
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Clin. Diabetes Healthc. , 02 May 2024
Sec. Diabetes Clinical Epidemiology
Volume 5 - 2024 | https://doi.org/10.3389/fcdhc.2024.1319840
 Khisimusi Debree Maluleke
Khisimusi Debree Maluleke Cairo Bruce Ntimana*
Cairo Bruce Ntimana* Reneilwe Given Mashaba
Reneilwe Given Mashaba Kagiso Peace Seakamela
Kagiso Peace Seakamela Eric Maimela
Eric MaimelaBackground: Diabetic retinopathy (DR) is the major cause of vision impairment or blindness in individuals who have diabetes. It has accounted for 2.6% of all cases of blindness, and 1.9% of all cases of vision impairments globally. There is a lack of data on the prevalence of diabetic retinopathy and its associated factors amongst diabetic rural populations. Hence, the current study aimed to determine factors associated with diabetic retinopathy (DR) among diabetes mellitus (DM) patients undergoing diabetic therapy.
Methods: The study was cross-sectional in design and the participants were selected using convenient sampling. STATA version 15 software was used for data analysis. Chi-square was used to compare proportions. Logistic regression was used to determine the relationship between DR and associated risk factors.
Results: The prevalence of DR was 35.3%, of which 32% were mild and 3.4% were moderate non-proliferative DR (NPDR). Females were more unemployed than males (32.1% versus 16.8%, p=0.0058). Males were found to drink alcohol (21.8% versus 1.9%, p<0.001) and smoke cigarettes (4% versus 0.3%, p=0.0034) more than females. Being aged ≥ 55 years (OR: 2.7, 95% CI: 1.6-4.4), with matric qualification (OR: 0.6; 95% CI: 0.4-1.0); employed (OR: 1.4, 95% CI: 1.2-1.6); having high systolic blood pressure (OR=1.4, 95%CI=1.1-1.7) were the independent determinants of DR.
Conclusions: The prevalence of diabetic retinopathy was 34%. DR was determined by high systolic blood pressure, old age, and employment. Although not statistically significant, gender, hyperglycemic state, poor glycemic control, smoking, and increased body mass index (BMI) were associated with increased risk of developing DR.
Diabetic retinopathy (DR) is the major cause of vision impairment or blindness in individuals who have diabetes (1). It is caused by persistent high blood sugar levels, which destroy the blood vessels or capillaries in the retina, resulting in a blockage or leakage from the retinal capillaries (2). In response to intra-retinal hemorrhage and blockage, the retina promotes the formation of new vessels to compensate for the malfunctioning ones (3). The formation of new vessels and/or the release of blood-borne components from damaged vessels can cause substantial vision loss and scarring (4, 5).
In 2010, roughly 800,000 diabetics were deemed blind, while 3.7 million diabetics were identified as visually impaired due to DR, with DR rising rapidly from 27% in 1990 to 64% in 2012 worldwide (6). Diabetic retinopathy has accounted for 2.6% of all cases of blindness, and 1.9% of all cases of vision impairments globally, which is an increase from 2.1% of blindness and 1.3% of vision impairments (6–8)
Risk factors influencing the rapid development of DR in diabetic people include; age (2); duration of diabetes mellitus (DM) (9), poorly controlled DM (10), and elevated BMI (11). Hypertension (12–16), type of DM (11), pregnancy (17), lipedema (9), nephropathy (18), and smoking (1) are also predisposing factors for DR. Other variables that contribute to the occurrence of DR include socioeconomic challenges such as insufficient access to medical care, inadequate facilities, poor loss gross domestic products (GDPs), and insufficient finance for the healthcare (19–22). Moreover, unhealthy lifestyles, physical inactivity, and bad eating habits are reported to be associated with DR development (23, 24).
