
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell Dev. Biol., 10 December 2019
Sec. Stem Cell Research
Volume 7 - 2019 | https://doi.org/10.3389/fcell.2019.00323
This article is part of the Research TopicImmunomodulatory Properties of Adult Stem Cells: Implications for the Niche Microenvironment in Homeostasis and DiseaseView all 9 articles
 Elena Stocco1,2,3
Elena Stocco1,2,3 Silvia Barbon1,2,3
Silvia Barbon1,2,3 Monica Piccione4
Monica Piccione4 Elisa Belluzzi5,6
Elisa Belluzzi5,6 Lucia Petrelli1
Lucia Petrelli1 Assunta Pozzuoli5,6
Assunta Pozzuoli5,6 Roberta Ramonda7
Roberta Ramonda7 Marco Rossato8
Marco Rossato8 Marta Favero7
Marta Favero7 Pietro Ruggieri6
Pietro Ruggieri6 Andrea Porzionato1,2
Andrea Porzionato1,2 Rosa Di Liddo3,4*
Rosa Di Liddo3,4* Raffaele De Caro1,2*†
Raffaele De Caro1,2*† Veronica Macchi1,2†
Veronica Macchi1,2†Recently, infrapatellar fat pad (IFP) has been considered as a source of stem cells for cartilage regeneration in osteoarthritis (OA) due to their ability for differentiation into chondrocytes. However, stressful conditions, like that related to OA, may induce a pathogenic reprograming. The aim of this study was to characterize the structural and functional properties of a new population of stem cells isolated from osteoarthritic infrapatellar fat pad (OA-IFP). Nine OA patients undergoing total knee arthroplasty (TKA) were enrolled in this study [median age = 74 years, interquartile range (IQR) = 78.25-67.7; median body mass index = 29.4 Kg/m2, IQR = 31.7-27.4]. OA-IFP stem cells were isolated and characterized for morphology, stemness, metabolic profile and multi-differentiative potential by transmission electron microscopy, flow cytometric analysis, gene expression study and cytochemistry. OA-IFP stem cells displayed a spindle-like morphology, self-renewal potential and responsiveness (CD44, CD105, VEGFR2, FGFR2, IL1R, and IL6R) to microenvironmental stimuli. Characterized by high grade of stemness (STAT3, NOTCH1, c-Myc, OCT-4, KLF4, and NANOG), the cells showed peculiar immunophenotypic properties (CD73+/CD39+/CD90+/CD105+/CD44–/+/CD45–). The expression of HLA-DR, CD34, Fas and FasL was indicative of a possible phenotypic reprograming induced by inflammation. Moreover, the response to mechanical stimuli together with high expression level of COL1A1 gene, suggested their possible protective response against in vivo mechanical overloading. Conversely, the low expression of CD38/NADase was indicative of their inability to counteract NAD+-mediated OA inflammation. Based on the ultrastructural, immunophenotypic and functional characterization, OA-IFP stem cells were hypothesized to be primed by the pathological environment and to exert incomplete protective activity from OA inflammation.
In osteoarthritis (OA) the clinical signs suggesting the occurrence of an inflammatory process are generally evident. These include manifestations such as swelling, heat, stiffness and pain, which are clearly recognizable since the disease onset and continue along its course (Favero et al., 2015). To date, arthroplasty is considered the only decisive option in OA; the other therapeutic strategies for early OA (e.g., weight reduction, exercise, braces, non-steroidal anti-inflammatory drugs, symptomatic slow-acting drugs, intra-articular injections of glucocorticoid or hyaluronic acid) can only slow its progression without any significant effect on the cartilage integrity (Im, 2018). Worldwide, approximately 9.6% of men and 18% of women aged ≥ 60 years are estimated to have symptomatic OA (Michaud et al., 1996); hence, the extent of the disease, the significant impairment of patients’ quality of life and the related costs require a better characterization of the pathophysiological mechanisms involved in its development. This would ensure more effective therapeutic approaches to OA, overcoming the limitations of the current ones, also suggesting alternative and/or tailored options for disease management.
Currently, it is commonly accepted that OA is not merely a cartilage disease but a whole joint disease involving different structures such as meniscus, synovial membrane, and infrapatellar fat pad (IFP) (Belluzzi et al., 2019). In the recent years, the potential role of the IFP in OA has been suggested by many researchers. For a long time, this peculiar extra-synovial adipose tissue was simplistically retained a deformable space filler endowed with shock absorber properties in the knee joint and a bystander in knee OA; conversely, it is now considered an emerging player in knee OA with a multifunctional role in knee joint homeostasis, also mediating joint pain and inflammation (Ioan-Facsinay and Kloppenburg, 2013; Belluzzi et al., 2017, 2019; Macchi et al., 2018). This occurs through an active secretion of cytokines and adipokines (Ioan-Facsinay and Kloppenburg, 2013) whose levels are gender dependent, being normally higher in women than in men even after adjusting for body mass index (BMI) (Poonpet and Honsawek, 2014). Therefore, it can be assumed that the IFP, similar to subcutaneous and visceral adipose tissue, is an active organ whose products contribute to inflammatory and degenerative processes underlying common joint diseases (Toussirot et al., 2007).
Furthermore, the IFP itself is affected by immune cells in OA; in fact, compared to healthy-IFPs, it shows an increase in inflammatory infiltration, vascularization and fibrosis with thickened interlobular septa also supported by higher levels of VEGF, MCP-1, and IL-6 proteins (Favero et al., 2017; Fontanella et al., 2018).
In addition to constitutive stromal vascular cells represented by hematopoietic cells (i.e., macrophages, T cells, mast cells, and B cells) and endothelial cells, also progenitor cells with mesenchymal stem cell-like characteristics have been recognized in the IFP (Hindle et al., 2017; Huri et al., 2018). Stem cells regenerate tissues in homeostasis but they also sense and respond to stressful conditions (Naik et al., 2018) undergoing a possible pathogenic reprograming under long-lasting inflammation (Michael et al., 2016). Thus, the purpose of this study was to isolate OA-IFP stem cells from patients undergoing total knee arthroplasty (TKA) and provide a broad characterization of such cell populations. A better understanding of their origin and pathophysiological role in OA may suggest a possible novel interpretive key to explain histopathological features commonly encountered in OA joints.
This study was performed after receiving institutional review board approval (CESC Code: 4510/AO/18). Patients affected by OA undergoing to TKA, showing good health, without tumor and/or knee comorbidities and not suffering from infective conditions were eligible. Nine patients were enrolled in this study after signing the informed consent [median age = 74 years, interquartile range (IQR) = 78.25-67.7; median body mass index = 29.4 Kg/m2, IQR = 31.7-27.4]; demographic data are reported in Table 1. At the moment of sample collection, the patients were naïve from therapies. Tissue specimens were collected during TKA and then rapidly processed for the isolation of stromal cells.
Osteoarthritic infrapatellar fat pad stem cells were isolated according to a modified protocol by Hindle et al. (2017). After washing tissues in phosphate buffered saline (PBS) supplemented with 2% penicillin/streptomycin (P/S; Sigma-Aldrich), enzymatic digestion was performed overnight at 37°C in a medium consisting of Dulbecco’s Modified Eagle Medium High Glucose (DMEM HG, Gibco, Thermo Fisher Scientific, Waltham, MA, United States), 1% collagenase B (Roche, Basel, Switzerland) and 1% P/S. The cell suspension was filtered through 40-μm cell strainer (BD Falcon, Franklin Lakes, NJ, United States) and centrifuged before seeding the pellet in proliferation medium (DMEM HG, 10% FBS, 1% P/S).
The proliferation rate of OA-IFP stem cells was evaluated from 8th to 20th generation after seeding in a 6-well plate (seeding density of 5 × 103 cells/cm2). At intervals of 24 h, the cells were detached by trypsin-EDTA solution and counted with the automatic cell counter. Average cell number per well and standard deviation (SD) were considered to describe the population doubling level (PDL). At first, the population doubling (PD) was calculated according to the equation:
where Nt is the number of cells counted at the time point considered; and N0 is the number of cells seeded at t = 0 (Ferroni et al., 2019). Then, the PDL of every passage was determined by addition of the PD relative to that passage to the PD of previous passages (Marrelli et al., 2013).
Cell viability was also assessed at 8th, 14th, and 20th generations using the Apoptotic/Necrotic/Healthy Cells Detection Kit (Promokine, Cat No. PK-CA707-30018, Heidelberg, Germany) following the Manufacturer’s instructions. The detection of stained cells was performed with a DMR microscope (Leica, Wetzlar Germany).
To assess OA-IFP cells metabolic activity, the subcultures were treated with 3-(4,5-dimethylthiazol-2-yl)-2,5-dimethyltetrazolium bromide (MTT) (0.5 mg/mL) for 4 h, at 1, 2, 5, 7, and 14 days from seeding. Formazan precipitates were dissolved in 2-propanol acid (0.04 M HCl in 2-propanol) and optical density was measured at 570 nm, using a Microplate autoreader EL 13. Results were expressed as number of cells grown on seeded surface (Grandi et al., 2018). Determining cell number was allowed by the standard curve previously developed (Gerlier and Thomasset, 1986).
