
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell Dev. Biol., 03 December 2019
Sec. Molecular and Cellular Pathology
Volume 7 - 2019 | https://doi.org/10.3389/fcell.2019.00314
This article is part of the Research TopicMolecular Mechanisms in Chronic Kidney DiseaseView all 20 articles
 Michele Provenzano1
Michele Provenzano1 Giuseppe Coppolino1*
Giuseppe Coppolino1* Luca De Nicola2
Luca De Nicola2 Raffaele Serra3
Raffaele Serra3 Carlo Garofalo2
Carlo Garofalo2 Michele Andreucci1
Michele Andreucci1 Davide Bolignano4
Davide Bolignano4Chronic kidney disease (CKD), defined by an estimated glomerular filtration rate <60 ml/min/1.73 m2 and/or an increase in urine protein excretion (i.e., albuminuria), is an important public health problem. Prevalence and incidence of CKD have risen by 87 and 89%, worldwide, over the last three decades. The onset of either albuminuria and eGFR reduction has found to predict higher cardiovascular (CV) risk, being this association strong, independent from traditional CV risk factors and reproducible across different setting of patients. Indeed, this relationship is present not only in high risk cohorts of CKD patients under regular nephrology care and in those with hypertension or type 2 diabetes, but also in general, otherwise healthy population. As underlying mechanisms of damage, it has hypothesized and partially proved that eGFR reduction and albuminuria can directly promote endothelial dysfunction, accelerate atherosclerosis and the deleterious effects of hypertension. Moreover, the predictive accuracy of risk prediction models was consistently improved when eGFR and albuminuria have been added to the traditional CV risk factors (i.e., Framingham risk score). These important findings led to consider CKD as an equivalent CV risk. Although it is hard to accept this definition in absence of additional reports from scientific Literature, a great effort has been done to reduce the CV risk in CKD patients. A large number of clinical trials have tested the effect of drugs on CV risk reduction. The targets used in these trials were different, including blood pressure, lipids, albuminuria, inflammation, and glucose. All these trials have determined an overall better control of CV risk, performed by clinicians. However, a non-negligible residual risk is still present and has been attributed to: (1) missed response to study treatment in a consistent portion of patients, (2) role of many CV risk factors in CKD patients not yet completely investigated. These combined observations provide a strong argument that kidney measures should be regularly included in individual prediction models for improving CV risk stratification. Further studies are needed to identify high risk patients and novel therapeutic targets to improve CV protection in CKD patients.
Chronic kidney disease (CKD) is defined as the presence of kidney damage, mainly albuminuria, and/or decreased kidney function (estimated glomerular filtration rate [eGFR] <60 mL/min/1.73 m2) for at least 3 months (Levey and Coresh, 2012). Since the new onset of eGFR reduction and/or albuminuria is often asymptomatic, CKD is considered as part of non-communicable diseases, together with type 2 diabetes, hypertension and obesity (Jha et al., 2013). In the past decades, the epidemiology of communicable and non-communicable diseases has completely changed. From one side, the drop of mortality due to the main communicable diseases represented the “public health triumph” of the 20th century (Mensah et al., 2017). Indeed, by 1960, the improvement of Health System organization and the development of vaccines and new antibiotics have dramatically decreased mortality from infectious disease and raised life expectancy by an average of 20 years in the United States population (Murray and Lopez, 2013). From around the same period, a sharp decline in cardiovascular (CV) mortality has also been observed in the United States population and worldwide (Centers for Disease Control and Prevention, 1999). In United States, using the 1940 population as reference, the annual heart disease mortality rate declined by 56% in 1996, whereas the mortality rate for stroke fell by 70% in the same period. Similar findings were observed in many other populations (Lozano et al., 2012). The descending trend in CV mortality has been attributed to the improvement in specific prevention strategies, such as better blood pressure control, reduction in total cholesterol plasma levels, smoking cessation, increased physical activity (Ford et al., 2007). On the other side, the burden of non-communicable diseases has dramatically increased in the last quarter century. The number of adults with diabetes has raised from 108 million in 1980 to 422 million in 2014 and that of hypertensive subjects almost duplicated in the period 1975–2015 (NCD Risk Factor Collaboration [NCD-RisC], 2016, 2017). The increased prevalence of these comorbidities together with the population growth and aging, as the longer survival in CV disease, have shaped the CKD epidemiology trend. Indeed, the global prevalence and incidence of CKD has risen by 87 and 89%, respectively, over the period 1990–2016 (Xie et al., 2018). Intriguingly, although the age-standardized mortality rate has shown to be improved for most communicable and non-communicable disease (Ford et al., 2007; Lozano et al., 2012; Mortality and Causes of Death Collaborators, 2015), CKD is among a small number of diseases for which death rates have essentially risen in the last three decades. Specifically, age-standardized death rate due to CKD has increased by 4.39% from 1990 to 2017 (Xie et al., 2018). To date, it has been not completely clarified the specific role of clinical and demographic variables as risk factors for CKD development, maintenance and progression, as well as their effects on the three main endpoints measured in CKD patients: kidney function decline, mortality, CV events. This is, for example, the case of body mass index (BMI) and hypertension. Two recent meta-analyses showed that obesity and not only hypertension, but also pre-hypertension, were significant predictors of new onset CKD (Garofalo et al., 2016, 2017). Further analyses showed that high BMI increases also the risk for dialysis initiation but not for CV events (Russo et al., 2014). These important findings confirm that epidemiologic analyses may provide different results, given the different populations examined.
Aim of this Narrative Review article is to elucidate the main epidemiologic evidences about the association between Kidney damage and CV disease, the mechanisms responsible for this association, available strategies to reduce CV risk in patients suffering from CKD and future directions to answer the unmet needs around this topic.
Cardiovascular events remain responsible for a large proportion of unfavorable outcomes in CKD patients. Indeed, among several CKD cohorts, mortality overcomes End-Stage-Kidney-Disease (ESKD), which is considered the “natural” endpoint in these population (Go et al., 2004). Among patients with CKD alone or with CKD and type 2 diabetes of the United States Medicare population, risks of death and atherosclerotic vascular disease exceeded that of ESKD (Foley et al., 2005). Similar findings were reported from individuals with eGFR <60 ml/min/1.73 m2 selected from insurance-based health organizations (i.e., Kaiser Permanente Northwest), or Veterans Affair elderly with proteinuria or type 2 diabetes (Keith et al., 2004; Patel et al., 2005). However, these cohorts included patients with advanced age. In the Medicare population, prevalence of patients older than 70, in the subset with CKD and diabetes or CKD alone, was 88 and 91%, respectively (Foley et al., 2005). Cohorts which enrolled patients with a broad range of age, primary renal diseases as well as cohorts of patients referred to Nephrologists (the so-called Nephrology care), showed different results. In a cohort of referred CKD, the rate of ESKD overcame death (including CV death) up to 60 years age or 65 years (only if eGFR was <30 ml/min/1.73 m2), being the risk of death more frequent in patients older than 60 years with mild reduced kidney function. Thus, age and kidney function are the two main moderators of mortality and ESKD risks in CKD patients. Moreover, in patients under nephrology care, at variance with unreferred, the ESKD incidence rate is higher than mortality and CV rates (Coppolino et al., 2010; De Nicola et al., 2012, 2017). Nevertheless, CV risk remains relevant in this setting of patients in clinical studies that have been carried-out from different Countries. The presence of previous CV events, defined as myocardial infarction, stroke, heart failure, peripheral vascular disease or revascularization, ranged from 10 to 30% in the NephroTest (France), Mild-to-Moderate Kidney Disease (MMKD) study (Germany, Austria, and South Tyrol) and the British Columbia Study (Canada) (Levin et al., 2008; Dieplinger et al., 2009; Moranne et al., 2009). This prevalence rose to 30–50% in CKD populations of MASTERPLAN (Netherlands) (van Zuilen et al., 2006), CRIB (Chronic Renal Impairment in Birmingham, United Kingdom) (Landray et al., 2001), AASK (African Americans) Study (Norris et al., 2006) and CKD-Multicohort (Italy) (Minutolo et al., 2018); in this latter study, which enrolled CKD patients referred to 40 Italian nephrology clinics, ESKD, CV fatal/non-fatal events and mortality rates were 5.26, 4.52, and 3.76 per 100/pt/years, respectively (Minutolo et al., 2018).
Although all these studies evidenced a significant association between CV disease and CKD, the frequency of CV risk varies due to the different inclusion criteria used to recruit patients, particularly with regards of eGFR and proteinuria levels (Astor et al., 2011). An extended estimate of the prevalence of CV risk in CKD patients has been recently realized by the Global Burden of Disease Investigators (Thomas et al., 2017). By analyzing more than 1,000 surveys of CKD patients from around the world, it was found that, in the year 2013, 1,207,453 out 2,163,699 deaths (more than half, 55.8%) were secondary to CV disease. Interestingly, the age-standardized CV disease mortality rate was slightly higher in developing vs. developed world (Figure 1). This phenomenon is partly related to the increase, in developing Countries, of risk factors that can accelerate CV disease development, such as the aging of the population and the change in dietary habit toward high caloric and fatty foods compared to still limited prevention and treatment strategies (Barquera et al., 2015). All these evidences confirm the burden of CV disease in CKD patients and reinforce the need to improve and better understanding how to reduce CV risk in these frailty patients (Piccoli et al., 2018) as well as to ameliorate health care access and infrastructure in developing countries (Perico et al., 2005). Moreover, these findings also encourage incorporating CV disease in the evaluation of the benefits of CKD screening worldwide. Indeed, cost-effectiveness CKD screening studies have concluded that screening is cost effective whether CV fatal and non-fatal events have been included in the analysis at variance with studies that measured the effect on ESKD only (Vos et al., 2010; Crews et al., 2011).
