- 1Department of Morphology, Surgery, and Experimental Medicine, University of Ferrara, Ferrara, Italy
- 2Institute of Science and Technology for Ceramics, National Research Council, Faenza, Italy
The regeneration of bone fractures, resulting from trauma, osteoporosis or tumors, is a major problem in our super-aging society. Bone regeneration is one of the main topics of concern in regenerative medicine. In recent years, stem cells have been employed in regenerative medicine with interesting results due to their self-renewal and differentiation capacity. Moreover, stem cells are able to secrete bioactive molecules and regulate the behavior of other cells in different host tissues. Bone regeneration process may improve effectively and rapidly when stem cells are used. To this purpose, stem cells are often employed with biomaterials/scaffolds and growth factors to accelerate bone healing at the fracture site. Briefly, this review will describe bone structure and the osteogenic differentiation of stem cells. In addition, the role of mesenchymal stem cells for bone repair/regrowth in the tissue engineering field and their recent progress in clinical applications will be discussed.
Introduction
Bone disorders are seen on a daily basis in clinical management, with remarkable health, social and economic outcomes (Figliomeni et al., 2018). Annually, more than 20 million individuals are affected by loss of bone tissue (Habibovic, 2017). Bone repair after fracture is a complex process that leads to new bone formation through sequential cellular and molecular events regulated by systemic and local factors (Arvidson et al., 2011).
Although bone fracture repair usually restores the damaged skeletal organ to its pre-injury status, about 10% of fractures will not heal normally (Einhorn and Gerstenfeld, 2015). Indeed, in some cases, the bone regeneration process could fail in extensive bone resections due to osteosarcoma, osteoporosis, osteomalacia, osteomyelitis, avascular necrosis, and atrophic non-union (Gao et al., 2014; Ferracini et al., 2018).
In particular, osteosarcoma and Ewing sarcoma are the two most common types of bone cancers diagnosed in young subjects (Ward et al., 2014). Indeed, unlike other tumors which usually affect elderly people (Tognon et al., 2015; Mazzoni et al., 2016, 2017b; Rotondo et al., 2016, 2018), osteosarcoma and Ewing sarcoma are mainly diagnosed in children/adolescents and young adults with a prevalence of 56 and 33%, respectively (Ward et al., 2014). Current osteosarcoma treatment includes surgical resection in association with chemotherapy (Harrison et al., 2018). On the other hand, osteoporosis is a chronic disease that leads patients to an increased risk of developing fractures (Schumacher et al., 2013). This pathology is characterized by high morbidity and mortality in aging populations (Migliaccio et al., 2017). Affected bones can be restored to normal conditions in clinical practice using bone grafts, such as auto-grafts, allo-grafts, or xeno-grafts (Rasch et al., 2019). Autologous grafts represent the clinical gold standard (Cypher and Grossman, 1996) in improving bone regeneration due to perfect histocompatibilty, as well as osteoinductive and osteoconductive proprieties. However, auto-grafts still show some disadvantages resulting from the limited amount of bone available for grafting and donor site morbidity. Conversely, allo-grafts and xeno-grafts represent an alternative approach to bone grafts as they solve the problem of limited autologous bone supply and do not require an additional surgical site for graft harvesting (Delloye et al., 2007). However, allo- and xeno-grafts present some drawbacks, such as donor scarcity, high costs, infectious agent transmission risk or immune reactions (Ferracini et al., 2018; Ho-Shui-Ling et al., 2018). For these reasons, a more efficient clinical therapeutic strategy is needed. To this end, tissue engineering has employed new osteoconductive and osteoinductive biomaterials/scaffolds, stem cells, and growth factors to improve bone repair/regrowth (Iaquinta et al., 2019). Stem cells, in particular MSCs, are characterized by sustained self-renewal and expansion, multi-potentiality, anti-inflammatory and immune-modulatory effects, in addition to the secretion of molecules that can start or support tissue regeneration/substitution (Caplan and Dennis, 2006). Despite having been used in clinical applications for more than 20 years, the characteristics and potential for bone repair of stem cells are yet to be fully elucidated (Jin and Lee, 2018). Specifically, stem cells have been considered in several medical fields to repair defective tissues and organs including bone, ligament and the heart (Abdel Meguid et al., 2018). Thus, this review will focus on potential applications for stem cells, in particular MSCs, to improve the regeneration of bone tissue.
Bone Structure
Bone is a rigid and highly dynamic tissue that supports and protects several organs in the body. Moreover, bone tissue provides the environment for red and white blood cell production, plays an important role in mineral homeostasis, such as calcium and phosphorus, and gives a solid base for skeletal muscles (Abdel Meguid et al., 2018). Two types of osseous tissue can be identified: solid cortical or compact bone, which represents 80% of bone mass, and trabecular bone, the remainder (Vico et al., 2017). Cortical bone is the hard-outer layer of the bone, while trabecular bone architecture is organized to optimize load transfer (Bayraktar et al., 2004). Trabecular bone can be found at the end of long bones, as well as in pelvic bones, the skull, ribs, and vertebrae. Furthermore, it contains red bone marrow where hematopoiesis takes place (Gdyczynski et al., 2014; Abdel Meguid et al., 2018).
Cortical and trabecular bones are subjected to bone remodeling (see below), a life-dominant process that plays an important role in bone mass balance and mineral homeostasis (Tolar et al., 2004). Moreover, two different phases can be distinguished in bone tissue (i) bone matrix and (ii) an organic phase that includes cellular elements, such as osteoblasts, osteoclasts, and osteocytes (Farbod et al., 2014) (Figure 1).
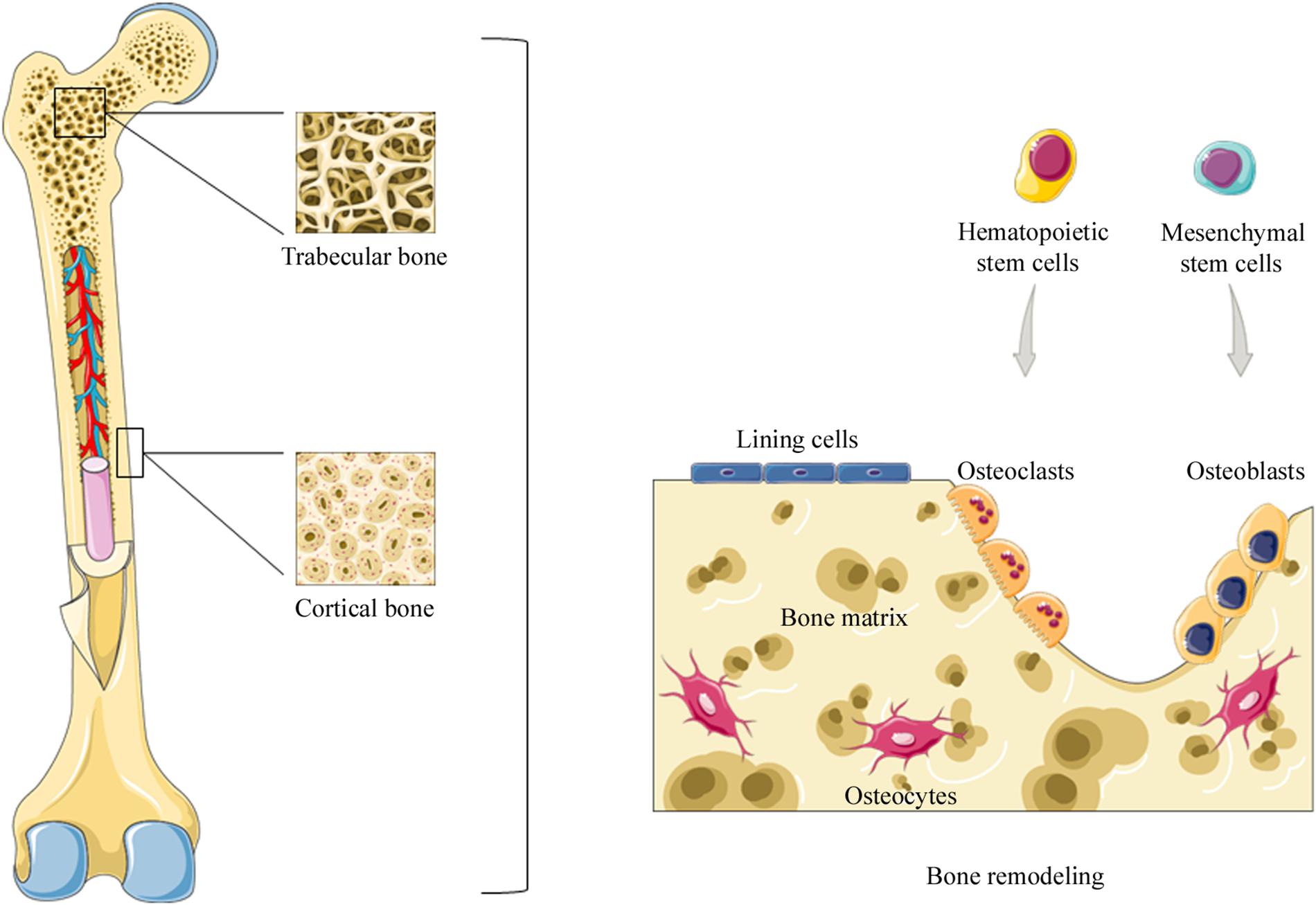
Figure 1. Representation of bone structure. Two types of osseous tissue can be identified: compact bone and trabecular bone. Bone tissue is subjected to bone remodeling, a life-dominant process that plays an important role in bone mass balance and mineral homeostasis. During bone remodeling osteoclasts, derived from hematopoietic stem cells, resorb old, or damaged bone. Subsequently, osteoblasts, derived from mesenchymal stem cells, are recruited to the damaged area in order to replace bone removed by osteoclasts. Instead, osteocytes derived from osteoblasts suspend their activity when buried in the bone matrix.
Bone Matrix
Bone matrix is a dynamic network that represents the intercellular substance of bone tissue. It is made up of several organic and inorganic components, such as collagen type I, which is the most abundant protein in bone tissue, elastin, polysaccharides, and calcium phosphate (Schönherr and Hausser, 2000; Fujisawa and Tamura, 2012; Farbod et al., 2014). The principal non-collagenous proteins of bone matrix are sialoprotein, osteonectin, osteopontin, and osteocalcin (Palmer et al., 2008), which contain aspartic acid (Asp) and glutamicacid (Glu) residues, with a high affinity for calcium ions (Ca2+) due to their charged carboxyl groups (Palmer et al., 2008). The polyglutamic acid segments in bone sialoprotein are responsible for binding the protein to apatite, while in osteopontin the same role is performed by polyaspartic acid segments (Ganss et al., 1999; Fantner et al., 2007). Osteonectin, a protein rich in cysteine amino acid, is expressed at a high concentration in mineralized tissues. Osteonectin is involved in osteoblast differentiation and osteoclast activity (Rosset and Bradshaw, 2016). Osteocalcin, also known as bone γ-carboxyglutamate protein (BGLAP), is expressed by osteoblasts and is commonly used as a clinical marker of bone turnover (Lambert et al., 2016). Bone matrix can regulate cell proliferation and differentiation through soluble growth factors and cytokines (Rosso et al., 2004). On the other hand, the inorganic component of bone matrix is an ion reservoir (Weatherholt et al., 2012). Hydroxylapatite [HA; Ca10(PO4)6(OH)2] is the most abundant inorganic crystal phase, containing citrate, carbonate and ions such as F–, K+, Sr2+, Pb2+, Zn2+, Cu2+, and Fe2+ (Marques et al., 2014).
In tissue engineering, knowledge of bone nanostructure and interactions between inorganic and organic phases are crucial for the production of biomaterials with structural and functional properties similar to natural bone tissue. Generally, these interactions involve anionic and/or cationic functional groups, which are found in the organic matrix and in turn exhibit strong affinity for either calcium or phosphate ions from the mineral phase of bone. Anionic functional groups, i.e., carboxyl-containing and calcium-binding moieties, including proteins, peptide sequences, single amino acids, and COOH groups, are the most extensively investigated chemical groups, which are considered to be instrumental to improving inorganic and organic phase interaction in synthetic nano-composites for bone regeneration (Farbod et al., 2014).
