumatoid arthritis; ROS, reactive oxygen species; Runx2, Runt-related transcription factor 2; SDF-1, stromal cell-derived factor-1; s-GAG, sulphated glycosaminoglycan; SMSCs, synovial mesenchymal stem cells; Sp1, specificity Protein 1; TGF- β 1, transforming growth factor β 1; TEM, transmission electron microscopy; Th1, T helper cell type 1; Th17, T helper cell type 17; Th2, T helper cell type 2; TMJ-OA, temporomandibular joint osteoarthritis; TNF- α, tumor necrosis factor- α; TRPS, tunable-resistive pulse sensing; uMSCs, umbilical cord mesenchymal stem cells; VEGF, vascular endothelial growth factor; YAP, yes-associated protein.
Footnotes
- ^ http://www.exocarta.org
- ^ http://www.microvesicles.org
- ^ www.systembio.com
- ^ www.clinicaltrials.gov
References
Baglio, S. R., Rooijers, K., Koppers-Lalic, D., Verweij, F. J., Perez Lanzon, M., Zini, N., et al. (2015). Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 6:127. doi: 10.1186/s13287-015-0116-z
Baron, R., and Kneissel, M. (2013). WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat. Med. 19, 179–192. doi: 10.1038/nm.3074
Batiz, L. F., Castro, M. A., Burgos, P. V., Velasquez, Z. D., Munoz, R. I., Lafourcade, C. A., et al. (2015). Exosomes as novel regulators of adult neurogenic niches. Front. Cell. Neurosci. 9:501. doi: 10.3389/fncel.2015.00501
Beyer, C., and Pisetsky, D. S. (2010). The role of microparticles in the pathogenesis of rheumatic diseases. Nat. Rev. Rheumatol. 6, 21–29. doi: 10.1038/nrrheum.2009.229
Biswas, S., Mandal, G., Roy Chowdhury, S., Purohit, S., Payne, K. K., Anadon, C., et al. (2019). Exosomes produced by mesenchymal stem cells drive differentiation of myeloid cells into immunosuppressive M2-polarized macrophages in breast cancer. J. Immunol. 203, 3447–3460. doi: 10.4049/jimmunol.1900692
Blanco, F. J., Rego, I., and Ruiz-Romero, C. (2011). The role of mitochondria in osteoarthritis. Nat. Rev. Rheumatol. 7, 161–169. doi: 10.1038/nrrheum.2010.213
Borger, V., Bremer, M., Ferrer-Tur, R., Gockeln, L., Stambouli, O., Becic, A., et al. (2017). Mesenchymal stem/stromal cell-derived extracellular vesicles and their potential as novel immunomodulatory therapeutic agents. Int. J. Mol. Sci. 18:1450. doi: 10.3390/ijms18071450
Breitbach, M., Bostani, T., Roell, W., Xia, Y., Dewald, O., Nygren, J. M., et al. (2007). Potential risks of bone marrow cell transplantation into infarcted hearts. Blood 110, 1362–1369. doi: 10.1182/blood-2006-12-063412
Bruno, S., Grange, C., Deregibus, M. C., Calogero, R. A., Saviozzi, S., Collino, F., et al. (2009). Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J. Am. Soc. Nephrol. 20, 1053–1067. doi: 10.1681/ASN.2008070798
Burrello, J., Monticone, S., Gai, C., Gomez, Y., Kholia, S., and Camussi, G. (2016). Stem cell-derived extracellular vesicles and immune-modulation. Front. Cell Dev. Biol. 4:83. doi: 10.3389/fcell.2016.00083
Cao, H., Cheng, Y., Gao, H., Zhuang, J., Zhang, W., Bian, Q., et al. (2020). In vivo tracking of mesenchymal stem cell-derived extracellular vesicles improving mitochondrial function in renal ischemia-reperfusion injury. ACS Nano 14, 4014–4026. doi: 10.1021/acsnano.9b08207
Chakari-Khiavi, F., Dolati, S., Chakari-Khiavi, A., Abbaszadeh, H., Aghebati-Maleki, L., Pourlak, T., et al. (2019). Prospects for the application of mesenchymal stem cells in Alzheimer’s disease treatment. Life Sci. 231:116564. doi: 10.1016/j.lfs.2019.116564
Chang, Y. H., Wu, K. C., Harn, H. J., Lin, S. Z., and Ding, D. C. (2018). Exosomes and stem cells in degenerative disease diagnosis and therapy. Cell Transpl. 27, 349–363. doi: 10.1177/0963689717723636
