
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Clin. Diabetes Healthc. , 05 July 2023
Sec. Diabetes, Lifestyle and Metabolic Syndrome
Volume 4 - 2023 | https://doi.org/10.3389/fcdhc.2023.1181998
This article is part of the Research Topic Safety and Side Effects of Psychotropic Medications, Volume II View all 11 articles
Background: Use of psychotropic drugs (PD) may be associated with impairment of physical function. However, few studies have assessed the impact of PD on health outcomes in patients with type 2 diabetes. This study aimed to examine the associations between psychotropic drug use and handgrip strength (HGS) and between the use of PD and hospitalization in patients with type 2 diabetes.
Methods: From April 2013 to December 2015, we conducted a retrospective cohort study in patients with type 2 diabetes at the National Center for Global Health and Medicine Kohnodai Hospital. Patients aged 20 years and over who can measure HGS were included. All participants received nutritional guidance regarding diet therapy for type 2 diabetes at baseline. Nonpsychotropic drug users were matched one-to-one with the PD users using propensity score matching method with respect to their baseline covariates. The differences in HGS and the number of patients who had hospitalizations during the study period were examined. By Cox proportional hazard regression analysis, the association between the use of PD and repeated hospitalizations was estimated.
Results: A total of 1,282 patients were enrolled and followed up for 2.36 ± 0.73 years. In the propensity score matching cohort, HGS was significantly lower (p = 0.006) in PD users than non-PD users. PD users had more hospitalizations than non-PD users. Cox proportional hazard regression analysis confirmed the association of repeated hospitalizations with the use of PD (hazard ratio = 2.138; 95% confidence interval, 1.144–3.995, p = 0.017)). In addition, HGS was significantly and inversely correlated with the number of hospitalizations (r = −0.143, p = 0.013).
Conclusions: The use of PD could increase the risk of repeated hospitalizations. Skeletal muscle may play a role in reducing the risk of hospitalization in patients who are treated with PD.
Patients with diabetes mellitus may suffer from psychological disorders, such as depression, anxiety, eating disorders, and schizophrenia (1). Although the definition of psychological disorders/syndromes in patients with diabetes varies across clinical studies, ranging from self-reported symptoms to using formal diagnostic criteria such as Diagnostic and Statistical Manual of Mental Disorders, interventions for psychological problems in patients with diabetes result in an improvement in the management of diabetes (1). Chronic insomnia with a sleep duration ≤ 5 h has been associated with an increased risk of diabetes (2). A meta-analysis showed that depression was associated with a 60% increase in the risk of development of type 2 diabetes (3). The prevalence of type 2 diabetes is 10% in patients with schizophrenia, and the relative risk of developing diabetes is 2.5 times higher in patients with schizophrenia than that in the general population (4). Therefore, clinicians should pay attention to such psychiatric comorbidities in patients with diabetes.
Antipsychotic drugs probably increase the risk of diabetes (5, 6) through causing obesity (7) and a reduced insulin sensitivity (8). Furthermore, although psychotropic drugs (PD) are widely used even in the absence of a confirmed diagnosis of psychiatric disorders, epidemiological studies have shown that antipsychotic drug use is associated with an increased mortality risk in patients with Parkinson’s disease (9) and Alzheimer’s (10). In addition, high-dose benzodiazepines use has a dose–response relationship with mortality in patients with schizophrenia (11); similarly, antipsychotic drug use in combination with benzodiazepines is associated with an increased risk of mortality in patients with dementia (12). Furthermore, a systematic review and meta-analysis has shown that antipsychotics, antidepressants, and benzodiazepines were consistently associated with a higher risk of falls (13). On the other hand, the relationship between the use of benzodiazepines, antidepressants, and antipsychotics and serious diseases, such as pneumonia, cancer and cardiovascular (CV) disease is controversial (14–18). In the literature, few studies have assessed the impact of PD on such health outcomes in patients with type 2 diabetes.
Generally, patients with type 2 diabetes have a lower energy expenditure, physical activity duration (19), and muscle strength (20, 21) than healthy individuals. Recently, we showed that handgrip strength (HGS), which is a simple and cost-effective method for evaluating muscle strength, predicts hospitalization, occurrence of CV events, and death among Japanese patients with type 2 diabetes (22). van Milligen et al. reported that women with depression and anxiety disorder had lower HGS compared with healthy controls (23). Antipsychotic drugs also decrease physical activity, whole body balance, and cardiorespiratory endurance (24). We hypothesize that PD, including benzodiazepine, antidepressant, and antipsychotic use, is associated with impairment of physical function such as HGS and subsequently leads to an increased risk of hospitalization. Thus, in this study, we examined the association of PD use with HGS and deaths, CV events, and hospitalization in patients with type 2 diabetes.
