- Department of Biological Sciences, St. John’s University, Jamaica, NY, United States
The concept of biological cell death—that is, cell death that is neither accidental nor chaotic—has existed and has been obvious since at least the beginning of the 20th C, but it was noticed by other than specialists apt choices of words that caught the spirit of the time, “programmed cell death” and “apoptosis” caught the attention of a wider range of scientists. Then, by the early 1990s the recognition of at least two genes that were important to cancer and other diseases by controlling cell death (p53, Bcl-2, and Fas); recognition that cell death could be controlled by a highly conserved family of proteases; and the development of rapid and easy means of measuring cell death, led to the explosion of the field as a subject of research. Today we recognize many variations on the theme of biological cell death, but many mysteries remain. The most important of these remaining mysteries is that we recognize many of the penultimate and ultimate steps to kill cells, but it is rarely clear how and why these steps are activated. Most likely they are activated by an interaction of several metabolic steps, but we will need more high-powered analysis to determine how this interaction functions.
Introduction
The 50th anniversary of the appearance of the term “apoptosis” (Lockshin and Williams, 1964; Kerr et al., 1972), approximately the 30th anniversary of the sequencing of the Caenorhabditis gene ced-3 and the realization that it was a homolog of a mammalian protease (Ellis et al., 1991), and approaching the 60th anniversary of the term “programmed cell death” (doctoral thesis 1963; and (Lockshin and Williams, 1965a; Lockshin and Williams, 1965b; Lockshin and Williams, 1965c; Lockshin and Williams, 1965d; Maghsoudi et al., 2012)) publications 1964–1965) is an occasion to reflect on what it meant, where we are today, and where we are going. It turns out that an important theme 60 years ago is returning as a theme for at least the immediate future: Acknowledge and accept the obvious. In the past, we had to accept the idea that cell death was a natural, biological, and sometimes planned event, not often a catastrophic and unexpected failure. Today we face the realization that even the most accepted agents of death—for instance, caspase-3—themselves are usually activated through primary but mostly occult failures in metabolism, leading to a cascade that ultimately activates the protease. In such a situation, inhibition of the protease may delay but will not in the long run prevent the death. The short term in which most experiments were done gave a false sense of certainty. As always, science proves to be an onion: Peel off one layer only to find a new layer underneath. Study of the mechanisms of cell death is still very much warranted, as manipulation of the processes has high potential medical value, but we should move forward with a more skeptical approach. This brief description of the history of the field hopefully will illustrate the point.
The past: the environment
Some elements of the graduate school environment of the 1960s no longer exist. In a growing economy, we tended to be well supported and, at the beginning of the space age, expected to find employment primarily in a university setting. Other elements are more problematic: using analog equipment, we recalibrated our measuring devices daily and understood well the meaning of a calibration curve (In the modern era, I have yet to encounter a student who is not confounded by a question such as, “Have you ever done a standard curve to verify that the optical measurement that you are using quantitatively is in fact linear?” It generally is not). But two characteristics that are important to any graduate program are a can-do and make-do attitude, and camaraderie. Perhaps the weirdest examples of make-do were using exposed and developed photographic film to detect small amounts of protease, and, thanks to a helpful packrat who ran the supply room, an adaptation to create a chronic intermittent neural stimulator. This latter consisted of an analog wall clock laid horizontally, in which the circuit was completed for a few seconds each minute as the minute hand contacted mercury droplets placed at the minute marks. The camaraderie was among graduate students who were the night staff or night rats of the laboratory, as we called ourselves, and many experiments were done, without the knowledge and sometimes permission, of the faculty, in collaboration with fellow students or postdocs who had access to equipment and understanding that was not available in our home laboratories. That cooperation, born perhaps in a more abundant and optimistic era, greatly expanded the range of our capabilities.