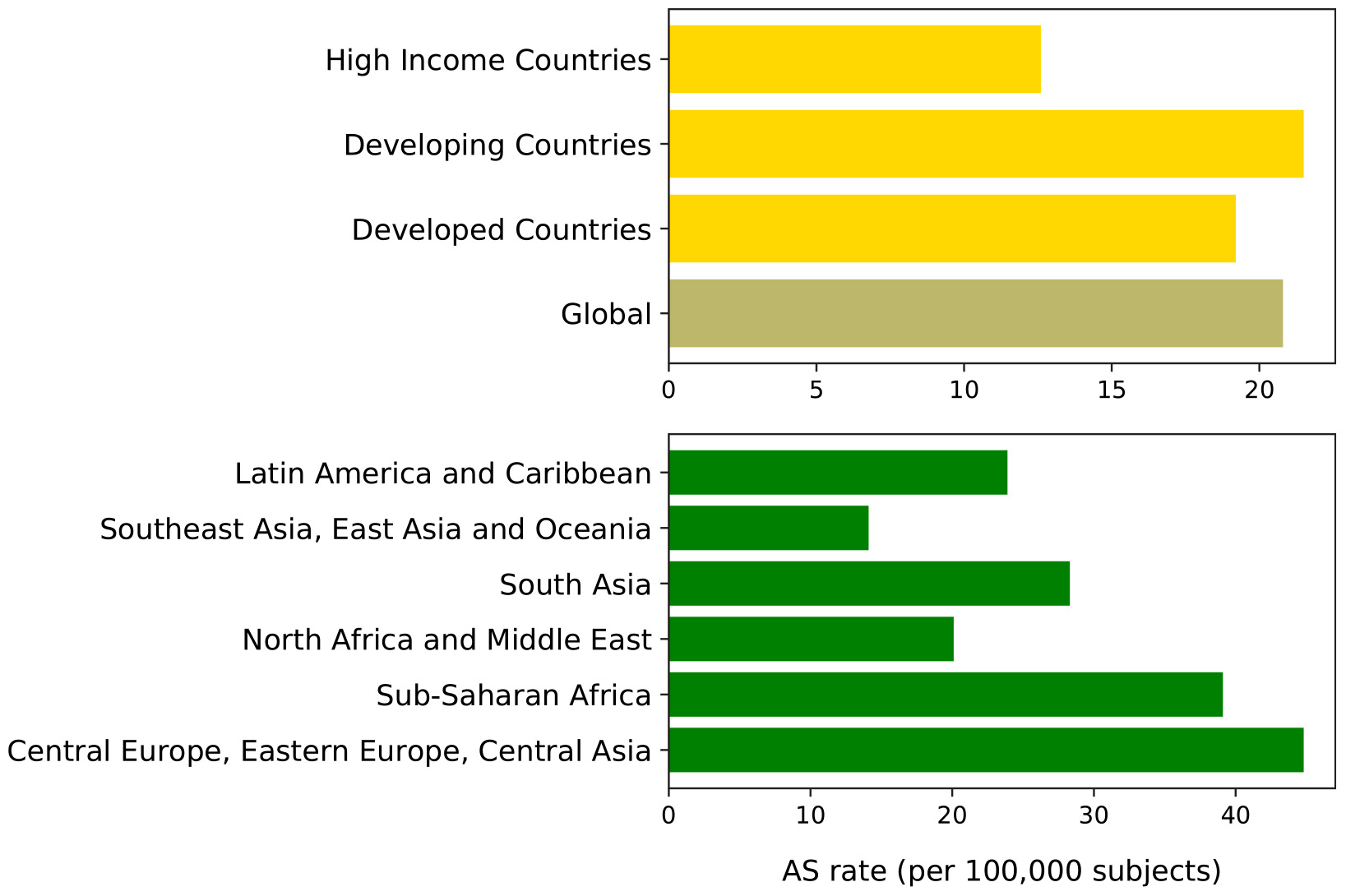
Figure 1. Age-standardized (AS) cardiovascular diseases mortality rate (per 100,000 subjects) worldwide in CKD patients in 2013 (Thomas et al., 2017). Top plot shows rates by development status; bottom plot shows rates by geographic macro-areas. Global, which represents the average rate, is darkly colored. Developed countries are defined as sovereign states that have a developed economy and technologically advanced infrastructure when compared to other countries. (Definition and list at: http://worldpopulationreview.com/countries/developed-countries/). Developing countries are characterized by being less developed industrially with a lower Human Development Index when compared to other countries. (Definition and list at: http://worldpopulationreview.com/countries/developing-countries/). High-income refers to Asia Pacific, Australia, Western Europe, Southern Latin America, North America; sub-Saharan Africa refers to Central sub-Saharan Africa, Eastern sub-Saharan Africa, Southern sub-Saharan Africa, Western sub-Saharan Africa.
In the past decades, several studies affirmed the importance of CKD as a risk factor for the onset of CV events such as myocardial infarction, stroke, chronic heart failure, peripheral vascular disease, CV death. These studies have been carried out in general population and high-risk cohorts (for example diabetic patients, CKD patients and those with a previous CV disease). In a recent meta-analysis, which enrolled more than 100,000 individuals from the general population, both a reduction in kidney function (eGFR) and the presence of kidney damage (albuminuria) were independently and strictly associated with an increased risk of death and CV death, regardless of many potential confounders such as age, gender, and traditional CV risk factors (Chronic Kidney Disease Prognosis Consortium Matsushita et al., 2010). In particular, mortality risk started to increase for eGFR level ≤60 ml/min/1.73 m2 and was two-fold higher at 30–45 ml/min/1.73 m2 as compared to normal eGFR levels. Regarding the albuminuria-related CV risk, this doubled by moving from 5 to 100 mg/g of urinary albumin/creatinine ratio, thus demonstrating that even a slight increase in albuminuria can herald patients’ higher CV and mortality risk. Similar findings were obtained from high risk population with type 2 diabetes and hypertension (Fox et al., 2012; Mahmoodi et al., 2012; Simeoni et al., 2016; Coppolino et al., 2017). A large meta-analysis from the CKD-Prognosis Consortium assessed the risk of CV mortality by type 2 diabetes status. CV risk was higher in diabetic vs. non-diabetic patients across the whole range of eGFR and albuminuria. However, Authors reported that interaction between eGFR (or albuminuria) and diabetes for all-cause mortality and CV-mortality endpoints was not significant (Fox et al., 2012). This means that, after establishing a reference point on the eGFR or albuminuria scale, the relative risks of the two health outcomes did not differ in diabetic vs. non-diabetic patients. Similar results were obtained when comparing hypertensive vs. non-hypertensive patients (Mahmoodi et al., 2012). An observational analysis of the ADVANCE (Action in Diabetes and Vascular disease: preterAx and diamicroN-MR Controlled Evaluation) clinical trial provided additional useful evidences on CV risk in type 2 diabetes patients. In that study, where more than 10,000 type 2 diabetic patients aged 55 (or older) were enrolled, higher albuminuria and lower eGFR levels were associated with an increased risk of all CV events (CV death, non-fatal myocardial infarction and non-fatal stroke) (Ninomiya et al., 2009).
According to these previous studies, both the two kidney measures (i.e., albuminuria and eGFR) are both strongly associated to the development of CV events. This association is independent from the presence of hypertension and diabetes, thus confirming the crucial role of CKD per se.
A first pioneering hypothesis about how albuminuria may testify high CV risk came from the group of researchers of the Steno Memorial Hospital, in Denmark, and was thus called the Steno hypothesis (Deckert et al., 1989). Owing the observation that in diabetic patients with increased albuminuria, this marker was associated to an increased transcapillary escape rate of fibrinogen and increased levels of von Willebrand factor, they suggested that albuminuria might reflect a general endothelial dysfunction and systemic vascular damage. Indeed, the leakage of albumin in the vessel-wall may trigger an inflammatory response, thus accelerating the atherosclerotic process. More recently, multiple experimental and clinical studies elucidated that the presence of albuminuria witnesses abnormalities in endothelial glycocalyx, as well as other endothelial structures (Stehouwer et al., 1992; Coppolino et al., 2009; Perticone et al., 2016). Perticone et al. (2015) have also found a significant inverse relationship between alkaline phosphatase and endothelium-dependent vasodilation, which can be mediated by an increase in fibroblast growth factor-23, an early marker of endothelial dysfunction in CKD patients. Moreover, in patients at increased risk for CKD, such as diabetic or hypertensive patients, the microvascular pressure and flows are increased (Ruggenenti and Remuzzi, 2006). This (also called hemodynamic hypothesis) can contribute to the development of albuminuria and the concurrent vascular damage in other organs, such as the heart and the eyes, with the onset of impaired coronary hemodynamics, left ventricular hypertrophy and retinopathy, respectively (Gavin et al., 1998; Liang et al., 2013; De Nicola et al., 2015a).
The contribution of eGFR to the increased CV risk has not completely understood yet, but has raised at the same time an increasing levels of clinical research attention. Indeed, in a survey conducted in the metropolitan area of Kyushu Island, in Southern Japan, heart tissue obtained from 482 individuals who underwent autopsies was examined. The severity of coronary atherosclerosis correlated with the grade of impairment in their kidney function (Nakano et al., 2010). Moreover, the presence of a significant coronary artery stenosis has been found, by angiography, in about half of pre-dialysis patients with extremely low levels of eGFR (Ohtake et al., 2005). Improving management of atherosclerotic risk factors, before reaching an advanced CKD stage, is therefore becoming one of the main targets of Nephrology care.
