- Department of Pathology and Immunology, Faculty of Medicine, CMU, University of Geneva, Geneva, Switzerland
Rare hematopoietic stem cells (HSCs) can self-renew, establish the entire blood system and represent the basis of regenerative medicine applied to hematological disorders. Clinical use of HSCs is however limited by their inefficient expansion ex vivo, creating a need to further understand HSC expansion in vivo. After embryonic HSCs are born from the hemogenic endothelium, they migrate to the embryonic/fetal niche, where the future adult HSC pool is established by considerable expansion. This takes place at different anatomical sites and is controlled by numerous signals. HSCs then migrate to their adult niche, where they are maintained throughout adulthood. Exactly how HSC expansion is controlled during embryogenesis remains to be characterized and is an important step to improve the therapeutic use of HSCs. We will review the current knowledge of HSC expansion in the different fetal niches across several model organisms and highlight possible clinical applications.
Introduction
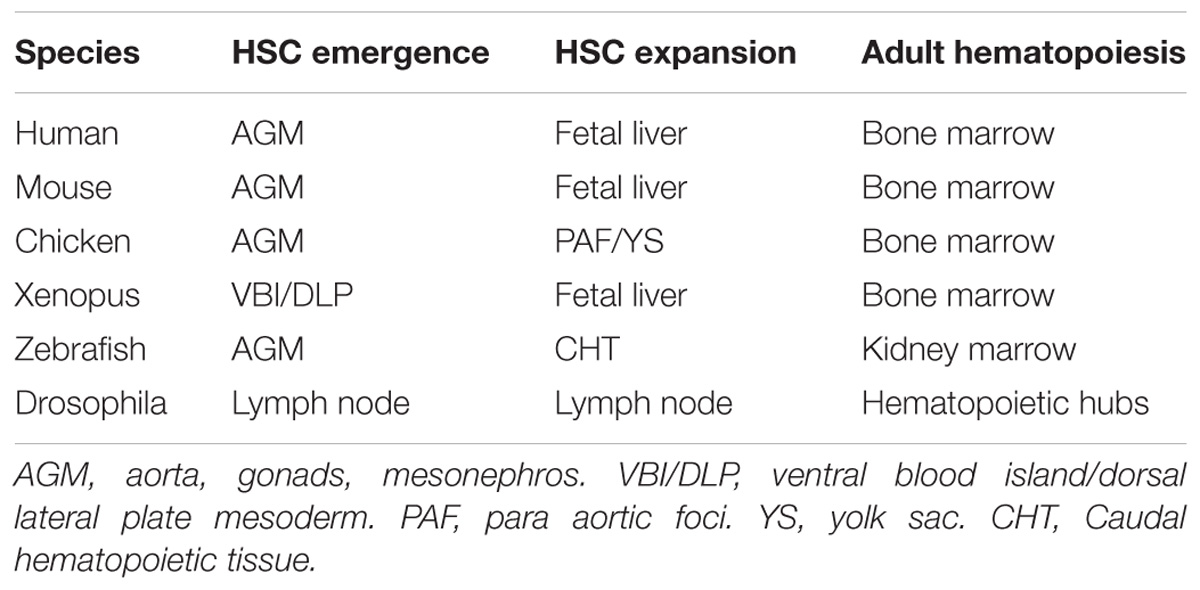
Hematopoiesis is a highly conserved process across many organisms that culminates with the emergence of hematopoietic stem cells (HSCs). In zebrafish and mammals, hematopoiesis initiates with the emergence of primitive myeloid and erythroid cells (Palis et al., 2001; Bertrand et al., 2005; Palis, 2014; McGrath et al., 2015). Similar cells, prohemocytes, are also detected in drosophila larvae that give rise to plasmatocytes (macrophage-like cells) and crystal cells (platelet-like cells) (Lebestky et al., 2000). Primitive myeloid and erythroid cells are also detected in xenopus embryos (Ciau-Uitz et al., 2014). Following this, definitive hematopoiesis then occurs in two distinct waves in vertebrates. The first wave is characterized by the transient erythro-myeloid precursors (EMPs) that arise in the yolk sac in mice and humans (Bertrand et al., 2005; McGrath et al., 2015), the posterior blood island in zebrafish (Bertrand et al., 2007) and the posterior-lateral ventral blood island in xenopus (Ciau-Uitz et al., 2014). The appearance of EMPs in chicken embryos remains to be determined. The second wave consists of HSC specification from the aortic hemogenic endothelium by the highly conserved process of endothelial-to-hematopoietic transition (EHT). The formation of the hemogenic endothelium requires the correct balance of extrinsic and intrinsic factors to initiate the expression of specific transcription factors, such as runx1 and gata2. During mammalian and avian development, HSC specification occurs in the aorta-gonads-mesonephros (AGM) region where they form intra-aortic clusters (Jaffredo et al., 2000; Bollerot et al., 2005a,b; Zovein et al., 2008; Chen et al., 2009; Boisset et al., 2010) between embryonic (E) day 9.5 and 11.5 in mice, between E26 and E40 in humans and between E3-4 in chickens. During zebrafish development, HSCs emerge between 32 to 60 hours post fertilization (hpf), from the hemogenic endothelium in the dorsal aortal (Bertrand et al., 2010; Kissa and Herbomel, 2010), a process that requires inflammatory cytokines produced by neutrophils (Espin-Palazon et al., 2014) and extracellular matrix (ECM) degradation by macrophages to allow HSCs to enter circulation (Travnickova et al., 2015). HSCs are first detected in the ventral blood island and then later in the dorsal lateral plate mesoderm in xenopus (Ciau-Uitz et al., 2000) and transient cells with HSC characteristics are closely associated to the cardiac tube in drosophila (Dey et al., 2016).
In all these organisms, the initial specification from endothelial cells (ECs) results in a limited number of HSCs that must mature and expand. This is achieved by migrating through different niches, each in distinct anatomical locations that contain specific microenvironments. The first niche that expands HSCs in mouse, humans and xenopus is the fetal liver (FL) (Ema and Nakauchi, 2000; Ciau-Uitz et al., 2014) before they migrate to the bone marrow (BM). In contrast, zebrafish HSCs expand in the caudal hematopoietic tissue (CHT) (Tamplin et al., 2015) and then migrate to the kidney marrow (KM) and chicken HSCs expand in the para-aortic foci (PAF) before seeding the BM (Dunon and Imhof, 2000; Jaffredo et al., 2000; Bollerot et al., 2005a,b). This process is different in drosophila where an initial wave of HSCs (derived from head mesoderm) arises early during larvae development, followed by a second wave of HSCs found in the lymph gland (Lebestky et al., 2000; Dey et al., 2016). HSCs are then seeded in hematopoietic clusters in the dorsal abdomen of adult drosophila (Ghosh et al., 2015; Dey et al., 2016).
Mouse and zebrafish studies have shown that HSCs physically interact with ECs that promote their proliferation in the fetal niche (Tamplin et al., 2015; Khan et al., 2016). We, and others, have shown that this expansion depends on the expression of several cytokines produced by stromal cells and caudal ECs (cECs) (Tamplin et al., 2015; Mahony et al., 2016, 2018). Additional signals are also important for their expansion, which will be discussed in this review. This embryonic expansion is an essential step in the formation of the adult HSC pool and the correct maturation of HSCs.
The self-renewing and multipotent properties of HSCs make these cells an excellent target for regenerative medicine protocols (Cavazzana-Calvo et al., 2000; Walasek et al., 2012; Aiuti et al., 2013). Many therapies currently make use of ex vivo expansion of autologous human HSCs, using a cytokine cocktail, but always with limited efficiency (Petzer et al., 1996; Cavazzana-Calvo et al., 2000; Schuster et al., 2012). Therefore a better understanding of the different combinations of cytokines present in the niche along with the additional mechanisms and signaling pathways that normally expand HSCs is required to improve clinical treatment of a range of hematopoietic diseases. Here, we review the recent literature that describes the extrinsic signals important for HSC homing, expansion and finally release from the embryonic niche across zebrafish, xenopus, chicken, and mammals. The anatomical sites where hematopoiesis occurs in these organisms are summarized in Table 1. We will then briefly discuss the possible clinical implications of this current knowledge.
