
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell Dev. Biol. , 22 August 2016
Sec. Membrane Traffic and Organelle Dynamics
Volume 4 - 2016 | https://doi.org/10.3389/fcell.2016.00083
This article is part of the Research Topic Extracellular microvesicles in immune cell communication View all 10 articles
Extra-cellular vesicles (EVs) are bilayer membrane structures enriched with proteins, nucleic acids, and other active molecules and have been implicated in many physiological and pathological processes over the past decade. Recently, evidence suggests EVs to play a more dichotomic role in the regulation of the immune system, whereby an immune response may be enhanced or supressed by EVs depending on their cell of origin and its functional state. EVs derived from antigen (Ag)-presenting cells for instance, have been involved in both innate and acquired (or adaptive) immune responses, as Ag carriers or presenters, or as vehicles for delivering active signaling molecules. On the other hand, tumor and stem cell derived EVs have been identified to exert an inhibitory effect on immune responses by carrying immuno-modulatory effectors, such as transcriptional factors, non-coding RNA (Species), and cytokines. In addition, stem cell-derived EVs have also been reported to impair dendritic cell maturation and to regulate the activation, differentiation, and proliferation of B cells. They have been shown to control natural killer cell activity and to suppress the innate immune response (IIR). Studies reporting the role of EVs on T lymphocyte modulation are controversial. Discrepancy in literature may be due to stem cell culture conditions, methods of EV purification, EV molecular content, and functional state of both parental and target cells. However, mesenchymal stem cell-derived EVs were shown to play a more suppressive role by shifting T cells from an activated to a T regulatory phenotype. In this review, we will discuss how stem cell-derived EVs may contribute toward the modulation of the immune response. Collectively, stem cell-derived EVs mainly exhibit an inhibitory effect on the immune system.
Extra-cellular vesicles (EVs) are bilayer membranal structures released by cells as a means of transferring their content to and from other cells, now acknowledged as a novel mechanism of intercellular communication. These vesicles comprise of both membrane and cytoplasmic components including: proteins, nucleic acids, and other active molecules and can be sub classified according to size, biogenesis, and composition into exosomes or microvesicles (also defined as shedding vesicles; Théry et al., 2009; EL Andaloussi et al., 2013; Yáñez-Mó et al., 2015; see Figure 1).
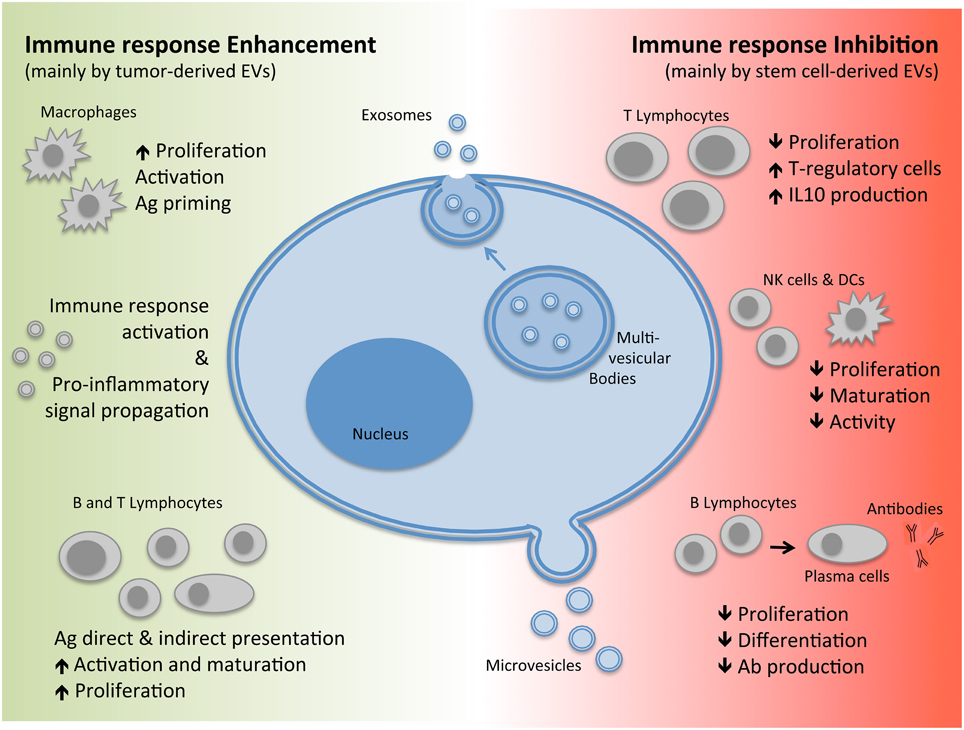
Figure 1. Biogenesis of EVs and their immuno-modulatory effects. EVs, Extracellular Vesicles; Ag, Antigen; IL, Interleukin; Ab, Antibodies.
The term “microvesicle” generally refers to both vesicles released by healthy cells as well as pre-apoptotic vesicles. They are commonly heterogeneous in size ranging from 50 to 1000 nm in diameter depending on the state of the cell during release by either direct shedding or budding from the plasma membrane (Figure 1). Exosomes, on the other hand, are generated through an invagination process of the endosomal membrane of multi-vesicular bodies (MVBs) found within the cells forming vesicles. This is followed by fusion of the MVBs with the plasma membrane leading to exocytosis and release of exosomes which are homogenous in size with a diameter ranging from 40 to 200 nm (Figure 1; Yáñez-Mó et al., 2015). Since exosomes and microvesicles share several molecular and functional characteristics and are released concomitantly by the same cell types, the term EVs will be used to collectively indicate the two subtypes throughout this review.
EVs carry a plethora of molecules that influence their mode of action. These include a variety of receptors, adhesion molecules, proteins involved in cell trafficking, and/or intra-cellular signal transduction, cytoskeletal proteins, cytoplasmic enzymes, cytokines, chemokines, as well as cell-specific antigens (Ags). Moreover, they are also enriched with a range of nucleic acids, including mRNA, long non-coding RNAs, microRNAs (miRNA), and even extra-chromosomal DNA (Robbins and Morelli, 2014; Yáñez-Mó et al., 2015). The content carried however may vary depending on the type of cell and the state of activation. Once released, these EVs may interact with neighboring cells or diffuse through and circulate in the bloodstream or other organic fluids such as: breast milk, semen, saliva, urine, and sputum. Their state of ubiquity makes them important effectors of cell-to-cell communication, which may occur in a paracrine, autocrine, exocrine, and or endocrine manner (Yáñez-Mó et al., 2015).
