
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell Dev. Biol. , 05 May 2020
Sec. Stem Cell Research
Volume 8 - 2020 | https://doi.org/10.3389/fcell.2020.00258
This article is part of the Research Topic Mesenchymal Stem Cell Senescence and Rejuvenation View all 13 articles
Mesenchymal stem cells (MSCs) are multipotent cells capable of self-renewal and differentiation. There is increasing evidence of the therapeutic value of MSCs in various clinical situations, however, these cells gradually lose their regenerative potential with age, with a concomitant increase in cellular dysfunction. Stem cell aging and replicative exhaustion are considered as hallmarks of aging and functional attrition in organisms. MSCs do not proliferate infinitely but undergo only a limited number of population doublings before becoming senescent. This greatly hinders their clinical application, given that cultures must be expanded to obtain a sufficient number of cells for cell-based therapy. Here, we review the current knowledge of the phenotypic and functional characteristics of senescent MSCs, molecular mechanisms underlying MSCs aging, and strategies to rejuvenate senescent MSCs, which can broaden their range of therapeutic applications.
Mesenchymal stem cells (MSCs) were originally isolated from bone marrow (Friedenstein et al., 1968) but have since been detected in many tissues including dental pulp (Gronthos et al., 2002), adipose tissue (Zuk et al., 2002), and umbilical cord blood (Wang et al., 2004). The essential features of this heterogeneous cell population as defined by the International Society for Cellular Therapy (ISCT) in 2006 are adherence to plastic under culture conditions; expression of the cell surface markers CD44, CD90, CD105, and CD73; absence of the hematopoietic markers CD45, CD34, CD14, CD11b, CD79α, CD19, and human leukocyte antigen-DR; and multi-differentiation potential, with the capacity to generate osteoblasts, chondroblasts, and adipocytes (Dominici et al., 2006). According to the recently published ISCT position statement, although the classic set of markers still applies to in vitro-expanded MSCs, surface markers are evolving (Viswanathan et al., 2019). For example, while the definition of MSCs includes CD34 negativity, MSCs can be positive for this marker in vivo (Bellagamba et al., 2018). MSCs can differentiate into cells of ectodermal and endodermal parentage (Al-Nbaheen et al., 2013) and novel surface markers (CD165, CD276, and CD82) have been identified (Shammaa et al., 2020). Moreover, surface marker expression can change under certain culture conditions or when stimulated by a molecule (i.e., interferon-γ) (Stagg et al., 2006). Stringent functional criteria must be met for the designation of a cell as a “stem” cell (Viswanathan et al., 2019; Nolta et al., 2020). MSCs can be safely transplanted autologously or allogeneically as they have low immunogenicity, and thus have many potential applications in cell-based therapy for various disease states (Squillaro et al., 2016). To be clinically useful, MSCs must be expanded in vitro over several population doublings (PDs) to obtain a sufficient number of cells for immediate administration. The age of donors is a major factor determining the lifespan and quality of MSCs (Sethe et al., 2006; Baker et al., 2015); cells from aged donors perform less well than those from young donors because of their reduced proliferative capacity and differentiation potential. For patients with age-related diseases, allogeneic MSCs from healthy young donors are clearly preferable to autologous MSCs. On the other hand, regardless of donor age or whether the cells are autologous or allogeneic, MSCs inevitably acquire a senescent phenotype after prolonged in vitro expansion (Dimmeler and Leri, 2008; Li et al., 2017). In vivo aging refers to donor age, which affects the lifespan of MSCs; in vitro aging is the loss of stem cell characteristics by MSCs as they enter senescence during expansion in culture; and senescence is a state where cells stop dividing, which negatively affects their immunomodulatory and differentiation capacities, leading to reduced efficacy following administration (Fan et al., 2010; Turinetto et al., 2016). Thus, for MSCs to be clinically effective, it is essential to monitor senescence and understand the molecular basis of MSC aging. In this review, we discuss changes that occur in senescent MSCs, current strategies for monitoring senescence and the molecular mechanisms involved, and interventions that can potentially slow or even reverse this process.
Mesenchymal stem cells were first used therapeutically in human patients in 1995 (Galipeau and Sensebe, 2018) and has since been applied to the treatment of a broad spectrum of diseases. As of January 2020, there were 767 MSC-based trials registered at www.ClinicalTrials.gov, most of which are at an early phase (phase I or I/II) (Figure 1A). Although MSCs have been obtained from a variety of human sources, those derived from bone marrow, umbilical cord, and adipose tissue are preferred for clinical applications and account for approximately 65% of MSCs being used (Figure 1B). Due to their multi-differentiation potential and immunomodulatory and paracrine effects, MSCs have been extensively applied in various diseases (Figure 1C). Interestingly, although autologous transplantation was initially favored over allogeneic MSCs, there has been a notable increase in the use of the latter over the past decade (Figure 1D); for example, 11 out of 19 industry-sponsored phase III clinical trials of MSCs used allogeneic transplantation (Wang et al., 2016; Galipeau and Sensebe, 2018). One reason for this popularity is their low immunogenicity—that is, allogeneic MSCs can be safely transplanted without a high risk of rejection by the recipient (Wang D. et al., 2013; Lee et al., 2016). Additionally, candidate patients for cell-based therapy usually have age-related diseases. While the regenerative capacity of MSCs declines markedly with age (Kretlow et al., 2008; Yu et al., 2011), autologous transplantation is not the best option for these patients. However, robust immunologic data from clinical trials using allogeneic MSCs are still lacking. Although MSCs are considered as immunoprivileged, their transdifferentiation into other cell types—a basic property of MSCs–can increase the risk of immunogenicity (Mukonoweshuro et al., 2014; Ryan et al., 2014). Thus, there is still much to learn and optimize in terms of in vivo MSC interactions in pathologic states, which can lead to a better understanding of MSC aging and improve the long-term safety and outcome of MSC engraftment.
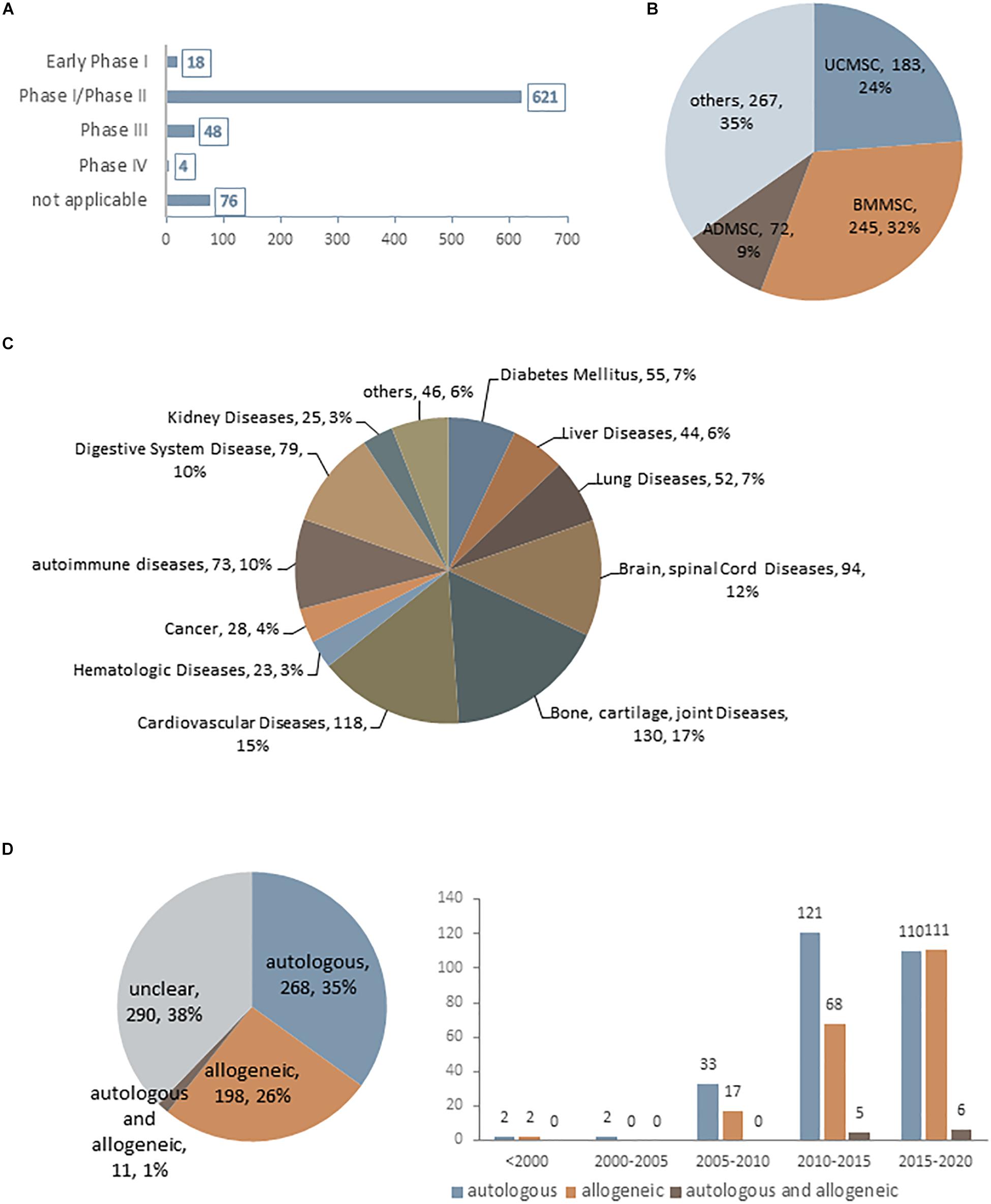
Figure 1. Current statistical data for MSC-based clinical trials as of January 2020 (data accessed from ClinicalTrials.gov ∼2020.1). (A–D) Statistics for MSC-based clinical trials in different phases (A), using difference cell sources (B), in different disease states (C), and using autologous or allogeneic transplantation (D).
