
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Clin. Diabetes Healthc. , 31 August 2023
Sec. Diabetes Therapies
Volume 4 - 2023 | https://doi.org/10.3389/fcdhc.2023.1204133
This article is part of the Research Topic Frontiers in Clinical Diabetes and Healthcare - Rising Stars Africa View all 10 articles
 Rahel Asres Shimelash1*†
Rahel Asres Shimelash1*† Getaneh Mulualem Belay2†
Getaneh Mulualem Belay2† Worknesh Aknaw2
Worknesh Aknaw2 Aster Tadesse Shibabaw1
Aster Tadesse Shibabaw1 Aderajew Agmas Adebabay3
Aderajew Agmas Adebabay3 Gezahagn Demsu Gedefaw4
Gezahagn Demsu Gedefaw4 Tadele Derbew Kassie5
Tadele Derbew Kassie5 Alemu Birara Zemariam6†
Alemu Birara Zemariam6†Background: Diabetic ketoacidosis is one of the major life-threatening conditions associated with acute metabolic complications. It remains a major public health problem in developing countries such as Ethiopia.
Objective: To assess the incidence and prediction of mortality in children with diabetic ketoacidosis in West Amhara Region Comprehensive Specialized Referral Hospitals, Northwest Ethiopia, in 2022.
Methods: An institution-based retrospective follow-up study was conducted among 423 study participants with a confirmed diagnosis of diabetic ketoacidosis from 01/01/2017 to 31/12/2021. Data were entered, coded, cleaned, and checked using Epi-Data version 4.6 and exported to Stata version 14 for data analysis.
Results: A total of 401 child records were included in the final analysis and were followed for 3781 days during the study period. The overall mortality of children with diabetic ketoacidosis was 10.6 per 1000 person-days observed (95% CI: 7.8-14.4) during the entire follow-up period. Hypoglycemia (AHR=4.6; 95% CI: 2.13-10.1), rural residence (AHR=2.9; 95% CI=1.01-8.11), age younger than five (AHR=4.4; 95% CI=1.4-13.7) or between five and 10 (AHR=3.1; 95% CI=1.1-8.8), and female gender (AHR=2.6; 95% CI=1.1-5.8) were significant predictors of mortality.
Conclusions: The incidence rate of mortality in children with diabetic ketoacidosis was relatively high. Age, rural residence, female gender, and hypoglycemia were significantly predictive of mortality. Community education or mass campaigns about the signs and symptoms of diabetic ketoacidosis may reduce the mortality rate in children.
Diabetic ketoacidosis (DKA) is a common complication of type 1 diabetes mellitus in children (1). It was defined as hyperglycemia (blood glucose level greater than 13.9 mmol/L or greater than 250mg/dl and any of the following: venous PH less than 7.3 or bicarbonate less than 15 mmol/L). In addition, clinical signs and symptoms of volume depletion, Kussmaul breathing, cerebral edema, acetone or fruity breath odor, mental status alterations, vomiting, nausea, and infection are indicators of DKA (2, 3).
DKA is becoming increasingly common in children (4). It has a mortality rate of 24% (5). According to the World Health Organization report, DKA is the leading cause of death in the 21st century, with a mortality rate of 10% per patient each year in children (6, 7). DKA accounts for 20% of all pediatric deaths in the United States (8). According to the CDC’s United States Surveillance System (USSS), the mortality rate of children with DKA was 6.3% (9). In Africa, the mortality rate of children with DKA was reported to be nearly 7% (10).
The global economic impact of DKA on the healthcare system is significant. Because of the direct and indirect economic impact of DKA, there will be a total global loss of US$ 1.7 trillion from 2011 to 2030, with US$ 900 billion in high-income nations and US$ 800 billion in low- and middle-income countries, while the exact impact in Africa is unknown (11, 12). DKA also represents a crisis in terms of healthcare costs, with a single admission costing between US $4125 and US $11,196 (13).
Several studies found that different factors were significantly associated with the mortality rate of DKA in children, such as infections and discontinuation of insulin therapy (14). Also, another study showed that the main significant predictors of mortality in children with DKA are altered sensation, shock, delayed diagnosis, renal failure, and sepsis (15). Early recognition of complications, especially cerebral edema, could reduce the serious consequences in children (16). It is preferable to raise awareness among healthcare workers, teachers, children, and parents (15). According to a recent study, early detection, assistance, and supervision are important for better outcomes in children with DKA. Healthcare workers who treat children are recommended to follow the treatment guidelines properly (17).
The Ethiopian Federal Ministry Of Health (FMOH) has established a National Strategic Action Plan (NSAP) for four priority non-communicable diseases (NCDs), which include DKA. However, limited resources such as low socioeconomic status and fast urbanization have increased morbidity, disability, and mortality among children with DKA (18). DKA remains a major public health concern in underdeveloped countries and a leading cause of death in children in Sub-Saharan Africa, including Ethiopia (19). The fatality rate has not been investigated in Ethiopia, even in the study areas. Hence, the main aim of this study was to determine the incidence and predictors of mortality among children in DKA.
An institution-based retrospective follow-up study was conducted to review five-year medical records of children with DKA in the Comprehensive Specialized Referral Hospitals of West Amhara Region from 01/01/2017 to 31/12/2021. Data were retrieved from 01/05/2022 to 30/05/2022.
The University of Gondar, and the Felege-Hiwot, Tibebe-Ghion, Debre-Markos, and Debre-Tabor Comprehensive Specialized Referral Hospitals were the study settings. All children with DKA admitted at referral hospitals in West Amhara Region were the source population, and among them, those diagnosed with DKA from 01/01/2017 to 31/12/2021 were the study population.
All children with DKA less than 15 years of age with follow-up from 01/01/2017 to 31/12/2021 were included in this study. Children with incomplete baseline clinical data, lost medical records, and outcome variables not recorded in their medical records during the data collection period were excluded.
