- 1Center for Neuroscience, Korea Institute of Science and Technology, Seoul, South Korea
- 2Department of Biotechnology and Genetic Engineering, Global Biotechnology & Biomedical Research Network, Islamic University, Kushtia, Bangladesh
- 3Department of Stem Cell and Regenerative Biotechnology, Konkuk University, Seoul, South Korea
- 4Department of Gynecology and Obstetrics, Johns Hopkins School of Medicine, Baltimore, MD, United States
- 5Department of Animal Science & Technology and BET Research Institute, Chung-Ang University, Anseong, South Korea
- 6Graduate School of Pharmaceutical Sciences, College of Pharmacy, Ewha Womans University, Seoul, South Korea
- 7ABEx Bio-Research Center, Dhaka, Bangladesh
- 8Department of Pharmacy, Southeast University, Dhaka, Bangladesh
- 9Pharmakon Neuroscience Research Network, Dhaka, Bangladesh
- 10Division of Bio-Medical Science and Technology, KIST School, Korea University of Science and Technology, Seoul, South Korea
Autophagy, a cellular self-digestion process that is activated in response to stress, has a functional role in tumor formation and progression. Cancer stem cells (CSCs) accounting for a minor proportion of total cancer cells-have distinct self-renewal and differentiation abilities and promote metastasis. Researchers have shown that a numeral number of natural products using traditional experimental methods have been revealed to target CSCs. However, the specific role of autophagy with respect to CSCs and tumorigenesis using natural products are still unknown. Currently, CSCs are considered to be one of the causative reasons underlying the failure of anticancer treatment as a result of tumor recurrence, metastasis, and chemo- or radio-resistance. Autophagy may play a dual role in CSC-related resistance to anticancer treatment; it is responsible for cell fate determination and the targeted degradation of transcription factors via growth arrest. It has been established that autophagy promotes drug resistance, dormancy, and stemness and maintenance of CSCs. Surprisingly, numerous studies have also suggested that autophagy can facilitate the loss of stemness in CSCs. Here, we review current progress in research related to the multifaceted connections between autophagy modulation and CSCs control using natural products. Overall, we emphasize the importance of understanding the role of autophagy in the maintenance of different CSCs and implications of this connection for the development of new strategies for cancer treatment targeting natural products.
Introduction
Autophagy is an intra-cellular molecular mechanism and pathway for self-digestion, in which unwanted cytoplasmic components, such as proteins, toxic compounds, injured/damaged organelles, lipid molecules, and mitochondria, are sequestered into membrane-bound vesicles (phagophore), which eventually form autophagosomes in order to recycle and degrade these substances (Rahman and Rhim, 2017). In normal conditions, autophagy is essential for the conservation of the overall cellular homeostasis, and contributing to protein and organelle quality control (Uddin et al., 2018). It also contributes to the stress response (for example, in conditions like hypoxia, chemical exposure, and starvation). The initiation and regulation of autophagy signaling are described in Figure 1. Recent research has emphasized the important role of autophagy in cancer cell adaptability, growth, and survival in the tumor microenvironment (Lei et al., 2017). Autophagy has dual roles in cancer cells, including tumor-stimulating as well as tumor-suppressive functions (Avalos et al., 2014), with a wide range of effects on tumor progression and oncogenesis (Avalos et al., 2014). Recent research has clearly established that autophagy is an important target for the control and management of cancer. However, the control factors depend on the stage and type of tumor, host microenvironment, systemic cellular location, and management strategy (Ojha et al., 2015; Sohn et al., 2017). Overall, autophagy has both stimulatory and suppressive effects on cancer and cancer stem cells (CSCs) and contributes to metastasis.
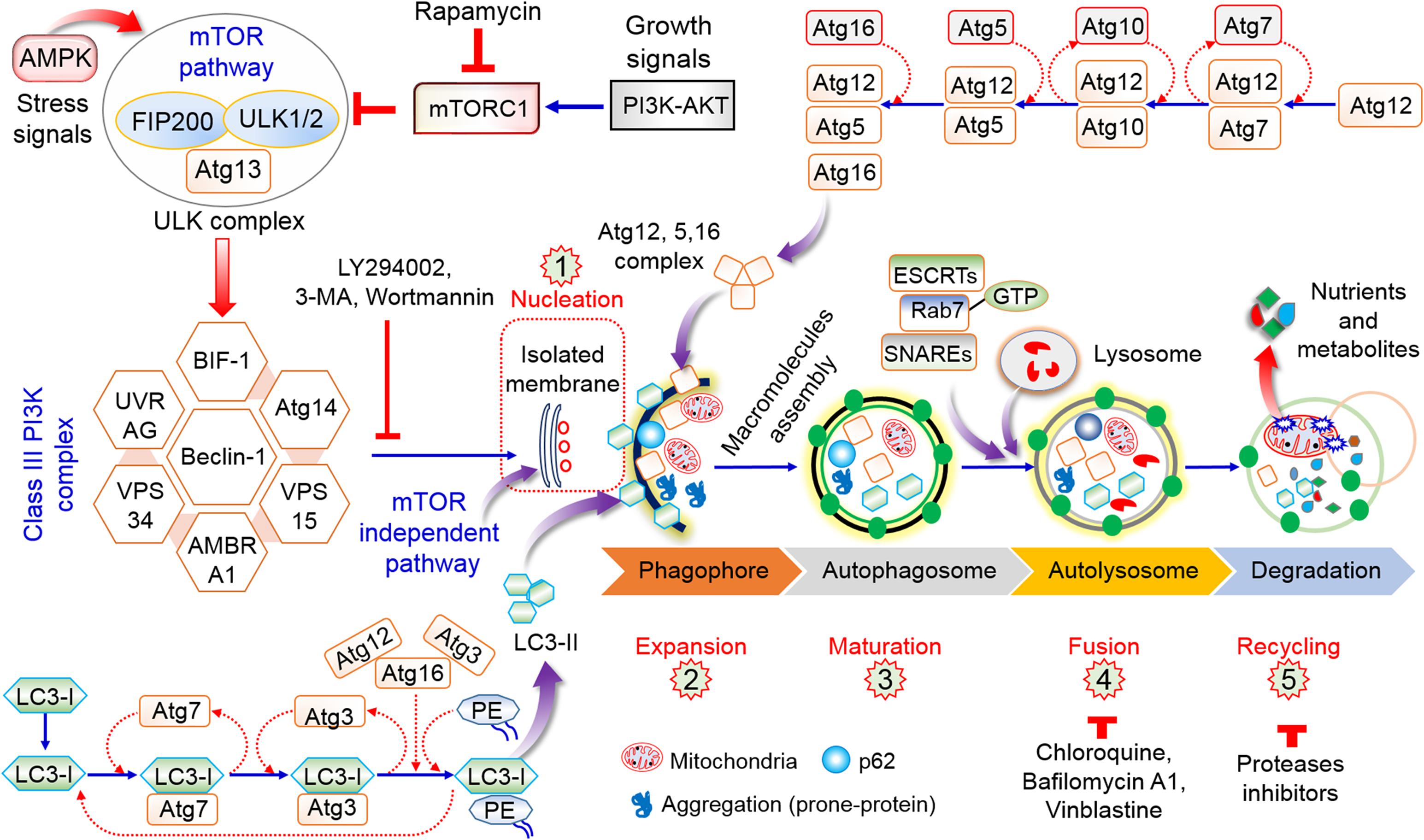
Figure 1. Role and regulation of the overall autophagy process. The initiation of autophagy is dependent on nutrient deprivation or growth factors that activate AMPK or inhibit mTORC1, thus stimulating the ULK1 complex (ATG13 and FIP200). The phosphorylation of Beclin-1 leads to the activation of VPS34, which stimulates phagophore initiation and formation. Atg5–Atg12 conjugation stimulates Atg7 and Atg10 to form the Atg12–Atg5–Atg16 complex, which ultimately influences phagophore formation. The Atg7, Atg3, and Atg16 complex has a function similar to that of E3 in the direction of the LC3-PE association (LC3-II) for formation of the autophagosome. The maturation of the autophagosome involves fusion to lysosomes via ESCRT, SNARE, and Rab7. Lysosome binds to autophagosome by several lysosomal proteins leading to cargo degradation as well as metabolite production and recycling of nutrient products (Rahman and Rhim, 2017).
Cancer stem cells exhibit features of normal stem cells, including self-renewal, differentiation, and tumorigenicity. Tumor formation and recurrence might be mainly be regulated by CSCs (Figure 2) (Gupta et al., 2009a; Suresh et al., 2016). Therefore, advances in anticancer therapies require the elucidation of the mechanisms underlying the survival and stemness of CSCs. Targeted therapy is a major strategy to reprogram CSCs into normal stem cells (Eun et al., 2017; Saha et al., 2017, 2018). In particular, targeting autophagy is a potential strategy to regulate CSCs (Nazio et al., 2019; Smith and Macleod, 2019). Although stimulation of autophagy exhibits side effects in some chemotherapies (Apel et al., 2008; Liu et al., 2011), autophagy has a useful and valuable therapeutic function in many cancers by mediating initiation of immunogenic cell death and clearance (Galluzzi et al., 2017). Therefore, to exploit autophagy stimulation and suppression for cancer prevention and management, it is essential to consider the precise role of this process in individual types of cancer and to determine the efficacy of autophagy regulation in cancer therapy. However, the critical mechanisms underlying CSC maintenance and functions with respect to autophagy have not been clearly described. This review emphasizes the role of autophagy in CSC biology and considers how targeting this process might impair CSC formation and survival. Furthermore, we discuss how autophagy contributes to each step of CSC physiology, including differentiation, generation, invasion, and migration, as well as pharmacological control by natural products.
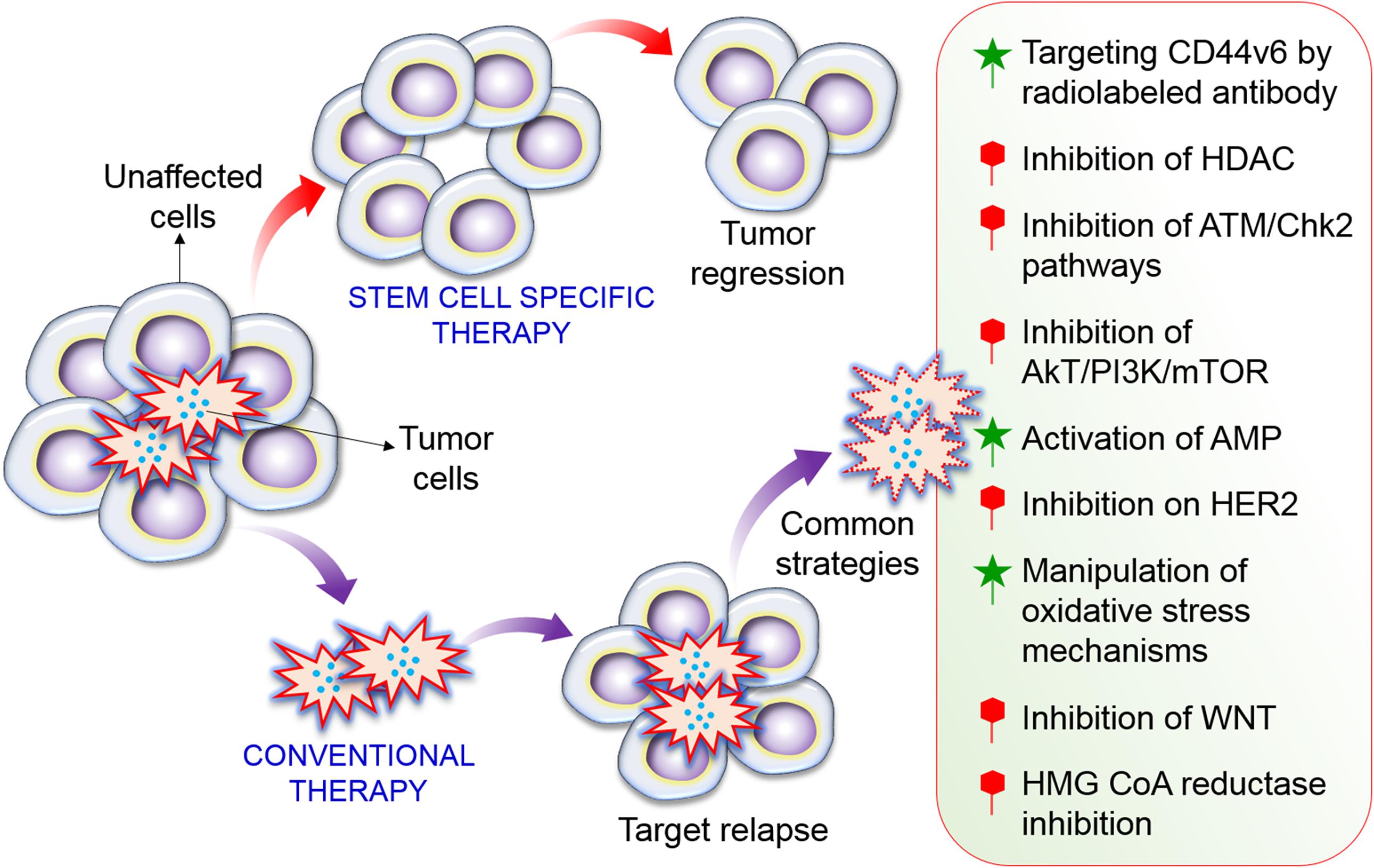
Figure 2. Formation of CSC and precise targets of CSC pathways. A high dose of radiotherapy has the potential to cure solid tumors via the inactivation of all CSCs. Recurrence can generally be attributed to one or a few remaining CSCs. A combined treatment strategy that precisely targets CSCs or several key molecular signaling pathways in CSC processes to control tumor cell growth would be expected to improve local tumor control. Among of them inhibition of HDAC, Akt/mTOR, HER2, WNT, and HMG CoA reductase inhibition is important to regulate CSCs. While activation of AMPK, manipulation of oxidative stress, and targeting CD44v6 also important of CSC pathways.