The global prevalence of DR was estimated to be between 27% and 28.1% (25, 26). The regional prevalence of DR is 20.6% in Europe, 12.5% in South East Asia, 36.2% in the Western Pacific, and 33.8% in Africa and the Middle East region (26). The continued increase in the prevalence of DM in low-to-middle-income countries has contributed to the increased prevalence of DR (27). The prevalence of DR in South Africa was reported to be at 71% in a study conducted in KwaZulu-Natal (28), however, there is little information on the prevalence of DR and its contributing factors among the rural black population in diabetic patients. Given the ongoing rise in diabetes mellitus, which may eventually result in DR, the current study aimed to determine the prevalence and risk factors of retinopathy (DR) among diabetes mellitus (DM) patients undergoing diabetic therapy in the Maruleng sub-district in Limpopo Province.
This cross-sectional survey was undertaken in all ten community clinics and the District Hospital in the Maruleng sub-district, Mopani District, Limpopo Province, South Africa. The Turfloop Research and Ethics Committee (TREC/28/2020: PG) of the University of Limpopo in South Africa gave ethical approval. The Limpopo Department of Health’s Provincial Health Research and Ethics Committee approved permission for this study to be conducted in the specified public healthcare facilities
The study consisted of 416 DM patients aged 18 years or older. Participants were selected using convenient sampling. Participants with gestational diabetes were excluded.
A piloted structured questionnaire was used to collect data, the questionnaire consisted of socio-demographics, behavioral (cigarette and alcohol drinking habits), history of DM, and treatment compliance information questions. The questionnaire was validated using the following steps: A draft of the questionnaire and a validation form for the validation procedures were submitted to the researchers who are aspect in validating questionnaires. Each item on the form was given a score based on its relevance (1 = not relevant, 2 = somewhat relevant, 3 = quite relevant, and 4 = extremely important), as well as its clarity (1 = not clear, 2 = somewhat clear, 3 = quite clear, and 4 = highly clear). The level of agreement and content validity index were calculated using these scores, and a mean content validity index of 0.80 or higher was considered acceptable (29). The questionnaire was considered valid after it was able to measure the intended parameters when repeated several times. Furthermore, the questionnaire underwent additional validation through a pilot survey involving the initial 8 participants. Blood pressure (BP), glucose, height, and weight were measured by incumbent nursing personnel at the reception area of every facility during the time of the study.
Eye care personnel performed a fundus examination using indirect ophthalmoscopy with 20 diopters (D) Volk lens to assess retinal integrity on dilated pupils to obtain a detailed view using 1% of Tropicamide eye drops. The presence of retinal abnormalities such as microaneurysms, hemorrhages, hard exudates, cotton wool spots, retinal venous beading, and other retinal microvascular abnormalities like neovascularization (new blood vessel growth) within one-disc diameter (NVD) or elsewhere on the retina (NVE) was used as a diagnostic criterion. Mild non-proliferative DR was diagnosed by the presence of at least one microaneurysm. Moderate non-proliferative DR was diagnosed by the presence of multiple microaneurysms plus hemorrhages. Severe non-proliferative DR was diagnosed by the presence of multiple microaneurysms, hemorrhage or hard exudates or cotton wool spots or venous beadings. Proliferative DR (PDR) was diagnosed by the presence of visible new blood vessels growing on the disc or elsewhere on the retina, etc., based on the Early Treatment Diabetic Retinopathy Study (ETDRS) (1) criteria for international classification/grading of DR.
Data analysis was carried out using the STATA version 15 for Windows (STATA Corp LP, College Station, TX, USA). Categorical variables were presented as numbers and percentages, which were reported as proportions at a 95% confidence interval (CI). The strength of association between the dependent variable (DR) and the independent variables (age, gender, educational and employment status, systolic blood pressure, hyperglycemia, smoking, and body mass index) was determined using logistic regression analysis, and the results were reported as odds ratios at 95% confidence intervals.
A total of 416 DM patients receiving diabetic chronic treatment were enrolled in this study, of which the majority (76%) were females, and only 24% were males. Table 1 below shows that significantly more females than males were unemployed (32.1% versus 16.8%, p=0.0058). Males were significantly more likely to drink alcohol (21.8% versus 1.9%, p<0.001) and smoke cigarettes (4% versus 0.3%, p=0.0034) as compared to females.
The overall prevalence of DR among 416 patients was 35.4%, comprising 32% of mild non-proliferative DR (NPDR) and 3.4% of moderate NPDR (Figure 1).
