- 1Department of Periodontics and Oral Medicine, School of Dentistry, University of Michigan, Ann Arbor, MI, United States
- 2Department of Periodontics and Preventive Dentistry, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, United States
This Perspective provides a brief summary of the scientific evidence for the two-way links between periodontal diseases and hyperglycemia (diabetes mellitus [DM] and pre-DM). It delivers in a nutshell current scientific evidence for manifestations of hyperglycemia on periodontal health status and effects of periodontal diseases on blood glucose levels and in turn incidence, progression, and complications of diabetes. Of outmost importance is presentation of scientific evidence for the potential of routine periodontal treatment to lower blood glucose levels, providing a novel, economical tool in DM management. Non-surgical periodontal treatment (“deep cleaning”) can be provided by dental hygienists or dentists in general dental offices, although severe cases should be referred to specialists. Such therapy can decrease the costs of DM care and other health care costs for people with DM. The great importance of a healthy oral cavity free of infection and subsequent inflammation – especially periodontitis that if untreated will cause loosening and eventually loss of affected teeth – has largely gone unnoticed by the medical community as the health care curricula are largely void of content regarding the bi-directional links between oral health and systemic health, despite elevation of blood glucose levels being an integral part of the general systemic inflammation response. The importance of keeping disease-free, natural teeth for proper biting and chewing, smiling, self-esteem, and pain avoidance cannot be overestimated. Medical and dental professionals are strongly encouraged to collaborate in patient-centered care for their mutual patients with – or at risk for – hyperglycemia.
1 Introduction
Periodontitis and diabetes mellitus (DM) often co-occur: People with periodontitis have greater risk of hyperglycemia and those with hyperglycemia have greater risk of periodontitis. This is expected because these conditions are both chronic, inflammation-associated diseases that share the same modifiable (1) and non-modifiable risk factors (2) as other non-communicable inflammation related chronic diseases (3, 4) and both are independently associated with DM complications and mortality (5, 6). Moreover, research has demonstrated causal effects between hyperglycemia and periodontitis in a dose-response relationship in both directions (two-way or bi-directional relationship) (7–14). DM and periodontitis are prevalent globally and consume huge human, medical/dental, and financial resources, described in the Supplementary Material.
2 Periodontal diseases
Periodontal diseases affect the soft and hard tissues surrounding the teeth (15).
2.1 Gingivitis
Gingivitis is mostly dental plaque (bacteria and food remnants attached to teeth)-induced inflammation of the soft gingiva (gum tissue surrounding the teeth) and is reversible by home oral hygiene measures (tooth brushing, flossing, etc.) (16–18). Dental plaque can calcify above and below the gum line, causing inflammatory responses in the surrounding tissues due to both its hardness and to harboring microbes lodged in niches in its rough surface, causing inflammation (17–19).
2.1.1 Effect of hyperglycemia: biology
Nonetheless, in hyperglycemia, gingivitis can occur without dental plaque initiation (8, 9, 20–23) because excess blood glucose is toxic and induces mitochondrial stress and respiratory bursts in inflammatory stress that in turn activates proinflammatory mediator cascades (24). Additionally, advanced glycation end‐products (AGEs) and their receptors (RAGE) on the cell surface induce proinflammatory signaling cascades (10, 25).
2.1.2 Effect of hyperglycemia: prevalence
Persons with DM have much greater prevalence of gingivitis than non-DM (26).
2.2 Chronic periodontitis
2.2.1 Biology
In especially susceptible individuals with compromised immune systems, gingivitis can progress to periodontitis, a chronic, irreversible breakdown of both gingiva and jaw bone, the intensity and severity of which depend more on the host responses than on specific bacterial agents (27, 28). Deepened spaces (pockets) around the teeth harbor at least 700 (~1,000)? difference species of bacteria plus virus, fungi, and archaea, several of which easily penetrate the inflamed, swollen gingival tissues and travel everywhere in the host via all body fluids (blood, saliva, lymph, tears, urine) and along nerves (29). Porphyromonas gingivalis (Pg) (30), “a master of immune subversion” (31) has developed sophisticated mechanisms to avoid detection/dissolution by the host’s immune system and survives during its body-wide travels (29, 32), resulting in Pg and its byproducts (polysaccharides) causing local and systemic inflammation (27–29).
2.2.2 Effect of hyperglycemia: biology
The structure and relative abundance (composition) of bacteria in the subgingival microbiome are significantly different in normoglycemia and hyperglycemia (33–40) and predicts glucose change in non-DM (41).
Hyperglycemia leads to inflammatory response disturbances (42), enhancing pro-inflammatory cytokines and matrix metalloproteinases (MMP) expression (35), so the periodontal tissue metabolism adversely affects blood vessels and promotes periodontitis, which develops more rapidly and more intensely (43), with hyperglycemia enhancing its progression (44, 45), even at pre-DM levels (46). Importantly, hyperglycemia severity, not the DM diagnosis, affects the periodontium (47, 48).
2.2.3 Effect of hyperglycemia: prevalence
Periodontitis was proclaimed DM’s 6th complication in 1993 (49)–without much attention from the medical community. Hyperglycemia is a risk driver for incident periodontitis (1, 5–8, 22, 23, 45, 50–52), and increases its severity (37) (Supplementary Material).
2.3 Tooth loss due to periodontitis
If left untreated, periodontitis may result in loosening and eventual loss of the tooth (15), being one of 2 major causes (with caries) of tooth loss in adults (53–57).
2.3.1 Effects of hyperglycemia and tooth loss
People with DM have an impaired immune system and experience 1.3-5-fold greater tooth loss (53, 58–63), including losing all teeth (edentulism) (61, 64, 65), compared to those with normoglycemia. Loose or missing teeth decrease quality of life (66–72) and impair masticatory function (73, 74), preventing biting/chewing crisp or hard healthy diet components recommended (74, 75), leading to consumption of soft food items typically containing few fibers and nutrients (76, 77), laden with sugar, fat, and salt.
2.3.2 Tooth substitutes: dental implants and removable prostheses
A crown-restored dental implant placed in the jaw bone may replace a lost tooth, but implants suffer from diseases parallel to the natural teeth, namely the reversible peri-implant mucositis and the irreversible peri-implantitis, the latter being extremely challenging to treat successfully. A more economical alternative is a removable prosthesis that rests on some natural teeth. Both options are costly and beyond reach for many DM patients.
2.3.3 Effect of hyperglycemia: peri-implantitis
The composition of the microbiome around implants is significantly different from that around teeth, especially in the deep peri-implant pockets (78). Those with hyperglycemia experience much greater risk for peri-implantitis (79–83)–including metabolic syndrome with 15-fold risk reported (84)–and for implant loss (79), with failure rates greater in hyperglycemia than in other diseases (85, 86), and greater in D1T than in D2T (79).
3 DM/hyperglycemia
DM is a carbohydrate-lipid metabolic disorder caused by insufficient insulin production, insensitivity to normal amounts of insulin, or both, resulting in abnormally high blood glucose levels (hyperglycemia, dysglycemia) (85) (Supplementary Material).
3.1 Effect of periodontitis: biology
The chronic, repetitious discharge of periodontal microbes and their byproducts into the bloodstream causes inflammatory markers to circulate and hence create or exacerbate insulin resistance (87–91) and DM complications (92, 93).
3.2 Effects of periodontitis: incidence of T2D, glycemic control in existing DM, and DM complications
Periodontitis negatively influences glucose control in existing T2D, contributes to incident T2D and to DM complications in a dose-response manner (87–89, 94).
4 Mechanisms underlying the bidirectional links between periodontitis and DM
Figure 1 shows conceptual models of the mechanisms underlying the two-way effects linking periodontitis and DM (11), shown differently in References (7, 12, 13).