We conducted a retrospective cohort study in patients with type 2 diabetes who were treated at the National Center for Global Health and Medicine Kohnodai Hospital between April 2013 and December 2015. Baseline is the date on which each patient’s data, such as medical history, anthropometric, physiological, and biochemical data, were collected. A total of 1,327 individuals aged >20 years with type 2 diabetes whose medical history, i.e., regular treatment with benzodiazepines, antidepressants, and antipsychotics, was collected at first examination were eligible for inclusion in our analyses. Patients aged <20 years (n = 2) with type 1 diabetes (n = 16) and without medication information (n = 24) were ineligible for inclusion. Additionally, patients whose HGS could not be measured because of disabilities, such as cerebral infarction sequelae (n = 3), were also excluded (Figure 1). The PD patient group was defined as follows (1): Patients who were prescribed at least one PD (benzodiazepines, antidepressants, and antipsychotics) at the first examination during the study period; (2) Patients who continuously received PD and confirmed medication adherence during the study period. Patients who were not prescribed PD at the start of follow-up did not receive any new PD during the course of the study.
The study protocol was approved by the Medical Ethics Committee of the National Center for Global Health and Medicine (Reference No. NCGM-G-002052), and the study was performed in accordance with the Declaration of Helsinki.
At the first clinical visit, patients were instructed to consume a calorie-restricted diet of 25–30 kcal/kg (ideal body weight) each day by certified nutritional educators as diet therapy for diabetes and to continue the diet during the study period. The dietary adherence of patients was confirmed on every consultation day on a monthly basis. All patients were evaluated and followed up until death or at the end of follow-up in May 2016. At the end of the follow-up, the information on hospitalization was collected from medical record review. Next, the number of hospitalizations was calculated in all subjects, and hospitalization for two or more times was defined as repeated hospitalization. In other words, repeated hospitalization refers to any hospitalization that occurred after the initial hospitalization during the study period.
Participants’ height was measured using a rigid stadiometer (TTM stadiometer; Tsutsumi Co., Ltd., Tokyo, Japan). Their weights were measured using calibrated scales (AD-6107NW; A&D Medical Co., Ltd., Tokyo, Japan). Body mass index (BMI) was calculated as body weight in kilograms divided by the square of the body height in meters. Waist circumference was measured with the participant in a standing position at the level of the umbilicus at the end of exhalation. HGS was measured twice using a Smedley analog hand dynamometer (No. 04125; MIS, Tokyo, Japan) using both hands in a standing position. We used the average HGS in kilograms in our final analyses. Blood pressure was measured with the participant in a seated position using an automatic sphygmomanometer (HBP-9020; Omron Co., Ltd, Tokyo, Japan).
To collect participants’ baseline characteristics, trained technicians at the Clinical Research Center of the National Center for Global Health and Medicine at Kohnodai Hospital asked participants at the outpatient clinic about their physical activity levels, smoking and drinking habits, sleep duration, and medication use. The Brinkman index (number of cigarettes per day multiplied by the number of years) was calculated to quantify patients’ smoking habits (25). Using patients’ regular exercise habits, we calculated the exercise time per day based on exercise sessions per day × exercise duration per session.
Blood samples were taken from the antecubital vein at the enrolment when HGS was measured. We measured plasma hemoglobin A1c (HbA1c) by high-performance liquid chromatography (HA-8180; Arkray, Tokyo, Japan). We calculated estimated glomerular filtration rate (eGFR) using the revised equation adjusted for the Japanese population (26).
Sample size calculation was performed using G*Power (https://www.psychologie.hhu.de/arbeitsgruppen/allgemeine-psychologie-und-arbeitspsychologie/gpower). Our sample size had sufficient power to detect statistical significance (Supplementary File).
Continuous variables were expressed as the mean ± standard deviation (SD). Categorical variables were expressed as numbers and patient groups compared using χ2 test. Student’s t test or the Mann–Whitney test, depending on whether the variables followed normal or nonnormal distribution, was performed to detect significant differences between patients treated with PD and those treated without PD, as appropriate. Additionally, the relationship between the number of hospitalizations and HGS (in kilograms) was assessed using Spearman’s rank correlation coefficient. We also compared patient outcomes i.e., hospitalizations, CV events, and deaths for patients treated with and without PD before and after propensity score matching using χ2 tests.
A propensity score-matched analysis was performed to balance the characteristics of subjects at baseline between groups. Covariates were age, gender, BMI, alcohol consumption, exercise time, sleep duration, systolic blood pressure, and HbA1c levels. Based on the propensity score, PD users were matched to non-PD users by the nearest neighbor matching that is based on the greedy matching algorithm at a 1:1 ratio to create a propensity score-matched cohort (27). Subjects were matched based on the logit of the propensity score using a caliper width of 0.2 of SD. Standardized differences in each variable were calculated to confirm the balance between groups. In addition, the c-statistic for evaluating the goodness of fit was calculated. Subjects were then compared based on number of hospitalizations, CV events, and deaths.
