- 1Science and Technology Facilities Council, Rutherford Appleton Laboratory, Central Laser Facility, Research Complex at Harwell, Harwell, UK
- 2Institute of Applied Physics, Technische Universität Wien, Wien, Austria
- 3MRC Human Immunology Unit, Weatherall Institute of Molecular Medicine, University of Oxford, Headley Way, UK
- 4Department of Biophysical Chemistry, J.Heyrovsky Institute of Physical Chemistry, Czech Academy of Sciences, Prague, Czech Republic
Ever since technologies enabled the characterization of eukaryotic plasma membranes, heterogeneities in the distributions of its constituents were observed. Over the years this led to the proposal of various models describing the plasma membrane organization such as lipid shells, picket-and-fences, lipid rafts, or protein islands, as addressed in numerous publications and reviews. Instead of emphasizing on one model we in this review give a brief overview over current models and highlight how current experimental work in one or the other way do not support the existence of a single overarching model. Instead, we highlight the vast variety of membrane properties and components, their influences and impacts. We believe that highlighting such controversial discoveries will stimulate unbiased research on plasma membrane organization and functionality, leading to a better understanding of this essential cellular structure.
Membranes are one of the key structures in cell biology. Besides being instrumental in compartmentalizing and protecting cells, their role as organizing centers for tasks such as metabolism or signaling is increasingly recognized. In fact, a majority of cellular processes are associated with membranes (Stryer, 1995). Membranes provide useful docks for correct localisation of proteins which is essential for their function (Miosge and Zamoyska, 2007; Grecco et al., 2011; Hung and Link, 2011). Importantly, in humans, mislocalization of membrane proteins leads to the loss-of-function and, frequently, can develop into diseases (Edwards et al., 2000; Matsuda et al., 2008; Hung and Link, 2011; Schaeffer et al., 2014). Nevertheless, the presence of proteins at a particular membrane is usually not sufficient for their function. Often, the nanoscopic localization, oligomerisation and/or clustering of membrane proteins can affect the efficiency of cellular processes (Cebecauer et al., 2010; Matthews, 2012; Nussinov, 2013; Garcia-Parajo et al., 2014). Membranes, the lipid environment and membrane properties in general, influence nanoscale organization and function of these molecules. It is, therefore, important to understand molecular details of membrane structure and mechanisms responsible for its dynamics organization.
Here, we review membrane properties, models of membrane organization and useful techniques for studies of membrane organization and dynamics, with a special focus on the plasma membrane of higher eukaryotes (mammals). Our specific aim is to re-emphasize currently omitted or underestimated biophysical principles and discuss their role in dynamic membrane organization. We attempt to provide a comprehensive description of membrane complexity and suggestions how to avoid interpretation of membrane-associated phenomena within the borders of a single theory. As a reader will see, we believe that there is no universal model of the plasma membrane dynamic lateral organization. These more general issues will be discussed in the last section. First, let us start with the very basic structure of membranes.
Basic Structure of Cell Membranes
A lipid bilayer forms the basis of all cellular membranes. It is a lamellar structure with a hydrophobic core and a polar headgroup region on both sides (Figure 1A). In cells, it is composed of hundreds, if not thousands, of different phospholipid species. These differ in their polar headgroup moiety but mainly in the length and saturation of acyl chains forming a hydrophobic core of a lipid bilayer. Other lipid and fatty acid species add to this complexity. Of those, sterols (cholesterol in mammals) are the most abundant in the plasma membrane and can represent up to 40% of total lipid (van Meer and de Kroon, 2011). Cholesterol has a special structure (Figure 1A) enabling strong impact on basic membrane properties such as viscosity or interleaflet coupling, as described multiple times in comprehensive articles (Ipsen et al., 1987; Mouritsen and Zuckermann, 2004; Maxfield and van Meer, 2010).
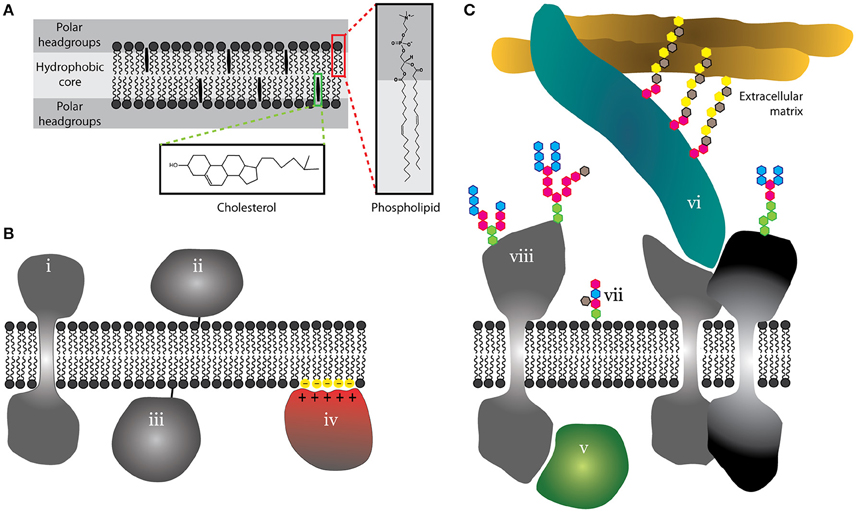
Figure 1. Schematic illustration of the basic structure of lipid bilayer and proteo-lipidic membranes. (A) Cell membranes are lamellar structures with a hydrophobic core and a polar headgroup space. As examples, phospholipids and cholesterol are shown with almost atomistic detail (red and green boxes). (B) Membrane proteins can integrate into membranes (i), but can use lipid anchors (ii and iii) or peripherally associate with membranes via electrostatic interactions (iv). (C) Proteins can further associate with membranes via protein-protein interactions on the cytosolic side (v) or at the interface between the plasma membrane and the extracellular matrix (vi). Outer leaflet lipids (vii) and extracellular domains of proteins (viii) are often glycosylated.
Proteins constitute approximately half of the total plasma membrane mass (Dupuy and Engelman, 2008). We distinguish integral and peripheral membrane proteins depending on their anchorage into a lipid bilayer via transmembrane domain(s) or a lipid moiety, respectively (Figure 1B). In addition, some proteins may associate with the membrane via electrostatic interactions with lipid headgroups (Figure 1B; McLaughlin and Murray, 2005) or a variety of protein-protein or protein-glycan interactions (Figure 1C; Stryer, 1995). Such proteins are commonly termed as “membrane-associated.” Extracellular parts of lipids and proteins are frequently glycosylated (Figure 1C). Indeed, glycans form a dense structure at the outer surface of the plasma membrane (Berrier and Yamada, 2007). This molecular complexity of membranes has probably evolved to serve as a selective barrier and organizing center with a high fidelity and robustness (Cebecauer et al., 2010). But what are those unique properties which were selected in the process of evolution to control critical cellular processes with such efficiency?
Intrinsic Properties of Cell Membranes Essential for Their Function
Early definitions, of which the “fluid mosaic model” of Singer and Nicolson (SN model Singer and Nicolson, 1971, 1972) is the best known, highlighted fluidity as one of the most critical membrane features. Indeed, fluidity of membranes provides important advantage over other cellular structures such as the cytoskeleton or ribonucleoproteins. It forms the basis for the highly dynamic character of membrane-associated (bio)chemical reactions and other cellular processes. Membrane fluidity enables the majority of molecules to diffuse freely over long distances and rotate or re-orientate to adopt optimal conformation. Membranes can be considered as two-dimensional solutions. This two-dimensional character also distinguishes membranes from other three-dimensional cellular solutes (e.g., the cytosol). The fundamental importance of fluidity is, for example, underlined by the fact that cells modify the saturation of their lipid acyl-chains to keep their membranes fluid when adapting to the environment, e.g., different temperatures (Fraenkel and Hopf, 1940; Buda et al., 1994).
Although membranes are fluid, they have higher viscosity (Box 1) than the cytosol (Luby-Phelps et al., 1993). This has a direct impact on the mobility of membrane molecules. Membrane viscosity can be modified by lipid composition or other factors, such as the presence of proteins or poorly mobile structures, and will thus vary over space and time.
Box 1. Membrane Fluidity, Viscosity and Mobility
Viscosity is a macroscopic parameter describing the behavior of a large, rigid sphere in a Newtonian fluid. Its use for membranes is imperfect and should be treated with care (Valeur and Berberan-Santos, 2012; Olšinová et al., 2014). Membranes are nanoscopic structures with 2D character and highly heterogeneous composition in terms of size and chemistry. Due to a lack of a better parameter, we use the term “viscosity” to describe membrane properties such as membrane lateral compressibility and acyl chain ordering, which influence the mobility of membrane components. Other terms, e.g., “microviscosity” or “rigidity” were also used in literature to cover these properties in one word (Shinitzky and Inbar, 1976; Kowalska and Cierniewski, 1983; Gut et al., 1985; Sherbet, 1989).
The term “fluidity” is frequently used to replace “viscosity” for biological membranes or other highly heterogeneous materials (Valeur and Berberan-Santos, 2012). We use term “fluidity” in this work to distinguish membranes from other cellular structures which exhibit much higher stability (e.g., nucleoproteins), thereby limiting rapid, long-range mobility of associated compounds.
Efforts to measure viscosity of cellular membranes are associated with serious technical difficulties (Valeur and Berberan-Santos, 2012; Olšinová et al., 2014). Instead, measurements of rotational or lateral diffusion were successfully applied to characterize membrane viscosity. In cellular membranes, lateral diffusion is frequently substituted with the term “mobility.” Mobility of membrane molecules can be influenced by many different factors, such as (i) membrane ordering or, in the other terms, how densely lipids and proteins are packed in the membrane (Kahya et al., 2003), (ii) lateral pressure of the membrane which is partially linked to ordering but also membrane hydration (polarity) and directly influences bilayer compressibility and elasticity (Marsh, 1996; Cantor, 1999), and (iii) macromolecular crowding (Saxton, 1987; Guigas and Weiss, 2015). Mobility of membrane components is further influenced by other intrinsic and extrinsic factors as described in the main text.
To illustrate dramatic differences in the mobility of molecules in synthetic and cellular environments, we provide a few values of diffusion coefficients in Table 1. These should be considered as a simple guideline due to differences in the precision with which these values were measured. We also provide the time scale a molecule requires to traverse the distance of 20 μm (longitudal size of HeLa cells) by random (Brownian) 2D motion. This should underline dramatic differences in the mobility of molecules in real space.
Another property emphasized in the SN model is continuity of the plasma membrane (Singer and Nicolson, 1972). The plasma membrane fully covers the cell surface. Its continuity is especially important for membrane receptors or effector molecules which need rapidly to re-localize, e.g., when a cell is changing its direction of chemotactic mobility (Janetopoulos and Firtel, 2008). Continuity also supports intermolecular interactions or the formation of multi-molecular assemblies within or at the surface of membranes. In some cells, membrane continuity is limited to the apical or basal side due to the presence of tight junctions eliminating free mobility of membrane molecules (Balda and Matter, 2008). We will discuss viscosity and continuity, and their impact on the organization of membranes in more detail further in the text.
Almost all molecules can interact and influence each other in cellular membranes. As a consequence, coexistence of molecules in membranes has cooperative character. Cooperativity of molecules was already mentioned for fluid cellular membranes in the SN model (Singer and Nicolson, 1972) but seems to be recently overseen. This property has a dramatic impact on experiments, in which systemic perturbance of membranes (e.g., by chemical or genetic treatment) was employed to support specific models of membrane organization.
Lipid membranes undergo interleaflet coupling, meaning that acyl chains of lipids in one leaflet interdigitate into the space of the other leaflet (Figure 2A; Nickels et al., 2015). Theoretical predictions suggest that interleaflet coupling can coordinate the organization of molecules between the two leaflets (Schmidt et al., 1978; Duzgunes et al., 1988; Merkel et al., 1989; Kiessling et al., 2006; Raghupathy et al., 2015; Williamson and Olmsted, 2015). Yet, White and co-workers recently provided an alternative view (Mihailescu et al., 2011; Capponi et al., 2016). They do not negate the existence of strong coupling between the two leaflets of lipid bilayer but observed no direct complementarity between the opposite acyl chains (Capponi et al., 2016). Cholesterol, which was predicted to intensify interleaflet coupling in membrane lipid domains, was found to reduce the level of acyl-chain interdigitation (Mihailescu et al., 2011). These works indicate that we need more experimental data in order to better understand the effect of interleaflet coupling in lipid bilayers.
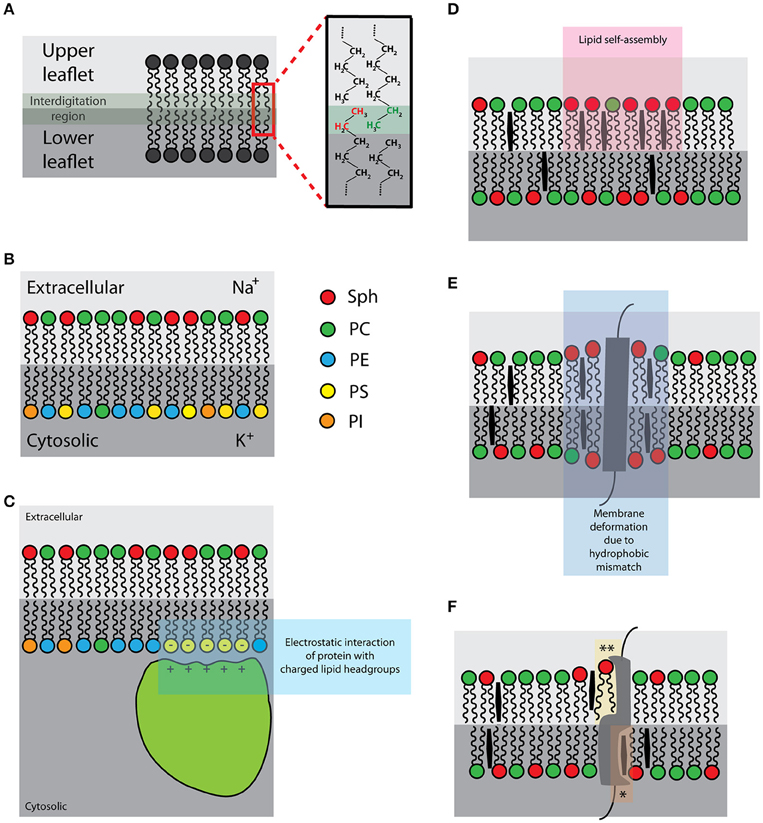
Figure 2. Schematic illustrations of the selected intrinsic membrane properties: (A) Interleaflet coupling [interdigitating lipid acyl chains in green-gray; zoom: interdigitating ethyl groups of upper (green) and lower (red) leaflets]; (B) Asymmetric distribution of lipids and ions [right hand-side: color-coding of lipid species]; (C) Negatively charged lipids (yellow) of the plasma membrane inner leaflet [for the association of proteins with basic-rich domains (light green)]; (D) Lipid self-assemblies (pink); (E) Hydrophobic mismatch (purple); (F) Protein-lipid interactions [*sphingolipid- and **cholesterol-binding pockets].