The sample size was calculated differently based on incidence and prediction. Since the incidence of death among children with DKA is not yet studied in Ethiopia, the minimum required sample size was calculated using proportion (50%). Then the sample size was determined based on the single population proportion formula N= (Z∝/2)2 * p (1-p)/(d) 2Z where N= sample size, Z∝/2 corresponding Z-score of 95% CI (1.96), and d=margin of error (5%). Rearranging the given formula, N= (1.96)2 * 0.5 * 0.5/(0.05)2 = 384.16. Finally, assuming 10% incomplete data (38,416), the final sample size for incidence was calculated as 422,576 .
For prediction, the sample size was determined by using the double population proportion formula and considering predictive variables of DKA mortality using the Epi-Info version 7 statistical package. It was calculated using a two-sided significant level () of 5%, power (ZB) of 80%, and a 1:1 ratio of exposed to non-exposed at 95% CI. Two predictive variables, infection and missed insulin doses, were used to calculate the sample size.
In this study, P1= percent of death outcomes in exposed children, and P2= percent of death outcomes in non-exposed children, Z : is taking CI 95%, ZB is power, and r = 1:1. Based on the exposure status of the infection, p1 = 50.9% and p2 = 27.4%, respectively. After entering these data into Epi-Info, considering 10% incomplete data, the total sample size was =165. In terms of missed insulin doses, p1 = 48.5%, and p2 = 33.2%; by adding 10% incomplete data, the total sample size was 386.8 (20). Finally, a large sample size of N=423 was selected for this study.
Initially, the number of medical records of children with DKA was obtained from each hospital, and 670 children with DKA between 01/01/2017 and 31/12/2021 from five referral hospitals. Then, the sample size was proportionally allocated to each hospital. Based on this, 107 out of 170 from the University of Gondar Hospital, 103 out of 163 from Felege-Hiwot Hospital, 93 out of 147 from Debre Markos Hospital, 44 out of 70 from Tibebe-Ghion Hospital, and 76 out of 120 from Debre Tabor Hospital were taken popotionally. Finally, a computer-generated simple random sampling technique was employed to recruit the study participants. A total of 423 medical charts were reviewed, of which 22 records were discarded due to the exclusion criteria. In total, 401 medical records were used for the final analysis.
Event: Death of the child at the specific time of follow-up, as confirmed by the physician.
Diabetic ketoacidosis: Defined as hyperglycemia (blood glucose > 250 mg/dl or 13.9 mmol/L and any of the following: venous PH<7.3 or bicarbonate< 15 mmol/L) (21).
Baseline clinical data: An initial measurement of a condition taken early and used for comparison over time to look for changes (22).
The data extraction tool was adapted from reviewed literature sources (23, 24). The questionnaire was given to different experts working in different specialties, like neonatology and pediatric nursing, to check its internal validity. Data were collected from patient charts. The charts were accessed based on their medical record number. Then, all DKA medical record numbers (MRN) were selected. Following the lottery method, simple random sampling was used. Three BSc nurses were recruited for supervision, and one BSc nurse was recruited for data collection for each hospital. Training was given for one day to data collectors and supervisors on data collection tools and procedures.
Data were entered, coded, cleaned, and checked using Epi-Data version 4.6, then checked for completeness and consistency and exported to Stata version 14 for data analysis. Descriptive measures were used to characterize the data. Missing data were handled by multiple imputations. Kaplan-Meier and log-rank tests were used to estimate and compare survival times. The incidence rate of DKA mortality during the follow-up period was calculated and presented per 1000 person-day observations. The overall cumulative survival rate from the total number of child person-days observed during the follow-up period was 3781 person-days, with a median time of 31 days. Children were followed for a minimum of 1 and a maximum of 33 days in the inpatient department. The assumption of the proportional hazards model was tested by using the Schoenfeld residuals test together with the graphical test. In addition, the overall goodness of the model fit was checked via the Cox-Snell residuals. So, it can be concluded that the final model fits the data well. To investigate the prediction of mortality in children with DKA, the Cox proportional-hazards model was employed, and bivariable Cox regression analysis was performed: variables with P< 0.25 levels were eligible for multivariable analysis. The AHR, 95% CI, and P-value were used to assess the strength of the association and statistical significance. All variables with a P-value< 0.05 in the multivariable analysis were considered statistically significant in predicting DKA mortality.
A total of 401 children were enrolled in the study. The completion rate was 95%. Nearly half of the children, 215 (53.62%), were female. The mean age of the children with DKA was 8.5 (± 2) years. Nearly two-thirds of the children, 249 (62.09%) had primary and higher education, and 252 (62.84%) were from rural areas (Table 1).