The OA-IFP stem cells and subcultures were observed by an optical microscope DM/IL (Leica, Wetzlar, Germany) equipped with a camera Nikon Digital Sight Ds-SMCc (Nikon Corporation, Tokyo, Japan). For ultrastructural studies, the cells were fixed with 2.5% glutaraldehyde (Polysciences Europe GmbH, Hirschberg an der Bergstrasse - Germany) in 0.1 M phosphate buffer and post-fixed with 1% osmium tetroxide (Agar Scientific Elektron Technology, United Kingdom) in 0.1 M phosphate buffer. After dehydration in a graded alcohol series, the samples were embedded in epoxy resin (Epoxy Embedding Medium Kit, Sigma-Aldrich, Switzerland) and processed as previously described (Stocco et al., 2018). Both 1% Toluidine blue staining and analysis by a Hitachi H-300 Transmission Electron Microscope (Hitachi, Krefeld, Germany) occurred.
Genes related to stemness (TERT, REX1, SOX2, STAT3, NOTCH1, c-Myc, OCT-4, KLF4, and NANOG) and metabolic activity (CD38 and CALR) of OA-IFP stem cells were characterized by RT-qPCR. Cells from 8th passage at a sub-confluent state (i.e., 70–80%) were used to extract RNA with TRI Reagent® solution (Zymo Research, United States). After RNA quantification using the NANODROP 2000 (Thermo Fisher Scientific, Waltham, MA, United States), gene expression study was carried out in a single-step reaction, using 12.5 ng of RNA, oligonucleotides (Thermo Fisher Scientific, Inc) listed in Table 2, qPCRBIO SyGreen 1-Step Go Lo-ROX (PCR Biosystems Ltd., London, United Kingdom) and Magnetic Induction Cycler (Bio Molecular Systems, Upper Coomera, QLD, Australia). To obtain statistical significance, three independent preparations were obtained to analyze target genes and hypoxanthine phosphoribosyltransferase 1 (HPRT1) housekeeping marker listed in Table 2. For data analysis, the comparative Ct method (2–Δ Ct) was adopted. Human leukocytes isolated by a standard density gradient separation from healthy donors were used as control for the analysis of CD38 and CALR.
Sub-confluent (i.e., 70–80%) OA-IFP stem cells from 8th generation were analyzed by single staining with FACSCanto II Flow cytometer (BD Biosciences, San Diego, CA, United States) at 24 h after seeding, using anti-human antibodies (Table 3). FCM analysis were performed to evaluate the expression of markers related to mesenchymal stem cell immunophenotype, hematopoietic lineage commitment, enzyme/signaling molecules, cell–matrix adhesion and cytokine/growth factor receptors. For each marker, data from three experimental replicas were analyzed with FlowJoTM software (BD) and then were reported as mean percentage of positive cells. Samples treated with only secondary antibodies or isotype control antibodies (Table 3) were considered as controls. No exclusion of dead cells from analysis was performed.
To confirm FCM data, a CD45 and HLA-DR double staining was performed on OA-IFPs tissue sections from samples used for stem cells isolation.
Osteoarthritic infrapatellar fat pad specimens were fixed in 10% formalin in PBS, paraffin-embedded and cut into 4 μm-thick sections. After deparaffinization, the sections were treated with EDTA (pH = 8; for Mouse Anti-Human-CD45) and citrate buffer (pH = 6; for Mouse Anti-Human HLA-DR) in microwave oven heating. After washing in PBS, the specimens were incubated with Dual Endogenous Enzyme block (ready to use – Dako, Milan, Italy) to assure endogenous peroxidase and alkaline phosphatase inhibition, rinsed in PBS, blocked in 0.2% BSA (Bovine Serum Albumin) and incubated with Mouse Anti-Human CD45 1:100 (Biocare CM016) overnight at 4°C. After washing, the samples were incubated with the secondary antibody (HRP-conjugated Goat Anti-Mouse – Jackson ImmunoResearch, Cambridgeshire, United Kingdom) and the reaction was detected with diaminobenzidine, Dako (brown stain). Thus, the sections were rinsed in PBS and incubated with Mouse Anti-Human HLA-DR 1:100 (Clone TAL.1B5 – Dako) overnight at 4°C. The slides were subsequently rinsed in PBS, covered with Mouse AP Polymer and HLA-DR was visualized with GBI-Permanent Red solution (Red stain). The samples were counterstained with Harris modified hematoxylin (Sigma, Milan, Italy) before mounting.
IFP specimens from OA patients are demonstrated to contain high level of fibrosis (Favero et al., 2017) and to be characterized by cells endowed with chondrogenic and osteogenic potential (Hindle et al., 2017). Thus, a gene expression study by qPCR was carried out using subcultures under resting conditions and at a sub-confluence state, in order to define the expression of extracellular-matrix related proteins of cartilage/bone tissue (COL1A1, COL2A1, COL9A3, COL10A1, SPARC), pro-fibrogenic (CTTN) markers and glucose transporters (GLUT1, GLUT4) as above reported, using oligo primers listed in Table 2.
Osteoarthritic infrapatellar fat pad stem cells ability to be used for creating an in vitro model of adipose tissue, resembling the in vivo environment, was verified. Thus, OA-IFP stem cells were seeded in a 24 well plate (5 × 103 cells/cm2) and cultured in proliferative medium for 3 days. To mimic the IFP environment, sub-confluent cells where then exposed to adipogenic soluble stimuli: insulin (10 μg/mL), dexamethasone (1 μM); indomethacin (60 μM), 3-isobutyl-1-methyxantine (0.5 mM) (all from Sigma-Aldrich) in basal medium (DMEM HG, 10% FBS, 1% P/S); cells under resting conditions were used as controls. At days 7 and 14, the cells were fixed with a 10% formalin solution (Sigma-Aldrich) and stained with Oil Red O (5 mg/ml in isopropanol) according to standard protocol. After mounting with glycerol, all samples were analyzed by a Leica DMR microscope endowed with a Nikon Digital Sight Ds-SMCc camera (Nikon Corporation).
In order to evaluate the response of OA-IFP stem cells to pathological environment, an in vitro system was set up. In details, endothelial medium [EBMTM-2 medium (Lonza Group AG, Switzerland), 5% FBS, 1% P/S] enriched of angiogenic factors [vascular endothelial growth factor (VEGF)], human basic fibroblast growth factor (hFGF-b), epidermal growth factor (EGF), insulin-like growth factor-1 (IGF-1), heparin, ascorbic acid was used in combination with stiff (glass coverslip) or soft (BD Matrigel diluted 1:10 in EBMTM-2 medium) substrates. The soluble factors were used to simulate the vascular-related signals, while soft or stiff substrates were employed to reproduce mechanical loading forces. It is expected that cells may respond differently according to the specific stimuli they exposed to. OA-IFP stem cells seeded on plastic surface of 24 well plate (5 × 103 cells/cm2) and cultured in proliferation medium represented the control-group.
At 3 and 7 days, the cultures were observed by a Leica DMR microscope endowed with a Nikon Digital Sight Ds-SMCc camera (Nikon Corporation) and pictures at 10x were processed through ImageJ software as described by Guidolin et al., 2004 to detect the branches (i.e., number and length) and the branching points.
Statistical analysis was performed with GraphPad Prism 6.0 (GraphPad Software, Sand Diego, CA, United States). Data referring to age and body mass index of the patients were expressed as median and interquartile range. All experimental data were expressed as average of three different experiments (technical replicates) ±Standard Deviation (SD) referring to nine patients. Statistical significance was determined by unpaired t-test and results were considered significant when P < 0.05.
IFP tissue samples were obtained from both male (n = 3) and female (n = 6) patients undergoing TKA (Figures 1A,B). The samples showed the presence of a highly vascularized synovial membrane, which was removed before tissue processing in order to obtain only IFP-native stem cells. Tissues had a fibrous consistency; in particular, a certain resistance during fragmentation was encountered in the external layer. On the contrary, the inner layer was less fibrotic.
