
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Cell Dev. Biol., 03 March 2020
Sec. Signaling
Volume 8 - 2020 | https://doi.org/10.3389/fcell.2020.00037
This article is part of the Research TopicPerspectives in Mammary Gland Development and Breast Cancer ResearchView all 13 articles
Delayed relapses at distant sites are a common clinical observation for certain types of cancers after removal of primary tumor, such as breast and prostate cancer. This evidence has been explained by postulating a long period during which disseminated cancer cells (DCCs) survive in a foreign environment without developing into overt metastasis. Because of the asymptomatic nature of this phenomenon, isolation, and analysis of disseminated dormant cancer cells from clinically disease-free patients is ethically and technically highly problematic and currently these data are largely limited to the bone marrow. That said, detecting, profiling and treating indolent metastatic lesions before the onset of relapse is the imperative. To overcome this major limitation many laboratories developed in vitro models of the metastatic niche for different organs and different types of cancers. In this review we focus specifically on in vitro models designed to study metastatic dormancy of breast cancer cells (BCCs). We provide an overview of the BCCs employed in the different organotypic systems and address the components of the metastatic microenvironment that have been shown to impact on the dormant phenotype: tissue architecture, stromal cells, biochemical environment, oxygen levels, cell density. A brief description of the organ-specific in vitro models for bone, liver, and lung is provided. Finally, we discuss the strategies employed so far for the validation of the different systems.
Dormancy is an old concept that describes a clinical phenomenon (Klein, 2011; Uhr and Pantel, 2011), i.e., the relapse of a cancer after surgical removal of the primary tumor in a patient considered clinically disease-free for a long time. This implies that cancer cells disseminated prior to surgery and persisted as Minimal Residual Disease (MRD) for a prolonged time (arbitrarily defined, but usually longer than 5 years) before switching to aggressive growth and overt metastasis. The recurrence can be at the primary site (primary tumor dormancy) or at a secondary site (metastatic dormancy). The mechanisms underlying the two types of dormancy are likely to be partially overlapping if involving cell intrinsic genetic/epigenetic mechanisms, or distinct, if dependent on the tissue microenvironment. Clinical dormancy is common for breast, prostate, melanoma, renal, and thyroid cancers, while it is rarely observed in lung and colon cancers (Uhr and Pantel, 2011). In breast cancers, estrogen receptor (ER) status seems to profoundly influence the rate of relapse: ER− patients tend to recur within the first 5 years following primary tumor diagnosis, while ER+ patients have increased risk between 5 and 20 years (Pan et al., 2017; Pantel and Hayes, 2018). While anti-estrogen therapy significantly improved patient outcomes, a significant fraction of them still develops distant relapses and extending the duration of the treatment beyond 5 years yields little benefit (Pan et al., 2017; Bense et al., 2018; Pantel and Hayes, 2018).
In this review we specifically focus on in vitro models developed to study metastatic dormancy. Upon dissemination in a secondary organ, metastatic breast cancer cells (BCCs) can undergo three fates: death, dormancy, or growth. Dormancy does not have a clear biological definition, it has been proposed a classification of dormant phenotypes into cellular dormancy (entering into reversible quiescence in G0) and tumor mass dormancy (a small cluster of cells where proliferation is counterbalanced by apoptosis due to lack of nutrients, blood supply or because of immune surveillance) (Linde et al., 2016; Goddard et al., 2018; Weidenfeld and Barkan, 2018; Lan et al., 2019). However, these states are likely to coexist within the same patients and probably the same cells can dynamically fluctuate between these different states.
Growth arrest mechanisms generally fall into three main categories: quiescence, terminal differentiation and senescence (Pack et al., 2019). While the former is reversible upon withdrawal of restrictive signals, the latter is associated with permanent exit from cell cycle and persistent activation of stress signals. Cyclin-dependent kinases (CDKs) coupled with cyclins promote cellular proliferation by inhibiting pocket protein family (Rb, p107, p130), conversely CDK-cyclin couples are inhibited by CIP/KIP inhibitors (p21, p27, p57) and INK4 inhibitors (like p16). Intrinsic and extrinsic factors are integrated into the regulation of this core machinery, for example, serum starvation triggers upregulation of p27 and exit from proliferation, while CDKs are induced by mitogenic signals. DNA damage is the strongest internal signal regulating proliferation and mediates growth arrest via stabilization of p53 and its target p21. Apart from the prominent role of p27 (Bragado et al., 2013; Touny et al., 2014), little is known about the role of cell-cycle machinery in the different stages of metastatic dormant phenotype and whether dormant cells lie closer to quiescence or senescence in the growth arrest spectrum.
Several strategies have been implemented to visualize dormant disseminated cells in vivo. The easiest methods are staining of fixed tissues for the proliferation-associated protein Ki67, growth arrest marker p27 or for DNA-incorporated synthetic nucleosides (such as BrdU or EdU) (Ghajar et al., 2013; Fluegen et al., 2017; Carlson et al., 2019; Montagner et al., 2020). The main limit of these methods is that they are not compatible with tissue viability and don’t allow isolation of non-proliferating cells. To circumvent this problem, De Cock et al. (2016) utilized an intracellular fluorescent vital dye to label cells prior to injection into mice. The dye is diluted at each cell division, allowing for isolation of cells that didn’t proliferate (Cock et al., 2016). Similarly, Fluegen et al. (2017) generated metastatic cells stably expressing a photoconvertible fluorescent protein, Histone 2B-Dendra2. This is photoconverted from green to red before injecting cells in mice, and nuclear red fluorescent signal decreases at each cell division, similar to a vital dye (Fluegen et al., 2017). The fluorescence ubiquitination–based cell cycle indicator (FUCCI) system has also been applied (Albrengues et al., 2018) which allows dynamic visualization of each phase of the cell cycle during in vivo imaging.
Whether dormant cells are quiescent or undergo a balanced proliferation (where proliferation rate is compensated by apoptosis) has a profound impact on the design of new therapies (Wells et al., 2013), because it is assumed that dormant cells are inherently resistant to conventional chemotherapy as they are not cycling (Wells et al., 2013; Linde et al., 2016). This is not entirely correct as recent data show that chemoresistance is in part actively supported by the metastatic niche and is not just a consequence of cell-cycle arrest (Carlson et al., 2019). Moreover, it has been recently shown that several patients with bone marrow-disseminated cancer cells (DCCs) that resisted treatment with FEC (fluorouracil, epirubicin, and cyclophosphamide), benefited from additional treatment with docetaxel; as this drug induces microtubule stabilization, cell-cycle arrest in the G(2)M phase and apoptosis, this suggests that a considerable fraction of dormant cells still has proliferative activity (Naume et al., 2014; Goddard et al., 2018). Notably, patients with dormant DCCs that persisted after the second therapy had worst prognosis, further supporting the idea that metastatic lesions develop from pre-existing dormant DCCs (Braun et al., 2005; Naume et al., 2014).
Despite the fact that metastatic lesions account for the vast majority of cancer-related deaths, metastatic colonization is an extremely inefficient process (Massagué and Obenauf, 2016). Each step of the hematogenous metastatic cascade of epithelial cancers (loss of polarity, detachment from primary tumor, migration through basal membrane and stromal layers, intravasation, survival in the blood stream, extravasation) represents a significant hurdle that contributes to the selection of aggressive cancer cell clones. Even focusing on the steps that follow intravasation, less than 0.01% of cells will eventually develop metastatic lesions and not even in all patients (Naumov et al., 2002; Braun et al., 2005). These numbers are confirmed by experimental models of metastatic dissemination (Valastyan and Weinberg, 2011), with the switch from micrometastasis to macrometastasis estimated to happen with a frequency lower than 0.02% for liver metastases from melanoma cells (Luzzi et al., 1998; Cameron et al., 2000). From these numbers the expectation might be that persistence in secondary organs is a feature restricted to few highly aggressive cells (seed) and/or to target organs with a peculiar permissive environment (soil). Yet, clinical and experimental evidence of early dissemination of breast cancers have been reported (Goddard et al., 2018), indicating that even cells from early stage disease can disseminate and persist. Moreover, several registries reported people who have developed cancers following organ transplants (Buell et al., 2005; Klein, 2011), indicating that disseminated cells survived in a quiescent state in different organs of donors with prior undiagnosed or cured cancers. Of note, transplanted organs were not the most common sites of metastasis, such as kidney or heart. This evidence supports the idea that survival after metastatic spreading might not be limited, per se, to highly aggressive cells or few target organs, and that indolent disease can seed additional sites.