HSC Emergence and Homing to the Embryonic Niche
Mammals
Mammalian HSCs are produced from the floor of the embryonic aorta in the AGM region (Zovein et al., 2008; Chen et al., 2009; Ivanovs et al., 2011) but also in the vitelline and umbilical arteries (de Bruijn et al., 2000). Additionally, human and murine studies have detected HSCs in the placenta that arise independently from and in parallel with the HSCs from the AGM (Gekas et al., 2005; Ottersbach and Dzierzak, 2005; Rhodes et al., 2008; Gekas et al., 2010). However it remains unknown what contribution placenta-derived HSCs make to the adult stem cell pool. HSCs then colonize the FL from E11 in mice and E28 in humans, mainly in response to CXCL12 (Chou and Lodish, 2010). CXCL12, released from ECs, stromal cells and mesenchymal progenitors, is well characterized for its role in HSC homing, retention and survival in the niche (Ara et al., 2003; Christensen et al., 2004; Sugiyama et al., 2006; Sawitza et al., 2009; Greenbaum et al., 2013). CXCL12 enhances migration of FL-HSCs in combination with stem cell factor (SCF), when compared to BM-HSCs (Christensen et al., 2004). Furthermore, mice lacking CXCL12 or its receptor (CXCR4) display normal FL hematopoiesis but aberrant spleen and BM colonization, suggesting that specific and distinct signaling environments attract and maintain HSCs (Nagasawa et al., 1996; Ara et al., 2003). In the FL, HSCs are found closely associated with ECs and stromal cells that promote HSC expansion (Tamplin et al., 2015; Khan et al., 2016).
In addition to cytokine secretion, a direct contact between the different cells within the FL and hematopoietic progenitors is also important to maintain and expand HSCs (Nanno et al., 1994; Corlu et al., 1998). HSCs express a number of integrins and adhesion receptors that are critical for the correct trafficking of HSCs to the FL and could mediate cell contact. For example, VE-Cadherin (CD144), α2b-integrin (CD41), β1-integrin (CD29), cKIT and CXCR4 are well established trafficking molecules expressed by HSCs and play a key role in HSC guidance to the fetal niche (Mazo et al., 2011).
Further studies have demonstrated that umbilical HSCs have a higher affinity to adhere to adult BM than embryonic HSCs, which is due to a specific shift in the expression of specific integrins by HSCs. This suggests that integrin expression is required during development to mediate homing to specific niches (Roy and Verfaillie, 1999). Integrins (mainly α4-integrin) are implicated in mediating HSC interaction with the vascular niche in the BM (Mazo et al., 1998), and their inhibition mobilizes HSCs from the FL (Kim et al., 2016). Human endothelium-derived HSCs also express and use a myeloid adhesion factor, glycosylphosphatidylinositol-anchored surface protein (GPI-80; also known as Vanin-2, or VNN2) (Prashad et al., 2015), to facilitate their migration and expansion in the fetal niche.
Several ECM, cell adhesion and cytoskeleton pathways are enriched in the AFT024 murine FL fibroblast-derived stromal cell line [a cell line that supports HSC expansion in vitro (Nolta et al., 2002)] and within HSCs, permitting HSC migration and anchoring to their niches (Charbord and Moore, 2005).
Chicken
Definitive chicken hematopoiesis is initiated at E3-4 by the emergence of intra-aortic clusters of HSCs derived from endothelium (as seen in mammals) (Jaffredo et al., 2000; Bollerot et al., 2005a,b; Yvernogeau and Robin, 2017). HSCs then migrate to the neighboring mesenchyme, ventral to the aorta and located in the PAFs, that support the development of CD45+ cells (Cormier, 1993; Geerts et al., 1993), such as myeloerythroid progenitor cells and immature thymic precursors (that have not yet undergone T-cell receptor rearrangements) (Lampisuo et al., 1999; Jaffredo et al., 2000; Liippo et al., 2000; Saynajakangas et al., 2009). An additional site of embryonic hematopoiesis includes the yolk sac, which also contributes to the expansion and maturation of erythroid and myeloid cells (Guedes et al., 2014). However, the homing signals to the chicken PAFs remain unidentified. Although little is known about the microenvironment that would support HSCs in the chicken PAFs, differential expression of integrins may play an important role in supporting HSCs (Corbel, 2002).
Xenopus
Fate-mapping and grafting experiments showed that bona fide HSCs are generated in the dorsal lateral plate (DLP), the equivalent of the mammalian AGM (Turpen et al., 1981; Maeno et al., 1985; Ciau-Uitz et al., 2000; Clements and Traver, 2013). In larval stages, DLP-derived HSCs reach maturity and seed the FL where they produce erythrocytes that will replace embryonic primitive erythrocytes. The FL is the main site of HSC expansion and differentiation during embryogenesis, i.e., before metamorphosis (Chen and Turpen, 1995). Classical studies made use of kidney and liver sections from bullfrog tadpoles to reveal hematopoietic microenvironments, supporting red blood cell development (Broyles et al., 1981). After metamorphosis, the majority of the blood cells are DLP-derived (Ciau-Uitz et al., 2014).
Zebrafish
During zebrafish development, gata2b (the earliest hemogenic endothelium marker) is expressed in ECs in the floor of the dorsal aorta (Butko et al., 2015). HSCs are then specified through the expression of runx1 and cmyb, and can be observed undergoing EHT from the floor of the dorsal aorta between 32–60 hpf (Bertrand et al., 2010; Kissa and Herbomel, 2010; Mahony et al., 2018). Contrary to mammals, zebrafish HSCs then migrate toward the vein, where they enter circulation to migrate to the CHT (Bertrand et al., 2010). Within the CHT they considerably expand between 3 and 4 days post fertilization (dpf) (Murayama et al., 2006; Tamplin et al., 2015; Mahony et al., 2016; Staal et al., 2016). HSCs migrate to the CHT embryonic niche in response to cxcl12a, expressed from stromal cells (Tamplin et al., 2015). Further zebrafish studies have identified klf6a as an important transcription factor that directly regulates ccl25b expression in ECs (Xue et al., 2015, 2017). Xue et al. (2017) demonstrated that ccl25b is expressed in the CHT at 48hpf and is an important cytokine for HSC chemoattraction to and expansion within the CHT niche. These results were further corroborated by the ex vivo culture of murine HSCs in the presence of Ccl21 (murine ortholog of ccl25b) that enhanced HSC expansion through activation of its receptor, Ccr7 (Xue et al., 2017). Upon arrival in the CHT niche, VCAM+ macrophages are also required to direct HSCs (through binding to α4-integrin expressed by HSCs) toward venous capillaries and retain them in their embryonic niche (Li et al., 2018).
Non-Cell-Autonomous Mediators of Hsc Expansion in the Embryonic Niche
The HSC pool first undergoes expansion shortly after HSC emergence from the AGM (Taoudi et al., 2008; Rybtsov et al., 2016), before migrating to their fetal niche. The number of HSCs then greatly expands to around 38 times their original number, peaking at around E14 in mice and ceasing around 2–4 days postnatal (Morrison et al., 1995; Ema and Nakauchi, 2000; Baumann et al., 2004; Lessard et al., 2004; Chen et al., 2009; Payushina, 2012). Therefore, fully characterizing the different cells and environmental cues that expand HSCs in different organisms is required to improve the currently limited regenerative therapies. We will hereafter describe the different elements of the microenvironment that contribute to this expansion, across the vertebrate phylum.
Stromal Cells
In the mouse embryo, HSCs are closely associated with Nestin+ periportal stromal cells that express many HSC expansion factors, such as angptl2 (Khan et al., 2016). Many different supportive stromal cell lines also have been derived from the mouse FL, such as AFT024, that support HSCs in vitro (Nolta et al., 2002), and the KM3 cell line, that supports human embryonic stem cells (Hu et al., 2012). The analysis of the AFT024 cell line revealed an enrichment in secreted factors such as insulin like growth factor, SCF, angiopoietin-3, Wnts and Ephrin2a that support HSCs (Charbord and Moore, 2005).
A subtype of stromal cells, stellate cells, are fat storing hepatic sinusoid cells that appear around E10-11 in the mouse embryo and express a number of cytokines, ECM and adhesion molecules (Ramadori and Saile, 2002; Tan et al., 2017). Stellate cells are Desmin-positive and are found in close proximity to HSCs (Kiassov et al., 1995). These cells express many different supportive hematopoietic cytokines such as OSM, Csf1, THPO, EPO, Igf1, SCF/KitL and Cxcl12 (Fujio et al., 1994; Kubota et al., 2007; Tan et al., 2017). Stellate cells also express VCAM, fibronectin1, vitronectin1, Lamb1-1 (Laminin-b1-1) and Lamc1 (Laminin-c1) (Kubota et al., 2007; Tan et al., 2017). In vitro, adult hepatic stellate cells can maintain HSCs in a similar manner to BM mesenchymal stem cells (Kordes et al., 2013). Stellate cells may therefore play an important role in maintaining the liver microenvironment and expanding the HSC pool.
Recent studies have also focused on zebrafish stromal cells, derived from the somites (Murayama et al., 2015). Isolated CHT zebrafish stromal cells [caudal hematopoietic embryonic stromal tissue (CHEST)] from 3dpf embryos express a range of hematopoietic cytokines, some of which are not present in isolated kidney cells from adult fish (such as gcsfb, il11a, il11b, and fgf21). Furthermore CHEST cells were able to expand cultured HSCs and stimulate HSC differentiation in vitro (Wolf et al., 2017). The development of these zebrafish stromal cell lines is an important step and represents a valuable tool to study hematopoiesis in this model. Indeed, by comparing the transcriptome of CHEST, ZKS (zebrafish kidney stromal cells) and ZEST (zebrafish embryonic stromal trunk cells), Berrun and colleagues highlighted the hematopoietic role of isthmin 1 (ism1), a secreted protein required for HSC development as well as erythro-myeloid differentiation (Berrun et al., 2018).