Virtually all cell types have the ability to release EVs including cells of the immune system such as: macrophages, antigen presenting cells (APCs), dendritic cells (DCs), B and T cells (both CD4+ T helper and CD8+ cytotoxic T lymphocytes), natural killer cells (NKs), as well as cells complementing the immune system such as platelets, mast cells, fibroblasts, epithelial cells, and stem cells. Recently, there has been enough growing evidence to suggest that EVs play an essential role in immuno-modulation (Robbins and Morelli, 2014), influencing both the activation and suppression of the immune response. Furthermore, a role of EVs has also been implicated in inflammation, autoimmune, infectious, and cancer diseases (Yáñez-Mó et al., 2015). This review will however focus on the interactions of stem-cell derived EVs with the immune system.
The immune system can be divided into two branches: the innate immune response (IIR)—an evolutionary conserved system common to all multicellular organisms, and the acquired or adaptive immune response (AIR)—an exclusive feature developed in vertebrates (Sirisinha, 2014).
Within the IIR system, EVs act as paracrine messengers, allowing the propagation of pro-inflammatory signals (Mastronardi et al., 2011; Wang et al., 2011; Prakash et al., 2012; Cloutier et al., 2013). However, they have also been reported to contribute as negative regulators of the inflammatory response primarily by carrying molecules such as Transforming Growth factor-β (TGF-β), and other immuno-suppressive mediators (Gasser and Schifferli, 2004). The role of EVs in the regulation of IIR is complex and has not yet been fully elucidated. Many studies so far have investigated the composition and function of EVs from innate immune cells cultured in-vitro. For instance, macrophages treated in vitro with EVs isolated from cells infected with Mycobacterium tuberculosis released cytokines and chemokines that contributed toward the activation of the immune response (Walters et al., 2013). On the other hand, macrophages infected with the Leishmania parasite secreted EVs enriched with the Leishmania surface protein gp63, which down-regulated the inflammatory response, favoring parasite invasion (Hassani and Olivier, 2013).
Whereas, IIR is a non-specific first line of defense against microbial pathogens and other tissue injuries, AIR is a specific response induced after Ag recognition by adaptive immune cells followed by activation and clonal expansion of immune cells carrying the recognized Ag-specific receptors (Schenten and Medzhitov, 2011; Zhang et al., 2014). In this setting, EVs may act not only as Ag carriers (since they may transfer bacterial, viral, and tumoral components to APCs; O'Neill and Quah, 2008; Walker et al., 2009; Testa et al., 2010), but also as modulators of direct and indirect Ag presentation. Furthermore, this property of EVs to carry Ags from parental cells can allow them to act as reporters of foreign agents in the organism both for the host immune system as well as from a diagnostic point of view (Yáñez-Mó et al., 2015). For example, tumor-derived EVs carry tumor-Ags, which can be taken up and processed by DCs and then cross-presented to tumor-specific cytotoxic T-lymphocytes (CTLs; Wolfers et al., 2001; Andre et al., 2002). This has been demonstrated for EVs isolated from ascites of tumoral patients as well as other tumoral cell lines (Wolfers et al., 2001; Andre et al., 2002; Morelli et al., 2004). This hypothesis is supported by the fact that vaccination of mice with tumor peptide-pulsed DC-derived EVs induces a potent CD8+ T cell-mediated anti-tumoral effect (Wolfers et al., 2001). On the basis of these findings, it can be speculated that tumor-derived EVs carry tumor-specific Ags and that they could be used to stimulate or inhibit the immune anti-tumoral surveillance (Robbins and Morelli, 2014). In this regard, ongoing studies are exploring their potential role in the field of anti-tumor vaccination, as reviewed by Kunigelis et al. (Kunigelis and Graner, 2015). Furthermore, APC-derived EVs can also act as “Ag-presenting vesicles” for in vitro T-cell clones (Théry et al., 2002; Muntasell et al., 2007; Nolte-'t Hoen et al., 2009), however this activity appears to be 10–20 times less efficient to that of corresponding APCs probably due to: the small size, vesicle diffusion, and limited number of MHC molecules per vesicle (Zitvogel et al., 1998; Vincent-Schneider et al., 2002; Qazi et al., 2009).
Many recent studies on EVs have focused on the dichotomic effects they have on the immune system (see Figure 1). There are studies that have reported that EVs are able to promote the immune response by carrying foreign Ags (Bhatnagar and Schorey, 2007; Robbins and Morelli, 2014) as well as inflammatory cytokines (Pizzirani, 2007; Zuccato et al., 2007) and therefore also play a role in mediating chronic inflammatory and autoimmune diseases. For instance, EVs derived from synovial fluid of patients with rheumatoid arthritis (RA) have higher levels of TNF-alpha compared to healthy controls (Zhang et al., 2006). Furthermore, these EVs are able to delay activated T-cell mediated cell death, thereby contributing to the pathogenesis of RA (Zhang et al., 2006). Similarly, EVs isolated from broncho-alveolar fluid of patients with sarcoidosis display pro-inflammatory activity (Qazi et al., 2010). On the other hand, EVs can also have the opposite effect, mediating immuno-suppression. For instance, EVs derived from stimulated T cells are found to be enriched with major histocompatibility complex (MHC) molecules, T-cell receptors (TCR), APO2 ligand, Fas ligand (FasL), and Natural-Killer Group-2 Member-D receptor (NKG2D), which have the ability to not only inhibit Natural killer cell (NK) cytotoxicity but also promote apoptosis of T-cells and down-regulation of Ag processing by APCs (Monleón et al., 2001; Busch et al., 2008; Xie et al., 2010; Hedlund et al., 2011). Although, the function of EVs to either promote or suppress the immune response depends on the type of cell they are released from, including tumors, those derived from stem cells have mainly demonstrated suppressive properties.
Advances in stem cell (SC) technology have opened interesting perspectives in the field of translational medicine in terms of regenerative and therapeutic strategies. Classically, stem cells can be classified into two main categories: embryonic stem cells (ESCs) and adult (or tissue) SCs (Nawaz et al., 2016).
ESCs are pluripotent cells that have the ability to differentiate into cells from any of the three embryonic germ layers: mesoderm, ectoderm, and endoderm. Furthermore, they can self-renew and proliferate indefinitely. As they are derived from embryos at 5–6 days post conception; their use is fairly limited due to ethical concerns on availability as well as for the risk of forming teratomas (Alvarez et al., 2012; Nawaz et al., 2016). Another sub type of SCs is the recently described induced pluripotent stem cells (iPS) which are adult cells that are engineered by manipulating the expression of certain genes to induce re-programming of the cell back to a pluripotent state (Takahashi and Yamanaka, 2006; Alvarez et al., 2012).
