- 1Department of Diabetes, Nutrition and Metabolic Diseases, University of Medicine and Pharmacy “Carol Davila”, Bucharest, Romania
- 2Department of Internal Medicine, University of Medicine and Pharmacy “Carol Davila”, Bucharest, Romania
- 3Department of Gastroenterology, Fundeni Clinical Institute, Bucharest, Romania
- 4Department of Gastroenterology, University of Medicine and Pharmacy “Carol Davila”, Bucharest, Romania
- 5National Institute of Diabetes, Nutrition and Metabolic Diseases, Bucharest, Romania
Non-alcoholic fatty liver disease (NAFLD) has an important role in the pathogenesis of cardiovascular diseases in the population with diabetes and it is highly prevalent in end-stage renal disease (ESRD) patients. This case series describes NAFLD associated factors and survival in type 2 diabetes patients (T2DM) who have ESRD treated with hemodialysis. NAFLD prevalence in patients with T2DM and ESRD is 69.2%. A high number of patients (15 out of 18) have obesity evaluated by calculating body mass index (BMI) and bioimpedance measurements. Patients with NAFLD have higher cardiovascular mortality risk, 13 of 18 patients were already diagnosed with coronary heart disease, 6 of 18 had cerebrovascular disease, and 6 of 18 had peripheral artery disease. Fourteen patients were treated with insulin, two patients with sitagliptin (renal adjusted dose of 25mg/day) and two patients with medical nutrition therapy, with an HbA1c ranging from 4.4 to 9.0%. After one-year follow-up 7 of 18 patients died, the causes having roughly equal proportions: myocardial infarction, SARS-CoV2 infection, and pulmonary edema. In conclusion, our population of type 2 diabetic patients with ESRD in hemodialysis had a prevalence of ultrasound-diagnosed NAFLD of 69.2%. Also, this population had a high death rate at one-year follow-up, cardiovascular causes being among the most common.
Introduction
In the last two decades, the incidence of NAFLD increased significantly, and is a common liver disease spread worldwide (1, 2). It can vary from steatosis to non-alcoholic steatohepatitis and can lead to cirrhosis and finally to hepatocellular carcinoma (2, 3). NAFLD is frequently associated with dyslipidemia, type 2 diabetes, and obesity, and is considered to be a manifestation of metabolic syndrome (4). Primary care systems faced the increase in the burden of the previously mentioned diseases and are responsible for managing cases with low risk of progression of NAFLD, but patients at high risk are referred to gastroenterology (5). When the medical systems are dealing with complex cases, such as the situation of advanced chronic kidney disease (CKD) or dialysis patients, the management of NAFLD includes the nephrologists. The latter consult these categories of patients more often, and recognizing the impact of NAFLD by them is essential for decreasing mortality.
Oxidative stress (6), lipotoxicity, inflammation, and insulin resistance are common pathogenic mechanisms for NAFLD and chronic kidney disease and together are linked to a higher risk of cardiovascular disease (CVD) (2, 7). Also, an alteration in hepatic lipoprotein metabolism occurs, thus accelerating the atherogenic process in patients with a combination of these two pathologies (8).
The decline in glomerular filtration rate (eGFR) evaluated by the annual percent change was higher in patients with NAFLD (−0.79% per year, 95% confidence interval [CI], [−1.31%, −0.27%] versus controls (0.30%, 95% CI [−0.14%, 0.76%]; p = 0.002). A higher fibrosis score, proteinuria, hypertension, smoking status and eGFR below 45 ml/min/1.73 m2 are associated with CKD progression in NAFLD (9). A previous systematic review (10) concluded that the influence of NAFLD within the CKD population regarding the major adverse clinical outcomes needs further research. The results of the observational studies included were conflicting, and the effect of NAFLD is difficult to individualize from other metabolic factors (10).
In view of the even smaller number of studies including subjects with end-stage renal disease (ESRD) and NAFLD, more research needs to address this topic. Firstly, NAFLD in patients undergoing hemodialysis (HD) has a high prevalence ranging from 50.5% to 86% depending on the method used for diagnosis (11–14). Secondly, NAFLD increased the chance of CVD by three times in previous studies that included elderly hemodialysis patients (11). Also, the carotid intima-media thickness is a marker of CVD that was significantly higher in subjects with NAFLD and HD (1.430 ± 0.3) compared with CKD alone (1.310 ± 0.2), and normal controls (0.864 ± 0.1) (14). Therefore, this case series aimed to describe the associated NAFLD factors and survival in type 2 diabetes patients (T2DM) who have ESRD.