In Table 2. Participants aged 55 and above had the highest proportion of diabetic retinopathy. The majority of participants in the aged group < 45-54 years had no diabetic retinopathy as compared to those with diabetic retinopathy. The proportion of participants with no matric certificates, and pensioners was higher in diabetic retinopathy as compared to non-diabetic retinopathy respectively (91.2 vs 81.4, p=0.010), (71.4 vs 9.7, p=<0.001). The proportion of unemployed, participants with matric certificates and alcohol consumption was higher in non-diabetic retinopathy as compared to diabetic retinopathy respectively (33.1 vs 19.7, p=0.004), (10.8 vs 3.4, p=0.008), (9.7 vs 7.1, p= 0.001).
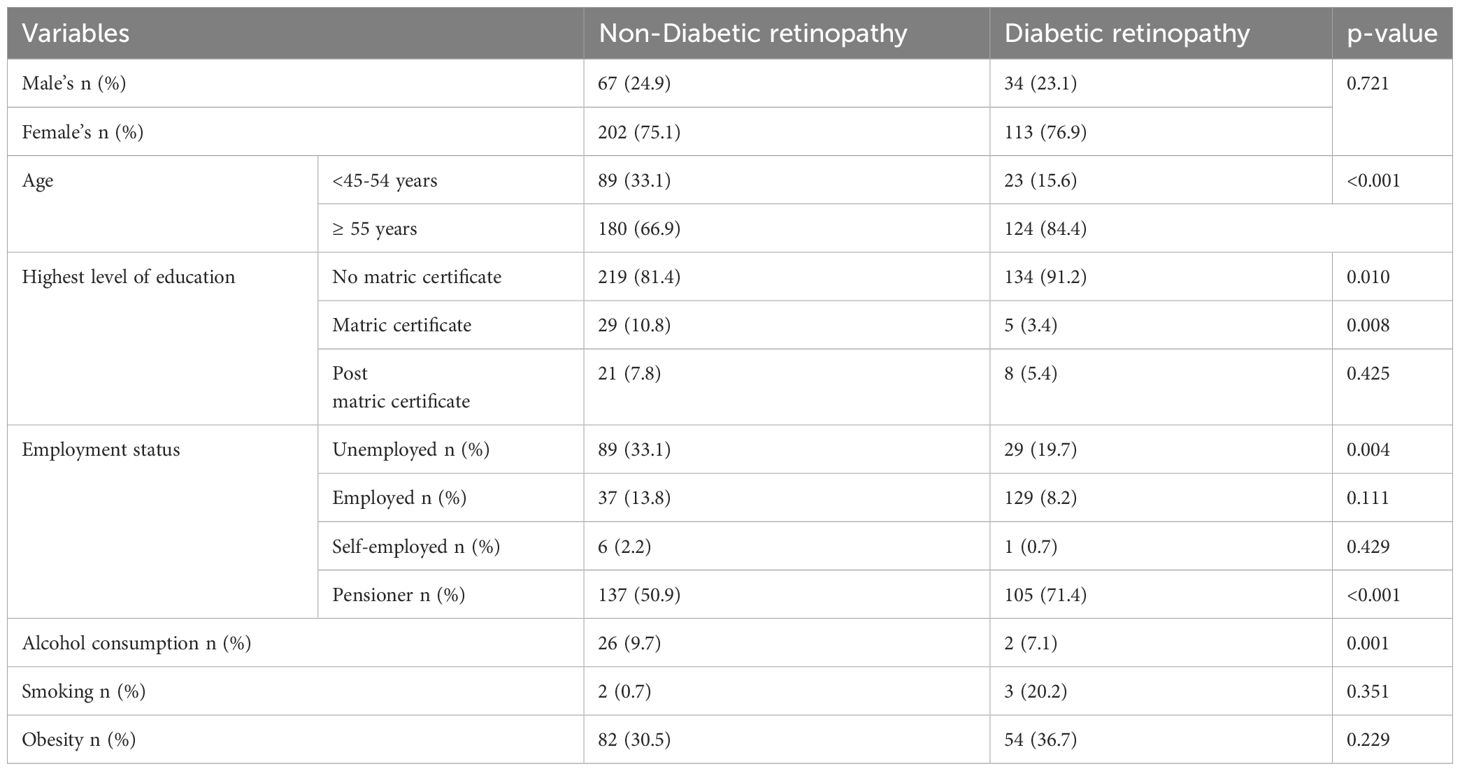
Table 2 Comparison between socio-demographic profiles of participants with diabetic retinopathy and those without diabetic retinopathy.