Transgenic mouse models of dormant/indolent metastatic mammary cancers have been described over the years (Li et al., 2000; Hüsemann et al., 2008) and have been recently used to discover the roles of progesterone receptor, Her2 and partial-EMT into early dissemination (Harper et al., 2016; Hosseini et al., 2016). However, these models also have significant limitations, such as the hurdles associated with tracking asynchronous disseminated metastatic cells. Moreover, dormancy is often the result of the crosstalk between the cancer cells and the metastatic stroma; thus, parameters should be modulated at single cell resolution, which is often impossible in vivo. Lastly, removing single stromal populations in vivo to prove their requirement into control of dormancy is incompatible with animal viability; the design of in vitro models is a valuable strategy to bypass these limitations.
The development of reliable in vitro models to investigate dormancy is hampered by the limited data from patients (Chéry et al., 2014; Vishnoi et al., 2015). Scattered dormant DCCs lie far below the radar of current diagnostic tools and significant advancements in that direction will be challenging and will run the risk of detecting lesions that would never progress (Srivastava et al., 2019). Thus, together with new tools for detection of metastatic clusters at single-cell resolution, development of markers for dangerous vs. harmless disseminated cells are highly desirable. Over the last decade, in parallel with advances in microfluidic technologies, biomaterials and biofabrication techniques, many groups developed and optimized in vitro tools to explore the issue of metastatic dormancy with different objectives, from discovery of basic mechanisms of survival to platforms for high-throughput drug discovery (Pradhan et al., 2018; Rao et al., 2019). Even though these in vitro models are increasing in number and complexity, their descriptive and/or predictive power is unknown, given the paucity of markers, metrics and expression data from patients. Nevertheless, there are common themes emerging from different models that led to the approval of clinical trials (Goddard et al., 2018) and to the development of tools to predict likelihood of relapse (Borgen et al., 2018). Moreover, recent publications provided explanations for epidemiological data linking inflammation with higher risk of breast cancer relapse (Cock et al., 2016; Albrengues et al., 2018). Recent reviews have covered in depth the history, evolution and recent advances in the dormancy field (Giancotti, 2013; Ghajar, 2015; Linde et al., 2016; Aguirre-Ghiso and Sosa, 2018; Goddard et al., 2018), this review focuses instead on in vitro models for breast cancer metastatic dormancy that have been more extensively validated and that, regardless of their complexity, led to discoveries supported by independent in vitro systems, animal models or by data from patients. Moreover, we provide a framework for the development of further in vitro models, by critically discussing metrics and parameters that should ideally be integrated to tightly anchor new and old models with data from animal models or breast cancer patients with the hope of circumventing the limitations discussed above (Figure 1).
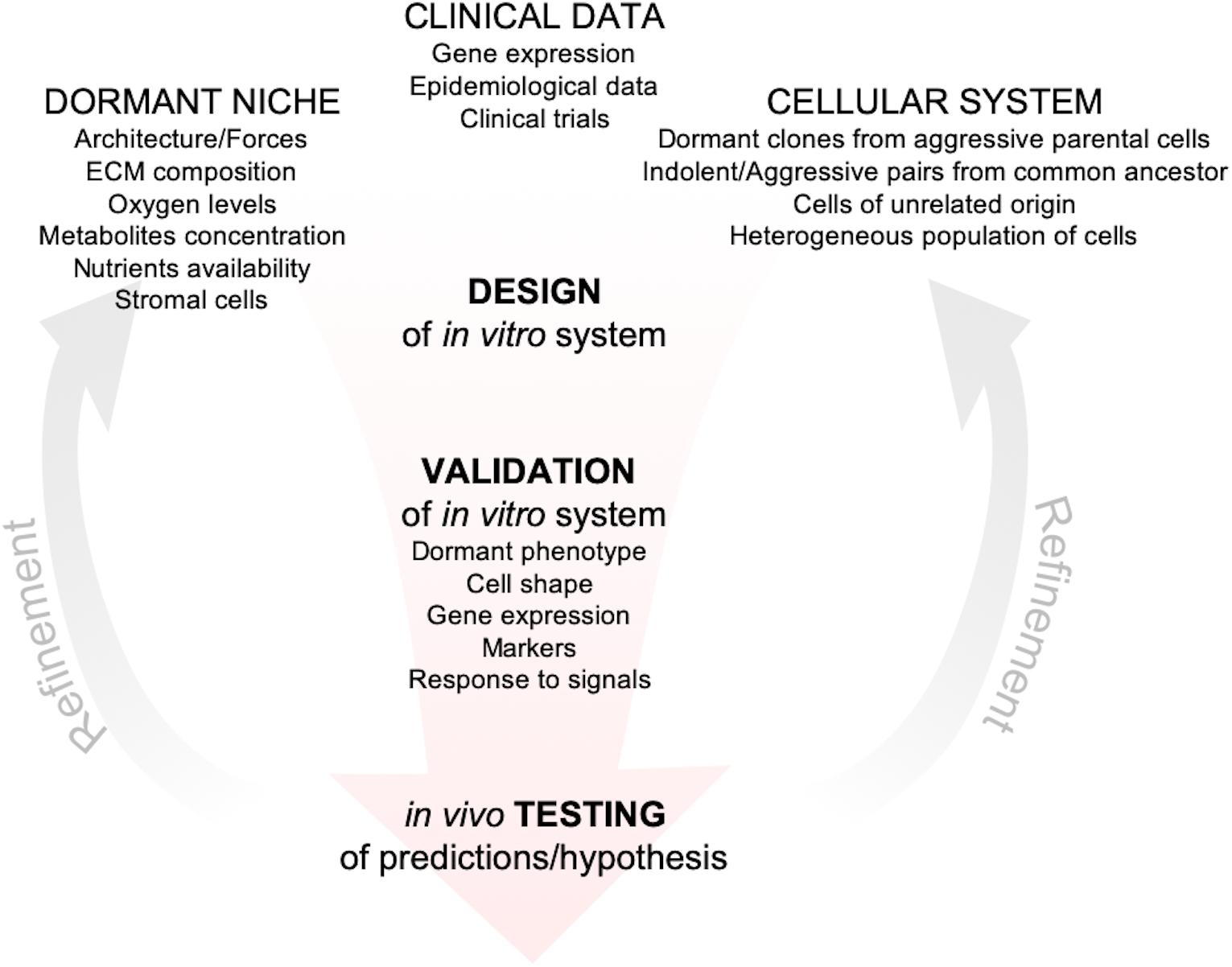
Figure 1. Framework for the development of in vitro models of metastatic dormancy. In vitro models should include one or more features of the dormant niche starting from measurements/observations of the target metastatic tissue in vivo. Then cellular systems have to be chosen based on clinical evidences that support their specific use, i.e., correct metastatic tropism, correct gene expression (when available), correct response to signals (such as inflammation). After the initial setup, the in vitro system must be validated and the cell lines, as well as the microenvironment, should exhibit one or more features specific of the dormant phenotype (obtained from animal models or clinical data). The in vitro system should then be exploited to generate hypothesis and prediction that could be tested experimentally in animal models or from clinical data. Finally, according to the feedbacks from the in vivo validation, the system can be refined for more accurate predictions and hypothesis.
To establish in vitro models that reflect the in vivo situation, it is first necessary to have cells that exhibit dormant behavior in vivo, several BCCs lines with different dormant proclivity and tropism have been generated.
The first option is the use of cell lines derived from in vivo selection of dormant clones from an aggressive parental cell line (comparison between parental and selected subclones). A list of those cellular variants is provided in Supplementary Table S1, alongside with the selection strategy. The fact that subclones with stable dormant phenotype can be isolated from the aggressive parental cell line is something more than a technical opportunity, but might reveal something more profound about the biology of the dormant phenotype, i.e., that heritable characteristics of single cells, most likely epigenetically specified, are as important as the dormant microenvironment to dictate the choice between quiescence and proliferation.