The past: the importance of language
The idea that cell death was a natural event was evident in the 19th C. It was indeed obvious, as anyone who reflected on arthropod or amphibian metamorphosis would have said. Elie Metchnikov noted, in his ground-breaking studies of the immune system and phagocytosis, that many of these cells died, and many embryologists and developmental biologists observed that cells died as organs changed. Rita Levi-Montalcini and Viktor Hamburger observed that the sympathetic ganglia of developing chicks were larger in the segments that bore limbs, not because of increased mitosis of neural precursors but because of many neural precursors in the unfavored segments spontaneously died; and John Saunders demonstrated that patches of dying cells in chick limbs would die even if excised prematurely and grown in culture (Maghsoudi et al., 2012). In my training, my mentor Carroll M. Williams was legendary for the use of colorful language—“a moth is a flying machine devoted to sex” as well as some less printable comments—and as students we all tried to emulate him. His group met late every afternoon for tea, where conversation often involved batting around each other’s ideas. In this context, the nascent world of computers was often mentioned and, perhaps the suggestion of a fellow student, we started to discuss my results as “programmed cell death”. Despite some later confusion about how rigid a program might be, we used the term precisely because we could define it by our ability to block it, in the instance of muscles dying during metamorphosis of an insect, by interfering with endocrine and neural signals. The history is recounted in (Clarke and Clarke, 1996; Lockshin and Zakeri, 2001; Vaux, 2002).
We chose the term because it was new and eye-catching. The choice was propitious. Although it meant something obvious—that, at least in developmental situations, if the death of a particular cell could be predicted to occur at a given location and time, it ultimately had to be part of the genome as much as the eruption of teeth, puberty, the involution of the thymus, or eye color—the phrase brought the idea to the attention of others in a way that more pallid language did not. Thus, from the 1960s onward, developmental deaths began to be described as “programmed”.
In 1972 Kerr, Wyllie, and Currie introduced a wider view. John Kerr, a pathologist, had been noting for some time that many dying cells did not explode, as would be expected if failure of metabolic resources such as oxygen led to accumulation of lactic acid and osmotic intake of water; but rather, they collapsed, and the nuclei became very dense and dark. He called this type of death “shrinkage necrosis”. We were aware of each other’s work since the insect cells we studied underwent a similar collapse, though lysosomes were much more evident in dying insect tissue. In 1972, Kerr on sabbatical leave in the laboratory of Alastair Currie and his graduate student Andrew Wyllie, sought a term to emphasize the generality of this type of death. Consulting a scholar of Greek, they chose “apoptosis,” meaning “falling of leaves,” to emphasize the idea that it could be the bookend to “mitosis” and distinct from other types of cell death such as lysing cells or coagulative necrosis. Again, a term struck the right chord, and the term “apoptosis” began to percolate through the literature. Arends, Morris, and Wyllie (Arends et al., 1990) picking up on earlier suggestions that the peculiar condensation of chromatin characteristic of apoptosis might result from inter-nucleosome cleavage of DNA by a bacterial-type DNase, demonstrated that apoptosis could be detected by electrophoresing DNA and observing a ladder of DNA fragments representing cleavage between nucleosomes. The assay was cheap and easy, leading others to detect apoptosis in situations where the short survival time of an apoptotic cell would normally obscure the existence of apoptosis. Finally, in the late 1980s, groups beginning with Sidney Brenner, Charles Sulston, and H. Robert Horvitz identified genes that actually controlled cell death in roundworms, one of which was quickly recognized to be a protease homologous to human genes (Yuan et al., 1993; Xue et al., 1996); while insects proved valuable in identifying inhibitors of apoptosis (IAPs) (Crook et al., 1993) including p35 (Sahdev et al., 2003); groups in Japan (Itoh et al., 1991) and Germany (Trauth et al., 1989) recognized that cells of the immune system that could kill other cells did so with a molecule (Fas Ligand) that attached to a receptor on the target cell (Fas), activating a death pathway; B-cell lymphoma was recognized as arising from a mutation in a gene (Bcl-2) that normally functioned to prevent activation of apoptosis but could not, in the mutation, be turned off (Korsmeyer et al., 1990; Tsujimoto and Shimizu, 2007); and another gene, found to be mutated in many cancers, was identified as a gene that, when activated, killed potentially cancerous cells (p53: (Yonish-Rouach et al., 1991; Lowe et al., 1994). The newly-developed accessibility of genes, by identification and manipulation, that if not exclusively then at least primarily, affected cell death in cancer, led to the explosive expansion of the field. Thus the idea finally achieved broad recognition: cells commit suicide, and the process was potentially manipulatable for medical purposes.