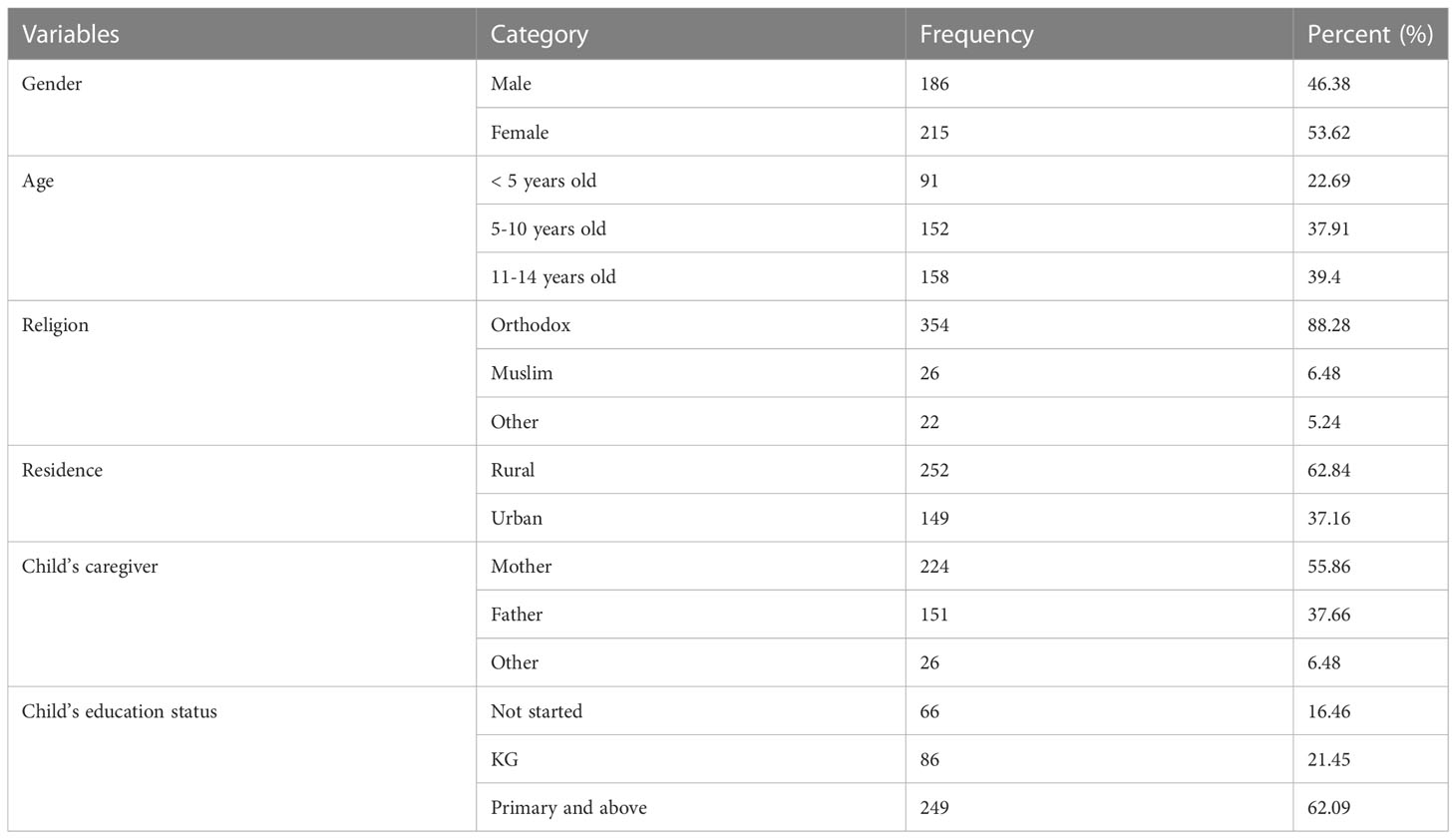
Table 1 Socio-demographic characteristics for prediction of mortality in children with diabetic ketoacidosis in the Comprehensive Specialized Referral Hospitals of West Amhara Region, Northwest Ethiopia, 2022 (N=401).
Nearly half of the children, 214 (53.37%), had good medication adherence, while 229 (57.11%) had improper medication storage, and almost half of the children, 198 (49.38%), had discontinued medication (Table 2).
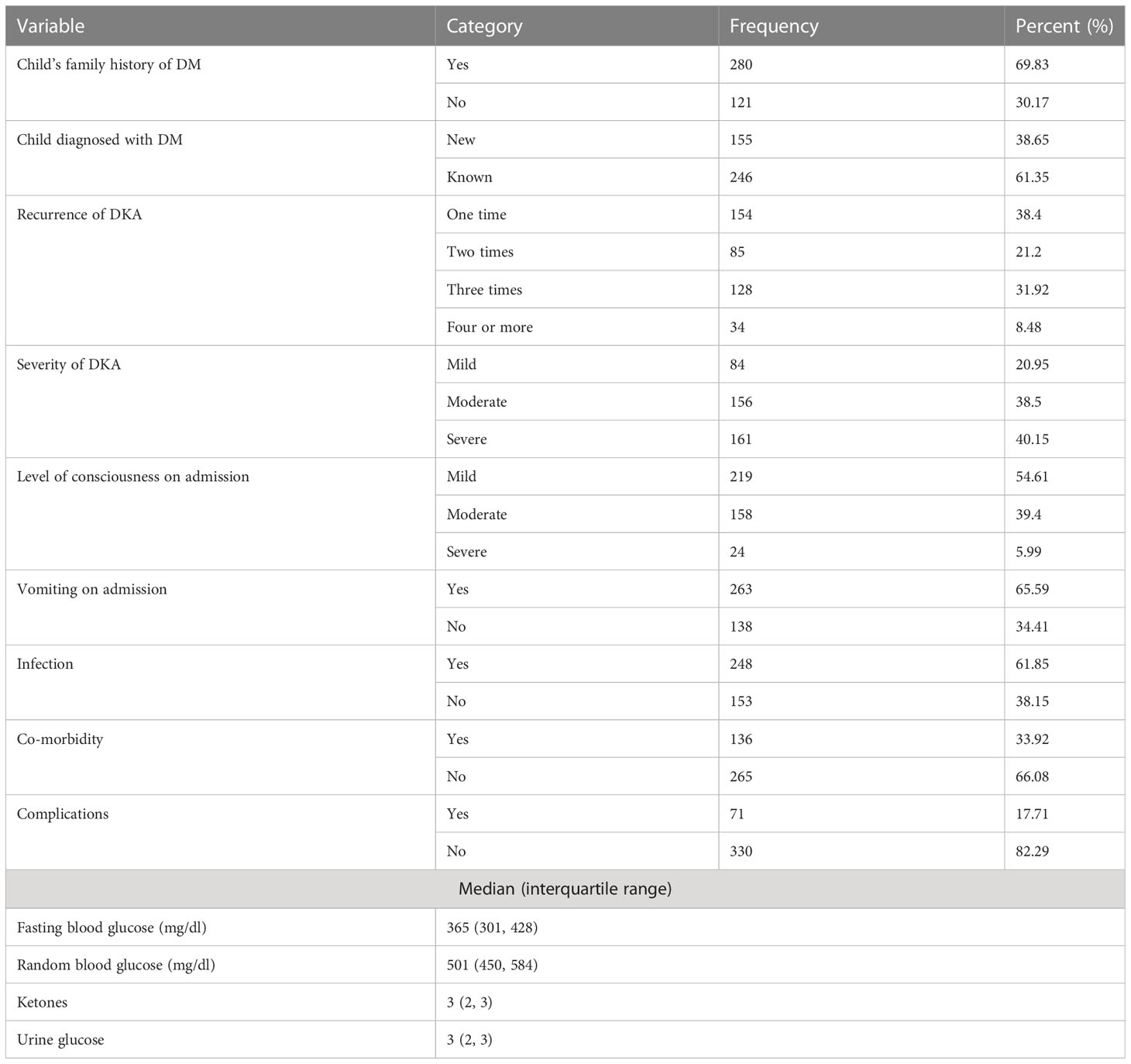
Table 2 Clinical characteristics and baseline data for predicting mortality in children with diabetic ketoacidosis in the Comprehensive Specialized Referral Hospitals of West Amhara Region, Northwest Ethiopia, 2022 (N=401).