A common way to measure a patient’s risk of developing a CV event consists in calculating a 10-year risk based on a combination of some predictors. The Framingham risk score computes the 10-year risk (%) of coronary heart disease for a subject given the exact value of age, gender, systolic blood pressure, total and HDL cholesterol, and smoking status. Once the score has been computed, it should modify the clinical management in accordance to the 10-year CV risk: ≥ 10% defines a very-high risk of CV risk; 5–10% a high risk; 1–5% a moderate risk; < 1% a low risk. However, when the predictive value of the traditional scores have been tested in CKD patients, the risk estimation was suboptimal and, definitely, not well calibrated (Weiner et al., 2007). This means that, traditional CV risk factors are able to explain only a portion of the total CV risk in CKD patients. Therefore, research has recently focused on assessing whether adding the CKD measures (i.e., albuminuria and eGFR) can ameliorate prediction models. A recent meta-analysis of general and high-risk populations, has showed that adding eGFR, albumin-to creatinine ratio (ACR), or both, to the traditional CV risk factors significantly improves the model performance for the prediction of all CV endpoints: CV mortality, coronary heart disease, stroke, and heart failure (Matsushita et al., 2015). More interestingly, the contribution of albuminuria, revealed as ACR, to the overall model performance was greater than the contribution of traditional risk factors. These results are particularly significant and suggest that in population with a higher CV risk, such as CKD patients, the assessment of both eGFR and albuminuria is the essential step. Two additional separate analyses, performed in the general populations of Alberta kidney disease (AKD) and atherosclerosis risk in communities (ARIC), provided important evidences on the relevant contribution of CKD to the development of CV events. Indeed, the AKD showed that the rate of myocardial infarction was significantly higher in patients with CKD, but without diabetes (6.9 per 1,000 person-years), than in those with diabetes, but without CKD (5.4 per 1,000 person-years), being the results similar when the GFR, estimated by the MDRD equation, and sex-specific serum creatinine cutoff points were used to identify reduced GFR (Tonelli et al., 2012). Moreover, the ARIC study evidenced that the adjusted risk for heart failure (HF) was about two-fold higher in patients with eGFR <60 mL/min/1.73 m2 compared to those with eGFR ≥90 mL/min/1.73 m2 regardless of the presence of coronary heart disease at baseline (Kottgen et al., 2007). Details of the principal observational cohort studies assessing the risk for CV fatal and non-fatal events among CKD patients are shown in Table 1. These results have led guidelines to consider CKD as coronary heart disease risk equivalent. The 2016 ESC/ESH guidelines classified patients with eGFR reduction at high risk (10-year CV mortality risk ≥ 10%), even in absence of albuminuria (Ponikowski et al., 2016). Similarly, KDIGO considered CKD patients older than 50 years old at high risk for CV events (Kidney Disease Improving Global Outcomes Work Group, 2013). Despite the great enthusiasm of such discoveries, several concerns have been raised about considering CKD as a CV disease risk equivalent. Indeed, the CV risk, which was estimated in AKD and ARIC studies, is profoundly influenced by the presence of an established CV disease, that is the strongest cause of a new CV event. Therefore, debate is still ongoing on whether considering CKD as risk equivalent of CV disease or not; in that case more evidence must be obtained to reach that conclusion. We need, in fact, to develop more accurate (i.e., in CKD patients only) risk prediction models on patients’ CV risk and their target treatment, based on these risk scores. New studies in referred CKD patients should comply with this scope.
Chronic kidney disease patients under stable “Nephrology care” represent a specific high-risk population that differs from general population and other high-risk populations, such as patients with hypertension or diabetes, in terms of basal risk, management and prognosis. In a multi-cohort analysis of about 4,000 patients referred to Nephrology Units in Italy, we have investigated the frequency of comorbidities and prognosis (Provenzano et al., 2018). Population was characterized by old age on average (67 years), and a high prevalence of diabetes (29%) and CV disease [heart failure, myocardial infarction, peripheral vascular disease, and stroke (34%)]. Therefore, this CKD population, in a large portion of cases, suffers from other comorbidities and consequently is at extremely high risk of worse outcome (Figure 2). A higher CV disease frequency has been found among CKD patients, who were diagnosed on diabetic nephropathy or hypertensive nephropathy, thus demonstrating the presence of a combination of multiple risk factors simultaneously in these patients (Figure 3). On the other hand, among over 400,000 Italian patients, followed by general practitioners, the frequency of diabetes and CV disease was 4.7 and 7.9%, respectively (Minutolo et al., 2008).
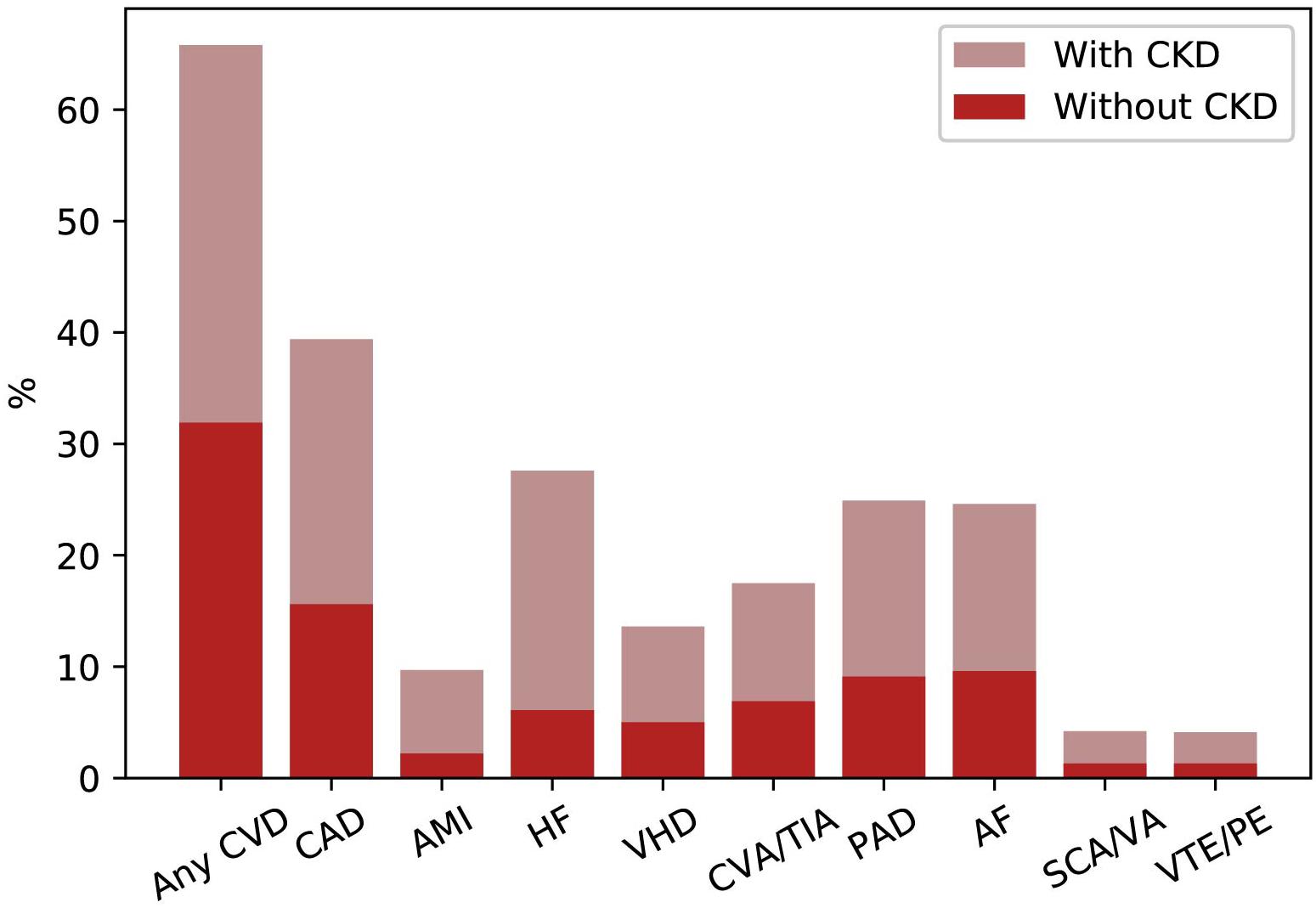
Figure 2. Prevalence of common cardiovascular diseases in patients with or without CKD in United States (2015). AF, atrial fibrillation; AMI, acute myocardial infarction; CAD, coronary artery disease; CKD, chronic kidney disease; CVA/TIA, cerebrovascular accident/transient ischemic attack; CVD, cardiovascular disease; HF, heart failure; PAD, peripheral arterial disease; SCA/VA, sudden cardiac arrest and ventricular arrhythmias; VHD, valvular heart disease; VTE/PE, venous thromboembolism and pulmonary embolism.