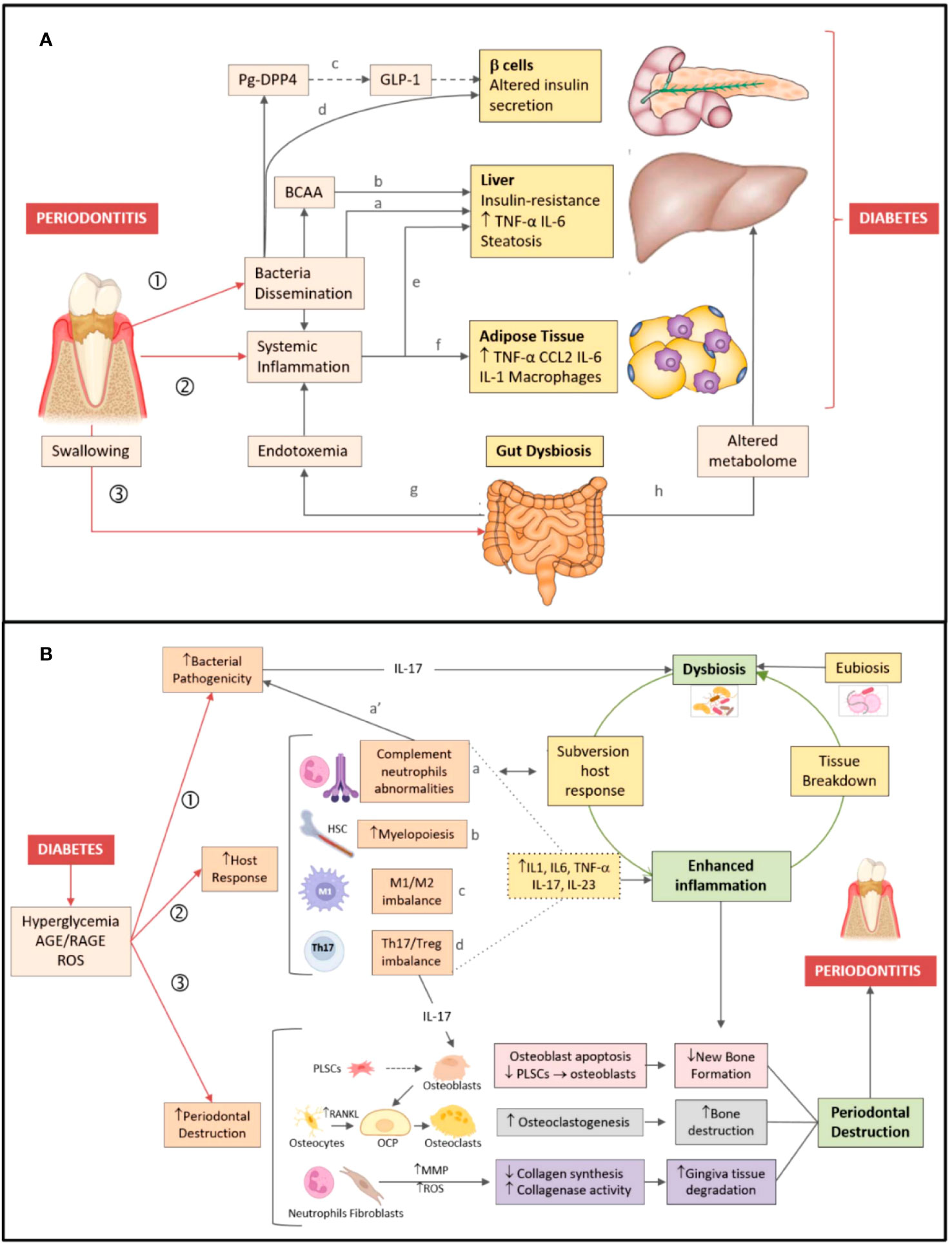
Figure 1 Bidirectional relationship between periodontitis and diabetes. (A) Periodontitis → diabetes direction. Periodontitis favors development/worsening of type 2 diabetes by three major mechanisms: (1) Dissemination of periodontal bacteria/bacterial products into the bloodstream. Bacteria/bacterial products can induce insulin resistance (a) by inhibiting hepatic glycogen synthesis, increasing hepatic gluconeogenesis, and (b) blocking the insulin receptor substrate via production of branched-chain amino acids (BCAA). (c) Dipeptidyl peptidase-4 (DPP4) produced by P. gingivalis (Pg-DPP4) can reduce glucose-induced insulin production by enhancing glucagon-like peptide 1 (GLP-1) degradation. (d) P. gingivalis may alter insulin production by inducing β cell dedifferentiation. (2) Induction/magnification of systemic inflammation, favoring both (e) hepatic and (f) adipose tissue insulin resistance. (3) Gut dysbiosis induced by swallowed periodontal bacteria, favoring both (g) endotoxemia and (h) changes in the blood metabolome. (B) Diabetes → periodontitis direction. Pathogenesis of periodontitis is depicted on the right-hand side of the figure. Dysbiosis, inflammation, and destruction of the periodontium (green boxes) are characteristic features of periodontitis. Dysbiotic bacteria reduce the efficacy of the host immune response, while fueling inflammation (open green arrow). In turn, inflammation-induced tissue breakdown favors dysbiosis (closed green arrow) closing the vicious cycle. Mechanisms linking diabetes to periodontitis are shown on the left-hand side of the figure. Diabetes favors development/worsening of periodontitis by three major mechanisms: (1) Increasing periodontal dysbiosis and bacterial pathogenicity via IL-17; (2) Enhancing the host response to the bacterial challenge. Diabetes (a) alters complement and neutrophil function (which also affects susceptibility to infection a’), (b) increases myelopoiesis, enhances (c) the M1/M2 macrophage ratio, (d) the Th17/Treg lymphocyte ratio, thus raising inflammatory cytokines levels (dotted lines) and fueling inflammation. (3) Increasing periodontal destruction. Diabetes reduces new bone formation by enhancing apoptosis of bone-forming cells and by lowering periodontal ligament stem cells (PLSCs) proliferation and differentiation in osteoblasts (pink boxes). Diabetes enhances osteoclastogenesis by increasing RANKL release by osteocytes/osteoblasts, leading to osteoclast precursor (OCP) differentiation in osteoclasts (grey boxes). Diabetes augments gingiva tissue degradation by increasing release of metalloproteinases (MMP) and reactive oxygen species (ROS) by neutrophils and fibroblasts (violet boxes) (11). Figure and legend created and copyrighted by Barutta et al. in 2022 (11), and reproduced here without any changes. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8774037/figure/biomedicines-10-00178-f001/. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution 4.0 International (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
5 Effects of periodontal treatment
5.1 Blood glucose level
Removal of soft and hardened plaque (calculus, “tartar”) above and below the gum line (non-surgical periodontal treatment, scaling and root planing, a. k. a. “deep cleaning”) can decrease HbA1c levels with at least 26 prior systematic reviews with meta-analyses and umbrella reviews calculating and reporting such HbA1c decrease to be around 0.5 percentage point from 3 to 12 months after periodontal therapy (7, 13, 95–118). This decrease is in the order of magnitude of the expected effect of adding a second oral anti-diabetic medication to metformin (119) and is hence clinically significant (13, 120). For a clinical perspective, 1 percentage point HbA1c reduction may reduce DM mortality by 21%, myocardial infarction by 14%, and DM microvascular complications by 37% (121). Non-surgical periodontal treatment reduces insulin resistance, improving insulin sensitivity in T2D (122).
Greater effect is seen with higher baseline HbA1c level (98), but decreases with increasing age (123), due to inflammaging or immunosenescence caused by diminishing immune defense efficiency in older ages (124–133).
5.2 Inflammatory markers
Periodontal treatment decreases levels of several inflammatory biomarkers, many of which in turn are risk factors for DM complications like atherosclerosis and myocardial infarction, such as c-reactive protein (134) interleukin-(IL)-1beta (91), IL-6 (91), tumor necrosis factor-alpha (TNF-alpha) (91). Statistically significant decreases in the concentration of the active inflammatory marker, c-reactive protein, from baseline to each 3-monthly visit up to 1 year upon extraction of terminally periodontally diseased teeth and scaling and root planing the remaining dentition are reported (134).
6 Interprofessional collaboration
The call for patient centered, interprofessional, transdisciplinary, and interdisciplinary collaboration is increasingly loud in an abundance of scientific papers and guidelines (4, 13, 135–147). Please see the APPENDIX and online-only Supplementary Material.
6.1 Hyperglycemia/DM in the dental setting
Because almost half of people with DM (148) and 90% of those with pre-DM are unaware thereof (148), the dental setting can be helpful in identifying these individuals, which is urgent for early diagnosis and prevention of DM complications (148, 149) Globally, dental professionals are increasingly aware of the two-directional association between hyperglycemia and periodontitis and they include DM in anamneses (150). They support chair-side screening for hyperglycemia despite citing barriers like time constraint, patient cooperation, cost/insurance coverage, and lack of equipment (150).
Two systematic reviews and meta-analyses of studies involving finger prick blood sampling among dental patients denying hyperglycemia calculated a T2D prevalence of 1.7%-46.4% and of pre-DM 23.3%-68.0% (151, 152). Gingival crevicular blood from the pocket is also a valid screening tool (153–157) because of its high correlation glucose concentration with serum. Hyperglycemia screening in the dental setting is found cost-effective (158, 159), and general physicians are receptive to receive referrals from dentists (160). Few teeth/edentulism and periodontitis are significantly associated with undiagnosed DM (63, 161) and hence predict potential hyperglycemia.
DM is such a strong risk driver for periodontitis that DM is incorporated in the grading of the stages of the most recent European Federation of Periodontology (EFP)/American Academy of Periodontology (AAP) periodontitis classification (162, 163). The Finnish Diabetes Risk Score (FINDRISC) and a periodontal disease risk score are significantly linearly correlated (164).
Federation Dentaire International (FDI) dedicated its entire 2017 World Forum to periodontitis and created action items to enhance global awareness of its connection to DM and other non-communicable diseases to promote general health (165) in line with the United Nations and World Health Organization (166).
A crude search on “diabetes” at the American Dental Association (ADA)’s home page (https://www.ada.org/) resulted in 93 hits.
The American Academy of Periodontology (AAP) recently created a 38-page report and 3 infographics that includes DM (167).
6.2 Periodontitis in DM management in the medical setting
The International Diabetes Federation (IDF) acknowledged the importance of good oral health in DM management by including an oral health section in the 9th edition of the IDF Diabetes Atlas (168), along with more detailed description in its scientific journal (120). Importantly, IDF and EFP published a consensus document with guidelines upon a joint workshop published in the respective organizations’ journals (5, 6).
In its 3rd edition of the mammoth work, “Diabetes in America” (169), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) included with great enthusiasm a 51-page long (versus 6 pages in the 2nd edition from 1995 (170)) chapter titled 31. Oral health and diabetes” (7), in which the periodontitis-DM links are prominently described, both by existing literature and by new analyses of National Health and Nutrition Examination Survey (NHANES) data specifically for this chapter.
A search for “oral health” at the American Medical Association (AMA)’s homepage (https://www.ama-assn.org/) turned up 2 hits, one of which links to a paper by physician Hugh Silk, a long-time, avid advocate for integration of primary care and oral health (139, 171–176).
6.2.1 American Diabetes Association (AD[M]A)
In contrast to all the global medical and dental organizations’ websites, there is no mention of oral health, let alone periodontitis, anywhere at AD[M]A’s website–despite:
1) periodontitis is an established risk factor for DM;
2) periodontitis is a DM complication;
3) international acceptance of the strong, current scientific evidence for the bi-directional causal relationship between periodontitis and hyperglycemia in both Medicine and Dentistry (5–7, 13, 120);
4) extraction of severely periodontally diseased teeth can decrease HbA1c concentration clinically significantly;
5) non-surgical periodontal therapy can decrease HbA1c concentration clinically significantly;
6) periodontal therapy decreases costs for both DM and general care, hospitalizations, and pharmaceuticals in patients with DM;
7) AD[M]A emphasizes a holistic approach to DM care, including in its consensus statement with the European Association for the Study of Diabetes (EASD) (177); and
8) initially poorly controlled DM patients improve their glucose control with dental visits (178).
6.2.1.1 “Standards of care in diabetes”
Every year, AD(M)A updates its “Standards of Care in Diabetes” guidelines for diagnosing and treating DM, published as a supplement to the January issue of its journal Diabetes Care. The 2023 edition consists of 20 individual articles totaling 302 pages (179). Regrettably, it still cites dated oral health related references published from 2011 to 2019 (180–183) under “2. Classification and Diagnosis of DM” (85) (exactly the same 4 references as in 2022 (184)) and in 2006-2018 (14, 88, 113, 185–187) under “4. Comorbidities” (188), respectively. The 2023 Writing Group even had the audacity to totally deny any relationship between DM and periodontitis or any other oral disease, as they write: “ASSESSMENT OF COMORBIDITIES: Besides assessing diabetes-related complications, clinicians and people with diabetes need to be aware of common comorbidities that affect people with diabetes and that may complicate management (14, 88, 113, 185–187). Diabetes comorbidities are conditions that affect people with diabetes more often than age-matched people without diabetes” (188). Note: There is not even any mention of oral diseases, which the attentive reader needs to discover by consulting the bibliography. In “Table 4.4—Referrals for initial care management,” it still says “Dentist for comprehensive dental and periodontal examination” (188), identically to in 2022 (189). The relevant text passage reads: “People with diabetes should receive recommended preventive care services (e.g., immunizations, cancer screening); smoking cessation counseling; and ophthalmological, dental, and podiatric referrals, as needed” (188).
