- 1Population Health Laboratory (#PopHealthLab), University of Fribourg, Fribourg, Switzerland
- 2Department of Epidemiology and Health Services, Center for Primary Care and Public Health (UNISANTÉ), University of Lausanne, Lausanne, Switzerland
- 3Paediatric Cardiology Unit, Woman-Mother-Child Department, Lausanne University Hospital (CHUV), Lausanne, Switzerland
- 4Institute of Primary Health Care (BIHAM), University of Bern, Bern, Switzerland
- 5Institute of Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada
- 6School of Population and Global Health, McGill University, Montréal, Canada
Introduction: Hyperglycemia is associated with a higher cardiovascular risk, as evidenced by increased carotid-intima media thickness (CIMT) in youth with diabetes. We conducted a systematic review and meta-analysis to assess the effect of pharmacological or non-pharmacological interventions on CIMT in children and adolescents with prediabetes or diabetes.
Methods: We conducted systematic searches of MEDLINE, EMBASE, and CENTRAL, together with supplementary searches in trial registers and other sources for studies completed up to September 2019. Interventional studies assessing ultrasound CIMT in children and adolescents with prediabetes or diabetes were considered for inclusion. Where appropriate, data were pooled across studies using random-effect meta-analysis. Quality was assessed using The Cochrane Collaboration’s risk-of-bias tool and a CIMT reliability tool.
Results: Six studies involving 644 children with type 1 diabetes mellitus were included. No study involved children with prediabetes or type 2 diabetes. Three randomized controlled trials (RCTs) evaluated the effects of metformin, quinapril, and atorvastatin. Three non-randomized studies, with a before-and-after design, evaluated the effects of physical exercise and continuous subcutaneous insulin infusion (CSII). The mean CIMT at baseline ranged from 0.40 to 0.51 mm. The pooled difference in CIMT was -0.01 mm (95% CI: -0.04 to 0.01) for metformin compared to placebo (2 studies; 135 participants; I2: 0%). The difference in CIMT was -0.01 mm (95% CI: -0.03 to 0.01) for quinapril compared to placebo (1 study; 406 participants). The mean change from baseline in CIMT was -0.03 mm (95% CI: -0.14 to 0.08) after physical exercise (1 study; 7 participants). Inconsistent results were reported for CSII or for atorvastatin. CIMT measurement was rated at a higher quality on all reliability domains in 3 (50%) studies. The confidence in results is limited by the low number of RCTs and their small sample sizes, as well as the high risk of bias in before-and-after studies.
Conclusions: Some pharmacological interventions may decrease CIMT in children with type 1 diabetes. However, there is great uncertainty with respect to their effects and no strong conclusions can be drawn. Further evidence from larger RCTs is required.
Systematic Review Registration: PROSPERO, CRD42017075169
1 Introduction
Children and adolescents with diabetes have a high long-term cardiovascular risk (1, 2), as evidenced by signs of subclinical atherosclerosis and increased carotid-intima media thickness (CIMT) (3–6). Prevalence of both type 1 and type 2 diabetes has increased in the past decades (2, 7). This is particularly worrisome because diabetes clusters with several other risk factors, for instance, hypertension, hyperlipidemia, or microalbuminuria (8), which may track into adulthood (9, 10) and accelerate the process of atherosclerosis (11, 12). Also, increased CIMT in adulthood is associated with cardiovascular disease (CVD) events, such as heart attack and stroke (8, 13, 14). Early intervention for CVD prevention in children with diabetes is therefore paramount, yet complex and relatively understudied.
Clinical trials evaluating cardiovascular treatment efficacy in early life use surrogate markers of CVD, such as ultrasound CIMT. Several studies in adults showed that drug treatments or dietary interventions may slow progression of CIMT (15–17), which in turn may be associated with a reduction in CVD risk (15). Likewise, clinical trials in high-risk children with obesity or familial hyperlipidemia showed that exercise training (18) or statin therapy (18) may decrease CIMT. However, in children with diabetes, data on effective interventions are limited and clinical recommendations are largely based on expert opinions (19–21). A few clinical trials using CIMT were performed recently, but they were not part of a systematic review and meta-analysis. Additionally, CIMT measurement methods are heterogeneous at young ages and inconsistent findings across studies may be partly explained by a low measurement reliability (22).
We therefore conducted a systematic review and meta-analysis (1) to assess the effect of pharmacological or non-pharmacological interventions on CIMT in children and adolescents with prediabetes or diabetes and (2) to assess the characteristics and reliability of CIMT measurement methods used in the included studies.
2 Methods
2.1 Protocol Development and Reporting
This study is part of a larger systematic review project that focuses on prenatal and postnatal exposures or interventions and CIMT in children and adolescents (22, 23). We followed methods outlined in the research protocol for this systematic review project, which was also registered with the International Prospective Register of Systematic Reviews (PROSPERO) (registration number CRD42017075169) and published (23). The reporting of this paper complies with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (24).
2.2 Eligibility Criteria
2.2.1 Study Designs
Interventional studies with a randomized or non-randomized, controlled or non-controlled design were considered for inclusion.
2.2.2 Participants
We considered for inclusion studies in children with a mean age ≤18 years at study entry and either prediabetes (e.g., impaired glucose tolerance, impaired fasting glucose) or diabetes mellitus (e.g., type 1 diabetes, type 2 diabetes, diabetes secondary to diseases of the exocrine pancreas, endocrinopathies, or drug-induced diabetes mellitus).
2.2.3 Interventions
No restrictions were posed. Both pharmacological and non-pharmacological interventions were deemed equally eligible.
2.2.4 Comparators
No restrictions were posed. Where applicable, the comparator could be a pharmacological or non-pharmacological intervention, usual care, placebo, or no intervention.
2.2.5 Outcome Measures
The outcome was the intima-media thickness of the carotid artery measured by ultrasonography.
2.2.6 Time Frame and Setting
No restrictions were posed.
2.2.7 Language
Studies in English and French were considered for inclusion.
2.3 Search Strategy
Systematic searches were conducted in the Medical Literature Analysis and Retrieval System Online (MEDLINE) database, Excerpta Medica database (EMBASE), and Cochrane Central Register of Controlled Trials (CENTRAL) from inception to March 2019. Supplementary searches were performed in September 2019 and consisted of (1) a manual search of reference lists and other reviews on the topic, (2) forward citation tracking on Web of Science based on retrieved eligible reports, and (3) personalized search queries in Google Scholar and trial registers. The strategies for the systematic searches are provided in the published study protocol (23) and those for the supplementary searches in Table S1 in Supplementary Material.
2.4 Study Selection Process
Study references were managed with Endnote (version X8.1) and deduplicated according to the method of Bramer et al. (25). Study screening was performed initially based on titles and abstracts and then based on full texts retained in the first step. Each report was screened independently by 2 reviewers using Covidence (26). Disagreements were resolved by discussion or, if necessary, by a third reviewer. The investigators of completed studies identified through supplementary searches in trial registers were contacted by email, but no supplementary data could be provided for this systematic review (Table S2 in Supplementary Material).
2.5 Data Extraction
Data were extracted independently by 2 reviewers using an electronic form in Microsoft Excel (version 2016). Extracted information concerned (1) study and population characteristics, (2) CIMT measurement method and reliability, (3) intervention characteristics, (4) adjusted and unadjusted effect sizes, (5) methodological quality (or risk of bias). The methodological quality was evaluated using the Cochrane’s collaboration risk-of-bias tool for randomized studies (22, 27). This tool classifies studies at low, high, and unclear risk of bias for study design and conduction, which made us conclude on high, low, and unclear methodological quality, respectively (28). The quality of the CIMT measurement method was evaluated using the tool published in the study protocol (23), which evaluates (1) the site of measurement, (2) the image analysis methods, and (3) the assessment of measurement reproducibility. This tool classifies measurements at higher, lower, and unclear reliability, which made us conclude on higher, lower, and unclear quality, respectively. Disagreements between reviewers were resolved by discussion or with the arbitration of a third reviewer. Essential missing information was searched by checking additional references related to that study, such as the published research protocol. The corresponding author of one study was contacted by e-mail and provided complementary information for computing the effect size estimate (29). The certainty of the evidence was rated for each intervention type by 1 reviewer using GRADE (Grading of Recommendations, Assessment, Development and Evaluations) (30). The GRADE rating (high, moderate, low, very low certainty) specifies the extent to which one can be confident that an estimate of effect is close to the true effect and involves consideration of limitations within and across studies with regard to methodological quality, inconsistencies and imprecision in effects, indirectness of the evidence, or publication bias.