Characteristics of Cancer Stem Cells
Cancer stem cells exhibit self-renewal and can differentiate into various cell types, including tumor cells (Mackillop et al., 1983). These cells are a small population of cancer cells that are responsible for tumor growth and heterogeneity, and contribute to metastatic activity and resistance against conventional anticancer therapies (based on in vivo or in vitro analyses) (Lobo et al., 2007). CSCs have been identified as subpopulations of acute myeloid leukemia (AML) cells that express CD34, a specific surface marker. Though initially recognized in AML, CSCs have since been detected in various solid and difficult-to-treat cancers, such as pancreatic, brain, ovarian, colon, lung, melanoma, and breast cancers (Singh et al., 2004; Hermann et al., 2007; Li et al., 2007; O’Brien et al., 2007; Ricci-Vitiani et al., 2007; Eramo et al., 2008; Schatton et al., 2008; Zhang et al., 2008; Boiko et al., 2010). Importantly, CSCs are likely involved in tumor growth, with astonishing self-renewal and differentiation abilities that give rise to diverse cell phenotypes. They are characterized by the presence of particular cell surface markers, which could be used to differentiate these cells from normal and other tumor-forming cells. Therefore, these markers provide a basis for the establishment of several in vitro as well as in vivo approaches to separate, manipulate, and control CSCs. Additional essential characteristics of CSCs can explain unusual malignancies in an immune-deficient mouse model (Lobo et al., 2007). Breast cancer is a well-described human solid and condense tumor comprised of various resident cells, including CSCs and non-CSCs. The subpopulation of CSCs (CD44+ and CD24–/low) has been detected in the early stages of tumor progression in mice deficient in immune response factors (Al-Hajj et al., 2003). However, the lack of success of traditional treatment strategies is closely associated with the plasticity of CSCs due to their unrestricted self-renewal and differentiation characteristics, potential proliferative activity, and ability to inactivate components of the cell pool. An understanding of the molecular and cellular mechanisms underlying CSC proliferation and survival remains critical for expanding the usefulness of current therapeutic approaches.
Two key models have been proposed to explain the tumor cell source and heterogeneity. According to the stochastic model, all cancer cells can induce new tumors cells by transforming from non-CSCs to the CSC phenotype via an energetic mechanism in response to particular stimuli, such as mutations. The second model is the hierarchical model, in which a single group of CSCs contributes to tumor occurrence and increases heterogeneity by producing differentiated and inactive cancer cells (Figure 3). While these phenotypes and models appear to be mutually exclusive, it is possible that a combination of the two models explains the observed patterns.
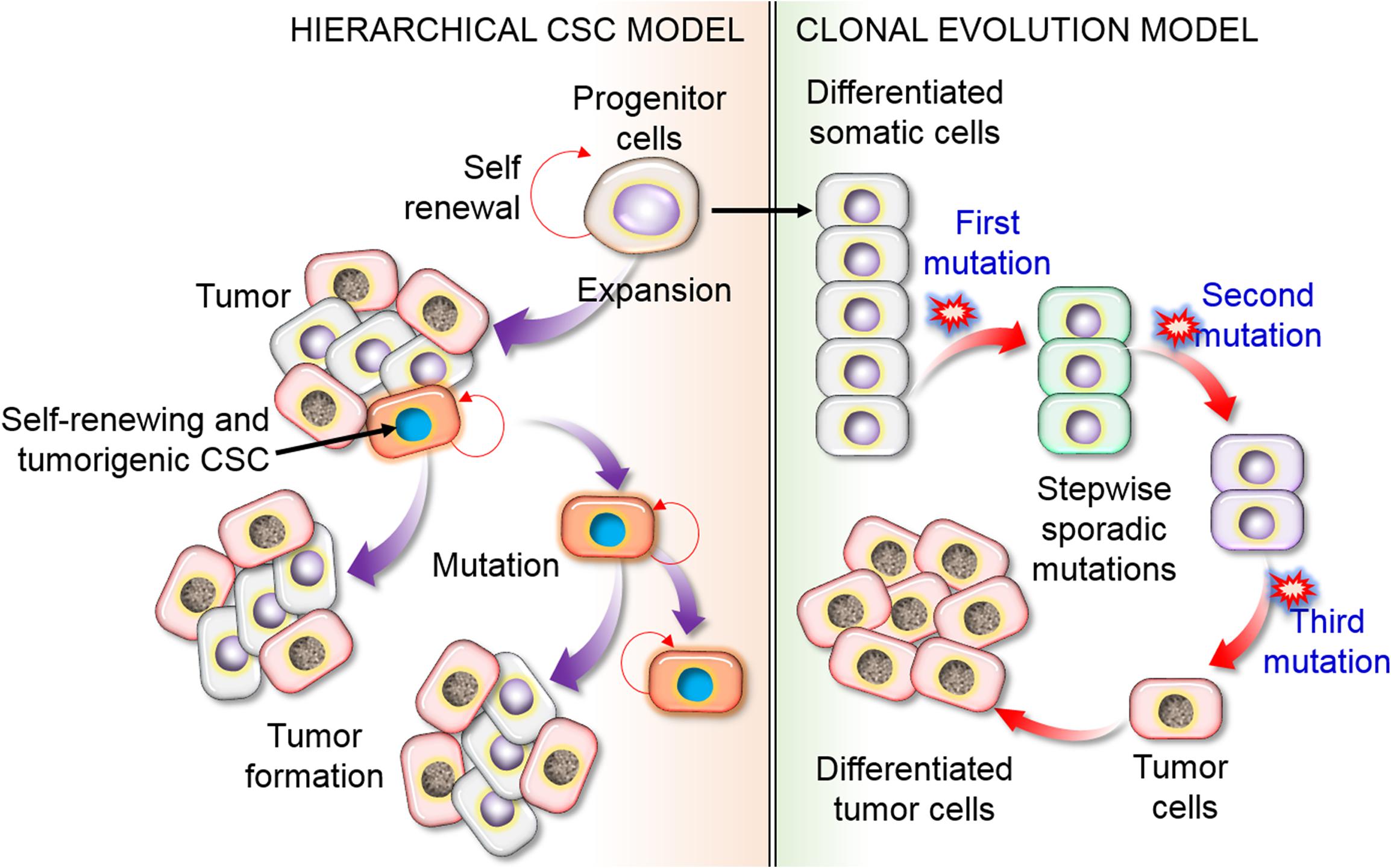
Figure 3. Schematic representation of the hierarchical CSC model of CSCs versus the clonal evolution or stochastic model of tumor cell heterogeneity. The hierarchical model proposes that only limited subpopulations of CSCs have the ability to initiate the development of cancer, with particular (intrinsic) features that could be recognized and targeted to destroy a tumor. In the stochastic model, to form cancerous cells, it is necessary to undergo a substantial series of DNA modifications. In this process, stepwise mutation causes tumor cells. Mutations could happen in any cell, resulting in cancer formation. This concept fundamentally suggests that all cells have the capacity to be tumorigenic with self-renewal or differentiation ability, leading to tumor heterogeneity, and other cells are differentiated as non-CSCs.
Maintenance and Survival of Cancer Stem Cells by Autophagy
The maintenance and aggressiveness of CSCs are fundamentally related to autophagy. CSCs are characterized by their self-renewal capacity and differentiation ability as compared to normal stem cells (Lobo et al., 2007). However, pluripotency is a fundamental characteristic of CSCs that permits unlimited growth and division as well as the maintenance of undifferentiated cells. The manipulation of autophagy is essential for the regulation of cancer cells and CSCs (Figure 4). CSCs were first identified in AML based on the expression of the cell surface markers, CD34 and CD38 (CD34+ and CD38–), using fluorescence-activated cell sorting (Zhang et al., 2008). However, studies on breast CSCs have shown that autophagy maintenance and homeostasis are fundamental requirements for the conservation of pluripotency in several pathophysiological conditions (Han et al., 2018; Nazio et al., 2019). Autophagy is enhanced in mammospheres and is correlated with adherent cells expressing Beclin-1 as well as Atg4. These two important autophagy proteins are required for the conservation, maintenance, and expansion of cells (Gong et al., 2012). Recent studies have demonstrated that autophagy is associated with CSCs in various types of cancer, including liver, pancreatic, breast, ovarian, glioblastoma, and osteosarcoma (Chaterjee and van Golen, 2011; Gong et al., 2013; Song et al., 2013; Zhang et al., 2016; Peng et al., 2017; Buccarelli et al., 2018). However, in hematological malignancies, autophagy can act as a tumor-suppressive or chemoresistance factor. Thus, depending on the kind of progenitors as well as the disease condition, autophagy might have opposite functions. Several autophagy-related genes (Beclin-1, Agt4, and Atg5) are upregulated, and the silencing of these genes negatively affects cell survival; as a result, the extent of autophagy in chronic myeloid leukemia (CML) appears to be related to the status of CSCs in the tumor (Rothe et al., 2014; Karvela et al., 2016). In contrast, efficient autophagy is necessary to protect against the progression of myelodysplastic syndrome into AML, and several additional autophagy-related genes (Atgs) are mutated or deregulated in patients with AML (Houwerzijl et al., 2009). Alternatively, blockage of autophagy leads to reduction in the levels of TFGβ2 and TGFβ3, thereby inhibiting the Smad pathway that is crucial for the CD29hiCD61+ CSC phenotype. Moreover, inhibition of autophagy downregulates IL-6 secretion, possibly via the JAK2/STAT3 signaling, in triple-negative autophagy-dependent breast cancer stem cells (BCSCs) (Maycotte et al., 2015). The secretion of IL-6 is an important factor for CSC conservation as well as maintenance, and promotes the CD44+/CD24 low phenotype in breast cancer (Iliopoulos et al., 2011). The IL-6-JAK2-STAT3 pathway might play an essential role in the transformation from non-CSCs to CSCs.
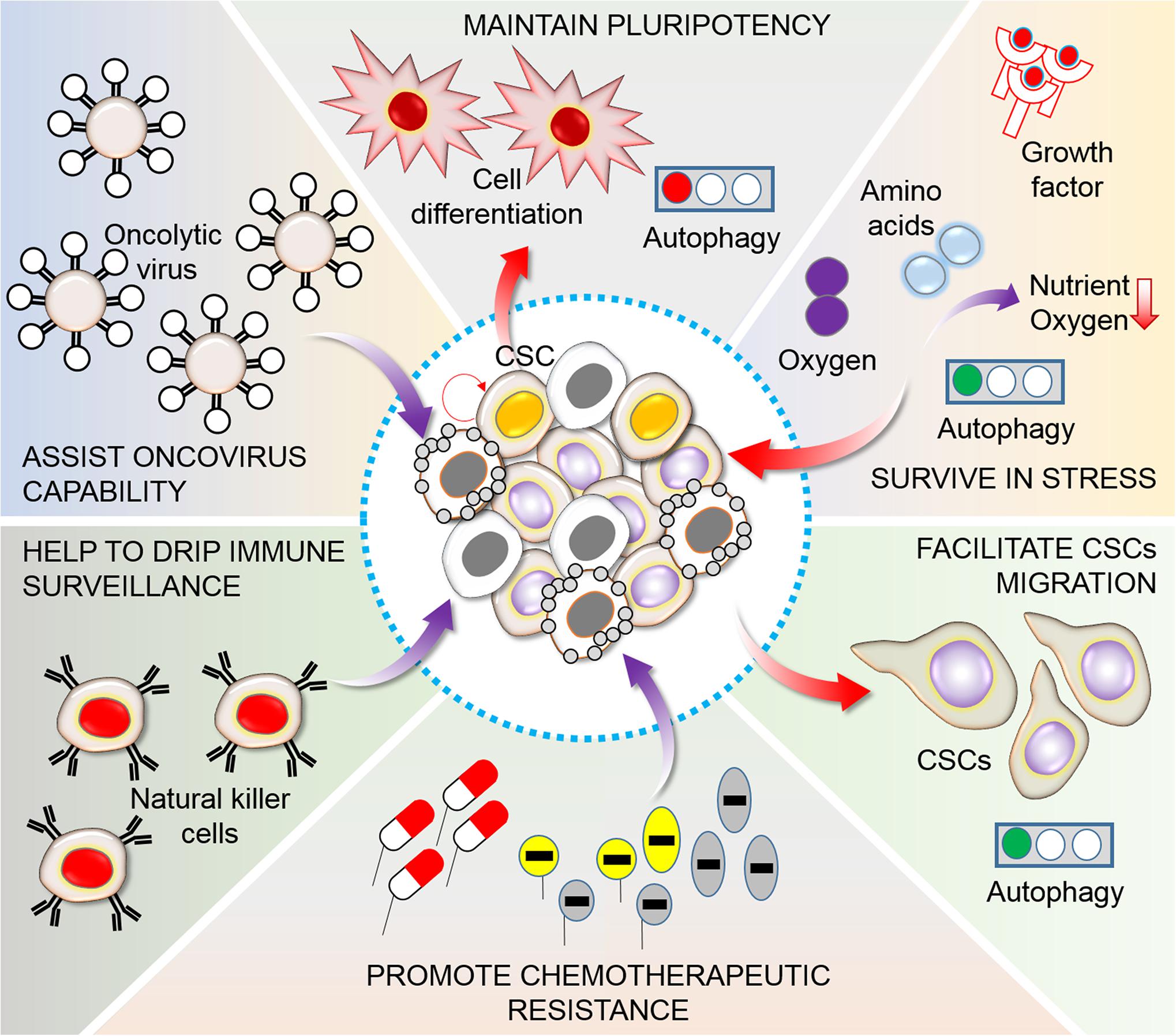
Figure 4. Functions of autophagy in CSC maintenance. A heterogeneous population of tumor cells generally consists of a small CSC population. CSCs are frequently related to an elevation of autophagy levels, which sustains pluripotency, promotes survival in limited nutrient conditions and hypoxia in the tumor microenvironment, controls migration and invasion, and stimulates chemotherapy resistance, escape from immunosurveillance of natural killer (NK) cells, and maintenance oncovirus competence (Nazio et al., 2019). In this consequence, manipulation of autophagy is found to be essential for targeting of cancer cells. Generally, during this pathway autophagy act in addition to contribute CSC differentiation, plasticity, generation, physiology, migration and invasion, viral and immune-resistance, and pharmacological properties.
Additional studies have proposed a role for the Forkhead box protein O (FOXO) in controlling the fate of CSCs (van Doeselaar and Burgering, 2018). FOXO-mediated transcriptional regulation is essential for homeostasis of stem cells in embryos as well as in adult cells (Gargini et al., 2015). However, it is important to distinguish how FOXO transcriptional activity contributes to CSC functions and maintenance. The silencing of FOXO3 improves the CSC renewal ability in breast, ovarian, colorectal, prostate, and liver cancer, and in glioblastoma cells (Dubrovska et al., 2009; Ning et al., 2014; Prabhu et al., 2015; Smit et al., 2016). Leukemia-initiating cells require FOXO3 for the maintenance of stem cell homeostasis (Pellicano et al., 2014). Conversely, from the perspective of autophagy, FOXOs regulate various Atgs, such as Beclin-1, LC3, ULK1, ATG5, ATG8, GABARAPL1, ATG12, ATG14, and BNIP3 (van Doeselaar and Burgering, 2018), and cytosolic FOXOs contribute to autophagy control and regulation. Most recently, it has been shown that AMBRA1, a pro-autophagic protein, is fundamental for controlling T-cell differentiation and for maintaining homeostasis, regulating the FOXO3–FOXOP3 interaction. Therefore, this might be involved in the FOXO3-dependent autophagy modulation in various cellular processes (Becher et al., 2018). Additional studies are needed to precisely determine how the FOXO-mediated control of stemness as well as autophagy are interrelated in tumorigenesis. Interestingly, recent studies have suggested that there is crosstalk between autophagy and staminal markers as well as the biosynthetic pathway of NAD+. Inefficiencies in normal autophagy created by autophagy inducers as well as inhibitors decrease the pluripotency of CSCs in teratocarcinoma, promoting differentiation in addition to cellular senescence (Ali Azouaou et al., 2015). Overall, accumulating evidence supports the complexity of the autophagy-mediated modulation of CSCs. A unique relationship between autophagy and stemness has recently been identified in ovarian cancer stem cells (OCSCs) (Peng et al., 2017). The overexpression of Forkhead Box A2 (FOXA2) in OCSCs is regulated through the autophagy pathway. The blockage or inhibition of autophagy via both pharmacological and genetic procedures promotes FOXA2 downregulation and the subsequent impairment of the self-renewal ability of cells (Peng et al., 2017). Another study has identified a pathway for autophagy regulation and the control of chromosome immovability by synchronizing the ATR checkpoint and the double-strand-break pathway (Robert et al., 2011). Therefore, CSCs might exploit autophagy to inhibit additional DNA damage and thereby improve survival.