Bone Cellular Elements and Bone Remodeling
The majority of bone tissue cells in the organic phase are osteoblasts and osteoclasts (Kartsogiannis and Ng, 2004). In particular osteoclasts, cells that derive from the myeloid lineage of hematopoietic precursors of bone marrow and specialized in bone resorption (Charles and Aliprantis, 2014) can also circulate in the bloodstream. On the other hand, osteoblasts derived from mesenchymal stem cells (MSCs) in bone marrow, blood and also from pericytes are involved in bone formation and replace bone removed by osteoclasts (Sims and Civitelli, 2014). It has been reported that MSCs migration to the bone surface is a significant step in bone formation and fracture healing. Indeed, alterations in MSC migration can lead to abnormal bone imbalances. However, MSC migration is a complex mechanism, whose regulation system is yet to be elucidated (Su et al., 2018). Moreover, other osteoblast-derived cells reside in bone tissue, such as bone lining cells and osteocytes. Bone lining cells cover the bone surface, where bone resorption or bone formation is not requested (Miller et al., 1989) while osteocytes derived from osteoblasts suspend their activity when buried in the matrix. It has been suggested that this mechanism represents a form of stress sensor (van Oers et al., 2015). When considered together, these cells are organized into temporary anatomical structures called basic multicellular units (BMUs). BMUs are key grouping cells that carry out bone remodeling, a biological process that leads to structural changes and skeletal renewal (Sims and Martin, 2015). Osteocytes recognize old or damaged bone areas and recruit osteoclast precursors at the remodeling site (Lerner et al., 2019). Bone remodeling consists in some sequential steps: (i) initiation, (ii) reversal, and (iii) termination phases. During the initiation phase, osteoclast precursors are recruited and differentiated into mature osteoclasts to allow bone resorption. Osteoclastogenesis requires specific key mediators, such as the macrophage colony-stimulating factor (M-CSF or CSF-1) and the receptor activator of nuclear factor-kB ligand (RANKL or TNFSF11). In particular, M-CSF is produced by osteoblasts and many other cell types; it is necessary for the proliferation of osteoclast precursors, as well as their differentiation and fusion into osteoclasts (Jiao et al., 2015). On the other hand, RANKL binds its receptor RANK, which is localized on the surface of osteoclast precursors to allow fusion, maturation, survival, and osteoclasts activation (Jimi et al., 1999; Levaot et al., 2015). In this contest, some authors have shown that osteocytes are the main source of the RANKL required for osteoclast formation (Xiong et al., 2015). During bone resorption, several factors that lead to MSCs recruitment and differentiation are released through bone remodeling to enable bone formation in the bone marrow microenvironment (Crane and Cao, 2014). The next transient phase, or reversal phase, consists in bone resorption inhibition in addition to osteoblasts recruitment and the subsequent differentiation that leads to bone formation. Osteoblasts can produce a protein called osteoprotegerin (OPG), which is a decoy receptor for RANKL. Thus, this protein prevents RANKL from binding to RANK, with the consequent inhibiting of osteoclast differentiation and activation (Boyce, 2013). The final step in the remodeling cycle is represented by the termination phase, when an equal amount of resorbed bone has been replaced (Raggatt and Partridge, 2010). Osteocytes contribute to ending the remodeling process by producing sclerostin, which inhibits the bone formation induced by Wnt signaling in osteoblasts (van Bezooijen et al., 2005). At the end of the process, mature osteoblasts undergo apoptosis, become bone lining cells or differentiate into osteocytes (Sims and Martin, 2015). Human skeleton integrity is maintained by a delicate balance between bone resorption and bone formation. Alterations to this mechanism result in several skeletal diseases, such as osteoporosis (McClung, 2018), due to excessive bone resorption, or osteopetrosis caused by excessive bone formation (Sobacchi et al., 2013).
Stem Cells in Tissue Engineering
In regenerative medicine, stem cells/progenitor cells should have the following important characteristics: (i) availability in large amounts, (ii) multiple differentiation, (iii) painless isolation methods, (iv) use in autologous or allogeneic transplant, (v) agreement with Good Manufacturing Practice guidelines (GMP) (Trohatou and Roubelakis, 2017).
Interestingly, in recent years, many researchers have focused their attention on the analysis of stem cells secretome. Indeed, different reports have been published demonstrating that significant biological functions, such as proliferation, differentiation, communication and migration can be regulated by cellular secreted molecules (Makridakis et al., 2013). Indeed, in clinical practice, MSC secreted molecules may direct different mature cells to differentiate. This differentiation process occurs in specific conditions, such as in the presence of medium composition and/or biomaterials proprieties (Trávníčková and Bačáková, 2018). To this end, biomaterials should be biocompatible, biodegradable and osteoinductive/osteoconductive to allow cellular proliferation and osteogenic differentiation in the healing site (Liu et al., 2010; Perez et al., 2018). Several biomaterials, inspired to facilitate bone composition and structure have been developed and employed in tissue engineering for bone repair (Fillingham and Jacobs, 2016), as reported for ceramics, polymers, and composite scaffolds (Iaquinta et al., 2019).
The presence of stem cells was first reported on in bone marrow (Drela et al., 2019). At present, several types of stem cells have been put forward as a source of osteoblast progenitors (Mushahary et al., 2018). Examples are human embryonic stem cells (hESCs), induced pluripotent stem cells (iPSCs), and human mesenchymal stem cells (hMSCs) (Perez et al., 2018). hESCs in vitro cultures were first established in Thomson et al. (1998). hESCs are pluripotent human embryonic stem cells derived from human blastocysts (Kwon et al., 2018). These cells maintain developmental potential for all three embryonic germ layers (endoderm, mesoderm, and ectoderm) even after months of proliferation in vitro, differentiating into specific cell types by controlling culture conditions (Thomson et al., 1998). hESC isolation requires human embryo destruction. For this reason, the use of hESCs is considered highly objectionable (Johnson, 2008). Indeed, in many countries, a ban on hESCs has negatively affected hESC research progress, as many governments around the world have not supported research funding. Nevertheless, in certain counties some progress has been made in isolating, culturing, and characterizing hESCs using different strategies (Khan et al., 2018). In order to circumvent ethical issues, proposals have been made to isolate hESCs from a single blastomere, without destroying the human embryo. This goal can be reached using a technique similar to that employed in pre-implantation genetic diagnosis (Chung et al., 2008). Several studies have reported on hESC proliferation and osteogenic compatibility with different biomaterials (Chen et al., 2018). Tang et al. (2012), evaluated the behavior of hESCs in vitro when associated with calcium phosphate cement (CPC) showing good cell viability and hESC osteogenic differentiation. Moreover, Liu and his collaborators have studied hESCs seeded onto macroporus CPC for bone regeneration in vivo in critical-sized cranial defects in rats (Liu et al., 2014). Similarly, Kim et al. (2008), have shown that hESCs in association with poly (D,L-lactic-co-glycolic acid)/hydroxylapatite composite scaffolds can be used for bone regeneration in vivo. However, major drawbacks in the use of hESCs include these significant matters: (i) potential unexpected differentiation, (ii) putative teratoma formation, (iii) culture conditions set up, (iv) immune reactions and (v) the ethical and religious debate (Cunningham et al., 2012). In this context, Takahashi and Yamanaka have developed iPSCs through the use of lentivirus containing four transcription factors (c-Myc, Oct3/4, Sox2, and Klf4), which induced a pluripotent state comparable to hESC (Takahashi and Yamanaka, 2006). Specifically, iPSC reprogramed cells, derived from adult somatic cells as skin fibroblasts, have the ability to re-differentiate virtually into any cell type (Perez et al., 2018). iPSCs show some advantages as a result of (i) by-passing the use of human embryos, (ii) showing morphology and growth properties specific to embryonic cells, (iii) expressing the same hESC marker genes, whereas (iv) these cells can be transplanted into the same patient without the adverse effects of the immune rejection (Takahashi and Yamanaka, 2006; Fu, 2014) as a result of being autologous. In tissue engineering, hiPSCs represent an interesting cell source since patient- or disease-specific mesenchymal/monocyte/macrophage precursors can be generated, which may differentiate into osteoblasts or osteoclasts, respectively (Lou, 2015). Ji et al. (2016), have established that hiPSCs from human gingival fibroblasts isolated from discarded gingival tissues combined with nano-hydroxylapatite/chitosan/gelatine scaffolds could be a potential innovative approach for bone tissue engineering. Moreover, Jeon et al. (2016), have shown that hiPSC-mesenchymal stem cells and macrophages differentiated into osteoblasts and osteoclasts, respectively, when co-cultured on hydroxylapatite-coated poly(lactic-co-glycolic acid)/poly(L-lactic acid) scaffolds. In another study, conducted by Xie et al. (2016) a biomimetic hydroxylapatite/collagen/chitosan (HAp/Col/CTS) scaffold was employed to induce osteogenic differentiation in iPSCs in vivo. Their data have shown that combined system iPSCs-HAp/Col/CTS can be used to create personalized and efficacious bone regeneration (Xie et al., 2016).
This review intends to highlight MSCs involvement in regenerative medicine and their potential application with or without scaffolds in clinical practice.
Mesenchymal Stem Cells
Mesenchymal stem cells are “fibroblastic-like” cells, which form clusters defined as fibroblast-colony forming units (CFU-F) (Friedenstein et al., 1970). MSCs are adherent cells positive for CD73, CD90, and CD105 markers (>95%) and negative for other specific antigens, such as CD45, CD34, CD14, CD79, and HLA class II (<2%) as defined by the International Society for Cellular Therapy (ISCT) (Dominici et al., 2006). MSCs can renew themselves through cell division, whereas they differentiate into osteoblasts, adipocytes, and chondrocytes after exposure to specific soluble factors in the microenvironment (Pittenger et al., 1999; Manfrini et al., 2013; Fitzsimmons et al., 2018). In vitro, osteogenic differentiation of stem cells typically involves the use of dexamethasone, β-glycerolphosphate, and ascorbic acid (Manfrini et al., 2013). Moreover, MSCs seem to have potent anti-inflammatory and immunomodulatory properties, in addition to their ability to form cartilage and bone. As a result of these characteristics, MSCs could be employed for the treatment of autoimmune diseases like rheumatoid arthritis, whereas further clinical studies are needed to produce sound evidence (Ansboro et al., 2017; Rotondo et al., 2017).
The ISCT criteria do not provide information about MSCs potential as therapeutic cell sources. Therefore, different comparative studies have been carried out to evaluate the potential of MSCs from various origins in order to select the best source for cell-based therapy (Jin et al., 2013). MSCs can be obtained from different tissues, such as amniotic fluid (AF-MSCs), dental pulp tissues (DPSCs), placental-derived MSCs (PD-MSCs), bone marrow (BM-MSCs) and adipose tissues (ADSCs) (Ullah et al., 2015). It has been reported that about 90% of AF-MSCs express some ESC markers, such as octamer-binding transcription factor 4 (Oct-4). Thus, AF-MSCs can be considered an intermediate stage between embryonic and adult stem cells (Rossi et al., 2014; Markmee et al., 2017). Osteogenic differentiation of AF-MSCs leads to Wnt signaling pathway activation, while Wnt signaling inhibition through selective inhibitor Dickkopf-1 (DKK-1) promotes adipogenesis (D’Alimonte et al., 2013).
Dental pulp tissues were reported to be the first human dental MSCs identified from pulp tissues (Pisciotta et al., 2015). Subsequently, other dental MSCs have been discovered, deriving from human exfoliated deciduous teeth (SHED), tooth germ progenitor cells (TGPCs), dental follicle progenitor cells (DFPCs), periodontal ligament (PDLSCs), alveolar bone-derived MSCs (ABMSCs), apical papilla (SCAP), and gingival MSCs (GMSCs) (Liu et al., 2015). These MSCs, derived from dental tissues, show some proprieties, such as self-renewal, multi-differentiation potential, immunomodulatory functions, as well as an effective capacity for tissue regeneration, including bone tissue (Liu et al., 2015).
The osteogenic differentiation capacity of MSCs derived from placental tissues, i.e., amniotic membrane MSCs (AM-MSCs), umbilical cord MSCs (UC-MSCs), chorionic membrane MSCs (CM-MSCs) and deciduas MSCs (DC-MSCs) have also been studied (Shen et al., 2019). These data have demonstratedthat AM-MSCs and UC-MSCs contain higher osteogenic potential and, therefore, are good sources for bone reconstruction tissue engineering (Shen et al., 2019). Other recent studies have reported that UC-MSCs, deriving from Wharton’s jelly region, show better osteogenic differentiation than other cordon regions (Mennan et al., 2013), while another research has compared UC-MSCs derived from Wharton’s jelly with ADSCs, demonstrating that ADSCs have a higher osteogenic differentiation capacity compared to UC-MSCs derived from Wharton’s jelly after 21 days of osteogenic differentiation (Zajdel et al., 2017).
Adipose tissues and BM-MSCs are probably the most common MSCs used in clinical practice (Fitzsimmons et al., 2018). Donor characteristics, such as age or body weight, can play a role in both the quality and quantity of collected MSCs (Marędziak et al., 2016). Specifically, it has been reported that BM-MSCs show altered proliferation and senescence with increasing age, while ADSCs do not demonstrate these negative age-related effects (Zhu et al., 2008; Beane et al., 2014).
It has been reported that biomarker levels in relation to senescence at ADSCs and BM-MSCs passages 6 and 10, such as SA-gal activity and p21 gene expression, were lower in ADSCs compared to BM-MSCs, which may contribute in part to the higher proliferation rate and differentiation potential of ADSCs (Chen et al., 2012).
On the other hand, Choudhery et al. (2014), showed that ADSC functions are influenced by advancing age. Furthermore, it has been reported (Liu et al., 2018) that transfected-BM-MSCs with microRNA miR-26a, which were previously investigated by Luzi et al. (2012) as a possible target for bone disease RNA-based therapy, improved the bone repair process of cranial bone defects in mice. Moreover, some MSC factors have been shown to be able to influence the differentiation abilities of these cells (Wang et al., 2015). It has been reported that UC-MSCs secretion factors can initiate osteogenesis of BM-MSCs in rat calvarial bone critical defects (Wang et al., 2015).
Mesenchymal stem cells substantially can be obtained from almost any tissue of the human body. However, stem cells collecting process and donor characteristics can represent practical drawbacks. For these reasons, the operator must consider the difficulty in obtaining samples and the potential adverse effects in collecting the cells from the donor in order to select an adequate cell source (Vishnubalaji et al., 2012). For example, collecting BM-MSCs from the donor can be painful, or resulting in bleeding and infection. On the other hand, ADSCs are abundant, while representing one of the main stem cell source. In addition, collecting ADSCs is much less painful procedure compared to other stem cell sources (Zare et al., 2018).