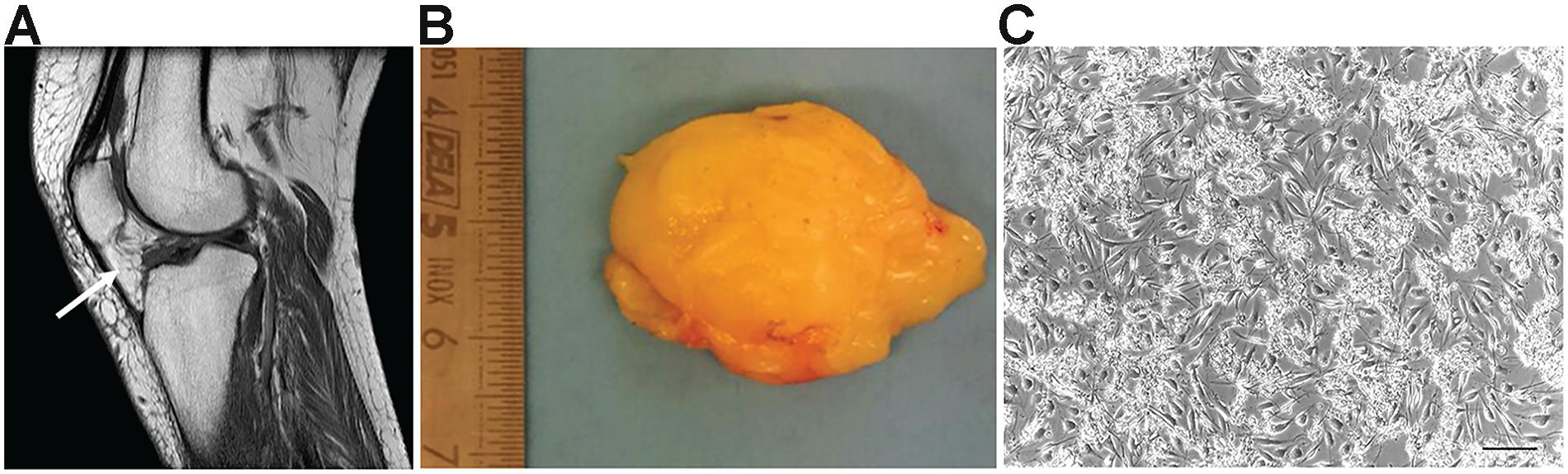
Figure 1. Osteoarthritic infrapatellar fat pad (OA-IFP) and derived cells. (A) Magnetic resonance imaging of the knee in sagittal section, showing the infrapatellar fat pad (IFP – white arrow) before total knee arthroplasty(TKA). (B) Gross appearance of the IFP after surgical excision and before tissue enzymatic digestion for cells isolation. (C) Representative optical microscope image of the IFP stem cells at P0 in culture. P, passage. Scale bar, 100 μm.
The whole isolation process was completed in 14 h, considering the over-night incubation period in the presence of the digesting enzyme collagenase B. At the end of the isolation protocol, the cells were resuspended in the proliferation medium and seeded at high density. It was not possible to accurately determine the mean total number of cells extracted from each sample due to the presence of extracellular matrix (ECM) fragments in suspension hindering the total cell count. However, high cell density as well as the maintenance of matrix residues were necessary to optimize cell adhesion and survival in monolayer culture (Figure 1C); tissue debris were then easily removed by subsequent changes of medium and passages in culture.
In culture, OA-IFP stem cells showed a typical spindle-like morphology which was maintained along passages in culture (Figure 2A). The proliferation rate of OA-IFP cells was evaluated in 12 consecutive generations to assess their ability to sustain long-term culture without reaching senescence. As reported by Figure 2B, from passage 8th to 20th the stem cells had performed 11.9 ± 8.3 population doublings. Vitality was also confirmed by specific staining using fluorescent dyes (Figure 2C).
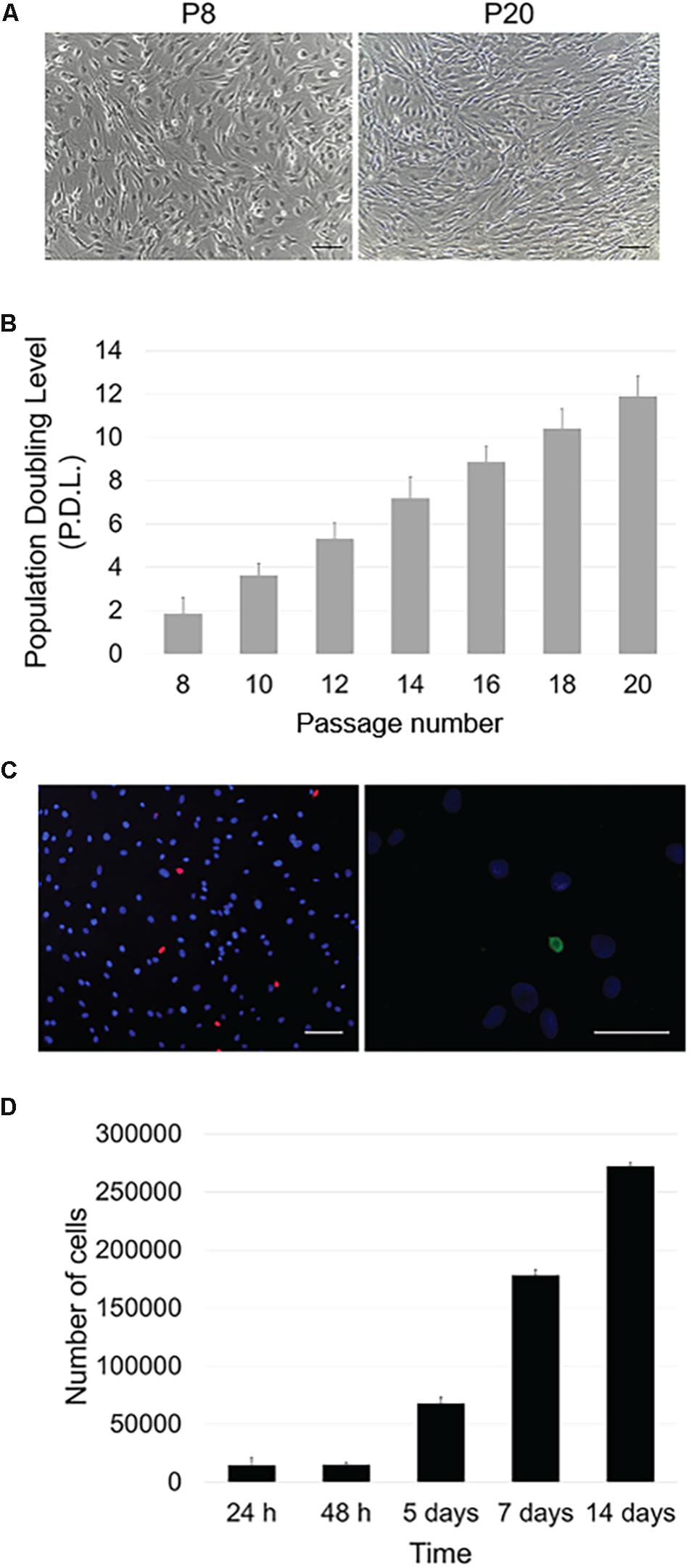
Figure 2. Proliferative potential and metabolic activity. (A) Optical microscope images of OA-IFP cells in proliferative medium at P8 and P20. P: passage. Scale bars, 100 μm. (B) Population Doubling Level (PDL) calculated throughout 12 generations; cells performed 11.9 ± 8.3 population doublings. (C) Vitality/necrosis/apoptosis test after treatment with fluorescent dyes. Blue dye stained viable cells while red elements corresponded to necrotic cells (scale bar, 100 μm); apoptotic cells were colored in green (scale bar, 50 μm). The images are representative of cells at 8th, 14th, and 20th generations. (D) Metabolic activity (MTT assay) of OA-IFP cells from 24 h to 14 days in culture. For the panels (B,D), results are the average of 3 technical replicates each referring to 9 patients.
MTT assay was used to assess OA-IFP stem cells metabolic activity also demonstrating a progressive cell growth at different endpoints (Figure 2D). From 24 to 48 h after seeding, the cell number varied moderately due to an initial slow expansion. Since day 5th, the metabolic activity and the cell number increased and the same trend was observed at day 7.
At 14 days from seeding, the contact inhibition between cells at confluence influenced the metabolic activity resulting in a slower cell growth.
Toluidine blue staining displayed cells with a regular appearance and deeply blue-stained vesicles distributed in perinuclear areas (Figure 3A). At TEM analysis, ultrastructural cell constituents like mitochondria, rough endoplasmic reticulum, Golgi apparatus, secretion vesicles were clearly identified in the cytoplasmic area surrounding cell nucleus which appeared voluminous and bordered by a thin and regular membrane (Figures 3B,C). Lysosomes were also recognized within the cytoplasm appearing as rounded formations of electron-dense material corresponding to those deeply stained with toluidine-blue in Figure 3A. Interestingly, TEM analysis also allowed to discriminate apparently empty round vesicles surrounding the dense ones. Such non-electron dense elements showed the presence of a thin honeycomb-like structure; in some contact areas, a partial fusion between the external membranes of the two vesicles types occurred. Filamentous and disorganized cell secretion products were localized in proximity to cell membrane (Figures 3D,E).