Autophagy-Induced Resistance of Cancer Stem Cells to Chemotherapy
Diverse molecular mechanisms contribute to CSC resistance to drug treatment, including cellular plasticity, highly efficient DNA damage repair, expression of genes related to multi-drug resistance, and prevention of apoptosis. A correlation between CSCs and drug resistance has also been observed in numerous human cancers, including breast, pancreatic, melanoma, leukemia, colorectal, and brain cancers (Abdullah and Chow, 2013). Among various cellular and molecular mechanisms involved in resistance to chemotherapeutics, autophagy appears to be critical (Ojha et al., 2015) (Figure 5). Furthermore, it has been well-documented that chemotherapeutic treatments are intrinsically able to prompt autophagy in cancer cells (Sui et al., 2013). Numerous studies using various investigational methods have shown that combinations of cytotoxic agents and autophagy mediators enhance CSC sensitivity. In neuronal glioblastoma (GBM) stem cells, an EGFR inhibitor [bevacizumab or temozolomide (TMZ)] combined with a late-stage autophagy blocker, chloroquine (CQ), improves drug toxicity, leading to the impairment of GBM CSC proliferation and survival (Golden et al., 2014; Huang et al., 2018). In gastric CSCs, CQ and 5-fluorouracil block Notch signaling and decrease viability (Li L. Q. et al., 2018). However, JAK-induced autophagy is involved in progression in cisplatin-resistant bladder cancer cells (Ojha et al., 2016b). In AML CSCs or glioma stem cells (GSCs), ATG7 knockdown potentiates the inhibitory effect of salinomycin on cell proliferation and existence (Yue et al., 2013). In particular, a combination of autophagy blockers, bafilomycin A1 or CQ, with tyrosine kinase inhibitors (TKIs) disrupts CML cell proliferation and existence (Bellodi et al., 2009). Additional studies have indicated that autophagy might function in drug-induced cytotoxicity (Rahman et al., 2016, 2017). Resveratrol, a polyphenolic compound, affects breast CSC growth and survival by blocking Wnt signaling, which promotes autophagy (Rahman et al., 2012a; Fu et al., 2014). The inhibition of mTOR promotes neuroblastoma as well as the differentiation of glioma CSCs (Zeng and Zhou, 2008; Zhao et al., 2010; Rahman et al., 2016, 2017). Overall, the specific involvement of autophagy in the drug resistance of CSCs provides a basis for the development of different antineoplastic therapies.
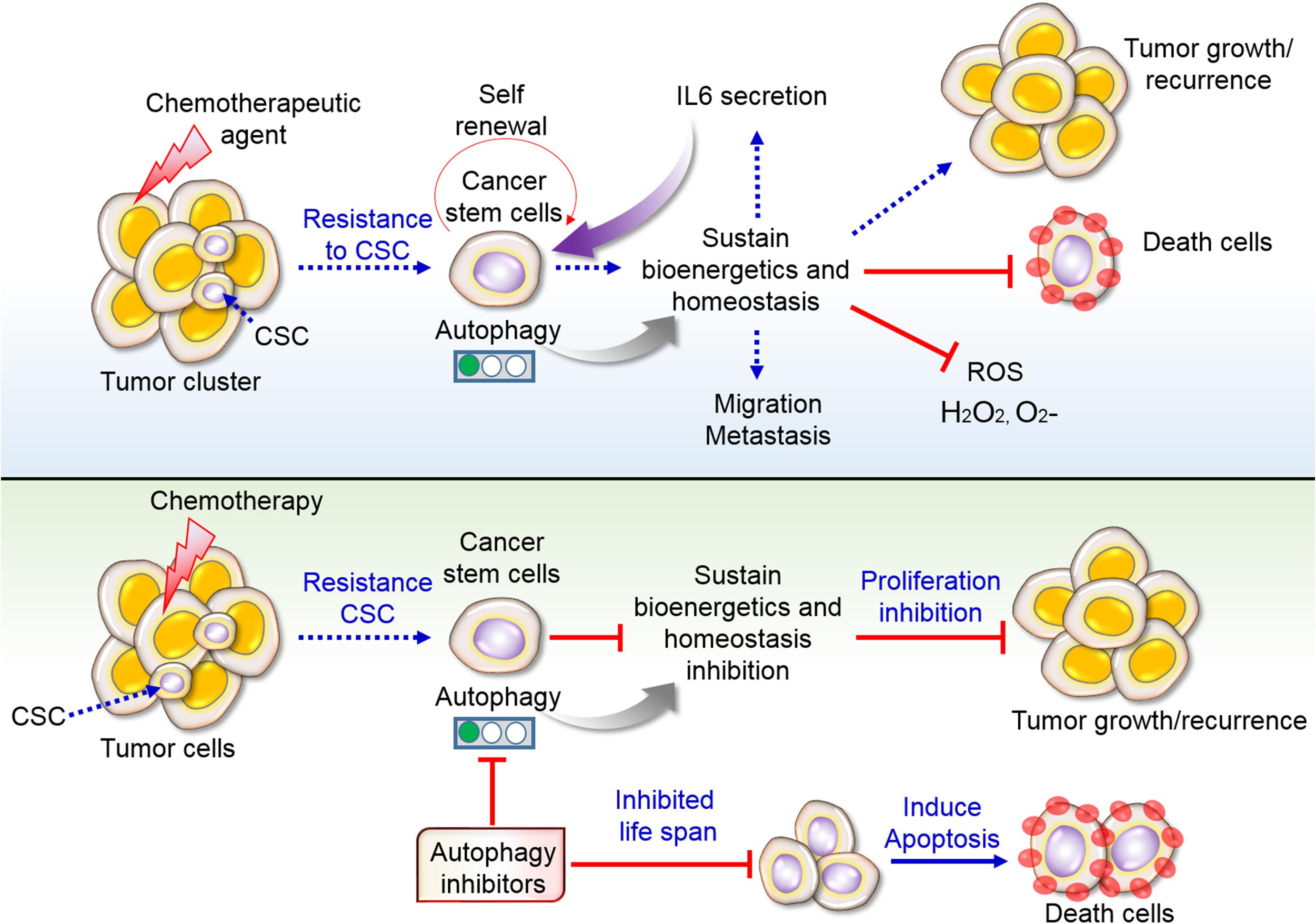
Figure 5. Resistance of CSCs to chemotherapy via autophagy. Conventional chemotherapy disrupts cell growth; however, CSCs remain unaffected by chemotherapy and sustain autophagy-mediated survival. Sustain cells further recurrence and make tumor heterogeneity along with migration and metastasis. Some cells become death due to therapeutic agents. Secretion of interleukin 6 (IL6) stimulates cancer cells self-renewal and proliferation. Inhibition of autophagy prevents cellular life span in addition to influence apoptotic cell death by chemotherapy. For that reason, targeting autophagy inhibitors in tumors and CSCs might overcome the resistance as well as inhibit tumor growth.
Role of Autophagy in the Control of Different Cancer Stem Cells With Natural Products
As both autophagy and CSCs are critical modulators in resistance to anticancer therapy, it is important to explore their correlation. A combination of gemcitabine (GC) and autophagy inhibitors (e.g., CQ) augments the propensity of pancreatic CSCs (Yang et al., 2015). A reduction of autophagy may hinder the preservation of CSCs. Salinomycin is more efficient than paclitaxel for reducing breast CSCs (Yue et al., 2013). Blocking autophagy by CQ sensitizes triple-negative breast cancer (TNBC) cells to paclitaxel by the modulation of JAK2 and DNMT1, thereby decreasing the levels of CD44+/CD24–/low CSCs. Autophagy also induces resistance to photodynamic therapy in CSCs in colorectal cancer (Wei et al., 2014). As a result, autophagy is expected to increase the efficacy of anticancer therapy. It is a prospective target for shifting anticancer treatment resistance in CSCs. The outcome of the inhibition of autophagy in CSCs is summarized in Figure 6. Additionally, mechanism of various natural compound in different CSCs regulation in relation to autophagy are presented in Table 1 and summarized the following points.
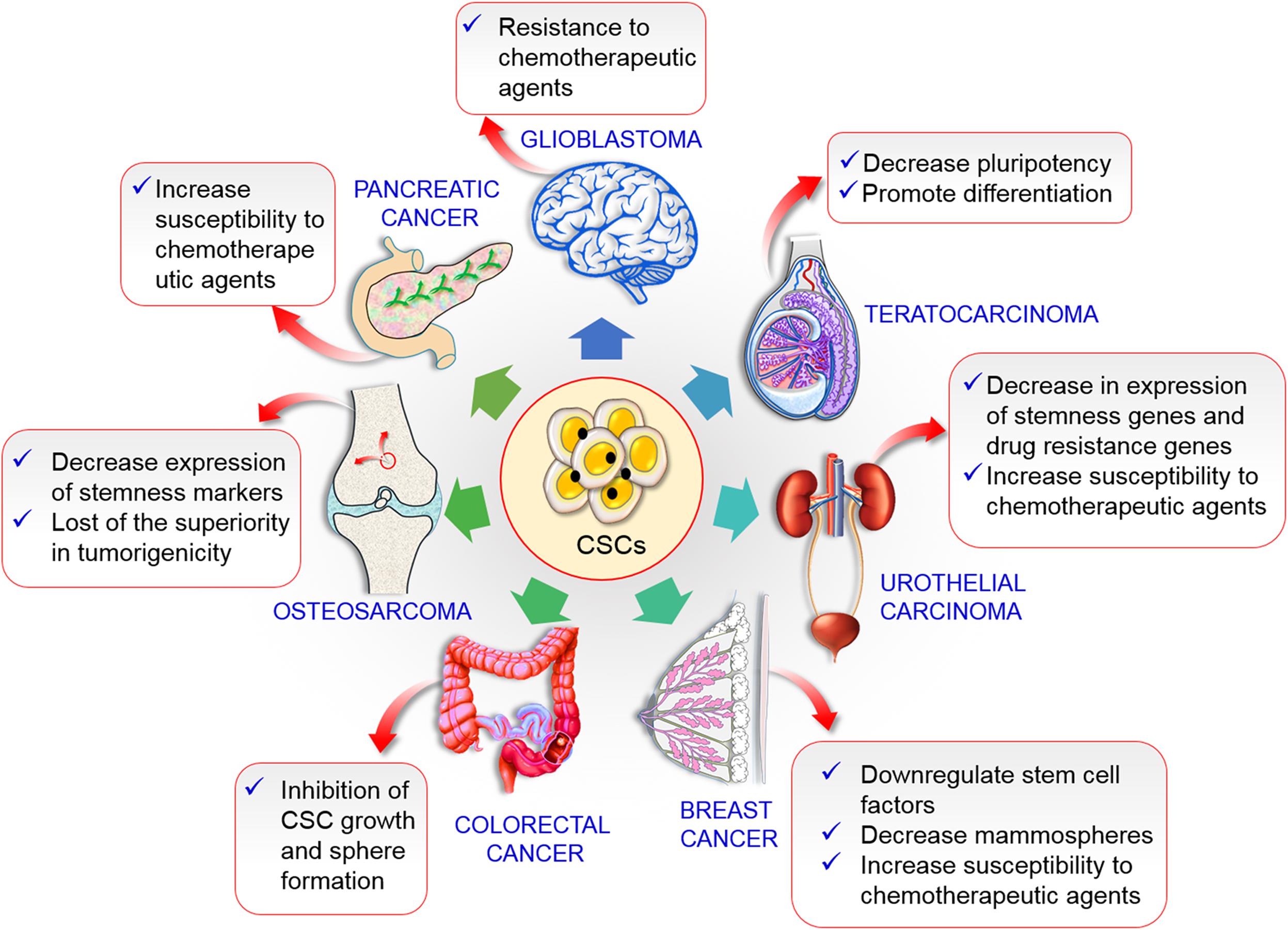
Figure 6. Prospective outcomes inhibition of autophagy various CSCs. In breast cancer decreases stem cell factors and mammospheres along with increases chemotherapeutic agents susceptibility. Inhibition of autophagy colorectal CSC causes growth inhibition. Tumorigenicity was lost in osteosarcoma CSC. Increase susceptibility to chemotherapies in pancreatic and urothelial CSCs autophagy inhibition. Autophagy reduction cause glioblastoma cells chemotherapies resistance.