The plasma membrane of eukaryotic cells is asymmetric (Figure 2B) in terms of lipid and surface ion composition, as well as the presence of specific proteins (Rothman and Lenard, 1977; van Meer et al., 2008). The lipid asymmetry is maintained by flippases and other lipid translocating or transport proteins (Canagarajah et al., 2008; Devaux et al., 2008). Chemical asymmetry, a gradient of ions, drives a number of vital cellular processes (e.g., generation of chemical energy and metabolism). On the other hand, lipid asymmetry further adds to the diversity and complexity of cellular compartments, thereby helping to optimize cellular processes. For example, negatively charged lipid headgroups in the inner leaflet provide the binding surface for proteins with specific binding domains (Figure 2C; McLaughlin and Murray, 2005). This can cause protein relocalisation often leading to the initiation of signaling events (Yeung et al., 2008). In addition, chemical asymmetry and the presence of ions induces heterogeneous distribution of lipids, at least in simulations and in model systems (Vácha et al., 2009; Jurkiewicz et al., 2012). Whether this effect contributes to the organization of plasma membrane in living cells is experimentally difficult to test; an asymmetric membrane is indispensable for cell viability. At the same time, the formation of asymmetric model membranes in vitro is a rather delicate process and was successfully performed only in a few cases in past (Kiessling et al., 2006; Collins and Keller, 2008; Chiantia et al., 2011). Therefore, data demonstrating lateral (re)organization due to membrane asymmetry are still rare.
Even though lipids interact only weakly, preferential self-assemblies of certain lipid species or conformations (Figure 2D) were demonstrated in model lipid mixtures (Björkbom et al., 2010; Ivankin et al., 2010). Under certain circumstances, lipid self-assembling may extensively reduce miscibility of its molecules, i.e., generate physico-chemical heterogeneities. A well-known example of lipid self-assembly and segregation is the formation of separated lipid phases in vesicles composed of two or more lipid species with different melting points (Bagatolli and Gratton, 1999; Korlach et al., 1999; Bernardino de la Serna et al., 2004; Veatch and Keller, 2005). Importantly, lipids are prone to phase separation or miscibility transitions also in cell membrane-derived vesicles and blebs, as well as artificial vesicles generated from lipids extracts and from native membranes (Bernardino de la Serna et al., 2004; Baumgart et al., 2007; Veatch et al., 2008). All these observations were achieved using equilibrated membranes; however, cells are non-equilibrium systems (Stryer, 1995). Indeed, no miscibility phase transitions were observed in living cells over a wide range of temperatures (Lee et al., 2015). Putative impact of lipid self-assembly and ordered lipid membranes on cell membranes is discussed in the section “Plasma membrane organization–general models and concepts.”
Hydrophobic thickness of a lipid bilayer is defined mainly by the length and saturation of acyl chains and the presence of sterols. Bilayer lipids interact non-specifically and transiently with transmembrane domains of integral proteins (Marsh, 1993). Imparity of the hydrophobic thickness of the bilayer and the hydrophobic length of TMD (s) is called hydrophobic mismatch (Figure 2E). Hydrophobic mismatch was proposed to induce molecular aggregation/segregation in lipid bilayers, as described in the mattress model (Mouritsen and Bloom, 1984). For example, lipids with longer and more saturated acyl chains will preferentially reside in the annulus of helical TMD with long hydrophobic length. More about the mattress model is discussed in the section “Plasma membrane organization–general models and concepts.”
Lipids can also interact with proteins in a more specific manner (Haberkant et al., 2008; Fantini and Barrantes, 2013; Yeagle, 2014). Several proteins carry lipid-binding domains (Ernst et al., 2010; Contreras et al., 2011; Fantini and Barrantes, 2013) to which lipids bind with a higher affinity compared to the lipids of the first shell interacting with transmembrane domains non-specifically. Such protein-lipid interactions (Figure 2F) can be highly specific in a way that lipid headgroup, acyl chain length and its saturation determine the affinity of such interactions (Contreras et al., 2012). Specific protein-lipid interactions have been shown to modulate protein stability and its function (Uittenbogaard and Smart, 2000; Hanson et al., 2008; Contreras et al., 2012) or are directly involved in transport of lipids between subcellular compartments (Kwon et al., 2009). But what is their impact on the lateral organization of plasma membrane is to date unclear.
The abovementioned intrinsic properties can be ascribed to any proteo-lipid membranes, independent of whether these are artificial or cellular structures. But what is so specific about membranes of living cells? Can “clever” use of these intrinsic properties, their local amplification, reduction and/or combination lead to such limitless concert of events such as metabolism and signal transduction? Or is there a need for extrinsic factors to support those basal membrane properties?
Extrinsic Factors Influencing the Plasma Membrane Organisation
The plasma membrane is built to interact with surrounding structures such as cortical actin, the extracellular matrix or a variety of ligand molecules. These form the basis of extrinsic factors which can shape the plasma membrane.
We assigned protein-protein interactions to the section of extrinsic factors, given the fact that extra-membranous (extracellular and cytosolic) domains are the predominant structures involved in persistent associations of proteins. Further, since these interactions often involve non-membranous protein scaffolds, we believe that protein-protein interactions have, to some extent, extrinsic character.
In contrast to lipids, proteins can interact with high affinity and thus form relatively stable structures (Figure 3A) within a sea of lipid molecules. Indeed, the interaction of proteins is a common process associated, for example, with leukocyte signaling or cellular adhesion, both taking place at the surface of cells (Douglass and Vale, 2005; Rossier et al., 2012). Supramolecular complexes of proteins can be relatively large and can further interact with other cellular components such as the cytoskeleton, thereby forming protein networks which can have local or systemic impact on membranes (see below).
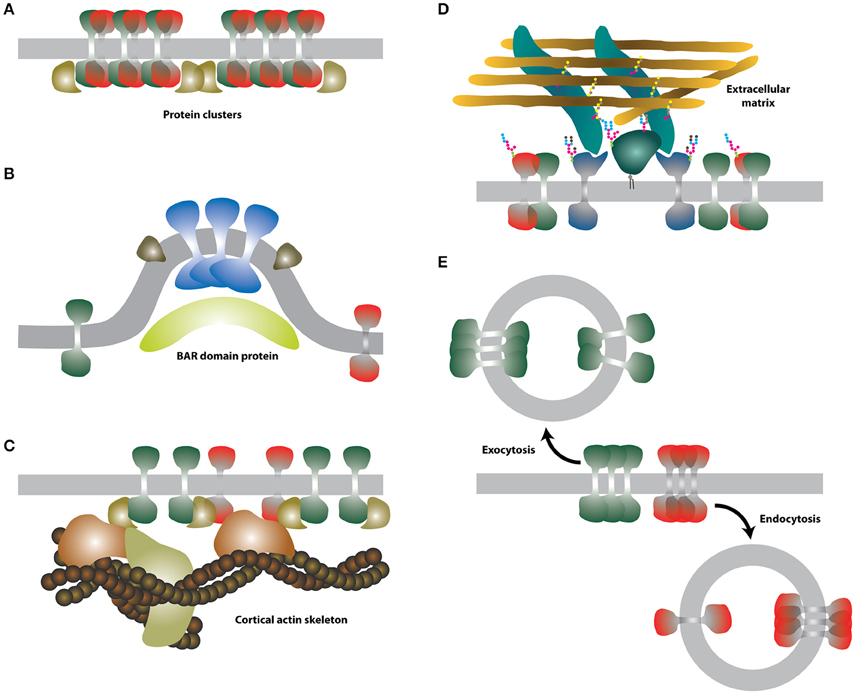
Figure 3. Schematic illustrations of the selected extrinsic membrane properties. (A) Protein-protein interactions. Putative membrane proteins (green and red) forming heterodimers can assemble into larger clusters and be stabilized by further interaction with cytosolic proteins (dark yellow). (B) Membrane curvature. Certain proteins (blue and green-brown) may prefer curved membranes. Curved membranes can be stabilized e.g., by BAR proteins (light yellow). (C) Intracellular cortical actin skeleton. Actin-binding proteins (dark yellow and orange) can associate with integral membrane proteins and form larger assemblies with reduced mobility. (D) Extracellular glycocalyx. Interaction of certain membrane proteins (dark blue and dark green) with the extracellular matrix may lead to the formation of larger assemblies with reduced mobility. (E) Endo-/exocytosis. Membrane lipids and proteins are rapidly endocytosed (red) or exocytosed (green).
More recently, a concept of protein islands was presented based on the fact that proteins were detected in distinct domains interspaced with protein-free areas, when membrane patches were imaged by electron microscopy (Wilson et al., 2000; Lillemeier et al., 2006). Heterogeneous distribution of proteins in entities reminiscent of such “protein islands” were often found by super-resolution fluorescence imaging of the cellular plasma membrane (for example Sieber et al., 2007; Lillemeier et al., 2010; Letschert et al., 2014; Saka et al., 2014; see also Figure 4). However, it is not yet clear whether such entities are created and stabilized by protein-protein interactions or other mechanisms are involved. The impact of the underlying actin cytoskeleton on protein islands was reported in the past (Wilson et al., 2001; Lillemeier et al., 2006).
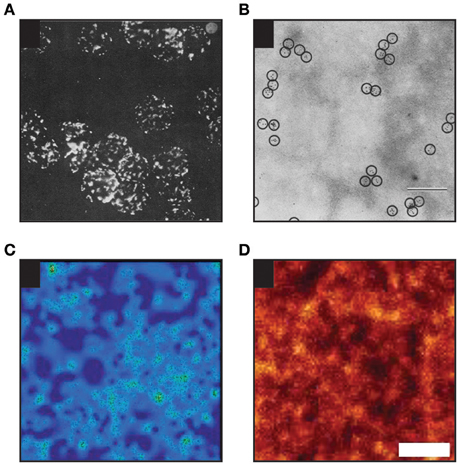
Figure 4. Examples of heterogenous distribution of proteins in the plasma membrane of mammalian cells. (A) Heterogenous distribution of MHC molecules as observed on murine lymphoid cells in 1967 by Cerottini and Brunner using epifluorescence microscopy (Adapted by permission from John Wiley and Sons Ltd; (Cerottini and Brunner, 1967). (B) MHC clustering on murine red blood cells as detected in 1971 by Nicolson and colleagues by electron microscopy (EM; scale bar: 200 nm; Adapted by permission from RUPress: ©1971 Nicolson et al., 1971). (C) Distribution of individual TCR molecules on activated primary human T cells analyzed by dSTORM (Adapted by permission from Macmillan Publishers Ltd: Nature Methods (Rubin-Delanchy et al., 2015), copyright 2015). Showing 3 × 3 μm area. (D) Distribution of proteins in membrane sheets derived from a neuroendocrine cell line as revealed by STED microscopy [Adapted by permission from Macmillan Publishers Ltd: Nature Communications (Saka et al., 2014), copyright 2015]. Scale bar: 500 nm.
Certain cytosolic proteins can interact with headgroups of selected lipid species (e.g., negatively charged phosphatidylserines and phosphoinositols) via electrostatic interactions (Figure 2C; McLaughlin and Murray, 2005). In cells, binding of proteins to charged headgroups of inner leaflet lipids is well-documented to control important cellular processes, e.g., phagocytosis (Botelho et al., 2000). In addition, peripheral protein interactions at the inner leaflet of the plasma membrane modulate localization and mobility of charged lipids, as well as some other, probably associated, molecules (Golebiewska et al., 2008, 2011; Yeung et al., 2008). But whether such peripheral interactions can modulate the mobility of other membrane components (e.g., at the outer leaflet) and have a more general impact on the plasma membrane organization awaits its direct proof.
Due to membrane plasticity and flexibity, certain lipids with non-conical shape, e.g., lysophospholipids or phosphatidylethanolamines found also in cell membranes, can deform the planar structure of lipid bilayers by bending (Figure 3B), thereby changing its curvature (Šachl et al., 2011). Similar to protein-protein interactions, curvature is not a typical extrinsic factor. But in cells, highly curved membranes are prevalently generated by curvature-forming proteins (e.g., BAR-domain containing proteins) or cytoskeleton-induced mechanical forces (McMahon and Gallop, 2005; Mattila and Lappalainen, 2008). Both these processes are externally regulated and require energy and/or cofactors (Mima et al., 2008; Frolov et al., 2010). For example, processes of endo/exocytosis are initiated by protein-induced membrane bending and ATP/GTP hydrolysis (Vilmart-Seuwen et al., 1986; Hansen and Nichols, 2009; Stachowiak et al., 2013). The plasma membrane has the capacity to form specialized extensions with high curvature to accomplish some of its specific functions, e.g., the formation of microvilli in polarized cells for the efficient uptake of nutrients (Crawley et al., 2014), or of membrane nanotubes for the inter-cellular communication (Onfelt et al., 2004). In theory, curvature can modulate the distribution of membrane molecules (Bozic et al., 2006; Wu and Liang, 2014). Indeed, some proteins accumulate in curved or filamentous membranes in cells, but the mechanisms responsible for such diversity are probably based on targeted delivery of molecules to these specific structures and partial impermeability of the basal region of such membrane extensions, e.g., of cilia (Trimble and Grinstein, 2015). In model membranes, specific proteins undergo curvature-driven sorting while others do not (Hatzakis et al., 2009; Aimon et al., 2014; Quemeneur et al., 2014). Specific intermolecular interactions between lipids and proteins were suggested to be responsible for such selectivity (Callan-Jones et al., 2011), nonetheless it is still unclear whether curvature-based protein and lipid sorting can occur in highly dynamic membranes of cells.