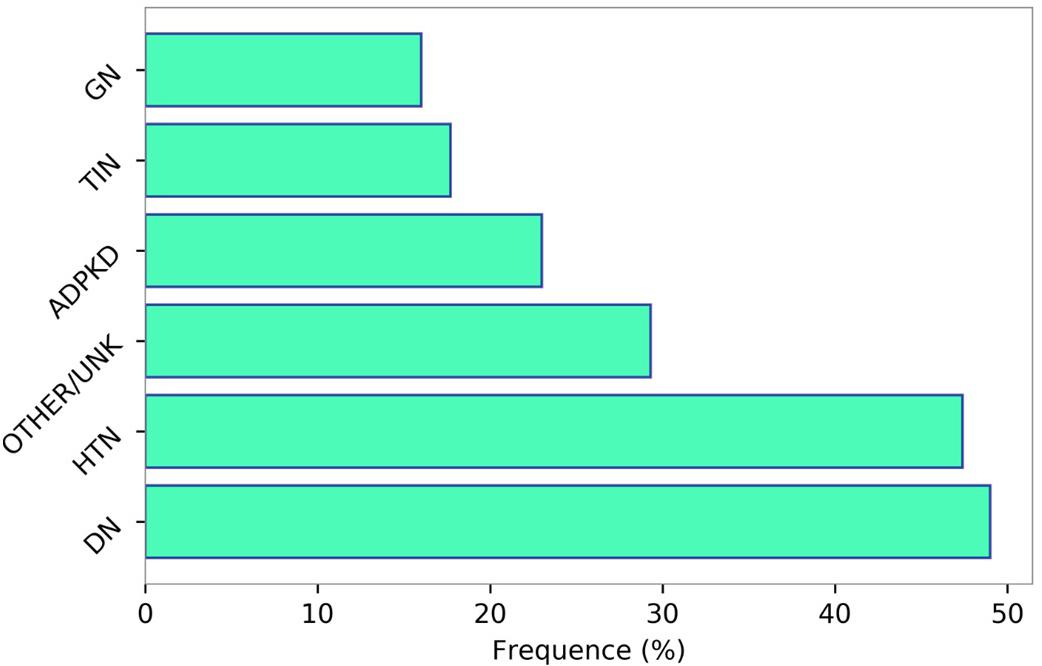
Figure 3. Prevalence of cardiovascular disease (myocardial infarction, stroke, peripheral vascular disease, chronic heart failure, and angina) according to the primary renal disease categories. Data source: 3,957 patients selected from the Italian multicohort of CKD patients referred to nephrologists (Provenzano et al., 2018). HTN, hypertensive nephropathy; DN, diabetic nephropathy; GN, glomerulonephritis; TIN, tubulo-interstitial nephropaties; ADPKD, autosomal dominant polycystic kidney disease; OTHER/UNK, other or unknown diagnosis.
Moreover, it has also been shown that in referred CKD patients, CV disease is poorly controlled. In the TABLE-CKD (TArget Blood Pressure Levels), a multicenter study which enrolled patients with stage 3–5 of CKD and at least 6-month follow up of Nephrology care, less than 15% of patients had blood pressure at target (<130/80 mmHg) and more than 80% patients showed an excessive salt intake not counterbalanced by an adequate treatment with loop diuretics, usually prescribed successfully by clinicians (Andreucci et al., 1999). However, the frequency of patients who had a better controlled hypertension significantly increased (up to 75% of patients) after 12 months of Nephrology care, which is generally considered a sufficient time interval for Nephrologists to intervene on the main co-morbidities (Russo et al., 2005; De Nicola et al., 2015a). Similarly, prevalence of hypercholesterolemia reached 50–60% depending on the stage of CKD (De Nicola et al., 2006). In another cohort of patients followed by Nephrologists, the SIR-SIN (Italian study on multiple predictors of outcome- epidemiology of chronic renal insufficiency in Italy) study, the therapeutic inertia for LDL control, defined as the percentage of patients not receiving statin prescription despite being recommended by current guideline, was as high as 61.3% (De Nicola et al., 2015b). These findings confirm, once again, the importance of referring high-risk patients to nephrologists.
As described for the general population and for diabetic/hypertensive patients, kidney measures have been confirmed as potent predictors of CV outcomes in tertiary nephrology care. The presence of proteinuria is an independent predictor of CV fatal and non-fatal events, with the risk starting to increase from 0.500 g/24 h and from 0.150 g/24 h in non-diabetic-CKD and diabetic-CKD patients, respectively (Coppolino et al., 2017; Minutolo et al., 2018). However, hazard ratio for CV events increased in all patients, selected from this cohort, also for slight increments of 24 h-proteinuria levels (Figure 4). This confirms how the effect of proteinuria on CV outcomes is consistent, strong and independent of other comorbidities, as described in the CKD-Prognosis Consortium. The risk of all-cause and CV death was increased as the eGFR declines (De Nicola et al., 2012). Smoking habit has been considered an independent predictor of CV events in general and high-risk cohorts (Cedillo-Couvert and Ricardo, 2016).
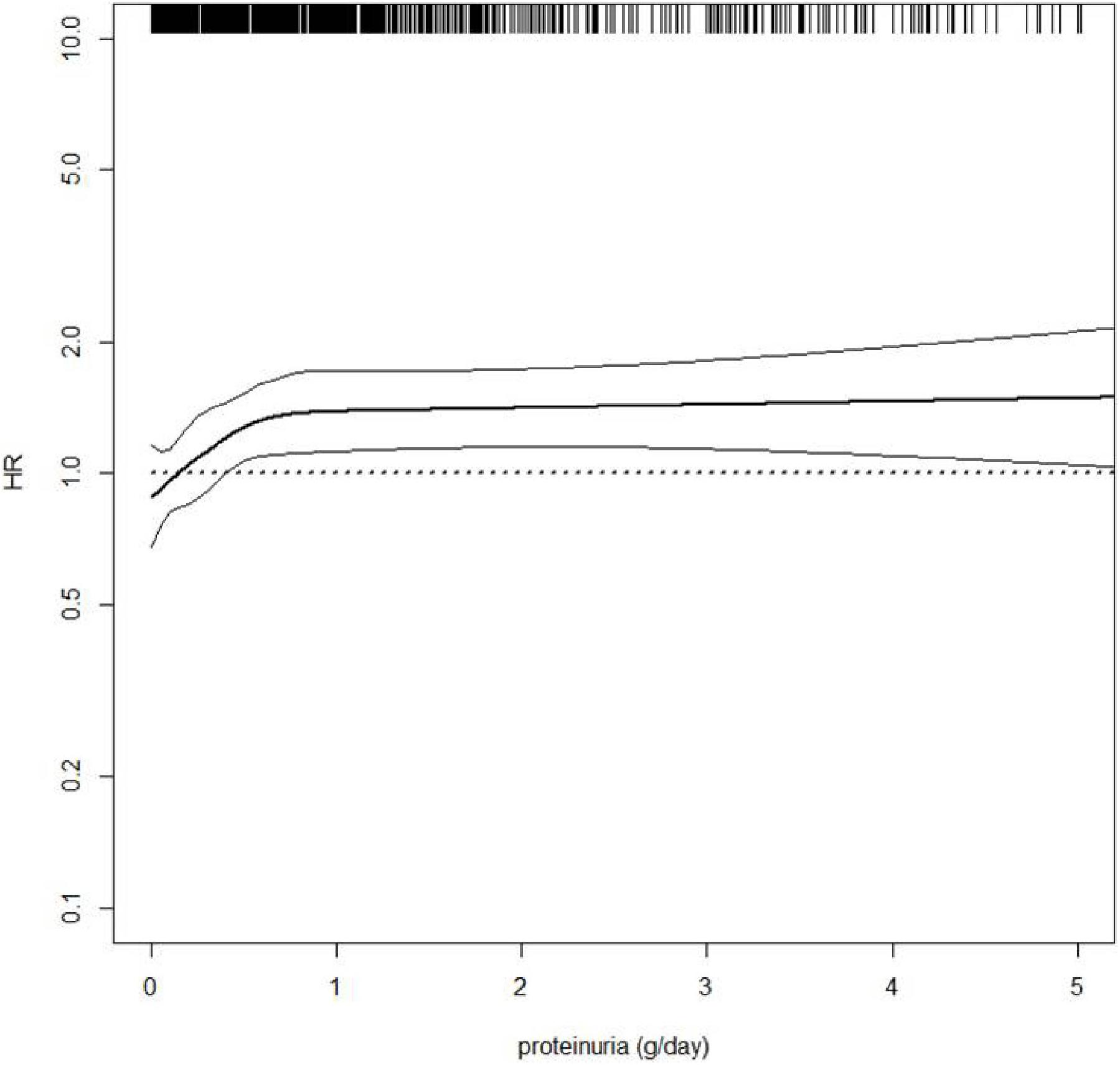
Figure 4. Hazard ratio (HR, solid thick line) and 95% confidence intervals (solid thin lines) for cardiovascular (CV) fatal and non-fatal events (new onset of myocardial infarction, congestive heart failure, stroke, revascularization, peripheral vascular disease, non-traumatic amputation or CV death) by 24 h-proteinuria level. HR was modeled by means of restricted cubic spline (RCS) due to the non-linear association between proteinuria and CV events. Four knots were located at 0, 25th, 50th, and 75th percentiles of proteinuria, whereas HR estimate was adjusted for age, gender, and eGFR value. Rug plot on the x axis at the top represents the distribution of observations. Data source: pooled analysis of four cohorts of CKD patients referred to Italian nephrology clinics (Minutolo et al., 2018).
Moreover, current and former smokers have been found at increased risk of developing albuminuria over time as compared to never smokers in the general population (Halimi et al., 2000). However, even though a recent meta-analysis has also shown that smoking is a predictor of new onset of CKD in the general population, the prognostic role of cigarette smoking or the combination of smoking and albuminuria, in patients with already established CKD, remains not completely quantified (Orth and Hallan, 2008; Bolignano et al., 2015; Xia et al., 2017; Coppolino et al., 2018a).
By considering CKD as CV risk equivalent can lead to a reclassification of patients for CV prevention. However, the general opinion, given the differences in basal risks between CKD populations vs. general and high-risk cohorts, is that more studies clarifying the exact role of CV risk factors in referred CKD patients are needed (Nagler et al., 2014). Moreover, despite the fact that to compute a 10-year risk for each patient could be cumbersome, this method can have a greater accuracy in the prediction of CV events in the future, unlike to consider all CKD patients with any degree of proteinuria, eGFR and comorbidities with the same CV risk.
In the last two decades, a growing number of intervention studies have been carried out, with the aim of achieving a better control of CV risk in CKD patients (Mann et al., 2001; Berl et al., 2003; Asselbergs et al., 2004; Norris et al., 2006; Rahman et al., 2006; Solomon et al., 2006; Brugts et al., 2007; Hallan et al., 2007; Kottgen et al., 2007; Perkovic et al., 2007; Bolignano et al., 2008, 2010; Anand et al., 2009; Heerspink et al., 2010; Shiga et al., 2010; Baigent et al., 2011; Parving et al., 2012; Tonelli et al., 2012; Wanner et al., 2016). This point is extremely important because, in the previous CV endpoints-trials, patients with impaired kidney function were regularly excluded (Zoccali et al., 2019). Nowadays, CV risk is also investigated with the aim to find novel biomarkers related to omics, imaging techniques and clinical data, which may help physicians in order to improve their knowledge and management (Serra et al., 2018).