Subsequently, Cox proportional hazard regression analysis was performed to assess the independent associations of mortality, CV events, and hospitalization with the use of PD in propensity score matched cohorts. Furthermore, multiple regression analysis was performed to assess relationship between hospitalization and HGS and use of PD.
P values of <0.05 determined by performing a two-sided test were considered statistically significance. Statistical analyses were performed using SPSS version 25 (IBM Co., Ltd., Chicago, IL).
This study enrolled 1,282 patients (709 men and 573 women) with type 2 diabetes. Of these, 379 (29.6%) patients were treated with PD, whereas 903 (70.4%) patients did not receive PD. A total of 314 patients (24.5%) received benzodiazepines, antidepressants, and/or antipsychotics; 175 (13.7%) received benzodiazepines; 93 (7.3%) received antidepressants; and 168 received antipsychotics (13.1%). The mean age and BMI of patients treated with PD were 60.1 ± 14.4) years and 26.9 ± 6 kg/m2, respectively. Patients with PD consume more alcohol (14.5 ± 26.7 g/day), engage in less exercise (9.5 ± 33.1 min/day), and sleep longer (7.8 ± 2.1 hours) compared to patients without PD. Additionally, patients with PD had lower systolic blood pressure (129.2 ± 18.5 mmHg), HbA1c levels (7.1 ± 1.4%), and HGS (22.3 ± 9.7 kg) compared to those without PD. Patients’ characteristics are listed in Supplementary Table 1.
Age, duration since diagnosis of diabetes, alcohol consumption, exercise time, systolic blood pressure, HbA1c, and HGS were lower in patients treated with PD than in those treated without PD. In contrast, BMI, sleep duration, and eGFR were higher in patients treated with PD. Patient groups did not differ by smoking status and diastolic blood pressure.
After a propensity score-matched analysis, two groups of 254 well-matched patients were generated. The c-statistic was 0.717 (95% confidence interval [CI], 0.683–0.750), suggesting that the performance of the propensity score-matched model was acceptable. Of these, 76 patients (29.9%) received benzodiazepines only; 27 patients (10.6%) received antidepressants only; and 44 patients received antipsychotics (17.3%) only. Patient’s characteristics at baseline were balanced (Table 1); however, HGS was still lower in patients treated with PD that in those treated without PD.
During a mean follow-up of 861 ± 265 days, 9 patients (1.8%) died, 4 (0.8%) experienced CV events, and 336 (66.1%) were admitted to our hospital in the matched cohort. All deceased patients had one or more hospitalizations during the study period. Among the PD group, 6 patients died, one experienced CV events and 119 were admitted. The total number of hospitalizations was 482. Of these, 185 (38.4%) were in the Diabetes and Endocrinology ward, 81 (16.8%) were in the Surgery ward, 55 (11.4%) were in the Internal Medicine ward, 34 (7.1%) were in the Hepatology ward, 27 (5.6%) were in the Gastroenterology ward, 24 (5.0%) were in the Ophthalmology ward, and 40 (8.3%) were in the Psychiatry ward. No significant difference in hospitalization was observed between groups; however, the number of patients treated with PD who were admitted to our hospital more than once was significantly higher than those treated without PD (Table 2).
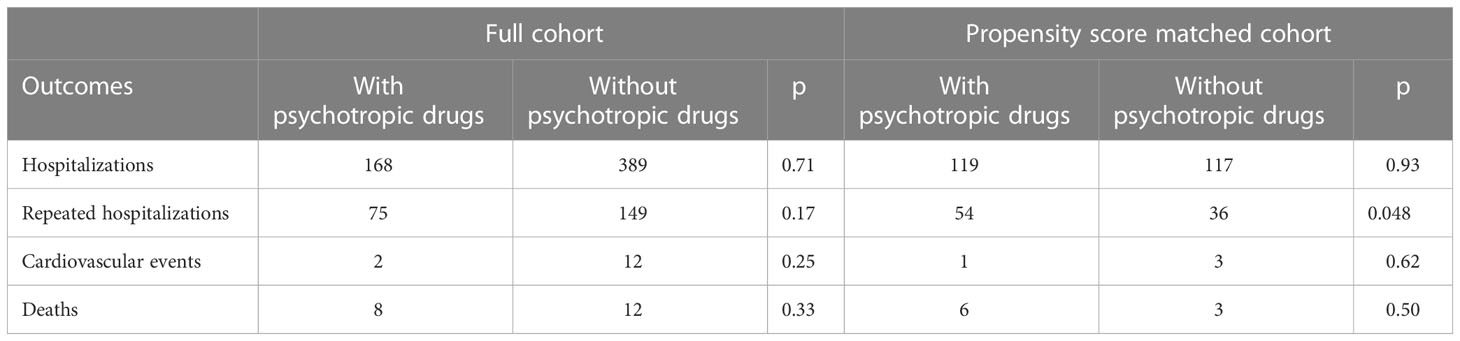
Table 2 Comparison of health outcomes between patients with or without psychotropic drugs in the full and matched cohorts.