Table 3. Presents a comparison of the sociodemographic characteristics between participants with diabetes retinopathy and those without non-diabetic retinopathy. Type 2 DM participants with diabetic retinopathy had a higher proportion of pensioners, and participants with no matric as compared to those with non-diabetic retinopathy respectively (72.3 vs 51.1, p=0.002), (91.5 vs 81.7, p=0.008). Type 2 DM participants with non-diabetic retinopathy had a higher proportion of unemployment and alcohol consumption, and participants with matric as their highest level of education as compared to those with diabetic retinopathy respectively (33.2 vs 18.4, p=0.002), (9.2 vs 1.4, p=0.002), and (11.1 vs 3.3, p=0.008). In type 1 DM, there was no significant difference in the socio-demographic profiles between with participant’s diabetic retinopathy and those without diabetic retinopathy.
The predictors of DR are shown in Table 4 after a logistic regression analysis model was used to assess the strength of a relationship between the dependent variable (i.e. DR) and independent factors. Old diabetic participants aged 55 and above, employed dietetic, and dietetic participants who were hypertensive were more likely to have diabetic retinopathy. DM participants with matric or more and DM participants who were alcohol consumers were less likely to have DR. Diabetic individuals who were smoking, hyperglycemic, poor glycemic control, and obese were associated with DR however, not statistically significant.
This study comprised type 1 and type 2 diabetes patients seeking treatment at public healthcare facilities in Maruleng, the local municipality of Mopani District, Limpopo Province. The overall proportion of diabetic retinopathy (DR) was 35.3%. DR in this study was more prevalent in females, type 2 DM patients, and older patients as in the other reported study in Spain (30) but contrary to other reported studies in Ethiopia (31), and Saudi Arabia (16), wherein the DR was more prevalent in males. However, only type 2 DM and patients over the age of 60 were consistent in these investigations. These findings are comparable to a systematic meta-analysis which reported a global prevalence of DR to be between 27% and 28.1% (25, 26), which was lower than the prevalence of our study, and this difference could be due to unequal healthcare systems around the world or year of publication (25).
The present study has shown a higher prevalence of DR than the prevalence of DR in the region of Europe, South East Asia, the Middle East, and North Africa, which was reported between 12.5% and 33.8% (32) contrary to a higher prevalence of DR in the region of Western Pacific (26). This could be due to the high prevalence of diabetes and other risk factors for non-communicable diseases in South Africa (33). Previous studies in South Africa carried out at the National Hospital in Bloemfontein (34), and Tshwane district (35) indicated the prevalence of DR to be 10.8% and 24.9%, respectively, which is lower than the proportion of DR in the current study. However, Mahomed et al. reported a higher prevalence of DR (71%) in their study which was carried out in KwaZulu-Natal at a provincial Hospital (28). This could be owing to the research methodology or kind of sample utilized to collect data, notably, the use of the diagnoses was categorized into 11 broad groups of eye conditions previous study (28), where there was a high possibility of participants over-reporting the prevalence. Thus the study elaborating the importance of using clinical confirmed diagnosis of DR
There was no relationship between DR and gender (both sexes) in this study. In accordance with the present Zhang et. (36), reported similar findings. However, Wat et al. reported DR to be prevalent in males (11). Similarly, several studies reported a positive association between the male gender and DR (37, 38). The inconsistencies between the present study and the study by Cui et al. (37), and Yin et al. (38), may be due to the difference in sample size and different geographical locations. In addition, the present study was conducted among participants aged 18 and above but the study by Cui et al, only considered participants aged 40 and above.