The main intervention studies assessing the CV risk reduction in CKD patients, are listed in Table 2. Interventions differed between studies, with the effect of anti-hypertensive drugs, diuretics, albuminuria lowering agents and statins being tested (Bolignano et al., 2015). The CV benefits of drugs intervening in the renin-angiotensin-aldosterone-system (RAAS-I) are well established. The heart outcomes protection evaluation (HOPE) (Mann et al., 2001)and perindopril protection against recurrent stroke study (PROGRESS) (Perkovic et al., 2007) studies demonstrated, in patients with CKD, that blood pressure lowering using RAAS-I can significantly reduce the subsequent risk for CV events, such as stroke, coronary heart disease, and CV death. Intriguingly, the magnitude of the absolute risk reduction, achieved with that therapy, was greater in patients with CKD than in those without CKD, testifying that the obtained benefit is more pronounced if basal risk is higher. This concept contains a very important “public health” message, namely the priority of identifying CKD patients at increased CV risk that need more intensive management in terms of number of visits and therapy as well (Perkovic et al., 2007). This may also explain why several trials enrolling patients at less increased risk did not show any significant CV protection from the treatment with RAAS-I (Asselbergs et al., 2004; Norris et al., 2006; Bolignano et al., 2016). The most part of these trials have also shown that the risk reduction after blood pressure lowering intervention is strictly dependent on the concomitant albuminuria reduction. An observational analysis of the Reduction in Endpoints in Non-insulin dependent diabetes mellitus with the Angiotensin II Antagonist Losartan (RENAAL) study, a double-blind, randomized clinical trial testing the effect of losartan vs. placebo on cardio-renal outcomes, showed that the change in albuminuria, 6 months after the basal visit, was the strongest determinant of the CV risk reduction (de Zeeuw et al., 2004). Later on, a meta-analysis of 32 RCTs provided a stronger evidence by showing a 13% to 29% risk reduction of CV endpoints for each 10% decrease in albuminuria levels, following different treatments (Savarese et al., 2014). Hence, the CV risk effect of novel antialbuminuric drugs has been tested (Bolignano et al., 2015; Leporini et al., 2016). For this aim, Sodium–glucose cotransporter 2 (SGLT-2) inhibitors are promising agents (Savarese et al., 2014; Wanner et al., 2016; Coppolino et al., 2018b; Garofalo et al., 2019). SGLT-2 inhibitors act by reducing the reabsorption of glucose in the renal proximal tubule (Garofalo et al., 2019). They determine both hemodynamic and non-hemodynamic changes in the kidney, the first being mainly represented by the reduction in intraglomerular pressure and eGFR through tubuloglomerular feedback, whereas the second by a consistent reduction in oxidant stress (up to 60%), a reduction in NLRP3 inflammasome activity and an increase in intrarenal angiotensinogen levels (De Nicola et al., 2014; Bakris, 2019). Large randomized clinical trials assessing the effect of SGLT-2 inhibitors on CV and renal endpoints have shown that they not only can reduce CV risk, but also the progression of renal function decline in patients with CKD and type 2 diabetes. The Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial aimed at evaluating the effects of the SGLT2 inhibitor canagliflozin primarily on kidney endpoints in patients with type 2 diabetes and albuminuric CKD. The results of this study have been recently published (Perkovic et al., 2019) and showed that the risk of the primary endpoints (i.e., ESKD, doubling of creatinine, or death from kidney or CV causes) was reduced by 30% with canagliflozin treatment compared to placebo (hazard ratio: 0.70; 95% confidence interval: 0.59–0.82; p < 0.001). Findings for the kidney-specific composite endpoint (i.e., ESKD, doubling of creatinine, or kidney-related death) were positive as well (hazard ratio: 0.66; 95% confidence interval: 0.53–0.81; p < 0.001). Canagliflozin treatment was also associated with a lower risk for several CV related endpoints, such as the onset of CV death, myocardial infarction, hospitalization for heart failure or stroke (Perkovic et al., 2019).
Interestingly, since these drugs modify pathways (mainly the intraglomerular hypertension) that are shared between diabetic and non-diabetic CKD patients, it has been started the attempt of evaluating their benefits even in CKD patients without diabetes. Two large RCTs are actually ongoing and their results are eagerly expected. The Dapa-CKD clinical trial (ClinicalTrials.gov Identifier: NCT03036150) is testing the effects of the SGLT-2 inhibitor dapagliflozin on kidney endpoints and CV mortality in patients with CKD with and without type 2 diabetes vs. placebo, whereas the EMPA-KIDNEY trial is exploring empagliflozin vs. placebo in patients with CKD in order to answer the question whether SGLT-2 inhibitors are effective in a wide range of patients with CKD, such as patients with albuminuria or with reduced eGFR regardless of albuminuria levels (Herrington et al., 2018).
One of the reasons why the current available treatments for CKD patients can reduce, but not completely delete, the CV risk is due to the wide variability in response to treatments. It has been demonstrated from a pooled analysis of clinical trials that almost 30% of patients do not respond to antialbuminuric or blood pressure lowering drugs (Minutolo et al., 2000; Petrykiv et al., 2017). Statins have demonstrated to reduce CV risk in CKD patients. In the SHARP clinical trial, patients with CKD, who underwent statin treatment, were significantly protected from CV events as compared to placebo group, regardless of the basal level of LDL cholesterol (Baigent et al., 2011). KDIGO guidelines have therefore recommended to start treatment based on the CV risk and eGFR level, rather than on the LDL-cholesterol target. Evidences providing protective LDL targets, in CKD patients, deserve a further research effort.
In conclusion, knowledge on the association between CKD and CV risk has improved in the last years: (1) CKD is an independent risk factor of CV outcomes; the “public health” dimension of this concept is enormous because prevalence of CKD has dramatically increased (Provenzano et al., 2019); (2) identifying high risk patients is a priority; further observational studies are needed to gain more insights on the role of the main risk factors, specifically assessed in CKD; (3) common CV risk calculators are not useful in CKD patients; since CKD patients manifest different CV risk according to the presence of comorbidities, computing risk calculators specific to CKD is required. This may help in risk stratification and clinical decision making; (4) new clinical trials, aimed to reduce the CV risk excess in CKD patients are ongoing. In this regard, efforts are required to reduce variability in response to the available nephro- and cardio-protective treatments.
DB, GC, LD, RS, and CG contributed to the research idea. MP and GC wrote the manuscript. MA and DB critically revised the content of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer GR declared a past co-authorship with several of the authors MP, LD, and CG to the handling Editor.
Anand, I. S., Bishu, K., Rector, T. S., Ishani, A., Kuskowski, M. A., and Cohn, J. N. (2009). Proteinuria, chronic kidney disease, and the effect of an angiotensin receptor blocker in addition to an angiotensin-converting enzyme inhibitor in patients with moderate to severe heart failure. Circulation 120, 1577–1584. doi: 10.1161/CIRCULATIONAHA.109.853648
Andreucci, M., Russo, D., Fuiano, G., Minutolo, R., and Andreucci, V. E. (1999). Diuretics in renal failure. Miner Electrolyte Metab. 25, 32–38. doi: 10.1159/000057416
Asselbergs, F. W., Diercks, G. F., Hillege, H. L., van Boven, A. J., Janssen, W. M., Voors, A. A., et al. (2004). Effects of fosinopril and pravastatin on cardiovascular events in subjects with microalbuminuria. Circulation 110, 2809–2816. doi: 10.1161/01.CIR.0000146378.65439.7A
Astor, B. C., Matsushita, K., Gansevoort, R. T., van der Velde, M., Woodward, M., Levey, A. S., et al. (2011). Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. a collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 79, 1331–1340. doi: 10.1038/ki.2010.550
Baigent, C., Landray, M. J., Reith, C., Emberson, J., Wheeler, D. C., Tomson, C., et al. (2011). The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (study of heart and renal protection): a randomised placebo-controlled trial. Lancet 377, 2181–2192. doi: 10.1016/S0140-6736(11)60739-60733
Bakris, G. L. (2019). Major advancements in slowing diabetic kidney disease progression: focus on SGLT2 inhibitors. Am. J. Kidney Dis. 74, 573–575. doi: 10.1053/j.ajkd.2019.05.009
Barquera, S., Pedroza-Tobias, A., Medina, C., Hernandez-Barrera, L., Bibbins-Domingo, K., Lozano, R., et al. (2015). Global overview of the epidemiology of atherosclerotic cardiovascular disease. Arch. Med. Res. 46, 328–338. doi: 10.1016/j.arcmed.2015.06.006
Berl, T., Hunsicker, L. G., Lewis, J. B., Pfeffer, M. A., Porush, J. G., Rouleau, J. L., et al. (2003). Cardiovascular outcomes in the irbesartan diabetic nephropathy trial of patients with type 2 diabetes and overt nephropathy. Ann. Intern. Med. 138, 542–549. doi: 10.7326/0003-4819-138-7-200304010-200304010
Bolignano, D., Coppolino, G., Aloisi, C., Romeo, A., Nicocia, G., and Buemi, M. (2008). Effect of a single intravenous immunoglobulin infusion on neutrophil gelatinase-associated lipocalin levels in proteinuric patients with normal renal function. J. Investig. Med. 56, 997–1003. doi: 10.2310/JIM.0b013e31818e7e95
Bolignano, D., Coppolino, G., Romeo, A., Lacquaniti, A., and Buemi, M. (2010). Neutrophil gelatinase-associated lipocalin levels in chronic haemodialysis patients. Nephrology 15, 23–26. doi: 10.1111/j.1440-1797.2009.01163.x
Bolignano, D., D’Arrigo, G., Pisano, A., and Coppolino, G. (2015). Pentoxifylline for anemia in chronic kidney disease: a systematic review and meta-analysis. PLoS One 10:e0134104. doi: 10.1371/journal.pone.0134104
Bolignano, D., Pisano, A., and Coppolino, G. (2016). The dark side of blocking RAS in diabetic patients with incipient or manifested nephropathy. Exp. Clin. Endocrinol. Diabetes 124, 350–360. doi: 10.1055/s-0035-1550017
Brantsma, A. H., Bakker, S. J., de Zeeuw, D., de Jong, P. E., Gansevoort, R. T., and Group, P. S. (2008). Extended prognostic value of urinary albumin excretion for cardiovascular events. J. Am. Soc. Nephrol. 19, 1785–1791. doi: 10.1681/ASN.2007101065
Brugts, J. J., Boersma, E., Chonchol, M., Deckers, J. W., Bertrand, M., Remme, W. J., et al. (2007). The cardioprotective effects of the angiotensin-converting enzyme inhibitor perindopril in patients with stable coronary artery disease are not modified by mild to moderate renal insufficiency: insights from the EUROPA trial. J. Am. Coll. Cardiol. 50, 2148–2155. doi: 10.1016/j.jacc.2007.08.029
Cedillo-Couvert, E., and Ricardo, A. C. (2016). Smoking, vascular events, and ESRD in patients with CKD. Am. J. Kidney Dis. 68, 338–340. doi: 10.1053/j.ajkd.2016.06.004
Centers for Disease Control and Prevention, (1999). Decline in deaths from heart disease and stroke–United States, 1900-1999. MMWR Morb. Mortal. Wkly. Rep. 48, 649–656.
