Introduction
Approximately half a billion people live with diabetes mellitus (DM) worldwide. (1) Additionally, the prevalence of both DM and obesity, a major risk factor for type 2 DM, has doubled by 2015 compared to 1980 (2, 3). Given its high burden of morbidity and complications, DM has been ranked the ninth leading cause of death globally in 2019 (4). Furthermore, diabetes mellitus and obesity co-exist with a nexus of conditions, among others, hypertension, dyslipidemia, coronary artery disease (CAD), heart failure (HF), resulting in what is referred to as cardiometabolic syndrome (CMS) with a recent analysis revealing that half of type 2 DM patients suffers from at least three cardio-renal-metabolic conditions (5). On the other hand, patients with DM are over-represented in cardiology outpatient clinics, coronary care units, and cardiovascular disease (CVD) remains the leading cause of death within this population. (6) Besides, abnormal glucose regulation is underdiagnosed in the CVD population; a large observational study in Europe has demonstrated that patients with CVD had abnormal glucose regulation in about two-thirds of the cases (7). This situation with multiple systemic conditions impacts the clinical course, quality of life, and long-term survival of patients with DM. Therefore, there is a substantial necessity for cardiometabolic multidisciplinary clinics (CMC) as a forum of collaboration for multiple specialities to achieve optimal CMS management.
Cardiometabolic Health Care Economics
DM exhausted health care resources in 2019 with an expenditure of USD 760 billion (1). In addition, the effect of suffering from multiple simultaneous cardiometabolic morbidities on health expenditure has been widely researched with estimated projections that obese patients with three cardiovascular risk factors (CRF) have 14-fold annual health costs compared to obese patients with no CRF (USD 12,190 vs. 838) (8). Another meta-analysis of 383,420 individuals with one or more components of CMS (hypertension, diabetes, dyslipidaemia, adiposity) revealed an adjusted total annual health care cost of USD 5,564 per individual in patients with one component of CMS, while the cost of care for those with four components came to USD 12,287 (8). Nevertheless, early detection is essential in improving short- and long-term outcomes; it worth to be mentioned that in a recent analysis of the INTEGRATE randomised controlled trial, the implementation of primary care-based cardiometabolic risk prevention programs failed to show cost-efficacy in the long-term (9). In the light of the above-mentioned economic burden of CMS, there remains a need for a new comprehensive model that will foster more effective interventions with sustained health effects at reasonable costs.
Challenging CMS Patient Management in a Fragmented Consultative Model
In the current dominating consultative model of care, ease of access to health services in networked systems has facilitated patient referrals. Subsequently, patients see more than one healthcare professional, resulting in a significant economic burden reflected by high reimbursement rates (10). Such impact is more pronounced in CMS, in which several obstacles can be identified. First, there is no centralisation of care associated with one physician or clinic, as patients may rotate between multiple providers (primary care practitioner, endocrinologist, cardiologist), with a considerable overlapping in CRF management (11). This leads to interference in deciding the optimal drug class, dosing, drug interactions and may confuse patients receiving misaligned recommendations from multiple providers, resulting in ineffective follow-ups, and even eroding trust, especially when inter-provider communication is lacking (11).
Furthermore, approaching such patients from a single speciality perspective and not overviewing the complex picture may result in underestimating adverse outcomes and long-term complications of DM, as the risk of CVD is increased by two to four folds (12). When it comes to acute coronary syndrome and HF patients, DM and co-existing comorbidity management in a patient-centred fashion is vital. Other essential aspects of cardiometabolic therapy, such as lifestyle modification and psychological counselling, are inadequately addressed in the conventional model of care (11, 13). As a result, CMS patients receive fragmented care featured by redundant diagnostics at higher costs and are at risk of drug-drug interactions and, most importantly, adverse CVD events (11).
The Role of Cardiometabolic Clinics in Eliminating Current Practice Gaps
Despite the strong evidence supporting the efficacy of current CV preventive therapies in avoiding adverse events and improving survival, there is still a lack of adherence to these agents, causing inadequate control of major CRF (14, 15). Evidence has revealed that therapeutic targets of the major three components of CMS (hypertension, hyperlipidemia, diabetes mellitus) were simultaneously achieved only in 7-19% of the patients (16, 17).