Irrespective of their source, MSCs enter a state of replicative senescence (i.e., in vitro aging, also known as the Hayflick limit) after repeated serial passage in culture when the cells stop dividing after a certain number of PDs (Hayflick and Moorhead, 1961). The maximum number of PDs that can be achieved by MSCs is estimated to be 30 to 40 (Banfi et al., 2002; Baxter et al., 2004). No clear information on passage number has been provided for the 15 MSC products approved to date for clinical use (Table 1). However, given that the differentiation potential of MSCs decreases after extended passages, low-passage cultures are recommended for clinical-scale expansion of cultures (Lechanteur et al., 2016). In the following sections, we discuss heterogeneity and biological and functional changes in MSC senescence (Figure 2).
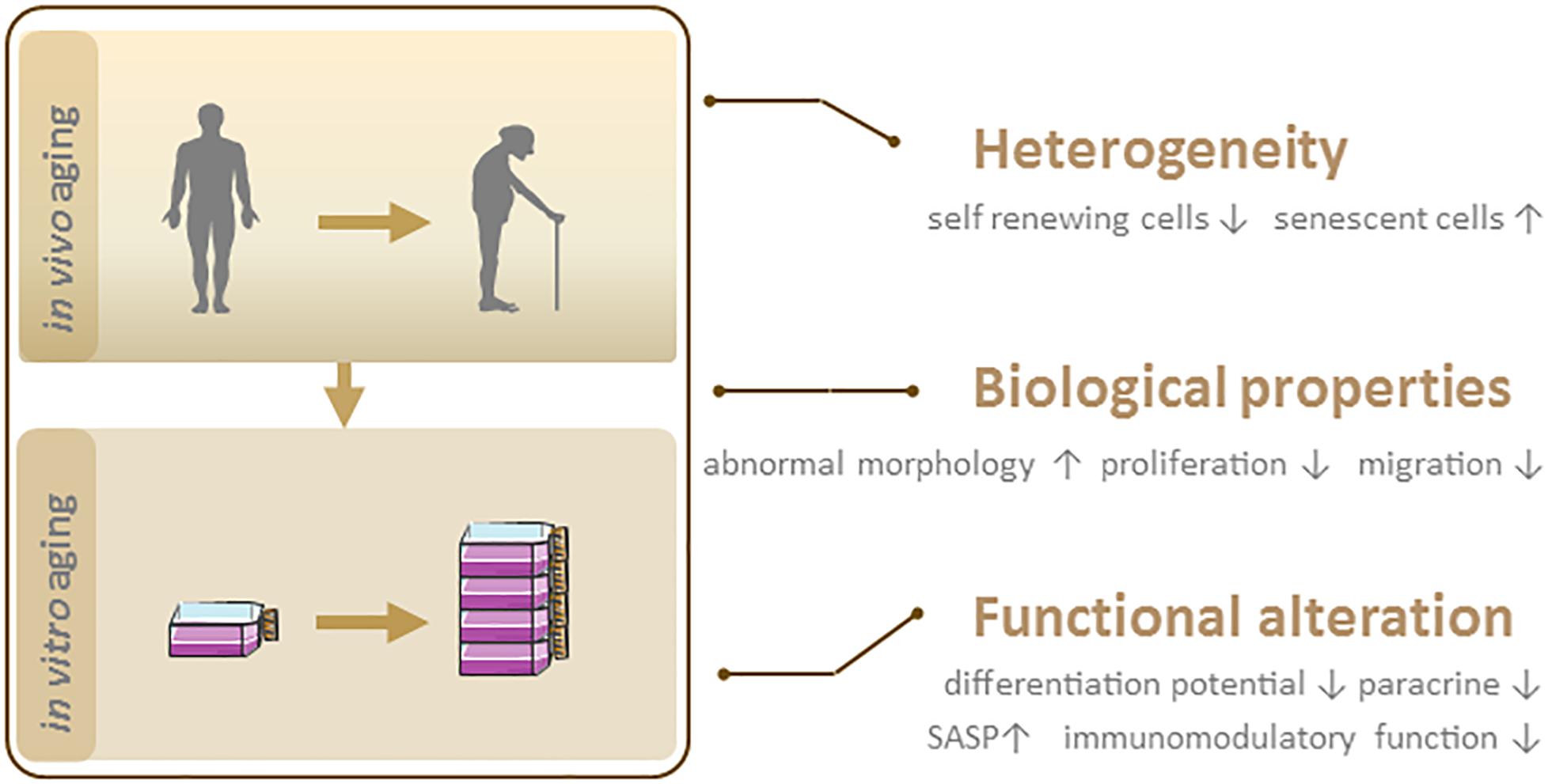
Figure 2. Phenotypic features of senescent MSCs. In vivo and in vitro aging lead to MSC senescence, which is characterized by heterogeneity, biological and functional changes.
Despite their global features as defined by the ICST, MSCs are complex cell populations that exhibit heterogeneity depending on the donor, tissue source, and whether they are clonal populations or single cells (Phinney, 2012). MSC heterogeneity comprises proliferation rate, morphology, immunophenotype, multilineage differentiation potential, and senescence (Schellenberg et al., 2012). In symmetric cell division, a self-renewing parent cell divides into two daughter cells with comparable shape and differentiation potential. In contrast, asymmetric cell division yields a self-renewing cell and a non-dividing cell that becomes senescent in culture. These dynamics result in an initially dominant cell population being overtaken by other clonal populations after multiple passages (Figure 3). Heterogeneity in the proliferation potential of cultured MSCs manifests morphologically as subpopulations of small, round, rapidly proliferating cells and slowly dividing, large flattened cells (Mets and Verdonk, 1981; Colter et al., 2001). Using the limiting dilution assay at later passages, it was determined that not every cell is capable of clonal expansion and colony formation at the time of culture establishment (Schellenberg et al., 2012). More importantly, the number of colony-forming unit (CFU) fibroblasts decreased continuously during culture expansion, and were scarcely detected after >20 passages (Schellenberg et al., 2012). Likewise, clonal analysis of single-cell–derived colonies has suggested that not every cell has trilineage (i.e., osteogenic, adipogenic, and chondrogenic) potential (Wagner et al., 2008), and subsets with high differentiation potential rapidly decline in number after a few passages (Schellenberg et al., 2012). The age-related heterogeneity of MSCs is thought to be associated with epigenetic status; subpopulations with variable expression of stem cell antigen (Sca)-1 regained the Sca-1 profile of the parent cell after 4–8 days of culture, which was accompanied by epigenetic changes at the lymphocyte antigen 6 complex promoter (Hamidouche et al., 2017). Given the relevance to clinical efficacy, molecular markers of aging in cultured MSCs are needed to reflect senescence-associated alterations. The current understanding of aging-related cellular changes is based primarily on homogeneous bulk cell-derived data; emerging tools for single-cell analysis can help to define the heterogeneity of MSCs.
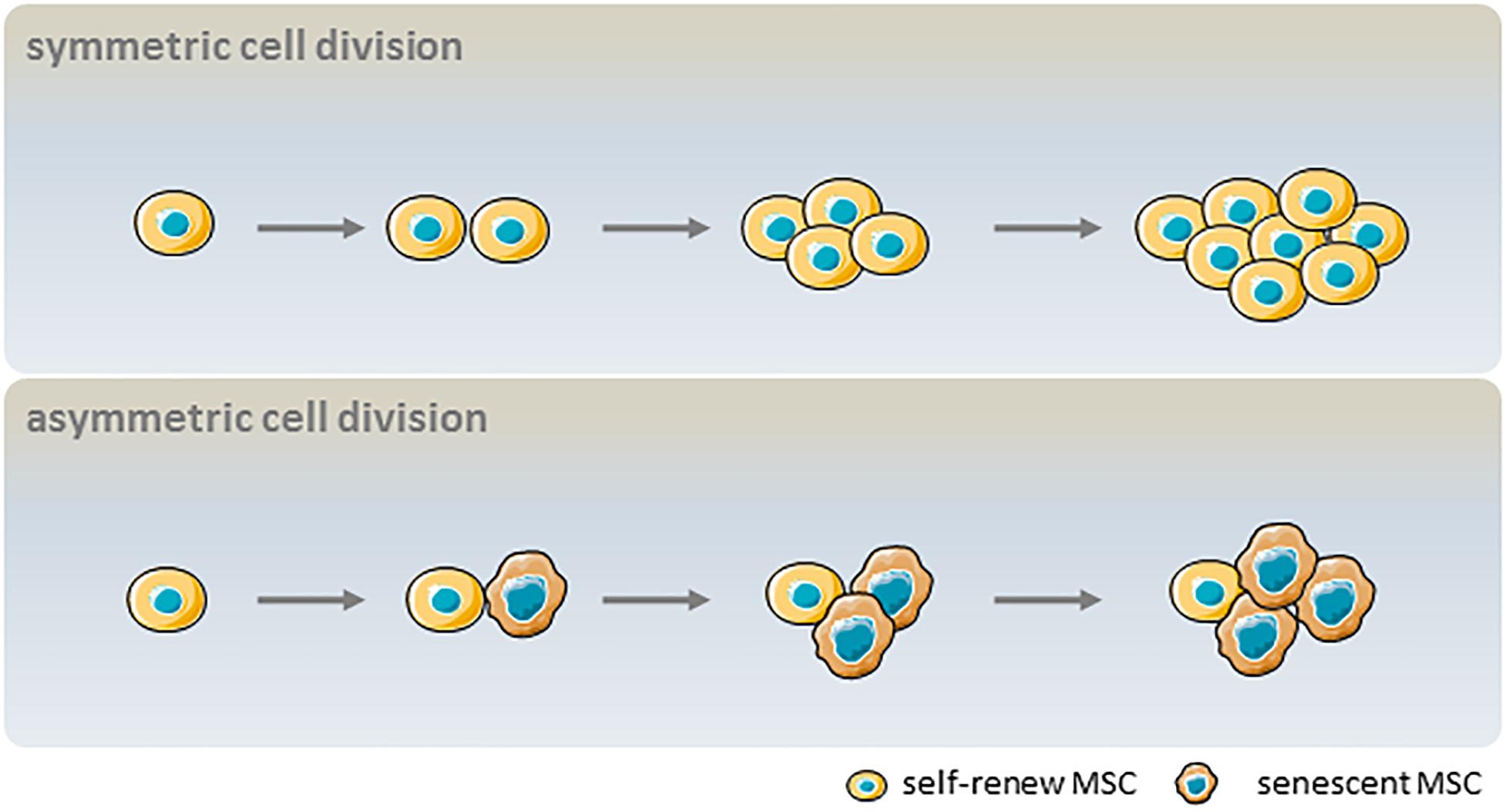
Figure 3. Influence of asymmetric cell division kinetics on the heterogeneity of MSC senescence. In symmetric cell division, a parent MSC with self-renewal capacity divides into two daughter MSCs that can also self-renew. In asymmetric cell division, a parent MSC with self-renewal capacity divides into one self-renewing and one non-dividing cell that becomes senescent in culture.