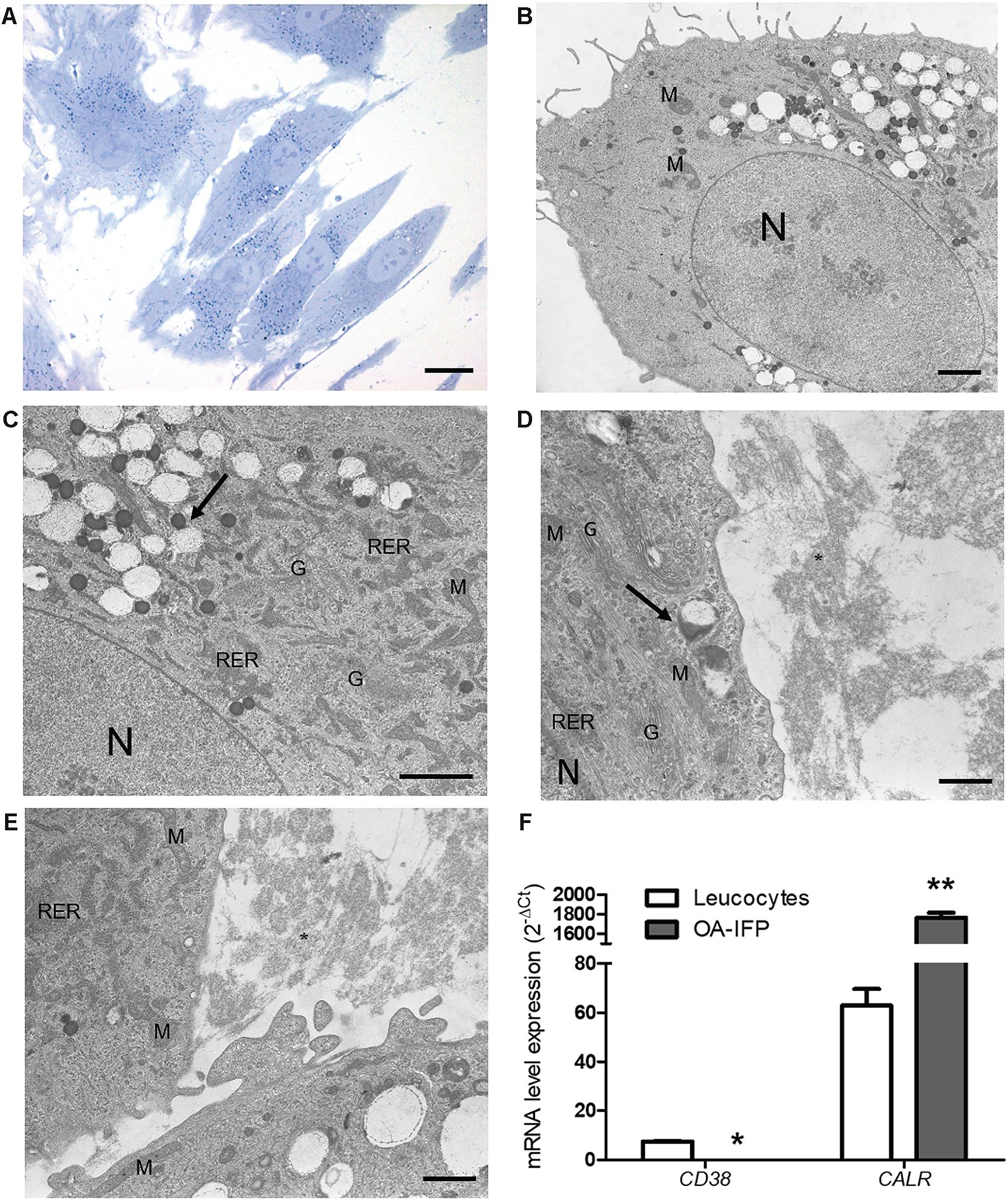
Figure 3. Ultrastructural characterization of OA-IFP stem cells. Toluidine Blue staining images (A) and TEM micrographs (B–E) of OA-IFP cells at P8 in culture. Black arrows in (C) and (D) show electron dense lysosomes and empty vesicles. Black asterisk in (E) shows secretion material resembling collagen fibrils outside the cytoplasm. M, mitochondria; N, nucleus; G, golgi apparatus; RER, rough endoplasmic reticulum. Scale bars, (A) 20 μm; (B) and (C) 2 μm; (D) and (E) 1 μm. (F) mRNA expression levels of CD38 and CALR in OA-IFP stem cells compared to human leukocytes (∗P < 0.5; ∗∗P < 0.01). For panel (F), results are the average of 3 technical replicates referring to 9 patients.
From the metabolic point of view, the analysis by qPCR on OA-IFP stem cells versus leukocytes, showed null-expression of mRNA for CD38 and significantly (P < 0.01) higher levels of calreticulin gene (Figure 3F).
During in vitro short and prolonged expansion, the active mRNA expression of NANOG, c-Myc, NOTCH1, KLF4 and STAT3 (Figure 4A) proved a multipotential grade of OA-IFP stem cells. By FACS analysis (Figures 4B,C) OA-IFP populations evidenced an almost homogenous immunophenotypic profile. In particular, the samples showed to be over 90% positive for the mesenchymal stem cell marker CD73, FGFR2, and IL6R suggesting both immunomodulatory activity and cellular responsivity to IFP- and OA-microenvironmental secreted factors. The cellular multipotency was confirmed by the detection of CD105, CD90, and CD44, whose variable expression among donors was hypothesized to be related to the age or inflammation state of patients. Due to their significant expression of HLA-DR (≥90%), Fas/FasL (≥98%), CD39, VEGFR2, and, only in some cases, of CD34, OA-IFP stem cells could represent a cellular compartment primed by chronic inflammatory conditions (Figures 4C,D). The null expression of CD45, CD117, and PDGFRβ excluded their possible derivation from hematopoietic/endothelial progenitors or perivascular stem cell niche (Figures 4B,C). Double immunohistochemistry on OA-IFP tissue samples confirmed the presence of cellular elements (Figure 4E) simultaneously positive to HLA-DR and negative to CD45 (Figure 4C). Taken together, our collected data evidenced distinctive immunophenotypic properties of OA-IFP compared to other multipotent populations isolated from IFP.
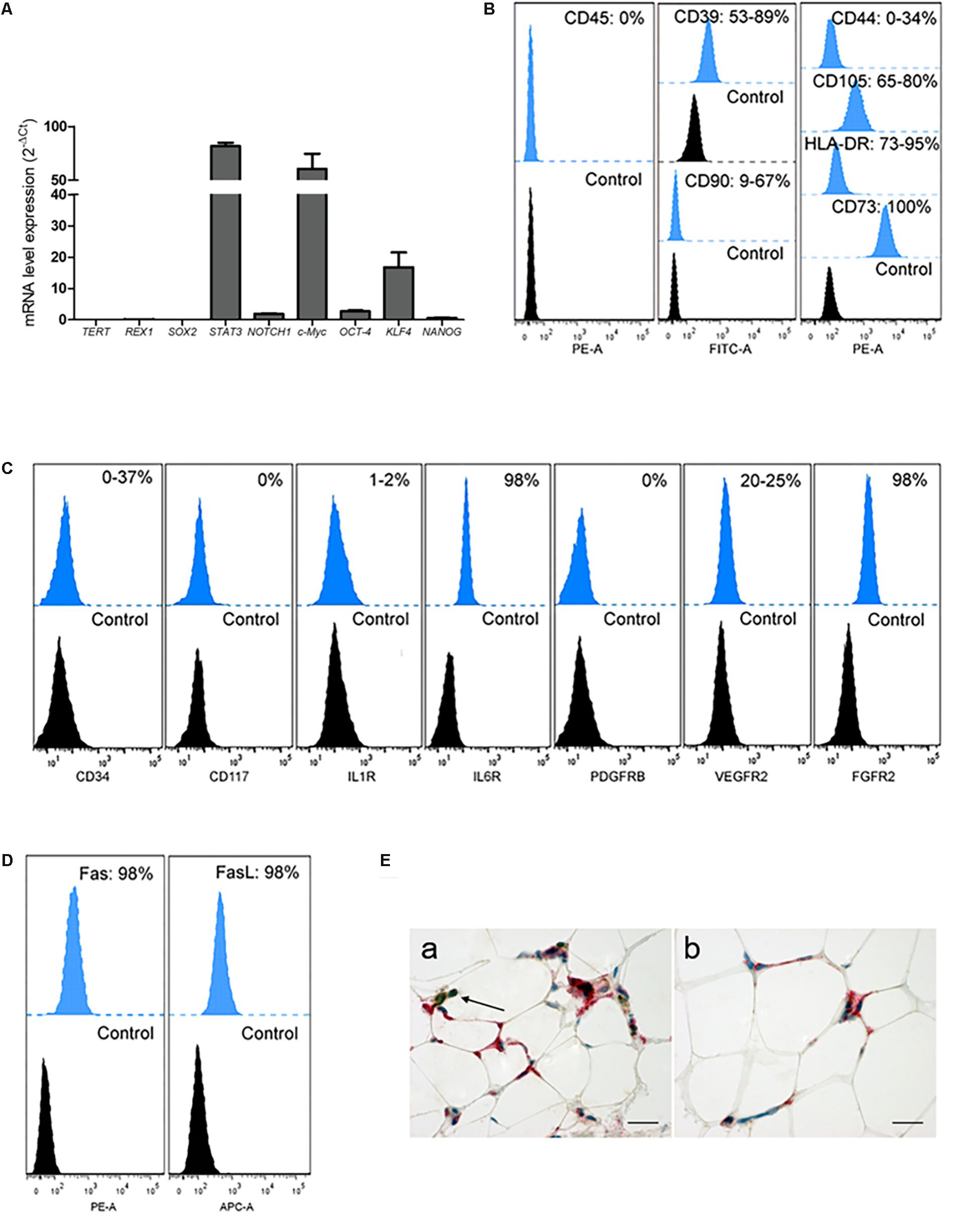
Figure 4. Analysis of OA-IFP by qPCR, FCM and immunohistochemistry. OA-IFP subcultures from 8th generation and under proliferative conditions were analyzed at a sub-confluence state for stemness (A,B) or cellular responsivity markers (C,D) by qPCR (A) and FCM [FacsDiva and FlowJo software (B,D)] (B–D) where the histograms show the mean percentage of positive cells; samples treated with only secondary antibodies or isotype control antibodies were used as references. Three technical replicates for each sample were analyzed. (E) Double immunohistochemical analysis of OA-IFP tissue samples showing cellular elements positive to HLA-DR (red) and negative to CD45 (brown). Lymphocytic elements, positive to only CD45 (brown), were considered as an internal control of the method (black arrow, E,a) (Scale bar: 25 μm). FCM, flow cytometry analysis.