Autophagy in Breast Cancer Stem Cells
The genetic inhibition of autophagy decreases the proportion of breast cancer cells with the CD44+/CD24–/low CSC-like phenotype, signifying a role of autophagy in maintaining typical CSCs in breast cancer (Cufi et al., 2011). Therefore, the inhibition of autophagic flux as well as lysosomal proteolytic function by K+/H+ ionophores by salinomycin efficiently decreases ALDH+ breast CSC population (Yue et al., 2013). It has also been established that the serum-starvation of mesenchymal stem cells (SD-MSCs) maintains MCF-7 tumor growth. SD-MSC tumors show high cellularity and differentiation as well as reduced apoptosis. Additionally, in vitro studies have revealed that SD-MSCs persist via autophagy, and the secretion of paracrine factors by these cells helps in the maintenance of tumor cells in nutrient/serum starvation conditions (Sanchez et al., 2011). However, Atg12, Atg5, and LC3B overexpression in quiescent stem cells has similar effects in breast cancer and the autophagy inhibitor 3-methyladenine restores the dormant phenotype (Chaterjee and van Golen, 2011). An increase in the expression of Beclin-1 in mammospheres has been detected in human breast cancer cells. Similar results have been obtained in additional breast cancer cell lines, such as BT474 and MCF-7. However, basal as well as a starvation-mediated autophagic fluxes are increased in aldehyde dehydrogenase 1-positive cells. These results confirmed that Beclin-1 is critical for CSC function and tumor growth, indicating that CSCs use autophagy for maintenance of growth and tumor persistence (Gong et al., 2013). Certainly, developed resistance to letrozole was decreased by treatment via a derivative of rapamycin RAD001, everolimus, and a dual PI3K/mTOR inhibitor, NVP-BEZ235, in breast cancer cells (Cavazzoni et al., 2012). Previously, it has been reported that FR122047 (FR), a known non-steroidal anti-inflammatory drug, can significantly induce the caspase-9 mediated cytoprotective autophagy but caspase7/8 mediated apoptosis in breast cancer cells (Jeong et al., 2011). However, salinomycin, a polyether ionophore antibiotic, eliminated BCSCs induction of lipid oxidation, mitochondrial dysfunction, and oxidative stress (Zhao et al., 2019). Thymoquinone, derived from Nigella sativa, increases the cytotoxicity in breast cancer cell lines via autophagy stimulation (Bashmail et al., 2018). Curcumin, natural phytochemical, preventing the proliferation of BCSCs along with decreasing the bulk breast cancer cells (Li and Zhang, 2014). Curcumin repressed BCSCs proliferation and migration through inhibiting β-catenin nuclear translocation as well as decreasing β-catenin expression transcriptional targets containing pro-EMT factors (Mukherjee et al., 2014). Resveratrol, polyphenols compound with anti-cancer activity, also repressed BCSCs mammospheres formation as well as tumorigenicity and facilitate autophagy by disrupting Wnt/β-catenin pathway (Fu et al., 2014). Genistein, isoflavone phytoestrogen dietary found in soybeans, repressed mammospheres formation through MCF-7 in addition to MDA-MB-231 cells moderately via decreasing PTEN/PI3K/Akt pathway (Montales et al., 2012). Quercetin, a dietary flavonoid from plants, could down-regulate P-glycoprotein and inhibit Y-box binding protein 1 nuclear translocation, thus improving chemosensitivity of breast cancer cells along with inhibiting BCSCs (Li S. Z. et al., 2018). 6-Shogaol, polyphenolic compounds derived ginger, disrupt Akt/GSK3β and hedgehog signaling pathway, thus mitigating stemness of BCSCs by autophagy (Wu et al., 2015).
Autophagy in Colon Cancer Stem Cells
Colon cancer stem cells (CCSC) are chemo/radiotherapy-resistant and numerous CCSC markers with extracellular domains, such as DCLK1 (May et al., 2008), Lgr5 (Schepers et al., 2012), and CD44 (Park et al., 2012), have been identified. Immortalized embryonic epithelial cells overexpressing progastrin (HEKmGAS) exhibit improved metastatic/tumorigenic potential (Sarkar et al., 2012). However, non-tumorigenic (HEKC) cells do not express stem cell markers and transformed CSCs HEKmGAS coexpress DCLK1 and CD44. HCT-116, HT-29, and DLD-1 colorectal cancer cells coexpress DCLK1/CD44 stem cell markers, in a manner similar to that observed in HEKmGAS tumorigenic cells (Sarkar et al., 2012). It has been found that curcumin increases the persistence of CSCs in colon. Furthermore, optimal curcumin concentrations significantly decreased the levels of stem cell markers. Contrary to expectations, curcumin augments the proliferation and survival of autophagic CSCs. These results suggest that the cell survival benefits from autophagy, with implications for the long-term resolution of colorectal cancer (Kantara et al., 2014). Antroquinonol, isolation from mushroom Antrodia camphorata, triggered PI3K/Akt/β-catenin signaling and could be a favorable cancer prevention agent for colon CSCs (Lin et al., 2017). Bitter melon extracts, isolated from Momordica charantia, inhibit CCSCs by affecting energy homeostasis in addition to increase Beclin-1, Atg7 and 12 autophagic protein in HT-29, and SW480 colon cancer cells (Kwatra et al., 2013).
Autophagy in Chronic Myeloid Leukemia Cancer Stem Cells
NSAID treatment increases LC3-II levels and decreases p62 levels, with a simultaneous decrease in the levels of numerous stemness-related markers, including Oct4, CD44, c-Myc, and mutant p53 in CD44highK562 cells, indicating that NSAIDs promote autophagy in CML cells (Moon et al., 2019). The dysregulation of autophagy-associated genes improves imatinib mesylate (IM)-induced cell death in cell lines as well as in primary CML cells. Combinatorial treatment with autophagy blockers and TKIs, such as IM, dasatinib, or nilotinib, results in the complete elimination of phenotypically as well as functionally distinct CML stem cells (Bellodi et al., 2009). It has previously been established that CML stem cells overexpressing p210BCR/ABL are inherently resistant to Das, IM, bosutinib, and nilotinib (Copland et al., 2006; Konig et al., 2008). Therefore, alternative methods that combine a TKI with an additional agent are necessary for CML CSC therapy. Thus, autophagy inhibitors might improve the therapeutic effects of TKIs in CML. Recently, it has been showed that imatinib, a small molecule kinase inhibitor, facilitates K63-linked ULK1 ubiquitination subsequent in autophagy stimulation is critical for CML and perspectives for the treatment of CML CSCs treatment (Han et al., 2019). Resveratrol, a polyphenol compound, is an attractive agent that prompts autophagic cell death through preventing the AMPK/mTOR pathway in CML cells (Puissant and Auberger, 2010). Resveratrol also activated autophagic cell death in CML cells through together with AMPK and JNK-mediated p62/SQSTM1 expression (Banerji and Gibson, 2012). Metformin, isolated from Galega officinalis, treatment reduced the melanoma tumor growth of CML CSC in mice in addition to induce apoptosis and autophagy (Tomic et al., 2011).
Autophagy in Brain Tumor Cancer Stem Cells
Glioblastoma is the most lethal tumor of the central nervous system (CNS) (Wen and Kesari, 2008). However, specific GBM types are highly distinct, with significant cellular heterogeneity, including minor subpopulations of GSCs. GSCs contribute to tumor initiation, malignant phenotypes, recurrence, and resistance to cancer therapy (Lathia et al., 2015). Higher levels of autophagy mesenchymal (MES) GSCs than that in proneural GSCs is related to increased resistance to therapy and tumorigenicity of MES GSCs (Huang et al., 2017). However, irradiation (IR) and chemotherapy in GBM, particularly with TMZ, increase autophagic activity (Kroemer, 2015; Liu et al., 2013). GBM uses autophagy as an escape machinery for cell survival in response to cytotoxic agents, including IR and TMZ, which are standard and effective first-line therapies (Kondo et al., 2005; White, 2015). However, the anti-glioma agent Delta-24-RGD promotes cell death by increasing autophagic protein expression as well as vacuole formation in brain stem cell lines derived from patients with GBM (Jiang et al., 2007). Delta-24-RGD-treated stem cells of brain tumor xenografts display enhanced persistence in a glioma-containing mouse model with higher levels of Atg5. Additionally, increasing data suggest that microRNAs (miRNAs) are dysregulated in cancer cells and that CSCs show different miRNA expression profiles in numerous types of tumors, including GBM. Context-dependent miRNA properties may control the response to therapy and tumorigenicity (Cascio et al., 2010; Liu et al., 2013; Huang et al., 2016a). Recently, it has been shown that miRNA-93, which is differentially expressed in MES and PN GBM subtypes, is related to autophagy in response to IR and TMZ (Huang et al., 2019). Therefore, the autophagy inhibitor CQ stimulates γIR-mediated cell death in extremely radioresistant patient-derived GSCs (Firat et al., 2012). Recently, study provided an understanding into the possible inhibitory mechanism of isatin, a plant compound, on neuroblastoma metastasis in vivo as well as in vitro (Cong et al., 2019). Natural products such as resveratrol, oxyresveratrol, aneglicin, gambogic acid, and 18α-Glycyrrhetinic acid induces apoptosis and autophagy in brain tumor neuroblastoma cells (Rahman et al., 2012a, 2013, 2016, 2017). Resveratrol expressively reduced TMZ resistance through downregulating NF-κB dependent signaling in T98G GBM cells (Huang et al., 2012) and activate AMPK pathway and mTOR signaling inhibition (Yuan et al., 2012). A bicyclic naphthoquinone plumbagin, found in the roots of Droseraceae, modulates numerous signaling pathways comprising Akt/mTOR, JNK, and NF-κB activates apoptosis and autophagy along with induces DNA damage as well as cell death in human brain tumor cells (Khaw et al., 2015). Andrographis paniculata, a medicinal herb cross the BBB, has been demonstrated that inhibits U87 as well as U251 GBM cell proliferation through inducing cell cycle arrest by reduced Cdk1 and Cdc25C expression and also displayed inhibition of PI3K/Akt/mTOR signaling pathway (Li et al., 2012). Celastrus orbiculatus extract was exposed to prevent cell proliferation, migration, and adhesion of human U87 and U251 GBM cells through PI3K/Akt/mTOR signaling pathway via autophagy signaling (Gu et al., 2016). Gintonin (GT), a glycolipoprotein isolated from the root of Panax ginseng Meyer, activates autophagy in a dose- and time-dependently manner via upregulated LC3-II expression in human U87MG GBM cells (Rahman et al., 2020a).
Autophagy in Pancreatic Cancer Stem Cells
High rates of autophagy have been detected under basal conditions in pancreatic cancer cells (Yang et al., 2011; Endo et al., 2017); however, the relation between pancreatic CSCs and autophagy remains to be explored. Pancreatic CSCs are characterized by numerous putative markers, such as CD24, CD44, CD133, EpCAM, and ALDH1 (Li et al., 2007; Kim et al., 2011). In particular, the presence of CSCs in pancreatic cancer is related to poor outcomes (Rajeshkumar et al., 2010) as well as increased metastatic activity and chemoresistance (Rajeshkumar et al., 2010; Van den Broeck et al., 2012). Consequently, drugs that selectively target CSCs are promising treatments for pancreatic cancer. Zhu et al. (2013) found that HIF-1α as well as autophagy control contribute to the transition from pancreatic cancer non-stem cells to stem cells and further showed that high autophagic flux is related to enhanced HIF-1α expression. They suggested that a combination of HIF-1α and autophagy contributes to the regulation of equilibrium between CSCs and non-CSCs (Zhu et al., 2013). This observation highlighted the significance of therapeutic approaches directed at CSCs and the tumor microenvironment. The autophagy-mediated induction of OPN/NF-κB signaling is essential for pancreatic CSC maintenance (Yang et al., 2015), and the combination of GC and autophagy inhibitors is a favorable therapeutic approach for the control of pancreatic CSCs. Recently, some marine organisms could be used a favorable source to recognize novel pharmacologically dynamic substances to treat pancreatic CSCs via autophagy induction (Geisen et al., 2015). However, rottlerin, polyphenol natural product isolated from the Asian tree Mallotus philippensis, a protein kinase C-delta (PKC-δ) inhibitor, can encourage autophagic cell death in pancreatic CSCs (Singh et al., 2012). A bioactive constituent, thymoquinone, isolated from the volatile oil of the black seed Nigella sativa, triggered apoptosis as well as tumor growth prevention in pancreatic CSCs both in vitro and in vivo via Notch1 and PI3K/Akt/mTOR regulated autophagy signaling pathways (Mu et al., 2015).
Autophagy in Urinary Bladder Cancer Stem Cells
Urinary bladder cancer, urothelial carcinoma (UC), is among the most common urogenital cancers worldwide (Witjes et al., 2014). The difficulty associated with the reversion of UC makes it amongst the most expensive human malignancies to manage and treat (Knowles and Hurst, 2015). CSCs are believed to be responsible for the initiation of UC as well as its progression and relapse (Ning et al., 2009). Autophagy signaling contributes to the survival response in bulk populations and in CSCs of urinary bladder cancer lines T24 and UM-UC-3 (Ojha et al., 2014). Hence, autophagy is related to cell survival in bladder carcinoma and is a potential target for the effective management of UC, and by extension to improved patient survival. Therefore, synergistic effects of GC and mitomycin (MM) cytotoxicity and the inhibition of autophagy potentially with a glycolytic inhibitor (2-deoxyglucose) might be helpful in improving the overall outcomes in patients with urinary bladder cancer (Ojha et al., 2016a). Alisertib, aurora kinase A inhibitor, returns balance of N-cadherin and E-cadherin which prevents phosphorylation of aurora kinase A as well as suppresses Akt/mTOR and induces G2/M phase arrest, autophagy, and apoptosis of urinary bladder CSCs (Ding et al., 2015). GC and MM are common DNA intercalating chemicals used to treat several UC types (Cheung-Ong et al., 2013). Additionally, both small interfering RNA and pharmacological autophagy inhibitors potentiate the effects of the chemotherapeutic agents, MM, GC, and cisplatin in urinary bladder CSCs from T24 and UM-UC-3 lines (Ojha et al., 2014). A bioactive polyphenol isolated from green tea, epigallocatechin-3-gallate (EGCG), repressed bladder cancer tumorspheres along with downregulated stem cell markers and inhibited proliferation-associated proteins and stimulated the apoptosis of bladder CSCs (Sun et al., 2019).
Autophagy in Prostate Cancer Stem Cells
Prostate cancer is one of the important reasons of death in some cases of males. Natural plant extracts of Berberis libanotic Ehrenb has been found to reduce the capability of prostate CSCs originating from PC3, DU145, and 22Ru1 cells strongly downregulate prostate CSCs markers such as Oct4, Sox2, CD44, Nanog, and CD166. The invasion as well as migration abilities were expressively decreased in a concentration in addition to time-dependent mode (El-Merahbi et al., 2014). In fact, trehalose augmented LC3 in addition to p62 accumulation along with induction of LC3 puncta in prostate CSCs cells such as PC3, LNCaP, as well as DU145 (Cristofani et al., 2018). Koenimbin, a natural dietary constituent of Murraya koenigii (L) Spreng, suppressed of PC-3 cells in addition to target PC-3-derived CSCs via apoptotic as well as CSC signaling pathways in vitro (Kamalidehghan et al., 2018).