Moreover, Cox proportional hazard regression analysis confirmed the association of repeated hospitalizations (three or more times) with the use of PD (hazard ratio [HR] = 2.138; 95% CI, 1.144–3.995, p = 0.017), while there are no significant associations between the use of PD and all-cause mortality and CV events (Table 3).
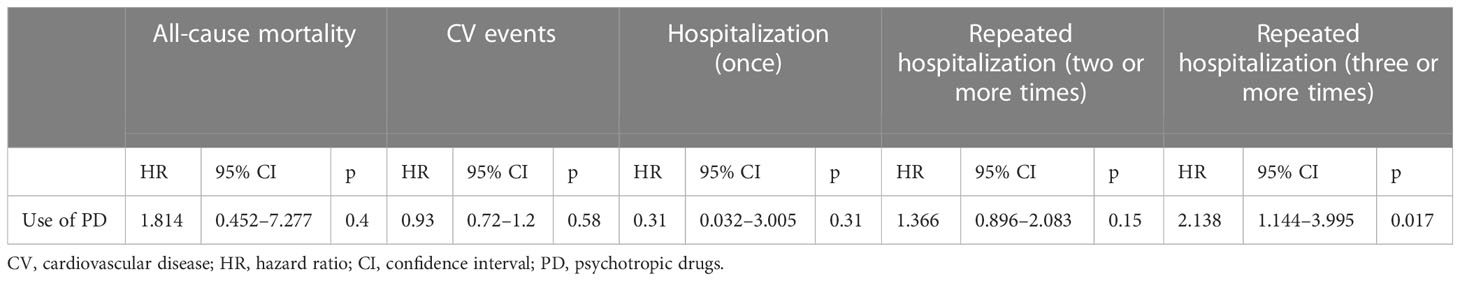
Table 3 Cox proportional hazard regression analysis for evaluating the associations of the use of psychotropic drugs with all-cause mortality, cardiovascular events, hospitalization, and repeated hospitalizations in patients with type 2 diabetes.
There is a negative correlation between HGS and the number of hospitalizations (r = −0.143, p = 0.013) (Figure 2). Furthermore, multiple regression analysis identified a positive association between repeated hospitalizations (three or more times) and the use of PD (β = 0.119, p = 0.007) (Table 4).
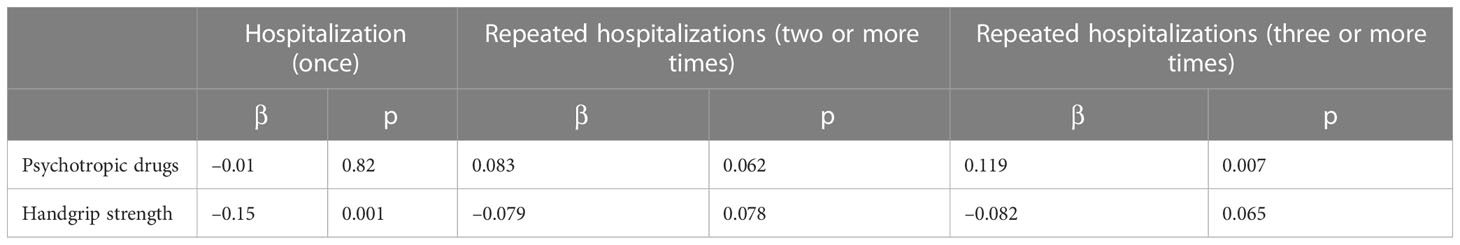
Table 4 Multiple regression analysis for evaluating the associations of the use of psychotropic drugs with hospitalization and repeated hospitalizations in patients with type 2 diabetes.
The main aim of this study was to examine the association of PD use with HGS and hospitalization in patients with type 2 diabetes. We demonstrated that PD users had lower HGS and more hospitalizations in a type 2 diabetes population than non-PD users. To the best of our knowledge, this study is the first to demonstrate that patients with type 2 diabetes receiving PD have decreased HGS, and such medication is associated with repeated hospitalizations in patients with type 2 diabetes.
No significant association between the use of PD and all-cause mortality was observed in this study; however, hospitalization and death share a commonality in indicating a decline in physical function. The average number of hospitalizations was higher in deceased patients than in surviving patients (3.44 ± 4.1 times vs. 0.88 ± 1.78 times, p <0.001 by Mann-Whitney U test). The number of repeated hospitalizations was also higher in deceased patients than in surviving patients (two or more times; p = 0.001; three or more times; p = 0.006 by χ2 test). In this study, six out of nine deceased patients were treated with PD, suggesting that the inappropriate use of PD could cause serious harm to physical health.