On health care professional level, a recent USA based data describing how frequent type 2 DM patients visit their health providers showed a two-fold higher rate of cardiology follow-ups vs. endocrinology reviews among type 2 DM patients and that increased even up to 4 folds in CMS patients, highlighting the possible contribution that cardiology specialists can make in prescribing guideline-directed therapies, especially, those with cardio-metabolic-renal merits (18). However, this contribution is still limited in prescribing FDA-approved sodium-glucose co-transporter (SGLT-2i) inhibitors since cardiologists are responsible for only 5% of prescriptions, according to a recent retrospective study in Massachusetts, USA (19). A recent study featured a 3-fold increase in the prescription of glucagon-like peptide-1 receptor agonists (GLP-1RA) in the USA between 2014 and 2019; however, cardiologists contributed the least to this increase (<1% each year) (20). Another USA insurance database analysis highlighted that administration of antihyperglycemic drugs with CV benefits (SGLT-2i, GLP-1RA) has increased between 2014 and 2019 for CVD patients, yet underutilised, and these drug applications were more likely in younger with higher socioeconomic status compared to administration of metformin (21). Overall, the current adoption of cardio-beneficial glucose-lowering agents is limited, and <20% of patients with DM and atherosclerotic cardiovascular burdens were prescribed such agents, according to a large cohort study (22). On top of that, many studies have demonstrated the cost-effectiveness of these new agents; according to a recent systematic review, these new antihyperglycemic agents were reported to be cost-effective in 26 of 30 studies compared with insulin, and 13 of 15 studies compared with sulfonylureas (23). In patients with HF, a recent study reported that dapagliflozin provided an intermediate value (mainly driven by a reduction in cardiovascular mortality) compared to the standard of care which includes appropriate treatment with angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or sacubitril-valsartan plus a β-blocker (24). These underutilised and cost-effective agents emphasise the importance of reconsidering the current siloed care model and a shift to a multidisciplinary clinic.
Preliminary data regarding the initiation of such multidisciplinary clinic have revealed that SGLT-2 inhibitors or GLP-1 agonists were initiated/up-titrated in half of the patients, resulting in an effective reduction in HbA1c levels in one-third at the follow-up visit (25).
Another experience of a developing cardiometabolic centre of excellence in the USA has demonstrated high efficacy within a short timeframe in comparison to the conventional model of care, reflected by a higher rate of guideline-directed medical therapy reception, defined as a high-intensity statin, antiplatelet or anticoagulant, ACE inhibitor/ARB, and either SGLT-2i or GLP-1RA (41.1% vs. 2.3%). A higher rate of administration of the following was experienced, including ACE inhibitor (30.2% vs. 9.1%), high-intensity statins (86% vs. 77.7%), and SGLT-2i or GLP-1RA (96.1% vs. 25.7%) in patients managed at the CMC as compared to propensity-matched group managed in the conventional care settings (15). In addition, patients enrolled in the CMC had a greater reduction of weight (−10.9 vs. −1.5 lbs, p<0.001), HbA1c (−0.5% vs. −0.2%, p=0.02), systolic blood pressure (−3.6 vs. +1.4 mmHg, p<0.01), LDL level (12.1 vs. −2.8 mg/dL, p<0.01), and total daily insulin dose (−31.6 vs.+1.1 units, p<0.001) as compared with the conventional care group (15).