In the present study, participants aged 55 and above had the highest proportion of diabetic retinopathy as compared to those without it, and the participants aged <45-54 years had no diabetic retinopathy as compared to those with diabetic retinopathy. Binary logistic regression showed that participants aged 55 and above were 27 times more likely to have DR. In agreement with the present study Learned and Pieramici (2), reported old age to be positively associated with DR. Old age is reported to be associated with diabetes and hypertension (39). Diabetes and hypertension are reported to co-exist together which can ultimately lead to DR (11).
The proportion of unemployed participants was higher in non-diabetic retinopathy as compared to diabetic retinopathy. However, the proportion of pensioners was higher in diabetic retinopathy as compared to non-diabetic retinopathy. In the Logistic regression employed, participants were significantly associated with DR which concurs with the finding of the previous study reported by Thomas et al. (26). However, other studies reported different findings (31, 32).
The proportion of alcohol consumption was higher in non-diabetic retinopathy as compared to diabetic retinopathy. Logistic regression showed a negative association between alcohol consumption and DR. The findings of the present study concur with the study by Gupta et al. (40), who reported alcohol consumption to be negatively associated with DR (40). Similarly, Xu et al. (41) noted an association between baseline alcohol consumption and a lower prevalence of DR (41). However, other studies reported no association between alcohol consumption and DR (42, 43). The difference between the present study and other studies that reported the insignificant association between alcohol consumption and DR may be due to that in the present study there was no measurement of the alcohol quantity. The present study found no association between smoking and DR. These findings are similar to several studies that reported a non-significant association between smoking and DR (11, 44, 45).
A high systolic blood pressure (or hypertension) in the present study was significantly associated with DR, and this concurs with the findings of the other previously reported studies (12–16). The relationship between hypertension and diabetic retinopathy may be explained by the clinical observation that hypertension and diabetes usually coexist (11). Hypertension causes blood vessels in the eye to rupture and bleed, damaging the nerves in the eye, which ultimately results in the blockage of arteries and veins that carry blood to the retina and out of the retina (46, 47).
The prevalence of diabetic retinopathy among DM participants receiving chronic diabetic treatment from public or state-owned healthcare in Maruleng, Mopani district of Limpopo province in South Africa was 34%. DR was more prevalent in females, type 2 DM patients, and older DM patients. The age of DM patients, employment status, and high systolic blood pressure (hypertension) were significantly associated with an increased risk of developing DR.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Turfloop Research Ethics Committee (TREC) (TREC/320/2017:PG). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
DKM: Conceptualization, Methodology, Statistical analysis, Validation, Writing – review & editing. CBN: Conceptualization, Methodology, Statistical analysis, Validation, Writing - review & editing. RGM: Conceptualization, Validation, Writing - review & editing. KPS: Conceptualization, Validation, Writing - review & editing. EM: Conceptualization, Methodology, Writing - review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The financial support to conduct the research was provided by the Health and Welfare Sector Education and Training Authority (HWSETA).
The authors would like to acknowledge the health professionals of Marulaneng Hospital for their assistance in the data collection process, and the health care faculties management for hosting the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kanski JJ, Bowling B. Kanski’s clinical ophthalmology e-book: a systematic approach. Australia: Elsevier Health Sciences (2015).
2. Learned D, Pieramici DJ. Epidemiology and natural history of diabetic retinopathy, in: Current management of diabetic retinopathy (2018). Elsevier. Available online at: https://linkinghub.elsevier.com/retrieve/pii/B9780323484527000019 (Accessed 2023 Jul 17).