Cross-sectional studies have shown that female patients with depressive or anxiety disorders had lower HGS (23) and patients with schizophrenia have more impairments in muscular strength, endurance, and flexibility compared with healthy controls (28). In addition, higher antipsychotic dosages were associated with poor physical function (28), and benzodiazepines were found to increase the risk of fall by muscle relaxant effect in older adults (29). The findings of previous studies suggest that psychotropic medication decreases muscle strength and physical fitness.
Although the causal relationship between physical function and psychotropic medication is unknown, benzodiazepines, antidepressants, and antipsychotics may induce muscle weakness and increase the risk of hospitalization. Recently, Sandvik et al. (30) reported that the use of PD was significantly associated with reduced handgrip strength in older hospitalized patients.
We cannot reveal the mechanism underlying the unfavorable impact of PD on muscle strength and physical function based on the findings of this study alone; however, PDs have the possibility of damaging skeletal muscle. PDs have a variety of adverse health effects, such as dizziness, drowsiness, unconsciousness, fatigue, and sleep disturbances. In addition, benzodiazepines are well known as having skeletal muscle relaxant effects (31). Although the underlying mechanism is unclear, use of antipsychotics is associated with the elevation of creatinine kinase and rhabdomyolysis (32). Furthermore, a benzodiazepine, namely, diazepam that enhances the activity of the GABAA receptors increases muscle sympathetic nerve activity and blood pressure during handgrip exercise in humans (33). Sympathetic nerve and arterial blood pressure responses to exercise is exaggerated in type 2 diabetes (34); thus, patients with type 2 diabetes might be prone to adverse effects of benzodiazepines. Such sympathetic nervous dysfunction decreases skeletal muscle blood flow, which might cause muscle weakness (35).
To emphasize, few studies have examined the association between PD and hospitalization in patients with diabetes. An observational cohort study in a nursing facility reported that psychotropic and psychoactive drugs were associated with an increase in the rate of hospitalization; however, the reasons for hospitalization were various, and how such drugs affect the risk of hospitalization was not clarified (36). However, hypoglycemia may also be associated with reduced physical fitness, which results in the increased risk of hospitalization in patients with PD. Indeed, the use of antipsychotics is significantly associated with an increased risk of hypoglycemia in older adults (37). Severe hypoglycemia is strongly associated with all-cause mortality (HR = 2.69; 95% CI, 1.97 to 3.67), cardiovascular mortality (HR = 2.68; 95% CI, 1.72 to 4.19), and other health outcomes, including cancer and respiratory and digestive diseases (38). Ogama et al. (39) reported that glucose fluctuations were independently and significantly associated with low HGS and muscle mass after adjusting for HbA1c levels. Ørngreen et al. (40) showed that decreased muscle mass could increase the risk of hypoglycemia in patients with neuromuscular disease. Skeletal muscle is an important source of gluconeogenesis in the fasting state and plays a crucial role in the regulation of glucose homeostasis (41). In this study, patients with PD might have experienced hypoglycemia due to decreased muscle fitness, resulting in some impairment of physical function and hospitalizations. However, we did not investigate whether study participants experienced hypoglycemia during the study period. Therefore, further investigations are warranted.
Some limitations need to be addressed in the present study. First, we enrolled subjects who regularly received psychotropic medications; however, we did not investigate whether they were diagnosed with mental disorders, such as schizophrenia, depression, bipolar, and anxiety disorder. Thus, our findings cannot refer to the association of mental disorders with hospitalization in patients with type 2 diabetes. However, the number of patients admitted to the psychiatric ward was small (n = 16) during the 3-year follow-up, suggesting that the number of patients with severe mental disorders was relatively few in this study cohort. However, this issue is the most critical when assessing the effect of PD other than the mental illness itself. Further studies which examine the relationship of health outcomes with the use of PD and the existence of mental disorders separately are required. Second, the matched cohort is limited by small sample size and relatively short follow-up period to identify the significant difference in CV events and deaths between groups and limited generalizability. Considering that psychiatric disorders are common in patients with diabetes (42), multi-institutional or population-based studies may be required to obtain a larger cohort. Third, we did not investigate detailed causes of hospitalizations (e.g., name of disease, severity of disease); therefore, how the use of PD was associated with hospitalization is unknown. Finally, we grouped benzodiazepines, antidepressants, and antipsychotics together as PD in this study; however, each drug class should be investigated separately in future studies. Each drug has different physical and mental effects depending on the type of drug. Not all PDs, but second-generation antipsychotics, cause weight gain and reduce insulin sensitivity and glucose tolerance, which may lead to the development of type 2 diabetes (43). Antidepressants may exert a cardioprotective effect and reduce the risk of CV events (18). In this study, 27 patients took only antidepressants, which could have possibly affected the study results. Despite these limitations, our findings suggest that the use of PD leads to unfavorable health outcomes in patients with type 2 diabetes.