The complexity of managing obesity, a chronic and relapsing disease that impairs metabolism and CV health, and challenges traditional primary care practices that have been established to treat simple or less complicated conditions, necessitates the need for more efficient multidisciplinary interventions (26, 27). In the view of lifestyle interventions efficacy in obesity and associated CRF management, guidelines strongly recommend a combination of lifestyle interventions and medical treatment for patients who are overweight (26, 28–30). A recent cluster-randomized trial by Höchsmann et al. compared obesity intensive lifestyle interventions vs. usual care. Patients who received intensive lifestyle interventions lost more weight at 24 months (mean difference, −4.51% [95% CI, −5.93 to −3.10]; p<0.01), achieved better fasting glucose control at 12 months (mean change, −7.1 mg/dL [95% CI, −12.0 to −2.1]; p<0.01), and had higher levels of high-density lipoprotein cholesterol at 24 months (mean difference at 24 months: 4.6 mg/dL [95% CI, 2.9–6.3]; p<0.01) in comparison to those receiving standard care (30). However, the rate of long-term adherence to such lifestyle modifications is still low (26). Other lifestyle modifications, such as smoking cessation programs, have proven short-term efficacy in improving glycemic control and CRF in patients with type 2 DM (31).
Dietary patterns such as Mediterranean or DASH (dietary approach to stop hypertension) diet have been widely advocated and are associated with superior control of CRF (32). Additionally, recently, medical nutrition therapy (MNT) interventions have been proven effective in patients with type 2 DM (33). Given the limited access to effective lifestyle interventions in the current model, offering lifestyle counselling in a multi-speciality clinic will facilitate the implementation of such strategies and surely improve patient outcomes (13).
Finally, an important implication of the multidisciplinary model in the CMC is to enhance screening and treatment of obstructive sleep apnea (OSA), given its high prevalence among cardiometabolic patients (∼60%), the evidence of improved patient-centred outcomes and quality of life with OSA treatment in patients with CVD, and last but not least, OSA independent association with high levels of glucose and triglyceride levels in addition to markers of inflammation, arterial stiffness, and atherosclerosis (34, 35).
The Multidisciplinary Cardiometabolic Clinic Model
The desired CMC consists of multidisciplinary staff members, including cardiologists, endocrinologists, clinical pharmacists, specialised nursing staff, nutritionists, exercise physiologists, behavioural specialists, and genetic counsellors. Nurse practitioners with dedicated training in the field play a crucial role in the CMC hence being responsible for surveillance for CRF, electronic medical record follow-up, regular vital parameter assessment, discussing laboratory and imaging outcomes with the patients, and supporting physicians coordinating inter-disciplinary therapeutic plans and effective appointment scheduling (32). Evidence revealed that diabetes and CVD care quality provided by specialised nurse practitioners was comparable to physician-based care, and it is established that a nurse navigator role is essential for the success of CMC (15, 36).
A multitude of services can be provided in the CMC to address risk factors, diagnose, perform multiple types of prevention and manage these patients depending on disease stage and therapeutic goals (Figure 1) (32, 37). In case the patient is at high risk or in the early disease stage, the care would include non-invasive CAD testing (echocardiography, stress testing, and coronary computed tomography), cardio-preventive and weight loss medications administration, behavioural counselling in addition to diet and lifestyle interventions (high fibre/vegan diet, aerobic training and smoking cessation) (32, 37, 38). The care for advanced CMS patients would extend to managing CAD and HF, improving quality of life, reducing symptoms, and disability burdens. In addition, careful clinical assessment, avoiding over-medicalization, and unnecessary harmful interventions are paramount.