Chaput, N., and Thery, C. (2011). Exosomes: immune properties and potential clinical implementations. Semin. Immunopathol. 33, 419–440. doi: 10.1007/s00281-010-0233-9
Chen, C., Skog, J., Hsu, C. H., Lessard, R. T., Balaj, L., Wurdinger, T., et al. (2010). Microfluidic isolation and transcriptome analysis of serum microvesicles. Lab Chip 10, 505–511. doi: 10.1039/b916199f
Chen, P., Zheng, L., Wang, Y., Tao, M., Xie, Z., Xia, C., et al. (2019). Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics 9, 2439–2459. doi: 10.7150/thno.31017
Chen, T. S., Lai, R. C., Lee, M. M., Choo, A. B., Lee, C. N., and Lim, S. K. (2010). Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 38, 215–224. doi: 10.1093/nar/gkp857
Chen, W., Huang, Y., Han, J., Yu, L., Li, Y., Lu, Z., et al. (2016). Immunomodulatory effects of mesenchymal stromal cells-derived exosome. Immunol. Res. 64, 831–840. doi: 10.1007/s12026-016-8798-6
Chen, Z., Wang, H. Q., Xia, Y., Yan, F. H., and Lu, Y. (2018). Therapeutic potential of mesenchymal cell-derived miRNA-150-5p-expressing exosomes in rheumatoid arthritis mediated by the modulation of MMP14 and VEGF. J. Immunol. 201, 2472–2482. doi: 10.4049/jimmunol.1800304
Cocucci, E., Racchetti, G., and Meldolesi, J. (2009). Shedding microvesicles: artefacts no more. Trends Cell Biol. 19, 43–51. doi: 10.1016/j.tcb.2008.11.003
Colombo, M., Raposo, G., and Thery, C. (2014). Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 30, 255–289. doi: 10.1146/annurev-cellbio-101512-122326
Contreras-Naranjo, J. C., Wu, H. J., and Ugaz, V. M. (2017). Microfluidics for exosome isolation and analysis: enabling liquid biopsy for personalized medicine. Lab Chip 17, 3558–3577. doi: 10.1039/c7lc00592j
Cosenza, S., Ruiz, M., Toupet, K., Jorgensen, C., and Noel, D. (2017). Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci. Rep. 7:16214. doi: 10.1038/s41598-017-15376-8
Cosenza, S., Toupet, K., Maumus, M., Luz-Crawford, P., Blanc-Brude, O., Jorgensen, C., et al. (2018). Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics 8, 1399–1410. doi: 10.7150/thno.21072
Crossland, R. E., Norden, J., Bibby, L. A., Davis, J., and Dickinson, A. M. (2016). Evaluation of optimal extracellular vesicle small RNA isolation and qRT-PCR normalisation for serum and urine. J. Immunol. Methods 429, 39–49. doi: 10.1016/j.jim.2015.12.011
da Silva Meirelles, L., Chagastelles, P. C., and Nardi, N. B. (2006). Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 119(Pt 11), 2204–2213. doi: 10.1242/jcs.02932
Davatchi, F., Sadeghi Abdollahi, B., Mohyeddin, M., and Nikbin, B. (2016). Mesenchymal stem cell therapy for knee osteoarthritis: 5 years follow-up of three patients. Int. J. Rheum. Dis. 19, 219–225. doi: 10.1111/1756-185x.12670
Del Fattore, A., Luciano, R., Saracino, R., Battafarano, G., Rizzo, C., Pascucci, L., et al. (2015). Differential effects of extracellular vesicles secreted by mesenchymal stem cells from different sources on glioblastoma cells. Expert Opin. Biol. Ther. 15, 495–504. doi: 10.1517/14712598.2015.997706
Dominici, M., Le Blanc, K., Mueller, I., Slaper-Cortenbach, I., Marini, F., Krause, D., et al. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 8, 315–317. doi: 10.1080/14653240600855905
Dong, R., Liu, Y., Yang, Y., Wang, H., Xu, Y., and Zhang, Z. (2019). MSC-derived exosomes-based therapy for peripheral nerve injury: a novel therapeutic strategy. Biomed. Res. Int. 2019:6458237. doi: 10.1155/2019/6458237
D’souza, N., Rossignoli, F., Golinelli, G., Grisendi, G., Spano, C., Candini, O., et al. (2015). Mesenchymal stem/stromal cells as a delivery platform in cell and gene therapies. BMC Medicine 13:186. doi: 10.1186/S12916-015-0426-0