Controlling Cancer Stem Cells Through Apoptosis Signaling Pathway With Natural Products
Program cell death or apoptosis is an acute cellular mechanism which facilitates survival as well as death via a multiple signaling transduction pathway (Elmore, 2007; Rahman et al., 2020b). Generally, the apoptotic pathway is damaged during cancer progression and development (Plati et al., 2011). However, apoptosis induction in CSC also require an additional attention for cancer therapy (Dragu et al., 2015). For that reason, numerous synthetic and natural compounds have been utilized to target extrinsic as well as intrinsic apoptotic signaling pathways. Treatment with TRAIL, tumor necrosis factor-related apoptosis-inducing ligand, in association with numerous anticancer compounds was stated to be effective in removing CSCs (Fox et al., 2010). Co-treatment of cisplatin was designated to be very important effective in decreasing triple negative breast CSCs via Wnt signaling inhibition as well as apoptosis induction (Yin et al., 2011a, b). Similarly, TRAIL treatment with daunorubicin or cytarabine has been revealed to reduce acute myeloid progenitor’s growth (Plasilova et al., 2002). Additionally, Bortezomib, a proteasome inhibitor, along with TRAIL, was exposed to trigger GBM stem cells apoptosis (Unterkircher et al., 2011). Besides, MSCs express TRAIL via lentiviral vector transduction was used to stimulate apoptosis in lung and squamous CSC population in holding tumors of nude mice (Loebinger et al., 2010). NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells, in another apoptosis inducing target of CSCs. Oxyresveratrol, derived from the Morus alba root extract, has been shown to cause accumulation ROS and induction of autophagic, as well as apoptotic, cell death through the FOXO-caspase 3-mediated pathway in neuroblastoma cells (Kwon et al., 2015; Rahman et al., 2017). Human neuroblastoma induced by angelicin and gambogic acid shown apoptosis cell death (Rahman et al., 2012b, 2013). Predominantly, NF-κB prevents apoptosis as a result stimulates cell proliferation, tumor progression, angiogenesis inflammation, as well as metastasis (Park and Hong, 2016). However, NF-κB pharmacologically inhibited via parthenolide, pyrrolidinedithiocarbamate as well as its analog diethyldithiocarbamate specially aim to control breast CSCs (Zhou et al., 2008). These observations emphasize that NF-κB is an important to conserve and maintain tumor-initiating CSCs survival (Zhou et al., 2008). Furthermore, NF-κB inhibition with MG-132, proteasome inhibitor, along with idarubicin stimulated apoptosis of leukemic stem cells with significantly lesser that in normal hematopoietic stem cells (Guzman et al., 2002). Natural plant extracts from Melandrium firmum, Dioscorea nipponica Makino, and Saussurea lappa Clarke has been shown to have anti-proliferative and apoptotic effects in neuroblastoma cells (Rahman et al., 2013, 2014, 2015). Therefore, apoptosis signaling to CSC control in preclinical examination to propose the opportunity to eradicate cancers treatment.
Therapeutic Aspects of Autophagy for the Cancer Stem Cells Treatment
One of the most important point is the drug resistance that outcomes in tumor relapse remains a main impediment to development survival of cancer patient. Besides, drug resistance might be owing to the existence of CSCs. Table 1 premises the various studies that have examined natural compounds that possible target CSCs via autophagy regulation. Targeting signaling of these compounds comprises inhibitors of Akt, mTOR, tyrosine kinases, HDAC, proteasome, IL6 signaling, farnesyl transferase, hypoxia, Notch, Wnt signaling, as well as hedgehog signaling. However, depending on the kind of cancer in addition the compound, induction or inhibition of autophagy has been considered for therapeutic target in CSCs treatment. For complete eradication of cancer, it might be essential to target non-CSC and CSCs populations. For instance, in one study shown that CQ, autophagy inhibitor, targets pancreatic CSCs through CXCR4 inhibition as well as independently of autophagy in hedgehog signaling (Balic et al., 2014). One of the few drugs, for example, metformin (Hirsch et al., 2009) and salinomycin (Gupta et al., 2009b) have been recognized to target CSCs control in vitro as well as in vivo effectively to eliminate CSCs alone or in combination by conventional chemotherapeutic drugs (Vidal et al., 2014). Pharmacological inhibitor such as PTC-209 and BMI-1 also revealed to successfully prevent self-renewal of CSC in vitro in addition to efficiently block tumor growth and progression of mouse xenografts model (Kreso et al., 2014).
Based on current studies, a combination of modulators of autophagy and standard chemotherapeutic agents could represent an effective anticancer treatment strategy. However, there are numerous bottlenecks in the development of an effective anticancer treatment targeting autophagy, as its reaction differs according to the origin and type of cell, stimulus, and stress conditions. However, the effects of autophagy in the tumor microenvironment should be validated in further studies. Furthermore, novel as well as reliable approaches for regulating autophagy in clinical models need to be established. Accordingly, an understanding of the functional role of autophagy in the response to therapies might contribute to overcoming the resistance to chemotherapy and sensitizing CSCs to anticancer treatments. There is growing evidence that autophagy is a promising target for stabilizing CSC invasion and aggressiveness. It is also essential to consider that CQ in addition to its derivatives, such as hydroxychloroquine, have been evaluated in numerous clinical trials and may be effective in combination with traditional anticancer therapies (Chen and Chan, 2017). Most importantly, CSC heterogeneity in addition to patient-specificity presents an increase in complexity that has not been previously understood. We are far from establishing unique and well-known combinations of drugs able to eliminate CSCs or, at minimum, to prevent and block their proliferation and growth. However, global efforts are focused on the incorporation of recent discoveries into novel therapeutic approaches. It has been propose that both the inhibition and activation of autophagy are targets for sensitizing and maintaining CSCs. The outcomes of CSC management and the effectiveness of CQ in anti-CSC therapies might depend on tumor growth properties, and autophagy-mediated CSC processes may be important determinants. Considerably more precise and stronger lysosome blockers than CQ are being identified. These drugs provide an opportunity to develop comprehensive combinations of treatments for CSCs, including pepstatin A (which blocks cathepsins D and E), E64d (blocker of cathepsins B, H, and L), or concanamycin A (a selective blocker of V-ATPase, which inhibits fusion between lysosomes as well as endosome acidification) (Yang et al., 2013). Conversely, the inhibition of autophagosome elimination and degradation does not disturb cargo sequestration and autophagosome maturation or formation. Therefore, drugs that are effective against the early phases of autophagy, such as a VPS34 inhibitor (SAR405) or PIK-III or ULK1 inhibitor (MRT68921), might provide better results with respect to controlling CSCs (Dowdle et al., 2014; Petherick et al., 2015). Additional examinations of the molecular mechanism and mode of action of CSC specific drugs along with non-CSC plasticity and role of autophagy are required before vastly effective treatments might be well-known for patients in all aspects of cancer.
Clinical Implications of Autophagy in Cancer Stem Cell Control
Therefore, regulatory factors along with molecular and cellular mechanisms by which autophagy exerts a functional role in CSCs are essential for the development of effective and safe antitumor approaches. The combined use of autophagy blockers/activators with chemotherapeutic drugs, the examination of the role of autophagy in the immune response, as well as virotherapy might be critical to develop novel approaches against CSCs as alternatives to conventional treatment. Furthermore, it is important to consider that solid tumors generally develop in environments with low oxygen levels, creating a niche to defend against CSCs, with additional destructive effects as well as resistant to cell death. Additional studies are therefore required to characterize the functions of autophagy in interference among stromal cells, adaptive immune cells, endothelial cells, and tumor-infiltrating innate immune cells. Notably, these kinds of cells might have different requirements for autophagy, making it difficult to develop autophagic therapies for CSCs. Further studies are also required for the development of novel and reliable approaches for measuring autophagic flux activation in patient samples in clinically. Undoubtedly, the sequestration of CSCs from the blood is an effective technique for observing autophagy. In future, RNA sequencing, a groundbreaking tool for transcriptome analyses, is promising for understanding the molecular determinants of the initiation and stimulation of autophagy.
Additionally, recent results enable us to identify novel and interesting scenarios for the modulation and control of autophagy in the microenvironment adjacent to CSCs. Malignant cells stimulate autophagy in the microenvironment in addition to distal tissues for the maintenance of self-growth and proliferation by enhancing the accessibility of reprocessed nutrients. However, blocking or reducing autophagy in the tumor yields adequate results with respect to tumor invasion and development, whereas the prevention of autophagy by the oral administration of CQ results in an additional visible decline in tumor progression, proliferation, as well as invasion (Katheder et al., 2017). Therefore, metabolic crosstalk between non-CSCs and CSCs as well as other cancer-associated fibroblasts (CAFs) establishes additional relationships (Yoshida, 2017). As a result, it could be hypothesized that targeting non-CSCs as well as CAFs by blocking autophagy might decrease nutrient accessibility and might negatively influence the intrinsic resistance of CSCs and mechanisms against chemotherapeutic strategies. Nonetheless, interventions that hinder autophagy might have unintended adverse side effects related to immune surveillance. Recent studies have shown that caloric restriction and fasting-dependent autophagy stimulation affect anticancer immune surveillance, thereby promoting tumor cell growth properties as well as improvements in responses to chemotherapy (Pietrocola et al., 2016).
Concluding Remarks
Recent studies suggest that enhancing CSC subpopulations can improve the outcomes of cancer therapy and prevent tumor recurrence after chemotherapy. The existence of CSCs in tumors expressively contributes to chemo- and radioresistance of tumors as well as metastasis. Autophagy eradicates and reprocesses unwanted cellular components. It promotes resistance to anticancer therapy. An easy method for controlling CSCs via autophagy has not been developed. Many investigators believe that autophagy functions to maintain CSC stemness, and thereby results in the failure of anticancer treatment. Autophagy may contribute to the loss of stemness in certain CSCs. Consequently, autophagy could serve as a prospective target for reducing the resistance of CSCs to anticancer treatment. Reducing the autophagic flux may not only improve chemotherapeutic properties, but may also deliver additional nutrient sources for cancer cells. The optimal doses of autophagy inhibitors have not been determined. Studies are based on cell lines as well as animal models. Additional clinical studies are required to verify the results of these investigations. Various additional factors must be considered before clinical applications. Nonetheless, the treatment of CSCs with autophagy modulators is a potentially beneficial approach for anticancer treatment. Therefore, it is crucial to perform additional experiments aimed at targeting autophagy signaling for controlling CSCs. More specifically, the biological effects of autophagy are still not fully understood. However, the effects of autophagy might depend on several factors, such as the cell type, stimulus, and microenvironment. Thus, an exciting and novel modulator of autophagy that is effective and safe is required for the treatment of CSCs.
Author Contributions
This work was collaboration among all of the authors. MAR and SS designed outlines and wrote the draft of the manuscript. MSR prepared the figures. MJU and MSU wrote the initial draft of the manuscript. M-GP reviewed the manuscript. HR and S-GC proposed the original idea and reviewed the scientific contents described in the manuscript. All authors read and approved the final submitted version of the manuscript.
Funding
The authors acknowledge the support by the Korea Research Fellowship (KRF) Program (2016H1D3A1908615 and 2017H1D3A1A02013844) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT, South Korea. This is also acknowledged by the RP-Grant 2020 of Ewha Womans University, South Korea. This study was supported by grants from the NRF funded by the Korean government (Grant No. 2019M3A9H1030682 and 2015R1A5A1009701).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abdullah, L. N., and Chow, E. K. (2013). Mechanisms of chemoresistance in cancer stem cells. Clin. Transl. Med. 2:3. doi: 10.1186/2001-1326-2-3
Al-Hajj, M., Wicha, M. S., Benito-Hernandez, A., Morrison, S. J., and Clarke, M. F. (2003). Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. U.S.A. 100, 3983–3988.
Ali Azouaou, S., Emhemmed, F., Idris-Khodja, N., Lobstein, A., Schini-Kerth, V., Muller, C. D., et al. (2015). Selective ROS-dependent p53-associated anticancer effects of the hypoxoside derivative rooperol on human teratocarcinomal cancer stem-like cells. Invest. New Drugs 33, 64–74. doi: 10.1007/s10637-014-0182-6
Apel, A., Herr, I., Schwarz, H., Rodemann, H. P., and Mayer, A. (2008). Blocked autophagy sensitizes resistant carcinoma cells to radiation therapy. Cancer Res. 68, 1485–1494. doi: 10.1158/0008-5472.CAN-07-0562
Avalos, Y., Canales, J., Bravo-Sagua, R., Criollo, A., Lavandero, S., and Quest, A. F. (2014). Tumor suppression and promotion by autophagy. Biomed Res. Int. 2014:603980. doi: 10.1155/2014/603980
Balic, A., Sorensen, M. D., Trabulo, S. M., Sainz, B., Cioffi, M., Vieira, C. R., et al. (2014). Chloroquine targets pancreatic cancer stem cells via inhibition of CXCR4 and hedgehog signaling. Mol. Cancer Ther. 13, 1758–1771. doi: 10.1158/1535-7163.MCT-13-0948
Banerji, V., and Gibson, S. B. (2012). Targeting metabolism and autophagy in the context of haematologic malignancies. Int. J. Cell Biol. 2012:595976. doi: 10.1155/2012/595976
Bashmail, H. A., Alamoudi, A. A., Noorwali, A., Hegazy, G. A., AJabnoor, G., Choudhry, H., et al. (2018). Thymoquinone synergizes gemcitabine anti-breast cancer activity via modulating its apoptotic and autophagic activities. Sci. Rep. 8:11674. doi: 10.1038/s41598-018-30046-z
Becher, J., Simula, L., Volpe, E., Procaccini, C., La Rocca, C., D’Acunzo, P., et al. (2018). AMBRA1 controls regulatory T-cell differentiation and homeostasis upstream of the FOXO3-FOXP3 axis. Dev. Cell 47, 592–607.e6. doi: 10.1016/j.devcel.2018.11.010.
Bellodi, C., Lidonnici, M. R., Hamilton, A., Helgason, G. V., Soliera, A. R., Ronchetti, M., et al. (2009). Targeting autophagy potentiates tyrosine kinase inhibitor-induced cell death in Philadelphia chromosome-positive cells, including primary CML stem cells. J. Clin. Invest. 119, 1109–1123. doi: 10.1172/JCI35660
Boiko, A. D., Razorenova, O. V., van de Rijn, M., Swetter, S. M., Johnson, D. L., Ly, D. P., et al. (2010). Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature 466, 133–137. doi: 10.1038/nature09161
Buccarelli, M., Marconi, M., Pacioni, S., De Pascalis, I., D’Alessandris, Q. G., Martini, M., et al. (2018). Inhibition of autophagy increases susceptibility of glioblastoma stem cells to temozolomide by igniting ferroptosis. Cell Death Dis. 9:841. doi: 10.1038/s41419-018-0864-7
Cascio, S., D’Andrea, A., Ferla, R., Surmacz, E., Gulotta, E., Amodeo, V., et al. (2010). miR-20b modulates VEGF expression by targeting HIF-1 alpha and STAT3 in MCF-7 breast cancer cells. J. Cell. Physiol. 224, 242–249. doi: 10.1002/jcp.22126
Cavazzoni, A., Bonelli, M. A., Fumarola, C., La Monica, S., Airoud, K., Bertoni, R., et al. (2012). Overcoming acquired resistance to letrozole by targeting the PI3K/AKT/mTOR pathway in breast cancer cell clones. Cancer Lett. 323, 77–87. doi: 10.1016/j.canlet.2012.03.034
Chaterjee, M., and van Golen, K. L. (2011). Breast cancer stem cells survive periods of farnesyl-transferase inhibitor-induced dormancy by undergoing autophagy. Bone Marrow Res. 2011:362938. doi: 10.1155/2011/362938
Chen, H., and Chan, D. C. (2017). Mitochondrial dynamics in regulating the unique phenotypes of cancer and stem cells. Cell Metab. 26, 39–48. doi: 10.1016/j.cmet.2017.05.016
Cheong, J. W., Min, Y. H., Eom, J. I., Kim, S. J., Jeung, H. K., and Kim, J. S. (2010). Inhibition of CK2 alpha and PI3K/Akt synergistically induces apoptosis of CD34(+)CD38(-) Leukaemia cells while sparing haematopoietic stem cells. Anticancer. Res. 30, 4625–4634.