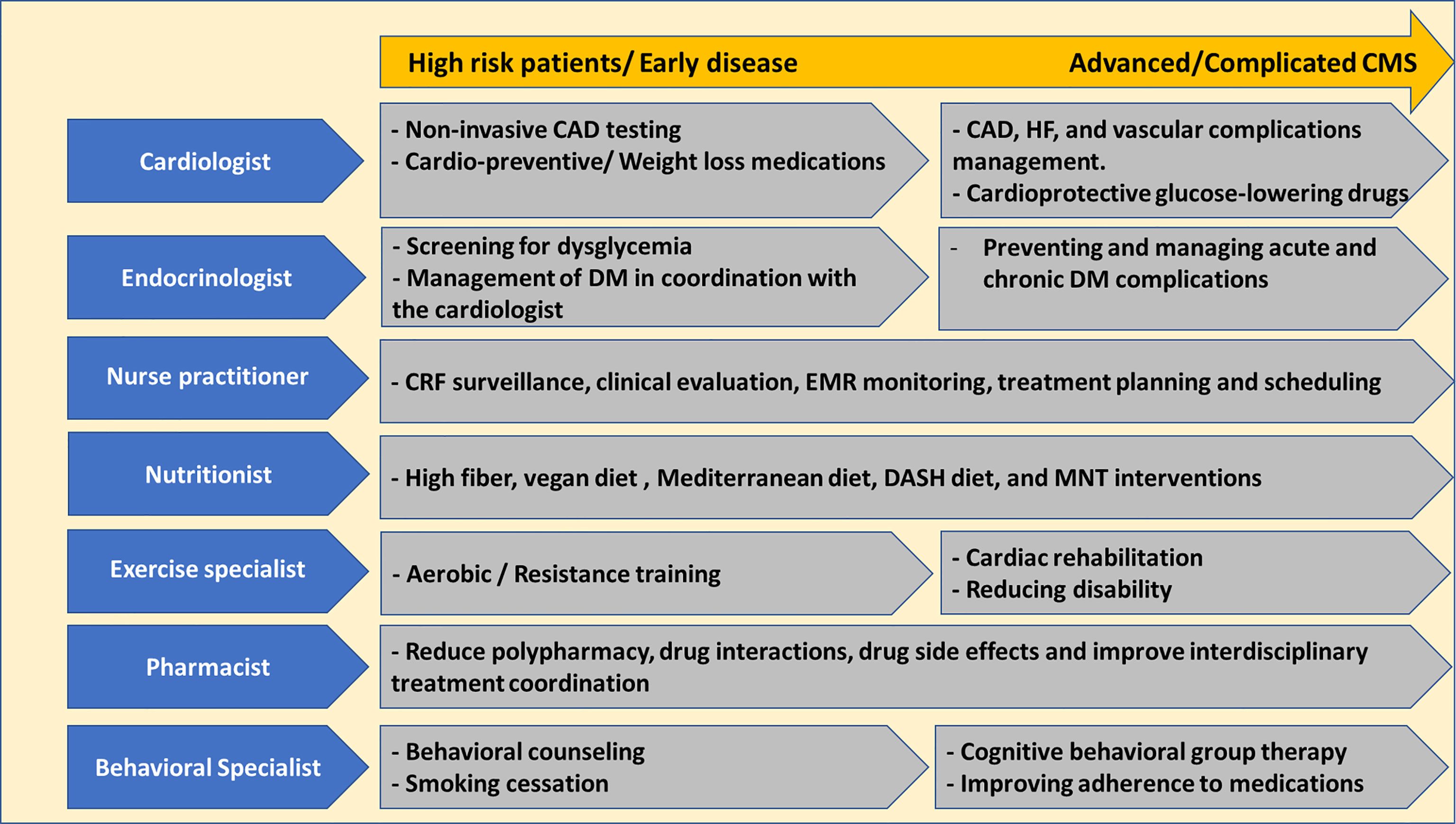
Figure 1 Multimodal services offered at the Cardiometabolic Clinic. CAD, coronary artery disease; CMS, cardiometabolic syndrome; CRF, cardiovascular risk factors; DASH, dietary approach to prevent hypertension; DM, diabetes mellitus; EMR, electronic medical records; MNT, medical nutrition therapy.
Challenges and Future Aims
Currently, the successful implementation of the CMC model is still limited. Financial costs, an excessive number of patients, and a shortage of specialised health care professionals are among the main obstacles towards the enforcement of the CMC model (11). Therefore; several strategies are suggested to enhance CMC adoption, starting by promoting for the success of such programs around the world to replicate further and implement such strategies, featuring cardiometabolic medicine on all levels of medical training (medical school, residency, fellowships). An additional layer of postgraduate development courses in cardiometabolic medicine or even board certification in cardiometabolic and lifestyle medicine is warranted by professional societies such as the American Diabetic Association and the American College of Lifestyle Medicine for health care providers to update the knowledge on this rapidly evolving medical field (39).