The second option is the use of cell line series generated from a common precursor, but then selected independently from different animals (Supplementary Table S1). A notable example of these cell lines is the D2 series (D2.A1, D2.1, D2.0R) established by Fred Miller lab and characterized by Ann Chambers lab in her pioneering works on cancer dormancy (Mahoney et al., 1985; Aslakson and Miller, 1992; Rak et al., 1992; Morris et al., 1993, 1994). These cells have been cloned from spontaneously growing tumor in different BALB/cfC3H mice transplanted with a D2 hyperplastic alveolar nodule (HAN) line (Medina, 1976). D2.0R and D2.A1 cells grow with comparable rate on plastic, but with extremely different dynamics in 3D systems, coculture models and in vivo: A1 form overt metastases in lung and liver, OR lie dormant in the same organs for several months (Naumov et al., 2002; Barkan et al., 2008; Shibue and Weinberg, 2009; Touny et al., 2014; Montagner et al., 2020). Notably, another breast cancer cell series of great interest has been developed by the same laboratory in BALB/c mice. These cells show progressive acquisition of aggressive traits, from primary tumor growth, local invasion, intravasation, lung homing, overt metastasis (67NR > 168FARN > 4T07 > 4T1) (Aslakson and Miller, 1992). Often used in studies about dormancy, the cell line 4T07 was generated by sequential intravenous injection and isolation from lungs of a thioguanine- and ouabain-resistant cell line (Dexter et al., 1978; Blazar et al., 1980; Miller et al., 1987). The comparison between the two cell lines has led to the discovery of important molecules involved in the dormant state of lung, bone and brain disseminated cells (Gao et al., 2012, 2016).
A third option is the comparison among cell lines from completely different origin. Examples of these classes are the widely used triple negative cell line MDA-MB-231 (on the aggressive side of the spectrum) and the ER+ cells MCF7, T47D, ZR-75-1 that form quiescent metastatic lesions upon intravenous injection (Harrell et al., 2006; Holen et al., 2016; Wright et al., 2016; Gawrzak et al., 2018). Recently, bone metastatic versions of MCF7 cell line have been developed (Pavlovic et al., 2015; Clements and Johnson, 2019).
The last option is the comparison within the same cell line. This approach is a valuable alternative whenever the question is related to the drivers of cellular heterogeneity within the same population in vitro (Ghajar et al., 2013).
During the last two decades, the role of the microenvironment has been gaining importance in understanding several steps of the malignant transformation. For metastatic dormancy, the context where cells disseminate is key, as these cells are likely not to gain further mutations once they have entered quiescence. Components of the dormant niche include, but are not limited to: tissue architecture (geometry and stiffness, adhesion, cell density, ECM), biophysical (shear stress, tissue stiffness) and biochemical (oxygen levels, ROS concentration, nutrients, metabolites) environment, stromal populations. Examples and details of in vitro systems including tissue architecture and stromal cells is provided in Figure 2 and Supplementary Table S2.
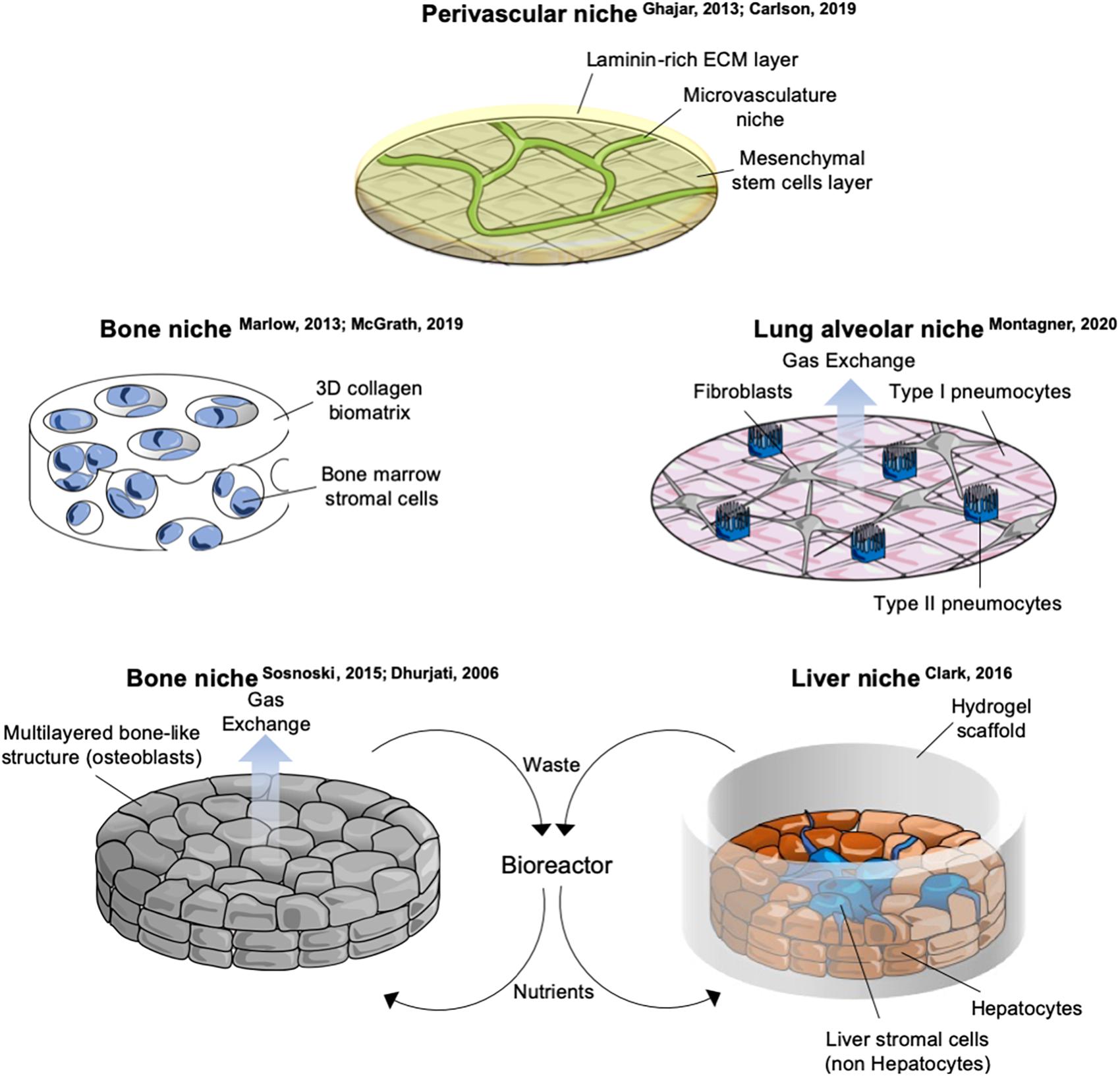
Figure 2. Schematics of selected in vitro organotypic systems designed for studying breast cancer cells (BCCs) metastatic dormancy. Schemes of in vitro systems developed to mimic microenvironments of specific metastatic organs. Details and references of each system are provided in Supplementary Table S2.
The rapid development of bioengineering and a better understanding of the principles behind mechanotransduction (Iskratsch et al., 2014; Piccolo et al., 2014; Dupont, 2016; Montagner and Dupont, 2020) has led to several in vitro approaches to study metastatic dormancy of BCC. Models involving scaffolds of natural or artificial biomaterials, microfabrication, microfluidics, bioreactors, implantable niches have been developed (extensively reviewed in Pradhan et al., 2018; Rao et al., 2019). 3D spheroids can be generated simply as clusters of cells floating into medium (Wenzel et al., 2014; Cavnar et al., 2015; Imamura et al., 2015) or by employing natural (collagen-I, hyaluronic acid, and Cultrex) (Barkan et al., 2008; Fang et al., 2016; Kassim et al., 2017) or synthetic biomaterials (hydrogels of silica-polyethylene, polycaprolactone scaffolds) (Guiro et al., 2015; Preciado et al., 2017). Cells within these structures showed different degrees of quiescence, apoptosis, hypoxia and have been tested for their sensitivity to drugs. However, same caveats apply and, although informative, these models require more validation to address if their findings translate in a dormant phenotype. A notable exception is the well-known 3D system developed in Green laboratory. In this in vitro model, D2 cells lie on top of a stiff layer of basement membrane matrix and are embedded in a second layer of diluted basal matrix. Under these conditions D2.0R cells remain dormant in round structures, while D2.A1 continuously grow and invade surrounding territories. This conformation can then be further functionalized by adding other ECM proteins, such as collagen-I that drives the proliferative switch of otherwise dormant cells (Barkan et al., 2008, 2010). This system has been extensively validated in vivo and by other laboratories as well (Shibue and Weinberg, 2009; Shibue et al., 2012), and led to the discovery of the integrin-Src-ERK axis in the dormant-to-proliferation switch (Barkan et al., 2008, 2010). The use of ECM proteins can be combined with other niche components such as stromal cells to increase the complexity of the system (Ghajar et al., 2013).