3. Simmons AB, Merrill MM, Reed JC, Deans MR, Edwards MM, Fuerst PG. Defective angiogenesis and intraretinal bleeding in mouse models with disrupted inner retinal lamination. Invest Ophthalmol Vis Sci. (2016) 57:1563–77. doi: 10.1167/iovs.15-18395
4. Armoon B, Karimy M. Epidemiology of childhood overweight, obesity and their related factors in a sample of preschool children from Central Iran. BMC Pediatr. (2019) 19:1–8. doi: 10.1186/s12887-019-1540-5
5. Campbell M, Doyle SL. Current perspectives on established and novel therapies for pathological neovascularization in retinal disease. Biochem Pharmacol. (2019) 164:321–5. doi: 10.1016/j.bcp.2019.04.029
6. Leasher JL, Bourne RR, Flaxman SR, Jonas JB, Keeffe J, Naidoo K, et al. Global estimates on the number of people blind or visually impaired by diabetic retinopathy: a meta-analysis from 1990 to 2010. Diabetes Care. (2016) 39:1643–9. doi: 10.2337/dc15-2171
7. Jonas JB, Sabanayagam C. Epidemiology and risk factors for diabetic retinopathy. In: Diabetic retinopathy and cardiovascular disease. Ghana: Karger Publishers (2019). p. 20–37.
8. Nuertey BD, Amissah-Arthur KN, Addai J, Adongo V, Nuertey AD, Kabutey C, et al. Prevalence, causes, and factors associated with visual impairment and blindness among registered pensioners in Ghana. J Ophthalmol. (2019) 2019. doi: 10.1155/2019/1717464
9. Zhang G, Chen H, Chen W, Zhang M. Prevalence and risk factors for diabetic retinopathy in China: a multi-hospital-based cross-sectional study. Br J Ophthalmol. (2017) 101:1591–5. doi: 10.1136/bjophthalmol-2017-310316
10. Kaur P, Kotru S, Singh S, Munshi A. miRNA signatures in diabetic retinopathy and nephropathy: delineating underlying mechanisms. J Physiol Biochem. (2022) 78:19–37. doi: 10.1007/s13105-021-00867-0
11. Wat N, Wong RL, Wong IY. Associations between diabetic retinopathy and systemic risk factors. Hong Kong Med J. (2016) 22:589. doi: 10.12809/hkmj164869
12. Cleland CR, Burton MJ, Hall C, Hall A, Courtright P, Makupa WU, et al. Diabetic retinopathy in Tanzania: prevalence and risk factors at entry into a regional screening programme. Trop Med Int Health. (2016) 21:417–26. doi: 10.1111/tmi.12652
13. Lewis AD, Hogg RE, Chandran M, Musonda L, North L, Chakravarthy U, et al. Prevalence of diabetic retinopathy and visual impairment in patients with diabetes mellitus in Zambia through the implementation of a mobile diabetic retinopathy screening project in the Copperbelt province: a cross-sectional study. Eye. (2018) 32:1201–8. doi: 10.1038/s41433-018-0055-x
14. Shiferaw WS, Akalu TY, Desta M, Kassie AM, Petrucka PM, Assefa HK, et al. Glycated hemoglobin A1C level and the risk of diabetic retinopathy in Africa: A systematic review and meta-analysis. Diabetes Metab Syndrome: Clin Res Rev. (2020) 14:1941–9. doi: 10.1016/j.dsx.2020.10.003
15. Tan GS, Gan A, Sabanayagam C, Tham YC, Neelam K, Mitchell P, et al. Ethnic differences in the prevalence and risk factors of diabetic retinopathy: the Singapore epidemiology of eye diseases study. Ophthalmology. (2018) 125:529–36. doi: 10.1016/j.ophtha.2017.10.026
16. Yasir ZH, Hassan AD, Rajiv K. Diabetic retinopathy (DR) among 40 years and older Saudi population with diabetes in Riyadh governorate, Saudi Arabia–A population based survey. Saudi J Ophthalmol. (2019) 33:363–8. doi: 10.1016/j.sjopt.2019.03.001
17. Chandrasekaran PR, Madanagopalan VG, Narayanan R. Diabetic retinopathy in pregnancy-A review. Indian J Ophthalmol. (2021) 69:3015. doi: 10.4103/ijo.IJO_1377_21
18. Wang J, Xin X, Luo W, Wang R, Wang X, Si S, et al. Anemia and diabetic kidney disease had joint effect on diabetic retinopathy among patients with type 2 diabetes. Invest Ophthalmol Vis Sci. (2020) 61:25–5. doi: 10.1167/iovs.61.14.25
19. Bhatia M, Kumar M, Dixit P, Dwivedi LK. Diagnosis and treatment of hypertension among people aged 45 years and over in India: a sub-national analysis of the variation in performance of Indian states. Front Public Health. (2021) 9:766458. doi: 10.3389/fpubh.2021.766458
21. Nunes C, Pereira AM, Morais-Almeida M. Asthma costs and social impact. Asthma Res Practice. (2017) 3:1–11. doi: 10.1186/s40733-016-0029-3
22. Sahoo H, Govil D, James KS, Prasad RD. Health issues, health care utilization and health care expenditure among elderly in India: Thematic review of literature. Aging Health Res. (2021) 1:100012. doi: 10.1016/j.ahr.2021.100012
23. Candari CJ, Cylus J, Nolte E. Assessing the economic costs of unhealthy diets and low physical activity: An evidence review and proposed framework. WHO Regional Office for Europe (2017). vol. 47.