Mesenchymal Stem Cells in Cell Therapies
In the bone regeneration field, cell-based therapies using MSCs can provide solutions to several problems relating to bone fractures due to trauma or bone diseases (Grayson et al., 2015). When bone is subjected to inflammatory stimuli, a cascade of inflammatory and regenerative events occur to allow local repair and bone healing (Loi et al., 2016). This process includes some sequential events, such as the local and systemic release of pro-inflammatory cytokines, recruitment of immune cells to the damaged site, soft-tissue inflammation and edema, differentiation of osteogenic progenitor cells, the local release of bone morphogenetic proteins, callus formation and bone remodeling (Grayson et al., 2015). During bone remodeling, MSCs are known to differentiate into osteoblasts to enable bone formation (Crane and Cao, 2014). Endogenous or exogenous MSCs migration to the bone injury site is a crucial step in treating bone disease (Su et al., 2018). In particular, endogenous MSC recruitment is influenced by inflammatory mediators secreted by immune cells (Ren et al., 2010; Almeida et al., 2012), TGF-β1 released by the bone matrix (Wan et al., 2012) or chemokines, such as stromal cell-derived factor 1 (SDF-1 also known as CXCL12) (Kitaori et al., 2009). It has been shown that CXCL12 is found at high levels both in human MSCs and primary osteoblasts; CXCL12 seems to be regulated by Slug, a member of a superfamily of zinc-finger transcription factors required for osteoblast differentiation (Piva et al., 2011). In addition, other chemotactic factors are involved in this process, such as cytokines (e.g., IL-6, TNF-a, and IL-1b) and growth factors (IGF-1, PDGF-BB, TGF-β, and HGF) (Li and Jiang, 2011).
Immediate use of exogenous MSCs after acute injury leads to a decrease in local and systemic inflammatory responses (Grayson et al., 2015). Many studies have reported that MSCs can regulate immune systems by suppressing T cells, reducing activation and proliferation of B-cell and NK cells, while promoting regulatory T cell generation (Yagi et al., 2010; Gao et al., 2014). In contrast, MSCs administrated in intermediate periods after injury, participate in bone repair due to differentiation into chondrocytes and osteoblasts, thus stimulating local endogenous osteoprogenitor cell recruitment (Grayson et al., 2015).
In MSCs-based therapy, a potential limitation is that MSCs do not persist following infusion. Some authors have sustained that a more active immunological process is also responsible for the limited persistence of allo-MSCs. Indeed, Ankrum et al. (2014), have supported the idea that MSCs are “immune evasive” and not “immune privileged,” as defined by others authors (Paterson et al., 2014), since allogenic MSCs from donors may cause immune rejection (Ankrum et al., 2014). The ISCT defined human MSCs as MHC I positive and MHC II negative (Dominici et al., 2006); Le Blanc et al. (2003), have demonstrated that undifferentiated MSCs express low levels of MHC class I and are negative for MHC class II, while differentiated MSCs or MSCs exposed to IFN-γ can express significantly more MHC I and MHC II.
Mesenchymal stem cells harvested from specific tissue in cell therapies, can be utilized with or without culture expansion (Verboket et al., 2018). Moreover, MSCs can be delivered to the injured area of the bone through: (i) systematic or local injections and (ii) engineering techniques (Oryan et al., 2017) (Figure 2).
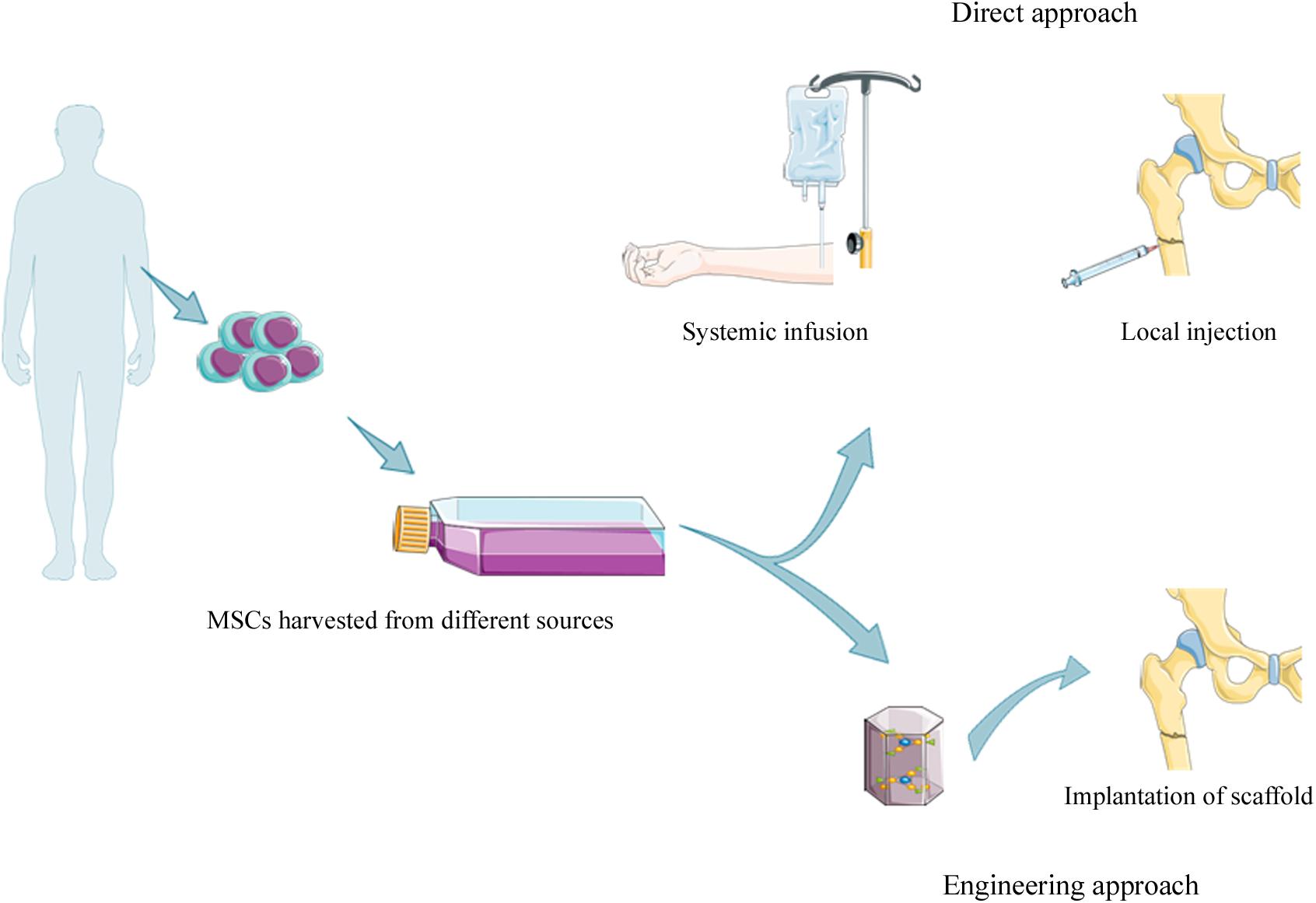
Figure 2. Strategies for MSCs based therapy. MSCs can be isolated from different sources [e.g., amniotic fluid (AF-MSCs), dental pulp tissue (DPSCs), placental-derived MSCs (PD-MSCs), bone marrow tissue (BM-MSCs), and adipose tissue (ADSCs)] with or without culture expansion before clinical application. MSCs can be introduced intravenously by systemic infusion or local injection into fracture site (direct approach), or loaded on scaffold (e.g., ceramics, polymers, and composite) before the implantation into damaged area.
Direct Injection Approach
The direct injection approach includes systemic MSCs administration through intravascular injection (e.g., intravenous and intra-arterial injection). This method enables MSCs to be widely distributed throughout the body using specific cell numbers defined as the effective cell dose (ECD), which is the minimum cell number required to observe a therapeutic effect (Horwitz et al., 2001). However, some limitations exist relating to the formation of microemboli (Boltze et al., 2015). Specifically, since MSC diameters are large (ranging from 15 to 30 μm), MSCs could be trapped in small vessels causing the “first pass effect,” for example, in lungs (Fischer et al., 2009).
The dynamic distribution of MSCs administrated through intra-artery, intravenous and intraperitoneal cavity infusions has been monitored by real-time imaging, immediately after infusion and at 48 h post-infusion in rat. The results showed that MSCs have been detected in the first phase in lungs and then in the liver and other organs, including long bones (Gao et al., 2001). Many researchers suppose that local injection of MSCs is more effective than systemic injections since all MSCs are lost when they are trapped in the lungs after systemic infusion (Oryan et al., 2017).
Direct injection of MSCs involves the bone marrow aspiration mainly from the iliac crest. The aspirated bone marrow, containing many MSCs, is called bone marrow aspirate concentrate (BMAC) (Qin et al., 2014). BMAC is usually reduced in volume to increase MSCs content. During this process red cells and plasma are removed before BMAC injection into damaged areas (Hernigou and Beaujean, 2002). Hernigou et al. (2005), suggested that autologous MSCs could be used for treating fractures in patients with atrophic non-union of the tibia through percutaneous injection of BMAC. A recent study has reported that BM-MSCs injection on day 7 after fracture can improve bone healing in a murine model (Wang et al., 2018). Huang et al. (2015), have demonstrated that systemic and local administration of allogenic BM-MSCs can improve callus formation in fracture healing in rats.
In conclusion, systemic MSCs injection is useful for treating injuries present at multiple sites. Some limitations/problems may arise in patients because of possible microemboli formation. Local MSCs injection is a non-invasive procedure, which is more advisable for a single injury than complex fractures (Raynaud and Rafii, 2013; Abazari et al., 2019).
MSCs, Biomaterials, Growth Factors in the Tissue Engineering Approach
Defects less than 50 mm in length might be repaired with autologous bone grafting, while this procedure is inefficient in larger defects (Dumic-Cule et al., 2015). As a result, alternative tissue-engineering strategies combining biomaterials/scaffolds, MSCs and growth factors are used in order to improve bone repair in fractures greater than 50 mm (Decambron et al., 2017). Scaffolds are considered structures, which improve cellular adhesion, proliferation and osteogenic differentiation. Another important characteristic of scaffolds concerns interconnected porosity (the optimal pore size is 200–350 μm) to enable the successful diffusion of nutrients, oxygen, and cellular waste products (Murphy et al., 2010).
It has been established that ECM contains growth factors and cytokines. For these characteristics ECM was employed, as potential therapeutic biomaterial, to promote cell proliferation and differentiation (Frantz et al., 2010). The excised tissue must undergo decellularization. This procedure, which is a combination of physical stress and chemical/enzymatic treatments, allows to remove cells without destroying essential ECM components. Then, decellularized extracellular matrix (dECM) can be used for therapeutic applications. It should be recalled that the composition and spatial orientation of ECM varies from tissue to tissue (Badylak et al., 2015). As mentioned above, bone ECM consists of an organic phase with types I, II, V collagen and non-collagenous proteins. The organic phase constitutes approximately 20% of bone mass, together with the mineral phase. Moreover, bone ECM also contains pro-inflammatory cytokines and several growth factors, such as bone morphogenic proteins (BMPs), vascular endothelial growth factor (VEGF), transforming growth factor β(TGF-β), platelet-derived growth factor (PDGF), and fibroblast growth factors (FGFs) (Papadimitropoulos et al., 2015).
The dECM can be processed for different tissue engineering applications. Thus, dECM can be used as scaffold in order to maintain its original geometry, as bio-ink (see below) or hydrogels (Kim et al., 2019). A recent study reported that tissue-specific hydrogels derived from decellularized bovine bone extracellular matrix (bECM) owns specific mechanical and biological characteristics, including osteogenic potential for clinical use (Sawkins et al., 2013). Moreover, in other investigations, dealing with bone tissue engineering, reported that the combination of bECM hydrogels with DPSCs, which is a source of potential stem cells, is sufficient to induce their osteogenic differentiation (Tatullo et al., 2015) without requiring additional osteogenic factors (Paduano et al., 2017). Thus, dECM is able to mimic in full the complex interactions that take place within the tissue. In addition, dECM because the cellular DNA is almost completely removed during the decellularization process has a lower risk of activating the immune response (Kim et al., 2019). However, one of the main dECM limitation is the lack of standardized procedures. Tissue sources and storage conditions employed before decellularization may influence the quality of dECM, resulting in batch-to-batch differences even within the same tissue type (Kim et al., 2019). To promote bone regeneration, limitations could be circumvented employing also synthetic bone graft substitutes that present specific physic-chemical properties (Ciocca et al., 2017).
Materials employed for bone repair include (i) metals and metal alloys, such as cobalt-chromium, zirconium, titanium, (ii) ceramics and bioactive glasses, which include calcium phosphate (CaP), tricalcium phosphate (TCP) and hydroxylapatite (HA)-derived scaffolds, (iii) biological materials, e.g., collagen and chitosan or synthetic polymers, including polylactic acid (PLA), polyglycolide (PGA) and the copolymer of poly-(DL-lactic-co-glycolic-acid) (PLGA), (iv) composite materials derived from the combination of polymer and ceramic scaffolds, such as HA-collagen scaffolds (Iaquinta et al., 2019).