Resident organ parenchymal cells are an essential component of the dormant niche contributing to each step of quiescence-to-proliferative switch. Difficulties in the coculture of BCCs together with stromal cells are primarily two: (i) availability of organ-specific stromal cells and (ii) finding the culturing protocol that allows survival of all the cellular components. Moreover, the cellular composition of a tissue is often dynamic and heterogeneous, including different lineages of the same cell type as well as specific resident and transient immune populations. This complexity is often not captured by current in vitro models. Cocultures developed so far involving BCCs include osteoclasts (Lu et al., 2011), osteoblasts (Sosnoski et al., 2015), lung alveolar cells (Montagner et al., 2020), endothelial cells (Ghajar et al., 2013; Carlson et al., 2019), hepatocytes and non-hepatocytes liver stromal cells (Wheeler et al., 2014), bone marrow stromal cells (Ghajar et al., 2013; Marlow et al., 2013; Carpenter et al., 2018), neutrophils (Albrengues et al., 2018), peripheral blood mononuclear cells (Carpenter et al., 2018; Figure 2 and Supplementary Table S2). While for some populations primary cells are available [lung and bone marrow stromal cells (Ghajar et al., 2013; Marlow et al., 2013; Carpenter et al., 2018), hepatocytes and non-hepatocytes (Clark et al., 2016), NK cells (Malladi et al., 2016), neutrophils (Albrengues et al., 2018), human osteoblasts (Sosnoski et al., 2015), mouse SNO osteoblast-like cells (Capulli et al., 2019)], other cells require immortalization [endothelial cells (Ghajar et al., 2013), fibroblasts and type1-like pneumocytes (Montagner et al., 2020), human fetal osteoblasts and mesenchymal cells of bone marrow origin (Marlow et al., 2013), spontaneously immortalized mouse calvaria osteoblasts (Sosnoski et al., 2015)] or transformation to be cultivated [murine preosteoclasts (Lu et al., 2011), type2-like pneumocytes (Montagner et al., 2020)] and this might influence the correct crosstalk with the dormant BCC. Moreover, it has been shown that fibroblasts and endothelial cells have organ-specific gene expression (Chang et al., 2002; Nolan et al., 2013) and thus using unmatched stromal cells might overlook organ specific signaling. On the other side, the use of immortalized, homogeneous stromal cells allows a precise and repeatable experimental setup compared to deriving primary cells. An important detail in the in vitro models cited above is the use of a very low number of BCCs relative to stromal cells (Ghajar et al., 2013; Wheeler et al., 2014; Montagner et al., 2020).
Mitogens and nutrients directly impact on cell-cycle machinery (Pack et al., 2019), it is not surprising that decreasing their concentration in culture medium to a more physiological level already has an effect on proliferation. In a recently developed lung organotypic system, we used a Mitogen Low Nutrient Low medium (MLNL) that didn’t have a different effect on D2 cells per se, but that allowed to pinpoint some factors of the signaling network after stromal cells were added (Montagner et al., 2020). Mitogen Low Medium (MLM) alone had a remarkable effect on HCC1954-LCC1 (Latency Competent Cells) cells instead (Malladi et al., 2016). Cultivating LCC1 subclones in MLM medium drove expression of quiescence genes, such as Sox9, downregulation of several mediators of anti-tumor responses from NK cells and downregulation of Wnt, myc, NF-kB pathways, higher TGFβ response and lower P-ERK/P-p38 ratio (Malladi et al., 2016).
Oxygen concentration for most of the tissues oscillates between 5 and 7%, compared to the 20% in air at normal atmospheric pressure (McKeown, 2014). Bone marrow is a particularly hypoxic environment (Spencer et al., 2014) and a favorable metastatic site for BCCs. The use of physiological oxygen levels decreases proliferation for most of cells (Hubbi and Semenza, 2015) and, as with low serum, it might not be specific to dormant cells (de Prati et al., 2017; Lee et al., 2018). However, hypoxia has been implicated in dormancy in two studies where it has been shown to repress LIFR-STAT3 pathway leading to metastatic outgrowth (Johnson et al., 2016) and to preset primary tumor cells with a dormant program, then manifested after dissemination (Fluegen et al., 2017).
Plating cells at a clonogenic density in vitro is already sufficient to induce heterogeneous growth arrest in BCCs. The Wieder laboratory developed an in vitro system of bone marrow dormancy that, despite its simplicity, has been shown to recall several aspects of quiescence validated in other laboratories. BCCs that are plated onto fibronectin-coated plates undergo quiescence in presence of FGF2 and activation of integrin α5β1, PI3K and ERK pathways. These cells express partial EMT markers and can re-enter proliferation upon treatment with IL6/8 and TGFβ) (Korah et al., 2004; Najmi et al., 2005; Barrios and Wieder, 2009; Tivari et al., 2015).
What are we really modeling? This is the first question when designing any model and although this is an issue not unique to the topic of this review, the limited availability of clinical data makes it harder to unambiguously describe a dormant cell in vitro. Because an unequivocal list of dormant cells’ features is unavailable, several groups have validated their models by looking at a number of aspects that justified the parallel between the proposed in vitro model and the in vivo evidences, although a single model encompassing all of them has not yet been developed (Figure 1).
The most important behavior underlying the dormant phenotype is growth arrest, and most of the in vitro models discussed in this review successfully achieve cell-cycle arrest of cells that can be reversed upon changing experimental conditions, such as serum levels, oxygen tension or with specific signals, such as inflammation. However, this does not demonstrate the relevance of the model. For example, it has been shown that adjusting the mechanical properties of the cell culture surface (using ECM-conjugated polyacrylamide gels) alone has a dramatic impact on cellular proliferation in vitro (Tilghman et al., 2012), but this does not imply that changes in local tissue mechanics are cause of entry and exit from dormancy. Ideally, the model conditions should be based on appropriate measurements of the in vivo environment in which dormant cells are found in terms of biophysical, biochemical and cellular composition of the niche (ECM composition and architecture, nutrients and metabolites concentration, ligands concentration, communication with stromal cells). However, this information is hard to determine at single-cell resolution in murine models and even harder to measure in clinical material. To distinguish between quiescence and senescence (or even apoptosis), cells must re-enter the proliferative state upon withdrawal of the factors used to trigger dormancy or upon treatment with signals able to drive exit from dormancy. Examples of such signals for BCCs are inflammation (LPS, smoke) (Cock et al., 2016; Albrengues et al., 2018), POSTN (Montagner et al., 2020), TGFβ1 (Ghajar et al., 2013), RTKs (Tivari et al., 2015; Montagner et al., 2020), IL6, Collagen I (Barkan et al., 2010), Src (Barkan et al., 2010; Montagner et al., 2020), SFRP2 (Montagner et al., 2020), IKKβ (Lamiaa et al., 2017), integrins activation (as discussed below); while examples of inhibitors are: TSP1 (Ghajar et al., 2013), p38 (Marlow et al., 2013), Alk5 (Marlow et al., 2013), BMP2 (Gao et al., 2012), TGFβ2 (Bragado et al., 2013), MSK1 (Gawrzak et al., 2018), IFN-β (Lan et al., 2019).