As MSC populations with a large proportion of senescent cells are less effective when transplanted, it is critical to detect senescent MSCs during expansion. Surface marker profiling is one approach for identifying and purifying senescent cells from a culture. For example, the expression of CD146—also known as melanoma cell adhesion molecule (MCAM)–was downregulated in MSCs derived from aged donors compared to those from young donors, as well as in MSCs after prolonged in vitro expansion (Gnani et al., 2019). Furthermore, low but not high CD146 expression was associated with a senescent phenotype in MSCs (Jin et al., 2016), and CD146+ MSCs showed increased migratory potential toward degenerating tissues (Wangler et al., 2019). CD264 is another surface marker of in vitro aging in MSCs that is unrelated to the chronologic age of the donor (Madsen et al., 2017); cells expressing this protein exhibit increased senescence-associated β-galactosidase (SA-β-gal) activity and reduced differentiation potential and colony-forming efficiency compared to CD264– MSCs (Madsen et al., 2020). Other surface markers that show altered expression with in vitro/in vivo aging are summarized in Table 2. It should be noted that although these molecules are expressed to varying degrees during the aging process and may be associated with functional changes, there is presently no consensus on whether they can serve as the gold standard for selective purification of young vs. old MSC populations.
Early-passage MSCs are small and have a fibroblast-like spindle shape but acquire a hypertrophic and flat morphology with more podia and actin stress fibers upon extended culture (Stenderup et al., 2003; Mauney et al., 2004; Stolzing and Scutt, 2006). MSCs from passages 1 to 3 have a uniform size but begin to enlarge at passage 5, such that cells at passages 6–9 are 4.8-fold larger than passage 1 cells (Oja et al., 2018). Morphologic features can predict how well cells can adapt to a given condition. Cell and nuclear morphology in MSCs in the first 3 days of osteogenic induction was found to be closely correlated with their long-term (35-day) mineralization capacity (Marklein et al., 2016). A subset of aged MSCs with a small cell size had ATP levels equivalent to those in young MSCs, whereas levels in large-sized cells were comparable to those in the aged parent MSC population (Block et al., 2017). Increased cell size and granularity were positively correlated with MSC autofluorescence; the latter has therefore been proposed as a non-invasive, real-time quantifiable marker for cellular senescence (Bertolo et al., 2019).
Animal cells sense and correct deviations in size by adjusting cell cycle length as well as growth rate (Miettinen et al., 2014), which is increased in small cells and reduced in large cells (Ginzberg et al., 2018). Loss of cell size uniformity can indicate abnormal biosynthesis or cell cycle progression. While the regulation of size homeostasis in relation to senescence is not fully understood, cell enlargement can distinguish senescent subpopulations in culture. Among aged MSCs, SA-β-gal activity is increased in large as compared to small-sized cells (Block et al., 2017). Notably, MSCs immortalized by SV40 (Negishi et al., 2000) or telomerase transfection (Kobune et al., 2003) are significantly smaller than their parent cells.
The enlargement of aging cells and their transformation to a hypertrophic morphology is accompanied by biological changes. A decline in proliferative capacity was reported in MSCs derived from old patients as compared to their healthy young counterparts (Banfi et al., 2002) and in long-term MSC cultures regardless of the cell source. MSCs from young donors had greater mitotic activity (41 ± 10 vs. 24 ± 11 PDs), slower progression to senescence, and an increased rate of proliferation (0.09 ± 0.02 vs. 0.05 ± 0.02 PDs/day) than those from old donors (Stenderup et al., 2003). CFU is a retrospective parameter describing the clonogenic potential of a single cell; decreases in CFU and average colony size are correlated with MSC aging in vitro (Liu et al., 2004).
In addition to impaired proliferation, aging negatively affects MSC migration and homing ability (Liu et al., 2017). Directed migration toward stimuli by MSCs is critical for better functional outcomes in cell-based therapy. Impaired migratory capacity in senescent MSCs in response to pro-inflammatory signals was found to be closely associated with activator protein (AP)-1 pathway inhibition (Sepulveda et al., 2014). Cell migration involves the reorganization of the actin cytoskeleton (Le Clainche and Carlier, 2008). MSCs derived from old donors exhibit reduced response to biological and mechanical signals because their actin cytoskeleton is less dynamic (Kasper et al., 2009). Gene expression profiling has identified several cytokines and chemokines and their receptors important for cell migration–including stromal cell-derived factor 1 (SDF-1) and its receptor chemokine receptor type 4 (CXCR4), tumor necrosis factor receptor (TNFR), IFN-γ receptor (IFNGR), and C-C motif chemokine receptor 7 (CCR7)—that are downregulated in aged MSCs as compared to younger cells (Geissler et al., 2012; Bustos et al., 2014).
A basic strategy for MSC-based regeneration is to replace cells that are lost or impaired by disease with functional cells. It was previously thought that MSCs exert their therapeutic effect through trans-differentiation. During in vitro culture, MSCs progressively lose their capacity to differentiate into adipogenic and osteogenic lineages although the preferred fate is debated, with some studies suggesting that aging shifts the balance in favor of adipocytes at the expense of osteoblastogenesis (Stolzing et al., 2008), and others reporting that osteogenic activity is preserved or even increased in late passages (Wagner et al., 2008) or that both osteogenic and adipogenic potential is lost (Geissler et al., 2012). Age-associated changes in differentiation potential may be related to altered susceptibility to reactive oxidative species (ROS) and apoptosis (Bruedigam et al., 2010); MSCs undergoing osteoblast differentiation showed dose-dependent increases in apoptosis and ROS accumulation upon treatment with rosiglitazone, whereas adipogenesis was unaffected (Jiang et al., 2008). Lineage bias in differentiation is regulated by key signaling pathways, intracellular oxidative stress, and transcriptional and post-transcriptional mechanisms. Gene expression analysis has revealed an age-related downregulation of osteoblast transcription factors such as core binding factor α1 (CBFA1), runt-related transcription factor 2 (Runx2), and distal-less homeobox 5 (DIx5) as well as collagen and osteocalcin, and upregulation of adipogenic factors such as peroxisome proliferator-activated receptor-γ (PPAR-γ) and adipocyte fatty acid-binding protein (aP2) (Jiang et al., 2008). The target genes activated by PPAR-γ are related to lipid metabolism and adipocyte differentiation. Thus, the age-related increase in PPAR-γ expression shifts the fate of MSCs toward adipogenesis, and Wnt/β-catenin signaling regulates MSC differentiation by suppressing PPAR-γ and biasing differentiation toward osteoblastogenesis (Xu et al., 2016).
Aging cells acquire a senescence-associated secretory phenotype (SASP) involving the secretion of proteins that can affect the behavior of neighboring cells via autocrine/paracrine mechanisms (Borodkina et al., 2018; Campisi et al., 2019). MSCs have potent anti-inflammatory and immunosuppressive functions and thus have therapeutic potential for inflammation-related diseases. Aged MSCs have a diminished capacity for inhibiting the proliferation of allogeneic peripheral blood mononuclear cells compared to younger cells (Gnani et al., 2019). The activation of SASP factors such as interleukin 6 (IL-6), IL-8, and monocyte chemotactic protein 1 (MCP1) in the conditioned medium of aged MSCs was shown to be increased compared to young MSC cultures at early passages, an effect that was exacerbated at late passages (Gnani et al., 2019). Secretion of SASP-related chemokines/cytokines not only drives responses that reinforce senescence in a cell-autonomous manner but also acts on neighboring cells via a paracrine mechanism to accelerate senescence. For example, factors secreted by aged MSCs were shown to activate pro-inflammatory gene expression in young hematopoietic stem cells and decreased their clonogenic potential (Gnani et al., 2019).
The beneficial effects of MSC-based therapy are attributable to the action of pro-angiogenic paracrine factors. In aged MSCs, the secretion of these factors—including vascular endothelial growth factor (VEGF), placental growth factor (PGF), and hepatic growth factor (HGF)—is reduced, whereas that of anti-angiogenic factors such as thrombospondin-1 (TBS1) and plasminogen activator inhibitor-1 (PAI-1) is increased. Thus, age negatively affects angiogenesis and directly undermines the therapeutic efficacy of MSCs (Efimenko et al., 2011; Khan et al., 2011).
β-D-Galactosidase (β-Gal) is a eukaryotic hydrolase localized in the lysosome that is active at the optimal pH (6.0) in senescent cells but is absent in proliferating cells (Dimri et al., 1995). SA-β-gal activity is suggested as the gold standard for evaluating senescence in cells and can be detected by cytochemistry/histochemistry and fluorescence-based methods. However, when used in combination with other markers, it can yield false-positive/negative results in quiescent cells or upon stress (de Magalhaes et al., 2004; Yang and Hu, 2005). Senescence-associated lysosomal α-L-fucosidase (SA-α-Fuc) has recently been identified as a more robust biomarker in all types of cellular senescence (Hildebrand et al., 2013; Singh and Piekorz, 2013), but there is still limited evidence for its sensitivity and specificity in distinguishing senescent MSCs.