In addition to hematopoietic cytokines, lipid metabolism is also important for HSC expansion and development during embryogenesis. This was recently demonstrated through the study of lipoprotein lipase (lpl), an enzyme expressed by stromal and/or ECs in the CHT, and required for fatty acid metabolism. Both lpl and its cofactor apolipoprotein c2 (apoc2) controlled the release of an essential fatty acid, (Docosahexaenoic acid), which was identified as a novel HSC expansion factor (Liu et al., 2018). This study highlighted an additional possible pathway important for improving HSC expansion ex vivo.
The anterior lobe of the drosophila lymph gland consists of a medullary zone (MZ), a cortical zone (CZ) and the posterior signaling center (PSC). Prohemocytes are located in the MZ and give rise to mature hemocytes, plasmatocytes, crystal cells and rare lamellocytes, in response to immune challenge (Evans et al., 2003). These progeny cells will then colonize the CZ. The drosophila PSC is an important signaling niche that controls blood cell production and maturation and has been associated to mammalian stromal cells (Lebestky et al., 2003). The PSC is a source of Hedgehog signal that activates the zinc finger transcription factor Cubitus interruptus (Ci) (homolog of the vertebrate Gli proteins) in cells located in the MZ to maintain quiescence of blood progenitors and fine tune differentiation upon immune challenge, independently of the EBF transcription factor Col within blood progenitors (Mandal et al., 2007; Benmimoun et al., 2015a,b; Pennetier et al., 2012). Further studies have suggested that the PSC may interact with nearby cells directly though thin processes that extend into the MZ (Mandal et al., 2007). Additional signals that affect PSC signaling and HSC maintenance include odorants that stimulate γ-aminobutyric acid release from the brain and drosophila insulin-like growth factor 2 by adipocytes (Yu et al., 2018).
Endothelial Cells
HSCs arrive in the zebrafish CHT niche and progressively colonize this tissue from 48 hpf until 80 hpf (Tamplin et al., 2015). Although some HSCs are still present at 96 hpf, the majority have proliferated and left the CHT niche (Mahony et al., 2016). To accommodate HSCs within the CHT, the vascular niche is remodeled to improve stem cell seeding, a process that is controlled by cxcr1 (Blaser et al., 2017). Upon arrival to the CHT niche, HSCs trigger a “cuddling” behavior from caudal endothelial cells (cECs). This cuddling allows the cECs to maintain close proximity between an HSC and a stromal cell, which induces HSC proliferation (Tamplin et al., 2015). The cuddled HSC then undergoes cell division where either both daughter cells will leave the niche, one will leave and the one most proximal to the stromal cell will stay or both will stay. Strikingly, pharmacological stimulation of HSC niche engraftment leads to an overall increase in the number of adult stem cells, as shown by lineage tracing (Tamplin et al., 2015). This indicates the contribution of HSC embryonic niche engraftment to increasing the adult stem cell pool size, and highlights the importance of fully understanding the cytokines expressed by ECs that expand HSCs.
Direct physical contacts between HSCs and ECs have been described in the mouse embryo, similarly to the zebrafish CHT (Tamplin et al., 2015), and may be mediated by E-selectin and VCAM1 (Schweitzer et al., 1996; Wittig et al., 2010). Murine FL-HSCs express the endothelial protein C receptor that can bind the activated protein C. This induces protease-activated receptor 1 signaling which inhibits apoptosis and maintains self-renewal activity (Iwasaki et al., 2010). In contrast to murine HSCs, human HSCs express endothelial protein C receptor in the FL, but lose its expression once they have migrated to the BM (Subramaniam et al., 2018).
Fetal liver ECs also support hematopoiesis ex vivo and promote HSC differentiation (Ohneda and Bautch, 1997; Wittig et al., 2010). FL ECs support hematopoiesis through the expression SCF/KitL that is normally membrane-bound. The conversion of this cytokine to its soluble form occurs under the control of MMP9, which transcription is regulated by Ezh2, a transcriptional repressor expressed by FL ECs. This process was only described in the context of erythropoiesis (Neo et al., 2018) but might be relevant for HSC expansion.
These studies underscore the importance of ECs in expanding HSCs in the zebrafish CHT and the FL in mammals. However, many other cell types support HSC expansion during embryogenesis.
HSC Regulation by Their Hematopoietic Progeny
Regulation of HSC expansion by their progeny has been described in the drosophila lymph gland. PDGF and VEGF-related factor- 1 (Pvf1) is produced by the PSC and activates its receptor (PVR) on differentiated cells in the cortical zone. This activates JAK/STAT signaling and consequently controls the expression and secretion of adenosine deaminase Growth Factor-A (Adgf-A) from differentiated cells. This enzyme converts adenosine into inosine that promotes progenitor maintenance, through the PKA intracellular pathway (Mondal et al., 2011). Similarly in mammals, the placental microenvironment supports hematopoiesis through PDGF-B expression to overall inhibit HSC differentiation (Chhabra et al., 2012). Drosophila bip1 (bric-à-brac interacting protein 1) and Nucleoporin 98 (Nup98) are additional genes identified in controlling blood progenitor proliferation that control PVR expression (Mondal et al., 2014). The negative feedback of differentiated cells into HSCs has been evidenced in the adult mouse BM, but remains undocumented in the FL.
Mammalian Hepatocytes
Hepatocytes contribute to most of the liver mass and therefore also play a role in the HSC niche. The analysis of human FL samples has revealed the presence of E-selectin positive bipotent FL hepatoblasts, capable of differentiating into hepatic or biliary epithelial cells (Terrace et al., 2007). Murine hepatoblasts have been characterized by their expression of Protein delta homolog 1 (DLK-1) and a range of important hematopoietic cytokines, such as Epo, Thpo, Il6, SCF, and Flt3l. Hepatoblasts also express high levels of ECM molecules, including vitronectin, fibronectin and tenascin C that are expressed under the control of TBGβ1 signaling (Sugiyama et al., 2013). The importance of hepatocytes in expanding HSCs was demonstrated by the co-culture of hepatocyte cell lines with FL cells, which resulted in a large increase in the number of HSCs (Aiuti et al., 1998). Their importance was further underscored by the analysis of Map2k4-mutant mouse embryos, lacking hepatoblasts, where hematopoiesis was strongly affected following a decrease in cytokines expression in the FL, such as EPO and SCF. Accordingly, these embryos displayed a strong decrease in the number of HSCs (Sugiyama et al., 2011).
Another study showed that DLK1-positive hepatocyte progenitors (or hepatoblasts) were the main contributor in the FL niche, as they were the main source of SCF, IGF-2, Cxcl12 as well as angptl2 and angptl3. Initially, these cells were identified as T cells as they were stained with the anti-CD3 antibody (Chou and Lodish, 2010). Ex vivo co-culture of these FL hepatoblasts with BM-HSCs leads to enhanced HSC long-term repopulation and ex vivo expansion of HSCs (Zhang and Lodish, 2004; Zhang et al., 2006). It was further suggested that Angptl2 could mediate its effects through the LILRB2 receptor (Deng et al., 2014).
Hypoxia and ROS
Hypoxia is an important maturation signal in the microenvironment. In drosophila, low oxygen levels are sensed by Sima, the ortholog of hypoxia-inducible factor 1-α (Hif1-α) that induces crystal cell differentiation. Moderate ROS levels are required for proliferation of early progenitors. Fine-tuning levels of ROS is also required to balance progenitor differentiation in the MZ by regulating E-cadherin levels (Gao et al., 2014). Although ROS levels and hypoxia are well-described cues relevant to the adult BM niche, very little is known about their role during embryonic HSC expansion in any other models.
Transcription Factors Controlling the HSC Niche
Understanding the genetic network that controls the specification and maintenance of the niche will allow building or reproducing these niches in vitro. Transcription factors control many aspects of the niche, from how the niche attracts HSCs to their nurturing and release. As mentioned earlier, klf6a is a transcription factor that controls the expression of ccl25b in cECs from the zebrafish CHT. Therefore, in the absence of klf6a, HSCs cannot colonize the CHT and do not expand (Xue et al., 2017).