Together with a reversible growth arrest, expression of gene/protein marker of dormancy should be addressed. Not many well-established markers are available for BCCs, those that have been widely validated in vitro and in vivo so far include DEC2/SHARP1, p27, NR2F1 and the ratio between P-ERK/P-p38 proteins (Touny et al., 2014; Johnson et al., 2016; Linde et al., 2016; Malladi et al., 2016; Borgen et al., 2018). We recently reported an RNA-seq analysis of lung-disseminated dormant BCCs that will hopefully provide new markers for the characterization of these cells in vitro (Montagner et al., 2020).
Regardless of the metrics adopted, the predictive power of an in vitro system represents its best validation and testing the predictions generated in mice or patients is the ultimately goal (Figure 1).
Whether the same mechanisms for quiescence or reawakening are shared among different organs in vivo is unknown. The observations that dormant subclones isolated from one organ show quiescence in other organs suggests that there might be some overlap and thus either intrinsic genetic/epigenetic mechanisms dominate over microenvironmental cues or there are common traits in very different niches. For example, D2.0R cells are dormant in liver and lung, HCC1954-LCC1 are derived from brain disseminated cells, but are found latent in lungs as well (Malladi et al., 2016), T47D-DBM have been isolated as bone dormant variant (Gawrzak et al., 2018), but they survive in quiescent state in lungs as well (Montagner et al., 2020). Mechanistic similarities between dormancy in different organs will aid the development of universal clinical strategies with the ability to eliminate dormant cells regardless of their anatomical site.
Activation of ERK and p38, associated with metastatic outgrowth and quiescence, respectively, have been consistently observed in bone and lungs (Barkan et al., 2010; Touny et al., 2014; Linde et al., 2016; Malladi et al., 2016; Gawrzak et al., 2018). THBS1 from PVN (perivascular niche) was shown to induce dormancy in lung and bone marrow (Ghajar et al., 2013). Src has been validated as BCCs survival signal in bone and lungs (Barkan et al., 2010; Zhang et al., 2013; Montagner et al., 2020). Similarly, Akt was found as a factor supporting survival or outgrowth of BCCs in bone in vivo (Zhang et al., 2013) and in vitro (Korah et al., 2004) and in an in vitro model of lung (Montagner et al., 2020). The proinflammatory cytokine LPS induces outgrowth of quiescent cells in lung (Cock et al., 2016; Albrengues et al., 2018) and in an in vitro model of dormancy in the liver (Clark et al., 2018).
A recurring theme in several models of BCCs dormancy is the importance of integrins into survival or chemoresistance. Many groups independently reported the key role of different integrin dimers. Integrinβ1-dependent activation of Src and ERK downstream of collagen-I has been found to drive exit from quiescence in vitro and in vivo (Barkan et al., 2010; Touny et al., 2014), to sustain reawakening of dormant cells following NET proteolysis of laminin in vivo [in the α3β1 form (Albrengues et al., 2018)] and to support survival after engagement of fibronectin in in vitro bone dormancy models [in the α5β1 form (Barrios and Wieder, 2009; Barney et al., 2019)]. Perivascular-driven chemoresistance of dormant BCCs also relies on α5β3 and α4β1 activation by von Willebrand Factor and VCAM-1 in endothelium, respectively. By using blocking antibodies against those isoforms in combination with doxorubicin and cyclophosphamide Carlson et al. (2019) were able to circumvent chemoresistance and decrease tumor burden in bone marrow. Finally, we recently found that acute treatment of mice with cilengitide (inhibitor of αvβ3, αvβ5 and α5β1 integrins) effectively reduced lung-disseminated dormant BCCs (Montagner et al., 2020). In sum, quiescent BCCs seem to rely on integrins in many ways and might prove more sensitive to integrin inhibitors then established or actively growing cancer cells.
So far, the battle to defeat metastatic breast cancer has achieved only limited advances since the advent of hormone target therapies. For some types of cancer, the period metastatic dormancy offers an opportunity to eliminate disease before it resumes aggressive growth, but the inherent lack of data from patients slows down the development of new therapies. The development of in vitro models to bypass this limitation has been the goal for several laboratories during the last decade, and common themes in the survival and growth of disseminated BCCs in different organs are starting to emerge. Here we present cellular models and microenvironmental factors implemented so far, together with a critical discussion on validation strategies. The discovery of new markers from patients and validation of same mechanisms among different systems will give confidence to translate these findings into clinical trials and hope to finally impact on the origin and development of metastatic breast cancer.
MM and ES equally contributed to conceiving and writing the review.
ES and MM were funded by the Francis Crick Institute, which receives its core funding from Cancer Research UK (FC001144), the UK Medical Research Council (FC001144), and the Wellcome Trust (FC001144). MM also received funding from Marie Curie Actions-Intra-European Fellowships no. 625496 and BIRD Seed grant from Department of Molecular Medicine (University of Padua).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2020.00037/full#supplementary-material
Aguirre-Ghiso, J. A., and Sosa, M. S. (2018). Emerging topics on disseminated cancer cell dormancy and the paradigm of metastasis. Annu. Rev. Cancer Biol. 2, 377–393. doi: 10.1146/annurev-cancerbio-030617-050446
Albrengues, J., Shields, M. A., Ng, D., Park, C. G., Ambrico, A., Poindexter, M. E., et al. (2018). Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 361:eaao4227. doi: 10.1126/science.aao4227
Aslakson, C. J., and Miller, F. R. (1992). Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 52, 1399–1405.
Barkan, D., El Touny, L. H., Michalowski, A. M., Smith, J. A., Chu, I., Davis, A. S., et al. (2010). Metastatic growth from dormant cells induced by a Col-I-enriched fibrotic environment. Cancer Res. 70, 5706–5716. doi: 10.1158/0008-5472.CAN-09-2356
Barkan, D., Kleinman, H., Simmons, J. L., Asmussen, H., Kamaraju, A. K., Hoenorhoff, M. J., et al. (2008). Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer Res. 68, 6241–6250. doi: 10.1158/0008-5472.CAN-07-6849
Barney, L. E., Hall, C. L., Schwartz, A. D., Parks, A. N., Sparages, C., Galarza, S., et al. (2019). Tumor cell-organized fibronectin is required to maintain a dormant breast cancer population. bioRxiv [Preprint]. doi: 10.1101/686527
Barrios, J., and Wieder, R. (2009). Dual FGF-2 and intergrin alpha5beta1 signaling mediate GRAF-induced RhoA inactivation in a model of breast cancer dormancy. Cancer Microenviron. 2, 33–47. doi: 10.1007/s12307-009-0019-6
Bense, R. D., Qiu, S. Q., de Vries, E. G. E., Schröder, C. P., and Fehrmann, R. S. N. (2018). Considering the biology of late recurrences in selecting patients for extended endocrine therapy in breast cancer. Cancer Treat. Rev. 70, 118–126. doi: 10.1016/j.ctrv.2018.07.015
Blazar, B. A., Laing, C. A., Miller, F. R., and Heppner, G. H. (1980). Activity of lymphoid cells separated from mammary tumors in blastogenesis and Winn assays. J. Natl. Cancer Inst. 65, 405–410. doi: 10.1093/jnci/65.2.405
Borgen, E., Rypdal, M. C., Sosa, M. S., Renolen, A., Schlichting, E., Lønning, P. E., et al. (2018). NR2F1 stratifies dormant disseminated tumor cells in breast cancer patients 11 medical and health sciences 1112 oncology and carcinogenesis. Breast Cancer Res. 20:120. doi: 10.1186/s13058-018-1049-0
Bragado, P., Estrada, Y., Parikh, F., Krause, S., Capobianco, C., Farina, H. G., et al. (2013). TGF-β2 dictates disseminated tumour cell fate in target organs through TGF-β-RIII and p38α/β signalling. Nat. Cell Biol. 15, 1351–1361. doi: 10.1038/ncb2861
Braun, S., Vogl, F. D., Naume, B., Janni, W., Osborne, M. P., Coombes, R. C., et al. (2005). A pooled analysis of bone marrow micrometastasis in breast cancer. N. Engl. J. Med. 353, 793–802. doi: 10.1056/NEJMoa050434
Briand, P., and Lykkesfeldt, A. E. (2001). An in vitro model of human breast carcinogenesis: epigenetic aspects. Breast Cancer Res. Treat. 65, 179–187. doi: 10.1023/A:1006434503061
Buell, J. F., Hanaway, M. J., Thomas, M., Alloway, R. R., and Woodle, E. S. (2005). Skin cancer following transplantation: the israel penn international transplant tumor registry experience. Transplant. Proc. 37, 962–963. doi: 10.1016/j.transproceed.2004.12.062
Cameron, M. D., Schmidt, E. E., Kerkvliet, N., Nadkarni, K. V., Morris, V. L., Groom, A. C., et al. (2000). Temporal progression of metastasis in lung: cell survival, dormancy, and location dependence of metastatic inefficiency. Cancer Res. 60, 2541–2546.