Under resting conditions, OA-IFP stem cells also demonstrated to have a differentiation potential into chondrogenic, and osteogenic lineages producing mRNAs of COL1A1, SPARC, and GLUT1; in addition, the significant expression of cortactin/CTTN gene suggested that the differentiation of OA-IFP stem cells might be regulated by mechano-transduction (Figure 5A). When treated with adipogenic stimuli, the cells acquired distinct functionality as demonstrated by the cytoplasmic accumulation of lipid droplets (Figure 5B). Moreover, combining specific soluble (endothelial medium enriched with angiogenic factors) and mechanical stimuli (stiff or soft), OA-IFP cells showed different behaviors (Figure 5C). On glass (i.e., stiff support) cells acquired an elongated morphology, describing cord-like structures; cell density remained comparable at both end-points (3 and 7 days). On Matrigel (i.e., soft support), an initial response similar to that previously described occurred at day 3. Thereafter cell density increased and the network was no more identifiable. In contrast, the control group did not show any specific cell orientation nor reduction in proliferation.
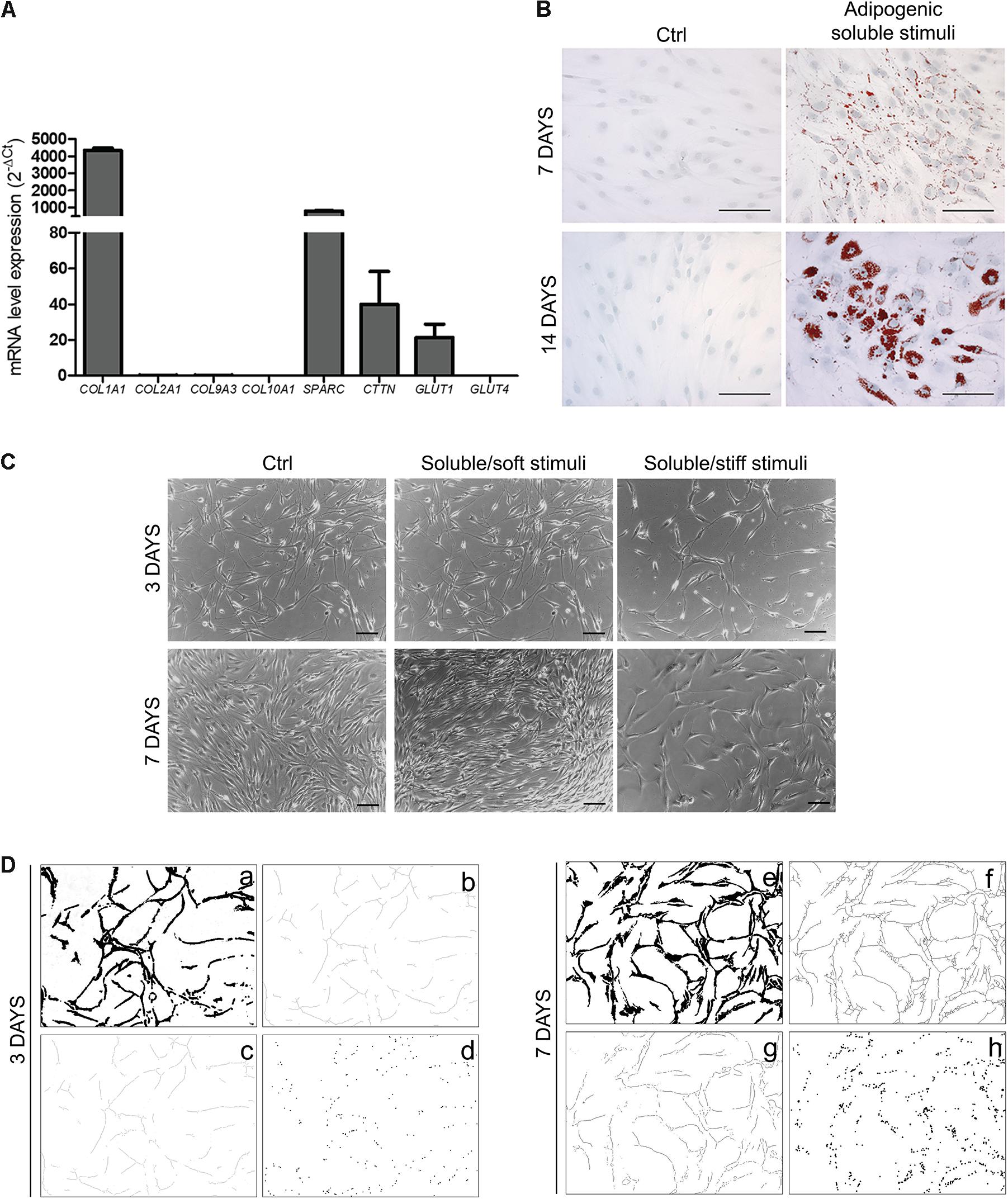
Figure 5. OA-IFP stem cells responsiveness to environmental stimuli. (A) qPCR analysis of mRNA expression levels of COL1A1, SPARC, GLUT1, and CTTN gene on OA-IFP stem cells at a sub-confluence state; null-expression of GLUT4, CTTN, COL2A1, COL9A3, COL10A1 mRNAs was detected. (B) Response of OA-IFP stem cells to adipogenic stimuli. After 7 and 14 days from stimulation, the cells showed cytoplasmic red-stained lipid droplets; scale bars, 100 μm. (C) Response of OA-IFP stem cells to soluble (endothelial medium enriched of angiogenic factors) and mechanical stimuli (stiff and soft support) at 3 and 7 days from stimulation. OA-IFP stem cells seeded on plastic surface and cultured in proliferation medium represented the control-group; scale bars, 100 μm. (D) Image processing steps of images referring to OA-IFP stem cells cultured on stiff support, at 3 and 7 days from stimulation. After edge detection and threshold binary tools (a,e) the skeletons were calculated (b,f), before the analysis of branches (i.e., number and length) (c,g) and branching points (d,h). The processed images highlight the formation of the cord-like structures. For panel (A), results are the average of 3 technical replicates for each sample referring to 9 patients.
After processing the images referring to OA-IFP stem cells cultured on stiff support (Figure 5D), it was observed a progressive increase in the number and length of the branches (+59.25 and +76.49%, respectively) and in the number of the branching points (+55.28%). The number of the branches increased from 132.43 ± 11.89 (day 3) to 223.50 ± 13.05 (day 7) per square unit (μm2); the length of the branches (expressed as linear value - μm) increased from 8830.86 ± 639.29 (day 3) to 11543.92 ± 1156.96 (day 7). A variation in the number of the branching points was also observed, from 87.70 ± 11.19 to 158.63 ± 13.44 per square unit (μm2) at day 3 and 7, respectively.
To date, IFP stem cells have been proposed as an alternative to subcutaneous adipose-derived stem cells and an attractive cell source for cartilage regeneration due to their ability for differentiation into chondrocytes, minor immunological rejection and constitutive immunomodulatory properties (Felimban et al., 2014; Ye et al., 2014, 2016; Tangchitphisut et al., 2016; Arora et al., 2017; Sun et al., 2018; Radhakrishnan et al., 2019). Inflammatory processes, microenvironmental changes, chemical factors and physical cues evoke a tissue adaptive and protective response acting in concert on immune cells and/or endogenous stem cells. In contrast to López-Ruiz et al. (2013), in this study we have demonstrated that in OA, IFP-derived stem cells might be reprogramed by the inflammatory environment preserving multi-differentiative plasticity. Thus, they might exert a protective role in counteracting mechanical overloading through deposition of collagenous matrix.