2.6 Data Analysis
Data analysis was performed in Stata (version 16), with graphical output from Stata (version 16) or R studio (version 4.1.2). Analyses were performed for each intervention type according to the study protocol (23) and recommendations and formulae provided in the Cochrane Handbook for Systematic Reviews (28, 31), Lipsey and Wilson (32), Wan and colleagues (33), Fu and colleagues (34), Sullivan (35), and Reichenbach and colleagues (36). Descriptive statistics about study participants are presented as means and standard deviations. For controlled studies, effect sizes are presented as differences in mean scores at follow-up or differences in mean change scores from baseline to follow-up with 95% confidence intervals (CI). For non-controlled studies, effect sizes are presented as mean change scores from baseline to follow-up with 95% CI. When necessary, data transformations were done: (1) means and standard deviations were estimated from medians and interquartile ranges (33); (2) the mean change score from baseline to follow-up for a single arm was calculated as mean score at follow-up – mean score at baseline; differences between arms were calculated as intervention – comparison; and (3) CIs were calculated from standard errors or estimated from p-values (28, 32, 35, 36). To perform the meta-analysis, additional data simplifications were done: (1) if a study reported on both mean and maximum CIMT, mean CIMT was included in the analysis; (2) if a study reported on both systolic and diastolic CIMT, the diastolic CIMT was included in the analysis; and (3) if a study provided effect estimates with different levels of adjustment, most adjusted estimates were used in the analyses. To report hemoglobin A1c (HbA1c) values as percentage (%) and mmol/mol, conversions were performed according to the National Glycohemoglobin Standardization Program’s (NGSP) converters (37) and underlying equations (38):
(1)H/tiffb/tiffA1cm/tiffm/tiffo/tiffl/m/tiffo/tiffl=(H/tiffb/tiffA1c%×10.929)−23.5
(2)
The meta-analysis was performed using the DerSimonian–Laird random-effect model, with the difference in CIMT in mm between intervention and comparison arms as the intervention effect. Pooling was not feasible for all studies because of the different intervention types assessed across studies. As previously shown to be valid, we pooled together outcomes reported as mean score at follow-up and mean change score from baseline to follow-up in the same meta-analysis (31, 39). The heterogeneity was assessed by the Cochran’s Q test, I2, and tau2 statistics (28, 40, 41). We planned to assess publication bias using funnel plots and Egger’s test (23), but this was not feasible due to the small number of included studies. We interpreted the point estimate as the best average treatment effect and reported alongside it the 95% confidence interval, which provides the uncertainty around the point estimate, as per the PRISMA guidelines (24) and recommendations of the Cochrane Collaboration (28, 31).
3 Results
3.1 Description of Studies and Baseline Characteristics of Participants
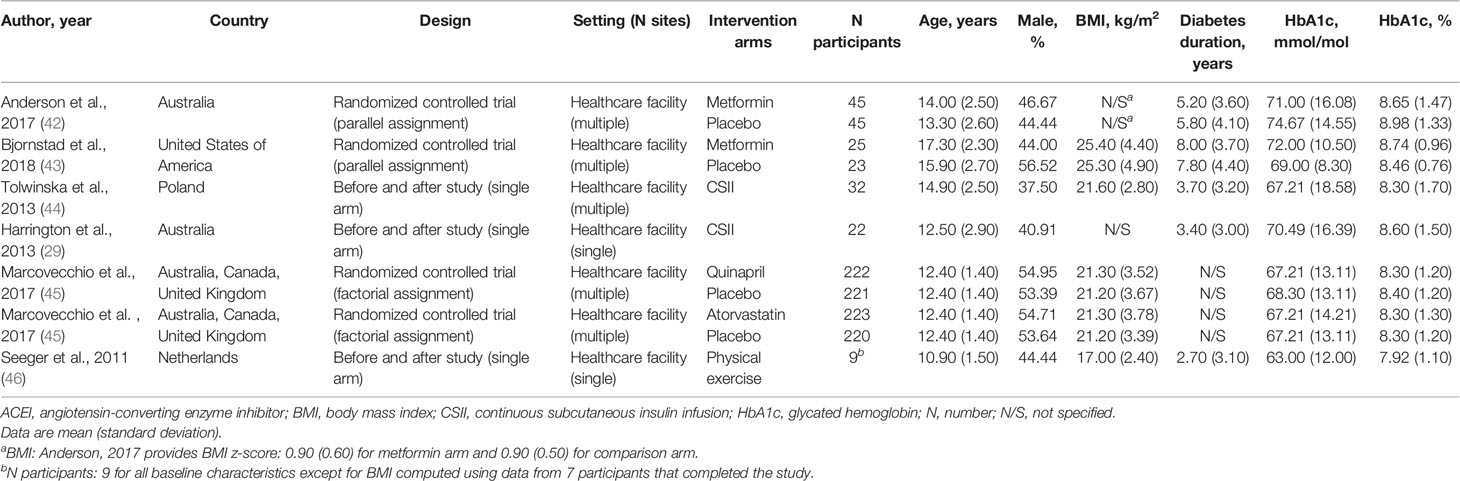
A total of 6,199 reports were screened based on titles and abstracts, and 22 were selected for full-text reviewing (Figure 1). Seven full texts, pertaining to 6 studies, with a randomized controlled design (n = 3) or a non-randomized non-controlled design (n = 3), were included in the systematic review. Studies were conducted in healthcare facilities in Europe (n = 2), Australia (n = 2), North America (n = 1), or cross-continentally (n = 1) (Table 1). Some 644 boys and girls (mean age between 10.9 and 17.3 years) with type 1 diabetes mellitus (mean time since diagnosis between 2.7 and 8.0 years) were included across studies. Mean HbA1c at baseline ranged from 63 to 74.67 mmol/mol (7.92 to 8.98%) (Table 1). Mean CIMT at baseline ranged from 0.40 to 0.51 mm. No study included children with prediabetes or type 2 diabetes (Table S3 in Supplementary Material).