The Schoenfield residuals were used as the basis for the global test of the proportional hazard assumption, which had a P-value of 0.67. When examining several multivariable Cox regression analyses, the minimum and maximum P-values were 0.06 and 0.92, respectively.
Out of 401 children, 40 (10%) died during the follow-up period. The overall cumulative survival rate from the total days of observation of the children during the follow-up period was 3781 person-days, with a median time of 31 days. Children were followed for a minimum of 1 and a maximum of 33 days in the inpatient department. Throughout the entire follow-up period, the overall mortality rate for children with DKA was 10.6 per 1000 person-days of observation (95% CI: 7.8-14.4). The overall Kaplan-Meier failure function indicated that the probability of death increased during the follow-up period. The cumulative odds of death at 10, 20, and 30 days were 0.08, 0.16, and 0.36, respectively (Figure 1).
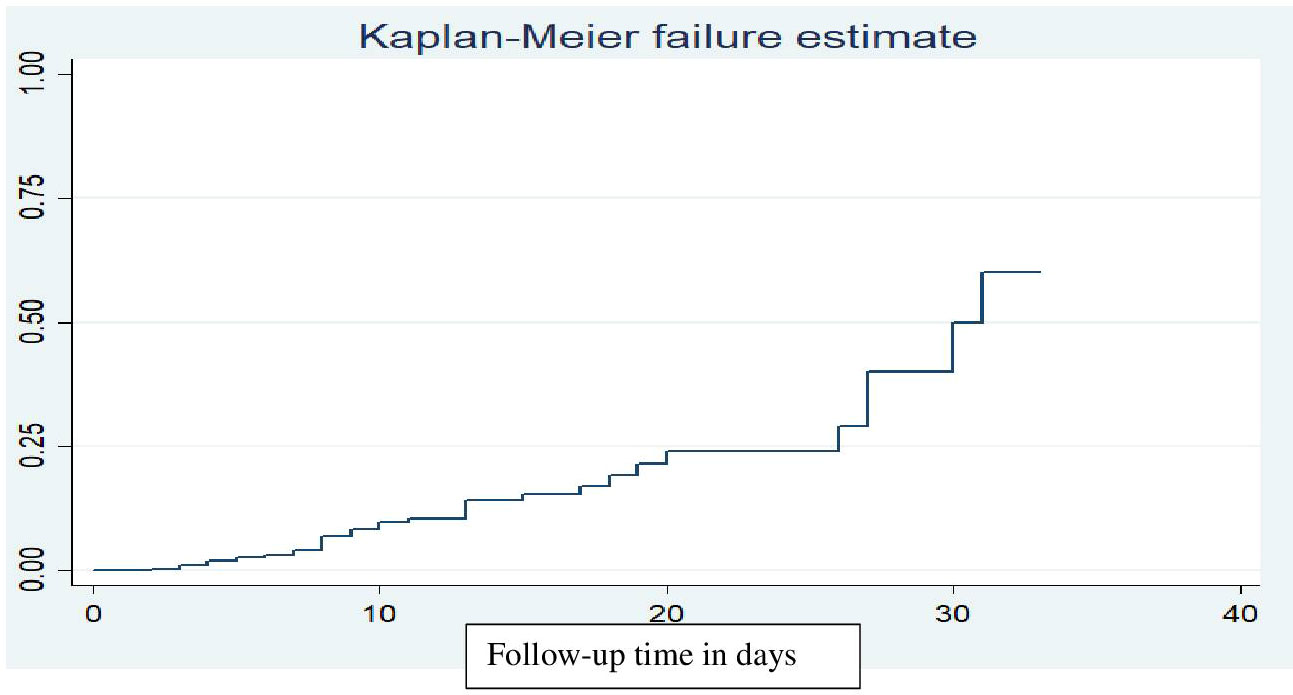
Figure 1 Kaplan-Meier failure function estimate of mortality in children with diabetic ketoacidosis in the Comprehensive Specialized Referral Hospitals of West Amhara Region, Northwest Ethiopia, 2022 (N=401).
The fitness of the model was checked graphically using the Cox–Snell residuals and the Nelson-Aalen cumulative hazard graph, as the line follows 45 degrees (Figure 2).