The cortical actin (CA) skeleton (Figure 3C) helps to keep and modulate the shape of living cells (Murase et al., 2004). In addition, it is involved in the regulation of membrane trafficking and signaling (for example Suzuki et al., 2007; Jaqaman et al., 2011; Gowrishankar et al., 2012; Johnson et al., 2012). The impact of the CA on the organization of the plasma membrane is well described and forms the basis of a key model discussed in the following section.
Similarly, the glycocalyx (Figure 3D), a part of the extracellular matrix (ECM) in vertebrates, forms a dense structure at the surface of eukaryotic cells (Stryer, 1995). Glycosaminoglycans and associated glycoproteins and proteoglycans of the glycocalyx were shown to modulate signaling by direct association with surface receptors (Bass et al., 2007; Morgan et al., 2007) or by binding of ligands (Hynes, 2009). In this way, the glycocalyx and the extracellular matrix regulate the shape of multicellular organisms. The glycocalyx was also predicted to influence the general organization of the plasma membrane (Jacobson et al., 1987). Indeed, glycosylated extracellular domains were shown to modulate the organization (Anderson and Fambrough, 1983) and mobility (Wier and Edidin, 1986; Zhang et al., 1991; Hartel et al., 2015) of membrane proteins. The molecular mechanism is still unknown and, to our knowledge, has not been studied in detail.
Cells keep their membranes “healthy” by a rapid turnover of its components. This is achieved mainly by vesicular transport—endo-/exocytosis (Figure 3E)—but also by a less well understood protein-mediated lipid transport mechanism(s) (Lev, 2012). Each exo-/endocytic event delivers or removes a material equivalent to a surface area of ≈ 30.000 nm2 (estimated for the average diameter of exo-/endocytic vesicles to be ~100 nm). Therefore, every such event can transiently, but dramatically change the local membrane composition and, thereby, organization. Whereas no preferred sites of exo-/endocytosis were reported under resting conditions (Schmoranzer et al., 2000), stimulation of cells can result in more localized vesicular transport and fusion/fission hotspots (Stinchcombe et al., 2001; Gaffield et al., 2009). This can further accelerate changes in the plasma membrane.
Alternatively, membrane components (specifically lipids) can be delivered to the plasma membrane by specific lipid transporters (Raychaudhuri et al., 2006; Voelker, 2009; Tarling et al., 2013). These may travel through the cytosol by diffusion or, more probably, such events can take place at membrane contact sites between the endoplasmic reticulum (ER) and plasma membrane (see Figure 5 in Fernández-Busnadiego et al., 2015). These sites are responsible for the synthesis, transport of lipids between the ER and plasma membrane (e.g., by Osh sterol transporters Raychaudhuri et al., 2006) and regulation of lipid metabolism in the plasma membrane (Stefan et al., 2011).
Vesicular and protein-mediated transport are the two main mechanisms responsible for a rapid turnover of membrane molecules (estimated to exchange almost all of its components within 1 h), but other routes such as free diffusion of small molecules (e.g., glucose, ions Cortizo et al., 1990) or infection of cells by viruses and other pathogens (Mazzon and Mercer, 2014) can further modulate membrane composition and organization.
Active transport of protons and ions, together with chemical asymmetry, generates an electrostatic potential across the plasma membrane of living cells (Hodgkin and Huxley, 1952). In addition to the function of the membrane potential in metabolism and transport of essential molecules in and out of cells, it has an impact on properties of model and cell membranes (O'Shea et al., 1984; Grossmann et al., 2007; Herman et al., 2015). Even in the absence of ions, asymmetric distribution of lipids in the bilayer can generate a transmembrane potential (Gurtovenko and Vattulainen, 2007). As a consequence, it is technically challenging to uncouple membrane potential and asymmetry. Of note, the available theoretical and experimental evidence related to the electrostatic potential and the organization of cell membranes was recently reviewed (Malinsky et al., 2016).
On their own, extrinsic factors do not have the capability to control all plasma membrane processes. Hence, more holistic hypotheses combining intrinsic and extrinsic factors are needed. In the following section, we will briefly describe a more general concept and five most popular models. A reader will find more detailed descriptions of these models and some alternative views in recently published reviews (e.g., Lingwood and Simons, 2010; Owen et al., 2010; Klammt and Lillemeier, 2012; Klotzsch and Schütz, 2013; Nicolson, 2014; Rao and Mayor, 2014; Mouritsen and Bagatolli, 2015; Sevcsik and Schütz, 2016).
Plasma Membrane Organisation–General Models and Concepts
Let us begin this section with a brief inspection of the mobility of membrane components. This will indicate how simple concepts highlighting intrinsic properties, namely viscosity and continuity can, to some extent, explain certain puzzles related to the plasma membrane organization and function. Measurements of lateral diffusion of membrane components over the last few decades uncovered much slower molecular mobility of molecules in cell membranes compared to their model counterparts (Wier and Edidin, 1986; Jacobson et al., 1987; Lippincott-Schwartz et al., 2001). On average, lipid tracers (e.g., DiI or BODIPY-DPPE) diffuse about four times faster in model membranes than in the plasma membrane of living cells (Box 1; Table 1). This difference can be explained by the compositional complexity of the plasma membrane. The large proportion of lipids with long and saturated acyl chains and cholesterol (van Meer et al., 2008) cause a higher rigidity (Sezgin et al., 2015) and, thereby, viscosity of membranes (Kucik et al., 1999). In addition, the presence of integral membrane proteins further increases the local viscosity in their immediate environment, which reduces the mobility of membrane constituents in general (Peters and Cherry, 1982; Chazotte and Hackenbrock, 1988; Frick et al., 2007; Saxton, 2008; Niemelä et al., 2010). A plethora of lipid-lipid and lipid-protein interactions, heterogeneities in general, can further contribute to this reduction in mobility.
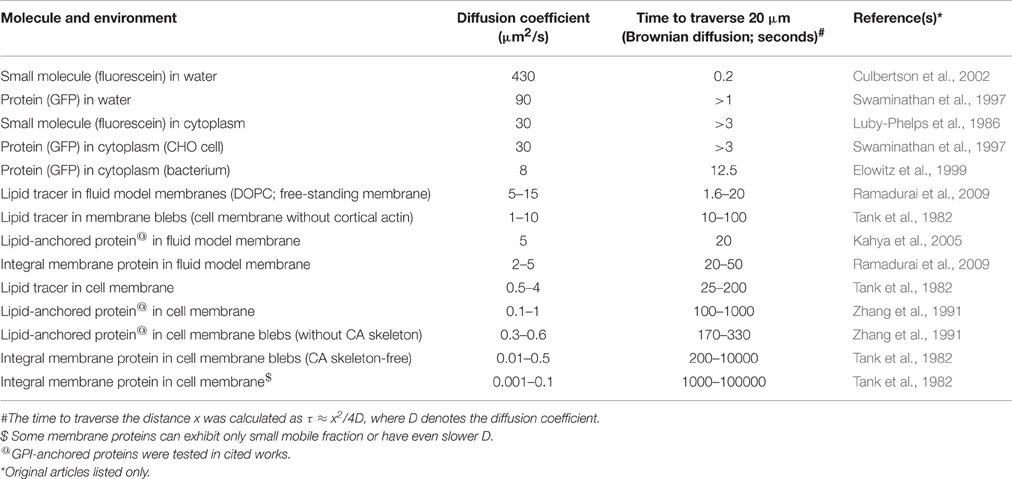
Table 1. Examples of diffusion coefficients and their translation to the times needed to traverse a distance of 20 μm (e.g., HeLa cell).
Therefore, intrinsic properties, particularly viscosity, can be responsible for the reduced long-range diffusion rates measured for lipids in cell membranes. Since membranes are continuous, all of its lipid components should be influenced similarly and equally throughout the whole area. For lipids which do not comply with this statement, localization and mobility is regulated by other factors such as proteins interacting with charged lipid headgroups, endocytosis,…etc. This simple concept works for lipids. But the extremely slow mobility of many plasma membrane proteins—one-to-two orders of magnitude lower compared to model membranes–calls for a more elaborate explanation.
Fluid Mosaic Model (SN Model; Figure 5A). The SN model in a large detail summarizes the understanding of the plasma membrane composition, structure and thermodynamics 45 years ago (Singer and Nicolson, 1972). The emphasis is placed on the fluidity of the membrane and coexistence of lipids and proteins in this essential cellular structures. We have already described crucial issues of this model in the previous sections. Here, we would like to underline that the word “mosaic” in the SN model was primarily used to accent a mixed character of cell membranes where diverse lipids and proteins coexist in a single lamellar structure. Later, this was frequently misinterpreted as homogeneous or random distribution of molecules. But heterogeneity of cell membranes was observed and reported as early as in 1960s (Figures 4A,B; Cerottini and Brunner, 1967; Aoki et al., 1969; Kourilsky et al., 1971; Nicolson et al., 1971). Indeed, Nicolson described putative mechanisms responsible for clustering of proteins (or formation of domains) in his pillar work already in 1979 (Nicolson, 1979 and Figure 4 therein). These assumptions are still valid almost 40 years later (Nicolson, 2014).
Hydrodynamic Model (Figure 5B). The mobility of transmembrane proteins and their aggregates in cell membranes can be defined by the hydrodynamic model (Saffman and Delbrück, 1975). This model hypothesizes that molecular diffusion rates depend mainly on membrane viscosity and thickness, and only weakly on the size of proteins and aggregates. This model was later updated many times (e.g., for arbitrary viscosity of membranes and solutes (Hughes et al., 1981) or asymmetric membranes Evans and Sackmann, 1988), and experimentally confirmed in model membranes (e.g., Ramadurai et al., 2009). However, it applies only for freely moving molecules absent of interactions with objects which do not co-diffuse as a single entity. The model is further limited by the density of objects in the membrane and their lipid environment. First, the presence of slowly moving obstacles and molecular crowding can strongly influence the mobility of membrane components (Saxton, 2008; Guigas and Weiss, 2015). Second, lipids in the vicinity of TMDs of integral membrane proteins (annular lipids or lipid shells) exhibit reduced lateral diffusion (Meier et al., 1987; Anderson and Jacobson, 2002). This is probably caused by the fact that TMDs form relatively large and rigid structure in the bilayer (Meier et al., 1987; Niemelä et al., 2010) but also due to the rough surface of TMDs. Therefore, the complexity of cell membranes evidently does not allow the application of hydrodynamic model or its variants as a general model of the plasma membrane organization. Nevertheless, it can provide a useful alternative to more complex models for local changes (nanoscale; see below).
Self-Assemblies of Lipids and Ordered Lipid Domains (Figure 5C). Observation of protein clusters (DePierre and Karnovsky, 1973), lipid segregation (Shimshick and McConnell, 1973a,b; Klausner et al., 1980) and heterogeneous distribution of certain lipids and proteins between apical and basal membranes of polarized cells (van Meer and Simons, 1982) led to the suggestions that lipids and their self-assemblies can determine the fate of newly synthesized or recycled membrane molecules (Karnovsky et al., 1982; Simons and van Meer, 1988). This concept was modified by Simons and Ikonen (Simons and Ikonen, 1997) who proposed “lipid rafts” as the plasma membrane platforms of high molecular order enriched in cholesterol and sphingolipids, in which proteins involved in signaling can selectively interact with effector molecules. In parallel, biochemical analyses revealed inefficient solubilisation of some, but not all, membrane proteins and lipids in mild detergents, forming the basis of detergent resistant membranes (DRMs). Throughout the years, the ordered lipid character of “model lipid rafts” was emphasized and suggested to correspond to domains present in the plasma membrane of cells. All these terms, lipid rafts, DRMs and ordered lipid domains, were used inconsistently and frequently led to misinterpretations which were highlighted in recent reviews (Cebecauer et al., 2009; Owen et al., 2010; Kraft, 2013; Sevcsik and Schütz, 2016). In addition, the data supporting spontaneous formation of lipid domains in living cells are rather controversial and inconclusive (e.g., Eggeling et al., 2009; Brameshuber et al., 2010; Owen et al., 2012; Honigmann et al., 2014; Sevcsik et al., 2015). On the other hand, an undisputable capacity of certain lipids (e.g., gangliosides) to self-aggregate (Fujita et al., 2007; Chen et al., 2008), anomalous diffusion and/or distribution of lipids in highly complex mixtures (Kusumi et al., 2005; Eggeling et al., 2009; He and Marguet, 2011; Jeon et al., 2012) and spontaneous formation of fluid nanoclusters (van Zanten et al., 2010; Amaro et al., 2016) were demonstrated in silico, in model membranes as well as in living cells. Such fluctuations can potentially contribute to the overall heterogeneity of the plasma membrane and the peculiar mobility of certain lipids and proteins therein. Yet, the direct observation of such anomaly remains challenging due the required spatial and temporal resolution to disclose molecular-scale objects at sub-millisecond rates, albeit recent advances in super-resolution optical microscopy and ultrafast single-molecule tracking indicate remedies to this limitation.
Mattress Model (Figure 5D). As mentioned above, lipids in the vicinity of TMDs exhibit abnormal behavior (Lee, 2004; Niemelä et al., 2010), particularly in cell membranes with a large variety of lipid species and TMDs. The average membrane hydrophobic thickness increases between the ER, Golgi apparatus and plasma membrane (Mitra et al., 2004). During protein translation, proteins with long TMDs are incorporated into the relatively thin membrane of the ER, causing hydrophobic mismatch. Lipids with longer and saturated acyl chains can form metastable shells surrounding such TMDs, thereby generating heterogeneity in the membrane of the ER. At a larger scale, hydrophobic mismatch was proposed to induce the formation of lipid/protein domains also in the plasma membrane (Mouritsen and Bloom, 1993; Anderson and Jacobson, 2002; Kaiser et al., 2011). Significant impact of hydrophobic mismatch is well-documented for the sorting of proteins in cell membranes (Munro, 1995; Sharpe et al., 2010; Chum et al., 2016). But whether similar “sorting” of lipids and proteins due to hydrophobic mismatch contributes to the nanoscale organization of the plasma membrane in living cells has so far not been experimentally proven, mainly due to aforementioned limitations on spatial and temporal resolution of potential direct observation methods.