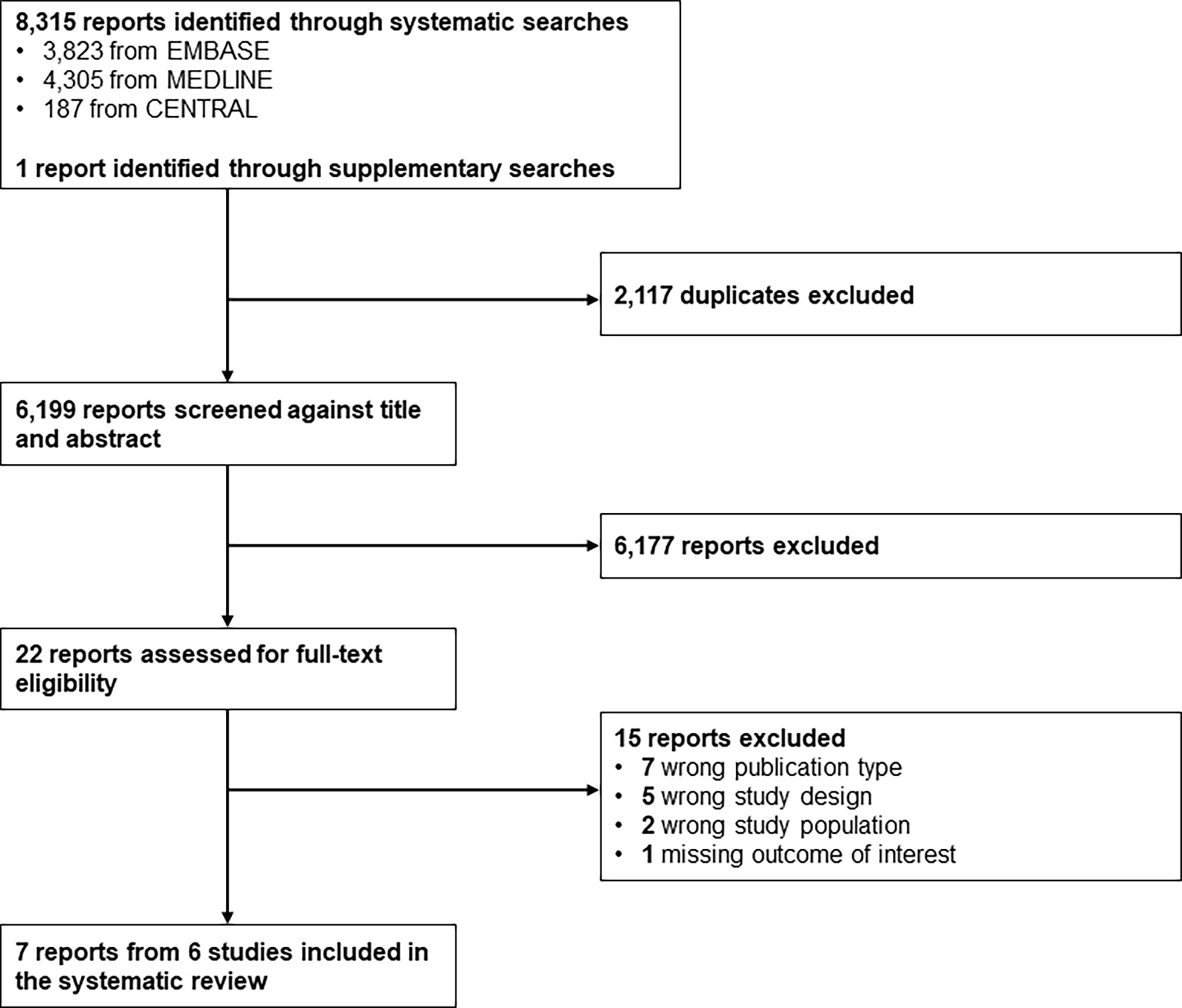
Figure 1 Study selection flow. CENTRAL, Cochrane Central Register of Controlled Trials; EMBASE, Excerpta Medica database; MEDLINE, Medical Literature Analysis and Retrieval System Online.
3.2 Description of CIMT Measurement Methods
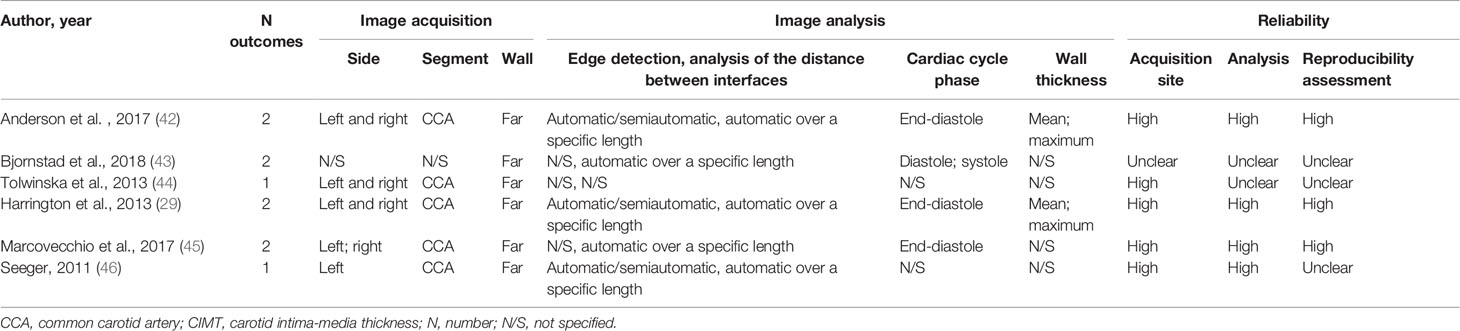
Image acquisition and analysis were relatively uniform across studies (Tables 2, S4 in Supplementary Material). The CIMT was primarily measured on the common carotid artery (CCA) far wall, with the distances between the intima and media interfaces assessed automatically over a specific length. A total of 1 to 2 CIMT outcomes were reported in each study. Two studies reported both mean and maximum wall thickness, and one study reported both diastolic and systolic CIMT. Measurements of the left or right or combined left and right carotid sides were analyzed, with one study reporting left CIMT and right CIMT as 2 separate outcomes. Three studies were judged to be at higher CIMT reliability on all domains of measurement quality.
3.3 Effects of Interventions
The pharmacological interventions evaluated were metformin (antidiabetic drug), quinapril (angiotensin-converting enzyme inhibitor (ACEI) drug), atorvastatin (lipid-lowering drug), and continuous subcutaneous insulin infusion (CSII) (antidiabetic device). Physical exercise was the only non-pharmacological intervention evaluated (Table 3). Trials varied in their methodological quality, but the evidence from the 3 RCTs was generally at low risk of bias, with 1 or 2 domains at unclear risk of bias. One RCT was rated at low risk of bias on all domains (Figures 2, S1 in supplementary material).
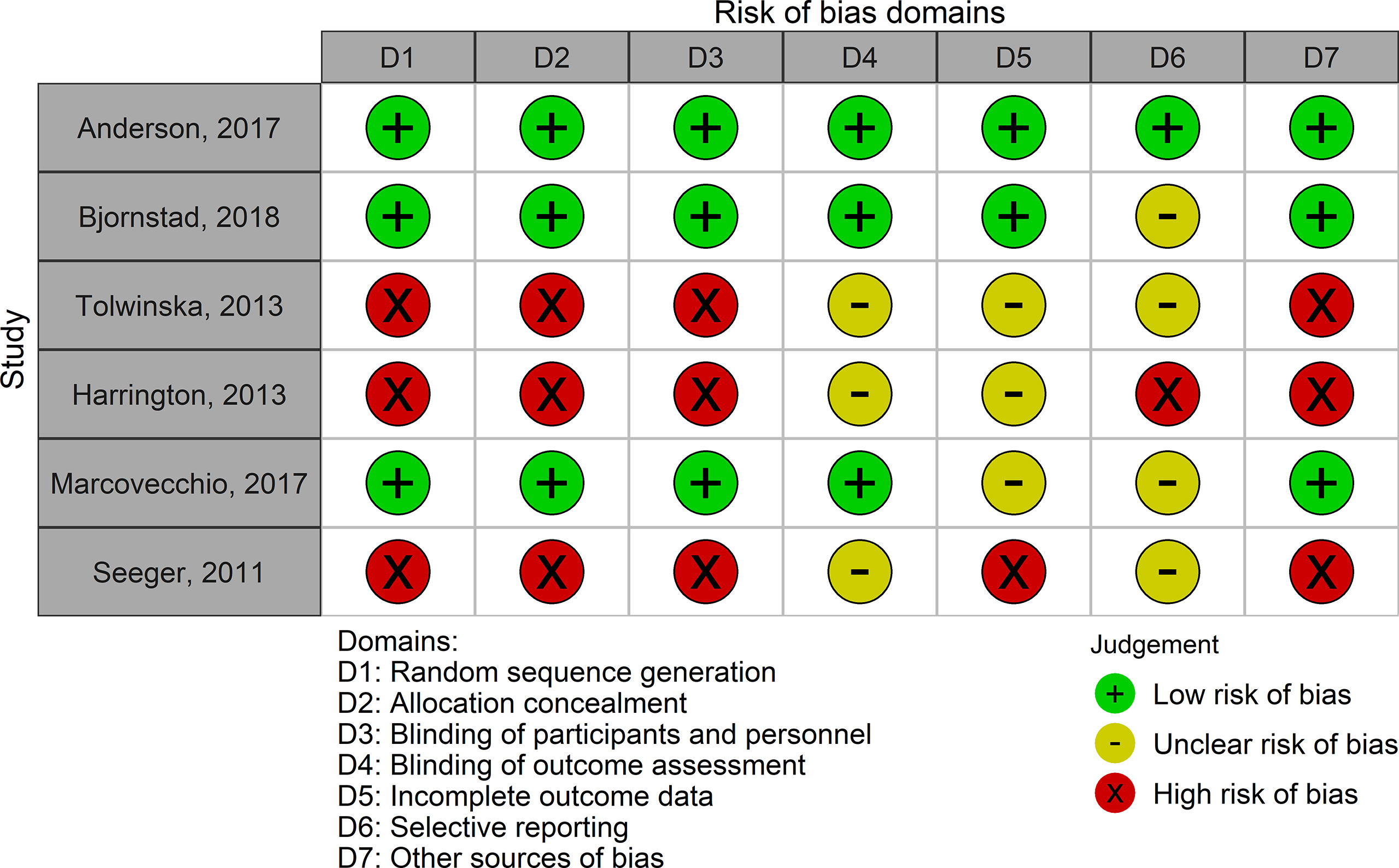
Figure 2 Risk of bias in each study included in the systematic review. Low risk of bias corresponds to high methodological quality. High risk of bias corresponds to low methodological quality.