To date, novel bioactive nanomaterials and nanofabrication techniques allow to control the physical and structural characteristics of new scaffolds (Cross et al., 2016). Indeed, it has been reported that two-dimensional (2D) synthetic nanosilicates (Laponite, Na+0.7[(Mg5.5Li0.3)Si8O20(OH)4]–0.7) induce hMSCs to the osteogenic differentiation through dissolution products, such as Na+, Mg2+, Li+, and Si(OH)4 (Gaharwar et al., 2013). It has been demonstrated that the incorporation of nanosilicates can improve physical integrity with an increase of the scaffold mechanical strength without any osteogenic supplements (Kerativitayanan et al., 2017).
Overall, it is difficult to create the complex structure of scaffolds with precise conventional techniques. A large variety of methods, i.e., solvent casting/particulate leaching, gas foaming, phase separation or electrospinning, have been used in the fabrication of 3D scaffolds, either as a single procedure or in combination (Turnbull et al., 2018). Moreover, 3D printing is a valid alternative technique developed for the production of scaffolds. Indeed, 3D printing allows to produce scaffolds, layer-by-layer, using powder, liquid or solid material substrates (Mironov, 2003). The microstructure of a 3D printed-scaffold can be obtained by a computer-aided design (CAD) model loaded onto a 3D printer (Do et al., 2015). Bioprinting is a 3D technique employed for the realization of constructs by depositing biological elements, such as cells and growth factors, in order to repair or replace damaged tissues (Skardal and Atala, 2015). To this purpose, bioprinting requires (i) the bio-ink, containing scaffolds in which biological components are encapsulated and (ii) a 3D bio-plotter, which is a 3D printer system used to extrude the bio-ink. Cells for printing can be obtained from tissue biopsies, blood samples or other sources, then expanded in vitro to maximize cell density on bioprinting. Cells are encapsulated within the biomaterial to realize the 3D biological construct to be implanted in vivo. The interface between cells and scaffolds has a crucial role in tissue regeneration, which is a complex and dynamic microenvironment (Murphy et al., 2014). The different properties of scaffold, such as stiffness and nanostructure, can affect stem cell responses, while enhancing the osteogenic differentiation for bone repair (Zhang et al., 2018). Many efforts have been carried out to design “smart” scaffolds with specific physical/chemical properties incorporating bioactive molecules and nanoparticles, such as growth factors or extracellular matrix (ECM)-like molecules (Motamedian et al., 2015). This approach improves the interactions with cells thus enhancing bone regeneration. Growth factors play a significant role in bone repair/regrowth. Several growth factors and biomolecules control the new bone formation and the ECM deposition. Indeed, growth factors, such as BMPs, VEGF, TGF-β, PDGF, IGF-1, and FGFs have frequently been included in scaffolds (Iaquinta et al., 2019). In regenerative medicine, platelet-rich plasma (PRP) derived from blood plasma and its derivatives are employed to potentiate stem cell proliferation, migration, and differentiation (Santos et al., 2018). However, PRP is not considered to be osteoinductive, whereas the addition of PRP to specific bone graft substitutes can improve the bone healing process (Malhotra et al., 2013). For example, PRP and autologous BM-MSCs synergism when seeded onto macroporous CPC can promote bone regeneration in mini pigs (Qiu et al., 2018).
In a recent study, the regenerative potential of two MSCs, i.e., AF-MSCs and BM-MSCs were compared in vitro and in vivo (Mohammed et al., 2019). This research demonstrated that AF-MSCs loaded on gel-foam scaffolds performed better during in vivo bone healing than BM-MSCs (Mohammed et al., 2019). Osteogenic differentiation of human ADSCs (Figure 3) loaded onto HA/type I collagen scaffold (Coll/Pro Osten 200®), a biomaterial used in maxillofacial surgery for zygomatic augmentation (D’Agostino et al., 2016), was tested in vitro to evaluate the expression of specific genes involved in osteogenic differentiation (e.g., SP7 and ALP), as well as adhesion molecules gene expression, such as ECM (Mazzoni et al., 2017a, 2019).
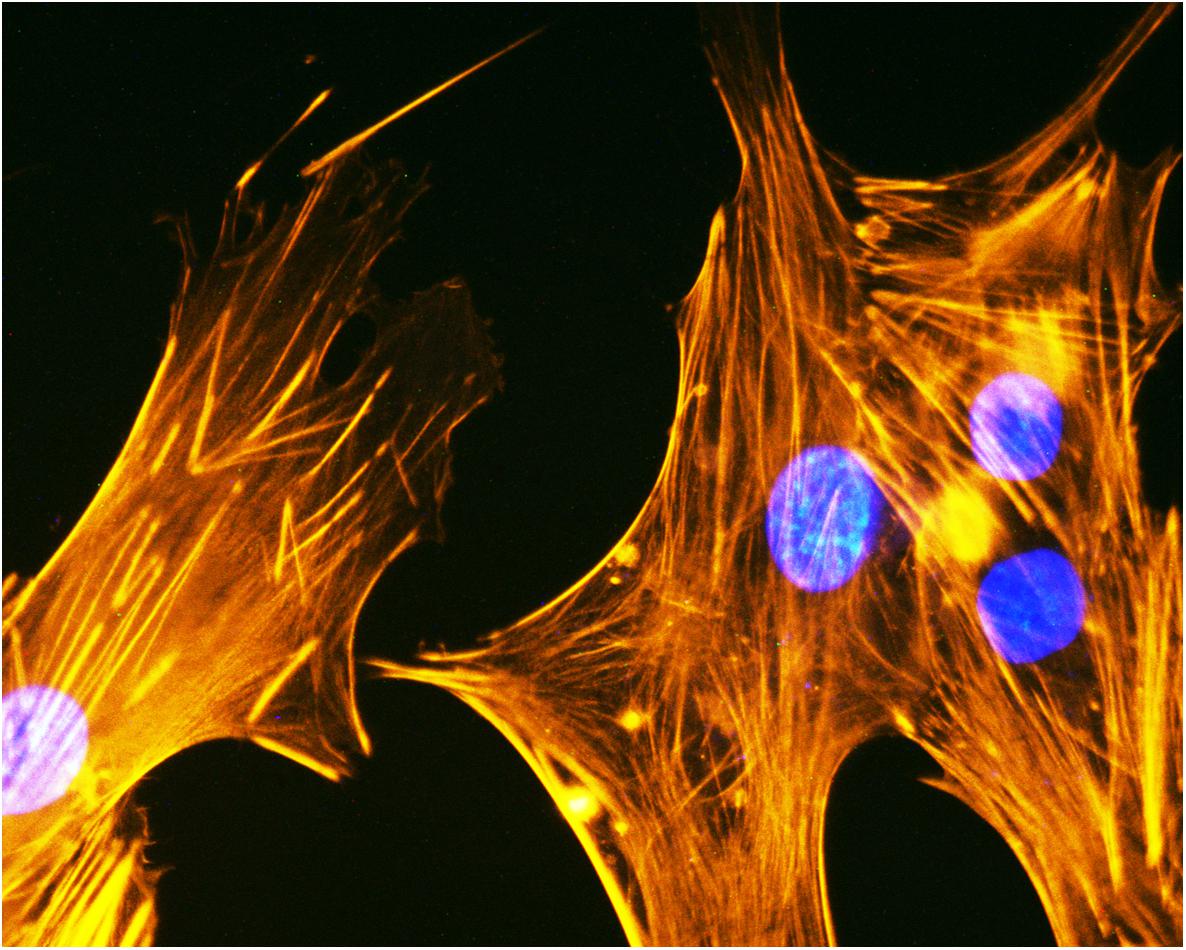
Figure 3. Cytoskeleton analysis of human ADSCs. Cytoskeleton analysis by phalloidin TRITC (tetramethylrhodamineisothiocyanate) staining of human ADSCs grown on the biomaterial (magnification 40x). Cellular nuclei were stained with 0.5 mg/ml DAPI.
In addition to human ADSCs, engineered human osteoblast-like cells, Saos-eGFP, were employed to evaluate the biocompatibility and bioactivity of HA/collagen-derived scaffolding in vitro (Manfrini et al., 2015). Interestingly, it has been reported that HA-derived scaffolding co-doped with gallium, magnesium, and carbonate showed osteogenic and antibacterial abilities. Specifically, doping with gallium can induce antibacterial effects without negative consequences for human ADSCs viability in vitro (Ballardini et al., 2018). Further stimulating work has reported that autologous ADSCs, when harvested in accordance with GMP guidelines, were employed to treat 13 cases of cranio-maxillofacial hard-tissue defects (Sándor et al., 2014). These defects were repaired with ADSCs seeded onto bioactive glass or β-TCP scaffolds and, in some cases, with additional recombinant bone morphogenetic protein-2 (BMP-2). Clinical evaluation showed successful integration of the constructs in 10 out of 13 cases (Sándor et al., 2014). A recent study in vitro compared ADSCs and BMSCs osteogenic capabilities when seeded onto Bioglass-based scaffolds. Data showed that both ADSCs and BMSCs have similar characteristics, whereas ADSCs seeded onto Bioglass-based scaffolds can differentiate into osteogenic lineage without the use of an osteogenic medium, compared to BMSCs (Rath et al., 2016). On the other hand, another study has revealed that BMSCs seeded onto nanocomposite bioactive glass/gelatine scaffold had higher osteogenesis capacities than UC-MSCs and ADSCs both in vitro and in vivo (Kargozar et al., 2018). An alternative approach to scaffold-based tissue engineering is the so called “cell sheet” technique, which was used for the first time in 1970 to create tissue from cultured cells (Green et al., 1979). This technique was based on cell sheets derived from hyperconfluent cell cultures characterized by extensive cell-to-cell interaction and its own ECM (Nakao et al., 2019). In addition, the cell sheet can be detached using a temperature-responsive culture dish grafted with a poly(N-isopropylacrylamide) in order to preserve cell–cell interactions (Kwon et al., 2018). Indeed, Kushida et al. (2000), have described that MSCs seeded onto temperature-responsive culture dishes can be harvested by reducing the temperature without enzymatic digestion. In tissue engineering, MSC sheets have been used for regenerating different types of organs/tissues including bone tissue, as recently described by Ueyama et al. (2016). Long et al. (2014), have reported that MSC sheets show improved osteogenicity inducing prolonged cartilage and callus formation during critical-sized bone defect repair in mice. Finally, in a pioneering study, Kim et al. (2016), associated the benefits of MSC sheets (derived from canine adipose-derived MSCs) with composite polymer/ceramic scaffolds, such as poly-ε-caprolactone (PCL)/β-tricalcium phosphate (β-TCP). Their results have shown that MSC sheets combined with composite scaffold strongly stimulate and accelerate new bone formation in a critical-sized bone defect in vitro (Kim et al., 2016). Additionally, in engineering tissue genetically modified-MSCs which express specific proteins, radioisotopes or microRNAs can be used as anti-tumor vectors owing to their ability to migrate to sites of active primary or meta-static cancers (Belmar-Lopez et al., 2013). Moreover, it is possible to induce modified-MSCs to produce osteogenic and angiogenic growth factors to promote bone regeneration (Nauth et al., 2010).
Clinical Trials
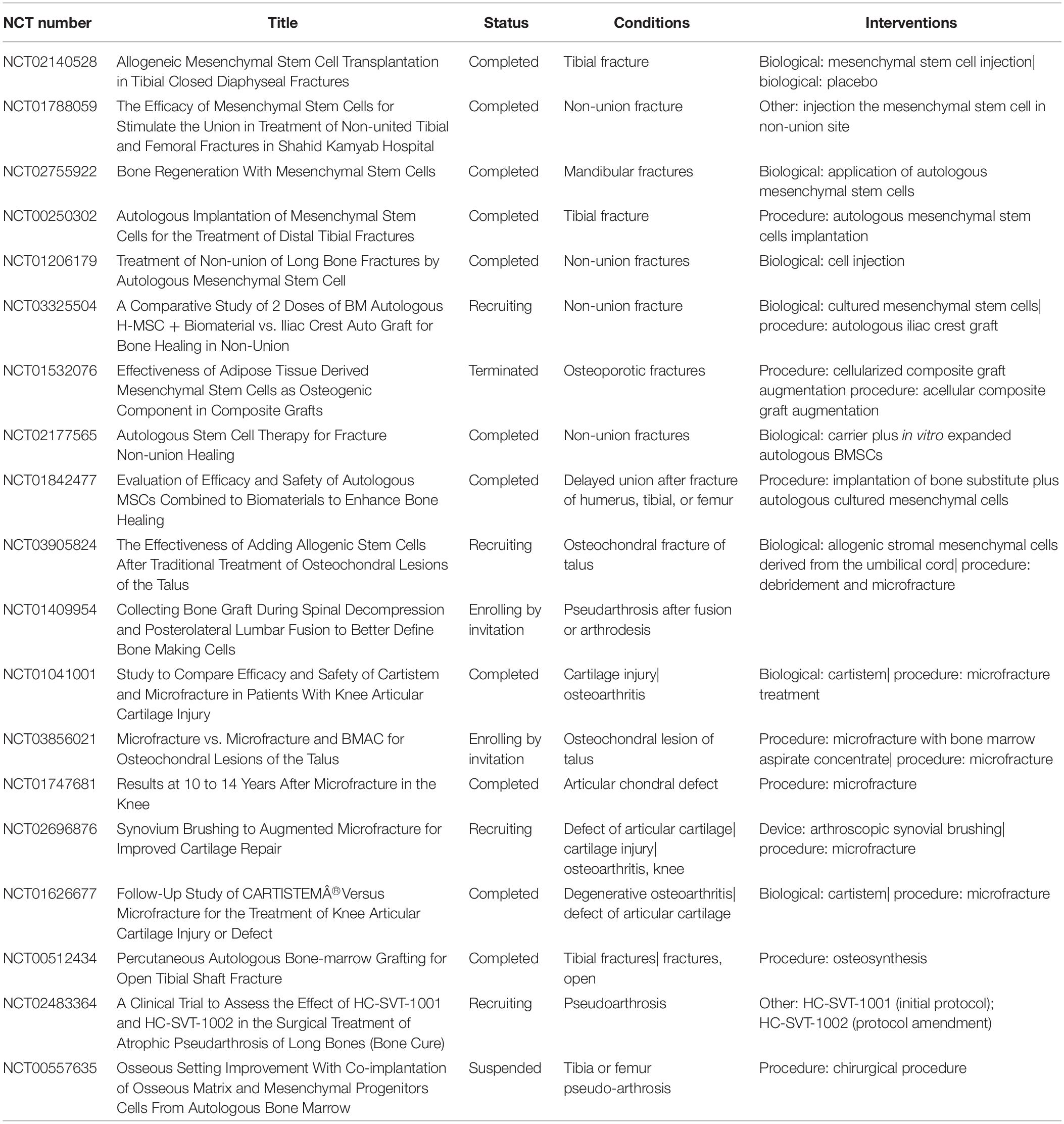
Mesenchymal stem cells have been studied in a large variety of animal species (e.g., sheep or rabbit) (Gallego et al., 2015; Erdogan et al., 2016). Thus, animal models can be used for understanding mechanisms and applications in clinical settings (Oryan et al., 2017). The clinical trials, which have been made available in the public domain, obtained from the ClinicalTrials.gov database1 (June 2019), are listed in Table 1 in order to show the current status of clinical MSC therapy for bone repair.