Telomeres are specialized nucleoprotein caps containing repetitive nucleotide sequences that protect chromosomes from end-to-end fusion and prevent the loss of genetic information during DNA replication (Sahin and Depinho, 2010). Telomeres shorten with every cell division and senescence is triggered when they reach a critical length (Baird et al., 2003). As such, telomere length has been used to estimate replicative history and predict senescence in MSCs (Montpetit et al., 2014). There is increasing evidence of an association between diminished proliferative capacity and telomere shortening in MSCs. However, the exact telomere length in senescent MSCs and whether it differs according to cell source, culture conditions, and measurement method is unclear. For example, a telomere length of 10 kb was proposed as a threshold for senescence (Baxter et al., 2004), although another study reported a length of 6.8 ± 0.6 kb in senescent cells (Oja et al., 2018). In addition, a recent study described a mechanism of senescence that is independent of cell division and telomere length, involving activation of classical senescence-associated pathways and yielding a non-canonical SASP (Anderson et al., 2019). Although this phenomenon was first reported in post-mitotic cardiomyocytes, it may also occur in MSCs. Thus, telomere shortening has limitations for the measurement of senescence, and other markers may be more informative under certain conditions.
The senescent state is characterized by cell cycle arrest. Senescence-associated growth arrest is maintained by the activation of several pathways including phosphorylated inhibitor of cyclin-dependent kinase 4A (p16INK4A)/phosphorylated retinoblastoma (pRb) and p53/p21WAF1 signaling (Campisi, 2005). p16INK4A is an inhibitor of cyclin-dependent kinase (CDK) and induces premature cell senescence via telomere-dependent and -independent mechanisms (Serrano et al., 1993). p16INK4A level was shown to increase with chronological age or PDs of MSCs in culture, and a large proportion of the p16INK4A-positive cells were negative for the proliferation marker Ki67 and positive for SA-β-gal. Inhibiting p16INK4A reduced the number of senescent MSCs and conferred cells with the ability to proliferate (Shibata et al., 2007). Similarly, overexpressing p21WAF1—a CDK inhibitor that acts by dephosphorylating pRb–increases cellular senescence, as evidenced by elevated SA-β-gal activity and telomere shortening (Huang et al., 2004), while inhibiting p21WAF1 in senescent cells restored their replicative capacity. However, p21WAF1 depletion was less efficient at preventing senescence than p53 depletion, suggesting that the latter acts through p21WAF1-independent mechanisms to exert this effect (Gire and Dulic, 2015).
Mesenchymal stem cells-derived microvesicles (MSC-MVs) that mimic the senescent state of the parent MSC have recently emerged as a potential cell-free biomarker for cellular senescence. Senescent late-passage MSCs secrete larger amounts of MSC-MVs of smaller size than those in early passages, and CD105 expression in MSC-MVs decreased with senescence in parent MSCs. RNA sequencing results suggest that most genes that are highly expressed in senescent MSC-MVs are involved in aging-related diseases (Lei et al., 2017). Functionally, senescent MSC-MVs have a lower capacity to promote osteogenesis (Lei et al., 2017) and recruit macrophages and fail to alter macrophage phenotypes (Huang et al., 2019).
Besides the abovementioned markers, new tools and approaches have been proposed to monitor MSC aging such as SiR-actin, a fluorogenic F-actin specific probe that can be used to evaluate actin turnover (Mishra et al., 2019). CyBC9 (another fluorescent probe) combined with high-throughput screening revealed accumulation of mitochondria in senescent MSCs that presumably resulted from the loss of membrane potential (Ang et al., 2019). Thus, senescence can be characterized not by a universal biomarker, but by a set of non-exclusive markers in conjunction with specific biological features.
Senescence is a multistep process involving various mechanisms that have not been fully elucidated. One of these is irreversible cell cycle arrest—typically in response to DNA damage—in the presence of growth-promoting stimuli. DNA damage accumulates throughout the lifetime of an organism as a result of DNA replication errors and exposure to endogenous and exogenous mutagens. The DNA damage response (DDR) network can sense and initiate repair of mutations and thereby slow their accumulation. Activation of the DDR network can transiently halt cell cycle progression through stabilization of p53 and transcriptional activation of the CDK inhibitor p21. However, if DNA damage persists, p16INK4A is activated via p38-mitogen-activated protein kinase-mediated mitochondrial dysfunction and ROS production. This results in CDK inhibition and activation of the tumor suppressor Rb1, which induces the onset of senescence (Figure 4; Zhang et al., 2007; Ciccia and Elledge, 2010).
Reactive oxidative species are a group of oxygen-containing small molecules. Approximately 90% of ROS are generated endogenously by the mitochondrial electron transport machinery (Poyton et al., 2009) but extrinsic factors such as radiation, ultraviolet light, hypoxia, and low temperature can also increase their production. As a metabolic by-product, ROS induce oxidation and various cellular responses through the generation of reactive secondary metabolites. While physiologic levels of ROS are necessary for proliferation and differentiation, an excess can trigger cellular senescence, including in MSCs (Sart et al., 2015). ROS induce DNA damage and accelerates telomere erosion, both of which activate the DDR. Senescent MSCs have elevated levels of ROS compared to normal cells that cause persistent DDR activation, thereby forming a positive feedback loop in the progression of senescence (Figure 4; Yang et al., 2015).
Reactive oxidative species accumulate with advancing age, leading to decreased mitochondrial metabolism. Mitochondrial DNA (mtDNA) encodes 13 polypeptides of the mitochondrial oxidative phosphorylation (OXPHOS) enzyme complexes, 22 tRNAs for mitochondrial biosynthesis, and 16S and 18S rRNA for mitochondria. Because of the absence of efficient repair mechanisms, the mutation rate of mtDNA is higher than that of nuclear DNA. A point mutation in mitochondria-encoded subunit 3 of cytochrome c oxidase was found to be associated with enhanced tissue degeneration and risk of premature aging (Niemann et al., 2017). The mitochondrial free radical theory of aging posits that age-dependent accumulation of mtDNA abnormalities, impaired OXPHOS, and altered expression of antioxidant enzymes lead to increased ROS production, which, in turn, results in progressive mitochondrial dysfunction and global cellular damage in a positive feedback loop (Figure 4; Wang C. H. et al., 2013). However, other studies suggest that mtDNA mutations and mitochondrial dysfunction affect aging independently of ROS production, based on the unexpected observation that genetic manipulations that increased mitochondrial ROS levels and oxidative damage did not accelerate aging in mice (Zhang et al., 2009), whereas those that impaired mitochondrial function without increasing ROS enhanced aging (Kujoth et al., 2005).
Autophagy is a critical process for maintaining cellular homeostasis under physiologic and pathologic conditions. By removing damaged cellular components including proteins and mitochondria, autophagy prevents age-related cellular injury (Wirawan et al., 2012) and allows stem cells to avoid the transformation from a reversible quiescent (G0) state to irreversible senescence (Garcia-Prat et al., 2016). Although ROS are known to stimulate autophagy, the age-related increase in ROS levels reduces autophagic capacity (Yamamoto et al., 2016). In fact, aged MSCs exhibit reduced autophagy, which is correlated with diminished self-renewal capacity and regenerative potential and replicative exhaustion. From a mechanistic standpoint, decreased autophagy results in the loss of proteostasis and increases mitochondrial activity, oxidative stress, and metabolism state in MSCs (Figure 4; Revuelta and Matheu, 2017).
Key aspects of the mechanisms of MSC aging remain unknown, but epigenetic changes (i.e., those occurring in the absence of DNA sequence alterations) such as DNA methylation, histone modification, and chromatin remodeling may play a role (Ozkul and Galderisi, 2016; Cakouros and Gronthos, 2019). For example, dysregulated expression of Brahma-related gene 1 (BRG1), a component of ATP-dependent chromatin remodeling complexes, was associated with senescence in MSCs via regulation of NANOG methylation status (Squillaro et al., 2015). Additionally, microenvironmental and hormonal conditions are important factors contributing to MSC aging in vivo. As MSCs exist in a semi-static state, replicative exhaustion is unlikely to occur (Ganguly et al., 2017). There is increasing evidence that the in vivo cellular aging process is caused by chronologic aging of the host and is accelerated by conditions such as obesity and systemic inflammation (Frasca et al., 2017; Franceschi et al., 2018).
Strategies allowing the generation of large numbers of MSCs that have retained their stemness are needed for clinical applications. Here, we summarize current research efforts to prevent MSC senescence.
Induced pluripotent stem cell (iPSC)-derived MSCs (iMSCs) can be passaged more than 40 times without exhibiting features of senescence (Sabapathy and Kumar, 2016). iMSCs retain a donor-specific DNA methylation profile while tissue-specific, senescence-associated, and age-related patterns are erased during reprogramming (Frobel et al., 2014). Recent studies have demonstrated that iMSCs have superior regenerative capacity compared to tissue-derived MSCs in preclinical degenerative disease models (Lian et al., 2010; Chen et al., 2019; Wang et al., 2019). However, the generation of iMSCs from iPSCs requires a significant degree of molecular manipulation, and there are safety concerns regarding the self-renewal and pluripotency of iPSC-derived cells after in vivo transplantation, which have the risk of tumorigenicity and genomic instability (Hynes et al., 2013). In addition, each independent iPSC line has a unique genetic and epigenetic profile that must be characterized. The concept of iMSCs is at its infancy and requires validation from preclinical and clinical studies before it can be clinically useful.