24. Gale R, Scanlon PH, Evans M, Ghanchi F, Yang Y, Silvestri G, et al. Action on diabetic macular oedema: achieving optimal patient management in treating visual impairment due to diabetic eye disease. Eye. (2017) 31:S1–20. doi: 10.1038/eye.2017.53
25. Hashemi H, Rezvan F, Pakzad R, Ansaripour A, Heydarian S, Yekta A, et al. Global and regional prevalence of diabetic retinopathy; a comprehensive systematic review and meta-analysis, in: Seminars in Ophthalmology. (2022). pp. 291–306. Taylor & Francis.
26. Thomas RL, Halim S, Gurudas S, Sivaprasad S, Owens DR. IDF Diabetes Atlas: A review of studies utilising retinal photography on the global prevalence of diabetes related retinopathy between 2015 and 2018. Diabetes Res Clin Practice. (2019) 157:107840. doi: 10.1016/j.diabres.2019.107840
27. Vujosevic S, Aldington SJ, Silva P, Hernández C, Scanlon P, Peto T, et al. Screening for diabetic retinopathy: new perspectives and challenges. Lancet Diabetes Endocrinol. (2020) 8:337–47. doi: 10.1016/S2213-8587(19)30411-5
28. Mahomed S, Verwey VF. Burden of eye conditions at a specialised eye hospital in KwaZulu-Natal, South Africa. Afr Vision Eye Health. (2020) 79:1–5. doi: 10.4102/aveh.v79i1.518
29. Ferreira R de MM, Junior LA, Correa KM, Alvares M, Mendonça R, Vieira RA, et al. Development and validity evidence of a questionnaire to assess the risk of hypertension in primary health care. Population Med. (2023) 5. doi: 10.18332/popmed/164466
30. López M, Cos FX, Álvarez-Guisasola F, Fuster E. Prevalence of diabetic retinopathy and its relationship with glomerular filtration rate and other risk factors in patients with type 2 diabetes mellitus in Spain. DM2 HOPE study. J Clin Trans Endocrinology. (2017) 9:61–5. doi: 10.1016/j.jcte.2017.07.004
31. Shibru T, Aga F, Boka A. Prevalence of diabetic retinopathy and associated factors among type 2 diabetes patients at tikur anbessa hospital Ethiopia. J Diabetes Metab. (2019) 10. doi: 10.35248/diabetes-metabolism
32. Valizadeh R, Moosazadeh M, Bahaadini K, Vali L, Lashkari T, Amiresmaili M. Determining the prevalence of retinopathy and its related factors among patients with type 2 diabetes in Kerman, Iran. Osong Public Health Res Perspectives. (2016) 7:296–300. doi: 10.1016/j.phrp.2016.08.004
33. van Heerden A, Barnabas RV, Norris SA, Micklesfield LK, van Rooyen H, Celum C. High prevalence of HIV and non-communicable disease (NCD) risk factors in rural KwaZulu-Natal, South Africa. J Int AIDS Society. (2017) 20:e25012. doi: 10.1002/jia2.25012
34. Cairncross JP, Steinberg WJ, Labuschagne MJ. Prevalence of eye pathology in a group of diabetic patients at National District Hospital Outpatient Department in Bloemfontein, South Africa. Afr J Primary Health Care Family Med. (2017) 9:1–7. doi: 10.4102/phcfm.v9i1.1440
35. Webb EM, Rheeder P, Roux P. Screening in primary care for diabetic retinopathy, maculopathy and visual loss in South Africa. Ophthalmologica. (2016) 235:141–9. doi: 10.1159/000443972