Clearly, periodontitis is not considered part of ongoing DM management (179).
6.2.1.2 Why?
A crude PubMed search: “diabetes and (periodontitis or ‘oral health’)” resulted in 22,149 hits. Why does AD[M]A totally and purposefully deny the large body of scientific literature regarding roles of periodontitis and any other inflammatory oral disease, whose treatment provides a scientifically sound and uncomplicated, straightforward tool to prevent and manage hyperglycemia DM?
Due to the global reach of AD[M]A, this is extremely detrimental to both the global medical community and the patients they serve worldwide, especially in under-resourced settings.
6.2.1.2.1 Incorporation of periodontitis into medical management of DM
Despite primary care providers expressing positive attitudes towards medical-dental care models, integrated care is rarely practiced (190–192), with Marshfield Clinic Health System (MCHS) being an exception (146, 191, 193, 194).
6.2.1.2.2 Lack of incorporation of periodontitis into medical management of DM
The degree of tooth loss decreases with increasing numbers of dental visits (61). Despite international medical/endocrinologic guidelines (except the AD[M]A’s) recommending at least annual dental checkups, DM patients are globally and consistently reported to have fewer dental checkup visits than in normoglycemia (59, 195–202).
6.2.1.3 Periodontal care reduces cost of medical care in DM
Persons with DM have greater dental (203) and medical care costs (204, 205) than their normoglycemic peers. (Supplementary Material). However, periodontal therapy and maintenance visits decrease expenses for both DM care (206–209) and other medical care, including pharmaceuticals and hospitalizations (206, 207, 210–213).
6.2.1.4 Malpractice lawsuits
Due to the impaired immune response in DM, “dental clearance” prior to any invasive procedure may be prudent to minimize the risk for periodontitis/loose teeth serving as reservoirs for bacteria causing infection in remote locations (29, 214).
Patients are increasingly aware of the importance of periodontitis for general health and of the potentially fatal consequences of its neglect. For example, when the DM complication myocardial infarct occurs, medical care providers may experience legal consequences by the survivor or the deceased’s estate for neglecting referral for proper dental examination and therapy – just like the dentists neglecting examining for and managing periodontitis (215). Hence, legal liability is another reason for medical and dental care professionals to take periodontitis seriously.
7 Discussion
In a nutshell: Periodontitis and diabetes are strongly linked in a two-way causal relationship. Hyperglycemia negatively affects the periodontal soft and hard tissues, facilitating their breakdown and impairing their healing. Chronic periodontitis-associated bacteremia and subsequent local and systemic inflammation increase blood glucose levels, which in turn can be decreased by periodontal treatment by a clinically relevant magnitude around 0.5 percentage point HbA1c after up to 12 months of maintenance visits after the initial therapy.
Therefore, it would be prudent for medical and dental professionals to collaborate in patient-centered, interprofessional management teams caring for their mutual patients with hyperglycemia.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
WSB: Conceptualization, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The fee for the license to re-publish the content of the APPENDIX: “Guidelines for medical & dental professionals & their patients” was covered by the author’s private funds.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcdhc.2023.1257087/full#supplementary-material
References
1. Borgnakke WS. Modifiable risk factors for periodontitis and diabetes. Curr Oral Health Rep (2016) 3:254–69. doi: 10.1007/s40496-016-0099-6
2. Borgnakke WS. “Non-modifiable” risk factors for periodontitis and diabetes. Curr Oral Health Rep (2016) 3:270–81. doi: 10.1007/s40496-016-0098-7
3. Sheiham A, Watt RG. The common risk factor approach: a rational basis for promoting oral health. Community Dent Oral Epidemiol (2000) 28:399–406. doi: 10.1034/j.1600-0528.2000.028006399.x
4. Larvin H, Kang J, Aggarwal VR, Pavitt S, Wu J. Systemic multimorbidity clusters in people with periodontitis. J Dent Res (2022) 101:1335–42. doi: 10.1177/00220345221098910
5. Sanz M, Ceriello A, Buysschaert M, Chapple I, Demmer RT, Graziani F, et al. Scientific evidence on the links between periodontal diseases and diabetes: consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J Clin Periodontol (2018) 45:138–49. doi: 10.1111/jcpe.12808
6. Sanz M, Ceriello A, Buysschaert M, Chapple I, Demmer RT, Graziani F, et al. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International diabetes Federation and the European Federation of Periodontology. Diabetes Res Clin Pract (2018) 137:231–41. doi: 10.1016/j.diabres.2017.12.001
7. Borgnakke WS, Genco RJ, Eke PI, Taylor GW. Ch 31. Oral health and diabetes. In: Cowie C, Casagrande S, Menke A, Cissell MA, Eberhardt MS, Meigs JB, Gregg EW, Knowler WC, Barrett-Connor E, Becker DJ, Brancati FL, Boyko EJ, Herman WH, Howard BV, Narayan KMV, Rewers M, Fradkin JE, editors. Diabetes in america. Bethesda, MD: National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (NIH/NIDDK), (2018) 2018:31.01–31.51. NIH Pub No. 17-1468. Available at: https://www.ncbi.nlm.nih.gov/books/NBK567975/.
8. Chapple IL, Genco RJ, Working Group 2 of Joint EFP/AAP Workshop, Berglundh T, Borgnakke W, Eickholz P, et al. Diabetes and periodontal diseases: consensus report of the joint EFP/AAP workshop on periodontitis and systemic diseases. J Clin Periodontol (2013) 40 Suppl 14:S106–12. doi: 10.1111/jcpe.12077
9. Chapple IL, Genco RJ, Working Group 2 of Joint EFP/AAP Workshop, Berglundh T, Borgnakke W, Eickholz P, et al. diabetes and periodontal diseases: consensus report of the joint EFP/AAP workshop on periodontitis and systemic diseases. J Periodontol (2013) 84:S106–12. doi: 10.1902/jop.2013.1340011
10. Lalla E, Papapanou PN. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol (2011) 7:738–48. doi: 10.1038/nrendo.2011.106
11. Barutta F, Bellini S, Durazzo M, Gruden G. Novel Insight into the mechanisms of the bidirectional relationship between diabetes and periodontitis. Biomedicines (2022) 10:178. doi: 10.3390/biomedicines10010178
12. Borgnakke WS. Does treatment of periodontal disease influence systemic disease? Dent Clin North Am (2015) 59:885–917. doi: 10.1016/j.cden.2015.06.007
13. Borgnakke WS, Poudel P. Diabetes and oral health: summary of current scientific evidence for why transdisciplinary collaboration is needed. Front Dent Med (2021) 2:709831. doi: 10.3389/fdmed.2021.709831
14. Casanova L, Hughes FJ, Preshaw PM. Diabetes and periodontal disease: a two-way relationship. Br Dent J (2014) 217:433–37. doi: 10.1038/sj.bdj.2014.907
15. Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet (2005) 366:1809–20. doi: 10.1016/S0140-6736(05)67728-8
16. Fernandez-Gutierrez MM, Imangaliyev S, Prodan A, Loos BG, Keijser BJF, Kleerebezem M. A salivary metabolite signature that reflects gingival host-microbe interactions: instability predicts gingivitis susceptibility. Sci Rep (2020) 10:3008. doi: 10.1038/s41598-020-59988-z
17. Stone SJ, Kumar PS, Offenbacher S, Heasman PA, McCracken GI. Exploring a temporal relationship between biofilm microbiota and inflammatory mediators during resolution of naturally occurring gingivitis. J Periodontol (2019) 90:627–36. doi: 10.1002/JPER.18-0156
18. Zemouri C, Jakubovics NS, Crielaard W, Zaura E, Dodds M, Schelkle B, et al. Resistance and resilience to experimental gingivitis: a systematic scoping review. BMC Oral Health (2019) 19:212. doi: 10.1186/s12903-019-0889-z
19. Bamashmous S, Kotsakis GA, Kerns KA, Leroux BG, Zenobia C, Chen D, et al. Human variation in gingival inflammation. Proc Natl Acad Sci U S A (2021) 118:e2012578118. doi: 10.1073/pnas.2012578118
20. Chapple ILC, Mealey BL, Van Dyke TE, Bartold PM, Dommisch H, Eickholz P, et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol (2018) 89 Suppl 1:S74–84. doi: 10.1002/jper.17-0719
21. Chapple ILC, Mealey BL, Van Dyke TE, Bartold PM, Dommisch H, Eickholz P, et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Clin Periodontol (2018) 45 Suppl 20:S68–77. doi: 10.1111/jcpe.12940
22. Jepsen S, Caton JG, Albandar JM, Bissada NF, Bouchard P, Cortellini P, et al. Periodontal manifestations of systemic diseases and developmental and acquired conditions: consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Clin Periodontol (2018) 45:S219–S29. doi: 10.1111/jcpe.12951
23. Jepsen S, Caton JG, Albandar JM, Bissada NF, Bouchard P, Cortellini P, et al. Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol (2018) 89:S237–S48. doi: 10.1002/jper.17-0733
24. Keles S, Anik A, Cevik O, Abas BI, Anik A. Gingival crevicular fluid levels of interleukin-18 and tumor necrosis factor-alpha in type 1 diabetic children with gingivitis. Clin Oral Investig (2020) 24:3623–31. doi: 10.1007/s00784-020-03238-z
25. Lalla E, Lamster IB, Drury S, Fu C, Schmidt AM. Hyperglycemia, glycoxidation and receptor for advanced glycation endproducts: potential mechanisms underlying diabetic complications, including diabetes-associated periodontitis. Periodontol 2000 (2000) 23:50–62. doi: 10.1034/j.1600-0757.2000.2230104.x
26. Al Qahtani NA, Joseph B, Deepthi A, Vijayakumari BK. Prevalence of chronic periodontitis and its risk determinants among female patients in the Aseer Region of KSA. J Taibah Univ Med Sci (2017) 12:241–48. doi: 10.1016/j.jtumed.2016.11.012
27. Bartold PM, Van Dyke TE. An appraisal of the role of specific bacteria in the initial pathogenesis of periodontitis. J Clin Periodontol (2019) 46:6–11. doi: 10.1111/jcpe.13046
28. Bartold PM, Van Dyke TE. Host modulation: controlling the inflammation to control the infection. Periodontol 2000 (2017) 75:317–29. doi: 10.1111/prd.12169
29. Borgnakke WS. Ch 3. The traveling oral microbiome. In: Glick M, editor. The oral-systemic health connection: a guide to patient care. Chicago, IL: Quintessence (2019). p. 38–85.