Fan, X.-L., Zhang, Y., Li, X., and Fu, Q.-L. (2020). Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell Mol. Life Sci. 77, 2771–2794. doi: 10.1007/s00018-020-03454-6
Fernandez-Llama, P., Khositseth, S., Gonzales, P. A., Star, R. A., Pisitkun, T., and Knepper, M. A. (2010). Tamm-Horsfall protein and urinary exosome isolation. Kidney Int. 77, 736–742. doi: 10.1038/ki.2009.550
Fricke, A., Ullrich, P. V., Cimniak, A. F. V., Becherer, C., Follo, M., Heinz, J., et al. (2017). Levels of activated platelet-derived microvesicles in patients with soft tissue sarcoma correlate with an increased risk of venous thromboembolism. BMC Cancer 17:527. doi: 10.1186/s12885-017-3515-y
Frydrychowicz, M., Kolecka-Bednarczyk, A., Madejczyk, M., Yasar, S., and Dworacki, G. (2015). Exosomes - structure, biogenesis and biological role in non-small-cell lung cancer. Scand. J. Immunol. 81, 2–10. doi: 10.1111/sji.12247
Furlani, D., Ugurlucan, M., Ong, L., Bieback, K., Pittermann, E., Westien, I., et al. (2009). Is the intravascular administration of mesenchymal stem cells safe? Mesenchymal stem cells and intravital microscopy. Microvasc. Res. 77, 370–376. doi: 10.1016/j.mvr.2009.02.001
Furuta, T., Miyaki, S., Ishitobi, H., Ogura, T., Kato, Y., Kamei, N., et al. (2016). Mesenchymal stem cell-derived exosomes promote fracture healing in a mouse model. Stem Cells Transl. Med. 5, 1620–1630. doi: 10.5966/sctm.2015-0285
Galipeau, J., and Sensebe, L. (2018). Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell 22, 824–833. doi: 10.1016/j.stem.2018.05.004
Gamez-Valero, A., Monguio-Tortajada, M., Carreras-Planella, L., Franquesa, M., Beyer, K., and Borras, F. E. (2016). Size-exclusion chromatography-based isolation minimally alters extracellular Vesicles’ characteristics compared to precipitating agents. Sci. Rep. 6:33641. doi: 10.1038/srep33641
Giebel, B., Kordelas, L., and Borger, V. (2017). Clinical potential of mesenchymal stem/stromal cell-derived extracellular vesicles. Stem Cell Investig. 4:84. doi: 10.21037/sci.2017.09.06
Greening, D. W., Xu, R., Ji, H., Tauro, B. J., and Simpson, R. J. (2015). A protocol for exosome isolation and characterization: evaluation of ultracentrifugation, density-gradient separation, and immunoaffinity capture methods. Methods Mol. Biol. 1295, 179–209. doi: 10.1007/978-1-4939-2550-6_15
Gurunathan, S., Kang, M. H., Jeyaraj, M., Qasim, M., and Kim, J. H. (2019). Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells 8:307. doi: 10.3390/cells8040307
Hanson, P. I., and Cashikar, A. (2012). Multivesicular body morphogenesis. Annu. Rev. Cell Dev. Biol. 28, 337–362. doi: 10.1146/annurev-cellbio-092910-154152
Harding, C., Heuser, J., and Stahl, P. (1983). Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell Biol. 97, 329–339. doi: 10.1083/jcb.97.2.329
Heldens, G. T. H., Blaney Davidson, E. N., Vitters, E. L., Schreurs, B. W., Piek, E., van den Berg, W. B., et al. (2012). Catabolic factors and osteoarthritis-conditioned medium inhibit chondrogenesis of human mesenchymal stem cells. Tissue Eng. Part A 18, 45–54. doi: 10.1089/ten.TEA.2011.0083
Hu, C., Zhao, L., and Li, L. (2019). Current understanding of adipose-derived mesenchymal stem cell-based therapies in liver diseases. Stem Cell Res. Ther. 10:199. doi: 10.1186/s13287-019-1310-1
Hu, L., Yin, C., Zhao, F., Ali, A., Ma, J., and Qian, A. (2018). Mesenchymal stem cells: cell fate decision to osteoblast or adipocyte and application in osteoporosis treatment. Int. J. Mol. Sci. 19:360. doi: 10.3390/ijms19020360
Jeong, J. O., Han, J. W., Kim, J. M., Cho, H. J., Park, C., Lee, N., et al. (2011). Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ. Res. 108, 1340–1347. doi: 10.1161/CIRCRESAHA.110.239848