Author Contributions
YM and WM joint authors. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Williams R, Karuranga S, Malanda B, Saeedi P, Basit A, Besançon S, et al. Global and Regional Estimates and Projections of Diabetes-Related Health Expenditure: Results From the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res Clin Pract (2020) 162:1–16. doi: 10.1016/j.diabres.2020.108072
3. Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, et al. Health Effects of Overweight and Obesity in 195 Countries Over 25 Years. N Engl J Med. (2017) 377(1):13–27. doi: 10.1056/NEJMoa1614362
4. WHO. K Facts of Diabetes Mellitus. In: Diabetes. World Health Organization [Internet] (2017). p. 2. Available at: www.Who.Int.
5. Arnold SV, Kosiborod M, Wang J, Fenici P, Gannedahl G, LoCasale RJ. Burden of Cardio‐Renal‐Metabolic Conditions in Adults With Type 2 Diabetes Within the Diabetes Collaborative Registry. Diabetes Obes Metab (2018) 20(8):2000–3. doi: 10.1111/dom.13303
6. Kosiborod M. Hyperglycemia in Acute Coronary Syndromes: From Mechanisms to Prognostic Implications. Endocrinol Metab Clin North Am (2018) 47(1):185–202. doi: 10.1016/j.ecl.2017.11.002
7. Bartnik M, Rydén L, Ferrari R, Malmberg K, Pyörälä K, Simoons M, et al. The Prevalence of Abnormal Glucose Regulation in Patients With Coronary Artery Disease Across Europe The Euro Heart Survey on Diabetes and the Heart. Eur Heart J (2004) 25:1880–90. doi: 10.1016/j.ehj.2004.07.027
8. Kelli HM, Kassas I, Lattouf OM. Cardio Metabolic Syndrome: A Global Epidemic. J Diabetes Metab (2015) 6(3):2–14. doi: 10.4172/2155-6156.1000513
9. Stol DM, Over EAB, Badenbroek IF, Hollander M, Nielen MMJ, Kraaijenhagen RA, et al. Cost-Effectiveness of a Stepwise Cardiometabolic Disease Prevention Program: Results of a Randomized Controlled Trial in Primary Care. BMC Med (2021) 19(1):57. doi: 10.1186/s12916-021-01933-6
10. Shi L. The Impact of Primary Care: A Focused Review. Scientifica (Cairo) (2012) 2012:1–22. doi: 10.6064/2012/432892
11. Chang L-S, Vaduganathan M, Plutzky J, Aroda VR. Bridging the Gap for Patients With Diabetes and Cardiovascular Disease Through Cardiometabolic Collaboration. Curr Diabetes Rep (2019) 19(12):157. 10.1007/s11892-019-1260-0.
12. Bertoluci MC, Rocha VZ. Cardiovascular Risk Assessment in Patients With Diabetes. Diabetol Metab Syndr (2017) 9:25. doi: 10.1186/s13098-017-0225-1
13. Carvajal R, Wadden TA, Tsai AG, Peck K, Moran CH. Managing Obesity in Primary Care Practice: A Narrative Review. Ann N Y Acad Sci (2013) 1281(1):191–206. doi: 10.1111/nyas.12004
14. Gaede P, Lund-Andersen H, Parving H-H, Pedersen O. Effect of a Multifactorial Intervention on Mortality in Type 2 Diabetes. N Engl J Med (2008) 358(6):580–91. doi: 10.1056/NEJMoa0706245
15. Thomas M, Magwire M, Gosch K, Sammour Y, Mehta R, O’Keefe J, et al. Cardiometabolic Center of Excellence: A Novel Care Delivery Model for Secondary Prevention of Cardiovascular Disease in Type 2 Diabetes. Circulation. Cardiovasc Qual Outcomes (2021) 0(0):CIRCOUTCOMES.120.007682. doi: 10.1161/CIRCOUTCOMES.120.007682