In accordance with the peculiar clinical OA framework, the patients enrolled in the study suffered from obesity, hypertension, dyslipidemia and type 2 diabetes, that are all conditions of chronic stress (Courties et al., 2017) affecting joint structures, including IFP and its cellular compartments.
Showing adherent and spindle-like morphology, OA-IFP stem cells were proved to have a high metabolic activity and a population doubling time of 42.7 ± 3.8 h likely adipose stem cells (Peng et al., 2008).
As previously described by López-Ruiz et al. (2013), TEM analysis on OA-IFP stem cells evidenced a cytoplasm rich in transport/storage vesicles and deeply stained/electron dense structures, rough endoplasmic reticulum and mitochondria. Higher magnifications also suggested the presence of autolysosomes, a product of fusion of lysosomes (dark, electron dense vesicles) and autophagosomes (large, white vacuoles) that are deputed to autophagy (Revuelta and Matheu, 2017; Boya et al., 2018), a process that have been suggested to have a role in OA development (Deng et al., 2019). In fact, there is consensus in claiming that autophagy can be triggered by a variety of internal or external stimuli as well as hypoxia, Reactive Oxygen Species (ROS) and accumulation of unfolded proteins, which are all conditions typically encountered in OA (Hughes et al., 2017).
Acting like scavengers, autolysosomes catabolize undesirable components to provide energy and essential building materials to maintain cellular homeostasis. Hence, according to these assumptions, OA-IFP stem cells likely switch-on this mechanism to counteract the disease-related stress condition.
Stem cells take advantage from glycolysis to gain energy and regulate the undifferentiated status (Zhang et al., 2016), while mitochondrial function is linked to stem cells activation. The significant amount of mitochondria (Bravo et al., 2011) (as shown also for OA-IFP stem cells by TEM), correlated with the high expression levels of calreticulin gene (Ní Fhlathartaigh et al., 2013). Calreticulin was assumed as an indicator of cell stress condition, activated status and a switched nicotinamide adenine dinucleotide (NAD+)-dependent energy metabolism. In OA, it has been reported that the unbalanced biomechanics cause a self-perpetuating cycle of low-grade damage (Scanzello et al., 2008) that, in turn, leads to the production of damage-associated molecular patterns. Cartilage ECM breakdown products, fibronectin and hyaluronic acid (Okamura et al., 2001; Termeer et al., 2002; van Lent et al., 2012) and intracellular alarmins [HMGB1 (Li et al., 2011), S100 proteins (van Lent et al., 2012)] signal on synovial macrophages, synoviocytes, or chondrocytes promoting the local production of numerous inflammatory mediators [IL-1β (He et al., 2002), VEGF, TNFα, IL-6 (Ushiyama et al., 2003), PDGFs (Pohlers et al., 2006)]. Moreover, regulatory mechanisms involved in controlling NAD+ levels have been demonstrated to be affected in OA because of an increased expression of IL-β and a compromised activity of NADases (Kobayashi et al., 2017). Thus, endoplasmic reticulum stress and highly expressed vacuoles in concert with the expression of prognostic OA marker IL-1βR1 highlight the occurrence of a strict correlation between OA-IFP biology and the inflammatory microenvironment (Daheshia and Yao, 2008). In addition, CD38/NADase, that is commonly up-regulated to reduce glycolytic and mitochondrial metabolism in OA (Chang et al., 2014), is not expressed in OA-IFP stem cells suggesting an impairment of the regulative mechanisms responsible of metabolic activity. Compared to other IFP-derived multipotent cells (Wickham et al., 2003; López-Ruiz et al., 2013; Garcia et al., 2016; Courties et al., 2017; do Amaral et al., 2017; Hindle et al., 2017), the OA-IFP stem cells investigated in this study have demonstrated not only distinct functional abilities but also characteristic immunophenotypic properties. Differences may probably be attributable to peculiarities in the patients cohort (i.e., age and disease severity) and/or in the isolation protocol (i.e., digestion times and processing phases of the digested tissue). In particular, the population showed the expression of STAT3, NOTCH1, c-Myc, OCT-4, KLF4, and NANOG, suggesting to have high self-renewal potential (Graf and Stadtfeld, 2008) while confirming their inflammation-activated state through the expression of HLA-DR, Fas and FasL (Hashimoto et al., 1998; Kim et al., 2000; Wickham et al., 2003), as already observed in patients affected by OA. These data are in line with the hypothesis that the inflammatory nature of the fat pad could condition endogenous stem cell niche upregulating HLA-DR (Wickham et al., 2003), but not promoting the expression of costimulatory molecules (Rübenhagen et al., 2012; Greene and Loeser, 2015). Due to the expression of CD34, a derivation of OA-IFP stem cells from adipose tissue (Bourin et al., 2013) or vascular endothelial compartment (Traktuev et al., 2008) can be hypothesized. While a derivation from perivascular niche is unlikely, as the pericyte marker PDGFRβ expression was lacking. Moreover, the expression of adhesion molecules (CD44, CD105), receptors for growth factors (VEGFR2, FGFR2) and cytokines (IL1R, IL6R) together with mRNA transcript of cortactin (CTTN) suggested a high level of microenvironmental responsiveness, further supporting the hypothesis that they are an endogenous subpopulation primed by the inflammatory milieu and able to switch to adaptive fibrogenic reprograming under unbalanced mechanical stimuli or dysregulated mechano-transduction. It is known that extracellular ATP levels increase in response to tissue-disturbing events, including inflammation, hypoxia or ischemia (Di Virgilio and Adinolfi, 2017). Based on our data, despite expressing a typical anti-inflammatory signature mediated by CD39 and CD73 (Eltzschig, 2013), OA-IFP stem cells could be limited in vivo to control the complex immune response related to OA. OA-IFP tissue is characterized by highly vascularized adipose lobuli separated by thickened septa (Favero et al., 2017; Fontanella et al., 2017) that, in turn, could prime endogenous stem cell populations. In our study, OA-IFP stem cells responded to adipogenic stimuli by accumulating lipid cytoplasmic droplets; in parallel, when stimulated with combined endothelial and mechano-transduction factors, net-like structures were observed on stiff-surface while a disorganized cell distribution was evidenced on soft matrix. Thus, the formation of net-like structures correlated with the high expression level of COL1A1 gene may suggest the potential involvement of OA-IFP stem cells to exert in vivo a protective response against mechanical overloading by thickening the fibrous septa. This adaptive-behavior is supported by significant body of evidence in the literature; the cells, including mesenchymal stem cells, can sense mechanical stress and soluble stimuli and translate such information in adaptive responses including differentiation, changes in morphology and cytoskeletal dynamics, also varying gene expression (McBeath et al., 2004; Chiquet et al., 2007, 2009). In OA, it is conceivable that stem cells expressing ectonucleotides and adenosine deaminase like OA-IFP cells could contribute in vivo to the generation of extracellular adenosine, that is a recognized checkpoint mediator for immune suppression (English and Mahon, 2011). Based on the evidence that OA-IFP stem cells have a low expression of CD38/NADase, we speculated that they could not be effective to counteract NAD+-mediated inflammation which is an important signature of OA.
Future pre-clinical studies based on OA-IFP stem cells will be useful to highlight their active or passive role in chronic OA-inflammation. In particular, functional studies developed in animal models of OA with adequate control-population will allow for a more specific understanding of OA-IFP stem cells behavior and role.
The datasets generated for this study are available on request to the corresponding author.
This study was performed after receiving Institutional Review Board approval (CESC Code: 4510/AO/18). The patients/participants provided their written informed consent to participate in this study.
ES, AnP, RDL, RDC, and VM: conception and design of the study. ES, SB, MP, EB, LP, and AsP: data acquisition. ES, RR, MR, MF, PR, RDL, and VM: data analysis and interpretation. ES and MP: drafting the article. All authors aided in revising this manuscript for intellectual content and approved the final version to be published.
This work was supported by the LifeLab Program of the “Consorzio per la Ricerca Sanitaria (CORIS)” of the Veneto Region, Italy (DGR1017, 17 July 2018) and the Dotazione Ordinaria Ricerca (DOR) of Di Liddo, for the year 2018. The funding sources had no role in this research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank the “Consorzio per la Ricerca Sanitaria” (CORIS) of the Veneto Region, Italy (LifeLab Program) for financial support. The authors would also like to thank Mrs. Alessandra Dubrovic for her contribution in immunohistochemical analysis, Dr. Diego Guidolin, Dr. Enrico De Rose, Paola Tomić, and Silvia Schiavon for technical support.