Cortical Actin Skeleton (Figure 5E). Membrane-proximal positioning of the CA skeleton and its direct association with the plasma membrane via actin-binding proteins or complexes makes it the first-hand structure to influence the mobility of plasma membrane molecules and their lateral organization. Indeed, the actin skeleton was demonstrated to affect membrane molecules in numerous works employing a variety of experimental approaches (e.g., Golan and Veatch, 1980; Sheetz et al., 1980; Tank et al., 1982; Fujiwara et al., 2002; Ritchie et al., 2003; Murase et al., 2004; Mueller et al., 2011; Andrade et al., 2015). The effect of the CA skeleton is to date the most accepted model for membrane organization, independent of whether we speak about indirect sterical hindrance (picket-and-fence model; (Koppel et al., 1981; Jacobson et al., 1984; Sako and Kusumi, 1995; Machta et al., 2011) or direct interactions of proteins with the CA skeleton (Saxton, 1990; Sheetz et al., 2006; Mueller et al., 2011; Rao and Mayor, 2014). Its undisputable impact was described in more detail in current reviews (Kusumi et al., 2010; Rao and Mayor, 2014). On the other hand, the CA skeleton provides a good explanation for many, but probably not all membrane-associated phenomena (see below).
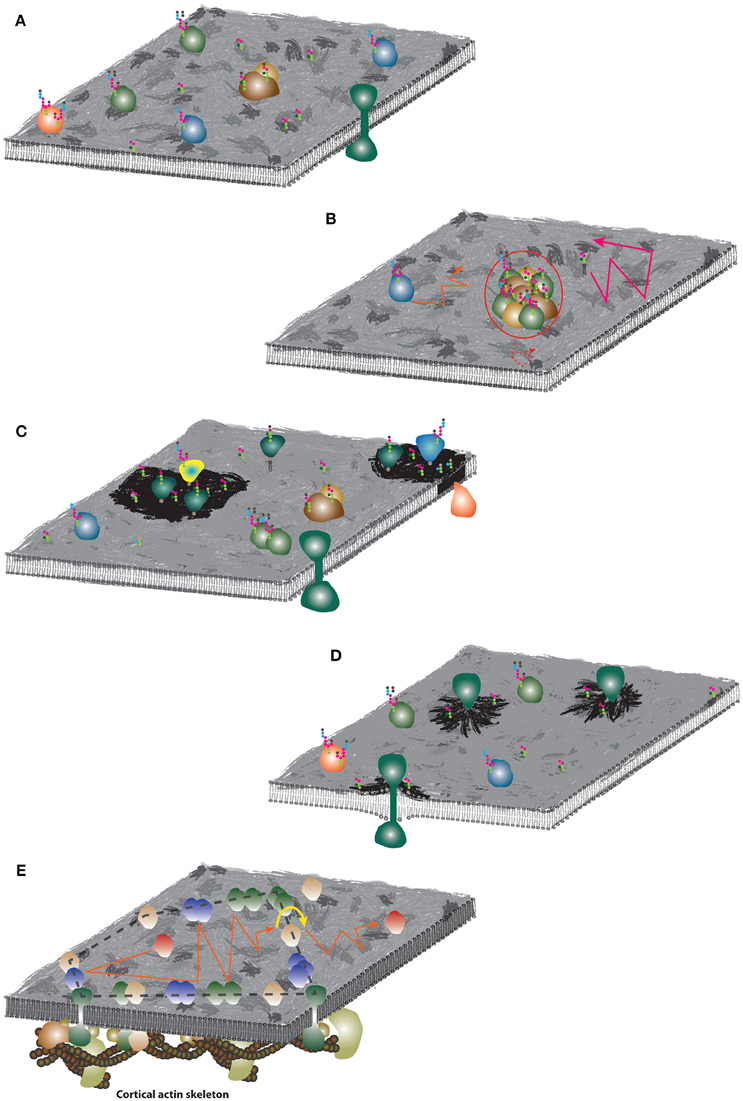
Figure 5. Schematic illustrations of the plasma membrane organization models. (A) Fluid mosaic model. The membrane surface was artistically decorated to indicate non-homogenous distribution of molecules. Colored objects represent various species of membrane proteins, strings of colored hexagons illustrate glycosylation of proteins and lipids. (B) Hydrodynamic model. Similar mobility of lipids and proteins are indicated by orange and pink trajectories. Large assemblies (red circle) with significantly larger radius can exhibit slower diffusion (dashed red trajectory). (C) Lipid membrane domains. Dark membrane patches indicate lipid self-assemblies and different lipid (and protein) composition. (D) Mattress model. Dark membrane patches indicate accumulation of lipid species due to increased hydrophobic length of protein TMDs. (E) Picket-and-fence model. Accumulation of proteins around the underlying CA skeleton and formation of fences (dashed black line) which may restrict “free” diffusion of non-associated proteins (red) to a limited area (red trajectory). For long-distance mobility, proteins have to “hop” over the fence (yellow arrow-line) which limits their long-range diffusion coefficient.
There is No Universal Model of the Plasma Membrane Lateral Organisation
Models listed in the previous sections, better or worse, contribute to the overall understanding how cells potentially organize molecules in their plasma membrane. Some of these models passed through their glorious periods, in which almost any article assumed the applicability of this one particular mechanism for the function and/or organization of the studied membrane molecule(s). A handful of recent experimental work (e.g., Kenworthy et al., 2004; Frisz et al., 2013; Honigmann et al., 2014; Letschert et al., 2014; Sevcsik et al., 2015; Wilson et al., 2015) and reviews (Kraft, 2013; Sevcsik and Schütz, 2016) argue against these universal theories. Improvements in technology for observing membrane studies have more and more reduced the affection for such a single, universal theory. A dynamic and complex plasma membrane is the environment where all molecules play in concert to achieve the optimal physiological output.
As a metaphor, one can think of human society. Similar to cell membranes, it is highly complex and dynamic, with activities difficult to investigate. As an example one can consider clustering. “Clustering” occurs in human society at the nanoscale (e.g., families), mesoscale (e.g., clubs, classes or other small interest groups), or macroscale (e.g., villages, cities, states). The formation of such “clusters” depends on intrinsic properties like affection or animosity, the local or global economic situation, but also the health and mobility of the individuals. As an analogy for extrinsic parameters we may consider the environmental situation (sunshine/rain, drought/flooding), local factors (alpine landscape vs. influence of the sea), but also the interaction with other “clusters.” As we know from experience, social systems may develop rather stable phenotypes at the macroscale (e.g., the current western society), which are still characterized by high dynamics at the nano—or mesoscale. On the contrary, there are periods in history, in which no stable situation was reached for many years. Our point is, that it is virtually impossible to predict the behavior of a large society from simple models, even if the intrinsic and extrinsic parameters are well-known at high detail. Or, if we return to the topic of cell membranes: currently, it seems impossible to explain the plasma membrane organization based on individual models described in the previous section. Hence, future challenges will include the clever combination of this principle models into more holistic meta-models to increase their predictive power. Or, in the other words, we believe there is no simple, universal mechanism underlying the organization of the plasma membrane of mammalian cells.
Why we believe this is so? And what are the consequences?
Starting with the first question, one has to look at the sections with the lists of intrinsic and extrinsic factors influencing the behavior of molecules in the plasma membrane. Both, intrinsic and extrinsic factors are highly interconnected and can occur at the same time or, more probably, in rapid, sequential events. If intrinsic properties should be considered as rather general factors, to which all molecules must adapt, extrinsic factors may have more specific effects. Tuning of intrinsic properties (e.g., fluidity or viscosity) requires significant changes in molecular composition. This can rapidly occur locally (at the nanoscale) and transiently (sub-second), but would require substantial costs of energy to induce large-scale and more stable changes. On the contrary, extrinsic factors (e.g., the CA skeleton or glycocalyx) can affect larger surface areas for longer periods of time with higher efficiency. It is, therefore, probably a combination of these factors which regulates behavior of molecules in the plasma membrane at a full spectrum of spatial and temporal scales.
This brings us to the second question about the consequences of the non-existence of omnipotent, universal model applicable to all plasma membrane components and events. First, when interpreting data acquired during the analysis of cell membranes, one should not ignore intrinsic membrane properties. Even though less visible (detectable), these form the basis of membrane organization and function. Extrinsic factors are important but may be consequential. In order to fully understand membrane-associated processes and avoid undesirable borders of a single theory, a careful analysis of sequential events, which may lead to the observed effect, needs to be performed (Box 2).
Box 2. Sequential Events Influencing the Plasma Membrane Organisation
Imaging techniques are excellent tools to monitor changes of the plasma membrane organization. High details can be explored using current advanced techniques (Eggeling, 2015). But all methods suffer from the fact that the preeminent feature (e.g., CA skeleton reorganization) can hide one or more less well detectable events (e.g., changes in local viscosity) accompanying an observed process. In some cases, these undetectable fluctuations may be the determining factors or triggers of a transformation process.
To illustrate the consequences of the abovementioned limitation(s), let's imagine a putative membrane-associated process: A ligand binds to its receptor which is followed by receptor oligomerisation or nanoscale clustering. Such increased protein density causes increased membrane viscosity which, in turn, reduces mobility of molecules in the vicinity of a cluster (Peters and Cherry, 1982; Niemelä et al., 2010). As a consequence, an actin-binding protein can collide with a receptor cluster, enhance low-affinity interactions by crosslinking cluster components and trigger CA skeleton reorganization. It is the last event which will stabilize the overall structure and, at the same time, it is the best detectable feature of this imaginary process. But the interpretation that the CA skeleton is responsible for the observed changes is only part of the story. In this case, the ability to detect small-scale viscosity fluctuations would help to better understand such process. Unfortunately, at present, such tools are not available for living cells.
Another concern with the interpretation of membrane-focused data is the systemic use of chemical and genetic tools as a proof of one or the other model of the plasma membrane organization. Specific side-effects of some of these treatments (e.g., detergents, methyl-β-cyclodextrin, cytochalasin D or temperature changes) have been described in past (Ailenberg and Silverman, 2003; Lichtenberg et al., 2005; Magee et al., 2005; Shvartsman et al., 2006; Zidovetzki and Levitan, 2007). Due to the fluidity and cooperativity, systemic treatment (both, chemical and genetic) will often influence the behavior of many (if not all) molecules present in or associated with the membrane, instead of only specific ones. In addition, the procedure of observing the system may potentially introduce artifacts, for example labels or intense light sources employed in fluorescence microscopy (Sezgin et al., 2012; Magidson and Khodjakov, 2013). Therefore, employment of treatments or observation techniques requires cautious interpretation and experiments performed with extensive number of controls. Leaving space for alternative interpretations and emphasis on possible side-effects should be a good practice in this kind of works.
In summary, we provide here a comprehensive list of membrane features and peripheral structures which were previously demonstrated or proposed to control lateral mobility and organization of the plasma membrane in mammalian cells. We also offer alternative views how to interpret results measured on the plasma membrane of living cells. We re-emphasize the impact of the intrinsic membrane properties which were discovered and characterized more than 20 years ago but were sometimes overlooked in more recent works. We finish with the hope that development of novel improved observation techniques such as fast single-molecule tracking (Ritchie et al., 2005; Ortega-Arroyo and Kukura, 2012), TOCSSL (Brameshuber et al., 2010), STED-FCS (Eggeling et al., 2009; Mueller et al., 2013; Eggeling, 2015), iMSD or related image correlation techniques (Hebert et al., 2005; Digman et al., 2009; Di Rienzo et al., 2013), will be rewarded with a more precise information about players responsible for the uniqueness of the plasma membrane. In case the improvements will be still insufficient, we should probably overpass the barrier (obstacle) between researchers studying mammalian cells and those focused on yeasts and plants. These organisms own membranes which behave much friendlier on temporal scale compared to the plasma membrane of mammalian cells. Such membranes are highly heterogeneous and can be imaged with the use of existing methods (Malínská et al., 2003; Spira et al., 2012). Cell cycle regulation and RNA interference were also discovered in yeast and plants.
Author Contributions
JBdlS, GS, CE, and MC defined the topic and wrote the manuscript.
Funding
This work was funded by Czech Science Foundation (15-06989S; MC), the Medical Research Council (MRC, grant number MC_UU_12010/unit programmes G0902418 and MC_UU_12025; CE), Marie Curie Career Integration Grant (JBdlS), and the Austrian Science Fund (FWF projects P 26337-B21, P 25730-B21).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Marie Olsinova, Daniela Glatzova, Tony Magee, Martin Hof, Lukasz Cwiklik, Piotr Jurkiewicz, and Tomas Chum for critical discussions which led to writing of this article.