30. Werheim ER, Senior KG, Shaffer CA, Cuadra GA. Oral pathogen Porphyromonas gingivalis can escape phagocytosis of mammalian macrophages. Microorganisms (2020) 8:1432. doi: 10.3390/microorganisms8091432
31. Olsen I, Singhrao SK. Is there a link between genetic defects in the complement cascade and Porphyromonas gingivalis Alzheimer’s disease? J Oral Microbiol (2020) 12:1676486. doi: 10.1080/20002297.2019.1676486
32. Lee JS, Yilmaz O. Key Elements of gingival epithelial homeostasis upon bacterial interaction. J Dent Res (2021) 100:333–40. doi: 10.1177/0022034520973012
33. Graves DT, Correa JD, Silva TA. The oral microbiota is modified by systemic diseases. J Dent Res (2019) 98:148–56. doi: 10.1177/0022034518805739
34. Graves DT, Ding Z, Yang Y. The impact of diabetes on periodontal diseases. Periodontol 2000 (2020) 82:214–24. doi: 10.1111/prd.12318
35. Lafleur S, Bodein A, Mbuya Malaika Mutombo J, Mathieu A, Joly Beauparlant C, Minne X, et al. Multi-omics data integration reveals key variables contributing to subgingival microbiome dysbiosis-induced inflammatory response in a hyperglycemic microenvironment. Int J Mol Sci (2023) 24:8832. doi: 10.3390/ijms24108832
36. Minty M, Canceil T, Serino M, Burcelin R, Terce F, Blasco-Baque V. Oral microbiota-induced periodontitis: a new risk factor of metabolic diseases. Rev Endocr Metab Disord (2019) 20:449–59. doi: 10.1007/s11154-019-09526-8
37. Portes J, Bullón B, Gallardo I, Fernandez-Riejos P, Quiles JL, Giampieri F, et al. Prevalence of undiagnosed diabetes and prediabetes related to periodontitis and its risk factors in elderly individuals. J Dent (2023) 132:104480. doi: 10.1016/j.jdent.2023.104480
38. Tang B, Yan C, Shen X, Li Y. The bidirectional biological interplay between microbiome and viruses in periodontitis and type-2 diabetes mellitus. Front Immunol (2022) 13:885029. doi: 10.3389/fimmu.2022.885029
39. Wade WG. Resilience of the oral microbiome. Periodontol 2000 (2021) 86:113–22. doi: 10.1111/prd.12365
40. Zhang D, Liu W, Peng L, Wang H, Lin M, Li Y, et al. Difference in oral microbial composition between chronic periodontitis patients with and without diabetic nephropathy. BMC Oral Health (2022) 22:12. doi: 10.1186/s12903-021-01985-3
41. Demmer RT, Trinh P, Rosenbaum M, Li G, LeDuc C, Leibel R, et al. Subgingival microbiota and longitudinal glucose change: The Oral Infections, Glucose Intolerance and Insulin Resistance Study (ORIGINS). J Dent Res (2019) 98:1488–96. doi: 10.1177/0022034519881978
42. Kumar PS, Monteiro MF, Dabdoub SM, Miranda GL, Casati MZ, Ribeiro FV, et al. Subgingival host-microbial interactions in hyperglycemic individuals. J Dent Res (2020) 99:650–57. doi: 10.1177/0022034520906842
43. Lu X, Liu T, Zhou J, Liu J, Yuan Z, Guo L. Subgingival microbiome in periodontitis and type 2 diabetes mellitus: an exploratory study using metagenomic sequencing. J Periodontal Implant Sci (2022) 52:282–97. doi: 10.5051/jpis.2103460173
44. Yu Y, Kim HJ, Song JM, Kang J, Lee H, Park HR, et al. Differential microbiota network in gingival tissues between periodontitis and periodontitis with diabetes. Front Cell Infect Microbiol (2022) 12:1061125. doi: 10.3389/fcimb.2022.1061125
45. Nascimento GG, Leite FRM, Vestergaard P, Scheutz F, Lopez R. Does diabetes increase the risk of periodontitis? a systematic review and meta-regression analysis of longitudinal prospective studies. Acta Diabetol (2018) 55:653–67. doi: 10.1007/s00592-018-1120-4
46. Banjar A, Alyafi R, AlGhamdi A, Assaggaf M, Almarghlani A, Hassan S, et al. The relationship between glycated hemoglobin level and the stage of periodontitis in individuals without diabetes. PloS One (2023) 18:e0279755. doi: 10.1371/journal.pone.0279755
47. Diz P, Scully C, Sanz M. Dental implants in the medically compromised patient. J Dent (2013) 41:195–206. doi: 10.1016/j.jdent.2012.12.008
48. Scully C, Hobkirk J, Dios PD. Dental endosseous implants in the medically compromised patient. J Oral Rehabil (2007) 34:590–9. doi: 10.1111/j.1365-2842.2007.01755.x
49. Löe H. Periodontal disease sixth complication Diabetes mellitus. Diabetes Care (1993) 16:329–34. doi: 10.2337/diacare.16.1.329
50. Eke PI, Wei L, Thornton-Evans GO, Borrell LN, Borgnakke WS, Dye B, et al. Risk indicators for periodontitis in us adults: NHANES 2009 to 2012. J Periodontol (2016) 87:1174–85. doi: 10.1902/jop.2016.160013
51. Genco RJ, Borgnakke WS. Risk factors for periodontal disease. Periodontol 2000 (2013) 62:59–94. doi: 10.1111/j.1600-0757.2012.00457.x
52. Genco RJ, Borgnakke WS. Diabetes as a potential risk for periodontitis: association studies. Periodontol 2000 (2020) 83:40–5. doi: 10.1111/prd.12270
53. Al-Shammari KF, Al-Khabbaz AK, Al-Ansari JM, Neiva R, Wang HL. Risk indicators for tooth loss due to periodontal disease. J Periodontol (2005) 76:1910–8. doi: 10.1902/jop.2005.76.11.1910
54. Martin JA, Page RC, Kaye EK, Hamed MT, Loeb CF. Periodontitis severity plus risk as a tooth loss predictor. J Periodontol (2009) 80:202–09. doi: 10.1902/jop.2009.080363
55. Phipps KR, Stevens VJ. Relative contribution of caries and periodontal disease in adult tooth loss for an HMO dental population. J Public Health Dent (1995) 55:250–2. doi: 10.1111/j.1752-7325.1995.tb02377.x
56. Raedel M, Noack B, Priess HW, Bohm S, Walter MH. Massive data analyses show negative impact of type 1 and 2 diabetes on the outcome of periodontal treatment. Clin Oral Investig (2021) 25:2037–43. doi: 10.1007/s00784-020-03512-0
57. Tiwari T, Tranby E, Thakkar-Samtani M, Frantsve-Hawley J. Determinants of tooth loss in a Medicaid adult population. JDR Clin Trans Res (2022) 7:289–97. doi: 10.1177/23800844211022277
58. Ahmadinia AR, Rahebi D, Mohammadi M, Ghelichi-Ghojogh M, Jafari A, Esmaielzadeh F, et al. Association between type 2 diabetes (T2D) and tooth loss: a systematic review and meta-analysis. BMC Endocr Disord (2022) 22:100. doi: 10.1186/s12902-022-01012-8
59. Vu GT, Little BB, Lai PC, Cheng GL. Tooth loss and uncontrolled diabetes among US adults. J Am Dent Assoc (2022) 153:542–51. doi: 10.1016/j.adaj.2021.11.008
60. Weijdijk LPM, Žiūkaitė L, van der Weijden GAF, Bakker EWP, Slot DE. The risk of tooth loss in patients with diabetes: a systematic review and meta-analysis. Int J Dent Hyg (2022) 20:145–66. doi: 10.1111/idh.12512
61. Yoo JJ, Kim DW, Kim MY, Kim YT, Yoon JH. The effect of diabetes on tooth loss caused by periodontal disease: a nationwide population-based cohort study in South Korea. J Periodontol (2019) 90:576–83. doi: 10.1002/JPER.18-0480
62. AlDukhail S, Alhazmi H, Riedy C, Barrow JR, Chamut S. Oral health outcomes among adults with diabetes served at HRSA-funded health centers. J Diabetes Complications (2021) 35:107979. doi: 10.1016/j.jdiacomp.2021.107979
63. Philips KH, Zhang S, Moss K, Ciarrocca K, Beck JD. Periodontal disease, undiagnosed diabetes, and body mass index: Implications for diabetes screening by dentists. J Am Dent Assoc (2021) 152:25–35. doi: 10.1016/j.adaj.2020.09.002
64. Raju K, Taylor GW, Tahir P, Hyde S. Association of tooth loss with morbidity and mortality by diabetes status in older adults: a systematic review. BMC Endocr Disord (2021) 21:205. doi: 10.1186/s12902-021-00830-6
65. Suzuki S, Noda T, Nishioka Y, Imamura T, Kamijo H, Sugihara N. Evaluation of tooth loss among patients with diabetes mellitus using the National Database of Health Insurance Claims and Specific Health Checkups of Japan. Int Dent J (2020) 70:308–15. doi: 10.1111/idj.12561
66. Schierz O, Baba K, Fueki K. Functional oral health-related quality of life impact: a systematic review in populations with tooth loss. J Oral Rehabil (2021) 48:256–70. doi: 10.1111/joor.12984
67. Matsuyama Y, Listl S, Jürges H, Watt RG, Aida J, Tsakos G. Causal effect of tooth loss on functional capacity in older adults in England: a natural experiment. J Am Geriatr Soc (2021) 69:1319–27. doi: 10.1111/jgs.17021
68. Lu TY, Chen JH, Du JK, Lin YC, Ho PS, Lee CH, et al. Dysphagia and masticatory performance as a mediator of the xerostomia to quality of life relation in the older population. BMC Geriatr (2020) 20:521. doi: 10.1186/s12877-020-01901-4
69. Zelig R, Jones VM, Touger-Decker R, Hoskin ER, Singer SR, Byham-Gray L, et al. The eating experience: adaptive and maladaptive strategies of older adults with tooth loss. JDR Clin Trans Res (2019) 4:217–28. doi: 10.1177/2380084419827532