Cheung-Ong, K., Giaever, G., and Nislow, C. (2013). DNA-damaging agents in cancer chemotherapy: serendipity and chemical biology. Chem. Biol. 20, 648–659. doi: 10.1016/j.chembiol.2013.04.007
Cong, S. B., Luo, H. Y., Li, X., Wang, F. L., Hua, Y. A., Zhang, L., et al. (2019). Isatin inhibits SH-SY5Y neuroblastoma cell invasion and metastasis through PTEN signaling. Int. J. Clin. Exp. Pathol. 12, 2446–2454.
Copland, M., Hamilton, A., EIrick, L. J., Baird, J. W., Allan, E. K., Jordanides, N., et al. (2006). Dasatinib (BMS-354825) targets an earlier progenitor population than imatinib in primary CML but does not eliminate the quiescent fraction. Blood 107, 4532–4539. doi: 10.1182/blood-2005-07-2947
Cristofani, R., Marelli, M. M., Cicardi, M. E., Fontana, F., Marzagalli, M., Limonta, P., et al. (2018). Dual role of autophagy on docetaxel-sensitivity in prostate cancer cells. Cell Death Dis. 9:889. doi: 10.1038/s41419-018-0866-5
Cufi, S., Vazquez-Martin, A., Oliveras-Ferraros, C., Martin-Castillo, B., Vellon, L., and Menendez, J. A. (2011). Autophagy positively regulates the CD44(+)CD24(-/low) breast cancer stem-like phenotype. Cell Cycle 10, 3871–3885. doi: 10.4161/cc.10.22.17976
Ding, Y. H., Zhou, Z. W., Ha, C. F., Zhang, X. Y., Pan, S. T., He, Z. X., et al. (2015). Alisertib, an Aurora kinase A inhibitor, induces apoptosis and autophagy but inhibits epithelial to mesenchymal transition in human epithelial ovarian cancer cells. Drug Des. Dev. Ther. 9, 425–464. doi: 10.2147/DDDT.S74062
Dowdle, W. E., Nyfeler, B., Nagel, J., Elling, R. A., Liu, S., Triantafellow, E., et al. (2014). Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo. Nat. Cell Biol. 16, 1069–1079. doi: 10.1038/ncb3053
Dragu, D. L., Necula, L. G., Bleotu, C., Diaconu, C. C., and Chivu-Economescu, M. (2015). Therapies targeting cancer stem cells: current trends and future challenges. World J. Stem Cells 7, 1185–1201. doi: 10.4252/wjsc.v7.i9.1185
Dubrovska, A., Kim, S., Salamone, R. J., Walker, J. R., Maira, S. M., Garcia-Echeverria, C., et al. (2009). The role of PTEN/Akt/PI3K signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc. Natl. Acad. Sci. U.S.A. 106, 268–273. doi: 10.1073/pnas.0810956106
El-Merahbi, R., Liu, Y. N., Eid, A., Daoud, G., Hosry, L., Monzer, A., et al. (2014). Berberis libanotica Ehrenb extract shows anti-neoplastic effects on prostate cancer stem/progenitor cells. PLoS One 9:e112453. doi: 10.1371/journal.pone.0112453
Endo, S., Nakata, K., Ohuchida, K., Takesue, S., Nakayama, H., Abe, T., et al. (2017). Autophagy is required for activation of pancreatic stellate cells, associated with pancreatic cancer progression and promotes growth of pancreatic tumors in mice. Gastroenterology 152, 1492–1506.e24. doi: 10.1053/j.gastro.2017.01.010
Eramo, A., Lotti, F., Sette, G., Pilozzi, E., Biffoni, M., Di Virgilio, A., et al. (2008). Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 15, 504–514. doi: 10.1038/sj.cdd.4402283
Eun, K., Ham, S. W., and Kim, H. (2017). Cancer stem cell heterogeneity: origin and new perspectives on CSC targeting. BMB Rep. 50, 117–125. doi: 10.5483/bmbrep.2017.50.3.222
Firat, E., Weyerbrock, A., Gaedicke, S., Grosu, A. L., and Niedermann, G. (2012). Chloroquine or chloroquine-PI3K/Akt pathway inhibitor combinations strongly promote gamma-irradiation-induced cell death in primary stem-like glioma cells. PLoS One 7: e47357. doi: 10.1371/journal.pone.0047357
Fox, N. L., Humphreys, R., Luster, T. A., Klein, J., and Gallant, G. (2010). Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) receptor-1 and receptor-2 agonists for cancer therapy. Expert Opin. Biol. Ther. 10, 1–18. doi: 10.1517/14712590903319656
Fu, Y., Chang, H., Peng, X., Bai, Q., Yi, L., Zhou, Y., et al. (2014). Resveratrol inhibits breast cancer stem-like cells and induces autophagy via suppressing Wnt/beta-catenin signaling pathway. PLoS One 9:e102535. doi: 10.1371/journal.pone.0102535
Galluzzi, L., Bravo-San Pedro, J. M., Demaria, S., Formenti, S. C., and Kroemer, G. (2017). Activating autophagy to potentiate immunogenic chemotherapy and radiation therapy. Nat. Rev. Clin. Oncol. 14, 247–258. doi: 10.1038/nrclinonc.2016.183
Gargini, R., Cerliani, J. P., Escoll, M., Anton, I. M., and Wandosell, F. (2015). Cancer stem cell-like phenotype and survival are coordinately regulated by Akt/FoxO/Bim pathway. Stem Cells 33, 646–660. doi: 10.1002/stem.1904
Geisen, U., Zenthoefer, M., Peipp, M., Kerber, J., Plenge, J., Manago, A., et al. (2015). Molecular mechanisms by which a Fucus vesiculosus extract mediates cell cycle inhibition and cell death in pancreatic cancer cells. Mar. Drugs 13, 4470–4491. doi: 10.3390/md13074470
Golden, E. B., Cho, H. Y., Jahanian, A., Hofman, F. M., Louie, S. G., Schonthal, A. H., et al. (2014). Chloroquine enhances temozolomide cytotoxicity in malignant gliomas by blocking autophagy. Neurosurg. Focus 37:E12. doi: 10.3171/2014.9.FOCUS14504
Gong, C., Bauvy, C., Tonelli, G., Yue, W., Delomenie, C., Nicolas, V., et al. (2013). Beclin 1 and autophagy are required for the tumorigenicity of breast cancer stem-like/progenitor cells. Oncogene 32, 2261–2272. doi: 10.1038/onc.2012.252
Gong, C., Song, E., Codogno, P., and Mehrpour, M. (2012). The roles of BECN1 and autophagy in cancer are context dependent. Autophagy 8, 1853–1855. doi: 10.4161/auto.21996
Gu, H., Feng, J., Wang, H., Qian, Y., Yang, L., Chen, J., et al. (2016). Celastrus orbiculatus extract inhibits the migration and invasion of human glioblastoma cells in vitro. BMC Complement. Altern. Med. 16:387.
Gupta, P. B., Chaffer, C. L., and Weinberg, R. A. (2009a). Cancer stem cells: mirage or reality? Nat. Med. 15, 1010–1012. doi: 10.1038/nm0909-1010
Gupta, P. B., Onder, T. T., Jiang, G. Z., Tao, K., Kuperwasser, C., Weinberg, R. A., et al. (2009b). Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 138, 645–659. doi: 10.1016/j.cell.2009.06.034
Guzman, M. L., Swiderski, C. F., Howard, D. S., Grimes, B. A., Rossi, R. M., Szilvassy, S. J., et al. (2002). Preferential induction of apoptosis for primary human leukemic stem cells. Proc. Natl. Acad. Sci. U.S.A. 99, 16220–16225. doi: 10.1073/pnas.252462599
Han, S. H., Korm, S., Han, Y. G., Choi, S. Y., Kim, S. H., Chung, H. J., et al. (2019). GCA links TRAF6-ULK1-dependent autophagy activation in resistant chronic myeloid leukemia. Autophagy 15, 2076–2090. doi: 10.1080/15548627.2019.1596492
Han, Y., Fan, S., Qin, T., Yang, J., Sun, Y., Lu, Y., et al. (2018). Role of autophagy in breast cancer and breast cancer stem cells (Review). Int. J. Oncol. 52, 1057–1070. doi: 10.3892/ijo.2018.4270
Hermann, P. C., Huber, S. L., Herrler, T., Aicher, A., Ellwart, J. W., Guba, M., et al. (2007). Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 1, 313–323. doi: 10.1016/j.stem.2007.06.002
Hirsch, H. A., Iliopoulos, D., Tsichlis, P. N., and Struhl, K. (2009). Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 69, 7507–7511. doi: 10.1158/0008-5472.can-09-2994
Houwerzijl, E. J., Pol, H. W., Blom, N. R., van der Want, J. J., de Wolf, J. T., and Vellenga, E. (2009). Erythroid precursors from patients with low-risk myelodysplasia demonstrate ultrastructural features of enhanced autophagy of mitochondria. Leukemia 23, 886–891. doi: 10.1038/leu.2008.389
Huang, H., Lin, H., Zhang, X., and Li, J. (2012). Resveratrol reverses temozolomide resistance by downregulation of MGMT in T98G glioblastoma cells by the NF-kappaB-dependent pathway. Oncol. Rep. 27, 2050–2056. doi: 10.3892/or.2012.1715
Huang, H., Song, J., Liu, Z., Pan, L., and Xu, G. (2018). Autophagy activation promotes bevacizumab resistance in glioblastoma by suppressing Akt/mTOR signaling pathway. Oncol. Lett. 15, 1487–1494. doi: 10.3892/ol.2017.7446
Huang, T. Z., Alvarez, A. A., Pangeni, R. P., Horbinski, C. M., Lu, S. J., Kim, S. H., et al. (2016). A regulatory circuit of miR-125b/miR-20b and Wnt signalling controls glioblastoma phenotypes through FZD6-modulated pathways. Nat. Commun. 7:12885. doi: 10.1038/ncomms12885
Huang, T. Z., Kim, C. K., Alvarez, A. A., Pangeni, R. P., Wan, X. C., Song, X., et al. (2017). MST4 phosphorylation of ATG4B regulates autophagic activity, tumorigenicity, and radioresistance in glioblastoma. Cancer Cell 32, 840–855.e8. doi: 10.1016/j.ccell.2017.11.005
Huang, T. Z., Wan, X. C., Alvarez, A. A., James, C. D., Song, X., Yang, Y. Y., et al. (2019). MIR93 (microRNA-93) regulates tumorigenicity and therapy response of glioblastoma by targeting autophagy. Autophagy 15, 1100–1111. doi: 10.1080/15548627.2019.1569947
Huang, Y. T., Lin, Y. W., Chiu, H. M., and Chiang, B. H. (2016). Curcumin induces apoptosis of colorectal cancer stem cells by coupling with CD44 marker. J. Agric. Food Chem. 64, 2247–2253. doi: 10.1021/acs.jafc.5b05649
Iliopoulos, D., Hirsch, H. A., Wang, G., and Struhl, K. (2011). Inducible formation of breast cancer stem cells and their dynamic equilibrium with non-stem cancer cells via IL6 secretion. Proc. Natl. Acad. Sci. U.S.A. 108, 1397–1402. doi: 10.1073/pnas.1018898108
Jeong, H. S., Choi, H. Y., Lee, E. R., Kim, J. H., Jeon, K., Lee, H. J., et al. (2011). Involvement of caspase-9 in autophagy-mediated cell survival pathway. Biochim. Biophys. Acta 1813, 80–90. doi: 10.1016/j.bbamcr.2010.09.016
Jiang, H., Gomez-Manzano, C., Aoki, H., Alonso, M. M., Kondo, S., McCormick, F., et al. (2007). Examination of the therapeutic potential of Delta-24-RGD in brain tumor stem cells: role of autophagic cell death. J. Natl. Cancer Inst. 99, 1410–1414. doi: 10.1093/jnci/djm102
Kamalidehghan, B., Ghafouri-Fard, S., Motevaseli, E., and Ahmadipour, F. (2018). Inhibition of human prostate cancer (PC-3) cells and targeting of PC-3-derived prostate cancer stem cells with koenimbin, a natural dietary compound from Murraya koenigii (L) Spreng. Drug Des. Dev. Ther. 12, 1119–1133. doi: 10.2147/DDDT.S156826
Kantara, C., O’Connell, M., Sarkar, S., Moya, S., Ullrich, R., and Singh, P. (2014). Curcumin promotes autophagic survival of a subset of colon cancer stem cells, which are ablated by DCLK1-siRNA. Cancer Res. 74, 2487–2498. doi: 10.1158/0008-5472.CAN-13-3536
Karvela, M., Baquero, P., Kuntz, E. M., Mukhopadhyay, A., Mitchell, R., Allan, E. K., et al. (2016). ATG7 regulates energy metabolism, differentiation and survival of Philadelphia-chromosome-positive cells. Autophagy 12, 936–948. doi: 10.1080/15548627.2016.1162359
Katheder, N. S., Khezri, R., O’Farrell, F., Schultz, S. W., Jain, A., Rahman, M. M., et al. (2017). Microenvironmental autophagy promotes tumour growth. Nature 541, 417–420. doi: 10.1038/nature20815
Khaw, A. K., Sameni, S., Venkatesan, S., Kalthur, G., and Hande, M. P. (2015). Plumbagin alters telomere dynamics, induces DNA damage and cell death in human brain tumour cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 793, 86–95. doi: 10.1016/j.mrgentox.2015.06.004
Kim, M. P., Fleming, J. B., Wang, H. M., Abbruzzese, J. L., Choi, W., Kopetz, S., et al. (2011). ALDH activity selectively defines an enhanced tumor-initiating cell population relative to CD133 expression in human pancreatic adenocarcinoma. PLoS One 6:e20636. doi: 10.1371/journal.pone.0020636
Knowles, M. A., and Hurst, C. D. (2015). Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat. Rev. Cancer 15, 25–41. doi: 10.1038/nrc3817
Kondo, Y., Kanzawa, T., Sawaya, R., and Kondo, S. (2005). The role of autophagy in cancer development and response to therapy. Nat. Rev. Cancer 5, 726–734. doi: 10.1038/nrc1692
Konig, H., Holtz, M., Modi, H., Manley, P., Holyoake, T. L., Forman, S. J., et al. (2008). Enhanced BCR-ABL kinase inhibition does not result in increased inhibition of downstream signaling pathways or increased growth suppression in CML progenitors. Leukemia 22, 748–755. doi: 10.1038/sj.leu.2405086
Kreso, A., van Galen, P., Pedley, N. M., Lima-Fernandes, E., Frelin, C., Davis, T., et al. (2014). Self-renewal as a therapeutic target in human colorectal cancer. Nat. Med. 20, 29–36. doi: 10.1038/nm.3418
Kroemer, G. (2015). Autophagy: a druggable process that is deregulated in aging and human disease. J. Clin. Investig. 125, 1–4. doi: 10.1172/JCI78652
Kwatra, D., Subramaniam, D., Ramamoorthy, P., Standing, D., Moran, E., Velayutham, R., et al. (2013). Methanolic extracts of bitter melon inhibit colon cancer stem cells by affecting energy homeostasis and autophagy. Evid. Based Complement. Alternat. Med. 2013:702869. doi: 10.1155/2013/702869
Kwon, Y. H., Bishayee, K., Rahman, M. A., Hong, J. S., Lim, S. S., and Huh, S. O. (2015). Morus alba accumulates reactive oxygen species to initiate apoptosis via FOXO-caspase 3-dependent pathway in neuroblastoma cells. Mol. Cells 38, 630–637. doi: 10.14348/molcells.2015.0030
Lathia, J. D., Mack, S. C., Mulkearns-Hubert, E. E., Valentim, C. L. L., and Rich, J. N. (2015). Cancer stem cells in glioblastoma. Gene Dev. 29, 1203–1217.