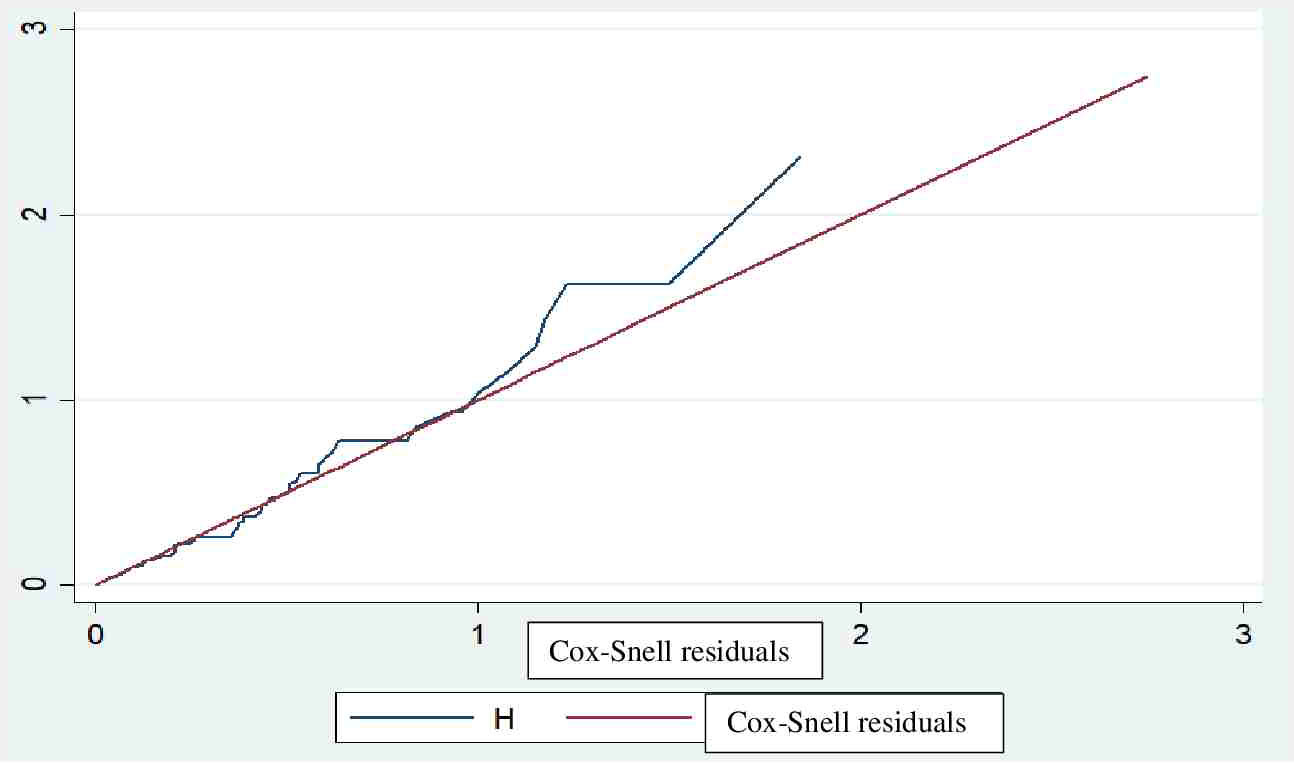
Figure 2 Fitness of model confirmed with Cox-Snell residuals for mortality in children with diabetic ketoacidosis in the Comprehensive Specialized Referral Hospitals of West Amhara Region, Northwest Ethiopia, 2022 (N=401).
In bivariate Cox regression, the severity of diabetic ketoacidosis, Glasgow Coma Scale, vomiting, complications, place of residence, medication discontinuation/skipping, infection, age, gender, co-morbidity, education status, drug adherence, and recurrent diabetic ketoacidosis were significantly predictive of mortality in DKA children at a P-value lower than or equal to 0.25. On the other hand, multivariate Cox regression analyses like hypoglycemia, living in a rural area, age, and female gender were significantly predictive of mortality in children with diabetic ketoacidosis at a P-value lower than 0.05 (Table 3).

Table 3 Bi-variable and multi-variable Cox regression analyses for predicting mortality in children with diabetic ketoacidosis in the Comprehensive Specialized Referral Hospitals of West Amhara Region, Northwest Ethiopia, 2022 (N=401).
The hazard of death in children with hypoglycemia was 4.6 times higher than in those without (AHR=4.6; 95% CI: 2.13-10.1). Rural residence increased the hazard of death by a factor of 2.9 compared to urban residence (AHR=2.9; 95% CI=1.01-8.11). In children younger than five years, the hazard of death increased 4.4-fold (AHR=4.4; 95% CI=1.4-13.7), and the hazard of death increased by a factor of 3.1 times in children aged five to 10 years compared to children aged 11 to 14 years (AHR=3.1; 95% CI=1.1-8.8). The hazard of death was 2.6 times higher in female children compared to male children (AHR=2.6; 95% CI=1.1-5.8).
In this study, the mortality rate was 10%. The overall mortality of children with diabetic ketoacidosis was 10.6 per 1000 child days observed (95% CI: 7.8-14.4) during the entire follow-up period. This finding was in line with studies conducted in Bangladesh, where the all-time mortality was 13.4/1000 (25), in Korea (11.8/1000) (26), and in South India (11/1000) (15). On the other hand, the results of the current study were relatively higher compared to other research conducted in the USA, where the all-time mortality was 0.21/1000 (27), Canada (0.18/1000) (28), UK (0.31/1000) (29), Iran (1.7/1000) (30), Kenya (6.9/1000) (17), and Pakistan (3.4/1000) (31). This discrepancy may be due to lifestyle differences between these populations and the population under study. Lifestyle modifications such as a healthy diet and regular physical activity might reduce the mortality rate of children with DKA in these countries. A low economic societal status, lack of access to health care facilities, and limited resources in the current study country may increase the mortality of children with DKA (32). Conversely, this finding was lower than a study conducted in Turkey, where the mortality of children with diabetic ketoacidosis was 24% (33). The possible reason for this may be due to the fact that the previous study was a prospective cohort and proper event registration may have occurred, but the current study was a retrospective cohort, and children who died due to DKA may have been overlooked.