3.3.1 Pharmacological Interventions
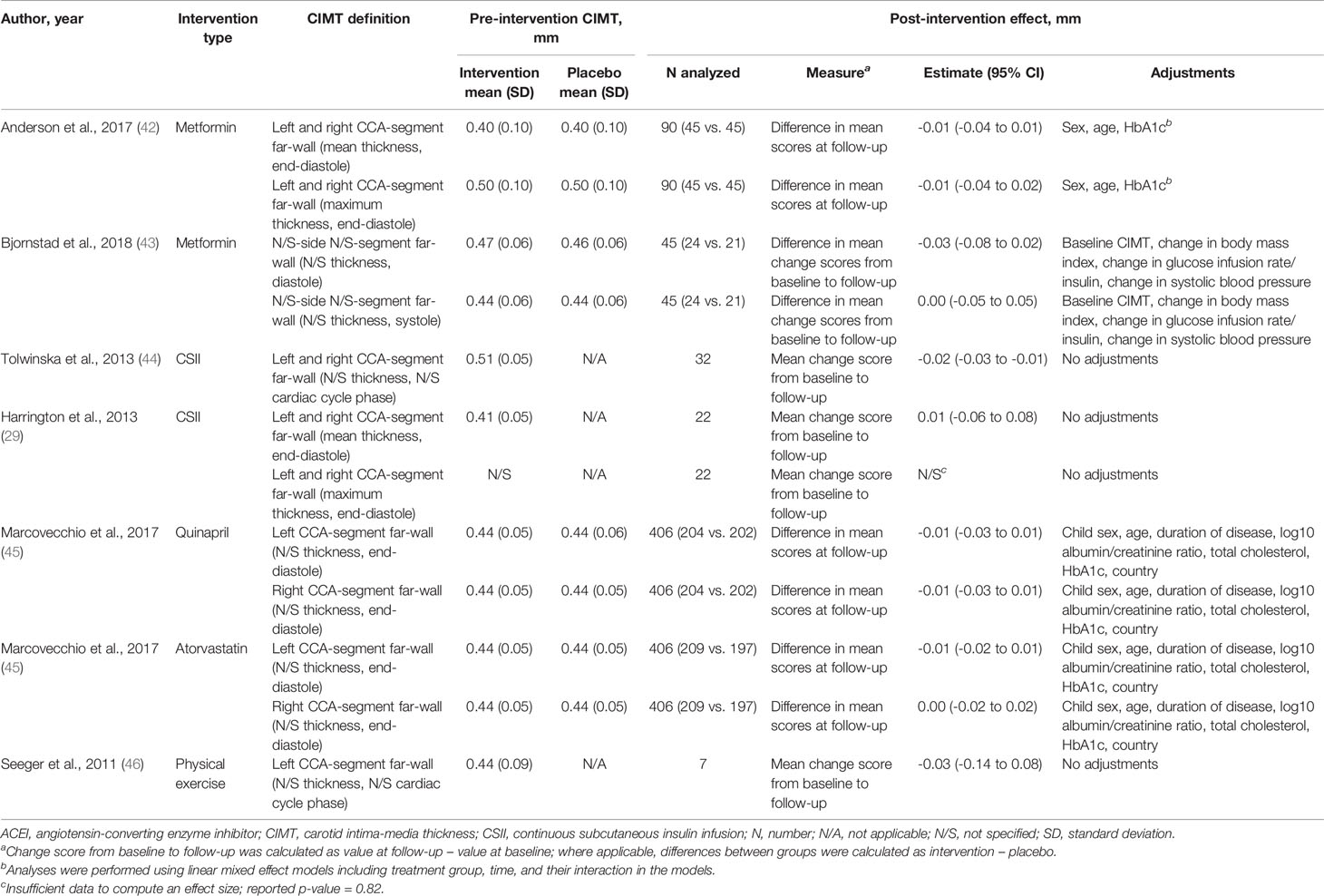
Two parallel-design RCTs compared metformin with placebo. The treatment duration ranged from 3 to 12 months (Table S3 in Supplementary Material). The pooled difference in CIMT was -0.01 mm (95% CI: -0.04 to 0.01) in favor of metformin (135 participants; I2: 0%; tau2:0) (Figure 3).