In the zebrafish as well, we have shown the role of tfec, a mitf family zinc finger transcription factor (Lister et al., 2011), in HSC expansion. Tfec is highly expressed in cECs of the CHT and controls the expression of several CHT niche cytokines (such as kitlgb, thpo, csf1a, and csf3b) (Mahony et al., 2016). These cytokines are known to promote HSC proliferation and survival, resulting in their expansion. Consequently, in tfec mutants, HSCs fail to mature, resulting in severe anemia (Mahony et al., 2016). However, this phenotype can be rescued by the overexpression of kitlgb (Mahony et al., 2016). In addition, tfec also controls the expression of oncostatin M (osm) from cECs that can synergistically enhance the expansion of HSCs with kitlgb (Mahony et al., 2018). Tfec is therefore a critical transcription factor required for the proper role of the embryonic hematopoietic niche. This role of tfec has probably been conserved in mammals. Indeed, in rats, the comparison of liver sinusoidal ECs to lung microvascular ECs revealed a specific combination of transcription factors that controlled liver ECs function and identity (Geraud et al., 2010). Tfec was identified as a liver specific transcription factor, together with Gata4, Lmo3, and Maf. Whereas the roles of Tfec, Lmo3, and Maf have not yet been investigated during mouse fetal hematopoiesis, Gata4 seems to be required for ECs to function as an HSC niche (Geraud et al., 2017). Further studies demonstrated that Gata4 is required for correct EC maturation in the embryonic liver and correct HSPC colonization, as Gata4 allows the specification of a discontinuous endothelium that allows HSC colonization. Therefore, when Gata4 was specifically deleted in liver sinusoidal ECs during development, the FL vasculature failed to develop normally and hematopoiesis was not supported by the FL (Geraud et al., 2017). The deletion of another transcription factor, activating transcription factor 4 (ATF4) impaired the ability of both endothelial and stromal cells to support FL HSC development. Further analysis revealed that Atf4 directly regulates the expression of Angptl3 to control the repopulating efficiency of HSCs (Zhao et al., 2015). Of note, Atf4 is also required at the cell-autonomous level for proper self-renewal of FL HSCs (Rieger, 2015).
HSC Release From the Embryonic Niche
The cellular processes governing mammalian HSC release from the embryonic niche remain to be fully characterized. However, a recent study has revealed that the FL-to-BM transition required correct formation of the Wiskott–Aldrich syndrome verprolin-homologous (WAVE) protein complex 2, mediated by Hem-1 (Shao et al., 2018). The disruption of this complex leads to a loss of the survival signal c-Abl in FL-HSCs, premature death and reduced BM colonization, highlighting a cell-intrinsic pathway that is required for FL-to-BM transition.
The HSC migration from FL to the BM is mediated by different cytokines, ECM signals and adhesion molecules (e.g., CXCL12, SCF, cadherins, integrins) (Ciriza et al., 2013). HSCs are then maintained in a quiescent state in BM throughout adulthood by many different signals and cells (Boulais and Frenette, 2015).
The mechanisms driving HSC release from the embryonic niche are similarly elusive in zebrafish. However, live imaging has revealed significant changes in the vascular architecture that normally accommodates HSCs. At 4 dpf, the number of HSCs present in the CHT decreases and they are no longer embedded in the vascular network, but they are instead found loosely associated to vascular cells (Tamplin et al., 2015; Mahony et al., 2016). Further studies have indicated that mmp9 expression in macrophages was required to modulate the accessibility of HSCs to cxcl12. Indeed, the inhibition of mmp9 resulted in the accumulation of HSCs in the CHT, which was rescued in cxcl12a-morphants (Theodore et al., 2017). Therefore the modulation of the vascular structure and the ECM by mmp9 is an important feature to control retention and release of HSCs from the embryonic niche.
Evolutionary Conserved Elements
Hematopoietic stem cell emergence and derivation from ECs is a highly conserved process across chick, zebrafish, mouse and humans (Jaffredo et al., 2000; Bollerot et al., 2005a,b; Zovein et al., 2008; Chen et al., 2009; Bertrand et al., 2010; Boisset et al., 2010). Even drosophila blood cells are found in close association to the cardiac tube (Dey et al., 2016). Furthermore, the cell autonomous expression of transcription factors (such as runx1 and gata2) required to achieve EHT is highly conserved. This knowledge gained from the study of different animal models has made it possible to generate inducible HSCs from ECs in vitro (Gomes et al., 2018).
Across the species discussed in this review there are several conserved processes but also many differences in how HSCs expand in their fetal niche. Understanding the main similarities and differences is crucial to fully understanding HSC expansion. Here we will summarize the main conserved elements across drosophila, zebrafish, mouse and human and how these have progressed throughout evolution.
The drosophila PSC consists of stromal cells in the lymph gland, and controls blood cell production and maturation (Lebestky et al., 2003). These cells are responsible for the signaling of a number of extrinsic factors to control HSC expansion (Figure 1 and Table 2). Throughout evolution, the structure of the embryonic niche has greatly changed. The vertebrate phylum coincides with the emergence of a closed circulatory system, dependent on vasculature. In the zebrafish, the role of vasculature becomes very important for HSC expansion (Tamplin et al., 2015) and remains so in higher vertebrate species (Table 2). In avian embryos, HSCs expand in the yolk sac, a highly vascularized structure (Guedes et al., 2014), and mouse HSCs are also closely associated to the growing vasculature in the FL that support HSCs (Ohneda and Bautch, 1997; Wittig et al., 2010; Tamplin et al., 2015) (Figure 1 and Table 2).

Figure 1. Summary of vertebrate HSC expansion in the embryonic niche. Following their derivation from aortic endothelium, HSCs home to their embryonic niche in response to several attractive cytokines, such as cxcl12 and ccl25b/Ccl21. HSCs are directed toward vascular cells by Mφ (macrophages). The vascular cells in the embryonic niche then remodel to accommodate the arriving HSCs. HSCs then become lodged in the fetal niche and undergo cell division to expand their initial number. This expansion is in response to several cytokines released from many different cell types. Endothelial cells release kitlg and osm, under the control of tfec. Stromal cells release cxcl12, under the control of atf4. Hepatoblasts release Kit-ligand and angiopoietins. After considerable expansion, the ECM is remodeled by Mmp9 released from neutrophils and HSCs leave their fetal niche to migrate to their adult niche. Outlined here is an overview of the main cell types involved in fetal HSC expansion along with some examples of important cytokines/signals that they secrete, although many others exist.

Table 2. Summary of the important cells and tissues required to mediate HSC expansion through evolution.
From zebrafish to mammals, HSCs expand in an increasingly more complex niche: whereas the CHT in teleosts is a transient vascularized tissue (Tamplin et al., 2015), HSCs will colonize the FL in mammals, a bona fide organ. With this, comes the addition of a new cellular layer to the HSC niche, i.e., the hepatocytes. As stromal and ECs, hepatoblasts will secrete similar survival and proliferative signals, such as SCF and other cytokines (Sugiyama et al., 2011, 2013) (Figure 1 and Table 2). It is interesting to note that even if the structures have changed, the genetic network seems to have been conserved. One such example is the transcription factor Tfec that is specifically expressed in the zebrafish CHT vasculature, as well as in sinusoidal ECs of the fetal and adult liver in rodents (Geraud et al., 2010; Mahony et al., 2016).
Clinical Implications
The recent use of drug screens has identified several promising candidates to improve the clinical use of HSCs in regenerative medicine. For example, StemRegenin-1 (SR1), an antagonist of the aryl hydrocarbon receptor (AHR) enhances the ex vivo expansion of CD34+ cells and improves long-term engraftment in murine models (Boitano et al., 2010). Recently, early phase clinical trials have further highlighted the effectiveness of this compound in improving the expansion of CD34+ cells from umbilical cord blood (Wagner et al., 2016). UM171, another compound, improves human cord blood cell expansion and engraftment, although the exact mechanism remains to be fully characterized (Fares et al., 2014). Zebrafish high throughput screens have also highlighted the role of dmPGE2 that confers a competitive advantage to treated HSCs and has been successful in subsequent clinical trials (North et al., 2007; Cutler et al., 2013; Goessling and North, 2014). Identifying additional compounds and combinations of cytokines will improve HSC expansion for therapeutic use.
The importance in further understanding the niche has been further underscored as it was shown that niche dysfunctions (for example, induced by mesenchymal infections) could lead to genotoxic stress and might sensitize HSCs to leukemia development (Zambetti et al., 2016; Passaro et al., 2017). It remains to be identified if aberrant cytokine signaling in the fetal niche can sensitize HSCs to different disorders.
Conclusion
Understanding the complete genetic and molecular program that controls HSC expansion in the embryonic niche remains an important goal to improve current protocols of regenerative medicine. The use of many animal models across phylogeny will concur to this aim, as most of the mechanisms involved in the control of HSC expansion by the embryonic niche appear to be conserved through evolution (Figure 1 and Table 2).
Author Contributions
CBM wrote the manuscript. JYB edited the manuscript.