Randomized controlled trials (RCTs) are considered the gold standard in evidence-based medicine research. However, there are situations where conducting such trials may not be feasible or ethical, necessitating the reliance on observational studies. It is unethical to investigate the effects of PD on hard endpoints, such as death or CV events, in patients who require medication through RCTs. In this context, propensity score matching, as used in this study, is a practical method for estimating causal effects in observational studies (44). Nevertheless, there may still be unknown and unmeasured confounding factors, despite the use of appropriate methods. Therefore, future studies should incorporate additional information about study participants, including the presence or absence of mental disorders, detailed disease conditions, educational level, socioeconomic status, and genetic information. For instance, pharmacogenetic variants have a significant impact on the metabolism of PD, and genetic testing is considered crucial in determining whether the use of PD is toxic or therapeutic for patients (45). Furthermore, the interactions between PDs should also be taken into account, as a high number of PD interactions can result in severe health issues in clinical practice (46). Ideally, researchers should examine both the individual and interaction effects of PD on health outcomes. Well-designed future studies of this nature are warranted.
In conclusion, the use of PD could increase the risk of repeated hospitalizations in patients with type 2 diabetes. An increase skeletal muscle strength may reduce the risk of hospitalization in patients treated with PD. Our findings suggest that clinicians should judiciously prescribe PD to patients with type 2 diabetes.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by The Medical Ethics Committee of the National Center for Global Health and Medicine. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
HH conducted the study, performed data analyses, drafted and revised the manuscript. HY critically reviewed the manuscript and the scientific interpretations of study results. All authors read and approved the final manuscript.
The authors appreciate the support of Tomoko Kaga and Izumi Omigawa who helped collect the data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcdhc.2023.1181998/full#supplementary-material
1. de Groot M, Golden SH, Wagner J. Psychological conditions in adults with diabetes. Am. Psychol. (2016) 71:552–62. doi: 10.1037/a0040408
2. Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep duration is associated with type 2 diabetes: a population-based study. Diabetes Care (2009) 32:1980–5. doi: 10.2337/dc09-0284
3. Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care (2008) 31:2383–90. doi: 10.2337/dc08-0985
4. Stubbs B, Vancampfort D, De Hert M, Mitchell AJ. The prevalence and predictors of type two diabetes mellitus in people with schizophrenia: a systematic review and comparative meta-analysis. Acta Psychiatr. Scand. (2015) 132:144–57. doi: 10.1111/acps.12439
5. Holt RI, Peveler RC. Association between antipsychotic drugs and diabetes. Diabetes Obes. Metab. (2006) 8(2):125–35. doi: 10.1111/j.1463-1326.2005.00495.x
6. Holt RIG. Association between antipsychotic medication use and diabetes. Curr. Diabetes Rep. (2019) 19(10):96. doi: 10.1007/s11892-019-1220-8
7. Holt RI, Peveler RC. Obesity, serious mental illness and antipsychotic drugs. Diabetes Obes. Metab. (2009) 11(7):665–79. doi: 10.1111/j.1463-1326.2009.01038.x
8. Hardy TA, Henry RR, Forrester TD, Kryzhanovskaya LA, Campbell GM, Marks DM, et al. Impact of olanzapine or risperidone treatment on insulin sensitivity in schizophrenia or schizoaffective disorder. Diabetes Obes. Metab. (2011) 13(8):726–35. doi: 10.1111/j.1463-1326.2011.01398.x
9. Weintraub D, Chiang C, Kim HM, Wilkinson J, Marras C, Stanislawski B, et al. Association of antipsychotic use with mortality risk in patients with Parkinson disease. JAMA Neurol. (2016) 73:535–41. doi: 10.1001/jamaneurol.2016.0031
10. Nielsen RE, Lolk A, Valentin JB, Andersen K. Cumulative dosages of antipsychotic drugs are associated with increased mortality rate in patients with alzheimer’s dementia. Acta Psychiatr. Scand. (2016) 134:314–20. doi: 10.1111/acps.12614