16. Bakke Å, Dalen I, Thue G, Cooper J, Skeie S, Berg TJ, et al. Variation in the Achievement of HbA1c, Blood Pressure and LDL Cholesterol Targets in Type 2 Diabetes in General Practice and Characteristics Associated With Risk Factor Control. Diabetic Med (2020) 37(9):1471–81. doi: 10.1111/dme.14159
17. Camara S, Bouenizabila E, Hermans MP, Ahn SA, Rousseau MF. Novel Determinants Preventing Achievement of Major Cardiovascular Targets in Type 2 Diabetes. Diabetes & Metabolic Syndrome. Clin Res Rev (2014) 8(3):145–51. doi: 10.1016/j.dsx.2014.04.037
18. Gunawan F, Nassif ME, Partridge C, Ahmad T, Kosiborod M, Inzucchi SE. Relative Frequency of Cardiology vs. Endocrinology Visits by Type 2 Diabetes Patients With Cardiovascular Disease in the USA: Implications for Implementing Evidence-Based Use of Glucose-Lowering Medications. Cardiovasc Endocrinol Metab (2020) 9(2):56–9. doi: 10.1097/XCE.0000000000000195
19. Vaduganathan M, Sathiyakumar V, Singh A, McCarthy CP, Qamar A, Januzzi JL, et al. Prescriber Patterns of SGLT2i After Expansions of U.S. Food and Drug Administration Labeling. J Am Coll Cardiol (2018) 72(25):3370–2. doi: 10.1016/j.jacc.2018.08.2202
20. Kosiborod M, Aroda VR, Broe Honore J, Husemoen LLN, Jensen AB, Matthiessen KS, et al. Trends in Initiation of GLP-1 RA in Patients With Type 2 Diabetes During 2014–2019: A US Database Study. Eur Heart J (2021) 42:2957(Supplement_1). doi: 10.1093/eurheartj/ehab724.2957
21. Shin H, Schneeweiss S, Glynn RJ, Patorno E. Trends in First-Line Glucose-Lowering Drug Use in Adults With Type 2 Diabetes in Light of Emerging Evidence for SGLT-2i and GLP-1ra. Diabetes Care (2021) 44(8):1774–82. doi: 10.2337/dc20-2926
22. Arnold SV, de Lemos JA, Rosenson RS, Ballantyne CM, Liu Y, Mues KE, et al. Use of Guideline-Recommended Risk Reduction Strategies Among Patients With Diabetes and Atherosclerotic Cardiovascular Disease. Circ (2019) 140(7):618–20. doi: 10.1161/CIRCULATIONAHA.119.041730
23. Hong D, Si L, Jiang M, Shao H, Ming W, Zhao Y, et al. Cost Effectiveness of Sodium-Glucose Cotransporter-2 (SGLT2) Inhibitors, Glucagon-Like Peptide-1 (GLP-1) Receptor Agonists, and Dipeptidyl Peptidase-4 (DPP-4) Inhibitors: A Systematic Review. Pharmacoecon (2019) 37(6):777–818. doi: 10.1007/s40273-019-00774-9
24. Parizo JT, Goldhaber-Fiebert JD, Salomon JA, Khush KK, Spertus JA, Heidenreich PA, et al. Cost-Effectiveness of Dapagliflozin for Treatment of Patients With Heart Failure With Reduced Ejection Fraction. JAMA Cardiol (2021) 6(8):926–35. doi: 10.1001/jamacardio.2021.1437
25. Bijman L, Narain R, Chen M, Joshi H. 197 Introduction of a Multidisciplinary Cardiac Metabolic Clinic in a UK Tertiary Cardiology Centre: Early Activity, Interventions and Potential for Cardiovascular Risk Optimisation. Heart (2021) 107:A152–3.