Funding
JYB is endorsed by a Chair in Life Sciences funded by the Gabriella Giorgi-Cavaglieri Foundation and is also funded by the Swiss National Fund (31003_166515).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Aiuti, A., Biasco, L., Scaramuzza, S., Ferrua, F., Cicalese, M. P., Baricordi, C., et al. (2013). Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science 341:1233151. doi: 10.1126/science.1233151
Aiuti, A., Cicchini, C., Bernardini, S., Fedele, G., Amicone, L., Fantoni, A., et al. (1998). Hematopoietic support and cytokine expression of murine-stable hepatocyte cell lines (MMH). Hepatology 28, 1645–1654. doi: 10.1002/hep.510280626
Ara, T., Tokoyoda, K., Sugiyama, T., Egawa, T., Kawabata, K., and Nagasawa, T. (2003). Long-term hematopoietic stem cells require stromal cell-derived factor-1 for colonizing bone marrow during ontogeny. Immunity 19, 257–267. doi: 10.1016/S1074-7613(03)00201-2
Baumann, C. I., Bailey, A. S., Li, W., Ferkowicz, M. J., Yoder, M. C., and Fleming, W. H. (2004). PECAM-1 is expressed on hematopoietic stem cells throughout ontogeny and identifies a population of erythroid progenitors. Blood 104, 1010–1016. doi: 10.1182/blood-2004-03-0989
Benmimoun, B., Haenlin, M., and Waltzer, L. (2015a). Haematopoietic progenitor maintenance by EBF/Collier: beyond the Niche. Cell Cycle 14, 3517–3518. doi: 10.1080/15384101.2015.1093449
Benmimoun, B., Polesello, C., Haenlin, M., and Waltzer, L. (2015b). The EBF transcription factor Collier directly promotes Drosophila blood cell progenitor maintenance independently of the niche. Proc. Natl. Acad. Sci. U.S.A. 112, 9052–9057. doi: 10.1073/pnas.1423967112
Berrun, A., Harris, E., and Stachura, D. L. (2018). Isthmin 1 (ism1) is required for normal hematopoiesis in developing zebrafish. PLoS One 13:e0196872. doi: 10.1371/journal.pone.0196872
Bertrand, J. Y., Chi, N. C., Santoso, B., Teng, S., Stainier, D. Y., and Traver, D. (2010). Haematopoietic stem cells derive directly from aortic endothelium during development. Nature 464, 108–111. doi: 10.1038/nature08738
Bertrand, J. Y., Jalil, A., Klaine, M., Jung, S., Cumano, A., and Godin, I. (2005). Three pathways to mature macrophages in the early mouse yolk sac. Blood 106, 3004–3011. doi: 10.1182/blood-2005-02-0461
Bertrand, J. Y., Kim, A. D., Violette, E. P., Stachura, D. L., Cisson, J. L., and Traver, D. (2007). Definitive hematopoiesis initiates through a committed erythromyeloid progenitor in the zebrafish embryo. Development 134, 4147–4156. doi: 10.1242/dev.012385
Blaser, B. W., Moore, J. L., Hagedorn, E. J., Li, B., Riquelme, R., Lichtig, A., et al. (2017). CXCR1 remodels the vascular niche to promote hematopoietic stem and progenitor cell engraftment. J. Exp. Med. 214, 1011–1027. doi: 10.1084/jem.20161616
Boisset, J. C., van Cappellen, W., Andrieu-Soler, C., Galjart, N., Dzierzak, E., and Robin, C. (2010). In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature 464, 116–120. doi: 10.1038/nature08764
Boitano, A. E., Wang, J., Romeo, R., Bouchez, L. C., Parker, A. E., Sutton, S. E., et al. (2010). Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science 329, 1345–1348. doi: 10.1126/science.1191536
Bollerot, K., Pouget, C., and Jaffredo, T. (2005a). The embryonic origins of hematopoietic stem cells: a tale of hemangioblast and hemogenic endothelium. APMIS 113, 790–803.
Bollerot, K., Romero, S., Dunon, D., and Jaffredo, T. (2005b). Core binding factor in the early avian embryo: cloning of Cbfbeta and combinatorial expression patterns with Runx1. Gene Expr. Patterns 6, 29–39.
Boulais, P. E., and Frenette, P. S. (2015). Making sense of hematopoietic stem cell niches. Blood 125, 2621–2629. doi: 10.1182/blood-2014-09-570192
Broyles, R. H., Johnson, G. M., Maples, P. B., and Kindell, G. R. (1981). Two erythropietic microenvironments and two larval red cell lines in bullfrog tadpoles. Dev. Biol. 81, 299–314. doi: 10.1016/0012-1606(81)90293-1
Butko, E., Distel, M., Pouget, C., Weijts, B., Kobayashi, I., Ng, K., et al. (2015). Gata2b is a restricted early regulator of hemogenic endothelium in the zebrafish embryo. Development 142, 1050–1061. doi: 10.1242/dev.119180
Cavazzana-Calvo, M., Hacein-Bey, S., de Saint Basile, G., Gross, F., Yvon, E., Nusbaum, P., et al. (2000). Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science 288, 669–672. doi: 10.1126/science.288.5466.669
Charbord, P., and Moore, K. (2005). Gene expression in stem cell-supporting stromal cell lines. Ann. N. Y. Acad. Sci. 1044, 159–167. doi: 10.1196/annals.1349.020
Chen, M. J., Yokomizo, T., Zeigler, B. M., Dzierzak, E., and Speck, N. A. (2009). Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature 457, 887–891. doi: 10.1038/nature07619
Chen, X. D., and Turpen, J. B. (1995). Intraembryonic origin of hepatic hematopoiesis in Xenopus laevis. J. Immunol. 154, 2557–2567.
Chhabra, A., Lechner, A. J., Ueno, M., Acharya, A., Van Handel, B., Wang, Y., et al. (2012). Trophoblasts regulate the placental hematopoietic niche through PDGF-B signaling. Dev. Cell 22, 651–659. doi: 10.1016/j.devcel.2011.12.022
Chou, S., and Lodish, H. F. (2010). Fetal liver hepatic progenitors are supportive stromal cells for hematopoietic stem cells. Proc. Natl. Acad. Sci. U.S.A. 107, 7799–7804. doi: 10.1073/pnas.1003586107
Christensen, J. L., Wright, D. E., Wagers, A. J., and Weissman, I. L. (2004). Circulation and chemotaxis of fetal hematopoietic stem cells. PLoS Biol. 2:E75. doi: 10.1371/journal.pbio.0020075
Ciau-Uitz, A., Monteiro, R., Kirmizitas, A., and Patient, R. (2014). Developmental hematopoiesis: ontogeny, genetic programming and conservation. Exp. Hematol. 42, 669–683. doi: 10.1016/j.exphem.2014.06.001
Ciau-Uitz, A., Walmsley, M., and Patient, R. (2000). Distinct origins of adult and embryonic blood in Xenopus. Cell 102, 787–796. doi: 10.1016/S0092-8674(00)00067-2
Ciriza, J., Thompson, H., Petrosian, R., Manilay, J. O., and Garcia-Ojeda, M. E. (2013). The migration of hematopoietic progenitors from the fetal liver to the fetal bone marrow: lessons learned and possible clinical applications. Exp. Hematol. 41, 411–423. doi: 10.1016/j.exphem.2013.01.009
Clements, W. K., and Traver, D. (2013). Signalling pathways that control vertebrate haematopoietic stem cell specification. Nat. Rev. Immunol. 13, 336–348. doi: 10.1038/nri3443
Corbel, C. (2002). Expression of alphaVbeta3 integrin in the chick embryo aortic endothelium. Int. J. Dev. Biol. 46, 827–830.
Corlu, A., Lamy, I., Ilyin, G. P., Fardel, O., Kneip, B., Le Jossic, C., et al. (1998). Hematopoiesis-promoting activity of rat liver biliary epithelial cells: involvement of a cell surface molecule, liver-regulating protein. Exp. Hematol. 26, 382–394.
Cormier, F. (1993). Avian pluripotent haemopoietic progenitor cells: detection and enrichment from the para-aortic region of the early embryo. J. Cell Sci. 105(Pt 3), 661–666.
Cutler, C., Multani, P., Robbins, D., Kim, H. T., Le, T., Hoggatt, J., et al. (2013). Prostaglandin-modulated umbilical cord blood hematopoietic stem cell transplantation. Blood 122, 3074–3081. doi: 10.1182/blood-2013-05-503177
de Bruijn, M. F., Speck, N. A., Peeters, M. C., and Dzierzak, E. (2000). Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. EMBO J. 19, 2465–2474. doi: 10.1093/emboj/19.11.2465
Deng, M., Lu, Z., Zheng, J., Wan, X., Chen, X., Hirayasu, K., et al. (2014). A motif in LILRB2 critical for Angptl2 binding and activation. Blood 124, 924–935. doi: 10.1182/blood-2014-01-549162
Dey, N. S., Ramesh, P., Chugh, M., Mandal, S., and Mandal, L. (2016). Dpp dependent Hematopoietic stem cells give rise to Hh dependent blood progenitors in larval lymph gland of Drosophila. Elife 5:e18295. doi: 10.7554/eLife.18295
Dunon, D., and Imhof, B. A. (2000). The role of cell traffic in the emergence of the T lymphoid system. Semin. Immunol. 12, 429–433. doi: 10.1006/smim.2000.0266
Ema, H., and Nakauchi, H. (2000). Expansion of hematopoietic stem cells in the developing liver of a mouse embryo. Blood 95, 2284–2288.