Adult stem cells are cells located in fetal and adult tissues; they have limited differentiation capabilities compared to ESCs, and are involved in the maintenance of cell turnover and tissue repair. Nomenclature of adult SCs depends mainly on the source of origin for example: SCs from the bone marrow are known as hematopoietic stem cells and mesenchymal stem/stromal cells (MSCs), and SCs from the nervous system as neural stem cells etc. (Alvarez et al., 2012).
The biological effect of SCs partly depends on a paracrine mechanism whereby, secreted factors including: growth factors, miRNAs, and EVs, influence stem cell regeneration and differentiation as well as mediate crosstalk to and from local and distant tissues (Lai et al., 2010; Zanotti et al., 2013; Wang et al., 2015a). SC-secreted factors have been reported to exhibit several properties such as: anti-apoptotic and angiogenic effects, cell-mobilization, maintenance of homeostasis as well as immune-modulation amongst others (Burdon et al., 2011; Tsuji and Kitamura, 2015). Furthermore, apart from secreted factors, stem cell-derived EVs have also been reported to mimic the effect of SCs after internalization by target cells (injured cells in the context of regeneration), mainly through receptor–ligand mediated interactions and/or direct fusion, leading to horizontal transfer of proteins and nucleic acids (including mRNA and non-coding RNA; Ratajczak et al., 2006a; Valadi et al., 2007; Camussi et al., 2010).
Many authors in the recent years have focused their research in studying the role of EVs (from various cellular origins) in immuno-modulation, however, very little studies have been reported that address the role of SC-derived EVs in the immune system particularly on IRR and AIR. Table 1 shows references of studies that have reported potential effectors identified within EVs that exhibit immuno-modulatory properties. The reason for such limited studies in the literature may partly be due to the fact that the role of SC-EVs as potential immune-modulatory effect is still novel (Nawaz et al., 2016) and partly due to the reporting of controversial results. Nevertheless, in order to elucidate the novel role(s) of SC-EVs on the immune system as well as to clarify the reported controversies in the results, further studies in this subject are only warranted.
Ratajczak et al. first reported that EVs released by ESCs might modulate the phenotype of target cells, supporting self-renewal of hematopoietic progenitors and multi-potency by transfer of growth factors and messenger-RNA (mRNA) (Ratajczak et al., 2006b). Subsequent studies confirmed this horizontal transfer of genetic material in other contexts, such as endothelial progenitor induced angiogenesis and modulation of bone-marrow cell phenotype by EVs released by injured tissues (Deregibus et al., 2007; Aliotta et al., 2010). Furthermore, ESC-derived EVs may not only contribute to cell-fate determination but also to tissue repair and various other physio-pathological processes, but their role as immuno-modulators has not yet been fully elucidated. Nonetheless, ESC-derived EVs have been reported to carry transcriptional factors and mRNAs such as: Nanog, octamer-binding transcription factor 4 (Oct-4), HoxB4, Rex-1, Oct4, and Sox2 that have immuno-modulatory properties (see Table 1). For instance, Nanog has been described in tumor cell immuno-escape (Ratajczak et al., 2006b; Noh et al., 2012), Oct-4 in the inhibition of IIR (Ratajczak et al., 2006b; Lee et al., 2012), and HoxB4 in impairment of DC maturation, and T-cell proliferation through a WNT-mediated mechanism (Ratajczak et al., 2006b; Staal et al., 2008). Whereas, Rex-1 and Sox2 have been shown to stimulate IIR (Weiner, 2002; Ratajczak et al., 2006b; Katsman et al., 2012; Xia et al., 2015). However, a direct role of ESC-derived EVs on the immune system through the above described effectors has yet to be reported. Similar to ESCs, little is known about the functions of iPS-derived EVs on the immune system. The available studies show that membrane proteins, such as CD81 and CD9, and miR-21 are carried by iPS-derived EVs and that these effectors may affect the immune response by regulating: cell adhesion, motility, activation and signal transduction (see Table 1; Levy et al., 1998; O'Connell et al., 2010; Hu et al., 2015; Wang et al., 2015b).
Amongst adult SCs, MSCs are certainly the most studied type (Pittenger et al., 1999; Bruno et al., 2015). In addition to regenerative effects in animal models (Syed and Evans, 2013), there is increasing evidence describing their role in both IIR and AIR modulation (MacDonald et al., 2011; Dalal et al., 2012). For instance, MSCs are able to not only inhibit NK proliferation and activity but also suppress T-/B-cell proliferation and DC maturation (Keating, 2012; Bruno et al., 2015). Furthermore, high levels of IFN-γ and TNF-α influence MSCs to develop immuno-suppressive properties toward IIR (affecting neutrophils, monocytes, and NKs) and AIR (T- and B-cells) (Corcione et al., 2006; Krampera et al., 2006; Spaggiari et al., 2006; Uccelli et al., 2008; Le Blanc and Mougiakakos, 2012). MSCs have also been reported to have a marked immuno-regulatory effect against autoimmune disorders and promising results have been observed in clinical trials on patients with Crohn' s disease and graft-vs.-host disease (Pittenger, 2009; Caplan and Correa, 2011).
It is still widely debated as to whether the immuno-modulatory action of MSCs relies on direct cell-to-cell interaction or on the paracrine action of soluble mediators released by these cells. Either one or both mechanisms are a possibility. Both, in vivo and in vitro studies have demonstrated that MSCs exhibit an immuno-suppressive role by suppressing T lymphocyte proliferation (Krampera et al., 2003; Aggarwal and Pittenger, 2005). A possible explanation for this role could be the presence of proteins and factors such as: TGF-β, hepatocyte growth factor (HGF), nitric oxide (NO), indoleamine 2,3-dioxygenase (IDO), human leukocyte antigen G (HLA-G), prostaglandin E2 (PGE-2), Inter Leukin (IL)-6, and IL-10 (Hwu et al., 2000; Aggarwal and Pittenger, 2005; Spaggiari et al., 2006; Djouad et al., 2007; Sato et al., 2007; English et al., 2009; Caplan and Correa, 2011) that are expressed highly by MSCs. For instance, It has been suggested, that IDO, PGE-2, and TGF-β1 are involved not only in the inhibition of NK cells but also in the inhibition of T-cell proliferation and activation (Aggarwal and Pittenger, 2005; Spaggiari et al., 2006). Furthermore, IL-6 has also been attributed to interfere with DC maturation (English et al., 2009). Finally, with a mechanism not completely known, MSCs have been reported to inhibit B-cells proliferation through the down-regulation of chemokine receptor CXCR4, CXCR5, and chemokine receptor type 7 (CCR7) (Corcione et al., 2006). The immuno-modulatory effects of MSCs can therefore be speculated to be achieved either through the release of the above mentioned factors and proteins directly into the extracellular milieu as soluble molecules, or they may be packaged into EVs together with nucleic acids and other post-transcriptional modulators which could influence the inflammatory response when released (Robbins and Morelli, 2014).