The present
There are currently over ½ million publications identified by the keywords “programmed cell death” or “apoptosis,” and more than 100 new publications are appearing every day. Comprehensive reviews can be found in many sources, and there is an extensive effort to normalize the language so that the numerous researchers use consistent and universally applicable terms. Such an effort is perhaps a Sisyphean one but serves at least to prevent serious misunderstanding.
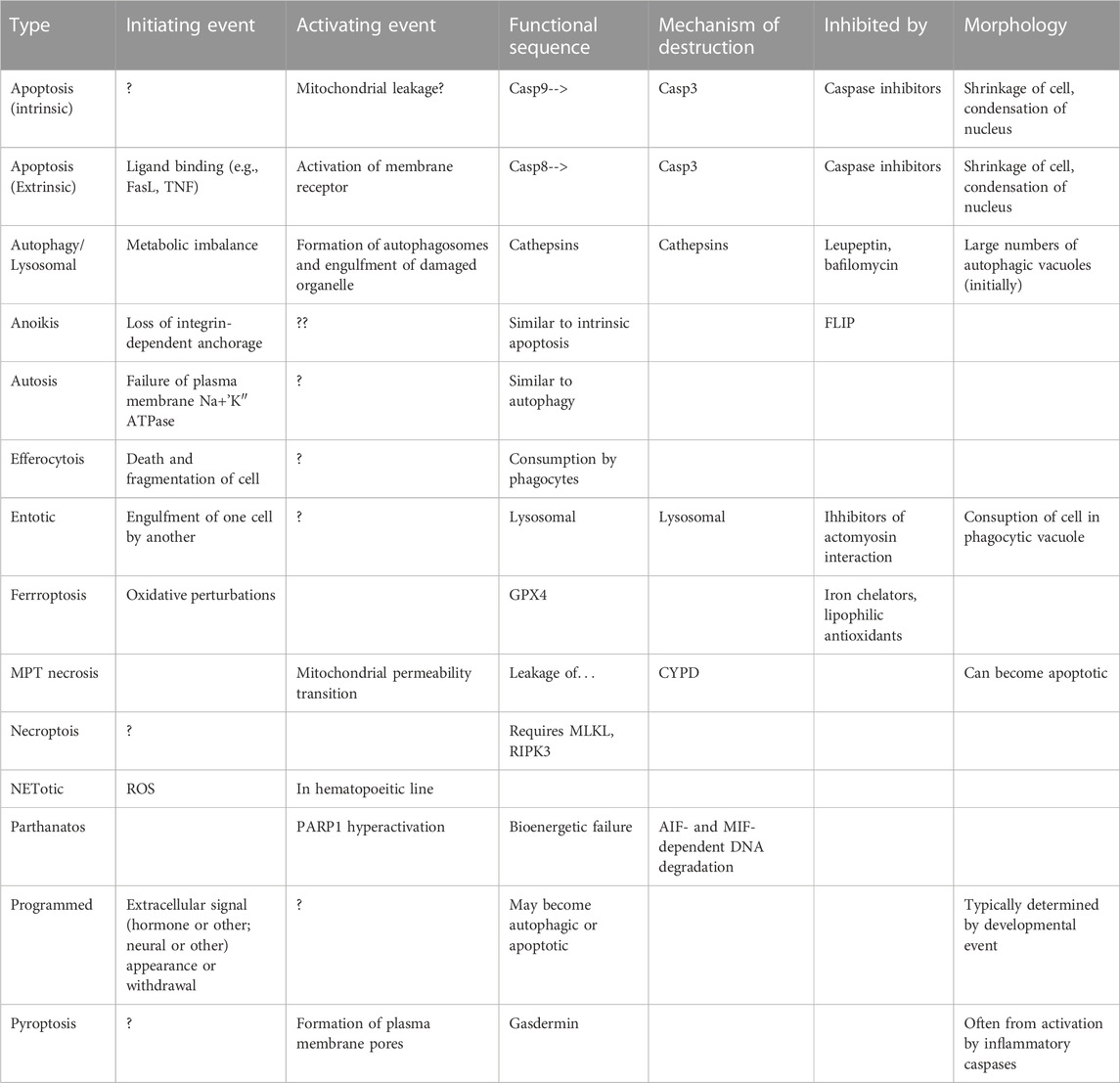
A current trend in the field is to identify other forms of controlled or regulated cell death, such as that caused or adjusted by other metabolites (autophagic cell death (Liu and Levine, 2015))—considered in the 1960s to be the major form of cell death--necroptosis, pyroptosis, ferroptosis… (Table 1) or otherwise constrained (Galluzzi et al., 2009; Klionsky et al., 2016; Galluzzi et al., 2018; Klionsky et al., 2021) and Galluzzi L et al.; an update to (Galluzzi et al., 2018) is in preparation). They all serve the purpose of identifying other components of dying cells, but the fundamental issue may be that cells have failsafe pathways, such that, should a critical metabolic process fail, the cell can safely dismantle itself. Apoptosis is the most common, and in developmental situations such failures are programmed into the developmental sequence, triggering failures at the appropriate time. Some of the failsafe mechanisms may be ancillary or hijacked mechanisms for enzymes originally evolve for other purposes.
Discussion
The future
Most interesting, many efforts have been made to identify cell death processes in plants (Joanna et al., 2021) yeast (Teng and Hardwick, 2015) and bacteria (Lee and Lee, 2019). Substantial progress has been made for yeast. Not unexpected, most of these processes bear insubstantial resemblance to apoptosis. For instance, the death of yeast cells involves considerably more activity of autophagosomes (Hardwick et al., 2022). Nevertheless, these diverse examples help us to better understand the generalities of cell death.
In recent years we have learned a great deal about the delicate balance among metabolic stability, autophagy, and apoptotic or non-apoptotic death (Akkoc and Gozuacik, 2020; Peker and Gozuacik, 2020; Akkoc et al., 2021) and remarkable new technology has taught us the complexity of regulation of mitochondrial transport and energetics (Luna-Vargas and Chipuk, 2016; Trotta and Chipuk, 2017; Rubio-Patiño et al., 2019; Chipuk et al., 2021). If we consider that cells are in perpetually metastable states, depending on the input, output, and balance among many reactions and ion distributions; and that each failure sequence can trigger an appropriate failsafe shutdown, then the future becomes more obvious if more branched and complicated: To identify the trigger conditions that initiate a failsafe shutdown such as activation of caspase-3, MLKL and RIPK3 for necroptosis, inflammatory caspases for pyroptosis, or other mechanisms for other named forms of cell death.