References
Ailenberg, M., and Silverman, M. (2003). Cytochalasin D disruption of actin filaments in 3T3 cells produces an anti-apoptotic response by activating gelatinase A extracellularly and initiating intracellular survival signals. Biochim. Biophys. Acta 1593, 249–258. doi: 10.1016/S0167-4889(02)00395-6
Aimon, S., Callan-Jones, A., Berthaud, A., Pinot, M., Toombes, G. E., and Bassereau, P. (2014). Membrane shape modulates transmembrane protein distribution. Dev. Cell 28, 212–218. doi: 10.1016/j.devcel.2013.12.012
Amaro, M., Šachl, R., Aydogan, G., Mikhalyov, II., Vácha, R., and Hof, M. (2016). GM1 Ganglioside inhibits β-Amyloid oligomerization induced by Sphingomyelin. Angew. Chem. 55, 9411–9415. doi: 10.1002/anie.201603178
Anderson, M. J., and Fambrough, D. M. (1983). Aggregates of acetylcholine receptors are associated with plaques of a basal lamina heparan sulfate proteoglycan on the surface of skeletal muscle fibers. J. Cell Biol. 97(5 Pt 1), 1396–1411. doi: 10.1083/jcb.97.5.1396
Anderson, R. G., and Jacobson, K. (2002). A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science 296, 1821–1825. doi: 10.1126/science.1068886
Andrade, D. M., Clausen, M. P., Keller, J., Mueller, V., Wu, C., Bear, J. E., et al. (2015). Cortical actin networks induce spatio-temporal confinement of phospholipids in the plasma membrane–a minimally invasive investigation by STED-FCS. Sci. Rep. 5:11454. doi: 10.1038/srep11454
Aoki, T., Hammerling, U., De Harven, E., Boyse, E. A., and Old, L. J. (1969). Antigenic structure of cell surfaces. An immunoferritin study of the occurrence and topography of H-2′ theta, and TL alloantigens on mouse cells. J. Exp. Med. 130, 979–1001. doi: 10.1084/jem.130.5.979
Bagatolli, L. A., and Gratton, E. (1999). Two-photon fluorescence microscopy observation of shape changes at the phase transition in phospholipid giant unilamellar vesicles. Biophys. J. 77, 2090–2101. doi: 10.1016/S0006-3495(99)77050-5
Balda, M. S., and Matter, K. (2008). Tight junctions at a glance. J. Cell Sci. 121(Pt 22), 3677–3682. doi: 10.1242/jcs.023887
Bass, M. D., Roach, K. A., Morgan, M. R., Mostafavi-Pour, Z., Schoen, T., Muramatsu, T., et al. (2007). Syndecan-4-dependent Rac1 regulation determines directional migration in response to the extracellular matrix. J. Cell Biol. 177, 527–538. doi: 10.1083/jcb.200610076
Baumgart, T., Hammond, A. T., Sengupta, P., Hess, S. T., Holowka, D. A., Baird, B. A., et al. (2007). Large-scale fluid/fluid phase separation of proteins and lipids in giant plasma membrane vesicles. Proc. Natl. Acad. Sci. U.S.A. 104, 3165–3170. doi: 10.1073/pnas.0611357104
Bernardino de la Serna, J., Perez-Gil, J., Simonsen, A. C., and Bagatolli, L. A. (2004). Cholesterol rules: direct observation of the coexistence of two fluid phases in native pulmonary surfactant membranes at physiological temperatures. J. Biol. Chem. 279, 40715–40722. doi: 10.1074/jbc.M404648200
Berrier, A. L., and Yamada, K. M. (2007). Cell-matrix adhesion. J. Cell. Physiol. 213, 565–573. doi: 10.1002/jcp.21237
Björkbom, A., Róg, T., Kaszuba, K., Kurita, M., Yamaguchi, S., Lönnfors, M., et al. (2010). Effect of sphingomyelin headgroup size on molecular properties and interactions with cholesterol. Biophys. J. 99, 3300–3308. doi: 10.1016/j.bpj.2010.09.049
Botelho, R. J., Teruel, M., Dierckman, R., Anderson, R., Wells, A., York, J. D., et al. (2000). Localized biphasic changes in phosphatidylinositol-4,5-bisphosphate at sites of phagocytosis. J. Cell Biol. 151, 1353–1368. doi: 10.1083/jcb.151.7.1353
Bozic, B., Kralj-Iglic, V., and Svetina, S. (2006). Coupling between vesicle shape and lateral distribution of mobile membrane inclusions. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 73(4 Pt 1):041915. doi: 10.1103/PhysRevE.73.041915
Brameshuber, M., Weghuber, J., Ruprecht, V., Gombos, I., Horváth, I., Vigh, L., et al. (2010). Imaging of mobile long-lived nanoplatforms in the live cell plasma membrane. J. Biol. Chem. 285, 41765–41771. doi: 10.1074/jbc.M110.182121
Buda, C., Dey, I., Balogh, N., Horvath, L. I., Maderspach, K., Juhasz, M., et al. (1994). Structural order of membranes and composition of phospholipids in fish brain-cells during thermal acclimatization. Proc. Natl. Acad. Sci. U.S.A. 91, 8234–8238. doi: 10.1073/pnas.91.17.8234
Callan-Jones, A., Sorre, B., and Bassereau, P. (2011). Curvature-driven lipid sorting in biomembranes. Cold Spring Harb. Perspect. Biol. 3:a004648. doi: 10.1101/cshperspect.a004648
Canagarajah, B. J., Hummer, G., Prinz, W. A., and Hurley, J. H. (2008). Dynamics of cholesterol exchange in the oxysterol binding protein family. J. Mol. Biol. 378, 737–748. doi: 10.1016/j.jmb.2008.01.075
Cantor, R. S. (1999). Lipid composition and the lateral pressure profile in membranes. Biophys. J. 76, A58–A58. doi: 10.1016/S0006-3495(99)77415-1
Capponi, S., Freites, J. A., Tobias, D. J., and White, S. H. (2016). Interleaflet mixing and coupling in liquid-disordered phospholipid bilayers. Biochim. Biophys. Acta 1858, 354–362. doi: 10.1016/j.bbamem.2015.11.024
Cebecauer, M., Owen, D. M., Markiewicz, A., and Magee, A. I. (2009). Lipid order and molecular assemblies in the plasma membrane of eukaryotic cells. Biochem. Soc. Trans. 37(Pt 5), 1056–1060. doi: 10.1042/BST0371056
Cebecauer, M., Spitaler, M., Sergè, A., and Magee, A. I. (2010). Signalling complexes and clusters: functional advantages and methodological hurdles. J. Cell Sci. 123(Pt 3), 309–320. doi: 10.1242/jcs.061739
Cerottini, J. C., and Brunner, K. T. (1967). Localization of mouse isoantigens on the cell surface as revealed by immunofluorescence. Immunology 13, 395–403.
Chazotte, B., and Hackenbrock, C. R. (1988). The multicollisional, obstructed, long-range diffusional nature of mitochondrial electron transport. J. Biol. Chem. 263, 14359–14367.
Chen, Y., Qin, J., and Chen, Z. W. (2008). Fluorescence-topographic NSOM directly visualizes peak-valley polarities of GM1/GM3 rafts in cell membrane fluctuations. J. Lipid Res. 49, 2268–2275. doi: 10.1194/jlr.D800031-JLR200
Chiantia, S., Schwille, P., Klymchenko, A. S., and London, E. (2011). Asymmetric GUVs prepared by MbetaCD-mediated lipid exchange: an FCS study. Biophys. J. 100, L1–L3. doi: 10.1016/j.bpj.2010.11.051
Chum, T., Glatzova, D., Kvicalova, Z., Malinsky, J., Brdicka, T., and Cebecauer, M. (2016). The role of palmitoylation and transmembrane domain in sorting of transmembrane adaptor proteins. J. Cell Sci. 129, 95–107. doi: 10.1242/jcs.194209
Collins, M. D., and Keller, S. L. (2008). Tuning lipid mixtures to induce or suppress domain formation across leaflets of unsupported asymmetric bilayers. Proc. Natl. Acad. Sci. U.S.A. 105, 124–128. doi: 10.1073/pnas.0702970105
Contreras, F. X., Ernst, A. M., Haberkant, P., Björkholm, P., Lindahl, E., Gönen, B., et al. (2012). Molecular recognition of a single sphingolipid species by a protein's transmembrane domain. Nature 481, 525–529. doi: 10.1038/nature10742
Contreras, F. X., Ernst, A. M., Wieland, F., and Brügger, B. (2011). Specificity of intramembrane protein-lipid interactions. Cold Spring Harb. Perspect. Biol. 3:a004705. doi: 10.1101/cshperspect.a004705
Cortizo, A. M., Paladini, A., Díaz, G. B., García, M. E., and Gagliardino, J. J. (1990). Changes induced by glucose in the plasma membrane properties of pancreatic islets. Mol. Cell. Endocrinol. 71, 49–54. doi: 10.1016/0303-7207(90)90074-I
Crawley, S. W., Mooseker, M. S., and Tyska, M. J. (2014). Shaping the intestinal brush border. J. Cell Biol. 207, 441–451. doi: 10.1083/jcb.201407015
Culbertson, C. T., Jacobson, S. C., and Michael Ramsey, J. (2002). Diffusion coefficient measurements in microfluidic devices. Talanta 56, 365–373. doi: 10.1016/S0039-9140(01)00602-6
DePierre, J. W., and Karnovsky, M. L. (1973). Plasma membranes of mammalian cells: a review of methods for their characterization and isolation. J. Cell Biol. 56, 275–303. doi: 10.1083/jcb.56.2.275
Devaux, P. F., Herrmann, A., Ohlwein, N., and Kozlov, M. M. (2008). How lipid flippases can modulate membrane structure. Biochim. Biophys. Acta 1778, 1591–1600. doi: 10.1016/j.bbamem.2008.03.007
Digman, M. A., Wiseman, P. W., Horwitz, A. R., and Gratton, E. (2009). Detecting protein complexes in living cells from laser scanning confocal image sequences by the cross correlation raster image spectroscopy method. Biophys. J. 96, 707–716. doi: 10.1016/j.bpj.2008.09.051
Di Rienzo, C., Gratton, E., Beltram, F., and Cardarelli, F. (2013). Fast spatiotemporal correlation spectroscopy to determine protein lateral diffusion laws in live cell membranes. Proc. Natl. Acad. Sci. U.S.A. 110, 12307–12312. doi: 10.1073/pnas.1222097110
Douglass, A. D., and Vale, R. D. (2005). Single-molecule microscopy reveals plasma membrane microdomains created by protein-protein networks that exclude or trap signaling molecules in T cells. Cell 121, 937–950. doi: 10.1016/j.cell.2005.04.009
Dupuy, A. D., and Engelman, D. M. (2008). Protein area occupancy at the center of the red blood cell membrane. Proc. Natl. Acad. Sci. U.S.A. 105, 2848–2852. doi: 10.1073/pnas.0712379105
Duzgunes, N., Newton, C., Fisher, K., Fedor, J., and Papahadjopoulos, D. (1988). Monolayer coupling in phosphatidylserine bilayers: distinct phase transitions induced by magnesium interacting with one or both monolayers. Biochim. Biophys. Acta 944, 391–398. doi: 10.1016/j.jcis.2016.09.007
Edwards, S. W., Tan, C. M., and Limbird, L. E. (2000). Localization of G-protein-coupled receptors in health and disease. Trends Pharmacol. Sci. 21, 304–308. doi: 10.1016/S0165-6147(00)01513-3
Eggeling, C. (2015). Super-resolution optical microscopy of lipid plasma membrane dynamics. Essays Biochem. 57, 69–80. doi: 10.1042/bse0570069
Eggeling, C., Ringemann, C., Medda, R., Schwarzmann, G., Sandhoff, K., Polyakova, S., et al. (2009). Direct observation of the nanoscale dynamics of membrane lipids in a living cell. Nature 457, 1159–1162. doi: 10.1038/nature07596
Elowitz, M. B., Surette, M. G., Wolf, P. E., Stock, J. B., and Leibler, S. (1999). Protein mobility in the cytoplasm of Escherichia coli. J. Bacteriol. 181, 197–203.