Chronic Kidney Disease Prognosis Consortium Matsushita, K., van der Velde, M., Astor, B. C., Woodward, M., Levey, A. S., et al. (2010). Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 375, 2073–2081. doi: 10.1016/S0140-6736(10)60674-60675
Coppolino, G., Buemi, A., Bolignano, D., Lacquaniti, A., La Spada, M., Stilo, F., et al. (2009). Perioperative iloprost and endothelial progenitor cells in uremic patients with severe limb ischemia undergoing peripheral revascularization. J. Surg. Res. 157, e129–e135. doi: 10.1016/j.jss.2008.07.017
Coppolino, G., Leonardi, G., Andreucci, M., and Bolignano, D. (2018a). Oxidative stress and kidney function: a brief update. Curr. Pharm. Des. 24, 4794–4799. doi: 10.2174/1381612825666190112165206
Coppolino, G., Leporini, C., Rivoli, L., Ursini, F., di Paola, E. D., Cernaro, V., et al. (2018b). Exploring the effects of DPP-4 inhibitors on the kidney from the bench to clinical trials. Pharmacol. Res. 129, 274–294. doi: 10.1016/j.phrs.2017.12.001
Coppolino, G., Lucisano, G., Bolignano, D., and Buemi, M. (2010). Acute cardiovascular complications of hemodialysis. Minerva Urol. Nefrol. 62, 67–80.
Coppolino, G., Pisano, A., Rivoli, L., and Bolignano, D. (2017). Renal denervation for resistant hypertension. Cochrane Database. Syst. Rev. 2:CD011499. doi: 10.1002/14651858.CD011499.pub2
Crews, D. C., Boulware, L. E., Gansevoort, R. T., and Jaar, B. G. (2011). Albuminuria: is it time to screen the general population? Adv. Chronic Kidney Dis. 18, 249–257. doi: 10.1053/j.ackd.2011.06.004
De Nicola, L., Gabbai, F. B., Liberti, M. E., Sagliocca, A., Conte, G., and Minutolo, R. (2014). Sodium/glucose cotransporter 2 inhibitors and prevention of diabetic nephropathy: targeting the renal tubule in diabetes. Am. J. Kidney Dis. 64, 16–24. doi: 10.1053/j.ajkd.2014.02.010
De Nicola, L., Minutolo, R., Chiodini, P., Borrelli, S., Zoccali, C., Postorino, M., et al. (2012). The effect of increasing age on the prognosis of non-dialysis patients with chronic kidney disease receiving stable nephrology care. Kidney Int. 82, 482–488. doi: 10.1038/ki.2012.174
De Nicola, L., Minutolo, R., Chiodini, P., Zoccali, C., Castellino, P., Donadio, C., et al. (2006). Global approach to cardiovascular risk in chronic kidney disease: reality and opportunities for intervention. Kidney Int. 69, 538–545. doi: 10.1038/sj.ki.5000085
De Nicola, L., Provenzano, M., Chiodini, P., Borrelli, S., Garofalo, C., Pacilio, M., et al. (2015a). Independent role of Underlying Kidney disease on renal prognosis of patients with chronic kidney disease under nephrology care. PLoS One 10:e0127071. doi: 10.1371/journal.pone.0127071
De Nicola, L., Provenzano, M., Chiodini, P., D’Arrigo, G., Tripepi, G., Del Vecchio, L., et al. (2015b). Prognostic role of LDL cholesterol in non-dialysis chronic kidney disease: multicenter prospective study in Italy. Nutr. Metab. Cardiovasc. Dis. 25, 756–762. doi: 10.1016/j.numecd.2015.04.001
De Nicola, L., Provenzano, M., Chiodini, P., Borrelli, S., Russo, L., Bellasi, A., et al. (2017). Epidemiology of low-proteinuric chronic kidney disease in renal clinics. PLoS One 12:e0172241. doi: 10.1371/journal.pone.0172241
de Zeeuw, D., Remuzzi, G., Parving, H. H., Keane, W. F., Zhang, Z., Shahinfar, S., et al. (2004). Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation 110, 921–927. doi: 10.1161/01.CIR.0000139860.33974.28
Deckert, T., Feldt-Rasmussen, B., Borch-Johnsen, K., Jensen, T., and Kofoed-Enevoldsen, A. (1989). Albuminuria reflects widespread vascular damage. Steno Hypothesis. Diabetol. 32, 219–226. doi: 10.1007/bf00285287
Dieplinger, B., Mueller, T., Kollerits, B., Struck, J., Ritz, E., von Eckardstein, A., et al. (2009). Pro-A-type natriuretic peptide and pro-adrenomedullin predict progression of chronic kidney disease: the MMKD study. Kidney Int. 75, 408–414. doi: 10.1038/ki.2008.560
Foley, R. N., Murray, A. M., Li, S., Herzog, C. A., McBean, A. M., Eggers, P. W., et al. (2005). Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J. Am. Soc. Nephrol. 16, 489–495. doi: 10.1681/ASN.2004030203
Ford, E. S., Ajani, U. A., Croft, J. B., Critchley, J. A., Labarthe, D. R., Kottke, T. E., et al. (2007). Explaining the decrease in U.S. deaths from coronary disease, 1980-2000. N. Engl. J. Med. 356, 2388–2398. doi: 10.1056/NEJMsa053935
Fox, C. S., Matsushita, K., Woodward, M., Bilo, H. J., Chalmers, J., Heerspink, H. J., et al. (2012). Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet 380, 1662–1673. doi: 10.1016/S0140-6736(12)61350-61356
Garofalo, C., Borrelli, S., Liberti, M. E., Andreucci, M., Conte, G., Minutolo, R., et al. (2019). SGLT2 inhibitors: nephroprotective efficacy and side effects. Medicina 55:E268. doi: 10.3390/medicina55060268
Garofalo, C., Borrelli, S., Minutolo, R., Chiodini, P., De Nicola, L., and Conte, G. (2017). A systematic review and meta-analysis suggests obesity predicts onset of chronic kidney disease in the general population. Kidney Int. 91, 1224–1235. doi: 10.1016/j.kint.2016.12.013
Garofalo, C., Borrelli, S., Pacilio, M., Minutolo, R., Chiodini, P., De Nicola, L., et al. (2016). Hypertension and prehypertension and prediction of development of decreased estimated GFR in the general population: a meta-analysis of cohort studies. Am. J. Kidney Dis. 67, 89–97. doi: 10.1053/j.ajkd.2015.08.027
Gavin, J. B., Maxwell, L., and Edgar, S. G. (1998). Microvascular involvement in cardiac pathology. J. Mol. Cell Cardiol. 30, 2531–2540. doi: 10.1006/jmcc.1998.0824
Go, A. S., Chertow, G. M., Fan, D., McCulloch, C. E., and Hsu, C. Y. (2004). Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 351, 1296–1305. doi: 10.1056/NEJMoa041031
Halimi, J. M., Giraudeau, B., Vol, S., Caces, E., Nivet, H., Lebranchu, Y., et al. (2000). Effects of current smoking and smoking discontinuation on renal function and proteinuria in the general population. Kidney Int. 58, 1285–1292. doi: 10.1046/j.1523-1755.2000.00284.x
Hallan, S., Astor, B., Romundstad, S., Aasarod, K., Kvenild, K., and Coresh, J. (2007). Association of kidney function and albuminuria with cardiovascular mortality in older vs younger individuals: the HUNT II study. Arch. Intern. Med. 167, 2490–2496. doi: 10.1001/archinte.167.22.2490
Heerspink, H. J., Ninomiya, T., Perkovic, V., Woodward, M., Zoungas, S., Cass, A., et al. (2010). Effects of a fixed combination of perindopril and indapamide in patients with type 2 diabetes and chronic kidney disease. Eur. Heart J. 31, 2888–2896. doi: 10.1093/eurheartj/ehq139