Aging is not a passive or random process but can be modulated through several key signaling molecules/pathways (Kenyon, 2010). Identification of age-related coordinating centers can provide novel targets for therapeutic interventions. Sirtuins (SIRTs) are a class of highly conserved nicotinamide adenine dinucleotide-dependent protein deacylases of which there are 7 (SIRT1–7) in mammals (Vassilopoulos et al., 2011). The role of SIRTs in aging is related to their regulation of energy metabolism, cell death, and circadian rhythm and maintenance of cellular and mitochondrial protein homeostasis (O’Callaghan and Vassilopoulos, 2017). Mitochondrial SIRTs (SIRT3–5) act as stress sensors and regulate protein networks to coordinate the stress response (van de Ven et al., 2017). Overexpression of SIRTs has been investigated as a potential strategy for preventing MSC aging. For instance, SIRT3 expression in MSCs decreased with prolonged culture and its overexpression in later-passage cells restored differentiation capacity and reduced aging-related senescence (Denu, 2017). SIRT1 is required for long-term growth of MSCs and SIRT1 overexpression was shown to delay senescence without loss of adipogenic or osteogenic potential (Yuan et al., 2012). Additionally, SIRT1 expression was shown to be spontaneously upregulated upon osteogenic differentiation and protected MSCs from extracellular oxidative stress (Li et al., 2018).
Genetic engineering has been used to slow MSC aging. Besides SIRTs, several molecules have been identified as potential targets for interventions to prevent senescence (Table 3). Ectopic expression of telomerase reverse transcriptase in MSCs extended their replicative lifespan, which preserved a normal karyotype, promoted telomere elongation, and abolished senescence without loss of differentiation potential (Simonsen et al., 2002). Introduction of Erb-B2 receptor tyrosine kinase 4 (ERBB4) in aged MSCs conferred resistance to oxidative stress-induced cell death and rescued the senescence phenotype (Liang et al., 2019). Knocking down macrophage migration inhibitory factor (MIF) in young MSCs induced senescence; conversely, its overexpression in aged MSCs rejuvenated the cells by activating autophagy (Zhang et al., 2019). However, the risk of malignant transformation remains a major barrier for the use of genetics-based approaches in clinical practice.
Pharmacologic approaches and cytokine supplementation have also shown promise for delaying senescence (Table 3). Inhibiting mechanistic target of rapamycin by rapamycin treatment enhanced autophagy and myogenic differentiation in aged stem cells (Takayama et al., 2017), while pretreatment with MIF rejuvenated MSCs in a state of age-induced senescence by interacting with CD74 and thereby activating 5′AMP-activated protein kinase-Forkhead box O3a signaling (Xia et al., 2015). Pharmacologic antagonism of lysophosphatidic acid, a ubiquitous metabolite in membrane phospholipid synthesis, extended the lifespan of MSCs in culture and increased their clonogenic potential while preserving their capacity for both osteogenic and adipogenic differentiation (Kanehira et al., 2012). However, as the effects of these approaches can vary–for instance, fibroblast growth factor supplementation resulted in the loss of osteogenic/adipocytic differentiation potential in long-term cultures (Gharibi and Hughes, 2012)–further research is needed to evaluate their long-term safety and efficacy.
Mesenchymal stem cells senescence both in vivo and in vitro can affect MSC characteristics, which has important clinical and safety implications. MSCs must be expanded for several PDs to meet clinical dose requirements, but cellular aging significantly hinders the generation of sufficient numbers of cells. In this review, we discussed the properties of senescent MSCs and the functional changes and cellular mechanisms involved, and highlighted potential rejuvenation strategies. However, the current knowledge of senescence is mainly based on bulk-cell data. Recent technical advances such as single-cell RNA sequencing, extended time-lapse in vivo imaging, and genetic lineage tracing will provide a more complete understanding of the MSC aging process, making it possible to slow senescence or even rejuvenate aged MSCs. Additionally, bioinformatics-based analyses of the genome–environment interactions involved in aging can provide potential drug targets for senescence intervention. Given that functional attrition and reduced regenerative potential in stem cells are an important aspect of aging in organisms, MSC rejuvenation holds considerable promise for broadening the applications of MSC-based therapy.
Mesenchymal stem cells-based therapy has several limitations, including the invasive process of collecting the cells and their inherent immunogenicity, as well as the large numbers required to achieve a clinically relevant effect (Berebichez-Fridman and Montero-Olvera, 2018). An increasing number of preclinical trials have reported therapeutic effects exerted by MSC-MVs via paracrine mechanisms in several disease models (Fujita et al., 2018). Whether MSC-MVs are senescent and how senescent MSC-MVs can be identified are outstanding issues to be addressed in future studies.
JL and YD searched the literature and drafted part of the manuscript. XL and ZL designed the whole study and revised the manuscript.
This research was supported in part by the National Natural Science Grant of China (No. 81500207 to XL), the Science and Technology Commission of Shanghai Municipality (Grant No. 17431906600), the National Key Research and Development Program of China (2017YFA0105600), and the Major Program of Development Fund for Shanghai Zhangjiang National Innovtaion Demonstration Zone (Stem Cell Strategic Biobank and Stem Cell Clinical Technology Transformation Platform) (ZJ2018-ZD-004).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Al-Nbaheen, M., Vishnubalaji, R., Ali, D., Bouslimi, A., Al-Jassir, F., Megges, M., et al. (2013). Human stromal (mesenchymal) stem cells from bone marrow, adipose tissue and skin exhibit differences in molecular phenotype and differentiation potential. Stem Cell Rev. Rep. 9, 32–43. doi: 10.1007/s12015-012-9365-8
Anderson, R., Lagnado, A., Maggiorani, D., Walaszczyk, A., Dookun, E., Chapman, J., et al. (2019). Length-independent telomere damage drives post-mitotic cardiomyocyte senescence. EMBO J. 38:e100492. doi: 10.15252/embj.2018100492
Ang, J., Lee, Y. A., Raghothaman, D., Jayaraman, P., Teo, K. L., Khan, F. J., et al. (2019). Rapid Detection of senescent mesenchymal stromal cells by a fluorescent probe. Biotechnol. J. 14:e1800691. doi: 10.1002/biot.201800691
Baird, D. M., Rowson, J., Wynford-Thomas, D., and Kipling, D. (2003). Extensive allelic variation and ultrashort telomeres in senescent human cells. Nat. Genet. 33, 203–207. doi: 10.1038/ng1084
Baker, N., Boyette, L. B., and Tuan, R. S. (2015). Characterization of bone marrow-derived mesenchymal stem cells in aging. Bone 70, 37–47. doi: 10.1016/j.bone.2014.10.014
Banfi, A., Bianchi, G., Notaro, R., Luzzatto, L., Cancedda, R., and Quarto, R. (2002). Replicative aging and gene expression in long-term cultures of human bone marrow stromal cells. Tissue Eng. 8, 901–910. doi: 10.1089/107632702320934001
Baxter, M. A., Wynn, R. F., Jowitt, S. N., Wraith, J. E., Fairbairn, L. J., and Bellantuono, I. (2004). Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells 22, 675–682. doi: 10.1634/stemcells.22-5-675
Bellagamba, B. C., Grudzinski, P. B., Ely, P. B., Nader, P. J. H., Nardi, N. B., and da Silva Meirelles, L. (2018). Induction of expression of CD271 and CD34 in mesenchymal stromal cells cultured as spheroids. Stem Cells Int. 2018:7357213. doi: 10.1155/2018/7357213
Berebichez-Fridman, R., and Montero-Olvera, P. R. (2018). Sources and clinical applications of mesenchymal stem cells: state-of-the-art review. Sultan Qaboos Univ. Med. J. 18, e264–e277. doi: 10.18295/squmj.2018.18.03.002
Bertolo, A., Baur, M., Guerrero, J., Potzel, T., and Stoyanov, J. (2019). Autofluorescence is a reliable in vitro marker of cellular senescence in human mesenchymal stromal cells. Sci. Rep. 9:2074. doi: 10.1038/s41598-019-38546-2
Block, T. J., Marinkovic, M., Tran, O. N., Gonzalez, A. O., Marshall, A., Dean, D. D., et al. (2017). Restoring the quantity and quality of elderly human mesenchymal stem cells for autologous cell-based therapies. Stem Cell Res. Ther. 8:239. doi: 10.1186/s13287-017-0688-x
Borodkina, A. V., Deryabin, P. I., Giukova, A. A., and Nikolsky, N. N. (2018). “Social life” of senescent cells: what is SASP and why study it? Acta Nat. 10, 4–14.