The ability of MSC-derived EVs to mimic the effect of the cell of origin has been studied on various different effector cells of the immune system (Blazquez et al., 2014; Conforti et al., 2014; Favaro et al., 2014, 2016; Zhang, 2014; Amarnath et al., 2015; Del Fattore et al., 2015; Gouveia de Andrade et al., 2015; Di Trapani et al., 2016; see Table 2).
The inhibitory effect of MSC-derived EVs on the activation of cells of the immune system has been demonstrated by various studies. For example, Conforti et al. reported an inhibitory activity of MSCs and MSC-derived EVs on B-cell proliferation, which was further confirmed by a study conducted by Di Trapani et al. (Conforti et al., 2014). Del Fattore et al. also demonstrated that MSC-derived EVs not only increased the ratio between regulatory T-cells and effector T-cells, but also increased the release of the immunosuppressive cytokine IL-10, however, IDO an established mediator of MSC immunosuppressive effects was not affected (Del Fattore et al., 2015). A possible way by which MSC-EVs exhibit these immunomodulatory properties could be through an adenosine A2A receptor mediated mechanism (Amarnath et al., 2015). In addition, the extracellular environment could also play a role, for instance it has been reported that an inflammatory environment (mimicked in culture by the presence of IFN-γ and TNF-α) may not only alter the release but also influence the biological activity of MSC-derived EVs toward a more immunosuppressive role. In fact MSC-derived EVs may polarize monocytes toward M2-like phenotype, which in turn induces CD4+ T cell to differentiate into regulatory T cells (Zhang, 2014). A study by Blazquez et al. also reported that EVs derived from human adipose stem cells had an inhibitory effect not only on the differentiation and activation of T cells but also on the release of IFN-γ (Blazquez et al., 2014). In addition it has been shown that IFN-α or -γ transferred by EVs are also able to activate immunological molecular pathways into target cells (Li, 2013; Cossetti, 2014).
A study carried out by Favaro et al. looking at the effect of MSC-EVs on peripheral blood mononuclear cells (PBMCs) from type 1 diabetic patients revealed some interesting results. They showed that, MSC-derived EVs inhibited IFN-γ production in PBMCs stimulated by the islet Ag glutamic acid decarboxylase 65 (GAD65) and significantly increased the production of immune-modulatory mediators such as PGE-2, TGF-β, IL-10, and IL-6. In addition, there was a reduction in the number of Th17 cells as reflected by the reduction in IL17 secretion and an increase in regulatory T-cells in the same setting, suggesting that T-cells switched to an anti-inflammatory phenotype in the presence of EVs (Favaro et al., 2014). Furthermore, in a later study they reported that DCs conditioned with MSC-derived EVs acquired an immature phenotype with increased IL-10 secretion, which contributed toward inhibition of inflammatory T-cell response against islet Ags (Di Nicola et al., 2002; Favaro et al., 2014, 2016).
Although some very interesting studies have been reported in the literature explaining the immuno-modulatory functions of EVs on T-cell proliferation, there are some that are controversial. For instance, Conforti et al. and Gouveia de Andrade et al. both reported that the immuno-modulatory effects of EVs on T-cells are absent or significantly lower compared to MSCs (Conforti et al., 2014; Gouveia de Andrade et al., 2015). However, the apparent discrepancy observed in the different studies may depend on different methodological approaches and the activation state of the EV cell of origin. Recently Krampera's group attempted to standardize the method for studying the immunomodulatory effects of MSC-derived EVs, comparing unfractionated PBMCs with purified T, B, and NK cells (Di Trapani et al., 2016). In this study, they demonstrated a direct correlation between the degree of EV-mediated immuno-suppression and EV uptake by immune effector cells. In the case of PBMCs, most of the EVs were internalized by monocytes rather than by B and T cells. Furthermore, MSC-derived EVs did not significantly inhibit T-cell proliferation, whereas down-regulated the proliferation of NK and B-cells. These effects were enhanced by MSC-priming with inflammatory cytokines (Di Trapani et al., 2016). On investigating they observed that priming with MSCs increased the level of immunomodulatory miRNAs, such as miRNA-155 and miRNA-146 and that EVs obtained from primed MSCs potentiated the immunosuppressive properties of resting MSCs on T-cells with a mechanism that was independent to a direct EV inhibition of T-cell proliferation (Di Trapani et al., 2016).
Taken together these studies suggest that EVs derived from MSCs are less effective than the cells themselves, as the latter may act either by direct cell-to-cell interaction as well as by releasing several active soluble factors. Moreover, the biological effect of EVs may vary depending extracellular micro-environment. A pro-inflammatory environment for instance may modify the composition of EVs and the consequent biological activities as well as the activation state of immune effector cells targeted by these EVs.
The ability of EVs to either enhance or suppress the immune response may be exploited in immuno-therapy. This dualistic effects of EVs makes them very flexible and lucrative, but, it also increases the risk of unpredictable adverse effects (Zhang et al., 2014). Several studies have reported how EV-dependent immuno-modulation can be correlated to the source type and the state of the cell of origin (activation and maturation degree). For example: APC and DC derived EVs can regulate Ag-specific and non-specific immune responses, both positively and negatively (Robbins and Morelli, 2014). The immunogenicity depends on the expression levels of MHC-class I and II, as well as co-stimulatory molecules such as CD80 and CD86 (Thomson and Robbins, 2008).
Although tumor-derived EVs seem to be mainly involved in immune-escape mechanisms, it has been demonstrated that APCs pulsed with EVs derived from tumor cells may cross-present Ags and stimulate the activation of an Ag-specific CTL-mediated anti-tumoral response (Escudier, 2005; Morse et al., 2005). This was also confirmed in a clinical trial conducted by Bu et al. on malignant gliomas (Bu et al., 2011). However, other clinical trials have also revealed the low immunogenicity of tumor-derived EVs, which represents the main limitation for this therapeutic strategy (Kunigelis and Graner, 2015).
The horizontal transfer of nucleic acid by EVs can be equally exploited in several therapeutic approaches (Robbins and Morelli, 2014). For example engineering EVs with miRNA or small interfering RNA (siRNA), capable of promoting or silencing the expression of particular transcripts, could be adopted in the future as therapeutic strategies.