Ernst, A. M., Contreras, F. X., Brügger, B., and Wieland, F. (2010). Determinants of specificity at the protein-lipid interface in membranes. FEBS Lett. 584, 1713–1720. doi: 10.1016/j.febslet.2009.12.060
Evans, E., and Sackmann, E. (1988). Translational and rotational drag coefficients for a disk moving in a liquid membrane-associated with a rigid substrate. J. Fluid Mech. 194, 553–561. doi: 10.1017/S0022112088003106
Fantini, J., and Barrantes, F. J. (2013). How cholesterol interacts with membrane proteins: an exploration of cholesterol-binding sites including CRAC, CARC, and tilted domains. Front. Physiol. 4:31. doi: 10.3389/fphys.2013.00031
Fernández-Busnadiego, R., Saheki, Y., and De Camilli, P. (2015). Three-dimensional architecture of extended synaptotagmin-mediated endoplasmic reticulum-plasma membrane contact sites. Proc. Natl. Acad. Sci. U.S.A. 112, E2004–E2013. doi: 10.1073/pnas.1503191112
Fraenkel, G., and Hopf, H. S. (1940). The physiological action of abnormally high temperatures on poikilothermic animals: temperature adaptation and the degree of saturation of the phosphatides. Biochem. J. 34, 1085–1092. doi: 10.1042/bj0341085
Frick, M., Schmidt, K., and Nichols, B. J. (2007). Modulation of lateral diffusion in the plasma membrane by protein density. Curr. Biol. 17, 462–467. doi: 10.1016/j.cub.2007.01.069
Frisz, J. F., Klitzing, H. A., Lou, K., Hutcheon, I. D., Weber, P. K., Zimmerberg, J., et al. (2013). Sphingolipid domains in the plasma membranes of fibroblasts are not enriched with cholesterol. J. Biol. Chem. 288, 16855–16861. doi: 10.1074/jbc.M113.473207
Frolov, V. A., Bashkirov, P. V., Akimov, S. A., and Zimmerberg, J. (2010). Membrane curvature and fission by dynamin: mechanics, dynamics and partners. Biophys. J. 98:2a. doi: 10.1016/j.bpj.2009.12.012
Fujita, A., Cheng, J., Hirakawa, M., Furukawa, K., Kusunoki, S., and Fujimoto, T. (2007). Gangliosides GM1 and GM3 in the living cell membrane form clusters susceptible to cholesterol depletion and chilling. Mol. Biol. Cell 18, 2112–2122. doi: 10.1091/mbc.E07-01-0071
Fujiwara, T., Ritchie, K., Murakoshi, H., Jacobson, K., and Kusumi, A. (2002). Phospholipids undergo hop diffusion in compartmentalized cell membrane. J. Cell Biol. 157, 1071–1081. doi: 10.1083/jcb.200202050
Gaffield, M. A., Tabares, L., and Betz, W. J. (2009). Preferred sites of exocytosis and endocytosis colocalize during high- but not lower-frequency stimulation in mouse motor nerve terminals. J. Neurosci. 29, 15308–15316. doi: 10.1523/JNEUROSCI.4646-09.2009
Garcia-Parajo, M. F., Cambi, A., Torreno-Pina, J. A., Thompson, N., and Jacobson, K. (2014). Nanoclustering as a dominant feature of plasma membrane organization. J. Cell Sci. 127, 4995–5005. doi: 10.1242/jcs.146340
Golan, D. E., and Veatch, W. (1980). Lateral mobility of band 3 in the human erythrocyte membrane studied by fluorescence photobleaching recovery: evidence for control by cytoskeletal interactions. Proc. Natl. Acad. Sci. U.S.A. 77, 2537–2541. doi: 10.1073/pnas.77.5.2537
Golebiewska, U., Kay, J. G., Masters, T., Grinstein, S., Im, W., Pastor, R. W., et al. (2011). Evidence for a fence that impedes the diffusion of phosphatidylinositol 4,5-bisphosphate out of the forming phagosomes of macrophages. Mol. Biol. Cell 22, 3498–3507. doi: 10.1091/mbc.E11-02-0114
Golebiewska, U., Nyako, M., Woturski, W., Zaitseva, I., and McLaughlin, S. (2008). Diffusion coefficient of fluorescent phosphatidylinositol 4,5-bisphosphate in the plasma membrane of cells. Mol. Biol. Cell 19, 1663–1669. doi: 10.1091/mbc.E07-12-1208
Gowrishankar, K., Ghosh, S., Saha, S., Rumamol, C., Mayor, S., and Rao, M. (2012). Active remodeling of cortical actin regulates spatiotemporal organization of cell surface molecules. Cell 149, 1353–1367. doi: 10.1016/j.cell.2012.05.008
Grecco, H. E., Schmick, M., and Bastiaens, P. I. (2011). Signaling from the living plasma membrane. Cell 144, 897–909. doi: 10.1016/j.cell.2011.01.029
Grossmann, G., Opekarová, M., Malinsky, J., Weig-Meckl, I., and Tanner, W. (2007). Membrane potential governs lateral segregation of plasma membrane proteins and lipids in yeast. EMBO J. 26, 1–8. doi: 10.1038/sj.emboj.7601466
Guigas, G., and Weiss, M. (2015). Effects of protein crowding on membrane systems. Biochim. Biophys. Acta 1858, 2441–2450. doi: 10.1016/j.bbamem.2015.12.021
Gurtovenko, A. A., and Vattulainen, I. (2007). Lipid transmembrane asymmetry and intrinsic membrane potential: two sides of the same coin. J. Am. Chem. Soc. 129, 5358–5359. doi: 10.1021/ja070949m
Gut, J., Kawato, S., Cherry, R. J., Winterhalter, K. H., and Richter, C. (1985). Lipid-Peroxidation decreases the rotational mobility of Cytochrome-P-450 in Rat-Liver microsomes. Biochim. Biophys. Acta 817, 217–228. doi: 10.1016/0005-2736(85)90023-9
Haberkant, P., Schmitt, O., Contreras, F. X., Thiele, C., Hanada, K., Sprong, H., et al. (2008). Protein-sphingolipid interactions within cellular membranes. J. Lipid Res. 49, 251–262. doi: 10.1194/jlr.D700023-JLR200
Hansen, C. G., and Nichols, B. J. (2009). Molecular mechanisms of clathrin-independent endocytosis. J. Cell Sci. 122(Pt 11), 1713–1721. doi: 10.1242/jcs.033951
Hanson, M. A., Cherezov, V., Griffith, M. T., Roth, C. B., Jaakola, V. P., Chien, E. Y., et al. (2008). A specific cholesterol binding site is established by the 2.8 A structure of the human beta2-adrenergic receptor. Structure 16, 897–905. doi: 10.1016/j.str.2008.05.001
Hartel, A. J., Glogger, M., Guigas, G., Jones, N. G., Fenz, S. F., Weiss, M., et al. (2015). The molecular size of the extra-membrane domain influences the diffusion of the GPI-anchored VSG on the trypanosome plasma membrane. Sci. Rep. 5:10394. doi: 10.1038/srep10394
Hatzakis, N. S., Bhatia, V. K., Larsen, J., Madsen, K. L., Bolinger, P. Y., Kunding, A. H., et al. (2009). How curved membranes recruit amphipathic helices and protein anchoring motifs. Nat. Chem. Biol. 5, 835–841. doi: 10.1038/nchembio.213
He, H. T., and Marguet, D. (2011). Detecting nanodomains in living cell membrane by fluorescence correlation spectroscopy. Annu. Rev. Phys. Chem. 62, 417–436. doi: 10.1146/annurev-physchem-032210-103402
Hebert, B., Costantino, S., and Wiseman, P. W. (2005). Spatiotemporal image correlation spectroscopy (STICS) theory, verification, and application to protein velocity mapping in living CHO cells. Biophys. J. 88, 3601–3614. doi: 10.1529/biophysj.104.054874
Herman, P., Vecer, J., Opekarova, M., Vesela, P., Jancikova, I., Zahumensky, J., et al. (2015). Depolarization affects the lateral microdomain structure of yeast plasma membrane. FEBS J. 282, 419–434. doi: 10.1111/febs.13156
Hodgkin, A. L., and Huxley, A. F. (1952). A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 117, 500–544. doi: 10.1113/jphysiol.1952.sp004764
Honigmann, A., Mueller, V., Ta, H., Schoenle, A., Sezgin, E., Hell, S. W., et al. (2014). Scanning STED-FCS reveals spatiotemporal heterogeneity of lipid interaction in the plasma membrane of living cells. Nat. Commun. 5:5412. doi: 10.1038/ncomms6412
Hughes, B. D., Pailthorpe, B. A., and White, L. R. (1981). The translational and rotational drag on a cylinder moving in a membrane. J. Fluid Mech. 110, 349–372. doi: 10.1017/S0022112081000785
Hung, M. C., and Link, W. (2011). Protein localization in disease and therapy. J. Cell Sci. 124(Pt 20), 3381–3392. doi: 10.1242/jcs.089110
Hynes, R. O. (2009). The extracellular matrix: not just pretty fibrils. Science 326, 1216–1219. doi: 10.1126/science.1176009
Ipsen, J. H., Karlström, G., Mouritsen, O. G., Wennerström, H., and Zuckermann, M. J. (1987). Phase equilibria in the phosphatidylcholine-cholesterol system. Biochim. Biophys. Acta 905, 162–172. doi: 10.1016/0005-2736(87)90020-4
Ivankin, A., Kuzmenko, I., and Gidalevitz, D. (2010). Cholesterol-phospholipid interactions: new insights from surface x-ray scattering data. Phys. Rev. Lett. 104, 108101–108104. doi: 10.1103/PhysRevLett.104.108101
Jacobson, K., Ishihara, A., and Inman, R. (1987). Lateral diffusion of proteins in membranes. Annu. Rev. Physiol. 49, 163–175. doi: 10.1146/annurev.ph.49.030187.001115
Jacobson, K., O'Dell, D., Holifield, B., Murphy, T. L., and August, J. T. (1984). Redistribution of a major cell surface glycoprotein during cell movement. J. Cell Biol. 99, 1613–1623. doi: 10.1083/jcb.99.5.1613
Janetopoulos, C., and Firtel, R. A. (2008). Directional sensing during chemotaxis. FEBS Lett. 582, 2075–2085. doi: 10.1016/j.febslet.2008.04.035
Jaqaman, K., Kuwata, H., Touret, N., Collins, R., Trimble, W. S., Danuser, G., et al. (2011). Cytoskeletal control of CD36 diffusion promotes its receptor and signaling function. Cell 146, 593–606. doi: 10.1016/j.cell.2011.06.049
Jeon, J. H., Monne, H. M., Javanainen, M., and Metzler, R. (2012). Anomalous diffusion of phospholipids and cholesterols in a lipid bilayer and its origins. Phys. Rev. Lett. 109:188103. doi: 10.1103/PhysRevLett.109.188103
Johnson, J. L., Monfregola, J., Napolitano, G., Kiosses, W. B., and Catz, S. D. (2012). Vesicular trafficking through cortical actin during exocytosis is regulated by the Rab27a effector JFC1/Slp1 and the RhoA-GTPase-activating protein Gem-interacting protein. Mol. Biol. Cell 23, 1902–1916. doi: 10.1091/mbc.E11-12-1001
Jurkiewicz, P., Cwiklik, L., Vojtíšková, A., Jungwirth, P., and Hof, M. (2012). Structure, dynamics, and hydration of POPC/POPS bilayers suspended in NaCl, KCl, and CsCl solutions. Biochim. Biophys. Acta 1818, 609–616. doi: 10.1016/j.bbamem.2011.11.033
Kahya, N., Brown, D. A., and Schwille, P. (2005). Raft partitioning and dynamic behavior of human placental alkaline phosphatase in giant unilamellar vesicles. Biochemistry 44, 7479–7489. doi: 10.1021/bi047429d
Kahya, N., Scherfeld, D., Bacia, K., Poolman, B., and Schwille, P. (2003). Probing lipid mobility of raft-exhibiting model membranes by fluorescence correlation spectroscopy. J. Biol. Chem. 278, 28109–28115. doi: 10.1074/jbc.M302969200
Kaiser, H. J., Orlowski, A., Róg, T., Nyholm, T. K., Chai, W., Feizi, T., et al. (2011). Lateral sorting in model membranes by cholesterol-mediated hydrophobic matching. Proc. Natl. Acad. Sci. U.S.A. 108, 16628–16633. doi: 10.1073/pnas.1103742108
Karnovsky, M. J., Kleinfeld, A. M., Hoover, R. L., Dawidowicz, E. A., McIntyre, D. E., Salzman, E. A., et al. (1982). Lipid domains in membranes. Ann. N. Y. Acad. Sci. 401, 61–75. doi: 10.1111/j.1749-6632.1982.tb25707.x
Kenworthy, A. K., Nichols, B. J., Remmert, C. L., Hendrix, G. M., Kumar, M., Zimmerberg, J., et al. (2004). Dynamics of putative raft-associated proteins at the cell surface. J. Cell Biol. 165, 735–746. doi: 10.1083/jcb.200312170
Kiessling, V., Crane, J. M., and Tamm, L. K. (2006). Transbilayer effects of raft-like lipid domains in asymmetric planar bilayers measured by single molecule tracking. Biophys. J. 91, 3313–3326. doi: 10.1529/biophysj.106.091421
Klammt, C., and Lillemeier, B. F. (2012). How membrane structures control T cell signaling. Front. Immunol. 3:291. doi: 10.3389/fimmu.2012.00291
Klausner, R. D., Kleinfeld, A. M., Hoover, R. L., and Karnovsky, M. J. (1980). Lipid domains in membranes. Evidence derived from structural perturbations induced by free fatty acids and lifetime heterogeneity analysis. J. Biol. Chem. 255, 1286–1295.
Klotzsch, E., and Schütz, G. J. (2013). A critical survey of methods to detect plasma membrane rafts. Philos. Trans. R. Soc. B Biol. Sci. 368:20120033. doi: 10.1098/rstb.2012.0033
Koppel, D. E., Sheetz, M. P., and Schindler, M. (1981). Matrix control of protein diffusion in biological membranes. Proc. Natl. Acad. Sci. U.S.A. 78, 3576–3580. doi: 10.1073/pnas.78.6.3576
Korlach, J., Schwille, P., Webb, W. W., and Feigenson, G. W. (1999). Characterization of lipid bilayer phases by confocal microscopy and fluorescence correlation spectroscopy. Proc. Natl. Acad. Sci. U.S.A. 96, 8461–8466. doi: 10.1073/pnas.96.15.8461
Kourilsky, F. M., Silvestre, D., Levy, J. P., Dausset, J., Nicolai, M. G., and Senik, A. (1971). Immunoferritin study of the distribution of HL-A antigens on human blood cells. J. Immunol. 106, 454–466.
Kowalska, M. A., and Cierniewski, C. S. (1983). Microenvironment changes of human-blood platelet membranes associated with fibrinogen binding. J. Membr. Biol. 75, 57–64. doi: 10.1007/BF01870799
Kraft, M. L. (2013). Plasma membrane organization and function: moving past lipid rafts. Mol. Biol. Cell 24, 2765–2768. doi: 10.1091/mbc.E13-03-0165
Kucik, D. F., Elson, E. L., and Sheetz, M. P. (1999). Weak dependence of mobility of membrane protein aggregates on aggregate size supports a viscous model of retardation of diffusion. Biophys. J. 76(1 Pt 1), 314–322. doi: 10.1016/S0006-3495(99)77198-5
Kusumi, A., Ike, H., Nakada, C., Murase, K., and Fujiwara, T. (2005). Single-molecule tracking of membrane molecules: plasma membrane compartmentalization and dynamic assembly of raft-philic signaling molecules. Semin. Immunol. 17, 3–21. doi: 10.1016/j.smim.2004.09.004
Kusumi, A., Shirai, Y. M., Koyama-Honda, I., Suzuki, K. G., and Fujiwara, T. K. (2010). Hierarchical organization of the plasma membrane: investigations by single-molecule tracking vs. fluorescence correlation spectroscopy. FEBS Lett. 584, 1814–1823. doi: 10.1016/j.febslet.2010.02.047
Kwon, H. J., Abi-Mosleh, L., Wang, M. L., Deisenhofer, J., Goldstein, J. L., Brown, M. S., et al. (2009). Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell 137, 1213–1224. doi: 10.1016/j.cell.2009.03.049
Lee, A. G. (2004). How lipids affect the activities of integral membrane proteins. Biochim. Biophys. Acta 1666, 62–87. doi: 10.1016/j.bbamem.2004.05.012
Lee, I. H., Saha, S., Polley, A., Huang, H., Mayor, S., Rao, M., et al. (2015). Live cell plasma membranes do not exhibit a miscibility phase transition over a wide range of temperatures. J. Phys. Chem. B 119, 4450–4459. doi: 10.1021/jp512839q
Letschert, S., Göhler, A., Franke, C., Bertleff-Zieschang, N., Memmel, E., Doose, S., et al. (2014). Super-resolution imaging of plasma membrane glycans. Angew. Chem. Int. Ed Engl. 53, 10921–10924. doi: 10.1002/anie.201406045
Lev, S. (2012). Nonvesicular lipid transfer from the endoplasmic reticulum. Cold Spring Harb. Perspect. Biol. 4:a013300. doi: 10.1101/cshperspect.a013300
Lichtenberg, D., Goñi, F. M., and Heerklotz, H. (2005). Detergent-resistant membranes should not be identified with membrane rafts. Trends Biochem. Sci. 30, 430–436. doi: 10.1016/j.tibs.2005.06.004
Lillemeier, B. F., Mörtelmaier, M. A., Forstner, M. B., Huppa, J. B., Groves, J. T., and Davis, M. M. (2010). TCR and Lat are expressed on separate protein islands on T cell membranes and concatenate during activation. Nat. Immunol. 11, 90–96. doi: 10.1038/ni.1832
Lillemeier, B. F., Pfeiffer, J. R., Surviladze, Z., Wilson, B. S., and Davis, M. M. (2006). Plasma membrane-associated proteins are clustered into islands attached to the cytoskeleton. Proc. Natl. Acad. Sci. U.S.A. 103, 18992–18997. doi: 10.1073/pnas.0609009103
Lingwood, D., and Simons, K. (2010). Lipid rafts as a membrane-organizing principle. Science 327, 46–50. doi: 10.1126/science.1174621
Lippincott-Schwartz, J., Snapp, E., and Kenworthy, A. (2001). Studying protein dynamics in living cells. Nat. Rev. Mol. Cell Biol. 2, 444–456. doi: 10.1038/35073068
Luby-Phelps, K., Castle, P., Taylor, D. L., and Lanni, F. (1986). Further evidence for the existence of a structural network in the cytoplasmic ground substance of living cells. J. Cell Biol. 103, A286–A286.