Jing, H., He, X., and Zheng, J. (2018). Exosomes and regenerative medicine: state of the art and perspectives. Transl. Res. 196, 1–16. doi: 10.1016/j.trsl.2018.01.005
Kanwar, S. S., Dunlay, C. J., Simeone, D. M., and Nagrath, S. (2014). Microfluidic device (ExoChip) for on-chip isolation, quantification and characterization of circulating exosomes. Lab Chip 14, 1891–1900. doi: 10.1039/c4lc00136b
Kim, J., Shin, H., Kim, J., Kim, J., and Park, J. (2015). Isolation of high-purity extracellular vesicles by extracting proteins using aqueous two-phase system. PLoS One 10:e0129760. doi: 10.1371/journal.pone.0129760
Konoshenko, M. Y., Lekchnov, E. A., Vlassov, A. V., and Laktionov, P. P. (2018). Isolation of extracellular vesicles: general methodologies and latest trends. Biomed. Res. Int. 2018:8545347. doi: 10.1155/2018/8545347
Kowal, J., Tkach, M., and Théry, C. (2014). Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 29, 116–125. doi: 10.1016/j.ceb.2014.05.004
Lai, R. C., Arslan, F., Lee, M. M., Sze, N. S., Choo, A., Chen, T. S., et al. (2010). Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 4, 214–222. doi: 10.1016/j.scr.2009.12.003
Lai, R. C., Tan, S. S., Teh, B. J., Sze, S. K., Arslan, F., de Kleijn, D. P., et al. (2012). Proteolytic potential of the MSC exosome proteome: implications for an exosome-mediated delivery of therapeutic proteasome. Int. J. Proteomics 2012:971907. doi: 10.1155/2012/971907
Lai, R. C., Tan, S. S., Yeo, R. W., Choo, A. B., Reiner, A. T., Su, Y., et al. (2016). MSC secretes at least 3 EV types each with a unique permutation of membrane lipid, protein and RNA. J. Extracell. Vesicles 5:29828. doi: 10.3402/jev.v5.29828
Lai, R. C., Yeo, R. W., and Lim, S. K. (2015). Mesenchymal stem cell exosomes. Semin. Cell Dev. Biol. 40, 82–88. doi: 10.1016/j.semcdb.2015.03.001
Li, T., Yan, Y., Wang, B., Qian, H., Zhang, X., Shen, L., et al. (2013). Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 22, 845–854. doi: 10.1089/scd.2012.0395
Liang, L. G., Kong, M. Q., Zhou, S., Sheng, Y. F., Wang, P., Yu, T., et al. (2017). An integrated double-filtration microfluidic device for isolation, enrichment and quantification of urinary extracellular vesicles for detection of bladder cancer. Sci. Rep. 7:46224. doi: 10.1038/srep46224
Lindblad, S., and Hedfors, E. (1987). Arthroscopic and immunohistologic characterization of knee joint synovitis in osteoarthritis. Arthritis Rheum. 30, 1081–1088. doi: 10.1002/art.1780301001
Liu, S., Liu, D., Chen, C., Hamamura, K., Moshaverinia, A., Yang, R., et al. (2015). MSC transplantation improves osteopenia via epigenetic regulation of notch signaling in lupus. Cell Metab. 22, 606–618. doi: 10.1016/j.cmet.2015.08.018
Liu, W., Li, L., Rong, Y., Qian, D., Chen, J., Zhou, Z., et al. (2020). Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater. 103, 196–212. doi: 10.1016/j.actbio.2019.12.020
Liu, Y., Zou, R., Wang, Z., Wen, C., Zhang, F., and Lin, F. (2018). Exosomal KLF3-AS1 from hMSCs promoted cartilage repair and chondrocyte proliferation in osteoarthritis. Biochem. J. 475, 3629–3638. doi: 10.1042/BCJ20180675
Liu-Bryan, R. (2015). Inflammation and intracellular metabolism: new targets in OA. Osteoarthr. Cartil. 23, 1835–1842. doi: 10.1016/j.joca.2014.12.016
Livshits, M. A., Khomyakova, E., Evtushenko, E. G., Lazarev, V. N., Kulemin, N. A., Semina, S. E., et al. (2015). Isolation of exosomes by differential centrifugation: theoretical analysis of a commonly used protocol. Sci. Rep. 5:17319. doi: 10.1038/srep17319