Espin-Palazon, R., Stachura, D. L., Campbell, C. A., Garcia-Moreno, D., Del Cid, N., Kim, A. D., et al. (2014). Proinflammatory signaling regulates hematopoietic stem cell emergence. Cell 159, 1070–1085. doi: 10.1016/j.cell.2014.10.031
Evans, C. J., Hartenstein, V., and Banerjee, U. (2003). Thicker than blood: conserved mechanisms in Drosophila and vertebrate hematopoiesis. Dev. Cell 5, 673–690. doi: 10.1016/S1534-5807(03)00335-6
Fares, I., Chagraoui, J., Gareau, Y., Gingras, S., Ruel, R., Mayotte, N., et al. (2014). Cord blood expansion. Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self-renewal. Science 345, 1509–1512. doi: 10.1126/science.1256337
Fujio, K., Evarts, R. P., Hu, Z., Marsden, E. R., and Thorgeirsson, S. S. (1994). Expression of stem cell factor and its receptor, c-kit, during liver regeneration from putative stem cells in adult rat. Lab. Invest. 70, 511–516.
Gao, H., Wu, X., Simon, L., and Fossett, N. (2014). Antioxidants maintain E-cadherin levels to limit Drosophila prohemocyte differentiation. PLoS One 9:e107768. doi: 10.1371/journal.pone.0107768
Geerts, W. J., Lamers, W. H., and Moorman, A. F. (1993). Differences in erythropoiesis in normal chicken and quail embryos. Histochem. J. 25, 280–290. doi: 10.1007/BF00159119
Gekas, C., Dieterlen-Lievre, F., Orkin, S. H., and Mikkola, H. K. (2005). The placenta is a niche for hematopoietic stem cells. Dev. Cell 8, 365–375. doi: 10.1016/j.devcel.2004.12.016
Gekas, C., Rhodes, K. E., Van Handel, B., Chhabra, A., Ueno, M., and Mikkola, H. K. (2010). Hematopoietic stem cell development in the placenta. Int. J. Dev. Biol. 54, 1089–1098. doi: 10.1387/ijdb.103070cg
Geraud, C., Koch, P. S., Zierow, J., Klapproth, K., Busch, K., Olsavszky, V., et al. (2017). GATA4-dependent organ-specific endothelial differentiation controls liver development and embryonic hematopoiesis. J. Clin. Invest. 127, 1099–1114. doi: 10.1172/JCI90086
Geraud, C., Schledzewski, K., Demory, A., Klein, D., Kaus, M., Peyre, F., et al. (2010). Liver sinusoidal endothelium: a microenvironment-dependent differentiation program in rat including the novel junctional protein liver endothelial differentiation-associated protein-1. Hepatology 52, 313–326. doi: 10.1002/hep.23618
Ghosh, S., Singh, A., Mandal, S., and Mandal, L. (2015). Active hematopoietic hubs in Drosophila adults generate hemocytes and contribute to immune response. Dev. Cell 33, 478–488. doi: 10.1016/j.devcel.2015.03.014
Goessling, W., and North, T. E. (2014). Repairing quite swimmingly: advances in regenerative medicine using zebrafish. Dis. Model. Mech. 7, 769–776. doi: 10.1242/dmm.016352
Gomes, A. M., Kurochkin, I., Chang, B., Daniel, M., Law, K., Satija, N., et al. (2018). Cooperative transcription factor induction mediates hemogenic reprogramming. Cell Rep. 25, 2821–2835.e7. doi: 10.1016/j.celrep.2018.11.032
Greenbaum, A., Hsu, Y. M., Day, R. B., Schuettpelz, L. G., Christopher, M. J., Borgerding, J. N., et al. (2013). CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 495, 227–230. doi: 10.1038/nature11926
Guedes, P. T., de Oliveira, B. C., Manso, P. P., Caputo, L. F., Cotta-Pereira, G., and Pelajo-Machado, M. (2014). Histological analyses demonstrate the temporary contribution of yolk sac, liver, and bone marrow to hematopoiesis during chicken development. PLoS One 9:e90975. doi: 10.1371/journal.pone.0090975
Hu, J., Hu, S., Ma, Q., Wang, X., Zhou, Z., Zhang, W., et al. (2012). Immortalized mouse fetal liver stromal cells support growth and maintenance of human embryonic stem cells. Oncol. Rep. 28, 1385–1391. doi: 10.3892/or.2012.1909
Ivanovs, A., Rybtsov, S., Welch, L., Anderson, R. A., Turner, M. L., and Medvinsky, A. (2011). Highly potent human hematopoietic stem cells first emerge in the intraembryonic aorta-gonad-mesonephros region. J. Exp. Med. 208, 2417–2427. doi: 10.1084/jem.20111688
Iwasaki, H., Arai, F., Kubota, Y., Dahl, M., and Suda, T. (2010). Endothelial protein C receptor-expressing hematopoietic stem cells reside in the perisinusoidal niche in fetal liver. Blood 116, 544–553. doi: 10.1182/blood-2009-08-240903
Jaffredo, T., Gautier, R., Brajeul, V., and Dieterlen-Lievre, F. (2000). Tracing the progeny of the aortic hemangioblast in the avian embryo. Dev. Biol. 224, 204–214. doi: 10.1006/dbio.2000.9799
Khan, J. A., Mendelson, A., Kunisaki, Y., Birbrair, A., Kou, Y., Arnal-Estape, A., et al. (2016). Fetal liver hematopoietic stem cell niches associate with portal vessels. Science 351, 176–180. doi: 10.1126/science.aad0084
Kiassov, A. P., Van Eyken, P., van Pelt, J. F., Depla, E., Fevery, J., Desmet, V. J., et al. (1995). Desmin expressing nonhematopoietic liver cells during rat liver development: an immunohistochemical and morphometric study. Differentiation 59, 253–258. doi: 10.1046/j.1432-0436.1995.5940253.x
Kim, A. G., Vrecenak, J. D., Boelig, M. M., Eissenberg, L., Rettig, M. P., Riley, J. S., et al. (2016). Enhanced in utero allogeneic engraftment in mice after mobilizing fetal HSCs by alpha4beta1/7 inhibition. Blood 128, 2457–2461. doi: 10.1182/blood-2016-06-723981
Kissa, K., and Herbomel, P. (2010). Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature 464, 112–115. doi: 10.1038/nature08761
Kordes, C., Sawitza, I., Gotze, S., and Haussinger, D. (2013). Hepatic stellate cells support hematopoiesis and are liver-resident mesenchymal stem cells. Cell. Physiol. Biochem. 31, 290–304. doi: 10.1159/000343368
Kubota, H., Yao, H. L., and Reid, L. M. (2007). Identification and characterization of vitamin A-storing cells in fetal liver: implications for functional importance of hepatic stellate cells in liver development and hematopoiesis. Stem Cells 25, 2339–2349. doi: 10.1634/stemcells.2006-0316
Lampisuo, M., Liippo, J., Vainio, O., McNagny, K. M., Kulmala, J., and Lassila, O. (1999). Characterization of prethymic progenitors within the chicken embryo. Int. Immunol. 11, 63–69. doi: 10.1093/intimm/11.1.63
Lebestky, T., Chang, T., Hartenstein, V., and Banerjee, U. (2000). Specification of Drosophila hematopoietic lineage by conserved transcription factors. Science 288, 146–149. doi: 10.1126/science.288.5463.146
Lebestky, T., Jung, S. H., and Banerjee, U. (2003). A Serrate-expressing signaling center controls Drosophila hematopoiesis. Genes Dev. 17, 348–353. doi: 10.1101/gad.1052803
Lessard, J., Faubert, A., and Sauvageau, G. (2004). Genetic programs regulating HSC specification, maintenance and expansion. Oncogene 23, 7199–7209. doi: 10.1038/sj.onc.1207940
Li, D., Xue, W., Li, M., Dong, M., Wang, J., Wang, X., et al. (2018). VCAM-1(+) macrophages guide the homing of HSPCs to a vascular niche. Nature 564, 119–124. doi: 10.1038/s41586-018-0709-7
Liippo, J., Koskela, K., and Lassila, O. (2000). Prethymic progenitors from the avian para-aortic mesoderm express GATA-3 and distinct chTcf isoforms but still lack T-cell receptor-gamma rearrangements. Scand. J. Immunol. 52, 502–509. doi: 10.1046/j.1365-3083.2000.00807.x
Lister, J. A., Lane, B. M., Nguyen, A., and Lunney, K. (2011). Embryonic expression of zebrafish MiT family genes tfe3b, tfeb, and tfec. Dev. Dyn. 240, 2529–2538. doi: 10.1002/dvdy.22743
Liu, C., Han, T., Stachura, D. L., Wang, H., Vaisman, B. L., Kim, J., et al. (2018). Lipoprotein lipase regulates hematopoietic stem progenitor cell maintenance through DHA supply. Nat. Commun. 9:1310. doi: 10.1038/s41467-018-03775-y
Maeno, M., Tochinai, S., and Katagiri, C. (1985). Differential participation of ventral and dorsolateral mesoderms in the hemopoiesis of Xenopus, as revealed in diploid-triploid or interspecific chimeras. Dev. Biol. 110, 503–508. doi: 10.1016/0012-1606(85)90108-3