Materials and methods
Design
This is a case series that initially included 26 patients, 11 females (42.3%) and 15 men (57.7%) that have type 2 diabetes mellitus and chronic end-stage renal disease. Patients were evaluated at the National Institute of Diabetes, Nutrition and Metabolic Diseases (INDNBM) N.C Paulescu and the followed-up for12 months (June 2020 - May 2021). This dialysis center is dedicated to patients with diabetes mellitus. The case series was approved by the Ethical Committee of NIDNMD and follows the recommendations of the Helsinki Declaration. All the patients agree to participate by signing the informed consent.
Clinical and anthropometric parameters
At enrollment, all the patients were evaluated by measuring height, weight, and clinical examination. The demographic and associated disease data were collected from dialysis protocols. Body composition was non-invasively analyzed using the Body Composition Monitor (BCM®) device from Fresenius Medical Care. The procedure was performed by applying skin electrodes at the level of the metacarpophalangeal joint, respectively at the level of the metatarsophalangeal joint. Through this procedure parameters such as lean tissue mass-LTM, fat mass-FM, and hydration status (total body water-TBW, overhydration-OH) are determined.
Biochemical tests
Blood samples were obtained before the HD session: serum creatinine, blood glucose, entry blood urea nitrogen, transaminases (AST- aspartate aminotransferase, ALT- alanine aminotransferase), blood count, lipid profile (total cholesterol/TC, HDL cholesterol/HDLc, LDL cholesterol/LDLc, triglycerides), blood gases.
Using these parameters, we calculated the Hepatic steatosis index (HSI): HSI = 8 × (ALT/AST ratio) + BMI (+2, if female; +2, if DM). This is a tool previously used for screening NAFLD in the general population (cut-off > 36) (15).
Ultrasound examination of the liver
An ultrasound was performed by the same examiner when presenting for an HD session or if the subject was hospitalized for other reasons (ultrasound equipment Aloka SSD-3500, Aloka Co. LTD). For the diagnosis we used the following ultrasound features: increased hepatorenal echogenicity, vascular blurring of the hepatic or portal vein, and bright hepatic echoes (16).
The inclusion criteria were: diabetes mellitus diagnosis based on the patient’s history and American Diabetes Association guidelines (17); End Stage Renal Disease diagnosis (18); an existing abdominal ultrasound within 6 months with criteria for NAFLD (16).
We excluded subjects with severe cognitive dysfunction, acute and chronic liver pathology (HBs antigen for hepatitis B and VHC antibodies for hepatitis C were tested 3-6 months before).
Statistics
The patients characteristics were synthesized in a database using SPSS® version 20 (IBM®). We considered that a descriptive statistic of the clinical and biological data will be relevant, and given the small number of subjects we expressed the results as a median and interquartile range (IQR). The correlation analysis was performed using the Spearman coefficient. The level of statistical significance was set at p <0.05.
Results
Of the 26 patients that agree to participate and had complete data, 69.2% had steatosis on US examination. Eighteen subjects with NAFLD, T2DM and ESRD were followed for 12 months in this case series. The female to male ratio was 1:1. The general characteristics of the population are presented in Table 1.
Regarding the complications of diabetes, 14 of 18 patients were diagnosed with retinopathy and 12 of them also had neuropathy. The majority had cardiovascular disease (CVD): coronary heart disease (13 of 18 patients), cerebrovascular disease (6 of 18 patients), and peripheral artery disease (6 of 18 patients).
All HSI values were above 36, with a median of 49.57 (11.74) at first visit, and 50.29 (11.76) at 12 months. There were no significant correlations between HSI and HbA1c, diabetes duration, or dialysis duration.
We observed that patients who receive insulin therapy (14 of 18) have a poorer control with a HbA1c 7.9 (1.8)%, than the others who were treated with oral antidiabetic drugs (sitagliptin 25mg/day) or a controlled diet - HbA1c 5.5 (0.5)%.