Immune-cell-based and stem cell based therapies currently present several risks and complications as well as technical problems such: culture-induced senescence, immune-mediated rejection, genetic instability, loss of functional properties, and malignant transformation that need to be solved to make them successful (Nawaz et al., 2016). EVs which mimic in part the effect of the stem cells from which they are derived may represent a novel cell-free solution, which could overcome some of the limitations mentioned above linked to cell based therapies (Zhang, 2014). Giebel et al. in a case report demonstrated the feasibility of an in vivo EV approach for the therapy of refractory graft vs. host disease (Kordelas et al., 2014). The results of this study suggested a beneficial effect of MSC-derived EVs as anti-inflammatory and immune-modulatory mediators in the context of a severe inflammatory and immune disease. Nevertheless, in order to develop an effective and successful immuno-therapy based on stem cell derived EVs warrants not only further studies to clarify the mechanism of action of EVs, but also the standardization of protocols for isolation and characterization (Witwer et al., 2013).
All authors listed, have made substantial, direct and intellectual contribution to the work, and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
EVs, Extra-cellular vesicles; Ag(s), Antigen(s); miRNA, MicroRNA; APCs, Antigen presenting cells; DCs, Dendritic cells; NKs, Natural killer cells; IIR, Innate immune response; AIR, Acquired immune response; TGF-β, Transforming Growth factor-β; CTLs, Cytotoxic T-lymphocytes; RA, Rheumatoid arthritis; SC(s), Stem cell(s); ESCs, Embryonic stem cells; MSCs, Mesenchymal stem/stromal cells; iPS, Induced pluripotent stem cells; IFN-γ, Interferon-γ; TNF-α, Tumor Necrosis Factor-α; HGF, Hepatocyte Growth Factor; NO, Nitric oxide; IDO, Indoleamine 2,3-dioxygenase; HLA-G, Human Leukocyte Antigen-G; PG, Prostaglandin; IL, Interleukin; CXCR4/CXCR5/CCR7, Chemokine Receptor Type 4/5–7; PBMCs, Peripheral Blood Mononuclear Cells; GAD65, Glutamic Acid Decarboxylase 65; NKG2D, Natural-Killer Group-2 Member-D receptor; MHC, Major Histocompatibility Complex; siRNA, Small interfering RNA; BM, Bone Marrow; ASCs, Adipose Stem Cells.
Aggarwal, S., and Pittenger, M. F. (2005). Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105, 1815–1822. doi: 10.1182/blood-2004-04-1559
Aliotta, J. M., Pereira, M., Johnson, K. W., de Paz, N., Dooner, M. S., Puente, N., et al. (2010). Microvesicle entry into marrow cells mediates tissue-specific changes in mRNA by direct delivery of mRNA and induction of transcription. Exp. Hematol. 38, 233–245. doi: 10.1016/j.exphem.2010.01.002
Alvarez, C. V., Garcia-Lavandeira, M., Garcia-Rendueles, M. E., Diaz-Rodriguez, E., Garcia-Rendueles, A. R., Perez-Romero, S., et al. (2012). Defining stem cell types: understanding the therapeutic potential of ESCs, ASCs, and iPS cells. J. Mol. Endocrinol. 49, R89–R111. doi: 10.1530/JME-12-0072
Amarnath, S., Foley, J. E., Farthing, D. E., Gress, R. E., Laurence, A., Eckhaus, M. A., et al. (2015). Bone marrow-derived mesenchymal stromal cells harness purinergenic signaling to tolerize human Th1 cells in vivo. Stem Cells 33, 1200–1212. doi: 10.1002/stem.1934
Andre, F., Schartz, N. E., Movassagh, M., Flament, C., Pautier, P., Morice, P., et al. (2002). Malignant effusions and immunogenic tumour-derived exosomes. Lancet 360, 295–305. doi: 10.1016/S0140-6736(02)09552-1
Bhatnagar, S., and Schorey, J. S. (2007). Exosomes released from infected macrophages contain mycobacterium avium glycopeptidolipids and are proinflammatory. J. Biol. Chem. 282, 25779–25789. doi: 10.1074/jbc.M702277200
Blazquez, R., Sanchez-Margallo, F. M., de la Rosa, O., Dalemans, W., Alvarez, V., Tarazona, R., et al. (2014). Immunomodulatory potential of human adipose mesenchymal stem cells derived exosomes on in vitro stimulated T cells. Front. Immunol. 5:556. doi: 10.3389/fimmu.2014.00556
Bruno, S., Deregibus, M. C., and Camussi, G. (2015). The secretome of mesenchymal stromal cells: role of extracellular vesicles in immunomodulation. Immunol. Lett. 168, 154–158. doi: 10.1016/j.imlet.2015.06.007
Bu, N., Wu, H., Sun, B., Zhang, G., Zhan, S., Zhang, R., et al. (2011). Exosome-loaded dendritic cells elicit tumor-specific CD8+ cytotoxic T cells in patients with glioma. J. Neurooncol. 104, 659–667. doi: 10.1007/s11060-011-0537-1
Burdon, T. J., Paul, A., Noiseux, N., Prakash, S., and Shum-Tim, D. (2011). Bone marrow stem cell derived paracrine factors for regenerative medicine: current perspectives and therapeutic potential. Bone Marrow Res. 12:7326. doi: 10.1155/2011/207326
Busch, A., Quast, T., Keller, S., Kolanus, W., Knolle, P., Altevogt, P., et al. (2008). Transfer of T cell surface molecules to dendritic cells upon CD4+ T cell priming involves two distinct mechanisms. J. Immunol. 181, 3965–3973. doi: 10.4049/jimmunol.181.6.3965
Camussi, G., Deregibus, M. C., Bruno, S., Cantaluppi, V., and Biancone, L. (2010). Exosomes/microvesicles as a mechanism of cell-to- cell communication. Kidney Int. 78, 838–848. doi: 10.1038/ki.2010.278
Caplan, A. I., and Correa, D. (2011). The MSC: an injury drugstore. Cell Stem Cell 9, 11–15. doi: 10.1016/j.stem.2011.06.008
Cloutier, N., Tan, S., Boudreau, L. H., Cramb, C., Subbaiah, R., Lahey, L., et al. (2013). The exposure of autoantigens by microparticles underlies the formation of potent inflammatory components: the microparticle-associated immune complexes. EMBO Mol. Med. 5, 235–249. doi: 10.1002/emmm.201201846