Herrington, W. G., Preiss, D., Haynes, R., von Eynatten, M., Staplin, N., Hauske, S. J., et al. (2018). The potential for improving cardio-renal outcomes by sodium-glucose co-transporter-2 inhibition in people with chronic kidney disease: a rationale for the EMPA-KIDNEY study. Clin. Kidney J. 11, 749–761. doi: 10.1093/ckj/sfy090
Jha, V., Garcia-Garcia, G., Iseki, K., Li, Z., Naicker, S., Plattner, B., et al. (2013). Chronic kidney disease: global dimension and perspectives. Lancet 382, 260–272. doi: 10.1016/S0140-6736(13)60687-X
Keith, D. S., Nichols, G. A., Gullion, C. M., Brown, J. B., and Smith, D. H. (2004). Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch. Intern. Med. 164, 659–663. doi: 10.1001/archinte.164.6.659
Kidney Disease Improving Global Outcomes Work Group (2013). Chapter 4: other complications of CKD: CVD, medication dosage, patient safety, infections, hospitalizations, and caveats for investigating complications of CKD. Kidney Int. Suppl. 3, 91–111. doi: 10.1038/kisup.2012.67
Kottgen, A., Russell, S. D., Loehr, L. R., Crainiceanu, C. M., Rosamond, W. D., Chang, P. P., et al. (2007). Reduced kidney function as a risk factor for incident heart failure: the atherosclerosis risk in communities (ARIC) study. J. Am. Soc. Nephrol. 18, 1307–1315. doi: 10.1681/ASN.2006101159
Landray, M. J., Thambyrajah, J., McGlynn, F. J., Jones, H. J., Baigent, C., Kendall, M. J., et al. (2001). Epidemiological evaluation of known and suspected cardiovascular risk factors in chronic renal impairment. Am. J. Kidney Dis. 38, 537–546. doi: 10.1053/ajkd.2001.26850
Leporini, C., Pisano, A., Russo, E., D Arrigo, G., de Sarro, G., Coppolino, G., et al. (2016). Effect of pentoxifylline on renal outcomes in chronic kidney disease patients: a systematic review and meta-analysis. Pharmacol. Res. 107, 315–332. doi: 10.1016/j.phrs.2016.03.001
Levey, A. S., and Coresh, J. (2012). Chronic kidney disease. Lancet 379, 165–180. doi: 10.1016/S0140-6736(11)60178-60175
Levin, A., Djurdjev, O., Beaulieu, M., and Er, L. (2008). Variability and risk factors for kidney disease progression and death following attainment of stage 4 CKD in a referred cohort. Am. J. Kidney Dis. 52, 661–671. doi: 10.1053/j.ajkd.2008.06.023
Liang, S., Zhang, X. G., Cai, G. Y., Zhu, H. Y., Zhou, J. H., Wu, J., et al. (2013). Identifying parameters to distinguish non-diabetic renal diseases from diabetic nephropathy in patients with type 2 diabetes mellitus: a meta-analysis. PLoS One 8:e64184. doi: 10.1371/journal.pone.0064184
Lozano, R., Naghavi, M., Foreman, K., Lim, S., Shibuya, K., Aboyans, V., et al. (2012). Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of disease study 2010. Lancet 380, 2095–2128. doi: 10.1016/S0140-6736(12)61728-61720
Mahmoodi, B. K., Matsushita, K., Woodward, M., Blankestijn, P. J., Cirillo, M., Ohkubo, T., et al. (2012). Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without hypertension: a meta-analysis. Lancet 380, 1649–1661. doi: 10.1016/S0140-6736(12)61272-61270
Mann, J. F., Gerstein, H. C., Pogue, J., Bosch, J., and Yusuf, S. (2001). Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Ann. Intern. Med. 134, 629–636. doi: 10.7326/0003-4819-134-8-200104170-200104177
Matsushita, K., Coresh, J., Sang, Y., Chalmers, J., Fox, C., Guallar, E., et al. (2015). Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 3, 514–525. doi: 10.1016/S2213-8587(15)00040-46
Mensah, G. A., Wei, G. S., Sorlie, P. D., Fine, L. J., Rosenberg, Y., Kaufmann, P. G., et al. (2017). Decline in cardiovascular mortality: possible causes and implications. Circ. Res. 120, 366–380. doi: 10.1161/CIRCRESAHA.116.309115
Minutolo, R., Agarwal, R., Borrelli, S., Chiodini, P., Bellizzi, V., Nappi, F., et al. (2011). Prognostic role of ambulatory blood pressure measurement in patients with nondialysis chronic kidney disease. Arch. Intern. Med. 171, 1090–1098. doi: 10.1001/archinternmed.2011.230
Minutolo, R., Andreucci, M., Balletta, M. M., and Russo, D. (2000). Effect of posture on sodium excretion and diuretic efficacy in nephrotic patients. Am. J. Kidney Dis. 36, 719–727. doi: 10.1053/ajkd.2000.17616
Minutolo, R., De Nicola, L., Mazzaglia, G., Postorino, M., Cricelli, C., Mantovani, L. G., et al. (2008). Detection and awareness of moderate to advanced CKD by primary care practitioners: a cross-sectional study from Italy. Am. J. Kidney Dis. 52, 444–453. doi: 10.1053/j.ajkd.2008.03.002
Minutolo, R., Gabbai, F. B., Provenzano, M., Chiodini, P., Borrelli, S., Garofalo, C., et al. (2018). Cardiorenal prognosis by residual proteinuria level in diabetic chronic kidney disease: pooled analysis of four cohort studies. Nephrol. Dial. Transplant. 33, 1942–1949. doi: 10.1093/ndt/gfy032
Minutolo, R., Locatelli, F., Gallieni, M., Bonofiglio, R., Fuiano, G., Oldrizzi, L., et al. (2013). Anaemia management in non-dialysis chronic kidney disease (CKD) patients: a multicentre prospective study in renal clinics. Nephrol. Dial. Transplant. 28, 3035–3045. doi: 10.1093/ndt/gft338
Minutolo, R., Sasso, F. C., Chiodini, P., Cianciaruso, B., Carbonara, O., Zamboli, P., et al. (2006). Management of cardiovascular risk factors in advanced type 2 diabetic nephropathy: a comparative analysis in nephrology, diabetology and primary care settings. J. Hypertens. 24, 1655–1661. doi: 10.1097/01.hjh.0000239303.93872.31
Moranne, O., Froissart, M., Rossert, J., Gauci, C., Boffa, J. J., Haymann, J. P., et al. (2009). Timing of onset of CKD-related metabolic complications. J. Am. Soc. Nephrol. 20, 164–171. doi: 10.1681/ASN.2008020159
Mortality, G. B. D. Causes of Death Collaborators, (2015). Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of disease study 2013. Lancet 385, 117–171. doi: 10.1016/S0140-6736(14)61682-61682
Murray, C. J., and Lopez, A. D. (2013). Measuring the global burden of disease. N. Engl. J. Med. 369, 448–457. doi: 10.1056/NEJMra1201534
Nagler, E. V., Webster, A. C., Bolignano, D., Haller, M. C., Nistor, I., van der Veer, S. N., et al. (2014). European renal best practice (ERBP) guideline development methodology: towards the best possible guidelines. Nephrol. Dial. Transplant. 29, 731–738. doi: 10.1093/ndt/gft407
Nakano, T., Ninomiya, T., Sumiyoshi, S., Fujii, H., Doi, Y., Hirakata, H., et al. (2010). Association of kidney function with coronary atherosclerosis and calcification in autopsy samples from Japanese elders: the Hisayama study. Am. J. Kidney Dis. 55, 21–30. doi: 10.1053/j.ajkd.2009.06.034
NCD Risk Factor Collaboration [NCD-RisC], (2016). Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 387, 1513–1530.
NCD Risk Factor Collaboration [NCD-RisC], (2017). Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19.1 million participants. Lancet 389, 37–55.