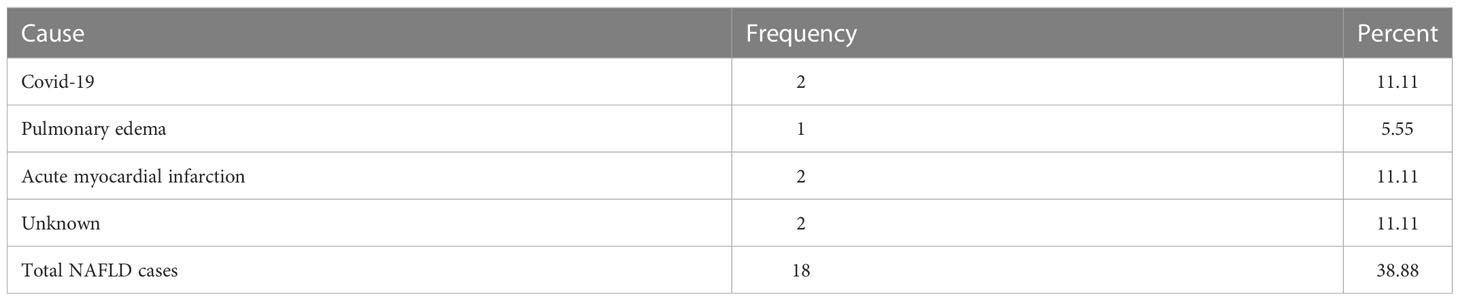
At one year follow-up, 7 patients died. The death causes are presented in Table 2. The study period overlaps with the COVID-19 pandemic, therefore 2 of 7 patients died after SARS-CoV2 infection.
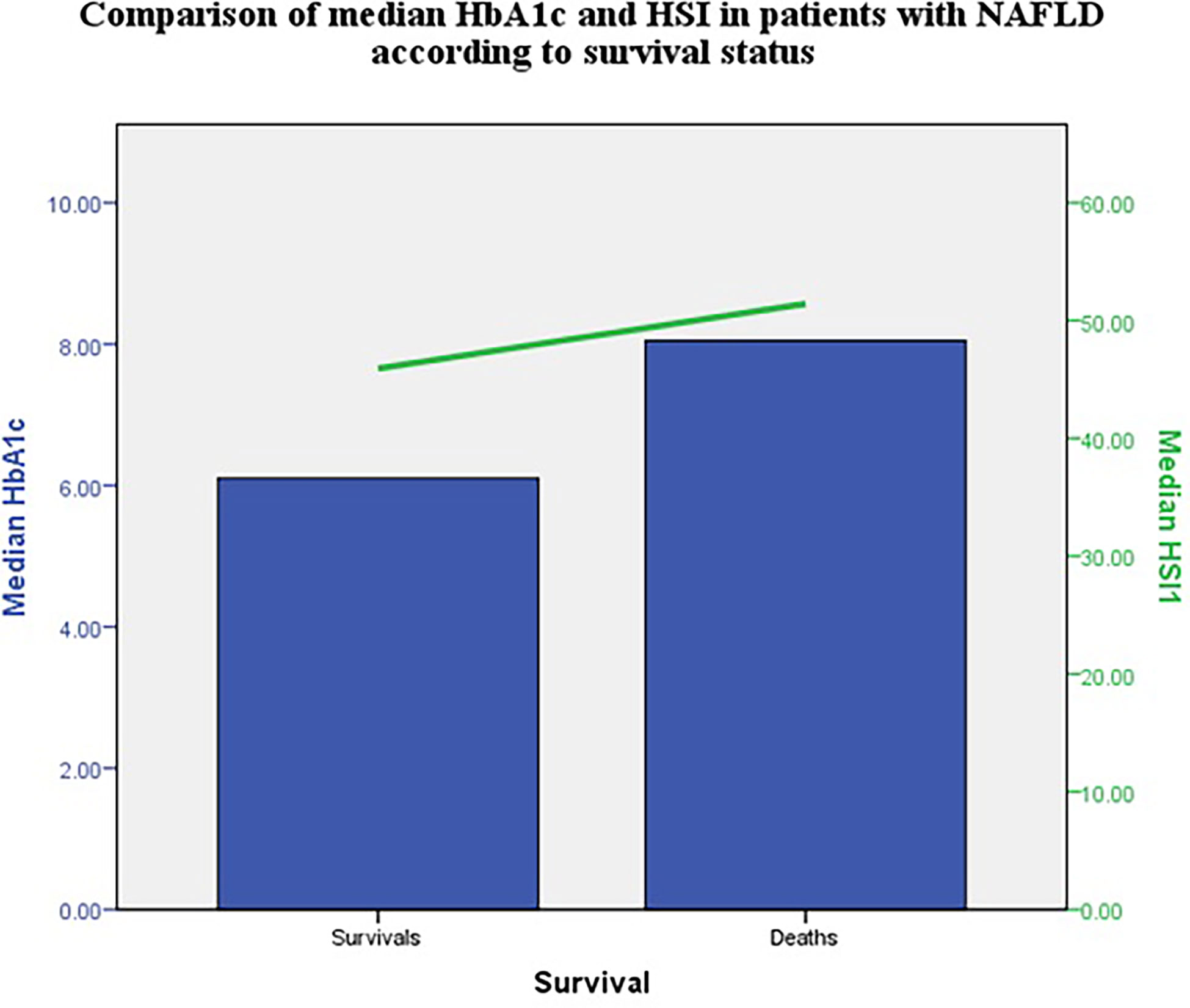
The median HbA1c and HSI were higher in patients with NAFLD that died compared with those that survived at 12 months (Figure 1).
Discussion
The association of NAFLD with diabetes and ESRD has been observed to increase the occurrence of CVD (6, 11). In our population, 69.2% of subjects were diagnosed with NAFLD and the majority had CVD as a consequence of long-standing diabetes. Although they have an extreme risk of mortality, the target guidelines for lipid profile (17) were not achieved with a median calculated LDLc of 87.65 (55.43) mg/dl, and triglycerides 163.00 (132.6) mg/dL. NAFLD could be the missing link between this atherogenic profile, inflammation, and increased mortality (19).
Median HSI was higher in those that did not survive. This non-invasive index has a good positive predictive value for NAFLD (20) and was associated with carotid atherosclerosis in diabetes (21). Thus, patients with higher values could have higher cardiovascular risk. HSI, together with other steatosis and fibrosis indexes should be evaluated in larger studies.
Inflammation evaluated by high sensitive C reactive protein (hs-CRP) was associated with a poor prognosis in HD patients (22). Also, Capone et al. observed a higher level of high sensitive cTnT in men that was moderately correlated with inflammation (23). Although hs-CRP was not evaluated in our case series, ferritin was available for most of the patients and had higher median levels. Malnutrition-inflammation syndrome could explain an elevated ferritin level above 200ng/ml (24). In previous studies in HD subjects, an U-shape association was observed between ferritin and all-cause mortality (25). Given the increased prevalence of microvascular and macrovascular complications of diabetes, patients have an extremely high mortality risk. Adding NAFLD might be the turning point that hastens the negative prognosis of these patients.