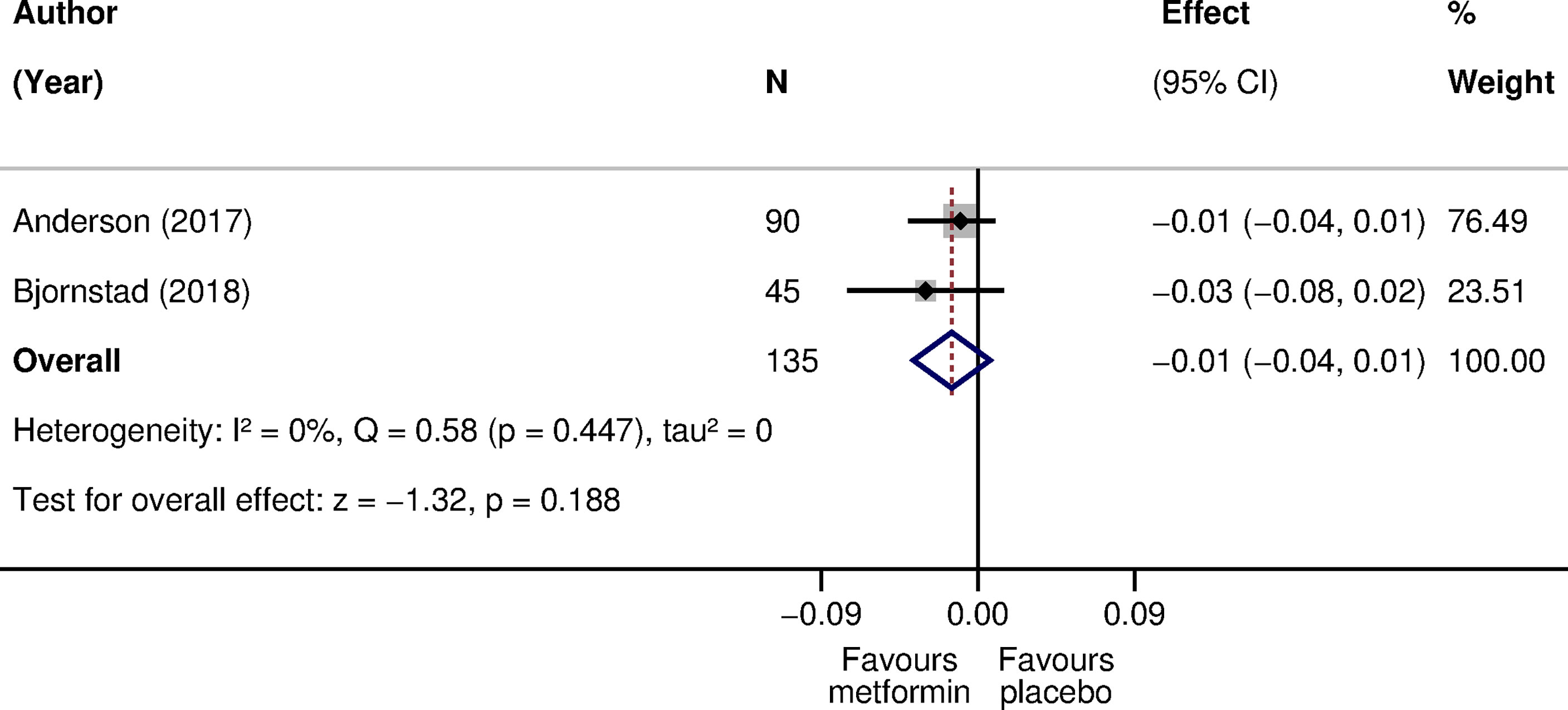
Figure 3 The effect of metformin compared with placebo on CIMT in children with type 1 diabetes. The effect is the difference in CIMT mean scores at follow-up (42) or in mean change scores from baseline to follow-up (43) in mm. A negative effect size corresponds to a lower CIMT in the metformin arm as opposed to the placebo arm. Weights are from the random-effects model. CI, confidence interval; CIMT, carotid intima-media thickness; N, sample size; Q, Cochran’s Q statistic; p, p-value; z, z statistic for the overall effect.
One RCT with a 2-by-2 factorial design compared quinapril or atorvastatin with placebo in 406 children at high risk for diabetic nephropathy. Treatment was provided over 2 to 4 years (Table S3). The difference in CIMT was -0.01 mm (95% CI: -0.03 to 0.01) in favor of quinapril for either left or right CIMT. The difference in CIMT was -0.01 mm (95% CI: -0.02 to 0.01) in favor of atorvastatin for left CIMT, but not for right CIMT [0.00 mm (95% CI: -0.02 to 0.02)] (Table 3).
Two non-randomized non-controlled studies compared CIMT before and after initiation of treatment with CSII. The treatment duration was 6 months in one study and was not specified in the other study (Table S3 in Supplementary Material). Effects in the opposite direction were reported. The mean change from baseline in CIMT was -0.02 mm (95% CI: -0.03 to -0.01) in one study (32 participants) and 0.01 mm (95% CI: -0.06 to 0.08) in the other study (22 participants) (Table 3).
3.3.2 Non-Pharmacological Interventions
A single non-randomized non-controlled study compared CIMT before and after physical exercise for 18 weeks (Table S3 in supplementary material). The mean change from baseline in CIMT was -0.03 mm (95% CI: -0.14 to 0.08) (7 participants) (Table 3).
3.4 Certainty of the Evidence
For metformin, quinapril, and atorvastatin, the evidence came from RCTs, but it was eventually rated at low certainty. For metformin, the evidence was downgraded due to very serious concerns related to imprecision in effect estimates (low number of participants; 95% CI of the pooled effect estimate crossing the line of no effect). For quinapril or atorvastatin, the evidence was downgraded due to serious concerns related to the comparator indirectness and imprecision in effect estimates. More specifically, quinapril and atorvastatin were evaluated in a 2-by-2 factorial design that assumed no interaction between the factorial comparisons. This means that quinapril was evaluated against a comparator comprising participants taking placebo and placebo or placebo and atorvastatin. Likewise, atorvastatin was evaluated against a comparator comprising participants taking placebo and placebo or placebo and quinapril. Regarding imprecision, the 95% CIs for the effect estimates indicate that no effect remains plausible despite the relatively large sample size (406 participants).
For CSII and physical exercise, the evidence came from non-randomized, non-controlled studies and it was rated at very low certainty. This rating was due to very serious concerns regarding the risk of bias (studies were rated at high or unclear risk of bias on all methodological domains), comparator indirectness (a single group of participants serving as their own controls), and imprecision. For CSII, the certainty of the evidence was also downgraded due to serious concerns related to inconsistency in results across studies.
4 Discussion
4.1 Summary of Main Results
In this systematic review of 6 interventional studies involving 644 children and adolescents with type 1 diabetes, we identified a small and statistically non-significant decrease in CIMT after metformin (low certainty), quinapril (low certainty), or physical exercise (very low certainty). Inconsistent results were reported for CSII or for atorvastatin. The CIMT measurement reliability was either higher or unclear. The confidence in results is limited by the low number of RCTs and their small sample sizes, as well as the high risk of bias in before and after studies.
4.2 Comparison With Other Studies
We found some evidence on the effect of medications in children with type 1 diabetes that was partially in line with findings among other children or adults at high CVD risk. Metformin was evaluated in a recent systematic review and meta-analysis that reported a pooled difference in CIMT of -0.053 mm (95% CI: -0.115 to 0.009) in favor of metformin among adults with prediabetes or diabetes (6 trials; 806 participants) (17). Our effect estimates in children with type 1 diabetes consistently pointed toward decreases in CIMT, but they were much smaller in magnitude and highly imprecise. The comparison of ACEI with placebo in a meta-analysis of 3 trials including 2,087 adults with impaired glucose tolerance, type 2 diabetes, or albuminuria showed no effect on CIMT (pooled difference 0.00 mm (95% CI: -0.01 to 0.00) (16). We found an effect estimate for ACEI in children with type 1 diabetes that was slightly higher in magnitude, but more imprecise (-0.01 mm; 95% CI: -0.03 to 0.01) (45). Likewise, the comparison of statins with placebo or usual care in a meta-analysis of 13 primary prevention trials showed a pooled difference in CIMT of 0.00 mm (95% CI: -0.01 to 0.01) in favor of statins (47). Nonetheless, one RCT among 211 children with familial hypercholesterolemia (48) showed that 2 years of pravastatin was associated with a -0.01mm (95% CI: -0.03 to 0.00) difference in CIMT. This latter trial was powered on CIMT, which was defined as the mean of the right and left CCA, carotid bulb, and internal carotid artery segments (49). Our results for the effect of atorvastatin on CIMT of the left (-0.01 mm; 95% CI: -0.02 to 0.01) and right (0.00 mm; 95% CI: -0.02 to 0.02) CCAs were inconsistent (45).
We found that the effect of dietary or lifestyle measures on CIMT is largely understudied in children with diabetes. We identified one non-controlled non-randomized study (7 participants) reporting a mean change in CIMT of -0.03 mm (95% CI: -0.14 to 0.08) following 18 weeks of exercise training. The study had several caveats, primarily related to the lack of an external control group and the extremely low sample size. Much stronger evidence exists in other populations. For instance, Garcia-Hermoso and colleagues (18) performed a systematic review and meta-analysis of 6 RCTs involving 303 children with overweight and obesity and reported that exercise training decreased CIMT by -0.31 standard deviation units (95% CI -0.54 to -0.07). Lifestyle interventions merit further consideration in future trials because they may be more acceptable to children and parents, may contribute to the development of healthy behaviors that track into adulthood (50), and have the potential to act on multiple mechanisms of atherosclerosis for instance, physical exercise may improve endothelial dysfunction and healthy diets may improve the lipid profile (51).