70. Park HE, Song HY, Han K, Cho KH, Kim YH. Number of remaining teeth and health-related quality of life: the Korean National Health and Nutrition Examination Survey 2010-2012. Health Qual Life Outcomes. (2019) 17:5. doi: 10.1186/s12955-019-1078-0
71. Iwasaki M, Borgnakke WS, Ogawa H, Yamaga T, Sato M, Minagawa K, et al. Effect of lifestyle on 6-year periodontitis incidence or progression and tooth loss in older adults. J Clin Periodontol (2018) 45:896–908. doi: 10.1111/jcpe.12920
72. Buset SL, Walter C, Friedmann A, Weiger R, Borgnakke WS, Zitzmann NU. Are periodontal diseases really silent? a systematic review of their effect on quality of life. J Clin Periodontol (2016) 43:333–44. doi: 10.1111/jcpe.12517
73. Abe T, Tominaga K, Ando Y, Toyama Y, Takeda M, Yamasaki M, et al. Number of teeth and masticatory function are associated with sarcopenia and diabetes mellitus status among community-dwelling older adults: a Shimane CoHRE study. PloS One (2021) 16:e0252625. doi: 10.1371/journal.pone.0252625
74. da Silveira DL, da Rosa Carlos Monteiro LE, da Silva Christofoli C, Schaan BD, Telo GH. Number of teeth lost on diet quality and glycemic control in patients with type 2 diabetes mellitus. Arch Endocrinol Metab (2022) 66:40–9. doi: 10.20945/2359-3997000000429
75. Ishikado A, Kondo K, Maegawa H, Morino K. Nutrition and periodontal health in the patients with diabetes mellitus: a review from the viewpoint of endothelial function. Curr Oral Health Rep (2021) 8:67–74. doi: 10.1007/s40496-021-00297-3
76. Shen J, Qian S, Huang L, Tao Y, Chen H, Deng K, et al. Association of the number of natural teeth with dietary diversity and nutritional status in older adults: a cross-sectional study in China. J Clin Periodontol (2023) 50:242–51. doi: 10.1111/jcpe.13728
77. Moynihan P, Varghese R. Impact of wearing dentures on dietary intake, nutritional status, and eating: a systematic review. JDR Clin Trans Res (2022) 7:334–51. doi: 10.1177/23800844211026608
78. Sabancı A, Eltas A, Celik B, Otlu B. The influence of diabetes mellitus on the peri-implant microflora: A cross-sectional study. J Oral Biol Craniofac Res (2022) 12:405–09. doi: 10.1016/j.jobcr.2022.05.007
79. Al Ansari Y, Shahwan H, Chrcanovic BR. Diabetes mellitus and dental implants: a systematic review and meta-analysis. Materials (Basel) (2022) 15(9):3227. doi: 10.3390/ma15093227
80. Monje A, Kan JY, Borgnakke WS. Impact of local predisposing/precipitating factors and systemic drivers on peri-implant diseases. Clin Implant Dent Relat Res (2023) 25:640–60. doi: 10.1111/cid.13155
81. Amerio E, Borgnakke WS. Ch. 8. Systemic confounders and deleterious habits associated with peri-implantitis. In: Monje A, Wang HL, editors. Unfolding peri-implantitis: diagnosis, prevention and management. Batavia, IL: Quintessence (2022). p. 256–93.
82. Monje A, Catena A, Borgnakke WS. Association between diabetes mellitus/hyperglycaemia and peri-implant diseases: systematic review and meta-analysis. J Clin Periodontol (2017) 44:636–48. doi: 10.1111/jcpe.12724
83. Lorean A, Ziv-On H, Perlis V, Ormianer Z. Marginal bone loss of dental implants in patients with type 2 diabetes mellitus with poorly controlled HbA1c values: a long-term retrospective study. Int J Oral Maxillofac Implants (2021) 36:355–60. doi: 10.11607/jomi.8476
84. Papi P, Di Murro B, Pranno N, Bisogni V, Saracino V, Letizia C, et al. Prevalence of peri-implant diseases among an Italian population of patients with metabolic syndrome: a cross-sectional study. J Periodontol (2019) 90:1374–82. doi: 10.1002/JPER.19-0077
85. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 2. Classification and diagnosis of diabetes: standards of care in diabetes-2023. Diabetes Care (2023) 46:S19–40. doi: 10.2337/dc23-S002
86. Parihar AS, Madhuri S, Devanna R, Sharma G, Singh R, Shetty K. Assessment of failure rate of dental implants in medically compromised patients. J Family Med Prim Care (2020) 9:883–85. doi: 10.4103/jfmpc.jfmpc_989_19
87. Borgnakke WS, Ylöstalo PV, Taylor GW, Genco RJ. Effect of periodontal disease on diabetes: systematic review of epidemiologic observational evidence. J Clin Periodontol (2013) 40:S135–52. doi: 10.1111/jcpe.12080
88. Borgnakke WS, Ylöstalo PV, Taylor GW, Genco RJ. Effect of periodontal disease on diabetes: systematic review of epidemiologic observational evidence. J Periodontol (2013) 84:S135–52. doi: 10.1902/jop.2013.1340013
89. Graziani F, Gennai S, Solini A, Petrini M. A systematic review and meta-analysis of epidemiologic observational evidence on the effect of periodontitis on diabetes; an update of the EFP-AAP review. J Clin Periodontol (2018) 45:167–87. doi: 10.1111/jcpe.12837
90. Chang Y, Lee JS, Lee KJ, Woo HG, Song TJ. Improved oral hygiene is associated with decreased risk of new-onset diabetes: a nationwide population-based cohort study. Diabetologia (2020) 63:924–33. doi: 10.1007/s00125-020-05112-9
91. Su Y, Ye L, Hu C, Zhang Y, Liu J, Shao L. Periodontitis as a promoting factor of T2D: current evidence and mechanisms. Int J Oral Sci (2023) 15:25. doi: 10.1038/s41368-023-00227-2
92. Borgnakke WS, Anderson PF, Shannon C, Jivanescu A. Is there a relationship between oral health and diabetic neuropathy? Curr Diabetes Rep (2015) 15:93. doi: 10.1007/s11892-015-0673-7
93. Glavind L, Lund B, Löe H. The relationship between periodontal state and diabetes duration, insulin dosage and retinal changes. J Periodontal Res (1969) 4:164–5.
94. Borgnakke WS. Gum disease can raise your blood sugar level. J Am Dent Assoc (2013) 144:860. doi: 10.14219/jada.archive.2013.0199
95. Ata-Ali F, Melo M, Cobo T, Nagasawa MA, Shibli JA, Ata-Ali J. Does non-surgical periodontal treatment improve glycemic control? a comprehensive review of meta-analyses. J Int Acad Periodontol (2020) 22:205–22.
96. Baeza M, Morales A, Cisterna C, Cavalla F, Jara G, Isamitt Y, et al. Effect of periodontal treatment in patients with periodontitis and diabetes: systematic review and meta-analysis. J Appl Oral Sci (2020) 28:e20190248. doi: 10.1590/1678-7757-2019-0248
97. Cao R, Li Q, Wu Q, Yao M, Chen Y, Zhou H. Effect of non-surgical periodontal therapy on glycemic control of type 2 diabetes mellitus: a systematic review and Bayesian network meta-analysis. BMC Oral Health (2019) 19:176. doi: 10.1186/s12903-019-0829-y
98. Chen YF, Zhan Q, Wu CZ, Yuan YH, Chen W, Yu FY, et al. Baseline HbA1c level influences the effect of periodontal therapy on glycemic control in people with type 2 diabetes and periodontitis: a systematic review on randomized controlled trails. Diabetes Ther (2021) 12:1249–78. doi: 10.1007/s13300-021-01000-6
99. Corbella S, Francetti L, Taschieri S, De Siena F, Fabbro MD. Effect of periodontal treatment on glycemic control of patients with diabetes: a systematic review and meta-analysis. J Diabetes Investig (2013) 4:502–9. doi: 10.1111/jdi.12088
100. Darré L, Vergnes JN, Gourdy P, Sixou M. Efficacy of periodontal treatment on glycaemic control in diabetic patients: a meta-analysis of interventional studies. Diabetes Metab (2008) 34:497–506. doi: 10.1016/j.diabet.2008.03.006
101. Elnour MAA, Mirghani HO. Periodontitis treatment (surgical and nonsurgical) effects on glycemic control: a review and meta-analysis. Ann Afr Med (2023) 22:131–35. doi: 10.4103/aam.aam_53_22
102. Engebretson S, Kocher T. Evidence that periodontal treatment improves diabetes outcomes: a systematic review and meta-analysis. J Clin Periodontol (2013) 40 Suppl 14:S153–63. doi: 10.1111/jcpe.12084
103. Engebretson S, Kocher T. Evidence that periodontal treatment improves diabetes outcomes: a systematic review and meta-analysis. J Periodontol (2013) 84:S153–63. doi: 10.1902/jop.2013.1340017
104. Jain A, Gupta J, Bansal D, Sood S, Gupta S, Jain A. Effect of scaling and root planing as monotherapy on glycemic control in patients of type 2 diabetes with chronic periodontitis: a systematic review and meta-analysis. J Indian Soc Periodontol (2019) 23:303–10. doi: 10.4103/jisp.jisp_417_18
105. Janket SJ, Wightman A, Baird AE, Van Dyke TE, Jones JA. Does periodontal treatment improve glycemic control in diabetic patients? a meta-analysis of intervention studies. J Dent Res (2005) 84:1154–9. doi: 10.1177/154405910508401212
106. Lavigne SE, Forrest JL. An umbrella review of systematic reviews examining the relationship between type 2 diabetes and periodontitis: position paper from the Canadian Dental Hygienists Association. Can J Dent Hyg (2021) 55:57–67.