Conforti, A., Scarsella, M., Starc, N., Giorda, E., Biagini, S., Proia, A., et al. (2014). Microvescicles derived from mesenchymal stromal cells are not as effective as their cellular counterpart in the ability to modulate immune responses in vitro. Stem Cell Dev. 23, 2591–2599. doi: 10.1089/scd.2014.0091
Corcione, A., Benvenuto, F., Ferretti, E., Giunti, D., Cappiello, V., Cazzanti, F., et al. (2006). Human mesenchymal stem cells modulate B-cell functions. Blood 107, 367–372. doi: 10.1182/blood-2005-07-2657
Cossetti, C. (2014). Extracellular vesicles from neural stem cells transfer IFN-gamma via Ifngr1 to activate Stat1 signaling in target cells. Mol. Cell 11, 589–593. doi: 10.1016/j.molcel.2014.08.020
Dalal, J., Gandy, K., and Domen, J. (2012). Role of mesenchymal stem cell therapy in Crohn's disease. Pediatr. Res. 71, 445–451. doi: 10.1038/pr.2011.56
Del Fattore, A., Luciano, R., Pascucci, L., Goffredo, B. M., Giorda, E., Scapaticci, M., et al. (2015). Immunoregulatory effects of mesenchymal stem cell-derived extracellular vesicles on T lymphocytes. Cell Transplant. 24, 2615–2627. doi: 10.3727/096368915X687543
Deregibus, M. C., Cantaluppi, V., Calogero, R., Lo Iacono, M., Tetta, C., Biancone, L., et al. (2007). Endothelial progenitor cell-derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood 7, 2440–2448. doi: 10.1182/blood-2007-03-078709
Di Nicola, M., Carlo-Stella, C., Magni, M., Milanesi, M., Longoni, P. D., Matteucci, P., et al. (2002). Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99, 3838–3843. doi: 10.1182/blood.V99.10.3838
Di Trapani, M., Bassi, G., Midolo, M., Gatti, A., Kamga, P. T., Cassaro, A., et al. (2016). Differential and transferable modulatory effects of mesenchymal stromal cell-derived extracellular vesicles on T, B and NK cell functions. Sci. Rep. 6:24120. doi: 10.1038/srep24120
Djouad, F., Charbonnier, L. M., Bouffi, C., Louis-Plence, P., Bony, C., Apparailly, F., et al. (2007). Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells 25, 2025–2032. doi: 10.1634/stemcells.2006-0548
EL Andaloussi, S., Mäger, I., Breakefield, X. O., and Wood, M. J. (2013). Extracellular vesicles: biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 12, 347–357. doi: 10.1038/nrd3978
English, K., Ryan, J. M., Tobin, L., Murphy, M. J., Barry, F. P., Mahon, B. P., et al. (2009). Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4 + CD25 (High) forkhead box P3 + regulatory T cells. Clin. Exp. Immunol. 156, 149–160. doi: 10.1111/j.1365-2249.2009.03874.x
Escudier, B. (2005). Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of the first phase I clinical trial. J. Transl. Med. 3, 10–17. doi: 10.1186/1479-5876-3-10
Favaro, E., Carpanetto, A., Caorsi, C., Giovarelli, M., Angelini, C., Cavallo-Perin, P., et al. (2016). Human mesenchymal stem cells and derived extracellular vesicles induce regulatory dendritic cells in type 1 diabetic patients. Diabetologia 59, 325–333. doi: 10.1007/s00125-015-3808-0
Favaro, E., Carpanetto, A., Lamorte, S., Fusco, A., Caorsi, C., Deregibus, M. C., et al. (2014). Human mesenchymal stem cell-derived microvesicles modulate T cell response to islet antigen glutamic acid decarboxylase in patients with type 1 diabetes. Diabetologia 57, 1664–1673. doi: 10.1007/s00125-014-3262-4
Gasser, O., and Schifferli, J. A. (2004). Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood 104, 2543–2548. doi: 10.1182/blood-2004-01-0361
Gouveia de Andrade, A. V., Bertolino, G., Riewaldt, J., Bieback, K., Karbanová, J., Odendahl, M., et al. (2015). Extracellular vesicles secreted by bone marrow- and adipose tissue-derived mesenchymal stromal cells fail to suppress lymphocyte proliferation. Stem Cells Dev. 6, 345–351. doi: 10.1089/scd.2014.0563
Hassani, K., and Olivier, M. (2013). Immunomodulatory impact of leishmania-induced macrophage exosomes: a comparative proteomic and functional analysis. PLoS Negl. Trop. Dis. 7:e2185. doi: 10.1371/journal.pntd.0002185
Hedlund, M., Nagaeva, O., Kargl, D., Baranov, V., and Mincheva-Nilsson, L. (2011). Thermal- and oxidative stress causes enhanced release of NKG2D ligand-bearing immuno-suppressive exosomes in leukemia/lymphoma T and B cells. PLoS ONE 6:e16899. doi: 10.1371/journal.pone.0016899
Hu, G. W., Li, Q., Niu, X., Hu, B., Liu, J., Zhou, S. M., et al. (2015). Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice. Stem Cell Res. Ther. 6, 10. doi: 10.1186/scrt546
Hwu, P., Du, M. X., Lapointe, R., Do, M., Taylor, M. W., and Young, H. A. (2000). Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J. Immunol. 164, 3596–3599. doi: 10.4049/jimmunol.164.7.3596
Katsman, D., Stackpole, E. J., Domin, D. R., and Farber, D. B. (2012). Embryonic stem cell-derived microvesicles induce gene expression changes in Müller cells of the retina. PLoS ONE 7:e50417. doi: 10.1371/journal.pone.0050417
Keating, A. (2012). Mesenchymal stromal cells: new directions. Cell Stem Cell 10, 709–716. doi: 10.1016/j.stem.2012.05.015
Kordelas, L., Rebmann, V., Ludwig, A. K., Radtke, S., Ruesing, J., Doeppner, T. R., et al. (2014). MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia 28, 970–973. doi: 10.1038/leu.2014.41
Krampera, M., Cosmi, L., Angeli, R., Pasini, A., Liotta, F., Andreini, A., et al. (2006). Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells 24, 386–398. doi: 10.1634/stemcells.2005-0008
Krampera, M., Glennie, S., Dyson, J., Scott, D., Laylor, R., Simpson, E., et al. (2003). Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 101, 3722–3729. doi: 10.1182/blood-2002-07-2104
Kunigelis, K. E., and Graner, M. W. (2015). The Dichotomy of Tumor Exosomes (TEX) in cancer immunity: is it all in the ConTEXt? Vaccines 3, 1019–1051. doi: 10.3390/vaccines3041019
Lai, R. C., Arslan, F., Lee, M. M., Sze, N. S., Choo, A., Chen, T. S., et al. (2010). Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 4, 214–222. doi: 10.1016/j.scr.2009.12.003