Adequately powered trials would be needed to identify suitable interventions to reduce CVD risk in children with type 1 diabetes. The RCTs included in our systematic review were not primarily designed to show an effect on CIMT, but on markers of endothelial dysfunction (flow-mediated dilation) (42), insulin sensitivity (steady-state glucose infusion rate/insulin) (43), or albuminuria (albumin-to-creatinine ratio) (45). However, they provide useful information to guide the design of future trials. Some of the interventions that were administered for at least 12 months, such as metformin or atorvastatin, resulted in point estimates for the treatment effect of about -0.01 mm. Although small, a decrease in CIMT of 0.01 mm/year might be clinically important on the long term as highlighted by the study of Willeit and colleagues (15). If we perform a rough estimation of the sample size required to have 80% power to detect a difference of 0.01 mm between 2 arms, at a 2-sided 0.05 α-level, when assuming a CIMT standard deviation of 0.05 mm, we would obtain a total of 788 participants (394 per arm). Increasing or decreasing the assumed value for the standard deviation of the outcome would result in a higher or lower sample size needed. The sources of variability for each study therefore need to be carefully considered in sample size planning (52).
4.3 Strengths and Limitations
To the best of our knowledge, this is the first quantitative synthesis of the effect of metformin on CIMT in children and adolescents with type 1 diabetes. Other strengths to be noted include the reporting of detailed characteristics of the CIMT measurement and broad searches, in multiple sources, to retrieve completed studies. However, the high imprecision in effects, together with the variation in trial designs and levels of methodological quality, limit the degree of confidence in results. Further, only children with type 1 diabetes were included in these studies. Our conclusions might not be applicable to patients with type 2 diabetes or prediabetes, although emerging evidence shows that the prevalence of increased CIMT is also high for adolescents and young adults with newly diagnosed type 2 diabetes (5). Next, measurement error in CIMT cannot be excluded and co-medications and co-interventions beyond the studied interventions were provided to participants, which may have influenced the observed effects (53). In fact, 3 out of 4 studies evaluating metformin, quinapril, and atorvastatin reported that insulin was continued during the study course and adjusted as per need or routinely recommended by the healthcare providers, which may have triggered imbalances between the intervention arms. One trial evaluating metformin for 12 months also reported providing dietary advice at baseline and 3 months, but this co-intervention was standardized and given to both the active and comparison arms. The risk of bias due to confounding is particularly important for non-randomized non-controlled studies due to the lack of a control group to account for time trends, no randomization and concealed allocation, and unadjusted effect estimates. However, the meta-analysis for metformin was performed using most adjusted estimates from 2 RCTs. Although the adjustment factors differed between the studies, there was no statistical heterogeneity associated with the pooled effect (I2: 0%; tau2: 0). Finally, the restriction to studies published in English or French, which were the languages spoken in common by the reviewers, is another limitation of this systematic review.
5 Conclusions
5.1 Implications for Practice and Research
Children with diabetes are at high risk of subclinical vascular complications, such as increased CIMT, and bear a disproportionate risk of clinical CVD in adulthood (12). This constitutes an important public health problem in the context of population aging and increased prevalence of diabetes worldwide (7), hence, early-life prevention of CVD has been advocated (2, 54). Multiple factors seem to contribute to their higher CVD risk, such as hyperglycemia, hypertension, dyslipidemia, or insulin resistance (55). Our meta-analysis suggests that the use of metformin as an adjunctive therapy may hold promise in CVD risk reduction in children with type 1 diabetes through improvements in CIMT. Provided the effect of metformin is confirmed in future trials, this is an important finding for clinical practice as vascular remodeling may constitute an additional treatment target. Given its insulin-sensitizing properties (43, 56), metformin may also have the ability to help with glycemic control during puberty when insulin resistance worsens and many youth with type 1 diabetes fail to meet clinical guidelines (57, 58).
Further evidence is needed to identify appropriate intervention strategies for maintaining a low CVD over the life course. The current evidence from RCTs reported on CIMT as a secondary endpoint and suffers from low certainty, mainly due to imprecision. The non-RCT evidence suffers from very low certainty, mainly due to high risk of bias and imprecision. Therefore, larger, adequately powered, and well-conducted RCTs, carried over longer time periods, in children with type 1 diabetes are warranted. Further evidence on the effect of nutrition, exercise, and psychosocial and behavioral interventions on preventing or improving vascular remodeling in youth with diabetes or prediabetes is also needed.
Author Contributions
AE, NS, and AC designed the study, with input from BC and SB. AE carried out the literature searches. AE and DA performed the duplicate study selection, data extraction, and quality assessments. AC and NS resolved the screening and data extraction conflicts. AE carried out the statistical analyses, with input and supervision from BC and AC. AE wrote the first draft of the manuscript. AC, BC, DA, NS, and SB made critical revisions to the manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Swiss National Science Foundation (www.snf.ch; project number 32003B-163240; grantee: AC). The funding body had no role in the study design, collection, analysis, and interpretation of data, or in the writing of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank librarians Thomas Brauchli (Data and Documentation Unit, Center for Primary Care and Public Health (UNISANTÉ), University of Lausanne, Lausanne, Switzerland) and Cécile Jaques (Medical Library, Lausanne University Hospital, University of Lausanne, Lausanne, Switzerland) for their input on developing and running the search strategies in the electronic databases. We also thank Magali Rios-Leyvraz (Center for Primary Care and Public Health (UNISANTÉ), University of Lausanne, Lausanne, Switzerland) for her help with study screening for the source project in which this systematic review on CIMT in children with diabetes is nested.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcdhc.2022.882504/full#supplementary-material
Abbreviations
ACEI, angiotensin-converting enzyme inhibitor; CCA, common carotid artery; CENTRAL, Cochrane Central Register of Controlled Trials; CI, confidence interval; CIMT, carotid-intima media thickness; CSII, continuous subcutaneous insulin infusion; CVD, cardiovascular disease; EMBASE, Excerpta Medica database; GRADE, Grading of Recommendations, Assessment, Development and Evaluations; HbA1c, Hemoglobin A1c; MEDLINE, Medical Literature Analysis and Retrieval System Online; NGSP, National Glycohemoglobin Standardization Program; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PROSPERO, International Prospective Register of Systematic Reviews; RCT, randomized controlled trial.
References
1. Maahs DM, Daniels SR, de Ferranti SD, Dichek HL, Flynn J, Goldstein BI, et al. Cardiovascular Disease Risk Factors in Youth With Diabetes Mellitus: A Scientific Statement From the American Heart Association. Circulation (2014) 130(17):1532–58. doi: 10.1161/CIR.0000000000000094
2. de Ferranti SD, Steinberger J, Ameduri R, Baker A, Gooding H, Kelly AS, et al. Cardiovascular Risk Reduction in High-Risk Pediatric Patients: A Scientific Statement From the American Heart Association. Circulation (2019) 139(13):e603–e34. doi: 10.1161/CIR.0000000000000618
3. Giannopoulou EZ, Doundoulakis I, Antza C, Christoforidis A, Haidich AB, Kotsis V, et al. Subclinical Arterial Damage in Children and Adolescents With Type 1 Diabetes: A Systematic Review and Meta-Analysis. Pediatr. Diab (2019) 20(6):668–77. doi: 10.1111/pedi.12874
4. Wang P, Xu YY, Lv TT, Guan SY, Li XM, Li XP, et al. Subclinical Atherosclerosis in Patients With Type 1 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Angiology (2019) 70(2):141–59. doi: 10.1177/0003319718787366
5. Gu W, Huang Y, Zhang Y, Hong J, Liu Y, Zhan W, et al. Adolescents and Young Adults With Newly Diagnosed Type 2 Diabetes Demonstrate Greater Carotid Intima-Media Thickness Than Those With Type 1 Diabetes. Diabetes Med. (2014) 31(1):84–91. doi: 10.1111/dme.12335
6. Ryder JR, Northrop E, Rudser KD, Kelly AS, Gao Z, Khoury PR, et al. Accelerated Early Vascular Aging Among Adolescents With Obesity and/or Type 2 Diabetes Mellitus. J Am Heart Assoc (2020) 9(10):e014891. doi: 10.1161/JAHA.119.014891
7. NCD Risk Factor Collaboration (NCD-RisC). Worldwide Trends in Diabetes Since 1980: A Pooled Analysis of 751 Population-Based Studies With 4.4 Million Participants. Lancet (London England) (2016) 387(10027):1513–30.