Conclusion
When we consider the truly biologically planned or programmed deaths, such as those occurring during development, metamorphosis, seasonal or cyclical activation and de-activation of primary and secondary sexual tissues, or perhaps even turnover of particular types of cells, it now seems apparent that the proximate cause of death may be a murder weapon such as caspase-3, but the ultimate cause is a change of metabolism, often relating to mitochondrial function and permeability, derived from an extracellular or other intracellular change. Extracellular changes may include change in circulating hormones or death ligands. Other intracellular changes are often unknown but suggest depletion of key nutrients leading to autophagocytosis of specific organelles. Considering that a defining moment in the story of programmed cell death and apoptosis was the recognition that the ced-3 (Cell Death abnormal) gene in Caenorhabditis was a protease with homologs in mammals, it is striking that we still do not have a clear sense of how ced-3 is activated. A temporal clock for the gene is highly unlikely; a clock based on number of divisions is highly unlikely, though an asymmetric division may play a role (Mishra et al., 2018). It remains likely that the activation of ced-3 depends on a concatenation of metabolic signals detected within the regulatory elements of the gene, as it is likely that other programmed deaths are ultimately triggered by metabolic events. For cell deaths not following a developmental program, mutations of regulatory genes can (bcl-2, p53) can lead to a failure of potentially malignant cells to self-destruct, creating a major source of cancer; and, in situations in which a crisis may be temporary, such as an infarct subject to intervention, blockage of caspases may delay death and salvage the affected cells (Jia et al., 2021), but for more delayed intervention or more grievous injury, the cell will follow other pathways to death. The questions of what controls the activation of caspases and what sorts of metabolic changes are required to activate apoptosis or other forms of cell death have been discussed, at least in lectures, since 2000, but are now appearing in the literature. We can now explore, via big data and artificial intelligence, the interactions between proteins and metabolites, or between different proteins (Jia et al., 2021), to determine what events trigger the activation of the death pathways. Another layer of the onion, perhaps approachable by a new generation of scientists.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary materials, further inquiries can be directed to the corresponding author.