Luby-Phelps, K., Mujumdar, S., Mujumdar, R. B., Ernst, L. A., Galbraith, W., and Waggoner, A. S. (1993). A novel fluorescence ratiometric method confirms the low solvent viscosity of the cytoplasm. Biophys. J. 65, 236–242. doi: 10.1016/S0006-3495(93)81075-0
Machta, B. B., Papanikolaou, S., Sethna, J. P., and Veatch, S. L. (2011). Minimal model of plasma membrane heterogeneity requires coupling cortical actin to criticality. Biophys. J. 100, 1668–1677. doi: 10.1016/j.bpj.2011.02.029
Magee, A. I., Adler, J., and Parmryd, I. (2005). Cold-induced coalescence of T-cell plasma membrane microdomains activates signalling pathways. J. Cell Sci. 118(Pt 14), 3141–3151. doi: 10.1242/jcs.02442
Magidson, V., and Khodjakov, A. (2013). Circumventing photodamage in live-cell microscopy. Methods Cell Biol. 114, 545–560. doi: 10.1016/B978-0-12-407761-4.00023-3
Malínská, K., Malínský, J., Opekarová, M., and Tanner, W. (2003). Visualization of protein compartmentation within the plasma membrane of living yeast cells. Mol. Biol. Cell 14, 4427–4436. doi: 10.1091/mbc.E03-04-0221
Malinsky, J., Tanner, W., and Opekarova, M. (2016). Transmembrane voltage: potential to induce lateral microdomains. Biochim. Biophys. Acta. 1861(8 Pt B), 806–811. doi: 10.1016/j.bbalip.2016.02.012
Marsh, D. (1993). “The nature of the lipid-protein interface and the influence of protein structure on protein-lipid interactions,” in Protein-Lipid Interactions, ed A. Watts (Amsterdam: Elsevier), 41–66.
Marsh, D. (1996). Lateral pressure in membranes. Biochim. Biophys. Acta 1286, 183–223. doi: 10.1016/S0304-4157(96)00009-3
Matsuda, S., Miura, E., Matsuda, K., Kakegawa, W., Kohda, K., Watanabe, M., et al. (2008). Accumulation of AMPA receptors in autophagosomes in neuronal axons lacking adaptor protein AP-4. Neuron 57, 730–745. doi: 10.1016/j.neuron.2008.02.012
Matthews, J. M. (2012). “Protein dimerization and oligomerization in biology,” in Advances in Experimental Medicine and Biology, ed M. M. Jacqueline (New York, NY: Springer-Verlag), V–Vi. doi: 10.1007/978-1-4614-3229-6
Mattila, P. K., and Lappalainen, P. (2008). Filopodia: molecular architecture and cellular functions. Nat. Rev. Mol. Cell Biol. 9, 446–454. doi: 10.1038/nrm2406
Maxfield, F. R., and van Meer, G. (2010). Cholesterol, the central lipid of mammalian cells. Curr. Opin. Cell Biol. 22, 422–429. doi: 10.1016/j.ceb.2010.05.004
Mazzon, M., and Mercer, J. (2014). Lipid interactions during virus entry and infection. Cell. Microbiol. 16, 1493–1502. doi: 10.1111/cmi.12340
McLaughlin, S., and Murray, D. (2005). Plasma membrane phosphoinositide organization by protein electrostatics. Nature 438, 605–611. doi: 10.1038/nature04398
McMahon, H. T., and Gallop, J. L. (2005). Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature 438, 590–596. doi: 10.1038/nature04396
Meier, P., Sachse, J. H., Brophy, P. J., Marsh, D., and Kothe, G. (1987). Integral membrane proteins significantly decrease the molecular motion in lipid bilayers: a deuteron NMR relaxation study of membranes containing myelin proteolipid apoprotein. Proc. Natl. Acad. Sci. U.S.A. 84, 3704–3708. doi: 10.1073/pnas.84.11.3704
Merkel, R., Sackmann, E., and Evans, E. (1989). Molecular friction and epitactic coupling between monolayers in supported bilayers. J. De Phys. 50, 1535–1555. doi: 10.1051/jphys:0198900500120153500
Mihailescu, M., Vaswani, R. G., Jardón-Valadez, E., Castro-Román, F., Freites, J. A., Worcester, D. L., et al. (2011). Acyl-chain methyl distributions of liquid-ordered and -disordered membranes. Biophys. J. 100, 1455–1462. doi: 10.1016/j.bpj.2011.01.035
Mima, J., Hickey, C. M., Xu, H., Jun, Y., and Wickner, W. (2008). Reconstituted membrane fusion requires regulatory lipids, SNAREs and synergistic SNARE chaperones. EMBO J. 27, 2031–2042. doi: 10.1038/emboj.2008.139
Miosge, L., and Zamoyska, R. (2007). Signalling in T-cell development: is it all location, location, location? Curr. Opin. Immunol. 19, 194–199. doi: 10.1016/j.coi.2007.02.008
Mitra, K., Ubarretxena-Belandia, I., Taguchi, T., Warren, G., and Engelman, D. M. (2004). Modulation of the bilayer thickness of exocytic pathway membranes by membrane proteins rather than cholesterol. Proc. Natl. Acad. Sci. U.S.A. 101, 4083–4088. doi: 10.1073/pnas.0307332101
Morgan, M. R., Humphries, M. J., and Bass, M. D. (2007). Synergistic control of cell adhesion by integrins and syndecans. Nat. Rev. Mol. Cell Biol. 8, 957–969. doi: 10.1038/nrm2289
Mouritsen, O. G., and Bagatolli, L. A. (2015). Lipid domains in model membranes: a brief historical perspective. Essays Biochem. 57, 1–19. doi: 10.1042/bse0570001
Mouritsen, O. G., and Bloom, M. (1984). Mattress model of lipid-protein interactions in membranes. Biophys. J. 46, 141–153. doi: 10.1016/S0006-3495(84)84007-2
Mouritsen, O. G., and Bloom, M. (1993). Models of Lipid-Protein Interactions in Membranes. Annu. Rev. Biophys. Biomol. Struct. 22, 145–171. doi: 10.1146/annurev.bb.22.060193.001045
Mouritsen, O. G., and Zuckermann, M. J. (2004). What's so special about cholesterol? Lipids 39, 1101–1113. doi: 10.1007/s11745-004-1336-x
Mueller, V., Honigmann, A., Ringemann, C., Medda, R., Schwarzmann, G., and Eggeling, C. (2013). FCS in STED microscopy: studying the nanoscale of lipid membrane dynamics. Meth. Enzymol. 519, 1–38. doi: 10.1016/B978-0-12-405539-1.00001-4
Mueller, V., Ringemann, C., Honigmann, A., Schwarzmann, G., Medda, R., Leutenegger, M., et al. (2011). STED nanoscopy reveals molecular details of cholesterol- and cytoskeleton-modulated lipid interactions in living cells. Biophys. J. 101, 1651–1660. doi: 10.1016/j.bpj.2011.09.006
Munro, S. (1995). An investigation of the role of transmembrane domains in Golgi protein retention. EMBO J. 14, 4695–4704.
Murase, K., Fujiwara, T., Umemura, Y., Suzuki, K., Iino, R., Yamashita, H., et al. (2004). Ultrafine membrane compartments for molecular diffusion as revealed by single molecule techniques. Biophys. J. 86, 4075–4093. doi: 10.1529/biophysj.103.035717
Nickels, J. D., Smith, J. C., and Cheng, X. (2015). Lateral organization, bilayer asymmetry, and inter-leaflet coupling of biological membranes. Chem. Phys. Lipids 192, 87–99. doi: 10.1016/j.chemphyslip.2015.07.012
Nicolson, G. L. (1979). Topographic display of cell surface components and their role in transmembrane signaling. Curr. Top. Dev. Biol. 13(Pt 1), 305–338. doi: 10.1016/S0070-2153(08)60700-0
Nicolson, G. L. (2014). The Fluid-Mosaic model of membrane structure: still relevant to understanding the structure, function and dynamics of biological membranes after more than 40 years. Biochim. Biophys. Acta 1838, 1451–1466. doi: 10.1016/j.bbamem.2013.10.019
Nicolson, G. L., Hyman, R., and Singer, S. J. (1971). The two-dimensional topographic distribution of H-2 histocompatibility alloantigens on mouse red blood cell membranes. J. Cell Biol. 50, 905–910. doi: 10.1083/jcb.50.3.905
Niemelä, P. S., Miettinen, M. S., Monticelli, L., Hammaren, H., Bjelkmar, P., Murtola, T., et al. (2010). Membrane proteins diffuse as dynamic complexes with lipids. J. Am. Chem. Soc. 132, 7574–7575. doi: 10.1021/ja101481b
Nussinov, R. (2013). The spatial structure of cell signaling systems. Phys. Biol. 10:045004. doi: 10.1088/1478-3975/10/4/045004
Olšinová, M., Jurkiewicz, P., Pozník, M., Šachl, R., Prausová, T., Hof, M., et al. (2014). Di- and tri-oxalkyl derivatives of a boron dipyrromethene (BODIPY) rotor dye in lipid bilayers. Phys. Chem. Chem. Phys. 16, 10688–10697. doi: 10.1039/C4CP00888J
Onfelt, B., Nedvetzki, S., Yanagi, K., and Davis, D. M. (2004). Cutting edge: membrane nanotubes connect immune cells. J. Immunol. 173, 1511–1513. doi: 10.4049/jimmunol.173.3.1511
Ortega-Arroyo, J., and Kukura, P. (2012). Interferometric scattering microscopy (iSCAT): new frontiers in ultrafast and ultrasensitive optical microscopy. Phys. Chem. Chem. Phys. 14, 15625–15636. doi: 10.1039/c2cp41013c
O'shea, P. S., Feuerstein-Thelen, S., and Azzi, A. (1984). Membrane-potential-dependent changes of the lipid microviscosity of mitochondria and phospholipid vesicles. Biochem. J. 220, 795–801. doi: 10.1042/bj2200795
Owen, D. M., Gaus, K., Magee, A. I., and Cebecauer, M. (2010). Dynamic organization of lymphocyte plasma membrane: lessons from advanced imaging methods. Immunology 131, 1–8. doi: 10.1111/j.1365-2567.2010.03319.x
Owen, D. M., Williamson, D. J., Magenau, A., and Gaus, K. (2012). Sub-resolution lipid domains exist in the plasma membrane and regulate protein diffusion and distribution. Nat. Commun. 3:1256. doi: 10.1038/ncomms2273
Peters, R., and Cherry, R. J. (1982). Lateral and rotational diffusion of bacteriorhodopsin in lipid bilayers: experimental test of the Saffman-Delbruck equations. Proc. Natl. Acad. Sci. U.S.A. 79, 4317–4321. doi: 10.1073/pnas.79.14.4317
Quemeneur, F., Sigurdsson, J. K., Renner, M., Atzberger, P. J., Bassereau, P., and Lacoste, D. (2014). Shape matters in protein mobility within membranes. Proc. Natl. Acad. Sci. U.S.A. 111, 5083–5087. doi: 10.1073/pnas.1321054111
Raghupathy, R., Anilkumar, A. A., Polley, A., Singh, P. P., Yadav, M., Johnson, C., et al. (2015). Transbilayer lipid interactions mediate nanoclustering of lipid-anchored proteins. Cell 161, 581–594. doi: 10.1016/j.cell.2015.03.048
Ramadurai, S., Holt, A., Krasnikov, V., van den Bogaart, G., Killian, J. A., and Poolman, B. (2009). Lateral diffusion of membrane proteins. J. Am. Chem. Soc. 131, 12650–12656. doi: 10.1021/ja902853g
Rao, M., and Mayor, S. (2014). Active organization of membrane constituents in living cells. Curr. Opin. Cell Biol. 29, 126–132. doi: 10.1016/j.ceb.2014.05.007
Raychaudhuri, S., Im, Y. J., Hurley, J. H., and Prinz, W. A. (2006). Nonvesicular sterol movement from plasma membrane to ER requires oxysterol-binding protein-related proteins and phosphoinositides. J. Cell Biol. 173, 107–119. doi: 10.1083/jcb.200510084
Ritchie, K., Iino, R., Fujiwara, T., Murase, K., and Kusumi, A. (2003). The fence and picket structure of the plasma membrane of live cells as revealed by single molecule techniques. Mol. Membr. Biol. 20, 13–18. doi: 10.1080/0968768021000055698
Ritchie, K., Shan, X. Y., Kondo, J., Iwasawa, K., Fujiwara, T., and Kusumi, A. (2005). Detection of non-Brownian diffusion in the cell membrane in single molecule tracking. Biophys. J. 88, 2266–2277. doi: 10.1529/biophysj.104.054106
Rossier, O., Octeau, V., Sibarita, J. B., Leduc, C., Tessier, B., Nair, D., et al. (2012). Integrins beta1 and beta3 exhibit distinct dynamic nanoscale organizations inside focal adhesions. Nat. Cell Biol. 14, 1057–1067. doi: 10.1038/ncb2588
Rubin-Delanchy, P., Burn, G. L., Griffié, J., Williamson, D. J., Heard, N. A., Cope, A. P., et al. (2015). Bayesian cluster identification in single-molecule localization microscopy data. Nat. Methods 12, 1072–1076. doi: 10.1038/nmeth.3612
Šachl, R., Mikhalyov, I., Gretskaya, N., Olzynska, A., Hof, M., and Johansson, L. B. (2011). Distribution of BODIPY-labelled phosphatidylethanolamines in lipid bilayers exhibiting different curvatures. Phys. Chem. Chem. Phys. 13, 11694–11701. doi: 10.1039/c1cp20608g
Saffman, P. G., and Delbrück, M. (1975). Brownian motion in biological membranes. Proc. Natl. Acad. Sci. U.S.A. 72, 3111–3113. doi: 10.1073/pnas.72.8.3111
Saka, S. K., Honigmann, A., Eggeling, C., Hell, S. W., Lang, T., and Rizzoli, S. O. (2014). Multi-protein assemblies underlie the mesoscale organization of the plasma membrane. Nat. Commun. 5, 4509. doi: 10.1038/ncomms5509