Mahony, C. B., Fish, R. J., Pasche, C., and Bertrand, J. Y. (2016). tfec controls the hematopoietic stem cell vascular niche during zebrafish embryogenesis. Blood 128, 1336–1345. doi: 10.1182/blood-2016-04-710137
Mahony, C. B., Pasche, C., and Bertrand, J. Y. (2018). Oncostatin M and kit-ligand control hematopoietic stem cell fate during zebrafish embryogenesis. Stem Cell Rep. 10, 1920–1934. doi: 10.1016/j.stemcr.2018.04.016
Mandal, L., Martinez-Agosto, J. A., Evans, C. J., Hartenstein, V., and Banerjee, U. (2007). A Hedgehog- and Antennapedia-dependent niche maintains Drosophila haematopoietic precursors. Nature 446, 320–324. doi: 10.1038/nature05585
Mazo, I. B., Gutierrez-Ramos, J. C., Frenette, P. S., Hynes, R. O., Wagner, D. D., and von Andrian, U. H. (1998). Hematopoietic progenitor cell rolling in bone marrow microvessels: parallel contributions by endothelial selectins and vascular cell adhesion molecule 1. J. Exp. Med. 188, 465–474. doi: 10.1084/jem.188.3.465
Mazo, I. B., Massberg, S., and von Andrian, U. H. (2011). Hematopoietic stem and progenitor cell trafficking. Trends Immunol. 32, 493–503. doi: 10.1016/j.it.2011.06.011
McGrath, K. E., Frame, J. M., Fegan, K. H., Bowen, J. R., Conway, S. J., Catherman, S. C., et al. (2015). Distinct sources of hematopoietic progenitors emerge before HSCs and provide functional blood cells in the mammalian embryo. Cell Rep 11, 1892–1904. doi: 10.1016/j.celrep.2015.05.036
Mondal, B. C., Mukherjee, T., Mandal, L., Evans, C. J., Sinenko, S. A., Martinez-Agosto, J. A., et al. (2011). Interaction between differentiating cell- and niche-derived signals in hematopoietic progenitor maintenance. Cell 147, 1589–1600. doi: 10.1016/j.cell.2011.11.041
Mondal, B. C., Shim, J., Evans, C. J., and Banerjee, U. (2014). Pvr expression regulators in equilibrium signal control and maintenance of Drosophila blood progenitors. Elife 3:e03626. doi: 10.7554/eLife.03626
Morrison, S. J., Hemmati, H. D., Wandycz, A. M., and Weissman, I. L. (1995). The purification and characterization of fetal liver hematopoietic stem cells. Proc. Natl. Acad. Sci. U.S.A. 92, 10302–10306. doi: 10.1073/pnas.92.22.10302
Murayama, E., Kissa, K., Zapata, A., Mordelet, E., Briolat, V., Lin, H. F., et al. (2006). Tracing hematopoietic precursor migration to successive hematopoietic organs during zebrafish development. Immunity 25, 963–975. doi: 10.1016/j.immuni.2006.10.015
Murayama, E., Sarris, M., Redd, M., Le Guyader, D., Vivier, C., Horsley, W., et al. (2015). NACA deficiency reveals the crucial role of somite-derived stromal cells in haematopoietic niche formation. Nat. Commun. 6:8375. doi: 10.1038/ncomms9375
Nagasawa, T., Hirota, S., Tachibana, K., Takakura, N., Nishikawa, S., Kitamura, Y., et al. (1996). Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 382, 635–638. doi: 10.1038/382635a0
Nanno, M., Hata, M., Doi, H., Satomi, S., Yagi, H., Sakata, T., et al. (1994). Stimulation of in vitro hematopoiesis by a murine fetal hepatocyte clone through cell-cell contact. J. Cell. Physiol. 160, 445–454. doi: 10.1002/jcp.1041600307
Neo, W. H., Booth, C. A. G., Azzoni, E., Chi, L., Delgado-Olguin, P., de Bruijn, M., et al. (2018). Cell-extrinsic hematopoietic impact of Ezh2 inactivation in fetal liver endothelial cells. Blood 131, 2223–2234. doi: 10.1182/blood-2017-10-811455
Nolta, J. A., Thiemann, F. T., Arakawa-Hoyt, J., Dao, M. A., Barsky, L. W., Moore, K. A., et al. (2002). The AFT024 stromal cell line supports long-term ex vivo maintenance of engrafting multipotent human hematopoietic progenitors. Leukemia 16, 352–361. doi: 10.1038/sj.leu.2402371
North, T. E., Goessling, W., Walkley, C. R., Lengerke, C., Kopani, K. R., Lord, A. M., et al. (2007). Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature 447, 1007–1011. doi: 10.1038/nature05883
Ohneda, O., and Bautch, V. L. (1997). Murine endothelial cells support fetal liver erythropoiesis and myelopoiesis via distinct interactions. Br. J. Haematol. 98, 798–808. doi: 10.1046/j.1365-2141.1997.3163133.x
Ottersbach, K., and Dzierzak, E. (2005). The murine placenta contains hematopoietic stem cells within the vascular labyrinth region. Dev. Cell 8, 377–387. doi: 10.1016/j.devcel.2005.02.001
Palis, J. (2014). Primitive and definitive erythropoiesis in mammals. Front. Physiol. 5:3. doi: 10.3389/fphys.2014.00003
Palis, J., Chan, R. J., Koniski, A., Patel, R., Starr, M., and Yoder, M. C. (2001). Spatial and temporal emergence of high proliferative potential hematopoietic precursors during murine embryogenesis. Proc. Natl. Acad. Sci. U.S.A. 98, 4528–4533. doi: 10.1073/pnas.071002398
Passaro, D., Di Tullio, A., Abarrategi, A., Rouault-Pierre, K., Foster, K., Ariza-McNaughton, L., et al. (2017). Increased vascular permeability in the bone marrow microenvironment contributes to disease progression and drug response in acute myeloid leukemia. Cancer Cell 32, 324–341.e6. doi: 10.1016/j.ccell.2017.08.001
Payushina, O. V. (2012). Hematopoietic microenvironment in the fetal liver: roles of different cell populations. ISRN Cell Biol. 2012:979480. doi: 10.5402/2012/979480
Pennetier, D., Oyallon, J., Morin-Poulard, I., Dejean, S., Vincent, A., and Crozatier, M. (2012). Size control of the Drosophila hematopoietic niche by bone morphogenetic protein signaling reveals parallels with mammals. Proc. Natl. Acad. Sci. U.S.A. 109, 3389–3394. doi: 10.1073/pnas.1109407109
Petzer, A. L., Zandstra, P. W., Piret, J. M., and Eaves, C. J. (1996). Differential cytokine effects on primitive (CD34+CD38-) human hematopoietic cells: novel responses to Flt3-ligand and thrombopoietin. J. Exp. Med. 183, 2551–2558. doi: 10.1084/jem.183.6.2551
Prashad, S. L., Calvanese, V., Yao, C. Y., Kaiser, J., Wang, Y., Sasidharan, R., et al. (2015). GPI-80 defines self-renewal ability in hematopoietic stem cells during human development. Cell Stem Cell 16, 80–87. doi: 10.1016/j.stem.2014.10.020
Ramadori, G., and Saile, B. (2002). Mesenchymal cells in the liver–one cell type or two? Liver 22, 283–294.
Rhodes, K. E., Gekas, C., Wang, Y., Lux, C. T., Francis, C. S., Chan, D. N., et al. (2008). The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell 2, 252–263. doi: 10.1016/j.stem.2008.01.001
Rieger, M. A. (2015). ATF4, a new player in fetal HSC expansion. Blood 126, 2351–2352. doi: 10.1182/blood-2015-10-671370
Roy, V., and Verfaillie, C. M. (1999). Expression and function of cell adhesion molecules on fetal liver, cord blood and bone marrow hematopoietic progenitors: implications for anatomical localization and developmental stage specific regulation of hematopoiesis. Exp. Hematol. 27, 302–312. doi: 10.1016/S0301-472X(98)00031-9
Rybtsov, S., Ivanovs, A., Zhao, S., and Medvinsky, A. (2016). Concealed expansion of immature precursors underpins acute burst of adult HSC activity in foetal liver. Development 143, 1284–1289. doi: 10.1242/dev.131193
Sawitza, I., Kordes, C., Reister, S., and Haussinger, D. (2009). The niche of stellate cells within rat liver. Hepatology 50, 1617–1624. doi: 10.1002/hep.23184
Saynajakangas, R., Uchida, T., and Vainio, O. (2009). Differential gene expression in CD45 cells at para-aortic foci stage of chicken haematopoiesis. Scand. J. Immunol. 70, 288–294. doi: 10.1111/j.1365-3083.2009.02304.x
Schuster, J. A., Stupnikov, M. R., Ma, G., Liao, W., Lai, R., Ma, Y., et al. (2012). Expansion of hematopoietic stem cells for transplantation: current perspectives. Exp. Hematol. Oncol. 1:12. doi: 10.1186/2162-3619-1-12
Schweitzer, K. M., Drager, A. M., van der Valk, P., Thijsen, S. F., Zevenbergen, A., Theijsmeijer, A. P., et al. (1996). Constitutive expression of E-selectin and vascular cell adhesion molecule-1 on endothelial cells of hematopoietic tissues. Am. J. Pathol. 148, 165–175.