8. Gourgari E, Dabelea D, Rother K. Modifiable Risk Factors for Cardiovascular Disease in Children With Type 1 Diabetes: Can Early Intervention Prevent Future Cardiovascular Events? Curr. Diabetes Rep. (2017) 17(12):134. doi: 10.1007/s11892-017-0968-y
9. Chen X, Wang Y. Tracking of Blood Pressure From Childhood to Adulthood: A Systematic Review and Meta-Regression Analysis. Circulation (2008) 117(25):3171–80. doi: 10.1161/CIRCULATIONAHA.107.730366
10. Webber LS, Srinivasan SR, Wattigney WA, Berenson GS. Tracking of Serum Lipids and Lipoproteins From Childhood to Adulthood. The Bogalusa Heart Study. Am. J. Epidemiol. (1991) 133(9):884–99. doi: 10.1093/oxfordjournals.aje.a115968
11. Lorenz MW, Gao L, Ziegelbauer K, Norata GD, Empana JP, Schmidtmann I, et al. Predictive Value for Cardiovascular Events of Common Carotid Intima Media Thickness and its Rate of Change in Individuals at High Cardiovascular Risk - Results From the PROG-IMT Collaboration. PloS One (2018) 13(4):e0191172.
12. Rawshani A, Sattar N, Franzén S, Rawshani A, Hattersley AT, Svensson AM, et al. Excess Mortality and Cardiovascular Disease in Young Adults With Type 1 Diabetes in Relation to Age at Onset: A Nationwide, Register-Based Cohort Study. Lancet (London England). (2018) 392(10146):477–86. doi: 10.1016/S0140-6736(18)31506-X
13. Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of Clinical Cardiovascular Events With Carotid Intima-Media Thickness: A Systematic Review and Meta-Analysis. Circulation (2007) 115(4):459–67. doi: 10.1161/CIRCULATIONAHA.106.628875
14. Eikendal AL, Groenewegen KA, Anderson TJ, Britton AR, Engstrom G, Evans GW, et al. Common Carotid Intima-Media Thickness Relates to Cardiovascular Events in Adults Aged <45 Years. Hypertension (2015) 65(4):707–13. doi: 10.1161/HYPERTENSIONAHA.114.04658
15. Willeit P, Tschiderer L, Allara E, Reuber K, Seekircher L, Gao L, et al. Carotid Intima-Media Thickness Progression as Surrogate Marker for Cardiovascular Risk: Meta-Analysis of 119 Clinical Trials Involving 100 667 Patients. Circulation (2020) 142(7):621–42. doi: 10.1161/CIRCULATIONAHA.120.046361
16. Huang R, Mills K, Romero J, Li Y, Hu Z, Cao Y, et al. Comparative Effects of Lipid Lowering, Hypoglycemic, Antihypertensive and Antiplatelet Medications on Carotid Artery Intima-Media Thickness Progression: A Network Meta-Analysis. Cardiovasc. Diabetol. (2019) 18(1):14. doi: 10.1186/s12933-019-0817-1
17. Chen Y, Li H, Ye Z, Găman MA, Tan SC, Zhu F. The Effect of Metformin on Carotid Intima-Media Thickness (CIMT): A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Eur. J. Pharmacol. (2020) 886:173458. doi: 10.1016/j.ejphar.2020.173458
18. Garcia-Hermoso A, Gonzalez-Ruiz K, Triana-Reina HR, Olloquequi J, Ramirez-Velez R. Effects of Exercise on Carotid Arterial Wall Thickness in Obese Pediatric Populations: A Meta-Analysis of Randomized Controlled Trials. Childhood Obes. (Print). (2017) 13(2):138–45. doi: 10.1089/chi.2016.0265
19. Chiang JL, Maahs DM, Garvey KC, Hood KK, Laffel LM, Weinzimer SA, et al. Type 1 Diabetes in Children and Adolescents: A Position Statement by the American Diabetes Association. Diabetes Care (2018) 41(9):2026–44. doi: 10.2337/dci18-0023
20. Maahs DM. Guidelines to Practice: Identifying Barriers to Cardiovascular Health Management in Pediatric Type 1 Diabetes. J. Pediatr. (2018) 197:14–5. doi: 10.1016/j.jpeds.2018.01.047
21. Bjornstad P, Donaghue KC, Maahs DM. Macrovascular Disease and Risk Factors in Youth With Type 1 Diabetes: Time to be More Attentive to Treatment? Lancet Diabetes Endocrinol. (2018) 6(10):809–20.
22. Epure AM, Rios-Leyvraz M, Anker D, Di Bernardo S, da Costa BR, Chiolero A, et al. Risk Factors During First 1,000 Days of Life for Carotid Intima-Media Thickness in Infants, Children, and Adolescents: A Systematic Review With Meta-Analyses. PloS Med. (2020) 17(11):e1003414. doi: 10.1371/journal.pmed.1003414
23. Epure AM, Leyvraz M, Mivelaz Y, Di Bernardo S, da Costa BR, Chiolero A, et al. Risk Factors and Determinants of Carotid Intima-Media Thickness in Children: Protocol for a Systematic Review and Meta-Analysis. BMJ Open (2018) 8(6):e019644. doi: 10.1136/bmjopen-2017-019644
24. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ (Clinical Res. ed). (2021) 372:n71.
25. Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T. De-Duplication of Database Search Results for Systematic Reviews in EndNote. J. Med. Library Assoc. JMLA. (2016) 104(3):240–3. doi: 10.3163/1536-5050.104.3.014
27. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's Tool for Assessing Risk of Bias in Randomised Trials. BMJ (Clinical Res. ed). (2011) 343:d5928. doi: 10.1136/bmj.d5928
28. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0: The Cochrane Collaboration (2011). Available at: www.handbook-5-1.cochrane.org.
29. Harrington J, Peña AS, Wilson L, Gent R, Dowling K, Baghurst P, et al. Vascular Function and Glucose Variability Improve Transiently Following Initiation of Continuous Subcutaneous Insulin Infusion in Children With Type 1 Diabetes. Pediatr. Diab (2013) 14(7):504–11. doi: 10.1111/pedi.12050
30. Schünemann H, Brożek J, Guyatt G, Oxman A. Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach (2013). Available at: http://gdt.guidelinedevelopment.org/app/handbook/handbook.html.
31. Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.1. Cochrane (2020). Available at: www.training.cochrane.org/handbook.
33. Wan X, Wang W, Liu J, Tong T. Estimating the Sample Mean and Standard Deviation From the Sample Size, Median, Range and/or Interquartile Range. BMC Med. Res. Method. (2014) 14:135.