Lobb, R. J., Becker, M., Wen Wen, S., Wong, C. S. F., Wiegmans, A. P., Leimgruber, A., et al. (2015). Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell. Vesicles 4:27031. doi: 10.3402/jev.v4.27031
Loeser, R. F., Goldring, S. R., Scanzello, C. R., and Goldring, M. B. (2012). Osteoarthritis: a disease of the joint as an organ. Arthr. Rheumatol. 64, 1697–1707. doi: 10.1002/art.34453
Lopez-Verrilli, M. A., Caviedes, A., Cabrera, A., Sandoval, S., Wyneken, U., and Khoury, M. (2016). Mesenchymal stem cell-derived exosomes from different sources selectively promote neuritic outgrowth. Neuroscience 320, 129–139. doi: 10.1016/j.neuroscience.2016.01.061
Mao, G., Zhang, Z., Hu, S., Zhang, Z., Chang, Z., Huang, Z., et al. (2018). Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res. Ther. 9:247. doi: 10.1186/s13287-018-1004-0
Mathivanan, S., Ji, H., and Simpson, R. J. (2010). Exosomes: extracellular organelles important in intercellular communication. J. Proteomics 73, 1907–1920. doi: 10.1016/j.jprot.2010.06.006
Meirelles Lda, S., Fontes, A. M., Covas, D. T., and Caplan, A. I. (2009). Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 20, 419–427. doi: 10.1016/j.cytogfr.2009.10.002
Missoum, A. (2020). Recent updates on mesenchymal stem cell based therapy for acute renal failure. Curr. Urol. 13, 189–199. doi: 10.1159/000499272
Naderi-Meshkin, H., Lai, X., Amirkhah, R., Vera, J., Rasko, J. E. J., and Schmitz, U. (2019). Exosomal lncRNAs and cancer: connecting the missing links. Bioinformatics 35, 352–360. doi: 10.1093/bioinformatics/bty527
Nold, P., Hackstein, H., Riedlinger, T., Kasper, C., Neumann, A., Mernberger, M., et al. (2015). Immunosuppressive capabilities of mesenchymal stromal cells are maintained under hypoxic growth conditions and after gamma irradiation. Cytotherapy 17, 152–162. doi: 10.1016/j.jcyt.2014.10.004
Ohno, S., Ishikawa, A., and Kuroda, M. (2013). Roles of exosomes and microvesicles in disease pathogenesis. Adv. Drug Deliv. Rev. 65, 398–401. doi: 10.1016/j.addr.2012.07.019
Pak, H. N., Qayyum, M., Kim, D. T., Hamabe, A., Miyauchi, Y., Lill, M. C., et al. (2003). Mesenchymal stem cell injection induces cardiac nerve sprouting and increased tenascin expression in a Swine model of myocardial infarction. J. Cardiovasc. Electrophysiol. 14, 841–848. doi: 10.1046/j.1540-8167.2003.03124.x
Pan, B. T., and Johnstone, R. M. (1983). Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell 33, 967–978. doi: 10.1016/0092-8674(83)90040-5
Park, K. S., Bandeira, E., Shelke, G. V., Lasser, C., and Lotvall, J. (2019). Enhancement of therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res. Ther. 10:288. doi: 10.1186/s13287-019-1398-3
Peterson, M. F., Otoc, N., Sethi, J. K., Gupta, A., and Antes, T. J. (2015). Integrated systems for exosome investigation. Methods 87, 31–45. doi: 10.1016/j.ymeth.2015.04.015
Pittenger, M. F., Mackay, A. M., Beck, S. C., Jaiswal, R. K., Douglas, R., Mosca, J. D., et al. (1999). Multilineage potential of adult human mesenchymal stem cells. Science 284, 143–147. doi: 10.1126/science.284.5411.143
Pozsgay, J., Szekanecz, Z., and Sarmay, G. (2017). Antigen-specific immunotherapies in rheumatic diseases. Nat. Rev. Rheumatol. 13, 525–537. doi: 10.1038/nrrheum.2017.107
Qi, H., Liu, D. P., Xiao, D. W., Tian, D. C., Su, Y. W., and Jin, S. F. (2019). Exosomes derived from mesenchymal stem cells inhibit mitochondrial dysfunction-induced apoptosis of chondrocytes via p38, ERK, and Akt pathways. In Vitro Cell Dev. Biol. Anim. 55, 203–210. doi: 10.1007/s11626-019-00330-x
Qi, X., Zhang, J. Y., Yuan, H., Xu, Z. L., Li, Q., Niu, X., et al. (2016). Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int. J. Biol. Sci. 12, 836–849. doi: 10.7150/ijbs.14809