Currently, there are 966 clinical trials available involving the use of MSCs for the treatment of several pathological conditions such as, respiratory syndrome, autoimmune diseases, or immune system diseases. In this review, firstly we considered all the clinical trials related to “mesenchymal stem cells” and “fractures”; subsequently, those labeled as “unknown status,” “withdrawn,” or “not yet recruiting” were excluded. Thus, 19 clinical trials were identified.
Among these, some clinical trials such as NCT01041001, NCT03856021, and NCT01747681 are related to cartilage engineering rather than bone fracture repair.
Mesenchymal stem cells were administrated in several procedures, such as direct injection or biomaterial implantation. Although many studies have been completed, the major limitation of these clinical trials is the absence of published data. In addition, many trials do not provide enough information about protocol, which would be required in order to reproduce this work in other centers/laboratories.
Conclusion
Mesenchymal stem cells are attractive candidates for cell-based therapy due to self-renewal, multipotent, immunosuppressive, and homing properties. Similarly, MSCs can regenerate damaged tissue, exert autocrine/paracrine effects and modified-MSCs can delivery therapeutic molecules/genes in bone disease treatment.
To date, the specific mechanisms of MSCs in bone healing are yet to be understood vis-à-vis evaluating their performance on large bone defects and defining the best approaches to be used in clinical practice. In particular, further research is required in order to avoid problems relating to unwanted MSC differentiation. To this purpose, standardized protocols are needed to enable the regulation of MSC growth conditions during ex vivo expansion. Other limitations in cell therapy concern ethical issues, possible immunological rejection, the limited amount of available stem cells or variability due to donor-related differences. In addition to MSCs isolation and expansion, another challenge for bone regenerative medicine is MSCs delivery to the bone injury. In this context, several osteoinductive/osteoconductive biomaterials have been employed to provide a 3D environment for MSCs at the site of bone fractures in order to promote MSCs angiogenesis and osteogenic differentiation. This approach can be improved by seeding MSCs on appropriate biomaterials in the presence of specific growth factors, such as BMPs (in particular BMP-2 and BMP-7) or PRP.
Moreover, tissue-derived MSC sheets could be used alone or in combination with different scaffolds in order to accelerate the bone healing process in orthopedic and traumatology cases.
However, despite MSC therapy being an interesting development in tissue engineering, further studies are needed to suggest new MSC therapies for bone repairs due to an absence of data from current completed clinical studies. In the future, further knowledge of MSC potential and the use of new therapeutic strategies could allow improved approaches to bone regeneration/healing to be developed.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
The work of the authors cited in this review was supported, in part, by the University of Ferrara FAR and FIR grants, Regione Emilia-Romagna POR FESR project “NIPROGEN,” and PRIN 2017 MIUR.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We wish to thank Dr. Georgia Emma Gili for revising the English text.
Footnotes
References
Abazari, M. F., Nejati, F., Nasiri, N., Khazeni, Z. A. S., Nazari, B., Enderami, S. E., et al. (2019). Platelet-rich plasma incorporated electrospun PVA-chitosan-HA nanofibers accelerates osteogenic differentiation and bone reconstruction. Gene 720:144096. doi: 10.1016/j.gene.2019.144096
Abdel Meguid, E., Ke, Y., Ji, J., and El-Hashash, A. H. K. (2018). Stem cells applications in bone and tooth repair and regeneration: new insights, tools, and hopes. J. Cell. Physiol. 233, 1825–1835. doi: 10.1002/jcp.25940
Almeida, C. R., Vasconcelos, D. P., Gonçalves, R. M., and Barbosa, M. A. (2012). Enhanced mesenchymal stromal cell recruitment via natural killer cells by incorporation of inflammatory signals in biomaterials. J. R. Soc. Interface 9, 261–271. doi: 10.1098/rsif.2011.0357
Ankrum, J. A., Ong, J. F., and Karp, J. M. (2014). Mesenchymal stem cells: immune evasive, not immune privileged. Nat. Biotechnol. 32, 252–260. doi: 10.1038/nbt.2816
Ansboro, S., Roelofs, A. J., and De Bari, C. (2017). Mesenchymal stem cells for the management of rheumatoid arthritis: immune modulation, repair or both? Curr. Opin. Rheumatol. 29, 201–207. doi: 10.1097/BOR.0000000000000370
Arvidson, K., Abdallah, B. M., Applegate, L. A., Baldini, N., Cenni, E., Gomez-Barrena, E., et al. (2011). Bone regeneration and stem cells. J. Cell. Mol. Med. 15, 718–746. doi: 10.1111/j.1582-4934.2010.01224.x
Badylak, S. F., Freytes, D. O., and Gilbert, T. W. (2015). Reprint of: extracellular matrix as a biological scaffold material: structure and function. Acta Biomater. 23(Suppl.), S17–S26. doi: 10.1016/j.actbio.2015.07.016
Ballardini, A., Montesi, M., Panseri, S., Vandini, A., Balboni, P. G., Tampieri, A., et al. (2018). New hydroxyapatite nanophases with enhanced osteogenic and anti-bacterial activity. J. Biomed. Mater. Res. A 106, 521–530. doi: 10.1002/jbm.a.36249
Bayraktar, H. H., Morgan, E. F., Niebur, G. L., Morris, G. E., Wong, E. K., and Keaveny, T. M. (2004). Comparison of the elastic and yield properties of human femoral trabecular and cortical bone tissue. J. Biomech. 37, 27–35. doi: 10.1016/s0021-9290(03)00257-4
Beane, O. S., Fonseca, V. C., Cooper, L. L., Koren, G., and Darling, E. M. (2014). Impact of aging on the regenerative properties of bone marrow-, muscle-, and adipose-derived mesenchymal stem/stromal cells. PLoS One 9:e115963. doi: 10.1371/journal.pone.0115963
Belmar-Lopez, C., Mendoza, G., Oberg, D., Burnet, J., Simon, C., Cervello, I., et al. (2013). Tissue-derived mesenchymal stromal cells used as vehicles for anti-tumor therapy exert different in vivo effects on migration capacity and tumor growth. BMC Med. 11:139. doi: 10.1186/1741-7015-11-139
Boltze, J., Arnold, A., Walczak, P., Jolkkonen, J., Cui, L., and Wagner, D.-C. (2015). The dark side of the force - constraints and complications of cell therapies for stroke. Front. Neurol. 6:155. doi: 10.3389/fneur.2015.00155
Boyce, B. F. (2013). Advances in osteoclast biology reveal potential new drug targets and new roles for osteoclasts. J. Bone Miner. Res. 28, 711–722. doi: 10.1002/jbmr.1885
Caplan, A. I., and Dennis, J. E. (2006). Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 98, 1076–1084. doi: 10.1002/jcb.20886
Charles, J. F., and Aliprantis, A. O. (2014). Osteoclasts: more than ‘bone eaters.’. Trends Mol Med. 20, 449–459. doi: 10.1016/j.molmed.2014.06.001
Chen, H.-T., Lee, M.-J., Chen, C.-H., Chuang, S.-C., Chang, L.-F., Ho, M.-L., et al. (2012). Proliferation and differentiation potential of human adipose-derived mesenchymal stem cells isolated from elderly patients with osteoporotic fractures. J. Cell. Mol. Med. 16, 582–593. doi: 10.1111/j.1582-4934.2011.01335.x
Chen, W., Liu, X., Chen, Q., Bao, C., Zhao, L., Zhu, Z., et al. (2018). Angiogenic and osteogenic regeneration in rats via calcium phosphate scaffold and endothelial cell coculture with hBMSCs, hUCMSCs, hiPSC-MSCs and hESC-MSCs. J. Tissue Eng. Regen. Med. 12, 191–203. doi: 10.1002/term.2395
Choudhery, M. S., Badowski, M., Muise, A., Pierce, J., and Harris, D. T. (2014). Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J. Transl. Med. 12, 8. doi: 10.1186/1479-5876-12-8
Chung, Y., Klimanskaya, I., Becker, S., Li, T., Maserati, M., Lu, S.-J., et al. (2008). Human embryonic stem cell lines generated without embryo destruction. Cell Stem Cell 2, 113–117. doi: 10.1016/j.stem.2007.12.013
Ciocca, L., Lesci, I. G., Mezini, O., Parrilli, A., Ragazzini, S., Rinnovati, R., et al. (2017). Customized hybrid biomimetic hydroxyapatite scaffold for bone tissue regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 105, 723–734. doi: 10.1002/jbm.b.33597
Crane, J. L., and Cao, X. (2014). Bone marrow mesenchymal stem cells and TGF-β signaling in bone remodeling. J. Clin. Invest. 124, 466–472. doi: 10.1172/JCI70050
Cross, L. M., Thakur, A., Jalili, N. A., Detamore, M., and Gaharwar, A. K. (2016). Nanoengineered biomaterials for repair and regeneration of orthopedic tissue interfaces. Acta Biomater. 42, 2–17. doi: 10.1016/j.actbio.2016.06.023
Cunningham, J. J., Ulbright, T. M., Pera, M. F., and Looijenga, L. H. J. (2012). Lessons from human teratomas to guide development of safe stem cell therapies. Nat. Biotechnol. 30, 849–857. doi: 10.1038/nbt.2329
Cypher, T. J., and Grossman, J. P. (1996). Biological principles of bone graft healing. J. Foot Ankle Surg. 35, 413–417. doi: 10.1016/s1067-2516(96)80061-5
D’Alimonte, I., Lannutti, A., Pipino, C., Di Tomo, P., Pierdomenico, L., Cianci, E., et al. (2013). Wnt signaling behaves as a “master regulator” in the osteogenic and adipogenic commitment of human amniotic fluid mesenchymal stem cells. Stem Cell Rev. 9, 642–654. doi: 10.1007/s12015-013-9436-5
Decambron, A., Fournet, A., Bensidhoum, M., Manassero, M., Sailhan, F., Petite, H., et al. (2017). Low-dose BMP-2 and MSC dual delivery onto coral scaffold for critical-size bone defect regeneration in sheep. J. Orthop. Res. 35, 2637–2645. doi: 10.1002/jor.23577
Delloye, C., Cornu, O., Druez, V., and Barbier, O. (2007). Bone allografts: what they can offer and what they cannot. J. Bone Joint Surg. Br. 89, 574–579. doi: 10.1302/0301-620X.89B5.19039
Do, A.-V., Khorsand, B., Geary, S. M., and Salem, A. K. (2015). 3D printing of scaffolds for tissue regeneration applications. Adv. Healthc Mater. 4, 1742–1762. doi: 10.1002/adhm.201500168
Dominici, M., Le Blanc, K., Mueller, I., Slaper-Cortenbach, I., Marini, F., Krause, D., et al. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 8, 315–317. doi: 10.1080/14653240600855905
Drela, K., Stanaszek, L., Nowakowski, A., Kuczynska, Z., and Lukomska, B. (2019). Experimental strategies of mesenchymal stem cell propagation: adverse events and potential risk of functional changes. Stem Cells Int. 2019:7012692. doi: 10.1155/2019/7012692
Dumic-Cule, I., Pecina, M., Jelic, M., Jankolija, M., Popek, I., Grgurevic, L., et al. (2015). Biological aspects of segmental bone defects management. Int. Orthop. 39, 1005–1011. doi: 10.1007/s00264-015-2728-4
D’Agostino, A., Trevisiol, L., Favero, V., Gunson, M. J., Pedica, F., Nocini, P. F., et al. (2016). Hydroxyapatite/Collagen composite is a reliable material for malar augmentation. J. Oral Maxillofac. Surg. 74, 1238.e1–1238.e15. doi: 10.1016/j.joms.2016.01.052
Einhorn, T. A., and Gerstenfeld, L. C. (2015). Fracture healing: mechanisms and interventions. Nat. Rev. Rheumatol. 11, 45–54. doi: 10.1038/nrrheum.2014.164
Erdogan, Ö, Supachawaroj, N., Soontornvipart, K., and Kheolamai, P. (2016). Treatment of peri-implant defects in the Rabbit’s tibia with adipose or bone marrow-derived mesenchymal stems cells. Clin. Implant. Dent. Relat. Res. 18, 1003–1014. doi: 10.1111/cid.12378
Fantner, G. E., Adams, J., Turner, P., Thurner, P. J., Fisher, L. W., and Hansma, P. K. (2007). Nanoscale ion mediated networks in bone: osteopontin can repeatedly dissipate large amounts of energy. Nano Lett. 7, 2491–2498. doi: 10.1021/nl0712769
Farbod, K., Nejadnik, M. R., Jansen, J. A., and Leeuwenburgh, S. C. G. (2014). Interactions between inorganic and organic phases in bone tissue as a source of inspiration for design of novel nanocomposites. Tissue Eng. Part B Rev. 20, 173–188. doi: 10.1089/ten.TEB.2013.0221
Ferracini, R., Martínez Herreros, I., Russo, A., Casalini, T., Rossi, F., and Perale, G. (2018). Scaffolds as structural tools for bone-targeted drug delivery. Pharmaceutics 10:E122. doi: 10.3390/pharmaceutics10030122
Figliomeni, A., Signorini, V., and Mazzantini, M. (2018). One year in review 2018: progress in osteoporosis treatment. Clin. Exp. Rheumatol. 36, 948–958.