Bruedigam, C., Eijken, M., Koedam, M., van de Peppel, J., Drabek, K., Chiba, H., et al. (2010). A new concept underlying stem cell lineage skewing that explains the detrimental effects of thiazolidinediones on bone. Stem Cells 28, 916–927. doi: 10.1002/stem.405
Bustos, M. L., Huleihel, L., Kapetanaki, M. G., Lino-Cardenas, C. L., Mroz, L., Ellis, B. M., et al. (2014). Aging mesenchymal stem cells fail to protect because of impaired migration and antiinflammatory response. Am. J. Respir. Crit. Care Med. 189, 787–798. doi: 10.1164/rccm.201306-1043OC
Cakouros, D., and Gronthos, S. (2019). Epigenetic regulation of bone marrow stem cell aging: revealing epigenetic signatures associated with hematopoietic and mesenchymal stem cell aging. Aging Dis. 10, 174–189. doi: 10.14336/AD.2017.1213
Campisi, J. (2005). Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120, 513–522. doi: 10.1016/j.cell.2005.02.003
Campisi, J., Kapahi, P., Lithgow, G. J., Melov, S., Newman, J. C., and Verdin, E. (2019). From discoveries in ageing research to therapeutics for healthy ageing. Nature 571, 183–192. doi: 10.1038/s41586-019-1365-2
Chen, Z., Han, X., Ouyang, X., Fang, J., Huang, X., and Wei, H. (2019). Transplantation of induced pluripotent stem cell-derived mesenchymal stem cells improved erectile dysfunction induced by cavernous nerve injury. Theranostics 9, 6354–6368. doi: 10.7150/thno.34008
Ciccia, A., and Elledge, S. J. (2010). The DNA damage response: making it safe to play with knives. Mol. Cell 40, 179–204. doi: 10.1016/j.molcel.2010.09.019
Colter, D. C., Sekiya, I., and Prockop, D. J. (2001). Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc. Natl. Acad. Sci. U.S.A. 98, 7841–7845. doi: 10.1073/pnas.141221698
de Magalhaes, J. P., Migeot, V., Mainfroid, V., de Longueville, F., Remacle, J., and Toussaint, O. (2004). No increase in senescence-associated beta-galactosidase activity in Werner syndrome fibroblasts after exposure to H2O2. Ann. N. Y. Acad. Sci. 1019, 375–378. doi: 10.1196/annals.1297.066
Denu, R. A. (2017). SIRT3 enhances mesenchymal stem cell longevity and differentiation. Oxid. Med. Cell Longev. 2017:5841716. doi: 10.1155/2017/5841716
Dimmeler, S., and Leri, A. (2008). Aging and disease as modifiers of efficacy of cell therapy. Circ. Res. 102, 1319–1330. doi: 10.1161/CIRCRESAHA.108.175943
Dimri, G. P., Lee, X., Basile, G., Acosta, M., Scott, G., Roskelley, C., et al. (1995). A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. U.S.A. 92, 9363–9367. doi: 10.1073/pnas.92.20.9363
Dominici, M., Le Blanc, K., Mueller, I., Slaper-Cortenbach, I., Marini, F., Krause, D., et al. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 8, 315–317. doi: 10.1080/14653240600855905
Efimenko, A., Starostina, E., Kalinina, N., and Stolzing, A. (2011). Angiogenic properties of aged adipose derived mesenchymal stem cells after hypoxic conditioning. J. Transl. Med. 9:10. doi: 10.1186/1479-5876-9-10
Fan, M., Chen, W., Liu, W., Du, G. Q., Jiang, S. L., Tian, W. C., et al. (2010). The effect of age on the efficacy of human mesenchymal stem cell transplantation after a myocardial infarction. Rejuvenation Res. 13, 429–438. doi: 10.1089/rej.2009.0986
Franceschi, C., Garagnani, P., Parini, P., Giuliani, C., and Santoro, A. (2018). Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 14, 576–590. doi: 10.1038/s41574-018-0059-4
Frasca, D., Blomberg, B. B., and Paganelli, R. (2017). Aging, obesity, and inflammatory age-related diseases. Front. Immunol. 8:1745. doi: 10.3389/fimmu.2017.01745
Friedenstein, A. J., Petrakova, K. V., Kurolesova, A. I., and Frolova, G. P. (1968). Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 6, 230–247.
Frobel, J., Hemeda, H., Lenz, M., Abagnale, G., Joussen, S., Denecke, B., et al. (2014). Epigenetic rejuvenation of mesenchymal stromal cells derived from induced pluripotent stem cells. Stem Cell Rep. 3, 414–422. doi: 10.1016/j.stemcr.2014.07.003
Fujita, Y., Kadota, T., Araya, J., Ochiya, T., and Kuwano, K. (2018). Clinical application of mesenchymal stem cell-derived extracellular vesicle-based therapeutics for inflammatory lung diseases. J. Clin. Med. 7:E355. doi: 10.3390/jcm7100355
Galipeau, J., and Sensebe, L. (2018). Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell 22, 824–833. doi: 10.1016/j.stem.2018.05.004
Ganguly, P., El-Jawhari, J. J., Giannoudis, P. V., Burska, A. N., Ponchel, F., and Jones, E. A. (2017). Age-related changes in bone marrow mesenchymal stromal cells: a potential impact on osteoporosis and osteoarthritis development. Cell Transplant. 26, 1520–1529. doi: 10.1177/0963689717721201
Garcia-Prat, L., Martinez-Vicente, M., Perdiguero, E., Ortet, L., Rodriguez-Ubreva, J., Rebollo, E., et al. (2016). Autophagy maintains stemness by preventing senescence. Nature 529, 37–42. doi: 10.1038/nature16187
Geissler, S., Textor, M., Kuhnisch, J., Konnig, D., Klein, O., Ode, A., et al. (2012). Functional comparison of chronological and in vitro aging: differential role of the cytoskeleton and mitochondria in mesenchymal stromal cells. PLoS One 7:e52700. doi: 10.1371/journal.pone.0052700
Gharibi, B., and Hughes, F. J. (2012). Effects of medium supplements on proliferation, differentiation potential, and in vitro expansion of mesenchymal stem cells. Stem Cells Transl. Med. 1, 771–782. doi: 10.5966/sctm.2010-0031
Ginzberg, M. B., Chang, N., D‘Souza, H., Patel, N., Kafri, R., and Kirschner, M. W. (2018). Cell size sensing in animal cells coordinates anabolic growth rates and cell cycle progression to maintain cell size uniformity. eLife 7:e26957. doi: 10.7554/eLife.26957
Gire, V., and Dulic, V. (2015). Senescence from G2 arrest, revisited. Cell Cycle 14, 297–304. doi: 10.1080/15384101.2014.1000134
Gnani, D., Crippa, S., Della Volpe, L., Rossella, V., Conti, A., Lettera, E., et al. (2019). An early-senescence state in aged mesenchymal stromal cells contributes to hematopoietic stem and progenitor cell clonogenic impairment through the activation of a pro-inflammatory program. Aging Cell 18:e12933. doi: 10.1111/acel.12933
Gronthos, S., Brahim, J., Li, W., Fisher, L. W., Cherman, N., Boyde, A., et al. (2002). Stem cell properties of human dental pulp stem cells. J. Dent. Res. 81, 531–535. doi: 10.1177/154405910208100806
Hamidouche, Z., Rother, K., Przybilla, J., Krinner, A., Clay, D., Hopp, L., et al. (2017). Bistable epigenetic states explain age-dependent decline in mesenchymal stem cell heterogeneity. Stem Cells 35, 694–704. doi: 10.1002/stem.2514
Hayflick, L., and Moorhead, P. S. (1961). The serial cultivation of human diploid cell strains. Exp. Cell Res. 25, 585–621. doi: 10.1016/0014-4827(61)90192-6
Hildebrand, D. G., Lehle, S., Borst, A., Haferkamp, S., Essmann, F., and Schulze-Osthoff, K. (2013). alpha-Fucosidase as a novel convenient biomarker for cellular senescence. Cell Cycle 12, 1922–1927. doi: 10.4161/cc.24944
Huang, R., Qin, C., Wang, J., Hu, Y., Zheng, G., Qiu, G., et al. (2019). Differential effects of extracellular vesicles from aging and young mesenchymal stem cells in acute lung injury. Aging (Albany NY) 11, 7996–8014. doi: 10.18632/aging.102314
Huang, Y., Corbley, M. J., Tang, Z., Yang, L., Peng, Y., Zhang, Z. Y., et al. (2004). Down-regulation of p21WAF1 promotes apoptosis in senescent human fibroblasts: involvement of retinoblastoma protein phosphorylation and delay of cellular aging. J. Cell Physiol. 201, 483–491. doi: 10.1002/jcp.20125
Hynes, K., Menicanin, D., Han, J., Marino, V., Mrozik, K., Gronthos, S., et al. (2013). Mesenchymal stem cells from iPS cells facilitate periodontal regeneration. J. Dent Res. 92, 833–839. doi: 10.1177/0022034513498258
Jiang, Y., Mishima, H., Sakai, S., Liu, Y. K., Ohyabu, Y., and Uemura, T. (2008). Gene expression analysis of major lineage-defining factors in human bone marrow cells: effect of aging, gender, and age-related disorders. J. Orthop. Res. 26, 910–917. doi: 10.1002/jor.20623
Jin, H. J., Kwon, J. H., Kim, M., Bae, Y. K., Choi, S. J., Oh, W., et al. (2016). Downregulation of melanoma cell adhesion molecule (MCAM/CD146) accelerates cellular senescence in human umbilical cord blood-derived mesenchymal stem cells. Stem Cells Transl. Med. 5, 427–439. doi: 10.5966/sctm.2015-0109
Kanehira, M., Kikuchi, T., Ohkouchi, S., Shibahara, T., Tode, N., Santoso, A., et al. (2012). Targeting lysophosphatidic acid signaling retards culture-associated senescence of human marrow stromal cells. PLoS One 7:e32185. doi: 10.1371/journal.pone.0032185
Kasper, G., Mao, L., Geissler, S., Draycheva, A., Trippens, J., Kuhnisch, J., et al. (2009). Insights into mesenchymal stem cell aging: involvement of antioxidant defense and actin cytoskeleton. Stem Cells 27, 1288–1297. doi: 10.1002/stem.49