Author contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Funding
Research by the author quoted here was funded by the National Science Foundation (United States) and National Institutes of Health (United States).
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AI, Artificial Intelligence; AIF, Apoptosis Inducing factor; Bcl-2, B Cell Lymphoma gene 2; Casp, Caspase; Ced-3, Cell Death Abnormal Gene 3; CYPD, Cyclophilin D; DNA, Deoxyribonucleic acid; Fas, FS7-associated surface antigen; FasL, Fas (QV) ligand; IAP, Inhibitor of apoptosis; MIF, Macrophage migration inhibitory factor; MLKL, Mixed lineage kinase domain like pseudokinase; MPT, Mitochondrial Permeability Transition; Na-K ATPase, Sodium-Potassium adenosine triphosphatase; p35, Anti-apoptotic protein of molecular mass 35 kDaltons; p53, Gene for protein of molecular mass 53 kDaltons; RIPK3, Receptor-interacting serine-threonine protein kinase 3; ROS, Reactive Oxygen Species; TNF, Tumor Necrosis Factor.
References
Akkoc, Y., and Gozuacik, D. (2020). MicroRNAs as major regulators of the autophagy pathway. Biochim. Biophys. Acta Mol. Cell Res. 1867 (5), 118662. doi:10.1016/j.bbamcr.2020.118662
Akkoc, Y., Peker, N., Akcay, A., and Gozuacik, D. (2021). Autophagy and cancer dormancy. Front. Oncol. 11, 627023. doi:10.3389/fonc.2021.627023
Arends, M. J., Morris, R. G., and Wyllie, A. H. (1990). Apoptosis: The role of the endonuclease. Am. J. Pathol. 136 (3), 593–608.
Chipuk, J. E., Mohammed, J. N., Gelles, J. D., and Chen, Y. (2021). Mechanistic connections between mitochondrial biology and regulated cell death. Dev. Cell 56 (9), 1221–1233. doi:10.1016/j.devcel.2021.03.033
Clarke, P. G. H., and Clarke, S. (1996). Nineteenth century research on naturally occurring cell death and related phenomena. Anat. Physiol. 193, 81–99. doi:10.1007/BF00214700
Crook, N. E., Clem, R. J., and Miller, L. K. (1993). An apoptosis-inhibiting baculovirus gene with a zinc finger-like motif. J. Virol. 67 (4), 2168–2174. doi:10.1128/JVI.67.4.2168-2174.1993
Ellis, R. E., Yuan, J. Y., and Horvitz, H. R. (1991). Mechanisms and functions of cell death. Annu. Rev. Cell Biol. 7, 663–698. doi:10.1146/annurev.cb.07.110191.003311
Galluzzi, L., Aaronson, S. A., Abrams, J., Alnemri, E. S., Andrews, D. W., Baehrecke, E. H., et al. (2009). Guidelines for the use and interpretation of assays for monitoring cell death in higher eukaryotes. Cell Death Differ. 16 (8), 1093–1107. doi:10.1038/cdd.2009.44
Galluzzi, L., Vitale, I., Aaronson, S. A., Abrams, J. M., Adam, D., Agostinis, P., et al. (2018). Molecular mechanisms of cell death: Recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 25 (3), 486–541. doi:10.1038/s41418-017-0012-4
Hardwick, J. M., Knorre, D., Palkova, Z., and Winderickx, J. (2022). Editorial: Yeast differentiation: From cell-to-cell heterogeneity to replicative aging and regulated cell death. Front. Cell Dev. Biol. 9, 823447. doi:10.3389/fcell.2021.823447
Itoh, N., Yonehara, S., Ishii, A., Yonehara, M., Mizushima, S., Sameshima, M., et al. (1991). The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell 66 (2), 233–243. doi:10.1016/0092-8674(91)90614-5
Jia, X. F., Liang, F. G., and Kitsis, R. N. (2021). Multiple cell death programs contribute to myocardial infarction. Circ. Res. 129 (3), 397–399. doi:10.1161/CIRCRESAHA.121.319584