Rural residence increased the hazard of death by a factor of 2.9. This finding was supported by a study conducted in Iraq (34). Rural residents were more susceptible to mortality compared to urban residents. Since most rural communities lack infrastructure such as health facilities and transportation, subjects may not be able to reach a health center as quickly as possible during their illness. Additionally, those living in remote areas with low socioeconomic levels may find it challenging to pay for transportation, medications, and medical monitoring equipment (35, 36). The other possible reason may be that people living in a rural setting may have lower health-seeking behaviors as compared with those living in urban areas (37).
Hypoglycemia was a significant predictor of mortality in children with DKA. Hypoglycemia increased the risk of death by a factor of 4.6. This finding was supported by a study conducted in India (38). The reason may be that if hypoglycemia is not treated in time, the condition may quickly deteriorate. Prolonged hypoglycemia leads to encephalopathy, brain damage, cardio-respiratory arrest, severe arrhythmia, and ischemic cardiovascular attack, along with loss of consciousness, coma, and death. (39).
Age was a significant predictor of mortality in children with DKA. Ages under five and between five and 10 increased the risk of death by 4.4 and 3.1 times, respectively, compared to ages between 11 to 14. This finding was supported by studies conducted in the whole of the UK (40) and in the city of Birmingham (41). The child’s age brings its own set of challenges when it comes to diabetes management. Because smaller children are more dependent on their caregivers from infancy through preschool, which results in non-adherence to medication and diet, difficulties in understanding clinical signs and symptoms, refusal to modify their lifestyle, and failure to convey their hypoglycemia and hyperglycemia symptoms, this could cause blood glucose level fluctuations and death (42–44).
Gender was one of the independent significant predictors of mortality in children with DKA. Female children had a 2.6-fold increased risk of death compared to male children. This finding was supported by a study conducted in Germany (45). One possible reason may be due to puberty-associated hormonal changes, such as a change in estrogen levels. Also, female children may have body image issues, particularly in adolescence, which results in skipping meals and insulin injections for weight loss (46). Furthermore, this may be due to lower sensitivity to insulin in female subjects than in male subjects. Female children have higher levels of glycosylated hemoglobin A1C (HgA1C), cholesterol, low-density lipoprotein (LDL), and triglycerides during the pre-pubertal and pubertal stages, leading to poorer glycemic control in female children (7, 47, 48).
Since our data were collected from secondary sources, the main limitation of this research consists of incomplete medical records. This may affect the outcomes of the study. Some important maternal and paternal predictive variables, such as educational level, and monthly income, in addition to baseline clinical data like PH, bicarbonate, and HgA1C were not reported in the medical records. Furthermore, since the data were collected in a hospital setting, child mortality in DKA patients may be underestimated. The child may die at home, and as a result, the mortality rate may be underreported.
The current study showed that the overall incidence of mortality in children with DKA was relatively high as compared to the reports on diabetes from the Ministry of Health in the Federal Democratic Republic of Ethiopia. Hypoglycemia, age below 10 years, rural residence, and female gender were significant predictors of mortality in children with DKA in the West Amhara Region. The key to achieving good outcomes and preventing mortality is the prevention of DKA through early detection, support, supervision, and proper blood glucose monitoring. Community education or mass campaigns on the signs and symptoms of DKA can reduce the death rate in children. Therefore, it is important that healthcare workers treating children with DKA follow guidelines. Finally, further prospective longitudinal studies would be recommended by incorporating important predictors of DKA mortality, such as baseline laboratory investigations of PH levels, bicarbonate, and HgA1C, and including other predictors like socio-economic status in addition to paternal or maternal educational levels.
Patients were not formally involved in the development of this specific study design.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethical approval was obtained from the School of Nursing Ethical Review Committee on behalf of the Institutional Research Ethical Committee of the University of Gondar, College of Medicine and Health Sciences, with reference number (248/2022) on 14/5/2022. Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
RS: design, methodology, software, analysis, resources, data curation, draft of the manuscript, editing, visualization, and supervision. GB and WA: collaborated in visualization, methodology, software, data curation, analysis, review of the manuscript draft, and supervision. AZ, AS, AA, GG, and TK: methodology, data curation, analysis, review of the manuscript draft, and supervision. All authors contributed to the article and approved the submitted version.
First, we would like to express our utmost gratitude to the University of Gondar, the College of Medicine and Health Sciences, and the School of Nursing for their unreserved support. Next, our great thanks go to the head of the diabetes mellitus care unit and all staff members at the Northwest Comprehensive Specialized Referral Hospitals in the Amhara Region for their cooperation in providing information about the study area and study population. Finally, our special thanks go to all of our friends for their support and their constructive feedback, and to the data collectors and supervisors for their unreserved efforts throughout our work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AHR, Adjusted Hazard Ratio; CHR, Crude Hazard Ratio; CI, Confidence Interval; DKA, Diabetic Ketoacidosis; DM, Diabetes Mellitus; FMOH, Federal Ministry of Health; T1DM, Type 1 Diabetes Mellitus; UK, United Kingdom; USA, United States of America; WHO, World Health Organization.
1. Wolfsdorf J, Craig ME, Daneman D, Dunger D, Edge J, Lee W, et al. Diabetic ketoacidosis. Pediatr. Diabetes (2007) 8:28–43.
2. Victor FM, d. Lima Andrade SR, Bandeira F. Diabetic ketoacidosis and hyperosmolar hyperglycemic state. Endocrinol. Diabetes (2022), 355–62. doi: 10.1007/978-3-030-90684-9_33
3. Ibald-Mulli A, Seufert J, Grimsmann JM, Laimer M, Bramlage P, Civet A, et al. Identification of predictive factors of diabetic ketoacidosis in type 1 diabetes using a subgroup discovery algorithm. Diab Obes. Metab. (2023). doi: 10.1111/dom.15039
4. Jefferies C, Cutfield SW, Derraik JG, Bhagvandas J, Albert BB, Hofman PL, et al. 15-year incidence of diabetic ketoacidosis at onset of type 1 diabetes in children from a regional setting (Auckland, New Zealand). Sci. Rep. (2015) 5:1–7. doi: 10.1038/srep10358
5. Edge J, Hawkins M, Winter D, Dunger D. The risk and outcome of cerebral oedema developing during diabetic ketoacidosis. Arch. Dis. childhood (2001) 85:16–22.