Khan, M., Mohsin, S., Khan, S. N., and Riazuddin, S. (2011). Repair of senescent myocardium by mesenchymal stem cells is dependent on the age of donor mice. J. Cell Mol. Med. 15, 1515–1527. doi: 10.1111/j.1582-4934.2009.00998.x
Kobune, M., Kawano, Y., Ito, Y., Chiba, H., Nakamura, K., Tsuda, H., et al. (2003). Telomerized human multipotent mesenchymal cells can differentiate into hematopoietic and cobblestone area-supporting cells. Exp. Hematol. 31, 715–722. doi: 10.1016/s0301-472x(03)00177-2
Kretlow, J. D., Jin, Y. Q., Liu, W., Zhang, W. J., Hong, T. H., Zhou, G., et al. (2008). Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol. 9:60. doi: 10.1186/1471-2121-9-60
Kujoth, G. C., Hiona, A., Pugh, T. D., Someya, S., Panzer, K., Wohlgemuth, S. E., et al. (2005). Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309, 481–484. doi: 10.1126/science.1112125
Le Clainche, C., and Carlier, M. F. (2008). Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol. Rev. 88, 489–513. doi: 10.1152/physrev.00021.2007
Lechanteur, C., Briquet, A., Giet, O., Delloye, O., Baudoux, E., and Beguin, Y. (2016). Clinical-scale expansion of mesenchymal stromal cells: a large banking experience. J. Transl. Med. 14:145. doi: 10.1186/s12967-016-0892-y
Lee, H. J., Kang, K. S., Kang, S. Y., Kim, H. S., Park, S. J., Lee, S. Y., et al. (2016). Immunologic properties of differentiated and undifferentiated mesenchymal stem cells derived from umbilical cord blood. J. Vet. Sci. 17, 289–297. doi: 10.4142/jvs.2016.17.3.289
Lei, Q., Liu, T., Gao, F., Xie, H., Sun, L., Zhao, A., et al. (2017). Microvesicles as potential biomarkers for the identification of senescence in human mesenchymal stem cells. Theranostics 7, 2673–2689. doi: 10.7150/thno.18915
Li, M., Yan, J., Chen, X., Tam, W., Zhou, L., Liu, T., et al. (2018). Spontaneous up-regulation of SIRT1 during osteogenesis contributes to stem cells‘ resistance to oxidative stress. J. Cell Biochem. 119, 4928–4944. doi: 10.1002/jcb.26730
Li, Y., Wu, Q., Wang, Y., Li, L., Bu, H., and Bao, J. (2017). Senescence of mesenchymal stem cells (Review). Int. J. Mol. Med. 39, 775–782. doi: 10.3892/ijmm.2017.2912
Lian, Q., Zhang, Y., Zhang, J., Zhang, H. K., Wu, X., Zhang, Y., et al. (2010). Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation 121, 1113–1123. doi: 10.1161/CIRCULATIONAHA.109.898312
Liang, X., Ding, Y., Lin, F., Zhang, Y., Zhou, X., Meng, Q., et al. (2019). Overexpression of ERBB4 rejuvenates aged mesenchymal stem cells and enhances angiogenesis via PI3K/AKT and MAPK/ERK pathways. FASEB J. 33, 4559–4570. doi: 10.1096/fj.201801690R
Liu, L., DiGirolamo, C. M., Navarro, P. A., Blasco, M. A., and Keefe, D. L. (2004). Telomerase deficiency impairs differentiation of mesenchymal stem cells. Exp. Cell Res. 294, 1–8. doi: 10.1016/j.yexcr.2003.10.031
Liu, M., Lei, H., Dong, P., Fu, X., Yang, Z., Yang, Y., et al. (2017). Adipose-derived mesenchymal stem cells from the elderly exhibit decreased migration and differentiation abilities with senescent properties. Cell Transplant. 26, 1505–1519. doi: 10.1177/0963689717721221
Madsen, S. D., Jones, S. H., Tucker, H. A., Giler, M. K., Muller, D. C., Discher, C. T., et al. (2020). Survival of aging CD264(+) and CD264(-) populations of human bone marrow mesenchymal stem cells is independent of colony-forming efficiency. Biotechnol. Bioeng. 117, 223–237. doi: 10.1002/bit.27195
Madsen, S. D., Russell, K. C., Tucker, H. A., Glowacki, J., Bunnell, B. A., and O‘Connor, K. C. (2017). Decoy TRAIL receptor CD264: a cell surface marker of cellular aging for human bone marrow-derived mesenchymal stem cells. Stem Cell Res. Ther. 8:201. doi: 10.1186/s13287-017-0649-4
Marklein, R. A., Lo Surdo, J. L., Bellayr, I. H., Godil, S. A., Puri, R. K., and Bauer, S. R. (2016). High content imaging of early morphological signatures predicts long term mineralization capacity of human mesenchymal stem cells upon osteogenic induction. Stem Cells 34, 935–947. doi: 10.1002/stem.2322
Mauney, J. R., Kaplan, D. L., and Volloch, V. (2004). Matrix-mediated retention of osteogenic differentiation potential by human adult bone marrow stromal cells during ex vivo expansion. Biomaterials 25, 3233–3243. doi: 10.1016/j.biomaterials.2003.10.005
Mets, T., and Verdonk, G. (1981). In vitro aging of human bone marrow derived stromal cells. Mech. Ageing Dev. 16, 81–89. doi: 10.1016/0047-6374(81)90035-x
Miettinen, T. P., Pessa, H. K., Caldez, M. J., Fuhrer, T., Diril, M. K., Sauer, U., et al. (2014). Identification of transcriptional and metabolic programs related to mammalian cell size. Curr. Biol. 24, 598–608. doi: 10.1016/j.cub.2014.01.071
Mishra, P., Martin, D. C., Androulakis, I. P., and Moghe, P. V. (2019). Fluorescence imaging of actin turnover parses early stem cell lineage divergence and senescence. Sci. Rep. 9:10377. doi: 10.1038/s41598-019-46682-y
Montpetit, A. J., Alhareeri, A. A., Montpetit, M., Starkweather, A. R., Elmore, L. W., Filler, K., et al. (2014). Telomere length: a review of methods for measurement. Nurs. Res. 63, 289–299. doi: 10.1097/NNR.0000000000000037
Mukonoweshuro, B., Brown, C. J., Fisher, J., and Ingham, E. (2014). Immunogenicity of undifferentiated and differentiated allogeneic mouse mesenchymal stem cells. J. Tissue Eng. 5:2041731414534255. doi: 10.1177/2041731414534255
Negishi, Y., Kudo, A., Obinata, A., Kawashima, K., Hirano, H., Yanai, N., et al. (2000). Multipotency of a bone marrow stromal cell line, TBR31-2, established from ts-SV40 T antigen gene transgenic mice. Biochem. Biophys. Res. Commun. 268, 450–455. doi: 10.1006/bbrc.2000.2076
Niemann, J., Johne, C., Schroder, S., Koch, F., Ibrahim, S. M., Schultz, J., et al. (2017). An mtDNA mutation accelerates liver aging by interfering with the ROS response and mitochondrial life cycle. Free Radic. Biol. Med. 102, 174–187. doi: 10.1016/j.freeradbiomed.2016.11.035
Nolta, J. A., Galipeau, J., and Phinney, D. G. (2020). Improving mesenchymal stem/stromal cell potency and survival: proceedings from the International Society of Cell Therapy (ISCT) MSC preconference held in May 2018, Palais des Congres de Montreal, organized by the ISCT MSC Scientific Committee. Cytotherapy 22, 123–126. doi: 10.1016/j.jcyt.2020.01.004
O’Callaghan, C., and Vassilopoulos, A. (2017). Sirtuins at the crossroads of stemness, aging, and cancer. Aging Cell 16, 1208–1218. doi: 10.1111/acel.12685
Oja, S., Komulainen, P., Penttila, A., Nystedt, J., and Korhonen, M. (2018). Automated image analysis detects aging in clinical-grade mesenchymal stromal cell cultures. Stem Cell Res. Ther. 9:6. doi: 10.1186/s13287-017-0740-x
Ozkul, Y., and Galderisi, U. (2016). The impact of epigenetics on mesenchymal stem cell biology. J. Cell Physiol. 231, 2393–2401. doi: 10.1002/jcp.25371
Phinney, D. G. (2012). Functional heterogeneity of mesenchymal stem cells: implications for cell therapy. J. Cell Biochem. 113, 2806–2812. doi: 10.1002/jcb.24166
Poyton, R. O., Ball, K. A., and Castello, P. R. (2009). Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol. Metab. 20, 332–340. doi: 10.1016/j.tem.2009.04.001
Revuelta, M., and Matheu, A. (2017). Autophagy in stem cell aging. Aging Cell 16, 912–915. doi: 10.1111/acel.12655
Ryan, A. E., Lohan, P., O‘Flynn, L., Treacy, O., Chen, X., Coleman, C., et al. (2014). Chondrogenic differentiation increases antidonor immune response to allogeneic mesenchymal stem cell transplantation. Mol. Ther. 22, 655–667. doi: 10.1038/mt.2013.261
Sabapathy, V., and Kumar, S. (2016). hiPSC-derived iMSCs: nextGen MSCs as an advanced therapeutically active cell resource for regenerative medicine. J. Cell Mol. Med. 20, 1571–1588. doi: 10.1111/jcmm.12839
Sahin, E., and Depinho, R. A. (2010). Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature 464, 520–528. doi: 10.1038/nature08982
Sart, S., Song, L., and Li, Y. (2015). Controlling redox status for stem cell survival, expansion, and differentiation. Oxid. Med. Cell Longev. 2015:105135. doi: 10.1155/2015/105135
Schellenberg, A., Stiehl, T., Horn, P., Joussen, S., Pallua, N., Ho, A. D., et al. (2012). Population dynamics of mesenchymal stromal cells during culture expansion. Cytotherapy 14, 401–411. doi: 10.3109/14653249.2011.640669
Sepulveda, J. C., Tome, M., Fernandez, M. E., Delgado, M., Campisi, J., Bernad, A., et al. (2014). Cell senescence abrogates the therapeutic potential of human mesenchymal stem cells in the lethal endotoxemia model. Stem Cells 32, 1865–1877. doi: 10.1002/stem.1654
Serrano, M., Hannon, G. J., and Beach, D. (1993). A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature 366, 704–707. doi: 10.1038/366704a0