107. Li Q, Hao S, Fang J, Xie J, Kong XH, Yang JX. Effect of non-surgical periodontal treatment on glycemic control of patients with diabetes: a meta-analysis of randomized controlled trials. Trials (2015) 16:291. doi: 10.1186/s13063-015-0810-2
108. Liew AK, Punnanithinont N, Lee YC, Yang J. Effect of non-surgical periodontal treatment on HbA1c: a meta-analysis of randomized controlled trials. Aust Dent J (2013) 58:350–7. doi: 10.1111/adj.12091
109. Ndjidda Bakari W, Diallo AM, Danwang C, Nzalie RNT, Benoist HM. Long-term effect of non-surgical periodontal treatment on glycaemic control in patients with diabetes with periodontitis: a systematic review and meta-analysis protocol. BMJ Open (2021) 11:e043250. doi: 10.1136/bmjopen-2020-043250
110. Oates TW, Guy V, Ni K, Ji C, Saito H, Shiau H, et al. Meta-regression analysis of study heterogeneity for systemic outcomes after periodontal therapy. JDR Clin Trans Res (2023) 8:6–15. doi: 10.1177/23800844211070467
111. Sgolastra F, Severino M, Pietropaoli D, Gatto R, Monaco A. Effectiveness of periodontal treatment to improve metabolic control in patients with chronic periodontitis and type 2 diabetes: a meta-analysis of randomized clinical trials. J Periodontol (2013) 84:958–73. doi: 10.1902/jop.2012.120377
112. Simpson TC, Needleman I, Wild SH, Moles DR, Mills EJ. Treatment of periodontal disease for glycaemic control in people with diabetes. Cochrane Database Syst Rev (2010) 5:CD004714. doi: 10.1002/14651858.CD004714.pub2
113. Simpson TC, Weldon JC, Worthington HV, Needleman I, Wild SH, Moles DR, et al. Treatment of periodontal disease for glycaemic control in people with diabetes mellitus. Cochrane Database Syst Rev (2015) 11:CD004714. doi: 10.1002/14651858.CD004714.pub3
114. Sun QY, Feng M, Zhang MZ, Zhang YQ, Cao MF, Bian LX, et al. Effects of periodontal treatment on glycemic control in type 2 diabetic patients: a meta-analysis of randomized controlled trials. Chin J Physiol (2014) 57:305–14. doi: 10.4077/cjp.2014.bac262
115. Teeuw WJ, Gerdes VE, Loos BG. Effect of periodontal treatment on glycemic control of diabetic patients: a systematic review and meta-analysis. Diabetes Care (2010) 33:421–7. doi: 10.2337/dc09-1378
116. Teshome A, Yitayeh A. The effect of periodontal therapy on glycemic control and fasting plasma glucose level in type 2 diabetic patients: systematic review and meta-analysis. BMC Oral Health (2016) 17:31. doi: 10.1186/s12903-016-0249-1
117. Wang TF, Jen IA, Chou C, Lei YP. Effects of periodontal therapy on metabolic control in patients with type 2 diabetes mellitus and periodontal disease: a meta-analysis. Med (2014) 93:e292. doi: 10.1097/MD.0000000000000292
118. Wang X, Han X, Guo X, Luo X, Wang D. The effect of periodontal treatment on hemoglobin A1c levels of diabetic patients: a systematic review and meta-analysis. PloS One (2014) 9:e108412. doi: 10.1371/journal.pone.0108412
119. Ibrahim M, Abu Al Magd M, Annabi FA, Assaad-Khalil S, Ba-Essa EM, Fahdil I, et al. Recommendations for management of diabetes during Ramadan: update 2015. BMJ Open Diabetes Res Care (2015) 3:e000108. doi: 10.1136/bmjdrc-2015-000108
120. Borgnakke WS. IDF diabetes atlas: diabetes and oral health - a two-way relationship of clinical importance. Diabetes Res Clin Pract (2019) 157:107839. doi: 10.1016/j.diabres.2019.107839
121. Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ (2000) 321:405–12. doi: 10.1136/bmj.321.7258.405
122. Mammen J, Vadakkekuttical RJ, George JM, Kaziyarakath JA, Radhakrishnan C. Effect of non-surgical periodontal therapy on insulin resistance in patients with type II diabetes mellitus and chronic periodontitis, as assessed by C-peptide and the homeostasis assessment index. J Investig Clin Dent (2017) 8:e12221. doi: 10.1111/jicd.12221
123. Zhu Z, Qi X, Zheng Y, Pei Y, Wu B. Age differences in the effects of multi-component periodontal treatments on oral and metabolic health among people with diabetes mellitus: a meta-epidemiological study. J Dent (2023) 135:104594. doi: 10.1016/j.jdent.2023.104594
124. Bertolini M, Clark D. Periodontal disease as a model to study chronic inflammation in aging. Geroscience (2023). doi: 10.1007/s11357-023-00835-0
125. Cancro MP. Age-associated B cells. Annu Rev Immunol (2020) 38:315–40. doi: 10.1146/annurev-immunol-092419-031130
126. Ebersole JL, Dawson DA 3rd, Emecen Huja P, Pandruvada S, Basu A, Nguyen L, et al. Age and periodontal health - immunological view. Curr Oral Health Rep (2018) 5:229–41. doi: 10.1007/s40496-018-0202-2
127. Feres M, Teles F, Teles R, Figueiredo LC, Faveri M. The subgingival periodontal microbiota of the aging mouth. Periodontol 2000 (2016) 72:30–53. doi: 10.1111/prd.12136
128. Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, et al. Inflamm-aging; an evolutionary perspective on immunosenescence. Ann N Y Acad Sci (2000) 908:244–54. doi: 10.1111/j.1749-6632.2000.tb06651.x
129. LaMonte MJ, Genco RJ, Zheng W, McSkimming DI, Andrews CA, Hovey KM, et al. Substantial differences in the subgingival microbiome measured by 16S metagenomics according to periodontitis status in older women. Dent J (Basel). (2018) 6(4):58. doi: 10.3390/dj6040058
130. Preshaw PM, Henne K, Taylor JJ, Valentine RA, Conrads G. Age-related changes in immune function (immune senescence) in caries and periodontal diseases: a systematic review. J Clin Periodontol (2017) 44 Suppl 18:S153–77. doi: 10.1111/jcpe.12675
131. Tang R, Ren Y, Zhang Y, Yin M, Ren X, Zhu Q, et al. Glucose-driven transformable complex eliminates biofilm and alleviates inflamm-aging for diabetic periodontitis therapy. Mater Today Bio (2023) 20:100678. doi: 10.1016/j.mtbio.2023.100678
132. Zhang P, Wang Q, Nie L, Zhu R, Zhou X, Zhao P, et al. Hyperglycemia-induced inflamm-aging accelerates gingival senescence via NLRC4 phosphorylation. J Biol Chem (2019) 294:18807–19. doi: 10.1074/jbc.RA119.010648
133. Zhao P, Yue Z, Nie L, Zhao Z, Wang Q, Chen J, et al. Hyperglycaemia-associated macrophage pyroptosis accelerates periodontal inflamm-aging. J Clin Periodontol (2021) 48:1379–92. doi: 10.1111/jcpe.13517
134. Rahman AU, Rashid S, Noon R, Samuel ZS, Lu B, Borgnakke WS, et al. Prospective evaluation of the systemic inflammatory marker C-reactive protein in patients with end-stage periodontitis getting teeth replaced with dental implants: a pilot investigation. Clin Oral Implants Res (2005) 16:128–31. doi: 10.1111/j.1600-0501.2004.01109.x
135. Slack-Smith L, Ng T, Macdonald ME, Durey A. Rethinking oral health in aging: ecosocial theory and intersectionality. J Dent Res (2023) 0:220345231175061. doi: 10.1177/00220345231175061
136. Sadowsky SJ, Landesman HM. A call for enhanced integration. J Prosthet Dent (2023) 129:1. doi: 10.1016/j.prosdent.2022.08.023
137. Reynolds EC. Transdisciplinary research: the virtuous cycle of research translation to improve oral health. J Dent Res (2022) 101:613–15. doi: 10.1177/00220345221090824
138. Okubo M, Kuraji R, Kamimura H, Numabe Y, Ito K, Sato T, et al. A case of necrotizing periodontitis in a care-requiring elderly person treated and managed by interprofessional collaboration. Dent J (Basel). (2022) 10:79. doi: 10.3390/dj10050079
139. Gill SA, Quinonez RB, Deutchman M, Conklin CE, Rizzolo D, Rabago D, et al. Integrating oral health into health professions school curricula. Med Educ Online (2022) 27:2090308. doi: 10.1080/10872981.2022.2090308
140. Vernon LT, Teng KA, Kaelber DC, Heintschel GP, Nelson S. Time to integrate oral health screening into medicine? a survey of primary care providers of older adults and an evidence-based rationale for integration. Gerodontology (2022) 39:231–40. doi: 10.1111/ger.12561
141. Verhulst MJL, Teeuw WJ, Gerdes VEA, Loos BG. Implementation of an oral care protocol for primary diabetes care: a pilot cluster-randomized controlled trial. Ann Family Med (2021) 19:197–206. doi: 10.1370/afm.2645
142. Valentim FB, Carneiro VC, Costa Gomes PD, Rosetti EP. The importance of integrated healthcare in the association between oral health and awareness of periodontitis and diabetes in type 2 diabetics. Oral Health Prev Dent (2021) 19:1–6. doi: 10.3290/j.ohpd.b875369
143. McGowan K, Phillips T, Gielis E, Dover T, Mitchell G, Mutch A, et al. Developing a prototype for integrated dental and diabetes care: understanding needs and priorities. Aust Dent J (2021) 66:41–8. doi: 10.1111/adj.12804
144. Jamil NA, Chau SH, Abdul Razak NI, Shamsul K II, Mohd-Said S, Rani H, et al. Development and evaluation of an integrated diabetes-periodontitis nutrition and health education module. BMC Med Educ (2021) 21:278. doi: 10.1186/s12909-021-02721-9
145. Ho BV, van der Maarel-Wierink CD, Rollman A, Weijenberg RAF, Lobbezoo F. ‘Don’t forget the mouth!’: a process evaluation of a public oral health project in community-dwelling frail older people. BMC Oral Health (2021) 21:536. doi: 10.1186/s12903-021-01884-7
146. Glurich I, Berg R, Panny A, Shimpi N, Steinmetz A, Nycz G, et al. Longitudinal observation of outcomes and patient access to integrated care following point-of-care glycemic screening in community health center dental safety net clinics. Front Oral Health (2021) 2:670355. doi: 10.3389/froh.2021.670355
147. Federation Dentaire International (FDI). The challenge of oral disease – a call for global action; the oral health atlas. 2nd ed. Benzian H, Williams D, editors. Geneva, Switzerland: FDI World Dental Federation (2015). p. 63. Available at: https://www.fdiworlddental.org/sites/default/files/2021-03/complete_oh_atlas-2_0.pdf.