Capulli, M., Hristova, D., Valbret, Z., Carys, K., Arjan, R., Maurizi, A., et al. (2019). Notch2 pathway mediates breast cancer cellular dormancy and mobilisation in bone and contributes to haematopoietic stem cell mimicry. Br. J. Cancer 121, 157–171. doi: 10.1038/s41416-019-0501-y
Carlson, P., Dasgupta, A., Grzelak, C. A., Kim, J., Barrett, A., Coleman, I. M., et al. (2019). Targeting the perivascular niche sensitizes disseminated tumour cells to chemotherapy. Nat. Cell Biol. 21, 238–250. doi: 10.1038/s41556-018-0267-0
Carpenter, R. A., Kwak, J.-G., Peyton, S. R., and Lee, J. (2018). Implantable pre-metastatic niches for the study of the microenvironmental regulation of disseminated human tumour cells. Nat. Biomed. Eng. 2, 915–929. doi: 10.1038/s41551-018-0307-x
Cavnar, S. P., Rickelmann, A. D., Meguiar, K. F., Xiao, A., Dosch, J., Leung, B. M., et al. (2015). Modeling selective elimination of quiescent cancer cells from bone marrow. Neoplasia 17, 625–633. doi: 10.1016/j.neo.2015.08.001
Chang, H. Y., Chi, J. T., Dudoit, S., Bondre, C., Van De Rijn, M., Botstein, D., et al. (2002). Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc. Natl. Acad. Sci. U.S.A. 99, 12877–12882. doi: 10.1073/pnas.162488599
Chéry, L., Lam, H. M., Coleman, I., Lakely, B., Coleman, R., Larson, S., et al. (2014). Characterization of single disseminated prostate cancer cells reveals tumor cell heterogeneity and identifies dormancy associated pathways. Oncotarget 5, 9939–9951. doi: 10.18632/oncotarget.2480
Clark, A. M., Kumar, M. P., Wheeler, S. E., Young, C. L., Venkataramanan, R., Stolz, D. B., et al. (2018). A model of dormant-emergent metastatic breast cancer progression enabling exploration of biomarker signatures. Mol. Cell. Proteom. 17, 619–630. doi: 10.1074/mcp.RA117.000370
Clark, A. M., Wheeler, S. E., Young, C. L., Stockdale, L., Shepard Neiman, J., Zhao, W., et al. (2016). A liver microphysiological system of tumor cell dormancy and inflammatory responsiveness is affected by scaffold properties. Lab. Chip. 17, 156–168. doi: 10.1039/c6lc01171c
Clements, M. E., and Johnson, R. W. (2019). PREX1 drives spontaneous bone dissemination of ER+ breast cancer cells. Oncogene 39, 1318–1334. doi: 10.1038/s41388-019-1064-3
Cock, J. M., Shibue, T., Dongre, A., Keckesova, Z., Reinhardt, F., Weinberg, R. A., et al. (2016). Inflammation triggers Zeb1-dependent escape from tumor latency. Cancer Res. 76, 6778–6784. doi: 10.1158/0008-5472.CAN-16-0608
de Prati, A., Butturini, E., Rigo, A., Oppici, E., Rossin, M., Boriero, D., et al. (2017). Metastatic breast cancer cells enter into dormant state and express cancer stem cells phenotype under chronic hypoxia. J. Cell. Biochem. 118, 3237–3248. doi: 10.1002/jcb.25972
Dexter, D. L., DeNucci, T., Miller, F. R., and Calabresi, P. (1978). Heterogeneity in drug sensitivity among tumor cell subpopulations of a single mammary tumor. Cancer Res. 38(11 Pt 1), 3758–3763.
Dhurjati, R., Liu, X., Gay, C. V., Mastro, A. M., and Vogler, E. A. (2006). Extended-term culture of bone cells in a compartmentalized bioreactor. Tissue Eng. 12, 3045–3054. doi: 10.1089/ten.2006.12.3045
Dupont, S. (2016). Role of YAP/TAZ in cell-matrix adhesion-mediated signalling and mechanotransduction. Exp. Cell Res. 343, 42–53. doi: 10.1016/j.yexcr.2015.10.034
Fang, J. Y., Tan, S. J., Wu, Y. C., Yang, Z., Hoang, B. X., and Han, B. (2016). From competency to dormancy: a 3D model to study cancer cells and drug responsiveness. J. Transl. Med. 14:38. doi: 10.1186/s12967-016-0798-8
Fluegen, G., Alvaro, A.-V., Wang, Y., Padgen, M. R., Williams, J. K., Nobre, A. R. R., et al. (2017). Phenotypic heterogeneity of disseminated tumour cells is preset by primary tumour hypoxic microenvironments. Nat. Cell Biol. 19, 120–132. doi: 10.1038/ncb3465
Gao, H., Chakraborty, G., Lee-Lim, A. P., Mo, Q., Decker, M., Vonica, A., et al. (2012). The BMP inhibitor Coco reactivates breast cancer cells at lung metastatic sites. Cell 150, 764–779. doi: 10.1016/j.cell.2012.06.035
Gao, H., Chakraborty, G., Zhang, Z., Akalay, I., Gadiya, M., Gao, Y., et al. (2016). Multi-organ site metastatic reactivation mediated by non-canonical discoidin domain receptor 1 signaling. Cell 166, 47–62. doi: 10.1016/j.cell.2016.06.009
Gawrzak, S., Rinaldi, L., Gregorio, S., Arenas, E. J., Salvador, F., Urosevic, J., et al. (2018). MSK1 regulates luminal cell differentiation and metastatic dormancy in ER + breast cancer. Nat. Cell Biol. 20, 211–221. doi: 10.1038/s41556-017-0021-z
Ghajar, C. M. (2015). Metastasis prevention by targeting the dormant niche. Nat. Rev. Cancer 15, 238–247. doi: 10.1038/nrc3910
Ghajar, C. M., Peinado, H., Mori, H., Matei, I. R., Evason, K. J., Brazier, H., et al. (2013). The perivascular niche regulates breast tumour dormancy. Nat. Cell Biol. 15, 807–817. doi: 10.1038/ncb2767
Giancotti, F. G. (2013). Mechanisms governing metastatic dormancy and reactivation. Cell 155, 750–764. doi: 10.1016/j.cell.2013.10.029
Goddard, E. T., Bozic, I., Riddell, S. R., and Ghajar, C. M. (2018). Dormant tumour cells, their niches and the influence of immunity. Nat. Cell Biol. 20, 1240–1249. doi: 10.1038/s41556-018-0214-0
Guiro, K., Patel, S. A., Greco, S. J., Rameshwar, P., and Arinzeh, T. L. (2015). Investigating breast cancer cell behavior using tissue engineering scaffolds. PLoS One 10:e0118724. doi: 10.1371/journal.pone.0118724
Harper, K. L., Sosa, M. S., Entenberg, D., Hosseini, H., Cheung, J. F., Nobre, R., et al. (2016). Mechanism of early dissemination and metastasis in Her2+ mammary cancer. Nature 540, 588–592. doi: 10.1038/nature20609
Harrell, J. C., Dye, W. W., Allred, D. C., Jedlicka, P., Spoelstra, N. S., Sartorius, C. A., et al. (2006). Estrogen receptor positive breast cancer metastasis: altered hormonal sensitivity and tumor aggressiveness in lymphatic vessels and lymph nodes. Cancer Res. 66, 9308–9315. doi: 10.1158/0008-5472.CAN-06-1769
Holen, I., Walker, M., Nutter, F., Fowles, A., Evans, C. A., Eaton, C. L., et al. (2016). Oestrogen receptor positive breast cancer metastasis to bone: inhibition by targeting the bone microenvironment in vivo. Clin. Exp. Metastasis 33, 211–224. doi: 10.1007/s10585-015-9770-x
Hosseini, H., Obradoviæ, M. M. S., Hoffmann, M., Harper, K. L., Sosa, M. S., Melanie, W.-K., et al. (2016). Early dissemination seeds metastasis in breast cancer. Nature 540, 552–558. doi: 10.1038/nature20785
Hubbi, M. E., and Semenza, G. L. (2015). Regulation of cell proliferation by hypoxia-inducible factors. Am. J. Physiol. Cell Physiol. 309, C775–C782. doi: 10.1152/ajpcell.00279.2015