6. Wolfsdorf JI, Allgrove J, Craig ME, Edge J, Glaser N, Jain V, et al. ISPAD Clinical Practice Consensus Guidelines 2014. Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr. Diabetes (2014) 15:154–79.
7. Onyiriuka AN, Ifebi E. Ketoacidosis at diagnosis of type 1 diabetes in children and adolescents: frequency and clinical characteristics. J. Diabetes Metab. Disord. (2013) 12:1–5.
8. Glaser NS, Ghetti S, Casper TC, Dean JM, Kuppermann N, P. E. C. A. R. N. D. F. S. Group. Pediatric diabetic ketoacidosis, fluid therapy, and cerebral injury: the design of a factorial randomized controlled trial. Pediatr. Diabetes (2013) 14:435–46.
9. Benoit SR, Zhang Y, Geiss LS, Gregg EW, Albright A. Trends in diabetic ketoacidosis hospitalizations and in-hospital mortality—United States, 2000–2014. Morbid Mortal Weekly Rep. (2018) 67:362. doi: 10.15585/mmwr.mm6712a3
10. Ahmed AM, Ahmed NH, Abdella ME. Pattern of hospital mortality among diabetic patients in Sudan. Pract. Diabetes Int. (2000) 17:41–3. doi: 10.1002/(SICI)1528-252X(200003/04)17:2<41::AID-PDI29>3.0.CO;2-S
11. Snowdon W, Waqa G, Raj A, Kanungo A, Robinson H. Non-communicable disease and health system response in Fiji. Series No. 34 The University of Melbourne | The Nossal Institute for Global Health, Melbourne. (2013).
13. Tieder JS, McLeod L, Keren R, Luan X, Localio R, Mahant S, et al. Variation in resource use and readmission for diabetic ketoacidosis in children’s hospitals. Pediatrics (2013) 132:229–36. doi: 10.1542/peds.2013-0359
14. Alijanpour Aghamaleki M, Shabanzadeh Z, Rezapour M, Bijani A, Aghajanpour F. Incidence, predisposing factors and complications of Diabetic Ketoacidosis in diabetic patients. Caspian J. Pediatr. (2016) 2:142–7.
15. Poovazhagi V. Factors associated with mortality in children with diabetic ketoacidosis (DKA) in South India. Int. J. Diabetes Developing Countries (2016) 36:295–302. doi: 10.1007/s13410-015-0441-x
16. Burcul I, Arambasic N, Polic B, Kovacevic T, Bartulovic I, Catipovic Ardalic T, et al. Characteristics of children with diabetic ketoacidosis treated in pediatric intensive care unit: two-center cross-sectional study in Croatia. Medicina (2019) 55:362. doi: 10.3390/medicina55070362
17. Musoma SN, Omar A, Mutai BC, Laigong P. Outcomes of children and adolescents admitted with diabetic ketoacidosis at Kenyatta National Hospital (KNH), Kenya. J. Diabetes Res. (2020) 2020:8987403. doi: 10.1155/2020/8987403
18. Non-Communicable O. National strategic action plan (nsap) for prevention & control of non-communicable diseases in Ethiopia. (2016), Google Scholar. Ministry of Health.
19. Schober E, Rami B, Waldhoer T. Diabetic ketoacidosis at diagnosis in Austrian children in 1989–2008: a population-based analysis. Diabetologia (2010) 53:1057–61. doi: 10.1007/s00125-010-1704-1
20. Mansour AA, Abdu-Alla M. A.-A.-K. Predictors of diabetic ketoacidosis among patients with type 1 diabetes mellitus seen in the emergency unit. Br. J. Med. Med. Res. (2016) 11:1.
21. Savage M, Dhatariya K, Kilvert A, Rayman G, Rees J, Courtney C, et al. Joint British Diabetes Societies guideline for the management of diabetic ketoacidosis. Diabetic medicine: J. Br. Diabetic Assoc. (2011) 28:508–15. doi: 10.1111/j.1464-5491.2011.03246.x
22. Malik MIK. A review of the efficacy of the Milwaukee Protocol in the treatment of ketoacidosis in pediatric intensive care unit patients at Rebro Hospital between 2009-2014. (2015).
23. Foster NC, Beck RW, Miller KM, Clements MA, Rickels MR, DiMeglio LA, et al. State of type 1 diabetes management and outcomes from the T1D Exchange in 2016–2018. Diabetes Technol. Ther. (2019) 21:66–72.
24. Semenkovich K, Berlin KS, Ankney RL, Klages KL, Keenan ME, Rybak TM, et al. Predictors of diabetic ketoacidosis hospitalizations and hemoglobin A1c among youth with Type 1 diabetes. Health Psychol. (2019) 38:577. doi: 10.1037/hea0000719
25. Tiwari LK, Muralindharan J, Singhi S. Risk factors for cerebral edema in diabetic ketoacidosis in a developing country: role of fluid refractory shock. Pediatr. Crit. Care Med. (2012) 13:e91–6. doi: 10.1097/PCC.0b013e3182196c6d
26. Ko SH, Lee WY, Lee JH, Kwon HS, Lee JM, Kim SR, et al. Clinical characteristics of diabetic ketoacidosis in Korea over the past two decades. Diabetic Med. (2005) 22:466–9. doi: 10.1111/j.1464-5491.2005.01450.x
27. Levitsky L. Death from diabetes (DM) in hospitalized children (1970-1988). Pediatr. Res. (1991) 29:A195.
28. Curtis JR, To T, Muirhead S, Cummings E, Daneman D. Recent trends in hospitalization for diabetic ketoacidosis in Ontario children. Diabetes Care (2002) 25:1591–6. doi: 10.2337/diacare.25.9.1591
29. Edge JA, Ford-Adams ME, Dunger DB. Causes of death in children with insulin dependent diabetes 1990–96. Arch. Dis. childhood (1999) 81:318–23.