26. Tak YJ, Lee SY. Long-Term Efficacy and Safety of Anti-Obesity Treatment: Where Do We Stand? Curr Obes Rep (2021) 10(1):14–30. doi: 10.1007/s13679-020-00422-w
27. Frood S, Johnston LM, Matteson CL, Finegood DT. Obesity, Complexity, and the Role of the Health System. Curr Obes Rep (2013) 2(4):320–6. doi: 10.1007/s13679-013-0072-9
28. Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, et al. Effect of Weight Loss With Lifestyle Intervention on Risk of Diabetes. Diabetes Care (2006) 29(9):2102–7. doi: 10.2337/dc06-0560
29. Wadden TA, Neiberg RH, Wing RR, Clark JM, Delahanty LM, Hill JO, et al. Four‐year Weight Losses in the Look AHEAD Study: Factors Associated With Long‐Term Success. Obesity (2011) 19(10):1987–98. doi: 10.1038/oby.2011.230
30. Höchsmann C, Dorling JL, Martin CK, Newton RL, Apolzan JW, Myers CA, et al. Effects of a 2-Year Primary Care Lifestyle Intervention on Cardiometabolic Risk Factors. Circulation (2021) 143(12):1202–14. doi: 10.1161/CIRCULATIONAHA.120.051328
31. Chen H-J, Huang W-H, Chan H-L, Hwang L-C. Improvement in Cardiometabolic Risk Factors During Smoking Cessation Treatment in Patients With Type 2 Diabetes: A Retrospective Cohort Study. Diabetes, Metabolic Syndrome and Obesity. Targets Ther (2021) 14:1695. doi: 10.2147/DMSO.S303446
32. Reiter-Brennan C, Dzaye O, Davis D, Blaha M, Eckel RH. Comprehensive Care Models for Cardiometabolic Disease. Curr Cardiol Rep (2021) 23(3):1–1. doi: 10.1007/s11886-021-01450-1
33. Franz MJ, MacLeod J, Evert A, Brown C, Gradwell E, Handu D, et al. Academy of Nutrition and Dietetics Nutrition Practice Guideline for Type 1 and Type 2 Diabetes in Adults: Systematic Review of Evidence for Medical Nutrition Therapy Effectiveness and Recommendations for Integration Into the Nutrition Care Process. J Acad Nutr Diet (2017) 117(10):1659–79. doi: 10.1016/j.jand.2017.03.022
34. Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G. Obstructive Sleep Apnea: A Cardiometabolic Risk in Obesity and the Metabolic Syndrome. J Am Coll Cardiol (2013) 62(7):569–76. doi: 10.1016/j.jacc.2013.05.045
35. Yeghiazarians Y, Jneid H, Tietjens JR, Redline S, Brown DL, El-Sherif N, et al. Obstructive Sleep Apnea and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation (2021) 144(3):e56–67. doi: 10.1161/CIR.0000000000000988
36. Virani SS, Akeroyd JM, Ramsey DJ, Chan WJ, Frazier L, Nasir K, et al. Comparative Effectiveness of Outpatient Cardiovascular Disease and Diabetes Care Delivery Between Advanced Practice Providers and Physician Providers in Primary Care: Implications for Care Under the Affordable Care Act. Am Heart J (2016) 181:74–82. doi: 10.1016/j.ahj.2016.07.020
37. Mechanick JI, Farkouh ME, Newman JD, Garvey WT. Cardiometabolic-Based Chronic Disease, Addressing Knowledge and Clinical Practice Gaps: JACC State-of-the-Art Review. J Am Coll Cardiol (2020) 75(5):539–55. doi: 10.1016/j.jacc.2019.11.046
38. Talebi Amri M, Bahraminasab M, Samkhaniyan E, Moini F, Kazemi Khobane Z. Effectiveness of Behavioral-Cognitive Group Therapy on Improvement of Quality of Life of Patients With Coronary Heart Disease. J Med Life (2015) 8(Spec Iss 4):301–6.
Keywords: cardiometabolic, clinics, multidisciplinary, diabetes, obesity
Citation: Manla Y and Almahmeed W (2022) Cardiometabolic Clinics: Is There a Need for a Multidisciplinary Clinic? Front. Clin. Diabetes Healthc. 3:880468. doi: 10.3389/fcdhc.2022.880468
Received: 21 February 2022; Accepted: 29 March 2022;
Published: 06 June 2022.
Edited by:
Manfredi Rizzo, University of Palermo, ItalyReviewed by:
Laura Gaita, Victor Babes University of Medicine and Pharmacy, RomaniaKalliopi Pafili, German Diabetes Center (DDZ), Germany
Copyright © 2022 Manla and Almahmeed. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wael Almahmeed, V21haG1lZWRAZ21haWwuY29t
 Yosef Manla
Yosef Manla Wael Almahmeed
Wael Almahmeed