Lei, Y., Zhang, D., Yu, J., Dong, H., Zhang, J., and Yang, S. (2017). Targeting autophagy in cancer stem cells as an anticancer therapy. Cancer Lett. 393, 33–39. doi: 10.1016/j.canlet.2017.02.012
Li, C., Heidt, D. G., Dalerba, P., Burant, C. F., Zhang, L., Adsay, V., et al. (2007). Identification of pancreatic cancer stem cells. Cancer Res. 67, 1030–1037.
Li, L. Q., Pan, D., Zhang, S. W., Xie, D. Y., Zheng, X. L., and Chen, H. (2018). Autophagy regulates chemoresistance of gastric cancer stem cells via the Notch signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 22, 3402–3407. doi: 10.26355/eurrev_201806_15162
Li, S. Z., Zhao, Q., Wang, B., Yuan, S., Wang, X. Y., and Li, K. (2018). Quercetin reversed MDR in breast cancer cells through down-regulating P-gp expression and eliminating cancer stem cells mediated by YB-1 nuclear translocation. Phytother. Res. 32, 1530–1536. doi: 10.1002/ptr.6081
Li, Y., Zhang, P., Qiu, F., Chen, L., Miao, C., Li, J., et al. (2012). Inactivation of PI3K/Akt signaling mediates proliferation inhibition and G2/M phase arrest induced by andrographolide in human glioblastoma cells. Life Sci. 90, 962–967. doi: 10.1016/j.lfs.2012.04.044
Li, Y., and Zhang, T. (2014). Targeting cancer stem cells by curcumin and clinical applications. Cancer Lett. 346, 197–205. doi: 10.1016/j.canlet.2014.01.012
Lin, H. C., Lin, M. H., Liao, J. H., Wu, T. H., Lee, T. H., Mi, F. L., et al. (2017). Antroquinonol, a ubiquinone derivative from the mushroom antrodia camphorata, inhibits colon cancer stem cell-like properties: insights into the molecular mechanism and inhibitory targets. J. Agric. Food Chem. 65, 51–59. doi: 10.1021/acs.jafc.6b04101
Liu, B., Bao, J. K., Yang, J. M., and Cheng, Y. (2013). Targeting autophagic pathways for cancer drug discovery. Chin J. Cancer 32, 113–120. doi: 10.5732/cjc.012.10010
Liu, D., Yang, Y., Liu, Q., and Wang, J. (2011). Inhibition of autophagy by 3-MA potentiates cisplatin-induced apoptosis in esophageal squamous cell carcinoma cells. Med. Oncol. 28, 105–111. doi: 10.1007/s12032-009-9397-3
Lobo, N. A., Shimono, Y., Qian, D., and Clarke, M. F. (2007). The biology of cancer stem cells. Annu. Rev. Cell Dev. Biol. 23, 675–699.
Loebinger, M. R., Sage, E. K., Davies, D., and Janes, S. M. (2010). TRAIL-expressing mesenchymal stem cells kill the putative cancer stem cell population. Br. J. Cancer 103, 1692–1697. doi: 10.1038/sj.bjc.6605952
Mackillop, W. J., Ciampi, A., Till, J. E., and Buick, R. N. (1983). A stem cell model of human tumor growth: implications for tumor cell clonogenic assays. J. Natl. Cancer Inst. 70, 9–16.
May, R., Riehl, T. E., Hunt, C., Sureban, S. M., Anant, S., and Houchen, C. W. (2008). Identification of a novel putative gastrointestinal stem cell and adenoma stem cell marker, doublecortin and CaM kinase-like-1, following radiation injury and in adenomatous polyposis coli/multiple intestinal neoplasia mice. Stem Cells 26, 630–637. doi: 10.1634/stemcells.2007-0621
Maycotte, P., Jones, K. L., Goodall, M. L., Thorburn, J., and Thorburn, A. (2015). Autophagy supports breast cancer stem cell maintenance by regulating IL6 secretion. Mol. Cancer Res. 13, 651–658. doi: 10.1158/1541-7786.MCR-14-0487
Miki, H., Uehara, N., Kimura, A., Sasaki, T., Yuri, T., Yoshizawa, K., et al. (2012). Resveratrol induces apoptosis via ROS-triggered autophagy in human colon cancer cells. Int. J. Oncol. 40, 1020–1028. doi: 10.3892/ijo.2012.1325
Montales, M. T., Rahal, O. M., Kang, J., Rogers, T. J., Prior, R. L., Wu, X., et al. (2012). Repression of mammosphere formation of human breast cancer cells by soy isoflavone genistein and blueberry polyphenolic acids suggests diet-mediated targeting of cancer stem-like/progenitor cells. Carcinogenesis 33, 652–660. doi: 10.1093/carcin/bgr317
Moon, H. J., Park, S. Y., Lee, S. H., Kang, C. D., and Kim, S. H. (2019). Nonsteroidal anti-inflammatory drugs sensitize CD44-overexpressing cancer cells to HSP90 inhibitor through autophagy activation. Oncol. Res. 27, 835–847. doi: 10.3727/096504019X15517850319579
Mu, G. G., Zhang, L. L., Li, H. Y., Liao, Y., and Yu, H. G. (2015). Thymoquinone pretreatment overcomes the insensitivity and potentiates the antitumor effect of gemcitabine through abrogation of Notch1, PI3K/Akt/mTOR regulated signaling pathways in pancreatic cancer. Dig. Dis. Sci. 60, 1067–1080. doi: 10.1007/s10620-014-3394-x
Mukherjee, S., Mazumdar, M., Chakraborty, S., Manna, A., Saha, S., Khan, P., et al. (2014). Curcumin inhibits breast cancer stem cell migration by amplifying the E-cadherin/beta-catenin negative feedback loop. Stem Cell Res. Ther. 5:116. doi: 10.1186/scrt506
Nazio, F., Bordi, M., Cianfanelli, V., Locatelli, F., and Cecconi, F. (2019). Autophagy and cancer stem cells: molecular mechanisms and therapeutic applications. Cell Death Differ. 26, 690–702. doi: 10.1038/s41418-019-0292-y
Ning, Y., Luo, C., Ren, K., Quan, M., and Cao, J. (2014). FOXO3a-mediated suppression of the self-renewal capacity of sphere-forming cells derived from the ovarian cancer SKOV3 cell line by 7-difluoromethoxyl-5,4’-di-n-octyl genistein. Mol. Med. Rep. 9, 1982–1988. doi: 10.3892/mmr.2014.2012
Ning, Z. F., Huang, Y. J., Lin, T. X., Zhou, Y. X., Jiang, C., Xu, K. W., et al. (2009). Subpopulations of stem-like cells in side population cells from the human bladder transitional cell cancer cell line T24. J. Int. Med. Res. 37, 621–630. doi: 10.1177/147323000903700304
O’Brien, C. A., Pollett, A., Gallinger, S., and Dick, J. E. (2007). A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 445, 106–110. doi: 10.1038/nature05372
Ojha, R., Bhattacharyya, S., and Singh, S. K. (2015). Autophagy in cancer stem cells: a potential link between chemoresistance, recurrence, and metastasis. Biores. Open Access 4, 97–108. doi: 10.1089/biores.2014.0035
Ojha, R., Jha, V., and Singh, S. K. (2016a). Gemcitabine and mitomycin induced autophagy regulates cancer stem cell pool in urothelial carcinoma cells. Biochim. Biophys. Acta 1863, 347–359. doi: 10.1016/j.bbamcr.2015.12.002
Ojha, R., Jha, V., Singh, S. K., and Bhattacharyya, S. (2014). Autophagy inhibition suppresses the tumorigenic potential of cancer stem cell enriched side population in bladder cancer. Biochim. Biophys. Acta 1842, 2073–2086. doi: 10.1016/j.bbadis.2014.07.007
Ojha, R., Singh, S. K., and Bhattacharyya, S. (2016b). JAK-mediated autophagy regulates stemness and cell survival in cisplatin resistant bladder cancer cells. Biochim. Biophys. Acta 1860, 2484–2497. doi: 10.1016/j.bbagen.2016.07.021
Park, M. H., and Hong, J. T. (2016). Roles of NF-kappa B in cancer and inflammatory diseases and their therapeutic approaches. Cells 5:E15.
Park, Y. S., Huh, J. W., Lee, J. H., and Kim, H. R. (2012). shRNA against CD44 inhibits cell proliferation, invasion and migration, and promotes apoptosis of colon carcinoma cells. Oncol. Rep. 27, 339–346. doi: 10.3892/or.2011.1532
Pellicano, F., Scott, M. T., Helgason, G. V., Hopcroft, L. E., Allan, E. K., Aspinall-O’Dea, M., et al. (2014). The antiproliferative activity of kinase inhibitors in chronic myeloid leukemia cells is mediated by FOXO transcription factors. Stem Cells 32, 2324–2337. doi: 10.1002/stem.1748
Peng, Q., Qin, J., Zhang, Y., Cheng, X., Wang, X., Lu, W., et al. (2017). Autophagy maintains the stemness of ovarian cancer stem cells by FOXA2. J. Exp. Clin. Cancer Res. 36:171. doi: 10.1186/s13046-017-0644-8
Petherick, K. J., Conway, O. J., Mpamhanga, C., Osborne, S. A., Kamal, A., Saxty, B., et al. (2015). Pharmacological inhibition of ULK1 kinase blocks mammalian target of rapamycin (mTOR)-dependent autophagy. J. Biol. Chem. 290, 11376–11383. doi: 10.1074/jbc.C114.627778
Pietrocola, F., Pol, J., and Kroemer, G. (2016). Fasting improves anticancer immunosurveillance via autophagy induction in malignant cells. Cell Cycle 15, 3327–3328. doi: 10.1080/15384101.2016.1224797
Plasilova, M., Zivny, J., Jelinek, J., Neuwirtova, R., Cermak, J., Necas, E., et al. (2002). TRAIL (Apo2L) suppresses growth of primary human leukemia and myelodysplasia progenitors. Leukemia 16, 67–73. doi: 10.1038/sj.leu.2402338
Plati, J., Bucur, O., and Khosravi-Far, R. (2011). Apoptotic cell signaling in cancer progression and therapy. Integr. Biol. 3, 279–296. doi: 10.1039/c0ib00144a
Prabhu, V. V., Allen, J. E., Dicker, D. T., and El-Deiry, W. S. (2015). Small-molecule ONC201/TIC10 targets chemotherapy-resistant colorectal cancer stem-like cells in an Akt/Foxo3a/TRAIL-Dependent manner. Cancer Res. 75, 1423–1432. doi: 10.1158/0008-5472.CAN-13-3451
Puissant, A., and Auberger, P. (2010). AMPK- and p62/SQSTM1-dependent autophagy mediate resveratrol-induced cell death in chronic myelogenous leukemia. Autophagy 6, 655–657. doi: 10.4161/auto.6.5.12126
Rahman, M. A., Bishayee, K., Habib, K., Sadra, A., and Huh, S. O. (2016). 18alpha-Glycyrrhetinic acid lethality for neuroblastoma cells via de-regulating the Beclin-1/Bcl-2 complex and inducing apoptosis. Biochem. Pharmacol. 117, 97–112. doi: 10.1016/j.bcp.2016.08.006
Rahman, M. A., Bishayee, K., Sadra, A., and Huh, S. O. (2017). Oxyresveratrol activates parallel apoptotic and autophagic cell death pathways in neuroblastoma cells. Biochim. Biophys. Acta Gen. Subj. 1861, 23–36. doi: 10.1016/j.bbagen.2016.10.025
Rahman, M. A., Hong, J. S., and Huh, S. O. (2015). Antiproliferative properties of Saussurea lappa clarke root extract in SH-SY5Y neuroblastoma cells via intrinsic apoptotic pathway. Anim. Cells Syst. 19, 119–126. doi: 10.1080/19768354.2015.1008041
Rahman, M. A., Hwang, H., Nah, S. Y., and Rhim, H. (2020a). Gintonin stimulates autophagic flux in primary cortical astrocytes. J. Ginseng Res. 44, 67–78. doi: 10.1016/j.jgr.2018.08.004
Rahman, M. A., Kim, N. H., and Huh, S. O. (2013). Cytotoxic effect of gambogic acid on SH-SY5Y neuroblastoma cells is mediated by intrinsic caspase-dependent signaling pathway. Mol. Cell. Biochem. 377, 187–196. doi: 10.1007/s11010-013-1584-z
Rahman, M. A., Kim, N. H., Kim, S. H., Oh, S. M., and Huh, S. O. (2012a). Antiproliferative and cytotoxic effects of resveratrol in mitochondria-mediated apoptosis in rat b103 neuroblastoma cells. Korean J. Physiol. Pharmacol. 16, 321–326. doi: 10.4196/kjpp.2012.16.5.321
Rahman, M. A., Kim, N. H., Yang, H., and Huh, S. O. (2012b). Angelicin induces apoptosis through intrinsic caspase-dependent pathway in human SH-SY5Y neuroblastoma cells. Mol. Cell. Biochem. 369, 95–104. doi: 10.1007/s11010-012-1372-1
Rahman, M. A., Rahman, M. R., Zaman, T., Uddin, M. S., Islam, R., Abdel-Daim, M. M., et al. (2020b). Emerging potential of naturally occurring autophagy modulator against neurodegeneration. Curr. Pharm. Des. 26, 772–779. doi: 10.2174/1381612826666200107142541
Rahman, M. A., and Rhim, H. (2017). Therapeutic implication of autophagy in neurodegenerative diseases. BMB Rep. 50, 345–354. doi: 10.5483/bmbrep.2017.50.7.069
Rahman, M. A., Yang, H., Kim, N. H., and Huh, S. O. (2014). Induction of apoptosis by Dioscorea nipponica Makino extracts in human SH-SY5Y neuroblastoma cells via mitochondria-mediated pathway. Anim. Cells Syst. 18, 41–51. doi: 10.1080/19768354.2014.880372