Qi, Y., Feng, G., and Yan, W. (2012). Mesenchymal stem cell-based treatment for cartilage defects in osteoarthritis. Mol. Biol. Rep. 39, 5683–5689. doi: 10.1007/s11033-011-1376-z
Ragni, E., Perucca Orfei, C., De Luca, P., Colombini, A., Vigano, M., and de Girolamo, L. (2020). Secreted factors and EV-miRNAs orchestrate the healing capacity of adipose mesenchymal stem cells for the treatment of knee osteoarthritis. Int. J. Mol. Sci. 21:1582. doi: 10.3390/ijms21051582
Ragni, E., Perucca Orfei, C., De Luca, P., Lugano, G., Vigano, M., Colombini, A., et al. (2019). Interaction with hyaluronan matrix and miRNA cargo as contributors for in vitro potential of mesenchymal stem cell-derived extracellular vesicles in a model of human osteoarthritic synoviocytes. Stem Cell Res. Ther. 10:109. doi: 10.1186/s13287-019-1215-z
Rani, S., Ryan, A. E., Griffin, M. D., and Ritter, T. (2015). Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol. Ther. 23, 812–823. doi: 10.1038/mt.2015.44
Raposo, G., and Stoorvogel, W. (2013). Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 200, 373–383. doi: 10.1083/jcb.201211138
Romanelli, P., Bieler, L., Scharler, C., Pachler, K., Kreutzer, C., Zaunmair, P., et al. (2019). Extracellular vesicles can deliver anti-inflammatory and anti-scarring activities of mesenchymal stromal cells after spinal cord injury. Front. Neurol. 10:1225. doi: 10.3389/fneur.2019.01225
Shao, H., Chung, J., and Issadore, D. (2015). Diagnostic technologies for circulating tumour cells and exosomes. Biosci. Rep. 36:e00292. doi: 10.1042/BSR20150180
Shenoda, B. B., and Ajit, S. K. (2016). Modulation of immune responses by exosomes derived from antigen-presenting cells. Clin. Med. Insights Pathol. 9(Suppl. 1) 1–8. doi: 10.4137/CPath.S39925
Squillaro, T., Peluso, G., and Galderisi, U. (2016). Clinical trials with mesenchymal stem cells: an update. Cell Transpl. 25, 829–848. doi: 10.3727/096368915x689622
Stiehler, M., Goronzy, J., and Gunther, K. P. (2015). [Total hip arthroplasty in overweight osteoarthritis patients]. Orthopade 44, 523–530. doi: 10.1007/s00132-015-3094-z
Takahashi, A., Okada, R., Nagao, K., Kawamata, Y., Hanyu, A., Yoshimoto, S., et al. (2017). Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat. Commun. 8:15287. doi: 10.1038/ncomms15287
Takeuchi, R., Katagiri, W., Endo, S., and Kobayashi, T. (2019). Exosomes from conditioned media of bone marrow-derived mesenchymal stem cells promote bone regeneration by enhancing angiogenesis. PLoS One 14:e0225472. doi: 10.1371/journal.pone.0225472
Tan, C. Y., Lai, R. C., Wong, W., Dan, Y. Y., Lim, S. K., and Ho, H. K. (2014). Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res. Ther. 5:76. doi: 10.1186/scrt465
Tao, S. C., Yuan, T., Zhang, Y. L., Yin, W. J., Guo, S. C., and Zhang, C. Q. (2017). Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics 7, 180–195. doi: 10.7150/thno.17133
Thery, C., Ostrowski, M., and Segura, E. (2009). Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 9, 581–593. doi: 10.1038/nri2567
Thery, C., Witwer, K. W., Aikawa, E., Alcaraz, M. J., Anderson, J. D., Andriantsitohaina, R., et al. (2018). Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 7:1535750. doi: 10.1080/20013078.2018.1535750
Tofiño-Vian, M., Guillén, M. I., Caz, P. D., Silvestre, A., and Alcaraz, M. J. (2018). Microvesicles from human adipose tissue-derived mesenchymal stem cells as a new protective strategy in osteoarthritic chondrocytes. Cell. Physiol. Biochem. 47, 11–25. doi: 10.1159/000489739
Toh, W. S., Lai, R. C., Hui, J. H. P., and Lim, S. K. (2017). MSC exosome as a cell-free MSC therapy for cartilage regeneration: implications for osteoarthritis treatment. Semin. Cell Dev. Biol. 67, 56–64. doi: 10.1016/j.semcdb.2016.11.008