In the 12-month follow-up period 7 deaths were recorded. Regarding the causes of this outcome, SARS-CoV2 infection and myocardial infarction were reported at the same rate. In the HEMO study, cardiac deaths accounted for approximately 40% of mortality on a mean follow-up of 3 years. Patients with cardiac disease at baseline had a relative risk of 2.57 for death (26). In our cases, 13 of 18 had coronary heart disease.
We aimed to determine the prevalence and associated factors with NAFLD in type 2 diabetes patients with ESRD in Romania. The evaluation of body composition, HSI index, and HbA1c dynamics in patients with NAFLD and HD was not described before. Their influence on cardiovascular mortality should be further investigated. The limitations consist of the unavailability of hepatic biopsy, Magnetic Resonance Imaging, or transient elastography with controlled attenuation parameter (12) for confirming steatosis, and the absence of the markers of inflammation.
Conclusion
The prevalence of NAFLD in our population of type 2 diabetes and ESRD hemodialysis patients was 69.2%. Diabetic patients in hemodialysis with ultrasound-diagnosed hepatic steatosis had a high death rate at one-year follow-up, cardiovascular causes being among the most common. Also, we found that the frail condition of these patients can predispose them to an unfavorable prognosis in the case of SARS-CoV-2 viral outbreaks.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by no 1/19.01.2020 Ethics Committee National Institute of Diabetes Nutrition and Metabolic Diseases. The patients/participants provided their written informed consent to participate in this study.