Joanna, K., ArunikaGunawardena, H. L. A. N., Bouteau, F., and McCabe, P. F. (2021). Editorial: Plant programmed cell death revisited. Sec. Plant Cell Biol. 12, 672465. doi:10.3389/fpls.2021.672465
Kerr, J. F., Wyllie, A. H., and Currie, A. R. (1972). Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 26 (4), 239–257. doi:10.1038/bjc.1972.33
Klionsky, D. J., Abdel-Aziz, A. K., Abdelfatah, S., Abdellatif, M., Abdoli, A., Abel, S., et al. (2021). Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition). Autophagy 17 (1), 1–382. doi:10.1080/15548627.2020.1797280
Klionsky, D. J., Abdelmohsen, K., Abe, A., Abedin, M. J., Abeliovich, H., Acevedo Arozena, A., et al. (2016). Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Erratum Autophagy 12 (1), 1–222. doi:10.1080/15548627.2015.1100356
Korsmeyer, S. J., McDonnell, T. J., Nunez, G., Hockenbery, D., and Young, R. (1990). Bcl-2: B cell life, death and neoplasia. Curr. Top. Microbiol. Immunol. 166, 203–207. doi:10.1007/978-3-642-75889-8_26
Lee, H., and Lee, D. G. (2019). Programmed cell death in bacterial community: Mechanisms of action, causes and consequences. J. Microbiol. Biotechnol. 29 (7), 1014–1021. doi:10.4014/jmb.1904.04017
Liu, Y., and Levine, B. (2015). Autosis and autophagic cell death: The dark side of autophagy. Cell Death Differ. 22 (3), 367–376. doi:10.1038/cdd.2014.143
Lockshin, R. A., and Williams, C. M. (1965b). Programmed cell death III Neural control of the breakdown of the instersegmental muscles of silkmoths. J. Insect Physiol. 11, 601–610. doi:10.1016/0022-1910(65)90142-3
Lockshin, R. A., and Williams, C. M. (1965d). Programmed cell death IV: The influence of drugs on the breakdown of the intersegmental muscles of silkmoths. J. Insect Physiol. 11, 803–809. doi:10.1016/0022-1910(65)90159-9
Lockshin, R. A., and Williams, C. M. (1965c). Programmed cell death V: Cytolytic enzymes in relation to the breakdown of the intersegmental muscles of silkmoths. J. Insect Physiol. 11 (7), 831–844. doi:10.1016/0022-1910(65)90186-1
Lockshin, R. A., and Williams, C. M. (1965a). Programmed cell death-i: Cytology of degeneration in the intersegmental muscles of the pernyi silkmoth. J. Insect Physiol. 11, 123–133. doi:10.1016/0022-1910(65)90099-5
Lockshin, R. A., and Williams, C. M. (1964). Programmed cell death—II: Endocrine potentiation of the breakdown of the intersegmental muscles of silkmoths. J. Insct Physiol. 10, 643–649. doi:10.1016/0022-1910(64)90034-4
Lockshin, R. A., and Zakeri, Z. (2001). Programmed cell death and apoptosis: Origins of the theory. Nat. Rev. Mol. Cell Biol. 2 (7), 545–550. doi:10.1038/35080097
Lowe, S. W., Bodis, S., Bardeesy, N., McClatchey, A., Remington, L., Ruley, H. E., et al. (1994). Apoptosis and the prognostic significance of p53 mutation. Cold Spring Harb. Symp. Quant. Biol. 59, 419–426. doi:10.1101/sqb.1994.059.01.047
Luna-Vargas, M. P. A., and Chipuk, J. E. (2016). Physiological and pharmacological control of BAK, BAX, and beyond. Trends Cell Biol. 26 (12), 906–917. doi:10.1016/j.tcb.2016.07.002
Maghsoudi, N., Zakeri, Z., and Lockshin, R. A. (2012). Programmed cell death and apoptosis-where it came from and where it is going: From elie metchnikoff to the control of caspases. Exp. Oncol. 34 (3), 146–152.