Sethe, S., Scutt, A., and Stolzing, A. (2006). Aging of mesenchymal stem cells. Ageing Res. Rev. 5, 91–116. doi: 10.1016/j.arr.2005.10.001
Shammaa, R., El-Kadiry, A. E., Abusarah, J., and Rafei, M. (2020). Mesenchymal stem cells beyond regenerative medicine. Front. Cell Dev. Biol. 8:72. doi: 10.3389/fcell.2020.00072
Shibata, K. R., Aoyama, T., Shima, Y., Fukiage, K., Otsuka, S., Furu, M., et al. (2007). Expression of the p16INK4A gene is associated closely with senescence of human mesenchymal stem cells and is potentially silenced by DNA methylation during in vitro expansion. Stem Cells 25, 2371–2382. doi: 10.1634/stemcells.2007-0225
Simonsen, J. L., Rosada, C., Serakinci, N., Justesen, J., Stenderup, K., Rattan, S. I., et al. (2002). Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat. Biotechnol. 20, 592–596. doi: 10.1038/nbt0602-592
Singh, M., and Piekorz, R. P. (2013). Senescence-associated lysosomal alpha-L-fucosidase (SA-alpha-Fuc): a sensitive and more robust biomarker for cellular senescence beyond SA-beta-Gal. Cell Cycle 12:1996. doi: 10.4161/cc.25318
Squillaro, T., Peluso, G., and Galderisi, U. (2016). Clinical trials with mesenchymal stem cells: an update. Cell Transplant. 25, 829–848. doi: 10.3727/096368915X689622
Squillaro, T., Severino, V., Alessio, N., Farina, A., Di Bernardo, G., Cipollaro, M., et al. (2015). De-regulated expression of the BRG1 chromatin remodeling factor in bone marrow mesenchymal stromal cells induces senescence associated with the silencing of NANOG and changes in the levels of chromatin proteins. Cell Cycle 14, 1315–1326. doi: 10.4161/15384101.2014.995053
Stagg, J., Pommey, S., Eliopoulos, N., and Galipeau, J. (2006). Interferon-gamma-stimulated marrow stromal cells: a new type of nonhematopoietic antigen-presenting cell. Blood 107, 2570–2577. doi: 10.1182/blood-2005-07-2793
Stenderup, K., Justesen, J., Clausen, C., and Kassem, M. (2003). Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone 33, 919–926. doi: 10.1016/j.bone.2003.07.005
Stolzing, A., Jones, E., McGonagle, D., and Scutt, A. (2008). Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech. Ageing Dev. 129, 163–173. doi: 10.1016/j.mad.2007.12.002
Stolzing, A., and Scutt, A. (2006). Age-related impairment of mesenchymal progenitor cell function. Aging Cell 5, 213–224. doi: 10.1111/j.1474-9726.2006.00213.x
Takayama, K., Kawakami, Y., Lavasani, M., Mu, X., Cummins, J. H., Yurube, T., et al. (2017). mTOR signaling plays a critical role in the defects observed in muscle-derived stem/progenitor cells isolated from a murine model of accelerated aging. J. Orthop. Res. 35, 1375–1382. doi: 10.1002/jor.23409
Turinetto, V., Vitale, E., and Giachino, C. (2016). Senescence in human mesenchymal stem cells: functional changes and implications in stem cell-based therapy. Int. J. Mol. Sci. 17:E1164. doi: 10.3390/ijms17071164
van de Ven, R. A. H., Santos, D., and Haigis, M. C. (2017). Mitochondrial sirtuins and molecular mechanisms of aging. Trends Mol. Med. 23, 320–331. doi: 10.1016/j.molmed.2017.02.005
Vassilopoulos, A., Fritz, K. S., Petersen, D. R., and Gius, D. (2011). The human sirtuin family: evolutionary divergences and functions. Hum. Genomics 5, 485–496. doi: 10.1186/1479-7364-5-5-485
Viswanathan, S., Shi, Y., Galipeau, J., Krampera, M., Leblanc, K., Martin, I., et al. (2019). Mesenchymal stem versus stromal cells: international society for cell & gene therapy (ISCT(R)) mesenchymal stromal cell committee position statement on nomenclature. Cytotherapy 21, 1019–1024. doi: 10.1016/j.jcyt.2019.08.002
Wagner, W., Horn, P., Castoldi, M., Diehlmann, A., Bork, S., Saffrich, R., et al. (2008). Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One 3:e2213. doi: 10.1371/journal.pone.0002213
Wang, C. H., Wu, S. B., Wu, Y. T., and Wei, Y. H. (2013). Oxidative stress response elicited by mitochondrial dysfunction: implication in the pathophysiology of aging. Exp. Biol. Med. (Maywood) 238, 450–460. doi: 10.1177/1535370213493069
Wang, D., Zhang, H., Liang, J., Li, X., Feng, X., Wang, H., et al. (2013). Allogeneic mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus: 4 years of experience. Cell Transplant. 22, 2267–2277. doi: 10.3727/096368911X582769c
Wang, H., Li, D., Zhai, Z., Zhang, X., Huang, W., Chen, X., et al. (2019). Characterization and therapeutic application of mesenchymal stem cells with neuromesodermal origin from human pluripotent stem cells. Theranostics 9, 1683–1697. doi: 10.7150/thno.30487
Wang, H. S., Hung, S. C., Peng, S. T., Huang, C. C., Wei, H. M., Guo, Y. J., et al. (2004). Mesenchymal stem cells in the Wharton‘s jelly of the human umbilical cord. Stem Cells 22, 1330–1337. doi: 10.1634/stemcells.2004-0013
Wang, L. T., Ting, C. H., Yen, M. L., Liu, K. J., Sytwu, H. K., Wu, K. K., et al. (2016). Human mesenchymal stem cells (MSCs) for treatment towards immune- and inflammation-mediated diseases: review of current clinical trials. J. Biomed. Sci. 23:76. doi: 10.1186/s12929-016-0289-5
Wangler, S., Menzel, U., Li, Z., Ma, J., Hoppe, S., Benneker, L. M., et al. (2019). CD146/MCAM distinguishes stem cell subpopulations with distinct migration and regenerative potential in degenerative intervertebral discs. Osteoarthritis Cartilage 27, 1094–1105. doi: 10.1016/j.joca.2019.04.002
Wirawan, E., Vanden Berghe, T., Lippens, S., Agostinis, P., and Vandenabeele, P. (2012). Autophagy: for better or for worse. Cell Res. 22, 43–61. doi: 10.1038/cr.2011.152
Xia, W., Zhang, F., Xie, C., Jiang, M., and Hou, M. (2015). Macrophage migration inhibitory factor confers resistance to senescence through CD74-dependent AMPK-FOXO3a signaling in mesenchymal stem cells. Stem Cell Res. Ther. 6:82. doi: 10.1186/s13287-015-0076-3
Xu, C., Wang, J., Zhu, T., Shen, Y., Tang, X., Fang, L., et al. (2016). Cross-talking between PPAR and WNT signaling and its regulation in mesenchymal stem cell differentiation. Curr. Stem Cell Res. Ther. 11, 247–254. doi: 10.2174/1574888x10666150723145707
Yamamoto, T., Takabatake, Y., Kimura, T., Takahashi, A., Namba, T., Matsuda, J., et al. (2016). Time-dependent dysregulation of autophagy: implications in aging and mitochondrial homeostasis in the kidney proximal tubule. Autophagy 12, 801–813. doi: 10.1080/15548627.2016.1159376
Yang, N. C., and Hu, M. L. (2005). The limitations and validities of senescence associated-beta-galactosidase activity as an aging marker for human foreskin fibroblast Hs68 cells. Exp. Gerontol. 40, 813–819. doi: 10.1016/j.exger.2005.07.011
Yang, S. R., Park, J. R., and Kang, K. S. (2015). Reactive oxygen species in mesenchymal stem cell aging: implication to lung diseases. Oxid. Med. Cell Longev. 2015:486263. doi: 10.1155/2015/486263
Yu, J. M., Wu, X., Gimble, J. M., Guan, X., Freitas, M. A., and Bunnell, B. A. (2011). Age-related changes in mesenchymal stem cells derived from rhesus macaque bone marrow. Aging Cell 10, 66–79. doi: 10.1111/j.1474-9726.2010.00646.x
Yuan, H. F., Zhai, C., Yan, X. L., Zhao, D. D., Wang, J. X., Zeng, Q., et al. (2012). SIRT1 is required for long-term growth of human mesenchymal stem cells. J. Mol. Med. (Berl.) 90, 389–400. doi: 10.1007/s00109-011-0825-4
Zhang, R., Chen, W., and Adams, P. D. (2007). Molecular dissection of formation of senescence-associated heterochromatin foci. Mol. Cell Biol. 27, 2343–2358. doi: 10.1128/MCB.02019-06
Zhang, Y., Ikeno, Y., Qi, W., Chaudhuri, A., Li, Y., Bokov, A., et al. (2009). Mice deficient in both Mn superoxide dismutase and glutathione peroxidase-1 have increased oxidative damage and a greater incidence of pathology but no reduction in longevity. J. Gerontol. A Biol. Sci. Med. Sci. 64, 1212–1220. doi: 10.1093/gerona/glp132
Zhang, Y., Zhu, W., He, H., Fan, B., Deng, R., Hong, Y., et al. (2019). Macrophage migration inhibitory factor rejuvenates aged human mesenchymal stem cells and improves myocardial repair. Aging (Albany NY) 11, 12641–12660. doi: 10.18632/aging.102592
Keywords: aging, mesenchymal stem cells, senescence, mechanism, rejuvenation
Citation: Liu J, Ding Y, Liu Z and Liang X (2020) Senescence in Mesenchymal Stem Cells: Functional Alterations, Molecular Mechanisms, and Rejuvenation Strategies. Front. Cell Dev. Biol. 8:258. doi: 10.3389/fcell.2020.00258
Received: 11 February 2020; Accepted: 27 March 2020;
Published: 05 May 2020.
Edited by:
Mark Hamrick, Augusta University, United StatesReviewed by:
Xingliang Fan, Sun Yat-sen University, ChinaCopyright © 2020 Liu, Ding, Liu and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongmin Liu, bGl1Lnpob25nbWluQHRvbmdqaS5lZHUuY24=; Xiaoting Liang, bGlhbmd4dEB0b25namkuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.