Author contributions
All persons who meet authorship criteria are listed as authors, and all authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript. Furthermore, each author certifies that this material or similar material has not been and will not be submitted to or published in any other publication before its appearance in Frontiers in Clinical Diabetes and Healthcare.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Alexander M, Loomis AK, Fairburn-Beech J, van der Lei J M, Duarte-Salles T, Prieto-Alhambra D, et al. Real-world data reveal a diagnostic gap in non-alcoholic fatty liver disease. BMC Med. (2018) 16:130. doi: 10.1186/s12916-018-1103-x
2. Kanbay M, Bulbul MC, Copur S, Afsar B, Sag AA, Siriopol D, et al. Therapeutic implications of shared mechanisms in non-alcoholic fatty liver disease and chronic kidney disease. J. Nephrol. (2021) 34:649–59. doi: 10.1007/s40620-020-00751-y
3. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. (2018) 15(1):11–20. doi: 10.1038/nrgastro.2017.109
4. Kasper P, Martin A, Lang S, Kütting F, Goeser T, Demir M, et al. NAFLD and cardiovascular diseases: A clinical review. Clin. Res. Cardiol. (2021) 110(7):921–37. doi: 10.1007/s00392-020-01709-7
5. Dokmak A, Lizaola-Mayo B, Trivedi HD. The impact of nonalcoholic fatty liver disease in primary care: A population health perspective. Am. J. Med. (2021) 134(1):23–9. doi: 10.1016/j.amjmed.2020.08.010
6. Wu PJ, Chen JB, Lee WC, Ng HY, Lien SC, Tsai PY, et al. Oxidative stress and nonalcoholic fatty liver disease in hemodialysis patients. BioMed. Res. Int. (2018) 2018:3961748. doi: 10.1155/2018/3961748
7. Mikolasevic I, Racki S, Zaputovic L, Lukenda V, Milic S, Orlic L. Nonalcoholic fatty liver disease (NAFLD): A new risk factor for adverse cardiovascular events in dialysis patients. Med. Hypotheses (2014) 82(2):205–8. doi: 10.1016/j.mehy.2013.11.039
8. Deprince A, Haas JT, Staels B. Dysregulated lipid metabolism links NAFLD to cardiovascular disease. Mol. Metab. (2020) 42:101092. doi: 10.1016/j.molmet.2020.101092
9. Jang HR, Kang D, Sinn DH, Gu S, Cho SJ, Lee JE, et al. Nonalcoholic fatty liver disease accelerates kidney function decline in patients with chronic kidney disease: A cohort study. Sci. Rep. (2018) 8(1):4718. doi: 10.1038/s41598-018-23014-0
10. Hydes T, Buchanan R, Kennedy OJ, Fraser S, Parkes J, Roderick P. Systematic review of the impact of non-alcoholic fatty liver disease on mortality and adverse clinical outcomes for individuals with chronic kidney disease. BMJ Open (2020) 10(9):e040970. doi: 10.1136/bmjopen-2020-040970
11. Stolic RV, Trajkovic GZ, Kostic MM, Sovtic SR, Odalovic AM, Krdzic BD, et al. Correlation between nonalcoholic fatty liver and cardiovascular disease in elderly hemodialysis patients. Int. Urol. Nephrol. (2016) 48(6):883–9. doi: 10.1007/s11255-016-1237-8
12. Cheng BC, Yen YH, Chen JF, Wu CK, Chang KC, Tseng PL, et al. Transient elastography as a screening tool for liver fibrosis in a large hemodialysis population. Sci. Rep. (2017) 7:46458. doi: 10.1038/srep46458
13. Behairy MA, Sherief AF, Hussein HA. Prevalence of non-alcoholic fatty liver disease among patients with non-diabetic chronic kidney disease detected by transient elastography. Int. Urol. Nephrol. (2021) 53(12):2593–601. doi: 10.1007/s11255-021-02815-9
14. Neri S, Signorelli SS, Scuderi R, Bruno M, Bertino G, Clementi A, et al. Carotid intima-media thickness and liver histology in hemodialysis patients with nonalcoholic fatty liver disease. Int. J. angiology (2011) 20(3):149–56. doi: 10.1055/s-0031-1283218
15. Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. (2010) 42(7):503–8. doi: 10.1016/j.dld.2009.08.002
16. Khov N, Sharma A, Riley TR. Bedside ultrasound in the diagnosis of nonalcoholic fatty liver disease. World J. Gastroenterol. (2014) 20(22):6821–5. doi: 10.3748/wjg.v20.i22.6821
17. American Diabetes Association. Classification and diagnosis of diabetes: Standards of medical care in diabetes – 2021. Diabetes Care (2020) 44:S15–33. doi: 10.2337/dc21-S002
18. Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. (2020) 98(4S):S1–S115. doi: 10.1016/j.kint.2020.06.019
19. Mikolasevic I, Stimac D, Racki S, Zaputovic L, Devcic B, Jelic I, et al. Relationship between non-alcoholic fatty liver disease and MIA syndrome. Hemodialysis Int. Int. Symposium Home Hemodialysis (2015) 19(3):472–81. doi: 10.1111/hdi.12280
20. Fennoun H, Mansouri SE, Tahiri M, Haraj NE, Aziz SE, Hadad F, et al. Interest of hepatic steatosis index (HSI) in screening for metabolic steatopathy in patients with type 2 diabetes. Pan Afr. Med. J. (2020) 37:270. doi: 10.11604/pamj.2020.37.270.9087
21. Wang C, Cai Z, Deng X, Li H, Zhao Z, Guo C, et al. Association of hepatic steatosis index and fatty liver index with carotid atherosclerosis in type 2 diabetes. Int. J. Med. Sci. (2021) 18(14):3280–9. doi: 10.7150/ijms.62010
22. Mikolasevic I, Lukenda V, Racki S, Milic S, Sladoje-Martinovic B, Orlic L. Nonalcoholic fatty liver disease (NAFLD) - a new factor that interplays between inflammation, malnutrition, and atherosclerosis in elderly hemodialysis patients. Clin. Interventions Aging (2014) 9:1295–303. doi: 10.2147/CIA.S65382
23. Capone D, Vinciguerra M, Ragosta A, Citro V, Tarantino G. Troponin levels relate to CRP concentrations in patients with NAFLD on maintenance haemodialysis: A retrospective study. Adv. Ther. (2020) 37(7):3337–47. doi: 10.1007/s12325-020-01385-z
24. Kalantar-Zadeh K, Rodriguez RA, Humphreys MH. Association between serum ferritin and measures of inflammation, nutrition and iron in haemodialysis patients. Nephrology dialysis Transplant. (2004) 19(1):141–9. doi: 10.1093/ndt/gfg493
25. Shoji T, Niihata K, Fukuma S, Fukuhara S, Akizawa T, Inaba M. Both low and high serum ferritin levels predict mortality risk in hemodialysis patients without inflammation. Clin. Exp. Nephrol. (2017) 21(4):685–93. doi: 10.1007/s10157-016-1317-1
Keywords: NAFLD, diabetes mellitus, hemodialysis, cardiovascular risk, mortality, bioimpedance (BIA), hepatic steatosis index (HSI)
Citation: Stoica RA, Tribus LC, Marin RI, David T, Preda CM, Bica IC and Serafinceanu C (2023) Non-alcoholic fatty liver disease in diabetes mellitus patients on chronic hemodialysis – A case series addressing cardiovascular and mortality risks. Front. Clin. Diabetes Healthc. 4:1113666. doi: 10.3389/fcdhc.2023.1113666
Received: 01 December 2022; Accepted: 25 January 2023;
Published: 09 February 2023.
Edited by:
Alessando Mattina, IRRCS ISMETT/UPMC Italy, ItalyReviewed by:
Bogdan Mihai, Grigore T. Popa University of Medicine and Pharmacy, RomaniaBogdan Timar, Victor Babes University of Medicine and Pharmacy, Romania
Giuseppe Lorello, University Hospital of Policlinico G. Martino, Italy
Copyright © 2023 Stoica, Tribus, Marin, David, Preda, Bica and Serafinceanu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Carina Tribus, bHRyaWJ1c3JvQHlhaG9vLmNvbQ==
 Roxana Adriana Stoica
Roxana Adriana Stoica Laura Carina Tribus2*
Laura Carina Tribus2* Cristian Serafinceanu
Cristian Serafinceanu