Arora, A., Sriram, M., Kothari, A., and Katti, D. S. (2017). Co-culture of infrapatellar fat pad-derived mesenchymal stromal cells and articular chondrocytes in plasma clot for cartilage tissue engineering. Cytotherapy 19, 881–894. doi: 10.1016/j.jcyt.2017.04.003
Belluzzi, E., El Hadi, H., Granzotto, M., Rossato, M., Ramonda, R., Macchi, V., et al. (2017). Systemic and local adipose tissue in knee osteoarthritis. J. Cell Physiol. 232, 1971–1978. doi: 10.1002/jcp.25716
Belluzzi, E., Stocco, E., Pozzuoli, A., Granzotto, M., Porzionato, A., Vettor, R., et al. (2019). Contribution of infrapatellar fat pad and synovial membrane to knee osteoarthritis pain. Biomed. Res. Int. 2019:6390182. doi: 10.1155/2019/6390182
Bourin, P., Bunnell, B. A., Casteilla, L., Dominici, M., Katz, A. J., March, K. L., et al. (2013). Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the international federation for adipose therapeutics and science (IFATS) and the international society for cellular therapy (ISCT). Cytotherapy 15, 641–648. doi: 10.1016/j.jcyt.2013.02.006
Boya, P., Codogno, P., and Rodriguez-Muela, N. (2018). Autophagy in stem cells: repair, remodelling and metabolic reprogramming. Development 145:dev146506. doi: 10.1242/dev.146506
Bravo, R., Vicencio, J. M., Parra, V., Troncoso, R., Munoz, J. P., Bui, M., et al. (2011). Increased ER-mitochondrial coupling promotes mitochondrial respiration and bioenergetics during early phases of ER stress. J. Cell Sci. 124, 2143–2152. doi: 10.1242/jcs.080762
Chang, X., Yue, L., Liu, W., Wang, Y., Wang, L., Xu, B., et al. (2014). CD38 and E2F transcription factor 2 have uniquely increased expression in rheumatoid arthritis synovial tissues. Clin. Exp. Immunol. 176, 222–231. doi: 10.1111/cei.12268
Chiquet, M., Gelman, L., Lutz, R., and Maier, S. (2009). From mechanotransduction to extracellular matrix gene expression in fibroblasts. Biochim. Biophys. Acta 1793, 911–920. doi: 10.1016/j.bbamcr.2009.01.012
Chiquet, M., Tunç-Civelek, V., and Sarasa-Renedo, A. (2007). Gene regulation by mechanotransduction in fibroblasts. Appl. Physiol. Nutr. Metab. 32, 967–973.
Courties, A., Sellam, J., and Berenbaum, F. (2017). Metabolic syndrome-associated osteoarthritis. Curr. Opin. Rheumatol. 29, 214–222. doi: 10.1097/BOR.0000000000000373
Daheshia, M., and Yao, J. Q. (2008). The interleukin 1beta pathway in the pathogenesis of osteoarthritis. J. Rheumatol. 35, 2306–2312.
Deng, Z., Li, Y., Liu, H., Xiao, S., Li, L., Tian, J., et al. (2019). The role of sirtuin 1 and its activator, resveratrol in osteoarthritis. Biosci. Rep. 39:BSR20190189. doi: 10.1042/BSR20190189
Di Virgilio, F., and Adinolfi, E. (2017). Extracellular purines, purinergic receptors and tumor growth. Oncogene 36, 293–303. doi: 10.1038/onc.2016.206
do Amaral, R. J. F. C., Almeida, H. V., Kelly, D. J., O’Brien, F. J., and Kearney, C. J. (2017). Infrapatellar fat pad stem cells: from developmental biology to cell therapy. Stem Cells Int. 2017:6843727. doi: 10.1155/2017/6843727
Eltzschig, H. K. (2013). Extracellular adenosine signaling in molecular medicine. J. Mol. Med. 91, 141–146.
English, K., and Mahon, B. P. (2011). Allogeneic mesenchymal stem cells: agents of immune modulation. J. Cell. Biochem. 112, 1963–1968. doi: 10.1002/jcb.23119
Favero, M., El-Hadi, H., Belluzzi, E., Granzotto, M., Porzionato, A., Sarasin, G., et al. (2017). Infrapatellar fat pad features in osteoarthritis: a histopathological and molecular study. Rheumatology 56, 1784–1793. doi: 10.1093/rheumatology/kex287
Favero, M., Ramonda, R., Goldring, M. B., Goldring, S. R., and Punzi, L. (2015). Early knee osteoarthritis. RMD Open 1:e000062. doi: 10.1136/rmdopen-2015-000062
Felimban, R., Ye, K., Traianedes, K., Di Bella, C., Crook, J., Wallace, G. G., et al. (2014). Differentiation of stem cells from human infrapatellar fat pad: characterization of cells undergoing chondrogenesis. Tissue Eng. Part A 20, 2213–2223. doi: 10.1089/ten.tea.2013.0657
Ferroni, L., Gardin, C., Dalla Paola, L., Campo, G., Cimaglia, P., Bellin, G., et al. (2019). Characterization of dermal stem cells of diabetic patients. Cells 8:E729. doi: 10.3390/cells8070729
Fontanella, C. G., Carniel, E. L., Frigo, A., Macchi, V., Porzionato, A., Sarasin, G., et al. (2017). Investigation of biomechanical response of hoffa’s fat pad and comparative characterization. J. Mech. Behav. Biomed. Mater. 67, 1–9. doi: 10.1016/j.jmbbm.2016.11.024
Fontanella, C. G., Macchi, V., Carniel, E. L., Frigo, A., Porzionato, A., Picardi, E. E. E., et al. (2018). Biomechanical behavior of hoffa’s fat pad in healthy and osteoarthritic conditions: histological and mechanical investigations. Australas. Phys. Eng. Sci. Med. 41, 657–667. doi: 10.1007/s13246-018-0661-8
Garcia, J., Wright, K., Roberts, S., Kuiper, J. H., Mangham, C., Richardson, J., et al. (2016). Characterisation of synovial fluid and infrapatellar fat pad derived mesenchymal stromal cells: the influence of tissue source and inflammatory stimulus. Sci. Rep. 6:24295. doi: 10.1038/srep24295
Gerlier, D., and Thomasset, N. (1986). Use of MTT colorimetric assay to measure cell activation. J. Immunol. Methods 94, 57–63.
Graf, T., and Stadtfeld, M. (2008). Heterogeneity of embryonic and adult stem cells. Cell Stem Cell 3, 480–483. doi: 10.1016/j.stem.2008.10.007
Grandi, F., Stocco, E., Barbon, S., Rambaldo, A., Contran, M., Fascetti Leon, F., et al. (2018). Composite scaffolds based on intestinal extracellular matrices and oxidized polyvinyl alcohol: a preliminary study for a new regenerative approach in short bowel syndrome. Biomed. Res. Int. 2018:7824757. doi: 10.1155/2018/7824757
Greene, M. A., and Loeser, R. F. (2015). Aging-related inflammation in osteoarthritis. Osteoarthritis Cartilage 23, 1966–1971. doi: 10.1016/j.joca.2015.01.008
Guidolin, D., Vacca, A., Nussdorfer, G. G., and Ribatti, D. (2004). A new image analysis method based on topological and fractal parameters to evaluate the angiostatic activity of docetaxel by using the matrigel assay in vitro. Microvasc. Res. 67, 117–124.
Hashimoto, H., Tanaka, M., Suda, T., Tomita, T., Hayashida, K., Takeuchi, E., et al. (1998). Soluble Fas ligand in the joints of patients with rheumatoid arthritis and osteoarthritis. Arthritis Rheum. 41, 657–662.
He, W., Pelletier, J. P., Martel-Pelletier, J., Laufer, S., and Di Battista, J. A. (2002). Synthesis of interleukin 1beta, tumor necrosis factor-alpha, and interstitial collagenase (MMP-1) is eicosanoid dependent in human osteoarthritis synovial membrane explants: interactions with antiinflammatory cytokines. J. Rheumatol. 29, 546–553.
Hindle, P., Khan, N., Biant, L., and Péault, B. (2017). The infrapatellar fat pad as a source of perivascular stem cells with increased chondrogenic potential for regenerative medicine. Stem Cells Transl. Med. 6, 77–87. doi: 10.5966/sctm.2016-0040
Hughes, A., Oxford, A. E., Tawara, K., Jorcyk, C. L., and Oxford, J. T. (2017). Endoplasmic reticulum stress and unfolded protein response in cartilage pathophysiology; contributing factors to apoptosis and osteoarthritis. Int. J. Mol. Sci. 20:18.
Huri, P. Y., Hamsici, S., Ergene, E., Huri, G., and Doral, M. N. (2018). Infrapatellar fat pad-derived stem cell-based regenerative strategies in orthopedic surgery. Knee Surg. Relat. Res. 30, 179–186. doi: 10.5792/ksrr.17.061
Im, G. I. (2018). Tissue engineering in osteoarthritis: current status and prospect of mesenchymal stem cell therapy. BioDrugs 32, 183–192. doi: 10.1007/s40259-018-0276-3
Ioan-Facsinay, A., and Kloppenburg, M. (2013). An emerging player in knee osteoarthritis: the infrapatellar fat pad. Arthritis Res. Ther. 15:225.
Kim, H. A., Lee, Y. J., Seong, S. C., Choe, K. W., and Song, Y. W. (2000). Apoptotic chondrocyte death in human osteoarthritis. J. Rheumatol. 27, 455–462.