30. Moravej H, Ilkhanipour H, Seratishirazi Z, Azargoon M, Amirhakimi A. Outcome of patients with diabetic ketoacidosis in South of Iran. J. Endocrinol. Diabetes (2017) 4:1–6.
31. Syed M, Khawaja FB, Saleem T, Khalid U, Rashid A, Humayun KN. Clinical profile and outcomes of paediatric patients with diabetic ketoacidosis at a tertiary care hospital in Pakistan. J. Pakistan Med. Assoc. (2011) 61:1082.
32. Jawaid A, Sohaila A, Mohammad N, Rabbani U. Frequency, clinical characteristics, biochemical findings and outcomes of DKA at the onset of type-1 DM in young children and adolescents living in a developing country–an experience from a pediatric emergency department. J. Pediatr. Endocrinol. Metab. (2019) 32:115–9. doi: 10.1515/jpem-2018-0324
33. Rewers A, Chase HP, Mackenzie T, Walravens P, Roback M, Rewers M, et al. Predictors of acute complications in children with type 1 diabetes. Jama (2002) 287:2511–8. doi: 10.1001/jama.287.19.2511
34. Sandahl K, Nielsen L, Svensson J, Johannesen J, Pociot F, Mortensen H, et al. Increased mortality in a Danish cohort of young people with type 1 diabetes mellitus followed for 24 years. Diabetic Med. (2017) 34:380–6. doi: 10.1111/dme.13124
36. Vaidya A, Shakya S, Krettek A. Obesity prevalence in Nepal: public health challenges in a low-income nation during an alarming worldwide trend. Int. J. Environ. Res. Public Health (2010) 7:2726–44.
37. Ghimire P, Dhamoon AS. Ketoacidosis. In: StatPearls. University of Kentucky Ag. Econ Staff Papers 366: StatPearls Publishing (2021).
38. Varadarajan P, Suresh S. Delayed diagnosis of diabetic ketoacidosis in children—a cause for concern. Int. J. Diabetes Developing Countries (2015) 35:66–70. doi: 10.1007/s13410-014-0245-4
39. A. D. A. W. o. Hypoglycemia. Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care (2005) 28:1245–9. doi: 10.2337/diacare.28.5.1245
40. Barski L, Harman-Boehm I, Nevzorov R, Rabaev E, Zektser M, Jotkowitz AB, et al. Gender-related differences in clinical characteristics and outcomes in patients with diabetic ketoacidosis. Gender Med. (2011) 8:372–7. doi: 10.1016/j.genm.2011.09.032
41. Wright J, Ruck K, Rabbitts R, Charlton M, De P, Barrett T, et al. Diabetic ketoacidosis (DKA) in Birmingham, UK, 2000—2009: an evaluation of risk factors for recurrence and mortality. Br. J. Diabetes Vasc. Dis. (2009) 9:278–82.
42. Westerberg DP. Diabetic ketoacidosis: evaluation and treatment. Am. Family phys (2013) 87:337–46.
43. Siminerio LM, Albanese-O’Neill A, Chiang JL, Hathaway K, Jackson CC, Weissberg-Benchell J, et al. Care of young children with diabetes in the child care setting: a position statement of the American Diabetes Association. Diabetes Care (2014) 37:2834–42. doi: 10.2337/dc14-1676
44. Vasireddy D, Sehgal M, Amritphale A. Risk factors, trends, and preventive measures for 30-day unplanned diabetic ketoacidosis readmissions in the pediatric population. Cureus (2021) 13:2021. doi: 10.7759/cureus.19205
45. Hammersen J, Tittel SR, Warncke K, Fritsch M, Placzek K, Pacaud D, et al. Previous diabetic ketoacidosis as a risk factor for recurrence in a large prospective contemporary pediatric cohort: Results from the DPV initiative. Pediatr. Diabetes (2021) 22:455–62.
46. Colton P, Rodin G, Bergenstal R, Parkin C. Eating disorders and diabetes: introduction and overview. Diabetes Spectr. (2009) 22:138–42. doi: 10.2337/diaspect.22.3.138
47. Zayed H. Epidemiology of diabetic ketoacidosis in Arab patients with type 1 diabetes: a systematic review. Int. J. Clin. Pract. (2016) 70:186–95. doi: 10.1111/ijcp.12777
Keywords: diabetic ketoacidosis, mortality, predictive factors, survival status, children
Citation: Shimelash RA, Belay GM, Aknaw W, Shibabaw AT, Adebabay AA, Gedefaw GD, Kassie TD and Zemariam AB (2023) Incidence and predictors of mortality in children with diabetic ketoacidosis in the comprehensive specialized referral hospitals of West Amhara Region, Northwest Ethiopia: a retrospective follow-up study. Front. Clin. Diabetes Healthc. 4:1204133. doi: 10.3389/fcdhc.2023.1204133
Received: 11 April 2023; Accepted: 20 July 2023;
Published: 31 August 2023.
Edited by:
Enoch Odame Anto, Kwame Nkrumah University of Science and Technology, GhanaReviewed by:
Andrea Enzo Scaramuzza, Istituti Ospitalieri di Cremona, ItalyCopyright © 2023 Shimelash, Belay, Aknaw, Shibabaw, Adebabay, Gedefaw, Kassie and Zemariam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rahel Asres Shimelash, cmFoZWxhc3JlczlAZ21haWwuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.