148. Magliano DJ, Boyko EJ IDF. Diabetes Atlas 10th Edition Scientific Committee. IDF diabetes atlas. 10th ed. Brussels, Belgium: International Diabetes Federation (2021). p. 141. Available at: https://diabetesatlas.org/.
149. Herman WH, Ye W, Griffin SJ, Simmons RK, Davies MJ, Khunti K, et al. Early detection and treatment of type 2 diabetes reduce cardiovascular morbidity and mortality: a simulation of the results of the Anglo-Danish-Dutch study of intensive treatment in people with screen-detected diabetes in primary care (ADDITION-Europe). Diabetes Care (2015) 38:1449–55. doi: 10.2337/dc14-2459
150. Shimpi N, Panny A, Glurich I, Chyou P-H, Acharya A. Knowledgeability, attitude and practice behaviors of dental providers toward provisions of integrated care delivery for patients with prediabetes/diabetes: Wisconsin statewide survey. Front Dent Med (2021) 2:674178. doi: 10.3389/fdmed.2021.674178
151. Chinnasamy A, Moodie M. Prevalence of undiagnosed diabetes and prediabetes in the dental setting: a systematic review and meta-analysis. Int J Dent (2020) 2020:2964020. doi: 10.1155/2020/2964020
152. Yonel Z, Cerullo E, Kroger AT, Gray LJ. Use of dental practices for the identification of adults with undiagnosed type 2 diabetes mellitus or non-diabetic hyperglycaemia: a systematic review. Diabetes Med (2020) 37:1443–53. doi: 10.1111/dme.14324
153. Elazazy O, Amr K, Abd El Fattah A, Abouzaid M. Evaluation of serum and gingival crevicular fluid microRNA-223, microRNA-203 and microRNA-200b expression in chronic periodontitis patients with and without diabetes type 2. Arch Oral Biol (2021) 121:104949. doi: 10.1016/j.archoralbio.2020.104949
154. Patel C, Dave B, Patel R, Kumar S, Dattani V, Joshi S, et al. Gingival crevicular blood glucose as a novel method for screening diabetes mellitus in periodontally compromised patients. Cureus (2023) 15:e39444. doi: 10.7759/cureus.39444
155. Rapone B, Ferrara E, Santacroce L, Topi S, Converti I, Gnoni A, et al. Gingival crevicular blood as a potential screening tool: a cross sectional comparative study. Int J Environ Res Public Health (2020) 17(20):7356. doi: 10.3390/ijerph17207356
156. Saeed Q, Memon S, Hosein M. Gingival crevicular blood glucose detection: screening for diabetes in the dental OPD. Pakistan J Med Dent (2020) 9:78–83. doi: 10.36283/PJMD9-4/014
157. Sande AR, Guru S, Guru R, Gaduputi S, Thati DK, Siddeshappa ST. Gingival crevicular blood glucose levels: is it a reliable tool for screening diabetes in a dental office? J Contemp Dent Pract (2020) 21:421–25.
158. Nasseh K, Greenberg B, Vujicic M, Glick M. The effect of chairside chronic disease screenings by oral health professionals on health care costs. Am J Public Health (2014) 104:744–50. doi: 10.2105/AJPH.2013.301644
159. Neidell M, Lamster IB, Shearer B. Cost-effectiveness of diabetes screening initiated through a dental visit. Community Dent Oral Epidemiol (2017) 45:275–80. doi: 10.1111/cdoe.12286
160. Panakhup M, Lertpanomwan I, Pajonklaew C, Arayapisit T, Yuma S, Pujarern P, et al. Attitude of physicians towards periodontal disease and diabetes mellitus screening in dental clinics in Thailand. Int J Environ Res Public Health (2021) 18(10):5383. doi: 10.3390/ijerph18105385
161. Yonel Z, Kocher T, Chapple ILC, Dietrich T, Völzke H, Nauck M, et al. Development and external validation of a multivariable prediction model to identify nondiabetic hyperglycemia and undiagnosed type 2 diabetes: diabetes risk assessment in dentistry score (DDS). J Dent Res (2023) 102:170–77. doi: 10.1177/00220345221129807
162. Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J Clin Periodontol (2018) 45 Suppl 20:S149–61. doi: 10.1111/jcpe.12945
163. Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J Periodontol (2018) 89:S159–72. doi: 10.1002/jper.18-0006
164. Salmerón D, Gomez Garcia F, Pons-Fuster E, Perez-Sayans M, Lorenzo-Pouso AI, Lopez-Jornet P. Screening for prediabetes and risk of periodontal disease. Diabetes Metab Syndr (2019) 13:1661–66. doi: 10.1016/j.dsx.2019.03.006
165. Glick M, Williams DM, Yahya IB, Bondioni E, Cheung WWM, Clark P, et al. Vision 2030: delivering optimal oral health for all. Geneva, Switzerland: World Dental Federation (IDF (2021). p. 52. Available at: https://www.fdiworlddental.org/sites/default/files/2021-02/Vision-30-Delivering%20Optimal-Oral-Health-for-All_0.pdf.
166. Federation Dentaire International (FDI). Global periodontal health: challenges, priorities and perspectives. Madrid, Spain: World Oral Health Forum 2017 (2017). p. 14. Available at: https://www.fdiworlddental.org/sites/default/files/2020-11/2017-wohf_on_gphp-proceedings.pdf.
167. Now is the time to take gum disease seriously: a roadmap for improving oral health in the United States. Economist Impact (2023). Available at: https://impact.economist.com/perspectives/sites/default/files/oral_b_report_us_letter-v7.pdf?fbclid=IwAR3-JFKL4EJu9bBfhjTIyMyW0_hsisfpENqFdY0fhXRnGrt_eeWITVQJLS0&mibextid=Zxz2cZ.
168. Borgnakke WS. Diabetes and oral health. In: IDF diabetes atlas, 9th edition, vol. 97-98. . Brussels, Belgium: International Diabetes Federation (IDF (2019). p. 102–4. Available at: https://diabetesatlas.org/atlas/ninth-edition/.
169. National Diabetes Data Group, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (NIH/NIDDK). Diabetes in america. 2nd edition. Bethesda, MD: NIH/NIDDK (1995). Available at: https://www.niddk.nih.gov/about-niddk/strategic-plans-reports/diabetes-in-america-2nd-edition.
170. Löe H, Genco RJ. Ch. 23: Oral complications in diabetes. In: NIH/NIDDK. Diabetes in america, 2nd edition. Bethesda, MD: NIH/NIDDK (1995). Available at: https://www.niddk.nih.gov/about-niddk/strategic-plans-reports/diabetes-in-america-2nd-edition.
171. Cardenas K, Weilnau T, Aguilar C, Ali A, Eidelman A, Ponnala S, et al. Partnering for integrated care: a learning collaborative for primary care and oral health teams. Ann Fam Med (2023) 21:S22–30. doi: 10.1370/afm.2918
172. Silk H. Integration of primary care and oral health. In: Basu S, Alpert JL, Phillips RS, editors. Primary care in the COVID-19 pandemic; improving access to high-quality primary care, accelerating transitions to alternative forms of care delivery, and addressing health disparities (2021). p. 237–49. Available at: https://www.carequest.org/system/files/Primary-Care-in-the-COVID-19-Pandemic-Harvard-Report.pdf.
173. Haber J, Dolce MC, Hartnett E, Savageau JA, Altman S, Lange-Kessler J, et al. Integrating oral health curricula into midwifery graduate programs: results of a US survey. J Midwifery Womens Health (2019) 64:462–71. doi: 10.1111/jmwh.12974
174. Ferullo A, Silk H, Savageau JA. Teaching oral health in U.S. medical schools: results of a national survey. Acad Med (2011) 86:226–30. doi: 10.1097/ACM.0b013e3182045a51
175. Romano J, Silk H. Why are family doctors still not addressing oral health? Ann Fam Med (2023) 21:S103–5. doi: 10.1370/afm.2929
176. Feierabend-Peters J, Silk H. Why should primary care clinicians learn to routinely examine the mouth? AMA J Ethics (2022) 24:E19–26. doi: 10.1001/amajethics.2022.19
177. Davies MJ, Aroda VR, Collins BS, Gabbay RA, Green J, Maruthur NM, et al. Management of hyperglycemia in type 2 diabetes, 2022. a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care (2022) 45:2753–86. doi: 10.2337/dci22-0034
178. Horbach AL, Baldisserotto J, Celeste RK. Association between dental visits at primary care and glycated hemoglobin level in patients with type 2 diabetes: a cohort study. Rev Bras Epidemiol (2021) 24:e210032. doi: 10.1590/1980-549720210032
179. American Diabetes Association. Standards of care – 2023. In: Diabetes care, vol. 46. (2023). p. 302. Available at: https://ada.silverchair-cdn.com/ada/content_public/journal/care/issue/46/supplement_1/21/standards-of-care-2023.pdf?Expires=1690749438&Signature=t5jsi11L8bv4Q7RcGzRnYptJgR6T-Uf-rh0FRVCQ5Sk~2lSupPjYtDATxuRQUvArG~FNZ1QM7QoyLencyR7Xje2lOLYA3OX6goWstIM3AcXnzAJ2KCdynBtWpIHy5q-mLGdTlQDMWYEaRVpFgBfeQDOVbdqa1mB7U2DcG11VNQoVz8hsq0JEfG4ToBmBUNnI2ttaRZ4uXA4FkP-CSIGwSa2Yh1iRM5HIINcZNE~HUAp6HG62MtajTEknaAysQ39WbTT6drBRAPDCTMXly25~sE0Z1c~wuEWGDGUeuGp9UW~Wk9a~FNDxxhDWlMliUoFXwoT2wzf8wbS8EpiZ3zzJBQ:&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA.