Kobayashi, H., Terauchi, K., Yui, N., Yatabel, K., Kamada, T., Fujiyal, H., et al. (2017). The nicotinamide adenine dinucleotide (n.d.)-dependent deacetylasesirtuin-1 regulates chondrocyte energy metabolism through themodulation of adenosine monophosphate-activated protein kinase(AMPK) in osteoarthritis (OA). J. Arthritis 6:2.
Li, Z., Cheng, G., Hu, K., Li, M., Zang, W., Dong, Y., et al. (2011). Correlation of synovial fluid HMGB-1 levels with radiographic severity of knee osteoarthritis. Clin. Invest. Med. 34:E298.
López-Ruiz, E., Perán, M., Cobo-Molinos, J., Jiménez, G., Picón, M., Bustamante, M., et al. (2013). Chondrocytes extract from patients with osteoarthritis induces chondrogenesis in infrapatellar fat pad-derived stem cells. Osteoarthritis Cartilage 21, 246–258. doi: 10.1016/j.joca.2012.10.007
Macchi, V., Stocco, E., Stecco, C., Belluzzi, E., Favero, M., Porzionato, A., et al. (2018). The infrapatellar fat pad and the synovial membrane: an anatomo-functional unit. J. Anat. 233, 146–154. doi: 10.1111/joa.12820
Marrelli, M., Paduano, F., and Tatullo, M. (2013). Cells isolated from human periapical cysts express mesenchymal stem cell-like properties. Int. J. Biol. Sci. 9, 1070–1078. doi: 10.7150/ijbs.6662
McBeath, R., Pirone, D. M., Nelson, C. M., Bhadriraju, K., and Chen, C. S. (2004). Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 6, 483–495.
Michael, S., Achilleos, C., Panayiotou, T., and Strati, K. (2016). Inflammation shapes stem cells and stemness during infection and beyond. Front. Cell Dev. Biol. 4:118. doi: 10.3389/fcell.2016.00118
Michaud, C. M., McKenna, M. T., Begg, S., Tomijima, N., Majmudar, M., Bulzacchelli, M. T., et al. (1996). The burden of disease and injury in the United States. Popul. Health Metr. 4:11.
Naik, S., Larsen, S. B., Cowley, C. J., and Fuchs, E. (2018). Two to tango: dialog between immunity and stem cells in health and disease. Cell 175, 908–920. doi: 10.1016/j.cell.2018.08.071
Ní Fhlathartaigh, M., McMahon, J., Reynolds, R., Connolly, D., Higgins, E., Counihan, T., et al. (2013). Calreticulin and other components of endoplasmic reticulum stress in rat and human inflammatory demyelination. Acta Neuropathol. Commun. 1:37. doi: 10.1186/2051-5960-1-37
Okamura, Y., Watari, M., Jerud, E. S., Young, D. W., Ishizaka, S. T., Rose, J., et al. (2001). The extra domain A of fibronectin activates Toll-like receptor 4. J. Biol. Chem. 276, 10229–10233.
Peng, L., Jia, Z., Yin, X., Zhang, X., Liu, Y., Chen, P., et al. (2008). Comparative analysis of mesenchymal stem cells from bone marrow, cartilage, and adipose tissue. Stem Cells Dev. 17, 761–773. doi: 10.1089/scd.2007.0217
Pohlers, D., Huber, R., Ukena, B., and Kinne, R. W. (2006). Expression of platelet-derived growth factors C and D in the synovial membrane of patients with rheumatoid arthritis and osteoarthritis. Arthritis Rheum. 54, 788–794.
Poonpet, T., and Honsawek, S. (2014). Adipokines: biomarkers for osteoarthritis? World J Orthop 5, 319–327. doi: 10.5312/wjo.v5.i3.319
Radhakrishnan, S., Trentz, O. A., Martin, C. A., Reddy, M. S., Rela, M., Chinnarasu, M., et al. (2019). Effect of passaging on the stemness of infrapatellar fat pad-derived stem cells and potential role of nucleostemin as a prognostic marker of impaired stemness. Mol. Med. Rep. 20, 813–829. doi: 10.3892/mmr.2019.10268
Revuelta, M., and Matheu, A. (2017). Autophagy in stem cell aging. Aging Cell 16, 912–915. doi: 10.1111/acel.12655
Rübenhagen, R., Schüttrumpf, J. P., Stürmer, K. M., and Frosch, K. H. (2012). Interleukin-7 levels in synovial fluid increase with age and MMP-1 levels decrease with progression of osteoarthritis. Acta Orthop. 83, 59–64. doi: 10.3109/17453674.2011.645195
Scanzello, C. R., Plaas, A., and Crow, M. K. (2008). Innate immune system activation in osteoarthritis: is osteoarthritis a chronic wound? Curr. Opin. Rheumatol. 20, 565–572. doi: 10.1097/BOR.0b013e32830aba34
Stocco, E., Barbon, S., Lora, L., Grandi, F., Sartore, L., Tiengo, C., et al. (2018). Partially oxidized polyvinyl alcohol conduit for peripheral nerve regeneration. Sci. Rep. 8:604. doi: 10.1038/s41598-017-19058-3
Sun, Y., Chen, S., and Pei, M. (2018). Comparative advantages of infrapatellar fat pad: an emerging stem cell source for regenerative medicine. Rheumatology 57, 2072–2086. doi: 10.1093/rheumatology/kex487
Tangchitphisut, P., Srikaew, N., Numhom, S., Tangprasittipap, A., Woratanarat, P., Wongsak, S., et al. (2016). Infrapatellar fat pad: an alternative source of adipose-derived mesenchymal stem cells. Arthritis 2016:4019873. doi: 10.1155/2016/4019873
Termeer, C., Benedix, F., Sleeman, J., Fieber, C., Voith, U., Ahrens, T., et al. (2002). Oligosaccharides of hyaluronan activate dendritic cells via toll-like receptor 4. J. Exp. Med. 195, 99–111.
Toussirot, E., Streit, G., and Wendling, D. (2007). The contribution of adipose tissue and adipokines to inflammation in joint diseases. Curr. Med. Chem. 14, 1095–1100.
Traktuev, D. O., Merfeld-Clauss, S., Li, J., Kolonin, M., Arap, W., Pasqualini, R., et al. (2008). A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ. Res. 102, 77–85.
Ushiyama, T., Chano, T., Inoue, K., and Matsusue, Y. (2003). Cytokine production in the infrapatellar fat pad: another source of cytokines in knee synovial fluids. Ann. Rheum. Dis. 62, 108–112.
van Lent, P. L., Blom, A. B., Schelbergen, R. F., Slöetjes, A., Lafeber, F. P., Lems, W. F., et al. (2012). Active involvement of alarmins S100A8 and S100A9 in the regulation of synovial activation and joint destruction during mouse and human osteoarthritis. Arthritis Rheum. 64, 1466–1476. doi: 10.1002/art.34315
Wickham, M. Q., Erickson, G. R., Gimble, J. M., Vail, T. P., and Guilak, F. (2003). Multipotent stromal cells derived from the infrapatellar fat pad of the knee. Clin. Orthop. Relat. Res. 412, 196–212.
Ye, K., Felimban, R., Traianedes, K., Moulton, S. E., Wallace, G. G., Chung, J., et al. (2014). Chondrogenesis of infrapatellar fat pad derived adipose stem cells in 3D printed chitosan scaffold. PLoS One 9:e99410. doi: 10.1371/journal.pone.0099410
Ye, K., Traianedes, K., Choong, P. F., and Myers, D. E. (2016). Chondrogenesis of human infrapatellar fat pad stem cells on acellular dermal matrix. Front. Surg. 3:3. doi: 10.3389/fsurg.2016.00003
Keywords: infrapatellar fat pad, osteoarthritis, stem cells, inflammation, reprograming
Citation: Stocco E, Barbon S, Piccione M, Belluzzi E, Petrelli L, Pozzuoli A, Ramonda R, Rossato M, Favero M, Ruggieri P, Porzionato A, Di Liddo R, De Caro R and Macchi V (2019) Infrapatellar Fat Pad Stem Cells Responsiveness to Microenvironment in Osteoarthritis: From Morphology to Function. Front. Cell Dev. Biol. 7:323. doi: 10.3389/fcell.2019.00323
Received: 01 August 2019; Accepted: 25 November 2019;
Published: 10 December 2019.
Edited by:
Gianluca Carnevale, University of Modena and Reggio Emilia, ItalyReviewed by:
Laura Bertoni, University of Modena and Reggio Emilia, ItalyCopyright © 2019 Stocco, Barbon, Piccione, Belluzzi, Petrelli, Pozzuoli, Ramonda, Rossato, Favero, Ruggieri, Porzionato, Di Liddo, De Caro and Macchi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rosa Di Liddo, cm9zYS5kaWxpZGRvQHVuaXBkLml0; Raffaele De Caro, cmFmZmFlbGUuZGVjYXJvQHVuaXBkLml0
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.