Sako, Y., and Kusumi, A. (1995). Barriers for lateral diffusion of transferrin receptor in the plasma membrane as characterized by receptor dragging by laser tweezers: fence versus tether. J. Cell Biol. 129, 1559–1574. doi: 10.1083/jcb.129.6.1559
Saxton, M. J. (1987). Lateral diffusion in an archipelago. The effect of mobile obstacles. Biophys. J. 52, 989–997. doi: 10.1016/S0006-3495(87)83291-5
Saxton, M. J. (1990). The membrane skeleton of erythrocytes. A percolation model. Biophys. J. 57, 1167–1177. doi: 10.1016/S0006-3495(90)82636-9
Saxton, M. J. (2008). A biological interpretation of transient anomalous subdiffusion. II. Reaction kinetics. Biophys. J. 94, 760–771. doi: 10.1529/biophysj.107.114074
Schaeffer, C., Creatore, A., and Rampoldi, L. (2014). Protein trafficking defects in inherited kidney diseases. Nephrol. Dial. Transplant 29(Suppl. 4), iv33–iv44. doi: 10.1093/ndt/gfu231
Schmidt, C. F., Barenholz, Y., Huang, C., and Thompson, T. E. (1978). Monolayer coupling in sphingomyelin bilayer systems. Nature 271, 775–777. doi: 10.1038/271775a0
Schmoranzer, J., Goulian, M., Axelrod, D., and Simon, S. M. (2000). Imaging constitutive exocytosis with total internal reflection fluorescence microscopy. J. Cell Biol. 149, 23–32. doi: 10.1083/jcb.149.1.23
Sevcsik, E., Brameshuber, M., Folser, M., Weghuber, J., Honigmann, A., and Schutz, G. J. (2015). GPI-anchored proteins do not reside in ordered domains in the live cell plasma membrane. Nat. Commun. 6:6969. doi: 10.1371/journal.pntd.0001708
Sevcsik, E., and Schütz, G. J. (2016). With or without rafts? Alternative views on cell membranes. Bioessays. 38, 129–139. doi: 10.1002/bies.201500150
Sezgin, E., Gutmann, T., Buhl, T., Dirkx, R., Grzybek, M., Coskun, Ü., et al. (2015). Adaptive lipid packing and bioactivity in membrane domains. PLoS ONE 10:e0123930. doi: 10.1371/journal.pone.0123930
Sezgin, E., Levental, I., Grzybek, M., Schwarzmann, G., Mueller, V., Honigmann, A., et al. (2012). Partitioning, diffusion, and ligand binding of raft lipid analogs in model and cellular plasma membranes. Biochim. Biophys. Acta 1818, 1777–1784. doi: 10.1016/j.bbamem.2012.03.007
Sharpe, H. J., Stevens, T. J., and Munro, S. (2010). A comprehensive comparison of transmembrane domains reveals organelle-specific properties. Cell 142, 158–169. doi: 10.1016/j.cell.2010.05.037
Sheetz, M. P., Sable, J. E., and Döbereiner, H. G. (2006). Continuous membrane-cytoskeleton adhesion requires continuous accommodation to lipid and cytoskeleton dynamics. Annu. Rev. Biophys. Biomol. Struct. 35, 417–434. doi: 10.1146/annurev.biophys.35.040405.102017
Sheetz, M. P., Schindler, M., and Koppel, D. E. (1980). Lateral mobility of integral membrane proteins is increased in spherocytic erythrocytes. Nature 285, 510–511. doi: 10.1038/285510a0
Sherbet, G. V. (1989). Membrane fluidity and cancer metastasis. Exp. Cell Biol. 57, 198–205. doi: 10.1159/000163526
Shimshick, E. J., and McConnell, H. M. (1973a). Lateral phase separation in phospholipid membranes. Biochemistry 12, 2351–2360. doi: 10.1021/bi00736a026
Shimshick, E. J., and McConnell, H. M. (1973b). Lateral phase separations in binary mixtures of cholesterol and phospholipids. Biochem. Biophys. Res. Commun. 53, 446–451. doi: 10.1016/0006-291X(73)90682-7
Shinitzky, M., and Inbar, M. (1976). Microviscosity parameters and protein mobility in biological-membranes. Biochim. Biophys. Acta 433, 133–149. doi: 10.1016/0005-2736(76)90183-8
Shvartsman, D. E., Gutman, O., Tietz, A., and Henis, Y. I. (2006). Cyclodextrins but not compactin inhibit the lateral diffusion of membrane proteins independent of cholesterol. Traffic 7, 917–926. doi: 10.3389/fphys.2016.00185
Sieber, J. J., Willig, K. I., Kutzner, C., Gerding-Reimers, C., Harke, B., Donnert, G., et al. (2007). Anatomy and dynamics of a supramolecular membrane protein cluster. Science 317, 1072–1076. doi: 10.1126/science.1141727
Simons, K., and Ikonen, E. (1997). Functional rafts in cell membranes. Nature 387, 569–572. doi: 10.1038/42408
Simons, K., and van Meer, G. (1988). Lipid sorting in epithelial cells. Biochemistry 27, 6197–6202. doi: 10.1021/bi00417a001
Singer, S. J., and Nicolson, G. L. (1971). The structure and chemistry of mammalian cell membranes. Am. J. Pathol. 65, 427–437.
Singer, S. J., and Nicolson, G. L. (1972). The fluid mosaic model of the structure of cell membranes. Science 175, 720–731. doi: 10.1126/science.175.4023.720
Spira, F., Mueller, N. S., Beck, G., von Olshausen, P., Beig, J., and Wedlich-Söldner, R. (2012). Patchwork organization of the yeast plasma membrane into numerous coexisting domains. Nat. Cell Biol. 14, 640–648. doi: 10.1038/ncb2487
Stachowiak, J. C., Brodsky, F. M., and Miller, E. A. (2013). A cost-benefit analysis of the physical mechanisms of membrane curvature. Nat. Cell Biol. 15, 1019–1027. doi: 10.1038/ncb2832
Stefan, C. J., Manford, A. G., Baird, D., Yamada-Hanff, J., Mao, Y., and Emr, S. D. (2011). Osh proteins regulate phosphoinositide metabolism at ER-plasma membrane contact sites. Cell 144, 389–401. doi: 10.1016/j.cell.2010.12.034
Stinchcombe, J. C., Bossi, G., Booth, S., and Griffiths, G. M. (2001). The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity 15, 751–761. doi: 10.1016/S1074-7613(01)00234-5
Suzuki, K. G., Fujiwara, T. K., Edidin, M., and Kusumi, A. (2007). Dynamic recruitment of phospholipase C gamma at transiently immobilized GPI-anchored receptor clusters induces IP3-Ca2+ signaling: single-molecule tracking study 2. J. Cell Biol. 177, 731–742. doi: 10.1083/jcb.200609175
Swaminathan, R., Hoang, C. P., and Verkman, A. S. (1997). Photobleaching recovery and anisotropy decay of green fluorescent protein GFP-S65T in solution and cells: cytoplasmic viscosity probed by green fluorescent protein translational and rotational diffusion. Biophys. J. 72, 1900–1907. doi: 10.1016/S0006-3495(97)78835-0
Tank, D. W., Wu, E. S., and Webb, W. W. (1982). Enhanced molecular diffusibility in muscle membrane blebs: release of lateral constraints. J. Cell Biol. 92, 207–212. doi: 10.1083/jcb.92.1.207
Tarling, E. J., de Aguiar Vallim, T. Q., and Edwards, P. A. (2013). Role of ABC transporters in lipid transport and human disease. Trends Endocrinol. Metab. 24, 342–350. doi: 10.1016/j.tem.2013.01.006
Trimble, W. S., and Grinstein, S. (2015). Barriers to the free diffusion of proteins and lipids in the plasma membrane. J. Cell Biol. 208, 259–271. doi: 10.1083/jcb.201410071
Uittenbogaard, A., and Smart, E. J. (2000). Palmitoylation of caveolin-1 is required for cholesterol binding, chaperone complex formation, and rapid transport of cholesterol to caveolae. J. Biol. Chem. 275, 25595–25599. doi: 10.1074/jbc.M003401200
Vácha, R., Berkowitz, M. L., and Jungwirth, P. (2009). Molecular model of a cell plasma membrane with an asymmetric multicomponent composition: water permeation and ion effects. Biophys. J. 96, 4493–4501. doi: 10.1016/j.bpj.2009.03.010
Valeur, B., and Berberan-Santos, M. N. (2012). Molecular Fluorescence. Principles and Applications. New York, NY: Wiley VCH.
van Meer, G., and de Kroon, A. I. (2011). Lipid map of the mammalian cell. J. Cell Sci. 124(Pt 1), 5–8. doi: 10.1242/jcs.071233
van Meer, G., and Simons, K. (1982). Viruses budding from either the apical or the basolateral plasma membrane domain of MDCK cells have unique phospholipid compositions. EMBO J. 1, 847–852.
van Meer, G., Voelker, D. R., and Feigenson, G. W. (2008). Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 9, 112–124. doi: 10.1038/nrm2330
van Zanten, T. S., Gómez, J., Manzo, C., Cambi, A., Buceta, J., Reigada, R., et al. (2010). Direct mapping of nanoscale compositional connectivity on intact cell membranes. Proc. Natl. Acad. Sci. U.S.A. 107, 15437–15442. doi: 10.1073/pnas.1003876107
Veatch, S. L., Cicuta, P., Sengupta, P., Honerkamp-Smith, A., Holowka, D., and Baird, B. (2008). Critical fluctuations in plasma membrane vesicles. ACS Chem. Biol. 3, 287–293. doi: 10.1021/cb800012x
Veatch, S. L., and Keller, S. L. (2005). Miscibility phase diagrams of giant vesicles containing sphingomyelin. Phys. Rev. Lett. 94:148101. doi: 10.1103/PhysRevLett.94.148101
Vilmart-Seuwen, J., Kersken, H., Stürzl, R., and Plattner, H. (1986). Atp keeps exocytosis sites in a primed state but is not required for Membrane-Fusion - an analysis with paramecium cells Invivo and Invitro. J. Cell Biol. 103, 1279–1288. doi: 10.1083/jcb.103.4.1279
Voelker, D. R. (2009). Genetic and biochemical analysis of non-vesicular lipid traffic. Annu. Rev. Biochem. 78, 827–856. doi: 10.1146/annurev.biochem.78.081307.112144
Wier, M. L., and Edidin, M. (1986). Effects of cell density and extracellular matrix on the lateral diffusion of major histocompatibility antigens in cultured fibroblasts. J. Cell Biol. 103, 215–222. doi: 10.1083/jcb.103.1.215
Williamson, J. J., and Olmsted, P. D. (2015). Kinetics of symmetry and asymmetry in a phase-separating bilayer membrane. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 92:052721. doi: 10.1103/PhysRevE.92.052721
Wilson, B. S., Pfeiffer, J. R., and Oliver, J. M. (2000). Observing FcepsilonRI signaling from the inside of the mast cell membrane. J. Cell Biol. 149, 1131–1142. doi: 10.1083/jcb.149.5.1131
Wilson, B. S., Pfeiffer, J. R., Surviladze, Z., Gaudet, E. A., and Oliver, J. M. (2001). High resolution mapping of mast cell membranes reveals primary and secondary domains of Fc(epsilon)RI and LAT. J. Cell Biol. 154, 645–658. doi: 10.1083/jcb.200104049
Wilson, R. L., Frisz, J. F., Klitzing, H. A., Zimmerberg, J., Weber, P. K., and Kraft, M. L. (2015). Hemagglutinin clusters in the plasma membrane are not enriched with cholesterol and sphingolipids. Biophys. J. 108, 1652–1659. doi: 10.1016/j.bpj.2015.02.026
Wu, Q. Y., and Liang, Q. (2014). Interplay between curvature and lateral organization of lipids and peptides/proteins in model membranes. Langmuir 30, 1116–1122. doi: 10.1021/la4039123
Yeagle, P. L. (2014). Non-covalent binding of membrane lipids to membrane proteins. Biochim. Biophys. Acta 1838, 1548–1559. doi: 10.1016/j.bbamem.2013.11.009
Yeung, T., Gilbert, G. E., Shi, J., Silvius, J., Kapus, A., and Grinstein, S. (2008). Membrane phosphatidylserine regulates surface charge and protein localization. Science 319, 210–213. doi: 10.1126/science.1152066
Zhang, F., Crise, B., Su, B., Hou, Y., Rose, J. K., Bothwell, A., et al. (1991). Lateral diffusion of membrane-spanning and glycosylphosphatidylinositol-linked proteins: toward establishing rules governing the lateral mobility of membrane proteins. J. Cell Biol. 115, 75–84. doi: 10.1083/jcb.115.1.75
Keywords: plasma membrane, membrane organization models, nanodomains, heterogenous distribution, membrane physical properties
Citation: Bernardino de la Serna J, Schütz GJ, Eggeling C and Cebecauer M (2016) There Is No Simple Model of the Plasma Membrane Organization. Front. Cell Dev. Biol. 4:106. doi: 10.3389/fcell.2016.00106
Received: 13 June 2016; Accepted: 14 September 2016;
Published: 29 September 2016.
Edited by:
Manuel Jose Prieto, Instituto Superior Técnico, PortugalReviewed by:
Dylan Myers Owen, University of New South Wales, AustraliaRichard M. Epand, McMaster University, Canada
Copyright © 2016 Bernardino de la Serna, Schütz, Eggeling and Cebecauer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marek Cebecauer, bWFyZWsuY2ViZWNhdWVyQGpoLWluc3QuY2FzLmN6
 Jorge Bernardino de la Serna
Jorge Bernardino de la Serna Gerhard J. Schütz
Gerhard J. Schütz Christian Eggeling
Christian Eggeling Marek Cebecauer
Marek Cebecauer