Rajeshkumar, N. V., Rasheed, Z. A., Garcia-Garcia, E., Lopez-Rios, F., Fujiwara, K., Matsui, W. H., et al. (2010). A combination of DR5 agonistic monoclonal antibody with gemcitabine targets pancreatic cancer stem cells and results in long-term disease control in human pancreatic cancer model. Mol. Cancer Ther. 9, 2582–2592. doi: 10.1158/1535-7163.MCT-10-0370
Ricci-Vitiani, L., Lombardi, D. G., Pilozzi, E., Biffoni, M., Todaro, M., Peschle, C., et al. (2007). Identification and expansion of human colon-cancer-initiating cells. Nature 445, 111–115. doi: 10.1038/nature05384
Robert, T., Vanoli, F., Chiolo, I., Shubassi, G., Bernstein, K. A., Rothstein, R., et al. (2011). HDACs link the DNA damage response, processing of double-strand breaks and autophagy. Nature 471, 74–79. doi: 10.1038/nature09803
Rothe, K., Lin, H., Lin, K. B., Leung, A., Wang, H. M., Malekesmaeili, M., et al. (2014). The core autophagy protein ATG4B is a potential biomarker and therapeutic target in CML stem/progenitor cells. Blood 123, 3622–3634. doi: 10.1182/blood-2013-07-516807
Saha, S. K., Choi, H. Y., Kim, B. W., Dayem, A. A., Yang, G. M., Kim, K. S., et al. (2017). KRT19 directly interacts with beta-catenin/RAC1 complex to regulate NUMB-dependent NOTCH signaling pathway and breast cancer properties. Oncogene 36, 332–349. doi: 10.1038/onc.2016.221
Saha, S. K., Kim, K., Yang, G. M., Choi, H. Y., and Cho, S. G. (2018). Cytokeratin 19 (KRT19) has a role in the reprogramming of cancer stem cell-like cells to less aggressive and more drug-sensitive cells. Int. J. Mol. Sci. 19:1423. doi: 10.3390/ijms19051423
Sanchez, C. G., Penfornis, P., Oskowitz, A. Z., Boonjindasup, A. G., Cai, D. Z., Dhule, S. S., et al. (2011). Activation of autophagy in mesenchymal stem cells provides tumor stromal support. Carcinogenesis 32, 964–972. doi: 10.1093/carcin/bgr029
Sarkar, S., Kantara, C., Ortiz, I., Swiercz, R., Kuo, J., Davey, R., et al. (2012). Progastrin overexpression imparts tumorigenic/metastatic potential to embryonic epithelial cells: phenotypic differences between transformed and nontransformed stem cells. Int. J. Cancer 131, E1088–E1099. doi: 10.1002/ijc.27615
Schatton, T., Murphy, G. F., Frank, N. Y., Yamaura, K., Waaga-Gasser, A. M., Gasser, M., et al. (2008). Identification of cells initiating human melanomas. Nature 451, 345–349. doi: 10.1038/nature06489
Schepers, A. G., Snippert, H. J., Stange, D. E., van den Born, M., van Es, J. H., van de Wetering, M., et al. (2012). Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science 337, 730–735. doi: 10.1126/science.1224676
Singh, B. N., Kumar, D., Shankar, S., and Srivastava, R. K. (2012). Rottlerin induces autophagy which leads to apoptotic cell death through inhibition of PI3K/Akt/mTOR pathway in human pancreatic cancer stem cells. Biochem. Pharmacol. 84, 1154–1163. doi: 10.1016/j.bcp.2012.08.007
Singh, S. K., Hawkins, C., Clarke, I. D., Squire, J. A., Bayani, J., Hide, T., et al. (2004). Identification of human brain tumour initiating cells. Nature 432, 396–401.
Smit, L., Berns, K., Spence, K., Ryder, W. D., Zeps, N., Madiredjo, M., et al. (2016). An integrated genomic approach identifies that the PI3K/AKT/FOXO pathway is involved in breast cancer tumor initiation. Oncotarget 7, 2596–2610. doi: 10.18632/oncotarget.6354
Smith, A. G., and Macleod, K. F. (2019). Autophagy, cancer stem cells and drug resistance. J. Pathol. 247, 708–718. doi: 10.1002/path.5222
Sohn, M., Kim, K., Uddin, M. J., Lee, G., Hwang, I., Kang, H., et al. (2017). Delayed treatment with fenofibrate protects against high-fat diet-induced kidney injury in mice: the possible role of AMPK autophagy. Am. J. Physiol. Renal Physiol. 312, F323–F334. doi: 10.1152/ajprenal.00596.2015
Song, Y. J., Zhang, S. S., Guo, X. L., Sun, K., Han, Z. P., Li, R., et al. (2013). Autophagy contributes to the survival of CD133+ liver cancer stem cells in the hypoxic and nutrient-deprived tumor microenvironment. Cancer Lett. 339, 70–81. doi: 10.1016/j.canlet.2013.07.021
Sui, X., Chen, R., Wang, Z., Huang, Z., Kong, N., Zhang, M., et al. (2013). Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death Dis. 4:e838. doi: 10.1038/cddis.2013.350
Sun, X. C., Song, J., Li, E. L., Geng, H., Li, Y., Yu, D. X., et al. (2019). (-)-Epigallocatechin-3-gallate inhibits bladder cancer stem cells via suppression of sonic hedgehog pathway. Oncol. Rep. 42, 425–435. doi: 10.3892/or.2019.7170
Suresh, R., Ali, S., Ahmad, A., Philip, P. A., and Sarkar, F. H. (2016). The role of cancer stem cells in recurrent and drug-resistant lung cancer. Adv. Exp. Med. Biol. 890, 57–74. doi: 10.1007/978-3-319-24932-2_4
Tomic, T., Botton, T., Cerezo, M., Robert, G., Luciano, F., Puissant, A., et al. (2011). Metformin inhibits melanoma development through autophagy and apoptosis mechanisms. Cell Death Dis. 2:e119. doi: 10.1038/cddis.2011.86
Uddin, M. S., Stachowiak, A., Mamun, A. A., Tzvetkov, N. T., Takeda, S., Atanasov, A. G., et al. (2018). Autophagy and Alzheimer’s disease: from molecular mechanisms to therapeutic implications. Front. Aging Neurosci. 10:04. doi: 10.3389/fnagi.2018.00004
Unterkircher, T., Cristofanon, S., and Fulda, S. (2011). Bortezomib primes glioblastoma including glioblastoma stem cells for TRAIL by increasing tBid stability and mitochondrial apoptosis. Klin. Padiatr. 223, 397–397. doi: 10.1158/1078-0432.CCR-11-0075
Van den Broeck, A., Gremeaux, L., Topal, B., and Vankelecom, H. (2012). Human pancreatic adenocarcinoma contains a side population resistant to gemcitabine. BMC Cancer 12:354. doi: 10.1186/1471-2407-12-354
van Doeselaar, S., and Burgering, B. M. T. (2018). FOXOs maintaining the equilibrium for better or for worse. Curr. Top. Dev. Biol. 127, 49–103. doi: 10.1016/bs.ctdb.2017.10.003
Vidal, S. J., Rodriguez-Bravo, V., Galsky, M., Cordon-Cardo, C., and Domingo-Domenech, J. (2014). Targeting cancer stem cells to suppress acquired chemotherapy resistance. Oncogene 33, 4451–4463. doi: 10.1038/onc.2013.411
Wei, M. F., Chen, M. W., Chen, K. C., Lou, P. J., Lin, S. Y., Hung, S. C., et al. (2014). Autophagy promotes resistance to photodynamic therapy-induced apoptosis selectively in colorectal cancer stem-like cells. Autophagy 10, 1179–1192. doi: 10.4161/auto.28679
Wen, P. Y., and Kesari, S. (2008). Malignant gliomas in adults. N. Engl. J. Med. 359, 492–507. doi: 10.1056/nejmra0708126
Witjes, J. A., Comperat, E., Cowan, N. C., De Santis, M., Gakis, G., Lebret, T., et al. (2014). EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur. Urol. 65, 778–792. doi: 10.1016/j.eururo.2013.11.046
Wu, C. H., Hong, B. H., Ho, C. T., and Yen, G. C. (2015). Targeting cancer stem cells in breast cancer: potential anticancer properties of 6-shogaol and pterostilbene. J. Agric. Food Chem. 63, 2432–2441. doi: 10.1021/acs.jafc.5b00002
Yang, M. C., Wang, H. C., Hou, Y. C., Tung, H. L., Chiu, T. J., and Shan, Y. S. (2015). Blockade of autophagy reduces pancreatic cancer stem cell activity and potentiates the tumoricidal effect of gemcitabine. Mol. Cancer 14:179. doi: 10.1186/s12943-015-0449-3
Yang, S. H., Wang, X. X., Contino, G., Liesa, M., Sahin, E., Ying, H. Q., et al. (2011). Pancreatic cancers require autophagy for tumor growth. Gene Dev. 25, 717–729. doi: 10.1101/gad.2016111
Yang, Y. P., Hu, L. F., Zheng, H. F., Mao, C. J., Hu, W. D., Xiong, K. P., et al. (2013). Application and interpretation of current autophagy inhibitors and activators. Acta Pharmacol. Sin. 34, 625–635. doi: 10.1038/aps.2013.5
Yin, S. P., Xu, L. P., Bandyopadhyay, S., Sethi, S., and Reddy, K. B. (2011a). Cisplatin and TRAIL enhance breast cancer stem cell death. Int. J. Oncol. 39, 891–898. doi: 10.3892/ijo.2011.1085
Yin, S. P., Xu, L. P., and Reddy, K. B. (2011b). Triple negative breast cancer: Cisplatin plus TRAIL treatment enhance tumor and cancer stem cell death in vitro and in vivo models. Cancer Res. 71:4098.
Yoshida, G. J. (2017). Therapeutic strategies of drug repositioning targeting autophagy to induce cancer cell death: from pathophysiology to treatment. J. Hematol. Oncol. 10:67. doi: 10.1186/s13045-017-0436-9
Yuan, Y., Xue, X., Guo, R. B., Sun, X. L., and Hu, G. (2012). Resveratrol enhances the antitumor effects of temozolomide in glioblastoma via ROS-dependent AMPK-TSC-mTOR signaling pathway. CNS Neurosci. Ther. 18, 536–546. doi: 10.1111/j.1755-5949.2012.00319.x
Yue, W., Hamai, A., Tonelli, G., Bauvy, C., Nicolas, V., Tharinger, H., et al. (2013). Inhibition of the autophagic flux by salinomycin in breast cancer stem-like/progenitor cells interferes with their maintenance. Autophagy 9, 714–729. doi: 10.4161/auto.23997
Zeng, M., and Zhou, J. N. (2008). Roles of autophagy and mTOR signaling in neuronal differentiation of mouse neuroblastoma cells. Cell. Signal. 20, 659–665. doi: 10.1016/j.cellsig.2007.11.015
Zhang, D., Zhao, Q., Sun, H., Yin, L., Wu, J., Xu, J., et al. (2016). Defective autophagy leads to the suppression of stem-like features of CD271(+) osteosarcoma cells. J. Biomed. Sci. 23:82.
Zhang, S., Balch, C., Chan, M. W., Lai, H. C., Matei, D., Schilder, J. M., et al. (2008). Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 68, 4311–4320. doi: 10.1158/0008-5472.CAN-08-0364
Zhao, Y., Huang, Q., Yang, J., Lou, M., Wang, A., Dong, J., et al. (2010). Autophagy impairment inhibits differentiation of glioma stem/progenitor cells. Brain Res. 1313, 250–258. doi: 10.1016/j.brainres.2009.12.004
Zhao, Y. M., Zhao, W., Lim, Y. C., and Liu, T. Q. (2019). Salinomycin-loaded gold nanoparticles for treating cancer stem cells by Ferroptosis-induced cell death. Mol. Pharmaceut. 16, 2532–2539. doi: 10.1021/acs.molpharmaceut.9b00132
Zhou, J. B., Zhang, H., Gu, P. H., Bai, J. N., Margolick, J. B., and Zhang, Y. (2008). NF-kappa B pathway inhibitors preferentially inhibit breast cancer stem-like cells. Breast Cancer Res. Treat. 111, 419–427. doi: 10.1007/s10549-007-9798-y
Keywords: autophagy, cancer stem cell, natural products, stemness, chemoresistance
Citation: Rahman MA, Saha SK, Rahman MS, Uddin MJ, Uddin MS, Pang M-G, Rhim H and Cho S-G (2020) Molecular Insights Into Therapeutic Potential of Autophagy Modulation by Natural Products for Cancer Stem Cells. Front. Cell Dev. Biol. 8:283. doi: 10.3389/fcell.2020.00283
Received: 31 January 2020; Accepted: 02 April 2020;
Published: 24 April 2020.
Edited by:
Bernd Kaina, Johannes Gutenberg University Mainz, GermanyReviewed by:
Dileep Kumar, The University of Utah, United StatesYounghun Jung, University of Michigan, United States
Copyright © 2020 Rahman, Saha, Rahman, Uddin, Uddin, Pang, Rhim and Cho. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Md. Ataur Rahman, YXRhdXIxOTgxcmFobWFuQGhvdG1haWwuY29t; Hyewhon Rhim, aHJoaW1Aa2lzdC5yZS5rcg==; Ssang-Goo Cho, c3Nhbmdvb0Brb25rdWsuYWMua3I=
†These authors have contributed equally to this work
 Md. Ataur Rahman
Md. Ataur Rahman Subbroto Kumar Saha
Subbroto Kumar Saha Md Saidur Rahman
Md Saidur Rahman Md Jamal Uddin
Md Jamal Uddin Md. Sahab Uddin
Md. Sahab Uddin Myung-Geol Pang
Myung-Geol Pang Hyewhon Rhim1,10*
Hyewhon Rhim1,10* Ssang-Goo Cho
Ssang-Goo Cho