Hüsemann, Y., Geigl, J. B., Schubert, F., Musiani, P., Meyer, M., Burghart, E., et al. (2008). Systemic spread is an early step in breast cancer. Cancer Cell 13, 58–68. doi: 10.1016/j.ccr.2007.12.003
Imamura, Y., Mukohara, T., Shimono, Y., Funakoshi, Y., Chayahara, N., Toyoda, M., et al. (2015). Comparison of 2D- and 3D-culture models as drug-testing platforms in breast cancer. Oncol. Rep. 33, 1837–1843. doi: 10.3892/or.2015.3767
Iskratsch, T., Wolfenson, H., and Sheetz, M. P. (2014). Appreciating force and shape-the rise of mechanotransduction in cell biology. Nat. Rev. Mol. Cell Biol. 15, 825–833. doi: 10.1038/nrm3903
Johnson, R. W., Finger, E. C., Olcina, M. M., Vilalta, M., Aguilera, T., Miao, Y., et al. (2016). Induction of LIFR confers a dormancy phenotype in breast cancer cells disseminated to the bone marrow. Nat. Cell Biol. 18, 1078–1089. doi: 10.1038/ncb3408
Kang, Y., Siegel, P. M., Shu, W., Drobnjak, M., Kakonen, S. M., Cordón-Cardo, C., et al. (2003). A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3, 537–549. doi: 10.1016/S1535-6108(03)00132-6
Kassim, Y. L., Tawil, E., Al Buquet, C., Cerf, D., and PierreVannier, J. (2017). Three dimensional tumor engineering by co-culture of breast tumor and endothelial cells using a hyaluronic acid hydrogel model. J. Clin. Exp. Oncol. 6:5. doi: 10.4172/2324-9110.1000194
Klein, C. A. (2011). Framework models of tumor dormancy from patient-derived observations. Curr. Opin. Genet. Dev. 21, 42–49. doi: 10.1016/j.gde.2010.10.011
Korah, R., Boots, M., and Wieder, R. (2004). Integrin α5β1 promotes survival of growth-arrested breast cancer cells: an in vitro paradigm for breast cancer dormancy in bone marrow. Cancer Res. 64, 4514–4522. doi: 10.1158/0008-5472.CAN-03-3853
Lamiaa, E.-S., Dubrovskyi, O., Kastrati, I., Danes, J. M., Zhang, Y., Whiteley, H. E., et al. (2017). Coactivation of estrogen receptor and IKK-β induces a dormant metastatic phenotype in ER-positive breast cancer. Cancer Res. 78, 974–984. doi: 10.1158/0008-5472.CAN-17-1686
Lan, Q., Peyvandi, S., Duffey, N., Huang, Y. T., Barras, D., Held, W., et al. (2019). Type I interferon/IRF7 axis instigates chemotherapy-induced immunological dormancy in breast cancer. Oncogene 38, 2814–2829. doi: 10.1038/s41388-018-0624-2
Lee, H. R., Leslie, F., and Azarin, S. M. (2018). A facile in vitro platform to study cancer cell dormancy under hypoxic microenvironments using CoCl2. J. Biol. Eng. 12:12. doi: 10.1186/s13036-018-0106-7
Li, Y., Hively, W. P., and Varmus, H. E. (2000). Use of MMTV-Wnt-1 transgenic mice for studying the genetic basis of breast cancer. Oncogene 19, 1002–1009. doi: 10.1038/sj.onc.1203273
Linde, N., Fluegen, G., and Aguirre-Ghiso, J. A. (2016). The Relationship Between Dormant Cancer Cells and Their Microenvironment. Adv. Cancer Res. 132, 45–71. doi: 10.1016/bs.acr.2016.07.002
Lu, X., Mu, E., Wei, Y., Riethdorf, S., Yang, Q., Yuan, M., et al. (2011). VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging α4β1-positive osteoclast progenitors. Cancer Cell 20, 701–714. doi: 10.1016/j.ccr.2011.11.002
Luzzi, K. J., MacDonald, I. C., Schmidt, E. E., Kerkvliet, N., Morris, V. L., Chambers, A. F., et al. (1998). Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am. J. Pathol. 153, 865–873. doi: 10.1016/S0002-9440(10)65628-3
Mahoney, K. H., Miller, B. E., and Heppner, G. H. (1985). FACS quantitation of leucine aminopeptidase and acid phosphatase on tumor-associated macrophages from metastatic and nonmetastatic mouse mammary tumors. J. Leukoc. Biol. 38, 573–585. doi: 10.1002/jlb.38.5.573
Malladi, S., MacAlinao, D. G., Jin, X., He, L., Basnet, H., Zou, Y., et al. (2016). Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell 165, 45–60. doi: 10.1016/j.cell.2016.02.025
Marlow, R., Honeth, G., Lombardi, S., Cariati, M., Hessey, S., Pipili, A., et al. (2013). A novel model of dormancy for bone metastatic breast cancer cells. Cancer Res. 73, 6886–6899. doi: 10.1158/0008-5472.CAN-13-0991
Massagué, J., and Obenauf, A. C. (2016). Metastatic colonization by circulating tumour cells. Nature 529, 298–306. doi: 10.1038/nature17038
McGrath, J., Panzica, L., Ransom, R., Withers, H. G., and Gelman, I. H. (2019). Identification of genes regulating breast cancer dormancy in 3D bone endosteal niche cultures. Mol. Cancer Res. 17, 860–869. doi: 10.1158/1541-7786.MCR-18-0956
McKeown, S. R. (2014). Defining normoxia, physoxia and hypoxia in tumours - implications for treatment response. Br. J. Radiol. 87:20130676. doi: 10.1259/bjr.20130676
Miller, B. E., Miller, F. R., Wilburn, D. J., and Heppner, G. H. (1987). Analysis of tumour cell composition in tumours composed of paired mixtures of mammary tumour cell lines. Br. J. Cancer 56, 561–569. doi: 10.1038/bjc.1987.242
Montagner, M., Bhome, R., Hooper, S., Chakravarty, P., Qin, X., Sufi, J., et al. (2020). Cross-talk with lung epithelial cells regulates Sfrp2-mediated latency in breast cancer dissemination. Nat. Cell Biol. 113, 1142–1151. doi: 10.1038/s41556-020-0474-3
Montagner, M., and Dupont, S. (2020). Mechanical forces as determinants of disseminated metastatic cell fate. Cells 9:250. doi: 10.3390/cells9010250
Morris, V. L., Koop, S., MacDonald, I. C., Schmidt, E. E., Grattan, M., Percy, D., et al. (1994). Mammary carcinoma cell lines of high and low metastatic potential differ not in extravasation but in subsequent migration and growth. Clin. Exp. Metastasis 12, 357–367. doi: 10.1007/BF01755879
Morris, V. L., Tuck, A. B., Wilson, S. M., Percy, D., and Chambers, A. F. (1993). Tumor progression and metastasis in murine D2 hyperplastic alveolar nodule mammary tumor cell lines. Clin. Exp. Metastasis 11, 103–112. doi: 10.1007/bf00880071
Najmi, S., Korah, R., Chandra, R., Abdellatif, M., and Wieder, R. (2005). Flavopiridol blocks integrin-mediated survival in dormant breast cancer cells. Clin. Cancer Res. 11, 2038–2046. doi: 10.1158/1078-0432.CCR-04-1083
Naume, B., Synnestvedt, M., Falk, R. S., Oncol, W.-G. J., Wiedswang, G., Weyde, K., et al. (2014). Clinical outcome with correlation to disseminated tumor cell (DTC) status after DTC-guided secondary adjuvant treatment with docetaxel in early breast cancer. J. Clin. Oncol. 32, 3848–3857. doi: 10.1200/JCO.2014.56.9327
Naumov, G. N., MacDonald, I. C., Weinmeister, P. M., Kerkvliet, N., Nadkarni, K. V., Wilson, S. M., et al. (2002). Persistence of solitary mammary carcinoma cells in a secondary site: a possible contributor to dormancy. Cancer Res. 62, 2162–2168.