11. Tiihonen J, Mittendorfer-Rutz E, Torniainen M, Alexanderson K, Tanskanen A. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow-up study. Am. J. Psychiatry (2016) 173:600–6. doi: 10.1176/appi.ajp.2015.15050618
12. Nørgaard A, Jensen-Dahm C, Gasse C, Wimberley T, Hansen ES, Waldemar G. Association of benzodiazepines and antidepressants with 180-day mortality among patients with dementia receiving antipsychotic pharmacotherapy: a nationwide registry-based study. J. Clin. Psychiatry (2020) 81:19m12828. doi: 10.4088/JCP.19m12828
13. Seppala LJ, Wermelink AMAT, de Vries M, Ploegmakers KJ, van de Glind EMM, Daams JG, et al. EUGMS task and finish group on fall-risk-increasing drugs. fall-risk-increasing drugs: a systematic review and meta-analysis: II. psychotropics. J. Am. Med. Dir Assoc. (2018) 19:371.e11–371.e17. doi: 10.1016/j.jamda.2017.12.098
14. Sun GQ, Zhang L, Zhang LN, Wu Z, Hu DF. Benzodiazepines or related drugs and risk of pneumonia: a systematic review and meta-analysis. Int. J. Geriatr. Psychiatry (2019) 34:513–21. doi: 10.1002/gps.5048
15. Kim HB, Myung SK, Park YC, Park B. Use of benzodiazepine and risk of cancer: a meta-analysis of observational studies. Int. J. Cancer (2017) 140:513–25. doi: 10.1002/ijc.30443
16. Pottegård A, Lash TL, Cronin-Fenton D, Ahern TP, Damkier P. Use of antipsychotics and risk of breast cancer: a Danish nationwide case-control study. Br. J. Clin. Pharmacol. (2018) 84:2152–61. doi: 10.1111/bcp.13661
17. Taipale H, Solmi M, Lähteenvuo M, Tanskanen A, Correll CU, Tiihonen J. Antipsychotic use and risk of breast cancer in women with schizophrenia: a nationwide nested case-control study in Finland. Lancet Psychiatry (2021) 8:883–91. doi: 10.1016/S2215-0366(21)00241-8
18. Lavoie KL, Paine NJ, Pelletier R, Arsenault A, Diodati JG, Campbell TS, et al. Relationship between antidepressant therapy and risk for cardiovascular events in patients with and without cardiovascular disease. Health Psychol. (2018) 37:989–99. doi: 10.1037/hea0000602
19. Fagour C, Gonzalez C, Pezzino S, Florenty S, Rosette-Narece M, Gin H, et al. Low physical activity in patients with type 2 diabetes: the role of obesity. Diabetes Metab. (2013) 39:85–7. doi: 10.1016/j.diabet.2012.09.003
20. Sayer AA, Dennison EM, Syddall HE, Gilbody HJ, Phillips DI, Cooper C. Type 2 diabetes, muscle strength, and impaired physical function: the tip of the iceberg? Diabetes Care (2005) 28:2541–2. doi: 10.2337/diacare.28.10.2541
21. Cetinus E, Buyukbese MA, Uzel M, Ekerbicer H, Karaoguz A. Hand grip strength in patients with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. (2005) 70:278–86. doi: 10.1016/j.diabres.2005.03.028
22. Hamasaki H, Kawashima Y, Katsuyama H, Sako A, Goto A, Yanai H. Association of handgrip strength with hospitalization, cardiovascular events, and mortality in Japanese patients with type 2 diabetes. Sci. Rep. (2017) 7:7041. doi: 10.1038/s41598-017-07438-8
23. van Milligen BA, Lamers F, de Hoop GT, Smit JH, Penninx BW. Objective physical functioning in patients with depressive and/or anxiety disorders. J. Affect. Disord. (2011) 131:193–9. doi: 10.1016/j.jad.2010.12.005
24. Vancampfort D, Probst M, Daenen A, Damme TV, De Hert M, Rosenbaum S, et al. Impact of antipsychotic medication on physical activity and physical fitness in adolescents: an exploratory study. Psychiatry Res. (2016) 242:192–7. doi: 10.1016/j.psychres.2016.05.042
25. Brinkman GL, Coates EO Jr. The effect of bronchitis, smoking, and occupation on ventilation. Am. Rev. Respir. Dis. (1963) 87:684–93. doi: 10.1164/arrd.1963.87.5.684
26. Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. (2009) 53:982–92. doi: 10.1053/j.ajkd.2008.12.034
27. Thoemmes F. Propensity score matching in SPSS. arXiv Preprint arXiv (2012) 1201:6385. doi: 10.48550/arXiv.1201.6385
28. Vancampfort D, Probst M, Scheewe T, De Herdt A, Sweers K, Knapen J, et al. Relationships between physical fitness, physical activity, smoking and metabolic and mental health parameters in people with schizophrenia. Psychiatry Res. (2013) 207:25–32. doi: 10.1016/j.psychres.2012.09.026
29. Airagnes G, Pelissolo A, Lavallée M, Flament M, Limosin F. Benzodiazepine misuse in the elderly: risk factors, consequences, and management. Curr. Psychiatry Rep. (2016) 18:89. doi: 10.1007/s11920-016-0727-9