Fillingham, Y., and Jacobs, J. (2016). Bone grafts and their substitutes. Bone Joint J. 98, 6–9. doi: 10.1302/0301-620X.98B.36350
Fischer, U. M., Harting, M. T., Jimenez, F., Monzon-Posadas, W. O., Xue, H., Savitz, S. I., et al. (2009). Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 18, 683–691. doi: 10.1089/scd.2008.0253
Fitzsimmons, R. E. B., Mazurek, M. S., Soos, A., and Simmons, C. A. (2018). Mesenchymal stromal/stem cells in regenerative medicine and tissue engineering. Stem Cells Int. 2018, 8031718. doi: 10.1155/2018/8031718
Frantz, C., Stewart, K. M., and Weaver, V. M. (2010). The extracellular matrix at a glance. J. Cell Sci. 123, 4195–4200. doi: 10.1242/jcs.023820
Friedenstein, A. J., Chailakhjan, R. K., and Lalykina, K. S. (1970). The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 3, 393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x
Fu, X. (2014). The immunogenicity of cells derived from induced pluripotent stem cells. Cell Mol. Immunol. 11, 14–16. doi: 10.1038/cmi.2013.60
Fujisawa, R., and Tamura, M. (2012). Acidic bone matrix proteins and their roles in calcification. Front. Biosci. 17:1891–1903. doi: 10.2741/4026
Gaharwar, A. K., Mihaila, S. M., Swami, A., Patel, A., Sant, S., Reis, R. L., et al. (2013). Bioactive silicate nanoplatelets for osteogenic differentiation of human mesenchymal stem cells. Adv. Mater. Weinheim 25, 3329–3336. doi: 10.1002/adma.201300584
Gallego, L., Pérez-Basterrechea, M., García-Consuegra, L., Álvarez-Viejo, M., Megías, J., Novoa, A., et al. (2015). Repair of segmental mandibular bone defects in sheep using bone marrow stromal cells and autologous serum scaffold: a pilot study. J. Clin. Periodontol. 42, 1143–1151. doi: 10.1111/jcpe.12480
Ganss, B., Kim, R. H., and Sodek, J. (1999). Bone sialoprotein. Crit. Rev. Oral Biol. Med. 10, 79–98.
Gao, C., Deng, Y., Feng, P., Mao, Z., Li, P., Yang, B., et al. (2014). Current progress in bioactive ceramic scaffolds for bone repair and regeneration. Int. J. Mol. Sci. 15, 4714–4732. doi: 10.3390/ijms15034714
Gao, J., Dennis, J. E., Muzic, R. F., Lundberg, M., and Caplan, A. I. (2001). The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs 169, 12–20. doi: 10.1159/000047856
Gdyczynski, C. M., Manbachi, A., Hashemi, S., Lashkari, B., and Cobbold, R. S. C. (2014). On estimating the directionality distribution in pedicle trabecular bone from micro-CT images. Physiol. Meas. 35, 2415–2428. doi: 10.1088/0967-3334/35/12/2415
Grayson, W. L., Bunnell, B. A., Martin, E., Frazier, T., Hung, B. P., and Gimble, J. M. (2015). Stromal cells and stem cells in clinical bone regeneration. Nat. Rev. Endocrinol. 11, 140–150. doi: 10.1038/nrendo.2014.234
Green, H., Kehinde, O., and Thomas, J. (1979). Growth of cultured human epidermal cells into multiple epithelia suitable for grafting. Proc. Natl. Acad. Sci. U.S.A. 76, 5665–5668. doi: 10.1073/pnas.76.11.5665
Habibovic, P. (2017). Strategic directions in osteoinduction and biomimetics. Tissue Eng. Part A 23, 1295–1296. doi: 10.1089/ten.TEA.2017.0430
Harrison, D. J., Geller, D. S., Gill, J. D., Lewis, V. O., and Gorlick, R. (2018). Current and future therapeutic approaches for osteosarcoma. Expert Rev. Anticancer Ther. 18, 39–50. doi: 10.1080/14737140.2018.1413939
Hernigou, P., Poignard, A., Beaujean, F., and Rouard, H. (2005). Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J. Bone Joint Surg. Am. 87, 1430–1437. doi: 10.2106/JBJS.D.02215
Hernigou, P., and Beaujean, F. (2002). Treatment of osteonecrosis with autologous bone marrow grafting. Clin. Orthop. Relat. Res. 405, 14–23. doi: 10.1097/00003086-200212000-00003
Horwitz, E. M., Prockop, D. J., Gordon, P. L., Koo, W. W., Fitzpatrick, L. A., Neel, M. D., et al. (2001). Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood 97, 1227–1231. doi: 10.1182/blood.v97.5.1227
Ho-Shui-Ling, A., Bolander, J., Rustom, L. E., Johnson, A. W., Luyten, F. P., and Picart, C. (2018). Bone regeneration strategies: engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials 180, 143–162. doi: 10.1016/j.biomaterials.2018.07.017
Huang, S., Xu, L., Zhang, Y., Sun, Y., and Li, G. (2015). Systemic and local administration of allogeneic bone marrow-derived mesenchymal stem cells promotes fracture healing in rats. Cell Transplant. 24, 2643–2655. doi: 10.3727/096368915X687219
Iaquinta, M., Mazzoni, E., Manfrini, M., D’Agostino, A., Trevisiol, L., Nocini, R., et al. (2019). Innovative biomaterials for bone regrowth. Int. J. f Mol. Sci. 20:E618.
Jeon, O. H., Panicker, L. M., Lu, Q., Chae, J. J., Feldman, R. A., and Elisseeff, J. H. (2016). Human iPSC-derived osteoblasts and osteoclasts together promote bone regeneration in 3D biomaterials. Sci. Rep. 6:26761. doi: 10.1038/srep26761
Ji, J., Tong, X., Huang, X., Zhang, J., Qin, H., and Hu, Q. (2016). Patient-derived human induced pluripotent stem cells from gingival fibroblasts composited with defined nanohydroxyapatite/chitosan/gelatin porous scaffolds as potential bone graft substitutes. Stem. Cells Transl. Med. 5, 95–105. doi: 10.5966/sctm.2015-0139
Jiao, H., Xiao, E., and Graves, D. T. (2015). Diabetes and its effect on bone and fracture healing. Curr. Osteoporos. Rep. 13, 327–335. doi: 10.1007/s11914-015-0286-8
Jimi, E., Akiyama, S., Tsurukai, T., Okahashi, N., Kobayashi, K., Udagawa, N., et al. (1999). Osteoclast differentiation factor acts as a multifunctional regulator in murine osteoclast differentiation and function. J. Immunol. 163, 434–442.
Jin, H. J., Bae, Y. K., Kim, M., Kwon, S.-J., Jeon, H. B., Choi, S. J., et al. (2013). Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int. J. Mol. Sci. 14, 17986–18001. doi: 10.3390/ijms140917986
Jin, Y.-Z., and Lee, J. H. (2018). Mesenchymal stem cell therapy for bone regeneration. Clin. Orthop. Surg. 10, 271–278. doi: 10.4055/cios.2018.10.3.271
Johnson, M. H. (2008). Human ES cells and a blastocyst from one embryo: exciting science but conflicting ethics? Cell Stem Cell 2, 103–104. doi: 10.1016/j.stem.2008.01.021
Kargozar, S., Mozafari, M., Hashemian, S. J., Brouki Milan, P., Hamzehlou, S., Soleimani, M., et al. (2018). Osteogenic potential of stem cells-seeded bioactive nanocomposite scaffolds: a comparative study between human mesenchymal stem cells derived from bone, umbilical cord Wharton’s jelly, and adipose tissue. J. Biomed. Mater. Res. Part B Appl. Biomater. 106, 61–72. doi: 10.1002/jbm.b.33814
Kartsogiannis, V., and Ng, K. W. (2004). Cell lines and primary cell cultures in the study of bone cell biology. Mol. Cell. Endocrinol. 228, 79–102. doi: 10.1016/j.mce.2003.06.002
Kerativitayanan, P., Tatullo, M., Khariton, M., Joshi, P., Perniconi, B., and Gaharwar, A. K. (2017). Nanoengineered osteoinductive and elastomeric scaffolds for bone tissue engineering. ACS Biomater. Sci. Eng. 3, 590–600. doi: 10.1021/acsbiomaterials.7b00029
Khan, F. A., Almohazey, D., Alomari, M., and Almofty, S. A. (2018). Isolation, culture, and functional characterization of human embryonic stem cells: current trends and challenges. Stem Cells Int. 2018:1429351. doi: 10.1155/2018/1429351
Kim, S., Kim, S.-S., Lee, S.-H., Eun Ahn, S., Gwak, S.-J., Song, J.-H., et al. (2008). In vivo bone formation from human embryonic stem cell-derived osteogenic cells in poly(d,l-lactic-co-glycolic acid)/hydroxyapatite composite scaffolds. Biomaterials 29, 1043–1053. doi: 10.1016/j.biomaterials.2007.11.005
Kim, Y., Lee, S. H., Kang, B.-J., Kim, W. H., Yun, H.-S., and Kweon, O.-K. (2016). Comparison of osteogenesis between adipose-derived mesenchymal stem cells and their sheets on Poly-ε-Caprolactone/β-tricalcium phosphate composite scaffolds in canine bone defects. Stem. Cells Int. 2016:8414715. doi: 10.1155/2016/8414715
Kim, Y. S., Majid, M., Melchiorri, A. J., and Mikos, A. G. (2019). Applications of decellularized extracellular matrix in bone and cartilage tissue engineering. Bioeng. Transl. Med. 4, 83–95. doi: 10.1002/btm2.10110
Kitaori, T., Ito, H., Schwarz, E. M., Tsutsumi, R., Yoshitomi, H., Oishi, S., et al. (2009). Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum 60, 813–823. doi: 10.1002/art.24330
Kushida, A., Yamato, M., Konno, C., Kikuchi, A., Sakurai, Y., and Okano, T. (2000). Temperature-responsive culture dishes allow nonenzymatic harvest of differentiated Madin-Darby canine kidney (MDCK) cell sheets. J. Biomed. Mater. Res. 51, 216–223. doi: 10.1002/(sici)1097-4636(200008)51:2<216::aid-jbm10>3.0.co;2-k
Kwon, S. G., Kwon, Y. W., Lee, T. W., Park, G. T., and Kim, J. H. (2018). Recent advances in stem cell therapeutics and tissue engineering strategies. Biomater. Res. 22:36. doi: 10.1186/s40824-018-0148-4
Lambert, L. J., Challa, A. K., Niu, A., Zhou, L., Tucholski, J., Johnson, M. S., et al. (2016). Increased trabecular bone and improved biomechanics in an osteocalcin-null rat model created by CRISPR/Cas9 technology. Dis. Model Mech. 9, 1169–1179. doi: 10.1242/dmm.025247
Le Blanc, K., Tammik, C., Rosendahl, K., Zetterberg, E., and Ringdén, O. (2003). HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp. Hematol. 31, 890–896. doi: 10.1016/s0301-472x(03)00110-3
Lerner, U. H., Kindstedt, E., and Lundberg, P. (2019). The critical interplay between bone resorbing and bone forming cells. J. Clin. Periodontol. 46(Suppl. 21), 33–51. doi: 10.1111/jcpe.13051
Levaot, N., Ottolenghi, A., Mann, M., Guterman-Ram, G., Kam, Z., and Geiger, B. (2015). Osteoclast fusion is initiated by a small subset of RANKL-stimulated monocyte progenitors, which can fuse to RANKL-unstimulated progenitors. Bone 79, 21–28. doi: 10.1016/j.bone.2015.05.021
Li, L., and Jiang, J. (2011). Regulatory factors of mesenchymal stem cell migration into injured tissues and their signal transduction mechanisms. Front. Med. 5:33–39. doi: 10.1007/s11684-011-0114-1
Liu, G., Li, Y., Sun, J., Zhou, H., Zhang, W., Cui, L., et al. (2010). In vitro and in vivo evaluation of osteogenesis of human umbilical cord blood-derived mesenchymal stem cells on partially demineralized bone matrix. Tissue Eng. Part A 16, 971–982. doi: 10.1089/ten.TEA.2009.0516
Liu, J., Yu, F., Sun, Y., Jiang, B., Zhang, W., Yang, J., et al. (2015). Concise reviews: characteristics and potential applications of human dental tissue-derived mesenchymal stem cells. Stem Cells 33, 627–638. doi: 10.1002/stem.1909
Liu, X., Wang, P., Chen, W., Weir, M. D., Bao, C., and Xu, H. H. K. (2014). Human embryonic stem cells and macroporous calcium phosphate construct for bone regeneration in cranial defects in rats. Acta Biomater 10, 4484–4493. doi: 10.1016/j.actbio.2014.06.027
Liu, Z., Chang, H., Hou, Y., Wang, Y., Zhou, Z., Wang, M., et al. (2018). Lentivirus-mediated microRNA-26a overexpression in bone mesenchymal stem cells facilitates bone regeneration in bone defects of calvaria in mice. Mol. Med. Rep. 18, 5317–5326. doi: 10.3892/mmr.2018.9596
Loi, F., Córdova, L. A., Pajarinen, J., Lin, T., Yao, Z., and Goodman, S. B. (2016). Inflammation, fracture and bone repair. Bone 86, 119–130. doi: 10.1016/j.bone.2016.02.020
Long, T., Zhu, Z., Awad, H. A., Schwarz, E. M., Hilton, M. J., and Dong, Y. (2014). The effect of mesenchymal stem cell sheets on structural allograft healing of critical sized femoral defects in mice. Biomaterials 35, 2752–2759. doi: 10.1016/j.biomaterials.2013.12.039
Lou, X. (2015). Induced pluripotent stem cells as a new strategy for osteogenesis and bone regeneration. Stem Cell Rev. 11, 645–651. doi: 10.1007/s12015-015-9594-8