180. Herman WH, Taylor GW, Jacobson JJ, Burke R, Brown MB. Screening for prediabetes and type 2 diabetes in dental offices. J Public Health Dent (2015) 75:175–82. doi: 10.1111/jphd.12082
181. Jadhav AN, Tarte PR, Puri SK. Dental clinic: potential source of high-risk screening for prediabetes and type 2 diabetes. Indian J Dent Res (2019) 30:851–54. doi: 10.4103/ijdr.IJDR_80_18
182. Lalla E, Cheng B, Kunzel C, Burkett S, Lamster IB. Dental findings and identification of undiagnosed hyperglycemia. J Dent Res (2013) 92:888–92. doi: 10.1177/0022034513502791
183. Lalla E, Kunzel C, Burkett S, Cheng B, Lamster IB. Identification of unrecognized diabetes and pre-diabetes in a dental setting. J Dent Res (2011) 90:855–60. doi: 10.1177/0022034511407069
184. American Diabetes Association Professional Practice Committee, Draznin B, VR A, Bakris G, Benson G, FM B, et al. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2022. Diabetes Care (2022) 45:S17–38. doi: 10.2337/dc22-S002
185. D’Aiuto F, Gkranias N, Bhowruth D, Khan T, Orlandi M, Suvan J, et al. Systemic effects of periodontitis treatment in patients with type 2 diabetes: a 12 month, single-centre, investigator-masked, randomised trial. Lancet Diabetes Endocrinol. (2018) 6:954–65. doi: 10.1016/S2213-8587(18)30038-X
186. Eke PI, Thornton-Evans GO, Wei L, Borgnakke WS, Dye BA, Genco RJ. Periodontitis in US adults: national health and nutrition examination survey 2009-2014. J Am Dent Assoc (2018) 149:576–88 & 88.e1-6. doi: 10.1016/j.adaj.2018.04.023
187. Khader YS, Dauod AS, El-Qaderi SS, Alkafajei A, Batayha WQ. Periodontal status of diabetics compared with nondiabetics: a meta-analysis. J Diabetes Complications (2006) 20:59–68. doi: 10.1016/j.jdiacomp.2005.05.006
188. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 4. Comprehensive medical evaluation and assessment of comorbidities: standards of care in diabetes-2023. Diabetes Care (2023) 46:S49–S67.2. doi: 10.2337/dc23-S004
189. American Diabetes Association Professional Practice Committee, Draznin B, VR A, Bakris G, Benson G, FM B, et al. 4. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes-2022. Diabetes Care (2022) 45:S46–59. doi: 10.2337/dc22-S004
190. Mathew JE, Jacob JJ, Kalra S. Periodontitis management in diabetes care. J Pak Med Assoc (2021) 71:2097–99.
191. Shimpi N, Schroeder D, Kilsdonk J, Chyou PH, Glurich I, Penniman E, et al. Medical providers’ oral health knowledgeability, attitudes, and practice behaviors: an opportunity for interprofessional collaboration. J Evid Based Dent Pract (2016) 16:19–29. doi: 10.1016/j.jebdp.2016.01.002
192. Shimpi N, Glurich I, Panny A, Acharya A. Knowledgeability, attitude, and practice behaviors of primary care providers toward managing patients’ oral health care in medical practice: Wisconsin statewide survey. J Am Dent Assoc (2019) 150:863–72. doi: 10.1016/j.adaj.2019.05.020
193. Shimpi N, Glurich I, Acharya A. Integrated care case study: Marshfield clinic health system. In: Acharya A, Powell V, Torres-Urquidy MH, Posteraro RH, Thyvalikakath TP, editors. Integration of medical and dental care and patient data. Switzerland: Springer Cham (2019). p. 315–26. doi: 10.1007/978-3-319-98298-4
194. Glurich I, Nycz G, Acharya A. Status update on translation of integrated primary dental-medical care delivery for management of diabetic patients. Clin Med Res (2017) 15(1-2):21–32. doi: 10.3121/cmr.2017.1348
195. Baccaglini L, Kusi Appiah A, Ray M, Yu F. US adults with diabetes mellitus: variability in oral healthcare utilization. PloS One (2021) 16:e0251120. doi: 10.1371/journal.pone.0251120
196. Nurminen M, Ratto H. Impact of diabetes diagnosis on dental care utilization: evidence from Finland. Health Econ Rev (2023) 13:26. doi: 10.1186/s13561-023-00440-z
197. Parbhakar KK, Rosella LC, Singhal S, Quinonez CR. Dental and medical care visits among persons with diabetes in Ontario, Canada, who self-report oral health status. Can J Dent Hyg (2022) 56:42–5.
198. Patel N, Fils-Aime R, Li CH, Lin M, Robison V. Prevalence of past-year dental visit among us adults aged 50 years or older, with selected chronic diseases, 2018. Prev Chronic Dis (2021) 18:E40. doi: 10.5888/pcd18.200576
199. Poudel P, Griffiths R, Arora A, Wong VW, Flack JR, Barker G, et al. Oral health status, knowledge, and behaviours of people with diabetes in Sydney, Australia. Int J Environ Res Public Health (2021) 18:3464. doi: 10.3390/ijerph18073464
200. Rekawek P, Carr BR, Boggess WJ, Coburn JF, Chuang SK, Panchal N, et al. Hygiene recall in diabetic and nondiabetic patients: a periodic prognostic factor in the protection against peri-implantitis? J Oral Maxillofac Surg (2021) 79:1038–43. doi: 10.1016/j.joms.2020.12.032
201. Schroeder SM, Adamsen C, Besse R. The relationship between diabetes and oral health status, and dental visits among American Indian, Alaska Native, and Native Hawaiian elders. J Am Dent Assoc (2021) 152:293–301. doi: 10.1016/j.adaj.2020.12.008
202. Zhang Y, Leveille SG, Shi L, Camhi SM. Disparities in preventive oral health care and periodontal health among adults with diabetes. Prev Chronic Dis (2021) 18:E47. doi: 10.5888/pcd18.200594
203. Chen Y, Zhang P, Luman ET, Griffin SO, Rolka DB. Incremental dental expenditures associated with diabetes among noninstitutionalized U.S. adults aged ≥18 years old in 2016-2017. Diabetes Care (2021) 44:1317–23. doi: 10.2337/dc20-2744
204. Iwasaki M, Sato M, Yoshihara A, Miyazaki H. Effects of periodontal diseases on diabetes-related medical expenditure. Curr Oral Health Rep (2016) 3:7–13. doi: 10.1007/s40496-016-0076-0
205. Shrestha SS, Honeycutt AA, Yang W, Zhang P, Khavjou OA, Poehler DC, et al. Economic costs attributable to diabetes in each U.S. state. Diabetes Care (2018) 41:2526–34. doi: 10.2337/dc18-1179
206. Nasseh K, Vujicic M, Glick M. The relationship between periodontal interventions and healthcare costs and utilization. evidence from an integrated dental, medical, and pharmacy commercial claims database. Health Econ (2017) 26:519–27. doi: 10.1002/hec.3316
207. Shin JH, Takada D, Kunisawa S, Imanaka Y. Effects of periodontal management for patients with type 2 diabetes on healthcare expenditure, hospitalization and worsening of diabetes: an observational study using medical, dental and pharmacy claims data in Japan. J Clin Periodontol (2021) 48:774–84. doi: 10.1111/jcpe.13441
208. Smits KPJ, Listl S, Plachokova AS, van der Galien O, Kalmus O. Effect of periodontal treatment on diabetes-related healthcare costs: a retrospective study. BMJ Open Diabetes Res Care (2020) 8(1):e001666. doi: 10.1136/bmjdrc-2020-001666
209. Thakkar-Samtani M, Heaton LJ, Kelly AL, Taylor SD, Vidone L, Tranby EP. Periodontal treatment associated with decreased diabetes mellitus-related treatment costs: an analysis of dental and medical claims data. J Am Dent Assoc (2023) 154:283–92; 292.e1. doi: 10.1016/j.adaj.2022.12.011
210. Blaschke K, Hellmich M, Samel C, Listl S, Schubert I. The impact of periodontal treatment on healthcare costs in newly diagnosed diabetes patients: evidence from a German claims database. Diabetes Res Clin Pract (2021) 172:108641. doi: 10.1016/j.diabres.2020.108641
211. Choi SE, Sima C, Pandya A. Impact of treating oral disease on preventing vascular diseases: a model-based cost-effectiveness analysis of periodontal treatment among patients with type 2 diabetes. Diabetes Care (2020) 43:563–71. doi: 10.2337/dc19-1201
212. National Association of Dental Plans (NADP). NADP analysis shows adults with medicaid preventive dental benefits have lower medical costs for chronic conditions. Dallas, TX: NADP (2017). p. 1. Available at: https://www.globenewswire.com/news-release/2017/11/16/1194435/0/en/NADP-Analysis-Shows-Adults-with-Medicaid-Preventive-Dental-Benefits-Have-Lower-Medical-Costs-for-Chronic-Conditions.html?print=1.
213. Suzuki S, Noda T, Nishioka Y, Myojin T, Kubo S, Imamura T, et al. Evaluation of public health expenditure by number of teeth among outpatients with diabetes mellitus. Bull Tokyo Dent Coll (2021) 62:55–60. doi: 10.2209/tdcpublication.2020-0035
214. Pallos D, Ruivo GF, Ferrari-Junior SH, Pannuti CS, Perozini C, Sarmento DJS, et al. Periodontal disease and detection of human herpesviruses in saliva and gingival crevicular fluid of chronic kidney disease patients. J Periodontol (2020) 91:1139–47. doi: 10.1002/jper.19-0583
Keywords: early diagnosis, glycated hemoglobin, health care costs, interprofessional relations, periodontal diseases, prevention and control, referral and consultation, surgical clearance
Citation: Borgnakke WS (2024) Current scientific evidence for why periodontitis should be included in diabetes management. Front. Clin. Diabetes Healthc. 4:1257087. doi: 10.3389/fcdhc.2023.1257087
Received: 11 July 2023; Accepted: 13 November 2023;
Published: 11 January 2024.
Edited by:
Zoe Xiaofang Zhu, Tufts University, United StatesCopyright © 2024 Borgnakke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenche Sylling Borgnakke, d3NiQHVtaWNoLmVkdQ==
†ORCID: Wenche Sylling Borgnakke, orcid.org/0000-0003-3593-093X
 Wenche Sylling Borgnakke
Wenche Sylling Borgnakke