34. Fu R, Vandermeer BW, Shamliyan TA, O’Neil ME, Yazdi F, Fox SH, et al. Handling Continuous Outcomes in Quantitative Synthesis. Methods Guide for Effectiveness and Comparative Effectiveness Reviews (Prepared by the Oregon Evidence-Based Practice Center Under Contract No 290-2007-10057-I). Rockville (MD: Agency for Healthcare Research and Quality (2013). AHRQ Publication No. 13-EHC103-EF.
35. Sullivan L. Confidence Intervals. In: MPH Online Learning Modules, Biostatistics. Boston University School of Public Health (2016). Available at: https://sphweb.bumc.bu.edu/otlt/MPH-Modules/BS/BS704_Confidence_Intervals.
36. Reichenbach S, Sterchi R, Scherer M, Trelle S, Bürgi E, Bürgi U, et al. Meta-Analysis: Chondroitin for Osteoarthritis of the Knee or Hip. Ann. Internal Med. (2007) 146(8):580–90. doi: 10.7326/0003-4819-146-8-200704170-00009
37. National Glycohemoglobin Standardization Program (NGSP). Convert Between NGSP, IFCC and eAG . Available at: www.ngsp.org/convert2.asp.
38. National Glycohemoglobin Standardization Program (NGSP). IFCC Standardization of HbA1c. Available at: http://www.ngsp.org/docs/IFCCstd.pdf.
39. da Costa BR, Nüesch E, Rutjes AW, Johnston BC, Reichenbach S, Trelle S, et al. Combining Follow-Up and Change Data is Valid in Meta-Analyses of Continuous Outcomes: A Meta-Epidemiological Study. J Clin Epidemiol (2013) 66(8):847–55. doi: 10.1016/j.jclinepi.2013.03.009
40. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. John Wiley & Sons, Ltd (2009).
41. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring Inconsistency in Meta-Analyses. BMJ (Clinical Res. ed). (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
42. Anderson JJA, Couper JJ, Giles LC, Leggett CE, Gent R, Coppin B, et al. Effect of Metformin on Vascular Function in Children With Type 1 Diabetes: A 12-Month Randomized Controlled Trial. J Clin Endocrinol Metab (2017) 102(12):4448–56. doi: 10.1210/jc.2017-00781
43. Bjornstad P, Schäfer M, Truong U, Cree-Green M, Pyle L, Baumgartner A, et al. Metformin Improves Insulin Sensitivity and Vascular Health in Youth With Type 1 Diabetes Mellitus. Circulation (2018) 138(25):2895–907. doi: 10.1161/CIRCULATIONAHA.118.035525
44. Tołwińska J, Głowińska-Olszewska B, Bossowski A. Insulin Therapy With Personal Insulin Pumps and Early Angiopathy in Children With Type 1 Diabetes Mellitus. Mediators Inflam. (2013) 2013:791283.
45. Marcovecchio ML, Chiesa ST, Bond S, Daneman D, Dawson S, Donaghue KC, et al. ACE Inhibitors and Statins in Adolescents With Type 1 Diabetes. New Engl. J. Med. (2017) 377(18):1733–45. doi: 10.1056/NEJMoa1703518
46. Seeger JP, Thijssen DH, Noordam K, Cranen ME, Hopman MT, Nijhuis-van der Sanden MW. Exercise Training Improves Physical Fitness and Vascular Function in Children With Type 1 Diabetes. Diabetes Obes. Metab. (2011) 13(4):382–4. doi: 10.1111/j.1463-1326.2011.01361.x
47. Huang Y, Li W, Dong L, Li R, Wu Y. Effect of Statin Therapy on the Progression of Common Carotid Artery Intima-Media Thickness: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Atheros Thromb. (2013) 20(1):108–21. doi: 10.5551/jat.14001
48. Wiegman A, Hutten BA, de Groot E, Rodenburg J, Bakker HD, Buller HR, et al. Efficacy and Safety of Statin Therapy in Children With Familial Hypercholesterolemia: A Randomized Controlled Trial. Jama (2004) 292(3):331–7. doi: 10.1001/jama.292.3.331
49. Vuorio A, Kuoppala J, Kovanen PT, Humphries SE, Tonstad S, Wiegman A, et al. Statins for Children With Familial Hypercholesterolemia. Cochrane Database System Rev. (2019) 2019(11). doi: 10.1002/14651858.CD006401.pub5
50. Epure AM, Chiolero A. From Detection Early in Life to the Primordial Prevention of Elevated Blood Pressure. J. Clin. Hyper (Greenwich Conn). (2019) 21(9):1350–1. doi: 10.1111/jch.13634
51. Genovesi S, Parati G. Cardiovascular Risk in Children: Focus on Pathophysiological Aspects. Int. J. Mol. Sci. (2020) 21(18). doi: 10.3390/ijms21186612
52. Lenth RV. Some Practical Guidelines for Effective Sample Size Determination. Am. Stat. (2001) 55(3):187–93. doi: 10.1198/000313001317098149
53. Manson JE, Shufelt CL, Robins JM. The Potential for Postrandomization Confounding in Randomized Clinical Trials. Jama (2016) 315(21):2273–4. doi: 10.1001/jama.2016.3676
54. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: Summary Report. Pediatrics (2011) 128(Suppl 5):S213–S56. doi: 10.1542/peds.2009-2107C
55. Pastore I, Bolla AM, Montefusco L, Lunati ME, Rossi A, Assi E, et al. The Impact of Diabetes Mellitus on Cardiovascular Risk Onset in Children and Adolescents. Int. J. Mol. Sci. (2020) 21(14). doi: 10.3390/ijms21144928
56. Tahrani AA, Bailey CJ, Del Prato S, Barnett AH. Management of Type 2 Diabetes: New and Future Developments in Treatment. Lancet (London England). (2011) 378(9786):182–97. doi: 10.1016/S0140-6736(11)60207-9
57. Foster NC, Beck RW, Miller KM, Clements MA, Rickels MR, DiMeglio LA, et al. State of Type 1 Diabetes Management and Outcomes From the T1D Exchange in 2016-2018. Diabetes Technol. Ther. (2019) 21(2):66–72. doi: 10.1089/dia.2018.0384
58. Wood JR, Miller KM, Maahs DM, Beck RW, DiMeglio LA, Libman IM, et al. Most Youth With Type 1 Diabetes in the T1D Exchange Clinic Registry do Not Meet American Diabetes Association or International Society for Pediatric and Adolescent Diabetes Clinical Guidelines. Diabetes Care (2013) 36(7):2035–7. doi: 10.2337/dc12-1959
Keywords: diabetes, atherosclerosis, carotid intima-media thickness, children, trials
Citation: Epure AM, Anker D, Di Bernardo S, da Costa BR, Sekarski N and Chiolero A (2022) Interventions to Decrease Carotid-Intima Media Thickness in Children and Adolescents With Type 1 Diabetes: A Systematic Review and Meta-Analysis. Front. Clin. Diabetes Healthc. 3:882504. doi: 10.3389/fcdhc.2022.882504
Received: 23 February 2022; Accepted: 29 March 2022;
Published: 04 July 2022.
Edited by:
Federica Fogacci, University of Bologna, ItalyReviewed by:
Simina Crisan, Victor Babes University of Medicine and Pharmacy, RomaniaAleksandra Jotic, University of Belgrade, Serbia
Irmak Sayın Alan, Güven Hospital, Turkey
Copyright © 2022 Epure, Anker, Di Bernardo, da Costa, Sekarski and Chiolero. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adina Mihaela Epure, YWRpbmEtbWloYWVsYS5lcHVyZUB1bmlmci5jaA==
†These authors have contributed equally to this work and share last authorship
 Adina Mihaela Epure
Adina Mihaela Epure Daniela Anker
Daniela Anker Stefano Di Bernardo
Stefano Di Bernardo Bruno R. da Costa4,5
Bruno R. da Costa4,5 Nicole Sekarski
Nicole Sekarski Arnaud Chiolero
Arnaud Chiolero