Luzi, E., Marini, F., Tognarini, I., Galli, G., Falchetti, A., and Brandi, M. L. (2012). The regulatory network menin-microRNA 26a as a possible target for RNA-based therapy of bone diseases. Nucleic Acid Ther. 22, 103–108. doi: 10.1089/nat.2012.0344
Makridakis, M., Roubelakis, M. G., and Vlahou, A. (2013). Stem cells: insights into the secretome. Biochim. Biophys. Acta 1834, 2380–2384. doi: 10.1016/j.bbapap.2013.01.032
Malhotra, A., Pelletier, M. H., Yu, Y., and Walsh, W. R. (2013). Can platelet-rich plasma (PRP) improve bone healing? A comparison between the theory and experimental outcomes. Arch. Orthop. Trauma. Surg. 133, 153–165. doi: 10.1007/s00402-012-1641-1
Manfrini, M., Di Bona, C., Canella, A., Lucarelli, E., Pellati, A., D’Agostino, A., et al. (2013). Mesenchymal stem cells from patients to assay bone graft substitutes. J. Cell. Physiol. 228, 1229–1237. doi: 10.1002/jcp.24276
Manfrini, M., Mazzoni, E., Barbanti-Brodano, G., Nocini, P., D’agostino, A., Trombelli, L., et al. (2015). Osteoconductivity of complex biomaterials assayed by fluorescent-engineered osteoblast-like cells. Cell Biochem. Biophys. 71, 1509–1515. doi: 10.1007/s12013-014-0374-x
Marędziak, M., Marycz, K., Tomaszewski, K. A., Kornicka, K., and Henry, B. M. (2016). The influence of aging on the regenerative potential of human adipose derived mesenchymal stem cells. Stem Cells Int. 2016:2152435. doi: 10.1155/2016/2152435
Markmee, R., Aungsuchawan, S., Narakornsak, S., Tancharoen, W., Bumrungkit, K., Pangchaidee, N., et al. (2017). Differentiation of mesenchymal stem cells from human amniotic fluid to cardiomyocyte-like cells. Mol. Med. Rep. 16, 6068–6076. doi: 10.3892/mmr.2017.7333
Marques, C., Ferreira, J. M., Andronescu, E., Ficai, D., Sonmez, M., and Ficai, A. (2014). Multifunctional materials for bone cancer treatment. Int. J. Nanomed. 9, 2713–2725. doi: 10.2147/IJN.S55943
Mazzoni, E., D’Agostino, A., Manfrini, M., Maniero, S., Puozzo, A., Bassi, E., et al. (2017a). Human adipose stem cells induced to osteogenic differentiation by an innovative collagen/hydroxylapatite hybrid scaffold. FASEB J. 31, 4555–4565. doi: 10.1096/fj.201601384R
Mazzoni, E., Rotondo, J. C., Marracino, L., Selvatici, R., Bononi, I., Torreggiani, E., et al. (2017b). Detection of merkel cell polyomavirus dna in serum samples of healthy blood donors. Front. Oncol. 7:294. doi: 10.3389/fonc.2017.00294
Mazzoni, E., Martini, F., Corallini, A., Taronna, A., Barbanti-Brodano, G., Querzoli, P., et al. (2016). Serologic investigation of undifferentiated nasopharyngeal carcinoma and simian virus 40 infection. Head Neck 38, 232–236. doi: 10.1002/hed.23879
Mazzoni, E., D’Agostino, A., Iaquinta, M. R., Bononi, I., Trevisiol, L., Rotondo, J. C., et al. (2019). Hydroxylapatite-collagen hybrid scaffold induces human adipose-derived mesenchymal stem cells (hASCs) to osteogenic differentiation in vitro and bone re-growth in patients. Stem Cells Transl. Med. SCT3_12636. doi: 10.1002/SCT3.12636
McClung, M. R. (2018). Romosozumab for the treatment of osteoporosis. Osteoporos. Sarcopenia 4, 11–15. doi: 10.1016/j.afos.2018.03.002
Mennan, C., Wright, K., Bhattacharjee, A., Balain, B., Richardson, J., and Roberts, S. (2013). Isolation and characterisation of mesenchymal stem cells from different regions of the human umbilical cord. Biomed. Res. Int. 2013:916136. doi: 10.1155/2013/916136
Migliaccio, S., Francomano, D., Romagnoli, E., Marocco, C., Fornari, R., Resmini, G., et al. (2017). Persistence with denosumab therapy in women affected by osteoporosis with fragility fractures: a multicenter observational real practice study in Italy. J. Endocrinol. Invest. 40, 1321–1326. doi: 10.1007/s40618-017-0701-3
Miller, S. C., de Saint-Georges, L., Bowman, B. M., and Jee, W. S. (1989). Bone lining cells: structure and function. Scanning Microsc. 3, 953–960.
Mironov, V. (2003). Printing technology to produce living tissue. Expert Opin Biol Ther 3, 701–704. doi: 10.1517/14712598.3.5.701
Mohammed, E. E. A., El-Zawahry, M., Farrag, A. R. H., Aziz, N. N. A., Sharaf-ElDin, W., Abu-Shahba, N., et al. (2019). Osteogenic Differentiation potential of human bone marrow and amniotic fluid-derived mesenchymal stem cells in vitro & in vivo. Open Access. Maced. J. Med. Sci. 7, 507–515. doi: 10.3889/oamjms.2019.124
Motamedian, S. R., Hosseinpour, S., Ahsaie, M. G., and Khojasteh, A. (2015). Smart scaffolds in bone tissue engineering: a systematic review of literature. World J. Stem. Cells 7, 657–668. doi: 10.4252/wjsc.v7.i3.657
Murphy, C. M., Haugh, M. G., and O’Brien, F. J. (2010). The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 31, 461–466. doi: 10.1016/j.biomaterials.2009.09.063
Murphy, W. L., McDevitt, T. C., and Engler, A. J. (2014). Materials as stem cell regulators. Nat. Mater 13, 547–557. doi: 10.1038/nmat3937
Mushahary, D., Spittler, A., Kasper, C., Weber, V., and Charwat, V. (2018). Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry A 93, 19–31. doi: 10.1002/cyto.a.23242
Nakao, M., Inanaga, D., Nagase, K., and Kanazawa, H. (2019). Characteristic differences of cell sheets composed of mesenchymal stem cells with different tissue origins. Regen. Ther. 11, 34–40. doi: 10.1016/j.reth.2019.01.002
Nauth, A., Miclau, T., Li, R., and Schemitsch, E. H. (2010). Gene therapy for fracture healing. J. Orthop. Trauma. 24(Suppl. 1), S17–S24. doi: 10.1097/BOT.0b013e3181cec6fb
Oryan, A., Kamali, A., Moshiri, A., and Baghaban Eslaminejad, M. (2017). Role of mesenchymal stem cells in bone regenerative medicine: what is the evidence? Cells Tissues Organs 204, 59–83. doi: 10.1159/000469704
Paduano, F., Marrelli, M., Alom, N., Amer, M., White, L. J., Shakesheff, K. M., et al. (2017). Decellularized bone extracellular matrix and human dental pulp stem cells as a construct for bone regeneration. J. Biomater. Sci. Polym. Ed. 28, 730–748. doi: 10.1080/09205063.2017.1301770
Palmer, L. C., Newcomb, C. J., Kaltz, S. R., Spoerke, E. D., and Stupp, S. I. (2008). Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem. Rev. 108, 4754–4783. doi: 10.1021/cr8004422
Papadimitropoulos, A., Scotti, C., Bourgine, P., Scherberich, A., and Martin, I. (2015). Engineered decellularized matrices to instruct bone regeneration processes. Bone 70, 66–72. doi: 10.1016/j.bone.2014.09.007
Paterson, Y. Z., Rash, N., Garvican, E. R., Paillot, R., and Guest, D. J. (2014). Equine mesenchymal stromal cells and embryo-derived stem cells are immune privileged in vitro. Stem Cell Res. Ther. 5:90. doi: 10.1186/scrt479
Perez, J. R., Kouroupis, D., Li, D. J., Best, T. M., Kaplan, L., and Correa, D. (2018). Tissue engineering and cell-based therapies for fractures and bone defects. Front. Bioeng. Biotechnol. 6:105. doi: 10.3389/fbioe.2018.00105
Pisciotta, A., Carnevale, G., Meloni, S., Riccio, M., De Biasi, S., Gibellini, L., et al. (2015). Human dental pulp stem cells (hDPSCs): isolation, enrichment and comparative differentiation of two sub-populations. BMC Dev. Biol. 15:14. doi: 10.1186/s12861-015-0065-x
Pittenger, M. F., Mackay, A. M., Beck, S. C., Jaiswal, R. K., Douglas, R., Mosca, J. D., et al. (1999). Multilineage potential of adult human mesenchymal stem cells. Science 284, 143–147. doi: 10.1126/science.284.5411.143
Piva, R., Manferdini, C., Lambertini, E., Torreggiani, E., Penolazzi, L., Gambari, R., et al. (2011). Slug contributes to the regulation of CXCL12 expression in human osteoblasts. Exp. Cell Res. 317, 1159–1168. doi: 10.1016/j.yexcr.2010.12.011
Qin, Y., Guan, J., and Zhang, C. (2014). Mesenchymal stem cells: mechanisms and role in bone regeneration. Postgrad. Med. J. 90, 643–647. doi: 10.1136/postgradmedj-2013-132387
Qiu, G., Shi, Z., Xu, H. H. K., Yang, B., Weir, M. D., Li, G., et al. (2018). Bone regeneration in minipigs via calcium phosphate cement scaffold delivering autologous bone marrow mesenchymal stem cells and platelet-rich plasma. J. Tissue Eng. Regen. Med. 12, e937–e948. doi: 10.1002/term.2416
Raggatt, L. J., and Partridge, N. C. (2010). Cellular and molecular mechanisms of bone remodeling. J. Biol. Chem. 285, 25103–25108. doi: 10.1074/jbc.R109.041087
Rasch, A., Naujokat, H., Wang, F., Seekamp, A., Fuchs, S., and Klüter, T. (2019). Evaluation of bone allograft processing methods: impact on decellularization efficacy, biocompatibility and mesenchymal stem cell functionality. PLoS One 14:e0218404. doi: 10.1371/journal.pone.0218404
Rath, S. N., Nooeaid, P., Arkudas, A., Beier, J. P., Strobel, L. A., Brandl, A., et al. (2016). Adipose- and bone marrow-derived mesenchymal stem cells display different osteogenic differentiation patterns in 3D bioactive glass-based scaffolds. J. Tissue Eng. Regen. Med. 10, E497–E509. doi: 10.1002/term.1849
Raynaud, C. M., and Rafii, A. (2013). The necessity of a systematic approach for the use of MSCs in the clinical setting. Stem Cells Int. 2013:892340. doi: 10.1155/2013/892340
Ren, G., Zhao, X., Zhang, L., Zhang, J., L’Huillier, A., Ling, W., et al. (2010). Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J. Immunol. 184, 2321–2328. doi: 10.4049/jimmunol.0902023
Rosset, E. M., and Bradshaw, A. D. (2016). SPARC/osteonectin in mineralized tissue. Matrix Biol. 52–54, 78–87. doi: 10.1016/j.matbio.2016.02.001
Rossi, B., Merlo, B., Colleoni, S., Iacono, E., Tazzari, P. L., Ricci, F., et al. (2014). Isolation and in vitro characterization of bovine amniotic fluid derived stem cells at different trimesters of pregnancy. Stem Cell Rev. 10, 712–724. doi: 10.1007/s12015-014-9525-0
Rosso, F., Giordano, A., Barbarisi, M., and Barbarisi, A. (2004). From cell-ECM interactions to tissue engineering. J. Cell. Physiol. 199, 174–180. doi: 10.1002/jcp.10471
Rotondo, J. C., Bononi, I., Puozzo, A., Govoni, M., Foschi, V., Lanza, G., et al. (2017). Merkel cell carcinomas arising in autoimmune disease affected patients treated with biologic drugs. Including Anti-TNF. Clin. Cancer Res. 23, 3929–3934. doi: 10.1158/1078-0432.CCR-162899
Rotondo, J. C., Borghi, A., Selvatici, R., Magri, E., Bianchini, E., Montinari, E., et al. (2016). Hypermethylation-induced inactivation of the IRF6 gene as a possible early event in progression of vulvar squamous cell carcinoma associated with Lichen Sclerosus. JAMA Dermatol. 152, 928–933. doi: 10.1001/jamadermatol.2016.1336
Rotondo, J. C., Borghi, A., Selvatici, R., Mazzoni, E., Bononi, I., Corazza, M., et al. (2018). Association of retinoic acid receptor β gene with onset and progression of lichen sclerosus-associated Vulvar Squamous cell carcinoma. JAMA Dermatol. 154, 819–823. doi: 10.1001/jamadermatol.2018.1373
Sándor, G. K., Numminen, J., Wolff, J., Thesleff, T., Miettinen, A., Tuovinen, V. J., et al. (2014). Adipose stem cells used to reconstruct 13 cases with cranio-maxillofacial hard-tissue defects. Stem Cells Transl. Med. 3, 530–540. doi: 10.5966/sctm.2013
Santos, S. C. N. D. S., Sigurjonsson, ÓE., Custódio, C. A., and Mano, J. F. C. D. L. (2018). Blood plasma derivatives for tissue engineering and regenerative medicine therapies. Tissue Eng. Part B Rev. 24, 454–462. doi: 10.1089/ten.TEB.2018.0008
Sawkins, M. J., Bowen, W., Dhadda, P., Markides, H., Sidney, L. E., Taylor, A. J., et al. (2013). Hydrogels derived from demineralized and decellularized bone extracellular matrix. Acta Biomater. 9, 7865–7873. doi: 10.1016/j.actbio.2013.04.029
Schönherr, E., and Hausser, H. J. (2000). Extracellular matrix and cytokines: a functional unit. Dev. Immunol. 7, 89–101.