30. Sandvik MK, Watne LO, Brugård A, Wang-Hansen MS, Kersten H. Association between psychotropic drug use and handgrip strength in older hospitalized patients. Eur. Geriatr. Med. (2021) 12:1213–20. doi: 10.1007/s41999-021-00511-6
31. Edinoff AN, Nix CA, Hollier J, Sagrera CE, Delacroix BM, Abubakar T, et al. Benzodiazepines: uses, dangers, and clinical considerations. Neurol. Int. (2021) 13:594–607. doi: 10.3390/neurolint13040059
32. Laoutidis ZG, Kioulos KT. Antipsychotic-induced elevation of creatine kinase: a systematic review of the literature and recommendations for the clinical practice. Psychopharmacol. (Berl) (2014) 231:4255–70. doi: 10.1007/s00213-014-3764-2
33. Teixeira AL, Fernandes IA, Vianna LC. GABAA receptors modulate sympathetic vasomotor outflow and the pressor response to skeletal muscle metaboreflex activation in humans. J. Physiol. (2019) 597:4139–50. doi: 10.1113/JP277929
34. Holwerda SW, Restaino RM, Manrique C, Lastra G, Fisher JP, Fadel PJ. Augmented pressor and sympathetic responses to skeletal muscle metaboreflex activation in type 2 diabetes patients. Am. J. Physiol. Heart Circ. Physiol. (2016) 310:H300–9. doi: 10.1152/ajpheart.00636.2015
35. DeLorey DS. Sympathetic vasoconstriction in skeletal muscle: modulatory effects of aging, exercise training, and sex. Appl. Physiol. Nutr. Metab. (2021) 46:1437–47. doi: 10.1139/apnm-2021-0399
36. Cooper JW, Freeman MH, Cook CL, Burfield AH. Psychotropic and psychoactive drugs and hospitalization rates in nursing facility residents. Pharm. Pract. (Granada) (2007) 5:140–4. doi: 10.4321/s1886-36552007000300008
37. van Keulen K, van der Linden PD, Souverein PC, Heerdink ER, Egberts AC, Knol W. Risk of hospitalization for hypoglycemia in older patients with diabetes using antipsychotic drugs. Am. J. Geriatr. Psychiatry (2015) 23:1144–53. doi: 10.1016/j.jagp.2015.04.006
38. Zoungas S, Patel A, Chalmers J, de Galan BE, Li Q, Billot L, et al. Severe hypoglycemia and risks of vascular events and death. N Engl. J. Med. (2010) 363:1410–8. doi: 10.1056/NEJMoa1003795
39. Ogama N, Sakurai T, Kawashima S, Tanikawa T, Tokuda H, Satake S, et al. Association of glucose fluctuations with sarcopenia in older adults with type 2 diabetes mellitus. J. Clin. Med. (2019) 8:319. doi: 10.3390/jcm8030319
40. Ørngreen MC, Zacho M, Hebert A, Laub M, Vissing J. Patients with severe muscle wasting are prone to develop hypoglycemia during fasting. Neurology (2003) 61:997–1000. doi: 10.1212/01.wnl.0000086813.59722.72
41. Sartori R, Romanello V, Sandri M. Mechanisms of muscle atrophy and hypertrophy: implications in health and disease. Nat. Commun. (2021) 12:330. doi: 10.1038/s41467-020-20123-1
42. Boden MT. Prevalence of mental disorders and related functioning and treatment engagement among people with diabetes. J. Psychosom Res. (2018) 106:62–9. doi: 10.1016/j.jpsychores.2018.01.001
43. Cernea S, Dima L, Correll CU, Manu P. Pharmacological management of glucose dysregulation in patients treated with second-generation antipsychotics. Drugs (2020) 80:1763–81. doi: 10.1007/s40265-020-01393-x
44. Ali MS, Prieto-Alhambra D, Lopes LC, Ramos D, Bispo N, Ichihara MY, et al. Propensity score methods in health technology assessment: principles, extended applications, and recent advances. Front. Pharmacol. (2019) 10:973. doi: 10.3389/fphar.2019.00973
45. Stingl JC, Brockmöller J, Viviani R. Genetic variability of drug-metabolizing enzymes: the dual impact on psychiatric therapy and regulation of brain function. Mol. Psychiatry (2013) 18:273–87. doi: 10.1038/mp.2012.42
Keywords: psychotropic drugs, type 2 diabetes, hospitalization, handgrip strength, skeletal muscle, psychiatric comorbidity
Citation: Hamasaki H and Yanai H (2023) Association of the use of psychotropic drugs with hospitalization, cardiovascular events, and mortality in patients with type 2 diabetes: a propensity score-matched cohort study. Front. Clin. Diabetes Healthc. 4:1181998. doi: 10.3389/fcdhc.2023.1181998
Received: 08 March 2023; Accepted: 16 June 2023;
Published: 05 July 2023.
Edited by:
José Pablo Miramontes González, Hospital Universitario Río Hortega, SpainReviewed by:
Neftali Eduardo Antonio-Villa, National Institute of Cardiology Ignacio Chavez, MexicoCopyright © 2023 Hamasaki and Yanai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hidetaka Hamasaki, aC1oYW1hc2FraUB1bWluLmFjLmpw; Hidekatsu Yanai, ZHlhbmFpQGhvc3BrLm5jZ20uZ28uanA=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.