36. Zhang L, Chen B, Tang L. Metabolic memory: mechanisms and implications for diabetic retinopathy. Diabetes Res Clin Practice. (2012) 96:286–93. doi: 10.1016/j.diabres.2011.12.006
37. Cui Y, Zhang M, Zhang L, Zhang L, Kuang J, Zhang G, et al. Prevalence and risk factors for diabetic retinopathy in a cross-sectional population-based study from rural southern China: Dongguan Eye Study. BMJ Open. (2019) 9:e023586. doi: 10.1136/bmjopen-2018-023586
38. Yin L, Zhang D, Ren Q, Su X, Sun Z. Prevalence and risk factors of diabetic retinopathy in diabetic patients: A community based cross-sectional study. Medicine. (2020) 99. doi: 10.1097/MD.0000000000019236
39. Kalyani RR, Golden SH, Cefalu WT. Diabetes and aging: unique considerations and goals of care. Diabetes Care. (2017) 40:440. doi: 10.2337/dci17-0005
40. Gupta P, Fenwick EK, Sabanayagam C, Gan ATL, Tham YC, Thakur S, et al. Association of alcohol intake with incidence and progression of diabetic retinopathy. Br J Ophthalmol. (2021) 105:538–42. doi: 10.1136/bjophthalmol-2020-316360
41. Xu L, You QS, Jonas JB. Prevalence of alcohol consumption and risk of ocular diseases in a general population: the Beijing Eye Study. Ophthalmology. (2009) 116:1872–9. doi: 10.1016/j.ophtha.2009.04.014
42. Lee CC, Stolk RP, Adler AI, Patel A, Chalmers J, Neal B, et al. Association between alcohol consumption and diabetic retinopathy and visual acuity-the AdRem Study. Diabetic Med. (2010) 27:1130–7. doi: 10.1111/j.1464-5491.2010.03080.x
43. Zhu W, Meng YF, Wu Y, Xu M, Lu J. Association of alcohol intake with risk of diabetic retinopathy: a meta-analysis of observational studies. Sci Rep. (2017) 7:4. doi: 10.1038/s41598-017-00034-w
44. Thapa R, Twyana SN, Paudyal G, Khanal S, Van Nispen R, Tan HS, et al. Prevalence and risk factors of diabetic retinopathy among an elderly population with diabetes in Nepal: the Bhaktapur Retina Study. Clin Ophthalmol. (2018) 12:561–8. doi: 10.2147/OPTH
45. Thapa R, Bajimaya S, Paudyal G, Khanal S, Tan S, Thapa SS, et al. Population awareness of diabetic eye disease and age related macular degeneration in Nepal: the Bhaktapur Retina Study. BMC Ophthalmol. (2015) 15:1–8. doi: 10.1186/s12886-015-0175-z
46. Srivastava BK, Rema M. Does hypertension play a role in diabetic retinopathy? J Assoc Physicians India. (2005) 53:803–8.
Keywords: prevalence, diabetic retinopathy, diabetes mellitus, blindness, non-proliferative
Citation: Maluleke KD, Ntimana CB, Mashaba RG, Seakamela KP and Maimela E (2024) Associated factors of diabetic retinopathy in type 1 and 2 diabetes in Limpopo province in South Africa. Front. Clin. Diabetes Healthc. 5:1319840. doi: 10.3389/fcdhc.2024.1319840
Received: 11 October 2023; Accepted: 15 March 2024;
Published: 02 May 2024.
Edited by:
Bayu Begashaw Bekele, Washington University in St. Louis, United StatesReviewed by:
Muhammad Akram, Government College University, Faisalabad, PakistanCopyright © 2024 Maluleke, Ntimana, Mashaba, Seakamela and Maimela. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cairo Bruce Ntimana, Y2Fpcm8ubnRpbWFuZUB1bC5hYy56YQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.