Toh, W. S., Lai, R. C., Zhang, B., and Lim, S. K. (2018). MSC exosome works through a protein-based mechanism of action. Biochem. Soc. Trans. 46, 843–853. doi: 10.1042/BST20180079
Tolar, J., Nauta, A. J., Osborn, M. J., Panoskaltsis Mortari, A., McElmurry, R. T., Bell, S., et al. (2007). Sarcoma derived from cultured mesenchymal stem cells. Stem Cells 25, 371–379. doi: 10.1634/stemcells.2005-0620
Trajkovic, K., Hsu, C., Chiantia, S., Rajendran, L., Wenzel, D., Wieland, F., et al. (2008). Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319, 1244–1247. doi: 10.1126/science.1153124
Trams, E. G., Lauter, C. J., Salem, N. Jr., and Heine, U. (1981). Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim. Biophys. Acta 645, 63–70. doi: 10.1016/0005-2736(81)90512-5
van der Pol, E., Böing, A. N., Harrison, P., Sturk, A., and Nieuwland, R. (2012). Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 64, 676–705. doi: 10.1124/pr.112.005983
van Niel, G., Porto-Carreiro, I., Simoes, S., and Raposo, G. (2006). Exosomes: a common pathway for a specialized function. J. Biochem. 140, 13–21. doi: 10.1093/jb/mvj128
Vega, A., Martin-Ferrero, M. A., Del Canto, F., Alberca, M., Garcia, V., Munar, A., et al. (2015). Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation 99, 1681–1690. doi: 10.1097/tp.0000000000000678
Wang, D., and Sun, W. (2014). Urinary extracellular microvesicles: isolation methods and prospects for urinary proteome. Proteomics 14, 1922–1932. doi: 10.1002/pmic.201300371
Wang, R., Xu, B., and Xu, H. (2018). TGF-beta1 promoted chondrocyte proliferation by regulating Sp1 through MSC-exosomes derived miR-135b. Cell Cycle 17, 2756–2765. doi: 10.1080/15384101.2018.1556063
Wang, Y. F., Yu, D. S., Liu, Z. M., Zhou, F., Dai, J., Wu, B. B., et al. (2017). Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res. Ther. 8:189. doi: 10.1186/S13287-017-0632-0
Wang, Z., Wang, Y., Wang, Z., Gutkind, J. S., Wang, Z., Wang, F., et al. (2015). Engineered mesenchymal stem cells with enhanced tropism and paracrine secretion of cytokines and growth factors to treat traumatic brain injury. Stem Cells 33, 456–467. doi: 10.1002/stem.1878
Wang, Z., Wu, H. J., Fine, D., Schmulen, J., Hu, Y., Godin, B., et al. (2013). Ciliated micropillars for the microfluidic-based isolation of nanoscale lipid vesicles. Lab Chip 13, 2879–2882. doi: 10.1039/c3lc41343h
Willis, G. R., Mitsialis, S. A., and Kourembanas, S. (2018). “Good things come in small packages”: application of exosome-based therapeutics in neonatal lung injury. Pediatr. Res. 83, 298–307. doi: 10.1038/pr.2017.256
Witwer, K. W., Buzas, E. I., Bemis, L. T., Bora, A., Lasser, C., Lotvall, J., et al. (2013). Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles 2:20360. doi: 10.3402/jev.v2i0.20360
Wu, J., Kuang, L., Chen, C., Yang, J., Zeng, W. N., Li, T., et al. (2019). miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials 206, 87–100. doi: 10.1016/j.biomaterials.2019.03.022
Wunsch, B. H., Smith, J. T., Gifford, S. M., Wang, C., Brink, M., Bruce, R. L., et al. (2016). Nanoscale lateral displacement arrays for the separation of exosomes and colloids down to 20 nm. Nat. Nanotechnol. 11, 936–940. doi: 10.1038/nnano.2016.134
Xin, H., Li, Y., Cui, Y., Yang, J. J., Zhang, Z. G., and Chopp, M. (2013). Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J. Cereb. Blood Flow Metab. 33, 1711–1715. doi: 10.1038/jcbfm.2013.152
Xin-Wei, L., Ying, Z., Liang-Bi, X., and Yu, W. (2015). Total hip arthroplasty: a review of advances, advantages and limitations. Int. J. Clin. Exp. Med. 8, 27–36.