Schumacher, M., Henß, A., Rohnke, M., and Gelinsky, M. (2013). A novel and easy-to-prepare strontium(II) modified calcium phosphate bone cement with enhanced mechanical properties. Acta Biomater. 9, 7536–7544. doi: 10.1016/j.actbio.2013.03.014
Shen, C., Yang, C., Xu, S., and Zhao, H. (2019). Comparison of osteogenic differentiation capacity in mesenchymal stem cells derived from human amniotic membrane (AM), umbilical cord (UC), chorionic membrane (CM), and decidua (DC). Cell Biosci. 9:17. doi: 10.1186/s13578-019-02813
Sims, N. A., and Civitelli, R. (2014). Cell-cell signaling: broadening our view of the basic multicellular unit. Calcif. Tissue Int. 94, 2–3. doi: 10.1007/s00223-013-9766-y
Sims, N. A., and Martin, T. J. (2015). Coupling signals between the osteoclast and osteoblast: how are messages transmitted between these temporary visitors to the bone surface? Front. Endocrinol. 6:41. doi: 10.3389/fendo.2015.00041
Skardal, A., and Atala, A. (2015). Biomaterials for integration with 3-D bioprinting. Ann. Biomed. Eng. 43, 730–746. doi: 10.1007/s10439-014-1207-1
Sobacchi, C., Schulz, A., Coxon, F. P., Villa, A., and Helfrich, M. H. (2013). Osteopetrosis: genetics, treatment and new insights into osteoclast function. Nat. Rev. Endocrinol. 9, 522–536. doi: 10.1038/nrendo.2013.137
Su, P., Tian, Y., Yang, C., Ma, X., Wang, X., Pei, J., et al. (2018). Mesenchymal stem cell migration during bone formation and bone diseases therapy. Int. J. Mol. Sci. 19:E2343. doi: 10.3390/ijms19082343
Takahashi, K., and Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676. doi: 10.1016/j.cell.2006.07.024
Tang, M., Chen, W., Weir, M. D., Thein-Han, W., and Xu, H. H. K. (2012). Human embryonic stem cell encapsulation in alginate microbeads in macroporous calcium phosphate cement for bone tissue engineering. Acta Biomater. 8, 3436–3445. doi: 10.1016/j.actbio.2012.05.016
Tatullo, M., Marrelli, M., Shakesheff, K. M., and White, L. J. (2015). Dental pulp stem cells: function, isolation and applications in regenerative medicine. J. Tissue Eng. Rege.n Med. 9, 1205–1216. doi: 10.1002/term.1899
Thomson, J. A., Itskovitz-Eldor, J., Shapiro, S. S., Waknitz, M. A., Swiergiel, J. J., Marshall, V. S., et al. (1998). Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147.
Tognon, M., Luppi, M., Corallini, A., Taronna, A., Barozzi, P., Rotondo, J. C., et al. (2015). Immunologic evidence of a strong association between non-Hodgkin lymphoma and simian virus 40. Cancer 121, 2618–2626. doi: 10.1002/cncr.29404
Tolar, J., Teitelbaum, S. L., and Orchard, P. J. (2004). Osteopetrosis. N. Engl. J. Med. 351, 2839–2849. doi: 10.1056/NEJMra040952
Trávníčková, M., and Bačáková, L. (2018). Application of adult mesenchymal stem cells in bone and vascular tissue engineering. Physiol. Res. 67, 831–850.
Trohatou, O., and Roubelakis, M. G. (2017). Mesenchymal stem/stromal cells in regenerative medicine: past. Present, and future. Cell Reprogram. 19, 217–224. doi: 10.1089/cell.2016.0062
Turnbull, G., Clarke, J., Picard, F., Riches, P., Jia, L., Han, F., et al. (2018). 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 3, 278–314. doi: 10.1016/j.bioactmat.2017.10.001
Ueyama, Y., Yagyuu, T., Maeda, M., Imada, M., Akahane, M., Kawate, K., et al. (2016). Maxillofacial bone regeneration with osteogenic matrix cell sheets: an experimental study in rats. Arch. Oral Biol. 72, 138–145. doi: 10.1016/j.archoralbio.2016.08.017
Ullah, I., Subbarao, R. B., and Rho, G. J. (2015). Human mesenchymal stem cells - current trends and future prospective. Biosci. Rep. 35, e00191. doi: 10.1042/BSR20150025
van Bezooijen, R. L., Papapoulos, S. E., and Löwik, C. W. G. (2005). Bone morphogenetic proteins and their antagonists: the sclerostin paradigm. J. Endocrinol. Invest. 28, 15–17.
van Oers, R. F. M., Wang, H., and Bacabac, R. G. (2015). Osteocyte shape and mechanical loading. Curr. Osteoporos. Rep. 13, 61–66. doi: 10.1007/s11914-015-0256-1
Verboket, R., Leiblein, M., Seebach, C., Nau, C., Janko, M., Bellen, M., et al. (2018). Autologous cell-based therapy for treatment of large bone defects: from bench to bedside. Eur. J. Trauma. Emerg. Surg. 44, 649–665. doi: 10.1007/s00068-018-0906-y
Vico, L., van Rietbergen, B., Vilayphiou, N., Linossier, M.-T., Locrelle, H., Normand, M., et al. (2017). Cortical and trabecular bone microstructure did not recover at weight-bearing skeletal sites and progressively deteriorated at non-weight-bearing sites during the year following international space station missions. J. Bone Miner. Res. 32, 2010–2021. doi: 10.1002/jbmr.3188
Vishnubalaji, R., Al-Nbaheen, M., Kadalmani, B., Aldahmash, A., and Ramesh, T. (2012). Comparative investigation of the differentiation capability of bone-marrow- and adipose-derived mesenchymal stem cells by qualitative and quantitative analysis. Cell Tissue Res. 347, 419–427. doi: 10.1007/s00441-011-13063
Wan, M., Li, C., Zhen, G., Jiao, K., He, W., Jia, X., et al. (2012). Injury-activated transforming growth factor β controls mobilization of mesenchymal stem cells for tissue remodeling. Stem Cells 30, 2498–2511. doi: 10.1002/stem.1208
Wang, K.-X., Xu, L.-L., Rui, Y.-F., Huang, S., Lin, S.-E., Xiong, J.-H., et al. (2015). The effects of secretion factors from umbilical cord derived mesenchymal stem cells on osteogenic differentiation of mesenchymal stem cells. PLoS One 10:e0120593. doi: 10.1371/journal.pone.0120593
Wang, X., Wang, C., Gou, W., Xu, X., Wang, Y., Wang, A., et al. (2018). The optimal time to inject bone mesenchymal stem cells for fracture healing in a murine model. Stem Cell Res. Ther. 9:272. doi: 10.1186/s13287-018-10347
Ward, E., DeSantis, C., Robbins, A., Kohler, B., and Jemal, A. (2014). Childhood and adolescent cancer statistics, 2014. CA Cancer J. Clin. 64, 83–103. doi: 10.3322/caac.21219
Weatherholt, A. M., Fuchs, R. K., and Warden, S. J. (2012). Specialized connective tissue: bone, the structural framework of the upper extremity. J. Hand. Ther. 25, 123–131. doi: 10.1016/j.jht.2011.08.003
Xie, J., Peng, C., Zhao, Q., Wang, X., Yuan, H., Yang, L., et al. (2016). Osteogenic differentiation and bone regeneration of iPSC-MSCs supported by a biomimetic nanofibrous scaffold. Acta Biomater. 29, 365–379. doi: 10.1016/j.actbio.2015.10.007
Xiong, J., Piemontese, M., Onal, M., Campbell, J., Goellner, J. J., Dusevich, V., et al. (2015). Osteocytes, not osteoblasts or lining cells, are the main source of the rankl required for osteoclast formation in remodeling bone. PLoS One 10:e0138189. doi: 10.1371/journal.pone.0138189
Yagi, H., Soto-Gutierrez, A., Parekkadan, B., Kitagawa, Y., Tompkins, R. G., Kobayashi, N., et al. (2010). Mesenchymal stem cells: mechanisms of immunomodulation and homing. Cell Transplant. 19, 667–679. doi: 10.3727/096368910X508762
Zajdel, A., Kałucka, M., Kokoszka-Mikołaj, E., and Wilczok, A. (2017). Osteogenic differentiation of human mesenchymal stem cells from adipose tissue and Wharton’s jelly of the umbilical cord. Acta Biochim. Pol. 64, 365–369. doi: 10.18388/abp.2016_1488
Zare, H., Jamshidi, S., Dehghan, M. M., Saheli, M., and Piryaei, A. (2018). Bone marrow or adipose tissue mesenchymal stem cells: comparison of the therapeutic potentials in mice model of acute liver failure. J. Cell. Biochem. 119, 5834–5842. doi: 10.1002/jcb.26772
Zhang, K., Wang, S., Zhou, C., Cheng, L., Gao, X., Xie, X., et al. (2018). Advanced smart biomaterials and constructs for hard tissue engineering and regeneration. Bone Res. 6:31. doi: 10.1038/s41413-018-00329
Keywords: stem cell, regenerative medicine, differentiation, bone, repair
Citation: Iaquinta MR, Mazzoni E, Bononi I, Rotondo JC, Mazziotta C, Montesi M, Sprio S, Tampieri A, Tognon M and Martini F (2019) Adult Stem Cells for Bone Regeneration and Repair. Front. Cell Dev. Biol. 7:268. doi: 10.3389/fcell.2019.00268
Received: 16 July 2019; Accepted: 21 October 2019;
Published: 12 November 2019.
Edited by:
Ming Li, Osaka University, JapanReviewed by:
Shree Ram Singh, National Cancer Institute at Frederick, United StatesMarco Tatullo, Tecnologica S.r.l., Italy
Copyright © 2019 Iaquinta, Mazzoni, Bononi, Rotondo, Mazziotta, Montesi, Sprio, Tampieri, Tognon and Martini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mauro Tognon, dGdtQHVuaWZlLml0; Fernanda Martini, bXJmQHVuaWZlLml0
†These authors have contributed equally to this work
 Maria Rosa Iaquinta
Maria Rosa Iaquinta Elisa Mazzoni
Elisa Mazzoni Ilaria Bononi
Ilaria Bononi John Charles Rotondo
John Charles Rotondo Chiara Mazziotta
Chiara Mazziotta Monica Montesi
Monica Montesi Simone Sprio
Simone Sprio Anna Tampieri
Anna Tampieri Mauro Tognon
Mauro Tognon Fernanda Martini
Fernanda Martini