Le Blanc, K., and Mougiakakos, D. (2012). Multipotent mesenchymal stromal cells and the innate immune system. Nat. Rev. Immunol. 12, 383–396. doi: 10.1038/nri3209
Lee, J., Sayed, N., Hunter, A., Au, K. F., Wong, W. H., Mocarski, E. S., et al. (2012). Activation of innate immunity is required for efficient nuclear reprogramming. Cell 151, 547–558. doi: 10.1016/j.cell.2012.09.034
Levy, S., Todd, S. C., and Maecker, H. T. (1998). CD81 (TAPA-1): a molecule involved in signal transduction and cell adhesion in the immune system. Annu. Rev. Immunol. 16, 89–109. doi: 10.1146/annurev.immunol.16.1.89
Li, J. (2013). Exosomes mediate the cell-to-cell transmission of IFN-α-induced antiviral activity. Nat. Immunol. 45, 456–465. doi: 10.1038/ni.2647
MacDonald, G. I., Augello, A., and De Bari, C. (2011). Role of mesenchymal stem cells in reestablishing immunologic tolerance in auto-immune Rheumatic diseases. Arthritis Rheum. 63, 2547–2557. doi: 10.1002/art.30474
Mastronardi, M. L., Mostefai, H. A., Meziani, F., Martinez, M. C., Asfar, P., and Andriantsitohaina, R. (2011). Circulating microparticles from septic shock patients exert differential tissue expression of enzymes related to inflammation and oxidative stress. Crit. Care Med. 39, 1739–1748. doi: 10.1097/CCM.0b013e3182190b4b
Monleón, I., Martínez-Lorenzo, M. J., Monteagudo, L., Lasierra, P., Taulés, M., Iturralde, M., et al. (2001). Differential secretion of Fas ligand- or APO2 ligand/TNF-related apoptosis-inducing ligand-carrying microvesicles during activation-induced death of human T cells. J. Immunol. 167, 6736–6744. doi: 10.4049/jimmunol.167.12.6736
Morelli, A. E., Larregina, A. T., Shufesky, W. J., Sullivan, M. L., Stolz, D. B., Papworth, G. D., et al. (2004). Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood 104, 3257–3266. doi: 10.1182/blood-2004-03-0824
Morse, M. A., Garst, J., Osada, T., Khan, S., Hobeika, A., Clay, T. M., et al. (2005). A phase I study of dexosome immunotherapy in patients with advanced non- small cell lung cancer. J. Transl. Med. 3, 9–13. doi: 10.1186/1479-5876-3-9
Muntasell, A., Berger, A. C., and Roche, P. A. (2007). T cell-induced secretion of MHC class II-peptide complexes on B cell exosomes. EMBO J. 26, 4263–4272. doi: 10.1038/sj.emboj.7601842
Nawaz, M., Fatima, F., Vallabhaneni, K. C., Penfornis, P., Valadi, H., Ekström, K., et al. (2016). Extracellular vesicles: evolving factors in stem cell biology. Stem Cells Int. 2016:1073140. doi: 10.1155/2016/1073140
Noh, K. H., Kim, B. W., Song, K. H., Cho, H., Lee, Y. H., Kim, J. H., et al. (2012). Nanog signaling in cancer promotes stem-like phenotype and immune evasion. J. Clin. Invest. 122, 4077–4093. doi: 10.1172/JCI64057
Nolte-'t Hoen, E. N., Buschow, S. I., Anderton, S. M., Stoorvogel, W., and Wauben, M. H. (2009). Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood 113, 1977–1981. doi: 10.1182/blood-2008-08-174094
O'Neill, H. C., and Quah, B. J. C. (2008). Exosomes secreted by bacterially infected macrophages are proinflammatory. Sci. Signal. 1:pe8. doi: 10.1126/stke.16pe8
O'Connell, R. M., Rao, D. S., Chaudhuri, A. A., and Baltimore, D. (2010). Physiological and pathological roles for microRNAs in the immune system. Nat. Rev. Immunol. 10, 111–122. doi: 10.1038/nri2708
Pittenger, M. (2009). Sleuthing the source of regeneration by MSCs. Cell Stem Cell 5, 8–10. doi: 10.1016/j.stem.2009.06.013
Pittenger, M. F., Mackay, A. M., Beck, S. C., Jaiswal, R. K., Douglas, R., Mosca, J. D., et al. (1999). Multilineage potential of adult human mesenchymal stem cells. Science 284, 143–147. doi: 10.1126/science.284.5411.143
Pizzirani, C. (2007). Stimulation of P2 receptors causes release of IL-1beta-loaded microvesicles from human dendritic cells. Blood 109, 3856–3864. doi: 10.1182/blood-2005-06-031377
Prakash, P. S., Caldwell, C. C., Lentsch, A. B., Pritts, T. A., and Robinson, B. R. H. (2012). Human microparticles generated during sepsis in patients with critical illness are neutrophil-derived and modulate the immune response. J. Trauma Acute Care Surg. 73, 401–406. doi: 10.1097/TA.0b013e31825a776d
Qazi, K. R., Gehrmann, U., Domange Jordö, E., Karlsson, M. C., and Gabrielsson, S. (2009). Antigen-loaded exosomes alone induce Th1-type memory through a B-cell-dependent mechanism. Blood 113, 2673–2683. doi: 10.1182/blood-2008-04-153536
Qazi, K. R., Torregrosa Paredes, P., Dahlberg, B., Grunewald, J., Eklund, A., and Gabrielsson, S. (2010). Proinflammatory exosomes in bronchoalveolar lavage fluid of patients with sarcoidosis. Thorax 65, 1016–1024. doi: 10.1136/thx.2009.132027
Ratajczak, J., Miekus, K., Kucia, M., Zhang, J., Reca, R., Dvorak, P., et al. (2006b). Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 20, 847–856. doi: 10.1038/sj.leu.2404132
Ratajczak, J., Wysoczynski, M., Hayek, F., Janowska-Wieczorek, A., and Ratajczak, M. Z. (2006a). Membrane-derived micro-vesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia 20, 1487–1495. doi: 10.1038/sj.leu.2404296
Robbins, P. D., and Morelli, A. E. (2014). Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 14, 195–208. doi: 10.1038/nri3622
Sato, K., Ozaki, K., Oh, I., Meguro, A., Hatanaka, K., Nagai, T., et al. (2007). Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood 109, 228–234. doi: 10.1182/blood-2006-02-002246
Schenten, D., and Medzhitov, R. (2011). The control of adaptive immune responses by the innate immune system. Adv. Immunol. 109, 87–124. doi: 10.1016/B978-0-12-387664-5.00003-0
Sirisinha, S. (2014). Evolutionary insights into the origin of innate and adaptive immune systems: different shades of grey. Asian Pac. J. Allergy Immunol. 32, 3–15.