Ninomiya, T., Perkovic, V., de Galan, B. E., Zoungas, S., Pillai, A., Jardine, M., et al. (2009). Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J. Am. Soc. Nephrol. 20, 1813–1821. doi: 10.1681/ASN.2008121270
Norris, K., Bourgoigne, J., Gassman, J., Hebert, L., Middleton, J., Phillips, R. A., et al. (2006). Cardiovascular outcomes in the African American study of kidney disease and hypertension (AASK) trial. Am. J. Kidney Dis. 48, 739–751. doi: 10.1053/j.ajkd.2006.08.004
Ohtake, T., Kobayashi, S., Moriya, H., Negishi, K., Okamoto, K., Maesato, K., et al. (2005). High prevalence of occult coronary artery stenosis in patients with chronic kidney disease at the initiation of renal replacement therapy: an angiographic examination. J. Am. Soc. Nephrol. 16, 1141–1148. doi: 10.1681/ASN.2004090765
Orth, S. R., and Hallan, S. I. (2008). Smoking: a risk factor for progression of chronic kidney disease and for cardiovascular morbidity and mortality in renal patients–absence of evidence or evidence of absence? Clin. J. Am. Soc. Nephrol. 3, 226–236. doi: 10.2215/CJN.03740907
Parving, H. H., Brenner, B. M., McMurray, J. J., de Zeeuw, D., Haffner, S. M., Solomon, S. D., et al. (2012). Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N. Engl. J. Med. 367, 2204–2213. doi: 10.1056/NEJMoa1208799
Patel, U. D., Young, E. W., Ojo, A. O., and Hayward, R. A. (2005). CKD progression and mortality among older patients with diabetes. Am. J. Kidney Dis. 46, 406–414. doi: 10.1053/j.ajkd.2005.05.027
Peeters, M. J., van Zuilen, A. D., van den Brand, J. A., Bots, M. L., van Buren, M., Ten Dam, M. A., et al. (2014). Nurse practitioner care improves renal outcome in patients with CKD. J. Am. Soc. Nephrol. 25, 390–398. doi: 10.1681/ASN.2012121222
Perico, N., Plata, R., Anabaya, A., Codreanu, I., Schieppati, A., Ruggenenti, P., et al. (2005). Strategies for national health care systems in emerging countries: the case of screening and prevention of renal disease progression in Bolivia. Kidney Int. Suppl. 97, S87–S94. doi: 10.1111/j.1523-1755.2005.09715.x
Perkovic, V., Jardine, M. J., Neal, B., Bompoint, S., Heerspink, H. J. L., Charytan, D. M., et al. (2019). Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N. Engl. J. Med. 380, 2295–2306. doi: 10.1056/NEJMoa1811744
Perkovic, V., Ninomiya, T., Arima, H., Gallagher, M., Jardine, M., Cass, A., et al. (2007). Chronic kidney disease, cardiovascular events, and the effects of perindopril-based blood pressure lowering: data from the PROGRESS study. J. Am. Soc. Nephrol. 18, 2766–2772. doi: 10.1681/ASN.2007020256
Perticone, F., Perticone, M., Maio, R., Sciacqua, A., Andreucci, M., Tripepi, G., et al. (2015). Serum alkaline phosphatase negatively affects endothelium-dependent vasodilation in naive hypertensive patients. Hypertension 66, 874–880. doi: 10.1161/HYPERTENSIONAHA.115.06117
Perticone, M., Maio, R., Sciacqua, A., Cimellaro, A., Andreucci, M., Tripepi, G., et al. (2016). Serum phosphorus levels are associated with endothelial dysfunction in hypertensive patients. Nutr. Metab. Cardiovasc. Dis. 26, 683–688. doi: 10.1016/j.numecd.2016.02.003
Petrykiv, S. I., de Zeeuw, D., Persson, F., Rossing, P., Gansevoort, R. T., Laverman, G. D., et al. (2017). Variability in response to albuminuria-lowering drugs: true or random? Br. J. Clin. Pharmacol. 83, 1197–1204. doi: 10.1111/bcp.13217
Piccoli, G. B., Breuer, C., Cabiddu, G., Testa, A., Jadeau, C., and Brunori, G. (2018). Where are you going, nephrology? considerations on models of care in an evolving discipline. J. Clin. Med. 7:E199. doi: 10.3390/jcm7080199
Ponikowski, P., Voors, A. A., Anker, S. D., Bueno, H., Cleland, J. G. F., Coats, A. J. S., et al. (2016). 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 37, 2129–2200. doi: 10.1093/eurheartj/ehw128
Provenzano, M., Chiodini, P., Minutolo, R., Zoccali, C., Bellizzi, V., Conte, G., et al. (2018). Reclassification of chronic kidney disease patients for end-stage renal disease risk by proteinuria indexed to estimated glomerular filtration rate: multicentre prospective study in nephrology clinics. Nephrol. Dial Transpl. doi: 10.1093/ndt/gfy217 [Epub ahead of print].
Provenzano, M., Mancuso, C., Garofalo, C., De Nicola, L., and Andreucci, M. (2019). [Temporal variation of chronic kidney Disease’s epidemiology]. G. Ital. Nefrol. 36:2019-vol2.
Rahman, M., Pressel, S., Davis, B. R., Nwachuku, C., Wright, JT Jr, Whelton, P. K., et al. (2006). Cardiovascular outcomes in high-risk hypertensive patients stratified by baseline glomerular filtration rate. Ann. Intern. Med. 144, 172–180. doi: 10.7326/0003-4819-144-3-200602070-200602075
Ruggenenti, P., and Remuzzi, G. (2006). Time to abandon microalbuminuria? Kidney Int. 70, 1214–1222. doi: 10.1038/sj.ki.5001729
Russo, D., Andreucci, M., De Blasio, A., Frattolillo, P., and Andreucci, V. E. (2005). Treatment of hypertension in Italian nephrology out-patient clinics: the THIN Study. Semin. Nephrol. 25, 431–434. doi: 10.1016/j.semnephrol.2005.05.015
Russo, D., Morrone, L. F., Errichiello, C., De Gregorio, M. G., Imbriaco, M., Battaglia, Y., et al. (2014). Impact of BMI on cardiovascular events, renal function, and coronary artery calcification. Blood Purif. 38, 1–6. doi: 10.1159/000362862
Savarese, G., Dei Cas, A., Rosano, G., D’Amore, C., Musella, F., Mosca, S., et al. (2014). Reduction of albumin urinary excretion is associated with reduced cardiovascular events in hypertensive and/or diabetic patients. a meta-regression analysis of 32 randomized trials. Int. J. Cardiol. 172, 403–410. doi: 10.1016/j.ijcard.2014.01.065
Serra, R., Ielapi, N., Barbetta, A., Andreucci, M., and de Franciscis, S. (2018). Novel biomarkers for cardiovascular risk. Biomark. Med. 12, 1015–1024. doi: 10.2217/bmm-2018-2056
Shiga, T., Kasanuki, H., Hagiwara, N., Sumiyoshi, T., Honda, T., Haze, K., et al. (2010). Angiotensin receptor blocker-based therapy and cardiovascular events in hypertensive patients with coronary artery disease and impaired renal function. Blood Press. 19, 359–365. doi: 10.3109/08037051003802475
Simeoni, M., Nicotera, R., Colao, M., Citraro, M. L., Pelagi, E., Cerantonio, A., et al. (2016). Direct inhibition of plasmatic renin activity with aliskiren: a promising but under-investigated therapeutic option for non-diabetic glomerulonephritis. Int. Urol. Nephrol. 48, 229–237. doi: 10.1007/s11255-015-1128-1124
Solomon, S. D., Rice, M. M., Solomon, K., Jose, P., Domanski, M., Sabatine, M., et al. (2006). Renal function and effectiveness of angiotensin-converting enzyme inhibitor therapy in patients with chronic stable coronary disease in the prevention of events with ACE inhibition (PEACE) trial. Circulation 114, 26–31. doi: 10.1161/CIRCULATIONAHA.105.592733
Stehouwer, C. D., Nauta, J. J., Zeldenrust, G. C., Hackeng, W. H., Donker, A. J., and den Ottolander, G. J. (1992). Urinary albumin excretion, cardiovascular disease, and endothelial dysfunction in non-insulin-dependent diabetes mellitus. Lancet 340, 319–323. doi: 10.1016/0140-6736(92)91401-s
Theilade, S., Lajer, M., Jorsal, A., Tarnow, L., Parving, H. H., and Rossing, P. (2012). Arterial stiffness and endothelial dysfunction independently and synergistically predict cardiovascular and renal outcome in patients with type 1 diabetes. Diabet. Med. 29, 990–994. doi: 10.1111/j.1464-5491.2012.03633.x
Thomas, B., Matsushita, K., Abate, K. H., Al-Aly, Z., Arnlov, J., Asayama, K., et al. (2017). Global cardiovascular and renal outcomes of reduced GFR. J. Am. Soc. Nephrol. 28, 2167–2179. doi: 10.1681/ASN.2016050562
Tonelli, M., Muntner, P., Lloyd, A., Manns, B. J., Klarenbach, S., Pannu, N., et al. (2012). Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet 380, 807–814. doi: 10.1016/S0140-6736(12)60572-60578
van Zuilen, A. D., van der Tweel, I., Blankestijn, P. J., Bots, M. L., van Buren, M., ten Dam, M. A., et al. (2006). multifactorial approach and superior treatment efficacy in renal patients with the aid of nurse practitioners. design of the masterplan study [isrctn73187232]. Trials 7:8. doi: 10.1186/1745-6215-7-8
Vos, T., Carter, R., Barendregt, J., Mihalopoulos, C., Veerman, J. L., and Magnus, A. (2010). Assessing Cost-Effectiveness in Prevention (ACE- Prevention): Final Report. Melbourne: Brisbane and Deakin University.
Wanner, C., Inzucchi, S. E., Lachin, J. M., Fitchett, D., von Eynatten, M., Mattheus, M., et al. (2016). Empagliflozin and progression of Kidney Disease in Type 2 diabetes. N. Engl. J. Med. 375, 323–334. doi: 10.1056/NEJMoa1515920
Weiner, D. E., Tighiouart, H., Elsayed, E. F., Griffith, J. L., Salem, D. N., Levey, A. S., et al. (2007). The framingham predictive instrument in chronic kidney disease. J. Am. Coll. Cardiol. 50, 217–224. doi: 10.1016/j.jacc.2007.03.037
Xia, J., Wang, L., Ma, Z., Zhong, L., Wang, Y., Gao, Y., et al. (2017). Cigarette smoking and chronic kidney disease in the general population: a systematic review and meta-analysis of prospective cohort studies. Nephrol. Dial. Transplant. 32, 475–487. doi: 10.1093/ndt/gfw452
Xie, Y., Bowe, B., Mokdad, A. H., Xian, H., Yan, Y., Li, T., et al. (2018). Analysis of the global burden of disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 94, 567–581. doi: 10.1016/j.kint.2018.04.011
Keywords: cardiovascular risk, epidemiology, chronic kidney disease, risk score, smoking habit, statins, eGFR, proteinuria
Citation: Provenzano M, Coppolino G, De Nicola L, Serra R, Garofalo C, Andreucci M and Bolignano D (2019) Unraveling Cardiovascular Risk in Renal Patients: A New Take on Old Tale. Front. Cell Dev. Biol. 7:314. doi: 10.3389/fcell.2019.00314
Received: 03 September 2019; Accepted: 18 November 2019;
Published: 03 December 2019.
Edited by:
Nejra Prohic, Clinical Center University of Sarajevo, Bosnia and HerzegovinaReviewed by:
Laszlo Rosivall, University of Debrecen, HungaryCopyright © 2019 Provenzano, Coppolino, De Nicola, Serra, Garofalo, Andreucci and Bolignano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giuseppe Coppolino, Z2NvcHBvbGlub0B1bmljei5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.