Nolan, D. J., Ginsberg, M., Israely, E., Palikuqi, B., Poulos, M. G., James, D., et al. (2013). Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev. Cell 26, 204–219. doi: 10.1016/j.devcel.2013.06.017
Pack, L. R., Daigh, L. H., and Meyer, T. (2019). Putting the brakes on the cell cycle: mechanisms of cellular growth arrest. Curr. Opin. Cell Biol. 60, 106–113. doi: 10.1016/j.ceb.2019.05.005
Pan, H., Gray, R., Braybrooke, J., Davies, C., Taylor, C., McGale, P., et al. (2017). 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N. Engl. J. Med. 377, 1836–1846. doi: 10.1056/NEJMoa1701830
Pantel, K., and Hayes, D. F. (2018). Disseminated breast tumour cells: biological and clinical meaning. Nat. Rev. Clin. Oncol. 15, 129–131. doi: 10.1038/nrclinonc.2017.174
Pavlovic, M., Arnal-Estapé, A., Rojo, F., Bellmunt, A., Tarragona, M., Guiu, M., et al. (2015). Enhanced MAF oncogene expression and breast cancer bone metastasis. J. Natl. Cancer Inst. 107:djv256. doi: 10.1093/jnci/djv256
Piccolo, S., Dupont, S., and Cordenonsi, M. (2014). The biology of YAP/TAZ: hippo signaling and beyond. Physiol. Rev. 94, 1287–1312. doi: 10.1152/physrev.00005.2014
Pradhan, S., Sperduto, J. L., Farino, C. J., and Slater, J. H. (2018). Engineered in vitro models of tumor dormancy and reactivation. J. Biol. Eng. 12:37. doi: 10.1186/s13036-018-0120-9
Preciado, J. A., Reátegui, E., Azarin, S. M., Lou, E., and Aksan, A. (2017). Immobilization platform to induce quiescence in dormancy-capable cancer cells. Technology 05, 129–138. doi: 10.1142/s2339547817500078
Rak, J. W., McEachern, D., and Miller, F. R. (1992). Sequential alteration of peanut agglutinin binding-glycoprotein expression during progression of murine mammary neoplasia. Br. J. Cancer 65, 641–648. doi: 10.1038/bjc.1992.138
Rao, S. S., Kondapaneni, R. V., and Narkhede, A. A. (2019). Bioengineered models to study tumor dormancy. J. Biol. Eng. 13:3. doi: 10.1186/s13036-018-0137-0
Shibue, T., Brooks, M. W., Fatih Inan, M., Reinhardt, F., Weinberg, R. A., Inan, F. M., et al. (2012). The outgrowth of micrometastases is enabled by the formation of filopodium-like protrusions. Cancer Discov. 2, 706–721. doi: 10.1158/2159-8290.CD-11-0239
Shibue, T., and Weinberg, R. A. (2009). Integrin beta1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proc. Natl. Acad. Sci. U.S.A. 106, 10290–10295. doi: 10.1073/pnas.0904227106
Sosnoski, D. M., Norgard, R. J., Grove, C. D., Foster, S. J., and Mastro, A. M. (2015). Dormancy and growth of metastatic breast cancer cells in a bone-like microenvironment. Clin. Exp. Metastasis 32, 335–344. doi: 10.1007/s10585-015-9710-9
Sowder, M. E., and Johnson, R. W. (2018). Enrichment and detection of bone disseminated tumor cells in models of low tumor burden. Sci. Rep. 8:14299. doi: 10.1038/s41598-018-32653-2
Spencer, J. A., Ferraro, F., Roussakis, E., Klein, A., Wu, J., Runnels, J. M., et al. (2014). Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 508, 269–273. doi: 10.1038/nature13034
Srivastava, S., Koay, E. J., Borowsky, A. D., De Marzo, A. M., Ghosh, S., Wagner, P. D., et al. (2019). Cancer overdiagnosis: a biological challenge and clinical dilemma. Nat. Rev. Cancer 19, 349–358. doi: 10.1038/s41568-019-0142-8
Tilghman, R. W., Blais, E. M., Cowan, C. R., Sherman, N. E., Grigera, P. R., Jeffery, E. D., et al. (2012). Matrix rigidity regulates cancer cell growth by modulating cellular metabolism and protein synthesis. PLoS One 7:e37231. doi: 10.1371/journal.pone.0037231
Tivari, S., Korah, R., Lindy, M., and Wieder, R. (2015). An in vitro dormancy model of estrogen-sensitive breast cancer in the bone marrow: a tool for molecular mechanism studies and hypothesis generation. J. Vis. Exp. 2015:e52672. doi: 10.3791/52672
Touny, L. H. T., Vieira, A., Mendoza, A., Khanna, C., Hoenerhoff, M. J. J., and Green, J. E. E. (2014). Combined SFK/MEK inhibition prevents metastatic outgrowth of dormant tumor cells. J. Clin. Invest. 124, 156–168. doi: 10.1172/JCI70259
Uhr, J. W., and Pantel, K. (2011). Controversies in clinical cancer dormancy. Proc. Natl. Acad. Sci. U.S.A. 108, 12396–12400. doi: 10.1073/pnas.1106613108
Valastyan, S., and Weinberg, R. A. (2011). Tumor metastasis: molecular insights and evolving paradigms. Cell 147, 275–292. doi: 10.1016/j.cell.2011.09.024
Vishnoi, M., Peddibhotla, S., Yin, W., Scamardo, A. T., George, G. C., Hong, D. S., et al. (2015). The isolation and characterization of CTC subsets related to breast cancer dormancy. Sci. Rep. 5:17533. doi: 10.1038/srep17533
Weidenfeld, K., and Barkan, D. (2018). EMT and stemness in tumor dormancy and outgrowth: are they intertwined processes? Front. Oncol. 8:381. doi: 10.3389/fonc.2018.00381
Wells, A., Griffith, L., Wells, J. Z., and Taylor, D. P. (2013). The dormancy dilemma: quiescence versus balanced proliferation. Cancer Res. 73, 3811–3816. doi: 10.1158/0008-5472.CAN-13-0356
Wenzel, C., Riefke, B., Gründemann, S., Krebs, A., Christian, S., Prinz, F., et al. (2014). 3D high-content screening for the identification of compounds that target cells in dormant tumor spheroid regions. Exp. Cell Res. 323, 131–143. doi: 10.1016/j.yexcr.2014.01.017
Wheeler, S. E., Clark, A. M., Taylor, D. P., Young, C. L., Pillai, V. C., Stolz, D. B., et al. (2014). Spontaneous dormancy of metastatic breast cancer cells in an all human liver microphysiologic system. Br. J. Cancer 111, 2342–2350. doi: 10.1038/bjc.2014.533
Wright, L. E., Ottewell, P. D., Rucci, N., Peyruchaud, O., Pagnotti, G. M., Chiechi, A., et al. (2016). Murine models of breast cancer bone metastasis. Bonekey Rep. 5:804. doi: 10.1038/bonekey.2016.31
Keywords: cancer dormancy, metastatic dormancy, in vitro models cancer, cancer metastasis, breast cancer, metastasis biology
Citation: Montagner M and Sahai E (2020) In vitro Models of Breast Cancer Metastatic Dormancy. Front. Cell Dev. Biol. 8:37. doi: 10.3389/fcell.2020.00037
Received: 31 October 2019; Accepted: 15 January 2020;
Published: 03 March 2020.
Edited by:
Emilia Peuhu, University of Turku, FinlandReviewed by:
Penelope Dawn Ottewell, The University of Sheffield, United KingdomCopyright © 2020 Montagner and Sahai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marco Montagner, bWFyY28ubW9udGFnbmVyQHVuaXBkLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.