Spaggiari, G. M., Capobianco, A., Becchetti, S., Mingari, M. C., and Moretta, L. (2006). Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood 107, 1484–1490. doi: 10.1182/blood-2005-07-2775
Staal, F. J., Luis, T. C., and Tiemessen, M. M. (2008). WNT signalling in the immune system: WNT is spreading its wings. Nat. Rev. Immunol. 8, 581–593. doi: 10.1038/nri2360
Syed, B. A., and Evans, J. B. (2013). Stem cell therapy market. Nat. Rev. Drug Discov. 12, 185–186. doi: 10.1038/nrd3953
Takahashi, K., and Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676. doi: 10.1016/j.cell.2006.07.024
Testa, J. S., Apcher, G. S., Comber, J. D., and Eisenlohr, L. C. (2010). Exosome-driven antigen transfer for MHC class II presentation facilitated by the receptor binding activity of influenza hemagglutinin. J. Immunol. 185, 6608–6616. doi: 10.4049/jimmunol.1001768
Théry, C., Duban, L., Segura, E., Véron, P., Lantz, O., and Amigorena, S. (2002). Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat. Immunol. 3, 1156–1162. doi: 10.1038/ni854
Théry, C., Ostrowski, M., and Segura, E. (2009). Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 9, 581–593. doi: 10.1038/nri2567
Thomson, A. W., and Robbins, P. D. (2008). Tolerogenic dendritic cells for autoimmune disease and transplantation. Ann. Rheum. Dis. 67(Suppl. 3):iii90–iii96. doi: 10.1136/ard.2008.099176
Tsuji, K., and Kitamura, S. (2015). Trophic factors from tissue stem cells for renal regeneration. Stem Cells Int. 7:e537204. doi: 10.1155/2015/537204
Uccelli, A., Moretta, L., and Pistoia, V. (2008). Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 8, 726–736. doi: 10.1038/nri2395
Valadi, H., Ekstrom, K., Bossios, A., Sjostrand, M., Lee, J. J., and Lotvall, J. O. (2007). Exosome-mediated transfer of mRNAs and micro- RNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659. doi: 10.1038/ncb1596
Vincent-Schneider, H., Stumptner-Cuvelette, P., Lankar, D., Pain, S., Raposo, G., Benaroch, P., et al. (2002). Exosomes bearing HLA-DR1 molecules need dendritic cells to efficiently stimulate specific T cells. Int. Immunol. 14, 713–722. doi: 10.1093/intimm/dxf048
Walker, J. D., Maier, C. L., and Pober, J. S. (2009). Cytomegalovirus-infected human endothelial cells can stimulate allogeneic CD4+ memory T cells by releasing antigenic exosomes. J. Immunol. 182, 1548–1559. doi: 10.4049/jimmunol.182.3.1548
Walters, S. B., Kieckbusch, J., Nagalingam, G., Swain, A., Latham, S. L., Grau, G. E., et al. (2013). Microparticles from mycobacteria- infected macrophages promote inflammation and cellular migration. J. Immunol. 190, 669–677. doi: 10.4049/jimmunol.1201856
Wang, J. G., Williams, J. C., Davis, B. K., Jacobson, K., Doerschuk, C. M., Ting, J. P. Y., et al. (2011). Monocytic microparticles activate endothelial cells in an IL-1 beta-dependent manner. Blood 118, 2366–2374. doi: 10.1182/blood-2011-01-330878
Wang, K. X., Xu, L. L., Rui, Y. F., Huang, S., Lin, S. E., Xiong, J. H., et al. (2015a). The effects of secretion factors from umbilical cord derived mesenchymal stem cells on osteogenic differentiation of mesenchymal stem cells. PLoS ONE 10:e0120593. doi: 10.1371/journal.pone.0120593
Wang, Y., Zhang, L., Li, Y., Chen, L., Wang, X., Guo, W., et al. (2015b). Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. Int. J. Cardiol. 192, 61–69. doi: 10.1016/j.ijcard.2015.05.020
Weiner, O. D. (2002). Rac activation: P-Rex1 - a convergence point for PIP3 and Gβγ? Curr. Biol. 12:R429. doi: 10.1371/journal.pone.0050417
Witwer, K. W., Buzás, E. I., Bemis, L. T., Bora, A., Lässer, C., Lötvall, J., et al. (2013). Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles 27:2. doi: 10.3402/jev.v2i0.20360
Wolfers, J., Lozier, A., Raposo, G., Regnault, A., Théry, C., Masurier, C., et al. (2001). Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat. Med. 7, 297–303. doi: 10.1038/85438
Xia, P., Wang, S., Ye, B., Du, Y., Huang, G., Zhu, P., et al. (2015). Sox2 functions as a sequence-specific DNA sensor in neutrophils to initiate innate immunity against microbial infection. Nat. Immunol. 16, 366–375. doi: 10.1038/ni.3117
Xie, Y., Zhang, H., Li, W., Deng, Y., Munegowda, M. A., Chibbar, R., et al. (2010). Dendritic cells recruit T cell exosomes via exosomal LFA-1 leading to inhibition of CD8+ CTL responses through downregulation of peptide/MHC class I and Fas ligand-mediated cytotoxicity. J. Immunol. 185, 5268–5278. doi: 10.4049/jimmunol.1000386
Yáñez-Mó, M., Siljander, P. R., Andreu, Z., Zavec, A. B., Borràs, F. E., Buzas, E. L., et al. (2015). Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 4:27066. doi: 10.3402/jev.v4.27066
Zanotti, L., Sarukhan, A., Dander, E., Castor, M., Cibella, J., Soldani, C., et al. (2013). Encapsulated mesenchymal stem cells for in vivo immunomodulation. Leukemia 27, 500–503. doi: 10.1038/leu.2012.202
Zhang, B. (2014). Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2, 175–179. doi: 10.1089/scd.2013.0479
Zhang, B., Yin, Y., Lai, R. C., and Lim, S. K. (2014). Immunotherapeutic potential of extracellular vesicles. Front. Immunol. 5:518. doi: 10.3389/fimmu.2014.00518
Zhang, H. G., Liu, C., Su, K., Yu, S., Zhang, L., Zhang, S., et al. (2006). A membrane form of TNF-alpha presented by exosomes delays T cell activation-induced cell death. J. Immunol. 176, 7385–7393. doi: 10.4049/jimmunol.176.12.7385
Zitvogel, L., Regnault, A., Lozier, A., Wolfers, J., Flament, C., Tenza, D., et al. (1998). Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat. Med. 4, 594–600. doi: 10.1038/nm0598-594
Keywords: extracellular vesicles, exosomes, stem cells, immune system, immuno-modulation
Citation: Burrello J, Monticone S, Gai C, Gomez Y, Kholia S and Camussi G (2016) Stem Cell-Derived Extracellular Vesicles and Immune-Modulation. Front. Cell Dev. Biol. 4:83. doi: 10.3389/fcell.2016.00083
Received: 19 May 2016; Accepted: 02 August 2016;
Published: 22 August 2016.
Edited by:
Kaushik Choudhuri, University of Michigan Health System, USACopyright © 2016 Burrello, Monticone, Gai, Gomez, Kholia and Camussi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giovanni Camussi, Z2lvdmFubmkuY2FtdXNzaUB1bml0by5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.