Shao, L., Chang, J., Feng, W., Wang, X., Williamson, E. A., Li, Y., et al. (2018). The Wave2 scaffold Hem-1 is required for transition of fetal liver hematopoiesis to bone marrow. Nat Commun 9, 2377. doi: 10.1038/s41467-018-04716-5
Staal, F. J., Spaink, H. P., and Fibbe, W. E. (2016). Visualizing human hematopoietic stem cell trafficking in vivo using a zebrafish xenograft model. Stem Cells Dev. 25, 360–365. doi: 10.1089/scd.2015.0195
Subramaniam, A., Safaee Talkhoncheh, M., Magnusson, M., and Larsson, J. (2018). Endothelial protein C receptor (EPCR) expression marks human fetal liver hematopoietic stem cells. Haematologica 104, e47–e50. doi: 10.3324/haematol.2018.198515
Sugiyama, D., Kulkeaw, K., and Mizuochi, C. (2013). TGF-beta-1 up-regulates extra-cellular matrix production in mouse hepatoblasts. Mech. Dev. 130, 195–206. doi: 10.1016/j.mod.2012.09.003
Sugiyama, D., Kulkeaw, K., Mizuochi, C., Horio, Y., and Okayama, S. (2011). Hepatoblasts comprise a niche for fetal liver erythropoiesis through cytokine production. Biochem. Biophys. Res. Commun. 410, 301–306. doi: 10.1016/j.bbrc.2011.05.137
Sugiyama, T., Kohara, H., Noda, M., and Nagasawa, T. (2006). Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 25, 977–988. doi: 10.1016/j.immuni.2006.10.016
Tamplin, O. J., Durand, E. M., Carr, L. A., Childs, S. J., Hagedorn, E. J., Li, P., et al. (2015). Hematopoietic stem cell arrival triggers dynamic remodeling of the perivascular niche. Cell 160, 241–252. doi: 10.1016/j.cell.2014.12.032
Tan, K. S., Kulkeaw, K., Nakanishi, Y., and Sugiyama, D. (2017). Expression of cytokine and extracellular matrix mRNAs in fetal hepatic stellate cells. Genes Cells 22, 836–844. doi: 10.1111/gtc.12517
Taoudi, S., Gonneau, C., Moore, K., Sheridan, J. M., Blackburn, C. C., Taylor, E., et al. (2008). Extensive hematopoietic stem cell generation in the AGM region via maturation of VE-cadherin+CD45+ pre-definitive HSCs. Cell Stem Cell 3, 99–108. doi: 10.1016/j.stem.2008.06.004
Terrace, J. D., Currie, I. S., Hay, D. C., Masson, N. M., Anderson, R. A., Forbes, S. J., et al. (2007). Progenitor cell characterization and location in the developing human liver. Stem Cells Dev. 16, 771–778. doi: 10.1089/scd.2007.0016
Theodore, L. N., Hagedorn, E. J., Cortes, M., Natsuhara, K., Liu, S. Y., Perlin, J. R., et al. (2017). Distinct roles for matrix metalloproteinases 2 and 9 in embryonic hematopoietic stem cell emergence, migration, and niche colonization. Stem Cell Rep. 8, 1226–1241. doi: 10.1016/j.stemcr.2017.03.016
Travnickova, J., Tran Chau, V., Julien, E., Mateos-Langerak, J., Gonzalez, C., Lelievre, E., et al. (2015). Primitive macrophages control HSPC mobilization and definitive haematopoiesis. Nat. Commun. 6:6227. doi: 10.1038/ncomms7227
Turpen, J. B., Knudson, C. M., and Hoefen, P. S. (1981). The early ontogeny of hematopoietic cells studied by grafting cytogenetically labeled tissue anlagen: localization of a prospective stem cell compartment. Dev. Biol. 85, 99–112. doi: 10.1016/0012-1606(81)90239-6
Wagner, JE Jr, Brunstein, C. G., Boitano, A. E., DeFor, T. E., McKenna, D., Sumstad, D., et al. (2016). Phase I/II trial of stemregenin-1 expanded umbilical cord blood hematopoietic stem cells supports testing as a stand-alone graft. Cell Stem Cell 18, 144–155. doi: 10.1016/j.stem.2015.10.004
Walasek, M. A., van Os, R., and de Haan, G. (2012). Hematopoietic stem cell expansion: challenges and opportunities. Ann. N. Y. Acad. Sci. 1266, 138–150. doi: 10.1111/j.1749-6632.2012.06549.x
Wittig, O., Paez-Cortez, J., and Cardier, J. E. (2010). Liver sinusoidal endothelial cells promote B lymphopoiesis from primitive hematopoietic cells. Stem Cells Dev. 19, 341–350. doi: 10.1089/scd.2009.0300
Wolf, A., Aggio, J., Campbell, C., Wright, F., Marquez, G., Traver, D., et al. (2017). Zebrafish Caudal Haematopoietic Embryonic Stromal Tissue (CHEST) Cells Support Haematopoiesis. Sci. Rep. 7:44644. doi: 10.1038/srep44644
Xue, Y., Gao, S., and Liu, F. (2015). Genome-wide analysis of the zebrafish Klf family identifies two genes important for erythroid maturation. Dev. Biol. 403, 115–127. doi: 10.1016/j.ydbio.2015.05.015
Xue, Y., Lv, J., Zhang, C., Wang, L., Ma, D., and Liu, F. (2017). The vascular niche regulates hematopoietic stem and progenitor cell lodgment and expansion via klf6a-ccl25b. Dev. Cell 42:e344. doi: 10.1016/j.devcel.2017.07.012
Yu, S., Luo, F., and Jin, L. H. (2018). The Drosophila lymph gland is an ideal model for studying hematopoiesis. Dev. Comp. Immunol. 83, 60–69. doi: 10.1016/j.dci.2017.11.017
Yvernogeau, L., and Robin, C. (2017). Restricted intra-embryonic origin of bona fide hematopoietic stem cells in the chicken. Development 144, 2352–2363. doi: 10.1242/dev.151613
Zambetti, N. A., Ping, Z., Chen, S., Kenswil, K. J. G., Mylona, M. A., Sanders, M. A., et al. (2016). Mesenchymal inflammation drives genotoxic stress in hematopoietic stem cells and predicts disease evolution in human pre-leukemia. Cell Stem Cell 19, 613–627. doi: 10.1016/j.stem.2016.08.021
Zhang, C. C., Kaba, M., Ge, G., Xie, K., Tong, W., Hug, C., et al. (2006). Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nat. Med. 12, 240–245. doi: 10.1038/nm1342
Zhang, C. C., and Lodish, H. F. (2004). Insulin-like growth factor 2 expressed in a novel fetal liver cell population is a growth factor for hematopoietic stem cells. Blood 103, 2513–2521. doi: 10.1182/blood-2003-08-2955
Zhao, Y., Zhou, J., Liu, D., Dong, F., Cheng, H., Wang, W., et al. (2015). ATF4 plays a pivotal role in the development of functional hematopoietic stem cells in mouse fetal liver. Blood 126, 2383–2391. doi: 10.1182/blood-2015-03-633354
Keywords: zebrafish, mammals, CHT, fetal liver, hematopoietic (stem) cells, caudal hematopoietic tissue, microenvironment
Citation: Mahony CB and Bertrand JY (2019) How HSCs Colonize and Expand in the Fetal Niche of the Vertebrate Embryo: An Evolutionary Perspective. Front. Cell Dev. Biol. 7:34. doi: 10.3389/fcell.2019.00034
Received: 23 November 2018; Accepted: 25 February 2019;
Published: 12 March 2019.
Edited by:
Hatem E. Sabaawy, Rutgers University, The State University of New Jersey, United StatesReviewed by:
César Nombela Arrieta, University of Zurich, SwitzerlandEirini Trompouki, Max-Planck-Institut für Immunbiologie und Epigenetik, Germany
Copyright © 2019 Mahony and Bertrand. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Julien Y. Bertrand, anVsaWVuLmJlcnRyYW5kQHVuaWdlLmNo
 Christopher B. Mahony
Christopher B. Mahony Julien Y. Bertrand
Julien Y. Bertrand