Yáñez-Mó, M., Siljander, P. R.-M., Andreu, Z., Zavec, A. B., Borràs, F. E., Buzas, E. I., et al. (2015). Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 4:27066. doi: 10.3402/jev.v4.27066
Yang, Y., Ye, Y., Su, X., He, J., Bai, W., and He, X. (2017). MSCs-derived exosomes and neuroinflammation, neurogenesis and therapy of traumatic brain injury. Front. Cell. Neurosci. 11:55. doi: 10.3389/fncel.2017.00055
Yeo, R. W., Lai, R. C., Zhang, B., Tan, S. S., Yin, Y., Teh, B. J., et al. (2013). Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv. Drug Deliv. Rev. 65, 336–341. doi: 10.1016/j.addr.2012.07.001
Yoon, Y. S., Park, J. S., Tkebuchava, T., Luedeman, C., and Losordo, D. W. (2004). Unexpected severe calcification after transplantation of bone marrow cells in acute myocardial infarction. Circulation 109, 3154–3157. doi: 10.1161/01.CIR.0000134696.08436.65
Zeringer, E., Barta, T., Li, M., and Vlassov, A. V. (2015). Strategies for isolation of exosomes. Cold Spring Harb. Protoc. 2015, 319–323. doi: 10.1101/pdb.top074476
Zhang, J. Y., Liu, X. L., Li, H. Y., Chen, C. Y., Hu, B., Niu, X., et al. (2016). Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res. Ther. 7:136. doi: 10.1186/S13287-016-0391-3
Zhang, S., Chu, W. C., Lai, R. C., Lim, S. K., Hui, J. H., and Toh, W. S. (2016). Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr. Cartil. 24, 2135–2140. doi: 10.1016/j.joca.2016.06.022
Zhang, S., Chuah, S. J., Lai, R. C., Hui, J. H. P., Lim, S. K., and Toh, W. S. (2018). MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials 156, 16–27. doi: 10.1016/j.biomaterials.2017.11.028
Zhang, S., Teo, K. Y. W., Chuah, S. J., Lai, R. C., Lim, S. K., and Toh, W. S. (2019). MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials 200, 35–47. doi: 10.1016/j.biomaterials.2019.02.006
Zhang, Y., Hao, Z., Wang, P., Xia, Y., Wu, J., Xia, D., et al. (2019). Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF-1alpha-mediated promotion of angiogenesis in a rat model of stabilized fracture. Cell Prolif. 52:e12570. doi: 10.1111/cpr.12570
Zhao, P., Xiao, L., Peng, J., Qian, Y. Q., and Huang, C. C. (2018). Exosomes derived from bone marrow mesenchymal stem cells improve osteoporosis through promoting osteoblast proliferation via MAPK pathway. Eur. Rev. Med. Pharm. Sci. 22, 3962–3970. doi: 10.26355/eurrev-201806-15280
Zhong, Z., Ethen, N. J., and Williams, B. O. (2014). WNT signaling in bone development and homeostasis. Wiley Interdiscip. Rev. Dev. Biol. 3, 489–500. doi: 10.1002/wdev.159
Zhou, J., Mukherjee, P., Gao, H., Luan, Q., and Papautsky, I. (2019). Label-free microfluidic sorting of microparticles. APL Bioeng. 3:041504. doi: 10.1063/1.5120501
Zhou, Y., Yamamoto, Y., Xiao, Z., and Ochiya, T. (2019). The immunomodulatory functions of mesenchymal stromal/stem cells mediated via paracrine activity. J. Clin. Med. 8:1025. doi: 10.3390/jcm8071025
Keywords: mesenchymal stem cells, extracellular vesicles, exosomes, osteoarthritis, rheumatoid arthritis, osteoporosis, bone fracture
Citation: Liu S, Xu X, Liang S, Chen Z, Zhang Y, Qian A and Hu L (2020) The Application of MSCs-Derived Extracellular Vesicles in Bone Disorders: Novel Cell-Free Therapeutic Strategy. Front. Cell Dev. Biol. 8:619. doi: 10.3389/fcell.2020.00619
Received: 09 March 2020; Accepted: 22 June 2020;
Published: 22 July 2020.
Edited by:
Chao Liang, Hong Kong Baptist University, Hong KongReviewed by:
Wei Seong Toh, National University of Singapore, SingaporeXu Zhang, Jiangsu University, China
Elena Jones, University of Leeds, United Kingdom
Copyright © 2020 Liu, Xu, Liang, Chen, Zhang, Qian and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Airong Qian, cWlhbmFpckBud3B1LmVkdS5jbg==; Lifang Hu, aHVsaWZhbmdAbndwdS5lZHUuY24=
†These authors have contributed equally to this work
 Shuyu Liu
Shuyu Liu Xia Xu
Xia Xu Shujing Liang
Shujing Liang Zhihao Chen
Zhihao Chen Yan Zhang
Yan Zhang Airong Qian
Airong Qian Lifang Hu
Lifang Hu