Mishra, N., Wei, H., and Conradt, B. (2018). Caenorhabditis elegans ced-3 caspase is required for asymmetric divisions that generate cells programmed to die. Genetics 210 (3), 983–998. doi:10.1534/genetics.118.301500
Peker, N., and Gozuacik, D. (2020). Autophagy as a cellular stress response mechanism in the nervous system. J. Mol. Biol. 432 (8), 2560–2588. doi:10.1016/j.jmb.2020.01.017
Rubio-Patiño, C., Trotta, A. P., and Chipuk, J. E. (2019). MDM2 and mitochondrial function: One complex intersection. Biochem. Pharmacol. 162, 14–20. doi:10.1016/j.bcp.2018.10.032
Sahdev, S., Taneja, T. K., Mohan, M., Sah, N. K., Khar, A. K., Hasnain, S. E., et al. (2003). Baculoviral p35 inhibits oxidant-induced activation of mitochondrial apoptotic pathway. Biochem. Biophys. Res. Commun. 307 (3), 483–490. doi:10.1016/s0006-291x(03)01224-5
Teng, X., and Hardwick, J. M. (2015). Cell death in genome evolution. Semin. Cell Dev. Biol. 39, 3–11. doi:10.1016/j.semcdb.2015.02.014
Trauth, B. C., Klas, C., Peters, A. M., Matzku, S., Möller, P., Falk, W., et al. (1989). Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science 245 (4915), 301–305. doi:10.1126/science.2787530
Trotta, A. P., and Chipuk, J. E. (2017). Mitochondrial dynamics as regulators of cancer biology. Cell Mol. Life Sci. 74 (11), 1999–2017. doi:10.1007/s00018-016-2451-3
Tsujimoto, Y., and Shimizu, S. (2007). Role of the mitochondrial membrane permeability transition in cell death. Apoptosis 12, 835–840. doi:10.1007/s10495-006-0525-7
Xue, D., Shaham, S., and Horvitz, H. R. (1996). The Caenorhabditis elegans cell-death protein CED-3 is a cysteine protease with substrate specificities similar to those of the human CPP32 protease. Genes Dev. 10 (9), 1073–1083. doi:10.1101/gad.10.9.1073
Yonish-Rouach, E., Resnftzky, D., Lotem, J., Sachs, L., Kimchi, A., and Oren, M. (1991). Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature 352, 345–347. doi:10.1038/352345a0
Keywords: apoptosis, programmed cell death, necroptosis, history, review, autophagy
Citation: Lockshin RA (2023) One-half century (or more) of study of cell death: origins, present, and perhaps future. Front. Cell. Death 2:1197400. doi: 10.3389/fceld.2023.1197400
Received: 31 March 2023; Accepted: 09 May 2023;
Published: 16 June 2023.
Edited by:
Lawrence M. Schwartz, University of Massachusetts Amherst, United StatesReviewed by:
Kimberly McCall, Boston University, United StatesCopyright © 2023 Lockshin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Richard A. Lockshin, cmxvY2tzaGluQGdtYWlsLmNvbQ==
 Richard A. Lockshin
Richard A. Lockshin